User login
There are a variety of well-established methods for diagnosing dermatophyte infections, including potassium hydroxide (KOH) preparations, fungal cultures, and skin biopsies. Each modality has its place in clinical practice, but they also have drawbacks. Reflectance confocal microscopy (RCM) is an emerging in vivo technology that could potentially serve as a sensitive, rapid, and noninvasive method of diagnosing dermatophytosis. Using near-infrared laser light scanning, RCM provides a quick noninvasive method of generating black-and-white, horizontal, quasipathology images that allow for the identification of cells and other structures similar to dermoscopy and histopathology.1 The images are obtained in a fully noninvasive fashion, as the device is placed in contact with the skin using a liquid medium. The process takes 5 to 15 minutes depending on the number of images obtained, and the images can then be displayed in real time on a computer screen or transmitted to a pathologist for evaluation.
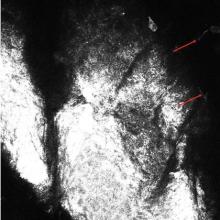
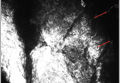
Most initial applications of RCM focused on evaluating melanocytic lesions with the primary goal of differentiating between benign nevi and melanomas, thus reducing the need for skin biopsies.2-4 Efforts to develop RCM diagnostic criteria for identification of other skin cancers5,6 as well as to aid in the diagnosis of nonneoplastic skin conditions are ongoing.7 The potential applications of RCM are virtually limitless, as this modality can (at least partially) take the place of biopsies in a variety of clinical scenarios.2,8 Few reports have documented the utility of RCM as a diagnostic tool for onychomycosis9,10 and dermatophytic infections of the skin.11,12 Hui et al13 reported use for RCM for microscopic evaluation of mycelium features. Turan et al14 found that RCM could not replace the current diagnostic standards for tinea incognito but may be successfully used as an in vivo noninvasive screening tool to facilitate diagnosis. Because it provides high-resolution horizontal images extending from the surface of the stratum corneum to the superficial reticular dermis, RCM could be an effective tool in the diagnosis of cutaneous dermatophyte infections, as organisms usually are located in the stratum corneum of the epidermis in this infection. Branching hyphae are readily visible in the stratum corneum on RCM (Figure).
We reviewed a series of 9 cases from a private practice setting in which RCM was used to diagnose dermatophytosis. We compared the diagnostic accuracy of RCM to results from other diagnostic methods and the ultimate clinical outcome to determine the usefulness of this new technology.
Methods
Our retrospective chart review included all cases in which RCM was used and the clinical differential diagnosis included tinea corporis over a 4-month period in a private, single-specialty dermatology practice. All patients were treated by the same dermatologist. The RCM images were taken using an imaging system that had a horizontal optical resolution of less than 1.25 μm and a vertical optical resolution of less than 5.0 μm. The imaging was performed by medical assistants who were trained by the device manufacturer.
The sample sites were cleaned with isopropyl alcohol and a translucent contact ring was affixed to the skin using a liquid medium. The imaging head of the device was connected to the imaging ring and the images were taken. Identical imaging protocol was used in all patients. Multiple sets of horizontal images and one stack of vertical images were obtained. Patients reported no discomfort during the procedure, and the entire process was usually completed within 15 minutes. The images were sent to the pathologist for evaluation using the manufacturer’s telepathology system and were returned with a diagnosis within 24 hours. (On-site, real-time diagnosis also is possible if the dermatologist is trained in interpreting the images.)
In the chart review we looked for other diagnostic methods used as well as clinical outcomes. A case was considered to be positive for dermatophytic infection if any of the other diagnostic modalities yielded positive results or if a definitive resolution of the condition could be achieved using antifungal treatments alone.
Results
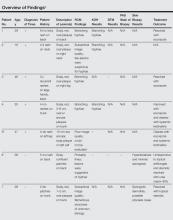
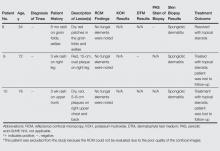
Ten patients (mean age, 43.1 years; age range, 16–76 years) with lesions that presented as possible dermatophytic infections underwent RCM analysis. In addition to RCM imaging, 5 patients underwent KOH testing of skin scrapings, 3 underwent analysis by fungal culture utilizing dermatophyte test medium (DTM), and 5 underwent biopsies. The findings are further summarized in the Table. One patient (patient 5) was excluded from the study because the RCM could not be evaluated due to the poor quality of the confocal images. Additionally, 2 patients (patients 2 and 7) had suboptimal imaging, which limited the evaluation.
Of the 9 evaluable cases, 4 (patients 1–4) were determined to be positive for the presence of dermatophytic infection through the fulfillment of criteria independent of RCM imaging. In each of those 4 cases, RCM images revealed the presence of hyphae, which indicated the presence of dermato-phytic infection. In these 4 cases, RCM and other diagnostic methods reached the same diagnosis.
In the other 5 cases (patients 6–10), the final diagnosis was not a dermatophytic infection. In 4 of those cases (patients 7–10), there were no signs of any structure resembling hyphae on the RCM images; however, in 1 case (patient 6), the RCM images showed structures that were consistent with the appearance of hyphae to the extent that the investigators, based solely on analysis of the RCM images, deemed a diagnosis indicating presence of a dermatophytic infection to be valid. In this case, a 38-year-old man presented with extensive scaly patches on the back of several months’ duration. Repeated skin biopsies showed hyperkeratosis and occasionally minimal spongiosis, while periodic acid–Schiff staining did not reveal fungal elements. Fungal cultures and KOH preparations were negative. Prior treatments with topical antifungals and steroids failed to improve the condition, which resolved rapidly with urea cream 40%. The interpretation of the RCM images in this patient did not match up with the results obtained from other methods of diagnosis and the clinical outcome; thus, we classified it as an incorrect diagnosis based on RCM analysis alone. In total, successful diagnosis using RCM imaging was achieved in 8 of 9 cases included in the analysis.
Comment
In this chart review, we evaluated the utility of using RCM in the diagnosis of dermatophytic infections of the skin by comparing findings noted on confocal imaging with those of other methods of diagnosis (Table). We included cases in which the clinical presentation raised the possibility of dermatophytic infection. Cases were considered positive for dermatophytes if KOH preparation, fungal culture, or skin biopsy (with or without periodic acid–Schiff staining) were positive or if there was a complete response to antifungal treatment alone. In this small number of cases, we found that RCM was 100% sensitive, as hyphae were readily seen in all cases of dermatophytic infections. In 1 RCM-positive case (patient 3), fungal culture with DTM was negative, but antifungal therapy was nonetheless given. Because the lesion resolved promptly with econazole, RCM proved to be true positive and DTM proved to be false negative (Table). Reflectance confocal microscopy imaging, however, was less specific. Of the 5 cases that showed no presence of dermatophytic infection, there was 1 case (patient 6) in which the pathologist could recognize structures that resembled fungal hyphae. There are various possible sources of structures masquerading as dermatophytes on confocal imaging, including the edges of nonnucleated loose keratinocytes, keratin fragments, and other foreign fibers. Evaluation by an experienced investigator can certainly help in limiting false-positive analyses, but a larger case study would be useful to develop a set of specific criteria to aid in the differentiation of fungal hyphae from other artifacts as well as to further define the sensitivity and specificity of RCM.
We also encountered difficulties with the technical aspects of RCM. One case (patient 5) was excluded from the analysis because the images were poor quality and could not be interpreted, and 2 cases (patients 2 and 7) had suboptimal images, in part due to operator error and in part due to equipment error that was recognized later on. The technical difficulties were problematic because no immediate review of image quality was available while patients were still present for possible reimaging. All of the images evaluated in this study were captured shortly after the RCM device was introduced to the practice. It is possible that with more training and a quick, on-site review of image quality, these technical problems could be avoided. Imaging protocols (ie, numbers and levels of scans taken by the confocal microscope) also could be adjusted so they include a large enough range to compensate for potential operator errors; however, these adjustments also could increase overall imaging time.
Conclusion
Based on our chart review of a small number of cases, we found that RCM can be a useful tool in diagnosing dermatophytic infections of the skin. With adequate training, dermatologists may be able to use RCM as an in-office tool to capture and evaluate images and subsequently diagnose or exclude dermatophytosis in a quick and noninvasive manner. However, further research and controlled studies of more cases will be required to develop accurate criteria for diagnosing fungal structures by RCM as well as to help determine the role of RCM in our diagnostic armamentarium.
1. Longo C, Farnetani F, Ciardo S, et al. Is confocal microscopy a valuable tool in diagnosing nodular lesions? a study of 140 cases. Br J Dermatol. 2013;169:58-67.
2. Debarbieux S, Dalle S, Depaepe L, et al. Second primary melanomas under BRAF blockers: study by reflectance confocal microscopy [published online a head of print April 1, 2013]. Br J Dermatol. 2013;168:1230-1235.
3. Schwartz RJ, Vera K, Navarrete N, et al. In vivo reflectance confocal microscopy of halo nevus. J Cutan Med Surg. 2013;17:33-38.
4. Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
5. Nori S, Rius-Diaz F, Cuevas J, et al. Sensitivity and specificity of reflectance-mode confocal micros-copy for in vivo diagnosis of basal cell carcinoma: a multicenter study. J Am Acad Dermatol. 2004;51:923-930.
6. Arzberger E, Komericki P, Ahlgrimm-Siess V, et al. Differentiation between balanitis and carcinoma in situ using reflectance confocal microscopy. JAMA Dermatol. 2013;149:440-445.
7. Ardigò M, Maliszewski I, Cota C, et al. Preliminary evaluation of in vivo reflectance confocal microscopy features of discoid lupus erythematosus. Br J Dermatol. 2007;156:1196-1203.
8. Longo C, Casari A, Pepe P, et al. Confocal microscopy insights into the treatment and cellular immune response of Basal cell carcinoma to photodynamic therapy. Dermatology. 2012;225:264-270.
9. Hongcharu W, Dwyer P, Gonzalez S, et al. Confirmation of onychomycosis by in vivo confocal microscopy. J Am Acad Dermatol. 2000;42:214-216.
10. Rothmund G, Sattler EC, Kaestle R, et al. Confocal laser scanning microscopy as a new valuable tool in the diagnosis of onychomycosis—comparison of six diagnostic methods [published online ahead of print April 23, 2012]. Mycoses. 2013;56:47-55.
11. Markus R, Huzaira M, Anderson RR, et al. A better potassium hydroxide preparation? Arch Dermatol. 2001;137:1076-1078.
12. Slutsky JB, Rabinovitz H, Grichnik JM, et al. Reflectance confocal microscopic features of dermatophytes, scabies, and Demodex. Arch Dermatol. 2011;147:1008.
13. Hui D, Xue-chang S, Ai-e X. Evaluation of reflectance confocal microscopy in dermatophytosis. Mycoses. 2013;56:130-133.
14. Turan E, Erdemir AT, Gurel MS, et al. A new diagnostic technique for tinea incognito: in vivo reflectance confocal microscopy. report of five cases. Skin Res Technol. 2013;19:e103-e107.
There are a variety of well-established methods for diagnosing dermatophyte infections, including potassium hydroxide (KOH) preparations, fungal cultures, and skin biopsies. Each modality has its place in clinical practice, but they also have drawbacks. Reflectance confocal microscopy (RCM) is an emerging in vivo technology that could potentially serve as a sensitive, rapid, and noninvasive method of diagnosing dermatophytosis. Using near-infrared laser light scanning, RCM provides a quick noninvasive method of generating black-and-white, horizontal, quasipathology images that allow for the identification of cells and other structures similar to dermoscopy and histopathology.1 The images are obtained in a fully noninvasive fashion, as the device is placed in contact with the skin using a liquid medium. The process takes 5 to 15 minutes depending on the number of images obtained, and the images can then be displayed in real time on a computer screen or transmitted to a pathologist for evaluation.
Most initial applications of RCM focused on evaluating melanocytic lesions with the primary goal of differentiating between benign nevi and melanomas, thus reducing the need for skin biopsies.2-4 Efforts to develop RCM diagnostic criteria for identification of other skin cancers5,6 as well as to aid in the diagnosis of nonneoplastic skin conditions are ongoing.7 The potential applications of RCM are virtually limitless, as this modality can (at least partially) take the place of biopsies in a variety of clinical scenarios.2,8 Few reports have documented the utility of RCM as a diagnostic tool for onychomycosis9,10 and dermatophytic infections of the skin.11,12 Hui et al13 reported use for RCM for microscopic evaluation of mycelium features. Turan et al14 found that RCM could not replace the current diagnostic standards for tinea incognito but may be successfully used as an in vivo noninvasive screening tool to facilitate diagnosis. Because it provides high-resolution horizontal images extending from the surface of the stratum corneum to the superficial reticular dermis, RCM could be an effective tool in the diagnosis of cutaneous dermatophyte infections, as organisms usually are located in the stratum corneum of the epidermis in this infection. Branching hyphae are readily visible in the stratum corneum on RCM (Figure).
We reviewed a series of 9 cases from a private practice setting in which RCM was used to diagnose dermatophytosis. We compared the diagnostic accuracy of RCM to results from other diagnostic methods and the ultimate clinical outcome to determine the usefulness of this new technology.
Methods
Our retrospective chart review included all cases in which RCM was used and the clinical differential diagnosis included tinea corporis over a 4-month period in a private, single-specialty dermatology practice. All patients were treated by the same dermatologist. The RCM images were taken using an imaging system that had a horizontal optical resolution of less than 1.25 μm and a vertical optical resolution of less than 5.0 μm. The imaging was performed by medical assistants who were trained by the device manufacturer.
The sample sites were cleaned with isopropyl alcohol and a translucent contact ring was affixed to the skin using a liquid medium. The imaging head of the device was connected to the imaging ring and the images were taken. Identical imaging protocol was used in all patients. Multiple sets of horizontal images and one stack of vertical images were obtained. Patients reported no discomfort during the procedure, and the entire process was usually completed within 15 minutes. The images were sent to the pathologist for evaluation using the manufacturer’s telepathology system and were returned with a diagnosis within 24 hours. (On-site, real-time diagnosis also is possible if the dermatologist is trained in interpreting the images.)
In the chart review we looked for other diagnostic methods used as well as clinical outcomes. A case was considered to be positive for dermatophytic infection if any of the other diagnostic modalities yielded positive results or if a definitive resolution of the condition could be achieved using antifungal treatments alone.
Results
Ten patients (mean age, 43.1 years; age range, 16–76 years) with lesions that presented as possible dermatophytic infections underwent RCM analysis. In addition to RCM imaging, 5 patients underwent KOH testing of skin scrapings, 3 underwent analysis by fungal culture utilizing dermatophyte test medium (DTM), and 5 underwent biopsies. The findings are further summarized in the Table. One patient (patient 5) was excluded from the study because the RCM could not be evaluated due to the poor quality of the confocal images. Additionally, 2 patients (patients 2 and 7) had suboptimal imaging, which limited the evaluation.
Of the 9 evaluable cases, 4 (patients 1–4) were determined to be positive for the presence of dermatophytic infection through the fulfillment of criteria independent of RCM imaging. In each of those 4 cases, RCM images revealed the presence of hyphae, which indicated the presence of dermato-phytic infection. In these 4 cases, RCM and other diagnostic methods reached the same diagnosis.
In the other 5 cases (patients 6–10), the final diagnosis was not a dermatophytic infection. In 4 of those cases (patients 7–10), there were no signs of any structure resembling hyphae on the RCM images; however, in 1 case (patient 6), the RCM images showed structures that were consistent with the appearance of hyphae to the extent that the investigators, based solely on analysis of the RCM images, deemed a diagnosis indicating presence of a dermatophytic infection to be valid. In this case, a 38-year-old man presented with extensive scaly patches on the back of several months’ duration. Repeated skin biopsies showed hyperkeratosis and occasionally minimal spongiosis, while periodic acid–Schiff staining did not reveal fungal elements. Fungal cultures and KOH preparations were negative. Prior treatments with topical antifungals and steroids failed to improve the condition, which resolved rapidly with urea cream 40%. The interpretation of the RCM images in this patient did not match up with the results obtained from other methods of diagnosis and the clinical outcome; thus, we classified it as an incorrect diagnosis based on RCM analysis alone. In total, successful diagnosis using RCM imaging was achieved in 8 of 9 cases included in the analysis.
Comment
In this chart review, we evaluated the utility of using RCM in the diagnosis of dermatophytic infections of the skin by comparing findings noted on confocal imaging with those of other methods of diagnosis (Table). We included cases in which the clinical presentation raised the possibility of dermatophytic infection. Cases were considered positive for dermatophytes if KOH preparation, fungal culture, or skin biopsy (with or without periodic acid–Schiff staining) were positive or if there was a complete response to antifungal treatment alone. In this small number of cases, we found that RCM was 100% sensitive, as hyphae were readily seen in all cases of dermatophytic infections. In 1 RCM-positive case (patient 3), fungal culture with DTM was negative, but antifungal therapy was nonetheless given. Because the lesion resolved promptly with econazole, RCM proved to be true positive and DTM proved to be false negative (Table). Reflectance confocal microscopy imaging, however, was less specific. Of the 5 cases that showed no presence of dermatophytic infection, there was 1 case (patient 6) in which the pathologist could recognize structures that resembled fungal hyphae. There are various possible sources of structures masquerading as dermatophytes on confocal imaging, including the edges of nonnucleated loose keratinocytes, keratin fragments, and other foreign fibers. Evaluation by an experienced investigator can certainly help in limiting false-positive analyses, but a larger case study would be useful to develop a set of specific criteria to aid in the differentiation of fungal hyphae from other artifacts as well as to further define the sensitivity and specificity of RCM.
We also encountered difficulties with the technical aspects of RCM. One case (patient 5) was excluded from the analysis because the images were poor quality and could not be interpreted, and 2 cases (patients 2 and 7) had suboptimal images, in part due to operator error and in part due to equipment error that was recognized later on. The technical difficulties were problematic because no immediate review of image quality was available while patients were still present for possible reimaging. All of the images evaluated in this study were captured shortly after the RCM device was introduced to the practice. It is possible that with more training and a quick, on-site review of image quality, these technical problems could be avoided. Imaging protocols (ie, numbers and levels of scans taken by the confocal microscope) also could be adjusted so they include a large enough range to compensate for potential operator errors; however, these adjustments also could increase overall imaging time.
Conclusion
Based on our chart review of a small number of cases, we found that RCM can be a useful tool in diagnosing dermatophytic infections of the skin. With adequate training, dermatologists may be able to use RCM as an in-office tool to capture and evaluate images and subsequently diagnose or exclude dermatophytosis in a quick and noninvasive manner. However, further research and controlled studies of more cases will be required to develop accurate criteria for diagnosing fungal structures by RCM as well as to help determine the role of RCM in our diagnostic armamentarium.
There are a variety of well-established methods for diagnosing dermatophyte infections, including potassium hydroxide (KOH) preparations, fungal cultures, and skin biopsies. Each modality has its place in clinical practice, but they also have drawbacks. Reflectance confocal microscopy (RCM) is an emerging in vivo technology that could potentially serve as a sensitive, rapid, and noninvasive method of diagnosing dermatophytosis. Using near-infrared laser light scanning, RCM provides a quick noninvasive method of generating black-and-white, horizontal, quasipathology images that allow for the identification of cells and other structures similar to dermoscopy and histopathology.1 The images are obtained in a fully noninvasive fashion, as the device is placed in contact with the skin using a liquid medium. The process takes 5 to 15 minutes depending on the number of images obtained, and the images can then be displayed in real time on a computer screen or transmitted to a pathologist for evaluation.
Most initial applications of RCM focused on evaluating melanocytic lesions with the primary goal of differentiating between benign nevi and melanomas, thus reducing the need for skin biopsies.2-4 Efforts to develop RCM diagnostic criteria for identification of other skin cancers5,6 as well as to aid in the diagnosis of nonneoplastic skin conditions are ongoing.7 The potential applications of RCM are virtually limitless, as this modality can (at least partially) take the place of biopsies in a variety of clinical scenarios.2,8 Few reports have documented the utility of RCM as a diagnostic tool for onychomycosis9,10 and dermatophytic infections of the skin.11,12 Hui et al13 reported use for RCM for microscopic evaluation of mycelium features. Turan et al14 found that RCM could not replace the current diagnostic standards for tinea incognito but may be successfully used as an in vivo noninvasive screening tool to facilitate diagnosis. Because it provides high-resolution horizontal images extending from the surface of the stratum corneum to the superficial reticular dermis, RCM could be an effective tool in the diagnosis of cutaneous dermatophyte infections, as organisms usually are located in the stratum corneum of the epidermis in this infection. Branching hyphae are readily visible in the stratum corneum on RCM (Figure).
We reviewed a series of 9 cases from a private practice setting in which RCM was used to diagnose dermatophytosis. We compared the diagnostic accuracy of RCM to results from other diagnostic methods and the ultimate clinical outcome to determine the usefulness of this new technology.
Methods
Our retrospective chart review included all cases in which RCM was used and the clinical differential diagnosis included tinea corporis over a 4-month period in a private, single-specialty dermatology practice. All patients were treated by the same dermatologist. The RCM images were taken using an imaging system that had a horizontal optical resolution of less than 1.25 μm and a vertical optical resolution of less than 5.0 μm. The imaging was performed by medical assistants who were trained by the device manufacturer.
The sample sites were cleaned with isopropyl alcohol and a translucent contact ring was affixed to the skin using a liquid medium. The imaging head of the device was connected to the imaging ring and the images were taken. Identical imaging protocol was used in all patients. Multiple sets of horizontal images and one stack of vertical images were obtained. Patients reported no discomfort during the procedure, and the entire process was usually completed within 15 minutes. The images were sent to the pathologist for evaluation using the manufacturer’s telepathology system and were returned with a diagnosis within 24 hours. (On-site, real-time diagnosis also is possible if the dermatologist is trained in interpreting the images.)
In the chart review we looked for other diagnostic methods used as well as clinical outcomes. A case was considered to be positive for dermatophytic infection if any of the other diagnostic modalities yielded positive results or if a definitive resolution of the condition could be achieved using antifungal treatments alone.
Results
Ten patients (mean age, 43.1 years; age range, 16–76 years) with lesions that presented as possible dermatophytic infections underwent RCM analysis. In addition to RCM imaging, 5 patients underwent KOH testing of skin scrapings, 3 underwent analysis by fungal culture utilizing dermatophyte test medium (DTM), and 5 underwent biopsies. The findings are further summarized in the Table. One patient (patient 5) was excluded from the study because the RCM could not be evaluated due to the poor quality of the confocal images. Additionally, 2 patients (patients 2 and 7) had suboptimal imaging, which limited the evaluation.
Of the 9 evaluable cases, 4 (patients 1–4) were determined to be positive for the presence of dermatophytic infection through the fulfillment of criteria independent of RCM imaging. In each of those 4 cases, RCM images revealed the presence of hyphae, which indicated the presence of dermato-phytic infection. In these 4 cases, RCM and other diagnostic methods reached the same diagnosis.
In the other 5 cases (patients 6–10), the final diagnosis was not a dermatophytic infection. In 4 of those cases (patients 7–10), there were no signs of any structure resembling hyphae on the RCM images; however, in 1 case (patient 6), the RCM images showed structures that were consistent with the appearance of hyphae to the extent that the investigators, based solely on analysis of the RCM images, deemed a diagnosis indicating presence of a dermatophytic infection to be valid. In this case, a 38-year-old man presented with extensive scaly patches on the back of several months’ duration. Repeated skin biopsies showed hyperkeratosis and occasionally minimal spongiosis, while periodic acid–Schiff staining did not reveal fungal elements. Fungal cultures and KOH preparations were negative. Prior treatments with topical antifungals and steroids failed to improve the condition, which resolved rapidly with urea cream 40%. The interpretation of the RCM images in this patient did not match up with the results obtained from other methods of diagnosis and the clinical outcome; thus, we classified it as an incorrect diagnosis based on RCM analysis alone. In total, successful diagnosis using RCM imaging was achieved in 8 of 9 cases included in the analysis.
Comment
In this chart review, we evaluated the utility of using RCM in the diagnosis of dermatophytic infections of the skin by comparing findings noted on confocal imaging with those of other methods of diagnosis (Table). We included cases in which the clinical presentation raised the possibility of dermatophytic infection. Cases were considered positive for dermatophytes if KOH preparation, fungal culture, or skin biopsy (with or without periodic acid–Schiff staining) were positive or if there was a complete response to antifungal treatment alone. In this small number of cases, we found that RCM was 100% sensitive, as hyphae were readily seen in all cases of dermatophytic infections. In 1 RCM-positive case (patient 3), fungal culture with DTM was negative, but antifungal therapy was nonetheless given. Because the lesion resolved promptly with econazole, RCM proved to be true positive and DTM proved to be false negative (Table). Reflectance confocal microscopy imaging, however, was less specific. Of the 5 cases that showed no presence of dermatophytic infection, there was 1 case (patient 6) in which the pathologist could recognize structures that resembled fungal hyphae. There are various possible sources of structures masquerading as dermatophytes on confocal imaging, including the edges of nonnucleated loose keratinocytes, keratin fragments, and other foreign fibers. Evaluation by an experienced investigator can certainly help in limiting false-positive analyses, but a larger case study would be useful to develop a set of specific criteria to aid in the differentiation of fungal hyphae from other artifacts as well as to further define the sensitivity and specificity of RCM.
We also encountered difficulties with the technical aspects of RCM. One case (patient 5) was excluded from the analysis because the images were poor quality and could not be interpreted, and 2 cases (patients 2 and 7) had suboptimal images, in part due to operator error and in part due to equipment error that was recognized later on. The technical difficulties were problematic because no immediate review of image quality was available while patients were still present for possible reimaging. All of the images evaluated in this study were captured shortly after the RCM device was introduced to the practice. It is possible that with more training and a quick, on-site review of image quality, these technical problems could be avoided. Imaging protocols (ie, numbers and levels of scans taken by the confocal microscope) also could be adjusted so they include a large enough range to compensate for potential operator errors; however, these adjustments also could increase overall imaging time.
Conclusion
Based on our chart review of a small number of cases, we found that RCM can be a useful tool in diagnosing dermatophytic infections of the skin. With adequate training, dermatologists may be able to use RCM as an in-office tool to capture and evaluate images and subsequently diagnose or exclude dermatophytosis in a quick and noninvasive manner. However, further research and controlled studies of more cases will be required to develop accurate criteria for diagnosing fungal structures by RCM as well as to help determine the role of RCM in our diagnostic armamentarium.
1. Longo C, Farnetani F, Ciardo S, et al. Is confocal microscopy a valuable tool in diagnosing nodular lesions? a study of 140 cases. Br J Dermatol. 2013;169:58-67.
2. Debarbieux S, Dalle S, Depaepe L, et al. Second primary melanomas under BRAF blockers: study by reflectance confocal microscopy [published online a head of print April 1, 2013]. Br J Dermatol. 2013;168:1230-1235.
3. Schwartz RJ, Vera K, Navarrete N, et al. In vivo reflectance confocal microscopy of halo nevus. J Cutan Med Surg. 2013;17:33-38.
4. Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
5. Nori S, Rius-Diaz F, Cuevas J, et al. Sensitivity and specificity of reflectance-mode confocal micros-copy for in vivo diagnosis of basal cell carcinoma: a multicenter study. J Am Acad Dermatol. 2004;51:923-930.
6. Arzberger E, Komericki P, Ahlgrimm-Siess V, et al. Differentiation between balanitis and carcinoma in situ using reflectance confocal microscopy. JAMA Dermatol. 2013;149:440-445.
7. Ardigò M, Maliszewski I, Cota C, et al. Preliminary evaluation of in vivo reflectance confocal microscopy features of discoid lupus erythematosus. Br J Dermatol. 2007;156:1196-1203.
8. Longo C, Casari A, Pepe P, et al. Confocal microscopy insights into the treatment and cellular immune response of Basal cell carcinoma to photodynamic therapy. Dermatology. 2012;225:264-270.
9. Hongcharu W, Dwyer P, Gonzalez S, et al. Confirmation of onychomycosis by in vivo confocal microscopy. J Am Acad Dermatol. 2000;42:214-216.
10. Rothmund G, Sattler EC, Kaestle R, et al. Confocal laser scanning microscopy as a new valuable tool in the diagnosis of onychomycosis—comparison of six diagnostic methods [published online ahead of print April 23, 2012]. Mycoses. 2013;56:47-55.
11. Markus R, Huzaira M, Anderson RR, et al. A better potassium hydroxide preparation? Arch Dermatol. 2001;137:1076-1078.
12. Slutsky JB, Rabinovitz H, Grichnik JM, et al. Reflectance confocal microscopic features of dermatophytes, scabies, and Demodex. Arch Dermatol. 2011;147:1008.
13. Hui D, Xue-chang S, Ai-e X. Evaluation of reflectance confocal microscopy in dermatophytosis. Mycoses. 2013;56:130-133.
14. Turan E, Erdemir AT, Gurel MS, et al. A new diagnostic technique for tinea incognito: in vivo reflectance confocal microscopy. report of five cases. Skin Res Technol. 2013;19:e103-e107.
1. Longo C, Farnetani F, Ciardo S, et al. Is confocal microscopy a valuable tool in diagnosing nodular lesions? a study of 140 cases. Br J Dermatol. 2013;169:58-67.
2. Debarbieux S, Dalle S, Depaepe L, et al. Second primary melanomas under BRAF blockers: study by reflectance confocal microscopy [published online a head of print April 1, 2013]. Br J Dermatol. 2013;168:1230-1235.
3. Schwartz RJ, Vera K, Navarrete N, et al. In vivo reflectance confocal microscopy of halo nevus. J Cutan Med Surg. 2013;17:33-38.
4. Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
5. Nori S, Rius-Diaz F, Cuevas J, et al. Sensitivity and specificity of reflectance-mode confocal micros-copy for in vivo diagnosis of basal cell carcinoma: a multicenter study. J Am Acad Dermatol. 2004;51:923-930.
6. Arzberger E, Komericki P, Ahlgrimm-Siess V, et al. Differentiation between balanitis and carcinoma in situ using reflectance confocal microscopy. JAMA Dermatol. 2013;149:440-445.
7. Ardigò M, Maliszewski I, Cota C, et al. Preliminary evaluation of in vivo reflectance confocal microscopy features of discoid lupus erythematosus. Br J Dermatol. 2007;156:1196-1203.
8. Longo C, Casari A, Pepe P, et al. Confocal microscopy insights into the treatment and cellular immune response of Basal cell carcinoma to photodynamic therapy. Dermatology. 2012;225:264-270.
9. Hongcharu W, Dwyer P, Gonzalez S, et al. Confirmation of onychomycosis by in vivo confocal microscopy. J Am Acad Dermatol. 2000;42:214-216.
10. Rothmund G, Sattler EC, Kaestle R, et al. Confocal laser scanning microscopy as a new valuable tool in the diagnosis of onychomycosis—comparison of six diagnostic methods [published online ahead of print April 23, 2012]. Mycoses. 2013;56:47-55.
11. Markus R, Huzaira M, Anderson RR, et al. A better potassium hydroxide preparation? Arch Dermatol. 2001;137:1076-1078.
12. Slutsky JB, Rabinovitz H, Grichnik JM, et al. Reflectance confocal microscopic features of dermatophytes, scabies, and Demodex. Arch Dermatol. 2011;147:1008.
13. Hui D, Xue-chang S, Ai-e X. Evaluation of reflectance confocal microscopy in dermatophytosis. Mycoses. 2013;56:130-133.
14. Turan E, Erdemir AT, Gurel MS, et al. A new diagnostic technique for tinea incognito: in vivo reflectance confocal microscopy. report of five cases. Skin Res Technol. 2013;19:e103-e107.
Practice Points
- Current methods for diagnosing dermatophytosis can be invasive, with variable sensitivity and/or slow turnaround time.
- Reflectance confocal microscopy is a promising option for rapid noninvasive diagnosis of dermatophytosis.