User login
What is the optimal interval for osteoporosis screening in postmenopausal women before fracture occurrence and osteoporosis treatment initiation?
Update on Osteoporosis
Steven R. Goldstein, MD (November 2011)
The optimal screening interval for bone density assessment in menopausal women is an extremely complicated but important issue because osteoporosis and fragility fracture are a major health concern. There are nearly 2 million osteoporotic fractures each year, accounting for 432,000 hospital admissions, 25 million office visits, and an increased risk of disability and death, all at a cost of up to $18 billion.1
There is no question that determination of bone mass (achieved through bone mineral density [BMD] testing by dual energy x-ray absorptiometry [DXA] and reported as T scores) will diagnose osteopenia and osteoporosis, correlate with fracture risk (the lower the bone mass, the higher the incidence of fracture), and monitor changes in bone mass over time.
Medicare allows for BMD testing every 23 months, and that has become standard for many clinicians.
Details of the trial
Gourlay and colleagues studied nearly 5,000 basically healthy women, the youngest of whom was 67 years of age. Women who had osteoporosis and who were taking medication for fracture reduction were excluded, as were women who had a history of pre-existing fracture.
The researchers concluded that the better the initial bone-density score at age 67, the longer it would take for a woman to develop osteoporosis. For instance, if a woman older than 67 years had a T score of –1.00 or better, it would take her 16.8 years (95% confidence interval [CI], 11.5 to 24.6) to reach osteoporosis. In contrast, a woman with a T score of –2.00 would reach osteoporosis in only 1.1 years. Current estrogen use was found to be significantly associated with higher BMD and a longer testing interval, although the authors did not recommend modifying the screening interval on the basis of estrogen use.
These findings certainly call into question the notion that all patients should be screened for osteoporosis every 23 months. Perhaps it is better to think of screening as a way to initially triage patients for decisions relative to follow-up.
Limitations and considerations
Some extremely important observations must be made:
- The article by Gourlay and colleagues created tremendous media attention, most of which implied that there is too much screening with DXA scans. Nothing can be further from the truth. Only 13% of women older than age 65 are actually getting a baseline DXA scan.2
- The data in this report apply only to white women older than 67 years who have no pre-existing fracture and are not taking any medications for osteoporosis. Extrapolation to younger women or other groups is not valid.
- We should not be interested in the development of an arbitrary T score for bone mass but rather the determination of whether a particular patient has a level of fracture risk that warrants pharmacologic intervention.
These observations support use of a model like FRAX (see “What is FRAX?”), which can be used annually even without an updated DXA of the hip. FRAX is much more appropriate than DXA testing every 23 months and should become the clinical standard of care.
The Fracture Risk Assessment (FRAX®) Tool1 has been developed by the World Health Organization (WHO). It is based on individual patient models that integrate the risks associated with clinical factors as well as bone mineral density at the femoral neck. FRAX models have been developed from studying population-based cohorts from Europe, North America, Asia, and Australia.
FRAX algorithms give the 10-year probability of hip fracture and of a major osteoporotic fracture (clinical spine, forearm, hip, or shoulder fracture).
WHO offers sophisticated computer-driven models or simplified, printable versions of FRAX for office use—available at http://www.who-frax.org/.
Reference
1. Welcome to FRAX. FRAX: WHO Fracture Risk Assessment Tool Web site. http://www.shef.ac.uk/FRAX/. Accessed July 7, 2012.
Remember, there are more fragility fractures in nonosteoporotic women than in osteoporotic women. The risk (incidence per 10,000 women) is greater in osteoporotic women, but the absolute number in the population is greater in women who have not yet reached that threshold.
In older healthy women, BMD follow-up arbitrarily at 23 months makes little sense. The interval before reassessment can substantially lengthen for some women with excellent initial T scores, while more frequent assessments should be performed for women with worse initial T scores. Furthermore, strict reliance solely on T scores is not the best method for predicting fracture risk or when to start pharmacologic intervention. Yearly assessment using a tool like FRAX should become the standard.
STEVEN R. GOLDSTEIN, MD
We want to hear from you! Tell us what you think.
1. Lewiecki EM, Laster AJ, Miller PF, Bilezikian JP. More bone density testing is needed, not less. J Bone Miner Res. 2012;27(4):739-742.
2. American Society for Bone and Mineral Research. The American Society for Bone and Mineral Research response to media coverage of New England Journal of Medicine study: “Bone Density Testing Interval and Transition to osteoporosis in older women.” http://www.asbmr.org/about/pressreleases/detail.aspx?cid=3801baff-0df3-47c0-874f-08a185d67001. Published February 1, 2012. Accessed April 9, 2012.
Update on Osteoporosis
Steven R. Goldstein, MD (November 2011)
The optimal screening interval for bone density assessment in menopausal women is an extremely complicated but important issue because osteoporosis and fragility fracture are a major health concern. There are nearly 2 million osteoporotic fractures each year, accounting for 432,000 hospital admissions, 25 million office visits, and an increased risk of disability and death, all at a cost of up to $18 billion.1
There is no question that determination of bone mass (achieved through bone mineral density [BMD] testing by dual energy x-ray absorptiometry [DXA] and reported as T scores) will diagnose osteopenia and osteoporosis, correlate with fracture risk (the lower the bone mass, the higher the incidence of fracture), and monitor changes in bone mass over time.
Medicare allows for BMD testing every 23 months, and that has become standard for many clinicians.
Details of the trial
Gourlay and colleagues studied nearly 5,000 basically healthy women, the youngest of whom was 67 years of age. Women who had osteoporosis and who were taking medication for fracture reduction were excluded, as were women who had a history of pre-existing fracture.
The researchers concluded that the better the initial bone-density score at age 67, the longer it would take for a woman to develop osteoporosis. For instance, if a woman older than 67 years had a T score of –1.00 or better, it would take her 16.8 years (95% confidence interval [CI], 11.5 to 24.6) to reach osteoporosis. In contrast, a woman with a T score of –2.00 would reach osteoporosis in only 1.1 years. Current estrogen use was found to be significantly associated with higher BMD and a longer testing interval, although the authors did not recommend modifying the screening interval on the basis of estrogen use.
These findings certainly call into question the notion that all patients should be screened for osteoporosis every 23 months. Perhaps it is better to think of screening as a way to initially triage patients for decisions relative to follow-up.
Limitations and considerations
Some extremely important observations must be made:
- The article by Gourlay and colleagues created tremendous media attention, most of which implied that there is too much screening with DXA scans. Nothing can be further from the truth. Only 13% of women older than age 65 are actually getting a baseline DXA scan.2
- The data in this report apply only to white women older than 67 years who have no pre-existing fracture and are not taking any medications for osteoporosis. Extrapolation to younger women or other groups is not valid.
- We should not be interested in the development of an arbitrary T score for bone mass but rather the determination of whether a particular patient has a level of fracture risk that warrants pharmacologic intervention.
These observations support use of a model like FRAX (see “What is FRAX?”), which can be used annually even without an updated DXA of the hip. FRAX is much more appropriate than DXA testing every 23 months and should become the clinical standard of care.
The Fracture Risk Assessment (FRAX®) Tool1 has been developed by the World Health Organization (WHO). It is based on individual patient models that integrate the risks associated with clinical factors as well as bone mineral density at the femoral neck. FRAX models have been developed from studying population-based cohorts from Europe, North America, Asia, and Australia.
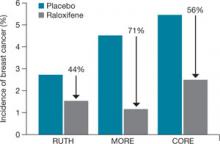
FRAX algorithms give the 10-year probability of hip fracture and of a major osteoporotic fracture (clinical spine, forearm, hip, or shoulder fracture).
WHO offers sophisticated computer-driven models or simplified, printable versions of FRAX for office use—available at http://www.who-frax.org/.
Reference
1. Welcome to FRAX. FRAX: WHO Fracture Risk Assessment Tool Web site. http://www.shef.ac.uk/FRAX/. Accessed July 7, 2012.
Remember, there are more fragility fractures in nonosteoporotic women than in osteoporotic women. The risk (incidence per 10,000 women) is greater in osteoporotic women, but the absolute number in the population is greater in women who have not yet reached that threshold.
In older healthy women, BMD follow-up arbitrarily at 23 months makes little sense. The interval before reassessment can substantially lengthen for some women with excellent initial T scores, while more frequent assessments should be performed for women with worse initial T scores. Furthermore, strict reliance solely on T scores is not the best method for predicting fracture risk or when to start pharmacologic intervention. Yearly assessment using a tool like FRAX should become the standard.
STEVEN R. GOLDSTEIN, MD
We want to hear from you! Tell us what you think.
Update on Osteoporosis
Steven R. Goldstein, MD (November 2011)
The optimal screening interval for bone density assessment in menopausal women is an extremely complicated but important issue because osteoporosis and fragility fracture are a major health concern. There are nearly 2 million osteoporotic fractures each year, accounting for 432,000 hospital admissions, 25 million office visits, and an increased risk of disability and death, all at a cost of up to $18 billion.1
There is no question that determination of bone mass (achieved through bone mineral density [BMD] testing by dual energy x-ray absorptiometry [DXA] and reported as T scores) will diagnose osteopenia and osteoporosis, correlate with fracture risk (the lower the bone mass, the higher the incidence of fracture), and monitor changes in bone mass over time.
Medicare allows for BMD testing every 23 months, and that has become standard for many clinicians.
Details of the trial
Gourlay and colleagues studied nearly 5,000 basically healthy women, the youngest of whom was 67 years of age. Women who had osteoporosis and who were taking medication for fracture reduction were excluded, as were women who had a history of pre-existing fracture.
The researchers concluded that the better the initial bone-density score at age 67, the longer it would take for a woman to develop osteoporosis. For instance, if a woman older than 67 years had a T score of –1.00 or better, it would take her 16.8 years (95% confidence interval [CI], 11.5 to 24.6) to reach osteoporosis. In contrast, a woman with a T score of –2.00 would reach osteoporosis in only 1.1 years. Current estrogen use was found to be significantly associated with higher BMD and a longer testing interval, although the authors did not recommend modifying the screening interval on the basis of estrogen use.
These findings certainly call into question the notion that all patients should be screened for osteoporosis every 23 months. Perhaps it is better to think of screening as a way to initially triage patients for decisions relative to follow-up.
Limitations and considerations
Some extremely important observations must be made:
- The article by Gourlay and colleagues created tremendous media attention, most of which implied that there is too much screening with DXA scans. Nothing can be further from the truth. Only 13% of women older than age 65 are actually getting a baseline DXA scan.2
- The data in this report apply only to white women older than 67 years who have no pre-existing fracture and are not taking any medications for osteoporosis. Extrapolation to younger women or other groups is not valid.
- We should not be interested in the development of an arbitrary T score for bone mass but rather the determination of whether a particular patient has a level of fracture risk that warrants pharmacologic intervention.
These observations support use of a model like FRAX (see “What is FRAX?”), which can be used annually even without an updated DXA of the hip. FRAX is much more appropriate than DXA testing every 23 months and should become the clinical standard of care.
The Fracture Risk Assessment (FRAX®) Tool1 has been developed by the World Health Organization (WHO). It is based on individual patient models that integrate the risks associated with clinical factors as well as bone mineral density at the femoral neck. FRAX models have been developed from studying population-based cohorts from Europe, North America, Asia, and Australia.
FRAX algorithms give the 10-year probability of hip fracture and of a major osteoporotic fracture (clinical spine, forearm, hip, or shoulder fracture).
WHO offers sophisticated computer-driven models or simplified, printable versions of FRAX for office use—available at http://www.who-frax.org/.
Reference
1. Welcome to FRAX. FRAX: WHO Fracture Risk Assessment Tool Web site. http://www.shef.ac.uk/FRAX/. Accessed July 7, 2012.
Remember, there are more fragility fractures in nonosteoporotic women than in osteoporotic women. The risk (incidence per 10,000 women) is greater in osteoporotic women, but the absolute number in the population is greater in women who have not yet reached that threshold.
In older healthy women, BMD follow-up arbitrarily at 23 months makes little sense. The interval before reassessment can substantially lengthen for some women with excellent initial T scores, while more frequent assessments should be performed for women with worse initial T scores. Furthermore, strict reliance solely on T scores is not the best method for predicting fracture risk or when to start pharmacologic intervention. Yearly assessment using a tool like FRAX should become the standard.
STEVEN R. GOLDSTEIN, MD
We want to hear from you! Tell us what you think.
1. Lewiecki EM, Laster AJ, Miller PF, Bilezikian JP. More bone density testing is needed, not less. J Bone Miner Res. 2012;27(4):739-742.
2. American Society for Bone and Mineral Research. The American Society for Bone and Mineral Research response to media coverage of New England Journal of Medicine study: “Bone Density Testing Interval and Transition to osteoporosis in older women.” http://www.asbmr.org/about/pressreleases/detail.aspx?cid=3801baff-0df3-47c0-874f-08a185d67001. Published February 1, 2012. Accessed April 9, 2012.
1. Lewiecki EM, Laster AJ, Miller PF, Bilezikian JP. More bone density testing is needed, not less. J Bone Miner Res. 2012;27(4):739-742.
2. American Society for Bone and Mineral Research. The American Society for Bone and Mineral Research response to media coverage of New England Journal of Medicine study: “Bone Density Testing Interval and Transition to osteoporosis in older women.” http://www.asbmr.org/about/pressreleases/detail.aspx?cid=3801baff-0df3-47c0-874f-08a185d67001. Published February 1, 2012. Accessed April 9, 2012.
UPDATE ON OSTEOPOROSIS
We, and our patients, are fortunate to have a robust armamentarium of osteoporosis preventives and treatments in the 21st century. Still, we have much to learn about the preservation and restoration of bone. Fine-tuning of individual therapies, and clarification of their attendant risks, are ongoing concerns.
In this article, I highlight several recent studies:
- an assessment of the periodontium in a group of postmenopausal women who were long-term users of bisphosphonates, versus nonusers, to determine the effects of these agents on periodontal health
- guidance from the American Society for Bone and Mineral Research on the risk of atypical femoral fracture in long-term users of bisphosphonates
- 2-year data on a new, delayed-release, weekly formulation of risedronate that can be taken with food
- two meta-analyses exploring the risk of fracture with use of a proton-pump inhibitor (PPI), as well as a recent summary of evidence
- a randomized trial of 2% nitroglycerin ointment to prevent fracture.
Bisphosphonates may reduce the risk of postmenopausal periodontal disease
Palomo L, Buencamino-Francisco MC, Carey JJ, Sivanandy M, Thacker H. Is long-term bisphosphonate therapy associated with benefits to the periodontium in postmenopausal women? Menopause. 2011;18(2):164–170.
The risk of periodontal disease increases in menopause. Inflammation can erode structures (i.e., periodontal ligament and alveolar bone) that attach the teeth into the jaw, leading, eventually, to loss of teeth (FIGURE).
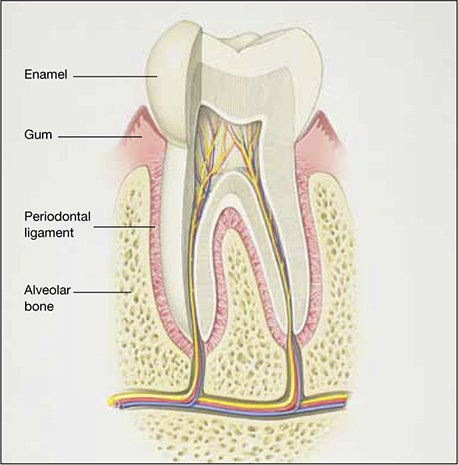
Anatomy of healthy periodontium
In periodontal disease, inflammation can erode structures, such as the periodontal ligament and alveolar bone, that attach the teeth into the jaw.In periodontal inflammation, a bacterial biofilm on tooth surfaces triggers a response by neutrophils and macrophages. In this respect, osteoporosis and periodontitis are mediated by common cytokines. Local production of cytokines seems to enhance osteoclast-mediated loss of skeletal and alveolar bone in estrogen-deficient women. In addition, generalized bone loss in postmenopausal osteoporosis renders the jaw susceptible to accelerated alveolar bone resorption and loss of periodontal attachment. For these reasons, physicians who care for postmenopausal women are advised to monitor their periodontal health; be vigilant for dental problems; and encourage them to practice good oral hygiene as a preventive measure against periodontitis and to seek regular dental care.
There has been tremendous publicity about the rare but very serious occurrence of osteonecrosis of the jaw in women who use bisphosphonates. In contrast, this study by Palomo and colleagues from the Case Western Reserve School of Dental Medicine seems to offer some preliminary good news about bisphosphonates and dental health: Long-term use appears to have some beneficial effects on the periodontium of postmenopausal women.
Details of the study
The aim of the study was to compare the periodontium in two groups of postmenopausal women known to have low bone mineral density (BMD): those who were long-term users (>2 years) of bisphosphonate therapy (n=28) and those who were not (n=28). The average age of participants in the study was 63 years, and the average T-score was –2.5. All women underwent cone-beam computed tomography of the jaw and a complete periodontal examination to determine the plaque score, periodontal probing depth, clinical attachment loss, bleeding on probing, and alveolar bone height.
Findings: Bisphosphonate users had a higher plaque score, a lower probing depth, and less loss of clinical attachment than did women in the control group. These differences were determined to be statistically significant. Bisphosphonate users also had less bleeding on probing and a higher alveolar bone height, but these differences were not significant. After adjustment for the plaque score, bisphosphonate use was a significant factor for probing depth but not for the other parameters.
Not all news about bisphosphonates is bad. Preliminary data seem to indicate that objective measures of periodontal disease are lower in bisphosphonate users who have low BMD than in nonusers.
Atypical femoral fracture is a real risk—but a rarity—with long-term use of antiresorptive drugs
Shane E, Burr D, Ebeling PR, et al; American Society for Bone and Mineral Research. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25(11):2267–2294.
As ObGyns, we are often the first-line providers of diagnostic services and treatment for postmenopausal women at risk of osteoporotic fracture. Oral and, more recently, parenteral bisphosphonates have been a mainstay of such treatment. Isolated reports of atypical femoral fracture in long-term users of bisphosphonates first surfaced around 2005.1 Since then, several case series have appeared, some with as many as 102 cases.2 In fact, atypical femoral fracture in bisphosphonate users has drawn so much attention that patients have begun to ask about these agents and express reservations about using them.
The issue has drawn the focus of the American Society for Bone and Mineral Research (ASBMR), which appointed a task force to address it. A multidisciplinary expert group reviewed pertinent published reports of atypical femoral fracture, as well as preclinical studies, that could provide insight into its pathogenesis.
What we know about this type of fracture
Preclinical data lend biologic plausibility to a potential association between long-term bisphosphonate use and atypical femoral fracture. These data highlight the effects of bisphosphonates on:
- collagen cross-linking and maturation
- accumulation of microdamage and advanced glycation end products
- heightened mineralization, remodeling, vascularity, and angiogenesis.
The task force concluded that bisphosphonates are highly effective at reducing the risk of spinal and nonspinal fractures, including typical and common femoral neck and intertrochanteric fractures. However, there is evidence of a relationship between long-term bisphosphonate use and a specific type of subtrochanteric and femoral shaft fracture. This type of fracture is characterized by unique radiographic features:
- transverse or short oblique orientation
- absence of comminution
- cortical thickening
- stress fracture or stress reaction on the symptomatic and/or contralateral side
- delayed healing.
This type of fracture also has unique clinical features—namely, prodromal pain and bilaterality.
The apparent increase in the risk of atypical femoral fracture in patients using glucocorticoids is a concern because bisphosphonates are the mainstay of prevention of glucocorticoid-induced osteoporotic fracture.
Bone biopsies from the iliac crest or fracture site, or both, generally show reduced bone formation consistent with bisphosphonate action. Paradoxically, some patients show biopsy evidence of enhanced bone resorption. Biochemical bone-turnover markers are often normal but may be increased.
Atypical femoral fracture can occur in patients who have not been treated with bisphosphonates, and its true incidence in treated and untreated patients is unknown. However, it appears to be more common in patients who have been exposed to long-term bisphosphonates—usually for longer than 3 years (median treatment: 7 years).
It must be emphasized that this type of fracture is rare, particularly considering the millions of patients who have used bisphosphonates and the much higher incidence of typical and common femoral neck and intertrochanteric fractures in untreated patients.
Bisphosphonates are important drugs for the prevention of common osteoporotic fractures. However, atypical femoral fracture is a concern, and more information is needed to help us identify patients at risk and to guide decision-making about the optimal duration of bisphosphonate therapy.
Any discussion with the patient about bisphosphonate use should begin with a mention of the millions of women who have been treated successfully with these agents and the thousands and thousands of hip, spinal, and other nonvertebral fractures that have been prevented. Only 249 cases of atypical femoral fracture have been reported worldwide.
The patient should also be reassured that research into this phenomenon is continuing. At present, the risk-benefit ratio greatly favors use of these medications in properly selected patients at heightened risk of osteoporotic fracture.
New delayed-release bisphosphonate can be taken with food
Benhamou CL, Zanchetta JR, Kaufman JM, et al. A novel delayed-release risedronate 35 mg once-a-week formulation taken with or without breakfast: 2 year BMD data. Osteoporosis Int. 2011;22:S337-S338.
Oral bisphosphonates are poorly absorbed from the gastrointestinal (GI) tract, with bioavailability of less than 1%. Absorption is further reduced when a bisphosphonate is taken with food, beverages other than plain water, medications, or supplements. Because of this limited absorption, oral bisphosphonates must be taken at least 30 to 60 minutes before the first food, drink, or other medication of the day. Noncompliance leads to reduced bioavailability and, potentially, decreased efficacy.
A new delayed-release formulation of risedronate (Atelvia) was approved by the FDA in late 2010 and made available for prescribing earlier this year. It is designed to improve absorption of risedronate in the presence of food, allowing for administration immediately after breakfast.
Two innovations were utilized in the manufacture of this tablet. First, it has an enteric coating to deliver risedronate beyond the stomach in the small intestine, with active drug released at a pH level above 5.5. Second, it contains a chelating agent—edetate disodium (EDTA)—which binds free divalent cations, such as calcium, magnesium, and iron.
Details of the study
Benhamou and colleagues compared three regimens of risedronate:
- 35 mg weekly of the delayed-release formulation, taken at least 30 minutes before breakfast
- 35 mg weekly of the delayed-release formulation, taken 30 minutes after breakfast
- 5 mg daily of the immediate-release risedronate formulation, taken 30 minutes before breakfast.
This phase 3, randomized, double-blind, active-controlled study involved 43 centers across North America, Europe, and South America. Characteristics of women in the study included:
- age of 50 years or older
- at least 5 years since the last menstrual period
- lumbar spine or total hip BMD corresponding to a T-score of –2.5 or worse, or a T-score of –2.0 or worse with at least one prevalent vertebral fracture.
The primary efficacy endpoint was the mean percentage of change in BMD at the lumbar spine.
Findings: At 2 years, the mean change for both delayed-release arms was significantly greater than for the 5-mg immediate-release dose. The authors concluded that the 35-mg weekly delayed-release regimen—whether taken at least 30 minutes before or after breakfast—is as effective and safe as the 5-mg daily immediate-release regimen.
Oral, delayed-release risedronate (35 mg weekly) is similar in efficacy and tolerability to the immediate-release formulation (5 mg daily). By minimizing the impact of concomitantly ingested food on the bioavailability of risedronate, the delayed-release formulation can make it easier for patients to accept and comply with the regimen, thereby maximizing absorption and improving efficacy of the drug in clinical practice.
Do proton-pump inhibitors increase the risk of fracture?
Targownik LE, Leslie WD. The relationship among proton pump inhibitors, bone disease and fracture [published online ahead of print May 20, 2011]. Expert Opin Drug Saf. doi: 10.1517/14740338.2011.586628.
Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med. 2011;124(6):519–526.
Ye X, Liu H, Wu C, et al. Proton pump inhibitors therapy and risk of hip fracture: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2011;23:794–800.
In May 2010, the FDA revised prescription and over-the-counter (OTC) labels for proton-pump inhibitors to include new safety information about a possible increased risk of fractures of the hip, wrist, and spine with use of these medications.
Proton pump inhibitors are so named because they prevent hydrogen ion (proton) secretion into the gastric lumen, thereby reducing acid in the stomach. Esomeprazole magnesium (Nexium), dexlansoprazole (Dexilant), omeprazole (Prilosec), omeprazole with sodium bicarbonate (Zegerid), lansoprazole (Prevacid), pantoprazole (Protonix), rabeprazole (Aciphex), and naproxen with esomeprazole magnesium (Vimovo) are available by prescription to treat conditions such as gastroesophageal reflux disease (GERD), ulcers of the stomach and small intestine, and inflammation of the esophagus. Delayed-release omeprazole magnesium (Prilosec OTC, Zegerid OTC) and delayed-release lansoprazole (Prevacid 24HR) are sold OTC for treatment of frequent heartburn.
The new safety information was based on an FDA review of several epidemiologic studies reporting an increased risk of fractures of the hip, wrist, and spine with PPI use. Some studies found that those at greatest risk of fracture received high doses of PPIs or used them for 1 year or longer. Most of the studies involved individuals 50 years and older; the increased risk of fracture was observed primarily in this age group.
Although the greatest risk of fracture involved people who had been taking a prescription PPI for at least 1 year or who had been taking a high dose of a prescription PPI, the FDA took the precaution of altering the “Drug facts” label on OTC agents as well. (Over-the-counter PPIs are indicated for 14 days of continuous use.) However, in March 2011, the FDA reversed its decision and retracted the wording on the label of OTC formulations. Following a thorough review of safety data, the agency concluded that the risk of fracture with short-term, low-dose use of PPIs is unlikely. Nevertheless, the FDA acknowledged that consumers sometimes use a PPI longer than the directions advise. Therefore, the agency recommends that health-care professionals be aware of the risk of fracture if the patient uses an OTC PPI at a higher dose or longer time than the label advises.
Two meta-analyses find a modestly increased risk of fracture
Yu and colleagues analyzed 11 observational case-control or cohort studies that involved mostly older adults. They found a modestly increased risk of hip fracture among individuals taking a PPI, compared with nonusers (relative risk [RR], 1.30; 95% CI, 1.19–1.43). They also found an increase in spinal (RR, 1.56; 95% CI, 1.31–1.85) and any-site fractures (RR, 1.16; 95% CI, 1.04–1.30) among PPI users. They acknowledged that residual confounding could not be excluded.
A separate meta-analysis by Ye and colleagues included seven studies. They found a statistically significant increase in the risk of hip fracture, compared with nonusers (odds ratio [OR], 1.24; 95% CI, 1.15–1.34). However, because of different effects of PPIs at different durations of therapy, Ye and colleagues concluded that evidence was insufficient to support a causal relationship between PPI use and hip fracture. Further investigation is warranted.
What we know about PPIs and bone
Because PPIs are widely used, it is of paramount importance to understand the precise effects through which use of a PPI may affect bone mineral metabolism and influence fracture risk.
Targownik and Leslie discuss the evidence supporting an association between PPIs and fragility fracture, the association between PPIs and calcium malabsorption, and the underlying condition that predisposes patients who have osteoporosis to fracture. They also explore the possible mechanisms by which PPIs may increase the risk of fracture. After conducting a PubMed search and review of the literature, they found limited evidence supporting the FDA’s assertion that PPIs may increase the risk of fracture. Other findings:
- Multiple analyses have demonstrated a modest association between chronic PPI use and an increased risk of fracture
- No studies have convincingly demonstrated that PPIs cause fracture
- Overall, PPI use does not interfere with calcium absorption in most instances; therefore, it is unlikely that PPIs influence the risk of fracture by interfering with the absorption of dietary calcium
- Dual-energy x-ray absorptiometry (DEXA) testing demonstrates no consistent effect of PPIs on BMD
- Preliminary evidence suggests that PPIs may interfere with normal osteoclastic function, although it is unclear whether such interference influences the risk of fracture.
PPIs are the most potent inhibitors of acid secretion available; standard once-daily dosing may lead to a 90% decrease in gastric acid secretion and will maintain an intragastric pH level above 4 for as long as 70% of any 24-hour period.
PPIs are known to be effective for treating symptoms of GERD and preventing GERD-related complications. These drugs are also the optimal agents for the treatment of peptic ulcer disease and prevention of complications of peptic ulcer disease in chronic users of NSAIDs.
The potency of PPIs makes them the agent of choice for a variety of patients. The drugs also have a long reputation for having a favorable side-effect profile.
Due to this combination of efficacy and safety, PPIs are among the most widely prescribed drugs in all of clinical medicine, trailing only antihypertensives and antidepressants in total number of prescriptions. This means that many of our postmenopausal patients are using these drugs. Those who are using a prescription-strength PPI should be aware of the potential risk of fracture, particularly if they are using it chronically. They also should be advised that exercise, calcium, and vitamin D are vital, as well as regular DEXA testing according to established guidelines.
Will nitroglycerin be the next treatment for osteoporosis?
Jamal SA, Hamilton CJ, Eastell R, Cummings SR. Effect of nitroglycerin ointment on bone density and strength in postmenopausal women: a randomized trial. JAMA. 2011;305(8):800–807.
Khosla S. Is nitroglycerin a novel and inexpensive treatment for osteoporosis? JAMA. 2011;305(8):826–827.
Data suggesting that nitrates may protect against fracture date back to 2006, when Rejnmark and colleagues explored the use of these agents in 124,655 individuals who sustained a fracture and 373,963 age- and sex-matched controls.3 After adjustment for possible confounders, use of nitrates was associated with an 11% reduction in the risk of any fracture (OR, 0.89; 95% CI, 0.86–0.92) and a 15% reduction in the risk of hip fracture (OR, 0.85; 95% CI, 0.79–0.92).3
However, in 2009, Wimalawansa and colleagues found no benefit of transdermal nitroglycerin in preventing bone loss in early postmenopausal women.4 In their 3-year, randomized, double-blind, placebo-controlled trial, 186 postmenopausal women (mean age, 56 years) were randomized to receive nitroglycerin ointment (22.5 mg/d) or placebo. After 36 months of therapy, changes in BMD at multiple sites were comparable between groups. Due to the significant incidence of headache related to nitroglycerin treatment, adherence was suboptimal (estimated at approximately 70%), perhaps contributing to the negative findings.4
Enter this study by Jamal and associates, who performed a double-blind, randomized, placebo-controlled trial 24 months in duration. Participants were 243 women with a mean age of 62 years and a lumbar spine T-score between 0 and –2.0. They were randomized to 15 mg daily of 2% nitroglycerin ointment (applied to the upper outer arm at bedtime) or placebo.
At 2 years, women in the nitroglycerin group had a significant increase in BMD at the:
- lumbar spine, from 1.05 to 1.14 g/cm2, for a percentage change of 6.7% (P<0.001) (placebo group change: 1.06 to 1.08 g/cm2)
- total hip, from 0.92 to 0.97 g/cm2, for a percentage change of 6.2% (P<0.001) (placebo group change: 0.93 to 0.92 g/cm2)
- femoral neck, from 0.88 to 0.93 g/cm2, for a percentage change of 7% (P<0.001) (placebo group change: 0.87 to 0.86 g/cm2).
Nitroglycerin also increased bone-specific alkaline phosphatase by 34.8% and decreased urine N-telopeptide by 54% (P<0.001). Incidence of serious adverse events did not differ between nitroglycerin and placebo groups (5 [42.5%]).
Among women who continued treatment for 24 months, headaches were reported by 40 (35%) nitroglycerin users versus six (5.4%) nonusers during the first month, decreasing substantially after 12 months.
At this time, no changes in clinical practice are warranted. However, the findings of Jamal and coworkers should set the stage for a larger, adequately powered study of nitroglycerin ointment, using fracture as an outcome. If such a study demonstrates fracture reduction, clinicians will have a novel and inexpensive therapy for osteoporosis. The findings of Jamal and coworkers also should prompt development of additional nitric oxide donors that have greater skeletal effects and a better adverse-effect profile—particularly in regard to headache.
We want to hear from you! Tell us what you think.
1. Husada G, Libberecht K, Peeters T, Populaire J. Bilateral mid-diaphyseal femoral stress fractures in the elderly. Eur J Trauma. 2005;31(1):68-71.Doi:10.1007/s00068-005-1421–5.
2. Dell R, Greene D, Ott S, et al. A retrospective analysis of all atypical femur fractures seen in a large California HMO from the years 2007 to 2009. Paper presented at ASBMR 2010 Annual Meeting; October 18, 2010; Toronto, Ontario, Canada. http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?filename="2311OBG_Update" aid=05caf316-b73e-47b8-a011-bf0766b062c0. Accessed Sept. 26, 2011.
3. Rejnmark L, Vestergaard P, Mosekilde L. Decreased fracture risk in users of organic nitrates: a nationwide case-control study. J Bone Miner Res. 2006;21(11):1811-1817.
4. Wimalawansa SJ, Grimes JP, Wilson AC, Hoover DR. Transdermal nitroglycerin therapy may not prevent early postmenopausal bone loss. J Clin Endocrinol Metab. 2009;94(9):3356-3364.
We, and our patients, are fortunate to have a robust armamentarium of osteoporosis preventives and treatments in the 21st century. Still, we have much to learn about the preservation and restoration of bone. Fine-tuning of individual therapies, and clarification of their attendant risks, are ongoing concerns.
In this article, I highlight several recent studies:
- an assessment of the periodontium in a group of postmenopausal women who were long-term users of bisphosphonates, versus nonusers, to determine the effects of these agents on periodontal health
- guidance from the American Society for Bone and Mineral Research on the risk of atypical femoral fracture in long-term users of bisphosphonates
- 2-year data on a new, delayed-release, weekly formulation of risedronate that can be taken with food
- two meta-analyses exploring the risk of fracture with use of a proton-pump inhibitor (PPI), as well as a recent summary of evidence
- a randomized trial of 2% nitroglycerin ointment to prevent fracture.
Bisphosphonates may reduce the risk of postmenopausal periodontal disease
Palomo L, Buencamino-Francisco MC, Carey JJ, Sivanandy M, Thacker H. Is long-term bisphosphonate therapy associated with benefits to the periodontium in postmenopausal women? Menopause. 2011;18(2):164–170.
The risk of periodontal disease increases in menopause. Inflammation can erode structures (i.e., periodontal ligament and alveolar bone) that attach the teeth into the jaw, leading, eventually, to loss of teeth (FIGURE).

Anatomy of healthy periodontium
In periodontal disease, inflammation can erode structures, such as the periodontal ligament and alveolar bone, that attach the teeth into the jaw.In periodontal inflammation, a bacterial biofilm on tooth surfaces triggers a response by neutrophils and macrophages. In this respect, osteoporosis and periodontitis are mediated by common cytokines. Local production of cytokines seems to enhance osteoclast-mediated loss of skeletal and alveolar bone in estrogen-deficient women. In addition, generalized bone loss in postmenopausal osteoporosis renders the jaw susceptible to accelerated alveolar bone resorption and loss of periodontal attachment. For these reasons, physicians who care for postmenopausal women are advised to monitor their periodontal health; be vigilant for dental problems; and encourage them to practice good oral hygiene as a preventive measure against periodontitis and to seek regular dental care.
There has been tremendous publicity about the rare but very serious occurrence of osteonecrosis of the jaw in women who use bisphosphonates. In contrast, this study by Palomo and colleagues from the Case Western Reserve School of Dental Medicine seems to offer some preliminary good news about bisphosphonates and dental health: Long-term use appears to have some beneficial effects on the periodontium of postmenopausal women.
Details of the study
The aim of the study was to compare the periodontium in two groups of postmenopausal women known to have low bone mineral density (BMD): those who were long-term users (>2 years) of bisphosphonate therapy (n=28) and those who were not (n=28). The average age of participants in the study was 63 years, and the average T-score was –2.5. All women underwent cone-beam computed tomography of the jaw and a complete periodontal examination to determine the plaque score, periodontal probing depth, clinical attachment loss, bleeding on probing, and alveolar bone height.
Findings: Bisphosphonate users had a higher plaque score, a lower probing depth, and less loss of clinical attachment than did women in the control group. These differences were determined to be statistically significant. Bisphosphonate users also had less bleeding on probing and a higher alveolar bone height, but these differences were not significant. After adjustment for the plaque score, bisphosphonate use was a significant factor for probing depth but not for the other parameters.
Not all news about bisphosphonates is bad. Preliminary data seem to indicate that objective measures of periodontal disease are lower in bisphosphonate users who have low BMD than in nonusers.
Atypical femoral fracture is a real risk—but a rarity—with long-term use of antiresorptive drugs
Shane E, Burr D, Ebeling PR, et al; American Society for Bone and Mineral Research. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25(11):2267–2294.
As ObGyns, we are often the first-line providers of diagnostic services and treatment for postmenopausal women at risk of osteoporotic fracture. Oral and, more recently, parenteral bisphosphonates have been a mainstay of such treatment. Isolated reports of atypical femoral fracture in long-term users of bisphosphonates first surfaced around 2005.1 Since then, several case series have appeared, some with as many as 102 cases.2 In fact, atypical femoral fracture in bisphosphonate users has drawn so much attention that patients have begun to ask about these agents and express reservations about using them.
The issue has drawn the focus of the American Society for Bone and Mineral Research (ASBMR), which appointed a task force to address it. A multidisciplinary expert group reviewed pertinent published reports of atypical femoral fracture, as well as preclinical studies, that could provide insight into its pathogenesis.
What we know about this type of fracture
Preclinical data lend biologic plausibility to a potential association between long-term bisphosphonate use and atypical femoral fracture. These data highlight the effects of bisphosphonates on:
- collagen cross-linking and maturation
- accumulation of microdamage and advanced glycation end products
- heightened mineralization, remodeling, vascularity, and angiogenesis.
The task force concluded that bisphosphonates are highly effective at reducing the risk of spinal and nonspinal fractures, including typical and common femoral neck and intertrochanteric fractures. However, there is evidence of a relationship between long-term bisphosphonate use and a specific type of subtrochanteric and femoral shaft fracture. This type of fracture is characterized by unique radiographic features:
- transverse or short oblique orientation
- absence of comminution
- cortical thickening
- stress fracture or stress reaction on the symptomatic and/or contralateral side
- delayed healing.
This type of fracture also has unique clinical features—namely, prodromal pain and bilaterality.
The apparent increase in the risk of atypical femoral fracture in patients using glucocorticoids is a concern because bisphosphonates are the mainstay of prevention of glucocorticoid-induced osteoporotic fracture.
Bone biopsies from the iliac crest or fracture site, or both, generally show reduced bone formation consistent with bisphosphonate action. Paradoxically, some patients show biopsy evidence of enhanced bone resorption. Biochemical bone-turnover markers are often normal but may be increased.
Atypical femoral fracture can occur in patients who have not been treated with bisphosphonates, and its true incidence in treated and untreated patients is unknown. However, it appears to be more common in patients who have been exposed to long-term bisphosphonates—usually for longer than 3 years (median treatment: 7 years).
It must be emphasized that this type of fracture is rare, particularly considering the millions of patients who have used bisphosphonates and the much higher incidence of typical and common femoral neck and intertrochanteric fractures in untreated patients.
Bisphosphonates are important drugs for the prevention of common osteoporotic fractures. However, atypical femoral fracture is a concern, and more information is needed to help us identify patients at risk and to guide decision-making about the optimal duration of bisphosphonate therapy.
Any discussion with the patient about bisphosphonate use should begin with a mention of the millions of women who have been treated successfully with these agents and the thousands and thousands of hip, spinal, and other nonvertebral fractures that have been prevented. Only 249 cases of atypical femoral fracture have been reported worldwide.
The patient should also be reassured that research into this phenomenon is continuing. At present, the risk-benefit ratio greatly favors use of these medications in properly selected patients at heightened risk of osteoporotic fracture.
New delayed-release bisphosphonate can be taken with food
Benhamou CL, Zanchetta JR, Kaufman JM, et al. A novel delayed-release risedronate 35 mg once-a-week formulation taken with or without breakfast: 2 year BMD data. Osteoporosis Int. 2011;22:S337-S338.
Oral bisphosphonates are poorly absorbed from the gastrointestinal (GI) tract, with bioavailability of less than 1%. Absorption is further reduced when a bisphosphonate is taken with food, beverages other than plain water, medications, or supplements. Because of this limited absorption, oral bisphosphonates must be taken at least 30 to 60 minutes before the first food, drink, or other medication of the day. Noncompliance leads to reduced bioavailability and, potentially, decreased efficacy.
A new delayed-release formulation of risedronate (Atelvia) was approved by the FDA in late 2010 and made available for prescribing earlier this year. It is designed to improve absorption of risedronate in the presence of food, allowing for administration immediately after breakfast.
Two innovations were utilized in the manufacture of this tablet. First, it has an enteric coating to deliver risedronate beyond the stomach in the small intestine, with active drug released at a pH level above 5.5. Second, it contains a chelating agent—edetate disodium (EDTA)—which binds free divalent cations, such as calcium, magnesium, and iron.
Details of the study
Benhamou and colleagues compared three regimens of risedronate:
- 35 mg weekly of the delayed-release formulation, taken at least 30 minutes before breakfast
- 35 mg weekly of the delayed-release formulation, taken 30 minutes after breakfast
- 5 mg daily of the immediate-release risedronate formulation, taken 30 minutes before breakfast.
This phase 3, randomized, double-blind, active-controlled study involved 43 centers across North America, Europe, and South America. Characteristics of women in the study included:
- age of 50 years or older
- at least 5 years since the last menstrual period
- lumbar spine or total hip BMD corresponding to a T-score of –2.5 or worse, or a T-score of –2.0 or worse with at least one prevalent vertebral fracture.
The primary efficacy endpoint was the mean percentage of change in BMD at the lumbar spine.
Findings: At 2 years, the mean change for both delayed-release arms was significantly greater than for the 5-mg immediate-release dose. The authors concluded that the 35-mg weekly delayed-release regimen—whether taken at least 30 minutes before or after breakfast—is as effective and safe as the 5-mg daily immediate-release regimen.
Oral, delayed-release risedronate (35 mg weekly) is similar in efficacy and tolerability to the immediate-release formulation (5 mg daily). By minimizing the impact of concomitantly ingested food on the bioavailability of risedronate, the delayed-release formulation can make it easier for patients to accept and comply with the regimen, thereby maximizing absorption and improving efficacy of the drug in clinical practice.
Do proton-pump inhibitors increase the risk of fracture?
Targownik LE, Leslie WD. The relationship among proton pump inhibitors, bone disease and fracture [published online ahead of print May 20, 2011]. Expert Opin Drug Saf. doi: 10.1517/14740338.2011.586628.
Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med. 2011;124(6):519–526.
Ye X, Liu H, Wu C, et al. Proton pump inhibitors therapy and risk of hip fracture: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2011;23:794–800.
In May 2010, the FDA revised prescription and over-the-counter (OTC) labels for proton-pump inhibitors to include new safety information about a possible increased risk of fractures of the hip, wrist, and spine with use of these medications.
Proton pump inhibitors are so named because they prevent hydrogen ion (proton) secretion into the gastric lumen, thereby reducing acid in the stomach. Esomeprazole magnesium (Nexium), dexlansoprazole (Dexilant), omeprazole (Prilosec), omeprazole with sodium bicarbonate (Zegerid), lansoprazole (Prevacid), pantoprazole (Protonix), rabeprazole (Aciphex), and naproxen with esomeprazole magnesium (Vimovo) are available by prescription to treat conditions such as gastroesophageal reflux disease (GERD), ulcers of the stomach and small intestine, and inflammation of the esophagus. Delayed-release omeprazole magnesium (Prilosec OTC, Zegerid OTC) and delayed-release lansoprazole (Prevacid 24HR) are sold OTC for treatment of frequent heartburn.
The new safety information was based on an FDA review of several epidemiologic studies reporting an increased risk of fractures of the hip, wrist, and spine with PPI use. Some studies found that those at greatest risk of fracture received high doses of PPIs or used them for 1 year or longer. Most of the studies involved individuals 50 years and older; the increased risk of fracture was observed primarily in this age group.
Although the greatest risk of fracture involved people who had been taking a prescription PPI for at least 1 year or who had been taking a high dose of a prescription PPI, the FDA took the precaution of altering the “Drug facts” label on OTC agents as well. (Over-the-counter PPIs are indicated for 14 days of continuous use.) However, in March 2011, the FDA reversed its decision and retracted the wording on the label of OTC formulations. Following a thorough review of safety data, the agency concluded that the risk of fracture with short-term, low-dose use of PPIs is unlikely. Nevertheless, the FDA acknowledged that consumers sometimes use a PPI longer than the directions advise. Therefore, the agency recommends that health-care professionals be aware of the risk of fracture if the patient uses an OTC PPI at a higher dose or longer time than the label advises.
Two meta-analyses find a modestly increased risk of fracture
Yu and colleagues analyzed 11 observational case-control or cohort studies that involved mostly older adults. They found a modestly increased risk of hip fracture among individuals taking a PPI, compared with nonusers (relative risk [RR], 1.30; 95% CI, 1.19–1.43). They also found an increase in spinal (RR, 1.56; 95% CI, 1.31–1.85) and any-site fractures (RR, 1.16; 95% CI, 1.04–1.30) among PPI users. They acknowledged that residual confounding could not be excluded.
A separate meta-analysis by Ye and colleagues included seven studies. They found a statistically significant increase in the risk of hip fracture, compared with nonusers (odds ratio [OR], 1.24; 95% CI, 1.15–1.34). However, because of different effects of PPIs at different durations of therapy, Ye and colleagues concluded that evidence was insufficient to support a causal relationship between PPI use and hip fracture. Further investigation is warranted.
What we know about PPIs and bone
Because PPIs are widely used, it is of paramount importance to understand the precise effects through which use of a PPI may affect bone mineral metabolism and influence fracture risk.
Targownik and Leslie discuss the evidence supporting an association between PPIs and fragility fracture, the association between PPIs and calcium malabsorption, and the underlying condition that predisposes patients who have osteoporosis to fracture. They also explore the possible mechanisms by which PPIs may increase the risk of fracture. After conducting a PubMed search and review of the literature, they found limited evidence supporting the FDA’s assertion that PPIs may increase the risk of fracture. Other findings:
- Multiple analyses have demonstrated a modest association between chronic PPI use and an increased risk of fracture
- No studies have convincingly demonstrated that PPIs cause fracture
- Overall, PPI use does not interfere with calcium absorption in most instances; therefore, it is unlikely that PPIs influence the risk of fracture by interfering with the absorption of dietary calcium
- Dual-energy x-ray absorptiometry (DEXA) testing demonstrates no consistent effect of PPIs on BMD
- Preliminary evidence suggests that PPIs may interfere with normal osteoclastic function, although it is unclear whether such interference influences the risk of fracture.
PPIs are the most potent inhibitors of acid secretion available; standard once-daily dosing may lead to a 90% decrease in gastric acid secretion and will maintain an intragastric pH level above 4 for as long as 70% of any 24-hour period.
PPIs are known to be effective for treating symptoms of GERD and preventing GERD-related complications. These drugs are also the optimal agents for the treatment of peptic ulcer disease and prevention of complications of peptic ulcer disease in chronic users of NSAIDs.
The potency of PPIs makes them the agent of choice for a variety of patients. The drugs also have a long reputation for having a favorable side-effect profile.
Due to this combination of efficacy and safety, PPIs are among the most widely prescribed drugs in all of clinical medicine, trailing only antihypertensives and antidepressants in total number of prescriptions. This means that many of our postmenopausal patients are using these drugs. Those who are using a prescription-strength PPI should be aware of the potential risk of fracture, particularly if they are using it chronically. They also should be advised that exercise, calcium, and vitamin D are vital, as well as regular DEXA testing according to established guidelines.
Will nitroglycerin be the next treatment for osteoporosis?
Jamal SA, Hamilton CJ, Eastell R, Cummings SR. Effect of nitroglycerin ointment on bone density and strength in postmenopausal women: a randomized trial. JAMA. 2011;305(8):800–807.
Khosla S. Is nitroglycerin a novel and inexpensive treatment for osteoporosis? JAMA. 2011;305(8):826–827.
Data suggesting that nitrates may protect against fracture date back to 2006, when Rejnmark and colleagues explored the use of these agents in 124,655 individuals who sustained a fracture and 373,963 age- and sex-matched controls.3 After adjustment for possible confounders, use of nitrates was associated with an 11% reduction in the risk of any fracture (OR, 0.89; 95% CI, 0.86–0.92) and a 15% reduction in the risk of hip fracture (OR, 0.85; 95% CI, 0.79–0.92).3
However, in 2009, Wimalawansa and colleagues found no benefit of transdermal nitroglycerin in preventing bone loss in early postmenopausal women.4 In their 3-year, randomized, double-blind, placebo-controlled trial, 186 postmenopausal women (mean age, 56 years) were randomized to receive nitroglycerin ointment (22.5 mg/d) or placebo. After 36 months of therapy, changes in BMD at multiple sites were comparable between groups. Due to the significant incidence of headache related to nitroglycerin treatment, adherence was suboptimal (estimated at approximately 70%), perhaps contributing to the negative findings.4
Enter this study by Jamal and associates, who performed a double-blind, randomized, placebo-controlled trial 24 months in duration. Participants were 243 women with a mean age of 62 years and a lumbar spine T-score between 0 and –2.0. They were randomized to 15 mg daily of 2% nitroglycerin ointment (applied to the upper outer arm at bedtime) or placebo.
At 2 years, women in the nitroglycerin group had a significant increase in BMD at the:
- lumbar spine, from 1.05 to 1.14 g/cm2, for a percentage change of 6.7% (P<0.001) (placebo group change: 1.06 to 1.08 g/cm2)
- total hip, from 0.92 to 0.97 g/cm2, for a percentage change of 6.2% (P<0.001) (placebo group change: 0.93 to 0.92 g/cm2)
- femoral neck, from 0.88 to 0.93 g/cm2, for a percentage change of 7% (P<0.001) (placebo group change: 0.87 to 0.86 g/cm2).
Nitroglycerin also increased bone-specific alkaline phosphatase by 34.8% and decreased urine N-telopeptide by 54% (P<0.001). Incidence of serious adverse events did not differ between nitroglycerin and placebo groups (5 [42.5%]).
Among women who continued treatment for 24 months, headaches were reported by 40 (35%) nitroglycerin users versus six (5.4%) nonusers during the first month, decreasing substantially after 12 months.
At this time, no changes in clinical practice are warranted. However, the findings of Jamal and coworkers should set the stage for a larger, adequately powered study of nitroglycerin ointment, using fracture as an outcome. If such a study demonstrates fracture reduction, clinicians will have a novel and inexpensive therapy for osteoporosis. The findings of Jamal and coworkers also should prompt development of additional nitric oxide donors that have greater skeletal effects and a better adverse-effect profile—particularly in regard to headache.
We want to hear from you! Tell us what you think.
We, and our patients, are fortunate to have a robust armamentarium of osteoporosis preventives and treatments in the 21st century. Still, we have much to learn about the preservation and restoration of bone. Fine-tuning of individual therapies, and clarification of their attendant risks, are ongoing concerns.
In this article, I highlight several recent studies:
- an assessment of the periodontium in a group of postmenopausal women who were long-term users of bisphosphonates, versus nonusers, to determine the effects of these agents on periodontal health
- guidance from the American Society for Bone and Mineral Research on the risk of atypical femoral fracture in long-term users of bisphosphonates
- 2-year data on a new, delayed-release, weekly formulation of risedronate that can be taken with food
- two meta-analyses exploring the risk of fracture with use of a proton-pump inhibitor (PPI), as well as a recent summary of evidence
- a randomized trial of 2% nitroglycerin ointment to prevent fracture.
Bisphosphonates may reduce the risk of postmenopausal periodontal disease
Palomo L, Buencamino-Francisco MC, Carey JJ, Sivanandy M, Thacker H. Is long-term bisphosphonate therapy associated with benefits to the periodontium in postmenopausal women? Menopause. 2011;18(2):164–170.
The risk of periodontal disease increases in menopause. Inflammation can erode structures (i.e., periodontal ligament and alveolar bone) that attach the teeth into the jaw, leading, eventually, to loss of teeth (FIGURE).

Anatomy of healthy periodontium
In periodontal disease, inflammation can erode structures, such as the periodontal ligament and alveolar bone, that attach the teeth into the jaw.In periodontal inflammation, a bacterial biofilm on tooth surfaces triggers a response by neutrophils and macrophages. In this respect, osteoporosis and periodontitis are mediated by common cytokines. Local production of cytokines seems to enhance osteoclast-mediated loss of skeletal and alveolar bone in estrogen-deficient women. In addition, generalized bone loss in postmenopausal osteoporosis renders the jaw susceptible to accelerated alveolar bone resorption and loss of periodontal attachment. For these reasons, physicians who care for postmenopausal women are advised to monitor their periodontal health; be vigilant for dental problems; and encourage them to practice good oral hygiene as a preventive measure against periodontitis and to seek regular dental care.
There has been tremendous publicity about the rare but very serious occurrence of osteonecrosis of the jaw in women who use bisphosphonates. In contrast, this study by Palomo and colleagues from the Case Western Reserve School of Dental Medicine seems to offer some preliminary good news about bisphosphonates and dental health: Long-term use appears to have some beneficial effects on the periodontium of postmenopausal women.
Details of the study
The aim of the study was to compare the periodontium in two groups of postmenopausal women known to have low bone mineral density (BMD): those who were long-term users (>2 years) of bisphosphonate therapy (n=28) and those who were not (n=28). The average age of participants in the study was 63 years, and the average T-score was –2.5. All women underwent cone-beam computed tomography of the jaw and a complete periodontal examination to determine the plaque score, periodontal probing depth, clinical attachment loss, bleeding on probing, and alveolar bone height.
Findings: Bisphosphonate users had a higher plaque score, a lower probing depth, and less loss of clinical attachment than did women in the control group. These differences were determined to be statistically significant. Bisphosphonate users also had less bleeding on probing and a higher alveolar bone height, but these differences were not significant. After adjustment for the plaque score, bisphosphonate use was a significant factor for probing depth but not for the other parameters.
Not all news about bisphosphonates is bad. Preliminary data seem to indicate that objective measures of periodontal disease are lower in bisphosphonate users who have low BMD than in nonusers.
Atypical femoral fracture is a real risk—but a rarity—with long-term use of antiresorptive drugs
Shane E, Burr D, Ebeling PR, et al; American Society for Bone and Mineral Research. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25(11):2267–2294.
As ObGyns, we are often the first-line providers of diagnostic services and treatment for postmenopausal women at risk of osteoporotic fracture. Oral and, more recently, parenteral bisphosphonates have been a mainstay of such treatment. Isolated reports of atypical femoral fracture in long-term users of bisphosphonates first surfaced around 2005.1 Since then, several case series have appeared, some with as many as 102 cases.2 In fact, atypical femoral fracture in bisphosphonate users has drawn so much attention that patients have begun to ask about these agents and express reservations about using them.
The issue has drawn the focus of the American Society for Bone and Mineral Research (ASBMR), which appointed a task force to address it. A multidisciplinary expert group reviewed pertinent published reports of atypical femoral fracture, as well as preclinical studies, that could provide insight into its pathogenesis.
What we know about this type of fracture
Preclinical data lend biologic plausibility to a potential association between long-term bisphosphonate use and atypical femoral fracture. These data highlight the effects of bisphosphonates on:
- collagen cross-linking and maturation
- accumulation of microdamage and advanced glycation end products
- heightened mineralization, remodeling, vascularity, and angiogenesis.
The task force concluded that bisphosphonates are highly effective at reducing the risk of spinal and nonspinal fractures, including typical and common femoral neck and intertrochanteric fractures. However, there is evidence of a relationship between long-term bisphosphonate use and a specific type of subtrochanteric and femoral shaft fracture. This type of fracture is characterized by unique radiographic features:
- transverse or short oblique orientation
- absence of comminution
- cortical thickening
- stress fracture or stress reaction on the symptomatic and/or contralateral side
- delayed healing.
This type of fracture also has unique clinical features—namely, prodromal pain and bilaterality.
The apparent increase in the risk of atypical femoral fracture in patients using glucocorticoids is a concern because bisphosphonates are the mainstay of prevention of glucocorticoid-induced osteoporotic fracture.
Bone biopsies from the iliac crest or fracture site, or both, generally show reduced bone formation consistent with bisphosphonate action. Paradoxically, some patients show biopsy evidence of enhanced bone resorption. Biochemical bone-turnover markers are often normal but may be increased.
Atypical femoral fracture can occur in patients who have not been treated with bisphosphonates, and its true incidence in treated and untreated patients is unknown. However, it appears to be more common in patients who have been exposed to long-term bisphosphonates—usually for longer than 3 years (median treatment: 7 years).
It must be emphasized that this type of fracture is rare, particularly considering the millions of patients who have used bisphosphonates and the much higher incidence of typical and common femoral neck and intertrochanteric fractures in untreated patients.
Bisphosphonates are important drugs for the prevention of common osteoporotic fractures. However, atypical femoral fracture is a concern, and more information is needed to help us identify patients at risk and to guide decision-making about the optimal duration of bisphosphonate therapy.
Any discussion with the patient about bisphosphonate use should begin with a mention of the millions of women who have been treated successfully with these agents and the thousands and thousands of hip, spinal, and other nonvertebral fractures that have been prevented. Only 249 cases of atypical femoral fracture have been reported worldwide.
The patient should also be reassured that research into this phenomenon is continuing. At present, the risk-benefit ratio greatly favors use of these medications in properly selected patients at heightened risk of osteoporotic fracture.
New delayed-release bisphosphonate can be taken with food
Benhamou CL, Zanchetta JR, Kaufman JM, et al. A novel delayed-release risedronate 35 mg once-a-week formulation taken with or without breakfast: 2 year BMD data. Osteoporosis Int. 2011;22:S337-S338.
Oral bisphosphonates are poorly absorbed from the gastrointestinal (GI) tract, with bioavailability of less than 1%. Absorption is further reduced when a bisphosphonate is taken with food, beverages other than plain water, medications, or supplements. Because of this limited absorption, oral bisphosphonates must be taken at least 30 to 60 minutes before the first food, drink, or other medication of the day. Noncompliance leads to reduced bioavailability and, potentially, decreased efficacy.
A new delayed-release formulation of risedronate (Atelvia) was approved by the FDA in late 2010 and made available for prescribing earlier this year. It is designed to improve absorption of risedronate in the presence of food, allowing for administration immediately after breakfast.
Two innovations were utilized in the manufacture of this tablet. First, it has an enteric coating to deliver risedronate beyond the stomach in the small intestine, with active drug released at a pH level above 5.5. Second, it contains a chelating agent—edetate disodium (EDTA)—which binds free divalent cations, such as calcium, magnesium, and iron.
Details of the study
Benhamou and colleagues compared three regimens of risedronate:
- 35 mg weekly of the delayed-release formulation, taken at least 30 minutes before breakfast
- 35 mg weekly of the delayed-release formulation, taken 30 minutes after breakfast
- 5 mg daily of the immediate-release risedronate formulation, taken 30 minutes before breakfast.
This phase 3, randomized, double-blind, active-controlled study involved 43 centers across North America, Europe, and South America. Characteristics of women in the study included:
- age of 50 years or older
- at least 5 years since the last menstrual period
- lumbar spine or total hip BMD corresponding to a T-score of –2.5 or worse, or a T-score of –2.0 or worse with at least one prevalent vertebral fracture.
The primary efficacy endpoint was the mean percentage of change in BMD at the lumbar spine.
Findings: At 2 years, the mean change for both delayed-release arms was significantly greater than for the 5-mg immediate-release dose. The authors concluded that the 35-mg weekly delayed-release regimen—whether taken at least 30 minutes before or after breakfast—is as effective and safe as the 5-mg daily immediate-release regimen.
Oral, delayed-release risedronate (35 mg weekly) is similar in efficacy and tolerability to the immediate-release formulation (5 mg daily). By minimizing the impact of concomitantly ingested food on the bioavailability of risedronate, the delayed-release formulation can make it easier for patients to accept and comply with the regimen, thereby maximizing absorption and improving efficacy of the drug in clinical practice.
Do proton-pump inhibitors increase the risk of fracture?
Targownik LE, Leslie WD. The relationship among proton pump inhibitors, bone disease and fracture [published online ahead of print May 20, 2011]. Expert Opin Drug Saf. doi: 10.1517/14740338.2011.586628.
Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med. 2011;124(6):519–526.
Ye X, Liu H, Wu C, et al. Proton pump inhibitors therapy and risk of hip fracture: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2011;23:794–800.
In May 2010, the FDA revised prescription and over-the-counter (OTC) labels for proton-pump inhibitors to include new safety information about a possible increased risk of fractures of the hip, wrist, and spine with use of these medications.
Proton pump inhibitors are so named because they prevent hydrogen ion (proton) secretion into the gastric lumen, thereby reducing acid in the stomach. Esomeprazole magnesium (Nexium), dexlansoprazole (Dexilant), omeprazole (Prilosec), omeprazole with sodium bicarbonate (Zegerid), lansoprazole (Prevacid), pantoprazole (Protonix), rabeprazole (Aciphex), and naproxen with esomeprazole magnesium (Vimovo) are available by prescription to treat conditions such as gastroesophageal reflux disease (GERD), ulcers of the stomach and small intestine, and inflammation of the esophagus. Delayed-release omeprazole magnesium (Prilosec OTC, Zegerid OTC) and delayed-release lansoprazole (Prevacid 24HR) are sold OTC for treatment of frequent heartburn.
The new safety information was based on an FDA review of several epidemiologic studies reporting an increased risk of fractures of the hip, wrist, and spine with PPI use. Some studies found that those at greatest risk of fracture received high doses of PPIs or used them for 1 year or longer. Most of the studies involved individuals 50 years and older; the increased risk of fracture was observed primarily in this age group.
Although the greatest risk of fracture involved people who had been taking a prescription PPI for at least 1 year or who had been taking a high dose of a prescription PPI, the FDA took the precaution of altering the “Drug facts” label on OTC agents as well. (Over-the-counter PPIs are indicated for 14 days of continuous use.) However, in March 2011, the FDA reversed its decision and retracted the wording on the label of OTC formulations. Following a thorough review of safety data, the agency concluded that the risk of fracture with short-term, low-dose use of PPIs is unlikely. Nevertheless, the FDA acknowledged that consumers sometimes use a PPI longer than the directions advise. Therefore, the agency recommends that health-care professionals be aware of the risk of fracture if the patient uses an OTC PPI at a higher dose or longer time than the label advises.
Two meta-analyses find a modestly increased risk of fracture
Yu and colleagues analyzed 11 observational case-control or cohort studies that involved mostly older adults. They found a modestly increased risk of hip fracture among individuals taking a PPI, compared with nonusers (relative risk [RR], 1.30; 95% CI, 1.19–1.43). They also found an increase in spinal (RR, 1.56; 95% CI, 1.31–1.85) and any-site fractures (RR, 1.16; 95% CI, 1.04–1.30) among PPI users. They acknowledged that residual confounding could not be excluded.
A separate meta-analysis by Ye and colleagues included seven studies. They found a statistically significant increase in the risk of hip fracture, compared with nonusers (odds ratio [OR], 1.24; 95% CI, 1.15–1.34). However, because of different effects of PPIs at different durations of therapy, Ye and colleagues concluded that evidence was insufficient to support a causal relationship between PPI use and hip fracture. Further investigation is warranted.
What we know about PPIs and bone
Because PPIs are widely used, it is of paramount importance to understand the precise effects through which use of a PPI may affect bone mineral metabolism and influence fracture risk.
Targownik and Leslie discuss the evidence supporting an association between PPIs and fragility fracture, the association between PPIs and calcium malabsorption, and the underlying condition that predisposes patients who have osteoporosis to fracture. They also explore the possible mechanisms by which PPIs may increase the risk of fracture. After conducting a PubMed search and review of the literature, they found limited evidence supporting the FDA’s assertion that PPIs may increase the risk of fracture. Other findings:
- Multiple analyses have demonstrated a modest association between chronic PPI use and an increased risk of fracture
- No studies have convincingly demonstrated that PPIs cause fracture
- Overall, PPI use does not interfere with calcium absorption in most instances; therefore, it is unlikely that PPIs influence the risk of fracture by interfering with the absorption of dietary calcium
- Dual-energy x-ray absorptiometry (DEXA) testing demonstrates no consistent effect of PPIs on BMD
- Preliminary evidence suggests that PPIs may interfere with normal osteoclastic function, although it is unclear whether such interference influences the risk of fracture.
PPIs are the most potent inhibitors of acid secretion available; standard once-daily dosing may lead to a 90% decrease in gastric acid secretion and will maintain an intragastric pH level above 4 for as long as 70% of any 24-hour period.
PPIs are known to be effective for treating symptoms of GERD and preventing GERD-related complications. These drugs are also the optimal agents for the treatment of peptic ulcer disease and prevention of complications of peptic ulcer disease in chronic users of NSAIDs.
The potency of PPIs makes them the agent of choice for a variety of patients. The drugs also have a long reputation for having a favorable side-effect profile.
Due to this combination of efficacy and safety, PPIs are among the most widely prescribed drugs in all of clinical medicine, trailing only antihypertensives and antidepressants in total number of prescriptions. This means that many of our postmenopausal patients are using these drugs. Those who are using a prescription-strength PPI should be aware of the potential risk of fracture, particularly if they are using it chronically. They also should be advised that exercise, calcium, and vitamin D are vital, as well as regular DEXA testing according to established guidelines.
Will nitroglycerin be the next treatment for osteoporosis?
Jamal SA, Hamilton CJ, Eastell R, Cummings SR. Effect of nitroglycerin ointment on bone density and strength in postmenopausal women: a randomized trial. JAMA. 2011;305(8):800–807.
Khosla S. Is nitroglycerin a novel and inexpensive treatment for osteoporosis? JAMA. 2011;305(8):826–827.
Data suggesting that nitrates may protect against fracture date back to 2006, when Rejnmark and colleagues explored the use of these agents in 124,655 individuals who sustained a fracture and 373,963 age- and sex-matched controls.3 After adjustment for possible confounders, use of nitrates was associated with an 11% reduction in the risk of any fracture (OR, 0.89; 95% CI, 0.86–0.92) and a 15% reduction in the risk of hip fracture (OR, 0.85; 95% CI, 0.79–0.92).3
However, in 2009, Wimalawansa and colleagues found no benefit of transdermal nitroglycerin in preventing bone loss in early postmenopausal women.4 In their 3-year, randomized, double-blind, placebo-controlled trial, 186 postmenopausal women (mean age, 56 years) were randomized to receive nitroglycerin ointment (22.5 mg/d) or placebo. After 36 months of therapy, changes in BMD at multiple sites were comparable between groups. Due to the significant incidence of headache related to nitroglycerin treatment, adherence was suboptimal (estimated at approximately 70%), perhaps contributing to the negative findings.4
Enter this study by Jamal and associates, who performed a double-blind, randomized, placebo-controlled trial 24 months in duration. Participants were 243 women with a mean age of 62 years and a lumbar spine T-score between 0 and –2.0. They were randomized to 15 mg daily of 2% nitroglycerin ointment (applied to the upper outer arm at bedtime) or placebo.
At 2 years, women in the nitroglycerin group had a significant increase in BMD at the:
- lumbar spine, from 1.05 to 1.14 g/cm2, for a percentage change of 6.7% (P<0.001) (placebo group change: 1.06 to 1.08 g/cm2)
- total hip, from 0.92 to 0.97 g/cm2, for a percentage change of 6.2% (P<0.001) (placebo group change: 0.93 to 0.92 g/cm2)
- femoral neck, from 0.88 to 0.93 g/cm2, for a percentage change of 7% (P<0.001) (placebo group change: 0.87 to 0.86 g/cm2).
Nitroglycerin also increased bone-specific alkaline phosphatase by 34.8% and decreased urine N-telopeptide by 54% (P<0.001). Incidence of serious adverse events did not differ between nitroglycerin and placebo groups (5 [42.5%]).
Among women who continued treatment for 24 months, headaches were reported by 40 (35%) nitroglycerin users versus six (5.4%) nonusers during the first month, decreasing substantially after 12 months.
At this time, no changes in clinical practice are warranted. However, the findings of Jamal and coworkers should set the stage for a larger, adequately powered study of nitroglycerin ointment, using fracture as an outcome. If such a study demonstrates fracture reduction, clinicians will have a novel and inexpensive therapy for osteoporosis. The findings of Jamal and coworkers also should prompt development of additional nitric oxide donors that have greater skeletal effects and a better adverse-effect profile—particularly in regard to headache.
We want to hear from you! Tell us what you think.
1. Husada G, Libberecht K, Peeters T, Populaire J. Bilateral mid-diaphyseal femoral stress fractures in the elderly. Eur J Trauma. 2005;31(1):68-71.Doi:10.1007/s00068-005-1421–5.
2. Dell R, Greene D, Ott S, et al. A retrospective analysis of all atypical femur fractures seen in a large California HMO from the years 2007 to 2009. Paper presented at ASBMR 2010 Annual Meeting; October 18, 2010; Toronto, Ontario, Canada. http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?filename="2311OBG_Update" aid=05caf316-b73e-47b8-a011-bf0766b062c0. Accessed Sept. 26, 2011.
3. Rejnmark L, Vestergaard P, Mosekilde L. Decreased fracture risk in users of organic nitrates: a nationwide case-control study. J Bone Miner Res. 2006;21(11):1811-1817.
4. Wimalawansa SJ, Grimes JP, Wilson AC, Hoover DR. Transdermal nitroglycerin therapy may not prevent early postmenopausal bone loss. J Clin Endocrinol Metab. 2009;94(9):3356-3364.
1. Husada G, Libberecht K, Peeters T, Populaire J. Bilateral mid-diaphyseal femoral stress fractures in the elderly. Eur J Trauma. 2005;31(1):68-71.Doi:10.1007/s00068-005-1421–5.
2. Dell R, Greene D, Ott S, et al. A retrospective analysis of all atypical femur fractures seen in a large California HMO from the years 2007 to 2009. Paper presented at ASBMR 2010 Annual Meeting; October 18, 2010; Toronto, Ontario, Canada. http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?filename="2311OBG_Update" aid=05caf316-b73e-47b8-a011-bf0766b062c0. Accessed Sept. 26, 2011.
3. Rejnmark L, Vestergaard P, Mosekilde L. Decreased fracture risk in users of organic nitrates: a nationwide case-control study. J Bone Miner Res. 2006;21(11):1811-1817.
4. Wimalawansa SJ, Grimes JP, Wilson AC, Hoover DR. Transdermal nitroglycerin therapy may not prevent early postmenopausal bone loss. J Clin Endocrinol Metab. 2009;94(9):3356-3364.
“Does cancer cause menorrhagia?” A lament on the decline of the art of medicine
“Does cancer cause menorrhagia?” I asked on morning rounds recently. The response?
Blank stares. From students and residents.
“Well, what is menorrhagia?” I ask.
A resident regurgitates the textbook definition: “Abnormally heavy and prolonged menstrual bleeding lasting longer than 7 days or with blood loss exceeding 80 mL.”
“OK. What is metrorrhagia?”
Another textbook definition: “Abnormal uterine bleeding at irregular intervals.”
“Do you think cancer causes menorrhagia?”
Silence and sideways glances ensue. After a while, a junior resident says, barely audibly, “I guess so.”
I pounce: “Do you really believe that cancer causes heavy bleeding every 28 days?”
The junior resident has fallen into my trap, which isn’t so difficult to set or spring these days. It seems that contemporary medical training teaches residents how to regurgitate information on demand, but it doesn’t do so well at showing them how to apply their knowledge to common scenarios.
Pay attention to the backstory
In my residents’ world, all abnormal bleeding is reduced to “menometrorrhagia.” In fact, this term is so common it has been affectionately shortened by these residents to “menomet.” When I ask them what causes abnormal uterine bleeding, they recite the entire shopping list from UpToDate, with little or no understanding of how to determine which pathologies are likely, based on the patient’s bleeding pattern.
We go through the entities that might increase bleeding at the “normal” time—a large cavity secondary to multiparity; uterine hypertrophy as a result of myomata (without a submucosal component); adenomyosis; a polyp in synchrony with the phases of the menstrual cycle.
Then we go over things likely to cause bleeding at an abnormal time—anovulation and all its causes; polyps; submucous myomas; hyperplasia; and carcinoma.
Last, we discuss situations where there might be overlap between the two, and the need to get as much reliable information from the patient as possible. After all, a patient can tell you a lot. Let me give you an example.
CASE
A 50-year-old patient taking unopposed estrogen reports that she had no menses for 4 months, followed by an episode of staining. Transvaginal ultrasonography reveals that she has an endometrial echo of 8 mm. The patient says that she had vasomotor symptoms that disappeared just before the staining. She is not obese but is mildly plump, without a personal or family history of diabetes. She is parous, with one normal spontaneous delivery.
We all know that unopposed estrogen in some women is a risk factor for hyperplasia and adenocarcinoma of the endometrium. Simple hyperplasia can be reversed with progestin administration, and the rate of endometrial cancer is reduced when a progestin is added to the regimen of a woman taking exogenous estrogen.
CASE CONTINUED
If I believed that this patient had experienced anovulation for 5 months, I would have chosen to administer progestin. But the history she reports (the vasomotor symptoms and amenorrhea) suggests 4 months of little or no ovarian function, followed by a resurrection of some ovarian function. As a result of the information she provides, and the several follicles visible during sonographic assessment, I am considerably less concerned about the unopposed estrogen than I might otherwise have been, and I am able to fine-tune my clinical management accordingly.
This type of assessment requires careful extraction of relevant information (i.e., a thorough history) and an understanding of the nuances of physiology and pathology. These skills are, I believe, no longer being emphasized in medical education.
That is a problem.
Algorithms can be deceptively simple
Clinical pathways in which decision trees are dichotomous have become the mainstay of clinical practice. Using one such tree, we conclude that an endometrial echo of 4 mm or less in a woman who experiences postmenopausal bleeding carries a cancer risk that is so low, no biopsy is necessary. (Notice now that this is postmenopausal bleeding, not menorrhagia or metrorrhagia!) This conclusion is based on excellent prospective data, but the cutoff of 4 mm is somewhat arbitrary. A higher cutoff allows more cancers to escape detection, and a lower number results in more interventions, such as dilatation and curettage, hysteroscopy, or saline-infusion sonohysterography (and, I hope, not blind suction piston biopsy, unless you are sure the process is indeed global and not focal).
We have ultrasonography machines that produce measurements down to hundredths of a centimeter! Some nights I wake in a cold sweat, worried that a clinician will get an ultrasound report of a 3.94-mm endometrial echo and conclude that the patient is fine, or that a report of 4.03 mm will prompt a clinician to go all the way to hysterectomy, if all else fails, just to get a bit of tissue! Where is the thought process—the art of medicine?
When I give third-year medical students their first didactic lecture of the clerkship, I implore them to ask, “Where does that come from?” “Who is the exception?” “Why?” Our patients expect us to be able to think, to understand why we do what we do, to realize who the outlier is or may be. Otherwise, why get a medical degree?
Patients often consult a physician out of fear of being the numerator. Maybe only 1 in every 305 women 35 years old will deliver a chromosomally abnormal baby. Or maybe only 3 to 7 of every 100 postmenopausal women who experience uterine bleeding will have endometrial cancer. The odds may be in the patient’s favor, but she is afraid that she might turn out to be that 1, or those 3 to 7, in the numerator of the equation.
Enter the EHR
With so much of clinical practice designed to be carried out by algorithm, our students are learning what to do but not why—so who will design newer and better algorithms in the future? The likely source of those new algorithms: the electronic health or medical record.
Computer experts, who need to know little or no pathophysiology, will be able to mine outcomes databases. For instance, they might analyze an institution’s last 3,000 cases of proven ovarian torsion, including dozens of parameters such as white blood cell count, size of the mass on ultrasonography, body temperature, and number of hours the patient experienced pain. Then they will perform a regression analysis on this data and develop a receiver-operating-characteristic (ROC) curve with the best “fit” for a manageable number of parameters. The physician will plug the data into a handheld device and, depending on which side of the curve the patient falls, will take her to the operating room or manage her expectantly.
There will be little need, or even tolerance, for judgment or experience.
When I describe this scenario to a colleague—he’s the safety officer on labor and delivery—he says we need protocols to protect patients because so many clinicians who rely on judgment and experience are doing a mediocre job. I argue that medicine by protocol narrows the bandwidth. It may bring the bottom up, but it also brings the top down. More people will get better care, but the outlier probably won’t.
But why do people go to the doctor? For fear of being the outlier!
Can we fix this problem?
Probably not.
The entire medical field is moving toward enhanced care for the majority at the expense of the few. Patient-safety systems and algorithms are the wave of the future.
A drop in the bucket
I still give that pep talk to third-year medical students as they enter our rotation, in the hope that it will resonate with even one or two of them, who may resolve to develop and cultivate judgment and experience even within the system that is enveloping us.
As for the rest of the students, they’ll squirm uncomfortably in their seats, or get confused on morning rounds—and, maybe, assert that cancer indeed causes menorrhagia.
We want to hear from you! Tell us what you think.
“Does cancer cause menorrhagia?” I asked on morning rounds recently. The response?
Blank stares. From students and residents.
“Well, what is menorrhagia?” I ask.
A resident regurgitates the textbook definition: “Abnormally heavy and prolonged menstrual bleeding lasting longer than 7 days or with blood loss exceeding 80 mL.”
“OK. What is metrorrhagia?”
Another textbook definition: “Abnormal uterine bleeding at irregular intervals.”
“Do you think cancer causes menorrhagia?”
Silence and sideways glances ensue. After a while, a junior resident says, barely audibly, “I guess so.”
I pounce: “Do you really believe that cancer causes heavy bleeding every 28 days?”
The junior resident has fallen into my trap, which isn’t so difficult to set or spring these days. It seems that contemporary medical training teaches residents how to regurgitate information on demand, but it doesn’t do so well at showing them how to apply their knowledge to common scenarios.
Pay attention to the backstory
In my residents’ world, all abnormal bleeding is reduced to “menometrorrhagia.” In fact, this term is so common it has been affectionately shortened by these residents to “menomet.” When I ask them what causes abnormal uterine bleeding, they recite the entire shopping list from UpToDate, with little or no understanding of how to determine which pathologies are likely, based on the patient’s bleeding pattern.
We go through the entities that might increase bleeding at the “normal” time—a large cavity secondary to multiparity; uterine hypertrophy as a result of myomata (without a submucosal component); adenomyosis; a polyp in synchrony with the phases of the menstrual cycle.
Then we go over things likely to cause bleeding at an abnormal time—anovulation and all its causes; polyps; submucous myomas; hyperplasia; and carcinoma.
Last, we discuss situations where there might be overlap between the two, and the need to get as much reliable information from the patient as possible. After all, a patient can tell you a lot. Let me give you an example.
CASE
A 50-year-old patient taking unopposed estrogen reports that she had no menses for 4 months, followed by an episode of staining. Transvaginal ultrasonography reveals that she has an endometrial echo of 8 mm. The patient says that she had vasomotor symptoms that disappeared just before the staining. She is not obese but is mildly plump, without a personal or family history of diabetes. She is parous, with one normal spontaneous delivery.
We all know that unopposed estrogen in some women is a risk factor for hyperplasia and adenocarcinoma of the endometrium. Simple hyperplasia can be reversed with progestin administration, and the rate of endometrial cancer is reduced when a progestin is added to the regimen of a woman taking exogenous estrogen.
CASE CONTINUED
If I believed that this patient had experienced anovulation for 5 months, I would have chosen to administer progestin. But the history she reports (the vasomotor symptoms and amenorrhea) suggests 4 months of little or no ovarian function, followed by a resurrection of some ovarian function. As a result of the information she provides, and the several follicles visible during sonographic assessment, I am considerably less concerned about the unopposed estrogen than I might otherwise have been, and I am able to fine-tune my clinical management accordingly.
This type of assessment requires careful extraction of relevant information (i.e., a thorough history) and an understanding of the nuances of physiology and pathology. These skills are, I believe, no longer being emphasized in medical education.
That is a problem.

Algorithms can be deceptively simple
Clinical pathways in which decision trees are dichotomous have become the mainstay of clinical practice. Using one such tree, we conclude that an endometrial echo of 4 mm or less in a woman who experiences postmenopausal bleeding carries a cancer risk that is so low, no biopsy is necessary. (Notice now that this is postmenopausal bleeding, not menorrhagia or metrorrhagia!) This conclusion is based on excellent prospective data, but the cutoff of 4 mm is somewhat arbitrary. A higher cutoff allows more cancers to escape detection, and a lower number results in more interventions, such as dilatation and curettage, hysteroscopy, or saline-infusion sonohysterography (and, I hope, not blind suction piston biopsy, unless you are sure the process is indeed global and not focal).
We have ultrasonography machines that produce measurements down to hundredths of a centimeter! Some nights I wake in a cold sweat, worried that a clinician will get an ultrasound report of a 3.94-mm endometrial echo and conclude that the patient is fine, or that a report of 4.03 mm will prompt a clinician to go all the way to hysterectomy, if all else fails, just to get a bit of tissue! Where is the thought process—the art of medicine?
When I give third-year medical students their first didactic lecture of the clerkship, I implore them to ask, “Where does that come from?” “Who is the exception?” “Why?” Our patients expect us to be able to think, to understand why we do what we do, to realize who the outlier is or may be. Otherwise, why get a medical degree?
Patients often consult a physician out of fear of being the numerator. Maybe only 1 in every 305 women 35 years old will deliver a chromosomally abnormal baby. Or maybe only 3 to 7 of every 100 postmenopausal women who experience uterine bleeding will have endometrial cancer. The odds may be in the patient’s favor, but she is afraid that she might turn out to be that 1, or those 3 to 7, in the numerator of the equation.
Enter the EHR
With so much of clinical practice designed to be carried out by algorithm, our students are learning what to do but not why—so who will design newer and better algorithms in the future? The likely source of those new algorithms: the electronic health or medical record.
Computer experts, who need to know little or no pathophysiology, will be able to mine outcomes databases. For instance, they might analyze an institution’s last 3,000 cases of proven ovarian torsion, including dozens of parameters such as white blood cell count, size of the mass on ultrasonography, body temperature, and number of hours the patient experienced pain. Then they will perform a regression analysis on this data and develop a receiver-operating-characteristic (ROC) curve with the best “fit” for a manageable number of parameters. The physician will plug the data into a handheld device and, depending on which side of the curve the patient falls, will take her to the operating room or manage her expectantly.
There will be little need, or even tolerance, for judgment or experience.
When I describe this scenario to a colleague—he’s the safety officer on labor and delivery—he says we need protocols to protect patients because so many clinicians who rely on judgment and experience are doing a mediocre job. I argue that medicine by protocol narrows the bandwidth. It may bring the bottom up, but it also brings the top down. More people will get better care, but the outlier probably won’t.
But why do people go to the doctor? For fear of being the outlier!
Can we fix this problem?
Probably not.
The entire medical field is moving toward enhanced care for the majority at the expense of the few. Patient-safety systems and algorithms are the wave of the future.
A drop in the bucket
I still give that pep talk to third-year medical students as they enter our rotation, in the hope that it will resonate with even one or two of them, who may resolve to develop and cultivate judgment and experience even within the system that is enveloping us.
As for the rest of the students, they’ll squirm uncomfortably in their seats, or get confused on morning rounds—and, maybe, assert that cancer indeed causes menorrhagia.
We want to hear from you! Tell us what you think.
“Does cancer cause menorrhagia?” I asked on morning rounds recently. The response?
Blank stares. From students and residents.
“Well, what is menorrhagia?” I ask.
A resident regurgitates the textbook definition: “Abnormally heavy and prolonged menstrual bleeding lasting longer than 7 days or with blood loss exceeding 80 mL.”
“OK. What is metrorrhagia?”
Another textbook definition: “Abnormal uterine bleeding at irregular intervals.”
“Do you think cancer causes menorrhagia?”
Silence and sideways glances ensue. After a while, a junior resident says, barely audibly, “I guess so.”
I pounce: “Do you really believe that cancer causes heavy bleeding every 28 days?”
The junior resident has fallen into my trap, which isn’t so difficult to set or spring these days. It seems that contemporary medical training teaches residents how to regurgitate information on demand, but it doesn’t do so well at showing them how to apply their knowledge to common scenarios.
Pay attention to the backstory
In my residents’ world, all abnormal bleeding is reduced to “menometrorrhagia.” In fact, this term is so common it has been affectionately shortened by these residents to “menomet.” When I ask them what causes abnormal uterine bleeding, they recite the entire shopping list from UpToDate, with little or no understanding of how to determine which pathologies are likely, based on the patient’s bleeding pattern.
We go through the entities that might increase bleeding at the “normal” time—a large cavity secondary to multiparity; uterine hypertrophy as a result of myomata (without a submucosal component); adenomyosis; a polyp in synchrony with the phases of the menstrual cycle.
Then we go over things likely to cause bleeding at an abnormal time—anovulation and all its causes; polyps; submucous myomas; hyperplasia; and carcinoma.
Last, we discuss situations where there might be overlap between the two, and the need to get as much reliable information from the patient as possible. After all, a patient can tell you a lot. Let me give you an example.
CASE
A 50-year-old patient taking unopposed estrogen reports that she had no menses for 4 months, followed by an episode of staining. Transvaginal ultrasonography reveals that she has an endometrial echo of 8 mm. The patient says that she had vasomotor symptoms that disappeared just before the staining. She is not obese but is mildly plump, without a personal or family history of diabetes. She is parous, with one normal spontaneous delivery.
We all know that unopposed estrogen in some women is a risk factor for hyperplasia and adenocarcinoma of the endometrium. Simple hyperplasia can be reversed with progestin administration, and the rate of endometrial cancer is reduced when a progestin is added to the regimen of a woman taking exogenous estrogen.
CASE CONTINUED
If I believed that this patient had experienced anovulation for 5 months, I would have chosen to administer progestin. But the history she reports (the vasomotor symptoms and amenorrhea) suggests 4 months of little or no ovarian function, followed by a resurrection of some ovarian function. As a result of the information she provides, and the several follicles visible during sonographic assessment, I am considerably less concerned about the unopposed estrogen than I might otherwise have been, and I am able to fine-tune my clinical management accordingly.
This type of assessment requires careful extraction of relevant information (i.e., a thorough history) and an understanding of the nuances of physiology and pathology. These skills are, I believe, no longer being emphasized in medical education.
That is a problem.

Algorithms can be deceptively simple
Clinical pathways in which decision trees are dichotomous have become the mainstay of clinical practice. Using one such tree, we conclude that an endometrial echo of 4 mm or less in a woman who experiences postmenopausal bleeding carries a cancer risk that is so low, no biopsy is necessary. (Notice now that this is postmenopausal bleeding, not menorrhagia or metrorrhagia!) This conclusion is based on excellent prospective data, but the cutoff of 4 mm is somewhat arbitrary. A higher cutoff allows more cancers to escape detection, and a lower number results in more interventions, such as dilatation and curettage, hysteroscopy, or saline-infusion sonohysterography (and, I hope, not blind suction piston biopsy, unless you are sure the process is indeed global and not focal).
We have ultrasonography machines that produce measurements down to hundredths of a centimeter! Some nights I wake in a cold sweat, worried that a clinician will get an ultrasound report of a 3.94-mm endometrial echo and conclude that the patient is fine, or that a report of 4.03 mm will prompt a clinician to go all the way to hysterectomy, if all else fails, just to get a bit of tissue! Where is the thought process—the art of medicine?
When I give third-year medical students their first didactic lecture of the clerkship, I implore them to ask, “Where does that come from?” “Who is the exception?” “Why?” Our patients expect us to be able to think, to understand why we do what we do, to realize who the outlier is or may be. Otherwise, why get a medical degree?
Patients often consult a physician out of fear of being the numerator. Maybe only 1 in every 305 women 35 years old will deliver a chromosomally abnormal baby. Or maybe only 3 to 7 of every 100 postmenopausal women who experience uterine bleeding will have endometrial cancer. The odds may be in the patient’s favor, but she is afraid that she might turn out to be that 1, or those 3 to 7, in the numerator of the equation.
Enter the EHR
With so much of clinical practice designed to be carried out by algorithm, our students are learning what to do but not why—so who will design newer and better algorithms in the future? The likely source of those new algorithms: the electronic health or medical record.
Computer experts, who need to know little or no pathophysiology, will be able to mine outcomes databases. For instance, they might analyze an institution’s last 3,000 cases of proven ovarian torsion, including dozens of parameters such as white blood cell count, size of the mass on ultrasonography, body temperature, and number of hours the patient experienced pain. Then they will perform a regression analysis on this data and develop a receiver-operating-characteristic (ROC) curve with the best “fit” for a manageable number of parameters. The physician will plug the data into a handheld device and, depending on which side of the curve the patient falls, will take her to the operating room or manage her expectantly.
There will be little need, or even tolerance, for judgment or experience.
When I describe this scenario to a colleague—he’s the safety officer on labor and delivery—he says we need protocols to protect patients because so many clinicians who rely on judgment and experience are doing a mediocre job. I argue that medicine by protocol narrows the bandwidth. It may bring the bottom up, but it also brings the top down. More people will get better care, but the outlier probably won’t.
But why do people go to the doctor? For fear of being the outlier!
Can we fix this problem?
Probably not.
The entire medical field is moving toward enhanced care for the majority at the expense of the few. Patient-safety systems and algorithms are the wave of the future.
A drop in the bucket
I still give that pep talk to third-year medical students as they enter our rotation, in the hope that it will resonate with even one or two of them, who may resolve to develop and cultivate judgment and experience even within the system that is enveloping us.
As for the rest of the students, they’ll squirm uncomfortably in their seats, or get confused on morning rounds—and, maybe, assert that cancer indeed causes menorrhagia.
We want to hear from you! Tell us what you think.
Skilled US imaging of the adnexae: The fallopian tubes
Part 1 A Starting Point (September 2010)
Part 2 The non-neoplastic ovarian mass (October 2010)
Part 3 Ovarian neoplasms (November 2010)
An imaging study of the adnexae would not be complete without thorough assessment of the fallopian tubes. Among the pathologies that may be identified or confirmed by ultrasonography are:
- ectopic pregnancy
- tubal inflammatory disease, or salpingitis
- chronic tubal disease, or hydrosalpinx
- tubo-ovarian complex
- tubo-ovarian abscess
- tubal and ovarian torsion
- cancer.
In this final installment of our four-part series on ultrasonographic (US) imaging of the adnexae, we take these entities as our focus.
Suspect ectopic pregnancy even if the hCG level is not yet available
A detailed discussion of ectopic pregnancy far exceeds the framework of this article. Suffice it to say that ectopic pregnancy should always be considered in a woman of reproductive age, especially one who complains of abdominal or pelvic pain, vaginal bleeding, or both. However, these signs and symptoms are present in only about 25% of women who have this condition. When these signs and symptoms are present, it is wise to be suspicious even if the results of human chorionic gonadotropin (hCG) measurement are not yet available.
A complete history is important in the diagnosis of ectopic pregnancy. Risk factors include a history of ectopic pregnancy, pelvic inflammatory disease (PID), or tubal surgery, or use of an intrauterine device.
Unequivocal US diagnosis of ectopic pregnancy is possible in only about 20% of cases, and depends on identification of an extrauterine pregnancy, which may not be visible in the early days of gestation. However, some grayscale ultrasonographic findings that may suggest ectopic pregnancy include:
- an empty uterus in a woman who has an hCG level above 1,000 to 1,500 mIu/mL (the discriminatory level)
- a thick, hyperechoic endometrial echo (decidualization)
- an adnexal mass other than a simple cyst
- echogenic fluid in the cul-de-sac (FIGURE 1A–1D).
Power Doppler can help the sonographer localize the ectopic pregnancy in the tubes by demonstrating the circular vascularization of the more or less typical “tubal ring” (FIGURE 1E–1G).
FIGURE 1 Ectopic pregnancy
A–D. Various cases of tubal ectopic gestation (arrows point to each gestation). E–G. Power Doppler localizes the ectopic pregnancy (arrows), side by side with the corpus luteum (CL).
A patient’s history may yield clues to tubal inflammatory disease
The diagnosis of acute salpingitis begins with a thorough patient history. Look for any report of PID, unexplained fever, foul vaginal discharge, sexually transmitted infection, or recent intrauterine procedures such as hysteroscopy, IUD insertion, endometrial biopsy, or saline infusion sonohysterography.
US diagnosis is based on the findings of a slightly dilated fallopian tube with low-level echogenic fluid content, thick tubal walls, and tenderness to the touch of the transvaginal probe.1
In cross section, the tube forms the “cogwheel sign” (FIGURE 2C). Power Doppler shows the subserosal blood vessels characteristic of this entity (FIGURE 2D).
FIGURE 2 Tubal disease
A–C. Grayscale images showing thick walls, low-level echoic fluid (pus?) and the “cogwheel sign” (CW). D. Subserosal vascularization typical of an inflammatory response in hollow abdominal viscera. E,F. Edematous fimbrial end (arrow) of the inflamed tubes, floating in a small amount of free pelvic fluid. G. Low-level echoic, fluid-filled, thick-walled tubes with incomplete septae (arrow) are the hallmarks of hydrosalpinx. H–J. Bilateral hydrosalpinx. Note the thin walls and anechoic fluid-filled forms (sausage-shaped) (CL = corpus luteum; OV = ovary; UT = uterus).
Look for fluid dilating the tube in chronic tubal disease
Hydrosalpinx is characterized on US by thin tubal walls with a relatively anechoic but large amount of fluid dilating the tube (FIGURE 2G–2J). The interior wall is studded with shallow, echogenic, mural nodules (without blood vessels) that assume the appearance of a tube or sausage. The small, shallow internal papillae give the cross section of the tube the appearance of beads on a string.
FIGURE 3 Tubo-ovarian complex
A. The tube (T) and ovary (OV) form an infectious conglomerate. B. Power Doppler appearance. C. Laparoscopic view.
Tubo-ovarian complex
When this complex arises, the anatomy and shape of the tube and the involved ovary are somewhat distorted but still largely discernible (FIGURE 3A AND 3B).
Tubo-ovarian abscess is a more advanced stage of a fast-progressing or neglected pelvic inflammatory process. In it, the tube and the ovary can barely be distinguished, and US signs of abscess appear, among them low-level echoic fluid and linear echogenicity (FIGURE 4).
FIGURE 4 Tubo-ovarian abscess
The tube and ovary are indistinguishable. The fluid is of low-level echogenicity (pus), and the walls are thick.
In ovarian torsion, the follicles press outward
Although torsion has distinct sonographic signs, it remains a clinical diagnosis that US findings may or may not support. Correct diagnosis often is the purview of expert sonographers and sonologists.
When ovarian torsion is present, the ovaries are enlarged and hyperechoic, their follicles pushed toward the surface (FIGURE 5A–5C). The ovaries are also tender to the touch and typically demonstrate no blood flow by Doppler interrogation. On occasion, when arterial flow is still present (venous flow is usually the first characteristic to vanish), a twisted arterial pattern may result, similar to the coil of a telephone cord. Some pelvic fluid may also appear.
Tubal torsion is harder to diagnose. US recognition depends on the finding of a normal ovary with intact blood flow beside a fluid-filled, thin-walled, tender, cystic structure with some of the previously mentioned sonomarkers of tubal occlusion such as the bead-on-a-string or cogwheel sign (FIGURE 5D–5G).
FIGURE 5 Torsion
A–C. Ovarian torsion. Hyperechoic, large ovary with follicles pushed toward the surface. Power Doppler reveals no blood flow in the ovary. D–F. Tubal torsion. Cystic dilatation with a small beak and a normal ovary. G. Intraoperative view of the tube (twisted three times; yellow arrows) and the normal ovary (white arrows).
Fluid in the cul-de-sac
In many cases, fluid may be present or trapped in the lesser pelvis, surrounded or blocked by the pelvic organs. If this fluid is the result or sequela of PID, thin, thread-like adhesive strands will be visible between the organs on US, betraying its pathogenesis (FIGURE 6). The “walls” of such loculated fluid are the pelvic wall itself and the surrounding organs.
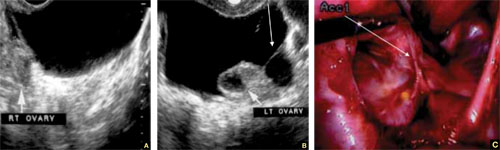
FIGURE 6 Fluid in the cul-de-sac
Sequelae of acute PID. A. Free pelvic fluid, also known as pelvic, peritoneal, loculated fluid. B. A normal ovary and an adhesive strand (arrow). C. Laparoscopic image of the adhesion (arrow).
Cancer of the tubes is unlikely, but it’s best to keep it in mind
Primary cancer of the fallopian tubes accounts for only 1% to 2% of all gynecologic cancers.2 Only 300 to 400 women are given this diagnosis each year in the United States— most of them postmenopausal.
Despite its rarity, fallopian-tube cancer is a major concern when a tubal mass is identified by palpation or imaging. In most cases, however, no palpable mass is found at the time of first examination, and tubal malignancy is diagnosed perioperatively or postoperatively.
US characteristics of tubal cancer are similar to those of ovarian cancer: a bizarre appearance, with extremely vascular tissue. At times, US attributes of tubal pathology, such as incomplete septae and tube-like fluid-filled structures, are apparent (FIGURE 7).
Consider cancer of the fallopian tube whenever an unexplained solid mass is palpated or imaged in the area of the tubes in conjunction with apparently normal ovaries.
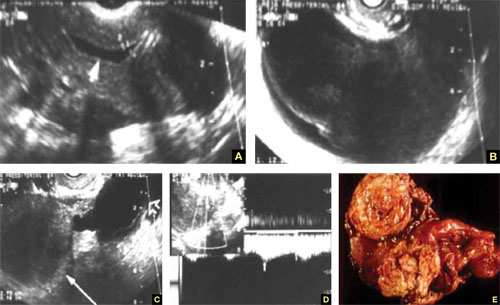
FIGURE 7 Fallopian tube cancer
A. Fluid-filled uterine cavity. B. Large cystic dilatation of the tube. C. A thickened tubal wall (arrow). D. Doppler interrogation reveals high diastolic flow (arrows). E. Macroscopic gross appearance.
The long view
As technology has advanced, so has ultrasonography. High-resolution transducers, color and power Doppler, and three-dimensional imaging make it possible for an experienced practitioner to identify and confirm the diagnosis of many adnexal masses and pathologies, from the corpus luteum to fallopian tube torsion. As the field continues to evolve, we expect that this modality will facilitate the diagnosis of adnexal abnormalities to an even greater degree.
In the meantime, this four-part tutorial offers guidance on the identification of adnexal masses. If we’ve helped ease coordination of care between the generalist ObGyn and the expert sonographer, we’ve accomplished our goal.
We want to hear from you! Tell us what you think.
1. Timor-Tritsch IE, Lerner JP, Monteagudo A, Murphy KE, Heller DS. Transvaginal sonographic markers of tubal inflammatory disease. Ultrasound Obstet Gynecol. 1998;12(1):56-66.
2. Goswami PK, Kerr-Wilson R, McCarthy K. Cancer of the fallopian tube. The Obstetrician & Gynaecologist. 2006;8(3):147-152.doi: 10.1576/toag.8.3.147.27249.
Part 1 A Starting Point (September 2010)
Part 2 The non-neoplastic ovarian mass (October 2010)
Part 3 Ovarian neoplasms (November 2010)
An imaging study of the adnexae would not be complete without thorough assessment of the fallopian tubes. Among the pathologies that may be identified or confirmed by ultrasonography are:
- ectopic pregnancy
- tubal inflammatory disease, or salpingitis
- chronic tubal disease, or hydrosalpinx
- tubo-ovarian complex
- tubo-ovarian abscess
- tubal and ovarian torsion
- cancer.
In this final installment of our four-part series on ultrasonographic (US) imaging of the adnexae, we take these entities as our focus.
Suspect ectopic pregnancy even if the hCG level is not yet available
A detailed discussion of ectopic pregnancy far exceeds the framework of this article. Suffice it to say that ectopic pregnancy should always be considered in a woman of reproductive age, especially one who complains of abdominal or pelvic pain, vaginal bleeding, or both. However, these signs and symptoms are present in only about 25% of women who have this condition. When these signs and symptoms are present, it is wise to be suspicious even if the results of human chorionic gonadotropin (hCG) measurement are not yet available.
A complete history is important in the diagnosis of ectopic pregnancy. Risk factors include a history of ectopic pregnancy, pelvic inflammatory disease (PID), or tubal surgery, or use of an intrauterine device.
Unequivocal US diagnosis of ectopic pregnancy is possible in only about 20% of cases, and depends on identification of an extrauterine pregnancy, which may not be visible in the early days of gestation. However, some grayscale ultrasonographic findings that may suggest ectopic pregnancy include:
- an empty uterus in a woman who has an hCG level above 1,000 to 1,500 mIu/mL (the discriminatory level)
- a thick, hyperechoic endometrial echo (decidualization)
- an adnexal mass other than a simple cyst
- echogenic fluid in the cul-de-sac (FIGURE 1A–1D).
Power Doppler can help the sonographer localize the ectopic pregnancy in the tubes by demonstrating the circular vascularization of the more or less typical “tubal ring” (FIGURE 1E–1G).

FIGURE 1 Ectopic pregnancy
A–D. Various cases of tubal ectopic gestation (arrows point to each gestation). E–G. Power Doppler localizes the ectopic pregnancy (arrows), side by side with the corpus luteum (CL).
A patient’s history may yield clues to tubal inflammatory disease
The diagnosis of acute salpingitis begins with a thorough patient history. Look for any report of PID, unexplained fever, foul vaginal discharge, sexually transmitted infection, or recent intrauterine procedures such as hysteroscopy, IUD insertion, endometrial biopsy, or saline infusion sonohysterography.
US diagnosis is based on the findings of a slightly dilated fallopian tube with low-level echogenic fluid content, thick tubal walls, and tenderness to the touch of the transvaginal probe.1
In cross section, the tube forms the “cogwheel sign” (FIGURE 2C). Power Doppler shows the subserosal blood vessels characteristic of this entity (FIGURE 2D).

FIGURE 2 Tubal disease
A–C. Grayscale images showing thick walls, low-level echoic fluid (pus?) and the “cogwheel sign” (CW). D. Subserosal vascularization typical of an inflammatory response in hollow abdominal viscera. E,F. Edematous fimbrial end (arrow) of the inflamed tubes, floating in a small amount of free pelvic fluid. G. Low-level echoic, fluid-filled, thick-walled tubes with incomplete septae (arrow) are the hallmarks of hydrosalpinx. H–J. Bilateral hydrosalpinx. Note the thin walls and anechoic fluid-filled forms (sausage-shaped) (CL = corpus luteum; OV = ovary; UT = uterus).
Look for fluid dilating the tube in chronic tubal disease
Hydrosalpinx is characterized on US by thin tubal walls with a relatively anechoic but large amount of fluid dilating the tube (FIGURE 2G–2J). The interior wall is studded with shallow, echogenic, mural nodules (without blood vessels) that assume the appearance of a tube or sausage. The small, shallow internal papillae give the cross section of the tube the appearance of beads on a string.

FIGURE 3 Tubo-ovarian complex
A. The tube (T) and ovary (OV) form an infectious conglomerate. B. Power Doppler appearance. C. Laparoscopic view.
Tubo-ovarian complex
When this complex arises, the anatomy and shape of the tube and the involved ovary are somewhat distorted but still largely discernible (FIGURE 3A AND 3B).
Tubo-ovarian abscess is a more advanced stage of a fast-progressing or neglected pelvic inflammatory process. In it, the tube and the ovary can barely be distinguished, and US signs of abscess appear, among them low-level echoic fluid and linear echogenicity (FIGURE 4).

FIGURE 4 Tubo-ovarian abscess
The tube and ovary are indistinguishable. The fluid is of low-level echogenicity (pus), and the walls are thick.
In ovarian torsion, the follicles press outward
Although torsion has distinct sonographic signs, it remains a clinical diagnosis that US findings may or may not support. Correct diagnosis often is the purview of expert sonographers and sonologists.
When ovarian torsion is present, the ovaries are enlarged and hyperechoic, their follicles pushed toward the surface (FIGURE 5A–5C). The ovaries are also tender to the touch and typically demonstrate no blood flow by Doppler interrogation. On occasion, when arterial flow is still present (venous flow is usually the first characteristic to vanish), a twisted arterial pattern may result, similar to the coil of a telephone cord. Some pelvic fluid may also appear.
Tubal torsion is harder to diagnose. US recognition depends on the finding of a normal ovary with intact blood flow beside a fluid-filled, thin-walled, tender, cystic structure with some of the previously mentioned sonomarkers of tubal occlusion such as the bead-on-a-string or cogwheel sign (FIGURE 5D–5G).

FIGURE 5 Torsion
A–C. Ovarian torsion. Hyperechoic, large ovary with follicles pushed toward the surface. Power Doppler reveals no blood flow in the ovary. D–F. Tubal torsion. Cystic dilatation with a small beak and a normal ovary. G. Intraoperative view of the tube (twisted three times; yellow arrows) and the normal ovary (white arrows).
Fluid in the cul-de-sac
In many cases, fluid may be present or trapped in the lesser pelvis, surrounded or blocked by the pelvic organs. If this fluid is the result or sequela of PID, thin, thread-like adhesive strands will be visible between the organs on US, betraying its pathogenesis (FIGURE 6). The “walls” of such loculated fluid are the pelvic wall itself and the surrounding organs.

FIGURE 6 Fluid in the cul-de-sac
Sequelae of acute PID. A. Free pelvic fluid, also known as pelvic, peritoneal, loculated fluid. B. A normal ovary and an adhesive strand (arrow). C. Laparoscopic image of the adhesion (arrow).
Cancer of the tubes is unlikely, but it’s best to keep it in mind
Primary cancer of the fallopian tubes accounts for only 1% to 2% of all gynecologic cancers.2 Only 300 to 400 women are given this diagnosis each year in the United States— most of them postmenopausal.
Despite its rarity, fallopian-tube cancer is a major concern when a tubal mass is identified by palpation or imaging. In most cases, however, no palpable mass is found at the time of first examination, and tubal malignancy is diagnosed perioperatively or postoperatively.
US characteristics of tubal cancer are similar to those of ovarian cancer: a bizarre appearance, with extremely vascular tissue. At times, US attributes of tubal pathology, such as incomplete septae and tube-like fluid-filled structures, are apparent (FIGURE 7).
Consider cancer of the fallopian tube whenever an unexplained solid mass is palpated or imaged in the area of the tubes in conjunction with apparently normal ovaries.

FIGURE 7 Fallopian tube cancer
A. Fluid-filled uterine cavity. B. Large cystic dilatation of the tube. C. A thickened tubal wall (arrow). D. Doppler interrogation reveals high diastolic flow (arrows). E. Macroscopic gross appearance.
The long view
As technology has advanced, so has ultrasonography. High-resolution transducers, color and power Doppler, and three-dimensional imaging make it possible for an experienced practitioner to identify and confirm the diagnosis of many adnexal masses and pathologies, from the corpus luteum to fallopian tube torsion. As the field continues to evolve, we expect that this modality will facilitate the diagnosis of adnexal abnormalities to an even greater degree.
In the meantime, this four-part tutorial offers guidance on the identification of adnexal masses. If we’ve helped ease coordination of care between the generalist ObGyn and the expert sonographer, we’ve accomplished our goal.
We want to hear from you! Tell us what you think.
Part 1 A Starting Point (September 2010)
Part 2 The non-neoplastic ovarian mass (October 2010)
Part 3 Ovarian neoplasms (November 2010)
An imaging study of the adnexae would not be complete without thorough assessment of the fallopian tubes. Among the pathologies that may be identified or confirmed by ultrasonography are:
- ectopic pregnancy
- tubal inflammatory disease, or salpingitis
- chronic tubal disease, or hydrosalpinx
- tubo-ovarian complex
- tubo-ovarian abscess
- tubal and ovarian torsion
- cancer.
In this final installment of our four-part series on ultrasonographic (US) imaging of the adnexae, we take these entities as our focus.
Suspect ectopic pregnancy even if the hCG level is not yet available
A detailed discussion of ectopic pregnancy far exceeds the framework of this article. Suffice it to say that ectopic pregnancy should always be considered in a woman of reproductive age, especially one who complains of abdominal or pelvic pain, vaginal bleeding, or both. However, these signs and symptoms are present in only about 25% of women who have this condition. When these signs and symptoms are present, it is wise to be suspicious even if the results of human chorionic gonadotropin (hCG) measurement are not yet available.
A complete history is important in the diagnosis of ectopic pregnancy. Risk factors include a history of ectopic pregnancy, pelvic inflammatory disease (PID), or tubal surgery, or use of an intrauterine device.
Unequivocal US diagnosis of ectopic pregnancy is possible in only about 20% of cases, and depends on identification of an extrauterine pregnancy, which may not be visible in the early days of gestation. However, some grayscale ultrasonographic findings that may suggest ectopic pregnancy include:
- an empty uterus in a woman who has an hCG level above 1,000 to 1,500 mIu/mL (the discriminatory level)
- a thick, hyperechoic endometrial echo (decidualization)
- an adnexal mass other than a simple cyst
- echogenic fluid in the cul-de-sac (FIGURE 1A–1D).
Power Doppler can help the sonographer localize the ectopic pregnancy in the tubes by demonstrating the circular vascularization of the more or less typical “tubal ring” (FIGURE 1E–1G).

FIGURE 1 Ectopic pregnancy
A–D. Various cases of tubal ectopic gestation (arrows point to each gestation). E–G. Power Doppler localizes the ectopic pregnancy (arrows), side by side with the corpus luteum (CL).
A patient’s history may yield clues to tubal inflammatory disease
The diagnosis of acute salpingitis begins with a thorough patient history. Look for any report of PID, unexplained fever, foul vaginal discharge, sexually transmitted infection, or recent intrauterine procedures such as hysteroscopy, IUD insertion, endometrial biopsy, or saline infusion sonohysterography.
US diagnosis is based on the findings of a slightly dilated fallopian tube with low-level echogenic fluid content, thick tubal walls, and tenderness to the touch of the transvaginal probe.1
In cross section, the tube forms the “cogwheel sign” (FIGURE 2C). Power Doppler shows the subserosal blood vessels characteristic of this entity (FIGURE 2D).

FIGURE 2 Tubal disease
A–C. Grayscale images showing thick walls, low-level echoic fluid (pus?) and the “cogwheel sign” (CW). D. Subserosal vascularization typical of an inflammatory response in hollow abdominal viscera. E,F. Edematous fimbrial end (arrow) of the inflamed tubes, floating in a small amount of free pelvic fluid. G. Low-level echoic, fluid-filled, thick-walled tubes with incomplete septae (arrow) are the hallmarks of hydrosalpinx. H–J. Bilateral hydrosalpinx. Note the thin walls and anechoic fluid-filled forms (sausage-shaped) (CL = corpus luteum; OV = ovary; UT = uterus).
Look for fluid dilating the tube in chronic tubal disease
Hydrosalpinx is characterized on US by thin tubal walls with a relatively anechoic but large amount of fluid dilating the tube (FIGURE 2G–2J). The interior wall is studded with shallow, echogenic, mural nodules (without blood vessels) that assume the appearance of a tube or sausage. The small, shallow internal papillae give the cross section of the tube the appearance of beads on a string.

FIGURE 3 Tubo-ovarian complex
A. The tube (T) and ovary (OV) form an infectious conglomerate. B. Power Doppler appearance. C. Laparoscopic view.
Tubo-ovarian complex
When this complex arises, the anatomy and shape of the tube and the involved ovary are somewhat distorted but still largely discernible (FIGURE 3A AND 3B).
Tubo-ovarian abscess is a more advanced stage of a fast-progressing or neglected pelvic inflammatory process. In it, the tube and the ovary can barely be distinguished, and US signs of abscess appear, among them low-level echoic fluid and linear echogenicity (FIGURE 4).

FIGURE 4 Tubo-ovarian abscess
The tube and ovary are indistinguishable. The fluid is of low-level echogenicity (pus), and the walls are thick.
In ovarian torsion, the follicles press outward
Although torsion has distinct sonographic signs, it remains a clinical diagnosis that US findings may or may not support. Correct diagnosis often is the purview of expert sonographers and sonologists.
When ovarian torsion is present, the ovaries are enlarged and hyperechoic, their follicles pushed toward the surface (FIGURE 5A–5C). The ovaries are also tender to the touch and typically demonstrate no blood flow by Doppler interrogation. On occasion, when arterial flow is still present (venous flow is usually the first characteristic to vanish), a twisted arterial pattern may result, similar to the coil of a telephone cord. Some pelvic fluid may also appear.
Tubal torsion is harder to diagnose. US recognition depends on the finding of a normal ovary with intact blood flow beside a fluid-filled, thin-walled, tender, cystic structure with some of the previously mentioned sonomarkers of tubal occlusion such as the bead-on-a-string or cogwheel sign (FIGURE 5D–5G).

FIGURE 5 Torsion
A–C. Ovarian torsion. Hyperechoic, large ovary with follicles pushed toward the surface. Power Doppler reveals no blood flow in the ovary. D–F. Tubal torsion. Cystic dilatation with a small beak and a normal ovary. G. Intraoperative view of the tube (twisted three times; yellow arrows) and the normal ovary (white arrows).
Fluid in the cul-de-sac
In many cases, fluid may be present or trapped in the lesser pelvis, surrounded or blocked by the pelvic organs. If this fluid is the result or sequela of PID, thin, thread-like adhesive strands will be visible between the organs on US, betraying its pathogenesis (FIGURE 6). The “walls” of such loculated fluid are the pelvic wall itself and the surrounding organs.

FIGURE 6 Fluid in the cul-de-sac
Sequelae of acute PID. A. Free pelvic fluid, also known as pelvic, peritoneal, loculated fluid. B. A normal ovary and an adhesive strand (arrow). C. Laparoscopic image of the adhesion (arrow).
Cancer of the tubes is unlikely, but it’s best to keep it in mind
Primary cancer of the fallopian tubes accounts for only 1% to 2% of all gynecologic cancers.2 Only 300 to 400 women are given this diagnosis each year in the United States— most of them postmenopausal.
Despite its rarity, fallopian-tube cancer is a major concern when a tubal mass is identified by palpation or imaging. In most cases, however, no palpable mass is found at the time of first examination, and tubal malignancy is diagnosed perioperatively or postoperatively.
US characteristics of tubal cancer are similar to those of ovarian cancer: a bizarre appearance, with extremely vascular tissue. At times, US attributes of tubal pathology, such as incomplete septae and tube-like fluid-filled structures, are apparent (FIGURE 7).
Consider cancer of the fallopian tube whenever an unexplained solid mass is palpated or imaged in the area of the tubes in conjunction with apparently normal ovaries.

FIGURE 7 Fallopian tube cancer
A. Fluid-filled uterine cavity. B. Large cystic dilatation of the tube. C. A thickened tubal wall (arrow). D. Doppler interrogation reveals high diastolic flow (arrows). E. Macroscopic gross appearance.
The long view
As technology has advanced, so has ultrasonography. High-resolution transducers, color and power Doppler, and three-dimensional imaging make it possible for an experienced practitioner to identify and confirm the diagnosis of many adnexal masses and pathologies, from the corpus luteum to fallopian tube torsion. As the field continues to evolve, we expect that this modality will facilitate the diagnosis of adnexal abnormalities to an even greater degree.
In the meantime, this four-part tutorial offers guidance on the identification of adnexal masses. If we’ve helped ease coordination of care between the generalist ObGyn and the expert sonographer, we’ve accomplished our goal.
We want to hear from you! Tell us what you think.
1. Timor-Tritsch IE, Lerner JP, Monteagudo A, Murphy KE, Heller DS. Transvaginal sonographic markers of tubal inflammatory disease. Ultrasound Obstet Gynecol. 1998;12(1):56-66.
2. Goswami PK, Kerr-Wilson R, McCarthy K. Cancer of the fallopian tube. The Obstetrician & Gynaecologist. 2006;8(3):147-152.doi: 10.1576/toag.8.3.147.27249.
1. Timor-Tritsch IE, Lerner JP, Monteagudo A, Murphy KE, Heller DS. Transvaginal sonographic markers of tubal inflammatory disease. Ultrasound Obstet Gynecol. 1998;12(1):56-66.
2. Goswami PK, Kerr-Wilson R, McCarthy K. Cancer of the fallopian tube. The Obstetrician & Gynaecologist. 2006;8(3):147-152.doi: 10.1576/toag.8.3.147.27249.
Skilled US imaging of the adnexae: Ovarian Neoplasms
Part 1: A Starting Point (September 2010)
Part 2: The non-neoplastic ovarian mass (October 2010)
Part 4: The fallopian tubes (December 2010)
Although roughly three quarters of ovarian neoplasms occur in premenopausal women, 87% of masses in this population are benign. The vast majority of malignant neoplasms—about 75%—are diagnosed in postmenopausal women.
These figures suggest that you have some discerning to do. Specifically, how do you identify the small percentage of masses in premenopausal women that are malignant—and winnow out the benign neoplasms in the postmenopausal population?
Now that we’ve equipped you with an understanding of the morphologic building blocks of adnexal masses, and how those masses are assessed using ultrasonography (US) (described in Part 2 of this four-part series), you can apply your skills of discernment to ovarian neoplasms. Specifically:
- teratoma (dermoid cyst)—one of the two most prevalent benign neoplasms of the ovary
- serous cystadenoma—the other most prevalent benign neoplasm
- hormone-secreting tumors
- malignant neoplasms.
Recall that Part 1 of this series offered a starting point for US imaging of the adnexae by describing (and showing) how basic pelvic structures appear in grayscale US and color and power Doppler. Part 2 focused on non-neoplastic ovarian masses. Part 4 will take as its subject tubal entities such as torsion, ectopic pregnancy, and cancer.
Teratomas present a variety of “faces”
Teratomas may appear to be solid, cystic, or both (FIGURE 1). At times, they have a bizarre or variable appearance. The overwhelming majority of teratomas can be recognized by shadowing, which may be extreme if the tumor contains a solid, echogenic central mass (FIGURE 1A). Such an echogenic core is sometimes called the “fried egg” sign when it is detected by transabdominal US.
FIGURE 1 Cystic and solid benign teratomas
A. Shadowing (small arrows) is apparent in a teratoma containing low-level echoic fluid. B. Several spherical “balls” floating in a cystic teratoma, with shadowing. C. Solid teratoma. D. A “typical” teratoma, with septation and multilocularity. E. Macroscopic view of an ovarian teratoma (arrow). F. Multiple sebaceous ball-shaped structures within a benign cystic teratoma (inset: macroscopic view).When the teratoma is cystic or partially cystic, it may contain a linear hyperechoic area consistent with sebaceous fluid and hair. Although magnetic resonance imaging (MRI) can confirm the fat content of a teratoma, US is very efficient in making the diagnosis, rendering MRI unnecessary.
As for blood vessels, teratomas are known to have scant or no apparent vascularity. A rule of thumb: If a bizarre adnexal structure with no vascularity is visible on US, and if it is cystic or solid in appearance (or both), benign teratoma should be included in the differential diagnosis.
Because an ovarian teratoma can assume almost any shape and form, three-dimensional (3D) US is almost useless in its evaluation.
Cystadenomas are relatively easy to identify on US
Benign cystadenomas—serous or mucinous—are extremely common. In at least 20% to 30% of cases, they are bilateral.
The US characteristics of these masses include:
- multilocularity, in many cases (although two thirds of simple unilocular cysts in postmenopausal women are serous cystadenomas)
- multiseptation, with the septae often fanning out from a central, apparently solid structure (FIGURE 2)
- anechoic nature when they contain fluid (in the serous variety) or with low-level echogenicity (in mucous cystadenomas).
FIGURE 2 Benign cystadenoma
A–C. Typical sonographic appearance of a benign cystadenoma, with septae fanning out from a solid area, creating an anechoic, fluid-filled, multilocular pattern. D. MRI appearance of the cyst (arrow points to solid area from which the septae fan out).As for vascularity, cystadenomas have a paucity of core vessels and have, if measured quantitatively, what we consider to be normal resistive and pulsatility indices and low peak systolic velocity. Histologically, they are benign. These neoplasms can be identified using US with relative ease and high confidence, rendering computed tomography (CT) and MRI (FIGURE 2D) virtually redundant.
When US characteristics overlap
Based on our 20 years of experience with US assessment of adnexal masses, and the potential overlap (on grayscale as well as color and power Doppler) between the US appearance of endometriomas, cystadenomas, and cystic teratomas, we recommend that, when a mass is not pathognomonic on US, this triad of entities be considered in the differential diagnosis. The entity that has the greatest number of relevant characteristics should be listed as the most likely and first possibility on the US report.
(For a description of the US appearance of endometriomas, see Part 2 of this series, which appeared in the October 2010 issue of OBG Management.)
Hormone-secreting tumors are small and symptomatic
Although hormone-secreting tumors are not malignant in the strict sense of the definition, they should be mentioned here because of the high probability that they can be diagnosed by transvaginal sonography (TVS). These tumors are small, hiding at times in an ovary of almost normal size. They are also vascular, featuring a characteristic ring-like pattern, much like that of the corpus luteum, on color or power Doppler. They also produce general and clear clinical symptoms and signs. For example, testosterone-like tumors cause male-pattern baldness, hirsutism, and voice changes.
Many providers suspect a hormone-secreting tumor based on its signs and symptoms, and seek US confirmation from us. In many of these cases, laboratory tests have been done and point to the possible diagnosis—e.g., a high testosterone level in the case of a Sertoli-Leydig cell tumor.
One typical estrogen-secreting tumor is the granulosa cell tumor (FIGURE 3). This tumor can usually be identified by the solid-appearing tissue surrounding multiple cysts of different sizes; it is typically richly supplied with blood vessels.
Another clue to the diagnosis is the state of the endometrium. Because a granulosa cell tumor secretes estrogen, it causes a thickened endometrial lining and, usually, abnormal uterine bleeding.
FIGURE 3 Granulosa cell tumor
A. Sagittal image of the uterus demonstrating a thick, hyperechoic endometrial echo under hormonal stimulation of the tumor. B. Multicystic and solid areas alternate in the enlarged uterus. Power Doppler demonstrates the typical increased vascularity. (The arrows point to the cystic area of the tumor.)
Malignant ovarian neoplasms are rare
As a rule, the larger the lesion, the more suspicious it is.
Malignant tumors usually have a complex appearance:
- thick walls (≥4 mm)
- heterogeneous texture
- multilocularity
- solid components
- papillary excrescences within the tumor as well as on the outer surface (FIGURE 4A and 4B).
FIGURE 4 Adenocarcinoma of the ovary
A. An enlarged right ovary containing several cystic structures. B. Right ovary and transverse section of the uterus. C, D. Power Doppler evaluation demonstrating rich vascularization. E. 3D orthogonal planes and volume calculation of the ovary (31.1 cc). F. 3D angiogram (lower right image) of the rich vascularization of the cancer. G. Relationship between the vascular right ovary and the uterus.Tumor vascularity is another marker suggestive of ovarian malignancy (FIGURE 4C and 4D). A fast-growing tumor requires a vascular “infrastructure,” a mesh of blood vessels that is laid down in expedited fashion and that is controlled by vascular growth factors. As explained in Part 2 of this series, the vessels in this vascular mesh lack the muscular layer of normal vessels. They frequently are intertwined, forming anastomoses and vascular lakes through which blood flows without much resistance. Look, therefore, for low resistance and high-velocity flow.
A new way to employ 3D US is to detect, measure, and quantify the blood supply to a tumor. FIGURE 4E shows how the vascularity and volume of an ovarian mass are calculated, with 3D angiographic display of the blood vessels contained within it demonstrated in FIGURE 4F. This vascular pattern can also be viewed in the context of the pelvic organs (FIGURE 4G), an approach that is useful in teaching.
Recently, Sladkevicus and colleagues used 3D US angiography to define tumor vascularity, identifying straight vessels, those that had changes in caliber, and bridging between vessels.1 They studied 104 patients who had 77 benign tumors, 6 borderline tumors, and 21 cancers. The researchers concluded that dense vessel patterns in the tumor made malignancy five times more likely. Widely dispersed straight vessels without branching were the strongest predictors of benign status, reducing the likelihood of malignancy by a factor of 10.1
We described the importance of a finding of blood vessels in an internal papillary structure as an accurate predictor of malignancy. We focused on a small volume of the mass, which was selected by a software program, and found that a preselected volume of 1 cc could reliably predict an increased, and pathological, vascular supply to an ovary containing cancer.2,3
Although ovarian cancer is rare, affecting 30 to 50 women of every 100,000, it is particularly deadly, with a 5-year survival rate (all stages) of 50%. If cancer is detected and treated during stage I, the 5-year survival rate rises substantially—to 95%. Sadly, only 25% of cases are detected while the cancer is still localized.
In stages III and IV, the 5-year survival rate is 28% or lower. It has been estimated that, if 75% of patients had their cancer detected during stage I, the mortality rate could be halved.
The lifetime risk of ovarian cancer in a woman who has no affected relative is 1.4% (1 case in every 70 women). When the patient has one affected first-degree relative, that risk rises to 5% (1 case in 20 women), and it rises to 7% (1 case in 14 women) when she has two or more affected first-degree relatives.
Stay tuned!
In the final installment of this series, coming next month, we discuss the use of US imaging to evaluate tubal anomalies, including torsion, ectopic pregnancy, and cancer.
We want to hear from you! Tell us what you think.
1. Sladkevicius P, Jokubkiene L, Valentin L. Contribution of morphological assessment of the vessel tree by three-dimensional ultrasound to a correct diagnosis of malignancy in ovarian masses. Ultrasound Obstet Gynecol. 2007;30(6):874.-
2. Kudla MJ, Timor-Tritsch IE, Hope JM, et al. Spherical tissue sampling in 3-dimensional power Doppler angiography: a new approach for evaluation of ovarian tumors. J Ultrasound Med. 2008;27(3):425-433.
3. Alcazar JL, Prka M. Evaluation of two different methods for vascular sampling by three-dimensional power Doppler angiography in solid and cystic-solid adnexal masses. Ultrasound Obstet Gynecol. 2009;33(3):349-354.
Part 1: A Starting Point (September 2010)
Part 2: The non-neoplastic ovarian mass (October 2010)
Part 4: The fallopian tubes (December 2010)
Although roughly three quarters of ovarian neoplasms occur in premenopausal women, 87% of masses in this population are benign. The vast majority of malignant neoplasms—about 75%—are diagnosed in postmenopausal women.
These figures suggest that you have some discerning to do. Specifically, how do you identify the small percentage of masses in premenopausal women that are malignant—and winnow out the benign neoplasms in the postmenopausal population?
Now that we’ve equipped you with an understanding of the morphologic building blocks of adnexal masses, and how those masses are assessed using ultrasonography (US) (described in Part 2 of this four-part series), you can apply your skills of discernment to ovarian neoplasms. Specifically:
- teratoma (dermoid cyst)—one of the two most prevalent benign neoplasms of the ovary
- serous cystadenoma—the other most prevalent benign neoplasm
- hormone-secreting tumors
- malignant neoplasms.
Recall that Part 1 of this series offered a starting point for US imaging of the adnexae by describing (and showing) how basic pelvic structures appear in grayscale US and color and power Doppler. Part 2 focused on non-neoplastic ovarian masses. Part 4 will take as its subject tubal entities such as torsion, ectopic pregnancy, and cancer.
Teratomas present a variety of “faces”
Teratomas may appear to be solid, cystic, or both (FIGURE 1). At times, they have a bizarre or variable appearance. The overwhelming majority of teratomas can be recognized by shadowing, which may be extreme if the tumor contains a solid, echogenic central mass (FIGURE 1A). Such an echogenic core is sometimes called the “fried egg” sign when it is detected by transabdominal US.
FIGURE 1 Cystic and solid benign teratomas
A. Shadowing (small arrows) is apparent in a teratoma containing low-level echoic fluid. B. Several spherical “balls” floating in a cystic teratoma, with shadowing. C. Solid teratoma. D. A “typical” teratoma, with septation and multilocularity. E. Macroscopic view of an ovarian teratoma (arrow). F. Multiple sebaceous ball-shaped structures within a benign cystic teratoma (inset: macroscopic view).When the teratoma is cystic or partially cystic, it may contain a linear hyperechoic area consistent with sebaceous fluid and hair. Although magnetic resonance imaging (MRI) can confirm the fat content of a teratoma, US is very efficient in making the diagnosis, rendering MRI unnecessary.
As for blood vessels, teratomas are known to have scant or no apparent vascularity. A rule of thumb: If a bizarre adnexal structure with no vascularity is visible on US, and if it is cystic or solid in appearance (or both), benign teratoma should be included in the differential diagnosis.
Because an ovarian teratoma can assume almost any shape and form, three-dimensional (3D) US is almost useless in its evaluation.
Cystadenomas are relatively easy to identify on US
Benign cystadenomas—serous or mucinous—are extremely common. In at least 20% to 30% of cases, they are bilateral.
The US characteristics of these masses include:
- multilocularity, in many cases (although two thirds of simple unilocular cysts in postmenopausal women are serous cystadenomas)
- multiseptation, with the septae often fanning out from a central, apparently solid structure (FIGURE 2)
- anechoic nature when they contain fluid (in the serous variety) or with low-level echogenicity (in mucous cystadenomas).
FIGURE 2 Benign cystadenoma
A–C. Typical sonographic appearance of a benign cystadenoma, with septae fanning out from a solid area, creating an anechoic, fluid-filled, multilocular pattern. D. MRI appearance of the cyst (arrow points to solid area from which the septae fan out).As for vascularity, cystadenomas have a paucity of core vessels and have, if measured quantitatively, what we consider to be normal resistive and pulsatility indices and low peak systolic velocity. Histologically, they are benign. These neoplasms can be identified using US with relative ease and high confidence, rendering computed tomography (CT) and MRI (FIGURE 2D) virtually redundant.
When US characteristics overlap
Based on our 20 years of experience with US assessment of adnexal masses, and the potential overlap (on grayscale as well as color and power Doppler) between the US appearance of endometriomas, cystadenomas, and cystic teratomas, we recommend that, when a mass is not pathognomonic on US, this triad of entities be considered in the differential diagnosis. The entity that has the greatest number of relevant characteristics should be listed as the most likely and first possibility on the US report.
(For a description of the US appearance of endometriomas, see Part 2 of this series, which appeared in the October 2010 issue of OBG Management.)
Hormone-secreting tumors are small and symptomatic
Although hormone-secreting tumors are not malignant in the strict sense of the definition, they should be mentioned here because of the high probability that they can be diagnosed by transvaginal sonography (TVS). These tumors are small, hiding at times in an ovary of almost normal size. They are also vascular, featuring a characteristic ring-like pattern, much like that of the corpus luteum, on color or power Doppler. They also produce general and clear clinical symptoms and signs. For example, testosterone-like tumors cause male-pattern baldness, hirsutism, and voice changes.
Many providers suspect a hormone-secreting tumor based on its signs and symptoms, and seek US confirmation from us. In many of these cases, laboratory tests have been done and point to the possible diagnosis—e.g., a high testosterone level in the case of a Sertoli-Leydig cell tumor.
One typical estrogen-secreting tumor is the granulosa cell tumor (FIGURE 3). This tumor can usually be identified by the solid-appearing tissue surrounding multiple cysts of different sizes; it is typically richly supplied with blood vessels.
Another clue to the diagnosis is the state of the endometrium. Because a granulosa cell tumor secretes estrogen, it causes a thickened endometrial lining and, usually, abnormal uterine bleeding.
FIGURE 3 Granulosa cell tumor
A. Sagittal image of the uterus demonstrating a thick, hyperechoic endometrial echo under hormonal stimulation of the tumor. B. Multicystic and solid areas alternate in the enlarged uterus. Power Doppler demonstrates the typical increased vascularity. (The arrows point to the cystic area of the tumor.)
Malignant ovarian neoplasms are rare
As a rule, the larger the lesion, the more suspicious it is.
Malignant tumors usually have a complex appearance:
- thick walls (≥4 mm)
- heterogeneous texture
- multilocularity
- solid components
- papillary excrescences within the tumor as well as on the outer surface (FIGURE 4A and 4B).
FIGURE 4 Adenocarcinoma of the ovary
A. An enlarged right ovary containing several cystic structures. B. Right ovary and transverse section of the uterus. C, D. Power Doppler evaluation demonstrating rich vascularization. E. 3D orthogonal planes and volume calculation of the ovary (31.1 cc). F. 3D angiogram (lower right image) of the rich vascularization of the cancer. G. Relationship between the vascular right ovary and the uterus.Tumor vascularity is another marker suggestive of ovarian malignancy (FIGURE 4C and 4D). A fast-growing tumor requires a vascular “infrastructure,” a mesh of blood vessels that is laid down in expedited fashion and that is controlled by vascular growth factors. As explained in Part 2 of this series, the vessels in this vascular mesh lack the muscular layer of normal vessels. They frequently are intertwined, forming anastomoses and vascular lakes through which blood flows without much resistance. Look, therefore, for low resistance and high-velocity flow.
A new way to employ 3D US is to detect, measure, and quantify the blood supply to a tumor. FIGURE 4E shows how the vascularity and volume of an ovarian mass are calculated, with 3D angiographic display of the blood vessels contained within it demonstrated in FIGURE 4F. This vascular pattern can also be viewed in the context of the pelvic organs (FIGURE 4G), an approach that is useful in teaching.
Recently, Sladkevicus and colleagues used 3D US angiography to define tumor vascularity, identifying straight vessels, those that had changes in caliber, and bridging between vessels.1 They studied 104 patients who had 77 benign tumors, 6 borderline tumors, and 21 cancers. The researchers concluded that dense vessel patterns in the tumor made malignancy five times more likely. Widely dispersed straight vessels without branching were the strongest predictors of benign status, reducing the likelihood of malignancy by a factor of 10.1
We described the importance of a finding of blood vessels in an internal papillary structure as an accurate predictor of malignancy. We focused on a small volume of the mass, which was selected by a software program, and found that a preselected volume of 1 cc could reliably predict an increased, and pathological, vascular supply to an ovary containing cancer.2,3
Although ovarian cancer is rare, affecting 30 to 50 women of every 100,000, it is particularly deadly, with a 5-year survival rate (all stages) of 50%. If cancer is detected and treated during stage I, the 5-year survival rate rises substantially—to 95%. Sadly, only 25% of cases are detected while the cancer is still localized.
In stages III and IV, the 5-year survival rate is 28% or lower. It has been estimated that, if 75% of patients had their cancer detected during stage I, the mortality rate could be halved.
The lifetime risk of ovarian cancer in a woman who has no affected relative is 1.4% (1 case in every 70 women). When the patient has one affected first-degree relative, that risk rises to 5% (1 case in 20 women), and it rises to 7% (1 case in 14 women) when she has two or more affected first-degree relatives.
Stay tuned!
In the final installment of this series, coming next month, we discuss the use of US imaging to evaluate tubal anomalies, including torsion, ectopic pregnancy, and cancer.
We want to hear from you! Tell us what you think.
Part 1: A Starting Point (September 2010)
Part 2: The non-neoplastic ovarian mass (October 2010)
Part 4: The fallopian tubes (December 2010)
Although roughly three quarters of ovarian neoplasms occur in premenopausal women, 87% of masses in this population are benign. The vast majority of malignant neoplasms—about 75%—are diagnosed in postmenopausal women.
These figures suggest that you have some discerning to do. Specifically, how do you identify the small percentage of masses in premenopausal women that are malignant—and winnow out the benign neoplasms in the postmenopausal population?
Now that we’ve equipped you with an understanding of the morphologic building blocks of adnexal masses, and how those masses are assessed using ultrasonography (US) (described in Part 2 of this four-part series), you can apply your skills of discernment to ovarian neoplasms. Specifically:
- teratoma (dermoid cyst)—one of the two most prevalent benign neoplasms of the ovary
- serous cystadenoma—the other most prevalent benign neoplasm
- hormone-secreting tumors
- malignant neoplasms.
Recall that Part 1 of this series offered a starting point for US imaging of the adnexae by describing (and showing) how basic pelvic structures appear in grayscale US and color and power Doppler. Part 2 focused on non-neoplastic ovarian masses. Part 4 will take as its subject tubal entities such as torsion, ectopic pregnancy, and cancer.
Teratomas present a variety of “faces”
Teratomas may appear to be solid, cystic, or both (FIGURE 1). At times, they have a bizarre or variable appearance. The overwhelming majority of teratomas can be recognized by shadowing, which may be extreme if the tumor contains a solid, echogenic central mass (FIGURE 1A). Such an echogenic core is sometimes called the “fried egg” sign when it is detected by transabdominal US.
FIGURE 1 Cystic and solid benign teratomas
A. Shadowing (small arrows) is apparent in a teratoma containing low-level echoic fluid. B. Several spherical “balls” floating in a cystic teratoma, with shadowing. C. Solid teratoma. D. A “typical” teratoma, with septation and multilocularity. E. Macroscopic view of an ovarian teratoma (arrow). F. Multiple sebaceous ball-shaped structures within a benign cystic teratoma (inset: macroscopic view).When the teratoma is cystic or partially cystic, it may contain a linear hyperechoic area consistent with sebaceous fluid and hair. Although magnetic resonance imaging (MRI) can confirm the fat content of a teratoma, US is very efficient in making the diagnosis, rendering MRI unnecessary.
As for blood vessels, teratomas are known to have scant or no apparent vascularity. A rule of thumb: If a bizarre adnexal structure with no vascularity is visible on US, and if it is cystic or solid in appearance (or both), benign teratoma should be included in the differential diagnosis.
Because an ovarian teratoma can assume almost any shape and form, three-dimensional (3D) US is almost useless in its evaluation.
Cystadenomas are relatively easy to identify on US
Benign cystadenomas—serous or mucinous—are extremely common. In at least 20% to 30% of cases, they are bilateral.
The US characteristics of these masses include:
- multilocularity, in many cases (although two thirds of simple unilocular cysts in postmenopausal women are serous cystadenomas)
- multiseptation, with the septae often fanning out from a central, apparently solid structure (FIGURE 2)
- anechoic nature when they contain fluid (in the serous variety) or with low-level echogenicity (in mucous cystadenomas).
FIGURE 2 Benign cystadenoma
A–C. Typical sonographic appearance of a benign cystadenoma, with septae fanning out from a solid area, creating an anechoic, fluid-filled, multilocular pattern. D. MRI appearance of the cyst (arrow points to solid area from which the septae fan out).As for vascularity, cystadenomas have a paucity of core vessels and have, if measured quantitatively, what we consider to be normal resistive and pulsatility indices and low peak systolic velocity. Histologically, they are benign. These neoplasms can be identified using US with relative ease and high confidence, rendering computed tomography (CT) and MRI (FIGURE 2D) virtually redundant.
When US characteristics overlap
Based on our 20 years of experience with US assessment of adnexal masses, and the potential overlap (on grayscale as well as color and power Doppler) between the US appearance of endometriomas, cystadenomas, and cystic teratomas, we recommend that, when a mass is not pathognomonic on US, this triad of entities be considered in the differential diagnosis. The entity that has the greatest number of relevant characteristics should be listed as the most likely and first possibility on the US report.
(For a description of the US appearance of endometriomas, see Part 2 of this series, which appeared in the October 2010 issue of OBG Management.)
Hormone-secreting tumors are small and symptomatic
Although hormone-secreting tumors are not malignant in the strict sense of the definition, they should be mentioned here because of the high probability that they can be diagnosed by transvaginal sonography (TVS). These tumors are small, hiding at times in an ovary of almost normal size. They are also vascular, featuring a characteristic ring-like pattern, much like that of the corpus luteum, on color or power Doppler. They also produce general and clear clinical symptoms and signs. For example, testosterone-like tumors cause male-pattern baldness, hirsutism, and voice changes.
Many providers suspect a hormone-secreting tumor based on its signs and symptoms, and seek US confirmation from us. In many of these cases, laboratory tests have been done and point to the possible diagnosis—e.g., a high testosterone level in the case of a Sertoli-Leydig cell tumor.
One typical estrogen-secreting tumor is the granulosa cell tumor (FIGURE 3). This tumor can usually be identified by the solid-appearing tissue surrounding multiple cysts of different sizes; it is typically richly supplied with blood vessels.
Another clue to the diagnosis is the state of the endometrium. Because a granulosa cell tumor secretes estrogen, it causes a thickened endometrial lining and, usually, abnormal uterine bleeding.
FIGURE 3 Granulosa cell tumor
A. Sagittal image of the uterus demonstrating a thick, hyperechoic endometrial echo under hormonal stimulation of the tumor. B. Multicystic and solid areas alternate in the enlarged uterus. Power Doppler demonstrates the typical increased vascularity. (The arrows point to the cystic area of the tumor.)
Malignant ovarian neoplasms are rare
As a rule, the larger the lesion, the more suspicious it is.
Malignant tumors usually have a complex appearance:
- thick walls (≥4 mm)
- heterogeneous texture
- multilocularity
- solid components
- papillary excrescences within the tumor as well as on the outer surface (FIGURE 4A and 4B).
FIGURE 4 Adenocarcinoma of the ovary
A. An enlarged right ovary containing several cystic structures. B. Right ovary and transverse section of the uterus. C, D. Power Doppler evaluation demonstrating rich vascularization. E. 3D orthogonal planes and volume calculation of the ovary (31.1 cc). F. 3D angiogram (lower right image) of the rich vascularization of the cancer. G. Relationship between the vascular right ovary and the uterus.Tumor vascularity is another marker suggestive of ovarian malignancy (FIGURE 4C and 4D). A fast-growing tumor requires a vascular “infrastructure,” a mesh of blood vessels that is laid down in expedited fashion and that is controlled by vascular growth factors. As explained in Part 2 of this series, the vessels in this vascular mesh lack the muscular layer of normal vessels. They frequently are intertwined, forming anastomoses and vascular lakes through which blood flows without much resistance. Look, therefore, for low resistance and high-velocity flow.
A new way to employ 3D US is to detect, measure, and quantify the blood supply to a tumor. FIGURE 4E shows how the vascularity and volume of an ovarian mass are calculated, with 3D angiographic display of the blood vessels contained within it demonstrated in FIGURE 4F. This vascular pattern can also be viewed in the context of the pelvic organs (FIGURE 4G), an approach that is useful in teaching.
Recently, Sladkevicus and colleagues used 3D US angiography to define tumor vascularity, identifying straight vessels, those that had changes in caliber, and bridging between vessels.1 They studied 104 patients who had 77 benign tumors, 6 borderline tumors, and 21 cancers. The researchers concluded that dense vessel patterns in the tumor made malignancy five times more likely. Widely dispersed straight vessels without branching were the strongest predictors of benign status, reducing the likelihood of malignancy by a factor of 10.1
We described the importance of a finding of blood vessels in an internal papillary structure as an accurate predictor of malignancy. We focused on a small volume of the mass, which was selected by a software program, and found that a preselected volume of 1 cc could reliably predict an increased, and pathological, vascular supply to an ovary containing cancer.2,3
Although ovarian cancer is rare, affecting 30 to 50 women of every 100,000, it is particularly deadly, with a 5-year survival rate (all stages) of 50%. If cancer is detected and treated during stage I, the 5-year survival rate rises substantially—to 95%. Sadly, only 25% of cases are detected while the cancer is still localized.
In stages III and IV, the 5-year survival rate is 28% or lower. It has been estimated that, if 75% of patients had their cancer detected during stage I, the mortality rate could be halved.
The lifetime risk of ovarian cancer in a woman who has no affected relative is 1.4% (1 case in every 70 women). When the patient has one affected first-degree relative, that risk rises to 5% (1 case in 20 women), and it rises to 7% (1 case in 14 women) when she has two or more affected first-degree relatives.
Stay tuned!
In the final installment of this series, coming next month, we discuss the use of US imaging to evaluate tubal anomalies, including torsion, ectopic pregnancy, and cancer.
We want to hear from you! Tell us what you think.
1. Sladkevicius P, Jokubkiene L, Valentin L. Contribution of morphological assessment of the vessel tree by three-dimensional ultrasound to a correct diagnosis of malignancy in ovarian masses. Ultrasound Obstet Gynecol. 2007;30(6):874.-
2. Kudla MJ, Timor-Tritsch IE, Hope JM, et al. Spherical tissue sampling in 3-dimensional power Doppler angiography: a new approach for evaluation of ovarian tumors. J Ultrasound Med. 2008;27(3):425-433.
3. Alcazar JL, Prka M. Evaluation of two different methods for vascular sampling by three-dimensional power Doppler angiography in solid and cystic-solid adnexal masses. Ultrasound Obstet Gynecol. 2009;33(3):349-354.
1. Sladkevicius P, Jokubkiene L, Valentin L. Contribution of morphological assessment of the vessel tree by three-dimensional ultrasound to a correct diagnosis of malignancy in ovarian masses. Ultrasound Obstet Gynecol. 2007;30(6):874.-
2. Kudla MJ, Timor-Tritsch IE, Hope JM, et al. Spherical tissue sampling in 3-dimensional power Doppler angiography: a new approach for evaluation of ovarian tumors. J Ultrasound Med. 2008;27(3):425-433.
3. Alcazar JL, Prka M. Evaluation of two different methods for vascular sampling by three-dimensional power Doppler angiography in solid and cystic-solid adnexal masses. Ultrasound Obstet Gynecol. 2009;33(3):349-354.
Skilled US imaging of the adnexae: The non-neoplastic mass
Part 1: A Starting Point (September 2010)
Part 3: Ovarian neoplasms (November 2010)
Part 4: The fallopian tubes (December 2010)
Scanning the ovaries is no simple task. As we mentioned in Part 1 of this four-part series, the practitioner must use the right equipment, take basic preparatory steps, be watchful for clues in the history, and reach a conclusion about what he or she sees. Not only that: The ultrasonographer must be extraordinarily vigilant, paying close attention to multiple characteristics of any mass, from thickness of the wall to the presence of papillations or a blood supply—signs of potential malignancy.
In this article, we detail the traits of various types of non-neoplastic ovarian masses, including:
- functional cysts—follicles, the corpus luteum, and theca lutein cysts
- nonfunctional cysts—serous masses and endometriomas
- cystadenofibromas. Although these masses are usually categorized histo-logically as neoplasms, we include them here due to their almost daily appearance in a busy gynecologic ultrasonographic (US) facility.
In Part 3, we will cover ovarian neoplasms, and in Part 4, our focus will be tubal entities such as ectopic pregnancy and torsion.
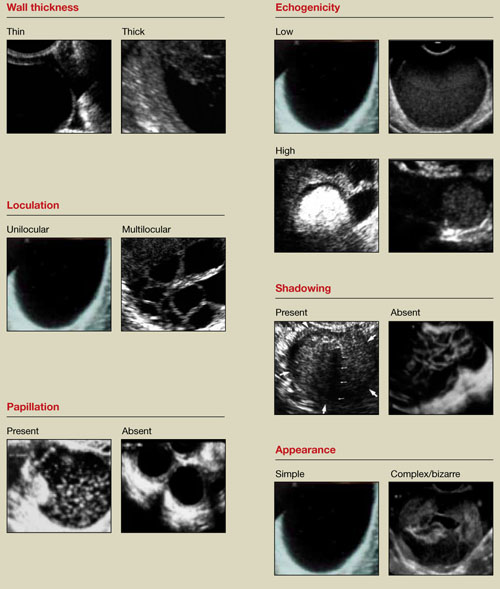
FIGURE 1 What is a mass made of? 6 morphologic building blocks
Take an inventory of the mass
Any adnexal mass should be assessed in light of its essential characteristics (Figure 1).
Wall structure. Pay attention to thickness. We use an arbitrary cutoff of 4 mm, giving extra scrutiny to thicknesses exceeding that measurement. In our experience, the thicker the wall, the more likely the mass is to be malignant.
Septation and loculation. A mass is typically unilocular or multilocular. Multilocularity is more common in tumors of low malignant potential and malignant neoplasms.
Papillation. Any internal or external papillae or excrescences should draw your attention. Papillarity in an ovarian mass renders that mass suspicious for malignancy.
Measure (height and width) any papillae that are identified, and document them. Because papillae are associated with ovarian malignancy, further assessment is warranted immediately. The first step is determining whether the papillations contain blood vessels—a task for which color and power Doppler are helpful. We prefer power Doppler because it is more sensitive, detecting blood-flow velocity in the lowest detectable range of 2 cm/s, and because it is not directionally influenced.
Papillae that contain blood vessels with detectable flow are suspicious for malignancy.
Exacoustos and colleagues found that papillae as large as 15 mm in height and 10 mm in width (base) were present in 48% of borderline ovarian tumors but in only 4% of benign and 4% of malignant tumors. However, when the intracystic solid tissue exceeded those dimensions, the lesions were present in 48% of invasive ovarian tumors, 18% of borderline ovarian tumors, and 7% of benign masses.1
Internal echo-structure. A mass can be anechoic, a finding that usually indicates the presence of clear fluid. Mostly solid masses are echogenic. And masses that contain particulate matter, such as blood, cellular matter, or even mucous material, usually have echogenicity of a low level, often described as a “ground-glass” appearance. A mass can also have mixed echo-genicity, a finding usually found in cases involving teratoma or malignancy.
Shadowing. If it is present, it may signify the presence of an extremely dense, solid tissue, such as bone or calcification. The diagnosis of a benign teratoma (i.e., dermoid cyst) should be entertained if shadowing is present in a hyperechoic nodule or mass. Malignant masses very rarely, if ever, display frank shadowing.
Overall appearance. On rare occasions, a bizarre shape or “complex” appearance (as it is termed in most radiology reports) may indicate a malignant mass. More likely it indicates the presence of a teratoma, cystadenoma, or even an atypical corpus luteum. In some reports generated by US laboratories, the term “complex” is applied to all structures other than simple cysts.
Size. The size of a mass can be misleading, as small ovarian lesions with the appropriate sonographic characteristics may be malignant and some larger ones without those characteristics may not be. However, it is understood that the larger an ovarian lesion, the more likely it is a tumor. One important distinction: The amount of fluid in a cystic structure or the amount of old blood in an endometrioma is not the disease process…it is the byproduct of the process. So an 8-cm endometrioma may create fewer pain or fertility issues than a 2- or 3-cm endometrioma. Similarly, the amount of “chocolate” fluid is not automatically indicative of the amount of active endometriotic glands or their sequelae!2
Ascites. If it is present, it should be recorded and investigated further because it may be caused by a malignant intra-abdominal tumor.
Motion tenderness. If the to-and-fro movement of the vaginal probe elicits any motion tenderness, it, too, should be documented. It may be a sign of pelvic peritonitis. In such cases, an “ominous appearing” adnexal finding may represent an inflammatory, rather than malignant, mass.
One of the components of extensive evaluation of the adnexae in general and ovaries in particular is color or power Doppler interrogation—or both.
Tumors contain a relatively large number of pathologic blood vessels that lack the muscular layer found in normal blood vessels and, as a result, demonstrate lower resistance to flow. Diastolic flow is high in these vessels, and resistance and pulsatility indices are low.
We also pay attention when these blood vessels have a tortuous appearance, changes in caliber, anastomoses, and vascular lakes.3 The more tortuous the vessels, with multiple inter-vessel connections and dilatations with changing calibers, the greater the risk of malignancy.4 No less important is the presence of a vessel within a “complex” ovarian mass. A centrally located vessel (also called a “lead vessel”) is suspicious for malignancy.5
A gallery of non-neoplastic ovarian masses
Non-neoplastic cysts are, by far, the most common structures of the ovary. They may be functional, as in the case of the follicles, corpus luteum, and theca lutein cysts, or they may be nonfunctional, as in serous cysts and endometriomas. (As we noted in Part 1, do not call the follicles and corpus luteum “cysts” because this designation suggests pathology.)6
FIGURE 2 Simple cyst
This cyst is anechoic and unilocular with thin walls and no papillae.
Functional cysts
Functional cysts, also known as “simple” cysts, may grow as large as 4 to 5 cm in diameter (Figure 2). They are typically unilocular, anechoic, and thin-walled, with no papillae, and almost never malignant. They usually resolve and require no treatment unless rupture or torsion occurs. Except for the corpus luteum, they have no increased blood flow, and need be viewed only by transvaginal ultrasonography (TVS).
The corpus luteum also can be recognized by TVS. It can exhibit any of a variety of internal structures and echo patterns, due to the multitude of shapes of the blood or clot that can be seen within it (Figure 3).
FIGURE 3 Corpus luteum
A–C. Gray-scale, color Doppler, and power Doppler images, respectively, of a typical corpus luteum. B and C show the enveloping vessels, or “ring of fire.” D. A rather typical gray-scale appearance with a mesh-like, linear internal texture. E. A common feature of the corpus luteum is a linear interphase (arrow) between the clot (c) and the liquified serum (s).
The corpus luteum is typically enveloped by blood vessels, visible on color Doppler as what is called a “ring of fire.” It regresses without intervention. In hyperstimulated ovaries, however, more than one may be present; this poses a real diagnostic challenge when ectopic pregnancy is suspected because it is difficult to differentiate the two entities.
Because the corpus luteum can sometimes resemble some types of ovarian tumors on TVS, imaging during the secretory phase of the cycle in a woman of reproductive age is not ideal. Instead, she should be scanned (or rescanned) between days 5 and 9 of the cycle.
FIGURE 4 Hormonally stimulated ovaries
A, B. The right and left ovaries stimulated by follicle-stimulating hormone preparation (arrow points to hilus). C. An ovary stimulated by clomiphene.
Lutein cysts may reach 5 to 10 cm in diameter. They generally have a thick wall, are multilocular, and typically occur after hormonal induction of ovulation (Figure 4). They also can occur in diabetes, molar pregnancy, and hydrops fetalis. We have seen a unilateral theca lutein cyst in a normal pregnancy (Figure 5). No treatment is necessary unless rupture or torsion occurs.
FIGURE 5 Lutein cysts
A–C. The typical “stained glass” appearance of three lutein cysts of the right ovary in a pregnant patient. D. Color Doppler image of the ovary demonstrating high-velocity flow (peak systolic velocity of 20.4 cm/s).
Serous cysts
These cysts can reach 4 cm in diameter, have smooth walls with no papillae, are unilocular, and occur most often during menopause. No pathological blood flow is visible in their walls. Most gynecologists follow them (Figure 6).1,7
FIGURE 6 Serous cyst
A. Right ovary containing the cyst. B. Normal left ovary. C. Power Doppler interrogation showing no particular flow in the walls of a serous cyst.
Endometriomas
After the simple cyst, the endometrioma is the most prevalent ovarian or adnexal cyst (Figure 7). It usually has a thick wall and is filled with homogeneous fluid with low-level echo-genicity. It can reach 10 cm in size, and many are bilateral. It is sometimes called a “chocolate” cyst because of its dark blood content.
FIGURE 7 Endometriomas
Endometriomas have low echogenicity. A. Unilateral, unilocular cyst with thin walls. B. Bilateral endometriomas. C. Blood flow in a solid or papillary component of the endometrioma is an occasional finding. It should be investigated further because of the risk that it represents endometrioid cancer.
Endometriomas do not resolve; they usually require surgical excision, although very small ones wholly contained within an ovary are often managed medically or expectantly.
These masses rarely (<1%) give rise to endometrioid carcinoma. Should an endometrioma contain papillae with blood vessels, it is extremely suspicious for endometrioid cancer.
FIGURE 8 Cystic fibromas
A. Sonographic image shows a thin wall and hyperechoic, small mural nodules. B. Macroscopic appearance of an area of internal papillary excrescences. C. Measurement of the small, mural nodules. D. Lack of blood flow in the small papillae, a typical finding on color or power Doppler. E, F. Blood flow in the wall of the cyst and in the mural nodules.
Ovarian fibromas
A fibroma is a slow-growing, benign, solid ovarian tumor. It usually has a cystic component and then is called a cystadenofibroma.
The cystic variety is filled with anechoic fluid and has a thin wall. However, its pathognomonic feature is the small (2–3 mm), extremely hyperechoic mural nodules (papillae) it contains (Figure 8A–C). In the overwhelming majority of cases, no blood vessels are detectable, and the mass is unilocular (Figure 8D–E). It can be recognized in the ovary by the semilunar shape of the tissue surrounding it (crescent sign). The differential diagnosis includes the simple (serous) cyst.
The solid fibroma has a myometrium-like texture, with few or no detectable blood vessels in the stroma. The differential diagnosis includes the Brenner tumor and the Krukenberg tumor.
According to a technology assessment from the Agency for Healthcare Research and Quality (AHRQ), “conventional gray-scale ultrasonography is the most common imaging modality used to differentiate benign from malignant adnexal masses. Especially with the advent of high-frequency transvaginal probes, the quality of the images allows description of the gross anatomic features of the lesion.”8 This descriptive ability is limited, however, “by the great variability of macroscopic characteristics of both benign and malignant masses. Furthermore, the technique is operator dependent.”8
To overcome these challenges, some experts have developed ultrasonographic (US) morphologic scoring systems, which assign a value to individual characteristics. Lerner and colleagues devised a 4-point system:
| Characteristic | Points | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Wall structure | Smooth or small irregularities (<3 mm) | Solid or not applicable | Papillarities larger than 3 mm | |
| Shadowing | Yes | No | ||
| Septation | None or thin (<3 mm) | Thick (≥3 mm) | ||
| Echogenicity | Sonolucent or low-level echo or echogenic core | Mixed or high | ||
The mean point value for benign masses was 1.8; for tumors of low malignant potential it was 3.9; and for malignant tumors it was 5.6 (P < .0005). Lerner and associates proposed a cutoff of 3. A score of 3 or higher, they felt, would be most predictive of malignancy, with sensitivity of 96.8% and specificity of 77%. Positive and negative predictive values were 29.4% and 99.6%, respectively.9
Almost all published scoring systems are based upon or derived from one reported by Sassone and coworkers.10 The most important and practical feature of all scoring systems is their ability to rule out malignancy.
Morphology and Doppler: A synergistic combination
As the same AHRQ report points out, “all of the diagnostic tests and scoring systems we evaluated exhibited a trade-off between sensitivity and specificity—studies of a given test that reported higher sensitivity had lower specificity, and vice versa.”8 Among evaluation methods, the combination of US morphology scores and Doppler imaging achieved the highest pooled sensitivity and specificity scores in distinguishing benign and malignant adnexal masses in postmenopausal women: 86% and 91%, respectively, according to the AHRQ report.8
Compare these figures with those of:
- Bimanual pelvic examination (45% and 90%, respectively)
- Doppler resistance index (72% and 90%)
- Doppler pulsatility index (80% and 73%)
- presence of blood vessels (88% and 78%).
The combination of US morphology scores and Doppler was comparable to the pooled sensitivity and specificity of magnetic resonance imaging (91% and 88%, respectively) and superior to computed tomography (90% and 75%, respectively).
Why the need to know?
Discrimination between benign and malignant masses serves a number of purposes, depending on the setting.
For example, if a symptomatic woman is found to have an adnexal mass, it is important to identify the type of mass causing the symptoms to determine the best course of treatment. And because surgery may be one of the treatment options, it is helpful to know whether a mass is likely to be malignant so that the patient can be referred to a specialist or center that has optimal surgical expertise.8
Some asymptomatic masses may be identified during the annual bimanual pelvic examination recommended by ACOG or during pregnancy-related US imaging. In this setting, it is important to ascertain whether the mass is likely to be malignant so that the patient can be referred to a specialist, if necessary. In addition, thorough assessment of the mass can help “avoid unnecessary diagnostic procedures, including surgery, and anxiety in women with asymptomatic, nonmalignant conditions. In some cases, there may be a rationale for removing certain asymptomatic benign lesions, including prevention of malignant transformation; prevention of ovarian torsion”; and prevention of rupture. Surgery may also be appropriate to avert the need for more complicated surgery in the future or to enhance fertility.8 —Janelle Yates, Senior Editor
Stay tuned!
Next issue, in Part 3 of this series, we will review the use of imaging in the investigation of ovarian neoplasms, both benign and malignant, with an abundance of US images to accompany our discussion.
We want to hear from you! Tell us what you think.
1. Exacoustos C, Romanini ME, Rinaldo D, et al. Preoperative sonographic features of borderline ovarian tumors. Ultrasound Obstet Gynecol. 2004;25(1):50-59.
2. Rulin MC, Preston AL. Adnexal masses in postmenopausal women. Obstet Gynecol. 1987;70(4):578-581.
3. Timor-Tritsch IE, Goldstein SR. The complexity of a complex mass and the simplicity of a simple cyst. J Ultraound Med. 2005;24(3):255-258.
4. Sladkevicius P, Jokubkiene L, Valentin L. Contribution of morphological assessment of the vessel tree by three-dimensional ultrasound to a correct diagnos is of malignancy in ovarian masses. Ultrasound Obstet Gynecol. 2007;30(6):874-882.
5. Testa AC, Mancari R, Di Legge A, et al. The “lead vessel”: a vascular ultrasound feature of metastasis in the ovaries. Ultrasound Obstet Gynecol. 2008;31:218-221.
6. Goldstein SR. Postmenopausal adnexal cysts: how clinical management has evolved. Am J Obstet Gynecol. 1996;175(6):1496-1501.
7. Levine D, Gosink BB, Wolf S, Feldesman MR, Pretorius D. Simple adnexal cysts: the natural history in postmenopausal women. Radiology. 1992;184(3):653-659.
8. Myers ER, Bastian LA, Havrilesky LJ, et al. Management of adnexal mass. Evidence Report Technol Assess. 2006;Feb;(130):1-145.
9. Lerner JP, Timor-Tritsch IE, Federman A, Abramovich G. Transvaginal ultrasonographic characterization of ovarian masses with an improved, weighted scoring system. Am J Obstet Gynecol. 1994;170(1 Pt 1):81-85.
10. Sassone AM, Timor-Tritsch IE, Artner A, et al. Transvaginal sonographic characterization of ovarian disease: evaluation of a new scoring system to predict ovarian malignancy. Obstet Gynecol. 2001;78:70-76.
Part 1: A Starting Point (September 2010)
Part 3: Ovarian neoplasms (November 2010)
Part 4: The fallopian tubes (December 2010)
Scanning the ovaries is no simple task. As we mentioned in Part 1 of this four-part series, the practitioner must use the right equipment, take basic preparatory steps, be watchful for clues in the history, and reach a conclusion about what he or she sees. Not only that: The ultrasonographer must be extraordinarily vigilant, paying close attention to multiple characteristics of any mass, from thickness of the wall to the presence of papillations or a blood supply—signs of potential malignancy.
In this article, we detail the traits of various types of non-neoplastic ovarian masses, including:
- functional cysts—follicles, the corpus luteum, and theca lutein cysts
- nonfunctional cysts—serous masses and endometriomas
- cystadenofibromas. Although these masses are usually categorized histo-logically as neoplasms, we include them here due to their almost daily appearance in a busy gynecologic ultrasonographic (US) facility.
In Part 3, we will cover ovarian neoplasms, and in Part 4, our focus will be tubal entities such as ectopic pregnancy and torsion.

FIGURE 1 What is a mass made of? 6 morphologic building blocks
Take an inventory of the mass
Any adnexal mass should be assessed in light of its essential characteristics (Figure 1).
Wall structure. Pay attention to thickness. We use an arbitrary cutoff of 4 mm, giving extra scrutiny to thicknesses exceeding that measurement. In our experience, the thicker the wall, the more likely the mass is to be malignant.
Septation and loculation. A mass is typically unilocular or multilocular. Multilocularity is more common in tumors of low malignant potential and malignant neoplasms.
Papillation. Any internal or external papillae or excrescences should draw your attention. Papillarity in an ovarian mass renders that mass suspicious for malignancy.
Measure (height and width) any papillae that are identified, and document them. Because papillae are associated with ovarian malignancy, further assessment is warranted immediately. The first step is determining whether the papillations contain blood vessels—a task for which color and power Doppler are helpful. We prefer power Doppler because it is more sensitive, detecting blood-flow velocity in the lowest detectable range of 2 cm/s, and because it is not directionally influenced.
Papillae that contain blood vessels with detectable flow are suspicious for malignancy.
Exacoustos and colleagues found that papillae as large as 15 mm in height and 10 mm in width (base) were present in 48% of borderline ovarian tumors but in only 4% of benign and 4% of malignant tumors. However, when the intracystic solid tissue exceeded those dimensions, the lesions were present in 48% of invasive ovarian tumors, 18% of borderline ovarian tumors, and 7% of benign masses.1
Internal echo-structure. A mass can be anechoic, a finding that usually indicates the presence of clear fluid. Mostly solid masses are echogenic. And masses that contain particulate matter, such as blood, cellular matter, or even mucous material, usually have echogenicity of a low level, often described as a “ground-glass” appearance. A mass can also have mixed echo-genicity, a finding usually found in cases involving teratoma or malignancy.
Shadowing. If it is present, it may signify the presence of an extremely dense, solid tissue, such as bone or calcification. The diagnosis of a benign teratoma (i.e., dermoid cyst) should be entertained if shadowing is present in a hyperechoic nodule or mass. Malignant masses very rarely, if ever, display frank shadowing.
Overall appearance. On rare occasions, a bizarre shape or “complex” appearance (as it is termed in most radiology reports) may indicate a malignant mass. More likely it indicates the presence of a teratoma, cystadenoma, or even an atypical corpus luteum. In some reports generated by US laboratories, the term “complex” is applied to all structures other than simple cysts.
Size. The size of a mass can be misleading, as small ovarian lesions with the appropriate sonographic characteristics may be malignant and some larger ones without those characteristics may not be. However, it is understood that the larger an ovarian lesion, the more likely it is a tumor. One important distinction: The amount of fluid in a cystic structure or the amount of old blood in an endometrioma is not the disease process…it is the byproduct of the process. So an 8-cm endometrioma may create fewer pain or fertility issues than a 2- or 3-cm endometrioma. Similarly, the amount of “chocolate” fluid is not automatically indicative of the amount of active endometriotic glands or their sequelae!2
Ascites. If it is present, it should be recorded and investigated further because it may be caused by a malignant intra-abdominal tumor.
Motion tenderness. If the to-and-fro movement of the vaginal probe elicits any motion tenderness, it, too, should be documented. It may be a sign of pelvic peritonitis. In such cases, an “ominous appearing” adnexal finding may represent an inflammatory, rather than malignant, mass.
One of the components of extensive evaluation of the adnexae in general and ovaries in particular is color or power Doppler interrogation—or both.
Tumors contain a relatively large number of pathologic blood vessels that lack the muscular layer found in normal blood vessels and, as a result, demonstrate lower resistance to flow. Diastolic flow is high in these vessels, and resistance and pulsatility indices are low.
We also pay attention when these blood vessels have a tortuous appearance, changes in caliber, anastomoses, and vascular lakes.3 The more tortuous the vessels, with multiple inter-vessel connections and dilatations with changing calibers, the greater the risk of malignancy.4 No less important is the presence of a vessel within a “complex” ovarian mass. A centrally located vessel (also called a “lead vessel”) is suspicious for malignancy.5
A gallery of non-neoplastic ovarian masses
Non-neoplastic cysts are, by far, the most common structures of the ovary. They may be functional, as in the case of the follicles, corpus luteum, and theca lutein cysts, or they may be nonfunctional, as in serous cysts and endometriomas. (As we noted in Part 1, do not call the follicles and corpus luteum “cysts” because this designation suggests pathology.)6

FIGURE 2 Simple cyst
This cyst is anechoic and unilocular with thin walls and no papillae.
Functional cysts
Functional cysts, also known as “simple” cysts, may grow as large as 4 to 5 cm in diameter (Figure 2). They are typically unilocular, anechoic, and thin-walled, with no papillae, and almost never malignant. They usually resolve and require no treatment unless rupture or torsion occurs. Except for the corpus luteum, they have no increased blood flow, and need be viewed only by transvaginal ultrasonography (TVS).
The corpus luteum also can be recognized by TVS. It can exhibit any of a variety of internal structures and echo patterns, due to the multitude of shapes of the blood or clot that can be seen within it (Figure 3).

FIGURE 3 Corpus luteum
A–C. Gray-scale, color Doppler, and power Doppler images, respectively, of a typical corpus luteum. B and C show the enveloping vessels, or “ring of fire.” D. A rather typical gray-scale appearance with a mesh-like, linear internal texture. E. A common feature of the corpus luteum is a linear interphase (arrow) between the clot (c) and the liquified serum (s).
The corpus luteum is typically enveloped by blood vessels, visible on color Doppler as what is called a “ring of fire.” It regresses without intervention. In hyperstimulated ovaries, however, more than one may be present; this poses a real diagnostic challenge when ectopic pregnancy is suspected because it is difficult to differentiate the two entities.
Because the corpus luteum can sometimes resemble some types of ovarian tumors on TVS, imaging during the secretory phase of the cycle in a woman of reproductive age is not ideal. Instead, she should be scanned (or rescanned) between days 5 and 9 of the cycle.

FIGURE 4 Hormonally stimulated ovaries
A, B. The right and left ovaries stimulated by follicle-stimulating hormone preparation (arrow points to hilus). C. An ovary stimulated by clomiphene.
Lutein cysts may reach 5 to 10 cm in diameter. They generally have a thick wall, are multilocular, and typically occur after hormonal induction of ovulation (Figure 4). They also can occur in diabetes, molar pregnancy, and hydrops fetalis. We have seen a unilateral theca lutein cyst in a normal pregnancy (Figure 5). No treatment is necessary unless rupture or torsion occurs.

FIGURE 5 Lutein cysts
A–C. The typical “stained glass” appearance of three lutein cysts of the right ovary in a pregnant patient. D. Color Doppler image of the ovary demonstrating high-velocity flow (peak systolic velocity of 20.4 cm/s).
Serous cysts
These cysts can reach 4 cm in diameter, have smooth walls with no papillae, are unilocular, and occur most often during menopause. No pathological blood flow is visible in their walls. Most gynecologists follow them (Figure 6).1,7

FIGURE 6 Serous cyst
A. Right ovary containing the cyst. B. Normal left ovary. C. Power Doppler interrogation showing no particular flow in the walls of a serous cyst.
Endometriomas
After the simple cyst, the endometrioma is the most prevalent ovarian or adnexal cyst (Figure 7). It usually has a thick wall and is filled with homogeneous fluid with low-level echo-genicity. It can reach 10 cm in size, and many are bilateral. It is sometimes called a “chocolate” cyst because of its dark blood content.

FIGURE 7 Endometriomas
Endometriomas have low echogenicity. A. Unilateral, unilocular cyst with thin walls. B. Bilateral endometriomas. C. Blood flow in a solid or papillary component of the endometrioma is an occasional finding. It should be investigated further because of the risk that it represents endometrioid cancer.
Endometriomas do not resolve; they usually require surgical excision, although very small ones wholly contained within an ovary are often managed medically or expectantly.
These masses rarely (<1%) give rise to endometrioid carcinoma. Should an endometrioma contain papillae with blood vessels, it is extremely suspicious for endometrioid cancer.

FIGURE 8 Cystic fibromas
A. Sonographic image shows a thin wall and hyperechoic, small mural nodules. B. Macroscopic appearance of an area of internal papillary excrescences. C. Measurement of the small, mural nodules. D. Lack of blood flow in the small papillae, a typical finding on color or power Doppler. E, F. Blood flow in the wall of the cyst and in the mural nodules.
Ovarian fibromas
A fibroma is a slow-growing, benign, solid ovarian tumor. It usually has a cystic component and then is called a cystadenofibroma.
The cystic variety is filled with anechoic fluid and has a thin wall. However, its pathognomonic feature is the small (2–3 mm), extremely hyperechoic mural nodules (papillae) it contains (Figure 8A–C). In the overwhelming majority of cases, no blood vessels are detectable, and the mass is unilocular (Figure 8D–E). It can be recognized in the ovary by the semilunar shape of the tissue surrounding it (crescent sign). The differential diagnosis includes the simple (serous) cyst.
The solid fibroma has a myometrium-like texture, with few or no detectable blood vessels in the stroma. The differential diagnosis includes the Brenner tumor and the Krukenberg tumor.
According to a technology assessment from the Agency for Healthcare Research and Quality (AHRQ), “conventional gray-scale ultrasonography is the most common imaging modality used to differentiate benign from malignant adnexal masses. Especially with the advent of high-frequency transvaginal probes, the quality of the images allows description of the gross anatomic features of the lesion.”8 This descriptive ability is limited, however, “by the great variability of macroscopic characteristics of both benign and malignant masses. Furthermore, the technique is operator dependent.”8
To overcome these challenges, some experts have developed ultrasonographic (US) morphologic scoring systems, which assign a value to individual characteristics. Lerner and colleagues devised a 4-point system:
| Characteristic | Points | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Wall structure | Smooth or small irregularities (<3 mm) | Solid or not applicable | Papillarities larger than 3 mm | |
| Shadowing | Yes | No | ||
| Septation | None or thin (<3 mm) | Thick (≥3 mm) | ||
| Echogenicity | Sonolucent or low-level echo or echogenic core | Mixed or high | ||
The mean point value for benign masses was 1.8; for tumors of low malignant potential it was 3.9; and for malignant tumors it was 5.6 (P < .0005). Lerner and associates proposed a cutoff of 3. A score of 3 or higher, they felt, would be most predictive of malignancy, with sensitivity of 96.8% and specificity of 77%. Positive and negative predictive values were 29.4% and 99.6%, respectively.9
Almost all published scoring systems are based upon or derived from one reported by Sassone and coworkers.10 The most important and practical feature of all scoring systems is their ability to rule out malignancy.
Morphology and Doppler: A synergistic combination
As the same AHRQ report points out, “all of the diagnostic tests and scoring systems we evaluated exhibited a trade-off between sensitivity and specificity—studies of a given test that reported higher sensitivity had lower specificity, and vice versa.”8 Among evaluation methods, the combination of US morphology scores and Doppler imaging achieved the highest pooled sensitivity and specificity scores in distinguishing benign and malignant adnexal masses in postmenopausal women: 86% and 91%, respectively, according to the AHRQ report.8
Compare these figures with those of:
- Bimanual pelvic examination (45% and 90%, respectively)
- Doppler resistance index (72% and 90%)
- Doppler pulsatility index (80% and 73%)
- presence of blood vessels (88% and 78%).
The combination of US morphology scores and Doppler was comparable to the pooled sensitivity and specificity of magnetic resonance imaging (91% and 88%, respectively) and superior to computed tomography (90% and 75%, respectively).
Why the need to know?
Discrimination between benign and malignant masses serves a number of purposes, depending on the setting.
For example, if a symptomatic woman is found to have an adnexal mass, it is important to identify the type of mass causing the symptoms to determine the best course of treatment. And because surgery may be one of the treatment options, it is helpful to know whether a mass is likely to be malignant so that the patient can be referred to a specialist or center that has optimal surgical expertise.8
Some asymptomatic masses may be identified during the annual bimanual pelvic examination recommended by ACOG or during pregnancy-related US imaging. In this setting, it is important to ascertain whether the mass is likely to be malignant so that the patient can be referred to a specialist, if necessary. In addition, thorough assessment of the mass can help “avoid unnecessary diagnostic procedures, including surgery, and anxiety in women with asymptomatic, nonmalignant conditions. In some cases, there may be a rationale for removing certain asymptomatic benign lesions, including prevention of malignant transformation; prevention of ovarian torsion”; and prevention of rupture. Surgery may also be appropriate to avert the need for more complicated surgery in the future or to enhance fertility.8 —Janelle Yates, Senior Editor
Stay tuned!
Next issue, in Part 3 of this series, we will review the use of imaging in the investigation of ovarian neoplasms, both benign and malignant, with an abundance of US images to accompany our discussion.
We want to hear from you! Tell us what you think.
Part 1: A Starting Point (September 2010)
Part 3: Ovarian neoplasms (November 2010)
Part 4: The fallopian tubes (December 2010)
Scanning the ovaries is no simple task. As we mentioned in Part 1 of this four-part series, the practitioner must use the right equipment, take basic preparatory steps, be watchful for clues in the history, and reach a conclusion about what he or she sees. Not only that: The ultrasonographer must be extraordinarily vigilant, paying close attention to multiple characteristics of any mass, from thickness of the wall to the presence of papillations or a blood supply—signs of potential malignancy.
In this article, we detail the traits of various types of non-neoplastic ovarian masses, including:
- functional cysts—follicles, the corpus luteum, and theca lutein cysts
- nonfunctional cysts—serous masses and endometriomas
- cystadenofibromas. Although these masses are usually categorized histo-logically as neoplasms, we include them here due to their almost daily appearance in a busy gynecologic ultrasonographic (US) facility.
In Part 3, we will cover ovarian neoplasms, and in Part 4, our focus will be tubal entities such as ectopic pregnancy and torsion.

FIGURE 1 What is a mass made of? 6 morphologic building blocks
Take an inventory of the mass
Any adnexal mass should be assessed in light of its essential characteristics (Figure 1).
Wall structure. Pay attention to thickness. We use an arbitrary cutoff of 4 mm, giving extra scrutiny to thicknesses exceeding that measurement. In our experience, the thicker the wall, the more likely the mass is to be malignant.
Septation and loculation. A mass is typically unilocular or multilocular. Multilocularity is more common in tumors of low malignant potential and malignant neoplasms.
Papillation. Any internal or external papillae or excrescences should draw your attention. Papillarity in an ovarian mass renders that mass suspicious for malignancy.
Measure (height and width) any papillae that are identified, and document them. Because papillae are associated with ovarian malignancy, further assessment is warranted immediately. The first step is determining whether the papillations contain blood vessels—a task for which color and power Doppler are helpful. We prefer power Doppler because it is more sensitive, detecting blood-flow velocity in the lowest detectable range of 2 cm/s, and because it is not directionally influenced.
Papillae that contain blood vessels with detectable flow are suspicious for malignancy.
Exacoustos and colleagues found that papillae as large as 15 mm in height and 10 mm in width (base) were present in 48% of borderline ovarian tumors but in only 4% of benign and 4% of malignant tumors. However, when the intracystic solid tissue exceeded those dimensions, the lesions were present in 48% of invasive ovarian tumors, 18% of borderline ovarian tumors, and 7% of benign masses.1
Internal echo-structure. A mass can be anechoic, a finding that usually indicates the presence of clear fluid. Mostly solid masses are echogenic. And masses that contain particulate matter, such as blood, cellular matter, or even mucous material, usually have echogenicity of a low level, often described as a “ground-glass” appearance. A mass can also have mixed echo-genicity, a finding usually found in cases involving teratoma or malignancy.
Shadowing. If it is present, it may signify the presence of an extremely dense, solid tissue, such as bone or calcification. The diagnosis of a benign teratoma (i.e., dermoid cyst) should be entertained if shadowing is present in a hyperechoic nodule or mass. Malignant masses very rarely, if ever, display frank shadowing.
Overall appearance. On rare occasions, a bizarre shape or “complex” appearance (as it is termed in most radiology reports) may indicate a malignant mass. More likely it indicates the presence of a teratoma, cystadenoma, or even an atypical corpus luteum. In some reports generated by US laboratories, the term “complex” is applied to all structures other than simple cysts.
Size. The size of a mass can be misleading, as small ovarian lesions with the appropriate sonographic characteristics may be malignant and some larger ones without those characteristics may not be. However, it is understood that the larger an ovarian lesion, the more likely it is a tumor. One important distinction: The amount of fluid in a cystic structure or the amount of old blood in an endometrioma is not the disease process…it is the byproduct of the process. So an 8-cm endometrioma may create fewer pain or fertility issues than a 2- or 3-cm endometrioma. Similarly, the amount of “chocolate” fluid is not automatically indicative of the amount of active endometriotic glands or their sequelae!2
Ascites. If it is present, it should be recorded and investigated further because it may be caused by a malignant intra-abdominal tumor.
Motion tenderness. If the to-and-fro movement of the vaginal probe elicits any motion tenderness, it, too, should be documented. It may be a sign of pelvic peritonitis. In such cases, an “ominous appearing” adnexal finding may represent an inflammatory, rather than malignant, mass.
One of the components of extensive evaluation of the adnexae in general and ovaries in particular is color or power Doppler interrogation—or both.
Tumors contain a relatively large number of pathologic blood vessels that lack the muscular layer found in normal blood vessels and, as a result, demonstrate lower resistance to flow. Diastolic flow is high in these vessels, and resistance and pulsatility indices are low.
We also pay attention when these blood vessels have a tortuous appearance, changes in caliber, anastomoses, and vascular lakes.3 The more tortuous the vessels, with multiple inter-vessel connections and dilatations with changing calibers, the greater the risk of malignancy.4 No less important is the presence of a vessel within a “complex” ovarian mass. A centrally located vessel (also called a “lead vessel”) is suspicious for malignancy.5
A gallery of non-neoplastic ovarian masses
Non-neoplastic cysts are, by far, the most common structures of the ovary. They may be functional, as in the case of the follicles, corpus luteum, and theca lutein cysts, or they may be nonfunctional, as in serous cysts and endometriomas. (As we noted in Part 1, do not call the follicles and corpus luteum “cysts” because this designation suggests pathology.)6

FIGURE 2 Simple cyst
This cyst is anechoic and unilocular with thin walls and no papillae.
Functional cysts
Functional cysts, also known as “simple” cysts, may grow as large as 4 to 5 cm in diameter (Figure 2). They are typically unilocular, anechoic, and thin-walled, with no papillae, and almost never malignant. They usually resolve and require no treatment unless rupture or torsion occurs. Except for the corpus luteum, they have no increased blood flow, and need be viewed only by transvaginal ultrasonography (TVS).
The corpus luteum also can be recognized by TVS. It can exhibit any of a variety of internal structures and echo patterns, due to the multitude of shapes of the blood or clot that can be seen within it (Figure 3).

FIGURE 3 Corpus luteum
A–C. Gray-scale, color Doppler, and power Doppler images, respectively, of a typical corpus luteum. B and C show the enveloping vessels, or “ring of fire.” D. A rather typical gray-scale appearance with a mesh-like, linear internal texture. E. A common feature of the corpus luteum is a linear interphase (arrow) between the clot (c) and the liquified serum (s).
The corpus luteum is typically enveloped by blood vessels, visible on color Doppler as what is called a “ring of fire.” It regresses without intervention. In hyperstimulated ovaries, however, more than one may be present; this poses a real diagnostic challenge when ectopic pregnancy is suspected because it is difficult to differentiate the two entities.
Because the corpus luteum can sometimes resemble some types of ovarian tumors on TVS, imaging during the secretory phase of the cycle in a woman of reproductive age is not ideal. Instead, she should be scanned (or rescanned) between days 5 and 9 of the cycle.

FIGURE 4 Hormonally stimulated ovaries
A, B. The right and left ovaries stimulated by follicle-stimulating hormone preparation (arrow points to hilus). C. An ovary stimulated by clomiphene.
Lutein cysts may reach 5 to 10 cm in diameter. They generally have a thick wall, are multilocular, and typically occur after hormonal induction of ovulation (Figure 4). They also can occur in diabetes, molar pregnancy, and hydrops fetalis. We have seen a unilateral theca lutein cyst in a normal pregnancy (Figure 5). No treatment is necessary unless rupture or torsion occurs.

FIGURE 5 Lutein cysts
A–C. The typical “stained glass” appearance of three lutein cysts of the right ovary in a pregnant patient. D. Color Doppler image of the ovary demonstrating high-velocity flow (peak systolic velocity of 20.4 cm/s).
Serous cysts
These cysts can reach 4 cm in diameter, have smooth walls with no papillae, are unilocular, and occur most often during menopause. No pathological blood flow is visible in their walls. Most gynecologists follow them (Figure 6).1,7

FIGURE 6 Serous cyst
A. Right ovary containing the cyst. B. Normal left ovary. C. Power Doppler interrogation showing no particular flow in the walls of a serous cyst.
Endometriomas
After the simple cyst, the endometrioma is the most prevalent ovarian or adnexal cyst (Figure 7). It usually has a thick wall and is filled with homogeneous fluid with low-level echo-genicity. It can reach 10 cm in size, and many are bilateral. It is sometimes called a “chocolate” cyst because of its dark blood content.

FIGURE 7 Endometriomas
Endometriomas have low echogenicity. A. Unilateral, unilocular cyst with thin walls. B. Bilateral endometriomas. C. Blood flow in a solid or papillary component of the endometrioma is an occasional finding. It should be investigated further because of the risk that it represents endometrioid cancer.
Endometriomas do not resolve; they usually require surgical excision, although very small ones wholly contained within an ovary are often managed medically or expectantly.
These masses rarely (<1%) give rise to endometrioid carcinoma. Should an endometrioma contain papillae with blood vessels, it is extremely suspicious for endometrioid cancer.

FIGURE 8 Cystic fibromas
A. Sonographic image shows a thin wall and hyperechoic, small mural nodules. B. Macroscopic appearance of an area of internal papillary excrescences. C. Measurement of the small, mural nodules. D. Lack of blood flow in the small papillae, a typical finding on color or power Doppler. E, F. Blood flow in the wall of the cyst and in the mural nodules.
Ovarian fibromas
A fibroma is a slow-growing, benign, solid ovarian tumor. It usually has a cystic component and then is called a cystadenofibroma.
The cystic variety is filled with anechoic fluid and has a thin wall. However, its pathognomonic feature is the small (2–3 mm), extremely hyperechoic mural nodules (papillae) it contains (Figure 8A–C). In the overwhelming majority of cases, no blood vessels are detectable, and the mass is unilocular (Figure 8D–E). It can be recognized in the ovary by the semilunar shape of the tissue surrounding it (crescent sign). The differential diagnosis includes the simple (serous) cyst.
The solid fibroma has a myometrium-like texture, with few or no detectable blood vessels in the stroma. The differential diagnosis includes the Brenner tumor and the Krukenberg tumor.
According to a technology assessment from the Agency for Healthcare Research and Quality (AHRQ), “conventional gray-scale ultrasonography is the most common imaging modality used to differentiate benign from malignant adnexal masses. Especially with the advent of high-frequency transvaginal probes, the quality of the images allows description of the gross anatomic features of the lesion.”8 This descriptive ability is limited, however, “by the great variability of macroscopic characteristics of both benign and malignant masses. Furthermore, the technique is operator dependent.”8
To overcome these challenges, some experts have developed ultrasonographic (US) morphologic scoring systems, which assign a value to individual characteristics. Lerner and colleagues devised a 4-point system:
| Characteristic | Points | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Wall structure | Smooth or small irregularities (<3 mm) | Solid or not applicable | Papillarities larger than 3 mm | |
| Shadowing | Yes | No | ||
| Septation | None or thin (<3 mm) | Thick (≥3 mm) | ||
| Echogenicity | Sonolucent or low-level echo or echogenic core | Mixed or high | ||
The mean point value for benign masses was 1.8; for tumors of low malignant potential it was 3.9; and for malignant tumors it was 5.6 (P < .0005). Lerner and associates proposed a cutoff of 3. A score of 3 or higher, they felt, would be most predictive of malignancy, with sensitivity of 96.8% and specificity of 77%. Positive and negative predictive values were 29.4% and 99.6%, respectively.9
Almost all published scoring systems are based upon or derived from one reported by Sassone and coworkers.10 The most important and practical feature of all scoring systems is their ability to rule out malignancy.
Morphology and Doppler: A synergistic combination
As the same AHRQ report points out, “all of the diagnostic tests and scoring systems we evaluated exhibited a trade-off between sensitivity and specificity—studies of a given test that reported higher sensitivity had lower specificity, and vice versa.”8 Among evaluation methods, the combination of US morphology scores and Doppler imaging achieved the highest pooled sensitivity and specificity scores in distinguishing benign and malignant adnexal masses in postmenopausal women: 86% and 91%, respectively, according to the AHRQ report.8
Compare these figures with those of:
- Bimanual pelvic examination (45% and 90%, respectively)
- Doppler resistance index (72% and 90%)
- Doppler pulsatility index (80% and 73%)
- presence of blood vessels (88% and 78%).
The combination of US morphology scores and Doppler was comparable to the pooled sensitivity and specificity of magnetic resonance imaging (91% and 88%, respectively) and superior to computed tomography (90% and 75%, respectively).
Why the need to know?
Discrimination between benign and malignant masses serves a number of purposes, depending on the setting.
For example, if a symptomatic woman is found to have an adnexal mass, it is important to identify the type of mass causing the symptoms to determine the best course of treatment. And because surgery may be one of the treatment options, it is helpful to know whether a mass is likely to be malignant so that the patient can be referred to a specialist or center that has optimal surgical expertise.8
Some asymptomatic masses may be identified during the annual bimanual pelvic examination recommended by ACOG or during pregnancy-related US imaging. In this setting, it is important to ascertain whether the mass is likely to be malignant so that the patient can be referred to a specialist, if necessary. In addition, thorough assessment of the mass can help “avoid unnecessary diagnostic procedures, including surgery, and anxiety in women with asymptomatic, nonmalignant conditions. In some cases, there may be a rationale for removing certain asymptomatic benign lesions, including prevention of malignant transformation; prevention of ovarian torsion”; and prevention of rupture. Surgery may also be appropriate to avert the need for more complicated surgery in the future or to enhance fertility.8 —Janelle Yates, Senior Editor
Stay tuned!
Next issue, in Part 3 of this series, we will review the use of imaging in the investigation of ovarian neoplasms, both benign and malignant, with an abundance of US images to accompany our discussion.
We want to hear from you! Tell us what you think.
1. Exacoustos C, Romanini ME, Rinaldo D, et al. Preoperative sonographic features of borderline ovarian tumors. Ultrasound Obstet Gynecol. 2004;25(1):50-59.
2. Rulin MC, Preston AL. Adnexal masses in postmenopausal women. Obstet Gynecol. 1987;70(4):578-581.
3. Timor-Tritsch IE, Goldstein SR. The complexity of a complex mass and the simplicity of a simple cyst. J Ultraound Med. 2005;24(3):255-258.
4. Sladkevicius P, Jokubkiene L, Valentin L. Contribution of morphological assessment of the vessel tree by three-dimensional ultrasound to a correct diagnos is of malignancy in ovarian masses. Ultrasound Obstet Gynecol. 2007;30(6):874-882.
5. Testa AC, Mancari R, Di Legge A, et al. The “lead vessel”: a vascular ultrasound feature of metastasis in the ovaries. Ultrasound Obstet Gynecol. 2008;31:218-221.
6. Goldstein SR. Postmenopausal adnexal cysts: how clinical management has evolved. Am J Obstet Gynecol. 1996;175(6):1496-1501.
7. Levine D, Gosink BB, Wolf S, Feldesman MR, Pretorius D. Simple adnexal cysts: the natural history in postmenopausal women. Radiology. 1992;184(3):653-659.
8. Myers ER, Bastian LA, Havrilesky LJ, et al. Management of adnexal mass. Evidence Report Technol Assess. 2006;Feb;(130):1-145.
9. Lerner JP, Timor-Tritsch IE, Federman A, Abramovich G. Transvaginal ultrasonographic characterization of ovarian masses with an improved, weighted scoring system. Am J Obstet Gynecol. 1994;170(1 Pt 1):81-85.
10. Sassone AM, Timor-Tritsch IE, Artner A, et al. Transvaginal sonographic characterization of ovarian disease: evaluation of a new scoring system to predict ovarian malignancy. Obstet Gynecol. 2001;78:70-76.
1. Exacoustos C, Romanini ME, Rinaldo D, et al. Preoperative sonographic features of borderline ovarian tumors. Ultrasound Obstet Gynecol. 2004;25(1):50-59.
2. Rulin MC, Preston AL. Adnexal masses in postmenopausal women. Obstet Gynecol. 1987;70(4):578-581.
3. Timor-Tritsch IE, Goldstein SR. The complexity of a complex mass and the simplicity of a simple cyst. J Ultraound Med. 2005;24(3):255-258.
4. Sladkevicius P, Jokubkiene L, Valentin L. Contribution of morphological assessment of the vessel tree by three-dimensional ultrasound to a correct diagnos is of malignancy in ovarian masses. Ultrasound Obstet Gynecol. 2007;30(6):874-882.
5. Testa AC, Mancari R, Di Legge A, et al. The “lead vessel”: a vascular ultrasound feature of metastasis in the ovaries. Ultrasound Obstet Gynecol. 2008;31:218-221.
6. Goldstein SR. Postmenopausal adnexal cysts: how clinical management has evolved. Am J Obstet Gynecol. 1996;175(6):1496-1501.
7. Levine D, Gosink BB, Wolf S, Feldesman MR, Pretorius D. Simple adnexal cysts: the natural history in postmenopausal women. Radiology. 1992;184(3):653-659.
8. Myers ER, Bastian LA, Havrilesky LJ, et al. Management of adnexal mass. Evidence Report Technol Assess. 2006;Feb;(130):1-145.
9. Lerner JP, Timor-Tritsch IE, Federman A, Abramovich G. Transvaginal ultrasonographic characterization of ovarian masses with an improved, weighted scoring system. Am J Obstet Gynecol. 1994;170(1 Pt 1):81-85.
10. Sassone AM, Timor-Tritsch IE, Artner A, et al. Transvaginal sonographic characterization of ovarian disease: evaluation of a new scoring system to predict ovarian malignancy. Obstet Gynecol. 2001;78:70-76.
Skilled US imaging of the adnexal mass: Starting point
No doubt about it: Scanning the adnexae is the most challenging task in gynecologic ultrasonography (US). There are many reasons for the difficulty, but probably none more important than the fact that you are expected to reach a conclusion about what you see—or at least narrow the differential diagnosis.
Some ultrasound laboratories try to hedge their bets, sending the referring physician a report that is nothing more than an exhaustive differential diagnosis, similar to what we see in textbooks. Such a list is useless to a referring clinician, who has probably already considered most of the possibilities and involved the lab to help narrow them down. Labs that send such reports are usually trying to protect themselves from litigation—typically involving cases in which ovarian cancer was missed—or attempting to accomplish a “self-referral” by encouraging further imaging.1
The referring physician is not perfect, either. In our practice, we often receive reports like the following terse description:
A complex cyst was seen in the adnexa. Ovarian malignancy cannot be ruled out.
That’s it. No description of the actual sonographic characteristics. No Doppler velocity flow studies. Yet, the few remarks include a mention of malignancy, and the provider often suggests that “additional imaging such as CT and MRI should be considered.”
When we scrutinize the sonographic images upon which these reports are based, we often discover a corpus luteum, cystic teratoma, benign cystadenoma, endometrioma, or, even, a simple cyst.
The need for competency is compelling
Now that gynecologic US has matured as a field in its own right, the referring physician should expect much more from a laboratory’s pelvic scan than a long recitation of potential diagnoses. And the lab should expect more basic information from the referring provider.
That is the primary reason for this four-part series—to help you identify some of the most prevalent adnexal masses, so that you can exclude cases that are no cause for concern, such as a corpus luteum, and refer patients who really do need additional imaging and expertise, providing as much information in the process as you can.
In Part 1 of the series, we introduce you to basic concepts, recommend equipment, and step you through numerous fundamental scans. Part 2 will focus on nonneoplastic ovarian masses, Part 3 on ovarian neoplasms, and Part 4 on tubal entities such as ectopic pregnancy and torsion.
As much as possible, we educate you by providing actual scans that represent real cases, pointing out the elements that should grab your attention. After all, a picture paints a thousand words.
Ultrasound reveals the polycystic nature of a patient’s ovary. The hilus is prominently hyperechoic.
A few fundamental practices enhance consistency and thoroughness
Before we shift our focus to scanning techniques and interpretation of images, we’d like to offer several basic pointers.
Establish, and document, the hormonal milieu. One of the most important requirements of US imaging, particularly during the reproductive years, is determining and documenting the date of the patient’s last menstrual period (LMP). The reason? Physiologic and pathologic processes involving the reproductive organs are driven by the menstrual cycle—or by therapeutic (or pathologic) hormonal stimulation. We mark each scan with the date of the LMP. If the patient is on hormone therapy, we also mark the scan “HT.” We make these marks on the screen in a way that prevents their erasure every time the picture is frozen and unfrozen. This makes it possible for us to look at the scan days, weeks, or even years later and know what day of the cycle it represents. Every finding must be judged in light of the patient’s hormonal status.
Use a transvaginal transducer. It provides a high-resolution view of any pathology. If need be, it can be combined with a trans-abdominal transducer to afford a more deeply penetrating, panoramic view of the pelvis. We use a variety of transducers to achieve depth, color, power Doppler, and three- dimensional (3D) US.
Take a history and examine the patient. Before scanning your own patient, take a short history and perform a bimanual, palpatory pelvic exam. You may need to examine her again after the scan to verify a sonographic finding.
It is doubly important to take a history if you are scanning a referred patient. Omitting this element is no excuse for overlooking a disease or pathology.
A bimanual, palpatory pelvic exam may also be recommended for some referred patients.
A transvaginal scan is not always possible. There are a number of reasons why the transvaginal approach may not be advisable for some patients, including virginal status, atrophic postmenopausal vagina, agenesis of the vagina, and transverse vaginal septae. In such cases, the best alternative is a transrectal scan, which makes it possible to image the pelvic organs from almost exactly the same vantage point as transvaginal US.2 With proper explanation (particularly with virginal patients), the initial reluctance and apprehension can usually be assuaged.
Don’t trust the referral slip. We recommend that you read, but do not overly trust, the referral slip. It often offers little useful information.
Helpful scanning techniques
Consider applying these maneuvers:
- place your non-scanning hand on the patient’s abdomen to help mobilize the pelvic contents as the transvaginal probe slides across the organs
- use the probe as an “eye” while your palpating finger touches the cervix, uterus, ovaries, and any adnexal mass. Observe the mobility of these structures in relation to each other and the pelvic wall. This technique yields what is often referred to as the “sliding organs” sign. It is possible to identify pelvic adhesions (if the structures do not slide freely) or rule them out (if they do)
- pinpoint the origin of any pain the patient may have by touching the ovary, cervix, and any adnexal mass. This technique is important in cases of ectopic pregnancy, adnexal torsion, or inflammatory disease of the pelvis or adnexae.
Start with a basic scan of key structures
On the way “in” toward the adnexae, take the time to look at the bladder and urethra (FIGURE 1). Some common pathologies of the bladder are diverticulae; calculi; and a thick and vascular bladder wall suggestive of cancer or endometrioma. Ask the patient whether she has experienced any hematuria if any of these pathologies are detected.
FIGURE 1 Imaging the bladder
(A, B) The bladder (bl), urethra (u), vagina (v), and rectum (r) appear in their proper relation in this sagittal view. The posterior angle of the bladder is also apparent (arrow closing an angle of about 110°). (C) Excessive thickness of the bladder wall suggests that this patient has cystitis. (D) Coronal view of the bladder and urethra (solid arrows).
Also take a look at the cervix, searching for Nabothian cysts, endocervical polyps, extreme vascularization (a possible indicator of cervical cancer), and prolapsing submucous myomas (FIGURE 2).
FIGURE 2 Uncommon pathology
A submucous myoma prolapses into the cervical canal in a 13-week intrauterine pregnancy. (A) Grayscale sagittal image and (B) outline view of the same image. (C,D) Color and power Doppler images show the blood supply to the myoma from the uterine cavity.
While you are looking, attempt to scan both kidneys and Morrison’s pouch. Large adnexal masses or fibroids of the uterus may put pressure on the ureter, causing various degrees of hydronephrosis.
Sometimes, when the right kidney is correctly imaged below the liver, you may detect fluid in the space between them (called Morrison’s space). This information has clear value that may aid in diagnosing the main pathology (i.e., ruptured tubal pregnancy, ascites, etc.).
Imaging of the ovaries
The best way to scan the ovaries is to use a high-frequency (4–9 MHz) transvaginal probe. In general, as the frequency of the probe increases, so does resolution of the image—but the ability to penetrate tissue diminishes. For this reason, for abdominal imaging, a 3-MHz probe is often used. For a transvaginal scan, in which the probe can be placed near an ovary, a 5-MHz probe is common. And for a scan of, say, the parathyroid gland, a 12-MHz probe is utilized.
During the reproductive years, the ovaries can be localized by their sonographic markers—the follicles (FIGURE 3A). The ovaries usually lie near the large hypogastric blood vessels (FIGURE 3B). During the secretory phase of the cycle, look for the corpus luteum, switching on the color or power Doppler mode to help locate it (FIGURES 3C, 3D).
The ovaries usually can be distinguished by their relative anechoic sono-texture in juxtaposition to the surrounding, constantly peristalsing small bowel. This strategy is the only help for spotting the ovaries in menopause, when they lose their follicles.
The size of the ovaries may be an important indicator of pathology. During the reproductive years, mean size is 8 mL (standard deviation [SD], 2–3 mL; range, 5–15 mL). Post-menopausal ovaries are small, with a mean size of 3.6 mL (SD, 1.4 mL; range, 1–14 mL).
FIGURE 3 How to spot the ovaries
(A) Anechoic follicles are markers of the ovary during the reproductive years. (B) The ovaries in relation to the hypogastric vessels. (C) Gray-scale image of the corpus luteum and the same image in (D) color Doppler.
A word about terminology: Don’t call follicles “cysts”
During a normal menstrual cycle, one or more follicles mature, reaching about 2 to 2.5 cm in diameter around mid-cycle. Do not call these follicles “cysts” or “follicular cysts.” They are follicles. Calling them cysts, or even including the word cyst in their description, suggests to many gynecology and radiology providers—and to patients themselves—the idea of pathology.1
An exception to that rule: An ovary that is larger than 12 to 14 mL and has a hyperechoic hilus and more than 12 small (4–5 mm), peripherally pushed follicles is usually called “polycystic” (FIGURE 4).3 However, not every ovary that fulfills these sonographic criteria is indeed polycystic. At times normal ovaries may contain multiple follicles without any of the clinical or laboratory indications of a polycystic ovary. In these cases, the ovary may be of normal size and may lack a hyperechoic hilus with rich hilar vascularity. We term such ovaries “multicystic” in their appearance.

FIGURE 4 The polycystic ovary
(A) Gray-scale image of a polycystic ovary. The typical hyperechoic hilus is evident (H). (B) Gross pathologic section of a polycystic ovary. (C) 3D orthogonal planes of a large ovary with a multitude of small follicles pushed peripherally by a voluminous hyperechoic hilus. (D) 3D inversion rendering of the same ovary.
We employ 3D inversion rendering to better see and count the number of follicles (FIGURE 4D).
An ovary can have a polycystic appearance in the following clinical situations:
- hyperthyroid state (36% of affected women)
- hyperprolactinemia (50%)
- hypothalamic hypogonadism (24%).
It also can appear polycystic for no apparent reason.
Stay tuned!
Next month, we continue our focus on adnexal imaging by describing (and showing) nonneoplastic ovarian masses.
We want to hear from you! Tell us what you think.
1. Timor-Tritsch IE, Goldstein SR. The complexity of a complex mass and the simplicity of a simple cyst. J Ultrasound Med. 2005;24(3):255-258.
2. Timor-Tritsch IE, Monteagudo A, Rebarber A, Goldstein SR, Tsymbal T. Transrectal scanning an alternative when transvaginal scanning is not feasible. Ultrasound Obstet Gynecol. 2003;21(5):443-479.
3. Abdel Gadir A, Khatim MS, Mowafi RS, Alnaser HM, Muharib NS, Shaw RW. Implications of ultrasonically diagnosed polycystic ovaries. II. Studies of dynamic and pulsatile hormonal patterns. Human Reprod. 1992;7(4):458-461.
No doubt about it: Scanning the adnexae is the most challenging task in gynecologic ultrasonography (US). There are many reasons for the difficulty, but probably none more important than the fact that you are expected to reach a conclusion about what you see—or at least narrow the differential diagnosis.
Some ultrasound laboratories try to hedge their bets, sending the referring physician a report that is nothing more than an exhaustive differential diagnosis, similar to what we see in textbooks. Such a list is useless to a referring clinician, who has probably already considered most of the possibilities and involved the lab to help narrow them down. Labs that send such reports are usually trying to protect themselves from litigation—typically involving cases in which ovarian cancer was missed—or attempting to accomplish a “self-referral” by encouraging further imaging.1
The referring physician is not perfect, either. In our practice, we often receive reports like the following terse description:
A complex cyst was seen in the adnexa. Ovarian malignancy cannot be ruled out.
That’s it. No description of the actual sonographic characteristics. No Doppler velocity flow studies. Yet, the few remarks include a mention of malignancy, and the provider often suggests that “additional imaging such as CT and MRI should be considered.”
When we scrutinize the sonographic images upon which these reports are based, we often discover a corpus luteum, cystic teratoma, benign cystadenoma, endometrioma, or, even, a simple cyst.
The need for competency is compelling
Now that gynecologic US has matured as a field in its own right, the referring physician should expect much more from a laboratory’s pelvic scan than a long recitation of potential diagnoses. And the lab should expect more basic information from the referring provider.
That is the primary reason for this four-part series—to help you identify some of the most prevalent adnexal masses, so that you can exclude cases that are no cause for concern, such as a corpus luteum, and refer patients who really do need additional imaging and expertise, providing as much information in the process as you can.
In Part 1 of the series, we introduce you to basic concepts, recommend equipment, and step you through numerous fundamental scans. Part 2 will focus on nonneoplastic ovarian masses, Part 3 on ovarian neoplasms, and Part 4 on tubal entities such as ectopic pregnancy and torsion.
As much as possible, we educate you by providing actual scans that represent real cases, pointing out the elements that should grab your attention. After all, a picture paints a thousand words.

Ultrasound reveals the polycystic nature of a patient’s ovary. The hilus is prominently hyperechoic.
A few fundamental practices enhance consistency and thoroughness
Before we shift our focus to scanning techniques and interpretation of images, we’d like to offer several basic pointers.
Establish, and document, the hormonal milieu. One of the most important requirements of US imaging, particularly during the reproductive years, is determining and documenting the date of the patient’s last menstrual period (LMP). The reason? Physiologic and pathologic processes involving the reproductive organs are driven by the menstrual cycle—or by therapeutic (or pathologic) hormonal stimulation. We mark each scan with the date of the LMP. If the patient is on hormone therapy, we also mark the scan “HT.” We make these marks on the screen in a way that prevents their erasure every time the picture is frozen and unfrozen. This makes it possible for us to look at the scan days, weeks, or even years later and know what day of the cycle it represents. Every finding must be judged in light of the patient’s hormonal status.
Use a transvaginal transducer. It provides a high-resolution view of any pathology. If need be, it can be combined with a trans-abdominal transducer to afford a more deeply penetrating, panoramic view of the pelvis. We use a variety of transducers to achieve depth, color, power Doppler, and three- dimensional (3D) US.
Take a history and examine the patient. Before scanning your own patient, take a short history and perform a bimanual, palpatory pelvic exam. You may need to examine her again after the scan to verify a sonographic finding.
It is doubly important to take a history if you are scanning a referred patient. Omitting this element is no excuse for overlooking a disease or pathology.
A bimanual, palpatory pelvic exam may also be recommended for some referred patients.
A transvaginal scan is not always possible. There are a number of reasons why the transvaginal approach may not be advisable for some patients, including virginal status, atrophic postmenopausal vagina, agenesis of the vagina, and transverse vaginal septae. In such cases, the best alternative is a transrectal scan, which makes it possible to image the pelvic organs from almost exactly the same vantage point as transvaginal US.2 With proper explanation (particularly with virginal patients), the initial reluctance and apprehension can usually be assuaged.
Don’t trust the referral slip. We recommend that you read, but do not overly trust, the referral slip. It often offers little useful information.
Helpful scanning techniques
Consider applying these maneuvers:
- place your non-scanning hand on the patient’s abdomen to help mobilize the pelvic contents as the transvaginal probe slides across the organs
- use the probe as an “eye” while your palpating finger touches the cervix, uterus, ovaries, and any adnexal mass. Observe the mobility of these structures in relation to each other and the pelvic wall. This technique yields what is often referred to as the “sliding organs” sign. It is possible to identify pelvic adhesions (if the structures do not slide freely) or rule them out (if they do)
- pinpoint the origin of any pain the patient may have by touching the ovary, cervix, and any adnexal mass. This technique is important in cases of ectopic pregnancy, adnexal torsion, or inflammatory disease of the pelvis or adnexae.
Start with a basic scan of key structures
On the way “in” toward the adnexae, take the time to look at the bladder and urethra (FIGURE 1). Some common pathologies of the bladder are diverticulae; calculi; and a thick and vascular bladder wall suggestive of cancer or endometrioma. Ask the patient whether she has experienced any hematuria if any of these pathologies are detected.

FIGURE 1 Imaging the bladder
(A, B) The bladder (bl), urethra (u), vagina (v), and rectum (r) appear in their proper relation in this sagittal view. The posterior angle of the bladder is also apparent (arrow closing an angle of about 110°). (C) Excessive thickness of the bladder wall suggests that this patient has cystitis. (D) Coronal view of the bladder and urethra (solid arrows).
Also take a look at the cervix, searching for Nabothian cysts, endocervical polyps, extreme vascularization (a possible indicator of cervical cancer), and prolapsing submucous myomas (FIGURE 2).

FIGURE 2 Uncommon pathology
A submucous myoma prolapses into the cervical canal in a 13-week intrauterine pregnancy. (A) Grayscale sagittal image and (B) outline view of the same image. (C,D) Color and power Doppler images show the blood supply to the myoma from the uterine cavity.
While you are looking, attempt to scan both kidneys and Morrison’s pouch. Large adnexal masses or fibroids of the uterus may put pressure on the ureter, causing various degrees of hydronephrosis.
Sometimes, when the right kidney is correctly imaged below the liver, you may detect fluid in the space between them (called Morrison’s space). This information has clear value that may aid in diagnosing the main pathology (i.e., ruptured tubal pregnancy, ascites, etc.).
Imaging of the ovaries
The best way to scan the ovaries is to use a high-frequency (4–9 MHz) transvaginal probe. In general, as the frequency of the probe increases, so does resolution of the image—but the ability to penetrate tissue diminishes. For this reason, for abdominal imaging, a 3-MHz probe is often used. For a transvaginal scan, in which the probe can be placed near an ovary, a 5-MHz probe is common. And for a scan of, say, the parathyroid gland, a 12-MHz probe is utilized.
During the reproductive years, the ovaries can be localized by their sonographic markers—the follicles (FIGURE 3A). The ovaries usually lie near the large hypogastric blood vessels (FIGURE 3B). During the secretory phase of the cycle, look for the corpus luteum, switching on the color or power Doppler mode to help locate it (FIGURES 3C, 3D).
The ovaries usually can be distinguished by their relative anechoic sono-texture in juxtaposition to the surrounding, constantly peristalsing small bowel. This strategy is the only help for spotting the ovaries in menopause, when they lose their follicles.
The size of the ovaries may be an important indicator of pathology. During the reproductive years, mean size is 8 mL (standard deviation [SD], 2–3 mL; range, 5–15 mL). Post-menopausal ovaries are small, with a mean size of 3.6 mL (SD, 1.4 mL; range, 1–14 mL).

FIGURE 3 How to spot the ovaries
(A) Anechoic follicles are markers of the ovary during the reproductive years. (B) The ovaries in relation to the hypogastric vessels. (C) Gray-scale image of the corpus luteum and the same image in (D) color Doppler.
A word about terminology: Don’t call follicles “cysts”
During a normal menstrual cycle, one or more follicles mature, reaching about 2 to 2.5 cm in diameter around mid-cycle. Do not call these follicles “cysts” or “follicular cysts.” They are follicles. Calling them cysts, or even including the word cyst in their description, suggests to many gynecology and radiology providers—and to patients themselves—the idea of pathology.1
An exception to that rule: An ovary that is larger than 12 to 14 mL and has a hyperechoic hilus and more than 12 small (4–5 mm), peripherally pushed follicles is usually called “polycystic” (FIGURE 4).3 However, not every ovary that fulfills these sonographic criteria is indeed polycystic. At times normal ovaries may contain multiple follicles without any of the clinical or laboratory indications of a polycystic ovary. In these cases, the ovary may be of normal size and may lack a hyperechoic hilus with rich hilar vascularity. We term such ovaries “multicystic” in their appearance.

FIGURE 4 The polycystic ovary
(A) Gray-scale image of a polycystic ovary. The typical hyperechoic hilus is evident (H). (B) Gross pathologic section of a polycystic ovary. (C) 3D orthogonal planes of a large ovary with a multitude of small follicles pushed peripherally by a voluminous hyperechoic hilus. (D) 3D inversion rendering of the same ovary.
We employ 3D inversion rendering to better see and count the number of follicles (FIGURE 4D).
An ovary can have a polycystic appearance in the following clinical situations:
- hyperthyroid state (36% of affected women)
- hyperprolactinemia (50%)
- hypothalamic hypogonadism (24%).
It also can appear polycystic for no apparent reason.
Stay tuned!
Next month, we continue our focus on adnexal imaging by describing (and showing) nonneoplastic ovarian masses.
We want to hear from you! Tell us what you think.
No doubt about it: Scanning the adnexae is the most challenging task in gynecologic ultrasonography (US). There are many reasons for the difficulty, but probably none more important than the fact that you are expected to reach a conclusion about what you see—or at least narrow the differential diagnosis.
Some ultrasound laboratories try to hedge their bets, sending the referring physician a report that is nothing more than an exhaustive differential diagnosis, similar to what we see in textbooks. Such a list is useless to a referring clinician, who has probably already considered most of the possibilities and involved the lab to help narrow them down. Labs that send such reports are usually trying to protect themselves from litigation—typically involving cases in which ovarian cancer was missed—or attempting to accomplish a “self-referral” by encouraging further imaging.1
The referring physician is not perfect, either. In our practice, we often receive reports like the following terse description:
A complex cyst was seen in the adnexa. Ovarian malignancy cannot be ruled out.
That’s it. No description of the actual sonographic characteristics. No Doppler velocity flow studies. Yet, the few remarks include a mention of malignancy, and the provider often suggests that “additional imaging such as CT and MRI should be considered.”
When we scrutinize the sonographic images upon which these reports are based, we often discover a corpus luteum, cystic teratoma, benign cystadenoma, endometrioma, or, even, a simple cyst.
The need for competency is compelling
Now that gynecologic US has matured as a field in its own right, the referring physician should expect much more from a laboratory’s pelvic scan than a long recitation of potential diagnoses. And the lab should expect more basic information from the referring provider.
That is the primary reason for this four-part series—to help you identify some of the most prevalent adnexal masses, so that you can exclude cases that are no cause for concern, such as a corpus luteum, and refer patients who really do need additional imaging and expertise, providing as much information in the process as you can.
In Part 1 of the series, we introduce you to basic concepts, recommend equipment, and step you through numerous fundamental scans. Part 2 will focus on nonneoplastic ovarian masses, Part 3 on ovarian neoplasms, and Part 4 on tubal entities such as ectopic pregnancy and torsion.
As much as possible, we educate you by providing actual scans that represent real cases, pointing out the elements that should grab your attention. After all, a picture paints a thousand words.

Ultrasound reveals the polycystic nature of a patient’s ovary. The hilus is prominently hyperechoic.
A few fundamental practices enhance consistency and thoroughness
Before we shift our focus to scanning techniques and interpretation of images, we’d like to offer several basic pointers.
Establish, and document, the hormonal milieu. One of the most important requirements of US imaging, particularly during the reproductive years, is determining and documenting the date of the patient’s last menstrual period (LMP). The reason? Physiologic and pathologic processes involving the reproductive organs are driven by the menstrual cycle—or by therapeutic (or pathologic) hormonal stimulation. We mark each scan with the date of the LMP. If the patient is on hormone therapy, we also mark the scan “HT.” We make these marks on the screen in a way that prevents their erasure every time the picture is frozen and unfrozen. This makes it possible for us to look at the scan days, weeks, or even years later and know what day of the cycle it represents. Every finding must be judged in light of the patient’s hormonal status.
Use a transvaginal transducer. It provides a high-resolution view of any pathology. If need be, it can be combined with a trans-abdominal transducer to afford a more deeply penetrating, panoramic view of the pelvis. We use a variety of transducers to achieve depth, color, power Doppler, and three- dimensional (3D) US.
Take a history and examine the patient. Before scanning your own patient, take a short history and perform a bimanual, palpatory pelvic exam. You may need to examine her again after the scan to verify a sonographic finding.
It is doubly important to take a history if you are scanning a referred patient. Omitting this element is no excuse for overlooking a disease or pathology.
A bimanual, palpatory pelvic exam may also be recommended for some referred patients.
A transvaginal scan is not always possible. There are a number of reasons why the transvaginal approach may not be advisable for some patients, including virginal status, atrophic postmenopausal vagina, agenesis of the vagina, and transverse vaginal septae. In such cases, the best alternative is a transrectal scan, which makes it possible to image the pelvic organs from almost exactly the same vantage point as transvaginal US.2 With proper explanation (particularly with virginal patients), the initial reluctance and apprehension can usually be assuaged.
Don’t trust the referral slip. We recommend that you read, but do not overly trust, the referral slip. It often offers little useful information.
Helpful scanning techniques
Consider applying these maneuvers:
- place your non-scanning hand on the patient’s abdomen to help mobilize the pelvic contents as the transvaginal probe slides across the organs
- use the probe as an “eye” while your palpating finger touches the cervix, uterus, ovaries, and any adnexal mass. Observe the mobility of these structures in relation to each other and the pelvic wall. This technique yields what is often referred to as the “sliding organs” sign. It is possible to identify pelvic adhesions (if the structures do not slide freely) or rule them out (if they do)
- pinpoint the origin of any pain the patient may have by touching the ovary, cervix, and any adnexal mass. This technique is important in cases of ectopic pregnancy, adnexal torsion, or inflammatory disease of the pelvis or adnexae.
Start with a basic scan of key structures
On the way “in” toward the adnexae, take the time to look at the bladder and urethra (FIGURE 1). Some common pathologies of the bladder are diverticulae; calculi; and a thick and vascular bladder wall suggestive of cancer or endometrioma. Ask the patient whether she has experienced any hematuria if any of these pathologies are detected.

FIGURE 1 Imaging the bladder
(A, B) The bladder (bl), urethra (u), vagina (v), and rectum (r) appear in their proper relation in this sagittal view. The posterior angle of the bladder is also apparent (arrow closing an angle of about 110°). (C) Excessive thickness of the bladder wall suggests that this patient has cystitis. (D) Coronal view of the bladder and urethra (solid arrows).
Also take a look at the cervix, searching for Nabothian cysts, endocervical polyps, extreme vascularization (a possible indicator of cervical cancer), and prolapsing submucous myomas (FIGURE 2).

FIGURE 2 Uncommon pathology
A submucous myoma prolapses into the cervical canal in a 13-week intrauterine pregnancy. (A) Grayscale sagittal image and (B) outline view of the same image. (C,D) Color and power Doppler images show the blood supply to the myoma from the uterine cavity.
While you are looking, attempt to scan both kidneys and Morrison’s pouch. Large adnexal masses or fibroids of the uterus may put pressure on the ureter, causing various degrees of hydronephrosis.
Sometimes, when the right kidney is correctly imaged below the liver, you may detect fluid in the space between them (called Morrison’s space). This information has clear value that may aid in diagnosing the main pathology (i.e., ruptured tubal pregnancy, ascites, etc.).
Imaging of the ovaries
The best way to scan the ovaries is to use a high-frequency (4–9 MHz) transvaginal probe. In general, as the frequency of the probe increases, so does resolution of the image—but the ability to penetrate tissue diminishes. For this reason, for abdominal imaging, a 3-MHz probe is often used. For a transvaginal scan, in which the probe can be placed near an ovary, a 5-MHz probe is common. And for a scan of, say, the parathyroid gland, a 12-MHz probe is utilized.
During the reproductive years, the ovaries can be localized by their sonographic markers—the follicles (FIGURE 3A). The ovaries usually lie near the large hypogastric blood vessels (FIGURE 3B). During the secretory phase of the cycle, look for the corpus luteum, switching on the color or power Doppler mode to help locate it (FIGURES 3C, 3D).
The ovaries usually can be distinguished by their relative anechoic sono-texture in juxtaposition to the surrounding, constantly peristalsing small bowel. This strategy is the only help for spotting the ovaries in menopause, when they lose their follicles.
The size of the ovaries may be an important indicator of pathology. During the reproductive years, mean size is 8 mL (standard deviation [SD], 2–3 mL; range, 5–15 mL). Post-menopausal ovaries are small, with a mean size of 3.6 mL (SD, 1.4 mL; range, 1–14 mL).

FIGURE 3 How to spot the ovaries
(A) Anechoic follicles are markers of the ovary during the reproductive years. (B) The ovaries in relation to the hypogastric vessels. (C) Gray-scale image of the corpus luteum and the same image in (D) color Doppler.
A word about terminology: Don’t call follicles “cysts”
During a normal menstrual cycle, one or more follicles mature, reaching about 2 to 2.5 cm in diameter around mid-cycle. Do not call these follicles “cysts” or “follicular cysts.” They are follicles. Calling them cysts, or even including the word cyst in their description, suggests to many gynecology and radiology providers—and to patients themselves—the idea of pathology.1
An exception to that rule: An ovary that is larger than 12 to 14 mL and has a hyperechoic hilus and more than 12 small (4–5 mm), peripherally pushed follicles is usually called “polycystic” (FIGURE 4).3 However, not every ovary that fulfills these sonographic criteria is indeed polycystic. At times normal ovaries may contain multiple follicles without any of the clinical or laboratory indications of a polycystic ovary. In these cases, the ovary may be of normal size and may lack a hyperechoic hilus with rich hilar vascularity. We term such ovaries “multicystic” in their appearance.

FIGURE 4 The polycystic ovary
(A) Gray-scale image of a polycystic ovary. The typical hyperechoic hilus is evident (H). (B) Gross pathologic section of a polycystic ovary. (C) 3D orthogonal planes of a large ovary with a multitude of small follicles pushed peripherally by a voluminous hyperechoic hilus. (D) 3D inversion rendering of the same ovary.
We employ 3D inversion rendering to better see and count the number of follicles (FIGURE 4D).
An ovary can have a polycystic appearance in the following clinical situations:
- hyperthyroid state (36% of affected women)
- hyperprolactinemia (50%)
- hypothalamic hypogonadism (24%).
It also can appear polycystic for no apparent reason.
Stay tuned!
Next month, we continue our focus on adnexal imaging by describing (and showing) nonneoplastic ovarian masses.
We want to hear from you! Tell us what you think.
1. Timor-Tritsch IE, Goldstein SR. The complexity of a complex mass and the simplicity of a simple cyst. J Ultrasound Med. 2005;24(3):255-258.
2. Timor-Tritsch IE, Monteagudo A, Rebarber A, Goldstein SR, Tsymbal T. Transrectal scanning an alternative when transvaginal scanning is not feasible. Ultrasound Obstet Gynecol. 2003;21(5):443-479.
3. Abdel Gadir A, Khatim MS, Mowafi RS, Alnaser HM, Muharib NS, Shaw RW. Implications of ultrasonically diagnosed polycystic ovaries. II. Studies of dynamic and pulsatile hormonal patterns. Human Reprod. 1992;7(4):458-461.
1. Timor-Tritsch IE, Goldstein SR. The complexity of a complex mass and the simplicity of a simple cyst. J Ultrasound Med. 2005;24(3):255-258.
2. Timor-Tritsch IE, Monteagudo A, Rebarber A, Goldstein SR, Tsymbal T. Transrectal scanning an alternative when transvaginal scanning is not feasible. Ultrasound Obstet Gynecol. 2003;21(5):443-479.
3. Abdel Gadir A, Khatim MS, Mowafi RS, Alnaser HM, Muharib NS, Shaw RW. Implications of ultrasonically diagnosed polycystic ovaries. II. Studies of dynamic and pulsatile hormonal patterns. Human Reprod. 1992;7(4):458-461.
OSTEOPOROSIS
The author reports that he serves on the advisory boards of Amgen, Boehringer Ingelheim, Depomed, Eli Lilly, and Novo Nordisk. He is a speaker for the Alliance for Bone Health, Eli Lilly, and Warner Chilcott.
Some hormonal contraceptives may affect bone density
Berenson AB, Rahman M, Breitkopf CR, Bi LX. Effects of depot medroxyprogesterone acetate and 20-microgram oral contraceptives on bone mineral density. Obstet Gynecol. 2008;112:788–799.
American College of Obstetricians and Gynecologists. ACOG Committee Opinion. No. 415. September 2008. Depot medroxyprogesterone acetate and bone effects. Obstet Gynecol. 2008;112:727–730.
A woman’s contraceptive choice may affect her bone mineral density (BMD)—particularly if she chooses depot medroxyprogesterone acetate (DMPA) or a very-low-dose oral contraceptive (OC) as her method.
In the case of DMPA, studies have shown that its use for 2 years significantly impairs BMD at the hip and spine, regardless of the patient’s age, although BMD usually rebounds after discontinuation of the drug.1
In the case of very-low-dose OCs, there is evidence that young women who use a pill that contains only 20 μg of ethinyl estradiol have a lower increase in BMD than do women the same age who do not use hormonal contraception. (OCs that contain a higher dosage of ethinyl estradiol have not been shown to hamper BMD.) A study by Polatti and colleagues reported a 7.8% increase in BMD over 5 years among women 19 to 22 years old who did not use OCs, compared with no change in BMD among women who used OCs containing 20 μg of ethinyl estradiol.2
DMPA, BMD, and the FDA
The deleterious effect of DMPA on BMD is particularly relevant in perimenopausal women, who have already begun to experience the age-related decline in BMD that starts around the age of 30. The effect is also troubling in adolescents, who normally experience a large accretion of bone during the teen and early adult years.
In 2004, the US Food and Drug Administration (FDA) added a boxed warning to DMPA labeling advising women to limit their use of the drug to 2 years. Since that time, other studies have found that BMD increases to a greater degree among past users of DMPA than among never users—suggesting that DMPA-related bone loss is reversible.
ACOG: DMPA can be used longer than 2 years in some
In September 2008, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion acknowledging the association between DMPA and BMD loss. The committee pointed out, however, that “current evidence suggests that partial or full recovery of BMD occurs at the spine and at least partial recovery occurs at the hip after discontinuation of DMPA” ( FIGURE ).
The ACOG opinion also noted that, “given the efficacy of DMPA, particularly for populations such as adolescents, for whom contraceptive adherence can be challenging, or for those who feel they could not comply with a daily contraceptive method or a method that must be used with each act of intercourse, the possible adverse effects of DMPA must be balanced against the significant personal and public health impact of unintended pregnancy.”
The committee recommended that, despite concerns about bone loss, practitioners should not hesitate to prescribe DMPA. Nor should they limit its use to 2 consecutive years or perform BMD monitoring solely in response to DMPA use. “Any observed short-term loss in BMD associated with DMPA use may be recovered and is unlikely to place a woman at risk of fracture during use or in later years,” the committee opinion noted.
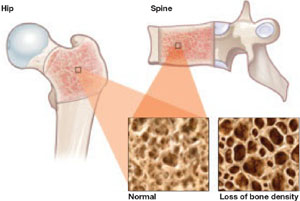
FIGURE DMPA-related bone loss is largely reversible
Depot medroxyprogesterone acetate (DMPA) is associated with a loss of bone mineral density at the hip and spine. Once the drug is discontinued, however, bone density appears to recover at least partially at both sites.
Study explores bone gains after DMPA
Berenson and associates measured BMD every 6 months for as long as 3 years in 703 white, African-American, and Hispanic women who used an OC, DMPA, or nonhormonal contraception. They also measured BMD for up to 2 additional years in 68 women who discontinued DMPA. They found no differences between races—although they did find the expected DMPA-associated bone loss.
They also found a small amount of bone loss (0.5% at the lumbar spine and 1.3% at the femoral neck) in users of very-low-dose OCs (20 μg ethinyl estradiol), compared with a gain of 1.9% at the lumbar spine and 0.6% at the femoral neck in nonusers of hormonal contraception.
Women who made a transition from DMPA to a very-low-dose OC recovered bone mass slowly. After DMPA was discontinued, women who selected nonhormonal contraception recovered BMD (4.9% at the spine, 3.2% at the femoral neck)—unlike those who chose a very-low-dose OC, who regained BMD at the spine (2.3%) but not the femoral neck (–0.7%).
Use of very-low-dose oral contraception (20 μg ethinyl estradiol) may lead to a small amount of bone loss or failure of bone accretion. Use of depot medroxyprogesterone acetate (DMPA) is associated with a greater degree of bone loss, but this loss is largely reversible at the spine. Use of a 20-μg oral contraceptive (OC) after discontinuation of DMPA may slow bone recovery.
As ACOG has indicated, concerns about skeletal health should not influence the decision to initiate or continue DMPA. Likewise, such concerns should not lead to restrictions on the use of OCs in teens or adult women. However, clinicians may wish to take bone effects into consideration when choosing the estrogen dosage of OCs for women younger than 30 who have yet to attain peak bone mass.
Denosumab nears FDA approval for treatment of osteoporosis
Cummings SR, San Martin J, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765.
Kendler DL, Roux C, Benhamou CL, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res. 2009 [Epub ahead of print].
Miller PD, Bolognese MA, Lewiecki EM, et al, for the Amg Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43:222–229.
An FDA panel advising the Division of Reproductive and Urologic Products voted to approve denosumab (proposed brand name: Prolia) as a treatment for osteoporosis. The drug is a fully human monoclonal antibody to the receptor activator of nuclear factor-B ligand (RANKL), a cytokine that is essential for the formation, function, and survival of osteoclasts. By binding RANKL, denosumab prevents interaction between RANKL and its receptor, RANK, on osteoclasts and osteoclast precursors and thereby inhibits osteoclast-mediated bone resorption. Its effects are reversible.
Trial: Denosumab versus placebo
Cummings and colleagues reported the findings of the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial, which involved 7,868 women 60 to 90 years old who had a BMD T-score between –2.5 and –4.0 at the lumbar spine or total hip. Participants were randomly assigned to receive 60 mg of denosumab or placebo subcutaneously every 6 months for 36 months. The primary endpoint was new vertebral fracture. Secondary endpoints included nonvertebral and hip fractures.
Findings included:
- Denosumab reduced the risk of new, radiographically detected vertebral fracture, with a cumulative incidence of 2.3%, versus 7.2% in the placebo group (risk ratio, 0.32; P<.001).
- Denosumab reduced the risk of hip fracture, with a cumulative incidence of 0.7% in the denosumab group, versus 1.2% in the placebo group (hazard ratio, 0.60; P=.04).
- Denosumab reduced the risk of nonvertebral fracture, with a cumulative incidence of 6.5% in the denosumab group, versus 8.0% in the placebo group (hazard ratio, 0.80; P=.01).
- There was no increase in the risk of cancer, infection, cardiovascular disease, delayed fracture healing, or hypocalcemia, and no cases of osteonecrosis of the jaw or adverse reaction to injection of the drug.
How does denosumab compare with alendronate?
Kendler and associates explored a clinically relevant question: What are the effects of switching from a bisphosphonate (in this case, alendronate) to denosumab?
They studied 504 postmenopausal women who were at least 55 years old, had a T-score between –2 and –4, and had been taking alendronate for at least 6 months. These women were randomized in double-blinded, double-dummy fashion to 60 mg of subcutaneous denosumab or a continuation of 70 mg of oral alendronate. Follow-up was 12 months.
Findings included:
- Among the women making a transition to denosumab, total hip BMD increased by 1.9% at 12 months, versus 1.05% in women continuing on alendronate (P<.0001).
- Women making a transition to denosumab also gained significantly more BMD at 12 months at the lumbar spine, femoral neck, and the distal third of the radius (all P<.0125).
- The transition to denosumab reduced bone turnover to a greater degree than did continuing alendronate.
What happens when denosumab is discontinued?
Miller and colleagues randomized postmenopausal women who had a lumbar spine T-score of –1.8 to –4.0 or a proximal femur T-score of –1.8 to –3.5 to one of the following arms:
- denosumab every 3 months (6, 14, or 30 mg)
- denosumab every 6 months (14, 60, 100, or 210 mg)
- open-label oral alendronate every week (70 mg)
- placebo.
After 24 months, women taking denosumab either:
- continued treatment at 60 mg every 6 months for an additional 24 months
- discontinued therapy
- discontinued treatment for 12 months and then reinitiated denosumab at 60 mg every 6 months for an additional 12 months.
The placebo cohort was maintained throughout this period.
Continuous, long-term denosumab increased BMD at the lumbar spine (9.4% to 11.8%) and total hip (4.0% to 6.1%). In contrast, discontinuation of denosumab was associated with a decrease in BMD of 6.6% at the lumbar spine and 5.3% at the total hip within the first 12 months. Retreatment with denosumab increased lumbar spine BMD by 9% from the original baseline values. Levels of bone turnover markers increased upon discontinuation of denosumab and decreased with retreatment. Adverse events occurred at similar rates in all treatment groups.
Among postmenopausal women who have low bone mineral density (BMD), long-term treatment with denosumab leads to gains in BMD and a reduction of markers of bone turnover. These effects are fully reversible upon discontinuation of the drug, but reoccur when treatment is restored.
In addition, switching from alendronate to denosumab produces a greater reduction in bone turnover than does continuation on the bisphosphonate.
Denosumab is a safe, extremely potent agent that will undoubtedly find a place in the ObGyn armamentarium, where it will join bisphosphonates, selective estrogen receptor modulators, and hormone therapy. The long dosing interval (every 6 months) should help increase compliance.
TSEC, a novel compound, enters development
Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045–1052.
Although raloxifene is the only selective estrogen receptor modulator (SERM) approved by the FDA for prevention and treatment of osteoporosis, numerous other SERMs have been explored for this application. One new category of drug is the tissue selective estrogen complex (TSEC), which pairs a SERM with an estrogen. This was previously attempted unsuccessfully with raloxifene and 17β-estradiol. The ideal SERM–estrogen combination would have the positive attributes of both classes of drugs with fewer, or none, of the undesired effects. An appropriate TSEC would therefore alleviate hot flushes, treat vulvar and vaginal atrophy, and protect against bone loss without stimulating the endometrium and increasing the risk of breast cancer.
One third-generation SERM that has been investigated for its utility in this regard is bazedoxifene (BZA), which produced endometrial thickness and amenorrhea rates comparable to those of placebo. It also produced greater gains in BMD at the lumbar spine in a 2-year, randomized, double-blind, placebo-controlled trial.3 The incidence of new vertebral fracture was significantly lower with BZA than with placebo in a study of postmenopausal women with osteoporosis.4
Given these favorable data, a TSEC containing BZA and conjugated equine estrogen was designed as a potential new comprehensive menopausal therapy.
Substudies suggest bazedoxifene has promise
Lindsay and associates reported on two osteoporosis substudies of the Selective estrogen Menopause and Response to Therapy (SMART) Trial. The main study, which evaluated the incidence of endometrial hyperplasia at 12 months, will be reported elsewhere; it was a multicenter, double-blind, randomized, placebo-controlled phase 3 trial that enrolled 3,397 women.
Substudy I involved women who were more than 5 years postmenopausal, and Substudy II included women who were between 1 and 5 years postmenopausal. Eligible screened subjects were randomly assigned to one of the following treatment groups:
- bazedoxifene (10, 20, or 40 mg), each with conjugated equine estrogen (0.625 or 0.45 mg)
- raloxifene (60 mg)
- placebo.
To maintain blinding, the combination of BZA and conjugated equine estrogen was provided as a single, encapsulated tablet to match the placebo, as was raloxifene. Subjects were directed to take one tablet orally at approximately the same time each day for 2 years. The primary outcome for both substudies was a change in BMD at the lumbar spine, as measured by dual-energy x-ray absorptiometry.
In both substudies, BMD increased to a greater degree at the lumbar spine and total hip at all BZA-estrogen dosages than with placebo, and it increased to a greater degree at the lumbar spine at most BZA-estrogen dosages, compared with raloxifene.
Osteocalcin and N-telopeptide significantly decreased at all BZA-estrogen dosages, compared with placebo, and at most BZA-estrogen dosages, compared with raloxifene.
Bazedoxifene is a potential therapeutic agent for menopausal women that may protect the endometrium while preventing bone loss in a population at higher risk of osteoporosis. It is not yet approved for this indication; further investigation is needed.
The combination of an estrogen and a selective estrogen receptor modulator to potentially relieve vasomotor symptoms, prevent vulvovaginal atrophy, and preserve bone mass without stimulating the endometrium or increasing the risk of breast cancer or venous thromboembolism is very exciting—and just might revolutionize treatment of menopausal women
Bolognese M, Krege JH, Utian WH, et al. Effects of arzoxifene on bone mineral density and endometrium in postmenopausal women with normal or low bone mass. J Clin Endocrinol Metab. 2009;94:2284–2289.
The benzothiophene derivative arzoxifene, which has been in development for the prevention and treatment of osteoporosis, as well as for reduction of the risk of invasive breast cancer in postmenopausal women, has been pulled from the regulatory approval process by its manufacturer, Eli Lilly.
This move is somewhat surprising because, in a phase 3 trial, arzoxifene significantly increased bone mineral density at the lumbar spine (2.9%) and total hip (2.2%), compared with placebo. It also decreased levels of biochemical markers of bone metabolism.
In the trial, Bolognese and associates randomly assigned 331 postmenopausal women who had normal or low bone mass to receive 20 mg of arzoxifene or placebo daily for 2 years.
The trial also found that changes in breast density were neutral or slightly decreased in the arzoxifene group, and there was no evidence of endometrial hyperplasia or carcinoma, based on central review of baseline and follow-up endometrial biopsies. Nor were there any significant differences between groups in endometrial thickness, as assessed by transvaginal ultrasonography, or in the incidence of uterine polyps, vaginal bleeding, and hot flushes.
Nevertheless, Lilly issued a press release in late August announcing that arzoxifene would not be submitted to the FDA for regulatory review.5 It appears that, although initial results from the “pivotal” 5-year, phase 3 GJAD “Generations” trial indicated that the drug had met the primary endpoints of significantly reducing the risk of vertebral fracture and invasive breast cancer in postmenopausal women, “the study failed to demonstrate a statistically significant difference in key secondary efficacy endpoints, such as nonvertebral fractures, clinical vertebral fractures, cardiovascular events, and cognitive function, compared with placebo.”
The release went on to say: “In addition, certain adverse events, including venous thromboembolic events, hot flushes, and gynecological-related events, were reported more frequently in the arzoxifene group, compared with placebo.”5
Arzoxifene therefore joins an expanding list of selective estrogen receptor modulators (SERMs) that did not make it out of clinical trials: idoxifene, droloxifene, levormeloxifene, and lasofoxifene (approved in Europe, however), to name a few. The fall of arzoxifene again underscores the notion that “not all SERMs are created equal.”6
1. Scholes D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med. 2005;159:139-144.
2. Polatti F, Perotti F, Filippa N, Gallina D, Nappi RE. Bone mass and long-term monophasic oral contraceptive treatment in young women. Contraception. 1995;51:221-224.
3. Miller PD, Chines AA, Christiansen C, et al. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-year results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res. 2008;23:525-535.
4. Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo- and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
5. Based on preliminary phase III GHAD study results, Lilly concludes arzoxifene’s clinical profile does not support regulatory submission [press release]. Available at http://newsroom.lilly.com/releasedetail.cfm?ReleaseID=403905. Accessed October 2, 2009.
6. Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.
The author reports that he serves on the advisory boards of Amgen, Boehringer Ingelheim, Depomed, Eli Lilly, and Novo Nordisk. He is a speaker for the Alliance for Bone Health, Eli Lilly, and Warner Chilcott.
Some hormonal contraceptives may affect bone density
Berenson AB, Rahman M, Breitkopf CR, Bi LX. Effects of depot medroxyprogesterone acetate and 20-microgram oral contraceptives on bone mineral density. Obstet Gynecol. 2008;112:788–799.
American College of Obstetricians and Gynecologists. ACOG Committee Opinion. No. 415. September 2008. Depot medroxyprogesterone acetate and bone effects. Obstet Gynecol. 2008;112:727–730.
A woman’s contraceptive choice may affect her bone mineral density (BMD)—particularly if she chooses depot medroxyprogesterone acetate (DMPA) or a very-low-dose oral contraceptive (OC) as her method.
In the case of DMPA, studies have shown that its use for 2 years significantly impairs BMD at the hip and spine, regardless of the patient’s age, although BMD usually rebounds after discontinuation of the drug.1
In the case of very-low-dose OCs, there is evidence that young women who use a pill that contains only 20 μg of ethinyl estradiol have a lower increase in BMD than do women the same age who do not use hormonal contraception. (OCs that contain a higher dosage of ethinyl estradiol have not been shown to hamper BMD.) A study by Polatti and colleagues reported a 7.8% increase in BMD over 5 years among women 19 to 22 years old who did not use OCs, compared with no change in BMD among women who used OCs containing 20 μg of ethinyl estradiol.2
DMPA, BMD, and the FDA
The deleterious effect of DMPA on BMD is particularly relevant in perimenopausal women, who have already begun to experience the age-related decline in BMD that starts around the age of 30. The effect is also troubling in adolescents, who normally experience a large accretion of bone during the teen and early adult years.
In 2004, the US Food and Drug Administration (FDA) added a boxed warning to DMPA labeling advising women to limit their use of the drug to 2 years. Since that time, other studies have found that BMD increases to a greater degree among past users of DMPA than among never users—suggesting that DMPA-related bone loss is reversible.
ACOG: DMPA can be used longer than 2 years in some
In September 2008, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion acknowledging the association between DMPA and BMD loss. The committee pointed out, however, that “current evidence suggests that partial or full recovery of BMD occurs at the spine and at least partial recovery occurs at the hip after discontinuation of DMPA” ( FIGURE ).
The ACOG opinion also noted that, “given the efficacy of DMPA, particularly for populations such as adolescents, for whom contraceptive adherence can be challenging, or for those who feel they could not comply with a daily contraceptive method or a method that must be used with each act of intercourse, the possible adverse effects of DMPA must be balanced against the significant personal and public health impact of unintended pregnancy.”
The committee recommended that, despite concerns about bone loss, practitioners should not hesitate to prescribe DMPA. Nor should they limit its use to 2 consecutive years or perform BMD monitoring solely in response to DMPA use. “Any observed short-term loss in BMD associated with DMPA use may be recovered and is unlikely to place a woman at risk of fracture during use or in later years,” the committee opinion noted.

FIGURE DMPA-related bone loss is largely reversible
Depot medroxyprogesterone acetate (DMPA) is associated with a loss of bone mineral density at the hip and spine. Once the drug is discontinued, however, bone density appears to recover at least partially at both sites.
Study explores bone gains after DMPA
Berenson and associates measured BMD every 6 months for as long as 3 years in 703 white, African-American, and Hispanic women who used an OC, DMPA, or nonhormonal contraception. They also measured BMD for up to 2 additional years in 68 women who discontinued DMPA. They found no differences between races—although they did find the expected DMPA-associated bone loss.
They also found a small amount of bone loss (0.5% at the lumbar spine and 1.3% at the femoral neck) in users of very-low-dose OCs (20 μg ethinyl estradiol), compared with a gain of 1.9% at the lumbar spine and 0.6% at the femoral neck in nonusers of hormonal contraception.
Women who made a transition from DMPA to a very-low-dose OC recovered bone mass slowly. After DMPA was discontinued, women who selected nonhormonal contraception recovered BMD (4.9% at the spine, 3.2% at the femoral neck)—unlike those who chose a very-low-dose OC, who regained BMD at the spine (2.3%) but not the femoral neck (–0.7%).
Use of very-low-dose oral contraception (20 μg ethinyl estradiol) may lead to a small amount of bone loss or failure of bone accretion. Use of depot medroxyprogesterone acetate (DMPA) is associated with a greater degree of bone loss, but this loss is largely reversible at the spine. Use of a 20-μg oral contraceptive (OC) after discontinuation of DMPA may slow bone recovery.
As ACOG has indicated, concerns about skeletal health should not influence the decision to initiate or continue DMPA. Likewise, such concerns should not lead to restrictions on the use of OCs in teens or adult women. However, clinicians may wish to take bone effects into consideration when choosing the estrogen dosage of OCs for women younger than 30 who have yet to attain peak bone mass.
Denosumab nears FDA approval for treatment of osteoporosis
Cummings SR, San Martin J, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765.
Kendler DL, Roux C, Benhamou CL, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res. 2009 [Epub ahead of print].
Miller PD, Bolognese MA, Lewiecki EM, et al, for the Amg Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43:222–229.
An FDA panel advising the Division of Reproductive and Urologic Products voted to approve denosumab (proposed brand name: Prolia) as a treatment for osteoporosis. The drug is a fully human monoclonal antibody to the receptor activator of nuclear factor-B ligand (RANKL), a cytokine that is essential for the formation, function, and survival of osteoclasts. By binding RANKL, denosumab prevents interaction between RANKL and its receptor, RANK, on osteoclasts and osteoclast precursors and thereby inhibits osteoclast-mediated bone resorption. Its effects are reversible.
Trial: Denosumab versus placebo
Cummings and colleagues reported the findings of the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial, which involved 7,868 women 60 to 90 years old who had a BMD T-score between –2.5 and –4.0 at the lumbar spine or total hip. Participants were randomly assigned to receive 60 mg of denosumab or placebo subcutaneously every 6 months for 36 months. The primary endpoint was new vertebral fracture. Secondary endpoints included nonvertebral and hip fractures.
Findings included:
- Denosumab reduced the risk of new, radiographically detected vertebral fracture, with a cumulative incidence of 2.3%, versus 7.2% in the placebo group (risk ratio, 0.32; P<.001).
- Denosumab reduced the risk of hip fracture, with a cumulative incidence of 0.7% in the denosumab group, versus 1.2% in the placebo group (hazard ratio, 0.60; P=.04).
- Denosumab reduced the risk of nonvertebral fracture, with a cumulative incidence of 6.5% in the denosumab group, versus 8.0% in the placebo group (hazard ratio, 0.80; P=.01).
- There was no increase in the risk of cancer, infection, cardiovascular disease, delayed fracture healing, or hypocalcemia, and no cases of osteonecrosis of the jaw or adverse reaction to injection of the drug.
How does denosumab compare with alendronate?
Kendler and associates explored a clinically relevant question: What are the effects of switching from a bisphosphonate (in this case, alendronate) to denosumab?
They studied 504 postmenopausal women who were at least 55 years old, had a T-score between –2 and –4, and had been taking alendronate for at least 6 months. These women were randomized in double-blinded, double-dummy fashion to 60 mg of subcutaneous denosumab or a continuation of 70 mg of oral alendronate. Follow-up was 12 months.
Findings included:
- Among the women making a transition to denosumab, total hip BMD increased by 1.9% at 12 months, versus 1.05% in women continuing on alendronate (P<.0001).
- Women making a transition to denosumab also gained significantly more BMD at 12 months at the lumbar spine, femoral neck, and the distal third of the radius (all P<.0125).
- The transition to denosumab reduced bone turnover to a greater degree than did continuing alendronate.
What happens when denosumab is discontinued?
Miller and colleagues randomized postmenopausal women who had a lumbar spine T-score of –1.8 to –4.0 or a proximal femur T-score of –1.8 to –3.5 to one of the following arms:
- denosumab every 3 months (6, 14, or 30 mg)
- denosumab every 6 months (14, 60, 100, or 210 mg)
- open-label oral alendronate every week (70 mg)
- placebo.
After 24 months, women taking denosumab either:
- continued treatment at 60 mg every 6 months for an additional 24 months
- discontinued therapy
- discontinued treatment for 12 months and then reinitiated denosumab at 60 mg every 6 months for an additional 12 months.
The placebo cohort was maintained throughout this period.
Continuous, long-term denosumab increased BMD at the lumbar spine (9.4% to 11.8%) and total hip (4.0% to 6.1%). In contrast, discontinuation of denosumab was associated with a decrease in BMD of 6.6% at the lumbar spine and 5.3% at the total hip within the first 12 months. Retreatment with denosumab increased lumbar spine BMD by 9% from the original baseline values. Levels of bone turnover markers increased upon discontinuation of denosumab and decreased with retreatment. Adverse events occurred at similar rates in all treatment groups.
Among postmenopausal women who have low bone mineral density (BMD), long-term treatment with denosumab leads to gains in BMD and a reduction of markers of bone turnover. These effects are fully reversible upon discontinuation of the drug, but reoccur when treatment is restored.
In addition, switching from alendronate to denosumab produces a greater reduction in bone turnover than does continuation on the bisphosphonate.
Denosumab is a safe, extremely potent agent that will undoubtedly find a place in the ObGyn armamentarium, where it will join bisphosphonates, selective estrogen receptor modulators, and hormone therapy. The long dosing interval (every 6 months) should help increase compliance.
TSEC, a novel compound, enters development
Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045–1052.
Although raloxifene is the only selective estrogen receptor modulator (SERM) approved by the FDA for prevention and treatment of osteoporosis, numerous other SERMs have been explored for this application. One new category of drug is the tissue selective estrogen complex (TSEC), which pairs a SERM with an estrogen. This was previously attempted unsuccessfully with raloxifene and 17β-estradiol. The ideal SERM–estrogen combination would have the positive attributes of both classes of drugs with fewer, or none, of the undesired effects. An appropriate TSEC would therefore alleviate hot flushes, treat vulvar and vaginal atrophy, and protect against bone loss without stimulating the endometrium and increasing the risk of breast cancer.
One third-generation SERM that has been investigated for its utility in this regard is bazedoxifene (BZA), which produced endometrial thickness and amenorrhea rates comparable to those of placebo. It also produced greater gains in BMD at the lumbar spine in a 2-year, randomized, double-blind, placebo-controlled trial.3 The incidence of new vertebral fracture was significantly lower with BZA than with placebo in a study of postmenopausal women with osteoporosis.4
Given these favorable data, a TSEC containing BZA and conjugated equine estrogen was designed as a potential new comprehensive menopausal therapy.
Substudies suggest bazedoxifene has promise
Lindsay and associates reported on two osteoporosis substudies of the Selective estrogen Menopause and Response to Therapy (SMART) Trial. The main study, which evaluated the incidence of endometrial hyperplasia at 12 months, will be reported elsewhere; it was a multicenter, double-blind, randomized, placebo-controlled phase 3 trial that enrolled 3,397 women.
Substudy I involved women who were more than 5 years postmenopausal, and Substudy II included women who were between 1 and 5 years postmenopausal. Eligible screened subjects were randomly assigned to one of the following treatment groups:
- bazedoxifene (10, 20, or 40 mg), each with conjugated equine estrogen (0.625 or 0.45 mg)
- raloxifene (60 mg)
- placebo.
To maintain blinding, the combination of BZA and conjugated equine estrogen was provided as a single, encapsulated tablet to match the placebo, as was raloxifene. Subjects were directed to take one tablet orally at approximately the same time each day for 2 years. The primary outcome for both substudies was a change in BMD at the lumbar spine, as measured by dual-energy x-ray absorptiometry.
In both substudies, BMD increased to a greater degree at the lumbar spine and total hip at all BZA-estrogen dosages than with placebo, and it increased to a greater degree at the lumbar spine at most BZA-estrogen dosages, compared with raloxifene.
Osteocalcin and N-telopeptide significantly decreased at all BZA-estrogen dosages, compared with placebo, and at most BZA-estrogen dosages, compared with raloxifene.
Bazedoxifene is a potential therapeutic agent for menopausal women that may protect the endometrium while preventing bone loss in a population at higher risk of osteoporosis. It is not yet approved for this indication; further investigation is needed.
The combination of an estrogen and a selective estrogen receptor modulator to potentially relieve vasomotor symptoms, prevent vulvovaginal atrophy, and preserve bone mass without stimulating the endometrium or increasing the risk of breast cancer or venous thromboembolism is very exciting—and just might revolutionize treatment of menopausal women
Bolognese M, Krege JH, Utian WH, et al. Effects of arzoxifene on bone mineral density and endometrium in postmenopausal women with normal or low bone mass. J Clin Endocrinol Metab. 2009;94:2284–2289.
The benzothiophene derivative arzoxifene, which has been in development for the prevention and treatment of osteoporosis, as well as for reduction of the risk of invasive breast cancer in postmenopausal women, has been pulled from the regulatory approval process by its manufacturer, Eli Lilly.
This move is somewhat surprising because, in a phase 3 trial, arzoxifene significantly increased bone mineral density at the lumbar spine (2.9%) and total hip (2.2%), compared with placebo. It also decreased levels of biochemical markers of bone metabolism.
In the trial, Bolognese and associates randomly assigned 331 postmenopausal women who had normal or low bone mass to receive 20 mg of arzoxifene or placebo daily for 2 years.
The trial also found that changes in breast density were neutral or slightly decreased in the arzoxifene group, and there was no evidence of endometrial hyperplasia or carcinoma, based on central review of baseline and follow-up endometrial biopsies. Nor were there any significant differences between groups in endometrial thickness, as assessed by transvaginal ultrasonography, or in the incidence of uterine polyps, vaginal bleeding, and hot flushes.
Nevertheless, Lilly issued a press release in late August announcing that arzoxifene would not be submitted to the FDA for regulatory review.5 It appears that, although initial results from the “pivotal” 5-year, phase 3 GJAD “Generations” trial indicated that the drug had met the primary endpoints of significantly reducing the risk of vertebral fracture and invasive breast cancer in postmenopausal women, “the study failed to demonstrate a statistically significant difference in key secondary efficacy endpoints, such as nonvertebral fractures, clinical vertebral fractures, cardiovascular events, and cognitive function, compared with placebo.”
The release went on to say: “In addition, certain adverse events, including venous thromboembolic events, hot flushes, and gynecological-related events, were reported more frequently in the arzoxifene group, compared with placebo.”5
Arzoxifene therefore joins an expanding list of selective estrogen receptor modulators (SERMs) that did not make it out of clinical trials: idoxifene, droloxifene, levormeloxifene, and lasofoxifene (approved in Europe, however), to name a few. The fall of arzoxifene again underscores the notion that “not all SERMs are created equal.”6
The author reports that he serves on the advisory boards of Amgen, Boehringer Ingelheim, Depomed, Eli Lilly, and Novo Nordisk. He is a speaker for the Alliance for Bone Health, Eli Lilly, and Warner Chilcott.
Some hormonal contraceptives may affect bone density
Berenson AB, Rahman M, Breitkopf CR, Bi LX. Effects of depot medroxyprogesterone acetate and 20-microgram oral contraceptives on bone mineral density. Obstet Gynecol. 2008;112:788–799.
American College of Obstetricians and Gynecologists. ACOG Committee Opinion. No. 415. September 2008. Depot medroxyprogesterone acetate and bone effects. Obstet Gynecol. 2008;112:727–730.
A woman’s contraceptive choice may affect her bone mineral density (BMD)—particularly if she chooses depot medroxyprogesterone acetate (DMPA) or a very-low-dose oral contraceptive (OC) as her method.
In the case of DMPA, studies have shown that its use for 2 years significantly impairs BMD at the hip and spine, regardless of the patient’s age, although BMD usually rebounds after discontinuation of the drug.1
In the case of very-low-dose OCs, there is evidence that young women who use a pill that contains only 20 μg of ethinyl estradiol have a lower increase in BMD than do women the same age who do not use hormonal contraception. (OCs that contain a higher dosage of ethinyl estradiol have not been shown to hamper BMD.) A study by Polatti and colleagues reported a 7.8% increase in BMD over 5 years among women 19 to 22 years old who did not use OCs, compared with no change in BMD among women who used OCs containing 20 μg of ethinyl estradiol.2
DMPA, BMD, and the FDA
The deleterious effect of DMPA on BMD is particularly relevant in perimenopausal women, who have already begun to experience the age-related decline in BMD that starts around the age of 30. The effect is also troubling in adolescents, who normally experience a large accretion of bone during the teen and early adult years.
In 2004, the US Food and Drug Administration (FDA) added a boxed warning to DMPA labeling advising women to limit their use of the drug to 2 years. Since that time, other studies have found that BMD increases to a greater degree among past users of DMPA than among never users—suggesting that DMPA-related bone loss is reversible.
ACOG: DMPA can be used longer than 2 years in some
In September 2008, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion acknowledging the association between DMPA and BMD loss. The committee pointed out, however, that “current evidence suggests that partial or full recovery of BMD occurs at the spine and at least partial recovery occurs at the hip after discontinuation of DMPA” ( FIGURE ).
The ACOG opinion also noted that, “given the efficacy of DMPA, particularly for populations such as adolescents, for whom contraceptive adherence can be challenging, or for those who feel they could not comply with a daily contraceptive method or a method that must be used with each act of intercourse, the possible adverse effects of DMPA must be balanced against the significant personal and public health impact of unintended pregnancy.”
The committee recommended that, despite concerns about bone loss, practitioners should not hesitate to prescribe DMPA. Nor should they limit its use to 2 consecutive years or perform BMD monitoring solely in response to DMPA use. “Any observed short-term loss in BMD associated with DMPA use may be recovered and is unlikely to place a woman at risk of fracture during use or in later years,” the committee opinion noted.

FIGURE DMPA-related bone loss is largely reversible
Depot medroxyprogesterone acetate (DMPA) is associated with a loss of bone mineral density at the hip and spine. Once the drug is discontinued, however, bone density appears to recover at least partially at both sites.
Study explores bone gains after DMPA
Berenson and associates measured BMD every 6 months for as long as 3 years in 703 white, African-American, and Hispanic women who used an OC, DMPA, or nonhormonal contraception. They also measured BMD for up to 2 additional years in 68 women who discontinued DMPA. They found no differences between races—although they did find the expected DMPA-associated bone loss.
They also found a small amount of bone loss (0.5% at the lumbar spine and 1.3% at the femoral neck) in users of very-low-dose OCs (20 μg ethinyl estradiol), compared with a gain of 1.9% at the lumbar spine and 0.6% at the femoral neck in nonusers of hormonal contraception.
Women who made a transition from DMPA to a very-low-dose OC recovered bone mass slowly. After DMPA was discontinued, women who selected nonhormonal contraception recovered BMD (4.9% at the spine, 3.2% at the femoral neck)—unlike those who chose a very-low-dose OC, who regained BMD at the spine (2.3%) but not the femoral neck (–0.7%).
Use of very-low-dose oral contraception (20 μg ethinyl estradiol) may lead to a small amount of bone loss or failure of bone accretion. Use of depot medroxyprogesterone acetate (DMPA) is associated with a greater degree of bone loss, but this loss is largely reversible at the spine. Use of a 20-μg oral contraceptive (OC) after discontinuation of DMPA may slow bone recovery.
As ACOG has indicated, concerns about skeletal health should not influence the decision to initiate or continue DMPA. Likewise, such concerns should not lead to restrictions on the use of OCs in teens or adult women. However, clinicians may wish to take bone effects into consideration when choosing the estrogen dosage of OCs for women younger than 30 who have yet to attain peak bone mass.
Denosumab nears FDA approval for treatment of osteoporosis
Cummings SR, San Martin J, McClung MR, et al; FREEDOM trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765.
Kendler DL, Roux C, Benhamou CL, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res. 2009 [Epub ahead of print].
Miller PD, Bolognese MA, Lewiecki EM, et al, for the Amg Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43:222–229.
An FDA panel advising the Division of Reproductive and Urologic Products voted to approve denosumab (proposed brand name: Prolia) as a treatment for osteoporosis. The drug is a fully human monoclonal antibody to the receptor activator of nuclear factor-B ligand (RANKL), a cytokine that is essential for the formation, function, and survival of osteoclasts. By binding RANKL, denosumab prevents interaction between RANKL and its receptor, RANK, on osteoclasts and osteoclast precursors and thereby inhibits osteoclast-mediated bone resorption. Its effects are reversible.
Trial: Denosumab versus placebo
Cummings and colleagues reported the findings of the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial, which involved 7,868 women 60 to 90 years old who had a BMD T-score between –2.5 and –4.0 at the lumbar spine or total hip. Participants were randomly assigned to receive 60 mg of denosumab or placebo subcutaneously every 6 months for 36 months. The primary endpoint was new vertebral fracture. Secondary endpoints included nonvertebral and hip fractures.
Findings included:
- Denosumab reduced the risk of new, radiographically detected vertebral fracture, with a cumulative incidence of 2.3%, versus 7.2% in the placebo group (risk ratio, 0.32; P<.001).
- Denosumab reduced the risk of hip fracture, with a cumulative incidence of 0.7% in the denosumab group, versus 1.2% in the placebo group (hazard ratio, 0.60; P=.04).
- Denosumab reduced the risk of nonvertebral fracture, with a cumulative incidence of 6.5% in the denosumab group, versus 8.0% in the placebo group (hazard ratio, 0.80; P=.01).
- There was no increase in the risk of cancer, infection, cardiovascular disease, delayed fracture healing, or hypocalcemia, and no cases of osteonecrosis of the jaw or adverse reaction to injection of the drug.
How does denosumab compare with alendronate?
Kendler and associates explored a clinically relevant question: What are the effects of switching from a bisphosphonate (in this case, alendronate) to denosumab?
They studied 504 postmenopausal women who were at least 55 years old, had a T-score between –2 and –4, and had been taking alendronate for at least 6 months. These women were randomized in double-blinded, double-dummy fashion to 60 mg of subcutaneous denosumab or a continuation of 70 mg of oral alendronate. Follow-up was 12 months.
Findings included:
- Among the women making a transition to denosumab, total hip BMD increased by 1.9% at 12 months, versus 1.05% in women continuing on alendronate (P<.0001).
- Women making a transition to denosumab also gained significantly more BMD at 12 months at the lumbar spine, femoral neck, and the distal third of the radius (all P<.0125).
- The transition to denosumab reduced bone turnover to a greater degree than did continuing alendronate.
What happens when denosumab is discontinued?
Miller and colleagues randomized postmenopausal women who had a lumbar spine T-score of –1.8 to –4.0 or a proximal femur T-score of –1.8 to –3.5 to one of the following arms:
- denosumab every 3 months (6, 14, or 30 mg)
- denosumab every 6 months (14, 60, 100, or 210 mg)
- open-label oral alendronate every week (70 mg)
- placebo.
After 24 months, women taking denosumab either:
- continued treatment at 60 mg every 6 months for an additional 24 months
- discontinued therapy
- discontinued treatment for 12 months and then reinitiated denosumab at 60 mg every 6 months for an additional 12 months.
The placebo cohort was maintained throughout this period.
Continuous, long-term denosumab increased BMD at the lumbar spine (9.4% to 11.8%) and total hip (4.0% to 6.1%). In contrast, discontinuation of denosumab was associated with a decrease in BMD of 6.6% at the lumbar spine and 5.3% at the total hip within the first 12 months. Retreatment with denosumab increased lumbar spine BMD by 9% from the original baseline values. Levels of bone turnover markers increased upon discontinuation of denosumab and decreased with retreatment. Adverse events occurred at similar rates in all treatment groups.
Among postmenopausal women who have low bone mineral density (BMD), long-term treatment with denosumab leads to gains in BMD and a reduction of markers of bone turnover. These effects are fully reversible upon discontinuation of the drug, but reoccur when treatment is restored.
In addition, switching from alendronate to denosumab produces a greater reduction in bone turnover than does continuation on the bisphosphonate.
Denosumab is a safe, extremely potent agent that will undoubtedly find a place in the ObGyn armamentarium, where it will join bisphosphonates, selective estrogen receptor modulators, and hormone therapy. The long dosing interval (every 6 months) should help increase compliance.
TSEC, a novel compound, enters development
Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045–1052.
Although raloxifene is the only selective estrogen receptor modulator (SERM) approved by the FDA for prevention and treatment of osteoporosis, numerous other SERMs have been explored for this application. One new category of drug is the tissue selective estrogen complex (TSEC), which pairs a SERM with an estrogen. This was previously attempted unsuccessfully with raloxifene and 17β-estradiol. The ideal SERM–estrogen combination would have the positive attributes of both classes of drugs with fewer, or none, of the undesired effects. An appropriate TSEC would therefore alleviate hot flushes, treat vulvar and vaginal atrophy, and protect against bone loss without stimulating the endometrium and increasing the risk of breast cancer.
One third-generation SERM that has been investigated for its utility in this regard is bazedoxifene (BZA), which produced endometrial thickness and amenorrhea rates comparable to those of placebo. It also produced greater gains in BMD at the lumbar spine in a 2-year, randomized, double-blind, placebo-controlled trial.3 The incidence of new vertebral fracture was significantly lower with BZA than with placebo in a study of postmenopausal women with osteoporosis.4
Given these favorable data, a TSEC containing BZA and conjugated equine estrogen was designed as a potential new comprehensive menopausal therapy.
Substudies suggest bazedoxifene has promise
Lindsay and associates reported on two osteoporosis substudies of the Selective estrogen Menopause and Response to Therapy (SMART) Trial. The main study, which evaluated the incidence of endometrial hyperplasia at 12 months, will be reported elsewhere; it was a multicenter, double-blind, randomized, placebo-controlled phase 3 trial that enrolled 3,397 women.
Substudy I involved women who were more than 5 years postmenopausal, and Substudy II included women who were between 1 and 5 years postmenopausal. Eligible screened subjects were randomly assigned to one of the following treatment groups:
- bazedoxifene (10, 20, or 40 mg), each with conjugated equine estrogen (0.625 or 0.45 mg)
- raloxifene (60 mg)
- placebo.
To maintain blinding, the combination of BZA and conjugated equine estrogen was provided as a single, encapsulated tablet to match the placebo, as was raloxifene. Subjects were directed to take one tablet orally at approximately the same time each day for 2 years. The primary outcome for both substudies was a change in BMD at the lumbar spine, as measured by dual-energy x-ray absorptiometry.
In both substudies, BMD increased to a greater degree at the lumbar spine and total hip at all BZA-estrogen dosages than with placebo, and it increased to a greater degree at the lumbar spine at most BZA-estrogen dosages, compared with raloxifene.
Osteocalcin and N-telopeptide significantly decreased at all BZA-estrogen dosages, compared with placebo, and at most BZA-estrogen dosages, compared with raloxifene.
Bazedoxifene is a potential therapeutic agent for menopausal women that may protect the endometrium while preventing bone loss in a population at higher risk of osteoporosis. It is not yet approved for this indication; further investigation is needed.
The combination of an estrogen and a selective estrogen receptor modulator to potentially relieve vasomotor symptoms, prevent vulvovaginal atrophy, and preserve bone mass without stimulating the endometrium or increasing the risk of breast cancer or venous thromboembolism is very exciting—and just might revolutionize treatment of menopausal women
Bolognese M, Krege JH, Utian WH, et al. Effects of arzoxifene on bone mineral density and endometrium in postmenopausal women with normal or low bone mass. J Clin Endocrinol Metab. 2009;94:2284–2289.
The benzothiophene derivative arzoxifene, which has been in development for the prevention and treatment of osteoporosis, as well as for reduction of the risk of invasive breast cancer in postmenopausal women, has been pulled from the regulatory approval process by its manufacturer, Eli Lilly.
This move is somewhat surprising because, in a phase 3 trial, arzoxifene significantly increased bone mineral density at the lumbar spine (2.9%) and total hip (2.2%), compared with placebo. It also decreased levels of biochemical markers of bone metabolism.
In the trial, Bolognese and associates randomly assigned 331 postmenopausal women who had normal or low bone mass to receive 20 mg of arzoxifene or placebo daily for 2 years.
The trial also found that changes in breast density were neutral or slightly decreased in the arzoxifene group, and there was no evidence of endometrial hyperplasia or carcinoma, based on central review of baseline and follow-up endometrial biopsies. Nor were there any significant differences between groups in endometrial thickness, as assessed by transvaginal ultrasonography, or in the incidence of uterine polyps, vaginal bleeding, and hot flushes.
Nevertheless, Lilly issued a press release in late August announcing that arzoxifene would not be submitted to the FDA for regulatory review.5 It appears that, although initial results from the “pivotal” 5-year, phase 3 GJAD “Generations” trial indicated that the drug had met the primary endpoints of significantly reducing the risk of vertebral fracture and invasive breast cancer in postmenopausal women, “the study failed to demonstrate a statistically significant difference in key secondary efficacy endpoints, such as nonvertebral fractures, clinical vertebral fractures, cardiovascular events, and cognitive function, compared with placebo.”
The release went on to say: “In addition, certain adverse events, including venous thromboembolic events, hot flushes, and gynecological-related events, were reported more frequently in the arzoxifene group, compared with placebo.”5
Arzoxifene therefore joins an expanding list of selective estrogen receptor modulators (SERMs) that did not make it out of clinical trials: idoxifene, droloxifene, levormeloxifene, and lasofoxifene (approved in Europe, however), to name a few. The fall of arzoxifene again underscores the notion that “not all SERMs are created equal.”6
1. Scholes D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med. 2005;159:139-144.
2. Polatti F, Perotti F, Filippa N, Gallina D, Nappi RE. Bone mass and long-term monophasic oral contraceptive treatment in young women. Contraception. 1995;51:221-224.
3. Miller PD, Chines AA, Christiansen C, et al. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-year results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res. 2008;23:525-535.
4. Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo- and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
5. Based on preliminary phase III GHAD study results, Lilly concludes arzoxifene’s clinical profile does not support regulatory submission [press release]. Available at http://newsroom.lilly.com/releasedetail.cfm?ReleaseID=403905. Accessed October 2, 2009.
6. Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.
1. Scholes D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med. 2005;159:139-144.
2. Polatti F, Perotti F, Filippa N, Gallina D, Nappi RE. Bone mass and long-term monophasic oral contraceptive treatment in young women. Contraception. 1995;51:221-224.
3. Miller PD, Chines AA, Christiansen C, et al. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-year results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res. 2008;23:525-535.
4. Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo- and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923-1934.
5. Based on preliminary phase III GHAD study results, Lilly concludes arzoxifene’s clinical profile does not support regulatory submission [press release]. Available at http://newsroom.lilly.com/releasedetail.cfm?ReleaseID=403905. Accessed October 2, 2009.
6. Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.
The case for chemoprevention as a tool to avert breast cancer
The author reports that he is a consultant to Eli Lilly, Pfizer, and Wyeth, and a speaker for Eli Lilly and Wyeth.
CASE 1: Premenopausal woman
at high risk of breast cancer
R. J. is a 43-year-old, nulliparous woman who reached menarche at age 11. She has undergone two breast biopsies, the most recent of which revealed ductal hyperplasia with marked atypia.
R. J.’s sister had breast cancer at 49 years of age; her mother had breast cancer at 66 years. Because of R. J.’s family history, she underwent testing for a BRCA mutation. The result was negative.
R. J. has come to your office today to find out if she can do anything to reduce her risk of breast cancer. What options can you offer?
The most common method of “prevention” of breast cancer involves early detection and assessment of abnormalities through frequent surveillance with mammography. Some women who have dense breasts, a history of breast biopsy, or other risk factors for breast cancer may benefit from intensive surveillance with both mammography and ultrasonography—and, in some cases, magnetic resonance imaging.
More aggressive options include:
- the use of a chemopreventive agent such as tamoxifen or raloxifene
- in rare cases—usually when a BRCA mutation is present—prophylactic mastectomy.
Before it is possible to determine the optimal approach for a particular woman, it is necessary to conduct an individualized assessment of her risk—that is, to estimate the probability that she will develop breast cancer over a defined period of time. Such an estimate is also useful for designing prevention trials in high-risk subsets of the population. (Prevention trials differ from therapeutic clinical trials in that asymptomatic healthy women are exposed to potentially toxic interventions for prolonged periods to reduce their risk of breast cancer.)
This article describes chemopreventive options for women at high risk, based on individualized risk assessment using the Gail model.
(Editor’s note: For additional discussion of the important role ObGyns play in the fight against breast cancer, see Editor in Chief Dr. Robert L. Barbieri’s Editorial.)
You can estimate the likelihood that a woman like your patient may develop breast cancer using various individual risk factors ( TABLE 1 ), but estimates for combinations of risk factors are preferable. The Gail model takes into account some nongenetic factors, such as parity and age at menarche, but also genetic factors, such as family history. The model calculates a woman’s individualized breast cancer probability and yields a numerical risk (a percentage) that she will develop invasive breast cancer over the next 5 years; it also yields an estimate of her risk of developing the malignancy over the remainder of her life.1,2
A Gail-model 5-year estimate of 1.66% or higher denotes a high risk of developing breast cancer. That benchmark was the one employed in the Breast Cancer Prevention Trial (BCPT), conducted as part of the National Surgical Adjuvant Breast and Bowel Project (NSABP).3
TABLE 1
What are the risk factors for breast cancer?
And what degree of relative risk do they confer?
| Relative risk | ||
|---|---|---|
| <2 | 2–4 | >4 |
| • Age 25–34 years at first live birth • Early menarche • Late menopause • Benign proliferative disease • Postmenopausal obesity • Alcohol use • Hormone replacement therapy | • Age >35 years at first live birth • First-degree relative with breast cancer • Nulliparity • Radiation exposure • Personal history of breast cancer | • Gene mutation (BRCA 1 or 2) • Lobular carcinoma in situ • Ductal carcinoma in situ • Atypical hyperplasia |
| Adapted from Bilimoria and Morrow23 | ||
Weaknesses of the Gail model
The Gail model’s approach to estimating risk has some limitations. The model uses the number of prior breast biopsies in its assessment—but the relative risk associated with prior biopsy is smaller for women older than 50 years than it is for younger women.
Furthermore, data on which Gail bases its estimates were collected in the late 1970s and early 1980s. Since then, the increasing ease of breast histopathologic assessment—through fine-needle aspiration and outpatient core-needle biopsy—has confused the issue of just what constitutes a breast “biopsy.” (Most patients surveyed consider it to be any histologic sampling of the breast.)
As a result, the 1.66% cutoff becomes somewhat difficult to interpret in light of current practice.
Consider the following example. A 50-year-old nulliparous Caucasian woman reached menarche when she was 11 years old, has never had a biopsy, and has no first-degree relatives with breast cancer. According to the Gail model, her risk of developing breast cancer is 1.2% over the next 5 years and 10.8% in her lifetime. Therefore, she is not considered at high risk. If she were to give a history of three previous breast biopsies, however, none of them showing hyperplasia, her 5-year risk would rise to 1.8% and push her over the line into the high-risk category.
Compare her situation to that of R. J., the nulliparous woman described in Case 1. R. J. also reached menarche at 11 years, but she has had two breast biopsies (one of which showed atypical hyperplasia) and has two first-degree relatives who have had breast cancer. Her Gail score shows a 5-year risk of breast cancer of 13.5% (the norm for a 43-year-old woman is 0.8%), and a lifetime risk of 69.2%. Clearly, she has a high risk of breast cancer.
How do we improve an imperfect science?
We need to identify objective findings that are patient-specific but highly correlative with the development of breast cancer. Patient-specific biomarkers have been proposed, such as ultrasensitive measurement of the serum estradiol level in postmenopausal women. In the Multiple Outcomes of Raloxifene Evaluation, also known as the MORE trial, women who experienced the greatest reduction in the rate of breast cancer during treatment with raloxifene were a subgroup who had the highest baseline level of serum estradiol—although, overall, all patients had an estradiol level well within the postmenopausal range (≤20 pmol/L).4,5
How tamoxifen became a chemopreventive agent
Tamoxifen inhibits mammary tumors in mice and rats and suppresses hormone-dependent breast cancer cell lines in vitro.6 Clinical data from the Early Breast Cancer Trialists’ Collaborative Group yielded additional motivation for prevention trials with tamoxifen: Besides reducing the rate of recurrent breast cancer, tamoxifen reduced the risk of contralateral new-onset breast cancer by 47% after 5 years of adjuvant treatment.7 Preclinical findings in vitro and in animal models, coupled with clinical data and evidence of tamoxifen’s favorable effects on skeleton remodeling and lipid levels, led to a series of chemoprevention trials in the United States and Europe using tamoxifen.
In the aforementioned BCPT, launched in 1992, 13,388 women 35 years and older who were deemed to be at high risk of developing breast cancer were enrolled at numerous sites throughout the United States and Canada.3 The Gail model was used to select women for the trial—only those who had a 5-year risk of 1.66% or higher were included. Participants were randomly assigned to receive tamoxifen 20 mg or placebo daily for 5 years. The trial was terminated early because of the dramatic reduction in new-onset breast cancer with tamoxifen, compared with placebo.
The overall incidence of breast cancer in the tamoxifen group was 3.4 cases for every 1,000 women, compared with 6.8 cases for every 1,000 women receiving placebo.3 Overall, the reduction in invasive breast cancer with tamoxifen was 49% (P<.00001). When broken down by age group, the reduction was:
- 44% in women 35 to 49 years old
- 51% in women 50 to 59 years old
- 55% in women 60 years and older.
Even noninvasive breast cancer was reduced with tamoxifen
Tamoxifen decreased the incidence of noninvasive breast cancer (ductal carcinoma in situ [DCIS]) by 50%. Expanded use of mammography has increased the detection of DCIS. Most DCIS lesions appear to be estrogen-receptor positive.8
In addition, tamoxifen reduced breast cancer risk in women who had a history of lobular carcinoma in situ (LCIS), a precancer, by 56%, and it reduced the risk of breast cancer in women who had a history of atypical hyperplasia by 86%. Overall, tamoxifen reduced the occurrence of estrogen-positive tumors by 69%, but had no impact on the incidence of estrogen-receptor–negative tumors.
The BCPT was stopped 14 months before planned because the Data and Safety Monitoring Board felt it was unethical to continue to allow one half of such high-risk participants to take placebo in light of the dramatic reduction in both invasive and noninvasive breast cancer among women who took tamoxifen.
In postmenopausal women, tamoxifen increases some risks
Two secondary endpoints of the BCPT are worthy of consideration:
- The overall relative risk (RR) of endometrial cancer associated with tamoxifen therapy in healthy women was 2.53 (95% confidence interval [CI], 1.35, 4.97). However, further analysis by age yielded a RR of 4.01 in women who were older than 50 years (95% CI, 1.70, 10.90), compared with a RR of 1.21 in women 49 years and younger (95% CI, 0.41, 3.60).
- The same age distinction held true for deep venous thrombosis (DVT) and pulmonary embolus, with no statistically significant increases in either in women 49 years and younger, but a RR of 1.71 and 3.19, respectively, in women 50 years and older. It is unclear whether the trial was sufficiently powered for this particular secondary endpoint.
These findings suggest that serious adverse events do not occur at the same magnitude in women younger than 50 years that they do in women 50 and older. The difference in the risk–benefit profile between younger and older women has significant clinical implications for the care of perimenopausal patients.
Risk of other malignancies was not affected by tamoxifen
Overall, invasive cancers other than those of the breast and uterus occurred at the same rate in the tamoxifen and placebo groups of the BCPT. The RR of death from any cause was 0.81 (95% CI, 0.56–1.16). There was a slight increase in the risk of myocardial infarction (RR, 1.11; 95% CI, 0.65–1.92) and a slight decrease in the risk of severe angina (RR, 0.93; 95% CI, 0.40–2.14) in tamoxifen users, although neither of these risks was statistically significant.
The overall RR of fracture of the hip, spine, or radius was 0.81 (95% CI, 0.63–1.05). There was a statistically significant increase in the number of women who had cataracts who then underwent cataract surgery in the tamoxifen group (RR, 1.57; 95% CI, 1.16–2.14).
Tamoxifen is approved as a preventive for high-risk women only
Based on the results of the BCPT, the US Food and Drug Administration (FDA) approved tamoxifen in October 1998 for the primary prevention of breast cancer in women who are at high risk of the disease. The FDA recommends that use of tamoxifen be limited to women at high risk because of the potentially serious side effects seen in clinical trials, including the BCPT.
The FDA did not define “high risk,” but it did recommend that the decision to use tamoxifen as chemopreventive therapy be based on thorough evaluation of the patient’s personal, family, and medical histories; her age; and her understanding of the risks and benefits of treatment.
The FDA also required the following language in the package insert:
- You should not take tamoxifen to reduce the risk of breast cancer unless you are at high risk of breast cancer. Certain conditions put women at high risk, and it is possible to calculate this risk for any woman. Breast cancer risk-assessment tools to help calculate your risk of breast cancer have been developed and are available to your health-care professional. You should discuss your risk with your healthcare professional.
CASE 1 RESOLVED
You determine that R. J. is an excellent candidate for tamoxifen by virtue of her significant risk of breast cancer. You are able to reassure her that, as the BCPT demonstrated, tamoxifen should not increase the risk of uterine cancer, DVT, or pulmonary embolism in a woman her age.
Raloxifene
CASE 2: Patient worries about breasts and bones
S. T. is a 58-year-old Caucasian mother of two whose own mother had breast cancer when she was 74 years old, and whose older sister was given a diagnosis of the malignancy 4 years ago.
S. T. had her first period when she was 11 years old, delivered her first child when she was 31, and entered menopause when she was 52. She is 5 ft 5 in tall and weighs 144 lb.
Her main reason for visiting you today is a breast Mammotome biopsy that showed ductal hyperplasia with atypia. She has been tested for a BRCA mutation, but the result was negative. Her Gail-model score is a 9.7% risk of developing breast cancer over the next 5 years, and a lifetime risk of 44.2%.
She also asks about osteoporosis prevention, given that a dual-energy x-ray absorptiometry (DXA) scan 1 year ago yielded a T-score of –1.3 for her hip and –1.1 for her spine. Her World Health Organization FRAX 10-year risk of hip fracture is 0.7%, and her risk of major osteoporotic fracture is 8.6%.
How do you respond to her concerns?
This patient has a high risk of invasive breast cancer but does not meet criteria for pharmacotherapy for osteoporosis prevention. A good option for her would be raloxifene, a selective estrogen-receptor modulator (SERM) that has been shown to reduce the risk of breast cancer as well as osteoporosis. S. T. would benefit from it on the basis of its breast benefit alone.
The genesis of a drug with multiple benefits
Raloxifene is a benzothiophene derivative, unlike the triphenylethylene family from which tamoxifen is derived. Like tamoxifen, raloxifene was originally investigated as a treatment for advanced breast cancer.
Preclinical studies indicated that raloxifene had an antiproliferative effect on both estrogen-receptor–positive mammary tumors and estrogen-receptor–positive human breast cancer cell lines.9 In the 1980s, however, a small, phase-II trial revealed that raloxifene had no further antitumor effects in postmenopausal women with advanced breast cancer in whom tamoxifen had failed.10 After information surfaced about the neoplastic effect of tamoxifen on the uteri of postmenopausal women, interest in raloxifene revived.11
Raloxifene has estrogen-agonistic activity on bone remodeling and lipid metabolism and was approved by the FDA for prevention of osteoporosis in postmenopausal women in December 1997. Its indication was extended to treatment of osteoporosis 2 years later.
Raloxifene appears to have no effect on the endometrium of postmenopausal women, compared with placebo. In a 12-month comparative trial, there was no difference in endometrial thickness, endoluminal masses, proliferation, or hyperplasia between the raloxifene and placebo groups.12 This finding corroborates earlier evidence that raloxifene does not cause endometrial hyperplasia or cancer and is not associated with vaginal bleeding or increased endometrial thickness, as measured by transvaginal ultrasonography.
A big difference between raloxifene and tamoxifen, therefore, is their varying effect on the uterus of postmenopausal women.
Additional clinical trials confirm anticancer action of raloxifene
Preclinical data in animal models suggested that, like tamoxifen, raloxifene has potent antiestrogenic effects on breast tissue.9 The MORE trial involved 7,705 postmenopausal women up to 80 years old who had established osteoporosis.13 In that trial, participants were randomized to raloxifene or placebo. Bone mineral density (BMD) and fracture incidence were the primary endpoints; breast cancer was a secondary endpoint.
Over the 4 years of the trial, raloxifene significantly reduced the incidence of all invasive breast cancers by 72%, compared with placebo (RR, 0.28; 95% CI, 0.17–0.46). Raloxifene also significantly reduced the incidence of invasive estrogen-receptor–positive tumors by 84%, compared with placebo (RR, 0.16; 95% CI, 0.09–0.30), but had no effect on estrogen-receptor–negative tumors. The incidence of vaginal bleeding, breast pain, and endometrial cancer in the raloxifene group did not differ significantly from that of the placebo group.
Like tamoxifen, raloxifene appeared to be associated with an increased risk of thromboembolic disease, including DVT and pulmonary embolism, which developed in 1.1% of women taking raloxifene, compared with 0.5% of women in the placebo group (P=.003).
In a 4-year continuation of the MORE trial, known as the Continuing Outcomes Relevant to Evista, or CORE, trial, 5,231 women were randomized to continue raloxifene or placebo.14 Over the 8 years of the combined trials, the incidence of invasive breast cancer was reduced by 66% in the raloxifene group (RR, 0.34; 95% CI, 0.22–0.50). The 8-year data are extremely clinically relevant, in that raloxifene has no time limit, whereas tamoxifen is usually prescribed for no longer than 5 years.
Raloxifene is not approved for use in premenopausal women. SERM compounds, which are structurally similar to clomiphene citrate, seem to have different effects in premenopausal and postmenopausal women, as evidenced by tamoxifen’s differing effects by age in the BCPT.
Other investigations of raloxifene confirm its value in high-risk women
To compare the clinical safety and efficacy of tamoxifen and raloxifene in reducing the risk of breast cancer among healthy women, the Study of Tamoxifen and Raloxifene (STAR) was initiated in 1999.15 In that trial, 19,747 postmenopausal women older than 35 years were blindly assigned to raloxifene 60 mg or tamoxifen 20 mg daily.
Baseline characteristics of subjects in STAR are summarized in TABLE 2 . Mean age was 58.5 years. All women had a 5-year risk of developing breast cancer that exceeded 1.66%, according to the Gail model. The average Gail score was 4.03% (standard deviation, ±2.17%). Because it would have been unethical to subject high-risk women to a placebo group in light of the findings of the BCPT, there was no placebo control.
TABLE 2
Baseline characteristics of women
in the Study of Tamoxifen and Raloxifene (STAR) trial
| Characteristic | Value |
|---|---|
| Age (mean) | 58.5 years |
| Caucasian | 93% |
| Hysterectomy | 51% |
| At least one first-degree relative with breast cancer | 71% |
| Lobular carcinoma in situ | 9% |
| Atypical hyperplasia | 23% |
| 5-year risk of invasive breast cancer (mean)* | 4.03% |
| *As estimated with the Gail model Risk Calculator. | |
Here are noteworthy findings of the STAR trial:
- 163 cases of invasive breast cancer occurred in the tamoxifen group, compared with 168 among women taking raloxifene (RR, 1.02; 95% CI, 0.82–1.28).
- 36 cases of uterine cancer occurred in the tamoxifen group, compared with 23 among women taking raloxifene (RR, 0.62; 95% CI, 0.35–1.08). Earlier studies had shown a marked difference in the rate of uterine cancer between these agents. Although the difference here is not statistically significant, uterine cancer was not an endpoint of the study; nor was the study powered to explore this difference.
- The number of hysterectomies among women who were diagnosed with endometrial hyperplasia with or without atypia was, proportionally, significantly higher among women taking tamoxifen ( TABLE 3 ).
- No difference between groups was found for other invasive cancers, ischemic heart events, or stroke.
- Thromboembolic events occurred less frequently in the raloxifene group (RR, 0.70; 95% CI, 0.54–0.91). However, both raloxifene and tamoxifen have consistently been associated with a twofold to threefold increase in the risk of thromboembolic events, compared with placebo.
- Vasomotor symptoms and leg cramps increased in frequency and severity among women in both groups of the trial. These symptoms appear to be less common and less severe among women who are older and more remote from the onset of menopause.
TABLE 3
Relative risk of hysterectomy and uterine hyperplasia during STAR
| Characteristic | Women who took tamoxifen | Women who took raloxifene | Relative risk (95% confidence interval) |
|---|---|---|---|
| Hysterectomy during study | 246 | 92 | 0.37 (0.28, 0.47) |
| Hyperplasia • with atypia • without atypia | 100 15 85 | 17 2 15 | 0.17 (0.09, 0.28) 0.13 (0.01, 0.56) 0.17 (0.09, 0.30) |
What is raloxifene’s effect on the heart?
The Raloxifene Use for The Heart (RUTH) trial explored the primary endpoints of coronary artery disease (CAD) and breast cancer in more than 10,000 women who had CAD or multiple risk factors for it.16 This study began prior to the Women’s Health Initiative, at a time when hormone replacement therapy was widely believed to reduce CAD.
In the double-blinded, randomized, placebo-controlled RUTH trial, raloxifene had no significant effect on primary coronary events (533 vs 553; hazard ratio [HR], 0.95; 95% CI, 0.84–1.07). Even in this population, however, there was a 44% reduction in invasive breast cancer (40 vs 70 events; HR, 0.56; 95% CI, 0.38–0.83).
Based on these results, the FDA approved raloxifene for the “reduction in risk of invasive breast cancer in postmenopausal women at high risk for breast cancer,” as well as for the “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” ( FIGURE ).
FIGURE How raloxifene reduced invasive breast cancer in three trials
Raloxifene significantly reduced the risk of cancer, compared with placebo, in the Raloxifene Use for The Heart (RUTH), Multiple Outcomes of Raloxifene Evaluation (MORE), and Continuing Outcomes Relevant to Evista (CORE) trials.
CASE 2 RESOLVED
S. T. begins taking raloxifene 60 mg daily to lower her risk of invasive breast cancer. Although she temporarily experienced hot flashes after initiating the drug, they are only mildly bothersome, and she continues raloxifene therapy. She says she is grateful that there is an agent that can help her reduce the likelihood that she will develop breast cancer, and protection of her BMD is an added benefit.
CASE 3: At risk for both breast cancer and bone fracture
A. N., 63, is a nulliparous Caucasian woman who weighs 134 lb and stands 5 ft 4 in tall. She reached menarche when she was 12 years old and entered menopause at 49.
Although A. N. has never had a breast abnormality, her 59-year-old sister was just given a diagnosis of breast cancer. Her Gail score reveals that she has a 3.1% risk of developing breast cancer over the next 5 years.
In addition to her concerns about breast cancer, A. N. is worried about hip fracture—because her mother suffered one after menopause and because her T-score is –1.9 at the hip and –2.1 at the spine. A. N. has used steroids off and on for much of her life for asthma. Her FRAX score indicates that she has a 2.8% risk of hip fracture and a 25% risk of major osteoporotic fracture over the next 10 years.
What do you offer her?
Because of new FRAX criteria, this osteopenic woman is now a candidate for medication to reduce her risk of major osteoporotic fracture, and raloxifene is a good option. Her Gail score of 3.1% also makes her a good candidate for breast cancer risk reduction with raloxifene.
CASE 3 RESOLVED
Because A. N. needs an agent that benefits both breast and bone, you prescribe raloxifene. The drug should significantly reduce her risk of both invasive breast cancer and bone fracture, without increasing her risk of endometrial hyperplasia and cancer, both of which are associated with tamoxifen in her age group.
Aromatase inhibitors
A fairly new class of drugs being explored for their ability to reduce the risk of breast cancer is aromatase inhibitors. Substantial evidence suggests that estrogens facilitate the development of breast cancer in animals and in women, although the precise mechanism remains unknown.17 The most commonly held theory is that estrogen stimulates proliferation of breast cells and thereby increases the risk of genetic mutation that could lead to cancer.
Aromatase inhibitors block peripheral conversion of androstenedione to estrogens. In premenopausal women, the primary site of this action is in the ovary. In postmenopausal women, this conversion occurs primarily in extraovarian sites, including the adrenal glands, adipose tissue, liver, muscle, and skin.
Aromatase inhibitors may be more effective than SERMs in preventing breast cancer because of their dual role: blocking both the initiation and promotion of breast cancer.18 These agents reduce levels of the genotoxic metabolites of estradiol by lowering estradiol concentration in tissue. At the same time, aromatase inhibitors also block tumor promotion by lowering tissue levels of estrogen and preventing cell proliferation.
The main drawback of these agents—besides the fact that they are not FDA-approved for reducing risk—is their antiestrogenic effect on bone and lipid metabolism. They also induce vasomotor symptoms.
Studies of third-generation aromatase inhibitors in the prevention of breast cancer are under way in high-risk women. These agents include anastrozole, exemestane, and letrozole.
1. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
2. Breast Cancer Assessment Tool. Available at: www.cancer.gov/bcrisktool/Default.aspx. Accessed June 5, 2009.
3. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
4. Ruffin MT, 4th, August DA, Kelloff GJ, Boone CW, Weber BL, Brenner DE. Selection criteria for breast cancer chemoprevention subjects. J Cell Biochem Suppl. 1993;17G:234-241.
5. Cummings SR, Duong T, Kenyon E, Cauley JA, Whitehead M, Krueger KA. For the Multiple Outcomes of Raloxifene Evaluation (MORE) Trial. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220.
6. Jordan VC, Allen KE. Evaluation of the antitumor activity of the non-steroidal antioestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma mode. Eur J Cancer. 1980;16:239-251.
7. Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988;319:1681-1692.
8. Bur ME, Zimarowski MJ, Schnitt SJ, Baker S, Lew R. Estrogen receptor immunohistochemistry in carcinoma in situ of the breast. Cancer. 1992;69:1174-1181.
9. Hol T, Cox MB, Bryant HU, Draper MW. Selective estrogen receptor modulators and postmenopausal women’s health. J Womens Health. 1997;6:523-531.
10. Buzdar AU, Marcus C, Holmes F, Hug V, Hortobagyi G. Phase II evaluation of LY156758 in metastatic breast cancer. Oncology. 1988;45:344-345.
11. Neven P, De Muylder X, Van Belle Y, Vanderick G, De Muylder E. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
12. Goldstein SR, Scheele WH, Rajagopalan SK, Wilkie JL, Walsh BW, Parsons AK. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
13. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Breast Cancer Res Treat. 2001;65:125-134.
14. Martino S, Cauley JA, Barrett-Connor E, et al. For the CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751-1761.
15. Vogel VG, Costantino JP, Wickerham DL, et al. For the National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;21:2727-2741.
16. Barrett-Connor E, Mosca L, Collins P, et al. For the Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137.
17. Santen RJ, Yue W, Naftolin F, Mor G, Berstein L. The potential of aromatase inhibitors in breast cancer prevention. Endocr Relat Cancer. 1999;6:235-243.
18. Goss PE, Strasser K. Aromatase inhibitors in the treatment and prevention of breast cancer. J Clin Oncol. 2001;19:881-894.
19. Bryant HU, Dere WH. Selective estrogen receptor modulators: an alternative to hormone replacement therapy. Proc Soc Exp Biol Med. 1998;217:45-52.
20. Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol. 1995;85:304-313.
21. Miller BA, Feuer EJ, Hankey BF. The significance of the rising incidence of breast cancer in the United States. In: DeVita VT, Hellman S, Rosenberg SA, eds. Important Advances in Oncology. Philadelphia: Lippincott; 1994:193-207.
22. Spicer DV, Pike MC. Risk factors in breast cancer. In: Roses DF, ed. Breast Cancer. New York: Churchill Livingston; 1944.
23. Bilimoria MM, Morrow M. The woman at increased risk for breast cancer: evaluation and management strategies. CA Cancer J Clin. 1995;45:263-278.
The author reports that he is a consultant to Eli Lilly, Pfizer, and Wyeth, and a speaker for Eli Lilly and Wyeth.
CASE 1: Premenopausal woman
at high risk of breast cancer
R. J. is a 43-year-old, nulliparous woman who reached menarche at age 11. She has undergone two breast biopsies, the most recent of which revealed ductal hyperplasia with marked atypia.
R. J.’s sister had breast cancer at 49 years of age; her mother had breast cancer at 66 years. Because of R. J.’s family history, she underwent testing for a BRCA mutation. The result was negative.
R. J. has come to your office today to find out if she can do anything to reduce her risk of breast cancer. What options can you offer?
The most common method of “prevention” of breast cancer involves early detection and assessment of abnormalities through frequent surveillance with mammography. Some women who have dense breasts, a history of breast biopsy, or other risk factors for breast cancer may benefit from intensive surveillance with both mammography and ultrasonography—and, in some cases, magnetic resonance imaging.
More aggressive options include:
- the use of a chemopreventive agent such as tamoxifen or raloxifene
- in rare cases—usually when a BRCA mutation is present—prophylactic mastectomy.
Before it is possible to determine the optimal approach for a particular woman, it is necessary to conduct an individualized assessment of her risk—that is, to estimate the probability that she will develop breast cancer over a defined period of time. Such an estimate is also useful for designing prevention trials in high-risk subsets of the population. (Prevention trials differ from therapeutic clinical trials in that asymptomatic healthy women are exposed to potentially toxic interventions for prolonged periods to reduce their risk of breast cancer.)
This article describes chemopreventive options for women at high risk, based on individualized risk assessment using the Gail model.
(Editor’s note: For additional discussion of the important role ObGyns play in the fight against breast cancer, see Editor in Chief Dr. Robert L. Barbieri’s Editorial.)
You can estimate the likelihood that a woman like your patient may develop breast cancer using various individual risk factors ( TABLE 1 ), but estimates for combinations of risk factors are preferable. The Gail model takes into account some nongenetic factors, such as parity and age at menarche, but also genetic factors, such as family history. The model calculates a woman’s individualized breast cancer probability and yields a numerical risk (a percentage) that she will develop invasive breast cancer over the next 5 years; it also yields an estimate of her risk of developing the malignancy over the remainder of her life.1,2
A Gail-model 5-year estimate of 1.66% or higher denotes a high risk of developing breast cancer. That benchmark was the one employed in the Breast Cancer Prevention Trial (BCPT), conducted as part of the National Surgical Adjuvant Breast and Bowel Project (NSABP).3
TABLE 1
What are the risk factors for breast cancer?
And what degree of relative risk do they confer?
| Relative risk | ||
|---|---|---|
| <2 | 2–4 | >4 |
| • Age 25–34 years at first live birth • Early menarche • Late menopause • Benign proliferative disease • Postmenopausal obesity • Alcohol use • Hormone replacement therapy | • Age >35 years at first live birth • First-degree relative with breast cancer • Nulliparity • Radiation exposure • Personal history of breast cancer | • Gene mutation (BRCA 1 or 2) • Lobular carcinoma in situ • Ductal carcinoma in situ • Atypical hyperplasia |
| Adapted from Bilimoria and Morrow23 | ||
Weaknesses of the Gail model
The Gail model’s approach to estimating risk has some limitations. The model uses the number of prior breast biopsies in its assessment—but the relative risk associated with prior biopsy is smaller for women older than 50 years than it is for younger women.
Furthermore, data on which Gail bases its estimates were collected in the late 1970s and early 1980s. Since then, the increasing ease of breast histopathologic assessment—through fine-needle aspiration and outpatient core-needle biopsy—has confused the issue of just what constitutes a breast “biopsy.” (Most patients surveyed consider it to be any histologic sampling of the breast.)
As a result, the 1.66% cutoff becomes somewhat difficult to interpret in light of current practice.
Consider the following example. A 50-year-old nulliparous Caucasian woman reached menarche when she was 11 years old, has never had a biopsy, and has no first-degree relatives with breast cancer. According to the Gail model, her risk of developing breast cancer is 1.2% over the next 5 years and 10.8% in her lifetime. Therefore, she is not considered at high risk. If she were to give a history of three previous breast biopsies, however, none of them showing hyperplasia, her 5-year risk would rise to 1.8% and push her over the line into the high-risk category.
Compare her situation to that of R. J., the nulliparous woman described in Case 1. R. J. also reached menarche at 11 years, but she has had two breast biopsies (one of which showed atypical hyperplasia) and has two first-degree relatives who have had breast cancer. Her Gail score shows a 5-year risk of breast cancer of 13.5% (the norm for a 43-year-old woman is 0.8%), and a lifetime risk of 69.2%. Clearly, she has a high risk of breast cancer.
How do we improve an imperfect science?
We need to identify objective findings that are patient-specific but highly correlative with the development of breast cancer. Patient-specific biomarkers have been proposed, such as ultrasensitive measurement of the serum estradiol level in postmenopausal women. In the Multiple Outcomes of Raloxifene Evaluation, also known as the MORE trial, women who experienced the greatest reduction in the rate of breast cancer during treatment with raloxifene were a subgroup who had the highest baseline level of serum estradiol—although, overall, all patients had an estradiol level well within the postmenopausal range (≤20 pmol/L).4,5
How tamoxifen became a chemopreventive agent
Tamoxifen inhibits mammary tumors in mice and rats and suppresses hormone-dependent breast cancer cell lines in vitro.6 Clinical data from the Early Breast Cancer Trialists’ Collaborative Group yielded additional motivation for prevention trials with tamoxifen: Besides reducing the rate of recurrent breast cancer, tamoxifen reduced the risk of contralateral new-onset breast cancer by 47% after 5 years of adjuvant treatment.7 Preclinical findings in vitro and in animal models, coupled with clinical data and evidence of tamoxifen’s favorable effects on skeleton remodeling and lipid levels, led to a series of chemoprevention trials in the United States and Europe using tamoxifen.
In the aforementioned BCPT, launched in 1992, 13,388 women 35 years and older who were deemed to be at high risk of developing breast cancer were enrolled at numerous sites throughout the United States and Canada.3 The Gail model was used to select women for the trial—only those who had a 5-year risk of 1.66% or higher were included. Participants were randomly assigned to receive tamoxifen 20 mg or placebo daily for 5 years. The trial was terminated early because of the dramatic reduction in new-onset breast cancer with tamoxifen, compared with placebo.
The overall incidence of breast cancer in the tamoxifen group was 3.4 cases for every 1,000 women, compared with 6.8 cases for every 1,000 women receiving placebo.3 Overall, the reduction in invasive breast cancer with tamoxifen was 49% (P<.00001). When broken down by age group, the reduction was:
- 44% in women 35 to 49 years old
- 51% in women 50 to 59 years old
- 55% in women 60 years and older.
Even noninvasive breast cancer was reduced with tamoxifen
Tamoxifen decreased the incidence of noninvasive breast cancer (ductal carcinoma in situ [DCIS]) by 50%. Expanded use of mammography has increased the detection of DCIS. Most DCIS lesions appear to be estrogen-receptor positive.8
In addition, tamoxifen reduced breast cancer risk in women who had a history of lobular carcinoma in situ (LCIS), a precancer, by 56%, and it reduced the risk of breast cancer in women who had a history of atypical hyperplasia by 86%. Overall, tamoxifen reduced the occurrence of estrogen-positive tumors by 69%, but had no impact on the incidence of estrogen-receptor–negative tumors.
The BCPT was stopped 14 months before planned because the Data and Safety Monitoring Board felt it was unethical to continue to allow one half of such high-risk participants to take placebo in light of the dramatic reduction in both invasive and noninvasive breast cancer among women who took tamoxifen.
In postmenopausal women, tamoxifen increases some risks
Two secondary endpoints of the BCPT are worthy of consideration:
- The overall relative risk (RR) of endometrial cancer associated with tamoxifen therapy in healthy women was 2.53 (95% confidence interval [CI], 1.35, 4.97). However, further analysis by age yielded a RR of 4.01 in women who were older than 50 years (95% CI, 1.70, 10.90), compared with a RR of 1.21 in women 49 years and younger (95% CI, 0.41, 3.60).
- The same age distinction held true for deep venous thrombosis (DVT) and pulmonary embolus, with no statistically significant increases in either in women 49 years and younger, but a RR of 1.71 and 3.19, respectively, in women 50 years and older. It is unclear whether the trial was sufficiently powered for this particular secondary endpoint.
These findings suggest that serious adverse events do not occur at the same magnitude in women younger than 50 years that they do in women 50 and older. The difference in the risk–benefit profile between younger and older women has significant clinical implications for the care of perimenopausal patients.
Risk of other malignancies was not affected by tamoxifen
Overall, invasive cancers other than those of the breast and uterus occurred at the same rate in the tamoxifen and placebo groups of the BCPT. The RR of death from any cause was 0.81 (95% CI, 0.56–1.16). There was a slight increase in the risk of myocardial infarction (RR, 1.11; 95% CI, 0.65–1.92) and a slight decrease in the risk of severe angina (RR, 0.93; 95% CI, 0.40–2.14) in tamoxifen users, although neither of these risks was statistically significant.
The overall RR of fracture of the hip, spine, or radius was 0.81 (95% CI, 0.63–1.05). There was a statistically significant increase in the number of women who had cataracts who then underwent cataract surgery in the tamoxifen group (RR, 1.57; 95% CI, 1.16–2.14).
Tamoxifen is approved as a preventive for high-risk women only
Based on the results of the BCPT, the US Food and Drug Administration (FDA) approved tamoxifen in October 1998 for the primary prevention of breast cancer in women who are at high risk of the disease. The FDA recommends that use of tamoxifen be limited to women at high risk because of the potentially serious side effects seen in clinical trials, including the BCPT.
The FDA did not define “high risk,” but it did recommend that the decision to use tamoxifen as chemopreventive therapy be based on thorough evaluation of the patient’s personal, family, and medical histories; her age; and her understanding of the risks and benefits of treatment.
The FDA also required the following language in the package insert:
- You should not take tamoxifen to reduce the risk of breast cancer unless you are at high risk of breast cancer. Certain conditions put women at high risk, and it is possible to calculate this risk for any woman. Breast cancer risk-assessment tools to help calculate your risk of breast cancer have been developed and are available to your health-care professional. You should discuss your risk with your healthcare professional.
CASE 1 RESOLVED
You determine that R. J. is an excellent candidate for tamoxifen by virtue of her significant risk of breast cancer. You are able to reassure her that, as the BCPT demonstrated, tamoxifen should not increase the risk of uterine cancer, DVT, or pulmonary embolism in a woman her age.
Raloxifene
CASE 2: Patient worries about breasts and bones
S. T. is a 58-year-old Caucasian mother of two whose own mother had breast cancer when she was 74 years old, and whose older sister was given a diagnosis of the malignancy 4 years ago.
S. T. had her first period when she was 11 years old, delivered her first child when she was 31, and entered menopause when she was 52. She is 5 ft 5 in tall and weighs 144 lb.
Her main reason for visiting you today is a breast Mammotome biopsy that showed ductal hyperplasia with atypia. She has been tested for a BRCA mutation, but the result was negative. Her Gail-model score is a 9.7% risk of developing breast cancer over the next 5 years, and a lifetime risk of 44.2%.
She also asks about osteoporosis prevention, given that a dual-energy x-ray absorptiometry (DXA) scan 1 year ago yielded a T-score of –1.3 for her hip and –1.1 for her spine. Her World Health Organization FRAX 10-year risk of hip fracture is 0.7%, and her risk of major osteoporotic fracture is 8.6%.
How do you respond to her concerns?
This patient has a high risk of invasive breast cancer but does not meet criteria for pharmacotherapy for osteoporosis prevention. A good option for her would be raloxifene, a selective estrogen-receptor modulator (SERM) that has been shown to reduce the risk of breast cancer as well as osteoporosis. S. T. would benefit from it on the basis of its breast benefit alone.
The genesis of a drug with multiple benefits
Raloxifene is a benzothiophene derivative, unlike the triphenylethylene family from which tamoxifen is derived. Like tamoxifen, raloxifene was originally investigated as a treatment for advanced breast cancer.
Preclinical studies indicated that raloxifene had an antiproliferative effect on both estrogen-receptor–positive mammary tumors and estrogen-receptor–positive human breast cancer cell lines.9 In the 1980s, however, a small, phase-II trial revealed that raloxifene had no further antitumor effects in postmenopausal women with advanced breast cancer in whom tamoxifen had failed.10 After information surfaced about the neoplastic effect of tamoxifen on the uteri of postmenopausal women, interest in raloxifene revived.11
Raloxifene has estrogen-agonistic activity on bone remodeling and lipid metabolism and was approved by the FDA for prevention of osteoporosis in postmenopausal women in December 1997. Its indication was extended to treatment of osteoporosis 2 years later.
Raloxifene appears to have no effect on the endometrium of postmenopausal women, compared with placebo. In a 12-month comparative trial, there was no difference in endometrial thickness, endoluminal masses, proliferation, or hyperplasia between the raloxifene and placebo groups.12 This finding corroborates earlier evidence that raloxifene does not cause endometrial hyperplasia or cancer and is not associated with vaginal bleeding or increased endometrial thickness, as measured by transvaginal ultrasonography.
A big difference between raloxifene and tamoxifen, therefore, is their varying effect on the uterus of postmenopausal women.
Additional clinical trials confirm anticancer action of raloxifene
Preclinical data in animal models suggested that, like tamoxifen, raloxifene has potent antiestrogenic effects on breast tissue.9 The MORE trial involved 7,705 postmenopausal women up to 80 years old who had established osteoporosis.13 In that trial, participants were randomized to raloxifene or placebo. Bone mineral density (BMD) and fracture incidence were the primary endpoints; breast cancer was a secondary endpoint.
Over the 4 years of the trial, raloxifene significantly reduced the incidence of all invasive breast cancers by 72%, compared with placebo (RR, 0.28; 95% CI, 0.17–0.46). Raloxifene also significantly reduced the incidence of invasive estrogen-receptor–positive tumors by 84%, compared with placebo (RR, 0.16; 95% CI, 0.09–0.30), but had no effect on estrogen-receptor–negative tumors. The incidence of vaginal bleeding, breast pain, and endometrial cancer in the raloxifene group did not differ significantly from that of the placebo group.
Like tamoxifen, raloxifene appeared to be associated with an increased risk of thromboembolic disease, including DVT and pulmonary embolism, which developed in 1.1% of women taking raloxifene, compared with 0.5% of women in the placebo group (P=.003).
In a 4-year continuation of the MORE trial, known as the Continuing Outcomes Relevant to Evista, or CORE, trial, 5,231 women were randomized to continue raloxifene or placebo.14 Over the 8 years of the combined trials, the incidence of invasive breast cancer was reduced by 66% in the raloxifene group (RR, 0.34; 95% CI, 0.22–0.50). The 8-year data are extremely clinically relevant, in that raloxifene has no time limit, whereas tamoxifen is usually prescribed for no longer than 5 years.
Raloxifene is not approved for use in premenopausal women. SERM compounds, which are structurally similar to clomiphene citrate, seem to have different effects in premenopausal and postmenopausal women, as evidenced by tamoxifen’s differing effects by age in the BCPT.
Other investigations of raloxifene confirm its value in high-risk women
To compare the clinical safety and efficacy of tamoxifen and raloxifene in reducing the risk of breast cancer among healthy women, the Study of Tamoxifen and Raloxifene (STAR) was initiated in 1999.15 In that trial, 19,747 postmenopausal women older than 35 years were blindly assigned to raloxifene 60 mg or tamoxifen 20 mg daily.
Baseline characteristics of subjects in STAR are summarized in TABLE 2 . Mean age was 58.5 years. All women had a 5-year risk of developing breast cancer that exceeded 1.66%, according to the Gail model. The average Gail score was 4.03% (standard deviation, ±2.17%). Because it would have been unethical to subject high-risk women to a placebo group in light of the findings of the BCPT, there was no placebo control.
TABLE 2
Baseline characteristics of women
in the Study of Tamoxifen and Raloxifene (STAR) trial
| Characteristic | Value |
|---|---|
| Age (mean) | 58.5 years |
| Caucasian | 93% |
| Hysterectomy | 51% |
| At least one first-degree relative with breast cancer | 71% |
| Lobular carcinoma in situ | 9% |
| Atypical hyperplasia | 23% |
| 5-year risk of invasive breast cancer (mean)* | 4.03% |
| *As estimated with the Gail model Risk Calculator. | |
Here are noteworthy findings of the STAR trial:
- 163 cases of invasive breast cancer occurred in the tamoxifen group, compared with 168 among women taking raloxifene (RR, 1.02; 95% CI, 0.82–1.28).
- 36 cases of uterine cancer occurred in the tamoxifen group, compared with 23 among women taking raloxifene (RR, 0.62; 95% CI, 0.35–1.08). Earlier studies had shown a marked difference in the rate of uterine cancer between these agents. Although the difference here is not statistically significant, uterine cancer was not an endpoint of the study; nor was the study powered to explore this difference.
- The number of hysterectomies among women who were diagnosed with endometrial hyperplasia with or without atypia was, proportionally, significantly higher among women taking tamoxifen ( TABLE 3 ).
- No difference between groups was found for other invasive cancers, ischemic heart events, or stroke.
- Thromboembolic events occurred less frequently in the raloxifene group (RR, 0.70; 95% CI, 0.54–0.91). However, both raloxifene and tamoxifen have consistently been associated with a twofold to threefold increase in the risk of thromboembolic events, compared with placebo.
- Vasomotor symptoms and leg cramps increased in frequency and severity among women in both groups of the trial. These symptoms appear to be less common and less severe among women who are older and more remote from the onset of menopause.
TABLE 3
Relative risk of hysterectomy and uterine hyperplasia during STAR
| Characteristic | Women who took tamoxifen | Women who took raloxifene | Relative risk (95% confidence interval) |
|---|---|---|---|
| Hysterectomy during study | 246 | 92 | 0.37 (0.28, 0.47) |
| Hyperplasia • with atypia • without atypia | 100 15 85 | 17 2 15 | 0.17 (0.09, 0.28) 0.13 (0.01, 0.56) 0.17 (0.09, 0.30) |
What is raloxifene’s effect on the heart?
The Raloxifene Use for The Heart (RUTH) trial explored the primary endpoints of coronary artery disease (CAD) and breast cancer in more than 10,000 women who had CAD or multiple risk factors for it.16 This study began prior to the Women’s Health Initiative, at a time when hormone replacement therapy was widely believed to reduce CAD.
In the double-blinded, randomized, placebo-controlled RUTH trial, raloxifene had no significant effect on primary coronary events (533 vs 553; hazard ratio [HR], 0.95; 95% CI, 0.84–1.07). Even in this population, however, there was a 44% reduction in invasive breast cancer (40 vs 70 events; HR, 0.56; 95% CI, 0.38–0.83).
Based on these results, the FDA approved raloxifene for the “reduction in risk of invasive breast cancer in postmenopausal women at high risk for breast cancer,” as well as for the “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” ( FIGURE ).
FIGURE How raloxifene reduced invasive breast cancer in three trials
Raloxifene significantly reduced the risk of cancer, compared with placebo, in the Raloxifene Use for The Heart (RUTH), Multiple Outcomes of Raloxifene Evaluation (MORE), and Continuing Outcomes Relevant to Evista (CORE) trials.
CASE 2 RESOLVED
S. T. begins taking raloxifene 60 mg daily to lower her risk of invasive breast cancer. Although she temporarily experienced hot flashes after initiating the drug, they are only mildly bothersome, and she continues raloxifene therapy. She says she is grateful that there is an agent that can help her reduce the likelihood that she will develop breast cancer, and protection of her BMD is an added benefit.
CASE 3: At risk for both breast cancer and bone fracture
A. N., 63, is a nulliparous Caucasian woman who weighs 134 lb and stands 5 ft 4 in tall. She reached menarche when she was 12 years old and entered menopause at 49.
Although A. N. has never had a breast abnormality, her 59-year-old sister was just given a diagnosis of breast cancer. Her Gail score reveals that she has a 3.1% risk of developing breast cancer over the next 5 years.
In addition to her concerns about breast cancer, A. N. is worried about hip fracture—because her mother suffered one after menopause and because her T-score is –1.9 at the hip and –2.1 at the spine. A. N. has used steroids off and on for much of her life for asthma. Her FRAX score indicates that she has a 2.8% risk of hip fracture and a 25% risk of major osteoporotic fracture over the next 10 years.
What do you offer her?
Because of new FRAX criteria, this osteopenic woman is now a candidate for medication to reduce her risk of major osteoporotic fracture, and raloxifene is a good option. Her Gail score of 3.1% also makes her a good candidate for breast cancer risk reduction with raloxifene.
CASE 3 RESOLVED
Because A. N. needs an agent that benefits both breast and bone, you prescribe raloxifene. The drug should significantly reduce her risk of both invasive breast cancer and bone fracture, without increasing her risk of endometrial hyperplasia and cancer, both of which are associated with tamoxifen in her age group.
Aromatase inhibitors
A fairly new class of drugs being explored for their ability to reduce the risk of breast cancer is aromatase inhibitors. Substantial evidence suggests that estrogens facilitate the development of breast cancer in animals and in women, although the precise mechanism remains unknown.17 The most commonly held theory is that estrogen stimulates proliferation of breast cells and thereby increases the risk of genetic mutation that could lead to cancer.
Aromatase inhibitors block peripheral conversion of androstenedione to estrogens. In premenopausal women, the primary site of this action is in the ovary. In postmenopausal women, this conversion occurs primarily in extraovarian sites, including the adrenal glands, adipose tissue, liver, muscle, and skin.
Aromatase inhibitors may be more effective than SERMs in preventing breast cancer because of their dual role: blocking both the initiation and promotion of breast cancer.18 These agents reduce levels of the genotoxic metabolites of estradiol by lowering estradiol concentration in tissue. At the same time, aromatase inhibitors also block tumor promotion by lowering tissue levels of estrogen and preventing cell proliferation.
The main drawback of these agents—besides the fact that they are not FDA-approved for reducing risk—is their antiestrogenic effect on bone and lipid metabolism. They also induce vasomotor symptoms.
Studies of third-generation aromatase inhibitors in the prevention of breast cancer are under way in high-risk women. These agents include anastrozole, exemestane, and letrozole.
The author reports that he is a consultant to Eli Lilly, Pfizer, and Wyeth, and a speaker for Eli Lilly and Wyeth.
CASE 1: Premenopausal woman
at high risk of breast cancer
R. J. is a 43-year-old, nulliparous woman who reached menarche at age 11. She has undergone two breast biopsies, the most recent of which revealed ductal hyperplasia with marked atypia.
R. J.’s sister had breast cancer at 49 years of age; her mother had breast cancer at 66 years. Because of R. J.’s family history, she underwent testing for a BRCA mutation. The result was negative.
R. J. has come to your office today to find out if she can do anything to reduce her risk of breast cancer. What options can you offer?
The most common method of “prevention” of breast cancer involves early detection and assessment of abnormalities through frequent surveillance with mammography. Some women who have dense breasts, a history of breast biopsy, or other risk factors for breast cancer may benefit from intensive surveillance with both mammography and ultrasonography—and, in some cases, magnetic resonance imaging.
More aggressive options include:
- the use of a chemopreventive agent such as tamoxifen or raloxifene
- in rare cases—usually when a BRCA mutation is present—prophylactic mastectomy.
Before it is possible to determine the optimal approach for a particular woman, it is necessary to conduct an individualized assessment of her risk—that is, to estimate the probability that she will develop breast cancer over a defined period of time. Such an estimate is also useful for designing prevention trials in high-risk subsets of the population. (Prevention trials differ from therapeutic clinical trials in that asymptomatic healthy women are exposed to potentially toxic interventions for prolonged periods to reduce their risk of breast cancer.)
This article describes chemopreventive options for women at high risk, based on individualized risk assessment using the Gail model.
(Editor’s note: For additional discussion of the important role ObGyns play in the fight against breast cancer, see Editor in Chief Dr. Robert L. Barbieri’s Editorial.)
You can estimate the likelihood that a woman like your patient may develop breast cancer using various individual risk factors ( TABLE 1 ), but estimates for combinations of risk factors are preferable. The Gail model takes into account some nongenetic factors, such as parity and age at menarche, but also genetic factors, such as family history. The model calculates a woman’s individualized breast cancer probability and yields a numerical risk (a percentage) that she will develop invasive breast cancer over the next 5 years; it also yields an estimate of her risk of developing the malignancy over the remainder of her life.1,2
A Gail-model 5-year estimate of 1.66% or higher denotes a high risk of developing breast cancer. That benchmark was the one employed in the Breast Cancer Prevention Trial (BCPT), conducted as part of the National Surgical Adjuvant Breast and Bowel Project (NSABP).3
TABLE 1
What are the risk factors for breast cancer?
And what degree of relative risk do they confer?
| Relative risk | ||
|---|---|---|
| <2 | 2–4 | >4 |
| • Age 25–34 years at first live birth • Early menarche • Late menopause • Benign proliferative disease • Postmenopausal obesity • Alcohol use • Hormone replacement therapy | • Age >35 years at first live birth • First-degree relative with breast cancer • Nulliparity • Radiation exposure • Personal history of breast cancer | • Gene mutation (BRCA 1 or 2) • Lobular carcinoma in situ • Ductal carcinoma in situ • Atypical hyperplasia |
| Adapted from Bilimoria and Morrow23 | ||
Weaknesses of the Gail model
The Gail model’s approach to estimating risk has some limitations. The model uses the number of prior breast biopsies in its assessment—but the relative risk associated with prior biopsy is smaller for women older than 50 years than it is for younger women.
Furthermore, data on which Gail bases its estimates were collected in the late 1970s and early 1980s. Since then, the increasing ease of breast histopathologic assessment—through fine-needle aspiration and outpatient core-needle biopsy—has confused the issue of just what constitutes a breast “biopsy.” (Most patients surveyed consider it to be any histologic sampling of the breast.)
As a result, the 1.66% cutoff becomes somewhat difficult to interpret in light of current practice.
Consider the following example. A 50-year-old nulliparous Caucasian woman reached menarche when she was 11 years old, has never had a biopsy, and has no first-degree relatives with breast cancer. According to the Gail model, her risk of developing breast cancer is 1.2% over the next 5 years and 10.8% in her lifetime. Therefore, she is not considered at high risk. If she were to give a history of three previous breast biopsies, however, none of them showing hyperplasia, her 5-year risk would rise to 1.8% and push her over the line into the high-risk category.
Compare her situation to that of R. J., the nulliparous woman described in Case 1. R. J. also reached menarche at 11 years, but she has had two breast biopsies (one of which showed atypical hyperplasia) and has two first-degree relatives who have had breast cancer. Her Gail score shows a 5-year risk of breast cancer of 13.5% (the norm for a 43-year-old woman is 0.8%), and a lifetime risk of 69.2%. Clearly, she has a high risk of breast cancer.
How do we improve an imperfect science?
We need to identify objective findings that are patient-specific but highly correlative with the development of breast cancer. Patient-specific biomarkers have been proposed, such as ultrasensitive measurement of the serum estradiol level in postmenopausal women. In the Multiple Outcomes of Raloxifene Evaluation, also known as the MORE trial, women who experienced the greatest reduction in the rate of breast cancer during treatment with raloxifene were a subgroup who had the highest baseline level of serum estradiol—although, overall, all patients had an estradiol level well within the postmenopausal range (≤20 pmol/L).4,5
How tamoxifen became a chemopreventive agent
Tamoxifen inhibits mammary tumors in mice and rats and suppresses hormone-dependent breast cancer cell lines in vitro.6 Clinical data from the Early Breast Cancer Trialists’ Collaborative Group yielded additional motivation for prevention trials with tamoxifen: Besides reducing the rate of recurrent breast cancer, tamoxifen reduced the risk of contralateral new-onset breast cancer by 47% after 5 years of adjuvant treatment.7 Preclinical findings in vitro and in animal models, coupled with clinical data and evidence of tamoxifen’s favorable effects on skeleton remodeling and lipid levels, led to a series of chemoprevention trials in the United States and Europe using tamoxifen.
In the aforementioned BCPT, launched in 1992, 13,388 women 35 years and older who were deemed to be at high risk of developing breast cancer were enrolled at numerous sites throughout the United States and Canada.3 The Gail model was used to select women for the trial—only those who had a 5-year risk of 1.66% or higher were included. Participants were randomly assigned to receive tamoxifen 20 mg or placebo daily for 5 years. The trial was terminated early because of the dramatic reduction in new-onset breast cancer with tamoxifen, compared with placebo.
The overall incidence of breast cancer in the tamoxifen group was 3.4 cases for every 1,000 women, compared with 6.8 cases for every 1,000 women receiving placebo.3 Overall, the reduction in invasive breast cancer with tamoxifen was 49% (P<.00001). When broken down by age group, the reduction was:
- 44% in women 35 to 49 years old
- 51% in women 50 to 59 years old
- 55% in women 60 years and older.
Even noninvasive breast cancer was reduced with tamoxifen
Tamoxifen decreased the incidence of noninvasive breast cancer (ductal carcinoma in situ [DCIS]) by 50%. Expanded use of mammography has increased the detection of DCIS. Most DCIS lesions appear to be estrogen-receptor positive.8
In addition, tamoxifen reduced breast cancer risk in women who had a history of lobular carcinoma in situ (LCIS), a precancer, by 56%, and it reduced the risk of breast cancer in women who had a history of atypical hyperplasia by 86%. Overall, tamoxifen reduced the occurrence of estrogen-positive tumors by 69%, but had no impact on the incidence of estrogen-receptor–negative tumors.
The BCPT was stopped 14 months before planned because the Data and Safety Monitoring Board felt it was unethical to continue to allow one half of such high-risk participants to take placebo in light of the dramatic reduction in both invasive and noninvasive breast cancer among women who took tamoxifen.
In postmenopausal women, tamoxifen increases some risks
Two secondary endpoints of the BCPT are worthy of consideration:
- The overall relative risk (RR) of endometrial cancer associated with tamoxifen therapy in healthy women was 2.53 (95% confidence interval [CI], 1.35, 4.97). However, further analysis by age yielded a RR of 4.01 in women who were older than 50 years (95% CI, 1.70, 10.90), compared with a RR of 1.21 in women 49 years and younger (95% CI, 0.41, 3.60).
- The same age distinction held true for deep venous thrombosis (DVT) and pulmonary embolus, with no statistically significant increases in either in women 49 years and younger, but a RR of 1.71 and 3.19, respectively, in women 50 years and older. It is unclear whether the trial was sufficiently powered for this particular secondary endpoint.
These findings suggest that serious adverse events do not occur at the same magnitude in women younger than 50 years that they do in women 50 and older. The difference in the risk–benefit profile between younger and older women has significant clinical implications for the care of perimenopausal patients.
Risk of other malignancies was not affected by tamoxifen
Overall, invasive cancers other than those of the breast and uterus occurred at the same rate in the tamoxifen and placebo groups of the BCPT. The RR of death from any cause was 0.81 (95% CI, 0.56–1.16). There was a slight increase in the risk of myocardial infarction (RR, 1.11; 95% CI, 0.65–1.92) and a slight decrease in the risk of severe angina (RR, 0.93; 95% CI, 0.40–2.14) in tamoxifen users, although neither of these risks was statistically significant.
The overall RR of fracture of the hip, spine, or radius was 0.81 (95% CI, 0.63–1.05). There was a statistically significant increase in the number of women who had cataracts who then underwent cataract surgery in the tamoxifen group (RR, 1.57; 95% CI, 1.16–2.14).
Tamoxifen is approved as a preventive for high-risk women only
Based on the results of the BCPT, the US Food and Drug Administration (FDA) approved tamoxifen in October 1998 for the primary prevention of breast cancer in women who are at high risk of the disease. The FDA recommends that use of tamoxifen be limited to women at high risk because of the potentially serious side effects seen in clinical trials, including the BCPT.
The FDA did not define “high risk,” but it did recommend that the decision to use tamoxifen as chemopreventive therapy be based on thorough evaluation of the patient’s personal, family, and medical histories; her age; and her understanding of the risks and benefits of treatment.
The FDA also required the following language in the package insert:
- You should not take tamoxifen to reduce the risk of breast cancer unless you are at high risk of breast cancer. Certain conditions put women at high risk, and it is possible to calculate this risk for any woman. Breast cancer risk-assessment tools to help calculate your risk of breast cancer have been developed and are available to your health-care professional. You should discuss your risk with your healthcare professional.
CASE 1 RESOLVED
You determine that R. J. is an excellent candidate for tamoxifen by virtue of her significant risk of breast cancer. You are able to reassure her that, as the BCPT demonstrated, tamoxifen should not increase the risk of uterine cancer, DVT, or pulmonary embolism in a woman her age.
Raloxifene
CASE 2: Patient worries about breasts and bones
S. T. is a 58-year-old Caucasian mother of two whose own mother had breast cancer when she was 74 years old, and whose older sister was given a diagnosis of the malignancy 4 years ago.
S. T. had her first period when she was 11 years old, delivered her first child when she was 31, and entered menopause when she was 52. She is 5 ft 5 in tall and weighs 144 lb.
Her main reason for visiting you today is a breast Mammotome biopsy that showed ductal hyperplasia with atypia. She has been tested for a BRCA mutation, but the result was negative. Her Gail-model score is a 9.7% risk of developing breast cancer over the next 5 years, and a lifetime risk of 44.2%.
She also asks about osteoporosis prevention, given that a dual-energy x-ray absorptiometry (DXA) scan 1 year ago yielded a T-score of –1.3 for her hip and –1.1 for her spine. Her World Health Organization FRAX 10-year risk of hip fracture is 0.7%, and her risk of major osteoporotic fracture is 8.6%.
How do you respond to her concerns?
This patient has a high risk of invasive breast cancer but does not meet criteria for pharmacotherapy for osteoporosis prevention. A good option for her would be raloxifene, a selective estrogen-receptor modulator (SERM) that has been shown to reduce the risk of breast cancer as well as osteoporosis. S. T. would benefit from it on the basis of its breast benefit alone.
The genesis of a drug with multiple benefits
Raloxifene is a benzothiophene derivative, unlike the triphenylethylene family from which tamoxifen is derived. Like tamoxifen, raloxifene was originally investigated as a treatment for advanced breast cancer.
Preclinical studies indicated that raloxifene had an antiproliferative effect on both estrogen-receptor–positive mammary tumors and estrogen-receptor–positive human breast cancer cell lines.9 In the 1980s, however, a small, phase-II trial revealed that raloxifene had no further antitumor effects in postmenopausal women with advanced breast cancer in whom tamoxifen had failed.10 After information surfaced about the neoplastic effect of tamoxifen on the uteri of postmenopausal women, interest in raloxifene revived.11
Raloxifene has estrogen-agonistic activity on bone remodeling and lipid metabolism and was approved by the FDA for prevention of osteoporosis in postmenopausal women in December 1997. Its indication was extended to treatment of osteoporosis 2 years later.
Raloxifene appears to have no effect on the endometrium of postmenopausal women, compared with placebo. In a 12-month comparative trial, there was no difference in endometrial thickness, endoluminal masses, proliferation, or hyperplasia between the raloxifene and placebo groups.12 This finding corroborates earlier evidence that raloxifene does not cause endometrial hyperplasia or cancer and is not associated with vaginal bleeding or increased endometrial thickness, as measured by transvaginal ultrasonography.
A big difference between raloxifene and tamoxifen, therefore, is their varying effect on the uterus of postmenopausal women.
Additional clinical trials confirm anticancer action of raloxifene
Preclinical data in animal models suggested that, like tamoxifen, raloxifene has potent antiestrogenic effects on breast tissue.9 The MORE trial involved 7,705 postmenopausal women up to 80 years old who had established osteoporosis.13 In that trial, participants were randomized to raloxifene or placebo. Bone mineral density (BMD) and fracture incidence were the primary endpoints; breast cancer was a secondary endpoint.
Over the 4 years of the trial, raloxifene significantly reduced the incidence of all invasive breast cancers by 72%, compared with placebo (RR, 0.28; 95% CI, 0.17–0.46). Raloxifene also significantly reduced the incidence of invasive estrogen-receptor–positive tumors by 84%, compared with placebo (RR, 0.16; 95% CI, 0.09–0.30), but had no effect on estrogen-receptor–negative tumors. The incidence of vaginal bleeding, breast pain, and endometrial cancer in the raloxifene group did not differ significantly from that of the placebo group.
Like tamoxifen, raloxifene appeared to be associated with an increased risk of thromboembolic disease, including DVT and pulmonary embolism, which developed in 1.1% of women taking raloxifene, compared with 0.5% of women in the placebo group (P=.003).
In a 4-year continuation of the MORE trial, known as the Continuing Outcomes Relevant to Evista, or CORE, trial, 5,231 women were randomized to continue raloxifene or placebo.14 Over the 8 years of the combined trials, the incidence of invasive breast cancer was reduced by 66% in the raloxifene group (RR, 0.34; 95% CI, 0.22–0.50). The 8-year data are extremely clinically relevant, in that raloxifene has no time limit, whereas tamoxifen is usually prescribed for no longer than 5 years.
Raloxifene is not approved for use in premenopausal women. SERM compounds, which are structurally similar to clomiphene citrate, seem to have different effects in premenopausal and postmenopausal women, as evidenced by tamoxifen’s differing effects by age in the BCPT.
Other investigations of raloxifene confirm its value in high-risk women
To compare the clinical safety and efficacy of tamoxifen and raloxifene in reducing the risk of breast cancer among healthy women, the Study of Tamoxifen and Raloxifene (STAR) was initiated in 1999.15 In that trial, 19,747 postmenopausal women older than 35 years were blindly assigned to raloxifene 60 mg or tamoxifen 20 mg daily.
Baseline characteristics of subjects in STAR are summarized in TABLE 2 . Mean age was 58.5 years. All women had a 5-year risk of developing breast cancer that exceeded 1.66%, according to the Gail model. The average Gail score was 4.03% (standard deviation, ±2.17%). Because it would have been unethical to subject high-risk women to a placebo group in light of the findings of the BCPT, there was no placebo control.
TABLE 2
Baseline characteristics of women
in the Study of Tamoxifen and Raloxifene (STAR) trial
| Characteristic | Value |
|---|---|
| Age (mean) | 58.5 years |
| Caucasian | 93% |
| Hysterectomy | 51% |
| At least one first-degree relative with breast cancer | 71% |
| Lobular carcinoma in situ | 9% |
| Atypical hyperplasia | 23% |
| 5-year risk of invasive breast cancer (mean)* | 4.03% |
| *As estimated with the Gail model Risk Calculator. | |
Here are noteworthy findings of the STAR trial:
- 163 cases of invasive breast cancer occurred in the tamoxifen group, compared with 168 among women taking raloxifene (RR, 1.02; 95% CI, 0.82–1.28).
- 36 cases of uterine cancer occurred in the tamoxifen group, compared with 23 among women taking raloxifene (RR, 0.62; 95% CI, 0.35–1.08). Earlier studies had shown a marked difference in the rate of uterine cancer between these agents. Although the difference here is not statistically significant, uterine cancer was not an endpoint of the study; nor was the study powered to explore this difference.
- The number of hysterectomies among women who were diagnosed with endometrial hyperplasia with or without atypia was, proportionally, significantly higher among women taking tamoxifen ( TABLE 3 ).
- No difference between groups was found for other invasive cancers, ischemic heart events, or stroke.
- Thromboembolic events occurred less frequently in the raloxifene group (RR, 0.70; 95% CI, 0.54–0.91). However, both raloxifene and tamoxifen have consistently been associated with a twofold to threefold increase in the risk of thromboembolic events, compared with placebo.
- Vasomotor symptoms and leg cramps increased in frequency and severity among women in both groups of the trial. These symptoms appear to be less common and less severe among women who are older and more remote from the onset of menopause.
TABLE 3
Relative risk of hysterectomy and uterine hyperplasia during STAR
| Characteristic | Women who took tamoxifen | Women who took raloxifene | Relative risk (95% confidence interval) |
|---|---|---|---|
| Hysterectomy during study | 246 | 92 | 0.37 (0.28, 0.47) |
| Hyperplasia • with atypia • without atypia | 100 15 85 | 17 2 15 | 0.17 (0.09, 0.28) 0.13 (0.01, 0.56) 0.17 (0.09, 0.30) |
What is raloxifene’s effect on the heart?
The Raloxifene Use for The Heart (RUTH) trial explored the primary endpoints of coronary artery disease (CAD) and breast cancer in more than 10,000 women who had CAD or multiple risk factors for it.16 This study began prior to the Women’s Health Initiative, at a time when hormone replacement therapy was widely believed to reduce CAD.
In the double-blinded, randomized, placebo-controlled RUTH trial, raloxifene had no significant effect on primary coronary events (533 vs 553; hazard ratio [HR], 0.95; 95% CI, 0.84–1.07). Even in this population, however, there was a 44% reduction in invasive breast cancer (40 vs 70 events; HR, 0.56; 95% CI, 0.38–0.83).
Based on these results, the FDA approved raloxifene for the “reduction in risk of invasive breast cancer in postmenopausal women at high risk for breast cancer,” as well as for the “reduction in risk of invasive breast cancer in postmenopausal women with osteoporosis” ( FIGURE ).
FIGURE How raloxifene reduced invasive breast cancer in three trials
Raloxifene significantly reduced the risk of cancer, compared with placebo, in the Raloxifene Use for The Heart (RUTH), Multiple Outcomes of Raloxifene Evaluation (MORE), and Continuing Outcomes Relevant to Evista (CORE) trials.
CASE 2 RESOLVED
S. T. begins taking raloxifene 60 mg daily to lower her risk of invasive breast cancer. Although she temporarily experienced hot flashes after initiating the drug, they are only mildly bothersome, and she continues raloxifene therapy. She says she is grateful that there is an agent that can help her reduce the likelihood that she will develop breast cancer, and protection of her BMD is an added benefit.
CASE 3: At risk for both breast cancer and bone fracture
A. N., 63, is a nulliparous Caucasian woman who weighs 134 lb and stands 5 ft 4 in tall. She reached menarche when she was 12 years old and entered menopause at 49.
Although A. N. has never had a breast abnormality, her 59-year-old sister was just given a diagnosis of breast cancer. Her Gail score reveals that she has a 3.1% risk of developing breast cancer over the next 5 years.
In addition to her concerns about breast cancer, A. N. is worried about hip fracture—because her mother suffered one after menopause and because her T-score is –1.9 at the hip and –2.1 at the spine. A. N. has used steroids off and on for much of her life for asthma. Her FRAX score indicates that she has a 2.8% risk of hip fracture and a 25% risk of major osteoporotic fracture over the next 10 years.
What do you offer her?
Because of new FRAX criteria, this osteopenic woman is now a candidate for medication to reduce her risk of major osteoporotic fracture, and raloxifene is a good option. Her Gail score of 3.1% also makes her a good candidate for breast cancer risk reduction with raloxifene.
CASE 3 RESOLVED
Because A. N. needs an agent that benefits both breast and bone, you prescribe raloxifene. The drug should significantly reduce her risk of both invasive breast cancer and bone fracture, without increasing her risk of endometrial hyperplasia and cancer, both of which are associated with tamoxifen in her age group.
Aromatase inhibitors
A fairly new class of drugs being explored for their ability to reduce the risk of breast cancer is aromatase inhibitors. Substantial evidence suggests that estrogens facilitate the development of breast cancer in animals and in women, although the precise mechanism remains unknown.17 The most commonly held theory is that estrogen stimulates proliferation of breast cells and thereby increases the risk of genetic mutation that could lead to cancer.
Aromatase inhibitors block peripheral conversion of androstenedione to estrogens. In premenopausal women, the primary site of this action is in the ovary. In postmenopausal women, this conversion occurs primarily in extraovarian sites, including the adrenal glands, adipose tissue, liver, muscle, and skin.
Aromatase inhibitors may be more effective than SERMs in preventing breast cancer because of their dual role: blocking both the initiation and promotion of breast cancer.18 These agents reduce levels of the genotoxic metabolites of estradiol by lowering estradiol concentration in tissue. At the same time, aromatase inhibitors also block tumor promotion by lowering tissue levels of estrogen and preventing cell proliferation.
The main drawback of these agents—besides the fact that they are not FDA-approved for reducing risk—is their antiestrogenic effect on bone and lipid metabolism. They also induce vasomotor symptoms.
Studies of third-generation aromatase inhibitors in the prevention of breast cancer are under way in high-risk women. These agents include anastrozole, exemestane, and letrozole.
1. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
2. Breast Cancer Assessment Tool. Available at: www.cancer.gov/bcrisktool/Default.aspx. Accessed June 5, 2009.
3. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
4. Ruffin MT, 4th, August DA, Kelloff GJ, Boone CW, Weber BL, Brenner DE. Selection criteria for breast cancer chemoprevention subjects. J Cell Biochem Suppl. 1993;17G:234-241.
5. Cummings SR, Duong T, Kenyon E, Cauley JA, Whitehead M, Krueger KA. For the Multiple Outcomes of Raloxifene Evaluation (MORE) Trial. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220.
6. Jordan VC, Allen KE. Evaluation of the antitumor activity of the non-steroidal antioestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma mode. Eur J Cancer. 1980;16:239-251.
7. Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988;319:1681-1692.
8. Bur ME, Zimarowski MJ, Schnitt SJ, Baker S, Lew R. Estrogen receptor immunohistochemistry in carcinoma in situ of the breast. Cancer. 1992;69:1174-1181.
9. Hol T, Cox MB, Bryant HU, Draper MW. Selective estrogen receptor modulators and postmenopausal women’s health. J Womens Health. 1997;6:523-531.
10. Buzdar AU, Marcus C, Holmes F, Hug V, Hortobagyi G. Phase II evaluation of LY156758 in metastatic breast cancer. Oncology. 1988;45:344-345.
11. Neven P, De Muylder X, Van Belle Y, Vanderick G, De Muylder E. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
12. Goldstein SR, Scheele WH, Rajagopalan SK, Wilkie JL, Walsh BW, Parsons AK. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
13. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Breast Cancer Res Treat. 2001;65:125-134.
14. Martino S, Cauley JA, Barrett-Connor E, et al. For the CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751-1761.
15. Vogel VG, Costantino JP, Wickerham DL, et al. For the National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;21:2727-2741.
16. Barrett-Connor E, Mosca L, Collins P, et al. For the Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137.
17. Santen RJ, Yue W, Naftolin F, Mor G, Berstein L. The potential of aromatase inhibitors in breast cancer prevention. Endocr Relat Cancer. 1999;6:235-243.
18. Goss PE, Strasser K. Aromatase inhibitors in the treatment and prevention of breast cancer. J Clin Oncol. 2001;19:881-894.
19. Bryant HU, Dere WH. Selective estrogen receptor modulators: an alternative to hormone replacement therapy. Proc Soc Exp Biol Med. 1998;217:45-52.
20. Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol. 1995;85:304-313.
21. Miller BA, Feuer EJ, Hankey BF. The significance of the rising incidence of breast cancer in the United States. In: DeVita VT, Hellman S, Rosenberg SA, eds. Important Advances in Oncology. Philadelphia: Lippincott; 1994:193-207.
22. Spicer DV, Pike MC. Risk factors in breast cancer. In: Roses DF, ed. Breast Cancer. New York: Churchill Livingston; 1944.
23. Bilimoria MM, Morrow M. The woman at increased risk for breast cancer: evaluation and management strategies. CA Cancer J Clin. 1995;45:263-278.
1. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
2. Breast Cancer Assessment Tool. Available at: www.cancer.gov/bcrisktool/Default.aspx. Accessed June 5, 2009.
3. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
4. Ruffin MT, 4th, August DA, Kelloff GJ, Boone CW, Weber BL, Brenner DE. Selection criteria for breast cancer chemoprevention subjects. J Cell Biochem Suppl. 1993;17G:234-241.
5. Cummings SR, Duong T, Kenyon E, Cauley JA, Whitehead M, Krueger KA. For the Multiple Outcomes of Raloxifene Evaluation (MORE) Trial. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220.
6. Jordan VC, Allen KE. Evaluation of the antitumor activity of the non-steroidal antioestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma mode. Eur J Cancer. 1980;16:239-251.
7. Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988;319:1681-1692.
8. Bur ME, Zimarowski MJ, Schnitt SJ, Baker S, Lew R. Estrogen receptor immunohistochemistry in carcinoma in situ of the breast. Cancer. 1992;69:1174-1181.
9. Hol T, Cox MB, Bryant HU, Draper MW. Selective estrogen receptor modulators and postmenopausal women’s health. J Womens Health. 1997;6:523-531.
10. Buzdar AU, Marcus C, Holmes F, Hug V, Hortobagyi G. Phase II evaluation of LY156758 in metastatic breast cancer. Oncology. 1988;45:344-345.
11. Neven P, De Muylder X, Van Belle Y, Vanderick G, De Muylder E. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
12. Goldstein SR, Scheele WH, Rajagopalan SK, Wilkie JL, Walsh BW, Parsons AK. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
13. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Breast Cancer Res Treat. 2001;65:125-134.
14. Martino S, Cauley JA, Barrett-Connor E, et al. For the CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751-1761.
15. Vogel VG, Costantino JP, Wickerham DL, et al. For the National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;21:2727-2741.
16. Barrett-Connor E, Mosca L, Collins P, et al. For the Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137.
17. Santen RJ, Yue W, Naftolin F, Mor G, Berstein L. The potential of aromatase inhibitors in breast cancer prevention. Endocr Relat Cancer. 1999;6:235-243.
18. Goss PE, Strasser K. Aromatase inhibitors in the treatment and prevention of breast cancer. J Clin Oncol. 2001;19:881-894.
19. Bryant HU, Dere WH. Selective estrogen receptor modulators: an alternative to hormone replacement therapy. Proc Soc Exp Biol Med. 1998;217:45-52.
20. Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol. 1995;85:304-313.
21. Miller BA, Feuer EJ, Hankey BF. The significance of the rising incidence of breast cancer in the United States. In: DeVita VT, Hellman S, Rosenberg SA, eds. Important Advances in Oncology. Philadelphia: Lippincott; 1994:193-207.
22. Spicer DV, Pike MC. Risk factors in breast cancer. In: Roses DF, ed. Breast Cancer. New York: Churchill Livingston; 1944.
23. Bilimoria MM, Morrow M. The woman at increased risk for breast cancer: evaluation and management strategies. CA Cancer J Clin. 1995;45:263-278.
OSTEOPOROSIS
Dr. Goldstein serves on the advisory boards of Eli Lilly, Pfizer, GlaxoSmithKline, Novo Nordisk, Novartis, Procter & Gamble, Upsher Smith, and Wyeth; is a consultant for Cook ObGyn and Ackrad Labs (a Cooper Co.); and is a speaker for Eli Lilly, Novo Nordisk, Procter & Gamble, and Wyeth.
- release of the long-awaited fracture risk-assessment tool, FRAX, from the World Health Organization
- release of updated guidelines on osteoporosis treatment from the National Osteoporosis Foundation—the first revision since 2003
- investigations of a possible association between atrial fibrillation and oral bisphosphonates
- release of guidelines on diagnosis, risk identification, prevention, and management of bisphosphonate-associated osteonecrosis of the jaw
- reports of low-energy femoral-shaft fractures associated with long-term use of alendronate
- report of data from a comparison of alendronate and denosumab, a new antiresorptive agent.
Each of these is explored in detail in this review.
FRAX tool makes it possible to direct therapy to women who need it most
The World Health Organization (WHO) has finally released the FRAX risk-assessment tool, which enables clinicians to calculate a woman’s 10-year risk of developing a hip fracture or any major osteoporotic fracture. The tool (at www.shef.ac.uk/FRAX) should, ultimately, be available as part of all dual-energy x-ray absorptiometry (DXA) software so that, when bone mass is measured, the patient’s 10-year risk of hip fracture and overall osteoporotic fracture is reported along with bone density.
FRAX has different thresholds for treatment from country to country, depending on resources available. The tool uses age, weight, height, fracture history, parental fracture history, smoking status, glucocorticoid use, history of rheumatoid arthritis, alcohol consumption, and bone mineral density (BMD) of the femoral neck to determine a woman’s risk of fracture.
In many respects, this tool is a welcome change from the use of BMD measurements alone. I have long been concerned that many clinicians base treatment decisions solely on T-scores. Compare, for example, a 51-year-old newly menopausal woman who has a T-score of -2.0 at the hip with a 67-year-old woman who has the same T-score but who entered menopause at age 48 with a T-score of 0. These women have the same bone mass but very different degrees of bone quality and fracture risk.
Nevertheless, use of an arbitrary threshold (i.e., 3% risk of hip fracture and 20% risk of any osteoporotic fracture over the next 10 years) to determine who gets treatment has limitations. Virtually all bone experts would agree that a pharmacotherapeutic agent that reduces hip fracture by 50% is a “home run.” However, if we deny treatment until a woman’s 10-year risk of hip fracture reaches 3%, that is the same as saying that, for every 100 women who are treated, only 1.5 will fracture a hip instead of three. The health establishment may call that cost-effective, but it will not be acceptable to all patients.
Moreover, patients do not always understand the difference between risk reduction and prevention. It pays to remember these facts when counseling women.
NOF uses new risk-assessment tool to refine treatment guidelines
National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Available at: www.nof.org/professionals/clinicians_guide_landing_pg.htm. Accessed October 8, 2008.
Dawson-Hughes B, Tosteson ANA, Melton LJ 3rd, et al, for the National Osteoporosis Foundation Guide Committee. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int. 2008;19:449–458.
Siris E, Delmas PD. Assessment of 10-year absolute fracture risk: a new paradigm with worldwide application [editorial]. Osteoporos Int. 2008;19:383–384.
In February, the National Osteoporosis Foundation (NOF) updated its Clinician’s Guide to Prevention and Treatment of Osteoporosis, first published in 1999 and last revised (with minor changes) in 2003. The guidelines are available at www.nof.org/professionals/clinicians_guide_landing_pg.htm, along with a link to the WHO fracture risk-assessment tool, FRAX (www.shef.ac.uk/FRAX).
The previous NOF guidelines applied only to postmenopausal white women and based recommendations for intervention entirely on a patient’s T-score, with some modification of the level of intervention with the presence of clinical risk factors. The new guidelines make use of FRAX to focus recommendations on those at highest risk of fracture.
When to begin treatment
The new NOF guidelines advise the practitioner to:
- check for secondary causes of osteoporosis
- recommend BMD testing for women 65 years and older, for younger postmenopausal women when the risk-factor profile raises concern, and when there is a history of fracture
- initiate treatment in women who have had hip or vertebral fracture
- initiate treatment in women who have a DXA-based T-score ≤-2.5 at the femoral neck, total hip, or spine
- initiate treatment in postmenopausal women who have low bone mass (T-score >-2.5 but <-1.0) and a 10-year risk of hip fracture ≥3% or a 10-year probability of any major osteoporosis-related fracture >20%, based on the US-adopted WHO absolute fracture risk model
- measure BMD in DXA centers that use accepted quality assurance measures appropriate for monitoring bone loss every 2 years. For patients on pharmacotherapy, DXA BMD testing is typically performed 2 years after initiating therapy and at 2-year intervals thereafter.
New determinants of treatment
These guidelines replace earlier ones in which all postmenopausal women who had a T-score <-2.0 and those who had a T-score <-1.5 “with risk factors” were candidates for therapy.
Treatment shifts to older population
The new guidelines will probably shift some treatment from younger patients who have a modestly reduced BMD to an older population more likely to have a higher risk of fracture.
For example, consider the following patient—a 52-year-old Caucasian woman who:
- is 5 ft 4 in tall and weighs 130 lb
- has no family or personal history of fracture
- doesn’t smoke or use alcohol excessively
- doesn’t use glucocorticoids
- has no rheumatoid arthritis
- has a femoral-neck T-score of -2.1.
She has a 10-year risk of hip fracture of 1.5% and an 8.5% risk of any major osteoporotic fracture. Therefore, she is no longer a candidate for pharmacotherapy. (Under the previous guidelines, she was.)
Conversely, a 77-year-old woman who has the same height, weight, and history and a T-score of the femoral neck of -1.4, has a 10-year risk of hip fracture of 2.7% and a 23% risk of any major osteoporotic fracture. She is now a candidate for pharmacotherapy. (Under the previous guidelines, she was not a candidate.)
How to counsel the patient
The updated guidelines also include a range of recommendations on what information to include in patient counseling:
- the risk of osteoporosis and related fracture
- the need to get adequate calcium (1,200 mg/day) and vitamin D (800 to 1,000 IU/day)
- the importance of regular weight-bearing and muscle-strengthening exercise to reduce the risk of fall and fracture
- the need to avoid smoking and excess alcohol intake.
Oral bisphosphonates and atrial fibrillation—is there a link?
Heckbert SR, Li G, Cummings SR, Smith NL, Psaty BM. Use of alendronate and risk of incident atrial fibrillation in women. Arch Intern Med. 2008;168:826–831.
Black DM, Delmas PD, Eastell R, et al, for the HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822.
Sørensen HT, Christensen S, Mehnert F, et al. Use of bisphosphonates among women and risk of atrial fibrillation and flutter: population-based case-control study. BMJ. 2008;336:813–816.
Postmenopausal women who have osteoporosis and are treated with once-yearly IV zoledronic acid have a higher risk of serious atrial fibrillation than nonusers do, according to a recent publication from the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) trial. This finding was unexpected and had not been recognized previously. But does it indicate elevated risk with oral bisphosphonate use?
In the Fracture Intervention Trial (FIT) of alendronate for patients who have osteoporosis, the risk of serious atrial fibrillation was higher in alendronate recipients (1.5%, n=47) than in nonusers (1.0%, n=31).1 However, this difference did not quite reach statistical significance (p=.07).
One case-control study points to 3% risk
The findings in regard to annual infusion of zoledronic acid prompted further evaluation of oral bisphosphonates. Heckbert and colleagues conducted a population-based case-control study at Group Health, an integrated health-care delivery system in Washington state, and estimated that 3% of incident atrial fibrillation might be explained by alendronate use.
Over 3 years, they identified 719 women who had a confirmed history of atrial fibrillation and 966 controls who did not, selected at random from the Group Health enrollment but matched for age and presence or absence of treated hypertension. More atrial fibrillation case patients than controls had ever used alendronate (6.5% [n=47] vs 4.1% [n=40]; p=.03).
Compared with never users of any bisphosphonate, those who had used alendronate had a higher risk of incident atrial fibrillation (odds ratio, 1.86; 95% confidence interval [CI], 1.09–3.15) after adjustment for matching variables, a diagnosis of osteoporosis, and history of cardiovascular disease.
Second case-control study finds no elevated risk
Sørensen and associates conducted a case-control study using medical databases in Denmark and concluded that there is no increased risk of atrial fibrillation and flutter with use of an oral bisphosphonate. They identified 13,586 patients who had atrial fibrillation and flutter and 65,054 patients who did not. Of these, 435 cases (3.2%) and 1,958 controls (2.9%) were current users of a bisphosphonate for osteoporosis. Etidronate and alendronate were used with almost the same frequency among cases and controls. The adjusted relative risk of atrial fibrillation with current use of a bisphosphonate, compared with nonuse, was 0.95 (95% CI, 0.84–1.07). New users had a relative risk of 0.75 (95% CI, 0.49–1.16), broadly similar to the estimate for continuing users (relative risk, 0.96; 95% CI, 0.85–1.09).
Bottom line? There is no compelling evidence that oral bisphosphonates cause an increase in atrial fibrillation. Even in the smaller case-control study that found a suggestion of elevated risk, the authors think that, at most, 3% of cases of atrial fibrillation might be attributable to oral alendronate.
An approach to osteonecrosis of the jaw among bisphosphonate users
Khan AA, Sándor GK, Dore E, et al. Canadian consensus practice guidelines for bisphosphonate-associated osteonecrosis of the jaw. J Rheumatol. 2008;35:1391–1397.
Since 2003, when the first reports of osteonecrosis of the jaw (ONJ) in patients receiving bisphosphonates were published, there has been widespread uncertainty among patients, physicians, and oral surgeons about diagnosis, identification of individuals at risk, prevention, and management of this troubling disorder (FIGURE 1).
To address these concerns, a multidisciplinary task force was convened by the Canadian Association of Oral and Maxillofacial Surgeons to systematically review the data. The task force included representatives from national and international societies representing the disciplines of oral surgery, dentistry, oral pathology, oral medicine, endocrinology, rheumatology, and oncology.
After reviewing the data, the task force made the following recommendations:
- In all oncology patients, a thorough dental examination, including radiographs, should be completed before IV bisphosphonate therapy is initiated. In this population, any invasive dental procedure is ideally completed before the start of high-dose bisphosphonate therapy. For nonurgent procedures in current users of bisphosphonate therapy, the drug should be discontinued 3 to 6 months before the dental treatment.
- Nononcology patients who are starting oral or IV bisphosphonate therapy do not require a dental examination beforehand, provided dental care is appropriate and oral hygiene is good.
- All patients taking a bisphosponate should be encouraged to stop smoking, limit alcohol use, and maintain good oral hygiene.
- Patients who have already been diagnosed with ONJ are best managed with supportive care, including pain control, treatment of secondary infection, and removal of necrotic debris. Aggressive debridement is contraindicated.
These recommendations are extremely helpful, especially because they make it clear that the average patient who has osteoporosis does not need to discontinue therapy before undergoing a dental procedure. Nor do patients who are about to embark on therapy—oral or IV—need any special dental examination as long as they maintain good oral hygiene and dental self-care.
Task force members were identified on the basis of their knowledge and expertise in the diagnosis and management of ONJ.
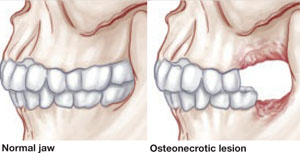
FIGURE 1 Osteonecrosis of the jaw
Blood flow to bone tissue is decreased in osteonecrosis of the jaw, leading to death of that tissue and the eventual collapse of bone.
ILLUSTRATIONS BY ROB FLEWELL FOR OBG MANAGEMENT
Distinctive fracture pattern linked to long-term alendronate
Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008;22:346–350.
Patients who sustain a fracture of the proximal femoral shaft after minimal or no trauma are likely to be long-term users of alendronate, according to a recent study. These fractures are characterized by a simple transverse pattern, “beaking” of the cortex on one side, and hypertrophy of the diaphyseal cortex (FIGURE 2).
In a retrospective study, Neviaser and colleagues blindly reviewed both radiographs and medical records of 59 patients who had femoral-shaft fractures. Among the 25 users of alendronate, 19 had experienced low- or no-trauma fractures with this distinctive pattern; only one nonuser had (odds ratio, 139.33; 95% CI, 19.0–939.4; p<.0001). This fracture pattern was 98% specific to alendronate use.
The average duration of alendronate use in patients who had this fracture pattern was significantly longer than in those who did not (6.9 years vs 2.5 years, respectively; p=.002). Only one patient with this fracture pattern had been taking alendronate for less than 4 years.
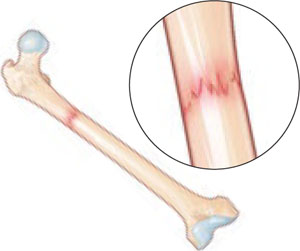
FIGURE 2 Low-impact femoral fracture
Simple transverse fractures of the proximal femur after low or no trauma have been linked to long-term alendronate use.
First reports came in 2005
Neviaser and associates mention case reports from 2005 that described nine patients who sustained spontaneous nontraumatic, nonpathologic fractures while on prolonged alendronate therapy (>3 years).2 In 2007, Goh and colleagues reported 13 subtrochanteric fractures, nine of which occurred in patients treated with alendronate. Of the nine, eight had a pattern associated with cortical hypertrophy.3
Cause-and-effect relationship remains unproven
The proximal femoral shaft is normally subjected to high stress, Neviaser and colleagues observe, and would not be expected to fracture from minimal trauma without underlying bone pathology.
In their study, 11 patients who had untreated osteoporosis had femoral-shaft fractures, but none had this specific pattern (unicortical beak, hypertrophied diaphyseal cortex). The authors hypothesize that adynamic metabolism from impaired resorption may be the underlying pathophysiology that leads to these fractures. They also point out that, although the pattern was 98% specific to alendronate users, this does not necessarily prove cause and effect—only an association. Clearly, further study is necessary.
Denosumab outperforms alendronate in phase 3 trial
Brown JP, Prince RL, Deal C, et al. Comparison of the effect of denosumab and alendronate on bone mineral density and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2008; Sep 3 [Epub ahead of print].
In the first head-to-head comparison of a nonbisphosphonate with alendronate, Brown and colleagues found significantly increased BMD at the total hip with denosumab after 12 months of use (3.5% vs 2.6%; p<.0001). This finding was reported at the American Society of Bone and Mineral Research annual meeting in Montreal in September.
Denosumab is an antiresorptive agent that inhibits osteoclast-mediated bone resorption and works through a different pathway than bisphosphonates. It is a fully human monoclonal antibody that neutralizes RANKL, a key mediator of osteoclast function, formation, and survival. Denosumab is injectable (subcutaneous) and is given every 6 months.
All sites showed improvement in BMD
In the phase 3 trial, 1,189 postmenopausal women who had a T-score at the total hip or lumbar spine ≤-2.0 were randomized to receive a subcutaneous injection of denosumab (60 mg every 6 months plus an oral placebo weekly) or oral alendronate (70 mg weekly plus a subcutaneous placebo injection every 6 months). Bone mineral density was monitored at various sites to detect any changes, as were bone-turnover markers at various times during the study.
In addition to BMD at the total hip, denosumab increased BMD at the following sites at 12 months, compared with alendronate:
- femoral neck, 0.6%
- trochanter, 1.0%
- lumbar spine, 1.1%
- distal radius, 0.6% (p≤.0002 at all sites).
Denosumab also was associated with a significantly greater reduction of bone-turnover markers than alendronate. The two groups had similar laboratory values and adverse events.
Although these preliminary results are extremely encouraging, we await data on fracture reduction from a study under way in postmenopausal women who have osteoporosis before definitive recommendations can be made about this agent.
1. Cummings SR, Schwartz AV, Black DM. Alendronate and atrial fibrillation [letter]. N Engl J Med. 2007;356:1895-1896.
2. Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294-1301.
3. Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349-353.
Dr. Goldstein serves on the advisory boards of Eli Lilly, Pfizer, GlaxoSmithKline, Novo Nordisk, Novartis, Procter & Gamble, Upsher Smith, and Wyeth; is a consultant for Cook ObGyn and Ackrad Labs (a Cooper Co.); and is a speaker for Eli Lilly, Novo Nordisk, Procter & Gamble, and Wyeth.
- release of the long-awaited fracture risk-assessment tool, FRAX, from the World Health Organization
- release of updated guidelines on osteoporosis treatment from the National Osteoporosis Foundation—the first revision since 2003
- investigations of a possible association between atrial fibrillation and oral bisphosphonates
- release of guidelines on diagnosis, risk identification, prevention, and management of bisphosphonate-associated osteonecrosis of the jaw
- reports of low-energy femoral-shaft fractures associated with long-term use of alendronate
- report of data from a comparison of alendronate and denosumab, a new antiresorptive agent.
Each of these is explored in detail in this review.
FRAX tool makes it possible to direct therapy to women who need it most
The World Health Organization (WHO) has finally released the FRAX risk-assessment tool, which enables clinicians to calculate a woman’s 10-year risk of developing a hip fracture or any major osteoporotic fracture. The tool (at www.shef.ac.uk/FRAX) should, ultimately, be available as part of all dual-energy x-ray absorptiometry (DXA) software so that, when bone mass is measured, the patient’s 10-year risk of hip fracture and overall osteoporotic fracture is reported along with bone density.
FRAX has different thresholds for treatment from country to country, depending on resources available. The tool uses age, weight, height, fracture history, parental fracture history, smoking status, glucocorticoid use, history of rheumatoid arthritis, alcohol consumption, and bone mineral density (BMD) of the femoral neck to determine a woman’s risk of fracture.
In many respects, this tool is a welcome change from the use of BMD measurements alone. I have long been concerned that many clinicians base treatment decisions solely on T-scores. Compare, for example, a 51-year-old newly menopausal woman who has a T-score of -2.0 at the hip with a 67-year-old woman who has the same T-score but who entered menopause at age 48 with a T-score of 0. These women have the same bone mass but very different degrees of bone quality and fracture risk.
Nevertheless, use of an arbitrary threshold (i.e., 3% risk of hip fracture and 20% risk of any osteoporotic fracture over the next 10 years) to determine who gets treatment has limitations. Virtually all bone experts would agree that a pharmacotherapeutic agent that reduces hip fracture by 50% is a “home run.” However, if we deny treatment until a woman’s 10-year risk of hip fracture reaches 3%, that is the same as saying that, for every 100 women who are treated, only 1.5 will fracture a hip instead of three. The health establishment may call that cost-effective, but it will not be acceptable to all patients.
Moreover, patients do not always understand the difference between risk reduction and prevention. It pays to remember these facts when counseling women.
NOF uses new risk-assessment tool to refine treatment guidelines
National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Available at: www.nof.org/professionals/clinicians_guide_landing_pg.htm. Accessed October 8, 2008.
Dawson-Hughes B, Tosteson ANA, Melton LJ 3rd, et al, for the National Osteoporosis Foundation Guide Committee. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int. 2008;19:449–458.
Siris E, Delmas PD. Assessment of 10-year absolute fracture risk: a new paradigm with worldwide application [editorial]. Osteoporos Int. 2008;19:383–384.
In February, the National Osteoporosis Foundation (NOF) updated its Clinician’s Guide to Prevention and Treatment of Osteoporosis, first published in 1999 and last revised (with minor changes) in 2003. The guidelines are available at www.nof.org/professionals/clinicians_guide_landing_pg.htm, along with a link to the WHO fracture risk-assessment tool, FRAX (www.shef.ac.uk/FRAX).
The previous NOF guidelines applied only to postmenopausal white women and based recommendations for intervention entirely on a patient’s T-score, with some modification of the level of intervention with the presence of clinical risk factors. The new guidelines make use of FRAX to focus recommendations on those at highest risk of fracture.
When to begin treatment
The new NOF guidelines advise the practitioner to:
- check for secondary causes of osteoporosis
- recommend BMD testing for women 65 years and older, for younger postmenopausal women when the risk-factor profile raises concern, and when there is a history of fracture
- initiate treatment in women who have had hip or vertebral fracture
- initiate treatment in women who have a DXA-based T-score ≤-2.5 at the femoral neck, total hip, or spine
- initiate treatment in postmenopausal women who have low bone mass (T-score >-2.5 but <-1.0) and a 10-year risk of hip fracture ≥3% or a 10-year probability of any major osteoporosis-related fracture >20%, based on the US-adopted WHO absolute fracture risk model
- measure BMD in DXA centers that use accepted quality assurance measures appropriate for monitoring bone loss every 2 years. For patients on pharmacotherapy, DXA BMD testing is typically performed 2 years after initiating therapy and at 2-year intervals thereafter.
New determinants of treatment
These guidelines replace earlier ones in which all postmenopausal women who had a T-score <-2.0 and those who had a T-score <-1.5 “with risk factors” were candidates for therapy.
Treatment shifts to older population
The new guidelines will probably shift some treatment from younger patients who have a modestly reduced BMD to an older population more likely to have a higher risk of fracture.
For example, consider the following patient—a 52-year-old Caucasian woman who:
- is 5 ft 4 in tall and weighs 130 lb
- has no family or personal history of fracture
- doesn’t smoke or use alcohol excessively
- doesn’t use glucocorticoids
- has no rheumatoid arthritis
- has a femoral-neck T-score of -2.1.
She has a 10-year risk of hip fracture of 1.5% and an 8.5% risk of any major osteoporotic fracture. Therefore, she is no longer a candidate for pharmacotherapy. (Under the previous guidelines, she was.)
Conversely, a 77-year-old woman who has the same height, weight, and history and a T-score of the femoral neck of -1.4, has a 10-year risk of hip fracture of 2.7% and a 23% risk of any major osteoporotic fracture. She is now a candidate for pharmacotherapy. (Under the previous guidelines, she was not a candidate.)
How to counsel the patient
The updated guidelines also include a range of recommendations on what information to include in patient counseling:
- the risk of osteoporosis and related fracture
- the need to get adequate calcium (1,200 mg/day) and vitamin D (800 to 1,000 IU/day)
- the importance of regular weight-bearing and muscle-strengthening exercise to reduce the risk of fall and fracture
- the need to avoid smoking and excess alcohol intake.
Oral bisphosphonates and atrial fibrillation—is there a link?
Heckbert SR, Li G, Cummings SR, Smith NL, Psaty BM. Use of alendronate and risk of incident atrial fibrillation in women. Arch Intern Med. 2008;168:826–831.
Black DM, Delmas PD, Eastell R, et al, for the HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822.
Sørensen HT, Christensen S, Mehnert F, et al. Use of bisphosphonates among women and risk of atrial fibrillation and flutter: population-based case-control study. BMJ. 2008;336:813–816.
Postmenopausal women who have osteoporosis and are treated with once-yearly IV zoledronic acid have a higher risk of serious atrial fibrillation than nonusers do, according to a recent publication from the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) trial. This finding was unexpected and had not been recognized previously. But does it indicate elevated risk with oral bisphosphonate use?
In the Fracture Intervention Trial (FIT) of alendronate for patients who have osteoporosis, the risk of serious atrial fibrillation was higher in alendronate recipients (1.5%, n=47) than in nonusers (1.0%, n=31).1 However, this difference did not quite reach statistical significance (p=.07).
One case-control study points to 3% risk
The findings in regard to annual infusion of zoledronic acid prompted further evaluation of oral bisphosphonates. Heckbert and colleagues conducted a population-based case-control study at Group Health, an integrated health-care delivery system in Washington state, and estimated that 3% of incident atrial fibrillation might be explained by alendronate use.
Over 3 years, they identified 719 women who had a confirmed history of atrial fibrillation and 966 controls who did not, selected at random from the Group Health enrollment but matched for age and presence or absence of treated hypertension. More atrial fibrillation case patients than controls had ever used alendronate (6.5% [n=47] vs 4.1% [n=40]; p=.03).
Compared with never users of any bisphosphonate, those who had used alendronate had a higher risk of incident atrial fibrillation (odds ratio, 1.86; 95% confidence interval [CI], 1.09–3.15) after adjustment for matching variables, a diagnosis of osteoporosis, and history of cardiovascular disease.
Second case-control study finds no elevated risk
Sørensen and associates conducted a case-control study using medical databases in Denmark and concluded that there is no increased risk of atrial fibrillation and flutter with use of an oral bisphosphonate. They identified 13,586 patients who had atrial fibrillation and flutter and 65,054 patients who did not. Of these, 435 cases (3.2%) and 1,958 controls (2.9%) were current users of a bisphosphonate for osteoporosis. Etidronate and alendronate were used with almost the same frequency among cases and controls. The adjusted relative risk of atrial fibrillation with current use of a bisphosphonate, compared with nonuse, was 0.95 (95% CI, 0.84–1.07). New users had a relative risk of 0.75 (95% CI, 0.49–1.16), broadly similar to the estimate for continuing users (relative risk, 0.96; 95% CI, 0.85–1.09).
Bottom line? There is no compelling evidence that oral bisphosphonates cause an increase in atrial fibrillation. Even in the smaller case-control study that found a suggestion of elevated risk, the authors think that, at most, 3% of cases of atrial fibrillation might be attributable to oral alendronate.
An approach to osteonecrosis of the jaw among bisphosphonate users
Khan AA, Sándor GK, Dore E, et al. Canadian consensus practice guidelines for bisphosphonate-associated osteonecrosis of the jaw. J Rheumatol. 2008;35:1391–1397.
Since 2003, when the first reports of osteonecrosis of the jaw (ONJ) in patients receiving bisphosphonates were published, there has been widespread uncertainty among patients, physicians, and oral surgeons about diagnosis, identification of individuals at risk, prevention, and management of this troubling disorder (FIGURE 1).
To address these concerns, a multidisciplinary task force was convened by the Canadian Association of Oral and Maxillofacial Surgeons to systematically review the data. The task force included representatives from national and international societies representing the disciplines of oral surgery, dentistry, oral pathology, oral medicine, endocrinology, rheumatology, and oncology.
After reviewing the data, the task force made the following recommendations:
- In all oncology patients, a thorough dental examination, including radiographs, should be completed before IV bisphosphonate therapy is initiated. In this population, any invasive dental procedure is ideally completed before the start of high-dose bisphosphonate therapy. For nonurgent procedures in current users of bisphosphonate therapy, the drug should be discontinued 3 to 6 months before the dental treatment.
- Nononcology patients who are starting oral or IV bisphosphonate therapy do not require a dental examination beforehand, provided dental care is appropriate and oral hygiene is good.
- All patients taking a bisphosponate should be encouraged to stop smoking, limit alcohol use, and maintain good oral hygiene.
- Patients who have already been diagnosed with ONJ are best managed with supportive care, including pain control, treatment of secondary infection, and removal of necrotic debris. Aggressive debridement is contraindicated.
These recommendations are extremely helpful, especially because they make it clear that the average patient who has osteoporosis does not need to discontinue therapy before undergoing a dental procedure. Nor do patients who are about to embark on therapy—oral or IV—need any special dental examination as long as they maintain good oral hygiene and dental self-care.
Task force members were identified on the basis of their knowledge and expertise in the diagnosis and management of ONJ.

FIGURE 1 Osteonecrosis of the jaw
Blood flow to bone tissue is decreased in osteonecrosis of the jaw, leading to death of that tissue and the eventual collapse of bone.
ILLUSTRATIONS BY ROB FLEWELL FOR OBG MANAGEMENT
Distinctive fracture pattern linked to long-term alendronate
Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008;22:346–350.
Patients who sustain a fracture of the proximal femoral shaft after minimal or no trauma are likely to be long-term users of alendronate, according to a recent study. These fractures are characterized by a simple transverse pattern, “beaking” of the cortex on one side, and hypertrophy of the diaphyseal cortex (FIGURE 2).
In a retrospective study, Neviaser and colleagues blindly reviewed both radiographs and medical records of 59 patients who had femoral-shaft fractures. Among the 25 users of alendronate, 19 had experienced low- or no-trauma fractures with this distinctive pattern; only one nonuser had (odds ratio, 139.33; 95% CI, 19.0–939.4; p<.0001). This fracture pattern was 98% specific to alendronate use.
The average duration of alendronate use in patients who had this fracture pattern was significantly longer than in those who did not (6.9 years vs 2.5 years, respectively; p=.002). Only one patient with this fracture pattern had been taking alendronate for less than 4 years.

FIGURE 2 Low-impact femoral fracture
Simple transverse fractures of the proximal femur after low or no trauma have been linked to long-term alendronate use.
First reports came in 2005
Neviaser and associates mention case reports from 2005 that described nine patients who sustained spontaneous nontraumatic, nonpathologic fractures while on prolonged alendronate therapy (>3 years).2 In 2007, Goh and colleagues reported 13 subtrochanteric fractures, nine of which occurred in patients treated with alendronate. Of the nine, eight had a pattern associated with cortical hypertrophy.3
Cause-and-effect relationship remains unproven
The proximal femoral shaft is normally subjected to high stress, Neviaser and colleagues observe, and would not be expected to fracture from minimal trauma without underlying bone pathology.
In their study, 11 patients who had untreated osteoporosis had femoral-shaft fractures, but none had this specific pattern (unicortical beak, hypertrophied diaphyseal cortex). The authors hypothesize that adynamic metabolism from impaired resorption may be the underlying pathophysiology that leads to these fractures. They also point out that, although the pattern was 98% specific to alendronate users, this does not necessarily prove cause and effect—only an association. Clearly, further study is necessary.
Denosumab outperforms alendronate in phase 3 trial
Brown JP, Prince RL, Deal C, et al. Comparison of the effect of denosumab and alendronate on bone mineral density and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2008; Sep 3 [Epub ahead of print].
In the first head-to-head comparison of a nonbisphosphonate with alendronate, Brown and colleagues found significantly increased BMD at the total hip with denosumab after 12 months of use (3.5% vs 2.6%; p<.0001). This finding was reported at the American Society of Bone and Mineral Research annual meeting in Montreal in September.
Denosumab is an antiresorptive agent that inhibits osteoclast-mediated bone resorption and works through a different pathway than bisphosphonates. It is a fully human monoclonal antibody that neutralizes RANKL, a key mediator of osteoclast function, formation, and survival. Denosumab is injectable (subcutaneous) and is given every 6 months.
All sites showed improvement in BMD
In the phase 3 trial, 1,189 postmenopausal women who had a T-score at the total hip or lumbar spine ≤-2.0 were randomized to receive a subcutaneous injection of denosumab (60 mg every 6 months plus an oral placebo weekly) or oral alendronate (70 mg weekly plus a subcutaneous placebo injection every 6 months). Bone mineral density was monitored at various sites to detect any changes, as were bone-turnover markers at various times during the study.
In addition to BMD at the total hip, denosumab increased BMD at the following sites at 12 months, compared with alendronate:
- femoral neck, 0.6%
- trochanter, 1.0%
- lumbar spine, 1.1%
- distal radius, 0.6% (p≤.0002 at all sites).
Denosumab also was associated with a significantly greater reduction of bone-turnover markers than alendronate. The two groups had similar laboratory values and adverse events.
Although these preliminary results are extremely encouraging, we await data on fracture reduction from a study under way in postmenopausal women who have osteoporosis before definitive recommendations can be made about this agent.
Dr. Goldstein serves on the advisory boards of Eli Lilly, Pfizer, GlaxoSmithKline, Novo Nordisk, Novartis, Procter & Gamble, Upsher Smith, and Wyeth; is a consultant for Cook ObGyn and Ackrad Labs (a Cooper Co.); and is a speaker for Eli Lilly, Novo Nordisk, Procter & Gamble, and Wyeth.
- release of the long-awaited fracture risk-assessment tool, FRAX, from the World Health Organization
- release of updated guidelines on osteoporosis treatment from the National Osteoporosis Foundation—the first revision since 2003
- investigations of a possible association between atrial fibrillation and oral bisphosphonates
- release of guidelines on diagnosis, risk identification, prevention, and management of bisphosphonate-associated osteonecrosis of the jaw
- reports of low-energy femoral-shaft fractures associated with long-term use of alendronate
- report of data from a comparison of alendronate and denosumab, a new antiresorptive agent.
Each of these is explored in detail in this review.
FRAX tool makes it possible to direct therapy to women who need it most
The World Health Organization (WHO) has finally released the FRAX risk-assessment tool, which enables clinicians to calculate a woman’s 10-year risk of developing a hip fracture or any major osteoporotic fracture. The tool (at www.shef.ac.uk/FRAX) should, ultimately, be available as part of all dual-energy x-ray absorptiometry (DXA) software so that, when bone mass is measured, the patient’s 10-year risk of hip fracture and overall osteoporotic fracture is reported along with bone density.
FRAX has different thresholds for treatment from country to country, depending on resources available. The tool uses age, weight, height, fracture history, parental fracture history, smoking status, glucocorticoid use, history of rheumatoid arthritis, alcohol consumption, and bone mineral density (BMD) of the femoral neck to determine a woman’s risk of fracture.
In many respects, this tool is a welcome change from the use of BMD measurements alone. I have long been concerned that many clinicians base treatment decisions solely on T-scores. Compare, for example, a 51-year-old newly menopausal woman who has a T-score of -2.0 at the hip with a 67-year-old woman who has the same T-score but who entered menopause at age 48 with a T-score of 0. These women have the same bone mass but very different degrees of bone quality and fracture risk.
Nevertheless, use of an arbitrary threshold (i.e., 3% risk of hip fracture and 20% risk of any osteoporotic fracture over the next 10 years) to determine who gets treatment has limitations. Virtually all bone experts would agree that a pharmacotherapeutic agent that reduces hip fracture by 50% is a “home run.” However, if we deny treatment until a woman’s 10-year risk of hip fracture reaches 3%, that is the same as saying that, for every 100 women who are treated, only 1.5 will fracture a hip instead of three. The health establishment may call that cost-effective, but it will not be acceptable to all patients.
Moreover, patients do not always understand the difference between risk reduction and prevention. It pays to remember these facts when counseling women.
NOF uses new risk-assessment tool to refine treatment guidelines
National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Available at: www.nof.org/professionals/clinicians_guide_landing_pg.htm. Accessed October 8, 2008.
Dawson-Hughes B, Tosteson ANA, Melton LJ 3rd, et al, for the National Osteoporosis Foundation Guide Committee. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int. 2008;19:449–458.
Siris E, Delmas PD. Assessment of 10-year absolute fracture risk: a new paradigm with worldwide application [editorial]. Osteoporos Int. 2008;19:383–384.
In February, the National Osteoporosis Foundation (NOF) updated its Clinician’s Guide to Prevention and Treatment of Osteoporosis, first published in 1999 and last revised (with minor changes) in 2003. The guidelines are available at www.nof.org/professionals/clinicians_guide_landing_pg.htm, along with a link to the WHO fracture risk-assessment tool, FRAX (www.shef.ac.uk/FRAX).
The previous NOF guidelines applied only to postmenopausal white women and based recommendations for intervention entirely on a patient’s T-score, with some modification of the level of intervention with the presence of clinical risk factors. The new guidelines make use of FRAX to focus recommendations on those at highest risk of fracture.
When to begin treatment
The new NOF guidelines advise the practitioner to:
- check for secondary causes of osteoporosis
- recommend BMD testing for women 65 years and older, for younger postmenopausal women when the risk-factor profile raises concern, and when there is a history of fracture
- initiate treatment in women who have had hip or vertebral fracture
- initiate treatment in women who have a DXA-based T-score ≤-2.5 at the femoral neck, total hip, or spine
- initiate treatment in postmenopausal women who have low bone mass (T-score >-2.5 but <-1.0) and a 10-year risk of hip fracture ≥3% or a 10-year probability of any major osteoporosis-related fracture >20%, based on the US-adopted WHO absolute fracture risk model
- measure BMD in DXA centers that use accepted quality assurance measures appropriate for monitoring bone loss every 2 years. For patients on pharmacotherapy, DXA BMD testing is typically performed 2 years after initiating therapy and at 2-year intervals thereafter.
New determinants of treatment
These guidelines replace earlier ones in which all postmenopausal women who had a T-score <-2.0 and those who had a T-score <-1.5 “with risk factors” were candidates for therapy.
Treatment shifts to older population
The new guidelines will probably shift some treatment from younger patients who have a modestly reduced BMD to an older population more likely to have a higher risk of fracture.
For example, consider the following patient—a 52-year-old Caucasian woman who:
- is 5 ft 4 in tall and weighs 130 lb
- has no family or personal history of fracture
- doesn’t smoke or use alcohol excessively
- doesn’t use glucocorticoids
- has no rheumatoid arthritis
- has a femoral-neck T-score of -2.1.
She has a 10-year risk of hip fracture of 1.5% and an 8.5% risk of any major osteoporotic fracture. Therefore, she is no longer a candidate for pharmacotherapy. (Under the previous guidelines, she was.)
Conversely, a 77-year-old woman who has the same height, weight, and history and a T-score of the femoral neck of -1.4, has a 10-year risk of hip fracture of 2.7% and a 23% risk of any major osteoporotic fracture. She is now a candidate for pharmacotherapy. (Under the previous guidelines, she was not a candidate.)
How to counsel the patient
The updated guidelines also include a range of recommendations on what information to include in patient counseling:
- the risk of osteoporosis and related fracture
- the need to get adequate calcium (1,200 mg/day) and vitamin D (800 to 1,000 IU/day)
- the importance of regular weight-bearing and muscle-strengthening exercise to reduce the risk of fall and fracture
- the need to avoid smoking and excess alcohol intake.
Oral bisphosphonates and atrial fibrillation—is there a link?
Heckbert SR, Li G, Cummings SR, Smith NL, Psaty BM. Use of alendronate and risk of incident atrial fibrillation in women. Arch Intern Med. 2008;168:826–831.
Black DM, Delmas PD, Eastell R, et al, for the HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822.
Sørensen HT, Christensen S, Mehnert F, et al. Use of bisphosphonates among women and risk of atrial fibrillation and flutter: population-based case-control study. BMJ. 2008;336:813–816.
Postmenopausal women who have osteoporosis and are treated with once-yearly IV zoledronic acid have a higher risk of serious atrial fibrillation than nonusers do, according to a recent publication from the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) trial. This finding was unexpected and had not been recognized previously. But does it indicate elevated risk with oral bisphosphonate use?
In the Fracture Intervention Trial (FIT) of alendronate for patients who have osteoporosis, the risk of serious atrial fibrillation was higher in alendronate recipients (1.5%, n=47) than in nonusers (1.0%, n=31).1 However, this difference did not quite reach statistical significance (p=.07).
One case-control study points to 3% risk
The findings in regard to annual infusion of zoledronic acid prompted further evaluation of oral bisphosphonates. Heckbert and colleagues conducted a population-based case-control study at Group Health, an integrated health-care delivery system in Washington state, and estimated that 3% of incident atrial fibrillation might be explained by alendronate use.
Over 3 years, they identified 719 women who had a confirmed history of atrial fibrillation and 966 controls who did not, selected at random from the Group Health enrollment but matched for age and presence or absence of treated hypertension. More atrial fibrillation case patients than controls had ever used alendronate (6.5% [n=47] vs 4.1% [n=40]; p=.03).
Compared with never users of any bisphosphonate, those who had used alendronate had a higher risk of incident atrial fibrillation (odds ratio, 1.86; 95% confidence interval [CI], 1.09–3.15) after adjustment for matching variables, a diagnosis of osteoporosis, and history of cardiovascular disease.
Second case-control study finds no elevated risk
Sørensen and associates conducted a case-control study using medical databases in Denmark and concluded that there is no increased risk of atrial fibrillation and flutter with use of an oral bisphosphonate. They identified 13,586 patients who had atrial fibrillation and flutter and 65,054 patients who did not. Of these, 435 cases (3.2%) and 1,958 controls (2.9%) were current users of a bisphosphonate for osteoporosis. Etidronate and alendronate were used with almost the same frequency among cases and controls. The adjusted relative risk of atrial fibrillation with current use of a bisphosphonate, compared with nonuse, was 0.95 (95% CI, 0.84–1.07). New users had a relative risk of 0.75 (95% CI, 0.49–1.16), broadly similar to the estimate for continuing users (relative risk, 0.96; 95% CI, 0.85–1.09).
Bottom line? There is no compelling evidence that oral bisphosphonates cause an increase in atrial fibrillation. Even in the smaller case-control study that found a suggestion of elevated risk, the authors think that, at most, 3% of cases of atrial fibrillation might be attributable to oral alendronate.
An approach to osteonecrosis of the jaw among bisphosphonate users
Khan AA, Sándor GK, Dore E, et al. Canadian consensus practice guidelines for bisphosphonate-associated osteonecrosis of the jaw. J Rheumatol. 2008;35:1391–1397.
Since 2003, when the first reports of osteonecrosis of the jaw (ONJ) in patients receiving bisphosphonates were published, there has been widespread uncertainty among patients, physicians, and oral surgeons about diagnosis, identification of individuals at risk, prevention, and management of this troubling disorder (FIGURE 1).
To address these concerns, a multidisciplinary task force was convened by the Canadian Association of Oral and Maxillofacial Surgeons to systematically review the data. The task force included representatives from national and international societies representing the disciplines of oral surgery, dentistry, oral pathology, oral medicine, endocrinology, rheumatology, and oncology.
After reviewing the data, the task force made the following recommendations:
- In all oncology patients, a thorough dental examination, including radiographs, should be completed before IV bisphosphonate therapy is initiated. In this population, any invasive dental procedure is ideally completed before the start of high-dose bisphosphonate therapy. For nonurgent procedures in current users of bisphosphonate therapy, the drug should be discontinued 3 to 6 months before the dental treatment.
- Nononcology patients who are starting oral or IV bisphosphonate therapy do not require a dental examination beforehand, provided dental care is appropriate and oral hygiene is good.
- All patients taking a bisphosponate should be encouraged to stop smoking, limit alcohol use, and maintain good oral hygiene.
- Patients who have already been diagnosed with ONJ are best managed with supportive care, including pain control, treatment of secondary infection, and removal of necrotic debris. Aggressive debridement is contraindicated.
These recommendations are extremely helpful, especially because they make it clear that the average patient who has osteoporosis does not need to discontinue therapy before undergoing a dental procedure. Nor do patients who are about to embark on therapy—oral or IV—need any special dental examination as long as they maintain good oral hygiene and dental self-care.
Task force members were identified on the basis of their knowledge and expertise in the diagnosis and management of ONJ.

FIGURE 1 Osteonecrosis of the jaw
Blood flow to bone tissue is decreased in osteonecrosis of the jaw, leading to death of that tissue and the eventual collapse of bone.
ILLUSTRATIONS BY ROB FLEWELL FOR OBG MANAGEMENT
Distinctive fracture pattern linked to long-term alendronate
Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008;22:346–350.
Patients who sustain a fracture of the proximal femoral shaft after minimal or no trauma are likely to be long-term users of alendronate, according to a recent study. These fractures are characterized by a simple transverse pattern, “beaking” of the cortex on one side, and hypertrophy of the diaphyseal cortex (FIGURE 2).
In a retrospective study, Neviaser and colleagues blindly reviewed both radiographs and medical records of 59 patients who had femoral-shaft fractures. Among the 25 users of alendronate, 19 had experienced low- or no-trauma fractures with this distinctive pattern; only one nonuser had (odds ratio, 139.33; 95% CI, 19.0–939.4; p<.0001). This fracture pattern was 98% specific to alendronate use.
The average duration of alendronate use in patients who had this fracture pattern was significantly longer than in those who did not (6.9 years vs 2.5 years, respectively; p=.002). Only one patient with this fracture pattern had been taking alendronate for less than 4 years.

FIGURE 2 Low-impact femoral fracture
Simple transverse fractures of the proximal femur after low or no trauma have been linked to long-term alendronate use.
First reports came in 2005
Neviaser and associates mention case reports from 2005 that described nine patients who sustained spontaneous nontraumatic, nonpathologic fractures while on prolonged alendronate therapy (>3 years).2 In 2007, Goh and colleagues reported 13 subtrochanteric fractures, nine of which occurred in patients treated with alendronate. Of the nine, eight had a pattern associated with cortical hypertrophy.3
Cause-and-effect relationship remains unproven
The proximal femoral shaft is normally subjected to high stress, Neviaser and colleagues observe, and would not be expected to fracture from minimal trauma without underlying bone pathology.
In their study, 11 patients who had untreated osteoporosis had femoral-shaft fractures, but none had this specific pattern (unicortical beak, hypertrophied diaphyseal cortex). The authors hypothesize that adynamic metabolism from impaired resorption may be the underlying pathophysiology that leads to these fractures. They also point out that, although the pattern was 98% specific to alendronate users, this does not necessarily prove cause and effect—only an association. Clearly, further study is necessary.
Denosumab outperforms alendronate in phase 3 trial
Brown JP, Prince RL, Deal C, et al. Comparison of the effect of denosumab and alendronate on bone mineral density and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2008; Sep 3 [Epub ahead of print].
In the first head-to-head comparison of a nonbisphosphonate with alendronate, Brown and colleagues found significantly increased BMD at the total hip with denosumab after 12 months of use (3.5% vs 2.6%; p<.0001). This finding was reported at the American Society of Bone and Mineral Research annual meeting in Montreal in September.
Denosumab is an antiresorptive agent that inhibits osteoclast-mediated bone resorption and works through a different pathway than bisphosphonates. It is a fully human monoclonal antibody that neutralizes RANKL, a key mediator of osteoclast function, formation, and survival. Denosumab is injectable (subcutaneous) and is given every 6 months.
All sites showed improvement in BMD
In the phase 3 trial, 1,189 postmenopausal women who had a T-score at the total hip or lumbar spine ≤-2.0 were randomized to receive a subcutaneous injection of denosumab (60 mg every 6 months plus an oral placebo weekly) or oral alendronate (70 mg weekly plus a subcutaneous placebo injection every 6 months). Bone mineral density was monitored at various sites to detect any changes, as were bone-turnover markers at various times during the study.
In addition to BMD at the total hip, denosumab increased BMD at the following sites at 12 months, compared with alendronate:
- femoral neck, 0.6%
- trochanter, 1.0%
- lumbar spine, 1.1%
- distal radius, 0.6% (p≤.0002 at all sites).
Denosumab also was associated with a significantly greater reduction of bone-turnover markers than alendronate. The two groups had similar laboratory values and adverse events.
Although these preliminary results are extremely encouraging, we await data on fracture reduction from a study under way in postmenopausal women who have osteoporosis before definitive recommendations can be made about this agent.
1. Cummings SR, Schwartz AV, Black DM. Alendronate and atrial fibrillation [letter]. N Engl J Med. 2007;356:1895-1896.
2. Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294-1301.
3. Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349-353.
1. Cummings SR, Schwartz AV, Black DM. Alendronate and atrial fibrillation [letter]. N Engl J Med. 2007;356:1895-1896.
2. Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294-1301.
3. Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007;89:349-353.