User login
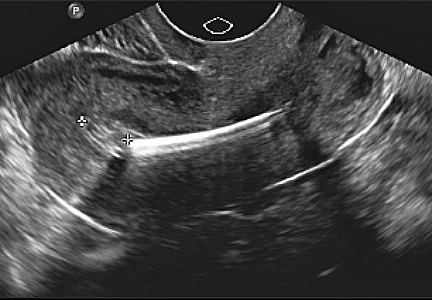
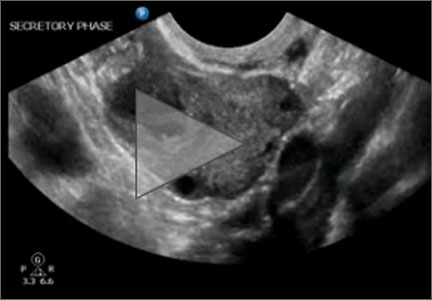
Using (dynamic) ultrasound to make an earlier diagnosis of endometriosis
Can you provide some background on endometriosis and the importance of early diagnosis?
Dr. Goldstein: Endometriosis is an inflammatory condition, characterized by endometrial tissue at sites outside the uterus—this definition comes from the World Endometriosis Society.
Endometriosis is said to affect about 10% of women of reproductive age, and if you look at a group, a subset of women with pelvic pain or infertility, the numbers rise to the range of 35% to 50%. It can present in a multitude of locations, mainly in the pelvis, although occasionally even in places like the lung. When it occurs in the uterus, it is known as adenomyosis; when it occurs inside the ovary, it can cause an endometrioma (or what is sometimes referred to as chocolate cyst of the ovary), but you can see endometriotic implants anywhere in the peritoneum—along the urinary tract, rectum, uterosacral ligaments, rectovaginal septum, and even the vaginal wall occasionally.
What I am really interested in is an earlier diagnosis of superficial endometriosis, and it should be apparent to the reader why this is important—the quality of life from pain from endometriosis can be debilitating. It can be a source of infertility, a source of menstrual irregularities, and a source of not only quality of life but also economic consequences. Many women can also undergo as much as a 7-year delay in diagnosis, so the need for a timely diagnosis and initiation of treatment is extremely important.
What is the role of ultrasound in endometriosis diagnostics?
Dr. Goldstein: In an article that I authored 31 years ago, I wrote that there was a difference between an ultrasound examination by referral and examining one’s patients with ultrasound. I coined a phrase: the “ultrasound-enhanced bimanual exam.” I believed that this term should become a routine part of the overall gynecologic exam. I wanted people to think about the bimanual that we had done for at least half a century, which, in my opinion, consists of 2 components:
- An objective component: Is this uterus normal? Is it enlarged or irregular in contour, suggesting maybe fibroids? Is an ovary enlarged? If so, does it feel cystic or solid?
- A subjective component: Does this patient have tenderness through the pelvis. Is there normal mobility of the pelvic organs?
Part of the thesis was that the objective portion could be replaced by an image that could be produced in seconds, dependent on the operator’s training and availability of equipment. The subjective portion, however, depended on the experience and, often, nuance of the examiner. Lately, I have been seeking to expand that thesis by having the imager use examination as part of their overall imaging—this is the concept of dynamic imaging.
Can you expand on the concept of dynamic ultrasound in this setting?
Dr. Goldstein: Presently, most imagers take a multitude of pictures, what I would call 2-dimensional snapshots, to illustrate anatomy. This is usually done by a sonographer, or a technician, who collects the images for viewing by the physician, who then often does so without holding the transducer. Increasing utilization of remote tools like teleradiology only makes this more likely, and for a minority of people who may use video clips instead of still images, they are still simply representations of anatomy. The guidelines for pelvic ultrasound are the underpinning of the expectation of those who are scanning the female pelvis. With dynamic imaging, the operator uses their other hand on the abdomen as well as some motion with the probe to see if they can elicit pain with the vaginal probe, checking for mobility, asking the patient to bear down. Whether you are a sonographer, a radiologist, or an ObGyn, dynamic imaging can bring the examination process into the imager’s hands.
Can you tell us more about the indications for pelvic sonography for endometriosis and what data can you give to support this?
Dr. Goldstein: There is a document titled “Ultrasound Examination of the Female Pelvis,” that was originally developed by the American Institute of Ultrasound in Medicine (AIUM). In this document, there are about 19 different indications for pelvic sonography (in no defined order), and it is interesting that the first indication listed is evaluation of pelvic pain. Well, I would ask you, how do you evaluate pelvic pain with a series of anatomic images? If you have a classic ovarian endometrioma, or you have a classic hydrosalpinx, you can surmise that these are the source of the pain that the patient is reporting. But how do you properly evaluate pain with just an anatomic image? Thus, the need to use dynamic assessment.
There was a concept first introduced by my colleague, Dr. Ilan Timor, known as the sliding organ sign, that was mainly used to determine if 2 structures were adherent or separate. This involved use of the abdominal hand, liberal use of the probe moving in and out, and under real-time vision, examining the patient with the ultrasound transducer; this is the concept of dynamic ultrasound. This practice can be expanded to verify if there is pelvic tenderness and can be a significant part of the nonlaparoscopic, presumptive diagnosis of endometriosis, even when there is no ovarian endometrioma.
To support this theory, I would point you toward a classic article by E Okaro and colleagues in the British Journal of OB-GYN. This study took 120 consecutive women with chronic pelvic pain who were scheduled for laparoscopy, but performed a transvaginal ultrasound prior, and they looked for anatomic abnormalities and divided this into hard markers and soft markers. Hard markers were obvious endometriomas and hydrosalpinges, while soft markers included things like reduced ovarian mobility, site-specific pelvic tenderness, and presence of loculated peritoneal fluid in the pelvis. These were typical of chronic pelvic pain patients that ranged from late teens to almost menopausal, as the average age was about 30 years old.
Patients had experienced pain for anywhere from 6 months to 12 years, but the average was about 4 years. At laparoscopy, 58% of these patients had pelvic pathology, and 42% had a normal pelvis. Of the 58% with pathology, the overwhelming majority—about 51 of 70 women—had endometriosis alone, and another 7 had endometriosis with adhesions. A normal ultrasound, based on the absence of hard markers, was found in 96 of 120 women. Thus, 24 of the 120 women had an abnormal scan based on the presence of these hard markers. At laparoscopy, all 24 women had abnormal laparoscopies. Of those 96 women who would have had a normal ultrasound, based on the anatomic absence of some pathology, 53% had an abnormal scan based on the presence of these soft markers while the remaining women had no soft- or hard-markers suggesting any pelvic pathology. At laparoscopy, 73% of the patients with soft markers had pelvic pathology and 27% had a normal laparoscopy. Of 45 patients who had a normal, transvaginal ultrasound, 9 were found to have small evidence of endometriosis without discrete endometriomas at laparoscopy.
To summarize the study data, 100% of patients with hard markers and chronic pelvic pain had abnormal anatomy at laparoscopy, but 73% of patients who had soft markers but otherwise would have been interpreted as normal anatomic findings had evidence of pelvic pathology. Such an approach, if used, could lead to a reduction in the number of unnecessary laparoscopies.
What it really boils down to is, if you have 100 women with chronic pelvic pain, are you willing to treat 100 patients without laparoscopy, knowing that 73 are going to have a positive laparoscopy and will require treatment anyway? You would treat 27% with a pharmaceutical agent that may provide relief of their pain, or may not, depending on what the true etiology was. I would be willing to do so, as a positive predictive value of 73% makes doing that worthwhile, and I believe a majority of clinicians would agree.
Do you have any other tips or ways to improve the reader’s understanding of transvaginal ultrasound?
Dr. Goldstein: Pelvic organs have mobility. If a premenopausal woman is examined in lithotomy position, if the ovaries are freely mobile, by gravity, they are going to go lateral to the uterus and are seen immediately adjacent to the iliac vessels. But remember, iliac vessels are retroperitoneal as they are outside the peritoneal cavity. If you were to turn that patient onto all fours, so that the ovaries are freely mobile, they are going to move somewhat toward the anterior abdominal wall. When an ovary is seen in a nonanatomic position, it could be normal or it could be held up by a loop of bowel, but it may indicate adhesions. This is where this sliding organ sign and liberal use of the other hand on the lower abdomen can be extremely important. The reader should also understand that our ability to localize ovaries on ultrasound depends on the amount of folliculogenesis. Follicles are black circles that are sonolucent, because they contain fluid, so they make it easy to localize ovaries, but also their anatomic position relative to the iliac vessels. However, there is a caveat—which is, sometimes an ovary might look like it is behind the uterus and not in its normal anatomic location. When dynamic imaging is used, you are able to cajole that ovary to move lateral and sit on top of the iliac vessels, which can enable you make the proper diagnosis.
Can you provide some background on endometriosis and the importance of early diagnosis?
Dr. Goldstein: Endometriosis is an inflammatory condition, characterized by endometrial tissue at sites outside the uterus—this definition comes from the World Endometriosis Society.
Endometriosis is said to affect about 10% of women of reproductive age, and if you look at a group, a subset of women with pelvic pain or infertility, the numbers rise to the range of 35% to 50%. It can present in a multitude of locations, mainly in the pelvis, although occasionally even in places like the lung. When it occurs in the uterus, it is known as adenomyosis; when it occurs inside the ovary, it can cause an endometrioma (or what is sometimes referred to as chocolate cyst of the ovary), but you can see endometriotic implants anywhere in the peritoneum—along the urinary tract, rectum, uterosacral ligaments, rectovaginal septum, and even the vaginal wall occasionally.
What I am really interested in is an earlier diagnosis of superficial endometriosis, and it should be apparent to the reader why this is important—the quality of life from pain from endometriosis can be debilitating. It can be a source of infertility, a source of menstrual irregularities, and a source of not only quality of life but also economic consequences. Many women can also undergo as much as a 7-year delay in diagnosis, so the need for a timely diagnosis and initiation of treatment is extremely important.
What is the role of ultrasound in endometriosis diagnostics?
Dr. Goldstein: In an article that I authored 31 years ago, I wrote that there was a difference between an ultrasound examination by referral and examining one’s patients with ultrasound. I coined a phrase: the “ultrasound-enhanced bimanual exam.” I believed that this term should become a routine part of the overall gynecologic exam. I wanted people to think about the bimanual that we had done for at least half a century, which, in my opinion, consists of 2 components:
- An objective component: Is this uterus normal? Is it enlarged or irregular in contour, suggesting maybe fibroids? Is an ovary enlarged? If so, does it feel cystic or solid?
- A subjective component: Does this patient have tenderness through the pelvis. Is there normal mobility of the pelvic organs?
Part of the thesis was that the objective portion could be replaced by an image that could be produced in seconds, dependent on the operator’s training and availability of equipment. The subjective portion, however, depended on the experience and, often, nuance of the examiner. Lately, I have been seeking to expand that thesis by having the imager use examination as part of their overall imaging—this is the concept of dynamic imaging.
Can you expand on the concept of dynamic ultrasound in this setting?
Dr. Goldstein: Presently, most imagers take a multitude of pictures, what I would call 2-dimensional snapshots, to illustrate anatomy. This is usually done by a sonographer, or a technician, who collects the images for viewing by the physician, who then often does so without holding the transducer. Increasing utilization of remote tools like teleradiology only makes this more likely, and for a minority of people who may use video clips instead of still images, they are still simply representations of anatomy. The guidelines for pelvic ultrasound are the underpinning of the expectation of those who are scanning the female pelvis. With dynamic imaging, the operator uses their other hand on the abdomen as well as some motion with the probe to see if they can elicit pain with the vaginal probe, checking for mobility, asking the patient to bear down. Whether you are a sonographer, a radiologist, or an ObGyn, dynamic imaging can bring the examination process into the imager’s hands.
Can you tell us more about the indications for pelvic sonography for endometriosis and what data can you give to support this?
Dr. Goldstein: There is a document titled “Ultrasound Examination of the Female Pelvis,” that was originally developed by the American Institute of Ultrasound in Medicine (AIUM). In this document, there are about 19 different indications for pelvic sonography (in no defined order), and it is interesting that the first indication listed is evaluation of pelvic pain. Well, I would ask you, how do you evaluate pelvic pain with a series of anatomic images? If you have a classic ovarian endometrioma, or you have a classic hydrosalpinx, you can surmise that these are the source of the pain that the patient is reporting. But how do you properly evaluate pain with just an anatomic image? Thus, the need to use dynamic assessment.
There was a concept first introduced by my colleague, Dr. Ilan Timor, known as the sliding organ sign, that was mainly used to determine if 2 structures were adherent or separate. This involved use of the abdominal hand, liberal use of the probe moving in and out, and under real-time vision, examining the patient with the ultrasound transducer; this is the concept of dynamic ultrasound. This practice can be expanded to verify if there is pelvic tenderness and can be a significant part of the nonlaparoscopic, presumptive diagnosis of endometriosis, even when there is no ovarian endometrioma.
To support this theory, I would point you toward a classic article by E Okaro and colleagues in the British Journal of OB-GYN. This study took 120 consecutive women with chronic pelvic pain who were scheduled for laparoscopy, but performed a transvaginal ultrasound prior, and they looked for anatomic abnormalities and divided this into hard markers and soft markers. Hard markers were obvious endometriomas and hydrosalpinges, while soft markers included things like reduced ovarian mobility, site-specific pelvic tenderness, and presence of loculated peritoneal fluid in the pelvis. These were typical of chronic pelvic pain patients that ranged from late teens to almost menopausal, as the average age was about 30 years old.
Patients had experienced pain for anywhere from 6 months to 12 years, but the average was about 4 years. At laparoscopy, 58% of these patients had pelvic pathology, and 42% had a normal pelvis. Of the 58% with pathology, the overwhelming majority—about 51 of 70 women—had endometriosis alone, and another 7 had endometriosis with adhesions. A normal ultrasound, based on the absence of hard markers, was found in 96 of 120 women. Thus, 24 of the 120 women had an abnormal scan based on the presence of these hard markers. At laparoscopy, all 24 women had abnormal laparoscopies. Of those 96 women who would have had a normal ultrasound, based on the anatomic absence of some pathology, 53% had an abnormal scan based on the presence of these soft markers while the remaining women had no soft- or hard-markers suggesting any pelvic pathology. At laparoscopy, 73% of the patients with soft markers had pelvic pathology and 27% had a normal laparoscopy. Of 45 patients who had a normal, transvaginal ultrasound, 9 were found to have small evidence of endometriosis without discrete endometriomas at laparoscopy.
To summarize the study data, 100% of patients with hard markers and chronic pelvic pain had abnormal anatomy at laparoscopy, but 73% of patients who had soft markers but otherwise would have been interpreted as normal anatomic findings had evidence of pelvic pathology. Such an approach, if used, could lead to a reduction in the number of unnecessary laparoscopies.
What it really boils down to is, if you have 100 women with chronic pelvic pain, are you willing to treat 100 patients without laparoscopy, knowing that 73 are going to have a positive laparoscopy and will require treatment anyway? You would treat 27% with a pharmaceutical agent that may provide relief of their pain, or may not, depending on what the true etiology was. I would be willing to do so, as a positive predictive value of 73% makes doing that worthwhile, and I believe a majority of clinicians would agree.
Do you have any other tips or ways to improve the reader’s understanding of transvaginal ultrasound?
Dr. Goldstein: Pelvic organs have mobility. If a premenopausal woman is examined in lithotomy position, if the ovaries are freely mobile, by gravity, they are going to go lateral to the uterus and are seen immediately adjacent to the iliac vessels. But remember, iliac vessels are retroperitoneal as they are outside the peritoneal cavity. If you were to turn that patient onto all fours, so that the ovaries are freely mobile, they are going to move somewhat toward the anterior abdominal wall. When an ovary is seen in a nonanatomic position, it could be normal or it could be held up by a loop of bowel, but it may indicate adhesions. This is where this sliding organ sign and liberal use of the other hand on the lower abdomen can be extremely important. The reader should also understand that our ability to localize ovaries on ultrasound depends on the amount of folliculogenesis. Follicles are black circles that are sonolucent, because they contain fluid, so they make it easy to localize ovaries, but also their anatomic position relative to the iliac vessels. However, there is a caveat—which is, sometimes an ovary might look like it is behind the uterus and not in its normal anatomic location. When dynamic imaging is used, you are able to cajole that ovary to move lateral and sit on top of the iliac vessels, which can enable you make the proper diagnosis.
Can you provide some background on endometriosis and the importance of early diagnosis?
Dr. Goldstein: Endometriosis is an inflammatory condition, characterized by endometrial tissue at sites outside the uterus—this definition comes from the World Endometriosis Society.
Endometriosis is said to affect about 10% of women of reproductive age, and if you look at a group, a subset of women with pelvic pain or infertility, the numbers rise to the range of 35% to 50%. It can present in a multitude of locations, mainly in the pelvis, although occasionally even in places like the lung. When it occurs in the uterus, it is known as adenomyosis; when it occurs inside the ovary, it can cause an endometrioma (or what is sometimes referred to as chocolate cyst of the ovary), but you can see endometriotic implants anywhere in the peritoneum—along the urinary tract, rectum, uterosacral ligaments, rectovaginal septum, and even the vaginal wall occasionally.
What I am really interested in is an earlier diagnosis of superficial endometriosis, and it should be apparent to the reader why this is important—the quality of life from pain from endometriosis can be debilitating. It can be a source of infertility, a source of menstrual irregularities, and a source of not only quality of life but also economic consequences. Many women can also undergo as much as a 7-year delay in diagnosis, so the need for a timely diagnosis and initiation of treatment is extremely important.
What is the role of ultrasound in endometriosis diagnostics?
Dr. Goldstein: In an article that I authored 31 years ago, I wrote that there was a difference between an ultrasound examination by referral and examining one’s patients with ultrasound. I coined a phrase: the “ultrasound-enhanced bimanual exam.” I believed that this term should become a routine part of the overall gynecologic exam. I wanted people to think about the bimanual that we had done for at least half a century, which, in my opinion, consists of 2 components:
- An objective component: Is this uterus normal? Is it enlarged or irregular in contour, suggesting maybe fibroids? Is an ovary enlarged? If so, does it feel cystic or solid?
- A subjective component: Does this patient have tenderness through the pelvis. Is there normal mobility of the pelvic organs?
Part of the thesis was that the objective portion could be replaced by an image that could be produced in seconds, dependent on the operator’s training and availability of equipment. The subjective portion, however, depended on the experience and, often, nuance of the examiner. Lately, I have been seeking to expand that thesis by having the imager use examination as part of their overall imaging—this is the concept of dynamic imaging.
Can you expand on the concept of dynamic ultrasound in this setting?
Dr. Goldstein: Presently, most imagers take a multitude of pictures, what I would call 2-dimensional snapshots, to illustrate anatomy. This is usually done by a sonographer, or a technician, who collects the images for viewing by the physician, who then often does so without holding the transducer. Increasing utilization of remote tools like teleradiology only makes this more likely, and for a minority of people who may use video clips instead of still images, they are still simply representations of anatomy. The guidelines for pelvic ultrasound are the underpinning of the expectation of those who are scanning the female pelvis. With dynamic imaging, the operator uses their other hand on the abdomen as well as some motion with the probe to see if they can elicit pain with the vaginal probe, checking for mobility, asking the patient to bear down. Whether you are a sonographer, a radiologist, or an ObGyn, dynamic imaging can bring the examination process into the imager’s hands.
Can you tell us more about the indications for pelvic sonography for endometriosis and what data can you give to support this?
Dr. Goldstein: There is a document titled “Ultrasound Examination of the Female Pelvis,” that was originally developed by the American Institute of Ultrasound in Medicine (AIUM). In this document, there are about 19 different indications for pelvic sonography (in no defined order), and it is interesting that the first indication listed is evaluation of pelvic pain. Well, I would ask you, how do you evaluate pelvic pain with a series of anatomic images? If you have a classic ovarian endometrioma, or you have a classic hydrosalpinx, you can surmise that these are the source of the pain that the patient is reporting. But how do you properly evaluate pain with just an anatomic image? Thus, the need to use dynamic assessment.
There was a concept first introduced by my colleague, Dr. Ilan Timor, known as the sliding organ sign, that was mainly used to determine if 2 structures were adherent or separate. This involved use of the abdominal hand, liberal use of the probe moving in and out, and under real-time vision, examining the patient with the ultrasound transducer; this is the concept of dynamic ultrasound. This practice can be expanded to verify if there is pelvic tenderness and can be a significant part of the nonlaparoscopic, presumptive diagnosis of endometriosis, even when there is no ovarian endometrioma.
To support this theory, I would point you toward a classic article by E Okaro and colleagues in the British Journal of OB-GYN. This study took 120 consecutive women with chronic pelvic pain who were scheduled for laparoscopy, but performed a transvaginal ultrasound prior, and they looked for anatomic abnormalities and divided this into hard markers and soft markers. Hard markers were obvious endometriomas and hydrosalpinges, while soft markers included things like reduced ovarian mobility, site-specific pelvic tenderness, and presence of loculated peritoneal fluid in the pelvis. These were typical of chronic pelvic pain patients that ranged from late teens to almost menopausal, as the average age was about 30 years old.
Patients had experienced pain for anywhere from 6 months to 12 years, but the average was about 4 years. At laparoscopy, 58% of these patients had pelvic pathology, and 42% had a normal pelvis. Of the 58% with pathology, the overwhelming majority—about 51 of 70 women—had endometriosis alone, and another 7 had endometriosis with adhesions. A normal ultrasound, based on the absence of hard markers, was found in 96 of 120 women. Thus, 24 of the 120 women had an abnormal scan based on the presence of these hard markers. At laparoscopy, all 24 women had abnormal laparoscopies. Of those 96 women who would have had a normal ultrasound, based on the anatomic absence of some pathology, 53% had an abnormal scan based on the presence of these soft markers while the remaining women had no soft- or hard-markers suggesting any pelvic pathology. At laparoscopy, 73% of the patients with soft markers had pelvic pathology and 27% had a normal laparoscopy. Of 45 patients who had a normal, transvaginal ultrasound, 9 were found to have small evidence of endometriosis without discrete endometriomas at laparoscopy.
To summarize the study data, 100% of patients with hard markers and chronic pelvic pain had abnormal anatomy at laparoscopy, but 73% of patients who had soft markers but otherwise would have been interpreted as normal anatomic findings had evidence of pelvic pathology. Such an approach, if used, could lead to a reduction in the number of unnecessary laparoscopies.
What it really boils down to is, if you have 100 women with chronic pelvic pain, are you willing to treat 100 patients without laparoscopy, knowing that 73 are going to have a positive laparoscopy and will require treatment anyway? You would treat 27% with a pharmaceutical agent that may provide relief of their pain, or may not, depending on what the true etiology was. I would be willing to do so, as a positive predictive value of 73% makes doing that worthwhile, and I believe a majority of clinicians would agree.
Do you have any other tips or ways to improve the reader’s understanding of transvaginal ultrasound?
Dr. Goldstein: Pelvic organs have mobility. If a premenopausal woman is examined in lithotomy position, if the ovaries are freely mobile, by gravity, they are going to go lateral to the uterus and are seen immediately adjacent to the iliac vessels. But remember, iliac vessels are retroperitoneal as they are outside the peritoneal cavity. If you were to turn that patient onto all fours, so that the ovaries are freely mobile, they are going to move somewhat toward the anterior abdominal wall. When an ovary is seen in a nonanatomic position, it could be normal or it could be held up by a loop of bowel, but it may indicate adhesions. This is where this sliding organ sign and liberal use of the other hand on the lower abdomen can be extremely important. The reader should also understand that our ability to localize ovaries on ultrasound depends on the amount of folliculogenesis. Follicles are black circles that are sonolucent, because they contain fluid, so they make it easy to localize ovaries, but also their anatomic position relative to the iliac vessels. However, there is a caveat—which is, sometimes an ovary might look like it is behind the uterus and not in its normal anatomic location. When dynamic imaging is used, you are able to cajole that ovary to move lateral and sit on top of the iliac vessels, which can enable you make the proper diagnosis.
Can a drug FDA approved for endometriosis become a mainstay for nonsurgical treatment of HMB in women with fibroids?
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Expert Commentary
Any women’s health care provider is extremely aware of how common uterine fibroids (leiomyomas) are in reproductive-aged women. Bleeding associated with such fibroids is a common source of medical morbidity and reduced quality of life for many patients. The mainstay treatment approach for such patients has been surgical, which over time has become minimally invasive. Finding a nonsurgical treatment for patients with fibroid-associated HMB is of huge importance. The recent failure of the selective progesterone receptor modulator ulipristal acetate to be approved by the US Food and Drug Administration (FDA) was a significant setback to finding an excellent option for medical management. A gonadotropin-releasing hormone (GnRH) antagonist like elagolix could become an incredibly important “arrow in the quiver” of women’s health clinicians.
Details about elagolix
As mentioned, elagolix was FDA approved in 2-dose regimens for the treatment of dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia associated with endometriosis. One would expect that such a GnRH antagonist would reduce or eliminate HMB in patients with fibroids, although formal study had never been undertaken. Previous studies of elagolix had shown the most common adverse reaction to be vasomotor symptoms—hot flashes and night sweats. In addition, the drug shows a dose-dependent decrease in bone mineral density (BMD), although its effect on long-term bone health and future fracture risk is unknown.1
Study specifics. The current study by Schlaff and colleagues was performed including 3 arms: a placebo arm, an elagolix 300 mg twice daily arm, and a third arm that received elagolix 300 mg twice daily and hormonal “add-back” therapy in the form of estradiol 1 mg and norethindrone acetate 0.5 mg daily. The authors actually report on two phase 3 six-month trials that were identical, double-blind, and randomized in nature. Both trials involved approximately 400 women. About 70% of the study participants overall were black, and the average age was approximately 42 years (range, 18 to 51). At baseline, BMD scores were mostly in the normal range. HMB for inclusion was defined as a volume of more than 80 mL per month.
The primary end point was menstrual blood loss volume less than 80 mL in the final month and at least a 50% reduction in menstrual blood loss from baseline to the final month. In the placebo group, only 9% and 10%, respectively, met these criteria.
Continue to: Results...
Results. In the first study group, 84% of those receiving elagolix alone achieved the primary end point, while the group that received elagolix plus add-back therapy had 69% success.
In the second study, both the elagolix group and the add-back group showed that 77% of patients met the primary end point criteria.
The incidences of hot flashes in the elagolix-alone groups were 64% and 43%, respectively, while with add-back therapy, they were 20% in both trials. In the placebo groups, 9% and 4% of participants reported hot flashes. At 6 months, the elagolix-only groups in both trials lost more BMD than the placebo groups, while BMD loss in both add-back groups was not statistically significant from the placebo groups.
Study strengths
Schlaff and colleagues conducted a very well-designed study. The two phase 3 clinical trials in preparation for drug approval were thorough and well reported. The authors are to be commended for including nearly 70% black women as study participants, since this is a racial group known to be affected by HMB resulting from fibroids.
Another strength was the addition of add-back therapy to the doses of elagolix. Concerns about bone loss from a health perspective and vasomotor symptoms from a quality-of-life perspective are not insignificant with elagolix-alone treatment, and proof that add-back therapy significantly diminishes or attenuates the efficacy of this entity is extremely important.
Elagolix is currently available (albeit not in the dosing regimen used in the current study or with built-in add-back therapy), and these study results offer an encouraging nonsurgical approach to HMB. The addition of add-back therapy to this oral GnRH antagonist will allow greater patient acceptance from a quality-of-life point of view because of diminution of vasomotor symptoms while maintaining BMD.
STEVEN R. GOLDSTEIN, MD
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Expert Commentary
Any women’s health care provider is extremely aware of how common uterine fibroids (leiomyomas) are in reproductive-aged women. Bleeding associated with such fibroids is a common source of medical morbidity and reduced quality of life for many patients. The mainstay treatment approach for such patients has been surgical, which over time has become minimally invasive. Finding a nonsurgical treatment for patients with fibroid-associated HMB is of huge importance. The recent failure of the selective progesterone receptor modulator ulipristal acetate to be approved by the US Food and Drug Administration (FDA) was a significant setback to finding an excellent option for medical management. A gonadotropin-releasing hormone (GnRH) antagonist like elagolix could become an incredibly important “arrow in the quiver” of women’s health clinicians.
Details about elagolix
As mentioned, elagolix was FDA approved in 2-dose regimens for the treatment of dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia associated with endometriosis. One would expect that such a GnRH antagonist would reduce or eliminate HMB in patients with fibroids, although formal study had never been undertaken. Previous studies of elagolix had shown the most common adverse reaction to be vasomotor symptoms—hot flashes and night sweats. In addition, the drug shows a dose-dependent decrease in bone mineral density (BMD), although its effect on long-term bone health and future fracture risk is unknown.1
Study specifics. The current study by Schlaff and colleagues was performed including 3 arms: a placebo arm, an elagolix 300 mg twice daily arm, and a third arm that received elagolix 300 mg twice daily and hormonal “add-back” therapy in the form of estradiol 1 mg and norethindrone acetate 0.5 mg daily. The authors actually report on two phase 3 six-month trials that were identical, double-blind, and randomized in nature. Both trials involved approximately 400 women. About 70% of the study participants overall were black, and the average age was approximately 42 years (range, 18 to 51). At baseline, BMD scores were mostly in the normal range. HMB for inclusion was defined as a volume of more than 80 mL per month.
The primary end point was menstrual blood loss volume less than 80 mL in the final month and at least a 50% reduction in menstrual blood loss from baseline to the final month. In the placebo group, only 9% and 10%, respectively, met these criteria.
Continue to: Results...
Results. In the first study group, 84% of those receiving elagolix alone achieved the primary end point, while the group that received elagolix plus add-back therapy had 69% success.
In the second study, both the elagolix group and the add-back group showed that 77% of patients met the primary end point criteria.
The incidences of hot flashes in the elagolix-alone groups were 64% and 43%, respectively, while with add-back therapy, they were 20% in both trials. In the placebo groups, 9% and 4% of participants reported hot flashes. At 6 months, the elagolix-only groups in both trials lost more BMD than the placebo groups, while BMD loss in both add-back groups was not statistically significant from the placebo groups.
Study strengths
Schlaff and colleagues conducted a very well-designed study. The two phase 3 clinical trials in preparation for drug approval were thorough and well reported. The authors are to be commended for including nearly 70% black women as study participants, since this is a racial group known to be affected by HMB resulting from fibroids.
Another strength was the addition of add-back therapy to the doses of elagolix. Concerns about bone loss from a health perspective and vasomotor symptoms from a quality-of-life perspective are not insignificant with elagolix-alone treatment, and proof that add-back therapy significantly diminishes or attenuates the efficacy of this entity is extremely important.
Elagolix is currently available (albeit not in the dosing regimen used in the current study or with built-in add-back therapy), and these study results offer an encouraging nonsurgical approach to HMB. The addition of add-back therapy to this oral GnRH antagonist will allow greater patient acceptance from a quality-of-life point of view because of diminution of vasomotor symptoms while maintaining BMD.
STEVEN R. GOLDSTEIN, MD
Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340.
Expert Commentary
Any women’s health care provider is extremely aware of how common uterine fibroids (leiomyomas) are in reproductive-aged women. Bleeding associated with such fibroids is a common source of medical morbidity and reduced quality of life for many patients. The mainstay treatment approach for such patients has been surgical, which over time has become minimally invasive. Finding a nonsurgical treatment for patients with fibroid-associated HMB is of huge importance. The recent failure of the selective progesterone receptor modulator ulipristal acetate to be approved by the US Food and Drug Administration (FDA) was a significant setback to finding an excellent option for medical management. A gonadotropin-releasing hormone (GnRH) antagonist like elagolix could become an incredibly important “arrow in the quiver” of women’s health clinicians.
Details about elagolix
As mentioned, elagolix was FDA approved in 2-dose regimens for the treatment of dysmenorrhea, nonmenstrual pelvic pain, and dyspareunia associated with endometriosis. One would expect that such a GnRH antagonist would reduce or eliminate HMB in patients with fibroids, although formal study had never been undertaken. Previous studies of elagolix had shown the most common adverse reaction to be vasomotor symptoms—hot flashes and night sweats. In addition, the drug shows a dose-dependent decrease in bone mineral density (BMD), although its effect on long-term bone health and future fracture risk is unknown.1
Study specifics. The current study by Schlaff and colleagues was performed including 3 arms: a placebo arm, an elagolix 300 mg twice daily arm, and a third arm that received elagolix 300 mg twice daily and hormonal “add-back” therapy in the form of estradiol 1 mg and norethindrone acetate 0.5 mg daily. The authors actually report on two phase 3 six-month trials that were identical, double-blind, and randomized in nature. Both trials involved approximately 400 women. About 70% of the study participants overall were black, and the average age was approximately 42 years (range, 18 to 51). At baseline, BMD scores were mostly in the normal range. HMB for inclusion was defined as a volume of more than 80 mL per month.
The primary end point was menstrual blood loss volume less than 80 mL in the final month and at least a 50% reduction in menstrual blood loss from baseline to the final month. In the placebo group, only 9% and 10%, respectively, met these criteria.
Continue to: Results...
Results. In the first study group, 84% of those receiving elagolix alone achieved the primary end point, while the group that received elagolix plus add-back therapy had 69% success.
In the second study, both the elagolix group and the add-back group showed that 77% of patients met the primary end point criteria.
The incidences of hot flashes in the elagolix-alone groups were 64% and 43%, respectively, while with add-back therapy, they were 20% in both trials. In the placebo groups, 9% and 4% of participants reported hot flashes. At 6 months, the elagolix-only groups in both trials lost more BMD than the placebo groups, while BMD loss in both add-back groups was not statistically significant from the placebo groups.
Study strengths
Schlaff and colleagues conducted a very well-designed study. The two phase 3 clinical trials in preparation for drug approval were thorough and well reported. The authors are to be commended for including nearly 70% black women as study participants, since this is a racial group known to be affected by HMB resulting from fibroids.
Another strength was the addition of add-back therapy to the doses of elagolix. Concerns about bone loss from a health perspective and vasomotor symptoms from a quality-of-life perspective are not insignificant with elagolix-alone treatment, and proof that add-back therapy significantly diminishes or attenuates the efficacy of this entity is extremely important.
Elagolix is currently available (albeit not in the dosing regimen used in the current study or with built-in add-back therapy), and these study results offer an encouraging nonsurgical approach to HMB. The addition of add-back therapy to this oral GnRH antagonist will allow greater patient acceptance from a quality-of-life point of view because of diminution of vasomotor symptoms while maintaining BMD.
STEVEN R. GOLDSTEIN, MD
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
2014 Update on osteoporosis
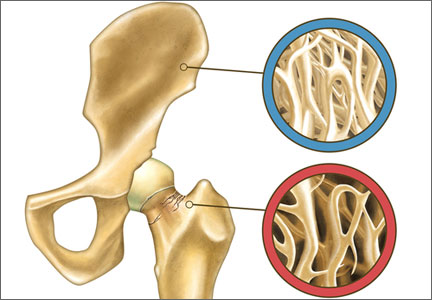
Gynecologists are “first-line” providers for the diagnosis and treatment of osteoporosis in women. Lest you doubt the importance of this fact, consider that there are more osteoporotic fractures annually in the United States than all myocardial infarctions, strokes, breast cancers, and gynecologic malignancies combined. It is our duty to stay abreast of current developments in the diagnosis and treatment of this potentially devastating skeletal disorder as our patients live longer and longer.
In this article, I present recent studies on:
- the use of conjugated estrogens and bazedoxifene (Duavee) to manage hot flashes and menopausal bone loss
- the need for adequate levels of vitamin D to maintain bone and overall health, with sunlight exposure remaining a viable option
- a reinterpretation of the findings on estrogen and fracture risk from the Women’s Health Initiative (WHI)
- the effects of selective serotonin reuptake inhibitors (SSRIs) on bone mineral density (BMD)
- development of blosozumab, a new agent in the fight against osteoporosis and fracture.
FIRST TISSUE-SELECTIVE ESTROGEN COMPLEX PROTECTS AGAINST BONE LOSS WITHOUT AFFECTING ENDOMETRIAL AND BREAST TISSUE
Komm BS, Mirkin S, Jenkins SN. Development of conjugated estrogens/bazedoxifene, the first tissue selective estrogen complex (TSEC) for management of menopausal hot flashes and postmenopausal bone loss. Steroids. 2014;90:71–81.
Pinkerton JV, Harvey JA, Lindsay R, et al; SMART-5 Investigators. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: a randomized trial. J Clin Endocrinol Metab. 2014;99(2):e189–e198.
Conjugated estrogens combined with the selective estrogen receptor modulator (SERM) bazedoxifene (Duavee) are a new option to alleviate menopausal symptoms and prevent postmenopausal bone loss. The rationale for development of the tissue-selective estrogen complex (TSEC) was to combine the benefits of conjugated estrogens with the SERM’s ability to offset estrogenic stimulation of the endometrium and breast.
TSECs offer a progestin-free alternative to traditional hormone therapy for women with a uterus. In preclinical studies, investigators found evidence to support bazedoxifene as the SERM of choice and demonstrated that, by combining it with conjugated estrogens, they could provide an optimal balance of estrogen-receptor agonist/antagonist activity, compared with other potential TSEC pairings. Clinical study results confirmed the efficacy of this combination in maintaining bone mass.
Given separately, conjugated estrogens and bazedoxifene each protect against the loss of BMD and help prevent fracture in postmenopausal women.
Findings in key populations
Komm and colleagues describe substudies of the Selective estrogens, Menopause, and Response to Therapy (SMART) trials to evaluate the combination of conjugated estrogens and SERMs to prevent osteoporosis in postmenopausal women with a uterus. One SMART-1 trial included two osteoporosis prevention substudies that evaluated the combination of conjugated estrogens and bazedoxifene in different subpopulations:
- women more than 5 years past the last menstrual period with a lumbar spine or hip BMD T-score between –1 and –2.5 plus one other risk factor for osteoporosis (n = 1,454)
- women 1 to 5 years past their last menstrual period (the interval during which bone loss is greatest) with at least one risk factor for osteoporosis (n = 861).
All doses of conjugated estrogens and bazedoxifene significantly increased the adjusted mean percentage of change in BMD of the lumbar spine from baseline to 24 months (a primary endpoint), compared with placebo, which was associated with decreases in BMD (P<.001). Findings were similar for total hip BMD.
In a separate study, Pinkerton and colleagues found that the dose of conjugated estrogens (0.45 mg) and bazedoxifene (20 mg) approved by the US Food and Drug Administration does not cause a change in breast density or thickness of the endometrium, nor does it increase breast pain, compared with placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This newly available TSEC—a combination of conjugated estrogens (0.45 mg) and bazedoxifene (20 mg)—is an effective, well-tolerated alternative to traditional estrogen-progestin hormone therapy for relief of menopausal symptoms and prevention of osteoporosis in postmenopausal women with a uterus.
DON’T EXCLUDE SUNLIGHT FROM THE BONE–HEALTH EQUATION
Holick MF. Sunlight, ultraviolet radiation, vitamin D, and skin cancer: how much sunlight do we need? Adv Exp Med Biol. 2014;810:1–16.
Many people think of vitamin D as the “sunshine vitamin.” During exposure to sunlight, ultraviolet photons enter the skin and convert 7-dehydrocholesterol to previtamin D3, which, in turn, is converted to vitamin D3.
Throughout most of human history, people have depended on sunlight for vitamin D. Variables such as skin pigmentation, sunscreen use, aging, time of day, season, and latitude dramatically affect previtamin synthesis.
Although vitamin D deficiency was thought to have been conquered, it is now recognized that more than 50% of the world’s population is at risk for vitamin D insufficiency or low levels of 25-hydroxyvitamin D. Among the reasons are inadequate fortification of foods with vitamin D and a misconception that most balanced diets contain adequate vitamin D.
Deficiency of this vitamin causes growth retardation and rickets in children and osteomalacia in adults and can precipitate and exacerbate osteopenia or osteoporosis and increase the risk of fracture in adults.
Some evidence also suggests that vitamin D deficiency may have other serious consequences, including an increased risk for common cancers and autoimmune, infectious, and cardiovascular diseases.
In this review, Holick argues that we need to remind our patients of the beneficial effects of moderate sunlight.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
There is no question that sufficient levels of vitamin D are vital to bone health, and perhaps to overall health in numerous other organ systems as well. The pendulum of our concern over skin cancers may have moved too far in the direction of sun avoidance. In reality, moderate sunlight as a source of vitamin D is still appropriate for many of our patients.
WHEN IT COMES TO ESTROGEN AND BONE, BENEFITS OUTWEIGH RISKS
de Villiers TJ. 8th Pieter van Keep Memorial Lecture. Estrogen and bone: have we completed a full circle? [published online ahead of print September 22, 2014]. Climacteric. 2014;17(suppl 2):4–7. doi:10.3109/13697137.2014.953047.
In the WHI estrogen-progestin arm, fracture rates were reported as hazard ratios:
- hip fracture, 0.66 (95% confidence interval [CI], 0.45–0.98)
- clinical vertebral fracture, 0.66 (95% CI, 0.44–0.98)
- nonvertebral fractures, 0.77 (95% CI, 0.69–0.86).
In the estrogen-only arm of the WHI, reductions in the rates of fracture were reported as percentages and were similar:
- 39% reduction in hip fracture, compared with placebo
- 38% reduction in clinical vertebral fracture
- 21% reduction in total fractures.
All of these reductions were statistically significant.
Despite the excellent anti-fracture efficacy demonstrated in the WHI, investigators concluded that the risks of hormone therapy outweighed the benefits in the general postmenopausal population.
Why we should reconsider estrogen for bone health
In his presidential address to the International Menopause Society (cited above), de Villiers observed that, in the WHI:
- Only clinical fractures were recorded. Unlike all other fracture trials, routine radiographs were not obtained to record morphometric fractures. This decision, he believes (and I concur), led to a significant understatement of estrogen’s protective effects against vertebral fracture.
- The general population studied had a low risk of fracture, with an average spinal T-score of –1.3. This, too, contributed to an understatement of estrogen’s protective effects, compared with the findings of other randomized controlled trials involving patients at much higher risk.
- From a bone-centric point of view, the WHI findings represent a favorable ratio of benefits to risks.
No bone-active drugs are completely free of potential adverse effects and restrictions, many of which become apparent only after FDA approval and general use of the drug. Bisphosphonates have been implicated in atrial fibrillation, osteonecrosis of the jaw, and atypical femur shaft fracture after extended use. Like estrogen, SERMs can increase the risk of death from deep venous thrombosis and stroke.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Estrogen is the only agent proved to be effective against all types of osteoporotic fractures during primary analysis of a large randomized controlled trial. This efficacy is of special importance for the patient with osteopenia who is at risk for fracture. Estrogen remains a serious option for the prevention of postmenopausal bone loss and osteoporosis-related fractures, especially in younger patients. Individualization of therapy is key.
COUNSEL SSRI AND SNRI USERS THAT BMD MAY DECLINE OVER THE LONG TERM
Ak E, Bulut SD, Bulut S, et al. Evaluation of the effect of selective serotonin reuptake inhibitors on bone mineral density: an observational cross-sectional study [published online ahead of print September 4, 2014]. Osteoporos Int. doi:10.10007/s00198-014-2859-2.
Moura C, Bernatsky S, Ambrahamowicz M, et al. Antidepressant use and 10-year incident fracture risk: the population-based Canadian Multicentre Osteoporosis Study (CaMoS). Osteoporos Int. 2014;25(5):1473–1481.
Bruyère O, Reginster J-V. Osteoporosis in patients taking selective serotonin reuptake inhibitors: a focus on fracture outcome [published online ahead of print August 5, 2014]. Endocrine. doi:10.1007/s12020-014-0357-0.
Evidence from longitudinal, cross-sectional, and prospective cohort studies suggests that the use of antidepressants at therapeutic doses is associated with a reduction in BMD and an increase in the risk of falls and fracture. These associations have been demonstrated in several distinct populations using various study designs, and with bone density, bone loss, or fractures as outcomes. They remain consistent even after adjustment for confounding variables such as age, body mass index, lifestyle factors such as alcohol and tobacco use, and fracture history.
Ak and colleagues recruited 60 patients given a diagnosis of generalized anxiety disorder and treated with paroxetine, sertraline, or citalopram for at least 12 months, comparing their BMD with that of 40 healthy volunteers. BMD was measured by dual-energy x-ray absorptiometry at the femoral and lumbar regions. BMD of the L2–L4 vertebrae, total lumbar vertebrae, and femoral intertrochanteric region, as well as total femoral Z-scores and femoral Ward’s region T-scores, were lower in the treatment group (P<.05). There was a significant negative correlation between the duration of treatment and the change in BMD values.
Moura and colleagues reviewed data from a large prospective Canadian cohort to assess the association between SSRIs, serotonin and norepinephrine reuptake inhibitors (SNRIs), and fracture in adults aged 50 and older. They used the Canadian Multicentre Osteoporosis Study (CaMos), a prospective, randomly selected, population-based community cohort.
Among 6,645 subjects, 192 (2.9%) were using SSRIs or SNRIs, or both, at baseline. During the 10-year study period, 978 participants (14.7%) experienced at least one fragility fracture. SSRI/SNRI use was associated with an increased risk of fragility fracture (hazard ratio [HR], 1.88; 95% CI, 1.48–2.39). After controlling for multiple risk factors, previous falls, and BMD of the hip and lumbar bone, the adjusted hazard ratio for current SSRI/SNRI use remained elevated (HR, 1.68; 95% CI, 1.32–2.14). The authors concluded that these results lend additional support to an association between SSRI/SNRI use and fragility fractures.
A few possible underlying mechanisms support the biological plausibility of these observations. One explanation is that increased fracture risk is mediated simply by falling. Another explanation could involve the influence of serotonin on bone. Besides their effects on balance, SSRIs may influence bone turnover and BMD. Whatever the mechanism, sufficient evidence exists to warrant the addition of SSRIs to the list of medications that contribute to osteoporosis.
Antidepressant use is not listed as a secondary cause of osteoporosis in the FRAX algorithm. Because the association between SSRI use and fracture risk appears to be independent of BMD, it may be useful to consider the possibility of including it in FRAX.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Consider BMD assessment for patients who take an SSRI, or who take an SSRI and have additional risk factors for fracture. Given the body of data on this issue, it seems appropriate to expect providers of SSRIs to conduct at least some discussion of bone health with patients.
IN THE PIPELINE: A HIGHLY EFFECTIVE AGENT TARGETING SCLEROSTIN
Recker R, Benson C, Matsumoto T, et al. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density [published online ahead of print September 5, 2014]. J Bone Miner Res. doi:10.1002/jbmr.2351.
Sclerostin is a protein secreted by osteocytes that negatively regulates the formation of mineralized bone matrix and bone mass. Recker and colleagues conducted a randomized, double-blind, placebo-
controlled, multicenter, phase 2 clinical trial of blosozumab, a humanized monoclonal antibody targeted against sclerostin. The year-long trial involved 120 postmenopausal women with low BMD (lumbar spine T-score, –2.0 to –3.5) who were randomly allocated to:
- subcutaneous blosozumab 180 mg every 4 weeks
- subcutaneous blosozumab 180 mg every 2 weeks
- subcutaneous blosozumab 270 mg every 2 weeks
- placebo.
All groups also received calcium and vitamin D and underwent serial measurement of spine and hip BMD and testing of biochemical markers of bone turnover. The mean age was 65.8 years, and the mean lumbar spine T-score was –2.8.
Women treated with blosozumab experienced statistically significant, dose-related increases in spine, femoral neck, and total hip BMD, compared with placebo. In the highest dose group, BMD increased 17.7% from baseline at the spine and 6.2% at the total hip. Biochemical markers of bone formation increased rapidly during treatment with blosozumab, trending toward pretreatment levels by the study’s end. CTX, a biochemical marker of bone resorption, decreased early during blosozumab treatment to a concentration lower than that in the placebo group by 2 weeks, and it remained low throughout treatment.
Mild injection-site reactions were reported more frequently with blosozumab than with placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although blosozumab is not yet available, clinicians should be aware of the potential of sclerostin-antibody therapies like it. Such therapies appear to have substantial anabolic effects on the skeleton and may become promising agents in the treatment of osteoporosis.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Gynecologists are “first-line” providers for the diagnosis and treatment of osteoporosis in women. Lest you doubt the importance of this fact, consider that there are more osteoporotic fractures annually in the United States than all myocardial infarctions, strokes, breast cancers, and gynecologic malignancies combined. It is our duty to stay abreast of current developments in the diagnosis and treatment of this potentially devastating skeletal disorder as our patients live longer and longer.
In this article, I present recent studies on:
- the use of conjugated estrogens and bazedoxifene (Duavee) to manage hot flashes and menopausal bone loss
- the need for adequate levels of vitamin D to maintain bone and overall health, with sunlight exposure remaining a viable option
- a reinterpretation of the findings on estrogen and fracture risk from the Women’s Health Initiative (WHI)
- the effects of selective serotonin reuptake inhibitors (SSRIs) on bone mineral density (BMD)
- development of blosozumab, a new agent in the fight against osteoporosis and fracture.
FIRST TISSUE-SELECTIVE ESTROGEN COMPLEX PROTECTS AGAINST BONE LOSS WITHOUT AFFECTING ENDOMETRIAL AND BREAST TISSUE
Komm BS, Mirkin S, Jenkins SN. Development of conjugated estrogens/bazedoxifene, the first tissue selective estrogen complex (TSEC) for management of menopausal hot flashes and postmenopausal bone loss. Steroids. 2014;90:71–81.
Pinkerton JV, Harvey JA, Lindsay R, et al; SMART-5 Investigators. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: a randomized trial. J Clin Endocrinol Metab. 2014;99(2):e189–e198.
Conjugated estrogens combined with the selective estrogen receptor modulator (SERM) bazedoxifene (Duavee) are a new option to alleviate menopausal symptoms and prevent postmenopausal bone loss. The rationale for development of the tissue-selective estrogen complex (TSEC) was to combine the benefits of conjugated estrogens with the SERM’s ability to offset estrogenic stimulation of the endometrium and breast.
TSECs offer a progestin-free alternative to traditional hormone therapy for women with a uterus. In preclinical studies, investigators found evidence to support bazedoxifene as the SERM of choice and demonstrated that, by combining it with conjugated estrogens, they could provide an optimal balance of estrogen-receptor agonist/antagonist activity, compared with other potential TSEC pairings. Clinical study results confirmed the efficacy of this combination in maintaining bone mass.
Given separately, conjugated estrogens and bazedoxifene each protect against the loss of BMD and help prevent fracture in postmenopausal women.
Findings in key populations
Komm and colleagues describe substudies of the Selective estrogens, Menopause, and Response to Therapy (SMART) trials to evaluate the combination of conjugated estrogens and SERMs to prevent osteoporosis in postmenopausal women with a uterus. One SMART-1 trial included two osteoporosis prevention substudies that evaluated the combination of conjugated estrogens and bazedoxifene in different subpopulations:
- women more than 5 years past the last menstrual period with a lumbar spine or hip BMD T-score between –1 and –2.5 plus one other risk factor for osteoporosis (n = 1,454)
- women 1 to 5 years past their last menstrual period (the interval during which bone loss is greatest) with at least one risk factor for osteoporosis (n = 861).
All doses of conjugated estrogens and bazedoxifene significantly increased the adjusted mean percentage of change in BMD of the lumbar spine from baseline to 24 months (a primary endpoint), compared with placebo, which was associated with decreases in BMD (P<.001). Findings were similar for total hip BMD.
In a separate study, Pinkerton and colleagues found that the dose of conjugated estrogens (0.45 mg) and bazedoxifene (20 mg) approved by the US Food and Drug Administration does not cause a change in breast density or thickness of the endometrium, nor does it increase breast pain, compared with placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This newly available TSEC—a combination of conjugated estrogens (0.45 mg) and bazedoxifene (20 mg)—is an effective, well-tolerated alternative to traditional estrogen-progestin hormone therapy for relief of menopausal symptoms and prevention of osteoporosis in postmenopausal women with a uterus.
DON’T EXCLUDE SUNLIGHT FROM THE BONE–HEALTH EQUATION
Holick MF. Sunlight, ultraviolet radiation, vitamin D, and skin cancer: how much sunlight do we need? Adv Exp Med Biol. 2014;810:1–16.
Many people think of vitamin D as the “sunshine vitamin.” During exposure to sunlight, ultraviolet photons enter the skin and convert 7-dehydrocholesterol to previtamin D3, which, in turn, is converted to vitamin D3.
Throughout most of human history, people have depended on sunlight for vitamin D. Variables such as skin pigmentation, sunscreen use, aging, time of day, season, and latitude dramatically affect previtamin synthesis.
Although vitamin D deficiency was thought to have been conquered, it is now recognized that more than 50% of the world’s population is at risk for vitamin D insufficiency or low levels of 25-hydroxyvitamin D. Among the reasons are inadequate fortification of foods with vitamin D and a misconception that most balanced diets contain adequate vitamin D.
Deficiency of this vitamin causes growth retardation and rickets in children and osteomalacia in adults and can precipitate and exacerbate osteopenia or osteoporosis and increase the risk of fracture in adults.
Some evidence also suggests that vitamin D deficiency may have other serious consequences, including an increased risk for common cancers and autoimmune, infectious, and cardiovascular diseases.
In this review, Holick argues that we need to remind our patients of the beneficial effects of moderate sunlight.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
There is no question that sufficient levels of vitamin D are vital to bone health, and perhaps to overall health in numerous other organ systems as well. The pendulum of our concern over skin cancers may have moved too far in the direction of sun avoidance. In reality, moderate sunlight as a source of vitamin D is still appropriate for many of our patients.
WHEN IT COMES TO ESTROGEN AND BONE, BENEFITS OUTWEIGH RISKS
de Villiers TJ. 8th Pieter van Keep Memorial Lecture. Estrogen and bone: have we completed a full circle? [published online ahead of print September 22, 2014]. Climacteric. 2014;17(suppl 2):4–7. doi:10.3109/13697137.2014.953047.
In the WHI estrogen-progestin arm, fracture rates were reported as hazard ratios:
- hip fracture, 0.66 (95% confidence interval [CI], 0.45–0.98)
- clinical vertebral fracture, 0.66 (95% CI, 0.44–0.98)
- nonvertebral fractures, 0.77 (95% CI, 0.69–0.86).
In the estrogen-only arm of the WHI, reductions in the rates of fracture were reported as percentages and were similar:
- 39% reduction in hip fracture, compared with placebo
- 38% reduction in clinical vertebral fracture
- 21% reduction in total fractures.
All of these reductions were statistically significant.
Despite the excellent anti-fracture efficacy demonstrated in the WHI, investigators concluded that the risks of hormone therapy outweighed the benefits in the general postmenopausal population.
Why we should reconsider estrogen for bone health
In his presidential address to the International Menopause Society (cited above), de Villiers observed that, in the WHI:
- Only clinical fractures were recorded. Unlike all other fracture trials, routine radiographs were not obtained to record morphometric fractures. This decision, he believes (and I concur), led to a significant understatement of estrogen’s protective effects against vertebral fracture.
- The general population studied had a low risk of fracture, with an average spinal T-score of –1.3. This, too, contributed to an understatement of estrogen’s protective effects, compared with the findings of other randomized controlled trials involving patients at much higher risk.
- From a bone-centric point of view, the WHI findings represent a favorable ratio of benefits to risks.
No bone-active drugs are completely free of potential adverse effects and restrictions, many of which become apparent only after FDA approval and general use of the drug. Bisphosphonates have been implicated in atrial fibrillation, osteonecrosis of the jaw, and atypical femur shaft fracture after extended use. Like estrogen, SERMs can increase the risk of death from deep venous thrombosis and stroke.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Estrogen is the only agent proved to be effective against all types of osteoporotic fractures during primary analysis of a large randomized controlled trial. This efficacy is of special importance for the patient with osteopenia who is at risk for fracture. Estrogen remains a serious option for the prevention of postmenopausal bone loss and osteoporosis-related fractures, especially in younger patients. Individualization of therapy is key.
COUNSEL SSRI AND SNRI USERS THAT BMD MAY DECLINE OVER THE LONG TERM
Ak E, Bulut SD, Bulut S, et al. Evaluation of the effect of selective serotonin reuptake inhibitors on bone mineral density: an observational cross-sectional study [published online ahead of print September 4, 2014]. Osteoporos Int. doi:10.10007/s00198-014-2859-2.
Moura C, Bernatsky S, Ambrahamowicz M, et al. Antidepressant use and 10-year incident fracture risk: the population-based Canadian Multicentre Osteoporosis Study (CaMoS). Osteoporos Int. 2014;25(5):1473–1481.
Bruyère O, Reginster J-V. Osteoporosis in patients taking selective serotonin reuptake inhibitors: a focus on fracture outcome [published online ahead of print August 5, 2014]. Endocrine. doi:10.1007/s12020-014-0357-0.
Evidence from longitudinal, cross-sectional, and prospective cohort studies suggests that the use of antidepressants at therapeutic doses is associated with a reduction in BMD and an increase in the risk of falls and fracture. These associations have been demonstrated in several distinct populations using various study designs, and with bone density, bone loss, or fractures as outcomes. They remain consistent even after adjustment for confounding variables such as age, body mass index, lifestyle factors such as alcohol and tobacco use, and fracture history.
Ak and colleagues recruited 60 patients given a diagnosis of generalized anxiety disorder and treated with paroxetine, sertraline, or citalopram for at least 12 months, comparing their BMD with that of 40 healthy volunteers. BMD was measured by dual-energy x-ray absorptiometry at the femoral and lumbar regions. BMD of the L2–L4 vertebrae, total lumbar vertebrae, and femoral intertrochanteric region, as well as total femoral Z-scores and femoral Ward’s region T-scores, were lower in the treatment group (P<.05). There was a significant negative correlation between the duration of treatment and the change in BMD values.
Moura and colleagues reviewed data from a large prospective Canadian cohort to assess the association between SSRIs, serotonin and norepinephrine reuptake inhibitors (SNRIs), and fracture in adults aged 50 and older. They used the Canadian Multicentre Osteoporosis Study (CaMos), a prospective, randomly selected, population-based community cohort.
Among 6,645 subjects, 192 (2.9%) were using SSRIs or SNRIs, or both, at baseline. During the 10-year study period, 978 participants (14.7%) experienced at least one fragility fracture. SSRI/SNRI use was associated with an increased risk of fragility fracture (hazard ratio [HR], 1.88; 95% CI, 1.48–2.39). After controlling for multiple risk factors, previous falls, and BMD of the hip and lumbar bone, the adjusted hazard ratio for current SSRI/SNRI use remained elevated (HR, 1.68; 95% CI, 1.32–2.14). The authors concluded that these results lend additional support to an association between SSRI/SNRI use and fragility fractures.
A few possible underlying mechanisms support the biological plausibility of these observations. One explanation is that increased fracture risk is mediated simply by falling. Another explanation could involve the influence of serotonin on bone. Besides their effects on balance, SSRIs may influence bone turnover and BMD. Whatever the mechanism, sufficient evidence exists to warrant the addition of SSRIs to the list of medications that contribute to osteoporosis.
Antidepressant use is not listed as a secondary cause of osteoporosis in the FRAX algorithm. Because the association between SSRI use and fracture risk appears to be independent of BMD, it may be useful to consider the possibility of including it in FRAX.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Consider BMD assessment for patients who take an SSRI, or who take an SSRI and have additional risk factors for fracture. Given the body of data on this issue, it seems appropriate to expect providers of SSRIs to conduct at least some discussion of bone health with patients.
IN THE PIPELINE: A HIGHLY EFFECTIVE AGENT TARGETING SCLEROSTIN
Recker R, Benson C, Matsumoto T, et al. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density [published online ahead of print September 5, 2014]. J Bone Miner Res. doi:10.1002/jbmr.2351.
Sclerostin is a protein secreted by osteocytes that negatively regulates the formation of mineralized bone matrix and bone mass. Recker and colleagues conducted a randomized, double-blind, placebo-
controlled, multicenter, phase 2 clinical trial of blosozumab, a humanized monoclonal antibody targeted against sclerostin. The year-long trial involved 120 postmenopausal women with low BMD (lumbar spine T-score, –2.0 to –3.5) who were randomly allocated to:
- subcutaneous blosozumab 180 mg every 4 weeks
- subcutaneous blosozumab 180 mg every 2 weeks
- subcutaneous blosozumab 270 mg every 2 weeks
- placebo.
All groups also received calcium and vitamin D and underwent serial measurement of spine and hip BMD and testing of biochemical markers of bone turnover. The mean age was 65.8 years, and the mean lumbar spine T-score was –2.8.
Women treated with blosozumab experienced statistically significant, dose-related increases in spine, femoral neck, and total hip BMD, compared with placebo. In the highest dose group, BMD increased 17.7% from baseline at the spine and 6.2% at the total hip. Biochemical markers of bone formation increased rapidly during treatment with blosozumab, trending toward pretreatment levels by the study’s end. CTX, a biochemical marker of bone resorption, decreased early during blosozumab treatment to a concentration lower than that in the placebo group by 2 weeks, and it remained low throughout treatment.
Mild injection-site reactions were reported more frequently with blosozumab than with placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although blosozumab is not yet available, clinicians should be aware of the potential of sclerostin-antibody therapies like it. Such therapies appear to have substantial anabolic effects on the skeleton and may become promising agents in the treatment of osteoporosis.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Gynecologists are “first-line” providers for the diagnosis and treatment of osteoporosis in women. Lest you doubt the importance of this fact, consider that there are more osteoporotic fractures annually in the United States than all myocardial infarctions, strokes, breast cancers, and gynecologic malignancies combined. It is our duty to stay abreast of current developments in the diagnosis and treatment of this potentially devastating skeletal disorder as our patients live longer and longer.
In this article, I present recent studies on:
- the use of conjugated estrogens and bazedoxifene (Duavee) to manage hot flashes and menopausal bone loss
- the need for adequate levels of vitamin D to maintain bone and overall health, with sunlight exposure remaining a viable option
- a reinterpretation of the findings on estrogen and fracture risk from the Women’s Health Initiative (WHI)
- the effects of selective serotonin reuptake inhibitors (SSRIs) on bone mineral density (BMD)
- development of blosozumab, a new agent in the fight against osteoporosis and fracture.
FIRST TISSUE-SELECTIVE ESTROGEN COMPLEX PROTECTS AGAINST BONE LOSS WITHOUT AFFECTING ENDOMETRIAL AND BREAST TISSUE
Komm BS, Mirkin S, Jenkins SN. Development of conjugated estrogens/bazedoxifene, the first tissue selective estrogen complex (TSEC) for management of menopausal hot flashes and postmenopausal bone loss. Steroids. 2014;90:71–81.
Pinkerton JV, Harvey JA, Lindsay R, et al; SMART-5 Investigators. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: a randomized trial. J Clin Endocrinol Metab. 2014;99(2):e189–e198.
Conjugated estrogens combined with the selective estrogen receptor modulator (SERM) bazedoxifene (Duavee) are a new option to alleviate menopausal symptoms and prevent postmenopausal bone loss. The rationale for development of the tissue-selective estrogen complex (TSEC) was to combine the benefits of conjugated estrogens with the SERM’s ability to offset estrogenic stimulation of the endometrium and breast.
TSECs offer a progestin-free alternative to traditional hormone therapy for women with a uterus. In preclinical studies, investigators found evidence to support bazedoxifene as the SERM of choice and demonstrated that, by combining it with conjugated estrogens, they could provide an optimal balance of estrogen-receptor agonist/antagonist activity, compared with other potential TSEC pairings. Clinical study results confirmed the efficacy of this combination in maintaining bone mass.
Given separately, conjugated estrogens and bazedoxifene each protect against the loss of BMD and help prevent fracture in postmenopausal women.
Findings in key populations
Komm and colleagues describe substudies of the Selective estrogens, Menopause, and Response to Therapy (SMART) trials to evaluate the combination of conjugated estrogens and SERMs to prevent osteoporosis in postmenopausal women with a uterus. One SMART-1 trial included two osteoporosis prevention substudies that evaluated the combination of conjugated estrogens and bazedoxifene in different subpopulations:
- women more than 5 years past the last menstrual period with a lumbar spine or hip BMD T-score between –1 and –2.5 plus one other risk factor for osteoporosis (n = 1,454)
- women 1 to 5 years past their last menstrual period (the interval during which bone loss is greatest) with at least one risk factor for osteoporosis (n = 861).
All doses of conjugated estrogens and bazedoxifene significantly increased the adjusted mean percentage of change in BMD of the lumbar spine from baseline to 24 months (a primary endpoint), compared with placebo, which was associated with decreases in BMD (P<.001). Findings were similar for total hip BMD.
In a separate study, Pinkerton and colleagues found that the dose of conjugated estrogens (0.45 mg) and bazedoxifene (20 mg) approved by the US Food and Drug Administration does not cause a change in breast density or thickness of the endometrium, nor does it increase breast pain, compared with placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This newly available TSEC—a combination of conjugated estrogens (0.45 mg) and bazedoxifene (20 mg)—is an effective, well-tolerated alternative to traditional estrogen-progestin hormone therapy for relief of menopausal symptoms and prevention of osteoporosis in postmenopausal women with a uterus.
DON’T EXCLUDE SUNLIGHT FROM THE BONE–HEALTH EQUATION
Holick MF. Sunlight, ultraviolet radiation, vitamin D, and skin cancer: how much sunlight do we need? Adv Exp Med Biol. 2014;810:1–16.
Many people think of vitamin D as the “sunshine vitamin.” During exposure to sunlight, ultraviolet photons enter the skin and convert 7-dehydrocholesterol to previtamin D3, which, in turn, is converted to vitamin D3.
Throughout most of human history, people have depended on sunlight for vitamin D. Variables such as skin pigmentation, sunscreen use, aging, time of day, season, and latitude dramatically affect previtamin synthesis.
Although vitamin D deficiency was thought to have been conquered, it is now recognized that more than 50% of the world’s population is at risk for vitamin D insufficiency or low levels of 25-hydroxyvitamin D. Among the reasons are inadequate fortification of foods with vitamin D and a misconception that most balanced diets contain adequate vitamin D.
Deficiency of this vitamin causes growth retardation and rickets in children and osteomalacia in adults and can precipitate and exacerbate osteopenia or osteoporosis and increase the risk of fracture in adults.
Some evidence also suggests that vitamin D deficiency may have other serious consequences, including an increased risk for common cancers and autoimmune, infectious, and cardiovascular diseases.
In this review, Holick argues that we need to remind our patients of the beneficial effects of moderate sunlight.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
There is no question that sufficient levels of vitamin D are vital to bone health, and perhaps to overall health in numerous other organ systems as well. The pendulum of our concern over skin cancers may have moved too far in the direction of sun avoidance. In reality, moderate sunlight as a source of vitamin D is still appropriate for many of our patients.
WHEN IT COMES TO ESTROGEN AND BONE, BENEFITS OUTWEIGH RISKS
de Villiers TJ. 8th Pieter van Keep Memorial Lecture. Estrogen and bone: have we completed a full circle? [published online ahead of print September 22, 2014]. Climacteric. 2014;17(suppl 2):4–7. doi:10.3109/13697137.2014.953047.
In the WHI estrogen-progestin arm, fracture rates were reported as hazard ratios:
- hip fracture, 0.66 (95% confidence interval [CI], 0.45–0.98)
- clinical vertebral fracture, 0.66 (95% CI, 0.44–0.98)
- nonvertebral fractures, 0.77 (95% CI, 0.69–0.86).
In the estrogen-only arm of the WHI, reductions in the rates of fracture were reported as percentages and were similar:
- 39% reduction in hip fracture, compared with placebo
- 38% reduction in clinical vertebral fracture
- 21% reduction in total fractures.
All of these reductions were statistically significant.
Despite the excellent anti-fracture efficacy demonstrated in the WHI, investigators concluded that the risks of hormone therapy outweighed the benefits in the general postmenopausal population.
Why we should reconsider estrogen for bone health
In his presidential address to the International Menopause Society (cited above), de Villiers observed that, in the WHI:
- Only clinical fractures were recorded. Unlike all other fracture trials, routine radiographs were not obtained to record morphometric fractures. This decision, he believes (and I concur), led to a significant understatement of estrogen’s protective effects against vertebral fracture.
- The general population studied had a low risk of fracture, with an average spinal T-score of –1.3. This, too, contributed to an understatement of estrogen’s protective effects, compared with the findings of other randomized controlled trials involving patients at much higher risk.
- From a bone-centric point of view, the WHI findings represent a favorable ratio of benefits to risks.
No bone-active drugs are completely free of potential adverse effects and restrictions, many of which become apparent only after FDA approval and general use of the drug. Bisphosphonates have been implicated in atrial fibrillation, osteonecrosis of the jaw, and atypical femur shaft fracture after extended use. Like estrogen, SERMs can increase the risk of death from deep venous thrombosis and stroke.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Estrogen is the only agent proved to be effective against all types of osteoporotic fractures during primary analysis of a large randomized controlled trial. This efficacy is of special importance for the patient with osteopenia who is at risk for fracture. Estrogen remains a serious option for the prevention of postmenopausal bone loss and osteoporosis-related fractures, especially in younger patients. Individualization of therapy is key.
COUNSEL SSRI AND SNRI USERS THAT BMD MAY DECLINE OVER THE LONG TERM
Ak E, Bulut SD, Bulut S, et al. Evaluation of the effect of selective serotonin reuptake inhibitors on bone mineral density: an observational cross-sectional study [published online ahead of print September 4, 2014]. Osteoporos Int. doi:10.10007/s00198-014-2859-2.
Moura C, Bernatsky S, Ambrahamowicz M, et al. Antidepressant use and 10-year incident fracture risk: the population-based Canadian Multicentre Osteoporosis Study (CaMoS). Osteoporos Int. 2014;25(5):1473–1481.
Bruyère O, Reginster J-V. Osteoporosis in patients taking selective serotonin reuptake inhibitors: a focus on fracture outcome [published online ahead of print August 5, 2014]. Endocrine. doi:10.1007/s12020-014-0357-0.
Evidence from longitudinal, cross-sectional, and prospective cohort studies suggests that the use of antidepressants at therapeutic doses is associated with a reduction in BMD and an increase in the risk of falls and fracture. These associations have been demonstrated in several distinct populations using various study designs, and with bone density, bone loss, or fractures as outcomes. They remain consistent even after adjustment for confounding variables such as age, body mass index, lifestyle factors such as alcohol and tobacco use, and fracture history.
Ak and colleagues recruited 60 patients given a diagnosis of generalized anxiety disorder and treated with paroxetine, sertraline, or citalopram for at least 12 months, comparing their BMD with that of 40 healthy volunteers. BMD was measured by dual-energy x-ray absorptiometry at the femoral and lumbar regions. BMD of the L2–L4 vertebrae, total lumbar vertebrae, and femoral intertrochanteric region, as well as total femoral Z-scores and femoral Ward’s region T-scores, were lower in the treatment group (P<.05). There was a significant negative correlation between the duration of treatment and the change in BMD values.
Moura and colleagues reviewed data from a large prospective Canadian cohort to assess the association between SSRIs, serotonin and norepinephrine reuptake inhibitors (SNRIs), and fracture in adults aged 50 and older. They used the Canadian Multicentre Osteoporosis Study (CaMos), a prospective, randomly selected, population-based community cohort.
Among 6,645 subjects, 192 (2.9%) were using SSRIs or SNRIs, or both, at baseline. During the 10-year study period, 978 participants (14.7%) experienced at least one fragility fracture. SSRI/SNRI use was associated with an increased risk of fragility fracture (hazard ratio [HR], 1.88; 95% CI, 1.48–2.39). After controlling for multiple risk factors, previous falls, and BMD of the hip and lumbar bone, the adjusted hazard ratio for current SSRI/SNRI use remained elevated (HR, 1.68; 95% CI, 1.32–2.14). The authors concluded that these results lend additional support to an association between SSRI/SNRI use and fragility fractures.
A few possible underlying mechanisms support the biological plausibility of these observations. One explanation is that increased fracture risk is mediated simply by falling. Another explanation could involve the influence of serotonin on bone. Besides their effects on balance, SSRIs may influence bone turnover and BMD. Whatever the mechanism, sufficient evidence exists to warrant the addition of SSRIs to the list of medications that contribute to osteoporosis.
Antidepressant use is not listed as a secondary cause of osteoporosis in the FRAX algorithm. Because the association between SSRI use and fracture risk appears to be independent of BMD, it may be useful to consider the possibility of including it in FRAX.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Consider BMD assessment for patients who take an SSRI, or who take an SSRI and have additional risk factors for fracture. Given the body of data on this issue, it seems appropriate to expect providers of SSRIs to conduct at least some discussion of bone health with patients.
IN THE PIPELINE: A HIGHLY EFFECTIVE AGENT TARGETING SCLEROSTIN
Recker R, Benson C, Matsumoto T, et al. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density [published online ahead of print September 5, 2014]. J Bone Miner Res. doi:10.1002/jbmr.2351.
Sclerostin is a protein secreted by osteocytes that negatively regulates the formation of mineralized bone matrix and bone mass. Recker and colleagues conducted a randomized, double-blind, placebo-
controlled, multicenter, phase 2 clinical trial of blosozumab, a humanized monoclonal antibody targeted against sclerostin. The year-long trial involved 120 postmenopausal women with low BMD (lumbar spine T-score, –2.0 to –3.5) who were randomly allocated to:
- subcutaneous blosozumab 180 mg every 4 weeks
- subcutaneous blosozumab 180 mg every 2 weeks
- subcutaneous blosozumab 270 mg every 2 weeks
- placebo.
All groups also received calcium and vitamin D and underwent serial measurement of spine and hip BMD and testing of biochemical markers of bone turnover. The mean age was 65.8 years, and the mean lumbar spine T-score was –2.8.
Women treated with blosozumab experienced statistically significant, dose-related increases in spine, femoral neck, and total hip BMD, compared with placebo. In the highest dose group, BMD increased 17.7% from baseline at the spine and 6.2% at the total hip. Biochemical markers of bone formation increased rapidly during treatment with blosozumab, trending toward pretreatment levels by the study’s end. CTX, a biochemical marker of bone resorption, decreased early during blosozumab treatment to a concentration lower than that in the placebo group by 2 weeks, and it remained low throughout treatment.
Mild injection-site reactions were reported more frequently with blosozumab than with placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although blosozumab is not yet available, clinicians should be aware of the potential of sclerostin-antibody therapies like it. Such therapies appear to have substantial anabolic effects on the skeleton and may become promising agents in the treatment of osteoporosis.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
STOP relying on 2D ultrasound for IUD localization
Several decades ago, the negative publicity associated with the Dalkon Shield brand of intrauterine device (IUD) caused a decline in the use of this form of long-acting reversible contraception (LARC). However, there has been a resurgence in the use of IUDs in the past few years;1 the current opinion of the American College of Obstetricians and Gynecologists encourages first-line use of LARC, and the IUD is popular with patients.2
Related articles:
Let's increase our use of IUDs and improve contraceptive effectiveness in this country. Robert L. Barbieri, MD (Editorial; August 2012)
5 IUD myths dispelled. Anne A. Moore, DNP, APN (September 2-13)
Some patients with IUDs will experience more painful periods, intramenstrual cramps or bleeding, or heavier menses. Until recently, many clinicians (including us) believed these possible adverse effects were not unexpected and often warned patients that it was not abnormal if one or more of these occurred.
More recently, however, results of an important study showed that patients with part of their IUD not totally located within the endometrial cavity (eg, protruded into the cervix or partially piercing the myometrium) had an increased rate of pain and bleeding.3 Of patients with any abnormally located part of their IUD, 36% had abnormal bleeding and 39% had pain, compared with 15% of women who reported abnormal bleeding and 19% who reported pain when their IUD was totally positioned within the endometrial cavity (P = .02 and .03, respectively).
Related article: Malpositioned IUDs: When you should intervene (and when you should not). Kari Braaten, MD, MPH, and Alisa B. Goldberg, MD, MPH (August 2012)
A 3D ultrasound can reveal malpositioning not identified on 2D
Prior to the widespread availability of ultrasonography, some practitioners will remember placing a sound in the uterus taped to a tenaculum and obtaining a flat plate and cross table lateral abdominal x-ray to ensure an IUD was indeed “intrauterine.” With the advent of transvaginal ultrasonography, a long-axis view with a centrally located IUD was thought to definitively locate the device as intrauterine (FIGURES 1A, 2A, AND 3A).
Related articles:
How to identify and localize IUDs on ultrasound. Michelle Stalnaker, MD, and Andrew Kaunitz, MD (Images in GYN ultrasound; August 2014)
Update on Contraception. Melissa Chen, MD, MPH, and Mitchell Creinin, MD (August 2014)
Now, with the advent of 3D transvaginal ultrasonography and the ability to construct a coronal plane, some IUDs, which appear to be totally normal on 2D sonography, actually show an arm that pierces the myometrium or protrudes into the cervix (FIGURES 1B, 2B, AND 3B). This in fact is probably the location that must exist at insertion for so-called “migration” to occur through the myometrium as uterine contractions, especially with menses, occur.
So the next time a patient with an IUD reports pain or bleeding, STOP doing only 2D ultrasound and START obtaining a 3D coronal reconstructive view.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
1. Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD [published correction appears in J Ultrasound Med. 2010;29(12):1848]. J Ultrasound Med. 2010;29(10):1453–1456.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114(6):1434–1438.
3. Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34(1):110–115.
Several decades ago, the negative publicity associated with the Dalkon Shield brand of intrauterine device (IUD) caused a decline in the use of this form of long-acting reversible contraception (LARC). However, there has been a resurgence in the use of IUDs in the past few years;1 the current opinion of the American College of Obstetricians and Gynecologists encourages first-line use of LARC, and the IUD is popular with patients.2
Related articles:
Let's increase our use of IUDs and improve contraceptive effectiveness in this country. Robert L. Barbieri, MD (Editorial; August 2012)
5 IUD myths dispelled. Anne A. Moore, DNP, APN (September 2-13)
Some patients with IUDs will experience more painful periods, intramenstrual cramps or bleeding, or heavier menses. Until recently, many clinicians (including us) believed these possible adverse effects were not unexpected and often warned patients that it was not abnormal if one or more of these occurred.
More recently, however, results of an important study showed that patients with part of their IUD not totally located within the endometrial cavity (eg, protruded into the cervix or partially piercing the myometrium) had an increased rate of pain and bleeding.3 Of patients with any abnormally located part of their IUD, 36% had abnormal bleeding and 39% had pain, compared with 15% of women who reported abnormal bleeding and 19% who reported pain when their IUD was totally positioned within the endometrial cavity (P = .02 and .03, respectively).
Related article: Malpositioned IUDs: When you should intervene (and when you should not). Kari Braaten, MD, MPH, and Alisa B. Goldberg, MD, MPH (August 2012)
A 3D ultrasound can reveal malpositioning not identified on 2D
Prior to the widespread availability of ultrasonography, some practitioners will remember placing a sound in the uterus taped to a tenaculum and obtaining a flat plate and cross table lateral abdominal x-ray to ensure an IUD was indeed “intrauterine.” With the advent of transvaginal ultrasonography, a long-axis view with a centrally located IUD was thought to definitively locate the device as intrauterine (FIGURES 1A, 2A, AND 3A).
Related articles:
How to identify and localize IUDs on ultrasound. Michelle Stalnaker, MD, and Andrew Kaunitz, MD (Images in GYN ultrasound; August 2014)
Update on Contraception. Melissa Chen, MD, MPH, and Mitchell Creinin, MD (August 2014)
Now, with the advent of 3D transvaginal ultrasonography and the ability to construct a coronal plane, some IUDs, which appear to be totally normal on 2D sonography, actually show an arm that pierces the myometrium or protrudes into the cervix (FIGURES 1B, 2B, AND 3B). This in fact is probably the location that must exist at insertion for so-called “migration” to occur through the myometrium as uterine contractions, especially with menses, occur.
So the next time a patient with an IUD reports pain or bleeding, STOP doing only 2D ultrasound and START obtaining a 3D coronal reconstructive view.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
Several decades ago, the negative publicity associated with the Dalkon Shield brand of intrauterine device (IUD) caused a decline in the use of this form of long-acting reversible contraception (LARC). However, there has been a resurgence in the use of IUDs in the past few years;1 the current opinion of the American College of Obstetricians and Gynecologists encourages first-line use of LARC, and the IUD is popular with patients.2
Related articles:
Let's increase our use of IUDs and improve contraceptive effectiveness in this country. Robert L. Barbieri, MD (Editorial; August 2012)
5 IUD myths dispelled. Anne A. Moore, DNP, APN (September 2-13)
Some patients with IUDs will experience more painful periods, intramenstrual cramps or bleeding, or heavier menses. Until recently, many clinicians (including us) believed these possible adverse effects were not unexpected and often warned patients that it was not abnormal if one or more of these occurred.
More recently, however, results of an important study showed that patients with part of their IUD not totally located within the endometrial cavity (eg, protruded into the cervix or partially piercing the myometrium) had an increased rate of pain and bleeding.3 Of patients with any abnormally located part of their IUD, 36% had abnormal bleeding and 39% had pain, compared with 15% of women who reported abnormal bleeding and 19% who reported pain when their IUD was totally positioned within the endometrial cavity (P = .02 and .03, respectively).
Related article: Malpositioned IUDs: When you should intervene (and when you should not). Kari Braaten, MD, MPH, and Alisa B. Goldberg, MD, MPH (August 2012)
A 3D ultrasound can reveal malpositioning not identified on 2D
Prior to the widespread availability of ultrasonography, some practitioners will remember placing a sound in the uterus taped to a tenaculum and obtaining a flat plate and cross table lateral abdominal x-ray to ensure an IUD was indeed “intrauterine.” With the advent of transvaginal ultrasonography, a long-axis view with a centrally located IUD was thought to definitively locate the device as intrauterine (FIGURES 1A, 2A, AND 3A).
Related articles:
How to identify and localize IUDs on ultrasound. Michelle Stalnaker, MD, and Andrew Kaunitz, MD (Images in GYN ultrasound; August 2014)
Update on Contraception. Melissa Chen, MD, MPH, and Mitchell Creinin, MD (August 2014)
Now, with the advent of 3D transvaginal ultrasonography and the ability to construct a coronal plane, some IUDs, which appear to be totally normal on 2D sonography, actually show an arm that pierces the myometrium or protrudes into the cervix (FIGURES 1B, 2B, AND 3B). This in fact is probably the location that must exist at insertion for so-called “migration” to occur through the myometrium as uterine contractions, especially with menses, occur.
So the next time a patient with an IUD reports pain or bleeding, STOP doing only 2D ultrasound and START obtaining a 3D coronal reconstructive view.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: rbarbieri@frontlinemedcom.com
1. Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD [published correction appears in J Ultrasound Med. 2010;29(12):1848]. J Ultrasound Med. 2010;29(10):1453–1456.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114(6):1434–1438.
3. Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34(1):110–115.
1. Shipp TD, Bromley B, Benacerraf BR. The width of the uterine cavity is narrower in patients with an embedded intrauterine device (IUD) compared to a normally positioned IUD [published correction appears in J Ultrasound Med. 2010;29(12):1848]. J Ultrasound Med. 2010;29(10):1453–1456.
2. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice; Long-Acting Reversible Contraception Working Group. ACOG Committee Opinion No. 450: Increasing use of contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2009;114(6):1434–1438.
3. Benacerraf BR, Shipp TD, Bromley B. Three-dimensional ultrasound detection of abnormally located intrauterine contraceptive devices that are a source of pelvic pain and abnormal bleeding. Ultrasound Obstet Gynecol. 2009;34(1):110–115.
Transvaginal ultrasonography of ovarian cyst
The "pill's" effects on bone accrual in young women
To ensure breast health and reduce the risk of venous thromboembolic events, drug makers have developed oral contraceptives (OCs) with lower and lower doses of ethinyl estradiol. In the process, however, the beneficial effects of endogenous estradiol on bone acquisition have been suppressed. Therefore, the lowest-dose OC may not necessarily be the most appropriate clinical choice for adolescents and young women seeking contraception.
In this audiocast, Dr. Goldstein discusses:
- How low-dose OCs may affect a young woman’s bone mass accrual
- How to assure that young women reach the goal of peak bone mass while taking OCs
- Why more clinicians aren't aware of this issue
- The contraceptive methods he recommends for a young woman who is still building bone mass
- For which populations does he recommend low-dose OCs?
Dr. Goldstein is the author of Update on Osteoporosis (December 2013).
To ensure breast health and reduce the risk of venous thromboembolic events, drug makers have developed oral contraceptives (OCs) with lower and lower doses of ethinyl estradiol. In the process, however, the beneficial effects of endogenous estradiol on bone acquisition have been suppressed. Therefore, the lowest-dose OC may not necessarily be the most appropriate clinical choice for adolescents and young women seeking contraception.
In this audiocast, Dr. Goldstein discusses:
- How low-dose OCs may affect a young woman’s bone mass accrual
- How to assure that young women reach the goal of peak bone mass while taking OCs
- Why more clinicians aren't aware of this issue
- The contraceptive methods he recommends for a young woman who is still building bone mass
- For which populations does he recommend low-dose OCs?
Dr. Goldstein is the author of Update on Osteoporosis (December 2013).
To ensure breast health and reduce the risk of venous thromboembolic events, drug makers have developed oral contraceptives (OCs) with lower and lower doses of ethinyl estradiol. In the process, however, the beneficial effects of endogenous estradiol on bone acquisition have been suppressed. Therefore, the lowest-dose OC may not necessarily be the most appropriate clinical choice for adolescents and young women seeking contraception.
In this audiocast, Dr. Goldstein discusses:
- How low-dose OCs may affect a young woman’s bone mass accrual
- How to assure that young women reach the goal of peak bone mass while taking OCs
- Why more clinicians aren't aware of this issue
- The contraceptive methods he recommends for a young woman who is still building bone mass
- For which populations does he recommend low-dose OCs?
Dr. Goldstein is the author of Update on Osteoporosis (December 2013).
Update on Osteoporosis
Because the low bone mass and deterioration of bone microarchitecture and quality that characterize osteoporosis can lead to fragility fracture, it is vital that we intervene in our patients’ health in a timely manner to reduce this risk. One way to accomplish this goal is to understand the role of age in determining a woman’s fracture risk. For example, an 80-year-old woman and a 50-year-old woman with a T-score of –2.5, as measured by dual x-ray absorptiometry (DXA), will have dramatically different fracture risks. According to the World Health Organization’s fracture-risk assessment tool (http://www.shef.ac.uk/FRAX/), the older woman has a 10-year probability of hip fracture approximately five times greater than the younger woman.
Although no new therapies have been approved during the past year, several important findings were published that affect clinical management of menopausal patients or suggest changes likely in the future.
In this article, I review:
- the latest guidance on osteoporosis from the American College of Obstetricians and Gynecologists (ACOG)
- the most recent indications for bone mineral density (BMD) testing from the International Society for Clinical Densitometry (ISCD)
- a study exploring the effect of oral hormonal contraception on the acquisition of peak BMD in adolescents and young women
- results of a randomized trial of the experimental agent odanacatib in postmenopausal women
- a pilot study of teriparatide (Forteo) for idiopathic osteoporosis in premenopausal women.
ACOG ISSUES RECOMMENDATIONS ON SCREENING, TREATMENT, AND LIFESTYLE
Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin #129: Osteoporosis. Obstet Gynecol. 2012;120(3):718–734.
This comprehensive review of management guidelines for ObGyns deserves “top billing” in this update. It offers recommendations on important interventions, from BMD measurement and subsequent monitoring to calcium and vitamin D supplementation.
When to initiate screening
- Begin BMD screening using DXA at age 65. DXA also may be appropriate for younger women if they are postmenopausal and have other significant risk factors for osteoporosis or fracture (Level A evidence – based on good and consistent scientific evidence).
- In the absence of new risk factors, do not perform DXA screening more frequently than every 2 years (Level B evidence – based on limited or inconsistent scientific evidence).
Which patients should be treated?
Treatment is recommended for:
- women with a T-score of –2.5 or lower
- women who have had a low-trauma fracture
- women with a T-score between –1 and –2.5 and a 10-year FRAX hip-fracture risk of 3% or higher or a 10-year FRAX risk of major osteoporotic fracture of 20% or higher, or both. A major osteoporotic fracture involves the forearm, hip, or shoulder, or a clinical vertebral fracture (Level A evidence).
Only therapies approved by the US FDA should be used for medical treatment. They are raloxifene (Evista), bisphosphonates (Actonel, Boniva, Fosamax, Reclast), parathyroid hormone, denosumab (Prolia), and calcitonin (Fortical, Miacalcin) (Level A evidence).
Monitoring of therapy
In the absence of new risk factors, do not repeat DXA monitoring of therapy once BMD has been determined to be stable or improved (Level B evidence).
Lifestyle recommendations
- Counsel women about lifestyle factors that may affect BMD and fracture risk, which include smoking, poor nutrition and excessive weight loss, weight-bearing and muscle-strengthening exercise, and fall prevention (Level B evidence).
- Advise patients of current recommendations for calcium and vitamin D intake from the Institute of Medicine, which are calcium 1,200 mg/day and vitamin D 600 IU/day for women aged 51 to 70 years (Level B evidence).
- Counsel girls and women of all ages about the effects of lifestyle on bone health (Level C evidence – based on consensus and expert opinion).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
By utilizing the FRAX risk-assessment tool, we can determine which patients truly require treatment. In the process, we should be able to reduce the overtreatment of younger women with low bone mass as well as the undertreatment of older women who appear to have less deranged bone mass.
ACOG also emphasizes the need to avoid the overutilization of DXA scans in various groups, as well as the importance of lifestyle adjustments to promote bone health in all age groups.
Related Article: STOP performing DXA scans in healthy, perimenopausal women Lisa Larkin, MD, and Andrew M. Kaunitz, MD (Stop/Start, Januaray 2013)
CLINICAL DENSITOMETRISTS WEIGH IN ON INDICATIONS FOR BMD ASSESSMENT
International Society for Clinical Densitometry (ISCD). Indications for bone mineral density (BMD) testing. http://www.iscd.org/official-positions/2013-iscd-official-positions-adult/. Updated August 15, 2013. Accessed November 7, 2013.
In its comprehensive review of BMD assessment, the ISCD elucidates the process, which typically involves DXA imaging.
Indications for BMD assessment
- The female patient is age 65 or older
- The postmenopausal patient is younger than age 65 but has a risk factor for low bone mass, such as low body weight, a history of fracture, use of a high-risk medication, or a disease or condition associated with bone loss
- The perimenopausal woman has clinical risk factors for fracture, such as low body weight, history of fracture, or use of a high-risk medication
- The adult sustains a fragility fracture
- The adult has a disease or condition associated with low bone mass or bone loss
- The adult is taking a medication associated with low bone mass or bone loss
- The patient is being considered for pharmacologic therapy
- The patient is being treated, to monitor effect
- The patient is not receiving therapy, but evidence of bone loss would lead to treatment.
When serial BMD assessment is appropriate
- When it is used to determine whether treatment should be initiated (in untreated patients) because of significant bone loss
- To monitor response to therapy by identifying an increase or stabilization of BMD
- To identify nonresponse by documenting a loss of BMD, suggesting the need for treatment re-evaluation and assessment for a secondary cause of osteoporosis
- To follow-up earlier assessment when the expected change in BMD equals or exceeds the least significant change
- When the interval is appropriate for the patient’s clinical status. (In general, BMD assessment is performed 1 year after initiation or change of therapy, with longer intervals once a therapeutic effect has been established.)
- When the patient is using a medication associated with rapid bone loss, such as glucocorticoid therapy. In such a patient, more frequent testing may be appropriate.
- Note that these recommendations differ slightly from ACOG’s statements regarding the use of DXA.
Diagnosis of osteoporosis
- According to the WHO international reference standard, osteoporosis can be diagnosed when a patient has a T-score of –2.5 or below at the femoral neck. The reference standard from which the T-score is calculated is the white female population aged 20 to 29 years in the National Health and Nutrition Examination Survey (NHANES) III database.
- Osteoporosis also may be diagnosed in postmenopausal women and men aged 50 or older when the T-score of the lumbar spine, total hip, or femoral neck is –2.5 or below. In some circumstances, the
33% radius (also called the 1/3 radius) may be utilized. - Other hip regions of interest, including Ward’s area and the greater trochanter, should not be used for diagnosis.
A move away from use of the term “osteopenia”
- The term may be retained, but “low bone mass” or “low bone density” is preferred
- People with low bone mass or low bone density do not necessarily have a high risk of fracture.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
DXA testing remains the cornerstone of diagnosis for patients at risk for fragility fracture. It also is the optimal method to determine the need for pharmacotherapy. In some instances, however, overutilization of DXA imaging has led to overtreatment, especially in younger women with low bone mass, when treatment is based on variables other than diminished bone quality (including, “small-boned” women, genetics, and failure to achieve peak bone mass as high as one’s peer group prior to menopause).
These recommendations help to clarify the rationale for follow-up DXA imaging for patients on therapy, an area in which scientific unanimity is lacking.
Related Article: What is the optimal interval for osteoporosis screening in postmenopausal women before fracture occurrence and osteoporosis treatment initiation? Steven R. Goldstein, MD (Examining the Evidence, August 2012)
IN ADOLESCENTS AND YOUNG WOMEN, CONSIDER THE BONE EFFECTS OF ORAL CONTRACEPTIVES
Ziglar S, Hunter TS. The effect of hormonal oral contraception on acquisition of peak bone mineral density of adolescents and young women. J Pharm Pract. 2012;25(3):331–340.
The bone loss observed in adolescents and young women who use depot medroxyprogesterone acetate (Depo-Provera) for contraception led to an FDA-mandated boxed warning on the medication’s package insert. The effect of oral contraceptives (OCs) on bone growth has received little publicity, however.
The best strategy to offset the natural loss of bone associated with aging and the menopausal transition is to ensure the development of maximal bone mass in youth. When maximal BMD is not achieved, the risk of osteoporosis is increased.
Adolescence is a critical period of bone mineralization, which is mediated by endogenous estradiol. The highest rate of bone mass accrual occurs 1 year before and 3 years after menarche. Young women who consume a diet low in calcium or who have an eating disorder, who fail to exercise, who smoke, or who have low estrogen status are most likely to have low peak bone mass.
OCs suppress endogenous estradiol production by interrupting the hypothalamic-pituitary-ovarian axis. By replacing endogenous estradiol with ethinyl estradiol (EE), OCs establish and maintain new hormone levels. Early initiation and use of very-low-dose EE increases the likelihood that the accrual of bone mass will be jeopardized at a critical time of bone mineralization.
Details of this meta-analysis
Ziglar and Hunter reviewed 11 prospective trials that showed a decrease in bone mass in adolescents and young women who used low-dose OCs, six trials that showed a neutral effect, and one trial that found an increase in bone mass. This last study involved only members of the US military whose level of daily exercise may not be representative of the general population of women the same age. Investigators also theorized that the use of norethindrone acetate as an androgenic progestin in this study may have exerted a positive effect on bone accrual.
Ziglar and Hunter concluded that the use of OCs containing 20 µg EE prevents adolescents and young women from attaining peak BMD. Evidence on the effect of contraceptives formulated with 30 to 35 µg EE is less definitive, but this dose may also impede BMD acquisition in adolescents.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
To ensure breast health and reduce the risk of venous thromboembolic events, drug makers have developed OCs with lower and lower doses of EE. In the process, however, the beneficial effects of endogenous estradiol on bone acquisition have been suppressed. Therefore, the lowest-dose OC may not necessarily be the most appropriate clinical choice for adolescents and young women seeking contraception.
Related Article: Osteoporosis treatment and breast cancer prevention: Two goals, one treatment? Robert L. Barbieri, MD (Editorial, November 2013)
EXPERIMENTAL DRUG REDUCES BONE RESORPTION WITHOUT IMPEDING BONE FORMATION
Brixen K, Chapurlat R, Cheung AM, et al. Bone density, turnover, and estimated strength in postmenopausal women treated with odanacatib: a randomized trial. J Clin Endocrinol Metab. 2013;98(2):571–580.
Current treatments for osteoporosis include antiresorptive agents, such as bisphosphonates and denosumab, that preserve bone mass by reducing the rate of bone turnover. These drugs reduce the number or activity (or both) of bone-resorbing osteoclasts. Because osteoclasts play a role in stimulating bone formation by osteoblasts, these treatments indirectly lower bone formation.
Odanacatib is a drug in Phase 3 development for the treatment of postmenopausal osteoporosis. It is a highly selective and reversible oral inhibitor of the collagenase activity of cathepsin K, which is secreted by osteoclasts. Odanacatib reduces bone resorption without reducing the number of osteoclasts and, thus, appears to preserve bone formation.
Details of the trial
Brixen and colleagues conducted a randomized, double-blind, international, 2-year, Phase 3 trial comparing odancatib 50 mg once weekly with placebo in postmenopausal women treated with calcium and vitamin D. The primary endpoint was the change from baseline BMD at the lumbar spine at 1 year, as assessed by DXA. Secondary endpoints included the change from baseline BMD at the hip (total hip, femoral neck, and trochanter) at 1 year, the change from baseline BMD at the spine and hip at 2 years, and 1- and 2-year changes in bone-turnover markers. A total of 214 women were enrolled (average age: 64 years; average T-score of 1.8 at the lumbar spine, –1.8 at the femoral neck, and –1.3 at the total hip).
At 1 year, the change from baseline BMD at the lumbar spine was significantly higher (P <.001) in women receiving odanacatib, compared with placebo (treatment difference: 3.5%). At 2 years, the treatment difference was even higher (5.4%). The mean changes in BMD at the femoral neck, total hip, and trochanter also were significantly greater (P <.001) in women receiving odanacatib, with treatment differences at 2 years of 3.8%, 3.3%, and 5.5%, respectively.
During the first 6 months of the trial, serum concentrations of bone-turnover markers (CTX and P1NP) decreased significantly (P <.001) in odanacatib-treated women, compared with those given placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although no new agents for the treatment of osteoporosis have been introduced over the past year, cathepsin K inhibitors appear to offer great promise for the future. As clinicians, we need to keep abreast of new developments that may be of potential value to our patients.
PILOT STUDY: TERIPARATIDE WAS EFFECTIVE IN 81% OF PREMENOPAUSAL WOMEN WITH IDIOPATHIC OSTEOPOROSIS
Cohen A, Stein EM, Recker RR, et al. Teriparatide for idiopathic osteoporosis in premenopausal women: A pilot study. J Clin Endocrinol Metab. 2013;98(5):1971–1981.
Idiopathic osteoporosis (IOP) affects young, otherwise healthy men and women with intact gonadal function and no secondary cause of bone loss or fragility. Women with IOP have abnormal bone microarchitecture with thinner cortices; fewer, thinner, and more widely separated and heterogeneously distributed trabeculae; more rod-like trabecular structures; less trabecular stiffness; and a higher level of marrow fat.
The osteoanabolic agent teriperatide increases BMD and reduces the incidence of fracture in postmenopausal women and in patients with glucocorticoid-induced osteoporosis, and it increases BMD in men with IOP. This study explored its effect in premenopausal women with IOP.
Details of the study
Cohen and colleagues recruited premenopausal women aged 20 to 48 years who had one or both of the following traits:
- a history of at least one low-trauma fracture more than 6 months before enrollment
- low BMD of the spine or hip (Z-score of –2.0 or below), as assessed by DXA.
All participants had regular menses and early follicular-phase follicle-stimulating hormone (FSH) levels below 20 mIU/mL; none were using hormonal contraception. Women who had secondary osteoporosis related to estrogen deficiency, an eating disorder, an endocrinopathy, celiac or gastrointestinal disease, hyperparathyroidism, marked hypercalciuria, a low serum level of 25-hydroxyvitamin D (<20 ng/mL), and drug exposures were excluded.
All participants (n = 21) received teriparatide 20 µg daily in the morning or evening, according to preference. They also were given calcium 630 mg and vitamin D 800 IU daily.
BMD increased at the spine by 10.8% (standard deviation: 8.3%), total hip by 6.2% (5.6%), and femoral neck by 7.6% (3.4%) (all P <.001). Transiliac biopsies demonstrated significant increases in cortical width and porosity, and trabecular bone volume and number increased as well. Four women had no increase in BMD.
Overall, Cohen and colleagues concluded that teriparatide was associated with increased BMD at the spine and hip and improved trabecular microarchitecture and stiffness at the iliac crest in the majority of women with IOP.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although ObGyns rarely prescribe teriparatide, often leaving this option for metabolic bone experts to offer, we should keep premenopausal IOP in mind when younger patients sustain low-trauma fractures of the hip or vertebrae, as well as fracture of an upper or lower extremity or the ribs.
Teriparatide appears to be an excellent choice for the majority of premenopausal patients with IOP (81% of patients in this pilot study).
Because the low bone mass and deterioration of bone microarchitecture and quality that characterize osteoporosis can lead to fragility fracture, it is vital that we intervene in our patients’ health in a timely manner to reduce this risk. One way to accomplish this goal is to understand the role of age in determining a woman’s fracture risk. For example, an 80-year-old woman and a 50-year-old woman with a T-score of –2.5, as measured by dual x-ray absorptiometry (DXA), will have dramatically different fracture risks. According to the World Health Organization’s fracture-risk assessment tool (http://www.shef.ac.uk/FRAX/), the older woman has a 10-year probability of hip fracture approximately five times greater than the younger woman.
Although no new therapies have been approved during the past year, several important findings were published that affect clinical management of menopausal patients or suggest changes likely in the future.
In this article, I review:
- the latest guidance on osteoporosis from the American College of Obstetricians and Gynecologists (ACOG)
- the most recent indications for bone mineral density (BMD) testing from the International Society for Clinical Densitometry (ISCD)
- a study exploring the effect of oral hormonal contraception on the acquisition of peak BMD in adolescents and young women
- results of a randomized trial of the experimental agent odanacatib in postmenopausal women
- a pilot study of teriparatide (Forteo) for idiopathic osteoporosis in premenopausal women.
ACOG ISSUES RECOMMENDATIONS ON SCREENING, TREATMENT, AND LIFESTYLE
Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin #129: Osteoporosis. Obstet Gynecol. 2012;120(3):718–734.
This comprehensive review of management guidelines for ObGyns deserves “top billing” in this update. It offers recommendations on important interventions, from BMD measurement and subsequent monitoring to calcium and vitamin D supplementation.
When to initiate screening
- Begin BMD screening using DXA at age 65. DXA also may be appropriate for younger women if they are postmenopausal and have other significant risk factors for osteoporosis or fracture (Level A evidence – based on good and consistent scientific evidence).
- In the absence of new risk factors, do not perform DXA screening more frequently than every 2 years (Level B evidence – based on limited or inconsistent scientific evidence).
Which patients should be treated?
Treatment is recommended for:
- women with a T-score of –2.5 or lower
- women who have had a low-trauma fracture
- women with a T-score between –1 and –2.5 and a 10-year FRAX hip-fracture risk of 3% or higher or a 10-year FRAX risk of major osteoporotic fracture of 20% or higher, or both. A major osteoporotic fracture involves the forearm, hip, or shoulder, or a clinical vertebral fracture (Level A evidence).
Only therapies approved by the US FDA should be used for medical treatment. They are raloxifene (Evista), bisphosphonates (Actonel, Boniva, Fosamax, Reclast), parathyroid hormone, denosumab (Prolia), and calcitonin (Fortical, Miacalcin) (Level A evidence).
Monitoring of therapy
In the absence of new risk factors, do not repeat DXA monitoring of therapy once BMD has been determined to be stable or improved (Level B evidence).
Lifestyle recommendations
- Counsel women about lifestyle factors that may affect BMD and fracture risk, which include smoking, poor nutrition and excessive weight loss, weight-bearing and muscle-strengthening exercise, and fall prevention (Level B evidence).
- Advise patients of current recommendations for calcium and vitamin D intake from the Institute of Medicine, which are calcium 1,200 mg/day and vitamin D 600 IU/day for women aged 51 to 70 years (Level B evidence).
- Counsel girls and women of all ages about the effects of lifestyle on bone health (Level C evidence – based on consensus and expert opinion).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
By utilizing the FRAX risk-assessment tool, we can determine which patients truly require treatment. In the process, we should be able to reduce the overtreatment of younger women with low bone mass as well as the undertreatment of older women who appear to have less deranged bone mass.
ACOG also emphasizes the need to avoid the overutilization of DXA scans in various groups, as well as the importance of lifestyle adjustments to promote bone health in all age groups.
Related Article: STOP performing DXA scans in healthy, perimenopausal women Lisa Larkin, MD, and Andrew M. Kaunitz, MD (Stop/Start, Januaray 2013)
CLINICAL DENSITOMETRISTS WEIGH IN ON INDICATIONS FOR BMD ASSESSMENT
International Society for Clinical Densitometry (ISCD). Indications for bone mineral density (BMD) testing. http://www.iscd.org/official-positions/2013-iscd-official-positions-adult/. Updated August 15, 2013. Accessed November 7, 2013.
In its comprehensive review of BMD assessment, the ISCD elucidates the process, which typically involves DXA imaging.
Indications for BMD assessment
- The female patient is age 65 or older
- The postmenopausal patient is younger than age 65 but has a risk factor for low bone mass, such as low body weight, a history of fracture, use of a high-risk medication, or a disease or condition associated with bone loss
- The perimenopausal woman has clinical risk factors for fracture, such as low body weight, history of fracture, or use of a high-risk medication
- The adult sustains a fragility fracture
- The adult has a disease or condition associated with low bone mass or bone loss
- The adult is taking a medication associated with low bone mass or bone loss
- The patient is being considered for pharmacologic therapy
- The patient is being treated, to monitor effect
- The patient is not receiving therapy, but evidence of bone loss would lead to treatment.
When serial BMD assessment is appropriate
- When it is used to determine whether treatment should be initiated (in untreated patients) because of significant bone loss
- To monitor response to therapy by identifying an increase or stabilization of BMD
- To identify nonresponse by documenting a loss of BMD, suggesting the need for treatment re-evaluation and assessment for a secondary cause of osteoporosis
- To follow-up earlier assessment when the expected change in BMD equals or exceeds the least significant change
- When the interval is appropriate for the patient’s clinical status. (In general, BMD assessment is performed 1 year after initiation or change of therapy, with longer intervals once a therapeutic effect has been established.)
- When the patient is using a medication associated with rapid bone loss, such as glucocorticoid therapy. In such a patient, more frequent testing may be appropriate.
- Note that these recommendations differ slightly from ACOG’s statements regarding the use of DXA.
Diagnosis of osteoporosis
- According to the WHO international reference standard, osteoporosis can be diagnosed when a patient has a T-score of –2.5 or below at the femoral neck. The reference standard from which the T-score is calculated is the white female population aged 20 to 29 years in the National Health and Nutrition Examination Survey (NHANES) III database.
- Osteoporosis also may be diagnosed in postmenopausal women and men aged 50 or older when the T-score of the lumbar spine, total hip, or femoral neck is –2.5 or below. In some circumstances, the
33% radius (also called the 1/3 radius) may be utilized. - Other hip regions of interest, including Ward’s area and the greater trochanter, should not be used for diagnosis.
A move away from use of the term “osteopenia”
- The term may be retained, but “low bone mass” or “low bone density” is preferred
- People with low bone mass or low bone density do not necessarily have a high risk of fracture.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
DXA testing remains the cornerstone of diagnosis for patients at risk for fragility fracture. It also is the optimal method to determine the need for pharmacotherapy. In some instances, however, overutilization of DXA imaging has led to overtreatment, especially in younger women with low bone mass, when treatment is based on variables other than diminished bone quality (including, “small-boned” women, genetics, and failure to achieve peak bone mass as high as one’s peer group prior to menopause).
These recommendations help to clarify the rationale for follow-up DXA imaging for patients on therapy, an area in which scientific unanimity is lacking.
Related Article: What is the optimal interval for osteoporosis screening in postmenopausal women before fracture occurrence and osteoporosis treatment initiation? Steven R. Goldstein, MD (Examining the Evidence, August 2012)
IN ADOLESCENTS AND YOUNG WOMEN, CONSIDER THE BONE EFFECTS OF ORAL CONTRACEPTIVES
Ziglar S, Hunter TS. The effect of hormonal oral contraception on acquisition of peak bone mineral density of adolescents and young women. J Pharm Pract. 2012;25(3):331–340.
The bone loss observed in adolescents and young women who use depot medroxyprogesterone acetate (Depo-Provera) for contraception led to an FDA-mandated boxed warning on the medication’s package insert. The effect of oral contraceptives (OCs) on bone growth has received little publicity, however.
The best strategy to offset the natural loss of bone associated with aging and the menopausal transition is to ensure the development of maximal bone mass in youth. When maximal BMD is not achieved, the risk of osteoporosis is increased.
Adolescence is a critical period of bone mineralization, which is mediated by endogenous estradiol. The highest rate of bone mass accrual occurs 1 year before and 3 years after menarche. Young women who consume a diet low in calcium or who have an eating disorder, who fail to exercise, who smoke, or who have low estrogen status are most likely to have low peak bone mass.
OCs suppress endogenous estradiol production by interrupting the hypothalamic-pituitary-ovarian axis. By replacing endogenous estradiol with ethinyl estradiol (EE), OCs establish and maintain new hormone levels. Early initiation and use of very-low-dose EE increases the likelihood that the accrual of bone mass will be jeopardized at a critical time of bone mineralization.
Details of this meta-analysis
Ziglar and Hunter reviewed 11 prospective trials that showed a decrease in bone mass in adolescents and young women who used low-dose OCs, six trials that showed a neutral effect, and one trial that found an increase in bone mass. This last study involved only members of the US military whose level of daily exercise may not be representative of the general population of women the same age. Investigators also theorized that the use of norethindrone acetate as an androgenic progestin in this study may have exerted a positive effect on bone accrual.
Ziglar and Hunter concluded that the use of OCs containing 20 µg EE prevents adolescents and young women from attaining peak BMD. Evidence on the effect of contraceptives formulated with 30 to 35 µg EE is less definitive, but this dose may also impede BMD acquisition in adolescents.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
To ensure breast health and reduce the risk of venous thromboembolic events, drug makers have developed OCs with lower and lower doses of EE. In the process, however, the beneficial effects of endogenous estradiol on bone acquisition have been suppressed. Therefore, the lowest-dose OC may not necessarily be the most appropriate clinical choice for adolescents and young women seeking contraception.
Related Article: Osteoporosis treatment and breast cancer prevention: Two goals, one treatment? Robert L. Barbieri, MD (Editorial, November 2013)
EXPERIMENTAL DRUG REDUCES BONE RESORPTION WITHOUT IMPEDING BONE FORMATION
Brixen K, Chapurlat R, Cheung AM, et al. Bone density, turnover, and estimated strength in postmenopausal women treated with odanacatib: a randomized trial. J Clin Endocrinol Metab. 2013;98(2):571–580.
Current treatments for osteoporosis include antiresorptive agents, such as bisphosphonates and denosumab, that preserve bone mass by reducing the rate of bone turnover. These drugs reduce the number or activity (or both) of bone-resorbing osteoclasts. Because osteoclasts play a role in stimulating bone formation by osteoblasts, these treatments indirectly lower bone formation.
Odanacatib is a drug in Phase 3 development for the treatment of postmenopausal osteoporosis. It is a highly selective and reversible oral inhibitor of the collagenase activity of cathepsin K, which is secreted by osteoclasts. Odanacatib reduces bone resorption without reducing the number of osteoclasts and, thus, appears to preserve bone formation.
Details of the trial
Brixen and colleagues conducted a randomized, double-blind, international, 2-year, Phase 3 trial comparing odancatib 50 mg once weekly with placebo in postmenopausal women treated with calcium and vitamin D. The primary endpoint was the change from baseline BMD at the lumbar spine at 1 year, as assessed by DXA. Secondary endpoints included the change from baseline BMD at the hip (total hip, femoral neck, and trochanter) at 1 year, the change from baseline BMD at the spine and hip at 2 years, and 1- and 2-year changes in bone-turnover markers. A total of 214 women were enrolled (average age: 64 years; average T-score of 1.8 at the lumbar spine, –1.8 at the femoral neck, and –1.3 at the total hip).
At 1 year, the change from baseline BMD at the lumbar spine was significantly higher (P <.001) in women receiving odanacatib, compared with placebo (treatment difference: 3.5%). At 2 years, the treatment difference was even higher (5.4%). The mean changes in BMD at the femoral neck, total hip, and trochanter also were significantly greater (P <.001) in women receiving odanacatib, with treatment differences at 2 years of 3.8%, 3.3%, and 5.5%, respectively.
During the first 6 months of the trial, serum concentrations of bone-turnover markers (CTX and P1NP) decreased significantly (P <.001) in odanacatib-treated women, compared with those given placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although no new agents for the treatment of osteoporosis have been introduced over the past year, cathepsin K inhibitors appear to offer great promise for the future. As clinicians, we need to keep abreast of new developments that may be of potential value to our patients.
PILOT STUDY: TERIPARATIDE WAS EFFECTIVE IN 81% OF PREMENOPAUSAL WOMEN WITH IDIOPATHIC OSTEOPOROSIS
Cohen A, Stein EM, Recker RR, et al. Teriparatide for idiopathic osteoporosis in premenopausal women: A pilot study. J Clin Endocrinol Metab. 2013;98(5):1971–1981.
Idiopathic osteoporosis (IOP) affects young, otherwise healthy men and women with intact gonadal function and no secondary cause of bone loss or fragility. Women with IOP have abnormal bone microarchitecture with thinner cortices; fewer, thinner, and more widely separated and heterogeneously distributed trabeculae; more rod-like trabecular structures; less trabecular stiffness; and a higher level of marrow fat.
The osteoanabolic agent teriperatide increases BMD and reduces the incidence of fracture in postmenopausal women and in patients with glucocorticoid-induced osteoporosis, and it increases BMD in men with IOP. This study explored its effect in premenopausal women with IOP.
Details of the study
Cohen and colleagues recruited premenopausal women aged 20 to 48 years who had one or both of the following traits:
- a history of at least one low-trauma fracture more than 6 months before enrollment
- low BMD of the spine or hip (Z-score of –2.0 or below), as assessed by DXA.
All participants had regular menses and early follicular-phase follicle-stimulating hormone (FSH) levels below 20 mIU/mL; none were using hormonal contraception. Women who had secondary osteoporosis related to estrogen deficiency, an eating disorder, an endocrinopathy, celiac or gastrointestinal disease, hyperparathyroidism, marked hypercalciuria, a low serum level of 25-hydroxyvitamin D (<20 ng/mL), and drug exposures were excluded.
All participants (n = 21) received teriparatide 20 µg daily in the morning or evening, according to preference. They also were given calcium 630 mg and vitamin D 800 IU daily.
BMD increased at the spine by 10.8% (standard deviation: 8.3%), total hip by 6.2% (5.6%), and femoral neck by 7.6% (3.4%) (all P <.001). Transiliac biopsies demonstrated significant increases in cortical width and porosity, and trabecular bone volume and number increased as well. Four women had no increase in BMD.
Overall, Cohen and colleagues concluded that teriparatide was associated with increased BMD at the spine and hip and improved trabecular microarchitecture and stiffness at the iliac crest in the majority of women with IOP.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although ObGyns rarely prescribe teriparatide, often leaving this option for metabolic bone experts to offer, we should keep premenopausal IOP in mind when younger patients sustain low-trauma fractures of the hip or vertebrae, as well as fracture of an upper or lower extremity or the ribs.
Teriparatide appears to be an excellent choice for the majority of premenopausal patients with IOP (81% of patients in this pilot study).
Because the low bone mass and deterioration of bone microarchitecture and quality that characterize osteoporosis can lead to fragility fracture, it is vital that we intervene in our patients’ health in a timely manner to reduce this risk. One way to accomplish this goal is to understand the role of age in determining a woman’s fracture risk. For example, an 80-year-old woman and a 50-year-old woman with a T-score of –2.5, as measured by dual x-ray absorptiometry (DXA), will have dramatically different fracture risks. According to the World Health Organization’s fracture-risk assessment tool (http://www.shef.ac.uk/FRAX/), the older woman has a 10-year probability of hip fracture approximately five times greater than the younger woman.
Although no new therapies have been approved during the past year, several important findings were published that affect clinical management of menopausal patients or suggest changes likely in the future.
In this article, I review:
- the latest guidance on osteoporosis from the American College of Obstetricians and Gynecologists (ACOG)
- the most recent indications for bone mineral density (BMD) testing from the International Society for Clinical Densitometry (ISCD)
- a study exploring the effect of oral hormonal contraception on the acquisition of peak BMD in adolescents and young women
- results of a randomized trial of the experimental agent odanacatib in postmenopausal women
- a pilot study of teriparatide (Forteo) for idiopathic osteoporosis in premenopausal women.
ACOG ISSUES RECOMMENDATIONS ON SCREENING, TREATMENT, AND LIFESTYLE
Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin #129: Osteoporosis. Obstet Gynecol. 2012;120(3):718–734.
This comprehensive review of management guidelines for ObGyns deserves “top billing” in this update. It offers recommendations on important interventions, from BMD measurement and subsequent monitoring to calcium and vitamin D supplementation.
When to initiate screening
- Begin BMD screening using DXA at age 65. DXA also may be appropriate for younger women if they are postmenopausal and have other significant risk factors for osteoporosis or fracture (Level A evidence – based on good and consistent scientific evidence).
- In the absence of new risk factors, do not perform DXA screening more frequently than every 2 years (Level B evidence – based on limited or inconsistent scientific evidence).
Which patients should be treated?
Treatment is recommended for:
- women with a T-score of –2.5 or lower
- women who have had a low-trauma fracture
- women with a T-score between –1 and –2.5 and a 10-year FRAX hip-fracture risk of 3% or higher or a 10-year FRAX risk of major osteoporotic fracture of 20% or higher, or both. A major osteoporotic fracture involves the forearm, hip, or shoulder, or a clinical vertebral fracture (Level A evidence).
Only therapies approved by the US FDA should be used for medical treatment. They are raloxifene (Evista), bisphosphonates (Actonel, Boniva, Fosamax, Reclast), parathyroid hormone, denosumab (Prolia), and calcitonin (Fortical, Miacalcin) (Level A evidence).
Monitoring of therapy
In the absence of new risk factors, do not repeat DXA monitoring of therapy once BMD has been determined to be stable or improved (Level B evidence).
Lifestyle recommendations
- Counsel women about lifestyle factors that may affect BMD and fracture risk, which include smoking, poor nutrition and excessive weight loss, weight-bearing and muscle-strengthening exercise, and fall prevention (Level B evidence).
- Advise patients of current recommendations for calcium and vitamin D intake from the Institute of Medicine, which are calcium 1,200 mg/day and vitamin D 600 IU/day for women aged 51 to 70 years (Level B evidence).
- Counsel girls and women of all ages about the effects of lifestyle on bone health (Level C evidence – based on consensus and expert opinion).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
By utilizing the FRAX risk-assessment tool, we can determine which patients truly require treatment. In the process, we should be able to reduce the overtreatment of younger women with low bone mass as well as the undertreatment of older women who appear to have less deranged bone mass.
ACOG also emphasizes the need to avoid the overutilization of DXA scans in various groups, as well as the importance of lifestyle adjustments to promote bone health in all age groups.
Related Article: STOP performing DXA scans in healthy, perimenopausal women Lisa Larkin, MD, and Andrew M. Kaunitz, MD (Stop/Start, Januaray 2013)
CLINICAL DENSITOMETRISTS WEIGH IN ON INDICATIONS FOR BMD ASSESSMENT
International Society for Clinical Densitometry (ISCD). Indications for bone mineral density (BMD) testing. http://www.iscd.org/official-positions/2013-iscd-official-positions-adult/. Updated August 15, 2013. Accessed November 7, 2013.
In its comprehensive review of BMD assessment, the ISCD elucidates the process, which typically involves DXA imaging.
Indications for BMD assessment
- The female patient is age 65 or older
- The postmenopausal patient is younger than age 65 but has a risk factor for low bone mass, such as low body weight, a history of fracture, use of a high-risk medication, or a disease or condition associated with bone loss
- The perimenopausal woman has clinical risk factors for fracture, such as low body weight, history of fracture, or use of a high-risk medication
- The adult sustains a fragility fracture
- The adult has a disease or condition associated with low bone mass or bone loss
- The adult is taking a medication associated with low bone mass or bone loss
- The patient is being considered for pharmacologic therapy
- The patient is being treated, to monitor effect
- The patient is not receiving therapy, but evidence of bone loss would lead to treatment.
When serial BMD assessment is appropriate
- When it is used to determine whether treatment should be initiated (in untreated patients) because of significant bone loss
- To monitor response to therapy by identifying an increase or stabilization of BMD
- To identify nonresponse by documenting a loss of BMD, suggesting the need for treatment re-evaluation and assessment for a secondary cause of osteoporosis
- To follow-up earlier assessment when the expected change in BMD equals or exceeds the least significant change
- When the interval is appropriate for the patient’s clinical status. (In general, BMD assessment is performed 1 year after initiation or change of therapy, with longer intervals once a therapeutic effect has been established.)
- When the patient is using a medication associated with rapid bone loss, such as glucocorticoid therapy. In such a patient, more frequent testing may be appropriate.
- Note that these recommendations differ slightly from ACOG’s statements regarding the use of DXA.
Diagnosis of osteoporosis
- According to the WHO international reference standard, osteoporosis can be diagnosed when a patient has a T-score of –2.5 or below at the femoral neck. The reference standard from which the T-score is calculated is the white female population aged 20 to 29 years in the National Health and Nutrition Examination Survey (NHANES) III database.
- Osteoporosis also may be diagnosed in postmenopausal women and men aged 50 or older when the T-score of the lumbar spine, total hip, or femoral neck is –2.5 or below. In some circumstances, the
33% radius (also called the 1/3 radius) may be utilized. - Other hip regions of interest, including Ward’s area and the greater trochanter, should not be used for diagnosis.
A move away from use of the term “osteopenia”
- The term may be retained, but “low bone mass” or “low bone density” is preferred
- People with low bone mass or low bone density do not necessarily have a high risk of fracture.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
DXA testing remains the cornerstone of diagnosis for patients at risk for fragility fracture. It also is the optimal method to determine the need for pharmacotherapy. In some instances, however, overutilization of DXA imaging has led to overtreatment, especially in younger women with low bone mass, when treatment is based on variables other than diminished bone quality (including, “small-boned” women, genetics, and failure to achieve peak bone mass as high as one’s peer group prior to menopause).
These recommendations help to clarify the rationale for follow-up DXA imaging for patients on therapy, an area in which scientific unanimity is lacking.
Related Article: What is the optimal interval for osteoporosis screening in postmenopausal women before fracture occurrence and osteoporosis treatment initiation? Steven R. Goldstein, MD (Examining the Evidence, August 2012)
IN ADOLESCENTS AND YOUNG WOMEN, CONSIDER THE BONE EFFECTS OF ORAL CONTRACEPTIVES
Ziglar S, Hunter TS. The effect of hormonal oral contraception on acquisition of peak bone mineral density of adolescents and young women. J Pharm Pract. 2012;25(3):331–340.
The bone loss observed in adolescents and young women who use depot medroxyprogesterone acetate (Depo-Provera) for contraception led to an FDA-mandated boxed warning on the medication’s package insert. The effect of oral contraceptives (OCs) on bone growth has received little publicity, however.
The best strategy to offset the natural loss of bone associated with aging and the menopausal transition is to ensure the development of maximal bone mass in youth. When maximal BMD is not achieved, the risk of osteoporosis is increased.
Adolescence is a critical period of bone mineralization, which is mediated by endogenous estradiol. The highest rate of bone mass accrual occurs 1 year before and 3 years after menarche. Young women who consume a diet low in calcium or who have an eating disorder, who fail to exercise, who smoke, or who have low estrogen status are most likely to have low peak bone mass.
OCs suppress endogenous estradiol production by interrupting the hypothalamic-pituitary-ovarian axis. By replacing endogenous estradiol with ethinyl estradiol (EE), OCs establish and maintain new hormone levels. Early initiation and use of very-low-dose EE increases the likelihood that the accrual of bone mass will be jeopardized at a critical time of bone mineralization.
Details of this meta-analysis
Ziglar and Hunter reviewed 11 prospective trials that showed a decrease in bone mass in adolescents and young women who used low-dose OCs, six trials that showed a neutral effect, and one trial that found an increase in bone mass. This last study involved only members of the US military whose level of daily exercise may not be representative of the general population of women the same age. Investigators also theorized that the use of norethindrone acetate as an androgenic progestin in this study may have exerted a positive effect on bone accrual.
Ziglar and Hunter concluded that the use of OCs containing 20 µg EE prevents adolescents and young women from attaining peak BMD. Evidence on the effect of contraceptives formulated with 30 to 35 µg EE is less definitive, but this dose may also impede BMD acquisition in adolescents.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
To ensure breast health and reduce the risk of venous thromboembolic events, drug makers have developed OCs with lower and lower doses of EE. In the process, however, the beneficial effects of endogenous estradiol on bone acquisition have been suppressed. Therefore, the lowest-dose OC may not necessarily be the most appropriate clinical choice for adolescents and young women seeking contraception.
Related Article: Osteoporosis treatment and breast cancer prevention: Two goals, one treatment? Robert L. Barbieri, MD (Editorial, November 2013)
EXPERIMENTAL DRUG REDUCES BONE RESORPTION WITHOUT IMPEDING BONE FORMATION
Brixen K, Chapurlat R, Cheung AM, et al. Bone density, turnover, and estimated strength in postmenopausal women treated with odanacatib: a randomized trial. J Clin Endocrinol Metab. 2013;98(2):571–580.
Current treatments for osteoporosis include antiresorptive agents, such as bisphosphonates and denosumab, that preserve bone mass by reducing the rate of bone turnover. These drugs reduce the number or activity (or both) of bone-resorbing osteoclasts. Because osteoclasts play a role in stimulating bone formation by osteoblasts, these treatments indirectly lower bone formation.
Odanacatib is a drug in Phase 3 development for the treatment of postmenopausal osteoporosis. It is a highly selective and reversible oral inhibitor of the collagenase activity of cathepsin K, which is secreted by osteoclasts. Odanacatib reduces bone resorption without reducing the number of osteoclasts and, thus, appears to preserve bone formation.
Details of the trial
Brixen and colleagues conducted a randomized, double-blind, international, 2-year, Phase 3 trial comparing odancatib 50 mg once weekly with placebo in postmenopausal women treated with calcium and vitamin D. The primary endpoint was the change from baseline BMD at the lumbar spine at 1 year, as assessed by DXA. Secondary endpoints included the change from baseline BMD at the hip (total hip, femoral neck, and trochanter) at 1 year, the change from baseline BMD at the spine and hip at 2 years, and 1- and 2-year changes in bone-turnover markers. A total of 214 women were enrolled (average age: 64 years; average T-score of 1.8 at the lumbar spine, –1.8 at the femoral neck, and –1.3 at the total hip).
At 1 year, the change from baseline BMD at the lumbar spine was significantly higher (P <.001) in women receiving odanacatib, compared with placebo (treatment difference: 3.5%). At 2 years, the treatment difference was even higher (5.4%). The mean changes in BMD at the femoral neck, total hip, and trochanter also were significantly greater (P <.001) in women receiving odanacatib, with treatment differences at 2 years of 3.8%, 3.3%, and 5.5%, respectively.
During the first 6 months of the trial, serum concentrations of bone-turnover markers (CTX and P1NP) decreased significantly (P <.001) in odanacatib-treated women, compared with those given placebo.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although no new agents for the treatment of osteoporosis have been introduced over the past year, cathepsin K inhibitors appear to offer great promise for the future. As clinicians, we need to keep abreast of new developments that may be of potential value to our patients.
PILOT STUDY: TERIPARATIDE WAS EFFECTIVE IN 81% OF PREMENOPAUSAL WOMEN WITH IDIOPATHIC OSTEOPOROSIS
Cohen A, Stein EM, Recker RR, et al. Teriparatide for idiopathic osteoporosis in premenopausal women: A pilot study. J Clin Endocrinol Metab. 2013;98(5):1971–1981.
Idiopathic osteoporosis (IOP) affects young, otherwise healthy men and women with intact gonadal function and no secondary cause of bone loss or fragility. Women with IOP have abnormal bone microarchitecture with thinner cortices; fewer, thinner, and more widely separated and heterogeneously distributed trabeculae; more rod-like trabecular structures; less trabecular stiffness; and a higher level of marrow fat.
The osteoanabolic agent teriperatide increases BMD and reduces the incidence of fracture in postmenopausal women and in patients with glucocorticoid-induced osteoporosis, and it increases BMD in men with IOP. This study explored its effect in premenopausal women with IOP.
Details of the study
Cohen and colleagues recruited premenopausal women aged 20 to 48 years who had one or both of the following traits:
- a history of at least one low-trauma fracture more than 6 months before enrollment
- low BMD of the spine or hip (Z-score of –2.0 or below), as assessed by DXA.
All participants had regular menses and early follicular-phase follicle-stimulating hormone (FSH) levels below 20 mIU/mL; none were using hormonal contraception. Women who had secondary osteoporosis related to estrogen deficiency, an eating disorder, an endocrinopathy, celiac or gastrointestinal disease, hyperparathyroidism, marked hypercalciuria, a low serum level of 25-hydroxyvitamin D (<20 ng/mL), and drug exposures were excluded.
All participants (n = 21) received teriparatide 20 µg daily in the morning or evening, according to preference. They also were given calcium 630 mg and vitamin D 800 IU daily.
BMD increased at the spine by 10.8% (standard deviation: 8.3%), total hip by 6.2% (5.6%), and femoral neck by 7.6% (3.4%) (all P <.001). Transiliac biopsies demonstrated significant increases in cortical width and porosity, and trabecular bone volume and number increased as well. Four women had no increase in BMD.
Overall, Cohen and colleagues concluded that teriparatide was associated with increased BMD at the spine and hip and improved trabecular microarchitecture and stiffness at the iliac crest in the majority of women with IOP.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Although ObGyns rarely prescribe teriparatide, often leaving this option for metabolic bone experts to offer, we should keep premenopausal IOP in mind when younger patients sustain low-trauma fractures of the hip or vertebrae, as well as fracture of an upper or lower extremity or the ribs.
Teriparatide appears to be an excellent choice for the majority of premenopausal patients with IOP (81% of patients in this pilot study).

Steven R. Goldstein, MD (December 2013)
Which abnormal ovarian findings can be followed by serial TVUS?
Ovarian cancer causes more deaths than any other cancer affecting the female reproductive system.1 One reason it’s so deadly: It usually isn’t detected until it has reached an advanced stage. No clear-cut symptoms point definitively to ovarian malignancy, and no feasible screening strategy has been found to increase detection at an early stage.
Among the strategies that have been utilized to detect ovarian cancer are bimanual examination of the adnexae (primarily in postmenopausal women), measurement of cancer antigen (CA) 125, and transvaginal ultrasonography (TVUS) of the ovaries. The last two strategies sometimes are combined in high-risk women.
TVUS can highlight ovarian abnormalities and provide information about their structure. The question then becomes which abnormalities are likely to resolve without treatment, and which should be scrutinized more closely. In this study, Pavlik and colleagues reviewed TVUS findings from 39,337 women enrolled in the University of Kentucky Ovarian Cancer Screening Program, which involved 221,576 baseline and interval TVUS scans.
Details of the study
Women in this study were screened with annual TVUS scans between 1987 and 2002. The population included:
- asymptomatic women aged 50 or older
- asymptomatic women over age 25 who had a first- or second-degree relative with documented ovarian cancer.
The initial TVUS scan was normal in almost 90% of women, and only about 10% subsequently experienced an abnormal scan. About half (46.7%) of the ovarian abnormalities identified via TVUS were found on the very first scan. Of these, 63.2% resolved during follow-up with no treatment.
Approximately 80% of women had no abnormal TVUS findings at any time during the observation period. This is notable because participants had a high risk for ovarian cancer by virtue of advanced age or family history.
TVUS abnormalities had a higher prevalence in premenopausal women (35%) than in postmenopausal women (17%; P<.001). The incidence of ovarian cysts also was significantly higher among premenopausal women (15.3% vs 8.2%; P<.001). These differences are to be expected, owing to the functional nature of premenopausal ovaries in regard to folliculogenesis, ovulation, and endometriosis.
Positive predictive values ranged from 15.3% to 24.7%
Over the 25 years covered by this study, our understanding of the malignant potential of various ovarian masses has evolved considerably. We have long known that unilocular cysts are extremely unlikely to be malignant, but now we are aware that even septated cysts are unlikely to represent cancer.
As for the success of this ovarian cancer-screening program, which identified 85 true malignancies and 472 nonmalignancies in surgical specimens, it had an overall positive predictive value of 15.3%. After January 1, 2008, however, when serial observation expanded to include septated cysts (because published data confirmed these masses to have low malignant potential), positive predictive value improved to 24.7%.
Pavlik and colleagues also discussed findings from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, which relied on a single TVUS abnormality to trigger a recommendation for surgery, with a positive predictive value of only 5.1%.2
Most cancers were diagnosed at an early stage
Of the invasive epithelial cancers identified in this study, the stage distribution at diagnosis was:
- Stage 1: 45%
- Stage 2: 23%
- Stage 3: 32%
- Stage 4: None.
This finding is notable, given statistics from the “real world,” where about 80% of ovarian cancers are diagnosed at Stage 3 or Stage 4.
Among benign findings that were managed surgically, 47% were serous cystadenomas, 13% were hemorrhagic cysts, 9% were fibromas, thecomas, or Brenner tumors, and the rest were fairly equally divided between hydrosalpinx or paratubal cysts; endometriomas; and mucinous cystadenomas, leiomyomas, and cystic teratomas.
What this evidence means for practice
In general, unilocular or septate cysts can be followed every 6 months by TVUS. Although more complex tumors may resolve spontaneously, they should be followed with serial TVUS, with caution, at intervals of 6 weeks to 3 months. The findings of each scan should determine the subsequent course of action, which could involve further monitoring or surgical extirpation.
Regrettably, this study did not utilize color flow Doppler imaging. Because malignant tumors are rich in neovascularity, and the vessels laid down by such tumors often lack a normal media layer, they often exhibit very low resistance to flow. Although neovascularity is not a perfect diagnostic indicator of malignancy, the presence of abundant blood flow and low resistance can raise the index of suspicion. In my opinion, color flow Doppler should be incorporated into ultrasonographic evaluation of potential ovarian malignancies.
—Steven R. Goldstein, MD
Tell us what you think, at rbarbieri@frontlinemedcom.com. Please include your name and city and state.
1. American Cancer Society. Cancer Facts and Figures, 2013. http://www.cancer.org/acs/groups/content/@e p i d e m i o l o g y s u r v e i l a n c e / d o c u m e n t s / d o c u m e n t/acspc-036845.pdf. Accessed August 20, 2013.
2. Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality—the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305(22):2295–2303.
Ovarian cancer causes more deaths than any other cancer affecting the female reproductive system.1 One reason it’s so deadly: It usually isn’t detected until it has reached an advanced stage. No clear-cut symptoms point definitively to ovarian malignancy, and no feasible screening strategy has been found to increase detection at an early stage.
Among the strategies that have been utilized to detect ovarian cancer are bimanual examination of the adnexae (primarily in postmenopausal women), measurement of cancer antigen (CA) 125, and transvaginal ultrasonography (TVUS) of the ovaries. The last two strategies sometimes are combined in high-risk women.
TVUS can highlight ovarian abnormalities and provide information about their structure. The question then becomes which abnormalities are likely to resolve without treatment, and which should be scrutinized more closely. In this study, Pavlik and colleagues reviewed TVUS findings from 39,337 women enrolled in the University of Kentucky Ovarian Cancer Screening Program, which involved 221,576 baseline and interval TVUS scans.
Details of the study
Women in this study were screened with annual TVUS scans between 1987 and 2002. The population included:
- asymptomatic women aged 50 or older
- asymptomatic women over age 25 who had a first- or second-degree relative with documented ovarian cancer.
The initial TVUS scan was normal in almost 90% of women, and only about 10% subsequently experienced an abnormal scan. About half (46.7%) of the ovarian abnormalities identified via TVUS were found on the very first scan. Of these, 63.2% resolved during follow-up with no treatment.
Approximately 80% of women had no abnormal TVUS findings at any time during the observation period. This is notable because participants had a high risk for ovarian cancer by virtue of advanced age or family history.
TVUS abnormalities had a higher prevalence in premenopausal women (35%) than in postmenopausal women (17%; P<.001). The incidence of ovarian cysts also was significantly higher among premenopausal women (15.3% vs 8.2%; P<.001). These differences are to be expected, owing to the functional nature of premenopausal ovaries in regard to folliculogenesis, ovulation, and endometriosis.
Positive predictive values ranged from 15.3% to 24.7%
Over the 25 years covered by this study, our understanding of the malignant potential of various ovarian masses has evolved considerably. We have long known that unilocular cysts are extremely unlikely to be malignant, but now we are aware that even septated cysts are unlikely to represent cancer.
As for the success of this ovarian cancer-screening program, which identified 85 true malignancies and 472 nonmalignancies in surgical specimens, it had an overall positive predictive value of 15.3%. After January 1, 2008, however, when serial observation expanded to include septated cysts (because published data confirmed these masses to have low malignant potential), positive predictive value improved to 24.7%.
Pavlik and colleagues also discussed findings from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, which relied on a single TVUS abnormality to trigger a recommendation for surgery, with a positive predictive value of only 5.1%.2
Most cancers were diagnosed at an early stage
Of the invasive epithelial cancers identified in this study, the stage distribution at diagnosis was:
- Stage 1: 45%
- Stage 2: 23%
- Stage 3: 32%
- Stage 4: None.
This finding is notable, given statistics from the “real world,” where about 80% of ovarian cancers are diagnosed at Stage 3 or Stage 4.
Among benign findings that were managed surgically, 47% were serous cystadenomas, 13% were hemorrhagic cysts, 9% were fibromas, thecomas, or Brenner tumors, and the rest were fairly equally divided between hydrosalpinx or paratubal cysts; endometriomas; and mucinous cystadenomas, leiomyomas, and cystic teratomas.
What this evidence means for practice
In general, unilocular or septate cysts can be followed every 6 months by TVUS. Although more complex tumors may resolve spontaneously, they should be followed with serial TVUS, with caution, at intervals of 6 weeks to 3 months. The findings of each scan should determine the subsequent course of action, which could involve further monitoring or surgical extirpation.
Regrettably, this study did not utilize color flow Doppler imaging. Because malignant tumors are rich in neovascularity, and the vessels laid down by such tumors often lack a normal media layer, they often exhibit very low resistance to flow. Although neovascularity is not a perfect diagnostic indicator of malignancy, the presence of abundant blood flow and low resistance can raise the index of suspicion. In my opinion, color flow Doppler should be incorporated into ultrasonographic evaluation of potential ovarian malignancies.
—Steven R. Goldstein, MD
Tell us what you think, at rbarbieri@frontlinemedcom.com. Please include your name and city and state.
Ovarian cancer causes more deaths than any other cancer affecting the female reproductive system.1 One reason it’s so deadly: It usually isn’t detected until it has reached an advanced stage. No clear-cut symptoms point definitively to ovarian malignancy, and no feasible screening strategy has been found to increase detection at an early stage.
Among the strategies that have been utilized to detect ovarian cancer are bimanual examination of the adnexae (primarily in postmenopausal women), measurement of cancer antigen (CA) 125, and transvaginal ultrasonography (TVUS) of the ovaries. The last two strategies sometimes are combined in high-risk women.
TVUS can highlight ovarian abnormalities and provide information about their structure. The question then becomes which abnormalities are likely to resolve without treatment, and which should be scrutinized more closely. In this study, Pavlik and colleagues reviewed TVUS findings from 39,337 women enrolled in the University of Kentucky Ovarian Cancer Screening Program, which involved 221,576 baseline and interval TVUS scans.
Details of the study
Women in this study were screened with annual TVUS scans between 1987 and 2002. The population included:
- asymptomatic women aged 50 or older
- asymptomatic women over age 25 who had a first- or second-degree relative with documented ovarian cancer.
The initial TVUS scan was normal in almost 90% of women, and only about 10% subsequently experienced an abnormal scan. About half (46.7%) of the ovarian abnormalities identified via TVUS were found on the very first scan. Of these, 63.2% resolved during follow-up with no treatment.
Approximately 80% of women had no abnormal TVUS findings at any time during the observation period. This is notable because participants had a high risk for ovarian cancer by virtue of advanced age or family history.
TVUS abnormalities had a higher prevalence in premenopausal women (35%) than in postmenopausal women (17%; P<.001). The incidence of ovarian cysts also was significantly higher among premenopausal women (15.3% vs 8.2%; P<.001). These differences are to be expected, owing to the functional nature of premenopausal ovaries in regard to folliculogenesis, ovulation, and endometriosis.
Positive predictive values ranged from 15.3% to 24.7%
Over the 25 years covered by this study, our understanding of the malignant potential of various ovarian masses has evolved considerably. We have long known that unilocular cysts are extremely unlikely to be malignant, but now we are aware that even septated cysts are unlikely to represent cancer.
As for the success of this ovarian cancer-screening program, which identified 85 true malignancies and 472 nonmalignancies in surgical specimens, it had an overall positive predictive value of 15.3%. After January 1, 2008, however, when serial observation expanded to include septated cysts (because published data confirmed these masses to have low malignant potential), positive predictive value improved to 24.7%.
Pavlik and colleagues also discussed findings from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, which relied on a single TVUS abnormality to trigger a recommendation for surgery, with a positive predictive value of only 5.1%.2
Most cancers were diagnosed at an early stage
Of the invasive epithelial cancers identified in this study, the stage distribution at diagnosis was:
- Stage 1: 45%
- Stage 2: 23%
- Stage 3: 32%
- Stage 4: None.
This finding is notable, given statistics from the “real world,” where about 80% of ovarian cancers are diagnosed at Stage 3 or Stage 4.
Among benign findings that were managed surgically, 47% were serous cystadenomas, 13% were hemorrhagic cysts, 9% were fibromas, thecomas, or Brenner tumors, and the rest were fairly equally divided between hydrosalpinx or paratubal cysts; endometriomas; and mucinous cystadenomas, leiomyomas, and cystic teratomas.
What this evidence means for practice
In general, unilocular or septate cysts can be followed every 6 months by TVUS. Although more complex tumors may resolve spontaneously, they should be followed with serial TVUS, with caution, at intervals of 6 weeks to 3 months. The findings of each scan should determine the subsequent course of action, which could involve further monitoring or surgical extirpation.
Regrettably, this study did not utilize color flow Doppler imaging. Because malignant tumors are rich in neovascularity, and the vessels laid down by such tumors often lack a normal media layer, they often exhibit very low resistance to flow. Although neovascularity is not a perfect diagnostic indicator of malignancy, the presence of abundant blood flow and low resistance can raise the index of suspicion. In my opinion, color flow Doppler should be incorporated into ultrasonographic evaluation of potential ovarian malignancies.
—Steven R. Goldstein, MD
Tell us what you think, at rbarbieri@frontlinemedcom.com. Please include your name and city and state.
1. American Cancer Society. Cancer Facts and Figures, 2013. http://www.cancer.org/acs/groups/content/@e p i d e m i o l o g y s u r v e i l a n c e / d o c u m e n t s / d o c u m e n t/acspc-036845.pdf. Accessed August 20, 2013.
2. Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality—the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305(22):2295–2303.
1. American Cancer Society. Cancer Facts and Figures, 2013. http://www.cancer.org/acs/groups/content/@e p i d e m i o l o g y s u r v e i l a n c e / d o c u m e n t s / d o c u m e n t/acspc-036845.pdf. Accessed August 20, 2013.
2. Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality—the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305(22):2295–2303.
An evidence-based approach to BMD assessment
Related article: Update on Osteoporosis Steven R. Goldstein, MD (November 2012)
Related article: Update on Osteoporosis Steven R. Goldstein, MD (November 2012)
Related article: Update on Osteoporosis Steven R. Goldstein, MD (November 2012)
UPDATE ON OSTEOPOROSIS
What is the optimal interval for osteoporosis screening in postmenopausal women before fracture occurrence and osteoporosis?
Steven R. Goldstein, MD (Examining the Evidence, August 2012)
Update on Menopause
Andrew M. Kaunitz, MD (May 2012)
Update on Osteoporosis
Steven R. Goldstein, MD (November 2011)
An appeal to the FDA: Remove the black-box warning for depot medroxyprogesterone acetate?
Andrew M. Kaunitz, MD; David A. Grimes, MD (Guest Editorial, August 2011)
Osteoporosis is a significant health issue—and it is likely to remain so as more and more women live longer and longer. In fact, increasing age is the single biggest risk factor for osteoporotic fragility fracture.
Over the past year, important research has improved our understanding in diverse areas of bone health. In this Update, I highlight studies that:
- seek to elucidate the optimal frequency of dual-energy x-ray absorptiometry (DXA) imaging to assess bone mineral density
- review secondary causes of osteoporosis besides menopause-related estrogen deficiency
- explore the use of quantitative ultrasound (QUS) to predict the risk of fracture
- report on a new class of pharmaceutical agents that inhibit the bone-resorption enzyme Cathepsin K.
All of these issues are clinically relevant to the ObGyn specialty because, when it comes to our patients’ bone health, we often function as the primary care physician.
When is DXA indicated—and how often should it be repeated?
Gourlay ML, Fine JP, Preisser JS, et al; Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366(3):225–233.
Lewiecki EM, Laster AJ, Miller PD, Bilezikian JP. More bone density testing is needed, not less. J Bone Miner Res. 2012;27(4):739–742.
American Society for Bone and Mineral Research response to media coverage of New England Journal of Medicine study: “Bone density testing interval and transition to osteoporosis in older women” [press release]. http://www.asbmr.org/about/pressreleases/detail.aspx?cid=3801baff-0df3-47c0-874f-08a185d67001. Published February 1, 2012. Accessed October 15, 2012.
Recommendations from professional societies, such as the National Osteoporosis Foundation, the International Society of Clinical Densitometry, and the American College of Obstetricians and Gynecologists say virtually the same thing about DXA imaging: Screening is appropriate for women 65 years and older and for postmenopausal women younger than age 65 who have risk factors for fracture. Risk factors include:
- history of a fragility fracture
- body weight less than 127 lb
- medical causes of bone loss, such as medication or disease
- parental history of hip fracture
- current smoker
- alcoholism
- rheumatoid arthritis.
The measurement of bone mineral density (BMD) has been the cornerstone of the diagnosis of osteopenia and osteoporosis since these classifications were introduced by the World Health Organization (WHO) in 1994. Although we are now able to evaluate a woman’s fracture risk using the FRAX tool, which does not require BMD assessment, DXA scanning has become entrenched in routine clinical practice in the United States. In addition, patients who use drugs to reduce their fracture risk often demand periodic testing to see how they are doing. Even women who do not take medications often want periodic assessment to confirm that they are not “losing bone.” Medicare allows for testing every 23 months.
23-month screening interval does not fit all women
Gourlay and colleagues prospectively followed 4,957 women aged 67 years or older who had no history of hip or vertebral fracture and who were not being treated for osteoporosis. After follow-up for as long as 15 years, investigators found that the better a woman’s initial bone density, the longer it took for her to develop osteoporosis. For example, among women over 67 years of age who had a T-score of –1.0 or better, it would take 16.8 years for 10% of this population to develop osteoporosis. In contrast, among women over 67 years of age who had a T-score of –2.0, it would take only 1.1 years for 10% of this population to develop osteoporosis.
This finding certainly calls into question the notion that all patients should be screened every 23 months. It may be better to think of screening as a way of triaging patients for decisions relative to subsequent follow-up.
Media distorted take-home message
This study was the focus of considerable attention from the media, which implied that too much DXA screening is being performed. In reality, only 13% of women over the age of 65 undergo a baseline DXA scan. However, routine follow-up of all patients at 23-month intervals is clearly not appropriate.
Because this study primarily involved white women older than age 67, extrapolation of its findings to other groups may not be appropriate. Nevertheless, the study helps to underscore the fact that reliance on BMD measurement alone should not be used to determine the need for therapeutic intervention. The FRAX tool can be used on an annual basis to assess a woman’s risk of fracture and does not require follow-up DXA imaging at any arbitrary interval.
In healthy older women, an interval of 23 months for repeat BMD assessment makes little sense. For women who have excellent initial T-scores, clinicians can lengthen this interval significantly.
However, strict reliance on the T-score isn’t the best way to predict a woman’s fracture risk or determine when pharmacologic intervention is warranted. Rather, yearly assessment using a tool such as FRAX should become the standard of care.
Some secondary causes of osteoporosis are overlooked or underappreciated
Miller PD. Unrecognized and unappreciated secondary causes of osteoporosis. Endocrinol Metab Clin North Am. 2012;41(3):613–628.
The fractures traditionally associated with osteoporosis involve the hip and vertebrae, although low-trauma fractures of the humerus, forearm, femur shaft, tibia, and fibula are also associated with a high risk of future fracture in untreated women.
Once a clinician is confident that a patient has osteoporosis, the question is whether the diagnosis is postmenopausal osteoporosis—or some other form of the disease. Although estrogen deficiency is the most common cause of osteoporosis in postmenopausal women, many other conditions may accompany estrogen deficiency and contribute to impaired bone strength in this population.
Among the culprits are some conditions that are not often encountered in the average gynecologic practice: monoclonal gammopathy of undetermined significance (MGUS), multiple myeloma, celiac disease, Crohn’s disease, and other inflammatory bowel diseases. In addition, bariatric surgery, eating disorders, primary hyperparathyroidism, and a number of medications have been implicated in BMD loss or increased risk of fracture, or both. Among the problematic drugs of particular interest to us as gynecologists are aromatase inhibitors, depot medroxyprogesterone acetate, proton pump inhibitors, and gonadotropin-releasing hormone (GnRH) agonists.
Other medications that can affect BMD are glucocorticoids, unfractionated heparin, selective serotonin reuptake inhibitors, excessive amounts of thyroid replacement agents, and some antiseizure medications.
If you suspect a secondary cause of osteoporosis, be prepared to perform a basic workup that includes:
- a careful history and physical examination
- complete blood count
- a chemistry profile, including serum calcium, phosphorous, electrolytes, alkaline phosphatase, and creatinine.
In addition, measurement of 25-hydroxy vitamin D and thyroid-stimulating hormone (TSH) may be helpful, as may serum protein electrophoresis.
Patients who have clinical or laboratory abnormalities suggestive of a secondary cause of osteoporosis are usually referred to a metabolic bone specialist (endocrinology or rheumatology).
When a patient has any clinical history that suggests a secondary cause of bone loss other than menopause-related estrogen deficiency, simple laboratory tests are appropriate and may uncover a condition that necessitates referral to a metabolic bone expert.
Quantitative ultrasound assessment of bone can help predict a woman’s risk of fracture
Guglielmi G, Rossini M, Nicolosi MG, Tagno A, Lentini G, de Terlizzi F. Three-year prospective study on fracture risk in postmenopausal women by quantitative ultrasound at the phalanges [published online ahead of print August 15, 2012]. J Clin Densitom. doi:10.1016 /j.jocd.2012.07.006.
Chan MY, Nguyen ND, Center JR, Eisman JA, Nguyen TV. Quantitative ultrasound and fracture risk prediction in non-osteoporotic men and women as defined by WHO criteria [published online ahead of print August 10, 2012]. Osteoporos Int. doi:10.1007/s00198-012 -2001-2.
I became interested in bone health through my longstanding interest in ultrasound, when a manufacturer asked me to evaluate equipment designed to assess bone density of the heel through quantitative ultrasound (QUS). This modality is not the diagnostic imaging we are familiar with in obstetrics and gynecology. In QUS, the homogeneity of healthy bone promotes sound transmission, whereas the voids and discontinuity of osteoporotic bone impede it. Therefore, normal bone has a faster speed of sound than less healthy bone. The other important quantitative measure is broadband ultrasound attenuation (BUA). Healthy bone is dense and absorbs and scatters sound to a greater extent than osteoporotic bone does.
Two trials of QUS
In 2010, Guglielmi and colleagues contacted 2,210 Italian women who had undergone QUS of the phalanges in 2006–2007. These women had an average age of 60.9 years, entered menopause at an average age of 49.3 years, and had a mean body mass index (BMI) of 26.5 kg/m2. By 2010, this group had experienced 108 new major osteoporotic fractures, including 23 hip fractures and 56 vertebral fractures. Investigators found a statistically significant correlation between QUS findings and fracture risk.
Chan and colleagues focused on 312 women 62 to 92 years of age who had femoral neck BMD, as measured by DXA, of –2.5 or better. QUS was measured as BUA at the calcaneus. The incidence of any fragility fracture was ascertained by radiographic reports during the follow-up period from 1994 to 2011. Eighty women (26%) experienced at least one fragility fracture during follow-up. After adjustment for covariates, women were significantly more likely to experience any fracture if BUA was decreased (hazard ratio [HR], 1.50; 95% confidence interval [CI], 1.13–1.99).
When the models that included BUA were compared with those that used femoral neck BMD, they had a greater area under the curve (0.71, 0.85, 0.71 for any fracture, hip fracture, and vertebral fracture, respectively) and yielded a net reclassification improvement of 16.4% (P=.009) when combined with femoral neck BMD. These findings suggest that calcaneal BUA is an independent predictor of fracture risk in women who have nonosteoporotic BMD.
In an era of increasing pressure to reduce costs, QUS assessment of bone is a promising modality that may be useful as a screening tool. Although it measures different variables than DXA imaging (more microarchitecture, less true density), it seems to predict the risk of fracture at less cost without ionizing radiation.
In the pipeline: A drug that curbs bone resorption without diminishing bone formation
Williams SC. Potential first-in-class osteoporosis drug speeds through trials. Nat Med. 2012;18(8):1158.
Ng KW. Potential role of odanacatib in the treatment of osteoporosis. Clin Interv Aging. 2012;7:235–247.
Alendronate was the first of the oral bisphosphonates to be approved by the US Food and Drug Administration (FDA). Once it was approved in 1999, the drug quickly became the most widely used bone agent in clinical practice and was soon joined by other oral and intravenous bisphosphonates. Regrettably, highly publicized adverse effects have caused many patients to shy away from this class of drugs. Two years ago, the FDA approved denosumab, a subcutaneous injectable agent that is a RANK ligand inhibitor.
The bisphosphonates and denosumab increase bone mass by shutting down the osteoclasts responsible for bone resorption, but they also inhibit creation of new bone. A new category of drug that inhibits the bone-resorption enzyme Cathepsin K appears to inhibit bone resorption without diminishing bone formation. Trials of two previous agents in this class were halted because of adverse effects—particularly effects to the skin, where the enzyme is expressed in addition to bone. However, Phase 2 trials in which odanacatib was compared with alendronate found that the new drug increased BMD almost twice as much as alendronate did, with less reduction in serum markers of bone formation.
Phase 3 trials of odanacatib in 16,000 women older than age 65 recently were halted so that the manufacturer could pursue regulatory approval ahead of the previous schedule. Although Phase 3 data have not been published yet, odanacatib may prove to be an exciting alternative to existing therapies.
Odanacatib is not yet available. However, by discussing therapies that may be “around the corner” with our patients, we demonstrate that we are staying ahead of the curve of scientific development.
We want to hear from you! Tell us what you think.
What is the optimal interval for osteoporosis screening in postmenopausal women before fracture occurrence and osteoporosis?
Steven R. Goldstein, MD (Examining the Evidence, August 2012)
Update on Menopause
Andrew M. Kaunitz, MD (May 2012)
Update on Osteoporosis
Steven R. Goldstein, MD (November 2011)
An appeal to the FDA: Remove the black-box warning for depot medroxyprogesterone acetate?
Andrew M. Kaunitz, MD; David A. Grimes, MD (Guest Editorial, August 2011)
Osteoporosis is a significant health issue—and it is likely to remain so as more and more women live longer and longer. In fact, increasing age is the single biggest risk factor for osteoporotic fragility fracture.
Over the past year, important research has improved our understanding in diverse areas of bone health. In this Update, I highlight studies that:
- seek to elucidate the optimal frequency of dual-energy x-ray absorptiometry (DXA) imaging to assess bone mineral density
- review secondary causes of osteoporosis besides menopause-related estrogen deficiency
- explore the use of quantitative ultrasound (QUS) to predict the risk of fracture
- report on a new class of pharmaceutical agents that inhibit the bone-resorption enzyme Cathepsin K.
All of these issues are clinically relevant to the ObGyn specialty because, when it comes to our patients’ bone health, we often function as the primary care physician.
When is DXA indicated—and how often should it be repeated?
Gourlay ML, Fine JP, Preisser JS, et al; Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366(3):225–233.
Lewiecki EM, Laster AJ, Miller PD, Bilezikian JP. More bone density testing is needed, not less. J Bone Miner Res. 2012;27(4):739–742.
American Society for Bone and Mineral Research response to media coverage of New England Journal of Medicine study: “Bone density testing interval and transition to osteoporosis in older women” [press release]. http://www.asbmr.org/about/pressreleases/detail.aspx?cid=3801baff-0df3-47c0-874f-08a185d67001. Published February 1, 2012. Accessed October 15, 2012.
Recommendations from professional societies, such as the National Osteoporosis Foundation, the International Society of Clinical Densitometry, and the American College of Obstetricians and Gynecologists say virtually the same thing about DXA imaging: Screening is appropriate for women 65 years and older and for postmenopausal women younger than age 65 who have risk factors for fracture. Risk factors include:
- history of a fragility fracture
- body weight less than 127 lb
- medical causes of bone loss, such as medication or disease
- parental history of hip fracture
- current smoker
- alcoholism
- rheumatoid arthritis.
The measurement of bone mineral density (BMD) has been the cornerstone of the diagnosis of osteopenia and osteoporosis since these classifications were introduced by the World Health Organization (WHO) in 1994. Although we are now able to evaluate a woman’s fracture risk using the FRAX tool, which does not require BMD assessment, DXA scanning has become entrenched in routine clinical practice in the United States. In addition, patients who use drugs to reduce their fracture risk often demand periodic testing to see how they are doing. Even women who do not take medications often want periodic assessment to confirm that they are not “losing bone.” Medicare allows for testing every 23 months.
23-month screening interval does not fit all women
Gourlay and colleagues prospectively followed 4,957 women aged 67 years or older who had no history of hip or vertebral fracture and who were not being treated for osteoporosis. After follow-up for as long as 15 years, investigators found that the better a woman’s initial bone density, the longer it took for her to develop osteoporosis. For example, among women over 67 years of age who had a T-score of –1.0 or better, it would take 16.8 years for 10% of this population to develop osteoporosis. In contrast, among women over 67 years of age who had a T-score of –2.0, it would take only 1.1 years for 10% of this population to develop osteoporosis.
This finding certainly calls into question the notion that all patients should be screened every 23 months. It may be better to think of screening as a way of triaging patients for decisions relative to subsequent follow-up.
Media distorted take-home message
This study was the focus of considerable attention from the media, which implied that too much DXA screening is being performed. In reality, only 13% of women over the age of 65 undergo a baseline DXA scan. However, routine follow-up of all patients at 23-month intervals is clearly not appropriate.
Because this study primarily involved white women older than age 67, extrapolation of its findings to other groups may not be appropriate. Nevertheless, the study helps to underscore the fact that reliance on BMD measurement alone should not be used to determine the need for therapeutic intervention. The FRAX tool can be used on an annual basis to assess a woman’s risk of fracture and does not require follow-up DXA imaging at any arbitrary interval.
In healthy older women, an interval of 23 months for repeat BMD assessment makes little sense. For women who have excellent initial T-scores, clinicians can lengthen this interval significantly.
However, strict reliance on the T-score isn’t the best way to predict a woman’s fracture risk or determine when pharmacologic intervention is warranted. Rather, yearly assessment using a tool such as FRAX should become the standard of care.
Some secondary causes of osteoporosis are overlooked or underappreciated
Miller PD. Unrecognized and unappreciated secondary causes of osteoporosis. Endocrinol Metab Clin North Am. 2012;41(3):613–628.
The fractures traditionally associated with osteoporosis involve the hip and vertebrae, although low-trauma fractures of the humerus, forearm, femur shaft, tibia, and fibula are also associated with a high risk of future fracture in untreated women.
Once a clinician is confident that a patient has osteoporosis, the question is whether the diagnosis is postmenopausal osteoporosis—or some other form of the disease. Although estrogen deficiency is the most common cause of osteoporosis in postmenopausal women, many other conditions may accompany estrogen deficiency and contribute to impaired bone strength in this population.
Among the culprits are some conditions that are not often encountered in the average gynecologic practice: monoclonal gammopathy of undetermined significance (MGUS), multiple myeloma, celiac disease, Crohn’s disease, and other inflammatory bowel diseases. In addition, bariatric surgery, eating disorders, primary hyperparathyroidism, and a number of medications have been implicated in BMD loss or increased risk of fracture, or both. Among the problematic drugs of particular interest to us as gynecologists are aromatase inhibitors, depot medroxyprogesterone acetate, proton pump inhibitors, and gonadotropin-releasing hormone (GnRH) agonists.
Other medications that can affect BMD are glucocorticoids, unfractionated heparin, selective serotonin reuptake inhibitors, excessive amounts of thyroid replacement agents, and some antiseizure medications.
If you suspect a secondary cause of osteoporosis, be prepared to perform a basic workup that includes:
- a careful history and physical examination
- complete blood count
- a chemistry profile, including serum calcium, phosphorous, electrolytes, alkaline phosphatase, and creatinine.
In addition, measurement of 25-hydroxy vitamin D and thyroid-stimulating hormone (TSH) may be helpful, as may serum protein electrophoresis.
Patients who have clinical or laboratory abnormalities suggestive of a secondary cause of osteoporosis are usually referred to a metabolic bone specialist (endocrinology or rheumatology).
When a patient has any clinical history that suggests a secondary cause of bone loss other than menopause-related estrogen deficiency, simple laboratory tests are appropriate and may uncover a condition that necessitates referral to a metabolic bone expert.
Quantitative ultrasound assessment of bone can help predict a woman’s risk of fracture
Guglielmi G, Rossini M, Nicolosi MG, Tagno A, Lentini G, de Terlizzi F. Three-year prospective study on fracture risk in postmenopausal women by quantitative ultrasound at the phalanges [published online ahead of print August 15, 2012]. J Clin Densitom. doi:10.1016 /j.jocd.2012.07.006.
Chan MY, Nguyen ND, Center JR, Eisman JA, Nguyen TV. Quantitative ultrasound and fracture risk prediction in non-osteoporotic men and women as defined by WHO criteria [published online ahead of print August 10, 2012]. Osteoporos Int. doi:10.1007/s00198-012 -2001-2.
I became interested in bone health through my longstanding interest in ultrasound, when a manufacturer asked me to evaluate equipment designed to assess bone density of the heel through quantitative ultrasound (QUS). This modality is not the diagnostic imaging we are familiar with in obstetrics and gynecology. In QUS, the homogeneity of healthy bone promotes sound transmission, whereas the voids and discontinuity of osteoporotic bone impede it. Therefore, normal bone has a faster speed of sound than less healthy bone. The other important quantitative measure is broadband ultrasound attenuation (BUA). Healthy bone is dense and absorbs and scatters sound to a greater extent than osteoporotic bone does.
Two trials of QUS
In 2010, Guglielmi and colleagues contacted 2,210 Italian women who had undergone QUS of the phalanges in 2006–2007. These women had an average age of 60.9 years, entered menopause at an average age of 49.3 years, and had a mean body mass index (BMI) of 26.5 kg/m2. By 2010, this group had experienced 108 new major osteoporotic fractures, including 23 hip fractures and 56 vertebral fractures. Investigators found a statistically significant correlation between QUS findings and fracture risk.
Chan and colleagues focused on 312 women 62 to 92 years of age who had femoral neck BMD, as measured by DXA, of –2.5 or better. QUS was measured as BUA at the calcaneus. The incidence of any fragility fracture was ascertained by radiographic reports during the follow-up period from 1994 to 2011. Eighty women (26%) experienced at least one fragility fracture during follow-up. After adjustment for covariates, women were significantly more likely to experience any fracture if BUA was decreased (hazard ratio [HR], 1.50; 95% confidence interval [CI], 1.13–1.99).
When the models that included BUA were compared with those that used femoral neck BMD, they had a greater area under the curve (0.71, 0.85, 0.71 for any fracture, hip fracture, and vertebral fracture, respectively) and yielded a net reclassification improvement of 16.4% (P=.009) when combined with femoral neck BMD. These findings suggest that calcaneal BUA is an independent predictor of fracture risk in women who have nonosteoporotic BMD.
In an era of increasing pressure to reduce costs, QUS assessment of bone is a promising modality that may be useful as a screening tool. Although it measures different variables than DXA imaging (more microarchitecture, less true density), it seems to predict the risk of fracture at less cost without ionizing radiation.
In the pipeline: A drug that curbs bone resorption without diminishing bone formation
Williams SC. Potential first-in-class osteoporosis drug speeds through trials. Nat Med. 2012;18(8):1158.
Ng KW. Potential role of odanacatib in the treatment of osteoporosis. Clin Interv Aging. 2012;7:235–247.
Alendronate was the first of the oral bisphosphonates to be approved by the US Food and Drug Administration (FDA). Once it was approved in 1999, the drug quickly became the most widely used bone agent in clinical practice and was soon joined by other oral and intravenous bisphosphonates. Regrettably, highly publicized adverse effects have caused many patients to shy away from this class of drugs. Two years ago, the FDA approved denosumab, a subcutaneous injectable agent that is a RANK ligand inhibitor.
The bisphosphonates and denosumab increase bone mass by shutting down the osteoclasts responsible for bone resorption, but they also inhibit creation of new bone. A new category of drug that inhibits the bone-resorption enzyme Cathepsin K appears to inhibit bone resorption without diminishing bone formation. Trials of two previous agents in this class were halted because of adverse effects—particularly effects to the skin, where the enzyme is expressed in addition to bone. However, Phase 2 trials in which odanacatib was compared with alendronate found that the new drug increased BMD almost twice as much as alendronate did, with less reduction in serum markers of bone formation.
Phase 3 trials of odanacatib in 16,000 women older than age 65 recently were halted so that the manufacturer could pursue regulatory approval ahead of the previous schedule. Although Phase 3 data have not been published yet, odanacatib may prove to be an exciting alternative to existing therapies.
Odanacatib is not yet available. However, by discussing therapies that may be “around the corner” with our patients, we demonstrate that we are staying ahead of the curve of scientific development.
We want to hear from you! Tell us what you think.
What is the optimal interval for osteoporosis screening in postmenopausal women before fracture occurrence and osteoporosis?
Steven R. Goldstein, MD (Examining the Evidence, August 2012)
Update on Menopause
Andrew M. Kaunitz, MD (May 2012)
Update on Osteoporosis
Steven R. Goldstein, MD (November 2011)
An appeal to the FDA: Remove the black-box warning for depot medroxyprogesterone acetate?
Andrew M. Kaunitz, MD; David A. Grimes, MD (Guest Editorial, August 2011)
Osteoporosis is a significant health issue—and it is likely to remain so as more and more women live longer and longer. In fact, increasing age is the single biggest risk factor for osteoporotic fragility fracture.
Over the past year, important research has improved our understanding in diverse areas of bone health. In this Update, I highlight studies that:
- seek to elucidate the optimal frequency of dual-energy x-ray absorptiometry (DXA) imaging to assess bone mineral density
- review secondary causes of osteoporosis besides menopause-related estrogen deficiency
- explore the use of quantitative ultrasound (QUS) to predict the risk of fracture
- report on a new class of pharmaceutical agents that inhibit the bone-resorption enzyme Cathepsin K.
All of these issues are clinically relevant to the ObGyn specialty because, when it comes to our patients’ bone health, we often function as the primary care physician.
When is DXA indicated—and how often should it be repeated?
Gourlay ML, Fine JP, Preisser JS, et al; Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366(3):225–233.
Lewiecki EM, Laster AJ, Miller PD, Bilezikian JP. More bone density testing is needed, not less. J Bone Miner Res. 2012;27(4):739–742.
American Society for Bone and Mineral Research response to media coverage of New England Journal of Medicine study: “Bone density testing interval and transition to osteoporosis in older women” [press release]. http://www.asbmr.org/about/pressreleases/detail.aspx?cid=3801baff-0df3-47c0-874f-08a185d67001. Published February 1, 2012. Accessed October 15, 2012.
Recommendations from professional societies, such as the National Osteoporosis Foundation, the International Society of Clinical Densitometry, and the American College of Obstetricians and Gynecologists say virtually the same thing about DXA imaging: Screening is appropriate for women 65 years and older and for postmenopausal women younger than age 65 who have risk factors for fracture. Risk factors include:
- history of a fragility fracture
- body weight less than 127 lb
- medical causes of bone loss, such as medication or disease
- parental history of hip fracture
- current smoker
- alcoholism
- rheumatoid arthritis.
The measurement of bone mineral density (BMD) has been the cornerstone of the diagnosis of osteopenia and osteoporosis since these classifications were introduced by the World Health Organization (WHO) in 1994. Although we are now able to evaluate a woman’s fracture risk using the FRAX tool, which does not require BMD assessment, DXA scanning has become entrenched in routine clinical practice in the United States. In addition, patients who use drugs to reduce their fracture risk often demand periodic testing to see how they are doing. Even women who do not take medications often want periodic assessment to confirm that they are not “losing bone.” Medicare allows for testing every 23 months.
23-month screening interval does not fit all women
Gourlay and colleagues prospectively followed 4,957 women aged 67 years or older who had no history of hip or vertebral fracture and who were not being treated for osteoporosis. After follow-up for as long as 15 years, investigators found that the better a woman’s initial bone density, the longer it took for her to develop osteoporosis. For example, among women over 67 years of age who had a T-score of –1.0 or better, it would take 16.8 years for 10% of this population to develop osteoporosis. In contrast, among women over 67 years of age who had a T-score of –2.0, it would take only 1.1 years for 10% of this population to develop osteoporosis.
This finding certainly calls into question the notion that all patients should be screened every 23 months. It may be better to think of screening as a way of triaging patients for decisions relative to subsequent follow-up.
Media distorted take-home message
This study was the focus of considerable attention from the media, which implied that too much DXA screening is being performed. In reality, only 13% of women over the age of 65 undergo a baseline DXA scan. However, routine follow-up of all patients at 23-month intervals is clearly not appropriate.
Because this study primarily involved white women older than age 67, extrapolation of its findings to other groups may not be appropriate. Nevertheless, the study helps to underscore the fact that reliance on BMD measurement alone should not be used to determine the need for therapeutic intervention. The FRAX tool can be used on an annual basis to assess a woman’s risk of fracture and does not require follow-up DXA imaging at any arbitrary interval.
In healthy older women, an interval of 23 months for repeat BMD assessment makes little sense. For women who have excellent initial T-scores, clinicians can lengthen this interval significantly.
However, strict reliance on the T-score isn’t the best way to predict a woman’s fracture risk or determine when pharmacologic intervention is warranted. Rather, yearly assessment using a tool such as FRAX should become the standard of care.
Some secondary causes of osteoporosis are overlooked or underappreciated
Miller PD. Unrecognized and unappreciated secondary causes of osteoporosis. Endocrinol Metab Clin North Am. 2012;41(3):613–628.
The fractures traditionally associated with osteoporosis involve the hip and vertebrae, although low-trauma fractures of the humerus, forearm, femur shaft, tibia, and fibula are also associated with a high risk of future fracture in untreated women.
Once a clinician is confident that a patient has osteoporosis, the question is whether the diagnosis is postmenopausal osteoporosis—or some other form of the disease. Although estrogen deficiency is the most common cause of osteoporosis in postmenopausal women, many other conditions may accompany estrogen deficiency and contribute to impaired bone strength in this population.
Among the culprits are some conditions that are not often encountered in the average gynecologic practice: monoclonal gammopathy of undetermined significance (MGUS), multiple myeloma, celiac disease, Crohn’s disease, and other inflammatory bowel diseases. In addition, bariatric surgery, eating disorders, primary hyperparathyroidism, and a number of medications have been implicated in BMD loss or increased risk of fracture, or both. Among the problematic drugs of particular interest to us as gynecologists are aromatase inhibitors, depot medroxyprogesterone acetate, proton pump inhibitors, and gonadotropin-releasing hormone (GnRH) agonists.
Other medications that can affect BMD are glucocorticoids, unfractionated heparin, selective serotonin reuptake inhibitors, excessive amounts of thyroid replacement agents, and some antiseizure medications.
If you suspect a secondary cause of osteoporosis, be prepared to perform a basic workup that includes:
- a careful history and physical examination
- complete blood count
- a chemistry profile, including serum calcium, phosphorous, electrolytes, alkaline phosphatase, and creatinine.
In addition, measurement of 25-hydroxy vitamin D and thyroid-stimulating hormone (TSH) may be helpful, as may serum protein electrophoresis.
Patients who have clinical or laboratory abnormalities suggestive of a secondary cause of osteoporosis are usually referred to a metabolic bone specialist (endocrinology or rheumatology).
When a patient has any clinical history that suggests a secondary cause of bone loss other than menopause-related estrogen deficiency, simple laboratory tests are appropriate and may uncover a condition that necessitates referral to a metabolic bone expert.
Quantitative ultrasound assessment of bone can help predict a woman’s risk of fracture
Guglielmi G, Rossini M, Nicolosi MG, Tagno A, Lentini G, de Terlizzi F. Three-year prospective study on fracture risk in postmenopausal women by quantitative ultrasound at the phalanges [published online ahead of print August 15, 2012]. J Clin Densitom. doi:10.1016 /j.jocd.2012.07.006.
Chan MY, Nguyen ND, Center JR, Eisman JA, Nguyen TV. Quantitative ultrasound and fracture risk prediction in non-osteoporotic men and women as defined by WHO criteria [published online ahead of print August 10, 2012]. Osteoporos Int. doi:10.1007/s00198-012 -2001-2.
I became interested in bone health through my longstanding interest in ultrasound, when a manufacturer asked me to evaluate equipment designed to assess bone density of the heel through quantitative ultrasound (QUS). This modality is not the diagnostic imaging we are familiar with in obstetrics and gynecology. In QUS, the homogeneity of healthy bone promotes sound transmission, whereas the voids and discontinuity of osteoporotic bone impede it. Therefore, normal bone has a faster speed of sound than less healthy bone. The other important quantitative measure is broadband ultrasound attenuation (BUA). Healthy bone is dense and absorbs and scatters sound to a greater extent than osteoporotic bone does.
Two trials of QUS
In 2010, Guglielmi and colleagues contacted 2,210 Italian women who had undergone QUS of the phalanges in 2006–2007. These women had an average age of 60.9 years, entered menopause at an average age of 49.3 years, and had a mean body mass index (BMI) of 26.5 kg/m2. By 2010, this group had experienced 108 new major osteoporotic fractures, including 23 hip fractures and 56 vertebral fractures. Investigators found a statistically significant correlation between QUS findings and fracture risk.
Chan and colleagues focused on 312 women 62 to 92 years of age who had femoral neck BMD, as measured by DXA, of –2.5 or better. QUS was measured as BUA at the calcaneus. The incidence of any fragility fracture was ascertained by radiographic reports during the follow-up period from 1994 to 2011. Eighty women (26%) experienced at least one fragility fracture during follow-up. After adjustment for covariates, women were significantly more likely to experience any fracture if BUA was decreased (hazard ratio [HR], 1.50; 95% confidence interval [CI], 1.13–1.99).
When the models that included BUA were compared with those that used femoral neck BMD, they had a greater area under the curve (0.71, 0.85, 0.71 for any fracture, hip fracture, and vertebral fracture, respectively) and yielded a net reclassification improvement of 16.4% (P=.009) when combined with femoral neck BMD. These findings suggest that calcaneal BUA is an independent predictor of fracture risk in women who have nonosteoporotic BMD.
In an era of increasing pressure to reduce costs, QUS assessment of bone is a promising modality that may be useful as a screening tool. Although it measures different variables than DXA imaging (more microarchitecture, less true density), it seems to predict the risk of fracture at less cost without ionizing radiation.
In the pipeline: A drug that curbs bone resorption without diminishing bone formation
Williams SC. Potential first-in-class osteoporosis drug speeds through trials. Nat Med. 2012;18(8):1158.
Ng KW. Potential role of odanacatib in the treatment of osteoporosis. Clin Interv Aging. 2012;7:235–247.
Alendronate was the first of the oral bisphosphonates to be approved by the US Food and Drug Administration (FDA). Once it was approved in 1999, the drug quickly became the most widely used bone agent in clinical practice and was soon joined by other oral and intravenous bisphosphonates. Regrettably, highly publicized adverse effects have caused many patients to shy away from this class of drugs. Two years ago, the FDA approved denosumab, a subcutaneous injectable agent that is a RANK ligand inhibitor.
The bisphosphonates and denosumab increase bone mass by shutting down the osteoclasts responsible for bone resorption, but they also inhibit creation of new bone. A new category of drug that inhibits the bone-resorption enzyme Cathepsin K appears to inhibit bone resorption without diminishing bone formation. Trials of two previous agents in this class were halted because of adverse effects—particularly effects to the skin, where the enzyme is expressed in addition to bone. However, Phase 2 trials in which odanacatib was compared with alendronate found that the new drug increased BMD almost twice as much as alendronate did, with less reduction in serum markers of bone formation.
Phase 3 trials of odanacatib in 16,000 women older than age 65 recently were halted so that the manufacturer could pursue regulatory approval ahead of the previous schedule. Although Phase 3 data have not been published yet, odanacatib may prove to be an exciting alternative to existing therapies.
Odanacatib is not yet available. However, by discussing therapies that may be “around the corner” with our patients, we demonstrate that we are staying ahead of the curve of scientific development.
We want to hear from you! Tell us what you think.