User login
New research appears to explain how TET2 mutations increase the risk of hematologic malignancies.
In studying mouse models and patient samples, researchers found evidence to suggest that loss of TET2 opens the door for mutations that drive lymphoid and myeloid malignancies.
The researchers said loss of TET2 leads to hypermutagenicity in hematopoietic stem and progenitor cells (HSPCs), and although TET2-deficient HSPCs are likely not malignant, the higher mutation rates in these cells may result in additional driver mutations in TET2 target genes over time.
“If you lose TET2, it’s not a malignant state per se,” said Mingjiang Xu, MD, PhD, of the University of Miami Miller School of Medicine in Florida.
“But it’s creating a situation for other mutations to happen, leading to all types of blood cancer.”
Dr Xu and his colleagues reported these findings in Nature Communications.
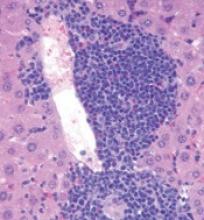
The researchers found that Tet2-knockout mice developed spontaneous, lethal hematologic malignancies. Most (92%) developed myeloid malignancies, but 3.5% developed T-cell malignancies, and 4.5% developed B-cell malignancies.
In sequencing tumor and non-tumor cells from the Tet2-knockout mice, the researchers observed that loss of Tet2 leads to hypermutagenicity in HSPCs.
The team identified 190 genes with recurrent single-nucleotide variants. This included genes that are recurrently altered in human hematologic malignancies—Apc, Nf1, Flt3, Cbl, Notch1, and Mll2.
The researchers also analyzed samples from patients with acute myeloid leukemia, myeloproliferative neoplasms, and myelodysplastic syndromes.
The team found that patients with TET2 mutations had “significantly more mutational events than patients with wild-type TET2.” And TET2 mutations were associated with subclonal events in APC, NF1, ASXL1, CBL, and ZRSR2, among other genes.
These findings suggest that targeting TET2 could potentially prevent the development of hematologic malignancies.
The researchers noted that TET2 mutations occur in healthy elderly individuals with clonal hematopoiesis, and these individuals would be ideal candidates for a preventive therapy targeting TET2.
“We are developing a method to target TET2,” Dr Xu said. “If we target that population [with TET2 mutations] for early therapy, we could potentially prevent those downstream mutations from happening.” ![]()
New research appears to explain how TET2 mutations increase the risk of hematologic malignancies.
In studying mouse models and patient samples, researchers found evidence to suggest that loss of TET2 opens the door for mutations that drive lymphoid and myeloid malignancies.
The researchers said loss of TET2 leads to hypermutagenicity in hematopoietic stem and progenitor cells (HSPCs), and although TET2-deficient HSPCs are likely not malignant, the higher mutation rates in these cells may result in additional driver mutations in TET2 target genes over time.
“If you lose TET2, it’s not a malignant state per se,” said Mingjiang Xu, MD, PhD, of the University of Miami Miller School of Medicine in Florida.
“But it’s creating a situation for other mutations to happen, leading to all types of blood cancer.”
Dr Xu and his colleagues reported these findings in Nature Communications.
The researchers found that Tet2-knockout mice developed spontaneous, lethal hematologic malignancies. Most (92%) developed myeloid malignancies, but 3.5% developed T-cell malignancies, and 4.5% developed B-cell malignancies.
In sequencing tumor and non-tumor cells from the Tet2-knockout mice, the researchers observed that loss of Tet2 leads to hypermutagenicity in HSPCs.
The team identified 190 genes with recurrent single-nucleotide variants. This included genes that are recurrently altered in human hematologic malignancies—Apc, Nf1, Flt3, Cbl, Notch1, and Mll2.
The researchers also analyzed samples from patients with acute myeloid leukemia, myeloproliferative neoplasms, and myelodysplastic syndromes.
The team found that patients with TET2 mutations had “significantly more mutational events than patients with wild-type TET2.” And TET2 mutations were associated with subclonal events in APC, NF1, ASXL1, CBL, and ZRSR2, among other genes.
These findings suggest that targeting TET2 could potentially prevent the development of hematologic malignancies.
The researchers noted that TET2 mutations occur in healthy elderly individuals with clonal hematopoiesis, and these individuals would be ideal candidates for a preventive therapy targeting TET2.
“We are developing a method to target TET2,” Dr Xu said. “If we target that population [with TET2 mutations] for early therapy, we could potentially prevent those downstream mutations from happening.” ![]()
New research appears to explain how TET2 mutations increase the risk of hematologic malignancies.
In studying mouse models and patient samples, researchers found evidence to suggest that loss of TET2 opens the door for mutations that drive lymphoid and myeloid malignancies.
The researchers said loss of TET2 leads to hypermutagenicity in hematopoietic stem and progenitor cells (HSPCs), and although TET2-deficient HSPCs are likely not malignant, the higher mutation rates in these cells may result in additional driver mutations in TET2 target genes over time.
“If you lose TET2, it’s not a malignant state per se,” said Mingjiang Xu, MD, PhD, of the University of Miami Miller School of Medicine in Florida.
“But it’s creating a situation for other mutations to happen, leading to all types of blood cancer.”
Dr Xu and his colleagues reported these findings in Nature Communications.
The researchers found that Tet2-knockout mice developed spontaneous, lethal hematologic malignancies. Most (92%) developed myeloid malignancies, but 3.5% developed T-cell malignancies, and 4.5% developed B-cell malignancies.
In sequencing tumor and non-tumor cells from the Tet2-knockout mice, the researchers observed that loss of Tet2 leads to hypermutagenicity in HSPCs.
The team identified 190 genes with recurrent single-nucleotide variants. This included genes that are recurrently altered in human hematologic malignancies—Apc, Nf1, Flt3, Cbl, Notch1, and Mll2.
The researchers also analyzed samples from patients with acute myeloid leukemia, myeloproliferative neoplasms, and myelodysplastic syndromes.
The team found that patients with TET2 mutations had “significantly more mutational events than patients with wild-type TET2.” And TET2 mutations were associated with subclonal events in APC, NF1, ASXL1, CBL, and ZRSR2, among other genes.
These findings suggest that targeting TET2 could potentially prevent the development of hematologic malignancies.
The researchers noted that TET2 mutations occur in healthy elderly individuals with clonal hematopoiesis, and these individuals would be ideal candidates for a preventive therapy targeting TET2.
“We are developing a method to target TET2,” Dr Xu said. “If we target that population [with TET2 mutations] for early therapy, we could potentially prevent those downstream mutations from happening.” ![]()