User login
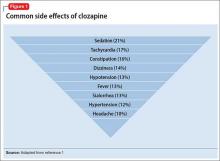
Clozapine is a highly effective antipsychotic with superior efficacy in treatment-resistant schizophrenia, but its side effect profile is daunting (Figure 1).1 Adverse reactions lead to approximately 17% of patients who take clozapine eventually discontinuing the medication.1 As we noted in Part 1 of this 3-part series,2 clozapine remains the most efficacious, but most tedious, antipsychotic available to psychiatrists because of its monitoring requirements and potential side effects.
A powerful rationale for prescribing clozapine, despite its drawbacks, is its association with a reduced risk of all-cause mortality.3,4 People with serious mental illness, including schizophrenia, have a median 10-year shorter life expectancy than the general population.5
A recent cohort study6 examined electronic health records to test whether intensive monitoring or lower suicide risk might account for the reduced mortality with clozapine. The authors found that the reduced mortality rate was not directly related to clozapine’s clinical monitoring or other confounding factors. They did find an association between clozapine use and reduced risk of death from both natural and unnatural causes.
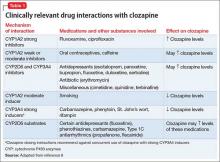
This second article in our series examines clozapine’s adverse effects from a systems perspective. Severe neutropenia, myocarditis, sedation, weight gain, orthostatic hypotension, and sialorrhea appear to be the most studied adverse effects, but myriad others can occur.7 We offer guidance to help the astute clinician continue this effective antipsychotic by monitoring carefully, treating side effects early, and managing potential drug interactions (Table 1).8
Hematologic eventsSevere neutropenia, defined as absolute neutrophil count (ANC) <500/µL, is a well-known adverse effect of clozapine that requires specific clinical monitoring, a requirement that was updated by the FDA in 2015.2 The incidence of severe neutropenia peaks in the first 2 months of clozapine therapy and tapers after 6 months, but some risk always remains.
Older efficacy studies in the United States gauged the 1-year cumulative incidence of severe clozapine-induced neutropenia to be 2%.9 A 1998 study of the effects of using a clozapine registry reported a lower incidence—0.38%—than the 2% noted above.10 Early recognition and recommended interventions can improve clinical outcomes.2
Drug interactions and neutropenia. A retrospective study of mental health inpatients taking clozapine concurrently with oseltamivir during an influenza outbreak found a statistically significant—but not clinically significant—change in ANC values.11 The authors noted that viral infection might lead to blood dyscrasia early in illness, and that oseltamivir has been associated with a small incidence of blood dyscrasia.11-13 This information might be useful when treating influenza in patients taking clozapine, although no specific change in management is recommended.
Similarly, concomitant treatment with clozapine and lithium can affect both white blood cell and ANC values.14,15 Lithium-treated patients often demonstrate increased circulating neutrophils via enhancement of granulocyte-colony stimulating factor.16 Case studies describe how initiating lithium treatment enabled some patients to continue clozapine after developing neutropenia.14,17 Leukocytosis can affect blood monitoring, possibly masking other blood dyscrasias, when lithium is used concurrently with clozapine.
Eosinophilia (blood eosinophil count >700/µL) occurs in approximately 1% of clozapine users, usually in the first 4 weeks of treatment.18 It can be benign and transient or a harbinger of a more rare adverse reaction such as myocarditis, pancreatitis, hepatitis, colitis, or nephritis.19 If a patient taking clozapine develops eosinophilia, clozapine’s package insert recommends that you:
- evaluate promptly for other systemic involvement (rash, other evidence of allergic reaction, myocarditis, other organ-specific disease)
- stop clozapine immediately if any of these are found.
If other causes of eosinophilia are identified (asthma, allergies, collagen vascular disease, parasitic infection, neoplasm), treat these and continue clozapine.
The manufacturer also mentions the occurrence of clozapine-related eosinophilia without organ involvement that can resolve without intervention, with careful monitoring over several weeks.8 In this scenario, there is flexibility to judge whether clozapine should be stopped or re-challenged, or if close monitoring is adequate. Consulting with an internal medicine or hematology specialist might be helpful.
Cardiovascular side effectsMost common events. Three of the 10 most common clozapine side effects are cardiac: tachycardia, hypotension, and hypertension (Figure 1).1 Orthostatic hypotension, bradycardia, and syncope also can occur, especially with rapid clozapine titration. Baseline electrocardiogram (ECG) can help differentiate whether abnormalities are clozapine-induced or related to a preexisting condition.
Reducing the dosage of clozapine or slowing titration could reverse cardiac side effects.8 If dosage reduction is not an option or is ineffective, first consider treating the side effect rather than discontinuing clozapine.20
Sinus tachycardia is one of the most common side effects of clozapine. First, rule out serious conditions—myocarditis, cardiomyopathy, neuroleptic malignant syndrome (NMS)—then consider waiting and monitoring for the first few months of clozapine treatment. If tachycardia continues, consider dosage reduction. Slower titration, or treatment with a cardio-selective beta blocker such as atenolol.21,22 Note that a recent Cochrane Review concluded that there is not enough randomized evidence to support any particular treatment for clozapine-induced tachycardia; the prescriber must therefore make a case-by-case clinical judgment.22
Similarly, orthostatic hypotension can be managed with a reduced dosage of clozapine or slower titration. Increased fluid intake, compression stockings, and, if necessary, fludrocortisone also can be initiated.20
Rare, potentially fatal events. Myocarditis, pericarditis, and cardiomyopathy are among the rare but potentially fatal adverse effects of clozapine. A recent study reported the incidence of myocarditis with clozapine at a range of 0.015% to 1.3%; cardiomyopathy was even more rare.23 Pulmonary embolism and deep venous thrombosis also are very rare possibilities; keep them in mind, however, when patients taking clozapine report new cardiovascular symptoms.
Patients with clozapine-induced cardiovascular effects most commonly report shortness of breath (60%), palpitations (36%), cough (16%), fatigue (16%), and chest pain (8%).7,24
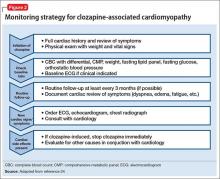
Clozapine’s “black-box” warning specifically recommends discontinuing clozapine and consulting cardiology when myocarditis or cardiomyopathy is suspected. In 50% of cases, myocarditis symptoms present in the first few weeks of clozapine treatment.23 The manufacturer states that myocarditis usually presents in the first 2 months, and cardiomyopathy after 8 weeks of treatment; however, either can present at any time.8Figure 2 provides a clinical reference for monitoring a clozapine patient for cardiomyopathy.24
Laboratory findings that support a diagnosis of clozapine-related myocarditis include:
- elevated C-reactive protein
- elevated troponin I or T
- elevated creatine kinase-MB
- peripheral eosinophilia.8,25
ECG, echocardiography, and cardiac MRI can be helpful in diagnosis, in consultation with a cardiologist.
Neurologic side effectsSeizures are listed in the “black-box” warning for clozapine. Seizure incidence with clozapine is 5% per year, with higher incidence at dosages ≥600 mg/d.8 Because clozapine-induced seizures are dosage-dependent, slow titration can mitigate this risk. Tonic-clonic seizures are the most common type associated with clozapine.
The manufacturer recommends caution when using clozapine in patients with a known seizure disorder, alcohol use disorder, or other CNS pathology.8 Patients with a seizure disorder may be at increased risk of experiencing clozapine-induced seizures, but this is not an absolute contraindication.26 Smoking cessation increases clozapine blood levels by an average of 57.4%, further increasing seizure risk.26,27
Discontinuing clozapine is unnecessary when a patient experiences a seizure. Instead, you can:
- halve the dosage prescribed at the time of the seizure (or at least reduce to the last seizure-free dosage)
- consider any medications or medical problems that might have contributed to a lower seizure threshold
- consider prophylaxis with an antiepileptic medication (eg, valproic acid has efficacy for both myoclonic and tonic-clonic seizures).20,26
Sedation is the most common side effect of clozapine.1 Patients experiencing severe sedation should not drive or operate heavy machinery. To reduce sedation, consider instructing the patient to take all or most of the clozapine dosage at bedtime. A critical review of modafinil for sedation caused by antipsychotics in schizophrenia found only 1 open-label study that showed any positive effects; the authors concluded that further study is needed.28
Cognitive and motor slowing are possible neurologic side effects of clozapine. Caution patients about the risk of participating in activities that require cognitive or motor performance until the individual effects of clozapine are known.8
Tardive dyskinesia. Clozapine carries some risk of tardive dyskinesia, although that risk is lower than with other antipsychotics. Similarly, all antipsychotics including clozapine are associated with a risk of NMS. In the rare case of clozapine-induced NMS, stop clozapine immediately and initiate supportive therapy. Clozapine-induced NMS is not an absolute contraindication to re-challenging a patient with clozapine, however, if doing so is clinically appropriate.20
Cerebrovascular events. In older people with dementia, the use of antipsychotics—including clozapine—has been shown to increase the risk of cerebrovascular events. Because most antipsychotics are not FDA-approved for treating psychosis associated with dementia (only pimavanserin is FDA-approved for symptoms of psychosis in Parkinson’s disease), a risk-benefit analysis should be documented when prescribing any antipsychotic in this population. In practice, clozapine’s benefits may outweigh the mortality risks in specific situations.29,30
CASE Sialorrhea puts progress at risk
Ms. B, age 40, has a history of treatment-resistant schizophrenia and is starting clozapine because of residual psychosis during trials of other antipsychotics. She develops severe persistent drooling, mostly at night, during clozapine titration. Sugar-free candy, multiple bed pillows, and changing the dosing schedule do not significantly improve the sialorrhea.
As a result, Ms. B is embarrassed to continue her usual activities. She asks to stop clozapine, even though her psychotic symptoms have improved and she is functioning at her highest level in years.
Ms. B already is taking trihexyphenidyl, 5 mg, 3 times daily, to manage extrapyramidal symptoms related to haloperidol decanoate treatment. After discussing other medication options for sialorrhea, she agrees to a trial of glycopyrrolate, 1 mg, twice daily. She experiences significant improvement and continues taking clozapine.
Sialorrhea develops in 13% of patients taking clozapine.1 As in Ms. B’s case, this side effect can be embarrassing, can limit social or occupational functioning, and might lead patients to discontinue clozapine treatment despite efficacy. Nonpharmacotherapeutic options include covering the pillow with a towel, lowering the clozapine dosage or titrating slowly (or both), and using sugarless gum or candy to increase swallowing.
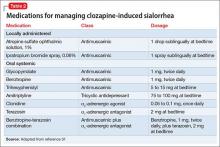
If the benefits of additional medications targeting side effects outweigh the risks, pharmacotherapeutic intervention may be appropriate. Options include the tricyclic antidepressant amitriptyline31; alpha-adrenergic agonists or antagonists (clonidine, terazosin); and anti-muscarinic medications (benztropine, atropine, trihexyphenidyl, glycopyrrolate) (Table 231). Scopolamine transdermal patch is another possible treatment strategy; however, the scopolamine patch was used for clozapine-induced sialorrhea in only a few case reports, and it is not considered a first-line treatment choice.30
When prescribing, consider the possibility of combined side effects with clozapine and adjunct medications having antimuscarinic or alpha-adrenergic activity, or both. Even atropine ophthalmic drops, administered sublingually, are readily absorbed and cross the blood–brain barrier.31 Another antimuscarinic agent, glycopyrrolate, is less likely to cross the blood–brain barrier and therefore is less likely to cause cognitive side effects. Glycopyrrolate is 5 times more potent at blocking the muscarinic receptor than atropine.31,32 Ipratropium bromide, another nonselective muscarinic receptor antagonist, has less systemic absorption than atropine drops, with less anticholinergic side effects when administered sublingually.
Limited evidence supports the efficacy of alpha-adrenergic medications for managing clozapine-induced sialorrhea. Monitor blood pressure when prescribing terazosin or clonidine, which could potentiate clozapine’s hypotensive effects.
Endocrine side effectsAmong antipsychotics, clozapine is associated with the greatest weight gain—averaging nearly 10% of body weight.33,34 Similarly, the risk of new-onset diabetes mellitus is highest with clozapine in relation to other antipsychotics: 43% reported in a 10-year naturalistic study.35 The risk of hyperlipidemia also increases with clozapine treatment.36 These metabolic changes increase the risk of cardiovascular-related death, with a 10-year mortality rate from cardiovascular disease reported at 9% in clozapine-treated patients.35
Despite clozapine’s metabolic side effects, patients with schizophrenia who are treated with clozapine show a significant reduction in overall mortality compared with patients not treated with clozapine.6 Effective identification and management of metabolic side effects can prevent the need to discontinue clozapine.
Behavioral weight management and exercise are recommended as initial therapy.20 If, based on clinical judgment, these alone are insufficient, data support the use of pharmacotherapeutic interventions. Metformin demonstrates a positive effect on body weight, insulin resistance, and lipids, making it the first choice for adjunctive treatment of clozapine-induced metabolic side effects.37-39
Gastrointestinal side effectsClozapine’s anticholinergic activity can lead to serious gastrointestinal (GI) side effects, including constipation, intestinal obstruction, fecal impaction, and paralytic ileus.8 Ileus has produced more fatal adverse reactions with clozapine than has severe neutropenia.20,40 Co-administered anticholinergic medications could increase the risk of ileus. Obtaining a GI review of systems and monitoring bowel movements (in inpatient or residential facilities) can aid in early identification and limit morbidity and mortality from GI adverse events. A high-fiber diet, adequate hydration, bulk laxatives in patients who can reliably maintain hydration, and GI consultation (if needed) may help manage GI side effects.20
Constitutional side effectsFever can occur with clozapine, most often in the first month of treatment, but the incidence is quite variable (0.5% to 55%).20,41 Although benign fever is common, agranulocytosis with infection, NMS, and other systemic illness must be ruled out. The recommended workup when a patient develops fever while taking clozapine includes physical examination and relevant testing (urinalysis, measurement of ANC and serum creatine kinase, chest radiograph, ECG, and, possibly, blood cultures).41
If evidence supports a serious adverse reaction, stop clozapine immediately.20 If benign clozapine-related fever is suspected, acetaminophen or another antipyretic might provide symptomatic relief; discontinuing clozapine is then unnecessary.41
Pregnancy. When a patient with schizophrenia requires clozapine treatment during pregnancy, reliable clinical guidance is limited. The American College of Obstetricians and Gynecologists Practice Bulletin on the use of psychiatric medications during pregnancy and lactation can be a useful resource.42
Be aware that the FDA very recently made major changes to the format and content of pregnancy and lactation labeling, removing the letter categories that have been used for medications approved on or after June 30, 2001. The manufacturers of medications (such as clozapine) that were approved before June 30, 2001, have 3 years to comply with new requirements.43
The FDA had rated clozapine a pregnancy risk category B medication, meaning no evidence of risk in humans. In 2011, the FDA issued a general warning that antipsychotic use in pregnancy can cause extrapyramidal symptoms and discontinuation symptoms in newborns.44,45
A 2015 review of psychotropic medications and pregnancy noted that approximately 60% of women with schizophrenia became pregnant, with an increased incidence of unplanned pregnancy. A high risk of psychotic relapse (65%) during pregnancy and in the postpartum period may lead to insufficient prenatal care, drug use, and obstetric complications.45 Some data suggest low fetal birth weight and an increased rate of therapeutic abortions in women with schizophrenia.42,46
When treating a pregnant patient, weigh the benefits of clozapine against the risks of adverse events, and clearly document the analysis. Clozapine treatment is not recommended during breast-feeding because of the risk of side effects for newborns.8
We highly recommend keeping updated on the literature regarding pregnancy and lactation information with antipsychotics, including clozapine, because prescribing information will likely be updated in the near future to comply with recent FDA labeling changes.
Final installment: Using clozapine off-labelClozapine is FDA-approved for refractory schizophrenia and for reducing the risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorder. In Part 3 of this series, we review off-label uses—such as managing bipolar disorder, borderline personality disorder, and aggressive behavior—that have varying degrees of scientific support.
1. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
2. Newman WJ, Newman BM. Rediscovering clozapine: after a turbulent history, current guidance on initiating and monitoring. Current Psychiatry. 2016;15(7):42-46,48-49.
3. Walker AM, Lanza LL, Arellano F, et al. Mortality in current and former users of clozapine. Epidemiology. 1997;8(6):671-677.
4. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
5. Walker E, McGee RE, Druss BG. Mortality in mental disorders and global disease burden Implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
6. Hayes RD, Downs J, Chang CK, et al. The effect of clozapine on premature mortality: an assessment of clinical monitoring and other potential confounders. Schizophr Bull. 2015;41(3):644-655.
7. De Fazio P, Gaetano R, Caroleo M, et al. Rare and very rare adverse effects of clozapine. Neuropsychiatr Dis Treat. 2015;11:1995-2003.
8. Novartis Pharmaceuticals Corporation. Clozaril (clozapine). Prescribing information. http://clozaril.com/wp-content/themes/eyesite/pi/Clozaril-2015A507-10022015-Approved.pdf. Accessed June 29, 2016.
9. Lieberman JA, Johns CA, Kane JM, et al. Clozapine-induced agranulocytosis: non-cross-reactivity with other psychotropic drugs. J Clin Psychiatry. 1988;49(7):271-277.
10. Honigfeld G, Arellano F, Sethi J, et al. Reducing clozapine-related morbidity and mortality: 5 years of experience with the Clozaril National Registry. J Clin Psychiatry. 1998;59(suppl 3):3-7.
11. Demler TL, Trigoboff E. Are clozapine patients at risk for blood dyscrasias with concomitant tamiflu use? Psychiatry (Edgmont). 2009;6(11):29-33.
12. Karalakulasingam R, Schacht RA, Lansing AM, et al. Influenza virus pneumonia after renal transplant. Postgrad Med. 1977;62(2):164-167.
13. Hoffman-La Roche Limited. Product monograph: Tamiflu. http://www.rochecanada.com/content/dam/roche_canada/en_CA/documents/Research/ClinicalTrialsForms/Products/ConsumerInformation/MonographsandPublicAdvisories/Tamiflu/Tamiflu_PM_E.pdf. Updated January 26, 2015. Accessed November 28, 2015.
14. Whiskey E, Taylor D. Restarting clozapine after neutropenia: evaluating the possibilities and practicalities. CNS Drugs. 2007;21(1):25-35.
15. Palominao A, Kukoyi O, Xiong GL. Leukocytosis after lithium and clozapine combination therapy. Ann Clin Psychiatry. 2010;22(3):205-206.
16. Focosi D, Azzarà A, Kast RE, et al. Lithium and hematology: established and proposed uses. J Leukoc Biol. 2009;85(1):20-28.
17. Papetti F, Darcourt G, Giordana JY, et al. Treatment of clozapine-induced granulocytopenia with lithium (two observations) [in French]. Encephale. 2004;30(6):578-582.
18. Hummer M, Sperner-Unterweger B, Kemmler G, et al. Does eosinophilia predict clozapine induced neutropenia? Psychopharmacology (Berl). 1996;124(1-2):201-204.
19. Aneja J, Sharma N, Mahajan S, et al. Eosinophilia induced by clozapine: a report of two cases and review of the literature. J Family Med Prim Care. 2015;4(1):127-129.
20. Nielsen J, Correll CU, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
21. Stryjer R, Timinsky I, Reznik, I, et al. Beta-adrenergic antagonists for the treatment of clozapine-induced sinus tachycardia: a retrospective study. Clin Neuropharmacol. 2009;32(5):290-292.
22. Lally J, Docherty MJ, MacCabe JH. Pharmacological interventions for clozapine-induced sinus tachycardia. Cochrane Database Syst Rev. 2016;9(6):CD011566.
23. Kamphuis H, Arends J, Timmerman L, et al. Myocarditis and cardiomyopathy: underestimated complications resulting from clozapine therapy [in Dutch]. Tijdschr Psychiatr. 2010;52(4):223-233.
24. Alawami M, Wasywich C, Cicovic A, et al. A systematic review of clozapine induced cardiomyopathy. Int J Cardiol. 2014;176(2):315-320.
25. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust N Z J Psychiatry. 2011;45(6):458-465.
26. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
27. Meyer JM. Individual changes in clozapine levels after smoking cessation: results and a predictive model. J Clin Psychopharmacol. 2001;21(6):569-574.
28. Saavedra-Velez C, Yusim A, Anbarasan D, et al. Modafinil as an adjunctive treatment of sedation, negative symptoms, and cognition in schizophrenia: a critical review. J Clin Psychiatry. 2009;70(1):104-112.
29. Klein C, Gordon J, Pollak L, et al. Clozapine in Parkinson’s disease psychosis: 5-year follow-up review. Clin Neuropharmacol. 2003;26(1):8-11.
30. Lutz UC, Sirfy A, Wiatr G, et al. Clozapine serum concentrations in dopamimetic psychosis in Parkinson’s disease and related disorders. Eur J Clin Pharmacol. 2014;70(12):1471-1476.
31. Bird AM, Smith TL, Walton AE. Current treatment strategies for clozapine-induced sialorrhea. Ann Pharmacother. 2011;45(5):667-675.
32. Duggal HS. Glycopyrrolate for clozapine-induced sialorrhea. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1546-1547.
33. Leadbetter R, Shutty M, Pavalonis D, et al. Clozapine-induced weight gain: prevalence and clinical relevance. Am J Psychiatry. 1992;149(1):68-72.
34. Lundblad W, Azzam PN, Gopalan, et al. Medical management of patients on clozapine: a guide for internists. J Hosp Med. 2015;10(8):537-543.
35. Henderson DC, Nguyen DD, Copeland PM, et al. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10-year naturalistic study. J Clin Psychiatry. 2005;66(9):1116-1121.
36. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166-173.
37. Carrizo E, Fernández V, Connell L, et al. Extended release metformin for metabolic control assistance during prolonged clozapine administration: a 14 week, double-blind, parallel group, placebo-controlled study. Schizophr Res. 2009;113(1):19-26.
38. Chen CH, Huang MC, Kao CF, et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine-treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(5):e424-e430.
39. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
40. Nielsen J, Meyer JM. Risk factors for ileus in patients with schizophrenia. Schizophr Bull. 2012;38(3):592-598.
41. Lowe CM, Grube RR, Scates AC. Characterization and clinical management of clozapine-induced fever. Ann Pharmacother. 2007;41(10):1700-1704.
42. ACOG Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111(4):1001-1020.
43. U.S. Food and Drug Administration. Pregnancy and Lactation Labeling (Drugs) Final Rule. https://s3.amazonaws.com/public-inspection.federalregister.gov/2014-28241.pdf. Published December 4, 2014. Accessed July 6, 2016.
44. Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation: a reference guide to fetal and neonatal risk. 9th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2011.
45. Larsen ER, Damkier P, Pedersen LH, et al; Danish Psychiatric Society; Danish Society of Obstetrics and Gynecology; Danish Paediatric Society; Danish Society of Clinical Pharmacology. Use of psychotropic drugs during pregnancy and breast-feeding. Acta Psychiatr Scand Suppl. 2015;(445):1-28.
46. McKenna K, Koren G, Tetelbaum M, et al. Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. J Clin Psychiatry. 2005;66(4):444-449.
Clozapine is a highly effective antipsychotic with superior efficacy in treatment-resistant schizophrenia, but its side effect profile is daunting (Figure 1).1 Adverse reactions lead to approximately 17% of patients who take clozapine eventually discontinuing the medication.1 As we noted in Part 1 of this 3-part series,2 clozapine remains the most efficacious, but most tedious, antipsychotic available to psychiatrists because of its monitoring requirements and potential side effects.
A powerful rationale for prescribing clozapine, despite its drawbacks, is its association with a reduced risk of all-cause mortality.3,4 People with serious mental illness, including schizophrenia, have a median 10-year shorter life expectancy than the general population.5
A recent cohort study6 examined electronic health records to test whether intensive monitoring or lower suicide risk might account for the reduced mortality with clozapine. The authors found that the reduced mortality rate was not directly related to clozapine’s clinical monitoring or other confounding factors. They did find an association between clozapine use and reduced risk of death from both natural and unnatural causes.
This second article in our series examines clozapine’s adverse effects from a systems perspective. Severe neutropenia, myocarditis, sedation, weight gain, orthostatic hypotension, and sialorrhea appear to be the most studied adverse effects, but myriad others can occur.7 We offer guidance to help the astute clinician continue this effective antipsychotic by monitoring carefully, treating side effects early, and managing potential drug interactions (Table 1).8
Hematologic eventsSevere neutropenia, defined as absolute neutrophil count (ANC) <500/µL, is a well-known adverse effect of clozapine that requires specific clinical monitoring, a requirement that was updated by the FDA in 2015.2 The incidence of severe neutropenia peaks in the first 2 months of clozapine therapy and tapers after 6 months, but some risk always remains.
Older efficacy studies in the United States gauged the 1-year cumulative incidence of severe clozapine-induced neutropenia to be 2%.9 A 1998 study of the effects of using a clozapine registry reported a lower incidence—0.38%—than the 2% noted above.10 Early recognition and recommended interventions can improve clinical outcomes.2
Drug interactions and neutropenia. A retrospective study of mental health inpatients taking clozapine concurrently with oseltamivir during an influenza outbreak found a statistically significant—but not clinically significant—change in ANC values.11 The authors noted that viral infection might lead to blood dyscrasia early in illness, and that oseltamivir has been associated with a small incidence of blood dyscrasia.11-13 This information might be useful when treating influenza in patients taking clozapine, although no specific change in management is recommended.
Similarly, concomitant treatment with clozapine and lithium can affect both white blood cell and ANC values.14,15 Lithium-treated patients often demonstrate increased circulating neutrophils via enhancement of granulocyte-colony stimulating factor.16 Case studies describe how initiating lithium treatment enabled some patients to continue clozapine after developing neutropenia.14,17 Leukocytosis can affect blood monitoring, possibly masking other blood dyscrasias, when lithium is used concurrently with clozapine.
Eosinophilia (blood eosinophil count >700/µL) occurs in approximately 1% of clozapine users, usually in the first 4 weeks of treatment.18 It can be benign and transient or a harbinger of a more rare adverse reaction such as myocarditis, pancreatitis, hepatitis, colitis, or nephritis.19 If a patient taking clozapine develops eosinophilia, clozapine’s package insert recommends that you:
- evaluate promptly for other systemic involvement (rash, other evidence of allergic reaction, myocarditis, other organ-specific disease)
- stop clozapine immediately if any of these are found.
If other causes of eosinophilia are identified (asthma, allergies, collagen vascular disease, parasitic infection, neoplasm), treat these and continue clozapine.
The manufacturer also mentions the occurrence of clozapine-related eosinophilia without organ involvement that can resolve without intervention, with careful monitoring over several weeks.8 In this scenario, there is flexibility to judge whether clozapine should be stopped or re-challenged, or if close monitoring is adequate. Consulting with an internal medicine or hematology specialist might be helpful.
Cardiovascular side effectsMost common events. Three of the 10 most common clozapine side effects are cardiac: tachycardia, hypotension, and hypertension (Figure 1).1 Orthostatic hypotension, bradycardia, and syncope also can occur, especially with rapid clozapine titration. Baseline electrocardiogram (ECG) can help differentiate whether abnormalities are clozapine-induced or related to a preexisting condition.
Reducing the dosage of clozapine or slowing titration could reverse cardiac side effects.8 If dosage reduction is not an option or is ineffective, first consider treating the side effect rather than discontinuing clozapine.20
Sinus tachycardia is one of the most common side effects of clozapine. First, rule out serious conditions—myocarditis, cardiomyopathy, neuroleptic malignant syndrome (NMS)—then consider waiting and monitoring for the first few months of clozapine treatment. If tachycardia continues, consider dosage reduction. Slower titration, or treatment with a cardio-selective beta blocker such as atenolol.21,22 Note that a recent Cochrane Review concluded that there is not enough randomized evidence to support any particular treatment for clozapine-induced tachycardia; the prescriber must therefore make a case-by-case clinical judgment.22
Similarly, orthostatic hypotension can be managed with a reduced dosage of clozapine or slower titration. Increased fluid intake, compression stockings, and, if necessary, fludrocortisone also can be initiated.20
Rare, potentially fatal events. Myocarditis, pericarditis, and cardiomyopathy are among the rare but potentially fatal adverse effects of clozapine. A recent study reported the incidence of myocarditis with clozapine at a range of 0.015% to 1.3%; cardiomyopathy was even more rare.23 Pulmonary embolism and deep venous thrombosis also are very rare possibilities; keep them in mind, however, when patients taking clozapine report new cardiovascular symptoms.
Patients with clozapine-induced cardiovascular effects most commonly report shortness of breath (60%), palpitations (36%), cough (16%), fatigue (16%), and chest pain (8%).7,24
Clozapine’s “black-box” warning specifically recommends discontinuing clozapine and consulting cardiology when myocarditis or cardiomyopathy is suspected. In 50% of cases, myocarditis symptoms present in the first few weeks of clozapine treatment.23 The manufacturer states that myocarditis usually presents in the first 2 months, and cardiomyopathy after 8 weeks of treatment; however, either can present at any time.8Figure 2 provides a clinical reference for monitoring a clozapine patient for cardiomyopathy.24
Laboratory findings that support a diagnosis of clozapine-related myocarditis include:
- elevated C-reactive protein
- elevated troponin I or T
- elevated creatine kinase-MB
- peripheral eosinophilia.8,25
ECG, echocardiography, and cardiac MRI can be helpful in diagnosis, in consultation with a cardiologist.
Neurologic side effectsSeizures are listed in the “black-box” warning for clozapine. Seizure incidence with clozapine is 5% per year, with higher incidence at dosages ≥600 mg/d.8 Because clozapine-induced seizures are dosage-dependent, slow titration can mitigate this risk. Tonic-clonic seizures are the most common type associated with clozapine.
The manufacturer recommends caution when using clozapine in patients with a known seizure disorder, alcohol use disorder, or other CNS pathology.8 Patients with a seizure disorder may be at increased risk of experiencing clozapine-induced seizures, but this is not an absolute contraindication.26 Smoking cessation increases clozapine blood levels by an average of 57.4%, further increasing seizure risk.26,27
Discontinuing clozapine is unnecessary when a patient experiences a seizure. Instead, you can:
- halve the dosage prescribed at the time of the seizure (or at least reduce to the last seizure-free dosage)
- consider any medications or medical problems that might have contributed to a lower seizure threshold
- consider prophylaxis with an antiepileptic medication (eg, valproic acid has efficacy for both myoclonic and tonic-clonic seizures).20,26
Sedation is the most common side effect of clozapine.1 Patients experiencing severe sedation should not drive or operate heavy machinery. To reduce sedation, consider instructing the patient to take all or most of the clozapine dosage at bedtime. A critical review of modafinil for sedation caused by antipsychotics in schizophrenia found only 1 open-label study that showed any positive effects; the authors concluded that further study is needed.28
Cognitive and motor slowing are possible neurologic side effects of clozapine. Caution patients about the risk of participating in activities that require cognitive or motor performance until the individual effects of clozapine are known.8
Tardive dyskinesia. Clozapine carries some risk of tardive dyskinesia, although that risk is lower than with other antipsychotics. Similarly, all antipsychotics including clozapine are associated with a risk of NMS. In the rare case of clozapine-induced NMS, stop clozapine immediately and initiate supportive therapy. Clozapine-induced NMS is not an absolute contraindication to re-challenging a patient with clozapine, however, if doing so is clinically appropriate.20
Cerebrovascular events. In older people with dementia, the use of antipsychotics—including clozapine—has been shown to increase the risk of cerebrovascular events. Because most antipsychotics are not FDA-approved for treating psychosis associated with dementia (only pimavanserin is FDA-approved for symptoms of psychosis in Parkinson’s disease), a risk-benefit analysis should be documented when prescribing any antipsychotic in this population. In practice, clozapine’s benefits may outweigh the mortality risks in specific situations.29,30
CASE Sialorrhea puts progress at risk
Ms. B, age 40, has a history of treatment-resistant schizophrenia and is starting clozapine because of residual psychosis during trials of other antipsychotics. She develops severe persistent drooling, mostly at night, during clozapine titration. Sugar-free candy, multiple bed pillows, and changing the dosing schedule do not significantly improve the sialorrhea.
As a result, Ms. B is embarrassed to continue her usual activities. She asks to stop clozapine, even though her psychotic symptoms have improved and she is functioning at her highest level in years.
Ms. B already is taking trihexyphenidyl, 5 mg, 3 times daily, to manage extrapyramidal symptoms related to haloperidol decanoate treatment. After discussing other medication options for sialorrhea, she agrees to a trial of glycopyrrolate, 1 mg, twice daily. She experiences significant improvement and continues taking clozapine.
Sialorrhea develops in 13% of patients taking clozapine.1 As in Ms. B’s case, this side effect can be embarrassing, can limit social or occupational functioning, and might lead patients to discontinue clozapine treatment despite efficacy. Nonpharmacotherapeutic options include covering the pillow with a towel, lowering the clozapine dosage or titrating slowly (or both), and using sugarless gum or candy to increase swallowing.
If the benefits of additional medications targeting side effects outweigh the risks, pharmacotherapeutic intervention may be appropriate. Options include the tricyclic antidepressant amitriptyline31; alpha-adrenergic agonists or antagonists (clonidine, terazosin); and anti-muscarinic medications (benztropine, atropine, trihexyphenidyl, glycopyrrolate) (Table 231). Scopolamine transdermal patch is another possible treatment strategy; however, the scopolamine patch was used for clozapine-induced sialorrhea in only a few case reports, and it is not considered a first-line treatment choice.30
When prescribing, consider the possibility of combined side effects with clozapine and adjunct medications having antimuscarinic or alpha-adrenergic activity, or both. Even atropine ophthalmic drops, administered sublingually, are readily absorbed and cross the blood–brain barrier.31 Another antimuscarinic agent, glycopyrrolate, is less likely to cross the blood–brain barrier and therefore is less likely to cause cognitive side effects. Glycopyrrolate is 5 times more potent at blocking the muscarinic receptor than atropine.31,32 Ipratropium bromide, another nonselective muscarinic receptor antagonist, has less systemic absorption than atropine drops, with less anticholinergic side effects when administered sublingually.
Limited evidence supports the efficacy of alpha-adrenergic medications for managing clozapine-induced sialorrhea. Monitor blood pressure when prescribing terazosin or clonidine, which could potentiate clozapine’s hypotensive effects.
Endocrine side effectsAmong antipsychotics, clozapine is associated with the greatest weight gain—averaging nearly 10% of body weight.33,34 Similarly, the risk of new-onset diabetes mellitus is highest with clozapine in relation to other antipsychotics: 43% reported in a 10-year naturalistic study.35 The risk of hyperlipidemia also increases with clozapine treatment.36 These metabolic changes increase the risk of cardiovascular-related death, with a 10-year mortality rate from cardiovascular disease reported at 9% in clozapine-treated patients.35
Despite clozapine’s metabolic side effects, patients with schizophrenia who are treated with clozapine show a significant reduction in overall mortality compared with patients not treated with clozapine.6 Effective identification and management of metabolic side effects can prevent the need to discontinue clozapine.
Behavioral weight management and exercise are recommended as initial therapy.20 If, based on clinical judgment, these alone are insufficient, data support the use of pharmacotherapeutic interventions. Metformin demonstrates a positive effect on body weight, insulin resistance, and lipids, making it the first choice for adjunctive treatment of clozapine-induced metabolic side effects.37-39
Gastrointestinal side effectsClozapine’s anticholinergic activity can lead to serious gastrointestinal (GI) side effects, including constipation, intestinal obstruction, fecal impaction, and paralytic ileus.8 Ileus has produced more fatal adverse reactions with clozapine than has severe neutropenia.20,40 Co-administered anticholinergic medications could increase the risk of ileus. Obtaining a GI review of systems and monitoring bowel movements (in inpatient or residential facilities) can aid in early identification and limit morbidity and mortality from GI adverse events. A high-fiber diet, adequate hydration, bulk laxatives in patients who can reliably maintain hydration, and GI consultation (if needed) may help manage GI side effects.20
Constitutional side effectsFever can occur with clozapine, most often in the first month of treatment, but the incidence is quite variable (0.5% to 55%).20,41 Although benign fever is common, agranulocytosis with infection, NMS, and other systemic illness must be ruled out. The recommended workup when a patient develops fever while taking clozapine includes physical examination and relevant testing (urinalysis, measurement of ANC and serum creatine kinase, chest radiograph, ECG, and, possibly, blood cultures).41
If evidence supports a serious adverse reaction, stop clozapine immediately.20 If benign clozapine-related fever is suspected, acetaminophen or another antipyretic might provide symptomatic relief; discontinuing clozapine is then unnecessary.41
Pregnancy. When a patient with schizophrenia requires clozapine treatment during pregnancy, reliable clinical guidance is limited. The American College of Obstetricians and Gynecologists Practice Bulletin on the use of psychiatric medications during pregnancy and lactation can be a useful resource.42
Be aware that the FDA very recently made major changes to the format and content of pregnancy and lactation labeling, removing the letter categories that have been used for medications approved on or after June 30, 2001. The manufacturers of medications (such as clozapine) that were approved before June 30, 2001, have 3 years to comply with new requirements.43
The FDA had rated clozapine a pregnancy risk category B medication, meaning no evidence of risk in humans. In 2011, the FDA issued a general warning that antipsychotic use in pregnancy can cause extrapyramidal symptoms and discontinuation symptoms in newborns.44,45
A 2015 review of psychotropic medications and pregnancy noted that approximately 60% of women with schizophrenia became pregnant, with an increased incidence of unplanned pregnancy. A high risk of psychotic relapse (65%) during pregnancy and in the postpartum period may lead to insufficient prenatal care, drug use, and obstetric complications.45 Some data suggest low fetal birth weight and an increased rate of therapeutic abortions in women with schizophrenia.42,46
When treating a pregnant patient, weigh the benefits of clozapine against the risks of adverse events, and clearly document the analysis. Clozapine treatment is not recommended during breast-feeding because of the risk of side effects for newborns.8
We highly recommend keeping updated on the literature regarding pregnancy and lactation information with antipsychotics, including clozapine, because prescribing information will likely be updated in the near future to comply with recent FDA labeling changes.
Final installment: Using clozapine off-labelClozapine is FDA-approved for refractory schizophrenia and for reducing the risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorder. In Part 3 of this series, we review off-label uses—such as managing bipolar disorder, borderline personality disorder, and aggressive behavior—that have varying degrees of scientific support.
Clozapine is a highly effective antipsychotic with superior efficacy in treatment-resistant schizophrenia, but its side effect profile is daunting (Figure 1).1 Adverse reactions lead to approximately 17% of patients who take clozapine eventually discontinuing the medication.1 As we noted in Part 1 of this 3-part series,2 clozapine remains the most efficacious, but most tedious, antipsychotic available to psychiatrists because of its monitoring requirements and potential side effects.
A powerful rationale for prescribing clozapine, despite its drawbacks, is its association with a reduced risk of all-cause mortality.3,4 People with serious mental illness, including schizophrenia, have a median 10-year shorter life expectancy than the general population.5
A recent cohort study6 examined electronic health records to test whether intensive monitoring or lower suicide risk might account for the reduced mortality with clozapine. The authors found that the reduced mortality rate was not directly related to clozapine’s clinical monitoring or other confounding factors. They did find an association between clozapine use and reduced risk of death from both natural and unnatural causes.
This second article in our series examines clozapine’s adverse effects from a systems perspective. Severe neutropenia, myocarditis, sedation, weight gain, orthostatic hypotension, and sialorrhea appear to be the most studied adverse effects, but myriad others can occur.7 We offer guidance to help the astute clinician continue this effective antipsychotic by monitoring carefully, treating side effects early, and managing potential drug interactions (Table 1).8
Hematologic eventsSevere neutropenia, defined as absolute neutrophil count (ANC) <500/µL, is a well-known adverse effect of clozapine that requires specific clinical monitoring, a requirement that was updated by the FDA in 2015.2 The incidence of severe neutropenia peaks in the first 2 months of clozapine therapy and tapers after 6 months, but some risk always remains.
Older efficacy studies in the United States gauged the 1-year cumulative incidence of severe clozapine-induced neutropenia to be 2%.9 A 1998 study of the effects of using a clozapine registry reported a lower incidence—0.38%—than the 2% noted above.10 Early recognition and recommended interventions can improve clinical outcomes.2
Drug interactions and neutropenia. A retrospective study of mental health inpatients taking clozapine concurrently with oseltamivir during an influenza outbreak found a statistically significant—but not clinically significant—change in ANC values.11 The authors noted that viral infection might lead to blood dyscrasia early in illness, and that oseltamivir has been associated with a small incidence of blood dyscrasia.11-13 This information might be useful when treating influenza in patients taking clozapine, although no specific change in management is recommended.
Similarly, concomitant treatment with clozapine and lithium can affect both white blood cell and ANC values.14,15 Lithium-treated patients often demonstrate increased circulating neutrophils via enhancement of granulocyte-colony stimulating factor.16 Case studies describe how initiating lithium treatment enabled some patients to continue clozapine after developing neutropenia.14,17 Leukocytosis can affect blood monitoring, possibly masking other blood dyscrasias, when lithium is used concurrently with clozapine.
Eosinophilia (blood eosinophil count >700/µL) occurs in approximately 1% of clozapine users, usually in the first 4 weeks of treatment.18 It can be benign and transient or a harbinger of a more rare adverse reaction such as myocarditis, pancreatitis, hepatitis, colitis, or nephritis.19 If a patient taking clozapine develops eosinophilia, clozapine’s package insert recommends that you:
- evaluate promptly for other systemic involvement (rash, other evidence of allergic reaction, myocarditis, other organ-specific disease)
- stop clozapine immediately if any of these are found.
If other causes of eosinophilia are identified (asthma, allergies, collagen vascular disease, parasitic infection, neoplasm), treat these and continue clozapine.
The manufacturer also mentions the occurrence of clozapine-related eosinophilia without organ involvement that can resolve without intervention, with careful monitoring over several weeks.8 In this scenario, there is flexibility to judge whether clozapine should be stopped or re-challenged, or if close monitoring is adequate. Consulting with an internal medicine or hematology specialist might be helpful.
Cardiovascular side effectsMost common events. Three of the 10 most common clozapine side effects are cardiac: tachycardia, hypotension, and hypertension (Figure 1).1 Orthostatic hypotension, bradycardia, and syncope also can occur, especially with rapid clozapine titration. Baseline electrocardiogram (ECG) can help differentiate whether abnormalities are clozapine-induced or related to a preexisting condition.
Reducing the dosage of clozapine or slowing titration could reverse cardiac side effects.8 If dosage reduction is not an option or is ineffective, first consider treating the side effect rather than discontinuing clozapine.20
Sinus tachycardia is one of the most common side effects of clozapine. First, rule out serious conditions—myocarditis, cardiomyopathy, neuroleptic malignant syndrome (NMS)—then consider waiting and monitoring for the first few months of clozapine treatment. If tachycardia continues, consider dosage reduction. Slower titration, or treatment with a cardio-selective beta blocker such as atenolol.21,22 Note that a recent Cochrane Review concluded that there is not enough randomized evidence to support any particular treatment for clozapine-induced tachycardia; the prescriber must therefore make a case-by-case clinical judgment.22
Similarly, orthostatic hypotension can be managed with a reduced dosage of clozapine or slower titration. Increased fluid intake, compression stockings, and, if necessary, fludrocortisone also can be initiated.20
Rare, potentially fatal events. Myocarditis, pericarditis, and cardiomyopathy are among the rare but potentially fatal adverse effects of clozapine. A recent study reported the incidence of myocarditis with clozapine at a range of 0.015% to 1.3%; cardiomyopathy was even more rare.23 Pulmonary embolism and deep venous thrombosis also are very rare possibilities; keep them in mind, however, when patients taking clozapine report new cardiovascular symptoms.
Patients with clozapine-induced cardiovascular effects most commonly report shortness of breath (60%), palpitations (36%), cough (16%), fatigue (16%), and chest pain (8%).7,24
Clozapine’s “black-box” warning specifically recommends discontinuing clozapine and consulting cardiology when myocarditis or cardiomyopathy is suspected. In 50% of cases, myocarditis symptoms present in the first few weeks of clozapine treatment.23 The manufacturer states that myocarditis usually presents in the first 2 months, and cardiomyopathy after 8 weeks of treatment; however, either can present at any time.8Figure 2 provides a clinical reference for monitoring a clozapine patient for cardiomyopathy.24
Laboratory findings that support a diagnosis of clozapine-related myocarditis include:
- elevated C-reactive protein
- elevated troponin I or T
- elevated creatine kinase-MB
- peripheral eosinophilia.8,25
ECG, echocardiography, and cardiac MRI can be helpful in diagnosis, in consultation with a cardiologist.
Neurologic side effectsSeizures are listed in the “black-box” warning for clozapine. Seizure incidence with clozapine is 5% per year, with higher incidence at dosages ≥600 mg/d.8 Because clozapine-induced seizures are dosage-dependent, slow titration can mitigate this risk. Tonic-clonic seizures are the most common type associated with clozapine.
The manufacturer recommends caution when using clozapine in patients with a known seizure disorder, alcohol use disorder, or other CNS pathology.8 Patients with a seizure disorder may be at increased risk of experiencing clozapine-induced seizures, but this is not an absolute contraindication.26 Smoking cessation increases clozapine blood levels by an average of 57.4%, further increasing seizure risk.26,27
Discontinuing clozapine is unnecessary when a patient experiences a seizure. Instead, you can:
- halve the dosage prescribed at the time of the seizure (or at least reduce to the last seizure-free dosage)
- consider any medications or medical problems that might have contributed to a lower seizure threshold
- consider prophylaxis with an antiepileptic medication (eg, valproic acid has efficacy for both myoclonic and tonic-clonic seizures).20,26
Sedation is the most common side effect of clozapine.1 Patients experiencing severe sedation should not drive or operate heavy machinery. To reduce sedation, consider instructing the patient to take all or most of the clozapine dosage at bedtime. A critical review of modafinil for sedation caused by antipsychotics in schizophrenia found only 1 open-label study that showed any positive effects; the authors concluded that further study is needed.28
Cognitive and motor slowing are possible neurologic side effects of clozapine. Caution patients about the risk of participating in activities that require cognitive or motor performance until the individual effects of clozapine are known.8
Tardive dyskinesia. Clozapine carries some risk of tardive dyskinesia, although that risk is lower than with other antipsychotics. Similarly, all antipsychotics including clozapine are associated with a risk of NMS. In the rare case of clozapine-induced NMS, stop clozapine immediately and initiate supportive therapy. Clozapine-induced NMS is not an absolute contraindication to re-challenging a patient with clozapine, however, if doing so is clinically appropriate.20
Cerebrovascular events. In older people with dementia, the use of antipsychotics—including clozapine—has been shown to increase the risk of cerebrovascular events. Because most antipsychotics are not FDA-approved for treating psychosis associated with dementia (only pimavanserin is FDA-approved for symptoms of psychosis in Parkinson’s disease), a risk-benefit analysis should be documented when prescribing any antipsychotic in this population. In practice, clozapine’s benefits may outweigh the mortality risks in specific situations.29,30
CASE Sialorrhea puts progress at risk
Ms. B, age 40, has a history of treatment-resistant schizophrenia and is starting clozapine because of residual psychosis during trials of other antipsychotics. She develops severe persistent drooling, mostly at night, during clozapine titration. Sugar-free candy, multiple bed pillows, and changing the dosing schedule do not significantly improve the sialorrhea.
As a result, Ms. B is embarrassed to continue her usual activities. She asks to stop clozapine, even though her psychotic symptoms have improved and she is functioning at her highest level in years.
Ms. B already is taking trihexyphenidyl, 5 mg, 3 times daily, to manage extrapyramidal symptoms related to haloperidol decanoate treatment. After discussing other medication options for sialorrhea, she agrees to a trial of glycopyrrolate, 1 mg, twice daily. She experiences significant improvement and continues taking clozapine.
Sialorrhea develops in 13% of patients taking clozapine.1 As in Ms. B’s case, this side effect can be embarrassing, can limit social or occupational functioning, and might lead patients to discontinue clozapine treatment despite efficacy. Nonpharmacotherapeutic options include covering the pillow with a towel, lowering the clozapine dosage or titrating slowly (or both), and using sugarless gum or candy to increase swallowing.
If the benefits of additional medications targeting side effects outweigh the risks, pharmacotherapeutic intervention may be appropriate. Options include the tricyclic antidepressant amitriptyline31; alpha-adrenergic agonists or antagonists (clonidine, terazosin); and anti-muscarinic medications (benztropine, atropine, trihexyphenidyl, glycopyrrolate) (Table 231). Scopolamine transdermal patch is another possible treatment strategy; however, the scopolamine patch was used for clozapine-induced sialorrhea in only a few case reports, and it is not considered a first-line treatment choice.30
When prescribing, consider the possibility of combined side effects with clozapine and adjunct medications having antimuscarinic or alpha-adrenergic activity, or both. Even atropine ophthalmic drops, administered sublingually, are readily absorbed and cross the blood–brain barrier.31 Another antimuscarinic agent, glycopyrrolate, is less likely to cross the blood–brain barrier and therefore is less likely to cause cognitive side effects. Glycopyrrolate is 5 times more potent at blocking the muscarinic receptor than atropine.31,32 Ipratropium bromide, another nonselective muscarinic receptor antagonist, has less systemic absorption than atropine drops, with less anticholinergic side effects when administered sublingually.
Limited evidence supports the efficacy of alpha-adrenergic medications for managing clozapine-induced sialorrhea. Monitor blood pressure when prescribing terazosin or clonidine, which could potentiate clozapine’s hypotensive effects.
Endocrine side effectsAmong antipsychotics, clozapine is associated with the greatest weight gain—averaging nearly 10% of body weight.33,34 Similarly, the risk of new-onset diabetes mellitus is highest with clozapine in relation to other antipsychotics: 43% reported in a 10-year naturalistic study.35 The risk of hyperlipidemia also increases with clozapine treatment.36 These metabolic changes increase the risk of cardiovascular-related death, with a 10-year mortality rate from cardiovascular disease reported at 9% in clozapine-treated patients.35
Despite clozapine’s metabolic side effects, patients with schizophrenia who are treated with clozapine show a significant reduction in overall mortality compared with patients not treated with clozapine.6 Effective identification and management of metabolic side effects can prevent the need to discontinue clozapine.
Behavioral weight management and exercise are recommended as initial therapy.20 If, based on clinical judgment, these alone are insufficient, data support the use of pharmacotherapeutic interventions. Metformin demonstrates a positive effect on body weight, insulin resistance, and lipids, making it the first choice for adjunctive treatment of clozapine-induced metabolic side effects.37-39
Gastrointestinal side effectsClozapine’s anticholinergic activity can lead to serious gastrointestinal (GI) side effects, including constipation, intestinal obstruction, fecal impaction, and paralytic ileus.8 Ileus has produced more fatal adverse reactions with clozapine than has severe neutropenia.20,40 Co-administered anticholinergic medications could increase the risk of ileus. Obtaining a GI review of systems and monitoring bowel movements (in inpatient or residential facilities) can aid in early identification and limit morbidity and mortality from GI adverse events. A high-fiber diet, adequate hydration, bulk laxatives in patients who can reliably maintain hydration, and GI consultation (if needed) may help manage GI side effects.20
Constitutional side effectsFever can occur with clozapine, most often in the first month of treatment, but the incidence is quite variable (0.5% to 55%).20,41 Although benign fever is common, agranulocytosis with infection, NMS, and other systemic illness must be ruled out. The recommended workup when a patient develops fever while taking clozapine includes physical examination and relevant testing (urinalysis, measurement of ANC and serum creatine kinase, chest radiograph, ECG, and, possibly, blood cultures).41
If evidence supports a serious adverse reaction, stop clozapine immediately.20 If benign clozapine-related fever is suspected, acetaminophen or another antipyretic might provide symptomatic relief; discontinuing clozapine is then unnecessary.41
Pregnancy. When a patient with schizophrenia requires clozapine treatment during pregnancy, reliable clinical guidance is limited. The American College of Obstetricians and Gynecologists Practice Bulletin on the use of psychiatric medications during pregnancy and lactation can be a useful resource.42
Be aware that the FDA very recently made major changes to the format and content of pregnancy and lactation labeling, removing the letter categories that have been used for medications approved on or after June 30, 2001. The manufacturers of medications (such as clozapine) that were approved before June 30, 2001, have 3 years to comply with new requirements.43
The FDA had rated clozapine a pregnancy risk category B medication, meaning no evidence of risk in humans. In 2011, the FDA issued a general warning that antipsychotic use in pregnancy can cause extrapyramidal symptoms and discontinuation symptoms in newborns.44,45
A 2015 review of psychotropic medications and pregnancy noted that approximately 60% of women with schizophrenia became pregnant, with an increased incidence of unplanned pregnancy. A high risk of psychotic relapse (65%) during pregnancy and in the postpartum period may lead to insufficient prenatal care, drug use, and obstetric complications.45 Some data suggest low fetal birth weight and an increased rate of therapeutic abortions in women with schizophrenia.42,46
When treating a pregnant patient, weigh the benefits of clozapine against the risks of adverse events, and clearly document the analysis. Clozapine treatment is not recommended during breast-feeding because of the risk of side effects for newborns.8
We highly recommend keeping updated on the literature regarding pregnancy and lactation information with antipsychotics, including clozapine, because prescribing information will likely be updated in the near future to comply with recent FDA labeling changes.
Final installment: Using clozapine off-labelClozapine is FDA-approved for refractory schizophrenia and for reducing the risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorder. In Part 3 of this series, we review off-label uses—such as managing bipolar disorder, borderline personality disorder, and aggressive behavior—that have varying degrees of scientific support.
1. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
2. Newman WJ, Newman BM. Rediscovering clozapine: after a turbulent history, current guidance on initiating and monitoring. Current Psychiatry. 2016;15(7):42-46,48-49.
3. Walker AM, Lanza LL, Arellano F, et al. Mortality in current and former users of clozapine. Epidemiology. 1997;8(6):671-677.
4. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
5. Walker E, McGee RE, Druss BG. Mortality in mental disorders and global disease burden Implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
6. Hayes RD, Downs J, Chang CK, et al. The effect of clozapine on premature mortality: an assessment of clinical monitoring and other potential confounders. Schizophr Bull. 2015;41(3):644-655.
7. De Fazio P, Gaetano R, Caroleo M, et al. Rare and very rare adverse effects of clozapine. Neuropsychiatr Dis Treat. 2015;11:1995-2003.
8. Novartis Pharmaceuticals Corporation. Clozaril (clozapine). Prescribing information. http://clozaril.com/wp-content/themes/eyesite/pi/Clozaril-2015A507-10022015-Approved.pdf. Accessed June 29, 2016.
9. Lieberman JA, Johns CA, Kane JM, et al. Clozapine-induced agranulocytosis: non-cross-reactivity with other psychotropic drugs. J Clin Psychiatry. 1988;49(7):271-277.
10. Honigfeld G, Arellano F, Sethi J, et al. Reducing clozapine-related morbidity and mortality: 5 years of experience with the Clozaril National Registry. J Clin Psychiatry. 1998;59(suppl 3):3-7.
11. Demler TL, Trigoboff E. Are clozapine patients at risk for blood dyscrasias with concomitant tamiflu use? Psychiatry (Edgmont). 2009;6(11):29-33.
12. Karalakulasingam R, Schacht RA, Lansing AM, et al. Influenza virus pneumonia after renal transplant. Postgrad Med. 1977;62(2):164-167.
13. Hoffman-La Roche Limited. Product monograph: Tamiflu. http://www.rochecanada.com/content/dam/roche_canada/en_CA/documents/Research/ClinicalTrialsForms/Products/ConsumerInformation/MonographsandPublicAdvisories/Tamiflu/Tamiflu_PM_E.pdf. Updated January 26, 2015. Accessed November 28, 2015.
14. Whiskey E, Taylor D. Restarting clozapine after neutropenia: evaluating the possibilities and practicalities. CNS Drugs. 2007;21(1):25-35.
15. Palominao A, Kukoyi O, Xiong GL. Leukocytosis after lithium and clozapine combination therapy. Ann Clin Psychiatry. 2010;22(3):205-206.
16. Focosi D, Azzarà A, Kast RE, et al. Lithium and hematology: established and proposed uses. J Leukoc Biol. 2009;85(1):20-28.
17. Papetti F, Darcourt G, Giordana JY, et al. Treatment of clozapine-induced granulocytopenia with lithium (two observations) [in French]. Encephale. 2004;30(6):578-582.
18. Hummer M, Sperner-Unterweger B, Kemmler G, et al. Does eosinophilia predict clozapine induced neutropenia? Psychopharmacology (Berl). 1996;124(1-2):201-204.
19. Aneja J, Sharma N, Mahajan S, et al. Eosinophilia induced by clozapine: a report of two cases and review of the literature. J Family Med Prim Care. 2015;4(1):127-129.
20. Nielsen J, Correll CU, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
21. Stryjer R, Timinsky I, Reznik, I, et al. Beta-adrenergic antagonists for the treatment of clozapine-induced sinus tachycardia: a retrospective study. Clin Neuropharmacol. 2009;32(5):290-292.
22. Lally J, Docherty MJ, MacCabe JH. Pharmacological interventions for clozapine-induced sinus tachycardia. Cochrane Database Syst Rev. 2016;9(6):CD011566.
23. Kamphuis H, Arends J, Timmerman L, et al. Myocarditis and cardiomyopathy: underestimated complications resulting from clozapine therapy [in Dutch]. Tijdschr Psychiatr. 2010;52(4):223-233.
24. Alawami M, Wasywich C, Cicovic A, et al. A systematic review of clozapine induced cardiomyopathy. Int J Cardiol. 2014;176(2):315-320.
25. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust N Z J Psychiatry. 2011;45(6):458-465.
26. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
27. Meyer JM. Individual changes in clozapine levels after smoking cessation: results and a predictive model. J Clin Psychopharmacol. 2001;21(6):569-574.
28. Saavedra-Velez C, Yusim A, Anbarasan D, et al. Modafinil as an adjunctive treatment of sedation, negative symptoms, and cognition in schizophrenia: a critical review. J Clin Psychiatry. 2009;70(1):104-112.
29. Klein C, Gordon J, Pollak L, et al. Clozapine in Parkinson’s disease psychosis: 5-year follow-up review. Clin Neuropharmacol. 2003;26(1):8-11.
30. Lutz UC, Sirfy A, Wiatr G, et al. Clozapine serum concentrations in dopamimetic psychosis in Parkinson’s disease and related disorders. Eur J Clin Pharmacol. 2014;70(12):1471-1476.
31. Bird AM, Smith TL, Walton AE. Current treatment strategies for clozapine-induced sialorrhea. Ann Pharmacother. 2011;45(5):667-675.
32. Duggal HS. Glycopyrrolate for clozapine-induced sialorrhea. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1546-1547.
33. Leadbetter R, Shutty M, Pavalonis D, et al. Clozapine-induced weight gain: prevalence and clinical relevance. Am J Psychiatry. 1992;149(1):68-72.
34. Lundblad W, Azzam PN, Gopalan, et al. Medical management of patients on clozapine: a guide for internists. J Hosp Med. 2015;10(8):537-543.
35. Henderson DC, Nguyen DD, Copeland PM, et al. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10-year naturalistic study. J Clin Psychiatry. 2005;66(9):1116-1121.
36. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166-173.
37. Carrizo E, Fernández V, Connell L, et al. Extended release metformin for metabolic control assistance during prolonged clozapine administration: a 14 week, double-blind, parallel group, placebo-controlled study. Schizophr Res. 2009;113(1):19-26.
38. Chen CH, Huang MC, Kao CF, et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine-treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(5):e424-e430.
39. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
40. Nielsen J, Meyer JM. Risk factors for ileus in patients with schizophrenia. Schizophr Bull. 2012;38(3):592-598.
41. Lowe CM, Grube RR, Scates AC. Characterization and clinical management of clozapine-induced fever. Ann Pharmacother. 2007;41(10):1700-1704.
42. ACOG Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111(4):1001-1020.
43. U.S. Food and Drug Administration. Pregnancy and Lactation Labeling (Drugs) Final Rule. https://s3.amazonaws.com/public-inspection.federalregister.gov/2014-28241.pdf. Published December 4, 2014. Accessed July 6, 2016.
44. Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation: a reference guide to fetal and neonatal risk. 9th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2011.
45. Larsen ER, Damkier P, Pedersen LH, et al; Danish Psychiatric Society; Danish Society of Obstetrics and Gynecology; Danish Paediatric Society; Danish Society of Clinical Pharmacology. Use of psychotropic drugs during pregnancy and breast-feeding. Acta Psychiatr Scand Suppl. 2015;(445):1-28.
46. McKenna K, Koren G, Tetelbaum M, et al. Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. J Clin Psychiatry. 2005;66(4):444-449.
1. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
2. Newman WJ, Newman BM. Rediscovering clozapine: after a turbulent history, current guidance on initiating and monitoring. Current Psychiatry. 2016;15(7):42-46,48-49.
3. Walker AM, Lanza LL, Arellano F, et al. Mortality in current and former users of clozapine. Epidemiology. 1997;8(6):671-677.
4. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
5. Walker E, McGee RE, Druss BG. Mortality in mental disorders and global disease burden Implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
6. Hayes RD, Downs J, Chang CK, et al. The effect of clozapine on premature mortality: an assessment of clinical monitoring and other potential confounders. Schizophr Bull. 2015;41(3):644-655.
7. De Fazio P, Gaetano R, Caroleo M, et al. Rare and very rare adverse effects of clozapine. Neuropsychiatr Dis Treat. 2015;11:1995-2003.
8. Novartis Pharmaceuticals Corporation. Clozaril (clozapine). Prescribing information. http://clozaril.com/wp-content/themes/eyesite/pi/Clozaril-2015A507-10022015-Approved.pdf. Accessed June 29, 2016.
9. Lieberman JA, Johns CA, Kane JM, et al. Clozapine-induced agranulocytosis: non-cross-reactivity with other psychotropic drugs. J Clin Psychiatry. 1988;49(7):271-277.
10. Honigfeld G, Arellano F, Sethi J, et al. Reducing clozapine-related morbidity and mortality: 5 years of experience with the Clozaril National Registry. J Clin Psychiatry. 1998;59(suppl 3):3-7.
11. Demler TL, Trigoboff E. Are clozapine patients at risk for blood dyscrasias with concomitant tamiflu use? Psychiatry (Edgmont). 2009;6(11):29-33.
12. Karalakulasingam R, Schacht RA, Lansing AM, et al. Influenza virus pneumonia after renal transplant. Postgrad Med. 1977;62(2):164-167.
13. Hoffman-La Roche Limited. Product monograph: Tamiflu. http://www.rochecanada.com/content/dam/roche_canada/en_CA/documents/Research/ClinicalTrialsForms/Products/ConsumerInformation/MonographsandPublicAdvisories/Tamiflu/Tamiflu_PM_E.pdf. Updated January 26, 2015. Accessed November 28, 2015.
14. Whiskey E, Taylor D. Restarting clozapine after neutropenia: evaluating the possibilities and practicalities. CNS Drugs. 2007;21(1):25-35.
15. Palominao A, Kukoyi O, Xiong GL. Leukocytosis after lithium and clozapine combination therapy. Ann Clin Psychiatry. 2010;22(3):205-206.
16. Focosi D, Azzarà A, Kast RE, et al. Lithium and hematology: established and proposed uses. J Leukoc Biol. 2009;85(1):20-28.
17. Papetti F, Darcourt G, Giordana JY, et al. Treatment of clozapine-induced granulocytopenia with lithium (two observations) [in French]. Encephale. 2004;30(6):578-582.
18. Hummer M, Sperner-Unterweger B, Kemmler G, et al. Does eosinophilia predict clozapine induced neutropenia? Psychopharmacology (Berl). 1996;124(1-2):201-204.
19. Aneja J, Sharma N, Mahajan S, et al. Eosinophilia induced by clozapine: a report of two cases and review of the literature. J Family Med Prim Care. 2015;4(1):127-129.
20. Nielsen J, Correll CU, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603-613.
21. Stryjer R, Timinsky I, Reznik, I, et al. Beta-adrenergic antagonists for the treatment of clozapine-induced sinus tachycardia: a retrospective study. Clin Neuropharmacol. 2009;32(5):290-292.
22. Lally J, Docherty MJ, MacCabe JH. Pharmacological interventions for clozapine-induced sinus tachycardia. Cochrane Database Syst Rev. 2016;9(6):CD011566.
23. Kamphuis H, Arends J, Timmerman L, et al. Myocarditis and cardiomyopathy: underestimated complications resulting from clozapine therapy [in Dutch]. Tijdschr Psychiatr. 2010;52(4):223-233.
24. Alawami M, Wasywich C, Cicovic A, et al. A systematic review of clozapine induced cardiomyopathy. Int J Cardiol. 2014;176(2):315-320.
25. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust N Z J Psychiatry. 2011;45(6):458-465.
26. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
27. Meyer JM. Individual changes in clozapine levels after smoking cessation: results and a predictive model. J Clin Psychopharmacol. 2001;21(6):569-574.
28. Saavedra-Velez C, Yusim A, Anbarasan D, et al. Modafinil as an adjunctive treatment of sedation, negative symptoms, and cognition in schizophrenia: a critical review. J Clin Psychiatry. 2009;70(1):104-112.
29. Klein C, Gordon J, Pollak L, et al. Clozapine in Parkinson’s disease psychosis: 5-year follow-up review. Clin Neuropharmacol. 2003;26(1):8-11.
30. Lutz UC, Sirfy A, Wiatr G, et al. Clozapine serum concentrations in dopamimetic psychosis in Parkinson’s disease and related disorders. Eur J Clin Pharmacol. 2014;70(12):1471-1476.
31. Bird AM, Smith TL, Walton AE. Current treatment strategies for clozapine-induced sialorrhea. Ann Pharmacother. 2011;45(5):667-675.
32. Duggal HS. Glycopyrrolate for clozapine-induced sialorrhea. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1546-1547.
33. Leadbetter R, Shutty M, Pavalonis D, et al. Clozapine-induced weight gain: prevalence and clinical relevance. Am J Psychiatry. 1992;149(1):68-72.
34. Lundblad W, Azzam PN, Gopalan, et al. Medical management of patients on clozapine: a guide for internists. J Hosp Med. 2015;10(8):537-543.
35. Henderson DC, Nguyen DD, Copeland PM, et al. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10-year naturalistic study. J Clin Psychiatry. 2005;66(9):1116-1121.
36. Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166-173.
37. Carrizo E, Fernández V, Connell L, et al. Extended release metformin for metabolic control assistance during prolonged clozapine administration: a 14 week, double-blind, parallel group, placebo-controlled study. Schizophr Res. 2009;113(1):19-26.
38. Chen CH, Huang MC, Kao CF, et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine-treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2013;74(5):e424-e430.
39. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
40. Nielsen J, Meyer JM. Risk factors for ileus in patients with schizophrenia. Schizophr Bull. 2012;38(3):592-598.
41. Lowe CM, Grube RR, Scates AC. Characterization and clinical management of clozapine-induced fever. Ann Pharmacother. 2007;41(10):1700-1704.
42. ACOG Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111(4):1001-1020.
43. U.S. Food and Drug Administration. Pregnancy and Lactation Labeling (Drugs) Final Rule. https://s3.amazonaws.com/public-inspection.federalregister.gov/2014-28241.pdf. Published December 4, 2014. Accessed July 6, 2016.
44. Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation: a reference guide to fetal and neonatal risk. 9th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2011.
45. Larsen ER, Damkier P, Pedersen LH, et al; Danish Psychiatric Society; Danish Society of Obstetrics and Gynecology; Danish Paediatric Society; Danish Society of Clinical Pharmacology. Use of psychotropic drugs during pregnancy and breast-feeding. Acta Psychiatr Scand Suppl. 2015;(445):1-28.
46. McKenna K, Koren G, Tetelbaum M, et al. Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. J Clin Psychiatry. 2005;66(4):444-449.