User login
Stenosis of the aortic valve has a long, latent, asymptomatic phase, but when symptoms finally occur, clinical deterioration can be rapid. For patients with severe stenosis, the standard treatment has long been replacement of the aortic valve via open heart surgery. But many patients with severe stenosis are considered too high-risk for this procedure.
Until about 5 years ago, these patients had no other option but medical therapy or percutaneous aortic balloon valvuloplasty as a palliative measure or as a bridge to open heart surgery. But 5 years of experience with percutaneous techniques to implant prosthetic aortic valves show that this less-invasive approach may become a viable option for patients with severe symptomatic aortic valve stenosis.
In this review, we discuss current prosthetic valves and percutaneous techniques and their relative advantages and limitations and the potential future role of this new treatment option.
THE NEED FOR A LESS-INVASIVE APPROACH
Calcific aortic stenosis is the most common valvular heart disease, affecting 2% to 4% of adults over age 65 in the United States alone.1,2 The aging of our population and the lack of drug therapies to prevent, halt, or effectively slow aortic valve stenosis are leading to a greater burden of this condition.1,3,4 Already in the United States more than 50,000 surgical aortic valve replacements are performed every year for severe aortic stenosis.1,2 The associated in-hospital death rate is 8.8% in patients over age 65 years, and as high as 13% in low-volume centers.1,5
The steady increase in the number of patients requiring aortic valve replacement, the high surgical risk in patients with multiple comorbidities, the reluctance of some patients to undergo the trauma and pain associated with open heart surgery via sternotomy, and the fact that percutaneous procedures are less traumatic and offer faster recovery and fewer hospital days—all these are forces that have been driving the development of percutaneous techniques for the treatment of aortic stenosis.6–11 In addition, a recent study12 showed that 33% of patients over age 75 were deemed too high-risk for open heart surgery and thus were left untreated.12
The evolution of percutaneous aortic valve replacement
The idea of percutaneous treatment of aortic stenosis was first put into clinical practice in 1985, when Cribier performed an aortic balloon valvuloplasty.6 This was followed in 200013 by the first successful implantation of a catheter-based stent valve in a human, and in 2002 by the first successful percutaneous aortic valve replacement in a human.13–15 In the following sections, we discuss the percutaneous approaches in current use for the treatment of degenerative aortic stenosis.
AORTIC BALLOON VALVULOPLASTY
Percutaneous aortic balloon valvuloplasty, partial dilation of the stenotic aortic valve with a balloon inserted via a catheter,1,16–19 improves symptoms but has failed to show a sustained benefit on rates of mortality or morbidity.1,16–18 The restenosis rate is high, and symptoms recur in most patients within months to a year.1,16–18 Procedural complication rates are about 10%, and complication rates at the catheter access site are even higher.1,16–18 The 30-day death rate in the National Heart, Lung, and Blood Institute’s Balloon Valvuloplasty Registry, which included more than 600 patients, was 14%.18 In a retrospective study of 212 patients who underwent single or repeat percutaneous aortic balloon valvuloplasty,20 the 1-year mortality rate was 36% for the entire cohort, with a median survival of 3 years. Patients who underwent a repeat procedure (33%) had 1-year mortality rate of 42%, compared with 16% in patients who did not undergo a repeat procedure.20
Percutaneous aortic balloon valvuloplasty serves best as palliative therapy in severely symptomatic patients, and as a bridge to surgery in hemodynamically unstable adult patients.21,22 Percutaneous aortic balloon valvuloplasty is not an option in patients who are good candidates for surgical valve replacement.1
PERCUTANEOUS AORTIC VALVE REPLACEMENT: THREE TECHNIQUES
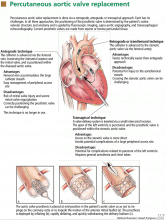
The antegrade technique
The retrograde technique
In the retrograde (ie, transfemoral) technique, access to the femoral artery is gained and the catheter with the prosthetic aortic valve is advanced to the stenotic aortic valve.8,11,26,28–30 This approach is faster and technically easier than the antegrade approach, but it can be associated with injury to the aortofemoral vessels and with failure of the prosthesis to cross the aortic arch or the stenotic aortic valve.11,23,30
The transapical technique
In the transapical technique, the valve delivery system is inserted via a small incision made between the ribs. The apex of the left ventricle is punctured with a needle, and the prosthetic valve is positioned within the stenotic aortic valve.27,31–33 The main advantage of this approach is that it allows more direct access to the aortic valve and eliminates the need for a large peripheral vascular access site in patients with peripheral vascular disease, small tortuous vasculature, or a history of major vascular complications or vascular repairs.31–33 Potential disadvantages are related to the left ventricular apical puncture and include adverse ventricular remodeling, left ventricular aneurysm or pseudoaneurysm, pericardial complications, pneumothorax, malignant ventricular arrhythmias, coronary artery injury, and the need for general anesthesia and chest tubes.27,31–35
Common features of the three approaches
The three percutaneous approaches have certain final steps in common.11,23,30,33 The position of final deployment of the prosthetic valve is determined by the patient’s native valvular structure and anatomy and is optimized by using fluoroscopic imaging of the native aortic valve calcification as an anatomical marker, along with guidance from supra-aortic angiography and transesophageal echocardiography.11,23,30,33 Ideally, the aortic valve prosthesis is placed at mid-position in the patient’s aortic valve, taking care to not to impinge on the coronary ostia or to impede the motion of the anterior mitral leaflet.11,23,30,33 In all three procedures, the prosthesis is then deployed by maximally inflating, rapidly deflating, and immediately withdrawing the delivery balloon. This final step is carried out during temporary high-rate right ventricular apical pacing, which produces ventricular tachycardia at 180 to 220 beats/min for up to 10 seconds.11,23,30,33 This leads to an immediate decrease in stroke volume, resulting in minimal forward flow through the aortic valve, which in turn facilitates precise positioning of the prosthetic valve.
So far, only the Cribier-Edwards valve has been deployed via all three techniques. The CoreValve has been deployed only via the retrograde technique. The Edwards SAPIEN valve has been deployed with retrograde and transapical approaches (see www.edwards.com/Products/TranscatheterValves/SapienTHV.htm and www.corevalve.com for animations depicting these techniques).
EXPERIENCE WITH THE CRIBIER-EDWARDS VALVE
The Cribier-Edwards valve has three leaflets made from equine pericardial tissue sutured inside a balloon-expandable stainless steel 14-mm stent (Table 1).11,23,33 With the use of a specially designed mechanical crimping device, the aortic valve prosthesis is mounted over a 3-cm-long balloon catheter, expandable to a diameter of 22 to 26 mm (NuMed Inc, Hopkinton, NY).11,23,30,33
After this prosthesis was tested in animal models,14,15 a trial for compassionate use in humans was begun, called the Initial Registry of Endovascular Implantation of Valves in Europe (I-REVIVE) trial. This trial was later continued as the Registry of Endovascular Critical Aortic Stenosis Treatment (RECAST) trial.23 All patients were formally evaluated by two cardio-thoracic surgeons and were deemed inappropriate for surgical aortic valve replacement.23
The success rate with the antegrade percutaneous approach was 85% (23 of 27 patients) and 57% for the retrograde approach (4 of 7 patients).11,23,30–33 Procedural limitations were migration or embolization of the prosthetic valve, failure to cross the stenotic aortic valve, and paravalvular aortic regurgitation.23 Anatomic and functional success was evidenced by improvement in aortic valve area, increase in left ventricular ejection fraction, and improved New York Heart Association functional class, all of which were sustained at up to 24 months.23
Webb et al11 reported similar results with retrograde implantation of the Cribier-Edwards valve in a cohort of 50 patients.11 The main difference between the two studies was the expected occurrence of aortofemoral complications with the retrograde approach.11,26 Procedural success increased from 76% in the first 25 patients to 96% in the second 25, and the 30-day mortality rate fell from 16% to 8%, which reflected the learning curve. Importantly, no patients needed conversion to open surgery during the first 30 days, and at a median follow-up of 359 days 35 (81%) of 43 patients who underwent successful transcatheter aortic valve replacement were still alive.11 Additionally, significant improvement was noted in left ventricular ejection fraction, mitral regurgitation, and New York Heart Association functional class, and these improvements persisted at 1 year.11
Lichtenstein et al31 and Walther et al32 successfully implanted the Cribier-Edwards valve using the transapical approach in a very high-risk elderly population with poor functional class. All patients were deemed unsuitable for standard surgical valve replacement and also for percutaneous transfemoral aortic valve implantation because of severe aorto-iliac disease. In both studies, the short-term and mid-term results were encouraging.
These experiences with the Cribier-Edwards valve showed that device- and technique-related shortcomings could be addressed. To date, more than 500 percutaneous aortic valve replacement procedures have been done with the Cribier-Edwards valve worldwide, with a greater than 95% technical success rate in the latest cohorts.36 Importantly, use of a larger (26-mm) prosthetic valve has been associated with a lower rate of prosthetic valve migration or embolization, and with a significantly lower rate of paravalvular aortic regurgitation.11,23
EXPERIENCE WITH THE COREVALVE SYSTEM
The CoreValve ReValving system is based on retrograde implantation of the CoreValve prosthesis—a self-expanding aortic valve prosthesis composed of three bovine pericardial leaflets mounted and sutured within a self-expanding 50-mm-long nitinol stent (Table 1).28–30 The inner diameter is 21 to 22 mm.28–30 This prosthesis has three distinct structural segments.28–30 The bottom portion exerts a high radial force that expands and pushes aside the calcified leaflets and avoids recoil; the central portion carries the valve, and it tapers to avoid the coronary artery ostia; and the upper portion flares to fixate and stabilize the deployed aortic valve prosthesis in the ascending aorta, thus preventing migration or embolization of the device.28–30 The main difference between the CoreValve and the Cribier-Edwards valve is that the Core-Valve is self-expanding, which theoretically permits it to conform to different aortic sizes and to anchor well in the aortic annulus.28–30 This feature allows the CoreValve to be used in patients with severe aortic insufficiency and other noncalcific aortic valvular conditions. The CoreValve has not yet been deployed via antegrade or transapical technique.
The first-generation CoreValve prosthesis was first implanted in a human recipient in 2005.29 Since then, improvements have been made, leading to the development of second- and third-generation devices. A pilot study of implantation of the first-generation CoreValve28 via the retrograde approach in elderly patients with poor functional class and severe aortic stenosis had a short-term procedural success rate of 84% (21 of 25 patients), with a significant reduction in the mean aortic valve gradient and improved functional class at 30-day follow-up.28 At 30 days, 17 (94%) of 18 patients had no or only mild aortic regurgitation.28 Procedural limitations and complications were similar to those with the Cribier-Edwards valve.
In a study of second- and third-generation devices (50 patients received a second-generation device, and 36 received a third-generation device),30 again in elderly patients with poor functional class and severe aortic stenosis, the short-term success rate of the device was 88% (76 of 86) in each group. After the procedure, the mean aortic valve gradient decreased significantly and functional class improved significantly.30 Immediate after implantation, no patient had more than moderate aortic regurgitation, and in 51 patients (66%) the aortic regurgitation remained unchanged or improved after CoreValve implantation.30 These results were maintained at 30-day follow-up.
CoreValve was approved in May 2007 for clinical use in Europe.36 Of note, CoreValve has also been used to treat severe aortic regurgitation of a degenerated bioprosthetic aortic valve in an 80-year-old man with multiple comorbidities.37
EXPERIENCE WITH THE EDWARDS SAPIEN VALVE
The Edwards SAPIEN valve is a modification of the initial Cribier-Edwards valve and is the latest percutaneous aortic valve prosthesis to enter clinical trials (Table 1). It is a trileaflet balloon-expandable stainless steel valve made from bovine pericardial tissue, available in two sizes (23 mm and 26 mm). In September 2007, it was approved for use in Europe with the RetroFlex transfemoral delivery system. The Ascendra transapical delivery system for the Edward SAPIEN valve has received approval in Europe.
The multicenter Placement of Aortic Transcatheter Valves (PARTNER) trial in North America is continuing to enroll patients, with enrollment projected to be complete by the end of 2008. The aim of this prospective randomized clinical trial is to enroll 1,040 patients in two separate treatment arms. The surgical arm of the trial is comparing the Edwards SAPIEN valve with standard surgical aortic valve replacement, with the objective of demonstrating non-inferiority. The medical management arm of the trial is comparing percutaneous valve replacement against medical therapy or balloon valvuloplasty in patients considered too high-risk for conventional surgical valve replacement.
The primary end point in both arms is death at 1 year; secondary end points focus on long-term (1-year) composite cardiovascular events, valve performance, and quality-of-life indicators. Preliminary data on the first 100 patients (74 via the transfemoral [ie, retrograde] and 26 via the transapical approach) who underwent percutaneous Edwards SAPIEN valve implantation for compassionate use showed device durability and symptom relief at up to 2 years.38 Overall procedural success was 91%, but, as with other trials, there was a steep learning curve, so that excluding the first 25 patients increased the procedural success rate to 96%.38 Aortic valve size and hemodynamics, left ventricular systolic function, mitral regurgitation, and functional class were all significantly improved. Mild aortic regurgitation was common, but none of the patients had severe aortic regurgitation. Importantly, the 15% 30-day death rate was significantly lower than the expected rate of 33%. The 6-month survival rate was 78%, but the 2-year rate was 60% in this high-risk elderly cohort.
Walther et al39 recently reported outcomes on their first 50 patients who underwent transapical implantation of the Edwards SAPIEN valve. The operators were able to implant the prosthesis in all 50 patients, but 3 required early conversion to open surgery with sternotomy. The overall survival at 30 days was 92%, but in the last 25 patients the 30-day survival rate was 96%, with a 1-year survival rate of 80%.
PUTTING THE DATA IN PERSPECTIVE
As noted in this review, a number of factors make a strong case for timely aortic valve replacement: the aging population, the increase in incidence and prevalence of aortic stenosis,1,3,4,27,40 the multiple comorbidities in older patients, and the eventually aggressive natural course of aortic stenosis.1,3,4,27,40–43 Yet current standards dictate not to proceed with standard surgical aortic valve replacement in patients who are truly asymptomatic and who have normal left ventricular systolic function,1,40 mainly because the risks of surgical valve replacement outweigh the benefits in this population.1,40 Aortic valve surgery carries a risk of early death of 15% for patients ages 80 to 84 and of 18% for patients age 85.3,9,10,12,43–45
These figures seem high when compared with death rates of 12% in recent studies of percutaneous valve replacement in similar patients.11,23,30,33 The rates become lower as the learning curve improves.11,21,23,27,30,33 Thus, as the design of aortic valve prostheses and the techniques to implant them are refined and tested for safety, the risk-benefit balance may change in favor of earlier intervention in aortic stenosis with a percutaneous approach.11,21,27,46 Some experts believe that in 10 years 10% to 30% of patients undergoing conventional valve replacement will be candidates for a percutaneous approach.
Of the techniques used to date, the retrograde approach seems most amenable to widespread acceptance, given its inherent advantage of being faster and easier.11,21,30 Limitations with the retrograde approach seen in earlier trials—challenges and complications associated with large-bore arterial vascular access, difficulty traversing the aortic arch with bulky devices, and the inability to cross the stenotic aortic valve to deploy the prosthesis even after balloon valvuloplasty11,21,30—are correctable with refinements in the devices and in technique.
New types of prosthetic aortic valves entering early human studies are improving on current devices, for example, by using collapsible, inflatable valve frames for retrievability before final deployment.
Surgical aortic valve replacement remains the gold standard treatment for patients with symptomatic aortic stenosis. And while studies of percutaneous aortic valve replacement show great promise for this less-invasive treat-men, enthusiasm about percutaneous aortic valve replacement should be tempered by an awareness of persistent limitations of this approach, such as vascular and mechanical complications and operator inexperience, which still need attention.
- Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006; 114:e84–231.
- Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 2005; 111:3316–3326.
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003; 24:1231–1243.
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368:1005–1011.
- Goodney PP, O'Connor GT, Wennberg DE, Birkmeyer JD. Do hospitals with low mortality rates in coronary artery bypass also perform well in valve replacement? Ann Thorac Surg 2003; 76:1131–1137.
- Cribier A, Savin T, Saoudi N, Rocha P, Berland J, Letac B. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet 1986; 1:63–67.
- Vahanian A, Palacios IF. Percutaneous approaches to valvular disease. Circulation 2004; 109:1572–1579.
- Webb JG, Munt B, Makkar RR, Naqvi TZ, Dang N. Percutaneous stent-mounted valve for treatment of aortic or pulmonary valve disease. Catheter Cardiovasc Interv 2004; 63:89–93.
- Alexander KP, Anstrom KJ, Muhlbaier LH, et al. Outcomes of cardiac surgery in patients =80 years: results from the National Cardiovascular Network. J Am Coll Cardiol 2000; 35:731–738.
- Mittermair RP, Muller LC. Quality of life after cardiac surgery in the elderly. J Cardiovasc Surg (Torino) 2002; 43:43–47.
- Webb JG, Pasupati S, Humphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007; 116:755–763.
- Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005; 26:2714–2720.
- Bonhoeffer P, Boudjemline Y, Saliba Z, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet 2000; 356:1403–1405.
- Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J 1992; 13:704–708.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002; 106:3006–3008.
- Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994; 89:642–650.
- Safian RD, Berman AD, Diver DJ, et al. Balloon aortic valvuloplasty in 170 consecutive patients. N Engl J Med 1988; 319:125–130.
- Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation 1991; 84:2383–2397.
- Safian RD, Mandell VS, Thurer RE, et al. Postmortem and intraoperative balloon valvuloplasty of calcific aortic stenosis in elderly patients: mechanisms of successful dilation. J Am Coll Cardiol 1987; 9:655–660.
- Agarwal A, Kini AS, Attanti S, et al. Results of repeat balloon valvuloplasty for treatment of aortic stenosis in patients aged 59 to 104 years. Am J Cardiol 2005; 95:43–47.
- Kapadia SR, Wazni OM, Tan WA, et al. Aortic valvuloplasty in 1990's: experience from a single center in United States. Circulation 1999; 100 18 suppl 1:1–448.
- Lieberman EB, Bashore TM, Hermiller JB, et al. Balloon aortic valvuloplasty in adults: failure of procedure to improve long-term survival. J Am Coll Cardiol 1995; 26:1522–1528.
- Cribier A, Eltchaninoff H, Tron C, et al. Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J Am Coll Cardiol 2006; 47:1214–1223.
- Cribier A, Eltchaninoff H, Tron C, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol 2004; 43:698–703.
- Rajagopal V, Kapadia SR, Tuzcu EM. Advances in the percutaneous treatment of aortic and mitral valve disease. Minerva Cardioangiol 2007; 55:83–94.
- Webb JG, Chandavimol M, Thompson CR, et al. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation 2006; 113:842–850.
- Salemi A. Percutaneous valve interventions. Curr Opin Anaesthesiol 2007; 20:70–74.
- Grube E, Laborde JC, Gerckens U, et al. Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study. Circulation 2006; 114:1616–1624.
- Grube E, Laborde JC, Zickmann B, et al. First report on a human percutaneous transluminal implantation of a self-expanding valve prosthesis for interventional treatment of aortic valve stenosis. Catheter Cardiovasc Interv 2005; 66:465–469.
- Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol 2007; 50:69–76.
- Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve implantation in humans: initial clinical experience. Circulation 2006; 114:591–596.
- Walther T, Falk V, Borger MA, et al. Minimally invasive transapical beating heart aortic valve implantation—proof of concept. Eur J Cardiothorac Surg 2007; 31:9–15.
- Ye J, Cheung A, Lichtenstein SV, et al. Six-month outcome of transapical transcatheter aortic valve implantation in the initial seven patients. Eur J Cardiothorac Surg 2007; 31:16–21.
- Turgut T, Deeb M, Moscucci M. Left ventricular apical puncture: a procedure surviving well into the new millennium. Catheter Cardiovasc Interv 2000; 49:68–73.
- Zuguchi M, Shindoh C, Chida K, et al. Safety and clinical benefits of transsubxiphoidal left ventricular puncture. Catheter Cardiovasc Interv 2002; 55:58–65.
- Sinha AK, Kini AS, Sharma SK. Percutaneous valve replacement: a paradigm shift. Curr Opin Cardiol 2007; 22:471–477.
- Wenaweser P, Buellesfeld L, Gerckens U, Grube E. Percutaneous aortic valve replacement for severe aortic regurgitation in degenerated bioprosthesis: the first valve in valve procedure using the CoreValve ReValving system. Catheter Cardiovasc Interv 2007; 70:760–764.
- Pasupati S, Humphries K, AlAli A, et al. Balloon expandable aortic valve (BEAV) implantation. The first 100 Canadian patients. Circulation 2007; 116 suppl:357.
- Walther T, Falk V, Kempfert J, et al. Transapical minimally invasive aortic valve implantation; the initial 50 patients. Eur J Cardiothorac Surg 2008; 33:983–988. Epub 2008 February 21.
- Carabello BA. Clinical practice. Aortic stenosis. N Engl J Med 2002; 346:677–682.
- Pellikka PA, Nishimura RA, Bailey KR, Tajik AJ. The natural history of adults with asymptomatic, hemodynamically significant aortic stenosis. J Am Coll Cardiol 1990; 15:1012–1017.
- Ross J, Braunwald E. Aortic stenosis. Circulation 1968; 38:61–67.
- Kvidal P, Bergstrom R, Horte LG, Stahle E. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol 2000; 35:747–756.
- Society of Thoracic Surgeons National Cardiac Surgery Database. Available at www.sts.org/documents/pdf/Spring2005STS-ExecutiveSummary.pdf. Accessed 9/11/2008.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002; 346:1128–1137.
- Wenger NK, Weber MA, Scheidt S. Valvular heart disease at elderly age: new vistas. Am J Geriatr Cardiol 2006; 15:273–274.
Stenosis of the aortic valve has a long, latent, asymptomatic phase, but when symptoms finally occur, clinical deterioration can be rapid. For patients with severe stenosis, the standard treatment has long been replacement of the aortic valve via open heart surgery. But many patients with severe stenosis are considered too high-risk for this procedure.
Until about 5 years ago, these patients had no other option but medical therapy or percutaneous aortic balloon valvuloplasty as a palliative measure or as a bridge to open heart surgery. But 5 years of experience with percutaneous techniques to implant prosthetic aortic valves show that this less-invasive approach may become a viable option for patients with severe symptomatic aortic valve stenosis.
In this review, we discuss current prosthetic valves and percutaneous techniques and their relative advantages and limitations and the potential future role of this new treatment option.
THE NEED FOR A LESS-INVASIVE APPROACH
Calcific aortic stenosis is the most common valvular heart disease, affecting 2% to 4% of adults over age 65 in the United States alone.1,2 The aging of our population and the lack of drug therapies to prevent, halt, or effectively slow aortic valve stenosis are leading to a greater burden of this condition.1,3,4 Already in the United States more than 50,000 surgical aortic valve replacements are performed every year for severe aortic stenosis.1,2 The associated in-hospital death rate is 8.8% in patients over age 65 years, and as high as 13% in low-volume centers.1,5
The steady increase in the number of patients requiring aortic valve replacement, the high surgical risk in patients with multiple comorbidities, the reluctance of some patients to undergo the trauma and pain associated with open heart surgery via sternotomy, and the fact that percutaneous procedures are less traumatic and offer faster recovery and fewer hospital days—all these are forces that have been driving the development of percutaneous techniques for the treatment of aortic stenosis.6–11 In addition, a recent study12 showed that 33% of patients over age 75 were deemed too high-risk for open heart surgery and thus were left untreated.12
The evolution of percutaneous aortic valve replacement
The idea of percutaneous treatment of aortic stenosis was first put into clinical practice in 1985, when Cribier performed an aortic balloon valvuloplasty.6 This was followed in 200013 by the first successful implantation of a catheter-based stent valve in a human, and in 2002 by the first successful percutaneous aortic valve replacement in a human.13–15 In the following sections, we discuss the percutaneous approaches in current use for the treatment of degenerative aortic stenosis.
AORTIC BALLOON VALVULOPLASTY
Percutaneous aortic balloon valvuloplasty, partial dilation of the stenotic aortic valve with a balloon inserted via a catheter,1,16–19 improves symptoms but has failed to show a sustained benefit on rates of mortality or morbidity.1,16–18 The restenosis rate is high, and symptoms recur in most patients within months to a year.1,16–18 Procedural complication rates are about 10%, and complication rates at the catheter access site are even higher.1,16–18 The 30-day death rate in the National Heart, Lung, and Blood Institute’s Balloon Valvuloplasty Registry, which included more than 600 patients, was 14%.18 In a retrospective study of 212 patients who underwent single or repeat percutaneous aortic balloon valvuloplasty,20 the 1-year mortality rate was 36% for the entire cohort, with a median survival of 3 years. Patients who underwent a repeat procedure (33%) had 1-year mortality rate of 42%, compared with 16% in patients who did not undergo a repeat procedure.20
Percutaneous aortic balloon valvuloplasty serves best as palliative therapy in severely symptomatic patients, and as a bridge to surgery in hemodynamically unstable adult patients.21,22 Percutaneous aortic balloon valvuloplasty is not an option in patients who are good candidates for surgical valve replacement.1
PERCUTANEOUS AORTIC VALVE REPLACEMENT: THREE TECHNIQUES
The antegrade technique
The retrograde technique
In the retrograde (ie, transfemoral) technique, access to the femoral artery is gained and the catheter with the prosthetic aortic valve is advanced to the stenotic aortic valve.8,11,26,28–30 This approach is faster and technically easier than the antegrade approach, but it can be associated with injury to the aortofemoral vessels and with failure of the prosthesis to cross the aortic arch or the stenotic aortic valve.11,23,30
The transapical technique
In the transapical technique, the valve delivery system is inserted via a small incision made between the ribs. The apex of the left ventricle is punctured with a needle, and the prosthetic valve is positioned within the stenotic aortic valve.27,31–33 The main advantage of this approach is that it allows more direct access to the aortic valve and eliminates the need for a large peripheral vascular access site in patients with peripheral vascular disease, small tortuous vasculature, or a history of major vascular complications or vascular repairs.31–33 Potential disadvantages are related to the left ventricular apical puncture and include adverse ventricular remodeling, left ventricular aneurysm or pseudoaneurysm, pericardial complications, pneumothorax, malignant ventricular arrhythmias, coronary artery injury, and the need for general anesthesia and chest tubes.27,31–35
Common features of the three approaches
The three percutaneous approaches have certain final steps in common.11,23,30,33 The position of final deployment of the prosthetic valve is determined by the patient’s native valvular structure and anatomy and is optimized by using fluoroscopic imaging of the native aortic valve calcification as an anatomical marker, along with guidance from supra-aortic angiography and transesophageal echocardiography.11,23,30,33 Ideally, the aortic valve prosthesis is placed at mid-position in the patient’s aortic valve, taking care to not to impinge on the coronary ostia or to impede the motion of the anterior mitral leaflet.11,23,30,33 In all three procedures, the prosthesis is then deployed by maximally inflating, rapidly deflating, and immediately withdrawing the delivery balloon. This final step is carried out during temporary high-rate right ventricular apical pacing, which produces ventricular tachycardia at 180 to 220 beats/min for up to 10 seconds.11,23,30,33 This leads to an immediate decrease in stroke volume, resulting in minimal forward flow through the aortic valve, which in turn facilitates precise positioning of the prosthetic valve.
So far, only the Cribier-Edwards valve has been deployed via all three techniques. The CoreValve has been deployed only via the retrograde technique. The Edwards SAPIEN valve has been deployed with retrograde and transapical approaches (see www.edwards.com/Products/TranscatheterValves/SapienTHV.htm and www.corevalve.com for animations depicting these techniques).
EXPERIENCE WITH THE CRIBIER-EDWARDS VALVE
The Cribier-Edwards valve has three leaflets made from equine pericardial tissue sutured inside a balloon-expandable stainless steel 14-mm stent (Table 1).11,23,33 With the use of a specially designed mechanical crimping device, the aortic valve prosthesis is mounted over a 3-cm-long balloon catheter, expandable to a diameter of 22 to 26 mm (NuMed Inc, Hopkinton, NY).11,23,30,33
After this prosthesis was tested in animal models,14,15 a trial for compassionate use in humans was begun, called the Initial Registry of Endovascular Implantation of Valves in Europe (I-REVIVE) trial. This trial was later continued as the Registry of Endovascular Critical Aortic Stenosis Treatment (RECAST) trial.23 All patients were formally evaluated by two cardio-thoracic surgeons and were deemed inappropriate for surgical aortic valve replacement.23
The success rate with the antegrade percutaneous approach was 85% (23 of 27 patients) and 57% for the retrograde approach (4 of 7 patients).11,23,30–33 Procedural limitations were migration or embolization of the prosthetic valve, failure to cross the stenotic aortic valve, and paravalvular aortic regurgitation.23 Anatomic and functional success was evidenced by improvement in aortic valve area, increase in left ventricular ejection fraction, and improved New York Heart Association functional class, all of which were sustained at up to 24 months.23
Webb et al11 reported similar results with retrograde implantation of the Cribier-Edwards valve in a cohort of 50 patients.11 The main difference between the two studies was the expected occurrence of aortofemoral complications with the retrograde approach.11,26 Procedural success increased from 76% in the first 25 patients to 96% in the second 25, and the 30-day mortality rate fell from 16% to 8%, which reflected the learning curve. Importantly, no patients needed conversion to open surgery during the first 30 days, and at a median follow-up of 359 days 35 (81%) of 43 patients who underwent successful transcatheter aortic valve replacement were still alive.11 Additionally, significant improvement was noted in left ventricular ejection fraction, mitral regurgitation, and New York Heart Association functional class, and these improvements persisted at 1 year.11
Lichtenstein et al31 and Walther et al32 successfully implanted the Cribier-Edwards valve using the transapical approach in a very high-risk elderly population with poor functional class. All patients were deemed unsuitable for standard surgical valve replacement and also for percutaneous transfemoral aortic valve implantation because of severe aorto-iliac disease. In both studies, the short-term and mid-term results were encouraging.
These experiences with the Cribier-Edwards valve showed that device- and technique-related shortcomings could be addressed. To date, more than 500 percutaneous aortic valve replacement procedures have been done with the Cribier-Edwards valve worldwide, with a greater than 95% technical success rate in the latest cohorts.36 Importantly, use of a larger (26-mm) prosthetic valve has been associated with a lower rate of prosthetic valve migration or embolization, and with a significantly lower rate of paravalvular aortic regurgitation.11,23
EXPERIENCE WITH THE COREVALVE SYSTEM
The CoreValve ReValving system is based on retrograde implantation of the CoreValve prosthesis—a self-expanding aortic valve prosthesis composed of three bovine pericardial leaflets mounted and sutured within a self-expanding 50-mm-long nitinol stent (Table 1).28–30 The inner diameter is 21 to 22 mm.28–30 This prosthesis has three distinct structural segments.28–30 The bottom portion exerts a high radial force that expands and pushes aside the calcified leaflets and avoids recoil; the central portion carries the valve, and it tapers to avoid the coronary artery ostia; and the upper portion flares to fixate and stabilize the deployed aortic valve prosthesis in the ascending aorta, thus preventing migration or embolization of the device.28–30 The main difference between the CoreValve and the Cribier-Edwards valve is that the Core-Valve is self-expanding, which theoretically permits it to conform to different aortic sizes and to anchor well in the aortic annulus.28–30 This feature allows the CoreValve to be used in patients with severe aortic insufficiency and other noncalcific aortic valvular conditions. The CoreValve has not yet been deployed via antegrade or transapical technique.
The first-generation CoreValve prosthesis was first implanted in a human recipient in 2005.29 Since then, improvements have been made, leading to the development of second- and third-generation devices. A pilot study of implantation of the first-generation CoreValve28 via the retrograde approach in elderly patients with poor functional class and severe aortic stenosis had a short-term procedural success rate of 84% (21 of 25 patients), with a significant reduction in the mean aortic valve gradient and improved functional class at 30-day follow-up.28 At 30 days, 17 (94%) of 18 patients had no or only mild aortic regurgitation.28 Procedural limitations and complications were similar to those with the Cribier-Edwards valve.
In a study of second- and third-generation devices (50 patients received a second-generation device, and 36 received a third-generation device),30 again in elderly patients with poor functional class and severe aortic stenosis, the short-term success rate of the device was 88% (76 of 86) in each group. After the procedure, the mean aortic valve gradient decreased significantly and functional class improved significantly.30 Immediate after implantation, no patient had more than moderate aortic regurgitation, and in 51 patients (66%) the aortic regurgitation remained unchanged or improved after CoreValve implantation.30 These results were maintained at 30-day follow-up.
CoreValve was approved in May 2007 for clinical use in Europe.36 Of note, CoreValve has also been used to treat severe aortic regurgitation of a degenerated bioprosthetic aortic valve in an 80-year-old man with multiple comorbidities.37
EXPERIENCE WITH THE EDWARDS SAPIEN VALVE
The Edwards SAPIEN valve is a modification of the initial Cribier-Edwards valve and is the latest percutaneous aortic valve prosthesis to enter clinical trials (Table 1). It is a trileaflet balloon-expandable stainless steel valve made from bovine pericardial tissue, available in two sizes (23 mm and 26 mm). In September 2007, it was approved for use in Europe with the RetroFlex transfemoral delivery system. The Ascendra transapical delivery system for the Edward SAPIEN valve has received approval in Europe.
The multicenter Placement of Aortic Transcatheter Valves (PARTNER) trial in North America is continuing to enroll patients, with enrollment projected to be complete by the end of 2008. The aim of this prospective randomized clinical trial is to enroll 1,040 patients in two separate treatment arms. The surgical arm of the trial is comparing the Edwards SAPIEN valve with standard surgical aortic valve replacement, with the objective of demonstrating non-inferiority. The medical management arm of the trial is comparing percutaneous valve replacement against medical therapy or balloon valvuloplasty in patients considered too high-risk for conventional surgical valve replacement.
The primary end point in both arms is death at 1 year; secondary end points focus on long-term (1-year) composite cardiovascular events, valve performance, and quality-of-life indicators. Preliminary data on the first 100 patients (74 via the transfemoral [ie, retrograde] and 26 via the transapical approach) who underwent percutaneous Edwards SAPIEN valve implantation for compassionate use showed device durability and symptom relief at up to 2 years.38 Overall procedural success was 91%, but, as with other trials, there was a steep learning curve, so that excluding the first 25 patients increased the procedural success rate to 96%.38 Aortic valve size and hemodynamics, left ventricular systolic function, mitral regurgitation, and functional class were all significantly improved. Mild aortic regurgitation was common, but none of the patients had severe aortic regurgitation. Importantly, the 15% 30-day death rate was significantly lower than the expected rate of 33%. The 6-month survival rate was 78%, but the 2-year rate was 60% in this high-risk elderly cohort.
Walther et al39 recently reported outcomes on their first 50 patients who underwent transapical implantation of the Edwards SAPIEN valve. The operators were able to implant the prosthesis in all 50 patients, but 3 required early conversion to open surgery with sternotomy. The overall survival at 30 days was 92%, but in the last 25 patients the 30-day survival rate was 96%, with a 1-year survival rate of 80%.
PUTTING THE DATA IN PERSPECTIVE
As noted in this review, a number of factors make a strong case for timely aortic valve replacement: the aging population, the increase in incidence and prevalence of aortic stenosis,1,3,4,27,40 the multiple comorbidities in older patients, and the eventually aggressive natural course of aortic stenosis.1,3,4,27,40–43 Yet current standards dictate not to proceed with standard surgical aortic valve replacement in patients who are truly asymptomatic and who have normal left ventricular systolic function,1,40 mainly because the risks of surgical valve replacement outweigh the benefits in this population.1,40 Aortic valve surgery carries a risk of early death of 15% for patients ages 80 to 84 and of 18% for patients age 85.3,9,10,12,43–45
These figures seem high when compared with death rates of 12% in recent studies of percutaneous valve replacement in similar patients.11,23,30,33 The rates become lower as the learning curve improves.11,21,23,27,30,33 Thus, as the design of aortic valve prostheses and the techniques to implant them are refined and tested for safety, the risk-benefit balance may change in favor of earlier intervention in aortic stenosis with a percutaneous approach.11,21,27,46 Some experts believe that in 10 years 10% to 30% of patients undergoing conventional valve replacement will be candidates for a percutaneous approach.
Of the techniques used to date, the retrograde approach seems most amenable to widespread acceptance, given its inherent advantage of being faster and easier.11,21,30 Limitations with the retrograde approach seen in earlier trials—challenges and complications associated with large-bore arterial vascular access, difficulty traversing the aortic arch with bulky devices, and the inability to cross the stenotic aortic valve to deploy the prosthesis even after balloon valvuloplasty11,21,30—are correctable with refinements in the devices and in technique.
New types of prosthetic aortic valves entering early human studies are improving on current devices, for example, by using collapsible, inflatable valve frames for retrievability before final deployment.
Surgical aortic valve replacement remains the gold standard treatment for patients with symptomatic aortic stenosis. And while studies of percutaneous aortic valve replacement show great promise for this less-invasive treat-men, enthusiasm about percutaneous aortic valve replacement should be tempered by an awareness of persistent limitations of this approach, such as vascular and mechanical complications and operator inexperience, which still need attention.
Stenosis of the aortic valve has a long, latent, asymptomatic phase, but when symptoms finally occur, clinical deterioration can be rapid. For patients with severe stenosis, the standard treatment has long been replacement of the aortic valve via open heart surgery. But many patients with severe stenosis are considered too high-risk for this procedure.
Until about 5 years ago, these patients had no other option but medical therapy or percutaneous aortic balloon valvuloplasty as a palliative measure or as a bridge to open heart surgery. But 5 years of experience with percutaneous techniques to implant prosthetic aortic valves show that this less-invasive approach may become a viable option for patients with severe symptomatic aortic valve stenosis.
In this review, we discuss current prosthetic valves and percutaneous techniques and their relative advantages and limitations and the potential future role of this new treatment option.
THE NEED FOR A LESS-INVASIVE APPROACH
Calcific aortic stenosis is the most common valvular heart disease, affecting 2% to 4% of adults over age 65 in the United States alone.1,2 The aging of our population and the lack of drug therapies to prevent, halt, or effectively slow aortic valve stenosis are leading to a greater burden of this condition.1,3,4 Already in the United States more than 50,000 surgical aortic valve replacements are performed every year for severe aortic stenosis.1,2 The associated in-hospital death rate is 8.8% in patients over age 65 years, and as high as 13% in low-volume centers.1,5
The steady increase in the number of patients requiring aortic valve replacement, the high surgical risk in patients with multiple comorbidities, the reluctance of some patients to undergo the trauma and pain associated with open heart surgery via sternotomy, and the fact that percutaneous procedures are less traumatic and offer faster recovery and fewer hospital days—all these are forces that have been driving the development of percutaneous techniques for the treatment of aortic stenosis.6–11 In addition, a recent study12 showed that 33% of patients over age 75 were deemed too high-risk for open heart surgery and thus were left untreated.12
The evolution of percutaneous aortic valve replacement
The idea of percutaneous treatment of aortic stenosis was first put into clinical practice in 1985, when Cribier performed an aortic balloon valvuloplasty.6 This was followed in 200013 by the first successful implantation of a catheter-based stent valve in a human, and in 2002 by the first successful percutaneous aortic valve replacement in a human.13–15 In the following sections, we discuss the percutaneous approaches in current use for the treatment of degenerative aortic stenosis.
AORTIC BALLOON VALVULOPLASTY
Percutaneous aortic balloon valvuloplasty, partial dilation of the stenotic aortic valve with a balloon inserted via a catheter,1,16–19 improves symptoms but has failed to show a sustained benefit on rates of mortality or morbidity.1,16–18 The restenosis rate is high, and symptoms recur in most patients within months to a year.1,16–18 Procedural complication rates are about 10%, and complication rates at the catheter access site are even higher.1,16–18 The 30-day death rate in the National Heart, Lung, and Blood Institute’s Balloon Valvuloplasty Registry, which included more than 600 patients, was 14%.18 In a retrospective study of 212 patients who underwent single or repeat percutaneous aortic balloon valvuloplasty,20 the 1-year mortality rate was 36% for the entire cohort, with a median survival of 3 years. Patients who underwent a repeat procedure (33%) had 1-year mortality rate of 42%, compared with 16% in patients who did not undergo a repeat procedure.20
Percutaneous aortic balloon valvuloplasty serves best as palliative therapy in severely symptomatic patients, and as a bridge to surgery in hemodynamically unstable adult patients.21,22 Percutaneous aortic balloon valvuloplasty is not an option in patients who are good candidates for surgical valve replacement.1
PERCUTANEOUS AORTIC VALVE REPLACEMENT: THREE TECHNIQUES
The antegrade technique
The retrograde technique
In the retrograde (ie, transfemoral) technique, access to the femoral artery is gained and the catheter with the prosthetic aortic valve is advanced to the stenotic aortic valve.8,11,26,28–30 This approach is faster and technically easier than the antegrade approach, but it can be associated with injury to the aortofemoral vessels and with failure of the prosthesis to cross the aortic arch or the stenotic aortic valve.11,23,30
The transapical technique
In the transapical technique, the valve delivery system is inserted via a small incision made between the ribs. The apex of the left ventricle is punctured with a needle, and the prosthetic valve is positioned within the stenotic aortic valve.27,31–33 The main advantage of this approach is that it allows more direct access to the aortic valve and eliminates the need for a large peripheral vascular access site in patients with peripheral vascular disease, small tortuous vasculature, or a history of major vascular complications or vascular repairs.31–33 Potential disadvantages are related to the left ventricular apical puncture and include adverse ventricular remodeling, left ventricular aneurysm or pseudoaneurysm, pericardial complications, pneumothorax, malignant ventricular arrhythmias, coronary artery injury, and the need for general anesthesia and chest tubes.27,31–35
Common features of the three approaches
The three percutaneous approaches have certain final steps in common.11,23,30,33 The position of final deployment of the prosthetic valve is determined by the patient’s native valvular structure and anatomy and is optimized by using fluoroscopic imaging of the native aortic valve calcification as an anatomical marker, along with guidance from supra-aortic angiography and transesophageal echocardiography.11,23,30,33 Ideally, the aortic valve prosthesis is placed at mid-position in the patient’s aortic valve, taking care to not to impinge on the coronary ostia or to impede the motion of the anterior mitral leaflet.11,23,30,33 In all three procedures, the prosthesis is then deployed by maximally inflating, rapidly deflating, and immediately withdrawing the delivery balloon. This final step is carried out during temporary high-rate right ventricular apical pacing, which produces ventricular tachycardia at 180 to 220 beats/min for up to 10 seconds.11,23,30,33 This leads to an immediate decrease in stroke volume, resulting in minimal forward flow through the aortic valve, which in turn facilitates precise positioning of the prosthetic valve.
So far, only the Cribier-Edwards valve has been deployed via all three techniques. The CoreValve has been deployed only via the retrograde technique. The Edwards SAPIEN valve has been deployed with retrograde and transapical approaches (see www.edwards.com/Products/TranscatheterValves/SapienTHV.htm and www.corevalve.com for animations depicting these techniques).
EXPERIENCE WITH THE CRIBIER-EDWARDS VALVE
The Cribier-Edwards valve has three leaflets made from equine pericardial tissue sutured inside a balloon-expandable stainless steel 14-mm stent (Table 1).11,23,33 With the use of a specially designed mechanical crimping device, the aortic valve prosthesis is mounted over a 3-cm-long balloon catheter, expandable to a diameter of 22 to 26 mm (NuMed Inc, Hopkinton, NY).11,23,30,33
After this prosthesis was tested in animal models,14,15 a trial for compassionate use in humans was begun, called the Initial Registry of Endovascular Implantation of Valves in Europe (I-REVIVE) trial. This trial was later continued as the Registry of Endovascular Critical Aortic Stenosis Treatment (RECAST) trial.23 All patients were formally evaluated by two cardio-thoracic surgeons and were deemed inappropriate for surgical aortic valve replacement.23
The success rate with the antegrade percutaneous approach was 85% (23 of 27 patients) and 57% for the retrograde approach (4 of 7 patients).11,23,30–33 Procedural limitations were migration or embolization of the prosthetic valve, failure to cross the stenotic aortic valve, and paravalvular aortic regurgitation.23 Anatomic and functional success was evidenced by improvement in aortic valve area, increase in left ventricular ejection fraction, and improved New York Heart Association functional class, all of which were sustained at up to 24 months.23
Webb et al11 reported similar results with retrograde implantation of the Cribier-Edwards valve in a cohort of 50 patients.11 The main difference between the two studies was the expected occurrence of aortofemoral complications with the retrograde approach.11,26 Procedural success increased from 76% in the first 25 patients to 96% in the second 25, and the 30-day mortality rate fell from 16% to 8%, which reflected the learning curve. Importantly, no patients needed conversion to open surgery during the first 30 days, and at a median follow-up of 359 days 35 (81%) of 43 patients who underwent successful transcatheter aortic valve replacement were still alive.11 Additionally, significant improvement was noted in left ventricular ejection fraction, mitral regurgitation, and New York Heart Association functional class, and these improvements persisted at 1 year.11
Lichtenstein et al31 and Walther et al32 successfully implanted the Cribier-Edwards valve using the transapical approach in a very high-risk elderly population with poor functional class. All patients were deemed unsuitable for standard surgical valve replacement and also for percutaneous transfemoral aortic valve implantation because of severe aorto-iliac disease. In both studies, the short-term and mid-term results were encouraging.
These experiences with the Cribier-Edwards valve showed that device- and technique-related shortcomings could be addressed. To date, more than 500 percutaneous aortic valve replacement procedures have been done with the Cribier-Edwards valve worldwide, with a greater than 95% technical success rate in the latest cohorts.36 Importantly, use of a larger (26-mm) prosthetic valve has been associated with a lower rate of prosthetic valve migration or embolization, and with a significantly lower rate of paravalvular aortic regurgitation.11,23
EXPERIENCE WITH THE COREVALVE SYSTEM
The CoreValve ReValving system is based on retrograde implantation of the CoreValve prosthesis—a self-expanding aortic valve prosthesis composed of three bovine pericardial leaflets mounted and sutured within a self-expanding 50-mm-long nitinol stent (Table 1).28–30 The inner diameter is 21 to 22 mm.28–30 This prosthesis has three distinct structural segments.28–30 The bottom portion exerts a high radial force that expands and pushes aside the calcified leaflets and avoids recoil; the central portion carries the valve, and it tapers to avoid the coronary artery ostia; and the upper portion flares to fixate and stabilize the deployed aortic valve prosthesis in the ascending aorta, thus preventing migration or embolization of the device.28–30 The main difference between the CoreValve and the Cribier-Edwards valve is that the Core-Valve is self-expanding, which theoretically permits it to conform to different aortic sizes and to anchor well in the aortic annulus.28–30 This feature allows the CoreValve to be used in patients with severe aortic insufficiency and other noncalcific aortic valvular conditions. The CoreValve has not yet been deployed via antegrade or transapical technique.
The first-generation CoreValve prosthesis was first implanted in a human recipient in 2005.29 Since then, improvements have been made, leading to the development of second- and third-generation devices. A pilot study of implantation of the first-generation CoreValve28 via the retrograde approach in elderly patients with poor functional class and severe aortic stenosis had a short-term procedural success rate of 84% (21 of 25 patients), with a significant reduction in the mean aortic valve gradient and improved functional class at 30-day follow-up.28 At 30 days, 17 (94%) of 18 patients had no or only mild aortic regurgitation.28 Procedural limitations and complications were similar to those with the Cribier-Edwards valve.
In a study of second- and third-generation devices (50 patients received a second-generation device, and 36 received a third-generation device),30 again in elderly patients with poor functional class and severe aortic stenosis, the short-term success rate of the device was 88% (76 of 86) in each group. After the procedure, the mean aortic valve gradient decreased significantly and functional class improved significantly.30 Immediate after implantation, no patient had more than moderate aortic regurgitation, and in 51 patients (66%) the aortic regurgitation remained unchanged or improved after CoreValve implantation.30 These results were maintained at 30-day follow-up.
CoreValve was approved in May 2007 for clinical use in Europe.36 Of note, CoreValve has also been used to treat severe aortic regurgitation of a degenerated bioprosthetic aortic valve in an 80-year-old man with multiple comorbidities.37
EXPERIENCE WITH THE EDWARDS SAPIEN VALVE
The Edwards SAPIEN valve is a modification of the initial Cribier-Edwards valve and is the latest percutaneous aortic valve prosthesis to enter clinical trials (Table 1). It is a trileaflet balloon-expandable stainless steel valve made from bovine pericardial tissue, available in two sizes (23 mm and 26 mm). In September 2007, it was approved for use in Europe with the RetroFlex transfemoral delivery system. The Ascendra transapical delivery system for the Edward SAPIEN valve has received approval in Europe.
The multicenter Placement of Aortic Transcatheter Valves (PARTNER) trial in North America is continuing to enroll patients, with enrollment projected to be complete by the end of 2008. The aim of this prospective randomized clinical trial is to enroll 1,040 patients in two separate treatment arms. The surgical arm of the trial is comparing the Edwards SAPIEN valve with standard surgical aortic valve replacement, with the objective of demonstrating non-inferiority. The medical management arm of the trial is comparing percutaneous valve replacement against medical therapy or balloon valvuloplasty in patients considered too high-risk for conventional surgical valve replacement.
The primary end point in both arms is death at 1 year; secondary end points focus on long-term (1-year) composite cardiovascular events, valve performance, and quality-of-life indicators. Preliminary data on the first 100 patients (74 via the transfemoral [ie, retrograde] and 26 via the transapical approach) who underwent percutaneous Edwards SAPIEN valve implantation for compassionate use showed device durability and symptom relief at up to 2 years.38 Overall procedural success was 91%, but, as with other trials, there was a steep learning curve, so that excluding the first 25 patients increased the procedural success rate to 96%.38 Aortic valve size and hemodynamics, left ventricular systolic function, mitral regurgitation, and functional class were all significantly improved. Mild aortic regurgitation was common, but none of the patients had severe aortic regurgitation. Importantly, the 15% 30-day death rate was significantly lower than the expected rate of 33%. The 6-month survival rate was 78%, but the 2-year rate was 60% in this high-risk elderly cohort.
Walther et al39 recently reported outcomes on their first 50 patients who underwent transapical implantation of the Edwards SAPIEN valve. The operators were able to implant the prosthesis in all 50 patients, but 3 required early conversion to open surgery with sternotomy. The overall survival at 30 days was 92%, but in the last 25 patients the 30-day survival rate was 96%, with a 1-year survival rate of 80%.
PUTTING THE DATA IN PERSPECTIVE
As noted in this review, a number of factors make a strong case for timely aortic valve replacement: the aging population, the increase in incidence and prevalence of aortic stenosis,1,3,4,27,40 the multiple comorbidities in older patients, and the eventually aggressive natural course of aortic stenosis.1,3,4,27,40–43 Yet current standards dictate not to proceed with standard surgical aortic valve replacement in patients who are truly asymptomatic and who have normal left ventricular systolic function,1,40 mainly because the risks of surgical valve replacement outweigh the benefits in this population.1,40 Aortic valve surgery carries a risk of early death of 15% for patients ages 80 to 84 and of 18% for patients age 85.3,9,10,12,43–45
These figures seem high when compared with death rates of 12% in recent studies of percutaneous valve replacement in similar patients.11,23,30,33 The rates become lower as the learning curve improves.11,21,23,27,30,33 Thus, as the design of aortic valve prostheses and the techniques to implant them are refined and tested for safety, the risk-benefit balance may change in favor of earlier intervention in aortic stenosis with a percutaneous approach.11,21,27,46 Some experts believe that in 10 years 10% to 30% of patients undergoing conventional valve replacement will be candidates for a percutaneous approach.
Of the techniques used to date, the retrograde approach seems most amenable to widespread acceptance, given its inherent advantage of being faster and easier.11,21,30 Limitations with the retrograde approach seen in earlier trials—challenges and complications associated with large-bore arterial vascular access, difficulty traversing the aortic arch with bulky devices, and the inability to cross the stenotic aortic valve to deploy the prosthesis even after balloon valvuloplasty11,21,30—are correctable with refinements in the devices and in technique.
New types of prosthetic aortic valves entering early human studies are improving on current devices, for example, by using collapsible, inflatable valve frames for retrievability before final deployment.
Surgical aortic valve replacement remains the gold standard treatment for patients with symptomatic aortic stenosis. And while studies of percutaneous aortic valve replacement show great promise for this less-invasive treat-men, enthusiasm about percutaneous aortic valve replacement should be tempered by an awareness of persistent limitations of this approach, such as vascular and mechanical complications and operator inexperience, which still need attention.
- Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006; 114:e84–231.
- Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 2005; 111:3316–3326.
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003; 24:1231–1243.
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368:1005–1011.
- Goodney PP, O'Connor GT, Wennberg DE, Birkmeyer JD. Do hospitals with low mortality rates in coronary artery bypass also perform well in valve replacement? Ann Thorac Surg 2003; 76:1131–1137.
- Cribier A, Savin T, Saoudi N, Rocha P, Berland J, Letac B. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet 1986; 1:63–67.
- Vahanian A, Palacios IF. Percutaneous approaches to valvular disease. Circulation 2004; 109:1572–1579.
- Webb JG, Munt B, Makkar RR, Naqvi TZ, Dang N. Percutaneous stent-mounted valve for treatment of aortic or pulmonary valve disease. Catheter Cardiovasc Interv 2004; 63:89–93.
- Alexander KP, Anstrom KJ, Muhlbaier LH, et al. Outcomes of cardiac surgery in patients =80 years: results from the National Cardiovascular Network. J Am Coll Cardiol 2000; 35:731–738.
- Mittermair RP, Muller LC. Quality of life after cardiac surgery in the elderly. J Cardiovasc Surg (Torino) 2002; 43:43–47.
- Webb JG, Pasupati S, Humphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007; 116:755–763.
- Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005; 26:2714–2720.
- Bonhoeffer P, Boudjemline Y, Saliba Z, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet 2000; 356:1403–1405.
- Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J 1992; 13:704–708.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002; 106:3006–3008.
- Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994; 89:642–650.
- Safian RD, Berman AD, Diver DJ, et al. Balloon aortic valvuloplasty in 170 consecutive patients. N Engl J Med 1988; 319:125–130.
- Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation 1991; 84:2383–2397.
- Safian RD, Mandell VS, Thurer RE, et al. Postmortem and intraoperative balloon valvuloplasty of calcific aortic stenosis in elderly patients: mechanisms of successful dilation. J Am Coll Cardiol 1987; 9:655–660.
- Agarwal A, Kini AS, Attanti S, et al. Results of repeat balloon valvuloplasty for treatment of aortic stenosis in patients aged 59 to 104 years. Am J Cardiol 2005; 95:43–47.
- Kapadia SR, Wazni OM, Tan WA, et al. Aortic valvuloplasty in 1990's: experience from a single center in United States. Circulation 1999; 100 18 suppl 1:1–448.
- Lieberman EB, Bashore TM, Hermiller JB, et al. Balloon aortic valvuloplasty in adults: failure of procedure to improve long-term survival. J Am Coll Cardiol 1995; 26:1522–1528.
- Cribier A, Eltchaninoff H, Tron C, et al. Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J Am Coll Cardiol 2006; 47:1214–1223.
- Cribier A, Eltchaninoff H, Tron C, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol 2004; 43:698–703.
- Rajagopal V, Kapadia SR, Tuzcu EM. Advances in the percutaneous treatment of aortic and mitral valve disease. Minerva Cardioangiol 2007; 55:83–94.
- Webb JG, Chandavimol M, Thompson CR, et al. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation 2006; 113:842–850.
- Salemi A. Percutaneous valve interventions. Curr Opin Anaesthesiol 2007; 20:70–74.
- Grube E, Laborde JC, Gerckens U, et al. Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study. Circulation 2006; 114:1616–1624.
- Grube E, Laborde JC, Zickmann B, et al. First report on a human percutaneous transluminal implantation of a self-expanding valve prosthesis for interventional treatment of aortic valve stenosis. Catheter Cardiovasc Interv 2005; 66:465–469.
- Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol 2007; 50:69–76.
- Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve implantation in humans: initial clinical experience. Circulation 2006; 114:591–596.
- Walther T, Falk V, Borger MA, et al. Minimally invasive transapical beating heart aortic valve implantation—proof of concept. Eur J Cardiothorac Surg 2007; 31:9–15.
- Ye J, Cheung A, Lichtenstein SV, et al. Six-month outcome of transapical transcatheter aortic valve implantation in the initial seven patients. Eur J Cardiothorac Surg 2007; 31:16–21.
- Turgut T, Deeb M, Moscucci M. Left ventricular apical puncture: a procedure surviving well into the new millennium. Catheter Cardiovasc Interv 2000; 49:68–73.
- Zuguchi M, Shindoh C, Chida K, et al. Safety and clinical benefits of transsubxiphoidal left ventricular puncture. Catheter Cardiovasc Interv 2002; 55:58–65.
- Sinha AK, Kini AS, Sharma SK. Percutaneous valve replacement: a paradigm shift. Curr Opin Cardiol 2007; 22:471–477.
- Wenaweser P, Buellesfeld L, Gerckens U, Grube E. Percutaneous aortic valve replacement for severe aortic regurgitation in degenerated bioprosthesis: the first valve in valve procedure using the CoreValve ReValving system. Catheter Cardiovasc Interv 2007; 70:760–764.
- Pasupati S, Humphries K, AlAli A, et al. Balloon expandable aortic valve (BEAV) implantation. The first 100 Canadian patients. Circulation 2007; 116 suppl:357.
- Walther T, Falk V, Kempfert J, et al. Transapical minimally invasive aortic valve implantation; the initial 50 patients. Eur J Cardiothorac Surg 2008; 33:983–988. Epub 2008 February 21.
- Carabello BA. Clinical practice. Aortic stenosis. N Engl J Med 2002; 346:677–682.
- Pellikka PA, Nishimura RA, Bailey KR, Tajik AJ. The natural history of adults with asymptomatic, hemodynamically significant aortic stenosis. J Am Coll Cardiol 1990; 15:1012–1017.
- Ross J, Braunwald E. Aortic stenosis. Circulation 1968; 38:61–67.
- Kvidal P, Bergstrom R, Horte LG, Stahle E. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol 2000; 35:747–756.
- Society of Thoracic Surgeons National Cardiac Surgery Database. Available at www.sts.org/documents/pdf/Spring2005STS-ExecutiveSummary.pdf. Accessed 9/11/2008.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002; 346:1128–1137.
- Wenger NK, Weber MA, Scheidt S. Valvular heart disease at elderly age: new vistas. Am J Geriatr Cardiol 2006; 15:273–274.
- Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 2006; 114:e84–231.
- Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 2005; 111:3316–3326.
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003; 24:1231–1243.
- Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006; 368:1005–1011.
- Goodney PP, O'Connor GT, Wennberg DE, Birkmeyer JD. Do hospitals with low mortality rates in coronary artery bypass also perform well in valve replacement? Ann Thorac Surg 2003; 76:1131–1137.
- Cribier A, Savin T, Saoudi N, Rocha P, Berland J, Letac B. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet 1986; 1:63–67.
- Vahanian A, Palacios IF. Percutaneous approaches to valvular disease. Circulation 2004; 109:1572–1579.
- Webb JG, Munt B, Makkar RR, Naqvi TZ, Dang N. Percutaneous stent-mounted valve for treatment of aortic or pulmonary valve disease. Catheter Cardiovasc Interv 2004; 63:89–93.
- Alexander KP, Anstrom KJ, Muhlbaier LH, et al. Outcomes of cardiac surgery in patients =80 years: results from the National Cardiovascular Network. J Am Coll Cardiol 2000; 35:731–738.
- Mittermair RP, Muller LC. Quality of life after cardiac surgery in the elderly. J Cardiovasc Surg (Torino) 2002; 43:43–47.
- Webb JG, Pasupati S, Humphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007; 116:755–763.
- Iung B, Cachier A, Baron G, et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005; 26:2714–2720.
- Bonhoeffer P, Boudjemline Y, Saliba Z, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet 2000; 356:1403–1405.
- Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J 1992; 13:704–708.
- Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002; 106:3006–3008.
- Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994; 89:642–650.
- Safian RD, Berman AD, Diver DJ, et al. Balloon aortic valvuloplasty in 170 consecutive patients. N Engl J Med 1988; 319:125–130.
- Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation 1991; 84:2383–2397.
- Safian RD, Mandell VS, Thurer RE, et al. Postmortem and intraoperative balloon valvuloplasty of calcific aortic stenosis in elderly patients: mechanisms of successful dilation. J Am Coll Cardiol 1987; 9:655–660.
- Agarwal A, Kini AS, Attanti S, et al. Results of repeat balloon valvuloplasty for treatment of aortic stenosis in patients aged 59 to 104 years. Am J Cardiol 2005; 95:43–47.
- Kapadia SR, Wazni OM, Tan WA, et al. Aortic valvuloplasty in 1990's: experience from a single center in United States. Circulation 1999; 100 18 suppl 1:1–448.
- Lieberman EB, Bashore TM, Hermiller JB, et al. Balloon aortic valvuloplasty in adults: failure of procedure to improve long-term survival. J Am Coll Cardiol 1995; 26:1522–1528.
- Cribier A, Eltchaninoff H, Tron C, et al. Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J Am Coll Cardiol 2006; 47:1214–1223.
- Cribier A, Eltchaninoff H, Tron C, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol 2004; 43:698–703.
- Rajagopal V, Kapadia SR, Tuzcu EM. Advances in the percutaneous treatment of aortic and mitral valve disease. Minerva Cardioangiol 2007; 55:83–94.
- Webb JG, Chandavimol M, Thompson CR, et al. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation 2006; 113:842–850.
- Salemi A. Percutaneous valve interventions. Curr Opin Anaesthesiol 2007; 20:70–74.
- Grube E, Laborde JC, Gerckens U, et al. Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study. Circulation 2006; 114:1616–1624.
- Grube E, Laborde JC, Zickmann B, et al. First report on a human percutaneous transluminal implantation of a self-expanding valve prosthesis for interventional treatment of aortic valve stenosis. Catheter Cardiovasc Interv 2005; 66:465–469.
- Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol 2007; 50:69–76.
- Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve implantation in humans: initial clinical experience. Circulation 2006; 114:591–596.
- Walther T, Falk V, Borger MA, et al. Minimally invasive transapical beating heart aortic valve implantation—proof of concept. Eur J Cardiothorac Surg 2007; 31:9–15.
- Ye J, Cheung A, Lichtenstein SV, et al. Six-month outcome of transapical transcatheter aortic valve implantation in the initial seven patients. Eur J Cardiothorac Surg 2007; 31:16–21.
- Turgut T, Deeb M, Moscucci M. Left ventricular apical puncture: a procedure surviving well into the new millennium. Catheter Cardiovasc Interv 2000; 49:68–73.
- Zuguchi M, Shindoh C, Chida K, et al. Safety and clinical benefits of transsubxiphoidal left ventricular puncture. Catheter Cardiovasc Interv 2002; 55:58–65.
- Sinha AK, Kini AS, Sharma SK. Percutaneous valve replacement: a paradigm shift. Curr Opin Cardiol 2007; 22:471–477.
- Wenaweser P, Buellesfeld L, Gerckens U, Grube E. Percutaneous aortic valve replacement for severe aortic regurgitation in degenerated bioprosthesis: the first valve in valve procedure using the CoreValve ReValving system. Catheter Cardiovasc Interv 2007; 70:760–764.
- Pasupati S, Humphries K, AlAli A, et al. Balloon expandable aortic valve (BEAV) implantation. The first 100 Canadian patients. Circulation 2007; 116 suppl:357.
- Walther T, Falk V, Kempfert J, et al. Transapical minimally invasive aortic valve implantation; the initial 50 patients. Eur J Cardiothorac Surg 2008; 33:983–988. Epub 2008 February 21.
- Carabello BA. Clinical practice. Aortic stenosis. N Engl J Med 2002; 346:677–682.
- Pellikka PA, Nishimura RA, Bailey KR, Tajik AJ. The natural history of adults with asymptomatic, hemodynamically significant aortic stenosis. J Am Coll Cardiol 1990; 15:1012–1017.
- Ross J, Braunwald E. Aortic stenosis. Circulation 1968; 38:61–67.
- Kvidal P, Bergstrom R, Horte LG, Stahle E. Observed and relative survival after aortic valve replacement. J Am Coll Cardiol 2000; 35:747–756.
- Society of Thoracic Surgeons National Cardiac Surgery Database. Available at www.sts.org/documents/pdf/Spring2005STS-ExecutiveSummary.pdf. Accessed 9/11/2008.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002; 346:1128–1137.
- Wenger NK, Weber MA, Scheidt S. Valvular heart disease at elderly age: new vistas. Am J Geriatr Cardiol 2006; 15:273–274.
KEY POINTS
- Aortic stenosis is the most common valvular condition, affecting 3% of the general population; its incidence and prevalence are increasing as the population ages.
- Many patients with severe aortic valve stenosis are considered too high-risk for standard surgical valve replacement but may be candidates for percutaneous valve replacement.
- Of the approaches now undergoing refinement, the most promising is retrograde (ie, femoral arterial) placement of the Edwards SAPIEN valve or the CoreValve.
- The technology is still evolving, and the learning curve is substantial, yet cautious enthusiasm about percutaneous aortic valve replacement is justified.