User login
Take-Home Points
- GCT of bones of the wrist is rare. This article is the only report of a wrist GCT during pregnancy that we could identify.
- Routine treatment usually consists of surgical excision with local adjuvant, and in the wrist, often results in reduced wrist motion.
- GCT of the wrist is more aggressive than the more common locations in long bones, with higher local recurrence rates if treated with surgery alone.
- Diagnosis is often delayed for GCT of the wrist, due to insufficient imaging, which should include CT or MRI.
- For pregnant women with GCT, local adjuvant treatments can be used in addition to surgery. Following pregnancy, denosumab can be used systemically, and can be effective with metastatic or unresectable disease.
Giant cell tumor (GCT) of bone accounts for about 5% of primary bone tumors.1-3 Only 3% to 5% of GCTs occur in the hand.4,5 Wrist involvement, which is rare, most often involves the hamate bone.5-7 Capitate bone involvement is exceedingly rare.8-11 Although histologically benign, GCT can recur locally after treatment with curettage alone, and lung metastases are found in 2% to 5% of cases.2,12-14 Therefore, en bloc tumor excision is preferred in the setting of cortical erosion or soft-tissue involvement.1,4,8 Wrist joint motion is inevitably reduced, and bone graft donor-site morbidity is significant.6-8
In the unusual case reported here, GCT presented in the capitate bone and, after the patient became pregnant, recurred in the hamate and trapezoid bones with soft-tissue extension and lung metastases. The capitate was excised en bloc and reconstructed with an interposition of polymethylmethacrylate bone cement. Pulmonary metastases developed, and the GCT expanded to involve multiple carpal bones and the bases of the second through fourth metacarpals. A 10-month course of systemic chemotherapy with the RANK ligand (RANKL) inhibitor denosumab was started after the pregnancy. After this treatment, the patient underwent both tumor resection and reconstruction with autogenous bicortical iliac crest bone graft (ICBG) carefully designed to preserve range of motion and maintain the fingers in anatomical position. Treatment with denosumab was continued after surgery. Although this case offers no endpoint for postoperative chemotherapy with denosumab, preoperative treatment dramatically reduced the GCT and permitted limb-sparing reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old right-handed woman with atraumatic swelling of the left wrist presented to an orthopedic surgeon at an outside facility. Physical examination revealed tender fullness on the dorsum of the wrist, slightly reduced range of motion and grip strength, and a neurovascularly intact wrist. The diagnosis was periarticular cyst, and the patient underwent physical therapy. Two years later, the swelling returned, tenderness was increasing, and symptoms did not resolve with cast immobilization. A radiograph showed a lytic lesion in the capitate bone (Figure 1).[[{"fid":"202332","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]
GCT was diagnosed with percutaneous needle biopsy. A preoperative chest radiograph was reported normal. For initial treatment, the capitate and trapezoid bones were resected en bloc through a dorsal approach. Reconstruction consisted of limited arthrodesis using bone cement without additional fixation.
At 6-month follow-up, the patient was pregnant, and there was a recurrence of the wrist lesion. During the first 2 months of pregnancy, swelling and pain rapidly progressed, and a palpable mass formed. Radiographs showed a lytic lesion extending into the hamate bone (Figure 2), and magnetic resonance imaging (MRI) showed articular extension of the lesion with involvement of the base of the fourth metacarpal. [[{"fid":"202334","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]Targeted anti-RANKL therapy was not recommended (and was not available at the patient’s home hospital). The patient deferred surgical treatment because of the pregnancy, which proved otherwise uneventful and ended with a full-term delivery.
After the pregnancy, radiographs of the wrist showed complete destruction of the hamate and trapezium bones, with erosion of the bases of the second through fourth metacarpals (Figure 3A). [[{"fid":"202335","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]The patient presented at our institution 4 years after initial diagnosis. Computed tomography (CT) of the chest showed numerous bilateral pulmonary nodular opacities. Wrist imaging showed soft-tissue extension (Figure 3B). The diagnosis of recurrent metastatic GCT was confirmed with needle biopsies of the wrist mass and the right lung nodule.
Systemic chemotherapy was initiated with 120 mg of denosumab, given subcutaneously on days 1, 8, and 15 and then monthly during the 10 months leading up to surgery. Serum calcium was monitored during treatment and remained within the normal range the entire time, except for once at the start of therapy, when it dropped to 6.8 mg/dL. After 8 months, the soft-tissue mass, originally 8 cm × 8 cm × 6 cm, shrunk and stabilized at 5 cm × 4 cm × 4 cm (Figure 3B), and a bony shell reformed around it. Nodules in both lung fields showed response to denosumab.
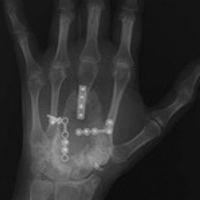
Histologic examination revealed scattered osteoclast-like, multinucleated giant cells, consistent with a recurrent lesion (Figure 4). [[{"fid":"202336","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":""}}}]]After 10 months of treatment with denosumab, the patient underwent resection (dorsal approach) of the residual cement, the soft-tissue mass, the affected carpal bones, half of the third metacarpal, and the second and fourth metacarpal bases. The proximal carpal row was preserved after no intra-articular involvement was verified. The closet margin was marginal; the tumor mass abutted without encompassing the flexor tendons and median nerve. The tumor was meticulously elevated from the neurovascular and tendinous structures, which were not sacrificed. Hydrogen peroxide was used for local adjuvant treatment. Bicortical autogenous ICBG was placed between the remaining scaphoid, lunate, and metacarpal bones. The second, third, and fourth metacarpal bases were stabilized on the overlapping outer table of ICBG with 2.0-mm plates and miniscrews (Figure 5A). Kirschner wires were used to stabilize the proximal bone graft and the scapholunate fossa. Cancellous bone graft was packed between the structural bone graft and neighboring unaffected carpal bones (Figure 5A). Immobilization with a short-arm thumb spica cast was maintained for 6 weeks after surgery and was followed by a 12-week rehabilitation program. The patient returned to normal activities when plain radiographs showed solid bony union (Figure 5B). Fourteen months after initial surgery, tenolysis was performed to free the extensor tendons (index, middle, and ring fingers on dorsum of left hand) from adhesions to the bone graft. At 37-month follow-up (Figure 5C), there was no clinical or radiographic evidence of progression in the wrist.[[{"fid":"202337","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 5.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 5.","field_file_image_credit[und][0][value]":""}}}]]
The patient had bilateral pulmonary metastases (Figures 6A, 6B). Treatment with denosumab produced an initial response (smaller pulmonary lesions) and subsequent stability. After 12 months of treatment with denosumab, the patient underwent left thoracotomy and wedge resection of pulmonary metastases on the left. Pathologic evaluation revealed pulmonary parenchyma with calcification and ossification and limited viable tumor. Given the dramatic effects on the left pulmonary metastases, denosumab was continued, and surgical intervention on the right was not attempted. Pulmonary metastases were stable afterward (Figure 6C).[[{"fid":"202338","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"6"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 6.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"6":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 6.","field_file_image_credit[und][0][value]":""}}}]]
At 54-month follow-up, systemic treatment with denosumab was continued. The patient had no pain in the wrist or hand and was able to use the left hand normally. There was some fissuring of the third and fourth digits over each other. However, the patient had good grip strength and was using eating utensils, picking up water bottles, and engaging in other activities without difficulty.
Discussion
GCT isolated to the carpus is rare. However, compared with GCT in the more common locations in long bones, it is also more aggressive, and its local recurrence rates are higher, probably 60% or more if treated with curettage alone.15 Therefore, excision augmented with adjuvant treatment is recommended.2,7 Use of bone cement in the hand is relatively uncommon.4,5,7-10
The diagnosis of GCT in the carpus is difficult and often delayed. The initial complaint is usually mild wrist pain after relatively mild trauma.5 The first reported case of GCT in the lunate bone was mistakenly thought to be Kienbock disease.5 Similarly, our patient was initially given a nononcologic diagnosis, which prompted conservative management.
Whether the biological behavior of GCT in the carpus differs from that of GCT in other sites is unclear. The high recurrence rates might be attributable in part to suboptimal curettage.5,6 En bloc resections of involved bone inevitably result in carpal instability or loss of wrist motion if arthrodesis is performed.4-7,11 In the present case, resection was followed by limited arthrodesis to mitigate motion losses.
Multifocal GCT in the carpal bones often affects younger patients and has a high rate of recurrence.7,16 In the present case, the patient’s pregnancy delayed treatment and allowed tumor extension into soft tissues and metacarpal bones. Given her young age, en bloc tumor resection was performed, with the proximal carpal row spared to preserve wrist motion. ICBG was carefully shaped to match the defect that remained after tumor resection.7 Supporting wrist height to prevent carpal collapse provided a stable base for remaining distal segments of the second through fourth metacarpals. After short-arm thumb spica casting and early rehabilitation, the patient recovered wrist motion and use of the involved fingers distal to the carpometacarpal joints.
In pregnant women, GCTs have been found primarily in the long bones and spine but are rare.17-21 A review of the literature (1950-present) revealed that the present article is the first report of GCT in the hand or wrist bones of a pregnant woman.18,20,21 There is no consensus as to whether surgical excision should be performed during pregnancy.18,20,21 In 1 unusual case, at 18 weeks’ gestation GCT in the distal femur was resected with curettage and bone grafting, and there were no complications.21 Therefore, pregnancy termination is not indicated for GCT.
The relationship between tumorigenesis and pregnancy is unclear.18,20,21 Empirically, pregnancy is thought to promote tumor growth.18,20 Estrogen and progesterone levels are elevated during pregnancy, potentially influencing tumor cells that are hormonally sensitive.18,20 An early report in which reverse transcription–polymerase chain reaction showed estrogen receptor expression in GCT osteoclast-like cells was followed by several studies that failed to find estrogen receptors at the protein level.19 In contrast, progesterone receptors were found in 50% of GCTs in a study.22 However, the etiopathogenic significance of this is unclear. In pregnant women, vascular endothelial growth factor, placental growth factor, and other growth factors induce osteoclast formation.23 ß-Human chorionic gonadotropin expression (ß-hCG) has been found in 58% of cases, with some showing ß-hCG elevation in the serum.24 Other studies have focused on an immunologic explanation for occurrence of GCT during pregnancy.18 Oncofetal antigens, which are similar to fetal antigens, have been found in fibrosarcoma and in an osteosarcoma cell line but not in GCT.18-20 Thus, though occurrence during pregnancy may be coincidental given the frequency of GCT in women of childbearing age, it is plausible that tumor growth may be enhanced by pregnancy. More studies are needed to understand the relationship between giant cell proliferation and pregnancy-related growth factors and hormones.
With GCT, the rate of pulmonary metastases ranges from 0% to 4%; these metastases are usually diagnosed at time of local recurrence, or 2 years to 3 years after initial GCT diagnosis.2,3,12,14,25 Lung metastases secondary to GCT in the hand or foot bones are rare; our literature review identified only 4 cases.12,14 Risk factors for lung metastasis include local recurrence, aggressive appearance (Enneking grade 3) on radiograph, Ki-67 antigen expression, and distal radius location.14 The mechanism of metastasis is unknown.12,14
Lung metastases are usually excised, but they may spontaneously evolve toward necrosis and ossification.12 In cases in which surgery is unfeasible, chemotherapy (eg, with doxorubicin) has been used to control progression.12,14 Radiation can cause sarcomatous transformation and is contraindicated. Interferon26-28 and other antiangiogenic strategies have been successfully used in systemic therapy for GCT of bone. More recently, bisphosphonates29-32 and denosumab33 have been investigated.29,32-36 The limited toxicity of denosumab makes the drug a very attractive treatment option for recurrent or unresectable GCT of bone.33 Reported rates of mortality from lung metastases have ranged from 0% to 40%.14 There is evidence that control of lung metastases during the first 3 years after diagnosis is important for favorable outcomes.2,3
Malignant stromal cells of GCT of bone have been known to secrete RANKL, which recruits osteoclasts and osteoclast precursor cells, which in turn generate aggressive osteolytic activity.33,37 Denosumab, a monoclonal antibody that inhibits RANKL, is effective in stopping osteoclastic activity. In a phase 2 trial of denosumab in the treatment of GCT of bone, 96% of treated patients with unresectable disease showed no progression at 13 months.38 In addition, 74% of treated patients who had resectable disease but were likely to have morbid surgery did not require surgery, and 62% of treated patients who underwent surgery were able to have a less morbid procedure. Forty-one percent to 58% of treated patients had a reduction in tumor size.
Denosumab is very well tolerated. The phase 2 trial found serious adverse events in 9% of patients, and in 5% of cases the drug was discontinued because of toxicity.38 Serious adverse events include osteonecrosis of jaw, hypocalcemia, and hypophosphatemia.37 Electrolyte changes with denosumab are easy to monitor and manage. Although the favorable toxicity profile of denosumab allows for long-term therapy, the data on therapy duration in patients with unresectable disease are unclear. Patients who discontinue therapy should be closely monitored, as disease can progress in this setting.37
In contrast to GCT of larger bones, GCT of the wrist is rare and typically more aggressive, and has higher local recurrence rates. In many cases, diagnosis is delayed by insufficient imaging, which optimally should include either CT or MRI (Table). [[{"fid":"202341","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"7"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"7":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":""}}}]]For pregnant women with GCT, options include surgical resection with curettage and local adjuvant treatment. After pregnancy, denosumab can be used systemically, and can be effective with metastatic or unresectable disease. Surgical treatment in the wrist can be challenging when partial or complete resections of carpal bones are required. Occupational therapy is recommended for optimization of hand function after surgery.
1. Balke M, Ahrens H, Streitbuerger A, et al. Treatment options for recurrent giant cell tumors of bone. J Cancer Res Clin Oncol. 2009;135(1):149-158.
2. Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Giant cell tumor of bone risk factors for recurrence. Clin Orthop Relat Res. 2011;469(2):591-599.
3. Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
4. Averill RM, Smith RJ, Campbell CJ. Giant-cell tumors of the bones of the hand. J Hand Surg Am. 1980;5(1):39-50.
5. Shigematsu K, Kobata Y, Yajima H, Kawamura K, Maegawa N, Takakura Y. Giant-cell tumors of the carpus. J Hand Surg Am. 2006;31(7):1214-1219.
6. Gupta GG, Lucas GL, Pirela-Cruz M. Multifocal giant cell tumor of the capitate, hamate, and triquetrum: a case report. J Hand Surg Am. 1995;20(6):1003-1006.
7. Tarng YW, Yang SW, Hsu CJ. Surgical treatment of multifocal giant cell tumor of carpal bones with preservation of wrist function: case report. J Hand Surg Am. 2009;34(2):262-265.
8. Angelini A, Mavrogenis AF, Ruggieri P. Giant cell tumor of the capitate. Musculoskelet Surg. 2011;95(1):45-48.
9. Howard FM, Lassen K. Giant cell tumor of the capitate. J Hand Surg Am. 1984;9(2):272-274.
10. McDonald DJ, Schajowicz F. Giant cell tumor of the capitate. A case report. Clin Orthop Relat Res. 1992(279):264-268.
11. Wilson SC, Cascio BM, Plauche HR. Giant-cell tumor of the capitate. Orthopedics. 2001;24(11):1085-1086.
12. Combalia-Aleu A, Sastre S, Fernández-de-Retana P, Tomás X, Palacin A. Giant cell tumor of the talus with pulmonary metastasis: seven years follow up. Foot. 2006;16(2):107-111.
13. Donthineni R, Boriani L, Ofluoglu O, Bandiera S. Metastatic behaviour of giant cell tumour of the spine. Int Orthop. 2009;33(2):497-501.
14. Jacopin S, Viehweger E, Glard Y, et al. Fatal lung metastasis secondary to index finger giant cell tumor in an 8-year-old child. Orthop Traumatol Surg Res. 2010;96(3):310-313.
15. Plate AM, Lee SJ, Steiner G, Posner MA. Tumor-like lesions and benign tumors of the hand and wrist. J Am Acad Orthop Surg. 2003;11(2):129-141.
16. Moreel P, Le Viet D. Failure of initial surgical treatment of a giant cell tumor of the capitate and its salvage: a case report [in French]. Chir Main. 2006;25(6):315-318.
17. Caillouette JC, Mattar N. Massive peripheral giant-cell reparative granuloma of the jaw: a pregnancy dependent tumor. Trans Pac Coast Obstet Gynecol Soc. 1978;45:78-81.
18. Kathiresan AS, Johnson JN, Hood BJ, Montoya SP, Vanni S, Gonzalez-Quintero VH. Giant cell bone tumor of the thoracic spine presenting in late pregnancy. Obstet Gynecol. 2011;118(2 pt 2):428-431.
19. Komiya S, Zenmyo M, Inoue A. Bone tumors in the pelvis presenting growth during pregnancy. Arch Orthop Trauma Surg. 1999;119(1-2):22-29.
20. Ross AE, Bojescul JA, Kuklo TR. Giant cell tumor: a case report of recurrence during pregnancy. Spine. 2005;30(12):E332-3E35.
21. Sharma JB, Chanana C, Rastogi, et al. Successful pregnancy outcome with elective caesarean section following two attempts of surgical excision of large giant cell tumor of the lower limb during pregnancy. Arch Gynecol Obstet. 2006;274(5):313-315.
22. Demertzis N, Kotsiandri F, Giotis I, Apostolikas N. Giant-cell tumors of bone and progesterone receptors. Orthopedics. 2003;26(12):1209-1212.
23. Taylor RM, Kashima TG, Knowles HJ, Athanasou NA. VEGF, FLT3 ligand, PlGF and HGF can substitute for M-CSF to induce human osteoclast formation: implications for giant cell tumour pathobiology. Lab Invest. 2012;92(10):1398-1406.
24. Lawless ME, Jour G, Hoch BL, Rendi MH. Beta-human chorionic gonadotropin expression in recurrent and metastatic giant cell tumors of bone: a potential mimicker of germ cell tumor. Int J Surg Pathol. 2014;22(7):617-622.
25. Viswanathan S, Jambhekar NA. Metastatic giant cell tumor of bone: are there associated factors and best treatment modalities? Clin Orthop Relat Res. 2010;468(3):827-833.
26. Kaban LB, Troulis MJ, Ebb D, August M, Hornicek FJ, Dodson TB. Antiangiogenic therapy with interferon alpha for giant cell lesions of the jaws. J Oral Maxillofac Surg. 2002;60(10):1103-1111.
27. Kaiser U, Neumann K, Havemann K. Generalised giant-cell tumour of bone: successful treatment of pulmonary metastases with interferon alpha, a case report. J Cancer Res Clin Oncol. 1993;119(5):301-303.
28. Dickerman JD. Interferon and giant cell tumors. Pediatrics. 1999;103(6 pt 1):1282-1283.
29. Balke M, Campanacci L, Gebert C, et al. Bisphosphonate treatment of aggressive primary, recurrent and metastatic giant cell tumour of bone. BMC Cancer. 2010;10:462.
30. Gille O, Oliveira Bde A, Guerin P, Lepreux S, Richez C, Vital JM. Regression of giant cell tumor of the cervical spine with bisphosphonate as single therapy. Spine. 2012;37(6):E396-E399.
31. Moriceau G, Ory B, Gobin B, et al. Therapeutic approach of primary bone tumours by bisphosphonates. Curr Pharm Des. 2010;16(27):2981-2987.
32. Tse LF, Wong KC, Kumta SM, Huang L, Chow TC, Griffith JF. Bisphosphonates reduce local recurrence in extremity giant cell tumor of bone: a case–control study. Bone. 2008;42(1):68-73.
33. Thomas D, Henshaw R, Skubitz K, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11(3):275-280.
34. Balke M, Hardes J. Denosumab: a breakthrough in treatment of giant-cell tumour of bone? Lancet Oncol. 2010;11(3):218-219.
35. Kyrgidis A, Toulis K. Safety and efficacy of denosumab in giant-cell tumour of bone. Lancet Oncol. 2010;11(6):513-514.
36. Thomas D, Carriere P, Jacobs I. Safety of denosumab in giant-cell tumour of bone. Lancet Oncol. 2010;11(9):815.
37. Skubitz KM. Giant cell tumor of bone: current treatment options. Curr Treat Options Oncol. 2014;15(3):507-518.
38. Chawla S, Henshaw R, Seeger L, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14(9):901-908.
Take-Home Points
- GCT of bones of the wrist is rare. This article is the only report of a wrist GCT during pregnancy that we could identify.
- Routine treatment usually consists of surgical excision with local adjuvant, and in the wrist, often results in reduced wrist motion.
- GCT of the wrist is more aggressive than the more common locations in long bones, with higher local recurrence rates if treated with surgery alone.
- Diagnosis is often delayed for GCT of the wrist, due to insufficient imaging, which should include CT or MRI.
- For pregnant women with GCT, local adjuvant treatments can be used in addition to surgery. Following pregnancy, denosumab can be used systemically, and can be effective with metastatic or unresectable disease.
Giant cell tumor (GCT) of bone accounts for about 5% of primary bone tumors.1-3 Only 3% to 5% of GCTs occur in the hand.4,5 Wrist involvement, which is rare, most often involves the hamate bone.5-7 Capitate bone involvement is exceedingly rare.8-11 Although histologically benign, GCT can recur locally after treatment with curettage alone, and lung metastases are found in 2% to 5% of cases.2,12-14 Therefore, en bloc tumor excision is preferred in the setting of cortical erosion or soft-tissue involvement.1,4,8 Wrist joint motion is inevitably reduced, and bone graft donor-site morbidity is significant.6-8
In the unusual case reported here, GCT presented in the capitate bone and, after the patient became pregnant, recurred in the hamate and trapezoid bones with soft-tissue extension and lung metastases. The capitate was excised en bloc and reconstructed with an interposition of polymethylmethacrylate bone cement. Pulmonary metastases developed, and the GCT expanded to involve multiple carpal bones and the bases of the second through fourth metacarpals. A 10-month course of systemic chemotherapy with the RANK ligand (RANKL) inhibitor denosumab was started after the pregnancy. After this treatment, the patient underwent both tumor resection and reconstruction with autogenous bicortical iliac crest bone graft (ICBG) carefully designed to preserve range of motion and maintain the fingers in anatomical position. Treatment with denosumab was continued after surgery. Although this case offers no endpoint for postoperative chemotherapy with denosumab, preoperative treatment dramatically reduced the GCT and permitted limb-sparing reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old right-handed woman with atraumatic swelling of the left wrist presented to an orthopedic surgeon at an outside facility. Physical examination revealed tender fullness on the dorsum of the wrist, slightly reduced range of motion and grip strength, and a neurovascularly intact wrist. The diagnosis was periarticular cyst, and the patient underwent physical therapy. Two years later, the swelling returned, tenderness was increasing, and symptoms did not resolve with cast immobilization. A radiograph showed a lytic lesion in the capitate bone (Figure 1).[[{"fid":"202332","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]
GCT was diagnosed with percutaneous needle biopsy. A preoperative chest radiograph was reported normal. For initial treatment, the capitate and trapezoid bones were resected en bloc through a dorsal approach. Reconstruction consisted of limited arthrodesis using bone cement without additional fixation.
At 6-month follow-up, the patient was pregnant, and there was a recurrence of the wrist lesion. During the first 2 months of pregnancy, swelling and pain rapidly progressed, and a palpable mass formed. Radiographs showed a lytic lesion extending into the hamate bone (Figure 2), and magnetic resonance imaging (MRI) showed articular extension of the lesion with involvement of the base of the fourth metacarpal. [[{"fid":"202334","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]Targeted anti-RANKL therapy was not recommended (and was not available at the patient’s home hospital). The patient deferred surgical treatment because of the pregnancy, which proved otherwise uneventful and ended with a full-term delivery.
After the pregnancy, radiographs of the wrist showed complete destruction of the hamate and trapezium bones, with erosion of the bases of the second through fourth metacarpals (Figure 3A). [[{"fid":"202335","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]The patient presented at our institution 4 years after initial diagnosis. Computed tomography (CT) of the chest showed numerous bilateral pulmonary nodular opacities. Wrist imaging showed soft-tissue extension (Figure 3B). The diagnosis of recurrent metastatic GCT was confirmed with needle biopsies of the wrist mass and the right lung nodule.
Systemic chemotherapy was initiated with 120 mg of denosumab, given subcutaneously on days 1, 8, and 15 and then monthly during the 10 months leading up to surgery. Serum calcium was monitored during treatment and remained within the normal range the entire time, except for once at the start of therapy, when it dropped to 6.8 mg/dL. After 8 months, the soft-tissue mass, originally 8 cm × 8 cm × 6 cm, shrunk and stabilized at 5 cm × 4 cm × 4 cm (Figure 3B), and a bony shell reformed around it. Nodules in both lung fields showed response to denosumab.
Histologic examination revealed scattered osteoclast-like, multinucleated giant cells, consistent with a recurrent lesion (Figure 4). [[{"fid":"202336","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":""}}}]]After 10 months of treatment with denosumab, the patient underwent resection (dorsal approach) of the residual cement, the soft-tissue mass, the affected carpal bones, half of the third metacarpal, and the second and fourth metacarpal bases. The proximal carpal row was preserved after no intra-articular involvement was verified. The closet margin was marginal; the tumor mass abutted without encompassing the flexor tendons and median nerve. The tumor was meticulously elevated from the neurovascular and tendinous structures, which were not sacrificed. Hydrogen peroxide was used for local adjuvant treatment. Bicortical autogenous ICBG was placed between the remaining scaphoid, lunate, and metacarpal bones. The second, third, and fourth metacarpal bases were stabilized on the overlapping outer table of ICBG with 2.0-mm plates and miniscrews (Figure 5A). Kirschner wires were used to stabilize the proximal bone graft and the scapholunate fossa. Cancellous bone graft was packed between the structural bone graft and neighboring unaffected carpal bones (Figure 5A). Immobilization with a short-arm thumb spica cast was maintained for 6 weeks after surgery and was followed by a 12-week rehabilitation program. The patient returned to normal activities when plain radiographs showed solid bony union (Figure 5B). Fourteen months after initial surgery, tenolysis was performed to free the extensor tendons (index, middle, and ring fingers on dorsum of left hand) from adhesions to the bone graft. At 37-month follow-up (Figure 5C), there was no clinical or radiographic evidence of progression in the wrist.[[{"fid":"202337","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 5.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 5.","field_file_image_credit[und][0][value]":""}}}]]
The patient had bilateral pulmonary metastases (Figures 6A, 6B). Treatment with denosumab produced an initial response (smaller pulmonary lesions) and subsequent stability. After 12 months of treatment with denosumab, the patient underwent left thoracotomy and wedge resection of pulmonary metastases on the left. Pathologic evaluation revealed pulmonary parenchyma with calcification and ossification and limited viable tumor. Given the dramatic effects on the left pulmonary metastases, denosumab was continued, and surgical intervention on the right was not attempted. Pulmonary metastases were stable afterward (Figure 6C).[[{"fid":"202338","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"6"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 6.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"6":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 6.","field_file_image_credit[und][0][value]":""}}}]]
At 54-month follow-up, systemic treatment with denosumab was continued. The patient had no pain in the wrist or hand and was able to use the left hand normally. There was some fissuring of the third and fourth digits over each other. However, the patient had good grip strength and was using eating utensils, picking up water bottles, and engaging in other activities without difficulty.
Discussion
GCT isolated to the carpus is rare. However, compared with GCT in the more common locations in long bones, it is also more aggressive, and its local recurrence rates are higher, probably 60% or more if treated with curettage alone.15 Therefore, excision augmented with adjuvant treatment is recommended.2,7 Use of bone cement in the hand is relatively uncommon.4,5,7-10
The diagnosis of GCT in the carpus is difficult and often delayed. The initial complaint is usually mild wrist pain after relatively mild trauma.5 The first reported case of GCT in the lunate bone was mistakenly thought to be Kienbock disease.5 Similarly, our patient was initially given a nononcologic diagnosis, which prompted conservative management.
Whether the biological behavior of GCT in the carpus differs from that of GCT in other sites is unclear. The high recurrence rates might be attributable in part to suboptimal curettage.5,6 En bloc resections of involved bone inevitably result in carpal instability or loss of wrist motion if arthrodesis is performed.4-7,11 In the present case, resection was followed by limited arthrodesis to mitigate motion losses.
Multifocal GCT in the carpal bones often affects younger patients and has a high rate of recurrence.7,16 In the present case, the patient’s pregnancy delayed treatment and allowed tumor extension into soft tissues and metacarpal bones. Given her young age, en bloc tumor resection was performed, with the proximal carpal row spared to preserve wrist motion. ICBG was carefully shaped to match the defect that remained after tumor resection.7 Supporting wrist height to prevent carpal collapse provided a stable base for remaining distal segments of the second through fourth metacarpals. After short-arm thumb spica casting and early rehabilitation, the patient recovered wrist motion and use of the involved fingers distal to the carpometacarpal joints.
In pregnant women, GCTs have been found primarily in the long bones and spine but are rare.17-21 A review of the literature (1950-present) revealed that the present article is the first report of GCT in the hand or wrist bones of a pregnant woman.18,20,21 There is no consensus as to whether surgical excision should be performed during pregnancy.18,20,21 In 1 unusual case, at 18 weeks’ gestation GCT in the distal femur was resected with curettage and bone grafting, and there were no complications.21 Therefore, pregnancy termination is not indicated for GCT.
The relationship between tumorigenesis and pregnancy is unclear.18,20,21 Empirically, pregnancy is thought to promote tumor growth.18,20 Estrogen and progesterone levels are elevated during pregnancy, potentially influencing tumor cells that are hormonally sensitive.18,20 An early report in which reverse transcription–polymerase chain reaction showed estrogen receptor expression in GCT osteoclast-like cells was followed by several studies that failed to find estrogen receptors at the protein level.19 In contrast, progesterone receptors were found in 50% of GCTs in a study.22 However, the etiopathogenic significance of this is unclear. In pregnant women, vascular endothelial growth factor, placental growth factor, and other growth factors induce osteoclast formation.23 ß-Human chorionic gonadotropin expression (ß-hCG) has been found in 58% of cases, with some showing ß-hCG elevation in the serum.24 Other studies have focused on an immunologic explanation for occurrence of GCT during pregnancy.18 Oncofetal antigens, which are similar to fetal antigens, have been found in fibrosarcoma and in an osteosarcoma cell line but not in GCT.18-20 Thus, though occurrence during pregnancy may be coincidental given the frequency of GCT in women of childbearing age, it is plausible that tumor growth may be enhanced by pregnancy. More studies are needed to understand the relationship between giant cell proliferation and pregnancy-related growth factors and hormones.
With GCT, the rate of pulmonary metastases ranges from 0% to 4%; these metastases are usually diagnosed at time of local recurrence, or 2 years to 3 years after initial GCT diagnosis.2,3,12,14,25 Lung metastases secondary to GCT in the hand or foot bones are rare; our literature review identified only 4 cases.12,14 Risk factors for lung metastasis include local recurrence, aggressive appearance (Enneking grade 3) on radiograph, Ki-67 antigen expression, and distal radius location.14 The mechanism of metastasis is unknown.12,14
Lung metastases are usually excised, but they may spontaneously evolve toward necrosis and ossification.12 In cases in which surgery is unfeasible, chemotherapy (eg, with doxorubicin) has been used to control progression.12,14 Radiation can cause sarcomatous transformation and is contraindicated. Interferon26-28 and other antiangiogenic strategies have been successfully used in systemic therapy for GCT of bone. More recently, bisphosphonates29-32 and denosumab33 have been investigated.29,32-36 The limited toxicity of denosumab makes the drug a very attractive treatment option for recurrent or unresectable GCT of bone.33 Reported rates of mortality from lung metastases have ranged from 0% to 40%.14 There is evidence that control of lung metastases during the first 3 years after diagnosis is important for favorable outcomes.2,3
Malignant stromal cells of GCT of bone have been known to secrete RANKL, which recruits osteoclasts and osteoclast precursor cells, which in turn generate aggressive osteolytic activity.33,37 Denosumab, a monoclonal antibody that inhibits RANKL, is effective in stopping osteoclastic activity. In a phase 2 trial of denosumab in the treatment of GCT of bone, 96% of treated patients with unresectable disease showed no progression at 13 months.38 In addition, 74% of treated patients who had resectable disease but were likely to have morbid surgery did not require surgery, and 62% of treated patients who underwent surgery were able to have a less morbid procedure. Forty-one percent to 58% of treated patients had a reduction in tumor size.
Denosumab is very well tolerated. The phase 2 trial found serious adverse events in 9% of patients, and in 5% of cases the drug was discontinued because of toxicity.38 Serious adverse events include osteonecrosis of jaw, hypocalcemia, and hypophosphatemia.37 Electrolyte changes with denosumab are easy to monitor and manage. Although the favorable toxicity profile of denosumab allows for long-term therapy, the data on therapy duration in patients with unresectable disease are unclear. Patients who discontinue therapy should be closely monitored, as disease can progress in this setting.37
In contrast to GCT of larger bones, GCT of the wrist is rare and typically more aggressive, and has higher local recurrence rates. In many cases, diagnosis is delayed by insufficient imaging, which optimally should include either CT or MRI (Table). [[{"fid":"202341","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"7"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"7":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":""}}}]]For pregnant women with GCT, options include surgical resection with curettage and local adjuvant treatment. After pregnancy, denosumab can be used systemically, and can be effective with metastatic or unresectable disease. Surgical treatment in the wrist can be challenging when partial or complete resections of carpal bones are required. Occupational therapy is recommended for optimization of hand function after surgery.
Take-Home Points
- GCT of bones of the wrist is rare. This article is the only report of a wrist GCT during pregnancy that we could identify.
- Routine treatment usually consists of surgical excision with local adjuvant, and in the wrist, often results in reduced wrist motion.
- GCT of the wrist is more aggressive than the more common locations in long bones, with higher local recurrence rates if treated with surgery alone.
- Diagnosis is often delayed for GCT of the wrist, due to insufficient imaging, which should include CT or MRI.
- For pregnant women with GCT, local adjuvant treatments can be used in addition to surgery. Following pregnancy, denosumab can be used systemically, and can be effective with metastatic or unresectable disease.
Giant cell tumor (GCT) of bone accounts for about 5% of primary bone tumors.1-3 Only 3% to 5% of GCTs occur in the hand.4,5 Wrist involvement, which is rare, most often involves the hamate bone.5-7 Capitate bone involvement is exceedingly rare.8-11 Although histologically benign, GCT can recur locally after treatment with curettage alone, and lung metastases are found in 2% to 5% of cases.2,12-14 Therefore, en bloc tumor excision is preferred in the setting of cortical erosion or soft-tissue involvement.1,4,8 Wrist joint motion is inevitably reduced, and bone graft donor-site morbidity is significant.6-8
In the unusual case reported here, GCT presented in the capitate bone and, after the patient became pregnant, recurred in the hamate and trapezoid bones with soft-tissue extension and lung metastases. The capitate was excised en bloc and reconstructed with an interposition of polymethylmethacrylate bone cement. Pulmonary metastases developed, and the GCT expanded to involve multiple carpal bones and the bases of the second through fourth metacarpals. A 10-month course of systemic chemotherapy with the RANK ligand (RANKL) inhibitor denosumab was started after the pregnancy. After this treatment, the patient underwent both tumor resection and reconstruction with autogenous bicortical iliac crest bone graft (ICBG) carefully designed to preserve range of motion and maintain the fingers in anatomical position. Treatment with denosumab was continued after surgery. Although this case offers no endpoint for postoperative chemotherapy with denosumab, preoperative treatment dramatically reduced the GCT and permitted limb-sparing reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old right-handed woman with atraumatic swelling of the left wrist presented to an orthopedic surgeon at an outside facility. Physical examination revealed tender fullness on the dorsum of the wrist, slightly reduced range of motion and grip strength, and a neurovascularly intact wrist. The diagnosis was periarticular cyst, and the patient underwent physical therapy. Two years later, the swelling returned, tenderness was increasing, and symptoms did not resolve with cast immobilization. A radiograph showed a lytic lesion in the capitate bone (Figure 1).[[{"fid":"202332","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 1.","field_file_image_credit[und][0][value]":""}}}]]
GCT was diagnosed with percutaneous needle biopsy. A preoperative chest radiograph was reported normal. For initial treatment, the capitate and trapezoid bones were resected en bloc through a dorsal approach. Reconstruction consisted of limited arthrodesis using bone cement without additional fixation.
At 6-month follow-up, the patient was pregnant, and there was a recurrence of the wrist lesion. During the first 2 months of pregnancy, swelling and pain rapidly progressed, and a palpable mass formed. Radiographs showed a lytic lesion extending into the hamate bone (Figure 2), and magnetic resonance imaging (MRI) showed articular extension of the lesion with involvement of the base of the fourth metacarpal. [[{"fid":"202334","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"2"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"2":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 2.","field_file_image_credit[und][0][value]":""}}}]]Targeted anti-RANKL therapy was not recommended (and was not available at the patient’s home hospital). The patient deferred surgical treatment because of the pregnancy, which proved otherwise uneventful and ended with a full-term delivery.
After the pregnancy, radiographs of the wrist showed complete destruction of the hamate and trapezium bones, with erosion of the bases of the second through fourth metacarpals (Figure 3A). [[{"fid":"202335","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"3"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"3":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 3.","field_file_image_credit[und][0][value]":""}}}]]The patient presented at our institution 4 years after initial diagnosis. Computed tomography (CT) of the chest showed numerous bilateral pulmonary nodular opacities. Wrist imaging showed soft-tissue extension (Figure 3B). The diagnosis of recurrent metastatic GCT was confirmed with needle biopsies of the wrist mass and the right lung nodule.
Systemic chemotherapy was initiated with 120 mg of denosumab, given subcutaneously on days 1, 8, and 15 and then monthly during the 10 months leading up to surgery. Serum calcium was monitored during treatment and remained within the normal range the entire time, except for once at the start of therapy, when it dropped to 6.8 mg/dL. After 8 months, the soft-tissue mass, originally 8 cm × 8 cm × 6 cm, shrunk and stabilized at 5 cm × 4 cm × 4 cm (Figure 3B), and a bony shell reformed around it. Nodules in both lung fields showed response to denosumab.
Histologic examination revealed scattered osteoclast-like, multinucleated giant cells, consistent with a recurrent lesion (Figure 4). [[{"fid":"202336","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"4"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"4":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 4.","field_file_image_credit[und][0][value]":""}}}]]After 10 months of treatment with denosumab, the patient underwent resection (dorsal approach) of the residual cement, the soft-tissue mass, the affected carpal bones, half of the third metacarpal, and the second and fourth metacarpal bases. The proximal carpal row was preserved after no intra-articular involvement was verified. The closet margin was marginal; the tumor mass abutted without encompassing the flexor tendons and median nerve. The tumor was meticulously elevated from the neurovascular and tendinous structures, which were not sacrificed. Hydrogen peroxide was used for local adjuvant treatment. Bicortical autogenous ICBG was placed between the remaining scaphoid, lunate, and metacarpal bones. The second, third, and fourth metacarpal bases were stabilized on the overlapping outer table of ICBG with 2.0-mm plates and miniscrews (Figure 5A). Kirschner wires were used to stabilize the proximal bone graft and the scapholunate fossa. Cancellous bone graft was packed between the structural bone graft and neighboring unaffected carpal bones (Figure 5A). Immobilization with a short-arm thumb spica cast was maintained for 6 weeks after surgery and was followed by a 12-week rehabilitation program. The patient returned to normal activities when plain radiographs showed solid bony union (Figure 5B). Fourteen months after initial surgery, tenolysis was performed to free the extensor tendons (index, middle, and ring fingers on dorsum of left hand) from adhesions to the bone graft. At 37-month follow-up (Figure 5C), there was no clinical or radiographic evidence of progression in the wrist.[[{"fid":"202337","view_mode":"medstat_image_flush_left","attributes":{"class":"media-element file-medstat-image-flush-left","data-delta":"5"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 5.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"5":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Figure 5.","field_file_image_credit[und][0][value]":""}}}]]
The patient had bilateral pulmonary metastases (Figures 6A, 6B). Treatment with denosumab produced an initial response (smaller pulmonary lesions) and subsequent stability. After 12 months of treatment with denosumab, the patient underwent left thoracotomy and wedge resection of pulmonary metastases on the left. Pathologic evaluation revealed pulmonary parenchyma with calcification and ossification and limited viable tumor. Given the dramatic effects on the left pulmonary metastases, denosumab was continued, and surgical intervention on the right was not attempted. Pulmonary metastases were stable afterward (Figure 6C).[[{"fid":"202338","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"6"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 6.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"6":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Figure 6.","field_file_image_credit[und][0][value]":""}}}]]
At 54-month follow-up, systemic treatment with denosumab was continued. The patient had no pain in the wrist or hand and was able to use the left hand normally. There was some fissuring of the third and fourth digits over each other. However, the patient had good grip strength and was using eating utensils, picking up water bottles, and engaging in other activities without difficulty.
Discussion
GCT isolated to the carpus is rare. However, compared with GCT in the more common locations in long bones, it is also more aggressive, and its local recurrence rates are higher, probably 60% or more if treated with curettage alone.15 Therefore, excision augmented with adjuvant treatment is recommended.2,7 Use of bone cement in the hand is relatively uncommon.4,5,7-10
The diagnosis of GCT in the carpus is difficult and often delayed. The initial complaint is usually mild wrist pain after relatively mild trauma.5 The first reported case of GCT in the lunate bone was mistakenly thought to be Kienbock disease.5 Similarly, our patient was initially given a nononcologic diagnosis, which prompted conservative management.
Whether the biological behavior of GCT in the carpus differs from that of GCT in other sites is unclear. The high recurrence rates might be attributable in part to suboptimal curettage.5,6 En bloc resections of involved bone inevitably result in carpal instability or loss of wrist motion if arthrodesis is performed.4-7,11 In the present case, resection was followed by limited arthrodesis to mitigate motion losses.
Multifocal GCT in the carpal bones often affects younger patients and has a high rate of recurrence.7,16 In the present case, the patient’s pregnancy delayed treatment and allowed tumor extension into soft tissues and metacarpal bones. Given her young age, en bloc tumor resection was performed, with the proximal carpal row spared to preserve wrist motion. ICBG was carefully shaped to match the defect that remained after tumor resection.7 Supporting wrist height to prevent carpal collapse provided a stable base for remaining distal segments of the second through fourth metacarpals. After short-arm thumb spica casting and early rehabilitation, the patient recovered wrist motion and use of the involved fingers distal to the carpometacarpal joints.
In pregnant women, GCTs have been found primarily in the long bones and spine but are rare.17-21 A review of the literature (1950-present) revealed that the present article is the first report of GCT in the hand or wrist bones of a pregnant woman.18,20,21 There is no consensus as to whether surgical excision should be performed during pregnancy.18,20,21 In 1 unusual case, at 18 weeks’ gestation GCT in the distal femur was resected with curettage and bone grafting, and there were no complications.21 Therefore, pregnancy termination is not indicated for GCT.
The relationship between tumorigenesis and pregnancy is unclear.18,20,21 Empirically, pregnancy is thought to promote tumor growth.18,20 Estrogen and progesterone levels are elevated during pregnancy, potentially influencing tumor cells that are hormonally sensitive.18,20 An early report in which reverse transcription–polymerase chain reaction showed estrogen receptor expression in GCT osteoclast-like cells was followed by several studies that failed to find estrogen receptors at the protein level.19 In contrast, progesterone receptors were found in 50% of GCTs in a study.22 However, the etiopathogenic significance of this is unclear. In pregnant women, vascular endothelial growth factor, placental growth factor, and other growth factors induce osteoclast formation.23 ß-Human chorionic gonadotropin expression (ß-hCG) has been found in 58% of cases, with some showing ß-hCG elevation in the serum.24 Other studies have focused on an immunologic explanation for occurrence of GCT during pregnancy.18 Oncofetal antigens, which are similar to fetal antigens, have been found in fibrosarcoma and in an osteosarcoma cell line but not in GCT.18-20 Thus, though occurrence during pregnancy may be coincidental given the frequency of GCT in women of childbearing age, it is plausible that tumor growth may be enhanced by pregnancy. More studies are needed to understand the relationship between giant cell proliferation and pregnancy-related growth factors and hormones.
With GCT, the rate of pulmonary metastases ranges from 0% to 4%; these metastases are usually diagnosed at time of local recurrence, or 2 years to 3 years after initial GCT diagnosis.2,3,12,14,25 Lung metastases secondary to GCT in the hand or foot bones are rare; our literature review identified only 4 cases.12,14 Risk factors for lung metastasis include local recurrence, aggressive appearance (Enneking grade 3) on radiograph, Ki-67 antigen expression, and distal radius location.14 The mechanism of metastasis is unknown.12,14
Lung metastases are usually excised, but they may spontaneously evolve toward necrosis and ossification.12 In cases in which surgery is unfeasible, chemotherapy (eg, with doxorubicin) has been used to control progression.12,14 Radiation can cause sarcomatous transformation and is contraindicated. Interferon26-28 and other antiangiogenic strategies have been successfully used in systemic therapy for GCT of bone. More recently, bisphosphonates29-32 and denosumab33 have been investigated.29,32-36 The limited toxicity of denosumab makes the drug a very attractive treatment option for recurrent or unresectable GCT of bone.33 Reported rates of mortality from lung metastases have ranged from 0% to 40%.14 There is evidence that control of lung metastases during the first 3 years after diagnosis is important for favorable outcomes.2,3
Malignant stromal cells of GCT of bone have been known to secrete RANKL, which recruits osteoclasts and osteoclast precursor cells, which in turn generate aggressive osteolytic activity.33,37 Denosumab, a monoclonal antibody that inhibits RANKL, is effective in stopping osteoclastic activity. In a phase 2 trial of denosumab in the treatment of GCT of bone, 96% of treated patients with unresectable disease showed no progression at 13 months.38 In addition, 74% of treated patients who had resectable disease but were likely to have morbid surgery did not require surgery, and 62% of treated patients who underwent surgery were able to have a less morbid procedure. Forty-one percent to 58% of treated patients had a reduction in tumor size.
Denosumab is very well tolerated. The phase 2 trial found serious adverse events in 9% of patients, and in 5% of cases the drug was discontinued because of toxicity.38 Serious adverse events include osteonecrosis of jaw, hypocalcemia, and hypophosphatemia.37 Electrolyte changes with denosumab are easy to monitor and manage. Although the favorable toxicity profile of denosumab allows for long-term therapy, the data on therapy duration in patients with unresectable disease are unclear. Patients who discontinue therapy should be closely monitored, as disease can progress in this setting.37
In contrast to GCT of larger bones, GCT of the wrist is rare and typically more aggressive, and has higher local recurrence rates. In many cases, diagnosis is delayed by insufficient imaging, which optimally should include either CT or MRI (Table). [[{"fid":"202341","view_mode":"medstat_image_flush_right","attributes":{"class":"media-element file-medstat-image-flush-right","data-delta":"7"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"7":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Table.","field_file_image_credit[und][0][value]":""}}}]]For pregnant women with GCT, options include surgical resection with curettage and local adjuvant treatment. After pregnancy, denosumab can be used systemically, and can be effective with metastatic or unresectable disease. Surgical treatment in the wrist can be challenging when partial or complete resections of carpal bones are required. Occupational therapy is recommended for optimization of hand function after surgery.
1. Balke M, Ahrens H, Streitbuerger A, et al. Treatment options for recurrent giant cell tumors of bone. J Cancer Res Clin Oncol. 2009;135(1):149-158.
2. Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Giant cell tumor of bone risk factors for recurrence. Clin Orthop Relat Res. 2011;469(2):591-599.
3. Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
4. Averill RM, Smith RJ, Campbell CJ. Giant-cell tumors of the bones of the hand. J Hand Surg Am. 1980;5(1):39-50.
5. Shigematsu K, Kobata Y, Yajima H, Kawamura K, Maegawa N, Takakura Y. Giant-cell tumors of the carpus. J Hand Surg Am. 2006;31(7):1214-1219.
6. Gupta GG, Lucas GL, Pirela-Cruz M. Multifocal giant cell tumor of the capitate, hamate, and triquetrum: a case report. J Hand Surg Am. 1995;20(6):1003-1006.
7. Tarng YW, Yang SW, Hsu CJ. Surgical treatment of multifocal giant cell tumor of carpal bones with preservation of wrist function: case report. J Hand Surg Am. 2009;34(2):262-265.
8. Angelini A, Mavrogenis AF, Ruggieri P. Giant cell tumor of the capitate. Musculoskelet Surg. 2011;95(1):45-48.
9. Howard FM, Lassen K. Giant cell tumor of the capitate. J Hand Surg Am. 1984;9(2):272-274.
10. McDonald DJ, Schajowicz F. Giant cell tumor of the capitate. A case report. Clin Orthop Relat Res. 1992(279):264-268.
11. Wilson SC, Cascio BM, Plauche HR. Giant-cell tumor of the capitate. Orthopedics. 2001;24(11):1085-1086.
12. Combalia-Aleu A, Sastre S, Fernández-de-Retana P, Tomás X, Palacin A. Giant cell tumor of the talus with pulmonary metastasis: seven years follow up. Foot. 2006;16(2):107-111.
13. Donthineni R, Boriani L, Ofluoglu O, Bandiera S. Metastatic behaviour of giant cell tumour of the spine. Int Orthop. 2009;33(2):497-501.
14. Jacopin S, Viehweger E, Glard Y, et al. Fatal lung metastasis secondary to index finger giant cell tumor in an 8-year-old child. Orthop Traumatol Surg Res. 2010;96(3):310-313.
15. Plate AM, Lee SJ, Steiner G, Posner MA. Tumor-like lesions and benign tumors of the hand and wrist. J Am Acad Orthop Surg. 2003;11(2):129-141.
16. Moreel P, Le Viet D. Failure of initial surgical treatment of a giant cell tumor of the capitate and its salvage: a case report [in French]. Chir Main. 2006;25(6):315-318.
17. Caillouette JC, Mattar N. Massive peripheral giant-cell reparative granuloma of the jaw: a pregnancy dependent tumor. Trans Pac Coast Obstet Gynecol Soc. 1978;45:78-81.
18. Kathiresan AS, Johnson JN, Hood BJ, Montoya SP, Vanni S, Gonzalez-Quintero VH. Giant cell bone tumor of the thoracic spine presenting in late pregnancy. Obstet Gynecol. 2011;118(2 pt 2):428-431.
19. Komiya S, Zenmyo M, Inoue A. Bone tumors in the pelvis presenting growth during pregnancy. Arch Orthop Trauma Surg. 1999;119(1-2):22-29.
20. Ross AE, Bojescul JA, Kuklo TR. Giant cell tumor: a case report of recurrence during pregnancy. Spine. 2005;30(12):E332-3E35.
21. Sharma JB, Chanana C, Rastogi, et al. Successful pregnancy outcome with elective caesarean section following two attempts of surgical excision of large giant cell tumor of the lower limb during pregnancy. Arch Gynecol Obstet. 2006;274(5):313-315.
22. Demertzis N, Kotsiandri F, Giotis I, Apostolikas N. Giant-cell tumors of bone and progesterone receptors. Orthopedics. 2003;26(12):1209-1212.
23. Taylor RM, Kashima TG, Knowles HJ, Athanasou NA. VEGF, FLT3 ligand, PlGF and HGF can substitute for M-CSF to induce human osteoclast formation: implications for giant cell tumour pathobiology. Lab Invest. 2012;92(10):1398-1406.
24. Lawless ME, Jour G, Hoch BL, Rendi MH. Beta-human chorionic gonadotropin expression in recurrent and metastatic giant cell tumors of bone: a potential mimicker of germ cell tumor. Int J Surg Pathol. 2014;22(7):617-622.
25. Viswanathan S, Jambhekar NA. Metastatic giant cell tumor of bone: are there associated factors and best treatment modalities? Clin Orthop Relat Res. 2010;468(3):827-833.
26. Kaban LB, Troulis MJ, Ebb D, August M, Hornicek FJ, Dodson TB. Antiangiogenic therapy with interferon alpha for giant cell lesions of the jaws. J Oral Maxillofac Surg. 2002;60(10):1103-1111.
27. Kaiser U, Neumann K, Havemann K. Generalised giant-cell tumour of bone: successful treatment of pulmonary metastases with interferon alpha, a case report. J Cancer Res Clin Oncol. 1993;119(5):301-303.
28. Dickerman JD. Interferon and giant cell tumors. Pediatrics. 1999;103(6 pt 1):1282-1283.
29. Balke M, Campanacci L, Gebert C, et al. Bisphosphonate treatment of aggressive primary, recurrent and metastatic giant cell tumour of bone. BMC Cancer. 2010;10:462.
30. Gille O, Oliveira Bde A, Guerin P, Lepreux S, Richez C, Vital JM. Regression of giant cell tumor of the cervical spine with bisphosphonate as single therapy. Spine. 2012;37(6):E396-E399.
31. Moriceau G, Ory B, Gobin B, et al. Therapeutic approach of primary bone tumours by bisphosphonates. Curr Pharm Des. 2010;16(27):2981-2987.
32. Tse LF, Wong KC, Kumta SM, Huang L, Chow TC, Griffith JF. Bisphosphonates reduce local recurrence in extremity giant cell tumor of bone: a case–control study. Bone. 2008;42(1):68-73.
33. Thomas D, Henshaw R, Skubitz K, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11(3):275-280.
34. Balke M, Hardes J. Denosumab: a breakthrough in treatment of giant-cell tumour of bone? Lancet Oncol. 2010;11(3):218-219.
35. Kyrgidis A, Toulis K. Safety and efficacy of denosumab in giant-cell tumour of bone. Lancet Oncol. 2010;11(6):513-514.
36. Thomas D, Carriere P, Jacobs I. Safety of denosumab in giant-cell tumour of bone. Lancet Oncol. 2010;11(9):815.
37. Skubitz KM. Giant cell tumor of bone: current treatment options. Curr Treat Options Oncol. 2014;15(3):507-518.
38. Chawla S, Henshaw R, Seeger L, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14(9):901-908.
1. Balke M, Ahrens H, Streitbuerger A, et al. Treatment options for recurrent giant cell tumors of bone. J Cancer Res Clin Oncol. 2009;135(1):149-158.
2. Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Giant cell tumor of bone risk factors for recurrence. Clin Orthop Relat Res. 2011;469(2):591-599.
3. Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
4. Averill RM, Smith RJ, Campbell CJ. Giant-cell tumors of the bones of the hand. J Hand Surg Am. 1980;5(1):39-50.
5. Shigematsu K, Kobata Y, Yajima H, Kawamura K, Maegawa N, Takakura Y. Giant-cell tumors of the carpus. J Hand Surg Am. 2006;31(7):1214-1219.
6. Gupta GG, Lucas GL, Pirela-Cruz M. Multifocal giant cell tumor of the capitate, hamate, and triquetrum: a case report. J Hand Surg Am. 1995;20(6):1003-1006.
7. Tarng YW, Yang SW, Hsu CJ. Surgical treatment of multifocal giant cell tumor of carpal bones with preservation of wrist function: case report. J Hand Surg Am. 2009;34(2):262-265.
8. Angelini A, Mavrogenis AF, Ruggieri P. Giant cell tumor of the capitate. Musculoskelet Surg. 2011;95(1):45-48.
9. Howard FM, Lassen K. Giant cell tumor of the capitate. J Hand Surg Am. 1984;9(2):272-274.
10. McDonald DJ, Schajowicz F. Giant cell tumor of the capitate. A case report. Clin Orthop Relat Res. 1992(279):264-268.
11. Wilson SC, Cascio BM, Plauche HR. Giant-cell tumor of the capitate. Orthopedics. 2001;24(11):1085-1086.
12. Combalia-Aleu A, Sastre S, Fernández-de-Retana P, Tomás X, Palacin A. Giant cell tumor of the talus with pulmonary metastasis: seven years follow up. Foot. 2006;16(2):107-111.
13. Donthineni R, Boriani L, Ofluoglu O, Bandiera S. Metastatic behaviour of giant cell tumour of the spine. Int Orthop. 2009;33(2):497-501.
14. Jacopin S, Viehweger E, Glard Y, et al. Fatal lung metastasis secondary to index finger giant cell tumor in an 8-year-old child. Orthop Traumatol Surg Res. 2010;96(3):310-313.
15. Plate AM, Lee SJ, Steiner G, Posner MA. Tumor-like lesions and benign tumors of the hand and wrist. J Am Acad Orthop Surg. 2003;11(2):129-141.
16. Moreel P, Le Viet D. Failure of initial surgical treatment of a giant cell tumor of the capitate and its salvage: a case report [in French]. Chir Main. 2006;25(6):315-318.
17. Caillouette JC, Mattar N. Massive peripheral giant-cell reparative granuloma of the jaw: a pregnancy dependent tumor. Trans Pac Coast Obstet Gynecol Soc. 1978;45:78-81.
18. Kathiresan AS, Johnson JN, Hood BJ, Montoya SP, Vanni S, Gonzalez-Quintero VH. Giant cell bone tumor of the thoracic spine presenting in late pregnancy. Obstet Gynecol. 2011;118(2 pt 2):428-431.
19. Komiya S, Zenmyo M, Inoue A. Bone tumors in the pelvis presenting growth during pregnancy. Arch Orthop Trauma Surg. 1999;119(1-2):22-29.
20. Ross AE, Bojescul JA, Kuklo TR. Giant cell tumor: a case report of recurrence during pregnancy. Spine. 2005;30(12):E332-3E35.
21. Sharma JB, Chanana C, Rastogi, et al. Successful pregnancy outcome with elective caesarean section following two attempts of surgical excision of large giant cell tumor of the lower limb during pregnancy. Arch Gynecol Obstet. 2006;274(5):313-315.
22. Demertzis N, Kotsiandri F, Giotis I, Apostolikas N. Giant-cell tumors of bone and progesterone receptors. Orthopedics. 2003;26(12):1209-1212.
23. Taylor RM, Kashima TG, Knowles HJ, Athanasou NA. VEGF, FLT3 ligand, PlGF and HGF can substitute for M-CSF to induce human osteoclast formation: implications for giant cell tumour pathobiology. Lab Invest. 2012;92(10):1398-1406.
24. Lawless ME, Jour G, Hoch BL, Rendi MH. Beta-human chorionic gonadotropin expression in recurrent and metastatic giant cell tumors of bone: a potential mimicker of germ cell tumor. Int J Surg Pathol. 2014;22(7):617-622.
25. Viswanathan S, Jambhekar NA. Metastatic giant cell tumor of bone: are there associated factors and best treatment modalities? Clin Orthop Relat Res. 2010;468(3):827-833.
26. Kaban LB, Troulis MJ, Ebb D, August M, Hornicek FJ, Dodson TB. Antiangiogenic therapy with interferon alpha for giant cell lesions of the jaws. J Oral Maxillofac Surg. 2002;60(10):1103-1111.
27. Kaiser U, Neumann K, Havemann K. Generalised giant-cell tumour of bone: successful treatment of pulmonary metastases with interferon alpha, a case report. J Cancer Res Clin Oncol. 1993;119(5):301-303.
28. Dickerman JD. Interferon and giant cell tumors. Pediatrics. 1999;103(6 pt 1):1282-1283.
29. Balke M, Campanacci L, Gebert C, et al. Bisphosphonate treatment of aggressive primary, recurrent and metastatic giant cell tumour of bone. BMC Cancer. 2010;10:462.
30. Gille O, Oliveira Bde A, Guerin P, Lepreux S, Richez C, Vital JM. Regression of giant cell tumor of the cervical spine with bisphosphonate as single therapy. Spine. 2012;37(6):E396-E399.
31. Moriceau G, Ory B, Gobin B, et al. Therapeutic approach of primary bone tumours by bisphosphonates. Curr Pharm Des. 2010;16(27):2981-2987.
32. Tse LF, Wong KC, Kumta SM, Huang L, Chow TC, Griffith JF. Bisphosphonates reduce local recurrence in extremity giant cell tumor of bone: a case–control study. Bone. 2008;42(1):68-73.
33. Thomas D, Henshaw R, Skubitz K, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11(3):275-280.
34. Balke M, Hardes J. Denosumab: a breakthrough in treatment of giant-cell tumour of bone? Lancet Oncol. 2010;11(3):218-219.
35. Kyrgidis A, Toulis K. Safety and efficacy of denosumab in giant-cell tumour of bone. Lancet Oncol. 2010;11(6):513-514.
36. Thomas D, Carriere P, Jacobs I. Safety of denosumab in giant-cell tumour of bone. Lancet Oncol. 2010;11(9):815.
37. Skubitz KM. Giant cell tumor of bone: current treatment options. Curr Treat Options Oncol. 2014;15(3):507-518.
38. Chawla S, Henshaw R, Seeger L, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14(9):901-908.