User login
If a patient receiving anticoagulant therapy suffers a bleeding event, the patient and physician must decide whether and how soon to restart the therapy, and with what agent.
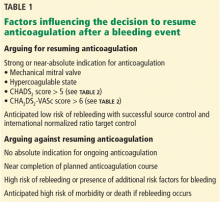
Foremost on our minds tends to be the risk of another hemorrhage. Subtler to appreciate immediately after an event is the continued risk of thrombosis, often from the same medical condition that prompted anticoagulation therapy in the first place (Table 1).
Complicating the decision, there may be a rebound effect: some thrombotic events such as pulmonary embolism and atrial fibrillation-related stroke may be more likely to occur in the first weeks after stopping warfarin than during similar intervals in patients who have not been taking it.1–3 The same thing may happen with the newer, target-specific oral anticoagulants.4–6
Although we have evidence-based guidelines for initiating and managing anticoagulant therapy, ample data on adverse events, and protocols for reversing anticoagulation if bleeding occurs, we do not have clear guidelines on restarting anticoagulation after a hemorrhagic event.
In this article, we outline a practical framework for approaching this clinical dilemma. Used in conjunction with consideration of a patient’s values and preferences as well as input from experts, this framework can help clinicians guide their patients through this challenging clinical decision. It consists of five questions:
- Why is the patient on anticoagulation, and what is the risk of thromboembolism without it?
- What was the clinical impact of the hemorrhage, and what is the risk of rebleeding if anticoagulation is resumed?
- What additional patient factors should be taken into consideration?
- How long should we wait before restarting anticoagulation?
- Would a newer drug be a better choice?
BLEEDING OCCURS IN 2% TO 3% OF PATIENTS PER YEAR
Most of our information on anticoagulation is about vitamin K antagonists—principally warfarin, in use since the 1950s. Among patients taking warfarin outside of clinical trials, the risk of major bleeding is estimated at 2% to 3% per year.7
However, the target-specific oral anticoagulants rivaroxaban (Xarelto), apixaban (Eliquis), dabigatran (Pradaxa) and edoxaban (Savaysa) are being used more and more, and we include them in our discussion insofar as we have information on them. The rates of bleeding with these new drugs in clinical trials have been comparable to or lower than those with warfarin.8 Postmarketing surveillance is under way.
WHY IS THE PATIENT ON ANTICOAGULATION? WHAT IS THE RISK WITHOUT IT?
Common, evidence-based indications for anticoagulation are to prevent complications in patients with venous thromboembolism and to prevent stroke in patients with atrial fibrillation or a mechanical heart valve. Other uses, such as in heart failure and its sequelae, pulmonary hypertension, and splanchnic or hepatic vein thrombosis, have less robust evidence to support them.
When anticoagulation-related bleeding occurs, it is essential to review why the patient is taking the drug and the risk of thromboembolism without it. Some indications pose a higher risk of thromboembolism than others and so argue more strongly for continuing the treatment.
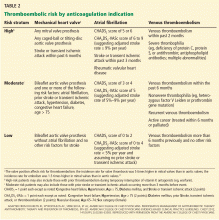
Douketis et al9 developed a risk-stratification scheme for perioperative thromboembolism. We have modified it by adding the CHA2DS2-VASc score (Table 2),9–11 and believe it can be used more widely.
High-risk indications
Conditions that pose a high risk of thrombosis almost always require restarting anticoagulation. Here, the most appropriate question nearly always is not if anticoagulation should be restarted, but when. Examples:
- A mechanical mitral valve
- Antiphospholipid antibody syndrome with recurrent thromboembolic events.
Lower-risk indications
Lower-risk indications allow more leeway in determining if anticoagulation should be resumed. The most straightforward cases fall well within established guidelines. Examples:
- Atrial fibrillation and a CHA2DS2-VASc score of 1. The 2014 guidelines from the American College of Cardiology, American Heart Association, and Heart Rhythm Society10 suggest that patients with nonvalvular atrial fibrillation and a CHA2DS2-VASc score of 1 have three options: an oral anticoagulant, aspirin, and no antithrombotic therapy. If such a patient on anticoagulant therapy subsequently experiences a major gastrointestinal hemorrhage requiring transfusion and intensive care and no definitively treatable source of bleeding is found on endoscopy, one can argue that the risks of continued anticoagulation (recurrent bleeding) now exceed the benefits and that the patient would be better served by aspirin or even no antithrombotic therapy.
- After 6 months of anticoagulation for unprovoked deep vein thrombosis. Several studies showed that aspirin reduced the risk of recurrent venous thromboembolism in patients who completed an initial 6-month course of anticoagulation.12–15 Though these studies did not specifically compare aspirin with warfarin or target-specific oral anticoagulants in preventing recurrent venous thromboembolism after a hemorrhage, it is reasonable to extrapolate their results to this situation.
If the risk of recurrent hemorrhage on anticoagulation is considered to be too great, then aspirin is an alternative to no anticoagulation, as it reduces the risk of recurrent venous thromboembolism.16 However, we advise caution if the bleeding lesion may be specifically exacerbated by aspirin, particularly upper gastrointestinal ulcers.
Moderate-risk indications
- After a partial course of anticoagulation for provoked venous thromboembolism. Suppose a patient in the 10th week of a planned 12-week course of anticoagulation for a surgically provoked, first deep vein thrombosis presents with abdominal pain and is found to have a retroperitoneal hematoma. In light of the risk of recurrent bleeding vs the benefit of resuming anticoagulation for the limited remaining period, her 12-week treatment course can reasonably be shortened to 10 weeks.
The risk of recurrent venous thromboembolism when a patient is off anticoagulation decreases with time from the initial event. The highest risk, estimated at 0.3% to 1.3% per day, is in the first 4 weeks, falling to 0.03% to 0.2% per day in weeks 5 through 12, and 0.05% per day thereafter.17–20
Additionally, a pooled analysis of seven randomized trials suggests that patients with isolated, distal deep vein thrombosis provoked by a temporary risk factor did not have a high risk of recurrence after being treated for 4 to 6 weeks.21 These analyses are based on vitamin K antagonists, though it seems reasonable to extrapolate this information to the target-specific oral anticoagulants.
More challenging are situations in which the evidence supporting the initial or continued need for anticoagulation is less robust, such as in heart failure, pulmonary hypertension, or splanchnic and hepatic vein thrombosis. In these cases, the lack of strong evidence supporting the use of anticoagulation should make us hesitate to resume it after bleeding.
WHAT WAS THE CLINICAL IMPACT? WHAT IS THE RISK OF REBLEEDING?
Different groups have defined major and minor bleeding in different ways.22,23 Several have proposed criteria to standardize how bleeding events (on warfarin and otherwise) are classified,23–25 but the definitions differ.
Specifically, all agree that a “major” bleeding event is one that is fatal, involves bleeding into a major organ, or leads to a substantial decline in hemoglobin level. However, the Thrombolysis in Myocardial Infarction trials use a decline of more than 5 g/dL in their definition,23,25 while the International Society on Thrombosis and Haemostasis uses 2 g/dL.24
Here, we review the clinical impact of the most common sources of anticoagulation-related hemorrhage—gastrointestinal, soft tissue, and urinary tract26—as well as intracerebral hemorrhage, a less common but more uniformly devastating event.27
Clinical impact of gastrointestinal hemorrhage
Each year, about 4.5% of patients taking warfarin have a gastrointestinal hemorrhage, though not all of these events are major.28 Evolving data suggest that the newer agents (particularly dabigatran, rivaroxaban, and edoxaban) pose a higher risk of gastrointestinal bleeding than warfarin.29 Patients may need plasma and blood transfusions and intravenous phytonadione, all of which carry risks, albeit small.
Frequently, endoscopy is needed to find the source of bleeding and to control it. If this does not work, angiographic intervention to infuse vasoconstrictors or embolic coils into the culprit artery may be required, and some patients need surgery. Each intervention carries its own risk.
Clinical impact of soft-tissue hemorrhage
Soft-tissue hemorrhage accounts for more than 20% of warfarin-related bleeding events26; as yet, we know of no data on the rate with the new drugs. Soft-tissue hemorrhage is often localized to the large muscles of the retroperitoneum and legs. Though retroperitoneal hemorrhage accounts for a relatively small portion of soft-tissue hemorrhages, it is associated with high rates of morbidity and death and will therefore be our focus.26
Much of the clinical impact of retroperitoneal hemorrhage is from a mass effect that causes abdominal compartment syndrome, hydroureter, ileus, abscess formation, and acute and chronic pain. At least 20% of cases are associated with femoral neuropathy. It can also lead to deep vein thrombosis from venous compression, coupled with hypercoagulability in response to bleeding. Brisk bleeding can lead to shock and death, and the mortality rate in retroperitoneal hemorrhage is estimated at 20% or higher.30
In many cases, the retroperitoneal hemorrhage will self-tamponade and the blood will be reabsorbed once the bleeding has stopped, but uncontrolled bleeding may require surgical or angiographic intervention.30
Clinical impact of urinary tract hemorrhage
Gross or microscopic hematuria can be found in an estimated 2% to 24% of patients taking warfarin31–33; data are lacking for the target-specific oral anticoagulants. Interventions required to manage urinary tract bleeding include bladder irrigation and, less often, transfusion.31 Since a significant number of cases of hematuria are due to neoplastic disease,32 a diagnostic workup with radiographic imaging of the upper tract and cystoscopy of the lower tract is usually required.31 While life-threatening hemorrhage is uncommon, complications such as transient urinary obstruction from clots may occur.
Clinical impact of intracranial hemorrhage
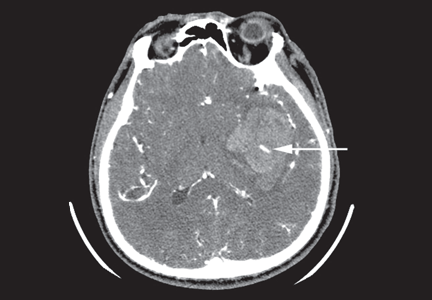
Intracranial hemorrhage is the most feared and deadly of the bleeding complications of anticoagulation. The incidence in patients on warfarin is estimated at 2% to 3% per year, which is markedly higher than the estimated incidence of 25 per 100,000 person-years in the general population.34 Emerging data indicate that the newer drugs are also associated with a risk of intracranial hemorrhage, though the risk is about half that with vitamin K antagonists.35 Intracranial hemorrhage leads to death or disability in 76% of cases, compared with 3% of cases of bleeding from the gastrointestinal or urinary tract.27
Regardless of the source of bleeding, hospitalization is likely to be required and may be prolonged, with attendant risks of nosocomial harms such as infection.
Risk of rebleeding
Given the scope and severity of anticoagulation-related bleeding, there is strong interest in predicting and preventing it. By some estimates, the incidence of recurrent bleeding after resuming vitamin K antagonists is 8% to 13%.22 Although there are several indices for predicting the risk of major bleeding when starting anticoagulation, there are currently no validated tools to estimate a patient’s risk of rebleeding.36
The patient factor that most consistently predicts major bleeding is a history of bleeding, particularly from the gastrointestinal tract. Finding and controlling the source of bleeding is important.26,37 For example, a patient with gross hematuria who is found on cystoscopy to have a urothelial papilloma is unlikely to have rebleeding if the tumor is successfully resected and serial follow-up shows no regrowth. In contrast, consider a patient with a major gastrointestinal hemorrhage, the source of which remains elusive after upper, lower, and capsule endoscopy or, alternatively, is suspected to be from one of multiple angiodysplastic lesions. Without definitive source management, this patient faces a high risk of rebleeding.
With or without anticoagulation, after a first intracranial hemorrhage the risk of another one is estimated at 2% to 4% per year.34 An observational study found a recurrence rate of 7.5% when vitamin K antagonist therapy was started after an intracranial hemorrhage (though not all patients were on a vitamin K antagonist at the time of the first hemorrhage).38
Patients with lobar hemorrhage and those with suspected cerebral amyloid angiopathy may be at particularly high risk if anticoagulation is resumed. Conversely, initial events attributed to uncontrolled hypertension that subsequently can be well controlled may portend a lower risk of rebleeding.34 For other types of intracranial hemorrhage, recurrence rates can be even higher. Irrespective of anticoagulation, one prospective study estimated the crude annual rebleeding rate with untreated arteriovenous malformations to be 7%.39 In chronic subdural hematoma, the recurrence rate after initial drainage has been estimated at 9.2% to 26.5%, with use of anticoagulants (in this case, vitamin K antagonists) being an independent predictor of recurrence.40
WHAT OTHER PATIENT FACTORS NEED CONSIDERATION?
Target INR on warfarin
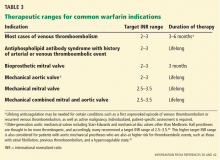
An important factor influencing the risk of bleeding with warfarin is the intensity of this therapy.37 A meta-analysis41 found that the risks of major hemorrhage and thromboembolism are minimized if the goal international normalized ratio (INR) is 2.0 to 3.0. When considering resuming anticoagulation after bleeding, make sure the therapeutic target is appropriate.37
Table 3 summarizes recommended therapeutic ranges for frequently encountered indications for warfarin.36,42,43
INR at time of the event and challenges in controlling it
The decision to resume anticoagulation in patients who bled while using warfarin must take into account the actual INR at the time of the event.
For example, consider a patient whose INR values are consistently in the therapeutic range. While on vacation, he receives ciprofloxacin for acute prostatitis from an urgent care team, and no adjustment to INR monitoring or warfarin dose is made. Several days later, he presents with lower gastrointestinal bleeding. His INR is 8, and colonoscopy reveals diverticulosis with a bleeding vessel, responsive to endoscopic therapy. After controlling the source of bleeding and reinforcing the need to always review new medications for potential interactions with anticoagulation, it is reasonable to expect that he once again will be able to keep his INR in the therapeutic range.
A patient on anticoagulation for the same indication but who has a history of repeated supratherapeutic levels, poor adherence, or poor access to INR monitoring poses very different concerns about resuming anticoagulation (as well as which agent to use, as we discuss below).
Of note, a high INR alone does not explain bleeding. It is estimated that a workup for gastrointestinal bleeding and gross hematuria uncovers previously undetected lesions in approximately one-third of cases involving warfarin.26 A similar malignancy-unmasking effect is now recognized in patients using the target-specific oral agents who experience gastrointestinal bleeding.44 Accordingly, we recommend a comprehensive source evaluation for any anticoagulation-related hemorrhage.
Comorbid conditions
Comorbid conditions associated with bleeding include cancer, end-stage renal disease, liver disease, arterial hypertension, prior stroke, and alcohol abuse.37,45 Gait instability, regardless of cause, may also increase the risk of trauma-related hemorrhage, but some have estimated that a patient would need to fall multiple times per week to contraindicate anticoagulation on the basis of falls alone.46
Concurrent medications
Concomitant therapies, including antiplatelet drugs and nonsteroidal anti-inflammatory drugs, increase bleeding risk.47,48 Aspirin and the nonsteroidals, in addition to having antiplatelet effects, also can cause gastric erosion.37 In evaluating whether and when to restart anticoagulation, it is advisable to review the role that concomitant therapies may have had in the index bleeding event and to evaluate the risks and benefits of these other agents.
Additionally, warfarin has many interactions. Although the newer drugs are lauded for having fewer interactions, they are not completely free of them, and the potential for interactions must always be reviewed.49 Further, unlike warfarin therapy, therapy with the newer agents is not routinely monitored with laboratory tests, so toxicity (or underdosing) may not be recognized until an adverse clinical event occurs. Ultimately, it may be safer to resume anticoagulation after a contributing drug can be safely discontinued.
Advanced age
The influence that the patient’s age should have on the decision to restart anticoagulation is unclear. Although the risk of intracranial hemorrhage increases with age, particularly after age 80, limited data exist in this population, particularly with regard to rebleeding. Further, age is a major risk factor for most thrombotic events, including venous thromboembolism and stroke from atrial fibrillation, so although the risks of anticoagulation may be higher, the benefits may also be higher than in younger patients.37,46 We discourage using age alone as a reason to withhold anticoagulation after a hemorrhage.
HOW LONG SHOULD WE WAIT TO RESTART ANTICOAGULATION?
We lack conclusive data on how long to wait to restart anticoagulation after an anticoagulation-associated hemorrhage.
The decision is complicated by evidence suggesting a rebound effect, with an increased risk of pulmonary embolism and atrial fibrillation-related stroke during the first 90 days of interruption of therapy with warfarin as well as with target-specific oral anticoagulants.3–8 In anticoagulation-associated retroperitoneal bleeding, there is increased risk of deep vein thrombosis from compression, even if venous thromboembolism was not the initial indication for anticoagulation.30
In patients with intracranial hemorrhage, evidence suggests that the intracranial hemorrhage itself increases the risk of arterial and venous thromboembolic events. Irrespective of whether a patient was previously on anticoagulation, the risk of arterial and venous thromboembolic events approaches 7% during the initial intracranial hemorrhage-related hospitalization and 9% during the first 90 days.34,50,51
To date, the only information we have about when to resume anticoagulation comes from patients taking vitamin K antagonists.
Timing after gastrointestinal bleeding
Small case series suggest that in the first 2 months after warfarin-associated gastrointestinal bleeding, there is substantial risk of rebleeding when anticoagulation is resumed—and of thrombosis when it is not.52,53 Two retrospective cohort studies may provide some guidance in this dilemma.28,54
Witt et al28 followed 442 patients who presented with gastrointestinal bleeding from any site during warfarin therapy for varied indications for up to 90 days after the index bleeding event. The risk of death was three times lower in patients who restarted warfarin than in those who did not, and their rate of thrombotic events was 10 times lower. The risk of recurrent gastrointestinal bleeding was statistically insignificant, and there were no fatal bleeding events. Anticoagulant therapy was generally resumed within 1 week of the bleeding event, at a median of 4 days.28,55
Qureshi et al54 performed a retrospective cohort study of 1,329 patients with nonvalvular atrial fibrillation who had experienced a gastrointestinal hemorrhage while taking warfarin. They found that resuming warfarin after 7 days was not associated with a higher risk of recurrent gastrointestinal bleeding and that the rates of death and thromboembolism were lower than in patients who resumed warfarin after 30 days. On the other hand, the risk of recurrent gastrointestinal bleeding was significantly greater if therapy was resumed within the first week.
In view of these studies, we believe that most patients should resume anticoagulation after 4 to 7 days of interruption after gastrointestinal bleeding.55
Timing after soft-tissue hemorrhage
The literature on resuming anticoagulation after soft-tissue hemorrhage is sparse. A retrospective study52 looked at this question in patients with spontaneous rectal sheath hematoma who had been receiving antiplatelet drugs, intravenous heparin, vitamin K antagonists, or a combination of these, but not target-specific agents. More than half of the patients were on vitamin K antagonists at the time of hemorrhage. Analysis suggested that when benefits of resuming anticoagulation are believed to outweigh risks, it is reasonable to resume anticoagulation 4 days after the index event.56
Timing after intracranial hemorrhage
Anticoagulation should not be considered within the first 24 hours after intracranial hemorrhage, as over 70% of patients develop some amount of hematoma expansion during this time.34,57 The period thereafter poses a challenge, as the risk of hematoma expansion decreases while the risk of arterial and venous thromboembolism is ongoing and cumulative.50
Perhaps surprisingly, national guidelines suggest starting prophylactic-dosed anticoagulation early in all intracranial hemorrhage patients, including those not previously on warfarin.58,59 In a randomized trial, Boeer et al60 concluded that starting low-dose subcutaneous heparin the day after an intracranial hemorrhage decreased the risk of thromboembolism without increasing the risk of rebleeding.60 Dickmann et al61 similarly concluded that there was no increased risk of rebleeding with early prophylactic-dosed subcutaneous heparin.61 Optimal mechanical thromboprophylaxis, including graduated compression stockings and intermittent pneumatic compression stockings, is also encouraged.34
Expert opinion remains divided on when and if anticoagulants should be resumed.34,62 The American Heart Association suggests that in nonvalvular atrial fibrillation, long-term anticoagulation should be avoided after spontaneous lobar hemorrhage; antiplatelet agents can be considered instead.58 In nonlobar hemorrhage, the American Heart Association suggests that anticoagulation be considered, depending on strength of indication, 7 to 10 days after the onset.58 The European Stroke Initiative suggests patients with strong indications for anticoagulation be restarted on warfarin 10 to 14 days after the event, depending on the risk of thromboembolism and recurrent intracranial hemorrhage.59 Others suggest delaying resumption to 10 to 30 weeks after an index intracranial hemorrhage.63
Overall, in the immediate acute period of intracranial hemorrhage, most patients will likely benefit from acute reversal of anticoagulation, followed by institution of prophylactic-dose anticoagulation after the first 24 hours. Going forward, patients who remain at higher risk of a recurrence of anticoagulant-related intracranial hemorrhage (such as those with lobar hemorrhage, suspected cerebral amyloid angiopathy, and other high-risk factors) than of thromboembolic events may be best managed without anticoagulants. Alternatively, patients with deep hemispheric intracranial hemorrhage, hypertension that can be well controlled, and a high risk of serious thromboembolism may experience net benefit from restarting anticoagulation.34
We recommend considering restarting anticoagulation 7 days after the onset of intracranial hemorrhage in patients at high risk of thromboembolism and after at least 14 days for patients at lower risk (Table 2). Discussions with neurologic and neurosurgical consultants should also inform this timing decision.
WOULD A NEWER DRUG BE A BETTER CHOICE?
The emergence of target-specific oral anticoagulants, including factor Xa inhibitors such as rivaroxaban, apixaban, and edoxaban and the direct thrombin inhibitor dabigatran etexilate, presents further challenges in managing anticoagulation after hemorrhage. Table 4 summarizes the current FDA-approved indications.64–67
These newer agents are attractive because, compared with warfarin, they have wider therapeutic windows, faster onset and offset of action, and fewer drug and food interactions.68 A meta-analysis of data available to date suggests that the new drugs, compared with warfarin, show a favorable risk-benefit profile with reductions in stroke, intracranial hemorrhage, and mortality with similar overall major bleeding rates, except for a possible increase in gastrointestinal bleeding.68
However, when managing anticoagulation after a bleeding event, the newer agents are challenging for two reasons: they may be associated with a higher incidence of gastrointestinal bleeding than warfarin, and they lack the typical reversal agents that can be used to manage an acute bleeding event.68,69
In individual studies comparing warfarin with dabigatran,70 rivaroxaban,71 apixaban,72 or edoxaban73 for stroke prevention in patients with atrial fibrillation, there was no significant difference in the rate of major bleeding between dabigatran in its higher dose (150 mg twice a day) or rivaroxaban compared with warfarin.70,71 The risk of major bleeding was actually lower with apixaban72 and edoxaban.73
In regard to specific types of major bleeding, the rate of intracranial hemorrhage was significantly lower with dabigatran, rivaroxaban, apixaban, and edoxaban than with warfarin.35,68–73 Some have proposed that since the brain is high in tissue factor, inhibition of tissue factor-factor VIIa complexes by vitamin K antagonists leaves the brain vulnerable to hemorrhage. Others suggest that the targeted mechanism of target-specific agents, as opposed to the multiple pathways in both the intrinsic and extrinsic coagulation cascade that vitamin K antagonists affect, may explain this difference.35,74,75
However, some studies suggest that rivaroxaban and the higher doses of dabigatran and edoxaban are associated with higher rates of major gastrointestinal bleeding compared with warfarin.69–71,76 But apixaban demonstrated no significant difference in gastrointestinal bleeding, and instead demonstrated rates of gastrointestinal bleeding comparable to that with aspirin for stroke prevention in atrial fibrillation.72
The new oral anticoagulants lack antidotes or reversal agents such as phytonadione and fresh-frozen plasma that are available to manage warfarin-associated bleeding events. Other proposed reversal options for the new agents include activated charcoal (if the drugs were taken recently enough to remain in the gastrointestinal tract) and concentrated clotting factor product, though research is ongoing in regards to the most appropriate use in clinical practice.37,69 Unlike rivaroxaban and apixaban, dabigatran has low plasma protein binding and is dialyzable, which provides another strategy in managing dabigatran-related bleeding.69
Of note, the above bleeding risk calculations relate to the first anticoagulant-related bleeding event, though presumably the same risk comparison across agents may be applicable to rebleeding events. Given the data above, when anticoagulation is to be resumed after an intracranial hemorrhage, the risk of rebleeding, particularly in the form of recurrent intracranial hemorrhage, may be lower if a target-specific oral anticoagulant is used.75 Similarly, when anticoagulation is to be resumed after a gastrointestinal bleeding event, reinitiation with warfarin or apixaban therapy may present the lowest risk of recurrent gastrointestinal rebleeding. In other sources of bleeding, such as retroperitoneal bleeding, we suggest consideration of transitioning to warfarin, given the availability of reversal agents in the event of recurrent bleeding.
Other important drug-specific factors that must be noted when selecting an agent with which to resume anticoagulation after a hemorrhage include the following:
- In patients with significant renal impairment, the choice of agent will be limited to a vitamin K antagonist.77
- A meta-analysis of randomized clinical trials suggests that in the elderly (age 75 and older) target-specific oral anticoagulants did not cause excess bleeding and were associated with at least equal efficacy compared with vitamin K antagonists.78
- Target-specific oral anticoagulants may be beneficial in patients who have challenges in achieving INR targets, as evidence suggests that switching to them is associated with a reduction in bleeding for patients who struggle to maintain an appropriately therapeutic INR.68 On the other hand, if there is concern that a patient may occasionally miss doses of an anticoagulant, given the rapid onset and offset of action of target-specific agents compared with warfarin, a missed dose of a target-specific agent may result in faster dissolution of anticoagulant effect and increased risk of thrombotic events, and lapses in anticoagulation will not be identified by routine drug monitoring.6–8,75 As such, it is vital to have a frank discussion with any patient who has difficulty maintaining therapeutic INRs on warfarin treatment to make sure that he or she is not missing doses.
- If there is no clear and compelling reason to select a particular agent, cost considerations should be taken into account. We have included estimated 30-day pricing for the various agents in Table 4.
- Jaffer AK, Brotman DJ, Bash LD, Mahmood SK, Lott B, White RH. Variations in perioperative warfarin management: outcomes and practice patterns at nine hospitals. Am J Med 2010; 123:141–150.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost 2010; 8:884–890.
- Raunsø J, Selmer C, Olesen JB, et al. Increased short-term risk of thrombo-embolism or death after interruption of warfarin treatment in patients with atrial fibrillation. Eur Heart J 2012; 33:1886–1892.
- Xarelto (rivaroxaban). Highlights of prescribing information. Jansen Pharmaceuticals, Inc. www.xareltohcp.com/sites/default/files/pdf/xarelto_0.pdf#zoom=100. Accessed March 9, 2015.
- Pradaxa (dabigatran etexilate mesylate). Highlights of prescribing information. Boehringer Ingelheim Pharmaceuticals, Inc. http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed March 9, 2015.
- Eliquis (apixaban). Highlights of prescribing information. Bristol-Myers Squibb Company. http://packageinserts.bms.com/pi/pi_eliquis.pdf. Accessed March 9, 2015.
- Schulman S, Beyth RJ, Kearon C, Levine MN; American College of Chest Physicians. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest 2008; 133(suppl 6):257S–298S.
- Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated bleeding. Blood 2014; 123:1152–1158.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Cannegieter SC, Rosendaal FR, Briët E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation 1994; 89:635–641.
- Warkentin TE. Aspirin for dual prevention of venous and arterial thrombosis. N Engl J Med 2012; 367:2039–2041.
- Simes J, Becattini C, Agnelli G, et al; INSPIRE Study Investigators* (International Collaboration of Aspirin Trials for Recurrent Venous Thromboembolism). Aspirin for the Prevention of Recurrent Venous Thromboembolism: The INSPIRE Collaboration. Circulation 2014; 130:1062–1071.
- Becattini C, Agnelli G, Schenone A, et al; WARFASA Investigators. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012; 366:1959–1967.
- Brighton TA, Eikelboom JW, Mann K, et al; ASPIRE Investigators. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012; 367:1979–1987.
- Wakefield TW, Obi AT, Henke PK. An aspirin a day to keep the clots away: can aspirin prevent recurrent thrombosis in extended treatment for venous thromboembolism? Circulation 2014; 130:1031–1033.
- Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med 1997; 336:1506–1511.
- Coon WW, Willis PW 3rd. Recurrence of venous thromboembolism. Surgery 1973; 73:823–827.
- Hull R, Delmore T, Genton E, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med 1979; 301:855–858.
- Jaffer AK, Brotman DJ, Chukwumerije N. When patients on warfarin need surgery. Cleve Clin J Med 2003; 70:973–984.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Guerrouij M, Uppal CS, Alklabi A, Douketis JD. The clinical impact of bleeding during oral anticoagulant therapy: assessment of morbidity, mortality and post-bleed anticoagulant management. J Thromb Thrombolysis 2011; 31:419–423.
- Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123:2736–2747.
- Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3:692–694.
- Wiviott SD, Antman EM, Gibson CM, et al; TRITON-TIMI 38 Investigators. Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet InhibitioN with prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38). Am Heart J 2006; 152:627–635.
- Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 1993; 95:315–328.
- Fang MC, Go AS, Chang Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med 2007; 120:700–705.
- Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med 2012; 172:1484–1491.
- Holster IL, Valkhoff VE, Kuipers EJ, Tjwa ET. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology 2013; 145:105-112.e15.
- Loor G, Bassiouny H, Valentin C, Shao MY, Funaki B, Desai T. Local and systemic consequences of large retroperitoneal clot burdens. World J Surg 2009; 33:1618–1625.
- Satasivam P, Reeves F, Lin M, et al. The effect of oral anticoagulation on the prevalence and management of haematuria in a contemporary Australian patient cohort. BJU Int 2012; 110(suppl 4):80–84.
- Van Savage JG, Fried FA. Anticoagulant associated hematuria: a prospective study. J Urol 1995; 153:1594–1596.
- Mosley DH, Schatz IJ, Breneman GM, Keyes JW. Long-term anticoagulant therapy. Complications and control in a review of 978 cases. JAMA 1963; 186:914–916.
- Goldstein JN, Greenberg SM. Should anticoagulation be resumed after intracerebral hemorrhage? Cleve Clin J Med 2010; 77:791–799.
- Caldeira D, Barra M, Pinto FJ, Ferreira JJ, Costa J. Intracranial hemorrhage risk with the new oral anticoagulants: a systematic review and meta-analysis. J Neurol 2014 Aug 14. [Epub ahead of print]
- Holbrook A, Schulman S, Witt DM, et al; American College of Chest Physicians. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e152S–e184S.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e44S–e88S.
- Poli D, Antonucci E, Dentali F, et al; Italian Federation of Anticoagulation Clinics (FCSA). Recurrence of ICH after resumption of anticoagulation with VK antagonists: CHIRONE study. Neurology 2014; 82:1020–1026.
- Choi JH, Mast H, Sciacca RR, et al. Clinical outcome after first and recurrent hemorrhage in patients with untreated brain arteriovenous malformation. Stroke 2006; 37:1243–1247.
- Chon KH, Lee JM, Koh EJ, Choi HY. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien) 2012; 154:1541–1548.
- Oake N, Jennings A, Forster AJ, et al. Anticoagulation intensity and outcomes among patients prescribed oral anticoagulant therapy: a systematic review and meta-analysis. CMAJ 2008; 179:235–244.
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH; American College of Chest Physicians. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e576S–e600S.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. Circulation 2008; 118:e523–e661.
- Clemens A, Strack A, Noack H, Konstantinides S, Brueckmann M, Lip GY. Anticoagulant-related gastrointestinal bleeding—could this facilitate early detection of benign or malignant gastrointestinal lesions? Ann Med 2014; 46:672–678.
- Khalid F, Qureshi W, Qureshi S, Alirhayim Z, Garikapati K, Patsias I. Impact of restarting warfarin therapy in renal disease anticoagulated patients with gastrointestinal hemorrhage. Ren Fail 2013; 35:1228–1235.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Davidson BL, Verheijen S, Lensing AW, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med 2014; 174:947–953.
- Knijff-Dutmer EA, Schut GA, van de Laar MA. Concomitant coumarin-NSAID therapy and risk for bleeding. Ann Pharmacother 2003; 37:12–16.
- Heidbuchel H, Verhamme P, Alings M, et al; European Heart Rhythm Association. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013; 15:625–651.
- Goldstein JN, Fazen LE, Wendell L, et al. Risk of thromboembolism following acute intracerebral hemorrhage. Neurocrit Care 2009; 10:28–34.
- Christensen MC, Dawson J, Vincent C. Risk of thromboembolic complications after intracerebral hemorrhage according to ethnicity. Adv Ther 2008; 25:831–841.
- Ananthasubramaniam K, Beattie JN, Rosman HS, Jayam V, Borzak S. How safely and for how long can warfarin therapy be withheld in prosthetic heart valve patients hospitalized with a major hemorrhage? Chest 2001; 119:478–484.
- Lee JK, Kang HW, Kim SG, Kim JS, Jung HC. Risks related with withholding and resuming anticoagulation in patients with non-variceal upper gastrointestinal bleeding while on warfarin therapy. Int J Clin Pract 2012; 66:64–68.
- Qureshi W, Mittal C, Patsias I, et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol 2014; 113:662–668.
- Brotman DJ, Jaffer AK. Resuming anticoagulation in the first week following gastrointestinal tract hemorrhage: should we adopt a 4-day rule? Arch Intern Med 2012; 172:1492–1493.
- Kunkala MR1, Kehl J, Zielinski MD. Spontaneous rectus sheath hematomas: when to restart anticoagulation? World J Surg 2013; 37:2555–2559.
- Davis SM, Broderick J, Hennerici M, et al; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006; 66:1175–1181.
- Broderick J, Connolly S, Feldmann E, et al; American Heart Association; American Stroke Association Stroke Council; High Blood Pressure Research Council; Quality of Care and Outcomes in Research Interdisciplinary Working Group. Guidelines for the management of spontaneous intracerebral hemorrhage in adults. Stroke 2007; 38:2001–2023.
- Steiner T, Kaste M, Forsting M, et al. Recommendations for the management of intracranial haemorrhage—part I: spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc Dis 2006; 22:294–316. Erratum in: Cerebrovasc Dis 2006; 22:461.
- Boeer A, Voth E, Henze T, Prange HW. Early heparin therapy in patients with spontaneous intracerebral haemorrhage. J Neurol Neurosurg Psychiatry 1991; 54:466–467.
- Dickmann U, Voth E, Schicha H, Henze T, Prange H, Emrich D. Heparin therapy, deep-vein thrombosis and pulmonary embolism after intracerebral hemorrhage. Klin Wochenschr 1988; 66:1182–1183.
- Aguilar MI, Hart RG, Kase CS, et al. Treatment of warfarin-associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc 2007; 82:82–92. Erratum in: Mayo Clin Proc 2007; 82:387.
- Majeed A, Kim YK, Roberts RS, Holmström M, Schulman S. Optimal timing of resumption of warfarin after intracranial hemorrhage. Stroke 2010; 41:2860–2866.
- US Food and Drug Administration. Drug Information. XARELTO (rivaroxaban) tablets, for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2013/022406s004lbl.pdf. Accessed March 9, 2015.
- US Food and Drug Administration. Drug Information. ELIQUIS® (apixaban) tablets for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2014/202155s009lbl.pdf. Accessed March 9, 2015.
- US Food and Drug Administration. Drug Information. PRADAXA® (dabigatran etexilate mesylate) capsules for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2014/022512s023lbl.pdf. Accessed March 9, 2015.
- New oral anticoagulants for acute venous thromboembolism. Med Lett Drugs Ther 2014; 56:3–4.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383:955–962.
- Nutescu EA, Dager WE, Kalus JS, Lewin JJ 3rd, Cipolle MD. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am J Health Syst Pharm 2013; 70:1914–1929.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151. Erratum in: N Engl J Med 2010; 363:1877.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg 2009; 108:1447–1452.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 2013; 70:1486–1490.
- Loffredo L, Perri L, Violi F. Impact of new oral anticoagulants on gastrointestinal bleeding in atrial fibrillation: a meta-analysis of interventional trials. Dig Liver Dis 2015 Feb 7. pii: S1590-8658(15)00189-9. doi: 10.1016/j.dld.2015.01.159. [Epub ahead of print]
- Thachil J. The newer direct oral anticoagulants: a practical guide. Clin Med 2014; 14:165–175.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
If a patient receiving anticoagulant therapy suffers a bleeding event, the patient and physician must decide whether and how soon to restart the therapy, and with what agent.
Foremost on our minds tends to be the risk of another hemorrhage. Subtler to appreciate immediately after an event is the continued risk of thrombosis, often from the same medical condition that prompted anticoagulation therapy in the first place (Table 1).
Complicating the decision, there may be a rebound effect: some thrombotic events such as pulmonary embolism and atrial fibrillation-related stroke may be more likely to occur in the first weeks after stopping warfarin than during similar intervals in patients who have not been taking it.1–3 The same thing may happen with the newer, target-specific oral anticoagulants.4–6
Although we have evidence-based guidelines for initiating and managing anticoagulant therapy, ample data on adverse events, and protocols for reversing anticoagulation if bleeding occurs, we do not have clear guidelines on restarting anticoagulation after a hemorrhagic event.
In this article, we outline a practical framework for approaching this clinical dilemma. Used in conjunction with consideration of a patient’s values and preferences as well as input from experts, this framework can help clinicians guide their patients through this challenging clinical decision. It consists of five questions:
- Why is the patient on anticoagulation, and what is the risk of thromboembolism without it?
- What was the clinical impact of the hemorrhage, and what is the risk of rebleeding if anticoagulation is resumed?
- What additional patient factors should be taken into consideration?
- How long should we wait before restarting anticoagulation?
- Would a newer drug be a better choice?
BLEEDING OCCURS IN 2% TO 3% OF PATIENTS PER YEAR
Most of our information on anticoagulation is about vitamin K antagonists—principally warfarin, in use since the 1950s. Among patients taking warfarin outside of clinical trials, the risk of major bleeding is estimated at 2% to 3% per year.7
However, the target-specific oral anticoagulants rivaroxaban (Xarelto), apixaban (Eliquis), dabigatran (Pradaxa) and edoxaban (Savaysa) are being used more and more, and we include them in our discussion insofar as we have information on them. The rates of bleeding with these new drugs in clinical trials have been comparable to or lower than those with warfarin.8 Postmarketing surveillance is under way.
WHY IS THE PATIENT ON ANTICOAGULATION? WHAT IS THE RISK WITHOUT IT?
Common, evidence-based indications for anticoagulation are to prevent complications in patients with venous thromboembolism and to prevent stroke in patients with atrial fibrillation or a mechanical heart valve. Other uses, such as in heart failure and its sequelae, pulmonary hypertension, and splanchnic or hepatic vein thrombosis, have less robust evidence to support them.
When anticoagulation-related bleeding occurs, it is essential to review why the patient is taking the drug and the risk of thromboembolism without it. Some indications pose a higher risk of thromboembolism than others and so argue more strongly for continuing the treatment.
Douketis et al9 developed a risk-stratification scheme for perioperative thromboembolism. We have modified it by adding the CHA2DS2-VASc score (Table 2),9–11 and believe it can be used more widely.
High-risk indications
Conditions that pose a high risk of thrombosis almost always require restarting anticoagulation. Here, the most appropriate question nearly always is not if anticoagulation should be restarted, but when. Examples:
- A mechanical mitral valve
- Antiphospholipid antibody syndrome with recurrent thromboembolic events.
Lower-risk indications
Lower-risk indications allow more leeway in determining if anticoagulation should be resumed. The most straightforward cases fall well within established guidelines. Examples:
- Atrial fibrillation and a CHA2DS2-VASc score of 1. The 2014 guidelines from the American College of Cardiology, American Heart Association, and Heart Rhythm Society10 suggest that patients with nonvalvular atrial fibrillation and a CHA2DS2-VASc score of 1 have three options: an oral anticoagulant, aspirin, and no antithrombotic therapy. If such a patient on anticoagulant therapy subsequently experiences a major gastrointestinal hemorrhage requiring transfusion and intensive care and no definitively treatable source of bleeding is found on endoscopy, one can argue that the risks of continued anticoagulation (recurrent bleeding) now exceed the benefits and that the patient would be better served by aspirin or even no antithrombotic therapy.
- After 6 months of anticoagulation for unprovoked deep vein thrombosis. Several studies showed that aspirin reduced the risk of recurrent venous thromboembolism in patients who completed an initial 6-month course of anticoagulation.12–15 Though these studies did not specifically compare aspirin with warfarin or target-specific oral anticoagulants in preventing recurrent venous thromboembolism after a hemorrhage, it is reasonable to extrapolate their results to this situation.
If the risk of recurrent hemorrhage on anticoagulation is considered to be too great, then aspirin is an alternative to no anticoagulation, as it reduces the risk of recurrent venous thromboembolism.16 However, we advise caution if the bleeding lesion may be specifically exacerbated by aspirin, particularly upper gastrointestinal ulcers.
Moderate-risk indications
- After a partial course of anticoagulation for provoked venous thromboembolism. Suppose a patient in the 10th week of a planned 12-week course of anticoagulation for a surgically provoked, first deep vein thrombosis presents with abdominal pain and is found to have a retroperitoneal hematoma. In light of the risk of recurrent bleeding vs the benefit of resuming anticoagulation for the limited remaining period, her 12-week treatment course can reasonably be shortened to 10 weeks.
The risk of recurrent venous thromboembolism when a patient is off anticoagulation decreases with time from the initial event. The highest risk, estimated at 0.3% to 1.3% per day, is in the first 4 weeks, falling to 0.03% to 0.2% per day in weeks 5 through 12, and 0.05% per day thereafter.17–20
Additionally, a pooled analysis of seven randomized trials suggests that patients with isolated, distal deep vein thrombosis provoked by a temporary risk factor did not have a high risk of recurrence after being treated for 4 to 6 weeks.21 These analyses are based on vitamin K antagonists, though it seems reasonable to extrapolate this information to the target-specific oral anticoagulants.
More challenging are situations in which the evidence supporting the initial or continued need for anticoagulation is less robust, such as in heart failure, pulmonary hypertension, or splanchnic and hepatic vein thrombosis. In these cases, the lack of strong evidence supporting the use of anticoagulation should make us hesitate to resume it after bleeding.
WHAT WAS THE CLINICAL IMPACT? WHAT IS THE RISK OF REBLEEDING?
Different groups have defined major and minor bleeding in different ways.22,23 Several have proposed criteria to standardize how bleeding events (on warfarin and otherwise) are classified,23–25 but the definitions differ.
Specifically, all agree that a “major” bleeding event is one that is fatal, involves bleeding into a major organ, or leads to a substantial decline in hemoglobin level. However, the Thrombolysis in Myocardial Infarction trials use a decline of more than 5 g/dL in their definition,23,25 while the International Society on Thrombosis and Haemostasis uses 2 g/dL.24
Here, we review the clinical impact of the most common sources of anticoagulation-related hemorrhage—gastrointestinal, soft tissue, and urinary tract26—as well as intracerebral hemorrhage, a less common but more uniformly devastating event.27
Clinical impact of gastrointestinal hemorrhage
Each year, about 4.5% of patients taking warfarin have a gastrointestinal hemorrhage, though not all of these events are major.28 Evolving data suggest that the newer agents (particularly dabigatran, rivaroxaban, and edoxaban) pose a higher risk of gastrointestinal bleeding than warfarin.29 Patients may need plasma and blood transfusions and intravenous phytonadione, all of which carry risks, albeit small.
Frequently, endoscopy is needed to find the source of bleeding and to control it. If this does not work, angiographic intervention to infuse vasoconstrictors or embolic coils into the culprit artery may be required, and some patients need surgery. Each intervention carries its own risk.
Clinical impact of soft-tissue hemorrhage
Soft-tissue hemorrhage accounts for more than 20% of warfarin-related bleeding events26; as yet, we know of no data on the rate with the new drugs. Soft-tissue hemorrhage is often localized to the large muscles of the retroperitoneum and legs. Though retroperitoneal hemorrhage accounts for a relatively small portion of soft-tissue hemorrhages, it is associated with high rates of morbidity and death and will therefore be our focus.26
Much of the clinical impact of retroperitoneal hemorrhage is from a mass effect that causes abdominal compartment syndrome, hydroureter, ileus, abscess formation, and acute and chronic pain. At least 20% of cases are associated with femoral neuropathy. It can also lead to deep vein thrombosis from venous compression, coupled with hypercoagulability in response to bleeding. Brisk bleeding can lead to shock and death, and the mortality rate in retroperitoneal hemorrhage is estimated at 20% or higher.30
In many cases, the retroperitoneal hemorrhage will self-tamponade and the blood will be reabsorbed once the bleeding has stopped, but uncontrolled bleeding may require surgical or angiographic intervention.30
Clinical impact of urinary tract hemorrhage
Gross or microscopic hematuria can be found in an estimated 2% to 24% of patients taking warfarin31–33; data are lacking for the target-specific oral anticoagulants. Interventions required to manage urinary tract bleeding include bladder irrigation and, less often, transfusion.31 Since a significant number of cases of hematuria are due to neoplastic disease,32 a diagnostic workup with radiographic imaging of the upper tract and cystoscopy of the lower tract is usually required.31 While life-threatening hemorrhage is uncommon, complications such as transient urinary obstruction from clots may occur.
Clinical impact of intracranial hemorrhage
Intracranial hemorrhage is the most feared and deadly of the bleeding complications of anticoagulation. The incidence in patients on warfarin is estimated at 2% to 3% per year, which is markedly higher than the estimated incidence of 25 per 100,000 person-years in the general population.34 Emerging data indicate that the newer drugs are also associated with a risk of intracranial hemorrhage, though the risk is about half that with vitamin K antagonists.35 Intracranial hemorrhage leads to death or disability in 76% of cases, compared with 3% of cases of bleeding from the gastrointestinal or urinary tract.27
Regardless of the source of bleeding, hospitalization is likely to be required and may be prolonged, with attendant risks of nosocomial harms such as infection.
Risk of rebleeding
Given the scope and severity of anticoagulation-related bleeding, there is strong interest in predicting and preventing it. By some estimates, the incidence of recurrent bleeding after resuming vitamin K antagonists is 8% to 13%.22 Although there are several indices for predicting the risk of major bleeding when starting anticoagulation, there are currently no validated tools to estimate a patient’s risk of rebleeding.36
The patient factor that most consistently predicts major bleeding is a history of bleeding, particularly from the gastrointestinal tract. Finding and controlling the source of bleeding is important.26,37 For example, a patient with gross hematuria who is found on cystoscopy to have a urothelial papilloma is unlikely to have rebleeding if the tumor is successfully resected and serial follow-up shows no regrowth. In contrast, consider a patient with a major gastrointestinal hemorrhage, the source of which remains elusive after upper, lower, and capsule endoscopy or, alternatively, is suspected to be from one of multiple angiodysplastic lesions. Without definitive source management, this patient faces a high risk of rebleeding.
With or without anticoagulation, after a first intracranial hemorrhage the risk of another one is estimated at 2% to 4% per year.34 An observational study found a recurrence rate of 7.5% when vitamin K antagonist therapy was started after an intracranial hemorrhage (though not all patients were on a vitamin K antagonist at the time of the first hemorrhage).38
Patients with lobar hemorrhage and those with suspected cerebral amyloid angiopathy may be at particularly high risk if anticoagulation is resumed. Conversely, initial events attributed to uncontrolled hypertension that subsequently can be well controlled may portend a lower risk of rebleeding.34 For other types of intracranial hemorrhage, recurrence rates can be even higher. Irrespective of anticoagulation, one prospective study estimated the crude annual rebleeding rate with untreated arteriovenous malformations to be 7%.39 In chronic subdural hematoma, the recurrence rate after initial drainage has been estimated at 9.2% to 26.5%, with use of anticoagulants (in this case, vitamin K antagonists) being an independent predictor of recurrence.40
WHAT OTHER PATIENT FACTORS NEED CONSIDERATION?
Target INR on warfarin
An important factor influencing the risk of bleeding with warfarin is the intensity of this therapy.37 A meta-analysis41 found that the risks of major hemorrhage and thromboembolism are minimized if the goal international normalized ratio (INR) is 2.0 to 3.0. When considering resuming anticoagulation after bleeding, make sure the therapeutic target is appropriate.37
Table 3 summarizes recommended therapeutic ranges for frequently encountered indications for warfarin.36,42,43
INR at time of the event and challenges in controlling it
The decision to resume anticoagulation in patients who bled while using warfarin must take into account the actual INR at the time of the event.
For example, consider a patient whose INR values are consistently in the therapeutic range. While on vacation, he receives ciprofloxacin for acute prostatitis from an urgent care team, and no adjustment to INR monitoring or warfarin dose is made. Several days later, he presents with lower gastrointestinal bleeding. His INR is 8, and colonoscopy reveals diverticulosis with a bleeding vessel, responsive to endoscopic therapy. After controlling the source of bleeding and reinforcing the need to always review new medications for potential interactions with anticoagulation, it is reasonable to expect that he once again will be able to keep his INR in the therapeutic range.
A patient on anticoagulation for the same indication but who has a history of repeated supratherapeutic levels, poor adherence, or poor access to INR monitoring poses very different concerns about resuming anticoagulation (as well as which agent to use, as we discuss below).
Of note, a high INR alone does not explain bleeding. It is estimated that a workup for gastrointestinal bleeding and gross hematuria uncovers previously undetected lesions in approximately one-third of cases involving warfarin.26 A similar malignancy-unmasking effect is now recognized in patients using the target-specific oral agents who experience gastrointestinal bleeding.44 Accordingly, we recommend a comprehensive source evaluation for any anticoagulation-related hemorrhage.
Comorbid conditions
Comorbid conditions associated with bleeding include cancer, end-stage renal disease, liver disease, arterial hypertension, prior stroke, and alcohol abuse.37,45 Gait instability, regardless of cause, may also increase the risk of trauma-related hemorrhage, but some have estimated that a patient would need to fall multiple times per week to contraindicate anticoagulation on the basis of falls alone.46
Concurrent medications
Concomitant therapies, including antiplatelet drugs and nonsteroidal anti-inflammatory drugs, increase bleeding risk.47,48 Aspirin and the nonsteroidals, in addition to having antiplatelet effects, also can cause gastric erosion.37 In evaluating whether and when to restart anticoagulation, it is advisable to review the role that concomitant therapies may have had in the index bleeding event and to evaluate the risks and benefits of these other agents.
Additionally, warfarin has many interactions. Although the newer drugs are lauded for having fewer interactions, they are not completely free of them, and the potential for interactions must always be reviewed.49 Further, unlike warfarin therapy, therapy with the newer agents is not routinely monitored with laboratory tests, so toxicity (or underdosing) may not be recognized until an adverse clinical event occurs. Ultimately, it may be safer to resume anticoagulation after a contributing drug can be safely discontinued.
Advanced age
The influence that the patient’s age should have on the decision to restart anticoagulation is unclear. Although the risk of intracranial hemorrhage increases with age, particularly after age 80, limited data exist in this population, particularly with regard to rebleeding. Further, age is a major risk factor for most thrombotic events, including venous thromboembolism and stroke from atrial fibrillation, so although the risks of anticoagulation may be higher, the benefits may also be higher than in younger patients.37,46 We discourage using age alone as a reason to withhold anticoagulation after a hemorrhage.
HOW LONG SHOULD WE WAIT TO RESTART ANTICOAGULATION?
We lack conclusive data on how long to wait to restart anticoagulation after an anticoagulation-associated hemorrhage.
The decision is complicated by evidence suggesting a rebound effect, with an increased risk of pulmonary embolism and atrial fibrillation-related stroke during the first 90 days of interruption of therapy with warfarin as well as with target-specific oral anticoagulants.3–8 In anticoagulation-associated retroperitoneal bleeding, there is increased risk of deep vein thrombosis from compression, even if venous thromboembolism was not the initial indication for anticoagulation.30
In patients with intracranial hemorrhage, evidence suggests that the intracranial hemorrhage itself increases the risk of arterial and venous thromboembolic events. Irrespective of whether a patient was previously on anticoagulation, the risk of arterial and venous thromboembolic events approaches 7% during the initial intracranial hemorrhage-related hospitalization and 9% during the first 90 days.34,50,51
To date, the only information we have about when to resume anticoagulation comes from patients taking vitamin K antagonists.
Timing after gastrointestinal bleeding
Small case series suggest that in the first 2 months after warfarin-associated gastrointestinal bleeding, there is substantial risk of rebleeding when anticoagulation is resumed—and of thrombosis when it is not.52,53 Two retrospective cohort studies may provide some guidance in this dilemma.28,54
Witt et al28 followed 442 patients who presented with gastrointestinal bleeding from any site during warfarin therapy for varied indications for up to 90 days after the index bleeding event. The risk of death was three times lower in patients who restarted warfarin than in those who did not, and their rate of thrombotic events was 10 times lower. The risk of recurrent gastrointestinal bleeding was statistically insignificant, and there were no fatal bleeding events. Anticoagulant therapy was generally resumed within 1 week of the bleeding event, at a median of 4 days.28,55
Qureshi et al54 performed a retrospective cohort study of 1,329 patients with nonvalvular atrial fibrillation who had experienced a gastrointestinal hemorrhage while taking warfarin. They found that resuming warfarin after 7 days was not associated with a higher risk of recurrent gastrointestinal bleeding and that the rates of death and thromboembolism were lower than in patients who resumed warfarin after 30 days. On the other hand, the risk of recurrent gastrointestinal bleeding was significantly greater if therapy was resumed within the first week.
In view of these studies, we believe that most patients should resume anticoagulation after 4 to 7 days of interruption after gastrointestinal bleeding.55
Timing after soft-tissue hemorrhage
The literature on resuming anticoagulation after soft-tissue hemorrhage is sparse. A retrospective study52 looked at this question in patients with spontaneous rectal sheath hematoma who had been receiving antiplatelet drugs, intravenous heparin, vitamin K antagonists, or a combination of these, but not target-specific agents. More than half of the patients were on vitamin K antagonists at the time of hemorrhage. Analysis suggested that when benefits of resuming anticoagulation are believed to outweigh risks, it is reasonable to resume anticoagulation 4 days after the index event.56
Timing after intracranial hemorrhage
Anticoagulation should not be considered within the first 24 hours after intracranial hemorrhage, as over 70% of patients develop some amount of hematoma expansion during this time.34,57 The period thereafter poses a challenge, as the risk of hematoma expansion decreases while the risk of arterial and venous thromboembolism is ongoing and cumulative.50
Perhaps surprisingly, national guidelines suggest starting prophylactic-dosed anticoagulation early in all intracranial hemorrhage patients, including those not previously on warfarin.58,59 In a randomized trial, Boeer et al60 concluded that starting low-dose subcutaneous heparin the day after an intracranial hemorrhage decreased the risk of thromboembolism without increasing the risk of rebleeding.60 Dickmann et al61 similarly concluded that there was no increased risk of rebleeding with early prophylactic-dosed subcutaneous heparin.61 Optimal mechanical thromboprophylaxis, including graduated compression stockings and intermittent pneumatic compression stockings, is also encouraged.34
Expert opinion remains divided on when and if anticoagulants should be resumed.34,62 The American Heart Association suggests that in nonvalvular atrial fibrillation, long-term anticoagulation should be avoided after spontaneous lobar hemorrhage; antiplatelet agents can be considered instead.58 In nonlobar hemorrhage, the American Heart Association suggests that anticoagulation be considered, depending on strength of indication, 7 to 10 days after the onset.58 The European Stroke Initiative suggests patients with strong indications for anticoagulation be restarted on warfarin 10 to 14 days after the event, depending on the risk of thromboembolism and recurrent intracranial hemorrhage.59 Others suggest delaying resumption to 10 to 30 weeks after an index intracranial hemorrhage.63
Overall, in the immediate acute period of intracranial hemorrhage, most patients will likely benefit from acute reversal of anticoagulation, followed by institution of prophylactic-dose anticoagulation after the first 24 hours. Going forward, patients who remain at higher risk of a recurrence of anticoagulant-related intracranial hemorrhage (such as those with lobar hemorrhage, suspected cerebral amyloid angiopathy, and other high-risk factors) than of thromboembolic events may be best managed without anticoagulants. Alternatively, patients with deep hemispheric intracranial hemorrhage, hypertension that can be well controlled, and a high risk of serious thromboembolism may experience net benefit from restarting anticoagulation.34
We recommend considering restarting anticoagulation 7 days after the onset of intracranial hemorrhage in patients at high risk of thromboembolism and after at least 14 days for patients at lower risk (Table 2). Discussions with neurologic and neurosurgical consultants should also inform this timing decision.
WOULD A NEWER DRUG BE A BETTER CHOICE?
The emergence of target-specific oral anticoagulants, including factor Xa inhibitors such as rivaroxaban, apixaban, and edoxaban and the direct thrombin inhibitor dabigatran etexilate, presents further challenges in managing anticoagulation after hemorrhage. Table 4 summarizes the current FDA-approved indications.64–67
These newer agents are attractive because, compared with warfarin, they have wider therapeutic windows, faster onset and offset of action, and fewer drug and food interactions.68 A meta-analysis of data available to date suggests that the new drugs, compared with warfarin, show a favorable risk-benefit profile with reductions in stroke, intracranial hemorrhage, and mortality with similar overall major bleeding rates, except for a possible increase in gastrointestinal bleeding.68
However, when managing anticoagulation after a bleeding event, the newer agents are challenging for two reasons: they may be associated with a higher incidence of gastrointestinal bleeding than warfarin, and they lack the typical reversal agents that can be used to manage an acute bleeding event.68,69
In individual studies comparing warfarin with dabigatran,70 rivaroxaban,71 apixaban,72 or edoxaban73 for stroke prevention in patients with atrial fibrillation, there was no significant difference in the rate of major bleeding between dabigatran in its higher dose (150 mg twice a day) or rivaroxaban compared with warfarin.70,71 The risk of major bleeding was actually lower with apixaban72 and edoxaban.73
In regard to specific types of major bleeding, the rate of intracranial hemorrhage was significantly lower with dabigatran, rivaroxaban, apixaban, and edoxaban than with warfarin.35,68–73 Some have proposed that since the brain is high in tissue factor, inhibition of tissue factor-factor VIIa complexes by vitamin K antagonists leaves the brain vulnerable to hemorrhage. Others suggest that the targeted mechanism of target-specific agents, as opposed to the multiple pathways in both the intrinsic and extrinsic coagulation cascade that vitamin K antagonists affect, may explain this difference.35,74,75
However, some studies suggest that rivaroxaban and the higher doses of dabigatran and edoxaban are associated with higher rates of major gastrointestinal bleeding compared with warfarin.69–71,76 But apixaban demonstrated no significant difference in gastrointestinal bleeding, and instead demonstrated rates of gastrointestinal bleeding comparable to that with aspirin for stroke prevention in atrial fibrillation.72
The new oral anticoagulants lack antidotes or reversal agents such as phytonadione and fresh-frozen plasma that are available to manage warfarin-associated bleeding events. Other proposed reversal options for the new agents include activated charcoal (if the drugs were taken recently enough to remain in the gastrointestinal tract) and concentrated clotting factor product, though research is ongoing in regards to the most appropriate use in clinical practice.37,69 Unlike rivaroxaban and apixaban, dabigatran has low plasma protein binding and is dialyzable, which provides another strategy in managing dabigatran-related bleeding.69
Of note, the above bleeding risk calculations relate to the first anticoagulant-related bleeding event, though presumably the same risk comparison across agents may be applicable to rebleeding events. Given the data above, when anticoagulation is to be resumed after an intracranial hemorrhage, the risk of rebleeding, particularly in the form of recurrent intracranial hemorrhage, may be lower if a target-specific oral anticoagulant is used.75 Similarly, when anticoagulation is to be resumed after a gastrointestinal bleeding event, reinitiation with warfarin or apixaban therapy may present the lowest risk of recurrent gastrointestinal rebleeding. In other sources of bleeding, such as retroperitoneal bleeding, we suggest consideration of transitioning to warfarin, given the availability of reversal agents in the event of recurrent bleeding.
Other important drug-specific factors that must be noted when selecting an agent with which to resume anticoagulation after a hemorrhage include the following:
- In patients with significant renal impairment, the choice of agent will be limited to a vitamin K antagonist.77
- A meta-analysis of randomized clinical trials suggests that in the elderly (age 75 and older) target-specific oral anticoagulants did not cause excess bleeding and were associated with at least equal efficacy compared with vitamin K antagonists.78
- Target-specific oral anticoagulants may be beneficial in patients who have challenges in achieving INR targets, as evidence suggests that switching to them is associated with a reduction in bleeding for patients who struggle to maintain an appropriately therapeutic INR.68 On the other hand, if there is concern that a patient may occasionally miss doses of an anticoagulant, given the rapid onset and offset of action of target-specific agents compared with warfarin, a missed dose of a target-specific agent may result in faster dissolution of anticoagulant effect and increased risk of thrombotic events, and lapses in anticoagulation will not be identified by routine drug monitoring.6–8,75 As such, it is vital to have a frank discussion with any patient who has difficulty maintaining therapeutic INRs on warfarin treatment to make sure that he or she is not missing doses.
- If there is no clear and compelling reason to select a particular agent, cost considerations should be taken into account. We have included estimated 30-day pricing for the various agents in Table 4.
If a patient receiving anticoagulant therapy suffers a bleeding event, the patient and physician must decide whether and how soon to restart the therapy, and with what agent.
Foremost on our minds tends to be the risk of another hemorrhage. Subtler to appreciate immediately after an event is the continued risk of thrombosis, often from the same medical condition that prompted anticoagulation therapy in the first place (Table 1).
Complicating the decision, there may be a rebound effect: some thrombotic events such as pulmonary embolism and atrial fibrillation-related stroke may be more likely to occur in the first weeks after stopping warfarin than during similar intervals in patients who have not been taking it.1–3 The same thing may happen with the newer, target-specific oral anticoagulants.4–6
Although we have evidence-based guidelines for initiating and managing anticoagulant therapy, ample data on adverse events, and protocols for reversing anticoagulation if bleeding occurs, we do not have clear guidelines on restarting anticoagulation after a hemorrhagic event.
In this article, we outline a practical framework for approaching this clinical dilemma. Used in conjunction with consideration of a patient’s values and preferences as well as input from experts, this framework can help clinicians guide their patients through this challenging clinical decision. It consists of five questions:
- Why is the patient on anticoagulation, and what is the risk of thromboembolism without it?
- What was the clinical impact of the hemorrhage, and what is the risk of rebleeding if anticoagulation is resumed?
- What additional patient factors should be taken into consideration?
- How long should we wait before restarting anticoagulation?
- Would a newer drug be a better choice?
BLEEDING OCCURS IN 2% TO 3% OF PATIENTS PER YEAR
Most of our information on anticoagulation is about vitamin K antagonists—principally warfarin, in use since the 1950s. Among patients taking warfarin outside of clinical trials, the risk of major bleeding is estimated at 2% to 3% per year.7
However, the target-specific oral anticoagulants rivaroxaban (Xarelto), apixaban (Eliquis), dabigatran (Pradaxa) and edoxaban (Savaysa) are being used more and more, and we include them in our discussion insofar as we have information on them. The rates of bleeding with these new drugs in clinical trials have been comparable to or lower than those with warfarin.8 Postmarketing surveillance is under way.
WHY IS THE PATIENT ON ANTICOAGULATION? WHAT IS THE RISK WITHOUT IT?
Common, evidence-based indications for anticoagulation are to prevent complications in patients with venous thromboembolism and to prevent stroke in patients with atrial fibrillation or a mechanical heart valve. Other uses, such as in heart failure and its sequelae, pulmonary hypertension, and splanchnic or hepatic vein thrombosis, have less robust evidence to support them.
When anticoagulation-related bleeding occurs, it is essential to review why the patient is taking the drug and the risk of thromboembolism without it. Some indications pose a higher risk of thromboembolism than others and so argue more strongly for continuing the treatment.
Douketis et al9 developed a risk-stratification scheme for perioperative thromboembolism. We have modified it by adding the CHA2DS2-VASc score (Table 2),9–11 and believe it can be used more widely.
High-risk indications
Conditions that pose a high risk of thrombosis almost always require restarting anticoagulation. Here, the most appropriate question nearly always is not if anticoagulation should be restarted, but when. Examples:
- A mechanical mitral valve
- Antiphospholipid antibody syndrome with recurrent thromboembolic events.
Lower-risk indications
Lower-risk indications allow more leeway in determining if anticoagulation should be resumed. The most straightforward cases fall well within established guidelines. Examples:
- Atrial fibrillation and a CHA2DS2-VASc score of 1. The 2014 guidelines from the American College of Cardiology, American Heart Association, and Heart Rhythm Society10 suggest that patients with nonvalvular atrial fibrillation and a CHA2DS2-VASc score of 1 have three options: an oral anticoagulant, aspirin, and no antithrombotic therapy. If such a patient on anticoagulant therapy subsequently experiences a major gastrointestinal hemorrhage requiring transfusion and intensive care and no definitively treatable source of bleeding is found on endoscopy, one can argue that the risks of continued anticoagulation (recurrent bleeding) now exceed the benefits and that the patient would be better served by aspirin or even no antithrombotic therapy.
- After 6 months of anticoagulation for unprovoked deep vein thrombosis. Several studies showed that aspirin reduced the risk of recurrent venous thromboembolism in patients who completed an initial 6-month course of anticoagulation.12–15 Though these studies did not specifically compare aspirin with warfarin or target-specific oral anticoagulants in preventing recurrent venous thromboembolism after a hemorrhage, it is reasonable to extrapolate their results to this situation.
If the risk of recurrent hemorrhage on anticoagulation is considered to be too great, then aspirin is an alternative to no anticoagulation, as it reduces the risk of recurrent venous thromboembolism.16 However, we advise caution if the bleeding lesion may be specifically exacerbated by aspirin, particularly upper gastrointestinal ulcers.
Moderate-risk indications
- After a partial course of anticoagulation for provoked venous thromboembolism. Suppose a patient in the 10th week of a planned 12-week course of anticoagulation for a surgically provoked, first deep vein thrombosis presents with abdominal pain and is found to have a retroperitoneal hematoma. In light of the risk of recurrent bleeding vs the benefit of resuming anticoagulation for the limited remaining period, her 12-week treatment course can reasonably be shortened to 10 weeks.
The risk of recurrent venous thromboembolism when a patient is off anticoagulation decreases with time from the initial event. The highest risk, estimated at 0.3% to 1.3% per day, is in the first 4 weeks, falling to 0.03% to 0.2% per day in weeks 5 through 12, and 0.05% per day thereafter.17–20
Additionally, a pooled analysis of seven randomized trials suggests that patients with isolated, distal deep vein thrombosis provoked by a temporary risk factor did not have a high risk of recurrence after being treated for 4 to 6 weeks.21 These analyses are based on vitamin K antagonists, though it seems reasonable to extrapolate this information to the target-specific oral anticoagulants.
More challenging are situations in which the evidence supporting the initial or continued need for anticoagulation is less robust, such as in heart failure, pulmonary hypertension, or splanchnic and hepatic vein thrombosis. In these cases, the lack of strong evidence supporting the use of anticoagulation should make us hesitate to resume it after bleeding.
WHAT WAS THE CLINICAL IMPACT? WHAT IS THE RISK OF REBLEEDING?
Different groups have defined major and minor bleeding in different ways.22,23 Several have proposed criteria to standardize how bleeding events (on warfarin and otherwise) are classified,23–25 but the definitions differ.
Specifically, all agree that a “major” bleeding event is one that is fatal, involves bleeding into a major organ, or leads to a substantial decline in hemoglobin level. However, the Thrombolysis in Myocardial Infarction trials use a decline of more than 5 g/dL in their definition,23,25 while the International Society on Thrombosis and Haemostasis uses 2 g/dL.24
Here, we review the clinical impact of the most common sources of anticoagulation-related hemorrhage—gastrointestinal, soft tissue, and urinary tract26—as well as intracerebral hemorrhage, a less common but more uniformly devastating event.27
Clinical impact of gastrointestinal hemorrhage
Each year, about 4.5% of patients taking warfarin have a gastrointestinal hemorrhage, though not all of these events are major.28 Evolving data suggest that the newer agents (particularly dabigatran, rivaroxaban, and edoxaban) pose a higher risk of gastrointestinal bleeding than warfarin.29 Patients may need plasma and blood transfusions and intravenous phytonadione, all of which carry risks, albeit small.
Frequently, endoscopy is needed to find the source of bleeding and to control it. If this does not work, angiographic intervention to infuse vasoconstrictors or embolic coils into the culprit artery may be required, and some patients need surgery. Each intervention carries its own risk.
Clinical impact of soft-tissue hemorrhage
Soft-tissue hemorrhage accounts for more than 20% of warfarin-related bleeding events26; as yet, we know of no data on the rate with the new drugs. Soft-tissue hemorrhage is often localized to the large muscles of the retroperitoneum and legs. Though retroperitoneal hemorrhage accounts for a relatively small portion of soft-tissue hemorrhages, it is associated with high rates of morbidity and death and will therefore be our focus.26
Much of the clinical impact of retroperitoneal hemorrhage is from a mass effect that causes abdominal compartment syndrome, hydroureter, ileus, abscess formation, and acute and chronic pain. At least 20% of cases are associated with femoral neuropathy. It can also lead to deep vein thrombosis from venous compression, coupled with hypercoagulability in response to bleeding. Brisk bleeding can lead to shock and death, and the mortality rate in retroperitoneal hemorrhage is estimated at 20% or higher.30
In many cases, the retroperitoneal hemorrhage will self-tamponade and the blood will be reabsorbed once the bleeding has stopped, but uncontrolled bleeding may require surgical or angiographic intervention.30
Clinical impact of urinary tract hemorrhage
Gross or microscopic hematuria can be found in an estimated 2% to 24% of patients taking warfarin31–33; data are lacking for the target-specific oral anticoagulants. Interventions required to manage urinary tract bleeding include bladder irrigation and, less often, transfusion.31 Since a significant number of cases of hematuria are due to neoplastic disease,32 a diagnostic workup with radiographic imaging of the upper tract and cystoscopy of the lower tract is usually required.31 While life-threatening hemorrhage is uncommon, complications such as transient urinary obstruction from clots may occur.
Clinical impact of intracranial hemorrhage
Intracranial hemorrhage is the most feared and deadly of the bleeding complications of anticoagulation. The incidence in patients on warfarin is estimated at 2% to 3% per year, which is markedly higher than the estimated incidence of 25 per 100,000 person-years in the general population.34 Emerging data indicate that the newer drugs are also associated with a risk of intracranial hemorrhage, though the risk is about half that with vitamin K antagonists.35 Intracranial hemorrhage leads to death or disability in 76% of cases, compared with 3% of cases of bleeding from the gastrointestinal or urinary tract.27
Regardless of the source of bleeding, hospitalization is likely to be required and may be prolonged, with attendant risks of nosocomial harms such as infection.
Risk of rebleeding
Given the scope and severity of anticoagulation-related bleeding, there is strong interest in predicting and preventing it. By some estimates, the incidence of recurrent bleeding after resuming vitamin K antagonists is 8% to 13%.22 Although there are several indices for predicting the risk of major bleeding when starting anticoagulation, there are currently no validated tools to estimate a patient’s risk of rebleeding.36
The patient factor that most consistently predicts major bleeding is a history of bleeding, particularly from the gastrointestinal tract. Finding and controlling the source of bleeding is important.26,37 For example, a patient with gross hematuria who is found on cystoscopy to have a urothelial papilloma is unlikely to have rebleeding if the tumor is successfully resected and serial follow-up shows no regrowth. In contrast, consider a patient with a major gastrointestinal hemorrhage, the source of which remains elusive after upper, lower, and capsule endoscopy or, alternatively, is suspected to be from one of multiple angiodysplastic lesions. Without definitive source management, this patient faces a high risk of rebleeding.
With or without anticoagulation, after a first intracranial hemorrhage the risk of another one is estimated at 2% to 4% per year.34 An observational study found a recurrence rate of 7.5% when vitamin K antagonist therapy was started after an intracranial hemorrhage (though not all patients were on a vitamin K antagonist at the time of the first hemorrhage).38
Patients with lobar hemorrhage and those with suspected cerebral amyloid angiopathy may be at particularly high risk if anticoagulation is resumed. Conversely, initial events attributed to uncontrolled hypertension that subsequently can be well controlled may portend a lower risk of rebleeding.34 For other types of intracranial hemorrhage, recurrence rates can be even higher. Irrespective of anticoagulation, one prospective study estimated the crude annual rebleeding rate with untreated arteriovenous malformations to be 7%.39 In chronic subdural hematoma, the recurrence rate after initial drainage has been estimated at 9.2% to 26.5%, with use of anticoagulants (in this case, vitamin K antagonists) being an independent predictor of recurrence.40
WHAT OTHER PATIENT FACTORS NEED CONSIDERATION?
Target INR on warfarin
An important factor influencing the risk of bleeding with warfarin is the intensity of this therapy.37 A meta-analysis41 found that the risks of major hemorrhage and thromboembolism are minimized if the goal international normalized ratio (INR) is 2.0 to 3.0. When considering resuming anticoagulation after bleeding, make sure the therapeutic target is appropriate.37
Table 3 summarizes recommended therapeutic ranges for frequently encountered indications for warfarin.36,42,43
INR at time of the event and challenges in controlling it
The decision to resume anticoagulation in patients who bled while using warfarin must take into account the actual INR at the time of the event.
For example, consider a patient whose INR values are consistently in the therapeutic range. While on vacation, he receives ciprofloxacin for acute prostatitis from an urgent care team, and no adjustment to INR monitoring or warfarin dose is made. Several days later, he presents with lower gastrointestinal bleeding. His INR is 8, and colonoscopy reveals diverticulosis with a bleeding vessel, responsive to endoscopic therapy. After controlling the source of bleeding and reinforcing the need to always review new medications for potential interactions with anticoagulation, it is reasonable to expect that he once again will be able to keep his INR in the therapeutic range.
A patient on anticoagulation for the same indication but who has a history of repeated supratherapeutic levels, poor adherence, or poor access to INR monitoring poses very different concerns about resuming anticoagulation (as well as which agent to use, as we discuss below).
Of note, a high INR alone does not explain bleeding. It is estimated that a workup for gastrointestinal bleeding and gross hematuria uncovers previously undetected lesions in approximately one-third of cases involving warfarin.26 A similar malignancy-unmasking effect is now recognized in patients using the target-specific oral agents who experience gastrointestinal bleeding.44 Accordingly, we recommend a comprehensive source evaluation for any anticoagulation-related hemorrhage.
Comorbid conditions
Comorbid conditions associated with bleeding include cancer, end-stage renal disease, liver disease, arterial hypertension, prior stroke, and alcohol abuse.37,45 Gait instability, regardless of cause, may also increase the risk of trauma-related hemorrhage, but some have estimated that a patient would need to fall multiple times per week to contraindicate anticoagulation on the basis of falls alone.46
Concurrent medications
Concomitant therapies, including antiplatelet drugs and nonsteroidal anti-inflammatory drugs, increase bleeding risk.47,48 Aspirin and the nonsteroidals, in addition to having antiplatelet effects, also can cause gastric erosion.37 In evaluating whether and when to restart anticoagulation, it is advisable to review the role that concomitant therapies may have had in the index bleeding event and to evaluate the risks and benefits of these other agents.
Additionally, warfarin has many interactions. Although the newer drugs are lauded for having fewer interactions, they are not completely free of them, and the potential for interactions must always be reviewed.49 Further, unlike warfarin therapy, therapy with the newer agents is not routinely monitored with laboratory tests, so toxicity (or underdosing) may not be recognized until an adverse clinical event occurs. Ultimately, it may be safer to resume anticoagulation after a contributing drug can be safely discontinued.
Advanced age
The influence that the patient’s age should have on the decision to restart anticoagulation is unclear. Although the risk of intracranial hemorrhage increases with age, particularly after age 80, limited data exist in this population, particularly with regard to rebleeding. Further, age is a major risk factor for most thrombotic events, including venous thromboembolism and stroke from atrial fibrillation, so although the risks of anticoagulation may be higher, the benefits may also be higher than in younger patients.37,46 We discourage using age alone as a reason to withhold anticoagulation after a hemorrhage.
HOW LONG SHOULD WE WAIT TO RESTART ANTICOAGULATION?
We lack conclusive data on how long to wait to restart anticoagulation after an anticoagulation-associated hemorrhage.
The decision is complicated by evidence suggesting a rebound effect, with an increased risk of pulmonary embolism and atrial fibrillation-related stroke during the first 90 days of interruption of therapy with warfarin as well as with target-specific oral anticoagulants.3–8 In anticoagulation-associated retroperitoneal bleeding, there is increased risk of deep vein thrombosis from compression, even if venous thromboembolism was not the initial indication for anticoagulation.30
In patients with intracranial hemorrhage, evidence suggests that the intracranial hemorrhage itself increases the risk of arterial and venous thromboembolic events. Irrespective of whether a patient was previously on anticoagulation, the risk of arterial and venous thromboembolic events approaches 7% during the initial intracranial hemorrhage-related hospitalization and 9% during the first 90 days.34,50,51
To date, the only information we have about when to resume anticoagulation comes from patients taking vitamin K antagonists.
Timing after gastrointestinal bleeding
Small case series suggest that in the first 2 months after warfarin-associated gastrointestinal bleeding, there is substantial risk of rebleeding when anticoagulation is resumed—and of thrombosis when it is not.52,53 Two retrospective cohort studies may provide some guidance in this dilemma.28,54
Witt et al28 followed 442 patients who presented with gastrointestinal bleeding from any site during warfarin therapy for varied indications for up to 90 days after the index bleeding event. The risk of death was three times lower in patients who restarted warfarin than in those who did not, and their rate of thrombotic events was 10 times lower. The risk of recurrent gastrointestinal bleeding was statistically insignificant, and there were no fatal bleeding events. Anticoagulant therapy was generally resumed within 1 week of the bleeding event, at a median of 4 days.28,55
Qureshi et al54 performed a retrospective cohort study of 1,329 patients with nonvalvular atrial fibrillation who had experienced a gastrointestinal hemorrhage while taking warfarin. They found that resuming warfarin after 7 days was not associated with a higher risk of recurrent gastrointestinal bleeding and that the rates of death and thromboembolism were lower than in patients who resumed warfarin after 30 days. On the other hand, the risk of recurrent gastrointestinal bleeding was significantly greater if therapy was resumed within the first week.
In view of these studies, we believe that most patients should resume anticoagulation after 4 to 7 days of interruption after gastrointestinal bleeding.55
Timing after soft-tissue hemorrhage
The literature on resuming anticoagulation after soft-tissue hemorrhage is sparse. A retrospective study52 looked at this question in patients with spontaneous rectal sheath hematoma who had been receiving antiplatelet drugs, intravenous heparin, vitamin K antagonists, or a combination of these, but not target-specific agents. More than half of the patients were on vitamin K antagonists at the time of hemorrhage. Analysis suggested that when benefits of resuming anticoagulation are believed to outweigh risks, it is reasonable to resume anticoagulation 4 days after the index event.56
Timing after intracranial hemorrhage
Anticoagulation should not be considered within the first 24 hours after intracranial hemorrhage, as over 70% of patients develop some amount of hematoma expansion during this time.34,57 The period thereafter poses a challenge, as the risk of hematoma expansion decreases while the risk of arterial and venous thromboembolism is ongoing and cumulative.50
Perhaps surprisingly, national guidelines suggest starting prophylactic-dosed anticoagulation early in all intracranial hemorrhage patients, including those not previously on warfarin.58,59 In a randomized trial, Boeer et al60 concluded that starting low-dose subcutaneous heparin the day after an intracranial hemorrhage decreased the risk of thromboembolism without increasing the risk of rebleeding.60 Dickmann et al61 similarly concluded that there was no increased risk of rebleeding with early prophylactic-dosed subcutaneous heparin.61 Optimal mechanical thromboprophylaxis, including graduated compression stockings and intermittent pneumatic compression stockings, is also encouraged.34
Expert opinion remains divided on when and if anticoagulants should be resumed.34,62 The American Heart Association suggests that in nonvalvular atrial fibrillation, long-term anticoagulation should be avoided after spontaneous lobar hemorrhage; antiplatelet agents can be considered instead.58 In nonlobar hemorrhage, the American Heart Association suggests that anticoagulation be considered, depending on strength of indication, 7 to 10 days after the onset.58 The European Stroke Initiative suggests patients with strong indications for anticoagulation be restarted on warfarin 10 to 14 days after the event, depending on the risk of thromboembolism and recurrent intracranial hemorrhage.59 Others suggest delaying resumption to 10 to 30 weeks after an index intracranial hemorrhage.63
Overall, in the immediate acute period of intracranial hemorrhage, most patients will likely benefit from acute reversal of anticoagulation, followed by institution of prophylactic-dose anticoagulation after the first 24 hours. Going forward, patients who remain at higher risk of a recurrence of anticoagulant-related intracranial hemorrhage (such as those with lobar hemorrhage, suspected cerebral amyloid angiopathy, and other high-risk factors) than of thromboembolic events may be best managed without anticoagulants. Alternatively, patients with deep hemispheric intracranial hemorrhage, hypertension that can be well controlled, and a high risk of serious thromboembolism may experience net benefit from restarting anticoagulation.34
We recommend considering restarting anticoagulation 7 days after the onset of intracranial hemorrhage in patients at high risk of thromboembolism and after at least 14 days for patients at lower risk (Table 2). Discussions with neurologic and neurosurgical consultants should also inform this timing decision.
WOULD A NEWER DRUG BE A BETTER CHOICE?
The emergence of target-specific oral anticoagulants, including factor Xa inhibitors such as rivaroxaban, apixaban, and edoxaban and the direct thrombin inhibitor dabigatran etexilate, presents further challenges in managing anticoagulation after hemorrhage. Table 4 summarizes the current FDA-approved indications.64–67
These newer agents are attractive because, compared with warfarin, they have wider therapeutic windows, faster onset and offset of action, and fewer drug and food interactions.68 A meta-analysis of data available to date suggests that the new drugs, compared with warfarin, show a favorable risk-benefit profile with reductions in stroke, intracranial hemorrhage, and mortality with similar overall major bleeding rates, except for a possible increase in gastrointestinal bleeding.68
However, when managing anticoagulation after a bleeding event, the newer agents are challenging for two reasons: they may be associated with a higher incidence of gastrointestinal bleeding than warfarin, and they lack the typical reversal agents that can be used to manage an acute bleeding event.68,69
In individual studies comparing warfarin with dabigatran,70 rivaroxaban,71 apixaban,72 or edoxaban73 for stroke prevention in patients with atrial fibrillation, there was no significant difference in the rate of major bleeding between dabigatran in its higher dose (150 mg twice a day) or rivaroxaban compared with warfarin.70,71 The risk of major bleeding was actually lower with apixaban72 and edoxaban.73
In regard to specific types of major bleeding, the rate of intracranial hemorrhage was significantly lower with dabigatran, rivaroxaban, apixaban, and edoxaban than with warfarin.35,68–73 Some have proposed that since the brain is high in tissue factor, inhibition of tissue factor-factor VIIa complexes by vitamin K antagonists leaves the brain vulnerable to hemorrhage. Others suggest that the targeted mechanism of target-specific agents, as opposed to the multiple pathways in both the intrinsic and extrinsic coagulation cascade that vitamin K antagonists affect, may explain this difference.35,74,75
However, some studies suggest that rivaroxaban and the higher doses of dabigatran and edoxaban are associated with higher rates of major gastrointestinal bleeding compared with warfarin.69–71,76 But apixaban demonstrated no significant difference in gastrointestinal bleeding, and instead demonstrated rates of gastrointestinal bleeding comparable to that with aspirin for stroke prevention in atrial fibrillation.72
The new oral anticoagulants lack antidotes or reversal agents such as phytonadione and fresh-frozen plasma that are available to manage warfarin-associated bleeding events. Other proposed reversal options for the new agents include activated charcoal (if the drugs were taken recently enough to remain in the gastrointestinal tract) and concentrated clotting factor product, though research is ongoing in regards to the most appropriate use in clinical practice.37,69 Unlike rivaroxaban and apixaban, dabigatran has low plasma protein binding and is dialyzable, which provides another strategy in managing dabigatran-related bleeding.69
Of note, the above bleeding risk calculations relate to the first anticoagulant-related bleeding event, though presumably the same risk comparison across agents may be applicable to rebleeding events. Given the data above, when anticoagulation is to be resumed after an intracranial hemorrhage, the risk of rebleeding, particularly in the form of recurrent intracranial hemorrhage, may be lower if a target-specific oral anticoagulant is used.75 Similarly, when anticoagulation is to be resumed after a gastrointestinal bleeding event, reinitiation with warfarin or apixaban therapy may present the lowest risk of recurrent gastrointestinal rebleeding. In other sources of bleeding, such as retroperitoneal bleeding, we suggest consideration of transitioning to warfarin, given the availability of reversal agents in the event of recurrent bleeding.
Other important drug-specific factors that must be noted when selecting an agent with which to resume anticoagulation after a hemorrhage include the following:
- In patients with significant renal impairment, the choice of agent will be limited to a vitamin K antagonist.77
- A meta-analysis of randomized clinical trials suggests that in the elderly (age 75 and older) target-specific oral anticoagulants did not cause excess bleeding and were associated with at least equal efficacy compared with vitamin K antagonists.78
- Target-specific oral anticoagulants may be beneficial in patients who have challenges in achieving INR targets, as evidence suggests that switching to them is associated with a reduction in bleeding for patients who struggle to maintain an appropriately therapeutic INR.68 On the other hand, if there is concern that a patient may occasionally miss doses of an anticoagulant, given the rapid onset and offset of action of target-specific agents compared with warfarin, a missed dose of a target-specific agent may result in faster dissolution of anticoagulant effect and increased risk of thrombotic events, and lapses in anticoagulation will not be identified by routine drug monitoring.6–8,75 As such, it is vital to have a frank discussion with any patient who has difficulty maintaining therapeutic INRs on warfarin treatment to make sure that he or she is not missing doses.
- If there is no clear and compelling reason to select a particular agent, cost considerations should be taken into account. We have included estimated 30-day pricing for the various agents in Table 4.
- Jaffer AK, Brotman DJ, Bash LD, Mahmood SK, Lott B, White RH. Variations in perioperative warfarin management: outcomes and practice patterns at nine hospitals. Am J Med 2010; 123:141–150.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost 2010; 8:884–890.
- Raunsø J, Selmer C, Olesen JB, et al. Increased short-term risk of thrombo-embolism or death after interruption of warfarin treatment in patients with atrial fibrillation. Eur Heart J 2012; 33:1886–1892.
- Xarelto (rivaroxaban). Highlights of prescribing information. Jansen Pharmaceuticals, Inc. www.xareltohcp.com/sites/default/files/pdf/xarelto_0.pdf#zoom=100. Accessed March 9, 2015.
- Pradaxa (dabigatran etexilate mesylate). Highlights of prescribing information. Boehringer Ingelheim Pharmaceuticals, Inc. http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed March 9, 2015.
- Eliquis (apixaban). Highlights of prescribing information. Bristol-Myers Squibb Company. http://packageinserts.bms.com/pi/pi_eliquis.pdf. Accessed March 9, 2015.
- Schulman S, Beyth RJ, Kearon C, Levine MN; American College of Chest Physicians. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest 2008; 133(suppl 6):257S–298S.
- Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated bleeding. Blood 2014; 123:1152–1158.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Cannegieter SC, Rosendaal FR, Briët E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation 1994; 89:635–641.
- Warkentin TE. Aspirin for dual prevention of venous and arterial thrombosis. N Engl J Med 2012; 367:2039–2041.
- Simes J, Becattini C, Agnelli G, et al; INSPIRE Study Investigators* (International Collaboration of Aspirin Trials for Recurrent Venous Thromboembolism). Aspirin for the Prevention of Recurrent Venous Thromboembolism: The INSPIRE Collaboration. Circulation 2014; 130:1062–1071.
- Becattini C, Agnelli G, Schenone A, et al; WARFASA Investigators. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012; 366:1959–1967.
- Brighton TA, Eikelboom JW, Mann K, et al; ASPIRE Investigators. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012; 367:1979–1987.
- Wakefield TW, Obi AT, Henke PK. An aspirin a day to keep the clots away: can aspirin prevent recurrent thrombosis in extended treatment for venous thromboembolism? Circulation 2014; 130:1031–1033.
- Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med 1997; 336:1506–1511.
- Coon WW, Willis PW 3rd. Recurrence of venous thromboembolism. Surgery 1973; 73:823–827.
- Hull R, Delmore T, Genton E, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med 1979; 301:855–858.
- Jaffer AK, Brotman DJ, Chukwumerije N. When patients on warfarin need surgery. Cleve Clin J Med 2003; 70:973–984.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Guerrouij M, Uppal CS, Alklabi A, Douketis JD. The clinical impact of bleeding during oral anticoagulant therapy: assessment of morbidity, mortality and post-bleed anticoagulant management. J Thromb Thrombolysis 2011; 31:419–423.
- Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123:2736–2747.
- Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3:692–694.
- Wiviott SD, Antman EM, Gibson CM, et al; TRITON-TIMI 38 Investigators. Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet InhibitioN with prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38). Am Heart J 2006; 152:627–635.
- Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 1993; 95:315–328.
- Fang MC, Go AS, Chang Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med 2007; 120:700–705.
- Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med 2012; 172:1484–1491.
- Holster IL, Valkhoff VE, Kuipers EJ, Tjwa ET. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology 2013; 145:105-112.e15.
- Loor G, Bassiouny H, Valentin C, Shao MY, Funaki B, Desai T. Local and systemic consequences of large retroperitoneal clot burdens. World J Surg 2009; 33:1618–1625.
- Satasivam P, Reeves F, Lin M, et al. The effect of oral anticoagulation on the prevalence and management of haematuria in a contemporary Australian patient cohort. BJU Int 2012; 110(suppl 4):80–84.
- Van Savage JG, Fried FA. Anticoagulant associated hematuria: a prospective study. J Urol 1995; 153:1594–1596.
- Mosley DH, Schatz IJ, Breneman GM, Keyes JW. Long-term anticoagulant therapy. Complications and control in a review of 978 cases. JAMA 1963; 186:914–916.
- Goldstein JN, Greenberg SM. Should anticoagulation be resumed after intracerebral hemorrhage? Cleve Clin J Med 2010; 77:791–799.
- Caldeira D, Barra M, Pinto FJ, Ferreira JJ, Costa J. Intracranial hemorrhage risk with the new oral anticoagulants: a systematic review and meta-analysis. J Neurol 2014 Aug 14. [Epub ahead of print]
- Holbrook A, Schulman S, Witt DM, et al; American College of Chest Physicians. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e152S–e184S.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e44S–e88S.
- Poli D, Antonucci E, Dentali F, et al; Italian Federation of Anticoagulation Clinics (FCSA). Recurrence of ICH after resumption of anticoagulation with VK antagonists: CHIRONE study. Neurology 2014; 82:1020–1026.
- Choi JH, Mast H, Sciacca RR, et al. Clinical outcome after first and recurrent hemorrhage in patients with untreated brain arteriovenous malformation. Stroke 2006; 37:1243–1247.
- Chon KH, Lee JM, Koh EJ, Choi HY. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien) 2012; 154:1541–1548.
- Oake N, Jennings A, Forster AJ, et al. Anticoagulation intensity and outcomes among patients prescribed oral anticoagulant therapy: a systematic review and meta-analysis. CMAJ 2008; 179:235–244.
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH; American College of Chest Physicians. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e576S–e600S.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. Circulation 2008; 118:e523–e661.
- Clemens A, Strack A, Noack H, Konstantinides S, Brueckmann M, Lip GY. Anticoagulant-related gastrointestinal bleeding—could this facilitate early detection of benign or malignant gastrointestinal lesions? Ann Med 2014; 46:672–678.
- Khalid F, Qureshi W, Qureshi S, Alirhayim Z, Garikapati K, Patsias I. Impact of restarting warfarin therapy in renal disease anticoagulated patients with gastrointestinal hemorrhage. Ren Fail 2013; 35:1228–1235.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Davidson BL, Verheijen S, Lensing AW, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med 2014; 174:947–953.
- Knijff-Dutmer EA, Schut GA, van de Laar MA. Concomitant coumarin-NSAID therapy and risk for bleeding. Ann Pharmacother 2003; 37:12–16.
- Heidbuchel H, Verhamme P, Alings M, et al; European Heart Rhythm Association. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013; 15:625–651.
- Goldstein JN, Fazen LE, Wendell L, et al. Risk of thromboembolism following acute intracerebral hemorrhage. Neurocrit Care 2009; 10:28–34.
- Christensen MC, Dawson J, Vincent C. Risk of thromboembolic complications after intracerebral hemorrhage according to ethnicity. Adv Ther 2008; 25:831–841.
- Ananthasubramaniam K, Beattie JN, Rosman HS, Jayam V, Borzak S. How safely and for how long can warfarin therapy be withheld in prosthetic heart valve patients hospitalized with a major hemorrhage? Chest 2001; 119:478–484.
- Lee JK, Kang HW, Kim SG, Kim JS, Jung HC. Risks related with withholding and resuming anticoagulation in patients with non-variceal upper gastrointestinal bleeding while on warfarin therapy. Int J Clin Pract 2012; 66:64–68.
- Qureshi W, Mittal C, Patsias I, et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol 2014; 113:662–668.
- Brotman DJ, Jaffer AK. Resuming anticoagulation in the first week following gastrointestinal tract hemorrhage: should we adopt a 4-day rule? Arch Intern Med 2012; 172:1492–1493.
- Kunkala MR1, Kehl J, Zielinski MD. Spontaneous rectus sheath hematomas: when to restart anticoagulation? World J Surg 2013; 37:2555–2559.
- Davis SM, Broderick J, Hennerici M, et al; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006; 66:1175–1181.
- Broderick J, Connolly S, Feldmann E, et al; American Heart Association; American Stroke Association Stroke Council; High Blood Pressure Research Council; Quality of Care and Outcomes in Research Interdisciplinary Working Group. Guidelines for the management of spontaneous intracerebral hemorrhage in adults. Stroke 2007; 38:2001–2023.
- Steiner T, Kaste M, Forsting M, et al. Recommendations for the management of intracranial haemorrhage—part I: spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc Dis 2006; 22:294–316. Erratum in: Cerebrovasc Dis 2006; 22:461.
- Boeer A, Voth E, Henze T, Prange HW. Early heparin therapy in patients with spontaneous intracerebral haemorrhage. J Neurol Neurosurg Psychiatry 1991; 54:466–467.
- Dickmann U, Voth E, Schicha H, Henze T, Prange H, Emrich D. Heparin therapy, deep-vein thrombosis and pulmonary embolism after intracerebral hemorrhage. Klin Wochenschr 1988; 66:1182–1183.
- Aguilar MI, Hart RG, Kase CS, et al. Treatment of warfarin-associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc 2007; 82:82–92. Erratum in: Mayo Clin Proc 2007; 82:387.
- Majeed A, Kim YK, Roberts RS, Holmström M, Schulman S. Optimal timing of resumption of warfarin after intracranial hemorrhage. Stroke 2010; 41:2860–2866.
- US Food and Drug Administration. Drug Information. XARELTO (rivaroxaban) tablets, for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2013/022406s004lbl.pdf. Accessed March 9, 2015.
- US Food and Drug Administration. Drug Information. ELIQUIS® (apixaban) tablets for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2014/202155s009lbl.pdf. Accessed March 9, 2015.
- US Food and Drug Administration. Drug Information. PRADAXA® (dabigatran etexilate mesylate) capsules for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2014/022512s023lbl.pdf. Accessed March 9, 2015.
- New oral anticoagulants for acute venous thromboembolism. Med Lett Drugs Ther 2014; 56:3–4.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383:955–962.
- Nutescu EA, Dager WE, Kalus JS, Lewin JJ 3rd, Cipolle MD. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am J Health Syst Pharm 2013; 70:1914–1929.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151. Erratum in: N Engl J Med 2010; 363:1877.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg 2009; 108:1447–1452.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 2013; 70:1486–1490.
- Loffredo L, Perri L, Violi F. Impact of new oral anticoagulants on gastrointestinal bleeding in atrial fibrillation: a meta-analysis of interventional trials. Dig Liver Dis 2015 Feb 7. pii: S1590-8658(15)00189-9. doi: 10.1016/j.dld.2015.01.159. [Epub ahead of print]
- Thachil J. The newer direct oral anticoagulants: a practical guide. Clin Med 2014; 14:165–175.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
- Jaffer AK, Brotman DJ, Bash LD, Mahmood SK, Lott B, White RH. Variations in perioperative warfarin management: outcomes and practice patterns at nine hospitals. Am J Med 2010; 123:141–150.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost 2010; 8:884–890.
- Raunsø J, Selmer C, Olesen JB, et al. Increased short-term risk of thrombo-embolism or death after interruption of warfarin treatment in patients with atrial fibrillation. Eur Heart J 2012; 33:1886–1892.
- Xarelto (rivaroxaban). Highlights of prescribing information. Jansen Pharmaceuticals, Inc. www.xareltohcp.com/sites/default/files/pdf/xarelto_0.pdf#zoom=100. Accessed March 9, 2015.
- Pradaxa (dabigatran etexilate mesylate). Highlights of prescribing information. Boehringer Ingelheim Pharmaceuticals, Inc. http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed March 9, 2015.
- Eliquis (apixaban). Highlights of prescribing information. Bristol-Myers Squibb Company. http://packageinserts.bms.com/pi/pi_eliquis.pdf. Accessed March 9, 2015.
- Schulman S, Beyth RJ, Kearon C, Levine MN; American College of Chest Physicians. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest 2008; 133(suppl 6):257S–298S.
- Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated bleeding. Blood 2014; 123:1152–1158.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Cannegieter SC, Rosendaal FR, Briët E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation 1994; 89:635–641.
- Warkentin TE. Aspirin for dual prevention of venous and arterial thrombosis. N Engl J Med 2012; 367:2039–2041.
- Simes J, Becattini C, Agnelli G, et al; INSPIRE Study Investigators* (International Collaboration of Aspirin Trials for Recurrent Venous Thromboembolism). Aspirin for the Prevention of Recurrent Venous Thromboembolism: The INSPIRE Collaboration. Circulation 2014; 130:1062–1071.
- Becattini C, Agnelli G, Schenone A, et al; WARFASA Investigators. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012; 366:1959–1967.
- Brighton TA, Eikelboom JW, Mann K, et al; ASPIRE Investigators. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012; 367:1979–1987.
- Wakefield TW, Obi AT, Henke PK. An aspirin a day to keep the clots away: can aspirin prevent recurrent thrombosis in extended treatment for venous thromboembolism? Circulation 2014; 130:1031–1033.
- Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med 1997; 336:1506–1511.
- Coon WW, Willis PW 3rd. Recurrence of venous thromboembolism. Surgery 1973; 73:823–827.
- Hull R, Delmore T, Genton E, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med 1979; 301:855–858.
- Jaffer AK, Brotman DJ, Chukwumerije N. When patients on warfarin need surgery. Cleve Clin J Med 2003; 70:973–984.
- Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011; 342:d3036.
- Guerrouij M, Uppal CS, Alklabi A, Douketis JD. The clinical impact of bleeding during oral anticoagulant therapy: assessment of morbidity, mortality and post-bleed anticoagulant management. J Thromb Thrombolysis 2011; 31:419–423.
- Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011; 123:2736–2747.
- Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3:692–694.
- Wiviott SD, Antman EM, Gibson CM, et al; TRITON-TIMI 38 Investigators. Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet InhibitioN with prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38). Am Heart J 2006; 152:627–635.
- Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 1993; 95:315–328.
- Fang MC, Go AS, Chang Y, et al. Death and disability from warfarin-associated intracranial and extracranial hemorrhages. Am J Med 2007; 120:700–705.
- Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med 2012; 172:1484–1491.
- Holster IL, Valkhoff VE, Kuipers EJ, Tjwa ET. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology 2013; 145:105-112.e15.
- Loor G, Bassiouny H, Valentin C, Shao MY, Funaki B, Desai T. Local and systemic consequences of large retroperitoneal clot burdens. World J Surg 2009; 33:1618–1625.
- Satasivam P, Reeves F, Lin M, et al. The effect of oral anticoagulation on the prevalence and management of haematuria in a contemporary Australian patient cohort. BJU Int 2012; 110(suppl 4):80–84.
- Van Savage JG, Fried FA. Anticoagulant associated hematuria: a prospective study. J Urol 1995; 153:1594–1596.
- Mosley DH, Schatz IJ, Breneman GM, Keyes JW. Long-term anticoagulant therapy. Complications and control in a review of 978 cases. JAMA 1963; 186:914–916.
- Goldstein JN, Greenberg SM. Should anticoagulation be resumed after intracerebral hemorrhage? Cleve Clin J Med 2010; 77:791–799.
- Caldeira D, Barra M, Pinto FJ, Ferreira JJ, Costa J. Intracranial hemorrhage risk with the new oral anticoagulants: a systematic review and meta-analysis. J Neurol 2014 Aug 14. [Epub ahead of print]
- Holbrook A, Schulman S, Witt DM, et al; American College of Chest Physicians. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e152S–e184S.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e44S–e88S.
- Poli D, Antonucci E, Dentali F, et al; Italian Federation of Anticoagulation Clinics (FCSA). Recurrence of ICH after resumption of anticoagulation with VK antagonists: CHIRONE study. Neurology 2014; 82:1020–1026.
- Choi JH, Mast H, Sciacca RR, et al. Clinical outcome after first and recurrent hemorrhage in patients with untreated brain arteriovenous malformation. Stroke 2006; 37:1243–1247.
- Chon KH, Lee JM, Koh EJ, Choi HY. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien) 2012; 154:1541–1548.
- Oake N, Jennings A, Forster AJ, et al. Anticoagulation intensity and outcomes among patients prescribed oral anticoagulant therapy: a systematic review and meta-analysis. CMAJ 2008; 179:235–244.
- Whitlock RP, Sun JC, Fremes SE, Rubens FD, Teoh KH; American College of Chest Physicians. Antithrombotic and thrombolytic therapy for valvular disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e576S–e600S.
- Bonow RO, Carabello BA, Chatterjee K, et al; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease. Circulation 2008; 118:e523–e661.
- Clemens A, Strack A, Noack H, Konstantinides S, Brueckmann M, Lip GY. Anticoagulant-related gastrointestinal bleeding—could this facilitate early detection of benign or malignant gastrointestinal lesions? Ann Med 2014; 46:672–678.
- Khalid F, Qureshi W, Qureshi S, Alirhayim Z, Garikapati K, Patsias I. Impact of restarting warfarin therapy in renal disease anticoagulated patients with gastrointestinal hemorrhage. Ren Fail 2013; 35:1228–1235.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Davidson BL, Verheijen S, Lensing AW, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med 2014; 174:947–953.
- Knijff-Dutmer EA, Schut GA, van de Laar MA. Concomitant coumarin-NSAID therapy and risk for bleeding. Ann Pharmacother 2003; 37:12–16.
- Heidbuchel H, Verhamme P, Alings M, et al; European Heart Rhythm Association. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013; 15:625–651.
- Goldstein JN, Fazen LE, Wendell L, et al. Risk of thromboembolism following acute intracerebral hemorrhage. Neurocrit Care 2009; 10:28–34.
- Christensen MC, Dawson J, Vincent C. Risk of thromboembolic complications after intracerebral hemorrhage according to ethnicity. Adv Ther 2008; 25:831–841.
- Ananthasubramaniam K, Beattie JN, Rosman HS, Jayam V, Borzak S. How safely and for how long can warfarin therapy be withheld in prosthetic heart valve patients hospitalized with a major hemorrhage? Chest 2001; 119:478–484.
- Lee JK, Kang HW, Kim SG, Kim JS, Jung HC. Risks related with withholding and resuming anticoagulation in patients with non-variceal upper gastrointestinal bleeding while on warfarin therapy. Int J Clin Pract 2012; 66:64–68.
- Qureshi W, Mittal C, Patsias I, et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol 2014; 113:662–668.
- Brotman DJ, Jaffer AK. Resuming anticoagulation in the first week following gastrointestinal tract hemorrhage: should we adopt a 4-day rule? Arch Intern Med 2012; 172:1492–1493.
- Kunkala MR1, Kehl J, Zielinski MD. Spontaneous rectus sheath hematomas: when to restart anticoagulation? World J Surg 2013; 37:2555–2559.
- Davis SM, Broderick J, Hennerici M, et al; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006; 66:1175–1181.
- Broderick J, Connolly S, Feldmann E, et al; American Heart Association; American Stroke Association Stroke Council; High Blood Pressure Research Council; Quality of Care and Outcomes in Research Interdisciplinary Working Group. Guidelines for the management of spontaneous intracerebral hemorrhage in adults. Stroke 2007; 38:2001–2023.
- Steiner T, Kaste M, Forsting M, et al. Recommendations for the management of intracranial haemorrhage—part I: spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc Dis 2006; 22:294–316. Erratum in: Cerebrovasc Dis 2006; 22:461.
- Boeer A, Voth E, Henze T, Prange HW. Early heparin therapy in patients with spontaneous intracerebral haemorrhage. J Neurol Neurosurg Psychiatry 1991; 54:466–467.
- Dickmann U, Voth E, Schicha H, Henze T, Prange H, Emrich D. Heparin therapy, deep-vein thrombosis and pulmonary embolism after intracerebral hemorrhage. Klin Wochenschr 1988; 66:1182–1183.
- Aguilar MI, Hart RG, Kase CS, et al. Treatment of warfarin-associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc 2007; 82:82–92. Erratum in: Mayo Clin Proc 2007; 82:387.
- Majeed A, Kim YK, Roberts RS, Holmström M, Schulman S. Optimal timing of resumption of warfarin after intracranial hemorrhage. Stroke 2010; 41:2860–2866.
- US Food and Drug Administration. Drug Information. XARELTO (rivaroxaban) tablets, for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2013/022406s004lbl.pdf. Accessed March 9, 2015.
- US Food and Drug Administration. Drug Information. ELIQUIS® (apixaban) tablets for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2014/202155s009lbl.pdf. Accessed March 9, 2015.
- US Food and Drug Administration. Drug Information. PRADAXA® (dabigatran etexilate mesylate) capsules for oral use. www.accessdata.fda.gov/drugsatfda_docs/label/2014/022512s023lbl.pdf. Accessed March 9, 2015.
- New oral anticoagulants for acute venous thromboembolism. Med Lett Drugs Ther 2014; 56:3–4.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014; 383:955–962.
- Nutescu EA, Dager WE, Kalus JS, Lewin JJ 3rd, Cipolle MD. Management of bleeding and reversal strategies for oral anticoagulants: clinical practice considerations. Am J Health Syst Pharm 2013; 70:1914–1929.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151. Erratum in: N Engl J Med 2010; 363:1877.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369:1406–1415.
- Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg 2009; 108:1447–1452.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol 2013; 70:1486–1490.
- Loffredo L, Perri L, Violi F. Impact of new oral anticoagulants on gastrointestinal bleeding in atrial fibrillation: a meta-analysis of interventional trials. Dig Liver Dis 2015 Feb 7. pii: S1590-8658(15)00189-9. doi: 10.1016/j.dld.2015.01.159. [Epub ahead of print]
- Thachil J. The newer direct oral anticoagulants: a practical guide. Clin Med 2014; 14:165–175.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
KEY POINTS
- Not all patients on anticoagulation at the time of a bleeding event have a strong indication to continue anticoagulation afterward.
- Important considerations when deciding whether to resume anticoagulation after hemorrhage are whether the source of bleeding has been found and controlled and, if the patient is receiving warfarin, whether he or she can be expected to maintain the target international normalized ratio.
- The newer oral anticoagulants, including factor Xa inhibitors and direct thrombin inhibitors, lack antidotes or reversal agents, and their risk of causing bleeding compared with warfarin varies by site of bleeding.