User login
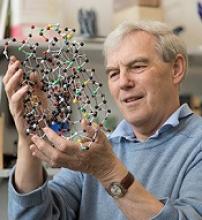
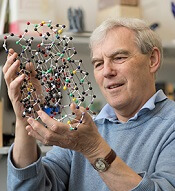
Three researchers have received the Nobel Prize in Chemistry 2017 for the development of cryo-electron microscopy (EM), which has simplified and improved the imaging of biomolecules.
Cryo-EM is a method used to image frozen biological molecules without the use of structure-altering dyes or fixatives or the need to coax the molecules into crystalline form.
This provides a simpler way to generate images of molecules in their normal states and greater understanding of biological function. It also aids the development of pharmaceuticals.
For developing cryo-EM, this year’s Nobel Prize in Chemistry* was awarded to:
- Jacques Dubochet, PhD, of University of Lausanne in Switzerland
- Joachim Frank, PhD, of Columbia University in New York, New York
- Richard Henderson, PhD, of MRC Laboratory of Molecular Biology in Cambridge, UK.
About the work
Electron microscopes were long believed to be suitable only for imaging dead matter because the electron beam destroys biological material.
However, in 1990, Dr Henderson succeeded in using an electron microscope to generate a 3-dimensional image of a protein at atomic resolution. This breakthrough proved the technology’s potential.
Dr Frank made the technology generally applicable. Between 1975 and 1986, he developed an image processing method in which the electron microscope’s fuzzy, 2-dimensional images are analyzed and merged to reveal a sharp, 3-dimensional structure.
Dr Dubochet added water to the mix. Liquid water evaporates in the electron microscope’s vacuum, which makes the biomolecules collapse.
In the early 1980s, Dr Dubochet succeeded in vitrifying water. He cooled water so rapidly that it solidified in its liquid form around a biological sample, allowing the biomolecules to retain their natural shape even in a vacuum.
Following these discoveries, the electron microscope’s every nut and bolt have been optimized. The desired atomic resolution was reached in 2013, and researchers can now routinely produce 3-dimensional structures of biomolecules.
In the past few years, the scientific literature has been filled with images of everything from proteins that cause antibiotic resistance to the surface of the Zika virus. ![]()
*The prize amount is 9 million Swedish krona to be shared equally among the Laureates.
Three researchers have received the Nobel Prize in Chemistry 2017 for the development of cryo-electron microscopy (EM), which has simplified and improved the imaging of biomolecules.
Cryo-EM is a method used to image frozen biological molecules without the use of structure-altering dyes or fixatives or the need to coax the molecules into crystalline form.
This provides a simpler way to generate images of molecules in their normal states and greater understanding of biological function. It also aids the development of pharmaceuticals.
For developing cryo-EM, this year’s Nobel Prize in Chemistry* was awarded to:
- Jacques Dubochet, PhD, of University of Lausanne in Switzerland
- Joachim Frank, PhD, of Columbia University in New York, New York
- Richard Henderson, PhD, of MRC Laboratory of Molecular Biology in Cambridge, UK.
About the work
Electron microscopes were long believed to be suitable only for imaging dead matter because the electron beam destroys biological material.
However, in 1990, Dr Henderson succeeded in using an electron microscope to generate a 3-dimensional image of a protein at atomic resolution. This breakthrough proved the technology’s potential.
Dr Frank made the technology generally applicable. Between 1975 and 1986, he developed an image processing method in which the electron microscope’s fuzzy, 2-dimensional images are analyzed and merged to reveal a sharp, 3-dimensional structure.
Dr Dubochet added water to the mix. Liquid water evaporates in the electron microscope’s vacuum, which makes the biomolecules collapse.
In the early 1980s, Dr Dubochet succeeded in vitrifying water. He cooled water so rapidly that it solidified in its liquid form around a biological sample, allowing the biomolecules to retain their natural shape even in a vacuum.
Following these discoveries, the electron microscope’s every nut and bolt have been optimized. The desired atomic resolution was reached in 2013, and researchers can now routinely produce 3-dimensional structures of biomolecules.
In the past few years, the scientific literature has been filled with images of everything from proteins that cause antibiotic resistance to the surface of the Zika virus. ![]()
*The prize amount is 9 million Swedish krona to be shared equally among the Laureates.
Three researchers have received the Nobel Prize in Chemistry 2017 for the development of cryo-electron microscopy (EM), which has simplified and improved the imaging of biomolecules.
Cryo-EM is a method used to image frozen biological molecules without the use of structure-altering dyes or fixatives or the need to coax the molecules into crystalline form.
This provides a simpler way to generate images of molecules in their normal states and greater understanding of biological function. It also aids the development of pharmaceuticals.
For developing cryo-EM, this year’s Nobel Prize in Chemistry* was awarded to:
- Jacques Dubochet, PhD, of University of Lausanne in Switzerland
- Joachim Frank, PhD, of Columbia University in New York, New York
- Richard Henderson, PhD, of MRC Laboratory of Molecular Biology in Cambridge, UK.
About the work
Electron microscopes were long believed to be suitable only for imaging dead matter because the electron beam destroys biological material.
However, in 1990, Dr Henderson succeeded in using an electron microscope to generate a 3-dimensional image of a protein at atomic resolution. This breakthrough proved the technology’s potential.
Dr Frank made the technology generally applicable. Between 1975 and 1986, he developed an image processing method in which the electron microscope’s fuzzy, 2-dimensional images are analyzed and merged to reveal a sharp, 3-dimensional structure.
Dr Dubochet added water to the mix. Liquid water evaporates in the electron microscope’s vacuum, which makes the biomolecules collapse.
In the early 1980s, Dr Dubochet succeeded in vitrifying water. He cooled water so rapidly that it solidified in its liquid form around a biological sample, allowing the biomolecules to retain their natural shape even in a vacuum.
Following these discoveries, the electron microscope’s every nut and bolt have been optimized. The desired atomic resolution was reached in 2013, and researchers can now routinely produce 3-dimensional structures of biomolecules.
In the past few years, the scientific literature has been filled with images of everything from proteins that cause antibiotic resistance to the surface of the Zika virus. ![]()
*The prize amount is 9 million Swedish krona to be shared equally among the Laureates.