User login
Clinical management of patients with medical and social comorbidities has become increasingly complex.[1, 2, 3, 4] This complexity stems from lack of data for these groups of patients who are often excluded from clinical trials.[5] There are data demonstrating that older patients and patients with multiple comorbidities including diabetes, renal disease, obesity, limited mobility, and poor access to healthcare have worse outcomes for specific conditions compared to otherwise equal counterparts, and that the cost of care for these patients is more expensive.[3, 4, 6] Moreover, traditional risk assessment of disease severity and outcomes are not accurate when applied to medically and socially complex patients.[7, 8]
Treatment decisions regarding antibiotic use add additional complexity. Specifically, physicians antibiotic prescribing decisions can promote the emergence of multidrug resistant pathogens in a hospital or population.[9, 10] Multidrug resistant organisms (MDROs) are particularly problematic, as their prevalence is increasing while the development of new antimicrobial agents is declining.[11] Infections caused by antibiotic‐resistant pathogens are associated with increased morbidity and mortality and healthcare costs.[12, 13, 14] Antibiotic use, although potentially lifesaving, can also result in severe complications such as Clostridium difficile‐associated diarrhea, acute kidney injury, and anaphylaxis, among other adverse events, particularly in older patients with medical comorbidities.[15, 16, 17, 18] Judicious antibiotic use is critical to halt the epidemic of MDROs and to minimize antibiotic‐associated adverse effects.[19, 20, 21]
Evidence‐based guidelines have the potential to assist physicians in choosing the antibiotic that achieves the best clinical outcome for a specific infection or situation.[11] This includes using the narrowest spectrum agent to minimize selection pressure on microorganisms and avoiding unneeded drugs to minimize adverse drug effects.[9, 11] Importantly, guideline adherence regarding antibiotic selection has been shown to be associated with increased clinical success and decreased mortality.[22, 23] Unfortunately, 30% to 50% of antibiotic use in hospitalized patients is inconsistent with national guidelines.[24, 25, 26] Reasons for physicians ordering of tests and treatments inconsistent with guidelines are not fully understood, and potentially include patient and physician factors, and the cultural and social context of the healthcare system.[27]
To optimize the use of antibiotics, it is important to understand how medical complexities (defined as demographic, comorbid, and limited healthcare access characteristics that are associated with suboptimal patient care and outcomes) influence physicians antibiotic prescribing practices.[28] We created 3 clinical vignettes for common diagnoses (dyspnea with initial concern for pneumonia, skin and soft tissue infection, and asymptomatic bacteriuria) among hospitalized patients. We selected these conditions because of their high prevalence, frequent management by hospitalists, generalist physicians, and noninfectious disease specialists, and because well‐documented evidence suggests either no antibiotics or narrower spectrum antibiotics are usually the treatments of choice. Using the Infectious Diseases Society of America (IDSA) guidelines relevant to each clinical vignette,[29, 30, 31] we assessed physicians recommendations for guideline‐appropriate antibiotic management for patients without and with medical complexities using an electronic multiple‐choice survey.
METHODS
Survey Participants
We surveyed internal medicine generalist and subspecialty inpatient physicians from 3 academic medical centers in the metropolitan Los Angeles, California area. Potential participants included attending and housestaff physicians in the departments of internal medicine and family medicine at the 3 medical centers associated with the University of California Los Angeles (UCLA) Clinical and Translational Science Institute: (1) Ronald ReaganUCLA Medical Center, a tertiary care academic medical center; (2) Harbor UCLA Medical Center, a county (public) medical center; and (3) CedarsSinai Medical Center, a tertiary care medical center. Each center was affiliated with a residency training program, although not all attending physicians were associated with the training programs. Physicians were eligible to perform the survey if they attended 2 weeks per year in the inpatient setting. We collected physician‐level information including level of training (resident/fellow vs attending), specialization or not, proportion of time spent working in the hospital, and proportion of time spent providing direct clinical care (compared to activities such as administration and research). All eligible participants were emailed a brief study description with a hyperlink to the electronic survey created in REDCap (Research Electronic Data Capture version 5.6.0, 2013). Administrative staff provided email lists for potential participants at 2 of the hospitals. Per hospital policy, an email list was not provided by the third hospital, and potential participants were emailed the survey link directly by the hospital administrative staff. We incentivized study participation by entering participants who completed the survey into a raffle to win either a $100 gift card or a computer tablet. Physicians had 3 months to complete the survey and were sent up to 5 emails encouraging them to complete it.
Survey
The survey consisted of 3 clinical vignettes describing common hospital‐based situations that required decision making about antibiotic use. The 3 clinical vignettes described: (1) a patient with dyspnea and no infiltrate on chest radiograph who is initially treated empirically with antibiotics for pneumonia but is ultimately diagnosed with a congestive heart failure exacerbation, (2) a patient admitted with a skin infection that grows methicillin‐sensitive Staphylococcus aureus, and (3) a patient with a urinary catheter who develops asymptomatic bacteriuria. The first vignette was chosen because congestive heart failure and pneumonia are among the most common reasons for hospitalization in the United States, and their overlapping syndromes can make the diagnosis challenging.[32, 33, 34, 35] The second vignette was chosen because skin infections are some of the most common infectious diseases, with an incidence that is twice that of urinary tract infections and 10 times that of pneumonia, and can lead to serious complications among hospitalized patients.[30, 36] The third vignette was chosen because the prevalence of asymptomatic bacteriuria approaches 100% among catheterized patients, and rates of unnecessary treatment for this condition are as high as 80%.[31, 37]
Each clinical vignette was then modified to include 1 of the 4 studied patient complexities (Table 1). Medical comorbidities were represented by modifying the baseline vignette to describe patients with poorly controlled diabetes, morbid obesity, chronic kidney disease, and/or heavy tobacco use (Table 2). Patients with poor functional status were described in the vignettes as having difficulty with ambulation, requiring a mobility device, or needing assistance with self‐care (Table 2). Clinical vignettes varied the age of the patient from 47 years in the baseline case to 86 years (Table 2). Patients expected to have limited postdischarge follow‐up were described as being uninsured, with the first available follow‐up occurring in a public clinic no sooner than 2 weeks after their hospital discharge (Table 2). These complexities were chosen because they are common among hospitalized patients and have been shown to be associated with worse outcomes in a variety of conditions.[38, 39, 40, 41, 42] The asymptomatic bacteriuria vignette did not have a question about limited postdischarge follow‐up, because the clinical decision making in this question only pertained to the initial diagnosis and management. All physicians were queried about antibiotic use in each of the 3 baseline vignettes and in the subsequent 4 modified vignettes for each baseline scenario, with each physician making antibiotic management decisions for 15 vignettes in total. Physicians responses were recorded using categorical responses describing treatment options.
|
| Dyspnea case (baseline scenario) |
| A 47‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath and a nonproductive cough. In the ED, he had a temp 99.6F, a HR of 120, and an RR 30. Exam was notable for bilateral crackles. CXR on admission was interpreted as having cardiomegaly, bilateral base atelectasis versus infiltrate and prominent pulmonary arteries with cephalization consistent with cardiogenic pulmonary edema. Admission laboratories were notable for an elevated BNP (950 pg/mL) and WBC (11.5 cells 10*9/L) with 75% neutrophils. Troponin and CK‐MB were not elevated. The patient was treated with diuretics, ceftriaxone, and azithromycin, and over the course of the next 48 hours has improved shortness of breath and no fevers. Because of his improvement he is prepared for hospital discharge. A repeat chest x‐ray shows cardiomegaly and bibasilar atelectasis. Blood cultures have been negative. He is insured and can follow with his primary physician within 3 days of discharge. Upon discharge you: |
| A. Discharge on his usual cardiac medications. |
| B. Discharge on his cardiac medications plus azithromycin to complete a 5‐day course of antibiotics. |
| C. Discharge on his cardiac medications plus azithromycin to complete a 7‐day course of antibiotics. |
| D. Discharge on usual cardiac medications plus levofloxacin to complete a 7‐day course of antibiotics. |
| Skin infection case (baseline scenario) |
| A 47‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. Admission temperature was 101.4F. In the ED, she was given vancomycin, and piperacillin/tazobactam and underwent incision and drainage of a thigh abscess, which drained a large amount of pus. The wound was packed. On day 3 she is afebrile, and the pain has improved. Cultures from the wound grow methicillin‐susceptible Staphylococcus aureus. Blood cultures are negative to date. She is ready for discharge. She has no allergies. She is insured and can be followed up with her primary physician within a few days of discharge. You: |
| A. Discharge on vancomycin and piperacillin/tazobactam through a PICC line to complete a 10‐day course. |
| B. Discharge on amoxicillin/clavulanate orally to complete a 10‐day course. |
| C. Discharge on cephalexin to complete a 10‐day course. |
| Asymptomatic bacteriuria case (baseline scenario) |
| A generally healthy 47‐year‐old female is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On post operative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. Labs from that day show a WBC of 8900 and a urinalysis with 12 WBCs/HPF and 2+ bacteria. She has surgical site discomfort and no dysuria or other lower urinary tract symptoms. She has not had a fever during her hospital stay. Two days later her urine culture grows 100,000 CFU/mL of Escherichia coli susceptible to ciprofloxacin but resistant to all other oral antibiotics. That day, the patient is still afebrile and has no dysuria or other lower urinary tract symptoms. Her Foley is changed and a repeat urinalysis shows that the urine has persistent leukocytes and bacteria. You: |
| A. Initiate intravenous ciprofloxacin. |
| B. Initiate oral ciprofloxacin. |
| C. Give no antibiotics. |
| |
| Dyspnea case | |
| Comorbidities | A 47‐year‐old male with a history of stage III congestive heart failure, moderate to heavy tobacco use, poorly controlled type 2 diabetes (last HbA1C=10.9), chronic renal insufficiency (baseline creatinine of 1.3), diabetic retinopathy, and diabetic neuropathy is hospitalized after presenting with shortness of breath. |
| Poor functional status | A 47‐year‐old male with a history of stage III congestive heart failure, and poor functional status with difficulty ambulating and with self‐care is hospitalized after presenting with shortness of breath. |
| Older age | An 86‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath. |
| Limited follow‐up | A 47‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath.Blood cultures have been negative. He is uninsured and will be referred for follow‐up to a public clinic, which has a 2‐week wait for the next available clinic appointment. |
| Skin infection case | |
| Comorbidities | A 47‐year‐old morbidly obese female (BMI=32.9) with type 2 diabetes is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Poor functional status | A 47‐year‐old obese female (BMI=32.9) and poor functional status due to her obesity (poor mobility, uses either a walker or an electric scooter at all times to move around) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Older age | An 86‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Limited follow‐up | A 47‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. She is uninsured and will be referred for follow‐up in a public clinic, which has a 2‐week wait for the next available clinic appointment. |
| Asymptomatic bacteriuria case | |
| Comorbidities | A 47‐year‐old female with a history type 2 diabetes with diabetic nephropathy and retinopathy is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On postoperative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
| Poor functional status | A 47‐year‐old female with a history of poor functional status (needs assistance with activities of daily living) due to her obesity and musculoskeletal comorbidities, such as osteoarthritis, is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On postoperative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
| Older age | A generally healthy 86‐year‐old female is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On post operative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
The institutional review boards at all 3 medical centers approved the study.
Statistical Analysis
We used physician self‐report of recommended antibiotic use stratified by baseline and modified clinical vignettes to calculate the proportion of physicians recommending antibiotic use. We examined how physicians antibiotic use varied according to both patient complexity (medical comorbidity, poor functional status, older age, and limited follow‐up after hospital discharge), and provider characteristics (level of training, degree of specialization, time spent working in the hospital, and time spent providing direct patient care).
Data were analyzed using SAS (version 9.1.3; SAS Institute, Cary, NC). Antibiotic use inconsistent with IDSA guideline recommendations was considered the outcome of interest for the data analysis. Statistical analyses were performed using [2] or Fischer exact test, Student t test, and analysis of variance, as appropriate.
RESULTS
Physician Survey
Of the 874 invited physicians, 255 (29%) responded to the survey. Of these, 8/255 (3.1%) responded that they did not spend 2 or more weeks in the inpatient setting and were thus ineligible for the survey. We analyzed data from the remaining 247 physician respondents. Most respondents (217/233, 93%) reported their primary role was direct clinical care (Table 3). Most (185/241, 77%) reported at least half of their clinical work occurred in the hospital. Approximately three‐quarters (183/241, 76%) of the respondents were residents; the remaining respondents were attending physicians (57/241, 24%). Almost half of attending physicians (46%) were internal medicine subspecialists.
| Physician Characteristic | No. (%) Completing the Survey | % of Physicians Not Adhering to Guidelines in Baseline Scenarios | P Value |
|---|---|---|---|
| |||
| Affiliated medical center, n =241 | |||
| Ronald Reagan UCLA | 47 (20%) | 37% | 0.37 |
| Harbor‐UCLA | 106 (44%) | 41% | |
| Cedars‐Sinai | 86 (35%) | 43% | |
| Primary professional activity, n=233 | |||
| Direct clinical care/teaching | 217 (93%) | 42% | 0.90 |
| Research/administration | 16 (7%) | 27% | |
| Percent of clinical duties in the hospital, n=241 | |||
| 1%25% | 57 (23%) | 41% | 0.71 |
| 51%75% | 93 (39%) | 42% | |
| 76%100% | 92 (38%) | 41% | |
| Level of training and subspecialization, n=241 | |||
| Resident/fellow | 183 (76%) | 43% | 0.05 |
| Attending | 58 (24%) | 34% | |
| Subspecialist | 27 (47%) | 34% | 0.90 |
| Hospitalist | 28 (48%) | 33% | |
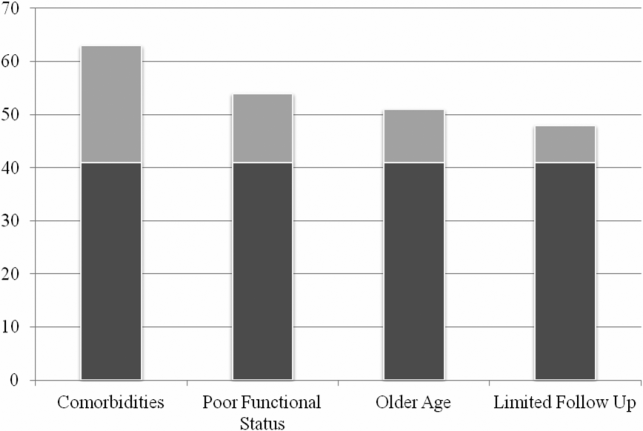
Physician recommendation for the use of antibiotics inconsistent with IDSA guidelines was prevalent in the baseline vignettes: 42% (303/729) overall, and 49% (120/246) for the dyspnea, 28% (68/242) for the skin infection, and 48% (115/241) for the asymptomatic bacteriuria cases. When the vignettes were modified to include patient complexities, the proportion of physicians recommending antibiotics increased significantly compared to the baseline vignette (63% (459/728), 54% (393/728), 51% (371/728), and 48% (232/487) for medical comorbidities, poor functional status, older age, and limited follow‐up respectively, P<0.001 for all comparisons) (Figure 1). The increase in the proportion of physicians recommending antibiotics inconsistent with guidelines for patients with medical complexities was the same when stratified by case (data not shown).

We found no association between provider characteristics (medical center, degree of attending physician specialization, percentage of clinical time spent practicing hospital‐based medicine, or percentage of time providing direct clinical care) and prescribing antibiotics in the baseline vignettes (Table 3). However, resident physicians (n=183) were more likely than attending physicians (n=57) to have recommended antibiotics in the baseline vignettes (43% vs 34%, P<0.05) (Table 3) and in all 4 vignettes with patient complexities (data not shown).
DISCUSSION
In our survey, almost half of the physician respondents recommended antibiotics that were inconsistent with national guidelines in the baseline vignettes. One explanation for this finding is that physicians may underestimate the risk associated with antibiotic use, such as the emergence of antimicrobial resistant pathogens and drug‐associated adverse effects. Although physicians generally agree that antibiotic resistance is an important problem, many believe that it is not a prominent issue in their practice or through their antibiotic prescribing practices.[43, 44] Others have shown that physicians underestimate the risk and severity of antibiotic‐associated complications such as C difficile.[45, 46] An accurate assessment and heightened awareness of the risks associated with antibiotics is important in clinical decision making, and should potentially be included not only in antibiotic stewardship educational efforts, but in national guideline recommendations as well.
Our survey also demonstrated that the tendency of physician respondents to recommend antibiotics was amplified for patients with medical complexities. This suggests that patient characteristics related to medical and social complexities play an important role in physicians clinical decision making about prescribing antibiotics. Previous investigations have shown that when physicians are deciding whether or not to prescribe antibiotics, they tend to deprioritize guideline recommendations and give greater weight to the risk of disease progression and complications that might occur if antibiotics are withheld.[47, 48, 49] Physicians are believed to prescribe antibiotics for complex patients more often, in part, because complex patients are more likely to suffer bad outcomes if undertreated.[50, 51] Axiomatically, patients with medical complexities are also at higher risk for antibiotic‐associated adverse effects including polypharmacy, drug‐drug interactions, and more severe side effects.[52, 53, 54]
An additional factor contributing to the overuse of antibiotics in our survey could be the lack of clear guideline recommendations for antibiotic management, especially among patients with complexities. We reviewed 20 national guidelines that addressed the medical decision making relevant to the survey's 3 clinical vignettes (see Supporting Information, Appendix 1, in the online version of this article). Fifteen of the guidelines provided recommendations for antibiotic management in the baseline vignettes, though most of the recommendations were not explicit about stopping or de‐escalating antibiotics. Furthermore, when antibiotic recommendations were present, they often lacked supporting data for the recommendation. Guidelines were even less complete for patients with medical complexities. For the asymptomatic bacteriuria vignette, 4 of 6 guidelines provided recommendations for patients with medical comorbidities described in our survey's modified vignettes.[31, 55, 56, 57] None of the guidelines related to the dyspnea or skin infection vignette provided specific antibiotic recommendations for complex patients with medical comorbidities, poor functional status, older age, or limited follow‐up (see Supporting Information, Appendix 1, in the online version of this article). Given these findings, along with evidence that medically complex patients are more likely to receive antibiotics compared to their less complex counterparts, there is a need for subsequent guidelines to more explicitly recommend best antibiotic practices for patients with medical and social complexities.
We also found that resident physicians were significantly more likely to recommend antibiotics inconsistent with guidelines compared to attending physicians. Although the underlying explanations for this finding were not explored in this study, possibilities include a lack of familiarity with guideline recommendations, less comfort in discontinuing antibiotics in the setting of clinical uncertainty, and/or a preference to accept the risks of overtreatment over the risks of undertreatment.
There are limitations to our study. First, although previous investigations have shown a high degree of correlation between actual antibiotic prescribing practices and antibiotic prescribing decisions self‐reported in clinical vignettes, physician responses in our survey may not reflect actual practices.[58] Second, we were not able to directly measure how physicians knowledge and interpretation of national guidelines influenced their antibiotic management decisions in the survey. Third, while our study was multisite including physicians from 3 different centers, not all invited physicians responded. Because of the confidentiality procedures surrounding the email distribution lists of potential participants, we were unable to obtain additional details about the non‐responders. Finally, because the large majority of respondents were trainees (76%), the generalizability of our findings to attending physicians may be limited. Nevertheless, because residents soon become staff physicians, resident‐reported data supplemented by that from attending physicians seems relevant to identifying opportunities for improving medical care.
In conclusion, we found that a large proportion of physicians recommended antibiotics that were not indicated based on IDSA guidelines for 3 vignettes depicting common hospital‐based clinical scenarios. This pattern of physicians recommending antibiotics inconsistent with guidelines was accentuated with significantly higher reported use for patients with medical comorbidities, poor functional status, older age, and limited healthcare access. Although good clinical judgment requires increased monitoring of patients with medical complexities, it is important for clinicians not to conflate the need for increased patient monitoring with the need for increased antibiotic use. Additional studies and corresponding guideline recommendations for frail, complex patients could be instrumental in reducing frequent use of antibiotics and the resultant cost, adverse effects, and emergence of antibiotic resistant pathogens. Educational efforts, particularly among trainees, regarding appropriate antibiotic use for clinical indications among patients without and with medical complexities would also likely contribute to these aims. Treatment guidelines should consider explicitly addressing medically complex patients in the context of management of infectious syndromes.
Disclosure
Nothing to report.
- , , , , , . Heart failure and chronic obstructive pulmonary disease multimorbidity at hospital discharge transition: a study of patient and carer experience [published online ahead of print May 16. 2014]. Health Expect. doi: 10.1111/hex.12208.
- , , , , , . Development of clinical practice guidelines for patients with comorbidity and multiple diseases [in Spanish]. Aten Primaria. 2014;46(7):385–392.
- , , , et al. Cardiac complications in patients with community‐acquired pneumonia: a systematic review and meta‐analysis of observational studies. PLoS Med. 2011;8(6):e1001048.
- , , , , , . Cost and incidence of social comorbidities in low‐risk patients with community‐acquired pneumonia admitted to a public hospital. Chest. 2003;124(6):2148–2155.
- , , , et al. Why do GPs exclude patients from participating in research? An exploration of adherence to and divergence from trial criteria. Fam Pract. 2014;31(3):364–370.
- , , , , . The impact of pre‐existing heart failure on pneumonia prognosis: population‐based cohort study. J Gen Intern Med. 2008;23(9):1407–1413.
- , , , et al. Severe community‐acquired pneumonia: use of intensive care services and evaluation of American and British Thoracic Society Diagnostic criteria. Am J Respir Crit Care Med. 2002;166(5):717–723.
- . Decisions about treating community‐acquired pneumonia. Ann Intern Med. 2005;142(3):215–216.
- , , , et al. The epidemic of antibiotic‐resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(2):155–164.
- Will antibiotic misuse now stop? Nat Rev Microbiol. 2003;1(2):85.
- , , , et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177.
- . Economic impact of antimicrobial resistance. Emerg Infect Dis. 2001;7(2):286–292.
- , , , , . Guideline‐concordant therapy and reduced mortality and length of stay in adults with community‐acquired pneumonia: playing by the rules. Arch Intern Med. 2009;169(16):1525–1531.
- , , , et al. Adherence to guidelines' empirical antibiotic recommendations and community‐acquired pneumonia outcome. Eur Respir J. 2008;32(4):892–901.
- , , , et al. Community‐associated Clostridium difficile infection and antibiotics: a meta‐analysis. J Antimicrob Chemother. 2013;68(9):1951–1961.
- , , , . Vancomycin‐induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. 2012;68(9):1243–1255.
- , . Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin Rev Allergy Immunol. 2013;45(1):131–142.
- , . Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol. 2011;72(3):381–393.
- , , , et al. A clinician's guide to the appropriate and accurate use of antibiotics: the Council for Appropriate and Rational Antibiotic Therapy (CARAT) criteria. Am J Med. 2005;118(suppl 7A):1S–6S.
- , , , , . Evaluation of rational antibiotic use. Int J Antimicrob Agents. 2000;15(2):131–135.
- , . Rational antibiotic prescribing. Challenges and successes [in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55(11–12):1418–1426.
- , , , et al. Antibiotic prescription for community‐acquired pneumonia in the intensive care unit: impact of adherence to Infectious Diseases Society of America guidelines on survival. Clin Infect Dis. 2005;41(12):1709–1716.
- , , . Guideline‐adherent initial intravenous antibiotic therapy for hospital‐acquired/ventilator‐associated pneumonia is clinically superior, saves lives and is cheaper than non guideline adherent therapy. Eur J Med Res. 2011;16(7):315–323.
- , , , , , . Antibiotic misuse: a prospective clinical audit in a French university hospital. Eur J Clin Microbiol Infect Dis. 2007;26(4):277–280.
- , , , , . Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163(8):972–978.
- , , , . A systematic review and meta‐analysis of misuse of antibiotic therapies in the community. Int J Antimicrob Agents. 2005;26(2):106–113.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. The complexity of care for patients with rheumatoid arthritis: metrics for better understanding chronic disease care. Med Care. 2007;45(1):55–65.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52.
- , , , , , . Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–654.
- , , , et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215.
- , , , et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29(19):2388–2442.
- , , , . Prevalence of negative chest radiography results in the emergency department patient with decompensated heart failure. Ann Emerg Med. 2006;47(1):13–18.
- , , , . Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117(5):305–311.
- , , , et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005–2010. BMC Infect Dis. In press.
- . Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2012;9(2):85–93.
- , , , et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312(12):1218–1226.
- , , , . Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814.
- , , , , . Frailty in elderly people. Lancet. 2013;381(9868):752–762.
- , , , , , . Risk factors for death in homeless adults in Boston. Arch Intern Med. 1998;158(13):1454–1460.
- . Taking it to the streets: homelessness, health, and health care in the United States. J Gen Intern Med. 2003;18(11):964–965.
- , , , , , . Antibiotic resistance: a survey of physician perceptions. Arch Intern Med. 2002;162(19):2210–2216.
- , , , et al. Primary care clinicians' perceptions of antibiotic resistance: a multi‐country qualitative interview study. J Antimicrob Chemother. 2013;68(1):237–243.
- , , , , , . Underestimation of Clostridium difficile infection among clinicians: an international survey. Eur J Clin Microbiol Infect Dis. 2012;31(9):2439–2444.
- , , , , , . Unnecessary antimicrobial use in patients with current or recent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2013;34(2):109–116.
- , , , , . Attitudes of primary care physicians to the prescribing of antibiotics and antimicrobial resistance: a qualitative study from Spain. Fam Pract. 2012;29(3):352–360.
- , , , , . Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents. 2013;41(3):203–212.
- , , , . Guidelines on uncomplicated urinary tract infections are difficult to follow: perceived barriers and suggested interventions. BMC Fam Pract. 2010;11:51.
- , , , . Why don't physicians adhere to guideline recommendations in practice? An analysis of barriers among Dutch general practitioners. Implement Sci. 2009;4:54.
- , , , , . Perceived barriers to guideline adherence: a survey among general practitioners. BMC Fam Pract. 2011;12:98.
- , , , . Clostridium difficile in acute and long‐stay elderly patients. Age Ageing. 1988;17(5):333–336.
- , , , , , . Pharmacodynamics of vancomycin in elderly patients aged 75 years or older with methicillin‐resistant Staphylococcus aureus hospital‐acquired pneumonia. Clin Interv Aging. 2013;8:1015–1021.
- , , , et al. Empiric guideline‐recommended weight‐based vancomycin dosing and nephrotoxicity rates in patients with methicillin‐resistant Staphylococcus aureus bacteremia: a retrospective cohort study. BMC Pharmacol Toxicol. 2013;14:12.
- , , , , , , , , . Guidelines on urological infections. Arnhem, The Netherlands: European Association of Urology (EAU); 2011. p. 15–27.
- Scottish Intercollegiate Guidelines Network. Management of suspected bacterial urinary tract infection in adults. Available at: http://www.sign.ac.uk/guidelines/fulltext/88/. Accessed on July 25, 2014.
- , , , et al. [Optimisation of the antibiotic policy in the Netherlands. X. The SWAB guideline for antimicrobial treatment of complicated urinary tract infections]. Ned Tijdschr Geneeskd 2006;150(43):2370–2376.
- , , , et al. Do case vignettes accurately reflect antibiotic prescription? Infect Control Hosp Epidemiol. 2011;32(10):1003–1009.
- The committee for The Japanese Respiratory Society guidelines in management of respiratory infections. Principles for the development of the guidelines. Respirology 2004;9(suppl 1):S1–S2.
Clinical management of patients with medical and social comorbidities has become increasingly complex.[1, 2, 3, 4] This complexity stems from lack of data for these groups of patients who are often excluded from clinical trials.[5] There are data demonstrating that older patients and patients with multiple comorbidities including diabetes, renal disease, obesity, limited mobility, and poor access to healthcare have worse outcomes for specific conditions compared to otherwise equal counterparts, and that the cost of care for these patients is more expensive.[3, 4, 6] Moreover, traditional risk assessment of disease severity and outcomes are not accurate when applied to medically and socially complex patients.[7, 8]
Treatment decisions regarding antibiotic use add additional complexity. Specifically, physicians antibiotic prescribing decisions can promote the emergence of multidrug resistant pathogens in a hospital or population.[9, 10] Multidrug resistant organisms (MDROs) are particularly problematic, as their prevalence is increasing while the development of new antimicrobial agents is declining.[11] Infections caused by antibiotic‐resistant pathogens are associated with increased morbidity and mortality and healthcare costs.[12, 13, 14] Antibiotic use, although potentially lifesaving, can also result in severe complications such as Clostridium difficile‐associated diarrhea, acute kidney injury, and anaphylaxis, among other adverse events, particularly in older patients with medical comorbidities.[15, 16, 17, 18] Judicious antibiotic use is critical to halt the epidemic of MDROs and to minimize antibiotic‐associated adverse effects.[19, 20, 21]
Evidence‐based guidelines have the potential to assist physicians in choosing the antibiotic that achieves the best clinical outcome for a specific infection or situation.[11] This includes using the narrowest spectrum agent to minimize selection pressure on microorganisms and avoiding unneeded drugs to minimize adverse drug effects.[9, 11] Importantly, guideline adherence regarding antibiotic selection has been shown to be associated with increased clinical success and decreased mortality.[22, 23] Unfortunately, 30% to 50% of antibiotic use in hospitalized patients is inconsistent with national guidelines.[24, 25, 26] Reasons for physicians ordering of tests and treatments inconsistent with guidelines are not fully understood, and potentially include patient and physician factors, and the cultural and social context of the healthcare system.[27]
To optimize the use of antibiotics, it is important to understand how medical complexities (defined as demographic, comorbid, and limited healthcare access characteristics that are associated with suboptimal patient care and outcomes) influence physicians antibiotic prescribing practices.[28] We created 3 clinical vignettes for common diagnoses (dyspnea with initial concern for pneumonia, skin and soft tissue infection, and asymptomatic bacteriuria) among hospitalized patients. We selected these conditions because of their high prevalence, frequent management by hospitalists, generalist physicians, and noninfectious disease specialists, and because well‐documented evidence suggests either no antibiotics or narrower spectrum antibiotics are usually the treatments of choice. Using the Infectious Diseases Society of America (IDSA) guidelines relevant to each clinical vignette,[29, 30, 31] we assessed physicians recommendations for guideline‐appropriate antibiotic management for patients without and with medical complexities using an electronic multiple‐choice survey.
METHODS
Survey Participants
We surveyed internal medicine generalist and subspecialty inpatient physicians from 3 academic medical centers in the metropolitan Los Angeles, California area. Potential participants included attending and housestaff physicians in the departments of internal medicine and family medicine at the 3 medical centers associated with the University of California Los Angeles (UCLA) Clinical and Translational Science Institute: (1) Ronald ReaganUCLA Medical Center, a tertiary care academic medical center; (2) Harbor UCLA Medical Center, a county (public) medical center; and (3) CedarsSinai Medical Center, a tertiary care medical center. Each center was affiliated with a residency training program, although not all attending physicians were associated with the training programs. Physicians were eligible to perform the survey if they attended 2 weeks per year in the inpatient setting. We collected physician‐level information including level of training (resident/fellow vs attending), specialization or not, proportion of time spent working in the hospital, and proportion of time spent providing direct clinical care (compared to activities such as administration and research). All eligible participants were emailed a brief study description with a hyperlink to the electronic survey created in REDCap (Research Electronic Data Capture version 5.6.0, 2013). Administrative staff provided email lists for potential participants at 2 of the hospitals. Per hospital policy, an email list was not provided by the third hospital, and potential participants were emailed the survey link directly by the hospital administrative staff. We incentivized study participation by entering participants who completed the survey into a raffle to win either a $100 gift card or a computer tablet. Physicians had 3 months to complete the survey and were sent up to 5 emails encouraging them to complete it.
Survey
The survey consisted of 3 clinical vignettes describing common hospital‐based situations that required decision making about antibiotic use. The 3 clinical vignettes described: (1) a patient with dyspnea and no infiltrate on chest radiograph who is initially treated empirically with antibiotics for pneumonia but is ultimately diagnosed with a congestive heart failure exacerbation, (2) a patient admitted with a skin infection that grows methicillin‐sensitive Staphylococcus aureus, and (3) a patient with a urinary catheter who develops asymptomatic bacteriuria. The first vignette was chosen because congestive heart failure and pneumonia are among the most common reasons for hospitalization in the United States, and their overlapping syndromes can make the diagnosis challenging.[32, 33, 34, 35] The second vignette was chosen because skin infections are some of the most common infectious diseases, with an incidence that is twice that of urinary tract infections and 10 times that of pneumonia, and can lead to serious complications among hospitalized patients.[30, 36] The third vignette was chosen because the prevalence of asymptomatic bacteriuria approaches 100% among catheterized patients, and rates of unnecessary treatment for this condition are as high as 80%.[31, 37]
Each clinical vignette was then modified to include 1 of the 4 studied patient complexities (Table 1). Medical comorbidities were represented by modifying the baseline vignette to describe patients with poorly controlled diabetes, morbid obesity, chronic kidney disease, and/or heavy tobacco use (Table 2). Patients with poor functional status were described in the vignettes as having difficulty with ambulation, requiring a mobility device, or needing assistance with self‐care (Table 2). Clinical vignettes varied the age of the patient from 47 years in the baseline case to 86 years (Table 2). Patients expected to have limited postdischarge follow‐up were described as being uninsured, with the first available follow‐up occurring in a public clinic no sooner than 2 weeks after their hospital discharge (Table 2). These complexities were chosen because they are common among hospitalized patients and have been shown to be associated with worse outcomes in a variety of conditions.[38, 39, 40, 41, 42] The asymptomatic bacteriuria vignette did not have a question about limited postdischarge follow‐up, because the clinical decision making in this question only pertained to the initial diagnosis and management. All physicians were queried about antibiotic use in each of the 3 baseline vignettes and in the subsequent 4 modified vignettes for each baseline scenario, with each physician making antibiotic management decisions for 15 vignettes in total. Physicians responses were recorded using categorical responses describing treatment options.
|
| Dyspnea case (baseline scenario) |
| A 47‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath and a nonproductive cough. In the ED, he had a temp 99.6F, a HR of 120, and an RR 30. Exam was notable for bilateral crackles. CXR on admission was interpreted as having cardiomegaly, bilateral base atelectasis versus infiltrate and prominent pulmonary arteries with cephalization consistent with cardiogenic pulmonary edema. Admission laboratories were notable for an elevated BNP (950 pg/mL) and WBC (11.5 cells 10*9/L) with 75% neutrophils. Troponin and CK‐MB were not elevated. The patient was treated with diuretics, ceftriaxone, and azithromycin, and over the course of the next 48 hours has improved shortness of breath and no fevers. Because of his improvement he is prepared for hospital discharge. A repeat chest x‐ray shows cardiomegaly and bibasilar atelectasis. Blood cultures have been negative. He is insured and can follow with his primary physician within 3 days of discharge. Upon discharge you: |
| A. Discharge on his usual cardiac medications. |
| B. Discharge on his cardiac medications plus azithromycin to complete a 5‐day course of antibiotics. |
| C. Discharge on his cardiac medications plus azithromycin to complete a 7‐day course of antibiotics. |
| D. Discharge on usual cardiac medications plus levofloxacin to complete a 7‐day course of antibiotics. |
| Skin infection case (baseline scenario) |
| A 47‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. Admission temperature was 101.4F. In the ED, she was given vancomycin, and piperacillin/tazobactam and underwent incision and drainage of a thigh abscess, which drained a large amount of pus. The wound was packed. On day 3 she is afebrile, and the pain has improved. Cultures from the wound grow methicillin‐susceptible Staphylococcus aureus. Blood cultures are negative to date. She is ready for discharge. She has no allergies. She is insured and can be followed up with her primary physician within a few days of discharge. You: |
| A. Discharge on vancomycin and piperacillin/tazobactam through a PICC line to complete a 10‐day course. |
| B. Discharge on amoxicillin/clavulanate orally to complete a 10‐day course. |
| C. Discharge on cephalexin to complete a 10‐day course. |
| Asymptomatic bacteriuria case (baseline scenario) |
| A generally healthy 47‐year‐old female is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On post operative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. Labs from that day show a WBC of 8900 and a urinalysis with 12 WBCs/HPF and 2+ bacteria. She has surgical site discomfort and no dysuria or other lower urinary tract symptoms. She has not had a fever during her hospital stay. Two days later her urine culture grows 100,000 CFU/mL of Escherichia coli susceptible to ciprofloxacin but resistant to all other oral antibiotics. That day, the patient is still afebrile and has no dysuria or other lower urinary tract symptoms. Her Foley is changed and a repeat urinalysis shows that the urine has persistent leukocytes and bacteria. You: |
| A. Initiate intravenous ciprofloxacin. |
| B. Initiate oral ciprofloxacin. |
| C. Give no antibiotics. |
| |
| Dyspnea case | |
| Comorbidities | A 47‐year‐old male with a history of stage III congestive heart failure, moderate to heavy tobacco use, poorly controlled type 2 diabetes (last HbA1C=10.9), chronic renal insufficiency (baseline creatinine of 1.3), diabetic retinopathy, and diabetic neuropathy is hospitalized after presenting with shortness of breath. |
| Poor functional status | A 47‐year‐old male with a history of stage III congestive heart failure, and poor functional status with difficulty ambulating and with self‐care is hospitalized after presenting with shortness of breath. |
| Older age | An 86‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath. |
| Limited follow‐up | A 47‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath.Blood cultures have been negative. He is uninsured and will be referred for follow‐up to a public clinic, which has a 2‐week wait for the next available clinic appointment. |
| Skin infection case | |
| Comorbidities | A 47‐year‐old morbidly obese female (BMI=32.9) with type 2 diabetes is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Poor functional status | A 47‐year‐old obese female (BMI=32.9) and poor functional status due to her obesity (poor mobility, uses either a walker or an electric scooter at all times to move around) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Older age | An 86‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Limited follow‐up | A 47‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. She is uninsured and will be referred for follow‐up in a public clinic, which has a 2‐week wait for the next available clinic appointment. |
| Asymptomatic bacteriuria case | |
| Comorbidities | A 47‐year‐old female with a history type 2 diabetes with diabetic nephropathy and retinopathy is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On postoperative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
| Poor functional status | A 47‐year‐old female with a history of poor functional status (needs assistance with activities of daily living) due to her obesity and musculoskeletal comorbidities, such as osteoarthritis, is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On postoperative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
| Older age | A generally healthy 86‐year‐old female is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On post operative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
The institutional review boards at all 3 medical centers approved the study.
Statistical Analysis
We used physician self‐report of recommended antibiotic use stratified by baseline and modified clinical vignettes to calculate the proportion of physicians recommending antibiotic use. We examined how physicians antibiotic use varied according to both patient complexity (medical comorbidity, poor functional status, older age, and limited follow‐up after hospital discharge), and provider characteristics (level of training, degree of specialization, time spent working in the hospital, and time spent providing direct patient care).
Data were analyzed using SAS (version 9.1.3; SAS Institute, Cary, NC). Antibiotic use inconsistent with IDSA guideline recommendations was considered the outcome of interest for the data analysis. Statistical analyses were performed using [2] or Fischer exact test, Student t test, and analysis of variance, as appropriate.
RESULTS
Physician Survey
Of the 874 invited physicians, 255 (29%) responded to the survey. Of these, 8/255 (3.1%) responded that they did not spend 2 or more weeks in the inpatient setting and were thus ineligible for the survey. We analyzed data from the remaining 247 physician respondents. Most respondents (217/233, 93%) reported their primary role was direct clinical care (Table 3). Most (185/241, 77%) reported at least half of their clinical work occurred in the hospital. Approximately three‐quarters (183/241, 76%) of the respondents were residents; the remaining respondents were attending physicians (57/241, 24%). Almost half of attending physicians (46%) were internal medicine subspecialists.
| Physician Characteristic | No. (%) Completing the Survey | % of Physicians Not Adhering to Guidelines in Baseline Scenarios | P Value |
|---|---|---|---|
| |||
| Affiliated medical center, n =241 | |||
| Ronald Reagan UCLA | 47 (20%) | 37% | 0.37 |
| Harbor‐UCLA | 106 (44%) | 41% | |
| Cedars‐Sinai | 86 (35%) | 43% | |
| Primary professional activity, n=233 | |||
| Direct clinical care/teaching | 217 (93%) | 42% | 0.90 |
| Research/administration | 16 (7%) | 27% | |
| Percent of clinical duties in the hospital, n=241 | |||
| 1%25% | 57 (23%) | 41% | 0.71 |
| 51%75% | 93 (39%) | 42% | |
| 76%100% | 92 (38%) | 41% | |
| Level of training and subspecialization, n=241 | |||
| Resident/fellow | 183 (76%) | 43% | 0.05 |
| Attending | 58 (24%) | 34% | |
| Subspecialist | 27 (47%) | 34% | 0.90 |
| Hospitalist | 28 (48%) | 33% | |
Physician recommendation for the use of antibiotics inconsistent with IDSA guidelines was prevalent in the baseline vignettes: 42% (303/729) overall, and 49% (120/246) for the dyspnea, 28% (68/242) for the skin infection, and 48% (115/241) for the asymptomatic bacteriuria cases. When the vignettes were modified to include patient complexities, the proportion of physicians recommending antibiotics increased significantly compared to the baseline vignette (63% (459/728), 54% (393/728), 51% (371/728), and 48% (232/487) for medical comorbidities, poor functional status, older age, and limited follow‐up respectively, P<0.001 for all comparisons) (Figure 1). The increase in the proportion of physicians recommending antibiotics inconsistent with guidelines for patients with medical complexities was the same when stratified by case (data not shown).

We found no association between provider characteristics (medical center, degree of attending physician specialization, percentage of clinical time spent practicing hospital‐based medicine, or percentage of time providing direct clinical care) and prescribing antibiotics in the baseline vignettes (Table 3). However, resident physicians (n=183) were more likely than attending physicians (n=57) to have recommended antibiotics in the baseline vignettes (43% vs 34%, P<0.05) (Table 3) and in all 4 vignettes with patient complexities (data not shown).
DISCUSSION
In our survey, almost half of the physician respondents recommended antibiotics that were inconsistent with national guidelines in the baseline vignettes. One explanation for this finding is that physicians may underestimate the risk associated with antibiotic use, such as the emergence of antimicrobial resistant pathogens and drug‐associated adverse effects. Although physicians generally agree that antibiotic resistance is an important problem, many believe that it is not a prominent issue in their practice or through their antibiotic prescribing practices.[43, 44] Others have shown that physicians underestimate the risk and severity of antibiotic‐associated complications such as C difficile.[45, 46] An accurate assessment and heightened awareness of the risks associated with antibiotics is important in clinical decision making, and should potentially be included not only in antibiotic stewardship educational efforts, but in national guideline recommendations as well.
Our survey also demonstrated that the tendency of physician respondents to recommend antibiotics was amplified for patients with medical complexities. This suggests that patient characteristics related to medical and social complexities play an important role in physicians clinical decision making about prescribing antibiotics. Previous investigations have shown that when physicians are deciding whether or not to prescribe antibiotics, they tend to deprioritize guideline recommendations and give greater weight to the risk of disease progression and complications that might occur if antibiotics are withheld.[47, 48, 49] Physicians are believed to prescribe antibiotics for complex patients more often, in part, because complex patients are more likely to suffer bad outcomes if undertreated.[50, 51] Axiomatically, patients with medical complexities are also at higher risk for antibiotic‐associated adverse effects including polypharmacy, drug‐drug interactions, and more severe side effects.[52, 53, 54]
An additional factor contributing to the overuse of antibiotics in our survey could be the lack of clear guideline recommendations for antibiotic management, especially among patients with complexities. We reviewed 20 national guidelines that addressed the medical decision making relevant to the survey's 3 clinical vignettes (see Supporting Information, Appendix 1, in the online version of this article). Fifteen of the guidelines provided recommendations for antibiotic management in the baseline vignettes, though most of the recommendations were not explicit about stopping or de‐escalating antibiotics. Furthermore, when antibiotic recommendations were present, they often lacked supporting data for the recommendation. Guidelines were even less complete for patients with medical complexities. For the asymptomatic bacteriuria vignette, 4 of 6 guidelines provided recommendations for patients with medical comorbidities described in our survey's modified vignettes.[31, 55, 56, 57] None of the guidelines related to the dyspnea or skin infection vignette provided specific antibiotic recommendations for complex patients with medical comorbidities, poor functional status, older age, or limited follow‐up (see Supporting Information, Appendix 1, in the online version of this article). Given these findings, along with evidence that medically complex patients are more likely to receive antibiotics compared to their less complex counterparts, there is a need for subsequent guidelines to more explicitly recommend best antibiotic practices for patients with medical and social complexities.
We also found that resident physicians were significantly more likely to recommend antibiotics inconsistent with guidelines compared to attending physicians. Although the underlying explanations for this finding were not explored in this study, possibilities include a lack of familiarity with guideline recommendations, less comfort in discontinuing antibiotics in the setting of clinical uncertainty, and/or a preference to accept the risks of overtreatment over the risks of undertreatment.
There are limitations to our study. First, although previous investigations have shown a high degree of correlation between actual antibiotic prescribing practices and antibiotic prescribing decisions self‐reported in clinical vignettes, physician responses in our survey may not reflect actual practices.[58] Second, we were not able to directly measure how physicians knowledge and interpretation of national guidelines influenced their antibiotic management decisions in the survey. Third, while our study was multisite including physicians from 3 different centers, not all invited physicians responded. Because of the confidentiality procedures surrounding the email distribution lists of potential participants, we were unable to obtain additional details about the non‐responders. Finally, because the large majority of respondents were trainees (76%), the generalizability of our findings to attending physicians may be limited. Nevertheless, because residents soon become staff physicians, resident‐reported data supplemented by that from attending physicians seems relevant to identifying opportunities for improving medical care.
In conclusion, we found that a large proportion of physicians recommended antibiotics that were not indicated based on IDSA guidelines for 3 vignettes depicting common hospital‐based clinical scenarios. This pattern of physicians recommending antibiotics inconsistent with guidelines was accentuated with significantly higher reported use for patients with medical comorbidities, poor functional status, older age, and limited healthcare access. Although good clinical judgment requires increased monitoring of patients with medical complexities, it is important for clinicians not to conflate the need for increased patient monitoring with the need for increased antibiotic use. Additional studies and corresponding guideline recommendations for frail, complex patients could be instrumental in reducing frequent use of antibiotics and the resultant cost, adverse effects, and emergence of antibiotic resistant pathogens. Educational efforts, particularly among trainees, regarding appropriate antibiotic use for clinical indications among patients without and with medical complexities would also likely contribute to these aims. Treatment guidelines should consider explicitly addressing medically complex patients in the context of management of infectious syndromes.
Disclosure
Nothing to report.
Clinical management of patients with medical and social comorbidities has become increasingly complex.[1, 2, 3, 4] This complexity stems from lack of data for these groups of patients who are often excluded from clinical trials.[5] There are data demonstrating that older patients and patients with multiple comorbidities including diabetes, renal disease, obesity, limited mobility, and poor access to healthcare have worse outcomes for specific conditions compared to otherwise equal counterparts, and that the cost of care for these patients is more expensive.[3, 4, 6] Moreover, traditional risk assessment of disease severity and outcomes are not accurate when applied to medically and socially complex patients.[7, 8]
Treatment decisions regarding antibiotic use add additional complexity. Specifically, physicians antibiotic prescribing decisions can promote the emergence of multidrug resistant pathogens in a hospital or population.[9, 10] Multidrug resistant organisms (MDROs) are particularly problematic, as their prevalence is increasing while the development of new antimicrobial agents is declining.[11] Infections caused by antibiotic‐resistant pathogens are associated with increased morbidity and mortality and healthcare costs.[12, 13, 14] Antibiotic use, although potentially lifesaving, can also result in severe complications such as Clostridium difficile‐associated diarrhea, acute kidney injury, and anaphylaxis, among other adverse events, particularly in older patients with medical comorbidities.[15, 16, 17, 18] Judicious antibiotic use is critical to halt the epidemic of MDROs and to minimize antibiotic‐associated adverse effects.[19, 20, 21]
Evidence‐based guidelines have the potential to assist physicians in choosing the antibiotic that achieves the best clinical outcome for a specific infection or situation.[11] This includes using the narrowest spectrum agent to minimize selection pressure on microorganisms and avoiding unneeded drugs to minimize adverse drug effects.[9, 11] Importantly, guideline adherence regarding antibiotic selection has been shown to be associated with increased clinical success and decreased mortality.[22, 23] Unfortunately, 30% to 50% of antibiotic use in hospitalized patients is inconsistent with national guidelines.[24, 25, 26] Reasons for physicians ordering of tests and treatments inconsistent with guidelines are not fully understood, and potentially include patient and physician factors, and the cultural and social context of the healthcare system.[27]
To optimize the use of antibiotics, it is important to understand how medical complexities (defined as demographic, comorbid, and limited healthcare access characteristics that are associated with suboptimal patient care and outcomes) influence physicians antibiotic prescribing practices.[28] We created 3 clinical vignettes for common diagnoses (dyspnea with initial concern for pneumonia, skin and soft tissue infection, and asymptomatic bacteriuria) among hospitalized patients. We selected these conditions because of their high prevalence, frequent management by hospitalists, generalist physicians, and noninfectious disease specialists, and because well‐documented evidence suggests either no antibiotics or narrower spectrum antibiotics are usually the treatments of choice. Using the Infectious Diseases Society of America (IDSA) guidelines relevant to each clinical vignette,[29, 30, 31] we assessed physicians recommendations for guideline‐appropriate antibiotic management for patients without and with medical complexities using an electronic multiple‐choice survey.
METHODS
Survey Participants
We surveyed internal medicine generalist and subspecialty inpatient physicians from 3 academic medical centers in the metropolitan Los Angeles, California area. Potential participants included attending and housestaff physicians in the departments of internal medicine and family medicine at the 3 medical centers associated with the University of California Los Angeles (UCLA) Clinical and Translational Science Institute: (1) Ronald ReaganUCLA Medical Center, a tertiary care academic medical center; (2) Harbor UCLA Medical Center, a county (public) medical center; and (3) CedarsSinai Medical Center, a tertiary care medical center. Each center was affiliated with a residency training program, although not all attending physicians were associated with the training programs. Physicians were eligible to perform the survey if they attended 2 weeks per year in the inpatient setting. We collected physician‐level information including level of training (resident/fellow vs attending), specialization or not, proportion of time spent working in the hospital, and proportion of time spent providing direct clinical care (compared to activities such as administration and research). All eligible participants were emailed a brief study description with a hyperlink to the electronic survey created in REDCap (Research Electronic Data Capture version 5.6.0, 2013). Administrative staff provided email lists for potential participants at 2 of the hospitals. Per hospital policy, an email list was not provided by the third hospital, and potential participants were emailed the survey link directly by the hospital administrative staff. We incentivized study participation by entering participants who completed the survey into a raffle to win either a $100 gift card or a computer tablet. Physicians had 3 months to complete the survey and were sent up to 5 emails encouraging them to complete it.
Survey
The survey consisted of 3 clinical vignettes describing common hospital‐based situations that required decision making about antibiotic use. The 3 clinical vignettes described: (1) a patient with dyspnea and no infiltrate on chest radiograph who is initially treated empirically with antibiotics for pneumonia but is ultimately diagnosed with a congestive heart failure exacerbation, (2) a patient admitted with a skin infection that grows methicillin‐sensitive Staphylococcus aureus, and (3) a patient with a urinary catheter who develops asymptomatic bacteriuria. The first vignette was chosen because congestive heart failure and pneumonia are among the most common reasons for hospitalization in the United States, and their overlapping syndromes can make the diagnosis challenging.[32, 33, 34, 35] The second vignette was chosen because skin infections are some of the most common infectious diseases, with an incidence that is twice that of urinary tract infections and 10 times that of pneumonia, and can lead to serious complications among hospitalized patients.[30, 36] The third vignette was chosen because the prevalence of asymptomatic bacteriuria approaches 100% among catheterized patients, and rates of unnecessary treatment for this condition are as high as 80%.[31, 37]
Each clinical vignette was then modified to include 1 of the 4 studied patient complexities (Table 1). Medical comorbidities were represented by modifying the baseline vignette to describe patients with poorly controlled diabetes, morbid obesity, chronic kidney disease, and/or heavy tobacco use (Table 2). Patients with poor functional status were described in the vignettes as having difficulty with ambulation, requiring a mobility device, or needing assistance with self‐care (Table 2). Clinical vignettes varied the age of the patient from 47 years in the baseline case to 86 years (Table 2). Patients expected to have limited postdischarge follow‐up were described as being uninsured, with the first available follow‐up occurring in a public clinic no sooner than 2 weeks after their hospital discharge (Table 2). These complexities were chosen because they are common among hospitalized patients and have been shown to be associated with worse outcomes in a variety of conditions.[38, 39, 40, 41, 42] The asymptomatic bacteriuria vignette did not have a question about limited postdischarge follow‐up, because the clinical decision making in this question only pertained to the initial diagnosis and management. All physicians were queried about antibiotic use in each of the 3 baseline vignettes and in the subsequent 4 modified vignettes for each baseline scenario, with each physician making antibiotic management decisions for 15 vignettes in total. Physicians responses were recorded using categorical responses describing treatment options.
|
| Dyspnea case (baseline scenario) |
| A 47‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath and a nonproductive cough. In the ED, he had a temp 99.6F, a HR of 120, and an RR 30. Exam was notable for bilateral crackles. CXR on admission was interpreted as having cardiomegaly, bilateral base atelectasis versus infiltrate and prominent pulmonary arteries with cephalization consistent with cardiogenic pulmonary edema. Admission laboratories were notable for an elevated BNP (950 pg/mL) and WBC (11.5 cells 10*9/L) with 75% neutrophils. Troponin and CK‐MB were not elevated. The patient was treated with diuretics, ceftriaxone, and azithromycin, and over the course of the next 48 hours has improved shortness of breath and no fevers. Because of his improvement he is prepared for hospital discharge. A repeat chest x‐ray shows cardiomegaly and bibasilar atelectasis. Blood cultures have been negative. He is insured and can follow with his primary physician within 3 days of discharge. Upon discharge you: |
| A. Discharge on his usual cardiac medications. |
| B. Discharge on his cardiac medications plus azithromycin to complete a 5‐day course of antibiotics. |
| C. Discharge on his cardiac medications plus azithromycin to complete a 7‐day course of antibiotics. |
| D. Discharge on usual cardiac medications plus levofloxacin to complete a 7‐day course of antibiotics. |
| Skin infection case (baseline scenario) |
| A 47‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. Admission temperature was 101.4F. In the ED, she was given vancomycin, and piperacillin/tazobactam and underwent incision and drainage of a thigh abscess, which drained a large amount of pus. The wound was packed. On day 3 she is afebrile, and the pain has improved. Cultures from the wound grow methicillin‐susceptible Staphylococcus aureus. Blood cultures are negative to date. She is ready for discharge. She has no allergies. She is insured and can be followed up with her primary physician within a few days of discharge. You: |
| A. Discharge on vancomycin and piperacillin/tazobactam through a PICC line to complete a 10‐day course. |
| B. Discharge on amoxicillin/clavulanate orally to complete a 10‐day course. |
| C. Discharge on cephalexin to complete a 10‐day course. |
| Asymptomatic bacteriuria case (baseline scenario) |
| A generally healthy 47‐year‐old female is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On post operative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. Labs from that day show a WBC of 8900 and a urinalysis with 12 WBCs/HPF and 2+ bacteria. She has surgical site discomfort and no dysuria or other lower urinary tract symptoms. She has not had a fever during her hospital stay. Two days later her urine culture grows 100,000 CFU/mL of Escherichia coli susceptible to ciprofloxacin but resistant to all other oral antibiotics. That day, the patient is still afebrile and has no dysuria or other lower urinary tract symptoms. Her Foley is changed and a repeat urinalysis shows that the urine has persistent leukocytes and bacteria. You: |
| A. Initiate intravenous ciprofloxacin. |
| B. Initiate oral ciprofloxacin. |
| C. Give no antibiotics. |
| |
| Dyspnea case | |
| Comorbidities | A 47‐year‐old male with a history of stage III congestive heart failure, moderate to heavy tobacco use, poorly controlled type 2 diabetes (last HbA1C=10.9), chronic renal insufficiency (baseline creatinine of 1.3), diabetic retinopathy, and diabetic neuropathy is hospitalized after presenting with shortness of breath. |
| Poor functional status | A 47‐year‐old male with a history of stage III congestive heart failure, and poor functional status with difficulty ambulating and with self‐care is hospitalized after presenting with shortness of breath. |
| Older age | An 86‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath. |
| Limited follow‐up | A 47‐year‐old male with a history of stage III congestive heart failure is hospitalized after presenting with shortness of breath.Blood cultures have been negative. He is uninsured and will be referred for follow‐up to a public clinic, which has a 2‐week wait for the next available clinic appointment. |
| Skin infection case | |
| Comorbidities | A 47‐year‐old morbidly obese female (BMI=32.9) with type 2 diabetes is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Poor functional status | A 47‐year‐old obese female (BMI=32.9) and poor functional status due to her obesity (poor mobility, uses either a walker or an electric scooter at all times to move around) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Older age | An 86‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. |
| Limited follow‐up | A 47‐year‐old obese female (BMI=32.9) is hospitalized after presenting with leg erythema, pain, and an 8 8‐cm fluctuant mass on her thigh. She is uninsured and will be referred for follow‐up in a public clinic, which has a 2‐week wait for the next available clinic appointment. |
| Asymptomatic bacteriuria case | |
| Comorbidities | A 47‐year‐old female with a history type 2 diabetes with diabetic nephropathy and retinopathy is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On postoperative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
| Poor functional status | A 47‐year‐old female with a history of poor functional status (needs assistance with activities of daily living) due to her obesity and musculoskeletal comorbidities, such as osteoarthritis, is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On postoperative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
| Older age | A generally healthy 86‐year‐old female is admitted to the hospital for a femoral head fracture and undergoes total hip replacement. On post operative day 2, the nurse sends a urinalysis and culture from the patient's Foley catheter because the patient's urine is cloudy. |
The institutional review boards at all 3 medical centers approved the study.
Statistical Analysis
We used physician self‐report of recommended antibiotic use stratified by baseline and modified clinical vignettes to calculate the proportion of physicians recommending antibiotic use. We examined how physicians antibiotic use varied according to both patient complexity (medical comorbidity, poor functional status, older age, and limited follow‐up after hospital discharge), and provider characteristics (level of training, degree of specialization, time spent working in the hospital, and time spent providing direct patient care).
Data were analyzed using SAS (version 9.1.3; SAS Institute, Cary, NC). Antibiotic use inconsistent with IDSA guideline recommendations was considered the outcome of interest for the data analysis. Statistical analyses were performed using [2] or Fischer exact test, Student t test, and analysis of variance, as appropriate.
RESULTS
Physician Survey
Of the 874 invited physicians, 255 (29%) responded to the survey. Of these, 8/255 (3.1%) responded that they did not spend 2 or more weeks in the inpatient setting and were thus ineligible for the survey. We analyzed data from the remaining 247 physician respondents. Most respondents (217/233, 93%) reported their primary role was direct clinical care (Table 3). Most (185/241, 77%) reported at least half of their clinical work occurred in the hospital. Approximately three‐quarters (183/241, 76%) of the respondents were residents; the remaining respondents were attending physicians (57/241, 24%). Almost half of attending physicians (46%) were internal medicine subspecialists.
| Physician Characteristic | No. (%) Completing the Survey | % of Physicians Not Adhering to Guidelines in Baseline Scenarios | P Value |
|---|---|---|---|
| |||
| Affiliated medical center, n =241 | |||
| Ronald Reagan UCLA | 47 (20%) | 37% | 0.37 |
| Harbor‐UCLA | 106 (44%) | 41% | |
| Cedars‐Sinai | 86 (35%) | 43% | |
| Primary professional activity, n=233 | |||
| Direct clinical care/teaching | 217 (93%) | 42% | 0.90 |
| Research/administration | 16 (7%) | 27% | |
| Percent of clinical duties in the hospital, n=241 | |||
| 1%25% | 57 (23%) | 41% | 0.71 |
| 51%75% | 93 (39%) | 42% | |
| 76%100% | 92 (38%) | 41% | |
| Level of training and subspecialization, n=241 | |||
| Resident/fellow | 183 (76%) | 43% | 0.05 |
| Attending | 58 (24%) | 34% | |
| Subspecialist | 27 (47%) | 34% | 0.90 |
| Hospitalist | 28 (48%) | 33% | |
Physician recommendation for the use of antibiotics inconsistent with IDSA guidelines was prevalent in the baseline vignettes: 42% (303/729) overall, and 49% (120/246) for the dyspnea, 28% (68/242) for the skin infection, and 48% (115/241) for the asymptomatic bacteriuria cases. When the vignettes were modified to include patient complexities, the proportion of physicians recommending antibiotics increased significantly compared to the baseline vignette (63% (459/728), 54% (393/728), 51% (371/728), and 48% (232/487) for medical comorbidities, poor functional status, older age, and limited follow‐up respectively, P<0.001 for all comparisons) (Figure 1). The increase in the proportion of physicians recommending antibiotics inconsistent with guidelines for patients with medical complexities was the same when stratified by case (data not shown).

We found no association between provider characteristics (medical center, degree of attending physician specialization, percentage of clinical time spent practicing hospital‐based medicine, or percentage of time providing direct clinical care) and prescribing antibiotics in the baseline vignettes (Table 3). However, resident physicians (n=183) were more likely than attending physicians (n=57) to have recommended antibiotics in the baseline vignettes (43% vs 34%, P<0.05) (Table 3) and in all 4 vignettes with patient complexities (data not shown).
DISCUSSION
In our survey, almost half of the physician respondents recommended antibiotics that were inconsistent with national guidelines in the baseline vignettes. One explanation for this finding is that physicians may underestimate the risk associated with antibiotic use, such as the emergence of antimicrobial resistant pathogens and drug‐associated adverse effects. Although physicians generally agree that antibiotic resistance is an important problem, many believe that it is not a prominent issue in their practice or through their antibiotic prescribing practices.[43, 44] Others have shown that physicians underestimate the risk and severity of antibiotic‐associated complications such as C difficile.[45, 46] An accurate assessment and heightened awareness of the risks associated with antibiotics is important in clinical decision making, and should potentially be included not only in antibiotic stewardship educational efforts, but in national guideline recommendations as well.
Our survey also demonstrated that the tendency of physician respondents to recommend antibiotics was amplified for patients with medical complexities. This suggests that patient characteristics related to medical and social complexities play an important role in physicians clinical decision making about prescribing antibiotics. Previous investigations have shown that when physicians are deciding whether or not to prescribe antibiotics, they tend to deprioritize guideline recommendations and give greater weight to the risk of disease progression and complications that might occur if antibiotics are withheld.[47, 48, 49] Physicians are believed to prescribe antibiotics for complex patients more often, in part, because complex patients are more likely to suffer bad outcomes if undertreated.[50, 51] Axiomatically, patients with medical complexities are also at higher risk for antibiotic‐associated adverse effects including polypharmacy, drug‐drug interactions, and more severe side effects.[52, 53, 54]
An additional factor contributing to the overuse of antibiotics in our survey could be the lack of clear guideline recommendations for antibiotic management, especially among patients with complexities. We reviewed 20 national guidelines that addressed the medical decision making relevant to the survey's 3 clinical vignettes (see Supporting Information, Appendix 1, in the online version of this article). Fifteen of the guidelines provided recommendations for antibiotic management in the baseline vignettes, though most of the recommendations were not explicit about stopping or de‐escalating antibiotics. Furthermore, when antibiotic recommendations were present, they often lacked supporting data for the recommendation. Guidelines were even less complete for patients with medical complexities. For the asymptomatic bacteriuria vignette, 4 of 6 guidelines provided recommendations for patients with medical comorbidities described in our survey's modified vignettes.[31, 55, 56, 57] None of the guidelines related to the dyspnea or skin infection vignette provided specific antibiotic recommendations for complex patients with medical comorbidities, poor functional status, older age, or limited follow‐up (see Supporting Information, Appendix 1, in the online version of this article). Given these findings, along with evidence that medically complex patients are more likely to receive antibiotics compared to their less complex counterparts, there is a need for subsequent guidelines to more explicitly recommend best antibiotic practices for patients with medical and social complexities.
We also found that resident physicians were significantly more likely to recommend antibiotics inconsistent with guidelines compared to attending physicians. Although the underlying explanations for this finding were not explored in this study, possibilities include a lack of familiarity with guideline recommendations, less comfort in discontinuing antibiotics in the setting of clinical uncertainty, and/or a preference to accept the risks of overtreatment over the risks of undertreatment.
There are limitations to our study. First, although previous investigations have shown a high degree of correlation between actual antibiotic prescribing practices and antibiotic prescribing decisions self‐reported in clinical vignettes, physician responses in our survey may not reflect actual practices.[58] Second, we were not able to directly measure how physicians knowledge and interpretation of national guidelines influenced their antibiotic management decisions in the survey. Third, while our study was multisite including physicians from 3 different centers, not all invited physicians responded. Because of the confidentiality procedures surrounding the email distribution lists of potential participants, we were unable to obtain additional details about the non‐responders. Finally, because the large majority of respondents were trainees (76%), the generalizability of our findings to attending physicians may be limited. Nevertheless, because residents soon become staff physicians, resident‐reported data supplemented by that from attending physicians seems relevant to identifying opportunities for improving medical care.
In conclusion, we found that a large proportion of physicians recommended antibiotics that were not indicated based on IDSA guidelines for 3 vignettes depicting common hospital‐based clinical scenarios. This pattern of physicians recommending antibiotics inconsistent with guidelines was accentuated with significantly higher reported use for patients with medical comorbidities, poor functional status, older age, and limited healthcare access. Although good clinical judgment requires increased monitoring of patients with medical complexities, it is important for clinicians not to conflate the need for increased patient monitoring with the need for increased antibiotic use. Additional studies and corresponding guideline recommendations for frail, complex patients could be instrumental in reducing frequent use of antibiotics and the resultant cost, adverse effects, and emergence of antibiotic resistant pathogens. Educational efforts, particularly among trainees, regarding appropriate antibiotic use for clinical indications among patients without and with medical complexities would also likely contribute to these aims. Treatment guidelines should consider explicitly addressing medically complex patients in the context of management of infectious syndromes.
Disclosure
Nothing to report.
- , , , , , . Heart failure and chronic obstructive pulmonary disease multimorbidity at hospital discharge transition: a study of patient and carer experience [published online ahead of print May 16. 2014]. Health Expect. doi: 10.1111/hex.12208.
- , , , , , . Development of clinical practice guidelines for patients with comorbidity and multiple diseases [in Spanish]. Aten Primaria. 2014;46(7):385–392.
- , , , et al. Cardiac complications in patients with community‐acquired pneumonia: a systematic review and meta‐analysis of observational studies. PLoS Med. 2011;8(6):e1001048.
- , , , , , . Cost and incidence of social comorbidities in low‐risk patients with community‐acquired pneumonia admitted to a public hospital. Chest. 2003;124(6):2148–2155.
- , , , et al. Why do GPs exclude patients from participating in research? An exploration of adherence to and divergence from trial criteria. Fam Pract. 2014;31(3):364–370.
- , , , , . The impact of pre‐existing heart failure on pneumonia prognosis: population‐based cohort study. J Gen Intern Med. 2008;23(9):1407–1413.
- , , , et al. Severe community‐acquired pneumonia: use of intensive care services and evaluation of American and British Thoracic Society Diagnostic criteria. Am J Respir Crit Care Med. 2002;166(5):717–723.
- . Decisions about treating community‐acquired pneumonia. Ann Intern Med. 2005;142(3):215–216.
- , , , et al. The epidemic of antibiotic‐resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(2):155–164.
- Will antibiotic misuse now stop? Nat Rev Microbiol. 2003;1(2):85.
- , , , et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177.
- . Economic impact of antimicrobial resistance. Emerg Infect Dis. 2001;7(2):286–292.
- , , , , . Guideline‐concordant therapy and reduced mortality and length of stay in adults with community‐acquired pneumonia: playing by the rules. Arch Intern Med. 2009;169(16):1525–1531.
- , , , et al. Adherence to guidelines' empirical antibiotic recommendations and community‐acquired pneumonia outcome. Eur Respir J. 2008;32(4):892–901.
- , , , et al. Community‐associated Clostridium difficile infection and antibiotics: a meta‐analysis. J Antimicrob Chemother. 2013;68(9):1951–1961.
- , , , . Vancomycin‐induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. 2012;68(9):1243–1255.
- , . Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin Rev Allergy Immunol. 2013;45(1):131–142.
- , . Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol. 2011;72(3):381–393.
- , , , et al. A clinician's guide to the appropriate and accurate use of antibiotics: the Council for Appropriate and Rational Antibiotic Therapy (CARAT) criteria. Am J Med. 2005;118(suppl 7A):1S–6S.
- , , , , . Evaluation of rational antibiotic use. Int J Antimicrob Agents. 2000;15(2):131–135.
- , . Rational antibiotic prescribing. Challenges and successes [in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55(11–12):1418–1426.
- , , , et al. Antibiotic prescription for community‐acquired pneumonia in the intensive care unit: impact of adherence to Infectious Diseases Society of America guidelines on survival. Clin Infect Dis. 2005;41(12):1709–1716.
- , , . Guideline‐adherent initial intravenous antibiotic therapy for hospital‐acquired/ventilator‐associated pneumonia is clinically superior, saves lives and is cheaper than non guideline adherent therapy. Eur J Med Res. 2011;16(7):315–323.
- , , , , , . Antibiotic misuse: a prospective clinical audit in a French university hospital. Eur J Clin Microbiol Infect Dis. 2007;26(4):277–280.
- , , , , . Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163(8):972–978.
- , , , . A systematic review and meta‐analysis of misuse of antibiotic therapies in the community. Int J Antimicrob Agents. 2005;26(2):106–113.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. The complexity of care for patients with rheumatoid arthritis: metrics for better understanding chronic disease care. Med Care. 2007;45(1):55–65.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52.
- , , , , , . Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–654.
- , , , et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215.
- , , , et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29(19):2388–2442.
- , , , . Prevalence of negative chest radiography results in the emergency department patient with decompensated heart failure. Ann Emerg Med. 2006;47(1):13–18.
- , , , . Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117(5):305–311.
- , , , et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005–2010. BMC Infect Dis. In press.
- . Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2012;9(2):85–93.
- , , , et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312(12):1218–1226.
- , , , . Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814.
- , , , , . Frailty in elderly people. Lancet. 2013;381(9868):752–762.
- , , , , , . Risk factors for death in homeless adults in Boston. Arch Intern Med. 1998;158(13):1454–1460.
- . Taking it to the streets: homelessness, health, and health care in the United States. J Gen Intern Med. 2003;18(11):964–965.
- , , , , , . Antibiotic resistance: a survey of physician perceptions. Arch Intern Med. 2002;162(19):2210–2216.
- , , , et al. Primary care clinicians' perceptions of antibiotic resistance: a multi‐country qualitative interview study. J Antimicrob Chemother. 2013;68(1):237–243.
- , , , , , . Underestimation of Clostridium difficile infection among clinicians: an international survey. Eur J Clin Microbiol Infect Dis. 2012;31(9):2439–2444.
- , , , , , . Unnecessary antimicrobial use in patients with current or recent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2013;34(2):109–116.
- , , , , . Attitudes of primary care physicians to the prescribing of antibiotics and antimicrobial resistance: a qualitative study from Spain. Fam Pract. 2012;29(3):352–360.
- , , , , . Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents. 2013;41(3):203–212.
- , , , . Guidelines on uncomplicated urinary tract infections are difficult to follow: perceived barriers and suggested interventions. BMC Fam Pract. 2010;11:51.
- , , , . Why don't physicians adhere to guideline recommendations in practice? An analysis of barriers among Dutch general practitioners. Implement Sci. 2009;4:54.
- , , , , . Perceived barriers to guideline adherence: a survey among general practitioners. BMC Fam Pract. 2011;12:98.
- , , , . Clostridium difficile in acute and long‐stay elderly patients. Age Ageing. 1988;17(5):333–336.
- , , , , , . Pharmacodynamics of vancomycin in elderly patients aged 75 years or older with methicillin‐resistant Staphylococcus aureus hospital‐acquired pneumonia. Clin Interv Aging. 2013;8:1015–1021.
- , , , et al. Empiric guideline‐recommended weight‐based vancomycin dosing and nephrotoxicity rates in patients with methicillin‐resistant Staphylococcus aureus bacteremia: a retrospective cohort study. BMC Pharmacol Toxicol. 2013;14:12.
- , , , , , , , , . Guidelines on urological infections. Arnhem, The Netherlands: European Association of Urology (EAU); 2011. p. 15–27.
- Scottish Intercollegiate Guidelines Network. Management of suspected bacterial urinary tract infection in adults. Available at: http://www.sign.ac.uk/guidelines/fulltext/88/. Accessed on July 25, 2014.
- , , , et al. [Optimisation of the antibiotic policy in the Netherlands. X. The SWAB guideline for antimicrobial treatment of complicated urinary tract infections]. Ned Tijdschr Geneeskd 2006;150(43):2370–2376.
- , , , et al. Do case vignettes accurately reflect antibiotic prescription? Infect Control Hosp Epidemiol. 2011;32(10):1003–1009.
- The committee for The Japanese Respiratory Society guidelines in management of respiratory infections. Principles for the development of the guidelines. Respirology 2004;9(suppl 1):S1–S2.
- , , , , , . Heart failure and chronic obstructive pulmonary disease multimorbidity at hospital discharge transition: a study of patient and carer experience [published online ahead of print May 16. 2014]. Health Expect. doi: 10.1111/hex.12208.
- , , , , , . Development of clinical practice guidelines for patients with comorbidity and multiple diseases [in Spanish]. Aten Primaria. 2014;46(7):385–392.
- , , , et al. Cardiac complications in patients with community‐acquired pneumonia: a systematic review and meta‐analysis of observational studies. PLoS Med. 2011;8(6):e1001048.
- , , , , , . Cost and incidence of social comorbidities in low‐risk patients with community‐acquired pneumonia admitted to a public hospital. Chest. 2003;124(6):2148–2155.
- , , , et al. Why do GPs exclude patients from participating in research? An exploration of adherence to and divergence from trial criteria. Fam Pract. 2014;31(3):364–370.
- , , , , . The impact of pre‐existing heart failure on pneumonia prognosis: population‐based cohort study. J Gen Intern Med. 2008;23(9):1407–1413.
- , , , et al. Severe community‐acquired pneumonia: use of intensive care services and evaluation of American and British Thoracic Society Diagnostic criteria. Am J Respir Crit Care Med. 2002;166(5):717–723.
- . Decisions about treating community‐acquired pneumonia. Ann Intern Med. 2005;142(3):215–216.
- , , , et al. The epidemic of antibiotic‐resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(2):155–164.
- Will antibiotic misuse now stop? Nat Rev Microbiol. 2003;1(2):85.
- , , , et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–177.
- . Economic impact of antimicrobial resistance. Emerg Infect Dis. 2001;7(2):286–292.
- , , , , . Guideline‐concordant therapy and reduced mortality and length of stay in adults with community‐acquired pneumonia: playing by the rules. Arch Intern Med. 2009;169(16):1525–1531.
- , , , et al. Adherence to guidelines' empirical antibiotic recommendations and community‐acquired pneumonia outcome. Eur Respir J. 2008;32(4):892–901.
- , , , et al. Community‐associated Clostridium difficile infection and antibiotics: a meta‐analysis. J Antimicrob Chemother. 2013;68(9):1951–1961.
- , , , . Vancomycin‐induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. 2012;68(9):1243–1255.
- , . Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin Rev Allergy Immunol. 2013;45(1):131–142.
- , . Neurotoxic effects associated with antibiotic use: management considerations. Br J Clin Pharmacol. 2011;72(3):381–393.
- , , , et al. A clinician's guide to the appropriate and accurate use of antibiotics: the Council for Appropriate and Rational Antibiotic Therapy (CARAT) criteria. Am J Med. 2005;118(suppl 7A):1S–6S.
- , , , , . Evaluation of rational antibiotic use. Int J Antimicrob Agents. 2000;15(2):131–135.
- , . Rational antibiotic prescribing. Challenges and successes [in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55(11–12):1418–1426.
- , , , et al. Antibiotic prescription for community‐acquired pneumonia in the intensive care unit: impact of adherence to Infectious Diseases Society of America guidelines on survival. Clin Infect Dis. 2005;41(12):1709–1716.
- , , . Guideline‐adherent initial intravenous antibiotic therapy for hospital‐acquired/ventilator‐associated pneumonia is clinically superior, saves lives and is cheaper than non guideline adherent therapy. Eur J Med Res. 2011;16(7):315–323.
- , , , , , . Antibiotic misuse: a prospective clinical audit in a French university hospital. Eur J Clin Microbiol Infect Dis. 2007;26(4):277–280.
- , , , , . Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163(8):972–978.
- , , , . A systematic review and meta‐analysis of misuse of antibiotic therapies in the community. Int J Antimicrob Agents. 2005;26(2):106–113.
- , . From best evidence to best practice: effective implementation of change in patients' care. Lancet. 2003;362(9391):1225–1230.
- , , , et al. The complexity of care for patients with rheumatoid arthritis: metrics for better understanding chronic disease care. Med Care. 2007;45(1):55–65.
- , , , et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52.
- , , , , , . Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–654.
- , , , et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215.
- , , , et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J. 2008;29(19):2388–2442.
- , , , . Prevalence of negative chest radiography results in the emergency department patient with decompensated heart failure. Ann Emerg Med. 2006;47(1):13–18.
- , , , . Patients admitted to hospital with suspected pneumonia and normal chest radiographs: epidemiology, microbiology, and outcomes. Am J Med. 2004;117(5):305–311.
- , , , et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005–2010. BMC Infect Dis. In press.
- . Asymptomatic bacteriuria: when the treatment is worse than the disease. Nat Rev Urol. 2012;9(2):85–93.
- , , , et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312(12):1218–1226.
- , , , . Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814.
- , , , , . Frailty in elderly people. Lancet. 2013;381(9868):752–762.
- , , , , , . Risk factors for death in homeless adults in Boston. Arch Intern Med. 1998;158(13):1454–1460.
- . Taking it to the streets: homelessness, health, and health care in the United States. J Gen Intern Med. 2003;18(11):964–965.
- , , , , , . Antibiotic resistance: a survey of physician perceptions. Arch Intern Med. 2002;162(19):2210–2216.
- , , , et al. Primary care clinicians' perceptions of antibiotic resistance: a multi‐country qualitative interview study. J Antimicrob Chemother. 2013;68(1):237–243.
- , , , , , . Underestimation of Clostridium difficile infection among clinicians: an international survey. Eur J Clin Microbiol Infect Dis. 2012;31(9):2439–2444.
- , , , , , . Unnecessary antimicrobial use in patients with current or recent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2013;34(2):109–116.
- , , , , . Attitudes of primary care physicians to the prescribing of antibiotics and antimicrobial resistance: a qualitative study from Spain. Fam Pract. 2012;29(3):352–360.
- , , , , . Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents. 2013;41(3):203–212.
- , , , . Guidelines on uncomplicated urinary tract infections are difficult to follow: perceived barriers and suggested interventions. BMC Fam Pract. 2010;11:51.
- , , , . Why don't physicians adhere to guideline recommendations in practice? An analysis of barriers among Dutch general practitioners. Implement Sci. 2009;4:54.
- , , , , . Perceived barriers to guideline adherence: a survey among general practitioners. BMC Fam Pract. 2011;12:98.
- , , , . Clostridium difficile in acute and long‐stay elderly patients. Age Ageing. 1988;17(5):333–336.
- , , , , , . Pharmacodynamics of vancomycin in elderly patients aged 75 years or older with methicillin‐resistant Staphylococcus aureus hospital‐acquired pneumonia. Clin Interv Aging. 2013;8:1015–1021.
- , , , et al. Empiric guideline‐recommended weight‐based vancomycin dosing and nephrotoxicity rates in patients with methicillin‐resistant Staphylococcus aureus bacteremia: a retrospective cohort study. BMC Pharmacol Toxicol. 2013;14:12.
- , , , , , , , , . Guidelines on urological infections. Arnhem, The Netherlands: European Association of Urology (EAU); 2011. p. 15–27.
- Scottish Intercollegiate Guidelines Network. Management of suspected bacterial urinary tract infection in adults. Available at: http://www.sign.ac.uk/guidelines/fulltext/88/. Accessed on July 25, 2014.
- , , , et al. [Optimisation of the antibiotic policy in the Netherlands. X. The SWAB guideline for antimicrobial treatment of complicated urinary tract infections]. Ned Tijdschr Geneeskd 2006;150(43):2370–2376.
- , , , et al. Do case vignettes accurately reflect antibiotic prescription? Infect Control Hosp Epidemiol. 2011;32(10):1003–1009.
- The committee for The Japanese Respiratory Society guidelines in management of respiratory infections. Principles for the development of the guidelines. Respirology 2004;9(suppl 1):S1–S2.
© 2015 Society of Hospital Medicine