User login
MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.
SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.
VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.
MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
Mesh kits for repairing prolapse are proliferating like crazy, just as they did for midurethral sling procedures. But mesh augmentation of prolapse surgeries requires more than a prepackaged assortment of tools and materials. In this article, moderator Mickey M. Karram, MD, and a panel of nationally recognized urogynecologists and urologists describe the literature on mesh augmentation and discuss indications, contraindications, techniques, applicable cases, and the considerable training required.
In Part 2, which will appear in the February issue of OBG Management, the panel tackles the thorny topic of complications, including erosion, extrusion, foreshortening of the vagina, dyspareunia, and pain. Their discussion focuses on ways to avoid these problems, and methods for correcting them.
Do we have enough data?
DR. KARRAM: To start, let’s quickly review the peer-reviewed literature on the use of mesh augmentation during surgery for pelvic organ prolapse.
DR. WALTERS: Until recently, most data concerned open abdominal sacrocolpopexy (ASC) using polypropylene or Merseline mesh. There is significant clinical experience with this operation, and multiple cohort studies show long-term cure rates of 78% to 100% for apical prolapse.1
At least two randomized controlled trials have compared open ASC with sutured vaginal colpopexy procedures, and ASC is certainly equal to—perhaps better than—all transvaginal sutured repairs.2,3
With ASC, most recurrences affect the distal half of the vagina and involve one or more of the following:
- anterior or posterior vaginal wall prolapse (or both)
- stress urinary incontinence (SUI)
- distal rectocele.1,3
Mesh erosion occurs in 3.4% of cases and is usually easily managed.1 Other complications, including bowel injury, tend to be related to access, regardless of whether the operation is performed via laparotomy or laparoscopy.
Robotic sacrocolpopexy has become popular in recent years, and we will probably see data on this approach as we gain experience.
When it comes to vaginal mesh kits, the peer-reviewed literature is just beginning to expand, with many studies being presented at international meetings. For anterior and, possibly, apical vaginal prolapse, the cure rate after use of a mesh kit appears to be as high as, or higher than, the rate for sutured repairs.4 This high rate of anatomic cure is balanced somewhat by additional cost and complications involving mesh and the kits.
For posterior vaginal wall prolapse and rectocele, I firmly believe, based on our research and that of others, that sutured repairs are superior to graft-augmented surgery.5
DR. KARRAM: What are the indications and contraindications for mesh augmentation of prolapse repair ( FIGURES 1 and 2 )?
DR. LUCENTE: I believe mesh is indicated in any patient in need of surgical repair of pelvic organ prolapse who is seeking optimal durability and is willing to accept the known risks of the surgery.
The issue becomes more complex when it comes to contraindications. Absolute contraindications are fairly obvious; they include medically unstable patients and those who may have an inactive infectious process within the pelvis or even undiagnosed abnormal uterine bleeding.
At our center, because the potential for dyspareunia and pelvic discomfort is our biggest concern, we have developed a profile of the patient who is more likely to develop these complaints. The profile includes any patient who has a chronic pain disorder of any type, but especially chronic pelvic pain disorders such as endometriosis and vulvodynia. Other risk factors appear to be a history of pelvic surgery involving any permanent material, suture or mesh, and young age.
So if we have a patient in her late 30s who has undergone reconstructive surgery using permanent sutures and who has an element of chronic pelvic pain, we would counsel her strongly to consider surgical options other than the use of synthetic mesh.
DR. WALTERS: The main indications for mesh-augmented prolapse repair are recurrent posthysterectomy vaginal vault prolapse, for which I usually perform ASC, and recurrent cystocele or anteriorapical prolapse, for which I use one of the anterior mesh kits.
I still think sutured repairs—by that, I mean uterosacral ligament or sacrospinous colpopexy with sutured rectocele repair—work best for recurrent posterior wall and posteriorapical prolapse. I don’t use mesh augmentation for rectocele.
The main contraindication to mesh augmentation, as I see it, is a history of mesh complications. If I am repairing a mesh complication such as erosion or pain, I do not place another mesh.
Medical issues that might increase mesh complications, such as diabetes, steroid use, or severe vaginal atrophy, would, at the very least, make me consider carefully whether mesh augmentation is appropriate. The literature is not clear on this, so mesh could still be used if the surgeon thinks it is necessary.
DR. KARRAM: I haven’t found a definitive indication for mesh augmentation. We have used biologic meshes empirically, but I am not convinced that they really add long-term durability, regardless of whether they are used in the anterior or posterior vaginal segment.
Our published durability rate for traditional suture-type repairs is in the range of 85% at 5 years out.6 Even if I assumed that mesh would give me 100% 5-year durability, this rate would have to be at the expense of some erosion, pain, and other complications unique to mesh. I do not think that the potential improvement in durability is worth these potential complications.
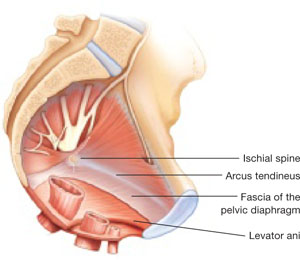
FIGURE 1 When the pelvic support system is intact, prolapse is rare
In the normal pelvis, organs are supported by a complex web of muscles, fascia, and connective tissue. 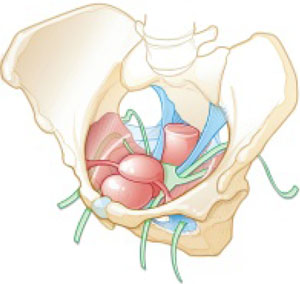
FIGURE 2 Mesh augmentation seeks to enhance the durability of repair
One type of mesh in final position. Mesh-augmented repair restores the vaginal apex and lends support to the walls of the vagina.
DR. KARRAM: If you are doing a lot of mesh repairs, you are obviously content with the results and feel that the few complications you are seeing are outweighed by the advantages mesh confers. How do you avoid extrusion and avert creation of a painful vagina?
DR. RAZ: Most of our cases are recurrent prolapse after failed vaginal or abdominal repair. I am indeed using a significant amount of soft polypropylene mesh for reconstructive procedures. As with the use of any other synthetic material, low-grade infection can develop after a few weeks or months. I use copious irrigation with antibiotic solution during reconstruction.
To avoid extrusion, I perform deep, rather than superficial, dissection of the vaginal wall to allow for better coverage of the mesh. For posterior mesh reconstruction, I cover the mesh with pararectal fascia to prevent erosion.
For mesh-augmented procedures, I cut the mesh myself in the operating room ( FIGURE 3 ). For a sling, I use a 10 cm × 1 cm soft polypropylene mesh. For a grade 3 or 4 cystocele, I use a trapezoid of soft polypropylene mesh with several points of fixation:
- at the sacrouterine ligament
- lateral to the obturator fascia
- distal to the bladder neck.
I always repair the vault at the same time.
For vault prolapse, I use a segment of soft polypropylene mesh in the shape of an apron with two arms (1 cm × 4 cm) and a central segment (4 cm × 7 cm). I support the vault using number 1-0 delayed absorbable suture and mesh. From outside the vaginal wall, in the posterolateral deep vaginal wall (inside the peritoneum), I incorporate the origin of the sacrouterine ligament and one arm of the mesh in the groove between the colon and levator ani, 15 cm from the introitus. I bring the suture 1 cm from the original entrance. A separate set of sutures brings the perirectal fascia together with the sacrouterine ligaments and perivesical fascia to close the peritoneal cavity. I tie the vault-suspension sutures, providing support to the cuff in a high posterior position (12 to 15 cm from the introitus).
In selected cases of significant recurrent rectocele, I use a rectangle of soft polypropylene mesh anchored to the origin of the sacrouterine ligament and distal to the perineal membrane. The mesh is covered by the pararectal fascia.
We have not seen vaginal, urethral, or bladder erosion in 1,800 cases of our distal urethral Prolene sling procedure using 10 cm × 1 cm soft mesh. In patients who have significant cystocele, vault prolapse, and recurrent rectocele, our vaginal erosion rate is 3%. We have never encountered rectal, bladder, or bowel perforation using our technique.
DR. LUCENTE: We often use mesh and are more than simply content with our results—we are extremely pleased, and so are our patients. Having said that, our techniques have definitely evolved over the past few years, as we’ve focused on how to decrease exposure and, more recently, optimize sexual function and vaginal comfort.
First, to avoid exposure, the most critical step is precise hydrodissection and distention of the true vesicovaginal space. This step can only be achieved through careful tactile guidance of the needle tip into the space, where it should remain while hydrodissection is performed. Always remember, sharp dissection “follows” hydrodissection. If you place the needle bevel within the vaginal wall, you will “split” the vaginal wall—as during standard colporrhaphy—which will lead to a high exposure rate.
Second, to avoid dyspareunia, it’s essential to pay close attention to POP-Q measurements, especially vaginal length, to ensure that the reconstruction restores the same length without foreshortening. This approach entails leaving the cervix in most patients who have a shorter vagina, and making sure that the mesh is secured above the ischial spine in younger, sexually active patients who have demonstrated a higher risk of postoperative deep, penetrating dyspareunia, compared with older, less sexually active patients.
Also paramount is to ensure that you have manually displaced the vagina inwardly as much as possible before deploying or setting the mesh. If you simply try to suture secure the mesh with the vagina incised open, without the ability to deploy the mesh with a closed, displaced vagina (to mimic deep penetration), it is difficult, if not impossible, to properly set the mesh for optimal comfort.
In the early days of midurethral pubovaginal slings using polypropylene, the adage was “looser is better than tighter.” This is even truer for transvaginal mesh.
DR. KARRAM: Dr. Walters, please describe your current surgical procedure of choice without mesh and explain why you haven’t adopted mesh for routine repairs.
DR. WALTERS: About 20% of my prolapse surgeries—usually for posthysterectomy or recurrent vaginal vault prolapse—involve ASC with placement of polypropylene mesh. I perform most of these cases through a Pfannenstiel incision, but I’ve also done them laparoscopically. Several of my partners perform ASC laparoscopically and robotically.
For the other 80% of my patients who have prolapse, I perform repairs transvaginally, usually using high bilateral uterosacralligament vaginal-vault suspension. We have learned to suture higher and slightly more medial on the uterosacral ligaments to attain greater vaginal depth and minimize ureteral obstruction. We use two or three sutures on each uterosacral ligament, usually a combination of permanent and delayed absorbable sutures.
I am also performing more sacrospinous ligament suspensions because this operation is being studied by the Pelvic Floor Disorders Network. Properly performed, it is an excellent surgery for apical prolapse. But, as with most of our surgeries for prolapse, recurrent anterior wall prolapse remains a problem.
Like you, Dr. Karram, we’ve studied our group’s anatomic and functional outcomes very carefully for more than 10 years and are mostly satisfied with our cure and complication rates. Although our anatomic outcomes with these surgeries are not always perfect, our reoperation rate for prolapse is only about 5%, with a high level of satisfaction in 88% to 92% of patients.
DR. RAZ: Unaugmented reconstruction fails in more than 30% of cases. Some patients who have significant prolapse and attenuated tissue think that this tissue will become healthier or stronger after reconstructive surgery, but that isn’t the case. In these situations, excision and plication make no clinical sense.
The problem is that we have yet to identify the ideal surrogate for poor-quality tissue. Most of us use polypropylene mesh in different variations. We need a better material that will be nonimmunogenic, well tolerated, and easily incorporated without erosion. Xenograft-like derivatives of dermis, or allografts such as cadaveric fascia, have failed over the long term because the body reabsorbs the graft without forming any new connective tissue.
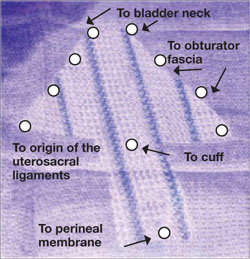
FIGURE 3 Mesh can be cut in the OR to custom-fit a patient
Hand-cut mesh and points of placement.
PHOTO: SHLOMO RAZ, MD
Is a kit a valuable aid?
DR. KARRAM: If a surgeon wants to augment a repair, what are the advantages of a packaged mesh kit, compared with simply cutting the mesh and performing surgery without a kit?
DR. WALTERS: The advantages of a packaged mesh kit are the convenience involved and the ability to consistently perform the same operation with the same product. That facilitates learning, teaching, and research. It also helps us understand the published literature a little better because “custom” prolapse repairs are operator-dependent and difficult to apply generally to a population of surgeons.
These advantages are most clearly apparent with midurethral sling mesh kits, which have almost revolutionized surgery for stress incontinence. I don’t believe mesh kits for prolapse are there yet, but they certainly have potential.
DR. RAZ: I’m opposed to the use of kits. They are industry-driven. One company has made $1 billion selling them. Imagine a patient who undergoes placement of a sling kit ($1,000), cystocele kit ($1,500), and posterior mesh kit ($1,500). How can our healthcare system sustain this burden, especially when there is no real evidence that a kit improves the operation, and given the incredible complication rate that we see?
Moreover, the kits contain a single-use needle and passer and a precut segment of polypropylene mesh. But every patient is different and requires a unique size or shape of mesh. I don’t believe that a surgeon who knows pelvic anatomy needs a kit to perform mesh-augmented reconstruction. We can buy the same segment of mesh for $200 to $400, cut it as needed, and perform the same operation advertised by industry.
For surgeons who prefer a kit, the tools that are included should be made reusable.
DR. LUCENTE: In my opinion, the primary advantage of a commercially available transvaginal mesh delivery system—notice, I avoided the word “kit,” because I think there are plenty of negative connotations associated with it—is the ability to deliver the mesh in a “tension”-free manner.
One alternative that many people pursue is cutting the mesh to size and using sutures to hold it in place while tissue ingrowth occurs. However, the hernia literature suggests that suturing mesh in place increases the risk of postoperative discomfort at the site of implantation. The true cause of the discomfort remains unclear, but it is thought to arise from nerve tethering or traction at the pre-committed points of attachment before the host tissue and mesh interface have adjusted or settled with tissue ingrowth.
All neuropathic complications of mesh implantation have been shown in the current hernia literature to be increased with the use of sutures.7 Also, as previously mentioned, it is extremely difficult to set or adjust the mesh with the vaginal incision remaining “open,” which is a downside to suture techniques.
What training is necessary to use a kit?
DR. KARRAM: Mesh kits are aggressively promoted by industry, with close to half a dozen different kits to be available soon. What is the minimum amount of training one should have before utilizing these kits?
DR. WALTERS: The surgeon should at least know how to perform traditional sutured prolapse repairs and SUI surgery and be able to perform cystoscopy. Ideally, the surgeon should undergo training on a cadaver with a skilled and experienced user of the mesh kit. The surgeon also should carefully review the risks and benefits of mesh kits with the patient and inform the patient that he or she is in the early learning curve of a particular surgery. The informed patient should have a right to refuse mesh-augmented prolapse surgery after the consent process.
DR. LUCENTE: I’m glad you asked this question. I strongly believe that surgical expertise and proficiency within gynecology need to be more effectively addressed by us all. We have a situation in our field in which techniques and technology are widening the gap between what is possible and what the surgeon is comfortable doing safely.
It’s incumbent on all of us, especially those who are in a leadership position as a chairperson or chief of a division, to work with our physician staff and faculty to optimize surgical skill and patient outcomes, including safety, with new technologies.
As for the minimal amount of training needed, that’s extremely variable. It depends on the current skill set of the physician and his or her ability to pick up the mechanics of the surgery as it is taught through a cadaver lab or preceptorship. It’s regrettable that some physicians lack the objectivity and insight to judge their own skill set. This, again, is the time for a chairperson or chief of a division to step up to the plate and ensure proper credentialing and demonstration of proficiency.
It is unrealistic to expect industry to decide who should or should not utilize this truly breakthrough technology. That is our responsibility as physicians.
DR. KARRAM: At a minimum, I think any surgeon utilizing a kit should have a firm understanding of pelvic floor anatomy and experience performing traditional repairs:
- intraperitoneal procedures such as Mc-Call culdoplasty and uterosacral suspension
- sacrospinous suspension
- retropubic procedures and anti-incontinence operations such as pubovaginal slings.
This three-dimensional understanding of the pelvic floor is mandatory if one is to assume that blind passage of trocars through potentially dangerous spaces is the wave of the future.
DR. RAZ: You need to be a pelvic surgeon, know your anatomy, and know how to manage complications if you are going to use one of these kits. You should stick to the surgery that works best in your hands. Industry cannot teach you to be a good pelvic surgeon; it takes lifelong experience.
DR. KARRAM: If you have a patient who is sexually inactive with pelvic organ prolapse, would you prefer a mesh repair or an obliterative procedure? And why?
DR. WALTERS: If the patient is sexually inactive—especially if she is older and definitely will not be in the future—it makes absolutely no sense to perform a mesh-augmented repair. A traditional, somewhat tight, sutured repair works fine in this setting and carries very low risk.
In fact, our group and others have found that, in carefully selected patients, partial colpectomy and colpocleisis procedures (without grafts) have among the highest cure and satisfaction rates of all surgeries we perform for prolapse; they also have relatively low risk.8 Recurrent prolapse after an obliterative procedure is rare; most of the dissatisfaction relates to postoperative voiding difficulties or persistent or de novo urinary incontinence.
DR. KARRAM: I also prefer an obliterative procedure. I see no reason to bring in the cost and potential for complications that mesh repair entails. An obliterative procedure should produce an anatomic success rate close to 100%, with minimal complications. It also can be performed quickly with minimal anesthesia and convalescence.
DR. LUCENTE: My response is based on a clinical study that my associate, Dr. Miles Murphy, has performed, comparing a transvaginal mesh procedure with a LaForte operation for severe pelvic organ prolapse.9 Both patient groups were well satisfied with the result, and success rates were comparable. However, the group that underwent the transvaginal mesh procedure had a shorter operative time.
As a result of these studies, we tend to prefer transvaginal mesh repair. Even though the woman may be sexually inactive, the procedure preserves vaginal function, and we all know that life has a way of being unpredictable. Her situation may change so that she once again desires sexual function.
However, for a very elderly woman—one in her late 80s or 90s—who has severe or extreme prolapse with a very large procidentia and vaginal length measuring, say, 13 cm beyond the introitus, I do prefer an obliterative procedure.
DR. RAZ: I agree. I would not offer a sexually inactive patient an obliterative procedure. You never know what the future will hold.
Mesh repair can be performed safely, provided the surgeon has good knowledge of anatomic landmarks and knows how to manage any potential complications that may arise.
1. Nygaard IE, McCreery R, Brubaker L, et al. Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805-823.
2. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1421;discussion 1421-1422.
3. Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol. 2004;190:20-26.
4. Murphy M. Society of Gynecologic Surgeons Systematic Review Group. Clinical practice guidelines on vaginal graft use from the Society of Gynecologic Surgeons. Obstet Gynecol. 2008;112:1123-1130.
5. Paraiso MF, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical procedures including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
6. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol 2006;108:255-263.
7. EU Hernia Trialists Collaboration. Repair of groin hernia with synthetic mesh: meta-analysis of randomized controlled trials. Ann Surg. 2002;235:322-332.
8. Barber MD, Amundsen C, Paraiso MFR, Weidner A, Romero A, Walters MD. Quality of life after surgery for genital prolapse in elderly women: obliterative and reconstructive surgery. Int Urogynecol J. 2007;18:799-806.
9. Murphy M, van Raalte H, Mercurio E, Haff R, Wiseman B, Lucente VR. Incontinence-related quality of life and sexual function following the tension-free vaginal tape vs the “inside-out” tension-free vaginal tape obturator. Int Urogynecol J. 2008;19:481-487.
MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.
SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.
VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.
MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
Mesh kits for repairing prolapse are proliferating like crazy, just as they did for midurethral sling procedures. But mesh augmentation of prolapse surgeries requires more than a prepackaged assortment of tools and materials. In this article, moderator Mickey M. Karram, MD, and a panel of nationally recognized urogynecologists and urologists describe the literature on mesh augmentation and discuss indications, contraindications, techniques, applicable cases, and the considerable training required.
In Part 2, which will appear in the February issue of OBG Management, the panel tackles the thorny topic of complications, including erosion, extrusion, foreshortening of the vagina, dyspareunia, and pain. Their discussion focuses on ways to avoid these problems, and methods for correcting them.
Do we have enough data?
DR. KARRAM: To start, let’s quickly review the peer-reviewed literature on the use of mesh augmentation during surgery for pelvic organ prolapse.
DR. WALTERS: Until recently, most data concerned open abdominal sacrocolpopexy (ASC) using polypropylene or Merseline mesh. There is significant clinical experience with this operation, and multiple cohort studies show long-term cure rates of 78% to 100% for apical prolapse.1
At least two randomized controlled trials have compared open ASC with sutured vaginal colpopexy procedures, and ASC is certainly equal to—perhaps better than—all transvaginal sutured repairs.2,3
With ASC, most recurrences affect the distal half of the vagina and involve one or more of the following:
- anterior or posterior vaginal wall prolapse (or both)
- stress urinary incontinence (SUI)
- distal rectocele.1,3
Mesh erosion occurs in 3.4% of cases and is usually easily managed.1 Other complications, including bowel injury, tend to be related to access, regardless of whether the operation is performed via laparotomy or laparoscopy.
Robotic sacrocolpopexy has become popular in recent years, and we will probably see data on this approach as we gain experience.
When it comes to vaginal mesh kits, the peer-reviewed literature is just beginning to expand, with many studies being presented at international meetings. For anterior and, possibly, apical vaginal prolapse, the cure rate after use of a mesh kit appears to be as high as, or higher than, the rate for sutured repairs.4 This high rate of anatomic cure is balanced somewhat by additional cost and complications involving mesh and the kits.
For posterior vaginal wall prolapse and rectocele, I firmly believe, based on our research and that of others, that sutured repairs are superior to graft-augmented surgery.5
DR. KARRAM: What are the indications and contraindications for mesh augmentation of prolapse repair ( FIGURES 1 and 2 )?
DR. LUCENTE: I believe mesh is indicated in any patient in need of surgical repair of pelvic organ prolapse who is seeking optimal durability and is willing to accept the known risks of the surgery.
The issue becomes more complex when it comes to contraindications. Absolute contraindications are fairly obvious; they include medically unstable patients and those who may have an inactive infectious process within the pelvis or even undiagnosed abnormal uterine bleeding.
At our center, because the potential for dyspareunia and pelvic discomfort is our biggest concern, we have developed a profile of the patient who is more likely to develop these complaints. The profile includes any patient who has a chronic pain disorder of any type, but especially chronic pelvic pain disorders such as endometriosis and vulvodynia. Other risk factors appear to be a history of pelvic surgery involving any permanent material, suture or mesh, and young age.
So if we have a patient in her late 30s who has undergone reconstructive surgery using permanent sutures and who has an element of chronic pelvic pain, we would counsel her strongly to consider surgical options other than the use of synthetic mesh.
DR. WALTERS: The main indications for mesh-augmented prolapse repair are recurrent posthysterectomy vaginal vault prolapse, for which I usually perform ASC, and recurrent cystocele or anteriorapical prolapse, for which I use one of the anterior mesh kits.
I still think sutured repairs—by that, I mean uterosacral ligament or sacrospinous colpopexy with sutured rectocele repair—work best for recurrent posterior wall and posteriorapical prolapse. I don’t use mesh augmentation for rectocele.
The main contraindication to mesh augmentation, as I see it, is a history of mesh complications. If I am repairing a mesh complication such as erosion or pain, I do not place another mesh.
Medical issues that might increase mesh complications, such as diabetes, steroid use, or severe vaginal atrophy, would, at the very least, make me consider carefully whether mesh augmentation is appropriate. The literature is not clear on this, so mesh could still be used if the surgeon thinks it is necessary.
DR. KARRAM: I haven’t found a definitive indication for mesh augmentation. We have used biologic meshes empirically, but I am not convinced that they really add long-term durability, regardless of whether they are used in the anterior or posterior vaginal segment.
Our published durability rate for traditional suture-type repairs is in the range of 85% at 5 years out.6 Even if I assumed that mesh would give me 100% 5-year durability, this rate would have to be at the expense of some erosion, pain, and other complications unique to mesh. I do not think that the potential improvement in durability is worth these potential complications.

FIGURE 1 When the pelvic support system is intact, prolapse is rare
In the normal pelvis, organs are supported by a complex web of muscles, fascia, and connective tissue. 
FIGURE 2 Mesh augmentation seeks to enhance the durability of repair
One type of mesh in final position. Mesh-augmented repair restores the vaginal apex and lends support to the walls of the vagina.
DR. KARRAM: If you are doing a lot of mesh repairs, you are obviously content with the results and feel that the few complications you are seeing are outweighed by the advantages mesh confers. How do you avoid extrusion and avert creation of a painful vagina?
DR. RAZ: Most of our cases are recurrent prolapse after failed vaginal or abdominal repair. I am indeed using a significant amount of soft polypropylene mesh for reconstructive procedures. As with the use of any other synthetic material, low-grade infection can develop after a few weeks or months. I use copious irrigation with antibiotic solution during reconstruction.
To avoid extrusion, I perform deep, rather than superficial, dissection of the vaginal wall to allow for better coverage of the mesh. For posterior mesh reconstruction, I cover the mesh with pararectal fascia to prevent erosion.
For mesh-augmented procedures, I cut the mesh myself in the operating room ( FIGURE 3 ). For a sling, I use a 10 cm × 1 cm soft polypropylene mesh. For a grade 3 or 4 cystocele, I use a trapezoid of soft polypropylene mesh with several points of fixation:
- at the sacrouterine ligament
- lateral to the obturator fascia
- distal to the bladder neck.
I always repair the vault at the same time.
For vault prolapse, I use a segment of soft polypropylene mesh in the shape of an apron with two arms (1 cm × 4 cm) and a central segment (4 cm × 7 cm). I support the vault using number 1-0 delayed absorbable suture and mesh. From outside the vaginal wall, in the posterolateral deep vaginal wall (inside the peritoneum), I incorporate the origin of the sacrouterine ligament and one arm of the mesh in the groove between the colon and levator ani, 15 cm from the introitus. I bring the suture 1 cm from the original entrance. A separate set of sutures brings the perirectal fascia together with the sacrouterine ligaments and perivesical fascia to close the peritoneal cavity. I tie the vault-suspension sutures, providing support to the cuff in a high posterior position (12 to 15 cm from the introitus).
In selected cases of significant recurrent rectocele, I use a rectangle of soft polypropylene mesh anchored to the origin of the sacrouterine ligament and distal to the perineal membrane. The mesh is covered by the pararectal fascia.
We have not seen vaginal, urethral, or bladder erosion in 1,800 cases of our distal urethral Prolene sling procedure using 10 cm × 1 cm soft mesh. In patients who have significant cystocele, vault prolapse, and recurrent rectocele, our vaginal erosion rate is 3%. We have never encountered rectal, bladder, or bowel perforation using our technique.
DR. LUCENTE: We often use mesh and are more than simply content with our results—we are extremely pleased, and so are our patients. Having said that, our techniques have definitely evolved over the past few years, as we’ve focused on how to decrease exposure and, more recently, optimize sexual function and vaginal comfort.
First, to avoid exposure, the most critical step is precise hydrodissection and distention of the true vesicovaginal space. This step can only be achieved through careful tactile guidance of the needle tip into the space, where it should remain while hydrodissection is performed. Always remember, sharp dissection “follows” hydrodissection. If you place the needle bevel within the vaginal wall, you will “split” the vaginal wall—as during standard colporrhaphy—which will lead to a high exposure rate.
Second, to avoid dyspareunia, it’s essential to pay close attention to POP-Q measurements, especially vaginal length, to ensure that the reconstruction restores the same length without foreshortening. This approach entails leaving the cervix in most patients who have a shorter vagina, and making sure that the mesh is secured above the ischial spine in younger, sexually active patients who have demonstrated a higher risk of postoperative deep, penetrating dyspareunia, compared with older, less sexually active patients.
Also paramount is to ensure that you have manually displaced the vagina inwardly as much as possible before deploying or setting the mesh. If you simply try to suture secure the mesh with the vagina incised open, without the ability to deploy the mesh with a closed, displaced vagina (to mimic deep penetration), it is difficult, if not impossible, to properly set the mesh for optimal comfort.
In the early days of midurethral pubovaginal slings using polypropylene, the adage was “looser is better than tighter.” This is even truer for transvaginal mesh.
DR. KARRAM: Dr. Walters, please describe your current surgical procedure of choice without mesh and explain why you haven’t adopted mesh for routine repairs.
DR. WALTERS: About 20% of my prolapse surgeries—usually for posthysterectomy or recurrent vaginal vault prolapse—involve ASC with placement of polypropylene mesh. I perform most of these cases through a Pfannenstiel incision, but I’ve also done them laparoscopically. Several of my partners perform ASC laparoscopically and robotically.
For the other 80% of my patients who have prolapse, I perform repairs transvaginally, usually using high bilateral uterosacralligament vaginal-vault suspension. We have learned to suture higher and slightly more medial on the uterosacral ligaments to attain greater vaginal depth and minimize ureteral obstruction. We use two or three sutures on each uterosacral ligament, usually a combination of permanent and delayed absorbable sutures.
I am also performing more sacrospinous ligament suspensions because this operation is being studied by the Pelvic Floor Disorders Network. Properly performed, it is an excellent surgery for apical prolapse. But, as with most of our surgeries for prolapse, recurrent anterior wall prolapse remains a problem.
Like you, Dr. Karram, we’ve studied our group’s anatomic and functional outcomes very carefully for more than 10 years and are mostly satisfied with our cure and complication rates. Although our anatomic outcomes with these surgeries are not always perfect, our reoperation rate for prolapse is only about 5%, with a high level of satisfaction in 88% to 92% of patients.
DR. RAZ: Unaugmented reconstruction fails in more than 30% of cases. Some patients who have significant prolapse and attenuated tissue think that this tissue will become healthier or stronger after reconstructive surgery, but that isn’t the case. In these situations, excision and plication make no clinical sense.
The problem is that we have yet to identify the ideal surrogate for poor-quality tissue. Most of us use polypropylene mesh in different variations. We need a better material that will be nonimmunogenic, well tolerated, and easily incorporated without erosion. Xenograft-like derivatives of dermis, or allografts such as cadaveric fascia, have failed over the long term because the body reabsorbs the graft without forming any new connective tissue.

FIGURE 3 Mesh can be cut in the OR to custom-fit a patient
Hand-cut mesh and points of placement.
PHOTO: SHLOMO RAZ, MD
Is a kit a valuable aid?
DR. KARRAM: If a surgeon wants to augment a repair, what are the advantages of a packaged mesh kit, compared with simply cutting the mesh and performing surgery without a kit?
DR. WALTERS: The advantages of a packaged mesh kit are the convenience involved and the ability to consistently perform the same operation with the same product. That facilitates learning, teaching, and research. It also helps us understand the published literature a little better because “custom” prolapse repairs are operator-dependent and difficult to apply generally to a population of surgeons.
These advantages are most clearly apparent with midurethral sling mesh kits, which have almost revolutionized surgery for stress incontinence. I don’t believe mesh kits for prolapse are there yet, but they certainly have potential.
DR. RAZ: I’m opposed to the use of kits. They are industry-driven. One company has made $1 billion selling them. Imagine a patient who undergoes placement of a sling kit ($1,000), cystocele kit ($1,500), and posterior mesh kit ($1,500). How can our healthcare system sustain this burden, especially when there is no real evidence that a kit improves the operation, and given the incredible complication rate that we see?
Moreover, the kits contain a single-use needle and passer and a precut segment of polypropylene mesh. But every patient is different and requires a unique size or shape of mesh. I don’t believe that a surgeon who knows pelvic anatomy needs a kit to perform mesh-augmented reconstruction. We can buy the same segment of mesh for $200 to $400, cut it as needed, and perform the same operation advertised by industry.
For surgeons who prefer a kit, the tools that are included should be made reusable.
DR. LUCENTE: In my opinion, the primary advantage of a commercially available transvaginal mesh delivery system—notice, I avoided the word “kit,” because I think there are plenty of negative connotations associated with it—is the ability to deliver the mesh in a “tension”-free manner.
One alternative that many people pursue is cutting the mesh to size and using sutures to hold it in place while tissue ingrowth occurs. However, the hernia literature suggests that suturing mesh in place increases the risk of postoperative discomfort at the site of implantation. The true cause of the discomfort remains unclear, but it is thought to arise from nerve tethering or traction at the pre-committed points of attachment before the host tissue and mesh interface have adjusted or settled with tissue ingrowth.
All neuropathic complications of mesh implantation have been shown in the current hernia literature to be increased with the use of sutures.7 Also, as previously mentioned, it is extremely difficult to set or adjust the mesh with the vaginal incision remaining “open,” which is a downside to suture techniques.
What training is necessary to use a kit?
DR. KARRAM: Mesh kits are aggressively promoted by industry, with close to half a dozen different kits to be available soon. What is the minimum amount of training one should have before utilizing these kits?
DR. WALTERS: The surgeon should at least know how to perform traditional sutured prolapse repairs and SUI surgery and be able to perform cystoscopy. Ideally, the surgeon should undergo training on a cadaver with a skilled and experienced user of the mesh kit. The surgeon also should carefully review the risks and benefits of mesh kits with the patient and inform the patient that he or she is in the early learning curve of a particular surgery. The informed patient should have a right to refuse mesh-augmented prolapse surgery after the consent process.
DR. LUCENTE: I’m glad you asked this question. I strongly believe that surgical expertise and proficiency within gynecology need to be more effectively addressed by us all. We have a situation in our field in which techniques and technology are widening the gap between what is possible and what the surgeon is comfortable doing safely.
It’s incumbent on all of us, especially those who are in a leadership position as a chairperson or chief of a division, to work with our physician staff and faculty to optimize surgical skill and patient outcomes, including safety, with new technologies.
As for the minimal amount of training needed, that’s extremely variable. It depends on the current skill set of the physician and his or her ability to pick up the mechanics of the surgery as it is taught through a cadaver lab or preceptorship. It’s regrettable that some physicians lack the objectivity and insight to judge their own skill set. This, again, is the time for a chairperson or chief of a division to step up to the plate and ensure proper credentialing and demonstration of proficiency.
It is unrealistic to expect industry to decide who should or should not utilize this truly breakthrough technology. That is our responsibility as physicians.
DR. KARRAM: At a minimum, I think any surgeon utilizing a kit should have a firm understanding of pelvic floor anatomy and experience performing traditional repairs:
- intraperitoneal procedures such as Mc-Call culdoplasty and uterosacral suspension
- sacrospinous suspension
- retropubic procedures and anti-incontinence operations such as pubovaginal slings.
This three-dimensional understanding of the pelvic floor is mandatory if one is to assume that blind passage of trocars through potentially dangerous spaces is the wave of the future.
DR. RAZ: You need to be a pelvic surgeon, know your anatomy, and know how to manage complications if you are going to use one of these kits. You should stick to the surgery that works best in your hands. Industry cannot teach you to be a good pelvic surgeon; it takes lifelong experience.
DR. KARRAM: If you have a patient who is sexually inactive with pelvic organ prolapse, would you prefer a mesh repair or an obliterative procedure? And why?
DR. WALTERS: If the patient is sexually inactive—especially if she is older and definitely will not be in the future—it makes absolutely no sense to perform a mesh-augmented repair. A traditional, somewhat tight, sutured repair works fine in this setting and carries very low risk.
In fact, our group and others have found that, in carefully selected patients, partial colpectomy and colpocleisis procedures (without grafts) have among the highest cure and satisfaction rates of all surgeries we perform for prolapse; they also have relatively low risk.8 Recurrent prolapse after an obliterative procedure is rare; most of the dissatisfaction relates to postoperative voiding difficulties or persistent or de novo urinary incontinence.
DR. KARRAM: I also prefer an obliterative procedure. I see no reason to bring in the cost and potential for complications that mesh repair entails. An obliterative procedure should produce an anatomic success rate close to 100%, with minimal complications. It also can be performed quickly with minimal anesthesia and convalescence.
DR. LUCENTE: My response is based on a clinical study that my associate, Dr. Miles Murphy, has performed, comparing a transvaginal mesh procedure with a LaForte operation for severe pelvic organ prolapse.9 Both patient groups were well satisfied with the result, and success rates were comparable. However, the group that underwent the transvaginal mesh procedure had a shorter operative time.
As a result of these studies, we tend to prefer transvaginal mesh repair. Even though the woman may be sexually inactive, the procedure preserves vaginal function, and we all know that life has a way of being unpredictable. Her situation may change so that she once again desires sexual function.
However, for a very elderly woman—one in her late 80s or 90s—who has severe or extreme prolapse with a very large procidentia and vaginal length measuring, say, 13 cm beyond the introitus, I do prefer an obliterative procedure.
DR. RAZ: I agree. I would not offer a sexually inactive patient an obliterative procedure. You never know what the future will hold.
Mesh repair can be performed safely, provided the surgeon has good knowledge of anatomic landmarks and knows how to manage any potential complications that may arise.
MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.
SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.
VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.
MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
Mesh kits for repairing prolapse are proliferating like crazy, just as they did for midurethral sling procedures. But mesh augmentation of prolapse surgeries requires more than a prepackaged assortment of tools and materials. In this article, moderator Mickey M. Karram, MD, and a panel of nationally recognized urogynecologists and urologists describe the literature on mesh augmentation and discuss indications, contraindications, techniques, applicable cases, and the considerable training required.
In Part 2, which will appear in the February issue of OBG Management, the panel tackles the thorny topic of complications, including erosion, extrusion, foreshortening of the vagina, dyspareunia, and pain. Their discussion focuses on ways to avoid these problems, and methods for correcting them.
Do we have enough data?
DR. KARRAM: To start, let’s quickly review the peer-reviewed literature on the use of mesh augmentation during surgery for pelvic organ prolapse.
DR. WALTERS: Until recently, most data concerned open abdominal sacrocolpopexy (ASC) using polypropylene or Merseline mesh. There is significant clinical experience with this operation, and multiple cohort studies show long-term cure rates of 78% to 100% for apical prolapse.1
At least two randomized controlled trials have compared open ASC with sutured vaginal colpopexy procedures, and ASC is certainly equal to—perhaps better than—all transvaginal sutured repairs.2,3
With ASC, most recurrences affect the distal half of the vagina and involve one or more of the following:
- anterior or posterior vaginal wall prolapse (or both)
- stress urinary incontinence (SUI)
- distal rectocele.1,3
Mesh erosion occurs in 3.4% of cases and is usually easily managed.1 Other complications, including bowel injury, tend to be related to access, regardless of whether the operation is performed via laparotomy or laparoscopy.
Robotic sacrocolpopexy has become popular in recent years, and we will probably see data on this approach as we gain experience.
When it comes to vaginal mesh kits, the peer-reviewed literature is just beginning to expand, with many studies being presented at international meetings. For anterior and, possibly, apical vaginal prolapse, the cure rate after use of a mesh kit appears to be as high as, or higher than, the rate for sutured repairs.4 This high rate of anatomic cure is balanced somewhat by additional cost and complications involving mesh and the kits.
For posterior vaginal wall prolapse and rectocele, I firmly believe, based on our research and that of others, that sutured repairs are superior to graft-augmented surgery.5
DR. KARRAM: What are the indications and contraindications for mesh augmentation of prolapse repair ( FIGURES 1 and 2 )?
DR. LUCENTE: I believe mesh is indicated in any patient in need of surgical repair of pelvic organ prolapse who is seeking optimal durability and is willing to accept the known risks of the surgery.
The issue becomes more complex when it comes to contraindications. Absolute contraindications are fairly obvious; they include medically unstable patients and those who may have an inactive infectious process within the pelvis or even undiagnosed abnormal uterine bleeding.
At our center, because the potential for dyspareunia and pelvic discomfort is our biggest concern, we have developed a profile of the patient who is more likely to develop these complaints. The profile includes any patient who has a chronic pain disorder of any type, but especially chronic pelvic pain disorders such as endometriosis and vulvodynia. Other risk factors appear to be a history of pelvic surgery involving any permanent material, suture or mesh, and young age.
So if we have a patient in her late 30s who has undergone reconstructive surgery using permanent sutures and who has an element of chronic pelvic pain, we would counsel her strongly to consider surgical options other than the use of synthetic mesh.
DR. WALTERS: The main indications for mesh-augmented prolapse repair are recurrent posthysterectomy vaginal vault prolapse, for which I usually perform ASC, and recurrent cystocele or anteriorapical prolapse, for which I use one of the anterior mesh kits.
I still think sutured repairs—by that, I mean uterosacral ligament or sacrospinous colpopexy with sutured rectocele repair—work best for recurrent posterior wall and posteriorapical prolapse. I don’t use mesh augmentation for rectocele.
The main contraindication to mesh augmentation, as I see it, is a history of mesh complications. If I am repairing a mesh complication such as erosion or pain, I do not place another mesh.
Medical issues that might increase mesh complications, such as diabetes, steroid use, or severe vaginal atrophy, would, at the very least, make me consider carefully whether mesh augmentation is appropriate. The literature is not clear on this, so mesh could still be used if the surgeon thinks it is necessary.
DR. KARRAM: I haven’t found a definitive indication for mesh augmentation. We have used biologic meshes empirically, but I am not convinced that they really add long-term durability, regardless of whether they are used in the anterior or posterior vaginal segment.
Our published durability rate for traditional suture-type repairs is in the range of 85% at 5 years out.6 Even if I assumed that mesh would give me 100% 5-year durability, this rate would have to be at the expense of some erosion, pain, and other complications unique to mesh. I do not think that the potential improvement in durability is worth these potential complications.

FIGURE 1 When the pelvic support system is intact, prolapse is rare
In the normal pelvis, organs are supported by a complex web of muscles, fascia, and connective tissue. 
FIGURE 2 Mesh augmentation seeks to enhance the durability of repair
One type of mesh in final position. Mesh-augmented repair restores the vaginal apex and lends support to the walls of the vagina.
DR. KARRAM: If you are doing a lot of mesh repairs, you are obviously content with the results and feel that the few complications you are seeing are outweighed by the advantages mesh confers. How do you avoid extrusion and avert creation of a painful vagina?
DR. RAZ: Most of our cases are recurrent prolapse after failed vaginal or abdominal repair. I am indeed using a significant amount of soft polypropylene mesh for reconstructive procedures. As with the use of any other synthetic material, low-grade infection can develop after a few weeks or months. I use copious irrigation with antibiotic solution during reconstruction.
To avoid extrusion, I perform deep, rather than superficial, dissection of the vaginal wall to allow for better coverage of the mesh. For posterior mesh reconstruction, I cover the mesh with pararectal fascia to prevent erosion.
For mesh-augmented procedures, I cut the mesh myself in the operating room ( FIGURE 3 ). For a sling, I use a 10 cm × 1 cm soft polypropylene mesh. For a grade 3 or 4 cystocele, I use a trapezoid of soft polypropylene mesh with several points of fixation:
- at the sacrouterine ligament
- lateral to the obturator fascia
- distal to the bladder neck.
I always repair the vault at the same time.
For vault prolapse, I use a segment of soft polypropylene mesh in the shape of an apron with two arms (1 cm × 4 cm) and a central segment (4 cm × 7 cm). I support the vault using number 1-0 delayed absorbable suture and mesh. From outside the vaginal wall, in the posterolateral deep vaginal wall (inside the peritoneum), I incorporate the origin of the sacrouterine ligament and one arm of the mesh in the groove between the colon and levator ani, 15 cm from the introitus. I bring the suture 1 cm from the original entrance. A separate set of sutures brings the perirectal fascia together with the sacrouterine ligaments and perivesical fascia to close the peritoneal cavity. I tie the vault-suspension sutures, providing support to the cuff in a high posterior position (12 to 15 cm from the introitus).
In selected cases of significant recurrent rectocele, I use a rectangle of soft polypropylene mesh anchored to the origin of the sacrouterine ligament and distal to the perineal membrane. The mesh is covered by the pararectal fascia.
We have not seen vaginal, urethral, or bladder erosion in 1,800 cases of our distal urethral Prolene sling procedure using 10 cm × 1 cm soft mesh. In patients who have significant cystocele, vault prolapse, and recurrent rectocele, our vaginal erosion rate is 3%. We have never encountered rectal, bladder, or bowel perforation using our technique.
DR. LUCENTE: We often use mesh and are more than simply content with our results—we are extremely pleased, and so are our patients. Having said that, our techniques have definitely evolved over the past few years, as we’ve focused on how to decrease exposure and, more recently, optimize sexual function and vaginal comfort.
First, to avoid exposure, the most critical step is precise hydrodissection and distention of the true vesicovaginal space. This step can only be achieved through careful tactile guidance of the needle tip into the space, where it should remain while hydrodissection is performed. Always remember, sharp dissection “follows” hydrodissection. If you place the needle bevel within the vaginal wall, you will “split” the vaginal wall—as during standard colporrhaphy—which will lead to a high exposure rate.
Second, to avoid dyspareunia, it’s essential to pay close attention to POP-Q measurements, especially vaginal length, to ensure that the reconstruction restores the same length without foreshortening. This approach entails leaving the cervix in most patients who have a shorter vagina, and making sure that the mesh is secured above the ischial spine in younger, sexually active patients who have demonstrated a higher risk of postoperative deep, penetrating dyspareunia, compared with older, less sexually active patients.
Also paramount is to ensure that you have manually displaced the vagina inwardly as much as possible before deploying or setting the mesh. If you simply try to suture secure the mesh with the vagina incised open, without the ability to deploy the mesh with a closed, displaced vagina (to mimic deep penetration), it is difficult, if not impossible, to properly set the mesh for optimal comfort.
In the early days of midurethral pubovaginal slings using polypropylene, the adage was “looser is better than tighter.” This is even truer for transvaginal mesh.
DR. KARRAM: Dr. Walters, please describe your current surgical procedure of choice without mesh and explain why you haven’t adopted mesh for routine repairs.
DR. WALTERS: About 20% of my prolapse surgeries—usually for posthysterectomy or recurrent vaginal vault prolapse—involve ASC with placement of polypropylene mesh. I perform most of these cases through a Pfannenstiel incision, but I’ve also done them laparoscopically. Several of my partners perform ASC laparoscopically and robotically.
For the other 80% of my patients who have prolapse, I perform repairs transvaginally, usually using high bilateral uterosacralligament vaginal-vault suspension. We have learned to suture higher and slightly more medial on the uterosacral ligaments to attain greater vaginal depth and minimize ureteral obstruction. We use two or three sutures on each uterosacral ligament, usually a combination of permanent and delayed absorbable sutures.
I am also performing more sacrospinous ligament suspensions because this operation is being studied by the Pelvic Floor Disorders Network. Properly performed, it is an excellent surgery for apical prolapse. But, as with most of our surgeries for prolapse, recurrent anterior wall prolapse remains a problem.
Like you, Dr. Karram, we’ve studied our group’s anatomic and functional outcomes very carefully for more than 10 years and are mostly satisfied with our cure and complication rates. Although our anatomic outcomes with these surgeries are not always perfect, our reoperation rate for prolapse is only about 5%, with a high level of satisfaction in 88% to 92% of patients.
DR. RAZ: Unaugmented reconstruction fails in more than 30% of cases. Some patients who have significant prolapse and attenuated tissue think that this tissue will become healthier or stronger after reconstructive surgery, but that isn’t the case. In these situations, excision and plication make no clinical sense.
The problem is that we have yet to identify the ideal surrogate for poor-quality tissue. Most of us use polypropylene mesh in different variations. We need a better material that will be nonimmunogenic, well tolerated, and easily incorporated without erosion. Xenograft-like derivatives of dermis, or allografts such as cadaveric fascia, have failed over the long term because the body reabsorbs the graft without forming any new connective tissue.

FIGURE 3 Mesh can be cut in the OR to custom-fit a patient
Hand-cut mesh and points of placement.
PHOTO: SHLOMO RAZ, MD
Is a kit a valuable aid?
DR. KARRAM: If a surgeon wants to augment a repair, what are the advantages of a packaged mesh kit, compared with simply cutting the mesh and performing surgery without a kit?
DR. WALTERS: The advantages of a packaged mesh kit are the convenience involved and the ability to consistently perform the same operation with the same product. That facilitates learning, teaching, and research. It also helps us understand the published literature a little better because “custom” prolapse repairs are operator-dependent and difficult to apply generally to a population of surgeons.
These advantages are most clearly apparent with midurethral sling mesh kits, which have almost revolutionized surgery for stress incontinence. I don’t believe mesh kits for prolapse are there yet, but they certainly have potential.
DR. RAZ: I’m opposed to the use of kits. They are industry-driven. One company has made $1 billion selling them. Imagine a patient who undergoes placement of a sling kit ($1,000), cystocele kit ($1,500), and posterior mesh kit ($1,500). How can our healthcare system sustain this burden, especially when there is no real evidence that a kit improves the operation, and given the incredible complication rate that we see?
Moreover, the kits contain a single-use needle and passer and a precut segment of polypropylene mesh. But every patient is different and requires a unique size or shape of mesh. I don’t believe that a surgeon who knows pelvic anatomy needs a kit to perform mesh-augmented reconstruction. We can buy the same segment of mesh for $200 to $400, cut it as needed, and perform the same operation advertised by industry.
For surgeons who prefer a kit, the tools that are included should be made reusable.
DR. LUCENTE: In my opinion, the primary advantage of a commercially available transvaginal mesh delivery system—notice, I avoided the word “kit,” because I think there are plenty of negative connotations associated with it—is the ability to deliver the mesh in a “tension”-free manner.
One alternative that many people pursue is cutting the mesh to size and using sutures to hold it in place while tissue ingrowth occurs. However, the hernia literature suggests that suturing mesh in place increases the risk of postoperative discomfort at the site of implantation. The true cause of the discomfort remains unclear, but it is thought to arise from nerve tethering or traction at the pre-committed points of attachment before the host tissue and mesh interface have adjusted or settled with tissue ingrowth.
All neuropathic complications of mesh implantation have been shown in the current hernia literature to be increased with the use of sutures.7 Also, as previously mentioned, it is extremely difficult to set or adjust the mesh with the vaginal incision remaining “open,” which is a downside to suture techniques.
What training is necessary to use a kit?
DR. KARRAM: Mesh kits are aggressively promoted by industry, with close to half a dozen different kits to be available soon. What is the minimum amount of training one should have before utilizing these kits?
DR. WALTERS: The surgeon should at least know how to perform traditional sutured prolapse repairs and SUI surgery and be able to perform cystoscopy. Ideally, the surgeon should undergo training on a cadaver with a skilled and experienced user of the mesh kit. The surgeon also should carefully review the risks and benefits of mesh kits with the patient and inform the patient that he or she is in the early learning curve of a particular surgery. The informed patient should have a right to refuse mesh-augmented prolapse surgery after the consent process.
DR. LUCENTE: I’m glad you asked this question. I strongly believe that surgical expertise and proficiency within gynecology need to be more effectively addressed by us all. We have a situation in our field in which techniques and technology are widening the gap between what is possible and what the surgeon is comfortable doing safely.
It’s incumbent on all of us, especially those who are in a leadership position as a chairperson or chief of a division, to work with our physician staff and faculty to optimize surgical skill and patient outcomes, including safety, with new technologies.
As for the minimal amount of training needed, that’s extremely variable. It depends on the current skill set of the physician and his or her ability to pick up the mechanics of the surgery as it is taught through a cadaver lab or preceptorship. It’s regrettable that some physicians lack the objectivity and insight to judge their own skill set. This, again, is the time for a chairperson or chief of a division to step up to the plate and ensure proper credentialing and demonstration of proficiency.
It is unrealistic to expect industry to decide who should or should not utilize this truly breakthrough technology. That is our responsibility as physicians.
DR. KARRAM: At a minimum, I think any surgeon utilizing a kit should have a firm understanding of pelvic floor anatomy and experience performing traditional repairs:
- intraperitoneal procedures such as Mc-Call culdoplasty and uterosacral suspension
- sacrospinous suspension
- retropubic procedures and anti-incontinence operations such as pubovaginal slings.
This three-dimensional understanding of the pelvic floor is mandatory if one is to assume that blind passage of trocars through potentially dangerous spaces is the wave of the future.
DR. RAZ: You need to be a pelvic surgeon, know your anatomy, and know how to manage complications if you are going to use one of these kits. You should stick to the surgery that works best in your hands. Industry cannot teach you to be a good pelvic surgeon; it takes lifelong experience.
DR. KARRAM: If you have a patient who is sexually inactive with pelvic organ prolapse, would you prefer a mesh repair or an obliterative procedure? And why?
DR. WALTERS: If the patient is sexually inactive—especially if she is older and definitely will not be in the future—it makes absolutely no sense to perform a mesh-augmented repair. A traditional, somewhat tight, sutured repair works fine in this setting and carries very low risk.
In fact, our group and others have found that, in carefully selected patients, partial colpectomy and colpocleisis procedures (without grafts) have among the highest cure and satisfaction rates of all surgeries we perform for prolapse; they also have relatively low risk.8 Recurrent prolapse after an obliterative procedure is rare; most of the dissatisfaction relates to postoperative voiding difficulties or persistent or de novo urinary incontinence.
DR. KARRAM: I also prefer an obliterative procedure. I see no reason to bring in the cost and potential for complications that mesh repair entails. An obliterative procedure should produce an anatomic success rate close to 100%, with minimal complications. It also can be performed quickly with minimal anesthesia and convalescence.
DR. LUCENTE: My response is based on a clinical study that my associate, Dr. Miles Murphy, has performed, comparing a transvaginal mesh procedure with a LaForte operation for severe pelvic organ prolapse.9 Both patient groups were well satisfied with the result, and success rates were comparable. However, the group that underwent the transvaginal mesh procedure had a shorter operative time.
As a result of these studies, we tend to prefer transvaginal mesh repair. Even though the woman may be sexually inactive, the procedure preserves vaginal function, and we all know that life has a way of being unpredictable. Her situation may change so that she once again desires sexual function.
However, for a very elderly woman—one in her late 80s or 90s—who has severe or extreme prolapse with a very large procidentia and vaginal length measuring, say, 13 cm beyond the introitus, I do prefer an obliterative procedure.
DR. RAZ: I agree. I would not offer a sexually inactive patient an obliterative procedure. You never know what the future will hold.
Mesh repair can be performed safely, provided the surgeon has good knowledge of anatomic landmarks and knows how to manage any potential complications that may arise.
1. Nygaard IE, McCreery R, Brubaker L, et al. Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805-823.
2. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1421;discussion 1421-1422.
3. Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol. 2004;190:20-26.
4. Murphy M. Society of Gynecologic Surgeons Systematic Review Group. Clinical practice guidelines on vaginal graft use from the Society of Gynecologic Surgeons. Obstet Gynecol. 2008;112:1123-1130.
5. Paraiso MF, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical procedures including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
6. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol 2006;108:255-263.
7. EU Hernia Trialists Collaboration. Repair of groin hernia with synthetic mesh: meta-analysis of randomized controlled trials. Ann Surg. 2002;235:322-332.
8. Barber MD, Amundsen C, Paraiso MFR, Weidner A, Romero A, Walters MD. Quality of life after surgery for genital prolapse in elderly women: obliterative and reconstructive surgery. Int Urogynecol J. 2007;18:799-806.
9. Murphy M, van Raalte H, Mercurio E, Haff R, Wiseman B, Lucente VR. Incontinence-related quality of life and sexual function following the tension-free vaginal tape vs the “inside-out” tension-free vaginal tape obturator. Int Urogynecol J. 2008;19:481-487.
1. Nygaard IE, McCreery R, Brubaker L, et al. Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805-823.
2. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1421;discussion 1421-1422.
3. Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol. 2004;190:20-26.
4. Murphy M. Society of Gynecologic Surgeons Systematic Review Group. Clinical practice guidelines on vaginal graft use from the Society of Gynecologic Surgeons. Obstet Gynecol. 2008;112:1123-1130.
5. Paraiso MF, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical procedures including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
6. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol 2006;108:255-263.
7. EU Hernia Trialists Collaboration. Repair of groin hernia with synthetic mesh: meta-analysis of randomized controlled trials. Ann Surg. 2002;235:322-332.
8. Barber MD, Amundsen C, Paraiso MFR, Weidner A, Romero A, Walters MD. Quality of life after surgery for genital prolapse in elderly women: obliterative and reconstructive surgery. Int Urogynecol J. 2007;18:799-806.
9. Murphy M, van Raalte H, Mercurio E, Haff R, Wiseman B, Lucente VR. Incontinence-related quality of life and sexual function following the tension-free vaginal tape vs the “inside-out” tension-free vaginal tape obturator. Int Urogynecol J. 2008;19:481-487.