User login
Take-Home Points
- Prescription opioid abuse and overdose-related deaths are on the rise in the United States.
- Following Open Reduction Internal Fixation (ORIF) of a distal radius fracture (DRF), patients consumed an average of 14.6 opioid pills. We recommend prescribing no more than 15-20 opioid pills after DRF ORIF.
- There was no difference in opioid consumption between patients who underwent general anesthesia vs regional anesthesia.
- There was a significant trend towards less opioid consumption with increasing age.
- There was a trend towards increased opioid consumption in patients with worsening fracture type as well as in self-pay/Medicaid patients.
Over the past 2 decades, prescription opioid abuse in the United States has risen steadily.1,2 Although use of opioid analgesics in the US far exceeds use in other countries, US patients do not report less pain or more satisfaction with pain relief.3-5 Between 1999 and 2002, oxycodone prescriptions increased by 50%, fentanyl prescriptions by 150%, and morphine prescriptions by 60%.6 Furthermore, the Centers for Disease Control and Prevention (CDC) reported in 2012 that, for every 100 people in the United States, US physicians wrote a mean of 82.5 opioid prescriptions and 37.6 benzodiazepine prescriptions; in total, US clinicians wrote 259 million opioid prescriptions in 2012, enough for every adult to have a bottle.7 The increase in prescription opioid abuse, not surprisingly, has paralleled a 124% increase in opioid overdose-related deaths.8 Cicero and colleagues2,9 recently found that, over the past 50 years, heroin use has dramatically shifted from being a problem mainly of urban centers and minorities toward one of older, suburban Caucasians with a previous history of prescription pain killer abuse. Deaths from prescription opioid overdoses now exceed deaths from heroin and cocaine overdoses combined.10 According to the CDC, emergency department visits related to nonmedical use of prescription opioid medications jumped 111% between 2004 and 2008.11
Opioid analgesics are often prescribed for the management of musculoskeletal pain and injuries.12-16 Orthopedic surgeons, who prescribe more opioids than physicians in any other surgical field, represent the third largest group of opioid prescribers, trailing only primary care physicians and internists, who far outnumber them.17 A study focused on opioid consumption after upper extremity surgery found that upper extremity surgeons tended to overprescribe opioids for postoperative analgesia.18 Many patients saved their remaining medication for later use and were never instructed on proper disposal. There is a developing consensus that opioid medication is not as safe and effective as once thought, and that a high-dose prescription or prolonged opioid therapy do not improve outcomes.19 In addition, patients may experience numerous opioid-associated adverse effects, including nausea, vomiting, constipation, lightheadedness, dizziness, blurred vision, headache, dry mouth, sweating, and itching.
In October 2012, patient satisfaction scores on the Hospital Consumer Assessment of Healthcare Providers and Systems started affecting Medicare reimbursements.20 By 2017, up to 6% of Medicare reimbursement will be at risk, given the poor outcomes caused by uncontrolled pain.21-24 The US healthcare culture has made it more important than ever for physicians to adequately manage postoperative pain while limiting opioid availability and the risk for abuse.
Distal radius fracture (DRF) open reduction and internal fixation (ORIF) is commonly performed by orthopedic surgeons and hand surgeons. Pain management and opioid consumption after DRF repair may be influenced by several variables. We conducted a study to investigate the impact of several clinical variables on postoperative opioid use; to test the hypothesis that post-DRF-ORIF opioid consumption would increase with worsening fracture classification and certain patient demographics; and to seek postoperative opioid consumption insights that would facilitate optimization of future opioid prescribing.
Materials and Methods
Institutional Review Board approval was obtained before initiation of the study. All outpatients who underwent DRF-ORIF (performed by 9 hand surgery fellowship-trained orthopedic surgeons) were consecutively enrolled over a 6-month period in 2014. All procedures were performed with a standard volar plating technique through a flexor carpi radialis approach. The postoperative rehabilitation protocol was standardized for all patients. Data collected on each patient included age, sex, payer type, fracture type, opioid prescribed, amount prescribed, amount consumed, reasons for stopping, adverse events, and any postoperative adjunctive pain medications. The data were taken from questionnaires completed by patients at their first visit within 2 weeks after surgery. Anesthesia type (general or regional) was noted as well. All fractures were classified by Dr. O’Neil using the AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification of long-bone fractures based on preoperative radiographs.
Amount of opioid analgesic consumed was converted into morphine equivalents to adjust for the different opioids prescribed after surgery: oxycodone/acetaminophen or oxycodone equivalent, hydrocodone/acetaminophen or hydrocodone equivalent, and acetaminophen/codeine.
Patients were excluded from the study if their procedure was performed on an inpatient basis, if they sustained other injuries or fractures from their trauma, or if an adjunctive procedure (including carpal tunnel release) was performed during the DRF repair.
We used the Spearman rank correlation coefficient and a count data model to examine the relationship between opioid use and age. The Kruskal-Wallis test was used to examine the relationships between opioid use and payer type, anesthesia type, and fracture type.
Results
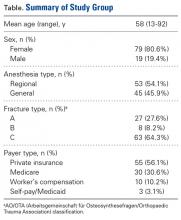
Of the 109 patients eligible for the study, 11 were excluded for incomplete postoperative questionnaires, leaving 98 patients (79 females, 19 males) for analysis. Mean age was 58 years (range, 13-92 years). Of the 98 patients, 45 received general anesthesia, and 53 received regional anesthesia with a single-shot peripheral nerve block before surgery and sedation perioperatively (Table).
Of the 98 study patients, 61 reported using over-the-counter adjunctive pain medications during the postoperative period, and 37 reported no use. Mean opioid consumption was 64.7 mg of morphine equivalents for the adjunctive medication users and 48.3 mg for the nonusers (P = .1947).
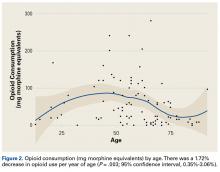
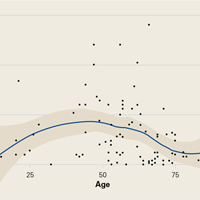
Demographic analysis revealed an inverse relationship between age and opioid use (Figure 2). The Spearman ρ between age and opioid consumption was –0.2958, which suggests decreased opioid use by older patients (P = .003).
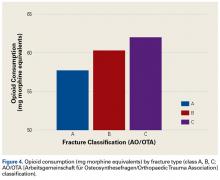
All fractures were graded with the AO/OTA long-bone fracture classification system. Mean opioid consumption for the 3 fracture-type groups was 57.7 mg (class A), 60.3 mg (class B), and 62.0 mg (class C) (Figure 4).
Discussion
The US healthcare culture has elevated physicians’ responsibility in adequately and aggressively managing their patients’ pain experience. Moreover, reimbursement may be affected by patient satisfaction scores, which are partly predicated on pain control.20-24 However, as rates of opioid use and abuse rise, it is important that physicians prescribe such medications judiciously. This is particularly germane to orthopedic surgeons, who prescribe more opioid analgesics than surgeons in any other field.17 Rodgers and colleagues18 found upper extremity surgeons, in particular, tended to overprescribe postoperative opioid analgesics. In the present study, we sought to identify the crucial risk factors that influence post-DRF-ORIF pain management and opioid consumption.
Mean postoperative opioid consumption (morphine equivalents) was 58.5 mg, roughly equivalent to 14.6 tabs of oxycodone/acetaminophen 5/325 mg, an opioid analgesic commonly used during the acute postoperative period. In addition, almost 70% of our patients required <75 mg of morphine equivalents, or <20 tabs of oxycodone/acetaminophen 5/325 mg. For upper extremity surgeons, these numbers may be better guides in determining the most appropriate amount of opioid to prescribe after DRF repair.
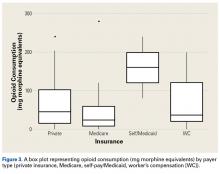
As for predicting levels of postoperative opioid medication, there was a significant trend toward less consumption with increasing age. Given this finding, surgeons prescribing for elderly patients should expect less opioid use. Regarding payer type, there was a trend toward more opioid use by self-pay/Medicaid patients; however, there were only 3 patients in this group. The situation in the study by Rodgers and colleagues18 is similar: Their finding that Medicaid patients consumed more pain pills after surgery was underpowered (only 5 patients in the group).
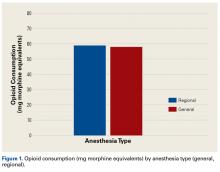
In the orthopedic community, support for use of regional anesthesia has been widespread for several reasons, including the belief that it reduces postoperative pain and therefore should reduce postoperative opioid consumption.25 However, we found no significant difference in postoperative opioid consumption between patients who received general anesthesia (with and without local anesthesia) and patients who received regional anesthesia (nerve block). Mean opioid consumption was 57.93 mg in the general anesthesia group and 58.98 mg in the regional anesthesia group. However, this finding could have been confounded by the variability in success and operator dependence inherent in regional anesthesia. In addition, the anatomical location for the peripheral nerve block and anesthetic could have affected the efficacy of the block and played a role in postoperative opioid consumption.
In this study, we tested the hypothesis that there would be more postoperative opioid consumption with worsening fracture type. Although our results did not reach statistical significance, there was a trend toward increased opioid consumption in patients with a complete intra-articular fracture (AO/OTA class C) vs patients with a partial articular fracture (class B) or an extra-articular fracture (class A). In addition, patients with a partial articular fracture tended to use more postoperative opioids than patients with an extra-articular fracture. In short, postoperative opioid consumption tended to be higher with increasing articular involvement of the fracture.
This study was limited in that it relied on patient self-reporting. Given the social stigma attached to opioid use, patients may have underreported their postoperative opioid consumption, been affected by recall bias, or both. The study also did not control for preoperative opioid use or history of opioid or substance abuse. Chronic preoperative opioid consumption may have affected postoperative opioid use. Other patient-related factors, such as body mass index (BMI) and hepatorenal dysfunction, can create tremendous variability in opioid metabolism across a population. Such factors were not controlled for in this study and therefore may have affected its results. That could help explain why older patients, who are more likely to have lower BMI and less efficient organ function for opioid metabolism, had lower postoperative opioid consumption. In addition, although we excluded patients with concomitant injuries and procedures, we did not screen patients for concomitant complex regional pain syndrome, fibromyalgia, or other medical conditions that might have had a significant impact on postoperative pain management needs. Last, some findings, such as the relationship between opioid use and payer type, were underpowered: Although self-pay/Medicaid patients had higher postoperative opioid consumption, they were few in number. The same was true of the Medicaid patients in the study by Rodgers and colleagues.18Our results demonstrated that post-DRF-ORIF opioid consumption decreased with age and was independent of type of perioperative anesthesia. There was a trend toward more opioid consumption with both self- and Medicaid payment and worsening fracture classification. It has become more important than ever for orthopedic surgeons to adequately manage postoperative pain while limiting opioid availability and the risk for abuse. Surgeons must remain aware of the variables in their patients’ postoperative pain experience in order to better optimize prescribing patterns and provide a safe and effective postoperative pain regimen.
Am J Orthop. 2017;46(1):E35-E40. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297(3):249-251.
2. Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71(7):821-826.
3. Helmerhorst GT, Lindenhovius AL, Vrahas M, Ring D, Kloen P. Satisfaction with pain relief after operative treatment of an ankle fracture. Injury. 2012;43(11):1958-1961.
4. Lindenhovius AL, Helmerhorst GT, Schnellen AC, Vrahas M, Ring D, Kloen P. Differences in prescription of narcotic pain medication after operative treatment of hip and ankle fractures in the United States and the Netherlands. J Trauma. 2009;67(1):160-164.
5. Seya MJ, Gelders SF, Achara OU, Milani B, Scholten WK. A first comparison between the consumption of and the need for opioid analgesics at country, regional, and global levels. J Pain Palliat Care Pharmacother. 2011;25(1):6-18.
6. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
7. Kuehn BM. CDC: major disparities in opioid prescribing among states: some states crack down on excess prescribing. JAMA. 2014;312(7):684-686.
8. Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15(9):618-627.
9. Cicero TJ, Kuehn BM. Driven by prescription drug abuse, heroin use increases among suburban and rural whites. JAMA. 2014;312(2):118-119.
10. Painkillers fuel growth in drug addiction. Harvard Ment Health Lett. Harvard Medical School website. http://www.health.harvard.edu/newsletter_article/painkillers-fuel-growth-in-drug-addiction. Published January 2011. Accessed March 18, 2015.
11. Cai R, Crane E, Poneleit K, Paulozzi L. Emergency department visits involving nonmedical use of selected prescription drugs in the United States, 2004-2008. J Pain Palliat Care Pharmacother. 2010;24(3):293-297.
12. Armaghani SJ, Lee DS, Bible JE, et al. Preoperative narcotic use and its relation to depression and anxiety in patients undergoing spine surgery. Spine. 2013;38(25):2196-2200.
13. Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109(3):514-519.
14. Deyo RA, Mirza SK, Turner JA, Martin BI. Overtreating chronic back pain: time to back off? J Am Board Fam Med. 2009;22(1):62-68.
15. Lee D, Armaghani S, Archer KR, et al. Preoperative opioid use as a predictor of adverse postoperative self-reported outcomes in patients undergoing spine surgery. J Bone Joint Surg Am. 2014;96(11):e89.
16. Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine. 2007;32(19):2127-2132.
17. Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SR. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299-1301.
18. Rodgers J, Cunningham K, Fitzgerald K, Finnerty E. Opioid consumption following outpatient upper extremity surgery. J Hand Surg Am. 2012;37(4):645-650.
19. Chen L, Vo T, Seefeld L, et al. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain. 2013;14(4):384-392.
20. Bush H. Doubling down on the patient experience. Hosp Health Netw. 2011;85(12):22-25, 1.
21. Centers for Medicare & Medicaid Services, US Department of Health and Human Services. Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and fiscal year 2013 rates; hospitals’ resident caps for graduate medical education payment purposes; quality reporting requirements for specific providers and for ambulatory surgical centers. Final rule. Fed Regist. 2012;77(170):53257-53750.
22. Centers for Medicare & Medicaid Services, US Department of Health and Human Services. Hospital Value-Based Purchasing. http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/Hospital_VBPurchasing_Fact_Sheet_ICN907664.pdf. Published September 2015. Accessed October 2015.
23. Manchikanti L, Singh V, Caraway DL, Benyamin RM, Falco FJ, Hirsch JA. Proposed physician payment schedule for 2013: guarded prognosis for interventional pain management. Pain Physician. 2012;15(5):E615-E627.
24. Bot AG, Bekkers S, Arnstein PM, Smith RM, Ring D. Opioid use after fracture surgery correlates with pain intensity and satisfaction with pain relief. Clin Orthop Relat Res. 2014;472(8):2542-2549.
25. Oldman M, McCartney CJ, Leung A, et al. A survey of orthopedic surgeons’ attitudes and knowledge regarding regional anesthesia. Anesth Analg. 2004;98(5):1486-1490.
Take-Home Points
- Prescription opioid abuse and overdose-related deaths are on the rise in the United States.
- Following Open Reduction Internal Fixation (ORIF) of a distal radius fracture (DRF), patients consumed an average of 14.6 opioid pills. We recommend prescribing no more than 15-20 opioid pills after DRF ORIF.
- There was no difference in opioid consumption between patients who underwent general anesthesia vs regional anesthesia.
- There was a significant trend towards less opioid consumption with increasing age.
- There was a trend towards increased opioid consumption in patients with worsening fracture type as well as in self-pay/Medicaid patients.
Over the past 2 decades, prescription opioid abuse in the United States has risen steadily.1,2 Although use of opioid analgesics in the US far exceeds use in other countries, US patients do not report less pain or more satisfaction with pain relief.3-5 Between 1999 and 2002, oxycodone prescriptions increased by 50%, fentanyl prescriptions by 150%, and morphine prescriptions by 60%.6 Furthermore, the Centers for Disease Control and Prevention (CDC) reported in 2012 that, for every 100 people in the United States, US physicians wrote a mean of 82.5 opioid prescriptions and 37.6 benzodiazepine prescriptions; in total, US clinicians wrote 259 million opioid prescriptions in 2012, enough for every adult to have a bottle.7 The increase in prescription opioid abuse, not surprisingly, has paralleled a 124% increase in opioid overdose-related deaths.8 Cicero and colleagues2,9 recently found that, over the past 50 years, heroin use has dramatically shifted from being a problem mainly of urban centers and minorities toward one of older, suburban Caucasians with a previous history of prescription pain killer abuse. Deaths from prescription opioid overdoses now exceed deaths from heroin and cocaine overdoses combined.10 According to the CDC, emergency department visits related to nonmedical use of prescription opioid medications jumped 111% between 2004 and 2008.11
Opioid analgesics are often prescribed for the management of musculoskeletal pain and injuries.12-16 Orthopedic surgeons, who prescribe more opioids than physicians in any other surgical field, represent the third largest group of opioid prescribers, trailing only primary care physicians and internists, who far outnumber them.17 A study focused on opioid consumption after upper extremity surgery found that upper extremity surgeons tended to overprescribe opioids for postoperative analgesia.18 Many patients saved their remaining medication for later use and were never instructed on proper disposal. There is a developing consensus that opioid medication is not as safe and effective as once thought, and that a high-dose prescription or prolonged opioid therapy do not improve outcomes.19 In addition, patients may experience numerous opioid-associated adverse effects, including nausea, vomiting, constipation, lightheadedness, dizziness, blurred vision, headache, dry mouth, sweating, and itching.
In October 2012, patient satisfaction scores on the Hospital Consumer Assessment of Healthcare Providers and Systems started affecting Medicare reimbursements.20 By 2017, up to 6% of Medicare reimbursement will be at risk, given the poor outcomes caused by uncontrolled pain.21-24 The US healthcare culture has made it more important than ever for physicians to adequately manage postoperative pain while limiting opioid availability and the risk for abuse.
Distal radius fracture (DRF) open reduction and internal fixation (ORIF) is commonly performed by orthopedic surgeons and hand surgeons. Pain management and opioid consumption after DRF repair may be influenced by several variables. We conducted a study to investigate the impact of several clinical variables on postoperative opioid use; to test the hypothesis that post-DRF-ORIF opioid consumption would increase with worsening fracture classification and certain patient demographics; and to seek postoperative opioid consumption insights that would facilitate optimization of future opioid prescribing.
Materials and Methods
Institutional Review Board approval was obtained before initiation of the study. All outpatients who underwent DRF-ORIF (performed by 9 hand surgery fellowship-trained orthopedic surgeons) were consecutively enrolled over a 6-month period in 2014. All procedures were performed with a standard volar plating technique through a flexor carpi radialis approach. The postoperative rehabilitation protocol was standardized for all patients. Data collected on each patient included age, sex, payer type, fracture type, opioid prescribed, amount prescribed, amount consumed, reasons for stopping, adverse events, and any postoperative adjunctive pain medications. The data were taken from questionnaires completed by patients at their first visit within 2 weeks after surgery. Anesthesia type (general or regional) was noted as well. All fractures were classified by Dr. O’Neil using the AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification of long-bone fractures based on preoperative radiographs.
Amount of opioid analgesic consumed was converted into morphine equivalents to adjust for the different opioids prescribed after surgery: oxycodone/acetaminophen or oxycodone equivalent, hydrocodone/acetaminophen or hydrocodone equivalent, and acetaminophen/codeine.
Patients were excluded from the study if their procedure was performed on an inpatient basis, if they sustained other injuries or fractures from their trauma, or if an adjunctive procedure (including carpal tunnel release) was performed during the DRF repair.
We used the Spearman rank correlation coefficient and a count data model to examine the relationship between opioid use and age. The Kruskal-Wallis test was used to examine the relationships between opioid use and payer type, anesthesia type, and fracture type.
Results
Of the 109 patients eligible for the study, 11 were excluded for incomplete postoperative questionnaires, leaving 98 patients (79 females, 19 males) for analysis. Mean age was 58 years (range, 13-92 years). Of the 98 patients, 45 received general anesthesia, and 53 received regional anesthesia with a single-shot peripheral nerve block before surgery and sedation perioperatively (Table).
Of the 98 study patients, 61 reported using over-the-counter adjunctive pain medications during the postoperative period, and 37 reported no use. Mean opioid consumption was 64.7 mg of morphine equivalents for the adjunctive medication users and 48.3 mg for the nonusers (P = .1947).
Demographic analysis revealed an inverse relationship between age and opioid use (Figure 2). The Spearman ρ between age and opioid consumption was –0.2958, which suggests decreased opioid use by older patients (P = .003).
All fractures were graded with the AO/OTA long-bone fracture classification system. Mean opioid consumption for the 3 fracture-type groups was 57.7 mg (class A), 60.3 mg (class B), and 62.0 mg (class C) (Figure 4).
Discussion
The US healthcare culture has elevated physicians’ responsibility in adequately and aggressively managing their patients’ pain experience. Moreover, reimbursement may be affected by patient satisfaction scores, which are partly predicated on pain control.20-24 However, as rates of opioid use and abuse rise, it is important that physicians prescribe such medications judiciously. This is particularly germane to orthopedic surgeons, who prescribe more opioid analgesics than surgeons in any other field.17 Rodgers and colleagues18 found upper extremity surgeons, in particular, tended to overprescribe postoperative opioid analgesics. In the present study, we sought to identify the crucial risk factors that influence post-DRF-ORIF pain management and opioid consumption.
Mean postoperative opioid consumption (morphine equivalents) was 58.5 mg, roughly equivalent to 14.6 tabs of oxycodone/acetaminophen 5/325 mg, an opioid analgesic commonly used during the acute postoperative period. In addition, almost 70% of our patients required <75 mg of morphine equivalents, or <20 tabs of oxycodone/acetaminophen 5/325 mg. For upper extremity surgeons, these numbers may be better guides in determining the most appropriate amount of opioid to prescribe after DRF repair.
As for predicting levels of postoperative opioid medication, there was a significant trend toward less consumption with increasing age. Given this finding, surgeons prescribing for elderly patients should expect less opioid use. Regarding payer type, there was a trend toward more opioid use by self-pay/Medicaid patients; however, there were only 3 patients in this group. The situation in the study by Rodgers and colleagues18 is similar: Their finding that Medicaid patients consumed more pain pills after surgery was underpowered (only 5 patients in the group).
In the orthopedic community, support for use of regional anesthesia has been widespread for several reasons, including the belief that it reduces postoperative pain and therefore should reduce postoperative opioid consumption.25 However, we found no significant difference in postoperative opioid consumption between patients who received general anesthesia (with and without local anesthesia) and patients who received regional anesthesia (nerve block). Mean opioid consumption was 57.93 mg in the general anesthesia group and 58.98 mg in the regional anesthesia group. However, this finding could have been confounded by the variability in success and operator dependence inherent in regional anesthesia. In addition, the anatomical location for the peripheral nerve block and anesthetic could have affected the efficacy of the block and played a role in postoperative opioid consumption.
In this study, we tested the hypothesis that there would be more postoperative opioid consumption with worsening fracture type. Although our results did not reach statistical significance, there was a trend toward increased opioid consumption in patients with a complete intra-articular fracture (AO/OTA class C) vs patients with a partial articular fracture (class B) or an extra-articular fracture (class A). In addition, patients with a partial articular fracture tended to use more postoperative opioids than patients with an extra-articular fracture. In short, postoperative opioid consumption tended to be higher with increasing articular involvement of the fracture.
This study was limited in that it relied on patient self-reporting. Given the social stigma attached to opioid use, patients may have underreported their postoperative opioid consumption, been affected by recall bias, or both. The study also did not control for preoperative opioid use or history of opioid or substance abuse. Chronic preoperative opioid consumption may have affected postoperative opioid use. Other patient-related factors, such as body mass index (BMI) and hepatorenal dysfunction, can create tremendous variability in opioid metabolism across a population. Such factors were not controlled for in this study and therefore may have affected its results. That could help explain why older patients, who are more likely to have lower BMI and less efficient organ function for opioid metabolism, had lower postoperative opioid consumption. In addition, although we excluded patients with concomitant injuries and procedures, we did not screen patients for concomitant complex regional pain syndrome, fibromyalgia, or other medical conditions that might have had a significant impact on postoperative pain management needs. Last, some findings, such as the relationship between opioid use and payer type, were underpowered: Although self-pay/Medicaid patients had higher postoperative opioid consumption, they were few in number. The same was true of the Medicaid patients in the study by Rodgers and colleagues.18Our results demonstrated that post-DRF-ORIF opioid consumption decreased with age and was independent of type of perioperative anesthesia. There was a trend toward more opioid consumption with both self- and Medicaid payment and worsening fracture classification. It has become more important than ever for orthopedic surgeons to adequately manage postoperative pain while limiting opioid availability and the risk for abuse. Surgeons must remain aware of the variables in their patients’ postoperative pain experience in order to better optimize prescribing patterns and provide a safe and effective postoperative pain regimen.
Am J Orthop. 2017;46(1):E35-E40. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Prescription opioid abuse and overdose-related deaths are on the rise in the United States.
- Following Open Reduction Internal Fixation (ORIF) of a distal radius fracture (DRF), patients consumed an average of 14.6 opioid pills. We recommend prescribing no more than 15-20 opioid pills after DRF ORIF.
- There was no difference in opioid consumption between patients who underwent general anesthesia vs regional anesthesia.
- There was a significant trend towards less opioid consumption with increasing age.
- There was a trend towards increased opioid consumption in patients with worsening fracture type as well as in self-pay/Medicaid patients.
Over the past 2 decades, prescription opioid abuse in the United States has risen steadily.1,2 Although use of opioid analgesics in the US far exceeds use in other countries, US patients do not report less pain or more satisfaction with pain relief.3-5 Between 1999 and 2002, oxycodone prescriptions increased by 50%, fentanyl prescriptions by 150%, and morphine prescriptions by 60%.6 Furthermore, the Centers for Disease Control and Prevention (CDC) reported in 2012 that, for every 100 people in the United States, US physicians wrote a mean of 82.5 opioid prescriptions and 37.6 benzodiazepine prescriptions; in total, US clinicians wrote 259 million opioid prescriptions in 2012, enough for every adult to have a bottle.7 The increase in prescription opioid abuse, not surprisingly, has paralleled a 124% increase in opioid overdose-related deaths.8 Cicero and colleagues2,9 recently found that, over the past 50 years, heroin use has dramatically shifted from being a problem mainly of urban centers and minorities toward one of older, suburban Caucasians with a previous history of prescription pain killer abuse. Deaths from prescription opioid overdoses now exceed deaths from heroin and cocaine overdoses combined.10 According to the CDC, emergency department visits related to nonmedical use of prescription opioid medications jumped 111% between 2004 and 2008.11
Opioid analgesics are often prescribed for the management of musculoskeletal pain and injuries.12-16 Orthopedic surgeons, who prescribe more opioids than physicians in any other surgical field, represent the third largest group of opioid prescribers, trailing only primary care physicians and internists, who far outnumber them.17 A study focused on opioid consumption after upper extremity surgery found that upper extremity surgeons tended to overprescribe opioids for postoperative analgesia.18 Many patients saved their remaining medication for later use and were never instructed on proper disposal. There is a developing consensus that opioid medication is not as safe and effective as once thought, and that a high-dose prescription or prolonged opioid therapy do not improve outcomes.19 In addition, patients may experience numerous opioid-associated adverse effects, including nausea, vomiting, constipation, lightheadedness, dizziness, blurred vision, headache, dry mouth, sweating, and itching.
In October 2012, patient satisfaction scores on the Hospital Consumer Assessment of Healthcare Providers and Systems started affecting Medicare reimbursements.20 By 2017, up to 6% of Medicare reimbursement will be at risk, given the poor outcomes caused by uncontrolled pain.21-24 The US healthcare culture has made it more important than ever for physicians to adequately manage postoperative pain while limiting opioid availability and the risk for abuse.
Distal radius fracture (DRF) open reduction and internal fixation (ORIF) is commonly performed by orthopedic surgeons and hand surgeons. Pain management and opioid consumption after DRF repair may be influenced by several variables. We conducted a study to investigate the impact of several clinical variables on postoperative opioid use; to test the hypothesis that post-DRF-ORIF opioid consumption would increase with worsening fracture classification and certain patient demographics; and to seek postoperative opioid consumption insights that would facilitate optimization of future opioid prescribing.
Materials and Methods
Institutional Review Board approval was obtained before initiation of the study. All outpatients who underwent DRF-ORIF (performed by 9 hand surgery fellowship-trained orthopedic surgeons) were consecutively enrolled over a 6-month period in 2014. All procedures were performed with a standard volar plating technique through a flexor carpi radialis approach. The postoperative rehabilitation protocol was standardized for all patients. Data collected on each patient included age, sex, payer type, fracture type, opioid prescribed, amount prescribed, amount consumed, reasons for stopping, adverse events, and any postoperative adjunctive pain medications. The data were taken from questionnaires completed by patients at their first visit within 2 weeks after surgery. Anesthesia type (general or regional) was noted as well. All fractures were classified by Dr. O’Neil using the AO/OTA (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association) classification of long-bone fractures based on preoperative radiographs.
Amount of opioid analgesic consumed was converted into morphine equivalents to adjust for the different opioids prescribed after surgery: oxycodone/acetaminophen or oxycodone equivalent, hydrocodone/acetaminophen or hydrocodone equivalent, and acetaminophen/codeine.
Patients were excluded from the study if their procedure was performed on an inpatient basis, if they sustained other injuries or fractures from their trauma, or if an adjunctive procedure (including carpal tunnel release) was performed during the DRF repair.
We used the Spearman rank correlation coefficient and a count data model to examine the relationship between opioid use and age. The Kruskal-Wallis test was used to examine the relationships between opioid use and payer type, anesthesia type, and fracture type.
Results
Of the 109 patients eligible for the study, 11 were excluded for incomplete postoperative questionnaires, leaving 98 patients (79 females, 19 males) for analysis. Mean age was 58 years (range, 13-92 years). Of the 98 patients, 45 received general anesthesia, and 53 received regional anesthesia with a single-shot peripheral nerve block before surgery and sedation perioperatively (Table).
Of the 98 study patients, 61 reported using over-the-counter adjunctive pain medications during the postoperative period, and 37 reported no use. Mean opioid consumption was 64.7 mg of morphine equivalents for the adjunctive medication users and 48.3 mg for the nonusers (P = .1947).
Demographic analysis revealed an inverse relationship between age and opioid use (Figure 2). The Spearman ρ between age and opioid consumption was –0.2958, which suggests decreased opioid use by older patients (P = .003).
All fractures were graded with the AO/OTA long-bone fracture classification system. Mean opioid consumption for the 3 fracture-type groups was 57.7 mg (class A), 60.3 mg (class B), and 62.0 mg (class C) (Figure 4).
Discussion
The US healthcare culture has elevated physicians’ responsibility in adequately and aggressively managing their patients’ pain experience. Moreover, reimbursement may be affected by patient satisfaction scores, which are partly predicated on pain control.20-24 However, as rates of opioid use and abuse rise, it is important that physicians prescribe such medications judiciously. This is particularly germane to orthopedic surgeons, who prescribe more opioid analgesics than surgeons in any other field.17 Rodgers and colleagues18 found upper extremity surgeons, in particular, tended to overprescribe postoperative opioid analgesics. In the present study, we sought to identify the crucial risk factors that influence post-DRF-ORIF pain management and opioid consumption.
Mean postoperative opioid consumption (morphine equivalents) was 58.5 mg, roughly equivalent to 14.6 tabs of oxycodone/acetaminophen 5/325 mg, an opioid analgesic commonly used during the acute postoperative period. In addition, almost 70% of our patients required <75 mg of morphine equivalents, or <20 tabs of oxycodone/acetaminophen 5/325 mg. For upper extremity surgeons, these numbers may be better guides in determining the most appropriate amount of opioid to prescribe after DRF repair.
As for predicting levels of postoperative opioid medication, there was a significant trend toward less consumption with increasing age. Given this finding, surgeons prescribing for elderly patients should expect less opioid use. Regarding payer type, there was a trend toward more opioid use by self-pay/Medicaid patients; however, there were only 3 patients in this group. The situation in the study by Rodgers and colleagues18 is similar: Their finding that Medicaid patients consumed more pain pills after surgery was underpowered (only 5 patients in the group).
In the orthopedic community, support for use of regional anesthesia has been widespread for several reasons, including the belief that it reduces postoperative pain and therefore should reduce postoperative opioid consumption.25 However, we found no significant difference in postoperative opioid consumption between patients who received general anesthesia (with and without local anesthesia) and patients who received regional anesthesia (nerve block). Mean opioid consumption was 57.93 mg in the general anesthesia group and 58.98 mg in the regional anesthesia group. However, this finding could have been confounded by the variability in success and operator dependence inherent in regional anesthesia. In addition, the anatomical location for the peripheral nerve block and anesthetic could have affected the efficacy of the block and played a role in postoperative opioid consumption.
In this study, we tested the hypothesis that there would be more postoperative opioid consumption with worsening fracture type. Although our results did not reach statistical significance, there was a trend toward increased opioid consumption in patients with a complete intra-articular fracture (AO/OTA class C) vs patients with a partial articular fracture (class B) or an extra-articular fracture (class A). In addition, patients with a partial articular fracture tended to use more postoperative opioids than patients with an extra-articular fracture. In short, postoperative opioid consumption tended to be higher with increasing articular involvement of the fracture.
This study was limited in that it relied on patient self-reporting. Given the social stigma attached to opioid use, patients may have underreported their postoperative opioid consumption, been affected by recall bias, or both. The study also did not control for preoperative opioid use or history of opioid or substance abuse. Chronic preoperative opioid consumption may have affected postoperative opioid use. Other patient-related factors, such as body mass index (BMI) and hepatorenal dysfunction, can create tremendous variability in opioid metabolism across a population. Such factors were not controlled for in this study and therefore may have affected its results. That could help explain why older patients, who are more likely to have lower BMI and less efficient organ function for opioid metabolism, had lower postoperative opioid consumption. In addition, although we excluded patients with concomitant injuries and procedures, we did not screen patients for concomitant complex regional pain syndrome, fibromyalgia, or other medical conditions that might have had a significant impact on postoperative pain management needs. Last, some findings, such as the relationship between opioid use and payer type, were underpowered: Although self-pay/Medicaid patients had higher postoperative opioid consumption, they were few in number. The same was true of the Medicaid patients in the study by Rodgers and colleagues.18Our results demonstrated that post-DRF-ORIF opioid consumption decreased with age and was independent of type of perioperative anesthesia. There was a trend toward more opioid consumption with both self- and Medicaid payment and worsening fracture classification. It has become more important than ever for orthopedic surgeons to adequately manage postoperative pain while limiting opioid availability and the risk for abuse. Surgeons must remain aware of the variables in their patients’ postoperative pain experience in order to better optimize prescribing patterns and provide a safe and effective postoperative pain regimen.
Am J Orthop. 2017;46(1):E35-E40. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297(3):249-251.
2. Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71(7):821-826.
3. Helmerhorst GT, Lindenhovius AL, Vrahas M, Ring D, Kloen P. Satisfaction with pain relief after operative treatment of an ankle fracture. Injury. 2012;43(11):1958-1961.
4. Lindenhovius AL, Helmerhorst GT, Schnellen AC, Vrahas M, Ring D, Kloen P. Differences in prescription of narcotic pain medication after operative treatment of hip and ankle fractures in the United States and the Netherlands. J Trauma. 2009;67(1):160-164.
5. Seya MJ, Gelders SF, Achara OU, Milani B, Scholten WK. A first comparison between the consumption of and the need for opioid analgesics at country, regional, and global levels. J Pain Palliat Care Pharmacother. 2011;25(1):6-18.
6. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
7. Kuehn BM. CDC: major disparities in opioid prescribing among states: some states crack down on excess prescribing. JAMA. 2014;312(7):684-686.
8. Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15(9):618-627.
9. Cicero TJ, Kuehn BM. Driven by prescription drug abuse, heroin use increases among suburban and rural whites. JAMA. 2014;312(2):118-119.
10. Painkillers fuel growth in drug addiction. Harvard Ment Health Lett. Harvard Medical School website. http://www.health.harvard.edu/newsletter_article/painkillers-fuel-growth-in-drug-addiction. Published January 2011. Accessed March 18, 2015.
11. Cai R, Crane E, Poneleit K, Paulozzi L. Emergency department visits involving nonmedical use of selected prescription drugs in the United States, 2004-2008. J Pain Palliat Care Pharmacother. 2010;24(3):293-297.
12. Armaghani SJ, Lee DS, Bible JE, et al. Preoperative narcotic use and its relation to depression and anxiety in patients undergoing spine surgery. Spine. 2013;38(25):2196-2200.
13. Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109(3):514-519.
14. Deyo RA, Mirza SK, Turner JA, Martin BI. Overtreating chronic back pain: time to back off? J Am Board Fam Med. 2009;22(1):62-68.
15. Lee D, Armaghani S, Archer KR, et al. Preoperative opioid use as a predictor of adverse postoperative self-reported outcomes in patients undergoing spine surgery. J Bone Joint Surg Am. 2014;96(11):e89.
16. Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine. 2007;32(19):2127-2132.
17. Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SR. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299-1301.
18. Rodgers J, Cunningham K, Fitzgerald K, Finnerty E. Opioid consumption following outpatient upper extremity surgery. J Hand Surg Am. 2012;37(4):645-650.
19. Chen L, Vo T, Seefeld L, et al. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain. 2013;14(4):384-392.
20. Bush H. Doubling down on the patient experience. Hosp Health Netw. 2011;85(12):22-25, 1.
21. Centers for Medicare & Medicaid Services, US Department of Health and Human Services. Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and fiscal year 2013 rates; hospitals’ resident caps for graduate medical education payment purposes; quality reporting requirements for specific providers and for ambulatory surgical centers. Final rule. Fed Regist. 2012;77(170):53257-53750.
22. Centers for Medicare & Medicaid Services, US Department of Health and Human Services. Hospital Value-Based Purchasing. http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/Hospital_VBPurchasing_Fact_Sheet_ICN907664.pdf. Published September 2015. Accessed October 2015.
23. Manchikanti L, Singh V, Caraway DL, Benyamin RM, Falco FJ, Hirsch JA. Proposed physician payment schedule for 2013: guarded prognosis for interventional pain management. Pain Physician. 2012;15(5):E615-E627.
24. Bot AG, Bekkers S, Arnstein PM, Smith RM, Ring D. Opioid use after fracture surgery correlates with pain intensity and satisfaction with pain relief. Clin Orthop Relat Res. 2014;472(8):2542-2549.
25. Oldman M, McCartney CJ, Leung A, et al. A survey of orthopedic surgeons’ attitudes and knowledge regarding regional anesthesia. Anesth Analg. 2004;98(5):1486-1490.
1. Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. JAMA. 2007;297(3):249-251.
2. Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71(7):821-826.
3. Helmerhorst GT, Lindenhovius AL, Vrahas M, Ring D, Kloen P. Satisfaction with pain relief after operative treatment of an ankle fracture. Injury. 2012;43(11):1958-1961.
4. Lindenhovius AL, Helmerhorst GT, Schnellen AC, Vrahas M, Ring D, Kloen P. Differences in prescription of narcotic pain medication after operative treatment of hip and ankle fractures in the United States and the Netherlands. J Trauma. 2009;67(1):160-164.
5. Seya MJ, Gelders SF, Achara OU, Milani B, Scholten WK. A first comparison between the consumption of and the need for opioid analgesics at country, regional, and global levels. J Pain Palliat Care Pharmacother. 2011;25(1):6-18.
6. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
7. Kuehn BM. CDC: major disparities in opioid prescribing among states: some states crack down on excess prescribing. JAMA. 2014;312(7):684-686.
8. Paulozzi LJ, Budnitz DS, Xi Y. Increasing deaths from opioid analgesics in the United States. Pharmacoepidemiol Drug Saf. 2006;15(9):618-627.
9. Cicero TJ, Kuehn BM. Driven by prescription drug abuse, heroin use increases among suburban and rural whites. JAMA. 2014;312(2):118-119.
10. Painkillers fuel growth in drug addiction. Harvard Ment Health Lett. Harvard Medical School website. http://www.health.harvard.edu/newsletter_article/painkillers-fuel-growth-in-drug-addiction. Published January 2011. Accessed March 18, 2015.
11. Cai R, Crane E, Poneleit K, Paulozzi L. Emergency department visits involving nonmedical use of selected prescription drugs in the United States, 2004-2008. J Pain Palliat Care Pharmacother. 2010;24(3):293-297.
12. Armaghani SJ, Lee DS, Bible JE, et al. Preoperative narcotic use and its relation to depression and anxiety in patients undergoing spine surgery. Spine. 2013;38(25):2196-2200.
13. Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000. Pain. 2004;109(3):514-519.
14. Deyo RA, Mirza SK, Turner JA, Martin BI. Overtreating chronic back pain: time to back off? J Am Board Fam Med. 2009;22(1):62-68.
15. Lee D, Armaghani S, Archer KR, et al. Preoperative opioid use as a predictor of adverse postoperative self-reported outcomes in patients undergoing spine surgery. J Bone Joint Surg Am. 2014;96(11):e89.
16. Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine. 2007;32(19):2127-2132.
17. Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SR. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299-1301.
18. Rodgers J, Cunningham K, Fitzgerald K, Finnerty E. Opioid consumption following outpatient upper extremity surgery. J Hand Surg Am. 2012;37(4):645-650.
19. Chen L, Vo T, Seefeld L, et al. Lack of correlation between opioid dose adjustment and pain score change in a group of chronic pain patients. J Pain. 2013;14(4):384-392.
20. Bush H. Doubling down on the patient experience. Hosp Health Netw. 2011;85(12):22-25, 1.
21. Centers for Medicare & Medicaid Services, US Department of Health and Human Services. Medicare program; hospital inpatient prospective payment systems for acute care hospitals and the long-term care hospital prospective payment system and fiscal year 2013 rates; hospitals’ resident caps for graduate medical education payment purposes; quality reporting requirements for specific providers and for ambulatory surgical centers. Final rule. Fed Regist. 2012;77(170):53257-53750.
22. Centers for Medicare & Medicaid Services, US Department of Health and Human Services. Hospital Value-Based Purchasing. http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/Hospital_VBPurchasing_Fact_Sheet_ICN907664.pdf. Published September 2015. Accessed October 2015.
23. Manchikanti L, Singh V, Caraway DL, Benyamin RM, Falco FJ, Hirsch JA. Proposed physician payment schedule for 2013: guarded prognosis for interventional pain management. Pain Physician. 2012;15(5):E615-E627.
24. Bot AG, Bekkers S, Arnstein PM, Smith RM, Ring D. Opioid use after fracture surgery correlates with pain intensity and satisfaction with pain relief. Clin Orthop Relat Res. 2014;472(8):2542-2549.
25. Oldman M, McCartney CJ, Leung A, et al. A survey of orthopedic surgeons’ attitudes and knowledge regarding regional anesthesia. Anesth Analg. 2004;98(5):1486-1490.