User login
Pustular psoriasis of pregnancy (PPP) is a rare but potentially serious dermatosis of pregnancy. Left untreated, PPP can be fatal for both the mother and the fetus.1,2 Contrary to many other pregnancy dermatoses, which are typically limited to the skin, systemic signs and symptoms often accompany PPP, including fatigue, fever, diarrhea, delirium, elevated markers of inflammation such as an increased erythrocyte sedimentation rate, and increased white blood cell counts.1,3,4 Progression of the rash to erythroderma with subsequent dangerous fluid and electrolyte imbalances, loss of thermoregulation in the skin, and the risk for secondary infection and sepsis can occur in severe cases.1,5
Increasing evidence suggests that PPP is likely a variant of generalized pustular psoriasis (GPP), which can be vulnerable to a variety of triggers, including metabolic disturbances, systemic steroid withdrawals, and pregnancy; however, classification of the disease as either a variant of disease or a distinct disease state remains controversial.1,6 Early recognition and prompt treatment are critically important given the potential for fetal and maternal morbidity and mortality that is associated with PPP.1,6,7
Clinical Presentation
Most cases of PPP involve presentation in the early part of the third trimester of pregnancy; postpartum PPP has been reported but is exceedingly rare.1 Typically, lesions develop in the skin folds and spread centrifugally.2,6 The lesions usually begin as erythematous plaques with a pustular ring with a central erosion. The face, palms, and soles of the feet usually are spared; occasionally, involvement of oral and esophageal mucosae is seen. Biopsy findings usually comprise spongiform pustules with neutrophil invasion into the epidermis. Characteristic laboratory findings include electrolyte derangements with elevated erythrocyte sedimentation rate and leukocytosis.2,8
As was seen in the case presentation, PPP largely resolves following childbirth; however, there is a significant risk for recurrence in subsequent pregnancies, which may be more severe and present earlier.1,6 Menstrual cycle changes and the use of oral contraceptives, particularly those containing progesterone, also have been associated with PPP flares.1,6 Although its pathophysiology is not entirely understood, the development of PPP is believed to be associated with the hormonal changes that occur in the third trimester, in particular elevated progesterone levels.6
Treatment
Oral corticosteroids remain the mainstay of treatment for PPP.6 Lower dosages ranging from 15 to 30 mg daily can be used for mild cases.1 More severe cases usually are treated with an initial trial of prednisone or prednisolone with dosages ranging from 30 mg daily to as high as 60 to 80 mg daily. Higher doses should be used with caution, however, as they may result in reduced fetal reactivity on fetal monitoring.1,9 Treatment at least throughout the remainder of a patient’s pregnancy usually is required, with subsequent gradual tapering of the medication.1
Although it was once reserved for severe or refractory PPP, in 2012, a task force from the National Psoriasis Foundation categorized cyclosporine as an appropriate first-line therapy for PPP.10 Published case reports have documented the successful treatment of PPP with cyclosporine in cases that did not respond to systemic steroids.6,11
Several case reports have documented the safe use of anti–tumor necrosis factors (TNFs), primarily infliximab, for PPP.12 Infliximab and other TNF-α antibodies are pregnancy category B, but limited controlled human data exist regarding their safety in pregnancy.1
Finally, the addition of narrowband UVB light therapy to oral corticosteroids also has been proposed as a safe treatment of refractory disease.6,13 Unlike psoralen plus UVA, which is usually reserved for postpartum use, narrowband UVB light therapy has been shown to be safe for use during pregnancy.1
Future Directions
Genetic and pathogenesis studies have described involvement of IL-1 and IL-36 cytokines in GPP.1 These interleukins are important in neutrophil chemotaxis leading to pustule formation. In addition, as in other psoriasis variants, TNF-α and IL-17α are important. Such findings may help to pave the way for the development of future therapies for both GPP and PPP.
Bottom Line
Clinicians should have a high index of suspicion for PPP in pregnant women who present with widespread cutaneous eruptions. Presently, oral corticosteroids paired with close involvement of obstetric care remains the cornerstone of treatment for PPP. As the case report illustrates, effective diagnosis, treatment, and monitoring are essential for safe outcomes for both mother and baby.
- Trivedi MK, Vaughn AR, Murase JE. Pustular psoriasis of pregnancy: current perspectives. Int J Womens Health. 2018;10:109-115.
- Kondo RN, Araújo FM, Pereira AM, et al. Pustular psoriasis of pregnancy (impetigo herpetiformis)—case report. An Bras Dermatol. 2013;88(6 suppl 1):186-189.
- Oumeish OY, Farraj SE, Bataineh AS. Some aspects of impetigo herpetiformis. Arch Dermatol. 1982;118:103-105.
- Flynn A, Burke N, Byrne B, et al. Two case reports of generalized pustular psoriasis of pregnancy: different outcomes. Obstet Med. 2016;9:55-59.
- Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine. BMJ Case Rep. 2011;2011:bcr0220113915.
- Pitch M, Somers K, Scott G, et al. A case of pustular psoriasis of pregnancy with positive maternal-fetal outcomes. Cutis. 2018;101:278-280.
- Namazi N, Dadkhahfar S. Impetigo herpetiformis: review of pathogenesis, complication, and treatment [published April 4, 2018]. Dermatol Res Pract. 2018;2018:5801280. doi:10.1155/2018/5801280. eCollection 2018.
- Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
- Ulubay M, Keskin U, Fidan U, et al. Case report of a rare dermatosis in pregnancy: impetigo herpetiformis. J Obstet Gynaecol Res. 2015;41:301-303.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
- Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
- Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
- Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrowband UVB [published online January 20, 2012]. Cutan Ocul Toxicol. 2012;31:67-69.
Pustular psoriasis of pregnancy (PPP) is a rare but potentially serious dermatosis of pregnancy. Left untreated, PPP can be fatal for both the mother and the fetus.1,2 Contrary to many other pregnancy dermatoses, which are typically limited to the skin, systemic signs and symptoms often accompany PPP, including fatigue, fever, diarrhea, delirium, elevated markers of inflammation such as an increased erythrocyte sedimentation rate, and increased white blood cell counts.1,3,4 Progression of the rash to erythroderma with subsequent dangerous fluid and electrolyte imbalances, loss of thermoregulation in the skin, and the risk for secondary infection and sepsis can occur in severe cases.1,5
Increasing evidence suggests that PPP is likely a variant of generalized pustular psoriasis (GPP), which can be vulnerable to a variety of triggers, including metabolic disturbances, systemic steroid withdrawals, and pregnancy; however, classification of the disease as either a variant of disease or a distinct disease state remains controversial.1,6 Early recognition and prompt treatment are critically important given the potential for fetal and maternal morbidity and mortality that is associated with PPP.1,6,7
Clinical Presentation
Most cases of PPP involve presentation in the early part of the third trimester of pregnancy; postpartum PPP has been reported but is exceedingly rare.1 Typically, lesions develop in the skin folds and spread centrifugally.2,6 The lesions usually begin as erythematous plaques with a pustular ring with a central erosion. The face, palms, and soles of the feet usually are spared; occasionally, involvement of oral and esophageal mucosae is seen. Biopsy findings usually comprise spongiform pustules with neutrophil invasion into the epidermis. Characteristic laboratory findings include electrolyte derangements with elevated erythrocyte sedimentation rate and leukocytosis.2,8
As was seen in the case presentation, PPP largely resolves following childbirth; however, there is a significant risk for recurrence in subsequent pregnancies, which may be more severe and present earlier.1,6 Menstrual cycle changes and the use of oral contraceptives, particularly those containing progesterone, also have been associated with PPP flares.1,6 Although its pathophysiology is not entirely understood, the development of PPP is believed to be associated with the hormonal changes that occur in the third trimester, in particular elevated progesterone levels.6
Treatment
Oral corticosteroids remain the mainstay of treatment for PPP.6 Lower dosages ranging from 15 to 30 mg daily can be used for mild cases.1 More severe cases usually are treated with an initial trial of prednisone or prednisolone with dosages ranging from 30 mg daily to as high as 60 to 80 mg daily. Higher doses should be used with caution, however, as they may result in reduced fetal reactivity on fetal monitoring.1,9 Treatment at least throughout the remainder of a patient’s pregnancy usually is required, with subsequent gradual tapering of the medication.1
Although it was once reserved for severe or refractory PPP, in 2012, a task force from the National Psoriasis Foundation categorized cyclosporine as an appropriate first-line therapy for PPP.10 Published case reports have documented the successful treatment of PPP with cyclosporine in cases that did not respond to systemic steroids.6,11
Several case reports have documented the safe use of anti–tumor necrosis factors (TNFs), primarily infliximab, for PPP.12 Infliximab and other TNF-α antibodies are pregnancy category B, but limited controlled human data exist regarding their safety in pregnancy.1
Finally, the addition of narrowband UVB light therapy to oral corticosteroids also has been proposed as a safe treatment of refractory disease.6,13 Unlike psoralen plus UVA, which is usually reserved for postpartum use, narrowband UVB light therapy has been shown to be safe for use during pregnancy.1
Future Directions
Genetic and pathogenesis studies have described involvement of IL-1 and IL-36 cytokines in GPP.1 These interleukins are important in neutrophil chemotaxis leading to pustule formation. In addition, as in other psoriasis variants, TNF-α and IL-17α are important. Such findings may help to pave the way for the development of future therapies for both GPP and PPP.
Bottom Line
Clinicians should have a high index of suspicion for PPP in pregnant women who present with widespread cutaneous eruptions. Presently, oral corticosteroids paired with close involvement of obstetric care remains the cornerstone of treatment for PPP. As the case report illustrates, effective diagnosis, treatment, and monitoring are essential for safe outcomes for both mother and baby.
Pustular psoriasis of pregnancy (PPP) is a rare but potentially serious dermatosis of pregnancy. Left untreated, PPP can be fatal for both the mother and the fetus.1,2 Contrary to many other pregnancy dermatoses, which are typically limited to the skin, systemic signs and symptoms often accompany PPP, including fatigue, fever, diarrhea, delirium, elevated markers of inflammation such as an increased erythrocyte sedimentation rate, and increased white blood cell counts.1,3,4 Progression of the rash to erythroderma with subsequent dangerous fluid and electrolyte imbalances, loss of thermoregulation in the skin, and the risk for secondary infection and sepsis can occur in severe cases.1,5
Increasing evidence suggests that PPP is likely a variant of generalized pustular psoriasis (GPP), which can be vulnerable to a variety of triggers, including metabolic disturbances, systemic steroid withdrawals, and pregnancy; however, classification of the disease as either a variant of disease or a distinct disease state remains controversial.1,6 Early recognition and prompt treatment are critically important given the potential for fetal and maternal morbidity and mortality that is associated with PPP.1,6,7
Clinical Presentation
Most cases of PPP involve presentation in the early part of the third trimester of pregnancy; postpartum PPP has been reported but is exceedingly rare.1 Typically, lesions develop in the skin folds and spread centrifugally.2,6 The lesions usually begin as erythematous plaques with a pustular ring with a central erosion. The face, palms, and soles of the feet usually are spared; occasionally, involvement of oral and esophageal mucosae is seen. Biopsy findings usually comprise spongiform pustules with neutrophil invasion into the epidermis. Characteristic laboratory findings include electrolyte derangements with elevated erythrocyte sedimentation rate and leukocytosis.2,8
As was seen in the case presentation, PPP largely resolves following childbirth; however, there is a significant risk for recurrence in subsequent pregnancies, which may be more severe and present earlier.1,6 Menstrual cycle changes and the use of oral contraceptives, particularly those containing progesterone, also have been associated with PPP flares.1,6 Although its pathophysiology is not entirely understood, the development of PPP is believed to be associated with the hormonal changes that occur in the third trimester, in particular elevated progesterone levels.6
Treatment
Oral corticosteroids remain the mainstay of treatment for PPP.6 Lower dosages ranging from 15 to 30 mg daily can be used for mild cases.1 More severe cases usually are treated with an initial trial of prednisone or prednisolone with dosages ranging from 30 mg daily to as high as 60 to 80 mg daily. Higher doses should be used with caution, however, as they may result in reduced fetal reactivity on fetal monitoring.1,9 Treatment at least throughout the remainder of a patient’s pregnancy usually is required, with subsequent gradual tapering of the medication.1
Although it was once reserved for severe or refractory PPP, in 2012, a task force from the National Psoriasis Foundation categorized cyclosporine as an appropriate first-line therapy for PPP.10 Published case reports have documented the successful treatment of PPP with cyclosporine in cases that did not respond to systemic steroids.6,11
Several case reports have documented the safe use of anti–tumor necrosis factors (TNFs), primarily infliximab, for PPP.12 Infliximab and other TNF-α antibodies are pregnancy category B, but limited controlled human data exist regarding their safety in pregnancy.1
Finally, the addition of narrowband UVB light therapy to oral corticosteroids also has been proposed as a safe treatment of refractory disease.6,13 Unlike psoralen plus UVA, which is usually reserved for postpartum use, narrowband UVB light therapy has been shown to be safe for use during pregnancy.1
Future Directions
Genetic and pathogenesis studies have described involvement of IL-1 and IL-36 cytokines in GPP.1 These interleukins are important in neutrophil chemotaxis leading to pustule formation. In addition, as in other psoriasis variants, TNF-α and IL-17α are important. Such findings may help to pave the way for the development of future therapies for both GPP and PPP.
Bottom Line
Clinicians should have a high index of suspicion for PPP in pregnant women who present with widespread cutaneous eruptions. Presently, oral corticosteroids paired with close involvement of obstetric care remains the cornerstone of treatment for PPP. As the case report illustrates, effective diagnosis, treatment, and monitoring are essential for safe outcomes for both mother and baby.
- Trivedi MK, Vaughn AR, Murase JE. Pustular psoriasis of pregnancy: current perspectives. Int J Womens Health. 2018;10:109-115.
- Kondo RN, Araújo FM, Pereira AM, et al. Pustular psoriasis of pregnancy (impetigo herpetiformis)—case report. An Bras Dermatol. 2013;88(6 suppl 1):186-189.
- Oumeish OY, Farraj SE, Bataineh AS. Some aspects of impetigo herpetiformis. Arch Dermatol. 1982;118:103-105.
- Flynn A, Burke N, Byrne B, et al. Two case reports of generalized pustular psoriasis of pregnancy: different outcomes. Obstet Med. 2016;9:55-59.
- Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine. BMJ Case Rep. 2011;2011:bcr0220113915.
- Pitch M, Somers K, Scott G, et al. A case of pustular psoriasis of pregnancy with positive maternal-fetal outcomes. Cutis. 2018;101:278-280.
- Namazi N, Dadkhahfar S. Impetigo herpetiformis: review of pathogenesis, complication, and treatment [published April 4, 2018]. Dermatol Res Pract. 2018;2018:5801280. doi:10.1155/2018/5801280. eCollection 2018.
- Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
- Ulubay M, Keskin U, Fidan U, et al. Case report of a rare dermatosis in pregnancy: impetigo herpetiformis. J Obstet Gynaecol Res. 2015;41:301-303.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
- Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
- Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
- Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrowband UVB [published online January 20, 2012]. Cutan Ocul Toxicol. 2012;31:67-69.
- Trivedi MK, Vaughn AR, Murase JE. Pustular psoriasis of pregnancy: current perspectives. Int J Womens Health. 2018;10:109-115.
- Kondo RN, Araújo FM, Pereira AM, et al. Pustular psoriasis of pregnancy (impetigo herpetiformis)—case report. An Bras Dermatol. 2013;88(6 suppl 1):186-189.
- Oumeish OY, Farraj SE, Bataineh AS. Some aspects of impetigo herpetiformis. Arch Dermatol. 1982;118:103-105.
- Flynn A, Burke N, Byrne B, et al. Two case reports of generalized pustular psoriasis of pregnancy: different outcomes. Obstet Med. 2016;9:55-59.
- Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine. BMJ Case Rep. 2011;2011:bcr0220113915.
- Pitch M, Somers K, Scott G, et al. A case of pustular psoriasis of pregnancy with positive maternal-fetal outcomes. Cutis. 2018;101:278-280.
- Namazi N, Dadkhahfar S. Impetigo herpetiformis: review of pathogenesis, complication, and treatment [published April 4, 2018]. Dermatol Res Pract. 2018;2018:5801280. doi:10.1155/2018/5801280. eCollection 2018.
- Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
- Ulubay M, Keskin U, Fidan U, et al. Case report of a rare dermatosis in pregnancy: impetigo herpetiformis. J Obstet Gynaecol Res. 2015;41:301-303.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
- Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
- Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
- Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrowband UVB [published online January 20, 2012]. Cutan Ocul Toxicol. 2012;31:67-69.
The Case
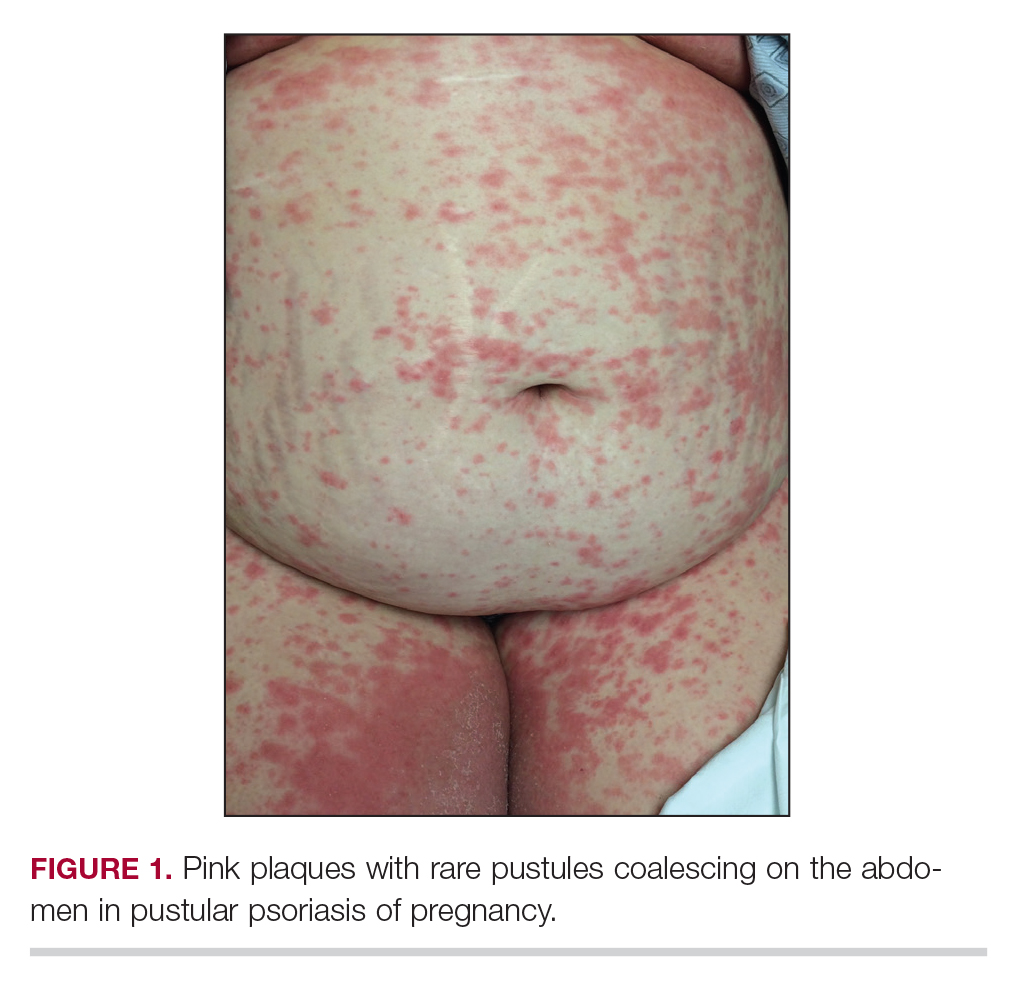
An otherwise healthy 29-year-old woman at 32 weeks’ gestation presented to the emergency department with a 1-week history of a pruritic burning rash that began on the thighs and then spread diffusely. She denied any similar rash in her prior pregnancy. The patient was not taking any medications except for prenatal vitamins, and she denied any systemic symptoms. Three days prior, treatment with methylprednisolone 50 mg once daily was initiated by the patient’s obstetrician for the rash, but the patient reported no improvement in symptoms. Physical examination revealed edematous pink plaques studded with 1- to 2-mm collarettes of scaling and sparse 1-mm pustules involving the arms, chest, abdomen, back, groin, buttocks, and legs (Figure 1). A peripheral rim of desquamative scaling was noted on the plaques on the back and inner thighs. There were pink macules on the palms, and superficial desquamation was noted on the lips; no other involvement of the oral mucosa was noted.
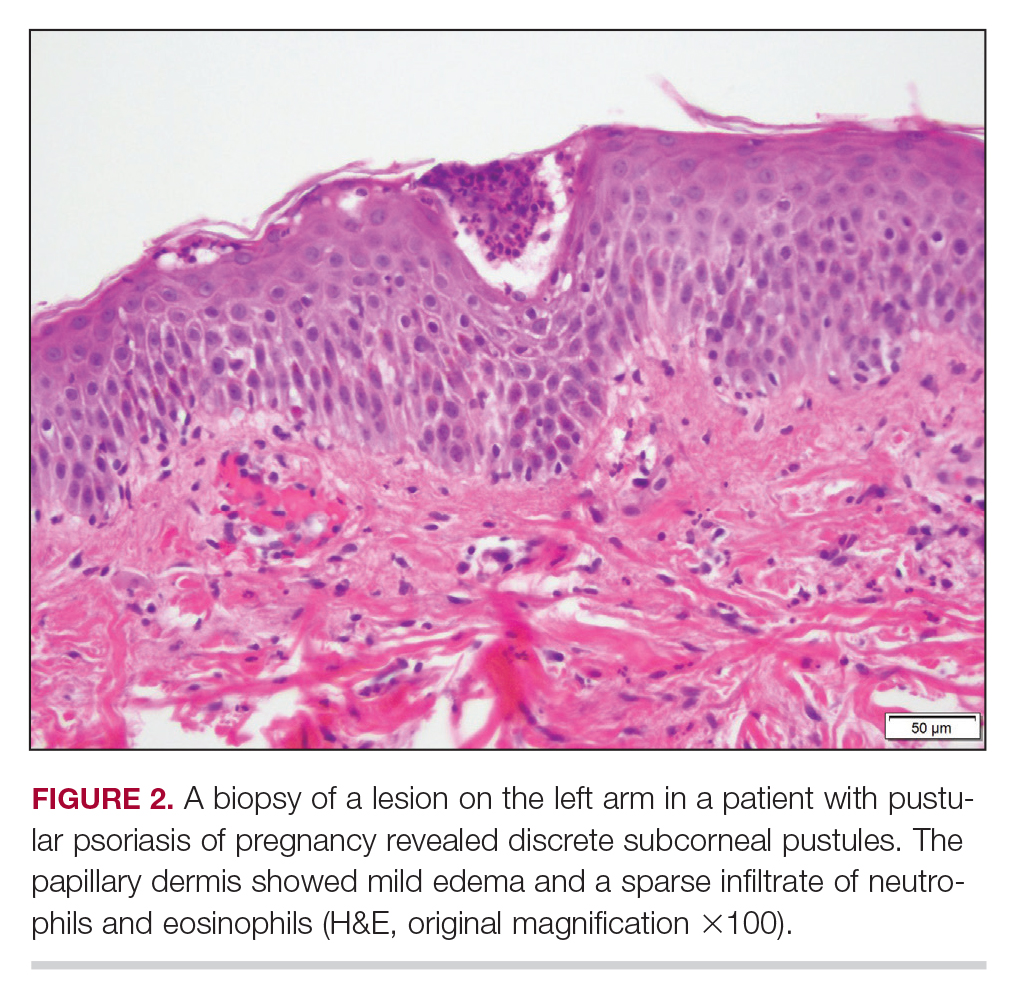
Biopsy specimens from the left arm revealed discrete subcorneal pustules with mild acanthosis of the epidermis with spongiosis (Figure 2). The papillary dermis showed a sparse infiltrate of neutrophils with numerous marginated neutrophils within vessels. Direct immunofluorescence was negative for human IgG, IgA, IgM, complement component 3, and fibrinogen. Laboratory workup revealed leukocytosis of 21.5×109/L (reference range, 4.5–11.0×109/L) with neutrophilic predominance of 73.6% (reference range, 56%), an elevated erythrocyte sedimentation rate of 40 mm/h (reference range, 0–20 mm/h), and normal calcium of 8.6 mg/dL (reference range, 8.2–10.2 mg/dL).
Treatment
The patient was started on methylprednisone 40 mg once daily with a plan to taper the dose by 8 mg every 5 days.
Patient Outcomes
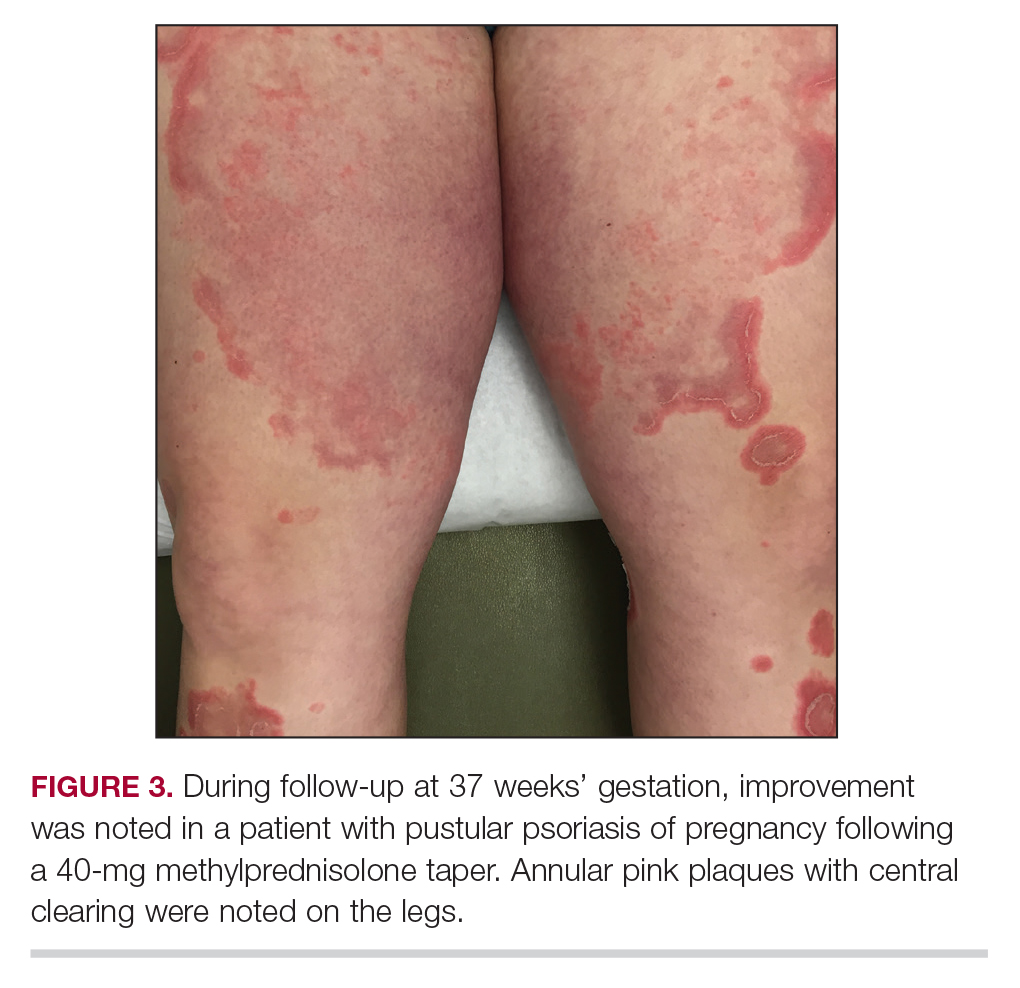
Three weeks following the initial presentation (35 weeks’ gestation), the patient continued to report pruritus and burning in the areas where the rash had developed. The morphology of the rash had changed considerably, as she now had prominent, annular, pink plaques with central clearing, trailing scaling, and a border of subtle pustules on the legs. Rings of desquamative scaling also were noted on the palms. During follow-up at 37 weeks’ gestation, the back, chest, and abdomen were improved from the initial presentation, and annular pink plaques with central clearing were noted on the legs (Figure 3). Given the clinical features and histopathologic findings, a diagnosis of pustular psoriasis of pregnancy (PPP) was made. Increased fetal surveillance with close obstetric follow-up was recommended. Weekly office visits with obstetrics and twice-weekly Doppler ultrasounds and fetal nonstress tests were deemed appropriate management. Given the risk for potential harm to the fetus PPP conveys, the patient was scheduled for induction at 39 weeks’ gestation. She was maintained on low-dose methylprednisolone 4 mg once daily for the duration of the pregnancy, and gradual improvement of the rash continued to be noted at the low treatment dose.
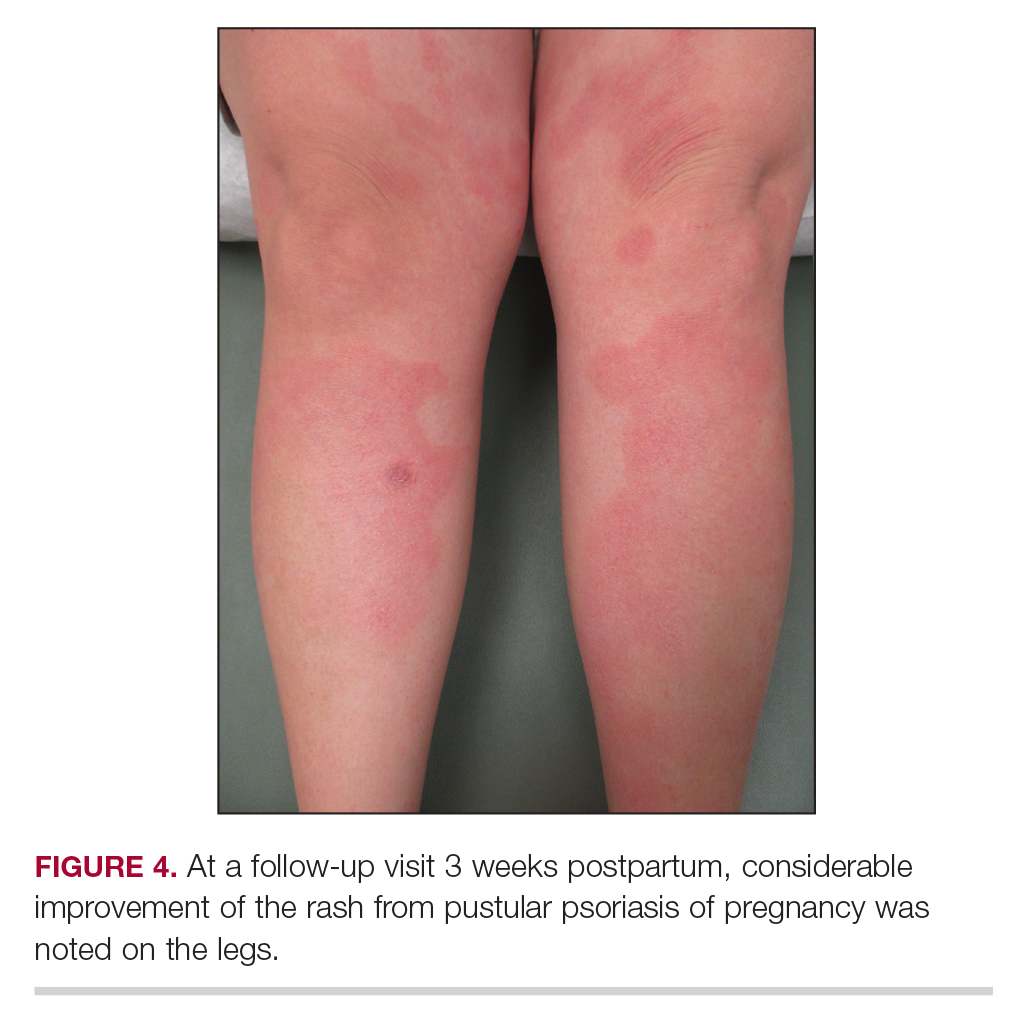
Following induction at 39 weeks’ gestation, the patient vaginally delivered a healthy, 6-lb male child at an outside hospital. She reported that the burning sensation improved within hours of delivery, and systemic steroids were stopped after delivery. At a follow-up visit 3 weeks postpartum, considerable improvement of the rash was noted with no evidence of pustules. Fading pink patches with a superficial scaling were noted on the back, chest, abdomen, arms, legs (Figure 4), and fingertips. The patient was counseled that PPP could recur in subsequent pregnancies and that she should be aware of the potential risks to the fetus.