User login
MD-IQ only
Does the Road to Treating Endometriosis Start in the Gut?
Researchers may be on track to develop a much-needed tool for studying endometriosis: A noninvasive stool test that could replace the current gold standard of laparoscopy.
Their approach, which focuses on the link between the gut microbiome and endometriosis, also identified a bacterial metabolite they said might be developed as an oral medication for the condition, which affects at least 11% of women.
In previous research, Rama Kommagani, PhD, an associate professor in the Department of Pathology & Immunology at Baylor College of Medicine in Houston, Texas, worked with a mouse model in which endometrial tissue from donor rodents was injected into the peritoneal space of healthy rodents to induce the disorder.
Transfer of fecal microbiota from mice with endometriosis to those without the condition induced the trademark lesions, suggesting the microbiome influences the development of endometriosis. Treating the animals with the antibiotic metronidazole inhibited the progression of endometrial lesions.
Kommagani speculated the microbes release metabolites that stimulate the growth of the endometrial lesions. “Bad bacteria release metabolites, you know, which actually promote the disease,” Kommagani said. “But the good bacteria might release some protective metabolites such as short-chain fatty acids.”
In a new study, Kommagani and his colleagues sought to identify a unique profile of bacteria-derived metabolites that could reliably diagnose endometriosis. Using stool specimens from 18 women with the condition and 31 without the disease, his team conducted whole metabolic profiling of the gut microbiota.
After identifying hundreds of metabolites in the samples, further analysis revealed a subset of 12 metabolites that consistently differentiated women with and without endometriosis.
These findings led to more questions. “If a metabolite is lower in women with endometriosis, does it have any functional relevance?” Kommagani said.
One candidate was 4-hydroxyindole (4HI), which was found in lower levels in the stool of patients. This substance is a little-understood derivative of its parent compound, indole, which occurs naturally in plants and has a wide range of therapeutic uses.
Using a mouse model, Kommagani’s lab demonstrated that feeding mice 4HI before receiving an endometrial transplant prevented the development of lesions typical of endometriosis. Mice given 4HI after they had developed endometriosis showed regression of lesions and decreased response to painful stimuli.
“In a nutshell, we found a specific set of bacterial metabolites in stool, which could be used towards a noninvasive diagnostic test,” Kommagani said. “But we also found this distinct, specific metabolite that could be used as a therapeutic molecule.”
Tatnai Burnett, MD, an assistant professor of obstetrics and gynecology at Mayo Clinic in Rochester, Minnesota, who is not associated with the study, said a noninvasive test would have several advantages over the current methods of diagnosing endometriosis. Clinicians could detect and treat women earlier in the course of their disease. Secondly, a test with sufficient negative predictive value would be helpful in deciding whether to initiate treatment with hormones or other oral medications or go straight to surgery. “I would choose not to do a surgery if I knew with enough certainty that I wasn’t going to find anything,” said Burnett.
Lastly, a test that was quantitative and showed a response to treatment could be used as a disease activity marker to monitor the course of someone’s treatment.
But Burnett said more data on the approach are necessary. “This is a fairly small study, as it goes, for developing a screening test,” he said. “We need to see what its positive predictive value, negative predictive value, sensitivity, and specificity are in a bigger group.”
The road to a cure is even longer than the path to developing a screening test. Kommagani’s lab is now conducting more studies in mice to elucidate the pharmacokinetics and toxicology of 4HI before human trials can be attempted.
And as Burnett pointed out, although mouse models are great for experimentation and generating hypotheses, “We’ve seen way too many times in the past where something’s really exciting in a mouse model or a rat model or a monkey model, and it just doesn’t pan out in humans.”
Kommagani received funding from National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development grants (R01HD102680, R01HD104813) and a Research Scholar Grant from the American Cancer Society. Burnett reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Researchers may be on track to develop a much-needed tool for studying endometriosis: A noninvasive stool test that could replace the current gold standard of laparoscopy.
Their approach, which focuses on the link between the gut microbiome and endometriosis, also identified a bacterial metabolite they said might be developed as an oral medication for the condition, which affects at least 11% of women.
In previous research, Rama Kommagani, PhD, an associate professor in the Department of Pathology & Immunology at Baylor College of Medicine in Houston, Texas, worked with a mouse model in which endometrial tissue from donor rodents was injected into the peritoneal space of healthy rodents to induce the disorder.
Transfer of fecal microbiota from mice with endometriosis to those without the condition induced the trademark lesions, suggesting the microbiome influences the development of endometriosis. Treating the animals with the antibiotic metronidazole inhibited the progression of endometrial lesions.
Kommagani speculated the microbes release metabolites that stimulate the growth of the endometrial lesions. “Bad bacteria release metabolites, you know, which actually promote the disease,” Kommagani said. “But the good bacteria might release some protective metabolites such as short-chain fatty acids.”
In a new study, Kommagani and his colleagues sought to identify a unique profile of bacteria-derived metabolites that could reliably diagnose endometriosis. Using stool specimens from 18 women with the condition and 31 without the disease, his team conducted whole metabolic profiling of the gut microbiota.
After identifying hundreds of metabolites in the samples, further analysis revealed a subset of 12 metabolites that consistently differentiated women with and without endometriosis.
These findings led to more questions. “If a metabolite is lower in women with endometriosis, does it have any functional relevance?” Kommagani said.
One candidate was 4-hydroxyindole (4HI), which was found in lower levels in the stool of patients. This substance is a little-understood derivative of its parent compound, indole, which occurs naturally in plants and has a wide range of therapeutic uses.
Using a mouse model, Kommagani’s lab demonstrated that feeding mice 4HI before receiving an endometrial transplant prevented the development of lesions typical of endometriosis. Mice given 4HI after they had developed endometriosis showed regression of lesions and decreased response to painful stimuli.
“In a nutshell, we found a specific set of bacterial metabolites in stool, which could be used towards a noninvasive diagnostic test,” Kommagani said. “But we also found this distinct, specific metabolite that could be used as a therapeutic molecule.”
Tatnai Burnett, MD, an assistant professor of obstetrics and gynecology at Mayo Clinic in Rochester, Minnesota, who is not associated with the study, said a noninvasive test would have several advantages over the current methods of diagnosing endometriosis. Clinicians could detect and treat women earlier in the course of their disease. Secondly, a test with sufficient negative predictive value would be helpful in deciding whether to initiate treatment with hormones or other oral medications or go straight to surgery. “I would choose not to do a surgery if I knew with enough certainty that I wasn’t going to find anything,” said Burnett.
Lastly, a test that was quantitative and showed a response to treatment could be used as a disease activity marker to monitor the course of someone’s treatment.
But Burnett said more data on the approach are necessary. “This is a fairly small study, as it goes, for developing a screening test,” he said. “We need to see what its positive predictive value, negative predictive value, sensitivity, and specificity are in a bigger group.”
The road to a cure is even longer than the path to developing a screening test. Kommagani’s lab is now conducting more studies in mice to elucidate the pharmacokinetics and toxicology of 4HI before human trials can be attempted.
And as Burnett pointed out, although mouse models are great for experimentation and generating hypotheses, “We’ve seen way too many times in the past where something’s really exciting in a mouse model or a rat model or a monkey model, and it just doesn’t pan out in humans.”
Kommagani received funding from National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development grants (R01HD102680, R01HD104813) and a Research Scholar Grant from the American Cancer Society. Burnett reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Researchers may be on track to develop a much-needed tool for studying endometriosis: A noninvasive stool test that could replace the current gold standard of laparoscopy.
Their approach, which focuses on the link between the gut microbiome and endometriosis, also identified a bacterial metabolite they said might be developed as an oral medication for the condition, which affects at least 11% of women.
In previous research, Rama Kommagani, PhD, an associate professor in the Department of Pathology & Immunology at Baylor College of Medicine in Houston, Texas, worked with a mouse model in which endometrial tissue from donor rodents was injected into the peritoneal space of healthy rodents to induce the disorder.
Transfer of fecal microbiota from mice with endometriosis to those without the condition induced the trademark lesions, suggesting the microbiome influences the development of endometriosis. Treating the animals with the antibiotic metronidazole inhibited the progression of endometrial lesions.
Kommagani speculated the microbes release metabolites that stimulate the growth of the endometrial lesions. “Bad bacteria release metabolites, you know, which actually promote the disease,” Kommagani said. “But the good bacteria might release some protective metabolites such as short-chain fatty acids.”
In a new study, Kommagani and his colleagues sought to identify a unique profile of bacteria-derived metabolites that could reliably diagnose endometriosis. Using stool specimens from 18 women with the condition and 31 without the disease, his team conducted whole metabolic profiling of the gut microbiota.
After identifying hundreds of metabolites in the samples, further analysis revealed a subset of 12 metabolites that consistently differentiated women with and without endometriosis.
These findings led to more questions. “If a metabolite is lower in women with endometriosis, does it have any functional relevance?” Kommagani said.
One candidate was 4-hydroxyindole (4HI), which was found in lower levels in the stool of patients. This substance is a little-understood derivative of its parent compound, indole, which occurs naturally in plants and has a wide range of therapeutic uses.
Using a mouse model, Kommagani’s lab demonstrated that feeding mice 4HI before receiving an endometrial transplant prevented the development of lesions typical of endometriosis. Mice given 4HI after they had developed endometriosis showed regression of lesions and decreased response to painful stimuli.
“In a nutshell, we found a specific set of bacterial metabolites in stool, which could be used towards a noninvasive diagnostic test,” Kommagani said. “But we also found this distinct, specific metabolite that could be used as a therapeutic molecule.”
Tatnai Burnett, MD, an assistant professor of obstetrics and gynecology at Mayo Clinic in Rochester, Minnesota, who is not associated with the study, said a noninvasive test would have several advantages over the current methods of diagnosing endometriosis. Clinicians could detect and treat women earlier in the course of their disease. Secondly, a test with sufficient negative predictive value would be helpful in deciding whether to initiate treatment with hormones or other oral medications or go straight to surgery. “I would choose not to do a surgery if I knew with enough certainty that I wasn’t going to find anything,” said Burnett.
Lastly, a test that was quantitative and showed a response to treatment could be used as a disease activity marker to monitor the course of someone’s treatment.
But Burnett said more data on the approach are necessary. “This is a fairly small study, as it goes, for developing a screening test,” he said. “We need to see what its positive predictive value, negative predictive value, sensitivity, and specificity are in a bigger group.”
The road to a cure is even longer than the path to developing a screening test. Kommagani’s lab is now conducting more studies in mice to elucidate the pharmacokinetics and toxicology of 4HI before human trials can be attempted.
And as Burnett pointed out, although mouse models are great for experimentation and generating hypotheses, “We’ve seen way too many times in the past where something’s really exciting in a mouse model or a rat model or a monkey model, and it just doesn’t pan out in humans.”
Kommagani received funding from National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development grants (R01HD102680, R01HD104813) and a Research Scholar Grant from the American Cancer Society. Burnett reported no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Endometriosis, Especially Severe Types, Boosts Ovarian Cancer Risk
Ovarian cancer risk was higher in women with endometriosis overall and markedly increased in those with severe forms, a large population-based cohort study found.
The findings, published in JAMA, suggest these women may benefit from counseling on ovarian cancer risk and prevention and potentially from targeted screening, according to a group led by Mollie E. Barnard, ScD, of the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
While the absolute increase in number of cases was small, endometriosis patients overall had a more than fourfold higher risk for any type of ovarian cancer. Those with more severe forms, such as ovarian endometriomas or deep infiltrating endometriosis, had a nearly 10-fold higher risk of any type of ovarian cancer. In addition, those with more severe endometriosis had a 19-fold higher risk of type 1 (slow-growing) ovarian cancer and almost three times the risk of the more aggressive type 2.
“Given the rarity of ovarian cancer, the excess risk was relatively small, with 10-20 additional cases per 10,000 women. Nevertheless, women with endometriosis, notably the more severe subtypes, may be an important population for targeted cancer screening and prevention studies,” said corresponding author Karen C. Schliep, PhD, MSPH, associate professor in the university’s Division of Public Health.
Prior studies have shown modest associations between endometriosis and ovarian cancer, Dr. Schliep said in an interview. A 2021 systematic review and meta-analysis found endometriosis conferred nearly double the risk of ovarian cancer, although associations varied by ovarian cancer histotype. Few studies have been large enough to assess associations between endometriosis types — including superficial or peritoneal endometriosis vs ovarian endometriomas or deep infiltrating endometriosis and ovarian cancer histotypes such as low-grade serous, endometrioid, clear cell, and mucinous carcinomas (type 1), and the most aggressive and lethal form, high-grade serous type 2, she said in an interview. “Our large health administrative database of over 11 million individuals with linked electronic health and cancer registry data allowed us to answer this as yet poorly studied research question.”
Study Details
Drawing on Utah electronic health records from 1992 to 2019, the investigators matched 78,893 women with endometriosis in a 1:5 ratio to unaffected women. Cases were categorized as superficial endometriosis, ovarian endometriomas, deep infiltrating endometriosis, or other, and the types of endometriosis were matched to ovarian cancer histotypes.
The mean age of patients at first endometriosis diagnosis was 36 and the mean follow-up was 12 years. Compared with controls, endometriosis patients were more likely to be nulliparous (31% vs 24%) and to have had a hysterectomy (39% vs 6%) during follow-up.
There were 596 reported cases of ovarian cancer in the cohort. Those with incident endometriosis were 4.2 times more likely to develop ovarian cancer (95% CI, 3.59-4.91), 7.48 times more likely to develop type 1 ovarian cancer (95% CI, 5.80-9.65), and 2.70 times more likely to develop type 2 ovarian cancer (95% CI, 2.09-3.49) compared with those without endometriosis.
The magnitudes of these associations varied by endometriosis subtype. Individuals diagnosed with deep infiltrating endometriosis and/or ovarian endometriomas had 9.66 times the risk of ovarian cancer vs individuals without endometriosis (95% CI, 7.77-12.00). “Women with, compared to without, more severe endometriosis had a 19-fold higher risk of type 1 ovarian cancer, including endometrioid, clear cell, mucinous, and low-grade serous,” Dr. Schliep said, with associated risk highest for malignant subtypes such as clear cell and endometrioid carcinoma (adjusted hazard ratios, 11.15 and 7.96, respectively.
According to Dr. Schliep, physicians should encourage endometriosis patients to be aware of but not worry about ovarian cancer risk because the likelihood of developing it remains low. For their part, patients can reduce their risk of cancer through a balanced diet with low intake of alcohol, regular exercise, a healthy weight, and abstention from smoking.
Her message for researchers is as follows: “We need more studies that explore how different types of endometriosis impact different types of ovarian cancer risk. These studies will guide improved ovarian cancer screening and prevention strategies among women with severe endometriosis, with or without other important ovarian cancer risk factors such as BRCA 1/2 variations.”
An accompanying editorial called the Utah study “eloquent” and noted its distinguishing contribution of observing associations between subtypes of endometriosis with overall risk for ovarian cancer as well as histologic subtypes of epithelial ovarian cancer.
Nevertheless, Michael T. McHale, MD, of the Department of Obstetrics, Gynecology, and Reproductive Sciences at Moores Cancer Center, UC San Diego Health, University of California, expressed some methodological concerns. Although the authors attempted to control for key confounders, he noted, the dataset could not provide details on the medical management of endometriosis, such as oral contraceptives or gonadotropin-releasing hormone agonists. “Additionally, there is a possibility that women in the control cohort could have had undiagnosed endometriosis,” he wrote.
Furthermore, making clinical recommendations from these reported observations, particularly with respect to deep infiltrating endometriosis, would require a clear and consistent definition of this type in the dataset over the entire study interval from 1992 to 2019 and for the state of Utah, which the authors did not provide.
“Despite this potential challenge, the increased risk associated with deep infiltrating and/or ovarian endometriosis was clearly significant,” Dr. McHale wrote.
And although the absolute number of ovarian cancers is limited, in his view, the increased risk is sufficiently significant to advise women who have completed childbearing or have alternative fertility options to consider “more definitive surgery.”
This study was supported by multiple not-for-profit agencies, including the National Cancer Institute, the University of Utah, the National Center for Research Resources, the Utah Department of Health and Human Services, the Utah Cancer Registry, the US Centers for Disease Control and Prevention, the Huntsman Cancer Foundation, the National Institutes of Health, and Doris Duke Foundation. Dr. Barnard reported grants from the National Cancer Institute during the conduct of the study and personal fees from Epi Excellence LLC outside the submitted work. Other coauthors reported similar funding from nonprofit agencies or private research organizations. Dr Schliep disclosed no competing interests. Dr McHale reported educational consulting for Eisai Training outside the submitted work.
Ovarian cancer risk was higher in women with endometriosis overall and markedly increased in those with severe forms, a large population-based cohort study found.
The findings, published in JAMA, suggest these women may benefit from counseling on ovarian cancer risk and prevention and potentially from targeted screening, according to a group led by Mollie E. Barnard, ScD, of the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
While the absolute increase in number of cases was small, endometriosis patients overall had a more than fourfold higher risk for any type of ovarian cancer. Those with more severe forms, such as ovarian endometriomas or deep infiltrating endometriosis, had a nearly 10-fold higher risk of any type of ovarian cancer. In addition, those with more severe endometriosis had a 19-fold higher risk of type 1 (slow-growing) ovarian cancer and almost three times the risk of the more aggressive type 2.
“Given the rarity of ovarian cancer, the excess risk was relatively small, with 10-20 additional cases per 10,000 women. Nevertheless, women with endometriosis, notably the more severe subtypes, may be an important population for targeted cancer screening and prevention studies,” said corresponding author Karen C. Schliep, PhD, MSPH, associate professor in the university’s Division of Public Health.
Prior studies have shown modest associations between endometriosis and ovarian cancer, Dr. Schliep said in an interview. A 2021 systematic review and meta-analysis found endometriosis conferred nearly double the risk of ovarian cancer, although associations varied by ovarian cancer histotype. Few studies have been large enough to assess associations between endometriosis types — including superficial or peritoneal endometriosis vs ovarian endometriomas or deep infiltrating endometriosis and ovarian cancer histotypes such as low-grade serous, endometrioid, clear cell, and mucinous carcinomas (type 1), and the most aggressive and lethal form, high-grade serous type 2, she said in an interview. “Our large health administrative database of over 11 million individuals with linked electronic health and cancer registry data allowed us to answer this as yet poorly studied research question.”
Study Details
Drawing on Utah electronic health records from 1992 to 2019, the investigators matched 78,893 women with endometriosis in a 1:5 ratio to unaffected women. Cases were categorized as superficial endometriosis, ovarian endometriomas, deep infiltrating endometriosis, or other, and the types of endometriosis were matched to ovarian cancer histotypes.
The mean age of patients at first endometriosis diagnosis was 36 and the mean follow-up was 12 years. Compared with controls, endometriosis patients were more likely to be nulliparous (31% vs 24%) and to have had a hysterectomy (39% vs 6%) during follow-up.
There were 596 reported cases of ovarian cancer in the cohort. Those with incident endometriosis were 4.2 times more likely to develop ovarian cancer (95% CI, 3.59-4.91), 7.48 times more likely to develop type 1 ovarian cancer (95% CI, 5.80-9.65), and 2.70 times more likely to develop type 2 ovarian cancer (95% CI, 2.09-3.49) compared with those without endometriosis.
The magnitudes of these associations varied by endometriosis subtype. Individuals diagnosed with deep infiltrating endometriosis and/or ovarian endometriomas had 9.66 times the risk of ovarian cancer vs individuals without endometriosis (95% CI, 7.77-12.00). “Women with, compared to without, more severe endometriosis had a 19-fold higher risk of type 1 ovarian cancer, including endometrioid, clear cell, mucinous, and low-grade serous,” Dr. Schliep said, with associated risk highest for malignant subtypes such as clear cell and endometrioid carcinoma (adjusted hazard ratios, 11.15 and 7.96, respectively.
According to Dr. Schliep, physicians should encourage endometriosis patients to be aware of but not worry about ovarian cancer risk because the likelihood of developing it remains low. For their part, patients can reduce their risk of cancer through a balanced diet with low intake of alcohol, regular exercise, a healthy weight, and abstention from smoking.
Her message for researchers is as follows: “We need more studies that explore how different types of endometriosis impact different types of ovarian cancer risk. These studies will guide improved ovarian cancer screening and prevention strategies among women with severe endometriosis, with or without other important ovarian cancer risk factors such as BRCA 1/2 variations.”
An accompanying editorial called the Utah study “eloquent” and noted its distinguishing contribution of observing associations between subtypes of endometriosis with overall risk for ovarian cancer as well as histologic subtypes of epithelial ovarian cancer.
Nevertheless, Michael T. McHale, MD, of the Department of Obstetrics, Gynecology, and Reproductive Sciences at Moores Cancer Center, UC San Diego Health, University of California, expressed some methodological concerns. Although the authors attempted to control for key confounders, he noted, the dataset could not provide details on the medical management of endometriosis, such as oral contraceptives or gonadotropin-releasing hormone agonists. “Additionally, there is a possibility that women in the control cohort could have had undiagnosed endometriosis,” he wrote.
Furthermore, making clinical recommendations from these reported observations, particularly with respect to deep infiltrating endometriosis, would require a clear and consistent definition of this type in the dataset over the entire study interval from 1992 to 2019 and for the state of Utah, which the authors did not provide.
“Despite this potential challenge, the increased risk associated with deep infiltrating and/or ovarian endometriosis was clearly significant,” Dr. McHale wrote.
And although the absolute number of ovarian cancers is limited, in his view, the increased risk is sufficiently significant to advise women who have completed childbearing or have alternative fertility options to consider “more definitive surgery.”
This study was supported by multiple not-for-profit agencies, including the National Cancer Institute, the University of Utah, the National Center for Research Resources, the Utah Department of Health and Human Services, the Utah Cancer Registry, the US Centers for Disease Control and Prevention, the Huntsman Cancer Foundation, the National Institutes of Health, and Doris Duke Foundation. Dr. Barnard reported grants from the National Cancer Institute during the conduct of the study and personal fees from Epi Excellence LLC outside the submitted work. Other coauthors reported similar funding from nonprofit agencies or private research organizations. Dr Schliep disclosed no competing interests. Dr McHale reported educational consulting for Eisai Training outside the submitted work.
Ovarian cancer risk was higher in women with endometriosis overall and markedly increased in those with severe forms, a large population-based cohort study found.
The findings, published in JAMA, suggest these women may benefit from counseling on ovarian cancer risk and prevention and potentially from targeted screening, according to a group led by Mollie E. Barnard, ScD, of the Huntsman Cancer Institute at the University of Utah in Salt Lake City.
While the absolute increase in number of cases was small, endometriosis patients overall had a more than fourfold higher risk for any type of ovarian cancer. Those with more severe forms, such as ovarian endometriomas or deep infiltrating endometriosis, had a nearly 10-fold higher risk of any type of ovarian cancer. In addition, those with more severe endometriosis had a 19-fold higher risk of type 1 (slow-growing) ovarian cancer and almost three times the risk of the more aggressive type 2.
“Given the rarity of ovarian cancer, the excess risk was relatively small, with 10-20 additional cases per 10,000 women. Nevertheless, women with endometriosis, notably the more severe subtypes, may be an important population for targeted cancer screening and prevention studies,” said corresponding author Karen C. Schliep, PhD, MSPH, associate professor in the university’s Division of Public Health.
Prior studies have shown modest associations between endometriosis and ovarian cancer, Dr. Schliep said in an interview. A 2021 systematic review and meta-analysis found endometriosis conferred nearly double the risk of ovarian cancer, although associations varied by ovarian cancer histotype. Few studies have been large enough to assess associations between endometriosis types — including superficial or peritoneal endometriosis vs ovarian endometriomas or deep infiltrating endometriosis and ovarian cancer histotypes such as low-grade serous, endometrioid, clear cell, and mucinous carcinomas (type 1), and the most aggressive and lethal form, high-grade serous type 2, she said in an interview. “Our large health administrative database of over 11 million individuals with linked electronic health and cancer registry data allowed us to answer this as yet poorly studied research question.”
Study Details
Drawing on Utah electronic health records from 1992 to 2019, the investigators matched 78,893 women with endometriosis in a 1:5 ratio to unaffected women. Cases were categorized as superficial endometriosis, ovarian endometriomas, deep infiltrating endometriosis, or other, and the types of endometriosis were matched to ovarian cancer histotypes.
The mean age of patients at first endometriosis diagnosis was 36 and the mean follow-up was 12 years. Compared with controls, endometriosis patients were more likely to be nulliparous (31% vs 24%) and to have had a hysterectomy (39% vs 6%) during follow-up.
There were 596 reported cases of ovarian cancer in the cohort. Those with incident endometriosis were 4.2 times more likely to develop ovarian cancer (95% CI, 3.59-4.91), 7.48 times more likely to develop type 1 ovarian cancer (95% CI, 5.80-9.65), and 2.70 times more likely to develop type 2 ovarian cancer (95% CI, 2.09-3.49) compared with those without endometriosis.
The magnitudes of these associations varied by endometriosis subtype. Individuals diagnosed with deep infiltrating endometriosis and/or ovarian endometriomas had 9.66 times the risk of ovarian cancer vs individuals without endometriosis (95% CI, 7.77-12.00). “Women with, compared to without, more severe endometriosis had a 19-fold higher risk of type 1 ovarian cancer, including endometrioid, clear cell, mucinous, and low-grade serous,” Dr. Schliep said, with associated risk highest for malignant subtypes such as clear cell and endometrioid carcinoma (adjusted hazard ratios, 11.15 and 7.96, respectively.
According to Dr. Schliep, physicians should encourage endometriosis patients to be aware of but not worry about ovarian cancer risk because the likelihood of developing it remains low. For their part, patients can reduce their risk of cancer through a balanced diet with low intake of alcohol, regular exercise, a healthy weight, and abstention from smoking.
Her message for researchers is as follows: “We need more studies that explore how different types of endometriosis impact different types of ovarian cancer risk. These studies will guide improved ovarian cancer screening and prevention strategies among women with severe endometriosis, with or without other important ovarian cancer risk factors such as BRCA 1/2 variations.”
An accompanying editorial called the Utah study “eloquent” and noted its distinguishing contribution of observing associations between subtypes of endometriosis with overall risk for ovarian cancer as well as histologic subtypes of epithelial ovarian cancer.
Nevertheless, Michael T. McHale, MD, of the Department of Obstetrics, Gynecology, and Reproductive Sciences at Moores Cancer Center, UC San Diego Health, University of California, expressed some methodological concerns. Although the authors attempted to control for key confounders, he noted, the dataset could not provide details on the medical management of endometriosis, such as oral contraceptives or gonadotropin-releasing hormone agonists. “Additionally, there is a possibility that women in the control cohort could have had undiagnosed endometriosis,” he wrote.
Furthermore, making clinical recommendations from these reported observations, particularly with respect to deep infiltrating endometriosis, would require a clear and consistent definition of this type in the dataset over the entire study interval from 1992 to 2019 and for the state of Utah, which the authors did not provide.
“Despite this potential challenge, the increased risk associated with deep infiltrating and/or ovarian endometriosis was clearly significant,” Dr. McHale wrote.
And although the absolute number of ovarian cancers is limited, in his view, the increased risk is sufficiently significant to advise women who have completed childbearing or have alternative fertility options to consider “more definitive surgery.”
This study was supported by multiple not-for-profit agencies, including the National Cancer Institute, the University of Utah, the National Center for Research Resources, the Utah Department of Health and Human Services, the Utah Cancer Registry, the US Centers for Disease Control and Prevention, the Huntsman Cancer Foundation, the National Institutes of Health, and Doris Duke Foundation. Dr. Barnard reported grants from the National Cancer Institute during the conduct of the study and personal fees from Epi Excellence LLC outside the submitted work. Other coauthors reported similar funding from nonprofit agencies or private research organizations. Dr Schliep disclosed no competing interests. Dr McHale reported educational consulting for Eisai Training outside the submitted work.
FROM JAMA
Benefit of Massage Therapy for Pain Unclear
The effectiveness of massage therapy for a range of painful adult health conditions remains uncertain. Despite hundreds of randomized clinical trials and dozens of systematic reviews, few studies have offered conclusions based on more than low-certainty evidence, a systematic review in JAMA Network Open has shown (doi: 10.1001/jamanetworkopen.2024.22259).
Some moderate-certainty evidence, however, suggested massage therapy may alleviate pain related to such conditions as low-back problems, labor, and breast cancer surgery, concluded a group led by Selene Mak, PhD, MPH, program manager in the Evidence Synthesis Program at the Veterans Health Administration Greater Los Angeles Healthcare System in Los Angeles, California.
“More high-quality randomized clinical trials are needed to provide a stronger evidence base to assess the effect of massage therapy on pain,” Dr. Mak and colleagues wrote.
The review updates a previous Veterans Affairs evidence map covering reviews of massage therapy for pain published through 2018.
To categorize the evidence base for decision-making by policymakers and practitioners, the VA requested an updated evidence map of reviews to answer the question: “What is the certainty of evidence in systematic reviews of massage therapy for pain?”
The Analysis
The current review included studies published from 2018 to 2023 with formal ratings of evidence quality or certainty, excluding other nonpharmacologic techniques such as sports massage therapy, osteopathy, dry cupping, dry needling, and internal massage therapy, and self-administered techniques such as foam rolling.
Of 129 systematic reviews, only 41 formally rated evidence quality, and 17 were evidence-mapped for pain across 13 health states: cancer, back, neck and mechanical neck issues, fibromyalgia, labor, myofascial, palliative care need, plantar fasciitis, postoperative, post breast cancer surgery, and post cesarean/postpartum.
The investigators found no conclusions based on a high certainty of evidence, while seven based conclusions on moderate-certainty evidence. All remaining conclusions were rated as having low- or very-low-certainty evidence.
The priority, they added, should be studies comparing massage therapy with other recommended, accepted, and active therapies for pain and should have sufficiently long follow-up to allow any nonspecific outcomes to dissipate, At least 6 months’ follow-up has been suggested for studies of chronic pain.
While massage therapy is considered safe, in patients with central sensitizations more aggressive treatments may cause a flare of myofascial pain.
This study was funded by the Department of Veterans Affairs Health Services Research and Development. The authors had no conflicts of interest to disclose.
The effectiveness of massage therapy for a range of painful adult health conditions remains uncertain. Despite hundreds of randomized clinical trials and dozens of systematic reviews, few studies have offered conclusions based on more than low-certainty evidence, a systematic review in JAMA Network Open has shown (doi: 10.1001/jamanetworkopen.2024.22259).
Some moderate-certainty evidence, however, suggested massage therapy may alleviate pain related to such conditions as low-back problems, labor, and breast cancer surgery, concluded a group led by Selene Mak, PhD, MPH, program manager in the Evidence Synthesis Program at the Veterans Health Administration Greater Los Angeles Healthcare System in Los Angeles, California.
“More high-quality randomized clinical trials are needed to provide a stronger evidence base to assess the effect of massage therapy on pain,” Dr. Mak and colleagues wrote.
The review updates a previous Veterans Affairs evidence map covering reviews of massage therapy for pain published through 2018.
To categorize the evidence base for decision-making by policymakers and practitioners, the VA requested an updated evidence map of reviews to answer the question: “What is the certainty of evidence in systematic reviews of massage therapy for pain?”
The Analysis
The current review included studies published from 2018 to 2023 with formal ratings of evidence quality or certainty, excluding other nonpharmacologic techniques such as sports massage therapy, osteopathy, dry cupping, dry needling, and internal massage therapy, and self-administered techniques such as foam rolling.
Of 129 systematic reviews, only 41 formally rated evidence quality, and 17 were evidence-mapped for pain across 13 health states: cancer, back, neck and mechanical neck issues, fibromyalgia, labor, myofascial, palliative care need, plantar fasciitis, postoperative, post breast cancer surgery, and post cesarean/postpartum.
The investigators found no conclusions based on a high certainty of evidence, while seven based conclusions on moderate-certainty evidence. All remaining conclusions were rated as having low- or very-low-certainty evidence.
The priority, they added, should be studies comparing massage therapy with other recommended, accepted, and active therapies for pain and should have sufficiently long follow-up to allow any nonspecific outcomes to dissipate, At least 6 months’ follow-up has been suggested for studies of chronic pain.
While massage therapy is considered safe, in patients with central sensitizations more aggressive treatments may cause a flare of myofascial pain.
This study was funded by the Department of Veterans Affairs Health Services Research and Development. The authors had no conflicts of interest to disclose.
The effectiveness of massage therapy for a range of painful adult health conditions remains uncertain. Despite hundreds of randomized clinical trials and dozens of systematic reviews, few studies have offered conclusions based on more than low-certainty evidence, a systematic review in JAMA Network Open has shown (doi: 10.1001/jamanetworkopen.2024.22259).
Some moderate-certainty evidence, however, suggested massage therapy may alleviate pain related to such conditions as low-back problems, labor, and breast cancer surgery, concluded a group led by Selene Mak, PhD, MPH, program manager in the Evidence Synthesis Program at the Veterans Health Administration Greater Los Angeles Healthcare System in Los Angeles, California.
“More high-quality randomized clinical trials are needed to provide a stronger evidence base to assess the effect of massage therapy on pain,” Dr. Mak and colleagues wrote.
The review updates a previous Veterans Affairs evidence map covering reviews of massage therapy for pain published through 2018.
To categorize the evidence base for decision-making by policymakers and practitioners, the VA requested an updated evidence map of reviews to answer the question: “What is the certainty of evidence in systematic reviews of massage therapy for pain?”
The Analysis
The current review included studies published from 2018 to 2023 with formal ratings of evidence quality or certainty, excluding other nonpharmacologic techniques such as sports massage therapy, osteopathy, dry cupping, dry needling, and internal massage therapy, and self-administered techniques such as foam rolling.
Of 129 systematic reviews, only 41 formally rated evidence quality, and 17 were evidence-mapped for pain across 13 health states: cancer, back, neck and mechanical neck issues, fibromyalgia, labor, myofascial, palliative care need, plantar fasciitis, postoperative, post breast cancer surgery, and post cesarean/postpartum.
The investigators found no conclusions based on a high certainty of evidence, while seven based conclusions on moderate-certainty evidence. All remaining conclusions were rated as having low- or very-low-certainty evidence.
The priority, they added, should be studies comparing massage therapy with other recommended, accepted, and active therapies for pain and should have sufficiently long follow-up to allow any nonspecific outcomes to dissipate, At least 6 months’ follow-up has been suggested for studies of chronic pain.
While massage therapy is considered safe, in patients with central sensitizations more aggressive treatments may cause a flare of myofascial pain.
This study was funded by the Department of Veterans Affairs Health Services Research and Development. The authors had no conflicts of interest to disclose.
FROM JAMA NETWORK OPEN
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Introduction: Imaging for Endometriosis — A Necessary Prerequisite
While the gold standard in the diagnosis of endometriosis remains laparoscopy, it is now recognized that thorough evaluation via ultrasound offers an acceptable, less expensive, and less invasive alternative. It is especially useful for the diagnosis of deep infiltrative disease, which penetrates more than 5 mm into the peritoneum, ovarian endometrioma, and when anatomic distortion occurs, such as to the path of the ureter.
Besides establishing the diagnosis, ultrasound imaging has become, along with MRI, the most important aid for proper preoperative planning. Not only does imaging provide the surgeon and patient with knowledge regarding the extent of the upcoming procedure, but it also allows the minimally invasive gynecologic (MIG) surgeon to involve colleagues, such as colorectal surgeons or urologists. For example, deep infiltrative endometriosis penetrating into the bowel mucosa will require a discoid or segmental bowel resection.
While many endometriosis experts rely on MRI, many MIG surgeons are dependent on ultrasound. I would not consider taking a patient with signs and symptoms suggestive of endometriosis to surgery without 2D/3D transvaginal ultrasound. If the patient possesses a uterus, a saline-infused sonogram is performed to potentially diagnose adenomyosis.
It is a pleasure and honor to welcome Professor Caterina Exacoustos MD, PhD, associate professor of ob.gyn. at the University of Rome “Tor Vergata,” to this edition of the Master Class in Gynecologic Surgery to discuss “Ultrasound and Its Role in the Diagnosis of and Management of Endometriosis, Including DIE.”
Prof. Exacoustos’ main areas of interest are endometriosis and benign diseases including uterine pathology and infertility. Her extensive body of work comprises over 120 scientific publications and numerous book chapters both in English and in Italian.
Prof. Exacoustos continues to be one of the most well respected lecturers speaking about ultrasound throughout the world.
Dr. Miller is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. Dr. Miller has no conflicts of interest to report.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Endometriosis affects approximately 10%-20% of premenopausal women worldwide. It is the leading cause of chronic pelvic pain, is often associated with infertility, and has a significant impact on quality of life. Although the natural history of endometriosis remains unknown, emerging evidence suggests that the pathophysiological steps of initiation and development of endometriosis must occur earlier in the lifespan. Most notably, the onset of endometriosis-associated pain symptoms is often reported during adolescence and young adulthood.1
While many patients with endometriosis are referred with dysmenorrhea at a young age, at age ≤ 25 years,2 symptoms are often highly underestimated and considered to be normal and transient.3,4 Clinical and pelvic exams are often negative in young women, and delays in endometriosis diagnosis are well known.
The presentation of primary dysmenorrhea with no anatomical cause embodies the paradigm that dysmenorrhea in adolescents is most often an insignificant disorder. This perspective is probably a root cause of delayed endometriosis diagnosis in young patients. However, another issue behind delayed diagnosis is the reluctance of the physician to perform a diagnostic laparoscopy — historically the gold standard for diagnosing endometriosis — for seemingly common symptoms such as dysmenorrhea in young patients.
Today we know that there are typical aspects of ultrasound imaging that identify endometriosis in the pelvis, and notably, the 2022 European Society for Human Reproduction and Embryology (ESHRE) endometriosis guideline5 recognizes imaging (ultrasound or MRI) as the standard for endometriosis diagnosis without requiring laparoscopic or histological confirmation.
An early and noninvasive method of diagnosis aids in timely diagnosis and provides for the timely initiation of medical management to improve quality of life and prevent progression of disease (Figure 1).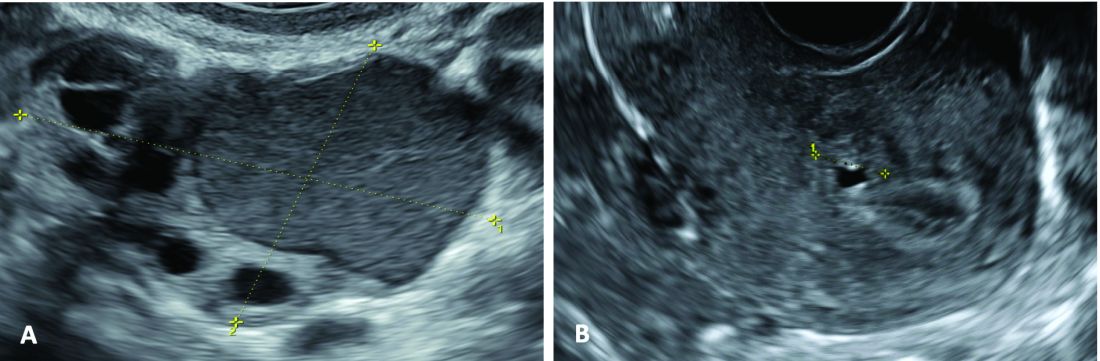
(A. Transvaginal ultrasound appearance of a small ovarian endometrioma in a 16-year-old girl. Note the unilocular cyst with ground glass echogenicity surrounded by multifollicular ovarian tissue. B. Ultrasound image of a retroverted uterus of an 18-year-old girl with focal adenomyosis of the posterior wall. Note the round cystic anechoic areas in the inner myometrium or junctional zone. The small intra-myometrial cyst is surrounded by a hyperechoic ring).
Indeed, the typical appearance of endometriotic pelvic lesions on transvaginal sonography, such as endometriomas and rectal deep infiltrating endometriosis (DIE) — as well as adenomyosis – can be medically treated without histologic confirmation .
When surgery is advisable, ultrasound findings also play a valuable role in presurgical staging, planning, and counseling for patients of all ages. Determining the extent and location of DIE preoperatively, for instance, facilitates the engagement of the appropriate surgical specialists so that multiple surgeries can be avoided. It also enables patients to be optimally informed before surgery of possible outcomes and complications.
Moreover, in the context of infertility, ultrasound can be a valuable tool for understanding uterine pathology and assessing for adenomyosis so that affected patients may be treated surgically or medically before turning to assisted reproductive technology.
Uniformity, Standardization in the Sonographic Assessment
In Europe, as in the United States, transvaginal sonography (TVS) is the first-line imaging tool for the diagnosis and management of endometriosis. In Europe, many ob.gyns. perform ultrasound themselves, as do treating surgeons. When diagnostic findings are negative but clinical suspicion is high, MRI is often utilized. Laparoscopy may then be considered in patients with negative imaging results.
Efforts to standardize terms, definitions, measurements, and sonographic features of different types of endometriosis have been made to make it easier for physicians to share data and communicate with each other. A lack of uniformity has contributed to variability in the reported diagnostic accuracy of TVS.
About 10 years ago, in one such effort, we assessed the accuracy of TVS for DIE by comparing TVS results with laparoscopic/histologic findings, and developed an ultrasound mapping system to accurately record the location, size and depth of lesions visualized by TVS. The accuracy of TVS ranged from 76% for the diagnosis of vaginal endometriosis to 97% for the diagnosis of bladder lesions and posterior cul-de-sac obliteration. Accuracy was 93% and 91% for detecting ureteral involvement (right and left); 87% for uterosacral ligament endometriotic lesions; and 87% for parametrial involvement.6
Shortly after, with a focus on DIE, expert sonographers and physician-sonographers from across Europe — as well as some experts from Australia, Japan, Brazil, Chile, and the United States (Y. Osuga from Brigham and Women’s Hospital and Harvard Medical School) — came together to agree on a uniform approach to the sonographic evaluation for suspected endometriosis and a standardization of terminology.
The consensus opinion from the International Deep Endometriosis Analysis (IDEA) group details four steps for examining women with suspected DIE: 1) Evaluation of the uterus and adnexa, 2) evaluation of transvaginal sonographic “soft markers” (ie. site-specific tenderness and ovarian mobility), 3) assessment of the status of the posterior cul-de-sac using real-time ultrasound-based “sliding sign,” and 4) assessment for DIE nodules in the anterior and posterior compartments.7
Our paper describing a mapping system and the IDEA paper describe how to detect deep endometriosis in the pelvis by utilizing an ultrasound view of normal anatomy and pelvic organ structure to provide landmarks for accurately defining the site of DIE lesions (Figure 2).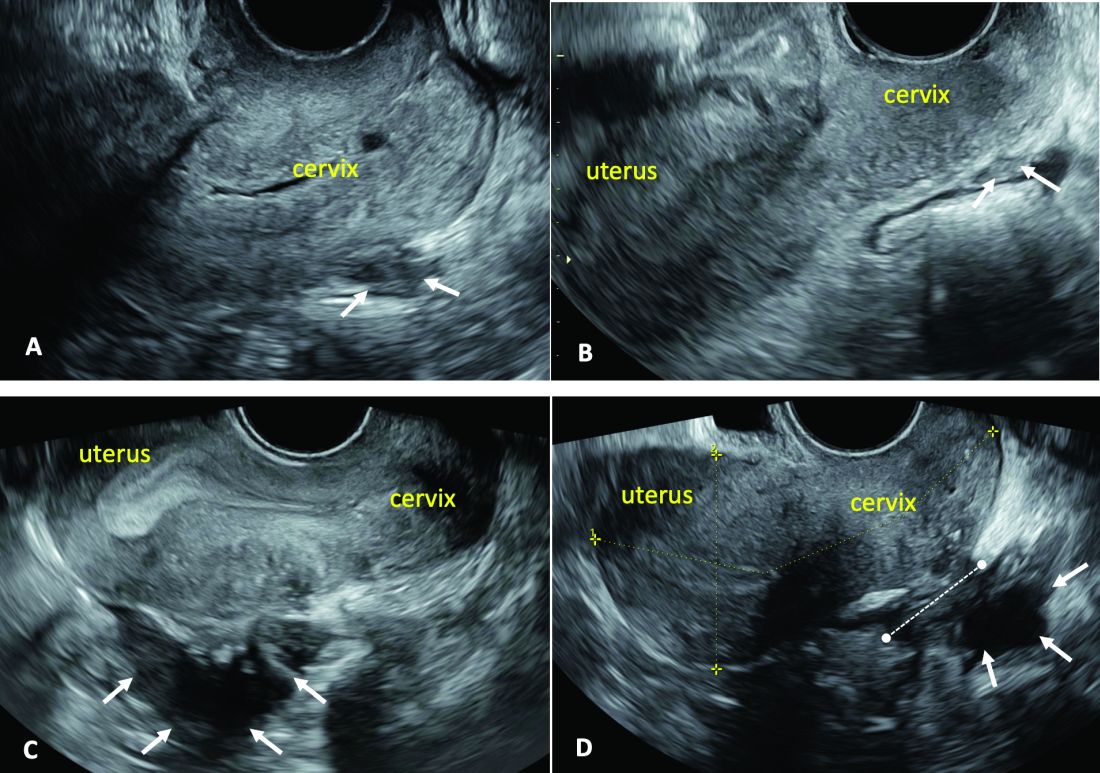
(A. Ultrasound appearance of a small DIE lesion of the retrocervical area [white arrows], which involved the torus uterinum and the right uterosacral ligament [USL]. The lesion appears as hypoechoic tissue with irregular margins caused by the fibrosis induced by the DIE. B. TVS appearance of small nodules of DIE of the left USL. Note the small retrocervical DIE lesion [white arrows], which appears hypoechoic due to the infiltration of the hyperechoic USL. C) Ultrasound appearance of a DIE nodule of the recto-sigmoid wall. Note the hypoechoic thickening of the muscular layers of the bowel wall attached to the corpus of the uterus and the adenomyosis of the posterior wall. The retrocervical area is free. D. TVS appearance of nodules of DIE of the lower rectal wall. Note the hypoechoic lesion [white arrows] of the rectum is attached to a retrocervical DIE fibrosis of the torus and USL [white dotted line]).
So-called rectovaginal endometriosis can be well assessed, for instance, since the involvement of the rectum, sigmoid colon, vaginal wall, rectovaginal septum, and posterior cul-de-sac uterosacral ligament can be seen by ultrasound as a single structure, making the location, size, and depth of any lesions discernible.
Again, this evaluation of the extent of disease is important for presurgical assessment so the surgeon can organize the right team and time of surgery and so the patient can be counseled on the advantages and possible complications of the treatment.
Notably, an accurate ultrasound description of pelvic endometriosis is helpful for accurate classification of disease. Endometriosis classification systems such as that of the American Association of Gynecologic Laparoscopists (AAGL)8 and the American Society of Reproductive Medicine (ASRM),9 as well as the #Enzian surgical description system,10 have been adapted to cover findings from ultrasound as well as MRI imaging.
A Systematic Evaluation
In keeping with the IDEA consensus opinion and based on our years of experience at the University of Rome, I advise that patients with typical pain symptoms of endometriosis or infertility undergo an accurate sonographic assessment of the pelvis with particular evaluation not only of the uterus and ovaries but of all pelvic retroperitoneal spaces.
The TVS examination should start with a slightly filled bladder, which permits a better evaluation of the bladder walls and the presence of endometriotic nodules. These nodules appear as hyperechoic linear or spherical lesions bulging toward the lumen and involving the serosa, muscularis, or (sub)mucosa of the bladder.
Then, an accurate evaluation of the uterus in 2D and 3D permits the diagnosis of adenomyosis. 3D sonographic evaluation of the myometrium and of the junctional zone are important; alteration and infiltration of the junctional zone and the presence of small adenomyotic cysts in the inner or outer myometrium are direct, specific signs of adenomyosis and should be ruled out in patients with dysmenorrhea, heavy menstrual bleeding, infertility, and pregnancy complications.
Endometriomas of the ovaries can be easily detected as having the typical appearance of a cyst with ground glass content. Adhesions of the ovaries and the uterus also should be evaluated with a dynamic ultrasound approach that utilizes the sliding sign and mobilization by palpation of the organs during the TVS scan.
Finally, the posterior and lateral retroperitoneal compartments should be carefully evaluated, with symptoms guiding the TVS examination whenever possible. Deep endometriotic nodules of the rectum appear as hypoechoic lesions or linear or nodular retroperitoneal thickening with irregular borders, penetrating into the intestinal wall and distorting its normal structure. In young patients, it seems very important to assess for small lesions below the peritoneum between the vagina and rectum, and in the parametria and around the ureter and nerves — lesions that, notably, would not be seen by diagnostic laparoscopy.
The Evaluation of Young Patients
In adolescent and young patients, endometriosis and adenomyosis are often present with small lesions and shallow tissue invasion, making a very careful and experienced approach to ultrasound essential for detection. Endometriomas are often of small diameter, and DIE is not always easily diagnosed because retroperitoneal lesions are similarly very small.
In a series of 270 adolescents (ages 12-20) who were referred to our outpatient gynecologic ultrasound unit over a 5-year period for various indications, at least one ultrasound feature of endometriosis was observed in 13.3%. In those with dysmenorrhea, the detection of endometriosis increased to 21%. Endometrioma was the most common type of endometriosis we found in the study, but DIE and adenomyosis were found in 4%-11%.
Although endometriotic lesions typically are small in young patients, they are often associated with severe pain symptoms, including chronic pelvic pain, dysmenorrhea, dyspareunia, dysuria, and dyschezia, all of which can have a serious effect on the quality of life of these young women. These symptoms keep them away from school during menstruation, away from sports, and cause painful intercourse and infertility. In young patients, an accurate TVS can provide a lot of information, and the ability to detect retroperitoneal endometriotic lesions and adenomyosis is probably better than with purely diagnostic laparoscopy, which would evaluate only superficial lesions.
TVS or, when needed, transrectal ultrasound, can enable adequate treatment and follow-up of the disease and its symptoms. There are no guidelines recommending adequate follow-up times to evaluate the effectiveness of medical therapy in patients with ultrasound signs of endometriosis. (Likewise, there are no indications for follow-up in patients with severe dysmenorrhea without ultrasound signs of endometriosis.) Certainly, our studies suggest careful evaluation over time of young patients with severe dysmenorrhea by serial ultrasound scans. With such follow-up, disease progress can be monitored and the medical or surgical treatment approach modified if needed.
The diagnosis of endometriosis at a young age has significant benefits not only in avoiding or reducing progression of the disease, but also in improving quality of life and aiding women in their desire for pregnancy.
Dr. Exacoustos is associate professor of ob.gyn. at the University of Rome “Tor Vergata.” She has no conflicts of interest to report.
References
1. Zondervan KT et al. N Engl J Med. 2020;382:1244-56.
2. Greene R et al. Fertil Steril. 2009;91:32-9.
3. Chapron C et al. J Pediatr Adolesc Gynecol. 2011;24:S7-12.
4. Randhawa AE et al. J Pediatr Adolesc Gynecol. 2021;34:643-8.
5. Becker CM et al. Hum Reprod Open. 2022(2):hoac009.
6. Exacoustos C et al. Fertil Steril. 2014;102:143-9. 7. Guerriero S et al. Ultrasound Obstet Gynecol. 2016;48(3):318-32.
8. Abrao MS et al. J Minim Invasive Gynecol. 2021;28:1941-50.9. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817-21. 10. Keckstein J et al. Acta Obstet Gynecol Scand. 2021;100:1165-75.
11. Martire FG et al. Fertil Steril. 2020;114(5):1049-57.
Introduction: Imaging for Endometriosis — A Necessary Prerequisite
While the gold standard in the diagnosis of endometriosis remains laparoscopy, it is now recognized that thorough evaluation via ultrasound offers an acceptable, less expensive, and less invasive alternative. It is especially useful for the diagnosis of deep infiltrative disease, which penetrates more than 5 mm into the peritoneum, ovarian endometrioma, and when anatomic distortion occurs, such as to the path of the ureter.
Besides establishing the diagnosis, ultrasound imaging has become, along with MRI, the most important aid for proper preoperative planning. Not only does imaging provide the surgeon and patient with knowledge regarding the extent of the upcoming procedure, but it also allows the minimally invasive gynecologic (MIG) surgeon to involve colleagues, such as colorectal surgeons or urologists. For example, deep infiltrative endometriosis penetrating into the bowel mucosa will require a discoid or segmental bowel resection.
While many endometriosis experts rely on MRI, many MIG surgeons are dependent on ultrasound. I would not consider taking a patient with signs and symptoms suggestive of endometriosis to surgery without 2D/3D transvaginal ultrasound. If the patient possesses a uterus, a saline-infused sonogram is performed to potentially diagnose adenomyosis.
It is a pleasure and honor to welcome Professor Caterina Exacoustos MD, PhD, associate professor of ob.gyn. at the University of Rome “Tor Vergata,” to this edition of the Master Class in Gynecologic Surgery to discuss “Ultrasound and Its Role in the Diagnosis of and Management of Endometriosis, Including DIE.”
Prof. Exacoustos’ main areas of interest are endometriosis and benign diseases including uterine pathology and infertility. Her extensive body of work comprises over 120 scientific publications and numerous book chapters both in English and in Italian.
Prof. Exacoustos continues to be one of the most well respected lecturers speaking about ultrasound throughout the world.
Dr. Miller is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. Dr. Miller has no conflicts of interest to report.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Endometriosis affects approximately 10%-20% of premenopausal women worldwide. It is the leading cause of chronic pelvic pain, is often associated with infertility, and has a significant impact on quality of life. Although the natural history of endometriosis remains unknown, emerging evidence suggests that the pathophysiological steps of initiation and development of endometriosis must occur earlier in the lifespan. Most notably, the onset of endometriosis-associated pain symptoms is often reported during adolescence and young adulthood.1
While many patients with endometriosis are referred with dysmenorrhea at a young age, at age ≤ 25 years,2 symptoms are often highly underestimated and considered to be normal and transient.3,4 Clinical and pelvic exams are often negative in young women, and delays in endometriosis diagnosis are well known.
The presentation of primary dysmenorrhea with no anatomical cause embodies the paradigm that dysmenorrhea in adolescents is most often an insignificant disorder. This perspective is probably a root cause of delayed endometriosis diagnosis in young patients. However, another issue behind delayed diagnosis is the reluctance of the physician to perform a diagnostic laparoscopy — historically the gold standard for diagnosing endometriosis — for seemingly common symptoms such as dysmenorrhea in young patients.
Today we know that there are typical aspects of ultrasound imaging that identify endometriosis in the pelvis, and notably, the 2022 European Society for Human Reproduction and Embryology (ESHRE) endometriosis guideline5 recognizes imaging (ultrasound or MRI) as the standard for endometriosis diagnosis without requiring laparoscopic or histological confirmation.
An early and noninvasive method of diagnosis aids in timely diagnosis and provides for the timely initiation of medical management to improve quality of life and prevent progression of disease (Figure 1).
(A. Transvaginal ultrasound appearance of a small ovarian endometrioma in a 16-year-old girl. Note the unilocular cyst with ground glass echogenicity surrounded by multifollicular ovarian tissue. B. Ultrasound image of a retroverted uterus of an 18-year-old girl with focal adenomyosis of the posterior wall. Note the round cystic anechoic areas in the inner myometrium or junctional zone. The small intra-myometrial cyst is surrounded by a hyperechoic ring).
Indeed, the typical appearance of endometriotic pelvic lesions on transvaginal sonography, such as endometriomas and rectal deep infiltrating endometriosis (DIE) — as well as adenomyosis – can be medically treated without histologic confirmation .
When surgery is advisable, ultrasound findings also play a valuable role in presurgical staging, planning, and counseling for patients of all ages. Determining the extent and location of DIE preoperatively, for instance, facilitates the engagement of the appropriate surgical specialists so that multiple surgeries can be avoided. It also enables patients to be optimally informed before surgery of possible outcomes and complications.
Moreover, in the context of infertility, ultrasound can be a valuable tool for understanding uterine pathology and assessing for adenomyosis so that affected patients may be treated surgically or medically before turning to assisted reproductive technology.
Uniformity, Standardization in the Sonographic Assessment
In Europe, as in the United States, transvaginal sonography (TVS) is the first-line imaging tool for the diagnosis and management of endometriosis. In Europe, many ob.gyns. perform ultrasound themselves, as do treating surgeons. When diagnostic findings are negative but clinical suspicion is high, MRI is often utilized. Laparoscopy may then be considered in patients with negative imaging results.
Efforts to standardize terms, definitions, measurements, and sonographic features of different types of endometriosis have been made to make it easier for physicians to share data and communicate with each other. A lack of uniformity has contributed to variability in the reported diagnostic accuracy of TVS.
About 10 years ago, in one such effort, we assessed the accuracy of TVS for DIE by comparing TVS results with laparoscopic/histologic findings, and developed an ultrasound mapping system to accurately record the location, size and depth of lesions visualized by TVS. The accuracy of TVS ranged from 76% for the diagnosis of vaginal endometriosis to 97% for the diagnosis of bladder lesions and posterior cul-de-sac obliteration. Accuracy was 93% and 91% for detecting ureteral involvement (right and left); 87% for uterosacral ligament endometriotic lesions; and 87% for parametrial involvement.6
Shortly after, with a focus on DIE, expert sonographers and physician-sonographers from across Europe — as well as some experts from Australia, Japan, Brazil, Chile, and the United States (Y. Osuga from Brigham and Women’s Hospital and Harvard Medical School) — came together to agree on a uniform approach to the sonographic evaluation for suspected endometriosis and a standardization of terminology.
The consensus opinion from the International Deep Endometriosis Analysis (IDEA) group details four steps for examining women with suspected DIE: 1) Evaluation of the uterus and adnexa, 2) evaluation of transvaginal sonographic “soft markers” (ie. site-specific tenderness and ovarian mobility), 3) assessment of the status of the posterior cul-de-sac using real-time ultrasound-based “sliding sign,” and 4) assessment for DIE nodules in the anterior and posterior compartments.7
Our paper describing a mapping system and the IDEA paper describe how to detect deep endometriosis in the pelvis by utilizing an ultrasound view of normal anatomy and pelvic organ structure to provide landmarks for accurately defining the site of DIE lesions (Figure 2).
(A. Ultrasound appearance of a small DIE lesion of the retrocervical area [white arrows], which involved the torus uterinum and the right uterosacral ligament [USL]. The lesion appears as hypoechoic tissue with irregular margins caused by the fibrosis induced by the DIE. B. TVS appearance of small nodules of DIE of the left USL. Note the small retrocervical DIE lesion [white arrows], which appears hypoechoic due to the infiltration of the hyperechoic USL. C) Ultrasound appearance of a DIE nodule of the recto-sigmoid wall. Note the hypoechoic thickening of the muscular layers of the bowel wall attached to the corpus of the uterus and the adenomyosis of the posterior wall. The retrocervical area is free. D. TVS appearance of nodules of DIE of the lower rectal wall. Note the hypoechoic lesion [white arrows] of the rectum is attached to a retrocervical DIE fibrosis of the torus and USL [white dotted line]).
So-called rectovaginal endometriosis can be well assessed, for instance, since the involvement of the rectum, sigmoid colon, vaginal wall, rectovaginal septum, and posterior cul-de-sac uterosacral ligament can be seen by ultrasound as a single structure, making the location, size, and depth of any lesions discernible.
Again, this evaluation of the extent of disease is important for presurgical assessment so the surgeon can organize the right team and time of surgery and so the patient can be counseled on the advantages and possible complications of the treatment.
Notably, an accurate ultrasound description of pelvic endometriosis is helpful for accurate classification of disease. Endometriosis classification systems such as that of the American Association of Gynecologic Laparoscopists (AAGL)8 and the American Society of Reproductive Medicine (ASRM),9 as well as the #Enzian surgical description system,10 have been adapted to cover findings from ultrasound as well as MRI imaging.
A Systematic Evaluation
In keeping with the IDEA consensus opinion and based on our years of experience at the University of Rome, I advise that patients with typical pain symptoms of endometriosis or infertility undergo an accurate sonographic assessment of the pelvis with particular evaluation not only of the uterus and ovaries but of all pelvic retroperitoneal spaces.
The TVS examination should start with a slightly filled bladder, which permits a better evaluation of the bladder walls and the presence of endometriotic nodules. These nodules appear as hyperechoic linear or spherical lesions bulging toward the lumen and involving the serosa, muscularis, or (sub)mucosa of the bladder.
Then, an accurate evaluation of the uterus in 2D and 3D permits the diagnosis of adenomyosis. 3D sonographic evaluation of the myometrium and of the junctional zone are important; alteration and infiltration of the junctional zone and the presence of small adenomyotic cysts in the inner or outer myometrium are direct, specific signs of adenomyosis and should be ruled out in patients with dysmenorrhea, heavy menstrual bleeding, infertility, and pregnancy complications.
Endometriomas of the ovaries can be easily detected as having the typical appearance of a cyst with ground glass content. Adhesions of the ovaries and the uterus also should be evaluated with a dynamic ultrasound approach that utilizes the sliding sign and mobilization by palpation of the organs during the TVS scan.
Finally, the posterior and lateral retroperitoneal compartments should be carefully evaluated, with symptoms guiding the TVS examination whenever possible. Deep endometriotic nodules of the rectum appear as hypoechoic lesions or linear or nodular retroperitoneal thickening with irregular borders, penetrating into the intestinal wall and distorting its normal structure. In young patients, it seems very important to assess for small lesions below the peritoneum between the vagina and rectum, and in the parametria and around the ureter and nerves — lesions that, notably, would not be seen by diagnostic laparoscopy.
The Evaluation of Young Patients
In adolescent and young patients, endometriosis and adenomyosis are often present with small lesions and shallow tissue invasion, making a very careful and experienced approach to ultrasound essential for detection. Endometriomas are often of small diameter, and DIE is not always easily diagnosed because retroperitoneal lesions are similarly very small.
In a series of 270 adolescents (ages 12-20) who were referred to our outpatient gynecologic ultrasound unit over a 5-year period for various indications, at least one ultrasound feature of endometriosis was observed in 13.3%. In those with dysmenorrhea, the detection of endometriosis increased to 21%. Endometrioma was the most common type of endometriosis we found in the study, but DIE and adenomyosis were found in 4%-11%.
Although endometriotic lesions typically are small in young patients, they are often associated with severe pain symptoms, including chronic pelvic pain, dysmenorrhea, dyspareunia, dysuria, and dyschezia, all of which can have a serious effect on the quality of life of these young women. These symptoms keep them away from school during menstruation, away from sports, and cause painful intercourse and infertility. In young patients, an accurate TVS can provide a lot of information, and the ability to detect retroperitoneal endometriotic lesions and adenomyosis is probably better than with purely diagnostic laparoscopy, which would evaluate only superficial lesions.
TVS or, when needed, transrectal ultrasound, can enable adequate treatment and follow-up of the disease and its symptoms. There are no guidelines recommending adequate follow-up times to evaluate the effectiveness of medical therapy in patients with ultrasound signs of endometriosis. (Likewise, there are no indications for follow-up in patients with severe dysmenorrhea without ultrasound signs of endometriosis.) Certainly, our studies suggest careful evaluation over time of young patients with severe dysmenorrhea by serial ultrasound scans. With such follow-up, disease progress can be monitored and the medical or surgical treatment approach modified if needed.
The diagnosis of endometriosis at a young age has significant benefits not only in avoiding or reducing progression of the disease, but also in improving quality of life and aiding women in their desire for pregnancy.
Dr. Exacoustos is associate professor of ob.gyn. at the University of Rome “Tor Vergata.” She has no conflicts of interest to report.
References
1. Zondervan KT et al. N Engl J Med. 2020;382:1244-56.
2. Greene R et al. Fertil Steril. 2009;91:32-9.
3. Chapron C et al. J Pediatr Adolesc Gynecol. 2011;24:S7-12.
4. Randhawa AE et al. J Pediatr Adolesc Gynecol. 2021;34:643-8.
5. Becker CM et al. Hum Reprod Open. 2022(2):hoac009.
6. Exacoustos C et al. Fertil Steril. 2014;102:143-9. 7. Guerriero S et al. Ultrasound Obstet Gynecol. 2016;48(3):318-32.
8. Abrao MS et al. J Minim Invasive Gynecol. 2021;28:1941-50.9. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817-21. 10. Keckstein J et al. Acta Obstet Gynecol Scand. 2021;100:1165-75.
11. Martire FG et al. Fertil Steril. 2020;114(5):1049-57.
Introduction: Imaging for Endometriosis — A Necessary Prerequisite
While the gold standard in the diagnosis of endometriosis remains laparoscopy, it is now recognized that thorough evaluation via ultrasound offers an acceptable, less expensive, and less invasive alternative. It is especially useful for the diagnosis of deep infiltrative disease, which penetrates more than 5 mm into the peritoneum, ovarian endometrioma, and when anatomic distortion occurs, such as to the path of the ureter.
Besides establishing the diagnosis, ultrasound imaging has become, along with MRI, the most important aid for proper preoperative planning. Not only does imaging provide the surgeon and patient with knowledge regarding the extent of the upcoming procedure, but it also allows the minimally invasive gynecologic (MIG) surgeon to involve colleagues, such as colorectal surgeons or urologists. For example, deep infiltrative endometriosis penetrating into the bowel mucosa will require a discoid or segmental bowel resection.
While many endometriosis experts rely on MRI, many MIG surgeons are dependent on ultrasound. I would not consider taking a patient with signs and symptoms suggestive of endometriosis to surgery without 2D/3D transvaginal ultrasound. If the patient possesses a uterus, a saline-infused sonogram is performed to potentially diagnose adenomyosis.
It is a pleasure and honor to welcome Professor Caterina Exacoustos MD, PhD, associate professor of ob.gyn. at the University of Rome “Tor Vergata,” to this edition of the Master Class in Gynecologic Surgery to discuss “Ultrasound and Its Role in the Diagnosis of and Management of Endometriosis, Including DIE.”
Prof. Exacoustos’ main areas of interest are endometriosis and benign diseases including uterine pathology and infertility. Her extensive body of work comprises over 120 scientific publications and numerous book chapters both in English and in Italian.
Prof. Exacoustos continues to be one of the most well respected lecturers speaking about ultrasound throughout the world.
Dr. Miller is professor of obstetrics and gynecology, department of clinical sciences, Rosalind Franklin University of Medicine and Science, North Chicago. Dr. Miller has no conflicts of interest to report.
Ultrasound and Its Role In Diagnosing and Managing Endometriosis
Endometriosis affects approximately 10%-20% of premenopausal women worldwide. It is the leading cause of chronic pelvic pain, is often associated with infertility, and has a significant impact on quality of life. Although the natural history of endometriosis remains unknown, emerging evidence suggests that the pathophysiological steps of initiation and development of endometriosis must occur earlier in the lifespan. Most notably, the onset of endometriosis-associated pain symptoms is often reported during adolescence and young adulthood.1
While many patients with endometriosis are referred with dysmenorrhea at a young age, at age ≤ 25 years,2 symptoms are often highly underestimated and considered to be normal and transient.3,4 Clinical and pelvic exams are often negative in young women, and delays in endometriosis diagnosis are well known.
The presentation of primary dysmenorrhea with no anatomical cause embodies the paradigm that dysmenorrhea in adolescents is most often an insignificant disorder. This perspective is probably a root cause of delayed endometriosis diagnosis in young patients. However, another issue behind delayed diagnosis is the reluctance of the physician to perform a diagnostic laparoscopy — historically the gold standard for diagnosing endometriosis — for seemingly common symptoms such as dysmenorrhea in young patients.
Today we know that there are typical aspects of ultrasound imaging that identify endometriosis in the pelvis, and notably, the 2022 European Society for Human Reproduction and Embryology (ESHRE) endometriosis guideline5 recognizes imaging (ultrasound or MRI) as the standard for endometriosis diagnosis without requiring laparoscopic or histological confirmation.
An early and noninvasive method of diagnosis aids in timely diagnosis and provides for the timely initiation of medical management to improve quality of life and prevent progression of disease (Figure 1).
(A. Transvaginal ultrasound appearance of a small ovarian endometrioma in a 16-year-old girl. Note the unilocular cyst with ground glass echogenicity surrounded by multifollicular ovarian tissue. B. Ultrasound image of a retroverted uterus of an 18-year-old girl with focal adenomyosis of the posterior wall. Note the round cystic anechoic areas in the inner myometrium or junctional zone. The small intra-myometrial cyst is surrounded by a hyperechoic ring).
Indeed, the typical appearance of endometriotic pelvic lesions on transvaginal sonography, such as endometriomas and rectal deep infiltrating endometriosis (DIE) — as well as adenomyosis – can be medically treated without histologic confirmation .
When surgery is advisable, ultrasound findings also play a valuable role in presurgical staging, planning, and counseling for patients of all ages. Determining the extent and location of DIE preoperatively, for instance, facilitates the engagement of the appropriate surgical specialists so that multiple surgeries can be avoided. It also enables patients to be optimally informed before surgery of possible outcomes and complications.
Moreover, in the context of infertility, ultrasound can be a valuable tool for understanding uterine pathology and assessing for adenomyosis so that affected patients may be treated surgically or medically before turning to assisted reproductive technology.
Uniformity, Standardization in the Sonographic Assessment
In Europe, as in the United States, transvaginal sonography (TVS) is the first-line imaging tool for the diagnosis and management of endometriosis. In Europe, many ob.gyns. perform ultrasound themselves, as do treating surgeons. When diagnostic findings are negative but clinical suspicion is high, MRI is often utilized. Laparoscopy may then be considered in patients with negative imaging results.
Efforts to standardize terms, definitions, measurements, and sonographic features of different types of endometriosis have been made to make it easier for physicians to share data and communicate with each other. A lack of uniformity has contributed to variability in the reported diagnostic accuracy of TVS.
About 10 years ago, in one such effort, we assessed the accuracy of TVS for DIE by comparing TVS results with laparoscopic/histologic findings, and developed an ultrasound mapping system to accurately record the location, size and depth of lesions visualized by TVS. The accuracy of TVS ranged from 76% for the diagnosis of vaginal endometriosis to 97% for the diagnosis of bladder lesions and posterior cul-de-sac obliteration. Accuracy was 93% and 91% for detecting ureteral involvement (right and left); 87% for uterosacral ligament endometriotic lesions; and 87% for parametrial involvement.6
Shortly after, with a focus on DIE, expert sonographers and physician-sonographers from across Europe — as well as some experts from Australia, Japan, Brazil, Chile, and the United States (Y. Osuga from Brigham and Women’s Hospital and Harvard Medical School) — came together to agree on a uniform approach to the sonographic evaluation for suspected endometriosis and a standardization of terminology.
The consensus opinion from the International Deep Endometriosis Analysis (IDEA) group details four steps for examining women with suspected DIE: 1) Evaluation of the uterus and adnexa, 2) evaluation of transvaginal sonographic “soft markers” (ie. site-specific tenderness and ovarian mobility), 3) assessment of the status of the posterior cul-de-sac using real-time ultrasound-based “sliding sign,” and 4) assessment for DIE nodules in the anterior and posterior compartments.7
Our paper describing a mapping system and the IDEA paper describe how to detect deep endometriosis in the pelvis by utilizing an ultrasound view of normal anatomy and pelvic organ structure to provide landmarks for accurately defining the site of DIE lesions (Figure 2).
(A. Ultrasound appearance of a small DIE lesion of the retrocervical area [white arrows], which involved the torus uterinum and the right uterosacral ligament [USL]. The lesion appears as hypoechoic tissue with irregular margins caused by the fibrosis induced by the DIE. B. TVS appearance of small nodules of DIE of the left USL. Note the small retrocervical DIE lesion [white arrows], which appears hypoechoic due to the infiltration of the hyperechoic USL. C) Ultrasound appearance of a DIE nodule of the recto-sigmoid wall. Note the hypoechoic thickening of the muscular layers of the bowel wall attached to the corpus of the uterus and the adenomyosis of the posterior wall. The retrocervical area is free. D. TVS appearance of nodules of DIE of the lower rectal wall. Note the hypoechoic lesion [white arrows] of the rectum is attached to a retrocervical DIE fibrosis of the torus and USL [white dotted line]).
So-called rectovaginal endometriosis can be well assessed, for instance, since the involvement of the rectum, sigmoid colon, vaginal wall, rectovaginal septum, and posterior cul-de-sac uterosacral ligament can be seen by ultrasound as a single structure, making the location, size, and depth of any lesions discernible.
Again, this evaluation of the extent of disease is important for presurgical assessment so the surgeon can organize the right team and time of surgery and so the patient can be counseled on the advantages and possible complications of the treatment.
Notably, an accurate ultrasound description of pelvic endometriosis is helpful for accurate classification of disease. Endometriosis classification systems such as that of the American Association of Gynecologic Laparoscopists (AAGL)8 and the American Society of Reproductive Medicine (ASRM),9 as well as the #Enzian surgical description system,10 have been adapted to cover findings from ultrasound as well as MRI imaging.
A Systematic Evaluation
In keeping with the IDEA consensus opinion and based on our years of experience at the University of Rome, I advise that patients with typical pain symptoms of endometriosis or infertility undergo an accurate sonographic assessment of the pelvis with particular evaluation not only of the uterus and ovaries but of all pelvic retroperitoneal spaces.
The TVS examination should start with a slightly filled bladder, which permits a better evaluation of the bladder walls and the presence of endometriotic nodules. These nodules appear as hyperechoic linear or spherical lesions bulging toward the lumen and involving the serosa, muscularis, or (sub)mucosa of the bladder.
Then, an accurate evaluation of the uterus in 2D and 3D permits the diagnosis of adenomyosis. 3D sonographic evaluation of the myometrium and of the junctional zone are important; alteration and infiltration of the junctional zone and the presence of small adenomyotic cysts in the inner or outer myometrium are direct, specific signs of adenomyosis and should be ruled out in patients with dysmenorrhea, heavy menstrual bleeding, infertility, and pregnancy complications.
Endometriomas of the ovaries can be easily detected as having the typical appearance of a cyst with ground glass content. Adhesions of the ovaries and the uterus also should be evaluated with a dynamic ultrasound approach that utilizes the sliding sign and mobilization by palpation of the organs during the TVS scan.
Finally, the posterior and lateral retroperitoneal compartments should be carefully evaluated, with symptoms guiding the TVS examination whenever possible. Deep endometriotic nodules of the rectum appear as hypoechoic lesions or linear or nodular retroperitoneal thickening with irregular borders, penetrating into the intestinal wall and distorting its normal structure. In young patients, it seems very important to assess for small lesions below the peritoneum between the vagina and rectum, and in the parametria and around the ureter and nerves — lesions that, notably, would not be seen by diagnostic laparoscopy.
The Evaluation of Young Patients
In adolescent and young patients, endometriosis and adenomyosis are often present with small lesions and shallow tissue invasion, making a very careful and experienced approach to ultrasound essential for detection. Endometriomas are often of small diameter, and DIE is not always easily diagnosed because retroperitoneal lesions are similarly very small.
In a series of 270 adolescents (ages 12-20) who were referred to our outpatient gynecologic ultrasound unit over a 5-year period for various indications, at least one ultrasound feature of endometriosis was observed in 13.3%. In those with dysmenorrhea, the detection of endometriosis increased to 21%. Endometrioma was the most common type of endometriosis we found in the study, but DIE and adenomyosis were found in 4%-11%.
Although endometriotic lesions typically are small in young patients, they are often associated with severe pain symptoms, including chronic pelvic pain, dysmenorrhea, dyspareunia, dysuria, and dyschezia, all of which can have a serious effect on the quality of life of these young women. These symptoms keep them away from school during menstruation, away from sports, and cause painful intercourse and infertility. In young patients, an accurate TVS can provide a lot of information, and the ability to detect retroperitoneal endometriotic lesions and adenomyosis is probably better than with purely diagnostic laparoscopy, which would evaluate only superficial lesions.
TVS or, when needed, transrectal ultrasound, can enable adequate treatment and follow-up of the disease and its symptoms. There are no guidelines recommending adequate follow-up times to evaluate the effectiveness of medical therapy in patients with ultrasound signs of endometriosis. (Likewise, there are no indications for follow-up in patients with severe dysmenorrhea without ultrasound signs of endometriosis.) Certainly, our studies suggest careful evaluation over time of young patients with severe dysmenorrhea by serial ultrasound scans. With such follow-up, disease progress can be monitored and the medical or surgical treatment approach modified if needed.
The diagnosis of endometriosis at a young age has significant benefits not only in avoiding or reducing progression of the disease, but also in improving quality of life and aiding women in their desire for pregnancy.
Dr. Exacoustos is associate professor of ob.gyn. at the University of Rome “Tor Vergata.” She has no conflicts of interest to report.
References
1. Zondervan KT et al. N Engl J Med. 2020;382:1244-56.
2. Greene R et al. Fertil Steril. 2009;91:32-9.
3. Chapron C et al. J Pediatr Adolesc Gynecol. 2011;24:S7-12.
4. Randhawa AE et al. J Pediatr Adolesc Gynecol. 2021;34:643-8.
5. Becker CM et al. Hum Reprod Open. 2022(2):hoac009.
6. Exacoustos C et al. Fertil Steril. 2014;102:143-9. 7. Guerriero S et al. Ultrasound Obstet Gynecol. 2016;48(3):318-32.
8. Abrao MS et al. J Minim Invasive Gynecol. 2021;28:1941-50.9. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817-21. 10. Keckstein J et al. Acta Obstet Gynecol Scand. 2021;100:1165-75.
11. Martire FG et al. Fertil Steril. 2020;114(5):1049-57.
A 27-year-old Haitian woman presented with a painful umbilical mass which had been growing in size for 5 months
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.
Elagolix curbs heavy bleeding linked to uterine leiomyomas
Uterine leiomyomas are common in premenopausal women, and 60% experience heavy menstrual bleeding, but nonsurgical options as an alternative to hysterectomy are limited, wrote Eric Brown, MD, of Gyn-Care, Atlanta, Georgia, and colleagues.
Elagolix sodium, an oral, short-acting nonpeptide, gonadotropin-releasing hormone antagonist, has been approved by the Food and Drug Administration at a dose of 300 mg twice daily with add-back therapy for up to 24 months of use. However, this treatment protocol is contraindicated or not preferable for some patients, the researchers said.
In a study published in Obstetrics & Gynecology , the researchers randomized 54 women to 150 mg of oral elagolix once daily, and 28 to a placebo for 6 months to investigate the safety and efficacy of the lower dose. The study population included women aged 18-51 years with a history of heavy menstrual bleeding association with uterine leiomyomas. Approximately two-thirds (65.9%) were Black.
The primary endpoint was the proportion of patients who met the criteria of menstrual blood loss volume less than 80 mL during the final month of treatment and menstrual blood loss volume reduction of 50% or more from baseline to the final month of treatment.
After 6 months, nearly half (49.4%) of the elagolix group met the study endpoint compared with 23.3% of the placebo group (P = .035).
Elagolix patients showed significantly greater reductions in both mean and median menstrual blood loss volumes compared with the placebo patients over the study period, and significant differences between the groups in the mean reduction of menstrual blood loss were evident after 1 month of treatment (P < .05 for months 1, 2, 3, and 5).
Results were similar in a further sensitivity analysis in which patients with incomplete final month data were considered nonresponders; 44.4% of elagolix patients and 21.4% of patients met the primary endpoint.
Overall, 51.9% of elagolix patients and 39.3% of placebo patients reported adverse events; the most common were headache and hot flush. Three patients (5.6%) in the elagolix group discontinued the drug because of adverse events. No serious or severe adverse events were reported in the elagolix group; both cases of reported serious adverse events (COVID-19 and an enlarged uvula) occurred in placebo patients.
Patient-reported outcomes were significantly greater in the elagolix patients, based on symptom severity score, 5 of 6 Uterine Fibroid Symptom and Quality of Life (UFS-QOL) health-related quality of life subscales, and the HRQOL total score at the end of the study.
The findings were limited by several factors including the small study population and lenient eligibility criteria that may have led to a higher placebo response rate, and the study did not monitor bone mineral density, the researchers noted.
However, the results suggest that elagolix at a 150-mg dose was well tolerated, with a safety profile similar to that seen in women who took the drug for endometriosis pain, and may be an option for women with contraindications to other therapy or for those who prefer once-daily dosing, they concluded.
The study was funded by AbbVie. Lead author Dr. Brown had no additional financial conflicts to disclose, but several coauthors disclosed relationships with AbbVie and other companies.
Uterine leiomyomas are common in premenopausal women, and 60% experience heavy menstrual bleeding, but nonsurgical options as an alternative to hysterectomy are limited, wrote Eric Brown, MD, of Gyn-Care, Atlanta, Georgia, and colleagues.
Elagolix sodium, an oral, short-acting nonpeptide, gonadotropin-releasing hormone antagonist, has been approved by the Food and Drug Administration at a dose of 300 mg twice daily with add-back therapy for up to 24 months of use. However, this treatment protocol is contraindicated or not preferable for some patients, the researchers said.
In a study published in Obstetrics & Gynecology , the researchers randomized 54 women to 150 mg of oral elagolix once daily, and 28 to a placebo for 6 months to investigate the safety and efficacy of the lower dose. The study population included women aged 18-51 years with a history of heavy menstrual bleeding association with uterine leiomyomas. Approximately two-thirds (65.9%) were Black.
The primary endpoint was the proportion of patients who met the criteria of menstrual blood loss volume less than 80 mL during the final month of treatment and menstrual blood loss volume reduction of 50% or more from baseline to the final month of treatment.
After 6 months, nearly half (49.4%) of the elagolix group met the study endpoint compared with 23.3% of the placebo group (P = .035).
Elagolix patients showed significantly greater reductions in both mean and median menstrual blood loss volumes compared with the placebo patients over the study period, and significant differences between the groups in the mean reduction of menstrual blood loss were evident after 1 month of treatment (P < .05 for months 1, 2, 3, and 5).
Results were similar in a further sensitivity analysis in which patients with incomplete final month data were considered nonresponders; 44.4% of elagolix patients and 21.4% of patients met the primary endpoint.
Overall, 51.9% of elagolix patients and 39.3% of placebo patients reported adverse events; the most common were headache and hot flush. Three patients (5.6%) in the elagolix group discontinued the drug because of adverse events. No serious or severe adverse events were reported in the elagolix group; both cases of reported serious adverse events (COVID-19 and an enlarged uvula) occurred in placebo patients.
Patient-reported outcomes were significantly greater in the elagolix patients, based on symptom severity score, 5 of 6 Uterine Fibroid Symptom and Quality of Life (UFS-QOL) health-related quality of life subscales, and the HRQOL total score at the end of the study.
The findings were limited by several factors including the small study population and lenient eligibility criteria that may have led to a higher placebo response rate, and the study did not monitor bone mineral density, the researchers noted.
However, the results suggest that elagolix at a 150-mg dose was well tolerated, with a safety profile similar to that seen in women who took the drug for endometriosis pain, and may be an option for women with contraindications to other therapy or for those who prefer once-daily dosing, they concluded.
The study was funded by AbbVie. Lead author Dr. Brown had no additional financial conflicts to disclose, but several coauthors disclosed relationships with AbbVie and other companies.
Uterine leiomyomas are common in premenopausal women, and 60% experience heavy menstrual bleeding, but nonsurgical options as an alternative to hysterectomy are limited, wrote Eric Brown, MD, of Gyn-Care, Atlanta, Georgia, and colleagues.
Elagolix sodium, an oral, short-acting nonpeptide, gonadotropin-releasing hormone antagonist, has been approved by the Food and Drug Administration at a dose of 300 mg twice daily with add-back therapy for up to 24 months of use. However, this treatment protocol is contraindicated or not preferable for some patients, the researchers said.
In a study published in Obstetrics & Gynecology , the researchers randomized 54 women to 150 mg of oral elagolix once daily, and 28 to a placebo for 6 months to investigate the safety and efficacy of the lower dose. The study population included women aged 18-51 years with a history of heavy menstrual bleeding association with uterine leiomyomas. Approximately two-thirds (65.9%) were Black.
The primary endpoint was the proportion of patients who met the criteria of menstrual blood loss volume less than 80 mL during the final month of treatment and menstrual blood loss volume reduction of 50% or more from baseline to the final month of treatment.
After 6 months, nearly half (49.4%) of the elagolix group met the study endpoint compared with 23.3% of the placebo group (P = .035).
Elagolix patients showed significantly greater reductions in both mean and median menstrual blood loss volumes compared with the placebo patients over the study period, and significant differences between the groups in the mean reduction of menstrual blood loss were evident after 1 month of treatment (P < .05 for months 1, 2, 3, and 5).
Results were similar in a further sensitivity analysis in which patients with incomplete final month data were considered nonresponders; 44.4% of elagolix patients and 21.4% of patients met the primary endpoint.
Overall, 51.9% of elagolix patients and 39.3% of placebo patients reported adverse events; the most common were headache and hot flush. Three patients (5.6%) in the elagolix group discontinued the drug because of adverse events. No serious or severe adverse events were reported in the elagolix group; both cases of reported serious adverse events (COVID-19 and an enlarged uvula) occurred in placebo patients.
Patient-reported outcomes were significantly greater in the elagolix patients, based on symptom severity score, 5 of 6 Uterine Fibroid Symptom and Quality of Life (UFS-QOL) health-related quality of life subscales, and the HRQOL total score at the end of the study.
The findings were limited by several factors including the small study population and lenient eligibility criteria that may have led to a higher placebo response rate, and the study did not monitor bone mineral density, the researchers noted.
However, the results suggest that elagolix at a 150-mg dose was well tolerated, with a safety profile similar to that seen in women who took the drug for endometriosis pain, and may be an option for women with contraindications to other therapy or for those who prefer once-daily dosing, they concluded.
The study was funded by AbbVie. Lead author Dr. Brown had no additional financial conflicts to disclose, but several coauthors disclosed relationships with AbbVie and other companies.
FROM OBSTETRICS & GYNECOLOGY
Two landmark papers change treatment paradigm for advanced endometrial cancer
I wanted to very briefly highlight a truly extraordinary event in my professional experience as a clinical investigator for almost 40 years in the area of the gynecologic malignancies:
In my career, of course, I’ve treated endometrial cancer, but the paradigm, the algorithms, and the strategies we’ve used have, for the most part, simply followed what we’ve done for ovarian cancer. If platinums worked in ovarian cancer, they probably worked in endometrial cancer, and that was true. If paclitaxel worked and had activity in ovarian cancer, it probably would in endometrial cancer, and that was true. It took some time, but basically, we use the same frontline chemotherapy in advanced or recurrent endometrial cancer as we’ve used in ovarian cancer, and on and on.
That world has changed, very much for the positive. Not only have pharmaceutical companies, academic investigators, and individual investigators in the community setting seen endometrial cancer as a major priority, but we have exciting new developments, and very specifically, of course, the immunotherapeutic agents known as checkpoint inhibitors.
One of these two papers was titled “Pembrolizumab Plus Chemotherapy in Advanced Endometrial Cancer” and the second one was titled “Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer.” Obviously, these were separate studies, but both used checkpoint inhibitor plus the chemotherapeutic agents carboplatin-paclitaxel, compared with chemotherapy alone as frontline therapy for advanced or recurrent ovarian cancer and demonstrated a statistically significant, and in my opinion, highly clinically meaningful improvement, in progression-free survival in favor of the regimen that included the checkpoint inhibitors.
Clearly, we will need longer follow-up to see both the overall magnitude of the effect of these therapies on overall survival and the duration of the effect – the shape of the curve. Do we cure many more people? Do we delay time to progression and death? That remains to be seen.
But the outcomes we have now are remarkably positive for patients and have absolutely changed the standard of care in the management of recurrent or advanced endometrial cancer.
I should note that this includes both patients who have evidence of mismatch repair deficiency and those patients who do not have evidence of deficiency, which is a large patient population. These studies demonstrated the benefit to the entire population of patients.
However, on the basis of the data that we have – not only in endometrial cancer, but in other tumor types – the greatest impact was seen in patients with evidence of mismatch repair deficiency, where the immunotherapy agent has been shown to be most relevant; not exclusively, but most relevant.
These are very important papers. If you have an interest in endometrial cancer or immunotherapy, I would encourage you to read these papers. They change the paradigm of management for advanced endometrial cancer, and they clearly point out directions for future research in the management of this class of gynecologic cancers.
Dr. Markman is a professor in the department of medical oncology and therapeutics research at City of Hope in Duarte, Calif., and the president of Medicine & Science at City of Hope Atlanta, Chicago, and Phoenix. He reported conflicts of interest with AstraZeneca and GlaxoSmithKline.
This transcript has been edited for clarity. A version of this article first appeared on Medscape.com.
I wanted to very briefly highlight a truly extraordinary event in my professional experience as a clinical investigator for almost 40 years in the area of the gynecologic malignancies:
In my career, of course, I’ve treated endometrial cancer, but the paradigm, the algorithms, and the strategies we’ve used have, for the most part, simply followed what we’ve done for ovarian cancer. If platinums worked in ovarian cancer, they probably worked in endometrial cancer, and that was true. If paclitaxel worked and had activity in ovarian cancer, it probably would in endometrial cancer, and that was true. It took some time, but basically, we use the same frontline chemotherapy in advanced or recurrent endometrial cancer as we’ve used in ovarian cancer, and on and on.
That world has changed, very much for the positive. Not only have pharmaceutical companies, academic investigators, and individual investigators in the community setting seen endometrial cancer as a major priority, but we have exciting new developments, and very specifically, of course, the immunotherapeutic agents known as checkpoint inhibitors.
One of these two papers was titled “Pembrolizumab Plus Chemotherapy in Advanced Endometrial Cancer” and the second one was titled “Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer.” Obviously, these were separate studies, but both used checkpoint inhibitor plus the chemotherapeutic agents carboplatin-paclitaxel, compared with chemotherapy alone as frontline therapy for advanced or recurrent ovarian cancer and demonstrated a statistically significant, and in my opinion, highly clinically meaningful improvement, in progression-free survival in favor of the regimen that included the checkpoint inhibitors.
Clearly, we will need longer follow-up to see both the overall magnitude of the effect of these therapies on overall survival and the duration of the effect – the shape of the curve. Do we cure many more people? Do we delay time to progression and death? That remains to be seen.
But the outcomes we have now are remarkably positive for patients and have absolutely changed the standard of care in the management of recurrent or advanced endometrial cancer.
I should note that this includes both patients who have evidence of mismatch repair deficiency and those patients who do not have evidence of deficiency, which is a large patient population. These studies demonstrated the benefit to the entire population of patients.
However, on the basis of the data that we have – not only in endometrial cancer, but in other tumor types – the greatest impact was seen in patients with evidence of mismatch repair deficiency, where the immunotherapy agent has been shown to be most relevant; not exclusively, but most relevant.
These are very important papers. If you have an interest in endometrial cancer or immunotherapy, I would encourage you to read these papers. They change the paradigm of management for advanced endometrial cancer, and they clearly point out directions for future research in the management of this class of gynecologic cancers.
Dr. Markman is a professor in the department of medical oncology and therapeutics research at City of Hope in Duarte, Calif., and the president of Medicine & Science at City of Hope Atlanta, Chicago, and Phoenix. He reported conflicts of interest with AstraZeneca and GlaxoSmithKline.
This transcript has been edited for clarity. A version of this article first appeared on Medscape.com.
I wanted to very briefly highlight a truly extraordinary event in my professional experience as a clinical investigator for almost 40 years in the area of the gynecologic malignancies:
In my career, of course, I’ve treated endometrial cancer, but the paradigm, the algorithms, and the strategies we’ve used have, for the most part, simply followed what we’ve done for ovarian cancer. If platinums worked in ovarian cancer, they probably worked in endometrial cancer, and that was true. If paclitaxel worked and had activity in ovarian cancer, it probably would in endometrial cancer, and that was true. It took some time, but basically, we use the same frontline chemotherapy in advanced or recurrent endometrial cancer as we’ve used in ovarian cancer, and on and on.
That world has changed, very much for the positive. Not only have pharmaceutical companies, academic investigators, and individual investigators in the community setting seen endometrial cancer as a major priority, but we have exciting new developments, and very specifically, of course, the immunotherapeutic agents known as checkpoint inhibitors.
One of these two papers was titled “Pembrolizumab Plus Chemotherapy in Advanced Endometrial Cancer” and the second one was titled “Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer.” Obviously, these were separate studies, but both used checkpoint inhibitor plus the chemotherapeutic agents carboplatin-paclitaxel, compared with chemotherapy alone as frontline therapy for advanced or recurrent ovarian cancer and demonstrated a statistically significant, and in my opinion, highly clinically meaningful improvement, in progression-free survival in favor of the regimen that included the checkpoint inhibitors.
Clearly, we will need longer follow-up to see both the overall magnitude of the effect of these therapies on overall survival and the duration of the effect – the shape of the curve. Do we cure many more people? Do we delay time to progression and death? That remains to be seen.
But the outcomes we have now are remarkably positive for patients and have absolutely changed the standard of care in the management of recurrent or advanced endometrial cancer.
I should note that this includes both patients who have evidence of mismatch repair deficiency and those patients who do not have evidence of deficiency, which is a large patient population. These studies demonstrated the benefit to the entire population of patients.
However, on the basis of the data that we have – not only in endometrial cancer, but in other tumor types – the greatest impact was seen in patients with evidence of mismatch repair deficiency, where the immunotherapy agent has been shown to be most relevant; not exclusively, but most relevant.
These are very important papers. If you have an interest in endometrial cancer or immunotherapy, I would encourage you to read these papers. They change the paradigm of management for advanced endometrial cancer, and they clearly point out directions for future research in the management of this class of gynecologic cancers.
Dr. Markman is a professor in the department of medical oncology and therapeutics research at City of Hope in Duarte, Calif., and the president of Medicine & Science at City of Hope Atlanta, Chicago, and Phoenix. He reported conflicts of interest with AstraZeneca and GlaxoSmithKline.
This transcript has been edited for clarity. A version of this article first appeared on Medscape.com.
New clues to an old mystery: Recent gains in endometriosis
In 1927, American gynecologist John Sampson published his theory of the etiology of endometriosis, postulating that retrograde flow of endometrial debris flows backward through the fallopian tubes during menses into the peritoneal cavity. Dr. Sampson’s notion remains the main paradigm today, mentioned still in recent articles on the topic, but it has a flaw: Although the theory may account for how endometrial tissue escapes the uterus, a 1984 study revealed that this phenomenon occurs in 90% of women. Why, then, do only 10% of women suffer from endometriosis?
Endometriosis describes a condition in which endometrial tissue lining the uterus is found outside the uterus. The disease can be painful, even crippling. As many as 30% of women in their reproductive years who have endometriosis are infertile as a consequence. The hallmarks of the condition are superficial peritoneal lesions of varying color, cysts in the ovaries, deeper nodules accompanied by scarring and adhesion, primarily in the pelvis but sometimes appearing outside the pelvis. The syndrome can be challenging to identify, requiring laparoscopy for definitive diagnosis.
John Sampson aside, scientists have struggled for the past century to identify the cause, or causes, of endometriosis. Hormones clearly play a role in its development, and women with endometriosis have an elevated risk of clear-cell and endometrioid ovarian cancer and autoimmune diseases. Immunodeficiency also could be to blame, if a faulty immune system fails to find and remove endometrial tissue outside of the uterus. A class of chemicals known as endocrine disruptors have been linked to endometriosis, but not definitively. Twin studies have demonstrated that as many as 50% of cases have a genetic basis, while mice with surgically induced endometriosis have been found to have a higher ratio of harmful to beneficial bacteria in their gut.
Several studies published this year point to new insights into the old mystery – with possible implications for ways to treat the disorder.
Perhaps the most surprising came out earlier this year in Science Translational Medicine, as a team of researchers in Japan reported that invasive infection by bacteria of the genus Fusobacterium may cause at least some cases of endometriosis.
Is Fusobacterium the new Helicobacter pylori?
The researchers, from Nagoya University, are the first to suggest that not only might a single bacterial genus cause endometriosis, but that antibiotic treatment could prevent progression of the disease. Using endometrial tissue obtained from 79 women undergoing hysterectomy for endometriosis and 76 women undergoing hysterectomy for other reasons (such as cervical cancer), the team started with gene expression profiling to explore differences between the two sets of samples.
They uncovered an interesting chain of cellular events: macrophages found in endometriotic lesions were secreting transforming growth factor-beta (TGF-beta). TGF-beta in turn stimulated high levels of expression of a gene called TAGLN in fibroblast cells from women with endometriosis but not in fibroblasts from women without endometriosis.
Turning on TAGLN transformed these previously inactive cells into active myofibroblasts, leading to increased proliferation, mobility, and attachment to mesothelial cells, the layer of cells that line body cavities and internal organs. In short, they identified some key players in an environment that seemed very favorable to the development of endometriosis.
“So, the question is: Why are macrophages activated?” said Yutaka Kondo, MD, PhD, the senior author of the study and a professor in the division of cancer biology at the Nagoya (Japan) University Graduate School of Medicine. “We think that there are always bacteria in the endometrium.”
After reviewing data from a previously published study, they used quantitative polymerase chain reaction to rule out one candidate, Erysipelothrix, but scored on their next attempt, identifying Fusobacterium species in endometrial tissue from 64% of the women with endometriosis, compared with fewer than 10% of the controls.
To confirm that the bacteria could cause disease and were not simply bystanders, Dr. Kondo’s team turned to a mouse model for endometriosis, in which endometrial cells are surgically removed from the uteri of mice and injected into the peritoneum of recipient mice, leading to the formation of endometriotic lesions. When mice received further injections of uterine tissue from mice that were infected with F. nucleatum, their lesions were more numerous when compared with mice that received injections of uninfected uterine tissue. Furthermore, antibiotic treatment with metronidazole or chloramphenicol immediately after surgery largely prevented progression to endometriosis, Dr. Kondo and his colleagues reported.
Dr. Kondo likened this relationship between Fusobacterium and endometriosis to that of the link between Helicobacter pylori and peptic ulcers but acknowledged that he doesn’t have all the answers.
“We need more clinical trials, and also we have to know what kind of treatment might be the most effective for the treatment of endometriosis,” Dr. Kondo said, pointing out that other therapies should still be pursued in addition to antibiotics, as not all the samples from women with endometriosis harbored Fusobacterium. “It might be possible that other mechanisms are also involved.”
Don’t write off gut microbiota
Ramakrishna Kommagani, PhD, associate professor of pathology and immunology at Baylor College of Medicine in Houston, agreed. “Endometriosis is a complex disease, which appears to be impacted by many factors, including genetic, epigenetic, and environmental factors,” Dr. Kommagani said.
A key difference between his work and Dr. Kondo’s is his focus on gut microbiota, whereas the Japanese team looked at bacteria in the vagina and endometrium. But Dr. Kommagani said he thinks both could play a role. “Maybe the vaginal microbiome might have a direct impact on disease similar to what we showed on the gut,” he said.
But he said at least part of the answer to why some women develop endometriosis may have to do more with the balance of beneficial and harmful bacteria in the gut rather than because of a single family of microbes like Fusobacterium.
Most recently, by dovetailing a mouse model for inducing endometriosis in mice treated with antibiotics to deplete their gut microbiome, Dr. Kommagani’s lab expanded on its previous work: They showed that the animals developed fewer of the typical lesions seen in endometriosis than those that did not receive antibiotics before all of the mice underwent the surgical procedure used by researchers to induce endometriosis – possibly because they had no bacteria in their gut triggering the inflammatory response required for the development of endometriosis.
But after oral feedings with fecal matter from mice without endometriosis, the microbiota-depleted rodents began developing lesions typical of endometriosis, suggesting that altered gut flora from mice with endometriosis appeared to promote the disorder. Meanwhile, their microbiota-depleted counterparts who were fed fecal matter from mice without endometriosis did not develop the typical lesions.
Dr. Kommagani’s team then compared metabolites from bacteria in stool from mice with and without endometriosis and investigated the in vitro effect of these metabolites on cells from human endometriotic lesions. One of them, quinic acid, increased the proliferation of human endometriotic epithelial cells.
“Some metabolites such as fiber-derived short-chain fatty acids have beneficial effects; they inhibit the disease,” Dr. Kommagani said. “But maybe an amino acid derivative such as quinic acid, [may] promote disease, and these are generated because there is a gut dysbiosis.”
This statement hints at some of the possible therapeutic approaches for endometriosis, such as a high-fiber diet to promote healthy gut flora, or perhaps antibiotics to eradicate unhealthy bacteria. But as with other conditions that have been linked to dysbiosis, like inflammatory bowel disease, use of antibiotics is a bit like balancing on a tightrope; although antibiotics may remove harmful bacteria, their use may negatively affect the beneficial bacteria.
Clues in genetic variants
Krina Zondervan, DPhil, professor and head of the department of reproductive and genomic epidemiology at the University of Oxford (England), focuses on genomic, molecular, and epidemiologic approaches to understanding endometriosis.
“It’s one thing identifying risk variants and the next question is, okay, well, what do those variants actually do in terms of biology?” Dr. Zondervan said. The Oxford team next explored how the identified genetic variants affect gene expression and the proteins generated, drawing on previously collected data on gene expression from samples of human blood and endometrial and uterine tissue.
They found many of the genes implicated in the risk for endometriosis code for proteins that affect sex hormones, uterine development, transformation of healthy cells into cancerous tissue, inflammatory adhesion molecules, and factors promoting development of new blood vessels. All of that, she said, explains how a few endometrial cells making their way into the pelvis can attach to ovaries, ligaments, and peritoneal surfaces; proliferate; and acquire a blood supply to ensure their survival.
“We were able to identify a whole host of things that were likely causal to the disease,” Dr. Zondervan said. And that finding led to her next question: “Are there particular genes or areas around them that can be targeted with certain medications?”
The surprising answer was that several of the genes linked to endometriosis share pathways with clinical syndromes that often occur in women with endometriosis. Many of these are chronic pain conditions – such as migraines, headaches, and back pain – but also include inflammatory illnesses such as asthma and osteoarthritis.
As Dr. Zondervan explained, “A lot of the variance that we see for endometriosis is also experienced for low back pain and migraine, and that clearly has something to do with pain perception and pain mechanisms.”
A connection between the development of neural pathways and endometriosis has been proposed before, as researchers have found that endometriotic lesions can develop their own nerve supply, creating a direct interaction between the lesions and the central nervous system. And some clinicians have been employing treatment strategies that employ multimodal therapies – employing physical therapists, mental health practitioners, nutritionists, and pain specialists prior to and following surgical removal of lesions – to improve overall success rates of treatment.
But Dr. Zondervan’s team is the first to uncover an important clue about how this happens.
The study findings also provide solid clues to researchers about which genes and proteins to focus on for drug target discovery. In particular, the gene pathways shared by endometriosis and various pain conditions could allow for repurposing of drugs developed for other conditions for treating endometriosis, reported Dr. Zondervan.
Dr. Zondervan’s other important conclusion, echoed by Dr. Kondo and Dr. Kommagani, is that endometriosis is not one disease. Rather, it appears to be akin to cancer in terms of the heterogeneity of how it presents and the different subtypes of diseases. The Oxford study corroborated this belief, identifying certain genes that were closely associated with cystic lesions in ovaries, but failing to turn up a genetic link to other types of lesions in the pelvis long considered to be part of the spectrum of endometriosis disease.
Dr. Zondervan agreed that the potential link with Fusobacterium is a fascinating area given the critical role of inflammation in the pathogenesis of endometriosis, although she’d like to see the work replicated with larger sample sizes. “From a personal point of view, I’d be really fascinated to see how genetics interplays with this,” she added.
What’s next?
The chief limitation of human studies looking at mechanisms of endometriosis is that they are correlational: Tissue samples are collected from women with and without endometriosis, often through an invasive procedure such as laparoscopy or biopsy, at one point in time. Currently, the best tools for proving causation are animal models of endometriosis, such as the those used by Dr. Kondo’s and Dr. Kommagani’s teams.
Better diagnostic tools would solve that problem. The ultimate goal is a noninvasive test for endometriosis that would allow clinicians to follow women over time and permit the monitoring of disease progression, or regression, without the need for painful procedures. Such a diagnostic tool would facilitate rigorous longitudinal studies evaluating mechanisms of disease, as well as monitoring outcomes of clinical trials of new treatments.
Could stool samples be the answer?
The Japanese team found that women harboring Fusobacterium in endometrial tissue also had Fusobacterium in vaginal samples taken at the time of their hysterectomy – and stool samples can pick up changes in the gut microbiome.
“Vaginal swab or stool tests are probably the best and easiest for noninvasive early detection,” Dr. Kommagani said.
Spit tests for DNA would be even easier to obtain. Polygenic risk scores could be developed to estimate an individual’s risk of disease based on the number of variants, but Dr. Zondervan cautioned that not all the genes that account for endometriosis are known.
“The things that we found altogether explain about 5% of disease variability, basically – which is still not an awful lot,” she said.
Dr. Kondo’s work was supported by the Grant-in-Aid for Scientific Research, the Japan Society for the Promotion of Science, and the Research Grant of the Princess Takamatsu Cancer Research Fund. A patent method for detecting bacteria of genus Fusobacterium in order to diagnose endometriosis (WO2023/ 042714), was submitted (international publication date, March 23, 2023).
Dr. Kommagani’s work was funded, in part, by National Institutes of Health/National Institute of Child Health and Human Development grants R01HD102680, R01HD065435, and R00HD080742. He has no other conflicts of interest. Dr. Zondervan received funding from the Wellcome Trust (216767; 104036; 084766; 212904; 076113 and 085475) and also reported grants from Bayer AG, AbbVie, Volition Rx, MDNA Life Sciences, and Roche Diagnostics outside the submitted work.
Dr. Thomas is a pediatrician and epidemiologist living in Portland, Ore.
A version of this article originally appeared on Medscape.com.
In 1927, American gynecologist John Sampson published his theory of the etiology of endometriosis, postulating that retrograde flow of endometrial debris flows backward through the fallopian tubes during menses into the peritoneal cavity. Dr. Sampson’s notion remains the main paradigm today, mentioned still in recent articles on the topic, but it has a flaw: Although the theory may account for how endometrial tissue escapes the uterus, a 1984 study revealed that this phenomenon occurs in 90% of women. Why, then, do only 10% of women suffer from endometriosis?
Endometriosis describes a condition in which endometrial tissue lining the uterus is found outside the uterus. The disease can be painful, even crippling. As many as 30% of women in their reproductive years who have endometriosis are infertile as a consequence. The hallmarks of the condition are superficial peritoneal lesions of varying color, cysts in the ovaries, deeper nodules accompanied by scarring and adhesion, primarily in the pelvis but sometimes appearing outside the pelvis. The syndrome can be challenging to identify, requiring laparoscopy for definitive diagnosis.
John Sampson aside, scientists have struggled for the past century to identify the cause, or causes, of endometriosis. Hormones clearly play a role in its development, and women with endometriosis have an elevated risk of clear-cell and endometrioid ovarian cancer and autoimmune diseases. Immunodeficiency also could be to blame, if a faulty immune system fails to find and remove endometrial tissue outside of the uterus. A class of chemicals known as endocrine disruptors have been linked to endometriosis, but not definitively. Twin studies have demonstrated that as many as 50% of cases have a genetic basis, while mice with surgically induced endometriosis have been found to have a higher ratio of harmful to beneficial bacteria in their gut.
Several studies published this year point to new insights into the old mystery – with possible implications for ways to treat the disorder.
Perhaps the most surprising came out earlier this year in Science Translational Medicine, as a team of researchers in Japan reported that invasive infection by bacteria of the genus Fusobacterium may cause at least some cases of endometriosis.
Is Fusobacterium the new Helicobacter pylori?
The researchers, from Nagoya University, are the first to suggest that not only might a single bacterial genus cause endometriosis, but that antibiotic treatment could prevent progression of the disease. Using endometrial tissue obtained from 79 women undergoing hysterectomy for endometriosis and 76 women undergoing hysterectomy for other reasons (such as cervical cancer), the team started with gene expression profiling to explore differences between the two sets of samples.
They uncovered an interesting chain of cellular events: macrophages found in endometriotic lesions were secreting transforming growth factor-beta (TGF-beta). TGF-beta in turn stimulated high levels of expression of a gene called TAGLN in fibroblast cells from women with endometriosis but not in fibroblasts from women without endometriosis.
Turning on TAGLN transformed these previously inactive cells into active myofibroblasts, leading to increased proliferation, mobility, and attachment to mesothelial cells, the layer of cells that line body cavities and internal organs. In short, they identified some key players in an environment that seemed very favorable to the development of endometriosis.
“So, the question is: Why are macrophages activated?” said Yutaka Kondo, MD, PhD, the senior author of the study and a professor in the division of cancer biology at the Nagoya (Japan) University Graduate School of Medicine. “We think that there are always bacteria in the endometrium.”
After reviewing data from a previously published study, they used quantitative polymerase chain reaction to rule out one candidate, Erysipelothrix, but scored on their next attempt, identifying Fusobacterium species in endometrial tissue from 64% of the women with endometriosis, compared with fewer than 10% of the controls.
To confirm that the bacteria could cause disease and were not simply bystanders, Dr. Kondo’s team turned to a mouse model for endometriosis, in which endometrial cells are surgically removed from the uteri of mice and injected into the peritoneum of recipient mice, leading to the formation of endometriotic lesions. When mice received further injections of uterine tissue from mice that were infected with F. nucleatum, their lesions were more numerous when compared with mice that received injections of uninfected uterine tissue. Furthermore, antibiotic treatment with metronidazole or chloramphenicol immediately after surgery largely prevented progression to endometriosis, Dr. Kondo and his colleagues reported.
Dr. Kondo likened this relationship between Fusobacterium and endometriosis to that of the link between Helicobacter pylori and peptic ulcers but acknowledged that he doesn’t have all the answers.
“We need more clinical trials, and also we have to know what kind of treatment might be the most effective for the treatment of endometriosis,” Dr. Kondo said, pointing out that other therapies should still be pursued in addition to antibiotics, as not all the samples from women with endometriosis harbored Fusobacterium. “It might be possible that other mechanisms are also involved.”
Don’t write off gut microbiota
Ramakrishna Kommagani, PhD, associate professor of pathology and immunology at Baylor College of Medicine in Houston, agreed. “Endometriosis is a complex disease, which appears to be impacted by many factors, including genetic, epigenetic, and environmental factors,” Dr. Kommagani said.
A key difference between his work and Dr. Kondo’s is his focus on gut microbiota, whereas the Japanese team looked at bacteria in the vagina and endometrium. But Dr. Kommagani said he thinks both could play a role. “Maybe the vaginal microbiome might have a direct impact on disease similar to what we showed on the gut,” he said.
But he said at least part of the answer to why some women develop endometriosis may have to do more with the balance of beneficial and harmful bacteria in the gut rather than because of a single family of microbes like Fusobacterium.
Most recently, by dovetailing a mouse model for inducing endometriosis in mice treated with antibiotics to deplete their gut microbiome, Dr. Kommagani’s lab expanded on its previous work: They showed that the animals developed fewer of the typical lesions seen in endometriosis than those that did not receive antibiotics before all of the mice underwent the surgical procedure used by researchers to induce endometriosis – possibly because they had no bacteria in their gut triggering the inflammatory response required for the development of endometriosis.
But after oral feedings with fecal matter from mice without endometriosis, the microbiota-depleted rodents began developing lesions typical of endometriosis, suggesting that altered gut flora from mice with endometriosis appeared to promote the disorder. Meanwhile, their microbiota-depleted counterparts who were fed fecal matter from mice without endometriosis did not develop the typical lesions.
Dr. Kommagani’s team then compared metabolites from bacteria in stool from mice with and without endometriosis and investigated the in vitro effect of these metabolites on cells from human endometriotic lesions. One of them, quinic acid, increased the proliferation of human endometriotic epithelial cells.
“Some metabolites such as fiber-derived short-chain fatty acids have beneficial effects; they inhibit the disease,” Dr. Kommagani said. “But maybe an amino acid derivative such as quinic acid, [may] promote disease, and these are generated because there is a gut dysbiosis.”
This statement hints at some of the possible therapeutic approaches for endometriosis, such as a high-fiber diet to promote healthy gut flora, or perhaps antibiotics to eradicate unhealthy bacteria. But as with other conditions that have been linked to dysbiosis, like inflammatory bowel disease, use of antibiotics is a bit like balancing on a tightrope; although antibiotics may remove harmful bacteria, their use may negatively affect the beneficial bacteria.
Clues in genetic variants
Krina Zondervan, DPhil, professor and head of the department of reproductive and genomic epidemiology at the University of Oxford (England), focuses on genomic, molecular, and epidemiologic approaches to understanding endometriosis.
“It’s one thing identifying risk variants and the next question is, okay, well, what do those variants actually do in terms of biology?” Dr. Zondervan said. The Oxford team next explored how the identified genetic variants affect gene expression and the proteins generated, drawing on previously collected data on gene expression from samples of human blood and endometrial and uterine tissue.
They found many of the genes implicated in the risk for endometriosis code for proteins that affect sex hormones, uterine development, transformation of healthy cells into cancerous tissue, inflammatory adhesion molecules, and factors promoting development of new blood vessels. All of that, she said, explains how a few endometrial cells making their way into the pelvis can attach to ovaries, ligaments, and peritoneal surfaces; proliferate; and acquire a blood supply to ensure their survival.
“We were able to identify a whole host of things that were likely causal to the disease,” Dr. Zondervan said. And that finding led to her next question: “Are there particular genes or areas around them that can be targeted with certain medications?”
The surprising answer was that several of the genes linked to endometriosis share pathways with clinical syndromes that often occur in women with endometriosis. Many of these are chronic pain conditions – such as migraines, headaches, and back pain – but also include inflammatory illnesses such as asthma and osteoarthritis.
As Dr. Zondervan explained, “A lot of the variance that we see for endometriosis is also experienced for low back pain and migraine, and that clearly has something to do with pain perception and pain mechanisms.”
A connection between the development of neural pathways and endometriosis has been proposed before, as researchers have found that endometriotic lesions can develop their own nerve supply, creating a direct interaction between the lesions and the central nervous system. And some clinicians have been employing treatment strategies that employ multimodal therapies – employing physical therapists, mental health practitioners, nutritionists, and pain specialists prior to and following surgical removal of lesions – to improve overall success rates of treatment.
But Dr. Zondervan’s team is the first to uncover an important clue about how this happens.
The study findings also provide solid clues to researchers about which genes and proteins to focus on for drug target discovery. In particular, the gene pathways shared by endometriosis and various pain conditions could allow for repurposing of drugs developed for other conditions for treating endometriosis, reported Dr. Zondervan.
Dr. Zondervan’s other important conclusion, echoed by Dr. Kondo and Dr. Kommagani, is that endometriosis is not one disease. Rather, it appears to be akin to cancer in terms of the heterogeneity of how it presents and the different subtypes of diseases. The Oxford study corroborated this belief, identifying certain genes that were closely associated with cystic lesions in ovaries, but failing to turn up a genetic link to other types of lesions in the pelvis long considered to be part of the spectrum of endometriosis disease.
Dr. Zondervan agreed that the potential link with Fusobacterium is a fascinating area given the critical role of inflammation in the pathogenesis of endometriosis, although she’d like to see the work replicated with larger sample sizes. “From a personal point of view, I’d be really fascinated to see how genetics interplays with this,” she added.
What’s next?
The chief limitation of human studies looking at mechanisms of endometriosis is that they are correlational: Tissue samples are collected from women with and without endometriosis, often through an invasive procedure such as laparoscopy or biopsy, at one point in time. Currently, the best tools for proving causation are animal models of endometriosis, such as the those used by Dr. Kondo’s and Dr. Kommagani’s teams.
Better diagnostic tools would solve that problem. The ultimate goal is a noninvasive test for endometriosis that would allow clinicians to follow women over time and permit the monitoring of disease progression, or regression, without the need for painful procedures. Such a diagnostic tool would facilitate rigorous longitudinal studies evaluating mechanisms of disease, as well as monitoring outcomes of clinical trials of new treatments.
Could stool samples be the answer?
The Japanese team found that women harboring Fusobacterium in endometrial tissue also had Fusobacterium in vaginal samples taken at the time of their hysterectomy – and stool samples can pick up changes in the gut microbiome.
“Vaginal swab or stool tests are probably the best and easiest for noninvasive early detection,” Dr. Kommagani said.
Spit tests for DNA would be even easier to obtain. Polygenic risk scores could be developed to estimate an individual’s risk of disease based on the number of variants, but Dr. Zondervan cautioned that not all the genes that account for endometriosis are known.
“The things that we found altogether explain about 5% of disease variability, basically – which is still not an awful lot,” she said.
Dr. Kondo’s work was supported by the Grant-in-Aid for Scientific Research, the Japan Society for the Promotion of Science, and the Research Grant of the Princess Takamatsu Cancer Research Fund. A patent method for detecting bacteria of genus Fusobacterium in order to diagnose endometriosis (WO2023/ 042714), was submitted (international publication date, March 23, 2023).
Dr. Kommagani’s work was funded, in part, by National Institutes of Health/National Institute of Child Health and Human Development grants R01HD102680, R01HD065435, and R00HD080742. He has no other conflicts of interest. Dr. Zondervan received funding from the Wellcome Trust (216767; 104036; 084766; 212904; 076113 and 085475) and also reported grants from Bayer AG, AbbVie, Volition Rx, MDNA Life Sciences, and Roche Diagnostics outside the submitted work.
Dr. Thomas is a pediatrician and epidemiologist living in Portland, Ore.
A version of this article originally appeared on Medscape.com.
In 1927, American gynecologist John Sampson published his theory of the etiology of endometriosis, postulating that retrograde flow of endometrial debris flows backward through the fallopian tubes during menses into the peritoneal cavity. Dr. Sampson’s notion remains the main paradigm today, mentioned still in recent articles on the topic, but it has a flaw: Although the theory may account for how endometrial tissue escapes the uterus, a 1984 study revealed that this phenomenon occurs in 90% of women. Why, then, do only 10% of women suffer from endometriosis?
Endometriosis describes a condition in which endometrial tissue lining the uterus is found outside the uterus. The disease can be painful, even crippling. As many as 30% of women in their reproductive years who have endometriosis are infertile as a consequence. The hallmarks of the condition are superficial peritoneal lesions of varying color, cysts in the ovaries, deeper nodules accompanied by scarring and adhesion, primarily in the pelvis but sometimes appearing outside the pelvis. The syndrome can be challenging to identify, requiring laparoscopy for definitive diagnosis.
John Sampson aside, scientists have struggled for the past century to identify the cause, or causes, of endometriosis. Hormones clearly play a role in its development, and women with endometriosis have an elevated risk of clear-cell and endometrioid ovarian cancer and autoimmune diseases. Immunodeficiency also could be to blame, if a faulty immune system fails to find and remove endometrial tissue outside of the uterus. A class of chemicals known as endocrine disruptors have been linked to endometriosis, but not definitively. Twin studies have demonstrated that as many as 50% of cases have a genetic basis, while mice with surgically induced endometriosis have been found to have a higher ratio of harmful to beneficial bacteria in their gut.
Several studies published this year point to new insights into the old mystery – with possible implications for ways to treat the disorder.
Perhaps the most surprising came out earlier this year in Science Translational Medicine, as a team of researchers in Japan reported that invasive infection by bacteria of the genus Fusobacterium may cause at least some cases of endometriosis.
Is Fusobacterium the new Helicobacter pylori?
The researchers, from Nagoya University, are the first to suggest that not only might a single bacterial genus cause endometriosis, but that antibiotic treatment could prevent progression of the disease. Using endometrial tissue obtained from 79 women undergoing hysterectomy for endometriosis and 76 women undergoing hysterectomy for other reasons (such as cervical cancer), the team started with gene expression profiling to explore differences between the two sets of samples.
They uncovered an interesting chain of cellular events: macrophages found in endometriotic lesions were secreting transforming growth factor-beta (TGF-beta). TGF-beta in turn stimulated high levels of expression of a gene called TAGLN in fibroblast cells from women with endometriosis but not in fibroblasts from women without endometriosis.
Turning on TAGLN transformed these previously inactive cells into active myofibroblasts, leading to increased proliferation, mobility, and attachment to mesothelial cells, the layer of cells that line body cavities and internal organs. In short, they identified some key players in an environment that seemed very favorable to the development of endometriosis.
“So, the question is: Why are macrophages activated?” said Yutaka Kondo, MD, PhD, the senior author of the study and a professor in the division of cancer biology at the Nagoya (Japan) University Graduate School of Medicine. “We think that there are always bacteria in the endometrium.”
After reviewing data from a previously published study, they used quantitative polymerase chain reaction to rule out one candidate, Erysipelothrix, but scored on their next attempt, identifying Fusobacterium species in endometrial tissue from 64% of the women with endometriosis, compared with fewer than 10% of the controls.
To confirm that the bacteria could cause disease and were not simply bystanders, Dr. Kondo’s team turned to a mouse model for endometriosis, in which endometrial cells are surgically removed from the uteri of mice and injected into the peritoneum of recipient mice, leading to the formation of endometriotic lesions. When mice received further injections of uterine tissue from mice that were infected with F. nucleatum, their lesions were more numerous when compared with mice that received injections of uninfected uterine tissue. Furthermore, antibiotic treatment with metronidazole or chloramphenicol immediately after surgery largely prevented progression to endometriosis, Dr. Kondo and his colleagues reported.
Dr. Kondo likened this relationship between Fusobacterium and endometriosis to that of the link between Helicobacter pylori and peptic ulcers but acknowledged that he doesn’t have all the answers.
“We need more clinical trials, and also we have to know what kind of treatment might be the most effective for the treatment of endometriosis,” Dr. Kondo said, pointing out that other therapies should still be pursued in addition to antibiotics, as not all the samples from women with endometriosis harbored Fusobacterium. “It might be possible that other mechanisms are also involved.”
Don’t write off gut microbiota
Ramakrishna Kommagani, PhD, associate professor of pathology and immunology at Baylor College of Medicine in Houston, agreed. “Endometriosis is a complex disease, which appears to be impacted by many factors, including genetic, epigenetic, and environmental factors,” Dr. Kommagani said.
A key difference between his work and Dr. Kondo’s is his focus on gut microbiota, whereas the Japanese team looked at bacteria in the vagina and endometrium. But Dr. Kommagani said he thinks both could play a role. “Maybe the vaginal microbiome might have a direct impact on disease similar to what we showed on the gut,” he said.
But he said at least part of the answer to why some women develop endometriosis may have to do more with the balance of beneficial and harmful bacteria in the gut rather than because of a single family of microbes like Fusobacterium.
Most recently, by dovetailing a mouse model for inducing endometriosis in mice treated with antibiotics to deplete their gut microbiome, Dr. Kommagani’s lab expanded on its previous work: They showed that the animals developed fewer of the typical lesions seen in endometriosis than those that did not receive antibiotics before all of the mice underwent the surgical procedure used by researchers to induce endometriosis – possibly because they had no bacteria in their gut triggering the inflammatory response required for the development of endometriosis.
But after oral feedings with fecal matter from mice without endometriosis, the microbiota-depleted rodents began developing lesions typical of endometriosis, suggesting that altered gut flora from mice with endometriosis appeared to promote the disorder. Meanwhile, their microbiota-depleted counterparts who were fed fecal matter from mice without endometriosis did not develop the typical lesions.
Dr. Kommagani’s team then compared metabolites from bacteria in stool from mice with and without endometriosis and investigated the in vitro effect of these metabolites on cells from human endometriotic lesions. One of them, quinic acid, increased the proliferation of human endometriotic epithelial cells.
“Some metabolites such as fiber-derived short-chain fatty acids have beneficial effects; they inhibit the disease,” Dr. Kommagani said. “But maybe an amino acid derivative such as quinic acid, [may] promote disease, and these are generated because there is a gut dysbiosis.”
This statement hints at some of the possible therapeutic approaches for endometriosis, such as a high-fiber diet to promote healthy gut flora, or perhaps antibiotics to eradicate unhealthy bacteria. But as with other conditions that have been linked to dysbiosis, like inflammatory bowel disease, use of antibiotics is a bit like balancing on a tightrope; although antibiotics may remove harmful bacteria, their use may negatively affect the beneficial bacteria.
Clues in genetic variants
Krina Zondervan, DPhil, professor and head of the department of reproductive and genomic epidemiology at the University of Oxford (England), focuses on genomic, molecular, and epidemiologic approaches to understanding endometriosis.
“It’s one thing identifying risk variants and the next question is, okay, well, what do those variants actually do in terms of biology?” Dr. Zondervan said. The Oxford team next explored how the identified genetic variants affect gene expression and the proteins generated, drawing on previously collected data on gene expression from samples of human blood and endometrial and uterine tissue.
They found many of the genes implicated in the risk for endometriosis code for proteins that affect sex hormones, uterine development, transformation of healthy cells into cancerous tissue, inflammatory adhesion molecules, and factors promoting development of new blood vessels. All of that, she said, explains how a few endometrial cells making their way into the pelvis can attach to ovaries, ligaments, and peritoneal surfaces; proliferate; and acquire a blood supply to ensure their survival.
“We were able to identify a whole host of things that were likely causal to the disease,” Dr. Zondervan said. And that finding led to her next question: “Are there particular genes or areas around them that can be targeted with certain medications?”
The surprising answer was that several of the genes linked to endometriosis share pathways with clinical syndromes that often occur in women with endometriosis. Many of these are chronic pain conditions – such as migraines, headaches, and back pain – but also include inflammatory illnesses such as asthma and osteoarthritis.
As Dr. Zondervan explained, “A lot of the variance that we see for endometriosis is also experienced for low back pain and migraine, and that clearly has something to do with pain perception and pain mechanisms.”
A connection between the development of neural pathways and endometriosis has been proposed before, as researchers have found that endometriotic lesions can develop their own nerve supply, creating a direct interaction between the lesions and the central nervous system. And some clinicians have been employing treatment strategies that employ multimodal therapies – employing physical therapists, mental health practitioners, nutritionists, and pain specialists prior to and following surgical removal of lesions – to improve overall success rates of treatment.
But Dr. Zondervan’s team is the first to uncover an important clue about how this happens.
The study findings also provide solid clues to researchers about which genes and proteins to focus on for drug target discovery. In particular, the gene pathways shared by endometriosis and various pain conditions could allow for repurposing of drugs developed for other conditions for treating endometriosis, reported Dr. Zondervan.
Dr. Zondervan’s other important conclusion, echoed by Dr. Kondo and Dr. Kommagani, is that endometriosis is not one disease. Rather, it appears to be akin to cancer in terms of the heterogeneity of how it presents and the different subtypes of diseases. The Oxford study corroborated this belief, identifying certain genes that were closely associated with cystic lesions in ovaries, but failing to turn up a genetic link to other types of lesions in the pelvis long considered to be part of the spectrum of endometriosis disease.
Dr. Zondervan agreed that the potential link with Fusobacterium is a fascinating area given the critical role of inflammation in the pathogenesis of endometriosis, although she’d like to see the work replicated with larger sample sizes. “From a personal point of view, I’d be really fascinated to see how genetics interplays with this,” she added.
What’s next?
The chief limitation of human studies looking at mechanisms of endometriosis is that they are correlational: Tissue samples are collected from women with and without endometriosis, often through an invasive procedure such as laparoscopy or biopsy, at one point in time. Currently, the best tools for proving causation are animal models of endometriosis, such as the those used by Dr. Kondo’s and Dr. Kommagani’s teams.
Better diagnostic tools would solve that problem. The ultimate goal is a noninvasive test for endometriosis that would allow clinicians to follow women over time and permit the monitoring of disease progression, or regression, without the need for painful procedures. Such a diagnostic tool would facilitate rigorous longitudinal studies evaluating mechanisms of disease, as well as monitoring outcomes of clinical trials of new treatments.
Could stool samples be the answer?
The Japanese team found that women harboring Fusobacterium in endometrial tissue also had Fusobacterium in vaginal samples taken at the time of their hysterectomy – and stool samples can pick up changes in the gut microbiome.
“Vaginal swab or stool tests are probably the best and easiest for noninvasive early detection,” Dr. Kommagani said.
Spit tests for DNA would be even easier to obtain. Polygenic risk scores could be developed to estimate an individual’s risk of disease based on the number of variants, but Dr. Zondervan cautioned that not all the genes that account for endometriosis are known.
“The things that we found altogether explain about 5% of disease variability, basically – which is still not an awful lot,” she said.
Dr. Kondo’s work was supported by the Grant-in-Aid for Scientific Research, the Japan Society for the Promotion of Science, and the Research Grant of the Princess Takamatsu Cancer Research Fund. A patent method for detecting bacteria of genus Fusobacterium in order to diagnose endometriosis (WO2023/ 042714), was submitted (international publication date, March 23, 2023).
Dr. Kommagani’s work was funded, in part, by National Institutes of Health/National Institute of Child Health and Human Development grants R01HD102680, R01HD065435, and R00HD080742. He has no other conflicts of interest. Dr. Zondervan received funding from the Wellcome Trust (216767; 104036; 084766; 212904; 076113 and 085475) and also reported grants from Bayer AG, AbbVie, Volition Rx, MDNA Life Sciences, and Roche Diagnostics outside the submitted work.
Dr. Thomas is a pediatrician and epidemiologist living in Portland, Ore.
A version of this article originally appeared on Medscape.com.
Noninvasive Methods for the Diagnosis of Endometriosis
What is the value of considering noninvasive methods for the diagnosis of endometriosis?
Dr. Flores: There is great value in noninvasive diagnostics for endometriosis. This is because while surgical diagnosis is the “gold standard,” surgery is invasive, and waiting until a surgical diagnosis can be made further contributes to delays in diagnosis. However, more recently there has been a shift toward utilizing noninvasive approaches to the diagnosis of endometriosis, with the primary one focusing on clinically diagnosing endometriosis.
One of the first things to remember is the importance of gathering a patient history and conducting a physical exam. We've all learned this in medical school, and it comes into play even more so with a condition such as endometriosis. Endometriosis is defined as a benign gynecologic disease characterized by endometrial-like tissue outside of the uterus, but this definition does not reflect the true scope and manifestations of endometriosis. Research over the years has demonstrated that endometriosis has systemic effects—affecting regions of the brain associated with anxiety/depression, altering pain sensitization, and having inflammatory effects that can not only affect the reproductive organs but also other organ systems. As such, our questions when evaluating patients for endometriosis need to focus on these various aspects of the disease.
Endometriosis usually leads to cyclic pain. This is because just as the lining of the uterus (the endometrium) grows and sheds every month in response to hormones, endometriotic lesions—which are endometrial-like tissue outside of the uterus—also grow and shed each month. However, there is no outflow for this shed tissue and, as a result, there is an inflammatory response as well as pain. Depending on where those lesions implant, symptoms can include not only cyclic pelvic pain but also cyclic bowel/bladder pain. I’ve also had patients complain of cyclic sharp/shooting leg pain.
Many times, patients present to us after having seen several different types of providers and having been diagnosed with conditions such as irritable bowel syndrome or painful bladder syndrome. However, if you talk to patients and ask them to tell you a little bit more about this bowel or bladder pain, they will frequently endorse that their symptoms are cyclic/most severe during their menses. With respect to pelvic pain, endometriosis-related pelvic pain is usually progressive—becoming progressively more painful over the years. These symptoms are strong indicators that endometriosis is the cause. A pelvic exam is also helpful as findings of nodularity or a fixed uterus may lend further support for endometriosis; a normal exam, however, does not rule out endometriosis.
What are the primary imaging techniques used to diagnose endometriosis?
Dr. Flores: While history and physical exam are the primary components of the clinical diagnosis, imaging can also be helpful. The 2 techniques most often used are pelvic ultrasound and magnetic resonance imaging (MRI).
While transvaginal ultrasound is sensitive and specific for diagnosing endometriomas (ovarian cysts of endometriotic tissue) and may also be able to accurately identify deep-infiltrating endometriosis, it is limited in its ability to visualize peritoneal disease. MRI can improve diagnosis of endometriosis and better estimate the depth of invasion of deep-infiltrating disease, as well as confirm diagnosis of an endometrioma. While MRI is an option for peritoneal endometriosis, superficial disease is usually not detected. Lastly, computed tomography imaging of the chest can be used when thoracic endometriosis is suspected but is otherwise not routinely recommended. Imaging is also helpful in ruling in/out other potential etiologies of pelvic pain such as fibroids and adenomyosis. It is important to recognize, however, that the absence of any findings of endometriosis on imaging does not rule out the disease.
What other best practices do you implement in your day-to-day to aid in diagnosis?
Dr. Flores: Take the time to listen to your patient. Often, they’ve seen several providers before ultimately seeing a provider who can diagnosis their endometriosis without the need for surgical evaluation. We have to ask questions related to their pain and when the pain occurs, and we can’t forget to also ask about pain during intercourse, as well as non-menstrual pelvic pain. Additionally, it is important to recognize that, for patients who may have been suffering from endometriosis for several years before reaching a diagnosis, they may present with chronic pelvic pain. In this case, it is important to ask what their menstrual cycles were like before the pelvic pain became chronic, and usually patients note cyclic pelvic pain that became progressive. We also know that patients who have a first-degree relative with endometriosis are 7 times more likely to be affected by the disease, so asking about a family history of endometriosis is important.
We have to think about endometriosis as a systemic disease. Previously, endometriosis was incorrectly thought of as solely a pelvic disease, but we've been learning more and more through research that it truly is a chronic, systemic disease with multifactorial effects throughout the body. For example, we have found that endometriosis affects regions of the brain associated with anxiety and depression, as well as causing changes in metabolism. For example, a common misconception is that women with a low body mass index (BMI) were at risk for endometriosis, when in fact it's just the opposite—it is the endometriosis that is causing changes in metabolism that lead to a decreased BMI. Patients with endometriosis also frequently struggle with mood disorders; therefore, we cannot dismiss this aspect of the disease process. It is imperative that we help patients feel heard and let them know that some of the mood symptoms they are experiencing may be related to their endometriosis. Expanding our view of endometriosis as a disease that extends beyond the pelvis and thinking about the systemic effects of endometriosis is key.
We have also identified small molecules (microRNAs) that are predictive of endometriosis. They are continuing to be investigated as a noninvasive biomarker of endometriosis.
Can you talk a little more about these biomarkers?
Dr. Flores: In terms of biomarkers, this is actually some exciting work I was fortunate to be involved in with Dr. Hugh Taylor at Yale. We studied circulating molecules known as microRNAs—these are small, noncoding RNAs that can modify gene expression. In endometriosis, we've identified several that, when combined, have a high sensitivity and specificity for diagnosing endometriosis. These specific microRNAs are undergoing continued studies to ensure that they are reliable in predicting endometriosis. Hopefully they will be available soon for clinical use, as this would be of great value to help shorten the time to diagnosis of endometriosis and ultimately avoid delays in endometriosis treatment.
What is the value of considering noninvasive methods for the diagnosis of endometriosis?
Dr. Flores: There is great value in noninvasive diagnostics for endometriosis. This is because while surgical diagnosis is the “gold standard,” surgery is invasive, and waiting until a surgical diagnosis can be made further contributes to delays in diagnosis. However, more recently there has been a shift toward utilizing noninvasive approaches to the diagnosis of endometriosis, with the primary one focusing on clinically diagnosing endometriosis.
One of the first things to remember is the importance of gathering a patient history and conducting a physical exam. We've all learned this in medical school, and it comes into play even more so with a condition such as endometriosis. Endometriosis is defined as a benign gynecologic disease characterized by endometrial-like tissue outside of the uterus, but this definition does not reflect the true scope and manifestations of endometriosis. Research over the years has demonstrated that endometriosis has systemic effects—affecting regions of the brain associated with anxiety/depression, altering pain sensitization, and having inflammatory effects that can not only affect the reproductive organs but also other organ systems. As such, our questions when evaluating patients for endometriosis need to focus on these various aspects of the disease.
Endometriosis usually leads to cyclic pain. This is because just as the lining of the uterus (the endometrium) grows and sheds every month in response to hormones, endometriotic lesions—which are endometrial-like tissue outside of the uterus—also grow and shed each month. However, there is no outflow for this shed tissue and, as a result, there is an inflammatory response as well as pain. Depending on where those lesions implant, symptoms can include not only cyclic pelvic pain but also cyclic bowel/bladder pain. I’ve also had patients complain of cyclic sharp/shooting leg pain.
Many times, patients present to us after having seen several different types of providers and having been diagnosed with conditions such as irritable bowel syndrome or painful bladder syndrome. However, if you talk to patients and ask them to tell you a little bit more about this bowel or bladder pain, they will frequently endorse that their symptoms are cyclic/most severe during their menses. With respect to pelvic pain, endometriosis-related pelvic pain is usually progressive—becoming progressively more painful over the years. These symptoms are strong indicators that endometriosis is the cause. A pelvic exam is also helpful as findings of nodularity or a fixed uterus may lend further support for endometriosis; a normal exam, however, does not rule out endometriosis.
What are the primary imaging techniques used to diagnose endometriosis?
Dr. Flores: While history and physical exam are the primary components of the clinical diagnosis, imaging can also be helpful. The 2 techniques most often used are pelvic ultrasound and magnetic resonance imaging (MRI).
While transvaginal ultrasound is sensitive and specific for diagnosing endometriomas (ovarian cysts of endometriotic tissue) and may also be able to accurately identify deep-infiltrating endometriosis, it is limited in its ability to visualize peritoneal disease. MRI can improve diagnosis of endometriosis and better estimate the depth of invasion of deep-infiltrating disease, as well as confirm diagnosis of an endometrioma. While MRI is an option for peritoneal endometriosis, superficial disease is usually not detected. Lastly, computed tomography imaging of the chest can be used when thoracic endometriosis is suspected but is otherwise not routinely recommended. Imaging is also helpful in ruling in/out other potential etiologies of pelvic pain such as fibroids and adenomyosis. It is important to recognize, however, that the absence of any findings of endometriosis on imaging does not rule out the disease.
What other best practices do you implement in your day-to-day to aid in diagnosis?
Dr. Flores: Take the time to listen to your patient. Often, they’ve seen several providers before ultimately seeing a provider who can diagnosis their endometriosis without the need for surgical evaluation. We have to ask questions related to their pain and when the pain occurs, and we can’t forget to also ask about pain during intercourse, as well as non-menstrual pelvic pain. Additionally, it is important to recognize that, for patients who may have been suffering from endometriosis for several years before reaching a diagnosis, they may present with chronic pelvic pain. In this case, it is important to ask what their menstrual cycles were like before the pelvic pain became chronic, and usually patients note cyclic pelvic pain that became progressive. We also know that patients who have a first-degree relative with endometriosis are 7 times more likely to be affected by the disease, so asking about a family history of endometriosis is important.
We have to think about endometriosis as a systemic disease. Previously, endometriosis was incorrectly thought of as solely a pelvic disease, but we've been learning more and more through research that it truly is a chronic, systemic disease with multifactorial effects throughout the body. For example, we have found that endometriosis affects regions of the brain associated with anxiety and depression, as well as causing changes in metabolism. For example, a common misconception is that women with a low body mass index (BMI) were at risk for endometriosis, when in fact it's just the opposite—it is the endometriosis that is causing changes in metabolism that lead to a decreased BMI. Patients with endometriosis also frequently struggle with mood disorders; therefore, we cannot dismiss this aspect of the disease process. It is imperative that we help patients feel heard and let them know that some of the mood symptoms they are experiencing may be related to their endometriosis. Expanding our view of endometriosis as a disease that extends beyond the pelvis and thinking about the systemic effects of endometriosis is key.
We have also identified small molecules (microRNAs) that are predictive of endometriosis. They are continuing to be investigated as a noninvasive biomarker of endometriosis.
Can you talk a little more about these biomarkers?
Dr. Flores: In terms of biomarkers, this is actually some exciting work I was fortunate to be involved in with Dr. Hugh Taylor at Yale. We studied circulating molecules known as microRNAs—these are small, noncoding RNAs that can modify gene expression. In endometriosis, we've identified several that, when combined, have a high sensitivity and specificity for diagnosing endometriosis. These specific microRNAs are undergoing continued studies to ensure that they are reliable in predicting endometriosis. Hopefully they will be available soon for clinical use, as this would be of great value to help shorten the time to diagnosis of endometriosis and ultimately avoid delays in endometriosis treatment.
What is the value of considering noninvasive methods for the diagnosis of endometriosis?
Dr. Flores: There is great value in noninvasive diagnostics for endometriosis. This is because while surgical diagnosis is the “gold standard,” surgery is invasive, and waiting until a surgical diagnosis can be made further contributes to delays in diagnosis. However, more recently there has been a shift toward utilizing noninvasive approaches to the diagnosis of endometriosis, with the primary one focusing on clinically diagnosing endometriosis.
One of the first things to remember is the importance of gathering a patient history and conducting a physical exam. We've all learned this in medical school, and it comes into play even more so with a condition such as endometriosis. Endometriosis is defined as a benign gynecologic disease characterized by endometrial-like tissue outside of the uterus, but this definition does not reflect the true scope and manifestations of endometriosis. Research over the years has demonstrated that endometriosis has systemic effects—affecting regions of the brain associated with anxiety/depression, altering pain sensitization, and having inflammatory effects that can not only affect the reproductive organs but also other organ systems. As such, our questions when evaluating patients for endometriosis need to focus on these various aspects of the disease.
Endometriosis usually leads to cyclic pain. This is because just as the lining of the uterus (the endometrium) grows and sheds every month in response to hormones, endometriotic lesions—which are endometrial-like tissue outside of the uterus—also grow and shed each month. However, there is no outflow for this shed tissue and, as a result, there is an inflammatory response as well as pain. Depending on where those lesions implant, symptoms can include not only cyclic pelvic pain but also cyclic bowel/bladder pain. I’ve also had patients complain of cyclic sharp/shooting leg pain.
Many times, patients present to us after having seen several different types of providers and having been diagnosed with conditions such as irritable bowel syndrome or painful bladder syndrome. However, if you talk to patients and ask them to tell you a little bit more about this bowel or bladder pain, they will frequently endorse that their symptoms are cyclic/most severe during their menses. With respect to pelvic pain, endometriosis-related pelvic pain is usually progressive—becoming progressively more painful over the years. These symptoms are strong indicators that endometriosis is the cause. A pelvic exam is also helpful as findings of nodularity or a fixed uterus may lend further support for endometriosis; a normal exam, however, does not rule out endometriosis.
What are the primary imaging techniques used to diagnose endometriosis?
Dr. Flores: While history and physical exam are the primary components of the clinical diagnosis, imaging can also be helpful. The 2 techniques most often used are pelvic ultrasound and magnetic resonance imaging (MRI).
While transvaginal ultrasound is sensitive and specific for diagnosing endometriomas (ovarian cysts of endometriotic tissue) and may also be able to accurately identify deep-infiltrating endometriosis, it is limited in its ability to visualize peritoneal disease. MRI can improve diagnosis of endometriosis and better estimate the depth of invasion of deep-infiltrating disease, as well as confirm diagnosis of an endometrioma. While MRI is an option for peritoneal endometriosis, superficial disease is usually not detected. Lastly, computed tomography imaging of the chest can be used when thoracic endometriosis is suspected but is otherwise not routinely recommended. Imaging is also helpful in ruling in/out other potential etiologies of pelvic pain such as fibroids and adenomyosis. It is important to recognize, however, that the absence of any findings of endometriosis on imaging does not rule out the disease.
What other best practices do you implement in your day-to-day to aid in diagnosis?
Dr. Flores: Take the time to listen to your patient. Often, they’ve seen several providers before ultimately seeing a provider who can diagnosis their endometriosis without the need for surgical evaluation. We have to ask questions related to their pain and when the pain occurs, and we can’t forget to also ask about pain during intercourse, as well as non-menstrual pelvic pain. Additionally, it is important to recognize that, for patients who may have been suffering from endometriosis for several years before reaching a diagnosis, they may present with chronic pelvic pain. In this case, it is important to ask what their menstrual cycles were like before the pelvic pain became chronic, and usually patients note cyclic pelvic pain that became progressive. We also know that patients who have a first-degree relative with endometriosis are 7 times more likely to be affected by the disease, so asking about a family history of endometriosis is important.
We have to think about endometriosis as a systemic disease. Previously, endometriosis was incorrectly thought of as solely a pelvic disease, but we've been learning more and more through research that it truly is a chronic, systemic disease with multifactorial effects throughout the body. For example, we have found that endometriosis affects regions of the brain associated with anxiety and depression, as well as causing changes in metabolism. For example, a common misconception is that women with a low body mass index (BMI) were at risk for endometriosis, when in fact it's just the opposite—it is the endometriosis that is causing changes in metabolism that lead to a decreased BMI. Patients with endometriosis also frequently struggle with mood disorders; therefore, we cannot dismiss this aspect of the disease process. It is imperative that we help patients feel heard and let them know that some of the mood symptoms they are experiencing may be related to their endometriosis. Expanding our view of endometriosis as a disease that extends beyond the pelvis and thinking about the systemic effects of endometriosis is key.
We have also identified small molecules (microRNAs) that are predictive of endometriosis. They are continuing to be investigated as a noninvasive biomarker of endometriosis.
Can you talk a little more about these biomarkers?
Dr. Flores: In terms of biomarkers, this is actually some exciting work I was fortunate to be involved in with Dr. Hugh Taylor at Yale. We studied circulating molecules known as microRNAs—these are small, noncoding RNAs that can modify gene expression. In endometriosis, we've identified several that, when combined, have a high sensitivity and specificity for diagnosing endometriosis. These specific microRNAs are undergoing continued studies to ensure that they are reliable in predicting endometriosis. Hopefully they will be available soon for clinical use, as this would be of great value to help shorten the time to diagnosis of endometriosis and ultimately avoid delays in endometriosis treatment.
Endometriosis and Abnormal Uterine Bleeding
What is the link between endometriosis and abnormal uterine bleeding?
Dr. Lager: This is an important question because when people first learn about endometriosis, common symptoms include pain with periods, pelvic pain, but not necessarily abnormal uterine bleeding. However, many patients do complain of abnormal uterine bleeding when presenting with endometriosis.
There are a couple of reasons why abnormal uterine bleeding is important to consider. Within the spectrum of endometriosis, vaginal endometriosis can contribute to abnormal vaginal bleeding, most commonly cyclic or postcoital. The bleeding could be rectal due to deeply infiltrative endometriosis, although gastrointestinal etiologies should be included in the differential. Another link is coexisting diagnoses such as fibroids, adenomyosis, and endometrial polyps. In fact, the rates for coexisting conditions with endometriosis can be high and vary from study to study.
As an example, some studies show rates between 7% and 11%, where adenomyosis coexists with endometriosis. Other studies look at magnetic resonance imaging for adenomyosis and deep infiltrative endometriosis and find that women younger than 36 years have rates as high as 90% for coexisting diagnoses, and 79% for all women, regardless of the diagnosis.
The overlap is high. When I think particularly about adenomyosis and endometriosis, in some ways, the conditions are along a spectrum where adenomyosis involves ectopic endometrial glands in the myometrium, whereas endometriosis involves ectopic tissue outside of the uterus, predominantly in reproductive organs, but can be anywhere outside of the endometrium. So, when I think about abnormal uterine bleeding particularly associated with dysmenorrhea or pelvic pain, this can often be included in the constellation of symptoms for endometriosis.
Furthermore, it is important to rule out other causes of abnormal uterine bleeding because they would potentially change the treatment.
What are the current treatment options for endometriosis and abnormal uterine bleeding?
Dr. Lager: Treatments for endometriosis are inclusive of any overlapping conditions and we use a multidisciplinary approach to address symptoms. Medical treatments include hormonal management, including birth control pills, etonogestrel implants (Nexplanon), levonorgestrel-releasing intrauterine devices, progestin-only pills, gonadotropin-releasing hormone (GnRH) agonists, GnRH antagonists, and combination medications. Some medications do overlap and work for both, such as combined GnRH antagonists, estradiol, and progesterone.
Surgical management includes diagnostic laparoscopy with excision of endometriosis. If there is another coexisting diagnosis that is structural in nature, such as endometrial polyps, adenomyosis, or fibroids, surgical management may include hysteroscopy, myomectomy, or hysterectomy as indicated. When we consider surgical and nonsurgical approaches, it is important to be clear on the etiology of abnormal uterine bleeding to appropriately counsel patients for what the surgery could entail.
Have you found there to be any age or racial disparities in endometriosis treatment?
Dr. Lager: One of the things that is important about endometriosis, and in medicine in general, is to really think about how we approach race as a social construct. In the past, medicine has included race as a risk factor for certain medical conditions. And physicians in training were taught to use these risk factors to determine a differential diagnosis. However, this strategy has limited us in understanding how historical and structural racism affected patient diagnosis and treatment.
If we think back to literature that was published in the 1950s or the 1970s, Dr. Meeks was one of the physicians who described a set of characteristics of patients with endometriosis. He commented that typical patients were women who were goal-oriented, had private insurance, and experienced delayed marriage, among other traits.
The problem with this characterization was that patients would then present with symptoms of endometriosis who did not fit the original phenotype as historically described and they would be misdiagnosed and thus treated incorrectly. This incorrect treatment further reinforced incorrect stereotypes of patient presentations. These misdiagnoses could lead to unfortunate consequences in their activities of daily living as well as reproductive outcomes. We do not have data on how many patients may have been misdiagnosed and treated for pelvic inflammatory disease because they were not White, did not have private insurance, or had children early. This is an example of areas where we need to recognize systemic racism and classism and work hard to simply do better for our patients.
Although misdiagnosing based on stereotypes has decreased over time, I still think that original thinking can certainly affect patient referrals. When we look at the data of patients who are diagnosed with endometriosis, we find a higher rate of White patients (17%) compared to Black (10.1%), Asian (11.3%), and Hispanic patients (7.4%). Ensuring that all of our patients are getting appropriate referrals and diagnosis should be a priority.
When we think about the timing to initial diagnosis, globally, we know that there is a delay in diagnosis anywhere from 7 to 12 years, and then on top of that, those social constructs decrease the rate of diagnosis for certain patient populations. Misdiagnosis based on social constructs is unacceptable and one aspect that I think is very important to point out.
In a more recent study of 12,000 patients in 2022, the rate of surgical complications associated with endometriosis surgery was higher in women who were Black, Asian and Pacific Islander, and Native American/American Indian than in women who were White. These groups have a much higher rate of complications and higher rates of laparotomy—an open procedure—versus laparoscopy. In younger women, there is a higher rate of oophorectomy at the time of surgery for endometriosis than in older women.
Are there any best practices you would like to share with your peers?
Dr. Lager: For patients with abnormal uterine bleeding, it is important to consider other diagnoses and not assume that abnormal bleeding is solely related to endometriosis, while considering deeply infiltrative endometriosis in the differential.
When patients do present with cyclical bleeding, especially, for example, after hysterectomy, it is important to examine for either vaginal or vaginal cuff endometriosis because there can be other reasons that patients will have abnormal uterine bleeding related to atypical endometriosis.
It is important to know the patient’s history and focus on each patient’s level of pain, how it affects their day-to-day activities, and how they are experiencing that pain.
We all should be working to improve our understanding of social history and systemic racism as best as we can and make sure all patients are getting the right care that they deserve.
What is the link between endometriosis and abnormal uterine bleeding?
Dr. Lager: This is an important question because when people first learn about endometriosis, common symptoms include pain with periods, pelvic pain, but not necessarily abnormal uterine bleeding. However, many patients do complain of abnormal uterine bleeding when presenting with endometriosis.
There are a couple of reasons why abnormal uterine bleeding is important to consider. Within the spectrum of endometriosis, vaginal endometriosis can contribute to abnormal vaginal bleeding, most commonly cyclic or postcoital. The bleeding could be rectal due to deeply infiltrative endometriosis, although gastrointestinal etiologies should be included in the differential. Another link is coexisting diagnoses such as fibroids, adenomyosis, and endometrial polyps. In fact, the rates for coexisting conditions with endometriosis can be high and vary from study to study.
As an example, some studies show rates between 7% and 11%, where adenomyosis coexists with endometriosis. Other studies look at magnetic resonance imaging for adenomyosis and deep infiltrative endometriosis and find that women younger than 36 years have rates as high as 90% for coexisting diagnoses, and 79% for all women, regardless of the diagnosis.
The overlap is high. When I think particularly about adenomyosis and endometriosis, in some ways, the conditions are along a spectrum where adenomyosis involves ectopic endometrial glands in the myometrium, whereas endometriosis involves ectopic tissue outside of the uterus, predominantly in reproductive organs, but can be anywhere outside of the endometrium. So, when I think about abnormal uterine bleeding particularly associated with dysmenorrhea or pelvic pain, this can often be included in the constellation of symptoms for endometriosis.
Furthermore, it is important to rule out other causes of abnormal uterine bleeding because they would potentially change the treatment.
What are the current treatment options for endometriosis and abnormal uterine bleeding?
Dr. Lager: Treatments for endometriosis are inclusive of any overlapping conditions and we use a multidisciplinary approach to address symptoms. Medical treatments include hormonal management, including birth control pills, etonogestrel implants (Nexplanon), levonorgestrel-releasing intrauterine devices, progestin-only pills, gonadotropin-releasing hormone (GnRH) agonists, GnRH antagonists, and combination medications. Some medications do overlap and work for both, such as combined GnRH antagonists, estradiol, and progesterone.
Surgical management includes diagnostic laparoscopy with excision of endometriosis. If there is another coexisting diagnosis that is structural in nature, such as endometrial polyps, adenomyosis, or fibroids, surgical management may include hysteroscopy, myomectomy, or hysterectomy as indicated. When we consider surgical and nonsurgical approaches, it is important to be clear on the etiology of abnormal uterine bleeding to appropriately counsel patients for what the surgery could entail.
Have you found there to be any age or racial disparities in endometriosis treatment?
Dr. Lager: One of the things that is important about endometriosis, and in medicine in general, is to really think about how we approach race as a social construct. In the past, medicine has included race as a risk factor for certain medical conditions. And physicians in training were taught to use these risk factors to determine a differential diagnosis. However, this strategy has limited us in understanding how historical and structural racism affected patient diagnosis and treatment.
If we think back to literature that was published in the 1950s or the 1970s, Dr. Meeks was one of the physicians who described a set of characteristics of patients with endometriosis. He commented that typical patients were women who were goal-oriented, had private insurance, and experienced delayed marriage, among other traits.
The problem with this characterization was that patients would then present with symptoms of endometriosis who did not fit the original phenotype as historically described and they would be misdiagnosed and thus treated incorrectly. This incorrect treatment further reinforced incorrect stereotypes of patient presentations. These misdiagnoses could lead to unfortunate consequences in their activities of daily living as well as reproductive outcomes. We do not have data on how many patients may have been misdiagnosed and treated for pelvic inflammatory disease because they were not White, did not have private insurance, or had children early. This is an example of areas where we need to recognize systemic racism and classism and work hard to simply do better for our patients.
Although misdiagnosing based on stereotypes has decreased over time, I still think that original thinking can certainly affect patient referrals. When we look at the data of patients who are diagnosed with endometriosis, we find a higher rate of White patients (17%) compared to Black (10.1%), Asian (11.3%), and Hispanic patients (7.4%). Ensuring that all of our patients are getting appropriate referrals and diagnosis should be a priority.
When we think about the timing to initial diagnosis, globally, we know that there is a delay in diagnosis anywhere from 7 to 12 years, and then on top of that, those social constructs decrease the rate of diagnosis for certain patient populations. Misdiagnosis based on social constructs is unacceptable and one aspect that I think is very important to point out.
In a more recent study of 12,000 patients in 2022, the rate of surgical complications associated with endometriosis surgery was higher in women who were Black, Asian and Pacific Islander, and Native American/American Indian than in women who were White. These groups have a much higher rate of complications and higher rates of laparotomy—an open procedure—versus laparoscopy. In younger women, there is a higher rate of oophorectomy at the time of surgery for endometriosis than in older women.
Are there any best practices you would like to share with your peers?
Dr. Lager: For patients with abnormal uterine bleeding, it is important to consider other diagnoses and not assume that abnormal bleeding is solely related to endometriosis, while considering deeply infiltrative endometriosis in the differential.
When patients do present with cyclical bleeding, especially, for example, after hysterectomy, it is important to examine for either vaginal or vaginal cuff endometriosis because there can be other reasons that patients will have abnormal uterine bleeding related to atypical endometriosis.
It is important to know the patient’s history and focus on each patient’s level of pain, how it affects their day-to-day activities, and how they are experiencing that pain.
We all should be working to improve our understanding of social history and systemic racism as best as we can and make sure all patients are getting the right care that they deserve.
What is the link between endometriosis and abnormal uterine bleeding?
Dr. Lager: This is an important question because when people first learn about endometriosis, common symptoms include pain with periods, pelvic pain, but not necessarily abnormal uterine bleeding. However, many patients do complain of abnormal uterine bleeding when presenting with endometriosis.
There are a couple of reasons why abnormal uterine bleeding is important to consider. Within the spectrum of endometriosis, vaginal endometriosis can contribute to abnormal vaginal bleeding, most commonly cyclic or postcoital. The bleeding could be rectal due to deeply infiltrative endometriosis, although gastrointestinal etiologies should be included in the differential. Another link is coexisting diagnoses such as fibroids, adenomyosis, and endometrial polyps. In fact, the rates for coexisting conditions with endometriosis can be high and vary from study to study.
As an example, some studies show rates between 7% and 11%, where adenomyosis coexists with endometriosis. Other studies look at magnetic resonance imaging for adenomyosis and deep infiltrative endometriosis and find that women younger than 36 years have rates as high as 90% for coexisting diagnoses, and 79% for all women, regardless of the diagnosis.
The overlap is high. When I think particularly about adenomyosis and endometriosis, in some ways, the conditions are along a spectrum where adenomyosis involves ectopic endometrial glands in the myometrium, whereas endometriosis involves ectopic tissue outside of the uterus, predominantly in reproductive organs, but can be anywhere outside of the endometrium. So, when I think about abnormal uterine bleeding particularly associated with dysmenorrhea or pelvic pain, this can often be included in the constellation of symptoms for endometriosis.
Furthermore, it is important to rule out other causes of abnormal uterine bleeding because they would potentially change the treatment.
What are the current treatment options for endometriosis and abnormal uterine bleeding?
Dr. Lager: Treatments for endometriosis are inclusive of any overlapping conditions and we use a multidisciplinary approach to address symptoms. Medical treatments include hormonal management, including birth control pills, etonogestrel implants (Nexplanon), levonorgestrel-releasing intrauterine devices, progestin-only pills, gonadotropin-releasing hormone (GnRH) agonists, GnRH antagonists, and combination medications. Some medications do overlap and work for both, such as combined GnRH antagonists, estradiol, and progesterone.
Surgical management includes diagnostic laparoscopy with excision of endometriosis. If there is another coexisting diagnosis that is structural in nature, such as endometrial polyps, adenomyosis, or fibroids, surgical management may include hysteroscopy, myomectomy, or hysterectomy as indicated. When we consider surgical and nonsurgical approaches, it is important to be clear on the etiology of abnormal uterine bleeding to appropriately counsel patients for what the surgery could entail.
Have you found there to be any age or racial disparities in endometriosis treatment?
Dr. Lager: One of the things that is important about endometriosis, and in medicine in general, is to really think about how we approach race as a social construct. In the past, medicine has included race as a risk factor for certain medical conditions. And physicians in training were taught to use these risk factors to determine a differential diagnosis. However, this strategy has limited us in understanding how historical and structural racism affected patient diagnosis and treatment.
If we think back to literature that was published in the 1950s or the 1970s, Dr. Meeks was one of the physicians who described a set of characteristics of patients with endometriosis. He commented that typical patients were women who were goal-oriented, had private insurance, and experienced delayed marriage, among other traits.
The problem with this characterization was that patients would then present with symptoms of endometriosis who did not fit the original phenotype as historically described and they would be misdiagnosed and thus treated incorrectly. This incorrect treatment further reinforced incorrect stereotypes of patient presentations. These misdiagnoses could lead to unfortunate consequences in their activities of daily living as well as reproductive outcomes. We do not have data on how many patients may have been misdiagnosed and treated for pelvic inflammatory disease because they were not White, did not have private insurance, or had children early. This is an example of areas where we need to recognize systemic racism and classism and work hard to simply do better for our patients.
Although misdiagnosing based on stereotypes has decreased over time, I still think that original thinking can certainly affect patient referrals. When we look at the data of patients who are diagnosed with endometriosis, we find a higher rate of White patients (17%) compared to Black (10.1%), Asian (11.3%), and Hispanic patients (7.4%). Ensuring that all of our patients are getting appropriate referrals and diagnosis should be a priority.
When we think about the timing to initial diagnosis, globally, we know that there is a delay in diagnosis anywhere from 7 to 12 years, and then on top of that, those social constructs decrease the rate of diagnosis for certain patient populations. Misdiagnosis based on social constructs is unacceptable and one aspect that I think is very important to point out.
In a more recent study of 12,000 patients in 2022, the rate of surgical complications associated with endometriosis surgery was higher in women who were Black, Asian and Pacific Islander, and Native American/American Indian than in women who were White. These groups have a much higher rate of complications and higher rates of laparotomy—an open procedure—versus laparoscopy. In younger women, there is a higher rate of oophorectomy at the time of surgery for endometriosis than in older women.
Are there any best practices you would like to share with your peers?
Dr. Lager: For patients with abnormal uterine bleeding, it is important to consider other diagnoses and not assume that abnormal bleeding is solely related to endometriosis, while considering deeply infiltrative endometriosis in the differential.
When patients do present with cyclical bleeding, especially, for example, after hysterectomy, it is important to examine for either vaginal or vaginal cuff endometriosis because there can be other reasons that patients will have abnormal uterine bleeding related to atypical endometriosis.
It is important to know the patient’s history and focus on each patient’s level of pain, how it affects their day-to-day activities, and how they are experiencing that pain.
We all should be working to improve our understanding of social history and systemic racism as best as we can and make sure all patients are getting the right care that they deserve.