User login
- In the Breast Cancer Prevention Trial, women taking tamoxifen experienced a 49% overall reduction in invasive breast cancer; the relative risk of endometrial cancer was 4.01 for women over 50 and 1.21 for women younger than 50.
- The risk of serious adverse effects with tamoxifen use appears to be lower in women under age 50.
- Preclinical animal data suggest that, like tamoxifen, raloxifene has potent antiestrogen effects on breast tissue.
- Because aromatase inhibitors block the peripheral conversion of androstenedione to estrogens, they inhibit both initiation and promotion of breast cancer. Thus, they may be more effective than selective estrogen receptor modulators in preventing the disease.
More than 28,000 additional breast cancers could be prevented over 5 years if all eligible women were given tamoxifen.1
This is just one of several important findings highlighted in the studies covered in this review of breast cancer risk assessment and chemopreventive options.
Because Ob/Gyns often treat tamoxifen users who experience uterine bleeding and worry about their risk of endometrial cancer (see “Endometrial screening and tamoxifen users: Going beyond the ACOG opinion,”), it is crucial that we have the latest data on preventive therapies for breast cancer—which include not only tamoxifen, but also, potentially, raloxifene and aromatase inhibitors—so that we may facilitate proper work-up and monitoring.
With the rising use of tamoxifen has come an increased need for vigilance for signs of endometrial cancer. To address the issue, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion on the subject in April 2000.1
In the opinion, ACOG observed that screening tests have not increased the early detection of endometrial cancer in women using tamoxifen and may lead to more invasive and costly diagnostic procedures. ACOG also recommended that Ob/Gyns:
- Educate women taking tamoxifen on the risks of endometrial proliferation, hyperplasia, and cancer and stress the importance of annual gynecologic exams.
- Closely monitor these patients for signs of endometrial hyperplasia or cancer.
- Encourage patients to promptly report abnormal vaginal symptoms, including bloody discharge, spotting, staining, or leukorrhea.
- Investigate any abnormal vaginal bleeding, bloody discharge, spotting, or staining.
- Limit tamoxifen therapy to 5 years, as no benefit has been established beyond this time frame.
- Initiate proper gynecologic management and reassess the use of tamoxifen if the patient develops atypical endometrial hyperplasia.
- Consider resuming tamoxifen therapy following hysterectomy for endometrial carcinoma, in consultation with the physician responsible for the woman’s breast care.1
Screening tests help determine risk. In light of data published over the last 5 years, I now perform endometrial screening on patients about to begin tamoxifen therapy—a practice that differs from the ACOG opinion outlined above. Here is why:
Work published by Berliere et al2 in 1998 and updated in 20003 suggest that, among women on tamoxifen therapy, there exists a group at high risk and a group at low risk for developing complex atypical hyperplasia of the endometrium.
Berliere and her group studied 575 asymptomatic postmenopausal women with recently diagnosed breast cancer about to begin tamoxifen therapy. Each woman received transvaginal ultrasound; if the endometrial echo was greater than 4 mm, office hysteroscopy was performed. Of the study population, 17.4% had initial benign polyps of the endometrium. All polyps were removed, tamoxifen therapy initiated, and follow-up carried out through 5 years.
Among the women with no initial polyp (whom I would classify as “squeaky clean”), 0.7% developed atypical hyperplasia over the 5-year study period—compared with 11.7% of those who had an initial polyp removed. In addition, 11.7% of squeaky clean patients experienced polyp formation, compared with 17.6% of those with initial polyps. Thus, the 17.4% with initial benign polyps had 18 times the risk of the squeaky clean group for developing atypical hyperplasia while on tamoxifen therapy.
These findings have caused me to rethink my approach toward endometrial surveillance for women taking tamoxifen.4
Here is my current practice:
- When patients are diagnosed with breast cancer and scheduled to begin tamoxifen therapy, I perform pretreatment screening.
- If no initial endometrial polyps are found, I follow ACOG’s recommendations. Further interventions are unnecessary (unless abnormal symptoms develop), since these patients are at no more risk for endometrial cancer than women not taking tamoxifen.
- For patients with an initial polyp (ie, the high-risk group), I remove the polyp prior to starting tamoxifen treatment and monitor them throughout the course of therapy, periodically utilizing transvaginal ultrasound and saline infusion sonohysterography.
REFERENCES
1. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion #232: Tamoxifen and Endometrial Cancer. Washington, DC: ACOG; April 2000.
2. Berliere M, Charles A, Galant C, Donnez J. Uterine side effects of tamoxifen: a need for systematic pretreatment screening. Obstet Gynecol. 1998;91(1):40-44.
3. Berliere M, Radikov G, Galant C, Piette P, Marbaix E, Donnez J. Identification of women at high risk of developing endometrial cancer on tamoxifen. Eur J Cancer. 2000;36(suppl 4):S35-S36.
4. Goldstein SR. Controversy about uterine effects and safety of SERMs: the saga continues. Menopause. 2002;9:381-384.
Assessing risk: The need for a new model
Although a number of breast cancer riskassessment models are available based on individual risk factors (TABLE 1), estimates based on combinations of factors are preferable. The Gail model,2 widely used to determine breast cancer risk, takes into account nongenetic (nulliparity, age at menarche) and genetic (family history) factors, as well as the number of previous breast biopsies. It assigns a smaller relative risk to women over age 50. A Web-based version, available at http://bcra.nci.nih.gov/brc, is useful for calculating a woman’s risk of developing invasive disease over the next 5 years, as well as over her remaining lifetime.
Limitations of the Gail model. Unfortunately, the data on which the model is based were collected in the late 1970s and early 1980s. Today, the greater ease of breast histopathologic assessment by fine-needle aspiration and outpatient core-needle biopsy has increased the rate of tissue sampling, creating confusion as to what constitutes a biopsy. Thus, the cutoff of 1.66% for high risk—the threshold adopted for the Breast Cancer Prevention Trial (BCPT)—loses some credibility.
Consider this example: A 50-year-old nulliparous Caucasian woman experienced menarche at age 11, has no first-degree relatives with a history of breast cancer, and has never had a breast biopsy. The Gail model would assign her a risk of developing breast cancer of 1.2% in the next 5 years and 10.8% over her lifetime. However, if the same patient had had 3 breast biopsies, her risk would rise to 1.8% in the next 5 years and 15.8% for her lifetime (placing her in the high-risk category), even if none of the biopsies revealed hyperplasia.
Biomarkers. Objective findings that are patient-specific but which correlate closely with breast cancer development are needed.
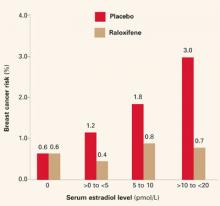
Biomarkers have been proposed; among them: ultrasensitive measurement of serum estradiol levels in postmenopausal women.3 In the Multiple Outcomes of Raloxifene Evaluation (MORE),4 the women who had the greatest reduction in breast cancer during treatment had the highest baseline serum estradiol levels (FIGURE 1)—although the baseline levels of all subjects were well within the postmenopausal range of 20 pmol/L or less.
TABLE 1
Breast cancer risk factors and their relative risks19
| RELATIVE RISK <2 | RELATIVE RISK 2–4 | RELATIVE RISK >4 |
|---|---|---|
|
|
|
FIGURE 1 Breast cancer risk in raloxifene users
Reprinted with permission from: Cummings SR, Duong T, Kenyon E, et al. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220. Copyright © 2002 American Medical Association. All rights reserved.
Chemoprevention: The rationale for tamoxifen
Data from preclinical animal and in vitro studies led to the use of tamoxifen, a selective estrogen receptor modulator (SERM), for primary prevention of breast cancer in healthy women. The drug was shown to inhibit mammary tumors in mice and rats and suppress hormone-dependent breast cancer cell lines in vitro.5
Clinical data from the Early Breast Cancer Trialists Collaborative Group also helped spur prevention trials with tamoxifen.6 Besides decreasing the risk of recurrent breast cancer, tamoxifen reduced the risk of contralateral, new-onset breast cancer by 47% after 5 years of adjuvant treatment (P = .00001) and by 26% after 2 years of treatment (P = .004). This, along with tamoxifen’s favorable effects on skeletal remodeling and lipid levels, led to a series of chemopreventive trials in the United States and Europe (TABLE 2).
TABLE 2
Results of tamoxifen trials
| BREAST CANCER PREVENTION TRIAL7 | ROYAL MARSDEN10 | ITALIAN11 | |
|---|---|---|---|
| Number of participants | 13,388 | 2,471 | 5,408 |
| Age ≤50 | 40% | 62% | 36% |
| One first-degree relative with breast cancer | 55% | 55% | 18% |
| >2 first-degree relatives with breast cancer | 13% | 17% | 2.5% |
| HRT users | 0% | 42% | 8% |
| Woman-years of follow-up | 46,858 | 12,355 | 20,731 |
| Cancer incidence/1,000 | |||
| Placebo | 6.8 | 5.0 | 2.3 |
| Tamoxifen | 3.4 | 4.7 | 2.1 |
Breast Cancer Prevention Trial: Tamoxifen reduces cancer incidence
In 1992, the National Surgical Adjuvant Breast and Bowel Project launched a prevention trial using tamoxifen: the Breast Cancer Prevention Trial.7 A total of 13,388 women aged 35 or older and at high risk for breast cancer were enrolled at numerous sites throughout the United States and Canada.
The Gail model was utilized to determine which women had sufficient risk—that is, a risk of developing invasive breast cancer within the next 5 years of 1.66% or greater—to be included in the trial.2 Subjects were randomly assigned to receive placebo or tamoxifen (20 mg/d) for 5 years.
The trial was terminated in April 1998, 14 months before its planned completion, due to the striking reduction in new-onset breast cancer in the tamoxifen group. The data safety monitoring board felt it would be unethical to allow one half of the participants, who were deemed to be at high risk, to continue taking placebo in light of the dramatic reduction in both invasive and noninvasive breast cancer with tamoxifen use.
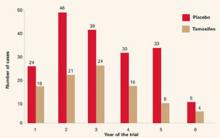
The overall incidence of breast cancer in the tamoxifen group was 3.4 cases per 1,000, compared with 6.8 cases per 1,000 in the placebo group. Overall, the reduction in invasive breast cancer was 49% (P<.000001). The reductions were 44% for women in the group aged 35 to 49 years, 51% for those aged 50 to 59, and 55% for those 60 years and older (FIGURE 2).
Tamoxifen decreased the incidence of noninvasive breast cancer (ductal carcinoma in situ) by 50%. (Expanded use of mammography has led to greater detection of this cancer. Most such lesions are estrogen-receptor–positive.8) In addition, tamoxifen reduced breast cancer risk in women with a history of lobular carcinoma in situ by 56% and atypical hyperplasia by 86%. Overall, tamoxifen decreased the occurrence of estrogen-receptor–positive tumors by 69%, but had no impact on tumors that were estrogen-receptor–negative.
Tamoxifen’s other effects in healthy women. The BCPT offered the first largescale data on the effects of tamoxifen in healthy women.7 (All previous studies included only women with breast cancer.) Several secondary endpoints merit consideration.
- Endometrial cancer risk. Researchers found the relative risk (RR) of endometrial cancer associated with tamoxifen therapy in healthy women was 2.53 (95% confidence intervals [CI], 1.35, 4.97). When this figure was calculated for the different age groups, it rose to 4.01 (95% CI, 1.70, 10.90) in women over 50, and declined to 1.21 for women ages 49 and under (95% CI, 0.41, 3.60).
- Thromboembolic event risk. The same age distinction was seen in relation to thromboembolic events. There were no statistically significant increases in pulmonary emboli or deep venous thrombosis in women 49 years of age or under. Although it is unclear whether the trial was sufficiently powered for this particular endpoint, the likelihood that serious adverse events will limit the potential benefits of tamoxifen appears to be lower in women under the age of 50. This has significant clinical consequences for physicians caring for perimenopausal patients.
- No change in incidence of other cancers. Overall, the incidence of invasive cancers other than those of the breast and uterus was the same for the tamoxifen and placebo groups.
- Other outcomes. The relative risk of death from any cause was 0.81 (95% CI, 0.56–1.16).
There was a slight increase in the risk of myocardial infarction (RR, 1.11; 95% CI, 0.65–1.92) and a slight decrease in the development of severe angina (RR, 0.93; 95% CI, 0.40–2.14) in tamoxifen users, although neither of these was statistically significant.
The overall relative risk of fractures at various sites (hip, spine, radius) was 0.81 (95% CI, 0.63–1.05).
A statistically significant increase was found in the number of women with cataracts who then underwent cataract surgery. That relative risk was 1.57 (95% CI, 1.16–2.14).
FIGURE 2 Breast Cancer Prevention Trial: Tamoxifen reduces incidence of breast cancer
The rate of cancer reduction for tamoxifen compared with placebo for years 1 through 6 was 33%, 55%, 39%, 49%, 69%, and 55%, respectively.
Reprinted from Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst.1998;90(18):1371-1388, by permission of Oxford University Press.
FDA approves tamoxifen for primary prevention
Based on the BCPT results, the US Food and Drug Administration (FDA) approved tamoxifen in October 1998 for primary prevention of breast cancer in women at high risk for the disease. It recommended that tamoxifen be limited to high-risk women because of the potentially serious side effects seen in clinical trials.
The FDA did not define high risk, but recommended that prophylactic use of tamoxifen be based on a thorough evaluation of a woman’s personal, family, and medical histories, as well as her age and understanding of the risks and benefits of treatment.
In addition, the FDA required that the package insert advise women to consult a health-care professional for breast cancer risk assessment and state that only women at high risk should take the drug (again, without defining high risk).
In 2002, the FDA added a “black box” warning to tamoxifen labeling that was directed at use of the drug for prevention rather than treatment. This warning concerned the occurrence of uterine sarcomas. The incidence of these cancers was found to be 0.17 per 1,000 women taking tamoxifen, compared with 0.015 per 1,000 controls.9
Underuse of tamoxifen? A recent study by Freedman et al1 calculated the number of women 35 to 79 years of age who were eligible for tamoxifen chemoprevention based on FDA criteria. They further calculated the number of women who would have a positive benefit-risk ratio for tamoxifen use, and concluded that, among white women alone, roughly 28,492 additional breast cancers could be prevented or deferred if these individuals took tamoxifen for the next 5 years.
European trials fail to confirm benefit of tamoxifen
The Royal Marsden Trial was one of 2 European trials that failed to replicate the BCPT results.10 This British study involved 2,471 healthy women between the ages of 30 and 70 who had a family history of breast cancer. The immediate follow-up was 70 months. No statistically significant decrease in breast cancer was found with tamoxifen use, compared with placebo.
One possibility for the discrepancy may be that eligibility for the Royal Marsden Trial was based predominantly on a strong family history of breast cancer. It also included a much larger proportion of women under age 50 (62%, compared with only 40% in the BCPT). Probably most importantly, 42% of the women received hormone replacement therapy (HRT) along with tamoxifen during the trial.
The Italian prevention study also failed to confirm the findings of the BCPT.11 Because it recruited participants from the general population, the overall risk of breast cancer was significantly lower than in the BCPT. Compliance rates in the Italian study were quite low: Approximately 26% of the subjects dropped out. Furthermore, the Italian trial included considerably fewer women over age 60 (12% versus 30% in the BCPT).
Raloxifene: Another cancer preventive?
Like tamoxifen, raloxifene is a SERM. It is a benzothiophene derivative; tamoxifen comes from the triphenylethylene family.
Like tamoxifen, raloxifene was originally investigated as a treatment for advanced breast cancer. Preclinical studies indicated that it had an antiproliferative effect on estrogen-receptor–positive mammary tumors and estrogen-receptor–positive human breast cancer cell lines.12 However, in the 1980s, a small phase II trial revealed that it had no further antitumor effects in postmenopausal women with advanced breast cancer in whom tamoxifen therapy had failed.13
Interest in raloxifene revived after tamoxifen’s neoplastic effects on the uteri of postmenopausal women became evident.14 As a SERM, raloxifene exhibits estrogen-agonist activity on bone remodeling and lipid metabolism. In December 1997, it won FDA approval for the prevention of osteoporosis in postmenopausal women. Its indication was extended to treatment in October 1999.
Studies found that raloxifene was similar to placebo in its effects on the endometrium of postmenopausal women.15 There were no differences in endometrial thickness, endoluminal masses, proliferation, or hyperplasia. This corroborated previous findings that raloxifene causes neither endometrial hyperplasia nor cancer and is not associated with vaginal bleeding or increased endometrial thickness (as measured by transvaginal ultrasound).
In addition, preclinical animal data suggest that, like tamoxifen, raloxifene has potent antiestrogen effects on breast tissue.12
The MORE trial involved 7,705 postmenopausal women up to age 80 with established osteoporosis who were randomized to receive raloxifene or placebo. Bone mineral density and fracture incidence were the primary endpoints; breast cancer was a secondary endpoint. At 4 years, raloxifene significantly reduced the incidence of all invasive breast cancers by 72%, compared with placebo (RR = 0.28; 95% CI, 0.17–0.46).16 It reduced the incidence of invasive estrogen-receptor–positive tumors by 84%, compared with placebo (RR = 0.16; 95% CI, 0.09–0.30), but had no effect on estrogen-receptor–negative tumors.
Because they block both initiation and promotion of breast cancer, aromatase inhibitors may be more effective than SERMs in preventing breast cancer.
The incidence of vaginal bleeding, breast pain, and endometrial cancer in the raloxifene group did not differ significantly from those of the placebo group. (Because women in the MORE trial were older and did not enter the study with an increased risk for breast cancer, these findings are not necessarily applicable to younger, high-risk women.)
Like tamoxifen, raloxifene slightly increased the risk of thromboembolic disease, including deep vein thrombosis. Pulmonary embolism developed in 1.1% of women in the raloxifene group, compared with 0.5% of subjects in the placebo group (P = .003).
Currently, there is no approved indication for raloxifene in premenopausal women. SERM compounds, which are structurally similar to clomiphene citrate, seem to have different effects in premenopausal and postmenopausal women, as tamoxifen did in the BCPT. Further study in the premenopausal population or with concomitant use of lowdose estrogen may be forthcoming.
Ongoing clinical trials
STAR. To directly compare the safety and efficacy of tamoxifen and raloxifene in reducing breast cancer risk among healthy women, the Study of Tamoxifen and Raloxifene (STAR) has been enrolling postmenopausal women 35 years of age or older who are at increased risk for breast cancer. This study began in 1999 and is expected to run for at least 7 years. It seeks to enroll 22,000 participants in its randomized, double-blind investigation. Participants will receive a daily dose of raloxifene (60 mg) or tamoxifen (20 mg).
RUTH. Raloxifene Use and the Heart (RUTH) is a double-blind, placebo-controlled trial of 60 mg of raloxifene that will include 10,000 women. Primary endpoints are coronary disease and invasive breast cancer. Trial enrollment ended in August 2000, and the study is expected to conclude in 2006.
Aromatase inhibitors: Another route of prevention
The evidence that estrogens facilitate breast cancer development in animals and women is substantial, although the precise mechanism is unknown.17 The most commonly held theory is that estrogen stimulates proliferation of breast cells. Thus, it statistically increases the chances for genetic mutations that could result in cancer.
Aromatase inhibitors block peripheral conversion of androstenedione to estrogens. In premenopausal patients, the primary site of this action is in the ovary. In post-menopausal women, it occurs predominantly in extraovarian sites, including the adrenal glands, adipose tissue, liver, muscle, and skin.
Because of their dual role, (blocking both the initiation and promotion of breast cancer), aromatase inhibitors may be more effective than SERMs in preventing breast cancer.18 By inhibiting the initiation process, they would reduce levels of genotoxic metabolites of estradiol by lowering estradiol concentration in tissue. They also would inhibit tumor promotion by lowering tissue levels of estradiol—thus blocking cell proliferation. However, because they are not selective, aromatase inhibitors would have an antiestrogen effect on bone and lipid metabolism and would induce vasomotor symptoms.
Dr. Goldstein serves on gynecology advisory boards for Eli Lilly and Company, Pfizer, AstraZeneca, and P&G Pharmaceuticals.
1. Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst. 2003;95:526-532.
2. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
3. Ruffin MT, August DA, Kelloff GJ, Boone CW, Weber BL, Brenner DE. Selection criteria for breast cancer chemoprevention subjects. J Cell Biochem Suppl. 1993;17G:234-241.
4. Cummings SR, Duong T, Kenyon E, et al. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220.
5. Jordan VC, Allen KE. Evaluation of the antitumor activity of the nonsteroidal antiestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma mode. Eur J Cancer. 1980;16:239-251.
6. Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer: an overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988;319:1681-1692.
7. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
8. Bur ME, Zimarowski MJ, Schnitt SJ, Baker S, Lew R. Estrogen receptor immunohistochemistry in carcinoma in situ of the breast. Cancer. 1992;69:1174-1181.
9. Nolvadex [package insert]. Wilmington, Del: AstraZeneca Pharmaceuticals; 2002.
10. Powles T, Eeles R, Ashley S, et al. Interim analysis of incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomized chemoprevention trial. Lancet. 1998;352:98-101.
11. Veronesi U, Maisonneuve P, Costa A, et al. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomized trial among hysterectomized women. Italian Prevention Study. Lancet. 1998;352:93-97.
12. Hol T, Cox MB, Bryant HU, Draper MW. Selective estrogen receptor modulators and postmenopausal women’s health. J Women’s Health. 1997;6:523-531.
13. Buzdar AU, Marcus C, Holmes F, Hug V, Hortobagyi G. Phase II evaluation of LY156758 in metastatic breast cancer. Oncology. 1988;45:344-345.
14. Neven P, Muylder X, Van Belle Y, Vanderick G, De Mylder E. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
15. Goldstein SR, Scheele WH, Rajagopalan SK, Wilke JL, Walsh BW, Parsons AK. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
16. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Breast Cancer Res Treat. 2001;65:125-134.
17. Santen RJ, Yue W, Nftolin F, Mor G, Berstein L. The potential of aromatase inhibitors in breast cancer prevention. Endocrine-Related Cancer. 1999;6:235-243.
18. Goss PE, Strasser K. Aromatase inhibitors in the treatment and prevention of breast cancer. J Clin Oncol. 2001;19:881-894.
19. Bilimoria MM, Morrow M. The woman at increased risk for breast cancer: evaluation and management strategies. CA Cancer J Clin. 1995;45:263-278.
- In the Breast Cancer Prevention Trial, women taking tamoxifen experienced a 49% overall reduction in invasive breast cancer; the relative risk of endometrial cancer was 4.01 for women over 50 and 1.21 for women younger than 50.
- The risk of serious adverse effects with tamoxifen use appears to be lower in women under age 50.
- Preclinical animal data suggest that, like tamoxifen, raloxifene has potent antiestrogen effects on breast tissue.
- Because aromatase inhibitors block the peripheral conversion of androstenedione to estrogens, they inhibit both initiation and promotion of breast cancer. Thus, they may be more effective than selective estrogen receptor modulators in preventing the disease.
More than 28,000 additional breast cancers could be prevented over 5 years if all eligible women were given tamoxifen.1
This is just one of several important findings highlighted in the studies covered in this review of breast cancer risk assessment and chemopreventive options.
Because Ob/Gyns often treat tamoxifen users who experience uterine bleeding and worry about their risk of endometrial cancer (see “Endometrial screening and tamoxifen users: Going beyond the ACOG opinion,”), it is crucial that we have the latest data on preventive therapies for breast cancer—which include not only tamoxifen, but also, potentially, raloxifene and aromatase inhibitors—so that we may facilitate proper work-up and monitoring.
With the rising use of tamoxifen has come an increased need for vigilance for signs of endometrial cancer. To address the issue, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion on the subject in April 2000.1
In the opinion, ACOG observed that screening tests have not increased the early detection of endometrial cancer in women using tamoxifen and may lead to more invasive and costly diagnostic procedures. ACOG also recommended that Ob/Gyns:
- Educate women taking tamoxifen on the risks of endometrial proliferation, hyperplasia, and cancer and stress the importance of annual gynecologic exams.
- Closely monitor these patients for signs of endometrial hyperplasia or cancer.
- Encourage patients to promptly report abnormal vaginal symptoms, including bloody discharge, spotting, staining, or leukorrhea.
- Investigate any abnormal vaginal bleeding, bloody discharge, spotting, or staining.
- Limit tamoxifen therapy to 5 years, as no benefit has been established beyond this time frame.
- Initiate proper gynecologic management and reassess the use of tamoxifen if the patient develops atypical endometrial hyperplasia.
- Consider resuming tamoxifen therapy following hysterectomy for endometrial carcinoma, in consultation with the physician responsible for the woman’s breast care.1
Screening tests help determine risk. In light of data published over the last 5 years, I now perform endometrial screening on patients about to begin tamoxifen therapy—a practice that differs from the ACOG opinion outlined above. Here is why:
Work published by Berliere et al2 in 1998 and updated in 20003 suggest that, among women on tamoxifen therapy, there exists a group at high risk and a group at low risk for developing complex atypical hyperplasia of the endometrium.
Berliere and her group studied 575 asymptomatic postmenopausal women with recently diagnosed breast cancer about to begin tamoxifen therapy. Each woman received transvaginal ultrasound; if the endometrial echo was greater than 4 mm, office hysteroscopy was performed. Of the study population, 17.4% had initial benign polyps of the endometrium. All polyps were removed, tamoxifen therapy initiated, and follow-up carried out through 5 years.
Among the women with no initial polyp (whom I would classify as “squeaky clean”), 0.7% developed atypical hyperplasia over the 5-year study period—compared with 11.7% of those who had an initial polyp removed. In addition, 11.7% of squeaky clean patients experienced polyp formation, compared with 17.6% of those with initial polyps. Thus, the 17.4% with initial benign polyps had 18 times the risk of the squeaky clean group for developing atypical hyperplasia while on tamoxifen therapy.
These findings have caused me to rethink my approach toward endometrial surveillance for women taking tamoxifen.4
Here is my current practice:
- When patients are diagnosed with breast cancer and scheduled to begin tamoxifen therapy, I perform pretreatment screening.
- If no initial endometrial polyps are found, I follow ACOG’s recommendations. Further interventions are unnecessary (unless abnormal symptoms develop), since these patients are at no more risk for endometrial cancer than women not taking tamoxifen.
- For patients with an initial polyp (ie, the high-risk group), I remove the polyp prior to starting tamoxifen treatment and monitor them throughout the course of therapy, periodically utilizing transvaginal ultrasound and saline infusion sonohysterography.
REFERENCES
1. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion #232: Tamoxifen and Endometrial Cancer. Washington, DC: ACOG; April 2000.
2. Berliere M, Charles A, Galant C, Donnez J. Uterine side effects of tamoxifen: a need for systematic pretreatment screening. Obstet Gynecol. 1998;91(1):40-44.
3. Berliere M, Radikov G, Galant C, Piette P, Marbaix E, Donnez J. Identification of women at high risk of developing endometrial cancer on tamoxifen. Eur J Cancer. 2000;36(suppl 4):S35-S36.
4. Goldstein SR. Controversy about uterine effects and safety of SERMs: the saga continues. Menopause. 2002;9:381-384.
Assessing risk: The need for a new model
Although a number of breast cancer riskassessment models are available based on individual risk factors (TABLE 1), estimates based on combinations of factors are preferable. The Gail model,2 widely used to determine breast cancer risk, takes into account nongenetic (nulliparity, age at menarche) and genetic (family history) factors, as well as the number of previous breast biopsies. It assigns a smaller relative risk to women over age 50. A Web-based version, available at http://bcra.nci.nih.gov/brc, is useful for calculating a woman’s risk of developing invasive disease over the next 5 years, as well as over her remaining lifetime.
Limitations of the Gail model. Unfortunately, the data on which the model is based were collected in the late 1970s and early 1980s. Today, the greater ease of breast histopathologic assessment by fine-needle aspiration and outpatient core-needle biopsy has increased the rate of tissue sampling, creating confusion as to what constitutes a biopsy. Thus, the cutoff of 1.66% for high risk—the threshold adopted for the Breast Cancer Prevention Trial (BCPT)—loses some credibility.
Consider this example: A 50-year-old nulliparous Caucasian woman experienced menarche at age 11, has no first-degree relatives with a history of breast cancer, and has never had a breast biopsy. The Gail model would assign her a risk of developing breast cancer of 1.2% in the next 5 years and 10.8% over her lifetime. However, if the same patient had had 3 breast biopsies, her risk would rise to 1.8% in the next 5 years and 15.8% for her lifetime (placing her in the high-risk category), even if none of the biopsies revealed hyperplasia.
Biomarkers. Objective findings that are patient-specific but which correlate closely with breast cancer development are needed.
Biomarkers have been proposed; among them: ultrasensitive measurement of serum estradiol levels in postmenopausal women.3 In the Multiple Outcomes of Raloxifene Evaluation (MORE),4 the women who had the greatest reduction in breast cancer during treatment had the highest baseline serum estradiol levels (FIGURE 1)—although the baseline levels of all subjects were well within the postmenopausal range of 20 pmol/L or less.
TABLE 1
Breast cancer risk factors and their relative risks19
| RELATIVE RISK <2 | RELATIVE RISK 2–4 | RELATIVE RISK >4 |
|---|---|---|
|
|
|
FIGURE 1 Breast cancer risk in raloxifene users
Reprinted with permission from: Cummings SR, Duong T, Kenyon E, et al. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220. Copyright © 2002 American Medical Association. All rights reserved.
Chemoprevention: The rationale for tamoxifen
Data from preclinical animal and in vitro studies led to the use of tamoxifen, a selective estrogen receptor modulator (SERM), for primary prevention of breast cancer in healthy women. The drug was shown to inhibit mammary tumors in mice and rats and suppress hormone-dependent breast cancer cell lines in vitro.5
Clinical data from the Early Breast Cancer Trialists Collaborative Group also helped spur prevention trials with tamoxifen.6 Besides decreasing the risk of recurrent breast cancer, tamoxifen reduced the risk of contralateral, new-onset breast cancer by 47% after 5 years of adjuvant treatment (P = .00001) and by 26% after 2 years of treatment (P = .004). This, along with tamoxifen’s favorable effects on skeletal remodeling and lipid levels, led to a series of chemopreventive trials in the United States and Europe (TABLE 2).
TABLE 2
Results of tamoxifen trials
| BREAST CANCER PREVENTION TRIAL7 | ROYAL MARSDEN10 | ITALIAN11 | |
|---|---|---|---|
| Number of participants | 13,388 | 2,471 | 5,408 |
| Age ≤50 | 40% | 62% | 36% |
| One first-degree relative with breast cancer | 55% | 55% | 18% |
| >2 first-degree relatives with breast cancer | 13% | 17% | 2.5% |
| HRT users | 0% | 42% | 8% |
| Woman-years of follow-up | 46,858 | 12,355 | 20,731 |
| Cancer incidence/1,000 | |||
| Placebo | 6.8 | 5.0 | 2.3 |
| Tamoxifen | 3.4 | 4.7 | 2.1 |
Breast Cancer Prevention Trial: Tamoxifen reduces cancer incidence
In 1992, the National Surgical Adjuvant Breast and Bowel Project launched a prevention trial using tamoxifen: the Breast Cancer Prevention Trial.7 A total of 13,388 women aged 35 or older and at high risk for breast cancer were enrolled at numerous sites throughout the United States and Canada.
The Gail model was utilized to determine which women had sufficient risk—that is, a risk of developing invasive breast cancer within the next 5 years of 1.66% or greater—to be included in the trial.2 Subjects were randomly assigned to receive placebo or tamoxifen (20 mg/d) for 5 years.
The trial was terminated in April 1998, 14 months before its planned completion, due to the striking reduction in new-onset breast cancer in the tamoxifen group. The data safety monitoring board felt it would be unethical to allow one half of the participants, who were deemed to be at high risk, to continue taking placebo in light of the dramatic reduction in both invasive and noninvasive breast cancer with tamoxifen use.
The overall incidence of breast cancer in the tamoxifen group was 3.4 cases per 1,000, compared with 6.8 cases per 1,000 in the placebo group. Overall, the reduction in invasive breast cancer was 49% (P<.000001). The reductions were 44% for women in the group aged 35 to 49 years, 51% for those aged 50 to 59, and 55% for those 60 years and older (FIGURE 2).
Tamoxifen decreased the incidence of noninvasive breast cancer (ductal carcinoma in situ) by 50%. (Expanded use of mammography has led to greater detection of this cancer. Most such lesions are estrogen-receptor–positive.8) In addition, tamoxifen reduced breast cancer risk in women with a history of lobular carcinoma in situ by 56% and atypical hyperplasia by 86%. Overall, tamoxifen decreased the occurrence of estrogen-receptor–positive tumors by 69%, but had no impact on tumors that were estrogen-receptor–negative.
Tamoxifen’s other effects in healthy women. The BCPT offered the first largescale data on the effects of tamoxifen in healthy women.7 (All previous studies included only women with breast cancer.) Several secondary endpoints merit consideration.
- Endometrial cancer risk. Researchers found the relative risk (RR) of endometrial cancer associated with tamoxifen therapy in healthy women was 2.53 (95% confidence intervals [CI], 1.35, 4.97). When this figure was calculated for the different age groups, it rose to 4.01 (95% CI, 1.70, 10.90) in women over 50, and declined to 1.21 for women ages 49 and under (95% CI, 0.41, 3.60).
- Thromboembolic event risk. The same age distinction was seen in relation to thromboembolic events. There were no statistically significant increases in pulmonary emboli or deep venous thrombosis in women 49 years of age or under. Although it is unclear whether the trial was sufficiently powered for this particular endpoint, the likelihood that serious adverse events will limit the potential benefits of tamoxifen appears to be lower in women under the age of 50. This has significant clinical consequences for physicians caring for perimenopausal patients.
- No change in incidence of other cancers. Overall, the incidence of invasive cancers other than those of the breast and uterus was the same for the tamoxifen and placebo groups.
- Other outcomes. The relative risk of death from any cause was 0.81 (95% CI, 0.56–1.16).
There was a slight increase in the risk of myocardial infarction (RR, 1.11; 95% CI, 0.65–1.92) and a slight decrease in the development of severe angina (RR, 0.93; 95% CI, 0.40–2.14) in tamoxifen users, although neither of these was statistically significant.
The overall relative risk of fractures at various sites (hip, spine, radius) was 0.81 (95% CI, 0.63–1.05).
A statistically significant increase was found in the number of women with cataracts who then underwent cataract surgery. That relative risk was 1.57 (95% CI, 1.16–2.14).
FIGURE 2 Breast Cancer Prevention Trial: Tamoxifen reduces incidence of breast cancer
The rate of cancer reduction for tamoxifen compared with placebo for years 1 through 6 was 33%, 55%, 39%, 49%, 69%, and 55%, respectively.
Reprinted from Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst.1998;90(18):1371-1388, by permission of Oxford University Press.
FDA approves tamoxifen for primary prevention
Based on the BCPT results, the US Food and Drug Administration (FDA) approved tamoxifen in October 1998 for primary prevention of breast cancer in women at high risk for the disease. It recommended that tamoxifen be limited to high-risk women because of the potentially serious side effects seen in clinical trials.
The FDA did not define high risk, but recommended that prophylactic use of tamoxifen be based on a thorough evaluation of a woman’s personal, family, and medical histories, as well as her age and understanding of the risks and benefits of treatment.
In addition, the FDA required that the package insert advise women to consult a health-care professional for breast cancer risk assessment and state that only women at high risk should take the drug (again, without defining high risk).
In 2002, the FDA added a “black box” warning to tamoxifen labeling that was directed at use of the drug for prevention rather than treatment. This warning concerned the occurrence of uterine sarcomas. The incidence of these cancers was found to be 0.17 per 1,000 women taking tamoxifen, compared with 0.015 per 1,000 controls.9
Underuse of tamoxifen? A recent study by Freedman et al1 calculated the number of women 35 to 79 years of age who were eligible for tamoxifen chemoprevention based on FDA criteria. They further calculated the number of women who would have a positive benefit-risk ratio for tamoxifen use, and concluded that, among white women alone, roughly 28,492 additional breast cancers could be prevented or deferred if these individuals took tamoxifen for the next 5 years.
European trials fail to confirm benefit of tamoxifen
The Royal Marsden Trial was one of 2 European trials that failed to replicate the BCPT results.10 This British study involved 2,471 healthy women between the ages of 30 and 70 who had a family history of breast cancer. The immediate follow-up was 70 months. No statistically significant decrease in breast cancer was found with tamoxifen use, compared with placebo.
One possibility for the discrepancy may be that eligibility for the Royal Marsden Trial was based predominantly on a strong family history of breast cancer. It also included a much larger proportion of women under age 50 (62%, compared with only 40% in the BCPT). Probably most importantly, 42% of the women received hormone replacement therapy (HRT) along with tamoxifen during the trial.
The Italian prevention study also failed to confirm the findings of the BCPT.11 Because it recruited participants from the general population, the overall risk of breast cancer was significantly lower than in the BCPT. Compliance rates in the Italian study were quite low: Approximately 26% of the subjects dropped out. Furthermore, the Italian trial included considerably fewer women over age 60 (12% versus 30% in the BCPT).
Raloxifene: Another cancer preventive?
Like tamoxifen, raloxifene is a SERM. It is a benzothiophene derivative; tamoxifen comes from the triphenylethylene family.
Like tamoxifen, raloxifene was originally investigated as a treatment for advanced breast cancer. Preclinical studies indicated that it had an antiproliferative effect on estrogen-receptor–positive mammary tumors and estrogen-receptor–positive human breast cancer cell lines.12 However, in the 1980s, a small phase II trial revealed that it had no further antitumor effects in postmenopausal women with advanced breast cancer in whom tamoxifen therapy had failed.13
Interest in raloxifene revived after tamoxifen’s neoplastic effects on the uteri of postmenopausal women became evident.14 As a SERM, raloxifene exhibits estrogen-agonist activity on bone remodeling and lipid metabolism. In December 1997, it won FDA approval for the prevention of osteoporosis in postmenopausal women. Its indication was extended to treatment in October 1999.
Studies found that raloxifene was similar to placebo in its effects on the endometrium of postmenopausal women.15 There were no differences in endometrial thickness, endoluminal masses, proliferation, or hyperplasia. This corroborated previous findings that raloxifene causes neither endometrial hyperplasia nor cancer and is not associated with vaginal bleeding or increased endometrial thickness (as measured by transvaginal ultrasound).
In addition, preclinical animal data suggest that, like tamoxifen, raloxifene has potent antiestrogen effects on breast tissue.12
The MORE trial involved 7,705 postmenopausal women up to age 80 with established osteoporosis who were randomized to receive raloxifene or placebo. Bone mineral density and fracture incidence were the primary endpoints; breast cancer was a secondary endpoint. At 4 years, raloxifene significantly reduced the incidence of all invasive breast cancers by 72%, compared with placebo (RR = 0.28; 95% CI, 0.17–0.46).16 It reduced the incidence of invasive estrogen-receptor–positive tumors by 84%, compared with placebo (RR = 0.16; 95% CI, 0.09–0.30), but had no effect on estrogen-receptor–negative tumors.
Because they block both initiation and promotion of breast cancer, aromatase inhibitors may be more effective than SERMs in preventing breast cancer.
The incidence of vaginal bleeding, breast pain, and endometrial cancer in the raloxifene group did not differ significantly from those of the placebo group. (Because women in the MORE trial were older and did not enter the study with an increased risk for breast cancer, these findings are not necessarily applicable to younger, high-risk women.)
Like tamoxifen, raloxifene slightly increased the risk of thromboembolic disease, including deep vein thrombosis. Pulmonary embolism developed in 1.1% of women in the raloxifene group, compared with 0.5% of subjects in the placebo group (P = .003).
Currently, there is no approved indication for raloxifene in premenopausal women. SERM compounds, which are structurally similar to clomiphene citrate, seem to have different effects in premenopausal and postmenopausal women, as tamoxifen did in the BCPT. Further study in the premenopausal population or with concomitant use of lowdose estrogen may be forthcoming.
Ongoing clinical trials
STAR. To directly compare the safety and efficacy of tamoxifen and raloxifene in reducing breast cancer risk among healthy women, the Study of Tamoxifen and Raloxifene (STAR) has been enrolling postmenopausal women 35 years of age or older who are at increased risk for breast cancer. This study began in 1999 and is expected to run for at least 7 years. It seeks to enroll 22,000 participants in its randomized, double-blind investigation. Participants will receive a daily dose of raloxifene (60 mg) or tamoxifen (20 mg).
RUTH. Raloxifene Use and the Heart (RUTH) is a double-blind, placebo-controlled trial of 60 mg of raloxifene that will include 10,000 women. Primary endpoints are coronary disease and invasive breast cancer. Trial enrollment ended in August 2000, and the study is expected to conclude in 2006.
Aromatase inhibitors: Another route of prevention
The evidence that estrogens facilitate breast cancer development in animals and women is substantial, although the precise mechanism is unknown.17 The most commonly held theory is that estrogen stimulates proliferation of breast cells. Thus, it statistically increases the chances for genetic mutations that could result in cancer.
Aromatase inhibitors block peripheral conversion of androstenedione to estrogens. In premenopausal patients, the primary site of this action is in the ovary. In post-menopausal women, it occurs predominantly in extraovarian sites, including the adrenal glands, adipose tissue, liver, muscle, and skin.
Because of their dual role, (blocking both the initiation and promotion of breast cancer), aromatase inhibitors may be more effective than SERMs in preventing breast cancer.18 By inhibiting the initiation process, they would reduce levels of genotoxic metabolites of estradiol by lowering estradiol concentration in tissue. They also would inhibit tumor promotion by lowering tissue levels of estradiol—thus blocking cell proliferation. However, because they are not selective, aromatase inhibitors would have an antiestrogen effect on bone and lipid metabolism and would induce vasomotor symptoms.
Dr. Goldstein serves on gynecology advisory boards for Eli Lilly and Company, Pfizer, AstraZeneca, and P&G Pharmaceuticals.
- In the Breast Cancer Prevention Trial, women taking tamoxifen experienced a 49% overall reduction in invasive breast cancer; the relative risk of endometrial cancer was 4.01 for women over 50 and 1.21 for women younger than 50.
- The risk of serious adverse effects with tamoxifen use appears to be lower in women under age 50.
- Preclinical animal data suggest that, like tamoxifen, raloxifene has potent antiestrogen effects on breast tissue.
- Because aromatase inhibitors block the peripheral conversion of androstenedione to estrogens, they inhibit both initiation and promotion of breast cancer. Thus, they may be more effective than selective estrogen receptor modulators in preventing the disease.
More than 28,000 additional breast cancers could be prevented over 5 years if all eligible women were given tamoxifen.1
This is just one of several important findings highlighted in the studies covered in this review of breast cancer risk assessment and chemopreventive options.
Because Ob/Gyns often treat tamoxifen users who experience uterine bleeding and worry about their risk of endometrial cancer (see “Endometrial screening and tamoxifen users: Going beyond the ACOG opinion,”), it is crucial that we have the latest data on preventive therapies for breast cancer—which include not only tamoxifen, but also, potentially, raloxifene and aromatase inhibitors—so that we may facilitate proper work-up and monitoring.
With the rising use of tamoxifen has come an increased need for vigilance for signs of endometrial cancer. To address the issue, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion on the subject in April 2000.1
In the opinion, ACOG observed that screening tests have not increased the early detection of endometrial cancer in women using tamoxifen and may lead to more invasive and costly diagnostic procedures. ACOG also recommended that Ob/Gyns:
- Educate women taking tamoxifen on the risks of endometrial proliferation, hyperplasia, and cancer and stress the importance of annual gynecologic exams.
- Closely monitor these patients for signs of endometrial hyperplasia or cancer.
- Encourage patients to promptly report abnormal vaginal symptoms, including bloody discharge, spotting, staining, or leukorrhea.
- Investigate any abnormal vaginal bleeding, bloody discharge, spotting, or staining.
- Limit tamoxifen therapy to 5 years, as no benefit has been established beyond this time frame.
- Initiate proper gynecologic management and reassess the use of tamoxifen if the patient develops atypical endometrial hyperplasia.
- Consider resuming tamoxifen therapy following hysterectomy for endometrial carcinoma, in consultation with the physician responsible for the woman’s breast care.1
Screening tests help determine risk. In light of data published over the last 5 years, I now perform endometrial screening on patients about to begin tamoxifen therapy—a practice that differs from the ACOG opinion outlined above. Here is why:
Work published by Berliere et al2 in 1998 and updated in 20003 suggest that, among women on tamoxifen therapy, there exists a group at high risk and a group at low risk for developing complex atypical hyperplasia of the endometrium.
Berliere and her group studied 575 asymptomatic postmenopausal women with recently diagnosed breast cancer about to begin tamoxifen therapy. Each woman received transvaginal ultrasound; if the endometrial echo was greater than 4 mm, office hysteroscopy was performed. Of the study population, 17.4% had initial benign polyps of the endometrium. All polyps were removed, tamoxifen therapy initiated, and follow-up carried out through 5 years.
Among the women with no initial polyp (whom I would classify as “squeaky clean”), 0.7% developed atypical hyperplasia over the 5-year study period—compared with 11.7% of those who had an initial polyp removed. In addition, 11.7% of squeaky clean patients experienced polyp formation, compared with 17.6% of those with initial polyps. Thus, the 17.4% with initial benign polyps had 18 times the risk of the squeaky clean group for developing atypical hyperplasia while on tamoxifen therapy.
These findings have caused me to rethink my approach toward endometrial surveillance for women taking tamoxifen.4
Here is my current practice:
- When patients are diagnosed with breast cancer and scheduled to begin tamoxifen therapy, I perform pretreatment screening.
- If no initial endometrial polyps are found, I follow ACOG’s recommendations. Further interventions are unnecessary (unless abnormal symptoms develop), since these patients are at no more risk for endometrial cancer than women not taking tamoxifen.
- For patients with an initial polyp (ie, the high-risk group), I remove the polyp prior to starting tamoxifen treatment and monitor them throughout the course of therapy, periodically utilizing transvaginal ultrasound and saline infusion sonohysterography.
REFERENCES
1. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion #232: Tamoxifen and Endometrial Cancer. Washington, DC: ACOG; April 2000.
2. Berliere M, Charles A, Galant C, Donnez J. Uterine side effects of tamoxifen: a need for systematic pretreatment screening. Obstet Gynecol. 1998;91(1):40-44.
3. Berliere M, Radikov G, Galant C, Piette P, Marbaix E, Donnez J. Identification of women at high risk of developing endometrial cancer on tamoxifen. Eur J Cancer. 2000;36(suppl 4):S35-S36.
4. Goldstein SR. Controversy about uterine effects and safety of SERMs: the saga continues. Menopause. 2002;9:381-384.
Assessing risk: The need for a new model
Although a number of breast cancer riskassessment models are available based on individual risk factors (TABLE 1), estimates based on combinations of factors are preferable. The Gail model,2 widely used to determine breast cancer risk, takes into account nongenetic (nulliparity, age at menarche) and genetic (family history) factors, as well as the number of previous breast biopsies. It assigns a smaller relative risk to women over age 50. A Web-based version, available at http://bcra.nci.nih.gov/brc, is useful for calculating a woman’s risk of developing invasive disease over the next 5 years, as well as over her remaining lifetime.
Limitations of the Gail model. Unfortunately, the data on which the model is based were collected in the late 1970s and early 1980s. Today, the greater ease of breast histopathologic assessment by fine-needle aspiration and outpatient core-needle biopsy has increased the rate of tissue sampling, creating confusion as to what constitutes a biopsy. Thus, the cutoff of 1.66% for high risk—the threshold adopted for the Breast Cancer Prevention Trial (BCPT)—loses some credibility.
Consider this example: A 50-year-old nulliparous Caucasian woman experienced menarche at age 11, has no first-degree relatives with a history of breast cancer, and has never had a breast biopsy. The Gail model would assign her a risk of developing breast cancer of 1.2% in the next 5 years and 10.8% over her lifetime. However, if the same patient had had 3 breast biopsies, her risk would rise to 1.8% in the next 5 years and 15.8% for her lifetime (placing her in the high-risk category), even if none of the biopsies revealed hyperplasia.
Biomarkers. Objective findings that are patient-specific but which correlate closely with breast cancer development are needed.
Biomarkers have been proposed; among them: ultrasensitive measurement of serum estradiol levels in postmenopausal women.3 In the Multiple Outcomes of Raloxifene Evaluation (MORE),4 the women who had the greatest reduction in breast cancer during treatment had the highest baseline serum estradiol levels (FIGURE 1)—although the baseline levels of all subjects were well within the postmenopausal range of 20 pmol/L or less.
TABLE 1
Breast cancer risk factors and their relative risks19
| RELATIVE RISK <2 | RELATIVE RISK 2–4 | RELATIVE RISK >4 |
|---|---|---|
|
|
|
FIGURE 1 Breast cancer risk in raloxifene users
Reprinted with permission from: Cummings SR, Duong T, Kenyon E, et al. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220. Copyright © 2002 American Medical Association. All rights reserved.
Chemoprevention: The rationale for tamoxifen
Data from preclinical animal and in vitro studies led to the use of tamoxifen, a selective estrogen receptor modulator (SERM), for primary prevention of breast cancer in healthy women. The drug was shown to inhibit mammary tumors in mice and rats and suppress hormone-dependent breast cancer cell lines in vitro.5
Clinical data from the Early Breast Cancer Trialists Collaborative Group also helped spur prevention trials with tamoxifen.6 Besides decreasing the risk of recurrent breast cancer, tamoxifen reduced the risk of contralateral, new-onset breast cancer by 47% after 5 years of adjuvant treatment (P = .00001) and by 26% after 2 years of treatment (P = .004). This, along with tamoxifen’s favorable effects on skeletal remodeling and lipid levels, led to a series of chemopreventive trials in the United States and Europe (TABLE 2).
TABLE 2
Results of tamoxifen trials
| BREAST CANCER PREVENTION TRIAL7 | ROYAL MARSDEN10 | ITALIAN11 | |
|---|---|---|---|
| Number of participants | 13,388 | 2,471 | 5,408 |
| Age ≤50 | 40% | 62% | 36% |
| One first-degree relative with breast cancer | 55% | 55% | 18% |
| >2 first-degree relatives with breast cancer | 13% | 17% | 2.5% |
| HRT users | 0% | 42% | 8% |
| Woman-years of follow-up | 46,858 | 12,355 | 20,731 |
| Cancer incidence/1,000 | |||
| Placebo | 6.8 | 5.0 | 2.3 |
| Tamoxifen | 3.4 | 4.7 | 2.1 |
Breast Cancer Prevention Trial: Tamoxifen reduces cancer incidence
In 1992, the National Surgical Adjuvant Breast and Bowel Project launched a prevention trial using tamoxifen: the Breast Cancer Prevention Trial.7 A total of 13,388 women aged 35 or older and at high risk for breast cancer were enrolled at numerous sites throughout the United States and Canada.
The Gail model was utilized to determine which women had sufficient risk—that is, a risk of developing invasive breast cancer within the next 5 years of 1.66% or greater—to be included in the trial.2 Subjects were randomly assigned to receive placebo or tamoxifen (20 mg/d) for 5 years.
The trial was terminated in April 1998, 14 months before its planned completion, due to the striking reduction in new-onset breast cancer in the tamoxifen group. The data safety monitoring board felt it would be unethical to allow one half of the participants, who were deemed to be at high risk, to continue taking placebo in light of the dramatic reduction in both invasive and noninvasive breast cancer with tamoxifen use.
The overall incidence of breast cancer in the tamoxifen group was 3.4 cases per 1,000, compared with 6.8 cases per 1,000 in the placebo group. Overall, the reduction in invasive breast cancer was 49% (P<.000001). The reductions were 44% for women in the group aged 35 to 49 years, 51% for those aged 50 to 59, and 55% for those 60 years and older (FIGURE 2).
Tamoxifen decreased the incidence of noninvasive breast cancer (ductal carcinoma in situ) by 50%. (Expanded use of mammography has led to greater detection of this cancer. Most such lesions are estrogen-receptor–positive.8) In addition, tamoxifen reduced breast cancer risk in women with a history of lobular carcinoma in situ by 56% and atypical hyperplasia by 86%. Overall, tamoxifen decreased the occurrence of estrogen-receptor–positive tumors by 69%, but had no impact on tumors that were estrogen-receptor–negative.
Tamoxifen’s other effects in healthy women. The BCPT offered the first largescale data on the effects of tamoxifen in healthy women.7 (All previous studies included only women with breast cancer.) Several secondary endpoints merit consideration.
- Endometrial cancer risk. Researchers found the relative risk (RR) of endometrial cancer associated with tamoxifen therapy in healthy women was 2.53 (95% confidence intervals [CI], 1.35, 4.97). When this figure was calculated for the different age groups, it rose to 4.01 (95% CI, 1.70, 10.90) in women over 50, and declined to 1.21 for women ages 49 and under (95% CI, 0.41, 3.60).
- Thromboembolic event risk. The same age distinction was seen in relation to thromboembolic events. There were no statistically significant increases in pulmonary emboli or deep venous thrombosis in women 49 years of age or under. Although it is unclear whether the trial was sufficiently powered for this particular endpoint, the likelihood that serious adverse events will limit the potential benefits of tamoxifen appears to be lower in women under the age of 50. This has significant clinical consequences for physicians caring for perimenopausal patients.
- No change in incidence of other cancers. Overall, the incidence of invasive cancers other than those of the breast and uterus was the same for the tamoxifen and placebo groups.
- Other outcomes. The relative risk of death from any cause was 0.81 (95% CI, 0.56–1.16).
There was a slight increase in the risk of myocardial infarction (RR, 1.11; 95% CI, 0.65–1.92) and a slight decrease in the development of severe angina (RR, 0.93; 95% CI, 0.40–2.14) in tamoxifen users, although neither of these was statistically significant.
The overall relative risk of fractures at various sites (hip, spine, radius) was 0.81 (95% CI, 0.63–1.05).
A statistically significant increase was found in the number of women with cataracts who then underwent cataract surgery. That relative risk was 1.57 (95% CI, 1.16–2.14).
FIGURE 2 Breast Cancer Prevention Trial: Tamoxifen reduces incidence of breast cancer
The rate of cancer reduction for tamoxifen compared with placebo for years 1 through 6 was 33%, 55%, 39%, 49%, 69%, and 55%, respectively.
Reprinted from Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst.1998;90(18):1371-1388, by permission of Oxford University Press.
FDA approves tamoxifen for primary prevention
Based on the BCPT results, the US Food and Drug Administration (FDA) approved tamoxifen in October 1998 for primary prevention of breast cancer in women at high risk for the disease. It recommended that tamoxifen be limited to high-risk women because of the potentially serious side effects seen in clinical trials.
The FDA did not define high risk, but recommended that prophylactic use of tamoxifen be based on a thorough evaluation of a woman’s personal, family, and medical histories, as well as her age and understanding of the risks and benefits of treatment.
In addition, the FDA required that the package insert advise women to consult a health-care professional for breast cancer risk assessment and state that only women at high risk should take the drug (again, without defining high risk).
In 2002, the FDA added a “black box” warning to tamoxifen labeling that was directed at use of the drug for prevention rather than treatment. This warning concerned the occurrence of uterine sarcomas. The incidence of these cancers was found to be 0.17 per 1,000 women taking tamoxifen, compared with 0.015 per 1,000 controls.9
Underuse of tamoxifen? A recent study by Freedman et al1 calculated the number of women 35 to 79 years of age who were eligible for tamoxifen chemoprevention based on FDA criteria. They further calculated the number of women who would have a positive benefit-risk ratio for tamoxifen use, and concluded that, among white women alone, roughly 28,492 additional breast cancers could be prevented or deferred if these individuals took tamoxifen for the next 5 years.
European trials fail to confirm benefit of tamoxifen
The Royal Marsden Trial was one of 2 European trials that failed to replicate the BCPT results.10 This British study involved 2,471 healthy women between the ages of 30 and 70 who had a family history of breast cancer. The immediate follow-up was 70 months. No statistically significant decrease in breast cancer was found with tamoxifen use, compared with placebo.
One possibility for the discrepancy may be that eligibility for the Royal Marsden Trial was based predominantly on a strong family history of breast cancer. It also included a much larger proportion of women under age 50 (62%, compared with only 40% in the BCPT). Probably most importantly, 42% of the women received hormone replacement therapy (HRT) along with tamoxifen during the trial.
The Italian prevention study also failed to confirm the findings of the BCPT.11 Because it recruited participants from the general population, the overall risk of breast cancer was significantly lower than in the BCPT. Compliance rates in the Italian study were quite low: Approximately 26% of the subjects dropped out. Furthermore, the Italian trial included considerably fewer women over age 60 (12% versus 30% in the BCPT).
Raloxifene: Another cancer preventive?
Like tamoxifen, raloxifene is a SERM. It is a benzothiophene derivative; tamoxifen comes from the triphenylethylene family.
Like tamoxifen, raloxifene was originally investigated as a treatment for advanced breast cancer. Preclinical studies indicated that it had an antiproliferative effect on estrogen-receptor–positive mammary tumors and estrogen-receptor–positive human breast cancer cell lines.12 However, in the 1980s, a small phase II trial revealed that it had no further antitumor effects in postmenopausal women with advanced breast cancer in whom tamoxifen therapy had failed.13
Interest in raloxifene revived after tamoxifen’s neoplastic effects on the uteri of postmenopausal women became evident.14 As a SERM, raloxifene exhibits estrogen-agonist activity on bone remodeling and lipid metabolism. In December 1997, it won FDA approval for the prevention of osteoporosis in postmenopausal women. Its indication was extended to treatment in October 1999.
Studies found that raloxifene was similar to placebo in its effects on the endometrium of postmenopausal women.15 There were no differences in endometrial thickness, endoluminal masses, proliferation, or hyperplasia. This corroborated previous findings that raloxifene causes neither endometrial hyperplasia nor cancer and is not associated with vaginal bleeding or increased endometrial thickness (as measured by transvaginal ultrasound).
In addition, preclinical animal data suggest that, like tamoxifen, raloxifene has potent antiestrogen effects on breast tissue.12
The MORE trial involved 7,705 postmenopausal women up to age 80 with established osteoporosis who were randomized to receive raloxifene or placebo. Bone mineral density and fracture incidence were the primary endpoints; breast cancer was a secondary endpoint. At 4 years, raloxifene significantly reduced the incidence of all invasive breast cancers by 72%, compared with placebo (RR = 0.28; 95% CI, 0.17–0.46).16 It reduced the incidence of invasive estrogen-receptor–positive tumors by 84%, compared with placebo (RR = 0.16; 95% CI, 0.09–0.30), but had no effect on estrogen-receptor–negative tumors.
Because they block both initiation and promotion of breast cancer, aromatase inhibitors may be more effective than SERMs in preventing breast cancer.
The incidence of vaginal bleeding, breast pain, and endometrial cancer in the raloxifene group did not differ significantly from those of the placebo group. (Because women in the MORE trial were older and did not enter the study with an increased risk for breast cancer, these findings are not necessarily applicable to younger, high-risk women.)
Like tamoxifen, raloxifene slightly increased the risk of thromboembolic disease, including deep vein thrombosis. Pulmonary embolism developed in 1.1% of women in the raloxifene group, compared with 0.5% of subjects in the placebo group (P = .003).
Currently, there is no approved indication for raloxifene in premenopausal women. SERM compounds, which are structurally similar to clomiphene citrate, seem to have different effects in premenopausal and postmenopausal women, as tamoxifen did in the BCPT. Further study in the premenopausal population or with concomitant use of lowdose estrogen may be forthcoming.
Ongoing clinical trials
STAR. To directly compare the safety and efficacy of tamoxifen and raloxifene in reducing breast cancer risk among healthy women, the Study of Tamoxifen and Raloxifene (STAR) has been enrolling postmenopausal women 35 years of age or older who are at increased risk for breast cancer. This study began in 1999 and is expected to run for at least 7 years. It seeks to enroll 22,000 participants in its randomized, double-blind investigation. Participants will receive a daily dose of raloxifene (60 mg) or tamoxifen (20 mg).
RUTH. Raloxifene Use and the Heart (RUTH) is a double-blind, placebo-controlled trial of 60 mg of raloxifene that will include 10,000 women. Primary endpoints are coronary disease and invasive breast cancer. Trial enrollment ended in August 2000, and the study is expected to conclude in 2006.
Aromatase inhibitors: Another route of prevention
The evidence that estrogens facilitate breast cancer development in animals and women is substantial, although the precise mechanism is unknown.17 The most commonly held theory is that estrogen stimulates proliferation of breast cells. Thus, it statistically increases the chances for genetic mutations that could result in cancer.
Aromatase inhibitors block peripheral conversion of androstenedione to estrogens. In premenopausal patients, the primary site of this action is in the ovary. In post-menopausal women, it occurs predominantly in extraovarian sites, including the adrenal glands, adipose tissue, liver, muscle, and skin.
Because of their dual role, (blocking both the initiation and promotion of breast cancer), aromatase inhibitors may be more effective than SERMs in preventing breast cancer.18 By inhibiting the initiation process, they would reduce levels of genotoxic metabolites of estradiol by lowering estradiol concentration in tissue. They also would inhibit tumor promotion by lowering tissue levels of estradiol—thus blocking cell proliferation. However, because they are not selective, aromatase inhibitors would have an antiestrogen effect on bone and lipid metabolism and would induce vasomotor symptoms.
Dr. Goldstein serves on gynecology advisory boards for Eli Lilly and Company, Pfizer, AstraZeneca, and P&G Pharmaceuticals.
1. Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst. 2003;95:526-532.
2. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
3. Ruffin MT, August DA, Kelloff GJ, Boone CW, Weber BL, Brenner DE. Selection criteria for breast cancer chemoprevention subjects. J Cell Biochem Suppl. 1993;17G:234-241.
4. Cummings SR, Duong T, Kenyon E, et al. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220.
5. Jordan VC, Allen KE. Evaluation of the antitumor activity of the nonsteroidal antiestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma mode. Eur J Cancer. 1980;16:239-251.
6. Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer: an overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988;319:1681-1692.
7. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
8. Bur ME, Zimarowski MJ, Schnitt SJ, Baker S, Lew R. Estrogen receptor immunohistochemistry in carcinoma in situ of the breast. Cancer. 1992;69:1174-1181.
9. Nolvadex [package insert]. Wilmington, Del: AstraZeneca Pharmaceuticals; 2002.
10. Powles T, Eeles R, Ashley S, et al. Interim analysis of incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomized chemoprevention trial. Lancet. 1998;352:98-101.
11. Veronesi U, Maisonneuve P, Costa A, et al. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomized trial among hysterectomized women. Italian Prevention Study. Lancet. 1998;352:93-97.
12. Hol T, Cox MB, Bryant HU, Draper MW. Selective estrogen receptor modulators and postmenopausal women’s health. J Women’s Health. 1997;6:523-531.
13. Buzdar AU, Marcus C, Holmes F, Hug V, Hortobagyi G. Phase II evaluation of LY156758 in metastatic breast cancer. Oncology. 1988;45:344-345.
14. Neven P, Muylder X, Van Belle Y, Vanderick G, De Mylder E. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
15. Goldstein SR, Scheele WH, Rajagopalan SK, Wilke JL, Walsh BW, Parsons AK. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
16. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Breast Cancer Res Treat. 2001;65:125-134.
17. Santen RJ, Yue W, Nftolin F, Mor G, Berstein L. The potential of aromatase inhibitors in breast cancer prevention. Endocrine-Related Cancer. 1999;6:235-243.
18. Goss PE, Strasser K. Aromatase inhibitors in the treatment and prevention of breast cancer. J Clin Oncol. 2001;19:881-894.
19. Bilimoria MM, Morrow M. The woman at increased risk for breast cancer: evaluation and management strategies. CA Cancer J Clin. 1995;45:263-278.
1. Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst. 2003;95:526-532.
2. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
3. Ruffin MT, August DA, Kelloff GJ, Boone CW, Weber BL, Brenner DE. Selection criteria for breast cancer chemoprevention subjects. J Cell Biochem Suppl. 1993;17G:234-241.
4. Cummings SR, Duong T, Kenyon E, et al. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220.
5. Jordan VC, Allen KE. Evaluation of the antitumor activity of the nonsteroidal antiestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma mode. Eur J Cancer. 1980;16:239-251.
6. Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer: an overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988;319:1681-1692.
7. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
8. Bur ME, Zimarowski MJ, Schnitt SJ, Baker S, Lew R. Estrogen receptor immunohistochemistry in carcinoma in situ of the breast. Cancer. 1992;69:1174-1181.
9. Nolvadex [package insert]. Wilmington, Del: AstraZeneca Pharmaceuticals; 2002.
10. Powles T, Eeles R, Ashley S, et al. Interim analysis of incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomized chemoprevention trial. Lancet. 1998;352:98-101.
11. Veronesi U, Maisonneuve P, Costa A, et al. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomized trial among hysterectomized women. Italian Prevention Study. Lancet. 1998;352:93-97.
12. Hol T, Cox MB, Bryant HU, Draper MW. Selective estrogen receptor modulators and postmenopausal women’s health. J Women’s Health. 1997;6:523-531.
13. Buzdar AU, Marcus C, Holmes F, Hug V, Hortobagyi G. Phase II evaluation of LY156758 in metastatic breast cancer. Oncology. 1988;45:344-345.
14. Neven P, Muylder X, Van Belle Y, Vanderick G, De Mylder E. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
15. Goldstein SR, Scheele WH, Rajagopalan SK, Wilke JL, Walsh BW, Parsons AK. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
16. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Breast Cancer Res Treat. 2001;65:125-134.
17. Santen RJ, Yue W, Nftolin F, Mor G, Berstein L. The potential of aromatase inhibitors in breast cancer prevention. Endocrine-Related Cancer. 1999;6:235-243.
18. Goss PE, Strasser K. Aromatase inhibitors in the treatment and prevention of breast cancer. J Clin Oncol. 2001;19:881-894.
19. Bilimoria MM, Morrow M. The woman at increased risk for breast cancer: evaluation and management strategies. CA Cancer J Clin. 1995;45:263-278.