User login
The last decade has witnessed important technological advances in the diagnosis of osteoporosis and an increase in therapeutic options. However, there is still considerable uncertainty about optimal strategies for screening and primary preventive treatment.
In 1994, a World Health Organization working group proposed that the diagnosis of osteoporosis be made when BMD, assessed by a dual-energy x-ray absorptiometry (DXA), is at least 2.5 standard deviations below the mean for young adult women (T-score) at the spine, hip, or wrist, or when a history of a traumatic fracture is present.2 A T-score between −1 and −2.5 is designated as osteopenia.
Osteoporosis is defined as “a skeletal disorder characterized by compromised bone strength predisposing to an increased risk of fracture.”1 While no accurate overall measurement of bone strength exists, bone mineral density (BMD) is frequently used as a proxy.
These facts underscore the importance of osteoporotic fractures:
- Only one third of patients regain their prior level of functioning after hip fracture, and one third are discharged to nursing homes.3
- About 1 in 5 patients dies within a year after a hip fracture.
- Vertebral fracture may result in chronic back pain and disability.4
- Existence of fracture greatly increases risk of subsequent fracture.5
- Direct medical costs for osteoporotic fractures are estimated at $13.8 billion in 1995 dollars.6
Prevalence of osteoporosis and fractures
Of American women over age 50 of all races, an estimated 15%, or 5 million, have osteoporosis (based on DXA T-score at the femoral neck) and an additional 40%, or 14 million, have osteopenia.7 In African Americans, the prevalence is about half that of whites.8 The prevalence of osteoporosis assessed by BMD testing increases with age—from 4% of white women aged 50 to 59 to 48% of women aged 80 to 89.9
At least 1 vertebral fracture, as indicated by radiographic criteria, has occurred in 5% of white women aged 50 to 59, and in 25% at age 80.3 The lifetime risk of hip fracture for 50-year-old white women and men is 14% and 5%, respectively; for African American women and men, 6% and 3%, respectively.3 Hip and symptomatic vertebral fractures occur mainly in women over 75,3,10 and the risk for wrist fractures increases starting in the late 50s.11
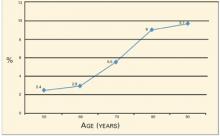
Age is a particularly important risk factor for hip fractures, reflecting deterioration in bone strength beyond that detectable with BMD testing. The National Osteoporosis Foundation12 observed that the 5-year risk of hip fracture for women with the same T-score (−3) increases dramatically with advancing age (Figure): from 2.4% at age 50 to 9.7% at age 90, with the steepest increase occurring during the 10 years between ages 70 (5.5%) and 80 (9%).
FIGURE
Five-year risk for hip fracture for women with T-score of −3 by age12
Bone mineral density testing
Screening recommendations
The clinical value of different screening strategies is not established, although recommendations have been made within guidelines and consensus statements that discuss prevention and treatment of osteoporosis. Guidelines are consistent in recommending that BMD screening be done only if results will influence treatment decisions. The US Preventive Services Task Force,13 The National Osteoporosis Foundation,14 and American Association of Clinical Endocrinologists15 recommend screening all women over 65, as well as younger women with risk factors for osteoporosis. The National Institutes of Health3 and the North American Menopause Society16 recommend an individualized decision-making approach to screening. The National Osteoporosis Foundation developed nomograms that integrate risk factors into decision-making for testing and treatment,12 which seem promising and merit testing in prospective studies.
Diagnostic testing
DXA. Although several technologies are available, DXA of the hip is considered the best predictor of hip fracture and an equivalent predictor of other fractures.10 The likelihood of making a diagnosis of osteoporosis based on BMD, however, varies and is related to type of test, equipment, anatomic site tested, number of sites tested, technique, and relevance of the reference range to the local population. For example, when the same group of people is tested with DXA equipment from different manufacturers, the proportion diagnosed with osteoporosis varies by as much as 15%.11
Quantitative ultrasound (QUS) and radiographic absorptiometry (RA). Testing by QUS of the heel and RA of the hand are less expensive than DXA and have become popular. While QUS of the heel has been shown to predict hip fracture and all nonvertebral fractures nearly as well as DXA,3,10 it does not highly correlate with DXA and appears to reflect other aspects of bone quality.10 Since QUS and DXA results frequently disagree and can cause confusion, DXA is the most appropriate test of BMD at present. If QUS and RA are used for screening, confirmation with DXA is recommended before therapy is initiated.
Calculations based on risk factors. In a comparison of strategies using risk factors to predict low BMD in postmenopausal women, 2 decision rules performed well: the Osteoporosis Risk Assessment Instrument, which is based on age and weight (Table 1),17 and the Simple Calculated Osteoporosis Risk Estimation (SCORE).17 Research to test these instruments with fracture rather than BMD as outcome is needed.18
Biochemical markers. Levels of markers in serum and/or urine reflect bone turnover and have potential use in diagnosing and monitoring therapy of osteoporosis. They are not yet widely available and have not been consistently associated with identifying patients at risk for fracture.10 They are not recommended at this time.
TABLE 1
Osteoporosis risk assessment instrument17
| Patient characteristic | Points |
|---|---|
| Age (years) | |
| 75 or older | 15 |
| 65 to 74 | 9 |
| 55 to 64 | 5 |
| 54 or younger | 0 |
| Weight | |
| <132 lb (60 kg) | 9 |
| 132 to 153.9 lb (60 to 70 kg) | 3 |
| >154 lb (>70 kg) | 0 |
| No current estrogen use | 2 |
| Total: | |
| Patients with a score of 9 or higher are at risk for diagnosis of osteoporosis by bone mineral density measurement. Sensitivity 97.5%, specificity 28%, positive predictive value 28%, negative predictive value 99.6%, given a 10% baseline risk of a bone mineral density 2.5 SD less than the mean. | |
Importance of primary prevention
At least half of bone strength is attributable to genetic factors12; modifiable factors may contribute almost equally as a group, and therefore warrant attention. Genetic risk factors include age, family history, female sex, low weight, small frame, and white or Asian race. Primary prevention efforts should begin in childhood and continue throughout the life span to maximize bone mass.3
Prevention efforts that target the modifiable factors described below should be a routine part of the health-maintenance visit.
Fall reduction
Falls are the direct cause of more than 90% of osteoporotic hip fractures,19 and the tendency to fall increases with age. Some studies have shown that, for women over age 70, the most important predictors of hip fractures are fall-related factors20,21 such as poor cognitive function, slow gait and otherwise impaired mobility, poor vision, drugs that impair alertness or balance, and history of falls. In women over 75, age and slow gait are equal to low BMD of the femoral neck as predictors of hip fracture.22 Unfortunately, labeling women as osteopenic or osteoporotic can cause fear of falling and lack of activity, leading to further acceleration of bone loss.10
Medications that interfere with balance or alertness should be avoided if possible. Environmental hazards such as loose rugs and uneven or slippery surfaces are also well-recognized modifiable risks for falls23,24 that should be eliminated. Hip protectors effectively reduce fractures in the frail elderly25 and can boost confidence for beneficial increases in physical activity levels,26 but they are often poorly accepted by patients.25,27 Other options include referral for gait training, home visits by a physician or nurse to identify problems in the home that increase the risk of falls, or providing information on home modification (such as installing bathtub rails, removing throw rugs, etc.).
Improvement of nutritional intake
Adequate consumption of calcium is essential for bone health. Calcium balance also can be adversely affected by dietary habits, including high intake of protein, phosphorus, and sodium, although these effects appear to be less important when dietary calcium is sufficient.3 The recommended calcium intake for postmenopausal women (1200–1500 mg/day)28 can be met with food sources, but supplements should be added if needed. Most postmenopausal women in the United States consume only about 600 mg/day.28 High-calcium foods include milk (290–300 mg/cup), sardines in oil, with bones (370 mg/3 oz), yogurt (300–500 mg depending on container size), cheese (165–270 mg/slice), canned salmon, with bones (170–210 mg/3 oz), broccoli (160–180 mg/cup), and tofu (144–155 mg/4 oz).15
Vitamin D is essential for intestinal absorption of calcium. The recommended intake for women is 400 IU/day for ages 51 to 70, 600 IU/day over age 70, and 800 IU/day for all high-risk women, including those who are homebound, institutionalized, on chronic glucocorticoids, or who live in northern latitudes and therefore have limited exposure to sunlight.29 Sources of vitamin D include sunlight, vitamin D–fortified foods, fish oils, and supplements. Multivitamins typically contain 400 IU of vitamin D.
Phytoestrogens, particularly in the form of soy products, have received attention for bone health. Overall, studies do not support the use of soy foods to prevent osteoporosis.3 A well-designed trial in postmenopausal women found that ipriflavone, a synthetic phytoestrogen, did not decrease bone loss.30 Furthermore, use was associated with subclinical lymphocytopenia.
Regular exercise
Weight-bearing physical activity such as walking or running in early life contributes to higher peak bone mass. Limited data suggest weight-bearing exercise in postmenopausal women produces small increases in bone density at the hip31 and improvement in balance and strength.32 For women with established osteoporosis, activities that place an anterior load on the vertebral bodies, such as forward flexion exercises, are associated with an increased incidence of new vertebral deformities, and patients should be advised to avoid them.33
Avoidance of adverse health habits
Current smoking, compared with never smoking, doubles the risk of hip fracture.34 Consumption of more than 1 alcoholic drink/day or more than 7/week is associated with osteoporosis and fracture, while moderate consumption of 1 drink/day or less is associated with decreased risk.35 Excessive caffeine intake is also associated with increased osteoporosis risk and should be avoided. This effect appears to result from substitution of calcium-containing beverages such as milk or fortified orange juice with caffeinated, non–calcium-containing beverages such as colas.
Treatment for fracture prevention and pain relief
The goals of therapy for osteoporosis are fracture prevention and pain relief to maximize physical function.15 Prior fracture is associated with a fivefold risk of future fractures.5 About 20% of women who experience a vertebral fracture have another fracture within 1 year.36 Currently available therapies (Table 2) are antiresorptive: they slow bone turnover and allow bone formation to exceed resorption. Trials of antiresorptive agents in elderly women with osteoporosis and baseline vertebral fracture demonstrate that 1 new vertebral fracture is prevented for each 12 to 35 women treated for 2 to 3 years.14,37Table 3 summarizes the results of key treatment studies and provides information on the number of women that need to be treated (NNT) for the study period to prevent 1 fracture.
TABLE 2
Drug therapy for prevention and treatment of postmenopausal osteoporosis
| Drug (trade name) | Indication and dosage | Possible side effects (% of patients) | Cost per month* |
|---|---|---|---|
| Calcium and vitamin D (generic, Tums, Citracal, and others) | Prevention and treatment: 1200–1500 mg/day calcium and 800 IU/day vitamin D | Nausea, dyspepsia (uncommon), constipation (10%) | $5 (both) |
| Estrogen† (Premarin, Ogen, Estrace, Estraderm, and others) | Prevention: 0.625 mg/day conjugated equine estrogen or the equivalent; 0.3 mg/day may be effective | Nausea, breast tenderness, vaginal bleeding, mood alterations, headache, bloating | $14–$28 |
| Alendronate (Fosamax) | Prevention and treatment: 5 mg/day or 35 mg/week | Nausea, dyspepsia esophageal irritation | $67 |
| Risedronate (Actonel) | Prevention and treatment: 5 mg/day or 35 mg/week | Abdominal pain, nausea, diarrhea | $67 |
| Raloxifene (Evista) | Treatment: 60 mg/day | Hot flashes (6%), leg cramps (3%) | $70 |
| Calcitonin nasal spray (Miacalcin) | Treatment: 200 IU/day (1 spray in 1 nostril per day) | Rhinitis (5%), epistaxis, sinusitis | $66 |
| *Average wholesale cost to the pharmacy for 30 days of therapy; (Drug Topics Red Book. Montvale, NJ; Medical Economics Co., Inc, 2002.) | |||
| †Women with a uterus need to take a progestin such as medroxyprogesterone acetate (Provera $30/month, generic $9/month) or a combination estrogen/progestin product (Prempro $33/month, FemHRT $26/month). | |||
TABLE 3
Clinical trials of drug therapy for the prevention of fracture in postmenopausal women with osteoporosis
| Trial, year | Therapy | Outcome prevented | Number needed to treat for n years |
|---|---|---|---|
| Elderly, postmenopausal women | |||
| Chapuy, 199267 | Calcium/vitamin D | Hip fracture | 48 women for 1.5 years |
| Postmenopausal women | |||
| WHI, 200242 | Hormone replacement therapy | Hip fracture | 2000 women for 5 years |
| Postmenopausal women with osteoporosis | |||
| Ettinger, 199956 | Raloxifene | Vertebral fracture | 29 women for 3 years |
| Liberman, 199554 | Alendronate | Vertebral fracture | 34 women for 3 years |
| Heaney, 200253 | Risendronate | Vertebral fracture | 15 women for 3 years |
| McClung, 200170 | Risedronate | Hip fracture | 91 women for 3 years |
| Postmenopausal women with osteoporosis and previous vertebral racture | |||
| Harris, 199951 | Risedronate | Vertebral fracture | 20 women for 3 years |
| Black, 199652 | Alendronate | Vertebral fracture | 35 women for 3 years |
| Black, 199652 | Alendronate | Hip fracture | 86 women for 3 years |
Calcium and vitamin D
Calcium with or without vitamin D has been reported to positively affect fracture incidence.14 Vitamin D alone does not decrease the incidence of hip fractures.38 Calcium, 1200 to 1500 mg/day, and vitamin D, 800 IU/day, should be used concurrently with other forms of pharmacologic treatment. Calcium supplements are best absorbed with meals; for maximum absorption, calcium should be taken in doses of 500 mg or less.29,39
Minor gastrointestinal adverse effects may occur (most often constipation, 10%),14 which is often resolved by switching to a different preparation.40 Calcium in doses up to 1500 mg/day does not increase the risk for renal calculi and may, in fact, decrease risk.40,41 Calcium interferes with the absorption of certain medications, including tetracycline and quinolone antibiotics, which should be taken several hours apart from calcium. Calcium carbonate requires an acidic environment to dissolve. Patients taking stomach acid–suppressant therapy should use calcium citrate because it does not require an acidic environment for dissolution or should take their calcium supplement with meals. Traces of lead may be present in natural sources of calcium (bone meal, oyster shell, limestone, and dolomite), but can be avoided by use of over-the-counter calcium carbonate tablets (Tums).
Estrogen
Data from the Women’s Health Initiative (WHI) demonstrated that hormone replacement therapy (HRT) combining an estrogen and a progestin reduced hip and vertebral fractures by 1 in 2000 women per year and reduced all fractures by 1 in 333 women per year.42 Estrogen has a positive effect on BMD whether given in early or late postmenopause.43 Rapid bone loss as assessed by BMD does not occur after stopping HRT.44 However, in elderly women who have never used HRT, BMD is similar to those who have used it for 10 years and then stopped for at least 10 years.43 The effect of short-term (< 5 years) HRT during perimenopause on lifetime risk of osteoporotic fracture is unknown.
Common side effects (Table 2) can often be addressed by altering dosages, specific products, or regimens. The risk for breast cancer increases with duration of treatment and with combination HRT, compared with estrogen-only preparations.42,45,46 Combination HRT products are, however, essential for endometrial protection in women who have a uterus. The WHI reported an increase in breast cancer cases of 1 in 1250 women per year during an average 5-year follow-up.42 An increased risk for venous thromboembolism of 1 in 555 women per year was also observed with HRT use. Both the Heart and Estrogen/Progestin Study47 and the WHI studies found that venous thromboembolism occurred more frequently in the first 2 years of HRT use. An increased risk of myocardial infarction in the first 2 years of use was also noted among women with coronary heart disease47 and those without heart disease (1 in 1429 women per year).42 A small increase in stroke risk has also been documented.42,48-50 Contraindications to estrogen use include active thromboembolism, estrogen-related cancers, and liver disease.
Bisphosphonates
Two bisphosphonates, alendronate and risedronate, are approved in the United States for both prevention and treatment of postmenopausal osteoporosis. Clinical trials have demonstrated that both rapidly reduce the risk for symptomatic fractures in women with previous fracture and osteoporosis.37,51,52 The extent of fracture reduction is significant: Recent studies have shown that, over 3 years, the number of patients who would need to be treated with risedronate to prevent a vertebral fracture is 15, with alendronate, 34.53,54 Prevention of fractures among women without a prior vertebral fracture is less well established. No published data demonstrate that one bisphosphonate is more effective than another at preventing clinical fractures. A bisphosphonate is, therefore, the drug of choice for severe osteoporosis.
Bisphosphonates are generally well tolerated.55,56 However, postmarketing surveillance has demonstrated esophagitis and esophageal ulcer associated with alendronate.55 A pooled analysis of trials of risedronate found no increase in upper gastrointestinal (GI) adverse events even in patients with history of peptic ulcers, heartburn, and esophagitis, or among those taking nonsteroidal anti-inflammatory drugs, including aspirin.57
Oral bisphosphonates are not well absorbed (less than 1% of each dose).55,56 Therefore, to maximize absorption and to decrease the likelihood of adverse GI effects, the manufacturers of both bisphosphonates recommend that patients take the medication with a full glass of water, remain upright (sitting or standing) for at least 30 minutes following the dose, and not recline until food is consumed. Both bisphosphonates should be used with caution in patients with active GI disorders. Bisphosphonates are eliminated via the kidney and are not recommended for patients with a creatinine clearance below 30 mL/min.
Selective estrogen receptor modulators (SERMs)
Raloxifene is currently the only selective estrogen receptor modulator approved in the United States for prevention and treatment of osteoporosis. It has been shown to significantly decrease new vertebral fractures in women with a previous history of fracture and osteoporosis.58 The magnitude of fracture reduction is similar to that of bisphosphonates, although improvement in BMD is less marked.59 Raloxifene may confer other benefits. It has been shown to reduce the risk of breast cancer60,61 except in women with low estradiol levels,60 and may reduce the risk of myocardial infarction in women at high risk.60 Raloxifene does not increase the risk for endometrial cancer.60
Hot flashes and leg cramps are relatively common side effects of raloxifene.60 The observed risk of venous thromboembolism, 1 in 465 women per year during 3 years of treatment, is similar to that observed with HRT.60 Raloxifene is teratogenic and should not be used in premenopausal women.
Salmon calcitonin
Salmon calcitonin has demonstrated an analgesic effect for osteoporotic fracture.63,64 A large trial of salmon calcitonin at dosages of 100, 200, and 400 IU/day versus placebo found that salmon calcitonin at 200 IU/day decreased new vertebral fractures among women with a previous osteoporotic vertebral fracture based on radiographic assessment.65 No benefit was observed at the 100 and 400 IU/day dosages. The effect of calcitonin on clinical (symptomatic) fractures has not been reported. Calcitonin is approved for use in treatment, but not prevention, of osteoporosis.
Nasal calcitonin can cause minor rhinitis symptoms.30 Saline nasal solution may be useful to prevent or resolve irritation and dryness. Administration using alternate nostrils helps minimize local side effects. Unopened bottles (14 doses) must be stored in the refrigerator. Open bottles are stable at room temperature for up to 30 days.
Monitoring therapy
The value of serial densitometry to monitor the therapy of individual patients has not been established by randomized trials comparing different monitoring intervals or monitoring versus no monitoring.10,66 One important limitation is the relative imprecision of BMD testing: it takes almost a year to detect a 3% change in BMD.10 Disconcerting decreases in BMD scores are seen in yearly testing and may be offset by larger increases later, without a change in therapy. In studies of alendronate and raloxifene, disproportionately large fracture reductions cannot be explained by improvement in BMD alone.66,68 Bone densitometry is therefore not recommended until the patient has been treated for 2 years, and is of uncertain value beyond that point.
1. Osteoporosis Prevention, Diagnosis, and Therapy. NIH Consensus Statement Online 2000 March 27-29; [cited 9/2/2]; 17(1):1-36.
2. World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO Technical Report Series 843. Geneva: World Health Organization; 1994.
3. NIH Consensus Development Panel on Osteoporosis Prevention Diagnosis and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
4. Gold DT. The clinical impact of vertebral fractures: quality of life in women with osteoporosis. Bone. 1996;18(suppl 3):185S-189S.
5. Black DM, Arden NK, Palermo I, Pearson J, Cummings SR. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1999;14:821-828.
6. Ray NF, Chan JK, Thamer M, Melton LJ, 3rd. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:24-35.
7. Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468-489.
8. Aloia JF, Vaswani A, Yeh JK, Flaster E. Risk for osteoporosis in black women. Calcif Tissue Int. 1996;59:415-423.
9. Melton LJ. How many women have osteoporosis now? J Bone Miner Res. 1995;10:175-177.
10. Nelson HD, Morris CD, Mahon S, Carney N, Nygren PM, Helfand M. Osteoporosis in postmenopausal women: diagnosis and monitoring. Evidence Report/Technology Assessment Number 28. Rockville, MD: Agency for Healthcare Research and Quality; November 2001. 01-E032.
11. Melton LJ, 3rd, Amadio PC, Crowson CS, O’Fallon WM. Long-term trends in the incidence of distal forearm fractures. Osteoporos Int. 1998;8:341-348.
12. National Osteoporosis Foundation. Osteoporosis: review of the evidence for prevention, diagnosis, and treatment and cost-effectiveness analysis. Osteoporos Int. 1998;8(suppl):S7-S80.
13. U.S. Preventive Services Task Force. Screening for Osteoporosis in Postmenopausal Women. September 2002. Originally in Annals of Internal Medicine 2002;137:526-8.Agency for Healthcare Research and Quality, Rockville, MD. http://www.ahrq.gov/clinic/3rduspstf/osteoporosis/osteorr.htm.
14. Physician’s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; 1998.
15. American Association of Clinical Endocrinologists 2001 medical guidelines for clinical practice for the prevention and management of osteoporosis. Endocr Pract. 2001;7:293-312.
16. North American Menopause Society. Management of postmenopausal osteoporosis: position statement of The North American Menopause Society. Menopause. 2002;9:84-101.
17. Cadarette SM, Jaglal SB, Murray TM, McIsaac WJ, Joseph L, Brown JP. Evaluation of decision rules for referring women for bone densitometry by dual energy x-ray absorptiometry. JAMA. 2001;286:57-63.
18. Wasnich RD. Consensus and the T-score fallacy. Clin Rheumatol. 1997;16:337-339.
19. Grisso JA, Kelsey JL, Strom BL, et al. Risk factors for falls as a cause of hip fracture in women.The Northeast Hip Fracture Study Group. N Engl J Med. 1991;324:1326-1331.
20. Cooper C, Barker DJ, Morris J, Briggs RS. Osteoporosis, falls, and age in fracture of the proximal femur. Br Med J (Clin Res Ed). 1987;295:13-15.
21. Gardsell P, Johnell O, Nilsson BE, Nilsson JA. The predictive value of fracture, disease, and falling tendency for fragility fractures in women. Calcif Tissue Int. 1989;45:327-330.
22. Dargent-Molina P, Schott AM, Hans D, et al. Separate and combined value of bone mass and gait speed measurements in screening for hip fracture risk: results from the EPIDOS study. Epidemiologie de l’Osteoporose. Osteoporos Int. 1999;9:188-192.
23. Clemson L, Cumming RG, Roland M. Case-control study of hazards in the home and risk of falls and hip fractures. Age Ageing. 1996;25:97-101.
24. Norton R, Campbell AJ, Lee-Joe T, Robinson E, Butler M. Circumstances of falls resulting in hip fractures among older people. J Am Geriatr Soc 1997;45:1108-1112.
25. Kannus P, Parkkari J, Niemi S, et al. Prevention of hip fracture in elderly people with use of a hip protector. N Engl J Med. 2000;343:1506-1513.
26. Cameron ID, Stafford B, Cumming RG, et al. Hip protectors improve falls self-efficacy. Age Ageing. 2000;29:57-62.
27. Hubacher M, Wettstein A. Acceptance of hip protectors for hip fracture prevention in nursing homes. Osteoporos Int. 2001;12:794-799.
28. NIH Consensus Development Panel on Optimal Calcium Intake. Optimal Calcium Intake. JAMA. 1994;272:1942-1948.
29. Morgan SL. Calcium and vitamin D in osteoporosis. Rheum Dis Clin North Am. 2001;27:101-130.
30. Tsourounis C. Clinical effects of phytoestrogens. Clin Obstet Gynecol. 2001;44:836-842.
31. Kelley GA. Aerobic exercise and bone density at the hip in postmenopausal women: a meta-analysis. Prev Med. 1998;27:798-807.
32. Marcus R. Role of exercise in preventing and treating osteoporosis. Rheum Dis Clin North Am. 2001;27:131-141,vi.-
33. Sinaki M, Mikkelsen BA. Postmenopausal spinal osteoporosis: flexion versus extension exercises. Arch Phys Med Rehabil. 1984;65:593-596.
34. Law M, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of major effect. BMJ. 1997;315:841-846.
35. Felson DT, Zhang Y, Hannan MT, Kannel WB, Kiel DP. Alcohol intake and bone mineral density in elderly men and women. The Framingham Study. Am J Epidemiol. 1995;142:485-492.
36. Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320-323.
37. Marcus R, Wong M, Heath H, 3rd, Stock JL. Antiresorptive treatment of postmenopausal osteoporosis: comparison of study designs and outcomes in large clinical trials with fracture as an endpoint. Endocr Rev. 2002;23:16-37.
38. Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM. Vitamin D supplementation and fracture incidence in elderly persons. A randomized, placebo-controlled clinical trial. Ann Intern Med. 1996;124:400-406.
39. Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346:77-84.
40. North American Menopause Society. The role of calcium in peri- and postmenopausal women; consensus opinion of The North American Menopause Society. Menopause. 2001;8:84-95.
41. Williams CP, Child DF, Hudson PR, et al. Why oral calcium supplements may reduce renal stone disease: report of a clinical pilot study. J Clin Pathol. 2001;54:54-62.
42. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results for the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321-333.
43. Barrett-Connor E, Hendrix S, Ettinger B. International position paper on women’s health and menopause: a comprehensive approach. National Heart, Lung, and Blood Institute; 2002.
44. Greendale GA, Espeland M, Slone S, Marcus R, Barrett-Connor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med. 2002;162:665-672.
45. Schairer C, Lubin J, Triosi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283:485-491.
46. Ross R, Paganini-Hill A, Wan PC, Pike MC. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst. 2000;92:328-332.
47. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
48. Oger E, Scarabin PY. Risk of stroke among users of hormone replacement therapy. Annals d’Endocrinologie. 1999;60:232-241.
49. Simon J, Hsia J, Cauley JA, et al. Postmenopausal hormone therapy and risk of stroke: the Heart and Estrogen/Progestin Replacement Study (HERS). Circulation. 2001;103:638-642.
50. Viscoli C, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243-1249.
51. Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344-1352.
52. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535-1541.
53. Heaney RP, Zizic TM, Fogelman I, et al. Risedronate reduces the risk of first vertebral fracture in osteoporotic women. Osteoporos Int. 2002;13:501-505.
54. Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333:1437-1443.
55. Sharpe M, Noble S, Spencer CM. Alendronate: an update of its use in osteoporosis. Drugs. 2001;61:999-1039.
56. Dunn CJ, Goa KL. Risedronate: a review of its pharmacological properties and clinical use in resorptive bone disease. Drugs. 2001;61:685-712.
57. Taggart H, Bolognese MA, Lindsay R, et al. Upper gastrointestinal tract safety of risedronate: a pooled analysis of 9 clinical trials. Mayo Clin Proc. 2002;77:262-270.
58. Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637-645.
59. Sarkar S, Mitlak B, Wong M, Stock J, Black D, Harper K. Raloxifene-induced fracture reductions not directly associated with BMD changes. J Bone Miner Res. 2002;1:1-10.
60. Barrett-Connor E. Raloxifene: risks and benefits. Ann N Y Acad Sci. 2001;949:295-303.
61. Cummings S, Duong T, Kenyon E, Cauley J, Whitehead M, Krueger K. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220.
62. Barrett-Connor E, Grady D, Sashegyi A, et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA. 2002;287:847-857.
63. Lyritis GP, Paspati I, Karachalios T, Ioakimidis D, Skarantavos G, Lyritis PG. Pain relief from nasal salmon calcitonin in osteoporotic vertebral crush fractures. A double blind, placebo-controlled clinical study. Acta Orthop Scand Suppl. 1997;275:112-114.
64. Pun KK, Chan LW. Analgesic effect of intranasal salmon calcitonin in the treatment of osteoporotic vertebral fractures. Clin Ther. 1989;11:205-209.
65. Chesnut CH, 3rd. Calcitonin in the prevention and treatment of osteoporosis. Osteoporos Int. 1993;3(suppl 1):206-207.
66. Crandall C. The role of serial bone mineral density testing for osteoporosis. J Womens Health Gend Based Med. 2001;10:887-895.
67. Cummings SR. The paradox of small changes in bone density and reductions in risk of fracture with raloxifene. Ann N Y Acad Sci. 2001;949:198-201.
68. Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002;112:281-289.
69. Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637-1642.
70. McClung M, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med. 2001;344:333-340.
The last decade has witnessed important technological advances in the diagnosis of osteoporosis and an increase in therapeutic options. However, there is still considerable uncertainty about optimal strategies for screening and primary preventive treatment.
In 1994, a World Health Organization working group proposed that the diagnosis of osteoporosis be made when BMD, assessed by a dual-energy x-ray absorptiometry (DXA), is at least 2.5 standard deviations below the mean for young adult women (T-score) at the spine, hip, or wrist, or when a history of a traumatic fracture is present.2 A T-score between −1 and −2.5 is designated as osteopenia.
Osteoporosis is defined as “a skeletal disorder characterized by compromised bone strength predisposing to an increased risk of fracture.”1 While no accurate overall measurement of bone strength exists, bone mineral density (BMD) is frequently used as a proxy.
These facts underscore the importance of osteoporotic fractures:
- Only one third of patients regain their prior level of functioning after hip fracture, and one third are discharged to nursing homes.3
- About 1 in 5 patients dies within a year after a hip fracture.
- Vertebral fracture may result in chronic back pain and disability.4
- Existence of fracture greatly increases risk of subsequent fracture.5
- Direct medical costs for osteoporotic fractures are estimated at $13.8 billion in 1995 dollars.6
Prevalence of osteoporosis and fractures
Of American women over age 50 of all races, an estimated 15%, or 5 million, have osteoporosis (based on DXA T-score at the femoral neck) and an additional 40%, or 14 million, have osteopenia.7 In African Americans, the prevalence is about half that of whites.8 The prevalence of osteoporosis assessed by BMD testing increases with age—from 4% of white women aged 50 to 59 to 48% of women aged 80 to 89.9
At least 1 vertebral fracture, as indicated by radiographic criteria, has occurred in 5% of white women aged 50 to 59, and in 25% at age 80.3 The lifetime risk of hip fracture for 50-year-old white women and men is 14% and 5%, respectively; for African American women and men, 6% and 3%, respectively.3 Hip and symptomatic vertebral fractures occur mainly in women over 75,3,10 and the risk for wrist fractures increases starting in the late 50s.11
Age is a particularly important risk factor for hip fractures, reflecting deterioration in bone strength beyond that detectable with BMD testing. The National Osteoporosis Foundation12 observed that the 5-year risk of hip fracture for women with the same T-score (−3) increases dramatically with advancing age (Figure): from 2.4% at age 50 to 9.7% at age 90, with the steepest increase occurring during the 10 years between ages 70 (5.5%) and 80 (9%).
FIGURE
Five-year risk for hip fracture for women with T-score of −3 by age12
Bone mineral density testing
Screening recommendations
The clinical value of different screening strategies is not established, although recommendations have been made within guidelines and consensus statements that discuss prevention and treatment of osteoporosis. Guidelines are consistent in recommending that BMD screening be done only if results will influence treatment decisions. The US Preventive Services Task Force,13 The National Osteoporosis Foundation,14 and American Association of Clinical Endocrinologists15 recommend screening all women over 65, as well as younger women with risk factors for osteoporosis. The National Institutes of Health3 and the North American Menopause Society16 recommend an individualized decision-making approach to screening. The National Osteoporosis Foundation developed nomograms that integrate risk factors into decision-making for testing and treatment,12 which seem promising and merit testing in prospective studies.
Diagnostic testing
DXA. Although several technologies are available, DXA of the hip is considered the best predictor of hip fracture and an equivalent predictor of other fractures.10 The likelihood of making a diagnosis of osteoporosis based on BMD, however, varies and is related to type of test, equipment, anatomic site tested, number of sites tested, technique, and relevance of the reference range to the local population. For example, when the same group of people is tested with DXA equipment from different manufacturers, the proportion diagnosed with osteoporosis varies by as much as 15%.11
Quantitative ultrasound (QUS) and radiographic absorptiometry (RA). Testing by QUS of the heel and RA of the hand are less expensive than DXA and have become popular. While QUS of the heel has been shown to predict hip fracture and all nonvertebral fractures nearly as well as DXA,3,10 it does not highly correlate with DXA and appears to reflect other aspects of bone quality.10 Since QUS and DXA results frequently disagree and can cause confusion, DXA is the most appropriate test of BMD at present. If QUS and RA are used for screening, confirmation with DXA is recommended before therapy is initiated.
Calculations based on risk factors. In a comparison of strategies using risk factors to predict low BMD in postmenopausal women, 2 decision rules performed well: the Osteoporosis Risk Assessment Instrument, which is based on age and weight (Table 1),17 and the Simple Calculated Osteoporosis Risk Estimation (SCORE).17 Research to test these instruments with fracture rather than BMD as outcome is needed.18
Biochemical markers. Levels of markers in serum and/or urine reflect bone turnover and have potential use in diagnosing and monitoring therapy of osteoporosis. They are not yet widely available and have not been consistently associated with identifying patients at risk for fracture.10 They are not recommended at this time.
TABLE 1
Osteoporosis risk assessment instrument17
| Patient characteristic | Points |
|---|---|
| Age (years) | |
| 75 or older | 15 |
| 65 to 74 | 9 |
| 55 to 64 | 5 |
| 54 or younger | 0 |
| Weight | |
| <132 lb (60 kg) | 9 |
| 132 to 153.9 lb (60 to 70 kg) | 3 |
| >154 lb (>70 kg) | 0 |
| No current estrogen use | 2 |
| Total: | |
| Patients with a score of 9 or higher are at risk for diagnosis of osteoporosis by bone mineral density measurement. Sensitivity 97.5%, specificity 28%, positive predictive value 28%, negative predictive value 99.6%, given a 10% baseline risk of a bone mineral density 2.5 SD less than the mean. | |
Importance of primary prevention
At least half of bone strength is attributable to genetic factors12; modifiable factors may contribute almost equally as a group, and therefore warrant attention. Genetic risk factors include age, family history, female sex, low weight, small frame, and white or Asian race. Primary prevention efforts should begin in childhood and continue throughout the life span to maximize bone mass.3
Prevention efforts that target the modifiable factors described below should be a routine part of the health-maintenance visit.
Fall reduction
Falls are the direct cause of more than 90% of osteoporotic hip fractures,19 and the tendency to fall increases with age. Some studies have shown that, for women over age 70, the most important predictors of hip fractures are fall-related factors20,21 such as poor cognitive function, slow gait and otherwise impaired mobility, poor vision, drugs that impair alertness or balance, and history of falls. In women over 75, age and slow gait are equal to low BMD of the femoral neck as predictors of hip fracture.22 Unfortunately, labeling women as osteopenic or osteoporotic can cause fear of falling and lack of activity, leading to further acceleration of bone loss.10
Medications that interfere with balance or alertness should be avoided if possible. Environmental hazards such as loose rugs and uneven or slippery surfaces are also well-recognized modifiable risks for falls23,24 that should be eliminated. Hip protectors effectively reduce fractures in the frail elderly25 and can boost confidence for beneficial increases in physical activity levels,26 but they are often poorly accepted by patients.25,27 Other options include referral for gait training, home visits by a physician or nurse to identify problems in the home that increase the risk of falls, or providing information on home modification (such as installing bathtub rails, removing throw rugs, etc.).
Improvement of nutritional intake
Adequate consumption of calcium is essential for bone health. Calcium balance also can be adversely affected by dietary habits, including high intake of protein, phosphorus, and sodium, although these effects appear to be less important when dietary calcium is sufficient.3 The recommended calcium intake for postmenopausal women (1200–1500 mg/day)28 can be met with food sources, but supplements should be added if needed. Most postmenopausal women in the United States consume only about 600 mg/day.28 High-calcium foods include milk (290–300 mg/cup), sardines in oil, with bones (370 mg/3 oz), yogurt (300–500 mg depending on container size), cheese (165–270 mg/slice), canned salmon, with bones (170–210 mg/3 oz), broccoli (160–180 mg/cup), and tofu (144–155 mg/4 oz).15
Vitamin D is essential for intestinal absorption of calcium. The recommended intake for women is 400 IU/day for ages 51 to 70, 600 IU/day over age 70, and 800 IU/day for all high-risk women, including those who are homebound, institutionalized, on chronic glucocorticoids, or who live in northern latitudes and therefore have limited exposure to sunlight.29 Sources of vitamin D include sunlight, vitamin D–fortified foods, fish oils, and supplements. Multivitamins typically contain 400 IU of vitamin D.
Phytoestrogens, particularly in the form of soy products, have received attention for bone health. Overall, studies do not support the use of soy foods to prevent osteoporosis.3 A well-designed trial in postmenopausal women found that ipriflavone, a synthetic phytoestrogen, did not decrease bone loss.30 Furthermore, use was associated with subclinical lymphocytopenia.
Regular exercise
Weight-bearing physical activity such as walking or running in early life contributes to higher peak bone mass. Limited data suggest weight-bearing exercise in postmenopausal women produces small increases in bone density at the hip31 and improvement in balance and strength.32 For women with established osteoporosis, activities that place an anterior load on the vertebral bodies, such as forward flexion exercises, are associated with an increased incidence of new vertebral deformities, and patients should be advised to avoid them.33
Avoidance of adverse health habits
Current smoking, compared with never smoking, doubles the risk of hip fracture.34 Consumption of more than 1 alcoholic drink/day or more than 7/week is associated with osteoporosis and fracture, while moderate consumption of 1 drink/day or less is associated with decreased risk.35 Excessive caffeine intake is also associated with increased osteoporosis risk and should be avoided. This effect appears to result from substitution of calcium-containing beverages such as milk or fortified orange juice with caffeinated, non–calcium-containing beverages such as colas.
Treatment for fracture prevention and pain relief
The goals of therapy for osteoporosis are fracture prevention and pain relief to maximize physical function.15 Prior fracture is associated with a fivefold risk of future fractures.5 About 20% of women who experience a vertebral fracture have another fracture within 1 year.36 Currently available therapies (Table 2) are antiresorptive: they slow bone turnover and allow bone formation to exceed resorption. Trials of antiresorptive agents in elderly women with osteoporosis and baseline vertebral fracture demonstrate that 1 new vertebral fracture is prevented for each 12 to 35 women treated for 2 to 3 years.14,37Table 3 summarizes the results of key treatment studies and provides information on the number of women that need to be treated (NNT) for the study period to prevent 1 fracture.
TABLE 2
Drug therapy for prevention and treatment of postmenopausal osteoporosis
| Drug (trade name) | Indication and dosage | Possible side effects (% of patients) | Cost per month* |
|---|---|---|---|
| Calcium and vitamin D (generic, Tums, Citracal, and others) | Prevention and treatment: 1200–1500 mg/day calcium and 800 IU/day vitamin D | Nausea, dyspepsia (uncommon), constipation (10%) | $5 (both) |
| Estrogen† (Premarin, Ogen, Estrace, Estraderm, and others) | Prevention: 0.625 mg/day conjugated equine estrogen or the equivalent; 0.3 mg/day may be effective | Nausea, breast tenderness, vaginal bleeding, mood alterations, headache, bloating | $14–$28 |
| Alendronate (Fosamax) | Prevention and treatment: 5 mg/day or 35 mg/week | Nausea, dyspepsia esophageal irritation | $67 |
| Risedronate (Actonel) | Prevention and treatment: 5 mg/day or 35 mg/week | Abdominal pain, nausea, diarrhea | $67 |
| Raloxifene (Evista) | Treatment: 60 mg/day | Hot flashes (6%), leg cramps (3%) | $70 |
| Calcitonin nasal spray (Miacalcin) | Treatment: 200 IU/day (1 spray in 1 nostril per day) | Rhinitis (5%), epistaxis, sinusitis | $66 |
| *Average wholesale cost to the pharmacy for 30 days of therapy; (Drug Topics Red Book. Montvale, NJ; Medical Economics Co., Inc, 2002.) | |||
| †Women with a uterus need to take a progestin such as medroxyprogesterone acetate (Provera $30/month, generic $9/month) or a combination estrogen/progestin product (Prempro $33/month, FemHRT $26/month). | |||
TABLE 3
Clinical trials of drug therapy for the prevention of fracture in postmenopausal women with osteoporosis
| Trial, year | Therapy | Outcome prevented | Number needed to treat for n years |
|---|---|---|---|
| Elderly, postmenopausal women | |||
| Chapuy, 199267 | Calcium/vitamin D | Hip fracture | 48 women for 1.5 years |
| Postmenopausal women | |||
| WHI, 200242 | Hormone replacement therapy | Hip fracture | 2000 women for 5 years |
| Postmenopausal women with osteoporosis | |||
| Ettinger, 199956 | Raloxifene | Vertebral fracture | 29 women for 3 years |
| Liberman, 199554 | Alendronate | Vertebral fracture | 34 women for 3 years |
| Heaney, 200253 | Risendronate | Vertebral fracture | 15 women for 3 years |
| McClung, 200170 | Risedronate | Hip fracture | 91 women for 3 years |
| Postmenopausal women with osteoporosis and previous vertebral racture | |||
| Harris, 199951 | Risedronate | Vertebral fracture | 20 women for 3 years |
| Black, 199652 | Alendronate | Vertebral fracture | 35 women for 3 years |
| Black, 199652 | Alendronate | Hip fracture | 86 women for 3 years |
Calcium and vitamin D
Calcium with or without vitamin D has been reported to positively affect fracture incidence.14 Vitamin D alone does not decrease the incidence of hip fractures.38 Calcium, 1200 to 1500 mg/day, and vitamin D, 800 IU/day, should be used concurrently with other forms of pharmacologic treatment. Calcium supplements are best absorbed with meals; for maximum absorption, calcium should be taken in doses of 500 mg or less.29,39
Minor gastrointestinal adverse effects may occur (most often constipation, 10%),14 which is often resolved by switching to a different preparation.40 Calcium in doses up to 1500 mg/day does not increase the risk for renal calculi and may, in fact, decrease risk.40,41 Calcium interferes with the absorption of certain medications, including tetracycline and quinolone antibiotics, which should be taken several hours apart from calcium. Calcium carbonate requires an acidic environment to dissolve. Patients taking stomach acid–suppressant therapy should use calcium citrate because it does not require an acidic environment for dissolution or should take their calcium supplement with meals. Traces of lead may be present in natural sources of calcium (bone meal, oyster shell, limestone, and dolomite), but can be avoided by use of over-the-counter calcium carbonate tablets (Tums).
Estrogen
Data from the Women’s Health Initiative (WHI) demonstrated that hormone replacement therapy (HRT) combining an estrogen and a progestin reduced hip and vertebral fractures by 1 in 2000 women per year and reduced all fractures by 1 in 333 women per year.42 Estrogen has a positive effect on BMD whether given in early or late postmenopause.43 Rapid bone loss as assessed by BMD does not occur after stopping HRT.44 However, in elderly women who have never used HRT, BMD is similar to those who have used it for 10 years and then stopped for at least 10 years.43 The effect of short-term (< 5 years) HRT during perimenopause on lifetime risk of osteoporotic fracture is unknown.
Common side effects (Table 2) can often be addressed by altering dosages, specific products, or regimens. The risk for breast cancer increases with duration of treatment and with combination HRT, compared with estrogen-only preparations.42,45,46 Combination HRT products are, however, essential for endometrial protection in women who have a uterus. The WHI reported an increase in breast cancer cases of 1 in 1250 women per year during an average 5-year follow-up.42 An increased risk for venous thromboembolism of 1 in 555 women per year was also observed with HRT use. Both the Heart and Estrogen/Progestin Study47 and the WHI studies found that venous thromboembolism occurred more frequently in the first 2 years of HRT use. An increased risk of myocardial infarction in the first 2 years of use was also noted among women with coronary heart disease47 and those without heart disease (1 in 1429 women per year).42 A small increase in stroke risk has also been documented.42,48-50 Contraindications to estrogen use include active thromboembolism, estrogen-related cancers, and liver disease.
Bisphosphonates
Two bisphosphonates, alendronate and risedronate, are approved in the United States for both prevention and treatment of postmenopausal osteoporosis. Clinical trials have demonstrated that both rapidly reduce the risk for symptomatic fractures in women with previous fracture and osteoporosis.37,51,52 The extent of fracture reduction is significant: Recent studies have shown that, over 3 years, the number of patients who would need to be treated with risedronate to prevent a vertebral fracture is 15, with alendronate, 34.53,54 Prevention of fractures among women without a prior vertebral fracture is less well established. No published data demonstrate that one bisphosphonate is more effective than another at preventing clinical fractures. A bisphosphonate is, therefore, the drug of choice for severe osteoporosis.
Bisphosphonates are generally well tolerated.55,56 However, postmarketing surveillance has demonstrated esophagitis and esophageal ulcer associated with alendronate.55 A pooled analysis of trials of risedronate found no increase in upper gastrointestinal (GI) adverse events even in patients with history of peptic ulcers, heartburn, and esophagitis, or among those taking nonsteroidal anti-inflammatory drugs, including aspirin.57
Oral bisphosphonates are not well absorbed (less than 1% of each dose).55,56 Therefore, to maximize absorption and to decrease the likelihood of adverse GI effects, the manufacturers of both bisphosphonates recommend that patients take the medication with a full glass of water, remain upright (sitting or standing) for at least 30 minutes following the dose, and not recline until food is consumed. Both bisphosphonates should be used with caution in patients with active GI disorders. Bisphosphonates are eliminated via the kidney and are not recommended for patients with a creatinine clearance below 30 mL/min.
Selective estrogen receptor modulators (SERMs)
Raloxifene is currently the only selective estrogen receptor modulator approved in the United States for prevention and treatment of osteoporosis. It has been shown to significantly decrease new vertebral fractures in women with a previous history of fracture and osteoporosis.58 The magnitude of fracture reduction is similar to that of bisphosphonates, although improvement in BMD is less marked.59 Raloxifene may confer other benefits. It has been shown to reduce the risk of breast cancer60,61 except in women with low estradiol levels,60 and may reduce the risk of myocardial infarction in women at high risk.60 Raloxifene does not increase the risk for endometrial cancer.60
Hot flashes and leg cramps are relatively common side effects of raloxifene.60 The observed risk of venous thromboembolism, 1 in 465 women per year during 3 years of treatment, is similar to that observed with HRT.60 Raloxifene is teratogenic and should not be used in premenopausal women.
Salmon calcitonin
Salmon calcitonin has demonstrated an analgesic effect for osteoporotic fracture.63,64 A large trial of salmon calcitonin at dosages of 100, 200, and 400 IU/day versus placebo found that salmon calcitonin at 200 IU/day decreased new vertebral fractures among women with a previous osteoporotic vertebral fracture based on radiographic assessment.65 No benefit was observed at the 100 and 400 IU/day dosages. The effect of calcitonin on clinical (symptomatic) fractures has not been reported. Calcitonin is approved for use in treatment, but not prevention, of osteoporosis.
Nasal calcitonin can cause minor rhinitis symptoms.30 Saline nasal solution may be useful to prevent or resolve irritation and dryness. Administration using alternate nostrils helps minimize local side effects. Unopened bottles (14 doses) must be stored in the refrigerator. Open bottles are stable at room temperature for up to 30 days.
Monitoring therapy
The value of serial densitometry to monitor the therapy of individual patients has not been established by randomized trials comparing different monitoring intervals or monitoring versus no monitoring.10,66 One important limitation is the relative imprecision of BMD testing: it takes almost a year to detect a 3% change in BMD.10 Disconcerting decreases in BMD scores are seen in yearly testing and may be offset by larger increases later, without a change in therapy. In studies of alendronate and raloxifene, disproportionately large fracture reductions cannot be explained by improvement in BMD alone.66,68 Bone densitometry is therefore not recommended until the patient has been treated for 2 years, and is of uncertain value beyond that point.
The last decade has witnessed important technological advances in the diagnosis of osteoporosis and an increase in therapeutic options. However, there is still considerable uncertainty about optimal strategies for screening and primary preventive treatment.
In 1994, a World Health Organization working group proposed that the diagnosis of osteoporosis be made when BMD, assessed by a dual-energy x-ray absorptiometry (DXA), is at least 2.5 standard deviations below the mean for young adult women (T-score) at the spine, hip, or wrist, or when a history of a traumatic fracture is present.2 A T-score between −1 and −2.5 is designated as osteopenia.
Osteoporosis is defined as “a skeletal disorder characterized by compromised bone strength predisposing to an increased risk of fracture.”1 While no accurate overall measurement of bone strength exists, bone mineral density (BMD) is frequently used as a proxy.
These facts underscore the importance of osteoporotic fractures:
- Only one third of patients regain their prior level of functioning after hip fracture, and one third are discharged to nursing homes.3
- About 1 in 5 patients dies within a year after a hip fracture.
- Vertebral fracture may result in chronic back pain and disability.4
- Existence of fracture greatly increases risk of subsequent fracture.5
- Direct medical costs for osteoporotic fractures are estimated at $13.8 billion in 1995 dollars.6
Prevalence of osteoporosis and fractures
Of American women over age 50 of all races, an estimated 15%, or 5 million, have osteoporosis (based on DXA T-score at the femoral neck) and an additional 40%, or 14 million, have osteopenia.7 In African Americans, the prevalence is about half that of whites.8 The prevalence of osteoporosis assessed by BMD testing increases with age—from 4% of white women aged 50 to 59 to 48% of women aged 80 to 89.9
At least 1 vertebral fracture, as indicated by radiographic criteria, has occurred in 5% of white women aged 50 to 59, and in 25% at age 80.3 The lifetime risk of hip fracture for 50-year-old white women and men is 14% and 5%, respectively; for African American women and men, 6% and 3%, respectively.3 Hip and symptomatic vertebral fractures occur mainly in women over 75,3,10 and the risk for wrist fractures increases starting in the late 50s.11
Age is a particularly important risk factor for hip fractures, reflecting deterioration in bone strength beyond that detectable with BMD testing. The National Osteoporosis Foundation12 observed that the 5-year risk of hip fracture for women with the same T-score (−3) increases dramatically with advancing age (Figure): from 2.4% at age 50 to 9.7% at age 90, with the steepest increase occurring during the 10 years between ages 70 (5.5%) and 80 (9%).
FIGURE
Five-year risk for hip fracture for women with T-score of −3 by age12
Bone mineral density testing
Screening recommendations
The clinical value of different screening strategies is not established, although recommendations have been made within guidelines and consensus statements that discuss prevention and treatment of osteoporosis. Guidelines are consistent in recommending that BMD screening be done only if results will influence treatment decisions. The US Preventive Services Task Force,13 The National Osteoporosis Foundation,14 and American Association of Clinical Endocrinologists15 recommend screening all women over 65, as well as younger women with risk factors for osteoporosis. The National Institutes of Health3 and the North American Menopause Society16 recommend an individualized decision-making approach to screening. The National Osteoporosis Foundation developed nomograms that integrate risk factors into decision-making for testing and treatment,12 which seem promising and merit testing in prospective studies.
Diagnostic testing
DXA. Although several technologies are available, DXA of the hip is considered the best predictor of hip fracture and an equivalent predictor of other fractures.10 The likelihood of making a diagnosis of osteoporosis based on BMD, however, varies and is related to type of test, equipment, anatomic site tested, number of sites tested, technique, and relevance of the reference range to the local population. For example, when the same group of people is tested with DXA equipment from different manufacturers, the proportion diagnosed with osteoporosis varies by as much as 15%.11
Quantitative ultrasound (QUS) and radiographic absorptiometry (RA). Testing by QUS of the heel and RA of the hand are less expensive than DXA and have become popular. While QUS of the heel has been shown to predict hip fracture and all nonvertebral fractures nearly as well as DXA,3,10 it does not highly correlate with DXA and appears to reflect other aspects of bone quality.10 Since QUS and DXA results frequently disagree and can cause confusion, DXA is the most appropriate test of BMD at present. If QUS and RA are used for screening, confirmation with DXA is recommended before therapy is initiated.
Calculations based on risk factors. In a comparison of strategies using risk factors to predict low BMD in postmenopausal women, 2 decision rules performed well: the Osteoporosis Risk Assessment Instrument, which is based on age and weight (Table 1),17 and the Simple Calculated Osteoporosis Risk Estimation (SCORE).17 Research to test these instruments with fracture rather than BMD as outcome is needed.18
Biochemical markers. Levels of markers in serum and/or urine reflect bone turnover and have potential use in diagnosing and monitoring therapy of osteoporosis. They are not yet widely available and have not been consistently associated with identifying patients at risk for fracture.10 They are not recommended at this time.
TABLE 1
Osteoporosis risk assessment instrument17
| Patient characteristic | Points |
|---|---|
| Age (years) | |
| 75 or older | 15 |
| 65 to 74 | 9 |
| 55 to 64 | 5 |
| 54 or younger | 0 |
| Weight | |
| <132 lb (60 kg) | 9 |
| 132 to 153.9 lb (60 to 70 kg) | 3 |
| >154 lb (>70 kg) | 0 |
| No current estrogen use | 2 |
| Total: | |
| Patients with a score of 9 or higher are at risk for diagnosis of osteoporosis by bone mineral density measurement. Sensitivity 97.5%, specificity 28%, positive predictive value 28%, negative predictive value 99.6%, given a 10% baseline risk of a bone mineral density 2.5 SD less than the mean. | |
Importance of primary prevention
At least half of bone strength is attributable to genetic factors12; modifiable factors may contribute almost equally as a group, and therefore warrant attention. Genetic risk factors include age, family history, female sex, low weight, small frame, and white or Asian race. Primary prevention efforts should begin in childhood and continue throughout the life span to maximize bone mass.3
Prevention efforts that target the modifiable factors described below should be a routine part of the health-maintenance visit.
Fall reduction
Falls are the direct cause of more than 90% of osteoporotic hip fractures,19 and the tendency to fall increases with age. Some studies have shown that, for women over age 70, the most important predictors of hip fractures are fall-related factors20,21 such as poor cognitive function, slow gait and otherwise impaired mobility, poor vision, drugs that impair alertness or balance, and history of falls. In women over 75, age and slow gait are equal to low BMD of the femoral neck as predictors of hip fracture.22 Unfortunately, labeling women as osteopenic or osteoporotic can cause fear of falling and lack of activity, leading to further acceleration of bone loss.10
Medications that interfere with balance or alertness should be avoided if possible. Environmental hazards such as loose rugs and uneven or slippery surfaces are also well-recognized modifiable risks for falls23,24 that should be eliminated. Hip protectors effectively reduce fractures in the frail elderly25 and can boost confidence for beneficial increases in physical activity levels,26 but they are often poorly accepted by patients.25,27 Other options include referral for gait training, home visits by a physician or nurse to identify problems in the home that increase the risk of falls, or providing information on home modification (such as installing bathtub rails, removing throw rugs, etc.).
Improvement of nutritional intake
Adequate consumption of calcium is essential for bone health. Calcium balance also can be adversely affected by dietary habits, including high intake of protein, phosphorus, and sodium, although these effects appear to be less important when dietary calcium is sufficient.3 The recommended calcium intake for postmenopausal women (1200–1500 mg/day)28 can be met with food sources, but supplements should be added if needed. Most postmenopausal women in the United States consume only about 600 mg/day.28 High-calcium foods include milk (290–300 mg/cup), sardines in oil, with bones (370 mg/3 oz), yogurt (300–500 mg depending on container size), cheese (165–270 mg/slice), canned salmon, with bones (170–210 mg/3 oz), broccoli (160–180 mg/cup), and tofu (144–155 mg/4 oz).15
Vitamin D is essential for intestinal absorption of calcium. The recommended intake for women is 400 IU/day for ages 51 to 70, 600 IU/day over age 70, and 800 IU/day for all high-risk women, including those who are homebound, institutionalized, on chronic glucocorticoids, or who live in northern latitudes and therefore have limited exposure to sunlight.29 Sources of vitamin D include sunlight, vitamin D–fortified foods, fish oils, and supplements. Multivitamins typically contain 400 IU of vitamin D.
Phytoestrogens, particularly in the form of soy products, have received attention for bone health. Overall, studies do not support the use of soy foods to prevent osteoporosis.3 A well-designed trial in postmenopausal women found that ipriflavone, a synthetic phytoestrogen, did not decrease bone loss.30 Furthermore, use was associated with subclinical lymphocytopenia.
Regular exercise
Weight-bearing physical activity such as walking or running in early life contributes to higher peak bone mass. Limited data suggest weight-bearing exercise in postmenopausal women produces small increases in bone density at the hip31 and improvement in balance and strength.32 For women with established osteoporosis, activities that place an anterior load on the vertebral bodies, such as forward flexion exercises, are associated with an increased incidence of new vertebral deformities, and patients should be advised to avoid them.33
Avoidance of adverse health habits
Current smoking, compared with never smoking, doubles the risk of hip fracture.34 Consumption of more than 1 alcoholic drink/day or more than 7/week is associated with osteoporosis and fracture, while moderate consumption of 1 drink/day or less is associated with decreased risk.35 Excessive caffeine intake is also associated with increased osteoporosis risk and should be avoided. This effect appears to result from substitution of calcium-containing beverages such as milk or fortified orange juice with caffeinated, non–calcium-containing beverages such as colas.
Treatment for fracture prevention and pain relief
The goals of therapy for osteoporosis are fracture prevention and pain relief to maximize physical function.15 Prior fracture is associated with a fivefold risk of future fractures.5 About 20% of women who experience a vertebral fracture have another fracture within 1 year.36 Currently available therapies (Table 2) are antiresorptive: they slow bone turnover and allow bone formation to exceed resorption. Trials of antiresorptive agents in elderly women with osteoporosis and baseline vertebral fracture demonstrate that 1 new vertebral fracture is prevented for each 12 to 35 women treated for 2 to 3 years.14,37Table 3 summarizes the results of key treatment studies and provides information on the number of women that need to be treated (NNT) for the study period to prevent 1 fracture.
TABLE 2
Drug therapy for prevention and treatment of postmenopausal osteoporosis
| Drug (trade name) | Indication and dosage | Possible side effects (% of patients) | Cost per month* |
|---|---|---|---|
| Calcium and vitamin D (generic, Tums, Citracal, and others) | Prevention and treatment: 1200–1500 mg/day calcium and 800 IU/day vitamin D | Nausea, dyspepsia (uncommon), constipation (10%) | $5 (both) |
| Estrogen† (Premarin, Ogen, Estrace, Estraderm, and others) | Prevention: 0.625 mg/day conjugated equine estrogen or the equivalent; 0.3 mg/day may be effective | Nausea, breast tenderness, vaginal bleeding, mood alterations, headache, bloating | $14–$28 |
| Alendronate (Fosamax) | Prevention and treatment: 5 mg/day or 35 mg/week | Nausea, dyspepsia esophageal irritation | $67 |
| Risedronate (Actonel) | Prevention and treatment: 5 mg/day or 35 mg/week | Abdominal pain, nausea, diarrhea | $67 |
| Raloxifene (Evista) | Treatment: 60 mg/day | Hot flashes (6%), leg cramps (3%) | $70 |
| Calcitonin nasal spray (Miacalcin) | Treatment: 200 IU/day (1 spray in 1 nostril per day) | Rhinitis (5%), epistaxis, sinusitis | $66 |
| *Average wholesale cost to the pharmacy for 30 days of therapy; (Drug Topics Red Book. Montvale, NJ; Medical Economics Co., Inc, 2002.) | |||
| †Women with a uterus need to take a progestin such as medroxyprogesterone acetate (Provera $30/month, generic $9/month) or a combination estrogen/progestin product (Prempro $33/month, FemHRT $26/month). | |||
TABLE 3
Clinical trials of drug therapy for the prevention of fracture in postmenopausal women with osteoporosis
| Trial, year | Therapy | Outcome prevented | Number needed to treat for n years |
|---|---|---|---|
| Elderly, postmenopausal women | |||
| Chapuy, 199267 | Calcium/vitamin D | Hip fracture | 48 women for 1.5 years |
| Postmenopausal women | |||
| WHI, 200242 | Hormone replacement therapy | Hip fracture | 2000 women for 5 years |
| Postmenopausal women with osteoporosis | |||
| Ettinger, 199956 | Raloxifene | Vertebral fracture | 29 women for 3 years |
| Liberman, 199554 | Alendronate | Vertebral fracture | 34 women for 3 years |
| Heaney, 200253 | Risendronate | Vertebral fracture | 15 women for 3 years |
| McClung, 200170 | Risedronate | Hip fracture | 91 women for 3 years |
| Postmenopausal women with osteoporosis and previous vertebral racture | |||
| Harris, 199951 | Risedronate | Vertebral fracture | 20 women for 3 years |
| Black, 199652 | Alendronate | Vertebral fracture | 35 women for 3 years |
| Black, 199652 | Alendronate | Hip fracture | 86 women for 3 years |
Calcium and vitamin D
Calcium with or without vitamin D has been reported to positively affect fracture incidence.14 Vitamin D alone does not decrease the incidence of hip fractures.38 Calcium, 1200 to 1500 mg/day, and vitamin D, 800 IU/day, should be used concurrently with other forms of pharmacologic treatment. Calcium supplements are best absorbed with meals; for maximum absorption, calcium should be taken in doses of 500 mg or less.29,39
Minor gastrointestinal adverse effects may occur (most often constipation, 10%),14 which is often resolved by switching to a different preparation.40 Calcium in doses up to 1500 mg/day does not increase the risk for renal calculi and may, in fact, decrease risk.40,41 Calcium interferes with the absorption of certain medications, including tetracycline and quinolone antibiotics, which should be taken several hours apart from calcium. Calcium carbonate requires an acidic environment to dissolve. Patients taking stomach acid–suppressant therapy should use calcium citrate because it does not require an acidic environment for dissolution or should take their calcium supplement with meals. Traces of lead may be present in natural sources of calcium (bone meal, oyster shell, limestone, and dolomite), but can be avoided by use of over-the-counter calcium carbonate tablets (Tums).
Estrogen
Data from the Women’s Health Initiative (WHI) demonstrated that hormone replacement therapy (HRT) combining an estrogen and a progestin reduced hip and vertebral fractures by 1 in 2000 women per year and reduced all fractures by 1 in 333 women per year.42 Estrogen has a positive effect on BMD whether given in early or late postmenopause.43 Rapid bone loss as assessed by BMD does not occur after stopping HRT.44 However, in elderly women who have never used HRT, BMD is similar to those who have used it for 10 years and then stopped for at least 10 years.43 The effect of short-term (< 5 years) HRT during perimenopause on lifetime risk of osteoporotic fracture is unknown.
Common side effects (Table 2) can often be addressed by altering dosages, specific products, or regimens. The risk for breast cancer increases with duration of treatment and with combination HRT, compared with estrogen-only preparations.42,45,46 Combination HRT products are, however, essential for endometrial protection in women who have a uterus. The WHI reported an increase in breast cancer cases of 1 in 1250 women per year during an average 5-year follow-up.42 An increased risk for venous thromboembolism of 1 in 555 women per year was also observed with HRT use. Both the Heart and Estrogen/Progestin Study47 and the WHI studies found that venous thromboembolism occurred more frequently in the first 2 years of HRT use. An increased risk of myocardial infarction in the first 2 years of use was also noted among women with coronary heart disease47 and those without heart disease (1 in 1429 women per year).42 A small increase in stroke risk has also been documented.42,48-50 Contraindications to estrogen use include active thromboembolism, estrogen-related cancers, and liver disease.
Bisphosphonates
Two bisphosphonates, alendronate and risedronate, are approved in the United States for both prevention and treatment of postmenopausal osteoporosis. Clinical trials have demonstrated that both rapidly reduce the risk for symptomatic fractures in women with previous fracture and osteoporosis.37,51,52 The extent of fracture reduction is significant: Recent studies have shown that, over 3 years, the number of patients who would need to be treated with risedronate to prevent a vertebral fracture is 15, with alendronate, 34.53,54 Prevention of fractures among women without a prior vertebral fracture is less well established. No published data demonstrate that one bisphosphonate is more effective than another at preventing clinical fractures. A bisphosphonate is, therefore, the drug of choice for severe osteoporosis.
Bisphosphonates are generally well tolerated.55,56 However, postmarketing surveillance has demonstrated esophagitis and esophageal ulcer associated with alendronate.55 A pooled analysis of trials of risedronate found no increase in upper gastrointestinal (GI) adverse events even in patients with history of peptic ulcers, heartburn, and esophagitis, or among those taking nonsteroidal anti-inflammatory drugs, including aspirin.57
Oral bisphosphonates are not well absorbed (less than 1% of each dose).55,56 Therefore, to maximize absorption and to decrease the likelihood of adverse GI effects, the manufacturers of both bisphosphonates recommend that patients take the medication with a full glass of water, remain upright (sitting or standing) for at least 30 minutes following the dose, and not recline until food is consumed. Both bisphosphonates should be used with caution in patients with active GI disorders. Bisphosphonates are eliminated via the kidney and are not recommended for patients with a creatinine clearance below 30 mL/min.
Selective estrogen receptor modulators (SERMs)
Raloxifene is currently the only selective estrogen receptor modulator approved in the United States for prevention and treatment of osteoporosis. It has been shown to significantly decrease new vertebral fractures in women with a previous history of fracture and osteoporosis.58 The magnitude of fracture reduction is similar to that of bisphosphonates, although improvement in BMD is less marked.59 Raloxifene may confer other benefits. It has been shown to reduce the risk of breast cancer60,61 except in women with low estradiol levels,60 and may reduce the risk of myocardial infarction in women at high risk.60 Raloxifene does not increase the risk for endometrial cancer.60
Hot flashes and leg cramps are relatively common side effects of raloxifene.60 The observed risk of venous thromboembolism, 1 in 465 women per year during 3 years of treatment, is similar to that observed with HRT.60 Raloxifene is teratogenic and should not be used in premenopausal women.
Salmon calcitonin
Salmon calcitonin has demonstrated an analgesic effect for osteoporotic fracture.63,64 A large trial of salmon calcitonin at dosages of 100, 200, and 400 IU/day versus placebo found that salmon calcitonin at 200 IU/day decreased new vertebral fractures among women with a previous osteoporotic vertebral fracture based on radiographic assessment.65 No benefit was observed at the 100 and 400 IU/day dosages. The effect of calcitonin on clinical (symptomatic) fractures has not been reported. Calcitonin is approved for use in treatment, but not prevention, of osteoporosis.
Nasal calcitonin can cause minor rhinitis symptoms.30 Saline nasal solution may be useful to prevent or resolve irritation and dryness. Administration using alternate nostrils helps minimize local side effects. Unopened bottles (14 doses) must be stored in the refrigerator. Open bottles are stable at room temperature for up to 30 days.
Monitoring therapy
The value of serial densitometry to monitor the therapy of individual patients has not been established by randomized trials comparing different monitoring intervals or monitoring versus no monitoring.10,66 One important limitation is the relative imprecision of BMD testing: it takes almost a year to detect a 3% change in BMD.10 Disconcerting decreases in BMD scores are seen in yearly testing and may be offset by larger increases later, without a change in therapy. In studies of alendronate and raloxifene, disproportionately large fracture reductions cannot be explained by improvement in BMD alone.66,68 Bone densitometry is therefore not recommended until the patient has been treated for 2 years, and is of uncertain value beyond that point.
1. Osteoporosis Prevention, Diagnosis, and Therapy. NIH Consensus Statement Online 2000 March 27-29; [cited 9/2/2]; 17(1):1-36.
2. World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO Technical Report Series 843. Geneva: World Health Organization; 1994.
3. NIH Consensus Development Panel on Osteoporosis Prevention Diagnosis and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
4. Gold DT. The clinical impact of vertebral fractures: quality of life in women with osteoporosis. Bone. 1996;18(suppl 3):185S-189S.
5. Black DM, Arden NK, Palermo I, Pearson J, Cummings SR. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1999;14:821-828.
6. Ray NF, Chan JK, Thamer M, Melton LJ, 3rd. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:24-35.
7. Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468-489.
8. Aloia JF, Vaswani A, Yeh JK, Flaster E. Risk for osteoporosis in black women. Calcif Tissue Int. 1996;59:415-423.
9. Melton LJ. How many women have osteoporosis now? J Bone Miner Res. 1995;10:175-177.
10. Nelson HD, Morris CD, Mahon S, Carney N, Nygren PM, Helfand M. Osteoporosis in postmenopausal women: diagnosis and monitoring. Evidence Report/Technology Assessment Number 28. Rockville, MD: Agency for Healthcare Research and Quality; November 2001. 01-E032.
11. Melton LJ, 3rd, Amadio PC, Crowson CS, O’Fallon WM. Long-term trends in the incidence of distal forearm fractures. Osteoporos Int. 1998;8:341-348.
12. National Osteoporosis Foundation. Osteoporosis: review of the evidence for prevention, diagnosis, and treatment and cost-effectiveness analysis. Osteoporos Int. 1998;8(suppl):S7-S80.
13. U.S. Preventive Services Task Force. Screening for Osteoporosis in Postmenopausal Women. September 2002. Originally in Annals of Internal Medicine 2002;137:526-8.Agency for Healthcare Research and Quality, Rockville, MD. http://www.ahrq.gov/clinic/3rduspstf/osteoporosis/osteorr.htm.
14. Physician’s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; 1998.
15. American Association of Clinical Endocrinologists 2001 medical guidelines for clinical practice for the prevention and management of osteoporosis. Endocr Pract. 2001;7:293-312.
16. North American Menopause Society. Management of postmenopausal osteoporosis: position statement of The North American Menopause Society. Menopause. 2002;9:84-101.
17. Cadarette SM, Jaglal SB, Murray TM, McIsaac WJ, Joseph L, Brown JP. Evaluation of decision rules for referring women for bone densitometry by dual energy x-ray absorptiometry. JAMA. 2001;286:57-63.
18. Wasnich RD. Consensus and the T-score fallacy. Clin Rheumatol. 1997;16:337-339.
19. Grisso JA, Kelsey JL, Strom BL, et al. Risk factors for falls as a cause of hip fracture in women.The Northeast Hip Fracture Study Group. N Engl J Med. 1991;324:1326-1331.
20. Cooper C, Barker DJ, Morris J, Briggs RS. Osteoporosis, falls, and age in fracture of the proximal femur. Br Med J (Clin Res Ed). 1987;295:13-15.
21. Gardsell P, Johnell O, Nilsson BE, Nilsson JA. The predictive value of fracture, disease, and falling tendency for fragility fractures in women. Calcif Tissue Int. 1989;45:327-330.
22. Dargent-Molina P, Schott AM, Hans D, et al. Separate and combined value of bone mass and gait speed measurements in screening for hip fracture risk: results from the EPIDOS study. Epidemiologie de l’Osteoporose. Osteoporos Int. 1999;9:188-192.
23. Clemson L, Cumming RG, Roland M. Case-control study of hazards in the home and risk of falls and hip fractures. Age Ageing. 1996;25:97-101.
24. Norton R, Campbell AJ, Lee-Joe T, Robinson E, Butler M. Circumstances of falls resulting in hip fractures among older people. J Am Geriatr Soc 1997;45:1108-1112.
25. Kannus P, Parkkari J, Niemi S, et al. Prevention of hip fracture in elderly people with use of a hip protector. N Engl J Med. 2000;343:1506-1513.
26. Cameron ID, Stafford B, Cumming RG, et al. Hip protectors improve falls self-efficacy. Age Ageing. 2000;29:57-62.
27. Hubacher M, Wettstein A. Acceptance of hip protectors for hip fracture prevention in nursing homes. Osteoporos Int. 2001;12:794-799.
28. NIH Consensus Development Panel on Optimal Calcium Intake. Optimal Calcium Intake. JAMA. 1994;272:1942-1948.
29. Morgan SL. Calcium and vitamin D in osteoporosis. Rheum Dis Clin North Am. 2001;27:101-130.
30. Tsourounis C. Clinical effects of phytoestrogens. Clin Obstet Gynecol. 2001;44:836-842.
31. Kelley GA. Aerobic exercise and bone density at the hip in postmenopausal women: a meta-analysis. Prev Med. 1998;27:798-807.
32. Marcus R. Role of exercise in preventing and treating osteoporosis. Rheum Dis Clin North Am. 2001;27:131-141,vi.-
33. Sinaki M, Mikkelsen BA. Postmenopausal spinal osteoporosis: flexion versus extension exercises. Arch Phys Med Rehabil. 1984;65:593-596.
34. Law M, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of major effect. BMJ. 1997;315:841-846.
35. Felson DT, Zhang Y, Hannan MT, Kannel WB, Kiel DP. Alcohol intake and bone mineral density in elderly men and women. The Framingham Study. Am J Epidemiol. 1995;142:485-492.
36. Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320-323.
37. Marcus R, Wong M, Heath H, 3rd, Stock JL. Antiresorptive treatment of postmenopausal osteoporosis: comparison of study designs and outcomes in large clinical trials with fracture as an endpoint. Endocr Rev. 2002;23:16-37.
38. Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM. Vitamin D supplementation and fracture incidence in elderly persons. A randomized, placebo-controlled clinical trial. Ann Intern Med. 1996;124:400-406.
39. Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346:77-84.
40. North American Menopause Society. The role of calcium in peri- and postmenopausal women; consensus opinion of The North American Menopause Society. Menopause. 2001;8:84-95.
41. Williams CP, Child DF, Hudson PR, et al. Why oral calcium supplements may reduce renal stone disease: report of a clinical pilot study. J Clin Pathol. 2001;54:54-62.
42. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results for the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321-333.
43. Barrett-Connor E, Hendrix S, Ettinger B. International position paper on women’s health and menopause: a comprehensive approach. National Heart, Lung, and Blood Institute; 2002.
44. Greendale GA, Espeland M, Slone S, Marcus R, Barrett-Connor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med. 2002;162:665-672.
45. Schairer C, Lubin J, Triosi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283:485-491.
46. Ross R, Paganini-Hill A, Wan PC, Pike MC. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst. 2000;92:328-332.
47. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
48. Oger E, Scarabin PY. Risk of stroke among users of hormone replacement therapy. Annals d’Endocrinologie. 1999;60:232-241.
49. Simon J, Hsia J, Cauley JA, et al. Postmenopausal hormone therapy and risk of stroke: the Heart and Estrogen/Progestin Replacement Study (HERS). Circulation. 2001;103:638-642.
50. Viscoli C, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243-1249.
51. Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344-1352.
52. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535-1541.
53. Heaney RP, Zizic TM, Fogelman I, et al. Risedronate reduces the risk of first vertebral fracture in osteoporotic women. Osteoporos Int. 2002;13:501-505.
54. Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333:1437-1443.
55. Sharpe M, Noble S, Spencer CM. Alendronate: an update of its use in osteoporosis. Drugs. 2001;61:999-1039.
56. Dunn CJ, Goa KL. Risedronate: a review of its pharmacological properties and clinical use in resorptive bone disease. Drugs. 2001;61:685-712.
57. Taggart H, Bolognese MA, Lindsay R, et al. Upper gastrointestinal tract safety of risedronate: a pooled analysis of 9 clinical trials. Mayo Clin Proc. 2002;77:262-270.
58. Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637-645.
59. Sarkar S, Mitlak B, Wong M, Stock J, Black D, Harper K. Raloxifene-induced fracture reductions not directly associated with BMD changes. J Bone Miner Res. 2002;1:1-10.
60. Barrett-Connor E. Raloxifene: risks and benefits. Ann N Y Acad Sci. 2001;949:295-303.
61. Cummings S, Duong T, Kenyon E, Cauley J, Whitehead M, Krueger K. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220.
62. Barrett-Connor E, Grady D, Sashegyi A, et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA. 2002;287:847-857.
63. Lyritis GP, Paspati I, Karachalios T, Ioakimidis D, Skarantavos G, Lyritis PG. Pain relief from nasal salmon calcitonin in osteoporotic vertebral crush fractures. A double blind, placebo-controlled clinical study. Acta Orthop Scand Suppl. 1997;275:112-114.
64. Pun KK, Chan LW. Analgesic effect of intranasal salmon calcitonin in the treatment of osteoporotic vertebral fractures. Clin Ther. 1989;11:205-209.
65. Chesnut CH, 3rd. Calcitonin in the prevention and treatment of osteoporosis. Osteoporos Int. 1993;3(suppl 1):206-207.
66. Crandall C. The role of serial bone mineral density testing for osteoporosis. J Womens Health Gend Based Med. 2001;10:887-895.
67. Cummings SR. The paradox of small changes in bone density and reductions in risk of fracture with raloxifene. Ann N Y Acad Sci. 2001;949:198-201.
68. Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002;112:281-289.
69. Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637-1642.
70. McClung M, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med. 2001;344:333-340.
1. Osteoporosis Prevention, Diagnosis, and Therapy. NIH Consensus Statement Online 2000 March 27-29; [cited 9/2/2]; 17(1):1-36.
2. World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO Technical Report Series 843. Geneva: World Health Organization; 1994.
3. NIH Consensus Development Panel on Osteoporosis Prevention Diagnosis and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
4. Gold DT. The clinical impact of vertebral fractures: quality of life in women with osteoporosis. Bone. 1996;18(suppl 3):185S-189S.
5. Black DM, Arden NK, Palermo I, Pearson J, Cummings SR. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1999;14:821-828.
6. Ray NF, Chan JK, Thamer M, Melton LJ, 3rd. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:24-35.
7. Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468-489.
8. Aloia JF, Vaswani A, Yeh JK, Flaster E. Risk for osteoporosis in black women. Calcif Tissue Int. 1996;59:415-423.
9. Melton LJ. How many women have osteoporosis now? J Bone Miner Res. 1995;10:175-177.
10. Nelson HD, Morris CD, Mahon S, Carney N, Nygren PM, Helfand M. Osteoporosis in postmenopausal women: diagnosis and monitoring. Evidence Report/Technology Assessment Number 28. Rockville, MD: Agency for Healthcare Research and Quality; November 2001. 01-E032.
11. Melton LJ, 3rd, Amadio PC, Crowson CS, O’Fallon WM. Long-term trends in the incidence of distal forearm fractures. Osteoporos Int. 1998;8:341-348.
12. National Osteoporosis Foundation. Osteoporosis: review of the evidence for prevention, diagnosis, and treatment and cost-effectiveness analysis. Osteoporos Int. 1998;8(suppl):S7-S80.
13. U.S. Preventive Services Task Force. Screening for Osteoporosis in Postmenopausal Women. September 2002. Originally in Annals of Internal Medicine 2002;137:526-8.Agency for Healthcare Research and Quality, Rockville, MD. http://www.ahrq.gov/clinic/3rduspstf/osteoporosis/osteorr.htm.
14. Physician’s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; 1998.
15. American Association of Clinical Endocrinologists 2001 medical guidelines for clinical practice for the prevention and management of osteoporosis. Endocr Pract. 2001;7:293-312.
16. North American Menopause Society. Management of postmenopausal osteoporosis: position statement of The North American Menopause Society. Menopause. 2002;9:84-101.
17. Cadarette SM, Jaglal SB, Murray TM, McIsaac WJ, Joseph L, Brown JP. Evaluation of decision rules for referring women for bone densitometry by dual energy x-ray absorptiometry. JAMA. 2001;286:57-63.
18. Wasnich RD. Consensus and the T-score fallacy. Clin Rheumatol. 1997;16:337-339.
19. Grisso JA, Kelsey JL, Strom BL, et al. Risk factors for falls as a cause of hip fracture in women.The Northeast Hip Fracture Study Group. N Engl J Med. 1991;324:1326-1331.
20. Cooper C, Barker DJ, Morris J, Briggs RS. Osteoporosis, falls, and age in fracture of the proximal femur. Br Med J (Clin Res Ed). 1987;295:13-15.
21. Gardsell P, Johnell O, Nilsson BE, Nilsson JA. The predictive value of fracture, disease, and falling tendency for fragility fractures in women. Calcif Tissue Int. 1989;45:327-330.
22. Dargent-Molina P, Schott AM, Hans D, et al. Separate and combined value of bone mass and gait speed measurements in screening for hip fracture risk: results from the EPIDOS study. Epidemiologie de l’Osteoporose. Osteoporos Int. 1999;9:188-192.
23. Clemson L, Cumming RG, Roland M. Case-control study of hazards in the home and risk of falls and hip fractures. Age Ageing. 1996;25:97-101.
24. Norton R, Campbell AJ, Lee-Joe T, Robinson E, Butler M. Circumstances of falls resulting in hip fractures among older people. J Am Geriatr Soc 1997;45:1108-1112.
25. Kannus P, Parkkari J, Niemi S, et al. Prevention of hip fracture in elderly people with use of a hip protector. N Engl J Med. 2000;343:1506-1513.
26. Cameron ID, Stafford B, Cumming RG, et al. Hip protectors improve falls self-efficacy. Age Ageing. 2000;29:57-62.
27. Hubacher M, Wettstein A. Acceptance of hip protectors for hip fracture prevention in nursing homes. Osteoporos Int. 2001;12:794-799.
28. NIH Consensus Development Panel on Optimal Calcium Intake. Optimal Calcium Intake. JAMA. 1994;272:1942-1948.
29. Morgan SL. Calcium and vitamin D in osteoporosis. Rheum Dis Clin North Am. 2001;27:101-130.
30. Tsourounis C. Clinical effects of phytoestrogens. Clin Obstet Gynecol. 2001;44:836-842.
31. Kelley GA. Aerobic exercise and bone density at the hip in postmenopausal women: a meta-analysis. Prev Med. 1998;27:798-807.
32. Marcus R. Role of exercise in preventing and treating osteoporosis. Rheum Dis Clin North Am. 2001;27:131-141,vi.-
33. Sinaki M, Mikkelsen BA. Postmenopausal spinal osteoporosis: flexion versus extension exercises. Arch Phys Med Rehabil. 1984;65:593-596.
34. Law M, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of major effect. BMJ. 1997;315:841-846.
35. Felson DT, Zhang Y, Hannan MT, Kannel WB, Kiel DP. Alcohol intake and bone mineral density in elderly men and women. The Framingham Study. Am J Epidemiol. 1995;142:485-492.
36. Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320-323.
37. Marcus R, Wong M, Heath H, 3rd, Stock JL. Antiresorptive treatment of postmenopausal osteoporosis: comparison of study designs and outcomes in large clinical trials with fracture as an endpoint. Endocr Rev. 2002;23:16-37.
38. Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM. Vitamin D supplementation and fracture incidence in elderly persons. A randomized, placebo-controlled clinical trial. Ann Intern Med. 1996;124:400-406.
39. Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346:77-84.
40. North American Menopause Society. The role of calcium in peri- and postmenopausal women; consensus opinion of The North American Menopause Society. Menopause. 2001;8:84-95.
41. Williams CP, Child DF, Hudson PR, et al. Why oral calcium supplements may reduce renal stone disease: report of a clinical pilot study. J Clin Pathol. 2001;54:54-62.
42. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results for the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321-333.
43. Barrett-Connor E, Hendrix S, Ettinger B. International position paper on women’s health and menopause: a comprehensive approach. National Heart, Lung, and Blood Institute; 2002.
44. Greendale GA, Espeland M, Slone S, Marcus R, Barrett-Connor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med. 2002;162:665-672.
45. Schairer C, Lubin J, Triosi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283:485-491.
46. Ross R, Paganini-Hill A, Wan PC, Pike MC. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst. 2000;92:328-332.
47. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
48. Oger E, Scarabin PY. Risk of stroke among users of hormone replacement therapy. Annals d’Endocrinologie. 1999;60:232-241.
49. Simon J, Hsia J, Cauley JA, et al. Postmenopausal hormone therapy and risk of stroke: the Heart and Estrogen/Progestin Replacement Study (HERS). Circulation. 2001;103:638-642.
50. Viscoli C, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243-1249.
51. Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344-1352.
52. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535-1541.
53. Heaney RP, Zizic TM, Fogelman I, et al. Risedronate reduces the risk of first vertebral fracture in osteoporotic women. Osteoporos Int. 2002;13:501-505.
54. Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333:1437-1443.
55. Sharpe M, Noble S, Spencer CM. Alendronate: an update of its use in osteoporosis. Drugs. 2001;61:999-1039.
56. Dunn CJ, Goa KL. Risedronate: a review of its pharmacological properties and clinical use in resorptive bone disease. Drugs. 2001;61:685-712.
57. Taggart H, Bolognese MA, Lindsay R, et al. Upper gastrointestinal tract safety of risedronate: a pooled analysis of 9 clinical trials. Mayo Clin Proc. 2002;77:262-270.
58. Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637-645.
59. Sarkar S, Mitlak B, Wong M, Stock J, Black D, Harper K. Raloxifene-induced fracture reductions not directly associated with BMD changes. J Bone Miner Res. 2002;1:1-10.
60. Barrett-Connor E. Raloxifene: risks and benefits. Ann N Y Acad Sci. 2001;949:295-303.
61. Cummings S, Duong T, Kenyon E, Cauley J, Whitehead M, Krueger K. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220.
62. Barrett-Connor E, Grady D, Sashegyi A, et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA. 2002;287:847-857.
63. Lyritis GP, Paspati I, Karachalios T, Ioakimidis D, Skarantavos G, Lyritis PG. Pain relief from nasal salmon calcitonin in osteoporotic vertebral crush fractures. A double blind, placebo-controlled clinical study. Acta Orthop Scand Suppl. 1997;275:112-114.
64. Pun KK, Chan LW. Analgesic effect of intranasal salmon calcitonin in the treatment of osteoporotic vertebral fractures. Clin Ther. 1989;11:205-209.
65. Chesnut CH, 3rd. Calcitonin in the prevention and treatment of osteoporosis. Osteoporos Int. 1993;3(suppl 1):206-207.
66. Crandall C. The role of serial bone mineral density testing for osteoporosis. J Womens Health Gend Based Med. 2001;10:887-895.
67. Cummings SR. The paradox of small changes in bone density and reductions in risk of fracture with raloxifene. Ann N Y Acad Sci. 2001;949:198-201.
68. Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002;112:281-289.
69. Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637-1642.
70. McClung M, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med. 2001;344:333-340.