User login
Acute pulmonary embolism (PE) is associated with significant morbidity and mortality.1 While expeditious diagnosis and management results in reduced mortality, the ability to rapidly and accurately identify those at increased risk for death remains elusive. Multiple studies have utilized various biomarkers as risk stratification tools, however, these approaches have proven to have many limitations. For example, both serum brain natriuretic peptide (BNP) and troponin levels have been studied as possible risk stratification tools. Those with elevated levels of these following a PE may have concomitant right ventricular (RV) dysfunction and/or hemodynamic instability. Thus, they may face a greater risk for cardiovascular collapse and death. The low positive predictive value of these biomarkers (14%‐44%) has limited their clinical utility.24 Furthermore, imaging modalities, such as echocardiography, which is considered the clinical gold standard for determining the presence of acute RV dysfunction in PE, may not be readily available and may require special expertise for interpretation.5
Conversely, the need to identify acute PE patients at low risk for death is just as important. Recent studies suggest that carefully selected patients can successfully be managed as outpatients which can subsequently lead to significant cost savings and patient satisfaction. Movement towards enhanced outpatient resources and the advent of subcutaneous anticoagulants have made outpatient management of acute PE an appealing possibility. However, proper education, close follow‐up, and a rigorous selection process to recognize those at minimal risk for a fatal complication must all be available before clinicians prematurely discharge these patients to home.
Recently, clinical scoring tools have been developed to aid in risk stratifying patients with acute PE to accurately determine patient outcome. The pulmonary embolism severity index (PESI) is a reproducible scoring system that accurately predicts 30‐day and 90‐day mortality.6, 7 It consists of 11 clinical variables that can be quickly assessed at the time of diagnosis (Table 1A). The fact that biomarkers and imaging technology, such as echocardiography, are unnecessary to compute a PESI score demonstrates the appeal of this system. Similar to the PESI, Sanchez et al.8 have proposed the prognosis in pulmonary embolism (PREP) score as an alternate clinical risk tool in PE (Table 1B). Contrary to PESI, the PREP only uses 3 clinical variables to accurately predict vital outcome with an area under the receiver operating characteristic (AUROC) curve of 0.73 (95% confidence interval [CI], 0.65‐0.82). While both scoring systems have been developed to predict 30‐day mortality in acute PE, the comparative validity of these prognostic tools has not been assessed.
| Predictors | Points Assigned |
|---|---|
| |
| Demographic characteristics | |
| Age (yr) | Age (yr) |
| Male sex | +10 |
| Comorbid conditions | |
| Cancer | +30 |
| Heart failure | +10 |
| Chronic lung disease | +10 |
| Clinical findings | |
| Pulse 110 beats/min | +20 |
| Systolic blood pressure <100 mm Hg | +30 |
| Respiratory rate 30 breaths/min | +20 |
| Temperature <36C | +20 |
| Altered mental status* | +60 |
| Arterial oxygen saturation <90% | +20 |
| Prognostic Factor | Points Assigned |
|---|---|
| |
| Altered mental status* | +10 |
| Cardiogenic shock (systolic blood pressure <90 mm Hg) | +6 |
| Cancer | +6 |
We hypothesized that the PESI more precisely risk stratifies the risk for death in acute PE compared to the PREP. Furthermore, we theorized that the PESI more reliably predicts not only 30‐day but also 90‐day mortality. To test our hypothesis, we performed a retrospective analysis, of all consecutive patients diagnosed with acute PE at our hospital, to compare the prognostic accuracy of these 2 scoring systems.
METHODS
Subjects and Definitions
Between October 2007 and February 2009, adults (age 18 years) diagnosed the day prior with acute PE were identified on a daily basis. This study cohort has been described elsewhere.7 Patients with newly diagnosed PE were eligible for enrollment. Those expected to die within 30 days of their acute PE, such as individuals suffering from a terminal condition (metastatic cancer) or critical illness being transitioned to comfort care, were excluded (n = 32). Patients with multiple admissions for acute PE were included only during the first episode. PE was diagnosed using objective criteria through 1 of the following modalities: high probability ventilation‐perfusion (V/Q) scintigraphy, computed tomography (CT) of the chest with PE protocol, or magnetic resonance imaging (MRI) of the chest. A list of patients who had the above imaging studies to evaluate for PE was provided to study personnel daily by the radiology department; this list was generated every morning and consisted of the day prior's studies. Patient management was not influenced by the research team and was the responsibility of the primary team. This study was approved by our local institutional review board and consent was not required.
We calculated the PESI as described by Aujesky and colleagues.6 For outpatients admitted with acute PE, clinical findings available just prior to, and after, diagnosis were used for scoring. For inpatients diagnosed with PE, clinical findings available during the 24 hours just prior to diagnosis were included. Raw PESI scores were converted to risk class (I‐V), and then further dichotomized into low‐risk (class I‐II) and high‐risk (class III‐V) groups (Table 2). The PREP score was computed based on the presence of altered mental status (AMS), cancer, and cardiogenic shock defined as a systolic blood pressure <90 mm Hg (Table 1B). A raw PREP score of <7 was then characterized as low risk for mortality, while scores 7 were considered high risk.
| PESI Score | Class | n | 30‐Day Mortality by Class (%) | 90‐Day Mortality by Class (%) | Low vs High Risk |
|---|---|---|---|---|---|
| |||||
| 65 | I | 49 | 0 (0.0) | 0 (0.0) | Low |
| 66‐85 | II | 59 | 0 (0.0) | 0 (0.0) | |
| 86‐105 | III | 60 | 0 (0.0) | 0 (0.0) | High |
| 106‐125 | IV | 56 | 2 (3.4) | 4 (6.9) | |
| >125 | V | 69 | 7 (9.2) | 8 (10.5) | |
Finally, the PESI and PREP scores were compared based on their ability to predict all‐cause 30‐day and 90‐day mortality. To determine vital status and date of death, we reviewed the Social Security Death Index 90 days after enrollment of all subjects was completed.
Statistical Analysis
To assess the predictive ability of the 2 scoring tools for death, we determined the negative predictive value and computed the AUROC curves for both scoring systems. AUROC curves were constructed for raw scores and when scores were further segregated by class and risk groups. Additionally, 95% CIs were estimated to determine the accuracy of the discriminatory power of the PESI score versus the PREP score.
Post hoc, we calculated the power of our study to assess whether the difference noted in AUROC curves between the PESI and PREP was adequate to truly determine statistical significance. We used methodology described by Hanley and McNeil to compare continuous values.9 Assuming an alpha of 0.05 and a 20% difference in the AUROC curves, as described in our results, the power in our study was 0.35. Therefore, an approximate sample size of 1000 would be necessary to determine statistical significance. This analysis was performed using Power Analysis and Sample Size (PASS) 11.
RESULTS
The final cohort included 302 subjects (mean age: 59.7 17.2 years; 44.0% males). As Table 3 reveals, the majority of PEs was diagnosed via CT scan (76%). On presentation, 6.6% had cardiogenic shock, while 5.0% had altered censorium. In terms of comorbid conditions, 25.2% had congestive heart failure, 25.2% had cancer, and 22.2% had a prior venous thromboembolic event. Overall, 3.0% and 4.0% met our primary outcomes of death within 30‐days and 90‐days of their acute PEs, respectively.
| |
| Demographics | |
| Age (yr), mean SD | 59.7 17.2 |
| Male sex, % | 44% |
| Diagnostic methodology | |
| CT chest, n (%) | 230 (76.2) |
| V/Q scan, n (%) | 71 (23.5) |
| MRA chest, n (%) | 1 (0.3) |
| Comorbidities | |
| Malignancy, n (%) | 76 (25.2) |
| Congestive heart failure, n (%) | 76 (25.2) |
| Chronic lung disease, n (%) | 72 (23.8) |
| Recent orthopedic surgery, n (%) | 22 (7.3) |
| Prior cerebrovascular accident, n (%) | 31 (10.3) |
| Prior venous thromboembolic disease, n (%) | 67 (22.2) |
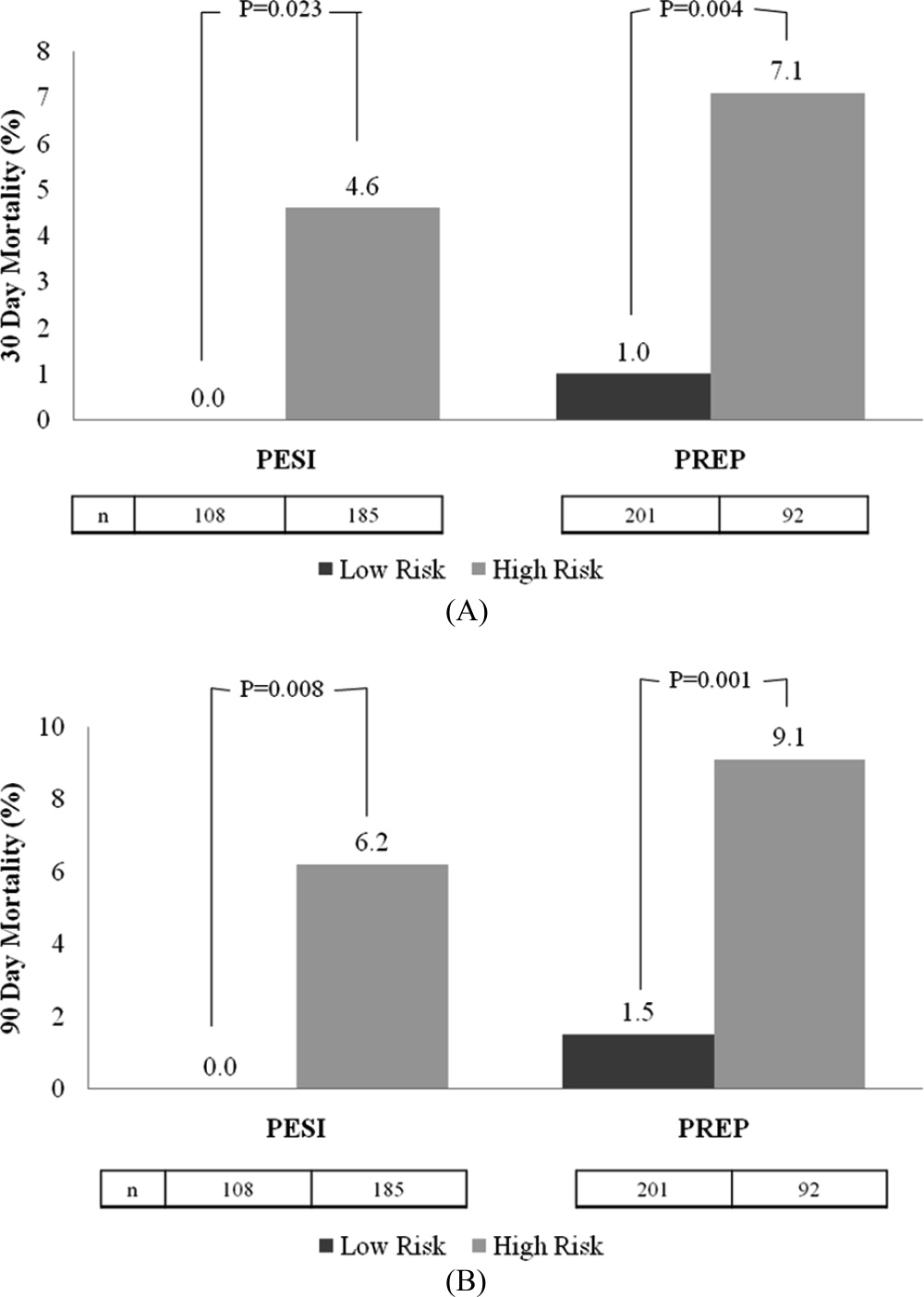
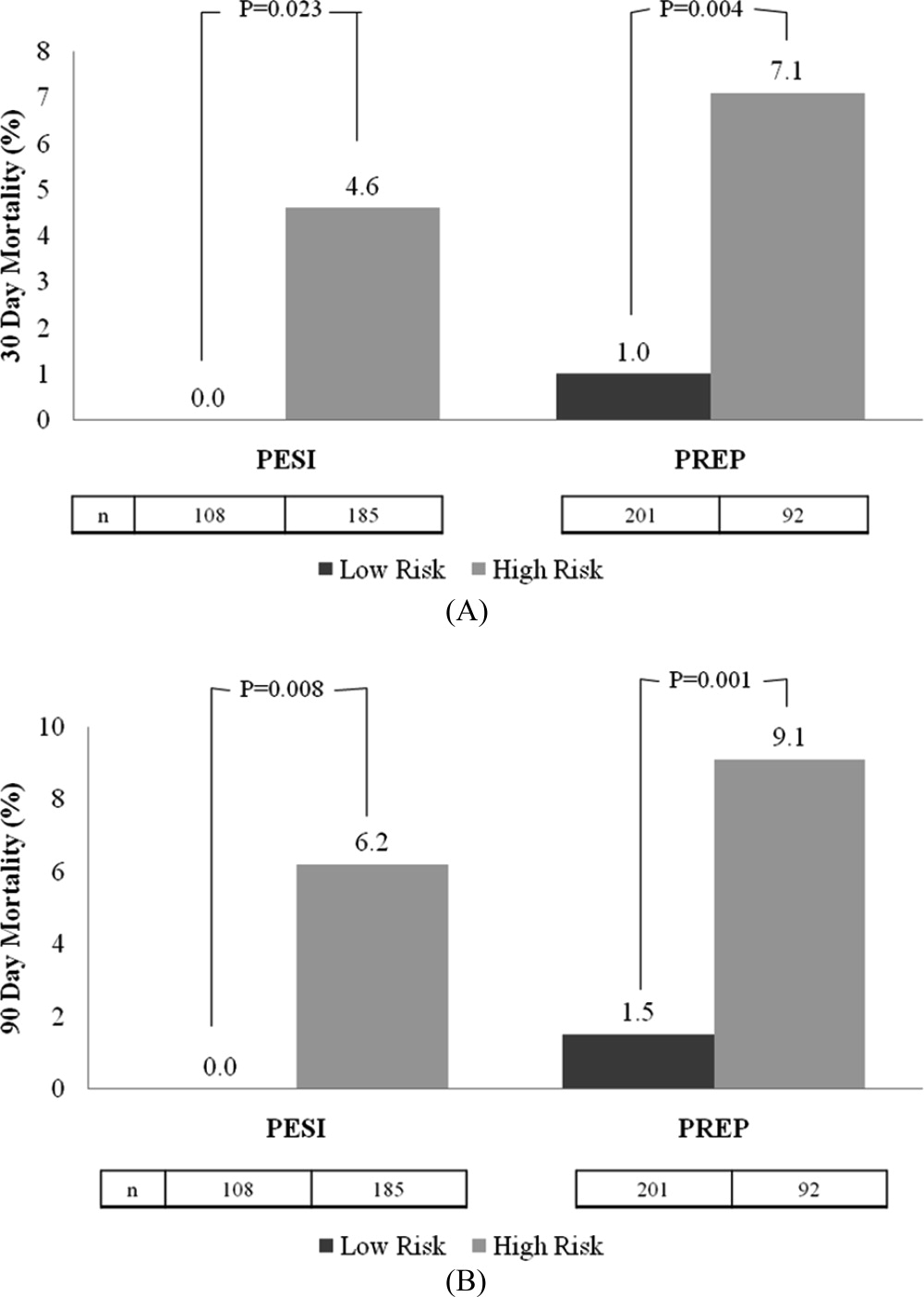
The rates of 30‐day and 90‐day mortality, respectively, increased with increasing score for both the PESI and the PREP. No patients in PESI class I died by either time point, while 9.2% of PESI class V subjects expired by 30 days (P < 0.0001) and 10.5% died by 90 days (P = 0.003) (Table 2). Based on PESI, 30‐day death rates were 4.6% in the high‐risk cohort versus 0% in the low‐risk group (P = 0.023). Conversely, 7.1% of high‐risk PREP subjects died by day 30 versus 1% of low‐risk subjects (P = 0.004) (Figure 1A). Those stratified into the PESI high‐risk group had a 90‐day mortality of 6.2% versus 0% for the low‐risk group (P = 0.008) versus 9.1% in those deemed high risk by PREP, as compared to 1.5% of those scored as low risk by PREP (P = 0.001) (Figure 1B).
Regarding the 30‐day mortality, the negative predictive value of the PESI was 100% (95% CI, 98.6%‐100%) while that for PREP was 99.0% (95% CI, 97.6%‐99.7%); the ability of the PREP to predict 30‐day mortality was similar to the PESI (Table 4). The AUROCs for PESI and PREP for predicting 30‐day death were also equivalent; for the raw PESI score, this measured 0.858 (95% CI, 0.773‐0.943), compared to 0.719 (95% CI, 0.563‐0.875) for PREP. When these scores were dichotomized to high‐risk versus low‐risk groups, the AUROC for the PESI was 0.684 (95% CI, 0.559‐0.810) and 0.732 (95% CI, 0.571‐0.893) for PREP.
| 30‐Day Mortality | 90‐Day Mortality | |||
|---|---|---|---|---|
| Scoring System | AUROC | 95% CI | AUROC | 95% CI |
| ||||
| Raw PESI | 0.858 | 0.773‐0.943 | 0.835 | 0.762‐0.907 |
| PESI class | 0.835 | 0.756‐0.914 | 0.813 | 0.738‐0.888 |
| PESI high vs low risk | 0.684 | 0.559‐0.810 | 0.686 | 0.576‐0.796 |
| Raw PREP | 0.719 | 0.563‐0.875 | 0.704 | 0.564‐0.844 |
| PREP high vs low risk | 0.732 | 0.571‐0.893 | 0.720 | 0.574‐0.865 |
In terms of 90‐day mortality, the negative predictive values of PESI and PREP did not change: 100% (95% CI, 97.4%‐100%) and 98.5% (95% CI, 96.9%‐99.5%), respectively. The ability of PESI and PREP as predictors of 90‐day mortality was equivalent (Table 4). Here, the AUROC for the raw PESI score remained excellent at 0.835 (97% CI, 0.762‐0.907). The AUROC for PREP was akin to that of PESI at 0.704 (95% CI, 0.564‐0.844). Segregating scores into high‐risk versus low‐risk groups demonstrated that the AUROC for PESI was 0.686 (95% CI, 0.576‐0.796) compared to 0.720 (95% CI, 0.574‐0.865) for PREP.
DISCUSSION
This retrospective analysis of patients with acute PE confirms that both the PESI and the PREP are accurate scoring tools for identifying patients at low risk of death. Under both rubrics, as the score increases, the likelihood of death also increases. More importantly, we demonstrate that the negative predictive value for both the PREP and PESI are excellent. Thus, these scoring tools can distinguish those at higher risk for death versus those at low risk in a simple‐to‐apply manner. In comparing these 2 scoring systems, the PREP comparably identifies acute PE patients at risk for death when contrasted with the PESI. Given the fewer required scoring points to calculate PREP and its ability to accurately predict clinically relevant outcomes, this simpler scoring system may have greater clinical utility.
Prior studies have validated the PESI as a risk stratification tool to predict 30‐day and 90‐day mortalities. In their original derivation of the PESI, Aujesky et al. demonstrated that higher PESI scores correlated with death at 30 days.6 Acute PE patients classified into risk class I had a short‐term mortality rate of 1.1% compared to nearly 25% of patients risk stratified into risk class V. The same authors subsequently verified that there is a linear relationship between PESI score and risk of death at 90 days.10 We have also confirmed the accuracy of the PESI for identifying persons at high risk for death and documented the limited interobserver variability in this tool.7 In combination, there is evidence that the PESI can accurately predict vital outcome. Despite the effectiveness of the PESI, it is a somewhat cumbersome scoring system. It requires gathering information on 11 clinical variables, each with a different score allocation to ultimately compute the PESI score. In contrast, the PREP only requires knowing 3 clinical variables: presence of cancer, mental status, and the presence of cardiogenic shock. Akin to the PESI, the PREP and mortality are linearly related, where higher PREP scores result in higher 30‐day and 90‐day mortalities.
Our analysis helps expand the evidence regarding clinical risk stratification in PE in several ways. First, we verify that both the PESI and PREP are accurate predictors of short‐term mortality. While this has been accomplished for the PESI in prior studies, to our knowledge, this is the first confirmatory study for PREP's utility as a risk stratification tool. Second, we demonstrate that PREP is also an accurate predictor of intermediate‐term mortality. If the eventual goal is to develop tools that allow for the initial outpatient management of acute PE, clinicians require data on longer‐term outcomes to ensure that later harms do not arise based on a decision to defer hospitalization. Prior observational studies and randomized controlled clinical trials have proven that appropriately selected individuals face similar rates of complications following acute PE, whether they are managed in or outside of a hospital setting.1116 The key limitation of these earlier efforts, though, was that there was no clear standardized approach to determining whom could be safely managed solely as an outpatient. Finally, our study is unique in that we compare the discriminatory power of these 2 risk‐scoring schemes and illustrate their equivalence. As a scoring system that only requires 3 variables, the PREP is easier and simpler, and may therefore have more clinical utility than the PESI. The high negative predictive value of the PREP suggests that it has potential in identifying patients with acute PE who can safely be managed on an outpatient basis. However, given the complexity of factors associated with the decision for early discharge, these scores should be used in conjunction with, and not supplant, clinical judgment for outpatient management. Of course, formal prospective management trials incorporating both the PREP and PESI are needed to validate this concept.
Why does PREP perform so well despite the fact that it focuses on so few clinical variables? Essentially, the PREP is an effective scoring tool for acute PE because of its ability to identify individuals at risk for progressing to shock. The presence of AMS in acute PE has been associated with a greater likelihood of death, as it likely arises as a consequence of severe shock or RV strain resulting in decreased cerebral blood flow. Alternatively, altered censorium could represent a manifestation of hypoxemia from significant V/Q mismatching and/or pulmonary shunting due to the obstructive clot. This, too, portends a poorer prognosis secondary to impending respiratory failure from hypoxemia. Thus, individuals with an acute PE presenting with altered mentation merit very close observation. Similarly, pending hemodynamic instability is a concerning manifestation that warrants inpatient monitoring.5, 17, 18 At the very minimum, these individuals have RV strain and should therefore be admitted to the hospital to potentially administer more aggressive treatment modalities (ie, thrombolytics or thrombectomy). The last clinical criteria involves the presence of malignancy. The presence of a cancer may serve as a surrogate marker for those at increased risk for early recurrent thromboembolic phenomena, since malignancy is associated with a hypercoagulable state.17, 19 Perhaps there is a threshold whereby accumulating clot resulting in RV strain ensues with subsequent poorer outcomes. Thus, it clinically and physiologically seems logical that, in the absence of any of these findings, patients with acute PE will have lower mortality rates.
Thus far, other methods used for risk stratification may either be expensive, not really obtainable, or not routinely available at the time of presentation. For example, confirmation of RV strain with an echocardiogram requires a skilled technician and interpreter. In contrast, both the PESI and PREP are scored based on multiple clinical findings. Hence, they are not dependent upon a single test to determine outcome, but on various clinical variables making these scoring tools comprehensive, simple, and reliable approaches of recognizing low‐risk patients.
Our analysis has several limitations. First, the retrospective nature of this analysis subjects it to multiple forms of bias. We attempted to eliminate these biases by defining, a priori, the time frame from which vital signs can be used during scoring. We also used all‐cause mortality as our primary endpoint to minimize the possibility of ascertainment bias. However, this type of bias could not be completely eliminated since data collected was not specifically for the purpose of this study. Second, this single‐center study may limit the generalizability of these findings; yet, the diversity of patients admitted to this 900‐bed, tertiary care facility, as well as the inclusion of both inpatients and outpatients, helps to mitigate this concern. Third, the exclusion of individuals with expectant deaths within <30 days limits the applicability of these findings to this group. We chose to exclude persons with anticipated short‐term mortality to reduce the tally of patients who did not receive therapeutic treatment (ie, those transitioned to comfort care). Fourth, the use of the Social Security Death Index objectively determines death status for all‐cause mortality but cannot delineate cause‐specific death. Consequently, death strictly due to PE could not be assessed. Fifth, the original investigators for PREP assessed the PREP score with and without BNP and left‐to‐right ventricular diameter ratios. Although their results demonstrated similar AUROCs for the PREP score with and without BNP to predict 30‐day outcomes, this was a finding we could not confirm due to inconsistencies in measuring BNP and echocardiograms in our cohort. Also, our post hoc power analysis demonstrates that our findings may be limited by sample size. The lack of statistically significant differences between the PESI and the PREP may, in fact, be due to the small sample size versus true effect. Finally, tolerance for medical therapy and compliance with treatment were not documented and, therefore, were immeasurable. Poor compliance to anticoagulants or intolerability increases risk for recurrent PE, while excessive anticoagulation increases likelihood of bleeding.
In summary, the PREP and PESI can both safely predict 30‐day and 90‐day outcomes. However, the simplicity of the PREP renders it more clinician friendly. The fact that only 3 clinical noninvasive variables are required would ultimately make it the preferred bedside tool to risk stratify patients for acute PE. The high negative predictive value and comparable AUROCs establishes the effectiveness of these 2 scoring systems in recognizing low‐risk patients. Irrespective of the clinician's choice to use 1 tool over the other, both have potential for clinical application at the bedside and in clinical trials. Nevertheless, further evidence is required before they are utilized to triage patients for outpatient therapy.
- .Pulmonary embolism: what have we learned since Virchow? Natural history, pathophysiology, and diagnosis.Chest.2002;122:1440–1456.
- ,,, et al.The incidence and prognostic significance of elevated cardiac troponins in patients with submassive pulmonary embolism.J Thromb Haemost.2005;3:508–513.
- ,,, et al.Biomarker‐based risk assessment model in acute pulmonary embolism.Eur Heart J.2005;26:2166–2172.
- ,.Cardiac biomarkers for risk stratification of patients with acute pulmonary embolism.Circulation.2003;108:2191–2194.
- ,,, et al.Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mm Hg or higher.Arch Intern Med.2005;165:1777–1781.
- ,,, et al.Derivation and validation of a prognostic model for pulmonary embolism.Am J Respir Crit Care Med.2005;172:1041–1046.
- ,,.The validation and reproducibility of the pulmonary embolism severity index.J Thromb Haemost.2010;8:1509–1514.
- ,,, et al.Prognostic factors for pulmonary embolism: the prep study, a prospective multicenter cohort study.Am J Respir Crit Care Med.2010;181:168–173.
- ,.A method of comparing the areas under receiver operating characteristic curves derived from the same cases.Radiology.1983;148:839–843.
- ,,, et al.Prospective validation of the pulmonary embolism severity index. A clinical prognostic model for pulmonary embolism.Thromb Haemost.2008;100:943–948.
- ,,, et al.Expanding eligibility for outpatient treatment of deep venous thrombosis and pulmonary embolism with low‐molecular‐weight heparin: a comparison of patient self‐injection with homecare injection.Arch Intern Med.1998;158:1809–1812.
- ,,, et al.Outpatient treatment of pulmonary embolism with dalteparin.Thromb Haemost.2000;83:209–211.
- ,,, et al.Outpatient treatment of pulmonary embolism is feasible and safe in a substantial proportion of patients.J Thromb Haemost.2003;1:186–187.
- ,,, et al.A randomized trial comparing 2 low‐molecular‐weight heparins for the outpatient treatment of deep vein thrombosis and pulmonary embolism.Arch Intern Med.2005;165:733–738.
- ,,, et al.Early discharge of patients with pulmonary embolism: a two‐phase observational study.Eur Respir J.2007;30:708–714.
- ,,, et al.Home treatment in pulmonary embolism.Thromb Res.2010;126:e1–e5.
- ,,.Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER).Lancet.1999;353:1386–1389.
- ,,, et al.Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta‐analysis of the randomized controlled trials.Circulation.2004;110:744–749.
- ,,, et al.Predictors of survival after deep vein thrombosis and pulmonary embolism: a population‐based, cohort study.Arch Intern Med.1999;159:445–453.
Acute pulmonary embolism (PE) is associated with significant morbidity and mortality.1 While expeditious diagnosis and management results in reduced mortality, the ability to rapidly and accurately identify those at increased risk for death remains elusive. Multiple studies have utilized various biomarkers as risk stratification tools, however, these approaches have proven to have many limitations. For example, both serum brain natriuretic peptide (BNP) and troponin levels have been studied as possible risk stratification tools. Those with elevated levels of these following a PE may have concomitant right ventricular (RV) dysfunction and/or hemodynamic instability. Thus, they may face a greater risk for cardiovascular collapse and death. The low positive predictive value of these biomarkers (14%‐44%) has limited their clinical utility.24 Furthermore, imaging modalities, such as echocardiography, which is considered the clinical gold standard for determining the presence of acute RV dysfunction in PE, may not be readily available and may require special expertise for interpretation.5
Conversely, the need to identify acute PE patients at low risk for death is just as important. Recent studies suggest that carefully selected patients can successfully be managed as outpatients which can subsequently lead to significant cost savings and patient satisfaction. Movement towards enhanced outpatient resources and the advent of subcutaneous anticoagulants have made outpatient management of acute PE an appealing possibility. However, proper education, close follow‐up, and a rigorous selection process to recognize those at minimal risk for a fatal complication must all be available before clinicians prematurely discharge these patients to home.
Recently, clinical scoring tools have been developed to aid in risk stratifying patients with acute PE to accurately determine patient outcome. The pulmonary embolism severity index (PESI) is a reproducible scoring system that accurately predicts 30‐day and 90‐day mortality.6, 7 It consists of 11 clinical variables that can be quickly assessed at the time of diagnosis (Table 1A). The fact that biomarkers and imaging technology, such as echocardiography, are unnecessary to compute a PESI score demonstrates the appeal of this system. Similar to the PESI, Sanchez et al.8 have proposed the prognosis in pulmonary embolism (PREP) score as an alternate clinical risk tool in PE (Table 1B). Contrary to PESI, the PREP only uses 3 clinical variables to accurately predict vital outcome with an area under the receiver operating characteristic (AUROC) curve of 0.73 (95% confidence interval [CI], 0.65‐0.82). While both scoring systems have been developed to predict 30‐day mortality in acute PE, the comparative validity of these prognostic tools has not been assessed.
| Predictors | Points Assigned |
|---|---|
| |
| Demographic characteristics | |
| Age (yr) | Age (yr) |
| Male sex | +10 |
| Comorbid conditions | |
| Cancer | +30 |
| Heart failure | +10 |
| Chronic lung disease | +10 |
| Clinical findings | |
| Pulse 110 beats/min | +20 |
| Systolic blood pressure <100 mm Hg | +30 |
| Respiratory rate 30 breaths/min | +20 |
| Temperature <36C | +20 |
| Altered mental status* | +60 |
| Arterial oxygen saturation <90% | +20 |
| Prognostic Factor | Points Assigned |
|---|---|
| |
| Altered mental status* | +10 |
| Cardiogenic shock (systolic blood pressure <90 mm Hg) | +6 |
| Cancer | +6 |
We hypothesized that the PESI more precisely risk stratifies the risk for death in acute PE compared to the PREP. Furthermore, we theorized that the PESI more reliably predicts not only 30‐day but also 90‐day mortality. To test our hypothesis, we performed a retrospective analysis, of all consecutive patients diagnosed with acute PE at our hospital, to compare the prognostic accuracy of these 2 scoring systems.
METHODS
Subjects and Definitions
Between October 2007 and February 2009, adults (age 18 years) diagnosed the day prior with acute PE were identified on a daily basis. This study cohort has been described elsewhere.7 Patients with newly diagnosed PE were eligible for enrollment. Those expected to die within 30 days of their acute PE, such as individuals suffering from a terminal condition (metastatic cancer) or critical illness being transitioned to comfort care, were excluded (n = 32). Patients with multiple admissions for acute PE were included only during the first episode. PE was diagnosed using objective criteria through 1 of the following modalities: high probability ventilation‐perfusion (V/Q) scintigraphy, computed tomography (CT) of the chest with PE protocol, or magnetic resonance imaging (MRI) of the chest. A list of patients who had the above imaging studies to evaluate for PE was provided to study personnel daily by the radiology department; this list was generated every morning and consisted of the day prior's studies. Patient management was not influenced by the research team and was the responsibility of the primary team. This study was approved by our local institutional review board and consent was not required.
We calculated the PESI as described by Aujesky and colleagues.6 For outpatients admitted with acute PE, clinical findings available just prior to, and after, diagnosis were used for scoring. For inpatients diagnosed with PE, clinical findings available during the 24 hours just prior to diagnosis were included. Raw PESI scores were converted to risk class (I‐V), and then further dichotomized into low‐risk (class I‐II) and high‐risk (class III‐V) groups (Table 2). The PREP score was computed based on the presence of altered mental status (AMS), cancer, and cardiogenic shock defined as a systolic blood pressure <90 mm Hg (Table 1B). A raw PREP score of <7 was then characterized as low risk for mortality, while scores 7 were considered high risk.
| PESI Score | Class | n | 30‐Day Mortality by Class (%) | 90‐Day Mortality by Class (%) | Low vs High Risk |
|---|---|---|---|---|---|
| |||||
| 65 | I | 49 | 0 (0.0) | 0 (0.0) | Low |
| 66‐85 | II | 59 | 0 (0.0) | 0 (0.0) | |
| 86‐105 | III | 60 | 0 (0.0) | 0 (0.0) | High |
| 106‐125 | IV | 56 | 2 (3.4) | 4 (6.9) | |
| >125 | V | 69 | 7 (9.2) | 8 (10.5) | |
Finally, the PESI and PREP scores were compared based on their ability to predict all‐cause 30‐day and 90‐day mortality. To determine vital status and date of death, we reviewed the Social Security Death Index 90 days after enrollment of all subjects was completed.
Statistical Analysis
To assess the predictive ability of the 2 scoring tools for death, we determined the negative predictive value and computed the AUROC curves for both scoring systems. AUROC curves were constructed for raw scores and when scores were further segregated by class and risk groups. Additionally, 95% CIs were estimated to determine the accuracy of the discriminatory power of the PESI score versus the PREP score.
Post hoc, we calculated the power of our study to assess whether the difference noted in AUROC curves between the PESI and PREP was adequate to truly determine statistical significance. We used methodology described by Hanley and McNeil to compare continuous values.9 Assuming an alpha of 0.05 and a 20% difference in the AUROC curves, as described in our results, the power in our study was 0.35. Therefore, an approximate sample size of 1000 would be necessary to determine statistical significance. This analysis was performed using Power Analysis and Sample Size (PASS) 11.
RESULTS
The final cohort included 302 subjects (mean age: 59.7 17.2 years; 44.0% males). As Table 3 reveals, the majority of PEs was diagnosed via CT scan (76%). On presentation, 6.6% had cardiogenic shock, while 5.0% had altered censorium. In terms of comorbid conditions, 25.2% had congestive heart failure, 25.2% had cancer, and 22.2% had a prior venous thromboembolic event. Overall, 3.0% and 4.0% met our primary outcomes of death within 30‐days and 90‐days of their acute PEs, respectively.
| |
| Demographics | |
| Age (yr), mean SD | 59.7 17.2 |
| Male sex, % | 44% |
| Diagnostic methodology | |
| CT chest, n (%) | 230 (76.2) |
| V/Q scan, n (%) | 71 (23.5) |
| MRA chest, n (%) | 1 (0.3) |
| Comorbidities | |
| Malignancy, n (%) | 76 (25.2) |
| Congestive heart failure, n (%) | 76 (25.2) |
| Chronic lung disease, n (%) | 72 (23.8) |
| Recent orthopedic surgery, n (%) | 22 (7.3) |
| Prior cerebrovascular accident, n (%) | 31 (10.3) |
| Prior venous thromboembolic disease, n (%) | 67 (22.2) |
The rates of 30‐day and 90‐day mortality, respectively, increased with increasing score for both the PESI and the PREP. No patients in PESI class I died by either time point, while 9.2% of PESI class V subjects expired by 30 days (P < 0.0001) and 10.5% died by 90 days (P = 0.003) (Table 2). Based on PESI, 30‐day death rates were 4.6% in the high‐risk cohort versus 0% in the low‐risk group (P = 0.023). Conversely, 7.1% of high‐risk PREP subjects died by day 30 versus 1% of low‐risk subjects (P = 0.004) (Figure 1A). Those stratified into the PESI high‐risk group had a 90‐day mortality of 6.2% versus 0% for the low‐risk group (P = 0.008) versus 9.1% in those deemed high risk by PREP, as compared to 1.5% of those scored as low risk by PREP (P = 0.001) (Figure 1B).

Regarding the 30‐day mortality, the negative predictive value of the PESI was 100% (95% CI, 98.6%‐100%) while that for PREP was 99.0% (95% CI, 97.6%‐99.7%); the ability of the PREP to predict 30‐day mortality was similar to the PESI (Table 4). The AUROCs for PESI and PREP for predicting 30‐day death were also equivalent; for the raw PESI score, this measured 0.858 (95% CI, 0.773‐0.943), compared to 0.719 (95% CI, 0.563‐0.875) for PREP. When these scores were dichotomized to high‐risk versus low‐risk groups, the AUROC for the PESI was 0.684 (95% CI, 0.559‐0.810) and 0.732 (95% CI, 0.571‐0.893) for PREP.
| 30‐Day Mortality | 90‐Day Mortality | |||
|---|---|---|---|---|
| Scoring System | AUROC | 95% CI | AUROC | 95% CI |
| ||||
| Raw PESI | 0.858 | 0.773‐0.943 | 0.835 | 0.762‐0.907 |
| PESI class | 0.835 | 0.756‐0.914 | 0.813 | 0.738‐0.888 |
| PESI high vs low risk | 0.684 | 0.559‐0.810 | 0.686 | 0.576‐0.796 |
| Raw PREP | 0.719 | 0.563‐0.875 | 0.704 | 0.564‐0.844 |
| PREP high vs low risk | 0.732 | 0.571‐0.893 | 0.720 | 0.574‐0.865 |
In terms of 90‐day mortality, the negative predictive values of PESI and PREP did not change: 100% (95% CI, 97.4%‐100%) and 98.5% (95% CI, 96.9%‐99.5%), respectively. The ability of PESI and PREP as predictors of 90‐day mortality was equivalent (Table 4). Here, the AUROC for the raw PESI score remained excellent at 0.835 (97% CI, 0.762‐0.907). The AUROC for PREP was akin to that of PESI at 0.704 (95% CI, 0.564‐0.844). Segregating scores into high‐risk versus low‐risk groups demonstrated that the AUROC for PESI was 0.686 (95% CI, 0.576‐0.796) compared to 0.720 (95% CI, 0.574‐0.865) for PREP.
DISCUSSION
This retrospective analysis of patients with acute PE confirms that both the PESI and the PREP are accurate scoring tools for identifying patients at low risk of death. Under both rubrics, as the score increases, the likelihood of death also increases. More importantly, we demonstrate that the negative predictive value for both the PREP and PESI are excellent. Thus, these scoring tools can distinguish those at higher risk for death versus those at low risk in a simple‐to‐apply manner. In comparing these 2 scoring systems, the PREP comparably identifies acute PE patients at risk for death when contrasted with the PESI. Given the fewer required scoring points to calculate PREP and its ability to accurately predict clinically relevant outcomes, this simpler scoring system may have greater clinical utility.
Prior studies have validated the PESI as a risk stratification tool to predict 30‐day and 90‐day mortalities. In their original derivation of the PESI, Aujesky et al. demonstrated that higher PESI scores correlated with death at 30 days.6 Acute PE patients classified into risk class I had a short‐term mortality rate of 1.1% compared to nearly 25% of patients risk stratified into risk class V. The same authors subsequently verified that there is a linear relationship between PESI score and risk of death at 90 days.10 We have also confirmed the accuracy of the PESI for identifying persons at high risk for death and documented the limited interobserver variability in this tool.7 In combination, there is evidence that the PESI can accurately predict vital outcome. Despite the effectiveness of the PESI, it is a somewhat cumbersome scoring system. It requires gathering information on 11 clinical variables, each with a different score allocation to ultimately compute the PESI score. In contrast, the PREP only requires knowing 3 clinical variables: presence of cancer, mental status, and the presence of cardiogenic shock. Akin to the PESI, the PREP and mortality are linearly related, where higher PREP scores result in higher 30‐day and 90‐day mortalities.
Our analysis helps expand the evidence regarding clinical risk stratification in PE in several ways. First, we verify that both the PESI and PREP are accurate predictors of short‐term mortality. While this has been accomplished for the PESI in prior studies, to our knowledge, this is the first confirmatory study for PREP's utility as a risk stratification tool. Second, we demonstrate that PREP is also an accurate predictor of intermediate‐term mortality. If the eventual goal is to develop tools that allow for the initial outpatient management of acute PE, clinicians require data on longer‐term outcomes to ensure that later harms do not arise based on a decision to defer hospitalization. Prior observational studies and randomized controlled clinical trials have proven that appropriately selected individuals face similar rates of complications following acute PE, whether they are managed in or outside of a hospital setting.1116 The key limitation of these earlier efforts, though, was that there was no clear standardized approach to determining whom could be safely managed solely as an outpatient. Finally, our study is unique in that we compare the discriminatory power of these 2 risk‐scoring schemes and illustrate their equivalence. As a scoring system that only requires 3 variables, the PREP is easier and simpler, and may therefore have more clinical utility than the PESI. The high negative predictive value of the PREP suggests that it has potential in identifying patients with acute PE who can safely be managed on an outpatient basis. However, given the complexity of factors associated with the decision for early discharge, these scores should be used in conjunction with, and not supplant, clinical judgment for outpatient management. Of course, formal prospective management trials incorporating both the PREP and PESI are needed to validate this concept.
Why does PREP perform so well despite the fact that it focuses on so few clinical variables? Essentially, the PREP is an effective scoring tool for acute PE because of its ability to identify individuals at risk for progressing to shock. The presence of AMS in acute PE has been associated with a greater likelihood of death, as it likely arises as a consequence of severe shock or RV strain resulting in decreased cerebral blood flow. Alternatively, altered censorium could represent a manifestation of hypoxemia from significant V/Q mismatching and/or pulmonary shunting due to the obstructive clot. This, too, portends a poorer prognosis secondary to impending respiratory failure from hypoxemia. Thus, individuals with an acute PE presenting with altered mentation merit very close observation. Similarly, pending hemodynamic instability is a concerning manifestation that warrants inpatient monitoring.5, 17, 18 At the very minimum, these individuals have RV strain and should therefore be admitted to the hospital to potentially administer more aggressive treatment modalities (ie, thrombolytics or thrombectomy). The last clinical criteria involves the presence of malignancy. The presence of a cancer may serve as a surrogate marker for those at increased risk for early recurrent thromboembolic phenomena, since malignancy is associated with a hypercoagulable state.17, 19 Perhaps there is a threshold whereby accumulating clot resulting in RV strain ensues with subsequent poorer outcomes. Thus, it clinically and physiologically seems logical that, in the absence of any of these findings, patients with acute PE will have lower mortality rates.
Thus far, other methods used for risk stratification may either be expensive, not really obtainable, or not routinely available at the time of presentation. For example, confirmation of RV strain with an echocardiogram requires a skilled technician and interpreter. In contrast, both the PESI and PREP are scored based on multiple clinical findings. Hence, they are not dependent upon a single test to determine outcome, but on various clinical variables making these scoring tools comprehensive, simple, and reliable approaches of recognizing low‐risk patients.
Our analysis has several limitations. First, the retrospective nature of this analysis subjects it to multiple forms of bias. We attempted to eliminate these biases by defining, a priori, the time frame from which vital signs can be used during scoring. We also used all‐cause mortality as our primary endpoint to minimize the possibility of ascertainment bias. However, this type of bias could not be completely eliminated since data collected was not specifically for the purpose of this study. Second, this single‐center study may limit the generalizability of these findings; yet, the diversity of patients admitted to this 900‐bed, tertiary care facility, as well as the inclusion of both inpatients and outpatients, helps to mitigate this concern. Third, the exclusion of individuals with expectant deaths within <30 days limits the applicability of these findings to this group. We chose to exclude persons with anticipated short‐term mortality to reduce the tally of patients who did not receive therapeutic treatment (ie, those transitioned to comfort care). Fourth, the use of the Social Security Death Index objectively determines death status for all‐cause mortality but cannot delineate cause‐specific death. Consequently, death strictly due to PE could not be assessed. Fifth, the original investigators for PREP assessed the PREP score with and without BNP and left‐to‐right ventricular diameter ratios. Although their results demonstrated similar AUROCs for the PREP score with and without BNP to predict 30‐day outcomes, this was a finding we could not confirm due to inconsistencies in measuring BNP and echocardiograms in our cohort. Also, our post hoc power analysis demonstrates that our findings may be limited by sample size. The lack of statistically significant differences between the PESI and the PREP may, in fact, be due to the small sample size versus true effect. Finally, tolerance for medical therapy and compliance with treatment were not documented and, therefore, were immeasurable. Poor compliance to anticoagulants or intolerability increases risk for recurrent PE, while excessive anticoagulation increases likelihood of bleeding.
In summary, the PREP and PESI can both safely predict 30‐day and 90‐day outcomes. However, the simplicity of the PREP renders it more clinician friendly. The fact that only 3 clinical noninvasive variables are required would ultimately make it the preferred bedside tool to risk stratify patients for acute PE. The high negative predictive value and comparable AUROCs establishes the effectiveness of these 2 scoring systems in recognizing low‐risk patients. Irrespective of the clinician's choice to use 1 tool over the other, both have potential for clinical application at the bedside and in clinical trials. Nevertheless, further evidence is required before they are utilized to triage patients for outpatient therapy.
Acute pulmonary embolism (PE) is associated with significant morbidity and mortality.1 While expeditious diagnosis and management results in reduced mortality, the ability to rapidly and accurately identify those at increased risk for death remains elusive. Multiple studies have utilized various biomarkers as risk stratification tools, however, these approaches have proven to have many limitations. For example, both serum brain natriuretic peptide (BNP) and troponin levels have been studied as possible risk stratification tools. Those with elevated levels of these following a PE may have concomitant right ventricular (RV) dysfunction and/or hemodynamic instability. Thus, they may face a greater risk for cardiovascular collapse and death. The low positive predictive value of these biomarkers (14%‐44%) has limited their clinical utility.24 Furthermore, imaging modalities, such as echocardiography, which is considered the clinical gold standard for determining the presence of acute RV dysfunction in PE, may not be readily available and may require special expertise for interpretation.5
Conversely, the need to identify acute PE patients at low risk for death is just as important. Recent studies suggest that carefully selected patients can successfully be managed as outpatients which can subsequently lead to significant cost savings and patient satisfaction. Movement towards enhanced outpatient resources and the advent of subcutaneous anticoagulants have made outpatient management of acute PE an appealing possibility. However, proper education, close follow‐up, and a rigorous selection process to recognize those at minimal risk for a fatal complication must all be available before clinicians prematurely discharge these patients to home.
Recently, clinical scoring tools have been developed to aid in risk stratifying patients with acute PE to accurately determine patient outcome. The pulmonary embolism severity index (PESI) is a reproducible scoring system that accurately predicts 30‐day and 90‐day mortality.6, 7 It consists of 11 clinical variables that can be quickly assessed at the time of diagnosis (Table 1A). The fact that biomarkers and imaging technology, such as echocardiography, are unnecessary to compute a PESI score demonstrates the appeal of this system. Similar to the PESI, Sanchez et al.8 have proposed the prognosis in pulmonary embolism (PREP) score as an alternate clinical risk tool in PE (Table 1B). Contrary to PESI, the PREP only uses 3 clinical variables to accurately predict vital outcome with an area under the receiver operating characteristic (AUROC) curve of 0.73 (95% confidence interval [CI], 0.65‐0.82). While both scoring systems have been developed to predict 30‐day mortality in acute PE, the comparative validity of these prognostic tools has not been assessed.
| Predictors | Points Assigned |
|---|---|
| |
| Demographic characteristics | |
| Age (yr) | Age (yr) |
| Male sex | +10 |
| Comorbid conditions | |
| Cancer | +30 |
| Heart failure | +10 |
| Chronic lung disease | +10 |
| Clinical findings | |
| Pulse 110 beats/min | +20 |
| Systolic blood pressure <100 mm Hg | +30 |
| Respiratory rate 30 breaths/min | +20 |
| Temperature <36C | +20 |
| Altered mental status* | +60 |
| Arterial oxygen saturation <90% | +20 |
| Prognostic Factor | Points Assigned |
|---|---|
| |
| Altered mental status* | +10 |
| Cardiogenic shock (systolic blood pressure <90 mm Hg) | +6 |
| Cancer | +6 |
We hypothesized that the PESI more precisely risk stratifies the risk for death in acute PE compared to the PREP. Furthermore, we theorized that the PESI more reliably predicts not only 30‐day but also 90‐day mortality. To test our hypothesis, we performed a retrospective analysis, of all consecutive patients diagnosed with acute PE at our hospital, to compare the prognostic accuracy of these 2 scoring systems.
METHODS
Subjects and Definitions
Between October 2007 and February 2009, adults (age 18 years) diagnosed the day prior with acute PE were identified on a daily basis. This study cohort has been described elsewhere.7 Patients with newly diagnosed PE were eligible for enrollment. Those expected to die within 30 days of their acute PE, such as individuals suffering from a terminal condition (metastatic cancer) or critical illness being transitioned to comfort care, were excluded (n = 32). Patients with multiple admissions for acute PE were included only during the first episode. PE was diagnosed using objective criteria through 1 of the following modalities: high probability ventilation‐perfusion (V/Q) scintigraphy, computed tomography (CT) of the chest with PE protocol, or magnetic resonance imaging (MRI) of the chest. A list of patients who had the above imaging studies to evaluate for PE was provided to study personnel daily by the radiology department; this list was generated every morning and consisted of the day prior's studies. Patient management was not influenced by the research team and was the responsibility of the primary team. This study was approved by our local institutional review board and consent was not required.
We calculated the PESI as described by Aujesky and colleagues.6 For outpatients admitted with acute PE, clinical findings available just prior to, and after, diagnosis were used for scoring. For inpatients diagnosed with PE, clinical findings available during the 24 hours just prior to diagnosis were included. Raw PESI scores were converted to risk class (I‐V), and then further dichotomized into low‐risk (class I‐II) and high‐risk (class III‐V) groups (Table 2). The PREP score was computed based on the presence of altered mental status (AMS), cancer, and cardiogenic shock defined as a systolic blood pressure <90 mm Hg (Table 1B). A raw PREP score of <7 was then characterized as low risk for mortality, while scores 7 were considered high risk.
| PESI Score | Class | n | 30‐Day Mortality by Class (%) | 90‐Day Mortality by Class (%) | Low vs High Risk |
|---|---|---|---|---|---|
| |||||
| 65 | I | 49 | 0 (0.0) | 0 (0.0) | Low |
| 66‐85 | II | 59 | 0 (0.0) | 0 (0.0) | |
| 86‐105 | III | 60 | 0 (0.0) | 0 (0.0) | High |
| 106‐125 | IV | 56 | 2 (3.4) | 4 (6.9) | |
| >125 | V | 69 | 7 (9.2) | 8 (10.5) | |
Finally, the PESI and PREP scores were compared based on their ability to predict all‐cause 30‐day and 90‐day mortality. To determine vital status and date of death, we reviewed the Social Security Death Index 90 days after enrollment of all subjects was completed.
Statistical Analysis
To assess the predictive ability of the 2 scoring tools for death, we determined the negative predictive value and computed the AUROC curves for both scoring systems. AUROC curves were constructed for raw scores and when scores were further segregated by class and risk groups. Additionally, 95% CIs were estimated to determine the accuracy of the discriminatory power of the PESI score versus the PREP score.
Post hoc, we calculated the power of our study to assess whether the difference noted in AUROC curves between the PESI and PREP was adequate to truly determine statistical significance. We used methodology described by Hanley and McNeil to compare continuous values.9 Assuming an alpha of 0.05 and a 20% difference in the AUROC curves, as described in our results, the power in our study was 0.35. Therefore, an approximate sample size of 1000 would be necessary to determine statistical significance. This analysis was performed using Power Analysis and Sample Size (PASS) 11.
RESULTS
The final cohort included 302 subjects (mean age: 59.7 17.2 years; 44.0% males). As Table 3 reveals, the majority of PEs was diagnosed via CT scan (76%). On presentation, 6.6% had cardiogenic shock, while 5.0% had altered censorium. In terms of comorbid conditions, 25.2% had congestive heart failure, 25.2% had cancer, and 22.2% had a prior venous thromboembolic event. Overall, 3.0% and 4.0% met our primary outcomes of death within 30‐days and 90‐days of their acute PEs, respectively.
| |
| Demographics | |
| Age (yr), mean SD | 59.7 17.2 |
| Male sex, % | 44% |
| Diagnostic methodology | |
| CT chest, n (%) | 230 (76.2) |
| V/Q scan, n (%) | 71 (23.5) |
| MRA chest, n (%) | 1 (0.3) |
| Comorbidities | |
| Malignancy, n (%) | 76 (25.2) |
| Congestive heart failure, n (%) | 76 (25.2) |
| Chronic lung disease, n (%) | 72 (23.8) |
| Recent orthopedic surgery, n (%) | 22 (7.3) |
| Prior cerebrovascular accident, n (%) | 31 (10.3) |
| Prior venous thromboembolic disease, n (%) | 67 (22.2) |
The rates of 30‐day and 90‐day mortality, respectively, increased with increasing score for both the PESI and the PREP. No patients in PESI class I died by either time point, while 9.2% of PESI class V subjects expired by 30 days (P < 0.0001) and 10.5% died by 90 days (P = 0.003) (Table 2). Based on PESI, 30‐day death rates were 4.6% in the high‐risk cohort versus 0% in the low‐risk group (P = 0.023). Conversely, 7.1% of high‐risk PREP subjects died by day 30 versus 1% of low‐risk subjects (P = 0.004) (Figure 1A). Those stratified into the PESI high‐risk group had a 90‐day mortality of 6.2% versus 0% for the low‐risk group (P = 0.008) versus 9.1% in those deemed high risk by PREP, as compared to 1.5% of those scored as low risk by PREP (P = 0.001) (Figure 1B).

Regarding the 30‐day mortality, the negative predictive value of the PESI was 100% (95% CI, 98.6%‐100%) while that for PREP was 99.0% (95% CI, 97.6%‐99.7%); the ability of the PREP to predict 30‐day mortality was similar to the PESI (Table 4). The AUROCs for PESI and PREP for predicting 30‐day death were also equivalent; for the raw PESI score, this measured 0.858 (95% CI, 0.773‐0.943), compared to 0.719 (95% CI, 0.563‐0.875) for PREP. When these scores were dichotomized to high‐risk versus low‐risk groups, the AUROC for the PESI was 0.684 (95% CI, 0.559‐0.810) and 0.732 (95% CI, 0.571‐0.893) for PREP.
| 30‐Day Mortality | 90‐Day Mortality | |||
|---|---|---|---|---|
| Scoring System | AUROC | 95% CI | AUROC | 95% CI |
| ||||
| Raw PESI | 0.858 | 0.773‐0.943 | 0.835 | 0.762‐0.907 |
| PESI class | 0.835 | 0.756‐0.914 | 0.813 | 0.738‐0.888 |
| PESI high vs low risk | 0.684 | 0.559‐0.810 | 0.686 | 0.576‐0.796 |
| Raw PREP | 0.719 | 0.563‐0.875 | 0.704 | 0.564‐0.844 |
| PREP high vs low risk | 0.732 | 0.571‐0.893 | 0.720 | 0.574‐0.865 |
In terms of 90‐day mortality, the negative predictive values of PESI and PREP did not change: 100% (95% CI, 97.4%‐100%) and 98.5% (95% CI, 96.9%‐99.5%), respectively. The ability of PESI and PREP as predictors of 90‐day mortality was equivalent (Table 4). Here, the AUROC for the raw PESI score remained excellent at 0.835 (97% CI, 0.762‐0.907). The AUROC for PREP was akin to that of PESI at 0.704 (95% CI, 0.564‐0.844). Segregating scores into high‐risk versus low‐risk groups demonstrated that the AUROC for PESI was 0.686 (95% CI, 0.576‐0.796) compared to 0.720 (95% CI, 0.574‐0.865) for PREP.
DISCUSSION
This retrospective analysis of patients with acute PE confirms that both the PESI and the PREP are accurate scoring tools for identifying patients at low risk of death. Under both rubrics, as the score increases, the likelihood of death also increases. More importantly, we demonstrate that the negative predictive value for both the PREP and PESI are excellent. Thus, these scoring tools can distinguish those at higher risk for death versus those at low risk in a simple‐to‐apply manner. In comparing these 2 scoring systems, the PREP comparably identifies acute PE patients at risk for death when contrasted with the PESI. Given the fewer required scoring points to calculate PREP and its ability to accurately predict clinically relevant outcomes, this simpler scoring system may have greater clinical utility.
Prior studies have validated the PESI as a risk stratification tool to predict 30‐day and 90‐day mortalities. In their original derivation of the PESI, Aujesky et al. demonstrated that higher PESI scores correlated with death at 30 days.6 Acute PE patients classified into risk class I had a short‐term mortality rate of 1.1% compared to nearly 25% of patients risk stratified into risk class V. The same authors subsequently verified that there is a linear relationship between PESI score and risk of death at 90 days.10 We have also confirmed the accuracy of the PESI for identifying persons at high risk for death and documented the limited interobserver variability in this tool.7 In combination, there is evidence that the PESI can accurately predict vital outcome. Despite the effectiveness of the PESI, it is a somewhat cumbersome scoring system. It requires gathering information on 11 clinical variables, each with a different score allocation to ultimately compute the PESI score. In contrast, the PREP only requires knowing 3 clinical variables: presence of cancer, mental status, and the presence of cardiogenic shock. Akin to the PESI, the PREP and mortality are linearly related, where higher PREP scores result in higher 30‐day and 90‐day mortalities.
Our analysis helps expand the evidence regarding clinical risk stratification in PE in several ways. First, we verify that both the PESI and PREP are accurate predictors of short‐term mortality. While this has been accomplished for the PESI in prior studies, to our knowledge, this is the first confirmatory study for PREP's utility as a risk stratification tool. Second, we demonstrate that PREP is also an accurate predictor of intermediate‐term mortality. If the eventual goal is to develop tools that allow for the initial outpatient management of acute PE, clinicians require data on longer‐term outcomes to ensure that later harms do not arise based on a decision to defer hospitalization. Prior observational studies and randomized controlled clinical trials have proven that appropriately selected individuals face similar rates of complications following acute PE, whether they are managed in or outside of a hospital setting.1116 The key limitation of these earlier efforts, though, was that there was no clear standardized approach to determining whom could be safely managed solely as an outpatient. Finally, our study is unique in that we compare the discriminatory power of these 2 risk‐scoring schemes and illustrate their equivalence. As a scoring system that only requires 3 variables, the PREP is easier and simpler, and may therefore have more clinical utility than the PESI. The high negative predictive value of the PREP suggests that it has potential in identifying patients with acute PE who can safely be managed on an outpatient basis. However, given the complexity of factors associated with the decision for early discharge, these scores should be used in conjunction with, and not supplant, clinical judgment for outpatient management. Of course, formal prospective management trials incorporating both the PREP and PESI are needed to validate this concept.
Why does PREP perform so well despite the fact that it focuses on so few clinical variables? Essentially, the PREP is an effective scoring tool for acute PE because of its ability to identify individuals at risk for progressing to shock. The presence of AMS in acute PE has been associated with a greater likelihood of death, as it likely arises as a consequence of severe shock or RV strain resulting in decreased cerebral blood flow. Alternatively, altered censorium could represent a manifestation of hypoxemia from significant V/Q mismatching and/or pulmonary shunting due to the obstructive clot. This, too, portends a poorer prognosis secondary to impending respiratory failure from hypoxemia. Thus, individuals with an acute PE presenting with altered mentation merit very close observation. Similarly, pending hemodynamic instability is a concerning manifestation that warrants inpatient monitoring.5, 17, 18 At the very minimum, these individuals have RV strain and should therefore be admitted to the hospital to potentially administer more aggressive treatment modalities (ie, thrombolytics or thrombectomy). The last clinical criteria involves the presence of malignancy. The presence of a cancer may serve as a surrogate marker for those at increased risk for early recurrent thromboembolic phenomena, since malignancy is associated with a hypercoagulable state.17, 19 Perhaps there is a threshold whereby accumulating clot resulting in RV strain ensues with subsequent poorer outcomes. Thus, it clinically and physiologically seems logical that, in the absence of any of these findings, patients with acute PE will have lower mortality rates.
Thus far, other methods used for risk stratification may either be expensive, not really obtainable, or not routinely available at the time of presentation. For example, confirmation of RV strain with an echocardiogram requires a skilled technician and interpreter. In contrast, both the PESI and PREP are scored based on multiple clinical findings. Hence, they are not dependent upon a single test to determine outcome, but on various clinical variables making these scoring tools comprehensive, simple, and reliable approaches of recognizing low‐risk patients.
Our analysis has several limitations. First, the retrospective nature of this analysis subjects it to multiple forms of bias. We attempted to eliminate these biases by defining, a priori, the time frame from which vital signs can be used during scoring. We also used all‐cause mortality as our primary endpoint to minimize the possibility of ascertainment bias. However, this type of bias could not be completely eliminated since data collected was not specifically for the purpose of this study. Second, this single‐center study may limit the generalizability of these findings; yet, the diversity of patients admitted to this 900‐bed, tertiary care facility, as well as the inclusion of both inpatients and outpatients, helps to mitigate this concern. Third, the exclusion of individuals with expectant deaths within <30 days limits the applicability of these findings to this group. We chose to exclude persons with anticipated short‐term mortality to reduce the tally of patients who did not receive therapeutic treatment (ie, those transitioned to comfort care). Fourth, the use of the Social Security Death Index objectively determines death status for all‐cause mortality but cannot delineate cause‐specific death. Consequently, death strictly due to PE could not be assessed. Fifth, the original investigators for PREP assessed the PREP score with and without BNP and left‐to‐right ventricular diameter ratios. Although their results demonstrated similar AUROCs for the PREP score with and without BNP to predict 30‐day outcomes, this was a finding we could not confirm due to inconsistencies in measuring BNP and echocardiograms in our cohort. Also, our post hoc power analysis demonstrates that our findings may be limited by sample size. The lack of statistically significant differences between the PESI and the PREP may, in fact, be due to the small sample size versus true effect. Finally, tolerance for medical therapy and compliance with treatment were not documented and, therefore, were immeasurable. Poor compliance to anticoagulants or intolerability increases risk for recurrent PE, while excessive anticoagulation increases likelihood of bleeding.
In summary, the PREP and PESI can both safely predict 30‐day and 90‐day outcomes. However, the simplicity of the PREP renders it more clinician friendly. The fact that only 3 clinical noninvasive variables are required would ultimately make it the preferred bedside tool to risk stratify patients for acute PE. The high negative predictive value and comparable AUROCs establishes the effectiveness of these 2 scoring systems in recognizing low‐risk patients. Irrespective of the clinician's choice to use 1 tool over the other, both have potential for clinical application at the bedside and in clinical trials. Nevertheless, further evidence is required before they are utilized to triage patients for outpatient therapy.
- .Pulmonary embolism: what have we learned since Virchow? Natural history, pathophysiology, and diagnosis.Chest.2002;122:1440–1456.
- ,,, et al.The incidence and prognostic significance of elevated cardiac troponins in patients with submassive pulmonary embolism.J Thromb Haemost.2005;3:508–513.
- ,,, et al.Biomarker‐based risk assessment model in acute pulmonary embolism.Eur Heart J.2005;26:2166–2172.
- ,.Cardiac biomarkers for risk stratification of patients with acute pulmonary embolism.Circulation.2003;108:2191–2194.
- ,,, et al.Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mm Hg or higher.Arch Intern Med.2005;165:1777–1781.
- ,,, et al.Derivation and validation of a prognostic model for pulmonary embolism.Am J Respir Crit Care Med.2005;172:1041–1046.
- ,,.The validation and reproducibility of the pulmonary embolism severity index.J Thromb Haemost.2010;8:1509–1514.
- ,,, et al.Prognostic factors for pulmonary embolism: the prep study, a prospective multicenter cohort study.Am J Respir Crit Care Med.2010;181:168–173.
- ,.A method of comparing the areas under receiver operating characteristic curves derived from the same cases.Radiology.1983;148:839–843.
- ,,, et al.Prospective validation of the pulmonary embolism severity index. A clinical prognostic model for pulmonary embolism.Thromb Haemost.2008;100:943–948.
- ,,, et al.Expanding eligibility for outpatient treatment of deep venous thrombosis and pulmonary embolism with low‐molecular‐weight heparin: a comparison of patient self‐injection with homecare injection.Arch Intern Med.1998;158:1809–1812.
- ,,, et al.Outpatient treatment of pulmonary embolism with dalteparin.Thromb Haemost.2000;83:209–211.
- ,,, et al.Outpatient treatment of pulmonary embolism is feasible and safe in a substantial proportion of patients.J Thromb Haemost.2003;1:186–187.
- ,,, et al.A randomized trial comparing 2 low‐molecular‐weight heparins for the outpatient treatment of deep vein thrombosis and pulmonary embolism.Arch Intern Med.2005;165:733–738.
- ,,, et al.Early discharge of patients with pulmonary embolism: a two‐phase observational study.Eur Respir J.2007;30:708–714.
- ,,, et al.Home treatment in pulmonary embolism.Thromb Res.2010;126:e1–e5.
- ,,.Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER).Lancet.1999;353:1386–1389.
- ,,, et al.Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta‐analysis of the randomized controlled trials.Circulation.2004;110:744–749.
- ,,, et al.Predictors of survival after deep vein thrombosis and pulmonary embolism: a population‐based, cohort study.Arch Intern Med.1999;159:445–453.
- .Pulmonary embolism: what have we learned since Virchow? Natural history, pathophysiology, and diagnosis.Chest.2002;122:1440–1456.
- ,,, et al.The incidence and prognostic significance of elevated cardiac troponins in patients with submassive pulmonary embolism.J Thromb Haemost.2005;3:508–513.
- ,,, et al.Biomarker‐based risk assessment model in acute pulmonary embolism.Eur Heart J.2005;26:2166–2172.
- ,.Cardiac biomarkers for risk stratification of patients with acute pulmonary embolism.Circulation.2003;108:2191–2194.
- ,,, et al.Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mm Hg or higher.Arch Intern Med.2005;165:1777–1781.
- ,,, et al.Derivation and validation of a prognostic model for pulmonary embolism.Am J Respir Crit Care Med.2005;172:1041–1046.
- ,,.The validation and reproducibility of the pulmonary embolism severity index.J Thromb Haemost.2010;8:1509–1514.
- ,,, et al.Prognostic factors for pulmonary embolism: the prep study, a prospective multicenter cohort study.Am J Respir Crit Care Med.2010;181:168–173.
- ,.A method of comparing the areas under receiver operating characteristic curves derived from the same cases.Radiology.1983;148:839–843.
- ,,, et al.Prospective validation of the pulmonary embolism severity index. A clinical prognostic model for pulmonary embolism.Thromb Haemost.2008;100:943–948.
- ,,, et al.Expanding eligibility for outpatient treatment of deep venous thrombosis and pulmonary embolism with low‐molecular‐weight heparin: a comparison of patient self‐injection with homecare injection.Arch Intern Med.1998;158:1809–1812.
- ,,, et al.Outpatient treatment of pulmonary embolism with dalteparin.Thromb Haemost.2000;83:209–211.
- ,,, et al.Outpatient treatment of pulmonary embolism is feasible and safe in a substantial proportion of patients.J Thromb Haemost.2003;1:186–187.
- ,,, et al.A randomized trial comparing 2 low‐molecular‐weight heparins for the outpatient treatment of deep vein thrombosis and pulmonary embolism.Arch Intern Med.2005;165:733–738.
- ,,, et al.Early discharge of patients with pulmonary embolism: a two‐phase observational study.Eur Respir J.2007;30:708–714.
- ,,, et al.Home treatment in pulmonary embolism.Thromb Res.2010;126:e1–e5.
- ,,.Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER).Lancet.1999;353:1386–1389.
- ,,, et al.Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta‐analysis of the randomized controlled trials.Circulation.2004;110:744–749.
- ,,, et al.Predictors of survival after deep vein thrombosis and pulmonary embolism: a population‐based, cohort study.Arch Intern Med.1999;159:445–453.
Copyright © 2011 Society of Hospital Medicine