User login
A 45-year-old woman visited the clinic 6 weeks after having a stroke while on her motorcycle, which resulted in a crash. She had not been wearing a helmet and was uncertain if she had sustained a head injury. She said that during the hospital stay following the accident, she was diagnosed as hypertensive; she denied any other significant prior medical history.
Following the crash, she said she’d been experiencing weakness in her right arm and leg and had been unable to open her right eye. When her right eye was opened manually, she said she had double vision and sensitivity to light.
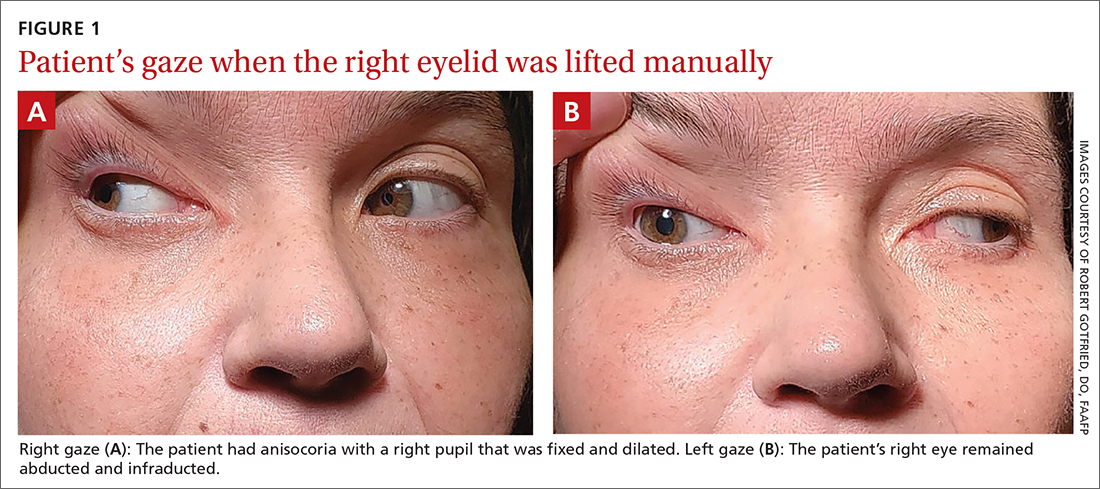
On exam, the patient had exotropia with hypotropia of her right eye. Additionally, she had anisocoria with an enlarged, nonreactive right pupil (FIGURE 1A). She was unable to adduct, supraduct, or infraduct her right eye (FIGURE 1B). Her cranial nerves were otherwise intact. On manual strength testing, she had 4/5 strength of both her right upper and lower extremities.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Third (oculomotor) nerve palsy
This patient had a complete third nerve palsy (TNP). This is defined as palsy involving all of the muscles innervated by the oculomotor nerve, with pupillary involvement.1 The oculomotor nerve supplies motor innervation to the levator palpebrae superioris, superior rectus, medial rectus, inferior rectus, and inferior oblique muscles and parasympathetic innervation to the pupillary constrictor and ciliary muscles.2 As a result, patients present with exotropia and hypotropia on exam with anisocoria. Diplopia, ptosis, and an enlarged pupil are classic symptoms of TNP.2
Computed tomography (CT) of the brain performed immediately after this patient’s accident demonstrated a 15-mm hemorrhage within the left basal ganglia with mild associated edema, and a small focus of hyperattenuation within the right aspect of the suprasellar cistern. There was no evidence of skull fracture. CT angiography (CTA) of the brain showed no evidence of aneurysm.
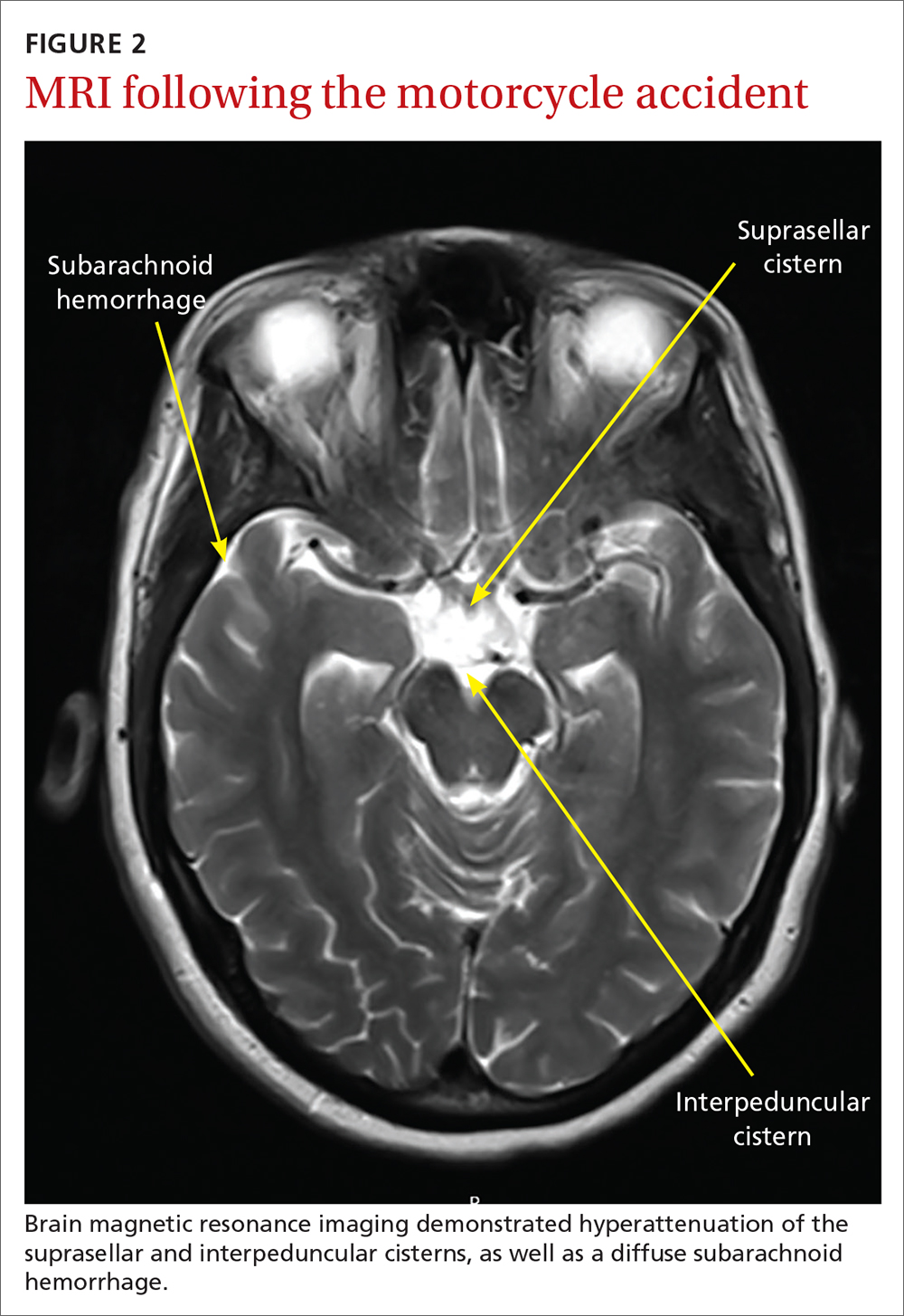
Several days later, magnetic resonance imaging (MRI) of the brain confirmed prior CT findings and revealed hemorrhagic contusions along the anterior and medial left temporal lobe. Additionally, the MRI showed subtle subdural hemorrhages along the midline falx and right parietal region, as well as diffuse subarachnoid hemorrhage around both hemispheres, the interpeduncular cistern, and the suprasellar cistern (FIGURE 2). The basal ganglia hemorrhage was believed to have been a result of uncontrolled hypertension. The hemorrhage was responsible for her right-sided weakness and was the presumed cause of the accident. The other findings were due to head trauma. Her TNP was most likely caused by both compression and irritation of the right oculomotor nerve.
An uncommon occurrence
A population-based study identified the annual incidence of TNP to be 4 per 100,000.1 The mean age of onset was 42 years. The incidence in patients older than 60 years was greater than the incidence in those younger than 60.2 Isolated TNP occurred in approximately 40% of cases.2
Complete TNP is typically indicative of compression of the ipsilateral third nerve.2 The most common region for third nerve injury is the subarachnoid space, where the oculomotor nerve is vulnerable to compression, often by an aneurysm arising from the junction of the internal carotid and posterior communicating arteries.3
Continue to: Incomplete TNP
Incomplete TNP is often microvascular in origin and requires evaluation for diabetes and hypertension. Microvascular TNP is frequently painful but usually self-resolves after 2 to 4 months.2 Giant cell arteritis may also cause an isolated, painful TNP.2
A varied differential diagnosis and a TNP link to COVID-19
The differential diagnosis for TNP includes the following:
Orbital apex injury is usually seen after high-energy craniofacial trauma.4 Orbital apex fractures present with different signs and symptoms, depending on the degree of injury to neural and vascular structures. Various syndromes come into play, the most common being superior orbital fissure syndrome, which is characterized by dysfunction of cranial nerves III, IV, V, and VI.4 Features include ophthalmoplegia, upper eyelid ptosis, a nonreactive dilated pupil, anesthesia over the ipsilateral forehead, loss of corneal reflex, orbital pain, and proptosis.4
In patients with suspected orbital apex fractures, it’s important to assess for the presence of an optic neuropathy, an evolving orbital compartment syndrome, or a ruptured globe, because these 3 things may demand acute intervention.4
Chronic progressive external ophthalmoplegia (CPEO) is a mitochondrial disorder characterized by a slow, progressive paralysis of the extraocular muscles.5 Patients usually experience bilateral, symmetrical, progressive ptosis, followed by ophthalmoparesis months to years later. Ciliary and iris muscles are not involved. CPEO often occurs with other systemic features of mitochondrial dysfunction that can cause significant morbidity and mortality.5
Continue to: Graves ophthalmopathy
Graves ophthalmopathy arises from soft-tissue enlargement in the orbit, leading to increased pressure within the bony cavity.6 Approximately 40% of patients with Graves ophthalmopathy present with restrictive extraocular myopathy; however > 90% have eyelid retraction, as opposed to ptosis.7
Guillain-Barré syndrome (GBS) is an acute, demyelinating immune-mediated polyneuropathy involving the spinal roots, peripheral nerves, and often the cranial nerves.8 The Miller Fisher variant of GBS is characterized by bilateral ophthalmoparesis, areflexia, and ataxia.8 At the early stage of illness, the presentation may be similar to TNP.8 Brain imaging is normal in patients with GBS; the diagnosis is established via characteristic electromyography and cerebrospinal fluid findings.8
Myasthenia gravis often manifests with variable ptosis associated with diplopia.9 Symptoms may be unilateral or bilateral. The ice-pack test has been identified as a simple, preliminary test for ocular myasthenia. The test involves the application of an ice-pack over the lids for 5 minutes. A 50% reduction in at least 1 component of ocular deviation is considered a positive response.10 Its specificity reportedly reaches 100%, with a sensitivity of 80%.10
COVID-19 infection may also include neurologic manifestations. There are an increasing number of case reports of central nervous system abnormalities including TNP.11,12
Trauma, tumors, or an aneurysm could be at work in TNP
TNP associated with trauma usually develops secondary to compression from an expanding hematoma, although it may also be a result of irritation of the nerve from blood in the subarachnoid space.13 Estimates of the incidence of TNP due to trauma range from 12% to 26% of cases.1,14 Vehicle-related injury is the most frequent cause of trauma-related TNP.14
Continue to: Pituitary tumors
Pituitary tumors most commonly involve the oculomotor nerve; 14% to 30% of pituitary tumors lead to TNP.13 Pituitary apoplexy secondary to infarction or hemorrhage is often associated with visual field defects and TNP.13
An underlying aneurysm manifests in a minority (10% to 15%) of patients presenting with TNP.3
Imaging is key to getting at the cause of TNP
The evaluation of patients presenting with acute TNP should be focused first on detecting an aneurysmal compressive lesion.3 CTA is the imaging modality of choice.
Once an aneurysm has been ruled out, the work-up should include a lumbar puncture and an erythrocyte sedimentation rate. Older patients should be assessed for conditions such as hypertension or diabetes that put them at risk for microvascular disease.3 If microvascular TNP is unlikely, MRI with MR angiography is recommended to exclude other potential etiologies of TNP.3 If the patient is younger than 50 years of age, consider potential infectious and inflammatory etiologies (eg, giant cell arteritis).3
Treatment options are varied
The treatment of patients with TNP is specific to the disease state. For those patients with vascular risk factors and a presumptive diagnosis of microvascular TNP, it is reasonable to observe the patient for 2 to 3 months.3 Antiplatelet therapy is usually initiated. Patching 1 eye is useful in alleviating diplopia, particularly in the short term. In most cases, deficits related to TNP resolve over weeks to months. Deficits that persist beyond 6 months may require surgical intervention.
Continue to: "The tip of the iceberg"
TNP: “The tip of the iceberg”
TNP may signal a neurologic emergency, such as an aneurysm, or other conditions such as pituitary disease or giant cell arteritis. Any patient presenting with acute onset of TNP should undergo a noninvasive neuroimaging study.3
Our patient was treated for hypertension; however, she was lost to follow-up.
1. Fang C, Leavitt JA, Hodge DO, et al. Incidence and etiologies of acquired third nerve palsy using a population-based method. JAMA Ophthalmol. 2017;135:23-28. doi: 10.1001/jamaophthalmol.2016.4456
2. Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol. 2007;27:257-268. doi: 10.1055/s-2007-979681
3. Margolin E, Freund P. A review of third nerve palsies. Int Ophthalmol Clin. 2019;59:99-112. doi: 10.1097/IIO.0000000000000279
4. Linnau KF, Hallam DK, Lomoschitz FM, et al. Orbital apex injury: trauma at the junction between the face and the cranium. Eur J Radiol. 2003;48:5-16. doi: 10.1016/s0720-048x(03)00203-1
5. McClelland C, Manousakis G, Lee MS. Progressive external ophthalmoplegia. Curr Neurol Neurosci Rep. 2016;16:53. doi: 10.1007/s11910-016-0652-7
6. Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362:726-738. doi: 10.1056/NEJMra0905750
7. Subetki I, Soewond P, Soebardi S, et al. Practical guidelines management of graves ophthalmopathy. Acta Med Indones. 2019;51:364-371.
8. Wijdicks EF, Klein CJ. Guillain-Barré syndrome. Mayo Clin Proc. 2017;92:467-479. doi: 10.1016/j.mayocp.2016.12.002
9. Beloor Suresh A, Asuncion RMD. Myasthenia Gravis. In: StatPearls [Internet]. StatPearls Publishing; 2021. Accessed April 26, 2021. www.ncbi.nlm.nih.gov/books/NBK559331/
10. Chatzistefanou KI, Kouris T, Iliakis E, et al. The ice pack test in the differential diagnosis of myasthenic diplopia. Ophthalmology. 2009;116:2236-2243. doi: 10.1016/j.ophtha.2009.04.039
11. Pascual-Prieto J, Narváez-Palazón C, Porta-Etessam J, et al. COVID-19 epidemic: should ophthalmologists be aware of oculomotor paresis? Arch Soc Esp Oftalmol. 2020;95:361-362. doi: 10.1016/j.oftal.2020.05.002
12. Collantes MEV, Espiritu AI, Sy MCC, et al. Neurological manifestations in COVID-19 infection: a systematic review and meta-analysis. Can J Neurol Sci. 2021;48:66-76. doi: 10.1017/cjn.2020.146
13. Raza HK, Chen H, Chansysouphanthong T, et al. The aetiologies of the unilateral oculomotor nerve palsy: a review of the literature. Somatosens Mot Res. 2018;35:229-239. doi :10.1080/08990220.2018.1547697
14. Keane J. Third nerve palsy: analysis of 1400 personally-examined inpatients. Can J Neurol Sci. 2010;37:662-670. doi: 10.1017/s0317167100010866
A 45-year-old woman visited the clinic 6 weeks after having a stroke while on her motorcycle, which resulted in a crash. She had not been wearing a helmet and was uncertain if she had sustained a head injury. She said that during the hospital stay following the accident, she was diagnosed as hypertensive; she denied any other significant prior medical history.
Following the crash, she said she’d been experiencing weakness in her right arm and leg and had been unable to open her right eye. When her right eye was opened manually, she said she had double vision and sensitivity to light.
On exam, the patient had exotropia with hypotropia of her right eye. Additionally, she had anisocoria with an enlarged, nonreactive right pupil (FIGURE 1A). She was unable to adduct, supraduct, or infraduct her right eye (FIGURE 1B). Her cranial nerves were otherwise intact. On manual strength testing, she had 4/5 strength of both her right upper and lower extremities.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Third (oculomotor) nerve palsy
This patient had a complete third nerve palsy (TNP). This is defined as palsy involving all of the muscles innervated by the oculomotor nerve, with pupillary involvement.1 The oculomotor nerve supplies motor innervation to the levator palpebrae superioris, superior rectus, medial rectus, inferior rectus, and inferior oblique muscles and parasympathetic innervation to the pupillary constrictor and ciliary muscles.2 As a result, patients present with exotropia and hypotropia on exam with anisocoria. Diplopia, ptosis, and an enlarged pupil are classic symptoms of TNP.2
Computed tomography (CT) of the brain performed immediately after this patient’s accident demonstrated a 15-mm hemorrhage within the left basal ganglia with mild associated edema, and a small focus of hyperattenuation within the right aspect of the suprasellar cistern. There was no evidence of skull fracture. CT angiography (CTA) of the brain showed no evidence of aneurysm.
Several days later, magnetic resonance imaging (MRI) of the brain confirmed prior CT findings and revealed hemorrhagic contusions along the anterior and medial left temporal lobe. Additionally, the MRI showed subtle subdural hemorrhages along the midline falx and right parietal region, as well as diffuse subarachnoid hemorrhage around both hemispheres, the interpeduncular cistern, and the suprasellar cistern (FIGURE 2). The basal ganglia hemorrhage was believed to have been a result of uncontrolled hypertension. The hemorrhage was responsible for her right-sided weakness and was the presumed cause of the accident. The other findings were due to head trauma. Her TNP was most likely caused by both compression and irritation of the right oculomotor nerve.
An uncommon occurrence
A population-based study identified the annual incidence of TNP to be 4 per 100,000.1 The mean age of onset was 42 years. The incidence in patients older than 60 years was greater than the incidence in those younger than 60.2 Isolated TNP occurred in approximately 40% of cases.2
Complete TNP is typically indicative of compression of the ipsilateral third nerve.2 The most common region for third nerve injury is the subarachnoid space, where the oculomotor nerve is vulnerable to compression, often by an aneurysm arising from the junction of the internal carotid and posterior communicating arteries.3
Continue to: Incomplete TNP
Incomplete TNP is often microvascular in origin and requires evaluation for diabetes and hypertension. Microvascular TNP is frequently painful but usually self-resolves after 2 to 4 months.2 Giant cell arteritis may also cause an isolated, painful TNP.2
A varied differential diagnosis and a TNP link to COVID-19
The differential diagnosis for TNP includes the following:
Orbital apex injury is usually seen after high-energy craniofacial trauma.4 Orbital apex fractures present with different signs and symptoms, depending on the degree of injury to neural and vascular structures. Various syndromes come into play, the most common being superior orbital fissure syndrome, which is characterized by dysfunction of cranial nerves III, IV, V, and VI.4 Features include ophthalmoplegia, upper eyelid ptosis, a nonreactive dilated pupil, anesthesia over the ipsilateral forehead, loss of corneal reflex, orbital pain, and proptosis.4
In patients with suspected orbital apex fractures, it’s important to assess for the presence of an optic neuropathy, an evolving orbital compartment syndrome, or a ruptured globe, because these 3 things may demand acute intervention.4
Chronic progressive external ophthalmoplegia (CPEO) is a mitochondrial disorder characterized by a slow, progressive paralysis of the extraocular muscles.5 Patients usually experience bilateral, symmetrical, progressive ptosis, followed by ophthalmoparesis months to years later. Ciliary and iris muscles are not involved. CPEO often occurs with other systemic features of mitochondrial dysfunction that can cause significant morbidity and mortality.5
Continue to: Graves ophthalmopathy
Graves ophthalmopathy arises from soft-tissue enlargement in the orbit, leading to increased pressure within the bony cavity.6 Approximately 40% of patients with Graves ophthalmopathy present with restrictive extraocular myopathy; however > 90% have eyelid retraction, as opposed to ptosis.7
Guillain-Barré syndrome (GBS) is an acute, demyelinating immune-mediated polyneuropathy involving the spinal roots, peripheral nerves, and often the cranial nerves.8 The Miller Fisher variant of GBS is characterized by bilateral ophthalmoparesis, areflexia, and ataxia.8 At the early stage of illness, the presentation may be similar to TNP.8 Brain imaging is normal in patients with GBS; the diagnosis is established via characteristic electromyography and cerebrospinal fluid findings.8
Myasthenia gravis often manifests with variable ptosis associated with diplopia.9 Symptoms may be unilateral or bilateral. The ice-pack test has been identified as a simple, preliminary test for ocular myasthenia. The test involves the application of an ice-pack over the lids for 5 minutes. A 50% reduction in at least 1 component of ocular deviation is considered a positive response.10 Its specificity reportedly reaches 100%, with a sensitivity of 80%.10
COVID-19 infection may also include neurologic manifestations. There are an increasing number of case reports of central nervous system abnormalities including TNP.11,12
Trauma, tumors, or an aneurysm could be at work in TNP
TNP associated with trauma usually develops secondary to compression from an expanding hematoma, although it may also be a result of irritation of the nerve from blood in the subarachnoid space.13 Estimates of the incidence of TNP due to trauma range from 12% to 26% of cases.1,14 Vehicle-related injury is the most frequent cause of trauma-related TNP.14
Continue to: Pituitary tumors
Pituitary tumors most commonly involve the oculomotor nerve; 14% to 30% of pituitary tumors lead to TNP.13 Pituitary apoplexy secondary to infarction or hemorrhage is often associated with visual field defects and TNP.13
An underlying aneurysm manifests in a minority (10% to 15%) of patients presenting with TNP.3
Imaging is key to getting at the cause of TNP
The evaluation of patients presenting with acute TNP should be focused first on detecting an aneurysmal compressive lesion.3 CTA is the imaging modality of choice.
Once an aneurysm has been ruled out, the work-up should include a lumbar puncture and an erythrocyte sedimentation rate. Older patients should be assessed for conditions such as hypertension or diabetes that put them at risk for microvascular disease.3 If microvascular TNP is unlikely, MRI with MR angiography is recommended to exclude other potential etiologies of TNP.3 If the patient is younger than 50 years of age, consider potential infectious and inflammatory etiologies (eg, giant cell arteritis).3
Treatment options are varied
The treatment of patients with TNP is specific to the disease state. For those patients with vascular risk factors and a presumptive diagnosis of microvascular TNP, it is reasonable to observe the patient for 2 to 3 months.3 Antiplatelet therapy is usually initiated. Patching 1 eye is useful in alleviating diplopia, particularly in the short term. In most cases, deficits related to TNP resolve over weeks to months. Deficits that persist beyond 6 months may require surgical intervention.
Continue to: "The tip of the iceberg"
TNP: “The tip of the iceberg”
TNP may signal a neurologic emergency, such as an aneurysm, or other conditions such as pituitary disease or giant cell arteritis. Any patient presenting with acute onset of TNP should undergo a noninvasive neuroimaging study.3
Our patient was treated for hypertension; however, she was lost to follow-up.
A 45-year-old woman visited the clinic 6 weeks after having a stroke while on her motorcycle, which resulted in a crash. She had not been wearing a helmet and was uncertain if she had sustained a head injury. She said that during the hospital stay following the accident, she was diagnosed as hypertensive; she denied any other significant prior medical history.
Following the crash, she said she’d been experiencing weakness in her right arm and leg and had been unable to open her right eye. When her right eye was opened manually, she said she had double vision and sensitivity to light.
On exam, the patient had exotropia with hypotropia of her right eye. Additionally, she had anisocoria with an enlarged, nonreactive right pupil (FIGURE 1A). She was unable to adduct, supraduct, or infraduct her right eye (FIGURE 1B). Her cranial nerves were otherwise intact. On manual strength testing, she had 4/5 strength of both her right upper and lower extremities.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Third (oculomotor) nerve palsy
This patient had a complete third nerve palsy (TNP). This is defined as palsy involving all of the muscles innervated by the oculomotor nerve, with pupillary involvement.1 The oculomotor nerve supplies motor innervation to the levator palpebrae superioris, superior rectus, medial rectus, inferior rectus, and inferior oblique muscles and parasympathetic innervation to the pupillary constrictor and ciliary muscles.2 As a result, patients present with exotropia and hypotropia on exam with anisocoria. Diplopia, ptosis, and an enlarged pupil are classic symptoms of TNP.2
Computed tomography (CT) of the brain performed immediately after this patient’s accident demonstrated a 15-mm hemorrhage within the left basal ganglia with mild associated edema, and a small focus of hyperattenuation within the right aspect of the suprasellar cistern. There was no evidence of skull fracture. CT angiography (CTA) of the brain showed no evidence of aneurysm.
Several days later, magnetic resonance imaging (MRI) of the brain confirmed prior CT findings and revealed hemorrhagic contusions along the anterior and medial left temporal lobe. Additionally, the MRI showed subtle subdural hemorrhages along the midline falx and right parietal region, as well as diffuse subarachnoid hemorrhage around both hemispheres, the interpeduncular cistern, and the suprasellar cistern (FIGURE 2). The basal ganglia hemorrhage was believed to have been a result of uncontrolled hypertension. The hemorrhage was responsible for her right-sided weakness and was the presumed cause of the accident. The other findings were due to head trauma. Her TNP was most likely caused by both compression and irritation of the right oculomotor nerve.
An uncommon occurrence
A population-based study identified the annual incidence of TNP to be 4 per 100,000.1 The mean age of onset was 42 years. The incidence in patients older than 60 years was greater than the incidence in those younger than 60.2 Isolated TNP occurred in approximately 40% of cases.2
Complete TNP is typically indicative of compression of the ipsilateral third nerve.2 The most common region for third nerve injury is the subarachnoid space, where the oculomotor nerve is vulnerable to compression, often by an aneurysm arising from the junction of the internal carotid and posterior communicating arteries.3
Continue to: Incomplete TNP
Incomplete TNP is often microvascular in origin and requires evaluation for diabetes and hypertension. Microvascular TNP is frequently painful but usually self-resolves after 2 to 4 months.2 Giant cell arteritis may also cause an isolated, painful TNP.2
A varied differential diagnosis and a TNP link to COVID-19
The differential diagnosis for TNP includes the following:
Orbital apex injury is usually seen after high-energy craniofacial trauma.4 Orbital apex fractures present with different signs and symptoms, depending on the degree of injury to neural and vascular structures. Various syndromes come into play, the most common being superior orbital fissure syndrome, which is characterized by dysfunction of cranial nerves III, IV, V, and VI.4 Features include ophthalmoplegia, upper eyelid ptosis, a nonreactive dilated pupil, anesthesia over the ipsilateral forehead, loss of corneal reflex, orbital pain, and proptosis.4
In patients with suspected orbital apex fractures, it’s important to assess for the presence of an optic neuropathy, an evolving orbital compartment syndrome, or a ruptured globe, because these 3 things may demand acute intervention.4
Chronic progressive external ophthalmoplegia (CPEO) is a mitochondrial disorder characterized by a slow, progressive paralysis of the extraocular muscles.5 Patients usually experience bilateral, symmetrical, progressive ptosis, followed by ophthalmoparesis months to years later. Ciliary and iris muscles are not involved. CPEO often occurs with other systemic features of mitochondrial dysfunction that can cause significant morbidity and mortality.5
Continue to: Graves ophthalmopathy
Graves ophthalmopathy arises from soft-tissue enlargement in the orbit, leading to increased pressure within the bony cavity.6 Approximately 40% of patients with Graves ophthalmopathy present with restrictive extraocular myopathy; however > 90% have eyelid retraction, as opposed to ptosis.7
Guillain-Barré syndrome (GBS) is an acute, demyelinating immune-mediated polyneuropathy involving the spinal roots, peripheral nerves, and often the cranial nerves.8 The Miller Fisher variant of GBS is characterized by bilateral ophthalmoparesis, areflexia, and ataxia.8 At the early stage of illness, the presentation may be similar to TNP.8 Brain imaging is normal in patients with GBS; the diagnosis is established via characteristic electromyography and cerebrospinal fluid findings.8
Myasthenia gravis often manifests with variable ptosis associated with diplopia.9 Symptoms may be unilateral or bilateral. The ice-pack test has been identified as a simple, preliminary test for ocular myasthenia. The test involves the application of an ice-pack over the lids for 5 minutes. A 50% reduction in at least 1 component of ocular deviation is considered a positive response.10 Its specificity reportedly reaches 100%, with a sensitivity of 80%.10
COVID-19 infection may also include neurologic manifestations. There are an increasing number of case reports of central nervous system abnormalities including TNP.11,12
Trauma, tumors, or an aneurysm could be at work in TNP
TNP associated with trauma usually develops secondary to compression from an expanding hematoma, although it may also be a result of irritation of the nerve from blood in the subarachnoid space.13 Estimates of the incidence of TNP due to trauma range from 12% to 26% of cases.1,14 Vehicle-related injury is the most frequent cause of trauma-related TNP.14
Continue to: Pituitary tumors
Pituitary tumors most commonly involve the oculomotor nerve; 14% to 30% of pituitary tumors lead to TNP.13 Pituitary apoplexy secondary to infarction or hemorrhage is often associated with visual field defects and TNP.13
An underlying aneurysm manifests in a minority (10% to 15%) of patients presenting with TNP.3
Imaging is key to getting at the cause of TNP
The evaluation of patients presenting with acute TNP should be focused first on detecting an aneurysmal compressive lesion.3 CTA is the imaging modality of choice.
Once an aneurysm has been ruled out, the work-up should include a lumbar puncture and an erythrocyte sedimentation rate. Older patients should be assessed for conditions such as hypertension or diabetes that put them at risk for microvascular disease.3 If microvascular TNP is unlikely, MRI with MR angiography is recommended to exclude other potential etiologies of TNP.3 If the patient is younger than 50 years of age, consider potential infectious and inflammatory etiologies (eg, giant cell arteritis).3
Treatment options are varied
The treatment of patients with TNP is specific to the disease state. For those patients with vascular risk factors and a presumptive diagnosis of microvascular TNP, it is reasonable to observe the patient for 2 to 3 months.3 Antiplatelet therapy is usually initiated. Patching 1 eye is useful in alleviating diplopia, particularly in the short term. In most cases, deficits related to TNP resolve over weeks to months. Deficits that persist beyond 6 months may require surgical intervention.
Continue to: "The tip of the iceberg"
TNP: “The tip of the iceberg”
TNP may signal a neurologic emergency, such as an aneurysm, or other conditions such as pituitary disease or giant cell arteritis. Any patient presenting with acute onset of TNP should undergo a noninvasive neuroimaging study.3
Our patient was treated for hypertension; however, she was lost to follow-up.
1. Fang C, Leavitt JA, Hodge DO, et al. Incidence and etiologies of acquired third nerve palsy using a population-based method. JAMA Ophthalmol. 2017;135:23-28. doi: 10.1001/jamaophthalmol.2016.4456
2. Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol. 2007;27:257-268. doi: 10.1055/s-2007-979681
3. Margolin E, Freund P. A review of third nerve palsies. Int Ophthalmol Clin. 2019;59:99-112. doi: 10.1097/IIO.0000000000000279
4. Linnau KF, Hallam DK, Lomoschitz FM, et al. Orbital apex injury: trauma at the junction between the face and the cranium. Eur J Radiol. 2003;48:5-16. doi: 10.1016/s0720-048x(03)00203-1
5. McClelland C, Manousakis G, Lee MS. Progressive external ophthalmoplegia. Curr Neurol Neurosci Rep. 2016;16:53. doi: 10.1007/s11910-016-0652-7
6. Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362:726-738. doi: 10.1056/NEJMra0905750
7. Subetki I, Soewond P, Soebardi S, et al. Practical guidelines management of graves ophthalmopathy. Acta Med Indones. 2019;51:364-371.
8. Wijdicks EF, Klein CJ. Guillain-Barré syndrome. Mayo Clin Proc. 2017;92:467-479. doi: 10.1016/j.mayocp.2016.12.002
9. Beloor Suresh A, Asuncion RMD. Myasthenia Gravis. In: StatPearls [Internet]. StatPearls Publishing; 2021. Accessed April 26, 2021. www.ncbi.nlm.nih.gov/books/NBK559331/
10. Chatzistefanou KI, Kouris T, Iliakis E, et al. The ice pack test in the differential diagnosis of myasthenic diplopia. Ophthalmology. 2009;116:2236-2243. doi: 10.1016/j.ophtha.2009.04.039
11. Pascual-Prieto J, Narváez-Palazón C, Porta-Etessam J, et al. COVID-19 epidemic: should ophthalmologists be aware of oculomotor paresis? Arch Soc Esp Oftalmol. 2020;95:361-362. doi: 10.1016/j.oftal.2020.05.002
12. Collantes MEV, Espiritu AI, Sy MCC, et al. Neurological manifestations in COVID-19 infection: a systematic review and meta-analysis. Can J Neurol Sci. 2021;48:66-76. doi: 10.1017/cjn.2020.146
13. Raza HK, Chen H, Chansysouphanthong T, et al. The aetiologies of the unilateral oculomotor nerve palsy: a review of the literature. Somatosens Mot Res. 2018;35:229-239. doi :10.1080/08990220.2018.1547697
14. Keane J. Third nerve palsy: analysis of 1400 personally-examined inpatients. Can J Neurol Sci. 2010;37:662-670. doi: 10.1017/s0317167100010866
1. Fang C, Leavitt JA, Hodge DO, et al. Incidence and etiologies of acquired third nerve palsy using a population-based method. JAMA Ophthalmol. 2017;135:23-28. doi: 10.1001/jamaophthalmol.2016.4456
2. Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol. 2007;27:257-268. doi: 10.1055/s-2007-979681
3. Margolin E, Freund P. A review of third nerve palsies. Int Ophthalmol Clin. 2019;59:99-112. doi: 10.1097/IIO.0000000000000279
4. Linnau KF, Hallam DK, Lomoschitz FM, et al. Orbital apex injury: trauma at the junction between the face and the cranium. Eur J Radiol. 2003;48:5-16. doi: 10.1016/s0720-048x(03)00203-1
5. McClelland C, Manousakis G, Lee MS. Progressive external ophthalmoplegia. Curr Neurol Neurosci Rep. 2016;16:53. doi: 10.1007/s11910-016-0652-7
6. Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362:726-738. doi: 10.1056/NEJMra0905750
7. Subetki I, Soewond P, Soebardi S, et al. Practical guidelines management of graves ophthalmopathy. Acta Med Indones. 2019;51:364-371.
8. Wijdicks EF, Klein CJ. Guillain-Barré syndrome. Mayo Clin Proc. 2017;92:467-479. doi: 10.1016/j.mayocp.2016.12.002
9. Beloor Suresh A, Asuncion RMD. Myasthenia Gravis. In: StatPearls [Internet]. StatPearls Publishing; 2021. Accessed April 26, 2021. www.ncbi.nlm.nih.gov/books/NBK559331/
10. Chatzistefanou KI, Kouris T, Iliakis E, et al. The ice pack test in the differential diagnosis of myasthenic diplopia. Ophthalmology. 2009;116:2236-2243. doi: 10.1016/j.ophtha.2009.04.039
11. Pascual-Prieto J, Narváez-Palazón C, Porta-Etessam J, et al. COVID-19 epidemic: should ophthalmologists be aware of oculomotor paresis? Arch Soc Esp Oftalmol. 2020;95:361-362. doi: 10.1016/j.oftal.2020.05.002
12. Collantes MEV, Espiritu AI, Sy MCC, et al. Neurological manifestations in COVID-19 infection: a systematic review and meta-analysis. Can J Neurol Sci. 2021;48:66-76. doi: 10.1017/cjn.2020.146
13. Raza HK, Chen H, Chansysouphanthong T, et al. The aetiologies of the unilateral oculomotor nerve palsy: a review of the literature. Somatosens Mot Res. 2018;35:229-239. doi :10.1080/08990220.2018.1547697
14. Keane J. Third nerve palsy: analysis of 1400 personally-examined inpatients. Can J Neurol Sci. 2010;37:662-670. doi: 10.1017/s0317167100010866