User login
To the Editor:
A 47-year-old man presented to the dermatology service with an asymptomatic plaque on the right thigh of 2 months’ duration. He had a medical history of HIV and Kaposi sarcoma as well as a recently relapsed primary effusion lymphoma (PEL) subsequent to an allogeneic bone marrow transplant. He initially was diagnosed with PEL 3 years prior to the current presentation during a workup for fever and weight loss. Imaging at the time demonstrated a bladder mass, which was biopsied and demonstrated PEL. Further imaging demonstrated both sinus and bone marrow involvement. Prior to dermatologic consultation, he had been treated with 6 cycles of etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH); 6 cycles of brentuximab; 4 cycles of rituximab with gemcitabine and oxaliplatin; and 2 cycles of ifosfamide, carboplatin, and etoposide. Despite these therapies, he had 3 relapses, and oncology determined the need for a matched unrelated donor allogeneic stem cell transplant for his PEL.
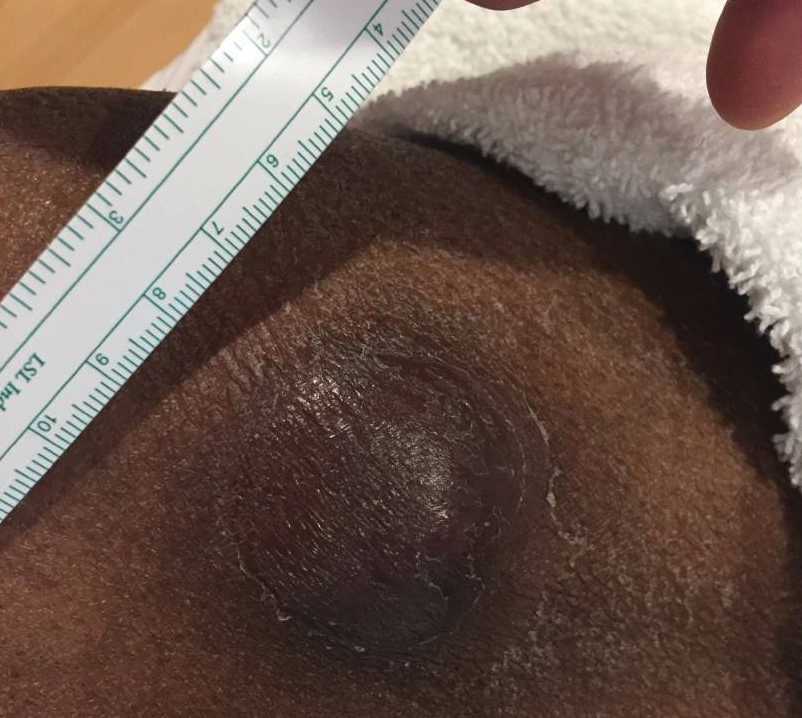
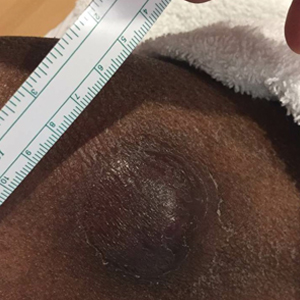
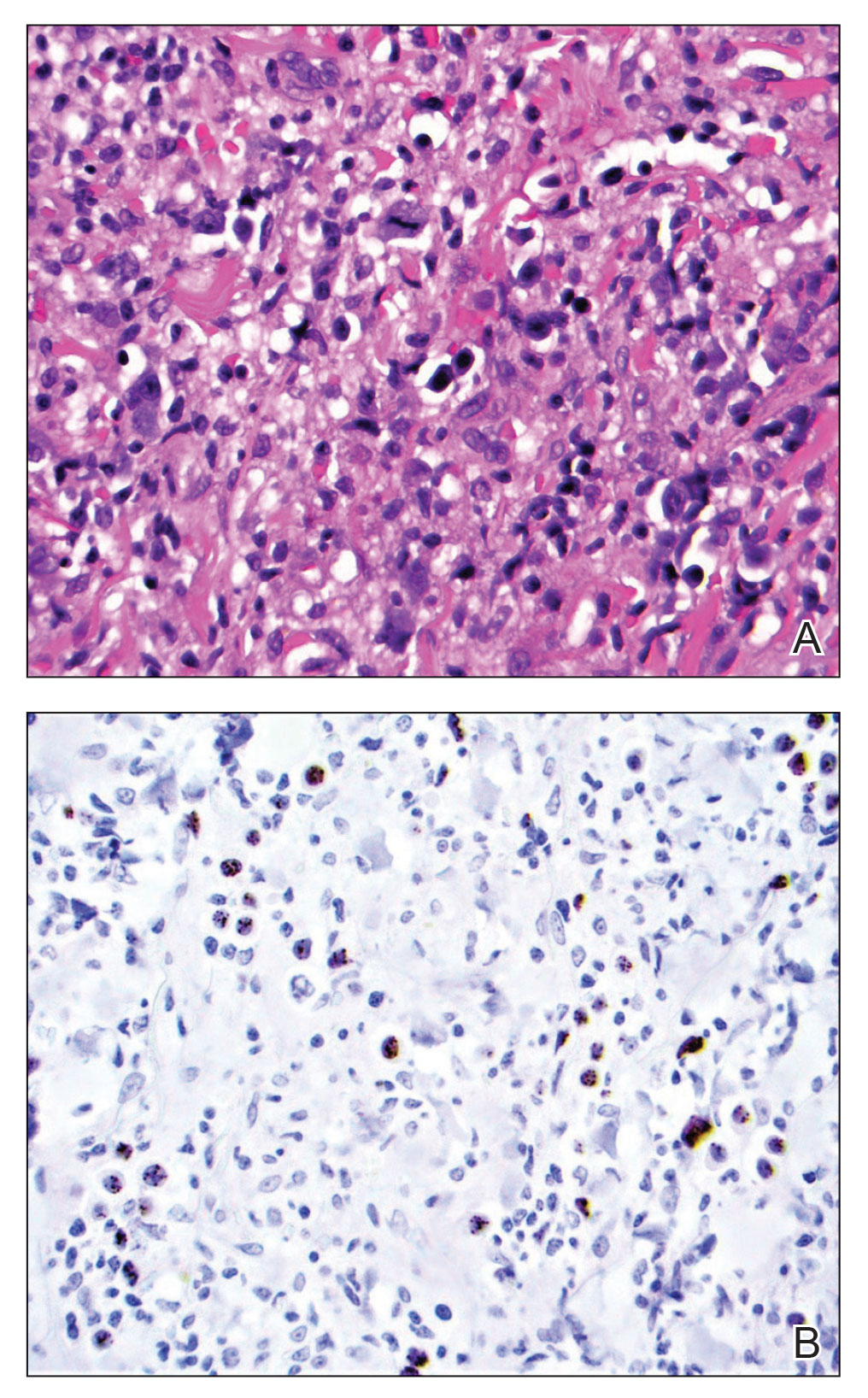
At the time of dermatology consultation, the patient was being managed on daratumumab and bortezomib. Physical examination revealed an infiltrative plaque on the right inferomedial thigh measuring approximately 6.0 cm (largest dimension) with a small amount of peripheral scale (Figure 1). An ultrasound revealed notable subcutaneous tissue edema and increased vascularity without a discrete mass or fluid collection. A 4-mm punch biopsy demonstrated a dense infiltrate comprised of collections of histiocytes admixed with scattered plasma cells and mature lymphoid aggregates. Additionally, rare enlarged plasmablastic cells with scant basophilic cytoplasm and slightly irregular nuclear contours were visualized (Figure 2A). Immunohistochemistry was positive for CD3 with a normal CD4:CD8 ratio, CD68-highlighted histiocytes within the lymphoid aggregates, and human herpesvirus 8 (HHV-8)(or Kaposi sarcoma–associated herpesvirus) demonstrated stippled nuclear staining within the scattered large cells (Figure 2B). Epstein-Barr virus–encoded RNA staining was negative, though the area of interest was lost on deeper sectioning of the tissue block. The histopathologic findings were consistent with cutaneous extracavitary PEL. Shortly after this diagnosis, he died from disease complications.
Primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that was first described by Knowles et al1 in 1989. Primary effusion lymphoma occurs exclusively in the setting of HHV-8 infection and typically is associated with chronic immunosuppression related to HIV/AIDS. Cases that are negative for HIV-1 are rare but have been reported in organ transplant recipients and elderly men from areas with a high prevalence of HHV-8 infections. Most HIV-associated cases show concurrent Epstein-Barr virus infection, though the pathogenic meaning of this co-infection remains unclear.2,3
Primary effusion lymphoma classically presents as an isolated effusion of malignant lymphoid cells within body cavities in the absence of solid tumor masses. The pleural, peritoneal, and pericardial spaces most commonly are involved. Extracavitary PEL, a rare variant, may present as a solid mass without effusion. In general, extracavitary tumors may occur in the setting of de novo malignancy or recurrent PEL.4 Cutaneous manifestations associated with extracavitary PEL are rare; 4 cases have been described in which skin lesions were the heralding sign of the disease.3 Interestingly, despite obligatory underlying HHV-8 infection, a review by Pielasinski et al3 noted only 2 patients with cutaneous PEL who had prior or concurrent Kaposi sarcoma. This heterogeneity in HHV-8–related phenotypes may be related to differences in microRNA expression, but further study is needed.5
The diagnosis of PEL relies on histologic, immunophenotypic, and molecular analysis of the affected tissue. The malignant cells typically are large with round to irregular nuclei. These cells may demonstrate a variety of appearances, including anaplastic, plasmablastic, and immunoblastic morphologies.6,7 The immunophenotype displays CD45 positivity and markers of lymphocyte activation (CD30, CD38, CD71), while typical B-cell (CD19, CD20, CD79a) and T-cell (CD3, CD4, CD8) markers often are absent.6-8 Human herpesvirus 8 detection by polymerase chain reaction testing of the peripheral blood or by immunohistochemistry staining of the affected tissue is required for diagnosis.6,7 Epstein-Barr virus infection may be detected via in situ hybridization, though it is not required for diagnosis.
The overall prognosis for PEL is poor; Brimo et al6 reported a median survival of less than 6 months, and Guillet et al9 reported 5-year overall survival (OS) for PEL vs extracavitary PEL to be 43% vs 39%. Another review noted variation in survival contingent on the number of body cavities involved; patients with a single body cavity involved experienced a median OS of 18 months, whereas patients with multiple involved cavities experienced a median OS of 4 months,7 possibly due to the limited study of treatment regimens or disease aggressiveness. Even in cases of successful initial treatment, relapse within 6 to 8 months is common. Extracavitary PEL may have improved disease-free survival relative to classic PEL, though the data were less clear for OS.9 Limitations of the Guillet et al9 study included a small sample size, the impossibility to randomize to disease type, and loss of power on the log-rank test for OS in the setting of possible nonproportional hazards (crossing survival curves). Overall, prognostic differences between the groups may be challenging to ascertain until further data are obtained.
As with many HIV-associated neoplasms, antiretroviral treatment (ART) for HIV-positive patients affords a better prognosis when used in addition to therapy directed at malignancy.7 The general approach is for concurrent ART with systemic therapies such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone for the rare CD20+ cases, and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or dose-adjusted EPOCH therapy in the more common CD20− PEL cases. Narkhede et al7 suggested avoidance of methotrexate in patients with effusions because of increased toxicity, but it is unclear if this recommendation is applicable in extracavitary PEL patients without an effusion. Additionally, second-line treatment modalities include radiation for solid PEL masses, HHV-8–targeted antivirals, and stem cell transplantation, though evidence is limited. Of note, there is a phase I-II trial (ClinicalTrials.gov identifier NCT02911142) ongoing for treatment-naïve PEL patients involving the experimental treatment DA-EPOCH-R plus lenalidomide, but the trial is ongoing.10
We report a case of cutaneous PEL in a patient with a history of Kaposi sarcoma. The patient’s deterioration and ultimate death despite initial treatment with EPOCH and bone marrow transplantation followed by final management with daratumumab and bortezomib confirm other reports that PEL has a poor prognosis and that optimal treatments are not well delineated for these patients. In general, the current approach is to utilize ART for HIV-positive patients and to then implement chemotherapy such as CHOP. Without continued research and careful planning of treatments, data will remain limited on how best to serve patients with PEL.
- Knowles DM, Inghirami G, Ubriaco A, et al. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792-799.
- Kugasia IAR, Kumar A, Khatri A, et al. Primary effusion lymphoma of the pleural space: report of a rare complication of cardiac transplant with review of the literature. Transpl Infect Dis. 2019;21:E13005.
- Pielasinski U, Santonja C, Rodriguez-Pinilla SM, et al. Extracavitary primary effusion lymphoma presenting as a cutaneous tumor: a case report and literature review. J Cutan Pathol. 2014;41:745-753.
- Boulanger E, Meignin V, Afonso PV, et al. Extracavitary tumor after primary effusion lymphoma: relapse or second distinct lymphoma? Haematologica. 2007;92:1275-1276.
- Goncalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS. 2017;31:1903-1916.
- Brimo F, Michel RP, Khetani K, et al. Primary effusion lymphoma: a series of 4 cases and review of the literature with emphasis on cytomorphologic and immunocytochemical differential diagnosis. Cancer. 2007;111:224-233.
- Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther. 2018;11:3747-3754.
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569-576.
- Guillet S, Gerard L, Meignin V, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016;91:233-237.
To the Editor:
A 47-year-old man presented to the dermatology service with an asymptomatic plaque on the right thigh of 2 months’ duration. He had a medical history of HIV and Kaposi sarcoma as well as a recently relapsed primary effusion lymphoma (PEL) subsequent to an allogeneic bone marrow transplant. He initially was diagnosed with PEL 3 years prior to the current presentation during a workup for fever and weight loss. Imaging at the time demonstrated a bladder mass, which was biopsied and demonstrated PEL. Further imaging demonstrated both sinus and bone marrow involvement. Prior to dermatologic consultation, he had been treated with 6 cycles of etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH); 6 cycles of brentuximab; 4 cycles of rituximab with gemcitabine and oxaliplatin; and 2 cycles of ifosfamide, carboplatin, and etoposide. Despite these therapies, he had 3 relapses, and oncology determined the need for a matched unrelated donor allogeneic stem cell transplant for his PEL.

At the time of dermatology consultation, the patient was being managed on daratumumab and bortezomib. Physical examination revealed an infiltrative plaque on the right inferomedial thigh measuring approximately 6.0 cm (largest dimension) with a small amount of peripheral scale (Figure 1). An ultrasound revealed notable subcutaneous tissue edema and increased vascularity without a discrete mass or fluid collection. A 4-mm punch biopsy demonstrated a dense infiltrate comprised of collections of histiocytes admixed with scattered plasma cells and mature lymphoid aggregates. Additionally, rare enlarged plasmablastic cells with scant basophilic cytoplasm and slightly irregular nuclear contours were visualized (Figure 2A). Immunohistochemistry was positive for CD3 with a normal CD4:CD8 ratio, CD68-highlighted histiocytes within the lymphoid aggregates, and human herpesvirus 8 (HHV-8)(or Kaposi sarcoma–associated herpesvirus) demonstrated stippled nuclear staining within the scattered large cells (Figure 2B). Epstein-Barr virus–encoded RNA staining was negative, though the area of interest was lost on deeper sectioning of the tissue block. The histopathologic findings were consistent with cutaneous extracavitary PEL. Shortly after this diagnosis, he died from disease complications.

Primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that was first described by Knowles et al1 in 1989. Primary effusion lymphoma occurs exclusively in the setting of HHV-8 infection and typically is associated with chronic immunosuppression related to HIV/AIDS. Cases that are negative for HIV-1 are rare but have been reported in organ transplant recipients and elderly men from areas with a high prevalence of HHV-8 infections. Most HIV-associated cases show concurrent Epstein-Barr virus infection, though the pathogenic meaning of this co-infection remains unclear.2,3
Primary effusion lymphoma classically presents as an isolated effusion of malignant lymphoid cells within body cavities in the absence of solid tumor masses. The pleural, peritoneal, and pericardial spaces most commonly are involved. Extracavitary PEL, a rare variant, may present as a solid mass without effusion. In general, extracavitary tumors may occur in the setting of de novo malignancy or recurrent PEL.4 Cutaneous manifestations associated with extracavitary PEL are rare; 4 cases have been described in which skin lesions were the heralding sign of the disease.3 Interestingly, despite obligatory underlying HHV-8 infection, a review by Pielasinski et al3 noted only 2 patients with cutaneous PEL who had prior or concurrent Kaposi sarcoma. This heterogeneity in HHV-8–related phenotypes may be related to differences in microRNA expression, but further study is needed.5
The diagnosis of PEL relies on histologic, immunophenotypic, and molecular analysis of the affected tissue. The malignant cells typically are large with round to irregular nuclei. These cells may demonstrate a variety of appearances, including anaplastic, plasmablastic, and immunoblastic morphologies.6,7 The immunophenotype displays CD45 positivity and markers of lymphocyte activation (CD30, CD38, CD71), while typical B-cell (CD19, CD20, CD79a) and T-cell (CD3, CD4, CD8) markers often are absent.6-8 Human herpesvirus 8 detection by polymerase chain reaction testing of the peripheral blood or by immunohistochemistry staining of the affected tissue is required for diagnosis.6,7 Epstein-Barr virus infection may be detected via in situ hybridization, though it is not required for diagnosis.
The overall prognosis for PEL is poor; Brimo et al6 reported a median survival of less than 6 months, and Guillet et al9 reported 5-year overall survival (OS) for PEL vs extracavitary PEL to be 43% vs 39%. Another review noted variation in survival contingent on the number of body cavities involved; patients with a single body cavity involved experienced a median OS of 18 months, whereas patients with multiple involved cavities experienced a median OS of 4 months,7 possibly due to the limited study of treatment regimens or disease aggressiveness. Even in cases of successful initial treatment, relapse within 6 to 8 months is common. Extracavitary PEL may have improved disease-free survival relative to classic PEL, though the data were less clear for OS.9 Limitations of the Guillet et al9 study included a small sample size, the impossibility to randomize to disease type, and loss of power on the log-rank test for OS in the setting of possible nonproportional hazards (crossing survival curves). Overall, prognostic differences between the groups may be challenging to ascertain until further data are obtained.
As with many HIV-associated neoplasms, antiretroviral treatment (ART) for HIV-positive patients affords a better prognosis when used in addition to therapy directed at malignancy.7 The general approach is for concurrent ART with systemic therapies such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone for the rare CD20+ cases, and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or dose-adjusted EPOCH therapy in the more common CD20− PEL cases. Narkhede et al7 suggested avoidance of methotrexate in patients with effusions because of increased toxicity, but it is unclear if this recommendation is applicable in extracavitary PEL patients without an effusion. Additionally, second-line treatment modalities include radiation for solid PEL masses, HHV-8–targeted antivirals, and stem cell transplantation, though evidence is limited. Of note, there is a phase I-II trial (ClinicalTrials.gov identifier NCT02911142) ongoing for treatment-naïve PEL patients involving the experimental treatment DA-EPOCH-R plus lenalidomide, but the trial is ongoing.10
We report a case of cutaneous PEL in a patient with a history of Kaposi sarcoma. The patient’s deterioration and ultimate death despite initial treatment with EPOCH and bone marrow transplantation followed by final management with daratumumab and bortezomib confirm other reports that PEL has a poor prognosis and that optimal treatments are not well delineated for these patients. In general, the current approach is to utilize ART for HIV-positive patients and to then implement chemotherapy such as CHOP. Without continued research and careful planning of treatments, data will remain limited on how best to serve patients with PEL.
To the Editor:
A 47-year-old man presented to the dermatology service with an asymptomatic plaque on the right thigh of 2 months’ duration. He had a medical history of HIV and Kaposi sarcoma as well as a recently relapsed primary effusion lymphoma (PEL) subsequent to an allogeneic bone marrow transplant. He initially was diagnosed with PEL 3 years prior to the current presentation during a workup for fever and weight loss. Imaging at the time demonstrated a bladder mass, which was biopsied and demonstrated PEL. Further imaging demonstrated both sinus and bone marrow involvement. Prior to dermatologic consultation, he had been treated with 6 cycles of etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin (EPOCH); 6 cycles of brentuximab; 4 cycles of rituximab with gemcitabine and oxaliplatin; and 2 cycles of ifosfamide, carboplatin, and etoposide. Despite these therapies, he had 3 relapses, and oncology determined the need for a matched unrelated donor allogeneic stem cell transplant for his PEL.

At the time of dermatology consultation, the patient was being managed on daratumumab and bortezomib. Physical examination revealed an infiltrative plaque on the right inferomedial thigh measuring approximately 6.0 cm (largest dimension) with a small amount of peripheral scale (Figure 1). An ultrasound revealed notable subcutaneous tissue edema and increased vascularity without a discrete mass or fluid collection. A 4-mm punch biopsy demonstrated a dense infiltrate comprised of collections of histiocytes admixed with scattered plasma cells and mature lymphoid aggregates. Additionally, rare enlarged plasmablastic cells with scant basophilic cytoplasm and slightly irregular nuclear contours were visualized (Figure 2A). Immunohistochemistry was positive for CD3 with a normal CD4:CD8 ratio, CD68-highlighted histiocytes within the lymphoid aggregates, and human herpesvirus 8 (HHV-8)(or Kaposi sarcoma–associated herpesvirus) demonstrated stippled nuclear staining within the scattered large cells (Figure 2B). Epstein-Barr virus–encoded RNA staining was negative, though the area of interest was lost on deeper sectioning of the tissue block. The histopathologic findings were consistent with cutaneous extracavitary PEL. Shortly after this diagnosis, he died from disease complications.

Primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that was first described by Knowles et al1 in 1989. Primary effusion lymphoma occurs exclusively in the setting of HHV-8 infection and typically is associated with chronic immunosuppression related to HIV/AIDS. Cases that are negative for HIV-1 are rare but have been reported in organ transplant recipients and elderly men from areas with a high prevalence of HHV-8 infections. Most HIV-associated cases show concurrent Epstein-Barr virus infection, though the pathogenic meaning of this co-infection remains unclear.2,3
Primary effusion lymphoma classically presents as an isolated effusion of malignant lymphoid cells within body cavities in the absence of solid tumor masses. The pleural, peritoneal, and pericardial spaces most commonly are involved. Extracavitary PEL, a rare variant, may present as a solid mass without effusion. In general, extracavitary tumors may occur in the setting of de novo malignancy or recurrent PEL.4 Cutaneous manifestations associated with extracavitary PEL are rare; 4 cases have been described in which skin lesions were the heralding sign of the disease.3 Interestingly, despite obligatory underlying HHV-8 infection, a review by Pielasinski et al3 noted only 2 patients with cutaneous PEL who had prior or concurrent Kaposi sarcoma. This heterogeneity in HHV-8–related phenotypes may be related to differences in microRNA expression, but further study is needed.5
The diagnosis of PEL relies on histologic, immunophenotypic, and molecular analysis of the affected tissue. The malignant cells typically are large with round to irregular nuclei. These cells may demonstrate a variety of appearances, including anaplastic, plasmablastic, and immunoblastic morphologies.6,7 The immunophenotype displays CD45 positivity and markers of lymphocyte activation (CD30, CD38, CD71), while typical B-cell (CD19, CD20, CD79a) and T-cell (CD3, CD4, CD8) markers often are absent.6-8 Human herpesvirus 8 detection by polymerase chain reaction testing of the peripheral blood or by immunohistochemistry staining of the affected tissue is required for diagnosis.6,7 Epstein-Barr virus infection may be detected via in situ hybridization, though it is not required for diagnosis.
The overall prognosis for PEL is poor; Brimo et al6 reported a median survival of less than 6 months, and Guillet et al9 reported 5-year overall survival (OS) for PEL vs extracavitary PEL to be 43% vs 39%. Another review noted variation in survival contingent on the number of body cavities involved; patients with a single body cavity involved experienced a median OS of 18 months, whereas patients with multiple involved cavities experienced a median OS of 4 months,7 possibly due to the limited study of treatment regimens or disease aggressiveness. Even in cases of successful initial treatment, relapse within 6 to 8 months is common. Extracavitary PEL may have improved disease-free survival relative to classic PEL, though the data were less clear for OS.9 Limitations of the Guillet et al9 study included a small sample size, the impossibility to randomize to disease type, and loss of power on the log-rank test for OS in the setting of possible nonproportional hazards (crossing survival curves). Overall, prognostic differences between the groups may be challenging to ascertain until further data are obtained.
As with many HIV-associated neoplasms, antiretroviral treatment (ART) for HIV-positive patients affords a better prognosis when used in addition to therapy directed at malignancy.7 The general approach is for concurrent ART with systemic therapies such as rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone for the rare CD20+ cases, and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or dose-adjusted EPOCH therapy in the more common CD20− PEL cases. Narkhede et al7 suggested avoidance of methotrexate in patients with effusions because of increased toxicity, but it is unclear if this recommendation is applicable in extracavitary PEL patients without an effusion. Additionally, second-line treatment modalities include radiation for solid PEL masses, HHV-8–targeted antivirals, and stem cell transplantation, though evidence is limited. Of note, there is a phase I-II trial (ClinicalTrials.gov identifier NCT02911142) ongoing for treatment-naïve PEL patients involving the experimental treatment DA-EPOCH-R plus lenalidomide, but the trial is ongoing.10
We report a case of cutaneous PEL in a patient with a history of Kaposi sarcoma. The patient’s deterioration and ultimate death despite initial treatment with EPOCH and bone marrow transplantation followed by final management with daratumumab and bortezomib confirm other reports that PEL has a poor prognosis and that optimal treatments are not well delineated for these patients. In general, the current approach is to utilize ART for HIV-positive patients and to then implement chemotherapy such as CHOP. Without continued research and careful planning of treatments, data will remain limited on how best to serve patients with PEL.
- Knowles DM, Inghirami G, Ubriaco A, et al. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792-799.
- Kugasia IAR, Kumar A, Khatri A, et al. Primary effusion lymphoma of the pleural space: report of a rare complication of cardiac transplant with review of the literature. Transpl Infect Dis. 2019;21:E13005.
- Pielasinski U, Santonja C, Rodriguez-Pinilla SM, et al. Extracavitary primary effusion lymphoma presenting as a cutaneous tumor: a case report and literature review. J Cutan Pathol. 2014;41:745-753.
- Boulanger E, Meignin V, Afonso PV, et al. Extracavitary tumor after primary effusion lymphoma: relapse or second distinct lymphoma? Haematologica. 2007;92:1275-1276.
- Goncalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS. 2017;31:1903-1916.
- Brimo F, Michel RP, Khetani K, et al. Primary effusion lymphoma: a series of 4 cases and review of the literature with emphasis on cytomorphologic and immunocytochemical differential diagnosis. Cancer. 2007;111:224-233.
- Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther. 2018;11:3747-3754.
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569-576.
- Guillet S, Gerard L, Meignin V, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016;91:233-237.
- Knowles DM, Inghirami G, Ubriaco A, et al. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792-799.
- Kugasia IAR, Kumar A, Khatri A, et al. Primary effusion lymphoma of the pleural space: report of a rare complication of cardiac transplant with review of the literature. Transpl Infect Dis. 2019;21:E13005.
- Pielasinski U, Santonja C, Rodriguez-Pinilla SM, et al. Extracavitary primary effusion lymphoma presenting as a cutaneous tumor: a case report and literature review. J Cutan Pathol. 2014;41:745-753.
- Boulanger E, Meignin V, Afonso PV, et al. Extracavitary tumor after primary effusion lymphoma: relapse or second distinct lymphoma? Haematologica. 2007;92:1275-1276.
- Goncalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS. 2017;31:1903-1916.
- Brimo F, Michel RP, Khetani K, et al. Primary effusion lymphoma: a series of 4 cases and review of the literature with emphasis on cytomorphologic and immunocytochemical differential diagnosis. Cancer. 2007;111:224-233.
- Narkhede M, Arora S, Ujjani C. Primary effusion lymphoma: current perspectives. Onco Targets Ther. 2018;11:3747-3754.
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569-576.
- Guillet S, Gerard L, Meignin V, et al. Classic and extracavitary primary effusion lymphoma in 51 HIV-infected patients from a single institution. Am J Hematol. 2016;91:233-237.
Practice Points
- Extracavitary primary effusion lymphoma is an aggressive non-Hodgkin B-cell lymphoma that occurs solely in the presence of human herpesvirus 8 infection and typically is associated with HIV/AIDS.
- Diagnosis necessitates a thorough workup and correlation of histologic, molecular, and immunophenotypic analysis.
- Antiretroviral therapy in HIV-positive patients and intensive chemotherapy regimens are the current recommended treatments. Despite newer targeted agents, the prognosis remains poor.