User login
In the United States an estimated 73,870 new cases of melanoma will be diagnosed in 2015.1 Although melanoma accounts for less than 2% of all US skin cancer cases, it is responsible for the vast majority of skin cancer deaths. From 2007 to 2011, melanoma mortality rates decreased by 2.6% per year in individuals younger than 50 years but increased by 0.6% per year among those 50 years and older.1 Reports of the direct annual treatment costs for melanoma in the United States have ranged from $44.9 million for Medicare recipients with existing cases of melanoma to $932.5 million for newly diagnosed melanomas across all age groups.2
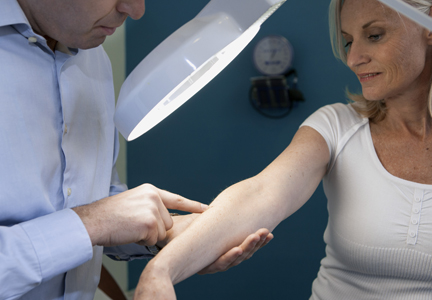
Melanoma survival rates are inversely related to tumor thickness at the time of diagnosis.3 Melanoma can be cured if caught early and properly treated. Secondary preventative measures include physician skin examinations (PSEs), which may increase the likelihood of detecting melanomas in earlier stages, thereby potentially increasing survival rates and quality of life as well as decreasing treatment costs. Physician skin examinations are performed in the physician’s office and are safe, noninvasive, and painless. Patients with suspicious lesions should subsequently undergo a skin biopsy, which is a low-risk procedure. False-positives from biopsies do not lead to extreme patient morbidity, and false-negatives will hopefully be detected at a subsequent visit.
There is a lack of consensus regarding recommendations for PSEs for skin cancer screening. Due to a lack of randomized controlled trials on the effects of skin cancer screening on patient morbidity and mortality, the US Preventive Services Task Force (USPSTF) has concluded that there is insufficient evidence to recommend for or against such screening4; however, other organizations including the American Cancer Society and the American Academy of Dermatology recommend periodic skin cancer screening examinations.1,5 In a rapidly changing health care climate and with the rollout of the Patient Protection and Affordable Care Act, a USPSTF recommendation for skin screening with PSEs for skin cancer would have a large impact on clinical practice in the United States.
This article provides a systematic review of the current domestic and international data regarding the impact of PSEs on melanoma tumor thickness at the time of diagnosis as well as mortality from melanoma.
Methods
Search Strategy
A systematic search of PubMed articles indexed for MEDLINE and Embase for studies related to melanoma and PSEs was performed for the period from each database’s inception to November 8, 2014. One of the authors (S.L.M.) designed a broad search strategy with assistance from a medical librarian who had expertise in searching research bibliographies. Articles were excluded if they had a cross-sectional study design or were editorials or review articles. Search terms included skin neoplasm, skin cancer, or melanoma in combination with any of the following: skin examination, mass screening, screening, and secondary prevention.
Study Selection
All published studies reporting outcomes and correlations with PSEs and cutaneous melanoma in adult patients were screened. If multiple studies were published describing the same study, follow-up studies were included for data extraction, but the original study was the primary resource. Observational studies were a focus in this review, as these types of studies are much more common in this subject area.
One of the authors (S.L.M.) screened the titles and abstracts of identified studies for eligibility. If the reviewer considered a study potentially eligible based on the abstract review, a full-text review was carried out. The reference lists of eligible studies were manually searched to identify additional studies.
Data Extraction, Quality Assessment, and Data Synthesis
Data items to be extracted were agreed on before search implementation and were extracted by one investigator (S.L.M.) following criteria developed by review of the Cochrane Handbook for Systematic Reviews of Interventions.6 Study population, design, sample size, and outcomes were extracted. Risk of bias of individual articles was evaluated using a tool developed from the RTI item bank (RTI International) for determining the risk of bias and precision of eligible observational studies.7 Studies ultimately were classified into 3 categories based on the risk of bias: (1) low risk of bias, (2) medium risk of bias, and (3) high risk of bias. The strength of evidence of included studies was evaluated by the following items: risk of bias, consistency, directness, precision, and overall conclusion. Data from the included studies was synthesized qualitatively in a narrative format. This review adhered to guidelines in the Cochrane Handbook for Systematic Reviews of Interventions6 and the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines.8
Results
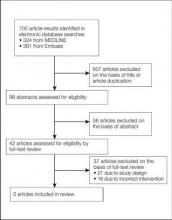
A total of 705 titles were screened, 98 abstracts were assessed for eligibility, 42 full-text reviews were carried out, and 5 eligible studies were identified (Figure 1). Five observational studies were included in the final review. A summary of the results is presented in Table 1.
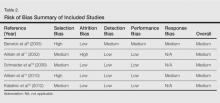
Included studies were assessed for several types of biases, including selection bias, attrition bias, detection bias, performance bias, and response bias. The judgments were given for each domain (Table 2). There was heterogeneity in study design, reporting of total-body skin examination methods, and reporting of outcomes among all 5 studies. All 5 studies were assessed as having a medium risk of bias.
Physician Skin Examination Impact
One article by Berwick et al9 reanalyzed data from a 1996 study10 and provided no significant evidence regarding the benefits of PSEs in the reduction of melanoma mortality. Data for 650 patients with newly diagnosed melanomas were obtained from the Connecticut Tumor Registry, a site for the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program, along with 549 age- and sex-frequency matched controls from the general population.10 Participants were followed biannually for a mean of 5.4 years. Of the original 650 case patients, 122 were excluded from the study with reasons provided. Physician skin examination was defined as a positive response to the following questionnaire item: “[Before your recent biopsy] did the doctor examine your skin during any of your visits?”9 Data analysis showed no significant association between PSE and death from melanoma. Upon univariate analysis, the hazard ratio for physician screening was 0.7 (95% confidence interval [CI], 0.4-1.3).9
The SCREEN (Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany) project, which was undertaken in Schleswig-Holstein, Germany, is the world’s largest systematic population-based skin cancer screening program.15 The participation rate was
19% (N=360,288) of the eligible population (citizens aged ≥20 years with statutory health insurance). Screening was a 2-step process performed by trained physicians: initial general practitioner whole-body skin examination followed by referral to a dermatologist for evaluation of suspicious skin findings. Five years after the SCREEN program was conducted, melanoma mortality declined by 47% per 100,000 men and by 49% per 100,000 women. The annual percentage change in the most recent 10-year period (2000-2009) was 7.5% (95% CI, –14.0 to –0.5; P<.05) for men and 7.1% for women (95% CI,
–10.5 to –2.9; P<.05). Simultaneously, the melanoma mortality rates in the 4 unscreened adjacent regions and the rest of Germany were stable, significantly (P<.05) different from the decline in mortality observed in Schleswig-Holstein.15
A community-based, prospective cohort study investigated the impact of an employee melanoma screening program at the Lawrence Livermore National Laboratory (Livermore, California) (1984-1996) demonstrated an impact on melanoma thickness and mortality rates.12 The cohort (approximately 5100 participants) was followed over 3 phases of surveillance: (1) preawareness (1969-1975), (2) early awareness of increased melanoma risk (1976-1984), and (3) screening program (1984-1996). The screening program encouraged employees to self-examine their skin for “suggestive lesions”; if a suggestive lesion was found, a full-body skin examination was performed by a physician. After being evaluated, participants with melanoma, dysplastic nevi, 50 or more moles, or a family history of melanoma were offered a periodic full-body examination every 3 to 24 months, often with
full-body photography and dermoscopy. Physician skin screening resulted in a reduction in crude incidence of thicker melanomas (defined as
>0.75 mm) during the 3 study phases. Compared with the early-awareness period (phase 2), a 69% reduction in the diagnosis of thick melanomas was reported in the screening program period (phase 3)(P=.0001). During the screening period, no eligible melanoma deaths occurred in the study population, whereas the expected number of deaths was 3.39 (P=.034) based on observed melanoma mortality in 5 San Francisco/Oakland Bay–area counties in California as reported to the SEER program from 1984 to 1996.12
The strongest evidence for reduced thickness of melanomas detected via PSEs was reported in a population-based, case-control study by Aitken et al14 of all residents in Queensland, Australia, aged 20 to 75 years with a histologically confirmed first primary invasive cutaneous melanoma diagnosed between January 2000 and December 2003. Whole-body PSE in the 3 years before diagnosis was inversely associated with tumor thickness at diagnosis (χ2=44.37; P<.001), including a 14% lower risk of diagnosis of a thick melanoma (>0.75 mm)(odds ratio [OR], 0.86; 95% CI, 0.75-0.98) and a 40% lower risk of diagnosis of a melanoma that was 3 mm or larger (OR, 0.60; 95% CI, 0.43-0.83). The investigators applied melanoma thickness-specific survival estimates to the thickness distribution of the screened and unscreened cases in their sample to estimate melanoma deaths within 5 and 10 years of diagnosis. Compared to the unscreened cases, they estimated that the screened cases would have 26% fewer melanoma deaths within 5 years of diagnosis and 23% fewer deaths within 10 years.14
Another prospective cohort study in Queensland was designed to detect a 20% reduction in mortality from melanoma during a 15-year intervention period in communities that received a screening program.11 A total of 44 communities (aggregate population, 560,000 adults aged ≥30 years) were randomized into intervention or control groups to receive a community-based melanoma screening program for 3 years versus usual medical care.Overall, thinner melanomas were identified in communities with the screening program versus neighboring communities without it.11 Of the 33 melanomas found through the screening program, 39% (13/33) were in situ lesions, 55% (18/33) were thin (<1 mm) invasive lesions, and 6% (2/33) were 1-mm thick or greater.16 Within the population of Queensland during the period from 1999 through 2002, 36% were in situ lesions, 48% were invasive thin melanomas, and 16% were invasive melanomas 1-mm thick or more, indicating that melanomas found through screening were thinner or less advanced.17
Comment
Our review identified 5 studies describing the impact of PSEs for melanoma screening on tumor thickness at diagnosis and melanoma mortality. Key findings are highlighted in Figure 2. Our findings suggest that PSEs are associated with a decline in melanoma tumor thickness and melanoma-specific mortality. Our findings are qualitatively similar to prior reviews that supported the use of PSEs to detect thinner melanomas and improve mortality outcomes.18-20
The greatest evidence for population-based screening programs was provided by the SCREEN study. This landmark study documented that screening programs utilizing primary care physicians (PCPs) and dermatologists can lead to a reduction in melanoma mortality.15 Findings from the study led to the countrywide expansion of the screening program in 2008, leading to 45 million Germans eligible for skin cancer screenings every 2 years.21 Nearly two-thirds of dermatologists (N=1348) were satisfied with routine PSE and 83% perceived a better quality of health care for skin with the 2008 expansion.22
Data suggest that physician-detected melanomas through PSEs or routine physical examinations are thinner at the time of diagnosis than those found by patients or their partners.14,23-26 Terushkin and Halpern20 analyzed 9 worldwide studies encompassing more than 7500 patients and found that physician-detected melanomas were 0.55 mm thinner than those detected by patients or their significant others. The workplace screening and education program reviewed herein also reported a reduction in thicker melanomas and melanoma mortality during the study period.12
Not all Americans have a regular dermatologist. As such, educating PCPs in skin cancer detection has been a recent area of study. The premise is that the skin examination can be integrated into routine physical examinations conducted by PCPs. The previously discussed studies, particularly Aitken et al,14 Schneider et al,12 and Katalinic et al,15 as well as the SCREEN program studies,15 suggest that integration of the skin examination into the routine physical examination may be a feasible method to reduce melanoma thickness and mortality. Furthermore, the SCREEN study15 identified participants with risk factors for melanoma, finding that approximately half of men and women (N=360,288) had at least one melanoma risk factor, which suggests that it may be more practical to design screening practices around high-risk participants.
Several studies were excluded from our analysis on the basis of study design, including cross-sectional observational studies; however, it is worth briefly commenting on the findings of the excluded studies here, as they add to the body of literature. A community-based, multi-institutional study of 566 adults with invasive melanoma assessed the role of PSEs in the year prior to diagnosis by interviewing participants in clinic within 3 months of melanoma diagnosis.24 Patients who underwent full-body PSE in the year prior to diagnosis were more than 2 times more likely to have thinner (≤1 mm) melanomas (OR, 2.51; 95% CI, 1.62-3.87]). Notably, men older than 60 years appeared to benefit the most from this practice; men in this age group contributed greatly to the observed effect, likely because they had 4 times the odds of a thinner melanoma (OR, 4.09; 95% CI, 1.88-8.89]). Thinner melanomas also were associated with an age of 60 years or younger, female sex, and higher education level.24
Pollitt et al27 analyzed the association between prediagnosis Medicaid enrollment status and melanoma tumor thickness. The study found that men and women who intermittently enrolled in Medicaid or were not enrolled until the month of diagnosis had an increased chance of late-stage melanoma when compared to other patients. Patients who continuously enrolled during the year prior to diagnosis had lower odds for thicker melanomas, suggesting that these patients had greater access to screening examinations.27
Roetzheim et al28 analyzed data from the SEER-Medicare linked dataset to investigate patterns of dermatologist and PCP visits in the 2 years before melanoma diagnosis. Medicare beneficiaries seeing both a dermatologist and a PCP prior to melanoma diagnosis had greater odds of a thinner melanoma and lower melanoma mortality compared to patients without such visits.28
Durbec et al29 conducted a retrospective, population-based study of 650 patients in France who were seen by a dermatologist for melanoma. The thinnest melanomas were reported in patients seeing a dermatologist for prospective follow-up of nevi or consulting a dermatologist for other diseases. Patients referred to a dermatologist by PCPs tended to be older and had the highest frequency of thick (>3 mm), nodular, and/or ulcerated melanomas,29 which could be interpreted as a need for greater PCP education in melanoma screening.
Rates of skin examinations have been increasing since the year 2000, both overall and among high-risk groups as reported by a recent study on skin cancer screening trends. Prevalence of having at least one total-body skin examination increased from 14.5% in 2000 to 16.5% in 2005 to 19.8% in 2010 (P<.0001).30 One study revealed a practice gap in which more than 3 in 10 PCPs and 1 in 10 dermatologists reported not screening more than half their high-risk patients for skin cancer.31 The major obstacle to narrowing the identified practice gap involves establishing a national strategy to screen high-risk individuals for skin cancer and requires partnerships among patients, PCPs, specialists, policy makers, and government sponsors.
Lack of evidence that screening for skin cancer with PSEs reduces overall mortality does not mean there is a lack of lifesaving potential of screenings. The resources required to execute a randomized controlled trial with adequate power are vast, as the USPSTF estimated 800,000 participants would be needed.4 Barriers to conducting a randomized clinical trial for skin cancer screening include the large sample size required, prolonged follow-up, and various ethical issues such as withholding screening for a cancer that is potentially curable in early stages. Lessons from screenings for breast and prostate cancers have taught us that such randomized controlled trials assessing cancer screening are costly and do not always produce definitive answers.32
Conclusion
Although proof of improved health outcomes from randomized controlled trials is still required, there is evidence to support targeted screening programs for the detection of thinner melanomas and, by proxy, reduced melanoma mortality. Amidst the health care climate change and payment reform, recommendations from national organizations on melanoma screenings are paramount. Clinicians should continue to offer regular skin examinations as the body of evidence continues to grow in support of PSEs for melanoma screening.
Acknowledgments—We are grateful to Mary Butler, PhD, and Robert Kane, MD, both from Minneapolis, Minnesota, for their guidance and consultation.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. http: //www.cancer.org/Research/CancerFactsStatistics/cancer factsfigures2015/cancer-facts-and-figures-2015. Accessed July 6, 2015.
2. Guy G Jr, Ekwueme D, Tangka F, et al. Melanoma treatment costs: a systematic review of the literature, 1990-2011. Am J Prev. 2012;43:537-545.
3. Margolis D, Halpern A, Rebbeck T, et al. Validation of a melanoma prognostic model. Arch Dermatol. 1998;134:1597-1601.
4. Wolff T, Tai E, Miller T. Screening for skin cancer: an update of the evidence for the U.S. Preventative Services Task Force. Ann Intern Med. 2009;150:194-198.
5. American Academy of Dermatology. Melanoma
Monday. http://www.aad.org/spot-skin-cancer
/community-programs-events/melanoma-monday. Accessed August 19, 2015.
6. Higgins JPT, Green S, eds. Cochrane Handbook for
Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. http:
//www.cochrane-handbook.org. Updated March 2011. Accessed November 10, 2014.
7. Viswanathan M, Berkman N. Development of the RTI item bank on risk of bias and precision of observational studies. J Clin Epidemiol. 2012;65:163-178.
8. Moher D, Liberati A, Tetzlaff J, et al; PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement [published online ahead of print July 23, 2009]. J Clin Epidemiol. 2009;62:1006-1012.
9. Berwick M, Armstrong B, Ben-Porat L. Sun exposure and mortality from melanoma. J Natl Cancer Inst. 2005;97:195-199.
10. Berwick M, Begg C, Fine J, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23.
11. Aitken J, Elwood J, Lowe J, et al. A randomised trial of population screening for melanoma. J Med Screen. 2002;9:33-37.
12. Schneider J, Moore D, Mendelsohn M. Screening program reduced melanoma mortality at the Lawrence Livermore National Laboratory, 1984 to 1996. J Am Acad Dermatol. 2008;58:741-749.
13. Expert Health Data Programming Inc. Health data software and health statistics. Available from: http: //www.ehdp.com. Accessed April 1, 2001. Cited by: Schneider J, Moore D, Mendelsohn M. Screening program reduced melanoma mortality at the Lawrence Livermore National Laboratory, 1984 to 1996. J Am Acad Dermatol. 2008;58:741-749.
14. Aitken J, Elwood M, Baade P, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010;126:450-458.
15. Katalinic A, Waldmann A, Weinstock M, et al. Does skin cancer screening save lives? an observational study comparing trends in melanoma mortality in regions with and without screening. Cancer. 2012;118:5395-5402.
16. Aitken J, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006;54:105-114.
17. Coory M, Baade P, Aitken JF, et al. Trends for in-situ and invasive melanoma in Queensland, Australia, 1982 to 2002. Cancer Causes Control. 2006;17:21-27.
18. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: part II. screening, education, and future directions. J Am Acad Dermatol. 2014;71:611.e1-611.e10; quiz, 621-622.
19. Curiel-Lewandrowski C, Chen S, Swetter S, et al. Screening and prevention measures for melanoma: is there a survival advantage? Curr Oncol Rep. 2012;14:458-467.
20. Terushkin V, Halpern A. Melanoma early detection. Hematol Oncol Clin North Am. 2009;23:481-500.
21. Geller A, Greinert R, Sinclair C, et al. A nationwide population-based skin cancer screening in Germany: proceedings of the first meeting of the International Task Force on Skin Cancer Screening and Prevention (September 24 and 25, 2009) [published online ahead of print April 8, 2010]. Cancer Epidemiol. 2010;34:355-358.
22. Kornek T, Schafer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists’ perspective. Dermatology. 2012;225:289-293.
23. De Giorgi V, Grazzini M, Rossari S, et al. Is skin self-examination for cutaneous melanoma detection still adequate? a retrospective study. Dermatology. 2012;225:31-36.
24. Swetter S, Johnson T, Miller D, et al. Melanoma in middle-aged and older men: a multi-institutional survey study of factors related to tumor thickness. Arch Dermatol. 2009;145:397-404.
25. Kantor J, Kantor D. Routine dermatologist-performed full-body skin examination and early melanoma detection. Arch Dermatol. 2009;145:873-876.
26. Kovalyshyn I, Dusza S, Siamas K, et al. The impact of physician screening on melanoma detection. Arch Dermatol. 2011;147:1269-1275.
27. Pollitt R, Clarke C, Shema S, et al. California Medicaid enrollment and melanoma stage at diagnosis: a population-based study. Am J Prev Med. 2008;35:7-13.
28. Roetzheim R, Lee J, Ferrante J, et al. The influence of dermatologist and primary care physician visits on melanoma outcomes among Medicare beneficiaries. J Am Board Fam Med. 2013;26:637-647.
29. Durbec F, Vitry F, Granel-Brocard F, et al. The role of circumstances of diagnosis and access to dermatological care in early diagnosis of cutaneous melanoma: a population-based study in France. Arch Dermatol. 2010;146:240-246.
30. Lakhani N, Saraiya M, Thompson T, et al. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med. 2014;61:75-80.
31. Oliveria SA, Heneghan MK, Cushman LF, et al. Skin cancer screening by dermatologists, family practitioners, and internists: barriers and facilitating factors. Arch Dermatol. 2011;147:39-44.
32. Bigby M. Why the evidence for skin cancer screening is insufficient: lessons from prostate cancer screening. Arch Dermatol. 2010;146:322-324.
In the United States an estimated 73,870 new cases of melanoma will be diagnosed in 2015.1 Although melanoma accounts for less than 2% of all US skin cancer cases, it is responsible for the vast majority of skin cancer deaths. From 2007 to 2011, melanoma mortality rates decreased by 2.6% per year in individuals younger than 50 years but increased by 0.6% per year among those 50 years and older.1 Reports of the direct annual treatment costs for melanoma in the United States have ranged from $44.9 million for Medicare recipients with existing cases of melanoma to $932.5 million for newly diagnosed melanomas across all age groups.2
Melanoma survival rates are inversely related to tumor thickness at the time of diagnosis.3 Melanoma can be cured if caught early and properly treated. Secondary preventative measures include physician skin examinations (PSEs), which may increase the likelihood of detecting melanomas in earlier stages, thereby potentially increasing survival rates and quality of life as well as decreasing treatment costs. Physician skin examinations are performed in the physician’s office and are safe, noninvasive, and painless. Patients with suspicious lesions should subsequently undergo a skin biopsy, which is a low-risk procedure. False-positives from biopsies do not lead to extreme patient morbidity, and false-negatives will hopefully be detected at a subsequent visit.
There is a lack of consensus regarding recommendations for PSEs for skin cancer screening. Due to a lack of randomized controlled trials on the effects of skin cancer screening on patient morbidity and mortality, the US Preventive Services Task Force (USPSTF) has concluded that there is insufficient evidence to recommend for or against such screening4; however, other organizations including the American Cancer Society and the American Academy of Dermatology recommend periodic skin cancer screening examinations.1,5 In a rapidly changing health care climate and with the rollout of the Patient Protection and Affordable Care Act, a USPSTF recommendation for skin screening with PSEs for skin cancer would have a large impact on clinical practice in the United States.
This article provides a systematic review of the current domestic and international data regarding the impact of PSEs on melanoma tumor thickness at the time of diagnosis as well as mortality from melanoma.
Methods
Search Strategy
A systematic search of PubMed articles indexed for MEDLINE and Embase for studies related to melanoma and PSEs was performed for the period from each database’s inception to November 8, 2014. One of the authors (S.L.M.) designed a broad search strategy with assistance from a medical librarian who had expertise in searching research bibliographies. Articles were excluded if they had a cross-sectional study design or were editorials or review articles. Search terms included skin neoplasm, skin cancer, or melanoma in combination with any of the following: skin examination, mass screening, screening, and secondary prevention.
Study Selection
All published studies reporting outcomes and correlations with PSEs and cutaneous melanoma in adult patients were screened. If multiple studies were published describing the same study, follow-up studies were included for data extraction, but the original study was the primary resource. Observational studies were a focus in this review, as these types of studies are much more common in this subject area.
One of the authors (S.L.M.) screened the titles and abstracts of identified studies for eligibility. If the reviewer considered a study potentially eligible based on the abstract review, a full-text review was carried out. The reference lists of eligible studies were manually searched to identify additional studies.
Data Extraction, Quality Assessment, and Data Synthesis
Data items to be extracted were agreed on before search implementation and were extracted by one investigator (S.L.M.) following criteria developed by review of the Cochrane Handbook for Systematic Reviews of Interventions.6 Study population, design, sample size, and outcomes were extracted. Risk of bias of individual articles was evaluated using a tool developed from the RTI item bank (RTI International) for determining the risk of bias and precision of eligible observational studies.7 Studies ultimately were classified into 3 categories based on the risk of bias: (1) low risk of bias, (2) medium risk of bias, and (3) high risk of bias. The strength of evidence of included studies was evaluated by the following items: risk of bias, consistency, directness, precision, and overall conclusion. Data from the included studies was synthesized qualitatively in a narrative format. This review adhered to guidelines in the Cochrane Handbook for Systematic Reviews of Interventions6 and the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines.8
Results
A total of 705 titles were screened, 98 abstracts were assessed for eligibility, 42 full-text reviews were carried out, and 5 eligible studies were identified (Figure 1). Five observational studies were included in the final review. A summary of the results is presented in Table 1.
Included studies were assessed for several types of biases, including selection bias, attrition bias, detection bias, performance bias, and response bias. The judgments were given for each domain (Table 2). There was heterogeneity in study design, reporting of total-body skin examination methods, and reporting of outcomes among all 5 studies. All 5 studies were assessed as having a medium risk of bias.
Physician Skin Examination Impact
One article by Berwick et al9 reanalyzed data from a 1996 study10 and provided no significant evidence regarding the benefits of PSEs in the reduction of melanoma mortality. Data for 650 patients with newly diagnosed melanomas were obtained from the Connecticut Tumor Registry, a site for the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program, along with 549 age- and sex-frequency matched controls from the general population.10 Participants were followed biannually for a mean of 5.4 years. Of the original 650 case patients, 122 were excluded from the study with reasons provided. Physician skin examination was defined as a positive response to the following questionnaire item: “[Before your recent biopsy] did the doctor examine your skin during any of your visits?”9 Data analysis showed no significant association between PSE and death from melanoma. Upon univariate analysis, the hazard ratio for physician screening was 0.7 (95% confidence interval [CI], 0.4-1.3).9
The SCREEN (Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany) project, which was undertaken in Schleswig-Holstein, Germany, is the world’s largest systematic population-based skin cancer screening program.15 The participation rate was
19% (N=360,288) of the eligible population (citizens aged ≥20 years with statutory health insurance). Screening was a 2-step process performed by trained physicians: initial general practitioner whole-body skin examination followed by referral to a dermatologist for evaluation of suspicious skin findings. Five years after the SCREEN program was conducted, melanoma mortality declined by 47% per 100,000 men and by 49% per 100,000 women. The annual percentage change in the most recent 10-year period (2000-2009) was 7.5% (95% CI, –14.0 to –0.5; P<.05) for men and 7.1% for women (95% CI,
–10.5 to –2.9; P<.05). Simultaneously, the melanoma mortality rates in the 4 unscreened adjacent regions and the rest of Germany were stable, significantly (P<.05) different from the decline in mortality observed in Schleswig-Holstein.15
A community-based, prospective cohort study investigated the impact of an employee melanoma screening program at the Lawrence Livermore National Laboratory (Livermore, California) (1984-1996) demonstrated an impact on melanoma thickness and mortality rates.12 The cohort (approximately 5100 participants) was followed over 3 phases of surveillance: (1) preawareness (1969-1975), (2) early awareness of increased melanoma risk (1976-1984), and (3) screening program (1984-1996). The screening program encouraged employees to self-examine their skin for “suggestive lesions”; if a suggestive lesion was found, a full-body skin examination was performed by a physician. After being evaluated, participants with melanoma, dysplastic nevi, 50 or more moles, or a family history of melanoma were offered a periodic full-body examination every 3 to 24 months, often with
full-body photography and dermoscopy. Physician skin screening resulted in a reduction in crude incidence of thicker melanomas (defined as
>0.75 mm) during the 3 study phases. Compared with the early-awareness period (phase 2), a 69% reduction in the diagnosis of thick melanomas was reported in the screening program period (phase 3)(P=.0001). During the screening period, no eligible melanoma deaths occurred in the study population, whereas the expected number of deaths was 3.39 (P=.034) based on observed melanoma mortality in 5 San Francisco/Oakland Bay–area counties in California as reported to the SEER program from 1984 to 1996.12
The strongest evidence for reduced thickness of melanomas detected via PSEs was reported in a population-based, case-control study by Aitken et al14 of all residents in Queensland, Australia, aged 20 to 75 years with a histologically confirmed first primary invasive cutaneous melanoma diagnosed between January 2000 and December 2003. Whole-body PSE in the 3 years before diagnosis was inversely associated with tumor thickness at diagnosis (χ2=44.37; P<.001), including a 14% lower risk of diagnosis of a thick melanoma (>0.75 mm)(odds ratio [OR], 0.86; 95% CI, 0.75-0.98) and a 40% lower risk of diagnosis of a melanoma that was 3 mm or larger (OR, 0.60; 95% CI, 0.43-0.83). The investigators applied melanoma thickness-specific survival estimates to the thickness distribution of the screened and unscreened cases in their sample to estimate melanoma deaths within 5 and 10 years of diagnosis. Compared to the unscreened cases, they estimated that the screened cases would have 26% fewer melanoma deaths within 5 years of diagnosis and 23% fewer deaths within 10 years.14
Another prospective cohort study in Queensland was designed to detect a 20% reduction in mortality from melanoma during a 15-year intervention period in communities that received a screening program.11 A total of 44 communities (aggregate population, 560,000 adults aged ≥30 years) were randomized into intervention or control groups to receive a community-based melanoma screening program for 3 years versus usual medical care.Overall, thinner melanomas were identified in communities with the screening program versus neighboring communities without it.11 Of the 33 melanomas found through the screening program, 39% (13/33) were in situ lesions, 55% (18/33) were thin (<1 mm) invasive lesions, and 6% (2/33) were 1-mm thick or greater.16 Within the population of Queensland during the period from 1999 through 2002, 36% were in situ lesions, 48% were invasive thin melanomas, and 16% were invasive melanomas 1-mm thick or more, indicating that melanomas found through screening were thinner or less advanced.17
Comment
Our review identified 5 studies describing the impact of PSEs for melanoma screening on tumor thickness at diagnosis and melanoma mortality. Key findings are highlighted in Figure 2. Our findings suggest that PSEs are associated with a decline in melanoma tumor thickness and melanoma-specific mortality. Our findings are qualitatively similar to prior reviews that supported the use of PSEs to detect thinner melanomas and improve mortality outcomes.18-20
The greatest evidence for population-based screening programs was provided by the SCREEN study. This landmark study documented that screening programs utilizing primary care physicians (PCPs) and dermatologists can lead to a reduction in melanoma mortality.15 Findings from the study led to the countrywide expansion of the screening program in 2008, leading to 45 million Germans eligible for skin cancer screenings every 2 years.21 Nearly two-thirds of dermatologists (N=1348) were satisfied with routine PSE and 83% perceived a better quality of health care for skin with the 2008 expansion.22
Data suggest that physician-detected melanomas through PSEs or routine physical examinations are thinner at the time of diagnosis than those found by patients or their partners.14,23-26 Terushkin and Halpern20 analyzed 9 worldwide studies encompassing more than 7500 patients and found that physician-detected melanomas were 0.55 mm thinner than those detected by patients or their significant others. The workplace screening and education program reviewed herein also reported a reduction in thicker melanomas and melanoma mortality during the study period.12
Not all Americans have a regular dermatologist. As such, educating PCPs in skin cancer detection has been a recent area of study. The premise is that the skin examination can be integrated into routine physical examinations conducted by PCPs. The previously discussed studies, particularly Aitken et al,14 Schneider et al,12 and Katalinic et al,15 as well as the SCREEN program studies,15 suggest that integration of the skin examination into the routine physical examination may be a feasible method to reduce melanoma thickness and mortality. Furthermore, the SCREEN study15 identified participants with risk factors for melanoma, finding that approximately half of men and women (N=360,288) had at least one melanoma risk factor, which suggests that it may be more practical to design screening practices around high-risk participants.
Several studies were excluded from our analysis on the basis of study design, including cross-sectional observational studies; however, it is worth briefly commenting on the findings of the excluded studies here, as they add to the body of literature. A community-based, multi-institutional study of 566 adults with invasive melanoma assessed the role of PSEs in the year prior to diagnosis by interviewing participants in clinic within 3 months of melanoma diagnosis.24 Patients who underwent full-body PSE in the year prior to diagnosis were more than 2 times more likely to have thinner (≤1 mm) melanomas (OR, 2.51; 95% CI, 1.62-3.87]). Notably, men older than 60 years appeared to benefit the most from this practice; men in this age group contributed greatly to the observed effect, likely because they had 4 times the odds of a thinner melanoma (OR, 4.09; 95% CI, 1.88-8.89]). Thinner melanomas also were associated with an age of 60 years or younger, female sex, and higher education level.24
Pollitt et al27 analyzed the association between prediagnosis Medicaid enrollment status and melanoma tumor thickness. The study found that men and women who intermittently enrolled in Medicaid or were not enrolled until the month of diagnosis had an increased chance of late-stage melanoma when compared to other patients. Patients who continuously enrolled during the year prior to diagnosis had lower odds for thicker melanomas, suggesting that these patients had greater access to screening examinations.27
Roetzheim et al28 analyzed data from the SEER-Medicare linked dataset to investigate patterns of dermatologist and PCP visits in the 2 years before melanoma diagnosis. Medicare beneficiaries seeing both a dermatologist and a PCP prior to melanoma diagnosis had greater odds of a thinner melanoma and lower melanoma mortality compared to patients without such visits.28
Durbec et al29 conducted a retrospective, population-based study of 650 patients in France who were seen by a dermatologist for melanoma. The thinnest melanomas were reported in patients seeing a dermatologist for prospective follow-up of nevi or consulting a dermatologist for other diseases. Patients referred to a dermatologist by PCPs tended to be older and had the highest frequency of thick (>3 mm), nodular, and/or ulcerated melanomas,29 which could be interpreted as a need for greater PCP education in melanoma screening.
Rates of skin examinations have been increasing since the year 2000, both overall and among high-risk groups as reported by a recent study on skin cancer screening trends. Prevalence of having at least one total-body skin examination increased from 14.5% in 2000 to 16.5% in 2005 to 19.8% in 2010 (P<.0001).30 One study revealed a practice gap in which more than 3 in 10 PCPs and 1 in 10 dermatologists reported not screening more than half their high-risk patients for skin cancer.31 The major obstacle to narrowing the identified practice gap involves establishing a national strategy to screen high-risk individuals for skin cancer and requires partnerships among patients, PCPs, specialists, policy makers, and government sponsors.
Lack of evidence that screening for skin cancer with PSEs reduces overall mortality does not mean there is a lack of lifesaving potential of screenings. The resources required to execute a randomized controlled trial with adequate power are vast, as the USPSTF estimated 800,000 participants would be needed.4 Barriers to conducting a randomized clinical trial for skin cancer screening include the large sample size required, prolonged follow-up, and various ethical issues such as withholding screening for a cancer that is potentially curable in early stages. Lessons from screenings for breast and prostate cancers have taught us that such randomized controlled trials assessing cancer screening are costly and do not always produce definitive answers.32
Conclusion
Although proof of improved health outcomes from randomized controlled trials is still required, there is evidence to support targeted screening programs for the detection of thinner melanomas and, by proxy, reduced melanoma mortality. Amidst the health care climate change and payment reform, recommendations from national organizations on melanoma screenings are paramount. Clinicians should continue to offer regular skin examinations as the body of evidence continues to grow in support of PSEs for melanoma screening.
Acknowledgments—We are grateful to Mary Butler, PhD, and Robert Kane, MD, both from Minneapolis, Minnesota, for their guidance and consultation.
In the United States an estimated 73,870 new cases of melanoma will be diagnosed in 2015.1 Although melanoma accounts for less than 2% of all US skin cancer cases, it is responsible for the vast majority of skin cancer deaths. From 2007 to 2011, melanoma mortality rates decreased by 2.6% per year in individuals younger than 50 years but increased by 0.6% per year among those 50 years and older.1 Reports of the direct annual treatment costs for melanoma in the United States have ranged from $44.9 million for Medicare recipients with existing cases of melanoma to $932.5 million for newly diagnosed melanomas across all age groups.2
Melanoma survival rates are inversely related to tumor thickness at the time of diagnosis.3 Melanoma can be cured if caught early and properly treated. Secondary preventative measures include physician skin examinations (PSEs), which may increase the likelihood of detecting melanomas in earlier stages, thereby potentially increasing survival rates and quality of life as well as decreasing treatment costs. Physician skin examinations are performed in the physician’s office and are safe, noninvasive, and painless. Patients with suspicious lesions should subsequently undergo a skin biopsy, which is a low-risk procedure. False-positives from biopsies do not lead to extreme patient morbidity, and false-negatives will hopefully be detected at a subsequent visit.
There is a lack of consensus regarding recommendations for PSEs for skin cancer screening. Due to a lack of randomized controlled trials on the effects of skin cancer screening on patient morbidity and mortality, the US Preventive Services Task Force (USPSTF) has concluded that there is insufficient evidence to recommend for or against such screening4; however, other organizations including the American Cancer Society and the American Academy of Dermatology recommend periodic skin cancer screening examinations.1,5 In a rapidly changing health care climate and with the rollout of the Patient Protection and Affordable Care Act, a USPSTF recommendation for skin screening with PSEs for skin cancer would have a large impact on clinical practice in the United States.
This article provides a systematic review of the current domestic and international data regarding the impact of PSEs on melanoma tumor thickness at the time of diagnosis as well as mortality from melanoma.
Methods
Search Strategy
A systematic search of PubMed articles indexed for MEDLINE and Embase for studies related to melanoma and PSEs was performed for the period from each database’s inception to November 8, 2014. One of the authors (S.L.M.) designed a broad search strategy with assistance from a medical librarian who had expertise in searching research bibliographies. Articles were excluded if they had a cross-sectional study design or were editorials or review articles. Search terms included skin neoplasm, skin cancer, or melanoma in combination with any of the following: skin examination, mass screening, screening, and secondary prevention.
Study Selection
All published studies reporting outcomes and correlations with PSEs and cutaneous melanoma in adult patients were screened. If multiple studies were published describing the same study, follow-up studies were included for data extraction, but the original study was the primary resource. Observational studies were a focus in this review, as these types of studies are much more common in this subject area.
One of the authors (S.L.M.) screened the titles and abstracts of identified studies for eligibility. If the reviewer considered a study potentially eligible based on the abstract review, a full-text review was carried out. The reference lists of eligible studies were manually searched to identify additional studies.
Data Extraction, Quality Assessment, and Data Synthesis
Data items to be extracted were agreed on before search implementation and were extracted by one investigator (S.L.M.) following criteria developed by review of the Cochrane Handbook for Systematic Reviews of Interventions.6 Study population, design, sample size, and outcomes were extracted. Risk of bias of individual articles was evaluated using a tool developed from the RTI item bank (RTI International) for determining the risk of bias and precision of eligible observational studies.7 Studies ultimately were classified into 3 categories based on the risk of bias: (1) low risk of bias, (2) medium risk of bias, and (3) high risk of bias. The strength of evidence of included studies was evaluated by the following items: risk of bias, consistency, directness, precision, and overall conclusion. Data from the included studies was synthesized qualitatively in a narrative format. This review adhered to guidelines in the Cochrane Handbook for Systematic Reviews of Interventions6 and the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines.8
Results
A total of 705 titles were screened, 98 abstracts were assessed for eligibility, 42 full-text reviews were carried out, and 5 eligible studies were identified (Figure 1). Five observational studies were included in the final review. A summary of the results is presented in Table 1.
Included studies were assessed for several types of biases, including selection bias, attrition bias, detection bias, performance bias, and response bias. The judgments were given for each domain (Table 2). There was heterogeneity in study design, reporting of total-body skin examination methods, and reporting of outcomes among all 5 studies. All 5 studies were assessed as having a medium risk of bias.
Physician Skin Examination Impact
One article by Berwick et al9 reanalyzed data from a 1996 study10 and provided no significant evidence regarding the benefits of PSEs in the reduction of melanoma mortality. Data for 650 patients with newly diagnosed melanomas were obtained from the Connecticut Tumor Registry, a site for the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program, along with 549 age- and sex-frequency matched controls from the general population.10 Participants were followed biannually for a mean of 5.4 years. Of the original 650 case patients, 122 were excluded from the study with reasons provided. Physician skin examination was defined as a positive response to the following questionnaire item: “[Before your recent biopsy] did the doctor examine your skin during any of your visits?”9 Data analysis showed no significant association between PSE and death from melanoma. Upon univariate analysis, the hazard ratio for physician screening was 0.7 (95% confidence interval [CI], 0.4-1.3).9
The SCREEN (Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany) project, which was undertaken in Schleswig-Holstein, Germany, is the world’s largest systematic population-based skin cancer screening program.15 The participation rate was
19% (N=360,288) of the eligible population (citizens aged ≥20 years with statutory health insurance). Screening was a 2-step process performed by trained physicians: initial general practitioner whole-body skin examination followed by referral to a dermatologist for evaluation of suspicious skin findings. Five years after the SCREEN program was conducted, melanoma mortality declined by 47% per 100,000 men and by 49% per 100,000 women. The annual percentage change in the most recent 10-year period (2000-2009) was 7.5% (95% CI, –14.0 to –0.5; P<.05) for men and 7.1% for women (95% CI,
–10.5 to –2.9; P<.05). Simultaneously, the melanoma mortality rates in the 4 unscreened adjacent regions and the rest of Germany were stable, significantly (P<.05) different from the decline in mortality observed in Schleswig-Holstein.15
A community-based, prospective cohort study investigated the impact of an employee melanoma screening program at the Lawrence Livermore National Laboratory (Livermore, California) (1984-1996) demonstrated an impact on melanoma thickness and mortality rates.12 The cohort (approximately 5100 participants) was followed over 3 phases of surveillance: (1) preawareness (1969-1975), (2) early awareness of increased melanoma risk (1976-1984), and (3) screening program (1984-1996). The screening program encouraged employees to self-examine their skin for “suggestive lesions”; if a suggestive lesion was found, a full-body skin examination was performed by a physician. After being evaluated, participants with melanoma, dysplastic nevi, 50 or more moles, or a family history of melanoma were offered a periodic full-body examination every 3 to 24 months, often with
full-body photography and dermoscopy. Physician skin screening resulted in a reduction in crude incidence of thicker melanomas (defined as
>0.75 mm) during the 3 study phases. Compared with the early-awareness period (phase 2), a 69% reduction in the diagnosis of thick melanomas was reported in the screening program period (phase 3)(P=.0001). During the screening period, no eligible melanoma deaths occurred in the study population, whereas the expected number of deaths was 3.39 (P=.034) based on observed melanoma mortality in 5 San Francisco/Oakland Bay–area counties in California as reported to the SEER program from 1984 to 1996.12
The strongest evidence for reduced thickness of melanomas detected via PSEs was reported in a population-based, case-control study by Aitken et al14 of all residents in Queensland, Australia, aged 20 to 75 years with a histologically confirmed first primary invasive cutaneous melanoma diagnosed between January 2000 and December 2003. Whole-body PSE in the 3 years before diagnosis was inversely associated with tumor thickness at diagnosis (χ2=44.37; P<.001), including a 14% lower risk of diagnosis of a thick melanoma (>0.75 mm)(odds ratio [OR], 0.86; 95% CI, 0.75-0.98) and a 40% lower risk of diagnosis of a melanoma that was 3 mm or larger (OR, 0.60; 95% CI, 0.43-0.83). The investigators applied melanoma thickness-specific survival estimates to the thickness distribution of the screened and unscreened cases in their sample to estimate melanoma deaths within 5 and 10 years of diagnosis. Compared to the unscreened cases, they estimated that the screened cases would have 26% fewer melanoma deaths within 5 years of diagnosis and 23% fewer deaths within 10 years.14
Another prospective cohort study in Queensland was designed to detect a 20% reduction in mortality from melanoma during a 15-year intervention period in communities that received a screening program.11 A total of 44 communities (aggregate population, 560,000 adults aged ≥30 years) were randomized into intervention or control groups to receive a community-based melanoma screening program for 3 years versus usual medical care.Overall, thinner melanomas were identified in communities with the screening program versus neighboring communities without it.11 Of the 33 melanomas found through the screening program, 39% (13/33) were in situ lesions, 55% (18/33) were thin (<1 mm) invasive lesions, and 6% (2/33) were 1-mm thick or greater.16 Within the population of Queensland during the period from 1999 through 2002, 36% were in situ lesions, 48% were invasive thin melanomas, and 16% were invasive melanomas 1-mm thick or more, indicating that melanomas found through screening were thinner or less advanced.17
Comment
Our review identified 5 studies describing the impact of PSEs for melanoma screening on tumor thickness at diagnosis and melanoma mortality. Key findings are highlighted in Figure 2. Our findings suggest that PSEs are associated with a decline in melanoma tumor thickness and melanoma-specific mortality. Our findings are qualitatively similar to prior reviews that supported the use of PSEs to detect thinner melanomas and improve mortality outcomes.18-20
The greatest evidence for population-based screening programs was provided by the SCREEN study. This landmark study documented that screening programs utilizing primary care physicians (PCPs) and dermatologists can lead to a reduction in melanoma mortality.15 Findings from the study led to the countrywide expansion of the screening program in 2008, leading to 45 million Germans eligible for skin cancer screenings every 2 years.21 Nearly two-thirds of dermatologists (N=1348) were satisfied with routine PSE and 83% perceived a better quality of health care for skin with the 2008 expansion.22
Data suggest that physician-detected melanomas through PSEs or routine physical examinations are thinner at the time of diagnosis than those found by patients or their partners.14,23-26 Terushkin and Halpern20 analyzed 9 worldwide studies encompassing more than 7500 patients and found that physician-detected melanomas were 0.55 mm thinner than those detected by patients or their significant others. The workplace screening and education program reviewed herein also reported a reduction in thicker melanomas and melanoma mortality during the study period.12
Not all Americans have a regular dermatologist. As such, educating PCPs in skin cancer detection has been a recent area of study. The premise is that the skin examination can be integrated into routine physical examinations conducted by PCPs. The previously discussed studies, particularly Aitken et al,14 Schneider et al,12 and Katalinic et al,15 as well as the SCREEN program studies,15 suggest that integration of the skin examination into the routine physical examination may be a feasible method to reduce melanoma thickness and mortality. Furthermore, the SCREEN study15 identified participants with risk factors for melanoma, finding that approximately half of men and women (N=360,288) had at least one melanoma risk factor, which suggests that it may be more practical to design screening practices around high-risk participants.
Several studies were excluded from our analysis on the basis of study design, including cross-sectional observational studies; however, it is worth briefly commenting on the findings of the excluded studies here, as they add to the body of literature. A community-based, multi-institutional study of 566 adults with invasive melanoma assessed the role of PSEs in the year prior to diagnosis by interviewing participants in clinic within 3 months of melanoma diagnosis.24 Patients who underwent full-body PSE in the year prior to diagnosis were more than 2 times more likely to have thinner (≤1 mm) melanomas (OR, 2.51; 95% CI, 1.62-3.87]). Notably, men older than 60 years appeared to benefit the most from this practice; men in this age group contributed greatly to the observed effect, likely because they had 4 times the odds of a thinner melanoma (OR, 4.09; 95% CI, 1.88-8.89]). Thinner melanomas also were associated with an age of 60 years or younger, female sex, and higher education level.24
Pollitt et al27 analyzed the association between prediagnosis Medicaid enrollment status and melanoma tumor thickness. The study found that men and women who intermittently enrolled in Medicaid or were not enrolled until the month of diagnosis had an increased chance of late-stage melanoma when compared to other patients. Patients who continuously enrolled during the year prior to diagnosis had lower odds for thicker melanomas, suggesting that these patients had greater access to screening examinations.27
Roetzheim et al28 analyzed data from the SEER-Medicare linked dataset to investigate patterns of dermatologist and PCP visits in the 2 years before melanoma diagnosis. Medicare beneficiaries seeing both a dermatologist and a PCP prior to melanoma diagnosis had greater odds of a thinner melanoma and lower melanoma mortality compared to patients without such visits.28
Durbec et al29 conducted a retrospective, population-based study of 650 patients in France who were seen by a dermatologist for melanoma. The thinnest melanomas were reported in patients seeing a dermatologist for prospective follow-up of nevi or consulting a dermatologist for other diseases. Patients referred to a dermatologist by PCPs tended to be older and had the highest frequency of thick (>3 mm), nodular, and/or ulcerated melanomas,29 which could be interpreted as a need for greater PCP education in melanoma screening.
Rates of skin examinations have been increasing since the year 2000, both overall and among high-risk groups as reported by a recent study on skin cancer screening trends. Prevalence of having at least one total-body skin examination increased from 14.5% in 2000 to 16.5% in 2005 to 19.8% in 2010 (P<.0001).30 One study revealed a practice gap in which more than 3 in 10 PCPs and 1 in 10 dermatologists reported not screening more than half their high-risk patients for skin cancer.31 The major obstacle to narrowing the identified practice gap involves establishing a national strategy to screen high-risk individuals for skin cancer and requires partnerships among patients, PCPs, specialists, policy makers, and government sponsors.
Lack of evidence that screening for skin cancer with PSEs reduces overall mortality does not mean there is a lack of lifesaving potential of screenings. The resources required to execute a randomized controlled trial with adequate power are vast, as the USPSTF estimated 800,000 participants would be needed.4 Barriers to conducting a randomized clinical trial for skin cancer screening include the large sample size required, prolonged follow-up, and various ethical issues such as withholding screening for a cancer that is potentially curable in early stages. Lessons from screenings for breast and prostate cancers have taught us that such randomized controlled trials assessing cancer screening are costly and do not always produce definitive answers.32
Conclusion
Although proof of improved health outcomes from randomized controlled trials is still required, there is evidence to support targeted screening programs for the detection of thinner melanomas and, by proxy, reduced melanoma mortality. Amidst the health care climate change and payment reform, recommendations from national organizations on melanoma screenings are paramount. Clinicians should continue to offer regular skin examinations as the body of evidence continues to grow in support of PSEs for melanoma screening.
Acknowledgments—We are grateful to Mary Butler, PhD, and Robert Kane, MD, both from Minneapolis, Minnesota, for their guidance and consultation.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. http: //www.cancer.org/Research/CancerFactsStatistics/cancer factsfigures2015/cancer-facts-and-figures-2015. Accessed July 6, 2015.
2. Guy G Jr, Ekwueme D, Tangka F, et al. Melanoma treatment costs: a systematic review of the literature, 1990-2011. Am J Prev. 2012;43:537-545.
3. Margolis D, Halpern A, Rebbeck T, et al. Validation of a melanoma prognostic model. Arch Dermatol. 1998;134:1597-1601.
4. Wolff T, Tai E, Miller T. Screening for skin cancer: an update of the evidence for the U.S. Preventative Services Task Force. Ann Intern Med. 2009;150:194-198.
5. American Academy of Dermatology. Melanoma
Monday. http://www.aad.org/spot-skin-cancer
/community-programs-events/melanoma-monday. Accessed August 19, 2015.
6. Higgins JPT, Green S, eds. Cochrane Handbook for
Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. http:
//www.cochrane-handbook.org. Updated March 2011. Accessed November 10, 2014.
7. Viswanathan M, Berkman N. Development of the RTI item bank on risk of bias and precision of observational studies. J Clin Epidemiol. 2012;65:163-178.
8. Moher D, Liberati A, Tetzlaff J, et al; PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement [published online ahead of print July 23, 2009]. J Clin Epidemiol. 2009;62:1006-1012.
9. Berwick M, Armstrong B, Ben-Porat L. Sun exposure and mortality from melanoma. J Natl Cancer Inst. 2005;97:195-199.
10. Berwick M, Begg C, Fine J, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23.
11. Aitken J, Elwood J, Lowe J, et al. A randomised trial of population screening for melanoma. J Med Screen. 2002;9:33-37.
12. Schneider J, Moore D, Mendelsohn M. Screening program reduced melanoma mortality at the Lawrence Livermore National Laboratory, 1984 to 1996. J Am Acad Dermatol. 2008;58:741-749.
13. Expert Health Data Programming Inc. Health data software and health statistics. Available from: http: //www.ehdp.com. Accessed April 1, 2001. Cited by: Schneider J, Moore D, Mendelsohn M. Screening program reduced melanoma mortality at the Lawrence Livermore National Laboratory, 1984 to 1996. J Am Acad Dermatol. 2008;58:741-749.
14. Aitken J, Elwood M, Baade P, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010;126:450-458.
15. Katalinic A, Waldmann A, Weinstock M, et al. Does skin cancer screening save lives? an observational study comparing trends in melanoma mortality in regions with and without screening. Cancer. 2012;118:5395-5402.
16. Aitken J, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006;54:105-114.
17. Coory M, Baade P, Aitken JF, et al. Trends for in-situ and invasive melanoma in Queensland, Australia, 1982 to 2002. Cancer Causes Control. 2006;17:21-27.
18. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: part II. screening, education, and future directions. J Am Acad Dermatol. 2014;71:611.e1-611.e10; quiz, 621-622.
19. Curiel-Lewandrowski C, Chen S, Swetter S, et al. Screening and prevention measures for melanoma: is there a survival advantage? Curr Oncol Rep. 2012;14:458-467.
20. Terushkin V, Halpern A. Melanoma early detection. Hematol Oncol Clin North Am. 2009;23:481-500.
21. Geller A, Greinert R, Sinclair C, et al. A nationwide population-based skin cancer screening in Germany: proceedings of the first meeting of the International Task Force on Skin Cancer Screening and Prevention (September 24 and 25, 2009) [published online ahead of print April 8, 2010]. Cancer Epidemiol. 2010;34:355-358.
22. Kornek T, Schafer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists’ perspective. Dermatology. 2012;225:289-293.
23. De Giorgi V, Grazzini M, Rossari S, et al. Is skin self-examination for cutaneous melanoma detection still adequate? a retrospective study. Dermatology. 2012;225:31-36.
24. Swetter S, Johnson T, Miller D, et al. Melanoma in middle-aged and older men: a multi-institutional survey study of factors related to tumor thickness. Arch Dermatol. 2009;145:397-404.
25. Kantor J, Kantor D. Routine dermatologist-performed full-body skin examination and early melanoma detection. Arch Dermatol. 2009;145:873-876.
26. Kovalyshyn I, Dusza S, Siamas K, et al. The impact of physician screening on melanoma detection. Arch Dermatol. 2011;147:1269-1275.
27. Pollitt R, Clarke C, Shema S, et al. California Medicaid enrollment and melanoma stage at diagnosis: a population-based study. Am J Prev Med. 2008;35:7-13.
28. Roetzheim R, Lee J, Ferrante J, et al. The influence of dermatologist and primary care physician visits on melanoma outcomes among Medicare beneficiaries. J Am Board Fam Med. 2013;26:637-647.
29. Durbec F, Vitry F, Granel-Brocard F, et al. The role of circumstances of diagnosis and access to dermatological care in early diagnosis of cutaneous melanoma: a population-based study in France. Arch Dermatol. 2010;146:240-246.
30. Lakhani N, Saraiya M, Thompson T, et al. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med. 2014;61:75-80.
31. Oliveria SA, Heneghan MK, Cushman LF, et al. Skin cancer screening by dermatologists, family practitioners, and internists: barriers and facilitating factors. Arch Dermatol. 2011;147:39-44.
32. Bigby M. Why the evidence for skin cancer screening is insufficient: lessons from prostate cancer screening. Arch Dermatol. 2010;146:322-324.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. http: //www.cancer.org/Research/CancerFactsStatistics/cancer factsfigures2015/cancer-facts-and-figures-2015. Accessed July 6, 2015.
2. Guy G Jr, Ekwueme D, Tangka F, et al. Melanoma treatment costs: a systematic review of the literature, 1990-2011. Am J Prev. 2012;43:537-545.
3. Margolis D, Halpern A, Rebbeck T, et al. Validation of a melanoma prognostic model. Arch Dermatol. 1998;134:1597-1601.
4. Wolff T, Tai E, Miller T. Screening for skin cancer: an update of the evidence for the U.S. Preventative Services Task Force. Ann Intern Med. 2009;150:194-198.
5. American Academy of Dermatology. Melanoma
Monday. http://www.aad.org/spot-skin-cancer
/community-programs-events/melanoma-monday. Accessed August 19, 2015.
6. Higgins JPT, Green S, eds. Cochrane Handbook for
Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. http:
//www.cochrane-handbook.org. Updated March 2011. Accessed November 10, 2014.
7. Viswanathan M, Berkman N. Development of the RTI item bank on risk of bias and precision of observational studies. J Clin Epidemiol. 2012;65:163-178.
8. Moher D, Liberati A, Tetzlaff J, et al; PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement [published online ahead of print July 23, 2009]. J Clin Epidemiol. 2009;62:1006-1012.
9. Berwick M, Armstrong B, Ben-Porat L. Sun exposure and mortality from melanoma. J Natl Cancer Inst. 2005;97:195-199.
10. Berwick M, Begg C, Fine J, et al. Screening for cutaneous melanoma by skin self-examination. J Natl Cancer Inst. 1996;88:17-23.
11. Aitken J, Elwood J, Lowe J, et al. A randomised trial of population screening for melanoma. J Med Screen. 2002;9:33-37.
12. Schneider J, Moore D, Mendelsohn M. Screening program reduced melanoma mortality at the Lawrence Livermore National Laboratory, 1984 to 1996. J Am Acad Dermatol. 2008;58:741-749.
13. Expert Health Data Programming Inc. Health data software and health statistics. Available from: http: //www.ehdp.com. Accessed April 1, 2001. Cited by: Schneider J, Moore D, Mendelsohn M. Screening program reduced melanoma mortality at the Lawrence Livermore National Laboratory, 1984 to 1996. J Am Acad Dermatol. 2008;58:741-749.
14. Aitken J, Elwood M, Baade P, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010;126:450-458.
15. Katalinic A, Waldmann A, Weinstock M, et al. Does skin cancer screening save lives? an observational study comparing trends in melanoma mortality in regions with and without screening. Cancer. 2012;118:5395-5402.
16. Aitken J, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006;54:105-114.
17. Coory M, Baade P, Aitken JF, et al. Trends for in-situ and invasive melanoma in Queensland, Australia, 1982 to 2002. Cancer Causes Control. 2006;17:21-27.
18. Mayer JE, Swetter SM, Fu T, et al. Screening, early detection, education, and trends for melanoma: current status (2007-2013) and future directions: part II. screening, education, and future directions. J Am Acad Dermatol. 2014;71:611.e1-611.e10; quiz, 621-622.
19. Curiel-Lewandrowski C, Chen S, Swetter S, et al. Screening and prevention measures for melanoma: is there a survival advantage? Curr Oncol Rep. 2012;14:458-467.
20. Terushkin V, Halpern A. Melanoma early detection. Hematol Oncol Clin North Am. 2009;23:481-500.
21. Geller A, Greinert R, Sinclair C, et al. A nationwide population-based skin cancer screening in Germany: proceedings of the first meeting of the International Task Force on Skin Cancer Screening and Prevention (September 24 and 25, 2009) [published online ahead of print April 8, 2010]. Cancer Epidemiol. 2010;34:355-358.
22. Kornek T, Schafer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists’ perspective. Dermatology. 2012;225:289-293.
23. De Giorgi V, Grazzini M, Rossari S, et al. Is skin self-examination for cutaneous melanoma detection still adequate? a retrospective study. Dermatology. 2012;225:31-36.
24. Swetter S, Johnson T, Miller D, et al. Melanoma in middle-aged and older men: a multi-institutional survey study of factors related to tumor thickness. Arch Dermatol. 2009;145:397-404.
25. Kantor J, Kantor D. Routine dermatologist-performed full-body skin examination and early melanoma detection. Arch Dermatol. 2009;145:873-876.
26. Kovalyshyn I, Dusza S, Siamas K, et al. The impact of physician screening on melanoma detection. Arch Dermatol. 2011;147:1269-1275.
27. Pollitt R, Clarke C, Shema S, et al. California Medicaid enrollment and melanoma stage at diagnosis: a population-based study. Am J Prev Med. 2008;35:7-13.
28. Roetzheim R, Lee J, Ferrante J, et al. The influence of dermatologist and primary care physician visits on melanoma outcomes among Medicare beneficiaries. J Am Board Fam Med. 2013;26:637-647.
29. Durbec F, Vitry F, Granel-Brocard F, et al. The role of circumstances of diagnosis and access to dermatological care in early diagnosis of cutaneous melanoma: a population-based study in France. Arch Dermatol. 2010;146:240-246.
30. Lakhani N, Saraiya M, Thompson T, et al. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med. 2014;61:75-80.
31. Oliveria SA, Heneghan MK, Cushman LF, et al. Skin cancer screening by dermatologists, family practitioners, and internists: barriers and facilitating factors. Arch Dermatol. 2011;147:39-44.
32. Bigby M. Why the evidence for skin cancer screening is insufficient: lessons from prostate cancer screening. Arch Dermatol. 2010;146:322-324.
Practice Points
- Current guidelines regarding melanoma screening are inconsistent.
- There is a growing pool of evidence supporting screening to improve melanoma outcomes.