User login
The pulmonary manifestations of small-vessel vasculitis are nonspecific and often overlap with other conditions. Consequently, the diagnosis and management of pulmonary vasculitis are complex and require special attention to detail. This article reviews clinical experience with vasculitis as it manifests in the pulmonary setting, with the goal of providing a sound clinical approach to diagnosis and management.
DIAGNOSTIC CONSIDERATIONS
Accurate diagnosis is enhanced with imaging technology, judicious use of bronchoscopy, and awareness of disorders that mimic or masquerade as pulmonary vasculitis. The diagnosis can be approached on the basis of pattern recognition. For example, microscopic polyangiitis (MPA) is characterized solely by alveolar hemorrhage syndrome. However, other diagnostic possibilities must be considered, such as infection, acute respiratory distress syndrome, and complications of medicines. The hallmark manifestation of granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is necrotizing granulomatous inflammations, but the pulmonary manifestations can include nodules, cavitary masses, airway stenosis, and alveolar hemorrhage. Asthma with eosinophilia is the distinguishing feature of eosinophilic GPA (Churg-Strauss syndrome, EGPA), and Goodpasture syndrome involves deposition of complement and immunoglobulins.
The use of imaging
The best imaging tool for suspected pulmonary vasculitides is high-resolution computed tomography (CT). As a general rule, CT for patients with suspected vasculitis should be ordered without contrast medium as contrast is not needed to assess the lung parenchyma. Vasculitis patients often have renal insufficiency, and contrast-free CT will help protect the kidneys. Another option, which will enhance evaluation of the distribution and location of pulmonary disease, is multiplanar reconstructions of images with virtual bronchoscopy or airway reconstruction. Certain findings on imaging will help to differentiate the vasculitides from one another as well as from mimicking diagnoses.
Eosinophilic GPA. Chest images of patients with EGPA appear as patchy, nonsegmental, often peripheral consolidations of ground-glass opacity. These tend to reside in all lobes of the lungs, close to the surface and occasionally accompanied by septal markings.
Microscopic polyangiitis. Although classically a disease of alveolar hemorrhage, MPA often does not manifest with hemoptysis. Approximately one-third of patients with MPA do not cough up blood, even after a large amount of hemorrhage directly into the parenchyma. Patients may present with nonspecific symptoms such as fatigue and shortness of breath. Chest imaging will enhance diagnostic accuracy, particularly when considered in conjunction with laboratory test results. MPA patients usually have low hematocrit levels and may actually have an increased diffusing capacity of the lung for carbon monoxide (Dlco).
Granulomatosis with polyangiitis. This form of vasculitis has characteristic nodules, cavitary lesions, and, in the worst cases, multifocal masses in the lungs. These can be identified with contrast-free CT, with examination for possible airway involvement.
Multiple lung cavity nodules and pronounced airway narrowing are significant diagnostic clues for GPA. Nodules up to 10 cm in diameter tend to be near sub-pleural and peripheral areas. Microbes and fungus may complicate the nodules’ primary presentation. While bronchoscopy may be helpful with imaging, surgical biopsy remains the gold standard to rule out infections.
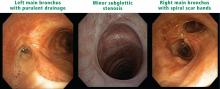
The disease may be multifocal, occurring outside the lungs from the larynx to bronchi and anywhere in the lung. Subglottic stenosis caused by inflammation and scarring affects 16% of patients with GPA, but it also often develops independently of other features of GPA and may have its own course independent of systemic symptoms.1
Bronchoscopy
Bronchoscopy is a relatively low-risk way to assess airways and nodules, but it has had a limited role in the diagnosis of nonfocal interstitial lung disease and rheumatologic lung disease in general. New technologies that augment traditional bronchoscopy and enhance its utility for diagnosis for focal entities are described below.
Electromagnetic navigation bronchoscopy (ENB) uses electromagnetic technology to localize and guide a catheter through the bronchial pathways. With the help of a virtual, 3-dimensional bronchial map reconstructed from a chest CT, the clinician can navigate to a desired location within the lung for biopsy and diagnosis of pulmonary nodules. The result is a diagnostic yield per nodule of nearly 80%.2 Seijo et al showed that diagnostic yields by ENB increase with the presence of the bronchus sign, or a bronchus leading directly to a peripheral lung lesion, as viewed on CT imaging.2 If nodules are bronchocentric, or surround airways, there is greater likelihood of reaching a diagnosis without resorting to surgery.
In peripheral radial ultrasound, a catheter is threaded through another catheter sheath in order to visualize the lesion. This technology can precisely localize lung lesions and often give some clues about the final pathology.
Bronchoscopic confocal fluorescence microscopy3 is a new form of microscopy that uses a fiberoptic miniprobe instead of an objective lens. High-quality images are achieved by the use of autofluorescence. Researchers have used the technology to detect changes in the respiratory bronchioles and other structures, but a clear atlas of many disease states does not yet exist. Oddly, endobronchial GPA images have been catalogued.3
Virtual bronchoscopy is a 3-dimensional image reconstruction and display technique that converts standard CT images into multiplanar images, which can be stacked. Virtual bronchoscopy augments conventional CT because of its ability to enhance detection in the subglottic region and more accurately measure stenosis.4 The technique cannot replace traditional bronchoscopy, however, because mucus and secretions can appear as abnormalities and cause false-positive results.
Detecting mimics
Diagnoses that masquerade as EGPA include chronic eosinophilic pneumonia, bronchiolitis obliterans with organizing pneumonia, and other interstitial lung diseases. Allergic bronchopulmonary aspergillosis—an asthma syndrome sometimes associated with eosinophilia and high immunoglobulin-E levels—also mimics EGPA. This diagnostic possibility is particularly relevant if the patient is taking immunosuppressive agents or corticosteroids.
Although alveolar hemorrhage is the sole pulmonary manifestation of MPA, the diagnosis is not limited to MPA alone. Alveolar hemorrhage may have other causes, including infection or acute respiratory distress syndrome. Bronchial lavage is recommended for accurate diagnosis, with the introduction of successive volumes of saline into the lungs and examination for increasing amounts of heme in each of the aliquots of alveolar lavage fluid.
Several diagnoses can mimic GPA. Many infections, including those caused by mycobacteria and Cryptococcus, can mimic endobronchial GPA. Biopsy of all new ulcers is recommended to minimize the possibility of missing these diagnoses. Tuberculosis in its latent form can closely resemble scarred GPA. Other mimickers of cavitary lung lesions can include metastatic melanoma, metastatic renal and thyroid cancers, squamous cell carcinoma, and rheumatoid arthritis with necrobiotic nodules that open in the lungs.
TREATMENT STRATEGIES
Medications
Although many patients with GPA are surgical candidates because of dyspnea related to fixed endobronchial or endotracheal obstructions, any surgical treatment carries the risk of inciting further flares. Treatment should focus first on mitigating the systemic inflammatory disorder with pharmacologic intervention. Standard pharmacologic therapy includes corticosteroids, azathioprine, cyclophosphamide, and rituximab. Patients with subglottic stenosis are frequently unresponsive to standard immunosuppressive therapy (glucocorticoids in combination with a cytotoxic agent).1
Surgical reconstruction
When medication falls short and surgery is needed to reverse strictures, a number of tools are at our disposal. Some involve heat, such as laser, cauterization, and argon plasma coagulation. In argon plasma coagulation, a jet of ionized argon gas (plasma) is directed through a probe passed through an endoscope. Other techniques rely on cold: cryoprobes, microdebriders, and rigid scissors. In general, freeze therapies cause less scarring than heat therapy. With any surgical technique, there is risk of scars that will contract and cause structural collapse, resulting in restenosis.
Dilation
The high rate of stenosis relapse has spurred interest in alternatives to surgical treatment. One of these, dilation via endoscopy, also may mitigate the wound healing process. Other techniques for clearing the obstructed area include rigid bronchoscopy, the use of bougies (increasingly larger dilators), and balloon dilation. Balloon dilation has some advantages over the other techniques. It permits maximal radial direction and pressure, causes less damage to trachea wall mucosa, and achieves better overall results; however, the procedure usually needs to be repeated.5 It must be done quickly, and it requires flawless communication between the otolaryngologist or pulmonologist and anesthesiologist in order to stabilize the airway below the vocal cords.
Intratracheal dilation-injection therapy
Dilation can be augmented with glucocorticoid injections. In 1991, researchers at the National Institutes of Health utilized a combination dilation-injection therapy for 20 patients who had GPA and subglottic stenosis.1 Patients were first treated with mercury-filled dilators coated with 1% triamcinolone cream. Methylprednisolone acetate was then injected into the stenotic area. None of the patients treated with intratracheal dilation-injection therapy required a tracheostomy and six who already had tracheostomies were decannulated. In contrast, 56% of patients who received standard immunosuppressive therapy and no intratracheal dilation-injection therapy required tracheostomy. Intratracheal dilation-injection therapy is considered a safe and effective treatment of GPA-associated subglottic stenosis and, in the absence of major organ disease activity, could be used without systemic immunosuppressive agents.
Mitomycin-C is a controversial alternative to corticosteroids during dilation. Mitomycin-C is an alkylating agent that inhibits fibroblast proliferation and extracellular matrix protein synthesis, with the potential for reduced scarring. In a recent trial of 26 patients, two doses given 3 to 4 weeks apart reduced the rate of stenosis for 2 to 3 years compared with a single dose.6 Restenosis occurred in both groups, however, and after 5 years, the relapse rates were the same.
Nd:YAG laser photoresection versus endobronchial electrosurgery
One of the most effective therapies for treating obstructive lesions is Nd:YAG laser photoresection (LPR) in which a laser that utilizes the crystal neodymium-doped yttrium aluminum garnet (Nd:Y3Al5O12) is paired with a flexible bronchoscope. The procedure can produce favorable outcomes,7 but it has not gained favor because of perceptions that the lasers require rigid bronchoscopy, expensive equipment, and special training. There are also concerns about complications.
The lower-cost endobronchial electrosurgery (EBES) also failed to gain acceptance because of cumbersome delivery systems and complications associated with power units. Recently, engineers have spawned a new generation of electrosurgical devices, prompting renewed interest in EBES.
A recent study compared LPR and EBES in patients who represented 118 evaluations for LPR.8 Forty percent were considered amenable to EBES and so did not go on to receive the more costly LPR. Of those, 89% achieved success in alleviating the obstruction. The authors concluded that EBES can potentially eliminate the need for LPR in 36% of procedures, and that it could achieve significant savings in cost and time. We use these ablative therapies only in dire circumstances; we use non–heat-based therapies, including repeated dilation, prior to considering use of other therapies.
Cryotherapy
Cryotherapy spray was initially thought to have great therapeutic potential, but the high pressures of the spray caused complications. This modality remains under investigation, however. Some probe-based cryotherapy techniques have been effective anecdotally. These use a metal-tipped probe attached to a cryogen; the Joule-Thompson effect causes delayed tissue destruction.
Stents
A small number of case reports note patient improvement after stenting.9,10 We use stents in rare circumstances, but because complications are frequent and sometimes severe, we consider stenting a last-resort option. In 2005, the US Food and Drug Administration mandated a Black Box warning against the use of metallic stents in patients who have benign tracheal strictures.
Multimodality therapies
In general, when intervention is required to salvage airways, a combination of dilation and steroid injection with or without topical mitomycin-C is standard. We try to avoid use of thermal therapy with laser or electrocautery because of the risk of exuberant inflammation and restenosis from thermal injury. No specific standard of care exists in these cases; reliance on clinical judgment is critical because of the presentation and variety of airway lesions. Further, no large-scale randomized trials exist to guide therapy, so it is best to work with a multidisciplinary team whose members have experience in managing these complex patients.
CONCLUSION
The differential diagnosis of pulmonary manifestations of small-vessel vasculitis is complex. Several diagnoses can mimic various forms of pulmonary vasculitis, and the manifestations and symptoms often overlap with other organ systems.
Imaging is useful for analysis of common patterns of small and midsize vasculitis, although the results may be confounded by disorders that mimic pulmonary vasculitis. To enhance diagnostic accuracy, laboratory and clinical findings should be considered along with images. Ideally, treatment will be minimally destructive and mucosa-sparing. Dilation therapies can be augmented with corticosteroid injections or, possibly, mitomycin-C.
- Langford CA, Sneller MC, Hallahan CW, et al. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Arthritis Rheum 1996; 39:1754–1760.
- Seijo LM, de Torres JP, Lozano MD, et al. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a bronchus sign on CT imaging: results from a prospective study. Chest 2010; 138:1316–1321.
- Filner JJ, Bonura EJ, Lau ST, et al. Bronchoscopic fibered confocal fluorescence microscopy image characteristics and pathologic correlations. J Bronchol Intervent Pulmonol 2011; 18:23–30.
- Summers RM, Aggarwal NR, Sneller MC, et al. CT virtual bronchoscopy of the central airway in patients with Wegener’s granulomatosis. Chest 2002; 121:242–250.
- Schokkenbroek AA, Franssen CFM, Dikkers FG. Dilatation tracheoscopy for laryngeal and tracheal stenosis in patients with Wegener’s granulomatosis. Eur Arch Otorhinolaryngol 2008; 265:549–555.
- Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope 2009; 119:272–283.
- Shvero J, Shitrit D, Koren R, Shalomi D, Kramer MR. Endoscopic laser surgery for subglottic stenosis in Wegener’s granulomatosis. Yonsei Med J 2007; 48:748–753.
- Coulter TD, Mehta AC. The heat is on: impact of endobronchial electrosurgery on the need for Nd-YAG laser photoresection. Chest 2000; 118:516–521.
- Tierman J, Shah C, Elborn JS. Successful stenting in endobronchial Wegener’s granulomatosis. Ulster Med J 2006; 75:155–157.
- Daum TE, Specks U, Colby TV, et al. Tracheobronchial involvement in Wegener’s granulomatosis. Am J Respir Crit Care Med 1995; 151:522–526.
The pulmonary manifestations of small-vessel vasculitis are nonspecific and often overlap with other conditions. Consequently, the diagnosis and management of pulmonary vasculitis are complex and require special attention to detail. This article reviews clinical experience with vasculitis as it manifests in the pulmonary setting, with the goal of providing a sound clinical approach to diagnosis and management.
DIAGNOSTIC CONSIDERATIONS
Accurate diagnosis is enhanced with imaging technology, judicious use of bronchoscopy, and awareness of disorders that mimic or masquerade as pulmonary vasculitis. The diagnosis can be approached on the basis of pattern recognition. For example, microscopic polyangiitis (MPA) is characterized solely by alveolar hemorrhage syndrome. However, other diagnostic possibilities must be considered, such as infection, acute respiratory distress syndrome, and complications of medicines. The hallmark manifestation of granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is necrotizing granulomatous inflammations, but the pulmonary manifestations can include nodules, cavitary masses, airway stenosis, and alveolar hemorrhage. Asthma with eosinophilia is the distinguishing feature of eosinophilic GPA (Churg-Strauss syndrome, EGPA), and Goodpasture syndrome involves deposition of complement and immunoglobulins.
The use of imaging
The best imaging tool for suspected pulmonary vasculitides is high-resolution computed tomography (CT). As a general rule, CT for patients with suspected vasculitis should be ordered without contrast medium as contrast is not needed to assess the lung parenchyma. Vasculitis patients often have renal insufficiency, and contrast-free CT will help protect the kidneys. Another option, which will enhance evaluation of the distribution and location of pulmonary disease, is multiplanar reconstructions of images with virtual bronchoscopy or airway reconstruction. Certain findings on imaging will help to differentiate the vasculitides from one another as well as from mimicking diagnoses.
Eosinophilic GPA. Chest images of patients with EGPA appear as patchy, nonsegmental, often peripheral consolidations of ground-glass opacity. These tend to reside in all lobes of the lungs, close to the surface and occasionally accompanied by septal markings.
Microscopic polyangiitis. Although classically a disease of alveolar hemorrhage, MPA often does not manifest with hemoptysis. Approximately one-third of patients with MPA do not cough up blood, even after a large amount of hemorrhage directly into the parenchyma. Patients may present with nonspecific symptoms such as fatigue and shortness of breath. Chest imaging will enhance diagnostic accuracy, particularly when considered in conjunction with laboratory test results. MPA patients usually have low hematocrit levels and may actually have an increased diffusing capacity of the lung for carbon monoxide (Dlco).
Granulomatosis with polyangiitis. This form of vasculitis has characteristic nodules, cavitary lesions, and, in the worst cases, multifocal masses in the lungs. These can be identified with contrast-free CT, with examination for possible airway involvement.
Multiple lung cavity nodules and pronounced airway narrowing are significant diagnostic clues for GPA. Nodules up to 10 cm in diameter tend to be near sub-pleural and peripheral areas. Microbes and fungus may complicate the nodules’ primary presentation. While bronchoscopy may be helpful with imaging, surgical biopsy remains the gold standard to rule out infections.
The disease may be multifocal, occurring outside the lungs from the larynx to bronchi and anywhere in the lung. Subglottic stenosis caused by inflammation and scarring affects 16% of patients with GPA, but it also often develops independently of other features of GPA and may have its own course independent of systemic symptoms.1
Bronchoscopy
Bronchoscopy is a relatively low-risk way to assess airways and nodules, but it has had a limited role in the diagnosis of nonfocal interstitial lung disease and rheumatologic lung disease in general. New technologies that augment traditional bronchoscopy and enhance its utility for diagnosis for focal entities are described below.
Electromagnetic navigation bronchoscopy (ENB) uses electromagnetic technology to localize and guide a catheter through the bronchial pathways. With the help of a virtual, 3-dimensional bronchial map reconstructed from a chest CT, the clinician can navigate to a desired location within the lung for biopsy and diagnosis of pulmonary nodules. The result is a diagnostic yield per nodule of nearly 80%.2 Seijo et al showed that diagnostic yields by ENB increase with the presence of the bronchus sign, or a bronchus leading directly to a peripheral lung lesion, as viewed on CT imaging.2 If nodules are bronchocentric, or surround airways, there is greater likelihood of reaching a diagnosis without resorting to surgery.
In peripheral radial ultrasound, a catheter is threaded through another catheter sheath in order to visualize the lesion. This technology can precisely localize lung lesions and often give some clues about the final pathology.
Bronchoscopic confocal fluorescence microscopy3 is a new form of microscopy that uses a fiberoptic miniprobe instead of an objective lens. High-quality images are achieved by the use of autofluorescence. Researchers have used the technology to detect changes in the respiratory bronchioles and other structures, but a clear atlas of many disease states does not yet exist. Oddly, endobronchial GPA images have been catalogued.3
Virtual bronchoscopy is a 3-dimensional image reconstruction and display technique that converts standard CT images into multiplanar images, which can be stacked. Virtual bronchoscopy augments conventional CT because of its ability to enhance detection in the subglottic region and more accurately measure stenosis.4 The technique cannot replace traditional bronchoscopy, however, because mucus and secretions can appear as abnormalities and cause false-positive results.
Detecting mimics
Diagnoses that masquerade as EGPA include chronic eosinophilic pneumonia, bronchiolitis obliterans with organizing pneumonia, and other interstitial lung diseases. Allergic bronchopulmonary aspergillosis—an asthma syndrome sometimes associated with eosinophilia and high immunoglobulin-E levels—also mimics EGPA. This diagnostic possibility is particularly relevant if the patient is taking immunosuppressive agents or corticosteroids.
Although alveolar hemorrhage is the sole pulmonary manifestation of MPA, the diagnosis is not limited to MPA alone. Alveolar hemorrhage may have other causes, including infection or acute respiratory distress syndrome. Bronchial lavage is recommended for accurate diagnosis, with the introduction of successive volumes of saline into the lungs and examination for increasing amounts of heme in each of the aliquots of alveolar lavage fluid.
Several diagnoses can mimic GPA. Many infections, including those caused by mycobacteria and Cryptococcus, can mimic endobronchial GPA. Biopsy of all new ulcers is recommended to minimize the possibility of missing these diagnoses. Tuberculosis in its latent form can closely resemble scarred GPA. Other mimickers of cavitary lung lesions can include metastatic melanoma, metastatic renal and thyroid cancers, squamous cell carcinoma, and rheumatoid arthritis with necrobiotic nodules that open in the lungs.
TREATMENT STRATEGIES
Medications
Although many patients with GPA are surgical candidates because of dyspnea related to fixed endobronchial or endotracheal obstructions, any surgical treatment carries the risk of inciting further flares. Treatment should focus first on mitigating the systemic inflammatory disorder with pharmacologic intervention. Standard pharmacologic therapy includes corticosteroids, azathioprine, cyclophosphamide, and rituximab. Patients with subglottic stenosis are frequently unresponsive to standard immunosuppressive therapy (glucocorticoids in combination with a cytotoxic agent).1
Surgical reconstruction
When medication falls short and surgery is needed to reverse strictures, a number of tools are at our disposal. Some involve heat, such as laser, cauterization, and argon plasma coagulation. In argon plasma coagulation, a jet of ionized argon gas (plasma) is directed through a probe passed through an endoscope. Other techniques rely on cold: cryoprobes, microdebriders, and rigid scissors. In general, freeze therapies cause less scarring than heat therapy. With any surgical technique, there is risk of scars that will contract and cause structural collapse, resulting in restenosis.
Dilation
The high rate of stenosis relapse has spurred interest in alternatives to surgical treatment. One of these, dilation via endoscopy, also may mitigate the wound healing process. Other techniques for clearing the obstructed area include rigid bronchoscopy, the use of bougies (increasingly larger dilators), and balloon dilation. Balloon dilation has some advantages over the other techniques. It permits maximal radial direction and pressure, causes less damage to trachea wall mucosa, and achieves better overall results; however, the procedure usually needs to be repeated.5 It must be done quickly, and it requires flawless communication between the otolaryngologist or pulmonologist and anesthesiologist in order to stabilize the airway below the vocal cords.
Intratracheal dilation-injection therapy
Dilation can be augmented with glucocorticoid injections. In 1991, researchers at the National Institutes of Health utilized a combination dilation-injection therapy for 20 patients who had GPA and subglottic stenosis.1 Patients were first treated with mercury-filled dilators coated with 1% triamcinolone cream. Methylprednisolone acetate was then injected into the stenotic area. None of the patients treated with intratracheal dilation-injection therapy required a tracheostomy and six who already had tracheostomies were decannulated. In contrast, 56% of patients who received standard immunosuppressive therapy and no intratracheal dilation-injection therapy required tracheostomy. Intratracheal dilation-injection therapy is considered a safe and effective treatment of GPA-associated subglottic stenosis and, in the absence of major organ disease activity, could be used without systemic immunosuppressive agents.
Mitomycin-C is a controversial alternative to corticosteroids during dilation. Mitomycin-C is an alkylating agent that inhibits fibroblast proliferation and extracellular matrix protein synthesis, with the potential for reduced scarring. In a recent trial of 26 patients, two doses given 3 to 4 weeks apart reduced the rate of stenosis for 2 to 3 years compared with a single dose.6 Restenosis occurred in both groups, however, and after 5 years, the relapse rates were the same.
Nd:YAG laser photoresection versus endobronchial electrosurgery
One of the most effective therapies for treating obstructive lesions is Nd:YAG laser photoresection (LPR) in which a laser that utilizes the crystal neodymium-doped yttrium aluminum garnet (Nd:Y3Al5O12) is paired with a flexible bronchoscope. The procedure can produce favorable outcomes,7 but it has not gained favor because of perceptions that the lasers require rigid bronchoscopy, expensive equipment, and special training. There are also concerns about complications.
The lower-cost endobronchial electrosurgery (EBES) also failed to gain acceptance because of cumbersome delivery systems and complications associated with power units. Recently, engineers have spawned a new generation of electrosurgical devices, prompting renewed interest in EBES.
A recent study compared LPR and EBES in patients who represented 118 evaluations for LPR.8 Forty percent were considered amenable to EBES and so did not go on to receive the more costly LPR. Of those, 89% achieved success in alleviating the obstruction. The authors concluded that EBES can potentially eliminate the need for LPR in 36% of procedures, and that it could achieve significant savings in cost and time. We use these ablative therapies only in dire circumstances; we use non–heat-based therapies, including repeated dilation, prior to considering use of other therapies.
Cryotherapy
Cryotherapy spray was initially thought to have great therapeutic potential, but the high pressures of the spray caused complications. This modality remains under investigation, however. Some probe-based cryotherapy techniques have been effective anecdotally. These use a metal-tipped probe attached to a cryogen; the Joule-Thompson effect causes delayed tissue destruction.
Stents
A small number of case reports note patient improvement after stenting.9,10 We use stents in rare circumstances, but because complications are frequent and sometimes severe, we consider stenting a last-resort option. In 2005, the US Food and Drug Administration mandated a Black Box warning against the use of metallic stents in patients who have benign tracheal strictures.
Multimodality therapies
In general, when intervention is required to salvage airways, a combination of dilation and steroid injection with or without topical mitomycin-C is standard. We try to avoid use of thermal therapy with laser or electrocautery because of the risk of exuberant inflammation and restenosis from thermal injury. No specific standard of care exists in these cases; reliance on clinical judgment is critical because of the presentation and variety of airway lesions. Further, no large-scale randomized trials exist to guide therapy, so it is best to work with a multidisciplinary team whose members have experience in managing these complex patients.
CONCLUSION
The differential diagnosis of pulmonary manifestations of small-vessel vasculitis is complex. Several diagnoses can mimic various forms of pulmonary vasculitis, and the manifestations and symptoms often overlap with other organ systems.
Imaging is useful for analysis of common patterns of small and midsize vasculitis, although the results may be confounded by disorders that mimic pulmonary vasculitis. To enhance diagnostic accuracy, laboratory and clinical findings should be considered along with images. Ideally, treatment will be minimally destructive and mucosa-sparing. Dilation therapies can be augmented with corticosteroid injections or, possibly, mitomycin-C.
The pulmonary manifestations of small-vessel vasculitis are nonspecific and often overlap with other conditions. Consequently, the diagnosis and management of pulmonary vasculitis are complex and require special attention to detail. This article reviews clinical experience with vasculitis as it manifests in the pulmonary setting, with the goal of providing a sound clinical approach to diagnosis and management.
DIAGNOSTIC CONSIDERATIONS
Accurate diagnosis is enhanced with imaging technology, judicious use of bronchoscopy, and awareness of disorders that mimic or masquerade as pulmonary vasculitis. The diagnosis can be approached on the basis of pattern recognition. For example, microscopic polyangiitis (MPA) is characterized solely by alveolar hemorrhage syndrome. However, other diagnostic possibilities must be considered, such as infection, acute respiratory distress syndrome, and complications of medicines. The hallmark manifestation of granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is necrotizing granulomatous inflammations, but the pulmonary manifestations can include nodules, cavitary masses, airway stenosis, and alveolar hemorrhage. Asthma with eosinophilia is the distinguishing feature of eosinophilic GPA (Churg-Strauss syndrome, EGPA), and Goodpasture syndrome involves deposition of complement and immunoglobulins.
The use of imaging
The best imaging tool for suspected pulmonary vasculitides is high-resolution computed tomography (CT). As a general rule, CT for patients with suspected vasculitis should be ordered without contrast medium as contrast is not needed to assess the lung parenchyma. Vasculitis patients often have renal insufficiency, and contrast-free CT will help protect the kidneys. Another option, which will enhance evaluation of the distribution and location of pulmonary disease, is multiplanar reconstructions of images with virtual bronchoscopy or airway reconstruction. Certain findings on imaging will help to differentiate the vasculitides from one another as well as from mimicking diagnoses.
Eosinophilic GPA. Chest images of patients with EGPA appear as patchy, nonsegmental, often peripheral consolidations of ground-glass opacity. These tend to reside in all lobes of the lungs, close to the surface and occasionally accompanied by septal markings.
Microscopic polyangiitis. Although classically a disease of alveolar hemorrhage, MPA often does not manifest with hemoptysis. Approximately one-third of patients with MPA do not cough up blood, even after a large amount of hemorrhage directly into the parenchyma. Patients may present with nonspecific symptoms such as fatigue and shortness of breath. Chest imaging will enhance diagnostic accuracy, particularly when considered in conjunction with laboratory test results. MPA patients usually have low hematocrit levels and may actually have an increased diffusing capacity of the lung for carbon monoxide (Dlco).
Granulomatosis with polyangiitis. This form of vasculitis has characteristic nodules, cavitary lesions, and, in the worst cases, multifocal masses in the lungs. These can be identified with contrast-free CT, with examination for possible airway involvement.
Multiple lung cavity nodules and pronounced airway narrowing are significant diagnostic clues for GPA. Nodules up to 10 cm in diameter tend to be near sub-pleural and peripheral areas. Microbes and fungus may complicate the nodules’ primary presentation. While bronchoscopy may be helpful with imaging, surgical biopsy remains the gold standard to rule out infections.
The disease may be multifocal, occurring outside the lungs from the larynx to bronchi and anywhere in the lung. Subglottic stenosis caused by inflammation and scarring affects 16% of patients with GPA, but it also often develops independently of other features of GPA and may have its own course independent of systemic symptoms.1
Bronchoscopy
Bronchoscopy is a relatively low-risk way to assess airways and nodules, but it has had a limited role in the diagnosis of nonfocal interstitial lung disease and rheumatologic lung disease in general. New technologies that augment traditional bronchoscopy and enhance its utility for diagnosis for focal entities are described below.
Electromagnetic navigation bronchoscopy (ENB) uses electromagnetic technology to localize and guide a catheter through the bronchial pathways. With the help of a virtual, 3-dimensional bronchial map reconstructed from a chest CT, the clinician can navigate to a desired location within the lung for biopsy and diagnosis of pulmonary nodules. The result is a diagnostic yield per nodule of nearly 80%.2 Seijo et al showed that diagnostic yields by ENB increase with the presence of the bronchus sign, or a bronchus leading directly to a peripheral lung lesion, as viewed on CT imaging.2 If nodules are bronchocentric, or surround airways, there is greater likelihood of reaching a diagnosis without resorting to surgery.
In peripheral radial ultrasound, a catheter is threaded through another catheter sheath in order to visualize the lesion. This technology can precisely localize lung lesions and often give some clues about the final pathology.
Bronchoscopic confocal fluorescence microscopy3 is a new form of microscopy that uses a fiberoptic miniprobe instead of an objective lens. High-quality images are achieved by the use of autofluorescence. Researchers have used the technology to detect changes in the respiratory bronchioles and other structures, but a clear atlas of many disease states does not yet exist. Oddly, endobronchial GPA images have been catalogued.3
Virtual bronchoscopy is a 3-dimensional image reconstruction and display technique that converts standard CT images into multiplanar images, which can be stacked. Virtual bronchoscopy augments conventional CT because of its ability to enhance detection in the subglottic region and more accurately measure stenosis.4 The technique cannot replace traditional bronchoscopy, however, because mucus and secretions can appear as abnormalities and cause false-positive results.
Detecting mimics
Diagnoses that masquerade as EGPA include chronic eosinophilic pneumonia, bronchiolitis obliterans with organizing pneumonia, and other interstitial lung diseases. Allergic bronchopulmonary aspergillosis—an asthma syndrome sometimes associated with eosinophilia and high immunoglobulin-E levels—also mimics EGPA. This diagnostic possibility is particularly relevant if the patient is taking immunosuppressive agents or corticosteroids.
Although alveolar hemorrhage is the sole pulmonary manifestation of MPA, the diagnosis is not limited to MPA alone. Alveolar hemorrhage may have other causes, including infection or acute respiratory distress syndrome. Bronchial lavage is recommended for accurate diagnosis, with the introduction of successive volumes of saline into the lungs and examination for increasing amounts of heme in each of the aliquots of alveolar lavage fluid.
Several diagnoses can mimic GPA. Many infections, including those caused by mycobacteria and Cryptococcus, can mimic endobronchial GPA. Biopsy of all new ulcers is recommended to minimize the possibility of missing these diagnoses. Tuberculosis in its latent form can closely resemble scarred GPA. Other mimickers of cavitary lung lesions can include metastatic melanoma, metastatic renal and thyroid cancers, squamous cell carcinoma, and rheumatoid arthritis with necrobiotic nodules that open in the lungs.
TREATMENT STRATEGIES
Medications
Although many patients with GPA are surgical candidates because of dyspnea related to fixed endobronchial or endotracheal obstructions, any surgical treatment carries the risk of inciting further flares. Treatment should focus first on mitigating the systemic inflammatory disorder with pharmacologic intervention. Standard pharmacologic therapy includes corticosteroids, azathioprine, cyclophosphamide, and rituximab. Patients with subglottic stenosis are frequently unresponsive to standard immunosuppressive therapy (glucocorticoids in combination with a cytotoxic agent).1
Surgical reconstruction
When medication falls short and surgery is needed to reverse strictures, a number of tools are at our disposal. Some involve heat, such as laser, cauterization, and argon plasma coagulation. In argon plasma coagulation, a jet of ionized argon gas (plasma) is directed through a probe passed through an endoscope. Other techniques rely on cold: cryoprobes, microdebriders, and rigid scissors. In general, freeze therapies cause less scarring than heat therapy. With any surgical technique, there is risk of scars that will contract and cause structural collapse, resulting in restenosis.
Dilation
The high rate of stenosis relapse has spurred interest in alternatives to surgical treatment. One of these, dilation via endoscopy, also may mitigate the wound healing process. Other techniques for clearing the obstructed area include rigid bronchoscopy, the use of bougies (increasingly larger dilators), and balloon dilation. Balloon dilation has some advantages over the other techniques. It permits maximal radial direction and pressure, causes less damage to trachea wall mucosa, and achieves better overall results; however, the procedure usually needs to be repeated.5 It must be done quickly, and it requires flawless communication between the otolaryngologist or pulmonologist and anesthesiologist in order to stabilize the airway below the vocal cords.
Intratracheal dilation-injection therapy
Dilation can be augmented with glucocorticoid injections. In 1991, researchers at the National Institutes of Health utilized a combination dilation-injection therapy for 20 patients who had GPA and subglottic stenosis.1 Patients were first treated with mercury-filled dilators coated with 1% triamcinolone cream. Methylprednisolone acetate was then injected into the stenotic area. None of the patients treated with intratracheal dilation-injection therapy required a tracheostomy and six who already had tracheostomies were decannulated. In contrast, 56% of patients who received standard immunosuppressive therapy and no intratracheal dilation-injection therapy required tracheostomy. Intratracheal dilation-injection therapy is considered a safe and effective treatment of GPA-associated subglottic stenosis and, in the absence of major organ disease activity, could be used without systemic immunosuppressive agents.
Mitomycin-C is a controversial alternative to corticosteroids during dilation. Mitomycin-C is an alkylating agent that inhibits fibroblast proliferation and extracellular matrix protein synthesis, with the potential for reduced scarring. In a recent trial of 26 patients, two doses given 3 to 4 weeks apart reduced the rate of stenosis for 2 to 3 years compared with a single dose.6 Restenosis occurred in both groups, however, and after 5 years, the relapse rates were the same.
Nd:YAG laser photoresection versus endobronchial electrosurgery
One of the most effective therapies for treating obstructive lesions is Nd:YAG laser photoresection (LPR) in which a laser that utilizes the crystal neodymium-doped yttrium aluminum garnet (Nd:Y3Al5O12) is paired with a flexible bronchoscope. The procedure can produce favorable outcomes,7 but it has not gained favor because of perceptions that the lasers require rigid bronchoscopy, expensive equipment, and special training. There are also concerns about complications.
The lower-cost endobronchial electrosurgery (EBES) also failed to gain acceptance because of cumbersome delivery systems and complications associated with power units. Recently, engineers have spawned a new generation of electrosurgical devices, prompting renewed interest in EBES.
A recent study compared LPR and EBES in patients who represented 118 evaluations for LPR.8 Forty percent were considered amenable to EBES and so did not go on to receive the more costly LPR. Of those, 89% achieved success in alleviating the obstruction. The authors concluded that EBES can potentially eliminate the need for LPR in 36% of procedures, and that it could achieve significant savings in cost and time. We use these ablative therapies only in dire circumstances; we use non–heat-based therapies, including repeated dilation, prior to considering use of other therapies.
Cryotherapy
Cryotherapy spray was initially thought to have great therapeutic potential, but the high pressures of the spray caused complications. This modality remains under investigation, however. Some probe-based cryotherapy techniques have been effective anecdotally. These use a metal-tipped probe attached to a cryogen; the Joule-Thompson effect causes delayed tissue destruction.
Stents
A small number of case reports note patient improvement after stenting.9,10 We use stents in rare circumstances, but because complications are frequent and sometimes severe, we consider stenting a last-resort option. In 2005, the US Food and Drug Administration mandated a Black Box warning against the use of metallic stents in patients who have benign tracheal strictures.
Multimodality therapies
In general, when intervention is required to salvage airways, a combination of dilation and steroid injection with or without topical mitomycin-C is standard. We try to avoid use of thermal therapy with laser or electrocautery because of the risk of exuberant inflammation and restenosis from thermal injury. No specific standard of care exists in these cases; reliance on clinical judgment is critical because of the presentation and variety of airway lesions. Further, no large-scale randomized trials exist to guide therapy, so it is best to work with a multidisciplinary team whose members have experience in managing these complex patients.
CONCLUSION
The differential diagnosis of pulmonary manifestations of small-vessel vasculitis is complex. Several diagnoses can mimic various forms of pulmonary vasculitis, and the manifestations and symptoms often overlap with other organ systems.
Imaging is useful for analysis of common patterns of small and midsize vasculitis, although the results may be confounded by disorders that mimic pulmonary vasculitis. To enhance diagnostic accuracy, laboratory and clinical findings should be considered along with images. Ideally, treatment will be minimally destructive and mucosa-sparing. Dilation therapies can be augmented with corticosteroid injections or, possibly, mitomycin-C.
- Langford CA, Sneller MC, Hallahan CW, et al. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Arthritis Rheum 1996; 39:1754–1760.
- Seijo LM, de Torres JP, Lozano MD, et al. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a bronchus sign on CT imaging: results from a prospective study. Chest 2010; 138:1316–1321.
- Filner JJ, Bonura EJ, Lau ST, et al. Bronchoscopic fibered confocal fluorescence microscopy image characteristics and pathologic correlations. J Bronchol Intervent Pulmonol 2011; 18:23–30.
- Summers RM, Aggarwal NR, Sneller MC, et al. CT virtual bronchoscopy of the central airway in patients with Wegener’s granulomatosis. Chest 2002; 121:242–250.
- Schokkenbroek AA, Franssen CFM, Dikkers FG. Dilatation tracheoscopy for laryngeal and tracheal stenosis in patients with Wegener’s granulomatosis. Eur Arch Otorhinolaryngol 2008; 265:549–555.
- Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope 2009; 119:272–283.
- Shvero J, Shitrit D, Koren R, Shalomi D, Kramer MR. Endoscopic laser surgery for subglottic stenosis in Wegener’s granulomatosis. Yonsei Med J 2007; 48:748–753.
- Coulter TD, Mehta AC. The heat is on: impact of endobronchial electrosurgery on the need for Nd-YAG laser photoresection. Chest 2000; 118:516–521.
- Tierman J, Shah C, Elborn JS. Successful stenting in endobronchial Wegener’s granulomatosis. Ulster Med J 2006; 75:155–157.
- Daum TE, Specks U, Colby TV, et al. Tracheobronchial involvement in Wegener’s granulomatosis. Am J Respir Crit Care Med 1995; 151:522–526.
- Langford CA, Sneller MC, Hallahan CW, et al. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Arthritis Rheum 1996; 39:1754–1760.
- Seijo LM, de Torres JP, Lozano MD, et al. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a bronchus sign on CT imaging: results from a prospective study. Chest 2010; 138:1316–1321.
- Filner JJ, Bonura EJ, Lau ST, et al. Bronchoscopic fibered confocal fluorescence microscopy image characteristics and pathologic correlations. J Bronchol Intervent Pulmonol 2011; 18:23–30.
- Summers RM, Aggarwal NR, Sneller MC, et al. CT virtual bronchoscopy of the central airway in patients with Wegener’s granulomatosis. Chest 2002; 121:242–250.
- Schokkenbroek AA, Franssen CFM, Dikkers FG. Dilatation tracheoscopy for laryngeal and tracheal stenosis in patients with Wegener’s granulomatosis. Eur Arch Otorhinolaryngol 2008; 265:549–555.
- Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope 2009; 119:272–283.
- Shvero J, Shitrit D, Koren R, Shalomi D, Kramer MR. Endoscopic laser surgery for subglottic stenosis in Wegener’s granulomatosis. Yonsei Med J 2007; 48:748–753.
- Coulter TD, Mehta AC. The heat is on: impact of endobronchial electrosurgery on the need for Nd-YAG laser photoresection. Chest 2000; 118:516–521.
- Tierman J, Shah C, Elborn JS. Successful stenting in endobronchial Wegener’s granulomatosis. Ulster Med J 2006; 75:155–157.
- Daum TE, Specks U, Colby TV, et al. Tracheobronchial involvement in Wegener’s granulomatosis. Am J Respir Crit Care Med 1995; 151:522–526.