User login
When should an indwelling pleural catheter be considered for malignant pleural effusion?
An indwelling pleural catheter should be considered when a malignant pleural effusion causes symptoms and recurs after thoracentesis, especially in patients with short to intermediate life expectancy or trapped lung, or who underwent unsuccessful pleurodesis.1
MALIGNANT PLEURAL EFFUSION
Malignant pleural effusion affects about 150,000 people in the United States each year. It occurs in 15% of patients with advanced malignancies, most often lung cancer, breast cancer, lymphoma, and ovarian cancer, which account for more than 50% of cases.2
In most patients with malignant pleural effusion, disabling dyspnea causes poor quality of life. The prognosis is unfavorable, with life expectancy of 3 to 12 months. Patients with poor performance status and lower glucose concentrations in the pleural fluid face a worse prognosis and a shorter life expectancy.2
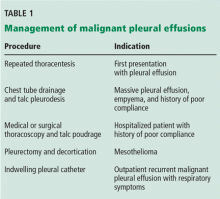
In general, management focuses on relieving symptoms rather than on cure. Symptoms can be controlled by thoracentesis, but if the effusion recurs, the patient needs repeated visits to the emergency room or clinic or a hospital admission to drain the fluid. Frequent hospital visits can be grueling for a patient with a poor functional status, and so can the adverse effects of repeated thoracentesis. For that reason, an early palliative approach to malignant pleural effusion in patients with cancer and a poor prognosis leads to better symptom control and a better quality of life.3 Multiple treatments can be offered to control the symptoms in patients with recurrent malignant pleural effusion (Table 1).
PLEURODESIS HAS BEEN THE TREATMENT OF CHOICE
Pleurodesis has been the treatment of choice for malignant pleural effusion for decades. In this procedure, adhesion of the visceral and parietal pleura is achxieved by inducing inflammation either mechanically or chemically between the pleural surfaces. Injection of a sclerosant into the pleural space generates the inflammation. The sclerosant can be introduced through a chest tube or thoracoscope such as in video-assisted thoracic surgery or medical pleuroscopy. The use of talc is associated with a higher success rate than other sclerosing agents such as bleomycin and doxycycline.4
The downside of this procedure is that pleural effusion recurs in 10% to 40% of cases, and patients require 2 to 4 days in the hospital. Also, the use of talc can lead to acute lung injury–acute respiratory distress syndrome, a rare but potentially life-threatening complication. The incidence of this complication may be related to particle size, with small particles posing a higher risk than large ones.5,6
PLACEMENT OF AN INDWELLING PLEURAL CATHETER
Indwelling pleural catheters are currently used as palliative therapy for patients with recurrent malignant pleural effusion who suffer from respiratory distress due to rapid reaccumulation of pleural fluids that require multiple thoracentesis procedures.
An indwelling pleural catheter is contraindicated in patients with uncontrolled coagulopathy, multiloculated pleural effusions, or extensive malignancy in the skin.3 Other factors that need to be considered are the patient’s social circumstances: ie, the patient must be in a clean and safe environment and must have insurance coverage for the supplies.
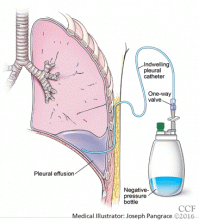
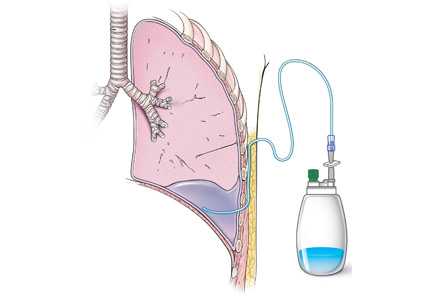
Catheters are 66 cm long and 15.5F and are made of silicone rubber with fenestrations along the distal 24 cm. They have a one-way valve at the proximal end that allows fluids and air to go out but not in (Figure 1).1 Several systems are commercially available in the United States.
The catheter is inserted and tunneled percutaneously with the patient under local anesthesia and conscious sedation (Figure 2). Insertion is a same-day outpatient procedure, and intermittent pleural fluid drainage can be done at home by a home heathcare provider or a trained family member.7
In a meta-analysis, insertion difficulties were reported in only 4% of cases, particularly in patients who underwent prior pleural interventions. Spontaneous pleurodesis occurred in 45% of patients at a mean of 52 days after insertion.8
After catheter insertion, the pleural space should be drained three times a week. No more than 1,000 mL of fluid should be removed at a time—or less if drainage causes chest pain or cough secondary to trapped lung (see below). When the drainage declines to 150 mL per session, the sessions can be reduced to twice a week. If the volume drops to less than 50 mL per session, imaging (computed tomography or bedside thoracic ultrasonography) is recommended to ensure the achievement of pleurodesis and to rule out catheter blockage.
A large multicenter randomized controlled trial9 compared indwelling pleural catheter therapy and chest tube insertion with talc pleurodesis. Both procedures relieved symptoms for the first 42 days, and there was no significant difference in quality of life. However, the median length of hospital stay was 4 days for the talc pleurodesis group compared with 0 days for the indwelling pleural catheter group. Twenty-two percent of the talc group required a further pleural procedure such as a video-assisted thoracic surgery or thoracoscopy, compared with 6% of the indwelling catheter group. On the other hand, 36% of those in the indwelling catheter group experienced nonserious adverse events such as pleural infections that mandated outpatient oral antibiotic therapy, cellulitis, and catheter blockage, compared with 7% of the talc group.9
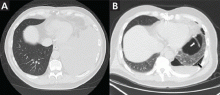
Symptomatic, inoperable trapped lung is another condition for which an indwelling pleural catheter is a reasonable strategy compared with pleurodesis. Trapped lung is a condition in which the lung fails to fully expand despite proper pleural fluid removal, creating a vacuum space between the parietal and visceral pleura (Figure 3).
Patients with trapped lung complain of severe dull or sharp pain during drainage of pleural fluids due to stretching of the visceral pleura against the intrathoracic vacuum space. Trapped lung can be detected objectively by using intrathoracic manometry while draining fluids, looking for more than a 20-cm H2O drop in the intrathoracic pressure. Radiographically, this may be identified as a pneumothorax ex vacuo10 (ie, caused by inability of the lung to expand to fill the thoracic cavity after pleural fluid has been drained) and is not a procedure complication.
Placement of an indwelling pleural catheter is the treatment of choice for trapped lung, since chemical pleurodesis is not feasible without the potential of parietal and visceral pleural apposition. In a retrospective study of indwelling catheter placement for palliative symptom control, a catheter relieved symptoms, improved quality of life, and afforded a substantial increase in mobility.1,11
In another multicenter pilot study,12 rapid pleurodesis was achieved in 30 patients with recurrent malignant pleural effusion by combining chemical pleurodesis and indwelling catheter placement. Both were done under direct vision with medical thoracoscopy. Pleurodesis succeeded in 92% of patients by day 8 after the procedure. The hospital stay was reduced to a mean of 2 days after the procedure. In the catheter group, fluids were drained three times in the first day after the procedure and twice a day on the second and third days. Of the 30 patients in this study, 2 had fever, 1 needed to have the catheter replaced, and 1 contracted empyema.
AN EFFECTIVE INITIAL TREATMENT
Placement of an indwelling pleural catheter is an effective initial treatment for recurrent malignant pleural effusion. Compared with chemical pleurodesis, it has a comparable success rate and complication rate. It offers the advantages of being a same-day surgical procedure entailing a shorter hospital stay and less need for further pleural intervention. This treatment should be considered for patients with symptomatic malignant pleural effusion, especially those in whom symptomatic malignant pleural effusion recurred after thoracentesis.8
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40.
- Thomas JM, Musani AI. Malignant pleural effusions: a review. Clin Chest Med 2013; 34:459–471.
- Thomas R, Francis R, Davies HE, Lee YC. Interventional therapies for malignant pleural effusions: the present and the future. Respirology 2014; 19:809–822.
- Rodriguez-Panadero F, Montes-Worboys A. Mechanisms of pleurodesis. Respiration 2012; 83:91–98.
- Gonzalez AV, Bezwada V, Beamis JF Jr, Villanueva AG. Lung injury following thoracoscopic talc insufflation: experience of a single North American center. Chest 2010; 137:1375–1381.
- Rossi VF, Vargas FS, Marchi E, et al. Acute inflammatory response secondary to intrapleural administration of two types of talc. Eur Respir J 2010; 35:396–401.
- Fysh ET, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142:394–400.
- Kheir F, Shawwa K, Alokla K, Omballi M, Alraiyes AH. Tunneled pleural catheter for the treatment of malignant pleural effusion: a systematic review and meta-analysis. Am J Ther 2015 Feb 2. [Epub ahead of print]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307:2383–2389.
- Ponrartana S, Laberge JM, Kerlan RK, Wilson MW, Gordon RL. Management of patients with “ex vacuo” pneumothorax after thoracentesis. Acad Radiol 2005; 12:980–986.
- Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg 2009; 9:961–964.
- Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011; 139:1419–1423.
An indwelling pleural catheter should be considered when a malignant pleural effusion causes symptoms and recurs after thoracentesis, especially in patients with short to intermediate life expectancy or trapped lung, or who underwent unsuccessful pleurodesis.1
MALIGNANT PLEURAL EFFUSION
Malignant pleural effusion affects about 150,000 people in the United States each year. It occurs in 15% of patients with advanced malignancies, most often lung cancer, breast cancer, lymphoma, and ovarian cancer, which account for more than 50% of cases.2
In most patients with malignant pleural effusion, disabling dyspnea causes poor quality of life. The prognosis is unfavorable, with life expectancy of 3 to 12 months. Patients with poor performance status and lower glucose concentrations in the pleural fluid face a worse prognosis and a shorter life expectancy.2
In general, management focuses on relieving symptoms rather than on cure. Symptoms can be controlled by thoracentesis, but if the effusion recurs, the patient needs repeated visits to the emergency room or clinic or a hospital admission to drain the fluid. Frequent hospital visits can be grueling for a patient with a poor functional status, and so can the adverse effects of repeated thoracentesis. For that reason, an early palliative approach to malignant pleural effusion in patients with cancer and a poor prognosis leads to better symptom control and a better quality of life.3 Multiple treatments can be offered to control the symptoms in patients with recurrent malignant pleural effusion (Table 1).
PLEURODESIS HAS BEEN THE TREATMENT OF CHOICE
Pleurodesis has been the treatment of choice for malignant pleural effusion for decades. In this procedure, adhesion of the visceral and parietal pleura is achxieved by inducing inflammation either mechanically or chemically between the pleural surfaces. Injection of a sclerosant into the pleural space generates the inflammation. The sclerosant can be introduced through a chest tube or thoracoscope such as in video-assisted thoracic surgery or medical pleuroscopy. The use of talc is associated with a higher success rate than other sclerosing agents such as bleomycin and doxycycline.4
The downside of this procedure is that pleural effusion recurs in 10% to 40% of cases, and patients require 2 to 4 days in the hospital. Also, the use of talc can lead to acute lung injury–acute respiratory distress syndrome, a rare but potentially life-threatening complication. The incidence of this complication may be related to particle size, with small particles posing a higher risk than large ones.5,6
PLACEMENT OF AN INDWELLING PLEURAL CATHETER
Indwelling pleural catheters are currently used as palliative therapy for patients with recurrent malignant pleural effusion who suffer from respiratory distress due to rapid reaccumulation of pleural fluids that require multiple thoracentesis procedures.
An indwelling pleural catheter is contraindicated in patients with uncontrolled coagulopathy, multiloculated pleural effusions, or extensive malignancy in the skin.3 Other factors that need to be considered are the patient’s social circumstances: ie, the patient must be in a clean and safe environment and must have insurance coverage for the supplies.
Catheters are 66 cm long and 15.5F and are made of silicone rubber with fenestrations along the distal 24 cm. They have a one-way valve at the proximal end that allows fluids and air to go out but not in (Figure 1).1 Several systems are commercially available in the United States.
The catheter is inserted and tunneled percutaneously with the patient under local anesthesia and conscious sedation (Figure 2). Insertion is a same-day outpatient procedure, and intermittent pleural fluid drainage can be done at home by a home heathcare provider or a trained family member.7
In a meta-analysis, insertion difficulties were reported in only 4% of cases, particularly in patients who underwent prior pleural interventions. Spontaneous pleurodesis occurred in 45% of patients at a mean of 52 days after insertion.8
After catheter insertion, the pleural space should be drained three times a week. No more than 1,000 mL of fluid should be removed at a time—or less if drainage causes chest pain or cough secondary to trapped lung (see below). When the drainage declines to 150 mL per session, the sessions can be reduced to twice a week. If the volume drops to less than 50 mL per session, imaging (computed tomography or bedside thoracic ultrasonography) is recommended to ensure the achievement of pleurodesis and to rule out catheter blockage.
A large multicenter randomized controlled trial9 compared indwelling pleural catheter therapy and chest tube insertion with talc pleurodesis. Both procedures relieved symptoms for the first 42 days, and there was no significant difference in quality of life. However, the median length of hospital stay was 4 days for the talc pleurodesis group compared with 0 days for the indwelling pleural catheter group. Twenty-two percent of the talc group required a further pleural procedure such as a video-assisted thoracic surgery or thoracoscopy, compared with 6% of the indwelling catheter group. On the other hand, 36% of those in the indwelling catheter group experienced nonserious adverse events such as pleural infections that mandated outpatient oral antibiotic therapy, cellulitis, and catheter blockage, compared with 7% of the talc group.9
Symptomatic, inoperable trapped lung is another condition for which an indwelling pleural catheter is a reasonable strategy compared with pleurodesis. Trapped lung is a condition in which the lung fails to fully expand despite proper pleural fluid removal, creating a vacuum space between the parietal and visceral pleura (Figure 3).
Patients with trapped lung complain of severe dull or sharp pain during drainage of pleural fluids due to stretching of the visceral pleura against the intrathoracic vacuum space. Trapped lung can be detected objectively by using intrathoracic manometry while draining fluids, looking for more than a 20-cm H2O drop in the intrathoracic pressure. Radiographically, this may be identified as a pneumothorax ex vacuo10 (ie, caused by inability of the lung to expand to fill the thoracic cavity after pleural fluid has been drained) and is not a procedure complication.
Placement of an indwelling pleural catheter is the treatment of choice for trapped lung, since chemical pleurodesis is not feasible without the potential of parietal and visceral pleural apposition. In a retrospective study of indwelling catheter placement for palliative symptom control, a catheter relieved symptoms, improved quality of life, and afforded a substantial increase in mobility.1,11
In another multicenter pilot study,12 rapid pleurodesis was achieved in 30 patients with recurrent malignant pleural effusion by combining chemical pleurodesis and indwelling catheter placement. Both were done under direct vision with medical thoracoscopy. Pleurodesis succeeded in 92% of patients by day 8 after the procedure. The hospital stay was reduced to a mean of 2 days after the procedure. In the catheter group, fluids were drained three times in the first day after the procedure and twice a day on the second and third days. Of the 30 patients in this study, 2 had fever, 1 needed to have the catheter replaced, and 1 contracted empyema.
AN EFFECTIVE INITIAL TREATMENT
Placement of an indwelling pleural catheter is an effective initial treatment for recurrent malignant pleural effusion. Compared with chemical pleurodesis, it has a comparable success rate and complication rate. It offers the advantages of being a same-day surgical procedure entailing a shorter hospital stay and less need for further pleural intervention. This treatment should be considered for patients with symptomatic malignant pleural effusion, especially those in whom symptomatic malignant pleural effusion recurred after thoracentesis.8
An indwelling pleural catheter should be considered when a malignant pleural effusion causes symptoms and recurs after thoracentesis, especially in patients with short to intermediate life expectancy or trapped lung, or who underwent unsuccessful pleurodesis.1
MALIGNANT PLEURAL EFFUSION
Malignant pleural effusion affects about 150,000 people in the United States each year. It occurs in 15% of patients with advanced malignancies, most often lung cancer, breast cancer, lymphoma, and ovarian cancer, which account for more than 50% of cases.2
In most patients with malignant pleural effusion, disabling dyspnea causes poor quality of life. The prognosis is unfavorable, with life expectancy of 3 to 12 months. Patients with poor performance status and lower glucose concentrations in the pleural fluid face a worse prognosis and a shorter life expectancy.2
In general, management focuses on relieving symptoms rather than on cure. Symptoms can be controlled by thoracentesis, but if the effusion recurs, the patient needs repeated visits to the emergency room or clinic or a hospital admission to drain the fluid. Frequent hospital visits can be grueling for a patient with a poor functional status, and so can the adverse effects of repeated thoracentesis. For that reason, an early palliative approach to malignant pleural effusion in patients with cancer and a poor prognosis leads to better symptom control and a better quality of life.3 Multiple treatments can be offered to control the symptoms in patients with recurrent malignant pleural effusion (Table 1).
PLEURODESIS HAS BEEN THE TREATMENT OF CHOICE
Pleurodesis has been the treatment of choice for malignant pleural effusion for decades. In this procedure, adhesion of the visceral and parietal pleura is achxieved by inducing inflammation either mechanically or chemically between the pleural surfaces. Injection of a sclerosant into the pleural space generates the inflammation. The sclerosant can be introduced through a chest tube or thoracoscope such as in video-assisted thoracic surgery or medical pleuroscopy. The use of talc is associated with a higher success rate than other sclerosing agents such as bleomycin and doxycycline.4
The downside of this procedure is that pleural effusion recurs in 10% to 40% of cases, and patients require 2 to 4 days in the hospital. Also, the use of talc can lead to acute lung injury–acute respiratory distress syndrome, a rare but potentially life-threatening complication. The incidence of this complication may be related to particle size, with small particles posing a higher risk than large ones.5,6
PLACEMENT OF AN INDWELLING PLEURAL CATHETER
Indwelling pleural catheters are currently used as palliative therapy for patients with recurrent malignant pleural effusion who suffer from respiratory distress due to rapid reaccumulation of pleural fluids that require multiple thoracentesis procedures.
An indwelling pleural catheter is contraindicated in patients with uncontrolled coagulopathy, multiloculated pleural effusions, or extensive malignancy in the skin.3 Other factors that need to be considered are the patient’s social circumstances: ie, the patient must be in a clean and safe environment and must have insurance coverage for the supplies.
Catheters are 66 cm long and 15.5F and are made of silicone rubber with fenestrations along the distal 24 cm. They have a one-way valve at the proximal end that allows fluids and air to go out but not in (Figure 1).1 Several systems are commercially available in the United States.
The catheter is inserted and tunneled percutaneously with the patient under local anesthesia and conscious sedation (Figure 2). Insertion is a same-day outpatient procedure, and intermittent pleural fluid drainage can be done at home by a home heathcare provider or a trained family member.7
In a meta-analysis, insertion difficulties were reported in only 4% of cases, particularly in patients who underwent prior pleural interventions. Spontaneous pleurodesis occurred in 45% of patients at a mean of 52 days after insertion.8
After catheter insertion, the pleural space should be drained three times a week. No more than 1,000 mL of fluid should be removed at a time—or less if drainage causes chest pain or cough secondary to trapped lung (see below). When the drainage declines to 150 mL per session, the sessions can be reduced to twice a week. If the volume drops to less than 50 mL per session, imaging (computed tomography or bedside thoracic ultrasonography) is recommended to ensure the achievement of pleurodesis and to rule out catheter blockage.
A large multicenter randomized controlled trial9 compared indwelling pleural catheter therapy and chest tube insertion with talc pleurodesis. Both procedures relieved symptoms for the first 42 days, and there was no significant difference in quality of life. However, the median length of hospital stay was 4 days for the talc pleurodesis group compared with 0 days for the indwelling pleural catheter group. Twenty-two percent of the talc group required a further pleural procedure such as a video-assisted thoracic surgery or thoracoscopy, compared with 6% of the indwelling catheter group. On the other hand, 36% of those in the indwelling catheter group experienced nonserious adverse events such as pleural infections that mandated outpatient oral antibiotic therapy, cellulitis, and catheter blockage, compared with 7% of the talc group.9
Symptomatic, inoperable trapped lung is another condition for which an indwelling pleural catheter is a reasonable strategy compared with pleurodesis. Trapped lung is a condition in which the lung fails to fully expand despite proper pleural fluid removal, creating a vacuum space between the parietal and visceral pleura (Figure 3).
Patients with trapped lung complain of severe dull or sharp pain during drainage of pleural fluids due to stretching of the visceral pleura against the intrathoracic vacuum space. Trapped lung can be detected objectively by using intrathoracic manometry while draining fluids, looking for more than a 20-cm H2O drop in the intrathoracic pressure. Radiographically, this may be identified as a pneumothorax ex vacuo10 (ie, caused by inability of the lung to expand to fill the thoracic cavity after pleural fluid has been drained) and is not a procedure complication.
Placement of an indwelling pleural catheter is the treatment of choice for trapped lung, since chemical pleurodesis is not feasible without the potential of parietal and visceral pleural apposition. In a retrospective study of indwelling catheter placement for palliative symptom control, a catheter relieved symptoms, improved quality of life, and afforded a substantial increase in mobility.1,11
In another multicenter pilot study,12 rapid pleurodesis was achieved in 30 patients with recurrent malignant pleural effusion by combining chemical pleurodesis and indwelling catheter placement. Both were done under direct vision with medical thoracoscopy. Pleurodesis succeeded in 92% of patients by day 8 after the procedure. The hospital stay was reduced to a mean of 2 days after the procedure. In the catheter group, fluids were drained three times in the first day after the procedure and twice a day on the second and third days. Of the 30 patients in this study, 2 had fever, 1 needed to have the catheter replaced, and 1 contracted empyema.
AN EFFECTIVE INITIAL TREATMENT
Placement of an indwelling pleural catheter is an effective initial treatment for recurrent malignant pleural effusion. Compared with chemical pleurodesis, it has a comparable success rate and complication rate. It offers the advantages of being a same-day surgical procedure entailing a shorter hospital stay and less need for further pleural intervention. This treatment should be considered for patients with symptomatic malignant pleural effusion, especially those in whom symptomatic malignant pleural effusion recurred after thoracentesis.8
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40.
- Thomas JM, Musani AI. Malignant pleural effusions: a review. Clin Chest Med 2013; 34:459–471.
- Thomas R, Francis R, Davies HE, Lee YC. Interventional therapies for malignant pleural effusions: the present and the future. Respirology 2014; 19:809–822.
- Rodriguez-Panadero F, Montes-Worboys A. Mechanisms of pleurodesis. Respiration 2012; 83:91–98.
- Gonzalez AV, Bezwada V, Beamis JF Jr, Villanueva AG. Lung injury following thoracoscopic talc insufflation: experience of a single North American center. Chest 2010; 137:1375–1381.
- Rossi VF, Vargas FS, Marchi E, et al. Acute inflammatory response secondary to intrapleural administration of two types of talc. Eur Respir J 2010; 35:396–401.
- Fysh ET, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142:394–400.
- Kheir F, Shawwa K, Alokla K, Omballi M, Alraiyes AH. Tunneled pleural catheter for the treatment of malignant pleural effusion: a systematic review and meta-analysis. Am J Ther 2015 Feb 2. [Epub ahead of print]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307:2383–2389.
- Ponrartana S, Laberge JM, Kerlan RK, Wilson MW, Gordon RL. Management of patients with “ex vacuo” pneumothorax after thoracentesis. Acad Radiol 2005; 12:980–986.
- Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg 2009; 9:961–964.
- Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011; 139:1419–1423.
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40.
- Thomas JM, Musani AI. Malignant pleural effusions: a review. Clin Chest Med 2013; 34:459–471.
- Thomas R, Francis R, Davies HE, Lee YC. Interventional therapies for malignant pleural effusions: the present and the future. Respirology 2014; 19:809–822.
- Rodriguez-Panadero F, Montes-Worboys A. Mechanisms of pleurodesis. Respiration 2012; 83:91–98.
- Gonzalez AV, Bezwada V, Beamis JF Jr, Villanueva AG. Lung injury following thoracoscopic talc insufflation: experience of a single North American center. Chest 2010; 137:1375–1381.
- Rossi VF, Vargas FS, Marchi E, et al. Acute inflammatory response secondary to intrapleural administration of two types of talc. Eur Respir J 2010; 35:396–401.
- Fysh ET, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142:394–400.
- Kheir F, Shawwa K, Alokla K, Omballi M, Alraiyes AH. Tunneled pleural catheter for the treatment of malignant pleural effusion: a systematic review and meta-analysis. Am J Ther 2015 Feb 2. [Epub ahead of print]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307:2383–2389.
- Ponrartana S, Laberge JM, Kerlan RK, Wilson MW, Gordon RL. Management of patients with “ex vacuo” pneumothorax after thoracentesis. Acad Radiol 2005; 12:980–986.
- Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg 2009; 9:961–964.
- Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011; 139:1419–1423.
Pulmonary disease in small-vessel vasculitis
The pulmonary manifestations of small-vessel vasculitis are nonspecific and often overlap with other conditions. Consequently, the diagnosis and management of pulmonary vasculitis are complex and require special attention to detail. This article reviews clinical experience with vasculitis as it manifests in the pulmonary setting, with the goal of providing a sound clinical approach to diagnosis and management.
DIAGNOSTIC CONSIDERATIONS
Accurate diagnosis is enhanced with imaging technology, judicious use of bronchoscopy, and awareness of disorders that mimic or masquerade as pulmonary vasculitis. The diagnosis can be approached on the basis of pattern recognition. For example, microscopic polyangiitis (MPA) is characterized solely by alveolar hemorrhage syndrome. However, other diagnostic possibilities must be considered, such as infection, acute respiratory distress syndrome, and complications of medicines. The hallmark manifestation of granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is necrotizing granulomatous inflammations, but the pulmonary manifestations can include nodules, cavitary masses, airway stenosis, and alveolar hemorrhage. Asthma with eosinophilia is the distinguishing feature of eosinophilic GPA (Churg-Strauss syndrome, EGPA), and Goodpasture syndrome involves deposition of complement and immunoglobulins.
The use of imaging
The best imaging tool for suspected pulmonary vasculitides is high-resolution computed tomography (CT). As a general rule, CT for patients with suspected vasculitis should be ordered without contrast medium as contrast is not needed to assess the lung parenchyma. Vasculitis patients often have renal insufficiency, and contrast-free CT will help protect the kidneys. Another option, which will enhance evaluation of the distribution and location of pulmonary disease, is multiplanar reconstructions of images with virtual bronchoscopy or airway reconstruction. Certain findings on imaging will help to differentiate the vasculitides from one another as well as from mimicking diagnoses.
Eosinophilic GPA. Chest images of patients with EGPA appear as patchy, nonsegmental, often peripheral consolidations of ground-glass opacity. These tend to reside in all lobes of the lungs, close to the surface and occasionally accompanied by septal markings.
Microscopic polyangiitis. Although classically a disease of alveolar hemorrhage, MPA often does not manifest with hemoptysis. Approximately one-third of patients with MPA do not cough up blood, even after a large amount of hemorrhage directly into the parenchyma. Patients may present with nonspecific symptoms such as fatigue and shortness of breath. Chest imaging will enhance diagnostic accuracy, particularly when considered in conjunction with laboratory test results. MPA patients usually have low hematocrit levels and may actually have an increased diffusing capacity of the lung for carbon monoxide (Dlco).
Granulomatosis with polyangiitis. This form of vasculitis has characteristic nodules, cavitary lesions, and, in the worst cases, multifocal masses in the lungs. These can be identified with contrast-free CT, with examination for possible airway involvement.
Multiple lung cavity nodules and pronounced airway narrowing are significant diagnostic clues for GPA. Nodules up to 10 cm in diameter tend to be near sub-pleural and peripheral areas. Microbes and fungus may complicate the nodules’ primary presentation. While bronchoscopy may be helpful with imaging, surgical biopsy remains the gold standard to rule out infections.
The disease may be multifocal, occurring outside the lungs from the larynx to bronchi and anywhere in the lung. Subglottic stenosis caused by inflammation and scarring affects 16% of patients with GPA, but it also often develops independently of other features of GPA and may have its own course independent of systemic symptoms.1
Bronchoscopy
Bronchoscopy is a relatively low-risk way to assess airways and nodules, but it has had a limited role in the diagnosis of nonfocal interstitial lung disease and rheumatologic lung disease in general. New technologies that augment traditional bronchoscopy and enhance its utility for diagnosis for focal entities are described below.
Electromagnetic navigation bronchoscopy (ENB) uses electromagnetic technology to localize and guide a catheter through the bronchial pathways. With the help of a virtual, 3-dimensional bronchial map reconstructed from a chest CT, the clinician can navigate to a desired location within the lung for biopsy and diagnosis of pulmonary nodules. The result is a diagnostic yield per nodule of nearly 80%.2 Seijo et al showed that diagnostic yields by ENB increase with the presence of the bronchus sign, or a bronchus leading directly to a peripheral lung lesion, as viewed on CT imaging.2 If nodules are bronchocentric, or surround airways, there is greater likelihood of reaching a diagnosis without resorting to surgery.
In peripheral radial ultrasound, a catheter is threaded through another catheter sheath in order to visualize the lesion. This technology can precisely localize lung lesions and often give some clues about the final pathology.
Bronchoscopic confocal fluorescence microscopy3 is a new form of microscopy that uses a fiberoptic miniprobe instead of an objective lens. High-quality images are achieved by the use of autofluorescence. Researchers have used the technology to detect changes in the respiratory bronchioles and other structures, but a clear atlas of many disease states does not yet exist. Oddly, endobronchial GPA images have been catalogued.3
Virtual bronchoscopy is a 3-dimensional image reconstruction and display technique that converts standard CT images into multiplanar images, which can be stacked. Virtual bronchoscopy augments conventional CT because of its ability to enhance detection in the subglottic region and more accurately measure stenosis.4 The technique cannot replace traditional bronchoscopy, however, because mucus and secretions can appear as abnormalities and cause false-positive results.
Detecting mimics
Diagnoses that masquerade as EGPA include chronic eosinophilic pneumonia, bronchiolitis obliterans with organizing pneumonia, and other interstitial lung diseases. Allergic bronchopulmonary aspergillosis—an asthma syndrome sometimes associated with eosinophilia and high immunoglobulin-E levels—also mimics EGPA. This diagnostic possibility is particularly relevant if the patient is taking immunosuppressive agents or corticosteroids.
Although alveolar hemorrhage is the sole pulmonary manifestation of MPA, the diagnosis is not limited to MPA alone. Alveolar hemorrhage may have other causes, including infection or acute respiratory distress syndrome. Bronchial lavage is recommended for accurate diagnosis, with the introduction of successive volumes of saline into the lungs and examination for increasing amounts of heme in each of the aliquots of alveolar lavage fluid.
Several diagnoses can mimic GPA. Many infections, including those caused by mycobacteria and Cryptococcus, can mimic endobronchial GPA. Biopsy of all new ulcers is recommended to minimize the possibility of missing these diagnoses. Tuberculosis in its latent form can closely resemble scarred GPA. Other mimickers of cavitary lung lesions can include metastatic melanoma, metastatic renal and thyroid cancers, squamous cell carcinoma, and rheumatoid arthritis with necrobiotic nodules that open in the lungs.
TREATMENT STRATEGIES
Medications
Although many patients with GPA are surgical candidates because of dyspnea related to fixed endobronchial or endotracheal obstructions, any surgical treatment carries the risk of inciting further flares. Treatment should focus first on mitigating the systemic inflammatory disorder with pharmacologic intervention. Standard pharmacologic therapy includes corticosteroids, azathioprine, cyclophosphamide, and rituximab. Patients with subglottic stenosis are frequently unresponsive to standard immunosuppressive therapy (glucocorticoids in combination with a cytotoxic agent).1
Surgical reconstruction
When medication falls short and surgery is needed to reverse strictures, a number of tools are at our disposal. Some involve heat, such as laser, cauterization, and argon plasma coagulation. In argon plasma coagulation, a jet of ionized argon gas (plasma) is directed through a probe passed through an endoscope. Other techniques rely on cold: cryoprobes, microdebriders, and rigid scissors. In general, freeze therapies cause less scarring than heat therapy. With any surgical technique, there is risk of scars that will contract and cause structural collapse, resulting in restenosis.
Dilation
The high rate of stenosis relapse has spurred interest in alternatives to surgical treatment. One of these, dilation via endoscopy, also may mitigate the wound healing process. Other techniques for clearing the obstructed area include rigid bronchoscopy, the use of bougies (increasingly larger dilators), and balloon dilation. Balloon dilation has some advantages over the other techniques. It permits maximal radial direction and pressure, causes less damage to trachea wall mucosa, and achieves better overall results; however, the procedure usually needs to be repeated.5 It must be done quickly, and it requires flawless communication between the otolaryngologist or pulmonologist and anesthesiologist in order to stabilize the airway below the vocal cords.
Intratracheal dilation-injection therapy
Dilation can be augmented with glucocorticoid injections. In 1991, researchers at the National Institutes of Health utilized a combination dilation-injection therapy for 20 patients who had GPA and subglottic stenosis.1 Patients were first treated with mercury-filled dilators coated with 1% triamcinolone cream. Methylprednisolone acetate was then injected into the stenotic area. None of the patients treated with intratracheal dilation-injection therapy required a tracheostomy and six who already had tracheostomies were decannulated. In contrast, 56% of patients who received standard immunosuppressive therapy and no intratracheal dilation-injection therapy required tracheostomy. Intratracheal dilation-injection therapy is considered a safe and effective treatment of GPA-associated subglottic stenosis and, in the absence of major organ disease activity, could be used without systemic immunosuppressive agents.
Mitomycin-C is a controversial alternative to corticosteroids during dilation. Mitomycin-C is an alkylating agent that inhibits fibroblast proliferation and extracellular matrix protein synthesis, with the potential for reduced scarring. In a recent trial of 26 patients, two doses given 3 to 4 weeks apart reduced the rate of stenosis for 2 to 3 years compared with a single dose.6 Restenosis occurred in both groups, however, and after 5 years, the relapse rates were the same.
Nd:YAG laser photoresection versus endobronchial electrosurgery
One of the most effective therapies for treating obstructive lesions is Nd:YAG laser photoresection (LPR) in which a laser that utilizes the crystal neodymium-doped yttrium aluminum garnet (Nd:Y3Al5O12) is paired with a flexible bronchoscope. The procedure can produce favorable outcomes,7 but it has not gained favor because of perceptions that the lasers require rigid bronchoscopy, expensive equipment, and special training. There are also concerns about complications.
The lower-cost endobronchial electrosurgery (EBES) also failed to gain acceptance because of cumbersome delivery systems and complications associated with power units. Recently, engineers have spawned a new generation of electrosurgical devices, prompting renewed interest in EBES.
A recent study compared LPR and EBES in patients who represented 118 evaluations for LPR.8 Forty percent were considered amenable to EBES and so did not go on to receive the more costly LPR. Of those, 89% achieved success in alleviating the obstruction. The authors concluded that EBES can potentially eliminate the need for LPR in 36% of procedures, and that it could achieve significant savings in cost and time. We use these ablative therapies only in dire circumstances; we use non–heat-based therapies, including repeated dilation, prior to considering use of other therapies.
Cryotherapy
Cryotherapy spray was initially thought to have great therapeutic potential, but the high pressures of the spray caused complications. This modality remains under investigation, however. Some probe-based cryotherapy techniques have been effective anecdotally. These use a metal-tipped probe attached to a cryogen; the Joule-Thompson effect causes delayed tissue destruction.
Stents
A small number of case reports note patient improvement after stenting.9,10 We use stents in rare circumstances, but because complications are frequent and sometimes severe, we consider stenting a last-resort option. In 2005, the US Food and Drug Administration mandated a Black Box warning against the use of metallic stents in patients who have benign tracheal strictures.
Multimodality therapies
In general, when intervention is required to salvage airways, a combination of dilation and steroid injection with or without topical mitomycin-C is standard. We try to avoid use of thermal therapy with laser or electrocautery because of the risk of exuberant inflammation and restenosis from thermal injury. No specific standard of care exists in these cases; reliance on clinical judgment is critical because of the presentation and variety of airway lesions. Further, no large-scale randomized trials exist to guide therapy, so it is best to work with a multidisciplinary team whose members have experience in managing these complex patients.
CONCLUSION
The differential diagnosis of pulmonary manifestations of small-vessel vasculitis is complex. Several diagnoses can mimic various forms of pulmonary vasculitis, and the manifestations and symptoms often overlap with other organ systems.
Imaging is useful for analysis of common patterns of small and midsize vasculitis, although the results may be confounded by disorders that mimic pulmonary vasculitis. To enhance diagnostic accuracy, laboratory and clinical findings should be considered along with images. Ideally, treatment will be minimally destructive and mucosa-sparing. Dilation therapies can be augmented with corticosteroid injections or, possibly, mitomycin-C.
- Langford CA, Sneller MC, Hallahan CW, et al. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Arthritis Rheum 1996; 39:1754–1760.
- Seijo LM, de Torres JP, Lozano MD, et al. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a bronchus sign on CT imaging: results from a prospective study. Chest 2010; 138:1316–1321.
- Filner JJ, Bonura EJ, Lau ST, et al. Bronchoscopic fibered confocal fluorescence microscopy image characteristics and pathologic correlations. J Bronchol Intervent Pulmonol 2011; 18:23–30.
- Summers RM, Aggarwal NR, Sneller MC, et al. CT virtual bronchoscopy of the central airway in patients with Wegener’s granulomatosis. Chest 2002; 121:242–250.
- Schokkenbroek AA, Franssen CFM, Dikkers FG. Dilatation tracheoscopy for laryngeal and tracheal stenosis in patients with Wegener’s granulomatosis. Eur Arch Otorhinolaryngol 2008; 265:549–555.
- Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope 2009; 119:272–283.
- Shvero J, Shitrit D, Koren R, Shalomi D, Kramer MR. Endoscopic laser surgery for subglottic stenosis in Wegener’s granulomatosis. Yonsei Med J 2007; 48:748–753.
- Coulter TD, Mehta AC. The heat is on: impact of endobronchial electrosurgery on the need for Nd-YAG laser photoresection. Chest 2000; 118:516–521.
- Tierman J, Shah C, Elborn JS. Successful stenting in endobronchial Wegener’s granulomatosis. Ulster Med J 2006; 75:155–157.
- Daum TE, Specks U, Colby TV, et al. Tracheobronchial involvement in Wegener’s granulomatosis. Am J Respir Crit Care Med 1995; 151:522–526.
The pulmonary manifestations of small-vessel vasculitis are nonspecific and often overlap with other conditions. Consequently, the diagnosis and management of pulmonary vasculitis are complex and require special attention to detail. This article reviews clinical experience with vasculitis as it manifests in the pulmonary setting, with the goal of providing a sound clinical approach to diagnosis and management.
DIAGNOSTIC CONSIDERATIONS
Accurate diagnosis is enhanced with imaging technology, judicious use of bronchoscopy, and awareness of disorders that mimic or masquerade as pulmonary vasculitis. The diagnosis can be approached on the basis of pattern recognition. For example, microscopic polyangiitis (MPA) is characterized solely by alveolar hemorrhage syndrome. However, other diagnostic possibilities must be considered, such as infection, acute respiratory distress syndrome, and complications of medicines. The hallmark manifestation of granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is necrotizing granulomatous inflammations, but the pulmonary manifestations can include nodules, cavitary masses, airway stenosis, and alveolar hemorrhage. Asthma with eosinophilia is the distinguishing feature of eosinophilic GPA (Churg-Strauss syndrome, EGPA), and Goodpasture syndrome involves deposition of complement and immunoglobulins.
The use of imaging
The best imaging tool for suspected pulmonary vasculitides is high-resolution computed tomography (CT). As a general rule, CT for patients with suspected vasculitis should be ordered without contrast medium as contrast is not needed to assess the lung parenchyma. Vasculitis patients often have renal insufficiency, and contrast-free CT will help protect the kidneys. Another option, which will enhance evaluation of the distribution and location of pulmonary disease, is multiplanar reconstructions of images with virtual bronchoscopy or airway reconstruction. Certain findings on imaging will help to differentiate the vasculitides from one another as well as from mimicking diagnoses.
Eosinophilic GPA. Chest images of patients with EGPA appear as patchy, nonsegmental, often peripheral consolidations of ground-glass opacity. These tend to reside in all lobes of the lungs, close to the surface and occasionally accompanied by septal markings.
Microscopic polyangiitis. Although classically a disease of alveolar hemorrhage, MPA often does not manifest with hemoptysis. Approximately one-third of patients with MPA do not cough up blood, even after a large amount of hemorrhage directly into the parenchyma. Patients may present with nonspecific symptoms such as fatigue and shortness of breath. Chest imaging will enhance diagnostic accuracy, particularly when considered in conjunction with laboratory test results. MPA patients usually have low hematocrit levels and may actually have an increased diffusing capacity of the lung for carbon monoxide (Dlco).
Granulomatosis with polyangiitis. This form of vasculitis has characteristic nodules, cavitary lesions, and, in the worst cases, multifocal masses in the lungs. These can be identified with contrast-free CT, with examination for possible airway involvement.
Multiple lung cavity nodules and pronounced airway narrowing are significant diagnostic clues for GPA. Nodules up to 10 cm in diameter tend to be near sub-pleural and peripheral areas. Microbes and fungus may complicate the nodules’ primary presentation. While bronchoscopy may be helpful with imaging, surgical biopsy remains the gold standard to rule out infections.
The disease may be multifocal, occurring outside the lungs from the larynx to bronchi and anywhere in the lung. Subglottic stenosis caused by inflammation and scarring affects 16% of patients with GPA, but it also often develops independently of other features of GPA and may have its own course independent of systemic symptoms.1
Bronchoscopy
Bronchoscopy is a relatively low-risk way to assess airways and nodules, but it has had a limited role in the diagnosis of nonfocal interstitial lung disease and rheumatologic lung disease in general. New technologies that augment traditional bronchoscopy and enhance its utility for diagnosis for focal entities are described below.
Electromagnetic navigation bronchoscopy (ENB) uses electromagnetic technology to localize and guide a catheter through the bronchial pathways. With the help of a virtual, 3-dimensional bronchial map reconstructed from a chest CT, the clinician can navigate to a desired location within the lung for biopsy and diagnosis of pulmonary nodules. The result is a diagnostic yield per nodule of nearly 80%.2 Seijo et al showed that diagnostic yields by ENB increase with the presence of the bronchus sign, or a bronchus leading directly to a peripheral lung lesion, as viewed on CT imaging.2 If nodules are bronchocentric, or surround airways, there is greater likelihood of reaching a diagnosis without resorting to surgery.
In peripheral radial ultrasound, a catheter is threaded through another catheter sheath in order to visualize the lesion. This technology can precisely localize lung lesions and often give some clues about the final pathology.
Bronchoscopic confocal fluorescence microscopy3 is a new form of microscopy that uses a fiberoptic miniprobe instead of an objective lens. High-quality images are achieved by the use of autofluorescence. Researchers have used the technology to detect changes in the respiratory bronchioles and other structures, but a clear atlas of many disease states does not yet exist. Oddly, endobronchial GPA images have been catalogued.3
Virtual bronchoscopy is a 3-dimensional image reconstruction and display technique that converts standard CT images into multiplanar images, which can be stacked. Virtual bronchoscopy augments conventional CT because of its ability to enhance detection in the subglottic region and more accurately measure stenosis.4 The technique cannot replace traditional bronchoscopy, however, because mucus and secretions can appear as abnormalities and cause false-positive results.
Detecting mimics
Diagnoses that masquerade as EGPA include chronic eosinophilic pneumonia, bronchiolitis obliterans with organizing pneumonia, and other interstitial lung diseases. Allergic bronchopulmonary aspergillosis—an asthma syndrome sometimes associated with eosinophilia and high immunoglobulin-E levels—also mimics EGPA. This diagnostic possibility is particularly relevant if the patient is taking immunosuppressive agents or corticosteroids.
Although alveolar hemorrhage is the sole pulmonary manifestation of MPA, the diagnosis is not limited to MPA alone. Alveolar hemorrhage may have other causes, including infection or acute respiratory distress syndrome. Bronchial lavage is recommended for accurate diagnosis, with the introduction of successive volumes of saline into the lungs and examination for increasing amounts of heme in each of the aliquots of alveolar lavage fluid.
Several diagnoses can mimic GPA. Many infections, including those caused by mycobacteria and Cryptococcus, can mimic endobronchial GPA. Biopsy of all new ulcers is recommended to minimize the possibility of missing these diagnoses. Tuberculosis in its latent form can closely resemble scarred GPA. Other mimickers of cavitary lung lesions can include metastatic melanoma, metastatic renal and thyroid cancers, squamous cell carcinoma, and rheumatoid arthritis with necrobiotic nodules that open in the lungs.
TREATMENT STRATEGIES
Medications
Although many patients with GPA are surgical candidates because of dyspnea related to fixed endobronchial or endotracheal obstructions, any surgical treatment carries the risk of inciting further flares. Treatment should focus first on mitigating the systemic inflammatory disorder with pharmacologic intervention. Standard pharmacologic therapy includes corticosteroids, azathioprine, cyclophosphamide, and rituximab. Patients with subglottic stenosis are frequently unresponsive to standard immunosuppressive therapy (glucocorticoids in combination with a cytotoxic agent).1
Surgical reconstruction
When medication falls short and surgery is needed to reverse strictures, a number of tools are at our disposal. Some involve heat, such as laser, cauterization, and argon plasma coagulation. In argon plasma coagulation, a jet of ionized argon gas (plasma) is directed through a probe passed through an endoscope. Other techniques rely on cold: cryoprobes, microdebriders, and rigid scissors. In general, freeze therapies cause less scarring than heat therapy. With any surgical technique, there is risk of scars that will contract and cause structural collapse, resulting in restenosis.
Dilation
The high rate of stenosis relapse has spurred interest in alternatives to surgical treatment. One of these, dilation via endoscopy, also may mitigate the wound healing process. Other techniques for clearing the obstructed area include rigid bronchoscopy, the use of bougies (increasingly larger dilators), and balloon dilation. Balloon dilation has some advantages over the other techniques. It permits maximal radial direction and pressure, causes less damage to trachea wall mucosa, and achieves better overall results; however, the procedure usually needs to be repeated.5 It must be done quickly, and it requires flawless communication between the otolaryngologist or pulmonologist and anesthesiologist in order to stabilize the airway below the vocal cords.
Intratracheal dilation-injection therapy
Dilation can be augmented with glucocorticoid injections. In 1991, researchers at the National Institutes of Health utilized a combination dilation-injection therapy for 20 patients who had GPA and subglottic stenosis.1 Patients were first treated with mercury-filled dilators coated with 1% triamcinolone cream. Methylprednisolone acetate was then injected into the stenotic area. None of the patients treated with intratracheal dilation-injection therapy required a tracheostomy and six who already had tracheostomies were decannulated. In contrast, 56% of patients who received standard immunosuppressive therapy and no intratracheal dilation-injection therapy required tracheostomy. Intratracheal dilation-injection therapy is considered a safe and effective treatment of GPA-associated subglottic stenosis and, in the absence of major organ disease activity, could be used without systemic immunosuppressive agents.
Mitomycin-C is a controversial alternative to corticosteroids during dilation. Mitomycin-C is an alkylating agent that inhibits fibroblast proliferation and extracellular matrix protein synthesis, with the potential for reduced scarring. In a recent trial of 26 patients, two doses given 3 to 4 weeks apart reduced the rate of stenosis for 2 to 3 years compared with a single dose.6 Restenosis occurred in both groups, however, and after 5 years, the relapse rates were the same.
Nd:YAG laser photoresection versus endobronchial electrosurgery
One of the most effective therapies for treating obstructive lesions is Nd:YAG laser photoresection (LPR) in which a laser that utilizes the crystal neodymium-doped yttrium aluminum garnet (Nd:Y3Al5O12) is paired with a flexible bronchoscope. The procedure can produce favorable outcomes,7 but it has not gained favor because of perceptions that the lasers require rigid bronchoscopy, expensive equipment, and special training. There are also concerns about complications.
The lower-cost endobronchial electrosurgery (EBES) also failed to gain acceptance because of cumbersome delivery systems and complications associated with power units. Recently, engineers have spawned a new generation of electrosurgical devices, prompting renewed interest in EBES.
A recent study compared LPR and EBES in patients who represented 118 evaluations for LPR.8 Forty percent were considered amenable to EBES and so did not go on to receive the more costly LPR. Of those, 89% achieved success in alleviating the obstruction. The authors concluded that EBES can potentially eliminate the need for LPR in 36% of procedures, and that it could achieve significant savings in cost and time. We use these ablative therapies only in dire circumstances; we use non–heat-based therapies, including repeated dilation, prior to considering use of other therapies.
Cryotherapy
Cryotherapy spray was initially thought to have great therapeutic potential, but the high pressures of the spray caused complications. This modality remains under investigation, however. Some probe-based cryotherapy techniques have been effective anecdotally. These use a metal-tipped probe attached to a cryogen; the Joule-Thompson effect causes delayed tissue destruction.
Stents
A small number of case reports note patient improvement after stenting.9,10 We use stents in rare circumstances, but because complications are frequent and sometimes severe, we consider stenting a last-resort option. In 2005, the US Food and Drug Administration mandated a Black Box warning against the use of metallic stents in patients who have benign tracheal strictures.
Multimodality therapies
In general, when intervention is required to salvage airways, a combination of dilation and steroid injection with or without topical mitomycin-C is standard. We try to avoid use of thermal therapy with laser or electrocautery because of the risk of exuberant inflammation and restenosis from thermal injury. No specific standard of care exists in these cases; reliance on clinical judgment is critical because of the presentation and variety of airway lesions. Further, no large-scale randomized trials exist to guide therapy, so it is best to work with a multidisciplinary team whose members have experience in managing these complex patients.
CONCLUSION
The differential diagnosis of pulmonary manifestations of small-vessel vasculitis is complex. Several diagnoses can mimic various forms of pulmonary vasculitis, and the manifestations and symptoms often overlap with other organ systems.
Imaging is useful for analysis of common patterns of small and midsize vasculitis, although the results may be confounded by disorders that mimic pulmonary vasculitis. To enhance diagnostic accuracy, laboratory and clinical findings should be considered along with images. Ideally, treatment will be minimally destructive and mucosa-sparing. Dilation therapies can be augmented with corticosteroid injections or, possibly, mitomycin-C.
The pulmonary manifestations of small-vessel vasculitis are nonspecific and often overlap with other conditions. Consequently, the diagnosis and management of pulmonary vasculitis are complex and require special attention to detail. This article reviews clinical experience with vasculitis as it manifests in the pulmonary setting, with the goal of providing a sound clinical approach to diagnosis and management.
DIAGNOSTIC CONSIDERATIONS
Accurate diagnosis is enhanced with imaging technology, judicious use of bronchoscopy, and awareness of disorders that mimic or masquerade as pulmonary vasculitis. The diagnosis can be approached on the basis of pattern recognition. For example, microscopic polyangiitis (MPA) is characterized solely by alveolar hemorrhage syndrome. However, other diagnostic possibilities must be considered, such as infection, acute respiratory distress syndrome, and complications of medicines. The hallmark manifestation of granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is necrotizing granulomatous inflammations, but the pulmonary manifestations can include nodules, cavitary masses, airway stenosis, and alveolar hemorrhage. Asthma with eosinophilia is the distinguishing feature of eosinophilic GPA (Churg-Strauss syndrome, EGPA), and Goodpasture syndrome involves deposition of complement and immunoglobulins.
The use of imaging
The best imaging tool for suspected pulmonary vasculitides is high-resolution computed tomography (CT). As a general rule, CT for patients with suspected vasculitis should be ordered without contrast medium as contrast is not needed to assess the lung parenchyma. Vasculitis patients often have renal insufficiency, and contrast-free CT will help protect the kidneys. Another option, which will enhance evaluation of the distribution and location of pulmonary disease, is multiplanar reconstructions of images with virtual bronchoscopy or airway reconstruction. Certain findings on imaging will help to differentiate the vasculitides from one another as well as from mimicking diagnoses.
Eosinophilic GPA. Chest images of patients with EGPA appear as patchy, nonsegmental, often peripheral consolidations of ground-glass opacity. These tend to reside in all lobes of the lungs, close to the surface and occasionally accompanied by septal markings.
Microscopic polyangiitis. Although classically a disease of alveolar hemorrhage, MPA often does not manifest with hemoptysis. Approximately one-third of patients with MPA do not cough up blood, even after a large amount of hemorrhage directly into the parenchyma. Patients may present with nonspecific symptoms such as fatigue and shortness of breath. Chest imaging will enhance diagnostic accuracy, particularly when considered in conjunction with laboratory test results. MPA patients usually have low hematocrit levels and may actually have an increased diffusing capacity of the lung for carbon monoxide (Dlco).
Granulomatosis with polyangiitis. This form of vasculitis has characteristic nodules, cavitary lesions, and, in the worst cases, multifocal masses in the lungs. These can be identified with contrast-free CT, with examination for possible airway involvement.
Multiple lung cavity nodules and pronounced airway narrowing are significant diagnostic clues for GPA. Nodules up to 10 cm in diameter tend to be near sub-pleural and peripheral areas. Microbes and fungus may complicate the nodules’ primary presentation. While bronchoscopy may be helpful with imaging, surgical biopsy remains the gold standard to rule out infections.
The disease may be multifocal, occurring outside the lungs from the larynx to bronchi and anywhere in the lung. Subglottic stenosis caused by inflammation and scarring affects 16% of patients with GPA, but it also often develops independently of other features of GPA and may have its own course independent of systemic symptoms.1
Bronchoscopy
Bronchoscopy is a relatively low-risk way to assess airways and nodules, but it has had a limited role in the diagnosis of nonfocal interstitial lung disease and rheumatologic lung disease in general. New technologies that augment traditional bronchoscopy and enhance its utility for diagnosis for focal entities are described below.
Electromagnetic navigation bronchoscopy (ENB) uses electromagnetic technology to localize and guide a catheter through the bronchial pathways. With the help of a virtual, 3-dimensional bronchial map reconstructed from a chest CT, the clinician can navigate to a desired location within the lung for biopsy and diagnosis of pulmonary nodules. The result is a diagnostic yield per nodule of nearly 80%.2 Seijo et al showed that diagnostic yields by ENB increase with the presence of the bronchus sign, or a bronchus leading directly to a peripheral lung lesion, as viewed on CT imaging.2 If nodules are bronchocentric, or surround airways, there is greater likelihood of reaching a diagnosis without resorting to surgery.
In peripheral radial ultrasound, a catheter is threaded through another catheter sheath in order to visualize the lesion. This technology can precisely localize lung lesions and often give some clues about the final pathology.
Bronchoscopic confocal fluorescence microscopy3 is a new form of microscopy that uses a fiberoptic miniprobe instead of an objective lens. High-quality images are achieved by the use of autofluorescence. Researchers have used the technology to detect changes in the respiratory bronchioles and other structures, but a clear atlas of many disease states does not yet exist. Oddly, endobronchial GPA images have been catalogued.3
Virtual bronchoscopy is a 3-dimensional image reconstruction and display technique that converts standard CT images into multiplanar images, which can be stacked. Virtual bronchoscopy augments conventional CT because of its ability to enhance detection in the subglottic region and more accurately measure stenosis.4 The technique cannot replace traditional bronchoscopy, however, because mucus and secretions can appear as abnormalities and cause false-positive results.
Detecting mimics
Diagnoses that masquerade as EGPA include chronic eosinophilic pneumonia, bronchiolitis obliterans with organizing pneumonia, and other interstitial lung diseases. Allergic bronchopulmonary aspergillosis—an asthma syndrome sometimes associated with eosinophilia and high immunoglobulin-E levels—also mimics EGPA. This diagnostic possibility is particularly relevant if the patient is taking immunosuppressive agents or corticosteroids.
Although alveolar hemorrhage is the sole pulmonary manifestation of MPA, the diagnosis is not limited to MPA alone. Alveolar hemorrhage may have other causes, including infection or acute respiratory distress syndrome. Bronchial lavage is recommended for accurate diagnosis, with the introduction of successive volumes of saline into the lungs and examination for increasing amounts of heme in each of the aliquots of alveolar lavage fluid.
Several diagnoses can mimic GPA. Many infections, including those caused by mycobacteria and Cryptococcus, can mimic endobronchial GPA. Biopsy of all new ulcers is recommended to minimize the possibility of missing these diagnoses. Tuberculosis in its latent form can closely resemble scarred GPA. Other mimickers of cavitary lung lesions can include metastatic melanoma, metastatic renal and thyroid cancers, squamous cell carcinoma, and rheumatoid arthritis with necrobiotic nodules that open in the lungs.
TREATMENT STRATEGIES
Medications
Although many patients with GPA are surgical candidates because of dyspnea related to fixed endobronchial or endotracheal obstructions, any surgical treatment carries the risk of inciting further flares. Treatment should focus first on mitigating the systemic inflammatory disorder with pharmacologic intervention. Standard pharmacologic therapy includes corticosteroids, azathioprine, cyclophosphamide, and rituximab. Patients with subglottic stenosis are frequently unresponsive to standard immunosuppressive therapy (glucocorticoids in combination with a cytotoxic agent).1
Surgical reconstruction
When medication falls short and surgery is needed to reverse strictures, a number of tools are at our disposal. Some involve heat, such as laser, cauterization, and argon plasma coagulation. In argon plasma coagulation, a jet of ionized argon gas (plasma) is directed through a probe passed through an endoscope. Other techniques rely on cold: cryoprobes, microdebriders, and rigid scissors. In general, freeze therapies cause less scarring than heat therapy. With any surgical technique, there is risk of scars that will contract and cause structural collapse, resulting in restenosis.
Dilation
The high rate of stenosis relapse has spurred interest in alternatives to surgical treatment. One of these, dilation via endoscopy, also may mitigate the wound healing process. Other techniques for clearing the obstructed area include rigid bronchoscopy, the use of bougies (increasingly larger dilators), and balloon dilation. Balloon dilation has some advantages over the other techniques. It permits maximal radial direction and pressure, causes less damage to trachea wall mucosa, and achieves better overall results; however, the procedure usually needs to be repeated.5 It must be done quickly, and it requires flawless communication between the otolaryngologist or pulmonologist and anesthesiologist in order to stabilize the airway below the vocal cords.
Intratracheal dilation-injection therapy
Dilation can be augmented with glucocorticoid injections. In 1991, researchers at the National Institutes of Health utilized a combination dilation-injection therapy for 20 patients who had GPA and subglottic stenosis.1 Patients were first treated with mercury-filled dilators coated with 1% triamcinolone cream. Methylprednisolone acetate was then injected into the stenotic area. None of the patients treated with intratracheal dilation-injection therapy required a tracheostomy and six who already had tracheostomies were decannulated. In contrast, 56% of patients who received standard immunosuppressive therapy and no intratracheal dilation-injection therapy required tracheostomy. Intratracheal dilation-injection therapy is considered a safe and effective treatment of GPA-associated subglottic stenosis and, in the absence of major organ disease activity, could be used without systemic immunosuppressive agents.
Mitomycin-C is a controversial alternative to corticosteroids during dilation. Mitomycin-C is an alkylating agent that inhibits fibroblast proliferation and extracellular matrix protein synthesis, with the potential for reduced scarring. In a recent trial of 26 patients, two doses given 3 to 4 weeks apart reduced the rate of stenosis for 2 to 3 years compared with a single dose.6 Restenosis occurred in both groups, however, and after 5 years, the relapse rates were the same.
Nd:YAG laser photoresection versus endobronchial electrosurgery
One of the most effective therapies for treating obstructive lesions is Nd:YAG laser photoresection (LPR) in which a laser that utilizes the crystal neodymium-doped yttrium aluminum garnet (Nd:Y3Al5O12) is paired with a flexible bronchoscope. The procedure can produce favorable outcomes,7 but it has not gained favor because of perceptions that the lasers require rigid bronchoscopy, expensive equipment, and special training. There are also concerns about complications.
The lower-cost endobronchial electrosurgery (EBES) also failed to gain acceptance because of cumbersome delivery systems and complications associated with power units. Recently, engineers have spawned a new generation of electrosurgical devices, prompting renewed interest in EBES.
A recent study compared LPR and EBES in patients who represented 118 evaluations for LPR.8 Forty percent were considered amenable to EBES and so did not go on to receive the more costly LPR. Of those, 89% achieved success in alleviating the obstruction. The authors concluded that EBES can potentially eliminate the need for LPR in 36% of procedures, and that it could achieve significant savings in cost and time. We use these ablative therapies only in dire circumstances; we use non–heat-based therapies, including repeated dilation, prior to considering use of other therapies.
Cryotherapy
Cryotherapy spray was initially thought to have great therapeutic potential, but the high pressures of the spray caused complications. This modality remains under investigation, however. Some probe-based cryotherapy techniques have been effective anecdotally. These use a metal-tipped probe attached to a cryogen; the Joule-Thompson effect causes delayed tissue destruction.
Stents
A small number of case reports note patient improvement after stenting.9,10 We use stents in rare circumstances, but because complications are frequent and sometimes severe, we consider stenting a last-resort option. In 2005, the US Food and Drug Administration mandated a Black Box warning against the use of metallic stents in patients who have benign tracheal strictures.
Multimodality therapies
In general, when intervention is required to salvage airways, a combination of dilation and steroid injection with or without topical mitomycin-C is standard. We try to avoid use of thermal therapy with laser or electrocautery because of the risk of exuberant inflammation and restenosis from thermal injury. No specific standard of care exists in these cases; reliance on clinical judgment is critical because of the presentation and variety of airway lesions. Further, no large-scale randomized trials exist to guide therapy, so it is best to work with a multidisciplinary team whose members have experience in managing these complex patients.
CONCLUSION
The differential diagnosis of pulmonary manifestations of small-vessel vasculitis is complex. Several diagnoses can mimic various forms of pulmonary vasculitis, and the manifestations and symptoms often overlap with other organ systems.
Imaging is useful for analysis of common patterns of small and midsize vasculitis, although the results may be confounded by disorders that mimic pulmonary vasculitis. To enhance diagnostic accuracy, laboratory and clinical findings should be considered along with images. Ideally, treatment will be minimally destructive and mucosa-sparing. Dilation therapies can be augmented with corticosteroid injections or, possibly, mitomycin-C.
- Langford CA, Sneller MC, Hallahan CW, et al. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Arthritis Rheum 1996; 39:1754–1760.
- Seijo LM, de Torres JP, Lozano MD, et al. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a bronchus sign on CT imaging: results from a prospective study. Chest 2010; 138:1316–1321.
- Filner JJ, Bonura EJ, Lau ST, et al. Bronchoscopic fibered confocal fluorescence microscopy image characteristics and pathologic correlations. J Bronchol Intervent Pulmonol 2011; 18:23–30.
- Summers RM, Aggarwal NR, Sneller MC, et al. CT virtual bronchoscopy of the central airway in patients with Wegener’s granulomatosis. Chest 2002; 121:242–250.
- Schokkenbroek AA, Franssen CFM, Dikkers FG. Dilatation tracheoscopy for laryngeal and tracheal stenosis in patients with Wegener’s granulomatosis. Eur Arch Otorhinolaryngol 2008; 265:549–555.
- Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope 2009; 119:272–283.
- Shvero J, Shitrit D, Koren R, Shalomi D, Kramer MR. Endoscopic laser surgery for subglottic stenosis in Wegener’s granulomatosis. Yonsei Med J 2007; 48:748–753.
- Coulter TD, Mehta AC. The heat is on: impact of endobronchial electrosurgery on the need for Nd-YAG laser photoresection. Chest 2000; 118:516–521.
- Tierman J, Shah C, Elborn JS. Successful stenting in endobronchial Wegener’s granulomatosis. Ulster Med J 2006; 75:155–157.
- Daum TE, Specks U, Colby TV, et al. Tracheobronchial involvement in Wegener’s granulomatosis. Am J Respir Crit Care Med 1995; 151:522–526.
- Langford CA, Sneller MC, Hallahan CW, et al. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Arthritis Rheum 1996; 39:1754–1760.
- Seijo LM, de Torres JP, Lozano MD, et al. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a bronchus sign on CT imaging: results from a prospective study. Chest 2010; 138:1316–1321.
- Filner JJ, Bonura EJ, Lau ST, et al. Bronchoscopic fibered confocal fluorescence microscopy image characteristics and pathologic correlations. J Bronchol Intervent Pulmonol 2011; 18:23–30.
- Summers RM, Aggarwal NR, Sneller MC, et al. CT virtual bronchoscopy of the central airway in patients with Wegener’s granulomatosis. Chest 2002; 121:242–250.
- Schokkenbroek AA, Franssen CFM, Dikkers FG. Dilatation tracheoscopy for laryngeal and tracheal stenosis in patients with Wegener’s granulomatosis. Eur Arch Otorhinolaryngol 2008; 265:549–555.
- Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope 2009; 119:272–283.
- Shvero J, Shitrit D, Koren R, Shalomi D, Kramer MR. Endoscopic laser surgery for subglottic stenosis in Wegener’s granulomatosis. Yonsei Med J 2007; 48:748–753.
- Coulter TD, Mehta AC. The heat is on: impact of endobronchial electrosurgery on the need for Nd-YAG laser photoresection. Chest 2000; 118:516–521.
- Tierman J, Shah C, Elborn JS. Successful stenting in endobronchial Wegener’s granulomatosis. Ulster Med J 2006; 75:155–157.
- Daum TE, Specks U, Colby TV, et al. Tracheobronchial involvement in Wegener’s granulomatosis. Am J Respir Crit Care Med 1995; 151:522–526.
Bronchial thermoplasty: A new treatment for severe refractory asthma
Asthma now has a new treatment, but it isn’t for everybody. Called bronchial thermoplasty, it is reserved for patients whose asthma is severe and refractory, as it involves three sessions of bronchoscopy, each lasting up to 1 hour, during which the smooth muscle layer is methodically ablated from the airway using radiofrequency energy.1,2
The US Food and Drug Administration (FDA) has approved bronchial thermoplasty,3 and although it does not cure asthma or completely eliminate its symptoms, patients with severe asthma that was not well controlled with medical therapy who underwent this procedure in clinical trials subsequently had fewer symptoms, enjoyed better quality of life, and needed less intensive health care (such as emergency room visits) than patients who did not undergo the procedure.4–6
Here, we present an overview of the pathophysiology of severe refractory asthma and the clinical trials of bronchial thermoplasty, its current protocols, and the status of this new treatment.
WHAT IS SEVERE REFRACTORY ASTHMA?
Asthma is a chronic inflammatory condition of the airways characterized by episodic symptoms of breathlessness, cough, and wheezing, which can wax and wane over time. Approximately 8.2% of the general population is affected.7
Our understanding of the pathophysiology of asthma has improved over the past 20 years, and with the publication of clinical guidelines from the National Asthma Education and Prevention Program in 1991,8 1997,9 and 2002,10 outcomes have improved. Most people with asthma can control their symptoms if they adhere to anti-inflammatory therapies and avoid triggers. Yet 5% to 10% of asthma patients have severe refractory disease, and asthma accounts for nearly half a million hospitalizations every year.11
The latest guidelines, published in 2007, emphasize the importance of assessing the severity of asthma, including the patient’s impairment (symptoms and limitations) and risk (likelihood of exacerbations).12
Workshop consensus definition of severe refractory asthma
A consensus group convened by the American Thoracic Society13 defined asthma as severe and refractory if the patient meets at least one of the following major criteria:
- Takes oral corticosteroids continuously or nearly continuously (> 50% of year)
- Takes high-dose inhaled corticosteroids.
In addition, the patient must meet at least two minor criteria, ie:
- Takes a controller medication such as a long-acting beta-agonist, theophylline, or a leukotriene antagonist every day
- Takes a short-acting beta agonist every day or nearly every day
- Has persistent airway obstruction, ie, a forced expiratory volume in 1 second (FEV1) less than 80% of predicted, or a peak expiratory flow that has a diurnal variability greater than 20%
- Has one or more urgent care visits for asthma per year
- Needs three or more oral corticosteroid “bursts” per year
- Has prompt deterioration when the dose of oral or inhaled corticosteroid is reduced by 25% or less
- Has had a near-fatal asthma event in the past.
Compared with people with mild asthma, people who have severe refractory asthma tend to be older, have fewer allergies, and make more use of intensive and urgent health care.14
Asthma is due to both inflammation and bronchoconstriction
The pathophysiology of asthma involves both chronic airway inflammation and bronchoconstriction, the latter characterized by a greater response to methacholine. Histologic findings include excessive mucus secretion, epithelial cell injury, and smooth muscle hypertrophy. These changes can lead to persistent airflow obstruction that can be difficult to control with medical therapies.12
Bronchoconstriction can be reversed temporarily with bronchodilators, but no longlasting therapy to reduce it has been available until now. Bronchial thermoplasty targets this gap in asthma management.
STUDIES OF BRONCHIAL THERMOPLASTY
Radiofrequency ablation has been used to treat other medical conditions such as lung cancer and cardiac arrhythmias.15,16 Its use to treat asthma by eradicating smooth muscle cells from the airway wall began with studies in animals.1 Later, studies were done in people without asthma,2 then in patients with mild to moderate asthma,17 and finally in patients with moderate to severe refractory asthma.4–6
These studies helped clarify which type of patients would be appropriate candidates and the outcomes to be anticipated, including adverse events.
Early studies
Danek et al,1 in a study in nonasthmatic dogs, found that thermoplasty at 65°C or 75°C (149°F or 167°F) attenuated the airway’s response to methacholine up to 3 years after treatment. As early as 1 week after treatment, airway smooth muscle was seen to be degenerating or absent, and the effect was inversely proportional to airway responsiveness.
Adverse effects of the procedure were cough, inflammatory edema of the airway wall, retained mucus, and blanching of the airway wall at the site of catheter contact. Three years later, there was no evidence of smooth muscle regeneration.
Miller et al2 next performed a feasibility study in eight patients, mean age 58 ± 8.3 years, who were scheduled to undergo lung resection for lung cancer. Five to 20 days before surgery, the investigators performed thermoplasty at 55°C or 65°C (131°F or 149°F) in three to nine sites per patient, 1 cm from known tumors but within areas to be resected. There were no significant adverse events such as hemoptysis, respiratory infections, or excess bronchial irritation.
A pilot study in mild to moderate asthma
Cox et al17 performed the first study of bronchial thermoplasty in patients with mild to moderate asthma. This was a prospective observational study in 16 patients who were younger than the patients in the previous study, with an average age of 30 years (range 24–58). They were given prednisone 30 or 50 mg the day before the procedure and on the day of the procedure. Three treatments were done, 3 weeks apart. The right middle lobe was not treated because the bronchus leading to it is relatively long and narrow, raising concern about damaging it.18
Results. The most frequent side effects were symptoms of airway irritation such as cough, dyspnea, wheezing, and bronchospasm. The mean time to onset was less than 1.7 days, and the mean time to resolution was 4.6 days after the most recent procedure. None of the patients needed to be hospitalized in the immediate postprocedure period.
In the 2 years after the procedure, there were 312 adverse events, mainly mild. Three (1%) of the adverse events were reported as severe, but they were deemed not related to the procedure. Yearly computed tomographic scans of the chest showed no structural changes such as bronchiectasis in the parenchyma or bronchial wall.
The FEV1 was higher at 12 weeks and at 1 year after thermoplasty than at baseline but was not significantly different from baseline at 2 years.
At baseline, the patients reported that 50% of their days were symptom-free; this increased to 73% at 12 weeks (P = .015).
In addition, airway hyperresponsiveness decreased significantly, and the effect persisted over 2 years. The provocative concentration of methacholine that caused a 20% reduction in FEV1 (the PC20) was:
- 0.92 mg/mL at baseline (95% confidence interval 0.42–1.99)
- 4.75 mg/mL at 12 weeks (2.51–8.85)
- 5.45 mg/mL at 1 year (1.54–19.32)
- 3.40 mg/mL at 2 years (1.35–8.52).
Limitations of this study include the relatively small number of patients enrolled and their relatively stable asthma.
The AIR trial: A randomized trial in moderate or persistent asthma
The first large multicenter trial of bronchial thermoplasty, the Asthma Intervention Research (AIR) trial,4 was prospective and randomized but not blinded. The aim was to determine whether bronchial thermoplasty would improve asthma control after long-acting beta agonists were discontinued.
Patients could be enrolled if they were 18 to 65 years old, had moderate or persistent asthma, and needed to take an inhaled corticosteroid (beclomethasone [Qvar] 200 μg or more or an equivalent drug) and a long-acting beta agonist (salmeterol [Serevent] 100 μg or more or an equivalent drug) every day. They also needed to have FEV1 values of 60% to 85% of predicted and airway reactivity (PC20 < 8 mg/mL), and their asthma had to have been stable for 6 weeks.
At baseline, the long-acting beta agonist was withdrawn temporarily; the final criterion for entry was that their asthma had to become worse when this was done.
Then, 112 patients were randomized to receive either bronchial thermoplasty with medical care (inhaled corticosteroids and long-acting beta agonists) or usual care, ie, medical therapy alone. Treatments were done in three sessions over 9 weeks, followed by attempts to discontinue their long-acting beta agonists at 3, 6, and 9 months after the procedure without exacerbations.
An exacerbation was defined as at least one of the following for 2 consecutive days: a reduction of peak flow by 20% of baseline average, the need for more than three additional puffs of rescue inhaler, or nocturnal awakenings caused by asthma symptoms. The patients kept a daily diary of their symptoms and rescue inhaler use, and they completed the Asthma Quality of Life Questionnaire (AQLQ) and the Asthma Control Questionnaire (ACQ).
Results. The number of mild (but not severe) exacerbations per week was significantly lower at 3 and 12 months than at baseline in the thermoplasty group, with 10 fewer mild exacerbations per patient per year, but was unchanged in the control group. There were significantly greater improvements in morning peak flow at 3, 6, and 12 months from baseline in the treatment group than in the usual-care group. Rescue medication use was also significantly less at 3 and 12 months. Symptom scores, AQLQ scores, and ACQ scores were all significantly better than at baseline as well.
Not surprisingly, in this cohort with unstable asthma, there were 407 adverse events in the treatment group and 106 adverse events in the control group. Most of these occurred within 1 day and resolved within 7 days after the procedure. There were more hospitalizations in the treatment group as well, for reasons that included exacerbations of asthma, collapse of the left lower lobe, and pleurisy.4
Therefore, this trial found that thermoplasty improved asthma symptoms within 3 months and that the effect lasted 1 year, with an encouraging reduction in the number of mild exacerbations. However, it was not blinded, and there is a strong placebo effect in asthma. Needed was a randomized trial in which the control group would undergo a sham treatment.
The RISA trial: A randomized trial in severe asthma
The Research in Severe Asthma (RISA) trial5 included patients with more severe asthma than those in the AIR trial. Entry criteria were:
- Taking high doses of an inhaled corticosteroid (> 750 μg of fluticasone or its equivalent per day)
- Taking prednisone (≤ 30 mg/day)
- An FEV1 of at least 50% of predicted without a bronchodilator
- A positive methacholine test.
Seventeen patients were randomized to undergo bronchial thermoplasty, and another 17 were randomized to receive medical treatment.
After a 2-week run-in period, the thermoplasty patients underwent three treatments, performed 3 weeks apart. For the next 16 weeks, the corticosteroid doses were kept stable in both groups, followed by a 14-week corticosteroid-weaning phase and then a 16-week reduced-corticosteroid phase. During this time, attempts were made to decrease the oral or inhaled corticosteroid doses according to a protocol (eg, a 20%–25% reduction every 2–4 weeks) unless there were mild exacerbations lasting more than 7 days.
Results. There were more adverse events in the thermoplasty group than in the medical management group in the treatment period, including seven hospitalizations for exacerbations of asthma and a partial collapse of the left lower lobe. There were no significant differences in adverse events between groups in the posttreatment period (up to 6 weeks after the last treatment). Forty-nine percent of the events were mild in each group; 10% of the events were severe in the thermoplasty group vs 4% in the control group.
During the steroid-stable phase, patients in the thermoplasty group used rescue inhalers significantly less than those in the control group, and their prebronchodilator FEV1 and AQLQ and ACQ scores were better. The differences in rescue inhaler use and questionnaire scores remained significant at 1 year.
Comment. As expected, serious adverse events occurred more often in patients with severe asthma in the treatment group than in the control group. However, 1 year after the procedure, the adverse-event rates were similar in the treatment and control groups, suggesting that this procedure can be safely performed in similar patient populations. Although there was significant potential for a placebo effect, these patients with severe persistent asthma showed significant improvement in clinical measures of asthma compared with the control group.
AIR2: A randomized, double-blind trial
The latest trial of this new therapy in severe asthma was the AIR2 trial.6 A major difference in its design compared with the earlier ones was that the control group underwent sham thermoplasty, allowing the trial to be truly double-blinded. (The bronchoscopy team knew which patients got which treatment, but the patients and the study physicians following them did not).
The primary outcome was the change in AQLQ score from baseline at 6, 9, and 12 months. Secondary outcomes included absolute changes in the asthma control scores, symptom scores, peak flows, rescue medication use, and FEV1.
The randomized groups (196 patients in the thermoplasty group and 101 in the sham treatment group) were well matched, and more than 80% in each group met the American Thoracic Society criteria for severe refractory asthma.
On the AQLQ, a change of more than 0.5 is considered clinically meaningful. Interestingly, there was a significant and clinically meaningful improvement in AQLQ in 64% of the sham treatment group, highlighting the placebo effect in asthma treatment.19 However, a larger proportion (79%) of the treated group had a clinically meaningful improvement on the AQLQ than in the sham treatment group.
Adverse events occurred in both groups; however, during the treatment phase, 16 patients in the bronchial thermoplasty group needed to be hospitalized for respiratory symptoms including worsening asthma, atelectasis, lower respiratory tract infections, decreased FEV1, and an aspirated tooth. One episode of hemoptysis required bronchial artery embolization. In contrast, only two patients in the sham treatment group needed hospitalization.
Therefore, this trial showed that patients with severe asthma treated with bronchial thermoplasty had a long-term improvement in quality of life and needed less health care.6
Translating these trials into practice
To summarize, these clinical trials showed that bronchial thermoplasty was feasible, was relatively safe, and produced better clinical outcomes in patients with severe asthma when medical therapies did not control their symptoms.
In practice, patient selection is likely to be important. A key question will be, Does the patient truly have severe refractory asthma, or is the patient not taking his or her medication? Adherence to therapy should be evaluated.
In addition, patients need to be observed and monitored closely during and after the treatment period, as airway complications and asthma exacerbations can occur up to 6 weeks after the last procedure. About 80% of all study patients had multiple symptoms of asthma and other symptoms in the treatment period. Rarely did these symptoms result in hospitalization, but they were more common in the treatment group in the AIR2 trial.
Long-term studies have evaluated the duration of effect and the safety of bronchial thermoplasty, and outcomes appear favorable.20,21
WHY DOES IT WORK?
The role of airway smooth muscle in asthma is yet to be fully elucidated. The trials outlined here showed that although asthma is a disease of the airways, including the small airways, treatment of airways 3 mm or larger improves asthma symptoms, quality of life, and health care utilization.6 Thus, the role of airway smooth muscle in asthma and as a target of therapy has not previously been fully realized.21
Early investigations into the mechanisms of airflow obstruction and airway resistance found that 75% of postnasal resistance occurs in the first six to eight generations (ie, branchings) of the airways, indicating that larger airways are involved.22 (The number of generations varies depending on the size of the person but it typically is 10 to 12.) Findings from the study in dogs introduced the idea that smooth muscle alterations contributed to the changes in airway resistance, and that subtle changes in airway smooth muscle could clinically benefit asthma patients.1
The speculated purpose of the airway smooth muscle layer is to support the airway, allow gas exchange, propel mucus for clearance, defend the airway, enhance cough, and promote lymphatic flow. However, the airway smooth muscle layer may also be vestigial. In asthma, airway smooth muscle adds to bronchoconstriction and hyperresponsiveness, and has a role in mediating inflammation and airway remodeling.21 No definitive studies have shown that eliminating airway smooth muscle greatly inhibits normal airway function.18
What exactly does thermoplasty do to the smooth muscle? Studies in smooth muscle from cows showed that high temperatures directly disrupt the actin-myosin interaction, likely through denaturation of motor proteins.23 This immediate loss of muscle cell function is not likely to be the result of apoptosis, autophagy, or necrosis, or mediated by heat-shock proteins, in view of the relatively quick muscle response and lack of progressive changes. Tissue responsiveness is substantially reduced a few seconds after application of 60°C of heat and is subsequently abolished within 5 minutes after treatment.23
The intervention appears to be dose-dependent. Responsiveness to cholinergic stimulation is lessened by treatment, and the desired effect is seen within seconds and does not progress.
Therefore, we can surmise that disruption of myosin function is likely the mechanism of the therapeutic effect, breaking the cascade of airway smooth muscle spasm. Now that we know about the airway smooth muscle as a possible target of therapy, and that it may play only a vestigial role, we can think about other therapies that focus on it.18,23
BRONCHIAL THERMOPLASTY PROTOCOLS
Patients are assessed before and on the day of the procedure to make sure their disease is stable (ie, their postbronchodilator FEV1 is within 15% of baseline values, and they have no evidence of asthma exacerbation or active infection), similar to the protocol used in the AIR2 trial,6 before proceeding with the treatment.
Patients are given 50 mg of prednisone 3 days before and again on the day of the procedure. Nebulized albuterol (2.5–5.0 mg) is given before the patients undergo screening spirometry and again before the procedure. If the preprocedure FEV1 is lower than 15% below baseline, we postpone the procedure to another day.
The procedure is performed with the patient under moderate conscious sedation, typically using fentanyl (Sublimaze), midazolam (Versed), and topical lidocaine in a monitored environment. The bronchoscope is inserted via either the mouth or nose, and supplemental oxygen is provided.
Thermoplasty is performed with the Alair system (Asthmatx, Inc., Sunnyvale, CA), which delivers a specific amount of radiofrequency (thermal) energy through a dedicated catheter. The catheter is deployed through a 2.0-mm channel of a flexible bronchoscope, starting in distal airways as small as 3 mm in diameter and working proximally to sequentially treat all airways to the mainstem lobar bronchi. The sites treated are meticulously recorded on a bronchial airway map to ensure that treatment sites are not skipped or overlapped (FIGURE 1).
An array of four electrodes is manually expanded to make contact with the airway walls; each electrode has 5 mm of exposed wire. As the energy is delivered, the control unit measures electrical resistance converted to thermal energy and turns off the current when an appropriate dosage is given. This thermal energy is what is responsible for altering the airway smooth muscle.
A full course of treatment requires three separate bronchoscopy sessions, each separated by 2 to 3 weeks. The left lower lobe and the right lower lobe are treated in separate procedures, and then both upper lobes are treated in a third procedure to minimize any respiratory symptoms. Each procedure usually requires 50 to 75 activations of the device and takes up to 60 minutes.
After each procedure the patient should be observed for 3 to 4 hours, and spirometry should be repeated to make sure the FEV1 (percent predicted) is within 20% of the baseline value. An additional 50-mg dose of prednisone is prescribed for the day after the procedure.24
FDA CLEARANCE AND LONG-TERM FOLLOW-UP
The FDA approved the Alair device for treating severe refractory asthma in early 2010.3 The indications for it are based on the study populations in the published trials. Patients can be evaluated for this treatment if they have well-documented severe persistent asthma not well controlled on inhaled corticosteroids and long-acting beta agonists and have no significant contraindications to bronchoscopy.
As part of the conditions of approval, the FDA required a postapproval study based on the long-term follow-up of the AIR2 trial. They specifically wanted to compare patients who have desirable long-term outcomes and those in whom any treatment effect wanes with time. Since we have only a few years of follow-up data, we still do not know all the possible late effects of the treatment; we have an opportunity to learn more.
Another question that needs to be studied is whether thermoplasty will help other forms of bronchospastic lung disease, such as chronic obstructive pulmonary disease.
A second postapproval study will be a prospective, open-label, single-arm, multicenter study conducted in the United States to assess the treatment effect and short-term and long-term safety profile of thermoplasty in asthma.
As experience with the procedure increases, we will be better able to characterize which patients may benefit from it. In addition, the knowledge gained by the longer-term study of airway smooth muscle function alterations will potentially drive the discovery of other innovative therapies for severe asthma.
- Danek CJ, Lombard CM, Dungworth DL, et al. Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. J Appl Physiol 2004; 97:1946–1953.
- Miller JD, Cox G, Vincic L, Lombard CM, Loomas BE, Danek CJ. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest 2005; 127:1999–2006.
- US Food and Drug Administration (FDA). Approval of Alair Bronchial Thermoplasty System: Alair Catheter and Alair RF Controller. 2010. www.accessdata.fda.gov/cdrh_docs/pdf8/P080032a.pdf. Accessed June 1, 2011.
- Cox G, Thomson NC, Rubin AS, et al; AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med 2007; 356:1327–1337.
- Pavord ID, Cox G, Thomson NC, et al; RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med 2007; 176:1185–1191.
- Castro M, Rubin AS, Laviolette M, et al; AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010; 181:116–124.
- Centers for Disease Control and Prevention. Vital signs: asthma prevalence, disease characteristics, and self-management education—United States, 2001–2009. MMWR Morb Mortal Wkly Rep 2011; 60( 17):547–552.
- Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute. National Asthma Education Program. Expert Panel Report. J Allergy Clin Immunol 1991; 88:425–534.
- US Department of Health and Human Services. Expert panel report 2 (EPR-2): Guidelines for the diagnosis and management of asthma, 1997. www.nhlbi.nih.gov/guidelines/archives/epr-2/index.htm. Accessed June 1, 2011.
- US Department of Health and Human Services. Expert panel report: Guidelines for the diagnosis and management of asthma—Update on selected topics 2002. www.nhlbi.nih.gov/guidelines/archives/epr-2_upd/index.htm. Accessed June 1, 2011.
- Akinbami L. Asthma prevalence, health care use and mortality: United States 2003–05, CDC National Center for Health Statistics, 2006. www.cdc.gov/nchs/data/hestat/asthma03-05/asthma03-05.htm. Accessed June 1, 2011.
- US Department of Health and Human Services. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma full report, 2007. www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed June 1, 2011.
- Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society Am J Respir Crit Care Med 2000; 162:2341–2351.
- Moore WC, Bleecker ER, Curran-Everett D, et al; National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol 2007; 119:405–413.
- Ambrogi MC, Fanucchi O, Lencioni R, Cioni R, Mussi A. Pulmonary radiofrequency ablation in a single lung patient. Thorax 2006; 61:828–829.
- Benussi S, Cini R, Gaynor SL, Alfieri O, Calafiore AM. Bipolar radiofrequency maze procedure through a transseptal approach. Ann Thorac Surg 2010; 90:1025–1027.
- Cox G, Miller JD, McWilliams A, Fitzgerald JM, Lam S. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med 2006; 173:965–969.
- Cox PG, Miller J, Mitzner W, Leff AR. Radiofrequency ablation of airway smooth muscle for sustained treatment of asthma: preliminary investigations. Eur Respir J 2004; 24:659–663.
- Wise RA, Bartlett SJ, Brown ED, et al; American Lung Association Asthma Clinical Research Centers. Randomized trial of the effect of drug presentation on asthma outcomes: the American Lung Association Asthma Clinical Research Centers. J Allergy Clin Immunol 2009; 124:436–444.
- Castro M, Rubin A, Laviolette M, Hanania NA, Armstrong B, Cox G; AIR2 Trial Study Group. Persistence of effectiveness of bronchial thermoplasty in patients with severe asthma. Ann Allergy Asthma Immunol 2011. doi: 10.1016/j.anai.2011.03.005.
- Thomson NC, Rubin AS, Niven RM, et al; AIR Trial Study Group. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med 2011; 11:8.
- Solway J, Irvin CG. Airway smooth muscle as a target for asthma therapy. N Engl J Med 2007; 356:1367–1369.
- Ingram RH, McFadden ER. Localization and mechanisms of airway responses. N Engl J Med 1977; 297:596–600.
- Dyrda P, Tazzeo T, DoHarris L, et al. Acute response of airway muscle to extreme temperature includes disruption of actin-myosin interaction. Am J Respir Cell Mol Biol 2011; 44:213–221.
- Mayse ML, Laviolette M, Rubin AS, et al. Clinical pearls for bronchial thermoplasty. J Bronchol 2007; 14:115–123.
Asthma now has a new treatment, but it isn’t for everybody. Called bronchial thermoplasty, it is reserved for patients whose asthma is severe and refractory, as it involves three sessions of bronchoscopy, each lasting up to 1 hour, during which the smooth muscle layer is methodically ablated from the airway using radiofrequency energy.1,2
The US Food and Drug Administration (FDA) has approved bronchial thermoplasty,3 and although it does not cure asthma or completely eliminate its symptoms, patients with severe asthma that was not well controlled with medical therapy who underwent this procedure in clinical trials subsequently had fewer symptoms, enjoyed better quality of life, and needed less intensive health care (such as emergency room visits) than patients who did not undergo the procedure.4–6
Here, we present an overview of the pathophysiology of severe refractory asthma and the clinical trials of bronchial thermoplasty, its current protocols, and the status of this new treatment.
WHAT IS SEVERE REFRACTORY ASTHMA?
Asthma is a chronic inflammatory condition of the airways characterized by episodic symptoms of breathlessness, cough, and wheezing, which can wax and wane over time. Approximately 8.2% of the general population is affected.7
Our understanding of the pathophysiology of asthma has improved over the past 20 years, and with the publication of clinical guidelines from the National Asthma Education and Prevention Program in 1991,8 1997,9 and 2002,10 outcomes have improved. Most people with asthma can control their symptoms if they adhere to anti-inflammatory therapies and avoid triggers. Yet 5% to 10% of asthma patients have severe refractory disease, and asthma accounts for nearly half a million hospitalizations every year.11
The latest guidelines, published in 2007, emphasize the importance of assessing the severity of asthma, including the patient’s impairment (symptoms and limitations) and risk (likelihood of exacerbations).12
Workshop consensus definition of severe refractory asthma
A consensus group convened by the American Thoracic Society13 defined asthma as severe and refractory if the patient meets at least one of the following major criteria:
- Takes oral corticosteroids continuously or nearly continuously (> 50% of year)
- Takes high-dose inhaled corticosteroids.
In addition, the patient must meet at least two minor criteria, ie:
- Takes a controller medication such as a long-acting beta-agonist, theophylline, or a leukotriene antagonist every day
- Takes a short-acting beta agonist every day or nearly every day
- Has persistent airway obstruction, ie, a forced expiratory volume in 1 second (FEV1) less than 80% of predicted, or a peak expiratory flow that has a diurnal variability greater than 20%
- Has one or more urgent care visits for asthma per year
- Needs three or more oral corticosteroid “bursts” per year
- Has prompt deterioration when the dose of oral or inhaled corticosteroid is reduced by 25% or less
- Has had a near-fatal asthma event in the past.
Compared with people with mild asthma, people who have severe refractory asthma tend to be older, have fewer allergies, and make more use of intensive and urgent health care.14
Asthma is due to both inflammation and bronchoconstriction
The pathophysiology of asthma involves both chronic airway inflammation and bronchoconstriction, the latter characterized by a greater response to methacholine. Histologic findings include excessive mucus secretion, epithelial cell injury, and smooth muscle hypertrophy. These changes can lead to persistent airflow obstruction that can be difficult to control with medical therapies.12
Bronchoconstriction can be reversed temporarily with bronchodilators, but no longlasting therapy to reduce it has been available until now. Bronchial thermoplasty targets this gap in asthma management.
STUDIES OF BRONCHIAL THERMOPLASTY
Radiofrequency ablation has been used to treat other medical conditions such as lung cancer and cardiac arrhythmias.15,16 Its use to treat asthma by eradicating smooth muscle cells from the airway wall began with studies in animals.1 Later, studies were done in people without asthma,2 then in patients with mild to moderate asthma,17 and finally in patients with moderate to severe refractory asthma.4–6
These studies helped clarify which type of patients would be appropriate candidates and the outcomes to be anticipated, including adverse events.
Early studies
Danek et al,1 in a study in nonasthmatic dogs, found that thermoplasty at 65°C or 75°C (149°F or 167°F) attenuated the airway’s response to methacholine up to 3 years after treatment. As early as 1 week after treatment, airway smooth muscle was seen to be degenerating or absent, and the effect was inversely proportional to airway responsiveness.
Adverse effects of the procedure were cough, inflammatory edema of the airway wall, retained mucus, and blanching of the airway wall at the site of catheter contact. Three years later, there was no evidence of smooth muscle regeneration.
Miller et al2 next performed a feasibility study in eight patients, mean age 58 ± 8.3 years, who were scheduled to undergo lung resection for lung cancer. Five to 20 days before surgery, the investigators performed thermoplasty at 55°C or 65°C (131°F or 149°F) in three to nine sites per patient, 1 cm from known tumors but within areas to be resected. There were no significant adverse events such as hemoptysis, respiratory infections, or excess bronchial irritation.
A pilot study in mild to moderate asthma
Cox et al17 performed the first study of bronchial thermoplasty in patients with mild to moderate asthma. This was a prospective observational study in 16 patients who were younger than the patients in the previous study, with an average age of 30 years (range 24–58). They were given prednisone 30 or 50 mg the day before the procedure and on the day of the procedure. Three treatments were done, 3 weeks apart. The right middle lobe was not treated because the bronchus leading to it is relatively long and narrow, raising concern about damaging it.18
Results. The most frequent side effects were symptoms of airway irritation such as cough, dyspnea, wheezing, and bronchospasm. The mean time to onset was less than 1.7 days, and the mean time to resolution was 4.6 days after the most recent procedure. None of the patients needed to be hospitalized in the immediate postprocedure period.
In the 2 years after the procedure, there were 312 adverse events, mainly mild. Three (1%) of the adverse events were reported as severe, but they were deemed not related to the procedure. Yearly computed tomographic scans of the chest showed no structural changes such as bronchiectasis in the parenchyma or bronchial wall.
The FEV1 was higher at 12 weeks and at 1 year after thermoplasty than at baseline but was not significantly different from baseline at 2 years.
At baseline, the patients reported that 50% of their days were symptom-free; this increased to 73% at 12 weeks (P = .015).
In addition, airway hyperresponsiveness decreased significantly, and the effect persisted over 2 years. The provocative concentration of methacholine that caused a 20% reduction in FEV1 (the PC20) was:
- 0.92 mg/mL at baseline (95% confidence interval 0.42–1.99)
- 4.75 mg/mL at 12 weeks (2.51–8.85)
- 5.45 mg/mL at 1 year (1.54–19.32)
- 3.40 mg/mL at 2 years (1.35–8.52).
Limitations of this study include the relatively small number of patients enrolled and their relatively stable asthma.
The AIR trial: A randomized trial in moderate or persistent asthma
The first large multicenter trial of bronchial thermoplasty, the Asthma Intervention Research (AIR) trial,4 was prospective and randomized but not blinded. The aim was to determine whether bronchial thermoplasty would improve asthma control after long-acting beta agonists were discontinued.
Patients could be enrolled if they were 18 to 65 years old, had moderate or persistent asthma, and needed to take an inhaled corticosteroid (beclomethasone [Qvar] 200 μg or more or an equivalent drug) and a long-acting beta agonist (salmeterol [Serevent] 100 μg or more or an equivalent drug) every day. They also needed to have FEV1 values of 60% to 85% of predicted and airway reactivity (PC20 < 8 mg/mL), and their asthma had to have been stable for 6 weeks.
At baseline, the long-acting beta agonist was withdrawn temporarily; the final criterion for entry was that their asthma had to become worse when this was done.
Then, 112 patients were randomized to receive either bronchial thermoplasty with medical care (inhaled corticosteroids and long-acting beta agonists) or usual care, ie, medical therapy alone. Treatments were done in three sessions over 9 weeks, followed by attempts to discontinue their long-acting beta agonists at 3, 6, and 9 months after the procedure without exacerbations.
An exacerbation was defined as at least one of the following for 2 consecutive days: a reduction of peak flow by 20% of baseline average, the need for more than three additional puffs of rescue inhaler, or nocturnal awakenings caused by asthma symptoms. The patients kept a daily diary of their symptoms and rescue inhaler use, and they completed the Asthma Quality of Life Questionnaire (AQLQ) and the Asthma Control Questionnaire (ACQ).
Results. The number of mild (but not severe) exacerbations per week was significantly lower at 3 and 12 months than at baseline in the thermoplasty group, with 10 fewer mild exacerbations per patient per year, but was unchanged in the control group. There were significantly greater improvements in morning peak flow at 3, 6, and 12 months from baseline in the treatment group than in the usual-care group. Rescue medication use was also significantly less at 3 and 12 months. Symptom scores, AQLQ scores, and ACQ scores were all significantly better than at baseline as well.
Not surprisingly, in this cohort with unstable asthma, there were 407 adverse events in the treatment group and 106 adverse events in the control group. Most of these occurred within 1 day and resolved within 7 days after the procedure. There were more hospitalizations in the treatment group as well, for reasons that included exacerbations of asthma, collapse of the left lower lobe, and pleurisy.4
Therefore, this trial found that thermoplasty improved asthma symptoms within 3 months and that the effect lasted 1 year, with an encouraging reduction in the number of mild exacerbations. However, it was not blinded, and there is a strong placebo effect in asthma. Needed was a randomized trial in which the control group would undergo a sham treatment.
The RISA trial: A randomized trial in severe asthma
The Research in Severe Asthma (RISA) trial5 included patients with more severe asthma than those in the AIR trial. Entry criteria were:
- Taking high doses of an inhaled corticosteroid (> 750 μg of fluticasone or its equivalent per day)
- Taking prednisone (≤ 30 mg/day)
- An FEV1 of at least 50% of predicted without a bronchodilator
- A positive methacholine test.
Seventeen patients were randomized to undergo bronchial thermoplasty, and another 17 were randomized to receive medical treatment.
After a 2-week run-in period, the thermoplasty patients underwent three treatments, performed 3 weeks apart. For the next 16 weeks, the corticosteroid doses were kept stable in both groups, followed by a 14-week corticosteroid-weaning phase and then a 16-week reduced-corticosteroid phase. During this time, attempts were made to decrease the oral or inhaled corticosteroid doses according to a protocol (eg, a 20%–25% reduction every 2–4 weeks) unless there were mild exacerbations lasting more than 7 days.
Results. There were more adverse events in the thermoplasty group than in the medical management group in the treatment period, including seven hospitalizations for exacerbations of asthma and a partial collapse of the left lower lobe. There were no significant differences in adverse events between groups in the posttreatment period (up to 6 weeks after the last treatment). Forty-nine percent of the events were mild in each group; 10% of the events were severe in the thermoplasty group vs 4% in the control group.
During the steroid-stable phase, patients in the thermoplasty group used rescue inhalers significantly less than those in the control group, and their prebronchodilator FEV1 and AQLQ and ACQ scores were better. The differences in rescue inhaler use and questionnaire scores remained significant at 1 year.
Comment. As expected, serious adverse events occurred more often in patients with severe asthma in the treatment group than in the control group. However, 1 year after the procedure, the adverse-event rates were similar in the treatment and control groups, suggesting that this procedure can be safely performed in similar patient populations. Although there was significant potential for a placebo effect, these patients with severe persistent asthma showed significant improvement in clinical measures of asthma compared with the control group.
AIR2: A randomized, double-blind trial
The latest trial of this new therapy in severe asthma was the AIR2 trial.6 A major difference in its design compared with the earlier ones was that the control group underwent sham thermoplasty, allowing the trial to be truly double-blinded. (The bronchoscopy team knew which patients got which treatment, but the patients and the study physicians following them did not).
The primary outcome was the change in AQLQ score from baseline at 6, 9, and 12 months. Secondary outcomes included absolute changes in the asthma control scores, symptom scores, peak flows, rescue medication use, and FEV1.
The randomized groups (196 patients in the thermoplasty group and 101 in the sham treatment group) were well matched, and more than 80% in each group met the American Thoracic Society criteria for severe refractory asthma.
On the AQLQ, a change of more than 0.5 is considered clinically meaningful. Interestingly, there was a significant and clinically meaningful improvement in AQLQ in 64% of the sham treatment group, highlighting the placebo effect in asthma treatment.19 However, a larger proportion (79%) of the treated group had a clinically meaningful improvement on the AQLQ than in the sham treatment group.
Adverse events occurred in both groups; however, during the treatment phase, 16 patients in the bronchial thermoplasty group needed to be hospitalized for respiratory symptoms including worsening asthma, atelectasis, lower respiratory tract infections, decreased FEV1, and an aspirated tooth. One episode of hemoptysis required bronchial artery embolization. In contrast, only two patients in the sham treatment group needed hospitalization.
Therefore, this trial showed that patients with severe asthma treated with bronchial thermoplasty had a long-term improvement in quality of life and needed less health care.6
Translating these trials into practice
To summarize, these clinical trials showed that bronchial thermoplasty was feasible, was relatively safe, and produced better clinical outcomes in patients with severe asthma when medical therapies did not control their symptoms.
In practice, patient selection is likely to be important. A key question will be, Does the patient truly have severe refractory asthma, or is the patient not taking his or her medication? Adherence to therapy should be evaluated.
In addition, patients need to be observed and monitored closely during and after the treatment period, as airway complications and asthma exacerbations can occur up to 6 weeks after the last procedure. About 80% of all study patients had multiple symptoms of asthma and other symptoms in the treatment period. Rarely did these symptoms result in hospitalization, but they were more common in the treatment group in the AIR2 trial.
Long-term studies have evaluated the duration of effect and the safety of bronchial thermoplasty, and outcomes appear favorable.20,21
WHY DOES IT WORK?
The role of airway smooth muscle in asthma is yet to be fully elucidated. The trials outlined here showed that although asthma is a disease of the airways, including the small airways, treatment of airways 3 mm or larger improves asthma symptoms, quality of life, and health care utilization.6 Thus, the role of airway smooth muscle in asthma and as a target of therapy has not previously been fully realized.21
Early investigations into the mechanisms of airflow obstruction and airway resistance found that 75% of postnasal resistance occurs in the first six to eight generations (ie, branchings) of the airways, indicating that larger airways are involved.22 (The number of generations varies depending on the size of the person but it typically is 10 to 12.) Findings from the study in dogs introduced the idea that smooth muscle alterations contributed to the changes in airway resistance, and that subtle changes in airway smooth muscle could clinically benefit asthma patients.1
The speculated purpose of the airway smooth muscle layer is to support the airway, allow gas exchange, propel mucus for clearance, defend the airway, enhance cough, and promote lymphatic flow. However, the airway smooth muscle layer may also be vestigial. In asthma, airway smooth muscle adds to bronchoconstriction and hyperresponsiveness, and has a role in mediating inflammation and airway remodeling.21 No definitive studies have shown that eliminating airway smooth muscle greatly inhibits normal airway function.18
What exactly does thermoplasty do to the smooth muscle? Studies in smooth muscle from cows showed that high temperatures directly disrupt the actin-myosin interaction, likely through denaturation of motor proteins.23 This immediate loss of muscle cell function is not likely to be the result of apoptosis, autophagy, or necrosis, or mediated by heat-shock proteins, in view of the relatively quick muscle response and lack of progressive changes. Tissue responsiveness is substantially reduced a few seconds after application of 60°C of heat and is subsequently abolished within 5 minutes after treatment.23
The intervention appears to be dose-dependent. Responsiveness to cholinergic stimulation is lessened by treatment, and the desired effect is seen within seconds and does not progress.
Therefore, we can surmise that disruption of myosin function is likely the mechanism of the therapeutic effect, breaking the cascade of airway smooth muscle spasm. Now that we know about the airway smooth muscle as a possible target of therapy, and that it may play only a vestigial role, we can think about other therapies that focus on it.18,23
BRONCHIAL THERMOPLASTY PROTOCOLS
Patients are assessed before and on the day of the procedure to make sure their disease is stable (ie, their postbronchodilator FEV1 is within 15% of baseline values, and they have no evidence of asthma exacerbation or active infection), similar to the protocol used in the AIR2 trial,6 before proceeding with the treatment.
Patients are given 50 mg of prednisone 3 days before and again on the day of the procedure. Nebulized albuterol (2.5–5.0 mg) is given before the patients undergo screening spirometry and again before the procedure. If the preprocedure FEV1 is lower than 15% below baseline, we postpone the procedure to another day.
The procedure is performed with the patient under moderate conscious sedation, typically using fentanyl (Sublimaze), midazolam (Versed), and topical lidocaine in a monitored environment. The bronchoscope is inserted via either the mouth or nose, and supplemental oxygen is provided.
Thermoplasty is performed with the Alair system (Asthmatx, Inc., Sunnyvale, CA), which delivers a specific amount of radiofrequency (thermal) energy through a dedicated catheter. The catheter is deployed through a 2.0-mm channel of a flexible bronchoscope, starting in distal airways as small as 3 mm in diameter and working proximally to sequentially treat all airways to the mainstem lobar bronchi. The sites treated are meticulously recorded on a bronchial airway map to ensure that treatment sites are not skipped or overlapped (FIGURE 1).
An array of four electrodes is manually expanded to make contact with the airway walls; each electrode has 5 mm of exposed wire. As the energy is delivered, the control unit measures electrical resistance converted to thermal energy and turns off the current when an appropriate dosage is given. This thermal energy is what is responsible for altering the airway smooth muscle.
A full course of treatment requires three separate bronchoscopy sessions, each separated by 2 to 3 weeks. The left lower lobe and the right lower lobe are treated in separate procedures, and then both upper lobes are treated in a third procedure to minimize any respiratory symptoms. Each procedure usually requires 50 to 75 activations of the device and takes up to 60 minutes.
After each procedure the patient should be observed for 3 to 4 hours, and spirometry should be repeated to make sure the FEV1 (percent predicted) is within 20% of the baseline value. An additional 50-mg dose of prednisone is prescribed for the day after the procedure.24
FDA CLEARANCE AND LONG-TERM FOLLOW-UP
The FDA approved the Alair device for treating severe refractory asthma in early 2010.3 The indications for it are based on the study populations in the published trials. Patients can be evaluated for this treatment if they have well-documented severe persistent asthma not well controlled on inhaled corticosteroids and long-acting beta agonists and have no significant contraindications to bronchoscopy.
As part of the conditions of approval, the FDA required a postapproval study based on the long-term follow-up of the AIR2 trial. They specifically wanted to compare patients who have desirable long-term outcomes and those in whom any treatment effect wanes with time. Since we have only a few years of follow-up data, we still do not know all the possible late effects of the treatment; we have an opportunity to learn more.
Another question that needs to be studied is whether thermoplasty will help other forms of bronchospastic lung disease, such as chronic obstructive pulmonary disease.
A second postapproval study will be a prospective, open-label, single-arm, multicenter study conducted in the United States to assess the treatment effect and short-term and long-term safety profile of thermoplasty in asthma.
As experience with the procedure increases, we will be better able to characterize which patients may benefit from it. In addition, the knowledge gained by the longer-term study of airway smooth muscle function alterations will potentially drive the discovery of other innovative therapies for severe asthma.
Asthma now has a new treatment, but it isn’t for everybody. Called bronchial thermoplasty, it is reserved for patients whose asthma is severe and refractory, as it involves three sessions of bronchoscopy, each lasting up to 1 hour, during which the smooth muscle layer is methodically ablated from the airway using radiofrequency energy.1,2
The US Food and Drug Administration (FDA) has approved bronchial thermoplasty,3 and although it does not cure asthma or completely eliminate its symptoms, patients with severe asthma that was not well controlled with medical therapy who underwent this procedure in clinical trials subsequently had fewer symptoms, enjoyed better quality of life, and needed less intensive health care (such as emergency room visits) than patients who did not undergo the procedure.4–6
Here, we present an overview of the pathophysiology of severe refractory asthma and the clinical trials of bronchial thermoplasty, its current protocols, and the status of this new treatment.
WHAT IS SEVERE REFRACTORY ASTHMA?
Asthma is a chronic inflammatory condition of the airways characterized by episodic symptoms of breathlessness, cough, and wheezing, which can wax and wane over time. Approximately 8.2% of the general population is affected.7
Our understanding of the pathophysiology of asthma has improved over the past 20 years, and with the publication of clinical guidelines from the National Asthma Education and Prevention Program in 1991,8 1997,9 and 2002,10 outcomes have improved. Most people with asthma can control their symptoms if they adhere to anti-inflammatory therapies and avoid triggers. Yet 5% to 10% of asthma patients have severe refractory disease, and asthma accounts for nearly half a million hospitalizations every year.11
The latest guidelines, published in 2007, emphasize the importance of assessing the severity of asthma, including the patient’s impairment (symptoms and limitations) and risk (likelihood of exacerbations).12
Workshop consensus definition of severe refractory asthma
A consensus group convened by the American Thoracic Society13 defined asthma as severe and refractory if the patient meets at least one of the following major criteria:
- Takes oral corticosteroids continuously or nearly continuously (> 50% of year)
- Takes high-dose inhaled corticosteroids.
In addition, the patient must meet at least two minor criteria, ie:
- Takes a controller medication such as a long-acting beta-agonist, theophylline, or a leukotriene antagonist every day
- Takes a short-acting beta agonist every day or nearly every day
- Has persistent airway obstruction, ie, a forced expiratory volume in 1 second (FEV1) less than 80% of predicted, or a peak expiratory flow that has a diurnal variability greater than 20%
- Has one or more urgent care visits for asthma per year
- Needs three or more oral corticosteroid “bursts” per year
- Has prompt deterioration when the dose of oral or inhaled corticosteroid is reduced by 25% or less
- Has had a near-fatal asthma event in the past.
Compared with people with mild asthma, people who have severe refractory asthma tend to be older, have fewer allergies, and make more use of intensive and urgent health care.14
Asthma is due to both inflammation and bronchoconstriction
The pathophysiology of asthma involves both chronic airway inflammation and bronchoconstriction, the latter characterized by a greater response to methacholine. Histologic findings include excessive mucus secretion, epithelial cell injury, and smooth muscle hypertrophy. These changes can lead to persistent airflow obstruction that can be difficult to control with medical therapies.12
Bronchoconstriction can be reversed temporarily with bronchodilators, but no longlasting therapy to reduce it has been available until now. Bronchial thermoplasty targets this gap in asthma management.
STUDIES OF BRONCHIAL THERMOPLASTY
Radiofrequency ablation has been used to treat other medical conditions such as lung cancer and cardiac arrhythmias.15,16 Its use to treat asthma by eradicating smooth muscle cells from the airway wall began with studies in animals.1 Later, studies were done in people without asthma,2 then in patients with mild to moderate asthma,17 and finally in patients with moderate to severe refractory asthma.4–6
These studies helped clarify which type of patients would be appropriate candidates and the outcomes to be anticipated, including adverse events.
Early studies
Danek et al,1 in a study in nonasthmatic dogs, found that thermoplasty at 65°C or 75°C (149°F or 167°F) attenuated the airway’s response to methacholine up to 3 years after treatment. As early as 1 week after treatment, airway smooth muscle was seen to be degenerating or absent, and the effect was inversely proportional to airway responsiveness.
Adverse effects of the procedure were cough, inflammatory edema of the airway wall, retained mucus, and blanching of the airway wall at the site of catheter contact. Three years later, there was no evidence of smooth muscle regeneration.
Miller et al2 next performed a feasibility study in eight patients, mean age 58 ± 8.3 years, who were scheduled to undergo lung resection for lung cancer. Five to 20 days before surgery, the investigators performed thermoplasty at 55°C or 65°C (131°F or 149°F) in three to nine sites per patient, 1 cm from known tumors but within areas to be resected. There were no significant adverse events such as hemoptysis, respiratory infections, or excess bronchial irritation.
A pilot study in mild to moderate asthma
Cox et al17 performed the first study of bronchial thermoplasty in patients with mild to moderate asthma. This was a prospective observational study in 16 patients who were younger than the patients in the previous study, with an average age of 30 years (range 24–58). They were given prednisone 30 or 50 mg the day before the procedure and on the day of the procedure. Three treatments were done, 3 weeks apart. The right middle lobe was not treated because the bronchus leading to it is relatively long and narrow, raising concern about damaging it.18
Results. The most frequent side effects were symptoms of airway irritation such as cough, dyspnea, wheezing, and bronchospasm. The mean time to onset was less than 1.7 days, and the mean time to resolution was 4.6 days after the most recent procedure. None of the patients needed to be hospitalized in the immediate postprocedure period.
In the 2 years after the procedure, there were 312 adverse events, mainly mild. Three (1%) of the adverse events were reported as severe, but they were deemed not related to the procedure. Yearly computed tomographic scans of the chest showed no structural changes such as bronchiectasis in the parenchyma or bronchial wall.
The FEV1 was higher at 12 weeks and at 1 year after thermoplasty than at baseline but was not significantly different from baseline at 2 years.
At baseline, the patients reported that 50% of their days were symptom-free; this increased to 73% at 12 weeks (P = .015).
In addition, airway hyperresponsiveness decreased significantly, and the effect persisted over 2 years. The provocative concentration of methacholine that caused a 20% reduction in FEV1 (the PC20) was:
- 0.92 mg/mL at baseline (95% confidence interval 0.42–1.99)
- 4.75 mg/mL at 12 weeks (2.51–8.85)
- 5.45 mg/mL at 1 year (1.54–19.32)
- 3.40 mg/mL at 2 years (1.35–8.52).
Limitations of this study include the relatively small number of patients enrolled and their relatively stable asthma.
The AIR trial: A randomized trial in moderate or persistent asthma
The first large multicenter trial of bronchial thermoplasty, the Asthma Intervention Research (AIR) trial,4 was prospective and randomized but not blinded. The aim was to determine whether bronchial thermoplasty would improve asthma control after long-acting beta agonists were discontinued.
Patients could be enrolled if they were 18 to 65 years old, had moderate or persistent asthma, and needed to take an inhaled corticosteroid (beclomethasone [Qvar] 200 μg or more or an equivalent drug) and a long-acting beta agonist (salmeterol [Serevent] 100 μg or more or an equivalent drug) every day. They also needed to have FEV1 values of 60% to 85% of predicted and airway reactivity (PC20 < 8 mg/mL), and their asthma had to have been stable for 6 weeks.
At baseline, the long-acting beta agonist was withdrawn temporarily; the final criterion for entry was that their asthma had to become worse when this was done.
Then, 112 patients were randomized to receive either bronchial thermoplasty with medical care (inhaled corticosteroids and long-acting beta agonists) or usual care, ie, medical therapy alone. Treatments were done in three sessions over 9 weeks, followed by attempts to discontinue their long-acting beta agonists at 3, 6, and 9 months after the procedure without exacerbations.
An exacerbation was defined as at least one of the following for 2 consecutive days: a reduction of peak flow by 20% of baseline average, the need for more than three additional puffs of rescue inhaler, or nocturnal awakenings caused by asthma symptoms. The patients kept a daily diary of their symptoms and rescue inhaler use, and they completed the Asthma Quality of Life Questionnaire (AQLQ) and the Asthma Control Questionnaire (ACQ).
Results. The number of mild (but not severe) exacerbations per week was significantly lower at 3 and 12 months than at baseline in the thermoplasty group, with 10 fewer mild exacerbations per patient per year, but was unchanged in the control group. There were significantly greater improvements in morning peak flow at 3, 6, and 12 months from baseline in the treatment group than in the usual-care group. Rescue medication use was also significantly less at 3 and 12 months. Symptom scores, AQLQ scores, and ACQ scores were all significantly better than at baseline as well.
Not surprisingly, in this cohort with unstable asthma, there were 407 adverse events in the treatment group and 106 adverse events in the control group. Most of these occurred within 1 day and resolved within 7 days after the procedure. There were more hospitalizations in the treatment group as well, for reasons that included exacerbations of asthma, collapse of the left lower lobe, and pleurisy.4
Therefore, this trial found that thermoplasty improved asthma symptoms within 3 months and that the effect lasted 1 year, with an encouraging reduction in the number of mild exacerbations. However, it was not blinded, and there is a strong placebo effect in asthma. Needed was a randomized trial in which the control group would undergo a sham treatment.
The RISA trial: A randomized trial in severe asthma
The Research in Severe Asthma (RISA) trial5 included patients with more severe asthma than those in the AIR trial. Entry criteria were:
- Taking high doses of an inhaled corticosteroid (> 750 μg of fluticasone or its equivalent per day)
- Taking prednisone (≤ 30 mg/day)
- An FEV1 of at least 50% of predicted without a bronchodilator
- A positive methacholine test.
Seventeen patients were randomized to undergo bronchial thermoplasty, and another 17 were randomized to receive medical treatment.
After a 2-week run-in period, the thermoplasty patients underwent three treatments, performed 3 weeks apart. For the next 16 weeks, the corticosteroid doses were kept stable in both groups, followed by a 14-week corticosteroid-weaning phase and then a 16-week reduced-corticosteroid phase. During this time, attempts were made to decrease the oral or inhaled corticosteroid doses according to a protocol (eg, a 20%–25% reduction every 2–4 weeks) unless there were mild exacerbations lasting more than 7 days.
Results. There were more adverse events in the thermoplasty group than in the medical management group in the treatment period, including seven hospitalizations for exacerbations of asthma and a partial collapse of the left lower lobe. There were no significant differences in adverse events between groups in the posttreatment period (up to 6 weeks after the last treatment). Forty-nine percent of the events were mild in each group; 10% of the events were severe in the thermoplasty group vs 4% in the control group.
During the steroid-stable phase, patients in the thermoplasty group used rescue inhalers significantly less than those in the control group, and their prebronchodilator FEV1 and AQLQ and ACQ scores were better. The differences in rescue inhaler use and questionnaire scores remained significant at 1 year.
Comment. As expected, serious adverse events occurred more often in patients with severe asthma in the treatment group than in the control group. However, 1 year after the procedure, the adverse-event rates were similar in the treatment and control groups, suggesting that this procedure can be safely performed in similar patient populations. Although there was significant potential for a placebo effect, these patients with severe persistent asthma showed significant improvement in clinical measures of asthma compared with the control group.
AIR2: A randomized, double-blind trial
The latest trial of this new therapy in severe asthma was the AIR2 trial.6 A major difference in its design compared with the earlier ones was that the control group underwent sham thermoplasty, allowing the trial to be truly double-blinded. (The bronchoscopy team knew which patients got which treatment, but the patients and the study physicians following them did not).
The primary outcome was the change in AQLQ score from baseline at 6, 9, and 12 months. Secondary outcomes included absolute changes in the asthma control scores, symptom scores, peak flows, rescue medication use, and FEV1.
The randomized groups (196 patients in the thermoplasty group and 101 in the sham treatment group) were well matched, and more than 80% in each group met the American Thoracic Society criteria for severe refractory asthma.
On the AQLQ, a change of more than 0.5 is considered clinically meaningful. Interestingly, there was a significant and clinically meaningful improvement in AQLQ in 64% of the sham treatment group, highlighting the placebo effect in asthma treatment.19 However, a larger proportion (79%) of the treated group had a clinically meaningful improvement on the AQLQ than in the sham treatment group.
Adverse events occurred in both groups; however, during the treatment phase, 16 patients in the bronchial thermoplasty group needed to be hospitalized for respiratory symptoms including worsening asthma, atelectasis, lower respiratory tract infections, decreased FEV1, and an aspirated tooth. One episode of hemoptysis required bronchial artery embolization. In contrast, only two patients in the sham treatment group needed hospitalization.
Therefore, this trial showed that patients with severe asthma treated with bronchial thermoplasty had a long-term improvement in quality of life and needed less health care.6
Translating these trials into practice
To summarize, these clinical trials showed that bronchial thermoplasty was feasible, was relatively safe, and produced better clinical outcomes in patients with severe asthma when medical therapies did not control their symptoms.
In practice, patient selection is likely to be important. A key question will be, Does the patient truly have severe refractory asthma, or is the patient not taking his or her medication? Adherence to therapy should be evaluated.
In addition, patients need to be observed and monitored closely during and after the treatment period, as airway complications and asthma exacerbations can occur up to 6 weeks after the last procedure. About 80% of all study patients had multiple symptoms of asthma and other symptoms in the treatment period. Rarely did these symptoms result in hospitalization, but they were more common in the treatment group in the AIR2 trial.
Long-term studies have evaluated the duration of effect and the safety of bronchial thermoplasty, and outcomes appear favorable.20,21
WHY DOES IT WORK?
The role of airway smooth muscle in asthma is yet to be fully elucidated. The trials outlined here showed that although asthma is a disease of the airways, including the small airways, treatment of airways 3 mm or larger improves asthma symptoms, quality of life, and health care utilization.6 Thus, the role of airway smooth muscle in asthma and as a target of therapy has not previously been fully realized.21
Early investigations into the mechanisms of airflow obstruction and airway resistance found that 75% of postnasal resistance occurs in the first six to eight generations (ie, branchings) of the airways, indicating that larger airways are involved.22 (The number of generations varies depending on the size of the person but it typically is 10 to 12.) Findings from the study in dogs introduced the idea that smooth muscle alterations contributed to the changes in airway resistance, and that subtle changes in airway smooth muscle could clinically benefit asthma patients.1
The speculated purpose of the airway smooth muscle layer is to support the airway, allow gas exchange, propel mucus for clearance, defend the airway, enhance cough, and promote lymphatic flow. However, the airway smooth muscle layer may also be vestigial. In asthma, airway smooth muscle adds to bronchoconstriction and hyperresponsiveness, and has a role in mediating inflammation and airway remodeling.21 No definitive studies have shown that eliminating airway smooth muscle greatly inhibits normal airway function.18
What exactly does thermoplasty do to the smooth muscle? Studies in smooth muscle from cows showed that high temperatures directly disrupt the actin-myosin interaction, likely through denaturation of motor proteins.23 This immediate loss of muscle cell function is not likely to be the result of apoptosis, autophagy, or necrosis, or mediated by heat-shock proteins, in view of the relatively quick muscle response and lack of progressive changes. Tissue responsiveness is substantially reduced a few seconds after application of 60°C of heat and is subsequently abolished within 5 minutes after treatment.23
The intervention appears to be dose-dependent. Responsiveness to cholinergic stimulation is lessened by treatment, and the desired effect is seen within seconds and does not progress.
Therefore, we can surmise that disruption of myosin function is likely the mechanism of the therapeutic effect, breaking the cascade of airway smooth muscle spasm. Now that we know about the airway smooth muscle as a possible target of therapy, and that it may play only a vestigial role, we can think about other therapies that focus on it.18,23
BRONCHIAL THERMOPLASTY PROTOCOLS
Patients are assessed before and on the day of the procedure to make sure their disease is stable (ie, their postbronchodilator FEV1 is within 15% of baseline values, and they have no evidence of asthma exacerbation or active infection), similar to the protocol used in the AIR2 trial,6 before proceeding with the treatment.
Patients are given 50 mg of prednisone 3 days before and again on the day of the procedure. Nebulized albuterol (2.5–5.0 mg) is given before the patients undergo screening spirometry and again before the procedure. If the preprocedure FEV1 is lower than 15% below baseline, we postpone the procedure to another day.
The procedure is performed with the patient under moderate conscious sedation, typically using fentanyl (Sublimaze), midazolam (Versed), and topical lidocaine in a monitored environment. The bronchoscope is inserted via either the mouth or nose, and supplemental oxygen is provided.
Thermoplasty is performed with the Alair system (Asthmatx, Inc., Sunnyvale, CA), which delivers a specific amount of radiofrequency (thermal) energy through a dedicated catheter. The catheter is deployed through a 2.0-mm channel of a flexible bronchoscope, starting in distal airways as small as 3 mm in diameter and working proximally to sequentially treat all airways to the mainstem lobar bronchi. The sites treated are meticulously recorded on a bronchial airway map to ensure that treatment sites are not skipped or overlapped (FIGURE 1).
An array of four electrodes is manually expanded to make contact with the airway walls; each electrode has 5 mm of exposed wire. As the energy is delivered, the control unit measures electrical resistance converted to thermal energy and turns off the current when an appropriate dosage is given. This thermal energy is what is responsible for altering the airway smooth muscle.
A full course of treatment requires three separate bronchoscopy sessions, each separated by 2 to 3 weeks. The left lower lobe and the right lower lobe are treated in separate procedures, and then both upper lobes are treated in a third procedure to minimize any respiratory symptoms. Each procedure usually requires 50 to 75 activations of the device and takes up to 60 minutes.
After each procedure the patient should be observed for 3 to 4 hours, and spirometry should be repeated to make sure the FEV1 (percent predicted) is within 20% of the baseline value. An additional 50-mg dose of prednisone is prescribed for the day after the procedure.24
FDA CLEARANCE AND LONG-TERM FOLLOW-UP
The FDA approved the Alair device for treating severe refractory asthma in early 2010.3 The indications for it are based on the study populations in the published trials. Patients can be evaluated for this treatment if they have well-documented severe persistent asthma not well controlled on inhaled corticosteroids and long-acting beta agonists and have no significant contraindications to bronchoscopy.
As part of the conditions of approval, the FDA required a postapproval study based on the long-term follow-up of the AIR2 trial. They specifically wanted to compare patients who have desirable long-term outcomes and those in whom any treatment effect wanes with time. Since we have only a few years of follow-up data, we still do not know all the possible late effects of the treatment; we have an opportunity to learn more.
Another question that needs to be studied is whether thermoplasty will help other forms of bronchospastic lung disease, such as chronic obstructive pulmonary disease.
A second postapproval study will be a prospective, open-label, single-arm, multicenter study conducted in the United States to assess the treatment effect and short-term and long-term safety profile of thermoplasty in asthma.
As experience with the procedure increases, we will be better able to characterize which patients may benefit from it. In addition, the knowledge gained by the longer-term study of airway smooth muscle function alterations will potentially drive the discovery of other innovative therapies for severe asthma.
- Danek CJ, Lombard CM, Dungworth DL, et al. Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. J Appl Physiol 2004; 97:1946–1953.
- Miller JD, Cox G, Vincic L, Lombard CM, Loomas BE, Danek CJ. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest 2005; 127:1999–2006.
- US Food and Drug Administration (FDA). Approval of Alair Bronchial Thermoplasty System: Alair Catheter and Alair RF Controller. 2010. www.accessdata.fda.gov/cdrh_docs/pdf8/P080032a.pdf. Accessed June 1, 2011.
- Cox G, Thomson NC, Rubin AS, et al; AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med 2007; 356:1327–1337.
- Pavord ID, Cox G, Thomson NC, et al; RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med 2007; 176:1185–1191.
- Castro M, Rubin AS, Laviolette M, et al; AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010; 181:116–124.
- Centers for Disease Control and Prevention. Vital signs: asthma prevalence, disease characteristics, and self-management education—United States, 2001–2009. MMWR Morb Mortal Wkly Rep 2011; 60( 17):547–552.
- Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute. National Asthma Education Program. Expert Panel Report. J Allergy Clin Immunol 1991; 88:425–534.
- US Department of Health and Human Services. Expert panel report 2 (EPR-2): Guidelines for the diagnosis and management of asthma, 1997. www.nhlbi.nih.gov/guidelines/archives/epr-2/index.htm. Accessed June 1, 2011.
- US Department of Health and Human Services. Expert panel report: Guidelines for the diagnosis and management of asthma—Update on selected topics 2002. www.nhlbi.nih.gov/guidelines/archives/epr-2_upd/index.htm. Accessed June 1, 2011.
- Akinbami L. Asthma prevalence, health care use and mortality: United States 2003–05, CDC National Center for Health Statistics, 2006. www.cdc.gov/nchs/data/hestat/asthma03-05/asthma03-05.htm. Accessed June 1, 2011.
- US Department of Health and Human Services. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma full report, 2007. www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed June 1, 2011.
- Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society Am J Respir Crit Care Med 2000; 162:2341–2351.
- Moore WC, Bleecker ER, Curran-Everett D, et al; National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol 2007; 119:405–413.
- Ambrogi MC, Fanucchi O, Lencioni R, Cioni R, Mussi A. Pulmonary radiofrequency ablation in a single lung patient. Thorax 2006; 61:828–829.
- Benussi S, Cini R, Gaynor SL, Alfieri O, Calafiore AM. Bipolar radiofrequency maze procedure through a transseptal approach. Ann Thorac Surg 2010; 90:1025–1027.
- Cox G, Miller JD, McWilliams A, Fitzgerald JM, Lam S. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med 2006; 173:965–969.
- Cox PG, Miller J, Mitzner W, Leff AR. Radiofrequency ablation of airway smooth muscle for sustained treatment of asthma: preliminary investigations. Eur Respir J 2004; 24:659–663.
- Wise RA, Bartlett SJ, Brown ED, et al; American Lung Association Asthma Clinical Research Centers. Randomized trial of the effect of drug presentation on asthma outcomes: the American Lung Association Asthma Clinical Research Centers. J Allergy Clin Immunol 2009; 124:436–444.
- Castro M, Rubin A, Laviolette M, Hanania NA, Armstrong B, Cox G; AIR2 Trial Study Group. Persistence of effectiveness of bronchial thermoplasty in patients with severe asthma. Ann Allergy Asthma Immunol 2011. doi: 10.1016/j.anai.2011.03.005.
- Thomson NC, Rubin AS, Niven RM, et al; AIR Trial Study Group. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med 2011; 11:8.
- Solway J, Irvin CG. Airway smooth muscle as a target for asthma therapy. N Engl J Med 2007; 356:1367–1369.
- Ingram RH, McFadden ER. Localization and mechanisms of airway responses. N Engl J Med 1977; 297:596–600.
- Dyrda P, Tazzeo T, DoHarris L, et al. Acute response of airway muscle to extreme temperature includes disruption of actin-myosin interaction. Am J Respir Cell Mol Biol 2011; 44:213–221.
- Mayse ML, Laviolette M, Rubin AS, et al. Clinical pearls for bronchial thermoplasty. J Bronchol 2007; 14:115–123.
- Danek CJ, Lombard CM, Dungworth DL, et al. Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. J Appl Physiol 2004; 97:1946–1953.
- Miller JD, Cox G, Vincic L, Lombard CM, Loomas BE, Danek CJ. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest 2005; 127:1999–2006.
- US Food and Drug Administration (FDA). Approval of Alair Bronchial Thermoplasty System: Alair Catheter and Alair RF Controller. 2010. www.accessdata.fda.gov/cdrh_docs/pdf8/P080032a.pdf. Accessed June 1, 2011.
- Cox G, Thomson NC, Rubin AS, et al; AIR Trial Study Group. Asthma control during the year after bronchial thermoplasty. N Engl J Med 2007; 356:1327–1337.
- Pavord ID, Cox G, Thomson NC, et al; RISA Trial Study Group. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med 2007; 176:1185–1191.
- Castro M, Rubin AS, Laviolette M, et al; AIR2 Trial Study Group. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010; 181:116–124.
- Centers for Disease Control and Prevention. Vital signs: asthma prevalence, disease characteristics, and self-management education—United States, 2001–2009. MMWR Morb Mortal Wkly Rep 2011; 60( 17):547–552.
- Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute. National Asthma Education Program. Expert Panel Report. J Allergy Clin Immunol 1991; 88:425–534.
- US Department of Health and Human Services. Expert panel report 2 (EPR-2): Guidelines for the diagnosis and management of asthma, 1997. www.nhlbi.nih.gov/guidelines/archives/epr-2/index.htm. Accessed June 1, 2011.
- US Department of Health and Human Services. Expert panel report: Guidelines for the diagnosis and management of asthma—Update on selected topics 2002. www.nhlbi.nih.gov/guidelines/archives/epr-2_upd/index.htm. Accessed June 1, 2011.
- Akinbami L. Asthma prevalence, health care use and mortality: United States 2003–05, CDC National Center for Health Statistics, 2006. www.cdc.gov/nchs/data/hestat/asthma03-05/asthma03-05.htm. Accessed June 1, 2011.
- US Department of Health and Human Services. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma full report, 2007. www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed June 1, 2011.
- Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society Am J Respir Crit Care Med 2000; 162:2341–2351.
- Moore WC, Bleecker ER, Curran-Everett D, et al; National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol 2007; 119:405–413.
- Ambrogi MC, Fanucchi O, Lencioni R, Cioni R, Mussi A. Pulmonary radiofrequency ablation in a single lung patient. Thorax 2006; 61:828–829.
- Benussi S, Cini R, Gaynor SL, Alfieri O, Calafiore AM. Bipolar radiofrequency maze procedure through a transseptal approach. Ann Thorac Surg 2010; 90:1025–1027.
- Cox G, Miller JD, McWilliams A, Fitzgerald JM, Lam S. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med 2006; 173:965–969.
- Cox PG, Miller J, Mitzner W, Leff AR. Radiofrequency ablation of airway smooth muscle for sustained treatment of asthma: preliminary investigations. Eur Respir J 2004; 24:659–663.
- Wise RA, Bartlett SJ, Brown ED, et al; American Lung Association Asthma Clinical Research Centers. Randomized trial of the effect of drug presentation on asthma outcomes: the American Lung Association Asthma Clinical Research Centers. J Allergy Clin Immunol 2009; 124:436–444.
- Castro M, Rubin A, Laviolette M, Hanania NA, Armstrong B, Cox G; AIR2 Trial Study Group. Persistence of effectiveness of bronchial thermoplasty in patients with severe asthma. Ann Allergy Asthma Immunol 2011. doi: 10.1016/j.anai.2011.03.005.
- Thomson NC, Rubin AS, Niven RM, et al; AIR Trial Study Group. Long-term (5 year) safety of bronchial thermoplasty: Asthma Intervention Research (AIR) trial. BMC Pulm Med 2011; 11:8.
- Solway J, Irvin CG. Airway smooth muscle as a target for asthma therapy. N Engl J Med 2007; 356:1367–1369.
- Ingram RH, McFadden ER. Localization and mechanisms of airway responses. N Engl J Med 1977; 297:596–600.
- Dyrda P, Tazzeo T, DoHarris L, et al. Acute response of airway muscle to extreme temperature includes disruption of actin-myosin interaction. Am J Respir Cell Mol Biol 2011; 44:213–221.
- Mayse ML, Laviolette M, Rubin AS, et al. Clinical pearls for bronchial thermoplasty. J Bronchol 2007; 14:115–123.
KEY POINTS
- Bronchial thermoplasty involves the application of radiofrequency energy to the airways distal to the mainstem bronchi down to airways as small as 3 mm in diameter.
- Treatments are done in three separate sessions, with careful monitoring before and after for respiratory complications that can occur in severe asthma. Airway complications and asthma exacerbations can occur up to 6 weeks after the last procedure, thus requiring close patient follow-up.
- In clinical trials, including a randomized trial in which the control group underwent sham thermoplasty, bronchial thermoplasty had an acceptable safety profile while improving asthma quality-of-life scores, symptoms, and health care utilization.