User login
When should an indwelling pleural catheter be considered for malignant pleural effusion?
An indwelling pleural catheter should be considered when a malignant pleural effusion causes symptoms and recurs after thoracentesis, especially in patients with short to intermediate life expectancy or trapped lung, or who underwent unsuccessful pleurodesis.1
MALIGNANT PLEURAL EFFUSION
Malignant pleural effusion affects about 150,000 people in the United States each year. It occurs in 15% of patients with advanced malignancies, most often lung cancer, breast cancer, lymphoma, and ovarian cancer, which account for more than 50% of cases.2
In most patients with malignant pleural effusion, disabling dyspnea causes poor quality of life. The prognosis is unfavorable, with life expectancy of 3 to 12 months. Patients with poor performance status and lower glucose concentrations in the pleural fluid face a worse prognosis and a shorter life expectancy.2
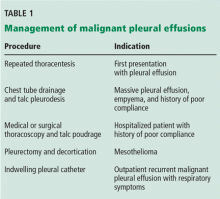
In general, management focuses on relieving symptoms rather than on cure. Symptoms can be controlled by thoracentesis, but if the effusion recurs, the patient needs repeated visits to the emergency room or clinic or a hospital admission to drain the fluid. Frequent hospital visits can be grueling for a patient with a poor functional status, and so can the adverse effects of repeated thoracentesis. For that reason, an early palliative approach to malignant pleural effusion in patients with cancer and a poor prognosis leads to better symptom control and a better quality of life.3 Multiple treatments can be offered to control the symptoms in patients with recurrent malignant pleural effusion (Table 1).
PLEURODESIS HAS BEEN THE TREATMENT OF CHOICE
Pleurodesis has been the treatment of choice for malignant pleural effusion for decades. In this procedure, adhesion of the visceral and parietal pleura is achxieved by inducing inflammation either mechanically or chemically between the pleural surfaces. Injection of a sclerosant into the pleural space generates the inflammation. The sclerosant can be introduced through a chest tube or thoracoscope such as in video-assisted thoracic surgery or medical pleuroscopy. The use of talc is associated with a higher success rate than other sclerosing agents such as bleomycin and doxycycline.4
The downside of this procedure is that pleural effusion recurs in 10% to 40% of cases, and patients require 2 to 4 days in the hospital. Also, the use of talc can lead to acute lung injury–acute respiratory distress syndrome, a rare but potentially life-threatening complication. The incidence of this complication may be related to particle size, with small particles posing a higher risk than large ones.5,6
PLACEMENT OF AN INDWELLING PLEURAL CATHETER
Indwelling pleural catheters are currently used as palliative therapy for patients with recurrent malignant pleural effusion who suffer from respiratory distress due to rapid reaccumulation of pleural fluids that require multiple thoracentesis procedures.
An indwelling pleural catheter is contraindicated in patients with uncontrolled coagulopathy, multiloculated pleural effusions, or extensive malignancy in the skin.3 Other factors that need to be considered are the patient’s social circumstances: ie, the patient must be in a clean and safe environment and must have insurance coverage for the supplies.
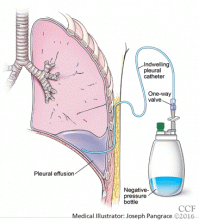
Catheters are 66 cm long and 15.5F and are made of silicone rubber with fenestrations along the distal 24 cm. They have a one-way valve at the proximal end that allows fluids and air to go out but not in (Figure 1).1 Several systems are commercially available in the United States.
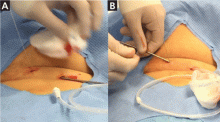
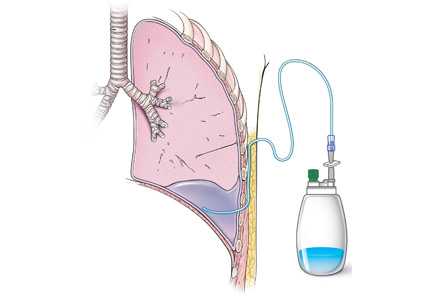
The catheter is inserted and tunneled percutaneously with the patient under local anesthesia and conscious sedation (Figure 2). Insertion is a same-day outpatient procedure, and intermittent pleural fluid drainage can be done at home by a home heathcare provider or a trained family member.7
In a meta-analysis, insertion difficulties were reported in only 4% of cases, particularly in patients who underwent prior pleural interventions. Spontaneous pleurodesis occurred in 45% of patients at a mean of 52 days after insertion.8
After catheter insertion, the pleural space should be drained three times a week. No more than 1,000 mL of fluid should be removed at a time—or less if drainage causes chest pain or cough secondary to trapped lung (see below). When the drainage declines to 150 mL per session, the sessions can be reduced to twice a week. If the volume drops to less than 50 mL per session, imaging (computed tomography or bedside thoracic ultrasonography) is recommended to ensure the achievement of pleurodesis and to rule out catheter blockage.
A large multicenter randomized controlled trial9 compared indwelling pleural catheter therapy and chest tube insertion with talc pleurodesis. Both procedures relieved symptoms for the first 42 days, and there was no significant difference in quality of life. However, the median length of hospital stay was 4 days for the talc pleurodesis group compared with 0 days for the indwelling pleural catheter group. Twenty-two percent of the talc group required a further pleural procedure such as a video-assisted thoracic surgery or thoracoscopy, compared with 6% of the indwelling catheter group. On the other hand, 36% of those in the indwelling catheter group experienced nonserious adverse events such as pleural infections that mandated outpatient oral antibiotic therapy, cellulitis, and catheter blockage, compared with 7% of the talc group.9
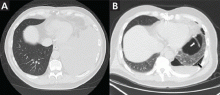
Symptomatic, inoperable trapped lung is another condition for which an indwelling pleural catheter is a reasonable strategy compared with pleurodesis. Trapped lung is a condition in which the lung fails to fully expand despite proper pleural fluid removal, creating a vacuum space between the parietal and visceral pleura (Figure 3).
Patients with trapped lung complain of severe dull or sharp pain during drainage of pleural fluids due to stretching of the visceral pleura against the intrathoracic vacuum space. Trapped lung can be detected objectively by using intrathoracic manometry while draining fluids, looking for more than a 20-cm H2O drop in the intrathoracic pressure. Radiographically, this may be identified as a pneumothorax ex vacuo10 (ie, caused by inability of the lung to expand to fill the thoracic cavity after pleural fluid has been drained) and is not a procedure complication.
Placement of an indwelling pleural catheter is the treatment of choice for trapped lung, since chemical pleurodesis is not feasible without the potential of parietal and visceral pleural apposition. In a retrospective study of indwelling catheter placement for palliative symptom control, a catheter relieved symptoms, improved quality of life, and afforded a substantial increase in mobility.1,11
In another multicenter pilot study,12 rapid pleurodesis was achieved in 30 patients with recurrent malignant pleural effusion by combining chemical pleurodesis and indwelling catheter placement. Both were done under direct vision with medical thoracoscopy. Pleurodesis succeeded in 92% of patients by day 8 after the procedure. The hospital stay was reduced to a mean of 2 days after the procedure. In the catheter group, fluids were drained three times in the first day after the procedure and twice a day on the second and third days. Of the 30 patients in this study, 2 had fever, 1 needed to have the catheter replaced, and 1 contracted empyema.
AN EFFECTIVE INITIAL TREATMENT
Placement of an indwelling pleural catheter is an effective initial treatment for recurrent malignant pleural effusion. Compared with chemical pleurodesis, it has a comparable success rate and complication rate. It offers the advantages of being a same-day surgical procedure entailing a shorter hospital stay and less need for further pleural intervention. This treatment should be considered for patients with symptomatic malignant pleural effusion, especially those in whom symptomatic malignant pleural effusion recurred after thoracentesis.8
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40.
- Thomas JM, Musani AI. Malignant pleural effusions: a review. Clin Chest Med 2013; 34:459–471.
- Thomas R, Francis R, Davies HE, Lee YC. Interventional therapies for malignant pleural effusions: the present and the future. Respirology 2014; 19:809–822.
- Rodriguez-Panadero F, Montes-Worboys A. Mechanisms of pleurodesis. Respiration 2012; 83:91–98.
- Gonzalez AV, Bezwada V, Beamis JF Jr, Villanueva AG. Lung injury following thoracoscopic talc insufflation: experience of a single North American center. Chest 2010; 137:1375–1381.
- Rossi VF, Vargas FS, Marchi E, et al. Acute inflammatory response secondary to intrapleural administration of two types of talc. Eur Respir J 2010; 35:396–401.
- Fysh ET, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142:394–400.
- Kheir F, Shawwa K, Alokla K, Omballi M, Alraiyes AH. Tunneled pleural catheter for the treatment of malignant pleural effusion: a systematic review and meta-analysis. Am J Ther 2015 Feb 2. [Epub ahead of print]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307:2383–2389.
- Ponrartana S, Laberge JM, Kerlan RK, Wilson MW, Gordon RL. Management of patients with “ex vacuo” pneumothorax after thoracentesis. Acad Radiol 2005; 12:980–986.
- Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg 2009; 9:961–964.
- Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011; 139:1419–1423.
An indwelling pleural catheter should be considered when a malignant pleural effusion causes symptoms and recurs after thoracentesis, especially in patients with short to intermediate life expectancy or trapped lung, or who underwent unsuccessful pleurodesis.1
MALIGNANT PLEURAL EFFUSION
Malignant pleural effusion affects about 150,000 people in the United States each year. It occurs in 15% of patients with advanced malignancies, most often lung cancer, breast cancer, lymphoma, and ovarian cancer, which account for more than 50% of cases.2
In most patients with malignant pleural effusion, disabling dyspnea causes poor quality of life. The prognosis is unfavorable, with life expectancy of 3 to 12 months. Patients with poor performance status and lower glucose concentrations in the pleural fluid face a worse prognosis and a shorter life expectancy.2
In general, management focuses on relieving symptoms rather than on cure. Symptoms can be controlled by thoracentesis, but if the effusion recurs, the patient needs repeated visits to the emergency room or clinic or a hospital admission to drain the fluid. Frequent hospital visits can be grueling for a patient with a poor functional status, and so can the adverse effects of repeated thoracentesis. For that reason, an early palliative approach to malignant pleural effusion in patients with cancer and a poor prognosis leads to better symptom control and a better quality of life.3 Multiple treatments can be offered to control the symptoms in patients with recurrent malignant pleural effusion (Table 1).
PLEURODESIS HAS BEEN THE TREATMENT OF CHOICE
Pleurodesis has been the treatment of choice for malignant pleural effusion for decades. In this procedure, adhesion of the visceral and parietal pleura is achxieved by inducing inflammation either mechanically or chemically between the pleural surfaces. Injection of a sclerosant into the pleural space generates the inflammation. The sclerosant can be introduced through a chest tube or thoracoscope such as in video-assisted thoracic surgery or medical pleuroscopy. The use of talc is associated with a higher success rate than other sclerosing agents such as bleomycin and doxycycline.4
The downside of this procedure is that pleural effusion recurs in 10% to 40% of cases, and patients require 2 to 4 days in the hospital. Also, the use of talc can lead to acute lung injury–acute respiratory distress syndrome, a rare but potentially life-threatening complication. The incidence of this complication may be related to particle size, with small particles posing a higher risk than large ones.5,6
PLACEMENT OF AN INDWELLING PLEURAL CATHETER
Indwelling pleural catheters are currently used as palliative therapy for patients with recurrent malignant pleural effusion who suffer from respiratory distress due to rapid reaccumulation of pleural fluids that require multiple thoracentesis procedures.
An indwelling pleural catheter is contraindicated in patients with uncontrolled coagulopathy, multiloculated pleural effusions, or extensive malignancy in the skin.3 Other factors that need to be considered are the patient’s social circumstances: ie, the patient must be in a clean and safe environment and must have insurance coverage for the supplies.
Catheters are 66 cm long and 15.5F and are made of silicone rubber with fenestrations along the distal 24 cm. They have a one-way valve at the proximal end that allows fluids and air to go out but not in (Figure 1).1 Several systems are commercially available in the United States.
The catheter is inserted and tunneled percutaneously with the patient under local anesthesia and conscious sedation (Figure 2). Insertion is a same-day outpatient procedure, and intermittent pleural fluid drainage can be done at home by a home heathcare provider or a trained family member.7
In a meta-analysis, insertion difficulties were reported in only 4% of cases, particularly in patients who underwent prior pleural interventions. Spontaneous pleurodesis occurred in 45% of patients at a mean of 52 days after insertion.8
After catheter insertion, the pleural space should be drained three times a week. No more than 1,000 mL of fluid should be removed at a time—or less if drainage causes chest pain or cough secondary to trapped lung (see below). When the drainage declines to 150 mL per session, the sessions can be reduced to twice a week. If the volume drops to less than 50 mL per session, imaging (computed tomography or bedside thoracic ultrasonography) is recommended to ensure the achievement of pleurodesis and to rule out catheter blockage.
A large multicenter randomized controlled trial9 compared indwelling pleural catheter therapy and chest tube insertion with talc pleurodesis. Both procedures relieved symptoms for the first 42 days, and there was no significant difference in quality of life. However, the median length of hospital stay was 4 days for the talc pleurodesis group compared with 0 days for the indwelling pleural catheter group. Twenty-two percent of the talc group required a further pleural procedure such as a video-assisted thoracic surgery or thoracoscopy, compared with 6% of the indwelling catheter group. On the other hand, 36% of those in the indwelling catheter group experienced nonserious adverse events such as pleural infections that mandated outpatient oral antibiotic therapy, cellulitis, and catheter blockage, compared with 7% of the talc group.9
Symptomatic, inoperable trapped lung is another condition for which an indwelling pleural catheter is a reasonable strategy compared with pleurodesis. Trapped lung is a condition in which the lung fails to fully expand despite proper pleural fluid removal, creating a vacuum space between the parietal and visceral pleura (Figure 3).
Patients with trapped lung complain of severe dull or sharp pain during drainage of pleural fluids due to stretching of the visceral pleura against the intrathoracic vacuum space. Trapped lung can be detected objectively by using intrathoracic manometry while draining fluids, looking for more than a 20-cm H2O drop in the intrathoracic pressure. Radiographically, this may be identified as a pneumothorax ex vacuo10 (ie, caused by inability of the lung to expand to fill the thoracic cavity after pleural fluid has been drained) and is not a procedure complication.
Placement of an indwelling pleural catheter is the treatment of choice for trapped lung, since chemical pleurodesis is not feasible without the potential of parietal and visceral pleural apposition. In a retrospective study of indwelling catheter placement for palliative symptom control, a catheter relieved symptoms, improved quality of life, and afforded a substantial increase in mobility.1,11
In another multicenter pilot study,12 rapid pleurodesis was achieved in 30 patients with recurrent malignant pleural effusion by combining chemical pleurodesis and indwelling catheter placement. Both were done under direct vision with medical thoracoscopy. Pleurodesis succeeded in 92% of patients by day 8 after the procedure. The hospital stay was reduced to a mean of 2 days after the procedure. In the catheter group, fluids were drained three times in the first day after the procedure and twice a day on the second and third days. Of the 30 patients in this study, 2 had fever, 1 needed to have the catheter replaced, and 1 contracted empyema.
AN EFFECTIVE INITIAL TREATMENT
Placement of an indwelling pleural catheter is an effective initial treatment for recurrent malignant pleural effusion. Compared with chemical pleurodesis, it has a comparable success rate and complication rate. It offers the advantages of being a same-day surgical procedure entailing a shorter hospital stay and less need for further pleural intervention. This treatment should be considered for patients with symptomatic malignant pleural effusion, especially those in whom symptomatic malignant pleural effusion recurred after thoracentesis.8
An indwelling pleural catheter should be considered when a malignant pleural effusion causes symptoms and recurs after thoracentesis, especially in patients with short to intermediate life expectancy or trapped lung, or who underwent unsuccessful pleurodesis.1
MALIGNANT PLEURAL EFFUSION
Malignant pleural effusion affects about 150,000 people in the United States each year. It occurs in 15% of patients with advanced malignancies, most often lung cancer, breast cancer, lymphoma, and ovarian cancer, which account for more than 50% of cases.2
In most patients with malignant pleural effusion, disabling dyspnea causes poor quality of life. The prognosis is unfavorable, with life expectancy of 3 to 12 months. Patients with poor performance status and lower glucose concentrations in the pleural fluid face a worse prognosis and a shorter life expectancy.2
In general, management focuses on relieving symptoms rather than on cure. Symptoms can be controlled by thoracentesis, but if the effusion recurs, the patient needs repeated visits to the emergency room or clinic or a hospital admission to drain the fluid. Frequent hospital visits can be grueling for a patient with a poor functional status, and so can the adverse effects of repeated thoracentesis. For that reason, an early palliative approach to malignant pleural effusion in patients with cancer and a poor prognosis leads to better symptom control and a better quality of life.3 Multiple treatments can be offered to control the symptoms in patients with recurrent malignant pleural effusion (Table 1).
PLEURODESIS HAS BEEN THE TREATMENT OF CHOICE
Pleurodesis has been the treatment of choice for malignant pleural effusion for decades. In this procedure, adhesion of the visceral and parietal pleura is achxieved by inducing inflammation either mechanically or chemically between the pleural surfaces. Injection of a sclerosant into the pleural space generates the inflammation. The sclerosant can be introduced through a chest tube or thoracoscope such as in video-assisted thoracic surgery or medical pleuroscopy. The use of talc is associated with a higher success rate than other sclerosing agents such as bleomycin and doxycycline.4
The downside of this procedure is that pleural effusion recurs in 10% to 40% of cases, and patients require 2 to 4 days in the hospital. Also, the use of talc can lead to acute lung injury–acute respiratory distress syndrome, a rare but potentially life-threatening complication. The incidence of this complication may be related to particle size, with small particles posing a higher risk than large ones.5,6
PLACEMENT OF AN INDWELLING PLEURAL CATHETER
Indwelling pleural catheters are currently used as palliative therapy for patients with recurrent malignant pleural effusion who suffer from respiratory distress due to rapid reaccumulation of pleural fluids that require multiple thoracentesis procedures.
An indwelling pleural catheter is contraindicated in patients with uncontrolled coagulopathy, multiloculated pleural effusions, or extensive malignancy in the skin.3 Other factors that need to be considered are the patient’s social circumstances: ie, the patient must be in a clean and safe environment and must have insurance coverage for the supplies.
Catheters are 66 cm long and 15.5F and are made of silicone rubber with fenestrations along the distal 24 cm. They have a one-way valve at the proximal end that allows fluids and air to go out but not in (Figure 1).1 Several systems are commercially available in the United States.
The catheter is inserted and tunneled percutaneously with the patient under local anesthesia and conscious sedation (Figure 2). Insertion is a same-day outpatient procedure, and intermittent pleural fluid drainage can be done at home by a home heathcare provider or a trained family member.7
In a meta-analysis, insertion difficulties were reported in only 4% of cases, particularly in patients who underwent prior pleural interventions. Spontaneous pleurodesis occurred in 45% of patients at a mean of 52 days after insertion.8
After catheter insertion, the pleural space should be drained three times a week. No more than 1,000 mL of fluid should be removed at a time—or less if drainage causes chest pain or cough secondary to trapped lung (see below). When the drainage declines to 150 mL per session, the sessions can be reduced to twice a week. If the volume drops to less than 50 mL per session, imaging (computed tomography or bedside thoracic ultrasonography) is recommended to ensure the achievement of pleurodesis and to rule out catheter blockage.
A large multicenter randomized controlled trial9 compared indwelling pleural catheter therapy and chest tube insertion with talc pleurodesis. Both procedures relieved symptoms for the first 42 days, and there was no significant difference in quality of life. However, the median length of hospital stay was 4 days for the talc pleurodesis group compared with 0 days for the indwelling pleural catheter group. Twenty-two percent of the talc group required a further pleural procedure such as a video-assisted thoracic surgery or thoracoscopy, compared with 6% of the indwelling catheter group. On the other hand, 36% of those in the indwelling catheter group experienced nonserious adverse events such as pleural infections that mandated outpatient oral antibiotic therapy, cellulitis, and catheter blockage, compared with 7% of the talc group.9
Symptomatic, inoperable trapped lung is another condition for which an indwelling pleural catheter is a reasonable strategy compared with pleurodesis. Trapped lung is a condition in which the lung fails to fully expand despite proper pleural fluid removal, creating a vacuum space between the parietal and visceral pleura (Figure 3).
Patients with trapped lung complain of severe dull or sharp pain during drainage of pleural fluids due to stretching of the visceral pleura against the intrathoracic vacuum space. Trapped lung can be detected objectively by using intrathoracic manometry while draining fluids, looking for more than a 20-cm H2O drop in the intrathoracic pressure. Radiographically, this may be identified as a pneumothorax ex vacuo10 (ie, caused by inability of the lung to expand to fill the thoracic cavity after pleural fluid has been drained) and is not a procedure complication.
Placement of an indwelling pleural catheter is the treatment of choice for trapped lung, since chemical pleurodesis is not feasible without the potential of parietal and visceral pleural apposition. In a retrospective study of indwelling catheter placement for palliative symptom control, a catheter relieved symptoms, improved quality of life, and afforded a substantial increase in mobility.1,11
In another multicenter pilot study,12 rapid pleurodesis was achieved in 30 patients with recurrent malignant pleural effusion by combining chemical pleurodesis and indwelling catheter placement. Both were done under direct vision with medical thoracoscopy. Pleurodesis succeeded in 92% of patients by day 8 after the procedure. The hospital stay was reduced to a mean of 2 days after the procedure. In the catheter group, fluids were drained three times in the first day after the procedure and twice a day on the second and third days. Of the 30 patients in this study, 2 had fever, 1 needed to have the catheter replaced, and 1 contracted empyema.
AN EFFECTIVE INITIAL TREATMENT
Placement of an indwelling pleural catheter is an effective initial treatment for recurrent malignant pleural effusion. Compared with chemical pleurodesis, it has a comparable success rate and complication rate. It offers the advantages of being a same-day surgical procedure entailing a shorter hospital stay and less need for further pleural intervention. This treatment should be considered for patients with symptomatic malignant pleural effusion, especially those in whom symptomatic malignant pleural effusion recurred after thoracentesis.8
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40.
- Thomas JM, Musani AI. Malignant pleural effusions: a review. Clin Chest Med 2013; 34:459–471.
- Thomas R, Francis R, Davies HE, Lee YC. Interventional therapies for malignant pleural effusions: the present and the future. Respirology 2014; 19:809–822.
- Rodriguez-Panadero F, Montes-Worboys A. Mechanisms of pleurodesis. Respiration 2012; 83:91–98.
- Gonzalez AV, Bezwada V, Beamis JF Jr, Villanueva AG. Lung injury following thoracoscopic talc insufflation: experience of a single North American center. Chest 2010; 137:1375–1381.
- Rossi VF, Vargas FS, Marchi E, et al. Acute inflammatory response secondary to intrapleural administration of two types of talc. Eur Respir J 2010; 35:396–401.
- Fysh ET, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142:394–400.
- Kheir F, Shawwa K, Alokla K, Omballi M, Alraiyes AH. Tunneled pleural catheter for the treatment of malignant pleural effusion: a systematic review and meta-analysis. Am J Ther 2015 Feb 2. [Epub ahead of print]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307:2383–2389.
- Ponrartana S, Laberge JM, Kerlan RK, Wilson MW, Gordon RL. Management of patients with “ex vacuo” pneumothorax after thoracentesis. Acad Radiol 2005; 12:980–986.
- Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg 2009; 9:961–964.
- Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011; 139:1419–1423.
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65(suppl 2):ii32–ii40.
- Thomas JM, Musani AI. Malignant pleural effusions: a review. Clin Chest Med 2013; 34:459–471.
- Thomas R, Francis R, Davies HE, Lee YC. Interventional therapies for malignant pleural effusions: the present and the future. Respirology 2014; 19:809–822.
- Rodriguez-Panadero F, Montes-Worboys A. Mechanisms of pleurodesis. Respiration 2012; 83:91–98.
- Gonzalez AV, Bezwada V, Beamis JF Jr, Villanueva AG. Lung injury following thoracoscopic talc insufflation: experience of a single North American center. Chest 2010; 137:1375–1381.
- Rossi VF, Vargas FS, Marchi E, et al. Acute inflammatory response secondary to intrapleural administration of two types of talc. Eur Respir J 2010; 35:396–401.
- Fysh ET, Waterer GW, Kendall PA, et al. Indwelling pleural catheters reduce inpatient days over pleurodesis for malignant pleural effusion. Chest 2012; 142:394–400.
- Kheir F, Shawwa K, Alokla K, Omballi M, Alraiyes AH. Tunneled pleural catheter for the treatment of malignant pleural effusion: a systematic review and meta-analysis. Am J Ther 2015 Feb 2. [Epub ahead of print]
- Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307:2383–2389.
- Ponrartana S, Laberge JM, Kerlan RK, Wilson MW, Gordon RL. Management of patients with “ex vacuo” pneumothorax after thoracentesis. Acad Radiol 2005; 12:980–986.
- Efthymiou CA, Masudi T, Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg 2009; 9:961–964.
- Reddy C, Ernst A, Lamb C, Feller-Kopman D. Rapid pleurodesis for malignant pleural effusions: a pilot study. Chest 2011; 139:1419–1423.