Article
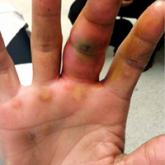
5 Points on Pyogenic Flexor Tenosynovitis of the Hand
- Author:
- Talia Chapman, MD
- Asif M. Ilyas, MD
Pyogenic flexor tenosynovitis (PFT) is a common closed space infection of the flexor tendon sheaths of the hand and remains one of the most...
Article
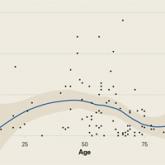
Prospective Evaluation of Opioid Consumption After Distal Radius Fracture Repair Surgery
- Author:
- Joseph T. O’Neil, MD
- Mark L. Wang, MD, PhD
- Nayoung Kim, BS
- Mitchell Maltenfort, PhD
- Asif M. Ilyas, MD
Pain management and opioid consumption after distal radius fracture (DRF) open reduction and internal fixation (ORIF) are highly variable and...
