User login
ABSTRACT
Osteoarthritis (OA) of the knee is a top cause of disability among the elderly. Total knee replacement (TKR) has been available as an effective and definite surgical method to treat severe OA of the knee. However, TKR is a significant procedure with potential risk for serious complications and high costs. Alternative lower risk therapies that can delay or obviate TKR are valuable to those who are poor candidates for surgery or wish to avoid TKR as long as possible. Given the chondroprotective effects of hyaluronic acid (HA) injections, they are a safe and effective treatment to improve pain, function, and longevity of the knee. Thus, HA features the potential to delay or obviate TKR.
We aim to study the safety and effectiveness of repeated courses of HA on the time to TKR over a 3-year period using data from a large US health plan administrative claims database.
Retrospective analyses were conducted by identifying knee OA patients during the selection period (2007-2010). The follow-up period was 36 months, post-index date of initial HA injection. Procedural outcomes and adverse events of interest were tabulated and analyzed. A Cox proportional hazards model was used to model the risk of TKR.
A total of 50,389 patients who received HA for treatment of knee OA and met the study inclusion criteria were analyzed. Successive courses of HA showed a good safety profile and led to high proportions of patients without TKR 3 years after treatment initiation. Multivariate statistical modeling showed that multiple courses of HA injections significantly decreased the rates of TKR (95.0% without TKR for ≥5 courses vs 71.6% without TKR for 1 course; hazard ratio, 0.138; P < .0001).
Repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effect of repeated HA courses on delaying TKR beyond a 3-year time horizon.
Continue to: Osteoarthritis (OA) of the knee...
Osteoarthritis (OA) of the knee has emerged as one of the main causes of disability in the United States. Although no currently known cure of OA can reverse the progression of the disease, total knee replacement (TKR) is an effective and definitive treatment. However, TKR is an invasive procedure with potential risk for serious complications, and it has imposed high costs on the US healthcare system, with expenses accounting for hospital expenditures of TKR estimated at $28.5 billion in 2009.1Alternative low-risk therapies that can delay or obviate TKR are valuable to a number of patients, especially the poor candidates for surgery or those who wish to avoid TKR.
Intra-articular (IA) hyaluronic acid (HA) injections have been available as a safe and effective treatment option to alleviate pain and to improve joint functions.2 Results of randomized double-blind controlled clinical trials have demonstrated the pain-relieving effect of IA HA injections.3-5 Furthermore, a recent network meta-analysis comparing various pharmacologic interventions for knee OA has confirmed the efficacy of IA HA injections, which outperformed other interventions when compared with oral placebos.6,7 IA therapies are more effective than oral therapies for knee OA pain, with IA HA injections demonstrating the most pain reduction, potentially due to the benefit associated with needle injection and aspiration. Recent experimental studies have also suggested that IA HA may provide cartilage protection, reduce inflammation, and boost the viscosity of synovial fluid;8 IA HA may also exert therapeutic effects by inhibiting bone formation in OA patients.9,10 HA possesses the potential to delay or obviate TKR. Previous research with a case series review of patients in an orthopedic specialty practice reported that the use of IA HA injections in patients with grade IV OA delayed TKR substantially.11 One study analyzed retrospective medical claims data from a single private insurer and discovered potential evidence for the modest benefit of IA HA injections in delaying TKR.12
More detailed research work on a large sample of patients with knee OA and the requirement of TKR as a condition for inclusion using US administrative claims data has demonstrated the TKR-delaying effects of IA HA injections in comparison with a control group without claims for IA HA injections.13,14 This study also uses real-world US administrative data but utilizes a different approach by starting with a sample of patients with knee OA and evidence of IA HA injections and then assessing the effect of repeated courses of HA treatment on the delay of TKR, without TKR as a mandatory condition for inclusion. All patients with knee OA within the time window were included, regardless of the need for TKR compared with previous studies which only considered patients who ultimately received TKR. Safety information and effectiveness information were examined to achieve a balanced risk-benefit assessment. We also analyzed how multiple courses of HA treatment and other potentially relevant covariates at baseline affected the risk of receiving TKR in a multivariate survival model. We aimed to achieve a realistic assessment of the clinical utility of HA injections in delaying TKR in a real-world setting using both safety and effectiveness data.
METHODS
DATA SOURCE
A retrospective cohort observational study using IMS Health’s PharMetrics Plus Health Plan Claims Database was conducted by identifying knee OA patients with claims indicating initiation of HA injection at an index date during the selection period (July 1, 2007 to June 30, 2010). All common HA agents in the US market during this period (Euflexxa, Hyalgan, Orthovisc, Supartz, and Synvisc) were selected via the corresponding J-codes and pooled for investigation of HA class effects. The follow-up period was 36 months, post-index date of the initial HA injection. Outcomes were measured, and adverse events were identified during this period. The time window for identification of adverse events was within 2 weeks from any injection during the course of therapy (evidence of an emergency room visit and/or physician office visit with requisite code). The data during the 12-month pre-index baseline period from the claims database was used to obtain information about baseline patient characteristics, such as age, gender, type of coverage, physician specialty, Charlson Comorbidity Index (CCI), major comorbidities, and major medications of interest commonly used among patients with knee OA.
STUDY SAMPLE SELECTION
The eligible patients required an outpatient claim indicating the initiation of HA injection. The date of the first claim for the patient within the selection window was defined as their index date. Patients had to be ≥18 years of age in the year of their index date. They had to present at least 1 clinical knee OA diagnosis at any point in the 12-month pre-index period (including the index date), and only patients who were continuously enrolled from 12 months pre-index to 36 months post-index date were evaluated. Among these patients (approximately 1.4 million), the following were excluded to minimize complications in data analysis and interpretation: patients with evidence of any HA use in the pre-index period; patients with evidence of a different kind of HA index medication in the post-index period; patients with evidence of TKR within 30 days of the index event during the post-index period; patients with evidence of 2 different kinds of HA index medications on the index date; and patients with evidence of diagnosis of hip OA, fibromyalgia, rheumatoid arthritis, lupus, or gout during the pre-index period.
Five patient cohorts were defined according to the number of courses of IA HA injections over the entire post-index period.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc.). Descriptive statistics such as means, standard deviations, medians, and 25% and 75% percentiles (Q1 and Q3, respectively) were provided for the continuous variables. Numbers and percentages were provided for the categorical variables. For statistical testing, Student’s t-tests were applied for the continuous variables and chi-square tests for the categorical variables. All the statistical tests were two-tailed. The sample sizes in this database study are remarkably large, such that differences that are not clinically important could still be statistically significant at the conventional alpha level of 0.05. Thus, we applied a more stringent requirement of the alpha level of 0.0001 to identify highly statistically significant results. The number and percentage of patients within each cohort with at least 1 instance of an adverse event of interest (those adverse events commonly expected for patients who receive IA injections for knee OA) were assessed. Times to TKR during the 36-month post-index period were analyzed and compared among different cohorts. Any patients who had not undergone TKR by the end of the post-index period were considered censored at 36 months. The Kaplan-Meier method was employed to model survival curves with time to TKR data, and log-rank tests were used to compare survival curves among different cohorts. A Cox proportional hazards model (PHM) was used to model the risk of TKR with a pre-specified set of covariates adjusted for baseline attributes, such as age, gender, comorbidities, and pre-index healthcare costs. Hazard ratios with 95% confidence intervals were used to examine the measures of event risk.
RESULTS
PATIENT CHARACTERISTICS
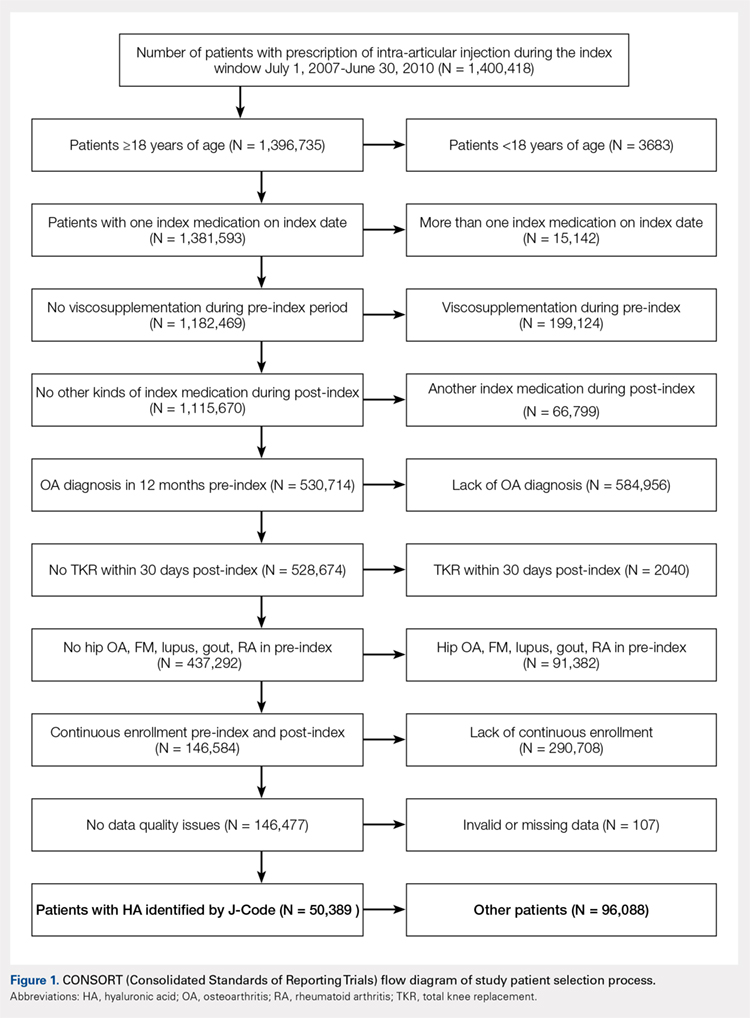
Applying study selection criteria to the claims database yielded 50,389 patients (Figure 1), providing an ample sample size for the statistical analysis. Only patients with evidence of knee OA and use of HA injections (the index medication of interest) were selected, regardless of whether they received TKR during the post-index period. The requirement for a knee OA diagnosis during the 12-month pre-index period resulted in the significant attrition of patients, with 584,956 patients being excluded. Among the 50,389 patients who received HA for treatment of knee OA, 36,260 (72.0%) received a single course of treatment, 8709 (17.3%) received 2 courses, 3179 (6.3%) received 3 courses, 1354 (2.7%) received 4 courses, and 887 (1.8%) received ≥5 courses of treatment.
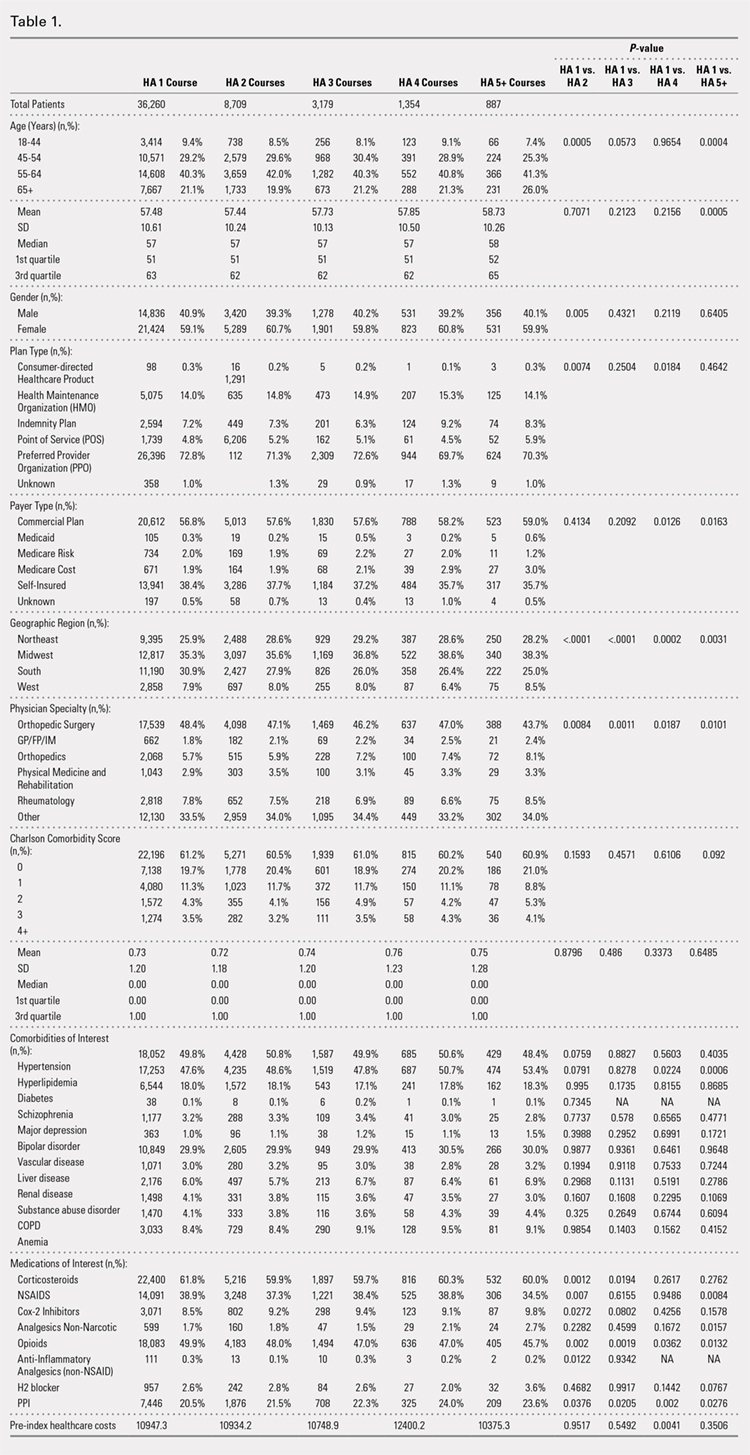
Comparison of baseline characteristics among the 5 IA HA cohorts showed the fairly similar baseline characteristics of all cohorts (Table 1). Geographic region, physician specialty, and opioid use showed differences among the cohorts. Cohorts with ≥5 HA courses presented lower proportions of patients from Southern US states, patients seeing orthopedic surgeons, and patients using opioids than cohorts with fewer HA courses.
PROCEDURES OF INTEREST
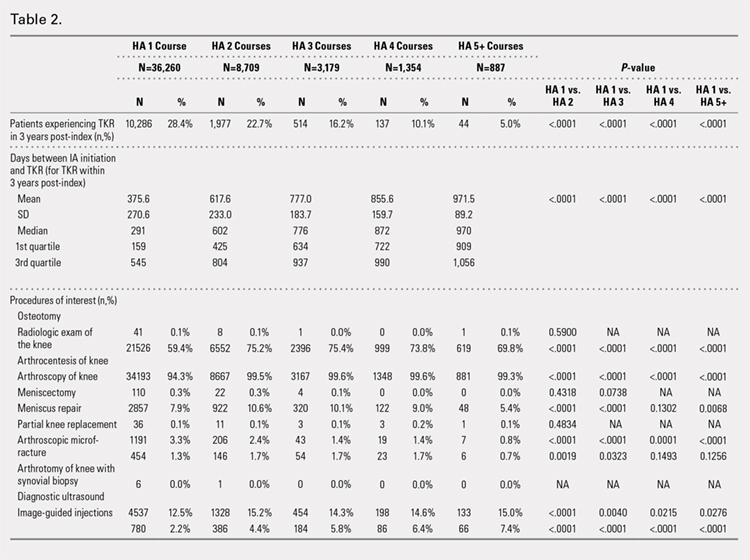
An analysis of the procedures patients received after HA treatment initiation showed that higher numbers of HA treatment courses resulted in lower proportions of patients receiving TKR within 3 years after HA treatment initiation (Table 2). With an increasing number of HA treatment courses, the proportion of patients with TKR within 3 years post-index consistently decreased from 28.4% (for 1 HA course) to 5.0% (for ≥5 HA courses), with all differences being highly statistically significant (P < .0001). Similarly, partial knee replacement exhibited a similar trend, with the proportion of patients decreasing from 3.3% (for 1 HA course) to 0.8% (for ≥5 HA courses; P < .0001). Among the patients with TKR within 3 years post-index, increasing numbers of treatment courses correlated with increasing time to TKR, with a mean of 375.6 days (for 1 HA course) rising to a mean of 971.5 days (for ≥5 HA courses; P < .0001). On the other hand, patients with multiple courses of HA treatment were more likely to undergo radiologic examinations of the knee, arthrocenteses, and image-guided injections than patients with only a single course of HA treatment (P < .0001).
ADVERSE EVENTS
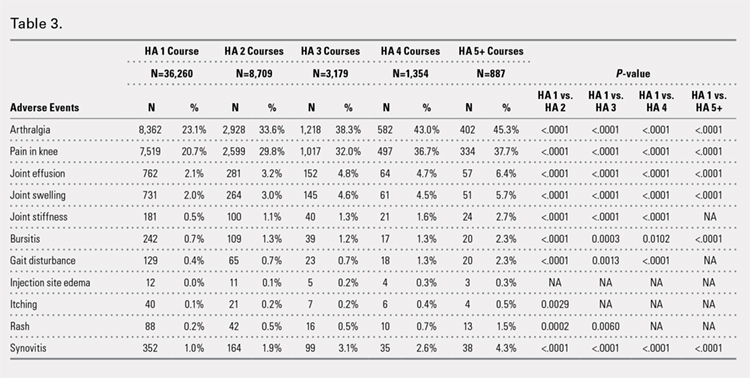
Arthralgia and joint pain in the knee were the most commonly recorded adverse events (Table 3). More courses of HA treatment were associated with higher rates of adverse events. Overall, the reported adverse events profile of repeated courses of HA treatment consisted of mostly common and mild adverse events and displayed no safety concern for patients with knee OA that was followed-up for 3 years. The causality of these adverse events directly related to HA injections vs a specific disease state cannot be determined from an administrative claims data set.
TIME TO TKR
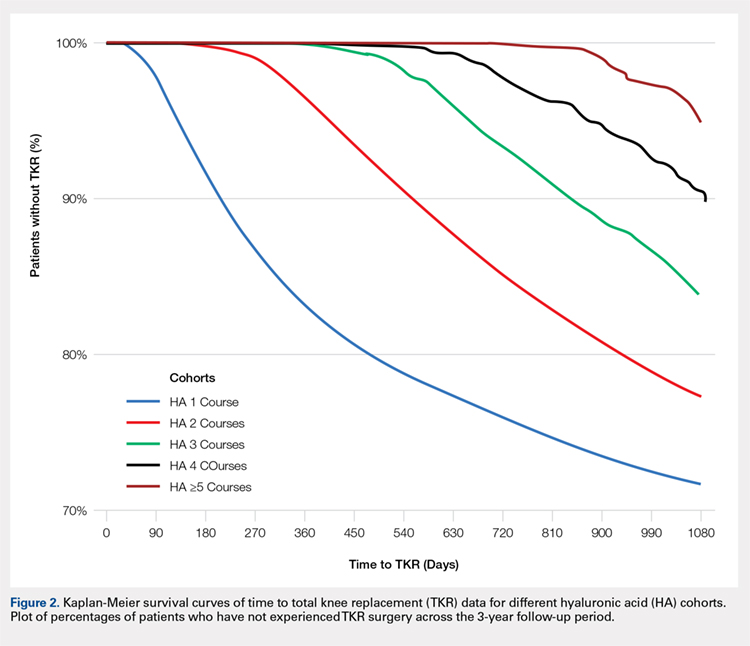
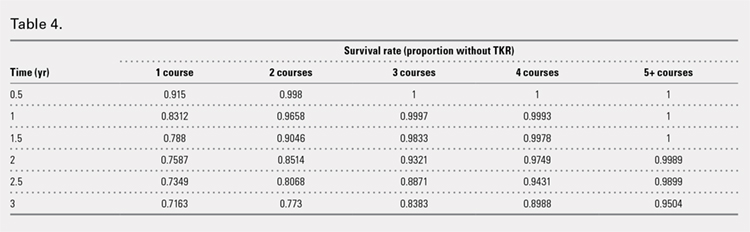
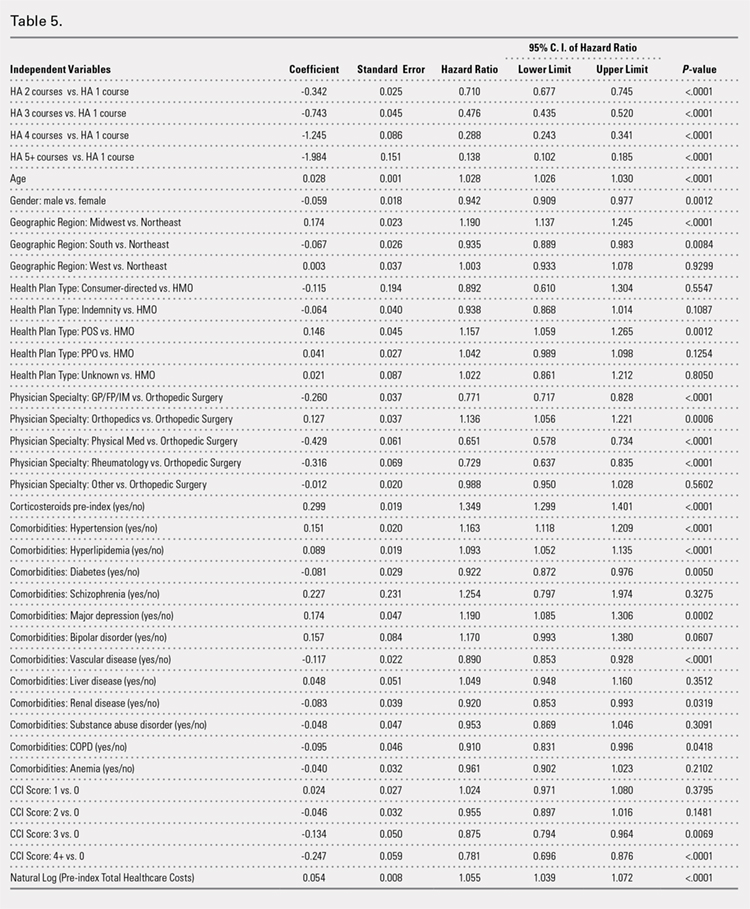
Successive courses of HA led to high proportions of patients without TKR 3 years after HA treatment initiation. This result is evident in the Kaplan-Meier survival curves of time to TKR for different HA cohorts (Figure 2), with log-rank tests of multiple courses vs a single course of HA (P < .0001) showing highly statistically significance. Tabulation of proportions of patients without TKR by various time points showed that increasing numbers of HA treatment courses correlated with higher proportions of patients without TKR at almost all time points (Table 4); within 3 years post-index, 71.6% of patients in the 1 HA course cohort exhibited no TKR, whereas 95.0% of patients in ≥5 HA courses cohort presented no TKR. We also performed a multivariate Cox PHM (Table 5) to account for baseline characteristics of different HA cohorts with covariates when estimating the risks of receiving TKR. The results of the Cox PHM showed that multiple courses of HA treatment significantly decreased the risk of TKR (hazard ratio, 0.138 for ≥5 HA courses vs 1 HA course; P < .0001). Inspection of other highly significant covariates showed that being older, living in the Midwest region of the US (vs the Northeast), receiving pre-index corticosteroids, having an orthopedic surgeon as a treating physician (vs a general practitioner, a rheumatologist, or a physical medicine and rehabilitation specialist), experiencing hypertension or hyperlipidemia, and higher pre-index total healthcare costs were associated with an increased risk of TKR (all P < .0001). Vascular disease and high CCI scores were associated with a decreased risk of TKR (P < .0001).
Continue to: Discussion...
DISCUSSION
This study demonstrated that multiple courses of HA treatment can delay the need for surgery for up to 3 years, with risk for both TKR and partial knee replacement decreasing in a dose-dependent manner. The potentially confounding effect of differences in baseline characteristics that could influence patients’ propensity to receive TKR in a database study was controlled by performing a multivariate analysis with covariate adjustment. The TKR-delaying effect of HA injection was more prominent in cohorts with a high number of HA treatment courses: 19 out of 20 patients in the cohort of ≥5 HA courses were free of TKR at the end of the 3-year post-index period. Such a high proportion of patients avoiding TKR with repeated courses of HA suggests that some patients may be able to successfully delay TKR well beyond the 3-year time span. This finding is counter-evidence to the frequently made assumption15 that all patients with knee OA will eventually progress to a state of disability, making TKR inevitable. The patients with end-stage radiographic knee OA can also benefit from IA HA injections for an extended period of time;16 the latest evidence indicates that nonoperative management can improve symptoms irrespective of radiographic disease severity, implying that TKR needs not to be the only therapeutic option for patients with end-stage radiographic knee OA.17 This finding suggests that HA treatment should be considered an important clinical treatment option for patients with knee OA.
Although the incidence rates of certain adverse events, such as arthralgia/joint pain, are sizable, these temporary adverse events commonly occur among patients who receive IA injections for knee OA; most of these events may simply include symptoms of the remaining underlying knee OA. These results are consistent with those of previous literature reporting the safety of repeated treatment with IA HA injections in a prospective clinical trial18 and demonstrating that repeated courses of HA treatment pose no greater safety risk than a single course of HA treatment.
Multivariate modeling outcomes of factors influencing risk of receiving TKR are broadly consistent with the generally accepted notions that different levels of disease severity and patients’ willingness to consider TKR at baseline influence the likelihood and timing of receiving TKR.19,20 Age and obesity are common risk factors for progression of OA. Orthopedic surgeons are more likely to recommend surgery than non-surgeons. The pre-index use of corticosteroids and high pre-index healthcare costs could be associated with more severe symptoms at baseline. Patients with vascular disease or severe comorbidities, as evidenced by high CCI scores, make poor candidates for major elective surgeries such as TKR. These results are intuitive and validate the clinical insights of this study. Moreover, inclusion of these covariates in the analysis model allows for indirect adjustment of the most important prognostic factors for TKR at baseline, permitting proper statistical comparison of the results for different cohort groups.
Recently, the efficacy of HA injections for OA patients has become the subject of debate when the American Academy of Orthopaedic Surgeons (AAOS) revised its clinical practice guideline, recommending against the use of HA.21 The AAOS’ findings differ from those of other clinical societies, such as the American College of Rheumatology22 and the European League Against Rheumatism,23 which provide no strong recommendation against the use of HA injections. The announcement of the new guideline by AAOS caused concern among clinicians and payers who had valued IA HA injections as a means to control knee OA pain before patients progress to TKR;24 on the other hand, the demand for nonoperative treatment of knee OA remains high. Utilization rates of TKR have increased dramatically, and surgeries are now performed on younger patients with increasing burden on the healthcare system,25,26 in spite of the fact that as high as a third of TKR surgeries may have been performed in inappropriate patients.27 Part of the confusion surrounding clinical utility of HA stems from the fact that up until recently, relatively little research looked into the practical benefits of HA in actual clinical practice. Analyses of databases such as registries are now gaining attention to overcome that problem. Examination of large administrative databases maintained by commercial payers offers the benefit of probing realistically the safety and efficacy of treatments in actual clinical environments in a very large number of patients with heterogeneous backgrounds. Recently, the Agency for Healthcare Research and Quality’s Technology Assessment Program in the US called for such studies to determine whether HA injections can delay progression to TKR.28 The results of this study and several others11,13,14,16 suggest that use of HA to treat OA of the knee is associated with the delay of TKR, supporting the utility of HA in clinical practice and the healthcare system. Potential clinical benefits of delaying TKR may include the reduced risk of aseptic loosening if younger patients can wait for TKR or more time to allow the modification of risk factors in patients who will ultimately undergo TKR.
LIMITATIONS
Follow-up period was limited to 3 years post-index date because longer follow-up data were not available at the time of the study design. If an incorrect adverse event or OA diagnosis was listed in the medical record, or if the medical record was incomplete, then patients might have been misclassified, resulting in selection bias. The claims dataset includes no uninsured and Medicare patients, as the population in the database consisted primarily of commercially-insured patients in the US. Therefore, the results are most generalizable to other commercially-insured patients in the US. Generalizability to other populations may not be assured if they differ in their accessibility to physician services or prescriptions from the patients in this study. Other treatments such as the nonsteroidal anti-inflammatory drugs used by patients were not included within the pre-specified statistical model because their potential effects were assumed to be short-lived and much less than those of corticosteroid. Including these treatments would overload the statistical model with too many covariates, leading to potential computational instability. The database used provides no information on systemic factors, including plan limits on medication use, that could affect care. Given the large and diverse nature of the healthcare plans in the database. However, these factors should not have materially affected our study results. The claims database also lacks direct indicators of OA disease severity, such as Kellgren-Lawrence scores or patient-reported outcomes, including pain and function questionnaire scores. Our multivariate analysis indirectly makes up for this deficiency by considering other baseline characteristics or clinical indicators that may be correlated with information unavailable in a claims database. Patients who opt to undergo repeated courses of HA treatment may be more inclined to avoid surgery or may naturally experience OA disease progression more slowly, making them potentially different from patients who select to undergo surgery earlier without repeated courses of HA treatment. This condition may introduce a bias that causes difficulty in proving the causality between repeated HA use and delay of TKR.
CONCLUSION
Analysis of the knee OA patient data from a real-world database showed that repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effects of repeated HA courses on delaying TKR beyond a 3-year period.
- Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112(3 Suppl 1):S13-S19.
- Arnold W, Fullerton DS, Holder S, May CS. Viscosupplementation: managed care issues for osteoarthritis of the knee. J Manag Care Pharm. 2007;13(4 Suppl):S3-S19.
- Strand V, Conaghan PG, Lohmander LS, et al. An integrated analysis of five double-blind, randomized controlled trials evaluating the safety and efficacy of a hyaluronan product for intra-articular injection in osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14(9):859-866.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20(5):350-356.
- Strand V, McIntyre LF, Beach WR, Miller LE, Block JE. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
- Bannuru RR, Schmid CH, Kent DM, Vaysbrott EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54.
- Mandl LA, Losina E. Relative efficacy of knee osteoarthritis treatments: are all placebos created equal? Ann Intern Med. 2015;162(1):71-72.
- Kusayama Y, Akamatsu Y, Kumagai K, Kobayashi H, Aratake M, Saito T. Changes in synovial fluid biomarkers and clinical efficacy of intra-articular injections of hyaluronic acid for patients with knee osteoarthritis. J Exp Orthop. 2014;1(1):16. doi:10.1186/s40634-014-0016-7.
- Kaneko K, Higuchi C, Kunugiza Y, et al. Hyaluronan inhibits BMP-induced osteoblast differentiation. FEBS Lett. 2015;589(4):447-454. doi:10.1016/j.febslet.2014.
- Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. doi:10.1186/s12891-015-0775-z.
- Waddell DD, Bricker DC. Total knee replacement delayed with hylan G-F 20 use in patients with grade IV osteoarthritis. J Manag Care Pharm. 2007;13(2):113-121.
- Khan T, Nanchanatt G, Farber K, Jan S. Analysis of the effectiveness of hyaluronic acid in prevention of total knee replacement in osteoarthritis patients. J Manag Care Pharm. 2014;20:S49.
- Abbott T, Altman RD, Dimeff R, et al. Do hyaluronic acid injections delay total knee replacement surgery? Arthritis Rheum. 2013;65(Suppl 10):2139.
- Altman R, Lim S, Steen R, Dasa V. Intra-articular hyaluronic acid delays total knee replacement in patients with knee osteoarthritis: evidence from a large U.S. health claims database. Osteoarthritis Cartilage. 2015;23(Suppl 2):A403-A404.
- Mather RC 3rd, Hug KT, Orlando LA, et al. Economic evaluation of access to musculoskeletal care: the case of waiting for total knee arthroplasty. BMC Musculoskelet Disord. 2014;15:22. doi:10.1186/1471-2474-15-22.
- Waddell DD, Joseph B. Delayed total knee replacement with Hylan G-F 20. J Knee Surg. 2016;29(2):159-168. doi:10.1055/s-0034-1395281.
- Atukorala I, Makovey J, Williams M, Ochoa Albiztegui E, Eyles JP, Hunter DJ. If you have end-stage radiographic knee osteoarthritis can you respond to non-surgical management? Osteoarthritis Cartilage. 2015;23(Suppl 2):A329.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. Effectiveness and safety of a multicenter extension and retreatment trial of Gel-200 in patients with knee osteoarthritis. Cartilage. 2012;3(4):297-304. doi:10.1177/1947603512451024.
- Riddle DL, Kong X, Jiranek WA. Two-year incidence and predictors of future knee arthroplasty in persons with symptomatic knee osteoarthritis: preliminary analysis of longitudinal data from the osteoarthritis initiative. Knee. 2009;16(6):494-500.
- Hawker GA, Guan J, Croxford R, et al. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54(10):3212-3220.
- Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-576. doi:10.5435/JAAOS-21-09-571.
- Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465-474.
- Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT). Ann Rheum Dis. 2003;62(12):1145-1155.
- Bannuru RR, Vaysbrot EE, McIntyre LF. Did the American Academy of Orthopaedic Surgeons osteoarthritis guidelines miss the mark? Arthroscopy. 2014;30(1):86-89. doi:10.1016/j.arthro.2013.10.007.
- Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94(3):201-207. doi:10.2106/JBJS.J.01958.
- Weinstein AM, Rome BN, Reichmann WM, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95(5):385-392. doi:10.2106/JBJS.L.00206.
- Riddle DL, Jiranek WA, Hayes CW. Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheumatol. 2014;66(8):2134-2143. doi:10.1002/art.38685.
- NewBerry SJ, Fitzgerald JD, Maglione MA, et al. Agency for Healthcare Research and Quality Web site. Systematic Review for Effectiveness of Hyaluronic Acid in the Treatment of Severe Degenerative Joint Disease (DJD) of the Knee: Technology Assessment Report. http://www.ahrq.gov/research/findings/ta/call-for-public-review.html. Published July 23, 2015. Accessed December 22, 2014.
ABSTRACT
Osteoarthritis (OA) of the knee is a top cause of disability among the elderly. Total knee replacement (TKR) has been available as an effective and definite surgical method to treat severe OA of the knee. However, TKR is a significant procedure with potential risk for serious complications and high costs. Alternative lower risk therapies that can delay or obviate TKR are valuable to those who are poor candidates for surgery or wish to avoid TKR as long as possible. Given the chondroprotective effects of hyaluronic acid (HA) injections, they are a safe and effective treatment to improve pain, function, and longevity of the knee. Thus, HA features the potential to delay or obviate TKR.
We aim to study the safety and effectiveness of repeated courses of HA on the time to TKR over a 3-year period using data from a large US health plan administrative claims database.
Retrospective analyses were conducted by identifying knee OA patients during the selection period (2007-2010). The follow-up period was 36 months, post-index date of initial HA injection. Procedural outcomes and adverse events of interest were tabulated and analyzed. A Cox proportional hazards model was used to model the risk of TKR.
A total of 50,389 patients who received HA for treatment of knee OA and met the study inclusion criteria were analyzed. Successive courses of HA showed a good safety profile and led to high proportions of patients without TKR 3 years after treatment initiation. Multivariate statistical modeling showed that multiple courses of HA injections significantly decreased the rates of TKR (95.0% without TKR for ≥5 courses vs 71.6% without TKR for 1 course; hazard ratio, 0.138; P < .0001).
Repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effect of repeated HA courses on delaying TKR beyond a 3-year time horizon.
Continue to: Osteoarthritis (OA) of the knee...
Osteoarthritis (OA) of the knee has emerged as one of the main causes of disability in the United States. Although no currently known cure of OA can reverse the progression of the disease, total knee replacement (TKR) is an effective and definitive treatment. However, TKR is an invasive procedure with potential risk for serious complications, and it has imposed high costs on the US healthcare system, with expenses accounting for hospital expenditures of TKR estimated at $28.5 billion in 2009.1Alternative low-risk therapies that can delay or obviate TKR are valuable to a number of patients, especially the poor candidates for surgery or those who wish to avoid TKR.
Intra-articular (IA) hyaluronic acid (HA) injections have been available as a safe and effective treatment option to alleviate pain and to improve joint functions.2 Results of randomized double-blind controlled clinical trials have demonstrated the pain-relieving effect of IA HA injections.3-5 Furthermore, a recent network meta-analysis comparing various pharmacologic interventions for knee OA has confirmed the efficacy of IA HA injections, which outperformed other interventions when compared with oral placebos.6,7 IA therapies are more effective than oral therapies for knee OA pain, with IA HA injections demonstrating the most pain reduction, potentially due to the benefit associated with needle injection and aspiration. Recent experimental studies have also suggested that IA HA may provide cartilage protection, reduce inflammation, and boost the viscosity of synovial fluid;8 IA HA may also exert therapeutic effects by inhibiting bone formation in OA patients.9,10 HA possesses the potential to delay or obviate TKR. Previous research with a case series review of patients in an orthopedic specialty practice reported that the use of IA HA injections in patients with grade IV OA delayed TKR substantially.11 One study analyzed retrospective medical claims data from a single private insurer and discovered potential evidence for the modest benefit of IA HA injections in delaying TKR.12
More detailed research work on a large sample of patients with knee OA and the requirement of TKR as a condition for inclusion using US administrative claims data has demonstrated the TKR-delaying effects of IA HA injections in comparison with a control group without claims for IA HA injections.13,14 This study also uses real-world US administrative data but utilizes a different approach by starting with a sample of patients with knee OA and evidence of IA HA injections and then assessing the effect of repeated courses of HA treatment on the delay of TKR, without TKR as a mandatory condition for inclusion. All patients with knee OA within the time window were included, regardless of the need for TKR compared with previous studies which only considered patients who ultimately received TKR. Safety information and effectiveness information were examined to achieve a balanced risk-benefit assessment. We also analyzed how multiple courses of HA treatment and other potentially relevant covariates at baseline affected the risk of receiving TKR in a multivariate survival model. We aimed to achieve a realistic assessment of the clinical utility of HA injections in delaying TKR in a real-world setting using both safety and effectiveness data.
METHODS
DATA SOURCE
A retrospective cohort observational study using IMS Health’s PharMetrics Plus Health Plan Claims Database was conducted by identifying knee OA patients with claims indicating initiation of HA injection at an index date during the selection period (July 1, 2007 to June 30, 2010). All common HA agents in the US market during this period (Euflexxa, Hyalgan, Orthovisc, Supartz, and Synvisc) were selected via the corresponding J-codes and pooled for investigation of HA class effects. The follow-up period was 36 months, post-index date of the initial HA injection. Outcomes were measured, and adverse events were identified during this period. The time window for identification of adverse events was within 2 weeks from any injection during the course of therapy (evidence of an emergency room visit and/or physician office visit with requisite code). The data during the 12-month pre-index baseline period from the claims database was used to obtain information about baseline patient characteristics, such as age, gender, type of coverage, physician specialty, Charlson Comorbidity Index (CCI), major comorbidities, and major medications of interest commonly used among patients with knee OA.
STUDY SAMPLE SELECTION
The eligible patients required an outpatient claim indicating the initiation of HA injection. The date of the first claim for the patient within the selection window was defined as their index date. Patients had to be ≥18 years of age in the year of their index date. They had to present at least 1 clinical knee OA diagnosis at any point in the 12-month pre-index period (including the index date), and only patients who were continuously enrolled from 12 months pre-index to 36 months post-index date were evaluated. Among these patients (approximately 1.4 million), the following were excluded to minimize complications in data analysis and interpretation: patients with evidence of any HA use in the pre-index period; patients with evidence of a different kind of HA index medication in the post-index period; patients with evidence of TKR within 30 days of the index event during the post-index period; patients with evidence of 2 different kinds of HA index medications on the index date; and patients with evidence of diagnosis of hip OA, fibromyalgia, rheumatoid arthritis, lupus, or gout during the pre-index period.
Five patient cohorts were defined according to the number of courses of IA HA injections over the entire post-index period.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc.). Descriptive statistics such as means, standard deviations, medians, and 25% and 75% percentiles (Q1 and Q3, respectively) were provided for the continuous variables. Numbers and percentages were provided for the categorical variables. For statistical testing, Student’s t-tests were applied for the continuous variables and chi-square tests for the categorical variables. All the statistical tests were two-tailed. The sample sizes in this database study are remarkably large, such that differences that are not clinically important could still be statistically significant at the conventional alpha level of 0.05. Thus, we applied a more stringent requirement of the alpha level of 0.0001 to identify highly statistically significant results. The number and percentage of patients within each cohort with at least 1 instance of an adverse event of interest (those adverse events commonly expected for patients who receive IA injections for knee OA) were assessed. Times to TKR during the 36-month post-index period were analyzed and compared among different cohorts. Any patients who had not undergone TKR by the end of the post-index period were considered censored at 36 months. The Kaplan-Meier method was employed to model survival curves with time to TKR data, and log-rank tests were used to compare survival curves among different cohorts. A Cox proportional hazards model (PHM) was used to model the risk of TKR with a pre-specified set of covariates adjusted for baseline attributes, such as age, gender, comorbidities, and pre-index healthcare costs. Hazard ratios with 95% confidence intervals were used to examine the measures of event risk.
RESULTS
PATIENT CHARACTERISTICS
Applying study selection criteria to the claims database yielded 50,389 patients (Figure 1), providing an ample sample size for the statistical analysis. Only patients with evidence of knee OA and use of HA injections (the index medication of interest) were selected, regardless of whether they received TKR during the post-index period. The requirement for a knee OA diagnosis during the 12-month pre-index period resulted in the significant attrition of patients, with 584,956 patients being excluded. Among the 50,389 patients who received HA for treatment of knee OA, 36,260 (72.0%) received a single course of treatment, 8709 (17.3%) received 2 courses, 3179 (6.3%) received 3 courses, 1354 (2.7%) received 4 courses, and 887 (1.8%) received ≥5 courses of treatment.

Comparison of baseline characteristics among the 5 IA HA cohorts showed the fairly similar baseline characteristics of all cohorts (Table 1). Geographic region, physician specialty, and opioid use showed differences among the cohorts. Cohorts with ≥5 HA courses presented lower proportions of patients from Southern US states, patients seeing orthopedic surgeons, and patients using opioids than cohorts with fewer HA courses.

PROCEDURES OF INTEREST
An analysis of the procedures patients received after HA treatment initiation showed that higher numbers of HA treatment courses resulted in lower proportions of patients receiving TKR within 3 years after HA treatment initiation (Table 2). With an increasing number of HA treatment courses, the proportion of patients with TKR within 3 years post-index consistently decreased from 28.4% (for 1 HA course) to 5.0% (for ≥5 HA courses), with all differences being highly statistically significant (P < .0001). Similarly, partial knee replacement exhibited a similar trend, with the proportion of patients decreasing from 3.3% (for 1 HA course) to 0.8% (for ≥5 HA courses; P < .0001). Among the patients with TKR within 3 years post-index, increasing numbers of treatment courses correlated with increasing time to TKR, with a mean of 375.6 days (for 1 HA course) rising to a mean of 971.5 days (for ≥5 HA courses; P < .0001). On the other hand, patients with multiple courses of HA treatment were more likely to undergo radiologic examinations of the knee, arthrocenteses, and image-guided injections than patients with only a single course of HA treatment (P < .0001).

ADVERSE EVENTS
Arthralgia and joint pain in the knee were the most commonly recorded adverse events (Table 3). More courses of HA treatment were associated with higher rates of adverse events. Overall, the reported adverse events profile of repeated courses of HA treatment consisted of mostly common and mild adverse events and displayed no safety concern for patients with knee OA that was followed-up for 3 years. The causality of these adverse events directly related to HA injections vs a specific disease state cannot be determined from an administrative claims data set.

TIME TO TKR
Successive courses of HA led to high proportions of patients without TKR 3 years after HA treatment initiation. This result is evident in the Kaplan-Meier survival curves of time to TKR for different HA cohorts (Figure 2), with log-rank tests of multiple courses vs a single course of HA (P < .0001) showing highly statistically significance. Tabulation of proportions of patients without TKR by various time points showed that increasing numbers of HA treatment courses correlated with higher proportions of patients without TKR at almost all time points (Table 4); within 3 years post-index, 71.6% of patients in the 1 HA course cohort exhibited no TKR, whereas 95.0% of patients in ≥5 HA courses cohort presented no TKR. We also performed a multivariate Cox PHM (Table 5) to account for baseline characteristics of different HA cohorts with covariates when estimating the risks of receiving TKR. The results of the Cox PHM showed that multiple courses of HA treatment significantly decreased the risk of TKR (hazard ratio, 0.138 for ≥5 HA courses vs 1 HA course; P < .0001). Inspection of other highly significant covariates showed that being older, living in the Midwest region of the US (vs the Northeast), receiving pre-index corticosteroids, having an orthopedic surgeon as a treating physician (vs a general practitioner, a rheumatologist, or a physical medicine and rehabilitation specialist), experiencing hypertension or hyperlipidemia, and higher pre-index total healthcare costs were associated with an increased risk of TKR (all P < .0001). Vascular disease and high CCI scores were associated with a decreased risk of TKR (P < .0001).



Continue to: Discussion...
DISCUSSION
This study demonstrated that multiple courses of HA treatment can delay the need for surgery for up to 3 years, with risk for both TKR and partial knee replacement decreasing in a dose-dependent manner. The potentially confounding effect of differences in baseline characteristics that could influence patients’ propensity to receive TKR in a database study was controlled by performing a multivariate analysis with covariate adjustment. The TKR-delaying effect of HA injection was more prominent in cohorts with a high number of HA treatment courses: 19 out of 20 patients in the cohort of ≥5 HA courses were free of TKR at the end of the 3-year post-index period. Such a high proportion of patients avoiding TKR with repeated courses of HA suggests that some patients may be able to successfully delay TKR well beyond the 3-year time span. This finding is counter-evidence to the frequently made assumption15 that all patients with knee OA will eventually progress to a state of disability, making TKR inevitable. The patients with end-stage radiographic knee OA can also benefit from IA HA injections for an extended period of time;16 the latest evidence indicates that nonoperative management can improve symptoms irrespective of radiographic disease severity, implying that TKR needs not to be the only therapeutic option for patients with end-stage radiographic knee OA.17 This finding suggests that HA treatment should be considered an important clinical treatment option for patients with knee OA.
Although the incidence rates of certain adverse events, such as arthralgia/joint pain, are sizable, these temporary adverse events commonly occur among patients who receive IA injections for knee OA; most of these events may simply include symptoms of the remaining underlying knee OA. These results are consistent with those of previous literature reporting the safety of repeated treatment with IA HA injections in a prospective clinical trial18 and demonstrating that repeated courses of HA treatment pose no greater safety risk than a single course of HA treatment.
Multivariate modeling outcomes of factors influencing risk of receiving TKR are broadly consistent with the generally accepted notions that different levels of disease severity and patients’ willingness to consider TKR at baseline influence the likelihood and timing of receiving TKR.19,20 Age and obesity are common risk factors for progression of OA. Orthopedic surgeons are more likely to recommend surgery than non-surgeons. The pre-index use of corticosteroids and high pre-index healthcare costs could be associated with more severe symptoms at baseline. Patients with vascular disease or severe comorbidities, as evidenced by high CCI scores, make poor candidates for major elective surgeries such as TKR. These results are intuitive and validate the clinical insights of this study. Moreover, inclusion of these covariates in the analysis model allows for indirect adjustment of the most important prognostic factors for TKR at baseline, permitting proper statistical comparison of the results for different cohort groups.
Recently, the efficacy of HA injections for OA patients has become the subject of debate when the American Academy of Orthopaedic Surgeons (AAOS) revised its clinical practice guideline, recommending against the use of HA.21 The AAOS’ findings differ from those of other clinical societies, such as the American College of Rheumatology22 and the European League Against Rheumatism,23 which provide no strong recommendation against the use of HA injections. The announcement of the new guideline by AAOS caused concern among clinicians and payers who had valued IA HA injections as a means to control knee OA pain before patients progress to TKR;24 on the other hand, the demand for nonoperative treatment of knee OA remains high. Utilization rates of TKR have increased dramatically, and surgeries are now performed on younger patients with increasing burden on the healthcare system,25,26 in spite of the fact that as high as a third of TKR surgeries may have been performed in inappropriate patients.27 Part of the confusion surrounding clinical utility of HA stems from the fact that up until recently, relatively little research looked into the practical benefits of HA in actual clinical practice. Analyses of databases such as registries are now gaining attention to overcome that problem. Examination of large administrative databases maintained by commercial payers offers the benefit of probing realistically the safety and efficacy of treatments in actual clinical environments in a very large number of patients with heterogeneous backgrounds. Recently, the Agency for Healthcare Research and Quality’s Technology Assessment Program in the US called for such studies to determine whether HA injections can delay progression to TKR.28 The results of this study and several others11,13,14,16 suggest that use of HA to treat OA of the knee is associated with the delay of TKR, supporting the utility of HA in clinical practice and the healthcare system. Potential clinical benefits of delaying TKR may include the reduced risk of aseptic loosening if younger patients can wait for TKR or more time to allow the modification of risk factors in patients who will ultimately undergo TKR.
LIMITATIONS
Follow-up period was limited to 3 years post-index date because longer follow-up data were not available at the time of the study design. If an incorrect adverse event or OA diagnosis was listed in the medical record, or if the medical record was incomplete, then patients might have been misclassified, resulting in selection bias. The claims dataset includes no uninsured and Medicare patients, as the population in the database consisted primarily of commercially-insured patients in the US. Therefore, the results are most generalizable to other commercially-insured patients in the US. Generalizability to other populations may not be assured if they differ in their accessibility to physician services or prescriptions from the patients in this study. Other treatments such as the nonsteroidal anti-inflammatory drugs used by patients were not included within the pre-specified statistical model because their potential effects were assumed to be short-lived and much less than those of corticosteroid. Including these treatments would overload the statistical model with too many covariates, leading to potential computational instability. The database used provides no information on systemic factors, including plan limits on medication use, that could affect care. Given the large and diverse nature of the healthcare plans in the database. However, these factors should not have materially affected our study results. The claims database also lacks direct indicators of OA disease severity, such as Kellgren-Lawrence scores or patient-reported outcomes, including pain and function questionnaire scores. Our multivariate analysis indirectly makes up for this deficiency by considering other baseline characteristics or clinical indicators that may be correlated with information unavailable in a claims database. Patients who opt to undergo repeated courses of HA treatment may be more inclined to avoid surgery or may naturally experience OA disease progression more slowly, making them potentially different from patients who select to undergo surgery earlier without repeated courses of HA treatment. This condition may introduce a bias that causes difficulty in proving the causality between repeated HA use and delay of TKR.
CONCLUSION
Analysis of the knee OA patient data from a real-world database showed that repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effects of repeated HA courses on delaying TKR beyond a 3-year period.
ABSTRACT
Osteoarthritis (OA) of the knee is a top cause of disability among the elderly. Total knee replacement (TKR) has been available as an effective and definite surgical method to treat severe OA of the knee. However, TKR is a significant procedure with potential risk for serious complications and high costs. Alternative lower risk therapies that can delay or obviate TKR are valuable to those who are poor candidates for surgery or wish to avoid TKR as long as possible. Given the chondroprotective effects of hyaluronic acid (HA) injections, they are a safe and effective treatment to improve pain, function, and longevity of the knee. Thus, HA features the potential to delay or obviate TKR.
We aim to study the safety and effectiveness of repeated courses of HA on the time to TKR over a 3-year period using data from a large US health plan administrative claims database.
Retrospective analyses were conducted by identifying knee OA patients during the selection period (2007-2010). The follow-up period was 36 months, post-index date of initial HA injection. Procedural outcomes and adverse events of interest were tabulated and analyzed. A Cox proportional hazards model was used to model the risk of TKR.
A total of 50,389 patients who received HA for treatment of knee OA and met the study inclusion criteria were analyzed. Successive courses of HA showed a good safety profile and led to high proportions of patients without TKR 3 years after treatment initiation. Multivariate statistical modeling showed that multiple courses of HA injections significantly decreased the rates of TKR (95.0% without TKR for ≥5 courses vs 71.6% without TKR for 1 course; hazard ratio, 0.138; P < .0001).
Repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effect of repeated HA courses on delaying TKR beyond a 3-year time horizon.
Continue to: Osteoarthritis (OA) of the knee...
Osteoarthritis (OA) of the knee has emerged as one of the main causes of disability in the United States. Although no currently known cure of OA can reverse the progression of the disease, total knee replacement (TKR) is an effective and definitive treatment. However, TKR is an invasive procedure with potential risk for serious complications, and it has imposed high costs on the US healthcare system, with expenses accounting for hospital expenditures of TKR estimated at $28.5 billion in 2009.1Alternative low-risk therapies that can delay or obviate TKR are valuable to a number of patients, especially the poor candidates for surgery or those who wish to avoid TKR.
Intra-articular (IA) hyaluronic acid (HA) injections have been available as a safe and effective treatment option to alleviate pain and to improve joint functions.2 Results of randomized double-blind controlled clinical trials have demonstrated the pain-relieving effect of IA HA injections.3-5 Furthermore, a recent network meta-analysis comparing various pharmacologic interventions for knee OA has confirmed the efficacy of IA HA injections, which outperformed other interventions when compared with oral placebos.6,7 IA therapies are more effective than oral therapies for knee OA pain, with IA HA injections demonstrating the most pain reduction, potentially due to the benefit associated with needle injection and aspiration. Recent experimental studies have also suggested that IA HA may provide cartilage protection, reduce inflammation, and boost the viscosity of synovial fluid;8 IA HA may also exert therapeutic effects by inhibiting bone formation in OA patients.9,10 HA possesses the potential to delay or obviate TKR. Previous research with a case series review of patients in an orthopedic specialty practice reported that the use of IA HA injections in patients with grade IV OA delayed TKR substantially.11 One study analyzed retrospective medical claims data from a single private insurer and discovered potential evidence for the modest benefit of IA HA injections in delaying TKR.12
More detailed research work on a large sample of patients with knee OA and the requirement of TKR as a condition for inclusion using US administrative claims data has demonstrated the TKR-delaying effects of IA HA injections in comparison with a control group without claims for IA HA injections.13,14 This study also uses real-world US administrative data but utilizes a different approach by starting with a sample of patients with knee OA and evidence of IA HA injections and then assessing the effect of repeated courses of HA treatment on the delay of TKR, without TKR as a mandatory condition for inclusion. All patients with knee OA within the time window were included, regardless of the need for TKR compared with previous studies which only considered patients who ultimately received TKR. Safety information and effectiveness information were examined to achieve a balanced risk-benefit assessment. We also analyzed how multiple courses of HA treatment and other potentially relevant covariates at baseline affected the risk of receiving TKR in a multivariate survival model. We aimed to achieve a realistic assessment of the clinical utility of HA injections in delaying TKR in a real-world setting using both safety and effectiveness data.
METHODS
DATA SOURCE
A retrospective cohort observational study using IMS Health’s PharMetrics Plus Health Plan Claims Database was conducted by identifying knee OA patients with claims indicating initiation of HA injection at an index date during the selection period (July 1, 2007 to June 30, 2010). All common HA agents in the US market during this period (Euflexxa, Hyalgan, Orthovisc, Supartz, and Synvisc) were selected via the corresponding J-codes and pooled for investigation of HA class effects. The follow-up period was 36 months, post-index date of the initial HA injection. Outcomes were measured, and adverse events were identified during this period. The time window for identification of adverse events was within 2 weeks from any injection during the course of therapy (evidence of an emergency room visit and/or physician office visit with requisite code). The data during the 12-month pre-index baseline period from the claims database was used to obtain information about baseline patient characteristics, such as age, gender, type of coverage, physician specialty, Charlson Comorbidity Index (CCI), major comorbidities, and major medications of interest commonly used among patients with knee OA.
STUDY SAMPLE SELECTION
The eligible patients required an outpatient claim indicating the initiation of HA injection. The date of the first claim for the patient within the selection window was defined as their index date. Patients had to be ≥18 years of age in the year of their index date. They had to present at least 1 clinical knee OA diagnosis at any point in the 12-month pre-index period (including the index date), and only patients who were continuously enrolled from 12 months pre-index to 36 months post-index date were evaluated. Among these patients (approximately 1.4 million), the following were excluded to minimize complications in data analysis and interpretation: patients with evidence of any HA use in the pre-index period; patients with evidence of a different kind of HA index medication in the post-index period; patients with evidence of TKR within 30 days of the index event during the post-index period; patients with evidence of 2 different kinds of HA index medications on the index date; and patients with evidence of diagnosis of hip OA, fibromyalgia, rheumatoid arthritis, lupus, or gout during the pre-index period.
Five patient cohorts were defined according to the number of courses of IA HA injections over the entire post-index period.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc.). Descriptive statistics such as means, standard deviations, medians, and 25% and 75% percentiles (Q1 and Q3, respectively) were provided for the continuous variables. Numbers and percentages were provided for the categorical variables. For statistical testing, Student’s t-tests were applied for the continuous variables and chi-square tests for the categorical variables. All the statistical tests were two-tailed. The sample sizes in this database study are remarkably large, such that differences that are not clinically important could still be statistically significant at the conventional alpha level of 0.05. Thus, we applied a more stringent requirement of the alpha level of 0.0001 to identify highly statistically significant results. The number and percentage of patients within each cohort with at least 1 instance of an adverse event of interest (those adverse events commonly expected for patients who receive IA injections for knee OA) were assessed. Times to TKR during the 36-month post-index period were analyzed and compared among different cohorts. Any patients who had not undergone TKR by the end of the post-index period were considered censored at 36 months. The Kaplan-Meier method was employed to model survival curves with time to TKR data, and log-rank tests were used to compare survival curves among different cohorts. A Cox proportional hazards model (PHM) was used to model the risk of TKR with a pre-specified set of covariates adjusted for baseline attributes, such as age, gender, comorbidities, and pre-index healthcare costs. Hazard ratios with 95% confidence intervals were used to examine the measures of event risk.
RESULTS
PATIENT CHARACTERISTICS
Applying study selection criteria to the claims database yielded 50,389 patients (Figure 1), providing an ample sample size for the statistical analysis. Only patients with evidence of knee OA and use of HA injections (the index medication of interest) were selected, regardless of whether they received TKR during the post-index period. The requirement for a knee OA diagnosis during the 12-month pre-index period resulted in the significant attrition of patients, with 584,956 patients being excluded. Among the 50,389 patients who received HA for treatment of knee OA, 36,260 (72.0%) received a single course of treatment, 8709 (17.3%) received 2 courses, 3179 (6.3%) received 3 courses, 1354 (2.7%) received 4 courses, and 887 (1.8%) received ≥5 courses of treatment.

Comparison of baseline characteristics among the 5 IA HA cohorts showed the fairly similar baseline characteristics of all cohorts (Table 1). Geographic region, physician specialty, and opioid use showed differences among the cohorts. Cohorts with ≥5 HA courses presented lower proportions of patients from Southern US states, patients seeing orthopedic surgeons, and patients using opioids than cohorts with fewer HA courses.

PROCEDURES OF INTEREST
An analysis of the procedures patients received after HA treatment initiation showed that higher numbers of HA treatment courses resulted in lower proportions of patients receiving TKR within 3 years after HA treatment initiation (Table 2). With an increasing number of HA treatment courses, the proportion of patients with TKR within 3 years post-index consistently decreased from 28.4% (for 1 HA course) to 5.0% (for ≥5 HA courses), with all differences being highly statistically significant (P < .0001). Similarly, partial knee replacement exhibited a similar trend, with the proportion of patients decreasing from 3.3% (for 1 HA course) to 0.8% (for ≥5 HA courses; P < .0001). Among the patients with TKR within 3 years post-index, increasing numbers of treatment courses correlated with increasing time to TKR, with a mean of 375.6 days (for 1 HA course) rising to a mean of 971.5 days (for ≥5 HA courses; P < .0001). On the other hand, patients with multiple courses of HA treatment were more likely to undergo radiologic examinations of the knee, arthrocenteses, and image-guided injections than patients with only a single course of HA treatment (P < .0001).

ADVERSE EVENTS
Arthralgia and joint pain in the knee were the most commonly recorded adverse events (Table 3). More courses of HA treatment were associated with higher rates of adverse events. Overall, the reported adverse events profile of repeated courses of HA treatment consisted of mostly common and mild adverse events and displayed no safety concern for patients with knee OA that was followed-up for 3 years. The causality of these adverse events directly related to HA injections vs a specific disease state cannot be determined from an administrative claims data set.

TIME TO TKR
Successive courses of HA led to high proportions of patients without TKR 3 years after HA treatment initiation. This result is evident in the Kaplan-Meier survival curves of time to TKR for different HA cohorts (Figure 2), with log-rank tests of multiple courses vs a single course of HA (P < .0001) showing highly statistically significance. Tabulation of proportions of patients without TKR by various time points showed that increasing numbers of HA treatment courses correlated with higher proportions of patients without TKR at almost all time points (Table 4); within 3 years post-index, 71.6% of patients in the 1 HA course cohort exhibited no TKR, whereas 95.0% of patients in ≥5 HA courses cohort presented no TKR. We also performed a multivariate Cox PHM (Table 5) to account for baseline characteristics of different HA cohorts with covariates when estimating the risks of receiving TKR. The results of the Cox PHM showed that multiple courses of HA treatment significantly decreased the risk of TKR (hazard ratio, 0.138 for ≥5 HA courses vs 1 HA course; P < .0001). Inspection of other highly significant covariates showed that being older, living in the Midwest region of the US (vs the Northeast), receiving pre-index corticosteroids, having an orthopedic surgeon as a treating physician (vs a general practitioner, a rheumatologist, or a physical medicine and rehabilitation specialist), experiencing hypertension or hyperlipidemia, and higher pre-index total healthcare costs were associated with an increased risk of TKR (all P < .0001). Vascular disease and high CCI scores were associated with a decreased risk of TKR (P < .0001).



Continue to: Discussion...
DISCUSSION
This study demonstrated that multiple courses of HA treatment can delay the need for surgery for up to 3 years, with risk for both TKR and partial knee replacement decreasing in a dose-dependent manner. The potentially confounding effect of differences in baseline characteristics that could influence patients’ propensity to receive TKR in a database study was controlled by performing a multivariate analysis with covariate adjustment. The TKR-delaying effect of HA injection was more prominent in cohorts with a high number of HA treatment courses: 19 out of 20 patients in the cohort of ≥5 HA courses were free of TKR at the end of the 3-year post-index period. Such a high proportion of patients avoiding TKR with repeated courses of HA suggests that some patients may be able to successfully delay TKR well beyond the 3-year time span. This finding is counter-evidence to the frequently made assumption15 that all patients with knee OA will eventually progress to a state of disability, making TKR inevitable. The patients with end-stage radiographic knee OA can also benefit from IA HA injections for an extended period of time;16 the latest evidence indicates that nonoperative management can improve symptoms irrespective of radiographic disease severity, implying that TKR needs not to be the only therapeutic option for patients with end-stage radiographic knee OA.17 This finding suggests that HA treatment should be considered an important clinical treatment option for patients with knee OA.
Although the incidence rates of certain adverse events, such as arthralgia/joint pain, are sizable, these temporary adverse events commonly occur among patients who receive IA injections for knee OA; most of these events may simply include symptoms of the remaining underlying knee OA. These results are consistent with those of previous literature reporting the safety of repeated treatment with IA HA injections in a prospective clinical trial18 and demonstrating that repeated courses of HA treatment pose no greater safety risk than a single course of HA treatment.
Multivariate modeling outcomes of factors influencing risk of receiving TKR are broadly consistent with the generally accepted notions that different levels of disease severity and patients’ willingness to consider TKR at baseline influence the likelihood and timing of receiving TKR.19,20 Age and obesity are common risk factors for progression of OA. Orthopedic surgeons are more likely to recommend surgery than non-surgeons. The pre-index use of corticosteroids and high pre-index healthcare costs could be associated with more severe symptoms at baseline. Patients with vascular disease or severe comorbidities, as evidenced by high CCI scores, make poor candidates for major elective surgeries such as TKR. These results are intuitive and validate the clinical insights of this study. Moreover, inclusion of these covariates in the analysis model allows for indirect adjustment of the most important prognostic factors for TKR at baseline, permitting proper statistical comparison of the results for different cohort groups.
Recently, the efficacy of HA injections for OA patients has become the subject of debate when the American Academy of Orthopaedic Surgeons (AAOS) revised its clinical practice guideline, recommending against the use of HA.21 The AAOS’ findings differ from those of other clinical societies, such as the American College of Rheumatology22 and the European League Against Rheumatism,23 which provide no strong recommendation against the use of HA injections. The announcement of the new guideline by AAOS caused concern among clinicians and payers who had valued IA HA injections as a means to control knee OA pain before patients progress to TKR;24 on the other hand, the demand for nonoperative treatment of knee OA remains high. Utilization rates of TKR have increased dramatically, and surgeries are now performed on younger patients with increasing burden on the healthcare system,25,26 in spite of the fact that as high as a third of TKR surgeries may have been performed in inappropriate patients.27 Part of the confusion surrounding clinical utility of HA stems from the fact that up until recently, relatively little research looked into the practical benefits of HA in actual clinical practice. Analyses of databases such as registries are now gaining attention to overcome that problem. Examination of large administrative databases maintained by commercial payers offers the benefit of probing realistically the safety and efficacy of treatments in actual clinical environments in a very large number of patients with heterogeneous backgrounds. Recently, the Agency for Healthcare Research and Quality’s Technology Assessment Program in the US called for such studies to determine whether HA injections can delay progression to TKR.28 The results of this study and several others11,13,14,16 suggest that use of HA to treat OA of the knee is associated with the delay of TKR, supporting the utility of HA in clinical practice and the healthcare system. Potential clinical benefits of delaying TKR may include the reduced risk of aseptic loosening if younger patients can wait for TKR or more time to allow the modification of risk factors in patients who will ultimately undergo TKR.
LIMITATIONS
Follow-up period was limited to 3 years post-index date because longer follow-up data were not available at the time of the study design. If an incorrect adverse event or OA diagnosis was listed in the medical record, or if the medical record was incomplete, then patients might have been misclassified, resulting in selection bias. The claims dataset includes no uninsured and Medicare patients, as the population in the database consisted primarily of commercially-insured patients in the US. Therefore, the results are most generalizable to other commercially-insured patients in the US. Generalizability to other populations may not be assured if they differ in their accessibility to physician services or prescriptions from the patients in this study. Other treatments such as the nonsteroidal anti-inflammatory drugs used by patients were not included within the pre-specified statistical model because their potential effects were assumed to be short-lived and much less than those of corticosteroid. Including these treatments would overload the statistical model with too many covariates, leading to potential computational instability. The database used provides no information on systemic factors, including plan limits on medication use, that could affect care. Given the large and diverse nature of the healthcare plans in the database. However, these factors should not have materially affected our study results. The claims database also lacks direct indicators of OA disease severity, such as Kellgren-Lawrence scores or patient-reported outcomes, including pain and function questionnaire scores. Our multivariate analysis indirectly makes up for this deficiency by considering other baseline characteristics or clinical indicators that may be correlated with information unavailable in a claims database. Patients who opt to undergo repeated courses of HA treatment may be more inclined to avoid surgery or may naturally experience OA disease progression more slowly, making them potentially different from patients who select to undergo surgery earlier without repeated courses of HA treatment. This condition may introduce a bias that causes difficulty in proving the causality between repeated HA use and delay of TKR.
CONCLUSION
Analysis of the knee OA patient data from a real-world database showed that repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effects of repeated HA courses on delaying TKR beyond a 3-year period.
- Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112(3 Suppl 1):S13-S19.
- Arnold W, Fullerton DS, Holder S, May CS. Viscosupplementation: managed care issues for osteoarthritis of the knee. J Manag Care Pharm. 2007;13(4 Suppl):S3-S19.
- Strand V, Conaghan PG, Lohmander LS, et al. An integrated analysis of five double-blind, randomized controlled trials evaluating the safety and efficacy of a hyaluronan product for intra-articular injection in osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14(9):859-866.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20(5):350-356.
- Strand V, McIntyre LF, Beach WR, Miller LE, Block JE. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
- Bannuru RR, Schmid CH, Kent DM, Vaysbrott EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54.
- Mandl LA, Losina E. Relative efficacy of knee osteoarthritis treatments: are all placebos created equal? Ann Intern Med. 2015;162(1):71-72.
- Kusayama Y, Akamatsu Y, Kumagai K, Kobayashi H, Aratake M, Saito T. Changes in synovial fluid biomarkers and clinical efficacy of intra-articular injections of hyaluronic acid for patients with knee osteoarthritis. J Exp Orthop. 2014;1(1):16. doi:10.1186/s40634-014-0016-7.
- Kaneko K, Higuchi C, Kunugiza Y, et al. Hyaluronan inhibits BMP-induced osteoblast differentiation. FEBS Lett. 2015;589(4):447-454. doi:10.1016/j.febslet.2014.
- Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. doi:10.1186/s12891-015-0775-z.
- Waddell DD, Bricker DC. Total knee replacement delayed with hylan G-F 20 use in patients with grade IV osteoarthritis. J Manag Care Pharm. 2007;13(2):113-121.
- Khan T, Nanchanatt G, Farber K, Jan S. Analysis of the effectiveness of hyaluronic acid in prevention of total knee replacement in osteoarthritis patients. J Manag Care Pharm. 2014;20:S49.
- Abbott T, Altman RD, Dimeff R, et al. Do hyaluronic acid injections delay total knee replacement surgery? Arthritis Rheum. 2013;65(Suppl 10):2139.
- Altman R, Lim S, Steen R, Dasa V. Intra-articular hyaluronic acid delays total knee replacement in patients with knee osteoarthritis: evidence from a large U.S. health claims database. Osteoarthritis Cartilage. 2015;23(Suppl 2):A403-A404.
- Mather RC 3rd, Hug KT, Orlando LA, et al. Economic evaluation of access to musculoskeletal care: the case of waiting for total knee arthroplasty. BMC Musculoskelet Disord. 2014;15:22. doi:10.1186/1471-2474-15-22.
- Waddell DD, Joseph B. Delayed total knee replacement with Hylan G-F 20. J Knee Surg. 2016;29(2):159-168. doi:10.1055/s-0034-1395281.
- Atukorala I, Makovey J, Williams M, Ochoa Albiztegui E, Eyles JP, Hunter DJ. If you have end-stage radiographic knee osteoarthritis can you respond to non-surgical management? Osteoarthritis Cartilage. 2015;23(Suppl 2):A329.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. Effectiveness and safety of a multicenter extension and retreatment trial of Gel-200 in patients with knee osteoarthritis. Cartilage. 2012;3(4):297-304. doi:10.1177/1947603512451024.
- Riddle DL, Kong X, Jiranek WA. Two-year incidence and predictors of future knee arthroplasty in persons with symptomatic knee osteoarthritis: preliminary analysis of longitudinal data from the osteoarthritis initiative. Knee. 2009;16(6):494-500.
- Hawker GA, Guan J, Croxford R, et al. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54(10):3212-3220.
- Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-576. doi:10.5435/JAAOS-21-09-571.
- Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465-474.
- Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT). Ann Rheum Dis. 2003;62(12):1145-1155.
- Bannuru RR, Vaysbrot EE, McIntyre LF. Did the American Academy of Orthopaedic Surgeons osteoarthritis guidelines miss the mark? Arthroscopy. 2014;30(1):86-89. doi:10.1016/j.arthro.2013.10.007.
- Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94(3):201-207. doi:10.2106/JBJS.J.01958.
- Weinstein AM, Rome BN, Reichmann WM, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95(5):385-392. doi:10.2106/JBJS.L.00206.
- Riddle DL, Jiranek WA, Hayes CW. Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheumatol. 2014;66(8):2134-2143. doi:10.1002/art.38685.
- NewBerry SJ, Fitzgerald JD, Maglione MA, et al. Agency for Healthcare Research and Quality Web site. Systematic Review for Effectiveness of Hyaluronic Acid in the Treatment of Severe Degenerative Joint Disease (DJD) of the Knee: Technology Assessment Report. http://www.ahrq.gov/research/findings/ta/call-for-public-review.html. Published July 23, 2015. Accessed December 22, 2014.
- Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112(3 Suppl 1):S13-S19.
- Arnold W, Fullerton DS, Holder S, May CS. Viscosupplementation: managed care issues for osteoarthritis of the knee. J Manag Care Pharm. 2007;13(4 Suppl):S3-S19.
- Strand V, Conaghan PG, Lohmander LS, et al. An integrated analysis of five double-blind, randomized controlled trials evaluating the safety and efficacy of a hyaluronan product for intra-articular injection in osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14(9):859-866.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20(5):350-356.
- Strand V, McIntyre LF, Beach WR, Miller LE, Block JE. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
- Bannuru RR, Schmid CH, Kent DM, Vaysbrott EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54.
- Mandl LA, Losina E. Relative efficacy of knee osteoarthritis treatments: are all placebos created equal? Ann Intern Med. 2015;162(1):71-72.
- Kusayama Y, Akamatsu Y, Kumagai K, Kobayashi H, Aratake M, Saito T. Changes in synovial fluid biomarkers and clinical efficacy of intra-articular injections of hyaluronic acid for patients with knee osteoarthritis. J Exp Orthop. 2014;1(1):16. doi:10.1186/s40634-014-0016-7.
- Kaneko K, Higuchi C, Kunugiza Y, et al. Hyaluronan inhibits BMP-induced osteoblast differentiation. FEBS Lett. 2015;589(4):447-454. doi:10.1016/j.febslet.2014.
- Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. doi:10.1186/s12891-015-0775-z.
- Waddell DD, Bricker DC. Total knee replacement delayed with hylan G-F 20 use in patients with grade IV osteoarthritis. J Manag Care Pharm. 2007;13(2):113-121.
- Khan T, Nanchanatt G, Farber K, Jan S. Analysis of the effectiveness of hyaluronic acid in prevention of total knee replacement in osteoarthritis patients. J Manag Care Pharm. 2014;20:S49.
- Abbott T, Altman RD, Dimeff R, et al. Do hyaluronic acid injections delay total knee replacement surgery? Arthritis Rheum. 2013;65(Suppl 10):2139.
- Altman R, Lim S, Steen R, Dasa V. Intra-articular hyaluronic acid delays total knee replacement in patients with knee osteoarthritis: evidence from a large U.S. health claims database. Osteoarthritis Cartilage. 2015;23(Suppl 2):A403-A404.
- Mather RC 3rd, Hug KT, Orlando LA, et al. Economic evaluation of access to musculoskeletal care: the case of waiting for total knee arthroplasty. BMC Musculoskelet Disord. 2014;15:22. doi:10.1186/1471-2474-15-22.
- Waddell DD, Joseph B. Delayed total knee replacement with Hylan G-F 20. J Knee Surg. 2016;29(2):159-168. doi:10.1055/s-0034-1395281.
- Atukorala I, Makovey J, Williams M, Ochoa Albiztegui E, Eyles JP, Hunter DJ. If you have end-stage radiographic knee osteoarthritis can you respond to non-surgical management? Osteoarthritis Cartilage. 2015;23(Suppl 2):A329.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. Effectiveness and safety of a multicenter extension and retreatment trial of Gel-200 in patients with knee osteoarthritis. Cartilage. 2012;3(4):297-304. doi:10.1177/1947603512451024.
- Riddle DL, Kong X, Jiranek WA. Two-year incidence and predictors of future knee arthroplasty in persons with symptomatic knee osteoarthritis: preliminary analysis of longitudinal data from the osteoarthritis initiative. Knee. 2009;16(6):494-500.
- Hawker GA, Guan J, Croxford R, et al. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54(10):3212-3220.
- Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-576. doi:10.5435/JAAOS-21-09-571.
- Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465-474.
- Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT). Ann Rheum Dis. 2003;62(12):1145-1155.
- Bannuru RR, Vaysbrot EE, McIntyre LF. Did the American Academy of Orthopaedic Surgeons osteoarthritis guidelines miss the mark? Arthroscopy. 2014;30(1):86-89. doi:10.1016/j.arthro.2013.10.007.
- Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94(3):201-207. doi:10.2106/JBJS.J.01958.
- Weinstein AM, Rome BN, Reichmann WM, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95(5):385-392. doi:10.2106/JBJS.L.00206.
- Riddle DL, Jiranek WA, Hayes CW. Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheumatol. 2014;66(8):2134-2143. doi:10.1002/art.38685.
- NewBerry SJ, Fitzgerald JD, Maglione MA, et al. Agency for Healthcare Research and Quality Web site. Systematic Review for Effectiveness of Hyaluronic Acid in the Treatment of Severe Degenerative Joint Disease (DJD) of the Knee: Technology Assessment Report. http://www.ahrq.gov/research/findings/ta/call-for-public-review.html. Published July 23, 2015. Accessed December 22, 2014.
TAKE-HOME POINTS
- Repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years.
- HA treatment should be considered an important clinical treatment option for patients with knee OA.
- Repeated courses of treatment with HA are safe.
- Repeated courses of HA treatment pose no greater safety risk than a single course of HA treatment.
- Additional research is needed to evaluate the effects of repeated HA courses on delaying TKR beyond a 3-year period.