User login
To the Editor: I read with interest the review of the new oral anticoagulants by Fawole et al1 and agree with their comments on the prevention of bleeding and the importance of monitoring renal function in managing patients on the new classes of oral anticoagulants. However, no specifics were given on how to proceed. Thus, I recommend that renal risk stratification be done before and 1 week after starting these new drugs.
Originally, the US Food and Drug Administration approved dabigatran (Pradaxa) at a dose of 150 mg orally twice daily in patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2. This dosing corresponded to the estimated glomerular filtration rate (eGFR) in patients with stage 4 chronic kidney disease, but this dosing is contraindicated in other guidelines worldwide (Canada, Europe, the United Kingdom, Japan, Australia, and New Zealand).2 Not unexpectedly, 3,781 serious adverse effects were noted in the 2011 US postmarketing experience with dabigatran. These included death (542 cases), hemorrhage (2,367 cases), acute renal failure (291 cases), stroke (644 cases), and suspected liver failure (15 cases).3 Thirteen months after dabigatran’s approval in the United States, Boehringer Ingelheim changed the dosage and product guidelines.2–4 The new dosage4 is 75 mg twice daily for patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2.
Therefore, I suggest a nephrologic “way out”5 when using the new oral anticoagulants to avoid the problems with dabigatran noted above.
First, if these drugs are to be used in nonvalvular atrial fibrillation, risk factors should be determined using the CHADS2 or the CHADS2-VASc score. Special attention should be given to patients age 75 and older, women, and patients with a history of stroke, transient ischemic attack, or systemic embolism. All of these have been noted to be major risk factors.6,7
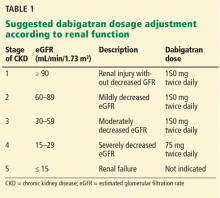
Second, renal risk stratification8 should be done using a comprehensive metabolic panel before and 1 week after starting new oral anticoagulants, or if there is a change in the patient’s clinical condition. Most US laboratories now provide an eGFR and the stage of chronic kidney disease.3,5 For example (Table 1), if dabigatran is used, one should follow current dosing guidelines for chronic kidney disease stages 1 through 3, ie, 150 mg twice daily. If stage 4 chronic kidney disease is detected (creatinine clearance 15–29 mL/min/1.73 m2), the updated recommended dosage is 75 mg twice daily. If stage 5 is noted (eGFR ≤ 15 mL/min/1.73 m2), dabigatran is not indicated. Similar steps can be done using current guidelines for the other new oral anticoagulants.
This simple renal risk stratification guideline should help avoid some of the problems noted in the dabigatran postmarketing experience, which were aggravated by the lack of approval of a 110-mg dose and by misleading advertising, claiming that no blood monitoring was required.2–5 Thus, the new oral anticoagulants should be a welcome addition to our armamentarium in patients who need them, and we hope to avoid the risks, morbidity, mortality, and expense of trying to reverse adverse effects.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants, dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013, 80:443–451.
- Pazmiño PA. Dabigatran associated acute renal failure (DAARF). El Paso Physician 2011; 34:7–9.
- Pazmiño PA. Adverse effects of dabigatran (Letter). Ann Intern Med 2012; 157:916.
- Pradaxa (prescribing information). Ridgefield, CT. Boehringer Ingelheim Pharmaceuticals 2011.
- Pazmiño PA. Dabigatran: a nephrological way out. Am J Med 2013; 126;e21–e22.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. The Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- Reinecke H, Brand E, Mesters R, et al. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol 2009; 20:705–711.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic disease: evaluation, classification and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
To the Editor: I read with interest the review of the new oral anticoagulants by Fawole et al1 and agree with their comments on the prevention of bleeding and the importance of monitoring renal function in managing patients on the new classes of oral anticoagulants. However, no specifics were given on how to proceed. Thus, I recommend that renal risk stratification be done before and 1 week after starting these new drugs.
Originally, the US Food and Drug Administration approved dabigatran (Pradaxa) at a dose of 150 mg orally twice daily in patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2. This dosing corresponded to the estimated glomerular filtration rate (eGFR) in patients with stage 4 chronic kidney disease, but this dosing is contraindicated in other guidelines worldwide (Canada, Europe, the United Kingdom, Japan, Australia, and New Zealand).2 Not unexpectedly, 3,781 serious adverse effects were noted in the 2011 US postmarketing experience with dabigatran. These included death (542 cases), hemorrhage (2,367 cases), acute renal failure (291 cases), stroke (644 cases), and suspected liver failure (15 cases).3 Thirteen months after dabigatran’s approval in the United States, Boehringer Ingelheim changed the dosage and product guidelines.2–4 The new dosage4 is 75 mg twice daily for patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2.
Therefore, I suggest a nephrologic “way out”5 when using the new oral anticoagulants to avoid the problems with dabigatran noted above.
First, if these drugs are to be used in nonvalvular atrial fibrillation, risk factors should be determined using the CHADS2 or the CHADS2-VASc score. Special attention should be given to patients age 75 and older, women, and patients with a history of stroke, transient ischemic attack, or systemic embolism. All of these have been noted to be major risk factors.6,7
Second, renal risk stratification8 should be done using a comprehensive metabolic panel before and 1 week after starting new oral anticoagulants, or if there is a change in the patient’s clinical condition. Most US laboratories now provide an eGFR and the stage of chronic kidney disease.3,5 For example (Table 1), if dabigatran is used, one should follow current dosing guidelines for chronic kidney disease stages 1 through 3, ie, 150 mg twice daily. If stage 4 chronic kidney disease is detected (creatinine clearance 15–29 mL/min/1.73 m2), the updated recommended dosage is 75 mg twice daily. If stage 5 is noted (eGFR ≤ 15 mL/min/1.73 m2), dabigatran is not indicated. Similar steps can be done using current guidelines for the other new oral anticoagulants.
This simple renal risk stratification guideline should help avoid some of the problems noted in the dabigatran postmarketing experience, which were aggravated by the lack of approval of a 110-mg dose and by misleading advertising, claiming that no blood monitoring was required.2–5 Thus, the new oral anticoagulants should be a welcome addition to our armamentarium in patients who need them, and we hope to avoid the risks, morbidity, mortality, and expense of trying to reverse adverse effects.
To the Editor: I read with interest the review of the new oral anticoagulants by Fawole et al1 and agree with their comments on the prevention of bleeding and the importance of monitoring renal function in managing patients on the new classes of oral anticoagulants. However, no specifics were given on how to proceed. Thus, I recommend that renal risk stratification be done before and 1 week after starting these new drugs.
Originally, the US Food and Drug Administration approved dabigatran (Pradaxa) at a dose of 150 mg orally twice daily in patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2. This dosing corresponded to the estimated glomerular filtration rate (eGFR) in patients with stage 4 chronic kidney disease, but this dosing is contraindicated in other guidelines worldwide (Canada, Europe, the United Kingdom, Japan, Australia, and New Zealand).2 Not unexpectedly, 3,781 serious adverse effects were noted in the 2011 US postmarketing experience with dabigatran. These included death (542 cases), hemorrhage (2,367 cases), acute renal failure (291 cases), stroke (644 cases), and suspected liver failure (15 cases).3 Thirteen months after dabigatran’s approval in the United States, Boehringer Ingelheim changed the dosage and product guidelines.2–4 The new dosage4 is 75 mg twice daily for patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2.
Therefore, I suggest a nephrologic “way out”5 when using the new oral anticoagulants to avoid the problems with dabigatran noted above.
First, if these drugs are to be used in nonvalvular atrial fibrillation, risk factors should be determined using the CHADS2 or the CHADS2-VASc score. Special attention should be given to patients age 75 and older, women, and patients with a history of stroke, transient ischemic attack, or systemic embolism. All of these have been noted to be major risk factors.6,7
Second, renal risk stratification8 should be done using a comprehensive metabolic panel before and 1 week after starting new oral anticoagulants, or if there is a change in the patient’s clinical condition. Most US laboratories now provide an eGFR and the stage of chronic kidney disease.3,5 For example (Table 1), if dabigatran is used, one should follow current dosing guidelines for chronic kidney disease stages 1 through 3, ie, 150 mg twice daily. If stage 4 chronic kidney disease is detected (creatinine clearance 15–29 mL/min/1.73 m2), the updated recommended dosage is 75 mg twice daily. If stage 5 is noted (eGFR ≤ 15 mL/min/1.73 m2), dabigatran is not indicated. Similar steps can be done using current guidelines for the other new oral anticoagulants.
This simple renal risk stratification guideline should help avoid some of the problems noted in the dabigatran postmarketing experience, which were aggravated by the lack of approval of a 110-mg dose and by misleading advertising, claiming that no blood monitoring was required.2–5 Thus, the new oral anticoagulants should be a welcome addition to our armamentarium in patients who need them, and we hope to avoid the risks, morbidity, mortality, and expense of trying to reverse adverse effects.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants, dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013, 80:443–451.
- Pazmiño PA. Dabigatran associated acute renal failure (DAARF). El Paso Physician 2011; 34:7–9.
- Pazmiño PA. Adverse effects of dabigatran (Letter). Ann Intern Med 2012; 157:916.
- Pradaxa (prescribing information). Ridgefield, CT. Boehringer Ingelheim Pharmaceuticals 2011.
- Pazmiño PA. Dabigatran: a nephrological way out. Am J Med 2013; 126;e21–e22.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. The Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- Reinecke H, Brand E, Mesters R, et al. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol 2009; 20:705–711.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic disease: evaluation, classification and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants, dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013, 80:443–451.
- Pazmiño PA. Dabigatran associated acute renal failure (DAARF). El Paso Physician 2011; 34:7–9.
- Pazmiño PA. Adverse effects of dabigatran (Letter). Ann Intern Med 2012; 157:916.
- Pradaxa (prescribing information). Ridgefield, CT. Boehringer Ingelheim Pharmaceuticals 2011.
- Pazmiño PA. Dabigatran: a nephrological way out. Am J Med 2013; 126;e21–e22.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. The Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- Reinecke H, Brand E, Mesters R, et al. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol 2009; 20:705–711.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic disease: evaluation, classification and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.