User login
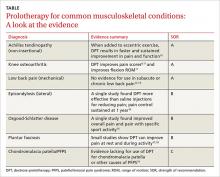
› Advise patients with Achilles tendinopathy that a combination of prolotherapy and eccentric exercise is likely to provide more rapid and sustained pain relief than either option alone. A
› Offer a third round of prolotherapy to a patient whose pain and/or function has not improved or has returned after 2 treatments. C
› Consider prolotherapy administered by a physician with expertise in the technique for adolescents with recalcitrant Osgood-Schlatter disease. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Over the past several years, prolotherapy has been gaining support as an option for patients with tendinopathies and painful osteoarthritic conditions. Yet the technique lacks both a consistent definition and an abundance of evidence.
Because the prefix “prolo” is thought to refer to proliferation or regeneration, some physicians prefer the term “sclerotherapy” when injecting sclerosing agents. Others point out that “prolotherapy” refers to the proliferation of tissue that the injections provoke, which has never been proven. We believe that the material injected should dictate the term used to describe it—dextrose prolotherapy (DPT) or platelet-rich plasma therapy (PRP), for example.
In this update, we focus on DPT—the injection of a solution containing hypertonic dextrose into ligaments, tendons, and joints to promote healing. You’ll find an overview of the proposed mechanism of action and a description of the technique (see “How DPT works”1-9), as well as a look at the evidence of its effectiveness for a variety of musculoskeletal conditions in the text and TABLE9-19 that follow. Our review is limited by the dearth of large, definitive studies, and consists mainly of anecdotal evidence, case reports, and other low-quality studies.
Considering DPT—for which patients?
Even for conditions for which the evidence of its efficacy is unequivocal, DPT is only one part of a comprehensive treatment plan. Functional assessment and correction of any weaknesses, inflexibilities, and/or training errors are also essential.
There are a number of other considerations, as well. For one thing, DPT is rarely covered by health insurance20 and is often considered only after conservative treatment has failed. What’s more, it is not suited to every patient.
Absolute contraindications include acute infections at the injection site, such as cellulitis, abscess, or septic arthritis. Relative contraindications include acute gout flare and acute fracture near the site.6
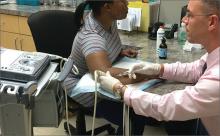
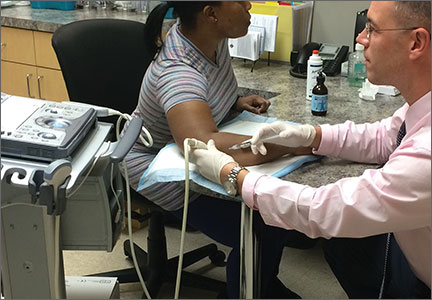
When DPT is a viable alternative, keep in mind that the procedure should only be done by a physician experienced in the technique—and that ultrasound guidance should be used to ensure precise anatomical delivery (FIGURE 1).21 Consent must be obtained and documented, and universal precautions observed.
Read on to find out whether to consider DPT for particular patients.
Achilles tendinopathy: DPT decreases pain, improves function (SOR A)
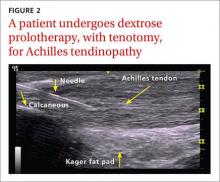
Non-insertional Achilles tendinopathy can be treated with prolotherapy to decrease pain and tendon thickness (FIGURE 2). A small, single blind randomized trial compared the effectiveness of eccentric exercise (ie, contractions performed to lengthen the muscle), DPT alone, and a combination of DPT and exercise for patients with chronic Achilles tendinopathy.10
The investigators found greater improvement in the Victorian Institute of Sport Assessment-Achilles (VISA-A) score at 12 months with the combined therapy (41.1 on a 0-100 scale) vs either eccentric exercise (23.7) or DPT (27.5) alone. The increase from baseline was greater for those who received combination therapy at 6 weeks (+11.7) compared with the eccentric-only group.10 Adding DPT (injected into the tender points of the subcutaneous tissues adjacent to the Achilles tendon) to eccentric exercise resulted in a more rapid and sustained improvement in pain, function, and stiffness.
In an earlier observational study, researchers achieved improvement in pain scores using a different DPT technique.22 Here, patients with chronic Achilles tendinosis received ultrasound-guided intratendinous dextrose injections every 6 weeks until symptoms resolved. Pain scores, calculated using a visual analogue scale (VAS), showed a mean reduction at rest (88%), during normal daily activities (84%), and during physical activity (78%). The mean number of treatment sessions was 4, and the mean time to achieve results was 30 weeks.22
Studies have shown that inflammatory changes are infrequently associated with chronic painful tendon conditions.1,2 Instead, the changes are degenerative in nature, and can occur in the main body of the tendon, in its bony insertion site, and in the structures surrounding the tendon.3 While the exact mechanism of action for DPT is unknown, studies have shown that cells exposed to hypertonic dextrose undergo osmotic lysis, creating a proinflammatory environment. This leads to recruitment of several growth factors that promote the healing of tendons, ligaments, and cartilage.4-6
Neovascularity and neuronal ingrowth, also present in tendinopathies, are believed to be a source of pain, as well. The injection of hypertonic dextrose may destroy the neovasculature, thus removing a nidus, or focal point, for pain.7
Concentrations of dextrose used may range from 10% to 50% and be combined with an injectable anesthetic alone or with other proliferants/sclerosing agents.6 We prefer a 50/50 mixture of 50% dextrose and 2% xylocaine without epinephrine, resulting in a final injection concentration of 25% dextrose and 1% xylocaine.
Techniques for tendinopathies vary from bathing the tendon without tenotomy to performing multiple tenotomies (with or without injection material into the tenotomy). For knee osteoarthritis, for example, both extra- and intra-articular approaches can be used alone or in combination.8,9 The extra-articular injections are done either at tender locations around the knee joint or at ligamentous attachment sites. The number of injection sessions can vary, as well. Variations in both the concentrations and techniques contribute to the difficulty in interpreting existing evidence.
Knee osteoarthritis: Pain level and movement improve (SOR A)
In a study of patients with knee osteoarthritis (OA) and pain lasting 6 months or more, participants received bimonthly injections of either DPT with lidocaine or lidocaine alone. At 12 months, only those in the DPT group had achieved significant improvement in VAS pain score (44%), self-reported swelling (63%), and knee flexion (14%).11
A more recent study randomized 90 adults with painful knee OA of at least 3 months’ duration to blinded injection (either DPT or saline) or at-home exercise.9 The injections involved both intra- and extra-articular techniques, performed monthly for a total of 3 to 5 injections. At 52 weeks, the DPT group had improved scores on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) by 15.3 points compared with the saline group (7.6 points) and the exercise-only group (8.2 points).
Half of those receiving DPT improved by 12 or more points, compared with less than a third of those receiving saline and a quarter of those treated with exercise alone. Knee Pain Scale (KPS)-based pain frequency and severity were also significantly reduced in the DPT group vs both comparison groups.9
Finger OA. One small randomized study tested the efficacy of DPT in patients with symptomatic finger OA affecting the distal or proximal interphalangeal joint or the trapeziometacarpal (thumb) joint.23 Participants received either DPT with xylocaine or xylocaine alone. Injections were done on the medial and lateral aspects of the affected joints at baseline, 2, and 4 months. Pain (VAS score) during active finger movement improved by 45% in the DPT group vs 15% in the group treated with xylocaine alone. After 6 months, those in the xylocaine-only group received the DPT protocol, and their pain reduction scores rose, on average, from 18% to 54%.23
Low back pain: Little help for chronic condition (SOR A)
Early studies of DPT for the treatment of low back pain had conflicting results. In 2004, the largest (N=110) and most rigorous study of DPT for chronic non-specific low back pain to date12 found no significant improvement.
Participants received either DPT or normal saline injections into tender lumbopelvic ligaments every 2 weeks for a total of 6 treatments. They were then randomized to either core and low back strengthening exercises or normal activity for 6 months. At 12 months, VAS pain and disability scores significantly decreased from baseline in all the groups, with a decline ranging from 26% to 44% for pain and 30% to 44% for disability. However, at no point were there significant differences between injection groups or activity groups.12
A 2007 Cochrane review found insufficient evidence to support the use of DPT alone for the treatment of non-specific low back pain but suggested that, as an adjunct, it may improve pain and disability scores.13 And in 2011, a Cochrane review confirmed that there was insufficient evidence for the use of DPT in sub-acute and chronic low back pain.14 Other studies on the use of DPT for specific low back conditions, including sacroiliac joint pain,24,25 coccydynia,26 and degenerative disc disease,27 have shown trends toward improvement in pain scores24-27 and disability,25 but only one of these was a randomized controlled trial (RCT).25
Lateral epicondylosis: More effective than saline (SOR B)
A single RCT compared DPT to placebo in patients with 6 months of moderate to severe lateral epicondylosis who had failed conservative treatment. Patients received 3 injections of either hypertonic dextrose or saline tendon insertions every 4 weeks, with needle touching bone at the supracondylar ridge, lateral epicondyle, and annular ligament.15 Patients randomly assigned to DPT experienced significant pain relief from baseline to 16 weeks, with a Likert score decline from 5.1 to 0.5, compared with the saline group (4.5 at baseline and 3.5 at 16 weeks). Clinical improvement was maintained at 52-week follow-up.15
Osgood-Schlatter: DPT improves pain relief (SOR B)
In one of the few studies of prolotherapy for adolescents, patients with recalcitrant Osgood-Schlatter disease were randomized to either structured physical therapy or 3 monthly injections of lidocaine, with or without dextrose, over the apophysis and patellar tendon origin.16 Injections began at the most distal point of tenderness and were repeated at 1 cm intervals for a total of 3 to 4 midline injections. The proximal injections were deep to the patellar tendon, on the tibia above the tuberosity.
Pain scores, measured by the Nirschl Pain Phase Scale (0-7), improved significantly more in the DPT group (3.9) compared with either the lidocaine group (2.4) or the exercise group (1.2). Dextrose-treated knees were significantly more likely than knees treated with lidocaine (14 of 21 vs 5 of 22) to be asymptomatic with sport activity. After 3 months, patients in the lidocaine and exercise groups who had not responded adequately were given the option of receiving DPT; those who underwent the 3-month DPT protocol achieved the same level of improvement as the initial DPT group.16
When considering or recommending DPT for an adolescent with Osgood-Schlatter disease, however, it is particularly important that he or she be referred to a physician with expertise in prolotherapy.
Plantar fasciosis: A possibility when conservative treatment fails (SOR B)
An early case series showed that DPT effectively improved pain at rest and during activity in patients with chronic plantar fasciosis refractory to conservative care.17 A small RCT recently compared PRP with DPT in such patients.18
Pain, disability, and activity limitation were measured by the Foot Functional Index. The PRP group improved by 29.7%, 26.6%, and 28% in pain, disability, and activity limitation, respectively, vs improvements of 17%, 14.5%, and 12.4% in the DPT group. Although there was a trend for PRP to be superior, the results were not statistically significant.18 This suggests that DPT may be an additional treatment option for patients with plantar fasciosis when conservative treatment fails.
Chondromalacia patella: Not enough is known (SOR C)
One study showed that DPT improved self-reported pain and function scores in patients with chronic knee pain secondary to chondromalacia patella. However, the study had no control group and no standardized injected solution; rather, the solution was tailored for each individual.19 Thus, there is insufficient data to make recommendations regarding the effectiveness of DPT in treating chondromalacia patella or other causes of patellofemoral pain syndrome.
What to tell patients about recovery and adverse effects
Injection of dextrose into ligaments, tendons, and joints carries the theoretical risks of light-headedness, allergic reaction, infection, and structural damage. However, there have been no reports of serious or significant adverse events associated with DPT when used for peripheral joint indications.
The most common risks of DPT are needle trauma-induced pain, mild bleeding, and bruising. A sense of fullness, stiffness, and occasional numbness at the site at the time of injection are common, benign, and typically self-limiting.6 If post-procedure numbness continues, the patient should follow up in 48 to 72 hours to rule out nerve damage.
Post-injection pain flare during the first 72 hours may occur. In a study of prolotherapy for knee OA pain, 10% to 20% of patients experienced such flares.15 Most patients respond well to acetaminophen and experience resolution of pain within a week. Non-steroidal anti-inflammatory drugs should not be used to treat post-procedure pain because they may interfere with the local inflammatory response needed for healing. Regular activities can be resumed immediately after an injection into a large joint, such as the knee, or after full sensation and proprioception returns if an anesthetic was used in combination with the hypertonic dextrose.
There is a theoretical risk of tendon weakening and rupture with tenotomy/intra-substance injections into weight-bearing tendons, but there are no known published reports of this complication with DPT. Nonetheless, we recommend that patients limit ballistic weight bearing or full strength activity for 48 hours after an injection into a non-weight bearing tendon and for 5 to7 days for injection into a weight-bearing tendon.
Physical/occupational therapy is important in chronic tendinopathy, and we encourage rapid return (24-48 hours) to low-intensity rehabilitation with gradual return (5-7 days) to full rehabilitation exercises.
The number of DPT injection sessions is variable. We recommend follow-up between 3 and 6 weeks for reevaluation. If the patient’s pain and/or function has not improved after 2 sets of injections—or DPT is initially successful but pain or dysfunction returns—another round of treatment should be offered in 3 to 6 weeks.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Fort Belvoir Community Hospital, Sports Medicine, Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; carlton.covey@usuhs.edu.
1. Khan KM, Cook J, Bonar F, et al. Histopathology of common tendinopathies: update and implications for clinical management. Sports Med. 1999;27:393-408.
2. Streit JJ, Shishani Y, Rodgers M, et al. Tendinopathy of the long head of the biceps tendon: histopathologic analysis of the extraarticular biceps tendon and tenosynovium. Open Access J Sports Med. 2015;10:63-70.
3. Maganaris CN, Narici MV, Almekinders LC, et al. Biomechanics and pathophysiology of overuse tendon injuries. Sports Med. 2004;34:1005-1017.
4. Jensen KT, Rabago DP, Zgierska A, et al. Response of knee ligaments to prolotherapy in a rat injury model. Am J Sports Med. 2008;36:1347-1357.
5. Kim SR, Stitik TP, Foye PM, et al. Critical review of prolotherapy for osteoarthritis, low back pain, and other musculoskeletal conditions: A physiatric perspective. Am J Phys Med Rehabil. 2004;83:379–389.
6. Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37:65-80.
7. Joseph MF, Denegar CR. Treating tendinopathy: perspective on anti-inflammatory intervention and therapeutic exercise. Clin Sports Med. 2015;34:363-374.
8. Eslamian F, Amouzandeh B. Therapeutic effects of prolotherapy with intra-articular dextrose injection in patients with osteoarthritis: a single-arm study with 6 months follow up. Ther Adv Musculoskelet Dis. 2015;7:35-44.
9. Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
10. Yelland MJ, Sweeting KR, Lyftogt JA, et al. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomised trial. Br J Sports Med. 2011;45:421-428.
11. Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68–74.
12. Yelland MJ, Glasziou PP, Bogduk N, et al. Prolotherapy injections, saline injections, and exercises for chronic low back pain: a randomized control trial. Spine. 2004;29:9-16.
13. Dagenais S, Yelland MJ, Del Mar C, et al. Prolotherapy injections for chronic low back pain. Cochrane Database Syst Rev. 2007;18(2):CD004059.
14. Staal JB, de Bie R, de Vet HCW, et al. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev. 2011;(3):CD001824.
15. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18:248.
16. Topol GA, Podesta LA, Reeves KD, et al. Hyperosmolar dextrose injection for recalcitrant Osgood-Schlatter disease. Pediatrics. 2011;128:e1121-e1128.
17. Ryan MB, Wong AD, Gillies JH, et al. Sonographically guided intratendinous injections of hyperosmolar dextrose/lidocaine: a pilot study for the treatment of chronic plantar fasciitis. Br J Sports Med. 2009;43:3003-3006.
18. Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PMR. 2014;6:152-158.
19. Hauser RA, Sprague IS. Outcomes of prolotherapy in chondromalacia patella patients: improvements in pain level and function. Clin Med Insights Arthritis Musculoskelet Disord. 2014;17:13-20.
20. United Healthcare medical policy. Prolotherapy for musculoskeletal indications. Available at: https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/Medical%20Policies/Medical%20Policies/Prolotherapy_for_Musculoskeletal_Indications.pdf. Accessed October 26, 2015.
21. Davidson J, Javaraman S. Guided interventions in musculoskeletal ultrasound: where’s the evidence? Clin Radiol. 2011;66:140-152.
22. Maxwell NJ, Ryan MB, Taunton JE, et al. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. Am J Roentgenol. 2007;189:W215.
23. Reeves KD, Hassanein K. Randomized, perspective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DTaP, PIP and Traneziometacarpal) joints: Evidence of clinical efficacy. J Altern Complem Med. 2000;6:311-320.
24. Cusi M, Saunders J, Hungerford B, et al. The use of prolotherapy in the sacroiliac joint. Br J Sports Med. 2010;44:100-104.
25. Kim WM, Lee HG, Jeong CW, et al. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med. 2010;16:1285-1290.
26. Khan SA, Kumar A, Varshney MK, et al. Dextrose prolotherapy for recalcitrant coccygodynia. J Orthop Surg. (Hong Kong) 2008;16:27–29.
27. Miller MR, Mathews RS, Reeves KD. Treatment of painful advanced internal lumbar disc derangement with intradiscal injection of hypertonic dextrose. Pain Physician. 2006;9:115–121.
› Advise patients with Achilles tendinopathy that a combination of prolotherapy and eccentric exercise is likely to provide more rapid and sustained pain relief than either option alone. A
› Offer a third round of prolotherapy to a patient whose pain and/or function has not improved or has returned after 2 treatments. C
› Consider prolotherapy administered by a physician with expertise in the technique for adolescents with recalcitrant Osgood-Schlatter disease. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Over the past several years, prolotherapy has been gaining support as an option for patients with tendinopathies and painful osteoarthritic conditions. Yet the technique lacks both a consistent definition and an abundance of evidence.
Because the prefix “prolo” is thought to refer to proliferation or regeneration, some physicians prefer the term “sclerotherapy” when injecting sclerosing agents. Others point out that “prolotherapy” refers to the proliferation of tissue that the injections provoke, which has never been proven. We believe that the material injected should dictate the term used to describe it—dextrose prolotherapy (DPT) or platelet-rich plasma therapy (PRP), for example.
In this update, we focus on DPT—the injection of a solution containing hypertonic dextrose into ligaments, tendons, and joints to promote healing. You’ll find an overview of the proposed mechanism of action and a description of the technique (see “How DPT works”1-9), as well as a look at the evidence of its effectiveness for a variety of musculoskeletal conditions in the text and TABLE9-19 that follow. Our review is limited by the dearth of large, definitive studies, and consists mainly of anecdotal evidence, case reports, and other low-quality studies.
Considering DPT—for which patients?
Even for conditions for which the evidence of its efficacy is unequivocal, DPT is only one part of a comprehensive treatment plan. Functional assessment and correction of any weaknesses, inflexibilities, and/or training errors are also essential.
There are a number of other considerations, as well. For one thing, DPT is rarely covered by health insurance20 and is often considered only after conservative treatment has failed. What’s more, it is not suited to every patient.
Absolute contraindications include acute infections at the injection site, such as cellulitis, abscess, or septic arthritis. Relative contraindications include acute gout flare and acute fracture near the site.6
When DPT is a viable alternative, keep in mind that the procedure should only be done by a physician experienced in the technique—and that ultrasound guidance should be used to ensure precise anatomical delivery (FIGURE 1).21 Consent must be obtained and documented, and universal precautions observed.
Read on to find out whether to consider DPT for particular patients.
Achilles tendinopathy: DPT decreases pain, improves function (SOR A)
Non-insertional Achilles tendinopathy can be treated with prolotherapy to decrease pain and tendon thickness (FIGURE 2). A small, single blind randomized trial compared the effectiveness of eccentric exercise (ie, contractions performed to lengthen the muscle), DPT alone, and a combination of DPT and exercise for patients with chronic Achilles tendinopathy.10
The investigators found greater improvement in the Victorian Institute of Sport Assessment-Achilles (VISA-A) score at 12 months with the combined therapy (41.1 on a 0-100 scale) vs either eccentric exercise (23.7) or DPT (27.5) alone. The increase from baseline was greater for those who received combination therapy at 6 weeks (+11.7) compared with the eccentric-only group.10 Adding DPT (injected into the tender points of the subcutaneous tissues adjacent to the Achilles tendon) to eccentric exercise resulted in a more rapid and sustained improvement in pain, function, and stiffness.
In an earlier observational study, researchers achieved improvement in pain scores using a different DPT technique.22 Here, patients with chronic Achilles tendinosis received ultrasound-guided intratendinous dextrose injections every 6 weeks until symptoms resolved. Pain scores, calculated using a visual analogue scale (VAS), showed a mean reduction at rest (88%), during normal daily activities (84%), and during physical activity (78%). The mean number of treatment sessions was 4, and the mean time to achieve results was 30 weeks.22
Studies have shown that inflammatory changes are infrequently associated with chronic painful tendon conditions.1,2 Instead, the changes are degenerative in nature, and can occur in the main body of the tendon, in its bony insertion site, and in the structures surrounding the tendon.3 While the exact mechanism of action for DPT is unknown, studies have shown that cells exposed to hypertonic dextrose undergo osmotic lysis, creating a proinflammatory environment. This leads to recruitment of several growth factors that promote the healing of tendons, ligaments, and cartilage.4-6
Neovascularity and neuronal ingrowth, also present in tendinopathies, are believed to be a source of pain, as well. The injection of hypertonic dextrose may destroy the neovasculature, thus removing a nidus, or focal point, for pain.7
Concentrations of dextrose used may range from 10% to 50% and be combined with an injectable anesthetic alone or with other proliferants/sclerosing agents.6 We prefer a 50/50 mixture of 50% dextrose and 2% xylocaine without epinephrine, resulting in a final injection concentration of 25% dextrose and 1% xylocaine.
Techniques for tendinopathies vary from bathing the tendon without tenotomy to performing multiple tenotomies (with or without injection material into the tenotomy). For knee osteoarthritis, for example, both extra- and intra-articular approaches can be used alone or in combination.8,9 The extra-articular injections are done either at tender locations around the knee joint or at ligamentous attachment sites. The number of injection sessions can vary, as well. Variations in both the concentrations and techniques contribute to the difficulty in interpreting existing evidence.
Knee osteoarthritis: Pain level and movement improve (SOR A)
In a study of patients with knee osteoarthritis (OA) and pain lasting 6 months or more, participants received bimonthly injections of either DPT with lidocaine or lidocaine alone. At 12 months, only those in the DPT group had achieved significant improvement in VAS pain score (44%), self-reported swelling (63%), and knee flexion (14%).11
A more recent study randomized 90 adults with painful knee OA of at least 3 months’ duration to blinded injection (either DPT or saline) or at-home exercise.9 The injections involved both intra- and extra-articular techniques, performed monthly for a total of 3 to 5 injections. At 52 weeks, the DPT group had improved scores on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) by 15.3 points compared with the saline group (7.6 points) and the exercise-only group (8.2 points).
Half of those receiving DPT improved by 12 or more points, compared with less than a third of those receiving saline and a quarter of those treated with exercise alone. Knee Pain Scale (KPS)-based pain frequency and severity were also significantly reduced in the DPT group vs both comparison groups.9
Finger OA. One small randomized study tested the efficacy of DPT in patients with symptomatic finger OA affecting the distal or proximal interphalangeal joint or the trapeziometacarpal (thumb) joint.23 Participants received either DPT with xylocaine or xylocaine alone. Injections were done on the medial and lateral aspects of the affected joints at baseline, 2, and 4 months. Pain (VAS score) during active finger movement improved by 45% in the DPT group vs 15% in the group treated with xylocaine alone. After 6 months, those in the xylocaine-only group received the DPT protocol, and their pain reduction scores rose, on average, from 18% to 54%.23
Low back pain: Little help for chronic condition (SOR A)
Early studies of DPT for the treatment of low back pain had conflicting results. In 2004, the largest (N=110) and most rigorous study of DPT for chronic non-specific low back pain to date12 found no significant improvement.
Participants received either DPT or normal saline injections into tender lumbopelvic ligaments every 2 weeks for a total of 6 treatments. They were then randomized to either core and low back strengthening exercises or normal activity for 6 months. At 12 months, VAS pain and disability scores significantly decreased from baseline in all the groups, with a decline ranging from 26% to 44% for pain and 30% to 44% for disability. However, at no point were there significant differences between injection groups or activity groups.12
A 2007 Cochrane review found insufficient evidence to support the use of DPT alone for the treatment of non-specific low back pain but suggested that, as an adjunct, it may improve pain and disability scores.13 And in 2011, a Cochrane review confirmed that there was insufficient evidence for the use of DPT in sub-acute and chronic low back pain.14 Other studies on the use of DPT for specific low back conditions, including sacroiliac joint pain,24,25 coccydynia,26 and degenerative disc disease,27 have shown trends toward improvement in pain scores24-27 and disability,25 but only one of these was a randomized controlled trial (RCT).25
Lateral epicondylosis: More effective than saline (SOR B)
A single RCT compared DPT to placebo in patients with 6 months of moderate to severe lateral epicondylosis who had failed conservative treatment. Patients received 3 injections of either hypertonic dextrose or saline tendon insertions every 4 weeks, with needle touching bone at the supracondylar ridge, lateral epicondyle, and annular ligament.15 Patients randomly assigned to DPT experienced significant pain relief from baseline to 16 weeks, with a Likert score decline from 5.1 to 0.5, compared with the saline group (4.5 at baseline and 3.5 at 16 weeks). Clinical improvement was maintained at 52-week follow-up.15
Osgood-Schlatter: DPT improves pain relief (SOR B)
In one of the few studies of prolotherapy for adolescents, patients with recalcitrant Osgood-Schlatter disease were randomized to either structured physical therapy or 3 monthly injections of lidocaine, with or without dextrose, over the apophysis and patellar tendon origin.16 Injections began at the most distal point of tenderness and were repeated at 1 cm intervals for a total of 3 to 4 midline injections. The proximal injections were deep to the patellar tendon, on the tibia above the tuberosity.
Pain scores, measured by the Nirschl Pain Phase Scale (0-7), improved significantly more in the DPT group (3.9) compared with either the lidocaine group (2.4) or the exercise group (1.2). Dextrose-treated knees were significantly more likely than knees treated with lidocaine (14 of 21 vs 5 of 22) to be asymptomatic with sport activity. After 3 months, patients in the lidocaine and exercise groups who had not responded adequately were given the option of receiving DPT; those who underwent the 3-month DPT protocol achieved the same level of improvement as the initial DPT group.16
When considering or recommending DPT for an adolescent with Osgood-Schlatter disease, however, it is particularly important that he or she be referred to a physician with expertise in prolotherapy.
Plantar fasciosis: A possibility when conservative treatment fails (SOR B)
An early case series showed that DPT effectively improved pain at rest and during activity in patients with chronic plantar fasciosis refractory to conservative care.17 A small RCT recently compared PRP with DPT in such patients.18
Pain, disability, and activity limitation were measured by the Foot Functional Index. The PRP group improved by 29.7%, 26.6%, and 28% in pain, disability, and activity limitation, respectively, vs improvements of 17%, 14.5%, and 12.4% in the DPT group. Although there was a trend for PRP to be superior, the results were not statistically significant.18 This suggests that DPT may be an additional treatment option for patients with plantar fasciosis when conservative treatment fails.
Chondromalacia patella: Not enough is known (SOR C)
One study showed that DPT improved self-reported pain and function scores in patients with chronic knee pain secondary to chondromalacia patella. However, the study had no control group and no standardized injected solution; rather, the solution was tailored for each individual.19 Thus, there is insufficient data to make recommendations regarding the effectiveness of DPT in treating chondromalacia patella or other causes of patellofemoral pain syndrome.
What to tell patients about recovery and adverse effects
Injection of dextrose into ligaments, tendons, and joints carries the theoretical risks of light-headedness, allergic reaction, infection, and structural damage. However, there have been no reports of serious or significant adverse events associated with DPT when used for peripheral joint indications.
The most common risks of DPT are needle trauma-induced pain, mild bleeding, and bruising. A sense of fullness, stiffness, and occasional numbness at the site at the time of injection are common, benign, and typically self-limiting.6 If post-procedure numbness continues, the patient should follow up in 48 to 72 hours to rule out nerve damage.
Post-injection pain flare during the first 72 hours may occur. In a study of prolotherapy for knee OA pain, 10% to 20% of patients experienced such flares.15 Most patients respond well to acetaminophen and experience resolution of pain within a week. Non-steroidal anti-inflammatory drugs should not be used to treat post-procedure pain because they may interfere with the local inflammatory response needed for healing. Regular activities can be resumed immediately after an injection into a large joint, such as the knee, or after full sensation and proprioception returns if an anesthetic was used in combination with the hypertonic dextrose.
There is a theoretical risk of tendon weakening and rupture with tenotomy/intra-substance injections into weight-bearing tendons, but there are no known published reports of this complication with DPT. Nonetheless, we recommend that patients limit ballistic weight bearing or full strength activity for 48 hours after an injection into a non-weight bearing tendon and for 5 to7 days for injection into a weight-bearing tendon.
Physical/occupational therapy is important in chronic tendinopathy, and we encourage rapid return (24-48 hours) to low-intensity rehabilitation with gradual return (5-7 days) to full rehabilitation exercises.
The number of DPT injection sessions is variable. We recommend follow-up between 3 and 6 weeks for reevaluation. If the patient’s pain and/or function has not improved after 2 sets of injections—or DPT is initially successful but pain or dysfunction returns—another round of treatment should be offered in 3 to 6 weeks.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Fort Belvoir Community Hospital, Sports Medicine, Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; carlton.covey@usuhs.edu.
› Advise patients with Achilles tendinopathy that a combination of prolotherapy and eccentric exercise is likely to provide more rapid and sustained pain relief than either option alone. A
› Offer a third round of prolotherapy to a patient whose pain and/or function has not improved or has returned after 2 treatments. C
› Consider prolotherapy administered by a physician with expertise in the technique for adolescents with recalcitrant Osgood-Schlatter disease. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Over the past several years, prolotherapy has been gaining support as an option for patients with tendinopathies and painful osteoarthritic conditions. Yet the technique lacks both a consistent definition and an abundance of evidence.
Because the prefix “prolo” is thought to refer to proliferation or regeneration, some physicians prefer the term “sclerotherapy” when injecting sclerosing agents. Others point out that “prolotherapy” refers to the proliferation of tissue that the injections provoke, which has never been proven. We believe that the material injected should dictate the term used to describe it—dextrose prolotherapy (DPT) or platelet-rich plasma therapy (PRP), for example.
In this update, we focus on DPT—the injection of a solution containing hypertonic dextrose into ligaments, tendons, and joints to promote healing. You’ll find an overview of the proposed mechanism of action and a description of the technique (see “How DPT works”1-9), as well as a look at the evidence of its effectiveness for a variety of musculoskeletal conditions in the text and TABLE9-19 that follow. Our review is limited by the dearth of large, definitive studies, and consists mainly of anecdotal evidence, case reports, and other low-quality studies.
Considering DPT—for which patients?
Even for conditions for which the evidence of its efficacy is unequivocal, DPT is only one part of a comprehensive treatment plan. Functional assessment and correction of any weaknesses, inflexibilities, and/or training errors are also essential.
There are a number of other considerations, as well. For one thing, DPT is rarely covered by health insurance20 and is often considered only after conservative treatment has failed. What’s more, it is not suited to every patient.
Absolute contraindications include acute infections at the injection site, such as cellulitis, abscess, or septic arthritis. Relative contraindications include acute gout flare and acute fracture near the site.6
When DPT is a viable alternative, keep in mind that the procedure should only be done by a physician experienced in the technique—and that ultrasound guidance should be used to ensure precise anatomical delivery (FIGURE 1).21 Consent must be obtained and documented, and universal precautions observed.
Read on to find out whether to consider DPT for particular patients.
Achilles tendinopathy: DPT decreases pain, improves function (SOR A)
Non-insertional Achilles tendinopathy can be treated with prolotherapy to decrease pain and tendon thickness (FIGURE 2). A small, single blind randomized trial compared the effectiveness of eccentric exercise (ie, contractions performed to lengthen the muscle), DPT alone, and a combination of DPT and exercise for patients with chronic Achilles tendinopathy.10
The investigators found greater improvement in the Victorian Institute of Sport Assessment-Achilles (VISA-A) score at 12 months with the combined therapy (41.1 on a 0-100 scale) vs either eccentric exercise (23.7) or DPT (27.5) alone. The increase from baseline was greater for those who received combination therapy at 6 weeks (+11.7) compared with the eccentric-only group.10 Adding DPT (injected into the tender points of the subcutaneous tissues adjacent to the Achilles tendon) to eccentric exercise resulted in a more rapid and sustained improvement in pain, function, and stiffness.
In an earlier observational study, researchers achieved improvement in pain scores using a different DPT technique.22 Here, patients with chronic Achilles tendinosis received ultrasound-guided intratendinous dextrose injections every 6 weeks until symptoms resolved. Pain scores, calculated using a visual analogue scale (VAS), showed a mean reduction at rest (88%), during normal daily activities (84%), and during physical activity (78%). The mean number of treatment sessions was 4, and the mean time to achieve results was 30 weeks.22
Studies have shown that inflammatory changes are infrequently associated with chronic painful tendon conditions.1,2 Instead, the changes are degenerative in nature, and can occur in the main body of the tendon, in its bony insertion site, and in the structures surrounding the tendon.3 While the exact mechanism of action for DPT is unknown, studies have shown that cells exposed to hypertonic dextrose undergo osmotic lysis, creating a proinflammatory environment. This leads to recruitment of several growth factors that promote the healing of tendons, ligaments, and cartilage.4-6
Neovascularity and neuronal ingrowth, also present in tendinopathies, are believed to be a source of pain, as well. The injection of hypertonic dextrose may destroy the neovasculature, thus removing a nidus, or focal point, for pain.7
Concentrations of dextrose used may range from 10% to 50% and be combined with an injectable anesthetic alone or with other proliferants/sclerosing agents.6 We prefer a 50/50 mixture of 50% dextrose and 2% xylocaine without epinephrine, resulting in a final injection concentration of 25% dextrose and 1% xylocaine.
Techniques for tendinopathies vary from bathing the tendon without tenotomy to performing multiple tenotomies (with or without injection material into the tenotomy). For knee osteoarthritis, for example, both extra- and intra-articular approaches can be used alone or in combination.8,9 The extra-articular injections are done either at tender locations around the knee joint or at ligamentous attachment sites. The number of injection sessions can vary, as well. Variations in both the concentrations and techniques contribute to the difficulty in interpreting existing evidence.
Knee osteoarthritis: Pain level and movement improve (SOR A)
In a study of patients with knee osteoarthritis (OA) and pain lasting 6 months or more, participants received bimonthly injections of either DPT with lidocaine or lidocaine alone. At 12 months, only those in the DPT group had achieved significant improvement in VAS pain score (44%), self-reported swelling (63%), and knee flexion (14%).11
A more recent study randomized 90 adults with painful knee OA of at least 3 months’ duration to blinded injection (either DPT or saline) or at-home exercise.9 The injections involved both intra- and extra-articular techniques, performed monthly for a total of 3 to 5 injections. At 52 weeks, the DPT group had improved scores on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) by 15.3 points compared with the saline group (7.6 points) and the exercise-only group (8.2 points).
Half of those receiving DPT improved by 12 or more points, compared with less than a third of those receiving saline and a quarter of those treated with exercise alone. Knee Pain Scale (KPS)-based pain frequency and severity were also significantly reduced in the DPT group vs both comparison groups.9
Finger OA. One small randomized study tested the efficacy of DPT in patients with symptomatic finger OA affecting the distal or proximal interphalangeal joint or the trapeziometacarpal (thumb) joint.23 Participants received either DPT with xylocaine or xylocaine alone. Injections were done on the medial and lateral aspects of the affected joints at baseline, 2, and 4 months. Pain (VAS score) during active finger movement improved by 45% in the DPT group vs 15% in the group treated with xylocaine alone. After 6 months, those in the xylocaine-only group received the DPT protocol, and their pain reduction scores rose, on average, from 18% to 54%.23
Low back pain: Little help for chronic condition (SOR A)
Early studies of DPT for the treatment of low back pain had conflicting results. In 2004, the largest (N=110) and most rigorous study of DPT for chronic non-specific low back pain to date12 found no significant improvement.
Participants received either DPT or normal saline injections into tender lumbopelvic ligaments every 2 weeks for a total of 6 treatments. They were then randomized to either core and low back strengthening exercises or normal activity for 6 months. At 12 months, VAS pain and disability scores significantly decreased from baseline in all the groups, with a decline ranging from 26% to 44% for pain and 30% to 44% for disability. However, at no point were there significant differences between injection groups or activity groups.12
A 2007 Cochrane review found insufficient evidence to support the use of DPT alone for the treatment of non-specific low back pain but suggested that, as an adjunct, it may improve pain and disability scores.13 And in 2011, a Cochrane review confirmed that there was insufficient evidence for the use of DPT in sub-acute and chronic low back pain.14 Other studies on the use of DPT for specific low back conditions, including sacroiliac joint pain,24,25 coccydynia,26 and degenerative disc disease,27 have shown trends toward improvement in pain scores24-27 and disability,25 but only one of these was a randomized controlled trial (RCT).25
Lateral epicondylosis: More effective than saline (SOR B)
A single RCT compared DPT to placebo in patients with 6 months of moderate to severe lateral epicondylosis who had failed conservative treatment. Patients received 3 injections of either hypertonic dextrose or saline tendon insertions every 4 weeks, with needle touching bone at the supracondylar ridge, lateral epicondyle, and annular ligament.15 Patients randomly assigned to DPT experienced significant pain relief from baseline to 16 weeks, with a Likert score decline from 5.1 to 0.5, compared with the saline group (4.5 at baseline and 3.5 at 16 weeks). Clinical improvement was maintained at 52-week follow-up.15
Osgood-Schlatter: DPT improves pain relief (SOR B)
In one of the few studies of prolotherapy for adolescents, patients with recalcitrant Osgood-Schlatter disease were randomized to either structured physical therapy or 3 monthly injections of lidocaine, with or without dextrose, over the apophysis and patellar tendon origin.16 Injections began at the most distal point of tenderness and were repeated at 1 cm intervals for a total of 3 to 4 midline injections. The proximal injections were deep to the patellar tendon, on the tibia above the tuberosity.
Pain scores, measured by the Nirschl Pain Phase Scale (0-7), improved significantly more in the DPT group (3.9) compared with either the lidocaine group (2.4) or the exercise group (1.2). Dextrose-treated knees were significantly more likely than knees treated with lidocaine (14 of 21 vs 5 of 22) to be asymptomatic with sport activity. After 3 months, patients in the lidocaine and exercise groups who had not responded adequately were given the option of receiving DPT; those who underwent the 3-month DPT protocol achieved the same level of improvement as the initial DPT group.16
When considering or recommending DPT for an adolescent with Osgood-Schlatter disease, however, it is particularly important that he or she be referred to a physician with expertise in prolotherapy.
Plantar fasciosis: A possibility when conservative treatment fails (SOR B)
An early case series showed that DPT effectively improved pain at rest and during activity in patients with chronic plantar fasciosis refractory to conservative care.17 A small RCT recently compared PRP with DPT in such patients.18
Pain, disability, and activity limitation were measured by the Foot Functional Index. The PRP group improved by 29.7%, 26.6%, and 28% in pain, disability, and activity limitation, respectively, vs improvements of 17%, 14.5%, and 12.4% in the DPT group. Although there was a trend for PRP to be superior, the results were not statistically significant.18 This suggests that DPT may be an additional treatment option for patients with plantar fasciosis when conservative treatment fails.
Chondromalacia patella: Not enough is known (SOR C)
One study showed that DPT improved self-reported pain and function scores in patients with chronic knee pain secondary to chondromalacia patella. However, the study had no control group and no standardized injected solution; rather, the solution was tailored for each individual.19 Thus, there is insufficient data to make recommendations regarding the effectiveness of DPT in treating chondromalacia patella or other causes of patellofemoral pain syndrome.
What to tell patients about recovery and adverse effects
Injection of dextrose into ligaments, tendons, and joints carries the theoretical risks of light-headedness, allergic reaction, infection, and structural damage. However, there have been no reports of serious or significant adverse events associated with DPT when used for peripheral joint indications.
The most common risks of DPT are needle trauma-induced pain, mild bleeding, and bruising. A sense of fullness, stiffness, and occasional numbness at the site at the time of injection are common, benign, and typically self-limiting.6 If post-procedure numbness continues, the patient should follow up in 48 to 72 hours to rule out nerve damage.
Post-injection pain flare during the first 72 hours may occur. In a study of prolotherapy for knee OA pain, 10% to 20% of patients experienced such flares.15 Most patients respond well to acetaminophen and experience resolution of pain within a week. Non-steroidal anti-inflammatory drugs should not be used to treat post-procedure pain because they may interfere with the local inflammatory response needed for healing. Regular activities can be resumed immediately after an injection into a large joint, such as the knee, or after full sensation and proprioception returns if an anesthetic was used in combination with the hypertonic dextrose.
There is a theoretical risk of tendon weakening and rupture with tenotomy/intra-substance injections into weight-bearing tendons, but there are no known published reports of this complication with DPT. Nonetheless, we recommend that patients limit ballistic weight bearing or full strength activity for 48 hours after an injection into a non-weight bearing tendon and for 5 to7 days for injection into a weight-bearing tendon.
Physical/occupational therapy is important in chronic tendinopathy, and we encourage rapid return (24-48 hours) to low-intensity rehabilitation with gradual return (5-7 days) to full rehabilitation exercises.
The number of DPT injection sessions is variable. We recommend follow-up between 3 and 6 weeks for reevaluation. If the patient’s pain and/or function has not improved after 2 sets of injections—or DPT is initially successful but pain or dysfunction returns—another round of treatment should be offered in 3 to 6 weeks.
CORRESPONDENCE
Carlton J. Covey, MD, FAAFP, Fort Belvoir Community Hospital, Sports Medicine, Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; carlton.covey@usuhs.edu.
1. Khan KM, Cook J, Bonar F, et al. Histopathology of common tendinopathies: update and implications for clinical management. Sports Med. 1999;27:393-408.
2. Streit JJ, Shishani Y, Rodgers M, et al. Tendinopathy of the long head of the biceps tendon: histopathologic analysis of the extraarticular biceps tendon and tenosynovium. Open Access J Sports Med. 2015;10:63-70.
3. Maganaris CN, Narici MV, Almekinders LC, et al. Biomechanics and pathophysiology of overuse tendon injuries. Sports Med. 2004;34:1005-1017.
4. Jensen KT, Rabago DP, Zgierska A, et al. Response of knee ligaments to prolotherapy in a rat injury model. Am J Sports Med. 2008;36:1347-1357.
5. Kim SR, Stitik TP, Foye PM, et al. Critical review of prolotherapy for osteoarthritis, low back pain, and other musculoskeletal conditions: A physiatric perspective. Am J Phys Med Rehabil. 2004;83:379–389.
6. Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37:65-80.
7. Joseph MF, Denegar CR. Treating tendinopathy: perspective on anti-inflammatory intervention and therapeutic exercise. Clin Sports Med. 2015;34:363-374.
8. Eslamian F, Amouzandeh B. Therapeutic effects of prolotherapy with intra-articular dextrose injection in patients with osteoarthritis: a single-arm study with 6 months follow up. Ther Adv Musculoskelet Dis. 2015;7:35-44.
9. Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
10. Yelland MJ, Sweeting KR, Lyftogt JA, et al. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomised trial. Br J Sports Med. 2011;45:421-428.
11. Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68–74.
12. Yelland MJ, Glasziou PP, Bogduk N, et al. Prolotherapy injections, saline injections, and exercises for chronic low back pain: a randomized control trial. Spine. 2004;29:9-16.
13. Dagenais S, Yelland MJ, Del Mar C, et al. Prolotherapy injections for chronic low back pain. Cochrane Database Syst Rev. 2007;18(2):CD004059.
14. Staal JB, de Bie R, de Vet HCW, et al. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev. 2011;(3):CD001824.
15. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18:248.
16. Topol GA, Podesta LA, Reeves KD, et al. Hyperosmolar dextrose injection for recalcitrant Osgood-Schlatter disease. Pediatrics. 2011;128:e1121-e1128.
17. Ryan MB, Wong AD, Gillies JH, et al. Sonographically guided intratendinous injections of hyperosmolar dextrose/lidocaine: a pilot study for the treatment of chronic plantar fasciitis. Br J Sports Med. 2009;43:3003-3006.
18. Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PMR. 2014;6:152-158.
19. Hauser RA, Sprague IS. Outcomes of prolotherapy in chondromalacia patella patients: improvements in pain level and function. Clin Med Insights Arthritis Musculoskelet Disord. 2014;17:13-20.
20. United Healthcare medical policy. Prolotherapy for musculoskeletal indications. Available at: https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/Medical%20Policies/Medical%20Policies/Prolotherapy_for_Musculoskeletal_Indications.pdf. Accessed October 26, 2015.
21. Davidson J, Javaraman S. Guided interventions in musculoskeletal ultrasound: where’s the evidence? Clin Radiol. 2011;66:140-152.
22. Maxwell NJ, Ryan MB, Taunton JE, et al. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. Am J Roentgenol. 2007;189:W215.
23. Reeves KD, Hassanein K. Randomized, perspective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DTaP, PIP and Traneziometacarpal) joints: Evidence of clinical efficacy. J Altern Complem Med. 2000;6:311-320.
24. Cusi M, Saunders J, Hungerford B, et al. The use of prolotherapy in the sacroiliac joint. Br J Sports Med. 2010;44:100-104.
25. Kim WM, Lee HG, Jeong CW, et al. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med. 2010;16:1285-1290.
26. Khan SA, Kumar A, Varshney MK, et al. Dextrose prolotherapy for recalcitrant coccygodynia. J Orthop Surg. (Hong Kong) 2008;16:27–29.
27. Miller MR, Mathews RS, Reeves KD. Treatment of painful advanced internal lumbar disc derangement with intradiscal injection of hypertonic dextrose. Pain Physician. 2006;9:115–121.
1. Khan KM, Cook J, Bonar F, et al. Histopathology of common tendinopathies: update and implications for clinical management. Sports Med. 1999;27:393-408.
2. Streit JJ, Shishani Y, Rodgers M, et al. Tendinopathy of the long head of the biceps tendon: histopathologic analysis of the extraarticular biceps tendon and tenosynovium. Open Access J Sports Med. 2015;10:63-70.
3. Maganaris CN, Narici MV, Almekinders LC, et al. Biomechanics and pathophysiology of overuse tendon injuries. Sports Med. 2004;34:1005-1017.
4. Jensen KT, Rabago DP, Zgierska A, et al. Response of knee ligaments to prolotherapy in a rat injury model. Am J Sports Med. 2008;36:1347-1357.
5. Kim SR, Stitik TP, Foye PM, et al. Critical review of prolotherapy for osteoarthritis, low back pain, and other musculoskeletal conditions: A physiatric perspective. Am J Phys Med Rehabil. 2004;83:379–389.
6. Rabago D, Slattengren A, Zgierska A. Prolotherapy in primary care practice. Prim Care. 2010;37:65-80.
7. Joseph MF, Denegar CR. Treating tendinopathy: perspective on anti-inflammatory intervention and therapeutic exercise. Clin Sports Med. 2015;34:363-374.
8. Eslamian F, Amouzandeh B. Therapeutic effects of prolotherapy with intra-articular dextrose injection in patients with osteoarthritis: a single-arm study with 6 months follow up. Ther Adv Musculoskelet Dis. 2015;7:35-44.
9. Rabago D, Patterson JJ, Mundt M, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013;11:229-237.
10. Yelland MJ, Sweeting KR, Lyftogt JA, et al. Prolotherapy injections and eccentric loading exercises for painful Achilles tendinosis: a randomised trial. Br J Sports Med. 2011;45:421-428.
11. Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000;6:68–74.
12. Yelland MJ, Glasziou PP, Bogduk N, et al. Prolotherapy injections, saline injections, and exercises for chronic low back pain: a randomized control trial. Spine. 2004;29:9-16.
13. Dagenais S, Yelland MJ, Del Mar C, et al. Prolotherapy injections for chronic low back pain. Cochrane Database Syst Rev. 2007;18(2):CD004059.
14. Staal JB, de Bie R, de Vet HCW, et al. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev. 2011;(3):CD001824.
15. Scarpone M, Rabago DP, Zgierska A, et al. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008;18:248.
16. Topol GA, Podesta LA, Reeves KD, et al. Hyperosmolar dextrose injection for recalcitrant Osgood-Schlatter disease. Pediatrics. 2011;128:e1121-e1128.
17. Ryan MB, Wong AD, Gillies JH, et al. Sonographically guided intratendinous injections of hyperosmolar dextrose/lidocaine: a pilot study for the treatment of chronic plantar fasciitis. Br J Sports Med. 2009;43:3003-3006.
18. Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PMR. 2014;6:152-158.
19. Hauser RA, Sprague IS. Outcomes of prolotherapy in chondromalacia patella patients: improvements in pain level and function. Clin Med Insights Arthritis Musculoskelet Disord. 2014;17:13-20.
20. United Healthcare medical policy. Prolotherapy for musculoskeletal indications. Available at: https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Policies%20and%20Protocols/Medical%20Policies/Medical%20Policies/Prolotherapy_for_Musculoskeletal_Indications.pdf. Accessed October 26, 2015.
21. Davidson J, Javaraman S. Guided interventions in musculoskeletal ultrasound: where’s the evidence? Clin Radiol. 2011;66:140-152.
22. Maxwell NJ, Ryan MB, Taunton JE, et al. Sonographically guided intratendinous injection of hyperosmolar dextrose to treat chronic tendinosis of the Achilles tendon: a pilot study. Am J Roentgenol. 2007;189:W215.
23. Reeves KD, Hassanein K. Randomized, perspective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DTaP, PIP and Traneziometacarpal) joints: Evidence of clinical efficacy. J Altern Complem Med. 2000;6:311-320.
24. Cusi M, Saunders J, Hungerford B, et al. The use of prolotherapy in the sacroiliac joint. Br J Sports Med. 2010;44:100-104.
25. Kim WM, Lee HG, Jeong CW, et al. A randomized controlled trial of intra-articular prolotherapy versus steroid injection for sacroiliac joint pain. J Altern Complement Med. 2010;16:1285-1290.
26. Khan SA, Kumar A, Varshney MK, et al. Dextrose prolotherapy for recalcitrant coccygodynia. J Orthop Surg. (Hong Kong) 2008;16:27–29.
27. Miller MR, Mathews RS, Reeves KD. Treatment of painful advanced internal lumbar disc derangement with intradiscal injection of hypertonic dextrose. Pain Physician. 2006;9:115–121.