User login
Few Women Know Uterine Fibroid Risk, Treatment Options
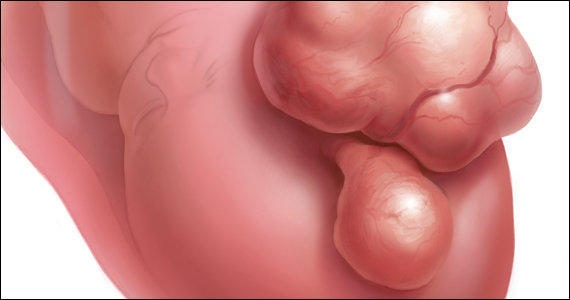
Most women (72%) are not aware they are at risk for developing uterine fibroids, though up to 77% of women will develop them in their lifetime, results of a new survey indicate.
Data from The Harris Poll, conducted on behalf of the Society of Interventional Radiology, also found that 17% of women mistakenly think a hysterectomy is the only treatment option, including more than one in four women (27%) who are between the ages of 18 and 34. Results were shared in a press release. The survey included 1,122 US women, some who have been diagnosed with uterine fibroids.
Fibroids may not cause symptoms for some, but some women may have heavy, prolonged, debilitating bleeding. Some women experience pelvic pain, a diminished sex life, and declining energy. However, the growths do not spread to other body regions and typically are not dangerous.
Hysterectomy Is Only One Option
Among the women in the survey who had been diagnosed with fibroids, 53% were presented the option of hysterectomy and 20% were told about other, less-invasive options, including over-the-counter NSAIDs (19%); uterine fibroid embolization (UFE) (17%); oral contraceptives (17%); and endometrial ablation (17%).
“Women need to be informed about the complete range of options available for treating their uterine fibroids, not just the surgical options as is most commonly done by gynecologists,” John C. Lipman, MD, founder and medical director of the Atlanta Fibroid Center in Smyrna, Georgia, said in the press release.
The survey also found that:
- More than half of women ages 18-34 (56%) and women ages 35-44 (51%) were either not familiar with uterine fibroids or never heard of them.
- Awareness was particularly low among Hispanic women, as 50% of Hispanic women say they’ve never heard of or aren’t familiar with the condition, compared with 37% of Black women who answered that way.
- More than one third (36%) of Black women and 22% of Hispanic women mistakenly think they are not at risk for developing fibroids, yet research has shown that uterine fibroids are three times more common in Black women and two times more common in Hispanic women than in White women.
For this study, the full sample data is accurate to within +/– 3.2 percentage points using a 95% confidence level. The data are part of the report “The Fibroid Fix: What Women Need to Know,” published on July 9 by the Society of Interventional Radiology.
Linda Fan, MD, chief of gynecology at Yale University in New Haven, Connecticut, said she is not surprised by those numbers. She says many patients are referred to her department who have not been given the full array of medical options for their fibroids or have not had thorough discussions with their providers, such as whether they want to preserve their fertility, or how they feel about an incision, undergoing anesthesia, or having their uterus removed.
Sometimes the hysterectomy choice is clear, she said — for instance, if there are indications of the rare cancer leiomyosarcoma, or if a postmenopausal woman has rapid growth of fibroids or heavy bleeding. Fibroids should not start growing after menopause, she said.
Additional options include radiofrequency ablation, performed while a patient is under anesthesia, by laparoscopy or hysteroscopy. The procedure uses ultrasound to watch a probe as it shrinks the fibroids with heat.
Currently, if a woman wants large fibroids removed and wants to keep her fertility options open, Dr. Fan says, myomectomy or medication are best “because we have the most information or data on (those options).”
When treating patients who don’t prioritize fertility, she said, UFE is a good option that doesn’t need incisions or anesthesia. But patients sometimes require a lot of pain medication afterward, Dr. Fan said. With radiofrequency ablation, specifically the Acessa and Sonata procedures, she said, “patients don’t experience a lot of pain after the procedure because the shrinking happens when they’re asleep under anesthesia.”
Uterine Fibroid Embolization a Nonsurgical Option
The report describes how UFE works but the Harris Poll showed that 60% of women who have heard of UFE did not hear about it first from a healthcare provider.
“UFE is a nonsurgical treatment, performed by interventional radiologists, that has been proven to significantly reduce heavy menstrual bleeding, relieve uterine pain, and improve energy levels,” the authors write. “Through a tiny incision in the wrist or thigh, a catheter is guided via imaging to the vessels leading to the fibroids. Through this catheter, small clear particles are injected to block the blood flow leading to the fibroids causing them to shrink and disappear.”
After UFE, most women leave the hospital the day of or the day after treatment, according to the report authors, who add that many patients also report they can resume normal activity in about 2 weeks, more quickly than with surgical treatments.
In some cases, watchful waiting will be the best option, the report notes, and that may require repeated checkups and scans.
Dr. Lipman is an adviser on The Fibroid Fix report.
Most women (72%) are not aware they are at risk for developing uterine fibroids, though up to 77% of women will develop them in their lifetime, results of a new survey indicate.
Data from The Harris Poll, conducted on behalf of the Society of Interventional Radiology, also found that 17% of women mistakenly think a hysterectomy is the only treatment option, including more than one in four women (27%) who are between the ages of 18 and 34. Results were shared in a press release. The survey included 1,122 US women, some who have been diagnosed with uterine fibroids.
Fibroids may not cause symptoms for some, but some women may have heavy, prolonged, debilitating bleeding. Some women experience pelvic pain, a diminished sex life, and declining energy. However, the growths do not spread to other body regions and typically are not dangerous.
Hysterectomy Is Only One Option
Among the women in the survey who had been diagnosed with fibroids, 53% were presented the option of hysterectomy and 20% were told about other, less-invasive options, including over-the-counter NSAIDs (19%); uterine fibroid embolization (UFE) (17%); oral contraceptives (17%); and endometrial ablation (17%).
“Women need to be informed about the complete range of options available for treating their uterine fibroids, not just the surgical options as is most commonly done by gynecologists,” John C. Lipman, MD, founder and medical director of the Atlanta Fibroid Center in Smyrna, Georgia, said in the press release.
The survey also found that:
- More than half of women ages 18-34 (56%) and women ages 35-44 (51%) were either not familiar with uterine fibroids or never heard of them.
- Awareness was particularly low among Hispanic women, as 50% of Hispanic women say they’ve never heard of or aren’t familiar with the condition, compared with 37% of Black women who answered that way.
- More than one third (36%) of Black women and 22% of Hispanic women mistakenly think they are not at risk for developing fibroids, yet research has shown that uterine fibroids are three times more common in Black women and two times more common in Hispanic women than in White women.
For this study, the full sample data is accurate to within +/– 3.2 percentage points using a 95% confidence level. The data are part of the report “The Fibroid Fix: What Women Need to Know,” published on July 9 by the Society of Interventional Radiology.
Linda Fan, MD, chief of gynecology at Yale University in New Haven, Connecticut, said she is not surprised by those numbers. She says many patients are referred to her department who have not been given the full array of medical options for their fibroids or have not had thorough discussions with their providers, such as whether they want to preserve their fertility, or how they feel about an incision, undergoing anesthesia, or having their uterus removed.
Sometimes the hysterectomy choice is clear, she said — for instance, if there are indications of the rare cancer leiomyosarcoma, or if a postmenopausal woman has rapid growth of fibroids or heavy bleeding. Fibroids should not start growing after menopause, she said.
Additional options include radiofrequency ablation, performed while a patient is under anesthesia, by laparoscopy or hysteroscopy. The procedure uses ultrasound to watch a probe as it shrinks the fibroids with heat.
Currently, if a woman wants large fibroids removed and wants to keep her fertility options open, Dr. Fan says, myomectomy or medication are best “because we have the most information or data on (those options).”
When treating patients who don’t prioritize fertility, she said, UFE is a good option that doesn’t need incisions or anesthesia. But patients sometimes require a lot of pain medication afterward, Dr. Fan said. With radiofrequency ablation, specifically the Acessa and Sonata procedures, she said, “patients don’t experience a lot of pain after the procedure because the shrinking happens when they’re asleep under anesthesia.”
Uterine Fibroid Embolization a Nonsurgical Option
The report describes how UFE works but the Harris Poll showed that 60% of women who have heard of UFE did not hear about it first from a healthcare provider.
“UFE is a nonsurgical treatment, performed by interventional radiologists, that has been proven to significantly reduce heavy menstrual bleeding, relieve uterine pain, and improve energy levels,” the authors write. “Through a tiny incision in the wrist or thigh, a catheter is guided via imaging to the vessels leading to the fibroids. Through this catheter, small clear particles are injected to block the blood flow leading to the fibroids causing them to shrink and disappear.”
After UFE, most women leave the hospital the day of or the day after treatment, according to the report authors, who add that many patients also report they can resume normal activity in about 2 weeks, more quickly than with surgical treatments.
In some cases, watchful waiting will be the best option, the report notes, and that may require repeated checkups and scans.
Dr. Lipman is an adviser on The Fibroid Fix report.
Most women (72%) are not aware they are at risk for developing uterine fibroids, though up to 77% of women will develop them in their lifetime, results of a new survey indicate.
Data from The Harris Poll, conducted on behalf of the Society of Interventional Radiology, also found that 17% of women mistakenly think a hysterectomy is the only treatment option, including more than one in four women (27%) who are between the ages of 18 and 34. Results were shared in a press release. The survey included 1,122 US women, some who have been diagnosed with uterine fibroids.
Fibroids may not cause symptoms for some, but some women may have heavy, prolonged, debilitating bleeding. Some women experience pelvic pain, a diminished sex life, and declining energy. However, the growths do not spread to other body regions and typically are not dangerous.
Hysterectomy Is Only One Option
Among the women in the survey who had been diagnosed with fibroids, 53% were presented the option of hysterectomy and 20% were told about other, less-invasive options, including over-the-counter NSAIDs (19%); uterine fibroid embolization (UFE) (17%); oral contraceptives (17%); and endometrial ablation (17%).
“Women need to be informed about the complete range of options available for treating their uterine fibroids, not just the surgical options as is most commonly done by gynecologists,” John C. Lipman, MD, founder and medical director of the Atlanta Fibroid Center in Smyrna, Georgia, said in the press release.
The survey also found that:
- More than half of women ages 18-34 (56%) and women ages 35-44 (51%) were either not familiar with uterine fibroids or never heard of them.
- Awareness was particularly low among Hispanic women, as 50% of Hispanic women say they’ve never heard of or aren’t familiar with the condition, compared with 37% of Black women who answered that way.
- More than one third (36%) of Black women and 22% of Hispanic women mistakenly think they are not at risk for developing fibroids, yet research has shown that uterine fibroids are three times more common in Black women and two times more common in Hispanic women than in White women.
For this study, the full sample data is accurate to within +/– 3.2 percentage points using a 95% confidence level. The data are part of the report “The Fibroid Fix: What Women Need to Know,” published on July 9 by the Society of Interventional Radiology.
Linda Fan, MD, chief of gynecology at Yale University in New Haven, Connecticut, said she is not surprised by those numbers. She says many patients are referred to her department who have not been given the full array of medical options for their fibroids or have not had thorough discussions with their providers, such as whether they want to preserve their fertility, or how they feel about an incision, undergoing anesthesia, or having their uterus removed.
Sometimes the hysterectomy choice is clear, she said — for instance, if there are indications of the rare cancer leiomyosarcoma, or if a postmenopausal woman has rapid growth of fibroids or heavy bleeding. Fibroids should not start growing after menopause, she said.
Additional options include radiofrequency ablation, performed while a patient is under anesthesia, by laparoscopy or hysteroscopy. The procedure uses ultrasound to watch a probe as it shrinks the fibroids with heat.
Currently, if a woman wants large fibroids removed and wants to keep her fertility options open, Dr. Fan says, myomectomy or medication are best “because we have the most information or data on (those options).”
When treating patients who don’t prioritize fertility, she said, UFE is a good option that doesn’t need incisions or anesthesia. But patients sometimes require a lot of pain medication afterward, Dr. Fan said. With radiofrequency ablation, specifically the Acessa and Sonata procedures, she said, “patients don’t experience a lot of pain after the procedure because the shrinking happens when they’re asleep under anesthesia.”
Uterine Fibroid Embolization a Nonsurgical Option
The report describes how UFE works but the Harris Poll showed that 60% of women who have heard of UFE did not hear about it first from a healthcare provider.
“UFE is a nonsurgical treatment, performed by interventional radiologists, that has been proven to significantly reduce heavy menstrual bleeding, relieve uterine pain, and improve energy levels,” the authors write. “Through a tiny incision in the wrist or thigh, a catheter is guided via imaging to the vessels leading to the fibroids. Through this catheter, small clear particles are injected to block the blood flow leading to the fibroids causing them to shrink and disappear.”
After UFE, most women leave the hospital the day of or the day after treatment, according to the report authors, who add that many patients also report they can resume normal activity in about 2 weeks, more quickly than with surgical treatments.
In some cases, watchful waiting will be the best option, the report notes, and that may require repeated checkups and scans.
Dr. Lipman is an adviser on The Fibroid Fix report.
Myomectomy best for avoiding reintervention after fibroid procedures
Reintervention rates after uterus-preserving surgery for leiomyomata were lowest after vaginal myomectomy, the most frequent among four therapeutic approaches, a large cohort study reported.
Accounting for censoring, the 7-year reintervention risk for vaginal myomectomy was 20.6%, followed by uterine artery embolization (26%), endometrial ablation (35.5%), and hysteroscopic myomectomy (37%).
Hysterectomies accounted for 63.2% of reinterventions according to lead author Susanna D. Mitro, PhD, a research scientist in the Division of Research and Department of Obstetrics and Gynecology at Kaiser Permanente Northern California, Oakland, and colleagues.
Risk did not vary by body mass index, race/ethnicity, or Neighborhood Deprivation Index, but did vary for some procedures by age and parity,
These findings generally align with earlier research and “illustrate clinically meaningful long-term differences in reintervention rates after a first uterus-preserving treatment for leiomyomas,” the researchers wrote in Obstetrics & Gynecology.
The Study
In a cohort of 10,324 patients ages 18-50, 19.9% were Asian, 21.2% Black, 21.3% Hispanic, and 32.5% White, with 5.2% of other races and ethnicities. The most affected age groups were 41-45 and 46-50 years. All participants underwent a first uterus-preserving procedure after leiomyoma diagnosis according to 2009-2021 electronic health records at Kaiser Permanente Northern California.
Reintervention referred to a second uterus-preserving procedure or hysterectomy. Median follow-up was 3.8 years (interquartile range, 1.8-7.4 years), and the proportions of index procedures were as follows: 18% (1857) for hysteroscopic myomectomy; 16.2% (1669) for uterine artery embolization; 21.4% (2211) for endometrial ablations; and 44.4% (4,587) for myomectomy.
Reintervention rates were higher in younger patients after uterine artery embolization, with patients ages 18-35 at the index procedure having 1.4-3.7 times greater reintervention rates than patients ages 46-50 years. Reintervention rates for hysteroscopic myomectomy varied by parity, with multiparous patients at 35% greater risk than their nulliparous counterparts.
On the age issue, the authors note that symptom recurrence may be less common in older patients, perhaps because of the onset of menopause. “Alternatively, findings may be explained by age-specific care strategies: Older patients experiencing symptom recurrence may prefer to wait until the onset of menopause rather than pursuing another surgical treatment,” they wrote.
A recent study with 7 years’ follow-up reported a 2.4 times greater risk of hysterectomy after uterine artery embolization versus myomectomy. Reintervention rates may be lower after myomectomy because otherwise asymptomatic patients pursue myomectomy to treat infertility, the authors wrote. Alternatively, myomectomy may more completely remove leiomyomas.
These common benign tumors take a toll on healthcare resources, in 2012 costing up to $9.4 billion annually (in 2010 dollars) for related surgeries, medications, and procedures. Leiomyomas are reportedly the most frequent reason for hysterectomy.
Robust data on the optimal therapeutic approach to fibroids have been sparse, however, with a 2017 comparative-effectiveness review from the Agency for Healthcare Research and Quality reporting that evidence on leiomyoma treatments was insufficient to guide clinical care. Few well-conducted trials of leiomyoma treatment have directly compared different treatment options, the authors noted.
The rate of myomectomy is reported to be 9.2 per 10,000 woman-years in Black women and 1.3 per 10,000 woman years in White women, and the recurrence rate after myomectomy can be as great as 60% when patients are followed up to 5 years.
The authors said their findings “may be a reference to discuss expectations for treatment outcomes when choosing initial uterus-preserving treatment for leiomyomas, especially for patients receiving treatment years before the likely onset of menopause.”
This research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health. Coauthor Dr. Lauren Wise is a paid consultant for AbbVie and has received in-kind donations from Swiss Precision Diagnostics and Kindara.com; she has also received payment from the Gates Foundation.
Reintervention rates after uterus-preserving surgery for leiomyomata were lowest after vaginal myomectomy, the most frequent among four therapeutic approaches, a large cohort study reported.
Accounting for censoring, the 7-year reintervention risk for vaginal myomectomy was 20.6%, followed by uterine artery embolization (26%), endometrial ablation (35.5%), and hysteroscopic myomectomy (37%).
Hysterectomies accounted for 63.2% of reinterventions according to lead author Susanna D. Mitro, PhD, a research scientist in the Division of Research and Department of Obstetrics and Gynecology at Kaiser Permanente Northern California, Oakland, and colleagues.
Risk did not vary by body mass index, race/ethnicity, or Neighborhood Deprivation Index, but did vary for some procedures by age and parity,
These findings generally align with earlier research and “illustrate clinically meaningful long-term differences in reintervention rates after a first uterus-preserving treatment for leiomyomas,” the researchers wrote in Obstetrics & Gynecology.
The Study
In a cohort of 10,324 patients ages 18-50, 19.9% were Asian, 21.2% Black, 21.3% Hispanic, and 32.5% White, with 5.2% of other races and ethnicities. The most affected age groups were 41-45 and 46-50 years. All participants underwent a first uterus-preserving procedure after leiomyoma diagnosis according to 2009-2021 electronic health records at Kaiser Permanente Northern California.
Reintervention referred to a second uterus-preserving procedure or hysterectomy. Median follow-up was 3.8 years (interquartile range, 1.8-7.4 years), and the proportions of index procedures were as follows: 18% (1857) for hysteroscopic myomectomy; 16.2% (1669) for uterine artery embolization; 21.4% (2211) for endometrial ablations; and 44.4% (4,587) for myomectomy.
Reintervention rates were higher in younger patients after uterine artery embolization, with patients ages 18-35 at the index procedure having 1.4-3.7 times greater reintervention rates than patients ages 46-50 years. Reintervention rates for hysteroscopic myomectomy varied by parity, with multiparous patients at 35% greater risk than their nulliparous counterparts.
On the age issue, the authors note that symptom recurrence may be less common in older patients, perhaps because of the onset of menopause. “Alternatively, findings may be explained by age-specific care strategies: Older patients experiencing symptom recurrence may prefer to wait until the onset of menopause rather than pursuing another surgical treatment,” they wrote.
A recent study with 7 years’ follow-up reported a 2.4 times greater risk of hysterectomy after uterine artery embolization versus myomectomy. Reintervention rates may be lower after myomectomy because otherwise asymptomatic patients pursue myomectomy to treat infertility, the authors wrote. Alternatively, myomectomy may more completely remove leiomyomas.
These common benign tumors take a toll on healthcare resources, in 2012 costing up to $9.4 billion annually (in 2010 dollars) for related surgeries, medications, and procedures. Leiomyomas are reportedly the most frequent reason for hysterectomy.
Robust data on the optimal therapeutic approach to fibroids have been sparse, however, with a 2017 comparative-effectiveness review from the Agency for Healthcare Research and Quality reporting that evidence on leiomyoma treatments was insufficient to guide clinical care. Few well-conducted trials of leiomyoma treatment have directly compared different treatment options, the authors noted.
The rate of myomectomy is reported to be 9.2 per 10,000 woman-years in Black women and 1.3 per 10,000 woman years in White women, and the recurrence rate after myomectomy can be as great as 60% when patients are followed up to 5 years.
The authors said their findings “may be a reference to discuss expectations for treatment outcomes when choosing initial uterus-preserving treatment for leiomyomas, especially for patients receiving treatment years before the likely onset of menopause.”
This research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health. Coauthor Dr. Lauren Wise is a paid consultant for AbbVie and has received in-kind donations from Swiss Precision Diagnostics and Kindara.com; she has also received payment from the Gates Foundation.
Reintervention rates after uterus-preserving surgery for leiomyomata were lowest after vaginal myomectomy, the most frequent among four therapeutic approaches, a large cohort study reported.
Accounting for censoring, the 7-year reintervention risk for vaginal myomectomy was 20.6%, followed by uterine artery embolization (26%), endometrial ablation (35.5%), and hysteroscopic myomectomy (37%).
Hysterectomies accounted for 63.2% of reinterventions according to lead author Susanna D. Mitro, PhD, a research scientist in the Division of Research and Department of Obstetrics and Gynecology at Kaiser Permanente Northern California, Oakland, and colleagues.
Risk did not vary by body mass index, race/ethnicity, or Neighborhood Deprivation Index, but did vary for some procedures by age and parity,
These findings generally align with earlier research and “illustrate clinically meaningful long-term differences in reintervention rates after a first uterus-preserving treatment for leiomyomas,” the researchers wrote in Obstetrics & Gynecology.
The Study
In a cohort of 10,324 patients ages 18-50, 19.9% were Asian, 21.2% Black, 21.3% Hispanic, and 32.5% White, with 5.2% of other races and ethnicities. The most affected age groups were 41-45 and 46-50 years. All participants underwent a first uterus-preserving procedure after leiomyoma diagnosis according to 2009-2021 electronic health records at Kaiser Permanente Northern California.
Reintervention referred to a second uterus-preserving procedure or hysterectomy. Median follow-up was 3.8 years (interquartile range, 1.8-7.4 years), and the proportions of index procedures were as follows: 18% (1857) for hysteroscopic myomectomy; 16.2% (1669) for uterine artery embolization; 21.4% (2211) for endometrial ablations; and 44.4% (4,587) for myomectomy.
Reintervention rates were higher in younger patients after uterine artery embolization, with patients ages 18-35 at the index procedure having 1.4-3.7 times greater reintervention rates than patients ages 46-50 years. Reintervention rates for hysteroscopic myomectomy varied by parity, with multiparous patients at 35% greater risk than their nulliparous counterparts.
On the age issue, the authors note that symptom recurrence may be less common in older patients, perhaps because of the onset of menopause. “Alternatively, findings may be explained by age-specific care strategies: Older patients experiencing symptom recurrence may prefer to wait until the onset of menopause rather than pursuing another surgical treatment,” they wrote.
A recent study with 7 years’ follow-up reported a 2.4 times greater risk of hysterectomy after uterine artery embolization versus myomectomy. Reintervention rates may be lower after myomectomy because otherwise asymptomatic patients pursue myomectomy to treat infertility, the authors wrote. Alternatively, myomectomy may more completely remove leiomyomas.
These common benign tumors take a toll on healthcare resources, in 2012 costing up to $9.4 billion annually (in 2010 dollars) for related surgeries, medications, and procedures. Leiomyomas are reportedly the most frequent reason for hysterectomy.
Robust data on the optimal therapeutic approach to fibroids have been sparse, however, with a 2017 comparative-effectiveness review from the Agency for Healthcare Research and Quality reporting that evidence on leiomyoma treatments was insufficient to guide clinical care. Few well-conducted trials of leiomyoma treatment have directly compared different treatment options, the authors noted.
The rate of myomectomy is reported to be 9.2 per 10,000 woman-years in Black women and 1.3 per 10,000 woman years in White women, and the recurrence rate after myomectomy can be as great as 60% when patients are followed up to 5 years.
The authors said their findings “may be a reference to discuss expectations for treatment outcomes when choosing initial uterus-preserving treatment for leiomyomas, especially for patients receiving treatment years before the likely onset of menopause.”
This research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health. Coauthor Dr. Lauren Wise is a paid consultant for AbbVie and has received in-kind donations from Swiss Precision Diagnostics and Kindara.com; she has also received payment from the Gates Foundation.
FROM OBSTETRICS & GYNECOLOGY
Despite effective therapies, fibroid care still lacking
In 2022, two colleagues from Johns Hopkins University, Baltimore, Bhuchitra Singh, MD, MPH, MS, MBA, and James Segars Jr., MD, reviewed the available literature to evaluate the effectiveness of newer minimally invasive therapies in reducing bleeding and improving the quality of life and control of symptoms linked to uterine fibroids.
Their goal, according to Dr. Segars, a professor of obstetrics and gynecology and director of the division of women’s health research at Johns Hopkins, was to help guide clinicians and patients in making decisions about the use of the newer therapies, including radiofrequency ablation and ultrasound-guided removal of lesions.
But he and Dr. Singh, the director of clinical research at the Howard W. and Georgeanna Seegar Jones Laboratory of Reproductive Sciences and Women’s Health Research, were surprised by their findings. “The outcomes were relatively the same,” Dr. Segars said. “All of the modalities lead to significant reduction in bleeding and other fibroid-related symptoms.”
The data on long-term complications and risk for recurrence are sparse for some of the newer approaches, and not enough high-quality long-term studies have been conducted for the Food and Drug Administration to approve them as fertility-sparing treatments.
But perhaps, the biggest challenge now is to ensure that women can take advantage of these newer therapies, with large gaps in both the diagnosis of fibroids and geographic access to minimally invasive treatments.
A widespread condition widely underdiagnosed
Uterine fibroids occur in most women (the incidence rises with age) and can be found in up to 70% of women by the time they reach menopause. Risk factors include family history, increasing interval since last birth, hypertension, and obesity. Increasing parity and use of oral contraceptives are protective.
But as many as 50% of cases go undiagnosed, and one reason for this is the failure of clinicians to dig deeply enough into women’s menstrual histories to diagnose fibroids.
“The most common cause of anemia is heavy menstrual bleeding,” said Shannon Laughlin-Tommaso, MD, MPH, a professor of obstetrics and gynecology at Mayo Clinic in Rochester, Minn. She frequently sees patients who have already undergone colonoscopy to work-up the source of their anemia before anyone suspects that fibroids are the culprit.
“When women tell us about their periods, what they’ve been told is normal [bleeding] – or what they’ve always had and considered normal – is actually kind of on the heavier spectrum,” she said.
Ideally, treatment for uterine fibroids would fix abnormally prolonged or heavy menstrual bleeding, relieve pain, and ameliorate symptoms associated with an enlarged uterus, such as pelvic pressure, urinary frequency, and constipation. And the fibroids would never recur.
By those measures, hysterectomy fits the bill: Success rates in relieving symptoms are high, and the risk for recurrence is zero. But the procedure carries significant drawbacks: short-term complications of surgery, including infection, bleeding, and injury to the bowels and bladder along with potential long-term risks for cardiovascular disease, cancer, ovarian failure and premature menopause, depression, and decline in cognitive function. Those factors loom even larger for women who still hope to have children.
For that reason, the American College of Obstetricians and Gynecologists recommends myomectomy, or surgical removal of individual fibroids, for women who desire uterine preservation or future pregnancy. And the literature here is solid, according to Dr. Singh, who found that 95% of myomectomy patients achieved control of their bleeding symptoms, whether it was via laparoscopy, hysteroscopy, or laparotomy. Up to 40% of women may develop new fibroids, or leiomyomas, within 3 years, although only 12.2% required a second surgery up to after 5 years.
But myomectomy is invasive, requiring general anesthesia, incisions in the uterus, and stitches to close the organ.
Newer techniques have emerged that can effectively treat symptoms of fibroids without requiring surgery. Uterine artery embolization (UAE), which involves passing a catheter into the femoral artery, or laparoscopic uterine artery occlusion can be used to cut off the blood supply of the fibroid. Other techniques, including focused ultrasound surgery and radiofrequency ablation (RFA), use various forms of energy to heat and ablate fibroids. The latter two can be performed in outpatient settings and often without general anesthesia.
Approved for use in 1994, UAE has the most data available, with reduction in the volume of fibroids and uterine tissue lasting up to 5 years, and rates of reintervention of 19%-38% between 2 and 5 years after the procedure. Dr. Singh’s review found that 79%-98.5% of recipients of the procedure reported declines in bleeding that persisted for several years, which is comparable to myomectomy. Quality of life and pain scores also showed good improvement, with follow-up in the different studies ranging from 12 months to over 5 years, the analysis showed.
UAE does have its drawbacks. In rare cases, embolization can deprive the entire uterus and ovaries of blood, which can cause ovarian dysfunction and potentially result in premature menopause, although this outcome is most common in women who are older than 45 years. The procedure can often also be painful enough that overnight hospitalization is required.
Focused ultrasound surgeries, which include magnetic resonance–guided focused ultrasound surgery (MRgFUS) and high-intensity focused ultrasound (HIFU), were approved by the FDA in 2004. Focused ultrasound waves pass through the abdominal wall and produce significant heating, causing a burn that destroys the targeted tissue without damaging surrounding tissue. As with UAE, improvements in fibroid-associated bleeding and measures of quality of life were similar to those after myomectomy up to 3 years later.
But Dr. Singh noted that both focused ultrasound and RFA can damage the skin or internal organs. “[As] always with the thermal interventions, there is the probability of skin as well as internal organs that might get the thermal energy if it’s not focused correctly on to the fibroid itself,” he said. In addition, MRgFUS is not an option for women who are not good candidates to undergo an MRI, such as those with claustrophobia or pacemakers.
Also, with focus ultrasound and RFA, “we do worry about that fibroid getting blood flow back,” which can lead to recurrence of heavy menstrual bleeding, Dr. Laughlin-Tommaso noted.
Although data on RFA are limited to 12 months of follow-up, most women reported meaningful reductions in bleeding symptoms. Longer follow-up has been reported for bleeding symptoms after MRgFUS, with similar results up to 3 years later.
For Leslie Hansen-Lindner, MD, chief of obstetrics and gynecology at Atrium Health in Charlotte, N.C., choosing the right procedure starts with a patient-centered conversation weighing the pros and cons of the options and the woman’s goals.
“Is their goal to reduce the size and impact of their fibroid, bleed less, and have a better quality of life on their period?” Dr. Hansen-Lindner said. “Or is their goal to have the entire fibroid removed?”
If the former, an RFA is appealing to many women. If the latter, laparoscopic or mini-laparotomy myomectomy might be a better choice. Although fewer than 10% of patients require surgical reintervention at 3 years of follow-up for RFA, myomectomy has more consistent long-term evidence showing that fewer women require re-intervention and preserve their fertility, she added.
Age also plays a role in the decision: The closer a woman is to menopause, the less likely she is to experience a recurrence, so a less-invasive procedure is preferable. But for younger women hoping to become pregnant, the lower risk for recurrence and good prognosis for future fertility might sway the choice toward myomectomy.
The first laparoscopic RFA procedures were approved for uterine fibroids in 2012. Dr. Hansen-Lindner is a proponent of transcervical fibroid ablation (TFA), a newer RFA procedure that the FDA approved in 2018. Performed through the cervix, TFA requires no incisions and can generally be done without general anesthesia. Eligible candidates would be any woman with symptomatic fibroids, such as heavy menstrual bleeding, pain, or bulk symptoms. The contraindications are few.
“It’s going to come down to size and location of fibroids, and whether or not they would be accessible by the TFA,” Dr. Hansen-Lindner said. “I have to make sure that there isn’t a fibroid blocking their cervix and that the fibroids are accessible with this device.”
TFA also is not suitable for removing most submucosal lesions, which typically must be removed by hysteroscopic myomectomy. Dr. Hansen-Lindner said that she often uses TFA in conjunction with hysteroscopic myomectomy for this scenario. Although data on pregnancy after RFA (including TFA), MRgFUS, and HIFU are lacking, Gynesonics, the manufacturer of the Sonata System (the device that delivers radiofrequency energy to shrink the fibroid) has documented 79 pregnancies among the 2,200 women who have undergone TFA in the United States since 2018.
Disparities hampering care
Uterine fibroids are a particular problem for Black women, whose symptoms are more likely to be ignored by clinicians, according to Jodie Katon, PhD, a core investigator at the Veterans Affairs Greater Los Angeles Center for the Study of Healthcare Innovation, Implementation and Policy. Dr. Katon cited studies in which Black women interviewed about their experiences reported a consistent theme: Clinicians dismissed their symptoms, told them these were nothing to worry about, and advised them to lose weight. Those interactions not only delayed diagnosis among Black women but also led many of them to mistrust clinicians and avoid the health care system altogether.
The failure of clinicians to take their complaints seriously is just one of the disparities affecting Black women. In reviewing the literature, Dr. Laughlin-Tommaso, who also serves as the associate dean for Education Diversity, Equity, and Inclusion at the Mayo Clinic, found that African American women experience two to three times the risk for fibroids, compared with White women, as well as earlier onset and more severe disease, as measured by number and size of the lesions.
According to Dr. Katon, the etiology of fibroids is still poorly understood. “What we do know is that Black women are disproportionately exposed to a variety of factors that we have shown through observational studies are associated with increased risk of development of uterine fibroids.”
The list includes factors like stress; interpersonal racism; early age at menarche; various indicators of poor diets, such as vitamin D deficiency; the use of certain beauty products, specifically hair straighteners; as well as exposure to air pollution and other environmental toxins.
Laughlin-Tommaso also pointed to historical disparities in management, citing a doubled risk for hysterectomy for Black women in a study published in 2007 despite survey data suggesting that Black women report being more interested in uterine-preserving therapies rather than a hysterectomy.
Breaking down barriers of access to new treatments
Dr. Laughlin-Tommaso looked at more recent trends in the management of fibroids using data from the multicenter COMPARE-UF study, which enrolled women between 2015 and 2020 undergoing fibroid treatment into a longitudinal registry to track their outcomes. She found that Black women underwent hysterectomies at a lower rate than did White women and were instead more likely to undergo myomectomy or UAE.
Some of the change may reflect lack of approved minimally invasive procedures before 2000. “But now that we have expanded options, I think most women are opting not to have a hysterectomy,” Dr. Laughlin-Tommaso said.
Dr. Katon has research funding from the VA to look more closely at racial disparities in the treatment of fibroids. In a study published in April 2023, she reported some surprising trends.
During the period from 2010 to 2018, she found that Black veterans diagnosed with fibroids were less likely than White veterans were to receive treatment, regardless of their age or the severity of their symptoms. This finding held even among women with anemia, which should have been a clear indication for treatment.
But, as in the COMPARE-UF study, the subset of Black veterans who received an interventional treatment were less likely than their White peers were to undergo hysterectomy in favor of a fertility-sparing treatment as their initial procedure. Dr. Katon called it a “welcome but unexpected finding.”
But another significant barrier remains: The two newest types of procedures, RFA and guided focused ultrasound, are not commonly performed outside of tertiary care facilities. However, studies have found that all these procedures are cost effective (studies for myomectomy, UAE, MRgFUS, and TFA). The implementation of a category 1 billing code for laparoscopic RFA in 2017 has led more insurance companies to cover the service, and a category 1 code will be available for TFA effective January 2024.
Although RFA does require investment in specialized equipment, which limits facilities from offering the procedure, any gynecologist who routinely performs hysteroscopy can easily learn to do TFA. And the VA, which is committed to eliminating disparities in women’s health, established a 2-year advanced fellowship in minimally invasive gynecologic surgery in 2022 to help expand their capacity to offer these procedures.
The VA has been rapidly expanding their gynecology services, and Katon said that she is confident that ultrasound-guided procedures and RFA will become more available within the system. “I would say we’re keeping pace. And in some ways, you know, as a national system we may be positioned to actually outpace the rest of the U.S.”
Dr. Segars reported prior research funding for clinical trials from BioSpecifics Technologies, Bayer, Allergan, AbbVie, and ObsEva and currently receives funding from Myovant Sciences. Dr. Hansen-Lindner reported personal fees from Gynesonics. Dr. Singh, Dr. Laughlin-Tommaso, and Dr. Katon reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
In 2022, two colleagues from Johns Hopkins University, Baltimore, Bhuchitra Singh, MD, MPH, MS, MBA, and James Segars Jr., MD, reviewed the available literature to evaluate the effectiveness of newer minimally invasive therapies in reducing bleeding and improving the quality of life and control of symptoms linked to uterine fibroids.
Their goal, according to Dr. Segars, a professor of obstetrics and gynecology and director of the division of women’s health research at Johns Hopkins, was to help guide clinicians and patients in making decisions about the use of the newer therapies, including radiofrequency ablation and ultrasound-guided removal of lesions.
But he and Dr. Singh, the director of clinical research at the Howard W. and Georgeanna Seegar Jones Laboratory of Reproductive Sciences and Women’s Health Research, were surprised by their findings. “The outcomes were relatively the same,” Dr. Segars said. “All of the modalities lead to significant reduction in bleeding and other fibroid-related symptoms.”
The data on long-term complications and risk for recurrence are sparse for some of the newer approaches, and not enough high-quality long-term studies have been conducted for the Food and Drug Administration to approve them as fertility-sparing treatments.
But perhaps, the biggest challenge now is to ensure that women can take advantage of these newer therapies, with large gaps in both the diagnosis of fibroids and geographic access to minimally invasive treatments.
A widespread condition widely underdiagnosed
Uterine fibroids occur in most women (the incidence rises with age) and can be found in up to 70% of women by the time they reach menopause. Risk factors include family history, increasing interval since last birth, hypertension, and obesity. Increasing parity and use of oral contraceptives are protective.
But as many as 50% of cases go undiagnosed, and one reason for this is the failure of clinicians to dig deeply enough into women’s menstrual histories to diagnose fibroids.
“The most common cause of anemia is heavy menstrual bleeding,” said Shannon Laughlin-Tommaso, MD, MPH, a professor of obstetrics and gynecology at Mayo Clinic in Rochester, Minn. She frequently sees patients who have already undergone colonoscopy to work-up the source of their anemia before anyone suspects that fibroids are the culprit.
“When women tell us about their periods, what they’ve been told is normal [bleeding] – or what they’ve always had and considered normal – is actually kind of on the heavier spectrum,” she said.
Ideally, treatment for uterine fibroids would fix abnormally prolonged or heavy menstrual bleeding, relieve pain, and ameliorate symptoms associated with an enlarged uterus, such as pelvic pressure, urinary frequency, and constipation. And the fibroids would never recur.
By those measures, hysterectomy fits the bill: Success rates in relieving symptoms are high, and the risk for recurrence is zero. But the procedure carries significant drawbacks: short-term complications of surgery, including infection, bleeding, and injury to the bowels and bladder along with potential long-term risks for cardiovascular disease, cancer, ovarian failure and premature menopause, depression, and decline in cognitive function. Those factors loom even larger for women who still hope to have children.
For that reason, the American College of Obstetricians and Gynecologists recommends myomectomy, or surgical removal of individual fibroids, for women who desire uterine preservation or future pregnancy. And the literature here is solid, according to Dr. Singh, who found that 95% of myomectomy patients achieved control of their bleeding symptoms, whether it was via laparoscopy, hysteroscopy, or laparotomy. Up to 40% of women may develop new fibroids, or leiomyomas, within 3 years, although only 12.2% required a second surgery up to after 5 years.
But myomectomy is invasive, requiring general anesthesia, incisions in the uterus, and stitches to close the organ.
Newer techniques have emerged that can effectively treat symptoms of fibroids without requiring surgery. Uterine artery embolization (UAE), which involves passing a catheter into the femoral artery, or laparoscopic uterine artery occlusion can be used to cut off the blood supply of the fibroid. Other techniques, including focused ultrasound surgery and radiofrequency ablation (RFA), use various forms of energy to heat and ablate fibroids. The latter two can be performed in outpatient settings and often without general anesthesia.
Approved for use in 1994, UAE has the most data available, with reduction in the volume of fibroids and uterine tissue lasting up to 5 years, and rates of reintervention of 19%-38% between 2 and 5 years after the procedure. Dr. Singh’s review found that 79%-98.5% of recipients of the procedure reported declines in bleeding that persisted for several years, which is comparable to myomectomy. Quality of life and pain scores also showed good improvement, with follow-up in the different studies ranging from 12 months to over 5 years, the analysis showed.
UAE does have its drawbacks. In rare cases, embolization can deprive the entire uterus and ovaries of blood, which can cause ovarian dysfunction and potentially result in premature menopause, although this outcome is most common in women who are older than 45 years. The procedure can often also be painful enough that overnight hospitalization is required.
Focused ultrasound surgeries, which include magnetic resonance–guided focused ultrasound surgery (MRgFUS) and high-intensity focused ultrasound (HIFU), were approved by the FDA in 2004. Focused ultrasound waves pass through the abdominal wall and produce significant heating, causing a burn that destroys the targeted tissue without damaging surrounding tissue. As with UAE, improvements in fibroid-associated bleeding and measures of quality of life were similar to those after myomectomy up to 3 years later.
But Dr. Singh noted that both focused ultrasound and RFA can damage the skin or internal organs. “[As] always with the thermal interventions, there is the probability of skin as well as internal organs that might get the thermal energy if it’s not focused correctly on to the fibroid itself,” he said. In addition, MRgFUS is not an option for women who are not good candidates to undergo an MRI, such as those with claustrophobia or pacemakers.
Also, with focus ultrasound and RFA, “we do worry about that fibroid getting blood flow back,” which can lead to recurrence of heavy menstrual bleeding, Dr. Laughlin-Tommaso noted.
Although data on RFA are limited to 12 months of follow-up, most women reported meaningful reductions in bleeding symptoms. Longer follow-up has been reported for bleeding symptoms after MRgFUS, with similar results up to 3 years later.
For Leslie Hansen-Lindner, MD, chief of obstetrics and gynecology at Atrium Health in Charlotte, N.C., choosing the right procedure starts with a patient-centered conversation weighing the pros and cons of the options and the woman’s goals.
“Is their goal to reduce the size and impact of their fibroid, bleed less, and have a better quality of life on their period?” Dr. Hansen-Lindner said. “Or is their goal to have the entire fibroid removed?”
If the former, an RFA is appealing to many women. If the latter, laparoscopic or mini-laparotomy myomectomy might be a better choice. Although fewer than 10% of patients require surgical reintervention at 3 years of follow-up for RFA, myomectomy has more consistent long-term evidence showing that fewer women require re-intervention and preserve their fertility, she added.
Age also plays a role in the decision: The closer a woman is to menopause, the less likely she is to experience a recurrence, so a less-invasive procedure is preferable. But for younger women hoping to become pregnant, the lower risk for recurrence and good prognosis for future fertility might sway the choice toward myomectomy.
The first laparoscopic RFA procedures were approved for uterine fibroids in 2012. Dr. Hansen-Lindner is a proponent of transcervical fibroid ablation (TFA), a newer RFA procedure that the FDA approved in 2018. Performed through the cervix, TFA requires no incisions and can generally be done without general anesthesia. Eligible candidates would be any woman with symptomatic fibroids, such as heavy menstrual bleeding, pain, or bulk symptoms. The contraindications are few.
“It’s going to come down to size and location of fibroids, and whether or not they would be accessible by the TFA,” Dr. Hansen-Lindner said. “I have to make sure that there isn’t a fibroid blocking their cervix and that the fibroids are accessible with this device.”
TFA also is not suitable for removing most submucosal lesions, which typically must be removed by hysteroscopic myomectomy. Dr. Hansen-Lindner said that she often uses TFA in conjunction with hysteroscopic myomectomy for this scenario. Although data on pregnancy after RFA (including TFA), MRgFUS, and HIFU are lacking, Gynesonics, the manufacturer of the Sonata System (the device that delivers radiofrequency energy to shrink the fibroid) has documented 79 pregnancies among the 2,200 women who have undergone TFA in the United States since 2018.
Disparities hampering care
Uterine fibroids are a particular problem for Black women, whose symptoms are more likely to be ignored by clinicians, according to Jodie Katon, PhD, a core investigator at the Veterans Affairs Greater Los Angeles Center for the Study of Healthcare Innovation, Implementation and Policy. Dr. Katon cited studies in which Black women interviewed about their experiences reported a consistent theme: Clinicians dismissed their symptoms, told them these were nothing to worry about, and advised them to lose weight. Those interactions not only delayed diagnosis among Black women but also led many of them to mistrust clinicians and avoid the health care system altogether.
The failure of clinicians to take their complaints seriously is just one of the disparities affecting Black women. In reviewing the literature, Dr. Laughlin-Tommaso, who also serves as the associate dean for Education Diversity, Equity, and Inclusion at the Mayo Clinic, found that African American women experience two to three times the risk for fibroids, compared with White women, as well as earlier onset and more severe disease, as measured by number and size of the lesions.
According to Dr. Katon, the etiology of fibroids is still poorly understood. “What we do know is that Black women are disproportionately exposed to a variety of factors that we have shown through observational studies are associated with increased risk of development of uterine fibroids.”
The list includes factors like stress; interpersonal racism; early age at menarche; various indicators of poor diets, such as vitamin D deficiency; the use of certain beauty products, specifically hair straighteners; as well as exposure to air pollution and other environmental toxins.
Laughlin-Tommaso also pointed to historical disparities in management, citing a doubled risk for hysterectomy for Black women in a study published in 2007 despite survey data suggesting that Black women report being more interested in uterine-preserving therapies rather than a hysterectomy.
Breaking down barriers of access to new treatments
Dr. Laughlin-Tommaso looked at more recent trends in the management of fibroids using data from the multicenter COMPARE-UF study, which enrolled women between 2015 and 2020 undergoing fibroid treatment into a longitudinal registry to track their outcomes. She found that Black women underwent hysterectomies at a lower rate than did White women and were instead more likely to undergo myomectomy or UAE.
Some of the change may reflect lack of approved minimally invasive procedures before 2000. “But now that we have expanded options, I think most women are opting not to have a hysterectomy,” Dr. Laughlin-Tommaso said.
Dr. Katon has research funding from the VA to look more closely at racial disparities in the treatment of fibroids. In a study published in April 2023, she reported some surprising trends.
During the period from 2010 to 2018, she found that Black veterans diagnosed with fibroids were less likely than White veterans were to receive treatment, regardless of their age or the severity of their symptoms. This finding held even among women with anemia, which should have been a clear indication for treatment.
But, as in the COMPARE-UF study, the subset of Black veterans who received an interventional treatment were less likely than their White peers were to undergo hysterectomy in favor of a fertility-sparing treatment as their initial procedure. Dr. Katon called it a “welcome but unexpected finding.”
But another significant barrier remains: The two newest types of procedures, RFA and guided focused ultrasound, are not commonly performed outside of tertiary care facilities. However, studies have found that all these procedures are cost effective (studies for myomectomy, UAE, MRgFUS, and TFA). The implementation of a category 1 billing code for laparoscopic RFA in 2017 has led more insurance companies to cover the service, and a category 1 code will be available for TFA effective January 2024.
Although RFA does require investment in specialized equipment, which limits facilities from offering the procedure, any gynecologist who routinely performs hysteroscopy can easily learn to do TFA. And the VA, which is committed to eliminating disparities in women’s health, established a 2-year advanced fellowship in minimally invasive gynecologic surgery in 2022 to help expand their capacity to offer these procedures.
The VA has been rapidly expanding their gynecology services, and Katon said that she is confident that ultrasound-guided procedures and RFA will become more available within the system. “I would say we’re keeping pace. And in some ways, you know, as a national system we may be positioned to actually outpace the rest of the U.S.”
Dr. Segars reported prior research funding for clinical trials from BioSpecifics Technologies, Bayer, Allergan, AbbVie, and ObsEva and currently receives funding from Myovant Sciences. Dr. Hansen-Lindner reported personal fees from Gynesonics. Dr. Singh, Dr. Laughlin-Tommaso, and Dr. Katon reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
In 2022, two colleagues from Johns Hopkins University, Baltimore, Bhuchitra Singh, MD, MPH, MS, MBA, and James Segars Jr., MD, reviewed the available literature to evaluate the effectiveness of newer minimally invasive therapies in reducing bleeding and improving the quality of life and control of symptoms linked to uterine fibroids.
Their goal, according to Dr. Segars, a professor of obstetrics and gynecology and director of the division of women’s health research at Johns Hopkins, was to help guide clinicians and patients in making decisions about the use of the newer therapies, including radiofrequency ablation and ultrasound-guided removal of lesions.
But he and Dr. Singh, the director of clinical research at the Howard W. and Georgeanna Seegar Jones Laboratory of Reproductive Sciences and Women’s Health Research, were surprised by their findings. “The outcomes were relatively the same,” Dr. Segars said. “All of the modalities lead to significant reduction in bleeding and other fibroid-related symptoms.”
The data on long-term complications and risk for recurrence are sparse for some of the newer approaches, and not enough high-quality long-term studies have been conducted for the Food and Drug Administration to approve them as fertility-sparing treatments.
But perhaps, the biggest challenge now is to ensure that women can take advantage of these newer therapies, with large gaps in both the diagnosis of fibroids and geographic access to minimally invasive treatments.
A widespread condition widely underdiagnosed
Uterine fibroids occur in most women (the incidence rises with age) and can be found in up to 70% of women by the time they reach menopause. Risk factors include family history, increasing interval since last birth, hypertension, and obesity. Increasing parity and use of oral contraceptives are protective.
But as many as 50% of cases go undiagnosed, and one reason for this is the failure of clinicians to dig deeply enough into women’s menstrual histories to diagnose fibroids.
“The most common cause of anemia is heavy menstrual bleeding,” said Shannon Laughlin-Tommaso, MD, MPH, a professor of obstetrics and gynecology at Mayo Clinic in Rochester, Minn. She frequently sees patients who have already undergone colonoscopy to work-up the source of their anemia before anyone suspects that fibroids are the culprit.
“When women tell us about their periods, what they’ve been told is normal [bleeding] – or what they’ve always had and considered normal – is actually kind of on the heavier spectrum,” she said.
Ideally, treatment for uterine fibroids would fix abnormally prolonged or heavy menstrual bleeding, relieve pain, and ameliorate symptoms associated with an enlarged uterus, such as pelvic pressure, urinary frequency, and constipation. And the fibroids would never recur.
By those measures, hysterectomy fits the bill: Success rates in relieving symptoms are high, and the risk for recurrence is zero. But the procedure carries significant drawbacks: short-term complications of surgery, including infection, bleeding, and injury to the bowels and bladder along with potential long-term risks for cardiovascular disease, cancer, ovarian failure and premature menopause, depression, and decline in cognitive function. Those factors loom even larger for women who still hope to have children.
For that reason, the American College of Obstetricians and Gynecologists recommends myomectomy, or surgical removal of individual fibroids, for women who desire uterine preservation or future pregnancy. And the literature here is solid, according to Dr. Singh, who found that 95% of myomectomy patients achieved control of their bleeding symptoms, whether it was via laparoscopy, hysteroscopy, or laparotomy. Up to 40% of women may develop new fibroids, or leiomyomas, within 3 years, although only 12.2% required a second surgery up to after 5 years.
But myomectomy is invasive, requiring general anesthesia, incisions in the uterus, and stitches to close the organ.
Newer techniques have emerged that can effectively treat symptoms of fibroids without requiring surgery. Uterine artery embolization (UAE), which involves passing a catheter into the femoral artery, or laparoscopic uterine artery occlusion can be used to cut off the blood supply of the fibroid. Other techniques, including focused ultrasound surgery and radiofrequency ablation (RFA), use various forms of energy to heat and ablate fibroids. The latter two can be performed in outpatient settings and often without general anesthesia.
Approved for use in 1994, UAE has the most data available, with reduction in the volume of fibroids and uterine tissue lasting up to 5 years, and rates of reintervention of 19%-38% between 2 and 5 years after the procedure. Dr. Singh’s review found that 79%-98.5% of recipients of the procedure reported declines in bleeding that persisted for several years, which is comparable to myomectomy. Quality of life and pain scores also showed good improvement, with follow-up in the different studies ranging from 12 months to over 5 years, the analysis showed.
UAE does have its drawbacks. In rare cases, embolization can deprive the entire uterus and ovaries of blood, which can cause ovarian dysfunction and potentially result in premature menopause, although this outcome is most common in women who are older than 45 years. The procedure can often also be painful enough that overnight hospitalization is required.
Focused ultrasound surgeries, which include magnetic resonance–guided focused ultrasound surgery (MRgFUS) and high-intensity focused ultrasound (HIFU), were approved by the FDA in 2004. Focused ultrasound waves pass through the abdominal wall and produce significant heating, causing a burn that destroys the targeted tissue without damaging surrounding tissue. As with UAE, improvements in fibroid-associated bleeding and measures of quality of life were similar to those after myomectomy up to 3 years later.
But Dr. Singh noted that both focused ultrasound and RFA can damage the skin or internal organs. “[As] always with the thermal interventions, there is the probability of skin as well as internal organs that might get the thermal energy if it’s not focused correctly on to the fibroid itself,” he said. In addition, MRgFUS is not an option for women who are not good candidates to undergo an MRI, such as those with claustrophobia or pacemakers.
Also, with focus ultrasound and RFA, “we do worry about that fibroid getting blood flow back,” which can lead to recurrence of heavy menstrual bleeding, Dr. Laughlin-Tommaso noted.
Although data on RFA are limited to 12 months of follow-up, most women reported meaningful reductions in bleeding symptoms. Longer follow-up has been reported for bleeding symptoms after MRgFUS, with similar results up to 3 years later.
For Leslie Hansen-Lindner, MD, chief of obstetrics and gynecology at Atrium Health in Charlotte, N.C., choosing the right procedure starts with a patient-centered conversation weighing the pros and cons of the options and the woman’s goals.
“Is their goal to reduce the size and impact of their fibroid, bleed less, and have a better quality of life on their period?” Dr. Hansen-Lindner said. “Or is their goal to have the entire fibroid removed?”
If the former, an RFA is appealing to many women. If the latter, laparoscopic or mini-laparotomy myomectomy might be a better choice. Although fewer than 10% of patients require surgical reintervention at 3 years of follow-up for RFA, myomectomy has more consistent long-term evidence showing that fewer women require re-intervention and preserve their fertility, she added.
Age also plays a role in the decision: The closer a woman is to menopause, the less likely she is to experience a recurrence, so a less-invasive procedure is preferable. But for younger women hoping to become pregnant, the lower risk for recurrence and good prognosis for future fertility might sway the choice toward myomectomy.
The first laparoscopic RFA procedures were approved for uterine fibroids in 2012. Dr. Hansen-Lindner is a proponent of transcervical fibroid ablation (TFA), a newer RFA procedure that the FDA approved in 2018. Performed through the cervix, TFA requires no incisions and can generally be done without general anesthesia. Eligible candidates would be any woman with symptomatic fibroids, such as heavy menstrual bleeding, pain, or bulk symptoms. The contraindications are few.
“It’s going to come down to size and location of fibroids, and whether or not they would be accessible by the TFA,” Dr. Hansen-Lindner said. “I have to make sure that there isn’t a fibroid blocking their cervix and that the fibroids are accessible with this device.”
TFA also is not suitable for removing most submucosal lesions, which typically must be removed by hysteroscopic myomectomy. Dr. Hansen-Lindner said that she often uses TFA in conjunction with hysteroscopic myomectomy for this scenario. Although data on pregnancy after RFA (including TFA), MRgFUS, and HIFU are lacking, Gynesonics, the manufacturer of the Sonata System (the device that delivers radiofrequency energy to shrink the fibroid) has documented 79 pregnancies among the 2,200 women who have undergone TFA in the United States since 2018.
Disparities hampering care
Uterine fibroids are a particular problem for Black women, whose symptoms are more likely to be ignored by clinicians, according to Jodie Katon, PhD, a core investigator at the Veterans Affairs Greater Los Angeles Center for the Study of Healthcare Innovation, Implementation and Policy. Dr. Katon cited studies in which Black women interviewed about their experiences reported a consistent theme: Clinicians dismissed their symptoms, told them these were nothing to worry about, and advised them to lose weight. Those interactions not only delayed diagnosis among Black women but also led many of them to mistrust clinicians and avoid the health care system altogether.
The failure of clinicians to take their complaints seriously is just one of the disparities affecting Black women. In reviewing the literature, Dr. Laughlin-Tommaso, who also serves as the associate dean for Education Diversity, Equity, and Inclusion at the Mayo Clinic, found that African American women experience two to three times the risk for fibroids, compared with White women, as well as earlier onset and more severe disease, as measured by number and size of the lesions.
According to Dr. Katon, the etiology of fibroids is still poorly understood. “What we do know is that Black women are disproportionately exposed to a variety of factors that we have shown through observational studies are associated with increased risk of development of uterine fibroids.”
The list includes factors like stress; interpersonal racism; early age at menarche; various indicators of poor diets, such as vitamin D deficiency; the use of certain beauty products, specifically hair straighteners; as well as exposure to air pollution and other environmental toxins.
Laughlin-Tommaso also pointed to historical disparities in management, citing a doubled risk for hysterectomy for Black women in a study published in 2007 despite survey data suggesting that Black women report being more interested in uterine-preserving therapies rather than a hysterectomy.
Breaking down barriers of access to new treatments
Dr. Laughlin-Tommaso looked at more recent trends in the management of fibroids using data from the multicenter COMPARE-UF study, which enrolled women between 2015 and 2020 undergoing fibroid treatment into a longitudinal registry to track their outcomes. She found that Black women underwent hysterectomies at a lower rate than did White women and were instead more likely to undergo myomectomy or UAE.
Some of the change may reflect lack of approved minimally invasive procedures before 2000. “But now that we have expanded options, I think most women are opting not to have a hysterectomy,” Dr. Laughlin-Tommaso said.
Dr. Katon has research funding from the VA to look more closely at racial disparities in the treatment of fibroids. In a study published in April 2023, she reported some surprising trends.
During the period from 2010 to 2018, she found that Black veterans diagnosed with fibroids were less likely than White veterans were to receive treatment, regardless of their age or the severity of their symptoms. This finding held even among women with anemia, which should have been a clear indication for treatment.
But, as in the COMPARE-UF study, the subset of Black veterans who received an interventional treatment were less likely than their White peers were to undergo hysterectomy in favor of a fertility-sparing treatment as their initial procedure. Dr. Katon called it a “welcome but unexpected finding.”
But another significant barrier remains: The two newest types of procedures, RFA and guided focused ultrasound, are not commonly performed outside of tertiary care facilities. However, studies have found that all these procedures are cost effective (studies for myomectomy, UAE, MRgFUS, and TFA). The implementation of a category 1 billing code for laparoscopic RFA in 2017 has led more insurance companies to cover the service, and a category 1 code will be available for TFA effective January 2024.
Although RFA does require investment in specialized equipment, which limits facilities from offering the procedure, any gynecologist who routinely performs hysteroscopy can easily learn to do TFA. And the VA, which is committed to eliminating disparities in women’s health, established a 2-year advanced fellowship in minimally invasive gynecologic surgery in 2022 to help expand their capacity to offer these procedures.
The VA has been rapidly expanding their gynecology services, and Katon said that she is confident that ultrasound-guided procedures and RFA will become more available within the system. “I would say we’re keeping pace. And in some ways, you know, as a national system we may be positioned to actually outpace the rest of the U.S.”
Dr. Segars reported prior research funding for clinical trials from BioSpecifics Technologies, Bayer, Allergan, AbbVie, and ObsEva and currently receives funding from Myovant Sciences. Dr. Hansen-Lindner reported personal fees from Gynesonics. Dr. Singh, Dr. Laughlin-Tommaso, and Dr. Katon reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Fibroids: Growing management options for a prevalent problem
OBG Manag. 33(12). | doi 10.12788/obgm.0169
Fibroids: Is surgery the only management approach?
Two chronic gynecologic conditions notably affect a woman’s quality of life (QoL), including fertility – one is endometriosis, and the other is a fibroid uterus. For a benign tumor, fibroids have an impressive prevalence found in approximately 50%-60% of women during their reproductive years. By menopause, it is estimated that 70% of woman have a fibroid, yet the true incidence is unknown given that only 25% of women experience symptoms bothersome enough to warrant intervention. This month’s article reviews the burden of fibroids and the latest management options that may potentially avoid surgery.
Background
Fibroids are monoclonal tumors of uterine smooth muscle that originate from the myometrium. Risk factors include family history, being premenopausal, increasing time since last delivery, obesity, and hypertension (ACOG Practice Bulletin no. 228 Jun 2021: Obstet Gynecol. 2021 Jun 1;137[6]:e100-e15) but oral hormonal contraception, depot medroxyprogesterone acetate (MPA), and increased parity reduce the risk of fibroids. Compared with White women, Black women have a 2-3 times higher prevalence of fibroids, develop them at a younger age, and present with larger fibroids.
The FIGO leiomyoma classification is the agreed upon system for identifying fibroid location. Symptoms are all too familiar to gynecologists, with life-threatening hemorrhage with severe anemia being the most feared, particularly for FIGO types 1-5. Transvaginal ultrasound is the simplest imaging tool for evaluation.
Fibroids and fertility
Fibroids can impair fertility in several ways: alteration of local anatomy, including the detrimental effects of abnormal uterine bleeding; functional changes by increasing uterine contractions and impairing endometrium and myometrial blood supply; and changes to the local hormonal environment that could impair egg/sperm transport, or embryo implantation (Hum Reprod Update. 2017;22:665-86).
Prior to consideration of surgery, saline infusion sonogram can determine the degree of impact on the endometrium, which is most applicable to the infertility patient, but can also allow guidance toward the appropriate surgical approach.
Treatment options – medical
Management of fibroids is based on a woman’s age, desire for fertility, symptoms, and location of the fibroid(s). Expectant observation of a woman with fibroids may be a reasonable approach, provided the lack of symptoms impairing QoL and of anemia. Typically, there is no change in fibroid size during the short term, considered less than 1 year. Regarding fertility, studies are heterogeneous so there is no definitive conclusion that fibroids impair natural fertility (Reprod Biomed Online. 2021;43:100-10). Spontaneous regression, defined by a reduction in fibroid volume of greater than 20%, has been noted to occur in 7.0% of fibroids (Curr Obstet Gynecol Rep. 2018;7[3]:117-21).
When fertility is not desired, medical management of fibroids is the initial conservative approach. GnRH agonists have been utilized for temporary relief of menometrorrhagia because of fibroids and to reduce their volume, particularly preoperatively. However, extended treatment can induce bone mineral density loss. Add-back therapy (tibolone, raloxifene, estriol, and ipriflavone) is of value in reducing bone loss while MPA and tibolone may manage vasomotor symptoms. More recently, the use of a GnRH antagonist (elagolix) along with add-back therapy has been approved for up to 24 months by the Food and Drug Administration and has demonstrated a more than 50% amenorrhea rate at 12 months (Obstet Gynecol. 2020;135:1313-26).
Progesterone plays an important role in fibroid growth, but the mechanism is unclear. Although not FDA approved, selective progesterone receptor modulators (SPRM) act directly on fibroid size reduction at the level of the pituitary to induce amenorrhea through inhibition of ovulation. Also, more than one course of SPRMs can provide benefit for bleeding control and volume reduction. The SPRM ulipristal acetate for four courses of 3 months demonstrated 73.5% of patients experienced a fibroid volume reduction of greater than 25% and were amenorrheic (Fertil Steril. 2017;108:416-25). GnRH agonists or SPRMs may benefit women if the fibroid is larger than 3 cm or anemia exists, thereby precluding immediate surgery.
Other medication options include the levonorgestrel IUD, combined hormonal contraceptives, and tranexamic acid – all of which have limited data on effective results of treating abnormal uterine bleeding.
Treatment options – surgical
Fibroids are the most common reason for hysterectomy as they are the contributing indication in approximately one-third of surgeries. When future fertility is desired, current surgical options include hysteroscopic and laparoscopic (including robotic) myomectomy. Hysteroscopy is the standard approach for FIGO type 1 fibroids and can also manage some type 2 fibroids provided they are less than 3 cm and the latter is greater than 5 mm from the serosa. Type 2 fibroids may benefit from a “two-step” removal to allow the myometrium to contract and extrude the fibroid. In light of the risk of fluid overload with nonelectrolyte solutions that enable the use of monopolar cautery, many procedures are now performed with bipolar cautery or morcellators.
Laparoscopy (including robotic) has outcomes similar to those of laparotomy although the risk of uterine rupture with the former requires careful attention to thorough closure of the myometrial defect. Robotic myomectomy has outcomes similar to those of standard laparoscopy with less blood loss, but operating times may be prolonged (Best Pract Res Clin Obstet Gynaecol. 2018;46:113-9).
The rate of myomectomy is reported to be 9.2 per 10,000 woman-years in Black women and 1.3 per 10,000 woman years in White women (Fertil Steril 2017;108;416-25). The rate of recurrence after myomectomy can be as great as 60% when patients are followed up to 5 years. Intramural fibroids greater than 2.85 cm and not distorting the uterine cavity may decrease in vitro fertilization (IVF) success (Fertil Steril 2014;101:716-21).
Noninvasive treatment modalities
Uterine artery embolization (UAE) is the most popular minimally invasive alternative to surgical myomectomy. Risks include postembolization syndrome (pain, fever, nausea, leukocytosis, and occasionally malaise), infection, and damage to fertility. Rarely, loss of ovarian function can occur, particularly in women above age 45. Because of the disruption of uterine blood flow, UAE increases the risk of accelerating ovarian aging and infertility as well as atrophic endometrium. In addition, pregnancy complications are increased including miscarriage, preterm labor, and postpartum hemorrhage. There is debate regarding the need for cesarean section at time of delivery given the potential for weakening of the uterine wall following UAE.
High-intensity focused ultrasound (HIFU) is guided by ultrasound or MRI and involves a high-energy-density ultrasound wave passing through the skin. The wave is absorbed and transformed into heat, causing the tissue protein to coagulate, and to be absorbed by the body. The procedure is scarless, carries a minimal risk of infection, and offers less pain compared with traditional approaches. However, HIFU is time consuming, and skin burns and unintentional tissue injury are a risk. A meta-analysis demonstrated improved symptoms of fibroids at 6 and 12 months (J Min Invasive Gynecol. 2021 in press).
Ultrasound-guided microwave ablation (MWA) uses an ablative electrode that is directly inserted into the target tissue via transcutaneous or transcervical approach via ultrasound guidance using microwave to produce heat for tissue coagulation necrosis. The advantages of MWA compared with HIFU and RFA are a higher tissue temperature, larger ablation volume, shorter operating time, less pain and no adverse major events (J Min Invasive Gynecol. 2021, in press).
Conclusion
The current literature cannot conclude that fibroids reduce the likelihood of achieving pregnancy with or without fertility treatment, based on a specific size, number, or location (not including submucosal or cavity-distorting intramural fibroids). Definitive evidence on the efficacy of myomectomy to improve fertility remains limited. Hysteroscopic myomectomy presumably improves pregnancy rates, but there is uncertainty as to its role in reducing miscarriage. Novel nonsurgical modalities are available and are expected to continue being developed but clarity on fertility outcomes is needed.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He has no conflicts of interests. Please contact him at obnews@mdedge.com.
Two chronic gynecologic conditions notably affect a woman’s quality of life (QoL), including fertility – one is endometriosis, and the other is a fibroid uterus. For a benign tumor, fibroids have an impressive prevalence found in approximately 50%-60% of women during their reproductive years. By menopause, it is estimated that 70% of woman have a fibroid, yet the true incidence is unknown given that only 25% of women experience symptoms bothersome enough to warrant intervention. This month’s article reviews the burden of fibroids and the latest management options that may potentially avoid surgery.
Background
Fibroids are monoclonal tumors of uterine smooth muscle that originate from the myometrium. Risk factors include family history, being premenopausal, increasing time since last delivery, obesity, and hypertension (ACOG Practice Bulletin no. 228 Jun 2021: Obstet Gynecol. 2021 Jun 1;137[6]:e100-e15) but oral hormonal contraception, depot medroxyprogesterone acetate (MPA), and increased parity reduce the risk of fibroids. Compared with White women, Black women have a 2-3 times higher prevalence of fibroids, develop them at a younger age, and present with larger fibroids.
The FIGO leiomyoma classification is the agreed upon system for identifying fibroid location. Symptoms are all too familiar to gynecologists, with life-threatening hemorrhage with severe anemia being the most feared, particularly for FIGO types 1-5. Transvaginal ultrasound is the simplest imaging tool for evaluation.
Fibroids and fertility
Fibroids can impair fertility in several ways: alteration of local anatomy, including the detrimental effects of abnormal uterine bleeding; functional changes by increasing uterine contractions and impairing endometrium and myometrial blood supply; and changes to the local hormonal environment that could impair egg/sperm transport, or embryo implantation (Hum Reprod Update. 2017;22:665-86).
Prior to consideration of surgery, saline infusion sonogram can determine the degree of impact on the endometrium, which is most applicable to the infertility patient, but can also allow guidance toward the appropriate surgical approach.
Treatment options – medical
Management of fibroids is based on a woman’s age, desire for fertility, symptoms, and location of the fibroid(s). Expectant observation of a woman with fibroids may be a reasonable approach, provided the lack of symptoms impairing QoL and of anemia. Typically, there is no change in fibroid size during the short term, considered less than 1 year. Regarding fertility, studies are heterogeneous so there is no definitive conclusion that fibroids impair natural fertility (Reprod Biomed Online. 2021;43:100-10). Spontaneous regression, defined by a reduction in fibroid volume of greater than 20%, has been noted to occur in 7.0% of fibroids (Curr Obstet Gynecol Rep. 2018;7[3]:117-21).
When fertility is not desired, medical management of fibroids is the initial conservative approach. GnRH agonists have been utilized for temporary relief of menometrorrhagia because of fibroids and to reduce their volume, particularly preoperatively. However, extended treatment can induce bone mineral density loss. Add-back therapy (tibolone, raloxifene, estriol, and ipriflavone) is of value in reducing bone loss while MPA and tibolone may manage vasomotor symptoms. More recently, the use of a GnRH antagonist (elagolix) along with add-back therapy has been approved for up to 24 months by the Food and Drug Administration and has demonstrated a more than 50% amenorrhea rate at 12 months (Obstet Gynecol. 2020;135:1313-26).
Progesterone plays an important role in fibroid growth, but the mechanism is unclear. Although not FDA approved, selective progesterone receptor modulators (SPRM) act directly on fibroid size reduction at the level of the pituitary to induce amenorrhea through inhibition of ovulation. Also, more than one course of SPRMs can provide benefit for bleeding control and volume reduction. The SPRM ulipristal acetate for four courses of 3 months demonstrated 73.5% of patients experienced a fibroid volume reduction of greater than 25% and were amenorrheic (Fertil Steril. 2017;108:416-25). GnRH agonists or SPRMs may benefit women if the fibroid is larger than 3 cm or anemia exists, thereby precluding immediate surgery.
Other medication options include the levonorgestrel IUD, combined hormonal contraceptives, and tranexamic acid – all of which have limited data on effective results of treating abnormal uterine bleeding.
Treatment options – surgical
Fibroids are the most common reason for hysterectomy as they are the contributing indication in approximately one-third of surgeries. When future fertility is desired, current surgical options include hysteroscopic and laparoscopic (including robotic) myomectomy. Hysteroscopy is the standard approach for FIGO type 1 fibroids and can also manage some type 2 fibroids provided they are less than 3 cm and the latter is greater than 5 mm from the serosa. Type 2 fibroids may benefit from a “two-step” removal to allow the myometrium to contract and extrude the fibroid. In light of the risk of fluid overload with nonelectrolyte solutions that enable the use of monopolar cautery, many procedures are now performed with bipolar cautery or morcellators.
Laparoscopy (including robotic) has outcomes similar to those of laparotomy although the risk of uterine rupture with the former requires careful attention to thorough closure of the myometrial defect. Robotic myomectomy has outcomes similar to those of standard laparoscopy with less blood loss, but operating times may be prolonged (Best Pract Res Clin Obstet Gynaecol. 2018;46:113-9).
The rate of myomectomy is reported to be 9.2 per 10,000 woman-years in Black women and 1.3 per 10,000 woman years in White women (Fertil Steril 2017;108;416-25). The rate of recurrence after myomectomy can be as great as 60% when patients are followed up to 5 years. Intramural fibroids greater than 2.85 cm and not distorting the uterine cavity may decrease in vitro fertilization (IVF) success (Fertil Steril 2014;101:716-21).
Noninvasive treatment modalities
Uterine artery embolization (UAE) is the most popular minimally invasive alternative to surgical myomectomy. Risks include postembolization syndrome (pain, fever, nausea, leukocytosis, and occasionally malaise), infection, and damage to fertility. Rarely, loss of ovarian function can occur, particularly in women above age 45. Because of the disruption of uterine blood flow, UAE increases the risk of accelerating ovarian aging and infertility as well as atrophic endometrium. In addition, pregnancy complications are increased including miscarriage, preterm labor, and postpartum hemorrhage. There is debate regarding the need for cesarean section at time of delivery given the potential for weakening of the uterine wall following UAE.
High-intensity focused ultrasound (HIFU) is guided by ultrasound or MRI and involves a high-energy-density ultrasound wave passing through the skin. The wave is absorbed and transformed into heat, causing the tissue protein to coagulate, and to be absorbed by the body. The procedure is scarless, carries a minimal risk of infection, and offers less pain compared with traditional approaches. However, HIFU is time consuming, and skin burns and unintentional tissue injury are a risk. A meta-analysis demonstrated improved symptoms of fibroids at 6 and 12 months (J Min Invasive Gynecol. 2021 in press).
Ultrasound-guided microwave ablation (MWA) uses an ablative electrode that is directly inserted into the target tissue via transcutaneous or transcervical approach via ultrasound guidance using microwave to produce heat for tissue coagulation necrosis. The advantages of MWA compared with HIFU and RFA are a higher tissue temperature, larger ablation volume, shorter operating time, less pain and no adverse major events (J Min Invasive Gynecol. 2021, in press).
Conclusion
The current literature cannot conclude that fibroids reduce the likelihood of achieving pregnancy with or without fertility treatment, based on a specific size, number, or location (not including submucosal or cavity-distorting intramural fibroids). Definitive evidence on the efficacy of myomectomy to improve fertility remains limited. Hysteroscopic myomectomy presumably improves pregnancy rates, but there is uncertainty as to its role in reducing miscarriage. Novel nonsurgical modalities are available and are expected to continue being developed but clarity on fertility outcomes is needed.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He has no conflicts of interests. Please contact him at obnews@mdedge.com.
Two chronic gynecologic conditions notably affect a woman’s quality of life (QoL), including fertility – one is endometriosis, and the other is a fibroid uterus. For a benign tumor, fibroids have an impressive prevalence found in approximately 50%-60% of women during their reproductive years. By menopause, it is estimated that 70% of woman have a fibroid, yet the true incidence is unknown given that only 25% of women experience symptoms bothersome enough to warrant intervention. This month’s article reviews the burden of fibroids and the latest management options that may potentially avoid surgery.
Background
Fibroids are monoclonal tumors of uterine smooth muscle that originate from the myometrium. Risk factors include family history, being premenopausal, increasing time since last delivery, obesity, and hypertension (ACOG Practice Bulletin no. 228 Jun 2021: Obstet Gynecol. 2021 Jun 1;137[6]:e100-e15) but oral hormonal contraception, depot medroxyprogesterone acetate (MPA), and increased parity reduce the risk of fibroids. Compared with White women, Black women have a 2-3 times higher prevalence of fibroids, develop them at a younger age, and present with larger fibroids.
The FIGO leiomyoma classification is the agreed upon system for identifying fibroid location. Symptoms are all too familiar to gynecologists, with life-threatening hemorrhage with severe anemia being the most feared, particularly for FIGO types 1-5. Transvaginal ultrasound is the simplest imaging tool for evaluation.
Fibroids and fertility
Fibroids can impair fertility in several ways: alteration of local anatomy, including the detrimental effects of abnormal uterine bleeding; functional changes by increasing uterine contractions and impairing endometrium and myometrial blood supply; and changes to the local hormonal environment that could impair egg/sperm transport, or embryo implantation (Hum Reprod Update. 2017;22:665-86).
Prior to consideration of surgery, saline infusion sonogram can determine the degree of impact on the endometrium, which is most applicable to the infertility patient, but can also allow guidance toward the appropriate surgical approach.
Treatment options – medical
Management of fibroids is based on a woman’s age, desire for fertility, symptoms, and location of the fibroid(s). Expectant observation of a woman with fibroids may be a reasonable approach, provided the lack of symptoms impairing QoL and of anemia. Typically, there is no change in fibroid size during the short term, considered less than 1 year. Regarding fertility, studies are heterogeneous so there is no definitive conclusion that fibroids impair natural fertility (Reprod Biomed Online. 2021;43:100-10). Spontaneous regression, defined by a reduction in fibroid volume of greater than 20%, has been noted to occur in 7.0% of fibroids (Curr Obstet Gynecol Rep. 2018;7[3]:117-21).
When fertility is not desired, medical management of fibroids is the initial conservative approach. GnRH agonists have been utilized for temporary relief of menometrorrhagia because of fibroids and to reduce their volume, particularly preoperatively. However, extended treatment can induce bone mineral density loss. Add-back therapy (tibolone, raloxifene, estriol, and ipriflavone) is of value in reducing bone loss while MPA and tibolone may manage vasomotor symptoms. More recently, the use of a GnRH antagonist (elagolix) along with add-back therapy has been approved for up to 24 months by the Food and Drug Administration and has demonstrated a more than 50% amenorrhea rate at 12 months (Obstet Gynecol. 2020;135:1313-26).
Progesterone plays an important role in fibroid growth, but the mechanism is unclear. Although not FDA approved, selective progesterone receptor modulators (SPRM) act directly on fibroid size reduction at the level of the pituitary to induce amenorrhea through inhibition of ovulation. Also, more than one course of SPRMs can provide benefit for bleeding control and volume reduction. The SPRM ulipristal acetate for four courses of 3 months demonstrated 73.5% of patients experienced a fibroid volume reduction of greater than 25% and were amenorrheic (Fertil Steril. 2017;108:416-25). GnRH agonists or SPRMs may benefit women if the fibroid is larger than 3 cm or anemia exists, thereby precluding immediate surgery.
Other medication options include the levonorgestrel IUD, combined hormonal contraceptives, and tranexamic acid – all of which have limited data on effective results of treating abnormal uterine bleeding.
Treatment options – surgical
Fibroids are the most common reason for hysterectomy as they are the contributing indication in approximately one-third of surgeries. When future fertility is desired, current surgical options include hysteroscopic and laparoscopic (including robotic) myomectomy. Hysteroscopy is the standard approach for FIGO type 1 fibroids and can also manage some type 2 fibroids provided they are less than 3 cm and the latter is greater than 5 mm from the serosa. Type 2 fibroids may benefit from a “two-step” removal to allow the myometrium to contract and extrude the fibroid. In light of the risk of fluid overload with nonelectrolyte solutions that enable the use of monopolar cautery, many procedures are now performed with bipolar cautery or morcellators.
Laparoscopy (including robotic) has outcomes similar to those of laparotomy although the risk of uterine rupture with the former requires careful attention to thorough closure of the myometrial defect. Robotic myomectomy has outcomes similar to those of standard laparoscopy with less blood loss, but operating times may be prolonged (Best Pract Res Clin Obstet Gynaecol. 2018;46:113-9).
The rate of myomectomy is reported to be 9.2 per 10,000 woman-years in Black women and 1.3 per 10,000 woman years in White women (Fertil Steril 2017;108;416-25). The rate of recurrence after myomectomy can be as great as 60% when patients are followed up to 5 years. Intramural fibroids greater than 2.85 cm and not distorting the uterine cavity may decrease in vitro fertilization (IVF) success (Fertil Steril 2014;101:716-21).
Noninvasive treatment modalities
Uterine artery embolization (UAE) is the most popular minimally invasive alternative to surgical myomectomy. Risks include postembolization syndrome (pain, fever, nausea, leukocytosis, and occasionally malaise), infection, and damage to fertility. Rarely, loss of ovarian function can occur, particularly in women above age 45. Because of the disruption of uterine blood flow, UAE increases the risk of accelerating ovarian aging and infertility as well as atrophic endometrium. In addition, pregnancy complications are increased including miscarriage, preterm labor, and postpartum hemorrhage. There is debate regarding the need for cesarean section at time of delivery given the potential for weakening of the uterine wall following UAE.
High-intensity focused ultrasound (HIFU) is guided by ultrasound or MRI and involves a high-energy-density ultrasound wave passing through the skin. The wave is absorbed and transformed into heat, causing the tissue protein to coagulate, and to be absorbed by the body. The procedure is scarless, carries a minimal risk of infection, and offers less pain compared with traditional approaches. However, HIFU is time consuming, and skin burns and unintentional tissue injury are a risk. A meta-analysis demonstrated improved symptoms of fibroids at 6 and 12 months (J Min Invasive Gynecol. 2021 in press).
Ultrasound-guided microwave ablation (MWA) uses an ablative electrode that is directly inserted into the target tissue via transcutaneous or transcervical approach via ultrasound guidance using microwave to produce heat for tissue coagulation necrosis. The advantages of MWA compared with HIFU and RFA are a higher tissue temperature, larger ablation volume, shorter operating time, less pain and no adverse major events (J Min Invasive Gynecol. 2021, in press).
Conclusion
The current literature cannot conclude that fibroids reduce the likelihood of achieving pregnancy with or without fertility treatment, based on a specific size, number, or location (not including submucosal or cavity-distorting intramural fibroids). Definitive evidence on the efficacy of myomectomy to improve fertility remains limited. Hysteroscopic myomectomy presumably improves pregnancy rates, but there is uncertainty as to its role in reducing miscarriage. Novel nonsurgical modalities are available and are expected to continue being developed but clarity on fertility outcomes is needed.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He has no conflicts of interests. Please contact him at obnews@mdedge.com.
Closing the racial gap in minimally invasive gyn hysterectomy and myomectomy
The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.


- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.


The historical mistreatment of Black bodies in gynecologic care has bled into present day inequities—from surgeries performed on enslaved Black women and sterilization of low-income Black women under federally funded programs, to higher rates of adverse health-related outcomes among Black women compared with their non-Black counterparts.1-3 Not only is the foundation of gynecology imperfect, so too is its current-day structure.
It is not enough to identify and describe racial inequities in health care; action plans to provide equitable care are called for. In this report, we aim to 1) contextualize the data on disparities in minimally invasive gynecologic surgery, specifically hysterectomy and myomectomy candidates and postsurgical outcomes, and 2) provide recommendations to close racial gaps in gynecologic treatment for more equitable experiences for minority women.
Black women and uterine fibroids
Uterine leiomyomas, or fibroids, are not only the most common benign pelvic tumor but they also cause a significant medical and financial burden in the United States, with estimated direct costs of $4.1 ̶ 9.4 billion.4 Fibroids can affect fertility and cause pain, bulk symptoms, heavy bleeding, anemia requiring blood transfusion, and poor pregnancy outcomes. The burden of disease for uterine fibroids is greatest for Black women.
The incidence of fibroids is 2 to 3 times higher in Black women compared with White women.5 According to ultrasound-based studies, the prevalence of fibroids among women aged 18 to 30 years was 26% among Black and 7% among White asymptomatic women.6 Earlier onset and more severe symptoms mean that there is a larger potential for impact on fertility for Black women. This coupled with the historical context of mistreatment of Black bodies makes the need for personalized medicine and culturally sensitive care critical.
Inequitable management of uterine fibroids
Although tumor size, location, and patient risk factors are used to determine the best treatment approach, the American College of Obstetricians and Gynecologists (ACOG) guidelines suggest that the use of alternative treatments to surgery should be first-line management instead of hysterectomy for most benign conditions.9 Conservative management will often help alleviate symptoms, slow the growth of fibroid(s), or bridge women to menopause, and treatment options include hormonal contraception, gonadotropin-releasing hormone agonists, hysteroscopic resection, uterine artery embolization, magnetic resonance-guided focused ultrasound, and myomectomy.
The rate of conservative management prior to hysterectomy varies by setting, reflecting potential bias in treatment decisions. Some medical settings have reported a 29% alternative management rate prior to hysterectomy, while others report much higher rates.10 A study using patient data from Kaiser Permanente Northern California (KPNC) showed that, within a large, diverse, and integrated health care system, more than 80% of patients received alternative treatments before undergoing hysterectomy; for those with symptomatic leiomyomas, 74.1% used alternative treatments prior to hysterectomy, and in logistic regression there was not a difference by race.11 Nationally, Black women are more likely to have hysterectomy or myomectomy compared with a nonsurgical uterine-sparing therapy.12,13
With about 600,000 cases per year within the United States, the hysterectomy is the most frequently performed benign gynecologic surgery.14 The most common indication is for “symptomatic fibroid uterus.” The approach to decision making for route of hysterectomy involves multiple patient and surgeon factors, including history of vaginal delivery, body mass index, history of previous surgery, uterine size, informed patient preference, and surgeon volume.15-17 ACOG recommends a minimally invasive hysterectomy (MIH) whenever feasible given its benefits in postoperative pain, recovery time, and blood loss. Myomectomy, particularly among women in their reproductive years desiring management of leiomyomas, is a uterine-sparing procedure versus hysterectomy. Minimally invasive myomectomy (MIM), compared with an open abdominal route, provides for lower drop in hemoglobin levels, shorter hospital stay, less adhesion formation, and decreased postoperative pain.18
Racial variations in hysterectomy rates persist overall and according to hysterectomy type. Black women are 2 to 3 times more likely to undergo hysterectomy for leiomyomas than other racial groups.19 These differences in rates have been shown to persist even when burden of disease is the same. One study found that Black women had increased odds of hysterectomy compared with their White counterparts even when there was no difference in mean fibroid volume by race,20 calling into question provider bias. Even in a universal insurance setting, Black patients have been found to have higher rates of open hysterectomies.21 Previous studies found that, despite growing frequency of laparoscopic and robotic-assisted hysterectomies, patients of a minority race had decreased odds of undergoing a MIH compared with their White counterparts.22
While little data exist on route of myomectomy by race, a recent study found minority women were more likely to undergo abdominal myomectomy compared with White women; Black women were twice as likely to undergo abdominal myomectomy (adjusted odds ratio [aOR], 1.9; 95% confidence interval [CI], 1.7–2.0), Asian American women were more than twice as likely (aOR, 2.3; 95% CI, 1.8–2.8), and Hispanic American women were 50% more likely to undergo abdominal myomectomy (aOR, 1.5; 95% CI, 1.2–1.9) when compared with White women.23 These differences remained after controlling for potential confounders, and there appeared to be an interaction between race and fibroid weight such that racial bias alone may not explain the differences.
Finally, Black women have higher perioperative complication rates compared with non-Black women. Postoperative complications including blood transfusion after myomectomy have been shown to be twice as high among Black women compared with White women. However, once uterine size, comorbidities, and fibroid number were controlled, race was not associated with higher complications. Black women, compared with White women, have been found to have 50% increased odds of morbidity after an abdominal myomectomy.24
Continue to: How to ensure that BIPOC women get the best management...
How to ensure that BIPOC women get the best management
Eliminating disparities and providing equitable and patient-centered care for Black, Indigenous, and people of color (BIPOC) women will require research, education, training, and targeted quality improvement initiatives.
Research into fibroids and comparative treatment outcomes
Uterine fibroids, despite their major public health impact, remain understudied. With Black women carrying the highest fibroid prevalence and severity burden, especially in their childbearing years, it is imperative that research efforts be focused on outcomes by race and ethnicity. Given the significant economic impact of fibroids, more efforts should be directed toward primary prevention of fibroid formation as well as secondary prevention and limitation of fibroid growth by affordable, effective, and safe means. For example, Bratka and colleagues researched the role of vitamin D in inhibiting growth of leiomyoma cells in animal models.25 Other innovative forms of management under investigation include aromatase inhibitors, green tea, cabergoline, elagolix, paricalcitol, and epigallocatechin gallate.26 Considerations such as stress, diet, and environmental risk factors have yet to be investigated in large studies.
Research contributing to evidence-based guidelines that address the needs of different patient populations affected by uterine fibroids is critical.8 Additionally, research conducted by Black women about Black women should be prioritized. In March 2021, the Stephanie Tubbs Jones Uterine Fibroid Research and Education Act of 2021 was introduced to fund $150 million in research supported by the National Institutes of Health (NIH). This is an opportunity to develop a research database to inform evidence-based culturally informed care regarding fertility counseling, medical management, and optimal surgical approach, as well as to award funding to minority researchers. There are disparities in distribution of funds from the NIH to minority researchers. Under-represented minorities are awarded fewer NIH grants compared with their counterparts despite initiatives to increase funding. Furthermore, in 2011, Black applicants for NIH funding were two-thirds as likely as White applicants to receive grants from 2000 ̶ 2006, even when accounting for publication record and training.27 Funding BIPOC researchers fuels diversity-driven investigation and can be useful in the charge to increase fibroid research.
Education and training: Changing the work force
Achieving equity requires change in provider work force. In a study of trends across multiple specialties including obstetrics and gynecology, Blacks and Latinx are more under-represented in 2016 than in 1990 across all specialties except for Black women in obstetrics and gynecology.28 It is well documented that under-represented minorities are more likely to engage in practice, research, service, and mentorship activities aligned with their identity.29 As a higher proportion of under-represented minority obstetricians and gynecologists practice in medically underserved areas,30 this presents a unique opportunity for gynecologists to improve care for and increase research involvement among BIPOC women.
Increasing BIPOC representation in medical and health care institutions and practices is not enough, however, to achieve health equity. Data from the Association of American Medical Colleges demonstrate that between 1978 and 2017 the total number of full-time obstetrics and gynecology faculty rose nearly fourfold from 1,688 to 6,347; however, the greatest rise in proportion of faculty who were nontenured was among women who were under-represented minorities.31 Additionally, there are disparities in wage by race even after controlling for hours worked and state of residence.32 Medical and academic centers and health care institutions and practices should proactively and systematically engage in the recruitment and retention of under-represented minority physicians and people in leadership roles. This will involve creating safe and inclusive work environments, with equal pay and promotion structures.
Quality initiatives to address provider bias
Provider bias should be addressed in clinical decision making and counseling of patients. Studies focused on ultrasonography have shown an estimated cumulative incidence of fibroids by age 50 of greater than 80% for Black women and nearly 70% for White women.5 Due to the prevalence and burden of fibroids among Black women there may be a provider bias in approach to management. Addressing this bias requires quality improvement efforts and investigation into patient and provider factors in management of fibroids. Black women have been a vulnerable population in medicine due to instances of mistreatment, and often times mistrust can play a role in how a patient views his or her care decisions. A patient-centered strategy allows patient factors such as age, uterine size, and cultural background to be considered such that a provider can tailor an approach that is best for the patient. Previous minority women focus groups have demonstrated that women have a strong desire for elective treatment;33 therefore, providers should listen openly to patients about their values and their perspectives on how fibroids affect their lives. Provider bias toward surgical volume, incentive for surgery, and implicit bias need to be addressed at every institution to work toward equitable and cost-effective care.
Integrated health care systems like Southern and Northern California Permanente Medical Group, using quality initiatives, have increased their minimally invasive surgery rates. Southern California Permanente Medical Group reached a 78% rate of MIH in a system of more than 350 surgeons performing benign indication hysterectomies as reported in 2011.34 Similarly, a study within KPNC, an institution with an MIH rate greater than 95%,35 found that racial disparities in route of MIH were eliminated through a quality improvement initiative described in detail in 2018 (FIGURE and TABLE).36
Conclusions
There are recognized successes in the gynecology field’s efforts to address racial disparities. Prior studies provide insight into opportunities to improve care in medical management of leiomyomas, minimally invasive route of hysterectomy and myomectomy, postsurgical outcomes, and institutional leadership. Particularly, when systemwide approaches are taken in the delivery of health care it is possible to significantly diminish racial disparities in gynecology.35 Much work remains to be done for our health care systems to provide equitable care.


- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
- Ojanuga D. The medical ethics of the ‘father of gynaecology,’ Dr J Marion Sims. J Med Ethics. 1993;19:28-31. doi: 10.1136/jme.19.1.28.
- Borrero S, Zite N, Creinin MD. Federally funded sterilization: time to rethink policy? Am J Public Health. 2012;102:1822-1825.
- Eaglehouse YL, Georg MW, Shriver CD, et al. Racial differences in time to breast cancer surgery and overall survival in the US Military Health System. JAMA Surg. 2019;154:e185113. doi: 10.1001/jamasurg.2018.5113.
- Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141-160.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967-973. doi: 10.1016/s0029-7844(97)00534-6.
- Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3-12. doi: 10.1016/j.bpobgyn.2015.11.018.
- Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med. 2017;35:473-480. doi: 10.1055/s-0037-1607264.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Corona LE, Swenson CW, Sheetz KH, et al. Use of other treatments before hysterectomy for benign conditions in a statewide hospital collaborative. Am J Obstet Gynecol. 2015;212:304.e1-e7. doi: 10.1016/j.ajog.2014.11.031.
- Nguyen NT, Merchant M, Ritterman Weintraub ML, et al. Alternative treatment utilization before hysterectomy for benign gynecologic conditions at a large integrated health system. J Minim Invasive Gynecol. 2019;26:847-855. doi: 10.1016/j.jmig.2018.08.013.
- Laughlin-Tommaso SK, Jacoby VL, Myers ER. Disparities in fibroid incidence, prognosis, and management. Obstet Gynecol Clin North Am. 2017;44:81-94. doi: 10.1016/j.ogc.2016.11.007.
- Borah BJ, Laughlin-Tommaso SK, Myers ER, et al. Association between patient characteristics and treatment procedure among patients with uterine leiomyomas. Obstet Gynecol. 2016;127:67-77.
- Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000-2004. Am J Obstet Gynecol. 2008;198:34.e1-e7. doi:10.1016/j.ajog.2007.05.039.
- Bardens D, Solomayer E, Baum S, et al. The impact of the body mass index (BMI) on laparoscopic hysterectomy for benign disease. Arch Gynecol Obstet. 2014;289:803-807. doi: 10.1007/s00404-013-3050-2.
- Seracchioli R, Venturoli S, Vianello F, et al. Total laparoscopic hysterectomy compared with abdominal hysterectomy in the presence of a large uterus. J Am Assoc Gynecol Laparosc. 2002;9:333-338. doi: 10.1016/s1074-3804(05)60413.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915. doi: 10.1097/AOG.0b013e3181f395d9.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145:14-21. doi: 10.1016/j.ejogrb.2009.03.009.
- Wechter ME, Stewart EA, Myers ER, et al. Leiomyoma-related hospitalization and surgery: prevalence and predicted growth based on population trends. Am J Obstet Gynecol. 2011;205:492.e1-e5. doi: 10.1016/j.ajog.2011.07.008.
- Bower JK, Schreiner PJ, Sternfeld B, et al. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300-307. doi: 10.2105/AJPH.2008.133702.
- Ranjit A, Sharma M, Romano A, et al. Does universal insurance mitigate racial differences in minimally invasive hysterectomy? J Minim Invasive Gynecol. 2017;24. doi:10.1016/j.jmig.2017.03.016.
- Pollack LM, Olsen MA, Gehlert SJ, et al. Racial/ethnic disparities/differences in hysterectomy route in women likely eligible for minimally invasive surgery. J Minim Invasive Gynecol. 2020;27:1167-1177.e2. doi:10.1016/j.jmig.2019.09.003.
- Stentz NC, Cooney LG, Sammel MD, et al. Association of patient race with surgical practice and perioperative morbidity after myomectomy. Obstet Gynecol. 2018;132:291-297. doi: 10.1097/AOG.0000000000002738.
- Roth TM, Gustilo-Ashby T, Barber MD, et al. Effects of race and clinical factors on short-term outcomes of abdominal myomectomy. Obstet Gynecol. 2003;101(5 pt 1):881-884. doi: 10.1016/s0029-7844(03)00015-2.
- Bratka S, Diamond JS, Al-Hendy A, et al. The role of vitamin D in uterine fibroid biology. Fertil Steril. 2015;104:698-706. doi: 10.1016/j.fertnstert.2015.05.031.
- Ciebiera M, Łukaszuk K, Męczekalski B, et al. Alternative oral agents in prophylaxis and therapy of uterine fibroids—an up-to-date review. Int J Mol Sci. 2017;18:2586. doi:10.3390/ijms18122586.
- Hayden EC. Racial bias haunts NIH funding. Nature. 2015;527:145.
- Lett LA, Orji WU, Sebro R. Declining racial and ethnic representation in clinical academic medicine: a longitudinal study of 16 US medical specialties. PLoS One. 2018;13:e0207274. doi: 10.1371/journal.pone.0207274.
- Sánchez JP, Poll-Hunter N, Stern N, et al. Balancing two cultures: American Indian/Alaska Native medical students’ perceptions of academic medicine careers. J Community Health. 2016;41:871-880.
- Rayburn WF, Xierali IM, Castillo-Page L, et al. Racial and ethnic differences between obstetrician-gynecologists and other adult medical specialists. Obstet Gynecol. 2016;127:148-152. doi: 10.1097/AOG.0000000000001184.
- Esters D, Xierali IM, Nivet MA, et al. The rise of nontenured faculty in obstetrics and gynecology by sex and underrepresented in medicine status. Obstet Gynecol. 2019;134 suppl 1:34S-39S. doi: 10.1097/AOG.0000000000003484.
- Ly DP, Seabury SA, Jena AB. Differences in incomes of physicians in the United States by race and sex: observational study. BMJ. 2016;I2923. doi:10.1136/bmj.i2923.
- Groff JY, Mullen PD, Byrd T, et al. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9 suppl 2:S39-50. doi: 10.1089/152460900318759.
- Andryjowicz E, Wray T. Regional expansion of minimally invasive surgery for hysterectomy: implementation and methodology in a large multispecialty group. Perm J. 2011;15:42-46.
- Zaritsky E, Ojo A, Tucker LY, et al. Racial disparities in route of hysterectomy for benign indications within an integrated health care system. JAMA Netw Open. 2019;2:e1917004. doi: 10.1001/jamanetworkopen.2019.17004.
- Abel MK, Kho KA, Walter A, et al. Measuring quality in minimally invasive gynecologic surgery: what, how, and why? J Minim Invasive Gynecol. 2019;26:321-326. doi: 10.1016/j.jmig.2018.11.013.
FDA and power morcellation, gel for vaginal odor, and an intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
FDA guidance for power morcellation
“The FDA has granted marketing authorization for one containment system and continues to encourage innovation in this area” said the report. Olympus’ Pneumoliner is the only FDA cleared containment device to provide a laparoscopic option for appropriately identified patients undergoing myomectomy and hysterectomy. The containment system is sold with Olympus’ PK Morcellator, but the company says that it has made the Pneumoliner available to physicians choosing an alternate to the PK Morcellator, provided that there is device compatibility. The Pneumoliner “reduces the spread of benign tissue into the abdominal cavity, in which pathologies, like fibroids, may regrow when tissue or cells are inadvertently left behind,” according to Olympus.
Vaginal odor elimination gel
The gel is sold in 7 single-day applications, with a single tube used per day at bedtime to eliminate unwanted odor. To maintain freshness and comfort, a single tube of Relactagel can be used for 2 to 3 days after a woman’s menstrual cycle, says Kora Healthcare. The company warns that mild irritation can occur with product use during fungal infections or when small tears are present in the vaginal tissue and that use should be discontinued if irritation occurs. In addition, if trying to become pregnant Relatagel should not be used, advises Kora Healthcare, although the gel is not a contraceptive.
Intrauterine electrosurgery system
Cesarean myomectomy: Safe operation or surgical folly?

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.
Prophylactic antibiotics for myomectomy?
In the 1990s, researchers found that patients undergoing any type of surgical procedure were more than twice as likely to die if they developed postsurgical infection.1 Work to reduce surgical site infection (SSI) has and does continue, with perioperative antibiotics representing a good part of that effort. The American College of Obstetricians and Gynecologists currently recommends such antibiotic therapy for women undergoing laparotomy and laparoscopic hysterectomy.2 ACOG does not, however, recommend prophylactic antibiotics for myomectomy procedures.3 Rates of infection for hysterectomy have been reported to be 3.9% for abdominal and 1.4% for minimally invasive approaches.4
To determine the current use of antibiotics during myomectomy and associated rates of SSI at their institutions, Dipti Banerjee, MD, and colleagues conducted a retrospective analysis of women undergoing laparoscopic or abdominal myomectomy between February 2013 and December 2017 at the University of California, Los Angeles and Hoag Memorial Hospital in Orange County, California. They presented their study results at AAGL’s 49th Global Congress on MIGS, held virtually November 6-14, 2020.3
Rate of SSI after myomectomy
A total of 620 women underwent laparoscopic myomectomy and 563 underwent open myomectomy during the study period. Antibiotics were used in 76.9% of cases. SSI developed within 6 weeks of surgery in 34 women (2.9%) overall. The women undergoing abdominal myomectomy without antibiotics were more likely to experience SSI than the women who received antibiotics (odds ratio [OR], 4.89; confidence interval [CI], 1.80–13.27; P = .0006). For laparoscopic myomectomy, antibiotic use did not affect the odds of developing SSI (OR, 1.08; CI, 0.35–3.35).
Antibiotics were more likely to be used in certain cases
Antibiotics were more likely to be administered for patients who:
- were obese (body mass index ≥30 kg/m2) (P = .009)
- underwent previous abdominal surgery (P = .001)
- underwent laparotomy (P <.0001)
- had endometrial cavity entry (P <.0001)
- had >1 fibroid (P = .0004) or an aggregate fibroid weight >500 g (P <.0001).
More data on antibiotics for myomectomy
In a retrospective study conducted at 2 academic hospitals in Boston, Massachusetts, 1,211 women underwent myomectomy from 2009 to 2016. (Exclusions were use of vaginal or hysteroscopic myomectomy, chromopertubation, or conversion to hysterectomy.) More than 92% of the women received perioperative antibiotics at the time of surgery. Although demographics were similar between women receiving and not receiving antibiotics, women who received antibiotics were more likely to have longer operative times (median 140 vs 85 min), a greater myoma burden (7 vs 2 myomas removed and weight 255 vs 53 g), and lose blood during the procedure (137 vs 50 mL). These women also were 4 times less likely to have surgical site infection (adjusted OR, 3.77; 95% CI, 1.30–10.97; P = .015).5,6
Banerjee and colleagues say that their California study demonstrates “that the majority of surgeons elect to use antibiotics prophylactically” during myomectomy, despite current ACOG guidelines, and that their findings of benefit for abdominal myomectomy but not for laparoscopic myomectomy should inform future guidance on antibiotics for myomectomy surgery.3
- Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-730.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:e172-e189.
- Banerjee D, Dejbakhsh S, Patel HH, et al. Perioperative antibiotic prophylaxis in myomectomy surgery. Paper presented at 49th Annual Meeting of the AAGL; November 2020.
- Uppal S, Harris J, Al-Niaimi A. Prophylactic antibiotic choice and risk of surgical site infection after hysterectomy. Obstet Gynecol. 2016;127:321-329.
- Kim AJ, Clark NV, Jansen LJ, et al. Perioperative antibiotic use and associated infectious outcomes at the time of myomectomy. Obstet Gynecol. 2019;133:626-635.
- Rebar RW. Should perioperative antibiotics at myomectomy be universal? NEJM J Watch. March 11, 2019.
In the 1990s, researchers found that patients undergoing any type of surgical procedure were more than twice as likely to die if they developed postsurgical infection.1 Work to reduce surgical site infection (SSI) has and does continue, with perioperative antibiotics representing a good part of that effort. The American College of Obstetricians and Gynecologists currently recommends such antibiotic therapy for women undergoing laparotomy and laparoscopic hysterectomy.2 ACOG does not, however, recommend prophylactic antibiotics for myomectomy procedures.3 Rates of infection for hysterectomy have been reported to be 3.9% for abdominal and 1.4% for minimally invasive approaches.4
To determine the current use of antibiotics during myomectomy and associated rates of SSI at their institutions, Dipti Banerjee, MD, and colleagues conducted a retrospective analysis of women undergoing laparoscopic or abdominal myomectomy between February 2013 and December 2017 at the University of California, Los Angeles and Hoag Memorial Hospital in Orange County, California. They presented their study results at AAGL’s 49th Global Congress on MIGS, held virtually November 6-14, 2020.3
Rate of SSI after myomectomy
A total of 620 women underwent laparoscopic myomectomy and 563 underwent open myomectomy during the study period. Antibiotics were used in 76.9% of cases. SSI developed within 6 weeks of surgery in 34 women (2.9%) overall. The women undergoing abdominal myomectomy without antibiotics were more likely to experience SSI than the women who received antibiotics (odds ratio [OR], 4.89; confidence interval [CI], 1.80–13.27; P = .0006). For laparoscopic myomectomy, antibiotic use did not affect the odds of developing SSI (OR, 1.08; CI, 0.35–3.35).
Antibiotics were more likely to be used in certain cases
Antibiotics were more likely to be administered for patients who:
- were obese (body mass index ≥30 kg/m2) (P = .009)
- underwent previous abdominal surgery (P = .001)
- underwent laparotomy (P <.0001)
- had endometrial cavity entry (P <.0001)
- had >1 fibroid (P = .0004) or an aggregate fibroid weight >500 g (P <.0001).
More data on antibiotics for myomectomy
In a retrospective study conducted at 2 academic hospitals in Boston, Massachusetts, 1,211 women underwent myomectomy from 2009 to 2016. (Exclusions were use of vaginal or hysteroscopic myomectomy, chromopertubation, or conversion to hysterectomy.) More than 92% of the women received perioperative antibiotics at the time of surgery. Although demographics were similar between women receiving and not receiving antibiotics, women who received antibiotics were more likely to have longer operative times (median 140 vs 85 min), a greater myoma burden (7 vs 2 myomas removed and weight 255 vs 53 g), and lose blood during the procedure (137 vs 50 mL). These women also were 4 times less likely to have surgical site infection (adjusted OR, 3.77; 95% CI, 1.30–10.97; P = .015).5,6
Banerjee and colleagues say that their California study demonstrates “that the majority of surgeons elect to use antibiotics prophylactically” during myomectomy, despite current ACOG guidelines, and that their findings of benefit for abdominal myomectomy but not for laparoscopic myomectomy should inform future guidance on antibiotics for myomectomy surgery.3
In the 1990s, researchers found that patients undergoing any type of surgical procedure were more than twice as likely to die if they developed postsurgical infection.1 Work to reduce surgical site infection (SSI) has and does continue, with perioperative antibiotics representing a good part of that effort. The American College of Obstetricians and Gynecologists currently recommends such antibiotic therapy for women undergoing laparotomy and laparoscopic hysterectomy.2 ACOG does not, however, recommend prophylactic antibiotics for myomectomy procedures.3 Rates of infection for hysterectomy have been reported to be 3.9% for abdominal and 1.4% for minimally invasive approaches.4
To determine the current use of antibiotics during myomectomy and associated rates of SSI at their institutions, Dipti Banerjee, MD, and colleagues conducted a retrospective analysis of women undergoing laparoscopic or abdominal myomectomy between February 2013 and December 2017 at the University of California, Los Angeles and Hoag Memorial Hospital in Orange County, California. They presented their study results at AAGL’s 49th Global Congress on MIGS, held virtually November 6-14, 2020.3
Rate of SSI after myomectomy
A total of 620 women underwent laparoscopic myomectomy and 563 underwent open myomectomy during the study period. Antibiotics were used in 76.9% of cases. SSI developed within 6 weeks of surgery in 34 women (2.9%) overall. The women undergoing abdominal myomectomy without antibiotics were more likely to experience SSI than the women who received antibiotics (odds ratio [OR], 4.89; confidence interval [CI], 1.80–13.27; P = .0006). For laparoscopic myomectomy, antibiotic use did not affect the odds of developing SSI (OR, 1.08; CI, 0.35–3.35).
Antibiotics were more likely to be used in certain cases
Antibiotics were more likely to be administered for patients who:
- were obese (body mass index ≥30 kg/m2) (P = .009)
- underwent previous abdominal surgery (P = .001)
- underwent laparotomy (P <.0001)
- had endometrial cavity entry (P <.0001)
- had >1 fibroid (P = .0004) or an aggregate fibroid weight >500 g (P <.0001).
More data on antibiotics for myomectomy
In a retrospective study conducted at 2 academic hospitals in Boston, Massachusetts, 1,211 women underwent myomectomy from 2009 to 2016. (Exclusions were use of vaginal or hysteroscopic myomectomy, chromopertubation, or conversion to hysterectomy.) More than 92% of the women received perioperative antibiotics at the time of surgery. Although demographics were similar between women receiving and not receiving antibiotics, women who received antibiotics were more likely to have longer operative times (median 140 vs 85 min), a greater myoma burden (7 vs 2 myomas removed and weight 255 vs 53 g), and lose blood during the procedure (137 vs 50 mL). These women also were 4 times less likely to have surgical site infection (adjusted OR, 3.77; 95% CI, 1.30–10.97; P = .015).5,6
Banerjee and colleagues say that their California study demonstrates “that the majority of surgeons elect to use antibiotics prophylactically” during myomectomy, despite current ACOG guidelines, and that their findings of benefit for abdominal myomectomy but not for laparoscopic myomectomy should inform future guidance on antibiotics for myomectomy surgery.3
- Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-730.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:e172-e189.
- Banerjee D, Dejbakhsh S, Patel HH, et al. Perioperative antibiotic prophylaxis in myomectomy surgery. Paper presented at 49th Annual Meeting of the AAGL; November 2020.
- Uppal S, Harris J, Al-Niaimi A. Prophylactic antibiotic choice and risk of surgical site infection after hysterectomy. Obstet Gynecol. 2016;127:321-329.
- Kim AJ, Clark NV, Jansen LJ, et al. Perioperative antibiotic use and associated infectious outcomes at the time of myomectomy. Obstet Gynecol. 2019;133:626-635.
- Rebar RW. Should perioperative antibiotics at myomectomy be universal? NEJM J Watch. March 11, 2019.
- Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-730.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:e172-e189.
- Banerjee D, Dejbakhsh S, Patel HH, et al. Perioperative antibiotic prophylaxis in myomectomy surgery. Paper presented at 49th Annual Meeting of the AAGL; November 2020.
- Uppal S, Harris J, Al-Niaimi A. Prophylactic antibiotic choice and risk of surgical site infection after hysterectomy. Obstet Gynecol. 2016;127:321-329.
- Kim AJ, Clark NV, Jansen LJ, et al. Perioperative antibiotic use and associated infectious outcomes at the time of myomectomy. Obstet Gynecol. 2019;133:626-635.
- Rebar RW. Should perioperative antibiotics at myomectomy be universal? NEJM J Watch. March 11, 2019.
Hysteroscopy and COVID-19: Have recommended techniques changed due to the pandemic?
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13

Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13

Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
The emergence of the coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) in December 2019, has resulted in a global pandemic that has challenged the medical community and will continue to represent a public health emergency for the next several months.1 It has rapidly spread globally, infecting many individuals in an unprecedented rate of infection and worldwide reach. On March 11, 2020, the World Health Organization designated COVID-19 as a pandemic. While the majority of infected individuals are asymptomatic or develop only mild symptoms, some have an unfortunate clinical course resulting in multi-organ failure and death.2
It is accepted that the virus mainly spreads during close contact and via respiratory droplets.3 The average time from infection to onset of symptoms ranges from 2 to 14 days, with an average of 5 days.4 Recommended measures to prevent the spread of the infection include social distancing (at least 6 feet from others), meticulous hand hygiene, and wearing a mask covering the mouth and nose when in public.5 Aiming to mitigate the risk of viral dissemination for patients and health care providers, and to preserve hospital resources, all nonessential medical interventions were initially suspended. Recently, the American College of Surgeons in a joint statement with 9 women’s health care societies have provided recommendations on how to resume clinical activities as we recover from the pandemic.6
As we reinitiate clinical activities, gynecologists have been alerted of the potential risk of viral dissemination during gynecologic minimally invasive surgical procedures due to the presence of the virus in blood, stool, and the potential risk of aerosolization of the virus, especially when using smoke-generating devices.7,8 This risk is not limited to intubation and extubation of the airway during anesthesia; the risk also presents itself during other aerosol-generating procedures, such as laparoscopy or robotic surgery.9,10
Hysteroscopy is considered the gold standard procedure for the diagnosis and management of intrauterine pathologies.11 It is frequently performed in an office setting without the use of anesthesia.11,12 It is usually well tolerated, with only a few patients reporting discomfort.12 It allows for immediate treatment (using the “see and treat” approach) while avoiding not only the risk of anesthesia, as stated, but also the need for intubation—which has a high risk of droplet contamination in COVID-19–infected individuals.13

Is there risk of viral dissemination during hysteroscopic procedures?
The novel and rapidly changing nature of the COVID-19 pandemic present many challenges to the gynecologist. Significant concerns have been raised regarding potential risk of viral dissemination during laparoscopic surgery due to aerosolization of viral particles and the presence of the virus in blood and the gastrointestinal tract of infected patients.7 Diagnostic, and some simple, hysteroscopic procedures are commonly performed in an outpatient setting, with the patient awake. Complex hysteroscopic interventions, however, are generally performed in the operating room, typically with the use of general anesthesia. Hysteroscopy has the theoretical risks of viral dissemination when performed in COVID-19–positive patients. Two important questions must be addressed to better understand the potential risk of COVID-19 viral dissemination during hysteroscopic procedures.
Continue to: 1. Is the virus present in the vaginal fluid of women infected with COVID-19?...
1. Is the virus present in the vaginal fluid of women infected with COVID-19?
Recent studies have confirmed the presence of viral particles in urine, feces, blood, and tears in addition to the respiratory tract in patients infected with COVID-19.3,14,15 The presence of the SARS-CoV-2 virus in the female genital system is currently unknown. Previous studies, of other epidemic viral infections, have demonstrated the presence of the virus in the female genital tract in affected patients of Zika virus and Ebola.16,17 However, 2 recent studies have failed to demonstrate the presence of the SARS-CoV-2 virus in the vaginal fluid of pregnant14 and not pregnant18 women with severe COVID-19 infection.
2. Is there risk of viral dissemination during hysteroscopy if using electrosurgery?
There are significant concerns with possible risk of COVID-19 transmission to health care providers in direct contact with infected patients during minimally invasive gynecologic procedures due to direct contamination and aerosolization of the virus.10,19 Current data on COVID-19 transmission during surgery are limited. However, it is important to recognize that viral aerosolization has been documented with other viral diseases, such as human papillomavirus and hepatitis B.20 A recent report called for awareness in the surgical community about the potential risks of COVID-19 viral dissemination during laparoscopic surgery. Among other recommendations, international experts advised minimizing the use of electrosurgery to reduce the creation of surgical plume, decreasing the pneumoperitoneum pressure to minimum levels, and using suction devices in a closed system.21 Although these preventive measures apply to laparoscopic surgery, it is important to consider that hysteroscopy is performed in a unique environment.
During hysteroscopy the uterine cavity is distended with a liquid medium (normal saline or electrolyte-free solutions); this is opposed to gynecologic laparoscopy, in which the peritoneal cavity is distended with carbon dioxide.22 The smoke produced with the use of hysteroscopic electrosurgical instruments generates bubbles that are immediately cooled down to the temperature of the distention media and subsequently dissolve into it. Therefore, there are no bubbles generated during hysteroscopic surgery that are subsequently released into the air. This results in a low risk for viral dissemination during hysteroscopic procedures. Nevertheless, the necessary precautions to minimize the risk of COVID-19 transmission during hysteroscopic intervention are extremely important.
Recommendations for hysteroscopic procedures during the COVID-19 pandemic
We provide our overall recommendations for hysteroscopy, as well as those specific to the office and hospital setting.
Recommendations: General
Limit hysteroscopic procedures to COVID-19–negative patients and to those patients in whom delaying the procedure could result in adverse clinical outcomes.23
Universally screen for potential COVID-19 infection. When possible, a phone interview to triage patients based on their symptoms and infection exposure status should take place before the patient arrives to the health care center. Patients with suspected or confirmed COVID-19 infection who require immediate evaluation should be directed to COVID-19–designated emergency areas.
Universally test for SARS-CoV-2 before procedures performed in the operating room (OR). Using nasopharyngeal swabs for the detection of viral RNA, employing molecular methods such as polymerase chain reaction (PCR), within 48 to 72 hours prior to all OR hysteroscopic procedures is strongly recommended. Adopting this testing strategy will aid to identify asymptomatic SARS-CoV-2‒infected patients, allowing to defer the procedure, if possible, among patients testing positive. If tests are limited, testing only patients scheduled for hysteroscopic procedures in which general or regional anesthesia will be required is acceptable.
Universal SARS-CoV-2 testing of patients undergoing in-office hysteroscopic diagnostic or minor operative procedures without the use of anesthesia is not required.
Limit the presence of a companion. It is understood that visitor policies may vary at the discretion of each institution’s guidelines. Children and individuals over the age of 60 years should not be granted access to the center. Companions will be subjected to the same screening criteria as patients.
Provide for social distancing and other precautionary measures. If more than one patient is scheduled to be at the facility at the same time, ensure that the facility provides adequate space to allow the appropriate social distancing recommendations between patients. Hand sanitizers and facemasks should be available for patients and companions.
Provide PPE for clinicians. All health care providers in close contact with the patient must wear personal protective equipment (PPE), which includes an apron and gown, a surgical mask, eye protection, and gloves. Health care providers should wear PPE deemed appropriate by their regulatory institutions following their local and national guidelines during clinical patient interactions.
Restrict surgical attendees to vital personnel. The participation of learners by physical presence in the office or operating room should be restricted.
Continue to: Recommendations: Office setting...
Recommendations: Office setting
Preprocedural recommendations
- Advise patients to come to the office alone. If the patient requires a companion, a maximum of one adult companion under the age of 60 should be accepted.
- Limit the number of health care team members present in the procedure room.
Intraprocedural recommendations
- Choose the appropriate device(s) that will allow for an effective and fast procedure.
- Use the recommended PPE for all clinicians.
- Limit the movement of staff members in and out of the procedure room.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same procedure room, allow enough time in between cases to grant a thorough OR decontamination.
- Allow for patients to recover from the procedure in the same room as the procedure took place in order to avoid potential contamination of multiple rooms.
- Expedite patient discharge.
- Follow up after the procedure by phone or telemedicine.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Continue to: Recommendations: Operating room setting...
Recommendations: Operating room setting
Preprocedural recommendations
- Perform adequate patient screening for potential COVID-19 infection. (Screening should be independent of symptoms and not be limited to those with clinical symptoms.)
- Limit the number of health care team members in the operating procedure room.
- To minimize unnecessary staff exposure, have surgeons and staff not needed for intubation remain outside the OR until intubation is completed and leave the OR before extubation.
Intraprocedure recommendations
- Limit personnel in the OR to a minimum.
- Staff should not enter or leave the room during the procedure.
- When possible, use conscious sedation or regional anesthesia to avoid the risk of viral dissemination at the time of intubation/extubation.
- Choose the device that will allow an effective and fast procedure.
- Favor non–smoke-generating devices, such as hysteroscopic scissors, graspers, and tissue retrieval systems.
- Connect active suction to the outflow, especially when using smoke-generating instruments, to facilitate the extraction of surgical smoke.
Postprocedure recommendations
- When more than one case is scheduled to be performed in the same room, allow enough time in between cases to grant a thorough OR decontamination.
- Expedite postprocedure recovery and patient discharge.
- After completion of the procedure, staff should remove scrubs and change into clean clothing.
- Use standard endoscope disinfection procedures, as they are effective and should not be modified.
Conclusions
The COVID-19 pandemic has caused a global health emergency. Our knowledge of this devastating virus is constantly evolving as we continue to fight this overwhelming disease. Theoretical risk of “viral” dissemination is considered extremely low, or negligible, during hysterosocopy. Hysteroscopic procedures in COVID-19–positive patients with life-threatening conditions or in patients in whom delaying the procedure could worsen outcomes should be performed taking appropriate measures. Patients who test negative for COVID-19 (confirmed by PCR) and require hysteroscopic procedures, should be treated using universal precautions. ●
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.
- Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25:e936-e945.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. February 24, 2020. doi:10.1001/jama.2020.2648.
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843-1844.
- Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793-798.
- Prem K, Liu Y, Russell TW, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261-e270.
- American College of Surgeons, American Society of Aesthesiologists, Association of periOperative Registered Nurses, American Hospital Association. Joint Statement: Roadmap for resuming elective surgery after COVID-19 pandemic. April 16, 2020. https://www.aorn.org/guidelines/aorn-support/roadmap-for-resuming-elective-surgery-after-covid-19. Accessed August 27, 2020.
- Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9:386-389.
- Mowbray NG, Ansell J, Horwood J, et al. Safe management of surgical smoke in the age of COVID-19. Br J Surg. May 3, 2020. doi: 10.1002/bjs.11679.
- Cohen SL, Liu G, Abrao M, et al. Perspectives on surgery in the time of COVID-19: safety first. J Minim Invasive Gynecol. 2020;27:792-793.
- COVID-19: protecting health-care workers. Lancet. 2020;395:922.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17:703-708.
- Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-576.
- Aslan MM, Yuvaci HU, Köse O, et al. SARS-CoV-2 is not present in the vaginal fluid of pregnant women with COVID-19. J Matern Fetal Neonatal Med. 2020:1-3. doi: 10.1080/14767058.2020.1793318.
- Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840.
- Prisant N, Bujan L, Benichou H, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000-1001.
- Rodriguez LL, De Roo A, Guimard Y, et al. Persistence and genetic stability of Ebola virus during the outbreak in Kikwit, Democratic Republic of the Congo, 1995. J Infect Dis. 1999;179 Suppl 1:S170-S176.
- Qiu L, Liu X, Xiao M, et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis. 2020;71:813-817.
- Brat GA, Hersey S, Chhabra K, et al. Protecting surgical teams during the COVID-19 outbreak: a narrative review and clinical considerations. Ann Surg. April 17, 2020. doi: 10.1097/SLA.0000000000003926.
- Kwak HD, Kim SH, Seo YS, et al. Detecting hepatitis B virus in surgical smoke emitted during laparoscopic surgery. Occup Environ Med. 2016;73:857-863.
- Zheng MH, Boni L, Fingerhut A. Minimally invasive surgery and the novel coronavirus outbreak: lessons learned in China and Italy. Ann Surg. 2020;272:e5-e6.
- Catena U. Surgical smoke in hysteroscopic surgery: does it really matter in COVID-19 times? Facts Views Vis Obgyn. 2020;12:67-68.
- Carugno J, Di Spiezio Sardo A, Alonso L, et al. COVID-19 pandemic. Impact on hysteroscopic procedures: a consensus statement from the Global Congress of Hysteroscopy Scientific Committee. J Minim Invasive Gynecol. 2020;27:988-992.