User login
In the past year, there has been significant progress in the availability of interventions to prevent respiratory syncytial virus (RSV) and its complications. Four products have been approved by the US Food and Drug Administration (FDA) and recommended by the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP). They include 2 vaccines for adults ages 60 years and older, a monoclonal antibody for infants and high-risk children, and a maternal vaccine to prevent RSV infection in newborns.
RSV in adults
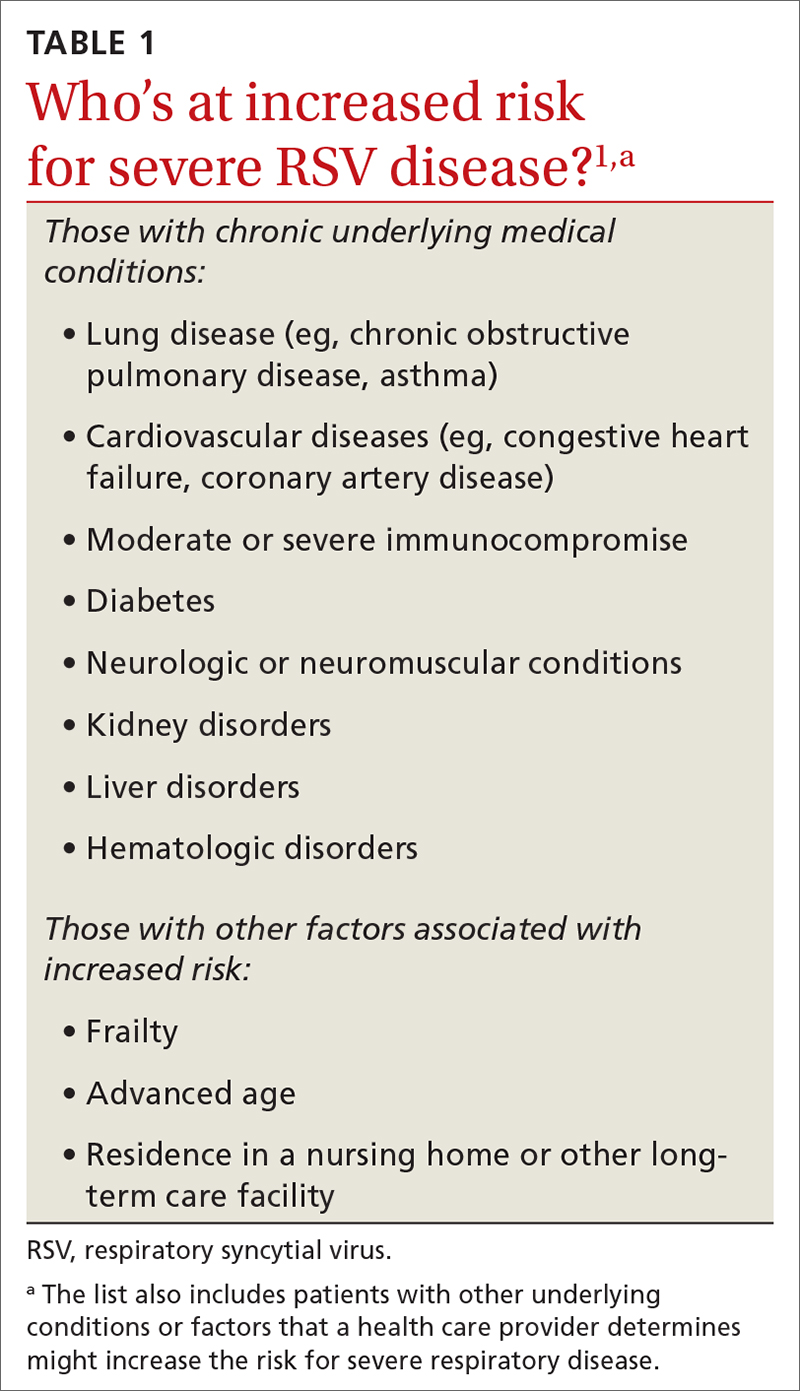
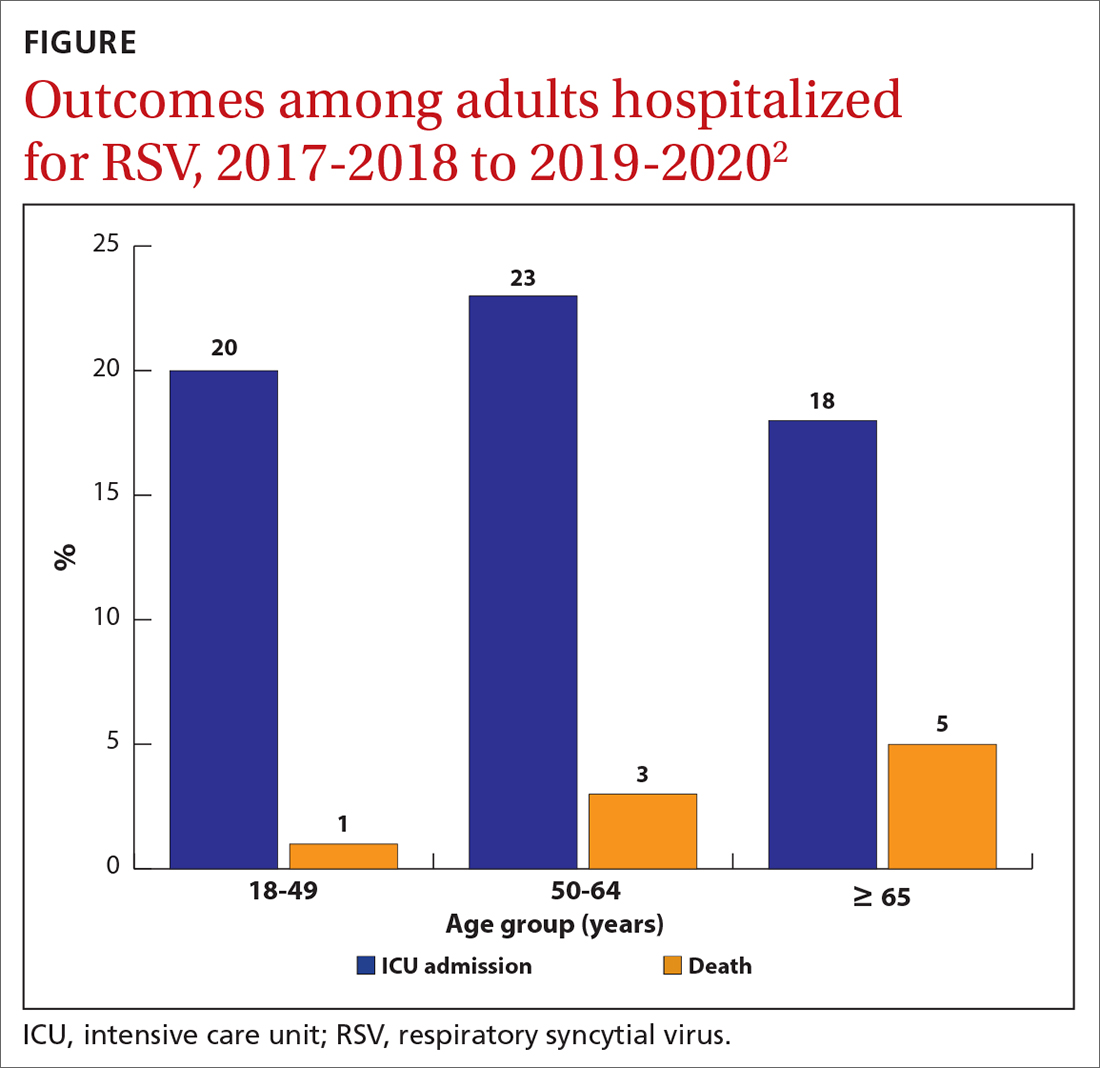
While there is some uncertainty about the total burden of RSV in adults in the United States, the CDC estimates that each year it causes 0.9 to 1.4 million medical encounters, 60,000 to 160,000 hospitalizations, and 6000 to 10,000 deaths.1 The rate of RSV-caused hospitalization increases with age,2 and the infection is more severe in those with certain chronic medical conditions (TABLE 11). The FIGURE2 demonstrates the outcomes of adults who are hospitalized for RSV. Adults older than 65 years have a 5% mortality rate if hospitalized for RSV infection.2
Vaccine options for adults
Two vaccines were recently approved for the prevention of RSV-associated lower respiratory tract disease (LRTD) in those ages 60 years and older: RSVPreF3 (Arexvy, GSK), which is an adjuvanted recombinant F protein vaccine, and RSVpreF (Abrysvo, Pfizer), which is a recombinant stabilized vaccine. Both require only a single dose (0.5 mL IM), which provides protection for 2 years.
The efficacy of the GSK vaccine in preventing laboratory-confirmed, RSV-associated LRTD was 82.6% during the first RSV season and 56.1% during the second season. The efficacy of the Pfizer vaccine in preventing symptomatic, laboratory-confirmed LRTD was 88.9% during the first RSV season and 78.6% during the second season.1 However, the trials leading to licensure of both vaccines were underpowered to show efficacy in the oldest adults and those who are frail or to show efficacy against RSV-caused hospitalization.
Safety of the adult RSV vaccines. The safety trials for both vaccines had a total of 38,177 participants. There were a total of 6 neurologic inflammatory conditions that developed within 42 days of vaccination, including 2 cases of suspected Guillain-Barré syndrome (GBS), 2 cases of possible acute disseminated encephalomyelitis, and 1 case each of chronic inflammatory demyelinating polyneuropathy and undifferentiated motor-sensory axonal polyneuropathy.1 That is a rate of 1 case of a neurologic inflammatory condition for every 6363 people vaccinated. Since the trials were not powered to determine whether the small number of cases were due to chance, postmarketing surveillance will be needed to clarify the true risk for GBS or other neurologic inflammatory events from RSV vaccination.
The lack of efficacy data for the most vulnerable older adults and the lingering questions about safety prompted the ACIP to recommend that adults ages 60 years and older may receive a single dose of RSV vaccine, using shared clinical decision-making—which is different from a routine or risk-based vaccine recommendation. For RSV vaccination, the decision to vaccinate should be based on a risk/benefit discussion between the clinician and the patient. Those most likely to benefit from the vaccine are listed in TABLE 1.1
While data on coadministration of RSV vaccines with other adult vaccines are sparse, the ACIP states that co-administration with other vaccines is acceptable.1 It is not known yet whether boosters will be needed after 2 years.
Continue to: RSV in infants and children
RSV in infants and children
RSV is the most common cause of hospitalization among infants and children in the United States. The CDC estimates that each year in children younger than 5 years, RSV is responsible for 1.5 million outpatient clinic visits, 520,000 emergency department visits, 58,000 to 80,000 hospitalizations, and 100 to 200 deaths.3 The risk for hospitalization from RSV is highest in the second and third months of life and decreases with increasing age.3
There are racial disparities in RSV severity: Intensive care unit admission rates are 1.2 to 1.6 times higher among non-Hispanic Black infants younger than 6 months than among non-Hispanic White infants, and hospitalization rates are up to 5 times higher in American Indian and Alaska Native populations.3
The months of highest RSV transmission in most locations are December through February, but this can vary. For practical purposes, RSV season runs from October through March.
Prevention in infants and children
The monoclonal antibody nirsevimab is now available for use in infants younger than 8 months born during or entering their first RSV season and children ages 8 to 19 months who are at increased risk for severe RSV disease and entering their second RSV season. Details regarding the use of this product were described in a recent Practice Alert Brief.4
Early studies on nirsevimab demonstrated 79% effectiveness in preventing medical-attended LRTD, 80.6% effectiveness in preventing hospitalization, and 90% effectiveness in preventing ICU admission. The number needed to immunize with nirsevimab to prevent an outpatient visit is estimated to be 17; to prevent an ED visit, 48; and to prevent an inpatient admission, 128. Due to the low RSV death rate, the studies were not able to demonstrate reduced mortality.5
Continue to: RSV vaccine in pregnancy
RSV vaccine in pregnancy
In August, the FDA approved Pfizer’s RSVpreF vaccine for use during pregnancy—as a single dose given at 32 to 36 weeks’ gestation—for the prevention of RSV LRTD in infants in the first 6 months of life. In the clinical trials, the vaccine was given at 24 to 36 weeks’ gestation. However, there was a statistically nonsignificant increase in preterm births in the RSVpreF group compared to the placebo group.6 While there were insufficient data to prove or rule out a causal relationship, the FDA advisory committee was more comfortable approving the vaccine for use only later in pregnancy, to avoid the possibility of very early preterm births after vaccination. The ACIP agreed.
From time of maternal vaccination, at least 14 days are needed to develop and transfer maternal antibodies across the placenta to protect the infant. Therefore, infants born less than 14 days after maternal vaccination should be considered unprotected.
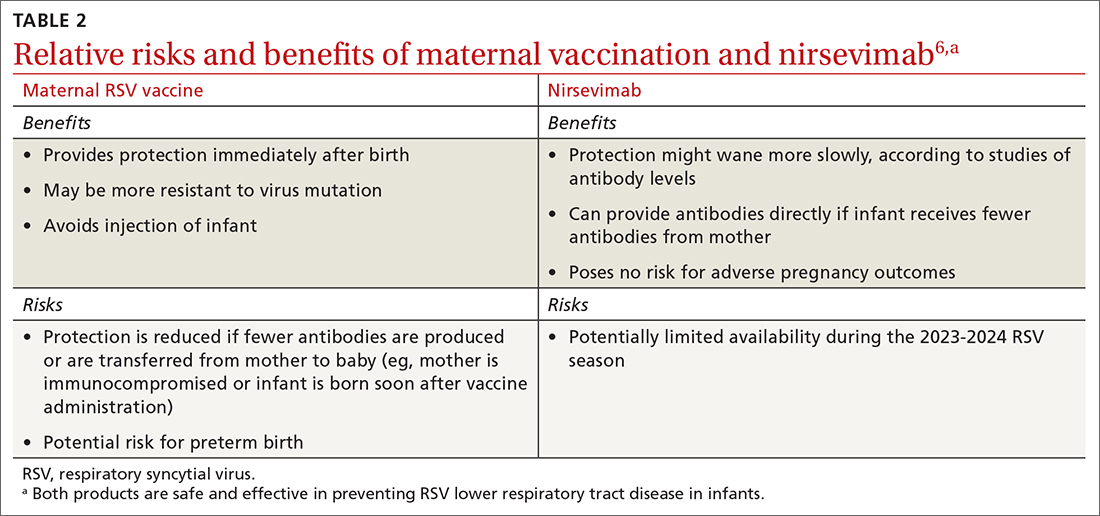
Both maternal vaccination with RSVpreF and infant injection with nirsevimab are now options to protect newborns and infants from RSV. However, use of both products is not needed, since combined they do not offer significant added protection compared to either product alone (exceptions to be discussed shortly).6 When the estimated due date will occur in the RSV season, maternity clinicians should provide information on both products and assist the mother in deciding whether to be vaccinated or rely on administration of nirsevimab to the infant after birth. The benefits and risks of these 2 options are listed in TABLE 2.6
There are some rare situations in which use of both products is recommended, and they include6:
- When the baby is born less than 14 days from the time of maternal vaccination
- When the mother has a condition that could produce an inadequate response to the vaccine
- When the infant has had cardiopulmonary bypass, which would lead to loss of maternal antibodies
- When the infant has severe disease placing them at increased risk for severe RSV.
Conclusion
All of these new RSV preventive products should soon be widely available and covered with no out-of-pocket expense by commercial and government payers. The exception might be nirsevimab—because of the time needed to produce it, it might not be universally available in the 2023-2024 season.
1. Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccine in older adults: recommendation of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72:793-801.
2. Melgar M. Evidence to recommendation framework. RSV in adults. Presented to the ACIP on February 23, 2023. Accessed November 7, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-02/slides-02-23/RSV-Adults-04-Melgar-508.pdf
3. Jones JM, Fleming-Dutra KE, Prill MM, et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: recommendation of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72:90-925.
4. Campos-Outcalt D. Are you ready for RSV season? There’s a new preventive option. J Fam Pract. 2023;72. doi: 10.12788/jfp.0663
5. Jones J. Evidence to recommendation framework: nirsevimab updates. Presented to the ACIP on August 3, 2023. Accessed August 23, 2023. https://stacks.cdc.gov/view/cdc/131586
6. Jones J. Clinical considerations for maternal RSVPreF vaccine and nirsevimab. Presented to the ACIP on September 25, 2023. Accessed November 8, 2023. www2.cdc.gov/vaccines/ed/ciinc/archives/23/09/ciiw_RSV2/CIIW%20RSV%20maternal%20vaccine%20mAb%209.27.23.pdf
In the past year, there has been significant progress in the availability of interventions to prevent respiratory syncytial virus (RSV) and its complications. Four products have been approved by the US Food and Drug Administration (FDA) and recommended by the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP). They include 2 vaccines for adults ages 60 years and older, a monoclonal antibody for infants and high-risk children, and a maternal vaccine to prevent RSV infection in newborns.

RSV in adults
While there is some uncertainty about the total burden of RSV in adults in the United States, the CDC estimates that each year it causes 0.9 to 1.4 million medical encounters, 60,000 to 160,000 hospitalizations, and 6000 to 10,000 deaths.1 The rate of RSV-caused hospitalization increases with age,2 and the infection is more severe in those with certain chronic medical conditions (TABLE 11). The FIGURE2 demonstrates the outcomes of adults who are hospitalized for RSV. Adults older than 65 years have a 5% mortality rate if hospitalized for RSV infection.2

Vaccine options for adults
Two vaccines were recently approved for the prevention of RSV-associated lower respiratory tract disease (LRTD) in those ages 60 years and older: RSVPreF3 (Arexvy, GSK), which is an adjuvanted recombinant F protein vaccine, and RSVpreF (Abrysvo, Pfizer), which is a recombinant stabilized vaccine. Both require only a single dose (0.5 mL IM), which provides protection for 2 years.
The efficacy of the GSK vaccine in preventing laboratory-confirmed, RSV-associated LRTD was 82.6% during the first RSV season and 56.1% during the second season. The efficacy of the Pfizer vaccine in preventing symptomatic, laboratory-confirmed LRTD was 88.9% during the first RSV season and 78.6% during the second season.1 However, the trials leading to licensure of both vaccines were underpowered to show efficacy in the oldest adults and those who are frail or to show efficacy against RSV-caused hospitalization.
Safety of the adult RSV vaccines. The safety trials for both vaccines had a total of 38,177 participants. There were a total of 6 neurologic inflammatory conditions that developed within 42 days of vaccination, including 2 cases of suspected Guillain-Barré syndrome (GBS), 2 cases of possible acute disseminated encephalomyelitis, and 1 case each of chronic inflammatory demyelinating polyneuropathy and undifferentiated motor-sensory axonal polyneuropathy.1 That is a rate of 1 case of a neurologic inflammatory condition for every 6363 people vaccinated. Since the trials were not powered to determine whether the small number of cases were due to chance, postmarketing surveillance will be needed to clarify the true risk for GBS or other neurologic inflammatory events from RSV vaccination.
The lack of efficacy data for the most vulnerable older adults and the lingering questions about safety prompted the ACIP to recommend that adults ages 60 years and older may receive a single dose of RSV vaccine, using shared clinical decision-making—which is different from a routine or risk-based vaccine recommendation. For RSV vaccination, the decision to vaccinate should be based on a risk/benefit discussion between the clinician and the patient. Those most likely to benefit from the vaccine are listed in TABLE 1.1
While data on coadministration of RSV vaccines with other adult vaccines are sparse, the ACIP states that co-administration with other vaccines is acceptable.1 It is not known yet whether boosters will be needed after 2 years.
Continue to: RSV in infants and children
RSV in infants and children
RSV is the most common cause of hospitalization among infants and children in the United States. The CDC estimates that each year in children younger than 5 years, RSV is responsible for 1.5 million outpatient clinic visits, 520,000 emergency department visits, 58,000 to 80,000 hospitalizations, and 100 to 200 deaths.3 The risk for hospitalization from RSV is highest in the second and third months of life and decreases with increasing age.3
There are racial disparities in RSV severity: Intensive care unit admission rates are 1.2 to 1.6 times higher among non-Hispanic Black infants younger than 6 months than among non-Hispanic White infants, and hospitalization rates are up to 5 times higher in American Indian and Alaska Native populations.3
The months of highest RSV transmission in most locations are December through February, but this can vary. For practical purposes, RSV season runs from October through March.
Prevention in infants and children
The monoclonal antibody nirsevimab is now available for use in infants younger than 8 months born during or entering their first RSV season and children ages 8 to 19 months who are at increased risk for severe RSV disease and entering their second RSV season. Details regarding the use of this product were described in a recent Practice Alert Brief.4
Early studies on nirsevimab demonstrated 79% effectiveness in preventing medical-attended LRTD, 80.6% effectiveness in preventing hospitalization, and 90% effectiveness in preventing ICU admission. The number needed to immunize with nirsevimab to prevent an outpatient visit is estimated to be 17; to prevent an ED visit, 48; and to prevent an inpatient admission, 128. Due to the low RSV death rate, the studies were not able to demonstrate reduced mortality.5
Continue to: RSV vaccine in pregnancy
RSV vaccine in pregnancy
In August, the FDA approved Pfizer’s RSVpreF vaccine for use during pregnancy—as a single dose given at 32 to 36 weeks’ gestation—for the prevention of RSV LRTD in infants in the first 6 months of life. In the clinical trials, the vaccine was given at 24 to 36 weeks’ gestation. However, there was a statistically nonsignificant increase in preterm births in the RSVpreF group compared to the placebo group.6 While there were insufficient data to prove or rule out a causal relationship, the FDA advisory committee was more comfortable approving the vaccine for use only later in pregnancy, to avoid the possibility of very early preterm births after vaccination. The ACIP agreed.
From time of maternal vaccination, at least 14 days are needed to develop and transfer maternal antibodies across the placenta to protect the infant. Therefore, infants born less than 14 days after maternal vaccination should be considered unprotected.
Both maternal vaccination with RSVpreF and infant injection with nirsevimab are now options to protect newborns and infants from RSV. However, use of both products is not needed, since combined they do not offer significant added protection compared to either product alone (exceptions to be discussed shortly).6 When the estimated due date will occur in the RSV season, maternity clinicians should provide information on both products and assist the mother in deciding whether to be vaccinated or rely on administration of nirsevimab to the infant after birth. The benefits and risks of these 2 options are listed in TABLE 2.6

There are some rare situations in which use of both products is recommended, and they include6:
- When the baby is born less than 14 days from the time of maternal vaccination
- When the mother has a condition that could produce an inadequate response to the vaccine
- When the infant has had cardiopulmonary bypass, which would lead to loss of maternal antibodies
- When the infant has severe disease placing them at increased risk for severe RSV.
Conclusion
All of these new RSV preventive products should soon be widely available and covered with no out-of-pocket expense by commercial and government payers. The exception might be nirsevimab—because of the time needed to produce it, it might not be universally available in the 2023-2024 season.
In the past year, there has been significant progress in the availability of interventions to prevent respiratory syncytial virus (RSV) and its complications. Four products have been approved by the US Food and Drug Administration (FDA) and recommended by the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP). They include 2 vaccines for adults ages 60 years and older, a monoclonal antibody for infants and high-risk children, and a maternal vaccine to prevent RSV infection in newborns.

RSV in adults
While there is some uncertainty about the total burden of RSV in adults in the United States, the CDC estimates that each year it causes 0.9 to 1.4 million medical encounters, 60,000 to 160,000 hospitalizations, and 6000 to 10,000 deaths.1 The rate of RSV-caused hospitalization increases with age,2 and the infection is more severe in those with certain chronic medical conditions (TABLE 11). The FIGURE2 demonstrates the outcomes of adults who are hospitalized for RSV. Adults older than 65 years have a 5% mortality rate if hospitalized for RSV infection.2

Vaccine options for adults
Two vaccines were recently approved for the prevention of RSV-associated lower respiratory tract disease (LRTD) in those ages 60 years and older: RSVPreF3 (Arexvy, GSK), which is an adjuvanted recombinant F protein vaccine, and RSVpreF (Abrysvo, Pfizer), which is a recombinant stabilized vaccine. Both require only a single dose (0.5 mL IM), which provides protection for 2 years.
The efficacy of the GSK vaccine in preventing laboratory-confirmed, RSV-associated LRTD was 82.6% during the first RSV season and 56.1% during the second season. The efficacy of the Pfizer vaccine in preventing symptomatic, laboratory-confirmed LRTD was 88.9% during the first RSV season and 78.6% during the second season.1 However, the trials leading to licensure of both vaccines were underpowered to show efficacy in the oldest adults and those who are frail or to show efficacy against RSV-caused hospitalization.
Safety of the adult RSV vaccines. The safety trials for both vaccines had a total of 38,177 participants. There were a total of 6 neurologic inflammatory conditions that developed within 42 days of vaccination, including 2 cases of suspected Guillain-Barré syndrome (GBS), 2 cases of possible acute disseminated encephalomyelitis, and 1 case each of chronic inflammatory demyelinating polyneuropathy and undifferentiated motor-sensory axonal polyneuropathy.1 That is a rate of 1 case of a neurologic inflammatory condition for every 6363 people vaccinated. Since the trials were not powered to determine whether the small number of cases were due to chance, postmarketing surveillance will be needed to clarify the true risk for GBS or other neurologic inflammatory events from RSV vaccination.
The lack of efficacy data for the most vulnerable older adults and the lingering questions about safety prompted the ACIP to recommend that adults ages 60 years and older may receive a single dose of RSV vaccine, using shared clinical decision-making—which is different from a routine or risk-based vaccine recommendation. For RSV vaccination, the decision to vaccinate should be based on a risk/benefit discussion between the clinician and the patient. Those most likely to benefit from the vaccine are listed in TABLE 1.1
While data on coadministration of RSV vaccines with other adult vaccines are sparse, the ACIP states that co-administration with other vaccines is acceptable.1 It is not known yet whether boosters will be needed after 2 years.
Continue to: RSV in infants and children
RSV in infants and children
RSV is the most common cause of hospitalization among infants and children in the United States. The CDC estimates that each year in children younger than 5 years, RSV is responsible for 1.5 million outpatient clinic visits, 520,000 emergency department visits, 58,000 to 80,000 hospitalizations, and 100 to 200 deaths.3 The risk for hospitalization from RSV is highest in the second and third months of life and decreases with increasing age.3
There are racial disparities in RSV severity: Intensive care unit admission rates are 1.2 to 1.6 times higher among non-Hispanic Black infants younger than 6 months than among non-Hispanic White infants, and hospitalization rates are up to 5 times higher in American Indian and Alaska Native populations.3
The months of highest RSV transmission in most locations are December through February, but this can vary. For practical purposes, RSV season runs from October through March.
Prevention in infants and children
The monoclonal antibody nirsevimab is now available for use in infants younger than 8 months born during or entering their first RSV season and children ages 8 to 19 months who are at increased risk for severe RSV disease and entering their second RSV season. Details regarding the use of this product were described in a recent Practice Alert Brief.4
Early studies on nirsevimab demonstrated 79% effectiveness in preventing medical-attended LRTD, 80.6% effectiveness in preventing hospitalization, and 90% effectiveness in preventing ICU admission. The number needed to immunize with nirsevimab to prevent an outpatient visit is estimated to be 17; to prevent an ED visit, 48; and to prevent an inpatient admission, 128. Due to the low RSV death rate, the studies were not able to demonstrate reduced mortality.5
Continue to: RSV vaccine in pregnancy
RSV vaccine in pregnancy
In August, the FDA approved Pfizer’s RSVpreF vaccine for use during pregnancy—as a single dose given at 32 to 36 weeks’ gestation—for the prevention of RSV LRTD in infants in the first 6 months of life. In the clinical trials, the vaccine was given at 24 to 36 weeks’ gestation. However, there was a statistically nonsignificant increase in preterm births in the RSVpreF group compared to the placebo group.6 While there were insufficient data to prove or rule out a causal relationship, the FDA advisory committee was more comfortable approving the vaccine for use only later in pregnancy, to avoid the possibility of very early preterm births after vaccination. The ACIP agreed.
From time of maternal vaccination, at least 14 days are needed to develop and transfer maternal antibodies across the placenta to protect the infant. Therefore, infants born less than 14 days after maternal vaccination should be considered unprotected.
Both maternal vaccination with RSVpreF and infant injection with nirsevimab are now options to protect newborns and infants from RSV. However, use of both products is not needed, since combined they do not offer significant added protection compared to either product alone (exceptions to be discussed shortly).6 When the estimated due date will occur in the RSV season, maternity clinicians should provide information on both products and assist the mother in deciding whether to be vaccinated or rely on administration of nirsevimab to the infant after birth. The benefits and risks of these 2 options are listed in TABLE 2.6

There are some rare situations in which use of both products is recommended, and they include6:
- When the baby is born less than 14 days from the time of maternal vaccination
- When the mother has a condition that could produce an inadequate response to the vaccine
- When the infant has had cardiopulmonary bypass, which would lead to loss of maternal antibodies
- When the infant has severe disease placing them at increased risk for severe RSV.
Conclusion
All of these new RSV preventive products should soon be widely available and covered with no out-of-pocket expense by commercial and government payers. The exception might be nirsevimab—because of the time needed to produce it, it might not be universally available in the 2023-2024 season.
1. Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccine in older adults: recommendation of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72:793-801.
2. Melgar M. Evidence to recommendation framework. RSV in adults. Presented to the ACIP on February 23, 2023. Accessed November 7, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-02/slides-02-23/RSV-Adults-04-Melgar-508.pdf
3. Jones JM, Fleming-Dutra KE, Prill MM, et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: recommendation of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72:90-925.
4. Campos-Outcalt D. Are you ready for RSV season? There’s a new preventive option. J Fam Pract. 2023;72. doi: 10.12788/jfp.0663
5. Jones J. Evidence to recommendation framework: nirsevimab updates. Presented to the ACIP on August 3, 2023. Accessed August 23, 2023. https://stacks.cdc.gov/view/cdc/131586
6. Jones J. Clinical considerations for maternal RSVPreF vaccine and nirsevimab. Presented to the ACIP on September 25, 2023. Accessed November 8, 2023. www2.cdc.gov/vaccines/ed/ciinc/archives/23/09/ciiw_RSV2/CIIW%20RSV%20maternal%20vaccine%20mAb%209.27.23.pdf
1. Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccine in older adults: recommendation of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72:793-801.
2. Melgar M. Evidence to recommendation framework. RSV in adults. Presented to the ACIP on February 23, 2023. Accessed November 7, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-02/slides-02-23/RSV-Adults-04-Melgar-508.pdf
3. Jones JM, Fleming-Dutra KE, Prill MM, et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: recommendation of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72:90-925.
4. Campos-Outcalt D. Are you ready for RSV season? There’s a new preventive option. J Fam Pract. 2023;72. doi: 10.12788/jfp.0663
5. Jones J. Evidence to recommendation framework: nirsevimab updates. Presented to the ACIP on August 3, 2023. Accessed August 23, 2023. https://stacks.cdc.gov/view/cdc/131586
6. Jones J. Clinical considerations for maternal RSVPreF vaccine and nirsevimab. Presented to the ACIP on September 25, 2023. Accessed November 8, 2023. www2.cdc.gov/vaccines/ed/ciinc/archives/23/09/ciiw_RSV2/CIIW%20RSV%20maternal%20vaccine%20mAb%209.27.23.pdf