User login
Early detection of breast cancer is vital to reducing the morbidity and mortality associated with this disease. After a brief overview of breast cancer epidemiology and risk assessment, this article describes screening and diagnostic imaging techniques as they are currently practiced to promote early breast cancer detection. We conclude with a review of image-guided needle biopsy techniques and a recommended approach to breast cancer screening in the general population.
EPIDEMIOLOGY OF BREAST CANCER: DAUNTING BUT SLOWLY IMPROVING
After nonmelanoma skin cancers, breast cancer is the most common form of cancer in women today, accounting for more than 1 in 4 cancers diagnosed in US women.1 If the current incidence of breast cancer remains constant, US females born today have an average risk of 12.7% of being diagnosed with breast cancer during their lifetime (ie, 1-in-8 lifetime risk), based on National Cancer Institute statistics.2,3 The American Cancer Society estimated that 178,480 new cases of invasive breast cancer and 62,030 new cases of in situ breast cancer would be diagnosed in the United States in 2007, and that 40,460 US women would die from breast cancer that year.1 Only lung cancer accounts for more cancer deaths in women.
The role of race and ethnicity
Breast cancer risk varies by race and ethnicity in the United States. After age 40 years, white women have a higher incidence of breast cancer compared with African American women; conversely, before age 40, African American women have a higher incidence compared with white women. African American women are more likely than their white counterparts to die from their breast cancer at any age. Incidence and death rates from breast cancer are lower among Asian American, American Indian, and Hispanic women compared with both white and African American women.1
Recent hopeful trends
Despite the daunting incidence numbers reviewed above, recent years have seen encouraging trends in US breast cancer epidemiology.
For invasive breast cancer, the growth in incidence rates slowed during the 1990s, and rates actually declined by 3.5% per year during the period 2001–2004.1 These changes are likely attributable to multiple factors, including variations in rates of mammography screening and decreased use of hormone replacement therapy after the 2002 publication of results from the Women’s Health Initiative trial. Still, the trend is encouraging.
Incidence rates of in situ breast cancer rose rapidly during the 1980s and 1990s, largely due to increased diagnosis by mammography, but have plateaued since 2000 among women aged 50 years or older while continuing to rise modestly in younger women.1
Meanwhile, the overall death rate from breast cancer in women declined by 2.2% annually from 1990 to 2004.1
RISK FACTORS AND RISK MODELING
Risk factors for breast cancer have been well described and include the following:
- Age ( ≥ 65 years vs < 65 years, although risk increases across all ages up to 80 years)
- Family history of breast cancer
- Late age at first full-term pregnancy (> 30 years)
- Never having a full-term pregnancy
- Early menarche and/or late menopause
- Certain genetic mutations for breast cancer (eg, in the BRCA1, BRCA2, ATM, and CHEK2 genes)
- Certain breast disorders, such as atypical hyperplasia or lobular carcinoma in situ
- High breast tissue density
- High bone density (postmenopausal)
- High-dose radiation to the chest.
The above risk factors are, in general, fixed. More elusive risk factors, in that they are variable and modifiable, include obesity, use of exogenous hormones (recent and long-term hormone replacement therapy; recent oral contraceptive use), alcohol use, tobacco use, diet, and a low level of physical activity. Breast implants are not a risk factor for breast cancer, though their presence does obscure breast tissue on imaging, limiting the detectability of a tumor when it does develop (see “Screening the Surgically Altered Breast” below).
Women with a genetic predisposition to breast cancer merit special consideration. Hereditary breast cancers account for about 5% to 10% of breast cancer cases, and the BRCA1 and BRCA2 mutations are responsible for 80% to 90% of these cases, while other gene mutations (noted above) or genetic syndromes account for the rest. Clinical options for managing women with a genetic predisposition include surveillance, chemoprevention, and prophylactic surgery.4 Detailed discussion of the management of these women is beyond the scope of this article, but readers are referred to www.nccn.org/professionals/physician_gls/PDF/ genetics_screening.pdf for practice guidelines from the National Comprehensive Cancer Network.5
Tools for risk assessment
Several tools are available to predict a woman’s risk of developing breast cancer. Probably the most widely used is the Gail model,6 which was published in 1989 and forms the statistical basis for the National Cancer Institute’s Breast Cancer Risk Assessment Tool, which is available for downloading at www.cancer.gov/bcrisktool.7 The model uses a woman’s personal medical and reproductive histories and her family history of breast cancer to predict her 5-year and lifetime risk of developing invasive breast cancer. Factors included in the risk calculation are age, race, number of first-degree relatives with a history of breast cancer, age at first live birth (or nulliparity), age at menarche, number of breast biopsies, and presence or absence of a history of atypical hyperplasia. The relative risk for each of these factors is multiplied to generate a composite risk. The Gail model has been validated for white women but has been shown to underestimate breast cancer risk in African American women; it remains to be validated for Hispanic women, Asian women, and other subgroups of women.7
The commonly taught “triple test” for palpable breast lesions is another risk model that incorporates clinical findings. It consists of a physical examination, mammography, and fine-needle aspiration8 (in the “modified triple test,” ultrasonography replaces mammography9). When all three elements of the test are concordant (either all benign or all malignant), the triple test has been reported to have 100% diagnostic accuracy.8,9
A WORD ABOUT BREAST EXAMINATION
Breast self-examination
Clinical breast examination
As noted in Table 1, regular clinical breast examinations are recommended by the American Cancer Society for asymptomatic women at average risk for breast cancer, with the recommended frequency depending on the woman’s age.10 The US Preventive Services Task Force takes the stance that there is insufficient evidence to recommend for or against breast cancer screening with clinical breast examination alone.11 While it is unclear precisely what contribution clinical breast exams make to the detection of breast cancer, they certainly provide clinicians an opportunity to raise awareness about breast cancer and educate patients about breast symptoms, risk factors, and new detection technologies.10
SCREENING MAMMOGRAPHY
Screening mammography is the single most effective method of early breast cancer detection,1 and the American Cancer Society recommends that women at average risk for breast cancer have annual screening mammograms beginning at age 40 years (Table 1).10
The evidence base
The primary evidence supporting the recommendation for screening mammography comes from eight randomized trials that studied the effectiveness of screening mammography for cancer detection in Sweden,12,13 the United States,14 Canada,15,16 and the United Kingdom.17 Overall, breast cancers detected by screening mammography are smaller and have a more favorable history and tumor biology than those detected clinically without the use of imaging. A pooled analysis of the most recent data from all randomized trials of screening mammography in women aged 39 to 74 years showed a 24% reduction in mortality (95% CI, 18% to 30%) in women undergoing screening mammography, although not all individual trials showed a statistically significant mortality reduction.10
The screening procedure at a glance
Analog vs digital
Digital mammograms are radiographs that are acquired digitally and allow digital enhancement to aid in interpretation. When receiving a digital mammogram, the woman being screened still undergoes compression and positioning as for a conventional film-screen mammogram, and the images are still produced with x-rays. However, digitization allows manipulation of the images as they are being interpreted, enabling the radiologist to focus on areas of interest or to “window” and “level” the image, similar to adjusting the tint and contrast on a television set.
Research trials comparing digital and film mammography, such as the Digital Mammographic Imaging Screening Trial (DMIST),19 have found digital mammography to be especially helpful in women with extremely dense breasts, who have an elevated risk for breast cancer. For women with fatty breasts the differences between the types of mammogram are less significant.
For further detail on digital mammography, readers are referred to the recent review by D’Orsi and Newell.20
SCREENING THE SURGICALLY ALTERED BREAST
Following surgical cancer treatment or reconstructive surgery, screening of remaining breast tissue for cancer is still performed and is just as essential to patient care as presurgery screening. The first line of defense for any patient with a surgically altered breast is mammography.
When a patient has had breast reconstruction following mastectomy, it is presumed that very little breast tissue remains. There is no standard of care for screening the nonbreast tissue introduced by the reconstructive procedure. Nonetheless, at our institution we perform a single mediolateral oblique projection on any flap-reconstructed breast in light of rare anecdotal accounts of cancer found in and around the reconstructed breast. When problem-solving is needed to evaluate a new palpable abnormality, special angled views (tangential) and directed ultrasonography can be used. We do not routinely perform screening mammography on mastectomy patients who have had reconstruction with implants, but we can investigate areas of clinical concern (eg, due to palpable masses) with directed ultrasonography.21
The cosmetically altered breast presents its own issues in cancer detection. Both silicone-gel and saline implants obscure breast tissue that could contain cancer. For this reason, special implant-displaced views are performed that allow visualization of a larger portion of breast tissue beyond that allowed by standard mammograms. Therefore, an asymptomatic patient with implants who presents for screening mammography will have eight mammography views obtained instead of the routine four views.22
Patients who have had breast reduction, excisional biopsy, or prior breast conservation surgery (lumpectomy and radiation) are screened in a routine manner with mammography.23 Patients who have had prior surgical procedures often have architectural distortion at the surgical site, which is generally stable over time. Any prior surgical procedure can predispose the patient to the development of fat necrosis, which is a benign entity but can mimic cancer in its early phases through the development of calcifications and, occasionally, a new palpable lump. We most commonly confront this issue in the period 2 to 4 years after the operation.24 Occasionally the findings are such that a biopsy is needed to determine whether fat necrosis is the cause. In this population, magnetic resonance imaging (MRI) can also be used as an adjunctive tool, and can sometimes clarify the presence of fat necrosis and other postoperative findings, such as seroma, hematoma, or inflammation. In other instances, only a biopsy can determine what a particular finding represents.
DIAGNOSTIC MAMMOGRAPHY
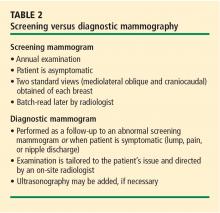
Any mammography performed for a problem-solving purpose is considered diagnostic mammography (Table 2); the exam is tailored to the patient’s individual issue.25 Diagnostic mammography requires the presence of a qualified radiologist at the time of imaging. The goal is to come to a final conclusion about the mammographic or clinical finding at the time of the patient’s visit. Special views are usually performed that include, but are not limited to, spot-compression or spot-magnification views, depending on the finding.26 The patient is then given a same-day written account of the results at the conclusion of the study.
Examples of problems that may prompt diagnostic mammography include patient-reported palpable findings, screening mammography findings that are recalled for further investigation, or physician-detected findings. Often, ultrasonography is also used at the same visit and its results are integrated with the mammography findings to arrive at the final impression.
BREAST ULTRASONOGRAPHY AND BREAST MRI
Ultrasonography and MRI are two very useful adjunctive tools for breast lesion detection and analysis. At this time, however, neither is a replacement for screening mammography as a primary screening modality; rather, each is used in a complementary fashion for lesion analysis and biopsy guidance.10,27
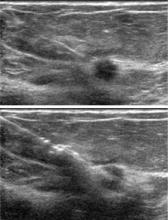
Ultrasonography: Best for further study of areas of interest
Ultrasonography uses high-frequency sound waves to create a picture using a probe directed to an area of interest in the breast. The optimal probe for breast imaging is one typically operating in a frequency of 12 to 18 MHz and 4 cm in scanning width.
Because ultrasonography provides views of only a small area of breast tissue at a time, it is operator and patient dependent. It is best used when a known area of interest needs further evaluation, such as when a patient reports a palpable abnormality or when a mass is detected on mammography.
Ultrasonography uses no ionizing radiation, so it is especially helpful in young or pregnant women who present with a palpable abnormality. It is also useful for patients who have recently undergone a surgical procedure. As ultrasonography is currently used, no compression is needed and it can be performed easily in patients with limited mobility. Needle biopsies are most easily performed using ultrasonographic guidance.
MRI: An emerging adjunct under study in high-risk patients
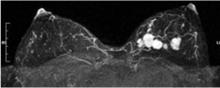
Breast MRI is an emerging modality under active research that shows promise for adjunctive breast imaging. It is commonly being used as a tool for local staging in women with newly diagnosed breast cancer.28,29 Current research is focused on its suitability as a screening modality, in conjunction with mammography, in high-risk populations based on family history and other factors addressed in the Gail model6 and similar risk models.
The limitations of breast MRI include its high cost, unsuitability for some patients (eg, the obese [due to table weight constraints], patients with pacemakers, patients with renal failure), the potential for unnecessary biopsies due to decreased specificity, lack of portability, and the length of time required for imaging.
When a lesion is initially detected with MRI, an attempt is usually made to identify it with ultrasonography as well, owing to the ease of ultrasonography-guided biopsy.32 It is important, however, for an imaging center that performs breast MRI to be able to perform biopsies using MRI guidance since not all lesions are identifiable by other modalities.33 Breast MRI studies are not easily portable between imaging facilities since a typical study contains a thousand or more images that are best viewed on a site-specific workstation monitor.
HISTOLOGIC CONFIRMATION
Once an abnormality is detected on imaging, a confirmatory histologic diagnosis is needed before embarking on medical or surgical treatments. Image-guided biopsy plays a critical role in this regard. In our breast imaging section, we perform ultrasonography-guided core needle biopsy and aspiration, stereotactic needle biopsy, and MRI-guided needle biopsy, as well as wire localizations on the day of surgery. All procedures performed are considered minimally invasive and are suitable for a vast majority of patients for whom they are recommended.34
Ultrasonography-guided procedures
Ultrasonography-guided fine-needle aspiration is an additional option for patients when core biopsy cannot be performed because the lesion is located adjacent to sensitive structures, such as implants or the pectoralis muscle. Fine-needle aspiration is also used to evaluate complicated breast cysts and, occasionally, lymph nodes. Drawbacks of fine-needle aspiration (relative to larger core needle biopsy) are that it is limited to cytologic, not histologic, examination and that it yields a higher false-negative rate.
Stereotactically guided procedures
Stereotactic core biopsy is performed when lesions—usually calcifications, but sometimes masses—are visible only on mammography.36,37 “Stereotactic” refers to the means by which the target is localized, ie, with a “stereo pair” of digital mammogram pictures with a small field of view. The patient is placed in a prone position with the breast of interest placed through a hole at the undersurface of the table in a light compression. The biopsy unit is attached to a dedicated computer that calculates coordinates. The needle is then brought to the coordinate position for sampling to take place.
The biopsy needle used for this procedure is vacuum-assisted, which means the needle is placed only one time, and samples in the vicinity of the target are vacuumed into a reservoir for retrieval. If the target is calcifications, a specimen radiograph is routinely performed to verify adequate sample acquisition before the patient leaves the biopsy table.38 When the original target is no longer visible, a titanium marker clip is often placed. This facilitates localization of the biopsied area should surgery be needed.
Stereotactic biopsy has several limitations that ultrasonography-guided biopsy does not. The patient must be cooperative and mobile enough to get on the table and hold a prone position for the duration of the procedure (about 45 minutes). If the patient is taking warfarin or has a bleeding diathesis, preprocedure steps such as clinical evaluation to check the international normalized ratio and prothrombin time, or even stopping the warfarin temporarily, may be needed to minimize bleeding during the procedure, as a 9- or 12-gauge needle is used. Stereotactic biopsy is also limited by lesion position. A far posterior lesion may not be accessible if it does not reach through the hole in the table. Also, there is a limit to the compressed thinness of breast tissue that can be biopsied. Finally, most tables used for stereotactic biopsy have a functioning weight limit of 300 pounds.
Open surgical biopsy
A final option is open surgical biopsy, which is used when the more minimally invasive techniques are equivocal, discordant, or impossible due to the limitations noted above, or when atypical cells are found.
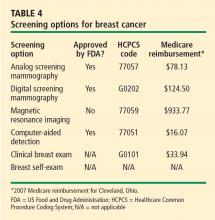
HOW SHOULD WE SCREEN OUR PATIENTS?
The various screening options for breast cancer are listed in Table 4, along with their market approval status and Medicare reimbursement levels.
For women at average risk for breast cancer, the American Cancer Society recommends an annual mammogram and clinical breast examination by a physician beginning at age 40 (Table 1).10
In conclusion, the process of finding breast cancer includes regular screening with mammography and clinical breast examination (plus MRI in high-risk women) and the diagnostic modalities of ultrasonography, MRI, and diagnostic mammography. Our ultimate goal is to find cancer at the earliest time possible by all means necessary for the individual patient.
- American Cancer Society. Breast Cancer Facts & Figures 2007-2008. Atlanta, GA: American Cancer Society, Inc. http://www.cancer.org/ downloads/STT/BCFF-Final.pdf. Accessed January 14, 2008.
- Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2003. Bethesda, MD: National Cancer Institute; 2006.
- National Cancer Institute fact sheet: probability of breast cancer in American women. National Cancer Institute Web site. http://www.cancer.gov/cancertopics/factsheet/Detection/probability-breast-cancer. Accessed January 18, 2008.
- Thull DL, Vogel VG. Recognition and management of hereditary breast cancer syndromes. Oncologist 2004; 9:13–24.
- National Comprehensive Cancer Network. NCCN Clinical practice guidelines in oncology: genetic/familial high-risk assessment: breast and ovarian. Available at: http://www.nccn.org/professionals/physician_gls/PDF/genetics_screening.pdf. Accessed January 28, 2008.
- Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989; 81:1879–1886.
- Breast cancer risk assessment tool. An interactive tool for measuring the risk of invasive breast cancer. National Cancer Institute Web site. http://www.cancer.gov/bcrisktool/. Accessed January 21, 2008.
- Vetto J, Pommier R, Schmidt W, et al. Use of the “triple test” for palpable breast lesions yields high diagnostic accuracy and cost savings. Am J Surg 1995; 169:519–522.
- Vetto JT, Pommier RF, Schmidt WA, Eppich H, Alexander PW. Diagnosis of palpable breast lesions in younger women by the modified triple test is accurate and cost-effective. Arch Surg 1996; 131:967–974.
- Smith RA, Saslow D, Sawyer KA, et al. American Cancer Society guidelines for breast cancer screening: update 2003. CA Cancer J Clin 2003; 53:141–169.
- U.S. Preventive Services Task Force. Screening for breast cancer: recommendations and rationale. Ann Intern Med 2002; 137:344–346.
- Nyström L, Andersson I, Bjurstam N, et al. Long-term effects of mammography screening: updated overview of the Swedish randomized trials. Lancet 2002; 359:909–919.
- Tabar L, Fagerberg G, Chen HH, et al. Efficacy of breast cancer screening by age: new results from the Swedish Two-County Trial. Cancer 1995; 75:2507–2517.
- Shapiro S, Venet W, Strax P, Venet L. Periodic Screening for Breast Cancer: The Health Insurance Plan Project and Its Sequelae, 1963-1986. Baltimore, MD: Johns Hopkins University Press; 1988.
- Miller AB, To T, Baines CJ, Wall C. Canadian National Breast Screening Study-2: 13-year results of a randomized trial in women aged 50-59 years. J Natl Cancer Inst 2000; 92:1490–1499.
- Miller AB, To T, Baines CJ, Wall C. The Canadian National Breast Screening Study-1: breast cancer mortality after 11 to 16 years of follow-up: a randomized screening trial of mammography in women age 40 to 49 years. Ann Intern Med 2002; 137:305–312.
- Alexander FE, Anderson TJ, Brown HK, et al. 14 years of follow-up from the Edinburgh randomized trial of breast-cancer screening. Lancet 1999; 353:1903–1908.
- Eklund GW, Cardenosa G. The art of mammographic positioning. Radiol Clin North Am 1992; 30:21–53.
- Pisano E, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast cancer screening. N Engl J Med 2005; 353:1773–1783.
- D’Orsi CJ, Newell MS. Digital mammography: clinical implementation and clinical trials. Semin Roentgenol 2007; 42:236–242.
- Fajardo LL, Roberts CC, Hunt KR. Mammographic surveillance of breast cancer patients: should the masectomy site be imaged? AJR Am J Roentgenol 1993; 161:953–955.
- Eklund GW, Busby RC, Miller SH, Job JS. Improved imaging of the augmented breast. AJR Am J Roentgenol 1988; 151:469–473.
- Mendelson EB. Evaluation of the postoperative breast. Radiol Clin North Am 1992; 30:107–138.
- Philpotts LE, Lee CH, Haffty BG, et al. Mammographic findings of recurrent breast cancer after l
- ACR practice guideline for the performance of diagnostic mammography. American College of Radiology Web site. http:// www.acr.org/SecondaryMainMenuCategories/quality_safety/guidelines/breast/diagnostic_mammography.aspx. Accessed January 14, 2008.
- Sickles EA. Practical solutions to common mammographic problems: tailoring the examination. AJR Am J Roentgenol 1988; 151:31–39.
- Jackson VP. The role of US in breast imaging. Radiology 1990; 177:305–311.
- Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med 2007; 356:1295–1303.
- Liberman L. Breast MR imaging in assessing extent of disease. Magn Reson Imaging Clin N Am 2006; 14:339–349.
- Kuhl C. The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology 2007; 244:356–378.
- Flickinger FW, Allison JD, Sherry RM, Wright JC. Differentiation of benign from malignant breast masses by time-intensity evaluation of contrast-enhanced MRI. Magn Reson Imaging 1993; 11:617–620.
- Chellman-Jeffers MR, Listinsky J, Dinunzio A, Lieber M, Rim A. Utility of second look ultrasound as an adjunct to contrast-enhanced MRI of the breast. Paper presented at: American Roentgen Ray Society Meeting; May 4, 2006; Vancouver, BC. Abstract 269.
- Orel SG, Schnall MD, Newman RW, Powell CM, Torosian MH, Rosato EF. MR imaging-guided localization and biopsy of breast lesions: initial experience. Radiology 1994; 193:97–102.
- Liberman L. Percutaneous imaging-guided core breast biopsy: state of the art at the millennium. AJR Am J Roentgenol 2000; 174:1191–1199.
- Fornage BD, Coan JD, David CL. Ultrasound-guided needle biopsy of the breast and other interventional procedures. Radiol Clin North Am 1992; 30:167–185.
- Parker SH, Lovin JD, Jobe WE, et al. Nonpalpable breast lesions: stereotactic automated large-core biopsies. Radiology 1991; 180:403–407.
- Parker SH, Burbank F, Jackman RJ, et al. Percutaneous large-core breast biopsy: a multi-institutional study. Radiology 1994; 193:3 59–364.
- Liberman L, Evans WP III, Dershaw DD, et al. Radiography of microcalcifications in stereotaxic mammary core biopsy specimens. Radiology 1994; 190:223–225.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007; 57:75–89.
Early detection of breast cancer is vital to reducing the morbidity and mortality associated with this disease. After a brief overview of breast cancer epidemiology and risk assessment, this article describes screening and diagnostic imaging techniques as they are currently practiced to promote early breast cancer detection. We conclude with a review of image-guided needle biopsy techniques and a recommended approach to breast cancer screening in the general population.
EPIDEMIOLOGY OF BREAST CANCER: DAUNTING BUT SLOWLY IMPROVING
After nonmelanoma skin cancers, breast cancer is the most common form of cancer in women today, accounting for more than 1 in 4 cancers diagnosed in US women.1 If the current incidence of breast cancer remains constant, US females born today have an average risk of 12.7% of being diagnosed with breast cancer during their lifetime (ie, 1-in-8 lifetime risk), based on National Cancer Institute statistics.2,3 The American Cancer Society estimated that 178,480 new cases of invasive breast cancer and 62,030 new cases of in situ breast cancer would be diagnosed in the United States in 2007, and that 40,460 US women would die from breast cancer that year.1 Only lung cancer accounts for more cancer deaths in women.
The role of race and ethnicity
Breast cancer risk varies by race and ethnicity in the United States. After age 40 years, white women have a higher incidence of breast cancer compared with African American women; conversely, before age 40, African American women have a higher incidence compared with white women. African American women are more likely than their white counterparts to die from their breast cancer at any age. Incidence and death rates from breast cancer are lower among Asian American, American Indian, and Hispanic women compared with both white and African American women.1
Recent hopeful trends
Despite the daunting incidence numbers reviewed above, recent years have seen encouraging trends in US breast cancer epidemiology.
For invasive breast cancer, the growth in incidence rates slowed during the 1990s, and rates actually declined by 3.5% per year during the period 2001–2004.1 These changes are likely attributable to multiple factors, including variations in rates of mammography screening and decreased use of hormone replacement therapy after the 2002 publication of results from the Women’s Health Initiative trial. Still, the trend is encouraging.
Incidence rates of in situ breast cancer rose rapidly during the 1980s and 1990s, largely due to increased diagnosis by mammography, but have plateaued since 2000 among women aged 50 years or older while continuing to rise modestly in younger women.1
Meanwhile, the overall death rate from breast cancer in women declined by 2.2% annually from 1990 to 2004.1
RISK FACTORS AND RISK MODELING
Risk factors for breast cancer have been well described and include the following:
- Age ( ≥ 65 years vs < 65 years, although risk increases across all ages up to 80 years)
- Family history of breast cancer
- Late age at first full-term pregnancy (> 30 years)
- Never having a full-term pregnancy
- Early menarche and/or late menopause
- Certain genetic mutations for breast cancer (eg, in the BRCA1, BRCA2, ATM, and CHEK2 genes)
- Certain breast disorders, such as atypical hyperplasia or lobular carcinoma in situ
- High breast tissue density
- High bone density (postmenopausal)
- High-dose radiation to the chest.
The above risk factors are, in general, fixed. More elusive risk factors, in that they are variable and modifiable, include obesity, use of exogenous hormones (recent and long-term hormone replacement therapy; recent oral contraceptive use), alcohol use, tobacco use, diet, and a low level of physical activity. Breast implants are not a risk factor for breast cancer, though their presence does obscure breast tissue on imaging, limiting the detectability of a tumor when it does develop (see “Screening the Surgically Altered Breast” below).
Women with a genetic predisposition to breast cancer merit special consideration. Hereditary breast cancers account for about 5% to 10% of breast cancer cases, and the BRCA1 and BRCA2 mutations are responsible for 80% to 90% of these cases, while other gene mutations (noted above) or genetic syndromes account for the rest. Clinical options for managing women with a genetic predisposition include surveillance, chemoprevention, and prophylactic surgery.4 Detailed discussion of the management of these women is beyond the scope of this article, but readers are referred to www.nccn.org/professionals/physician_gls/PDF/ genetics_screening.pdf for practice guidelines from the National Comprehensive Cancer Network.5
Tools for risk assessment
Several tools are available to predict a woman’s risk of developing breast cancer. Probably the most widely used is the Gail model,6 which was published in 1989 and forms the statistical basis for the National Cancer Institute’s Breast Cancer Risk Assessment Tool, which is available for downloading at www.cancer.gov/bcrisktool.7 The model uses a woman’s personal medical and reproductive histories and her family history of breast cancer to predict her 5-year and lifetime risk of developing invasive breast cancer. Factors included in the risk calculation are age, race, number of first-degree relatives with a history of breast cancer, age at first live birth (or nulliparity), age at menarche, number of breast biopsies, and presence or absence of a history of atypical hyperplasia. The relative risk for each of these factors is multiplied to generate a composite risk. The Gail model has been validated for white women but has been shown to underestimate breast cancer risk in African American women; it remains to be validated for Hispanic women, Asian women, and other subgroups of women.7
The commonly taught “triple test” for palpable breast lesions is another risk model that incorporates clinical findings. It consists of a physical examination, mammography, and fine-needle aspiration8 (in the “modified triple test,” ultrasonography replaces mammography9). When all three elements of the test are concordant (either all benign or all malignant), the triple test has been reported to have 100% diagnostic accuracy.8,9
A WORD ABOUT BREAST EXAMINATION
Breast self-examination
Clinical breast examination
As noted in Table 1, regular clinical breast examinations are recommended by the American Cancer Society for asymptomatic women at average risk for breast cancer, with the recommended frequency depending on the woman’s age.10 The US Preventive Services Task Force takes the stance that there is insufficient evidence to recommend for or against breast cancer screening with clinical breast examination alone.11 While it is unclear precisely what contribution clinical breast exams make to the detection of breast cancer, they certainly provide clinicians an opportunity to raise awareness about breast cancer and educate patients about breast symptoms, risk factors, and new detection technologies.10
SCREENING MAMMOGRAPHY
Screening mammography is the single most effective method of early breast cancer detection,1 and the American Cancer Society recommends that women at average risk for breast cancer have annual screening mammograms beginning at age 40 years (Table 1).10
The evidence base
The primary evidence supporting the recommendation for screening mammography comes from eight randomized trials that studied the effectiveness of screening mammography for cancer detection in Sweden,12,13 the United States,14 Canada,15,16 and the United Kingdom.17 Overall, breast cancers detected by screening mammography are smaller and have a more favorable history and tumor biology than those detected clinically without the use of imaging. A pooled analysis of the most recent data from all randomized trials of screening mammography in women aged 39 to 74 years showed a 24% reduction in mortality (95% CI, 18% to 30%) in women undergoing screening mammography, although not all individual trials showed a statistically significant mortality reduction.10
The screening procedure at a glance
Analog vs digital
Digital mammograms are radiographs that are acquired digitally and allow digital enhancement to aid in interpretation. When receiving a digital mammogram, the woman being screened still undergoes compression and positioning as for a conventional film-screen mammogram, and the images are still produced with x-rays. However, digitization allows manipulation of the images as they are being interpreted, enabling the radiologist to focus on areas of interest or to “window” and “level” the image, similar to adjusting the tint and contrast on a television set.
Research trials comparing digital and film mammography, such as the Digital Mammographic Imaging Screening Trial (DMIST),19 have found digital mammography to be especially helpful in women with extremely dense breasts, who have an elevated risk for breast cancer. For women with fatty breasts the differences between the types of mammogram are less significant.
For further detail on digital mammography, readers are referred to the recent review by D’Orsi and Newell.20
SCREENING THE SURGICALLY ALTERED BREAST
Following surgical cancer treatment or reconstructive surgery, screening of remaining breast tissue for cancer is still performed and is just as essential to patient care as presurgery screening. The first line of defense for any patient with a surgically altered breast is mammography.
When a patient has had breast reconstruction following mastectomy, it is presumed that very little breast tissue remains. There is no standard of care for screening the nonbreast tissue introduced by the reconstructive procedure. Nonetheless, at our institution we perform a single mediolateral oblique projection on any flap-reconstructed breast in light of rare anecdotal accounts of cancer found in and around the reconstructed breast. When problem-solving is needed to evaluate a new palpable abnormality, special angled views (tangential) and directed ultrasonography can be used. We do not routinely perform screening mammography on mastectomy patients who have had reconstruction with implants, but we can investigate areas of clinical concern (eg, due to palpable masses) with directed ultrasonography.21
The cosmetically altered breast presents its own issues in cancer detection. Both silicone-gel and saline implants obscure breast tissue that could contain cancer. For this reason, special implant-displaced views are performed that allow visualization of a larger portion of breast tissue beyond that allowed by standard mammograms. Therefore, an asymptomatic patient with implants who presents for screening mammography will have eight mammography views obtained instead of the routine four views.22
Patients who have had breast reduction, excisional biopsy, or prior breast conservation surgery (lumpectomy and radiation) are screened in a routine manner with mammography.23 Patients who have had prior surgical procedures often have architectural distortion at the surgical site, which is generally stable over time. Any prior surgical procedure can predispose the patient to the development of fat necrosis, which is a benign entity but can mimic cancer in its early phases through the development of calcifications and, occasionally, a new palpable lump. We most commonly confront this issue in the period 2 to 4 years after the operation.24 Occasionally the findings are such that a biopsy is needed to determine whether fat necrosis is the cause. In this population, magnetic resonance imaging (MRI) can also be used as an adjunctive tool, and can sometimes clarify the presence of fat necrosis and other postoperative findings, such as seroma, hematoma, or inflammation. In other instances, only a biopsy can determine what a particular finding represents.
DIAGNOSTIC MAMMOGRAPHY
Any mammography performed for a problem-solving purpose is considered diagnostic mammography (Table 2); the exam is tailored to the patient’s individual issue.25 Diagnostic mammography requires the presence of a qualified radiologist at the time of imaging. The goal is to come to a final conclusion about the mammographic or clinical finding at the time of the patient’s visit. Special views are usually performed that include, but are not limited to, spot-compression or spot-magnification views, depending on the finding.26 The patient is then given a same-day written account of the results at the conclusion of the study.
Examples of problems that may prompt diagnostic mammography include patient-reported palpable findings, screening mammography findings that are recalled for further investigation, or physician-detected findings. Often, ultrasonography is also used at the same visit and its results are integrated with the mammography findings to arrive at the final impression.
BREAST ULTRASONOGRAPHY AND BREAST MRI
Ultrasonography and MRI are two very useful adjunctive tools for breast lesion detection and analysis. At this time, however, neither is a replacement for screening mammography as a primary screening modality; rather, each is used in a complementary fashion for lesion analysis and biopsy guidance.10,27
Ultrasonography: Best for further study of areas of interest
Ultrasonography uses high-frequency sound waves to create a picture using a probe directed to an area of interest in the breast. The optimal probe for breast imaging is one typically operating in a frequency of 12 to 18 MHz and 4 cm in scanning width.
Because ultrasonography provides views of only a small area of breast tissue at a time, it is operator and patient dependent. It is best used when a known area of interest needs further evaluation, such as when a patient reports a palpable abnormality or when a mass is detected on mammography.
Ultrasonography uses no ionizing radiation, so it is especially helpful in young or pregnant women who present with a palpable abnormality. It is also useful for patients who have recently undergone a surgical procedure. As ultrasonography is currently used, no compression is needed and it can be performed easily in patients with limited mobility. Needle biopsies are most easily performed using ultrasonographic guidance.
MRI: An emerging adjunct under study in high-risk patients
Breast MRI is an emerging modality under active research that shows promise for adjunctive breast imaging. It is commonly being used as a tool for local staging in women with newly diagnosed breast cancer.28,29 Current research is focused on its suitability as a screening modality, in conjunction with mammography, in high-risk populations based on family history and other factors addressed in the Gail model6 and similar risk models.
The limitations of breast MRI include its high cost, unsuitability for some patients (eg, the obese [due to table weight constraints], patients with pacemakers, patients with renal failure), the potential for unnecessary biopsies due to decreased specificity, lack of portability, and the length of time required for imaging.
When a lesion is initially detected with MRI, an attempt is usually made to identify it with ultrasonography as well, owing to the ease of ultrasonography-guided biopsy.32 It is important, however, for an imaging center that performs breast MRI to be able to perform biopsies using MRI guidance since not all lesions are identifiable by other modalities.33 Breast MRI studies are not easily portable between imaging facilities since a typical study contains a thousand or more images that are best viewed on a site-specific workstation monitor.
HISTOLOGIC CONFIRMATION
Once an abnormality is detected on imaging, a confirmatory histologic diagnosis is needed before embarking on medical or surgical treatments. Image-guided biopsy plays a critical role in this regard. In our breast imaging section, we perform ultrasonography-guided core needle biopsy and aspiration, stereotactic needle biopsy, and MRI-guided needle biopsy, as well as wire localizations on the day of surgery. All procedures performed are considered minimally invasive and are suitable for a vast majority of patients for whom they are recommended.34
Ultrasonography-guided procedures
Ultrasonography-guided fine-needle aspiration is an additional option for patients when core biopsy cannot be performed because the lesion is located adjacent to sensitive structures, such as implants or the pectoralis muscle. Fine-needle aspiration is also used to evaluate complicated breast cysts and, occasionally, lymph nodes. Drawbacks of fine-needle aspiration (relative to larger core needle biopsy) are that it is limited to cytologic, not histologic, examination and that it yields a higher false-negative rate.
Stereotactically guided procedures
Stereotactic core biopsy is performed when lesions—usually calcifications, but sometimes masses—are visible only on mammography.36,37 “Stereotactic” refers to the means by which the target is localized, ie, with a “stereo pair” of digital mammogram pictures with a small field of view. The patient is placed in a prone position with the breast of interest placed through a hole at the undersurface of the table in a light compression. The biopsy unit is attached to a dedicated computer that calculates coordinates. The needle is then brought to the coordinate position for sampling to take place.
The biopsy needle used for this procedure is vacuum-assisted, which means the needle is placed only one time, and samples in the vicinity of the target are vacuumed into a reservoir for retrieval. If the target is calcifications, a specimen radiograph is routinely performed to verify adequate sample acquisition before the patient leaves the biopsy table.38 When the original target is no longer visible, a titanium marker clip is often placed. This facilitates localization of the biopsied area should surgery be needed.
Stereotactic biopsy has several limitations that ultrasonography-guided biopsy does not. The patient must be cooperative and mobile enough to get on the table and hold a prone position for the duration of the procedure (about 45 minutes). If the patient is taking warfarin or has a bleeding diathesis, preprocedure steps such as clinical evaluation to check the international normalized ratio and prothrombin time, or even stopping the warfarin temporarily, may be needed to minimize bleeding during the procedure, as a 9- or 12-gauge needle is used. Stereotactic biopsy is also limited by lesion position. A far posterior lesion may not be accessible if it does not reach through the hole in the table. Also, there is a limit to the compressed thinness of breast tissue that can be biopsied. Finally, most tables used for stereotactic biopsy have a functioning weight limit of 300 pounds.
Open surgical biopsy
A final option is open surgical biopsy, which is used when the more minimally invasive techniques are equivocal, discordant, or impossible due to the limitations noted above, or when atypical cells are found.
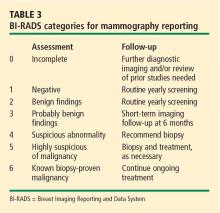
HOW SHOULD WE SCREEN OUR PATIENTS?
The various screening options for breast cancer are listed in Table 4, along with their market approval status and Medicare reimbursement levels.
For women at average risk for breast cancer, the American Cancer Society recommends an annual mammogram and clinical breast examination by a physician beginning at age 40 (Table 1).10
In conclusion, the process of finding breast cancer includes regular screening with mammography and clinical breast examination (plus MRI in high-risk women) and the diagnostic modalities of ultrasonography, MRI, and diagnostic mammography. Our ultimate goal is to find cancer at the earliest time possible by all means necessary for the individual patient.
Early detection of breast cancer is vital to reducing the morbidity and mortality associated with this disease. After a brief overview of breast cancer epidemiology and risk assessment, this article describes screening and diagnostic imaging techniques as they are currently practiced to promote early breast cancer detection. We conclude with a review of image-guided needle biopsy techniques and a recommended approach to breast cancer screening in the general population.
EPIDEMIOLOGY OF BREAST CANCER: DAUNTING BUT SLOWLY IMPROVING
After nonmelanoma skin cancers, breast cancer is the most common form of cancer in women today, accounting for more than 1 in 4 cancers diagnosed in US women.1 If the current incidence of breast cancer remains constant, US females born today have an average risk of 12.7% of being diagnosed with breast cancer during their lifetime (ie, 1-in-8 lifetime risk), based on National Cancer Institute statistics.2,3 The American Cancer Society estimated that 178,480 new cases of invasive breast cancer and 62,030 new cases of in situ breast cancer would be diagnosed in the United States in 2007, and that 40,460 US women would die from breast cancer that year.1 Only lung cancer accounts for more cancer deaths in women.
The role of race and ethnicity
Breast cancer risk varies by race and ethnicity in the United States. After age 40 years, white women have a higher incidence of breast cancer compared with African American women; conversely, before age 40, African American women have a higher incidence compared with white women. African American women are more likely than their white counterparts to die from their breast cancer at any age. Incidence and death rates from breast cancer are lower among Asian American, American Indian, and Hispanic women compared with both white and African American women.1
Recent hopeful trends
Despite the daunting incidence numbers reviewed above, recent years have seen encouraging trends in US breast cancer epidemiology.
For invasive breast cancer, the growth in incidence rates slowed during the 1990s, and rates actually declined by 3.5% per year during the period 2001–2004.1 These changes are likely attributable to multiple factors, including variations in rates of mammography screening and decreased use of hormone replacement therapy after the 2002 publication of results from the Women’s Health Initiative trial. Still, the trend is encouraging.
Incidence rates of in situ breast cancer rose rapidly during the 1980s and 1990s, largely due to increased diagnosis by mammography, but have plateaued since 2000 among women aged 50 years or older while continuing to rise modestly in younger women.1
Meanwhile, the overall death rate from breast cancer in women declined by 2.2% annually from 1990 to 2004.1
RISK FACTORS AND RISK MODELING
Risk factors for breast cancer have been well described and include the following:
- Age ( ≥ 65 years vs < 65 years, although risk increases across all ages up to 80 years)
- Family history of breast cancer
- Late age at first full-term pregnancy (> 30 years)
- Never having a full-term pregnancy
- Early menarche and/or late menopause
- Certain genetic mutations for breast cancer (eg, in the BRCA1, BRCA2, ATM, and CHEK2 genes)
- Certain breast disorders, such as atypical hyperplasia or lobular carcinoma in situ
- High breast tissue density
- High bone density (postmenopausal)
- High-dose radiation to the chest.
The above risk factors are, in general, fixed. More elusive risk factors, in that they are variable and modifiable, include obesity, use of exogenous hormones (recent and long-term hormone replacement therapy; recent oral contraceptive use), alcohol use, tobacco use, diet, and a low level of physical activity. Breast implants are not a risk factor for breast cancer, though their presence does obscure breast tissue on imaging, limiting the detectability of a tumor when it does develop (see “Screening the Surgically Altered Breast” below).
Women with a genetic predisposition to breast cancer merit special consideration. Hereditary breast cancers account for about 5% to 10% of breast cancer cases, and the BRCA1 and BRCA2 mutations are responsible for 80% to 90% of these cases, while other gene mutations (noted above) or genetic syndromes account for the rest. Clinical options for managing women with a genetic predisposition include surveillance, chemoprevention, and prophylactic surgery.4 Detailed discussion of the management of these women is beyond the scope of this article, but readers are referred to www.nccn.org/professionals/physician_gls/PDF/ genetics_screening.pdf for practice guidelines from the National Comprehensive Cancer Network.5
Tools for risk assessment
Several tools are available to predict a woman’s risk of developing breast cancer. Probably the most widely used is the Gail model,6 which was published in 1989 and forms the statistical basis for the National Cancer Institute’s Breast Cancer Risk Assessment Tool, which is available for downloading at www.cancer.gov/bcrisktool.7 The model uses a woman’s personal medical and reproductive histories and her family history of breast cancer to predict her 5-year and lifetime risk of developing invasive breast cancer. Factors included in the risk calculation are age, race, number of first-degree relatives with a history of breast cancer, age at first live birth (or nulliparity), age at menarche, number of breast biopsies, and presence or absence of a history of atypical hyperplasia. The relative risk for each of these factors is multiplied to generate a composite risk. The Gail model has been validated for white women but has been shown to underestimate breast cancer risk in African American women; it remains to be validated for Hispanic women, Asian women, and other subgroups of women.7
The commonly taught “triple test” for palpable breast lesions is another risk model that incorporates clinical findings. It consists of a physical examination, mammography, and fine-needle aspiration8 (in the “modified triple test,” ultrasonography replaces mammography9). When all three elements of the test are concordant (either all benign or all malignant), the triple test has been reported to have 100% diagnostic accuracy.8,9
A WORD ABOUT BREAST EXAMINATION
Breast self-examination
Clinical breast examination
As noted in Table 1, regular clinical breast examinations are recommended by the American Cancer Society for asymptomatic women at average risk for breast cancer, with the recommended frequency depending on the woman’s age.10 The US Preventive Services Task Force takes the stance that there is insufficient evidence to recommend for or against breast cancer screening with clinical breast examination alone.11 While it is unclear precisely what contribution clinical breast exams make to the detection of breast cancer, they certainly provide clinicians an opportunity to raise awareness about breast cancer and educate patients about breast symptoms, risk factors, and new detection technologies.10
SCREENING MAMMOGRAPHY
Screening mammography is the single most effective method of early breast cancer detection,1 and the American Cancer Society recommends that women at average risk for breast cancer have annual screening mammograms beginning at age 40 years (Table 1).10
The evidence base
The primary evidence supporting the recommendation for screening mammography comes from eight randomized trials that studied the effectiveness of screening mammography for cancer detection in Sweden,12,13 the United States,14 Canada,15,16 and the United Kingdom.17 Overall, breast cancers detected by screening mammography are smaller and have a more favorable history and tumor biology than those detected clinically without the use of imaging. A pooled analysis of the most recent data from all randomized trials of screening mammography in women aged 39 to 74 years showed a 24% reduction in mortality (95% CI, 18% to 30%) in women undergoing screening mammography, although not all individual trials showed a statistically significant mortality reduction.10
The screening procedure at a glance
Analog vs digital
Digital mammograms are radiographs that are acquired digitally and allow digital enhancement to aid in interpretation. When receiving a digital mammogram, the woman being screened still undergoes compression and positioning as for a conventional film-screen mammogram, and the images are still produced with x-rays. However, digitization allows manipulation of the images as they are being interpreted, enabling the radiologist to focus on areas of interest or to “window” and “level” the image, similar to adjusting the tint and contrast on a television set.
Research trials comparing digital and film mammography, such as the Digital Mammographic Imaging Screening Trial (DMIST),19 have found digital mammography to be especially helpful in women with extremely dense breasts, who have an elevated risk for breast cancer. For women with fatty breasts the differences between the types of mammogram are less significant.
For further detail on digital mammography, readers are referred to the recent review by D’Orsi and Newell.20
SCREENING THE SURGICALLY ALTERED BREAST
Following surgical cancer treatment or reconstructive surgery, screening of remaining breast tissue for cancer is still performed and is just as essential to patient care as presurgery screening. The first line of defense for any patient with a surgically altered breast is mammography.
When a patient has had breast reconstruction following mastectomy, it is presumed that very little breast tissue remains. There is no standard of care for screening the nonbreast tissue introduced by the reconstructive procedure. Nonetheless, at our institution we perform a single mediolateral oblique projection on any flap-reconstructed breast in light of rare anecdotal accounts of cancer found in and around the reconstructed breast. When problem-solving is needed to evaluate a new palpable abnormality, special angled views (tangential) and directed ultrasonography can be used. We do not routinely perform screening mammography on mastectomy patients who have had reconstruction with implants, but we can investigate areas of clinical concern (eg, due to palpable masses) with directed ultrasonography.21
The cosmetically altered breast presents its own issues in cancer detection. Both silicone-gel and saline implants obscure breast tissue that could contain cancer. For this reason, special implant-displaced views are performed that allow visualization of a larger portion of breast tissue beyond that allowed by standard mammograms. Therefore, an asymptomatic patient with implants who presents for screening mammography will have eight mammography views obtained instead of the routine four views.22
Patients who have had breast reduction, excisional biopsy, or prior breast conservation surgery (lumpectomy and radiation) are screened in a routine manner with mammography.23 Patients who have had prior surgical procedures often have architectural distortion at the surgical site, which is generally stable over time. Any prior surgical procedure can predispose the patient to the development of fat necrosis, which is a benign entity but can mimic cancer in its early phases through the development of calcifications and, occasionally, a new palpable lump. We most commonly confront this issue in the period 2 to 4 years after the operation.24 Occasionally the findings are such that a biopsy is needed to determine whether fat necrosis is the cause. In this population, magnetic resonance imaging (MRI) can also be used as an adjunctive tool, and can sometimes clarify the presence of fat necrosis and other postoperative findings, such as seroma, hematoma, or inflammation. In other instances, only a biopsy can determine what a particular finding represents.
DIAGNOSTIC MAMMOGRAPHY
Any mammography performed for a problem-solving purpose is considered diagnostic mammography (Table 2); the exam is tailored to the patient’s individual issue.25 Diagnostic mammography requires the presence of a qualified radiologist at the time of imaging. The goal is to come to a final conclusion about the mammographic or clinical finding at the time of the patient’s visit. Special views are usually performed that include, but are not limited to, spot-compression or spot-magnification views, depending on the finding.26 The patient is then given a same-day written account of the results at the conclusion of the study.
Examples of problems that may prompt diagnostic mammography include patient-reported palpable findings, screening mammography findings that are recalled for further investigation, or physician-detected findings. Often, ultrasonography is also used at the same visit and its results are integrated with the mammography findings to arrive at the final impression.
BREAST ULTRASONOGRAPHY AND BREAST MRI
Ultrasonography and MRI are two very useful adjunctive tools for breast lesion detection and analysis. At this time, however, neither is a replacement for screening mammography as a primary screening modality; rather, each is used in a complementary fashion for lesion analysis and biopsy guidance.10,27
Ultrasonography: Best for further study of areas of interest
Ultrasonography uses high-frequency sound waves to create a picture using a probe directed to an area of interest in the breast. The optimal probe for breast imaging is one typically operating in a frequency of 12 to 18 MHz and 4 cm in scanning width.
Because ultrasonography provides views of only a small area of breast tissue at a time, it is operator and patient dependent. It is best used when a known area of interest needs further evaluation, such as when a patient reports a palpable abnormality or when a mass is detected on mammography.
Ultrasonography uses no ionizing radiation, so it is especially helpful in young or pregnant women who present with a palpable abnormality. It is also useful for patients who have recently undergone a surgical procedure. As ultrasonography is currently used, no compression is needed and it can be performed easily in patients with limited mobility. Needle biopsies are most easily performed using ultrasonographic guidance.
MRI: An emerging adjunct under study in high-risk patients
Breast MRI is an emerging modality under active research that shows promise for adjunctive breast imaging. It is commonly being used as a tool for local staging in women with newly diagnosed breast cancer.28,29 Current research is focused on its suitability as a screening modality, in conjunction with mammography, in high-risk populations based on family history and other factors addressed in the Gail model6 and similar risk models.
The limitations of breast MRI include its high cost, unsuitability for some patients (eg, the obese [due to table weight constraints], patients with pacemakers, patients with renal failure), the potential for unnecessary biopsies due to decreased specificity, lack of portability, and the length of time required for imaging.
When a lesion is initially detected with MRI, an attempt is usually made to identify it with ultrasonography as well, owing to the ease of ultrasonography-guided biopsy.32 It is important, however, for an imaging center that performs breast MRI to be able to perform biopsies using MRI guidance since not all lesions are identifiable by other modalities.33 Breast MRI studies are not easily portable between imaging facilities since a typical study contains a thousand or more images that are best viewed on a site-specific workstation monitor.
HISTOLOGIC CONFIRMATION
Once an abnormality is detected on imaging, a confirmatory histologic diagnosis is needed before embarking on medical or surgical treatments. Image-guided biopsy plays a critical role in this regard. In our breast imaging section, we perform ultrasonography-guided core needle biopsy and aspiration, stereotactic needle biopsy, and MRI-guided needle biopsy, as well as wire localizations on the day of surgery. All procedures performed are considered minimally invasive and are suitable for a vast majority of patients for whom they are recommended.34
Ultrasonography-guided procedures
Ultrasonography-guided fine-needle aspiration is an additional option for patients when core biopsy cannot be performed because the lesion is located adjacent to sensitive structures, such as implants or the pectoralis muscle. Fine-needle aspiration is also used to evaluate complicated breast cysts and, occasionally, lymph nodes. Drawbacks of fine-needle aspiration (relative to larger core needle biopsy) are that it is limited to cytologic, not histologic, examination and that it yields a higher false-negative rate.
Stereotactically guided procedures
Stereotactic core biopsy is performed when lesions—usually calcifications, but sometimes masses—are visible only on mammography.36,37 “Stereotactic” refers to the means by which the target is localized, ie, with a “stereo pair” of digital mammogram pictures with a small field of view. The patient is placed in a prone position with the breast of interest placed through a hole at the undersurface of the table in a light compression. The biopsy unit is attached to a dedicated computer that calculates coordinates. The needle is then brought to the coordinate position for sampling to take place.
The biopsy needle used for this procedure is vacuum-assisted, which means the needle is placed only one time, and samples in the vicinity of the target are vacuumed into a reservoir for retrieval. If the target is calcifications, a specimen radiograph is routinely performed to verify adequate sample acquisition before the patient leaves the biopsy table.38 When the original target is no longer visible, a titanium marker clip is often placed. This facilitates localization of the biopsied area should surgery be needed.
Stereotactic biopsy has several limitations that ultrasonography-guided biopsy does not. The patient must be cooperative and mobile enough to get on the table and hold a prone position for the duration of the procedure (about 45 minutes). If the patient is taking warfarin or has a bleeding diathesis, preprocedure steps such as clinical evaluation to check the international normalized ratio and prothrombin time, or even stopping the warfarin temporarily, may be needed to minimize bleeding during the procedure, as a 9- or 12-gauge needle is used. Stereotactic biopsy is also limited by lesion position. A far posterior lesion may not be accessible if it does not reach through the hole in the table. Also, there is a limit to the compressed thinness of breast tissue that can be biopsied. Finally, most tables used for stereotactic biopsy have a functioning weight limit of 300 pounds.
Open surgical biopsy
A final option is open surgical biopsy, which is used when the more minimally invasive techniques are equivocal, discordant, or impossible due to the limitations noted above, or when atypical cells are found.
HOW SHOULD WE SCREEN OUR PATIENTS?
The various screening options for breast cancer are listed in Table 4, along with their market approval status and Medicare reimbursement levels.
For women at average risk for breast cancer, the American Cancer Society recommends an annual mammogram and clinical breast examination by a physician beginning at age 40 (Table 1).10
In conclusion, the process of finding breast cancer includes regular screening with mammography and clinical breast examination (plus MRI in high-risk women) and the diagnostic modalities of ultrasonography, MRI, and diagnostic mammography. Our ultimate goal is to find cancer at the earliest time possible by all means necessary for the individual patient.
- American Cancer Society. Breast Cancer Facts & Figures 2007-2008. Atlanta, GA: American Cancer Society, Inc. http://www.cancer.org/ downloads/STT/BCFF-Final.pdf. Accessed January 14, 2008.
- Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2003. Bethesda, MD: National Cancer Institute; 2006.
- National Cancer Institute fact sheet: probability of breast cancer in American women. National Cancer Institute Web site. http://www.cancer.gov/cancertopics/factsheet/Detection/probability-breast-cancer. Accessed January 18, 2008.
- Thull DL, Vogel VG. Recognition and management of hereditary breast cancer syndromes. Oncologist 2004; 9:13–24.
- National Comprehensive Cancer Network. NCCN Clinical practice guidelines in oncology: genetic/familial high-risk assessment: breast and ovarian. Available at: http://www.nccn.org/professionals/physician_gls/PDF/genetics_screening.pdf. Accessed January 28, 2008.
- Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989; 81:1879–1886.
- Breast cancer risk assessment tool. An interactive tool for measuring the risk of invasive breast cancer. National Cancer Institute Web site. http://www.cancer.gov/bcrisktool/. Accessed January 21, 2008.
- Vetto J, Pommier R, Schmidt W, et al. Use of the “triple test” for palpable breast lesions yields high diagnostic accuracy and cost savings. Am J Surg 1995; 169:519–522.
- Vetto JT, Pommier RF, Schmidt WA, Eppich H, Alexander PW. Diagnosis of palpable breast lesions in younger women by the modified triple test is accurate and cost-effective. Arch Surg 1996; 131:967–974.
- Smith RA, Saslow D, Sawyer KA, et al. American Cancer Society guidelines for breast cancer screening: update 2003. CA Cancer J Clin 2003; 53:141–169.
- U.S. Preventive Services Task Force. Screening for breast cancer: recommendations and rationale. Ann Intern Med 2002; 137:344–346.
- Nyström L, Andersson I, Bjurstam N, et al. Long-term effects of mammography screening: updated overview of the Swedish randomized trials. Lancet 2002; 359:909–919.
- Tabar L, Fagerberg G, Chen HH, et al. Efficacy of breast cancer screening by age: new results from the Swedish Two-County Trial. Cancer 1995; 75:2507–2517.
- Shapiro S, Venet W, Strax P, Venet L. Periodic Screening for Breast Cancer: The Health Insurance Plan Project and Its Sequelae, 1963-1986. Baltimore, MD: Johns Hopkins University Press; 1988.
- Miller AB, To T, Baines CJ, Wall C. Canadian National Breast Screening Study-2: 13-year results of a randomized trial in women aged 50-59 years. J Natl Cancer Inst 2000; 92:1490–1499.
- Miller AB, To T, Baines CJ, Wall C. The Canadian National Breast Screening Study-1: breast cancer mortality after 11 to 16 years of follow-up: a randomized screening trial of mammography in women age 40 to 49 years. Ann Intern Med 2002; 137:305–312.
- Alexander FE, Anderson TJ, Brown HK, et al. 14 years of follow-up from the Edinburgh randomized trial of breast-cancer screening. Lancet 1999; 353:1903–1908.
- Eklund GW, Cardenosa G. The art of mammographic positioning. Radiol Clin North Am 1992; 30:21–53.
- Pisano E, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast cancer screening. N Engl J Med 2005; 353:1773–1783.
- D’Orsi CJ, Newell MS. Digital mammography: clinical implementation and clinical trials. Semin Roentgenol 2007; 42:236–242.
- Fajardo LL, Roberts CC, Hunt KR. Mammographic surveillance of breast cancer patients: should the masectomy site be imaged? AJR Am J Roentgenol 1993; 161:953–955.
- Eklund GW, Busby RC, Miller SH, Job JS. Improved imaging of the augmented breast. AJR Am J Roentgenol 1988; 151:469–473.
- Mendelson EB. Evaluation of the postoperative breast. Radiol Clin North Am 1992; 30:107–138.
- Philpotts LE, Lee CH, Haffty BG, et al. Mammographic findings of recurrent breast cancer after l
- ACR practice guideline for the performance of diagnostic mammography. American College of Radiology Web site. http:// www.acr.org/SecondaryMainMenuCategories/quality_safety/guidelines/breast/diagnostic_mammography.aspx. Accessed January 14, 2008.
- Sickles EA. Practical solutions to common mammographic problems: tailoring the examination. AJR Am J Roentgenol 1988; 151:31–39.
- Jackson VP. The role of US in breast imaging. Radiology 1990; 177:305–311.
- Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med 2007; 356:1295–1303.
- Liberman L. Breast MR imaging in assessing extent of disease. Magn Reson Imaging Clin N Am 2006; 14:339–349.
- Kuhl C. The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology 2007; 244:356–378.
- Flickinger FW, Allison JD, Sherry RM, Wright JC. Differentiation of benign from malignant breast masses by time-intensity evaluation of contrast-enhanced MRI. Magn Reson Imaging 1993; 11:617–620.
- Chellman-Jeffers MR, Listinsky J, Dinunzio A, Lieber M, Rim A. Utility of second look ultrasound as an adjunct to contrast-enhanced MRI of the breast. Paper presented at: American Roentgen Ray Society Meeting; May 4, 2006; Vancouver, BC. Abstract 269.
- Orel SG, Schnall MD, Newman RW, Powell CM, Torosian MH, Rosato EF. MR imaging-guided localization and biopsy of breast lesions: initial experience. Radiology 1994; 193:97–102.
- Liberman L. Percutaneous imaging-guided core breast biopsy: state of the art at the millennium. AJR Am J Roentgenol 2000; 174:1191–1199.
- Fornage BD, Coan JD, David CL. Ultrasound-guided needle biopsy of the breast and other interventional procedures. Radiol Clin North Am 1992; 30:167–185.
- Parker SH, Lovin JD, Jobe WE, et al. Nonpalpable breast lesions: stereotactic automated large-core biopsies. Radiology 1991; 180:403–407.
- Parker SH, Burbank F, Jackman RJ, et al. Percutaneous large-core breast biopsy: a multi-institutional study. Radiology 1994; 193:3 59–364.
- Liberman L, Evans WP III, Dershaw DD, et al. Radiography of microcalcifications in stereotaxic mammary core biopsy specimens. Radiology 1994; 190:223–225.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007; 57:75–89.
- American Cancer Society. Breast Cancer Facts & Figures 2007-2008. Atlanta, GA: American Cancer Society, Inc. http://www.cancer.org/ downloads/STT/BCFF-Final.pdf. Accessed January 14, 2008.
- Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2003. Bethesda, MD: National Cancer Institute; 2006.
- National Cancer Institute fact sheet: probability of breast cancer in American women. National Cancer Institute Web site. http://www.cancer.gov/cancertopics/factsheet/Detection/probability-breast-cancer. Accessed January 18, 2008.
- Thull DL, Vogel VG. Recognition and management of hereditary breast cancer syndromes. Oncologist 2004; 9:13–24.
- National Comprehensive Cancer Network. NCCN Clinical practice guidelines in oncology: genetic/familial high-risk assessment: breast and ovarian. Available at: http://www.nccn.org/professionals/physician_gls/PDF/genetics_screening.pdf. Accessed January 28, 2008.
- Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989; 81:1879–1886.
- Breast cancer risk assessment tool. An interactive tool for measuring the risk of invasive breast cancer. National Cancer Institute Web site. http://www.cancer.gov/bcrisktool/. Accessed January 21, 2008.
- Vetto J, Pommier R, Schmidt W, et al. Use of the “triple test” for palpable breast lesions yields high diagnostic accuracy and cost savings. Am J Surg 1995; 169:519–522.
- Vetto JT, Pommier RF, Schmidt WA, Eppich H, Alexander PW. Diagnosis of palpable breast lesions in younger women by the modified triple test is accurate and cost-effective. Arch Surg 1996; 131:967–974.
- Smith RA, Saslow D, Sawyer KA, et al. American Cancer Society guidelines for breast cancer screening: update 2003. CA Cancer J Clin 2003; 53:141–169.
- U.S. Preventive Services Task Force. Screening for breast cancer: recommendations and rationale. Ann Intern Med 2002; 137:344–346.
- Nyström L, Andersson I, Bjurstam N, et al. Long-term effects of mammography screening: updated overview of the Swedish randomized trials. Lancet 2002; 359:909–919.
- Tabar L, Fagerberg G, Chen HH, et al. Efficacy of breast cancer screening by age: new results from the Swedish Two-County Trial. Cancer 1995; 75:2507–2517.
- Shapiro S, Venet W, Strax P, Venet L. Periodic Screening for Breast Cancer: The Health Insurance Plan Project and Its Sequelae, 1963-1986. Baltimore, MD: Johns Hopkins University Press; 1988.
- Miller AB, To T, Baines CJ, Wall C. Canadian National Breast Screening Study-2: 13-year results of a randomized trial in women aged 50-59 years. J Natl Cancer Inst 2000; 92:1490–1499.
- Miller AB, To T, Baines CJ, Wall C. The Canadian National Breast Screening Study-1: breast cancer mortality after 11 to 16 years of follow-up: a randomized screening trial of mammography in women age 40 to 49 years. Ann Intern Med 2002; 137:305–312.
- Alexander FE, Anderson TJ, Brown HK, et al. 14 years of follow-up from the Edinburgh randomized trial of breast-cancer screening. Lancet 1999; 353:1903–1908.
- Eklund GW, Cardenosa G. The art of mammographic positioning. Radiol Clin North Am 1992; 30:21–53.
- Pisano E, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast cancer screening. N Engl J Med 2005; 353:1773–1783.
- D’Orsi CJ, Newell MS. Digital mammography: clinical implementation and clinical trials. Semin Roentgenol 2007; 42:236–242.
- Fajardo LL, Roberts CC, Hunt KR. Mammographic surveillance of breast cancer patients: should the masectomy site be imaged? AJR Am J Roentgenol 1993; 161:953–955.
- Eklund GW, Busby RC, Miller SH, Job JS. Improved imaging of the augmented breast. AJR Am J Roentgenol 1988; 151:469–473.
- Mendelson EB. Evaluation of the postoperative breast. Radiol Clin North Am 1992; 30:107–138.
- Philpotts LE, Lee CH, Haffty BG, et al. Mammographic findings of recurrent breast cancer after l
- ACR practice guideline for the performance of diagnostic mammography. American College of Radiology Web site. http:// www.acr.org/SecondaryMainMenuCategories/quality_safety/guidelines/breast/diagnostic_mammography.aspx. Accessed January 14, 2008.
- Sickles EA. Practical solutions to common mammographic problems: tailoring the examination. AJR Am J Roentgenol 1988; 151:31–39.
- Jackson VP. The role of US in breast imaging. Radiology 1990; 177:305–311.
- Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med 2007; 356:1295–1303.
- Liberman L. Breast MR imaging in assessing extent of disease. Magn Reson Imaging Clin N Am 2006; 14:339–349.
- Kuhl C. The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology 2007; 244:356–378.
- Flickinger FW, Allison JD, Sherry RM, Wright JC. Differentiation of benign from malignant breast masses by time-intensity evaluation of contrast-enhanced MRI. Magn Reson Imaging 1993; 11:617–620.
- Chellman-Jeffers MR, Listinsky J, Dinunzio A, Lieber M, Rim A. Utility of second look ultrasound as an adjunct to contrast-enhanced MRI of the breast. Paper presented at: American Roentgen Ray Society Meeting; May 4, 2006; Vancouver, BC. Abstract 269.
- Orel SG, Schnall MD, Newman RW, Powell CM, Torosian MH, Rosato EF. MR imaging-guided localization and biopsy of breast lesions: initial experience. Radiology 1994; 193:97–102.
- Liberman L. Percutaneous imaging-guided core breast biopsy: state of the art at the millennium. AJR Am J Roentgenol 2000; 174:1191–1199.
- Fornage BD, Coan JD, David CL. Ultrasound-guided needle biopsy of the breast and other interventional procedures. Radiol Clin North Am 1992; 30:167–185.
- Parker SH, Lovin JD, Jobe WE, et al. Nonpalpable breast lesions: stereotactic automated large-core biopsies. Radiology 1991; 180:403–407.
- Parker SH, Burbank F, Jackman RJ, et al. Percutaneous large-core breast biopsy: a multi-institutional study. Radiology 1994; 193:3 59–364.
- Liberman L, Evans WP III, Dershaw DD, et al. Radiography of microcalcifications in stereotaxic mammary core biopsy specimens. Radiology 1994; 190:223–225.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007; 57:75–89.