User login
Venous thromboembolism (VTE) is a major complication of cancer, occurring in 4% to 20% of patients,1 and is one of the leading causes of death in cancer patients, although these figures are believed to be underestimates, given the low autopsy rates among cancer patients.2 In hospitalized cancer patients specifically, VTE is the second leading cause of death.3,4 The risk of VTE in cancer patients undergoing surgery is three to five times greater than that in surgical patients without cancer.4 Moreover, cancer patients with symptomatic deep vein thrombosis (DVT) exhibit a high risk of recurrent VTE that may persist for many years after the index event.5
VTE PREVENTION POSES PARTICULAR CHALLENGES IN CANCER PATIENTS
Until recently, data on VTE prevention specific to cancer patients have been sparse. Cancer patients have represented only a small subset (< 20%) of participants in most of the largest clinical trials of VTE prophylaxis. Until the past 2 or 3 years, clinicians largely have had to extrapolate their approach to VTE prophylaxis in cancer patients from data in patients without cancer, bearing in mind that cancer patients are among the populations at highest risk of developing VTE.
High rates of VTE, even with prophylaxis
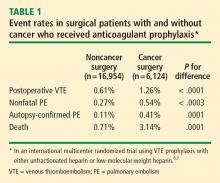
Further insights have come from the @RISTOS project, a Web-based prospective registry of patients undergoing general, urologic, or gynecologic surgery for cancer at multiple centers in Italy.8 Of the 2,372 patients tracked in this study, 82% received in-hospital VTE prophylaxis and 31% received prophylaxis following discharge. Despite this relatively high frequency of prophylaxis, however, the incidence of clinically overt VTE was 2.1% and the incidence of fatal VTE was 0.8%. Notably, most VTE events occurred after hospital discharge, and VTE was the most common cause of 30-day postoperative death in this registry.
RISK FACTORS: CANCER TYPE AND TREATMENT LOOM LARGE
Both the type and stage of a patient’s cancer are important in assessing the risk of VTE. For men, cancers of the prostate, colon, brain, and lung have been associated with an increased risk of VTE. Among women, cancers of the breast, ovary, and lung have been especially implicated as risk factors for VTE.9,10
The type of cancer therapy also influences VTE risk:
- Surgery. Among patients who undergo cancer-related surgery, the rate of proximal DVT is 10% to 20%, the rate of clinically evident PE is 4% to 10%, and the incidence of fatal PE is 0.2% to 5%.8,11
- Systemic treatments, including chemotherapy and hormone therapy, are also associated with an increased risk of VTE.12–15
- Central venous catheters. Approximately 4% of cancer patients who have central venous catheters placed develop clinically relevant VTE.16,17
In addition to the above risks related to cancer treatments, the following have been identified as risk factors for VTE in surgical oncology patients:
- Age greater than 40 years (risk also increases steeply after age 60 and again after age 75)
- Cancer procoagulants
- Thrombophilia
- Length and complications of cancer surgery (ie, often involving tissue trauma and immobilization)
- Debilitation and slow recovery.
Another risk factor worth noting is perioperative transfusion, as illustrated in a recent study of 14,104 adults undergoing colorectal cancer resection.18 The overall incidence of VTE in these patients was 1.0%, and the risk of death was nearly four times as great in patients who developed VTE as in those who did not. Notably, the need for transfusion was a marker of increased risk of VTE, particularly in women: women who received perioperative transfusions had almost double the risk of developing VTE compared with women who did not receive transfusions (P = .004).
CLINICAL TRIALS OF PROPHYLAXIS IN CANCER SURGERY PATIENTS
LMWH vs UFH for in-hospital prophylaxis
Two large randomized, double-blind trials have compared low-molecular-weight heparin (LMWH) with low-dose unfractionated heparin (UFH) for VTE prophylaxis in surgical patients with cancer—the Enoxaparin and Cancer (ENOXACAN) study19 and the Canadian Colorectal Surgery DVT Prophylaxis Trial.20 Patients in these studies underwent surgery for abdominal or pelvic cancer (mostly colorectal cancer). Both studies compared 40 mg of the LMWH enoxaparin given once daily with 5,000 U of UFH given three times daily for 7 to 10 days postoperatively. Outcome measures were the presence of DVT determined by venography on day 7 to 10 and the incidence of symptomatic VTE. Rates of VTE were statistically equivalent between the two treatment arms in both ENOXACAN (14.7% with LMWH vs 18.2% with UFH) and the Canadian Colorectal Surgery study (9.4% with both therapies), as were rates of major bleeding (4.1% with LMWH vs 2.9% with UFH in ENOXACAN; 2.7% with LMWH vs 1.5% with UFH in the Canadian study).
These findings are consistent with a 2001 meta-analysis by Mismetti et al of all available randomized trials comparing LMWH with placebo or with UFH for VTE prophylaxis in general surgery.21 This analysis found no differences in rates of asymptomatic DVT, clinical PE, clinical thromboembolism, death, major hemorrhage, total hemorrhage, wound hematoma, or need for transfusion between LMWH and UFH in patients undergoing either cancer-related surgery or surgery not related to cancer.
Fondaparinux for in-hospital prophylaxis
Subgroup analysis of the large randomized trial known as PEGASUS22 sheds some light on the efficacy of the factor Xa inhibitor fondaparinux relative to LMWH for thromboprophylaxis in cancer surgery patients. PEGASUS compared fondaparinux 2.5 mg once daily with the LMWH dalteparin 5,000 IU once daily for 5 to 9 days in patients undergoing high-risk abdominal surgery. Among the study’s 1,408 patients undergoing surgery for cancer, rates of VTE were 4.7% in the fondaparinux group compared with 7.7% in the LMWH group, a relative risk reduction of 38.6% with fondaparinux (95% CI, 6.7% to 59.6%). In contrast, in the rest of the PEGASUS population (patients undergoing abdominal surgery for reasons other than cancer), LMWH was nonsignificantly more efficacious at preventing VTE than was fondaparinux. Rates of major bleeding in this cancer subgroup were comparable between the two treatments.
Extended prophylaxis
Two additional randomized trials have evaluated extended prophylaxis with LMWH in surgical cancer patients—ENOXACAN II23 and the Fragmin After Major Abdominal Surgery (FAME) study.24
In ENOXACAN II, patients undergoing surgery for abdominal or pelvic cancer first received 6 to 10 days of prophylaxis with enoxaparin 40 mg once daily and then were randomized in a double-blind fashion to an additional 21 days of enoxaparin or placebo.23 Among 332 patients in the intent-to-treat analysis, the rate of VTE at the end of the double-blind phase was reduced from 12.0% with placebo to 4.8% with extended-duration enoxaparin (P = .02), an effect that was maintained at 3-month follow-up (P = .01). There was no significant difference between the two groups in rates of major bleeding events or any bleeding events.
In FAME, patients received 5,000 IU of dalteparin once daily for 1 week following major abdominal surgery and then were randomized in open-label fashion to either placebo or extended prophylaxis with dalteparin for 3 more weeks; a subanalysis examined outcomes in the 198 FAME participants whose abdominal surgery was for cancer.24 Among these 198 cancer surgery patients, the rate of venography-documented VTE at 4 weeks was reduced from 19.6% with placebo to 8.8% with extended-duration dalteparin, a relative reduction of 55% (P = .03). The rate of proximal DVT was reduced from 10.4% to 2.2% with extended prophylaxis, a relative reduction of 79% (P = .02).
The number needed to treat with extended LMWH prophylaxis to prevent one VTE event was 14 in ENOXACAN II23 and 9 in the FAME subanalysis of cancer surgery patients.24
New systematic review of relevant trials
Leonardi et al recently published a systematic review of 26 randomized controlled trials of DVT prophylaxis in 7,639 cancer surgery patients.25 They found the overall incidence of DVT to be 12.7% in those who received pharmacologic prophylaxis compared with 35.2% in controls. They also found high-dose LMWH therapy (> 3,400 U daily) to be associated with a significantly lower incidence of DVT than low-dose LMWH therapy (≤ 3,400 U daily) (7.9% vs 14.5%, respectively; P < .01). No differences were demonstrated between LMWH and UFH in preventing DVT, DVT location, or bleeding. Bleeding complications requiring discontinuation of pharmacologic prophylaxis occurred in 3% of patients overall.
Implications of HIT
The sequelae of heparin-induced thrombocytopenia (HIT) can have major consequences for cancer surgery patients. The incidence of HIT is markedly lower with LMWH than with UFH, as demonstrated in a nested case-control study by Creekmore et al.26 These researchers also found that the average cost of an admission during which HIT developed was nearly four times as great as the average cost of an admission during which UFH or LMWH was given without development of HIT ($56,364 vs $15,231; P < .001).
EVIDENCE IN SPECIFIC ONCOLOGIC POPULATIONS
Most of the patients in the trials reviewed above underwent abdominal surgery for malignancy. Although studies of VTE prophylaxis in patients undergoing nonabdominal cancer surgery are relatively few, some data are available for a few other specific oncologic populations, as reviewed below.
Surgery for gynecologic cancer
There is a paucity of randomized controlled trials or prospective observational studies on VTE and its prevention in the gynecologic cancer surgery population. Based on small historical studies, the postoperative risk of VTE in this population varies from 12% to 35%.27,28 Twice-daily administration of UFH 5,000 U appears to be ineffective as VTE prophylaxis in this population, but increasing the frequency to three times daily reduces VTE risk by 50% to 60% compared with placebo. Once-daily LMWH is comparable to three-times-daily UFH in efficacy and safety in this population.
A systematic Cochrane review of eight randomized controlled trials in patients undergoing major gynecologic surgery revealed that heparin prophylaxis (either UFH or LMWH) reduces the risk of DVT by 70% compared with no prophylaxis, with an identical risk reduction specifically among women with malignancy (odds ratio, 0.30; 95% CI, 0.10 to 0.89).29 This review found no evidence that anticoagulation reduces the risk of PE following major gynecologic surgery. LMWH and UFH were similar in efficacy for preventing DVT and had a comparable risk of bleeding complications.
Surgery for urologic cancer
The risk of VTE and the benefits of thromboprophylaxis also are poorly studied in patients undergoing surgery for urologic cancer.
The risk of VTE varies with the type of urologic surgery and the method used to diagnose VTE. For instance, patients undergoing radical retropubic prostatectomy have been reported to develop DVT at rates of 1% to 3%, PE at rates of 1% to 3%, and fatal PE at a rate of 0.6%, whereas the incidences of these events are somewhat higher in patients undergoing cystectomy: 8% for DVT, 2% to 4% for PE, and 2% for fatal PE. Radiologic diagnosis of thromboembolism in pelvic surgery patients has yielded higher incidences, with DVT rates of 21% to 51% and PE rates of 11% to 22%.30
Small studies suggest that prophylaxis with either low-dose UFH or LMWH is both effective in reducing VTE risk and safe in urologic cancer surgery patients, although pharmacologic prophylaxis poses a possible increased risk of pelvic hematoma and lymphocele formation in this population.30
Neurosurgery
Most neurosurgical procedures are performed for malignancies. The risk of venography-confirmed VTE in patients undergoing neurosurgery is approximately 30% to 40%.31,32 Likewise, the risks of intracranial or intraspinal hemorrhage in these patients are high. For this reason, mechanical methods of VTE prophylaxis are preferred in these patients. The use of anticoagulant prophylaxis remains controversial in this setting, although more recent data suggest that it might be safer than previously recognized.
GUIDELINES FOR VTE PROPHYLAXIS IN THE CANCER SURGERY PATIENT
American College of Chest Physicians
National Comprehensive Cancer Network
The National Comprehensive Cancer Network (NCCN) recently published clinical practice guidelines on venous thromboembolic disease in cancer patients.35 The defined at-risk population for these guidelines is the adult cancer inpatient with a diagnosis of (or clinical suspicion for) cancer. The guidelines recommend prophylactic anticoagulation (category 1 recommendation) with or without a sequential compression device as initial prophylaxis, unless the patient has a relative contraindication to anticoagulation, in which case mechanical prophylaxis (sequential compression device or graduated compression stockings) is recommended. (A category 1 recommendation indicates “uniform NCCN consensus, based on high-level evidence.”)
The NCCN guidelines include a specific recommended risk-factor assessment, which includes noting the patient’s age (VTE risk increases beginning at age 40 and then steeply again at age 75), any prior VTE, the presence of familial thrombophilia or active cancer, the use of medications associated with increased VTE risk (chemotherapy, exogenous estrogen compounds, and thalidomide or lenalidomide), and a number of other risk factors for VTE as outlined in the prior two articles in this supplement. The NCCN guidelines explicitly call for assessment of modifiable risk factors for VTE (ie, smoking or other tobacco use, obesity, and a low level of activity or lack of exercise) and call for active patient education on these factors.
American Society of Clinical Oncology
Our recommended algorithm
LINGERING CHALLENGE OF UNDERUTILIZATION
Despite this consensus on ways to reduce thromboembolic risk in this population and the clear evidence of the benefit of VTE prophylaxis in patients with cancer, data from several registries confirm a persistently low utilization of prophylaxis in patients with cancer.36–38 The global Fundamental Research in Oncology and Thrombosis (FRONTLINE) study surveyed 3,891 clinicians who treat cancer patients regarding their practices with respect to VTE in those patients.36 The survey found that only 52% of respondents routinely used thromboprophylaxis for their surgical patients with cancer. More striking, however, was the finding that most respondents routinely considered thrombo-prophylaxis in only 5% of their medical oncology patients. These data are echoed by findings of other retrospective medical record reviews in patients undergoing major abdominal or abdominothoracic surgery (in many cases for cancer), with VTE prophylaxis rates ranging from 38% to 75%.37,38
SUMMARY
Patients undergoing surgery for cancer have an increased risk of VTE and fatal PE, even when thromboprophylaxis is used. Nevertheless, prophylaxis with either LMWH or UFH does reduce venographic VTE event rates in these patients. If UFH is chosen for prophylaxis, a three-times-daily regimen should be used in this population. In specific surgical cancer populations, especially those undergoing abdominal surgery, out-of-hospital prophylaxis with once-daily LMWH is warranted. Current registries reveal that compliance with established guidelines for VTE prophylaxis in this population is low.


Dr. Jaffer: Dr. Amin, based on your study on thrombo-prophylaxis rates in US medical centers, will you comment on rates of prophylaxis for cancer surgery patients?
Dr. Amin: The overall study included approximately 200,000 medical patients and about 80,000 surgical patients enrolled over more than a 3-year period between 2002 and 2005.39,40 Our goal was to assess rates of prophylaxis and, when it was provided, whether it was appropriate (in terms of type, dosage, and duration) based on the ACCP guidelines. A subanalysis assessed medical cancer patients and surgical cancer patients separately. Medical cancer patients received thromboprophylaxis 56% of the time but received appropriate prophylaxis only 28% of the time. Among surgical cancer patients, appropriate prophylaxis was given only about 24% of the time for those undergoing gynecologic surgery and about 12% of the time for those undergoing neurosurgery. These percentages are consistent with data from other national registries, such as the IMPROVE registry, which documented prophylaxis rates on the order of 45% in medical patients with cancer.41 We also analyzed the data according to individual practitioners and found that medical oncologists use prophylaxis about 25% of the time, which is relatively consistent with other providers, such as internists and surgeons.
So there is a huge opportunity to improve rates of prophylaxis for this group of patients that national guidelines say are at high risk. Why is prophylaxis so underutilized in the cancer population? One factor may be a misperception about the risk of bleeding with anticoagulants. Yet several studies have shown that the rate of bleeding from prophylaxis is extremely low, whether LMWH or UFH is used, so more awareness of actual bleeding risk is needed. Another factor is the obvious focus among internists and oncologists on treating the patient, with perhaps a reduced consideration of prophylaxis and prevention. A third factor may be a concern about thrombocytopenia. However, in our study of prophylaxis rates in US medical centers, we excluded patients who had thrombocytopenia, yet rates of prophylaxis were still low. Nothing in the literature indicates that anticoagulants cannot be used in patients with platelet counts of 50,000 to 150,000 cells/µL or higher, so this suggests that we need to do more education.
Dr. Jaffer: Dr. Brotman, can you tell us more about how clinicians in practice should use prophylaxis in their neurosurgery patients, such as those undergoing craniotomy or spine surgery for cancer? What is the safest and most efficacious way to prevent DVT in these patients?
Dr. Brotman: First, it’s important to recognize that some sort of prophylaxis needs to be used. Neurosurgery patients are at an extremely high risk for thromboembolic events, and such events are often fatal in these patients. Having said that, the jury is still out on whether the prophylaxis in these patients should be compression devices or anticoagulation. This gives physicians some latitude in their decisions. They can decide not to use pharmacologic prophylaxis so long as they use pneumatic compression devices consistently, perhaps even starting during the operation and certainly throughout hospitalization when the patient is immobilized.
Certainly, the concerns about using full-dose anticoagulation in the immediate postoperative setting in neurosurgery patients are valid. Yet these patients are at very high risk for thromboembolic events, and if we take too cautious an approach to prophylaxis in the immediate perioperative setting, more patients are going to have thromboembolic events, at which point management decisions become much more difficult. The risk of intracranial bleeding with anticoagulation to treat a patient who develops a DVT at postoperative day 10 will certainly be higher than it would have been with lower-dose perioperative prophylactic anticoagulation. Plus, if you put in a filter at that point, the outcomes tend to be poor. Therefore, I believe there is some degree of risk that we should be willing to take with regard to perioperative bleeding, even in neurosurgery patients.
Dr. McKean: I’d like to make a point about combination prophylaxis. At many institutions, compression stockings and sequential compression devices are used preoperatively and intraoperatively, and then pharmacologic prophylaxis—for example, twice-daily UFH—is used postoperatively. There is concern that these patients are hypercoagulable, and most clinicians believe that mechanical prophylaxis alone, even with sequential compression devices plus compression stockings, is not aggressive enough in these high-risk patients.
Dr. Jaffer: Dr. Spyropoulos, what is the optimal duration of pharmacologic prophylaxis for cancer surgery patients?
Dr. Spyropoulos: First let’s consider in-hospital prophylaxis. The supportive data for in-hospital prophylaxis are strong, and the duration of therapy used in the major in-hospital prophylaxis trials was 7 to 10 days. With regard to extended prophylaxis, we have at least two moderately sized randomized controlled trials, ENOXACAN II23 and the substudy of FAME,24 that demonstrated that extending prophylaxis with LMWH at doses of 3,400 U once daily (5,000 IU of dalteparin; 40 mg of enoxaparin) reduces VTE risk at postoperative day 30. Also, recent data from the @RISTOS registry show that in cancer surgery patients, especially those having abdominal or pelvic procedures, the leading cause of 30-day mortality was VTE.8 This registry also shows that despite prophylaxis, the rate of symptomatic VTE can be as high as 2%, with the rate of fatal VTE approaching 1%. Thus, in cancer patients undergoing abdominal or pelvic surgery, physicians should strongly consider prophylaxis of up to 30 days’ duration.
Dr. Jaffer: One striking finding from the @RISTOS registry was that 40% of VTE events in these cancer surgery patients occurred after postoperative day 21. This really underscores the need to consider prophylaxis for at least 4 weeks in these patients in real-world practice.
Dr. Brotman: The other striking finding from that registry was that the in-hospital prophylaxis rate was quite high, about 80%, and the rate of extended prophylaxis approached 35%. These are rates that are rarely achieved in clinical practice. Yet despite these high levels of prophylaxis, patients in this registry still had a high incidence of morbidity and mortality from VTE. This suggests that we need to improve our out-of-hospital VTE prevention paradigms.
Dr. Jaffer: Dr. Deitelzweig, oncologists and internists are often unsure about whether their ambulatory cancer patients who are receiving chemotherapy should be on any form of prophylaxis. What is your opinion?
Dr. Deitelzweig: That question comes up regularly because these patients are encountered across many medical specialties. At this point, all of the large organizations, including ASCO and NCCN, are advocating that prophylaxis is not indicated for such patients.
- Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007; 25:5490–5505.
- Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 2007; 5:632–634.
- Ambrus JL, Ambrus CM, Mink IB, Pickren JW. Causes of death in cancer patients. J Med 1975; 6:61–64.
- Donati MB. Cancer and thrombosis. Haemostasis 1994; 24:128–131.
- Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med 1996; 125:1–7.
- Haas S, Wolf H, Kakkar AK, Fareed J, Encke A. Prevention of fatal pulmonary embolism and mortality in surgical patients: a randomized double-blind comparison of LMWH with unfractionated heparin. Thromb Haemost 2005; 94:814–819.
- Kakkar AK, Haas S, Wolf H, Encke A. Evaluation of perioperative fatal pulmonary embolism and death in cancer surgical patients: the MC-4 cancer substudy. Thromb Haemost 2005; 94:867–871.
- Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg 2006; 243:89–95.
- Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy: risk analysis using Medicare claims data. Medicine (Baltimore) 1999; 78:285–291.
- Levine M, Gent M, Hirsh J, et al. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med 1996; 334:677–681.
- Bergqvist D. Risk of venous thromboembolism in patients undergoing cancer surgery and options for thromboprophylaxis. J Surg Oncol 2007; 95:167–174.
- Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000; 160:809–815.
- Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: determination of frequency and characteristics. Thromb Haemost 2002; 87:575–579.
- Kröger K, Weiland D, Ose C, et al. Risk factors for venous thromboembolic events in cancer patients. Ann Oncol 2006; 17:297–303.
- Blom JW, Vanderschoot JP, Oostindiër MJ, et al. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost 2006; 4:529–535.
- Couban S, Simpson DR, Barnett MJ, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood 2002; 100:1525–1531.
- Walshe LJ, Malak SF, Eagan J, Sepkowitz KA. Complication rates among cancer patients with peripherally inserted central catheters. J Clin Oncol 2002; 20:3276–3281.
- Nilsson KR, Berenholtz SM, Garrett-Mayer E, et al. Association between venous thromboembolism and perioperative allogeneic transfusion. Arch Surg 2007; 142:126–133.
- Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: a double-blind randomized multicentre trial with venographic assessment. ENOXACAN Study Group. Br J Surg 1997; 84:1099–1103.
- McLeod RS, Geerts WH, Sniderman KW, et al. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian Colorectal DVT Prophylaxis Trial: a randomized, double-blind trial. Ann Surg 2001; 233:438–444.
- Mismetti P, Laporte S, Darmon JY, Buchmüller A, Decousus H. Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg 2001; 88:913–930.
- Agnelli G, Bergqvist D, Cohen AT, et al, on behalf of the PEGASUS investigators. Randomized clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high-risk abdominal surgery. Br J Surg 2005; 92:1212–1220.
- Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002; 346:975–980.
- Rasmussen MS, Wille-Jorgensen P, Jorgensen LN, et al. Prolonged thromboprophylaxis with low molecular weight heparin (dalteparin) following major abdominal surgery for malignancy [abstract 186]. Blood 2003; 102:56a.
- Leonardi MJ, McGory ML, Ko CY. A systematic review of deep venous thrombosis prophylaxis in cancer patients: implications for improving quality. Ann Surg Oncol 2007; 14:929–936.
- Creekmore FM, Oderda GM, Pendleton RC, Brixner DI. Incidence and economic implications of heparin-induced thrombocytopenia in medical patients receiving prophylaxis for venous thromboembolism. Pharmacotherapy 2006; 26:1438–1445.
- Walsh JJ, Bonnar J, Wright FW. A study of pulmonary embolism and deep leg vein thrombosis after major gynaecological surgery using labeled fibrinogen-phlebography and lung scanning. J Obstet Gynaecol Br Commonw 1974; 81:311–316.
- Clarke-Pearson DL, Synan IS, Coleman RE, et al. The natural history of postoperative venous thromboemboli in gynecologic oncology: a prospective study of 382 patients. Am J Obstet Gynecol 1984; 148:1051–1054.
- Oates-Whitehead RM, D’Angelo A, Mol B. Anticoagulant and aspirin prophylaxis for preventing thromboembolism after major gynaecological surgery. Cochrane Database Syst Rev 2003; (4):CD003679.
- Kibel AS, Loughlin KR. Pathogenesis and prophylaxis of postoperative thromboembolic disease in urological pelvic surgery. J Urol 1995; 153:1763–1774.
- Agnelli G, Piovella F, Buoncristiani P, et al. Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N Engl J Med 1998; 339:80–85.
- Semrad TJ, O’Donnell R, Wun T, et al. Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg 2007; 106:601–608.
- Iorio A, Agnelli G. Low-molecular-weight and unfractionated heparin for prevention of venous thromboembolism in neurosurgery: a meta-analysis. Arch Intern Med 2000; 160:2327–2332.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Venous thromboembolic disease. V.1.2007. http://www.nccn.org/professionals/physician_gls/PDF/vte.pdf. Accessed December 5, 2007.
- Kakkar AK, Levine M, Pinedo HM, Wolff R, Wong J. Venous thrombosis in cancer patients: insights from the FRONTLINE survey. Oncologist 2003; 8:381–388.
- Stratton MA, Anderson FA, Bussey HI, et al. Prevention of venous thromboembolism: adherence to the 1995 American College of Chest Physicians consensus guidelines for surgical patients. Arch Intern Med 2000; 160:334–340.
- Bratzler DW, Raskob GE, Murray CK, Bumpus LJ, Piatt DS. Underuse of venous thromboembolism prophylaxis for general surgery patients: physician practices in the community hospital setting. Arch Intern Med 1998; 158:1909–1912.
- Amin A, Stemkowski S, Lin J, et al. Thromboprophylaxis rates in US medical centers: success or failure? J Thromb Haemost 2007; 5:1610–1616.
- Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis in US hospitals: adherence to the 6th American College of Chest Physicians’ recommendations for at-risk medical and surgical patients. Abstract presented at: 41st Midyear Clinical Meeting of the American Society of Health-System Pharmacists; December 3–7, 2006; Anaheim, CA.
- Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest 2007; 132:936–945.
- Clarke-Pearson DL, Synan IS, Dodge R, et al. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol 1993; 168:1146–1154.
- Einstein MH, Pritts EA, Hartenbach EM. Venous thromboembolism prevention in gynecologic cancer surgery: a systematic review. Gynecol Oncol 2007; 105:813–819.
- Clarke-Pearson DL, Synan IS, Hinshaw WM, Coleman RE, Creasman WT. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol 1984; 63:92–98.
- Ruff RL, Posner JB. Incidence and treatment of peripheral venous thrombosis in patients with glioma. Ann Neurol 1983; 13:334–336.
- Levin JM, Schiff D, Loeffler JS, Fine HA, Black PM, Wen PY. Complications of therapy for venous thromboembolic disease in patients with brain tumors. Neurology 1993; 43:1111–1114.
Venous thromboembolism (VTE) is a major complication of cancer, occurring in 4% to 20% of patients,1 and is one of the leading causes of death in cancer patients, although these figures are believed to be underestimates, given the low autopsy rates among cancer patients.2 In hospitalized cancer patients specifically, VTE is the second leading cause of death.3,4 The risk of VTE in cancer patients undergoing surgery is three to five times greater than that in surgical patients without cancer.4 Moreover, cancer patients with symptomatic deep vein thrombosis (DVT) exhibit a high risk of recurrent VTE that may persist for many years after the index event.5
VTE PREVENTION POSES PARTICULAR CHALLENGES IN CANCER PATIENTS
Until recently, data on VTE prevention specific to cancer patients have been sparse. Cancer patients have represented only a small subset (< 20%) of participants in most of the largest clinical trials of VTE prophylaxis. Until the past 2 or 3 years, clinicians largely have had to extrapolate their approach to VTE prophylaxis in cancer patients from data in patients without cancer, bearing in mind that cancer patients are among the populations at highest risk of developing VTE.
High rates of VTE, even with prophylaxis
Further insights have come from the @RISTOS project, a Web-based prospective registry of patients undergoing general, urologic, or gynecologic surgery for cancer at multiple centers in Italy.8 Of the 2,372 patients tracked in this study, 82% received in-hospital VTE prophylaxis and 31% received prophylaxis following discharge. Despite this relatively high frequency of prophylaxis, however, the incidence of clinically overt VTE was 2.1% and the incidence of fatal VTE was 0.8%. Notably, most VTE events occurred after hospital discharge, and VTE was the most common cause of 30-day postoperative death in this registry.
RISK FACTORS: CANCER TYPE AND TREATMENT LOOM LARGE
Both the type and stage of a patient’s cancer are important in assessing the risk of VTE. For men, cancers of the prostate, colon, brain, and lung have been associated with an increased risk of VTE. Among women, cancers of the breast, ovary, and lung have been especially implicated as risk factors for VTE.9,10
The type of cancer therapy also influences VTE risk:
- Surgery. Among patients who undergo cancer-related surgery, the rate of proximal DVT is 10% to 20%, the rate of clinically evident PE is 4% to 10%, and the incidence of fatal PE is 0.2% to 5%.8,11
- Systemic treatments, including chemotherapy and hormone therapy, are also associated with an increased risk of VTE.12–15
- Central venous catheters. Approximately 4% of cancer patients who have central venous catheters placed develop clinically relevant VTE.16,17
In addition to the above risks related to cancer treatments, the following have been identified as risk factors for VTE in surgical oncology patients:
- Age greater than 40 years (risk also increases steeply after age 60 and again after age 75)
- Cancer procoagulants
- Thrombophilia
- Length and complications of cancer surgery (ie, often involving tissue trauma and immobilization)
- Debilitation and slow recovery.
Another risk factor worth noting is perioperative transfusion, as illustrated in a recent study of 14,104 adults undergoing colorectal cancer resection.18 The overall incidence of VTE in these patients was 1.0%, and the risk of death was nearly four times as great in patients who developed VTE as in those who did not. Notably, the need for transfusion was a marker of increased risk of VTE, particularly in women: women who received perioperative transfusions had almost double the risk of developing VTE compared with women who did not receive transfusions (P = .004).
CLINICAL TRIALS OF PROPHYLAXIS IN CANCER SURGERY PATIENTS
LMWH vs UFH for in-hospital prophylaxis
Two large randomized, double-blind trials have compared low-molecular-weight heparin (LMWH) with low-dose unfractionated heparin (UFH) for VTE prophylaxis in surgical patients with cancer—the Enoxaparin and Cancer (ENOXACAN) study19 and the Canadian Colorectal Surgery DVT Prophylaxis Trial.20 Patients in these studies underwent surgery for abdominal or pelvic cancer (mostly colorectal cancer). Both studies compared 40 mg of the LMWH enoxaparin given once daily with 5,000 U of UFH given three times daily for 7 to 10 days postoperatively. Outcome measures were the presence of DVT determined by venography on day 7 to 10 and the incidence of symptomatic VTE. Rates of VTE were statistically equivalent between the two treatment arms in both ENOXACAN (14.7% with LMWH vs 18.2% with UFH) and the Canadian Colorectal Surgery study (9.4% with both therapies), as were rates of major bleeding (4.1% with LMWH vs 2.9% with UFH in ENOXACAN; 2.7% with LMWH vs 1.5% with UFH in the Canadian study).
These findings are consistent with a 2001 meta-analysis by Mismetti et al of all available randomized trials comparing LMWH with placebo or with UFH for VTE prophylaxis in general surgery.21 This analysis found no differences in rates of asymptomatic DVT, clinical PE, clinical thromboembolism, death, major hemorrhage, total hemorrhage, wound hematoma, or need for transfusion between LMWH and UFH in patients undergoing either cancer-related surgery or surgery not related to cancer.
Fondaparinux for in-hospital prophylaxis
Subgroup analysis of the large randomized trial known as PEGASUS22 sheds some light on the efficacy of the factor Xa inhibitor fondaparinux relative to LMWH for thromboprophylaxis in cancer surgery patients. PEGASUS compared fondaparinux 2.5 mg once daily with the LMWH dalteparin 5,000 IU once daily for 5 to 9 days in patients undergoing high-risk abdominal surgery. Among the study’s 1,408 patients undergoing surgery for cancer, rates of VTE were 4.7% in the fondaparinux group compared with 7.7% in the LMWH group, a relative risk reduction of 38.6% with fondaparinux (95% CI, 6.7% to 59.6%). In contrast, in the rest of the PEGASUS population (patients undergoing abdominal surgery for reasons other than cancer), LMWH was nonsignificantly more efficacious at preventing VTE than was fondaparinux. Rates of major bleeding in this cancer subgroup were comparable between the two treatments.
Extended prophylaxis
Two additional randomized trials have evaluated extended prophylaxis with LMWH in surgical cancer patients—ENOXACAN II23 and the Fragmin After Major Abdominal Surgery (FAME) study.24
In ENOXACAN II, patients undergoing surgery for abdominal or pelvic cancer first received 6 to 10 days of prophylaxis with enoxaparin 40 mg once daily and then were randomized in a double-blind fashion to an additional 21 days of enoxaparin or placebo.23 Among 332 patients in the intent-to-treat analysis, the rate of VTE at the end of the double-blind phase was reduced from 12.0% with placebo to 4.8% with extended-duration enoxaparin (P = .02), an effect that was maintained at 3-month follow-up (P = .01). There was no significant difference between the two groups in rates of major bleeding events or any bleeding events.
In FAME, patients received 5,000 IU of dalteparin once daily for 1 week following major abdominal surgery and then were randomized in open-label fashion to either placebo or extended prophylaxis with dalteparin for 3 more weeks; a subanalysis examined outcomes in the 198 FAME participants whose abdominal surgery was for cancer.24 Among these 198 cancer surgery patients, the rate of venography-documented VTE at 4 weeks was reduced from 19.6% with placebo to 8.8% with extended-duration dalteparin, a relative reduction of 55% (P = .03). The rate of proximal DVT was reduced from 10.4% to 2.2% with extended prophylaxis, a relative reduction of 79% (P = .02).
The number needed to treat with extended LMWH prophylaxis to prevent one VTE event was 14 in ENOXACAN II23 and 9 in the FAME subanalysis of cancer surgery patients.24
New systematic review of relevant trials
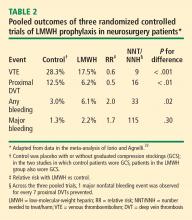
Leonardi et al recently published a systematic review of 26 randomized controlled trials of DVT prophylaxis in 7,639 cancer surgery patients.25 They found the overall incidence of DVT to be 12.7% in those who received pharmacologic prophylaxis compared with 35.2% in controls. They also found high-dose LMWH therapy (> 3,400 U daily) to be associated with a significantly lower incidence of DVT than low-dose LMWH therapy (≤ 3,400 U daily) (7.9% vs 14.5%, respectively; P < .01). No differences were demonstrated between LMWH and UFH in preventing DVT, DVT location, or bleeding. Bleeding complications requiring discontinuation of pharmacologic prophylaxis occurred in 3% of patients overall.
Implications of HIT
The sequelae of heparin-induced thrombocytopenia (HIT) can have major consequences for cancer surgery patients. The incidence of HIT is markedly lower with LMWH than with UFH, as demonstrated in a nested case-control study by Creekmore et al.26 These researchers also found that the average cost of an admission during which HIT developed was nearly four times as great as the average cost of an admission during which UFH or LMWH was given without development of HIT ($56,364 vs $15,231; P < .001).
EVIDENCE IN SPECIFIC ONCOLOGIC POPULATIONS
Most of the patients in the trials reviewed above underwent abdominal surgery for malignancy. Although studies of VTE prophylaxis in patients undergoing nonabdominal cancer surgery are relatively few, some data are available for a few other specific oncologic populations, as reviewed below.
Surgery for gynecologic cancer
There is a paucity of randomized controlled trials or prospective observational studies on VTE and its prevention in the gynecologic cancer surgery population. Based on small historical studies, the postoperative risk of VTE in this population varies from 12% to 35%.27,28 Twice-daily administration of UFH 5,000 U appears to be ineffective as VTE prophylaxis in this population, but increasing the frequency to three times daily reduces VTE risk by 50% to 60% compared with placebo. Once-daily LMWH is comparable to three-times-daily UFH in efficacy and safety in this population.
A systematic Cochrane review of eight randomized controlled trials in patients undergoing major gynecologic surgery revealed that heparin prophylaxis (either UFH or LMWH) reduces the risk of DVT by 70% compared with no prophylaxis, with an identical risk reduction specifically among women with malignancy (odds ratio, 0.30; 95% CI, 0.10 to 0.89).29 This review found no evidence that anticoagulation reduces the risk of PE following major gynecologic surgery. LMWH and UFH were similar in efficacy for preventing DVT and had a comparable risk of bleeding complications.
Surgery for urologic cancer
The risk of VTE and the benefits of thromboprophylaxis also are poorly studied in patients undergoing surgery for urologic cancer.
The risk of VTE varies with the type of urologic surgery and the method used to diagnose VTE. For instance, patients undergoing radical retropubic prostatectomy have been reported to develop DVT at rates of 1% to 3%, PE at rates of 1% to 3%, and fatal PE at a rate of 0.6%, whereas the incidences of these events are somewhat higher in patients undergoing cystectomy: 8% for DVT, 2% to 4% for PE, and 2% for fatal PE. Radiologic diagnosis of thromboembolism in pelvic surgery patients has yielded higher incidences, with DVT rates of 21% to 51% and PE rates of 11% to 22%.30
Small studies suggest that prophylaxis with either low-dose UFH or LMWH is both effective in reducing VTE risk and safe in urologic cancer surgery patients, although pharmacologic prophylaxis poses a possible increased risk of pelvic hematoma and lymphocele formation in this population.30
Neurosurgery
Most neurosurgical procedures are performed for malignancies. The risk of venography-confirmed VTE in patients undergoing neurosurgery is approximately 30% to 40%.31,32 Likewise, the risks of intracranial or intraspinal hemorrhage in these patients are high. For this reason, mechanical methods of VTE prophylaxis are preferred in these patients. The use of anticoagulant prophylaxis remains controversial in this setting, although more recent data suggest that it might be safer than previously recognized.
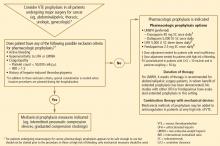
GUIDELINES FOR VTE PROPHYLAXIS IN THE CANCER SURGERY PATIENT
American College of Chest Physicians
National Comprehensive Cancer Network
The National Comprehensive Cancer Network (NCCN) recently published clinical practice guidelines on venous thromboembolic disease in cancer patients.35 The defined at-risk population for these guidelines is the adult cancer inpatient with a diagnosis of (or clinical suspicion for) cancer. The guidelines recommend prophylactic anticoagulation (category 1 recommendation) with or without a sequential compression device as initial prophylaxis, unless the patient has a relative contraindication to anticoagulation, in which case mechanical prophylaxis (sequential compression device or graduated compression stockings) is recommended. (A category 1 recommendation indicates “uniform NCCN consensus, based on high-level evidence.”)
The NCCN guidelines include a specific recommended risk-factor assessment, which includes noting the patient’s age (VTE risk increases beginning at age 40 and then steeply again at age 75), any prior VTE, the presence of familial thrombophilia or active cancer, the use of medications associated with increased VTE risk (chemotherapy, exogenous estrogen compounds, and thalidomide or lenalidomide), and a number of other risk factors for VTE as outlined in the prior two articles in this supplement. The NCCN guidelines explicitly call for assessment of modifiable risk factors for VTE (ie, smoking or other tobacco use, obesity, and a low level of activity or lack of exercise) and call for active patient education on these factors.
American Society of Clinical Oncology
Our recommended algorithm
LINGERING CHALLENGE OF UNDERUTILIZATION
Despite this consensus on ways to reduce thromboembolic risk in this population and the clear evidence of the benefit of VTE prophylaxis in patients with cancer, data from several registries confirm a persistently low utilization of prophylaxis in patients with cancer.36–38 The global Fundamental Research in Oncology and Thrombosis (FRONTLINE) study surveyed 3,891 clinicians who treat cancer patients regarding their practices with respect to VTE in those patients.36 The survey found that only 52% of respondents routinely used thromboprophylaxis for their surgical patients with cancer. More striking, however, was the finding that most respondents routinely considered thrombo-prophylaxis in only 5% of their medical oncology patients. These data are echoed by findings of other retrospective medical record reviews in patients undergoing major abdominal or abdominothoracic surgery (in many cases for cancer), with VTE prophylaxis rates ranging from 38% to 75%.37,38
SUMMARY
Patients undergoing surgery for cancer have an increased risk of VTE and fatal PE, even when thromboprophylaxis is used. Nevertheless, prophylaxis with either LMWH or UFH does reduce venographic VTE event rates in these patients. If UFH is chosen for prophylaxis, a three-times-daily regimen should be used in this population. In specific surgical cancer populations, especially those undergoing abdominal surgery, out-of-hospital prophylaxis with once-daily LMWH is warranted. Current registries reveal that compliance with established guidelines for VTE prophylaxis in this population is low.


Dr. Jaffer: Dr. Amin, based on your study on thrombo-prophylaxis rates in US medical centers, will you comment on rates of prophylaxis for cancer surgery patients?
Dr. Amin: The overall study included approximately 200,000 medical patients and about 80,000 surgical patients enrolled over more than a 3-year period between 2002 and 2005.39,40 Our goal was to assess rates of prophylaxis and, when it was provided, whether it was appropriate (in terms of type, dosage, and duration) based on the ACCP guidelines. A subanalysis assessed medical cancer patients and surgical cancer patients separately. Medical cancer patients received thromboprophylaxis 56% of the time but received appropriate prophylaxis only 28% of the time. Among surgical cancer patients, appropriate prophylaxis was given only about 24% of the time for those undergoing gynecologic surgery and about 12% of the time for those undergoing neurosurgery. These percentages are consistent with data from other national registries, such as the IMPROVE registry, which documented prophylaxis rates on the order of 45% in medical patients with cancer.41 We also analyzed the data according to individual practitioners and found that medical oncologists use prophylaxis about 25% of the time, which is relatively consistent with other providers, such as internists and surgeons.
So there is a huge opportunity to improve rates of prophylaxis for this group of patients that national guidelines say are at high risk. Why is prophylaxis so underutilized in the cancer population? One factor may be a misperception about the risk of bleeding with anticoagulants. Yet several studies have shown that the rate of bleeding from prophylaxis is extremely low, whether LMWH or UFH is used, so more awareness of actual bleeding risk is needed. Another factor is the obvious focus among internists and oncologists on treating the patient, with perhaps a reduced consideration of prophylaxis and prevention. A third factor may be a concern about thrombocytopenia. However, in our study of prophylaxis rates in US medical centers, we excluded patients who had thrombocytopenia, yet rates of prophylaxis were still low. Nothing in the literature indicates that anticoagulants cannot be used in patients with platelet counts of 50,000 to 150,000 cells/µL or higher, so this suggests that we need to do more education.
Dr. Jaffer: Dr. Brotman, can you tell us more about how clinicians in practice should use prophylaxis in their neurosurgery patients, such as those undergoing craniotomy or spine surgery for cancer? What is the safest and most efficacious way to prevent DVT in these patients?
Dr. Brotman: First, it’s important to recognize that some sort of prophylaxis needs to be used. Neurosurgery patients are at an extremely high risk for thromboembolic events, and such events are often fatal in these patients. Having said that, the jury is still out on whether the prophylaxis in these patients should be compression devices or anticoagulation. This gives physicians some latitude in their decisions. They can decide not to use pharmacologic prophylaxis so long as they use pneumatic compression devices consistently, perhaps even starting during the operation and certainly throughout hospitalization when the patient is immobilized.
Certainly, the concerns about using full-dose anticoagulation in the immediate postoperative setting in neurosurgery patients are valid. Yet these patients are at very high risk for thromboembolic events, and if we take too cautious an approach to prophylaxis in the immediate perioperative setting, more patients are going to have thromboembolic events, at which point management decisions become much more difficult. The risk of intracranial bleeding with anticoagulation to treat a patient who develops a DVT at postoperative day 10 will certainly be higher than it would have been with lower-dose perioperative prophylactic anticoagulation. Plus, if you put in a filter at that point, the outcomes tend to be poor. Therefore, I believe there is some degree of risk that we should be willing to take with regard to perioperative bleeding, even in neurosurgery patients.
Dr. McKean: I’d like to make a point about combination prophylaxis. At many institutions, compression stockings and sequential compression devices are used preoperatively and intraoperatively, and then pharmacologic prophylaxis—for example, twice-daily UFH—is used postoperatively. There is concern that these patients are hypercoagulable, and most clinicians believe that mechanical prophylaxis alone, even with sequential compression devices plus compression stockings, is not aggressive enough in these high-risk patients.
Dr. Jaffer: Dr. Spyropoulos, what is the optimal duration of pharmacologic prophylaxis for cancer surgery patients?
Dr. Spyropoulos: First let’s consider in-hospital prophylaxis. The supportive data for in-hospital prophylaxis are strong, and the duration of therapy used in the major in-hospital prophylaxis trials was 7 to 10 days. With regard to extended prophylaxis, we have at least two moderately sized randomized controlled trials, ENOXACAN II23 and the substudy of FAME,24 that demonstrated that extending prophylaxis with LMWH at doses of 3,400 U once daily (5,000 IU of dalteparin; 40 mg of enoxaparin) reduces VTE risk at postoperative day 30. Also, recent data from the @RISTOS registry show that in cancer surgery patients, especially those having abdominal or pelvic procedures, the leading cause of 30-day mortality was VTE.8 This registry also shows that despite prophylaxis, the rate of symptomatic VTE can be as high as 2%, with the rate of fatal VTE approaching 1%. Thus, in cancer patients undergoing abdominal or pelvic surgery, physicians should strongly consider prophylaxis of up to 30 days’ duration.
Dr. Jaffer: One striking finding from the @RISTOS registry was that 40% of VTE events in these cancer surgery patients occurred after postoperative day 21. This really underscores the need to consider prophylaxis for at least 4 weeks in these patients in real-world practice.
Dr. Brotman: The other striking finding from that registry was that the in-hospital prophylaxis rate was quite high, about 80%, and the rate of extended prophylaxis approached 35%. These are rates that are rarely achieved in clinical practice. Yet despite these high levels of prophylaxis, patients in this registry still had a high incidence of morbidity and mortality from VTE. This suggests that we need to improve our out-of-hospital VTE prevention paradigms.
Dr. Jaffer: Dr. Deitelzweig, oncologists and internists are often unsure about whether their ambulatory cancer patients who are receiving chemotherapy should be on any form of prophylaxis. What is your opinion?
Dr. Deitelzweig: That question comes up regularly because these patients are encountered across many medical specialties. At this point, all of the large organizations, including ASCO and NCCN, are advocating that prophylaxis is not indicated for such patients.
Venous thromboembolism (VTE) is a major complication of cancer, occurring in 4% to 20% of patients,1 and is one of the leading causes of death in cancer patients, although these figures are believed to be underestimates, given the low autopsy rates among cancer patients.2 In hospitalized cancer patients specifically, VTE is the second leading cause of death.3,4 The risk of VTE in cancer patients undergoing surgery is three to five times greater than that in surgical patients without cancer.4 Moreover, cancer patients with symptomatic deep vein thrombosis (DVT) exhibit a high risk of recurrent VTE that may persist for many years after the index event.5
VTE PREVENTION POSES PARTICULAR CHALLENGES IN CANCER PATIENTS
Until recently, data on VTE prevention specific to cancer patients have been sparse. Cancer patients have represented only a small subset (< 20%) of participants in most of the largest clinical trials of VTE prophylaxis. Until the past 2 or 3 years, clinicians largely have had to extrapolate their approach to VTE prophylaxis in cancer patients from data in patients without cancer, bearing in mind that cancer patients are among the populations at highest risk of developing VTE.
High rates of VTE, even with prophylaxis
Further insights have come from the @RISTOS project, a Web-based prospective registry of patients undergoing general, urologic, or gynecologic surgery for cancer at multiple centers in Italy.8 Of the 2,372 patients tracked in this study, 82% received in-hospital VTE prophylaxis and 31% received prophylaxis following discharge. Despite this relatively high frequency of prophylaxis, however, the incidence of clinically overt VTE was 2.1% and the incidence of fatal VTE was 0.8%. Notably, most VTE events occurred after hospital discharge, and VTE was the most common cause of 30-day postoperative death in this registry.
RISK FACTORS: CANCER TYPE AND TREATMENT LOOM LARGE
Both the type and stage of a patient’s cancer are important in assessing the risk of VTE. For men, cancers of the prostate, colon, brain, and lung have been associated with an increased risk of VTE. Among women, cancers of the breast, ovary, and lung have been especially implicated as risk factors for VTE.9,10
The type of cancer therapy also influences VTE risk:
- Surgery. Among patients who undergo cancer-related surgery, the rate of proximal DVT is 10% to 20%, the rate of clinically evident PE is 4% to 10%, and the incidence of fatal PE is 0.2% to 5%.8,11
- Systemic treatments, including chemotherapy and hormone therapy, are also associated with an increased risk of VTE.12–15
- Central venous catheters. Approximately 4% of cancer patients who have central venous catheters placed develop clinically relevant VTE.16,17
In addition to the above risks related to cancer treatments, the following have been identified as risk factors for VTE in surgical oncology patients:
- Age greater than 40 years (risk also increases steeply after age 60 and again after age 75)
- Cancer procoagulants
- Thrombophilia
- Length and complications of cancer surgery (ie, often involving tissue trauma and immobilization)
- Debilitation and slow recovery.
Another risk factor worth noting is perioperative transfusion, as illustrated in a recent study of 14,104 adults undergoing colorectal cancer resection.18 The overall incidence of VTE in these patients was 1.0%, and the risk of death was nearly four times as great in patients who developed VTE as in those who did not. Notably, the need for transfusion was a marker of increased risk of VTE, particularly in women: women who received perioperative transfusions had almost double the risk of developing VTE compared with women who did not receive transfusions (P = .004).
CLINICAL TRIALS OF PROPHYLAXIS IN CANCER SURGERY PATIENTS
LMWH vs UFH for in-hospital prophylaxis
Two large randomized, double-blind trials have compared low-molecular-weight heparin (LMWH) with low-dose unfractionated heparin (UFH) for VTE prophylaxis in surgical patients with cancer—the Enoxaparin and Cancer (ENOXACAN) study19 and the Canadian Colorectal Surgery DVT Prophylaxis Trial.20 Patients in these studies underwent surgery for abdominal or pelvic cancer (mostly colorectal cancer). Both studies compared 40 mg of the LMWH enoxaparin given once daily with 5,000 U of UFH given three times daily for 7 to 10 days postoperatively. Outcome measures were the presence of DVT determined by venography on day 7 to 10 and the incidence of symptomatic VTE. Rates of VTE were statistically equivalent between the two treatment arms in both ENOXACAN (14.7% with LMWH vs 18.2% with UFH) and the Canadian Colorectal Surgery study (9.4% with both therapies), as were rates of major bleeding (4.1% with LMWH vs 2.9% with UFH in ENOXACAN; 2.7% with LMWH vs 1.5% with UFH in the Canadian study).
These findings are consistent with a 2001 meta-analysis by Mismetti et al of all available randomized trials comparing LMWH with placebo or with UFH for VTE prophylaxis in general surgery.21 This analysis found no differences in rates of asymptomatic DVT, clinical PE, clinical thromboembolism, death, major hemorrhage, total hemorrhage, wound hematoma, or need for transfusion between LMWH and UFH in patients undergoing either cancer-related surgery or surgery not related to cancer.
Fondaparinux for in-hospital prophylaxis
Subgroup analysis of the large randomized trial known as PEGASUS22 sheds some light on the efficacy of the factor Xa inhibitor fondaparinux relative to LMWH for thromboprophylaxis in cancer surgery patients. PEGASUS compared fondaparinux 2.5 mg once daily with the LMWH dalteparin 5,000 IU once daily for 5 to 9 days in patients undergoing high-risk abdominal surgery. Among the study’s 1,408 patients undergoing surgery for cancer, rates of VTE were 4.7% in the fondaparinux group compared with 7.7% in the LMWH group, a relative risk reduction of 38.6% with fondaparinux (95% CI, 6.7% to 59.6%). In contrast, in the rest of the PEGASUS population (patients undergoing abdominal surgery for reasons other than cancer), LMWH was nonsignificantly more efficacious at preventing VTE than was fondaparinux. Rates of major bleeding in this cancer subgroup were comparable between the two treatments.
Extended prophylaxis
Two additional randomized trials have evaluated extended prophylaxis with LMWH in surgical cancer patients—ENOXACAN II23 and the Fragmin After Major Abdominal Surgery (FAME) study.24
In ENOXACAN II, patients undergoing surgery for abdominal or pelvic cancer first received 6 to 10 days of prophylaxis with enoxaparin 40 mg once daily and then were randomized in a double-blind fashion to an additional 21 days of enoxaparin or placebo.23 Among 332 patients in the intent-to-treat analysis, the rate of VTE at the end of the double-blind phase was reduced from 12.0% with placebo to 4.8% with extended-duration enoxaparin (P = .02), an effect that was maintained at 3-month follow-up (P = .01). There was no significant difference between the two groups in rates of major bleeding events or any bleeding events.
In FAME, patients received 5,000 IU of dalteparin once daily for 1 week following major abdominal surgery and then were randomized in open-label fashion to either placebo or extended prophylaxis with dalteparin for 3 more weeks; a subanalysis examined outcomes in the 198 FAME participants whose abdominal surgery was for cancer.24 Among these 198 cancer surgery patients, the rate of venography-documented VTE at 4 weeks was reduced from 19.6% with placebo to 8.8% with extended-duration dalteparin, a relative reduction of 55% (P = .03). The rate of proximal DVT was reduced from 10.4% to 2.2% with extended prophylaxis, a relative reduction of 79% (P = .02).
The number needed to treat with extended LMWH prophylaxis to prevent one VTE event was 14 in ENOXACAN II23 and 9 in the FAME subanalysis of cancer surgery patients.24
New systematic review of relevant trials
Leonardi et al recently published a systematic review of 26 randomized controlled trials of DVT prophylaxis in 7,639 cancer surgery patients.25 They found the overall incidence of DVT to be 12.7% in those who received pharmacologic prophylaxis compared with 35.2% in controls. They also found high-dose LMWH therapy (> 3,400 U daily) to be associated with a significantly lower incidence of DVT than low-dose LMWH therapy (≤ 3,400 U daily) (7.9% vs 14.5%, respectively; P < .01). No differences were demonstrated between LMWH and UFH in preventing DVT, DVT location, or bleeding. Bleeding complications requiring discontinuation of pharmacologic prophylaxis occurred in 3% of patients overall.
Implications of HIT
The sequelae of heparin-induced thrombocytopenia (HIT) can have major consequences for cancer surgery patients. The incidence of HIT is markedly lower with LMWH than with UFH, as demonstrated in a nested case-control study by Creekmore et al.26 These researchers also found that the average cost of an admission during which HIT developed was nearly four times as great as the average cost of an admission during which UFH or LMWH was given without development of HIT ($56,364 vs $15,231; P < .001).
EVIDENCE IN SPECIFIC ONCOLOGIC POPULATIONS
Most of the patients in the trials reviewed above underwent abdominal surgery for malignancy. Although studies of VTE prophylaxis in patients undergoing nonabdominal cancer surgery are relatively few, some data are available for a few other specific oncologic populations, as reviewed below.
Surgery for gynecologic cancer
There is a paucity of randomized controlled trials or prospective observational studies on VTE and its prevention in the gynecologic cancer surgery population. Based on small historical studies, the postoperative risk of VTE in this population varies from 12% to 35%.27,28 Twice-daily administration of UFH 5,000 U appears to be ineffective as VTE prophylaxis in this population, but increasing the frequency to three times daily reduces VTE risk by 50% to 60% compared with placebo. Once-daily LMWH is comparable to three-times-daily UFH in efficacy and safety in this population.
A systematic Cochrane review of eight randomized controlled trials in patients undergoing major gynecologic surgery revealed that heparin prophylaxis (either UFH or LMWH) reduces the risk of DVT by 70% compared with no prophylaxis, with an identical risk reduction specifically among women with malignancy (odds ratio, 0.30; 95% CI, 0.10 to 0.89).29 This review found no evidence that anticoagulation reduces the risk of PE following major gynecologic surgery. LMWH and UFH were similar in efficacy for preventing DVT and had a comparable risk of bleeding complications.
Surgery for urologic cancer
The risk of VTE and the benefits of thromboprophylaxis also are poorly studied in patients undergoing surgery for urologic cancer.
The risk of VTE varies with the type of urologic surgery and the method used to diagnose VTE. For instance, patients undergoing radical retropubic prostatectomy have been reported to develop DVT at rates of 1% to 3%, PE at rates of 1% to 3%, and fatal PE at a rate of 0.6%, whereas the incidences of these events are somewhat higher in patients undergoing cystectomy: 8% for DVT, 2% to 4% for PE, and 2% for fatal PE. Radiologic diagnosis of thromboembolism in pelvic surgery patients has yielded higher incidences, with DVT rates of 21% to 51% and PE rates of 11% to 22%.30
Small studies suggest that prophylaxis with either low-dose UFH or LMWH is both effective in reducing VTE risk and safe in urologic cancer surgery patients, although pharmacologic prophylaxis poses a possible increased risk of pelvic hematoma and lymphocele formation in this population.30
Neurosurgery
Most neurosurgical procedures are performed for malignancies. The risk of venography-confirmed VTE in patients undergoing neurosurgery is approximately 30% to 40%.31,32 Likewise, the risks of intracranial or intraspinal hemorrhage in these patients are high. For this reason, mechanical methods of VTE prophylaxis are preferred in these patients. The use of anticoagulant prophylaxis remains controversial in this setting, although more recent data suggest that it might be safer than previously recognized.
GUIDELINES FOR VTE PROPHYLAXIS IN THE CANCER SURGERY PATIENT
American College of Chest Physicians
National Comprehensive Cancer Network
The National Comprehensive Cancer Network (NCCN) recently published clinical practice guidelines on venous thromboembolic disease in cancer patients.35 The defined at-risk population for these guidelines is the adult cancer inpatient with a diagnosis of (or clinical suspicion for) cancer. The guidelines recommend prophylactic anticoagulation (category 1 recommendation) with or without a sequential compression device as initial prophylaxis, unless the patient has a relative contraindication to anticoagulation, in which case mechanical prophylaxis (sequential compression device or graduated compression stockings) is recommended. (A category 1 recommendation indicates “uniform NCCN consensus, based on high-level evidence.”)
The NCCN guidelines include a specific recommended risk-factor assessment, which includes noting the patient’s age (VTE risk increases beginning at age 40 and then steeply again at age 75), any prior VTE, the presence of familial thrombophilia or active cancer, the use of medications associated with increased VTE risk (chemotherapy, exogenous estrogen compounds, and thalidomide or lenalidomide), and a number of other risk factors for VTE as outlined in the prior two articles in this supplement. The NCCN guidelines explicitly call for assessment of modifiable risk factors for VTE (ie, smoking or other tobacco use, obesity, and a low level of activity or lack of exercise) and call for active patient education on these factors.
American Society of Clinical Oncology
Our recommended algorithm
LINGERING CHALLENGE OF UNDERUTILIZATION
Despite this consensus on ways to reduce thromboembolic risk in this population and the clear evidence of the benefit of VTE prophylaxis in patients with cancer, data from several registries confirm a persistently low utilization of prophylaxis in patients with cancer.36–38 The global Fundamental Research in Oncology and Thrombosis (FRONTLINE) study surveyed 3,891 clinicians who treat cancer patients regarding their practices with respect to VTE in those patients.36 The survey found that only 52% of respondents routinely used thromboprophylaxis for their surgical patients with cancer. More striking, however, was the finding that most respondents routinely considered thrombo-prophylaxis in only 5% of their medical oncology patients. These data are echoed by findings of other retrospective medical record reviews in patients undergoing major abdominal or abdominothoracic surgery (in many cases for cancer), with VTE prophylaxis rates ranging from 38% to 75%.37,38
SUMMARY
Patients undergoing surgery for cancer have an increased risk of VTE and fatal PE, even when thromboprophylaxis is used. Nevertheless, prophylaxis with either LMWH or UFH does reduce venographic VTE event rates in these patients. If UFH is chosen for prophylaxis, a three-times-daily regimen should be used in this population. In specific surgical cancer populations, especially those undergoing abdominal surgery, out-of-hospital prophylaxis with once-daily LMWH is warranted. Current registries reveal that compliance with established guidelines for VTE prophylaxis in this population is low.


Dr. Jaffer: Dr. Amin, based on your study on thrombo-prophylaxis rates in US medical centers, will you comment on rates of prophylaxis for cancer surgery patients?
Dr. Amin: The overall study included approximately 200,000 medical patients and about 80,000 surgical patients enrolled over more than a 3-year period between 2002 and 2005.39,40 Our goal was to assess rates of prophylaxis and, when it was provided, whether it was appropriate (in terms of type, dosage, and duration) based on the ACCP guidelines. A subanalysis assessed medical cancer patients and surgical cancer patients separately. Medical cancer patients received thromboprophylaxis 56% of the time but received appropriate prophylaxis only 28% of the time. Among surgical cancer patients, appropriate prophylaxis was given only about 24% of the time for those undergoing gynecologic surgery and about 12% of the time for those undergoing neurosurgery. These percentages are consistent with data from other national registries, such as the IMPROVE registry, which documented prophylaxis rates on the order of 45% in medical patients with cancer.41 We also analyzed the data according to individual practitioners and found that medical oncologists use prophylaxis about 25% of the time, which is relatively consistent with other providers, such as internists and surgeons.
So there is a huge opportunity to improve rates of prophylaxis for this group of patients that national guidelines say are at high risk. Why is prophylaxis so underutilized in the cancer population? One factor may be a misperception about the risk of bleeding with anticoagulants. Yet several studies have shown that the rate of bleeding from prophylaxis is extremely low, whether LMWH or UFH is used, so more awareness of actual bleeding risk is needed. Another factor is the obvious focus among internists and oncologists on treating the patient, with perhaps a reduced consideration of prophylaxis and prevention. A third factor may be a concern about thrombocytopenia. However, in our study of prophylaxis rates in US medical centers, we excluded patients who had thrombocytopenia, yet rates of prophylaxis were still low. Nothing in the literature indicates that anticoagulants cannot be used in patients with platelet counts of 50,000 to 150,000 cells/µL or higher, so this suggests that we need to do more education.
Dr. Jaffer: Dr. Brotman, can you tell us more about how clinicians in practice should use prophylaxis in their neurosurgery patients, such as those undergoing craniotomy or spine surgery for cancer? What is the safest and most efficacious way to prevent DVT in these patients?
Dr. Brotman: First, it’s important to recognize that some sort of prophylaxis needs to be used. Neurosurgery patients are at an extremely high risk for thromboembolic events, and such events are often fatal in these patients. Having said that, the jury is still out on whether the prophylaxis in these patients should be compression devices or anticoagulation. This gives physicians some latitude in their decisions. They can decide not to use pharmacologic prophylaxis so long as they use pneumatic compression devices consistently, perhaps even starting during the operation and certainly throughout hospitalization when the patient is immobilized.
Certainly, the concerns about using full-dose anticoagulation in the immediate postoperative setting in neurosurgery patients are valid. Yet these patients are at very high risk for thromboembolic events, and if we take too cautious an approach to prophylaxis in the immediate perioperative setting, more patients are going to have thromboembolic events, at which point management decisions become much more difficult. The risk of intracranial bleeding with anticoagulation to treat a patient who develops a DVT at postoperative day 10 will certainly be higher than it would have been with lower-dose perioperative prophylactic anticoagulation. Plus, if you put in a filter at that point, the outcomes tend to be poor. Therefore, I believe there is some degree of risk that we should be willing to take with regard to perioperative bleeding, even in neurosurgery patients.
Dr. McKean: I’d like to make a point about combination prophylaxis. At many institutions, compression stockings and sequential compression devices are used preoperatively and intraoperatively, and then pharmacologic prophylaxis—for example, twice-daily UFH—is used postoperatively. There is concern that these patients are hypercoagulable, and most clinicians believe that mechanical prophylaxis alone, even with sequential compression devices plus compression stockings, is not aggressive enough in these high-risk patients.
Dr. Jaffer: Dr. Spyropoulos, what is the optimal duration of pharmacologic prophylaxis for cancer surgery patients?
Dr. Spyropoulos: First let’s consider in-hospital prophylaxis. The supportive data for in-hospital prophylaxis are strong, and the duration of therapy used in the major in-hospital prophylaxis trials was 7 to 10 days. With regard to extended prophylaxis, we have at least two moderately sized randomized controlled trials, ENOXACAN II23 and the substudy of FAME,24 that demonstrated that extending prophylaxis with LMWH at doses of 3,400 U once daily (5,000 IU of dalteparin; 40 mg of enoxaparin) reduces VTE risk at postoperative day 30. Also, recent data from the @RISTOS registry show that in cancer surgery patients, especially those having abdominal or pelvic procedures, the leading cause of 30-day mortality was VTE.8 This registry also shows that despite prophylaxis, the rate of symptomatic VTE can be as high as 2%, with the rate of fatal VTE approaching 1%. Thus, in cancer patients undergoing abdominal or pelvic surgery, physicians should strongly consider prophylaxis of up to 30 days’ duration.
Dr. Jaffer: One striking finding from the @RISTOS registry was that 40% of VTE events in these cancer surgery patients occurred after postoperative day 21. This really underscores the need to consider prophylaxis for at least 4 weeks in these patients in real-world practice.
Dr. Brotman: The other striking finding from that registry was that the in-hospital prophylaxis rate was quite high, about 80%, and the rate of extended prophylaxis approached 35%. These are rates that are rarely achieved in clinical practice. Yet despite these high levels of prophylaxis, patients in this registry still had a high incidence of morbidity and mortality from VTE. This suggests that we need to improve our out-of-hospital VTE prevention paradigms.
Dr. Jaffer: Dr. Deitelzweig, oncologists and internists are often unsure about whether their ambulatory cancer patients who are receiving chemotherapy should be on any form of prophylaxis. What is your opinion?
Dr. Deitelzweig: That question comes up regularly because these patients are encountered across many medical specialties. At this point, all of the large organizations, including ASCO and NCCN, are advocating that prophylaxis is not indicated for such patients.
- Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007; 25:5490–5505.
- Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 2007; 5:632–634.
- Ambrus JL, Ambrus CM, Mink IB, Pickren JW. Causes of death in cancer patients. J Med 1975; 6:61–64.
- Donati MB. Cancer and thrombosis. Haemostasis 1994; 24:128–131.
- Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med 1996; 125:1–7.
- Haas S, Wolf H, Kakkar AK, Fareed J, Encke A. Prevention of fatal pulmonary embolism and mortality in surgical patients: a randomized double-blind comparison of LMWH with unfractionated heparin. Thromb Haemost 2005; 94:814–819.
- Kakkar AK, Haas S, Wolf H, Encke A. Evaluation of perioperative fatal pulmonary embolism and death in cancer surgical patients: the MC-4 cancer substudy. Thromb Haemost 2005; 94:867–871.
- Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg 2006; 243:89–95.
- Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy: risk analysis using Medicare claims data. Medicine (Baltimore) 1999; 78:285–291.
- Levine M, Gent M, Hirsh J, et al. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med 1996; 334:677–681.
- Bergqvist D. Risk of venous thromboembolism in patients undergoing cancer surgery and options for thromboprophylaxis. J Surg Oncol 2007; 95:167–174.
- Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000; 160:809–815.
- Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: determination of frequency and characteristics. Thromb Haemost 2002; 87:575–579.
- Kröger K, Weiland D, Ose C, et al. Risk factors for venous thromboembolic events in cancer patients. Ann Oncol 2006; 17:297–303.
- Blom JW, Vanderschoot JP, Oostindiër MJ, et al. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost 2006; 4:529–535.
- Couban S, Simpson DR, Barnett MJ, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood 2002; 100:1525–1531.
- Walshe LJ, Malak SF, Eagan J, Sepkowitz KA. Complication rates among cancer patients with peripherally inserted central catheters. J Clin Oncol 2002; 20:3276–3281.
- Nilsson KR, Berenholtz SM, Garrett-Mayer E, et al. Association between venous thromboembolism and perioperative allogeneic transfusion. Arch Surg 2007; 142:126–133.
- Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: a double-blind randomized multicentre trial with venographic assessment. ENOXACAN Study Group. Br J Surg 1997; 84:1099–1103.
- McLeod RS, Geerts WH, Sniderman KW, et al. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian Colorectal DVT Prophylaxis Trial: a randomized, double-blind trial. Ann Surg 2001; 233:438–444.
- Mismetti P, Laporte S, Darmon JY, Buchmüller A, Decousus H. Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg 2001; 88:913–930.
- Agnelli G, Bergqvist D, Cohen AT, et al, on behalf of the PEGASUS investigators. Randomized clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high-risk abdominal surgery. Br J Surg 2005; 92:1212–1220.
- Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002; 346:975–980.
- Rasmussen MS, Wille-Jorgensen P, Jorgensen LN, et al. Prolonged thromboprophylaxis with low molecular weight heparin (dalteparin) following major abdominal surgery for malignancy [abstract 186]. Blood 2003; 102:56a.
- Leonardi MJ, McGory ML, Ko CY. A systematic review of deep venous thrombosis prophylaxis in cancer patients: implications for improving quality. Ann Surg Oncol 2007; 14:929–936.
- Creekmore FM, Oderda GM, Pendleton RC, Brixner DI. Incidence and economic implications of heparin-induced thrombocytopenia in medical patients receiving prophylaxis for venous thromboembolism. Pharmacotherapy 2006; 26:1438–1445.
- Walsh JJ, Bonnar J, Wright FW. A study of pulmonary embolism and deep leg vein thrombosis after major gynaecological surgery using labeled fibrinogen-phlebography and lung scanning. J Obstet Gynaecol Br Commonw 1974; 81:311–316.
- Clarke-Pearson DL, Synan IS, Coleman RE, et al. The natural history of postoperative venous thromboemboli in gynecologic oncology: a prospective study of 382 patients. Am J Obstet Gynecol 1984; 148:1051–1054.
- Oates-Whitehead RM, D’Angelo A, Mol B. Anticoagulant and aspirin prophylaxis for preventing thromboembolism after major gynaecological surgery. Cochrane Database Syst Rev 2003; (4):CD003679.
- Kibel AS, Loughlin KR. Pathogenesis and prophylaxis of postoperative thromboembolic disease in urological pelvic surgery. J Urol 1995; 153:1763–1774.
- Agnelli G, Piovella F, Buoncristiani P, et al. Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N Engl J Med 1998; 339:80–85.
- Semrad TJ, O’Donnell R, Wun T, et al. Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg 2007; 106:601–608.
- Iorio A, Agnelli G. Low-molecular-weight and unfractionated heparin for prevention of venous thromboembolism in neurosurgery: a meta-analysis. Arch Intern Med 2000; 160:2327–2332.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Venous thromboembolic disease. V.1.2007. http://www.nccn.org/professionals/physician_gls/PDF/vte.pdf. Accessed December 5, 2007.
- Kakkar AK, Levine M, Pinedo HM, Wolff R, Wong J. Venous thrombosis in cancer patients: insights from the FRONTLINE survey. Oncologist 2003; 8:381–388.
- Stratton MA, Anderson FA, Bussey HI, et al. Prevention of venous thromboembolism: adherence to the 1995 American College of Chest Physicians consensus guidelines for surgical patients. Arch Intern Med 2000; 160:334–340.
- Bratzler DW, Raskob GE, Murray CK, Bumpus LJ, Piatt DS. Underuse of venous thromboembolism prophylaxis for general surgery patients: physician practices in the community hospital setting. Arch Intern Med 1998; 158:1909–1912.
- Amin A, Stemkowski S, Lin J, et al. Thromboprophylaxis rates in US medical centers: success or failure? J Thromb Haemost 2007; 5:1610–1616.
- Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis in US hospitals: adherence to the 6th American College of Chest Physicians’ recommendations for at-risk medical and surgical patients. Abstract presented at: 41st Midyear Clinical Meeting of the American Society of Health-System Pharmacists; December 3–7, 2006; Anaheim, CA.
- Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest 2007; 132:936–945.
- Clarke-Pearson DL, Synan IS, Dodge R, et al. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol 1993; 168:1146–1154.
- Einstein MH, Pritts EA, Hartenbach EM. Venous thromboembolism prevention in gynecologic cancer surgery: a systematic review. Gynecol Oncol 2007; 105:813–819.
- Clarke-Pearson DL, Synan IS, Hinshaw WM, Coleman RE, Creasman WT. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol 1984; 63:92–98.
- Ruff RL, Posner JB. Incidence and treatment of peripheral venous thrombosis in patients with glioma. Ann Neurol 1983; 13:334–336.
- Levin JM, Schiff D, Loeffler JS, Fine HA, Black PM, Wen PY. Complications of therapy for venous thromboembolic disease in patients with brain tumors. Neurology 1993; 43:1111–1114.
- Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007; 25:5490–5505.
- Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 2007; 5:632–634.
- Ambrus JL, Ambrus CM, Mink IB, Pickren JW. Causes of death in cancer patients. J Med 1975; 6:61–64.
- Donati MB. Cancer and thrombosis. Haemostasis 1994; 24:128–131.
- Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med 1996; 125:1–7.
- Haas S, Wolf H, Kakkar AK, Fareed J, Encke A. Prevention of fatal pulmonary embolism and mortality in surgical patients: a randomized double-blind comparison of LMWH with unfractionated heparin. Thromb Haemost 2005; 94:814–819.
- Kakkar AK, Haas S, Wolf H, Encke A. Evaluation of perioperative fatal pulmonary embolism and death in cancer surgical patients: the MC-4 cancer substudy. Thromb Haemost 2005; 94:867–871.
- Agnelli G, Bolis G, Capussotti L, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg 2006; 243:89–95.
- Levitan N, Dowlati A, Remick SC, et al. Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy: risk analysis using Medicare claims data. Medicine (Baltimore) 1999; 78:285–291.
- Levine M, Gent M, Hirsh J, et al. A comparison of low-molecular-weight heparin administered primarily at home with unfractionated heparin administered in the hospital for proximal deep-vein thrombosis. N Engl J Med 1996; 334:677–681.
- Bergqvist D. Risk of venous thromboembolism in patients undergoing cancer surgery and options for thromboprophylaxis. J Surg Oncol 2007; 95:167–174.
- Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000; 160:809–815.
- Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: determination of frequency and characteristics. Thromb Haemost 2002; 87:575–579.
- Kröger K, Weiland D, Ose C, et al. Risk factors for venous thromboembolic events in cancer patients. Ann Oncol 2006; 17:297–303.
- Blom JW, Vanderschoot JP, Oostindiër MJ, et al. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost 2006; 4:529–535.
- Couban S, Simpson DR, Barnett MJ, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood 2002; 100:1525–1531.
- Walshe LJ, Malak SF, Eagan J, Sepkowitz KA. Complication rates among cancer patients with peripherally inserted central catheters. J Clin Oncol 2002; 20:3276–3281.
- Nilsson KR, Berenholtz SM, Garrett-Mayer E, et al. Association between venous thromboembolism and perioperative allogeneic transfusion. Arch Surg 2007; 142:126–133.
- Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: a double-blind randomized multicentre trial with venographic assessment. ENOXACAN Study Group. Br J Surg 1997; 84:1099–1103.
- McLeod RS, Geerts WH, Sniderman KW, et al. Subcutaneous heparin versus low-molecular-weight heparin as thromboprophylaxis in patients undergoing colorectal surgery: results of the Canadian Colorectal DVT Prophylaxis Trial: a randomized, double-blind trial. Ann Surg 2001; 233:438–444.
- Mismetti P, Laporte S, Darmon JY, Buchmüller A, Decousus H. Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg 2001; 88:913–930.
- Agnelli G, Bergqvist D, Cohen AT, et al, on behalf of the PEGASUS investigators. Randomized clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high-risk abdominal surgery. Br J Surg 2005; 92:1212–1220.
- Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002; 346:975–980.
- Rasmussen MS, Wille-Jorgensen P, Jorgensen LN, et al. Prolonged thromboprophylaxis with low molecular weight heparin (dalteparin) following major abdominal surgery for malignancy [abstract 186]. Blood 2003; 102:56a.
- Leonardi MJ, McGory ML, Ko CY. A systematic review of deep venous thrombosis prophylaxis in cancer patients: implications for improving quality. Ann Surg Oncol 2007; 14:929–936.
- Creekmore FM, Oderda GM, Pendleton RC, Brixner DI. Incidence and economic implications of heparin-induced thrombocytopenia in medical patients receiving prophylaxis for venous thromboembolism. Pharmacotherapy 2006; 26:1438–1445.
- Walsh JJ, Bonnar J, Wright FW. A study of pulmonary embolism and deep leg vein thrombosis after major gynaecological surgery using labeled fibrinogen-phlebography and lung scanning. J Obstet Gynaecol Br Commonw 1974; 81:311–316.
- Clarke-Pearson DL, Synan IS, Coleman RE, et al. The natural history of postoperative venous thromboemboli in gynecologic oncology: a prospective study of 382 patients. Am J Obstet Gynecol 1984; 148:1051–1054.
- Oates-Whitehead RM, D’Angelo A, Mol B. Anticoagulant and aspirin prophylaxis for preventing thromboembolism after major gynaecological surgery. Cochrane Database Syst Rev 2003; (4):CD003679.
- Kibel AS, Loughlin KR. Pathogenesis and prophylaxis of postoperative thromboembolic disease in urological pelvic surgery. J Urol 1995; 153:1763–1774.
- Agnelli G, Piovella F, Buoncristiani P, et al. Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N Engl J Med 1998; 339:80–85.
- Semrad TJ, O’Donnell R, Wun T, et al. Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg 2007; 106:601–608.
- Iorio A, Agnelli G. Low-molecular-weight and unfractionated heparin for prevention of venous thromboembolism in neurosurgery: a meta-analysis. Arch Intern Med 2000; 160:2327–2332.
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):338S–400S.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Venous thromboembolic disease. V.1.2007. http://www.nccn.org/professionals/physician_gls/PDF/vte.pdf. Accessed December 5, 2007.
- Kakkar AK, Levine M, Pinedo HM, Wolff R, Wong J. Venous thrombosis in cancer patients: insights from the FRONTLINE survey. Oncologist 2003; 8:381–388.
- Stratton MA, Anderson FA, Bussey HI, et al. Prevention of venous thromboembolism: adherence to the 1995 American College of Chest Physicians consensus guidelines for surgical patients. Arch Intern Med 2000; 160:334–340.
- Bratzler DW, Raskob GE, Murray CK, Bumpus LJ, Piatt DS. Underuse of venous thromboembolism prophylaxis for general surgery patients: physician practices in the community hospital setting. Arch Intern Med 1998; 158:1909–1912.
- Amin A, Stemkowski S, Lin J, et al. Thromboprophylaxis rates in US medical centers: success or failure? J Thromb Haemost 2007; 5:1610–1616.
- Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis in US hospitals: adherence to the 6th American College of Chest Physicians’ recommendations for at-risk medical and surgical patients. Abstract presented at: 41st Midyear Clinical Meeting of the American Society of Health-System Pharmacists; December 3–7, 2006; Anaheim, CA.
- Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest 2007; 132:936–945.
- Clarke-Pearson DL, Synan IS, Dodge R, et al. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol 1993; 168:1146–1154.
- Einstein MH, Pritts EA, Hartenbach EM. Venous thromboembolism prevention in gynecologic cancer surgery: a systematic review. Gynecol Oncol 2007; 105:813–819.
- Clarke-Pearson DL, Synan IS, Hinshaw WM, Coleman RE, Creasman WT. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol 1984; 63:92–98.
- Ruff RL, Posner JB. Incidence and treatment of peripheral venous thrombosis in patients with glioma. Ann Neurol 1983; 13:334–336.
- Levin JM, Schiff D, Loeffler JS, Fine HA, Black PM, Wen PY. Complications of therapy for venous thromboembolic disease in patients with brain tumors. Neurology 1993; 43:1111–1114.