User login
OSTEOPOROSIS
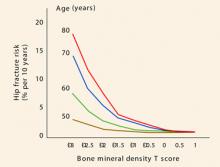
As 2007 draws to a close, we are still awaiting the World Health Organization’s fracture risk-assessment tool. The much-anticipated instrument will calculate 5- and 10-year fracture risks using an individual’s femoral neck T-score, age, history of low-trauma fracture, body mass index, steroid exposure, family history of hip fracture, smoking status, and alcohol intake. Once it is implemented, the tool will eliminate much of the confusion that arises when the T-score is the only variable used to determine the need for pharmacotherapy.
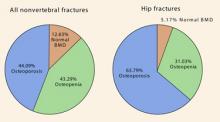
Why is the ability to stratify risk important? Although the incidence of fragility fractures is highest in osteoporotic women (as defined by the T-score), the absolute number of fractures is greater in those who have osteopenia. All clinicians should realize that the current definitions of normal bone density, osteopenia, and osteoporosis apply to the postmenopausal population only:
- normal – T-score above -1.0
- osteopenia – T-score below -1.0 but above -2.5
- osteoporosis – T-score below -2.5.
Indications added for raloxifene
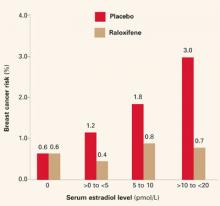
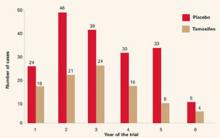
The year did bring new indications for raloxifene, based on data from the RUTH and STAR trials,1,2 which were mentioned in this Update 1 year ago. On September 14, the Food and Drug Administration approved two new indications:
- reduction of risk of invasive breast cancer in postmenopausal women with osteoporosis
- reduction of risk of invasive breast cancer in postmenopausal women at high risk of breast cancer.
These new indications are very important for clinicians who prescribe agents to prevent fragility fractures. Raloxifene should be considered for breast cancer risk reduction when deciding which agent to prescribe.
REAL study finds real advantage with risedronate
Silverman SL, Watts NB, Delmas PD, et al. Effectiveness of bisphosphonates on nonvertebral and hip fractures in the first year of therapy: the risedronate and alendronate (REAL) cohort study. Osteoporos Int. 2007;18(1):25–34.
Patients who take risedronate have lower rates of hip and nonvertebral fracture during their first year of therapy than do those who take alendronate. That is the finding of the RisedronatE and ALendronate (REAL) cohort study, a retrospective observation of the records of health-care utilization among women in the United States. Silverman and colleagues analyzed data sets for women older than age 65 who had ever used once-weekly dosing of risedronate or alendronate. The risedronate cohort included 12,215 women who were followed for a mean of 226 days of therapy. The alendronate cohort included 21,615 women followed for a mean of 238 days of therapy.
Risedronate group had more risk factors for fracture
At baseline, women taking risedronate had a statistically greater incidence of:
- advanced age
- use of concomitant medications
- glucocorticoid use
- rheumatoid arthritis.
Each of these characteristics might have been expected to increase the risk of fragility fracture. However, through 1 year of therapy, women using risedronate had an incidence of nonvertebral fractures 18% lower than those using alendronate (2.0% versus 2.3%; 95% confidence interval [CI], 0.02–0.32). They also had an incidence of hip fracture 43% lower than those using alendronate (0.4% versus 0.6%; 95% CI, 0.13–0.63). Overall, there were 507 nonvertebral fractures and 109 hip fractures.
Footnote: Large database analyses complement randomized trials
Randomized clinical trials (RCTs) are, of course, the gold standard for determining drug safety and efficacy and the key requisite for regulatory approval of new drugs. By design, RCTs have strict inclusion and exclusion criteria to meet the regulatory standard for evaluating drug efficacy and safety and to exclude internal bias. Often, the majority of patients are deemed ineligible for entry into an RCT because of comorbidity, concomitant medication use, age, or severity of disease. Therefore, database analyses are often more “real world” than RCTs.
As clinical practice data accumulate over time, observational, or outcomes, studies can be conducted to complement the data that have been generated by RCTs. For example, over the past decade, large databases of health-care utilization claims have become available in the United States and are being tapped to conduct effectiveness studies. These observational studies can supplement the efficacy measures obtained from the carefully controlled environment of a placebo-controlled RCT. They also provide a measure of effectiveness over a wide range of patients and health-care practices. Data generated from the REAL study are just another piece of the huge puzzle we must grapple with as we seek good information about agents to treat osteoporosis and prevent fragility fractures.
Annual infusion of zoledronic acid reduces risk of fracture
Black DM, Delmas PD, Eastell R, et al, for the HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822.
Lyles KW, Colon-Emeris C, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357. DOI: 10.1056/NEJMoa074941.
Zoledronic acid (Zometa) is indicated for the treatment of high levels of serum calcium associated with Paget’s disease and various malignancies (multiple myeloma, breast, prostate, and lung). These two studies explore use of this agent to prevent fracture in postmenopausal women with osteoporosis—a use for which it proved effective. Other benefits may include improved compliance and ease of administration in some women.
Black and colleagues conducted their randomized, double-blind, placebo-controlled trial to assess the effect of annual infusion of zoledronic acid on the risk of fracture over a 3-year period. A total of 3,889 postmenopausal women with osteoporosis (mean age, 73 years; range, 65–89 years) were assigned to receive a single 15-minute, 5-mg infusion of the drug at baseline, 12 months, and 24 months, and a total of 3,876 women received placebo. Approximately half the women were from Europe, and the other half were from North and South America and Asia. All women received oral daily calcium (1,000–1,500 mg) and vitamin D (400–1,200 U), and all were monitored for 36 months.
The risk of vertebral fracture was reduced in the treatment group by 70% over 3 years, compared with the placebo group (3.3% or 92 women in the treatment group versus 10.9% or 310 women receiving placebo). The risk of hip fracture was reduced by 41% in the treatment group (1.4% or 52 women receiving zoledronic acid versus 2.5% or 88 women in the placebo group). (For all comparisons, P<.001.)
The most common postdose symptoms, seen within 3 days of infusion, included fever, flu-like symptoms, myalgia, headache, and arthralgias. There were more serious adverse events related to atrial fibrillation in the women receiving zoledronic acid (50 women receiving zoledronic acid versus 20 in the placebo group; P<.001).
Treatment is a valuable option for carefully selected populations
The trial by Black and colleagues holds great promise for some patients, especially those who have (or appear to have) upper gastrointestinal intolerance of oral bisphosphonates or who may have difficulty adhering to the positional requirements (i.e., remaining upright) of oral therapy. This may be especially true of patients in nursing homes. Once-yearly intravenous (IV) infusion may also make compliance easier for these patients.
One important detail of this trial: Of almost 8,000 women studied, the youngest was age 65. The implication: Don’t automatically assume this regimen is an appropriate alternative for younger osteopenic women who perceive themselves to have acid reflux.
Women at extremely high risk of fracture also benefit
Fracture is most likely to occur in women who have already experienced it (FIGURE 1). Lyles and colleagues chose this population for their study of once-yearly infusion of zoledronic acid. The study involved 2,127 patients within 90 days after repair of low-trauma hip fracture. Subjects were randomized to receive 5-mg IV zoledronic acid annually or placebo in a blinded fashion. All patients also received vitamin D and calcium supplements. Mean age was 74.5 years, as might be expected in a hip-fracture cohort, and median follow-up was 1.9 years.
New clinical fractures occurred in 8.6% of women in the zoledronic acid group and 13.9% of the placebo group—a 35% risk reduction with IV zoledronic acid (P=.001). Deaths occurred in 101 of 1,054 patients (9.6%) in the zoledronic acid group and 141 of 1,057 patients (13.3%) in the placebo group. This was a reduction of 28% in death from any cause in the zoledronic acid group (P=.01).
The most frequent adverse events in patients receiving zoledronic acid were pyrexia, myalgia, and bone and musculoskeletal pain. No cases of osteonecrosis of the jaw were reported. Rates of atrial fibrillation and stroke were similar in both the zoledronic acid and placebo groups.
This study provides further evidence that, for extremely high-risk women (those who have already suffered hip fracture), yearly zoledronic acid may be an extremely useful tool, especially for elderly women, in whom compliance with any medication—weekly or monthly—may be difficult.
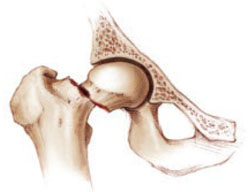
FIGURE 1 Once a fracture occurs, another is likely
Although the risk of new fractures is heightened in women who have already experienced one, Lyles and colleagues found a 35% risk reduction with zoledronic acid.
Parathyroid hormone reduces vertebral fractures in osteoporotic women
Greenspan SL, Bone HG, Ettinger MP, et al, for the Treatment of Osteoporosis with Parathyroid Hormone Study Group. Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146:326–339.
Teriparatide (Forteo) is a synthetic portion (1-34) of the parathyroid hormone (PTH) molecule that is identical in sequence to the biologically active segment of the 84-amino acid human PTH.
It has been shown to prevent fracture in women with low bone mineral density (BMD). Teriparatide is the only anabolic bone agent approved for clinical use; all other pharmacotherapies are antiresorptive.
The study by Greenspan and associates—the Treatment of Osteoporosis with Parathyroid Hormone, or TOP trial—involved the full-length PTH molecule (1–84) and provides evidence that it, too, can prevent vertebral fracture in women who have low BMD (FIGURE 2). This agent is used in Europe but not yet available in the United States.
TOP was an 18-month, randomized, double-blind, placebo-controlled, parallel-group study of 2,532 women with low BMD at the hip or lumbar spine. It was conducted at 168 centers in nine countries.
The primary outcome measure was new or worsened vertebral fracture; secondary outcomes were changes in BMD and safety. The trial investigated the safety of recombinant PTH and its effect on the incidence of vertebral fractures in postmenopausal women with osteoporosis.
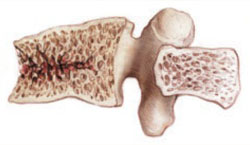
FIGURE 2 Fracture-prone bone responds to PTH
PTH prevented vertebral fracture in women with low BMD, existing fractures, or both.
Women had very low BMD, or low BMD and existing fractures
Participants were postmenopausal women aged 45 to 54 years. They had a BMD 3 standard deviations or more (T-score ≤ -3.0) below the mean peak bone mass of young adult women at the lumbar spine, femoral neck, or total hip, with no vertebral fractures, or they had a BMD T-score of -2.5 and 1 to 4 vertebral fractures. Postmenopausal women aged 55 years or older were included if their BMD T-score was -2.5 and they had no vertebral fractures, or if their BMD T-score was -2.0 and they had 1 to 4 vertebral fractures before enrollment. The women were given 100 μg of recombinant human PTH or placebo daily, as well as calcium (700 mg/day) and vitamin D3 (400 U/day).
PTH reduced the risk of new fractures or prevented worsening of existing fractures. The reduction in relative risk (RR) for vertebral fracture was 0.42 (95% CI, 0.24–0.72; P=.001). Women who received PTH had an increase in mean BMD of 6.9% at the spine (95% CI, 6.4–7.4) and 2.1% at the hip (95% CI, 1.7–2.5). Adverse events included a higher incidence of hypercalciuria, hypercalcemia, and nausea.
Although it is unlikely that many gynecologists will be ordering or monitoring injectable PTH therapy, we should be aware of the data. All too often such therapy is not even considered for women with severe osteoporosis (T-score < -2.5 and preexisting fracture), who may be excellent candidates.
Long-term alendronate users can sometimes take a “drug holiday”
Black DM, Schwartz AV, Ensrud KE, et al, for the FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment. The Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938.
As early as 2002, Greenspan and colleagues3 demonstrated that women on alendronate for 21 months maintained femoral-neck BMD through 15 months of crossover to placebo. This study by Black and associates, known as the FLEX trial, is a long-term extension of the Fracture Intervention Trial (FIT). A total of 1,099 women who had participated in FIT and taken alendronate for 5 years were then randomized to one of two doses of alendronate or placebo for an additional 5 years. To qualify for the FLEX trial, all women had to have low bone mass at the beginning of FIT. The average age of women in the FLEX trial was 73 years. The primary outcome was total hip BMD; secondary outcomes were BMD at other sites and biochemical markers.
Women who remained on alendronate maintained a higher BMD of the hip and spine than women on placebo, but all patients’ levels remained at or above pretreatment levels of 10 years earlier. The same was true for markers of bone remodeling. The cumulative risk of nonvertebral fractures did not differ between the two groups (19% for placebo, 18.9% for alendronate; RR, 1.0; 95% CI, 0.76–1.32). The risk of clinically recognized vertebral fractures was lower in the women who continued alendronate (5.3% for placebo and 2.4% for alendronate; RR, 0.45; 95% CI, 0.24–0.85), but there was no significant reduction in morphometric vertebral fractures.
Data can aid in determining duration of therapy
These data are extremely helpful, especially for clinicians who are trying to determine how long to continue bisphosphonate therapy and which patients may be candidates for a “drug holiday.” We have all had women whose response to a bisphosphonate has been so robust that follow-up BMD measurements have climbed to a range in which therapy would not have been initiated. This study clearly shows that the cumulative effect of 5 years of alendronate followed by 5 years of placebo is positive, compared with the bone loss one would expect in untreated women.
The answer isn’t clear, but women with a previous fracture, and those at high risk for spine fracture, are likely to benefit from continued treatment with alendronate. Patients with a lower risk of fracture are better candidates for the holiday.
As for when the drug holiday should end, that isn’t clear, either. Continued close monitoring of these lower-risk women using bone-density measurements may help identify that minority of patients who do not maintain bone mass off the medication.
1. Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137.
2. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes. The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;295:2727-2741.
3. Greenspan SL, Emkey RD, Bone HG, et al. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875-883.
As 2007 draws to a close, we are still awaiting the World Health Organization’s fracture risk-assessment tool. The much-anticipated instrument will calculate 5- and 10-year fracture risks using an individual’s femoral neck T-score, age, history of low-trauma fracture, body mass index, steroid exposure, family history of hip fracture, smoking status, and alcohol intake. Once it is implemented, the tool will eliminate much of the confusion that arises when the T-score is the only variable used to determine the need for pharmacotherapy.
Why is the ability to stratify risk important? Although the incidence of fragility fractures is highest in osteoporotic women (as defined by the T-score), the absolute number of fractures is greater in those who have osteopenia. All clinicians should realize that the current definitions of normal bone density, osteopenia, and osteoporosis apply to the postmenopausal population only:
- normal – T-score above -1.0
- osteopenia – T-score below -1.0 but above -2.5
- osteoporosis – T-score below -2.5.
Indications added for raloxifene
The year did bring new indications for raloxifene, based on data from the RUTH and STAR trials,1,2 which were mentioned in this Update 1 year ago. On September 14, the Food and Drug Administration approved two new indications:
- reduction of risk of invasive breast cancer in postmenopausal women with osteoporosis
- reduction of risk of invasive breast cancer in postmenopausal women at high risk of breast cancer.
These new indications are very important for clinicians who prescribe agents to prevent fragility fractures. Raloxifene should be considered for breast cancer risk reduction when deciding which agent to prescribe.
REAL study finds real advantage with risedronate
Silverman SL, Watts NB, Delmas PD, et al. Effectiveness of bisphosphonates on nonvertebral and hip fractures in the first year of therapy: the risedronate and alendronate (REAL) cohort study. Osteoporos Int. 2007;18(1):25–34.
Patients who take risedronate have lower rates of hip and nonvertebral fracture during their first year of therapy than do those who take alendronate. That is the finding of the RisedronatE and ALendronate (REAL) cohort study, a retrospective observation of the records of health-care utilization among women in the United States. Silverman and colleagues analyzed data sets for women older than age 65 who had ever used once-weekly dosing of risedronate or alendronate. The risedronate cohort included 12,215 women who were followed for a mean of 226 days of therapy. The alendronate cohort included 21,615 women followed for a mean of 238 days of therapy.
Risedronate group had more risk factors for fracture
At baseline, women taking risedronate had a statistically greater incidence of:
- advanced age
- use of concomitant medications
- glucocorticoid use
- rheumatoid arthritis.
Each of these characteristics might have been expected to increase the risk of fragility fracture. However, through 1 year of therapy, women using risedronate had an incidence of nonvertebral fractures 18% lower than those using alendronate (2.0% versus 2.3%; 95% confidence interval [CI], 0.02–0.32). They also had an incidence of hip fracture 43% lower than those using alendronate (0.4% versus 0.6%; 95% CI, 0.13–0.63). Overall, there were 507 nonvertebral fractures and 109 hip fractures.
Footnote: Large database analyses complement randomized trials
Randomized clinical trials (RCTs) are, of course, the gold standard for determining drug safety and efficacy and the key requisite for regulatory approval of new drugs. By design, RCTs have strict inclusion and exclusion criteria to meet the regulatory standard for evaluating drug efficacy and safety and to exclude internal bias. Often, the majority of patients are deemed ineligible for entry into an RCT because of comorbidity, concomitant medication use, age, or severity of disease. Therefore, database analyses are often more “real world” than RCTs.
As clinical practice data accumulate over time, observational, or outcomes, studies can be conducted to complement the data that have been generated by RCTs. For example, over the past decade, large databases of health-care utilization claims have become available in the United States and are being tapped to conduct effectiveness studies. These observational studies can supplement the efficacy measures obtained from the carefully controlled environment of a placebo-controlled RCT. They also provide a measure of effectiveness over a wide range of patients and health-care practices. Data generated from the REAL study are just another piece of the huge puzzle we must grapple with as we seek good information about agents to treat osteoporosis and prevent fragility fractures.
Annual infusion of zoledronic acid reduces risk of fracture
Black DM, Delmas PD, Eastell R, et al, for the HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822.
Lyles KW, Colon-Emeris C, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357. DOI: 10.1056/NEJMoa074941.
Zoledronic acid (Zometa) is indicated for the treatment of high levels of serum calcium associated with Paget’s disease and various malignancies (multiple myeloma, breast, prostate, and lung). These two studies explore use of this agent to prevent fracture in postmenopausal women with osteoporosis—a use for which it proved effective. Other benefits may include improved compliance and ease of administration in some women.
Black and colleagues conducted their randomized, double-blind, placebo-controlled trial to assess the effect of annual infusion of zoledronic acid on the risk of fracture over a 3-year period. A total of 3,889 postmenopausal women with osteoporosis (mean age, 73 years; range, 65–89 years) were assigned to receive a single 15-minute, 5-mg infusion of the drug at baseline, 12 months, and 24 months, and a total of 3,876 women received placebo. Approximately half the women were from Europe, and the other half were from North and South America and Asia. All women received oral daily calcium (1,000–1,500 mg) and vitamin D (400–1,200 U), and all were monitored for 36 months.
The risk of vertebral fracture was reduced in the treatment group by 70% over 3 years, compared with the placebo group (3.3% or 92 women in the treatment group versus 10.9% or 310 women receiving placebo). The risk of hip fracture was reduced by 41% in the treatment group (1.4% or 52 women receiving zoledronic acid versus 2.5% or 88 women in the placebo group). (For all comparisons, P<.001.)
The most common postdose symptoms, seen within 3 days of infusion, included fever, flu-like symptoms, myalgia, headache, and arthralgias. There were more serious adverse events related to atrial fibrillation in the women receiving zoledronic acid (50 women receiving zoledronic acid versus 20 in the placebo group; P<.001).
Treatment is a valuable option for carefully selected populations
The trial by Black and colleagues holds great promise for some patients, especially those who have (or appear to have) upper gastrointestinal intolerance of oral bisphosphonates or who may have difficulty adhering to the positional requirements (i.e., remaining upright) of oral therapy. This may be especially true of patients in nursing homes. Once-yearly intravenous (IV) infusion may also make compliance easier for these patients.
One important detail of this trial: Of almost 8,000 women studied, the youngest was age 65. The implication: Don’t automatically assume this regimen is an appropriate alternative for younger osteopenic women who perceive themselves to have acid reflux.
Women at extremely high risk of fracture also benefit
Fracture is most likely to occur in women who have already experienced it (FIGURE 1). Lyles and colleagues chose this population for their study of once-yearly infusion of zoledronic acid. The study involved 2,127 patients within 90 days after repair of low-trauma hip fracture. Subjects were randomized to receive 5-mg IV zoledronic acid annually or placebo in a blinded fashion. All patients also received vitamin D and calcium supplements. Mean age was 74.5 years, as might be expected in a hip-fracture cohort, and median follow-up was 1.9 years.
New clinical fractures occurred in 8.6% of women in the zoledronic acid group and 13.9% of the placebo group—a 35% risk reduction with IV zoledronic acid (P=.001). Deaths occurred in 101 of 1,054 patients (9.6%) in the zoledronic acid group and 141 of 1,057 patients (13.3%) in the placebo group. This was a reduction of 28% in death from any cause in the zoledronic acid group (P=.01).
The most frequent adverse events in patients receiving zoledronic acid were pyrexia, myalgia, and bone and musculoskeletal pain. No cases of osteonecrosis of the jaw were reported. Rates of atrial fibrillation and stroke were similar in both the zoledronic acid and placebo groups.
This study provides further evidence that, for extremely high-risk women (those who have already suffered hip fracture), yearly zoledronic acid may be an extremely useful tool, especially for elderly women, in whom compliance with any medication—weekly or monthly—may be difficult.

FIGURE 1 Once a fracture occurs, another is likely
Although the risk of new fractures is heightened in women who have already experienced one, Lyles and colleagues found a 35% risk reduction with zoledronic acid.
Parathyroid hormone reduces vertebral fractures in osteoporotic women
Greenspan SL, Bone HG, Ettinger MP, et al, for the Treatment of Osteoporosis with Parathyroid Hormone Study Group. Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146:326–339.
Teriparatide (Forteo) is a synthetic portion (1-34) of the parathyroid hormone (PTH) molecule that is identical in sequence to the biologically active segment of the 84-amino acid human PTH.
It has been shown to prevent fracture in women with low bone mineral density (BMD). Teriparatide is the only anabolic bone agent approved for clinical use; all other pharmacotherapies are antiresorptive.
The study by Greenspan and associates—the Treatment of Osteoporosis with Parathyroid Hormone, or TOP trial—involved the full-length PTH molecule (1–84) and provides evidence that it, too, can prevent vertebral fracture in women who have low BMD (FIGURE 2). This agent is used in Europe but not yet available in the United States.
TOP was an 18-month, randomized, double-blind, placebo-controlled, parallel-group study of 2,532 women with low BMD at the hip or lumbar spine. It was conducted at 168 centers in nine countries.
The primary outcome measure was new or worsened vertebral fracture; secondary outcomes were changes in BMD and safety. The trial investigated the safety of recombinant PTH and its effect on the incidence of vertebral fractures in postmenopausal women with osteoporosis.

FIGURE 2 Fracture-prone bone responds to PTH
PTH prevented vertebral fracture in women with low BMD, existing fractures, or both.
Women had very low BMD, or low BMD and existing fractures
Participants were postmenopausal women aged 45 to 54 years. They had a BMD 3 standard deviations or more (T-score ≤ -3.0) below the mean peak bone mass of young adult women at the lumbar spine, femoral neck, or total hip, with no vertebral fractures, or they had a BMD T-score of -2.5 and 1 to 4 vertebral fractures. Postmenopausal women aged 55 years or older were included if their BMD T-score was -2.5 and they had no vertebral fractures, or if their BMD T-score was -2.0 and they had 1 to 4 vertebral fractures before enrollment. The women were given 100 μg of recombinant human PTH or placebo daily, as well as calcium (700 mg/day) and vitamin D3 (400 U/day).
PTH reduced the risk of new fractures or prevented worsening of existing fractures. The reduction in relative risk (RR) for vertebral fracture was 0.42 (95% CI, 0.24–0.72; P=.001). Women who received PTH had an increase in mean BMD of 6.9% at the spine (95% CI, 6.4–7.4) and 2.1% at the hip (95% CI, 1.7–2.5). Adverse events included a higher incidence of hypercalciuria, hypercalcemia, and nausea.
Although it is unlikely that many gynecologists will be ordering or monitoring injectable PTH therapy, we should be aware of the data. All too often such therapy is not even considered for women with severe osteoporosis (T-score < -2.5 and preexisting fracture), who may be excellent candidates.
Long-term alendronate users can sometimes take a “drug holiday”
Black DM, Schwartz AV, Ensrud KE, et al, for the FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment. The Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938.
As early as 2002, Greenspan and colleagues3 demonstrated that women on alendronate for 21 months maintained femoral-neck BMD through 15 months of crossover to placebo. This study by Black and associates, known as the FLEX trial, is a long-term extension of the Fracture Intervention Trial (FIT). A total of 1,099 women who had participated in FIT and taken alendronate for 5 years were then randomized to one of two doses of alendronate or placebo for an additional 5 years. To qualify for the FLEX trial, all women had to have low bone mass at the beginning of FIT. The average age of women in the FLEX trial was 73 years. The primary outcome was total hip BMD; secondary outcomes were BMD at other sites and biochemical markers.
Women who remained on alendronate maintained a higher BMD of the hip and spine than women on placebo, but all patients’ levels remained at or above pretreatment levels of 10 years earlier. The same was true for markers of bone remodeling. The cumulative risk of nonvertebral fractures did not differ between the two groups (19% for placebo, 18.9% for alendronate; RR, 1.0; 95% CI, 0.76–1.32). The risk of clinically recognized vertebral fractures was lower in the women who continued alendronate (5.3% for placebo and 2.4% for alendronate; RR, 0.45; 95% CI, 0.24–0.85), but there was no significant reduction in morphometric vertebral fractures.
Data can aid in determining duration of therapy
These data are extremely helpful, especially for clinicians who are trying to determine how long to continue bisphosphonate therapy and which patients may be candidates for a “drug holiday.” We have all had women whose response to a bisphosphonate has been so robust that follow-up BMD measurements have climbed to a range in which therapy would not have been initiated. This study clearly shows that the cumulative effect of 5 years of alendronate followed by 5 years of placebo is positive, compared with the bone loss one would expect in untreated women.
The answer isn’t clear, but women with a previous fracture, and those at high risk for spine fracture, are likely to benefit from continued treatment with alendronate. Patients with a lower risk of fracture are better candidates for the holiday.
As for when the drug holiday should end, that isn’t clear, either. Continued close monitoring of these lower-risk women using bone-density measurements may help identify that minority of patients who do not maintain bone mass off the medication.
As 2007 draws to a close, we are still awaiting the World Health Organization’s fracture risk-assessment tool. The much-anticipated instrument will calculate 5- and 10-year fracture risks using an individual’s femoral neck T-score, age, history of low-trauma fracture, body mass index, steroid exposure, family history of hip fracture, smoking status, and alcohol intake. Once it is implemented, the tool will eliminate much of the confusion that arises when the T-score is the only variable used to determine the need for pharmacotherapy.
Why is the ability to stratify risk important? Although the incidence of fragility fractures is highest in osteoporotic women (as defined by the T-score), the absolute number of fractures is greater in those who have osteopenia. All clinicians should realize that the current definitions of normal bone density, osteopenia, and osteoporosis apply to the postmenopausal population only:
- normal – T-score above -1.0
- osteopenia – T-score below -1.0 but above -2.5
- osteoporosis – T-score below -2.5.
Indications added for raloxifene
The year did bring new indications for raloxifene, based on data from the RUTH and STAR trials,1,2 which were mentioned in this Update 1 year ago. On September 14, the Food and Drug Administration approved two new indications:
- reduction of risk of invasive breast cancer in postmenopausal women with osteoporosis
- reduction of risk of invasive breast cancer in postmenopausal women at high risk of breast cancer.
These new indications are very important for clinicians who prescribe agents to prevent fragility fractures. Raloxifene should be considered for breast cancer risk reduction when deciding which agent to prescribe.
REAL study finds real advantage with risedronate
Silverman SL, Watts NB, Delmas PD, et al. Effectiveness of bisphosphonates on nonvertebral and hip fractures in the first year of therapy: the risedronate and alendronate (REAL) cohort study. Osteoporos Int. 2007;18(1):25–34.
Patients who take risedronate have lower rates of hip and nonvertebral fracture during their first year of therapy than do those who take alendronate. That is the finding of the RisedronatE and ALendronate (REAL) cohort study, a retrospective observation of the records of health-care utilization among women in the United States. Silverman and colleagues analyzed data sets for women older than age 65 who had ever used once-weekly dosing of risedronate or alendronate. The risedronate cohort included 12,215 women who were followed for a mean of 226 days of therapy. The alendronate cohort included 21,615 women followed for a mean of 238 days of therapy.
Risedronate group had more risk factors for fracture
At baseline, women taking risedronate had a statistically greater incidence of:
- advanced age
- use of concomitant medications
- glucocorticoid use
- rheumatoid arthritis.
Each of these characteristics might have been expected to increase the risk of fragility fracture. However, through 1 year of therapy, women using risedronate had an incidence of nonvertebral fractures 18% lower than those using alendronate (2.0% versus 2.3%; 95% confidence interval [CI], 0.02–0.32). They also had an incidence of hip fracture 43% lower than those using alendronate (0.4% versus 0.6%; 95% CI, 0.13–0.63). Overall, there were 507 nonvertebral fractures and 109 hip fractures.
Footnote: Large database analyses complement randomized trials
Randomized clinical trials (RCTs) are, of course, the gold standard for determining drug safety and efficacy and the key requisite for regulatory approval of new drugs. By design, RCTs have strict inclusion and exclusion criteria to meet the regulatory standard for evaluating drug efficacy and safety and to exclude internal bias. Often, the majority of patients are deemed ineligible for entry into an RCT because of comorbidity, concomitant medication use, age, or severity of disease. Therefore, database analyses are often more “real world” than RCTs.
As clinical practice data accumulate over time, observational, or outcomes, studies can be conducted to complement the data that have been generated by RCTs. For example, over the past decade, large databases of health-care utilization claims have become available in the United States and are being tapped to conduct effectiveness studies. These observational studies can supplement the efficacy measures obtained from the carefully controlled environment of a placebo-controlled RCT. They also provide a measure of effectiveness over a wide range of patients and health-care practices. Data generated from the REAL study are just another piece of the huge puzzle we must grapple with as we seek good information about agents to treat osteoporosis and prevent fragility fractures.
Annual infusion of zoledronic acid reduces risk of fracture
Black DM, Delmas PD, Eastell R, et al, for the HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822.
Lyles KW, Colon-Emeris C, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357. DOI: 10.1056/NEJMoa074941.
Zoledronic acid (Zometa) is indicated for the treatment of high levels of serum calcium associated with Paget’s disease and various malignancies (multiple myeloma, breast, prostate, and lung). These two studies explore use of this agent to prevent fracture in postmenopausal women with osteoporosis—a use for which it proved effective. Other benefits may include improved compliance and ease of administration in some women.
Black and colleagues conducted their randomized, double-blind, placebo-controlled trial to assess the effect of annual infusion of zoledronic acid on the risk of fracture over a 3-year period. A total of 3,889 postmenopausal women with osteoporosis (mean age, 73 years; range, 65–89 years) were assigned to receive a single 15-minute, 5-mg infusion of the drug at baseline, 12 months, and 24 months, and a total of 3,876 women received placebo. Approximately half the women were from Europe, and the other half were from North and South America and Asia. All women received oral daily calcium (1,000–1,500 mg) and vitamin D (400–1,200 U), and all were monitored for 36 months.
The risk of vertebral fracture was reduced in the treatment group by 70% over 3 years, compared with the placebo group (3.3% or 92 women in the treatment group versus 10.9% or 310 women receiving placebo). The risk of hip fracture was reduced by 41% in the treatment group (1.4% or 52 women receiving zoledronic acid versus 2.5% or 88 women in the placebo group). (For all comparisons, P<.001.)
The most common postdose symptoms, seen within 3 days of infusion, included fever, flu-like symptoms, myalgia, headache, and arthralgias. There were more serious adverse events related to atrial fibrillation in the women receiving zoledronic acid (50 women receiving zoledronic acid versus 20 in the placebo group; P<.001).
Treatment is a valuable option for carefully selected populations
The trial by Black and colleagues holds great promise for some patients, especially those who have (or appear to have) upper gastrointestinal intolerance of oral bisphosphonates or who may have difficulty adhering to the positional requirements (i.e., remaining upright) of oral therapy. This may be especially true of patients in nursing homes. Once-yearly intravenous (IV) infusion may also make compliance easier for these patients.
One important detail of this trial: Of almost 8,000 women studied, the youngest was age 65. The implication: Don’t automatically assume this regimen is an appropriate alternative for younger osteopenic women who perceive themselves to have acid reflux.
Women at extremely high risk of fracture also benefit
Fracture is most likely to occur in women who have already experienced it (FIGURE 1). Lyles and colleagues chose this population for their study of once-yearly infusion of zoledronic acid. The study involved 2,127 patients within 90 days after repair of low-trauma hip fracture. Subjects were randomized to receive 5-mg IV zoledronic acid annually or placebo in a blinded fashion. All patients also received vitamin D and calcium supplements. Mean age was 74.5 years, as might be expected in a hip-fracture cohort, and median follow-up was 1.9 years.
New clinical fractures occurred in 8.6% of women in the zoledronic acid group and 13.9% of the placebo group—a 35% risk reduction with IV zoledronic acid (P=.001). Deaths occurred in 101 of 1,054 patients (9.6%) in the zoledronic acid group and 141 of 1,057 patients (13.3%) in the placebo group. This was a reduction of 28% in death from any cause in the zoledronic acid group (P=.01).
The most frequent adverse events in patients receiving zoledronic acid were pyrexia, myalgia, and bone and musculoskeletal pain. No cases of osteonecrosis of the jaw were reported. Rates of atrial fibrillation and stroke were similar in both the zoledronic acid and placebo groups.
This study provides further evidence that, for extremely high-risk women (those who have already suffered hip fracture), yearly zoledronic acid may be an extremely useful tool, especially for elderly women, in whom compliance with any medication—weekly or monthly—may be difficult.

FIGURE 1 Once a fracture occurs, another is likely
Although the risk of new fractures is heightened in women who have already experienced one, Lyles and colleagues found a 35% risk reduction with zoledronic acid.
Parathyroid hormone reduces vertebral fractures in osteoporotic women
Greenspan SL, Bone HG, Ettinger MP, et al, for the Treatment of Osteoporosis with Parathyroid Hormone Study Group. Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146:326–339.
Teriparatide (Forteo) is a synthetic portion (1-34) of the parathyroid hormone (PTH) molecule that is identical in sequence to the biologically active segment of the 84-amino acid human PTH.
It has been shown to prevent fracture in women with low bone mineral density (BMD). Teriparatide is the only anabolic bone agent approved for clinical use; all other pharmacotherapies are antiresorptive.
The study by Greenspan and associates—the Treatment of Osteoporosis with Parathyroid Hormone, or TOP trial—involved the full-length PTH molecule (1–84) and provides evidence that it, too, can prevent vertebral fracture in women who have low BMD (FIGURE 2). This agent is used in Europe but not yet available in the United States.
TOP was an 18-month, randomized, double-blind, placebo-controlled, parallel-group study of 2,532 women with low BMD at the hip or lumbar spine. It was conducted at 168 centers in nine countries.
The primary outcome measure was new or worsened vertebral fracture; secondary outcomes were changes in BMD and safety. The trial investigated the safety of recombinant PTH and its effect on the incidence of vertebral fractures in postmenopausal women with osteoporosis.

FIGURE 2 Fracture-prone bone responds to PTH
PTH prevented vertebral fracture in women with low BMD, existing fractures, or both.
Women had very low BMD, or low BMD and existing fractures
Participants were postmenopausal women aged 45 to 54 years. They had a BMD 3 standard deviations or more (T-score ≤ -3.0) below the mean peak bone mass of young adult women at the lumbar spine, femoral neck, or total hip, with no vertebral fractures, or they had a BMD T-score of -2.5 and 1 to 4 vertebral fractures. Postmenopausal women aged 55 years or older were included if their BMD T-score was -2.5 and they had no vertebral fractures, or if their BMD T-score was -2.0 and they had 1 to 4 vertebral fractures before enrollment. The women were given 100 μg of recombinant human PTH or placebo daily, as well as calcium (700 mg/day) and vitamin D3 (400 U/day).
PTH reduced the risk of new fractures or prevented worsening of existing fractures. The reduction in relative risk (RR) for vertebral fracture was 0.42 (95% CI, 0.24–0.72; P=.001). Women who received PTH had an increase in mean BMD of 6.9% at the spine (95% CI, 6.4–7.4) and 2.1% at the hip (95% CI, 1.7–2.5). Adverse events included a higher incidence of hypercalciuria, hypercalcemia, and nausea.
Although it is unlikely that many gynecologists will be ordering or monitoring injectable PTH therapy, we should be aware of the data. All too often such therapy is not even considered for women with severe osteoporosis (T-score < -2.5 and preexisting fracture), who may be excellent candidates.
Long-term alendronate users can sometimes take a “drug holiday”
Black DM, Schwartz AV, Ensrud KE, et al, for the FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment. The Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938.
As early as 2002, Greenspan and colleagues3 demonstrated that women on alendronate for 21 months maintained femoral-neck BMD through 15 months of crossover to placebo. This study by Black and associates, known as the FLEX trial, is a long-term extension of the Fracture Intervention Trial (FIT). A total of 1,099 women who had participated in FIT and taken alendronate for 5 years were then randomized to one of two doses of alendronate or placebo for an additional 5 years. To qualify for the FLEX trial, all women had to have low bone mass at the beginning of FIT. The average age of women in the FLEX trial was 73 years. The primary outcome was total hip BMD; secondary outcomes were BMD at other sites and biochemical markers.
Women who remained on alendronate maintained a higher BMD of the hip and spine than women on placebo, but all patients’ levels remained at or above pretreatment levels of 10 years earlier. The same was true for markers of bone remodeling. The cumulative risk of nonvertebral fractures did not differ between the two groups (19% for placebo, 18.9% for alendronate; RR, 1.0; 95% CI, 0.76–1.32). The risk of clinically recognized vertebral fractures was lower in the women who continued alendronate (5.3% for placebo and 2.4% for alendronate; RR, 0.45; 95% CI, 0.24–0.85), but there was no significant reduction in morphometric vertebral fractures.
Data can aid in determining duration of therapy
These data are extremely helpful, especially for clinicians who are trying to determine how long to continue bisphosphonate therapy and which patients may be candidates for a “drug holiday.” We have all had women whose response to a bisphosphonate has been so robust that follow-up BMD measurements have climbed to a range in which therapy would not have been initiated. This study clearly shows that the cumulative effect of 5 years of alendronate followed by 5 years of placebo is positive, compared with the bone loss one would expect in untreated women.
The answer isn’t clear, but women with a previous fracture, and those at high risk for spine fracture, are likely to benefit from continued treatment with alendronate. Patients with a lower risk of fracture are better candidates for the holiday.
As for when the drug holiday should end, that isn’t clear, either. Continued close monitoring of these lower-risk women using bone-density measurements may help identify that minority of patients who do not maintain bone mass off the medication.
1. Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137.
2. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes. The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;295:2727-2741.
3. Greenspan SL, Emkey RD, Bone HG, et al. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875-883.
1. Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125-137.
2. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes. The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial. JAMA. 2006;295:2727-2741.
3. Greenspan SL, Emkey RD, Bone HG, et al. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875-883.
Shoulder dystocia: Clarifying the care of an old problem
The author reports no financial relationships relevant to this article.
Brachial plexus injury is a dreaded sequela of shoulder dystocia, one that lies at the root of many medical liability disputes. Although brachial plexus injury cannot be prevented, most of the commonly used maneuvers for freeing a stuck shoulder are designed to maximize fetal safety and minimize injury.
What is the standard of care when dystocia occurs? Several respected sources have offered conflicting recommendations, particularly in regard to maternal pushing once dystocia is diagnosed. My aim in this article is to clarify the issue.
Endogenous force versus exogenous force
The introduction to the American College of Obstetricians and Gynecologists’ practice bulletin on shoulder dystocia, published in November 2002, provides a useful summary of much of our current knowledge:
Shoulder dystocia is most often an unpredictable and unpreventable obstetric emergency. Failure of the shoulders to deliver spontaneously places both the pregnant woman and fetus at risk for injury. Several maneuvers to release impacted shoulders have been developed, but the urgency of this event makes prospective studies impractical for comparing their effectiveness.1
Because prospective studies are unlikely ever to be performed, some investigators have turned to mathematical modeling to learn more about the forces exerted on the fetal neck overlying the roots of the brachial plexus when a shoulder is impacted against the symphysis pubis.
Gonik and colleagues2 performed elegant modeling of the pressure between the base of the fetal neck and symphysis pubis during dystocia. They utilized data on birth forces gathered by CaldeyroBarcia and Poseiro3 in their classic work on intrauterine pressure. Gonik and colleagues also used data from Allen and associates,4 who measured the force of clinician-applied traction after delivery of the head by having clinicians wear sensory gloves that recorded the force of traction applied.
Gonik and associates2 concluded that the pressure resulting from endogenous forces is four to nine times greater than the pressure generated by a clinician. “Neonatal brachial plexus injury is not a priori explained by iatrogenically induced excessive traction,” they wrote. “Spontaneous endogenous forces may contribute substantially to this type of neonatal trauma.”
How an understanding of endogenous forces alters management
Although fewer than 10% of cases of shoulder dystocia result in permanent brachial plexus injury,1 such injuries are a major source of malpractice litigation in obstetrics, as I noted at the beginning of this article. In most such cases, injury is blamed on excessive traction by the physician (FIGURE). Newer data, such as the study by Gonik and colleagues,2 may implicate expulsive force (ie, maternal pushing) as another, perhaps greater, cause.
As long ago as 1988, Acker and colleagues5 reported on their experience with Erb’s palsy, which was associated with rapid delivery and unusually forceful expulsive effort in one third of cases. Their findings suggest that, when shoulder dystocia occurs and additional maneuvers are necessary to deliver the impacted anterior shoulder, the contribution of potentially harmful endogenous forces should be kept in mind. Counterintuitive strategies, including having the mother stop pushing until the anterior shoulder is freed, may help limit injury.
FIGURE Maternal pushing may contribute to injury
When shoulder dystocia occurs, the progress of labor is interrupted and brachial plexus injury can result, a common cause of litigation. Until now, plaintiff’s attorneys have tended to blame these injuries on the obstetrician and “excess” traction, but it now appears that maternal pushing contributes as well—possibly, to a greater degree than any effort by the physician.
How confusion crept into the literature
The 21st (current) edition of Williams Obstetrics,6 in the section on management of shoulder dystocia, states that “an initial gentle attempt at traction assisted by maternal expulsive efforts is recommended.” There is no reference for this statement. However, a look at the 19th edition of the textbook7 reveals identical wording, and the reference cited is ACOG Technical Bulletin No. 152 (August 1991), entitled Operative Vaginal Delivery. A subsequent version of the same bulletin (no. 196 from August 1994) contains identical wording. By the time that version was replaced by ACOG Practice Bulletin No. 17 (June 2000), however, it no longer contained any information on shoulder dystocia. Instead, ACOG published Practice Pattern No. 7 (October 1997), entitled Shoulder Dystocia. This document did not recommend maternal expulsive force after a diagnosis of shoulder dystocia—in fact, maternal force was not even mentioned. Nor is it mentioned in the current practice bulletin (no. 40 from November 2002), which replaced the previous version of Shoulder Dystocia.
Confusion doesn’t end there
In its section on shoulder dystocia, ACOG’s publication Precis (1998) states:
Management of shoulder dystocia involves both anticipation of and preparation for problems. The key to preventing fetal injury is avoidance of excess traction on the fetal head. When shoulder dystocia is diagnosed, a deliberate and planned sequence of events should be initiated. Pushing should be halted and obstructive causes should be considered. Aggressive fundal pressure or continued pushing will only further impact the anterior shoulder.
We are left with the paradox that the current edition of Williams Obstetrics, in its discussion of shoulder dystocia, carries a statement recommending maternal pushing based on a 1994 ACOG document—a statement that subsequent ACOG documents no longer contain. In fact, one of those documents—Precis—tells us that pushing should be halted, an instruction supported by the mathematical modeling of Gonik and colleagues.2 And a popular online text (UpToDate.com) advises: “The mother should be told not to push during attempts to reposition the fetus.”8 Once the fetus is successfully repositioned, maternal pushing or traction, or both, can be reinstated.
Putting it all into clinical perspective
The current ACOG practice bulletin on shoulder dystocia (no. 40 from November 2002) observes that “retraction of the delivered fetal head against the maternal perineum (turtle sign) may be present and may assist in the diagnosis of shoulder dystocia.” When present, the turtle sign strongly suggests that the anterior shoulder is already impacted against the symphysis pubis. Maternal expulsive forces may have already put enough pressure on the nerve roots of the brachial plexus to cause damage. Any degree of traction or continued maternal pushing is likely to compound an already potentially serious problem.
In such cases, it is prudent to resort to known maneuvers, avoid encouraging continued maternal pushing, and simply support and guide the head without supplying any real traction.
When the turtle sign is absent, shoulder dystocia can be diagnosed only after the head is delivered, when the usual methods (ie, downward traction and continued maternal pushing) fail to advance delivery. Diagnosis in these cases requires recognition on the part of the delivering physician that shoulder dystocia is present. At that point, continued expulsive force and any real degree of traction no longer are appropriate.
1. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 40: Shoulder dystocia. Washington, DC: American College of Obstetricians and Gynecologists; 2002.
2. Gonik B, Walker A, Grimm M. Mathematic modeling of forces associated with shoulder dystocia: a comparison of endogenous and exogenous sources. Am J Obstet Gynecol. 2000;182:689-691.
3. Caldeyro-Barcia R, Poseiro JJ. Physiology of the uterine contraction. Clin Obstet Gynecol. 1960;3:386-392.
4. Allen R, Sorab J, Gonik B. Risk factors for shoulder dystocia: an engineering study of clinician-applied forces. Obstet Gynecol. 1991;77:352-355.
5. Acker DB, Gregory KD, Sachs BP, Friedman EA. Risk factors for Erb-Duchenne palsy. Obstet Gynecol. 1988;71:389-392.
6. Dystocia: abnormal presentation position and development of the fetus: shoulder dystocia. In: Cunningham FG, Gant NF, Leveno KJ, Gilstrap LC III, Hauth JC, Wenstrom KD (eds). Williams Obstetrics. 21st ed. New York: McGraw-Hill; 2001:459-464.
7. Dystocia due to abnormalities in presentation position or development of the fetus: shoulder dystocia. In: Cunningham FG, MacDonald PC, Gant NF (eds). Williams Obstetrics. 19th ed. Norwalk, Conn: Appleton & Lange; 1993:509-514.
8. Rodis JF. Management of fetal macrosomia and shoulder dystocia. UpToDate [serial online]. Waltham, MA; November 7, 2007.
The author reports no financial relationships relevant to this article.
Brachial plexus injury is a dreaded sequela of shoulder dystocia, one that lies at the root of many medical liability disputes. Although brachial plexus injury cannot be prevented, most of the commonly used maneuvers for freeing a stuck shoulder are designed to maximize fetal safety and minimize injury.
What is the standard of care when dystocia occurs? Several respected sources have offered conflicting recommendations, particularly in regard to maternal pushing once dystocia is diagnosed. My aim in this article is to clarify the issue.
Endogenous force versus exogenous force
The introduction to the American College of Obstetricians and Gynecologists’ practice bulletin on shoulder dystocia, published in November 2002, provides a useful summary of much of our current knowledge:
Shoulder dystocia is most often an unpredictable and unpreventable obstetric emergency. Failure of the shoulders to deliver spontaneously places both the pregnant woman and fetus at risk for injury. Several maneuvers to release impacted shoulders have been developed, but the urgency of this event makes prospective studies impractical for comparing their effectiveness.1
Because prospective studies are unlikely ever to be performed, some investigators have turned to mathematical modeling to learn more about the forces exerted on the fetal neck overlying the roots of the brachial plexus when a shoulder is impacted against the symphysis pubis.
Gonik and colleagues2 performed elegant modeling of the pressure between the base of the fetal neck and symphysis pubis during dystocia. They utilized data on birth forces gathered by CaldeyroBarcia and Poseiro3 in their classic work on intrauterine pressure. Gonik and colleagues also used data from Allen and associates,4 who measured the force of clinician-applied traction after delivery of the head by having clinicians wear sensory gloves that recorded the force of traction applied.
Gonik and associates2 concluded that the pressure resulting from endogenous forces is four to nine times greater than the pressure generated by a clinician. “Neonatal brachial plexus injury is not a priori explained by iatrogenically induced excessive traction,” they wrote. “Spontaneous endogenous forces may contribute substantially to this type of neonatal trauma.”
How an understanding of endogenous forces alters management
Although fewer than 10% of cases of shoulder dystocia result in permanent brachial plexus injury,1 such injuries are a major source of malpractice litigation in obstetrics, as I noted at the beginning of this article. In most such cases, injury is blamed on excessive traction by the physician (FIGURE). Newer data, such as the study by Gonik and colleagues,2 may implicate expulsive force (ie, maternal pushing) as another, perhaps greater, cause.
As long ago as 1988, Acker and colleagues5 reported on their experience with Erb’s palsy, which was associated with rapid delivery and unusually forceful expulsive effort in one third of cases. Their findings suggest that, when shoulder dystocia occurs and additional maneuvers are necessary to deliver the impacted anterior shoulder, the contribution of potentially harmful endogenous forces should be kept in mind. Counterintuitive strategies, including having the mother stop pushing until the anterior shoulder is freed, may help limit injury.
FIGURE Maternal pushing may contribute to injury
When shoulder dystocia occurs, the progress of labor is interrupted and brachial plexus injury can result, a common cause of litigation. Until now, plaintiff’s attorneys have tended to blame these injuries on the obstetrician and “excess” traction, but it now appears that maternal pushing contributes as well—possibly, to a greater degree than any effort by the physician.
How confusion crept into the literature
The 21st (current) edition of Williams Obstetrics,6 in the section on management of shoulder dystocia, states that “an initial gentle attempt at traction assisted by maternal expulsive efforts is recommended.” There is no reference for this statement. However, a look at the 19th edition of the textbook7 reveals identical wording, and the reference cited is ACOG Technical Bulletin No. 152 (August 1991), entitled Operative Vaginal Delivery. A subsequent version of the same bulletin (no. 196 from August 1994) contains identical wording. By the time that version was replaced by ACOG Practice Bulletin No. 17 (June 2000), however, it no longer contained any information on shoulder dystocia. Instead, ACOG published Practice Pattern No. 7 (October 1997), entitled Shoulder Dystocia. This document did not recommend maternal expulsive force after a diagnosis of shoulder dystocia—in fact, maternal force was not even mentioned. Nor is it mentioned in the current practice bulletin (no. 40 from November 2002), which replaced the previous version of Shoulder Dystocia.
Confusion doesn’t end there
In its section on shoulder dystocia, ACOG’s publication Precis (1998) states:
Management of shoulder dystocia involves both anticipation of and preparation for problems. The key to preventing fetal injury is avoidance of excess traction on the fetal head. When shoulder dystocia is diagnosed, a deliberate and planned sequence of events should be initiated. Pushing should be halted and obstructive causes should be considered. Aggressive fundal pressure or continued pushing will only further impact the anterior shoulder.
We are left with the paradox that the current edition of Williams Obstetrics, in its discussion of shoulder dystocia, carries a statement recommending maternal pushing based on a 1994 ACOG document—a statement that subsequent ACOG documents no longer contain. In fact, one of those documents—Precis—tells us that pushing should be halted, an instruction supported by the mathematical modeling of Gonik and colleagues.2 And a popular online text (UpToDate.com) advises: “The mother should be told not to push during attempts to reposition the fetus.”8 Once the fetus is successfully repositioned, maternal pushing or traction, or both, can be reinstated.
Putting it all into clinical perspective
The current ACOG practice bulletin on shoulder dystocia (no. 40 from November 2002) observes that “retraction of the delivered fetal head against the maternal perineum (turtle sign) may be present and may assist in the diagnosis of shoulder dystocia.” When present, the turtle sign strongly suggests that the anterior shoulder is already impacted against the symphysis pubis. Maternal expulsive forces may have already put enough pressure on the nerve roots of the brachial plexus to cause damage. Any degree of traction or continued maternal pushing is likely to compound an already potentially serious problem.
In such cases, it is prudent to resort to known maneuvers, avoid encouraging continued maternal pushing, and simply support and guide the head without supplying any real traction.
When the turtle sign is absent, shoulder dystocia can be diagnosed only after the head is delivered, when the usual methods (ie, downward traction and continued maternal pushing) fail to advance delivery. Diagnosis in these cases requires recognition on the part of the delivering physician that shoulder dystocia is present. At that point, continued expulsive force and any real degree of traction no longer are appropriate.
The author reports no financial relationships relevant to this article.
Brachial plexus injury is a dreaded sequela of shoulder dystocia, one that lies at the root of many medical liability disputes. Although brachial plexus injury cannot be prevented, most of the commonly used maneuvers for freeing a stuck shoulder are designed to maximize fetal safety and minimize injury.
What is the standard of care when dystocia occurs? Several respected sources have offered conflicting recommendations, particularly in regard to maternal pushing once dystocia is diagnosed. My aim in this article is to clarify the issue.
Endogenous force versus exogenous force
The introduction to the American College of Obstetricians and Gynecologists’ practice bulletin on shoulder dystocia, published in November 2002, provides a useful summary of much of our current knowledge:
Shoulder dystocia is most often an unpredictable and unpreventable obstetric emergency. Failure of the shoulders to deliver spontaneously places both the pregnant woman and fetus at risk for injury. Several maneuvers to release impacted shoulders have been developed, but the urgency of this event makes prospective studies impractical for comparing their effectiveness.1
Because prospective studies are unlikely ever to be performed, some investigators have turned to mathematical modeling to learn more about the forces exerted on the fetal neck overlying the roots of the brachial plexus when a shoulder is impacted against the symphysis pubis.
Gonik and colleagues2 performed elegant modeling of the pressure between the base of the fetal neck and symphysis pubis during dystocia. They utilized data on birth forces gathered by CaldeyroBarcia and Poseiro3 in their classic work on intrauterine pressure. Gonik and colleagues also used data from Allen and associates,4 who measured the force of clinician-applied traction after delivery of the head by having clinicians wear sensory gloves that recorded the force of traction applied.
Gonik and associates2 concluded that the pressure resulting from endogenous forces is four to nine times greater than the pressure generated by a clinician. “Neonatal brachial plexus injury is not a priori explained by iatrogenically induced excessive traction,” they wrote. “Spontaneous endogenous forces may contribute substantially to this type of neonatal trauma.”
How an understanding of endogenous forces alters management
Although fewer than 10% of cases of shoulder dystocia result in permanent brachial plexus injury,1 such injuries are a major source of malpractice litigation in obstetrics, as I noted at the beginning of this article. In most such cases, injury is blamed on excessive traction by the physician (FIGURE). Newer data, such as the study by Gonik and colleagues,2 may implicate expulsive force (ie, maternal pushing) as another, perhaps greater, cause.
As long ago as 1988, Acker and colleagues5 reported on their experience with Erb’s palsy, which was associated with rapid delivery and unusually forceful expulsive effort in one third of cases. Their findings suggest that, when shoulder dystocia occurs and additional maneuvers are necessary to deliver the impacted anterior shoulder, the contribution of potentially harmful endogenous forces should be kept in mind. Counterintuitive strategies, including having the mother stop pushing until the anterior shoulder is freed, may help limit injury.
FIGURE Maternal pushing may contribute to injury
When shoulder dystocia occurs, the progress of labor is interrupted and brachial plexus injury can result, a common cause of litigation. Until now, plaintiff’s attorneys have tended to blame these injuries on the obstetrician and “excess” traction, but it now appears that maternal pushing contributes as well—possibly, to a greater degree than any effort by the physician.
How confusion crept into the literature
The 21st (current) edition of Williams Obstetrics,6 in the section on management of shoulder dystocia, states that “an initial gentle attempt at traction assisted by maternal expulsive efforts is recommended.” There is no reference for this statement. However, a look at the 19th edition of the textbook7 reveals identical wording, and the reference cited is ACOG Technical Bulletin No. 152 (August 1991), entitled Operative Vaginal Delivery. A subsequent version of the same bulletin (no. 196 from August 1994) contains identical wording. By the time that version was replaced by ACOG Practice Bulletin No. 17 (June 2000), however, it no longer contained any information on shoulder dystocia. Instead, ACOG published Practice Pattern No. 7 (October 1997), entitled Shoulder Dystocia. This document did not recommend maternal expulsive force after a diagnosis of shoulder dystocia—in fact, maternal force was not even mentioned. Nor is it mentioned in the current practice bulletin (no. 40 from November 2002), which replaced the previous version of Shoulder Dystocia.
Confusion doesn’t end there
In its section on shoulder dystocia, ACOG’s publication Precis (1998) states:
Management of shoulder dystocia involves both anticipation of and preparation for problems. The key to preventing fetal injury is avoidance of excess traction on the fetal head. When shoulder dystocia is diagnosed, a deliberate and planned sequence of events should be initiated. Pushing should be halted and obstructive causes should be considered. Aggressive fundal pressure or continued pushing will only further impact the anterior shoulder.
We are left with the paradox that the current edition of Williams Obstetrics, in its discussion of shoulder dystocia, carries a statement recommending maternal pushing based on a 1994 ACOG document—a statement that subsequent ACOG documents no longer contain. In fact, one of those documents—Precis—tells us that pushing should be halted, an instruction supported by the mathematical modeling of Gonik and colleagues.2 And a popular online text (UpToDate.com) advises: “The mother should be told not to push during attempts to reposition the fetus.”8 Once the fetus is successfully repositioned, maternal pushing or traction, or both, can be reinstated.
Putting it all into clinical perspective
The current ACOG practice bulletin on shoulder dystocia (no. 40 from November 2002) observes that “retraction of the delivered fetal head against the maternal perineum (turtle sign) may be present and may assist in the diagnosis of shoulder dystocia.” When present, the turtle sign strongly suggests that the anterior shoulder is already impacted against the symphysis pubis. Maternal expulsive forces may have already put enough pressure on the nerve roots of the brachial plexus to cause damage. Any degree of traction or continued maternal pushing is likely to compound an already potentially serious problem.
In such cases, it is prudent to resort to known maneuvers, avoid encouraging continued maternal pushing, and simply support and guide the head without supplying any real traction.
When the turtle sign is absent, shoulder dystocia can be diagnosed only after the head is delivered, when the usual methods (ie, downward traction and continued maternal pushing) fail to advance delivery. Diagnosis in these cases requires recognition on the part of the delivering physician that shoulder dystocia is present. At that point, continued expulsive force and any real degree of traction no longer are appropriate.
1. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 40: Shoulder dystocia. Washington, DC: American College of Obstetricians and Gynecologists; 2002.
2. Gonik B, Walker A, Grimm M. Mathematic modeling of forces associated with shoulder dystocia: a comparison of endogenous and exogenous sources. Am J Obstet Gynecol. 2000;182:689-691.
3. Caldeyro-Barcia R, Poseiro JJ. Physiology of the uterine contraction. Clin Obstet Gynecol. 1960;3:386-392.
4. Allen R, Sorab J, Gonik B. Risk factors for shoulder dystocia: an engineering study of clinician-applied forces. Obstet Gynecol. 1991;77:352-355.
5. Acker DB, Gregory KD, Sachs BP, Friedman EA. Risk factors for Erb-Duchenne palsy. Obstet Gynecol. 1988;71:389-392.
6. Dystocia: abnormal presentation position and development of the fetus: shoulder dystocia. In: Cunningham FG, Gant NF, Leveno KJ, Gilstrap LC III, Hauth JC, Wenstrom KD (eds). Williams Obstetrics. 21st ed. New York: McGraw-Hill; 2001:459-464.
7. Dystocia due to abnormalities in presentation position or development of the fetus: shoulder dystocia. In: Cunningham FG, MacDonald PC, Gant NF (eds). Williams Obstetrics. 19th ed. Norwalk, Conn: Appleton & Lange; 1993:509-514.
8. Rodis JF. Management of fetal macrosomia and shoulder dystocia. UpToDate [serial online]. Waltham, MA; November 7, 2007.
1. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 40: Shoulder dystocia. Washington, DC: American College of Obstetricians and Gynecologists; 2002.
2. Gonik B, Walker A, Grimm M. Mathematic modeling of forces associated with shoulder dystocia: a comparison of endogenous and exogenous sources. Am J Obstet Gynecol. 2000;182:689-691.
3. Caldeyro-Barcia R, Poseiro JJ. Physiology of the uterine contraction. Clin Obstet Gynecol. 1960;3:386-392.
4. Allen R, Sorab J, Gonik B. Risk factors for shoulder dystocia: an engineering study of clinician-applied forces. Obstet Gynecol. 1991;77:352-355.
5. Acker DB, Gregory KD, Sachs BP, Friedman EA. Risk factors for Erb-Duchenne palsy. Obstet Gynecol. 1988;71:389-392.
6. Dystocia: abnormal presentation position and development of the fetus: shoulder dystocia. In: Cunningham FG, Gant NF, Leveno KJ, Gilstrap LC III, Hauth JC, Wenstrom KD (eds). Williams Obstetrics. 21st ed. New York: McGraw-Hill; 2001:459-464.
7. Dystocia due to abnormalities in presentation position or development of the fetus: shoulder dystocia. In: Cunningham FG, MacDonald PC, Gant NF (eds). Williams Obstetrics. 19th ed. Norwalk, Conn: Appleton & Lange; 1993:509-514.
8. Rodis JF. Management of fetal macrosomia and shoulder dystocia. UpToDate [serial online]. Waltham, MA; November 7, 2007.
Does bone loss resume when alendronate is discontinued?
Yes. But the amount of bone loss is clinically small (2% to 3%) in women who stop taking alendronate after 5 years of therapy. At 10 years after initiation of alendronate (5 years after discontinuation), bone mineral density remained well above baseline value.
Expert Commentary
This large, multicenter trial will help us better define clinical management with the bisphosphonates—although this study looked specifically at alendronate. An earlier and smaller study of 226 subjects by Greenspan and colleagues demonstrated that bone mineral density (BMD) maintains itself for 15 months after discontinuation of alendronate.1 This trial by Black and colleagues—the FLEX trial—is a 5-year extension of the Fracture Intervention Trial (FIT).2 It randomized 1,099 women who had taken alendronate for 5 years in FIT to alendronate 5 mg daily (n=329), 10 mg daily (n=333), or placebo (n=437) for 5 additional years. Women were excluded from FLEX if their T-score was less than -3.5 or their BMD was lower than at entry into FIT.
In the FLEX trial, women who switched to placebo after 5 years of alendronate lost a statistically significant but clinically small amount of BMD—approximately 2% to 3%—compared with those who continued taking alendronate for a full 10 years. In all groups, however, BMD levels remained well above baseline at the time of entry into FIT.
Similarities in fracture rates, too
Despite the small difference in BMD measurements between groups, there was no increase in overall clinical fractures or radiographically detected vertebral fractures among women in the placebo group. However, there was a statistically significant 2.9% increase in absolute risk for clinically detected vertebral fractures. One reason for these somewhat surprising findings may be that the trial was powered to detect BMD changes rather than fractures. Nevertheless, it appears that, for some women, 5 years of bisphosphonate therapy may be enough to realize fracture-reduction benefits.
The magnitude of the absolute reduction in clinical vertebral fractures was greatest in women with T-scores worse than -2.5 at the beginning of FLEX, as well as in those with a baseline vertebral fracture at entry. The authors conclude that women at high risk of vertebral fracture because of previous vertebral fractures may be considered for continued therapy. Obviously, a long-term study powered for fractures rather than BMD measurement would be ideal, if extraordinarily expensive.
Who can take a ‘drug holiday’?
Women who have a good response to 5 years of bisphosphonate therapy (ie, a 3–5% increase in hip BMD, 8–10% increase in spine BMD, and a T-score better than -3.5) do not appear to be at increased risk of vertebral fracture after a “drug holiday” of up to 5 years. Such an approach would clearly improve the cost-effectiveness of bisphosphonate therapy. However, it would also necessitate careful BMD monitoring because the BMD values listed above are mean findings. Close monitoring would identify women who might be rapidly losing BMD and who need to resume bisphosphonate therapy or an alternative. Therefore, the treatment center should be reliable, with use of the same dual-energy x-ray absorptiometry (DXA) machine whenever possible.
Today, almost all patients are treated with once-weekly dosing. Although this regimen appears to be equivalent to daily dosing,3 it could confound the findings of FLEX.
Bottom line: Consider stopping alendronate in selected patients
Findings from FIT and similar trials established that the initiation of bisphosphonate therapy in postmenopausal women with osteoporosis or a previous nontraumatic fracture substantially reduces their risk of vertebral and nonvertebral fractures.4 These new data from the FLEX trial will allow us to discontinue bisphosphonate therapy in some women after 5 years without exposing them to additional risk.5
1. Greenspan SL. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875-883.
2. Black DM, Cummings SR, Karpt DB, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535-1541.
3. Emkey R. Alendronate and risedronate for the treatment of postmenopausal osteoporosis: clinical profiles of the once-weekly and once-daily dosing formulations. Med Gen Med. 2004;204(3):6.-
4. Fink HA, Ensrud KE, Nelson DB, et al. Disability after clinical fracture in postmenopausal women with low bone density: the Fracture Intervention Trial (FIT). Osteoporosis Int. 2003;14:69-76.
5. Colon-Emerie CS. Ten vs five years of bisphosphonate treatment for postmenopausal osteoporosis. Enough of a good thing. JAMA. 2006;296:2968-2969.
Yes. But the amount of bone loss is clinically small (2% to 3%) in women who stop taking alendronate after 5 years of therapy. At 10 years after initiation of alendronate (5 years after discontinuation), bone mineral density remained well above baseline value.
Expert Commentary
This large, multicenter trial will help us better define clinical management with the bisphosphonates—although this study looked specifically at alendronate. An earlier and smaller study of 226 subjects by Greenspan and colleagues demonstrated that bone mineral density (BMD) maintains itself for 15 months after discontinuation of alendronate.1 This trial by Black and colleagues—the FLEX trial—is a 5-year extension of the Fracture Intervention Trial (FIT).2 It randomized 1,099 women who had taken alendronate for 5 years in FIT to alendronate 5 mg daily (n=329), 10 mg daily (n=333), or placebo (n=437) for 5 additional years. Women were excluded from FLEX if their T-score was less than -3.5 or their BMD was lower than at entry into FIT.
In the FLEX trial, women who switched to placebo after 5 years of alendronate lost a statistically significant but clinically small amount of BMD—approximately 2% to 3%—compared with those who continued taking alendronate for a full 10 years. In all groups, however, BMD levels remained well above baseline at the time of entry into FIT.
Similarities in fracture rates, too
Despite the small difference in BMD measurements between groups, there was no increase in overall clinical fractures or radiographically detected vertebral fractures among women in the placebo group. However, there was a statistically significant 2.9% increase in absolute risk for clinically detected vertebral fractures. One reason for these somewhat surprising findings may be that the trial was powered to detect BMD changes rather than fractures. Nevertheless, it appears that, for some women, 5 years of bisphosphonate therapy may be enough to realize fracture-reduction benefits.
The magnitude of the absolute reduction in clinical vertebral fractures was greatest in women with T-scores worse than -2.5 at the beginning of FLEX, as well as in those with a baseline vertebral fracture at entry. The authors conclude that women at high risk of vertebral fracture because of previous vertebral fractures may be considered for continued therapy. Obviously, a long-term study powered for fractures rather than BMD measurement would be ideal, if extraordinarily expensive.
Who can take a ‘drug holiday’?
Women who have a good response to 5 years of bisphosphonate therapy (ie, a 3–5% increase in hip BMD, 8–10% increase in spine BMD, and a T-score better than -3.5) do not appear to be at increased risk of vertebral fracture after a “drug holiday” of up to 5 years. Such an approach would clearly improve the cost-effectiveness of bisphosphonate therapy. However, it would also necessitate careful BMD monitoring because the BMD values listed above are mean findings. Close monitoring would identify women who might be rapidly losing BMD and who need to resume bisphosphonate therapy or an alternative. Therefore, the treatment center should be reliable, with use of the same dual-energy x-ray absorptiometry (DXA) machine whenever possible.
Today, almost all patients are treated with once-weekly dosing. Although this regimen appears to be equivalent to daily dosing,3 it could confound the findings of FLEX.
Bottom line: Consider stopping alendronate in selected patients
Findings from FIT and similar trials established that the initiation of bisphosphonate therapy in postmenopausal women with osteoporosis or a previous nontraumatic fracture substantially reduces their risk of vertebral and nonvertebral fractures.4 These new data from the FLEX trial will allow us to discontinue bisphosphonate therapy in some women after 5 years without exposing them to additional risk.5
Yes. But the amount of bone loss is clinically small (2% to 3%) in women who stop taking alendronate after 5 years of therapy. At 10 years after initiation of alendronate (5 years after discontinuation), bone mineral density remained well above baseline value.
Expert Commentary
This large, multicenter trial will help us better define clinical management with the bisphosphonates—although this study looked specifically at alendronate. An earlier and smaller study of 226 subjects by Greenspan and colleagues demonstrated that bone mineral density (BMD) maintains itself for 15 months after discontinuation of alendronate.1 This trial by Black and colleagues—the FLEX trial—is a 5-year extension of the Fracture Intervention Trial (FIT).2 It randomized 1,099 women who had taken alendronate for 5 years in FIT to alendronate 5 mg daily (n=329), 10 mg daily (n=333), or placebo (n=437) for 5 additional years. Women were excluded from FLEX if their T-score was less than -3.5 or their BMD was lower than at entry into FIT.
In the FLEX trial, women who switched to placebo after 5 years of alendronate lost a statistically significant but clinically small amount of BMD—approximately 2% to 3%—compared with those who continued taking alendronate for a full 10 years. In all groups, however, BMD levels remained well above baseline at the time of entry into FIT.
Similarities in fracture rates, too
Despite the small difference in BMD measurements between groups, there was no increase in overall clinical fractures or radiographically detected vertebral fractures among women in the placebo group. However, there was a statistically significant 2.9% increase in absolute risk for clinically detected vertebral fractures. One reason for these somewhat surprising findings may be that the trial was powered to detect BMD changes rather than fractures. Nevertheless, it appears that, for some women, 5 years of bisphosphonate therapy may be enough to realize fracture-reduction benefits.
The magnitude of the absolute reduction in clinical vertebral fractures was greatest in women with T-scores worse than -2.5 at the beginning of FLEX, as well as in those with a baseline vertebral fracture at entry. The authors conclude that women at high risk of vertebral fracture because of previous vertebral fractures may be considered for continued therapy. Obviously, a long-term study powered for fractures rather than BMD measurement would be ideal, if extraordinarily expensive.
Who can take a ‘drug holiday’?
Women who have a good response to 5 years of bisphosphonate therapy (ie, a 3–5% increase in hip BMD, 8–10% increase in spine BMD, and a T-score better than -3.5) do not appear to be at increased risk of vertebral fracture after a “drug holiday” of up to 5 years. Such an approach would clearly improve the cost-effectiveness of bisphosphonate therapy. However, it would also necessitate careful BMD monitoring because the BMD values listed above are mean findings. Close monitoring would identify women who might be rapidly losing BMD and who need to resume bisphosphonate therapy or an alternative. Therefore, the treatment center should be reliable, with use of the same dual-energy x-ray absorptiometry (DXA) machine whenever possible.
Today, almost all patients are treated with once-weekly dosing. Although this regimen appears to be equivalent to daily dosing,3 it could confound the findings of FLEX.
Bottom line: Consider stopping alendronate in selected patients
Findings from FIT and similar trials established that the initiation of bisphosphonate therapy in postmenopausal women with osteoporosis or a previous nontraumatic fracture substantially reduces their risk of vertebral and nonvertebral fractures.4 These new data from the FLEX trial will allow us to discontinue bisphosphonate therapy in some women after 5 years without exposing them to additional risk.5
1. Greenspan SL. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875-883.
2. Black DM, Cummings SR, Karpt DB, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535-1541.
3. Emkey R. Alendronate and risedronate for the treatment of postmenopausal osteoporosis: clinical profiles of the once-weekly and once-daily dosing formulations. Med Gen Med. 2004;204(3):6.-
4. Fink HA, Ensrud KE, Nelson DB, et al. Disability after clinical fracture in postmenopausal women with low bone density: the Fracture Intervention Trial (FIT). Osteoporosis Int. 2003;14:69-76.
5. Colon-Emerie CS. Ten vs five years of bisphosphonate treatment for postmenopausal osteoporosis. Enough of a good thing. JAMA. 2006;296:2968-2969.
1. Greenspan SL. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875-883.
2. Black DM, Cummings SR, Karpt DB, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535-1541.
3. Emkey R. Alendronate and risedronate for the treatment of postmenopausal osteoporosis: clinical profiles of the once-weekly and once-daily dosing formulations. Med Gen Med. 2004;204(3):6.-
4. Fink HA, Ensrud KE, Nelson DB, et al. Disability after clinical fracture in postmenopausal women with low bone density: the Fracture Intervention Trial (FIT). Osteoporosis Int. 2003;14:69-76.
5. Colon-Emerie CS. Ten vs five years of bisphosphonate treatment for postmenopausal osteoporosis. Enough of a good thing. JAMA. 2006;296:2968-2969.
Osteopenia: When to intervene?
In the decade or so since the World Health Organization (WHO) first characterized the terms osteoporosis and osteopenia, basing them on bone-density measurements from dual-energy x-ray absorptiometry (DXA), we have come to know the definitions well, thanks to attention in the lay and medical press (TABLE 1).1
What is the goal behind the heightened public awareness? To reduce the number of osteoporotic fractures.
Do the WHO definitions further this goal? Not really.
Rather than an isolated numerical value, a more useful and descriptive definition of osteoporosis is the following: a skeletal disease characterized by low bone mass and disruption of bone tissue architecture that reduces the mechanical strength of the skeleton and increases the risk of fragility fractures.
In many cases, this definition would encompass women now diagnosed as having osteopenia. Unfortunately, although risk factors for osteoporosis have been described and well promulgated (TABLE 2), the almost exclusive focus of diagnosis has been and continues to be DXA scanning and the WHO definitions, with their reliance on the term osteopenia to convey heightened risk short of full-blown osteoporosis.
This article explains why that way of assessing a woman’s risk of fracture is not the most informative. In fact, the term osteopenia has very little clinical relevance. Some women in the osteopenic range have a high risk of fracture in the short term, whereas others have a great deal of bone health. The hope is that this term will be retired in the near future and replaced with tools that enable us to calculate the absolute fracture risk in 5 to 10 years.
TABLE 1
World Health Organization’s diagnostic categories for bone mineral density
| BONE MINERAL DENSITY MEASUREMENT | DIAGNOSIS |
|---|---|
| Within 1 standard deviation of young adult mean | Normal |
| 1 to 2.5 standard deviations below young adult mean | Osteopenia |
| More than 2.5 standard deviations below young adult mean | Osteoporosis |
| More than 2.5 standard deviations below young adult mean, with fragility fractures | Severe osteoporosis |
| Source: WHO.1 | |
TABLE 2
13 major osteoporosis risk factors in postmenopausal women
|
Only 1 piece of the puzzle
Bone mineral density (BMD) measures bone mass, which is simply 1 component of bone strength. BMD does not assess bone microarchitecture, although it can facilitate a diagnosis of osteopenia or osteoporosis using the WHO definitions.
We use BMD to monitor risk of fracture, much as blood pressure predicts the risk of cardiovascular disease. Many patients with high blood pressure never have a heart attack or stroke, and many patients with normal blood pressure do—but overall, rising blood pressure and rising risk of cardiovascular disease go together.
We use BMD to monitor response to treatment, but it is accurate only if the concept of least-specific change (LSC) is taken into account: LSC=2.77×the precision error of the machine. Thus, in a good center, BMD measurement of the spine will be ±3%, and measurement of the hip will be ±5%.
Bone loss is a continuum, not a T score
Another limitation of the term osteopenia: There is a lot of distance under the curve from –1 to –2.49 standard deviations. Thus, when it comes to risk assessment, it is important to remember that loss of bone mass is a continuum. And because the risk of fracture is directly related to bone mass, fracture risk is a continuum, too. For every standard deviation of bone mass lost, the relative risk of fracture doubles, but absolute fracture risk is highly age-dependent (FIGURE 1).
In younger women, the relative risk of fracture is quite low, and it remains low even when doubled.
However, as careful inspection of FIGURE 1 reveals, the absolute fracture risk of a 50-year-old with a T score of –3 (a score most clinicians would be very concerned about) is exactly the same as the absolute fracture risk of an 80-year-old woman with a T score of –1 (a score many clinicians might consider excellent for a woman that age).
Thus, the T score is only part of the story.
Another example: A 38-year-old woman with a long history of poor calcium ingestion and several years of hypomenorrhea in her 20s has a T score of –2. This woman does not have the same fracture risk as a 63-year-old woman who also has a T score of –2, but who had a T score of 0 when she entered menopause at age 49. These 2 women have the same bone mass, but very different levels of bone quality and fracture risk.
So which women should have their bone mass tested?
Various organizations have issued guidelines for measuring BMD in women to assess risk of fracture (TABLE 3).
FIGURE 1 Risk of fracture increases with advancing age and continuous loss of bone
Adapted from Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12:989–995.TABLE 3
3 sets of guidelines on who needs a bone mineral density test
| ORGANIZATION | CRITERIA |
|---|---|
| National Osteoporosis Foundation2 |
|
| US Preventive Services Task Force3 |
|
| International Society for Clinical Densitometry4 |
|
| NOTE: Per guidelines of the International Society for Clinical Densitometry, women discontinuing estrogen should be considered for bone-density testing according to the indications listed above. | |
When to intervene?
It should be said that it is never too early to intervene when it comes to important lifestyle issues. Adequate calcium and vitamin D are essential throughout life for all women. Encouraging patients to quit smoking is crucial, as is fall prevention, especially for frail women and those with poor eyesight. It is beneficial for women to maintain flexibility, agility, mobility, and strength; these are important components of bone health and total health, and should be taught early in life.
Teriparatide is a bone builder…
This agent is the first parathyroid hormone analog that is anabolic and can build new bone. All the older, familiar agents (estrogen, bisphosphonates, selective estrogen receptor modulators) are antiresorptive. That is, they act by retarding the resorptive part of the dynamic lifelong process whereby bone is constantly laid down and taken away.
…not a magic bullet
When I first heard of anabolic compounds such as teriparatide several years ago, I naively thought it might be possible to modify our approach to bone health. Instead of treating patients to prevent osteoporosis, why not simply wait until patients developed the disease and then treat them with anabolic bone-building agents?
The problem with such reasoning is this: Although the risk of fracture is higher in women with osteoporosis, the number of fractures is greater in postmenopausal women with osteopenia because there are so many more women with osteopenia than with osteoporosis. In fact, the Surgeon General’s report on the state of bone health in the United States estimated that 34 million women have osteopenia and 10 million women have osteoporosis.5
Thus, it becomes obvious that we cannot simply wait until women have developed osteoporosis to treat them if we are going to prevent the majority of fragility fractures.
3 studies exposed risk of osteopenic fracture
The MORE trial
The Multiple Outcomes of Raloxifene Evaluation (MORE) trial6 involved 7,705 women less than 80 years of age in a randomized, placebo-controlled, multicenter, double-blind study of postmenopausal osteoporosis. One of the groups studied had T scores as low as –2.5 and no previous fractures. The other group had 1 or more vertebral fractures at baseline. Women were randomized to raloxifene or placebo for 3 years. Partway through the trial the relevant T-score database was corrected,6 which had the effect of recategorizing many women originally enrolled with “osteoporosis” as “osteopenic.”
In the placebo group of 1,152 osteopenic women with no preexisting vertebral fractures, 42 new vertebral fractures occurred (rate: 3.6%). In addition, of 298 women with osteoporosis, 19 new vertebral fractures occurred (rate: 6.4%). Thus, the fracture rate in osteoporotic women is greater, but the prevalence in osteopenic women is much higher. In this case, the ratio of osteopenic to osteoporotic women was 3.9:1. This ratio is not dissimilar to that of 3.4:1 cited in the Surgeon General’s report.5
The Rotterdam study
This trial7 followed 4,878 women older than 55 years by obtaining BMD measurements of the femoral neck through DXA scanning for an average of 6.8 years. More than one third of the hip fractures occurred in women without osteoporosis (FIGURE 2). In fact, 5% of the hip fractures occurred in women with normal BMD!
In terms of all nonvertebral fractures, more than half occurred in women without osteoporosis, and 12% occurred in women with normal BMD.
FIGURE 2 The majority of nonvertebral fractures and a significant minority of hip fractures occurred in women not yet osteoporotic—ROTTERDAM TRIAL
Source: Schuit SC, et al.7
The NORA trial
The National Osteoporosis Risk Assessment8 (NORA) also tells us a lot about osteopenic women. This trial was a longitudinal family-practice–based study involving slightly more than 200,000 women. It was a 3-year study with 1 year of follow-up. All women self-reported baseline characteristics and received a peripheral measurement of bone density, either by single-energy x-ray absorptiometry, peripheral DXA, or ultrasound.
The authors applied WHO guidelines for BMD measurement, recognizing that their values were peripheral and might therefore understate BMD values based on central DXA.
Ninety percent of the study population was white. The average age was 64.5 years (range 50–104 years), and 11% had previous fractures. (This fracture rate may underrepresent the actual number of previous fractures because the data were self-reported.) Twenty-two percent had a maternal history of osteoporosis.
These patients may have been healthier than the general population because, in order to be part of the study, they had to have a personal physician. Seven percent of the patients had osteoporosis and 40% had osteopenia, based on the peripheral BMD measurements.
The risk of fracture differed significantly by race. The various relative risks are shown in TABLE 4.
Of the postmenopausal women who sustained new fractures within 1 year of study entry, 82% had peripheral BMD measurements in the osteopenic range.
TABLE 4
Relative risk by race NORA trial
| RACE | RELATIVE RISK (RANGE) |
|---|---|
| Caucasian (reference group) | 1.00 |
| African American | 0.55 (0.48–0.62) |
| Hispanic | 1.31 (1.19–1.44) |
| Asian | 1.56 (1.32–1.85) |
| Source: Siris E, et al.8 | |
So whom do we treat?
As has been observed, osteopenic women clearly constitute the majority of women with fractures, not to mention a sizeable number of women in general. As noted, the T-score range of –1 to –2.49 is wide. It is not feasible to treat the entire osteopenic population. Thus, there is a need to stratify risk.
Miller et al9 attempted to solve this problem by identifying osteopenic women at increased short-term risk of fracture. They analyzed the records of more than 57,000 white women from the NORA trial with peripheral T scores that were osteopenic, and entered 32 risk factors for fracture into a regression-tree analysis. They found 1,130 new fractures that occurred within 1 year.
Signs of imminent fracture
The most important determinants of short-term fracture risk (1 year) were:
- previous fracture regardless of T score (4.1% risk),
- T score of less than –1.8 (2.2% risk),
- poor health status (2.2% risk), and
- poor mobility (1.9% risk).9
These 4 risk factors do not differ substantially from the current National Osteoporosis Foundation guidelines (TABLE 5), which recommend treating all women with previous fractures, T scores worse than –2, or T scores worse than –1.5 with additional risk factors.
TABLE 5
Protocol to prevent osteoporotic fractures National Osteoporosis Foundation
| Calcium intake 1,200 mg/day |
| Vitamin D 400–800 IU/day if risk is high |
| Regular weight-bearing, muscle-strengthening exercise |
| No smoking |
| Moderate alcohol consumption |
| Treatment of all vertebral and hip fractures |
Consider prophylactic treatment if:
|
Looking ahead
The WHO scientific group met in 2004 in Brussels, with representatives from leading organizations, including the American Society for Bone and Mineral Research, the International Osteoporosis Foundation, and the National Osteoporosis Foundation, to name a few. The hope is that we will soon have tools to calculate a 5- or 10-year absolute risk of fracture using multiple parameters such as age, body mass index, smoking, ever use of steroids, previous fracture, family history, and BMD. With such a tool, all we would need to do is establish the level of risk at which pharmacotherapy should be initiated.
Disclosure
Dr. Goldstein reports that he serves on the gynecology advisory boards for Eli Lilly, Merck, Pfizer, Procter & Gamble, and TAP Pharmaceuticals.
1. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129.
2. Physician’s Guide to Prevention and Treatment of Osteoporosis. 2nd ed. Washington, DC: National Osteoporosis Foundation; 2003.
3. Screening for osteoporosis in postmenopausal women: recommendations and rationale. Ann Intern Med. 2002;137:526-528.
4. Position statement: executive summary. The Writing Group for the International Society for Clinical Densitometry (ISCD). Position Development Conference. J Clin Densitom. 2004;4:7-12
5. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, Md: US Department of Health and Human Services; 2004.
6. Kanis JA, Johnell O, Black DM, et al. Effect of raloxifene on the risk of new vertebral fracture in post-menopausal women with osteopenia or osteoporosis: a reanalysis of the Multiple Outcomes of Raloxifene Evaluation trial. Bone. 2003;3:293-300
7. Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195-202
8. Siris E, Miller P, Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286:2815-2822
9. Miller PD, Barlas S, Brenneman SK, et al. An approach to identifying osteopenic women at increased short-term risk of fracture. Arch Intern Med. 2004;164:1113-1120
In the decade or so since the World Health Organization (WHO) first characterized the terms osteoporosis and osteopenia, basing them on bone-density measurements from dual-energy x-ray absorptiometry (DXA), we have come to know the definitions well, thanks to attention in the lay and medical press (TABLE 1).1
What is the goal behind the heightened public awareness? To reduce the number of osteoporotic fractures.
Do the WHO definitions further this goal? Not really.
Rather than an isolated numerical value, a more useful and descriptive definition of osteoporosis is the following: a skeletal disease characterized by low bone mass and disruption of bone tissue architecture that reduces the mechanical strength of the skeleton and increases the risk of fragility fractures.
In many cases, this definition would encompass women now diagnosed as having osteopenia. Unfortunately, although risk factors for osteoporosis have been described and well promulgated (TABLE 2), the almost exclusive focus of diagnosis has been and continues to be DXA scanning and the WHO definitions, with their reliance on the term osteopenia to convey heightened risk short of full-blown osteoporosis.
This article explains why that way of assessing a woman’s risk of fracture is not the most informative. In fact, the term osteopenia has very little clinical relevance. Some women in the osteopenic range have a high risk of fracture in the short term, whereas others have a great deal of bone health. The hope is that this term will be retired in the near future and replaced with tools that enable us to calculate the absolute fracture risk in 5 to 10 years.
TABLE 1
World Health Organization’s diagnostic categories for bone mineral density
| BONE MINERAL DENSITY MEASUREMENT | DIAGNOSIS |
|---|---|
| Within 1 standard deviation of young adult mean | Normal |
| 1 to 2.5 standard deviations below young adult mean | Osteopenia |
| More than 2.5 standard deviations below young adult mean | Osteoporosis |
| More than 2.5 standard deviations below young adult mean, with fragility fractures | Severe osteoporosis |
| Source: WHO.1 | |
TABLE 2
13 major osteoporosis risk factors in postmenopausal women
|
Only 1 piece of the puzzle
Bone mineral density (BMD) measures bone mass, which is simply 1 component of bone strength. BMD does not assess bone microarchitecture, although it can facilitate a diagnosis of osteopenia or osteoporosis using the WHO definitions.
We use BMD to monitor risk of fracture, much as blood pressure predicts the risk of cardiovascular disease. Many patients with high blood pressure never have a heart attack or stroke, and many patients with normal blood pressure do—but overall, rising blood pressure and rising risk of cardiovascular disease go together.
We use BMD to monitor response to treatment, but it is accurate only if the concept of least-specific change (LSC) is taken into account: LSC=2.77×the precision error of the machine. Thus, in a good center, BMD measurement of the spine will be ±3%, and measurement of the hip will be ±5%.
Bone loss is a continuum, not a T score
Another limitation of the term osteopenia: There is a lot of distance under the curve from –1 to –2.49 standard deviations. Thus, when it comes to risk assessment, it is important to remember that loss of bone mass is a continuum. And because the risk of fracture is directly related to bone mass, fracture risk is a continuum, too. For every standard deviation of bone mass lost, the relative risk of fracture doubles, but absolute fracture risk is highly age-dependent (FIGURE 1).
In younger women, the relative risk of fracture is quite low, and it remains low even when doubled.
However, as careful inspection of FIGURE 1 reveals, the absolute fracture risk of a 50-year-old with a T score of –3 (a score most clinicians would be very concerned about) is exactly the same as the absolute fracture risk of an 80-year-old woman with a T score of –1 (a score many clinicians might consider excellent for a woman that age).
Thus, the T score is only part of the story.
Another example: A 38-year-old woman with a long history of poor calcium ingestion and several years of hypomenorrhea in her 20s has a T score of –2. This woman does not have the same fracture risk as a 63-year-old woman who also has a T score of –2, but who had a T score of 0 when she entered menopause at age 49. These 2 women have the same bone mass, but very different levels of bone quality and fracture risk.
So which women should have their bone mass tested?
Various organizations have issued guidelines for measuring BMD in women to assess risk of fracture (TABLE 3).
FIGURE 1 Risk of fracture increases with advancing age and continuous loss of bone
Adapted from Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12:989–995.TABLE 3
3 sets of guidelines on who needs a bone mineral density test
| ORGANIZATION | CRITERIA |
|---|---|
| National Osteoporosis Foundation2 |
|
| US Preventive Services Task Force3 |
|
| International Society for Clinical Densitometry4 |
|
| NOTE: Per guidelines of the International Society for Clinical Densitometry, women discontinuing estrogen should be considered for bone-density testing according to the indications listed above. | |
When to intervene?
It should be said that it is never too early to intervene when it comes to important lifestyle issues. Adequate calcium and vitamin D are essential throughout life for all women. Encouraging patients to quit smoking is crucial, as is fall prevention, especially for frail women and those with poor eyesight. It is beneficial for women to maintain flexibility, agility, mobility, and strength; these are important components of bone health and total health, and should be taught early in life.
Teriparatide is a bone builder…
This agent is the first parathyroid hormone analog that is anabolic and can build new bone. All the older, familiar agents (estrogen, bisphosphonates, selective estrogen receptor modulators) are antiresorptive. That is, they act by retarding the resorptive part of the dynamic lifelong process whereby bone is constantly laid down and taken away.
…not a magic bullet
When I first heard of anabolic compounds such as teriparatide several years ago, I naively thought it might be possible to modify our approach to bone health. Instead of treating patients to prevent osteoporosis, why not simply wait until patients developed the disease and then treat them with anabolic bone-building agents?
The problem with such reasoning is this: Although the risk of fracture is higher in women with osteoporosis, the number of fractures is greater in postmenopausal women with osteopenia because there are so many more women with osteopenia than with osteoporosis. In fact, the Surgeon General’s report on the state of bone health in the United States estimated that 34 million women have osteopenia and 10 million women have osteoporosis.5
Thus, it becomes obvious that we cannot simply wait until women have developed osteoporosis to treat them if we are going to prevent the majority of fragility fractures.
3 studies exposed risk of osteopenic fracture
The MORE trial
The Multiple Outcomes of Raloxifene Evaluation (MORE) trial6 involved 7,705 women less than 80 years of age in a randomized, placebo-controlled, multicenter, double-blind study of postmenopausal osteoporosis. One of the groups studied had T scores as low as –2.5 and no previous fractures. The other group had 1 or more vertebral fractures at baseline. Women were randomized to raloxifene or placebo for 3 years. Partway through the trial the relevant T-score database was corrected,6 which had the effect of recategorizing many women originally enrolled with “osteoporosis” as “osteopenic.”
In the placebo group of 1,152 osteopenic women with no preexisting vertebral fractures, 42 new vertebral fractures occurred (rate: 3.6%). In addition, of 298 women with osteoporosis, 19 new vertebral fractures occurred (rate: 6.4%). Thus, the fracture rate in osteoporotic women is greater, but the prevalence in osteopenic women is much higher. In this case, the ratio of osteopenic to osteoporotic women was 3.9:1. This ratio is not dissimilar to that of 3.4:1 cited in the Surgeon General’s report.5
The Rotterdam study
This trial7 followed 4,878 women older than 55 years by obtaining BMD measurements of the femoral neck through DXA scanning for an average of 6.8 years. More than one third of the hip fractures occurred in women without osteoporosis (FIGURE 2). In fact, 5% of the hip fractures occurred in women with normal BMD!
In terms of all nonvertebral fractures, more than half occurred in women without osteoporosis, and 12% occurred in women with normal BMD.
FIGURE 2 The majority of nonvertebral fractures and a significant minority of hip fractures occurred in women not yet osteoporotic—ROTTERDAM TRIAL
Source: Schuit SC, et al.7
The NORA trial
The National Osteoporosis Risk Assessment8 (NORA) also tells us a lot about osteopenic women. This trial was a longitudinal family-practice–based study involving slightly more than 200,000 women. It was a 3-year study with 1 year of follow-up. All women self-reported baseline characteristics and received a peripheral measurement of bone density, either by single-energy x-ray absorptiometry, peripheral DXA, or ultrasound.
The authors applied WHO guidelines for BMD measurement, recognizing that their values were peripheral and might therefore understate BMD values based on central DXA.
Ninety percent of the study population was white. The average age was 64.5 years (range 50–104 years), and 11% had previous fractures. (This fracture rate may underrepresent the actual number of previous fractures because the data were self-reported.) Twenty-two percent had a maternal history of osteoporosis.
These patients may have been healthier than the general population because, in order to be part of the study, they had to have a personal physician. Seven percent of the patients had osteoporosis and 40% had osteopenia, based on the peripheral BMD measurements.
The risk of fracture differed significantly by race. The various relative risks are shown in TABLE 4.
Of the postmenopausal women who sustained new fractures within 1 year of study entry, 82% had peripheral BMD measurements in the osteopenic range.
TABLE 4
Relative risk by race NORA trial
| RACE | RELATIVE RISK (RANGE) |
|---|---|
| Caucasian (reference group) | 1.00 |
| African American | 0.55 (0.48–0.62) |
| Hispanic | 1.31 (1.19–1.44) |
| Asian | 1.56 (1.32–1.85) |
| Source: Siris E, et al.8 | |
So whom do we treat?
As has been observed, osteopenic women clearly constitute the majority of women with fractures, not to mention a sizeable number of women in general. As noted, the T-score range of –1 to –2.49 is wide. It is not feasible to treat the entire osteopenic population. Thus, there is a need to stratify risk.
Miller et al9 attempted to solve this problem by identifying osteopenic women at increased short-term risk of fracture. They analyzed the records of more than 57,000 white women from the NORA trial with peripheral T scores that were osteopenic, and entered 32 risk factors for fracture into a regression-tree analysis. They found 1,130 new fractures that occurred within 1 year.
Signs of imminent fracture
The most important determinants of short-term fracture risk (1 year) were:
- previous fracture regardless of T score (4.1% risk),
- T score of less than –1.8 (2.2% risk),
- poor health status (2.2% risk), and
- poor mobility (1.9% risk).9
These 4 risk factors do not differ substantially from the current National Osteoporosis Foundation guidelines (TABLE 5), which recommend treating all women with previous fractures, T scores worse than –2, or T scores worse than –1.5 with additional risk factors.
TABLE 5
Protocol to prevent osteoporotic fractures National Osteoporosis Foundation
| Calcium intake 1,200 mg/day |
| Vitamin D 400–800 IU/day if risk is high |
| Regular weight-bearing, muscle-strengthening exercise |
| No smoking |
| Moderate alcohol consumption |
| Treatment of all vertebral and hip fractures |
Consider prophylactic treatment if:
|
Looking ahead
The WHO scientific group met in 2004 in Brussels, with representatives from leading organizations, including the American Society for Bone and Mineral Research, the International Osteoporosis Foundation, and the National Osteoporosis Foundation, to name a few. The hope is that we will soon have tools to calculate a 5- or 10-year absolute risk of fracture using multiple parameters such as age, body mass index, smoking, ever use of steroids, previous fracture, family history, and BMD. With such a tool, all we would need to do is establish the level of risk at which pharmacotherapy should be initiated.
Disclosure
Dr. Goldstein reports that he serves on the gynecology advisory boards for Eli Lilly, Merck, Pfizer, Procter & Gamble, and TAP Pharmaceuticals.
In the decade or so since the World Health Organization (WHO) first characterized the terms osteoporosis and osteopenia, basing them on bone-density measurements from dual-energy x-ray absorptiometry (DXA), we have come to know the definitions well, thanks to attention in the lay and medical press (TABLE 1).1
What is the goal behind the heightened public awareness? To reduce the number of osteoporotic fractures.
Do the WHO definitions further this goal? Not really.
Rather than an isolated numerical value, a more useful and descriptive definition of osteoporosis is the following: a skeletal disease characterized by low bone mass and disruption of bone tissue architecture that reduces the mechanical strength of the skeleton and increases the risk of fragility fractures.
In many cases, this definition would encompass women now diagnosed as having osteopenia. Unfortunately, although risk factors for osteoporosis have been described and well promulgated (TABLE 2), the almost exclusive focus of diagnosis has been and continues to be DXA scanning and the WHO definitions, with their reliance on the term osteopenia to convey heightened risk short of full-blown osteoporosis.
This article explains why that way of assessing a woman’s risk of fracture is not the most informative. In fact, the term osteopenia has very little clinical relevance. Some women in the osteopenic range have a high risk of fracture in the short term, whereas others have a great deal of bone health. The hope is that this term will be retired in the near future and replaced with tools that enable us to calculate the absolute fracture risk in 5 to 10 years.
TABLE 1
World Health Organization’s diagnostic categories for bone mineral density
| BONE MINERAL DENSITY MEASUREMENT | DIAGNOSIS |
|---|---|
| Within 1 standard deviation of young adult mean | Normal |
| 1 to 2.5 standard deviations below young adult mean | Osteopenia |
| More than 2.5 standard deviations below young adult mean | Osteoporosis |
| More than 2.5 standard deviations below young adult mean, with fragility fractures | Severe osteoporosis |
| Source: WHO.1 | |
TABLE 2
13 major osteoporosis risk factors in postmenopausal women
|
Only 1 piece of the puzzle
Bone mineral density (BMD) measures bone mass, which is simply 1 component of bone strength. BMD does not assess bone microarchitecture, although it can facilitate a diagnosis of osteopenia or osteoporosis using the WHO definitions.
We use BMD to monitor risk of fracture, much as blood pressure predicts the risk of cardiovascular disease. Many patients with high blood pressure never have a heart attack or stroke, and many patients with normal blood pressure do—but overall, rising blood pressure and rising risk of cardiovascular disease go together.
We use BMD to monitor response to treatment, but it is accurate only if the concept of least-specific change (LSC) is taken into account: LSC=2.77×the precision error of the machine. Thus, in a good center, BMD measurement of the spine will be ±3%, and measurement of the hip will be ±5%.
Bone loss is a continuum, not a T score
Another limitation of the term osteopenia: There is a lot of distance under the curve from –1 to –2.49 standard deviations. Thus, when it comes to risk assessment, it is important to remember that loss of bone mass is a continuum. And because the risk of fracture is directly related to bone mass, fracture risk is a continuum, too. For every standard deviation of bone mass lost, the relative risk of fracture doubles, but absolute fracture risk is highly age-dependent (FIGURE 1).
In younger women, the relative risk of fracture is quite low, and it remains low even when doubled.
However, as careful inspection of FIGURE 1 reveals, the absolute fracture risk of a 50-year-old with a T score of –3 (a score most clinicians would be very concerned about) is exactly the same as the absolute fracture risk of an 80-year-old woman with a T score of –1 (a score many clinicians might consider excellent for a woman that age).
Thus, the T score is only part of the story.
Another example: A 38-year-old woman with a long history of poor calcium ingestion and several years of hypomenorrhea in her 20s has a T score of –2. This woman does not have the same fracture risk as a 63-year-old woman who also has a T score of –2, but who had a T score of 0 when she entered menopause at age 49. These 2 women have the same bone mass, but very different levels of bone quality and fracture risk.
So which women should have their bone mass tested?
Various organizations have issued guidelines for measuring BMD in women to assess risk of fracture (TABLE 3).
FIGURE 1 Risk of fracture increases with advancing age and continuous loss of bone
Adapted from Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12:989–995.TABLE 3
3 sets of guidelines on who needs a bone mineral density test
| ORGANIZATION | CRITERIA |
|---|---|
| National Osteoporosis Foundation2 |
|
| US Preventive Services Task Force3 |
|
| International Society for Clinical Densitometry4 |
|
| NOTE: Per guidelines of the International Society for Clinical Densitometry, women discontinuing estrogen should be considered for bone-density testing according to the indications listed above. | |
When to intervene?
It should be said that it is never too early to intervene when it comes to important lifestyle issues. Adequate calcium and vitamin D are essential throughout life for all women. Encouraging patients to quit smoking is crucial, as is fall prevention, especially for frail women and those with poor eyesight. It is beneficial for women to maintain flexibility, agility, mobility, and strength; these are important components of bone health and total health, and should be taught early in life.
Teriparatide is a bone builder…
This agent is the first parathyroid hormone analog that is anabolic and can build new bone. All the older, familiar agents (estrogen, bisphosphonates, selective estrogen receptor modulators) are antiresorptive. That is, they act by retarding the resorptive part of the dynamic lifelong process whereby bone is constantly laid down and taken away.
…not a magic bullet
When I first heard of anabolic compounds such as teriparatide several years ago, I naively thought it might be possible to modify our approach to bone health. Instead of treating patients to prevent osteoporosis, why not simply wait until patients developed the disease and then treat them with anabolic bone-building agents?
The problem with such reasoning is this: Although the risk of fracture is higher in women with osteoporosis, the number of fractures is greater in postmenopausal women with osteopenia because there are so many more women with osteopenia than with osteoporosis. In fact, the Surgeon General’s report on the state of bone health in the United States estimated that 34 million women have osteopenia and 10 million women have osteoporosis.5
Thus, it becomes obvious that we cannot simply wait until women have developed osteoporosis to treat them if we are going to prevent the majority of fragility fractures.
3 studies exposed risk of osteopenic fracture
The MORE trial
The Multiple Outcomes of Raloxifene Evaluation (MORE) trial6 involved 7,705 women less than 80 years of age in a randomized, placebo-controlled, multicenter, double-blind study of postmenopausal osteoporosis. One of the groups studied had T scores as low as –2.5 and no previous fractures. The other group had 1 or more vertebral fractures at baseline. Women were randomized to raloxifene or placebo for 3 years. Partway through the trial the relevant T-score database was corrected,6 which had the effect of recategorizing many women originally enrolled with “osteoporosis” as “osteopenic.”
In the placebo group of 1,152 osteopenic women with no preexisting vertebral fractures, 42 new vertebral fractures occurred (rate: 3.6%). In addition, of 298 women with osteoporosis, 19 new vertebral fractures occurred (rate: 6.4%). Thus, the fracture rate in osteoporotic women is greater, but the prevalence in osteopenic women is much higher. In this case, the ratio of osteopenic to osteoporotic women was 3.9:1. This ratio is not dissimilar to that of 3.4:1 cited in the Surgeon General’s report.5
The Rotterdam study
This trial7 followed 4,878 women older than 55 years by obtaining BMD measurements of the femoral neck through DXA scanning for an average of 6.8 years. More than one third of the hip fractures occurred in women without osteoporosis (FIGURE 2). In fact, 5% of the hip fractures occurred in women with normal BMD!
In terms of all nonvertebral fractures, more than half occurred in women without osteoporosis, and 12% occurred in women with normal BMD.
FIGURE 2 The majority of nonvertebral fractures and a significant minority of hip fractures occurred in women not yet osteoporotic—ROTTERDAM TRIAL
Source: Schuit SC, et al.7
The NORA trial
The National Osteoporosis Risk Assessment8 (NORA) also tells us a lot about osteopenic women. This trial was a longitudinal family-practice–based study involving slightly more than 200,000 women. It was a 3-year study with 1 year of follow-up. All women self-reported baseline characteristics and received a peripheral measurement of bone density, either by single-energy x-ray absorptiometry, peripheral DXA, or ultrasound.
The authors applied WHO guidelines for BMD measurement, recognizing that their values were peripheral and might therefore understate BMD values based on central DXA.
Ninety percent of the study population was white. The average age was 64.5 years (range 50–104 years), and 11% had previous fractures. (This fracture rate may underrepresent the actual number of previous fractures because the data were self-reported.) Twenty-two percent had a maternal history of osteoporosis.
These patients may have been healthier than the general population because, in order to be part of the study, they had to have a personal physician. Seven percent of the patients had osteoporosis and 40% had osteopenia, based on the peripheral BMD measurements.
The risk of fracture differed significantly by race. The various relative risks are shown in TABLE 4.
Of the postmenopausal women who sustained new fractures within 1 year of study entry, 82% had peripheral BMD measurements in the osteopenic range.
TABLE 4
Relative risk by race NORA trial
| RACE | RELATIVE RISK (RANGE) |
|---|---|
| Caucasian (reference group) | 1.00 |
| African American | 0.55 (0.48–0.62) |
| Hispanic | 1.31 (1.19–1.44) |
| Asian | 1.56 (1.32–1.85) |
| Source: Siris E, et al.8 | |
So whom do we treat?
As has been observed, osteopenic women clearly constitute the majority of women with fractures, not to mention a sizeable number of women in general. As noted, the T-score range of –1 to –2.49 is wide. It is not feasible to treat the entire osteopenic population. Thus, there is a need to stratify risk.
Miller et al9 attempted to solve this problem by identifying osteopenic women at increased short-term risk of fracture. They analyzed the records of more than 57,000 white women from the NORA trial with peripheral T scores that were osteopenic, and entered 32 risk factors for fracture into a regression-tree analysis. They found 1,130 new fractures that occurred within 1 year.
Signs of imminent fracture
The most important determinants of short-term fracture risk (1 year) were:
- previous fracture regardless of T score (4.1% risk),
- T score of less than –1.8 (2.2% risk),
- poor health status (2.2% risk), and
- poor mobility (1.9% risk).9
These 4 risk factors do not differ substantially from the current National Osteoporosis Foundation guidelines (TABLE 5), which recommend treating all women with previous fractures, T scores worse than –2, or T scores worse than –1.5 with additional risk factors.
TABLE 5
Protocol to prevent osteoporotic fractures National Osteoporosis Foundation
| Calcium intake 1,200 mg/day |
| Vitamin D 400–800 IU/day if risk is high |
| Regular weight-bearing, muscle-strengthening exercise |
| No smoking |
| Moderate alcohol consumption |
| Treatment of all vertebral and hip fractures |
Consider prophylactic treatment if:
|
Looking ahead
The WHO scientific group met in 2004 in Brussels, with representatives from leading organizations, including the American Society for Bone and Mineral Research, the International Osteoporosis Foundation, and the National Osteoporosis Foundation, to name a few. The hope is that we will soon have tools to calculate a 5- or 10-year absolute risk of fracture using multiple parameters such as age, body mass index, smoking, ever use of steroids, previous fracture, family history, and BMD. With such a tool, all we would need to do is establish the level of risk at which pharmacotherapy should be initiated.
Disclosure
Dr. Goldstein reports that he serves on the gynecology advisory boards for Eli Lilly, Merck, Pfizer, Procter & Gamble, and TAP Pharmaceuticals.
1. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129.
2. Physician’s Guide to Prevention and Treatment of Osteoporosis. 2nd ed. Washington, DC: National Osteoporosis Foundation; 2003.
3. Screening for osteoporosis in postmenopausal women: recommendations and rationale. Ann Intern Med. 2002;137:526-528.
4. Position statement: executive summary. The Writing Group for the International Society for Clinical Densitometry (ISCD). Position Development Conference. J Clin Densitom. 2004;4:7-12
5. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, Md: US Department of Health and Human Services; 2004.
6. Kanis JA, Johnell O, Black DM, et al. Effect of raloxifene on the risk of new vertebral fracture in post-menopausal women with osteopenia or osteoporosis: a reanalysis of the Multiple Outcomes of Raloxifene Evaluation trial. Bone. 2003;3:293-300
7. Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195-202
8. Siris E, Miller P, Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286:2815-2822
9. Miller PD, Barlas S, Brenneman SK, et al. An approach to identifying osteopenic women at increased short-term risk of fracture. Arch Intern Med. 2004;164:1113-1120
1. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1-129.
2. Physician’s Guide to Prevention and Treatment of Osteoporosis. 2nd ed. Washington, DC: National Osteoporosis Foundation; 2003.
3. Screening for osteoporosis in postmenopausal women: recommendations and rationale. Ann Intern Med. 2002;137:526-528.
4. Position statement: executive summary. The Writing Group for the International Society for Clinical Densitometry (ISCD). Position Development Conference. J Clin Densitom. 2004;4:7-12
5. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, Md: US Department of Health and Human Services; 2004.
6. Kanis JA, Johnell O, Black DM, et al. Effect of raloxifene on the risk of new vertebral fracture in post-menopausal women with osteopenia or osteoporosis: a reanalysis of the Multiple Outcomes of Raloxifene Evaluation trial. Bone. 2003;3:293-300
7. Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195-202
8. Siris E, Miller P, Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286:2815-2822
9. Miller PD, Barlas S, Brenneman SK, et al. An approach to identifying osteopenic women at increased short-term risk of fracture. Arch Intern Med. 2004;164:1113-1120
Preventing fragility fractures: Effective drugs and doses
The numbers tell why. The total number of fragility fractures in American women in a single year—1 million—out-numbers all heart attacks, strokes, breast cancers, and gynecologic cancers combined. A quality-of-life study by Toteson and Hammond found that 4 out of 10 Caucasian women over 50 will fracture a hip, spine, or wrist, sooner or later. One of every 5 who fracture a hip ends up in a nursing home. The direct care cost of osteoporotic fractures was $17 billion in 2001 dollars.
Now, we have more treatment options than ever. And 2005 has been a banner year for discoveries we can put into practice immediately, in our efforts to prevent fragility fractures.
Why so confusing?
McClung MR. The relationship between bone mineral density and fracture risk. Curr Osteoporos Rep. 2005;3:57–63.
- The terms osteopenia and osteoporosis are arbitrary cutoffs. Fracture risk is a continuum and involves multiple factors in addition to bone mass.
The clinically crucial part of that definition is…“increasing the risk of fragility fractures.” Certainly, low bone mass on DEXA is a risk factor. And guidelines from the World Health Organization (WHO), the National Osteoporosis Foundation, and the North American Menopause Society are based on T-scores. However, treatment that bases intervention on absolute fracture risk would be much more appropriate; in fact, the WHO is expected to shortly issue a method to calculate fracture risk. Factors are likely to include age, previous fracture, family history, body mass index, ever use of steroids, propensity for falling, eyesight, overall health, and bone mass (ie, BMD determinations).
We need to realize that WHO definitions of T-score categories are meant for postmenopausal women. Inappropriate use of DEXA scanning in a premenopausal patient may identify a woman with low bone mass, but her bone quality and risk of fragility fracture differ greatly from that of a distantly postmenopausal woman with the same T-score. It may seem counterintuitive, but a 50-year-old woman with a T-score of –3.0 has the same absolute fracture risk, going forward, as an 80-year-old woman with a T-score of –1.
Although the risk of fracture is greatest in women with osteoporosis, there are many more women with osteopenia who will have a fracture. But that doesn’t mean we should prescribe pharmacotherapy for every osteopenic woman in an attempt to prevent fractures. As the US Surgeon General’s report last October estimated, 34 million women have osteopenia and “only” 10 million have osteoporosis. Not every woman with osteopenia should be a candidate for pharmacotherapy, but these facts do underscore the need for a better way to assess absolute fracture risk.
ADDITIONAL REFERENCES
- Miller PD, Barlas S, Brenneman SK, et al. An approach to identifying osteopenic women at increased short-term risk of fracture. Arch Intern Med. 2004;164:1113–1120.
- Salkeld G, Cameron ID, Cumming RG, et al. Quality of life related to fear of falling and hip fracture in older women: a time trade off study. BMJ. 2000;320:341–346.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195–202.
- Tosteson AN, Hammond CS. Quality-of-life assessment in osteoporosis: health-status and preference-based measures. PharmacoEconomics. 2002;20:289–303.
Are all bisphosphonates created equal?
Rosen CJ, Hochberg MC, Bonnick SL, et al. Postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res. 2005;20:141–151.
- Antifracture efficacy at the spine appears to be indistinguishable among antiresorptive agents, despite differences in BMD and bone turnover. Gastrointestinal tolerability was similar in the FACT study.
Endpoints of the FACT study. The primary endpoint was change from baseline BMD at the hip trochanter at 12 months. Secondary endpoints included BMD at multiple sites, bone turnover markers, and drug tolerability. After 12 months, BMD increased 3.4% with alendronate and 2.1% with risedronate (P<.001 alendronate produced significantly greater reductions in bone markers. fracture data were collected as part of the safety monitoring: fractures group and risedronate group.>
Antiresorptives lower fracture risk even without increasing BMD
However, until a head-to-head antifracture efficacy study is done, we cannot infer whether alendronate or risedronate is more effective, based on surrogate endpoints. In fact, if one looks at observations on calcitonin and raloxifene, all 4 drugs provide a similar level of fracture protection, at least in the spine, despite marked differences in turnover markers and BMD. This similarity in antifracture efficacy is probably because antiresorptive drugs affect bone quality and microarchitecture, as well as bone mass.
Antiresorptive medications reduce fracture risk, even in the absence of substantial increases in BMD. This finding has significant implications for monitoring therapy. The misconception that efficacy depends on the amount of bone gained often prompts physicians to stop a drug or add a second drug if a patient’s bone density does not increase. The indication of treatment success, however, is absence of bone loss, not extent of bone gain.
The key to meaningful monitoring
Serial observations with DEXA scanning are fraught with error if one does not understand the concept of least specific change. Least specific change is defined as 2.77 times the precision error of the scanning machine used. Thus, in good centers, BMD measurement of the spine should vary no more than ±3%; measurement of the hip may vary as much as ±5%. For example, a patient who gains 2% over time in the hip and spine is no different statistically from a patient who loses 2% over time in the hip and spine. However, many patients and clinicians feel gratified by a modest increase—and consider an alternative or additional medication if there is a mild decrease. If we take into account the “least specific change,” it becomes evident that in both cases, the patients are in fact unchanged.
Daily pill more likely to get blamed for GI symptoms?
The perception among many clinicians prior to the FACT head-to-head trial was that risedronate had greater GI tolerability than alendronate. However, in the FACT trial no differences were noted in adverse events of the GI tract for either compound. When first introduced, alendronate was a daily regimen. Both alendronate and risedronate are now being given once per week, predominately, and it seems that this schedule has led to fewer complaints and fewer patients discontinuing medication because of GI symptoms. This change probably is because patients are not as likely to relate all of their GI symptoms to a pill taken a week ago, but are more likely to blame any GI complaint on a pill they take every day.
ADDITIONAL REFERENCES
- Bauer DC, Black DM, Garnero P, et al for the Fracture Intervention Trial Study Group. Change in bone turnover and hip, nonspine, and vertebral fracture in alendronate-treated women: the Fracture Intervention Trial. J Bone Miner Res. 2004;19:1250–1258.
- Watts NB, Cooper C, Lindsay R, et al. Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: greater increases in bone mineral density do not relate to greater decreases in fracture risk. J Clin Densitom. 2004;7:255–261.
Which is better, once-a-month or once-a-day ibandronate?
Miller PD, McClung MR, Macovei L, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res. 2005;20:1315–1322.
- “Monthly ibandronate is at least as effective and well tolerated as…the daily ibandronate regimen in postmenopausal osteoporosis.”
Which schedule will patients follow?
Virtually all clinicians would agree that patients prefer weekly to daily dosing, especially if the medication is somewhat inconvenient. Bisphosphonates should be taken with a full glass of water, and the patient should remain standing or sitting upright and avoid other food or drink for 1/2 hour (a full hour with ibandronate).
It remains to be seen. Once-a-month dosing may offer more appeal than weekly alendronate or risedronate, but whether adherence will be better or worse remains to be seen.
Does ibandronate prevent fractures?
Daily ibandronate, 2.5 mg, has been shown to improve bone density and bone turnover values and to reduce vertebral fractures.
There are no prospective data showing nonvertebral fracture reduction—as there are for alendronate and risedronate. However, there was a time when we had only vertebral fracture data on those compounds; a leap of faith was necessary to prescribe them for overall fracture prevention.
The MOBILE study employed a randomized, double-blind method referred to as a “noninferior” trial. A total of 1,609 women with osteoporosis were assigned to once-monthly or daily oral ibandronate. All monthly regimens proved “noninferior” to daily dosing, and the highest monthly dose (150 mg) proved superior to the daily regimen, in terms of lumbar spine BMD increase at 1 year. All regimens were similarly tolerated.
Those who would criticize this methodology will be interested to recall that noninferiority trials were exactly the mechanism that led the way from daily to weekly dosing for alendronate and risedronate.
Which patients are best suited to ibandronate?
Until nonvertebral fracture data become available, however, many clinicians may feel that ibandronate is best suited for these patients:
- women who feel that even once weekly dosing is too inconvenient, and
- younger postmenopausal women who are not at high or immediate risk for hip or other nonvertebral fractures.
- Chesnut CH III, Skag A, Christiansen C, et al for the Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249.
- Delmas PD, Recker RR, Chestnut CH III, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004;15:792–798.
- Reginster JY, Felsenberg D, Cooper C, et al. A new concept for bisphosphonate therapy: a rationale for the development of monthly oral dosing of ibandronate. Osteoporos Int. 2005 Jun 14; [Epub ahead of print].
Martino S, Cauley JA, Barrett-Connor E, et al for the CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–1761.
- In postmenopausal women at high risk for breast cancer who also need bone pharmacotherapy, raloxifene offers an additional benefit in the breast as well as in the skeleton.
How low can you go?
The MORE trial failed to show a reduction in hip fracture. However, the rate of hip fracture in the placebo group was very low (0.7%) compared to that of placebo groups in an alendronate trial known as FIT I (2.2% placebo group) and the risedronate trial (3.9% placebo group) conducted by McClung and colleagues. This finding underscores the notion that it is difficult to lower risk if a group’s risk level is initially low.
Efficacy after 8 years. The Continuing Outcomes Relevant to Evista (CORE) study, which included 5,213 women, extended the MORE trial for 4 years. The primary endpoint was new-onset invasive breast cancer. After 4 years of the original MORE trial, the incidence of invasive breast cancer among patients given raloxifene was reduced 72% compared to that among patients given placebo. At the end of 8 years, the incidence of invasive breast cancer and estrogen-receptor positive breast cancer were reduced by 66% and 76%, respectively, compared with placebo.
A second chance
Unlike tamoxifen (the original selective estrogen receptor modulator [SERM]) whose use in women with breast cancer is limited to 5 years, raloxifene has no time limit.
ADDITIONAL REFERENCES
- American College of Obstetricians and Gynecologists. Selective estrogen receptor modulators. ACOG Practice Bulletin No. 39. Obstet Gynecol. 2002;100:835:835–844.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541.
- Delmas PD, Ensrud KE, Adachi JD, et al for the Multiple Outcomes of Raloxifene Evaluation Investigators. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2002;87:3609–3617.
- McClung MR, Geusens P, Miller PD, et al for the Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–340.
Dr. Goldstein reports that he serves on the gynecology advisory boards for Eli Lilly, Merck, Pfizer, Procter & Gamble, and TAP Pharmaceuticals.
The numbers tell why. The total number of fragility fractures in American women in a single year—1 million—out-numbers all heart attacks, strokes, breast cancers, and gynecologic cancers combined. A quality-of-life study by Toteson and Hammond found that 4 out of 10 Caucasian women over 50 will fracture a hip, spine, or wrist, sooner or later. One of every 5 who fracture a hip ends up in a nursing home. The direct care cost of osteoporotic fractures was $17 billion in 2001 dollars.
Now, we have more treatment options than ever. And 2005 has been a banner year for discoveries we can put into practice immediately, in our efforts to prevent fragility fractures.
Why so confusing?
McClung MR. The relationship between bone mineral density and fracture risk. Curr Osteoporos Rep. 2005;3:57–63.
- The terms osteopenia and osteoporosis are arbitrary cutoffs. Fracture risk is a continuum and involves multiple factors in addition to bone mass.
The clinically crucial part of that definition is…“increasing the risk of fragility fractures.” Certainly, low bone mass on DEXA is a risk factor. And guidelines from the World Health Organization (WHO), the National Osteoporosis Foundation, and the North American Menopause Society are based on T-scores. However, treatment that bases intervention on absolute fracture risk would be much more appropriate; in fact, the WHO is expected to shortly issue a method to calculate fracture risk. Factors are likely to include age, previous fracture, family history, body mass index, ever use of steroids, propensity for falling, eyesight, overall health, and bone mass (ie, BMD determinations).
We need to realize that WHO definitions of T-score categories are meant for postmenopausal women. Inappropriate use of DEXA scanning in a premenopausal patient may identify a woman with low bone mass, but her bone quality and risk of fragility fracture differ greatly from that of a distantly postmenopausal woman with the same T-score. It may seem counterintuitive, but a 50-year-old woman with a T-score of –3.0 has the same absolute fracture risk, going forward, as an 80-year-old woman with a T-score of –1.
Although the risk of fracture is greatest in women with osteoporosis, there are many more women with osteopenia who will have a fracture. But that doesn’t mean we should prescribe pharmacotherapy for every osteopenic woman in an attempt to prevent fractures. As the US Surgeon General’s report last October estimated, 34 million women have osteopenia and “only” 10 million have osteoporosis. Not every woman with osteopenia should be a candidate for pharmacotherapy, but these facts do underscore the need for a better way to assess absolute fracture risk.
ADDITIONAL REFERENCES
- Miller PD, Barlas S, Brenneman SK, et al. An approach to identifying osteopenic women at increased short-term risk of fracture. Arch Intern Med. 2004;164:1113–1120.
- Salkeld G, Cameron ID, Cumming RG, et al. Quality of life related to fear of falling and hip fracture in older women: a time trade off study. BMJ. 2000;320:341–346.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195–202.
- Tosteson AN, Hammond CS. Quality-of-life assessment in osteoporosis: health-status and preference-based measures. PharmacoEconomics. 2002;20:289–303.
Are all bisphosphonates created equal?
Rosen CJ, Hochberg MC, Bonnick SL, et al. Postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res. 2005;20:141–151.
- Antifracture efficacy at the spine appears to be indistinguishable among antiresorptive agents, despite differences in BMD and bone turnover. Gastrointestinal tolerability was similar in the FACT study.
Endpoints of the FACT study. The primary endpoint was change from baseline BMD at the hip trochanter at 12 months. Secondary endpoints included BMD at multiple sites, bone turnover markers, and drug tolerability. After 12 months, BMD increased 3.4% with alendronate and 2.1% with risedronate (P<.001 alendronate produced significantly greater reductions in bone markers. fracture data were collected as part of the safety monitoring: fractures group and risedronate group.>
Antiresorptives lower fracture risk even without increasing BMD
However, until a head-to-head antifracture efficacy study is done, we cannot infer whether alendronate or risedronate is more effective, based on surrogate endpoints. In fact, if one looks at observations on calcitonin and raloxifene, all 4 drugs provide a similar level of fracture protection, at least in the spine, despite marked differences in turnover markers and BMD. This similarity in antifracture efficacy is probably because antiresorptive drugs affect bone quality and microarchitecture, as well as bone mass.
Antiresorptive medications reduce fracture risk, even in the absence of substantial increases in BMD. This finding has significant implications for monitoring therapy. The misconception that efficacy depends on the amount of bone gained often prompts physicians to stop a drug or add a second drug if a patient’s bone density does not increase. The indication of treatment success, however, is absence of bone loss, not extent of bone gain.
The key to meaningful monitoring
Serial observations with DEXA scanning are fraught with error if one does not understand the concept of least specific change. Least specific change is defined as 2.77 times the precision error of the scanning machine used. Thus, in good centers, BMD measurement of the spine should vary no more than ±3%; measurement of the hip may vary as much as ±5%. For example, a patient who gains 2% over time in the hip and spine is no different statistically from a patient who loses 2% over time in the hip and spine. However, many patients and clinicians feel gratified by a modest increase—and consider an alternative or additional medication if there is a mild decrease. If we take into account the “least specific change,” it becomes evident that in both cases, the patients are in fact unchanged.
Daily pill more likely to get blamed for GI symptoms?
The perception among many clinicians prior to the FACT head-to-head trial was that risedronate had greater GI tolerability than alendronate. However, in the FACT trial no differences were noted in adverse events of the GI tract for either compound. When first introduced, alendronate was a daily regimen. Both alendronate and risedronate are now being given once per week, predominately, and it seems that this schedule has led to fewer complaints and fewer patients discontinuing medication because of GI symptoms. This change probably is because patients are not as likely to relate all of their GI symptoms to a pill taken a week ago, but are more likely to blame any GI complaint on a pill they take every day.
ADDITIONAL REFERENCES
- Bauer DC, Black DM, Garnero P, et al for the Fracture Intervention Trial Study Group. Change in bone turnover and hip, nonspine, and vertebral fracture in alendronate-treated women: the Fracture Intervention Trial. J Bone Miner Res. 2004;19:1250–1258.
- Watts NB, Cooper C, Lindsay R, et al. Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: greater increases in bone mineral density do not relate to greater decreases in fracture risk. J Clin Densitom. 2004;7:255–261.
Which is better, once-a-month or once-a-day ibandronate?
Miller PD, McClung MR, Macovei L, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res. 2005;20:1315–1322.
- “Monthly ibandronate is at least as effective and well tolerated as…the daily ibandronate regimen in postmenopausal osteoporosis.”
Which schedule will patients follow?
Virtually all clinicians would agree that patients prefer weekly to daily dosing, especially if the medication is somewhat inconvenient. Bisphosphonates should be taken with a full glass of water, and the patient should remain standing or sitting upright and avoid other food or drink for 1/2 hour (a full hour with ibandronate).
It remains to be seen. Once-a-month dosing may offer more appeal than weekly alendronate or risedronate, but whether adherence will be better or worse remains to be seen.
Does ibandronate prevent fractures?
Daily ibandronate, 2.5 mg, has been shown to improve bone density and bone turnover values and to reduce vertebral fractures.
There are no prospective data showing nonvertebral fracture reduction—as there are for alendronate and risedronate. However, there was a time when we had only vertebral fracture data on those compounds; a leap of faith was necessary to prescribe them for overall fracture prevention.
The MOBILE study employed a randomized, double-blind method referred to as a “noninferior” trial. A total of 1,609 women with osteoporosis were assigned to once-monthly or daily oral ibandronate. All monthly regimens proved “noninferior” to daily dosing, and the highest monthly dose (150 mg) proved superior to the daily regimen, in terms of lumbar spine BMD increase at 1 year. All regimens were similarly tolerated.
Those who would criticize this methodology will be interested to recall that noninferiority trials were exactly the mechanism that led the way from daily to weekly dosing for alendronate and risedronate.
Which patients are best suited to ibandronate?
Until nonvertebral fracture data become available, however, many clinicians may feel that ibandronate is best suited for these patients:
- women who feel that even once weekly dosing is too inconvenient, and
- younger postmenopausal women who are not at high or immediate risk for hip or other nonvertebral fractures.
- Chesnut CH III, Skag A, Christiansen C, et al for the Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249.
- Delmas PD, Recker RR, Chestnut CH III, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004;15:792–798.
- Reginster JY, Felsenberg D, Cooper C, et al. A new concept for bisphosphonate therapy: a rationale for the development of monthly oral dosing of ibandronate. Osteoporos Int. 2005 Jun 14; [Epub ahead of print].
Martino S, Cauley JA, Barrett-Connor E, et al for the CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–1761.
- In postmenopausal women at high risk for breast cancer who also need bone pharmacotherapy, raloxifene offers an additional benefit in the breast as well as in the skeleton.
How low can you go?
The MORE trial failed to show a reduction in hip fracture. However, the rate of hip fracture in the placebo group was very low (0.7%) compared to that of placebo groups in an alendronate trial known as FIT I (2.2% placebo group) and the risedronate trial (3.9% placebo group) conducted by McClung and colleagues. This finding underscores the notion that it is difficult to lower risk if a group’s risk level is initially low.
Efficacy after 8 years. The Continuing Outcomes Relevant to Evista (CORE) study, which included 5,213 women, extended the MORE trial for 4 years. The primary endpoint was new-onset invasive breast cancer. After 4 years of the original MORE trial, the incidence of invasive breast cancer among patients given raloxifene was reduced 72% compared to that among patients given placebo. At the end of 8 years, the incidence of invasive breast cancer and estrogen-receptor positive breast cancer were reduced by 66% and 76%, respectively, compared with placebo.
A second chance
Unlike tamoxifen (the original selective estrogen receptor modulator [SERM]) whose use in women with breast cancer is limited to 5 years, raloxifene has no time limit.
ADDITIONAL REFERENCES
- American College of Obstetricians and Gynecologists. Selective estrogen receptor modulators. ACOG Practice Bulletin No. 39. Obstet Gynecol. 2002;100:835:835–844.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541.
- Delmas PD, Ensrud KE, Adachi JD, et al for the Multiple Outcomes of Raloxifene Evaluation Investigators. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2002;87:3609–3617.
- McClung MR, Geusens P, Miller PD, et al for the Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–340.
Dr. Goldstein reports that he serves on the gynecology advisory boards for Eli Lilly, Merck, Pfizer, Procter & Gamble, and TAP Pharmaceuticals.
The numbers tell why. The total number of fragility fractures in American women in a single year—1 million—out-numbers all heart attacks, strokes, breast cancers, and gynecologic cancers combined. A quality-of-life study by Toteson and Hammond found that 4 out of 10 Caucasian women over 50 will fracture a hip, spine, or wrist, sooner or later. One of every 5 who fracture a hip ends up in a nursing home. The direct care cost of osteoporotic fractures was $17 billion in 2001 dollars.
Now, we have more treatment options than ever. And 2005 has been a banner year for discoveries we can put into practice immediately, in our efforts to prevent fragility fractures.
Why so confusing?
McClung MR. The relationship between bone mineral density and fracture risk. Curr Osteoporos Rep. 2005;3:57–63.
- The terms osteopenia and osteoporosis are arbitrary cutoffs. Fracture risk is a continuum and involves multiple factors in addition to bone mass.
The clinically crucial part of that definition is…“increasing the risk of fragility fractures.” Certainly, low bone mass on DEXA is a risk factor. And guidelines from the World Health Organization (WHO), the National Osteoporosis Foundation, and the North American Menopause Society are based on T-scores. However, treatment that bases intervention on absolute fracture risk would be much more appropriate; in fact, the WHO is expected to shortly issue a method to calculate fracture risk. Factors are likely to include age, previous fracture, family history, body mass index, ever use of steroids, propensity for falling, eyesight, overall health, and bone mass (ie, BMD determinations).
We need to realize that WHO definitions of T-score categories are meant for postmenopausal women. Inappropriate use of DEXA scanning in a premenopausal patient may identify a woman with low bone mass, but her bone quality and risk of fragility fracture differ greatly from that of a distantly postmenopausal woman with the same T-score. It may seem counterintuitive, but a 50-year-old woman with a T-score of –3.0 has the same absolute fracture risk, going forward, as an 80-year-old woman with a T-score of –1.
Although the risk of fracture is greatest in women with osteoporosis, there are many more women with osteopenia who will have a fracture. But that doesn’t mean we should prescribe pharmacotherapy for every osteopenic woman in an attempt to prevent fractures. As the US Surgeon General’s report last October estimated, 34 million women have osteopenia and “only” 10 million have osteoporosis. Not every woman with osteopenia should be a candidate for pharmacotherapy, but these facts do underscore the need for a better way to assess absolute fracture risk.
ADDITIONAL REFERENCES
- Miller PD, Barlas S, Brenneman SK, et al. An approach to identifying osteopenic women at increased short-term risk of fracture. Arch Intern Med. 2004;164:1113–1120.
- Salkeld G, Cameron ID, Cumming RG, et al. Quality of life related to fear of falling and hip fracture in older women: a time trade off study. BMJ. 2000;320:341–346.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195–202.
- Tosteson AN, Hammond CS. Quality-of-life assessment in osteoporosis: health-status and preference-based measures. PharmacoEconomics. 2002;20:289–303.
Are all bisphosphonates created equal?
Rosen CJ, Hochberg MC, Bonnick SL, et al. Postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res. 2005;20:141–151.
- Antifracture efficacy at the spine appears to be indistinguishable among antiresorptive agents, despite differences in BMD and bone turnover. Gastrointestinal tolerability was similar in the FACT study.
Endpoints of the FACT study. The primary endpoint was change from baseline BMD at the hip trochanter at 12 months. Secondary endpoints included BMD at multiple sites, bone turnover markers, and drug tolerability. After 12 months, BMD increased 3.4% with alendronate and 2.1% with risedronate (P<.001 alendronate produced significantly greater reductions in bone markers. fracture data were collected as part of the safety monitoring: fractures group and risedronate group.>
Antiresorptives lower fracture risk even without increasing BMD
However, until a head-to-head antifracture efficacy study is done, we cannot infer whether alendronate or risedronate is more effective, based on surrogate endpoints. In fact, if one looks at observations on calcitonin and raloxifene, all 4 drugs provide a similar level of fracture protection, at least in the spine, despite marked differences in turnover markers and BMD. This similarity in antifracture efficacy is probably because antiresorptive drugs affect bone quality and microarchitecture, as well as bone mass.
Antiresorptive medications reduce fracture risk, even in the absence of substantial increases in BMD. This finding has significant implications for monitoring therapy. The misconception that efficacy depends on the amount of bone gained often prompts physicians to stop a drug or add a second drug if a patient’s bone density does not increase. The indication of treatment success, however, is absence of bone loss, not extent of bone gain.
The key to meaningful monitoring
Serial observations with DEXA scanning are fraught with error if one does not understand the concept of least specific change. Least specific change is defined as 2.77 times the precision error of the scanning machine used. Thus, in good centers, BMD measurement of the spine should vary no more than ±3%; measurement of the hip may vary as much as ±5%. For example, a patient who gains 2% over time in the hip and spine is no different statistically from a patient who loses 2% over time in the hip and spine. However, many patients and clinicians feel gratified by a modest increase—and consider an alternative or additional medication if there is a mild decrease. If we take into account the “least specific change,” it becomes evident that in both cases, the patients are in fact unchanged.
Daily pill more likely to get blamed for GI symptoms?
The perception among many clinicians prior to the FACT head-to-head trial was that risedronate had greater GI tolerability than alendronate. However, in the FACT trial no differences were noted in adverse events of the GI tract for either compound. When first introduced, alendronate was a daily regimen. Both alendronate and risedronate are now being given once per week, predominately, and it seems that this schedule has led to fewer complaints and fewer patients discontinuing medication because of GI symptoms. This change probably is because patients are not as likely to relate all of their GI symptoms to a pill taken a week ago, but are more likely to blame any GI complaint on a pill they take every day.
ADDITIONAL REFERENCES
- Bauer DC, Black DM, Garnero P, et al for the Fracture Intervention Trial Study Group. Change in bone turnover and hip, nonspine, and vertebral fracture in alendronate-treated women: the Fracture Intervention Trial. J Bone Miner Res. 2004;19:1250–1258.
- Watts NB, Cooper C, Lindsay R, et al. Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: greater increases in bone mineral density do not relate to greater decreases in fracture risk. J Clin Densitom. 2004;7:255–261.
Which is better, once-a-month or once-a-day ibandronate?
Miller PD, McClung MR, Macovei L, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res. 2005;20:1315–1322.
- “Monthly ibandronate is at least as effective and well tolerated as…the daily ibandronate regimen in postmenopausal osteoporosis.”
Which schedule will patients follow?
Virtually all clinicians would agree that patients prefer weekly to daily dosing, especially if the medication is somewhat inconvenient. Bisphosphonates should be taken with a full glass of water, and the patient should remain standing or sitting upright and avoid other food or drink for 1/2 hour (a full hour with ibandronate).
It remains to be seen. Once-a-month dosing may offer more appeal than weekly alendronate or risedronate, but whether adherence will be better or worse remains to be seen.
Does ibandronate prevent fractures?
Daily ibandronate, 2.5 mg, has been shown to improve bone density and bone turnover values and to reduce vertebral fractures.
There are no prospective data showing nonvertebral fracture reduction—as there are for alendronate and risedronate. However, there was a time when we had only vertebral fracture data on those compounds; a leap of faith was necessary to prescribe them for overall fracture prevention.
The MOBILE study employed a randomized, double-blind method referred to as a “noninferior” trial. A total of 1,609 women with osteoporosis were assigned to once-monthly or daily oral ibandronate. All monthly regimens proved “noninferior” to daily dosing, and the highest monthly dose (150 mg) proved superior to the daily regimen, in terms of lumbar spine BMD increase at 1 year. All regimens were similarly tolerated.
Those who would criticize this methodology will be interested to recall that noninferiority trials were exactly the mechanism that led the way from daily to weekly dosing for alendronate and risedronate.
Which patients are best suited to ibandronate?
Until nonvertebral fracture data become available, however, many clinicians may feel that ibandronate is best suited for these patients:
- women who feel that even once weekly dosing is too inconvenient, and
- younger postmenopausal women who are not at high or immediate risk for hip or other nonvertebral fractures.
- Chesnut CH III, Skag A, Christiansen C, et al for the Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–1249.
- Delmas PD, Recker RR, Chestnut CH III, et al. Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int. 2004;15:792–798.
- Reginster JY, Felsenberg D, Cooper C, et al. A new concept for bisphosphonate therapy: a rationale for the development of monthly oral dosing of ibandronate. Osteoporos Int. 2005 Jun 14; [Epub ahead of print].
Martino S, Cauley JA, Barrett-Connor E, et al for the CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–1761.
- In postmenopausal women at high risk for breast cancer who also need bone pharmacotherapy, raloxifene offers an additional benefit in the breast as well as in the skeleton.
How low can you go?
The MORE trial failed to show a reduction in hip fracture. However, the rate of hip fracture in the placebo group was very low (0.7%) compared to that of placebo groups in an alendronate trial known as FIT I (2.2% placebo group) and the risedronate trial (3.9% placebo group) conducted by McClung and colleagues. This finding underscores the notion that it is difficult to lower risk if a group’s risk level is initially low.
Efficacy after 8 years. The Continuing Outcomes Relevant to Evista (CORE) study, which included 5,213 women, extended the MORE trial for 4 years. The primary endpoint was new-onset invasive breast cancer. After 4 years of the original MORE trial, the incidence of invasive breast cancer among patients given raloxifene was reduced 72% compared to that among patients given placebo. At the end of 8 years, the incidence of invasive breast cancer and estrogen-receptor positive breast cancer were reduced by 66% and 76%, respectively, compared with placebo.
A second chance
Unlike tamoxifen (the original selective estrogen receptor modulator [SERM]) whose use in women with breast cancer is limited to 5 years, raloxifene has no time limit.
ADDITIONAL REFERENCES
- American College of Obstetricians and Gynecologists. Selective estrogen receptor modulators. ACOG Practice Bulletin No. 39. Obstet Gynecol. 2002;100:835:835–844.
- Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541.
- Delmas PD, Ensrud KE, Adachi JD, et al for the Multiple Outcomes of Raloxifene Evaluation Investigators. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2002;87:3609–3617.
- McClung MR, Geusens P, Miller PD, et al for the Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–340.
Dr. Goldstein reports that he serves on the gynecology advisory boards for Eli Lilly, Merck, Pfizer, Procter & Gamble, and TAP Pharmaceuticals.
Aromatase inhibitors and breast cancer? Don’t write off tamoxifen
What are the findings of 3 large randomized, controlled trials on adjuvant use of aromatase inhibitors?
Results
All 3 trials found improved disease-free survival with aromatase inhibitors in women with early-stage breast carcinoma, compared with tamoxifen.
Expert commentary
Each aromatase inhibitor should be offered in clinical scenarios similar to those in the studies, the investigators concluded from their analysis. Each of the 3 trials focused on a single aromatase inhibitor:
- Anastrozole: 4-year disease-free survival of 86.9%, versus 84.5% in the tamoxifen group. Arimidex, Tamoxifen Alone or in Combination study
- Exemestane: 3-year disease-free survival of 91.5% for women already disease-free after 2 to 3 years of tamoxifen, versus 86.8% with 3 years of additional tamoxifen. Intergroup Exemestane Study
- Letrozole: 4-year survival of 93% for women who had completed 5 years of tamoxifen, versus 87% for no further treatment. MA-17 trial
Too little long-term data
Morandi and colleagues acknowledge the immaturity of their safety data, but give short shrift to osteoporotic fractures. With more and more women diagnosed with stage I—and even stage 0—breast tumors, I believe we must consider long-term safety data, especially in terms of osteoporotic fractures, before we make a wholesale change from selective estrogen receptor modulators (ie, tamoxifen) to aromatase inhibitors.
Match the patient to the study
While aromatase inhibitors are clearly superior in women with advanced breast cancer and probably superior in women at highest risk of recurrence, I am concerned about using them in women at extremely low risk for recurrence or mortality. We must make certain, however, that the patient we are treating has the same characteristics as the patients in the study upon which we are basing our management.
Furthermore, the American Society of Clinical Oncology Technology Assessment Working Group1,2 found that the evidence of tamoxifen’s safety and efficacy is “compelling, extensive, and long-term.” When it came to anastrozole, however, they found “extensive supporting data very promising, but insufficient to change the standard of practice at this time.”
Bottom line
A 5-year course of adjuvant tamoxifen remains the standard for women with hormone-receptor–positive breast cancer.
1. Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for women with hormone receptor-positive breast cancer: status report 2002. J Clin Oncol. 2002;20:3317-3327.
2. Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment working group update: use of aromatase inhibitors in the adjuvant setting. J Clin Oncol. 2003;21:2597-2599.
What are the findings of 3 large randomized, controlled trials on adjuvant use of aromatase inhibitors?
Results
All 3 trials found improved disease-free survival with aromatase inhibitors in women with early-stage breast carcinoma, compared with tamoxifen.
Expert commentary
Each aromatase inhibitor should be offered in clinical scenarios similar to those in the studies, the investigators concluded from their analysis. Each of the 3 trials focused on a single aromatase inhibitor:
- Anastrozole: 4-year disease-free survival of 86.9%, versus 84.5% in the tamoxifen group. Arimidex, Tamoxifen Alone or in Combination study
- Exemestane: 3-year disease-free survival of 91.5% for women already disease-free after 2 to 3 years of tamoxifen, versus 86.8% with 3 years of additional tamoxifen. Intergroup Exemestane Study
- Letrozole: 4-year survival of 93% for women who had completed 5 years of tamoxifen, versus 87% for no further treatment. MA-17 trial
Too little long-term data
Morandi and colleagues acknowledge the immaturity of their safety data, but give short shrift to osteoporotic fractures. With more and more women diagnosed with stage I—and even stage 0—breast tumors, I believe we must consider long-term safety data, especially in terms of osteoporotic fractures, before we make a wholesale change from selective estrogen receptor modulators (ie, tamoxifen) to aromatase inhibitors.
Match the patient to the study
While aromatase inhibitors are clearly superior in women with advanced breast cancer and probably superior in women at highest risk of recurrence, I am concerned about using them in women at extremely low risk for recurrence or mortality. We must make certain, however, that the patient we are treating has the same characteristics as the patients in the study upon which we are basing our management.
Furthermore, the American Society of Clinical Oncology Technology Assessment Working Group1,2 found that the evidence of tamoxifen’s safety and efficacy is “compelling, extensive, and long-term.” When it came to anastrozole, however, they found “extensive supporting data very promising, but insufficient to change the standard of practice at this time.”
Bottom line
A 5-year course of adjuvant tamoxifen remains the standard for women with hormone-receptor–positive breast cancer.
What are the findings of 3 large randomized, controlled trials on adjuvant use of aromatase inhibitors?
Results
All 3 trials found improved disease-free survival with aromatase inhibitors in women with early-stage breast carcinoma, compared with tamoxifen.
Expert commentary
Each aromatase inhibitor should be offered in clinical scenarios similar to those in the studies, the investigators concluded from their analysis. Each of the 3 trials focused on a single aromatase inhibitor:
- Anastrozole: 4-year disease-free survival of 86.9%, versus 84.5% in the tamoxifen group. Arimidex, Tamoxifen Alone or in Combination study
- Exemestane: 3-year disease-free survival of 91.5% for women already disease-free after 2 to 3 years of tamoxifen, versus 86.8% with 3 years of additional tamoxifen. Intergroup Exemestane Study
- Letrozole: 4-year survival of 93% for women who had completed 5 years of tamoxifen, versus 87% for no further treatment. MA-17 trial
Too little long-term data
Morandi and colleagues acknowledge the immaturity of their safety data, but give short shrift to osteoporotic fractures. With more and more women diagnosed with stage I—and even stage 0—breast tumors, I believe we must consider long-term safety data, especially in terms of osteoporotic fractures, before we make a wholesale change from selective estrogen receptor modulators (ie, tamoxifen) to aromatase inhibitors.
Match the patient to the study
While aromatase inhibitors are clearly superior in women with advanced breast cancer and probably superior in women at highest risk of recurrence, I am concerned about using them in women at extremely low risk for recurrence or mortality. We must make certain, however, that the patient we are treating has the same characteristics as the patients in the study upon which we are basing our management.
Furthermore, the American Society of Clinical Oncology Technology Assessment Working Group1,2 found that the evidence of tamoxifen’s safety and efficacy is “compelling, extensive, and long-term.” When it came to anastrozole, however, they found “extensive supporting data very promising, but insufficient to change the standard of practice at this time.”
Bottom line
A 5-year course of adjuvant tamoxifen remains the standard for women with hormone-receptor–positive breast cancer.
1. Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for women with hormone receptor-positive breast cancer: status report 2002. J Clin Oncol. 2002;20:3317-3327.
2. Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment working group update: use of aromatase inhibitors in the adjuvant setting. J Clin Oncol. 2003;21:2597-2599.
1. Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for women with hormone receptor-positive breast cancer: status report 2002. J Clin Oncol. 2002;20:3317-3327.
2. Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment working group update: use of aromatase inhibitors in the adjuvant setting. J Clin Oncol. 2003;21:2597-2599.
OBG Management ©2005 Dowden Health Media
Reducing the risk of breast cancer: Key trials and their impact on practice
- In the Breast Cancer Prevention Trial, women taking tamoxifen experienced a 49% overall reduction in invasive breast cancer; the relative risk of endometrial cancer was 4.01 for women over 50 and 1.21 for women younger than 50.
- The risk of serious adverse effects with tamoxifen use appears to be lower in women under age 50.
- Preclinical animal data suggest that, like tamoxifen, raloxifene has potent antiestrogen effects on breast tissue.
- Because aromatase inhibitors block the peripheral conversion of androstenedione to estrogens, they inhibit both initiation and promotion of breast cancer. Thus, they may be more effective than selective estrogen receptor modulators in preventing the disease.
More than 28,000 additional breast cancers could be prevented over 5 years if all eligible women were given tamoxifen.1
This is just one of several important findings highlighted in the studies covered in this review of breast cancer risk assessment and chemopreventive options.
Because Ob/Gyns often treat tamoxifen users who experience uterine bleeding and worry about their risk of endometrial cancer (see “Endometrial screening and tamoxifen users: Going beyond the ACOG opinion,”), it is crucial that we have the latest data on preventive therapies for breast cancer—which include not only tamoxifen, but also, potentially, raloxifene and aromatase inhibitors—so that we may facilitate proper work-up and monitoring.
With the rising use of tamoxifen has come an increased need for vigilance for signs of endometrial cancer. To address the issue, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion on the subject in April 2000.1
In the opinion, ACOG observed that screening tests have not increased the early detection of endometrial cancer in women using tamoxifen and may lead to more invasive and costly diagnostic procedures. ACOG also recommended that Ob/Gyns:
- Educate women taking tamoxifen on the risks of endometrial proliferation, hyperplasia, and cancer and stress the importance of annual gynecologic exams.
- Closely monitor these patients for signs of endometrial hyperplasia or cancer.
- Encourage patients to promptly report abnormal vaginal symptoms, including bloody discharge, spotting, staining, or leukorrhea.
- Investigate any abnormal vaginal bleeding, bloody discharge, spotting, or staining.
- Limit tamoxifen therapy to 5 years, as no benefit has been established beyond this time frame.
- Initiate proper gynecologic management and reassess the use of tamoxifen if the patient develops atypical endometrial hyperplasia.
- Consider resuming tamoxifen therapy following hysterectomy for endometrial carcinoma, in consultation with the physician responsible for the woman’s breast care.1
Screening tests help determine risk. In light of data published over the last 5 years, I now perform endometrial screening on patients about to begin tamoxifen therapy—a practice that differs from the ACOG opinion outlined above. Here is why:
Work published by Berliere et al2 in 1998 and updated in 20003 suggest that, among women on tamoxifen therapy, there exists a group at high risk and a group at low risk for developing complex atypical hyperplasia of the endometrium.
Berliere and her group studied 575 asymptomatic postmenopausal women with recently diagnosed breast cancer about to begin tamoxifen therapy. Each woman received transvaginal ultrasound; if the endometrial echo was greater than 4 mm, office hysteroscopy was performed. Of the study population, 17.4% had initial benign polyps of the endometrium. All polyps were removed, tamoxifen therapy initiated, and follow-up carried out through 5 years.
Among the women with no initial polyp (whom I would classify as “squeaky clean”), 0.7% developed atypical hyperplasia over the 5-year study period—compared with 11.7% of those who had an initial polyp removed. In addition, 11.7% of squeaky clean patients experienced polyp formation, compared with 17.6% of those with initial polyps. Thus, the 17.4% with initial benign polyps had 18 times the risk of the squeaky clean group for developing atypical hyperplasia while on tamoxifen therapy.
These findings have caused me to rethink my approach toward endometrial surveillance for women taking tamoxifen.4
Here is my current practice:
- When patients are diagnosed with breast cancer and scheduled to begin tamoxifen therapy, I perform pretreatment screening.
- If no initial endometrial polyps are found, I follow ACOG’s recommendations. Further interventions are unnecessary (unless abnormal symptoms develop), since these patients are at no more risk for endometrial cancer than women not taking tamoxifen.
- For patients with an initial polyp (ie, the high-risk group), I remove the polyp prior to starting tamoxifen treatment and monitor them throughout the course of therapy, periodically utilizing transvaginal ultrasound and saline infusion sonohysterography.
REFERENCES
1. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion #232: Tamoxifen and Endometrial Cancer. Washington, DC: ACOG; April 2000.
2. Berliere M, Charles A, Galant C, Donnez J. Uterine side effects of tamoxifen: a need for systematic pretreatment screening. Obstet Gynecol. 1998;91(1):40-44.
3. Berliere M, Radikov G, Galant C, Piette P, Marbaix E, Donnez J. Identification of women at high risk of developing endometrial cancer on tamoxifen. Eur J Cancer. 2000;36(suppl 4):S35-S36.
4. Goldstein SR. Controversy about uterine effects and safety of SERMs: the saga continues. Menopause. 2002;9:381-384.
Assessing risk: The need for a new model
Although a number of breast cancer riskassessment models are available based on individual risk factors (TABLE 1), estimates based on combinations of factors are preferable. The Gail model,2 widely used to determine breast cancer risk, takes into account nongenetic (nulliparity, age at menarche) and genetic (family history) factors, as well as the number of previous breast biopsies. It assigns a smaller relative risk to women over age 50. A Web-based version, available at http://bcra.nci.nih.gov/brc, is useful for calculating a woman’s risk of developing invasive disease over the next 5 years, as well as over her remaining lifetime.
Limitations of the Gail model. Unfortunately, the data on which the model is based were collected in the late 1970s and early 1980s. Today, the greater ease of breast histopathologic assessment by fine-needle aspiration and outpatient core-needle biopsy has increased the rate of tissue sampling, creating confusion as to what constitutes a biopsy. Thus, the cutoff of 1.66% for high risk—the threshold adopted for the Breast Cancer Prevention Trial (BCPT)—loses some credibility.
Consider this example: A 50-year-old nulliparous Caucasian woman experienced menarche at age 11, has no first-degree relatives with a history of breast cancer, and has never had a breast biopsy. The Gail model would assign her a risk of developing breast cancer of 1.2% in the next 5 years and 10.8% over her lifetime. However, if the same patient had had 3 breast biopsies, her risk would rise to 1.8% in the next 5 years and 15.8% for her lifetime (placing her in the high-risk category), even if none of the biopsies revealed hyperplasia.
Biomarkers. Objective findings that are patient-specific but which correlate closely with breast cancer development are needed.
Biomarkers have been proposed; among them: ultrasensitive measurement of serum estradiol levels in postmenopausal women.3 In the Multiple Outcomes of Raloxifene Evaluation (MORE),4 the women who had the greatest reduction in breast cancer during treatment had the highest baseline serum estradiol levels (FIGURE 1)—although the baseline levels of all subjects were well within the postmenopausal range of 20 pmol/L or less.
TABLE 1
Breast cancer risk factors and their relative risks19
| RELATIVE RISK <2 | RELATIVE RISK 2–4 | RELATIVE RISK >4 |
|---|---|---|
|
|
|
FIGURE 1 Breast cancer risk in raloxifene users
Reprinted with permission from: Cummings SR, Duong T, Kenyon E, et al. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220. Copyright © 2002 American Medical Association. All rights reserved.
Chemoprevention: The rationale for tamoxifen
Data from preclinical animal and in vitro studies led to the use of tamoxifen, a selective estrogen receptor modulator (SERM), for primary prevention of breast cancer in healthy women. The drug was shown to inhibit mammary tumors in mice and rats and suppress hormone-dependent breast cancer cell lines in vitro.5
Clinical data from the Early Breast Cancer Trialists Collaborative Group also helped spur prevention trials with tamoxifen.6 Besides decreasing the risk of recurrent breast cancer, tamoxifen reduced the risk of contralateral, new-onset breast cancer by 47% after 5 years of adjuvant treatment (P = .00001) and by 26% after 2 years of treatment (P = .004). This, along with tamoxifen’s favorable effects on skeletal remodeling and lipid levels, led to a series of chemopreventive trials in the United States and Europe (TABLE 2).
TABLE 2
Results of tamoxifen trials
| BREAST CANCER PREVENTION TRIAL7 | ROYAL MARSDEN10 | ITALIAN11 | |
|---|---|---|---|
| Number of participants | 13,388 | 2,471 | 5,408 |
| Age ≤50 | 40% | 62% | 36% |
| One first-degree relative with breast cancer | 55% | 55% | 18% |
| >2 first-degree relatives with breast cancer | 13% | 17% | 2.5% |
| HRT users | 0% | 42% | 8% |
| Woman-years of follow-up | 46,858 | 12,355 | 20,731 |
| Cancer incidence/1,000 | |||
| Placebo | 6.8 | 5.0 | 2.3 |
| Tamoxifen | 3.4 | 4.7 | 2.1 |
Breast Cancer Prevention Trial: Tamoxifen reduces cancer incidence
In 1992, the National Surgical Adjuvant Breast and Bowel Project launched a prevention trial using tamoxifen: the Breast Cancer Prevention Trial.7 A total of 13,388 women aged 35 or older and at high risk for breast cancer were enrolled at numerous sites throughout the United States and Canada.
The Gail model was utilized to determine which women had sufficient risk—that is, a risk of developing invasive breast cancer within the next 5 years of 1.66% or greater—to be included in the trial.2 Subjects were randomly assigned to receive placebo or tamoxifen (20 mg/d) for 5 years.
The trial was terminated in April 1998, 14 months before its planned completion, due to the striking reduction in new-onset breast cancer in the tamoxifen group. The data safety monitoring board felt it would be unethical to allow one half of the participants, who were deemed to be at high risk, to continue taking placebo in light of the dramatic reduction in both invasive and noninvasive breast cancer with tamoxifen use.
The overall incidence of breast cancer in the tamoxifen group was 3.4 cases per 1,000, compared with 6.8 cases per 1,000 in the placebo group. Overall, the reduction in invasive breast cancer was 49% (P<.000001). The reductions were 44% for women in the group aged 35 to 49 years, 51% for those aged 50 to 59, and 55% for those 60 years and older (FIGURE 2).
Tamoxifen decreased the incidence of noninvasive breast cancer (ductal carcinoma in situ) by 50%. (Expanded use of mammography has led to greater detection of this cancer. Most such lesions are estrogen-receptor–positive.8) In addition, tamoxifen reduced breast cancer risk in women with a history of lobular carcinoma in situ by 56% and atypical hyperplasia by 86%. Overall, tamoxifen decreased the occurrence of estrogen-receptor–positive tumors by 69%, but had no impact on tumors that were estrogen-receptor–negative.
Tamoxifen’s other effects in healthy women. The BCPT offered the first largescale data on the effects of tamoxifen in healthy women.7 (All previous studies included only women with breast cancer.) Several secondary endpoints merit consideration.
- Endometrial cancer risk. Researchers found the relative risk (RR) of endometrial cancer associated with tamoxifen therapy in healthy women was 2.53 (95% confidence intervals [CI], 1.35, 4.97). When this figure was calculated for the different age groups, it rose to 4.01 (95% CI, 1.70, 10.90) in women over 50, and declined to 1.21 for women ages 49 and under (95% CI, 0.41, 3.60).
- Thromboembolic event risk. The same age distinction was seen in relation to thromboembolic events. There were no statistically significant increases in pulmonary emboli or deep venous thrombosis in women 49 years of age or under. Although it is unclear whether the trial was sufficiently powered for this particular endpoint, the likelihood that serious adverse events will limit the potential benefits of tamoxifen appears to be lower in women under the age of 50. This has significant clinical consequences for physicians caring for perimenopausal patients.
- No change in incidence of other cancers. Overall, the incidence of invasive cancers other than those of the breast and uterus was the same for the tamoxifen and placebo groups.
- Other outcomes. The relative risk of death from any cause was 0.81 (95% CI, 0.56–1.16).
There was a slight increase in the risk of myocardial infarction (RR, 1.11; 95% CI, 0.65–1.92) and a slight decrease in the development of severe angina (RR, 0.93; 95% CI, 0.40–2.14) in tamoxifen users, although neither of these was statistically significant.
The overall relative risk of fractures at various sites (hip, spine, radius) was 0.81 (95% CI, 0.63–1.05).
A statistically significant increase was found in the number of women with cataracts who then underwent cataract surgery. That relative risk was 1.57 (95% CI, 1.16–2.14).
FIGURE 2 Breast Cancer Prevention Trial: Tamoxifen reduces incidence of breast cancer
The rate of cancer reduction for tamoxifen compared with placebo for years 1 through 6 was 33%, 55%, 39%, 49%, 69%, and 55%, respectively.
Reprinted from Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst.1998;90(18):1371-1388, by permission of Oxford University Press.
FDA approves tamoxifen for primary prevention
Based on the BCPT results, the US Food and Drug Administration (FDA) approved tamoxifen in October 1998 for primary prevention of breast cancer in women at high risk for the disease. It recommended that tamoxifen be limited to high-risk women because of the potentially serious side effects seen in clinical trials.
The FDA did not define high risk, but recommended that prophylactic use of tamoxifen be based on a thorough evaluation of a woman’s personal, family, and medical histories, as well as her age and understanding of the risks and benefits of treatment.
In addition, the FDA required that the package insert advise women to consult a health-care professional for breast cancer risk assessment and state that only women at high risk should take the drug (again, without defining high risk).
In 2002, the FDA added a “black box” warning to tamoxifen labeling that was directed at use of the drug for prevention rather than treatment. This warning concerned the occurrence of uterine sarcomas. The incidence of these cancers was found to be 0.17 per 1,000 women taking tamoxifen, compared with 0.015 per 1,000 controls.9
Underuse of tamoxifen? A recent study by Freedman et al1 calculated the number of women 35 to 79 years of age who were eligible for tamoxifen chemoprevention based on FDA criteria. They further calculated the number of women who would have a positive benefit-risk ratio for tamoxifen use, and concluded that, among white women alone, roughly 28,492 additional breast cancers could be prevented or deferred if these individuals took tamoxifen for the next 5 years.
European trials fail to confirm benefit of tamoxifen
The Royal Marsden Trial was one of 2 European trials that failed to replicate the BCPT results.10 This British study involved 2,471 healthy women between the ages of 30 and 70 who had a family history of breast cancer. The immediate follow-up was 70 months. No statistically significant decrease in breast cancer was found with tamoxifen use, compared with placebo.
One possibility for the discrepancy may be that eligibility for the Royal Marsden Trial was based predominantly on a strong family history of breast cancer. It also included a much larger proportion of women under age 50 (62%, compared with only 40% in the BCPT). Probably most importantly, 42% of the women received hormone replacement therapy (HRT) along with tamoxifen during the trial.
The Italian prevention study also failed to confirm the findings of the BCPT.11 Because it recruited participants from the general population, the overall risk of breast cancer was significantly lower than in the BCPT. Compliance rates in the Italian study were quite low: Approximately 26% of the subjects dropped out. Furthermore, the Italian trial included considerably fewer women over age 60 (12% versus 30% in the BCPT).
Raloxifene: Another cancer preventive?
Like tamoxifen, raloxifene is a SERM. It is a benzothiophene derivative; tamoxifen comes from the triphenylethylene family.
Like tamoxifen, raloxifene was originally investigated as a treatment for advanced breast cancer. Preclinical studies indicated that it had an antiproliferative effect on estrogen-receptor–positive mammary tumors and estrogen-receptor–positive human breast cancer cell lines.12 However, in the 1980s, a small phase II trial revealed that it had no further antitumor effects in postmenopausal women with advanced breast cancer in whom tamoxifen therapy had failed.13
Interest in raloxifene revived after tamoxifen’s neoplastic effects on the uteri of postmenopausal women became evident.14 As a SERM, raloxifene exhibits estrogen-agonist activity on bone remodeling and lipid metabolism. In December 1997, it won FDA approval for the prevention of osteoporosis in postmenopausal women. Its indication was extended to treatment in October 1999.
Studies found that raloxifene was similar to placebo in its effects on the endometrium of postmenopausal women.15 There were no differences in endometrial thickness, endoluminal masses, proliferation, or hyperplasia. This corroborated previous findings that raloxifene causes neither endometrial hyperplasia nor cancer and is not associated with vaginal bleeding or increased endometrial thickness (as measured by transvaginal ultrasound).
In addition, preclinical animal data suggest that, like tamoxifen, raloxifene has potent antiestrogen effects on breast tissue.12
The MORE trial involved 7,705 postmenopausal women up to age 80 with established osteoporosis who were randomized to receive raloxifene or placebo. Bone mineral density and fracture incidence were the primary endpoints; breast cancer was a secondary endpoint. At 4 years, raloxifene significantly reduced the incidence of all invasive breast cancers by 72%, compared with placebo (RR = 0.28; 95% CI, 0.17–0.46).16 It reduced the incidence of invasive estrogen-receptor–positive tumors by 84%, compared with placebo (RR = 0.16; 95% CI, 0.09–0.30), but had no effect on estrogen-receptor–negative tumors.
Because they block both initiation and promotion of breast cancer, aromatase inhibitors may be more effective than SERMs in preventing breast cancer.
The incidence of vaginal bleeding, breast pain, and endometrial cancer in the raloxifene group did not differ significantly from those of the placebo group. (Because women in the MORE trial were older and did not enter the study with an increased risk for breast cancer, these findings are not necessarily applicable to younger, high-risk women.)
Like tamoxifen, raloxifene slightly increased the risk of thromboembolic disease, including deep vein thrombosis. Pulmonary embolism developed in 1.1% of women in the raloxifene group, compared with 0.5% of subjects in the placebo group (P = .003).
Currently, there is no approved indication for raloxifene in premenopausal women. SERM compounds, which are structurally similar to clomiphene citrate, seem to have different effects in premenopausal and postmenopausal women, as tamoxifen did in the BCPT. Further study in the premenopausal population or with concomitant use of lowdose estrogen may be forthcoming.
Ongoing clinical trials
STAR. To directly compare the safety and efficacy of tamoxifen and raloxifene in reducing breast cancer risk among healthy women, the Study of Tamoxifen and Raloxifene (STAR) has been enrolling postmenopausal women 35 years of age or older who are at increased risk for breast cancer. This study began in 1999 and is expected to run for at least 7 years. It seeks to enroll 22,000 participants in its randomized, double-blind investigation. Participants will receive a daily dose of raloxifene (60 mg) or tamoxifen (20 mg).
RUTH. Raloxifene Use and the Heart (RUTH) is a double-blind, placebo-controlled trial of 60 mg of raloxifene that will include 10,000 women. Primary endpoints are coronary disease and invasive breast cancer. Trial enrollment ended in August 2000, and the study is expected to conclude in 2006.
Aromatase inhibitors: Another route of prevention
The evidence that estrogens facilitate breast cancer development in animals and women is substantial, although the precise mechanism is unknown.17 The most commonly held theory is that estrogen stimulates proliferation of breast cells. Thus, it statistically increases the chances for genetic mutations that could result in cancer.
Aromatase inhibitors block peripheral conversion of androstenedione to estrogens. In premenopausal patients, the primary site of this action is in the ovary. In post-menopausal women, it occurs predominantly in extraovarian sites, including the adrenal glands, adipose tissue, liver, muscle, and skin.
Because of their dual role, (blocking both the initiation and promotion of breast cancer), aromatase inhibitors may be more effective than SERMs in preventing breast cancer.18 By inhibiting the initiation process, they would reduce levels of genotoxic metabolites of estradiol by lowering estradiol concentration in tissue. They also would inhibit tumor promotion by lowering tissue levels of estradiol—thus blocking cell proliferation. However, because they are not selective, aromatase inhibitors would have an antiestrogen effect on bone and lipid metabolism and would induce vasomotor symptoms.
Dr. Goldstein serves on gynecology advisory boards for Eli Lilly and Company, Pfizer, AstraZeneca, and P&G Pharmaceuticals.
1. Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst. 2003;95:526-532.
2. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
3. Ruffin MT, August DA, Kelloff GJ, Boone CW, Weber BL, Brenner DE. Selection criteria for breast cancer chemoprevention subjects. J Cell Biochem Suppl. 1993;17G:234-241.
4. Cummings SR, Duong T, Kenyon E, et al. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220.
5. Jordan VC, Allen KE. Evaluation of the antitumor activity of the nonsteroidal antiestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma mode. Eur J Cancer. 1980;16:239-251.
6. Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer: an overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988;319:1681-1692.
7. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
8. Bur ME, Zimarowski MJ, Schnitt SJ, Baker S, Lew R. Estrogen receptor immunohistochemistry in carcinoma in situ of the breast. Cancer. 1992;69:1174-1181.
9. Nolvadex [package insert]. Wilmington, Del: AstraZeneca Pharmaceuticals; 2002.
10. Powles T, Eeles R, Ashley S, et al. Interim analysis of incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomized chemoprevention trial. Lancet. 1998;352:98-101.
11. Veronesi U, Maisonneuve P, Costa A, et al. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomized trial among hysterectomized women. Italian Prevention Study. Lancet. 1998;352:93-97.
12. Hol T, Cox MB, Bryant HU, Draper MW. Selective estrogen receptor modulators and postmenopausal women’s health. J Women’s Health. 1997;6:523-531.
13. Buzdar AU, Marcus C, Holmes F, Hug V, Hortobagyi G. Phase II evaluation of LY156758 in metastatic breast cancer. Oncology. 1988;45:344-345.
14. Neven P, Muylder X, Van Belle Y, Vanderick G, De Mylder E. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
15. Goldstein SR, Scheele WH, Rajagopalan SK, Wilke JL, Walsh BW, Parsons AK. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
16. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Breast Cancer Res Treat. 2001;65:125-134.
17. Santen RJ, Yue W, Nftolin F, Mor G, Berstein L. The potential of aromatase inhibitors in breast cancer prevention. Endocrine-Related Cancer. 1999;6:235-243.
18. Goss PE, Strasser K. Aromatase inhibitors in the treatment and prevention of breast cancer. J Clin Oncol. 2001;19:881-894.
19. Bilimoria MM, Morrow M. The woman at increased risk for breast cancer: evaluation and management strategies. CA Cancer J Clin. 1995;45:263-278.
- In the Breast Cancer Prevention Trial, women taking tamoxifen experienced a 49% overall reduction in invasive breast cancer; the relative risk of endometrial cancer was 4.01 for women over 50 and 1.21 for women younger than 50.
- The risk of serious adverse effects with tamoxifen use appears to be lower in women under age 50.
- Preclinical animal data suggest that, like tamoxifen, raloxifene has potent antiestrogen effects on breast tissue.
- Because aromatase inhibitors block the peripheral conversion of androstenedione to estrogens, they inhibit both initiation and promotion of breast cancer. Thus, they may be more effective than selective estrogen receptor modulators in preventing the disease.
More than 28,000 additional breast cancers could be prevented over 5 years if all eligible women were given tamoxifen.1
This is just one of several important findings highlighted in the studies covered in this review of breast cancer risk assessment and chemopreventive options.
Because Ob/Gyns often treat tamoxifen users who experience uterine bleeding and worry about their risk of endometrial cancer (see “Endometrial screening and tamoxifen users: Going beyond the ACOG opinion,”), it is crucial that we have the latest data on preventive therapies for breast cancer—which include not only tamoxifen, but also, potentially, raloxifene and aromatase inhibitors—so that we may facilitate proper work-up and monitoring.
With the rising use of tamoxifen has come an increased need for vigilance for signs of endometrial cancer. To address the issue, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion on the subject in April 2000.1
In the opinion, ACOG observed that screening tests have not increased the early detection of endometrial cancer in women using tamoxifen and may lead to more invasive and costly diagnostic procedures. ACOG also recommended that Ob/Gyns:
- Educate women taking tamoxifen on the risks of endometrial proliferation, hyperplasia, and cancer and stress the importance of annual gynecologic exams.
- Closely monitor these patients for signs of endometrial hyperplasia or cancer.
- Encourage patients to promptly report abnormal vaginal symptoms, including bloody discharge, spotting, staining, or leukorrhea.
- Investigate any abnormal vaginal bleeding, bloody discharge, spotting, or staining.
- Limit tamoxifen therapy to 5 years, as no benefit has been established beyond this time frame.
- Initiate proper gynecologic management and reassess the use of tamoxifen if the patient develops atypical endometrial hyperplasia.
- Consider resuming tamoxifen therapy following hysterectomy for endometrial carcinoma, in consultation with the physician responsible for the woman’s breast care.1
Screening tests help determine risk. In light of data published over the last 5 years, I now perform endometrial screening on patients about to begin tamoxifen therapy—a practice that differs from the ACOG opinion outlined above. Here is why:
Work published by Berliere et al2 in 1998 and updated in 20003 suggest that, among women on tamoxifen therapy, there exists a group at high risk and a group at low risk for developing complex atypical hyperplasia of the endometrium.
Berliere and her group studied 575 asymptomatic postmenopausal women with recently diagnosed breast cancer about to begin tamoxifen therapy. Each woman received transvaginal ultrasound; if the endometrial echo was greater than 4 mm, office hysteroscopy was performed. Of the study population, 17.4% had initial benign polyps of the endometrium. All polyps were removed, tamoxifen therapy initiated, and follow-up carried out through 5 years.
Among the women with no initial polyp (whom I would classify as “squeaky clean”), 0.7% developed atypical hyperplasia over the 5-year study period—compared with 11.7% of those who had an initial polyp removed. In addition, 11.7% of squeaky clean patients experienced polyp formation, compared with 17.6% of those with initial polyps. Thus, the 17.4% with initial benign polyps had 18 times the risk of the squeaky clean group for developing atypical hyperplasia while on tamoxifen therapy.
These findings have caused me to rethink my approach toward endometrial surveillance for women taking tamoxifen.4
Here is my current practice:
- When patients are diagnosed with breast cancer and scheduled to begin tamoxifen therapy, I perform pretreatment screening.
- If no initial endometrial polyps are found, I follow ACOG’s recommendations. Further interventions are unnecessary (unless abnormal symptoms develop), since these patients are at no more risk for endometrial cancer than women not taking tamoxifen.
- For patients with an initial polyp (ie, the high-risk group), I remove the polyp prior to starting tamoxifen treatment and monitor them throughout the course of therapy, periodically utilizing transvaginal ultrasound and saline infusion sonohysterography.
REFERENCES
1. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion #232: Tamoxifen and Endometrial Cancer. Washington, DC: ACOG; April 2000.
2. Berliere M, Charles A, Galant C, Donnez J. Uterine side effects of tamoxifen: a need for systematic pretreatment screening. Obstet Gynecol. 1998;91(1):40-44.
3. Berliere M, Radikov G, Galant C, Piette P, Marbaix E, Donnez J. Identification of women at high risk of developing endometrial cancer on tamoxifen. Eur J Cancer. 2000;36(suppl 4):S35-S36.
4. Goldstein SR. Controversy about uterine effects and safety of SERMs: the saga continues. Menopause. 2002;9:381-384.
Assessing risk: The need for a new model
Although a number of breast cancer riskassessment models are available based on individual risk factors (TABLE 1), estimates based on combinations of factors are preferable. The Gail model,2 widely used to determine breast cancer risk, takes into account nongenetic (nulliparity, age at menarche) and genetic (family history) factors, as well as the number of previous breast biopsies. It assigns a smaller relative risk to women over age 50. A Web-based version, available at http://bcra.nci.nih.gov/brc, is useful for calculating a woman’s risk of developing invasive disease over the next 5 years, as well as over her remaining lifetime.
Limitations of the Gail model. Unfortunately, the data on which the model is based were collected in the late 1970s and early 1980s. Today, the greater ease of breast histopathologic assessment by fine-needle aspiration and outpatient core-needle biopsy has increased the rate of tissue sampling, creating confusion as to what constitutes a biopsy. Thus, the cutoff of 1.66% for high risk—the threshold adopted for the Breast Cancer Prevention Trial (BCPT)—loses some credibility.
Consider this example: A 50-year-old nulliparous Caucasian woman experienced menarche at age 11, has no first-degree relatives with a history of breast cancer, and has never had a breast biopsy. The Gail model would assign her a risk of developing breast cancer of 1.2% in the next 5 years and 10.8% over her lifetime. However, if the same patient had had 3 breast biopsies, her risk would rise to 1.8% in the next 5 years and 15.8% for her lifetime (placing her in the high-risk category), even if none of the biopsies revealed hyperplasia.
Biomarkers. Objective findings that are patient-specific but which correlate closely with breast cancer development are needed.
Biomarkers have been proposed; among them: ultrasensitive measurement of serum estradiol levels in postmenopausal women.3 In the Multiple Outcomes of Raloxifene Evaluation (MORE),4 the women who had the greatest reduction in breast cancer during treatment had the highest baseline serum estradiol levels (FIGURE 1)—although the baseline levels of all subjects were well within the postmenopausal range of 20 pmol/L or less.
TABLE 1
Breast cancer risk factors and their relative risks19
| RELATIVE RISK <2 | RELATIVE RISK 2–4 | RELATIVE RISK >4 |
|---|---|---|
|
|
|
FIGURE 1 Breast cancer risk in raloxifene users
Reprinted with permission from: Cummings SR, Duong T, Kenyon E, et al. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220. Copyright © 2002 American Medical Association. All rights reserved.
Chemoprevention: The rationale for tamoxifen
Data from preclinical animal and in vitro studies led to the use of tamoxifen, a selective estrogen receptor modulator (SERM), for primary prevention of breast cancer in healthy women. The drug was shown to inhibit mammary tumors in mice and rats and suppress hormone-dependent breast cancer cell lines in vitro.5
Clinical data from the Early Breast Cancer Trialists Collaborative Group also helped spur prevention trials with tamoxifen.6 Besides decreasing the risk of recurrent breast cancer, tamoxifen reduced the risk of contralateral, new-onset breast cancer by 47% after 5 years of adjuvant treatment (P = .00001) and by 26% after 2 years of treatment (P = .004). This, along with tamoxifen’s favorable effects on skeletal remodeling and lipid levels, led to a series of chemopreventive trials in the United States and Europe (TABLE 2).
TABLE 2
Results of tamoxifen trials
| BREAST CANCER PREVENTION TRIAL7 | ROYAL MARSDEN10 | ITALIAN11 | |
|---|---|---|---|
| Number of participants | 13,388 | 2,471 | 5,408 |
| Age ≤50 | 40% | 62% | 36% |
| One first-degree relative with breast cancer | 55% | 55% | 18% |
| >2 first-degree relatives with breast cancer | 13% | 17% | 2.5% |
| HRT users | 0% | 42% | 8% |
| Woman-years of follow-up | 46,858 | 12,355 | 20,731 |
| Cancer incidence/1,000 | |||
| Placebo | 6.8 | 5.0 | 2.3 |
| Tamoxifen | 3.4 | 4.7 | 2.1 |
Breast Cancer Prevention Trial: Tamoxifen reduces cancer incidence
In 1992, the National Surgical Adjuvant Breast and Bowel Project launched a prevention trial using tamoxifen: the Breast Cancer Prevention Trial.7 A total of 13,388 women aged 35 or older and at high risk for breast cancer were enrolled at numerous sites throughout the United States and Canada.
The Gail model was utilized to determine which women had sufficient risk—that is, a risk of developing invasive breast cancer within the next 5 years of 1.66% or greater—to be included in the trial.2 Subjects were randomly assigned to receive placebo or tamoxifen (20 mg/d) for 5 years.
The trial was terminated in April 1998, 14 months before its planned completion, due to the striking reduction in new-onset breast cancer in the tamoxifen group. The data safety monitoring board felt it would be unethical to allow one half of the participants, who were deemed to be at high risk, to continue taking placebo in light of the dramatic reduction in both invasive and noninvasive breast cancer with tamoxifen use.
The overall incidence of breast cancer in the tamoxifen group was 3.4 cases per 1,000, compared with 6.8 cases per 1,000 in the placebo group. Overall, the reduction in invasive breast cancer was 49% (P<.000001). The reductions were 44% for women in the group aged 35 to 49 years, 51% for those aged 50 to 59, and 55% for those 60 years and older (FIGURE 2).
Tamoxifen decreased the incidence of noninvasive breast cancer (ductal carcinoma in situ) by 50%. (Expanded use of mammography has led to greater detection of this cancer. Most such lesions are estrogen-receptor–positive.8) In addition, tamoxifen reduced breast cancer risk in women with a history of lobular carcinoma in situ by 56% and atypical hyperplasia by 86%. Overall, tamoxifen decreased the occurrence of estrogen-receptor–positive tumors by 69%, but had no impact on tumors that were estrogen-receptor–negative.
Tamoxifen’s other effects in healthy women. The BCPT offered the first largescale data on the effects of tamoxifen in healthy women.7 (All previous studies included only women with breast cancer.) Several secondary endpoints merit consideration.
- Endometrial cancer risk. Researchers found the relative risk (RR) of endometrial cancer associated with tamoxifen therapy in healthy women was 2.53 (95% confidence intervals [CI], 1.35, 4.97). When this figure was calculated for the different age groups, it rose to 4.01 (95% CI, 1.70, 10.90) in women over 50, and declined to 1.21 for women ages 49 and under (95% CI, 0.41, 3.60).
- Thromboembolic event risk. The same age distinction was seen in relation to thromboembolic events. There were no statistically significant increases in pulmonary emboli or deep venous thrombosis in women 49 years of age or under. Although it is unclear whether the trial was sufficiently powered for this particular endpoint, the likelihood that serious adverse events will limit the potential benefits of tamoxifen appears to be lower in women under the age of 50. This has significant clinical consequences for physicians caring for perimenopausal patients.
- No change in incidence of other cancers. Overall, the incidence of invasive cancers other than those of the breast and uterus was the same for the tamoxifen and placebo groups.
- Other outcomes. The relative risk of death from any cause was 0.81 (95% CI, 0.56–1.16).
There was a slight increase in the risk of myocardial infarction (RR, 1.11; 95% CI, 0.65–1.92) and a slight decrease in the development of severe angina (RR, 0.93; 95% CI, 0.40–2.14) in tamoxifen users, although neither of these was statistically significant.
The overall relative risk of fractures at various sites (hip, spine, radius) was 0.81 (95% CI, 0.63–1.05).
A statistically significant increase was found in the number of women with cataracts who then underwent cataract surgery. That relative risk was 1.57 (95% CI, 1.16–2.14).
FIGURE 2 Breast Cancer Prevention Trial: Tamoxifen reduces incidence of breast cancer
The rate of cancer reduction for tamoxifen compared with placebo for years 1 through 6 was 33%, 55%, 39%, 49%, 69%, and 55%, respectively.
Reprinted from Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst.1998;90(18):1371-1388, by permission of Oxford University Press.
FDA approves tamoxifen for primary prevention
Based on the BCPT results, the US Food and Drug Administration (FDA) approved tamoxifen in October 1998 for primary prevention of breast cancer in women at high risk for the disease. It recommended that tamoxifen be limited to high-risk women because of the potentially serious side effects seen in clinical trials.
The FDA did not define high risk, but recommended that prophylactic use of tamoxifen be based on a thorough evaluation of a woman’s personal, family, and medical histories, as well as her age and understanding of the risks and benefits of treatment.
In addition, the FDA required that the package insert advise women to consult a health-care professional for breast cancer risk assessment and state that only women at high risk should take the drug (again, without defining high risk).
In 2002, the FDA added a “black box” warning to tamoxifen labeling that was directed at use of the drug for prevention rather than treatment. This warning concerned the occurrence of uterine sarcomas. The incidence of these cancers was found to be 0.17 per 1,000 women taking tamoxifen, compared with 0.015 per 1,000 controls.9
Underuse of tamoxifen? A recent study by Freedman et al1 calculated the number of women 35 to 79 years of age who were eligible for tamoxifen chemoprevention based on FDA criteria. They further calculated the number of women who would have a positive benefit-risk ratio for tamoxifen use, and concluded that, among white women alone, roughly 28,492 additional breast cancers could be prevented or deferred if these individuals took tamoxifen for the next 5 years.
European trials fail to confirm benefit of tamoxifen
The Royal Marsden Trial was one of 2 European trials that failed to replicate the BCPT results.10 This British study involved 2,471 healthy women between the ages of 30 and 70 who had a family history of breast cancer. The immediate follow-up was 70 months. No statistically significant decrease in breast cancer was found with tamoxifen use, compared with placebo.
One possibility for the discrepancy may be that eligibility for the Royal Marsden Trial was based predominantly on a strong family history of breast cancer. It also included a much larger proportion of women under age 50 (62%, compared with only 40% in the BCPT). Probably most importantly, 42% of the women received hormone replacement therapy (HRT) along with tamoxifen during the trial.
The Italian prevention study also failed to confirm the findings of the BCPT.11 Because it recruited participants from the general population, the overall risk of breast cancer was significantly lower than in the BCPT. Compliance rates in the Italian study were quite low: Approximately 26% of the subjects dropped out. Furthermore, the Italian trial included considerably fewer women over age 60 (12% versus 30% in the BCPT).
Raloxifene: Another cancer preventive?
Like tamoxifen, raloxifene is a SERM. It is a benzothiophene derivative; tamoxifen comes from the triphenylethylene family.
Like tamoxifen, raloxifene was originally investigated as a treatment for advanced breast cancer. Preclinical studies indicated that it had an antiproliferative effect on estrogen-receptor–positive mammary tumors and estrogen-receptor–positive human breast cancer cell lines.12 However, in the 1980s, a small phase II trial revealed that it had no further antitumor effects in postmenopausal women with advanced breast cancer in whom tamoxifen therapy had failed.13
Interest in raloxifene revived after tamoxifen’s neoplastic effects on the uteri of postmenopausal women became evident.14 As a SERM, raloxifene exhibits estrogen-agonist activity on bone remodeling and lipid metabolism. In December 1997, it won FDA approval for the prevention of osteoporosis in postmenopausal women. Its indication was extended to treatment in October 1999.
Studies found that raloxifene was similar to placebo in its effects on the endometrium of postmenopausal women.15 There were no differences in endometrial thickness, endoluminal masses, proliferation, or hyperplasia. This corroborated previous findings that raloxifene causes neither endometrial hyperplasia nor cancer and is not associated with vaginal bleeding or increased endometrial thickness (as measured by transvaginal ultrasound).
In addition, preclinical animal data suggest that, like tamoxifen, raloxifene has potent antiestrogen effects on breast tissue.12
The MORE trial involved 7,705 postmenopausal women up to age 80 with established osteoporosis who were randomized to receive raloxifene or placebo. Bone mineral density and fracture incidence were the primary endpoints; breast cancer was a secondary endpoint. At 4 years, raloxifene significantly reduced the incidence of all invasive breast cancers by 72%, compared with placebo (RR = 0.28; 95% CI, 0.17–0.46).16 It reduced the incidence of invasive estrogen-receptor–positive tumors by 84%, compared with placebo (RR = 0.16; 95% CI, 0.09–0.30), but had no effect on estrogen-receptor–negative tumors.
Because they block both initiation and promotion of breast cancer, aromatase inhibitors may be more effective than SERMs in preventing breast cancer.
The incidence of vaginal bleeding, breast pain, and endometrial cancer in the raloxifene group did not differ significantly from those of the placebo group. (Because women in the MORE trial were older and did not enter the study with an increased risk for breast cancer, these findings are not necessarily applicable to younger, high-risk women.)
Like tamoxifen, raloxifene slightly increased the risk of thromboembolic disease, including deep vein thrombosis. Pulmonary embolism developed in 1.1% of women in the raloxifene group, compared with 0.5% of subjects in the placebo group (P = .003).
Currently, there is no approved indication for raloxifene in premenopausal women. SERM compounds, which are structurally similar to clomiphene citrate, seem to have different effects in premenopausal and postmenopausal women, as tamoxifen did in the BCPT. Further study in the premenopausal population or with concomitant use of lowdose estrogen may be forthcoming.
Ongoing clinical trials
STAR. To directly compare the safety and efficacy of tamoxifen and raloxifene in reducing breast cancer risk among healthy women, the Study of Tamoxifen and Raloxifene (STAR) has been enrolling postmenopausal women 35 years of age or older who are at increased risk for breast cancer. This study began in 1999 and is expected to run for at least 7 years. It seeks to enroll 22,000 participants in its randomized, double-blind investigation. Participants will receive a daily dose of raloxifene (60 mg) or tamoxifen (20 mg).
RUTH. Raloxifene Use and the Heart (RUTH) is a double-blind, placebo-controlled trial of 60 mg of raloxifene that will include 10,000 women. Primary endpoints are coronary disease and invasive breast cancer. Trial enrollment ended in August 2000, and the study is expected to conclude in 2006.
Aromatase inhibitors: Another route of prevention
The evidence that estrogens facilitate breast cancer development in animals and women is substantial, although the precise mechanism is unknown.17 The most commonly held theory is that estrogen stimulates proliferation of breast cells. Thus, it statistically increases the chances for genetic mutations that could result in cancer.
Aromatase inhibitors block peripheral conversion of androstenedione to estrogens. In premenopausal patients, the primary site of this action is in the ovary. In post-menopausal women, it occurs predominantly in extraovarian sites, including the adrenal glands, adipose tissue, liver, muscle, and skin.
Because of their dual role, (blocking both the initiation and promotion of breast cancer), aromatase inhibitors may be more effective than SERMs in preventing breast cancer.18 By inhibiting the initiation process, they would reduce levels of genotoxic metabolites of estradiol by lowering estradiol concentration in tissue. They also would inhibit tumor promotion by lowering tissue levels of estradiol—thus blocking cell proliferation. However, because they are not selective, aromatase inhibitors would have an antiestrogen effect on bone and lipid metabolism and would induce vasomotor symptoms.
Dr. Goldstein serves on gynecology advisory boards for Eli Lilly and Company, Pfizer, AstraZeneca, and P&G Pharmaceuticals.
- In the Breast Cancer Prevention Trial, women taking tamoxifen experienced a 49% overall reduction in invasive breast cancer; the relative risk of endometrial cancer was 4.01 for women over 50 and 1.21 for women younger than 50.
- The risk of serious adverse effects with tamoxifen use appears to be lower in women under age 50.
- Preclinical animal data suggest that, like tamoxifen, raloxifene has potent antiestrogen effects on breast tissue.
- Because aromatase inhibitors block the peripheral conversion of androstenedione to estrogens, they inhibit both initiation and promotion of breast cancer. Thus, they may be more effective than selective estrogen receptor modulators in preventing the disease.
More than 28,000 additional breast cancers could be prevented over 5 years if all eligible women were given tamoxifen.1
This is just one of several important findings highlighted in the studies covered in this review of breast cancer risk assessment and chemopreventive options.
Because Ob/Gyns often treat tamoxifen users who experience uterine bleeding and worry about their risk of endometrial cancer (see “Endometrial screening and tamoxifen users: Going beyond the ACOG opinion,”), it is crucial that we have the latest data on preventive therapies for breast cancer—which include not only tamoxifen, but also, potentially, raloxifene and aromatase inhibitors—so that we may facilitate proper work-up and monitoring.
With the rising use of tamoxifen has come an increased need for vigilance for signs of endometrial cancer. To address the issue, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion on the subject in April 2000.1
In the opinion, ACOG observed that screening tests have not increased the early detection of endometrial cancer in women using tamoxifen and may lead to more invasive and costly diagnostic procedures. ACOG also recommended that Ob/Gyns:
- Educate women taking tamoxifen on the risks of endometrial proliferation, hyperplasia, and cancer and stress the importance of annual gynecologic exams.
- Closely monitor these patients for signs of endometrial hyperplasia or cancer.
- Encourage patients to promptly report abnormal vaginal symptoms, including bloody discharge, spotting, staining, or leukorrhea.
- Investigate any abnormal vaginal bleeding, bloody discharge, spotting, or staining.
- Limit tamoxifen therapy to 5 years, as no benefit has been established beyond this time frame.
- Initiate proper gynecologic management and reassess the use of tamoxifen if the patient develops atypical endometrial hyperplasia.
- Consider resuming tamoxifen therapy following hysterectomy for endometrial carcinoma, in consultation with the physician responsible for the woman’s breast care.1
Screening tests help determine risk. In light of data published over the last 5 years, I now perform endometrial screening on patients about to begin tamoxifen therapy—a practice that differs from the ACOG opinion outlined above. Here is why:
Work published by Berliere et al2 in 1998 and updated in 20003 suggest that, among women on tamoxifen therapy, there exists a group at high risk and a group at low risk for developing complex atypical hyperplasia of the endometrium.
Berliere and her group studied 575 asymptomatic postmenopausal women with recently diagnosed breast cancer about to begin tamoxifen therapy. Each woman received transvaginal ultrasound; if the endometrial echo was greater than 4 mm, office hysteroscopy was performed. Of the study population, 17.4% had initial benign polyps of the endometrium. All polyps were removed, tamoxifen therapy initiated, and follow-up carried out through 5 years.
Among the women with no initial polyp (whom I would classify as “squeaky clean”), 0.7% developed atypical hyperplasia over the 5-year study period—compared with 11.7% of those who had an initial polyp removed. In addition, 11.7% of squeaky clean patients experienced polyp formation, compared with 17.6% of those with initial polyps. Thus, the 17.4% with initial benign polyps had 18 times the risk of the squeaky clean group for developing atypical hyperplasia while on tamoxifen therapy.
These findings have caused me to rethink my approach toward endometrial surveillance for women taking tamoxifen.4
Here is my current practice:
- When patients are diagnosed with breast cancer and scheduled to begin tamoxifen therapy, I perform pretreatment screening.
- If no initial endometrial polyps are found, I follow ACOG’s recommendations. Further interventions are unnecessary (unless abnormal symptoms develop), since these patients are at no more risk for endometrial cancer than women not taking tamoxifen.
- For patients with an initial polyp (ie, the high-risk group), I remove the polyp prior to starting tamoxifen treatment and monitor them throughout the course of therapy, periodically utilizing transvaginal ultrasound and saline infusion sonohysterography.
REFERENCES
1. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion #232: Tamoxifen and Endometrial Cancer. Washington, DC: ACOG; April 2000.
2. Berliere M, Charles A, Galant C, Donnez J. Uterine side effects of tamoxifen: a need for systematic pretreatment screening. Obstet Gynecol. 1998;91(1):40-44.
3. Berliere M, Radikov G, Galant C, Piette P, Marbaix E, Donnez J. Identification of women at high risk of developing endometrial cancer on tamoxifen. Eur J Cancer. 2000;36(suppl 4):S35-S36.
4. Goldstein SR. Controversy about uterine effects and safety of SERMs: the saga continues. Menopause. 2002;9:381-384.
Assessing risk: The need for a new model
Although a number of breast cancer riskassessment models are available based on individual risk factors (TABLE 1), estimates based on combinations of factors are preferable. The Gail model,2 widely used to determine breast cancer risk, takes into account nongenetic (nulliparity, age at menarche) and genetic (family history) factors, as well as the number of previous breast biopsies. It assigns a smaller relative risk to women over age 50. A Web-based version, available at http://bcra.nci.nih.gov/brc, is useful for calculating a woman’s risk of developing invasive disease over the next 5 years, as well as over her remaining lifetime.
Limitations of the Gail model. Unfortunately, the data on which the model is based were collected in the late 1970s and early 1980s. Today, the greater ease of breast histopathologic assessment by fine-needle aspiration and outpatient core-needle biopsy has increased the rate of tissue sampling, creating confusion as to what constitutes a biopsy. Thus, the cutoff of 1.66% for high risk—the threshold adopted for the Breast Cancer Prevention Trial (BCPT)—loses some credibility.
Consider this example: A 50-year-old nulliparous Caucasian woman experienced menarche at age 11, has no first-degree relatives with a history of breast cancer, and has never had a breast biopsy. The Gail model would assign her a risk of developing breast cancer of 1.2% in the next 5 years and 10.8% over her lifetime. However, if the same patient had had 3 breast biopsies, her risk would rise to 1.8% in the next 5 years and 15.8% for her lifetime (placing her in the high-risk category), even if none of the biopsies revealed hyperplasia.
Biomarkers. Objective findings that are patient-specific but which correlate closely with breast cancer development are needed.
Biomarkers have been proposed; among them: ultrasensitive measurement of serum estradiol levels in postmenopausal women.3 In the Multiple Outcomes of Raloxifene Evaluation (MORE),4 the women who had the greatest reduction in breast cancer during treatment had the highest baseline serum estradiol levels (FIGURE 1)—although the baseline levels of all subjects were well within the postmenopausal range of 20 pmol/L or less.
TABLE 1
Breast cancer risk factors and their relative risks19
| RELATIVE RISK <2 | RELATIVE RISK 2–4 | RELATIVE RISK >4 |
|---|---|---|
|
|
|
FIGURE 1 Breast cancer risk in raloxifene users
Reprinted with permission from: Cummings SR, Duong T, Kenyon E, et al. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220. Copyright © 2002 American Medical Association. All rights reserved.
Chemoprevention: The rationale for tamoxifen
Data from preclinical animal and in vitro studies led to the use of tamoxifen, a selective estrogen receptor modulator (SERM), for primary prevention of breast cancer in healthy women. The drug was shown to inhibit mammary tumors in mice and rats and suppress hormone-dependent breast cancer cell lines in vitro.5
Clinical data from the Early Breast Cancer Trialists Collaborative Group also helped spur prevention trials with tamoxifen.6 Besides decreasing the risk of recurrent breast cancer, tamoxifen reduced the risk of contralateral, new-onset breast cancer by 47% after 5 years of adjuvant treatment (P = .00001) and by 26% after 2 years of treatment (P = .004). This, along with tamoxifen’s favorable effects on skeletal remodeling and lipid levels, led to a series of chemopreventive trials in the United States and Europe (TABLE 2).
TABLE 2
Results of tamoxifen trials
| BREAST CANCER PREVENTION TRIAL7 | ROYAL MARSDEN10 | ITALIAN11 | |
|---|---|---|---|
| Number of participants | 13,388 | 2,471 | 5,408 |
| Age ≤50 | 40% | 62% | 36% |
| One first-degree relative with breast cancer | 55% | 55% | 18% |
| >2 first-degree relatives with breast cancer | 13% | 17% | 2.5% |
| HRT users | 0% | 42% | 8% |
| Woman-years of follow-up | 46,858 | 12,355 | 20,731 |
| Cancer incidence/1,000 | |||
| Placebo | 6.8 | 5.0 | 2.3 |
| Tamoxifen | 3.4 | 4.7 | 2.1 |
Breast Cancer Prevention Trial: Tamoxifen reduces cancer incidence
In 1992, the National Surgical Adjuvant Breast and Bowel Project launched a prevention trial using tamoxifen: the Breast Cancer Prevention Trial.7 A total of 13,388 women aged 35 or older and at high risk for breast cancer were enrolled at numerous sites throughout the United States and Canada.
The Gail model was utilized to determine which women had sufficient risk—that is, a risk of developing invasive breast cancer within the next 5 years of 1.66% or greater—to be included in the trial.2 Subjects were randomly assigned to receive placebo or tamoxifen (20 mg/d) for 5 years.
The trial was terminated in April 1998, 14 months before its planned completion, due to the striking reduction in new-onset breast cancer in the tamoxifen group. The data safety monitoring board felt it would be unethical to allow one half of the participants, who were deemed to be at high risk, to continue taking placebo in light of the dramatic reduction in both invasive and noninvasive breast cancer with tamoxifen use.
The overall incidence of breast cancer in the tamoxifen group was 3.4 cases per 1,000, compared with 6.8 cases per 1,000 in the placebo group. Overall, the reduction in invasive breast cancer was 49% (P<.000001). The reductions were 44% for women in the group aged 35 to 49 years, 51% for those aged 50 to 59, and 55% for those 60 years and older (FIGURE 2).
Tamoxifen decreased the incidence of noninvasive breast cancer (ductal carcinoma in situ) by 50%. (Expanded use of mammography has led to greater detection of this cancer. Most such lesions are estrogen-receptor–positive.8) In addition, tamoxifen reduced breast cancer risk in women with a history of lobular carcinoma in situ by 56% and atypical hyperplasia by 86%. Overall, tamoxifen decreased the occurrence of estrogen-receptor–positive tumors by 69%, but had no impact on tumors that were estrogen-receptor–negative.
Tamoxifen’s other effects in healthy women. The BCPT offered the first largescale data on the effects of tamoxifen in healthy women.7 (All previous studies included only women with breast cancer.) Several secondary endpoints merit consideration.
- Endometrial cancer risk. Researchers found the relative risk (RR) of endometrial cancer associated with tamoxifen therapy in healthy women was 2.53 (95% confidence intervals [CI], 1.35, 4.97). When this figure was calculated for the different age groups, it rose to 4.01 (95% CI, 1.70, 10.90) in women over 50, and declined to 1.21 for women ages 49 and under (95% CI, 0.41, 3.60).
- Thromboembolic event risk. The same age distinction was seen in relation to thromboembolic events. There were no statistically significant increases in pulmonary emboli or deep venous thrombosis in women 49 years of age or under. Although it is unclear whether the trial was sufficiently powered for this particular endpoint, the likelihood that serious adverse events will limit the potential benefits of tamoxifen appears to be lower in women under the age of 50. This has significant clinical consequences for physicians caring for perimenopausal patients.
- No change in incidence of other cancers. Overall, the incidence of invasive cancers other than those of the breast and uterus was the same for the tamoxifen and placebo groups.
- Other outcomes. The relative risk of death from any cause was 0.81 (95% CI, 0.56–1.16).
There was a slight increase in the risk of myocardial infarction (RR, 1.11; 95% CI, 0.65–1.92) and a slight decrease in the development of severe angina (RR, 0.93; 95% CI, 0.40–2.14) in tamoxifen users, although neither of these was statistically significant.
The overall relative risk of fractures at various sites (hip, spine, radius) was 0.81 (95% CI, 0.63–1.05).
A statistically significant increase was found in the number of women with cataracts who then underwent cataract surgery. That relative risk was 1.57 (95% CI, 1.16–2.14).
FIGURE 2 Breast Cancer Prevention Trial: Tamoxifen reduces incidence of breast cancer
The rate of cancer reduction for tamoxifen compared with placebo for years 1 through 6 was 33%, 55%, 39%, 49%, 69%, and 55%, respectively.
Reprinted from Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst.1998;90(18):1371-1388, by permission of Oxford University Press.
FDA approves tamoxifen for primary prevention
Based on the BCPT results, the US Food and Drug Administration (FDA) approved tamoxifen in October 1998 for primary prevention of breast cancer in women at high risk for the disease. It recommended that tamoxifen be limited to high-risk women because of the potentially serious side effects seen in clinical trials.
The FDA did not define high risk, but recommended that prophylactic use of tamoxifen be based on a thorough evaluation of a woman’s personal, family, and medical histories, as well as her age and understanding of the risks and benefits of treatment.
In addition, the FDA required that the package insert advise women to consult a health-care professional for breast cancer risk assessment and state that only women at high risk should take the drug (again, without defining high risk).
In 2002, the FDA added a “black box” warning to tamoxifen labeling that was directed at use of the drug for prevention rather than treatment. This warning concerned the occurrence of uterine sarcomas. The incidence of these cancers was found to be 0.17 per 1,000 women taking tamoxifen, compared with 0.015 per 1,000 controls.9
Underuse of tamoxifen? A recent study by Freedman et al1 calculated the number of women 35 to 79 years of age who were eligible for tamoxifen chemoprevention based on FDA criteria. They further calculated the number of women who would have a positive benefit-risk ratio for tamoxifen use, and concluded that, among white women alone, roughly 28,492 additional breast cancers could be prevented or deferred if these individuals took tamoxifen for the next 5 years.
European trials fail to confirm benefit of tamoxifen
The Royal Marsden Trial was one of 2 European trials that failed to replicate the BCPT results.10 This British study involved 2,471 healthy women between the ages of 30 and 70 who had a family history of breast cancer. The immediate follow-up was 70 months. No statistically significant decrease in breast cancer was found with tamoxifen use, compared with placebo.
One possibility for the discrepancy may be that eligibility for the Royal Marsden Trial was based predominantly on a strong family history of breast cancer. It also included a much larger proportion of women under age 50 (62%, compared with only 40% in the BCPT). Probably most importantly, 42% of the women received hormone replacement therapy (HRT) along with tamoxifen during the trial.
The Italian prevention study also failed to confirm the findings of the BCPT.11 Because it recruited participants from the general population, the overall risk of breast cancer was significantly lower than in the BCPT. Compliance rates in the Italian study were quite low: Approximately 26% of the subjects dropped out. Furthermore, the Italian trial included considerably fewer women over age 60 (12% versus 30% in the BCPT).
Raloxifene: Another cancer preventive?
Like tamoxifen, raloxifene is a SERM. It is a benzothiophene derivative; tamoxifen comes from the triphenylethylene family.
Like tamoxifen, raloxifene was originally investigated as a treatment for advanced breast cancer. Preclinical studies indicated that it had an antiproliferative effect on estrogen-receptor–positive mammary tumors and estrogen-receptor–positive human breast cancer cell lines.12 However, in the 1980s, a small phase II trial revealed that it had no further antitumor effects in postmenopausal women with advanced breast cancer in whom tamoxifen therapy had failed.13
Interest in raloxifene revived after tamoxifen’s neoplastic effects on the uteri of postmenopausal women became evident.14 As a SERM, raloxifene exhibits estrogen-agonist activity on bone remodeling and lipid metabolism. In December 1997, it won FDA approval for the prevention of osteoporosis in postmenopausal women. Its indication was extended to treatment in October 1999.
Studies found that raloxifene was similar to placebo in its effects on the endometrium of postmenopausal women.15 There were no differences in endometrial thickness, endoluminal masses, proliferation, or hyperplasia. This corroborated previous findings that raloxifene causes neither endometrial hyperplasia nor cancer and is not associated with vaginal bleeding or increased endometrial thickness (as measured by transvaginal ultrasound).
In addition, preclinical animal data suggest that, like tamoxifen, raloxifene has potent antiestrogen effects on breast tissue.12
The MORE trial involved 7,705 postmenopausal women up to age 80 with established osteoporosis who were randomized to receive raloxifene or placebo. Bone mineral density and fracture incidence were the primary endpoints; breast cancer was a secondary endpoint. At 4 years, raloxifene significantly reduced the incidence of all invasive breast cancers by 72%, compared with placebo (RR = 0.28; 95% CI, 0.17–0.46).16 It reduced the incidence of invasive estrogen-receptor–positive tumors by 84%, compared with placebo (RR = 0.16; 95% CI, 0.09–0.30), but had no effect on estrogen-receptor–negative tumors.
Because they block both initiation and promotion of breast cancer, aromatase inhibitors may be more effective than SERMs in preventing breast cancer.
The incidence of vaginal bleeding, breast pain, and endometrial cancer in the raloxifene group did not differ significantly from those of the placebo group. (Because women in the MORE trial were older and did not enter the study with an increased risk for breast cancer, these findings are not necessarily applicable to younger, high-risk women.)
Like tamoxifen, raloxifene slightly increased the risk of thromboembolic disease, including deep vein thrombosis. Pulmonary embolism developed in 1.1% of women in the raloxifene group, compared with 0.5% of subjects in the placebo group (P = .003).
Currently, there is no approved indication for raloxifene in premenopausal women. SERM compounds, which are structurally similar to clomiphene citrate, seem to have different effects in premenopausal and postmenopausal women, as tamoxifen did in the BCPT. Further study in the premenopausal population or with concomitant use of lowdose estrogen may be forthcoming.
Ongoing clinical trials
STAR. To directly compare the safety and efficacy of tamoxifen and raloxifene in reducing breast cancer risk among healthy women, the Study of Tamoxifen and Raloxifene (STAR) has been enrolling postmenopausal women 35 years of age or older who are at increased risk for breast cancer. This study began in 1999 and is expected to run for at least 7 years. It seeks to enroll 22,000 participants in its randomized, double-blind investigation. Participants will receive a daily dose of raloxifene (60 mg) or tamoxifen (20 mg).
RUTH. Raloxifene Use and the Heart (RUTH) is a double-blind, placebo-controlled trial of 60 mg of raloxifene that will include 10,000 women. Primary endpoints are coronary disease and invasive breast cancer. Trial enrollment ended in August 2000, and the study is expected to conclude in 2006.
Aromatase inhibitors: Another route of prevention
The evidence that estrogens facilitate breast cancer development in animals and women is substantial, although the precise mechanism is unknown.17 The most commonly held theory is that estrogen stimulates proliferation of breast cells. Thus, it statistically increases the chances for genetic mutations that could result in cancer.
Aromatase inhibitors block peripheral conversion of androstenedione to estrogens. In premenopausal patients, the primary site of this action is in the ovary. In post-menopausal women, it occurs predominantly in extraovarian sites, including the adrenal glands, adipose tissue, liver, muscle, and skin.
Because of their dual role, (blocking both the initiation and promotion of breast cancer), aromatase inhibitors may be more effective than SERMs in preventing breast cancer.18 By inhibiting the initiation process, they would reduce levels of genotoxic metabolites of estradiol by lowering estradiol concentration in tissue. They also would inhibit tumor promotion by lowering tissue levels of estradiol—thus blocking cell proliferation. However, because they are not selective, aromatase inhibitors would have an antiestrogen effect on bone and lipid metabolism and would induce vasomotor symptoms.
Dr. Goldstein serves on gynecology advisory boards for Eli Lilly and Company, Pfizer, AstraZeneca, and P&G Pharmaceuticals.
1. Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst. 2003;95:526-532.
2. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
3. Ruffin MT, August DA, Kelloff GJ, Boone CW, Weber BL, Brenner DE. Selection criteria for breast cancer chemoprevention subjects. J Cell Biochem Suppl. 1993;17G:234-241.
4. Cummings SR, Duong T, Kenyon E, et al. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220.
5. Jordan VC, Allen KE. Evaluation of the antitumor activity of the nonsteroidal antiestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma mode. Eur J Cancer. 1980;16:239-251.
6. Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer: an overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988;319:1681-1692.
7. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
8. Bur ME, Zimarowski MJ, Schnitt SJ, Baker S, Lew R. Estrogen receptor immunohistochemistry in carcinoma in situ of the breast. Cancer. 1992;69:1174-1181.
9. Nolvadex [package insert]. Wilmington, Del: AstraZeneca Pharmaceuticals; 2002.
10. Powles T, Eeles R, Ashley S, et al. Interim analysis of incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomized chemoprevention trial. Lancet. 1998;352:98-101.
11. Veronesi U, Maisonneuve P, Costa A, et al. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomized trial among hysterectomized women. Italian Prevention Study. Lancet. 1998;352:93-97.
12. Hol T, Cox MB, Bryant HU, Draper MW. Selective estrogen receptor modulators and postmenopausal women’s health. J Women’s Health. 1997;6:523-531.
13. Buzdar AU, Marcus C, Holmes F, Hug V, Hortobagyi G. Phase II evaluation of LY156758 in metastatic breast cancer. Oncology. 1988;45:344-345.
14. Neven P, Muylder X, Van Belle Y, Vanderick G, De Mylder E. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
15. Goldstein SR, Scheele WH, Rajagopalan SK, Wilke JL, Walsh BW, Parsons AK. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
16. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Breast Cancer Res Treat. 2001;65:125-134.
17. Santen RJ, Yue W, Nftolin F, Mor G, Berstein L. The potential of aromatase inhibitors in breast cancer prevention. Endocrine-Related Cancer. 1999;6:235-243.
18. Goss PE, Strasser K. Aromatase inhibitors in the treatment and prevention of breast cancer. J Clin Oncol. 2001;19:881-894.
19. Bilimoria MM, Morrow M. The woman at increased risk for breast cancer: evaluation and management strategies. CA Cancer J Clin. 1995;45:263-278.
1. Freedman AN, Graubard BI, Rao SR, McCaskill-Stevens W, Ballard-Barbash R, Gail MH. Estimates of the number of US women who could benefit from tamoxifen for breast cancer chemoprevention. J Natl Cancer Inst. 2003;95:526-532.
2. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
3. Ruffin MT, August DA, Kelloff GJ, Boone CW, Weber BL, Brenner DE. Selection criteria for breast cancer chemoprevention subjects. J Cell Biochem Suppl. 1993;17G:234-241.
4. Cummings SR, Duong T, Kenyon E, et al. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220.
5. Jordan VC, Allen KE. Evaluation of the antitumor activity of the nonsteroidal antiestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma mode. Eur J Cancer. 1980;16:239-251.
6. Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer: an overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988;319:1681-1692.
7. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371-1388.
8. Bur ME, Zimarowski MJ, Schnitt SJ, Baker S, Lew R. Estrogen receptor immunohistochemistry in carcinoma in situ of the breast. Cancer. 1992;69:1174-1181.
9. Nolvadex [package insert]. Wilmington, Del: AstraZeneca Pharmaceuticals; 2002.
10. Powles T, Eeles R, Ashley S, et al. Interim analysis of incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomized chemoprevention trial. Lancet. 1998;352:98-101.
11. Veronesi U, Maisonneuve P, Costa A, et al. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomized trial among hysterectomized women. Italian Prevention Study. Lancet. 1998;352:93-97.
12. Hol T, Cox MB, Bryant HU, Draper MW. Selective estrogen receptor modulators and postmenopausal women’s health. J Women’s Health. 1997;6:523-531.
13. Buzdar AU, Marcus C, Holmes F, Hug V, Hortobagyi G. Phase II evaluation of LY156758 in metastatic breast cancer. Oncology. 1988;45:344-345.
14. Neven P, Muylder X, Van Belle Y, Vanderick G, De Mylder E. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
15. Goldstein SR, Scheele WH, Rajagopalan SK, Wilke JL, Walsh BW, Parsons AK. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
16. Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Breast Cancer Res Treat. 2001;65:125-134.
17. Santen RJ, Yue W, Nftolin F, Mor G, Berstein L. The potential of aromatase inhibitors in breast cancer prevention. Endocrine-Related Cancer. 1999;6:235-243.
18. Goss PE, Strasser K. Aromatase inhibitors in the treatment and prevention of breast cancer. J Clin Oncol. 2001;19:881-894.
19. Bilimoria MM, Morrow M. The woman at increased risk for breast cancer: evaluation and management strategies. CA Cancer J Clin. 1995;45:263-278.