User login
In the past several years, three new oral anticoagulants—dabigatran etexilate (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis)—have been approved for use in the United States. These long-awaited agents are appealing because they are easy to use, do not require laboratory monitoring, and have demonstrated equivalence, or in some cases, superiority to warfarin in preventing stroke or systemic embolism in at-risk populations.1–4 However, unlike warfarin, they have no specific reversal agents. How then should one manage spontaneous bleeding problems and those due to drug overdose, and how can we quickly reverse anticoagulation if emergency surgery is needed?
For these reasons, physicians and patients have been wary of these agents. However, with a systematic approach based on an understanding of the properties of these drugs, the appropriate use and interpretation of coagulation tests, and awareness of available therapeutic strategies, physicians can more confidently provide care for patients who require urgent reversal of anticoagulant effects.
Here, we review the available literature and suggest practical strategies for management based on an understanding of the pharmacokinetic and pharmacodynamic effects of these drugs and our current knowledge of the coagulation tests.
NEED FOR ANTICOAGULANTS
Anticoagulants are important in preventing systemic embolization in patients with atrial fibrillation and preventing pulmonary embolism in patients with venous thromboembolism.
And the numbers are staggering. The estimated prevalence of atrial fibrillation in the United States was 3.03 million in 2005 and is projected to increase to 7.56 million by 2050.5 Ischemic stroke is the most serious complication of atrial fibrillation, which accounts for 23.5% of strokes in patients ages 80 through 89 according to Framingham data.6 Venous thromboembolism accounts for 900,000 incident or recurrent fatal and nonfatal events in the United States yearly.7
HOW THE NEW AGENTS BLOCK COAGULATION
Thrombin (factor IIa), a serine protease, is central to the process of clot formation during hemostasis. It activates factors V, VIII, and XI (thus generating more thrombin), catalyzes the conversion of fibrinogen to fibrin, and stimulates platelet aggregation. Its role in the final steps of the coagulation cascade has made it a target for new direct thrombin inhibitors such as dabigatran.
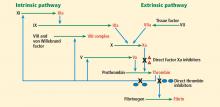
Factor Xa is a serine protease that plays a central role in the coagulation cascade. It is a desirable target for anticoagulation because it is the convergence point for the extrinsic and the intrinsic coagulation pathways. It converts prothrombin to thrombin. Rivaroxaban and apixaban are direct factor Xa inhibitors (Figure 1).
Dabigatran, a direct thrombin inhibitor
Dabigatran etexilate is a synthetic, orally available prodrug that is rapidly absorbed and converted by esterases to its active form, dabigatran, a potent direct inhibitor of both free thrombin and clot-bound thrombin.8
Plasma levels of dabigatran peak within 2 hours of administration, and its half-life is 14 to 17 hours.9 Dabigatran is eliminated mainly via the kidneys, with more that 80% of the drug excreted unchanged in the urine (Table 1).
Rivaroxaban, a factor Xa inhibitor
Rivaroxaban is a potent, selective, direct factor Xa inhibitor.
Plasma levels of rivaroxaban peak 2 to 3 hours after administration, and it is cleared with a terminal half-life of 7 to 11 hours.10,11
Rivaroxaban is eliminated by the kidneys and in the feces. The kidneys eliminate one-third of the active drug unchanged and another one-third as inactive metabolites. The remaining one-third is metabolized by the liver and then excreted in the feces. Rivaroxaban has a predictable and dose-dependent pharmacodynamic and pharmacokinetic profile that is not affected by age, sex, or body weight (Table 1).12
Apixaban, an oral factor Xa inhibitor
Apixaban is a selective, direct oral factor Xa inhibitor.
Plasma levels of apixaban peak about 3 hours after administration, and its terminal half-life is 8 to 14 hours.13 Apixaban is eliminated by oxidative metabolism, by the kidney, and in the feces. It has predictable pharmacodynamic and pharmacokinetic profiles and has the least renal dependence of the three agents (Table 1).
THE NEW ORAL ANTICOAGULANTS AND BLOOD COAGULATION ASSAYS
Assessment of the anticoagulant activity of the new oral anticoagulants is not necessary in routine clinical practice, but it may be useful in planning intervention in patients with major bleeding, those with drug overdose, or those who need emergency surgery.
The activated partial thromboplastin time
The activated partial thromboplastin time (aPTT) is a measure of the activity of the intrinsic pathway of the coagulation cascade.
Dabigatran. There is a curvilinear relationship between the aPTT and the plasma concentration of dabigatran and other direct thrombin inhibitors, although the aPTT prolongation appears to vary with different reagents and coagulometers.9,14,15 However, Stangier et al9 found a linear relationship between the aPTT and the square root of the dabigatran plasma concentration.
Rivaroxaban prolongs the aPTT in a dose-dependent manner, but there is no standard for calibration of this assay. Hence, the aPTT is not recommended for monitoring rivaroxaban in clinical practice.
Apixaban may also prolong the aPTT, but there are limited data on its reactivity with different reagents.
The prothrombin time and international normalized ratio
The prothrombin time and international normalized ratio (INR) are measures of the extrinsic pathway of the coagulation cascade.
Dabigatran. The INR has a linear response to the dabigatran concentration, but it is insensitive.9 Hence, it is not suitable for monitoring the anticoagulant effects of direct thrombin inhibitors.
Rivaroxaban. The prothrombin time correlates strongly with the plasma concentration of rivaroxaban in healthy trial participants11 and in patients undergoing total hip arthroplasty or total knee arthroplasty.16 Samama et al17 noted that, unlike with vitamin K antagonists, the INR cannot be used to monitor patients on rivaroxaban because the prothrombin time results varied with different reagents. They used a standard calibration curve to express the prothrombin time results in plasma concentrations of rivaroxaban rather than in seconds or the INR.
Apixaban increases the INR in a dose-dependent manner.18 Its effect on different reagents remains unknown.
The thrombin time
The thrombin time reflects the activity of thrombin in the plasma. The amount of thrombin and the concentration of thrombin inhibitors in the plasma sample determine the time to clot formation.
Dabigatran. The thrombin time displays a linear dose-response to dabigatran, but only over the range of therapeutic concentrations. At a dabigatran concentration greater than 600 ng/mL, the test often exceeds the maximum measurement time of coagulometers.9 Hence, this test is too sensitive for emergency monitoring, especially in cases of drug overdose. However, it is well suited for determining if any dabigatran is present.
Rivaroxaban and apixaban have no effect on the thrombin time.
The Hemoclot direct thrombin inhibitor assay and dabigatran
The Hemoclot direct thrombin inhibitor assay (Hyphen BioMed, France) is a sensitive diluted thrombin time assay that can be used for quantitative measurement of dabigatran activity in plasma. This test is based on inhibition of a constant amount of highly purified human alpha-thrombin by adding it to diluted test plasma (1:8 to 1:20) mixed with normal pooled human plasma.19,20
Stangier et al19 found that the Hemoclot assay was suitable for calculating a wide range of dabigatran concentrations up to 4,000 nmol/L (1,886 ng/mL). Although this finding has not been confirmed in larger studies, this test may provide a rapid and accurate assessment of dabigatran’s anticoagulant activity in cases of emergency surgery or overdose.
The ecarin clotting time and dabigatran
The ecarin clotting time is a measure of the activity of direct thrombin inhibitors, but not the factor Xa inhibitors.
Ecarin is a highly purified metalloprotease isolated from the venom of a snake, Echis carinatus, and it generates meizothrombin from prothrombin.21 Meizothrombin facilitates clot formation by converting fibrinogen to fibrin and, like thrombin, it can be inactivated by direct thrombin inhibitors, thereby prolonging the clotting time.
The limitations of the ecarin clotting time include dependence on the plasma levels of fibrinogen and prothrombin.
The ecarin chromogenic assay and dabigatran
The ecarin chromogenic assay is an improvement on the principle of the ecarin clotting time that can be used to measure the activity of direct thrombin inhibitors.22 In this test, ecarin is added to a plasma sample to generate meizothrombin, and the amidolytic activity of meizothrombin towards a chromogenic substrate is then determined.
Results of the ecarin chromogenic assay are not influenced by the levels of fibrinogen or prothrombin. Another advantage is that this assay can be used in automated and manual analyzers, thus enabling its use at the bedside. However, to our knowledge, it is not being regularly used to monitor direct thrombin inhibitors in the clinical setting, and there is no standard calibration of the ecarin clotting time method.
Assays of factor Xa activity
A variety of assays to monitor the anticoagulant activity of factor Xa inhibitors have been proposed.23–25 All measure inhibition of the activity of factor Xa using methods similar to those used in monitoring heparin levels. All require calibrators with a known concentration of the Xa inhibitor; many are easily adapted for laboratories currently providing measurement of factor Xa inhibition from heparin.23 These assays have been suggested as a better indicator of plasma concentration of factor Xa inhibitor drugs than the prothrombin time.25
CONTROLLING BLEEDING IN PATIENTS ON THE NEW ORAL ANTICOAGULANTS
Bleeding is an anticipated adverse event in patients taking anticoagulants. It is associated with significant morbidity and risk of death.26,27
Many physicians still have limited experience with using the new oral anticoagulants and managing the attendant bleeding risks. Hence, we recommend that every health institution have a treatment policy or algorithm to guide all clinical staff in the management of such emergencies.
Prevention of bleeding
Management of bleeding from these agents should begin with preventing bleeding in the first place.
The physician should adhere to the recommended dosages of these medications. Studies have shown that the plasma concentration of these drugs and the risk of bleeding increase with increasing dosage.1,28,29
In addition, these medications should be used for the shortest time for which anticoagulation is required, especially when used for preventing deep vein thrombosis. Prolonged use increases the risk of bleeding.30,31
Most patients who need anticoagulation have comorbidities such as heart failure, renal failure, diabetes mellitus, and hypertension. Although the kidneys play a major role in the excretion of dabigatran and, to some extent, rivaroxaban and apixaban, patients with severe renal impairment were excluded from the major trials of all three drugs.1–3 Hence, to avoid excessive drug accumulation and bleeding, these medications should not be used in such patients pending further studies. Further, patients taking these medications should be closely followed to detect new clinical situations, such as acute renal failure, that will necessitate their discontinuation or dose adjustment.
If surgery is needed
If a patient taking a new oral anticoagulant needs to undergo elective surgery, it is important to temporarily discontinue the drug, assess the risk of bleeding, and test for renal impairment.
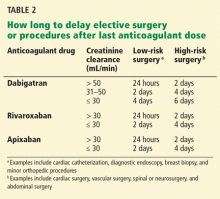
Renal impairment is particularly relevant in the case of dabigatran, since more than 80% of the unchanged drug is cleared by the kidneys. Decreasing the dose, prolonging the dosing interval, or both have been suggested as means to reduce the risk of bleeding in patients with renal impairment who are taking dabigatran.32,33 Patients with normal renal function undergoing low-risk surgery should discontinue dabigatran at least 24 hours before the surgery. If the creatinine clearance is 31 to 50 mL/min, inclusively, the last dose should be at least 48 hours before the procedure for low-risk surgery, and 4 days before a procedure that poses a high risk of bleeding.32–34 Some experts have given the same recommendations for rivaroxaban and apixaban (Table 2).34
The aPTT and prothrombin time are readily available tests, but they cannot determine the residual anticoagulant effects of dabigatran, rivaroxaban, or apixaban. However, in many (but not all) cases, a normal aPTT suggests that the hemostatic function is not impaired by dabigatran, and a normal prothrombin time or an absence of anti-factor Xa activity would similarly exclude hemostatic dysfunction caused by rivaroxaban or apixaban. These tests are potentially useful as adjuncts before surgical procedures that require complete hemostasis.
Furthermore, a normal thrombin time rules out the presence of a significant amount of dabigatran. Therefore, a normal thrombin time might be particularly useful in a patient undergoing a high-risk intervention such as epidural cannulation or neurosurgery and who is normally receiving dabigatran.
Managing overdose and bleeding complications
Assessing the severity of bleeding is the key to managing bleeding complications (Table 3).
Minor bleeding such as epistaxis and ecchymosis can be managed symptomatically (eg, with nasal packing), perhaps with short-term withdrawal of the anticoagulant. Moderate bleeding such as upper or lower gastrointestinal bleeding can be managed by withdrawal of the anticoagulant, clinical monitoring, blood transfusion if needed, and treatment directed at the etiology.
Major and life-threatening bleeding (eg, intracerebral hemorrhage) requires aggressive treatment in the intensive care unit, withdrawal of the anticoagulant, mechanical compression of the bleeding site if accessible, fluid replacement and blood transfusion as appropriate, and interventional procedures. Nonspecific reversal agents might be considered in patients with major or life-threatening bleeding.
The half-life of dabigatran after multiple doses is approximately 14 to 17 hours and is not dose-dependent.9 Hence, if there is no active bleeding after a dabigatran overdose, stopping the drug may be sufficient. Since the pharmacodynamic effect of dabigatran declines in parallel to its plasma concentration, urgent but not emergency surgery may need to be delayed for only about 12 hours from the last dose of dabigatran.
The 2011 American College of Cardiology Foundation/American Heart Association guidelines recommend that patients with severe hemorrhage resulting from dabigatran should receive supportive therapy, including transfusion of fresh-frozen plasma, transfusion of packed red blood cells, or surgical intervention if appropriate.35 However, transfusion of fresh-frozen plasma is debatable because there is no evidence to support its use in this situation. While fresh-frozen plasma may be useful in cases of coagulation factor depletion, it does not effectively reverse inhibition of coagulation factors.36
Off-label use of nonspecific hemostatic agents
To date, no specific agent has been demonstrated to reverse excessive bleeding in patients taking the new oral anticoagulants. However, in view of their procoagulant capabilities, nonspecific hemostatic agents have been suggested for use in reversal of major bleeding resulting from these drugs.37–39 Examples are:
Recombinant factor VIIa (NovoSeven) initiates thrombin generation by activating factor X.
Four-factor prothrombin complex concentrate (Beriplex, recently approved in the United States) contains relatively large amounts of four nonactive vitamin K-dependent procoagulant factors (factors II, VII, IX, and X) that stimulate thrombin formation.
Three-factor prothrombin complex concentrate (Bebulin VH and Profilnine SD) contains low amounts of nonactive factor VII relative to factors II, IX, and X. In some centers a four-factor equivalent is produced by transfusion of a three-factor product with the addition of small amounts of recombinant factor VIIa or fresh-frozen plasma to replace the missing factor VII.40
Activated prothrombin complex concentrate (FEIBA NF) contains activated factor VII and factors II, IX, and X, mainly in nonactivated form.36 Therefore, it combines the effect of both recombinant factor VIIa and four-factor prothrombin complex concentrate.37
Studies of nonspecific hemostatic agents
In a study of rats infused with high doses of dabigatran, van Ryn et al38 observed that activated prothrombin complex concentrate at a dose of 50 or 100 U/kg and recombinant factor VIIa at a dose of 0.1 or 0.5 mg/kg reduced the rat-tail bleeding time in a dose-dependent manner but not the blood loss, compared with controls, even with a higher dose of recombinant factor VIIa (1 mg/kg). Recombinant factor VIIa also reversed the prolonged aPTT induced by dabigatran, whereas activated prothrombin complex concentrate did not. They suggested that recombinant factor VIIa and activated prothrombin complex concentrate may be potential antidotes for dabigatran-induced severe bleeding in humans.
In an ex vivo study of healthy people who took a single dose of dabigatran 150 mg or rivaroxaban 20 mg, Marlu et al37 found that activated prothrombin complex concentrate and four-factor prothrombin complex concentrate could be reasonable antidotes to these drugs.
Dabigatran-associated bleeding after cardiac surgery in humans has been successfully managed with hemodialysis and recombinant factor VIIa, although the efficacy of the latter cannot be individually assessed in the study.41
In a randomized placebo-controlled trial aimed at reversing rivaroxaban and dabigatran in healthy participants, Eerenberg et al39 showed that four-factor prothrombin complex concentrate at a dose of 50 IU/kg reversed prolongation of the prothrombin time and decreased the endogenous thrombin potential in those who received rivaroxaban, but it failed to reverse the aPTT, the endogenous thrombin potential, and thrombin time in those who received dabigatran.
However, Marlu et al reported that four-factor prothrombin complex concentrate at three doses (12.5 U/kg, 25 U/kg, and 50 U/kg)—or better still, activated prothrombin complex concentrate (40–80 U/kg)—could be a useful antidote to dabigatran.37
It is important to note that the healthy participants in the Eerenberg et al study39 took dabigatran 150 mg twice daily and rivaroxaban 20 mg daily for 2.5 days, whereas those in the Marlu et al study37 took the same dose of each medication, but only once.
The three-factor prothrombin complex concentrate products have been shown to be less effective than four-factor ones in reversing supratherapeutic INRs in patients with warfarin overdose, but whether this will be true with the new oral anticoagulants remains unknown. Furthermore, the four-factor concentrates effectively reversed warfarin-induced coagulopathy and bleeding in patients,42 but to our knowledge, the same is yet to be demonstrated in bleeding related to the newer agents.
Other measures
Gastric lavage or the administration of activated charcoal (or in some cases both) may reduce drug absorption if done within 2 or 3 hours of drug ingestion (Table 1). Because it is lipophilic, more than 99.9% of dabigatran etexilate was adsorbed by activated charcoal from water prepared to simulate gastric fluid in an in vitro experiment by van Ryn et al.43 This has not been tested in patients, and no similar study has been done for rivaroxaban or apixaban. However, use of charcoal in cases of recent ingestion, particularly with intentional overdose of these agents, seems reasonable.
Hemodialysis may reverse the anticoagulant effects of dabigatran overdose or severe bleeding because only about 35% of dabigatran is bound to plasma proteins (Table 1). In a single-center study, 50 mg of dabigatran etexilate was given orally to six patients with end-stage renal disease before dialysis, and the mean fraction of the drug removed by the dialyzer was 62% at 2 hours and 68% at 4 hours.32 This study suggests that hemodialysis may be useful to accelerate the removal of the drug in cases of life-threatening bleeding.
Rivaroxaban and apixaban are not dialyzable: the plasma protein binding of rivaroxaban is 95% and that of apixaban is 87%.
FUTURE DIRECTIONS
Because the new oral anticoagulants, unlike warfarin, have a wide therapeutic window, routine anticoagulant monitoring is not needed and might be misleading. However, there are times when monitoring might be useful; at such times, a validated, widely available, easily understood test would be good to have—but we don’t have it—at least not yet.
Therapeutic ranges for the aPTT have been established empirically for heparin in various indications.44 Additional study is needed to determine if an appropriate aPTT range can be determined for the new oral anticoagulants, particularly dabigatran.
Similarly, as with low-molecular-weight heparins, anti-factor Xa activity monitoring may become a more available validated means of testing for exposure to rivaroxaban and apixaban. More promising, using concepts derived from the development of the INR for warfarin monitoring,45 Tripodi et al46 have derived normalized INR-like assays to report rivaroxaban levels. A standardized schema for reporting results is being developed.46 Studies are required to determine if and how this assay may be useful. Initial trials in this regard are encouraging.47
Finally, the thrombotic risk associated with the use of nonspecific prohemostatic agents is unknown.37,48 Additional studies are required to standardize their dosages, frequency of administration, and duration of action, as well as to quantify their complications in bleeding patients.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 2009; 104:1534–1539.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991; 22:983–988.
- Heit JA, Cohen AT, Anderson FA; on behalf of the VTE Impact Assessment Group. Estimated annual number of incident and recurrent, non-fatal and fatal venous thromboembolism (VTE) events in the US. Blood (ASH Annual Meeting Abstracts) 2005; 106:abstract 910.
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009; 15(suppl 1):9S–16S.
- Stangier J, Rathgen K, Stähle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007; 64:292–303.
- Kubitza D, Becka M, Wensing G, Voith B, Zuehlsdorf M. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939—an oral, direct factor Xa inhibitor—after multiple dosing in healthy male subjects. Eur J Clin Pharmacol 2005; 61:873–880.
- Mueck W, Becka M, Kubitza D, Voith B, Zuehlsdorf M. Population model of the pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct factor Xa inhibitor—in healthy subjects. Int J Clin Pharmacol Ther 2007; 45:335–344.
- Weitz JI, Eikelboom JW, Samama MM; American College of Chest Physicians. New antithrombotic drugs: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e120S–e151S.
- Raghavan N, Frost CE, Yu Z, et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos 2009; 37:74–81.
- Cullberg M, Eriksson UG, Larsson M, Karlsson MO. Population modelling of the effect of inogatran, at thrombin inhibitor, on ex vivo coagulation time (APTT) in healthy subjects and patients with coronary artery disease. Br J Clin Pharmacol 2001; 51:71–79.
- Carlsson SC, Mattsson C, Eriksson UG, et al. A review of the effects of the oral direct thrombin inhibitor ximelagatran on coagulation assays. Thromb Res 2005; 115:9–18.
- Mueck W, Eriksson BI, Bauer KA, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct factor Xa inhibitor—in patients undergoing major orthopaedic surgery. Clin Pharmacokinet 2008; 47:203–216.
- Samama MM, Martinoli JL, LeFlem L, et al. Assessment of laboratory assays to measure rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2010; 103:815–825.
- Wong PC, Crain EJ, Xin B, et al. Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic studies. J Thromb Haemost 2008; 6:820–829.
- Stangier J, Feuring M. Using the HEMOCLOT direct thrombin inhibitor assay to determine plasma concentrations of dabigatran. Blood Coagul Fibrinolysis 2012; 23:138–143.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Nowak G. The ecarin clotting time, a universal method to quantify direct thrombin inhibitors. Pathophysiol Haemost Thromb 2003–2004; 33:173–183.
- Lange U, Nowak G, Bucha E. Ecarin chromogenic assay—a new method for quantitative determination of direct thrombin inhibitors like hirudin. Pathophysiol Haemost Thromb 2003–2004; 33:184–191.
- Samama MM, Contant G, Spiro TE, et al; Rivaroxaban Anti-Factor Xa Chromogenic Assay Field Trial Laboratories. Evaluation of the anti-factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost 2012; 107:379–387.
- Miyares MA, Davis K. Newer oral anticoagulants: a review of laboratory monitoring options and reversal agents in the hemorrhagic patient. Am J Health Syst Pharm 2012; 69:1473–1484.
- Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost 2010; 104:1263–1271.
- Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006; 114:774–782.
- Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol 2007; 49:1362–1368.
- Perzborn E, Strassburger J, Wilmen A, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939—an oral, direct factor Xa inhibitor. J Thromb Haemost 2005; 3:514–521.
- Eriksson BI, Dahl OE, Rosencher N, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Borris LC, Friedman RJ, et al; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008; 358:2765–2775.
- Lassen MR, Ageno W, Borris LC, et al; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008; 358:2776–2786.
- Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010; 49:259–268.
- US Food and Drug Administration (FDA). Medication Guide: Pradaxa (dabigatran etexilate mesylate) capsules. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM231720.pdf. Accessed June 5, 2013.
- Schulman S, Crowther MA. How I treat with anticoagulants in 2012: new and old anticoagulants, and when and how to switch. Blood 2012; 119:3016–3023.
- Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/ AHA/ HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2011; 57:1330–1337.
- Crowther MA, Warkentin TE. Managing bleeding in anticoagulated patients with a focus on novel therapeutic agents. J Thromb Haemost 2009; 7(suppl 1):107–110.
- Marlu R, Hodaj E, Paris A, Albaladejo P, Cracowski JL, Pernod G. Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: a randomised crossover ex vivo study in healthy volunteers. Thromb Haemost 2012; 108:217–224.
- van Ryn J, Ruehl D, Priepke H, Hauel N, Wienen W. Reversibility of the anticoagulant effect of high doses of the direct thrombin inhibitor dabigatran, by recombinant factor VIIa or activated prothrombin complex concentrate. 13th Congress of the European Hematology Association, June 12–15, 2008. Hematologica 2008; 93( s1):148Abs.0370.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124:1573–1579.
- Holland L, Warkentin TE, Refaai M, Crowther MA, Johnston MA, Sarode R. Suboptimal effect of a three-factor prothrombin complex concentrate (Profilnine-SD) in correcting supratherapeutic international normalized ratio due to warfarin overdose. Transfusion 2009; 49:1171–1177.
- Warkentin TE, Margetts P, Connolly SJ, Lamy A, Ricci C, Eikelboom JW. Recombinant factor VIIa (rFVIIa) and hemodialysis to manage massive dabigatran-associated postcardiac surgery bleeding. Blood 2012; 119:2172–2174.
- Song MM, Warne CP, Crowther MA. Prothrombin complex concentrate (PCC, Octaplex) in patients requiring immediate reversal of vitamin K antagonist anticoagulation. Thromb Res 2012; 129:526–529.
- van Ryn J, Sieger P, Kink-Eiband M, Gansser D, Clemens A. Adsorption of dabigatran etexilate in water or dabigatran in pooled human plasma by activated charcoal in vitro. 51st ASH Annual Meeting and Exposition. Abstract no. 1065. http://ash.confex.com/ash/2009/webprogram/Paper21383.html. Accessed June 5, 2013.
- Hirsh J. Heparin. N Engl J Med 1991; 324:1565–1574.
- van den Besselaar AMHP, Poller L, Tripodi A. Guidelines for thromboplastins and plasmas used to control for oral anticoagulant therapy. WHO Technical Report Series 1999; 889:64–93.
- Tripodi A, Chantarangkul V, Guinet C, Samama MM. The international normalized ratio calibrated for rivaroxaban has the potential to normalize prothrombin time results for rivaroxaban-treated patients: Results of an in vitro study. J Thromb Haemost 2011; 9:226–228.
- Samama MM, Contant G, Spiro TE, et al; Rivaroxaban Prothrombin Time Field Trial Laboratories. Evaluation of the prothrombin time for measuring rivaroxaban plasma concentrations using calibrators and controls: results of a multicenter field trial. Clin Appl Thromb Hemost 2012; 18:150–158.
- Ehrlich HJ, Henzl MJ, Gomperts ED. Safety of factor VIII inhibitor bypass activity (FEIBA): 10-year compilation of thrombotic adverse events. Haemophilia 2002; 8:83–90.
In the past several years, three new oral anticoagulants—dabigatran etexilate (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis)—have been approved for use in the United States. These long-awaited agents are appealing because they are easy to use, do not require laboratory monitoring, and have demonstrated equivalence, or in some cases, superiority to warfarin in preventing stroke or systemic embolism in at-risk populations.1–4 However, unlike warfarin, they have no specific reversal agents. How then should one manage spontaneous bleeding problems and those due to drug overdose, and how can we quickly reverse anticoagulation if emergency surgery is needed?
For these reasons, physicians and patients have been wary of these agents. However, with a systematic approach based on an understanding of the properties of these drugs, the appropriate use and interpretation of coagulation tests, and awareness of available therapeutic strategies, physicians can more confidently provide care for patients who require urgent reversal of anticoagulant effects.
Here, we review the available literature and suggest practical strategies for management based on an understanding of the pharmacokinetic and pharmacodynamic effects of these drugs and our current knowledge of the coagulation tests.
NEED FOR ANTICOAGULANTS
Anticoagulants are important in preventing systemic embolization in patients with atrial fibrillation and preventing pulmonary embolism in patients with venous thromboembolism.
And the numbers are staggering. The estimated prevalence of atrial fibrillation in the United States was 3.03 million in 2005 and is projected to increase to 7.56 million by 2050.5 Ischemic stroke is the most serious complication of atrial fibrillation, which accounts for 23.5% of strokes in patients ages 80 through 89 according to Framingham data.6 Venous thromboembolism accounts for 900,000 incident or recurrent fatal and nonfatal events in the United States yearly.7
HOW THE NEW AGENTS BLOCK COAGULATION
Thrombin (factor IIa), a serine protease, is central to the process of clot formation during hemostasis. It activates factors V, VIII, and XI (thus generating more thrombin), catalyzes the conversion of fibrinogen to fibrin, and stimulates platelet aggregation. Its role in the final steps of the coagulation cascade has made it a target for new direct thrombin inhibitors such as dabigatran.
Factor Xa is a serine protease that plays a central role in the coagulation cascade. It is a desirable target for anticoagulation because it is the convergence point for the extrinsic and the intrinsic coagulation pathways. It converts prothrombin to thrombin. Rivaroxaban and apixaban are direct factor Xa inhibitors (Figure 1).
Dabigatran, a direct thrombin inhibitor
Dabigatran etexilate is a synthetic, orally available prodrug that is rapidly absorbed and converted by esterases to its active form, dabigatran, a potent direct inhibitor of both free thrombin and clot-bound thrombin.8
Plasma levels of dabigatran peak within 2 hours of administration, and its half-life is 14 to 17 hours.9 Dabigatran is eliminated mainly via the kidneys, with more that 80% of the drug excreted unchanged in the urine (Table 1).
Rivaroxaban, a factor Xa inhibitor
Rivaroxaban is a potent, selective, direct factor Xa inhibitor.
Plasma levels of rivaroxaban peak 2 to 3 hours after administration, and it is cleared with a terminal half-life of 7 to 11 hours.10,11
Rivaroxaban is eliminated by the kidneys and in the feces. The kidneys eliminate one-third of the active drug unchanged and another one-third as inactive metabolites. The remaining one-third is metabolized by the liver and then excreted in the feces. Rivaroxaban has a predictable and dose-dependent pharmacodynamic and pharmacokinetic profile that is not affected by age, sex, or body weight (Table 1).12
Apixaban, an oral factor Xa inhibitor
Apixaban is a selective, direct oral factor Xa inhibitor.
Plasma levels of apixaban peak about 3 hours after administration, and its terminal half-life is 8 to 14 hours.13 Apixaban is eliminated by oxidative metabolism, by the kidney, and in the feces. It has predictable pharmacodynamic and pharmacokinetic profiles and has the least renal dependence of the three agents (Table 1).
THE NEW ORAL ANTICOAGULANTS AND BLOOD COAGULATION ASSAYS
Assessment of the anticoagulant activity of the new oral anticoagulants is not necessary in routine clinical practice, but it may be useful in planning intervention in patients with major bleeding, those with drug overdose, or those who need emergency surgery.
The activated partial thromboplastin time
The activated partial thromboplastin time (aPTT) is a measure of the activity of the intrinsic pathway of the coagulation cascade.
Dabigatran. There is a curvilinear relationship between the aPTT and the plasma concentration of dabigatran and other direct thrombin inhibitors, although the aPTT prolongation appears to vary with different reagents and coagulometers.9,14,15 However, Stangier et al9 found a linear relationship between the aPTT and the square root of the dabigatran plasma concentration.
Rivaroxaban prolongs the aPTT in a dose-dependent manner, but there is no standard for calibration of this assay. Hence, the aPTT is not recommended for monitoring rivaroxaban in clinical practice.
Apixaban may also prolong the aPTT, but there are limited data on its reactivity with different reagents.
The prothrombin time and international normalized ratio
The prothrombin time and international normalized ratio (INR) are measures of the extrinsic pathway of the coagulation cascade.
Dabigatran. The INR has a linear response to the dabigatran concentration, but it is insensitive.9 Hence, it is not suitable for monitoring the anticoagulant effects of direct thrombin inhibitors.
Rivaroxaban. The prothrombin time correlates strongly with the plasma concentration of rivaroxaban in healthy trial participants11 and in patients undergoing total hip arthroplasty or total knee arthroplasty.16 Samama et al17 noted that, unlike with vitamin K antagonists, the INR cannot be used to monitor patients on rivaroxaban because the prothrombin time results varied with different reagents. They used a standard calibration curve to express the prothrombin time results in plasma concentrations of rivaroxaban rather than in seconds or the INR.
Apixaban increases the INR in a dose-dependent manner.18 Its effect on different reagents remains unknown.
The thrombin time
The thrombin time reflects the activity of thrombin in the plasma. The amount of thrombin and the concentration of thrombin inhibitors in the plasma sample determine the time to clot formation.
Dabigatran. The thrombin time displays a linear dose-response to dabigatran, but only over the range of therapeutic concentrations. At a dabigatran concentration greater than 600 ng/mL, the test often exceeds the maximum measurement time of coagulometers.9 Hence, this test is too sensitive for emergency monitoring, especially in cases of drug overdose. However, it is well suited for determining if any dabigatran is present.
Rivaroxaban and apixaban have no effect on the thrombin time.
The Hemoclot direct thrombin inhibitor assay and dabigatran
The Hemoclot direct thrombin inhibitor assay (Hyphen BioMed, France) is a sensitive diluted thrombin time assay that can be used for quantitative measurement of dabigatran activity in plasma. This test is based on inhibition of a constant amount of highly purified human alpha-thrombin by adding it to diluted test plasma (1:8 to 1:20) mixed with normal pooled human plasma.19,20
Stangier et al19 found that the Hemoclot assay was suitable for calculating a wide range of dabigatran concentrations up to 4,000 nmol/L (1,886 ng/mL). Although this finding has not been confirmed in larger studies, this test may provide a rapid and accurate assessment of dabigatran’s anticoagulant activity in cases of emergency surgery or overdose.
The ecarin clotting time and dabigatran
The ecarin clotting time is a measure of the activity of direct thrombin inhibitors, but not the factor Xa inhibitors.
Ecarin is a highly purified metalloprotease isolated from the venom of a snake, Echis carinatus, and it generates meizothrombin from prothrombin.21 Meizothrombin facilitates clot formation by converting fibrinogen to fibrin and, like thrombin, it can be inactivated by direct thrombin inhibitors, thereby prolonging the clotting time.
The limitations of the ecarin clotting time include dependence on the plasma levels of fibrinogen and prothrombin.
The ecarin chromogenic assay and dabigatran
The ecarin chromogenic assay is an improvement on the principle of the ecarin clotting time that can be used to measure the activity of direct thrombin inhibitors.22 In this test, ecarin is added to a plasma sample to generate meizothrombin, and the amidolytic activity of meizothrombin towards a chromogenic substrate is then determined.
Results of the ecarin chromogenic assay are not influenced by the levels of fibrinogen or prothrombin. Another advantage is that this assay can be used in automated and manual analyzers, thus enabling its use at the bedside. However, to our knowledge, it is not being regularly used to monitor direct thrombin inhibitors in the clinical setting, and there is no standard calibration of the ecarin clotting time method.
Assays of factor Xa activity
A variety of assays to monitor the anticoagulant activity of factor Xa inhibitors have been proposed.23–25 All measure inhibition of the activity of factor Xa using methods similar to those used in monitoring heparin levels. All require calibrators with a known concentration of the Xa inhibitor; many are easily adapted for laboratories currently providing measurement of factor Xa inhibition from heparin.23 These assays have been suggested as a better indicator of plasma concentration of factor Xa inhibitor drugs than the prothrombin time.25
CONTROLLING BLEEDING IN PATIENTS ON THE NEW ORAL ANTICOAGULANTS
Bleeding is an anticipated adverse event in patients taking anticoagulants. It is associated with significant morbidity and risk of death.26,27
Many physicians still have limited experience with using the new oral anticoagulants and managing the attendant bleeding risks. Hence, we recommend that every health institution have a treatment policy or algorithm to guide all clinical staff in the management of such emergencies.
Prevention of bleeding
Management of bleeding from these agents should begin with preventing bleeding in the first place.
The physician should adhere to the recommended dosages of these medications. Studies have shown that the plasma concentration of these drugs and the risk of bleeding increase with increasing dosage.1,28,29
In addition, these medications should be used for the shortest time for which anticoagulation is required, especially when used for preventing deep vein thrombosis. Prolonged use increases the risk of bleeding.30,31
Most patients who need anticoagulation have comorbidities such as heart failure, renal failure, diabetes mellitus, and hypertension. Although the kidneys play a major role in the excretion of dabigatran and, to some extent, rivaroxaban and apixaban, patients with severe renal impairment were excluded from the major trials of all three drugs.1–3 Hence, to avoid excessive drug accumulation and bleeding, these medications should not be used in such patients pending further studies. Further, patients taking these medications should be closely followed to detect new clinical situations, such as acute renal failure, that will necessitate their discontinuation or dose adjustment.
If surgery is needed
If a patient taking a new oral anticoagulant needs to undergo elective surgery, it is important to temporarily discontinue the drug, assess the risk of bleeding, and test for renal impairment.
Renal impairment is particularly relevant in the case of dabigatran, since more than 80% of the unchanged drug is cleared by the kidneys. Decreasing the dose, prolonging the dosing interval, or both have been suggested as means to reduce the risk of bleeding in patients with renal impairment who are taking dabigatran.32,33 Patients with normal renal function undergoing low-risk surgery should discontinue dabigatran at least 24 hours before the surgery. If the creatinine clearance is 31 to 50 mL/min, inclusively, the last dose should be at least 48 hours before the procedure for low-risk surgery, and 4 days before a procedure that poses a high risk of bleeding.32–34 Some experts have given the same recommendations for rivaroxaban and apixaban (Table 2).34
The aPTT and prothrombin time are readily available tests, but they cannot determine the residual anticoagulant effects of dabigatran, rivaroxaban, or apixaban. However, in many (but not all) cases, a normal aPTT suggests that the hemostatic function is not impaired by dabigatran, and a normal prothrombin time or an absence of anti-factor Xa activity would similarly exclude hemostatic dysfunction caused by rivaroxaban or apixaban. These tests are potentially useful as adjuncts before surgical procedures that require complete hemostasis.
Furthermore, a normal thrombin time rules out the presence of a significant amount of dabigatran. Therefore, a normal thrombin time might be particularly useful in a patient undergoing a high-risk intervention such as epidural cannulation or neurosurgery and who is normally receiving dabigatran.
Managing overdose and bleeding complications
Assessing the severity of bleeding is the key to managing bleeding complications (Table 3).
Minor bleeding such as epistaxis and ecchymosis can be managed symptomatically (eg, with nasal packing), perhaps with short-term withdrawal of the anticoagulant. Moderate bleeding such as upper or lower gastrointestinal bleeding can be managed by withdrawal of the anticoagulant, clinical monitoring, blood transfusion if needed, and treatment directed at the etiology.
Major and life-threatening bleeding (eg, intracerebral hemorrhage) requires aggressive treatment in the intensive care unit, withdrawal of the anticoagulant, mechanical compression of the bleeding site if accessible, fluid replacement and blood transfusion as appropriate, and interventional procedures. Nonspecific reversal agents might be considered in patients with major or life-threatening bleeding.
The half-life of dabigatran after multiple doses is approximately 14 to 17 hours and is not dose-dependent.9 Hence, if there is no active bleeding after a dabigatran overdose, stopping the drug may be sufficient. Since the pharmacodynamic effect of dabigatran declines in parallel to its plasma concentration, urgent but not emergency surgery may need to be delayed for only about 12 hours from the last dose of dabigatran.
The 2011 American College of Cardiology Foundation/American Heart Association guidelines recommend that patients with severe hemorrhage resulting from dabigatran should receive supportive therapy, including transfusion of fresh-frozen plasma, transfusion of packed red blood cells, or surgical intervention if appropriate.35 However, transfusion of fresh-frozen plasma is debatable because there is no evidence to support its use in this situation. While fresh-frozen plasma may be useful in cases of coagulation factor depletion, it does not effectively reverse inhibition of coagulation factors.36
Off-label use of nonspecific hemostatic agents
To date, no specific agent has been demonstrated to reverse excessive bleeding in patients taking the new oral anticoagulants. However, in view of their procoagulant capabilities, nonspecific hemostatic agents have been suggested for use in reversal of major bleeding resulting from these drugs.37–39 Examples are:
Recombinant factor VIIa (NovoSeven) initiates thrombin generation by activating factor X.
Four-factor prothrombin complex concentrate (Beriplex, recently approved in the United States) contains relatively large amounts of four nonactive vitamin K-dependent procoagulant factors (factors II, VII, IX, and X) that stimulate thrombin formation.
Three-factor prothrombin complex concentrate (Bebulin VH and Profilnine SD) contains low amounts of nonactive factor VII relative to factors II, IX, and X. In some centers a four-factor equivalent is produced by transfusion of a three-factor product with the addition of small amounts of recombinant factor VIIa or fresh-frozen plasma to replace the missing factor VII.40
Activated prothrombin complex concentrate (FEIBA NF) contains activated factor VII and factors II, IX, and X, mainly in nonactivated form.36 Therefore, it combines the effect of both recombinant factor VIIa and four-factor prothrombin complex concentrate.37
Studies of nonspecific hemostatic agents
In a study of rats infused with high doses of dabigatran, van Ryn et al38 observed that activated prothrombin complex concentrate at a dose of 50 or 100 U/kg and recombinant factor VIIa at a dose of 0.1 or 0.5 mg/kg reduced the rat-tail bleeding time in a dose-dependent manner but not the blood loss, compared with controls, even with a higher dose of recombinant factor VIIa (1 mg/kg). Recombinant factor VIIa also reversed the prolonged aPTT induced by dabigatran, whereas activated prothrombin complex concentrate did not. They suggested that recombinant factor VIIa and activated prothrombin complex concentrate may be potential antidotes for dabigatran-induced severe bleeding in humans.
In an ex vivo study of healthy people who took a single dose of dabigatran 150 mg or rivaroxaban 20 mg, Marlu et al37 found that activated prothrombin complex concentrate and four-factor prothrombin complex concentrate could be reasonable antidotes to these drugs.
Dabigatran-associated bleeding after cardiac surgery in humans has been successfully managed with hemodialysis and recombinant factor VIIa, although the efficacy of the latter cannot be individually assessed in the study.41
In a randomized placebo-controlled trial aimed at reversing rivaroxaban and dabigatran in healthy participants, Eerenberg et al39 showed that four-factor prothrombin complex concentrate at a dose of 50 IU/kg reversed prolongation of the prothrombin time and decreased the endogenous thrombin potential in those who received rivaroxaban, but it failed to reverse the aPTT, the endogenous thrombin potential, and thrombin time in those who received dabigatran.
However, Marlu et al reported that four-factor prothrombin complex concentrate at three doses (12.5 U/kg, 25 U/kg, and 50 U/kg)—or better still, activated prothrombin complex concentrate (40–80 U/kg)—could be a useful antidote to dabigatran.37
It is important to note that the healthy participants in the Eerenberg et al study39 took dabigatran 150 mg twice daily and rivaroxaban 20 mg daily for 2.5 days, whereas those in the Marlu et al study37 took the same dose of each medication, but only once.
The three-factor prothrombin complex concentrate products have been shown to be less effective than four-factor ones in reversing supratherapeutic INRs in patients with warfarin overdose, but whether this will be true with the new oral anticoagulants remains unknown. Furthermore, the four-factor concentrates effectively reversed warfarin-induced coagulopathy and bleeding in patients,42 but to our knowledge, the same is yet to be demonstrated in bleeding related to the newer agents.
Other measures
Gastric lavage or the administration of activated charcoal (or in some cases both) may reduce drug absorption if done within 2 or 3 hours of drug ingestion (Table 1). Because it is lipophilic, more than 99.9% of dabigatran etexilate was adsorbed by activated charcoal from water prepared to simulate gastric fluid in an in vitro experiment by van Ryn et al.43 This has not been tested in patients, and no similar study has been done for rivaroxaban or apixaban. However, use of charcoal in cases of recent ingestion, particularly with intentional overdose of these agents, seems reasonable.
Hemodialysis may reverse the anticoagulant effects of dabigatran overdose or severe bleeding because only about 35% of dabigatran is bound to plasma proteins (Table 1). In a single-center study, 50 mg of dabigatran etexilate was given orally to six patients with end-stage renal disease before dialysis, and the mean fraction of the drug removed by the dialyzer was 62% at 2 hours and 68% at 4 hours.32 This study suggests that hemodialysis may be useful to accelerate the removal of the drug in cases of life-threatening bleeding.
Rivaroxaban and apixaban are not dialyzable: the plasma protein binding of rivaroxaban is 95% and that of apixaban is 87%.
FUTURE DIRECTIONS
Because the new oral anticoagulants, unlike warfarin, have a wide therapeutic window, routine anticoagulant monitoring is not needed and might be misleading. However, there are times when monitoring might be useful; at such times, a validated, widely available, easily understood test would be good to have—but we don’t have it—at least not yet.
Therapeutic ranges for the aPTT have been established empirically for heparin in various indications.44 Additional study is needed to determine if an appropriate aPTT range can be determined for the new oral anticoagulants, particularly dabigatran.
Similarly, as with low-molecular-weight heparins, anti-factor Xa activity monitoring may become a more available validated means of testing for exposure to rivaroxaban and apixaban. More promising, using concepts derived from the development of the INR for warfarin monitoring,45 Tripodi et al46 have derived normalized INR-like assays to report rivaroxaban levels. A standardized schema for reporting results is being developed.46 Studies are required to determine if and how this assay may be useful. Initial trials in this regard are encouraging.47
Finally, the thrombotic risk associated with the use of nonspecific prohemostatic agents is unknown.37,48 Additional studies are required to standardize their dosages, frequency of administration, and duration of action, as well as to quantify their complications in bleeding patients.
In the past several years, three new oral anticoagulants—dabigatran etexilate (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis)—have been approved for use in the United States. These long-awaited agents are appealing because they are easy to use, do not require laboratory monitoring, and have demonstrated equivalence, or in some cases, superiority to warfarin in preventing stroke or systemic embolism in at-risk populations.1–4 However, unlike warfarin, they have no specific reversal agents. How then should one manage spontaneous bleeding problems and those due to drug overdose, and how can we quickly reverse anticoagulation if emergency surgery is needed?
For these reasons, physicians and patients have been wary of these agents. However, with a systematic approach based on an understanding of the properties of these drugs, the appropriate use and interpretation of coagulation tests, and awareness of available therapeutic strategies, physicians can more confidently provide care for patients who require urgent reversal of anticoagulant effects.
Here, we review the available literature and suggest practical strategies for management based on an understanding of the pharmacokinetic and pharmacodynamic effects of these drugs and our current knowledge of the coagulation tests.
NEED FOR ANTICOAGULANTS
Anticoagulants are important in preventing systemic embolization in patients with atrial fibrillation and preventing pulmonary embolism in patients with venous thromboembolism.
And the numbers are staggering. The estimated prevalence of atrial fibrillation in the United States was 3.03 million in 2005 and is projected to increase to 7.56 million by 2050.5 Ischemic stroke is the most serious complication of atrial fibrillation, which accounts for 23.5% of strokes in patients ages 80 through 89 according to Framingham data.6 Venous thromboembolism accounts for 900,000 incident or recurrent fatal and nonfatal events in the United States yearly.7
HOW THE NEW AGENTS BLOCK COAGULATION
Thrombin (factor IIa), a serine protease, is central to the process of clot formation during hemostasis. It activates factors V, VIII, and XI (thus generating more thrombin), catalyzes the conversion of fibrinogen to fibrin, and stimulates platelet aggregation. Its role in the final steps of the coagulation cascade has made it a target for new direct thrombin inhibitors such as dabigatran.
Factor Xa is a serine protease that plays a central role in the coagulation cascade. It is a desirable target for anticoagulation because it is the convergence point for the extrinsic and the intrinsic coagulation pathways. It converts prothrombin to thrombin. Rivaroxaban and apixaban are direct factor Xa inhibitors (Figure 1).
Dabigatran, a direct thrombin inhibitor
Dabigatran etexilate is a synthetic, orally available prodrug that is rapidly absorbed and converted by esterases to its active form, dabigatran, a potent direct inhibitor of both free thrombin and clot-bound thrombin.8
Plasma levels of dabigatran peak within 2 hours of administration, and its half-life is 14 to 17 hours.9 Dabigatran is eliminated mainly via the kidneys, with more that 80% of the drug excreted unchanged in the urine (Table 1).
Rivaroxaban, a factor Xa inhibitor
Rivaroxaban is a potent, selective, direct factor Xa inhibitor.
Plasma levels of rivaroxaban peak 2 to 3 hours after administration, and it is cleared with a terminal half-life of 7 to 11 hours.10,11
Rivaroxaban is eliminated by the kidneys and in the feces. The kidneys eliminate one-third of the active drug unchanged and another one-third as inactive metabolites. The remaining one-third is metabolized by the liver and then excreted in the feces. Rivaroxaban has a predictable and dose-dependent pharmacodynamic and pharmacokinetic profile that is not affected by age, sex, or body weight (Table 1).12
Apixaban, an oral factor Xa inhibitor
Apixaban is a selective, direct oral factor Xa inhibitor.
Plasma levels of apixaban peak about 3 hours after administration, and its terminal half-life is 8 to 14 hours.13 Apixaban is eliminated by oxidative metabolism, by the kidney, and in the feces. It has predictable pharmacodynamic and pharmacokinetic profiles and has the least renal dependence of the three agents (Table 1).
THE NEW ORAL ANTICOAGULANTS AND BLOOD COAGULATION ASSAYS
Assessment of the anticoagulant activity of the new oral anticoagulants is not necessary in routine clinical practice, but it may be useful in planning intervention in patients with major bleeding, those with drug overdose, or those who need emergency surgery.
The activated partial thromboplastin time
The activated partial thromboplastin time (aPTT) is a measure of the activity of the intrinsic pathway of the coagulation cascade.
Dabigatran. There is a curvilinear relationship between the aPTT and the plasma concentration of dabigatran and other direct thrombin inhibitors, although the aPTT prolongation appears to vary with different reagents and coagulometers.9,14,15 However, Stangier et al9 found a linear relationship between the aPTT and the square root of the dabigatran plasma concentration.
Rivaroxaban prolongs the aPTT in a dose-dependent manner, but there is no standard for calibration of this assay. Hence, the aPTT is not recommended for monitoring rivaroxaban in clinical practice.
Apixaban may also prolong the aPTT, but there are limited data on its reactivity with different reagents.
The prothrombin time and international normalized ratio
The prothrombin time and international normalized ratio (INR) are measures of the extrinsic pathway of the coagulation cascade.
Dabigatran. The INR has a linear response to the dabigatran concentration, but it is insensitive.9 Hence, it is not suitable for monitoring the anticoagulant effects of direct thrombin inhibitors.
Rivaroxaban. The prothrombin time correlates strongly with the plasma concentration of rivaroxaban in healthy trial participants11 and in patients undergoing total hip arthroplasty or total knee arthroplasty.16 Samama et al17 noted that, unlike with vitamin K antagonists, the INR cannot be used to monitor patients on rivaroxaban because the prothrombin time results varied with different reagents. They used a standard calibration curve to express the prothrombin time results in plasma concentrations of rivaroxaban rather than in seconds or the INR.
Apixaban increases the INR in a dose-dependent manner.18 Its effect on different reagents remains unknown.
The thrombin time
The thrombin time reflects the activity of thrombin in the plasma. The amount of thrombin and the concentration of thrombin inhibitors in the plasma sample determine the time to clot formation.
Dabigatran. The thrombin time displays a linear dose-response to dabigatran, but only over the range of therapeutic concentrations. At a dabigatran concentration greater than 600 ng/mL, the test often exceeds the maximum measurement time of coagulometers.9 Hence, this test is too sensitive for emergency monitoring, especially in cases of drug overdose. However, it is well suited for determining if any dabigatran is present.
Rivaroxaban and apixaban have no effect on the thrombin time.
The Hemoclot direct thrombin inhibitor assay and dabigatran
The Hemoclot direct thrombin inhibitor assay (Hyphen BioMed, France) is a sensitive diluted thrombin time assay that can be used for quantitative measurement of dabigatran activity in plasma. This test is based on inhibition of a constant amount of highly purified human alpha-thrombin by adding it to diluted test plasma (1:8 to 1:20) mixed with normal pooled human plasma.19,20
Stangier et al19 found that the Hemoclot assay was suitable for calculating a wide range of dabigatran concentrations up to 4,000 nmol/L (1,886 ng/mL). Although this finding has not been confirmed in larger studies, this test may provide a rapid and accurate assessment of dabigatran’s anticoagulant activity in cases of emergency surgery or overdose.
The ecarin clotting time and dabigatran
The ecarin clotting time is a measure of the activity of direct thrombin inhibitors, but not the factor Xa inhibitors.
Ecarin is a highly purified metalloprotease isolated from the venom of a snake, Echis carinatus, and it generates meizothrombin from prothrombin.21 Meizothrombin facilitates clot formation by converting fibrinogen to fibrin and, like thrombin, it can be inactivated by direct thrombin inhibitors, thereby prolonging the clotting time.
The limitations of the ecarin clotting time include dependence on the plasma levels of fibrinogen and prothrombin.
The ecarin chromogenic assay and dabigatran
The ecarin chromogenic assay is an improvement on the principle of the ecarin clotting time that can be used to measure the activity of direct thrombin inhibitors.22 In this test, ecarin is added to a plasma sample to generate meizothrombin, and the amidolytic activity of meizothrombin towards a chromogenic substrate is then determined.
Results of the ecarin chromogenic assay are not influenced by the levels of fibrinogen or prothrombin. Another advantage is that this assay can be used in automated and manual analyzers, thus enabling its use at the bedside. However, to our knowledge, it is not being regularly used to monitor direct thrombin inhibitors in the clinical setting, and there is no standard calibration of the ecarin clotting time method.
Assays of factor Xa activity
A variety of assays to monitor the anticoagulant activity of factor Xa inhibitors have been proposed.23–25 All measure inhibition of the activity of factor Xa using methods similar to those used in monitoring heparin levels. All require calibrators with a known concentration of the Xa inhibitor; many are easily adapted for laboratories currently providing measurement of factor Xa inhibition from heparin.23 These assays have been suggested as a better indicator of plasma concentration of factor Xa inhibitor drugs than the prothrombin time.25
CONTROLLING BLEEDING IN PATIENTS ON THE NEW ORAL ANTICOAGULANTS
Bleeding is an anticipated adverse event in patients taking anticoagulants. It is associated with significant morbidity and risk of death.26,27
Many physicians still have limited experience with using the new oral anticoagulants and managing the attendant bleeding risks. Hence, we recommend that every health institution have a treatment policy or algorithm to guide all clinical staff in the management of such emergencies.
Prevention of bleeding
Management of bleeding from these agents should begin with preventing bleeding in the first place.
The physician should adhere to the recommended dosages of these medications. Studies have shown that the plasma concentration of these drugs and the risk of bleeding increase with increasing dosage.1,28,29
In addition, these medications should be used for the shortest time for which anticoagulation is required, especially when used for preventing deep vein thrombosis. Prolonged use increases the risk of bleeding.30,31
Most patients who need anticoagulation have comorbidities such as heart failure, renal failure, diabetes mellitus, and hypertension. Although the kidneys play a major role in the excretion of dabigatran and, to some extent, rivaroxaban and apixaban, patients with severe renal impairment were excluded from the major trials of all three drugs.1–3 Hence, to avoid excessive drug accumulation and bleeding, these medications should not be used in such patients pending further studies. Further, patients taking these medications should be closely followed to detect new clinical situations, such as acute renal failure, that will necessitate their discontinuation or dose adjustment.
If surgery is needed
If a patient taking a new oral anticoagulant needs to undergo elective surgery, it is important to temporarily discontinue the drug, assess the risk of bleeding, and test for renal impairment.
Renal impairment is particularly relevant in the case of dabigatran, since more than 80% of the unchanged drug is cleared by the kidneys. Decreasing the dose, prolonging the dosing interval, or both have been suggested as means to reduce the risk of bleeding in patients with renal impairment who are taking dabigatran.32,33 Patients with normal renal function undergoing low-risk surgery should discontinue dabigatran at least 24 hours before the surgery. If the creatinine clearance is 31 to 50 mL/min, inclusively, the last dose should be at least 48 hours before the procedure for low-risk surgery, and 4 days before a procedure that poses a high risk of bleeding.32–34 Some experts have given the same recommendations for rivaroxaban and apixaban (Table 2).34
The aPTT and prothrombin time are readily available tests, but they cannot determine the residual anticoagulant effects of dabigatran, rivaroxaban, or apixaban. However, in many (but not all) cases, a normal aPTT suggests that the hemostatic function is not impaired by dabigatran, and a normal prothrombin time or an absence of anti-factor Xa activity would similarly exclude hemostatic dysfunction caused by rivaroxaban or apixaban. These tests are potentially useful as adjuncts before surgical procedures that require complete hemostasis.
Furthermore, a normal thrombin time rules out the presence of a significant amount of dabigatran. Therefore, a normal thrombin time might be particularly useful in a patient undergoing a high-risk intervention such as epidural cannulation or neurosurgery and who is normally receiving dabigatran.
Managing overdose and bleeding complications
Assessing the severity of bleeding is the key to managing bleeding complications (Table 3).
Minor bleeding such as epistaxis and ecchymosis can be managed symptomatically (eg, with nasal packing), perhaps with short-term withdrawal of the anticoagulant. Moderate bleeding such as upper or lower gastrointestinal bleeding can be managed by withdrawal of the anticoagulant, clinical monitoring, blood transfusion if needed, and treatment directed at the etiology.
Major and life-threatening bleeding (eg, intracerebral hemorrhage) requires aggressive treatment in the intensive care unit, withdrawal of the anticoagulant, mechanical compression of the bleeding site if accessible, fluid replacement and blood transfusion as appropriate, and interventional procedures. Nonspecific reversal agents might be considered in patients with major or life-threatening bleeding.
The half-life of dabigatran after multiple doses is approximately 14 to 17 hours and is not dose-dependent.9 Hence, if there is no active bleeding after a dabigatran overdose, stopping the drug may be sufficient. Since the pharmacodynamic effect of dabigatran declines in parallel to its plasma concentration, urgent but not emergency surgery may need to be delayed for only about 12 hours from the last dose of dabigatran.
The 2011 American College of Cardiology Foundation/American Heart Association guidelines recommend that patients with severe hemorrhage resulting from dabigatran should receive supportive therapy, including transfusion of fresh-frozen plasma, transfusion of packed red blood cells, or surgical intervention if appropriate.35 However, transfusion of fresh-frozen plasma is debatable because there is no evidence to support its use in this situation. While fresh-frozen plasma may be useful in cases of coagulation factor depletion, it does not effectively reverse inhibition of coagulation factors.36
Off-label use of nonspecific hemostatic agents
To date, no specific agent has been demonstrated to reverse excessive bleeding in patients taking the new oral anticoagulants. However, in view of their procoagulant capabilities, nonspecific hemostatic agents have been suggested for use in reversal of major bleeding resulting from these drugs.37–39 Examples are:
Recombinant factor VIIa (NovoSeven) initiates thrombin generation by activating factor X.
Four-factor prothrombin complex concentrate (Beriplex, recently approved in the United States) contains relatively large amounts of four nonactive vitamin K-dependent procoagulant factors (factors II, VII, IX, and X) that stimulate thrombin formation.
Three-factor prothrombin complex concentrate (Bebulin VH and Profilnine SD) contains low amounts of nonactive factor VII relative to factors II, IX, and X. In some centers a four-factor equivalent is produced by transfusion of a three-factor product with the addition of small amounts of recombinant factor VIIa or fresh-frozen plasma to replace the missing factor VII.40
Activated prothrombin complex concentrate (FEIBA NF) contains activated factor VII and factors II, IX, and X, mainly in nonactivated form.36 Therefore, it combines the effect of both recombinant factor VIIa and four-factor prothrombin complex concentrate.37
Studies of nonspecific hemostatic agents
In a study of rats infused with high doses of dabigatran, van Ryn et al38 observed that activated prothrombin complex concentrate at a dose of 50 or 100 U/kg and recombinant factor VIIa at a dose of 0.1 or 0.5 mg/kg reduced the rat-tail bleeding time in a dose-dependent manner but not the blood loss, compared with controls, even with a higher dose of recombinant factor VIIa (1 mg/kg). Recombinant factor VIIa also reversed the prolonged aPTT induced by dabigatran, whereas activated prothrombin complex concentrate did not. They suggested that recombinant factor VIIa and activated prothrombin complex concentrate may be potential antidotes for dabigatran-induced severe bleeding in humans.
In an ex vivo study of healthy people who took a single dose of dabigatran 150 mg or rivaroxaban 20 mg, Marlu et al37 found that activated prothrombin complex concentrate and four-factor prothrombin complex concentrate could be reasonable antidotes to these drugs.
Dabigatran-associated bleeding after cardiac surgery in humans has been successfully managed with hemodialysis and recombinant factor VIIa, although the efficacy of the latter cannot be individually assessed in the study.41
In a randomized placebo-controlled trial aimed at reversing rivaroxaban and dabigatran in healthy participants, Eerenberg et al39 showed that four-factor prothrombin complex concentrate at a dose of 50 IU/kg reversed prolongation of the prothrombin time and decreased the endogenous thrombin potential in those who received rivaroxaban, but it failed to reverse the aPTT, the endogenous thrombin potential, and thrombin time in those who received dabigatran.
However, Marlu et al reported that four-factor prothrombin complex concentrate at three doses (12.5 U/kg, 25 U/kg, and 50 U/kg)—or better still, activated prothrombin complex concentrate (40–80 U/kg)—could be a useful antidote to dabigatran.37
It is important to note that the healthy participants in the Eerenberg et al study39 took dabigatran 150 mg twice daily and rivaroxaban 20 mg daily for 2.5 days, whereas those in the Marlu et al study37 took the same dose of each medication, but only once.
The three-factor prothrombin complex concentrate products have been shown to be less effective than four-factor ones in reversing supratherapeutic INRs in patients with warfarin overdose, but whether this will be true with the new oral anticoagulants remains unknown. Furthermore, the four-factor concentrates effectively reversed warfarin-induced coagulopathy and bleeding in patients,42 but to our knowledge, the same is yet to be demonstrated in bleeding related to the newer agents.
Other measures
Gastric lavage or the administration of activated charcoal (or in some cases both) may reduce drug absorption if done within 2 or 3 hours of drug ingestion (Table 1). Because it is lipophilic, more than 99.9% of dabigatran etexilate was adsorbed by activated charcoal from water prepared to simulate gastric fluid in an in vitro experiment by van Ryn et al.43 This has not been tested in patients, and no similar study has been done for rivaroxaban or apixaban. However, use of charcoal in cases of recent ingestion, particularly with intentional overdose of these agents, seems reasonable.
Hemodialysis may reverse the anticoagulant effects of dabigatran overdose or severe bleeding because only about 35% of dabigatran is bound to plasma proteins (Table 1). In a single-center study, 50 mg of dabigatran etexilate was given orally to six patients with end-stage renal disease before dialysis, and the mean fraction of the drug removed by the dialyzer was 62% at 2 hours and 68% at 4 hours.32 This study suggests that hemodialysis may be useful to accelerate the removal of the drug in cases of life-threatening bleeding.
Rivaroxaban and apixaban are not dialyzable: the plasma protein binding of rivaroxaban is 95% and that of apixaban is 87%.
FUTURE DIRECTIONS
Because the new oral anticoagulants, unlike warfarin, have a wide therapeutic window, routine anticoagulant monitoring is not needed and might be misleading. However, there are times when monitoring might be useful; at such times, a validated, widely available, easily understood test would be good to have—but we don’t have it—at least not yet.
Therapeutic ranges for the aPTT have been established empirically for heparin in various indications.44 Additional study is needed to determine if an appropriate aPTT range can be determined for the new oral anticoagulants, particularly dabigatran.
Similarly, as with low-molecular-weight heparins, anti-factor Xa activity monitoring may become a more available validated means of testing for exposure to rivaroxaban and apixaban. More promising, using concepts derived from the development of the INR for warfarin monitoring,45 Tripodi et al46 have derived normalized INR-like assays to report rivaroxaban levels. A standardized schema for reporting results is being developed.46 Studies are required to determine if and how this assay may be useful. Initial trials in this regard are encouraging.47
Finally, the thrombotic risk associated with the use of nonspecific prohemostatic agents is unknown.37,48 Additional studies are required to standardize their dosages, frequency of administration, and duration of action, as well as to quantify their complications in bleeding patients.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 2009; 104:1534–1539.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991; 22:983–988.
- Heit JA, Cohen AT, Anderson FA; on behalf of the VTE Impact Assessment Group. Estimated annual number of incident and recurrent, non-fatal and fatal venous thromboembolism (VTE) events in the US. Blood (ASH Annual Meeting Abstracts) 2005; 106:abstract 910.
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009; 15(suppl 1):9S–16S.
- Stangier J, Rathgen K, Stähle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007; 64:292–303.
- Kubitza D, Becka M, Wensing G, Voith B, Zuehlsdorf M. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939—an oral, direct factor Xa inhibitor—after multiple dosing in healthy male subjects. Eur J Clin Pharmacol 2005; 61:873–880.
- Mueck W, Becka M, Kubitza D, Voith B, Zuehlsdorf M. Population model of the pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct factor Xa inhibitor—in healthy subjects. Int J Clin Pharmacol Ther 2007; 45:335–344.
- Weitz JI, Eikelboom JW, Samama MM; American College of Chest Physicians. New antithrombotic drugs: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e120S–e151S.
- Raghavan N, Frost CE, Yu Z, et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos 2009; 37:74–81.
- Cullberg M, Eriksson UG, Larsson M, Karlsson MO. Population modelling of the effect of inogatran, at thrombin inhibitor, on ex vivo coagulation time (APTT) in healthy subjects and patients with coronary artery disease. Br J Clin Pharmacol 2001; 51:71–79.
- Carlsson SC, Mattsson C, Eriksson UG, et al. A review of the effects of the oral direct thrombin inhibitor ximelagatran on coagulation assays. Thromb Res 2005; 115:9–18.
- Mueck W, Eriksson BI, Bauer KA, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct factor Xa inhibitor—in patients undergoing major orthopaedic surgery. Clin Pharmacokinet 2008; 47:203–216.
- Samama MM, Martinoli JL, LeFlem L, et al. Assessment of laboratory assays to measure rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2010; 103:815–825.
- Wong PC, Crain EJ, Xin B, et al. Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic studies. J Thromb Haemost 2008; 6:820–829.
- Stangier J, Feuring M. Using the HEMOCLOT direct thrombin inhibitor assay to determine plasma concentrations of dabigatran. Blood Coagul Fibrinolysis 2012; 23:138–143.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Nowak G. The ecarin clotting time, a universal method to quantify direct thrombin inhibitors. Pathophysiol Haemost Thromb 2003–2004; 33:173–183.
- Lange U, Nowak G, Bucha E. Ecarin chromogenic assay—a new method for quantitative determination of direct thrombin inhibitors like hirudin. Pathophysiol Haemost Thromb 2003–2004; 33:184–191.
- Samama MM, Contant G, Spiro TE, et al; Rivaroxaban Anti-Factor Xa Chromogenic Assay Field Trial Laboratories. Evaluation of the anti-factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost 2012; 107:379–387.
- Miyares MA, Davis K. Newer oral anticoagulants: a review of laboratory monitoring options and reversal agents in the hemorrhagic patient. Am J Health Syst Pharm 2012; 69:1473–1484.
- Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost 2010; 104:1263–1271.
- Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006; 114:774–782.
- Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol 2007; 49:1362–1368.
- Perzborn E, Strassburger J, Wilmen A, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939—an oral, direct factor Xa inhibitor. J Thromb Haemost 2005; 3:514–521.
- Eriksson BI, Dahl OE, Rosencher N, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Borris LC, Friedman RJ, et al; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008; 358:2765–2775.
- Lassen MR, Ageno W, Borris LC, et al; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008; 358:2776–2786.
- Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010; 49:259–268.
- US Food and Drug Administration (FDA). Medication Guide: Pradaxa (dabigatran etexilate mesylate) capsules. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM231720.pdf. Accessed June 5, 2013.
- Schulman S, Crowther MA. How I treat with anticoagulants in 2012: new and old anticoagulants, and when and how to switch. Blood 2012; 119:3016–3023.
- Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/ AHA/ HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2011; 57:1330–1337.
- Crowther MA, Warkentin TE. Managing bleeding in anticoagulated patients with a focus on novel therapeutic agents. J Thromb Haemost 2009; 7(suppl 1):107–110.
- Marlu R, Hodaj E, Paris A, Albaladejo P, Cracowski JL, Pernod G. Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: a randomised crossover ex vivo study in healthy volunteers. Thromb Haemost 2012; 108:217–224.
- van Ryn J, Ruehl D, Priepke H, Hauel N, Wienen W. Reversibility of the anticoagulant effect of high doses of the direct thrombin inhibitor dabigatran, by recombinant factor VIIa or activated prothrombin complex concentrate. 13th Congress of the European Hematology Association, June 12–15, 2008. Hematologica 2008; 93( s1):148Abs.0370.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124:1573–1579.
- Holland L, Warkentin TE, Refaai M, Crowther MA, Johnston MA, Sarode R. Suboptimal effect of a three-factor prothrombin complex concentrate (Profilnine-SD) in correcting supratherapeutic international normalized ratio due to warfarin overdose. Transfusion 2009; 49:1171–1177.
- Warkentin TE, Margetts P, Connolly SJ, Lamy A, Ricci C, Eikelboom JW. Recombinant factor VIIa (rFVIIa) and hemodialysis to manage massive dabigatran-associated postcardiac surgery bleeding. Blood 2012; 119:2172–2174.
- Song MM, Warne CP, Crowther MA. Prothrombin complex concentrate (PCC, Octaplex) in patients requiring immediate reversal of vitamin K antagonist anticoagulation. Thromb Res 2012; 129:526–529.
- van Ryn J, Sieger P, Kink-Eiband M, Gansser D, Clemens A. Adsorption of dabigatran etexilate in water or dabigatran in pooled human plasma by activated charcoal in vitro. 51st ASH Annual Meeting and Exposition. Abstract no. 1065. http://ash.confex.com/ash/2009/webprogram/Paper21383.html. Accessed June 5, 2013.
- Hirsh J. Heparin. N Engl J Med 1991; 324:1565–1574.
- van den Besselaar AMHP, Poller L, Tripodi A. Guidelines for thromboplastins and plasmas used to control for oral anticoagulant therapy. WHO Technical Report Series 1999; 889:64–93.
- Tripodi A, Chantarangkul V, Guinet C, Samama MM. The international normalized ratio calibrated for rivaroxaban has the potential to normalize prothrombin time results for rivaroxaban-treated patients: Results of an in vitro study. J Thromb Haemost 2011; 9:226–228.
- Samama MM, Contant G, Spiro TE, et al; Rivaroxaban Prothrombin Time Field Trial Laboratories. Evaluation of the prothrombin time for measuring rivaroxaban plasma concentrations using calibrators and controls: results of a multicenter field trial. Clin Appl Thromb Hemost 2012; 18:150–158.
- Ehrlich HJ, Henzl MJ, Gomperts ED. Safety of factor VIII inhibitor bypass activity (FEIBA): 10-year compilation of thrombotic adverse events. Haemophilia 2002; 8:83–90.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 2009; 104:1534–1539.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991; 22:983–988.
- Heit JA, Cohen AT, Anderson FA; on behalf of the VTE Impact Assessment Group. Estimated annual number of incident and recurrent, non-fatal and fatal venous thromboembolism (VTE) events in the US. Blood (ASH Annual Meeting Abstracts) 2005; 106:abstract 910.
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost 2009; 15(suppl 1):9S–16S.
- Stangier J, Rathgen K, Stähle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007; 64:292–303.
- Kubitza D, Becka M, Wensing G, Voith B, Zuehlsdorf M. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939—an oral, direct factor Xa inhibitor—after multiple dosing in healthy male subjects. Eur J Clin Pharmacol 2005; 61:873–880.
- Mueck W, Becka M, Kubitza D, Voith B, Zuehlsdorf M. Population model of the pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct factor Xa inhibitor—in healthy subjects. Int J Clin Pharmacol Ther 2007; 45:335–344.
- Weitz JI, Eikelboom JW, Samama MM; American College of Chest Physicians. New antithrombotic drugs: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e120S–e151S.
- Raghavan N, Frost CE, Yu Z, et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos 2009; 37:74–81.
- Cullberg M, Eriksson UG, Larsson M, Karlsson MO. Population modelling of the effect of inogatran, at thrombin inhibitor, on ex vivo coagulation time (APTT) in healthy subjects and patients with coronary artery disease. Br J Clin Pharmacol 2001; 51:71–79.
- Carlsson SC, Mattsson C, Eriksson UG, et al. A review of the effects of the oral direct thrombin inhibitor ximelagatran on coagulation assays. Thromb Res 2005; 115:9–18.
- Mueck W, Eriksson BI, Bauer KA, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct factor Xa inhibitor—in patients undergoing major orthopaedic surgery. Clin Pharmacokinet 2008; 47:203–216.
- Samama MM, Martinoli JL, LeFlem L, et al. Assessment of laboratory assays to measure rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2010; 103:815–825.
- Wong PC, Crain EJ, Xin B, et al. Apixaban, an oral, direct and highly selective factor Xa inhibitor: in vitro, antithrombotic and antihemostatic studies. J Thromb Haemost 2008; 6:820–829.
- Stangier J, Feuring M. Using the HEMOCLOT direct thrombin inhibitor assay to determine plasma concentrations of dabigatran. Blood Coagul Fibrinolysis 2012; 23:138–143.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Nowak G. The ecarin clotting time, a universal method to quantify direct thrombin inhibitors. Pathophysiol Haemost Thromb 2003–2004; 33:173–183.
- Lange U, Nowak G, Bucha E. Ecarin chromogenic assay—a new method for quantitative determination of direct thrombin inhibitors like hirudin. Pathophysiol Haemost Thromb 2003–2004; 33:184–191.
- Samama MM, Contant G, Spiro TE, et al; Rivaroxaban Anti-Factor Xa Chromogenic Assay Field Trial Laboratories. Evaluation of the anti-factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost 2012; 107:379–387.
- Miyares MA, Davis K. Newer oral anticoagulants: a review of laboratory monitoring options and reversal agents in the hemorrhagic patient. Am J Health Syst Pharm 2012; 69:1473–1484.
- Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost 2010; 104:1263–1271.
- Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006; 114:774–782.
- Manoukian SV, Feit F, Mehran R, et al. Impact of major bleeding on 30-day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol 2007; 49:1362–1368.
- Perzborn E, Strassburger J, Wilmen A, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939—an oral, direct factor Xa inhibitor. J Thromb Haemost 2005; 3:514–521.
- Eriksson BI, Dahl OE, Rosencher N, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Borris LC, Friedman RJ, et al; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008; 358:2765–2775.
- Lassen MR, Ageno W, Borris LC, et al; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008; 358:2776–2786.
- Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010; 49:259–268.
- US Food and Drug Administration (FDA). Medication Guide: Pradaxa (dabigatran etexilate mesylate) capsules. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM231720.pdf. Accessed June 5, 2013.
- Schulman S, Crowther MA. How I treat with anticoagulants in 2012: new and old anticoagulants, and when and how to switch. Blood 2012; 119:3016–3023.
- Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/ AHA/ HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2011; 57:1330–1337.
- Crowther MA, Warkentin TE. Managing bleeding in anticoagulated patients with a focus on novel therapeutic agents. J Thromb Haemost 2009; 7(suppl 1):107–110.
- Marlu R, Hodaj E, Paris A, Albaladejo P, Cracowski JL, Pernod G. Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: a randomised crossover ex vivo study in healthy volunteers. Thromb Haemost 2012; 108:217–224.
- van Ryn J, Ruehl D, Priepke H, Hauel N, Wienen W. Reversibility of the anticoagulant effect of high doses of the direct thrombin inhibitor dabigatran, by recombinant factor VIIa or activated prothrombin complex concentrate. 13th Congress of the European Hematology Association, June 12–15, 2008. Hematologica 2008; 93( s1):148Abs.0370.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124:1573–1579.
- Holland L, Warkentin TE, Refaai M, Crowther MA, Johnston MA, Sarode R. Suboptimal effect of a three-factor prothrombin complex concentrate (Profilnine-SD) in correcting supratherapeutic international normalized ratio due to warfarin overdose. Transfusion 2009; 49:1171–1177.
- Warkentin TE, Margetts P, Connolly SJ, Lamy A, Ricci C, Eikelboom JW. Recombinant factor VIIa (rFVIIa) and hemodialysis to manage massive dabigatran-associated postcardiac surgery bleeding. Blood 2012; 119:2172–2174.
- Song MM, Warne CP, Crowther MA. Prothrombin complex concentrate (PCC, Octaplex) in patients requiring immediate reversal of vitamin K antagonist anticoagulation. Thromb Res 2012; 129:526–529.
- van Ryn J, Sieger P, Kink-Eiband M, Gansser D, Clemens A. Adsorption of dabigatran etexilate in water or dabigatran in pooled human plasma by activated charcoal in vitro. 51st ASH Annual Meeting and Exposition. Abstract no. 1065. http://ash.confex.com/ash/2009/webprogram/Paper21383.html. Accessed June 5, 2013.
- Hirsh J. Heparin. N Engl J Med 1991; 324:1565–1574.
- van den Besselaar AMHP, Poller L, Tripodi A. Guidelines for thromboplastins and plasmas used to control for oral anticoagulant therapy. WHO Technical Report Series 1999; 889:64–93.
- Tripodi A, Chantarangkul V, Guinet C, Samama MM. The international normalized ratio calibrated for rivaroxaban has the potential to normalize prothrombin time results for rivaroxaban-treated patients: Results of an in vitro study. J Thromb Haemost 2011; 9:226–228.
- Samama MM, Contant G, Spiro TE, et al; Rivaroxaban Prothrombin Time Field Trial Laboratories. Evaluation of the prothrombin time for measuring rivaroxaban plasma concentrations using calibrators and controls: results of a multicenter field trial. Clin Appl Thromb Hemost 2012; 18:150–158.
- Ehrlich HJ, Henzl MJ, Gomperts ED. Safety of factor VIII inhibitor bypass activity (FEIBA): 10-year compilation of thrombotic adverse events. Haemophilia 2002; 8:83–90.
KEY POINTS
- Thromboprophylaxis with anticoagulants is an important aspect of managing patients at risk of systemic or pulmonary embolization.
- Dabigatran is a direct inhibitor of thrombin (factor IIa); rivaroxaban and apixaban inhibit factor Xa.
- Monitoring of coagulation function is not routinely necessary with the new drugs but may be useful in emergencies.
- Nonspecific hemostatic agents that have been suggested for off-label use in reversing excessive bleeding in patients taking the new oral anticoagulants include recombinant factor VIIa, three-factor and four-factor prothrombin complex concentrate, and activated prothrombin complex concentrate.