User login
Oncoplastic surgery refers to immediate or delayed breast reconstruction following partial mastectomy, also known as breast conservation therapy. The term was coined by Audretsch et al in 19981 and is now often referred to as oncoplasty. It involves four integral components:2
- Oncologically sound techniques of tumor removal
- Partial reconstruction of the breast to correct small defects
- Immediate reconstruction for larger defects using various principles of plastic surgery
- Creation of symmetry with the contralateral breast.
This article provides a brief overview of various procedures used for reconstruction following breast conservation therapy and the factors that guide selection among these procedures for individual patients. It concludes with a discussion of complications of oncoplastic procedures, patient counseling, and other general considerations in patient management.
THE RATIONALE FOR RECONSTRUCTION
Effects of radiation argue for immediate reconstruction
Although radiation therapy is integral to the comprehensive treatment of breast cancer after breast conservation therapy, radiation-induced changes to the breast are one of the greatest obstacles faced when delayed reconstruction is performed. Radiation results in deformation of the parenchyma, leading to retraction, fibrosis, vasculitis, and skin breakdown. The effects of radiation on breast tissue may possibly be a larger problem when reconstruction is delayed, as wound healing is inhibited and vascular supply is impaired. Therefore, immediate reconstruction should be undertaken whenever possible.7 (The timing of reconstruction is discussed in greater detail in the final article in this supplement, although mainly in the context of mastectomy.)
OPTIONS FOR RECONSTRUCTION
Various techniques of partial breast reconstruction can be used to achieve an aesthetically acceptable result. They can be thought of as volume-displacement procedures, such as local tissue rearrangement and reduction mammaplasty, or as volume-replacement procedures using flap reconstruction.8 Additionally, simple wound closure (primary closure) may be performed if small amounts of tissue can be removed without creating a noticeable defect, but simple closure is an option only for large breasts. The decision among techniques depends on a variety of factors, as delineated below.
Local tissue rearrangement
Local tissue rearrangement is defined as the use of local tissue (skin and subcutaneous and/or breast tissue) from either the breast or the axilla. This technique involves the transfer of adjacent breast parenchyma and skin to the area of the defect. It is dependent on a random blood supply and does not involve creating a parenchymal tissue pedicle.4,5 It does rely, however, on a balance between the amount of tissue resected and the available residual breast size and volume. This procedure is not suitable for patients who require large-volume resection with a small breast or limited breast tissue.
When local tissue rearrangement is to be performed, the surgical incision needs to be planned by both the oncologic surgeon and the plastic surgeon to ensure an appropriate cosmetic outcome and prevent displacement or distortion of the nipple-areola complex. If such planning is not done, the cosmetic outcome may be compromised, thereby undermining one of the reasons for breast conservation in the first place. When full-thickness excisions of tissue are removed from a certain area of the breast—termed “no man’s land” by Grisotti and Calabrese7—the nipple-areola complex shifts to an unnatural position. Therefore, resections in this area, located superiomedial to the nipple, should include little or no skin.
Other techniques of tissue transposition include circumareolar incisions for tumors located adjacent to the nipple-areola complex, radially designed resections for lateral tumors, and donut-shaped resections for superior or lateral tumors.8
Reduction mammaplasty
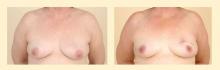
Standard breast reduction techniques are used on the contralateral (uninvolved) breast. This matching procedure can be performed at the same time as the initial cancer operation or as a delayed procedure. The matching procedure is usually performed at a later date for those who need to undergo radiation therapy, allowing time for healing and for final breast volume and shape to be achieved. Reduction of the contralateral breast does not increase its risk for cancer; in fact, reduction may improve body image and make breast self-examinations and follow-up mammography easier.
Therapeutic reduction mammaplasty is highly versatile and gives a better aesthetic result in the immediate setting when compared with flap reconstruction. However, it is usually limited to patients with a brassiere cup size of D or larger.4
An advantage of reduction mammaplasty is that reducing the size of the affected breast facilitates postoperative radiation therapy. Some radiation oncologists are reluctant to administer radiation to a large breast because of increased toxicity to the skin and the likelihood of a poor aesthetic outcome. With reduction mammaplasty, lower radiation doses are required and the delivery of radiation is more uniform.4
Reduction mammaplasty is ideal for women with moderate-sized or large breasts with ptosis (sagging), for whom a reduction in size would be considered a positive outcome.10 Patients with symptomatic macromastia likewise benefit from reduction in breast volume. An additional advantage is that the reduction procedure on the contralateral breast affords the opportunity for tissue sampling from this presumedly uninvolved breast; occult carcinomas in the contralateral breast have been identified in a small percentage of patients.11
At the same time, the exposure of the contralateral breast to surgery also constitutes the main disadvantage of this procedure, as both breasts are placed at risk for wound or nipple complications and the discomfort of surgery.9 Moreover, surgery time is also increased. Lastly, reduction mammaplasty can be offered only to patients who possess enough breast tissue to undergo reduction.12
Flap reconstruction
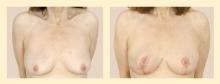
Flap reconstruction is indicated in patients who have significant breast volume deficit after resection and have insufficient adjacent tissue for local tissue recruitment and rearrangement. This method of reconstruction is based on an axial blood supply, which means that a specific vascular pedicle is responsible for a given distribution of tissue. For this purpose, flaps can be either myocutaneous (muscle-skin flaps), fasciocutaneous (fascia, subcutaneous tissue, and skin) or adipocutaneous (containing fat and skin). Examples include the latissimus dorsi myocutaneous flap, the transverse thoracoepigastric skin flap, and the lateral thoracic adipocutaneous flap.4–6
The latissimus dorsi myocutaneous flap is used most often, especially when more than 25% of the breast volume has been resected. Since a large volume of tissue is removed, either the tumor and a margin can be resected or a nipple-sparing subcutaneous mastectomy may be performed10 (nipple-sparing mastectomy would not be breast-conserving and has been discussed earlier in this supplement). This myocutaneous flap is based on the thoracodorsal vessels and was first described for volume replacement after breast-conserving surgery by Noguchi et al.13 A benefit of this flap is that most patients do not need reduction of the contralateral breast for symmetry, as the flap usually provides adequate tissue volume.4 This is beneficial for the patient, as she is not exposed to the potential complications of an operation on the contralateral breast.
The lateral thoracic adipocutaneous flap is another option. This flap has the benefit of sparing the muscle while using skin and fat from the axillary region. It can be based on one of three vascular pedicles that have been shown to be reliable as a sole blood supply. The most common pedicle for this technique is the thoracodorsal artery, as the main blood supply for the thoracodorsal artery perforator flap. This flap provides a potentially large amount of tissue for use and affords patients the chance to have a redundant roll of axillary tissue removed. This tissue can be used alone for reconstruction or in conjunction with a breast implant.6
One drawback of the latissimus dorsi flap is the potential for mismatch of skin color and texture when there is a need to address a significant skin deficit on the breast. Replacing a whole aesthetic unit, as opposed to only a small skin paddle, can minimize this potential; thus, using a larger amount of skin may provide a better aesthetic result. Rarely, if there is no skin defect, the muscle alone can be used, with no skin component.5 The lateral thoracic flap, on the other hand, may be more similar in skin color and texture to the native breast and may allow the scar to be better hidden in the axilla than is the case with the latissimus dorsi flap.6 Any type of flap presents potential donor site problems as well as breast complications (discussed below).
Flap reconstruction broadens the application of breast conservation therapy to women who would not otherwise be candidates because of the large volume of tissue they need to have removed.2 Oncoplasty reconstruction also allows the oncologic surgeon to be more aggressive with tissue removal without concerns about compromising the aesthetic outcome. Patients with small to moderate breasts are therefore candidates for flap reconstruction, as even modest resections in such patients result in a large volume of tissue loss and the need for additional tissue to reconstruct the breast.14 Any of the aforementioned flaps are advantageous, as they are in close proximity to the breast and can readily be used for reconstruction.6
CHOICE OF TECHNIQUE
Many factors contribute to the choice among reconstructive methods for a particular patient after breast conservation therapy.
Tumor location plays a significant role. Kronowitz et al described using breast reduction as their primary reconstructive modality, particularly for tumors of the upper inner, upper outer, and lower inner quadrants of the breast.4 They used flap reconstruction only for outer-quadrant tumors, and they found that tumors of the lower outer quadrant were the largest and lent themselves to local tissue rearrangement, often with axillary tissue.4 Centrally located tumors usually require removal of the nipple-areola complex and can be challenging to reconstruct. The techniques include either (1) direct closure with some degree of local tissue remodeling, or (2) reduction mammaplasty. The majority of patients with centrally located tumors will need contralateral breast reduction for symmetry14 and nipple-areola reconstruction at a later date.
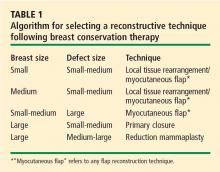
The size of the defect created by the tumor resection also significantly affects the choice of technique, as does the patient’s preoperative brassiere size. In the analysis by Kronowitz et al, defects smaller than 20% of the overall breast size were found to be amenable to breast reduction, whereas larger defects were reconstructed with flaps or local tissue rearrangement.4 Also, women with a brassiere cup size of D or larger tended to undergo breast reduction, whereas those with a size smaller than D underwent local tissue rearrangement or flap reconstruction.4
COMPLICATIONS
Complications of breast surgery include seromas (of the breast as well as the donor site when a flap is used), nipple necrosis, wound dehiscence, infection, hematoma, fat necrosis, and mastectomy flap necrosis. Postoperative hematomas and superficial wound infections tend to occur in the immediate postoperative period (usually within the first few days), whereas the other complications mentioned may take 1 to 2 weeks to develop. These complications are common to all breast operations and are not specific to reconstruction after breast conservation therapy.
Postoperative complications vary in frequency but are more common when reconstruction is delayed.4,7 They also vary depending on the reconstructive technique. Donor-site seromas and fat necrosis are most common with immediate reconstruction using a flap; wound dehiscence is most common with delayed local tissue rearrangement; and breast seroma is most common with delayed reduction mammaplasty.4
Other issues to consider include the possible delay in adjuvant therapy in patients who experience wound healing problems, especially in those who are obese, who smoke, or who undergo therapeutic mammaplasty.15,16 Moreover, operative time is increased with oncoplasty as compared with simple wide local excision, which increases patients’ exposure to anesthesia and thereby raises the risk of complications, particularly in older patients with comorbidities.16
Risk factors for complications
Certain patient characteristics carry an increased risk for postoperative complications. These include tobacco smoking, previous breast surgeries, comorbidities that impair wound healing, and obesity.4,15–17
The vasoconstrictive, thrombotic, and hypoxic effects of tobacco place patients who smoke at an increased risk for necrosis of the nipple-areola complex, as well as for pulmonary complications, when breast reduction is performed. The standard recommendation is cessation of smoking for 6 to 8 weeks preoperatively to reduce pulmonary risks, although rigorous scientific validation is lacking.17
Breasts that have been previously operated on have scarring of the skin and subcutaneous tissues, which may affect the surgical incision and technique. Additionally, vascular compromise of the underlying breast tissue and nipple-areola complex is a possibility in patients who have had previous breast operations.4 For these reasons, it is of utmost importance to obtain a full history of any previous breast procedures a patient has had.
Obesity is a risk factor for impaired wound healing, as delayed wound healing has been correlated with increased body mass index in patients undergoing breast reduction.15
What about positive margins?
Addressing positive margins can be problematic after breast conservation therapy with immediate reconstruction, as it is difficult to locate the resection margin after the breast tissue has been rearranged.4,5,12,14 Patients who have positive margins will usually need to undergo completion mastectomy and opt for immediate reconstruction with a transverse rectus abdominis myocutaneous (TRAM) flap or a latissimus dorsi flap with an implant. Therefore, use of a TRAM flap for initial reconstruction after breast conservation therapy is discouraged.4,14 If a TRAM flap is needed to restore the shape and contour of the breast after breast conservation, it is usually better to perform a mastectomy, as it provides a superior aesthetic result and reduces the risk of a subsequent malignancy since the breast tissue is removed.5
PATIENT COUNSELING, PREOPERATIVE PLANNING
The diagnosis of breast cancer is devastating for most women and is compounded by mental anguish associated with the anticipated changes in their appearance.10 There is a psychological advantage to having reconstruction performed during the same operation as resection because the breast’s preoperative form is immediately restored and little to no asymmetry is seen postoperatively.12 One study showed that breast cancer patients who underwent reconstructive surgery had better body images and felt they had more control over their treatment compared with patients who simply had breast conservation therapy or mastectomy without reconstruction; these perceptions also conferred a psychological benefit among the patients who had reconstructive procedures.18
At the same time, all patients need to be counseled about the potential drawbacks of reconstruction, including the possibility of reoperation for positive margins, wound complications, or a disappointing or unacceptable aesthetic outcome.
Oncoplastic surgery is a multispecialty collaboration. Good communication and preoperative planning is imperative and must include the general surgeon, plastic surgeon, oncologist, and, most importantly, the patient. Considerations in how to approach diagnostic biopsies, lymph node sampling, timing of contralateral breast symmetrizing procedures, and the possibility of positive margins all need to be discussed preoperatively.8,10
ADDITIONAL CONSIDERATIONS
Timing of reconstruction
Immediate reconstruction is preferred for many reasons, including a reduced incidence of wound healing problems, facility in administering postoperative radiation therapy, and better aesthetic results.3,4,11 A one-stage procedure also facilitates breast remodeling, as there is no scar tissue to deal with. Patients’ psychological trauma of coping with a deformity is also reduced because better symmetry is achieved with immediate reconstruction.10
Additionally, some authors have reported lower rates of local recurrence in breast conservation therapy patients who received immediate reconstruction, likely owing to the larger amount of tissue resected and subsequent lower incidence of positive margins.4,11,14 Local recurrence in patients undergoing breast conservation therapy and oncoplasty is between 2% and 9%, depending on the study.11,12
Postoperative surveillance
Postoperative surveillance can still be performed effectively despite the tissue transposition involved in any of the oncoplastic reconstruction techniques. A new baseline mammogram is obtained, to which future imaging studies are compared. Fat necrosis may appear to be new calcifications. Titanium clips may also be placed within the defect cavity so that it can be tracked to its new location. These clips also aid in localizing postoperative radiation therapy.11
Patient satisfaction
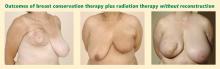
Several studies have assessed patient satisfaction with breast conservation therapy without and with reconstruction. Following breast conservation therapy without reconstruction, cosmetic results are rated as poor by 15% to 20% of patients.10 Patients notice breast asymmetry and are generally dissatisfied to some degree after breast conservation with radiation therapy and no further reconstruction.3 In contrast, a survey in a series of patients who had oncoplasty found that 95% reported good aesthetic results at short-term follow-up.10 Another series found that 88% of patients undergoing oncoplastic techniques reported fair to excellent outcomes at 2 years, and 82% did so at 5 years.12 When these patients were further analyzed, assessments of cosmetic outcomes were worse in those who received preoperative rather than postoperative radiation therapy.12
SUMMARY
Oncoplastic surgical approaches can be applied to the full spectrum of patients undergoing breast conservation therapy. They are particularly useful when a large defect is anticipated, when a symmetrizing procedure is desired for the contralateral breast, and when the tumor-to-breast volume ratio is unfavorable for simple closure.14 Immediate reconstruction is clearly preferred over delayed reconstruction, as it is associated with fewer complications, easier administration of postoperative radiation therapy, better aesthetic results, and possibly lower rates of local recurrence. Patients are more satisfied with the cosmetic outcome of oncoplastic procedures compared with breast conservation therapy alone. Successful oncoplasty requires thorough patient counseling and comprehensive preoperative planning among patient, oncologist, and general and plastic surgeons.
- Audretsch W, Rezai M, Kolotas C, et al. Tumor-specific immediate reconstruction in breast cancer patients. Perspect Plast Surg 1998; 11:71–100.
- Baildam AD. Oncoplastic surgery of the breast. Br J Surg 2002; 89:532–533.
- Bajaj AK, Kon PS, Oberg KC, Miles DA. Aesthetic outcomes in patients undergoing breast conservation therapy for the treatment of localized breast cancer. Plast Reconstr Surg 2004; 114:1442–1449.
- Kronowitz SJ, Feledy JA, Hunt KK, et al. Determining the optimal approach to breast reconstruction after partial mastectomy. Plast Reconstr Surg 2006; 117:1–11.
- Clough KB, Kroll SS, Audretsch W. An approach to the repair of partial mastectomy defects. Plast Reconstr Surg 1999; 104:409–420.
- Levine JL, Soucid NE, Allen RJ. Algorithm for autologous breast reconstruction for partial mastectomy defects. Plast Reconstr Surg 2005; 116:762–767.
- Grisotti A, Calabrese C. Conservative treatment of breast cancer: reconstructive issues. In: Spears S, ed. Surgery of the Breast: Principles and Art. 2nd ed. Philadelphia: Lippincott, Williams & Wilkins; 2006:147–178.
- Anderson BO, Masetti R, Silverstein MJ. Oncoplastic approaches to partial mastectomy: an overview of volume-displacement techniques. Lancet Oncol 2005; 6:145–157.
- McCulley SJ, Durani P, Macmillan RD. Therapeutic mammaplasty for centrally located breast tumors. Plast Reconstr Surg 2006; 117:366–373.
- Papp C, Wechselberger G, Schoeller T. Autologous breast reconstruction after breast-conserving cancer surgery. Plast Reconstr Surg 1998; 102:1932–1936.
- Losken A, Styblo TM, Carlson GW, et al. Management algorithm and outcome evaluation of partial mastectomy defects treated using reduction or mastopexy techniques. Ann Plast Surg 2007; 59:235–242.
- Clough KB, Lewis JS, Couturaud B, Fitoussi A, Nos C, Falcou MC. Oncoplastic techniques allow extensive resections for breast-conserving therapy of breast carcinomas. Ann Surg 2003; 237:26–34.
- Noguchi M, Taniya T, Miyazaki I, Saito Y. Immediate transposition of a latissimus dorsi muscle for correcting a postquadrantectomy breast deformity in Japanese patients. Int Surg 1990; 75:166–170.
- Huemer GM, Schrenk P, Moser F, Wagner E, Wayand W. Oncoplastic techniques allow breast-conserving treatment in centrally located breast cancers. Plast Reconstr Surg 2007; 120:390–398.
- Platt AJ, Mohan D, Baguley P. The effect of body mass index and wound irrigation on outcome after bilateral breast reduction. Ann Plast Surg 2003; 51:552–555.
- Iwuagwu OC. Additional considerations in the application of oncoplastic approaches [letter]. Lancet Oncol 2005; 6:356.
- Rohrich RJ, Coberly DM, Krueger JK, Brown SA. Planning elective operations on patients who smoke: survey of North American plastic surgeons. Plast Reconstr Surg 2002; 109:350–355.
- Nicholson RM, Leinster S, Sassoon EM. A comparison of the cosmetic and psychological outcome of breast reconstruction, breast conserving surgery and mastectomy without reconstruction. Breast 2007; 16:396–410.
Oncoplastic surgery refers to immediate or delayed breast reconstruction following partial mastectomy, also known as breast conservation therapy. The term was coined by Audretsch et al in 19981 and is now often referred to as oncoplasty. It involves four integral components:2
- Oncologically sound techniques of tumor removal
- Partial reconstruction of the breast to correct small defects
- Immediate reconstruction for larger defects using various principles of plastic surgery
- Creation of symmetry with the contralateral breast.
This article provides a brief overview of various procedures used for reconstruction following breast conservation therapy and the factors that guide selection among these procedures for individual patients. It concludes with a discussion of complications of oncoplastic procedures, patient counseling, and other general considerations in patient management.
THE RATIONALE FOR RECONSTRUCTION
Effects of radiation argue for immediate reconstruction
Although radiation therapy is integral to the comprehensive treatment of breast cancer after breast conservation therapy, radiation-induced changes to the breast are one of the greatest obstacles faced when delayed reconstruction is performed. Radiation results in deformation of the parenchyma, leading to retraction, fibrosis, vasculitis, and skin breakdown. The effects of radiation on breast tissue may possibly be a larger problem when reconstruction is delayed, as wound healing is inhibited and vascular supply is impaired. Therefore, immediate reconstruction should be undertaken whenever possible.7 (The timing of reconstruction is discussed in greater detail in the final article in this supplement, although mainly in the context of mastectomy.)
OPTIONS FOR RECONSTRUCTION
Various techniques of partial breast reconstruction can be used to achieve an aesthetically acceptable result. They can be thought of as volume-displacement procedures, such as local tissue rearrangement and reduction mammaplasty, or as volume-replacement procedures using flap reconstruction.8 Additionally, simple wound closure (primary closure) may be performed if small amounts of tissue can be removed without creating a noticeable defect, but simple closure is an option only for large breasts. The decision among techniques depends on a variety of factors, as delineated below.
Local tissue rearrangement
Local tissue rearrangement is defined as the use of local tissue (skin and subcutaneous and/or breast tissue) from either the breast or the axilla. This technique involves the transfer of adjacent breast parenchyma and skin to the area of the defect. It is dependent on a random blood supply and does not involve creating a parenchymal tissue pedicle.4,5 It does rely, however, on a balance between the amount of tissue resected and the available residual breast size and volume. This procedure is not suitable for patients who require large-volume resection with a small breast or limited breast tissue.
When local tissue rearrangement is to be performed, the surgical incision needs to be planned by both the oncologic surgeon and the plastic surgeon to ensure an appropriate cosmetic outcome and prevent displacement or distortion of the nipple-areola complex. If such planning is not done, the cosmetic outcome may be compromised, thereby undermining one of the reasons for breast conservation in the first place. When full-thickness excisions of tissue are removed from a certain area of the breast—termed “no man’s land” by Grisotti and Calabrese7—the nipple-areola complex shifts to an unnatural position. Therefore, resections in this area, located superiomedial to the nipple, should include little or no skin.
Other techniques of tissue transposition include circumareolar incisions for tumors located adjacent to the nipple-areola complex, radially designed resections for lateral tumors, and donut-shaped resections for superior or lateral tumors.8
Reduction mammaplasty
Standard breast reduction techniques are used on the contralateral (uninvolved) breast. This matching procedure can be performed at the same time as the initial cancer operation or as a delayed procedure. The matching procedure is usually performed at a later date for those who need to undergo radiation therapy, allowing time for healing and for final breast volume and shape to be achieved. Reduction of the contralateral breast does not increase its risk for cancer; in fact, reduction may improve body image and make breast self-examinations and follow-up mammography easier.
Therapeutic reduction mammaplasty is highly versatile and gives a better aesthetic result in the immediate setting when compared with flap reconstruction. However, it is usually limited to patients with a brassiere cup size of D or larger.4
An advantage of reduction mammaplasty is that reducing the size of the affected breast facilitates postoperative radiation therapy. Some radiation oncologists are reluctant to administer radiation to a large breast because of increased toxicity to the skin and the likelihood of a poor aesthetic outcome. With reduction mammaplasty, lower radiation doses are required and the delivery of radiation is more uniform.4
Reduction mammaplasty is ideal for women with moderate-sized or large breasts with ptosis (sagging), for whom a reduction in size would be considered a positive outcome.10 Patients with symptomatic macromastia likewise benefit from reduction in breast volume. An additional advantage is that the reduction procedure on the contralateral breast affords the opportunity for tissue sampling from this presumedly uninvolved breast; occult carcinomas in the contralateral breast have been identified in a small percentage of patients.11
At the same time, the exposure of the contralateral breast to surgery also constitutes the main disadvantage of this procedure, as both breasts are placed at risk for wound or nipple complications and the discomfort of surgery.9 Moreover, surgery time is also increased. Lastly, reduction mammaplasty can be offered only to patients who possess enough breast tissue to undergo reduction.12
Flap reconstruction
Flap reconstruction is indicated in patients who have significant breast volume deficit after resection and have insufficient adjacent tissue for local tissue recruitment and rearrangement. This method of reconstruction is based on an axial blood supply, which means that a specific vascular pedicle is responsible for a given distribution of tissue. For this purpose, flaps can be either myocutaneous (muscle-skin flaps), fasciocutaneous (fascia, subcutaneous tissue, and skin) or adipocutaneous (containing fat and skin). Examples include the latissimus dorsi myocutaneous flap, the transverse thoracoepigastric skin flap, and the lateral thoracic adipocutaneous flap.4–6
The latissimus dorsi myocutaneous flap is used most often, especially when more than 25% of the breast volume has been resected. Since a large volume of tissue is removed, either the tumor and a margin can be resected or a nipple-sparing subcutaneous mastectomy may be performed10 (nipple-sparing mastectomy would not be breast-conserving and has been discussed earlier in this supplement). This myocutaneous flap is based on the thoracodorsal vessels and was first described for volume replacement after breast-conserving surgery by Noguchi et al.13 A benefit of this flap is that most patients do not need reduction of the contralateral breast for symmetry, as the flap usually provides adequate tissue volume.4 This is beneficial for the patient, as she is not exposed to the potential complications of an operation on the contralateral breast.
The lateral thoracic adipocutaneous flap is another option. This flap has the benefit of sparing the muscle while using skin and fat from the axillary region. It can be based on one of three vascular pedicles that have been shown to be reliable as a sole blood supply. The most common pedicle for this technique is the thoracodorsal artery, as the main blood supply for the thoracodorsal artery perforator flap. This flap provides a potentially large amount of tissue for use and affords patients the chance to have a redundant roll of axillary tissue removed. This tissue can be used alone for reconstruction or in conjunction with a breast implant.6
One drawback of the latissimus dorsi flap is the potential for mismatch of skin color and texture when there is a need to address a significant skin deficit on the breast. Replacing a whole aesthetic unit, as opposed to only a small skin paddle, can minimize this potential; thus, using a larger amount of skin may provide a better aesthetic result. Rarely, if there is no skin defect, the muscle alone can be used, with no skin component.5 The lateral thoracic flap, on the other hand, may be more similar in skin color and texture to the native breast and may allow the scar to be better hidden in the axilla than is the case with the latissimus dorsi flap.6 Any type of flap presents potential donor site problems as well as breast complications (discussed below).
Flap reconstruction broadens the application of breast conservation therapy to women who would not otherwise be candidates because of the large volume of tissue they need to have removed.2 Oncoplasty reconstruction also allows the oncologic surgeon to be more aggressive with tissue removal without concerns about compromising the aesthetic outcome. Patients with small to moderate breasts are therefore candidates for flap reconstruction, as even modest resections in such patients result in a large volume of tissue loss and the need for additional tissue to reconstruct the breast.14 Any of the aforementioned flaps are advantageous, as they are in close proximity to the breast and can readily be used for reconstruction.6
CHOICE OF TECHNIQUE
Many factors contribute to the choice among reconstructive methods for a particular patient after breast conservation therapy.
Tumor location plays a significant role. Kronowitz et al described using breast reduction as their primary reconstructive modality, particularly for tumors of the upper inner, upper outer, and lower inner quadrants of the breast.4 They used flap reconstruction only for outer-quadrant tumors, and they found that tumors of the lower outer quadrant were the largest and lent themselves to local tissue rearrangement, often with axillary tissue.4 Centrally located tumors usually require removal of the nipple-areola complex and can be challenging to reconstruct. The techniques include either (1) direct closure with some degree of local tissue remodeling, or (2) reduction mammaplasty. The majority of patients with centrally located tumors will need contralateral breast reduction for symmetry14 and nipple-areola reconstruction at a later date.
The size of the defect created by the tumor resection also significantly affects the choice of technique, as does the patient’s preoperative brassiere size. In the analysis by Kronowitz et al, defects smaller than 20% of the overall breast size were found to be amenable to breast reduction, whereas larger defects were reconstructed with flaps or local tissue rearrangement.4 Also, women with a brassiere cup size of D or larger tended to undergo breast reduction, whereas those with a size smaller than D underwent local tissue rearrangement or flap reconstruction.4
COMPLICATIONS
Complications of breast surgery include seromas (of the breast as well as the donor site when a flap is used), nipple necrosis, wound dehiscence, infection, hematoma, fat necrosis, and mastectomy flap necrosis. Postoperative hematomas and superficial wound infections tend to occur in the immediate postoperative period (usually within the first few days), whereas the other complications mentioned may take 1 to 2 weeks to develop. These complications are common to all breast operations and are not specific to reconstruction after breast conservation therapy.
Postoperative complications vary in frequency but are more common when reconstruction is delayed.4,7 They also vary depending on the reconstructive technique. Donor-site seromas and fat necrosis are most common with immediate reconstruction using a flap; wound dehiscence is most common with delayed local tissue rearrangement; and breast seroma is most common with delayed reduction mammaplasty.4
Other issues to consider include the possible delay in adjuvant therapy in patients who experience wound healing problems, especially in those who are obese, who smoke, or who undergo therapeutic mammaplasty.15,16 Moreover, operative time is increased with oncoplasty as compared with simple wide local excision, which increases patients’ exposure to anesthesia and thereby raises the risk of complications, particularly in older patients with comorbidities.16
Risk factors for complications
Certain patient characteristics carry an increased risk for postoperative complications. These include tobacco smoking, previous breast surgeries, comorbidities that impair wound healing, and obesity.4,15–17
The vasoconstrictive, thrombotic, and hypoxic effects of tobacco place patients who smoke at an increased risk for necrosis of the nipple-areola complex, as well as for pulmonary complications, when breast reduction is performed. The standard recommendation is cessation of smoking for 6 to 8 weeks preoperatively to reduce pulmonary risks, although rigorous scientific validation is lacking.17
Breasts that have been previously operated on have scarring of the skin and subcutaneous tissues, which may affect the surgical incision and technique. Additionally, vascular compromise of the underlying breast tissue and nipple-areola complex is a possibility in patients who have had previous breast operations.4 For these reasons, it is of utmost importance to obtain a full history of any previous breast procedures a patient has had.
Obesity is a risk factor for impaired wound healing, as delayed wound healing has been correlated with increased body mass index in patients undergoing breast reduction.15
What about positive margins?
Addressing positive margins can be problematic after breast conservation therapy with immediate reconstruction, as it is difficult to locate the resection margin after the breast tissue has been rearranged.4,5,12,14 Patients who have positive margins will usually need to undergo completion mastectomy and opt for immediate reconstruction with a transverse rectus abdominis myocutaneous (TRAM) flap or a latissimus dorsi flap with an implant. Therefore, use of a TRAM flap for initial reconstruction after breast conservation therapy is discouraged.4,14 If a TRAM flap is needed to restore the shape and contour of the breast after breast conservation, it is usually better to perform a mastectomy, as it provides a superior aesthetic result and reduces the risk of a subsequent malignancy since the breast tissue is removed.5
PATIENT COUNSELING, PREOPERATIVE PLANNING
The diagnosis of breast cancer is devastating for most women and is compounded by mental anguish associated with the anticipated changes in their appearance.10 There is a psychological advantage to having reconstruction performed during the same operation as resection because the breast’s preoperative form is immediately restored and little to no asymmetry is seen postoperatively.12 One study showed that breast cancer patients who underwent reconstructive surgery had better body images and felt they had more control over their treatment compared with patients who simply had breast conservation therapy or mastectomy without reconstruction; these perceptions also conferred a psychological benefit among the patients who had reconstructive procedures.18
At the same time, all patients need to be counseled about the potential drawbacks of reconstruction, including the possibility of reoperation for positive margins, wound complications, or a disappointing or unacceptable aesthetic outcome.
Oncoplastic surgery is a multispecialty collaboration. Good communication and preoperative planning is imperative and must include the general surgeon, plastic surgeon, oncologist, and, most importantly, the patient. Considerations in how to approach diagnostic biopsies, lymph node sampling, timing of contralateral breast symmetrizing procedures, and the possibility of positive margins all need to be discussed preoperatively.8,10
ADDITIONAL CONSIDERATIONS
Timing of reconstruction
Immediate reconstruction is preferred for many reasons, including a reduced incidence of wound healing problems, facility in administering postoperative radiation therapy, and better aesthetic results.3,4,11 A one-stage procedure also facilitates breast remodeling, as there is no scar tissue to deal with. Patients’ psychological trauma of coping with a deformity is also reduced because better symmetry is achieved with immediate reconstruction.10
Additionally, some authors have reported lower rates of local recurrence in breast conservation therapy patients who received immediate reconstruction, likely owing to the larger amount of tissue resected and subsequent lower incidence of positive margins.4,11,14 Local recurrence in patients undergoing breast conservation therapy and oncoplasty is between 2% and 9%, depending on the study.11,12
Postoperative surveillance
Postoperative surveillance can still be performed effectively despite the tissue transposition involved in any of the oncoplastic reconstruction techniques. A new baseline mammogram is obtained, to which future imaging studies are compared. Fat necrosis may appear to be new calcifications. Titanium clips may also be placed within the defect cavity so that it can be tracked to its new location. These clips also aid in localizing postoperative radiation therapy.11
Patient satisfaction
Several studies have assessed patient satisfaction with breast conservation therapy without and with reconstruction. Following breast conservation therapy without reconstruction, cosmetic results are rated as poor by 15% to 20% of patients.10 Patients notice breast asymmetry and are generally dissatisfied to some degree after breast conservation with radiation therapy and no further reconstruction.3 In contrast, a survey in a series of patients who had oncoplasty found that 95% reported good aesthetic results at short-term follow-up.10 Another series found that 88% of patients undergoing oncoplastic techniques reported fair to excellent outcomes at 2 years, and 82% did so at 5 years.12 When these patients were further analyzed, assessments of cosmetic outcomes were worse in those who received preoperative rather than postoperative radiation therapy.12
SUMMARY
Oncoplastic surgical approaches can be applied to the full spectrum of patients undergoing breast conservation therapy. They are particularly useful when a large defect is anticipated, when a symmetrizing procedure is desired for the contralateral breast, and when the tumor-to-breast volume ratio is unfavorable for simple closure.14 Immediate reconstruction is clearly preferred over delayed reconstruction, as it is associated with fewer complications, easier administration of postoperative radiation therapy, better aesthetic results, and possibly lower rates of local recurrence. Patients are more satisfied with the cosmetic outcome of oncoplastic procedures compared with breast conservation therapy alone. Successful oncoplasty requires thorough patient counseling and comprehensive preoperative planning among patient, oncologist, and general and plastic surgeons.
Oncoplastic surgery refers to immediate or delayed breast reconstruction following partial mastectomy, also known as breast conservation therapy. The term was coined by Audretsch et al in 19981 and is now often referred to as oncoplasty. It involves four integral components:2
- Oncologically sound techniques of tumor removal
- Partial reconstruction of the breast to correct small defects
- Immediate reconstruction for larger defects using various principles of plastic surgery
- Creation of symmetry with the contralateral breast.
This article provides a brief overview of various procedures used for reconstruction following breast conservation therapy and the factors that guide selection among these procedures for individual patients. It concludes with a discussion of complications of oncoplastic procedures, patient counseling, and other general considerations in patient management.
THE RATIONALE FOR RECONSTRUCTION
Effects of radiation argue for immediate reconstruction
Although radiation therapy is integral to the comprehensive treatment of breast cancer after breast conservation therapy, radiation-induced changes to the breast are one of the greatest obstacles faced when delayed reconstruction is performed. Radiation results in deformation of the parenchyma, leading to retraction, fibrosis, vasculitis, and skin breakdown. The effects of radiation on breast tissue may possibly be a larger problem when reconstruction is delayed, as wound healing is inhibited and vascular supply is impaired. Therefore, immediate reconstruction should be undertaken whenever possible.7 (The timing of reconstruction is discussed in greater detail in the final article in this supplement, although mainly in the context of mastectomy.)
OPTIONS FOR RECONSTRUCTION
Various techniques of partial breast reconstruction can be used to achieve an aesthetically acceptable result. They can be thought of as volume-displacement procedures, such as local tissue rearrangement and reduction mammaplasty, or as volume-replacement procedures using flap reconstruction.8 Additionally, simple wound closure (primary closure) may be performed if small amounts of tissue can be removed without creating a noticeable defect, but simple closure is an option only for large breasts. The decision among techniques depends on a variety of factors, as delineated below.
Local tissue rearrangement
Local tissue rearrangement is defined as the use of local tissue (skin and subcutaneous and/or breast tissue) from either the breast or the axilla. This technique involves the transfer of adjacent breast parenchyma and skin to the area of the defect. It is dependent on a random blood supply and does not involve creating a parenchymal tissue pedicle.4,5 It does rely, however, on a balance between the amount of tissue resected and the available residual breast size and volume. This procedure is not suitable for patients who require large-volume resection with a small breast or limited breast tissue.
When local tissue rearrangement is to be performed, the surgical incision needs to be planned by both the oncologic surgeon and the plastic surgeon to ensure an appropriate cosmetic outcome and prevent displacement or distortion of the nipple-areola complex. If such planning is not done, the cosmetic outcome may be compromised, thereby undermining one of the reasons for breast conservation in the first place. When full-thickness excisions of tissue are removed from a certain area of the breast—termed “no man’s land” by Grisotti and Calabrese7—the nipple-areola complex shifts to an unnatural position. Therefore, resections in this area, located superiomedial to the nipple, should include little or no skin.
Other techniques of tissue transposition include circumareolar incisions for tumors located adjacent to the nipple-areola complex, radially designed resections for lateral tumors, and donut-shaped resections for superior or lateral tumors.8
Reduction mammaplasty
Standard breast reduction techniques are used on the contralateral (uninvolved) breast. This matching procedure can be performed at the same time as the initial cancer operation or as a delayed procedure. The matching procedure is usually performed at a later date for those who need to undergo radiation therapy, allowing time for healing and for final breast volume and shape to be achieved. Reduction of the contralateral breast does not increase its risk for cancer; in fact, reduction may improve body image and make breast self-examinations and follow-up mammography easier.
Therapeutic reduction mammaplasty is highly versatile and gives a better aesthetic result in the immediate setting when compared with flap reconstruction. However, it is usually limited to patients with a brassiere cup size of D or larger.4
An advantage of reduction mammaplasty is that reducing the size of the affected breast facilitates postoperative radiation therapy. Some radiation oncologists are reluctant to administer radiation to a large breast because of increased toxicity to the skin and the likelihood of a poor aesthetic outcome. With reduction mammaplasty, lower radiation doses are required and the delivery of radiation is more uniform.4
Reduction mammaplasty is ideal for women with moderate-sized or large breasts with ptosis (sagging), for whom a reduction in size would be considered a positive outcome.10 Patients with symptomatic macromastia likewise benefit from reduction in breast volume. An additional advantage is that the reduction procedure on the contralateral breast affords the opportunity for tissue sampling from this presumedly uninvolved breast; occult carcinomas in the contralateral breast have been identified in a small percentage of patients.11
At the same time, the exposure of the contralateral breast to surgery also constitutes the main disadvantage of this procedure, as both breasts are placed at risk for wound or nipple complications and the discomfort of surgery.9 Moreover, surgery time is also increased. Lastly, reduction mammaplasty can be offered only to patients who possess enough breast tissue to undergo reduction.12
Flap reconstruction
Flap reconstruction is indicated in patients who have significant breast volume deficit after resection and have insufficient adjacent tissue for local tissue recruitment and rearrangement. This method of reconstruction is based on an axial blood supply, which means that a specific vascular pedicle is responsible for a given distribution of tissue. For this purpose, flaps can be either myocutaneous (muscle-skin flaps), fasciocutaneous (fascia, subcutaneous tissue, and skin) or adipocutaneous (containing fat and skin). Examples include the latissimus dorsi myocutaneous flap, the transverse thoracoepigastric skin flap, and the lateral thoracic adipocutaneous flap.4–6
The latissimus dorsi myocutaneous flap is used most often, especially when more than 25% of the breast volume has been resected. Since a large volume of tissue is removed, either the tumor and a margin can be resected or a nipple-sparing subcutaneous mastectomy may be performed10 (nipple-sparing mastectomy would not be breast-conserving and has been discussed earlier in this supplement). This myocutaneous flap is based on the thoracodorsal vessels and was first described for volume replacement after breast-conserving surgery by Noguchi et al.13 A benefit of this flap is that most patients do not need reduction of the contralateral breast for symmetry, as the flap usually provides adequate tissue volume.4 This is beneficial for the patient, as she is not exposed to the potential complications of an operation on the contralateral breast.
The lateral thoracic adipocutaneous flap is another option. This flap has the benefit of sparing the muscle while using skin and fat from the axillary region. It can be based on one of three vascular pedicles that have been shown to be reliable as a sole blood supply. The most common pedicle for this technique is the thoracodorsal artery, as the main blood supply for the thoracodorsal artery perforator flap. This flap provides a potentially large amount of tissue for use and affords patients the chance to have a redundant roll of axillary tissue removed. This tissue can be used alone for reconstruction or in conjunction with a breast implant.6
One drawback of the latissimus dorsi flap is the potential for mismatch of skin color and texture when there is a need to address a significant skin deficit on the breast. Replacing a whole aesthetic unit, as opposed to only a small skin paddle, can minimize this potential; thus, using a larger amount of skin may provide a better aesthetic result. Rarely, if there is no skin defect, the muscle alone can be used, with no skin component.5 The lateral thoracic flap, on the other hand, may be more similar in skin color and texture to the native breast and may allow the scar to be better hidden in the axilla than is the case with the latissimus dorsi flap.6 Any type of flap presents potential donor site problems as well as breast complications (discussed below).
Flap reconstruction broadens the application of breast conservation therapy to women who would not otherwise be candidates because of the large volume of tissue they need to have removed.2 Oncoplasty reconstruction also allows the oncologic surgeon to be more aggressive with tissue removal without concerns about compromising the aesthetic outcome. Patients with small to moderate breasts are therefore candidates for flap reconstruction, as even modest resections in such patients result in a large volume of tissue loss and the need for additional tissue to reconstruct the breast.14 Any of the aforementioned flaps are advantageous, as they are in close proximity to the breast and can readily be used for reconstruction.6
CHOICE OF TECHNIQUE
Many factors contribute to the choice among reconstructive methods for a particular patient after breast conservation therapy.
Tumor location plays a significant role. Kronowitz et al described using breast reduction as their primary reconstructive modality, particularly for tumors of the upper inner, upper outer, and lower inner quadrants of the breast.4 They used flap reconstruction only for outer-quadrant tumors, and they found that tumors of the lower outer quadrant were the largest and lent themselves to local tissue rearrangement, often with axillary tissue.4 Centrally located tumors usually require removal of the nipple-areola complex and can be challenging to reconstruct. The techniques include either (1) direct closure with some degree of local tissue remodeling, or (2) reduction mammaplasty. The majority of patients with centrally located tumors will need contralateral breast reduction for symmetry14 and nipple-areola reconstruction at a later date.
The size of the defect created by the tumor resection also significantly affects the choice of technique, as does the patient’s preoperative brassiere size. In the analysis by Kronowitz et al, defects smaller than 20% of the overall breast size were found to be amenable to breast reduction, whereas larger defects were reconstructed with flaps or local tissue rearrangement.4 Also, women with a brassiere cup size of D or larger tended to undergo breast reduction, whereas those with a size smaller than D underwent local tissue rearrangement or flap reconstruction.4
COMPLICATIONS
Complications of breast surgery include seromas (of the breast as well as the donor site when a flap is used), nipple necrosis, wound dehiscence, infection, hematoma, fat necrosis, and mastectomy flap necrosis. Postoperative hematomas and superficial wound infections tend to occur in the immediate postoperative period (usually within the first few days), whereas the other complications mentioned may take 1 to 2 weeks to develop. These complications are common to all breast operations and are not specific to reconstruction after breast conservation therapy.
Postoperative complications vary in frequency but are more common when reconstruction is delayed.4,7 They also vary depending on the reconstructive technique. Donor-site seromas and fat necrosis are most common with immediate reconstruction using a flap; wound dehiscence is most common with delayed local tissue rearrangement; and breast seroma is most common with delayed reduction mammaplasty.4
Other issues to consider include the possible delay in adjuvant therapy in patients who experience wound healing problems, especially in those who are obese, who smoke, or who undergo therapeutic mammaplasty.15,16 Moreover, operative time is increased with oncoplasty as compared with simple wide local excision, which increases patients’ exposure to anesthesia and thereby raises the risk of complications, particularly in older patients with comorbidities.16
Risk factors for complications
Certain patient characteristics carry an increased risk for postoperative complications. These include tobacco smoking, previous breast surgeries, comorbidities that impair wound healing, and obesity.4,15–17
The vasoconstrictive, thrombotic, and hypoxic effects of tobacco place patients who smoke at an increased risk for necrosis of the nipple-areola complex, as well as for pulmonary complications, when breast reduction is performed. The standard recommendation is cessation of smoking for 6 to 8 weeks preoperatively to reduce pulmonary risks, although rigorous scientific validation is lacking.17
Breasts that have been previously operated on have scarring of the skin and subcutaneous tissues, which may affect the surgical incision and technique. Additionally, vascular compromise of the underlying breast tissue and nipple-areola complex is a possibility in patients who have had previous breast operations.4 For these reasons, it is of utmost importance to obtain a full history of any previous breast procedures a patient has had.
Obesity is a risk factor for impaired wound healing, as delayed wound healing has been correlated with increased body mass index in patients undergoing breast reduction.15
What about positive margins?
Addressing positive margins can be problematic after breast conservation therapy with immediate reconstruction, as it is difficult to locate the resection margin after the breast tissue has been rearranged.4,5,12,14 Patients who have positive margins will usually need to undergo completion mastectomy and opt for immediate reconstruction with a transverse rectus abdominis myocutaneous (TRAM) flap or a latissimus dorsi flap with an implant. Therefore, use of a TRAM flap for initial reconstruction after breast conservation therapy is discouraged.4,14 If a TRAM flap is needed to restore the shape and contour of the breast after breast conservation, it is usually better to perform a mastectomy, as it provides a superior aesthetic result and reduces the risk of a subsequent malignancy since the breast tissue is removed.5
PATIENT COUNSELING, PREOPERATIVE PLANNING
The diagnosis of breast cancer is devastating for most women and is compounded by mental anguish associated with the anticipated changes in their appearance.10 There is a psychological advantage to having reconstruction performed during the same operation as resection because the breast’s preoperative form is immediately restored and little to no asymmetry is seen postoperatively.12 One study showed that breast cancer patients who underwent reconstructive surgery had better body images and felt they had more control over their treatment compared with patients who simply had breast conservation therapy or mastectomy without reconstruction; these perceptions also conferred a psychological benefit among the patients who had reconstructive procedures.18
At the same time, all patients need to be counseled about the potential drawbacks of reconstruction, including the possibility of reoperation for positive margins, wound complications, or a disappointing or unacceptable aesthetic outcome.
Oncoplastic surgery is a multispecialty collaboration. Good communication and preoperative planning is imperative and must include the general surgeon, plastic surgeon, oncologist, and, most importantly, the patient. Considerations in how to approach diagnostic biopsies, lymph node sampling, timing of contralateral breast symmetrizing procedures, and the possibility of positive margins all need to be discussed preoperatively.8,10
ADDITIONAL CONSIDERATIONS
Timing of reconstruction
Immediate reconstruction is preferred for many reasons, including a reduced incidence of wound healing problems, facility in administering postoperative radiation therapy, and better aesthetic results.3,4,11 A one-stage procedure also facilitates breast remodeling, as there is no scar tissue to deal with. Patients’ psychological trauma of coping with a deformity is also reduced because better symmetry is achieved with immediate reconstruction.10
Additionally, some authors have reported lower rates of local recurrence in breast conservation therapy patients who received immediate reconstruction, likely owing to the larger amount of tissue resected and subsequent lower incidence of positive margins.4,11,14 Local recurrence in patients undergoing breast conservation therapy and oncoplasty is between 2% and 9%, depending on the study.11,12
Postoperative surveillance
Postoperative surveillance can still be performed effectively despite the tissue transposition involved in any of the oncoplastic reconstruction techniques. A new baseline mammogram is obtained, to which future imaging studies are compared. Fat necrosis may appear to be new calcifications. Titanium clips may also be placed within the defect cavity so that it can be tracked to its new location. These clips also aid in localizing postoperative radiation therapy.11
Patient satisfaction
Several studies have assessed patient satisfaction with breast conservation therapy without and with reconstruction. Following breast conservation therapy without reconstruction, cosmetic results are rated as poor by 15% to 20% of patients.10 Patients notice breast asymmetry and are generally dissatisfied to some degree after breast conservation with radiation therapy and no further reconstruction.3 In contrast, a survey in a series of patients who had oncoplasty found that 95% reported good aesthetic results at short-term follow-up.10 Another series found that 88% of patients undergoing oncoplastic techniques reported fair to excellent outcomes at 2 years, and 82% did so at 5 years.12 When these patients were further analyzed, assessments of cosmetic outcomes were worse in those who received preoperative rather than postoperative radiation therapy.12
SUMMARY
Oncoplastic surgical approaches can be applied to the full spectrum of patients undergoing breast conservation therapy. They are particularly useful when a large defect is anticipated, when a symmetrizing procedure is desired for the contralateral breast, and when the tumor-to-breast volume ratio is unfavorable for simple closure.14 Immediate reconstruction is clearly preferred over delayed reconstruction, as it is associated with fewer complications, easier administration of postoperative radiation therapy, better aesthetic results, and possibly lower rates of local recurrence. Patients are more satisfied with the cosmetic outcome of oncoplastic procedures compared with breast conservation therapy alone. Successful oncoplasty requires thorough patient counseling and comprehensive preoperative planning among patient, oncologist, and general and plastic surgeons.
- Audretsch W, Rezai M, Kolotas C, et al. Tumor-specific immediate reconstruction in breast cancer patients. Perspect Plast Surg 1998; 11:71–100.
- Baildam AD. Oncoplastic surgery of the breast. Br J Surg 2002; 89:532–533.
- Bajaj AK, Kon PS, Oberg KC, Miles DA. Aesthetic outcomes in patients undergoing breast conservation therapy for the treatment of localized breast cancer. Plast Reconstr Surg 2004; 114:1442–1449.
- Kronowitz SJ, Feledy JA, Hunt KK, et al. Determining the optimal approach to breast reconstruction after partial mastectomy. Plast Reconstr Surg 2006; 117:1–11.
- Clough KB, Kroll SS, Audretsch W. An approach to the repair of partial mastectomy defects. Plast Reconstr Surg 1999; 104:409–420.
- Levine JL, Soucid NE, Allen RJ. Algorithm for autologous breast reconstruction for partial mastectomy defects. Plast Reconstr Surg 2005; 116:762–767.
- Grisotti A, Calabrese C. Conservative treatment of breast cancer: reconstructive issues. In: Spears S, ed. Surgery of the Breast: Principles and Art. 2nd ed. Philadelphia: Lippincott, Williams & Wilkins; 2006:147–178.
- Anderson BO, Masetti R, Silverstein MJ. Oncoplastic approaches to partial mastectomy: an overview of volume-displacement techniques. Lancet Oncol 2005; 6:145–157.
- McCulley SJ, Durani P, Macmillan RD. Therapeutic mammaplasty for centrally located breast tumors. Plast Reconstr Surg 2006; 117:366–373.
- Papp C, Wechselberger G, Schoeller T. Autologous breast reconstruction after breast-conserving cancer surgery. Plast Reconstr Surg 1998; 102:1932–1936.
- Losken A, Styblo TM, Carlson GW, et al. Management algorithm and outcome evaluation of partial mastectomy defects treated using reduction or mastopexy techniques. Ann Plast Surg 2007; 59:235–242.
- Clough KB, Lewis JS, Couturaud B, Fitoussi A, Nos C, Falcou MC. Oncoplastic techniques allow extensive resections for breast-conserving therapy of breast carcinomas. Ann Surg 2003; 237:26–34.
- Noguchi M, Taniya T, Miyazaki I, Saito Y. Immediate transposition of a latissimus dorsi muscle for correcting a postquadrantectomy breast deformity in Japanese patients. Int Surg 1990; 75:166–170.
- Huemer GM, Schrenk P, Moser F, Wagner E, Wayand W. Oncoplastic techniques allow breast-conserving treatment in centrally located breast cancers. Plast Reconstr Surg 2007; 120:390–398.
- Platt AJ, Mohan D, Baguley P. The effect of body mass index and wound irrigation on outcome after bilateral breast reduction. Ann Plast Surg 2003; 51:552–555.
- Iwuagwu OC. Additional considerations in the application of oncoplastic approaches [letter]. Lancet Oncol 2005; 6:356.
- Rohrich RJ, Coberly DM, Krueger JK, Brown SA. Planning elective operations on patients who smoke: survey of North American plastic surgeons. Plast Reconstr Surg 2002; 109:350–355.
- Nicholson RM, Leinster S, Sassoon EM. A comparison of the cosmetic and psychological outcome of breast reconstruction, breast conserving surgery and mastectomy without reconstruction. Breast 2007; 16:396–410.
- Audretsch W, Rezai M, Kolotas C, et al. Tumor-specific immediate reconstruction in breast cancer patients. Perspect Plast Surg 1998; 11:71–100.
- Baildam AD. Oncoplastic surgery of the breast. Br J Surg 2002; 89:532–533.
- Bajaj AK, Kon PS, Oberg KC, Miles DA. Aesthetic outcomes in patients undergoing breast conservation therapy for the treatment of localized breast cancer. Plast Reconstr Surg 2004; 114:1442–1449.
- Kronowitz SJ, Feledy JA, Hunt KK, et al. Determining the optimal approach to breast reconstruction after partial mastectomy. Plast Reconstr Surg 2006; 117:1–11.
- Clough KB, Kroll SS, Audretsch W. An approach to the repair of partial mastectomy defects. Plast Reconstr Surg 1999; 104:409–420.
- Levine JL, Soucid NE, Allen RJ. Algorithm for autologous breast reconstruction for partial mastectomy defects. Plast Reconstr Surg 2005; 116:762–767.
- Grisotti A, Calabrese C. Conservative treatment of breast cancer: reconstructive issues. In: Spears S, ed. Surgery of the Breast: Principles and Art. 2nd ed. Philadelphia: Lippincott, Williams & Wilkins; 2006:147–178.
- Anderson BO, Masetti R, Silverstein MJ. Oncoplastic approaches to partial mastectomy: an overview of volume-displacement techniques. Lancet Oncol 2005; 6:145–157.
- McCulley SJ, Durani P, Macmillan RD. Therapeutic mammaplasty for centrally located breast tumors. Plast Reconstr Surg 2006; 117:366–373.
- Papp C, Wechselberger G, Schoeller T. Autologous breast reconstruction after breast-conserving cancer surgery. Plast Reconstr Surg 1998; 102:1932–1936.
- Losken A, Styblo TM, Carlson GW, et al. Management algorithm and outcome evaluation of partial mastectomy defects treated using reduction or mastopexy techniques. Ann Plast Surg 2007; 59:235–242.
- Clough KB, Lewis JS, Couturaud B, Fitoussi A, Nos C, Falcou MC. Oncoplastic techniques allow extensive resections for breast-conserving therapy of breast carcinomas. Ann Surg 2003; 237:26–34.
- Noguchi M, Taniya T, Miyazaki I, Saito Y. Immediate transposition of a latissimus dorsi muscle for correcting a postquadrantectomy breast deformity in Japanese patients. Int Surg 1990; 75:166–170.
- Huemer GM, Schrenk P, Moser F, Wagner E, Wayand W. Oncoplastic techniques allow breast-conserving treatment in centrally located breast cancers. Plast Reconstr Surg 2007; 120:390–398.
- Platt AJ, Mohan D, Baguley P. The effect of body mass index and wound irrigation on outcome after bilateral breast reduction. Ann Plast Surg 2003; 51:552–555.
- Iwuagwu OC. Additional considerations in the application of oncoplastic approaches [letter]. Lancet Oncol 2005; 6:356.
- Rohrich RJ, Coberly DM, Krueger JK, Brown SA. Planning elective operations on patients who smoke: survey of North American plastic surgeons. Plast Reconstr Surg 2002; 109:350–355.
- Nicholson RM, Leinster S, Sassoon EM. A comparison of the cosmetic and psychological outcome of breast reconstruction, breast conserving surgery and mastectomy without reconstruction. Breast 2007; 16:396–410.