User login
In 1984, a worker at a Pennsylvania nuclear power plant triggered the radiation detector as he was getting ready to go home. This would not be unusual for such a facility, but there was no nuclear fuel on site at the time. The alarm went off every time he left work.
One day, he triggered the alarm as he crossed the detector on arriving at the plant, leading him to suspect that he was bringing radiation from home. He eventually convinced the plant’s health physicists to check his home, although at first they were opposed to the idea. The results revealed high concentrations of radon everywhere, especially in his basement.
Radon was already known to be associated with health risks in underground miners at that time. This event revealed that a naturally occurring radioactive gas could be found in households at potentially hazardous concentrations.
The incident captured the public’s attention, and the Environmental Protection Agency (EPA) and the US Centers for Disease Control and Prevention (CDC) recommended that nearly all homes be tested.1,2 In 1988, the International Agency for Research on Cancer classified radon as a human carcinogen, and Congress passed the Indoor Radon Abatement Act in response to growing concern over health risks.3 This law funded state and federal measures to survey schools and federal buildings for radon levels, to educate citizens, and to develop programs for technical assistance. The long-term goal was to reduce indoor levels nationwide to no more than outdoor levels.
Radon is still considered an important public health hazard. From 15,000 to 21,000 people are estimated to die of lung cancer as a result of radon exposure each year in the United States, making it the second most common cause of lung cancer, behind smoking.4
Considering the relevance of this issue, this article will review the unique characteristics of radon as a risk factor for lung cancer.
WHAT IS RADON?
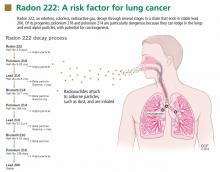
Radon is a noble gas that occurs naturally as a decay product of uranium 238 and thorium 232. It is colorless, tasteless, and imperceptible to our senses. Its most common isotope is radon 222 (222Rn), which has a half-life of 3.8 days and decays by emitting an alpha particle to become polonium 218. The decay chain continues through several intermediate steps until the stable isotope lead 206 is formed (Figure 1). Two of the isotopes in this chain, polonium 218 and polonium 214, also emit alpha particles.5–7
Radon is primarily formed in soil. Its most important precursor, uranium 238, is ubiquitous, found in most soils and rocks in various concentrations. Radon can also be found in surface water, metal mines (uranium, phosphorus, silver, gold), residue of coal combustion, and natural gas.
Outdoor levels are usually much lower than indoor levels, as radon dissipates very quickly. Indoor radon mostly comes from the soil under the house or building, but it can also originate from coal combustion, gas appliances, and water (especially from private wells). In municipal water systems or surface reservoirs, most of the radon dissipates into the air or decays before the water reaches homes.8,9
Radon’s only commercial application in the United States is in calibrating measuring instruments. In the past, it was used in radiography and to treat cancer but was later replaced by other radiation sources that cost less and pose less hazard of alpha radiation.10
HOW RADON CAN HARM
Alpha particles, emitted by radon 222 and its progenies polonium 218 and polonium 214, are highly effective in damaging tissues. Although they do not travel far or fast, with their two protons and two neutrons, alpha particles are heavy and therefore can cause considerable damage at short range. Although alpha particles can be stopped by a thin barrier such as a piece of paper or the skin, if the source is inhaled or ingested and lodges against mucosal linings, the alpha particles emitted can destroy cells.11
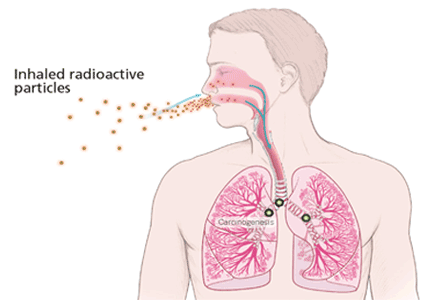
The main route of radon exposure is by inhalation. Since radon is biologically inert, it is readily exhaled after it reaches the lungs. However, radon’s progenies can also be inhaled, either as free particles or attached to airborne particles such as dust, which they tend to attract as a result of their charged state. This attached fraction is believed to be more carcinogenic because it tends to deposit on the respiratory epithelium, notably in the carinae of bronchi. The smaller the dust particle, the deeper it can travel into the lung. The radiation emissions damage the genetic material of cells lining the airways, with the potential to result in lung cancer if the repair process is incomplete.5,8,9
Other routes of exposure include ingestion and dermal exposure. Radon and its progenies can be swallowed in drinking water, passing through the stomach walls and bowels and entering the blood.12 Dermal exposure is not considered a significant route unless the dermis is exposed, since in usual circumstances the skin protects the body from alpha radiation.13
Possible biologic mechanisms by which radon exposure might increase the risk of cancer include gene mutations, chromosome aberrations, generation of reactive oxygen species, up- or down-regulation of cytokines, and production of proteins associated with cell-cycle regulation.14–16
HOW IS RADON MEASURED?
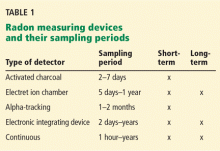
Several devices are commercially available to measure radon levels at home. The most common ones are activated charcoal detectors, electret ion chambers, alpha-track detectors, electronic integrating devices, and continuous monitors. There is no evidence that one device is better than another, but devices that measure radon gas are usually preferred over those that measure decay products because they are simpler to use and more cost-effective. These devices are divided into those used for short-term testing (2–90 days) and long-term testing (Table 1).17
Radon levels can be expressed as follows:
Working levels. One working level (WL) is any combination of radon progeny in 1 L of air that ultimately releases 1.3 × 105 MeV of alpha energy during decay. In studies of miners, the radon progeny concentrations are generally expressed in WL. The cumulative exposure of an individual to this concentration over a “working month” of 170 hours is defined as a working level month (WLM).
Picocuries per liter. In the United States, the rate of decay is commonly reported in picocuries per liter (pCi/L): 1 pCi/L translates to 0.005 WL under usual conditions. The outdoor radon level is normally around 0.4 pCi/L.
Becquerel per cubic meter (Bq/m3) is an International System unit of measure: 1 WL corresponds to 3.7 × 103 Bq/m3, and 1 pCi/L is equivalent to 37 Bq/m3.
Different areas have different radon levels
The Indoor Radon Abatement Act of 1988 helped identify areas in the United States that have the potential for elevated indoor radon levels. An estimated 6 million homes have concentrations greater than 4 pCi/L.
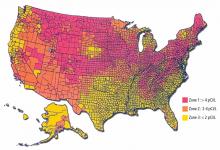
To assist in implementing radon-reducing strategies and allocation of resources, the EPA has created a map (Figure 2) that classifies counties according to the predicted indoor level.18
WHAT IS THE RELATIONSHIP BETWEEN RADON AND LUNG CANCER?
Determining the degree to which radon exposure contributes to lung cancer is a complex task. Radon can be found nearly everywhere, and there are diurnal, seasonal, and random year-to-year variations in the concentration of radon in indoor air.
A minority view
Not everyone agrees that radon is completely bad. For centuries, people have flocked to spas to “take the waters,” and the water at many of these spas has been found to contain radon. In the early 20th century, radiation was touted as having medicinal benefits, and people in many places in the world still go to “radon spas” (some of them in abandoned uranium mines) to help treat conditions such as arthritis and to feel invigorated and energized.
In 2006, a report by Zdrojewicz and Strzelczyk19 urged the medical community to keep an open mind about the possibility that radon exposure may be beneficial in very low doses, perhaps by stimulating repair mechanisms. This concept, called hormesis, differs from the mainstream view that cancer risk rises linearly with radiation dose, with no minimum threshold level (see below).
Risk in miners
As early as in the 16th century, metal miners in central Europe were noted to have a high rate of death from respiratory disease. Radon was discovered in 1900, and in the 20th century lung cancer was linked to high levels of radon detected in uranium mines.
Several small studies of underground miners exposed to high concentrations of radon consistently demonstrated an increased risk of lung cancer.
The Committee on the Biological Effects of Ionizing Radiation (BEIR VI 1999) reviewed 11 major cohort studies of miners. The studies included more than 60,000 miners in Europe, North America, Asia, and Australia, of whom 2,600 died of lung cancer. Lung cancer rates increased linearly with cumulative radon exposure, and the estimated average increase in the lung cancer death rate per WLM in the combined studies was 0.44% (95% confidence interval [CI] 0.20–1.00%). The percentage increase in the lung cancer death rate per WLM varied with time since exposure, with the highest increase in risk during the 5 to 14 years after exposure.4,17 Furthermore, the increase in risk was higher in younger miners, who were exposed to a relatively low radon concentration.
Risk in the general population
The magnitude of the risk in miners led to concern about radon exposure as a cause of lung cancer in the general population. Statistical models were generated that suggested a causal link between radon exposure and lung cancer. Although extrapolation of the results from miners caused controversy, the BEIR VI estimation of risk was validated by studies in the general population.7,20–23
Since the 1980s, several small case-control studies with limited power examined the relationship between indoor radon and lung cancer in the general population. In these studies, individuals who had developed lung cancer were compared with controls who had not developed the disease but who otherwise represented the population from which the cases of lung cancer came.
To improve the statistical power, the investigators of the major studies in Europe, North America, and China pooled the results in separate analyses (Table 2).7,20–23 The average radon concentration to which each individual had been exposed over the previous decades was estimated by measuring the radon concentration at their present and previous homes. On the basis of information from the uranium miners, these studies assumed that the period of exposure was the 30 years ending 5 years before the diagnosis or at death from lung cancer.
The results provided convincing evidence that radon exposure is a cause of lung cancer in the general population and substantiated the extrapolation from the studies of miners. Further, the results of all three pooled analyses were consistent with a linear dose-response relationship with no threshold, suggesting an increased risk of lung cancer even with a radon level below 4 pCi/L (200 Bq/m3), which is the concentration at which mitigation actions are recommended in many countries.17
The North American pooled analysis included 3,662 cases and 4,966 controls from seven studies in the United States and Canada. When data from all studies were combined, the risk of lung cancer was found to increase by 11% per 100-Bq/m3 (about 2.7-pCi/L) increase in measured radon concentration (95% CI 0%–28%). The estimated increase in lung cancer was independent of age, sex, or smoking history.7,20
The Chinese pooled data22 demonstrated a 13% (95% CI 1%–36%) increased risk per 100 Bq/m3.
In the European study, the risk of lung cancer increased by 8% per 100 Bq/m3 (95% CI 3%–16%). The European investigators repeated the analysis, taking into account the random year-to-year variability in measured radon concentration, finding the final estimated risk was an increase of 16% per 100 Bq/m3 using long-term average concentration.21
The combined estimate21,24 from the three pooling studies based on measured radon concentration is an increased risk of lung cancer of 10% per 100 Bq/m3.
Synergistic risk with smoking
Radon exposure was independently associated with lung cancer, and the relationship with cigarette smoking is believed to be synergistic. The radon progeny particles attach themselves to smoke and dust and are then deposited in the bronchial epithelium.25
In the pool of European case-control studies, the cancer risk for current smokers of 15 to 24 cigarettes per day relative to that in never-smokers was 25.8 (95% CI 21–31). Assuming that in the same analysis the lung cancer risk increased by 16% per 100 Bq/m3 of usual radon concentration regardless of smoking status, the cumulative absolute risk by age 75 would be 0.67% in those who never smoked and 16% in smokers at usual radon levels of 400 Bq/m3 (11 pCi/L).21
Rates of all lung cancer subtypes increased
Radon exposure is not associated with a specific histologic subtype of lung cancer. It has been speculated that the incidence of the small-cell subtype might be slightly increased because radon tends to deposit in the more central bronchial carinae.20,21 However, all subtypes have been described in association with radon, the most common being adenocarcinoma and squamous cell carcinoma.26–28
EFFECT OF MITIGATION MEASURES
The US Surgeon General and the EPA recommend that all homes be tested.18 Short-term tests should be used first, keeping in mind that diurnal and seasonal variations may occur.
The World Health Organization has proposed a reference level of 100 Bq/m3 (2.7 pCi/L) to minimize health hazards from indoor radon exposure.17 If this level cannot be reached under the country-specific conditions, the chosen reference level should not exceed 300 Bq/m3 (8 pCi/L).
In the United States, if the result of home testing is higher than 4 pCi/L, a follow-up measurement should be done using a different short-term test or a long-term test. If the follow-up result confirms a level of more than 4 pCi/L, mitigating actions are recommended. The goal is to reduce the indoor radon level as much as possible—down to zero or at least comparable to outdoor levels (national average 0.4 pCi/L).18
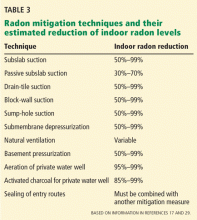
A variety of radon mitigation strategies have been used, with different rates of efficacy (Table 3). The optimal strategy depends on the likely source or cause, construction characteristics, soil, and climate.29 Table 4 lists resources for the general public about testing and mitigation measures.
How beneficial is radon mitigation?
Although it is logical to try to reduce the indoor radon concentration, there is no strong evidence yet that this intervention decreases the incidence of lung cancer in the general population.
Using the BEIR VI risk model, Méndez et al30 estimated a 21% reduction in the annual radon-related lung cancer mortality rate by 2100 if all households were compliant with government recommendations (mitigation actions at levels of 4 pCi/L) and assuming that the percentage of cigarette smokers remained constant.
On the other hand, if the number of smokers continues to decline, the benefits from radon mitigation may be less. The expected benefit from mitigation in this scenario is a reduction of 12% in annual radon-related deaths by the year 2100.30 However, it will be challenging to determine whether the expected decline in the incidence of lung cancer and lung cancer deaths is truly attributable to mitigation measures.
MANAGING PATIENTS EXPOSED TO RADON
Screen for lung cancer in smokers only
The National Lung Screening Study (NLST) was a large multicenter trial of annual low-dose computed tomography (CT) to screen for lung cancer in a cohort at high risk: age 55 to 74, at least a 30 pack-year history of smoking in a current smoker, or a former smoker who quit within the past 15 years. The trial demonstrated a 20% reduction in lung cancer deaths in the CT screening group.31
Since the publication of the NLST results, many societies have endorsed screening for lung cancer with low-dose CT using the study criteria. The National Comprehensive Cancer Network (NCCN) expanded these criteria and has recommended screening in patients over age 50 who have a history of smoking and one additional risk factor, such as radon exposure.
However, radon exposure has not been incorporated into a lung cancer risk-prediction model, and there is no empirical evidence suggesting that people who have such a history would benefit from screening.32,33 The joint guidelines of the American College of Chest Physicians and American Society of Clinical Oncology recommend annual low-dose CT screening only for patients who meet the NLST criteria.34
What to do about indeterminate lung nodules
The widely used guidelines from the Fleischner Society35 on how to manage small lung nodules stratify patients into groups at low and high risk of developing lung cancer on the basis of risk factors. The guidelines apply to adults age 35 and older in whom an indeterminate solid nodule was recently detected.
If a patient is at high risk, the recommended approach includes follow-up in shorter intervals depending on the nodule size. History of smoking is recognized as a major risk factor, and the statement also lists family history and exposure to asbestos, uranium, and radon.35
Although the association of radon with lung cancer has been shown in epidemiologic studies, radon exposure has not been included in validated statistical models that assess the probability that an indeterminate lung nodule is malignant. We would expect the risk to be higher in miners, who suffer a more intense exposure to higher levels of radon, than in the general population, which has a low and constantly variable residential exposure. Furthermore, there are no data to support a more aggressive follow-up approach in patients with indeterminate lung nodules and a history of radon exposure.
RADON AND OTHER CANCERS
When a person is exposed to radon, the bronchial epithelium receives the highest dose of ionizing radiation, but other organs such as the kidneys, stomach, and bone marrow may receive doses as well, although lower. Several studies have looked into possible associations, but there is no strong evidence to suggest an increased mortality rate related to radon from cancers other than lung.24,36 However, there seems to be a positive association between radon and the incidence of lymphoproliferative disorders in uranium miners.37,38
Radon can be measured in drinking water, and a few studies have looked at a possible association with gastrointestinal malignancies. The results did not reveal a consistent positive correlation.39,40 The risk of cancer from exposure to radon in the public water supply is likely small and mostly from the transfer of radon particles into the air and not from drinking the water. On the other hand, the risk could be higher with private wells, where radon levels are variable and are possibly higher than from public sources.41
DATA ARE INSUFFICIENT TO GUIDE MANAGEMENT
Radon is a naturally occurring and ubiquitous radioactive gas that can cause tissue damage. Cohort and case-control studies have demonstrated that radon exposure is associated with increased risk of lung cancer. It is recommended that radon levels be measured in every home in the United States and mitigation measures instituted if levels exceed 4 pCi/L.
There are insufficient data to help guide the management of patients with a history of radon exposure, and prospective studies are needed to better understand the individual risk of developing lung cancer and the appropriate management of such patients.
Smoking cessation is an integral part of lung cancer risk reduction from radon exposure.
- Berreby D. The radon raiders: turning perils into profits. The New York Times 1987. www.nytimes.com/1987/07/26/business/the-radon-raiders-turning-perils-into-profits.html?src=pm&pagewanted=1. Accessed August 5, 2014.
- Lewis RK. A history of radon—1470 to 1984. www.ohio-radonpro.com/Radon_History.html. Accessed August 5, 2014.
- World Health Organization (WHO). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Manmade mineral fibres and radon. Summary of data reported and evaluation. http://monographs.iarc.fr/ENG/Monographs/vol43/volume43.pdf. Accessed August 5, 2014.
- Committee on Health Risks of Exposure to Radon (BEIR VI). Health effects of exposure to radon: BEIR VI. Washington, DC: National Academies Press; 1999.
- Samet JM. Radon and lung cancer. J Natl Cancer Inst 1989; 81:745–757.
- Lewis RJ, Lewis Sr RJ. Hawley’s condensed chemical dictionary. 14thed. New York: Wiley-Interscience; 2001.
- Krewski D, Lubin JH, Zielinski JM, et al. Residential radon and risk of lung cancer: a combined analysis of 7 North American case-control studies. Epidemiology 2005; 16:137–145.
- Darby S, Hill D, Doll R. Radon: a likely carcinogen at all exposures. Ann Oncol 2001; 12:1341–1351.
- Sethi TK, El-Ghamry MN, Kloecker GH. Radon and lung cancer. Clin Adv Hematol Oncol 2012; 10:157–164.
- Morrison A. Use of radon for industrial radiography. Can J Res 1945; 23:413–419.
- Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res 1997; 57:3963–3971.
- Ishikawa T, Narazaki Y, Yasuoka Y, Tokonami S, Yamada Y. Bio-kinetics of radon ingested from drinking water. Radiat Prot Dosimetry 2003; 105:65–70.
- Ishikawa T, Yamada Y, Fukutsu K, Tokonami S. Deposition and clearance for radon progeny in the human respiratory tract. Radiat Prot Dosimetry 2003; 105:143–148.
- Farkas A, Hofmann W, Balásházy I, Szoke I, Madas BG, Moustafa M. Effect of site-specific bronchial radon progeny deposition on the spatial and temporal distributions of cellular responses. Radiat Environ Biophys 2011; 50:281–297.
- Robertson A, Allen J, Laney R, Curnow A. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci 2013; 14:14024–14063.
- Chauhan V, Howland M, Wilkins R. Effects of alpha-particle radiation on microRNA responses in human cell-lines. Open Biochem J 2012; 6:16–22.
- World Health Organization (WHO). WHO handbook on indoor radon: a public health perspective; 2009. www.nrsb.org/pdf/WHO%20Radon%20Handbook.pdf. Accessed August 5, 2014.
- United States Environmental Protection Agency (EPA). www.epa.gov/radon/. Accessed August 5, 2014.
- Zdrojewicz Z, Strzelczyk JJ. Radon treatment controversy. Dose Response 2006; 4:106–118.
- Krewski D, Lubin JH, Zielinski JM, et al. A combined analysis of North American case-control studies of residential radon and lung cancer. J Toxicol Environ Health A 2006; 69:533–597.
- Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ 2005; 330:223.
- Lubin JH, Wang ZY, Boice JD, et al. Risk of lung cancer and residential radon in China: pooled results of two studies. Int J Cancer 2004; 109:132–137.
- Darby S, Hill D, Deo H, et al. Residential radon and lung cancer—detailed results of a collaborative analysis of individual data on 7,148 persons with lung cancer and 14,208 persons without lung cancer from 13 epidemiologic studies in Europe. Scand J Work Environ Health 2006; 32(suppl 1):1–83.
- Darby SC, Whitley E, Howe GR, et al. Radon and cancers other than lung cancer in underground miners: a collaborative analysis of 11 studies. J Natl Cancer Inst 1995; 87:378–384.
- Baias PF, Hofmann W, Winkler-Heil R, Cosma C, Duliu OG. Lung dosimetry for inhaled radon progeny in smokers. Radiat Prot Dosimetry 2010; 138:111–118.
- Land CE, Shimosato Y, Saccomanno G, et al. Radiation-associated lung cancer: a comparison of the histology of lung cancers in uranium miners and survivors of the atomic bombings of Hiroshima and Nagasaki. Radiat Res 1993; 134:234–243.
- Kreuzer M, Müller KM, Brachner A, et al. Histopathologic findings of lung carcinoma in German uranium miners. Cancer 2000; 89:2613–2621.
- Saccomanno G, Auerbach O, Kuschner M, et al. A comparison between the localization of lung tumors in uranium miners and in nonminers from 1947 to 1991. Cancer 1996; 77:1278–1283.
- Rahman NM, Tracy BL. Radon control systems in existing and new construction: a review. Radiat Prot Dosimetry 2009; 135:243–255.
- Méndez D, Alshanqeety O, Warner KE, Lantz PM, Courant PN. The impact of declining smoking on radon-related lung cancer in the United States. Am J Public Health 2011; 101:310–314.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Wood DE, Eapen GA, Ettinger DS, et al. Lung cancer screening. J Natl Compr Canc Netw 2012; 10:240–265.
- Ettinger DS, Akerley W, Borghaei H, et al; NCCN (National Comprehensive Cancer Network). Non-small cell lung cancer. J Natl Compr Canc Netw 2012; 10:1236–1271.
- Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143(suppl 5):e78S–e92S.
- MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005; 237:395–400.
- Darby SC, Radford EP, Whitley E. Radon exposure and cancers other than lung cancer in Swedish iron miners. Environ Health Perspect 1995; 103(suppl 2):45–47.
- Laurier D, Tirmarche M, Mitton N, et al. An update of cancer mortality among the French cohort of uranium miners: extended follow-up and new source of data for causes of death. Eur J Epidemiol 2004; 19:139–146.
- Rericha V, Kulich M, Rericha R, Shore DL, Sandler DP. Incidence of leukemia, lymphoma, and multiple myeloma in Czech uranium miners: a case-cohort study. Environ Health Perspect 2006; 114:818–822.
- Auvinen A, Salonen L, Pekkanen J, Pukkala E, Ilus T, Kurttio P. Radon and other natural radionuclides in drinking water and risk of stomach cancer: a case-cohort study in Finland. Int J Cancer 2005; 114:109–113.
- Kjellberg S, Wiseman JS. The relationship of radon to gastrointestinal malignancies. Am Surg 1995; 61:822–825.
- Cappello MA, Ferraro A, Mendelsohn AB, Prehn AW. Radon-contaminated drinking water from private wells: an environmental health assessment examining a rural Colorado mountain community’s exposure. J Environ Health 2013; 76:18–24.
In 1984, a worker at a Pennsylvania nuclear power plant triggered the radiation detector as he was getting ready to go home. This would not be unusual for such a facility, but there was no nuclear fuel on site at the time. The alarm went off every time he left work.
One day, he triggered the alarm as he crossed the detector on arriving at the plant, leading him to suspect that he was bringing radiation from home. He eventually convinced the plant’s health physicists to check his home, although at first they were opposed to the idea. The results revealed high concentrations of radon everywhere, especially in his basement.
Radon was already known to be associated with health risks in underground miners at that time. This event revealed that a naturally occurring radioactive gas could be found in households at potentially hazardous concentrations.
The incident captured the public’s attention, and the Environmental Protection Agency (EPA) and the US Centers for Disease Control and Prevention (CDC) recommended that nearly all homes be tested.1,2 In 1988, the International Agency for Research on Cancer classified radon as a human carcinogen, and Congress passed the Indoor Radon Abatement Act in response to growing concern over health risks.3 This law funded state and federal measures to survey schools and federal buildings for radon levels, to educate citizens, and to develop programs for technical assistance. The long-term goal was to reduce indoor levels nationwide to no more than outdoor levels.
Radon is still considered an important public health hazard. From 15,000 to 21,000 people are estimated to die of lung cancer as a result of radon exposure each year in the United States, making it the second most common cause of lung cancer, behind smoking.4
Considering the relevance of this issue, this article will review the unique characteristics of radon as a risk factor for lung cancer.
WHAT IS RADON?
Radon is a noble gas that occurs naturally as a decay product of uranium 238 and thorium 232. It is colorless, tasteless, and imperceptible to our senses. Its most common isotope is radon 222 (222Rn), which has a half-life of 3.8 days and decays by emitting an alpha particle to become polonium 218. The decay chain continues through several intermediate steps until the stable isotope lead 206 is formed (Figure 1). Two of the isotopes in this chain, polonium 218 and polonium 214, also emit alpha particles.5–7
Radon is primarily formed in soil. Its most important precursor, uranium 238, is ubiquitous, found in most soils and rocks in various concentrations. Radon can also be found in surface water, metal mines (uranium, phosphorus, silver, gold), residue of coal combustion, and natural gas.
Outdoor levels are usually much lower than indoor levels, as radon dissipates very quickly. Indoor radon mostly comes from the soil under the house or building, but it can also originate from coal combustion, gas appliances, and water (especially from private wells). In municipal water systems or surface reservoirs, most of the radon dissipates into the air or decays before the water reaches homes.8,9
Radon’s only commercial application in the United States is in calibrating measuring instruments. In the past, it was used in radiography and to treat cancer but was later replaced by other radiation sources that cost less and pose less hazard of alpha radiation.10
HOW RADON CAN HARM
Alpha particles, emitted by radon 222 and its progenies polonium 218 and polonium 214, are highly effective in damaging tissues. Although they do not travel far or fast, with their two protons and two neutrons, alpha particles are heavy and therefore can cause considerable damage at short range. Although alpha particles can be stopped by a thin barrier such as a piece of paper or the skin, if the source is inhaled or ingested and lodges against mucosal linings, the alpha particles emitted can destroy cells.11
The main route of radon exposure is by inhalation. Since radon is biologically inert, it is readily exhaled after it reaches the lungs. However, radon’s progenies can also be inhaled, either as free particles or attached to airborne particles such as dust, which they tend to attract as a result of their charged state. This attached fraction is believed to be more carcinogenic because it tends to deposit on the respiratory epithelium, notably in the carinae of bronchi. The smaller the dust particle, the deeper it can travel into the lung. The radiation emissions damage the genetic material of cells lining the airways, with the potential to result in lung cancer if the repair process is incomplete.5,8,9
Other routes of exposure include ingestion and dermal exposure. Radon and its progenies can be swallowed in drinking water, passing through the stomach walls and bowels and entering the blood.12 Dermal exposure is not considered a significant route unless the dermis is exposed, since in usual circumstances the skin protects the body from alpha radiation.13
Possible biologic mechanisms by which radon exposure might increase the risk of cancer include gene mutations, chromosome aberrations, generation of reactive oxygen species, up- or down-regulation of cytokines, and production of proteins associated with cell-cycle regulation.14–16
HOW IS RADON MEASURED?
Several devices are commercially available to measure radon levels at home. The most common ones are activated charcoal detectors, electret ion chambers, alpha-track detectors, electronic integrating devices, and continuous monitors. There is no evidence that one device is better than another, but devices that measure radon gas are usually preferred over those that measure decay products because they are simpler to use and more cost-effective. These devices are divided into those used for short-term testing (2–90 days) and long-term testing (Table 1).17
Radon levels can be expressed as follows:
Working levels. One working level (WL) is any combination of radon progeny in 1 L of air that ultimately releases 1.3 × 105 MeV of alpha energy during decay. In studies of miners, the radon progeny concentrations are generally expressed in WL. The cumulative exposure of an individual to this concentration over a “working month” of 170 hours is defined as a working level month (WLM).
Picocuries per liter. In the United States, the rate of decay is commonly reported in picocuries per liter (pCi/L): 1 pCi/L translates to 0.005 WL under usual conditions. The outdoor radon level is normally around 0.4 pCi/L.
Becquerel per cubic meter (Bq/m3) is an International System unit of measure: 1 WL corresponds to 3.7 × 103 Bq/m3, and 1 pCi/L is equivalent to 37 Bq/m3.
Different areas have different radon levels
The Indoor Radon Abatement Act of 1988 helped identify areas in the United States that have the potential for elevated indoor radon levels. An estimated 6 million homes have concentrations greater than 4 pCi/L.
To assist in implementing radon-reducing strategies and allocation of resources, the EPA has created a map (Figure 2) that classifies counties according to the predicted indoor level.18
WHAT IS THE RELATIONSHIP BETWEEN RADON AND LUNG CANCER?
Determining the degree to which radon exposure contributes to lung cancer is a complex task. Radon can be found nearly everywhere, and there are diurnal, seasonal, and random year-to-year variations in the concentration of radon in indoor air.
A minority view
Not everyone agrees that radon is completely bad. For centuries, people have flocked to spas to “take the waters,” and the water at many of these spas has been found to contain radon. In the early 20th century, radiation was touted as having medicinal benefits, and people in many places in the world still go to “radon spas” (some of them in abandoned uranium mines) to help treat conditions such as arthritis and to feel invigorated and energized.
In 2006, a report by Zdrojewicz and Strzelczyk19 urged the medical community to keep an open mind about the possibility that radon exposure may be beneficial in very low doses, perhaps by stimulating repair mechanisms. This concept, called hormesis, differs from the mainstream view that cancer risk rises linearly with radiation dose, with no minimum threshold level (see below).
Risk in miners
As early as in the 16th century, metal miners in central Europe were noted to have a high rate of death from respiratory disease. Radon was discovered in 1900, and in the 20th century lung cancer was linked to high levels of radon detected in uranium mines.
Several small studies of underground miners exposed to high concentrations of radon consistently demonstrated an increased risk of lung cancer.
The Committee on the Biological Effects of Ionizing Radiation (BEIR VI 1999) reviewed 11 major cohort studies of miners. The studies included more than 60,000 miners in Europe, North America, Asia, and Australia, of whom 2,600 died of lung cancer. Lung cancer rates increased linearly with cumulative radon exposure, and the estimated average increase in the lung cancer death rate per WLM in the combined studies was 0.44% (95% confidence interval [CI] 0.20–1.00%). The percentage increase in the lung cancer death rate per WLM varied with time since exposure, with the highest increase in risk during the 5 to 14 years after exposure.4,17 Furthermore, the increase in risk was higher in younger miners, who were exposed to a relatively low radon concentration.
Risk in the general population
The magnitude of the risk in miners led to concern about radon exposure as a cause of lung cancer in the general population. Statistical models were generated that suggested a causal link between radon exposure and lung cancer. Although extrapolation of the results from miners caused controversy, the BEIR VI estimation of risk was validated by studies in the general population.7,20–23
Since the 1980s, several small case-control studies with limited power examined the relationship between indoor radon and lung cancer in the general population. In these studies, individuals who had developed lung cancer were compared with controls who had not developed the disease but who otherwise represented the population from which the cases of lung cancer came.
To improve the statistical power, the investigators of the major studies in Europe, North America, and China pooled the results in separate analyses (Table 2).7,20–23 The average radon concentration to which each individual had been exposed over the previous decades was estimated by measuring the radon concentration at their present and previous homes. On the basis of information from the uranium miners, these studies assumed that the period of exposure was the 30 years ending 5 years before the diagnosis or at death from lung cancer.
The results provided convincing evidence that radon exposure is a cause of lung cancer in the general population and substantiated the extrapolation from the studies of miners. Further, the results of all three pooled analyses were consistent with a linear dose-response relationship with no threshold, suggesting an increased risk of lung cancer even with a radon level below 4 pCi/L (200 Bq/m3), which is the concentration at which mitigation actions are recommended in many countries.17
The North American pooled analysis included 3,662 cases and 4,966 controls from seven studies in the United States and Canada. When data from all studies were combined, the risk of lung cancer was found to increase by 11% per 100-Bq/m3 (about 2.7-pCi/L) increase in measured radon concentration (95% CI 0%–28%). The estimated increase in lung cancer was independent of age, sex, or smoking history.7,20
The Chinese pooled data22 demonstrated a 13% (95% CI 1%–36%) increased risk per 100 Bq/m3.
In the European study, the risk of lung cancer increased by 8% per 100 Bq/m3 (95% CI 3%–16%). The European investigators repeated the analysis, taking into account the random year-to-year variability in measured radon concentration, finding the final estimated risk was an increase of 16% per 100 Bq/m3 using long-term average concentration.21
The combined estimate21,24 from the three pooling studies based on measured radon concentration is an increased risk of lung cancer of 10% per 100 Bq/m3.
Synergistic risk with smoking
Radon exposure was independently associated with lung cancer, and the relationship with cigarette smoking is believed to be synergistic. The radon progeny particles attach themselves to smoke and dust and are then deposited in the bronchial epithelium.25
In the pool of European case-control studies, the cancer risk for current smokers of 15 to 24 cigarettes per day relative to that in never-smokers was 25.8 (95% CI 21–31). Assuming that in the same analysis the lung cancer risk increased by 16% per 100 Bq/m3 of usual radon concentration regardless of smoking status, the cumulative absolute risk by age 75 would be 0.67% in those who never smoked and 16% in smokers at usual radon levels of 400 Bq/m3 (11 pCi/L).21
Rates of all lung cancer subtypes increased
Radon exposure is not associated with a specific histologic subtype of lung cancer. It has been speculated that the incidence of the small-cell subtype might be slightly increased because radon tends to deposit in the more central bronchial carinae.20,21 However, all subtypes have been described in association with radon, the most common being adenocarcinoma and squamous cell carcinoma.26–28
EFFECT OF MITIGATION MEASURES
The US Surgeon General and the EPA recommend that all homes be tested.18 Short-term tests should be used first, keeping in mind that diurnal and seasonal variations may occur.
The World Health Organization has proposed a reference level of 100 Bq/m3 (2.7 pCi/L) to minimize health hazards from indoor radon exposure.17 If this level cannot be reached under the country-specific conditions, the chosen reference level should not exceed 300 Bq/m3 (8 pCi/L).
In the United States, if the result of home testing is higher than 4 pCi/L, a follow-up measurement should be done using a different short-term test or a long-term test. If the follow-up result confirms a level of more than 4 pCi/L, mitigating actions are recommended. The goal is to reduce the indoor radon level as much as possible—down to zero or at least comparable to outdoor levels (national average 0.4 pCi/L).18
A variety of radon mitigation strategies have been used, with different rates of efficacy (Table 3). The optimal strategy depends on the likely source or cause, construction characteristics, soil, and climate.29 Table 4 lists resources for the general public about testing and mitigation measures.
How beneficial is radon mitigation?
Although it is logical to try to reduce the indoor radon concentration, there is no strong evidence yet that this intervention decreases the incidence of lung cancer in the general population.
Using the BEIR VI risk model, Méndez et al30 estimated a 21% reduction in the annual radon-related lung cancer mortality rate by 2100 if all households were compliant with government recommendations (mitigation actions at levels of 4 pCi/L) and assuming that the percentage of cigarette smokers remained constant.
On the other hand, if the number of smokers continues to decline, the benefits from radon mitigation may be less. The expected benefit from mitigation in this scenario is a reduction of 12% in annual radon-related deaths by the year 2100.30 However, it will be challenging to determine whether the expected decline in the incidence of lung cancer and lung cancer deaths is truly attributable to mitigation measures.
MANAGING PATIENTS EXPOSED TO RADON
Screen for lung cancer in smokers only
The National Lung Screening Study (NLST) was a large multicenter trial of annual low-dose computed tomography (CT) to screen for lung cancer in a cohort at high risk: age 55 to 74, at least a 30 pack-year history of smoking in a current smoker, or a former smoker who quit within the past 15 years. The trial demonstrated a 20% reduction in lung cancer deaths in the CT screening group.31
Since the publication of the NLST results, many societies have endorsed screening for lung cancer with low-dose CT using the study criteria. The National Comprehensive Cancer Network (NCCN) expanded these criteria and has recommended screening in patients over age 50 who have a history of smoking and one additional risk factor, such as radon exposure.
However, radon exposure has not been incorporated into a lung cancer risk-prediction model, and there is no empirical evidence suggesting that people who have such a history would benefit from screening.32,33 The joint guidelines of the American College of Chest Physicians and American Society of Clinical Oncology recommend annual low-dose CT screening only for patients who meet the NLST criteria.34
What to do about indeterminate lung nodules
The widely used guidelines from the Fleischner Society35 on how to manage small lung nodules stratify patients into groups at low and high risk of developing lung cancer on the basis of risk factors. The guidelines apply to adults age 35 and older in whom an indeterminate solid nodule was recently detected.
If a patient is at high risk, the recommended approach includes follow-up in shorter intervals depending on the nodule size. History of smoking is recognized as a major risk factor, and the statement also lists family history and exposure to asbestos, uranium, and radon.35
Although the association of radon with lung cancer has been shown in epidemiologic studies, radon exposure has not been included in validated statistical models that assess the probability that an indeterminate lung nodule is malignant. We would expect the risk to be higher in miners, who suffer a more intense exposure to higher levels of radon, than in the general population, which has a low and constantly variable residential exposure. Furthermore, there are no data to support a more aggressive follow-up approach in patients with indeterminate lung nodules and a history of radon exposure.
RADON AND OTHER CANCERS
When a person is exposed to radon, the bronchial epithelium receives the highest dose of ionizing radiation, but other organs such as the kidneys, stomach, and bone marrow may receive doses as well, although lower. Several studies have looked into possible associations, but there is no strong evidence to suggest an increased mortality rate related to radon from cancers other than lung.24,36 However, there seems to be a positive association between radon and the incidence of lymphoproliferative disorders in uranium miners.37,38
Radon can be measured in drinking water, and a few studies have looked at a possible association with gastrointestinal malignancies. The results did not reveal a consistent positive correlation.39,40 The risk of cancer from exposure to radon in the public water supply is likely small and mostly from the transfer of radon particles into the air and not from drinking the water. On the other hand, the risk could be higher with private wells, where radon levels are variable and are possibly higher than from public sources.41
DATA ARE INSUFFICIENT TO GUIDE MANAGEMENT
Radon is a naturally occurring and ubiquitous radioactive gas that can cause tissue damage. Cohort and case-control studies have demonstrated that radon exposure is associated with increased risk of lung cancer. It is recommended that radon levels be measured in every home in the United States and mitigation measures instituted if levels exceed 4 pCi/L.
There are insufficient data to help guide the management of patients with a history of radon exposure, and prospective studies are needed to better understand the individual risk of developing lung cancer and the appropriate management of such patients.
Smoking cessation is an integral part of lung cancer risk reduction from radon exposure.
In 1984, a worker at a Pennsylvania nuclear power plant triggered the radiation detector as he was getting ready to go home. This would not be unusual for such a facility, but there was no nuclear fuel on site at the time. The alarm went off every time he left work.
One day, he triggered the alarm as he crossed the detector on arriving at the plant, leading him to suspect that he was bringing radiation from home. He eventually convinced the plant’s health physicists to check his home, although at first they were opposed to the idea. The results revealed high concentrations of radon everywhere, especially in his basement.
Radon was already known to be associated with health risks in underground miners at that time. This event revealed that a naturally occurring radioactive gas could be found in households at potentially hazardous concentrations.
The incident captured the public’s attention, and the Environmental Protection Agency (EPA) and the US Centers for Disease Control and Prevention (CDC) recommended that nearly all homes be tested.1,2 In 1988, the International Agency for Research on Cancer classified radon as a human carcinogen, and Congress passed the Indoor Radon Abatement Act in response to growing concern over health risks.3 This law funded state and federal measures to survey schools and federal buildings for radon levels, to educate citizens, and to develop programs for technical assistance. The long-term goal was to reduce indoor levels nationwide to no more than outdoor levels.
Radon is still considered an important public health hazard. From 15,000 to 21,000 people are estimated to die of lung cancer as a result of radon exposure each year in the United States, making it the second most common cause of lung cancer, behind smoking.4
Considering the relevance of this issue, this article will review the unique characteristics of radon as a risk factor for lung cancer.
WHAT IS RADON?
Radon is a noble gas that occurs naturally as a decay product of uranium 238 and thorium 232. It is colorless, tasteless, and imperceptible to our senses. Its most common isotope is radon 222 (222Rn), which has a half-life of 3.8 days and decays by emitting an alpha particle to become polonium 218. The decay chain continues through several intermediate steps until the stable isotope lead 206 is formed (Figure 1). Two of the isotopes in this chain, polonium 218 and polonium 214, also emit alpha particles.5–7
Radon is primarily formed in soil. Its most important precursor, uranium 238, is ubiquitous, found in most soils and rocks in various concentrations. Radon can also be found in surface water, metal mines (uranium, phosphorus, silver, gold), residue of coal combustion, and natural gas.
Outdoor levels are usually much lower than indoor levels, as radon dissipates very quickly. Indoor radon mostly comes from the soil under the house or building, but it can also originate from coal combustion, gas appliances, and water (especially from private wells). In municipal water systems or surface reservoirs, most of the radon dissipates into the air or decays before the water reaches homes.8,9
Radon’s only commercial application in the United States is in calibrating measuring instruments. In the past, it was used in radiography and to treat cancer but was later replaced by other radiation sources that cost less and pose less hazard of alpha radiation.10
HOW RADON CAN HARM
Alpha particles, emitted by radon 222 and its progenies polonium 218 and polonium 214, are highly effective in damaging tissues. Although they do not travel far or fast, with their two protons and two neutrons, alpha particles are heavy and therefore can cause considerable damage at short range. Although alpha particles can be stopped by a thin barrier such as a piece of paper or the skin, if the source is inhaled or ingested and lodges against mucosal linings, the alpha particles emitted can destroy cells.11
The main route of radon exposure is by inhalation. Since radon is biologically inert, it is readily exhaled after it reaches the lungs. However, radon’s progenies can also be inhaled, either as free particles or attached to airborne particles such as dust, which they tend to attract as a result of their charged state. This attached fraction is believed to be more carcinogenic because it tends to deposit on the respiratory epithelium, notably in the carinae of bronchi. The smaller the dust particle, the deeper it can travel into the lung. The radiation emissions damage the genetic material of cells lining the airways, with the potential to result in lung cancer if the repair process is incomplete.5,8,9
Other routes of exposure include ingestion and dermal exposure. Radon and its progenies can be swallowed in drinking water, passing through the stomach walls and bowels and entering the blood.12 Dermal exposure is not considered a significant route unless the dermis is exposed, since in usual circumstances the skin protects the body from alpha radiation.13
Possible biologic mechanisms by which radon exposure might increase the risk of cancer include gene mutations, chromosome aberrations, generation of reactive oxygen species, up- or down-regulation of cytokines, and production of proteins associated with cell-cycle regulation.14–16
HOW IS RADON MEASURED?
Several devices are commercially available to measure radon levels at home. The most common ones are activated charcoal detectors, electret ion chambers, alpha-track detectors, electronic integrating devices, and continuous monitors. There is no evidence that one device is better than another, but devices that measure radon gas are usually preferred over those that measure decay products because they are simpler to use and more cost-effective. These devices are divided into those used for short-term testing (2–90 days) and long-term testing (Table 1).17
Radon levels can be expressed as follows:
Working levels. One working level (WL) is any combination of radon progeny in 1 L of air that ultimately releases 1.3 × 105 MeV of alpha energy during decay. In studies of miners, the radon progeny concentrations are generally expressed in WL. The cumulative exposure of an individual to this concentration over a “working month” of 170 hours is defined as a working level month (WLM).
Picocuries per liter. In the United States, the rate of decay is commonly reported in picocuries per liter (pCi/L): 1 pCi/L translates to 0.005 WL under usual conditions. The outdoor radon level is normally around 0.4 pCi/L.
Becquerel per cubic meter (Bq/m3) is an International System unit of measure: 1 WL corresponds to 3.7 × 103 Bq/m3, and 1 pCi/L is equivalent to 37 Bq/m3.
Different areas have different radon levels
The Indoor Radon Abatement Act of 1988 helped identify areas in the United States that have the potential for elevated indoor radon levels. An estimated 6 million homes have concentrations greater than 4 pCi/L.
To assist in implementing radon-reducing strategies and allocation of resources, the EPA has created a map (Figure 2) that classifies counties according to the predicted indoor level.18
WHAT IS THE RELATIONSHIP BETWEEN RADON AND LUNG CANCER?
Determining the degree to which radon exposure contributes to lung cancer is a complex task. Radon can be found nearly everywhere, and there are diurnal, seasonal, and random year-to-year variations in the concentration of radon in indoor air.
A minority view
Not everyone agrees that radon is completely bad. For centuries, people have flocked to spas to “take the waters,” and the water at many of these spas has been found to contain radon. In the early 20th century, radiation was touted as having medicinal benefits, and people in many places in the world still go to “radon spas” (some of them in abandoned uranium mines) to help treat conditions such as arthritis and to feel invigorated and energized.
In 2006, a report by Zdrojewicz and Strzelczyk19 urged the medical community to keep an open mind about the possibility that radon exposure may be beneficial in very low doses, perhaps by stimulating repair mechanisms. This concept, called hormesis, differs from the mainstream view that cancer risk rises linearly with radiation dose, with no minimum threshold level (see below).
Risk in miners
As early as in the 16th century, metal miners in central Europe were noted to have a high rate of death from respiratory disease. Radon was discovered in 1900, and in the 20th century lung cancer was linked to high levels of radon detected in uranium mines.
Several small studies of underground miners exposed to high concentrations of radon consistently demonstrated an increased risk of lung cancer.
The Committee on the Biological Effects of Ionizing Radiation (BEIR VI 1999) reviewed 11 major cohort studies of miners. The studies included more than 60,000 miners in Europe, North America, Asia, and Australia, of whom 2,600 died of lung cancer. Lung cancer rates increased linearly with cumulative radon exposure, and the estimated average increase in the lung cancer death rate per WLM in the combined studies was 0.44% (95% confidence interval [CI] 0.20–1.00%). The percentage increase in the lung cancer death rate per WLM varied with time since exposure, with the highest increase in risk during the 5 to 14 years after exposure.4,17 Furthermore, the increase in risk was higher in younger miners, who were exposed to a relatively low radon concentration.
Risk in the general population
The magnitude of the risk in miners led to concern about radon exposure as a cause of lung cancer in the general population. Statistical models were generated that suggested a causal link between radon exposure and lung cancer. Although extrapolation of the results from miners caused controversy, the BEIR VI estimation of risk was validated by studies in the general population.7,20–23
Since the 1980s, several small case-control studies with limited power examined the relationship between indoor radon and lung cancer in the general population. In these studies, individuals who had developed lung cancer were compared with controls who had not developed the disease but who otherwise represented the population from which the cases of lung cancer came.
To improve the statistical power, the investigators of the major studies in Europe, North America, and China pooled the results in separate analyses (Table 2).7,20–23 The average radon concentration to which each individual had been exposed over the previous decades was estimated by measuring the radon concentration at their present and previous homes. On the basis of information from the uranium miners, these studies assumed that the period of exposure was the 30 years ending 5 years before the diagnosis or at death from lung cancer.
The results provided convincing evidence that radon exposure is a cause of lung cancer in the general population and substantiated the extrapolation from the studies of miners. Further, the results of all three pooled analyses were consistent with a linear dose-response relationship with no threshold, suggesting an increased risk of lung cancer even with a radon level below 4 pCi/L (200 Bq/m3), which is the concentration at which mitigation actions are recommended in many countries.17
The North American pooled analysis included 3,662 cases and 4,966 controls from seven studies in the United States and Canada. When data from all studies were combined, the risk of lung cancer was found to increase by 11% per 100-Bq/m3 (about 2.7-pCi/L) increase in measured radon concentration (95% CI 0%–28%). The estimated increase in lung cancer was independent of age, sex, or smoking history.7,20
The Chinese pooled data22 demonstrated a 13% (95% CI 1%–36%) increased risk per 100 Bq/m3.
In the European study, the risk of lung cancer increased by 8% per 100 Bq/m3 (95% CI 3%–16%). The European investigators repeated the analysis, taking into account the random year-to-year variability in measured radon concentration, finding the final estimated risk was an increase of 16% per 100 Bq/m3 using long-term average concentration.21
The combined estimate21,24 from the three pooling studies based on measured radon concentration is an increased risk of lung cancer of 10% per 100 Bq/m3.
Synergistic risk with smoking
Radon exposure was independently associated with lung cancer, and the relationship with cigarette smoking is believed to be synergistic. The radon progeny particles attach themselves to smoke and dust and are then deposited in the bronchial epithelium.25
In the pool of European case-control studies, the cancer risk for current smokers of 15 to 24 cigarettes per day relative to that in never-smokers was 25.8 (95% CI 21–31). Assuming that in the same analysis the lung cancer risk increased by 16% per 100 Bq/m3 of usual radon concentration regardless of smoking status, the cumulative absolute risk by age 75 would be 0.67% in those who never smoked and 16% in smokers at usual radon levels of 400 Bq/m3 (11 pCi/L).21
Rates of all lung cancer subtypes increased
Radon exposure is not associated with a specific histologic subtype of lung cancer. It has been speculated that the incidence of the small-cell subtype might be slightly increased because radon tends to deposit in the more central bronchial carinae.20,21 However, all subtypes have been described in association with radon, the most common being adenocarcinoma and squamous cell carcinoma.26–28
EFFECT OF MITIGATION MEASURES
The US Surgeon General and the EPA recommend that all homes be tested.18 Short-term tests should be used first, keeping in mind that diurnal and seasonal variations may occur.
The World Health Organization has proposed a reference level of 100 Bq/m3 (2.7 pCi/L) to minimize health hazards from indoor radon exposure.17 If this level cannot be reached under the country-specific conditions, the chosen reference level should not exceed 300 Bq/m3 (8 pCi/L).
In the United States, if the result of home testing is higher than 4 pCi/L, a follow-up measurement should be done using a different short-term test or a long-term test. If the follow-up result confirms a level of more than 4 pCi/L, mitigating actions are recommended. The goal is to reduce the indoor radon level as much as possible—down to zero or at least comparable to outdoor levels (national average 0.4 pCi/L).18
A variety of radon mitigation strategies have been used, with different rates of efficacy (Table 3). The optimal strategy depends on the likely source or cause, construction characteristics, soil, and climate.29 Table 4 lists resources for the general public about testing and mitigation measures.
How beneficial is radon mitigation?
Although it is logical to try to reduce the indoor radon concentration, there is no strong evidence yet that this intervention decreases the incidence of lung cancer in the general population.
Using the BEIR VI risk model, Méndez et al30 estimated a 21% reduction in the annual radon-related lung cancer mortality rate by 2100 if all households were compliant with government recommendations (mitigation actions at levels of 4 pCi/L) and assuming that the percentage of cigarette smokers remained constant.
On the other hand, if the number of smokers continues to decline, the benefits from radon mitigation may be less. The expected benefit from mitigation in this scenario is a reduction of 12% in annual radon-related deaths by the year 2100.30 However, it will be challenging to determine whether the expected decline in the incidence of lung cancer and lung cancer deaths is truly attributable to mitigation measures.
MANAGING PATIENTS EXPOSED TO RADON
Screen for lung cancer in smokers only
The National Lung Screening Study (NLST) was a large multicenter trial of annual low-dose computed tomography (CT) to screen for lung cancer in a cohort at high risk: age 55 to 74, at least a 30 pack-year history of smoking in a current smoker, or a former smoker who quit within the past 15 years. The trial demonstrated a 20% reduction in lung cancer deaths in the CT screening group.31
Since the publication of the NLST results, many societies have endorsed screening for lung cancer with low-dose CT using the study criteria. The National Comprehensive Cancer Network (NCCN) expanded these criteria and has recommended screening in patients over age 50 who have a history of smoking and one additional risk factor, such as radon exposure.
However, radon exposure has not been incorporated into a lung cancer risk-prediction model, and there is no empirical evidence suggesting that people who have such a history would benefit from screening.32,33 The joint guidelines of the American College of Chest Physicians and American Society of Clinical Oncology recommend annual low-dose CT screening only for patients who meet the NLST criteria.34
What to do about indeterminate lung nodules
The widely used guidelines from the Fleischner Society35 on how to manage small lung nodules stratify patients into groups at low and high risk of developing lung cancer on the basis of risk factors. The guidelines apply to adults age 35 and older in whom an indeterminate solid nodule was recently detected.
If a patient is at high risk, the recommended approach includes follow-up in shorter intervals depending on the nodule size. History of smoking is recognized as a major risk factor, and the statement also lists family history and exposure to asbestos, uranium, and radon.35
Although the association of radon with lung cancer has been shown in epidemiologic studies, radon exposure has not been included in validated statistical models that assess the probability that an indeterminate lung nodule is malignant. We would expect the risk to be higher in miners, who suffer a more intense exposure to higher levels of radon, than in the general population, which has a low and constantly variable residential exposure. Furthermore, there are no data to support a more aggressive follow-up approach in patients with indeterminate lung nodules and a history of radon exposure.
RADON AND OTHER CANCERS
When a person is exposed to radon, the bronchial epithelium receives the highest dose of ionizing radiation, but other organs such as the kidneys, stomach, and bone marrow may receive doses as well, although lower. Several studies have looked into possible associations, but there is no strong evidence to suggest an increased mortality rate related to radon from cancers other than lung.24,36 However, there seems to be a positive association between radon and the incidence of lymphoproliferative disorders in uranium miners.37,38
Radon can be measured in drinking water, and a few studies have looked at a possible association with gastrointestinal malignancies. The results did not reveal a consistent positive correlation.39,40 The risk of cancer from exposure to radon in the public water supply is likely small and mostly from the transfer of radon particles into the air and not from drinking the water. On the other hand, the risk could be higher with private wells, where radon levels are variable and are possibly higher than from public sources.41
DATA ARE INSUFFICIENT TO GUIDE MANAGEMENT
Radon is a naturally occurring and ubiquitous radioactive gas that can cause tissue damage. Cohort and case-control studies have demonstrated that radon exposure is associated with increased risk of lung cancer. It is recommended that radon levels be measured in every home in the United States and mitigation measures instituted if levels exceed 4 pCi/L.
There are insufficient data to help guide the management of patients with a history of radon exposure, and prospective studies are needed to better understand the individual risk of developing lung cancer and the appropriate management of such patients.
Smoking cessation is an integral part of lung cancer risk reduction from radon exposure.
- Berreby D. The radon raiders: turning perils into profits. The New York Times 1987. www.nytimes.com/1987/07/26/business/the-radon-raiders-turning-perils-into-profits.html?src=pm&pagewanted=1. Accessed August 5, 2014.
- Lewis RK. A history of radon—1470 to 1984. www.ohio-radonpro.com/Radon_History.html. Accessed August 5, 2014.
- World Health Organization (WHO). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Manmade mineral fibres and radon. Summary of data reported and evaluation. http://monographs.iarc.fr/ENG/Monographs/vol43/volume43.pdf. Accessed August 5, 2014.
- Committee on Health Risks of Exposure to Radon (BEIR VI). Health effects of exposure to radon: BEIR VI. Washington, DC: National Academies Press; 1999.
- Samet JM. Radon and lung cancer. J Natl Cancer Inst 1989; 81:745–757.
- Lewis RJ, Lewis Sr RJ. Hawley’s condensed chemical dictionary. 14thed. New York: Wiley-Interscience; 2001.
- Krewski D, Lubin JH, Zielinski JM, et al. Residential radon and risk of lung cancer: a combined analysis of 7 North American case-control studies. Epidemiology 2005; 16:137–145.
- Darby S, Hill D, Doll R. Radon: a likely carcinogen at all exposures. Ann Oncol 2001; 12:1341–1351.
- Sethi TK, El-Ghamry MN, Kloecker GH. Radon and lung cancer. Clin Adv Hematol Oncol 2012; 10:157–164.
- Morrison A. Use of radon for industrial radiography. Can J Res 1945; 23:413–419.
- Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res 1997; 57:3963–3971.
- Ishikawa T, Narazaki Y, Yasuoka Y, Tokonami S, Yamada Y. Bio-kinetics of radon ingested from drinking water. Radiat Prot Dosimetry 2003; 105:65–70.
- Ishikawa T, Yamada Y, Fukutsu K, Tokonami S. Deposition and clearance for radon progeny in the human respiratory tract. Radiat Prot Dosimetry 2003; 105:143–148.
- Farkas A, Hofmann W, Balásházy I, Szoke I, Madas BG, Moustafa M. Effect of site-specific bronchial radon progeny deposition on the spatial and temporal distributions of cellular responses. Radiat Environ Biophys 2011; 50:281–297.
- Robertson A, Allen J, Laney R, Curnow A. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci 2013; 14:14024–14063.
- Chauhan V, Howland M, Wilkins R. Effects of alpha-particle radiation on microRNA responses in human cell-lines. Open Biochem J 2012; 6:16–22.
- World Health Organization (WHO). WHO handbook on indoor radon: a public health perspective; 2009. www.nrsb.org/pdf/WHO%20Radon%20Handbook.pdf. Accessed August 5, 2014.
- United States Environmental Protection Agency (EPA). www.epa.gov/radon/. Accessed August 5, 2014.
- Zdrojewicz Z, Strzelczyk JJ. Radon treatment controversy. Dose Response 2006; 4:106–118.
- Krewski D, Lubin JH, Zielinski JM, et al. A combined analysis of North American case-control studies of residential radon and lung cancer. J Toxicol Environ Health A 2006; 69:533–597.
- Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ 2005; 330:223.
- Lubin JH, Wang ZY, Boice JD, et al. Risk of lung cancer and residential radon in China: pooled results of two studies. Int J Cancer 2004; 109:132–137.
- Darby S, Hill D, Deo H, et al. Residential radon and lung cancer—detailed results of a collaborative analysis of individual data on 7,148 persons with lung cancer and 14,208 persons without lung cancer from 13 epidemiologic studies in Europe. Scand J Work Environ Health 2006; 32(suppl 1):1–83.
- Darby SC, Whitley E, Howe GR, et al. Radon and cancers other than lung cancer in underground miners: a collaborative analysis of 11 studies. J Natl Cancer Inst 1995; 87:378–384.
- Baias PF, Hofmann W, Winkler-Heil R, Cosma C, Duliu OG. Lung dosimetry for inhaled radon progeny in smokers. Radiat Prot Dosimetry 2010; 138:111–118.
- Land CE, Shimosato Y, Saccomanno G, et al. Radiation-associated lung cancer: a comparison of the histology of lung cancers in uranium miners and survivors of the atomic bombings of Hiroshima and Nagasaki. Radiat Res 1993; 134:234–243.
- Kreuzer M, Müller KM, Brachner A, et al. Histopathologic findings of lung carcinoma in German uranium miners. Cancer 2000; 89:2613–2621.
- Saccomanno G, Auerbach O, Kuschner M, et al. A comparison between the localization of lung tumors in uranium miners and in nonminers from 1947 to 1991. Cancer 1996; 77:1278–1283.
- Rahman NM, Tracy BL. Radon control systems in existing and new construction: a review. Radiat Prot Dosimetry 2009; 135:243–255.
- Méndez D, Alshanqeety O, Warner KE, Lantz PM, Courant PN. The impact of declining smoking on radon-related lung cancer in the United States. Am J Public Health 2011; 101:310–314.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Wood DE, Eapen GA, Ettinger DS, et al. Lung cancer screening. J Natl Compr Canc Netw 2012; 10:240–265.
- Ettinger DS, Akerley W, Borghaei H, et al; NCCN (National Comprehensive Cancer Network). Non-small cell lung cancer. J Natl Compr Canc Netw 2012; 10:1236–1271.
- Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143(suppl 5):e78S–e92S.
- MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005; 237:395–400.
- Darby SC, Radford EP, Whitley E. Radon exposure and cancers other than lung cancer in Swedish iron miners. Environ Health Perspect 1995; 103(suppl 2):45–47.
- Laurier D, Tirmarche M, Mitton N, et al. An update of cancer mortality among the French cohort of uranium miners: extended follow-up and new source of data for causes of death. Eur J Epidemiol 2004; 19:139–146.
- Rericha V, Kulich M, Rericha R, Shore DL, Sandler DP. Incidence of leukemia, lymphoma, and multiple myeloma in Czech uranium miners: a case-cohort study. Environ Health Perspect 2006; 114:818–822.
- Auvinen A, Salonen L, Pekkanen J, Pukkala E, Ilus T, Kurttio P. Radon and other natural radionuclides in drinking water and risk of stomach cancer: a case-cohort study in Finland. Int J Cancer 2005; 114:109–113.
- Kjellberg S, Wiseman JS. The relationship of radon to gastrointestinal malignancies. Am Surg 1995; 61:822–825.
- Cappello MA, Ferraro A, Mendelsohn AB, Prehn AW. Radon-contaminated drinking water from private wells: an environmental health assessment examining a rural Colorado mountain community’s exposure. J Environ Health 2013; 76:18–24.
- Berreby D. The radon raiders: turning perils into profits. The New York Times 1987. www.nytimes.com/1987/07/26/business/the-radon-raiders-turning-perils-into-profits.html?src=pm&pagewanted=1. Accessed August 5, 2014.
- Lewis RK. A history of radon—1470 to 1984. www.ohio-radonpro.com/Radon_History.html. Accessed August 5, 2014.
- World Health Organization (WHO). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Manmade mineral fibres and radon. Summary of data reported and evaluation. http://monographs.iarc.fr/ENG/Monographs/vol43/volume43.pdf. Accessed August 5, 2014.
- Committee on Health Risks of Exposure to Radon (BEIR VI). Health effects of exposure to radon: BEIR VI. Washington, DC: National Academies Press; 1999.
- Samet JM. Radon and lung cancer. J Natl Cancer Inst 1989; 81:745–757.
- Lewis RJ, Lewis Sr RJ. Hawley’s condensed chemical dictionary. 14thed. New York: Wiley-Interscience; 2001.
- Krewski D, Lubin JH, Zielinski JM, et al. Residential radon and risk of lung cancer: a combined analysis of 7 North American case-control studies. Epidemiology 2005; 16:137–145.
- Darby S, Hill D, Doll R. Radon: a likely carcinogen at all exposures. Ann Oncol 2001; 12:1341–1351.
- Sethi TK, El-Ghamry MN, Kloecker GH. Radon and lung cancer. Clin Adv Hematol Oncol 2012; 10:157–164.
- Morrison A. Use of radon for industrial radiography. Can J Res 1945; 23:413–419.
- Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res 1997; 57:3963–3971.
- Ishikawa T, Narazaki Y, Yasuoka Y, Tokonami S, Yamada Y. Bio-kinetics of radon ingested from drinking water. Radiat Prot Dosimetry 2003; 105:65–70.
- Ishikawa T, Yamada Y, Fukutsu K, Tokonami S. Deposition and clearance for radon progeny in the human respiratory tract. Radiat Prot Dosimetry 2003; 105:143–148.
- Farkas A, Hofmann W, Balásházy I, Szoke I, Madas BG, Moustafa M. Effect of site-specific bronchial radon progeny deposition on the spatial and temporal distributions of cellular responses. Radiat Environ Biophys 2011; 50:281–297.
- Robertson A, Allen J, Laney R, Curnow A. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci 2013; 14:14024–14063.
- Chauhan V, Howland M, Wilkins R. Effects of alpha-particle radiation on microRNA responses in human cell-lines. Open Biochem J 2012; 6:16–22.
- World Health Organization (WHO). WHO handbook on indoor radon: a public health perspective; 2009. www.nrsb.org/pdf/WHO%20Radon%20Handbook.pdf. Accessed August 5, 2014.
- United States Environmental Protection Agency (EPA). www.epa.gov/radon/. Accessed August 5, 2014.
- Zdrojewicz Z, Strzelczyk JJ. Radon treatment controversy. Dose Response 2006; 4:106–118.
- Krewski D, Lubin JH, Zielinski JM, et al. A combined analysis of North American case-control studies of residential radon and lung cancer. J Toxicol Environ Health A 2006; 69:533–597.
- Darby S, Hill D, Auvinen A, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ 2005; 330:223.
- Lubin JH, Wang ZY, Boice JD, et al. Risk of lung cancer and residential radon in China: pooled results of two studies. Int J Cancer 2004; 109:132–137.
- Darby S, Hill D, Deo H, et al. Residential radon and lung cancer—detailed results of a collaborative analysis of individual data on 7,148 persons with lung cancer and 14,208 persons without lung cancer from 13 epidemiologic studies in Europe. Scand J Work Environ Health 2006; 32(suppl 1):1–83.
- Darby SC, Whitley E, Howe GR, et al. Radon and cancers other than lung cancer in underground miners: a collaborative analysis of 11 studies. J Natl Cancer Inst 1995; 87:378–384.
- Baias PF, Hofmann W, Winkler-Heil R, Cosma C, Duliu OG. Lung dosimetry for inhaled radon progeny in smokers. Radiat Prot Dosimetry 2010; 138:111–118.
- Land CE, Shimosato Y, Saccomanno G, et al. Radiation-associated lung cancer: a comparison of the histology of lung cancers in uranium miners and survivors of the atomic bombings of Hiroshima and Nagasaki. Radiat Res 1993; 134:234–243.
- Kreuzer M, Müller KM, Brachner A, et al. Histopathologic findings of lung carcinoma in German uranium miners. Cancer 2000; 89:2613–2621.
- Saccomanno G, Auerbach O, Kuschner M, et al. A comparison between the localization of lung tumors in uranium miners and in nonminers from 1947 to 1991. Cancer 1996; 77:1278–1283.
- Rahman NM, Tracy BL. Radon control systems in existing and new construction: a review. Radiat Prot Dosimetry 2009; 135:243–255.
- Méndez D, Alshanqeety O, Warner KE, Lantz PM, Courant PN. The impact of declining smoking on radon-related lung cancer in the United States. Am J Public Health 2011; 101:310–314.
- National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365:395–409.
- Wood DE, Eapen GA, Ettinger DS, et al. Lung cancer screening. J Natl Compr Canc Netw 2012; 10:240–265.
- Ettinger DS, Akerley W, Borghaei H, et al; NCCN (National Comprehensive Cancer Network). Non-small cell lung cancer. J Natl Compr Canc Netw 2012; 10:1236–1271.
- Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143(suppl 5):e78S–e92S.
- MacMahon H, Austin JH, Gamsu G, et al; Fleischner Society. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005; 237:395–400.
- Darby SC, Radford EP, Whitley E. Radon exposure and cancers other than lung cancer in Swedish iron miners. Environ Health Perspect 1995; 103(suppl 2):45–47.
- Laurier D, Tirmarche M, Mitton N, et al. An update of cancer mortality among the French cohort of uranium miners: extended follow-up and new source of data for causes of death. Eur J Epidemiol 2004; 19:139–146.
- Rericha V, Kulich M, Rericha R, Shore DL, Sandler DP. Incidence of leukemia, lymphoma, and multiple myeloma in Czech uranium miners: a case-cohort study. Environ Health Perspect 2006; 114:818–822.
- Auvinen A, Salonen L, Pekkanen J, Pukkala E, Ilus T, Kurttio P. Radon and other natural radionuclides in drinking water and risk of stomach cancer: a case-cohort study in Finland. Int J Cancer 2005; 114:109–113.
- Kjellberg S, Wiseman JS. The relationship of radon to gastrointestinal malignancies. Am Surg 1995; 61:822–825.
- Cappello MA, Ferraro A, Mendelsohn AB, Prehn AW. Radon-contaminated drinking water from private wells: an environmental health assessment examining a rural Colorado mountain community’s exposure. J Environ Health 2013; 76:18–24.
KEY POINTS
- Radon is a noble gas that occurs naturally as a decay product of uranium 238 and thorium 232.
- Radon 222 decays to polonium 218 and then, after several intermediate steps, to polonium 214, both of which emit alpha particles, which are highly effective in damaging tissues.
- Radon exposure is associated with increased lung cancer incidence in underground miners. In the general population, it is estimated to be the second most common cause of lung cancer, after cigarette smoking.
- There is no evidence yet of a benefit of lung cancer screening based on radon exposure.