User login
Q: Which is the most likely diagnosis?
- Pulmonary Langerhans cell histiocytosis
- Cystic fibrosis
- Pneumocystis jirovecii pneumonia
- Alpha-1 antitrypsin deficiency
- Tuberous sclerosis complex with lymphangioleiomyomatosis
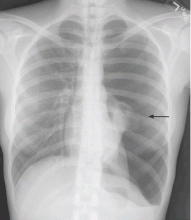
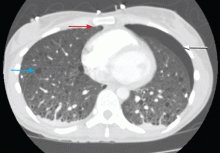
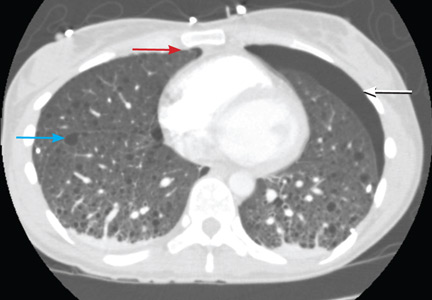
A: The correct diagnosis is tuberous sclerosis complex with lymphangioleiomyomatosis. The lymphangioleiomyomatosis was suggested by the CT findings, by the recurrence of pneumothorax, and, later, by biopsy results. Lymphangioleiomyomatosis occurs in about 30% of women with the tuberous sclerosis complex.1 However, 10% to 15% of women with lymphangioleiomyomatosis do not have tuberous sclerosis complex, 2 in which case the condition is called sporadic lymphangioleiomyomatosis.
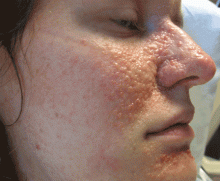
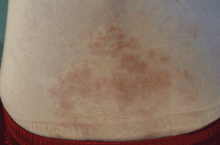
Tuberous sclerosis complex can involve the nerves (seizures, brain tumors), the lungs (lymphangioleiomyomatosis, causing pneumothorax or chylothorax), and the skin; skin lesions include facial angiofibromas (Figure 1), ash-leaf spot (Figure 2), and shagreen patch (Figure 3). It is also associated with abdominal involvement (lymphangiomyomas, renal angiomyolipomas).3
Lymphangioleiomyomatosis usually presents as spontaneous pneumothorax in women of childbearing age. After initial stabilization of pneumothorax with simple aspiration or thoracostomy, the patient should undergo ipsilateral chemical or surgical pleurodesis, as the risk of recurrent pneumothorax is greater than 70%.4 Single or bilateral lung transplantation has been accepted as therapy for end-stage pulmonary lymphangioleiomyomatosis, characterized by recurrent pneumothoraces and chylous pleural fluid collections causing respiratory failure (marked dyspnea, hypoxemia, and reductions in forced expiratory volume in the first second of expiration and in diffusing capacity for carbon monoxide. Recurrence of lymphangioleiomyomatosis in the allograft lung is rare.5 Hormone therapies such as intramuscular progesterone, oral progestins, or gonadotropin-releasing hormone agonists have been used for lymphangioleiomyomatosis (not pneumothorax), but they are no longer recommended.
Pulmonary Langerhans cell histiocytosis, cystic fibrosis, Pneumocystis jirovecii pneumonia, and alpha-1 antitrypsin deficiency have all been associated with spontaneous pneumothorax.
Pulmonary Langerhans cell histiocytosis can affect multiple systems, including lung, skin, bone, and the pituitary gland. More than 90% of patients have a history of smoking.6 Chest radiography reveals a reticulonodular pattern with involvement of the middle and upper lobes. Later, the nodules tend to cavitate and form contiguous cysts that may mimic lymphangioleiomyomatosis on a high-resolution chest CT.
Cystic fibrosis is most often diagnosed before the age of 3.7 In adults, it can present as sinus and pulmonary disease (chronic cough with sputum production, chronic sinusitis with nasal polyposis, radiographic evidence of bronchiectasis and, less commonly, pneumothorax); as a gastrointestinal tract and nutritional abnormality (pancreatic insufficiency, distal intestinal obstruction, focal biliary cirrhosis); and as male infertility.
Pneumocystis jiroveciipneumonia occurs mainly in patients on chronic immunosuppressive drugs or with immune deficiency due to HIV infection. Typical radiographic features are bilateral perihilar interstitial infiltrates that become increasingly homogeneous and diffuse as the disease progresses. Less common findings include solitary or multiple nodules, upper-lobe infiltrates in patients receiving aerosolized pentamidine (NebuPent), pneumatoceles, and pneumothorax.8
Alpha-1 antitrypsin is an inhibitor of neutrophil elastase. Deficiency is associated with severe, early-onset panacinar emphysema with a basilar predominance, with chronic liver disease including cirrhosis, and less commonly with panniculitis and vasculitis associated with antineutrophil cytoplasmic antibody.9 Coalescence of panacinar emphysema leads to the formation of bullae and is important in the development of spontaneous pneumothorax.10
The patient underwent bilateral talc pleurodesis. Lung biopsy at the same time confirmed lymphangioleiomyomatosis. One month later, the right pneumothorax recurred, and she underwent pleurodesis in the right hemithorax with tetracycline. Six months after the second pleurodesis, she was asymptomatic.
- Costello LC, Hartman TE, Ryu JH. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc 2000; 75:591–594.
- Strizheva GD, Carsillo T, Kruger WD, Sullivan EJ, Ryu JH, Henske EP. The spectrum of mutations in TSC1 and TSC2 in women with tuberous sclerosis and lymphangiomyomatosis. Am J Respir Crit Care Med 2001; 163:253–258.
- McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008; 133:507–516.
- Almoosa KF, Ryu JH, Mendez J, et al. Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications. Chest 2006; 129:1274–1281.
- Benden C, Rea F, Behr J, et al. Lung transplantation for lymphangioleiomyomatosis: the European Experience. J Heart Lung Transplant 2009; 28:1–7.
- Vassallo R, Ryu JH, Colby TV, Hartman T, Limper AH. Pulmonary Langerhans’-cell histiocytosis. N Engl J Med 2000; 342:1969–1978.
- Boyle MP. Adult cystic fibrosis. JAMA 2007; 298:1787–1793.
- Thomas CF, Limper AH. Pneumocystis pneumonia. N Engl J Med 2004; 350:2487–2498.
- Silverman EK, Sandhaus RA. Clinical practice. Alpha1-antitrypsin deficiency. N Engl J Med 2009; 360:2749–2757.
- Anderson AE, Furlaneto JA, Foraker AG. Bronchopulmonary derangements in nonsmokers. Am Rev Respir Dis 1970; 101:518–527.
Q: Which is the most likely diagnosis?
- Pulmonary Langerhans cell histiocytosis
- Cystic fibrosis
- Pneumocystis jirovecii pneumonia
- Alpha-1 antitrypsin deficiency
- Tuberous sclerosis complex with lymphangioleiomyomatosis
A: The correct diagnosis is tuberous sclerosis complex with lymphangioleiomyomatosis. The lymphangioleiomyomatosis was suggested by the CT findings, by the recurrence of pneumothorax, and, later, by biopsy results. Lymphangioleiomyomatosis occurs in about 30% of women with the tuberous sclerosis complex.1 However, 10% to 15% of women with lymphangioleiomyomatosis do not have tuberous sclerosis complex, 2 in which case the condition is called sporadic lymphangioleiomyomatosis.
Tuberous sclerosis complex can involve the nerves (seizures, brain tumors), the lungs (lymphangioleiomyomatosis, causing pneumothorax or chylothorax), and the skin; skin lesions include facial angiofibromas (Figure 1), ash-leaf spot (Figure 2), and shagreen patch (Figure 3). It is also associated with abdominal involvement (lymphangiomyomas, renal angiomyolipomas).3
Lymphangioleiomyomatosis usually presents as spontaneous pneumothorax in women of childbearing age. After initial stabilization of pneumothorax with simple aspiration or thoracostomy, the patient should undergo ipsilateral chemical or surgical pleurodesis, as the risk of recurrent pneumothorax is greater than 70%.4 Single or bilateral lung transplantation has been accepted as therapy for end-stage pulmonary lymphangioleiomyomatosis, characterized by recurrent pneumothoraces and chylous pleural fluid collections causing respiratory failure (marked dyspnea, hypoxemia, and reductions in forced expiratory volume in the first second of expiration and in diffusing capacity for carbon monoxide. Recurrence of lymphangioleiomyomatosis in the allograft lung is rare.5 Hormone therapies such as intramuscular progesterone, oral progestins, or gonadotropin-releasing hormone agonists have been used for lymphangioleiomyomatosis (not pneumothorax), but they are no longer recommended.
Pulmonary Langerhans cell histiocytosis, cystic fibrosis, Pneumocystis jirovecii pneumonia, and alpha-1 antitrypsin deficiency have all been associated with spontaneous pneumothorax.
Pulmonary Langerhans cell histiocytosis can affect multiple systems, including lung, skin, bone, and the pituitary gland. More than 90% of patients have a history of smoking.6 Chest radiography reveals a reticulonodular pattern with involvement of the middle and upper lobes. Later, the nodules tend to cavitate and form contiguous cysts that may mimic lymphangioleiomyomatosis on a high-resolution chest CT.
Cystic fibrosis is most often diagnosed before the age of 3.7 In adults, it can present as sinus and pulmonary disease (chronic cough with sputum production, chronic sinusitis with nasal polyposis, radiographic evidence of bronchiectasis and, less commonly, pneumothorax); as a gastrointestinal tract and nutritional abnormality (pancreatic insufficiency, distal intestinal obstruction, focal biliary cirrhosis); and as male infertility.
Pneumocystis jiroveciipneumonia occurs mainly in patients on chronic immunosuppressive drugs or with immune deficiency due to HIV infection. Typical radiographic features are bilateral perihilar interstitial infiltrates that become increasingly homogeneous and diffuse as the disease progresses. Less common findings include solitary or multiple nodules, upper-lobe infiltrates in patients receiving aerosolized pentamidine (NebuPent), pneumatoceles, and pneumothorax.8
Alpha-1 antitrypsin is an inhibitor of neutrophil elastase. Deficiency is associated with severe, early-onset panacinar emphysema with a basilar predominance, with chronic liver disease including cirrhosis, and less commonly with panniculitis and vasculitis associated with antineutrophil cytoplasmic antibody.9 Coalescence of panacinar emphysema leads to the formation of bullae and is important in the development of spontaneous pneumothorax.10
The patient underwent bilateral talc pleurodesis. Lung biopsy at the same time confirmed lymphangioleiomyomatosis. One month later, the right pneumothorax recurred, and she underwent pleurodesis in the right hemithorax with tetracycline. Six months after the second pleurodesis, she was asymptomatic.
Q: Which is the most likely diagnosis?
- Pulmonary Langerhans cell histiocytosis
- Cystic fibrosis
- Pneumocystis jirovecii pneumonia
- Alpha-1 antitrypsin deficiency
- Tuberous sclerosis complex with lymphangioleiomyomatosis
A: The correct diagnosis is tuberous sclerosis complex with lymphangioleiomyomatosis. The lymphangioleiomyomatosis was suggested by the CT findings, by the recurrence of pneumothorax, and, later, by biopsy results. Lymphangioleiomyomatosis occurs in about 30% of women with the tuberous sclerosis complex.1 However, 10% to 15% of women with lymphangioleiomyomatosis do not have tuberous sclerosis complex, 2 in which case the condition is called sporadic lymphangioleiomyomatosis.
Tuberous sclerosis complex can involve the nerves (seizures, brain tumors), the lungs (lymphangioleiomyomatosis, causing pneumothorax or chylothorax), and the skin; skin lesions include facial angiofibromas (Figure 1), ash-leaf spot (Figure 2), and shagreen patch (Figure 3). It is also associated with abdominal involvement (lymphangiomyomas, renal angiomyolipomas).3
Lymphangioleiomyomatosis usually presents as spontaneous pneumothorax in women of childbearing age. After initial stabilization of pneumothorax with simple aspiration or thoracostomy, the patient should undergo ipsilateral chemical or surgical pleurodesis, as the risk of recurrent pneumothorax is greater than 70%.4 Single or bilateral lung transplantation has been accepted as therapy for end-stage pulmonary lymphangioleiomyomatosis, characterized by recurrent pneumothoraces and chylous pleural fluid collections causing respiratory failure (marked dyspnea, hypoxemia, and reductions in forced expiratory volume in the first second of expiration and in diffusing capacity for carbon monoxide. Recurrence of lymphangioleiomyomatosis in the allograft lung is rare.5 Hormone therapies such as intramuscular progesterone, oral progestins, or gonadotropin-releasing hormone agonists have been used for lymphangioleiomyomatosis (not pneumothorax), but they are no longer recommended.
Pulmonary Langerhans cell histiocytosis, cystic fibrosis, Pneumocystis jirovecii pneumonia, and alpha-1 antitrypsin deficiency have all been associated with spontaneous pneumothorax.
Pulmonary Langerhans cell histiocytosis can affect multiple systems, including lung, skin, bone, and the pituitary gland. More than 90% of patients have a history of smoking.6 Chest radiography reveals a reticulonodular pattern with involvement of the middle and upper lobes. Later, the nodules tend to cavitate and form contiguous cysts that may mimic lymphangioleiomyomatosis on a high-resolution chest CT.
Cystic fibrosis is most often diagnosed before the age of 3.7 In adults, it can present as sinus and pulmonary disease (chronic cough with sputum production, chronic sinusitis with nasal polyposis, radiographic evidence of bronchiectasis and, less commonly, pneumothorax); as a gastrointestinal tract and nutritional abnormality (pancreatic insufficiency, distal intestinal obstruction, focal biliary cirrhosis); and as male infertility.
Pneumocystis jiroveciipneumonia occurs mainly in patients on chronic immunosuppressive drugs or with immune deficiency due to HIV infection. Typical radiographic features are bilateral perihilar interstitial infiltrates that become increasingly homogeneous and diffuse as the disease progresses. Less common findings include solitary or multiple nodules, upper-lobe infiltrates in patients receiving aerosolized pentamidine (NebuPent), pneumatoceles, and pneumothorax.8
Alpha-1 antitrypsin is an inhibitor of neutrophil elastase. Deficiency is associated with severe, early-onset panacinar emphysema with a basilar predominance, with chronic liver disease including cirrhosis, and less commonly with panniculitis and vasculitis associated with antineutrophil cytoplasmic antibody.9 Coalescence of panacinar emphysema leads to the formation of bullae and is important in the development of spontaneous pneumothorax.10
The patient underwent bilateral talc pleurodesis. Lung biopsy at the same time confirmed lymphangioleiomyomatosis. One month later, the right pneumothorax recurred, and she underwent pleurodesis in the right hemithorax with tetracycline. Six months after the second pleurodesis, she was asymptomatic.
- Costello LC, Hartman TE, Ryu JH. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc 2000; 75:591–594.
- Strizheva GD, Carsillo T, Kruger WD, Sullivan EJ, Ryu JH, Henske EP. The spectrum of mutations in TSC1 and TSC2 in women with tuberous sclerosis and lymphangiomyomatosis. Am J Respir Crit Care Med 2001; 163:253–258.
- McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008; 133:507–516.
- Almoosa KF, Ryu JH, Mendez J, et al. Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications. Chest 2006; 129:1274–1281.
- Benden C, Rea F, Behr J, et al. Lung transplantation for lymphangioleiomyomatosis: the European Experience. J Heart Lung Transplant 2009; 28:1–7.
- Vassallo R, Ryu JH, Colby TV, Hartman T, Limper AH. Pulmonary Langerhans’-cell histiocytosis. N Engl J Med 2000; 342:1969–1978.
- Boyle MP. Adult cystic fibrosis. JAMA 2007; 298:1787–1793.
- Thomas CF, Limper AH. Pneumocystis pneumonia. N Engl J Med 2004; 350:2487–2498.
- Silverman EK, Sandhaus RA. Clinical practice. Alpha1-antitrypsin deficiency. N Engl J Med 2009; 360:2749–2757.
- Anderson AE, Furlaneto JA, Foraker AG. Bronchopulmonary derangements in nonsmokers. Am Rev Respir Dis 1970; 101:518–527.
- Costello LC, Hartman TE, Ryu JH. High frequency of pulmonary lymphangioleiomyomatosis in women with tuberous sclerosis complex. Mayo Clin Proc 2000; 75:591–594.
- Strizheva GD, Carsillo T, Kruger WD, Sullivan EJ, Ryu JH, Henske EP. The spectrum of mutations in TSC1 and TSC2 in women with tuberous sclerosis and lymphangiomyomatosis. Am J Respir Crit Care Med 2001; 163:253–258.
- McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest 2008; 133:507–516.
- Almoosa KF, Ryu JH, Mendez J, et al. Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications. Chest 2006; 129:1274–1281.
- Benden C, Rea F, Behr J, et al. Lung transplantation for lymphangioleiomyomatosis: the European Experience. J Heart Lung Transplant 2009; 28:1–7.
- Vassallo R, Ryu JH, Colby TV, Hartman T, Limper AH. Pulmonary Langerhans’-cell histiocytosis. N Engl J Med 2000; 342:1969–1978.
- Boyle MP. Adult cystic fibrosis. JAMA 2007; 298:1787–1793.
- Thomas CF, Limper AH. Pneumocystis pneumonia. N Engl J Med 2004; 350:2487–2498.
- Silverman EK, Sandhaus RA. Clinical practice. Alpha1-antitrypsin deficiency. N Engl J Med 2009; 360:2749–2757.
- Anderson AE, Furlaneto JA, Foraker AG. Bronchopulmonary derangements in nonsmokers. Am Rev Respir Dis 1970; 101:518–527.