User login
In 1992, the American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics (AAP) published their first joint guidelines on the prevention of early-onset neonatal group B streptococcal (GBS) infection.1 In this initial statement, the organizations recommended universal culturing of obstetric patients at 28 weeks’ gestation and treatment of colonized women during labor if they had a recognized risk factor for neonatal GBS infection.
In 1996, the Centers for Disease Control and Prevention (CDC) published its first set of official guidelines on the topic and suggested that both universal screening and a risk-factor–based approach were reasonable options.2 The 2002 update of the CDC guidelines strongly recommended universal screening of all pregnant women at 35 to 37 weeks’ gestation and intrapartum prophylaxis for all colonized women regardless of risk factors.3
The third set of CDC guidelines was published in 2010.4 The key features of this version were the elimination of erythromycin as an alternative to penicillin in patients who are allergic to beta-lactam antibiotics and the establishment of 4 hours as the critical interval for administration of prophylaxis prior to delivery. The 2010 publication was the last such report from the CDC. Since then ACOG and AAP have been tasked with providing updated practice guidelines. To that end, ACOG recently issued a new Committee Opinion on “Prevention of Group B Streptococcal Early-Onset Disease in Newborns.”5 Here we will highlight the key features of our current strategy for preventing neonatal GBS infection.
CASE Pregnant patient presents with many questions about GBS
A 26-year-old primigravid woman presents for her first prenatal appointment at 9 weeks’ gestation. Her older sister recently delivered a term infant that died in the first week of life from GBS sepsis. Understandably, she has many questions.
1. Your patient first wants to know, “What is this streptococcal organism and how likely am I to have this infection?”
Streptococcus agalactiae, also known as GBS, is a gram-positive encapsulated bacterium that produces beta hemolysis when grown on blood agar. Approximately 25% of pregnant women harbor this organism in the lower genital tract and/or rectum.6
GBS is one of the most important causes of neonatal infection, particularly in preterm infants. The frequency of infection is now 0.23 per 1,000 live births in the US.5
Neonatal infection can be divided into early-onset infection (occurring within the first 7 days of life) and late-onset infection (occurring from after the first week until the third month of life). Approximately 80% to 85% of cases of neonatal GBS infections are early in onset. Virtually all of the early-onset infections result from vertical transmission during delivery from a colonized mother to her infant.5-7
2. “How dangerous is this infection to my baby and me? Are there certain factors that increase the risk of my baby becoming infected?”
GBS is responsible for approximately 2% to 3% of cases of either asymptomatic bacteriuria or acute cystitis. Women with urinary tract infections caused by GBS are at increased risk for preterm premature rupture of membranes and preterm delivery. Genital tract colonization also increases a woman’s risk for chorioamnionitis and endometritis, particularly after cesarean delivery (CD). In addition, GBS can be part of the polymicrobial flora in women who have a wound (incisional site) infection following CD.6,7
Continue to: In colonized women, several risk factors...
In colonized women, several risk factors have been identified that increase the probability of early-onset neonatal GBS infection. These factors include: preterm labor, especially when complicated by premature rupture of membranes; intrapartum maternal fever (usually due to chorioamnionitis); rupture of membranes greater than 18 hours before delivery; previous delivery of an infected infant; young age; and black or Hispanic ethnicity. Approximately 25% of colonized women will have one of these risk factors.5-7
These risk factors have a profound impact on neonatal attack rates and mortality. Without the interventions outlined below, the neonatal infection rate is 40% to 50% in the presence of a risk factor and less than 5% in the absence of a risk factor. In infected infants, neonatal mortality approaches 30% to 35% when a maternal risk factor is present, but is less than 5% when risk factors are absent.5-7
3. “What will you do to determine if I am colonized with this organism?”
The current guidelines set forth in the ACOG Committee Opinion recommend that selected high-risk patients (patients with preterm labor or preterm premature rupture of membranes) be tested for GBS at the time of initial presentation. All other women should be tested for GBS during the interval 36 0/7 to 37 6/7 weeks’ gestation.5 Testing at this point in pregnancy is almost 90% sensitive for identifying patients who will be colonized at the time of admission for labor if no more than 5 weeks elapse between the time the culture is obtained and labor begins. The positive predictive value of this test is 87%, and the negative predictive value is 96%.8
ACOG’s previous guidelines provided for testing at 35 rather than 36 weeks. The change in the recommendations was based on 2 factors. First, all women with unknown GBS status who may deliver before 37 weeks already should be targeted for prophylaxis. Second, the new 5-week window now will include women who deliver up to 41 weeks’ gestation. Given current obstetric practice in the US, delivery beyond 41 weeks is unlikely.5
At the present time, the best test for identification of GBS colonization is bacteriologic culture. A cotton swab is placed into the lower third of the vagina, streaked along the perineum, and then placed into the rectum. The swab is withdrawn, placed in a culturette tube, and transported to the laboratory. In the laboratory, the swab is cultured for approximately 24 hours in a nutrient broth and then subcultured on a selective blood agar plate. Failure to sample both the vagina and rectum or failure to use selective broth and selective blood agar will reduce the yield of positive cultures by approximately 50%.5-7
In recent years, researchers have become interested in the use of rapid nucleic acid amplification tests for the identification of GBS. These tests perform well if the test protocol provides for an 18- to 24-hour incubation in nutrient broth prior to application of the nucleic acid probe. When the tests are performed without this enrichment phase, sensitivities are inferior to those associated with bacteriologic culture. In addition, because the rapid tests do not isolate the organisms, they do not allow for antibiotic sensitivity testing.5-7
Continue to: “If I test positive for GBS, how and when will you treat me?”...
4. “If I test positive for GBS, how and when will you treat me?”
The current ACOG guidelines recommend that all colonized women be treated intrapartum with prophylactic antibiotics regardless of whether risk factors are present. Treatment should be started at the time of admission and continued until the infant is delivered.5
The drugs of choice for intrapartum prophylaxis are intravenous penicillin or ampicillin. If the patient has a mild allergy to penicillin, cefazolin is the appropriate alternative. If the patient has a severe allergy to penicillin, the 2 options are vancomycin or clindamycin. If the latter drug is used, the laboratory must perform sensitivity testing because 13% to 20% of strains of GBS may be resistant to clindamycin. The frequency of resistance to erythromycin now ranges from 25% to 32%. Thus, erythromycin is no longer used for intrapartum prophylaxis.5-7,9
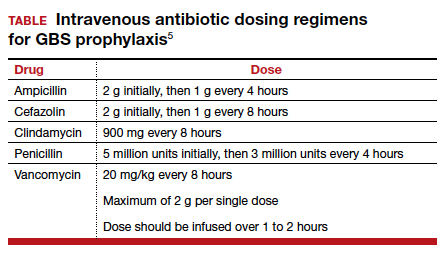
The appropriate intravenous dosages of these antibiotics are listed in the TABLE.5 The new ACOG guidelines have revised the previous recommendations for dosing of penicillin, eliminating the 2.5 million-unit dose. They also have revised the dosing recommendations for vancomyin, eliminating the previous recommendation of 1 g every 12 hours.5 The new recommendations regarding vancomycin are particularly important and are based, at least in part, on an interesting report from Onwuchuruba and colleagues.10 These authors studied maternal and cord blood concentrations of vancomycin in mother-infant dyads receiving either the original recommended dosage of vancomycin (1 g every 12 hours) or a dosage of 15 to 20 mg/kg every 8 hours. With standard dosing, only 9% of neonates had therapeutic vancomycin serum concentrations at delivery. With the 20 mg/kg dose of vancomycin, the percent of neonates with therapeutic serum concentrations of vancomycin increased to 80%.
5. “For how long must I be treated in labor before my baby will be protected by the antibiotics?”
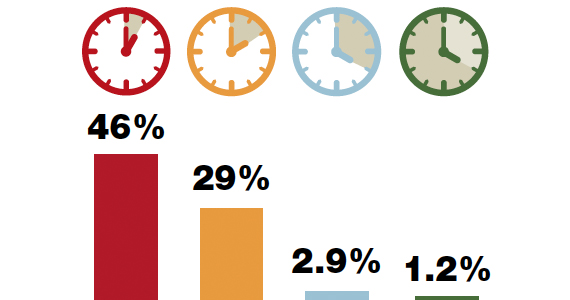
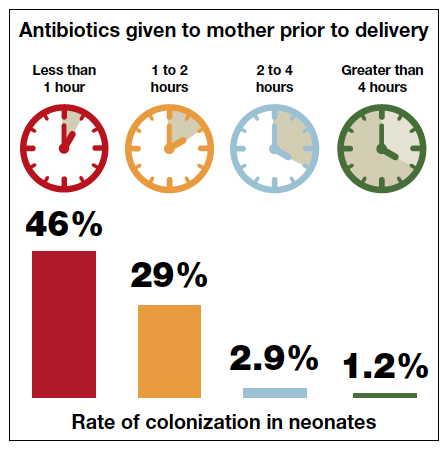
The current ACOG Committee Opinion stresses the importance of treating the colonized mother for at least 4 hours prior to delivery.5 This recommendation is based primarily on the landmark report by De Cueto and colleagues.11 These authors evaluated colonized women who received intrapartum prophylaxis at varying times prior to delivery. Their primary endpoint was the percentage of newborns who were colonized with GBS. If the mothers had received antibiotics for less than 1 hour prior to delivery, 46% of neonates were colonized. This figure was equal to the rate of colonization in neonates whose mothers received no antibiotics. When the interval was 1 to 2 hours, the percentage was 29%. When mothers had received antibiotics for 2 to 4 hours, the neonatal colonization rate fell to 2.9%. When antibiotics had been administered for greater than 4 hours, the rate of neonatal colonization was only 1.2%.
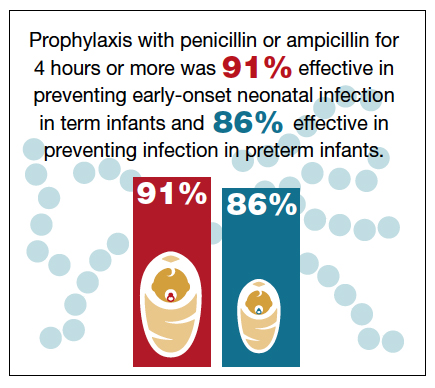
Fairlie and colleagues recently reported the results of another interesting investigation comparing the effectiveness of prophylaxis based on duration of treatment and choice of individual antibiotics.12 Prophylaxis with penicillin or ampicillin for 4 hours or more was 91% effective in preventing early-onset neonatal infection in term infants and 86% effective in preventing infection in preterm infants. These outcomes were superior to the outcomes in both term and preterm infants who received penicillin or ampicillin for less than 4 hours.
These observations agree with the findings of McNanley and colleagues who evaluated vaginal colony counts of GBS following different periods of antibiotic administration.13 These authors noted that mean colony counts decreased 5-fold within 2 hours of penicillin administration, 50-fold within 4 hours, and 1,000-fold within 6 hours.
Despite these compelling findings, the ACOG Committee Opinion stresses that obstetric interventions such as amniotomy and oxytocin augmentation should not be delayed simply to permit a certain time period of antibiotic administration.5
Continue to: “If I were to have a scheduled CD before the onset of labor and/or ruptured membranes, would I still need to receive antibiotics?”...
6. “If I were to have a scheduled CD before the onset of labor and/or ruptured membranes, would I still need to receive antibiotics?”
If a mother is scheduled to have a CD, for example because of a prior cesarean or because of a persistent fetal malpresentation, she should still have a GBS culture at 36 0/7 to 37 6/7 weeks’ gestation. The information obtained from this culture may be of value to both the obstetrician and pediatrician if the patient experiences labor or rupture of membranes prior to her scheduled surgery. If she does not experience spontaneous labor prior to her scheduled date of surgery, she does not require specific GBS prophylaxis at the time of her operation.5 Rather, she should receive prophylactic antibiotics to prevent post–cesarean infection, ideally, the combination of cefazolin (2 g IV) plus azithromycin (500 mg IV).14 Cefazolin, of course, provides excellent coverage of GBS.
7. “If I am colonized with GBS and I receive treatment during labor, will my baby be safe after delivery?”
The interventions outlined above will prevent almost 90% of early-onset GBS infections, but they are not foolproof.5-7,15,16 Successful management of the neonate is dependent upon several factors, including:5-7
- gestational age
- presence of maternal chorioamnionitis
- presence or absence of risk factors for early-onset infection
- duration (adequacy) of maternal treatment during labor
- presence of immediate clinical signs of infection in the neonate (such as fever, lethargy, hemodynamic instability, respiratory distress, or elevated or decreased white blood cell count).
If the mother is at term and receives intrapartum prophylaxis for at least 4 hours prior to delivery, the neonate usually will not require any special tests and simply will be observed for 24 to 48 hours for signs of infection.
If the mother delivers preterm and receives appropriate intrapartum prophylaxis, the pediatricians typically will obtain a complete blood count (CBC) and treat with prophylactic antibiotics (ampicillin plus gentamicin) for 48 hours if abnormalities are noted on the CBC or the baby exhibits signs of infection. If the CBC is normal and the baby shows no signs of infection, no treatment is indicated.
Regardless of gestational age, if the mother does not receive prophylaxis for at least 4 hours before delivery, the pediatricians usually will obtain a CBC and closely observe the baby in the hospital for signs of infection. If such signs develop or the CBC is abnormal, blood and cerebrospinal fluid cultures will be obtained. Antibiotic therapy (usually ampicillin plus gentamicin) is then initiated, and the drugs are continued until cultures return with no growth. If either culture is positive, antibiotics will then be continued for 7 to 10 days.
If the mother has documented chorioamnionitis and receives treatment intrapartum with appropriate antibiotics (usually ampicillin plus gentamicin), the pediatricians usually will obtain a CBC, C-reactive protein (CRP) level, and blood cultures and then start the infant on antibiotics, pending the result of the laboratory tests. If the CBC and CRP are reassuring, the cultures are negative after 48 hours, and the infant demonstrates no signs of clinical infection, many pediatricians will then discontinue antibiotics. Others may still continue the antibiotics for 7 to 10 days.
- Committee on Infectious Diseases and Committee on Fetus and Newborn. Guidelines for prevention of group B streptococcal (GBS) infection by chemoprophylaxis. Pediatrics. 1992;90:775-778.
- CDC. Prevention of perinatal group B streptococcal disease: a public health perspective. MMWR Recomm Rep. 1996;45(RR-7):1-24.
- Schrag S, Gorwitz R, Fultz-Butts K, et al. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51(RR-11):1-22.
- Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 2010;59:1-36.
- Prevention of group B streptococcal early-onset disease in newborns. ACOG Committee Opinion Summary, Number 782. Obstet Gynecol. 2019;134:206-210.
- Duff P, Birsner M. Maternal and perinatal infection in pregnancy: bacteria. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics. Normal and Problem Pregnancies. 7th ed. Philadelphia, PA: Elsevier; 2017.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019.
- Yancey MK, Schuchat A, Brown LK, et al. The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet Gynecol. 1996;88:811-815.
- Edwards RK, Clark P, Duff P. Intrapartum antibiotic prophylaxis 2: positive predictive value of antenatal group B streptococci cultures and antibiotic susceptibility of clinical isolates. Obstet Gynecol. 2002;100:540-544.
- Onwuchuruba CN, Towers CV, Howard BC, et al. Transplacental passage of vancomycin from mother to neonate. Am J Obstet Gynecol. 2014;210:352.e1-352.e4.
- de Cueto M, Sanchez MJ, Sampedro A, et al. Timing of intrapartum ampicillin and prevention of vertical transmission of group B streptococcus. Obstet Gynecol. 1998;91:112-114.
- Fairlie T, Zell ER, Schrag S. Effectiveness of intrapartum antibiotic prophylaxis for prevention of early-onset group B streptococcal disease. Obstet Gynecol. 2013;121:570-577.
- McNanley AR, Glantz JC, Hardy DJ, et al. The effect of intrapartum penicillin on vaginal group B streptococcus colony counts. Am J Obstet Gynecol. 2007;197:583.e1-583.e4.
- Tita AT, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
- Brozanski BS, Jones JG, Krohn MA, et al. Effect of a screening-based prevention policy on prevalence of early-onset group B streptococcal sepsis. Obstet Gynecol. 2000;95:496-501.
- Rosenstein NE, Schuchat A. Opportunities for prevention of perinatal group B streptococcal disease: a multistate surveillance analysis. The National Group B Streptococcal Disease Study Group. Obstet Gynecol. 1997;90:901-906.
In 1992, the American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics (AAP) published their first joint guidelines on the prevention of early-onset neonatal group B streptococcal (GBS) infection.1 In this initial statement, the organizations recommended universal culturing of obstetric patients at 28 weeks’ gestation and treatment of colonized women during labor if they had a recognized risk factor for neonatal GBS infection.
In 1996, the Centers for Disease Control and Prevention (CDC) published its first set of official guidelines on the topic and suggested that both universal screening and a risk-factor–based approach were reasonable options.2 The 2002 update of the CDC guidelines strongly recommended universal screening of all pregnant women at 35 to 37 weeks’ gestation and intrapartum prophylaxis for all colonized women regardless of risk factors.3
The third set of CDC guidelines was published in 2010.4 The key features of this version were the elimination of erythromycin as an alternative to penicillin in patients who are allergic to beta-lactam antibiotics and the establishment of 4 hours as the critical interval for administration of prophylaxis prior to delivery. The 2010 publication was the last such report from the CDC. Since then ACOG and AAP have been tasked with providing updated practice guidelines. To that end, ACOG recently issued a new Committee Opinion on “Prevention of Group B Streptococcal Early-Onset Disease in Newborns.”5 Here we will highlight the key features of our current strategy for preventing neonatal GBS infection.
CASE Pregnant patient presents with many questions about GBS
A 26-year-old primigravid woman presents for her first prenatal appointment at 9 weeks’ gestation. Her older sister recently delivered a term infant that died in the first week of life from GBS sepsis. Understandably, she has many questions.
1. Your patient first wants to know, “What is this streptococcal organism and how likely am I to have this infection?”
Streptococcus agalactiae, also known as GBS, is a gram-positive encapsulated bacterium that produces beta hemolysis when grown on blood agar. Approximately 25% of pregnant women harbor this organism in the lower genital tract and/or rectum.6
GBS is one of the most important causes of neonatal infection, particularly in preterm infants. The frequency of infection is now 0.23 per 1,000 live births in the US.5
Neonatal infection can be divided into early-onset infection (occurring within the first 7 days of life) and late-onset infection (occurring from after the first week until the third month of life). Approximately 80% to 85% of cases of neonatal GBS infections are early in onset. Virtually all of the early-onset infections result from vertical transmission during delivery from a colonized mother to her infant.5-7
2. “How dangerous is this infection to my baby and me? Are there certain factors that increase the risk of my baby becoming infected?”
GBS is responsible for approximately 2% to 3% of cases of either asymptomatic bacteriuria or acute cystitis. Women with urinary tract infections caused by GBS are at increased risk for preterm premature rupture of membranes and preterm delivery. Genital tract colonization also increases a woman’s risk for chorioamnionitis and endometritis, particularly after cesarean delivery (CD). In addition, GBS can be part of the polymicrobial flora in women who have a wound (incisional site) infection following CD.6,7
Continue to: In colonized women, several risk factors...
In colonized women, several risk factors have been identified that increase the probability of early-onset neonatal GBS infection. These factors include: preterm labor, especially when complicated by premature rupture of membranes; intrapartum maternal fever (usually due to chorioamnionitis); rupture of membranes greater than 18 hours before delivery; previous delivery of an infected infant; young age; and black or Hispanic ethnicity. Approximately 25% of colonized women will have one of these risk factors.5-7
These risk factors have a profound impact on neonatal attack rates and mortality. Without the interventions outlined below, the neonatal infection rate is 40% to 50% in the presence of a risk factor and less than 5% in the absence of a risk factor. In infected infants, neonatal mortality approaches 30% to 35% when a maternal risk factor is present, but is less than 5% when risk factors are absent.5-7
3. “What will you do to determine if I am colonized with this organism?”
The current guidelines set forth in the ACOG Committee Opinion recommend that selected high-risk patients (patients with preterm labor or preterm premature rupture of membranes) be tested for GBS at the time of initial presentation. All other women should be tested for GBS during the interval 36 0/7 to 37 6/7 weeks’ gestation.5 Testing at this point in pregnancy is almost 90% sensitive for identifying patients who will be colonized at the time of admission for labor if no more than 5 weeks elapse between the time the culture is obtained and labor begins. The positive predictive value of this test is 87%, and the negative predictive value is 96%.8
ACOG’s previous guidelines provided for testing at 35 rather than 36 weeks. The change in the recommendations was based on 2 factors. First, all women with unknown GBS status who may deliver before 37 weeks already should be targeted for prophylaxis. Second, the new 5-week window now will include women who deliver up to 41 weeks’ gestation. Given current obstetric practice in the US, delivery beyond 41 weeks is unlikely.5
At the present time, the best test for identification of GBS colonization is bacteriologic culture. A cotton swab is placed into the lower third of the vagina, streaked along the perineum, and then placed into the rectum. The swab is withdrawn, placed in a culturette tube, and transported to the laboratory. In the laboratory, the swab is cultured for approximately 24 hours in a nutrient broth and then subcultured on a selective blood agar plate. Failure to sample both the vagina and rectum or failure to use selective broth and selective blood agar will reduce the yield of positive cultures by approximately 50%.5-7
In recent years, researchers have become interested in the use of rapid nucleic acid amplification tests for the identification of GBS. These tests perform well if the test protocol provides for an 18- to 24-hour incubation in nutrient broth prior to application of the nucleic acid probe. When the tests are performed without this enrichment phase, sensitivities are inferior to those associated with bacteriologic culture. In addition, because the rapid tests do not isolate the organisms, they do not allow for antibiotic sensitivity testing.5-7
Continue to: “If I test positive for GBS, how and when will you treat me?”...
4. “If I test positive for GBS, how and when will you treat me?”
The current ACOG guidelines recommend that all colonized women be treated intrapartum with prophylactic antibiotics regardless of whether risk factors are present. Treatment should be started at the time of admission and continued until the infant is delivered.5
The drugs of choice for intrapartum prophylaxis are intravenous penicillin or ampicillin. If the patient has a mild allergy to penicillin, cefazolin is the appropriate alternative. If the patient has a severe allergy to penicillin, the 2 options are vancomycin or clindamycin. If the latter drug is used, the laboratory must perform sensitivity testing because 13% to 20% of strains of GBS may be resistant to clindamycin. The frequency of resistance to erythromycin now ranges from 25% to 32%. Thus, erythromycin is no longer used for intrapartum prophylaxis.5-7,9
The appropriate intravenous dosages of these antibiotics are listed in the TABLE.5 The new ACOG guidelines have revised the previous recommendations for dosing of penicillin, eliminating the 2.5 million-unit dose. They also have revised the dosing recommendations for vancomyin, eliminating the previous recommendation of 1 g every 12 hours.5 The new recommendations regarding vancomycin are particularly important and are based, at least in part, on an interesting report from Onwuchuruba and colleagues.10 These authors studied maternal and cord blood concentrations of vancomycin in mother-infant dyads receiving either the original recommended dosage of vancomycin (1 g every 12 hours) or a dosage of 15 to 20 mg/kg every 8 hours. With standard dosing, only 9% of neonates had therapeutic vancomycin serum concentrations at delivery. With the 20 mg/kg dose of vancomycin, the percent of neonates with therapeutic serum concentrations of vancomycin increased to 80%.

5. “For how long must I be treated in labor before my baby will be protected by the antibiotics?”
The current ACOG Committee Opinion stresses the importance of treating the colonized mother for at least 4 hours prior to delivery.5 This recommendation is based primarily on the landmark report by De Cueto and colleagues.11 These authors evaluated colonized women who received intrapartum prophylaxis at varying times prior to delivery. Their primary endpoint was the percentage of newborns who were colonized with GBS. If the mothers had received antibiotics for less than 1 hour prior to delivery, 46% of neonates were colonized. This figure was equal to the rate of colonization in neonates whose mothers received no antibiotics. When the interval was 1 to 2 hours, the percentage was 29%. When mothers had received antibiotics for 2 to 4 hours, the neonatal colonization rate fell to 2.9%. When antibiotics had been administered for greater than 4 hours, the rate of neonatal colonization was only 1.2%.

Fairlie and colleagues recently reported the results of another interesting investigation comparing the effectiveness of prophylaxis based on duration of treatment and choice of individual antibiotics.12 Prophylaxis with penicillin or ampicillin for 4 hours or more was 91% effective in preventing early-onset neonatal infection in term infants and 86% effective in preventing infection in preterm infants. These outcomes were superior to the outcomes in both term and preterm infants who received penicillin or ampicillin for less than 4 hours.

These observations agree with the findings of McNanley and colleagues who evaluated vaginal colony counts of GBS following different periods of antibiotic administration.13 These authors noted that mean colony counts decreased 5-fold within 2 hours of penicillin administration, 50-fold within 4 hours, and 1,000-fold within 6 hours.
Despite these compelling findings, the ACOG Committee Opinion stresses that obstetric interventions such as amniotomy and oxytocin augmentation should not be delayed simply to permit a certain time period of antibiotic administration.5
Continue to: “If I were to have a scheduled CD before the onset of labor and/or ruptured membranes, would I still need to receive antibiotics?”...
6. “If I were to have a scheduled CD before the onset of labor and/or ruptured membranes, would I still need to receive antibiotics?”
If a mother is scheduled to have a CD, for example because of a prior cesarean or because of a persistent fetal malpresentation, she should still have a GBS culture at 36 0/7 to 37 6/7 weeks’ gestation. The information obtained from this culture may be of value to both the obstetrician and pediatrician if the patient experiences labor or rupture of membranes prior to her scheduled surgery. If she does not experience spontaneous labor prior to her scheduled date of surgery, she does not require specific GBS prophylaxis at the time of her operation.5 Rather, she should receive prophylactic antibiotics to prevent post–cesarean infection, ideally, the combination of cefazolin (2 g IV) plus azithromycin (500 mg IV).14 Cefazolin, of course, provides excellent coverage of GBS.
7. “If I am colonized with GBS and I receive treatment during labor, will my baby be safe after delivery?”
The interventions outlined above will prevent almost 90% of early-onset GBS infections, but they are not foolproof.5-7,15,16 Successful management of the neonate is dependent upon several factors, including:5-7
- gestational age
- presence of maternal chorioamnionitis
- presence or absence of risk factors for early-onset infection
- duration (adequacy) of maternal treatment during labor
- presence of immediate clinical signs of infection in the neonate (such as fever, lethargy, hemodynamic instability, respiratory distress, or elevated or decreased white blood cell count).
If the mother is at term and receives intrapartum prophylaxis for at least 4 hours prior to delivery, the neonate usually will not require any special tests and simply will be observed for 24 to 48 hours for signs of infection.
If the mother delivers preterm and receives appropriate intrapartum prophylaxis, the pediatricians typically will obtain a complete blood count (CBC) and treat with prophylactic antibiotics (ampicillin plus gentamicin) for 48 hours if abnormalities are noted on the CBC or the baby exhibits signs of infection. If the CBC is normal and the baby shows no signs of infection, no treatment is indicated.
Regardless of gestational age, if the mother does not receive prophylaxis for at least 4 hours before delivery, the pediatricians usually will obtain a CBC and closely observe the baby in the hospital for signs of infection. If such signs develop or the CBC is abnormal, blood and cerebrospinal fluid cultures will be obtained. Antibiotic therapy (usually ampicillin plus gentamicin) is then initiated, and the drugs are continued until cultures return with no growth. If either culture is positive, antibiotics will then be continued for 7 to 10 days.
If the mother has documented chorioamnionitis and receives treatment intrapartum with appropriate antibiotics (usually ampicillin plus gentamicin), the pediatricians usually will obtain a CBC, C-reactive protein (CRP) level, and blood cultures and then start the infant on antibiotics, pending the result of the laboratory tests. If the CBC and CRP are reassuring, the cultures are negative after 48 hours, and the infant demonstrates no signs of clinical infection, many pediatricians will then discontinue antibiotics. Others may still continue the antibiotics for 7 to 10 days.
In 1992, the American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics (AAP) published their first joint guidelines on the prevention of early-onset neonatal group B streptococcal (GBS) infection.1 In this initial statement, the organizations recommended universal culturing of obstetric patients at 28 weeks’ gestation and treatment of colonized women during labor if they had a recognized risk factor for neonatal GBS infection.
In 1996, the Centers for Disease Control and Prevention (CDC) published its first set of official guidelines on the topic and suggested that both universal screening and a risk-factor–based approach were reasonable options.2 The 2002 update of the CDC guidelines strongly recommended universal screening of all pregnant women at 35 to 37 weeks’ gestation and intrapartum prophylaxis for all colonized women regardless of risk factors.3
The third set of CDC guidelines was published in 2010.4 The key features of this version were the elimination of erythromycin as an alternative to penicillin in patients who are allergic to beta-lactam antibiotics and the establishment of 4 hours as the critical interval for administration of prophylaxis prior to delivery. The 2010 publication was the last such report from the CDC. Since then ACOG and AAP have been tasked with providing updated practice guidelines. To that end, ACOG recently issued a new Committee Opinion on “Prevention of Group B Streptococcal Early-Onset Disease in Newborns.”5 Here we will highlight the key features of our current strategy for preventing neonatal GBS infection.
CASE Pregnant patient presents with many questions about GBS
A 26-year-old primigravid woman presents for her first prenatal appointment at 9 weeks’ gestation. Her older sister recently delivered a term infant that died in the first week of life from GBS sepsis. Understandably, she has many questions.
1. Your patient first wants to know, “What is this streptococcal organism and how likely am I to have this infection?”
Streptococcus agalactiae, also known as GBS, is a gram-positive encapsulated bacterium that produces beta hemolysis when grown on blood agar. Approximately 25% of pregnant women harbor this organism in the lower genital tract and/or rectum.6
GBS is one of the most important causes of neonatal infection, particularly in preterm infants. The frequency of infection is now 0.23 per 1,000 live births in the US.5
Neonatal infection can be divided into early-onset infection (occurring within the first 7 days of life) and late-onset infection (occurring from after the first week until the third month of life). Approximately 80% to 85% of cases of neonatal GBS infections are early in onset. Virtually all of the early-onset infections result from vertical transmission during delivery from a colonized mother to her infant.5-7
2. “How dangerous is this infection to my baby and me? Are there certain factors that increase the risk of my baby becoming infected?”
GBS is responsible for approximately 2% to 3% of cases of either asymptomatic bacteriuria or acute cystitis. Women with urinary tract infections caused by GBS are at increased risk for preterm premature rupture of membranes and preterm delivery. Genital tract colonization also increases a woman’s risk for chorioamnionitis and endometritis, particularly after cesarean delivery (CD). In addition, GBS can be part of the polymicrobial flora in women who have a wound (incisional site) infection following CD.6,7
Continue to: In colonized women, several risk factors...
In colonized women, several risk factors have been identified that increase the probability of early-onset neonatal GBS infection. These factors include: preterm labor, especially when complicated by premature rupture of membranes; intrapartum maternal fever (usually due to chorioamnionitis); rupture of membranes greater than 18 hours before delivery; previous delivery of an infected infant; young age; and black or Hispanic ethnicity. Approximately 25% of colonized women will have one of these risk factors.5-7
These risk factors have a profound impact on neonatal attack rates and mortality. Without the interventions outlined below, the neonatal infection rate is 40% to 50% in the presence of a risk factor and less than 5% in the absence of a risk factor. In infected infants, neonatal mortality approaches 30% to 35% when a maternal risk factor is present, but is less than 5% when risk factors are absent.5-7
3. “What will you do to determine if I am colonized with this organism?”
The current guidelines set forth in the ACOG Committee Opinion recommend that selected high-risk patients (patients with preterm labor or preterm premature rupture of membranes) be tested for GBS at the time of initial presentation. All other women should be tested for GBS during the interval 36 0/7 to 37 6/7 weeks’ gestation.5 Testing at this point in pregnancy is almost 90% sensitive for identifying patients who will be colonized at the time of admission for labor if no more than 5 weeks elapse between the time the culture is obtained and labor begins. The positive predictive value of this test is 87%, and the negative predictive value is 96%.8
ACOG’s previous guidelines provided for testing at 35 rather than 36 weeks. The change in the recommendations was based on 2 factors. First, all women with unknown GBS status who may deliver before 37 weeks already should be targeted for prophylaxis. Second, the new 5-week window now will include women who deliver up to 41 weeks’ gestation. Given current obstetric practice in the US, delivery beyond 41 weeks is unlikely.5
At the present time, the best test for identification of GBS colonization is bacteriologic culture. A cotton swab is placed into the lower third of the vagina, streaked along the perineum, and then placed into the rectum. The swab is withdrawn, placed in a culturette tube, and transported to the laboratory. In the laboratory, the swab is cultured for approximately 24 hours in a nutrient broth and then subcultured on a selective blood agar plate. Failure to sample both the vagina and rectum or failure to use selective broth and selective blood agar will reduce the yield of positive cultures by approximately 50%.5-7
In recent years, researchers have become interested in the use of rapid nucleic acid amplification tests for the identification of GBS. These tests perform well if the test protocol provides for an 18- to 24-hour incubation in nutrient broth prior to application of the nucleic acid probe. When the tests are performed without this enrichment phase, sensitivities are inferior to those associated with bacteriologic culture. In addition, because the rapid tests do not isolate the organisms, they do not allow for antibiotic sensitivity testing.5-7
Continue to: “If I test positive for GBS, how and when will you treat me?”...
4. “If I test positive for GBS, how and when will you treat me?”
The current ACOG guidelines recommend that all colonized women be treated intrapartum with prophylactic antibiotics regardless of whether risk factors are present. Treatment should be started at the time of admission and continued until the infant is delivered.5
The drugs of choice for intrapartum prophylaxis are intravenous penicillin or ampicillin. If the patient has a mild allergy to penicillin, cefazolin is the appropriate alternative. If the patient has a severe allergy to penicillin, the 2 options are vancomycin or clindamycin. If the latter drug is used, the laboratory must perform sensitivity testing because 13% to 20% of strains of GBS may be resistant to clindamycin. The frequency of resistance to erythromycin now ranges from 25% to 32%. Thus, erythromycin is no longer used for intrapartum prophylaxis.5-7,9
The appropriate intravenous dosages of these antibiotics are listed in the TABLE.5 The new ACOG guidelines have revised the previous recommendations for dosing of penicillin, eliminating the 2.5 million-unit dose. They also have revised the dosing recommendations for vancomyin, eliminating the previous recommendation of 1 g every 12 hours.5 The new recommendations regarding vancomycin are particularly important and are based, at least in part, on an interesting report from Onwuchuruba and colleagues.10 These authors studied maternal and cord blood concentrations of vancomycin in mother-infant dyads receiving either the original recommended dosage of vancomycin (1 g every 12 hours) or a dosage of 15 to 20 mg/kg every 8 hours. With standard dosing, only 9% of neonates had therapeutic vancomycin serum concentrations at delivery. With the 20 mg/kg dose of vancomycin, the percent of neonates with therapeutic serum concentrations of vancomycin increased to 80%.

5. “For how long must I be treated in labor before my baby will be protected by the antibiotics?”
The current ACOG Committee Opinion stresses the importance of treating the colonized mother for at least 4 hours prior to delivery.5 This recommendation is based primarily on the landmark report by De Cueto and colleagues.11 These authors evaluated colonized women who received intrapartum prophylaxis at varying times prior to delivery. Their primary endpoint was the percentage of newborns who were colonized with GBS. If the mothers had received antibiotics for less than 1 hour prior to delivery, 46% of neonates were colonized. This figure was equal to the rate of colonization in neonates whose mothers received no antibiotics. When the interval was 1 to 2 hours, the percentage was 29%. When mothers had received antibiotics for 2 to 4 hours, the neonatal colonization rate fell to 2.9%. When antibiotics had been administered for greater than 4 hours, the rate of neonatal colonization was only 1.2%.

Fairlie and colleagues recently reported the results of another interesting investigation comparing the effectiveness of prophylaxis based on duration of treatment and choice of individual antibiotics.12 Prophylaxis with penicillin or ampicillin for 4 hours or more was 91% effective in preventing early-onset neonatal infection in term infants and 86% effective in preventing infection in preterm infants. These outcomes were superior to the outcomes in both term and preterm infants who received penicillin or ampicillin for less than 4 hours.

These observations agree with the findings of McNanley and colleagues who evaluated vaginal colony counts of GBS following different periods of antibiotic administration.13 These authors noted that mean colony counts decreased 5-fold within 2 hours of penicillin administration, 50-fold within 4 hours, and 1,000-fold within 6 hours.
Despite these compelling findings, the ACOG Committee Opinion stresses that obstetric interventions such as amniotomy and oxytocin augmentation should not be delayed simply to permit a certain time period of antibiotic administration.5
Continue to: “If I were to have a scheduled CD before the onset of labor and/or ruptured membranes, would I still need to receive antibiotics?”...
6. “If I were to have a scheduled CD before the onset of labor and/or ruptured membranes, would I still need to receive antibiotics?”
If a mother is scheduled to have a CD, for example because of a prior cesarean or because of a persistent fetal malpresentation, she should still have a GBS culture at 36 0/7 to 37 6/7 weeks’ gestation. The information obtained from this culture may be of value to both the obstetrician and pediatrician if the patient experiences labor or rupture of membranes prior to her scheduled surgery. If she does not experience spontaneous labor prior to her scheduled date of surgery, she does not require specific GBS prophylaxis at the time of her operation.5 Rather, she should receive prophylactic antibiotics to prevent post–cesarean infection, ideally, the combination of cefazolin (2 g IV) plus azithromycin (500 mg IV).14 Cefazolin, of course, provides excellent coverage of GBS.
7. “If I am colonized with GBS and I receive treatment during labor, will my baby be safe after delivery?”
The interventions outlined above will prevent almost 90% of early-onset GBS infections, but they are not foolproof.5-7,15,16 Successful management of the neonate is dependent upon several factors, including:5-7
- gestational age
- presence of maternal chorioamnionitis
- presence or absence of risk factors for early-onset infection
- duration (adequacy) of maternal treatment during labor
- presence of immediate clinical signs of infection in the neonate (such as fever, lethargy, hemodynamic instability, respiratory distress, or elevated or decreased white blood cell count).
If the mother is at term and receives intrapartum prophylaxis for at least 4 hours prior to delivery, the neonate usually will not require any special tests and simply will be observed for 24 to 48 hours for signs of infection.
If the mother delivers preterm and receives appropriate intrapartum prophylaxis, the pediatricians typically will obtain a complete blood count (CBC) and treat with prophylactic antibiotics (ampicillin plus gentamicin) for 48 hours if abnormalities are noted on the CBC or the baby exhibits signs of infection. If the CBC is normal and the baby shows no signs of infection, no treatment is indicated.
Regardless of gestational age, if the mother does not receive prophylaxis for at least 4 hours before delivery, the pediatricians usually will obtain a CBC and closely observe the baby in the hospital for signs of infection. If such signs develop or the CBC is abnormal, blood and cerebrospinal fluid cultures will be obtained. Antibiotic therapy (usually ampicillin plus gentamicin) is then initiated, and the drugs are continued until cultures return with no growth. If either culture is positive, antibiotics will then be continued for 7 to 10 days.
If the mother has documented chorioamnionitis and receives treatment intrapartum with appropriate antibiotics (usually ampicillin plus gentamicin), the pediatricians usually will obtain a CBC, C-reactive protein (CRP) level, and blood cultures and then start the infant on antibiotics, pending the result of the laboratory tests. If the CBC and CRP are reassuring, the cultures are negative after 48 hours, and the infant demonstrates no signs of clinical infection, many pediatricians will then discontinue antibiotics. Others may still continue the antibiotics for 7 to 10 days.
- Committee on Infectious Diseases and Committee on Fetus and Newborn. Guidelines for prevention of group B streptococcal (GBS) infection by chemoprophylaxis. Pediatrics. 1992;90:775-778.
- CDC. Prevention of perinatal group B streptococcal disease: a public health perspective. MMWR Recomm Rep. 1996;45(RR-7):1-24.
- Schrag S, Gorwitz R, Fultz-Butts K, et al. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51(RR-11):1-22.
- Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 2010;59:1-36.
- Prevention of group B streptococcal early-onset disease in newborns. ACOG Committee Opinion Summary, Number 782. Obstet Gynecol. 2019;134:206-210.
- Duff P, Birsner M. Maternal and perinatal infection in pregnancy: bacteria. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics. Normal and Problem Pregnancies. 7th ed. Philadelphia, PA: Elsevier; 2017.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019.
- Yancey MK, Schuchat A, Brown LK, et al. The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet Gynecol. 1996;88:811-815.
- Edwards RK, Clark P, Duff P. Intrapartum antibiotic prophylaxis 2: positive predictive value of antenatal group B streptococci cultures and antibiotic susceptibility of clinical isolates. Obstet Gynecol. 2002;100:540-544.
- Onwuchuruba CN, Towers CV, Howard BC, et al. Transplacental passage of vancomycin from mother to neonate. Am J Obstet Gynecol. 2014;210:352.e1-352.e4.
- de Cueto M, Sanchez MJ, Sampedro A, et al. Timing of intrapartum ampicillin and prevention of vertical transmission of group B streptococcus. Obstet Gynecol. 1998;91:112-114.
- Fairlie T, Zell ER, Schrag S. Effectiveness of intrapartum antibiotic prophylaxis for prevention of early-onset group B streptococcal disease. Obstet Gynecol. 2013;121:570-577.
- McNanley AR, Glantz JC, Hardy DJ, et al. The effect of intrapartum penicillin on vaginal group B streptococcus colony counts. Am J Obstet Gynecol. 2007;197:583.e1-583.e4.
- Tita AT, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
- Brozanski BS, Jones JG, Krohn MA, et al. Effect of a screening-based prevention policy on prevalence of early-onset group B streptococcal sepsis. Obstet Gynecol. 2000;95:496-501.
- Rosenstein NE, Schuchat A. Opportunities for prevention of perinatal group B streptococcal disease: a multistate surveillance analysis. The National Group B Streptococcal Disease Study Group. Obstet Gynecol. 1997;90:901-906.
- Committee on Infectious Diseases and Committee on Fetus and Newborn. Guidelines for prevention of group B streptococcal (GBS) infection by chemoprophylaxis. Pediatrics. 1992;90:775-778.
- CDC. Prevention of perinatal group B streptococcal disease: a public health perspective. MMWR Recomm Rep. 1996;45(RR-7):1-24.
- Schrag S, Gorwitz R, Fultz-Butts K, et al. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51(RR-11):1-22.
- Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). MMWR Recomm Rep. 2010;59:1-36.
- Prevention of group B streptococcal early-onset disease in newborns. ACOG Committee Opinion Summary, Number 782. Obstet Gynecol. 2019;134:206-210.
- Duff P, Birsner M. Maternal and perinatal infection in pregnancy: bacteria. In: Gabbe SG, Niebyl JR, Simpson JL, et al, eds. Obstetrics. Normal and Problem Pregnancies. 7th ed. Philadelphia, PA: Elsevier; 2017.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019.
- Yancey MK, Schuchat A, Brown LK, et al. The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet Gynecol. 1996;88:811-815.
- Edwards RK, Clark P, Duff P. Intrapartum antibiotic prophylaxis 2: positive predictive value of antenatal group B streptococci cultures and antibiotic susceptibility of clinical isolates. Obstet Gynecol. 2002;100:540-544.
- Onwuchuruba CN, Towers CV, Howard BC, et al. Transplacental passage of vancomycin from mother to neonate. Am J Obstet Gynecol. 2014;210:352.e1-352.e4.
- de Cueto M, Sanchez MJ, Sampedro A, et al. Timing of intrapartum ampicillin and prevention of vertical transmission of group B streptococcus. Obstet Gynecol. 1998;91:112-114.
- Fairlie T, Zell ER, Schrag S. Effectiveness of intrapartum antibiotic prophylaxis for prevention of early-onset group B streptococcal disease. Obstet Gynecol. 2013;121:570-577.
- McNanley AR, Glantz JC, Hardy DJ, et al. The effect of intrapartum penicillin on vaginal group B streptococcus colony counts. Am J Obstet Gynecol. 2007;197:583.e1-583.e4.
- Tita AT, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
- Brozanski BS, Jones JG, Krohn MA, et al. Effect of a screening-based prevention policy on prevalence of early-onset group B streptococcal sepsis. Obstet Gynecol. 2000;95:496-501.
- Rosenstein NE, Schuchat A. Opportunities for prevention of perinatal group B streptococcal disease: a multistate surveillance analysis. The National Group B Streptococcal Disease Study Group. Obstet Gynecol. 1997;90:901-906.