User login
We, and our patients, are fortunate to have a robust armamentarium of osteoporosis preventives and treatments in the 21st century. Still, we have much to learn about the preservation and restoration of bone. Fine-tuning of individual therapies, and clarification of their attendant risks, are ongoing concerns.
In this article, I highlight several recent studies:
- an assessment of the periodontium in a group of postmenopausal women who were long-term users of bisphosphonates, versus nonusers, to determine the effects of these agents on periodontal health
- guidance from the American Society for Bone and Mineral Research on the risk of atypical femoral fracture in long-term users of bisphosphonates
- 2-year data on a new, delayed-release, weekly formulation of risedronate that can be taken with food
- two meta-analyses exploring the risk of fracture with use of a proton-pump inhibitor (PPI), as well as a recent summary of evidence
- a randomized trial of 2% nitroglycerin ointment to prevent fracture.
Bisphosphonates may reduce the risk of postmenopausal periodontal disease
Palomo L, Buencamino-Francisco MC, Carey JJ, Sivanandy M, Thacker H. Is long-term bisphosphonate therapy associated with benefits to the periodontium in postmenopausal women? Menopause. 2011;18(2):164–170.
The risk of periodontal disease increases in menopause. Inflammation can erode structures (i.e., periodontal ligament and alveolar bone) that attach the teeth into the jaw, leading, eventually, to loss of teeth (FIGURE).
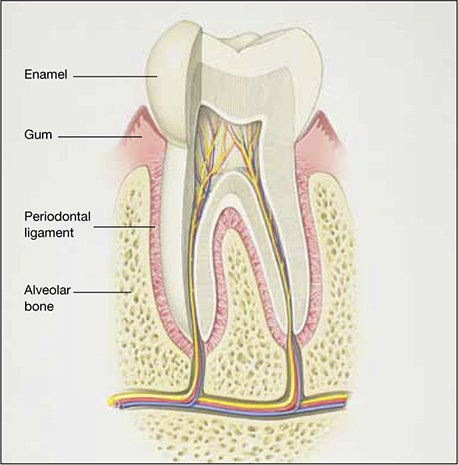
Anatomy of healthy periodontium
In periodontal disease, inflammation can erode structures, such as the periodontal ligament and alveolar bone, that attach the teeth into the jaw.In periodontal inflammation, a bacterial biofilm on tooth surfaces triggers a response by neutrophils and macrophages. In this respect, osteoporosis and periodontitis are mediated by common cytokines. Local production of cytokines seems to enhance osteoclast-mediated loss of skeletal and alveolar bone in estrogen-deficient women. In addition, generalized bone loss in postmenopausal osteoporosis renders the jaw susceptible to accelerated alveolar bone resorption and loss of periodontal attachment. For these reasons, physicians who care for postmenopausal women are advised to monitor their periodontal health; be vigilant for dental problems; and encourage them to practice good oral hygiene as a preventive measure against periodontitis and to seek regular dental care.
There has been tremendous publicity about the rare but very serious occurrence of osteonecrosis of the jaw in women who use bisphosphonates. In contrast, this study by Palomo and colleagues from the Case Western Reserve School of Dental Medicine seems to offer some preliminary good news about bisphosphonates and dental health: Long-term use appears to have some beneficial effects on the periodontium of postmenopausal women.
Details of the study
The aim of the study was to compare the periodontium in two groups of postmenopausal women known to have low bone mineral density (BMD): those who were long-term users (>2 years) of bisphosphonate therapy (n=28) and those who were not (n=28). The average age of participants in the study was 63 years, and the average T-score was –2.5. All women underwent cone-beam computed tomography of the jaw and a complete periodontal examination to determine the plaque score, periodontal probing depth, clinical attachment loss, bleeding on probing, and alveolar bone height.
Findings: Bisphosphonate users had a higher plaque score, a lower probing depth, and less loss of clinical attachment than did women in the control group. These differences were determined to be statistically significant. Bisphosphonate users also had less bleeding on probing and a higher alveolar bone height, but these differences were not significant. After adjustment for the plaque score, bisphosphonate use was a significant factor for probing depth but not for the other parameters.
Not all news about bisphosphonates is bad. Preliminary data seem to indicate that objective measures of periodontal disease are lower in bisphosphonate users who have low BMD than in nonusers.
Atypical femoral fracture is a real risk—but a rarity—with long-term use of antiresorptive drugs
Shane E, Burr D, Ebeling PR, et al; American Society for Bone and Mineral Research. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25(11):2267–2294.
As ObGyns, we are often the first-line providers of diagnostic services and treatment for postmenopausal women at risk of osteoporotic fracture. Oral and, more recently, parenteral bisphosphonates have been a mainstay of such treatment. Isolated reports of atypical femoral fracture in long-term users of bisphosphonates first surfaced around 2005.1 Since then, several case series have appeared, some with as many as 102 cases.2 In fact, atypical femoral fracture in bisphosphonate users has drawn so much attention that patients have begun to ask about these agents and express reservations about using them.
The issue has drawn the focus of the American Society for Bone and Mineral Research (ASBMR), which appointed a task force to address it. A multidisciplinary expert group reviewed pertinent published reports of atypical femoral fracture, as well as preclinical studies, that could provide insight into its pathogenesis.
What we know about this type of fracture
Preclinical data lend biologic plausibility to a potential association between long-term bisphosphonate use and atypical femoral fracture. These data highlight the effects of bisphosphonates on:
- collagen cross-linking and maturation
- accumulation of microdamage and advanced glycation end products
- heightened mineralization, remodeling, vascularity, and angiogenesis.
The task force concluded that bisphosphonates are highly effective at reducing the risk of spinal and nonspinal fractures, including typical and common femoral neck and intertrochanteric fractures. However, there is evidence of a relationship between long-term bisphosphonate use and a specific type of subtrochanteric and femoral shaft fracture. This type of fracture is characterized by unique radiographic features:
- transverse or short oblique orientation
- absence of comminution
- cortical thickening
- stress fracture or stress reaction on the symptomatic and/or contralateral side
- delayed healing.
This type of fracture also has unique clinical features—namely, prodromal pain and bilaterality.
The apparent increase in the risk of atypical femoral fracture in patients using glucocorticoids is a concern because bisphosphonates are the mainstay of prevention of glucocorticoid-induced osteoporotic fracture.
Bone biopsies from the iliac crest or fracture site, or both, generally show reduced bone formation consistent with bisphosphonate action. Paradoxically, some patients show biopsy evidence of enhanced bone resorption. Biochemical bone-turnover markers are often normal but may be increased.
Atypical femoral fracture can occur in patients who have not been treated with bisphosphonates, and its true incidence in treated and untreated patients is unknown. However, it appears to be more common in patients who have been exposed to long-term bisphosphonates—usually for longer than 3 years (median treatment: 7 years).
It must be emphasized that this type of fracture is rare, particularly considering the millions of patients who have used bisphosphonates and the much higher incidence of typical and common femoral neck and intertrochanteric fractures in untreated patients.
Bisphosphonates are important drugs for the prevention of common osteoporotic fractures. However, atypical femoral fracture is a concern, and more information is needed to help us identify patients at risk and to guide decision-making about the optimal duration of bisphosphonate therapy.
Any discussion with the patient about bisphosphonate use should begin with a mention of the millions of women who have been treated successfully with these agents and the thousands and thousands of hip, spinal, and other nonvertebral fractures that have been prevented. Only 249 cases of atypical femoral fracture have been reported worldwide.
The patient should also be reassured that research into this phenomenon is continuing. At present, the risk-benefit ratio greatly favors use of these medications in properly selected patients at heightened risk of osteoporotic fracture.
New delayed-release bisphosphonate can be taken with food
Benhamou CL, Zanchetta JR, Kaufman JM, et al. A novel delayed-release risedronate 35 mg once-a-week formulation taken with or without breakfast: 2 year BMD data. Osteoporosis Int. 2011;22:S337-S338.
Oral bisphosphonates are poorly absorbed from the gastrointestinal (GI) tract, with bioavailability of less than 1%. Absorption is further reduced when a bisphosphonate is taken with food, beverages other than plain water, medications, or supplements. Because of this limited absorption, oral bisphosphonates must be taken at least 30 to 60 minutes before the first food, drink, or other medication of the day. Noncompliance leads to reduced bioavailability and, potentially, decreased efficacy.
A new delayed-release formulation of risedronate (Atelvia) was approved by the FDA in late 2010 and made available for prescribing earlier this year. It is designed to improve absorption of risedronate in the presence of food, allowing for administration immediately after breakfast.
Two innovations were utilized in the manufacture of this tablet. First, it has an enteric coating to deliver risedronate beyond the stomach in the small intestine, with active drug released at a pH level above 5.5. Second, it contains a chelating agent—edetate disodium (EDTA)—which binds free divalent cations, such as calcium, magnesium, and iron.
Details of the study
Benhamou and colleagues compared three regimens of risedronate:
- 35 mg weekly of the delayed-release formulation, taken at least 30 minutes before breakfast
- 35 mg weekly of the delayed-release formulation, taken 30 minutes after breakfast
- 5 mg daily of the immediate-release risedronate formulation, taken 30 minutes before breakfast.
This phase 3, randomized, double-blind, active-controlled study involved 43 centers across North America, Europe, and South America. Characteristics of women in the study included:
- age of 50 years or older
- at least 5 years since the last menstrual period
- lumbar spine or total hip BMD corresponding to a T-score of –2.5 or worse, or a T-score of –2.0 or worse with at least one prevalent vertebral fracture.
The primary efficacy endpoint was the mean percentage of change in BMD at the lumbar spine.
Findings: At 2 years, the mean change for both delayed-release arms was significantly greater than for the 5-mg immediate-release dose. The authors concluded that the 35-mg weekly delayed-release regimen—whether taken at least 30 minutes before or after breakfast—is as effective and safe as the 5-mg daily immediate-release regimen.
Oral, delayed-release risedronate (35 mg weekly) is similar in efficacy and tolerability to the immediate-release formulation (5 mg daily). By minimizing the impact of concomitantly ingested food on the bioavailability of risedronate, the delayed-release formulation can make it easier for patients to accept and comply with the regimen, thereby maximizing absorption and improving efficacy of the drug in clinical practice.
Do proton-pump inhibitors increase the risk of fracture?
Targownik LE, Leslie WD. The relationship among proton pump inhibitors, bone disease and fracture [published online ahead of print May 20, 2011]. Expert Opin Drug Saf. doi: 10.1517/14740338.2011.586628.
Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med. 2011;124(6):519–526.
Ye X, Liu H, Wu C, et al. Proton pump inhibitors therapy and risk of hip fracture: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2011;23:794–800.
In May 2010, the FDA revised prescription and over-the-counter (OTC) labels for proton-pump inhibitors to include new safety information about a possible increased risk of fractures of the hip, wrist, and spine with use of these medications.
Proton pump inhibitors are so named because they prevent hydrogen ion (proton) secretion into the gastric lumen, thereby reducing acid in the stomach. Esomeprazole magnesium (Nexium), dexlansoprazole (Dexilant), omeprazole (Prilosec), omeprazole with sodium bicarbonate (Zegerid), lansoprazole (Prevacid), pantoprazole (Protonix), rabeprazole (Aciphex), and naproxen with esomeprazole magnesium (Vimovo) are available by prescription to treat conditions such as gastroesophageal reflux disease (GERD), ulcers of the stomach and small intestine, and inflammation of the esophagus. Delayed-release omeprazole magnesium (Prilosec OTC, Zegerid OTC) and delayed-release lansoprazole (Prevacid 24HR) are sold OTC for treatment of frequent heartburn.
The new safety information was based on an FDA review of several epidemiologic studies reporting an increased risk of fractures of the hip, wrist, and spine with PPI use. Some studies found that those at greatest risk of fracture received high doses of PPIs or used them for 1 year or longer. Most of the studies involved individuals 50 years and older; the increased risk of fracture was observed primarily in this age group.
Although the greatest risk of fracture involved people who had been taking a prescription PPI for at least 1 year or who had been taking a high dose of a prescription PPI, the FDA took the precaution of altering the “Drug facts” label on OTC agents as well. (Over-the-counter PPIs are indicated for 14 days of continuous use.) However, in March 2011, the FDA reversed its decision and retracted the wording on the label of OTC formulations. Following a thorough review of safety data, the agency concluded that the risk of fracture with short-term, low-dose use of PPIs is unlikely. Nevertheless, the FDA acknowledged that consumers sometimes use a PPI longer than the directions advise. Therefore, the agency recommends that health-care professionals be aware of the risk of fracture if the patient uses an OTC PPI at a higher dose or longer time than the label advises.
Two meta-analyses find a modestly increased risk of fracture
Yu and colleagues analyzed 11 observational case-control or cohort studies that involved mostly older adults. They found a modestly increased risk of hip fracture among individuals taking a PPI, compared with nonusers (relative risk [RR], 1.30; 95% CI, 1.19–1.43). They also found an increase in spinal (RR, 1.56; 95% CI, 1.31–1.85) and any-site fractures (RR, 1.16; 95% CI, 1.04–1.30) among PPI users. They acknowledged that residual confounding could not be excluded.
A separate meta-analysis by Ye and colleagues included seven studies. They found a statistically significant increase in the risk of hip fracture, compared with nonusers (odds ratio [OR], 1.24; 95% CI, 1.15–1.34). However, because of different effects of PPIs at different durations of therapy, Ye and colleagues concluded that evidence was insufficient to support a causal relationship between PPI use and hip fracture. Further investigation is warranted.
What we know about PPIs and bone
Because PPIs are widely used, it is of paramount importance to understand the precise effects through which use of a PPI may affect bone mineral metabolism and influence fracture risk.
Targownik and Leslie discuss the evidence supporting an association between PPIs and fragility fracture, the association between PPIs and calcium malabsorption, and the underlying condition that predisposes patients who have osteoporosis to fracture. They also explore the possible mechanisms by which PPIs may increase the risk of fracture. After conducting a PubMed search and review of the literature, they found limited evidence supporting the FDA’s assertion that PPIs may increase the risk of fracture. Other findings:
- Multiple analyses have demonstrated a modest association between chronic PPI use and an increased risk of fracture
- No studies have convincingly demonstrated that PPIs cause fracture
- Overall, PPI use does not interfere with calcium absorption in most instances; therefore, it is unlikely that PPIs influence the risk of fracture by interfering with the absorption of dietary calcium
- Dual-energy x-ray absorptiometry (DEXA) testing demonstrates no consistent effect of PPIs on BMD
- Preliminary evidence suggests that PPIs may interfere with normal osteoclastic function, although it is unclear whether such interference influences the risk of fracture.
PPIs are the most potent inhibitors of acid secretion available; standard once-daily dosing may lead to a 90% decrease in gastric acid secretion and will maintain an intragastric pH level above 4 for as long as 70% of any 24-hour period.
PPIs are known to be effective for treating symptoms of GERD and preventing GERD-related complications. These drugs are also the optimal agents for the treatment of peptic ulcer disease and prevention of complications of peptic ulcer disease in chronic users of NSAIDs.
The potency of PPIs makes them the agent of choice for a variety of patients. The drugs also have a long reputation for having a favorable side-effect profile.
Due to this combination of efficacy and safety, PPIs are among the most widely prescribed drugs in all of clinical medicine, trailing only antihypertensives and antidepressants in total number of prescriptions. This means that many of our postmenopausal patients are using these drugs. Those who are using a prescription-strength PPI should be aware of the potential risk of fracture, particularly if they are using it chronically. They also should be advised that exercise, calcium, and vitamin D are vital, as well as regular DEXA testing according to established guidelines.
Will nitroglycerin be the next treatment for osteoporosis?
Jamal SA, Hamilton CJ, Eastell R, Cummings SR. Effect of nitroglycerin ointment on bone density and strength in postmenopausal women: a randomized trial. JAMA. 2011;305(8):800–807.
Khosla S. Is nitroglycerin a novel and inexpensive treatment for osteoporosis? JAMA. 2011;305(8):826–827.
Data suggesting that nitrates may protect against fracture date back to 2006, when Rejnmark and colleagues explored the use of these agents in 124,655 individuals who sustained a fracture and 373,963 age- and sex-matched controls.3 After adjustment for possible confounders, use of nitrates was associated with an 11% reduction in the risk of any fracture (OR, 0.89; 95% CI, 0.86–0.92) and a 15% reduction in the risk of hip fracture (OR, 0.85; 95% CI, 0.79–0.92).3
However, in 2009, Wimalawansa and colleagues found no benefit of transdermal nitroglycerin in preventing bone loss in early postmenopausal women.4 In their 3-year, randomized, double-blind, placebo-controlled trial, 186 postmenopausal women (mean age, 56 years) were randomized to receive nitroglycerin ointment (22.5 mg/d) or placebo. After 36 months of therapy, changes in BMD at multiple sites were comparable between groups. Due to the significant incidence of headache related to nitroglycerin treatment, adherence was suboptimal (estimated at approximately 70%), perhaps contributing to the negative findings.4
Enter this study by Jamal and associates, who performed a double-blind, randomized, placebo-controlled trial 24 months in duration. Participants were 243 women with a mean age of 62 years and a lumbar spine T-score between 0 and –2.0. They were randomized to 15 mg daily of 2% nitroglycerin ointment (applied to the upper outer arm at bedtime) or placebo.
At 2 years, women in the nitroglycerin group had a significant increase in BMD at the:
- lumbar spine, from 1.05 to 1.14 g/cm2, for a percentage change of 6.7% (P<0.001) (placebo group change: 1.06 to 1.08 g/cm2)
- total hip, from 0.92 to 0.97 g/cm2, for a percentage change of 6.2% (P<0.001) (placebo group change: 0.93 to 0.92 g/cm2)
- femoral neck, from 0.88 to 0.93 g/cm2, for a percentage change of 7% (P<0.001) (placebo group change: 0.87 to 0.86 g/cm2).
Nitroglycerin also increased bone-specific alkaline phosphatase by 34.8% and decreased urine N-telopeptide by 54% (P<0.001). Incidence of serious adverse events did not differ between nitroglycerin and placebo groups (5 [42.5%]).
Among women who continued treatment for 24 months, headaches were reported by 40 (35%) nitroglycerin users versus six (5.4%) nonusers during the first month, decreasing substantially after 12 months.
At this time, no changes in clinical practice are warranted. However, the findings of Jamal and coworkers should set the stage for a larger, adequately powered study of nitroglycerin ointment, using fracture as an outcome. If such a study demonstrates fracture reduction, clinicians will have a novel and inexpensive therapy for osteoporosis. The findings of Jamal and coworkers also should prompt development of additional nitric oxide donors that have greater skeletal effects and a better adverse-effect profile—particularly in regard to headache.
We want to hear from you! Tell us what you think.
1. Husada G, Libberecht K, Peeters T, Populaire J. Bilateral mid-diaphyseal femoral stress fractures in the elderly. Eur J Trauma. 2005;31(1):68-71.Doi:10.1007/s00068-005-1421–5.
2. Dell R, Greene D, Ott S, et al. A retrospective analysis of all atypical femur fractures seen in a large California HMO from the years 2007 to 2009. Paper presented at ASBMR 2010 Annual Meeting; October 18, 2010; Toronto, Ontario, Canada. http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?filename="2311OBG_Update" aid=05caf316-b73e-47b8-a011-bf0766b062c0. Accessed Sept. 26, 2011.
3. Rejnmark L, Vestergaard P, Mosekilde L. Decreased fracture risk in users of organic nitrates: a nationwide case-control study. J Bone Miner Res. 2006;21(11):1811-1817.
4. Wimalawansa SJ, Grimes JP, Wilson AC, Hoover DR. Transdermal nitroglycerin therapy may not prevent early postmenopausal bone loss. J Clin Endocrinol Metab. 2009;94(9):3356-3364.
We, and our patients, are fortunate to have a robust armamentarium of osteoporosis preventives and treatments in the 21st century. Still, we have much to learn about the preservation and restoration of bone. Fine-tuning of individual therapies, and clarification of their attendant risks, are ongoing concerns.
In this article, I highlight several recent studies:
- an assessment of the periodontium in a group of postmenopausal women who were long-term users of bisphosphonates, versus nonusers, to determine the effects of these agents on periodontal health
- guidance from the American Society for Bone and Mineral Research on the risk of atypical femoral fracture in long-term users of bisphosphonates
- 2-year data on a new, delayed-release, weekly formulation of risedronate that can be taken with food
- two meta-analyses exploring the risk of fracture with use of a proton-pump inhibitor (PPI), as well as a recent summary of evidence
- a randomized trial of 2% nitroglycerin ointment to prevent fracture.
Bisphosphonates may reduce the risk of postmenopausal periodontal disease
Palomo L, Buencamino-Francisco MC, Carey JJ, Sivanandy M, Thacker H. Is long-term bisphosphonate therapy associated with benefits to the periodontium in postmenopausal women? Menopause. 2011;18(2):164–170.
The risk of periodontal disease increases in menopause. Inflammation can erode structures (i.e., periodontal ligament and alveolar bone) that attach the teeth into the jaw, leading, eventually, to loss of teeth (FIGURE).

Anatomy of healthy periodontium
In periodontal disease, inflammation can erode structures, such as the periodontal ligament and alveolar bone, that attach the teeth into the jaw.In periodontal inflammation, a bacterial biofilm on tooth surfaces triggers a response by neutrophils and macrophages. In this respect, osteoporosis and periodontitis are mediated by common cytokines. Local production of cytokines seems to enhance osteoclast-mediated loss of skeletal and alveolar bone in estrogen-deficient women. In addition, generalized bone loss in postmenopausal osteoporosis renders the jaw susceptible to accelerated alveolar bone resorption and loss of periodontal attachment. For these reasons, physicians who care for postmenopausal women are advised to monitor their periodontal health; be vigilant for dental problems; and encourage them to practice good oral hygiene as a preventive measure against periodontitis and to seek regular dental care.
There has been tremendous publicity about the rare but very serious occurrence of osteonecrosis of the jaw in women who use bisphosphonates. In contrast, this study by Palomo and colleagues from the Case Western Reserve School of Dental Medicine seems to offer some preliminary good news about bisphosphonates and dental health: Long-term use appears to have some beneficial effects on the periodontium of postmenopausal women.
Details of the study
The aim of the study was to compare the periodontium in two groups of postmenopausal women known to have low bone mineral density (BMD): those who were long-term users (>2 years) of bisphosphonate therapy (n=28) and those who were not (n=28). The average age of participants in the study was 63 years, and the average T-score was –2.5. All women underwent cone-beam computed tomography of the jaw and a complete periodontal examination to determine the plaque score, periodontal probing depth, clinical attachment loss, bleeding on probing, and alveolar bone height.
Findings: Bisphosphonate users had a higher plaque score, a lower probing depth, and less loss of clinical attachment than did women in the control group. These differences were determined to be statistically significant. Bisphosphonate users also had less bleeding on probing and a higher alveolar bone height, but these differences were not significant. After adjustment for the plaque score, bisphosphonate use was a significant factor for probing depth but not for the other parameters.
Not all news about bisphosphonates is bad. Preliminary data seem to indicate that objective measures of periodontal disease are lower in bisphosphonate users who have low BMD than in nonusers.
Atypical femoral fracture is a real risk—but a rarity—with long-term use of antiresorptive drugs
Shane E, Burr D, Ebeling PR, et al; American Society for Bone and Mineral Research. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25(11):2267–2294.
As ObGyns, we are often the first-line providers of diagnostic services and treatment for postmenopausal women at risk of osteoporotic fracture. Oral and, more recently, parenteral bisphosphonates have been a mainstay of such treatment. Isolated reports of atypical femoral fracture in long-term users of bisphosphonates first surfaced around 2005.1 Since then, several case series have appeared, some with as many as 102 cases.2 In fact, atypical femoral fracture in bisphosphonate users has drawn so much attention that patients have begun to ask about these agents and express reservations about using them.
The issue has drawn the focus of the American Society for Bone and Mineral Research (ASBMR), which appointed a task force to address it. A multidisciplinary expert group reviewed pertinent published reports of atypical femoral fracture, as well as preclinical studies, that could provide insight into its pathogenesis.
What we know about this type of fracture
Preclinical data lend biologic plausibility to a potential association between long-term bisphosphonate use and atypical femoral fracture. These data highlight the effects of bisphosphonates on:
- collagen cross-linking and maturation
- accumulation of microdamage and advanced glycation end products
- heightened mineralization, remodeling, vascularity, and angiogenesis.
The task force concluded that bisphosphonates are highly effective at reducing the risk of spinal and nonspinal fractures, including typical and common femoral neck and intertrochanteric fractures. However, there is evidence of a relationship between long-term bisphosphonate use and a specific type of subtrochanteric and femoral shaft fracture. This type of fracture is characterized by unique radiographic features:
- transverse or short oblique orientation
- absence of comminution
- cortical thickening
- stress fracture or stress reaction on the symptomatic and/or contralateral side
- delayed healing.
This type of fracture also has unique clinical features—namely, prodromal pain and bilaterality.
The apparent increase in the risk of atypical femoral fracture in patients using glucocorticoids is a concern because bisphosphonates are the mainstay of prevention of glucocorticoid-induced osteoporotic fracture.
Bone biopsies from the iliac crest or fracture site, or both, generally show reduced bone formation consistent with bisphosphonate action. Paradoxically, some patients show biopsy evidence of enhanced bone resorption. Biochemical bone-turnover markers are often normal but may be increased.
Atypical femoral fracture can occur in patients who have not been treated with bisphosphonates, and its true incidence in treated and untreated patients is unknown. However, it appears to be more common in patients who have been exposed to long-term bisphosphonates—usually for longer than 3 years (median treatment: 7 years).
It must be emphasized that this type of fracture is rare, particularly considering the millions of patients who have used bisphosphonates and the much higher incidence of typical and common femoral neck and intertrochanteric fractures in untreated patients.
Bisphosphonates are important drugs for the prevention of common osteoporotic fractures. However, atypical femoral fracture is a concern, and more information is needed to help us identify patients at risk and to guide decision-making about the optimal duration of bisphosphonate therapy.
Any discussion with the patient about bisphosphonate use should begin with a mention of the millions of women who have been treated successfully with these agents and the thousands and thousands of hip, spinal, and other nonvertebral fractures that have been prevented. Only 249 cases of atypical femoral fracture have been reported worldwide.
The patient should also be reassured that research into this phenomenon is continuing. At present, the risk-benefit ratio greatly favors use of these medications in properly selected patients at heightened risk of osteoporotic fracture.
New delayed-release bisphosphonate can be taken with food
Benhamou CL, Zanchetta JR, Kaufman JM, et al. A novel delayed-release risedronate 35 mg once-a-week formulation taken with or without breakfast: 2 year BMD data. Osteoporosis Int. 2011;22:S337-S338.
Oral bisphosphonates are poorly absorbed from the gastrointestinal (GI) tract, with bioavailability of less than 1%. Absorption is further reduced when a bisphosphonate is taken with food, beverages other than plain water, medications, or supplements. Because of this limited absorption, oral bisphosphonates must be taken at least 30 to 60 minutes before the first food, drink, or other medication of the day. Noncompliance leads to reduced bioavailability and, potentially, decreased efficacy.
A new delayed-release formulation of risedronate (Atelvia) was approved by the FDA in late 2010 and made available for prescribing earlier this year. It is designed to improve absorption of risedronate in the presence of food, allowing for administration immediately after breakfast.
Two innovations were utilized in the manufacture of this tablet. First, it has an enteric coating to deliver risedronate beyond the stomach in the small intestine, with active drug released at a pH level above 5.5. Second, it contains a chelating agent—edetate disodium (EDTA)—which binds free divalent cations, such as calcium, magnesium, and iron.
Details of the study
Benhamou and colleagues compared three regimens of risedronate:
- 35 mg weekly of the delayed-release formulation, taken at least 30 minutes before breakfast
- 35 mg weekly of the delayed-release formulation, taken 30 minutes after breakfast
- 5 mg daily of the immediate-release risedronate formulation, taken 30 minutes before breakfast.
This phase 3, randomized, double-blind, active-controlled study involved 43 centers across North America, Europe, and South America. Characteristics of women in the study included:
- age of 50 years or older
- at least 5 years since the last menstrual period
- lumbar spine or total hip BMD corresponding to a T-score of –2.5 or worse, or a T-score of –2.0 or worse with at least one prevalent vertebral fracture.
The primary efficacy endpoint was the mean percentage of change in BMD at the lumbar spine.
Findings: At 2 years, the mean change for both delayed-release arms was significantly greater than for the 5-mg immediate-release dose. The authors concluded that the 35-mg weekly delayed-release regimen—whether taken at least 30 minutes before or after breakfast—is as effective and safe as the 5-mg daily immediate-release regimen.
Oral, delayed-release risedronate (35 mg weekly) is similar in efficacy and tolerability to the immediate-release formulation (5 mg daily). By minimizing the impact of concomitantly ingested food on the bioavailability of risedronate, the delayed-release formulation can make it easier for patients to accept and comply with the regimen, thereby maximizing absorption and improving efficacy of the drug in clinical practice.
Do proton-pump inhibitors increase the risk of fracture?
Targownik LE, Leslie WD. The relationship among proton pump inhibitors, bone disease and fracture [published online ahead of print May 20, 2011]. Expert Opin Drug Saf. doi: 10.1517/14740338.2011.586628.
Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med. 2011;124(6):519–526.
Ye X, Liu H, Wu C, et al. Proton pump inhibitors therapy and risk of hip fracture: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2011;23:794–800.
In May 2010, the FDA revised prescription and over-the-counter (OTC) labels for proton-pump inhibitors to include new safety information about a possible increased risk of fractures of the hip, wrist, and spine with use of these medications.
Proton pump inhibitors are so named because they prevent hydrogen ion (proton) secretion into the gastric lumen, thereby reducing acid in the stomach. Esomeprazole magnesium (Nexium), dexlansoprazole (Dexilant), omeprazole (Prilosec), omeprazole with sodium bicarbonate (Zegerid), lansoprazole (Prevacid), pantoprazole (Protonix), rabeprazole (Aciphex), and naproxen with esomeprazole magnesium (Vimovo) are available by prescription to treat conditions such as gastroesophageal reflux disease (GERD), ulcers of the stomach and small intestine, and inflammation of the esophagus. Delayed-release omeprazole magnesium (Prilosec OTC, Zegerid OTC) and delayed-release lansoprazole (Prevacid 24HR) are sold OTC for treatment of frequent heartburn.
The new safety information was based on an FDA review of several epidemiologic studies reporting an increased risk of fractures of the hip, wrist, and spine with PPI use. Some studies found that those at greatest risk of fracture received high doses of PPIs or used them for 1 year or longer. Most of the studies involved individuals 50 years and older; the increased risk of fracture was observed primarily in this age group.
Although the greatest risk of fracture involved people who had been taking a prescription PPI for at least 1 year or who had been taking a high dose of a prescription PPI, the FDA took the precaution of altering the “Drug facts” label on OTC agents as well. (Over-the-counter PPIs are indicated for 14 days of continuous use.) However, in March 2011, the FDA reversed its decision and retracted the wording on the label of OTC formulations. Following a thorough review of safety data, the agency concluded that the risk of fracture with short-term, low-dose use of PPIs is unlikely. Nevertheless, the FDA acknowledged that consumers sometimes use a PPI longer than the directions advise. Therefore, the agency recommends that health-care professionals be aware of the risk of fracture if the patient uses an OTC PPI at a higher dose or longer time than the label advises.
Two meta-analyses find a modestly increased risk of fracture
Yu and colleagues analyzed 11 observational case-control or cohort studies that involved mostly older adults. They found a modestly increased risk of hip fracture among individuals taking a PPI, compared with nonusers (relative risk [RR], 1.30; 95% CI, 1.19–1.43). They also found an increase in spinal (RR, 1.56; 95% CI, 1.31–1.85) and any-site fractures (RR, 1.16; 95% CI, 1.04–1.30) among PPI users. They acknowledged that residual confounding could not be excluded.
A separate meta-analysis by Ye and colleagues included seven studies. They found a statistically significant increase in the risk of hip fracture, compared with nonusers (odds ratio [OR], 1.24; 95% CI, 1.15–1.34). However, because of different effects of PPIs at different durations of therapy, Ye and colleagues concluded that evidence was insufficient to support a causal relationship between PPI use and hip fracture. Further investigation is warranted.
What we know about PPIs and bone
Because PPIs are widely used, it is of paramount importance to understand the precise effects through which use of a PPI may affect bone mineral metabolism and influence fracture risk.
Targownik and Leslie discuss the evidence supporting an association between PPIs and fragility fracture, the association between PPIs and calcium malabsorption, and the underlying condition that predisposes patients who have osteoporosis to fracture. They also explore the possible mechanisms by which PPIs may increase the risk of fracture. After conducting a PubMed search and review of the literature, they found limited evidence supporting the FDA’s assertion that PPIs may increase the risk of fracture. Other findings:
- Multiple analyses have demonstrated a modest association between chronic PPI use and an increased risk of fracture
- No studies have convincingly demonstrated that PPIs cause fracture
- Overall, PPI use does not interfere with calcium absorption in most instances; therefore, it is unlikely that PPIs influence the risk of fracture by interfering with the absorption of dietary calcium
- Dual-energy x-ray absorptiometry (DEXA) testing demonstrates no consistent effect of PPIs on BMD
- Preliminary evidence suggests that PPIs may interfere with normal osteoclastic function, although it is unclear whether such interference influences the risk of fracture.
PPIs are the most potent inhibitors of acid secretion available; standard once-daily dosing may lead to a 90% decrease in gastric acid secretion and will maintain an intragastric pH level above 4 for as long as 70% of any 24-hour period.
PPIs are known to be effective for treating symptoms of GERD and preventing GERD-related complications. These drugs are also the optimal agents for the treatment of peptic ulcer disease and prevention of complications of peptic ulcer disease in chronic users of NSAIDs.
The potency of PPIs makes them the agent of choice for a variety of patients. The drugs also have a long reputation for having a favorable side-effect profile.
Due to this combination of efficacy and safety, PPIs are among the most widely prescribed drugs in all of clinical medicine, trailing only antihypertensives and antidepressants in total number of prescriptions. This means that many of our postmenopausal patients are using these drugs. Those who are using a prescription-strength PPI should be aware of the potential risk of fracture, particularly if they are using it chronically. They also should be advised that exercise, calcium, and vitamin D are vital, as well as regular DEXA testing according to established guidelines.
Will nitroglycerin be the next treatment for osteoporosis?
Jamal SA, Hamilton CJ, Eastell R, Cummings SR. Effect of nitroglycerin ointment on bone density and strength in postmenopausal women: a randomized trial. JAMA. 2011;305(8):800–807.
Khosla S. Is nitroglycerin a novel and inexpensive treatment for osteoporosis? JAMA. 2011;305(8):826–827.
Data suggesting that nitrates may protect against fracture date back to 2006, when Rejnmark and colleagues explored the use of these agents in 124,655 individuals who sustained a fracture and 373,963 age- and sex-matched controls.3 After adjustment for possible confounders, use of nitrates was associated with an 11% reduction in the risk of any fracture (OR, 0.89; 95% CI, 0.86–0.92) and a 15% reduction in the risk of hip fracture (OR, 0.85; 95% CI, 0.79–0.92).3
However, in 2009, Wimalawansa and colleagues found no benefit of transdermal nitroglycerin in preventing bone loss in early postmenopausal women.4 In their 3-year, randomized, double-blind, placebo-controlled trial, 186 postmenopausal women (mean age, 56 years) were randomized to receive nitroglycerin ointment (22.5 mg/d) or placebo. After 36 months of therapy, changes in BMD at multiple sites were comparable between groups. Due to the significant incidence of headache related to nitroglycerin treatment, adherence was suboptimal (estimated at approximately 70%), perhaps contributing to the negative findings.4
Enter this study by Jamal and associates, who performed a double-blind, randomized, placebo-controlled trial 24 months in duration. Participants were 243 women with a mean age of 62 years and a lumbar spine T-score between 0 and –2.0. They were randomized to 15 mg daily of 2% nitroglycerin ointment (applied to the upper outer arm at bedtime) or placebo.
At 2 years, women in the nitroglycerin group had a significant increase in BMD at the:
- lumbar spine, from 1.05 to 1.14 g/cm2, for a percentage change of 6.7% (P<0.001) (placebo group change: 1.06 to 1.08 g/cm2)
- total hip, from 0.92 to 0.97 g/cm2, for a percentage change of 6.2% (P<0.001) (placebo group change: 0.93 to 0.92 g/cm2)
- femoral neck, from 0.88 to 0.93 g/cm2, for a percentage change of 7% (P<0.001) (placebo group change: 0.87 to 0.86 g/cm2).
Nitroglycerin also increased bone-specific alkaline phosphatase by 34.8% and decreased urine N-telopeptide by 54% (P<0.001). Incidence of serious adverse events did not differ between nitroglycerin and placebo groups (5 [42.5%]).
Among women who continued treatment for 24 months, headaches were reported by 40 (35%) nitroglycerin users versus six (5.4%) nonusers during the first month, decreasing substantially after 12 months.
At this time, no changes in clinical practice are warranted. However, the findings of Jamal and coworkers should set the stage for a larger, adequately powered study of nitroglycerin ointment, using fracture as an outcome. If such a study demonstrates fracture reduction, clinicians will have a novel and inexpensive therapy for osteoporosis. The findings of Jamal and coworkers also should prompt development of additional nitric oxide donors that have greater skeletal effects and a better adverse-effect profile—particularly in regard to headache.
We want to hear from you! Tell us what you think.
We, and our patients, are fortunate to have a robust armamentarium of osteoporosis preventives and treatments in the 21st century. Still, we have much to learn about the preservation and restoration of bone. Fine-tuning of individual therapies, and clarification of their attendant risks, are ongoing concerns.
In this article, I highlight several recent studies:
- an assessment of the periodontium in a group of postmenopausal women who were long-term users of bisphosphonates, versus nonusers, to determine the effects of these agents on periodontal health
- guidance from the American Society for Bone and Mineral Research on the risk of atypical femoral fracture in long-term users of bisphosphonates
- 2-year data on a new, delayed-release, weekly formulation of risedronate that can be taken with food
- two meta-analyses exploring the risk of fracture with use of a proton-pump inhibitor (PPI), as well as a recent summary of evidence
- a randomized trial of 2% nitroglycerin ointment to prevent fracture.
Bisphosphonates may reduce the risk of postmenopausal periodontal disease
Palomo L, Buencamino-Francisco MC, Carey JJ, Sivanandy M, Thacker H. Is long-term bisphosphonate therapy associated with benefits to the periodontium in postmenopausal women? Menopause. 2011;18(2):164–170.
The risk of periodontal disease increases in menopause. Inflammation can erode structures (i.e., periodontal ligament and alveolar bone) that attach the teeth into the jaw, leading, eventually, to loss of teeth (FIGURE).

Anatomy of healthy periodontium
In periodontal disease, inflammation can erode structures, such as the periodontal ligament and alveolar bone, that attach the teeth into the jaw.In periodontal inflammation, a bacterial biofilm on tooth surfaces triggers a response by neutrophils and macrophages. In this respect, osteoporosis and periodontitis are mediated by common cytokines. Local production of cytokines seems to enhance osteoclast-mediated loss of skeletal and alveolar bone in estrogen-deficient women. In addition, generalized bone loss in postmenopausal osteoporosis renders the jaw susceptible to accelerated alveolar bone resorption and loss of periodontal attachment. For these reasons, physicians who care for postmenopausal women are advised to monitor their periodontal health; be vigilant for dental problems; and encourage them to practice good oral hygiene as a preventive measure against periodontitis and to seek regular dental care.
There has been tremendous publicity about the rare but very serious occurrence of osteonecrosis of the jaw in women who use bisphosphonates. In contrast, this study by Palomo and colleagues from the Case Western Reserve School of Dental Medicine seems to offer some preliminary good news about bisphosphonates and dental health: Long-term use appears to have some beneficial effects on the periodontium of postmenopausal women.
Details of the study
The aim of the study was to compare the periodontium in two groups of postmenopausal women known to have low bone mineral density (BMD): those who were long-term users (>2 years) of bisphosphonate therapy (n=28) and those who were not (n=28). The average age of participants in the study was 63 years, and the average T-score was –2.5. All women underwent cone-beam computed tomography of the jaw and a complete periodontal examination to determine the plaque score, periodontal probing depth, clinical attachment loss, bleeding on probing, and alveolar bone height.
Findings: Bisphosphonate users had a higher plaque score, a lower probing depth, and less loss of clinical attachment than did women in the control group. These differences were determined to be statistically significant. Bisphosphonate users also had less bleeding on probing and a higher alveolar bone height, but these differences were not significant. After adjustment for the plaque score, bisphosphonate use was a significant factor for probing depth but not for the other parameters.
Not all news about bisphosphonates is bad. Preliminary data seem to indicate that objective measures of periodontal disease are lower in bisphosphonate users who have low BMD than in nonusers.
Atypical femoral fracture is a real risk—but a rarity—with long-term use of antiresorptive drugs
Shane E, Burr D, Ebeling PR, et al; American Society for Bone and Mineral Research. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25(11):2267–2294.
As ObGyns, we are often the first-line providers of diagnostic services and treatment for postmenopausal women at risk of osteoporotic fracture. Oral and, more recently, parenteral bisphosphonates have been a mainstay of such treatment. Isolated reports of atypical femoral fracture in long-term users of bisphosphonates first surfaced around 2005.1 Since then, several case series have appeared, some with as many as 102 cases.2 In fact, atypical femoral fracture in bisphosphonate users has drawn so much attention that patients have begun to ask about these agents and express reservations about using them.
The issue has drawn the focus of the American Society for Bone and Mineral Research (ASBMR), which appointed a task force to address it. A multidisciplinary expert group reviewed pertinent published reports of atypical femoral fracture, as well as preclinical studies, that could provide insight into its pathogenesis.
What we know about this type of fracture
Preclinical data lend biologic plausibility to a potential association between long-term bisphosphonate use and atypical femoral fracture. These data highlight the effects of bisphosphonates on:
- collagen cross-linking and maturation
- accumulation of microdamage and advanced glycation end products
- heightened mineralization, remodeling, vascularity, and angiogenesis.
The task force concluded that bisphosphonates are highly effective at reducing the risk of spinal and nonspinal fractures, including typical and common femoral neck and intertrochanteric fractures. However, there is evidence of a relationship between long-term bisphosphonate use and a specific type of subtrochanteric and femoral shaft fracture. This type of fracture is characterized by unique radiographic features:
- transverse or short oblique orientation
- absence of comminution
- cortical thickening
- stress fracture or stress reaction on the symptomatic and/or contralateral side
- delayed healing.
This type of fracture also has unique clinical features—namely, prodromal pain and bilaterality.
The apparent increase in the risk of atypical femoral fracture in patients using glucocorticoids is a concern because bisphosphonates are the mainstay of prevention of glucocorticoid-induced osteoporotic fracture.
Bone biopsies from the iliac crest or fracture site, or both, generally show reduced bone formation consistent with bisphosphonate action. Paradoxically, some patients show biopsy evidence of enhanced bone resorption. Biochemical bone-turnover markers are often normal but may be increased.
Atypical femoral fracture can occur in patients who have not been treated with bisphosphonates, and its true incidence in treated and untreated patients is unknown. However, it appears to be more common in patients who have been exposed to long-term bisphosphonates—usually for longer than 3 years (median treatment: 7 years).
It must be emphasized that this type of fracture is rare, particularly considering the millions of patients who have used bisphosphonates and the much higher incidence of typical and common femoral neck and intertrochanteric fractures in untreated patients.
Bisphosphonates are important drugs for the prevention of common osteoporotic fractures. However, atypical femoral fracture is a concern, and more information is needed to help us identify patients at risk and to guide decision-making about the optimal duration of bisphosphonate therapy.
Any discussion with the patient about bisphosphonate use should begin with a mention of the millions of women who have been treated successfully with these agents and the thousands and thousands of hip, spinal, and other nonvertebral fractures that have been prevented. Only 249 cases of atypical femoral fracture have been reported worldwide.
The patient should also be reassured that research into this phenomenon is continuing. At present, the risk-benefit ratio greatly favors use of these medications in properly selected patients at heightened risk of osteoporotic fracture.
New delayed-release bisphosphonate can be taken with food
Benhamou CL, Zanchetta JR, Kaufman JM, et al. A novel delayed-release risedronate 35 mg once-a-week formulation taken with or without breakfast: 2 year BMD data. Osteoporosis Int. 2011;22:S337-S338.
Oral bisphosphonates are poorly absorbed from the gastrointestinal (GI) tract, with bioavailability of less than 1%. Absorption is further reduced when a bisphosphonate is taken with food, beverages other than plain water, medications, or supplements. Because of this limited absorption, oral bisphosphonates must be taken at least 30 to 60 minutes before the first food, drink, or other medication of the day. Noncompliance leads to reduced bioavailability and, potentially, decreased efficacy.
A new delayed-release formulation of risedronate (Atelvia) was approved by the FDA in late 2010 and made available for prescribing earlier this year. It is designed to improve absorption of risedronate in the presence of food, allowing for administration immediately after breakfast.
Two innovations were utilized in the manufacture of this tablet. First, it has an enteric coating to deliver risedronate beyond the stomach in the small intestine, with active drug released at a pH level above 5.5. Second, it contains a chelating agent—edetate disodium (EDTA)—which binds free divalent cations, such as calcium, magnesium, and iron.
Details of the study
Benhamou and colleagues compared three regimens of risedronate:
- 35 mg weekly of the delayed-release formulation, taken at least 30 minutes before breakfast
- 35 mg weekly of the delayed-release formulation, taken 30 minutes after breakfast
- 5 mg daily of the immediate-release risedronate formulation, taken 30 minutes before breakfast.
This phase 3, randomized, double-blind, active-controlled study involved 43 centers across North America, Europe, and South America. Characteristics of women in the study included:
- age of 50 years or older
- at least 5 years since the last menstrual period
- lumbar spine or total hip BMD corresponding to a T-score of –2.5 or worse, or a T-score of –2.0 or worse with at least one prevalent vertebral fracture.
The primary efficacy endpoint was the mean percentage of change in BMD at the lumbar spine.
Findings: At 2 years, the mean change for both delayed-release arms was significantly greater than for the 5-mg immediate-release dose. The authors concluded that the 35-mg weekly delayed-release regimen—whether taken at least 30 minutes before or after breakfast—is as effective and safe as the 5-mg daily immediate-release regimen.
Oral, delayed-release risedronate (35 mg weekly) is similar in efficacy and tolerability to the immediate-release formulation (5 mg daily). By minimizing the impact of concomitantly ingested food on the bioavailability of risedronate, the delayed-release formulation can make it easier for patients to accept and comply with the regimen, thereby maximizing absorption and improving efficacy of the drug in clinical practice.
Do proton-pump inhibitors increase the risk of fracture?
Targownik LE, Leslie WD. The relationship among proton pump inhibitors, bone disease and fracture [published online ahead of print May 20, 2011]. Expert Opin Drug Saf. doi: 10.1517/14740338.2011.586628.
Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med. 2011;124(6):519–526.
Ye X, Liu H, Wu C, et al. Proton pump inhibitors therapy and risk of hip fracture: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2011;23:794–800.
In May 2010, the FDA revised prescription and over-the-counter (OTC) labels for proton-pump inhibitors to include new safety information about a possible increased risk of fractures of the hip, wrist, and spine with use of these medications.
Proton pump inhibitors are so named because they prevent hydrogen ion (proton) secretion into the gastric lumen, thereby reducing acid in the stomach. Esomeprazole magnesium (Nexium), dexlansoprazole (Dexilant), omeprazole (Prilosec), omeprazole with sodium bicarbonate (Zegerid), lansoprazole (Prevacid), pantoprazole (Protonix), rabeprazole (Aciphex), and naproxen with esomeprazole magnesium (Vimovo) are available by prescription to treat conditions such as gastroesophageal reflux disease (GERD), ulcers of the stomach and small intestine, and inflammation of the esophagus. Delayed-release omeprazole magnesium (Prilosec OTC, Zegerid OTC) and delayed-release lansoprazole (Prevacid 24HR) are sold OTC for treatment of frequent heartburn.
The new safety information was based on an FDA review of several epidemiologic studies reporting an increased risk of fractures of the hip, wrist, and spine with PPI use. Some studies found that those at greatest risk of fracture received high doses of PPIs or used them for 1 year or longer. Most of the studies involved individuals 50 years and older; the increased risk of fracture was observed primarily in this age group.
Although the greatest risk of fracture involved people who had been taking a prescription PPI for at least 1 year or who had been taking a high dose of a prescription PPI, the FDA took the precaution of altering the “Drug facts” label on OTC agents as well. (Over-the-counter PPIs are indicated for 14 days of continuous use.) However, in March 2011, the FDA reversed its decision and retracted the wording on the label of OTC formulations. Following a thorough review of safety data, the agency concluded that the risk of fracture with short-term, low-dose use of PPIs is unlikely. Nevertheless, the FDA acknowledged that consumers sometimes use a PPI longer than the directions advise. Therefore, the agency recommends that health-care professionals be aware of the risk of fracture if the patient uses an OTC PPI at a higher dose or longer time than the label advises.
Two meta-analyses find a modestly increased risk of fracture
Yu and colleagues analyzed 11 observational case-control or cohort studies that involved mostly older adults. They found a modestly increased risk of hip fracture among individuals taking a PPI, compared with nonusers (relative risk [RR], 1.30; 95% CI, 1.19–1.43). They also found an increase in spinal (RR, 1.56; 95% CI, 1.31–1.85) and any-site fractures (RR, 1.16; 95% CI, 1.04–1.30) among PPI users. They acknowledged that residual confounding could not be excluded.
A separate meta-analysis by Ye and colleagues included seven studies. They found a statistically significant increase in the risk of hip fracture, compared with nonusers (odds ratio [OR], 1.24; 95% CI, 1.15–1.34). However, because of different effects of PPIs at different durations of therapy, Ye and colleagues concluded that evidence was insufficient to support a causal relationship between PPI use and hip fracture. Further investigation is warranted.
What we know about PPIs and bone
Because PPIs are widely used, it is of paramount importance to understand the precise effects through which use of a PPI may affect bone mineral metabolism and influence fracture risk.
Targownik and Leslie discuss the evidence supporting an association between PPIs and fragility fracture, the association between PPIs and calcium malabsorption, and the underlying condition that predisposes patients who have osteoporosis to fracture. They also explore the possible mechanisms by which PPIs may increase the risk of fracture. After conducting a PubMed search and review of the literature, they found limited evidence supporting the FDA’s assertion that PPIs may increase the risk of fracture. Other findings:
- Multiple analyses have demonstrated a modest association between chronic PPI use and an increased risk of fracture
- No studies have convincingly demonstrated that PPIs cause fracture
- Overall, PPI use does not interfere with calcium absorption in most instances; therefore, it is unlikely that PPIs influence the risk of fracture by interfering with the absorption of dietary calcium
- Dual-energy x-ray absorptiometry (DEXA) testing demonstrates no consistent effect of PPIs on BMD
- Preliminary evidence suggests that PPIs may interfere with normal osteoclastic function, although it is unclear whether such interference influences the risk of fracture.
PPIs are the most potent inhibitors of acid secretion available; standard once-daily dosing may lead to a 90% decrease in gastric acid secretion and will maintain an intragastric pH level above 4 for as long as 70% of any 24-hour period.
PPIs are known to be effective for treating symptoms of GERD and preventing GERD-related complications. These drugs are also the optimal agents for the treatment of peptic ulcer disease and prevention of complications of peptic ulcer disease in chronic users of NSAIDs.
The potency of PPIs makes them the agent of choice for a variety of patients. The drugs also have a long reputation for having a favorable side-effect profile.
Due to this combination of efficacy and safety, PPIs are among the most widely prescribed drugs in all of clinical medicine, trailing only antihypertensives and antidepressants in total number of prescriptions. This means that many of our postmenopausal patients are using these drugs. Those who are using a prescription-strength PPI should be aware of the potential risk of fracture, particularly if they are using it chronically. They also should be advised that exercise, calcium, and vitamin D are vital, as well as regular DEXA testing according to established guidelines.
Will nitroglycerin be the next treatment for osteoporosis?
Jamal SA, Hamilton CJ, Eastell R, Cummings SR. Effect of nitroglycerin ointment on bone density and strength in postmenopausal women: a randomized trial. JAMA. 2011;305(8):800–807.
Khosla S. Is nitroglycerin a novel and inexpensive treatment for osteoporosis? JAMA. 2011;305(8):826–827.
Data suggesting that nitrates may protect against fracture date back to 2006, when Rejnmark and colleagues explored the use of these agents in 124,655 individuals who sustained a fracture and 373,963 age- and sex-matched controls.3 After adjustment for possible confounders, use of nitrates was associated with an 11% reduction in the risk of any fracture (OR, 0.89; 95% CI, 0.86–0.92) and a 15% reduction in the risk of hip fracture (OR, 0.85; 95% CI, 0.79–0.92).3
However, in 2009, Wimalawansa and colleagues found no benefit of transdermal nitroglycerin in preventing bone loss in early postmenopausal women.4 In their 3-year, randomized, double-blind, placebo-controlled trial, 186 postmenopausal women (mean age, 56 years) were randomized to receive nitroglycerin ointment (22.5 mg/d) or placebo. After 36 months of therapy, changes in BMD at multiple sites were comparable between groups. Due to the significant incidence of headache related to nitroglycerin treatment, adherence was suboptimal (estimated at approximately 70%), perhaps contributing to the negative findings.4
Enter this study by Jamal and associates, who performed a double-blind, randomized, placebo-controlled trial 24 months in duration. Participants were 243 women with a mean age of 62 years and a lumbar spine T-score between 0 and –2.0. They were randomized to 15 mg daily of 2% nitroglycerin ointment (applied to the upper outer arm at bedtime) or placebo.
At 2 years, women in the nitroglycerin group had a significant increase in BMD at the:
- lumbar spine, from 1.05 to 1.14 g/cm2, for a percentage change of 6.7% (P<0.001) (placebo group change: 1.06 to 1.08 g/cm2)
- total hip, from 0.92 to 0.97 g/cm2, for a percentage change of 6.2% (P<0.001) (placebo group change: 0.93 to 0.92 g/cm2)
- femoral neck, from 0.88 to 0.93 g/cm2, for a percentage change of 7% (P<0.001) (placebo group change: 0.87 to 0.86 g/cm2).
Nitroglycerin also increased bone-specific alkaline phosphatase by 34.8% and decreased urine N-telopeptide by 54% (P<0.001). Incidence of serious adverse events did not differ between nitroglycerin and placebo groups (5 [42.5%]).
Among women who continued treatment for 24 months, headaches were reported by 40 (35%) nitroglycerin users versus six (5.4%) nonusers during the first month, decreasing substantially after 12 months.
At this time, no changes in clinical practice are warranted. However, the findings of Jamal and coworkers should set the stage for a larger, adequately powered study of nitroglycerin ointment, using fracture as an outcome. If such a study demonstrates fracture reduction, clinicians will have a novel and inexpensive therapy for osteoporosis. The findings of Jamal and coworkers also should prompt development of additional nitric oxide donors that have greater skeletal effects and a better adverse-effect profile—particularly in regard to headache.
We want to hear from you! Tell us what you think.
1. Husada G, Libberecht K, Peeters T, Populaire J. Bilateral mid-diaphyseal femoral stress fractures in the elderly. Eur J Trauma. 2005;31(1):68-71.Doi:10.1007/s00068-005-1421–5.
2. Dell R, Greene D, Ott S, et al. A retrospective analysis of all atypical femur fractures seen in a large California HMO from the years 2007 to 2009. Paper presented at ASBMR 2010 Annual Meeting; October 18, 2010; Toronto, Ontario, Canada. http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?filename="2311OBG_Update" aid=05caf316-b73e-47b8-a011-bf0766b062c0. Accessed Sept. 26, 2011.
3. Rejnmark L, Vestergaard P, Mosekilde L. Decreased fracture risk in users of organic nitrates: a nationwide case-control study. J Bone Miner Res. 2006;21(11):1811-1817.
4. Wimalawansa SJ, Grimes JP, Wilson AC, Hoover DR. Transdermal nitroglycerin therapy may not prevent early postmenopausal bone loss. J Clin Endocrinol Metab. 2009;94(9):3356-3364.
1. Husada G, Libberecht K, Peeters T, Populaire J. Bilateral mid-diaphyseal femoral stress fractures in the elderly. Eur J Trauma. 2005;31(1):68-71.Doi:10.1007/s00068-005-1421–5.
2. Dell R, Greene D, Ott S, et al. A retrospective analysis of all atypical femur fractures seen in a large California HMO from the years 2007 to 2009. Paper presented at ASBMR 2010 Annual Meeting; October 18, 2010; Toronto, Ontario, Canada. http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?filename="2311OBG_Update" aid=05caf316-b73e-47b8-a011-bf0766b062c0. Accessed Sept. 26, 2011.
3. Rejnmark L, Vestergaard P, Mosekilde L. Decreased fracture risk in users of organic nitrates: a nationwide case-control study. J Bone Miner Res. 2006;21(11):1811-1817.
4. Wimalawansa SJ, Grimes JP, Wilson AC, Hoover DR. Transdermal nitroglycerin therapy may not prevent early postmenopausal bone loss. J Clin Endocrinol Metab. 2009;94(9):3356-3364.
