User login
2016 Update on female sexual dysfunction
The age-adjusted prevalence of any sexual problem is 43% among US women. A full 22% of these women experience sexually related personal distress.1 With publication of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition2 has come a shift in classification and, at times, management approach for reported female sexual dysfunction. When women report to their clinicians decreased sexual desire or arousal or pain at penetration, the management is no longer guided by a linear model of sexual response (excitation, plateau, orgasm, and resolution) but rather by a more nuanced and complex biopsychosocial approach. In this model, diagnosis and management strategies to address bothersome sexual concerns consider the whole woman in the context of her physical and psychosocial health. The patient’s age, medical history, and relationship status are among the factors that could affect management of the problem. In an effort to explore this management approach, I used this Update on Female Sexual Dysfunction as an opportunity to convene a roundtable of several experts, representing varying backgrounds and practice vantage points, to discuss 5 cases of sexual problems that you as a busy clinician may encounter in your practice.

Genital atrophy in a sexually inactive 61-year-old woman
Barbara S. Levy, MD: Two years after her husband's death, which followed several years of illness, your 61-year-old patient mentions at her well woman visit that she anticipates becoming sexually active again. She has not used systemic or vaginal hormone therapy. During pelvic examination, atrophic external genital changes are present, and use of an ultrathin (thinner than a Pederson) speculum reveals vaginal epithelial atrophic changes. A single-digit bimanual exam can be performed with moderate patient discomfort; the patient cannot tolerate a 2-digit bimanual exam. She expresses concern about being able to engage in penile/vaginal sexual intercourse.
Dr. Kaunitz, what is important for you to ask this patient, and what concerns you most on her physical exam?
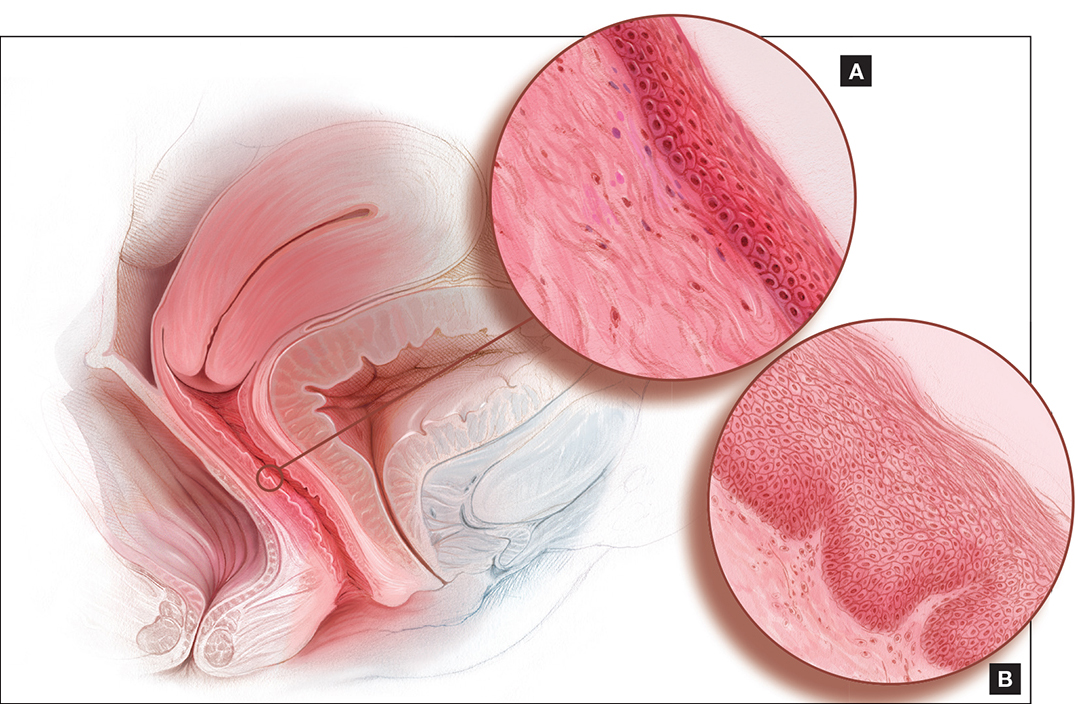
Andrew M. Kaunitz, MD: First, it is important to recognize the patient's expectations and desires. As the case suggests, but further questioning could clarify, she would like to be able to comfortably engage in sexual intercourse with a new partner, but penetration may be difficult (and definitely painful for her) unless treatment is pursued. This combination of mucosal and vestibular atrophic changes (genitourinary syndrome of menopause [GSM], or vulvovaginal atrophy [VVA]) plus the absence of penetration for many years can be a double whammy situation for menopausal women. In this case it has led to extensive contracture of the introitus, and if it is not addressed will cause sexual dysfunction.
Dr. Levy: In addition we need to clarify whether or not a history of breast cancer or some other thing may impact the care we provide. How would you approach talking with this patient in order to manage her care?
Dr. Kaunitz: One step is to see how motivated she is to address this, as it is not something that, as gynecologists, we can snap our fingers and the situation will be resolved. If the patient is motivated to treat the atrophic changes with medical treatment, in the form of low-dose vaginal estrogen, and dilation, either on her own if she's highly motivated to do so, or in my practice more commonly with the support of a women's physical therapist, over time she should be able to comfortably engage in sexual intercourse with penetration. If this is what she wants, we can help steer her in the right direction.
Sheryl Kingsberg, PhD: You know that this woman is motivated by virtue of her initiating the topic herself. Patients are often embarrassed talking about sexual issues, or they are not sure that their gynecologist is comfortable with it. After all, they think, if this is the right place to discuss sexual problems, why didn't he or she ask me? Clinicians must be aware that it is their responsibility to ask about sexual function and not leave it for the patient to open the door.
Dr. Kaunitz: Great point.
Cheryl B. Iglesia, MD: Gratefully, a lot of the atrophic changes this patient demonstrates are reversible. However, other autoimmune diseases (eg, lichen planus, which can affect the vaginal epithelium, or lichen sclerosus, which can affect the clitoris, labia, and vulva) can also cause constriction, and in severe cases, complete obliteration of the vagina and introitus. Women may not be sexually active, and for each annual exam their clinician uses a smaller and smaller speculum--to the point that they cannot even access the cervix anymore--and the vagina can close off. Clinicians may not realize that you need something other than estrogen; with lichen planus you need steroid suppository treatment, and with lichen sclerosus you need topical steroid treatment. So these autoimmune conditions should also be in the differential and, with appropriate treatment, the sexual effects can be reversible.
Michael Krychman, MD: I agree. The vulva can be a great mimicker and, according to the history and physical exam, at some point a vulvoscopy, and even potential biopsies, may be warranted as clinically indicated.
The concept of a comprehensive approach, as Dr. Kingsberg had previously mentioned, involves not only sexual medicine but also evaluating the patient's biopsychosocial variables that may impact her condition. We also need to set realistic expectations. Some women may benefit from off-label use of medications besides estrogen, including topical testosterone. Informed consent is very important with these treatments. I also have had much clinical success with intravaginal diazepam/lorazepam for pelvic floor hypertonus.
In addition, certainly I agree that pelvic floor physical therapy (PT) is a vital treatment component for this patient and, not to diminish its importance, but many women cannot afford, nor do they have the time or opportunity, to go to pelvic floor PT. As clinicians, we can develop and implement effective programs, even in the office, to educate the patient to help herself as well.
Dr. Kaunitz: Absolutely. Also, if, in a clinical setting consistent with atrophic changes, an ObGyn physician is comfortable that vulvovaginal changes noted on exam represent GSM/atrophic changes, I do not feel vulvoscopy is warranted.
Dr. Levy: In conclusion, we need to be aware that pelvic floor PT may not be available everywhere and that a woman's own digits and her partner can also be incorporated into this treatment.
Something that we have all talked about in other venues, but have not looked at in the larger sphere here, is whether there is value to seeing women annually and performing pelvic exams. As Dr. Kingsberg mentioned, this is a highly motivated patient. We have many patients out there who are silent sufferers. The physical exam is an opportunity for us to recognize and address this problem.
Dyspareunia and low sexual desire in a breast cancer survivor
Dr. Levy: In this case, a 36-year-old woman with BRCA1−positive breast cancer has vaginal dryness, painful intercourse, and lowered sexual interest since her treatment, which included chemotherapy after bilateral mastectomies. She has a bilateral salpingo-oophorectomy(BSO) scheduled for primary prevention of her ovarian cancer risk.
Dr. Kingsberg, what is important for you to know to help guide case management?
Dr. Kingsberg: This woman is actually presenting with 2 sexual problems: dyspareunia, which is probably secondary to VVA or GSM, and low sexual desire. Key questions are: 1) When was symptom onset--acquired after treatment or lifelong? 2) Did she develop the dyspareunia and as a result of having pain during sex lost desire to have sex? Or, did she lose desire and then, without it, had no arousal and therefore pain with penetration developed? It also could be that she has 2 distinct problems, VVA and hypoactive sexual desire disorder (HSDD), in which case you need to think about treating both. Finally, we do not actually know if she is having penetrative intercourse or even if she has a partner.
A vulvovaginal exam would give clues as to whether she has VVA, and hormone levels would indicate if she now has chemo-induced menopause. If she is not in menopause now, she certainly is going to be with her BSO. The hormonal changes due to menopause actually can be primarily responsible for both the dyspareunia and HSDD. Management of both symptoms really needs to be based on shared decision making with the patient--with which treatment for which conditions coming first, based on what is causing her the most distress.
I would encourage this woman to treat her VVA since GSM does have long-term physiologic consequences if untreated. The American College of Obstetricians and Gynecologists (ACOG) recommends nonhormonal treatments as first-line treatments, with vaginal estrogen considered if these therapies fail.3 If lubricants and moisturizers and other nonhormonal options are not sufficient, you could consider local estrogen, even though she is a breast cancer survivor, as well as ospemiphene.
If she is distressed by her loss of sexual desire, you can choose to treat her for HSDD. Flibanserin is the first FDA-approved treatment for HSDD. It is only approved in premenopausal women, so it would be considered off-label use if she is postmenopausal (even though she is quite young). You also could consider exogenous testosterone off-label, after consulting with her oncologist.
In addition to the obvious physiologic etiology of her pain and her low desire, the biopsychosocial aspects to consider are: 1) changes to her body image, as she has had bilateral mastectomies, 2) her anxiety about the cancer diagnosis, and 3) concerns about her relationship if she has one--her partner's reactions to her illness and the quality of the relationship outside the bedroom.
Dr. Iglesia: I am seeing here in our nation's capital a lot of advertisements for laser therapy for GSM. I caution women about this because providers are charging a lot of money for this therapy when we do not have long-term safety and effectiveness data for it.
Our group is currently conducting a randomized controlled trial, looking at vaginal estrogen cream versus laser therapy for GSM here at Medstar Health--one of the first in the country as part of a multisite trial. But the North American Menopause Society (NAMS) has come out with a pretty strong statement,4 as has ACOG,5 on this therapy, and I caution people about overzealously offering a very costly procedure targeted to a very vulnerable population, especially to women with personal histories of estrogen-sensitive cancers.
Dr. Krychman: I agree. Very often cancer patients are preyed upon by those offering emerging unproven technologies or medications. We have to work as a coordinated comprehensive team, whether it's a sexual medicine expert, psychologist, urogynecologist, gynecologist, or oncologist, and incorporate the patient's needs and expectations and risk tolerance coupled with treatment efficacy and safety.
Dr. Levy: This was a complex case. The biopsychosocial model is critical here. It's important that we are not siloes in our medical management approach and that we try to help this patient embrace the complexity of her situation. It's not only that she has cancer at age 36; there is a possible guilt factor if she has children and passed that gene on.
Another point that we began to talk about is the fact that in this country we tend to be early adopters of new technology. In our discussion with patients, we should focus on what we know and the risk of the unknowns related to some of the treatment options. But let's discuss lasers a little more later on.
Diminished arousal and orgasmic intensity in a patient taking SSRIs
Dr. Levy: In this next case, a 44-year-old woman in a 15-year marriage notices a change in her orgasmic intensity and latency. She has a supportive husband, and they are attentive to each other's sexual needs. However, she notices a change in her arousal and orgasmic intensity, which has diminished over the last year. She reports that the time to orgasm or latency has increased and both she and her partner are frustrated and getting concerned. She has a history of depression that has been managed by selective serotonin reuptake inhibitors for the past 5 years and has no depressive symptoms currently.
Dr. Krychman, what are you considering before beginning to talk with this patient?
Dr. Krychman: My approach really is a comprehensive one, looking not only at the underlying medical issues but also at the psychological and dynamic relationship facets. We of course also want to look at medications: Has she changed her dose or the timing of when she takes it? Is this a new onset? Finally, we want to know why this is coming to the forefront now. Is it because it is getting worse, or is it because there is some significant issue that is going on in the relationship?
Regarding the physical exam, it is important to rule out underlying genital pelvic pathology. Young women can get changes in the integrity of the pelvic floor, in what I would call the orgasmic matrix--the clitoral tissue, the body, the crura (or arms of the clitoris)--we want to examine and be reassured that her genital anatomy is normal and that there is no underlying pathology that could signal an underlying abnormal hormonal profile. Young women certainly can get lowered estrogen effects at the genital/pelvic tissues (including the labia and vulva), and intravaginally as well. Sometimes women will have pelvic floor hypertonus, as we see with other urinary issues. A thorough pelvic exam is quite vital.
Let's not forget the body that is attached to the genitals; we want to rule out chronic medical disease that may impact her: hypertension, diabetes, or hypercholesterolemia. Untreated, these conditions may directly impact the arousal physiologic mechanisms.
Dr. Levy: In doing this patient's physical exam I would be looking for significant weight gain, and even asking about her partner's weight. Body image can be a huge issue. If she has a history of depression, if she is suffering from a body image problem, she can be feeling unattractive. In my experience this can be a common thing to affect women in their mid-40s.
How would you manage this case?
Dr. Krychman: It is important to divide it up in terms of a conservative to aggressive approach. We want to find out about the relationship. For instance, is the sexual dynamic scripted (ie, boring and predictable)? Is she distracted and frustrated or is she getting enough of the type of stimulation that she likes and enjoys? There certainly are a lot of new devices that are available, whether a self-stimulator or vibrator, the Fiera, or other stimulating devices, that may be important to incorporate into the sexual repertoire. If there is underlying pathology, we want to evaluate and treat that. She may need to be primed, so to speak, with systemic hormones. And does she have issues related to other effects of hormonal deprivation, even local effects? Does she have clitoral atrophy?
There are neutraceuticals that are currently available, whether topical arousal gels or ointments, and we as clinicians need to be critical and evaluate their benefit/risk and look at the data concerning these products. In addition, women who have changes in arousal and in orgasmic intensity and latency may be very frustrated. They describe it as climbing up to a peak but never getting over the top, and this frustration may lead to participant spectatoring, so incorporating a certified sex therapist or counselor is sometimes very critical.
Finally, there are a lot of snake oils, charmers, and charlatan unproven procedures--injecting fillers or other substances into the clitoris are a few examples. I would be a critical clinician, examine the evidence, look at the benefit/risk before advocating an intervention that does not have good clinical data to support its use--a comprehensive approach of sexual medicine as well as sexual psychology.
Dr. Kingsberg: Additionally, we know she is in a long-term relationship--15 years; we want to acknowledge the partner. We talked about the partner's weight, but what about his erectile function? Does he have changes in sexual function that are affecting her, and she is the one carrying the "symptom"?
Looking at each piece separately helps a clinician from getting overwhelmed by the patient who comes in reporting distress with orgasmic dysfunction. We have no pharmacologic FDA-approved treatments, so it can feel off-putting for a clinician to try to fix the reported issue. Looking at each component to help her figure out the underlying cause can be helpful.
Dr. Iglesia: With aging, there can be changes in blood flow, not to mention the hormonal and even peripheral nerve changes, that require more stimulation in order to achieve the desired response. I echo concern about expensive procedures being offered with no evidence, such as the "O" or "G" shot, that can cost up to thousands of dollars.
The other procedure that gives me a lot of angst is clitoral unhooding. The 3 parts of the clitoris are sensitive in terms of innervation and blood flow, and cutting around that delicate tissue goes against the surgical principles required for preserving nerves and blood flow.
New onset pain post−prolapse surgery with TOT sling placement
Dr. Levy: For this case, let's consider a 42-year-old woman (P3) who is 6 months post− vaginal hysterectomy. The surgery included ovarian preservation combined with anterior and posterior repair for prolapse as well as apical uterosacral ligament suspension for stage 2 uterovaginal prolapse. A transobturator sling was used.
Extensive preop evaluation was performed, with confirmed symptomatic prolapse. She had no stress incontinence symptoms but did have confirmed occult stress incontinence.
Surgery was uneventful. She resumed intercourse at 8 weeks, but she now has pain with both initial entry and deep penetration. Lubricants and changes in position have not helped. She is in a stable relationship with her husband of 17 years, and she is worried that the sling mesh might be the culprit. On exam, she has no atrophy, pH is 4.5, vaginal length is 8 cm, and there is no prolapse. There is no mesh exposure noted, although she reports slight tenderness with palpation of the right sling arm beneath the right pubic bone.
Dr. Iglesia, what are the patient history questions important to ask here?
Dr. Iglesia: This is not an uncommon scenario--elective surgical correction of occult or latent stress incontinence after surgical correction for pelvic organ prolapse. Now this patient here has no more prolapse complaints; however, she has a new symptom. There are many different causes of dyspareunia; we cannot just assume it is the sling mesh (although with all the legal representation advertisements for those who have had mesh placed, it can certainly be at the top of the patient's mind, causing anxiety and fear).
Multiple trials have looked at prophylactic surgery for incontinence at the time of prolapse repairs. This woman happened to be one of those patients who did not have incontinence symptoms, and they put a sling in. A recent large trial examined women with vaginal prolapse who underwent hysterectomy and suspension.6 (They compared 2 different suspensions.) What is interesting is that 25% of women with prolapse do have baseline pain. However, at 24 months, de novo pain can occur in 10% of women--just from the apical suspension. So, here, it could be the prolapse suspension. Or, in terms of the transobturator sling, long-term data do tell us that the de novo dyspareunia rate ranges on the magnitude of 1% to 9%.7 What is important here is figuring out the cause of the dyspareunia.
Dr. Levy: One of the important points you raised already was that 25% of these women have preoperative pain. So figuring out what her functioning was before surgery and incorporating that into our assessment postop would be pretty important I would think.
Dr. Iglesia: Yes, you need to understand what her typical encounter was before the surgery and how things have changed now that the prolapse is not in the way. Changes obviously can occur with scar tissue, which over time will improve. If she is perimenopausal and starts to get epithelial changes, we can fix that. The question then becomes, "Is the pain emanating from the mesh?"
When examining this patient, it is not uncommon for me to be able to feel "banjo" strings if the mesh is too tight or close to the surface. It is not exposed but it's palpable, and the patient may feel a ridge during penetration. You can ask the patient if pain occurs with different penetration positions. In addition, explore associated neurologic symptoms (numbness or muscle pain in the thigh).
Dr. Kingsberg: There were 2 different sources of pain--on initial entry and at deep penetration. You want to make sure you address both. Importantly, did one precede the other? For instance, if women have pain with penetration they can then end up with an arousal disorder (the length of the vagina cannot increase as much as it might otherwise) and dystonia secondary to the pain with penetration. The timing of the pain--did it all happen at the same time, or did she start out with pain at one point and did it move to something else--is another critical piece of the history.
Dr. Iglesia: It does take a detailed history and physical exam to identify myofascial trigger points. In this case there seems to be pinpoint tenderness directly on the part of the mesh that is not exposed on the right. There are people who feel you should remove the whole mesh, including the arms. Others feel, okay, we can work on these trigger points, with injections, physical therapy, extra lubrication, and neuromodulatory medications. Only then would they think about potentially excising the sling or a portion thereof.
Dr. Krychman: Keep in mind that, even if you do remove the sling, her pain may not subside, if it is secondary to an underlying issue. Because of media sensationalism, she could be focusing on the sling. It is important to set realistic expectations. I often see vulvar pathology or even provoked vestibulodynia that can present with a deep dyspareunia. The concept of collision dyspareunia or introital discomfort or pain on insertion has far reaching implications. We need to look at the patient in totality, ruling out underlying issues related to the bladder, even the colon.
Patient inquires about the benefits of laser treatment for vaginal health
Dr. Levy: Let's move to this last case: A 47-year-old patient who reports lack of sexual satisfaction and attributes it to a loose vagina says, "I've heard about surgery and laser treatment and radiofrequency devices for vaginal health. What are the benefits and risks of these procedures, and will they correct the issues that I'm experiencing?"
How do you approach this patient?
Dr. Krychman: I want to know why this is of paramount importance to her. Is this her actual complaint or is it society's unrealistic expectation of sexual pleasure and performance placed upon her? Or is this a relationship issue surfacing, compounded by physiologic changes? With good communication techniques, like "ask, tell, ask" or effective use of silence (not interrupting), patients will lead us to the reason and the rationale.
Dr. Levy: I have seen a lot of advertising to women, now showing pictures of genitalia, perhaps creating an expectation that we should look infantile in some way. We are creating a sense of beauty and acceptablevisualization of the vagina and vulva that are completely unrealistic. It's fascinating on one hand but it is also disturbing in that some of the direct-to-consumer marketing going on is creating a sense of unease in women who are otherwise perfectly satisfied. Now they take a look at their sagging skin, maybe after having 2 or 3 children, and although they may not look the same they function not so badly perhaps. I think we are creating a distress and an illness model that is interesting to discuss.
Dr. Iglesia, you are in the midst of a randomized trial, giving an informed consent to participants about the expectations for this potential intervention. How do you explain to women what these laser and radiofrequency devices are expected to do and why they might or might not work?
Dr. Iglesia: We are doing a randomized trial for menopausal women who have GSM. We are comparing estrogen cream with fractional CO2 laser therapy. I also am involved in another randomized trial for lichen sclerosus. I am not involved in the cosmetic use of a laser for people who feel their vaginas are just "loose." Like you, Dr. Levy, I am very concerned about the images that women are seeing of the idealized vulva and vagina, and about the rise of cosmetic gynecology, much of which is being performed by plastic surgeons or dermatologists.
A recent article looked at the number of women who are doing pubic hair grooming; the prevalance is about 80% here in America.8 So people have a clear view of what is down there, and then they compare it to what they see in pornographic images on the Internet and want to look like a Barbie doll. That is disturbing because women, particularly young women, do not realize what happens with GSM changes to the vulva and vagina. On the other hand, these laser machines are very expensive, and some doctors are charging thousands of dollars and promising cosmetic and functional results for which we lack long-term, comparative data.
The laser that we are studying is one by Cynosure called Mona Lisa, which works with fractional CO2 and has very low depth of penetration. The concept is that, with microdot therapy (on the order of micrometers), pinpoint destruction will foster regeneration of new collagen and blood flow to the vagina and vulva. We are still in the midst of analyzing this.
Dr. Krychman: I caution people not to lump devices together. There is a significant difference between laser and radiofrequency--especially in the depth of tissue penetration, and level of evidence as well. There are companies performing randomized clinical trials, with well designed sham controls, and have demonstrated clinical efficacy. We need to be cautious of a procedure that is saying it's the best thing since sliced bread, curing interstitial cystitis, dyspareunia, and lichen sclerosus and improving orgasm, lubrication, and arousal. These far reaching, off-label claims are concerning and misleading.
Dr. Levy: I think the important things are 1) shared decision making with the patient and 2) disclosure of what we do not know, which are the long-term results and outcomes and possible downstream negative effects of some of these treatments, since the data we have are so short term.
Dr. Kingsberg: Basics are important. You talked about the pressure for cosmetic appearance, but is that really what is going on for this particular patient? Is she describing a sexual dysfunction when she talks about lack of satisfaction? You need to operationally define that term. Does she have problems with arousal or orgasm or desire and those are what underlie her lack of satisfaction? Is the key to management helping her come to terms with body image issues or to treat a sexual dysfunction? If it truly is a sexual dysfunction, then you can have the shared decision making on preferred treatment approach.
Dr. Levy: This has been an enlightening discussion. Thank all of you for your expertise and clinical acumen.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition. American Psychiatric Association Publishing: Arlington, VA; 2013.
- American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: The Use of Vaginal Estrogen in Women With a History of Estrogen-Dependent Breast Cancer. Obstet Gynecol. 2016;127(3):e93−e96.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society (NAMS). NAMS Menopause e-Consult: Laser treatment safe for vulvovaginal atrophy? 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed August 17, 2016.
- The American College of Obstetricians and Gynecologists and The American Congress of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and U.S. Food and Drug Administration clearance: Position Statement. http://www.acog.org/Resources-And-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Published May 2016. Accessed August 17, 2016.
- Lukacz ES, Warren LK, Richter HE, et al. Quality of life and sexual function 2 years after vaginal surgery for prolapse. Obstet Gynecol. 2016;127(6):1071−1079.
- Brubaker L, Chiang S, Zyczynski H, et al. The impact of stress incontinence surgery on female sexual function. Am J Obstet Gynecol. 2009;200(5):562.e1.
- Rowen TS, Gaither TW, Awad MA, Osterberg EC, Shindel AW, Breyer BN. Pubic hair grooming prevalence and motivation among women in the United States [published online ahead of print June 29, 2016]. JAMA Dermatol. doi:10.1001/jamader matol.2016.2154.
The age-adjusted prevalence of any sexual problem is 43% among US women. A full 22% of these women experience sexually related personal distress.1 With publication of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition2 has come a shift in classification and, at times, management approach for reported female sexual dysfunction. When women report to their clinicians decreased sexual desire or arousal or pain at penetration, the management is no longer guided by a linear model of sexual response (excitation, plateau, orgasm, and resolution) but rather by a more nuanced and complex biopsychosocial approach. In this model, diagnosis and management strategies to address bothersome sexual concerns consider the whole woman in the context of her physical and psychosocial health. The patient’s age, medical history, and relationship status are among the factors that could affect management of the problem. In an effort to explore this management approach, I used this Update on Female Sexual Dysfunction as an opportunity to convene a roundtable of several experts, representing varying backgrounds and practice vantage points, to discuss 5 cases of sexual problems that you as a busy clinician may encounter in your practice.

Genital atrophy in a sexually inactive 61-year-old woman
Barbara S. Levy, MD: Two years after her husband's death, which followed several years of illness, your 61-year-old patient mentions at her well woman visit that she anticipates becoming sexually active again. She has not used systemic or vaginal hormone therapy. During pelvic examination, atrophic external genital changes are present, and use of an ultrathin (thinner than a Pederson) speculum reveals vaginal epithelial atrophic changes. A single-digit bimanual exam can be performed with moderate patient discomfort; the patient cannot tolerate a 2-digit bimanual exam. She expresses concern about being able to engage in penile/vaginal sexual intercourse.
Dr. Kaunitz, what is important for you to ask this patient, and what concerns you most on her physical exam?
Andrew M. Kaunitz, MD: First, it is important to recognize the patient's expectations and desires. As the case suggests, but further questioning could clarify, she would like to be able to comfortably engage in sexual intercourse with a new partner, but penetration may be difficult (and definitely painful for her) unless treatment is pursued. This combination of mucosal and vestibular atrophic changes (genitourinary syndrome of menopause [GSM], or vulvovaginal atrophy [VVA]) plus the absence of penetration for many years can be a double whammy situation for menopausal women. In this case it has led to extensive contracture of the introitus, and if it is not addressed will cause sexual dysfunction.
Dr. Levy: In addition we need to clarify whether or not a history of breast cancer or some other thing may impact the care we provide. How would you approach talking with this patient in order to manage her care?
Dr. Kaunitz: One step is to see how motivated she is to address this, as it is not something that, as gynecologists, we can snap our fingers and the situation will be resolved. If the patient is motivated to treat the atrophic changes with medical treatment, in the form of low-dose vaginal estrogen, and dilation, either on her own if she's highly motivated to do so, or in my practice more commonly with the support of a women's physical therapist, over time she should be able to comfortably engage in sexual intercourse with penetration. If this is what she wants, we can help steer her in the right direction.
Sheryl Kingsberg, PhD: You know that this woman is motivated by virtue of her initiating the topic herself. Patients are often embarrassed talking about sexual issues, or they are not sure that their gynecologist is comfortable with it. After all, they think, if this is the right place to discuss sexual problems, why didn't he or she ask me? Clinicians must be aware that it is their responsibility to ask about sexual function and not leave it for the patient to open the door.
Dr. Kaunitz: Great point.
Cheryl B. Iglesia, MD: Gratefully, a lot of the atrophic changes this patient demonstrates are reversible. However, other autoimmune diseases (eg, lichen planus, which can affect the vaginal epithelium, or lichen sclerosus, which can affect the clitoris, labia, and vulva) can also cause constriction, and in severe cases, complete obliteration of the vagina and introitus. Women may not be sexually active, and for each annual exam their clinician uses a smaller and smaller speculum--to the point that they cannot even access the cervix anymore--and the vagina can close off. Clinicians may not realize that you need something other than estrogen; with lichen planus you need steroid suppository treatment, and with lichen sclerosus you need topical steroid treatment. So these autoimmune conditions should also be in the differential and, with appropriate treatment, the sexual effects can be reversible.
Michael Krychman, MD: I agree. The vulva can be a great mimicker and, according to the history and physical exam, at some point a vulvoscopy, and even potential biopsies, may be warranted as clinically indicated.
The concept of a comprehensive approach, as Dr. Kingsberg had previously mentioned, involves not only sexual medicine but also evaluating the patient's biopsychosocial variables that may impact her condition. We also need to set realistic expectations. Some women may benefit from off-label use of medications besides estrogen, including topical testosterone. Informed consent is very important with these treatments. I also have had much clinical success with intravaginal diazepam/lorazepam for pelvic floor hypertonus.
In addition, certainly I agree that pelvic floor physical therapy (PT) is a vital treatment component for this patient and, not to diminish its importance, but many women cannot afford, nor do they have the time or opportunity, to go to pelvic floor PT. As clinicians, we can develop and implement effective programs, even in the office, to educate the patient to help herself as well.
Dr. Kaunitz: Absolutely. Also, if, in a clinical setting consistent with atrophic changes, an ObGyn physician is comfortable that vulvovaginal changes noted on exam represent GSM/atrophic changes, I do not feel vulvoscopy is warranted.
Dr. Levy: In conclusion, we need to be aware that pelvic floor PT may not be available everywhere and that a woman's own digits and her partner can also be incorporated into this treatment.
Something that we have all talked about in other venues, but have not looked at in the larger sphere here, is whether there is value to seeing women annually and performing pelvic exams. As Dr. Kingsberg mentioned, this is a highly motivated patient. We have many patients out there who are silent sufferers. The physical exam is an opportunity for us to recognize and address this problem.

Dyspareunia and low sexual desire in a breast cancer survivor
Dr. Levy: In this case, a 36-year-old woman with BRCA1−positive breast cancer has vaginal dryness, painful intercourse, and lowered sexual interest since her treatment, which included chemotherapy after bilateral mastectomies. She has a bilateral salpingo-oophorectomy(BSO) scheduled for primary prevention of her ovarian cancer risk.
Dr. Kingsberg, what is important for you to know to help guide case management?
Dr. Kingsberg: This woman is actually presenting with 2 sexual problems: dyspareunia, which is probably secondary to VVA or GSM, and low sexual desire. Key questions are: 1) When was symptom onset--acquired after treatment or lifelong? 2) Did she develop the dyspareunia and as a result of having pain during sex lost desire to have sex? Or, did she lose desire and then, without it, had no arousal and therefore pain with penetration developed? It also could be that she has 2 distinct problems, VVA and hypoactive sexual desire disorder (HSDD), in which case you need to think about treating both. Finally, we do not actually know if she is having penetrative intercourse or even if she has a partner.
A vulvovaginal exam would give clues as to whether she has VVA, and hormone levels would indicate if she now has chemo-induced menopause. If she is not in menopause now, she certainly is going to be with her BSO. The hormonal changes due to menopause actually can be primarily responsible for both the dyspareunia and HSDD. Management of both symptoms really needs to be based on shared decision making with the patient--with which treatment for which conditions coming first, based on what is causing her the most distress.
I would encourage this woman to treat her VVA since GSM does have long-term physiologic consequences if untreated. The American College of Obstetricians and Gynecologists (ACOG) recommends nonhormonal treatments as first-line treatments, with vaginal estrogen considered if these therapies fail.3 If lubricants and moisturizers and other nonhormonal options are not sufficient, you could consider local estrogen, even though she is a breast cancer survivor, as well as ospemiphene.
If she is distressed by her loss of sexual desire, you can choose to treat her for HSDD. Flibanserin is the first FDA-approved treatment for HSDD. It is only approved in premenopausal women, so it would be considered off-label use if she is postmenopausal (even though she is quite young). You also could consider exogenous testosterone off-label, after consulting with her oncologist.
In addition to the obvious physiologic etiology of her pain and her low desire, the biopsychosocial aspects to consider are: 1) changes to her body image, as she has had bilateral mastectomies, 2) her anxiety about the cancer diagnosis, and 3) concerns about her relationship if she has one--her partner's reactions to her illness and the quality of the relationship outside the bedroom.
Dr. Iglesia: I am seeing here in our nation's capital a lot of advertisements for laser therapy for GSM. I caution women about this because providers are charging a lot of money for this therapy when we do not have long-term safety and effectiveness data for it.
Our group is currently conducting a randomized controlled trial, looking at vaginal estrogen cream versus laser therapy for GSM here at Medstar Health--one of the first in the country as part of a multisite trial. But the North American Menopause Society (NAMS) has come out with a pretty strong statement,4 as has ACOG,5 on this therapy, and I caution people about overzealously offering a very costly procedure targeted to a very vulnerable population, especially to women with personal histories of estrogen-sensitive cancers.
Dr. Krychman: I agree. Very often cancer patients are preyed upon by those offering emerging unproven technologies or medications. We have to work as a coordinated comprehensive team, whether it's a sexual medicine expert, psychologist, urogynecologist, gynecologist, or oncologist, and incorporate the patient's needs and expectations and risk tolerance coupled with treatment efficacy and safety.
Dr. Levy: This was a complex case. The biopsychosocial model is critical here. It's important that we are not siloes in our medical management approach and that we try to help this patient embrace the complexity of her situation. It's not only that she has cancer at age 36; there is a possible guilt factor if she has children and passed that gene on.
Another point that we began to talk about is the fact that in this country we tend to be early adopters of new technology. In our discussion with patients, we should focus on what we know and the risk of the unknowns related to some of the treatment options. But let's discuss lasers a little more later on.
Diminished arousal and orgasmic intensity in a patient taking SSRIs
Dr. Levy: In this next case, a 44-year-old woman in a 15-year marriage notices a change in her orgasmic intensity and latency. She has a supportive husband, and they are attentive to each other's sexual needs. However, she notices a change in her arousal and orgasmic intensity, which has diminished over the last year. She reports that the time to orgasm or latency has increased and both she and her partner are frustrated and getting concerned. She has a history of depression that has been managed by selective serotonin reuptake inhibitors for the past 5 years and has no depressive symptoms currently.
Dr. Krychman, what are you considering before beginning to talk with this patient?
Dr. Krychman: My approach really is a comprehensive one, looking not only at the underlying medical issues but also at the psychological and dynamic relationship facets. We of course also want to look at medications: Has she changed her dose or the timing of when she takes it? Is this a new onset? Finally, we want to know why this is coming to the forefront now. Is it because it is getting worse, or is it because there is some significant issue that is going on in the relationship?
Regarding the physical exam, it is important to rule out underlying genital pelvic pathology. Young women can get changes in the integrity of the pelvic floor, in what I would call the orgasmic matrix--the clitoral tissue, the body, the crura (or arms of the clitoris)--we want to examine and be reassured that her genital anatomy is normal and that there is no underlying pathology that could signal an underlying abnormal hormonal profile. Young women certainly can get lowered estrogen effects at the genital/pelvic tissues (including the labia and vulva), and intravaginally as well. Sometimes women will have pelvic floor hypertonus, as we see with other urinary issues. A thorough pelvic exam is quite vital.
Let's not forget the body that is attached to the genitals; we want to rule out chronic medical disease that may impact her: hypertension, diabetes, or hypercholesterolemia. Untreated, these conditions may directly impact the arousal physiologic mechanisms.
Dr. Levy: In doing this patient's physical exam I would be looking for significant weight gain, and even asking about her partner's weight. Body image can be a huge issue. If she has a history of depression, if she is suffering from a body image problem, she can be feeling unattractive. In my experience this can be a common thing to affect women in their mid-40s.
How would you manage this case?
Dr. Krychman: It is important to divide it up in terms of a conservative to aggressive approach. We want to find out about the relationship. For instance, is the sexual dynamic scripted (ie, boring and predictable)? Is she distracted and frustrated or is she getting enough of the type of stimulation that she likes and enjoys? There certainly are a lot of new devices that are available, whether a self-stimulator or vibrator, the Fiera, or other stimulating devices, that may be important to incorporate into the sexual repertoire. If there is underlying pathology, we want to evaluate and treat that. She may need to be primed, so to speak, with systemic hormones. And does she have issues related to other effects of hormonal deprivation, even local effects? Does she have clitoral atrophy?
There are neutraceuticals that are currently available, whether topical arousal gels or ointments, and we as clinicians need to be critical and evaluate their benefit/risk and look at the data concerning these products. In addition, women who have changes in arousal and in orgasmic intensity and latency may be very frustrated. They describe it as climbing up to a peak but never getting over the top, and this frustration may lead to participant spectatoring, so incorporating a certified sex therapist or counselor is sometimes very critical.
Finally, there are a lot of snake oils, charmers, and charlatan unproven procedures--injecting fillers or other substances into the clitoris are a few examples. I would be a critical clinician, examine the evidence, look at the benefit/risk before advocating an intervention that does not have good clinical data to support its use--a comprehensive approach of sexual medicine as well as sexual psychology.
Dr. Kingsberg: Additionally, we know she is in a long-term relationship--15 years; we want to acknowledge the partner. We talked about the partner's weight, but what about his erectile function? Does he have changes in sexual function that are affecting her, and she is the one carrying the "symptom"?
Looking at each piece separately helps a clinician from getting overwhelmed by the patient who comes in reporting distress with orgasmic dysfunction. We have no pharmacologic FDA-approved treatments, so it can feel off-putting for a clinician to try to fix the reported issue. Looking at each component to help her figure out the underlying cause can be helpful.
Dr. Iglesia: With aging, there can be changes in blood flow, not to mention the hormonal and even peripheral nerve changes, that require more stimulation in order to achieve the desired response. I echo concern about expensive procedures being offered with no evidence, such as the "O" or "G" shot, that can cost up to thousands of dollars.
The other procedure that gives me a lot of angst is clitoral unhooding. The 3 parts of the clitoris are sensitive in terms of innervation and blood flow, and cutting around that delicate tissue goes against the surgical principles required for preserving nerves and blood flow.
New onset pain post−prolapse surgery with TOT sling placement
Dr. Levy: For this case, let's consider a 42-year-old woman (P3) who is 6 months post− vaginal hysterectomy. The surgery included ovarian preservation combined with anterior and posterior repair for prolapse as well as apical uterosacral ligament suspension for stage 2 uterovaginal prolapse. A transobturator sling was used.
Extensive preop evaluation was performed, with confirmed symptomatic prolapse. She had no stress incontinence symptoms but did have confirmed occult stress incontinence.
Surgery was uneventful. She resumed intercourse at 8 weeks, but she now has pain with both initial entry and deep penetration. Lubricants and changes in position have not helped. She is in a stable relationship with her husband of 17 years, and she is worried that the sling mesh might be the culprit. On exam, she has no atrophy, pH is 4.5, vaginal length is 8 cm, and there is no prolapse. There is no mesh exposure noted, although she reports slight tenderness with palpation of the right sling arm beneath the right pubic bone.
Dr. Iglesia, what are the patient history questions important to ask here?
Dr. Iglesia: This is not an uncommon scenario--elective surgical correction of occult or latent stress incontinence after surgical correction for pelvic organ prolapse. Now this patient here has no more prolapse complaints; however, she has a new symptom. There are many different causes of dyspareunia; we cannot just assume it is the sling mesh (although with all the legal representation advertisements for those who have had mesh placed, it can certainly be at the top of the patient's mind, causing anxiety and fear).
Multiple trials have looked at prophylactic surgery for incontinence at the time of prolapse repairs. This woman happened to be one of those patients who did not have incontinence symptoms, and they put a sling in. A recent large trial examined women with vaginal prolapse who underwent hysterectomy and suspension.6 (They compared 2 different suspensions.) What is interesting is that 25% of women with prolapse do have baseline pain. However, at 24 months, de novo pain can occur in 10% of women--just from the apical suspension. So, here, it could be the prolapse suspension. Or, in terms of the transobturator sling, long-term data do tell us that the de novo dyspareunia rate ranges on the magnitude of 1% to 9%.7 What is important here is figuring out the cause of the dyspareunia.
Dr. Levy: One of the important points you raised already was that 25% of these women have preoperative pain. So figuring out what her functioning was before surgery and incorporating that into our assessment postop would be pretty important I would think.
Dr. Iglesia: Yes, you need to understand what her typical encounter was before the surgery and how things have changed now that the prolapse is not in the way. Changes obviously can occur with scar tissue, which over time will improve. If she is perimenopausal and starts to get epithelial changes, we can fix that. The question then becomes, "Is the pain emanating from the mesh?"
When examining this patient, it is not uncommon for me to be able to feel "banjo" strings if the mesh is too tight or close to the surface. It is not exposed but it's palpable, and the patient may feel a ridge during penetration. You can ask the patient if pain occurs with different penetration positions. In addition, explore associated neurologic symptoms (numbness or muscle pain in the thigh).
Dr. Kingsberg: There were 2 different sources of pain--on initial entry and at deep penetration. You want to make sure you address both. Importantly, did one precede the other? For instance, if women have pain with penetration they can then end up with an arousal disorder (the length of the vagina cannot increase as much as it might otherwise) and dystonia secondary to the pain with penetration. The timing of the pain--did it all happen at the same time, or did she start out with pain at one point and did it move to something else--is another critical piece of the history.
Dr. Iglesia: It does take a detailed history and physical exam to identify myofascial trigger points. In this case there seems to be pinpoint tenderness directly on the part of the mesh that is not exposed on the right. There are people who feel you should remove the whole mesh, including the arms. Others feel, okay, we can work on these trigger points, with injections, physical therapy, extra lubrication, and neuromodulatory medications. Only then would they think about potentially excising the sling or a portion thereof.
Dr. Krychman: Keep in mind that, even if you do remove the sling, her pain may not subside, if it is secondary to an underlying issue. Because of media sensationalism, she could be focusing on the sling. It is important to set realistic expectations. I often see vulvar pathology or even provoked vestibulodynia that can present with a deep dyspareunia. The concept of collision dyspareunia or introital discomfort or pain on insertion has far reaching implications. We need to look at the patient in totality, ruling out underlying issues related to the bladder, even the colon.
Patient inquires about the benefits of laser treatment for vaginal health
Dr. Levy: Let's move to this last case: A 47-year-old patient who reports lack of sexual satisfaction and attributes it to a loose vagina says, "I've heard about surgery and laser treatment and radiofrequency devices for vaginal health. What are the benefits and risks of these procedures, and will they correct the issues that I'm experiencing?"
How do you approach this patient?
Dr. Krychman: I want to know why this is of paramount importance to her. Is this her actual complaint or is it society's unrealistic expectation of sexual pleasure and performance placed upon her? Or is this a relationship issue surfacing, compounded by physiologic changes? With good communication techniques, like "ask, tell, ask" or effective use of silence (not interrupting), patients will lead us to the reason and the rationale.
Dr. Levy: I have seen a lot of advertising to women, now showing pictures of genitalia, perhaps creating an expectation that we should look infantile in some way. We are creating a sense of beauty and acceptablevisualization of the vagina and vulva that are completely unrealistic. It's fascinating on one hand but it is also disturbing in that some of the direct-to-consumer marketing going on is creating a sense of unease in women who are otherwise perfectly satisfied. Now they take a look at their sagging skin, maybe after having 2 or 3 children, and although they may not look the same they function not so badly perhaps. I think we are creating a distress and an illness model that is interesting to discuss.
Dr. Iglesia, you are in the midst of a randomized trial, giving an informed consent to participants about the expectations for this potential intervention. How do you explain to women what these laser and radiofrequency devices are expected to do and why they might or might not work?
Dr. Iglesia: We are doing a randomized trial for menopausal women who have GSM. We are comparing estrogen cream with fractional CO2 laser therapy. I also am involved in another randomized trial for lichen sclerosus. I am not involved in the cosmetic use of a laser for people who feel their vaginas are just "loose." Like you, Dr. Levy, I am very concerned about the images that women are seeing of the idealized vulva and vagina, and about the rise of cosmetic gynecology, much of which is being performed by plastic surgeons or dermatologists.
A recent article looked at the number of women who are doing pubic hair grooming; the prevalance is about 80% here in America.8 So people have a clear view of what is down there, and then they compare it to what they see in pornographic images on the Internet and want to look like a Barbie doll. That is disturbing because women, particularly young women, do not realize what happens with GSM changes to the vulva and vagina. On the other hand, these laser machines are very expensive, and some doctors are charging thousands of dollars and promising cosmetic and functional results for which we lack long-term, comparative data.
The laser that we are studying is one by Cynosure called Mona Lisa, which works with fractional CO2 and has very low depth of penetration. The concept is that, with microdot therapy (on the order of micrometers), pinpoint destruction will foster regeneration of new collagen and blood flow to the vagina and vulva. We are still in the midst of analyzing this.
Dr. Krychman: I caution people not to lump devices together. There is a significant difference between laser and radiofrequency--especially in the depth of tissue penetration, and level of evidence as well. There are companies performing randomized clinical trials, with well designed sham controls, and have demonstrated clinical efficacy. We need to be cautious of a procedure that is saying it's the best thing since sliced bread, curing interstitial cystitis, dyspareunia, and lichen sclerosus and improving orgasm, lubrication, and arousal. These far reaching, off-label claims are concerning and misleading.
Dr. Levy: I think the important things are 1) shared decision making with the patient and 2) disclosure of what we do not know, which are the long-term results and outcomes and possible downstream negative effects of some of these treatments, since the data we have are so short term.
Dr. Kingsberg: Basics are important. You talked about the pressure for cosmetic appearance, but is that really what is going on for this particular patient? Is she describing a sexual dysfunction when she talks about lack of satisfaction? You need to operationally define that term. Does she have problems with arousal or orgasm or desire and those are what underlie her lack of satisfaction? Is the key to management helping her come to terms with body image issues or to treat a sexual dysfunction? If it truly is a sexual dysfunction, then you can have the shared decision making on preferred treatment approach.
Dr. Levy: This has been an enlightening discussion. Thank all of you for your expertise and clinical acumen.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The age-adjusted prevalence of any sexual problem is 43% among US women. A full 22% of these women experience sexually related personal distress.1 With publication of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition2 has come a shift in classification and, at times, management approach for reported female sexual dysfunction. When women report to their clinicians decreased sexual desire or arousal or pain at penetration, the management is no longer guided by a linear model of sexual response (excitation, plateau, orgasm, and resolution) but rather by a more nuanced and complex biopsychosocial approach. In this model, diagnosis and management strategies to address bothersome sexual concerns consider the whole woman in the context of her physical and psychosocial health. The patient’s age, medical history, and relationship status are among the factors that could affect management of the problem. In an effort to explore this management approach, I used this Update on Female Sexual Dysfunction as an opportunity to convene a roundtable of several experts, representing varying backgrounds and practice vantage points, to discuss 5 cases of sexual problems that you as a busy clinician may encounter in your practice.

Genital atrophy in a sexually inactive 61-year-old woman
Barbara S. Levy, MD: Two years after her husband's death, which followed several years of illness, your 61-year-old patient mentions at her well woman visit that she anticipates becoming sexually active again. She has not used systemic or vaginal hormone therapy. During pelvic examination, atrophic external genital changes are present, and use of an ultrathin (thinner than a Pederson) speculum reveals vaginal epithelial atrophic changes. A single-digit bimanual exam can be performed with moderate patient discomfort; the patient cannot tolerate a 2-digit bimanual exam. She expresses concern about being able to engage in penile/vaginal sexual intercourse.
Dr. Kaunitz, what is important for you to ask this patient, and what concerns you most on her physical exam?
Andrew M. Kaunitz, MD: First, it is important to recognize the patient's expectations and desires. As the case suggests, but further questioning could clarify, she would like to be able to comfortably engage in sexual intercourse with a new partner, but penetration may be difficult (and definitely painful for her) unless treatment is pursued. This combination of mucosal and vestibular atrophic changes (genitourinary syndrome of menopause [GSM], or vulvovaginal atrophy [VVA]) plus the absence of penetration for many years can be a double whammy situation for menopausal women. In this case it has led to extensive contracture of the introitus, and if it is not addressed will cause sexual dysfunction.
Dr. Levy: In addition we need to clarify whether or not a history of breast cancer or some other thing may impact the care we provide. How would you approach talking with this patient in order to manage her care?
Dr. Kaunitz: One step is to see how motivated she is to address this, as it is not something that, as gynecologists, we can snap our fingers and the situation will be resolved. If the patient is motivated to treat the atrophic changes with medical treatment, in the form of low-dose vaginal estrogen, and dilation, either on her own if she's highly motivated to do so, or in my practice more commonly with the support of a women's physical therapist, over time she should be able to comfortably engage in sexual intercourse with penetration. If this is what she wants, we can help steer her in the right direction.
Sheryl Kingsberg, PhD: You know that this woman is motivated by virtue of her initiating the topic herself. Patients are often embarrassed talking about sexual issues, or they are not sure that their gynecologist is comfortable with it. After all, they think, if this is the right place to discuss sexual problems, why didn't he or she ask me? Clinicians must be aware that it is their responsibility to ask about sexual function and not leave it for the patient to open the door.
Dr. Kaunitz: Great point.
Cheryl B. Iglesia, MD: Gratefully, a lot of the atrophic changes this patient demonstrates are reversible. However, other autoimmune diseases (eg, lichen planus, which can affect the vaginal epithelium, or lichen sclerosus, which can affect the clitoris, labia, and vulva) can also cause constriction, and in severe cases, complete obliteration of the vagina and introitus. Women may not be sexually active, and for each annual exam their clinician uses a smaller and smaller speculum--to the point that they cannot even access the cervix anymore--and the vagina can close off. Clinicians may not realize that you need something other than estrogen; with lichen planus you need steroid suppository treatment, and with lichen sclerosus you need topical steroid treatment. So these autoimmune conditions should also be in the differential and, with appropriate treatment, the sexual effects can be reversible.
Michael Krychman, MD: I agree. The vulva can be a great mimicker and, according to the history and physical exam, at some point a vulvoscopy, and even potential biopsies, may be warranted as clinically indicated.
The concept of a comprehensive approach, as Dr. Kingsberg had previously mentioned, involves not only sexual medicine but also evaluating the patient's biopsychosocial variables that may impact her condition. We also need to set realistic expectations. Some women may benefit from off-label use of medications besides estrogen, including topical testosterone. Informed consent is very important with these treatments. I also have had much clinical success with intravaginal diazepam/lorazepam for pelvic floor hypertonus.
In addition, certainly I agree that pelvic floor physical therapy (PT) is a vital treatment component for this patient and, not to diminish its importance, but many women cannot afford, nor do they have the time or opportunity, to go to pelvic floor PT. As clinicians, we can develop and implement effective programs, even in the office, to educate the patient to help herself as well.
Dr. Kaunitz: Absolutely. Also, if, in a clinical setting consistent with atrophic changes, an ObGyn physician is comfortable that vulvovaginal changes noted on exam represent GSM/atrophic changes, I do not feel vulvoscopy is warranted.
Dr. Levy: In conclusion, we need to be aware that pelvic floor PT may not be available everywhere and that a woman's own digits and her partner can also be incorporated into this treatment.
Something that we have all talked about in other venues, but have not looked at in the larger sphere here, is whether there is value to seeing women annually and performing pelvic exams. As Dr. Kingsberg mentioned, this is a highly motivated patient. We have many patients out there who are silent sufferers. The physical exam is an opportunity for us to recognize and address this problem.

Dyspareunia and low sexual desire in a breast cancer survivor
Dr. Levy: In this case, a 36-year-old woman with BRCA1−positive breast cancer has vaginal dryness, painful intercourse, and lowered sexual interest since her treatment, which included chemotherapy after bilateral mastectomies. She has a bilateral salpingo-oophorectomy(BSO) scheduled for primary prevention of her ovarian cancer risk.
Dr. Kingsberg, what is important for you to know to help guide case management?
Dr. Kingsberg: This woman is actually presenting with 2 sexual problems: dyspareunia, which is probably secondary to VVA or GSM, and low sexual desire. Key questions are: 1) When was symptom onset--acquired after treatment or lifelong? 2) Did she develop the dyspareunia and as a result of having pain during sex lost desire to have sex? Or, did she lose desire and then, without it, had no arousal and therefore pain with penetration developed? It also could be that she has 2 distinct problems, VVA and hypoactive sexual desire disorder (HSDD), in which case you need to think about treating both. Finally, we do not actually know if she is having penetrative intercourse or even if she has a partner.
A vulvovaginal exam would give clues as to whether she has VVA, and hormone levels would indicate if she now has chemo-induced menopause. If she is not in menopause now, she certainly is going to be with her BSO. The hormonal changes due to menopause actually can be primarily responsible for both the dyspareunia and HSDD. Management of both symptoms really needs to be based on shared decision making with the patient--with which treatment for which conditions coming first, based on what is causing her the most distress.
I would encourage this woman to treat her VVA since GSM does have long-term physiologic consequences if untreated. The American College of Obstetricians and Gynecologists (ACOG) recommends nonhormonal treatments as first-line treatments, with vaginal estrogen considered if these therapies fail.3 If lubricants and moisturizers and other nonhormonal options are not sufficient, you could consider local estrogen, even though she is a breast cancer survivor, as well as ospemiphene.
If she is distressed by her loss of sexual desire, you can choose to treat her for HSDD. Flibanserin is the first FDA-approved treatment for HSDD. It is only approved in premenopausal women, so it would be considered off-label use if she is postmenopausal (even though she is quite young). You also could consider exogenous testosterone off-label, after consulting with her oncologist.
In addition to the obvious physiologic etiology of her pain and her low desire, the biopsychosocial aspects to consider are: 1) changes to her body image, as she has had bilateral mastectomies, 2) her anxiety about the cancer diagnosis, and 3) concerns about her relationship if she has one--her partner's reactions to her illness and the quality of the relationship outside the bedroom.
Dr. Iglesia: I am seeing here in our nation's capital a lot of advertisements for laser therapy for GSM. I caution women about this because providers are charging a lot of money for this therapy when we do not have long-term safety and effectiveness data for it.
Our group is currently conducting a randomized controlled trial, looking at vaginal estrogen cream versus laser therapy for GSM here at Medstar Health--one of the first in the country as part of a multisite trial. But the North American Menopause Society (NAMS) has come out with a pretty strong statement,4 as has ACOG,5 on this therapy, and I caution people about overzealously offering a very costly procedure targeted to a very vulnerable population, especially to women with personal histories of estrogen-sensitive cancers.
Dr. Krychman: I agree. Very often cancer patients are preyed upon by those offering emerging unproven technologies or medications. We have to work as a coordinated comprehensive team, whether it's a sexual medicine expert, psychologist, urogynecologist, gynecologist, or oncologist, and incorporate the patient's needs and expectations and risk tolerance coupled with treatment efficacy and safety.
Dr. Levy: This was a complex case. The biopsychosocial model is critical here. It's important that we are not siloes in our medical management approach and that we try to help this patient embrace the complexity of her situation. It's not only that she has cancer at age 36; there is a possible guilt factor if she has children and passed that gene on.
Another point that we began to talk about is the fact that in this country we tend to be early adopters of new technology. In our discussion with patients, we should focus on what we know and the risk of the unknowns related to some of the treatment options. But let's discuss lasers a little more later on.
Diminished arousal and orgasmic intensity in a patient taking SSRIs
Dr. Levy: In this next case, a 44-year-old woman in a 15-year marriage notices a change in her orgasmic intensity and latency. She has a supportive husband, and they are attentive to each other's sexual needs. However, she notices a change in her arousal and orgasmic intensity, which has diminished over the last year. She reports that the time to orgasm or latency has increased and both she and her partner are frustrated and getting concerned. She has a history of depression that has been managed by selective serotonin reuptake inhibitors for the past 5 years and has no depressive symptoms currently.
Dr. Krychman, what are you considering before beginning to talk with this patient?
Dr. Krychman: My approach really is a comprehensive one, looking not only at the underlying medical issues but also at the psychological and dynamic relationship facets. We of course also want to look at medications: Has she changed her dose or the timing of when she takes it? Is this a new onset? Finally, we want to know why this is coming to the forefront now. Is it because it is getting worse, or is it because there is some significant issue that is going on in the relationship?
Regarding the physical exam, it is important to rule out underlying genital pelvic pathology. Young women can get changes in the integrity of the pelvic floor, in what I would call the orgasmic matrix--the clitoral tissue, the body, the crura (or arms of the clitoris)--we want to examine and be reassured that her genital anatomy is normal and that there is no underlying pathology that could signal an underlying abnormal hormonal profile. Young women certainly can get lowered estrogen effects at the genital/pelvic tissues (including the labia and vulva), and intravaginally as well. Sometimes women will have pelvic floor hypertonus, as we see with other urinary issues. A thorough pelvic exam is quite vital.
Let's not forget the body that is attached to the genitals; we want to rule out chronic medical disease that may impact her: hypertension, diabetes, or hypercholesterolemia. Untreated, these conditions may directly impact the arousal physiologic mechanisms.
Dr. Levy: In doing this patient's physical exam I would be looking for significant weight gain, and even asking about her partner's weight. Body image can be a huge issue. If she has a history of depression, if she is suffering from a body image problem, she can be feeling unattractive. In my experience this can be a common thing to affect women in their mid-40s.
How would you manage this case?
Dr. Krychman: It is important to divide it up in terms of a conservative to aggressive approach. We want to find out about the relationship. For instance, is the sexual dynamic scripted (ie, boring and predictable)? Is she distracted and frustrated or is she getting enough of the type of stimulation that she likes and enjoys? There certainly are a lot of new devices that are available, whether a self-stimulator or vibrator, the Fiera, or other stimulating devices, that may be important to incorporate into the sexual repertoire. If there is underlying pathology, we want to evaluate and treat that. She may need to be primed, so to speak, with systemic hormones. And does she have issues related to other effects of hormonal deprivation, even local effects? Does she have clitoral atrophy?
There are neutraceuticals that are currently available, whether topical arousal gels or ointments, and we as clinicians need to be critical and evaluate their benefit/risk and look at the data concerning these products. In addition, women who have changes in arousal and in orgasmic intensity and latency may be very frustrated. They describe it as climbing up to a peak but never getting over the top, and this frustration may lead to participant spectatoring, so incorporating a certified sex therapist or counselor is sometimes very critical.
Finally, there are a lot of snake oils, charmers, and charlatan unproven procedures--injecting fillers or other substances into the clitoris are a few examples. I would be a critical clinician, examine the evidence, look at the benefit/risk before advocating an intervention that does not have good clinical data to support its use--a comprehensive approach of sexual medicine as well as sexual psychology.
Dr. Kingsberg: Additionally, we know she is in a long-term relationship--15 years; we want to acknowledge the partner. We talked about the partner's weight, but what about his erectile function? Does he have changes in sexual function that are affecting her, and she is the one carrying the "symptom"?
Looking at each piece separately helps a clinician from getting overwhelmed by the patient who comes in reporting distress with orgasmic dysfunction. We have no pharmacologic FDA-approved treatments, so it can feel off-putting for a clinician to try to fix the reported issue. Looking at each component to help her figure out the underlying cause can be helpful.
Dr. Iglesia: With aging, there can be changes in blood flow, not to mention the hormonal and even peripheral nerve changes, that require more stimulation in order to achieve the desired response. I echo concern about expensive procedures being offered with no evidence, such as the "O" or "G" shot, that can cost up to thousands of dollars.
The other procedure that gives me a lot of angst is clitoral unhooding. The 3 parts of the clitoris are sensitive in terms of innervation and blood flow, and cutting around that delicate tissue goes against the surgical principles required for preserving nerves and blood flow.
New onset pain post−prolapse surgery with TOT sling placement
Dr. Levy: For this case, let's consider a 42-year-old woman (P3) who is 6 months post− vaginal hysterectomy. The surgery included ovarian preservation combined with anterior and posterior repair for prolapse as well as apical uterosacral ligament suspension for stage 2 uterovaginal prolapse. A transobturator sling was used.
Extensive preop evaluation was performed, with confirmed symptomatic prolapse. She had no stress incontinence symptoms but did have confirmed occult stress incontinence.
Surgery was uneventful. She resumed intercourse at 8 weeks, but she now has pain with both initial entry and deep penetration. Lubricants and changes in position have not helped. She is in a stable relationship with her husband of 17 years, and she is worried that the sling mesh might be the culprit. On exam, she has no atrophy, pH is 4.5, vaginal length is 8 cm, and there is no prolapse. There is no mesh exposure noted, although she reports slight tenderness with palpation of the right sling arm beneath the right pubic bone.
Dr. Iglesia, what are the patient history questions important to ask here?
Dr. Iglesia: This is not an uncommon scenario--elective surgical correction of occult or latent stress incontinence after surgical correction for pelvic organ prolapse. Now this patient here has no more prolapse complaints; however, she has a new symptom. There are many different causes of dyspareunia; we cannot just assume it is the sling mesh (although with all the legal representation advertisements for those who have had mesh placed, it can certainly be at the top of the patient's mind, causing anxiety and fear).
Multiple trials have looked at prophylactic surgery for incontinence at the time of prolapse repairs. This woman happened to be one of those patients who did not have incontinence symptoms, and they put a sling in. A recent large trial examined women with vaginal prolapse who underwent hysterectomy and suspension.6 (They compared 2 different suspensions.) What is interesting is that 25% of women with prolapse do have baseline pain. However, at 24 months, de novo pain can occur in 10% of women--just from the apical suspension. So, here, it could be the prolapse suspension. Or, in terms of the transobturator sling, long-term data do tell us that the de novo dyspareunia rate ranges on the magnitude of 1% to 9%.7 What is important here is figuring out the cause of the dyspareunia.
Dr. Levy: One of the important points you raised already was that 25% of these women have preoperative pain. So figuring out what her functioning was before surgery and incorporating that into our assessment postop would be pretty important I would think.
Dr. Iglesia: Yes, you need to understand what her typical encounter was before the surgery and how things have changed now that the prolapse is not in the way. Changes obviously can occur with scar tissue, which over time will improve. If she is perimenopausal and starts to get epithelial changes, we can fix that. The question then becomes, "Is the pain emanating from the mesh?"
When examining this patient, it is not uncommon for me to be able to feel "banjo" strings if the mesh is too tight or close to the surface. It is not exposed but it's palpable, and the patient may feel a ridge during penetration. You can ask the patient if pain occurs with different penetration positions. In addition, explore associated neurologic symptoms (numbness or muscle pain in the thigh).
Dr. Kingsberg: There were 2 different sources of pain--on initial entry and at deep penetration. You want to make sure you address both. Importantly, did one precede the other? For instance, if women have pain with penetration they can then end up with an arousal disorder (the length of the vagina cannot increase as much as it might otherwise) and dystonia secondary to the pain with penetration. The timing of the pain--did it all happen at the same time, or did she start out with pain at one point and did it move to something else--is another critical piece of the history.
Dr. Iglesia: It does take a detailed history and physical exam to identify myofascial trigger points. In this case there seems to be pinpoint tenderness directly on the part of the mesh that is not exposed on the right. There are people who feel you should remove the whole mesh, including the arms. Others feel, okay, we can work on these trigger points, with injections, physical therapy, extra lubrication, and neuromodulatory medications. Only then would they think about potentially excising the sling or a portion thereof.
Dr. Krychman: Keep in mind that, even if you do remove the sling, her pain may not subside, if it is secondary to an underlying issue. Because of media sensationalism, she could be focusing on the sling. It is important to set realistic expectations. I often see vulvar pathology or even provoked vestibulodynia that can present with a deep dyspareunia. The concept of collision dyspareunia or introital discomfort or pain on insertion has far reaching implications. We need to look at the patient in totality, ruling out underlying issues related to the bladder, even the colon.
Patient inquires about the benefits of laser treatment for vaginal health
Dr. Levy: Let's move to this last case: A 47-year-old patient who reports lack of sexual satisfaction and attributes it to a loose vagina says, "I've heard about surgery and laser treatment and radiofrequency devices for vaginal health. What are the benefits and risks of these procedures, and will they correct the issues that I'm experiencing?"
How do you approach this patient?
Dr. Krychman: I want to know why this is of paramount importance to her. Is this her actual complaint or is it society's unrealistic expectation of sexual pleasure and performance placed upon her? Or is this a relationship issue surfacing, compounded by physiologic changes? With good communication techniques, like "ask, tell, ask" or effective use of silence (not interrupting), patients will lead us to the reason and the rationale.
Dr. Levy: I have seen a lot of advertising to women, now showing pictures of genitalia, perhaps creating an expectation that we should look infantile in some way. We are creating a sense of beauty and acceptablevisualization of the vagina and vulva that are completely unrealistic. It's fascinating on one hand but it is also disturbing in that some of the direct-to-consumer marketing going on is creating a sense of unease in women who are otherwise perfectly satisfied. Now they take a look at their sagging skin, maybe after having 2 or 3 children, and although they may not look the same they function not so badly perhaps. I think we are creating a distress and an illness model that is interesting to discuss.
Dr. Iglesia, you are in the midst of a randomized trial, giving an informed consent to participants about the expectations for this potential intervention. How do you explain to women what these laser and radiofrequency devices are expected to do and why they might or might not work?
Dr. Iglesia: We are doing a randomized trial for menopausal women who have GSM. We are comparing estrogen cream with fractional CO2 laser therapy. I also am involved in another randomized trial for lichen sclerosus. I am not involved in the cosmetic use of a laser for people who feel their vaginas are just "loose." Like you, Dr. Levy, I am very concerned about the images that women are seeing of the idealized vulva and vagina, and about the rise of cosmetic gynecology, much of which is being performed by plastic surgeons or dermatologists.
A recent article looked at the number of women who are doing pubic hair grooming; the prevalance is about 80% here in America.8 So people have a clear view of what is down there, and then they compare it to what they see in pornographic images on the Internet and want to look like a Barbie doll. That is disturbing because women, particularly young women, do not realize what happens with GSM changes to the vulva and vagina. On the other hand, these laser machines are very expensive, and some doctors are charging thousands of dollars and promising cosmetic and functional results for which we lack long-term, comparative data.
The laser that we are studying is one by Cynosure called Mona Lisa, which works with fractional CO2 and has very low depth of penetration. The concept is that, with microdot therapy (on the order of micrometers), pinpoint destruction will foster regeneration of new collagen and blood flow to the vagina and vulva. We are still in the midst of analyzing this.
Dr. Krychman: I caution people not to lump devices together. There is a significant difference between laser and radiofrequency--especially in the depth of tissue penetration, and level of evidence as well. There are companies performing randomized clinical trials, with well designed sham controls, and have demonstrated clinical efficacy. We need to be cautious of a procedure that is saying it's the best thing since sliced bread, curing interstitial cystitis, dyspareunia, and lichen sclerosus and improving orgasm, lubrication, and arousal. These far reaching, off-label claims are concerning and misleading.
Dr. Levy: I think the important things are 1) shared decision making with the patient and 2) disclosure of what we do not know, which are the long-term results and outcomes and possible downstream negative effects of some of these treatments, since the data we have are so short term.
Dr. Kingsberg: Basics are important. You talked about the pressure for cosmetic appearance, but is that really what is going on for this particular patient? Is she describing a sexual dysfunction when she talks about lack of satisfaction? You need to operationally define that term. Does she have problems with arousal or orgasm or desire and those are what underlie her lack of satisfaction? Is the key to management helping her come to terms with body image issues or to treat a sexual dysfunction? If it truly is a sexual dysfunction, then you can have the shared decision making on preferred treatment approach.
Dr. Levy: This has been an enlightening discussion. Thank all of you for your expertise and clinical acumen.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition. American Psychiatric Association Publishing: Arlington, VA; 2013.
- American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: The Use of Vaginal Estrogen in Women With a History of Estrogen-Dependent Breast Cancer. Obstet Gynecol. 2016;127(3):e93−e96.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society (NAMS). NAMS Menopause e-Consult: Laser treatment safe for vulvovaginal atrophy? 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed August 17, 2016.
- The American College of Obstetricians and Gynecologists and The American Congress of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and U.S. Food and Drug Administration clearance: Position Statement. http://www.acog.org/Resources-And-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Published May 2016. Accessed August 17, 2016.
- Lukacz ES, Warren LK, Richter HE, et al. Quality of life and sexual function 2 years after vaginal surgery for prolapse. Obstet Gynecol. 2016;127(6):1071−1079.
- Brubaker L, Chiang S, Zyczynski H, et al. The impact of stress incontinence surgery on female sexual function. Am J Obstet Gynecol. 2009;200(5):562.e1.
- Rowen TS, Gaither TW, Awad MA, Osterberg EC, Shindel AW, Breyer BN. Pubic hair grooming prevalence and motivation among women in the United States [published online ahead of print June 29, 2016]. JAMA Dermatol. doi:10.1001/jamader matol.2016.2154.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition. American Psychiatric Association Publishing: Arlington, VA; 2013.
- American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: The Use of Vaginal Estrogen in Women With a History of Estrogen-Dependent Breast Cancer. Obstet Gynecol. 2016;127(3):e93−e96.
- Krychman ML, Shifren JL, Liu JH, Kingsberg SL, Utian WH. The North American Menopause Society (NAMS). NAMS Menopause e-Consult: Laser treatment safe for vulvovaginal atrophy? 2015;11(3). http://www.medscape.com/viewarticle/846960. Accessed August 17, 2016.
- The American College of Obstetricians and Gynecologists and The American Congress of Obstetricians and Gynecologists. Fractional laser treatment of vulvovaginal atrophy and U.S. Food and Drug Administration clearance: Position Statement. http://www.acog.org/Resources-And-Publications/Position-Statements/Fractional-Laser-Treatment-of-Vulvovaginal-Atrophy-and-US-Food-and-Drug-Administration-Clearance. Published May 2016. Accessed August 17, 2016.
- Lukacz ES, Warren LK, Richter HE, et al. Quality of life and sexual function 2 years after vaginal surgery for prolapse. Obstet Gynecol. 2016;127(6):1071−1079.
- Brubaker L, Chiang S, Zyczynski H, et al. The impact of stress incontinence surgery on female sexual function. Am J Obstet Gynecol. 2009;200(5):562.e1.
- Rowen TS, Gaither TW, Awad MA, Osterberg EC, Shindel AW, Breyer BN. Pubic hair grooming prevalence and motivation among women in the United States [published online ahead of print June 29, 2016]. JAMA Dermatol. doi:10.1001/jamader matol.2016.2154.
In This Article
- This roundtable's expert panel
- Dyspareunia and low sexual desire in a breast cancer survivor
- Laser treatment and vaginal health
2 HPV vaccines, 7 questions that you need answered
Not long ago (in medical years), we were still trying to discover the cause of cervical cancer. Today, not only do we know that cause to be persistent human papillomavirus (HPV) infection, but we have two vaccines at our disposal to prevent the primary oncogenic strains of the virus.
We’ve come a long way.
The availability of two vaccines raises questions, however. What kind of data do we have on the bivalent (Cervarix, GlaxoSmithKline) and quadrivalent (Gardasil, Merck) vaccines so far? Is one of them clearly superior to the other? If not, what population is each vaccine best suited for—and how do we counsel patients about their options?
To address these and other questions, OBG Management Contributing Editor Neal M. Lonky, MD, MPH, assembled a panel of physicians who have expertise in cervical disease detection and prevention and asked them to sift the data that have accumulated thus far. In the discussion that follows, they touch on long-term efficacy, the likely impact of the vaccines on cervical cancer screening, and other aspects of disease prevention in the era of HPV vaccination.

Juan C. Felix, MD
Professor of Clinical Pathology and Obstetrics and Gynecology; Director of Cytopathology fellowship; and Chief of Gynecologic Pathology at the Keck School of Medicine, University of Southern California; and Chief of Cytopathology at Los Angeles County and University of Southern California Medical Center in Los Angeles.
Dr. Felix reports that he is a speaker for Merck and GlaxoSmithKline.

Diane M. Harper, MD, MS, MPH
Director of the Gynecologic Cancer Prevention Research Group and Professor of Obstetrics and Gynecology, Community and family Medicine, and Informatics and Personalized Medicine at the University of Missouri–Kansas City School of Medicine.
Dr. Harper reports that she has served as a speaker and advisor for Merck and GlaxoSmithKline, and that the institutions at which she conducted HPV vaccination trials have received funding from Merck and GlaxoSmithKline.

Warner K. Huh, MD
Associate Professor in the Department of Obstetrics and Gynecology, and Associate Scientist at the Comprehensive Cancer Center at the University of Alabama– Birmingham.
Dr. Huh reports that he receives grant or research support from and is a speaker and consultant to Merck and GlaxoSmithKline.

Karen K. Smith-McCune, MD, PhD
John Kerner Endowed Chair of Gynecologic Oncology, Director of the Dysplasia Clinic, and Professor of Obstetrics, Gynecology, and Reproductive Sciences at the University of California–San francisco.
Dr. Smith-McCune reports she has performed unpaid consulting for OncoHealth Inc. and is planning to join its Scientific Advisory Board.
1. How were the vaccines developed?
Neal M. Lonky, MD, MPH: What should clinicians know about the development, function, and mechanism of action of the two HPV vaccines?
Warner K. Huh, MD: The bivalent and quadrivalent vaccines are both excellent products, and their respective Phase-3 trials demonstrate that they provide impressive protection against HPV, particularly among women who test negative (by polymerase chain reaction) for the specific HPV types contained within the vaccines.1-3
Cervarix protects against HPV types 16 and 18, whereas Gardasil is effective against HPV types 6, 11, 16, and 18.
Dr. Lonky: Do the vaccines function similarly?
Diane M. Harper, MD, MS, MPH: Yes. Both stimulate an immediate antibody response in the woman who is not infected with the relevant virus and are effective in preventing cervical intraepithelial neoplasia grade 2 and higher (CIN 2+), as well as persistent infection, caused by vaccine-related and cross-protected HPV types. The quality of the antibody response is best for HPV 16 for both vaccines. The quality of the antibody response for HPV 6, 11, and 18 for Gardasil is much poorer than its response for HPV 16. Cervarix induces an equally high and sustained antibody response for HPV 18 as for HPV 16.
Juan C. Felix, MD: Both vaccines are based on the same virus-like particles (VLP). The functionality of the vaccines is, therefore, mainly dependent on the dosage of VLP and the adjuvant used. Gardasil uses a proprietary aluminum sulfate adjuvant, whereas Cervarix uses aluminum hydroxide and monophosphoryl lipid A.
Karen K. Smith-McCune, MD, PhD: Both adjuvants have an extensive track record of safety and efficacy in other vaccines. Because they have different structures, however, they may have varying effects on many components of the immune response elicited by the L1 antigens.
Dr. Harper: Both adjuvants contain aluminum, which has so far proved to be safe despite the newly established association between high aluminum intake and Alzheimer’s disease.
Dr. Lonky: Were there any notable challenges in developing the vaccines?
Dr. Harper: It was difficult to formulate the appropriate dosages of VLP in Gardasil. Higher dosages of HPV 11 and 16 were needed to prevent cross-inhibition by HPV 6 and 18. As a result, the antigenic protein component of Gardasil that is necessary to effect an immunologic antibody response is high, at 120 μg. In Cervarix, the antigenic VLP load is 20 μg each for HPV 16 and 18.
Dr. Lonky: What is the significance of the different VLP loads?
Dr. Harper: Side effects, such as autoimmune neurologic demyelination, albeit rare, have been associated with a higher antigenic protein load. Multiple reports of autoimmune demyelinating diseases—including paralysis, blindness, and death—have been published by neurologists in regard to Gardasil.4,5 Others have shown that young girls are more at risk than young boys for these neurologic side effects.6
Dr. Felix: Some data suggest that the two vaccine formulations interact differently with the human immune system. In a head-to-head trial funded by GlaxoSmithKline, Cervarix produced higher total and neutralizing antibody titers than Gardasil did.7
Although higher immunogenicity is generally thought to be beneficial, the ultimate determinant of a vaccine’s success is its efficacy—and duration of that efficacy—in clinical trials and follow-up of vaccinated populations. So far, Cervarix has demonstrated efficacy through 8.4 years in its follow-up cohort.8 Similarly, Gardasil has proved to be effective after 5 years of follow-up, with no incident cases of cervical cancer reported in the vaccinated arm.9
2. Does either vaccine offer “extra” immunity?
Dr. Lonky: What is the potential for overlapping immunity to other high-risk viral types with these vaccines?
Dr. Harper: It is quite clear from pivotal trials of both vaccines that Gardasil produces efficacy of 46% against persistent infection caused by HPV 31. Data from the pivotal Phase-3 trial of Gardasil also show that it offers no protection against persistent infection with HPV 45, an important cause of adenocarcinoma.10
In contrast, Cervarix demonstrates substantial efficacy against both persistent infection and CIN 2+ disease caused by HPV 31, 33, and 45.3
These findings mean that Cervarix is 91% effective against HPV types that cause adenocarcinoma and 83% effective overall against squamous cell carcinoma. Compare that with Gardasil, which is 78% effective overall against HPV types that cause adenocarcinoma and 73% effective against HPV types that cause squamous cell carcinoma.
The immune titers tell a supportive story. After vaccination with Gardasil, the antibody titer immediately declines for HPV 6, 11, and 18, reaching the baseline for natural infection within 18 months.7 HPV 18 shows continued, significant loss of seropositivity over time, and antibody titers for HPV 6 and 11 also decline. In the monovalent HPV 16 pre-Gardasil experimental vaccine, 14% of women no longer had measurable titers to HPV 16 after 8.5 years.11
After vaccination with Cervarix, antibody titers for HPV 16 and 18 remain more than seven times and more than four times higher, respectively, than natural infection titers for 8.4 years, with no loss of measurable antibody titer for either type. The antibody titers for HPV 31, 33, and 45 remain substantially higher than natural infection titers for at least 6.4 years. These titers correlate with the vaccine’s very high efficacy against CIN 2+ lesions caused by HPV 16, 18, 31, 33, and 45.
In other words, Cervarix generates an immune response (and efficacy) that indicates robust protection against five of the most common oncogenic HPV types, providing maximal protection against nearly 85% of all cervical cancers. Gardasil protects against 74% of all cervical cancers overall.12 This makes Cervarix the superior cervical cancer vaccine.
Gardasil is the superior vaccine against genital warts, although the duration of its protection is uncertain.
Dr. Huh: I’d just like to point out that there are no head-to-head trials comparing the vaccines in terms of efficacy. Antibody titers are higher with Cervarix than with Gardasil, as you noted, and it may be that, over time, the higher titers are more durable with Cervarix. However, we have yet to fully correlate clinical efficacy with antibody titers. In other words, immunogenicity does not equal clinical efficacy.
Dr. Felix: The data for Gardasil are particularly interesting because there have been no incident HPV-18 lesions detected despite the absence of detectable HPV-18 antibody titers in more than 20% of vaccinated women as soon as 2 years after immunization.9 These data strongly suggest that it is not antibody titer alone that grants protection against HPV-induced lesions of the cervix.
Dr. Harper: This speaks to the difficulty of running a trial to ensure both enough participants and a sufficient attack rate of HPV 18 to cause new lesions to be detected in vaccinated women. In the relevant trial, there were only 112 vaccinated women—not nearly enough women to overcome the very low attack rate of HPV 18 in the trial population—and they were followed for 5 years.9 We cannot be sure that the lack of incident HPV-18 lesions in the vaccinated women is the result of efficacy.
Dr. Felix: As for overlapping immunity to HPV types not included in the vaccines, it has been described for both Cervarix and Gardasil. In the case of Cervarix, the manufacturer demonstrated unexpectedly high rates of protection against all CIN 2+ and CIN 3+ lesions—70% and 87%, respectively. These rates were too high to be explained by protection against types 16, 18, 31, and 45 alone. It is possible, therefore, that Cervarix may protect against other high-risk HPV types.13
Gardasil has proved to be effective against HPV types 31, 33, 52, and others.10 When total protection against CIN 2+ and CIN 3+ lesions is examined from Phase-3 trials of the vaccine, however, the rates are only 42% and 43%, respectively. These data are difficult to interpret because HPV 16 and 18 together are thought to account for 70% of CIN 3. Some reassurance can be gained from the fact that the number of incident cases of CIN 2+ and CIN 3+ caused by HPV 16 and 18 in the vaccinated group in the Gardasil trial was identical to the number seen in the Cervarix trial.3,10 The reason for the discrepancy in total number of cases of CIN 2+ and CIN 3+ between the two trials—and, therefore, between the two vaccines—cannot be explained by cross-protection alone and is probably attributable to differences in study populations. The Gardasil trial had a higher baseline prevalence of HPV 16 and 18 (9% and 4%, respectively) than the Cervarix trial did (5% and 2%, respectively), a fact that may be explained by the different demographics of their respective populations.2,14
Ultimately, it is hazardous to compare trials, particularly when they are conducted in significantly different populations. On this issue, I concur with the World Health Organization (WHO), which recommended that such comparisons be avoided in the determination of which type of HPV vaccine to recommend.15
Dr. Huh: I agree that it would be inappropriate to make cross-trial comparisons, given differences in the way the trials were designed and conducted. To draw conclusions about clinical efficacy of these two excellent vaccines, based on a comparison of their trials, is completely unscientific. Only a true head-to-head study that has efficacy as its endpoint can tell us which vaccine is superior—and such a trial would require thousands (if not tens of thousands) of subjects and a considerable amount of time to complete. In my opinion, such a study would be counterproductive to our goal of vaccination.
Dr. Harper: I disagree. The whole purpose of this roundtable is to compare vaccines. It is not “unscientific” to compare the trials.
Dr. Huh: On the contrary—it is completely inappropriate to directly compare the Phase-3 clinical trials from Merck and GlaxoSmithKline. One can speculate about the differences between them, but any clinical trialist knows that a direct, scientific comparison cannot be made. Only a real head-to-head study powered for efficacy can do this.
- Both the bivalent and quadrivalent vaccines appear to be excellent products. Besides protecting against the main oncogenic strains of human papillomavirus (HPV) (types 16 and 18 for both vaccines, and the genital-wart-associated strains 6 and 11 for the quadrivalent vaccine), both Cervarix and Gardasil offer some degree of cross-protection against additional HPV strains.
- Vaccination of the sexually naïve patient with either vaccine provides significant protection against cervical intraepithelial neoplasia 2 (CIN 2) or worse.
- HPV vaccination is expected to reduce the rate of abnormal Pap tests and the need for common excisional treatments for cervical dysplasia in vaccinated women. It will do the same in the population as a whole if rates of vaccination are sufficient to provide “herd” immunity.
3. Is one vaccine more effective than the other?
Dr. Lonky: How do the vaccines compare in terms of efficacy?
Dr. Smith-McCune: In discussing efficacy, I think we should focus on CIN 3 because it is the immediate surrogate for cancer, whereas CIN 2 lesions can be transient in younger women. I think it is also important to focus on outcomes regardless of the HPV types associated with the lesions. This approach is more clinically relevant, as we don’t perform HPV typing of lesions in clinical practice. Nor do we manage lesions differently depending on the HPV type in the lesion.
That said, it is difficult to compare efficacy of the vaccines for several reasons, a few of which we have already discussed. For example, the bivalent and quadrivalent vaccines were studied in separate randomized trials. Although the study populations were similar, they were not identical. Women in both trials were relatively sexually naïve, but the cutoff for number of lifetime sexual partners was different (5 for Gardasil versus 7 for Cervarix). In trials of Gardasil, women who had a history of abnormal cytology or genital warts were excluded. In trials of Cervarix, women who had a history of colposcopy were excluded. In Gardasil trials, approximately 3% of women were from the Asian Pacific, versus 34% in the Cervarix trials, and so on.3,16
The trials also had different protocols for referral to colposcopy, which would affect disease detection. And the length of follow-up differs between trials.3,9
Dr. Lonky: Can we draw any conclusions about efficacy?
Dr. Smith-McCune: Yes. The trials defined outcomes in several populations of participants. In addition to the overall population (called the “intention-to-treat population” in the Gardasil trials and the “total vaccinated cohort” in the Cervarix trials), the trials defined a subpopulation of women naïve to oncogenic HPV types to gain information about the likely impact of vaccinating girls before the onset of sexual activity. The definitions of these “naïve” populations were slightly different, mainly in the number of HPV types tested, so again, some caution needs to be exercised in making comparisons.
End-of-trial data in the naïve population show a 43% reduction in CIN 3 lesions for Gardasil and 87% for Cervarix (for CIN 3 or worse). By inference, we can tell the sexually naïve patient that vaccination with either vaccine will provide significant protection against CIN 3 lesions, likely to result in significant protection against cervical cancer over time.
We can gather some estimates of efficacy in sexually non-naïve women by looking at results from all trial participants. Gardasil reduced overall CIN 3 lesions by 16% overall; Cervarix reduced CIN 3 or worse by 33%. When counseling an individual patient, if she has had a similarly low number of lifetime sexual partners (e.g., the median number in the Gardasil trials was 2), these results provide an estimate of her likely protection against CIN 3 with vaccination.
Common excisional treatments for cervical dysplasia are known to be associated with adverse perinatal outcomes.17 The ability to reduce the need for these treatments is an important outcome of vaccination. In the HPV-naïve populations, vaccination reduced definitive cervical therapies or excisions by 42% (Gardasil) and 69% (Cervarix). These figures are useful in counseling virginal patients about the long-term benefits of vaccination.
For sexually active patients 26 years and younger, HPV vaccination significantly reduced definitive cervical therapy or excisions by 23% (Gardasil) and 25% (Cervarix). Again, these figures are most applicable for counseling patients who have had relatively few lifetime sexual partners. So the exact extent of protection is likely to vary by the patient’s total number of lifetime sexual partners.
I expect that we will see more data on the effects of vaccination stratified by the number of lifetime sexual partners, because that information would be very useful in counseling individual sexually active women.
Dr. Harper: Both vaccines reduce the rate of abnormal Pap tests by 10% regardless of HPV type in that population of women.9,18
4. Are the two vaccines safe?
Dr. Lonky: What about safety of the vaccines? What do we know?
Dr. Felix: The safety profiles seen in clinical trials of both vaccines are very similar and consist almost entirely of nonserious adverse events.2,9 In the United States, a greater number of Gardasil doses has been administered, owing to its earlier development. As of January 1, 2010, more than 28 million doses had been distributed, and numerous major events had been recorded in the Vaccine Adverse Event Reporting System (VAERS). Of 15,829 adverse events reported, only 8% were considered serious by the CDC. CDC investigation, by expert panels, of all serious adverse events found no evidence linking Gardasil to any of them, including Guillain-Barré syndrome, blood clots, and death.19
Dr. Huh: A few other points to consider:
- The reporting rate for Gardasil is triple that for all other vaccines combined
- Because VAERS is a passive reporting system, under-reporting is distinctly possible
- Post-licensure safety surveillance is still underway
- Both products have pregnancy registries.
Dr. Harper: The current postmarketing commitment between Merck and the FDA is to recognize a rate of serious adverse events that exceeds 2 cases in every 10,000 women in a cohort of 44,000 women who have received all three doses of Gardasil. Although autoimmune neurologic sequelae have occurred after Gardasil administration, regulatory authorities are not required to evaluate these reactions, such as Guillain-Barré syndrome, because the frequency is lower than the agreed-upon threshold. Nevertheless, adverse events could be life threatening to some girls.
Any risk of death—even if it is lower than the agreed-upon threshold—should be presented to women as a possible risk of vaccination with Gardasil. In the United States, the same women could choose a lifetime of Pap screening and be afforded the same protection against cervical cancer as they would get from vaccination.
5. Is quadrivalent better than bivalent?
Dr. Lonky: Why would a clinician choose a bivalent vaccine when the quadrivalent vaccine protects not only against carcinogenic types 16 and 18, but also against HPV-associated genital warts?
Dr. Harper: A smart clinician would ask the patient what she values. The physician is obligated to present the evidence and let her choose!
Dr. Lonky: What does the evidence suggest?
Dr. Felix: A clear recommendation between Cervarix and Gardasil is very difficult to make at this time, for the reasons already stated. Both vaccines provide 98% protection against HPV 16 and 18 for the prevention of CIN 2+ lesions.3,9 As we have discussed, both vaccines also provide protection against high-risk strains of HPV other than types 16 and 18.
In the Cervarix trial, there was an overall reduction of all CIN 2+ lesions that was higher as a percentage of total lesions than the reduction seen in the Gardasil trial.3,9 However, unlike Cervarix, Gardasil significantly reduced the rate of vaginal intraepithelial neoplasia (VaIN) and vulvar intraepithelial neoplasia (VIN).9
Dr. Harper: GlaxoSmithKline is analyzing its data on vulvar and vaginal protection, and it is likely that Cervarix will demonstrate some efficacy in this regard, too. But the economic burden of noncervical cancers is estimated to be only 8% of the economic burden of all HPV-related diseases.20 The prevention of cervical cancer is the dominant clinical and economic force for vaccination.
Dr. Felix: Clearly, the protection against genital warts demonstrated in the Gardasil trial will not be realized with Cervarix, as it does not offer immunization or cross-protection against HPV 6 or 11. Gardasil’s protection against HPV 6 and 11 prompted FDA approval of the vaccine for boys and men.21
According to the WHO, when counseling girls and women about the HPV vaccine, the clinician should weigh the possible value of a deep reduction in total CIN 2+ lesions provided by Cervarix against the reduction in VaIN, VIN, and genital warts provided by Gardasil.15 Boys and men will see clinically proven benefits only from Gardasil for the prevention of external genital warts. Other benefits are strictly theoretical.22,23
Dr. Harper: Vaccine protection must last at least 15 years to reduce the rate of cervical cancer. Otherwise, the development of cervical cancer will only be postponed, if boosters are not implemented.
It is now widely recognized that Cervarix induces high antibody titers, offering 100% efficacy even after 8.4 years, making it very likely that the protection it provides will continue for at least 15 years. It is also widely acknowledged by immunologists that Gardasil-induced titers for HPV 6, 11, and 18 are much shorter-lived, so protection is likely to wane 5 to 10 years after vaccination.
That means that Gardasil provides excellent protection against one cancer-causing type of HPV. In addition, it protects against genital warts caused by HPV types 6 and 11 for at least 5 years. In comparison, Cervarix protects against five cancer-causing types of HPV, thereby preventing about 90% of cervical cancers, and is likely to remain effective for at least 15 years.
There are 10 times as many women who have an abnormal Pap test as there are women who have genital warts, so one would think that Cervarix would be the vaccine of choice in preventing the life-threatening disease of cervical cancer.
Dr. Smith-McCune: I would agree that the choice should be discussed with patients—and with parents. If the objective is primarily to protect against cervical cancer precursors, then the bivalent vaccine may be the better choice, with the caveat that we can’t really compare the results from the vaccine trials for reasons discussed earlier, and there are no data from a randomized head-to-head trial comparing the two vaccines. If the decision involves a desire to reduce genital warts or vulvar and vaginal dysplasia, then the quadrivalent vaccine would be the better choice.
6. What impact do the vaccines have on screening?
Dr. Lonky: Do the vaccines have varying effects on our need to screen for, triage, and treat cervical cancer precursors?
Dr. Harper: The Pap smear has reduced the rate of cervical cancer in the United States by 75%—that rate is now at an all-time low of 8 cases for every 100,000 women. But the Pap smear is not perfect; there is a 30% false-negative rate among women who develop cervical cancer, and a large false-positive rate that involves referral to colposcopy for minimally abnormal cytology reports. And when CIN 2+ disease is detected, treatment is not without risk. Surgery increases the risk of reproductive morbidity in future pregnancies. Having protection against this outcome could be tremendously valuable for some women.
Compare the HPV vaccine, which has probable benefit but also the potential for serious adverse events, including demyelinating diseases that cause blindness, paralysis, and death in a small number of recipients.
If women were to choose to be vaccinated with Gardasil and forgo further Pap screening, the rate of cervical cancer in the United States would rise from 8 to 14 cases for every 100,000 women. If they were to choose Cervarix instead, with no further Pap screening, the rate would rise from 8 to 9.5 cases for every 100,000 women.
If women were to choose both HPV vaccination and continued Pap screening, the rate of cervical cancer still would not decline from its current level of 8 cases for every 100,000 women. Instead, the benefit would be that fewer women have abnormal Pap tests, and fewer women would need to be treated for CIN 2+ disease.
Women and physicians must understand these facts. A woman who chooses to be vaccinated may gain individual protection, but the overall rate of cervical cancer will not be affected.
Dr. Huh: Regardless of the HPV vaccine selected, we need to seriously rethink how we screen women in the United States. One could easily argue that the combination of the vaccine and continued screening is too expensive. It might be wise to consider lengthening the screening interval—and, perhaps, further delaying initial screening to 25 years of age—to make cervical cancer prevention with both modalities more cost-effective.24 The most important thing to recognize is that women still need to be screened, even if they have been vaccinated.
As more women are vaccinated, we expect to see a decline in the prevalence of CIN 2+ and CIN 3+ lesions, and this will ultimately weaken the positive predictive value of cytology. Perhaps it is time to consider the HPV test as a primary screen, with triage to cytology in women who test HPV-positive.25
Dr. Smith-McCune: As we accumulate data over time about the effects of vaccination on the rates of CIN 3 and cancer, modeling will be helpful in determining the best screening algorithm for women who have been vaccinated against HPV.
It is important to remember that approximately 50% of women who are given a diagnosis of cervical cancer in the United States have never been screened. It is vital that we continue to reach out to the under-screened population and focus vaccination efforts on populations of girls who are likely to have limited access to care in the future.
7. Can we vaccinate every woman?
Dr. Lonky: Is universal vaccination of women achievable for either vaccine?
Dr. Felix: Universal vaccination against HPV would be achievable only via school mandates. Without them, vaccination will not approach the 80% threshold needed to produce herd immunity.
Despite the clear benefit of such mandates to the general population—particularly the medically under-served—the issue has become a political football. As a result, school mandates will probably never be realized.
Dr. Harper: I don’t believe it is ethical to mandate vaccination of all girls and women. It is a choice that women and parents, in conversation with their physicians and daughters, must make when considering how to be protected against cervical cancer. Herd immunity is a moot point because we are only vaccinating girls (50% of the population) and can never reach the theoretical 70% threshold for herd immunity to be apparent.
Dr. Lonky: Is the availability of two vaccines a boon or a hindrance?
Dr. Smith-McCune: I think it is always a good thing to have choices in medicine.
Dr. Huh: I see the availability of two vaccines as a boon. That availability means that two companies are now putting forth consistent educational messages about the importance of vaccination and, I hope, stimulating competition that will reduce the overall cost of the vaccine series. Having two vaccines can only promote awareness, access, and greater appreciation of the considerable protection these two vaccines provide.
Despite solid evidence that the quadrivalent (Gardasil) HPV vaccine and the bivalent (Cervarix) HPV vaccine protect against cervical cancer, only about one fifth of the female population between 11 and 26 years of age has received the full series of Gardasil since it won FDA approval in 2006. Barriers to vaccination are not financial alone, as the vaccination rate is similarly low among women who have health insurance.
Why isn’t the vaccination rate higher? I see eight barriers to full implementation:
- Economic disparities. Each vaccine costs roughly $400 (national average) for the full series of injections. Although women who do not get Pap screening are most likely to benefit from the vaccines, they usually cannot afford them. federal childhood immunization programs cover teens and young women until 18 years of age in most states, and until 21 years in a few. that leaves most women who seek vaccination from gynecologists without coverage.
- Fear. Pain at the injection site, syncope, and a slightly elevated incidence of thromboembolism are the adverse events most commonly associated with HPV vaccination in the literature. In the life cycle of a vaccine, reports of sudden death or neurologic injury (Guillain-Barré syndrome) occur in the early years, but are reported at a rate lower than 2 cases in every 10,000 women. Nevertheless, such events may create fear about undergoing immunization.
- Long latency period. Because the outcome of cancer prevention won’t become apparent for 20 to 40 years following vaccination, the need for immunization may seem less than urgent.
- Cultural and religious beliefs. Because carcinogenic HPV strains are sexually transmitted, some families may associate vaccination with the promotion of sexual activity. Even in states that mandate vaccination, the courts have upheld a parent’s right to refuse vaccination on these grounds.
- The premarket push. Aggressive promotion of vaccination by both manufacturers and a push by advocates for legislation to mandate the vaccine prior to completion of Phase-3 trials and gathering of robust safety data may have diminished trust in the vaccine and reduced its acceptance.
- Lack of legislation. In states that do not consider HPV vaccination to be a necessary public health intervention, the lack of mandates and funding reduce the vaccination rate. In addition, some legislators have been more active advocates of vaccination than others.
- Reduced involvement of the obGyn. ObGyns don’t routinely vaccinate patients; pediatricians do. Young women are slipping through the cracks because the conventional ObGyn practice does not have a vaccination program that ensures payment, reimbursement, and completion of the vaccine series. Many ObGyn practices are reluctant to institute such a program because the profit margin is small, there are associated risks, and the time required to counsel the patient and for follow-up is extensive.
- Failure to complete the series. Some women do not complete the full vaccine series, owing to cost or side effects, or both. Solid evidence that a single dose could be as protective as the full series would be compelling. A single-dose vaccine would also be less expensive.
The principal danger of a low vaccination rate is the loss of insurance coverage for immunization against HPV. On one hand, payers may begin to ask whether coverage is justified when so few girls and women are vaccinated, leaving the payer with two burdens: the expense of vaccination and the expense of conventional screening programs and treatment, although the costs of treatment would be reduced with vaccination. On the other hand, Gardasil’s protection against genital warts may provide incentive for payers to cover or discount the vaccine because of the reduction in the need to diagnose, triage, and treat condyloma.
Ultimately, HPV vaccination may become another optional intervention that is paid for by the individual, despite evidence in girls and women that cervical cancer can be prevented.
—NEAL M. LONKY, MD, MPH
We want to hear from you! Tell us what you think.
1. The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915-1927.
2. Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. HPV PATRICIA study group. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161-2170.
3. Paavonen J, Naud P, Salmeron J, et al. For the HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301-314.
4. DiMario FJ, Hajjar M, Ciesielski T. A 16-year-old girl with bilateral visual loss and left hemiparesis following an immunization against human papillomavirus. J Child Neurology. 2010;25(3):321-327.
5. Sutton I, Lahoria R, Tan I, Clouston P, Barnett M. CNS demyelination and quadrivalent HPV vaccination. Multiple Sclerosis. 2009;15(1):116-119.
6. Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10(5):338-349.
7. Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin. 2009;5(10):705-719.
8. GlaxoSmithKline Vaccine HPV-007 Study Group. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 8.4 years. Presented at: 28th Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID); May 4-8, 2010; Nice, France.
9. Muñoz N, Kjaer SK, Sigurdsson K, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102(5):325-339.
10. Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naïve women aged 16-26 years. J Infect Dis. 2009;199(7):926-935.
11. Rowhani-Rahbar A, Mao C, Hughes JP, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine. 2009;27(41):5612-5619.
12. Harper DM, Williams KB. Prophylactic HPV vaccines: current knowledge of impact on gynecologic premalignancies [published online ahead of print July 3, 2010]. Discovery Medicine. 2010;10(50).http://www.discoverymedicine.com/Diane-M-Harper/2010/07/03/prophylactichpv-vaccines-current-knowledge-of-impact-ongynecologic-premalignancies. Accessed July 13, 2010.
13. Cervarix [human papillomavirus bivalent (types 16 and 18) vaccine recombinant]. Food and Drug Administration. Initial US approval: 2009:1–12.
14. Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928-1943.
15. World Health Organization. Human papillomavirus vaccines: WHO position paper. Weekly Epidemiological Record. 2009;84(15):118-131.http://www.who.int/wer/2009/wer8415/en/index.html. Published April 10, 2009. Accessed July 13, 2010.
16. Ault KA. For Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on the risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369(9576):1861-1868.
17. Arbyn M, Kyrgiou M, Simoens C, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ. 2008;337:a1284.-Doi: 10.1136/bmj.a1284.
18. Paavonen J. For the HPV PATRICIA Study Group. Efficacy of HPV 16/18 ASO4-adjuvanted vaccine against abnormal cytology, colposcopy referrals, and cervical procedures. Abstract SS 4–1. Data presented at Cervical Cancer Prevention, EuroGin 2010. European Research Organisation on Genital Infection and Neoplasia (EUROGIN); February 17-20, 2010; Monte Carlo, Monaco.http://www.eurogin.com/2010/programoverview.html. Accessed July 13, 2010.
19. Centers for Disease Control and Prevention. Reports of health concerns following HPV vaccination. 2010;1-3.http://www.cdc.gov/vaccinesafety/Vaccines/HPV/gardasil.html. Published June 21, 2010. Accessed July 13, 2010.
20. Myers ER. The economic impact of HPV vaccines: not just cervical cancer. Am J Obstet Gynecol. 2008;198(5):487-488.
21. Gardasil [human papillomavirus quadrivalent (types 6, 11, 16, and 18) vaccine, recombinant]. Food and Drug Administration; 2009. Initial Approval 2006;1-26.
22. Olsson SE, Kjaer SK, Sigurdsson K, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin. 2009;5(10).
23. Pirotta M, Ung L, Stein A, et al. The psychosocial burden of human papillomavirus related disease and screening interventions. Sex Transm Infect. 2009;85(7):508-513.
24. Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359(8):821-832.
25. Cox JT. Is the HPV test effective as the primary screen for cervical cancer? Examining the Evidence. OBG Management. 2010;22(7):10-11.
Not long ago (in medical years), we were still trying to discover the cause of cervical cancer. Today, not only do we know that cause to be persistent human papillomavirus (HPV) infection, but we have two vaccines at our disposal to prevent the primary oncogenic strains of the virus.
We’ve come a long way.
The availability of two vaccines raises questions, however. What kind of data do we have on the bivalent (Cervarix, GlaxoSmithKline) and quadrivalent (Gardasil, Merck) vaccines so far? Is one of them clearly superior to the other? If not, what population is each vaccine best suited for—and how do we counsel patients about their options?
To address these and other questions, OBG Management Contributing Editor Neal M. Lonky, MD, MPH, assembled a panel of physicians who have expertise in cervical disease detection and prevention and asked them to sift the data that have accumulated thus far. In the discussion that follows, they touch on long-term efficacy, the likely impact of the vaccines on cervical cancer screening, and other aspects of disease prevention in the era of HPV vaccination.

Juan C. Felix, MD
Professor of Clinical Pathology and Obstetrics and Gynecology; Director of Cytopathology fellowship; and Chief of Gynecologic Pathology at the Keck School of Medicine, University of Southern California; and Chief of Cytopathology at Los Angeles County and University of Southern California Medical Center in Los Angeles.
Dr. Felix reports that he is a speaker for Merck and GlaxoSmithKline.

Diane M. Harper, MD, MS, MPH
Director of the Gynecologic Cancer Prevention Research Group and Professor of Obstetrics and Gynecology, Community and family Medicine, and Informatics and Personalized Medicine at the University of Missouri–Kansas City School of Medicine.
Dr. Harper reports that she has served as a speaker and advisor for Merck and GlaxoSmithKline, and that the institutions at which she conducted HPV vaccination trials have received funding from Merck and GlaxoSmithKline.

Warner K. Huh, MD
Associate Professor in the Department of Obstetrics and Gynecology, and Associate Scientist at the Comprehensive Cancer Center at the University of Alabama– Birmingham.
Dr. Huh reports that he receives grant or research support from and is a speaker and consultant to Merck and GlaxoSmithKline.

Karen K. Smith-McCune, MD, PhD
John Kerner Endowed Chair of Gynecologic Oncology, Director of the Dysplasia Clinic, and Professor of Obstetrics, Gynecology, and Reproductive Sciences at the University of California–San francisco.
Dr. Smith-McCune reports she has performed unpaid consulting for OncoHealth Inc. and is planning to join its Scientific Advisory Board.
1. How were the vaccines developed?
Neal M. Lonky, MD, MPH: What should clinicians know about the development, function, and mechanism of action of the two HPV vaccines?
Warner K. Huh, MD: The bivalent and quadrivalent vaccines are both excellent products, and their respective Phase-3 trials demonstrate that they provide impressive protection against HPV, particularly among women who test negative (by polymerase chain reaction) for the specific HPV types contained within the vaccines.1-3
Cervarix protects against HPV types 16 and 18, whereas Gardasil is effective against HPV types 6, 11, 16, and 18.
Dr. Lonky: Do the vaccines function similarly?
Diane M. Harper, MD, MS, MPH: Yes. Both stimulate an immediate antibody response in the woman who is not infected with the relevant virus and are effective in preventing cervical intraepithelial neoplasia grade 2 and higher (CIN 2+), as well as persistent infection, caused by vaccine-related and cross-protected HPV types. The quality of the antibody response is best for HPV 16 for both vaccines. The quality of the antibody response for HPV 6, 11, and 18 for Gardasil is much poorer than its response for HPV 16. Cervarix induces an equally high and sustained antibody response for HPV 18 as for HPV 16.
Juan C. Felix, MD: Both vaccines are based on the same virus-like particles (VLP). The functionality of the vaccines is, therefore, mainly dependent on the dosage of VLP and the adjuvant used. Gardasil uses a proprietary aluminum sulfate adjuvant, whereas Cervarix uses aluminum hydroxide and monophosphoryl lipid A.
Karen K. Smith-McCune, MD, PhD: Both adjuvants have an extensive track record of safety and efficacy in other vaccines. Because they have different structures, however, they may have varying effects on many components of the immune response elicited by the L1 antigens.
Dr. Harper: Both adjuvants contain aluminum, which has so far proved to be safe despite the newly established association between high aluminum intake and Alzheimer’s disease.
Dr. Lonky: Were there any notable challenges in developing the vaccines?
Dr. Harper: It was difficult to formulate the appropriate dosages of VLP in Gardasil. Higher dosages of HPV 11 and 16 were needed to prevent cross-inhibition by HPV 6 and 18. As a result, the antigenic protein component of Gardasil that is necessary to effect an immunologic antibody response is high, at 120 μg. In Cervarix, the antigenic VLP load is 20 μg each for HPV 16 and 18.
Dr. Lonky: What is the significance of the different VLP loads?
Dr. Harper: Side effects, such as autoimmune neurologic demyelination, albeit rare, have been associated with a higher antigenic protein load. Multiple reports of autoimmune demyelinating diseases—including paralysis, blindness, and death—have been published by neurologists in regard to Gardasil.4,5 Others have shown that young girls are more at risk than young boys for these neurologic side effects.6
Dr. Felix: Some data suggest that the two vaccine formulations interact differently with the human immune system. In a head-to-head trial funded by GlaxoSmithKline, Cervarix produced higher total and neutralizing antibody titers than Gardasil did.7
Although higher immunogenicity is generally thought to be beneficial, the ultimate determinant of a vaccine’s success is its efficacy—and duration of that efficacy—in clinical trials and follow-up of vaccinated populations. So far, Cervarix has demonstrated efficacy through 8.4 years in its follow-up cohort.8 Similarly, Gardasil has proved to be effective after 5 years of follow-up, with no incident cases of cervical cancer reported in the vaccinated arm.9

2. Does either vaccine offer “extra” immunity?
Dr. Lonky: What is the potential for overlapping immunity to other high-risk viral types with these vaccines?
Dr. Harper: It is quite clear from pivotal trials of both vaccines that Gardasil produces efficacy of 46% against persistent infection caused by HPV 31. Data from the pivotal Phase-3 trial of Gardasil also show that it offers no protection against persistent infection with HPV 45, an important cause of adenocarcinoma.10
In contrast, Cervarix demonstrates substantial efficacy against both persistent infection and CIN 2+ disease caused by HPV 31, 33, and 45.3
These findings mean that Cervarix is 91% effective against HPV types that cause adenocarcinoma and 83% effective overall against squamous cell carcinoma. Compare that with Gardasil, which is 78% effective overall against HPV types that cause adenocarcinoma and 73% effective against HPV types that cause squamous cell carcinoma.
The immune titers tell a supportive story. After vaccination with Gardasil, the antibody titer immediately declines for HPV 6, 11, and 18, reaching the baseline for natural infection within 18 months.7 HPV 18 shows continued, significant loss of seropositivity over time, and antibody titers for HPV 6 and 11 also decline. In the monovalent HPV 16 pre-Gardasil experimental vaccine, 14% of women no longer had measurable titers to HPV 16 after 8.5 years.11
After vaccination with Cervarix, antibody titers for HPV 16 and 18 remain more than seven times and more than four times higher, respectively, than natural infection titers for 8.4 years, with no loss of measurable antibody titer for either type. The antibody titers for HPV 31, 33, and 45 remain substantially higher than natural infection titers for at least 6.4 years. These titers correlate with the vaccine’s very high efficacy against CIN 2+ lesions caused by HPV 16, 18, 31, 33, and 45.
In other words, Cervarix generates an immune response (and efficacy) that indicates robust protection against five of the most common oncogenic HPV types, providing maximal protection against nearly 85% of all cervical cancers. Gardasil protects against 74% of all cervical cancers overall.12 This makes Cervarix the superior cervical cancer vaccine.
Gardasil is the superior vaccine against genital warts, although the duration of its protection is uncertain.
Dr. Huh: I’d just like to point out that there are no head-to-head trials comparing the vaccines in terms of efficacy. Antibody titers are higher with Cervarix than with Gardasil, as you noted, and it may be that, over time, the higher titers are more durable with Cervarix. However, we have yet to fully correlate clinical efficacy with antibody titers. In other words, immunogenicity does not equal clinical efficacy.
Dr. Felix: The data for Gardasil are particularly interesting because there have been no incident HPV-18 lesions detected despite the absence of detectable HPV-18 antibody titers in more than 20% of vaccinated women as soon as 2 years after immunization.9 These data strongly suggest that it is not antibody titer alone that grants protection against HPV-induced lesions of the cervix.
Dr. Harper: This speaks to the difficulty of running a trial to ensure both enough participants and a sufficient attack rate of HPV 18 to cause new lesions to be detected in vaccinated women. In the relevant trial, there were only 112 vaccinated women—not nearly enough women to overcome the very low attack rate of HPV 18 in the trial population—and they were followed for 5 years.9 We cannot be sure that the lack of incident HPV-18 lesions in the vaccinated women is the result of efficacy.
Dr. Felix: As for overlapping immunity to HPV types not included in the vaccines, it has been described for both Cervarix and Gardasil. In the case of Cervarix, the manufacturer demonstrated unexpectedly high rates of protection against all CIN 2+ and CIN 3+ lesions—70% and 87%, respectively. These rates were too high to be explained by protection against types 16, 18, 31, and 45 alone. It is possible, therefore, that Cervarix may protect against other high-risk HPV types.13
Gardasil has proved to be effective against HPV types 31, 33, 52, and others.10 When total protection against CIN 2+ and CIN 3+ lesions is examined from Phase-3 trials of the vaccine, however, the rates are only 42% and 43%, respectively. These data are difficult to interpret because HPV 16 and 18 together are thought to account for 70% of CIN 3. Some reassurance can be gained from the fact that the number of incident cases of CIN 2+ and CIN 3+ caused by HPV 16 and 18 in the vaccinated group in the Gardasil trial was identical to the number seen in the Cervarix trial.3,10 The reason for the discrepancy in total number of cases of CIN 2+ and CIN 3+ between the two trials—and, therefore, between the two vaccines—cannot be explained by cross-protection alone and is probably attributable to differences in study populations. The Gardasil trial had a higher baseline prevalence of HPV 16 and 18 (9% and 4%, respectively) than the Cervarix trial did (5% and 2%, respectively), a fact that may be explained by the different demographics of their respective populations.2,14
Ultimately, it is hazardous to compare trials, particularly when they are conducted in significantly different populations. On this issue, I concur with the World Health Organization (WHO), which recommended that such comparisons be avoided in the determination of which type of HPV vaccine to recommend.15
Dr. Huh: I agree that it would be inappropriate to make cross-trial comparisons, given differences in the way the trials were designed and conducted. To draw conclusions about clinical efficacy of these two excellent vaccines, based on a comparison of their trials, is completely unscientific. Only a true head-to-head study that has efficacy as its endpoint can tell us which vaccine is superior—and such a trial would require thousands (if not tens of thousands) of subjects and a considerable amount of time to complete. In my opinion, such a study would be counterproductive to our goal of vaccination.
Dr. Harper: I disagree. The whole purpose of this roundtable is to compare vaccines. It is not “unscientific” to compare the trials.
Dr. Huh: On the contrary—it is completely inappropriate to directly compare the Phase-3 clinical trials from Merck and GlaxoSmithKline. One can speculate about the differences between them, but any clinical trialist knows that a direct, scientific comparison cannot be made. Only a real head-to-head study powered for efficacy can do this.
- Both the bivalent and quadrivalent vaccines appear to be excellent products. Besides protecting against the main oncogenic strains of human papillomavirus (HPV) (types 16 and 18 for both vaccines, and the genital-wart-associated strains 6 and 11 for the quadrivalent vaccine), both Cervarix and Gardasil offer some degree of cross-protection against additional HPV strains.
- Vaccination of the sexually naïve patient with either vaccine provides significant protection against cervical intraepithelial neoplasia 2 (CIN 2) or worse.
- HPV vaccination is expected to reduce the rate of abnormal Pap tests and the need for common excisional treatments for cervical dysplasia in vaccinated women. It will do the same in the population as a whole if rates of vaccination are sufficient to provide “herd” immunity.
3. Is one vaccine more effective than the other?
Dr. Lonky: How do the vaccines compare in terms of efficacy?
Dr. Smith-McCune: In discussing efficacy, I think we should focus on CIN 3 because it is the immediate surrogate for cancer, whereas CIN 2 lesions can be transient in younger women. I think it is also important to focus on outcomes regardless of the HPV types associated with the lesions. This approach is more clinically relevant, as we don’t perform HPV typing of lesions in clinical practice. Nor do we manage lesions differently depending on the HPV type in the lesion.
That said, it is difficult to compare efficacy of the vaccines for several reasons, a few of which we have already discussed. For example, the bivalent and quadrivalent vaccines were studied in separate randomized trials. Although the study populations were similar, they were not identical. Women in both trials were relatively sexually naïve, but the cutoff for number of lifetime sexual partners was different (5 for Gardasil versus 7 for Cervarix). In trials of Gardasil, women who had a history of abnormal cytology or genital warts were excluded. In trials of Cervarix, women who had a history of colposcopy were excluded. In Gardasil trials, approximately 3% of women were from the Asian Pacific, versus 34% in the Cervarix trials, and so on.3,16
The trials also had different protocols for referral to colposcopy, which would affect disease detection. And the length of follow-up differs between trials.3,9
Dr. Lonky: Can we draw any conclusions about efficacy?
Dr. Smith-McCune: Yes. The trials defined outcomes in several populations of participants. In addition to the overall population (called the “intention-to-treat population” in the Gardasil trials and the “total vaccinated cohort” in the Cervarix trials), the trials defined a subpopulation of women naïve to oncogenic HPV types to gain information about the likely impact of vaccinating girls before the onset of sexual activity. The definitions of these “naïve” populations were slightly different, mainly in the number of HPV types tested, so again, some caution needs to be exercised in making comparisons.
End-of-trial data in the naïve population show a 43% reduction in CIN 3 lesions for Gardasil and 87% for Cervarix (for CIN 3 or worse). By inference, we can tell the sexually naïve patient that vaccination with either vaccine will provide significant protection against CIN 3 lesions, likely to result in significant protection against cervical cancer over time.
We can gather some estimates of efficacy in sexually non-naïve women by looking at results from all trial participants. Gardasil reduced overall CIN 3 lesions by 16% overall; Cervarix reduced CIN 3 or worse by 33%. When counseling an individual patient, if she has had a similarly low number of lifetime sexual partners (e.g., the median number in the Gardasil trials was 2), these results provide an estimate of her likely protection against CIN 3 with vaccination.
Common excisional treatments for cervical dysplasia are known to be associated with adverse perinatal outcomes.17 The ability to reduce the need for these treatments is an important outcome of vaccination. In the HPV-naïve populations, vaccination reduced definitive cervical therapies or excisions by 42% (Gardasil) and 69% (Cervarix). These figures are useful in counseling virginal patients about the long-term benefits of vaccination.
For sexually active patients 26 years and younger, HPV vaccination significantly reduced definitive cervical therapy or excisions by 23% (Gardasil) and 25% (Cervarix). Again, these figures are most applicable for counseling patients who have had relatively few lifetime sexual partners. So the exact extent of protection is likely to vary by the patient’s total number of lifetime sexual partners.
I expect that we will see more data on the effects of vaccination stratified by the number of lifetime sexual partners, because that information would be very useful in counseling individual sexually active women.
Dr. Harper: Both vaccines reduce the rate of abnormal Pap tests by 10% regardless of HPV type in that population of women.9,18
4. Are the two vaccines safe?
Dr. Lonky: What about safety of the vaccines? What do we know?
Dr. Felix: The safety profiles seen in clinical trials of both vaccines are very similar and consist almost entirely of nonserious adverse events.2,9 In the United States, a greater number of Gardasil doses has been administered, owing to its earlier development. As of January 1, 2010, more than 28 million doses had been distributed, and numerous major events had been recorded in the Vaccine Adverse Event Reporting System (VAERS). Of 15,829 adverse events reported, only 8% were considered serious by the CDC. CDC investigation, by expert panels, of all serious adverse events found no evidence linking Gardasil to any of them, including Guillain-Barré syndrome, blood clots, and death.19
Dr. Huh: A few other points to consider:
- The reporting rate for Gardasil is triple that for all other vaccines combined
- Because VAERS is a passive reporting system, under-reporting is distinctly possible
- Post-licensure safety surveillance is still underway
- Both products have pregnancy registries.
Dr. Harper: The current postmarketing commitment between Merck and the FDA is to recognize a rate of serious adverse events that exceeds 2 cases in every 10,000 women in a cohort of 44,000 women who have received all three doses of Gardasil. Although autoimmune neurologic sequelae have occurred after Gardasil administration, regulatory authorities are not required to evaluate these reactions, such as Guillain-Barré syndrome, because the frequency is lower than the agreed-upon threshold. Nevertheless, adverse events could be life threatening to some girls.
Any risk of death—even if it is lower than the agreed-upon threshold—should be presented to women as a possible risk of vaccination with Gardasil. In the United States, the same women could choose a lifetime of Pap screening and be afforded the same protection against cervical cancer as they would get from vaccination.
5. Is quadrivalent better than bivalent?
Dr. Lonky: Why would a clinician choose a bivalent vaccine when the quadrivalent vaccine protects not only against carcinogenic types 16 and 18, but also against HPV-associated genital warts?
Dr. Harper: A smart clinician would ask the patient what she values. The physician is obligated to present the evidence and let her choose!
Dr. Lonky: What does the evidence suggest?
Dr. Felix: A clear recommendation between Cervarix and Gardasil is very difficult to make at this time, for the reasons already stated. Both vaccines provide 98% protection against HPV 16 and 18 for the prevention of CIN 2+ lesions.3,9 As we have discussed, both vaccines also provide protection against high-risk strains of HPV other than types 16 and 18.
In the Cervarix trial, there was an overall reduction of all CIN 2+ lesions that was higher as a percentage of total lesions than the reduction seen in the Gardasil trial.3,9 However, unlike Cervarix, Gardasil significantly reduced the rate of vaginal intraepithelial neoplasia (VaIN) and vulvar intraepithelial neoplasia (VIN).9
Dr. Harper: GlaxoSmithKline is analyzing its data on vulvar and vaginal protection, and it is likely that Cervarix will demonstrate some efficacy in this regard, too. But the economic burden of noncervical cancers is estimated to be only 8% of the economic burden of all HPV-related diseases.20 The prevention of cervical cancer is the dominant clinical and economic force for vaccination.
Dr. Felix: Clearly, the protection against genital warts demonstrated in the Gardasil trial will not be realized with Cervarix, as it does not offer immunization or cross-protection against HPV 6 or 11. Gardasil’s protection against HPV 6 and 11 prompted FDA approval of the vaccine for boys and men.21
According to the WHO, when counseling girls and women about the HPV vaccine, the clinician should weigh the possible value of a deep reduction in total CIN 2+ lesions provided by Cervarix against the reduction in VaIN, VIN, and genital warts provided by Gardasil.15 Boys and men will see clinically proven benefits only from Gardasil for the prevention of external genital warts. Other benefits are strictly theoretical.22,23
Dr. Harper: Vaccine protection must last at least 15 years to reduce the rate of cervical cancer. Otherwise, the development of cervical cancer will only be postponed, if boosters are not implemented.
It is now widely recognized that Cervarix induces high antibody titers, offering 100% efficacy even after 8.4 years, making it very likely that the protection it provides will continue for at least 15 years. It is also widely acknowledged by immunologists that Gardasil-induced titers for HPV 6, 11, and 18 are much shorter-lived, so protection is likely to wane 5 to 10 years after vaccination.
That means that Gardasil provides excellent protection against one cancer-causing type of HPV. In addition, it protects against genital warts caused by HPV types 6 and 11 for at least 5 years. In comparison, Cervarix protects against five cancer-causing types of HPV, thereby preventing about 90% of cervical cancers, and is likely to remain effective for at least 15 years.
There are 10 times as many women who have an abnormal Pap test as there are women who have genital warts, so one would think that Cervarix would be the vaccine of choice in preventing the life-threatening disease of cervical cancer.
Dr. Smith-McCune: I would agree that the choice should be discussed with patients—and with parents. If the objective is primarily to protect against cervical cancer precursors, then the bivalent vaccine may be the better choice, with the caveat that we can’t really compare the results from the vaccine trials for reasons discussed earlier, and there are no data from a randomized head-to-head trial comparing the two vaccines. If the decision involves a desire to reduce genital warts or vulvar and vaginal dysplasia, then the quadrivalent vaccine would be the better choice.
6. What impact do the vaccines have on screening?
Dr. Lonky: Do the vaccines have varying effects on our need to screen for, triage, and treat cervical cancer precursors?
Dr. Harper: The Pap smear has reduced the rate of cervical cancer in the United States by 75%—that rate is now at an all-time low of 8 cases for every 100,000 women. But the Pap smear is not perfect; there is a 30% false-negative rate among women who develop cervical cancer, and a large false-positive rate that involves referral to colposcopy for minimally abnormal cytology reports. And when CIN 2+ disease is detected, treatment is not without risk. Surgery increases the risk of reproductive morbidity in future pregnancies. Having protection against this outcome could be tremendously valuable for some women.
Compare the HPV vaccine, which has probable benefit but also the potential for serious adverse events, including demyelinating diseases that cause blindness, paralysis, and death in a small number of recipients.
If women were to choose to be vaccinated with Gardasil and forgo further Pap screening, the rate of cervical cancer in the United States would rise from 8 to 14 cases for every 100,000 women. If they were to choose Cervarix instead, with no further Pap screening, the rate would rise from 8 to 9.5 cases for every 100,000 women.
If women were to choose both HPV vaccination and continued Pap screening, the rate of cervical cancer still would not decline from its current level of 8 cases for every 100,000 women. Instead, the benefit would be that fewer women have abnormal Pap tests, and fewer women would need to be treated for CIN 2+ disease.
Women and physicians must understand these facts. A woman who chooses to be vaccinated may gain individual protection, but the overall rate of cervical cancer will not be affected.
Dr. Huh: Regardless of the HPV vaccine selected, we need to seriously rethink how we screen women in the United States. One could easily argue that the combination of the vaccine and continued screening is too expensive. It might be wise to consider lengthening the screening interval—and, perhaps, further delaying initial screening to 25 years of age—to make cervical cancer prevention with both modalities more cost-effective.24 The most important thing to recognize is that women still need to be screened, even if they have been vaccinated.
As more women are vaccinated, we expect to see a decline in the prevalence of CIN 2+ and CIN 3+ lesions, and this will ultimately weaken the positive predictive value of cytology. Perhaps it is time to consider the HPV test as a primary screen, with triage to cytology in women who test HPV-positive.25
Dr. Smith-McCune: As we accumulate data over time about the effects of vaccination on the rates of CIN 3 and cancer, modeling will be helpful in determining the best screening algorithm for women who have been vaccinated against HPV.
It is important to remember that approximately 50% of women who are given a diagnosis of cervical cancer in the United States have never been screened. It is vital that we continue to reach out to the under-screened population and focus vaccination efforts on populations of girls who are likely to have limited access to care in the future.
7. Can we vaccinate every woman?
Dr. Lonky: Is universal vaccination of women achievable for either vaccine?
Dr. Felix: Universal vaccination against HPV would be achievable only via school mandates. Without them, vaccination will not approach the 80% threshold needed to produce herd immunity.
Despite the clear benefit of such mandates to the general population—particularly the medically under-served—the issue has become a political football. As a result, school mandates will probably never be realized.
Dr. Harper: I don’t believe it is ethical to mandate vaccination of all girls and women. It is a choice that women and parents, in conversation with their physicians and daughters, must make when considering how to be protected against cervical cancer. Herd immunity is a moot point because we are only vaccinating girls (50% of the population) and can never reach the theoretical 70% threshold for herd immunity to be apparent.
Dr. Lonky: Is the availability of two vaccines a boon or a hindrance?
Dr. Smith-McCune: I think it is always a good thing to have choices in medicine.
Dr. Huh: I see the availability of two vaccines as a boon. That availability means that two companies are now putting forth consistent educational messages about the importance of vaccination and, I hope, stimulating competition that will reduce the overall cost of the vaccine series. Having two vaccines can only promote awareness, access, and greater appreciation of the considerable protection these two vaccines provide.
Despite solid evidence that the quadrivalent (Gardasil) HPV vaccine and the bivalent (Cervarix) HPV vaccine protect against cervical cancer, only about one fifth of the female population between 11 and 26 years of age has received the full series of Gardasil since it won FDA approval in 2006. Barriers to vaccination are not financial alone, as the vaccination rate is similarly low among women who have health insurance.
Why isn’t the vaccination rate higher? I see eight barriers to full implementation:
- Economic disparities. Each vaccine costs roughly $400 (national average) for the full series of injections. Although women who do not get Pap screening are most likely to benefit from the vaccines, they usually cannot afford them. federal childhood immunization programs cover teens and young women until 18 years of age in most states, and until 21 years in a few. that leaves most women who seek vaccination from gynecologists without coverage.
- Fear. Pain at the injection site, syncope, and a slightly elevated incidence of thromboembolism are the adverse events most commonly associated with HPV vaccination in the literature. In the life cycle of a vaccine, reports of sudden death or neurologic injury (Guillain-Barré syndrome) occur in the early years, but are reported at a rate lower than 2 cases in every 10,000 women. Nevertheless, such events may create fear about undergoing immunization.
- Long latency period. Because the outcome of cancer prevention won’t become apparent for 20 to 40 years following vaccination, the need for immunization may seem less than urgent.
- Cultural and religious beliefs. Because carcinogenic HPV strains are sexually transmitted, some families may associate vaccination with the promotion of sexual activity. Even in states that mandate vaccination, the courts have upheld a parent’s right to refuse vaccination on these grounds.
- The premarket push. Aggressive promotion of vaccination by both manufacturers and a push by advocates for legislation to mandate the vaccine prior to completion of Phase-3 trials and gathering of robust safety data may have diminished trust in the vaccine and reduced its acceptance.
- Lack of legislation. In states that do not consider HPV vaccination to be a necessary public health intervention, the lack of mandates and funding reduce the vaccination rate. In addition, some legislators have been more active advocates of vaccination than others.
- Reduced involvement of the obGyn. ObGyns don’t routinely vaccinate patients; pediatricians do. Young women are slipping through the cracks because the conventional ObGyn practice does not have a vaccination program that ensures payment, reimbursement, and completion of the vaccine series. Many ObGyn practices are reluctant to institute such a program because the profit margin is small, there are associated risks, and the time required to counsel the patient and for follow-up is extensive.
- Failure to complete the series. Some women do not complete the full vaccine series, owing to cost or side effects, or both. Solid evidence that a single dose could be as protective as the full series would be compelling. A single-dose vaccine would also be less expensive.
The principal danger of a low vaccination rate is the loss of insurance coverage for immunization against HPV. On one hand, payers may begin to ask whether coverage is justified when so few girls and women are vaccinated, leaving the payer with two burdens: the expense of vaccination and the expense of conventional screening programs and treatment, although the costs of treatment would be reduced with vaccination. On the other hand, Gardasil’s protection against genital warts may provide incentive for payers to cover or discount the vaccine because of the reduction in the need to diagnose, triage, and treat condyloma.
Ultimately, HPV vaccination may become another optional intervention that is paid for by the individual, despite evidence in girls and women that cervical cancer can be prevented.
—NEAL M. LONKY, MD, MPH
We want to hear from you! Tell us what you think.
Not long ago (in medical years), we were still trying to discover the cause of cervical cancer. Today, not only do we know that cause to be persistent human papillomavirus (HPV) infection, but we have two vaccines at our disposal to prevent the primary oncogenic strains of the virus.
We’ve come a long way.
The availability of two vaccines raises questions, however. What kind of data do we have on the bivalent (Cervarix, GlaxoSmithKline) and quadrivalent (Gardasil, Merck) vaccines so far? Is one of them clearly superior to the other? If not, what population is each vaccine best suited for—and how do we counsel patients about their options?
To address these and other questions, OBG Management Contributing Editor Neal M. Lonky, MD, MPH, assembled a panel of physicians who have expertise in cervical disease detection and prevention and asked them to sift the data that have accumulated thus far. In the discussion that follows, they touch on long-term efficacy, the likely impact of the vaccines on cervical cancer screening, and other aspects of disease prevention in the era of HPV vaccination.

Juan C. Felix, MD
Professor of Clinical Pathology and Obstetrics and Gynecology; Director of Cytopathology fellowship; and Chief of Gynecologic Pathology at the Keck School of Medicine, University of Southern California; and Chief of Cytopathology at Los Angeles County and University of Southern California Medical Center in Los Angeles.
Dr. Felix reports that he is a speaker for Merck and GlaxoSmithKline.

Diane M. Harper, MD, MS, MPH
Director of the Gynecologic Cancer Prevention Research Group and Professor of Obstetrics and Gynecology, Community and family Medicine, and Informatics and Personalized Medicine at the University of Missouri–Kansas City School of Medicine.
Dr. Harper reports that she has served as a speaker and advisor for Merck and GlaxoSmithKline, and that the institutions at which she conducted HPV vaccination trials have received funding from Merck and GlaxoSmithKline.

Warner K. Huh, MD
Associate Professor in the Department of Obstetrics and Gynecology, and Associate Scientist at the Comprehensive Cancer Center at the University of Alabama– Birmingham.
Dr. Huh reports that he receives grant or research support from and is a speaker and consultant to Merck and GlaxoSmithKline.

Karen K. Smith-McCune, MD, PhD
John Kerner Endowed Chair of Gynecologic Oncology, Director of the Dysplasia Clinic, and Professor of Obstetrics, Gynecology, and Reproductive Sciences at the University of California–San francisco.
Dr. Smith-McCune reports she has performed unpaid consulting for OncoHealth Inc. and is planning to join its Scientific Advisory Board.
1. How were the vaccines developed?
Neal M. Lonky, MD, MPH: What should clinicians know about the development, function, and mechanism of action of the two HPV vaccines?
Warner K. Huh, MD: The bivalent and quadrivalent vaccines are both excellent products, and their respective Phase-3 trials demonstrate that they provide impressive protection against HPV, particularly among women who test negative (by polymerase chain reaction) for the specific HPV types contained within the vaccines.1-3
Cervarix protects against HPV types 16 and 18, whereas Gardasil is effective against HPV types 6, 11, 16, and 18.
Dr. Lonky: Do the vaccines function similarly?
Diane M. Harper, MD, MS, MPH: Yes. Both stimulate an immediate antibody response in the woman who is not infected with the relevant virus and are effective in preventing cervical intraepithelial neoplasia grade 2 and higher (CIN 2+), as well as persistent infection, caused by vaccine-related and cross-protected HPV types. The quality of the antibody response is best for HPV 16 for both vaccines. The quality of the antibody response for HPV 6, 11, and 18 for Gardasil is much poorer than its response for HPV 16. Cervarix induces an equally high and sustained antibody response for HPV 18 as for HPV 16.
Juan C. Felix, MD: Both vaccines are based on the same virus-like particles (VLP). The functionality of the vaccines is, therefore, mainly dependent on the dosage of VLP and the adjuvant used. Gardasil uses a proprietary aluminum sulfate adjuvant, whereas Cervarix uses aluminum hydroxide and monophosphoryl lipid A.
Karen K. Smith-McCune, MD, PhD: Both adjuvants have an extensive track record of safety and efficacy in other vaccines. Because they have different structures, however, they may have varying effects on many components of the immune response elicited by the L1 antigens.
Dr. Harper: Both adjuvants contain aluminum, which has so far proved to be safe despite the newly established association between high aluminum intake and Alzheimer’s disease.
Dr. Lonky: Were there any notable challenges in developing the vaccines?
Dr. Harper: It was difficult to formulate the appropriate dosages of VLP in Gardasil. Higher dosages of HPV 11 and 16 were needed to prevent cross-inhibition by HPV 6 and 18. As a result, the antigenic protein component of Gardasil that is necessary to effect an immunologic antibody response is high, at 120 μg. In Cervarix, the antigenic VLP load is 20 μg each for HPV 16 and 18.
Dr. Lonky: What is the significance of the different VLP loads?
Dr. Harper: Side effects, such as autoimmune neurologic demyelination, albeit rare, have been associated with a higher antigenic protein load. Multiple reports of autoimmune demyelinating diseases—including paralysis, blindness, and death—have been published by neurologists in regard to Gardasil.4,5 Others have shown that young girls are more at risk than young boys for these neurologic side effects.6
Dr. Felix: Some data suggest that the two vaccine formulations interact differently with the human immune system. In a head-to-head trial funded by GlaxoSmithKline, Cervarix produced higher total and neutralizing antibody titers than Gardasil did.7
Although higher immunogenicity is generally thought to be beneficial, the ultimate determinant of a vaccine’s success is its efficacy—and duration of that efficacy—in clinical trials and follow-up of vaccinated populations. So far, Cervarix has demonstrated efficacy through 8.4 years in its follow-up cohort.8 Similarly, Gardasil has proved to be effective after 5 years of follow-up, with no incident cases of cervical cancer reported in the vaccinated arm.9

2. Does either vaccine offer “extra” immunity?
Dr. Lonky: What is the potential for overlapping immunity to other high-risk viral types with these vaccines?
Dr. Harper: It is quite clear from pivotal trials of both vaccines that Gardasil produces efficacy of 46% against persistent infection caused by HPV 31. Data from the pivotal Phase-3 trial of Gardasil also show that it offers no protection against persistent infection with HPV 45, an important cause of adenocarcinoma.10
In contrast, Cervarix demonstrates substantial efficacy against both persistent infection and CIN 2+ disease caused by HPV 31, 33, and 45.3
These findings mean that Cervarix is 91% effective against HPV types that cause adenocarcinoma and 83% effective overall against squamous cell carcinoma. Compare that with Gardasil, which is 78% effective overall against HPV types that cause adenocarcinoma and 73% effective against HPV types that cause squamous cell carcinoma.
The immune titers tell a supportive story. After vaccination with Gardasil, the antibody titer immediately declines for HPV 6, 11, and 18, reaching the baseline for natural infection within 18 months.7 HPV 18 shows continued, significant loss of seropositivity over time, and antibody titers for HPV 6 and 11 also decline. In the monovalent HPV 16 pre-Gardasil experimental vaccine, 14% of women no longer had measurable titers to HPV 16 after 8.5 years.11
After vaccination with Cervarix, antibody titers for HPV 16 and 18 remain more than seven times and more than four times higher, respectively, than natural infection titers for 8.4 years, with no loss of measurable antibody titer for either type. The antibody titers for HPV 31, 33, and 45 remain substantially higher than natural infection titers for at least 6.4 years. These titers correlate with the vaccine’s very high efficacy against CIN 2+ lesions caused by HPV 16, 18, 31, 33, and 45.
In other words, Cervarix generates an immune response (and efficacy) that indicates robust protection against five of the most common oncogenic HPV types, providing maximal protection against nearly 85% of all cervical cancers. Gardasil protects against 74% of all cervical cancers overall.12 This makes Cervarix the superior cervical cancer vaccine.
Gardasil is the superior vaccine against genital warts, although the duration of its protection is uncertain.
Dr. Huh: I’d just like to point out that there are no head-to-head trials comparing the vaccines in terms of efficacy. Antibody titers are higher with Cervarix than with Gardasil, as you noted, and it may be that, over time, the higher titers are more durable with Cervarix. However, we have yet to fully correlate clinical efficacy with antibody titers. In other words, immunogenicity does not equal clinical efficacy.
Dr. Felix: The data for Gardasil are particularly interesting because there have been no incident HPV-18 lesions detected despite the absence of detectable HPV-18 antibody titers in more than 20% of vaccinated women as soon as 2 years after immunization.9 These data strongly suggest that it is not antibody titer alone that grants protection against HPV-induced lesions of the cervix.
Dr. Harper: This speaks to the difficulty of running a trial to ensure both enough participants and a sufficient attack rate of HPV 18 to cause new lesions to be detected in vaccinated women. In the relevant trial, there were only 112 vaccinated women—not nearly enough women to overcome the very low attack rate of HPV 18 in the trial population—and they were followed for 5 years.9 We cannot be sure that the lack of incident HPV-18 lesions in the vaccinated women is the result of efficacy.
Dr. Felix: As for overlapping immunity to HPV types not included in the vaccines, it has been described for both Cervarix and Gardasil. In the case of Cervarix, the manufacturer demonstrated unexpectedly high rates of protection against all CIN 2+ and CIN 3+ lesions—70% and 87%, respectively. These rates were too high to be explained by protection against types 16, 18, 31, and 45 alone. It is possible, therefore, that Cervarix may protect against other high-risk HPV types.13
Gardasil has proved to be effective against HPV types 31, 33, 52, and others.10 When total protection against CIN 2+ and CIN 3+ lesions is examined from Phase-3 trials of the vaccine, however, the rates are only 42% and 43%, respectively. These data are difficult to interpret because HPV 16 and 18 together are thought to account for 70% of CIN 3. Some reassurance can be gained from the fact that the number of incident cases of CIN 2+ and CIN 3+ caused by HPV 16 and 18 in the vaccinated group in the Gardasil trial was identical to the number seen in the Cervarix trial.3,10 The reason for the discrepancy in total number of cases of CIN 2+ and CIN 3+ between the two trials—and, therefore, between the two vaccines—cannot be explained by cross-protection alone and is probably attributable to differences in study populations. The Gardasil trial had a higher baseline prevalence of HPV 16 and 18 (9% and 4%, respectively) than the Cervarix trial did (5% and 2%, respectively), a fact that may be explained by the different demographics of their respective populations.2,14
Ultimately, it is hazardous to compare trials, particularly when they are conducted in significantly different populations. On this issue, I concur with the World Health Organization (WHO), which recommended that such comparisons be avoided in the determination of which type of HPV vaccine to recommend.15
Dr. Huh: I agree that it would be inappropriate to make cross-trial comparisons, given differences in the way the trials were designed and conducted. To draw conclusions about clinical efficacy of these two excellent vaccines, based on a comparison of their trials, is completely unscientific. Only a true head-to-head study that has efficacy as its endpoint can tell us which vaccine is superior—and such a trial would require thousands (if not tens of thousands) of subjects and a considerable amount of time to complete. In my opinion, such a study would be counterproductive to our goal of vaccination.
Dr. Harper: I disagree. The whole purpose of this roundtable is to compare vaccines. It is not “unscientific” to compare the trials.
Dr. Huh: On the contrary—it is completely inappropriate to directly compare the Phase-3 clinical trials from Merck and GlaxoSmithKline. One can speculate about the differences between them, but any clinical trialist knows that a direct, scientific comparison cannot be made. Only a real head-to-head study powered for efficacy can do this.
- Both the bivalent and quadrivalent vaccines appear to be excellent products. Besides protecting against the main oncogenic strains of human papillomavirus (HPV) (types 16 and 18 for both vaccines, and the genital-wart-associated strains 6 and 11 for the quadrivalent vaccine), both Cervarix and Gardasil offer some degree of cross-protection against additional HPV strains.
- Vaccination of the sexually naïve patient with either vaccine provides significant protection against cervical intraepithelial neoplasia 2 (CIN 2) or worse.
- HPV vaccination is expected to reduce the rate of abnormal Pap tests and the need for common excisional treatments for cervical dysplasia in vaccinated women. It will do the same in the population as a whole if rates of vaccination are sufficient to provide “herd” immunity.
3. Is one vaccine more effective than the other?
Dr. Lonky: How do the vaccines compare in terms of efficacy?
Dr. Smith-McCune: In discussing efficacy, I think we should focus on CIN 3 because it is the immediate surrogate for cancer, whereas CIN 2 lesions can be transient in younger women. I think it is also important to focus on outcomes regardless of the HPV types associated with the lesions. This approach is more clinically relevant, as we don’t perform HPV typing of lesions in clinical practice. Nor do we manage lesions differently depending on the HPV type in the lesion.
That said, it is difficult to compare efficacy of the vaccines for several reasons, a few of which we have already discussed. For example, the bivalent and quadrivalent vaccines were studied in separate randomized trials. Although the study populations were similar, they were not identical. Women in both trials were relatively sexually naïve, but the cutoff for number of lifetime sexual partners was different (5 for Gardasil versus 7 for Cervarix). In trials of Gardasil, women who had a history of abnormal cytology or genital warts were excluded. In trials of Cervarix, women who had a history of colposcopy were excluded. In Gardasil trials, approximately 3% of women were from the Asian Pacific, versus 34% in the Cervarix trials, and so on.3,16
The trials also had different protocols for referral to colposcopy, which would affect disease detection. And the length of follow-up differs between trials.3,9
Dr. Lonky: Can we draw any conclusions about efficacy?
Dr. Smith-McCune: Yes. The trials defined outcomes in several populations of participants. In addition to the overall population (called the “intention-to-treat population” in the Gardasil trials and the “total vaccinated cohort” in the Cervarix trials), the trials defined a subpopulation of women naïve to oncogenic HPV types to gain information about the likely impact of vaccinating girls before the onset of sexual activity. The definitions of these “naïve” populations were slightly different, mainly in the number of HPV types tested, so again, some caution needs to be exercised in making comparisons.
End-of-trial data in the naïve population show a 43% reduction in CIN 3 lesions for Gardasil and 87% for Cervarix (for CIN 3 or worse). By inference, we can tell the sexually naïve patient that vaccination with either vaccine will provide significant protection against CIN 3 lesions, likely to result in significant protection against cervical cancer over time.
We can gather some estimates of efficacy in sexually non-naïve women by looking at results from all trial participants. Gardasil reduced overall CIN 3 lesions by 16% overall; Cervarix reduced CIN 3 or worse by 33%. When counseling an individual patient, if she has had a similarly low number of lifetime sexual partners (e.g., the median number in the Gardasil trials was 2), these results provide an estimate of her likely protection against CIN 3 with vaccination.
Common excisional treatments for cervical dysplasia are known to be associated with adverse perinatal outcomes.17 The ability to reduce the need for these treatments is an important outcome of vaccination. In the HPV-naïve populations, vaccination reduced definitive cervical therapies or excisions by 42% (Gardasil) and 69% (Cervarix). These figures are useful in counseling virginal patients about the long-term benefits of vaccination.
For sexually active patients 26 years and younger, HPV vaccination significantly reduced definitive cervical therapy or excisions by 23% (Gardasil) and 25% (Cervarix). Again, these figures are most applicable for counseling patients who have had relatively few lifetime sexual partners. So the exact extent of protection is likely to vary by the patient’s total number of lifetime sexual partners.
I expect that we will see more data on the effects of vaccination stratified by the number of lifetime sexual partners, because that information would be very useful in counseling individual sexually active women.
Dr. Harper: Both vaccines reduce the rate of abnormal Pap tests by 10% regardless of HPV type in that population of women.9,18
4. Are the two vaccines safe?
Dr. Lonky: What about safety of the vaccines? What do we know?
Dr. Felix: The safety profiles seen in clinical trials of both vaccines are very similar and consist almost entirely of nonserious adverse events.2,9 In the United States, a greater number of Gardasil doses has been administered, owing to its earlier development. As of January 1, 2010, more than 28 million doses had been distributed, and numerous major events had been recorded in the Vaccine Adverse Event Reporting System (VAERS). Of 15,829 adverse events reported, only 8% were considered serious by the CDC. CDC investigation, by expert panels, of all serious adverse events found no evidence linking Gardasil to any of them, including Guillain-Barré syndrome, blood clots, and death.19
Dr. Huh: A few other points to consider:
- The reporting rate for Gardasil is triple that for all other vaccines combined
- Because VAERS is a passive reporting system, under-reporting is distinctly possible
- Post-licensure safety surveillance is still underway
- Both products have pregnancy registries.
Dr. Harper: The current postmarketing commitment between Merck and the FDA is to recognize a rate of serious adverse events that exceeds 2 cases in every 10,000 women in a cohort of 44,000 women who have received all three doses of Gardasil. Although autoimmune neurologic sequelae have occurred after Gardasil administration, regulatory authorities are not required to evaluate these reactions, such as Guillain-Barré syndrome, because the frequency is lower than the agreed-upon threshold. Nevertheless, adverse events could be life threatening to some girls.
Any risk of death—even if it is lower than the agreed-upon threshold—should be presented to women as a possible risk of vaccination with Gardasil. In the United States, the same women could choose a lifetime of Pap screening and be afforded the same protection against cervical cancer as they would get from vaccination.
5. Is quadrivalent better than bivalent?
Dr. Lonky: Why would a clinician choose a bivalent vaccine when the quadrivalent vaccine protects not only against carcinogenic types 16 and 18, but also against HPV-associated genital warts?
Dr. Harper: A smart clinician would ask the patient what she values. The physician is obligated to present the evidence and let her choose!
Dr. Lonky: What does the evidence suggest?
Dr. Felix: A clear recommendation between Cervarix and Gardasil is very difficult to make at this time, for the reasons already stated. Both vaccines provide 98% protection against HPV 16 and 18 for the prevention of CIN 2+ lesions.3,9 As we have discussed, both vaccines also provide protection against high-risk strains of HPV other than types 16 and 18.
In the Cervarix trial, there was an overall reduction of all CIN 2+ lesions that was higher as a percentage of total lesions than the reduction seen in the Gardasil trial.3,9 However, unlike Cervarix, Gardasil significantly reduced the rate of vaginal intraepithelial neoplasia (VaIN) and vulvar intraepithelial neoplasia (VIN).9
Dr. Harper: GlaxoSmithKline is analyzing its data on vulvar and vaginal protection, and it is likely that Cervarix will demonstrate some efficacy in this regard, too. But the economic burden of noncervical cancers is estimated to be only 8% of the economic burden of all HPV-related diseases.20 The prevention of cervical cancer is the dominant clinical and economic force for vaccination.
Dr. Felix: Clearly, the protection against genital warts demonstrated in the Gardasil trial will not be realized with Cervarix, as it does not offer immunization or cross-protection against HPV 6 or 11. Gardasil’s protection against HPV 6 and 11 prompted FDA approval of the vaccine for boys and men.21
According to the WHO, when counseling girls and women about the HPV vaccine, the clinician should weigh the possible value of a deep reduction in total CIN 2+ lesions provided by Cervarix against the reduction in VaIN, VIN, and genital warts provided by Gardasil.15 Boys and men will see clinically proven benefits only from Gardasil for the prevention of external genital warts. Other benefits are strictly theoretical.22,23
Dr. Harper: Vaccine protection must last at least 15 years to reduce the rate of cervical cancer. Otherwise, the development of cervical cancer will only be postponed, if boosters are not implemented.
It is now widely recognized that Cervarix induces high antibody titers, offering 100% efficacy even after 8.4 years, making it very likely that the protection it provides will continue for at least 15 years. It is also widely acknowledged by immunologists that Gardasil-induced titers for HPV 6, 11, and 18 are much shorter-lived, so protection is likely to wane 5 to 10 years after vaccination.
That means that Gardasil provides excellent protection against one cancer-causing type of HPV. In addition, it protects against genital warts caused by HPV types 6 and 11 for at least 5 years. In comparison, Cervarix protects against five cancer-causing types of HPV, thereby preventing about 90% of cervical cancers, and is likely to remain effective for at least 15 years.
There are 10 times as many women who have an abnormal Pap test as there are women who have genital warts, so one would think that Cervarix would be the vaccine of choice in preventing the life-threatening disease of cervical cancer.
Dr. Smith-McCune: I would agree that the choice should be discussed with patients—and with parents. If the objective is primarily to protect against cervical cancer precursors, then the bivalent vaccine may be the better choice, with the caveat that we can’t really compare the results from the vaccine trials for reasons discussed earlier, and there are no data from a randomized head-to-head trial comparing the two vaccines. If the decision involves a desire to reduce genital warts or vulvar and vaginal dysplasia, then the quadrivalent vaccine would be the better choice.
6. What impact do the vaccines have on screening?
Dr. Lonky: Do the vaccines have varying effects on our need to screen for, triage, and treat cervical cancer precursors?
Dr. Harper: The Pap smear has reduced the rate of cervical cancer in the United States by 75%—that rate is now at an all-time low of 8 cases for every 100,000 women. But the Pap smear is not perfect; there is a 30% false-negative rate among women who develop cervical cancer, and a large false-positive rate that involves referral to colposcopy for minimally abnormal cytology reports. And when CIN 2+ disease is detected, treatment is not without risk. Surgery increases the risk of reproductive morbidity in future pregnancies. Having protection against this outcome could be tremendously valuable for some women.
Compare the HPV vaccine, which has probable benefit but also the potential for serious adverse events, including demyelinating diseases that cause blindness, paralysis, and death in a small number of recipients.
If women were to choose to be vaccinated with Gardasil and forgo further Pap screening, the rate of cervical cancer in the United States would rise from 8 to 14 cases for every 100,000 women. If they were to choose Cervarix instead, with no further Pap screening, the rate would rise from 8 to 9.5 cases for every 100,000 women.
If women were to choose both HPV vaccination and continued Pap screening, the rate of cervical cancer still would not decline from its current level of 8 cases for every 100,000 women. Instead, the benefit would be that fewer women have abnormal Pap tests, and fewer women would need to be treated for CIN 2+ disease.
Women and physicians must understand these facts. A woman who chooses to be vaccinated may gain individual protection, but the overall rate of cervical cancer will not be affected.
Dr. Huh: Regardless of the HPV vaccine selected, we need to seriously rethink how we screen women in the United States. One could easily argue that the combination of the vaccine and continued screening is too expensive. It might be wise to consider lengthening the screening interval—and, perhaps, further delaying initial screening to 25 years of age—to make cervical cancer prevention with both modalities more cost-effective.24 The most important thing to recognize is that women still need to be screened, even if they have been vaccinated.
As more women are vaccinated, we expect to see a decline in the prevalence of CIN 2+ and CIN 3+ lesions, and this will ultimately weaken the positive predictive value of cytology. Perhaps it is time to consider the HPV test as a primary screen, with triage to cytology in women who test HPV-positive.25
Dr. Smith-McCune: As we accumulate data over time about the effects of vaccination on the rates of CIN 3 and cancer, modeling will be helpful in determining the best screening algorithm for women who have been vaccinated against HPV.
It is important to remember that approximately 50% of women who are given a diagnosis of cervical cancer in the United States have never been screened. It is vital that we continue to reach out to the under-screened population and focus vaccination efforts on populations of girls who are likely to have limited access to care in the future.
7. Can we vaccinate every woman?
Dr. Lonky: Is universal vaccination of women achievable for either vaccine?
Dr. Felix: Universal vaccination against HPV would be achievable only via school mandates. Without them, vaccination will not approach the 80% threshold needed to produce herd immunity.
Despite the clear benefit of such mandates to the general population—particularly the medically under-served—the issue has become a political football. As a result, school mandates will probably never be realized.
Dr. Harper: I don’t believe it is ethical to mandate vaccination of all girls and women. It is a choice that women and parents, in conversation with their physicians and daughters, must make when considering how to be protected against cervical cancer. Herd immunity is a moot point because we are only vaccinating girls (50% of the population) and can never reach the theoretical 70% threshold for herd immunity to be apparent.
Dr. Lonky: Is the availability of two vaccines a boon or a hindrance?
Dr. Smith-McCune: I think it is always a good thing to have choices in medicine.
Dr. Huh: I see the availability of two vaccines as a boon. That availability means that two companies are now putting forth consistent educational messages about the importance of vaccination and, I hope, stimulating competition that will reduce the overall cost of the vaccine series. Having two vaccines can only promote awareness, access, and greater appreciation of the considerable protection these two vaccines provide.
Despite solid evidence that the quadrivalent (Gardasil) HPV vaccine and the bivalent (Cervarix) HPV vaccine protect against cervical cancer, only about one fifth of the female population between 11 and 26 years of age has received the full series of Gardasil since it won FDA approval in 2006. Barriers to vaccination are not financial alone, as the vaccination rate is similarly low among women who have health insurance.
Why isn’t the vaccination rate higher? I see eight barriers to full implementation:
- Economic disparities. Each vaccine costs roughly $400 (national average) for the full series of injections. Although women who do not get Pap screening are most likely to benefit from the vaccines, they usually cannot afford them. federal childhood immunization programs cover teens and young women until 18 years of age in most states, and until 21 years in a few. that leaves most women who seek vaccination from gynecologists without coverage.
- Fear. Pain at the injection site, syncope, and a slightly elevated incidence of thromboembolism are the adverse events most commonly associated with HPV vaccination in the literature. In the life cycle of a vaccine, reports of sudden death or neurologic injury (Guillain-Barré syndrome) occur in the early years, but are reported at a rate lower than 2 cases in every 10,000 women. Nevertheless, such events may create fear about undergoing immunization.
- Long latency period. Because the outcome of cancer prevention won’t become apparent for 20 to 40 years following vaccination, the need for immunization may seem less than urgent.
- Cultural and religious beliefs. Because carcinogenic HPV strains are sexually transmitted, some families may associate vaccination with the promotion of sexual activity. Even in states that mandate vaccination, the courts have upheld a parent’s right to refuse vaccination on these grounds.
- The premarket push. Aggressive promotion of vaccination by both manufacturers and a push by advocates for legislation to mandate the vaccine prior to completion of Phase-3 trials and gathering of robust safety data may have diminished trust in the vaccine and reduced its acceptance.
- Lack of legislation. In states that do not consider HPV vaccination to be a necessary public health intervention, the lack of mandates and funding reduce the vaccination rate. In addition, some legislators have been more active advocates of vaccination than others.
- Reduced involvement of the obGyn. ObGyns don’t routinely vaccinate patients; pediatricians do. Young women are slipping through the cracks because the conventional ObGyn practice does not have a vaccination program that ensures payment, reimbursement, and completion of the vaccine series. Many ObGyn practices are reluctant to institute such a program because the profit margin is small, there are associated risks, and the time required to counsel the patient and for follow-up is extensive.
- Failure to complete the series. Some women do not complete the full vaccine series, owing to cost or side effects, or both. Solid evidence that a single dose could be as protective as the full series would be compelling. A single-dose vaccine would also be less expensive.
The principal danger of a low vaccination rate is the loss of insurance coverage for immunization against HPV. On one hand, payers may begin to ask whether coverage is justified when so few girls and women are vaccinated, leaving the payer with two burdens: the expense of vaccination and the expense of conventional screening programs and treatment, although the costs of treatment would be reduced with vaccination. On the other hand, Gardasil’s protection against genital warts may provide incentive for payers to cover or discount the vaccine because of the reduction in the need to diagnose, triage, and treat condyloma.
Ultimately, HPV vaccination may become another optional intervention that is paid for by the individual, despite evidence in girls and women that cervical cancer can be prevented.
—NEAL M. LONKY, MD, MPH
We want to hear from you! Tell us what you think.
1. The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915-1927.
2. Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. HPV PATRICIA study group. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161-2170.
3. Paavonen J, Naud P, Salmeron J, et al. For the HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301-314.
4. DiMario FJ, Hajjar M, Ciesielski T. A 16-year-old girl with bilateral visual loss and left hemiparesis following an immunization against human papillomavirus. J Child Neurology. 2010;25(3):321-327.
5. Sutton I, Lahoria R, Tan I, Clouston P, Barnett M. CNS demyelination and quadrivalent HPV vaccination. Multiple Sclerosis. 2009;15(1):116-119.
6. Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10(5):338-349.
7. Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin. 2009;5(10):705-719.
8. GlaxoSmithKline Vaccine HPV-007 Study Group. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 8.4 years. Presented at: 28th Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID); May 4-8, 2010; Nice, France.
9. Muñoz N, Kjaer SK, Sigurdsson K, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102(5):325-339.
10. Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naïve women aged 16-26 years. J Infect Dis. 2009;199(7):926-935.
11. Rowhani-Rahbar A, Mao C, Hughes JP, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine. 2009;27(41):5612-5619.
12. Harper DM, Williams KB. Prophylactic HPV vaccines: current knowledge of impact on gynecologic premalignancies [published online ahead of print July 3, 2010]. Discovery Medicine. 2010;10(50).http://www.discoverymedicine.com/Diane-M-Harper/2010/07/03/prophylactichpv-vaccines-current-knowledge-of-impact-ongynecologic-premalignancies. Accessed July 13, 2010.
13. Cervarix [human papillomavirus bivalent (types 16 and 18) vaccine recombinant]. Food and Drug Administration. Initial US approval: 2009:1–12.
14. Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928-1943.
15. World Health Organization. Human papillomavirus vaccines: WHO position paper. Weekly Epidemiological Record. 2009;84(15):118-131.http://www.who.int/wer/2009/wer8415/en/index.html. Published April 10, 2009. Accessed July 13, 2010.
16. Ault KA. For Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on the risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369(9576):1861-1868.
17. Arbyn M, Kyrgiou M, Simoens C, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ. 2008;337:a1284.-Doi: 10.1136/bmj.a1284.
18. Paavonen J. For the HPV PATRICIA Study Group. Efficacy of HPV 16/18 ASO4-adjuvanted vaccine against abnormal cytology, colposcopy referrals, and cervical procedures. Abstract SS 4–1. Data presented at Cervical Cancer Prevention, EuroGin 2010. European Research Organisation on Genital Infection and Neoplasia (EUROGIN); February 17-20, 2010; Monte Carlo, Monaco.http://www.eurogin.com/2010/programoverview.html. Accessed July 13, 2010.
19. Centers for Disease Control and Prevention. Reports of health concerns following HPV vaccination. 2010;1-3.http://www.cdc.gov/vaccinesafety/Vaccines/HPV/gardasil.html. Published June 21, 2010. Accessed July 13, 2010.
20. Myers ER. The economic impact of HPV vaccines: not just cervical cancer. Am J Obstet Gynecol. 2008;198(5):487-488.
21. Gardasil [human papillomavirus quadrivalent (types 6, 11, 16, and 18) vaccine, recombinant]. Food and Drug Administration; 2009. Initial Approval 2006;1-26.
22. Olsson SE, Kjaer SK, Sigurdsson K, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin. 2009;5(10).
23. Pirotta M, Ung L, Stein A, et al. The psychosocial burden of human papillomavirus related disease and screening interventions. Sex Transm Infect. 2009;85(7):508-513.
24. Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359(8):821-832.
25. Cox JT. Is the HPV test effective as the primary screen for cervical cancer? Examining the Evidence. OBG Management. 2010;22(7):10-11.
1. The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915-1927.
2. Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. HPV PATRICIA study group. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161-2170.
3. Paavonen J, Naud P, Salmeron J, et al. For the HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301-314.
4. DiMario FJ, Hajjar M, Ciesielski T. A 16-year-old girl with bilateral visual loss and left hemiparesis following an immunization against human papillomavirus. J Child Neurology. 2010;25(3):321-327.
5. Sutton I, Lahoria R, Tan I, Clouston P, Barnett M. CNS demyelination and quadrivalent HPV vaccination. Multiple Sclerosis. 2009;15(1):116-119.
6. Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10(5):338-349.
7. Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin. 2009;5(10):705-719.
8. GlaxoSmithKline Vaccine HPV-007 Study Group. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 8.4 years. Presented at: 28th Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID); May 4-8, 2010; Nice, France.
9. Muñoz N, Kjaer SK, Sigurdsson K, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102(5):325-339.
10. Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naïve women aged 16-26 years. J Infect Dis. 2009;199(7):926-935.
11. Rowhani-Rahbar A, Mao C, Hughes JP, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine. 2009;27(41):5612-5619.
12. Harper DM, Williams KB. Prophylactic HPV vaccines: current knowledge of impact on gynecologic premalignancies [published online ahead of print July 3, 2010]. Discovery Medicine. 2010;10(50).http://www.discoverymedicine.com/Diane-M-Harper/2010/07/03/prophylactichpv-vaccines-current-knowledge-of-impact-ongynecologic-premalignancies. Accessed July 13, 2010.
13. Cervarix [human papillomavirus bivalent (types 16 and 18) vaccine recombinant]. Food and Drug Administration. Initial US approval: 2009:1–12.
14. Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928-1943.
15. World Health Organization. Human papillomavirus vaccines: WHO position paper. Weekly Epidemiological Record. 2009;84(15):118-131.http://www.who.int/wer/2009/wer8415/en/index.html. Published April 10, 2009. Accessed July 13, 2010.
16. Ault KA. For Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on the risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369(9576):1861-1868.
17. Arbyn M, Kyrgiou M, Simoens C, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ. 2008;337:a1284.-Doi: 10.1136/bmj.a1284.
18. Paavonen J. For the HPV PATRICIA Study Group. Efficacy of HPV 16/18 ASO4-adjuvanted vaccine against abnormal cytology, colposcopy referrals, and cervical procedures. Abstract SS 4–1. Data presented at Cervical Cancer Prevention, EuroGin 2010. European Research Organisation on Genital Infection and Neoplasia (EUROGIN); February 17-20, 2010; Monte Carlo, Monaco.http://www.eurogin.com/2010/programoverview.html. Accessed July 13, 2010.
19. Centers for Disease Control and Prevention. Reports of health concerns following HPV vaccination. 2010;1-3.http://www.cdc.gov/vaccinesafety/Vaccines/HPV/gardasil.html. Published June 21, 2010. Accessed July 13, 2010.
20. Myers ER. The economic impact of HPV vaccines: not just cervical cancer. Am J Obstet Gynecol. 2008;198(5):487-488.
21. Gardasil [human papillomavirus quadrivalent (types 6, 11, 16, and 18) vaccine, recombinant]. Food and Drug Administration; 2009. Initial Approval 2006;1-26.
22. Olsson SE, Kjaer SK, Sigurdsson K, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin. 2009;5(10).
23. Pirotta M, Ung L, Stein A, et al. The psychosocial burden of human papillomavirus related disease and screening interventions. Sex Transm Infect. 2009;85(7):508-513.
24. Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359(8):821-832.
25. Cox JT. Is the HPV test effective as the primary screen for cervical cancer? Examining the Evidence. OBG Management. 2010;22(7):10-11.
ROUNDTABLE PART 2 OF 2: Using mesh to repair prolapse: Averting, managing complications
Hear Dr Phillips discuss the key points of this series
Vaginal placement of mesh for the correction of pelvic organ prolapse is not an entirely benign procedure. As Mickey M. Karram, MD, and an expert panel discuss in this article—the second of a two-part series—complications secondary to mesh placement can be a challenge to correct and often make life miserable for patients who experience them. Here, these experts address mesh erosion, extrusion, and other serious complications; discuss ways to prevent them; and offer strategies for managing them when they arise.
In Part 1, which appeared in the January 2009 issue of OBG Management, the panel discussed the increasing use of mesh in prolapse repair—in particular, the proliferation of mesh kits.
How common is erosion?
DR. KARRAM: The literature seems to indicate that, even in the best of hands, there is an extrusion, or erosion, rate of between 5% and 17% when mesh is used. Would you agree with this statistic?
DR. LUCENTE: Not completely. The vaginal exposure rate can be as low as 2%, as reported by our center and others, when the mesh is properly placed below all histologic layers of the vaginal wall, as it is when it is “delivered” to the pelvis via the transabdominal route.1,2
At the other end of the scale, an exposure rate above 17% has been reported when mesh is improperly placed within the vaginal wall—that is, just below the mucosa, as some surgeons have described in the methodology section of their abstract or article.3,4

MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.

SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.

VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.

MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
We have found that complete, full-thickness dissection of the vaginal wall into the true pelvic space (vesicovaginal and rectovaginal), utilizing small vaginal incisions and limiting hysterectomy and the trimming of vaginal mucosa, can promote a very low vaginal-exposure rate.
DR. WALTERS: Some surgeons tell me that their own extrusion or erosion rate is lower than the published rate of 5% to 17%, but it is impossible to be certain of the long-term outcome in any patient unless she is followed carefully. The patient may consult another physician about her complications. The primary surgeon—even an expert—often does not know the actual mesh complication rate.
That said, I am sure that some surgeons are particularly adept at using mesh kits for prolapse repair, thereby keeping their mesh complication rate low. The 5% to 17% number is what most gynecologic surgeons should expect for their patients.
DR. RAZ: The complication rates are clearly underreported since very few centers of excellence report on complications and the majority of users don’t report them. Also, the reported complication rate concerns short-term erosion. I imagine that, as time passes and vaginal tissue becomes more atrophic, the incidence of erosion will increase.
Are simple measures enough to resolve erosion?
DR. KARRAM: There seems to be a general perception that most extrusions or erosions can be easily managed in the office by placing estrogen or trimming. In our experience, that approach has been successful in a minority of cases only.
What have you seen?
DR. WALTERS: At the Cleveland Clinic, as at most tertiary care referral centers, we often see the worst cases of extrusion or erosion related to mesh. Estrogen helps in some cases of simple mesh exposure, especially after sacrocolpopexy. If estrogen is going to be effective, however, the problem should clear up relatively quickly; if it isn’t effective after a month or two of therapy, estrogen is unlikely to ever be successful.
When it comes to related problems, such as ridges or strictures in the vagina, dyspareunia, penile pain with insertion, and vaginal burning pain, I have not found simple trimming and estrogen to be effective.
DR. KARRAM: It’s also unlikely that simple excision or placement of estrogen will be successful over the long term. When an extrusion or erosion occurs, we are generally seeing only the tip of the iceberg. That’s because mesh is placed in a certain plane. Although only part of the mesh may be exposed, the entire mesh is likely to be affected because it lies in the same plane.
Also, because of the special nature of vaginal flora, it is unlikely that a foreign body is going to be successfully managed by simple excision or placement of estrogen.
DR. LUCENTE: Management of vaginal exposure really depends on the size of the exposure, its location, and whether there is underlying infection or ischemia of host tissue. When the exposure is small (<1 cm in diameter) and in the midline, with the mesh lying flat below the plane of the vaginal wall, we have been very successful using a conservative approach.
However, even the tiniest of exposures needs to be surgically excised if it traverses the vaginal sulcus. Obviously, any mesh erosion into viscera such as the bladder and bowel also requires surgical intervention. Host-tissue factors always play a contributing role.
I also want to point out that the manner in which exposure is managed depends to some extent on whether the mesh was properly placed. Exposures that arise when mesh is implanted improperly are difficult to correct and usually require complete removal.
Although we, too, started off with an exposure rate around 8%, it is now very low, thanks to technical advancements.
DR. RAZ: A very small vaginal erosion of a mesh sling can sometimes be managed in the office by excision. The cases referred to our service generally involve more extensive areas of exposure that will not be resolved by local treatment.
Is risk of injury operator-dependent?
DR. KARRAM: We’re all seeing very severe complications secondary to mesh placement. Would each of you give your opinion as to whether the severe complications such as significant pain, dyspareunia, and injury of important structures are mostly technical or inherent to mesh placement. Would they happen in the best of hands?
DR. LUCENTE: The more severe complications, for the most part, are very much related to technique. Not that they cannot happen in the very best of hands, but they are extremely rare when technique is meticulous.
Over a 4-year period, after well over 1,000 transvaginal mesh surgeries at our center, we had no death, ICU admission, or transfusion, and our intraoperative complication rate was only 3%, most commonly involving simple cystotomy without long-term consequence. This compares very favorably to the nearly 12% complication rate reported recently in the CARE trial for abdominal sacral colpopexy.5
Our primary challenge today is preventing postoperative dyspareunia. Our rate of new-onset dyspareunia is approximately 3.5%. This complication is, I think, more likely to be related to the inherent material properties of mesh, such as elasticity and flexural rigidity, and to host-tissue response to the material itself.
DR. RAZ: I think that the majority of complications are operator-dependent. Thin dissection of the vaginal wall and unrecognized bladder, urethral, and vaginal perforation are the most common reasons for the complications. Mesh does not move after surgery; if there is a problem, it means that the mesh was misplaced.
Another problem is that industry, in an effort to sell more kits, is pushing physicians who are unfamiliar with the principles of pelvic reconstruction to perform this complex procedure. Repair of major vaginal prolapse is not a simple sling procedure.
In addition, there is a greater likelihood of complications in patients who have severe atrophic tissues. These patients should not be candidates for mesh reconstruction.
DR. WALTERS: Many of the complications that we see with mesh are certainly operator-dependent. For example, mesh that is placed under too much tension leaves the vagina tight and stiff, and mesh that is placed with ripples and ridges causes irregularities in the vagina that are often painful, especially during intercourse.
I do not believe that mesh “erodes” into the bladder, urethra, or rectum, but that it is placed there inadvertently and overlooked intraoperatively (FIGURES 1 and 2), Visceral erosion can occur if the primary surgeon made a cystotomy or proctotomy before proceeding with the mesh kit, and the mesh eventually wore through the repaired area.
There are also some problems that are inherent to mesh, and that occur even in the best hands and after surgeries that are performed very competently. Some mesh exposures are inevitable, as are some cases of dyspareunia and rare cases of vaginal burning and pain. In addition, I am seeing more de novo SUI [stress urinary incontinence] with anterior mesh kits. Although this is not really a complication, it does lead to dissatisfaction in patients and merits efforts to prevent it.
DR. KARRAM: Yes. With the current state of mesh, I believe pain and dyspareunia are almost inevitable in some cases.
DR. LUCENTE: Another problem that is currently underaddressed is scar plating along the surface of the mesh. Such plating forms more readily in the absence of mechanical movement or distention during the early stages of wound healing. To make a comparison, even the best reconstructive orthopedic surgeons cannot achieve optimal functional outcomes with an implant surgery without intense postoperative physical therapy, which may simply involve range of motion or movement.
Most everyone is familiar with the capsular fibrosis and contraction that develop around a breast implant if there isn’t immediate postoperative massaging of the breast tissue and implant during wound repair. I am confident that the rate of dyspareunia will decline over time if specialists in reconstructive pelvic surgery pay closer attention to optimizing vaginal length, preserving the cervix (in women with relatively shorter vaginal length), and ensuring optimal apical attachment (that is, above the ischial spine) in younger, sexually active patients.
DR. RAZ: I think it is the surgeon rather than the surgery who causes most complications. In its effort to sell kits, industry sometimes puts them in the hands of surgeons who are not well prepared for the task. This operation can be quite complex, and you cannot create a pelvic surgeon from a physician who is unfamiliar with the anatomy. If you cannot manage the potential complications, you should not perform this type of surgery.
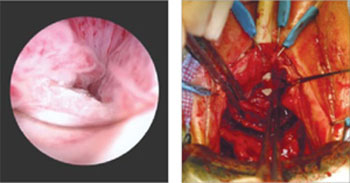
FIGURE 1 When mesh “erodes” into the urethra
Two images of mesh in the urethra. There is some uncertainty here whether mesh that has penetrated the urethra eroded through vaginal tissue or was placed there inadvertently and overlooked intraoperatively.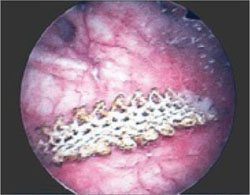
FIGURE 2 Mesh in the bladder
A segment of tension-free vaginal tape has penetrated into the bladder.
Should mesh be removed at the time of injury?
DR. KARRAM: As we discuss specific complications, let’s start with the most severe, which I would say relate to the inadvertent placement of mesh through important structures such as bowel, bladder, or ureters. If this were to happen and be diagnosed intraoperatively, what would you recommend that the surgeon do—abort the procedure or simply remove the mesh or trocar and attempt to pass it again safely?
DR. LUCENTE: That is a difficult question to answer because so much depends on various intraoperative factors.
I am much more comfortable proceeding with surgery after intraoperative bladder injury than after bowel or rectal injury. We have successfully corrected cystotomies that were small, did not encroach on the ureter, and were easily repaired without tension—and we have seen no fistula formation as a result.
The key is to maintain a high index of suspicion throughout the procedure. We have always diagnosed injuries before mesh is delivered—either during dissection or during passage of the needle or trocar. We have not experienced any ureteral injuries aside from “kinking” of one ureter, which was easily corrected with simple readjustment of the mesh.
If, at any time, we were concerned about potential infection, fistula, or a more severe complication that would be aggravated by proceeding with the operation, we would abort the procedure. However, we would be likely to proceed with an alternative operation to address the pelvic-support defect so that the patient would not awaken with intraoperative injury and no surgical treatment for her primary complaint.
We conduct informed consent in such a way as to preserve our flexibility to adapt the surgical plan to execute the reparative work that is necessary despite the development of a non–life-threatening complication during surgery. In the event of any injury to the bowel that would involve gross spillage of fecal material, of course, I would abort placement of synthetic mesh.
DR. WALTERS: If I placed one of the trocars through the bladder or bowel, I would probably remove it, reposition it, and continue with the surgery. With bladder perforation, this approach is generally no problem, but I would usually leave a Foley catheter in place for 1 week of continuous bladder drainage.
If I placed the trocar through the rectum, I would probably oversew the proctotomy, irrigate the space, and continue with the mesh repair. If I had an outright laceration in the bladder or rectum as part of the dissection, I would repair it and consider converting the surgery to prolapse repair without mesh.
The most dreaded complication: the foreshortened vagina
DR. KARRAM: It would seem that the most difficult complication to deal with is the foreshortened, firm, painful vagina. A patient who has these problems may be perceived, at times, as a pelvic “cripple.” Is this an accepted, albeit rare, complication? Or can it be avoided?
DR. LUCENTE: This is the most feared complication arising from the use of synthetic mesh. I do believe it can almost always be avoided—but I never say never. The key is to pay full attention to considerations of vaginal length before surgery, including, first, preservation of the cervix, and, second, placing the mesh loosely, properly sized, and attached with optimization of apical support to preserve vaginal length.
I also believe that use of second-generation meshes that are lighter, more elastic, and more flexible helps reduce this complication when the mesh is properly placed by a surgeon well trained in the technique.
When the vagina is foreshortened, the sooner it is revised, the better the chance that pain will resolve, whether the mesh is removed or released.
DR. RAZ: Mesh infection, capsular formation, dissection of a thin vaginal wall, and excess vaginal-wall excision lead to the short, firm, and painful vagina. The use and abuse of mesh has created a new subspecialty to manage mesh complications. The PFS syndrome (painful, firm, and short vagina) is one of the most difficult complications to treat because, in many cases, it cannot be reversed without major surgery.
DR. WALTERS: Women who have a foreshortened, firm, or painful vagina after mesh augmentation almost always need to have the mesh removed with reconstruction of the vaginal canal. I have never seen a successful outcome in this type of patient without complete or near-complete removal of the mesh.
1. van Raalte H, Lucente V, Haff R, Murphy M. Prolift: an innovative delivery system for transvaginal placement of synthetic grafts for the repair of pelvic organ prolapse. J Pelvic Med Surg .2007;13:351-360.
2. Murphy M, Raders JL, Haff R, Yeager M, Lucente V. Early U.S. experience with vaginal extraperitoneal colpopexy using propylene graft (Prolift) for the treatment of pelvic organ prolapse. J Pelvic Med Surg .2006;12:104-105.
3. Nguyen JM, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111:891-898.
4. Nieminen K, Hiltunen R, Heiskanen E, et al. Symptom resolution and sexual function after anterior vaginal wall repair with or without polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1611-1616.
5. Brubaker L, Nygaard I, Richter HE, et al. Two-year outcomes after sacrocolpopexy with and without Burch to prevent stress urinary incontinence. Obstet Gynecol. 2008;112:49-55.
Hear Dr Phillips discuss the key points of this series
Vaginal placement of mesh for the correction of pelvic organ prolapse is not an entirely benign procedure. As Mickey M. Karram, MD, and an expert panel discuss in this article—the second of a two-part series—complications secondary to mesh placement can be a challenge to correct and often make life miserable for patients who experience them. Here, these experts address mesh erosion, extrusion, and other serious complications; discuss ways to prevent them; and offer strategies for managing them when they arise.
In Part 1, which appeared in the January 2009 issue of OBG Management, the panel discussed the increasing use of mesh in prolapse repair—in particular, the proliferation of mesh kits.
How common is erosion?
DR. KARRAM: The literature seems to indicate that, even in the best of hands, there is an extrusion, or erosion, rate of between 5% and 17% when mesh is used. Would you agree with this statistic?
DR. LUCENTE: Not completely. The vaginal exposure rate can be as low as 2%, as reported by our center and others, when the mesh is properly placed below all histologic layers of the vaginal wall, as it is when it is “delivered” to the pelvis via the transabdominal route.1,2
At the other end of the scale, an exposure rate above 17% has been reported when mesh is improperly placed within the vaginal wall—that is, just below the mucosa, as some surgeons have described in the methodology section of their abstract or article.3,4

MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.

SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.

VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.

MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
We have found that complete, full-thickness dissection of the vaginal wall into the true pelvic space (vesicovaginal and rectovaginal), utilizing small vaginal incisions and limiting hysterectomy and the trimming of vaginal mucosa, can promote a very low vaginal-exposure rate.
DR. WALTERS: Some surgeons tell me that their own extrusion or erosion rate is lower than the published rate of 5% to 17%, but it is impossible to be certain of the long-term outcome in any patient unless she is followed carefully. The patient may consult another physician about her complications. The primary surgeon—even an expert—often does not know the actual mesh complication rate.
That said, I am sure that some surgeons are particularly adept at using mesh kits for prolapse repair, thereby keeping their mesh complication rate low. The 5% to 17% number is what most gynecologic surgeons should expect for their patients.
DR. RAZ: The complication rates are clearly underreported since very few centers of excellence report on complications and the majority of users don’t report them. Also, the reported complication rate concerns short-term erosion. I imagine that, as time passes and vaginal tissue becomes more atrophic, the incidence of erosion will increase.
Are simple measures enough to resolve erosion?
DR. KARRAM: There seems to be a general perception that most extrusions or erosions can be easily managed in the office by placing estrogen or trimming. In our experience, that approach has been successful in a minority of cases only.
What have you seen?
DR. WALTERS: At the Cleveland Clinic, as at most tertiary care referral centers, we often see the worst cases of extrusion or erosion related to mesh. Estrogen helps in some cases of simple mesh exposure, especially after sacrocolpopexy. If estrogen is going to be effective, however, the problem should clear up relatively quickly; if it isn’t effective after a month or two of therapy, estrogen is unlikely to ever be successful.
When it comes to related problems, such as ridges or strictures in the vagina, dyspareunia, penile pain with insertion, and vaginal burning pain, I have not found simple trimming and estrogen to be effective.
DR. KARRAM: It’s also unlikely that simple excision or placement of estrogen will be successful over the long term. When an extrusion or erosion occurs, we are generally seeing only the tip of the iceberg. That’s because mesh is placed in a certain plane. Although only part of the mesh may be exposed, the entire mesh is likely to be affected because it lies in the same plane.
Also, because of the special nature of vaginal flora, it is unlikely that a foreign body is going to be successfully managed by simple excision or placement of estrogen.
DR. LUCENTE: Management of vaginal exposure really depends on the size of the exposure, its location, and whether there is underlying infection or ischemia of host tissue. When the exposure is small (<1 cm in diameter) and in the midline, with the mesh lying flat below the plane of the vaginal wall, we have been very successful using a conservative approach.
However, even the tiniest of exposures needs to be surgically excised if it traverses the vaginal sulcus. Obviously, any mesh erosion into viscera such as the bladder and bowel also requires surgical intervention. Host-tissue factors always play a contributing role.
I also want to point out that the manner in which exposure is managed depends to some extent on whether the mesh was properly placed. Exposures that arise when mesh is implanted improperly are difficult to correct and usually require complete removal.
Although we, too, started off with an exposure rate around 8%, it is now very low, thanks to technical advancements.
DR. RAZ: A very small vaginal erosion of a mesh sling can sometimes be managed in the office by excision. The cases referred to our service generally involve more extensive areas of exposure that will not be resolved by local treatment.
Is risk of injury operator-dependent?
DR. KARRAM: We’re all seeing very severe complications secondary to mesh placement. Would each of you give your opinion as to whether the severe complications such as significant pain, dyspareunia, and injury of important structures are mostly technical or inherent to mesh placement. Would they happen in the best of hands?
DR. LUCENTE: The more severe complications, for the most part, are very much related to technique. Not that they cannot happen in the very best of hands, but they are extremely rare when technique is meticulous.
Over a 4-year period, after well over 1,000 transvaginal mesh surgeries at our center, we had no death, ICU admission, or transfusion, and our intraoperative complication rate was only 3%, most commonly involving simple cystotomy without long-term consequence. This compares very favorably to the nearly 12% complication rate reported recently in the CARE trial for abdominal sacral colpopexy.5
Our primary challenge today is preventing postoperative dyspareunia. Our rate of new-onset dyspareunia is approximately 3.5%. This complication is, I think, more likely to be related to the inherent material properties of mesh, such as elasticity and flexural rigidity, and to host-tissue response to the material itself.
DR. RAZ: I think that the majority of complications are operator-dependent. Thin dissection of the vaginal wall and unrecognized bladder, urethral, and vaginal perforation are the most common reasons for the complications. Mesh does not move after surgery; if there is a problem, it means that the mesh was misplaced.
Another problem is that industry, in an effort to sell more kits, is pushing physicians who are unfamiliar with the principles of pelvic reconstruction to perform this complex procedure. Repair of major vaginal prolapse is not a simple sling procedure.
In addition, there is a greater likelihood of complications in patients who have severe atrophic tissues. These patients should not be candidates for mesh reconstruction.
DR. WALTERS: Many of the complications that we see with mesh are certainly operator-dependent. For example, mesh that is placed under too much tension leaves the vagina tight and stiff, and mesh that is placed with ripples and ridges causes irregularities in the vagina that are often painful, especially during intercourse.
I do not believe that mesh “erodes” into the bladder, urethra, or rectum, but that it is placed there inadvertently and overlooked intraoperatively (FIGURES 1 and 2), Visceral erosion can occur if the primary surgeon made a cystotomy or proctotomy before proceeding with the mesh kit, and the mesh eventually wore through the repaired area.
There are also some problems that are inherent to mesh, and that occur even in the best hands and after surgeries that are performed very competently. Some mesh exposures are inevitable, as are some cases of dyspareunia and rare cases of vaginal burning and pain. In addition, I am seeing more de novo SUI [stress urinary incontinence] with anterior mesh kits. Although this is not really a complication, it does lead to dissatisfaction in patients and merits efforts to prevent it.
DR. KARRAM: Yes. With the current state of mesh, I believe pain and dyspareunia are almost inevitable in some cases.
DR. LUCENTE: Another problem that is currently underaddressed is scar plating along the surface of the mesh. Such plating forms more readily in the absence of mechanical movement or distention during the early stages of wound healing. To make a comparison, even the best reconstructive orthopedic surgeons cannot achieve optimal functional outcomes with an implant surgery without intense postoperative physical therapy, which may simply involve range of motion or movement.
Most everyone is familiar with the capsular fibrosis and contraction that develop around a breast implant if there isn’t immediate postoperative massaging of the breast tissue and implant during wound repair. I am confident that the rate of dyspareunia will decline over time if specialists in reconstructive pelvic surgery pay closer attention to optimizing vaginal length, preserving the cervix (in women with relatively shorter vaginal length), and ensuring optimal apical attachment (that is, above the ischial spine) in younger, sexually active patients.
DR. RAZ: I think it is the surgeon rather than the surgery who causes most complications. In its effort to sell kits, industry sometimes puts them in the hands of surgeons who are not well prepared for the task. This operation can be quite complex, and you cannot create a pelvic surgeon from a physician who is unfamiliar with the anatomy. If you cannot manage the potential complications, you should not perform this type of surgery.

FIGURE 1 When mesh “erodes” into the urethra
Two images of mesh in the urethra. There is some uncertainty here whether mesh that has penetrated the urethra eroded through vaginal tissue or was placed there inadvertently and overlooked intraoperatively.
FIGURE 2 Mesh in the bladder
A segment of tension-free vaginal tape has penetrated into the bladder.
Should mesh be removed at the time of injury?
DR. KARRAM: As we discuss specific complications, let’s start with the most severe, which I would say relate to the inadvertent placement of mesh through important structures such as bowel, bladder, or ureters. If this were to happen and be diagnosed intraoperatively, what would you recommend that the surgeon do—abort the procedure or simply remove the mesh or trocar and attempt to pass it again safely?
DR. LUCENTE: That is a difficult question to answer because so much depends on various intraoperative factors.
I am much more comfortable proceeding with surgery after intraoperative bladder injury than after bowel or rectal injury. We have successfully corrected cystotomies that were small, did not encroach on the ureter, and were easily repaired without tension—and we have seen no fistula formation as a result.
The key is to maintain a high index of suspicion throughout the procedure. We have always diagnosed injuries before mesh is delivered—either during dissection or during passage of the needle or trocar. We have not experienced any ureteral injuries aside from “kinking” of one ureter, which was easily corrected with simple readjustment of the mesh.
If, at any time, we were concerned about potential infection, fistula, or a more severe complication that would be aggravated by proceeding with the operation, we would abort the procedure. However, we would be likely to proceed with an alternative operation to address the pelvic-support defect so that the patient would not awaken with intraoperative injury and no surgical treatment for her primary complaint.
We conduct informed consent in such a way as to preserve our flexibility to adapt the surgical plan to execute the reparative work that is necessary despite the development of a non–life-threatening complication during surgery. In the event of any injury to the bowel that would involve gross spillage of fecal material, of course, I would abort placement of synthetic mesh.
DR. WALTERS: If I placed one of the trocars through the bladder or bowel, I would probably remove it, reposition it, and continue with the surgery. With bladder perforation, this approach is generally no problem, but I would usually leave a Foley catheter in place for 1 week of continuous bladder drainage.
If I placed the trocar through the rectum, I would probably oversew the proctotomy, irrigate the space, and continue with the mesh repair. If I had an outright laceration in the bladder or rectum as part of the dissection, I would repair it and consider converting the surgery to prolapse repair without mesh.
The most dreaded complication: the foreshortened vagina
DR. KARRAM: It would seem that the most difficult complication to deal with is the foreshortened, firm, painful vagina. A patient who has these problems may be perceived, at times, as a pelvic “cripple.” Is this an accepted, albeit rare, complication? Or can it be avoided?
DR. LUCENTE: This is the most feared complication arising from the use of synthetic mesh. I do believe it can almost always be avoided—but I never say never. The key is to pay full attention to considerations of vaginal length before surgery, including, first, preservation of the cervix, and, second, placing the mesh loosely, properly sized, and attached with optimization of apical support to preserve vaginal length.
I also believe that use of second-generation meshes that are lighter, more elastic, and more flexible helps reduce this complication when the mesh is properly placed by a surgeon well trained in the technique.
When the vagina is foreshortened, the sooner it is revised, the better the chance that pain will resolve, whether the mesh is removed or released.
DR. RAZ: Mesh infection, capsular formation, dissection of a thin vaginal wall, and excess vaginal-wall excision lead to the short, firm, and painful vagina. The use and abuse of mesh has created a new subspecialty to manage mesh complications. The PFS syndrome (painful, firm, and short vagina) is one of the most difficult complications to treat because, in many cases, it cannot be reversed without major surgery.
DR. WALTERS: Women who have a foreshortened, firm, or painful vagina after mesh augmentation almost always need to have the mesh removed with reconstruction of the vaginal canal. I have never seen a successful outcome in this type of patient without complete or near-complete removal of the mesh.
Hear Dr Phillips discuss the key points of this series
Vaginal placement of mesh for the correction of pelvic organ prolapse is not an entirely benign procedure. As Mickey M. Karram, MD, and an expert panel discuss in this article—the second of a two-part series—complications secondary to mesh placement can be a challenge to correct and often make life miserable for patients who experience them. Here, these experts address mesh erosion, extrusion, and other serious complications; discuss ways to prevent them; and offer strategies for managing them when they arise.
In Part 1, which appeared in the January 2009 issue of OBG Management, the panel discussed the increasing use of mesh in prolapse repair—in particular, the proliferation of mesh kits.
How common is erosion?
DR. KARRAM: The literature seems to indicate that, even in the best of hands, there is an extrusion, or erosion, rate of between 5% and 17% when mesh is used. Would you agree with this statistic?
DR. LUCENTE: Not completely. The vaginal exposure rate can be as low as 2%, as reported by our center and others, when the mesh is properly placed below all histologic layers of the vaginal wall, as it is when it is “delivered” to the pelvis via the transabdominal route.1,2
At the other end of the scale, an exposure rate above 17% has been reported when mesh is improperly placed within the vaginal wall—that is, just below the mucosa, as some surgeons have described in the methodology section of their abstract or article.3,4

MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.

SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.

VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.

MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
We have found that complete, full-thickness dissection of the vaginal wall into the true pelvic space (vesicovaginal and rectovaginal), utilizing small vaginal incisions and limiting hysterectomy and the trimming of vaginal mucosa, can promote a very low vaginal-exposure rate.
DR. WALTERS: Some surgeons tell me that their own extrusion or erosion rate is lower than the published rate of 5% to 17%, but it is impossible to be certain of the long-term outcome in any patient unless she is followed carefully. The patient may consult another physician about her complications. The primary surgeon—even an expert—often does not know the actual mesh complication rate.
That said, I am sure that some surgeons are particularly adept at using mesh kits for prolapse repair, thereby keeping their mesh complication rate low. The 5% to 17% number is what most gynecologic surgeons should expect for their patients.
DR. RAZ: The complication rates are clearly underreported since very few centers of excellence report on complications and the majority of users don’t report them. Also, the reported complication rate concerns short-term erosion. I imagine that, as time passes and vaginal tissue becomes more atrophic, the incidence of erosion will increase.
Are simple measures enough to resolve erosion?
DR. KARRAM: There seems to be a general perception that most extrusions or erosions can be easily managed in the office by placing estrogen or trimming. In our experience, that approach has been successful in a minority of cases only.
What have you seen?
DR. WALTERS: At the Cleveland Clinic, as at most tertiary care referral centers, we often see the worst cases of extrusion or erosion related to mesh. Estrogen helps in some cases of simple mesh exposure, especially after sacrocolpopexy. If estrogen is going to be effective, however, the problem should clear up relatively quickly; if it isn’t effective after a month or two of therapy, estrogen is unlikely to ever be successful.
When it comes to related problems, such as ridges or strictures in the vagina, dyspareunia, penile pain with insertion, and vaginal burning pain, I have not found simple trimming and estrogen to be effective.
DR. KARRAM: It’s also unlikely that simple excision or placement of estrogen will be successful over the long term. When an extrusion or erosion occurs, we are generally seeing only the tip of the iceberg. That’s because mesh is placed in a certain plane. Although only part of the mesh may be exposed, the entire mesh is likely to be affected because it lies in the same plane.
Also, because of the special nature of vaginal flora, it is unlikely that a foreign body is going to be successfully managed by simple excision or placement of estrogen.
DR. LUCENTE: Management of vaginal exposure really depends on the size of the exposure, its location, and whether there is underlying infection or ischemia of host tissue. When the exposure is small (<1 cm in diameter) and in the midline, with the mesh lying flat below the plane of the vaginal wall, we have been very successful using a conservative approach.
However, even the tiniest of exposures needs to be surgically excised if it traverses the vaginal sulcus. Obviously, any mesh erosion into viscera such as the bladder and bowel also requires surgical intervention. Host-tissue factors always play a contributing role.
I also want to point out that the manner in which exposure is managed depends to some extent on whether the mesh was properly placed. Exposures that arise when mesh is implanted improperly are difficult to correct and usually require complete removal.
Although we, too, started off with an exposure rate around 8%, it is now very low, thanks to technical advancements.
DR. RAZ: A very small vaginal erosion of a mesh sling can sometimes be managed in the office by excision. The cases referred to our service generally involve more extensive areas of exposure that will not be resolved by local treatment.
Is risk of injury operator-dependent?
DR. KARRAM: We’re all seeing very severe complications secondary to mesh placement. Would each of you give your opinion as to whether the severe complications such as significant pain, dyspareunia, and injury of important structures are mostly technical or inherent to mesh placement. Would they happen in the best of hands?
DR. LUCENTE: The more severe complications, for the most part, are very much related to technique. Not that they cannot happen in the very best of hands, but they are extremely rare when technique is meticulous.
Over a 4-year period, after well over 1,000 transvaginal mesh surgeries at our center, we had no death, ICU admission, or transfusion, and our intraoperative complication rate was only 3%, most commonly involving simple cystotomy without long-term consequence. This compares very favorably to the nearly 12% complication rate reported recently in the CARE trial for abdominal sacral colpopexy.5
Our primary challenge today is preventing postoperative dyspareunia. Our rate of new-onset dyspareunia is approximately 3.5%. This complication is, I think, more likely to be related to the inherent material properties of mesh, such as elasticity and flexural rigidity, and to host-tissue response to the material itself.
DR. RAZ: I think that the majority of complications are operator-dependent. Thin dissection of the vaginal wall and unrecognized bladder, urethral, and vaginal perforation are the most common reasons for the complications. Mesh does not move after surgery; if there is a problem, it means that the mesh was misplaced.
Another problem is that industry, in an effort to sell more kits, is pushing physicians who are unfamiliar with the principles of pelvic reconstruction to perform this complex procedure. Repair of major vaginal prolapse is not a simple sling procedure.
In addition, there is a greater likelihood of complications in patients who have severe atrophic tissues. These patients should not be candidates for mesh reconstruction.
DR. WALTERS: Many of the complications that we see with mesh are certainly operator-dependent. For example, mesh that is placed under too much tension leaves the vagina tight and stiff, and mesh that is placed with ripples and ridges causes irregularities in the vagina that are often painful, especially during intercourse.
I do not believe that mesh “erodes” into the bladder, urethra, or rectum, but that it is placed there inadvertently and overlooked intraoperatively (FIGURES 1 and 2), Visceral erosion can occur if the primary surgeon made a cystotomy or proctotomy before proceeding with the mesh kit, and the mesh eventually wore through the repaired area.
There are also some problems that are inherent to mesh, and that occur even in the best hands and after surgeries that are performed very competently. Some mesh exposures are inevitable, as are some cases of dyspareunia and rare cases of vaginal burning and pain. In addition, I am seeing more de novo SUI [stress urinary incontinence] with anterior mesh kits. Although this is not really a complication, it does lead to dissatisfaction in patients and merits efforts to prevent it.
DR. KARRAM: Yes. With the current state of mesh, I believe pain and dyspareunia are almost inevitable in some cases.
DR. LUCENTE: Another problem that is currently underaddressed is scar plating along the surface of the mesh. Such plating forms more readily in the absence of mechanical movement or distention during the early stages of wound healing. To make a comparison, even the best reconstructive orthopedic surgeons cannot achieve optimal functional outcomes with an implant surgery without intense postoperative physical therapy, which may simply involve range of motion or movement.
Most everyone is familiar with the capsular fibrosis and contraction that develop around a breast implant if there isn’t immediate postoperative massaging of the breast tissue and implant during wound repair. I am confident that the rate of dyspareunia will decline over time if specialists in reconstructive pelvic surgery pay closer attention to optimizing vaginal length, preserving the cervix (in women with relatively shorter vaginal length), and ensuring optimal apical attachment (that is, above the ischial spine) in younger, sexually active patients.
DR. RAZ: I think it is the surgeon rather than the surgery who causes most complications. In its effort to sell kits, industry sometimes puts them in the hands of surgeons who are not well prepared for the task. This operation can be quite complex, and you cannot create a pelvic surgeon from a physician who is unfamiliar with the anatomy. If you cannot manage the potential complications, you should not perform this type of surgery.

FIGURE 1 When mesh “erodes” into the urethra
Two images of mesh in the urethra. There is some uncertainty here whether mesh that has penetrated the urethra eroded through vaginal tissue or was placed there inadvertently and overlooked intraoperatively.
FIGURE 2 Mesh in the bladder
A segment of tension-free vaginal tape has penetrated into the bladder.
Should mesh be removed at the time of injury?
DR. KARRAM: As we discuss specific complications, let’s start with the most severe, which I would say relate to the inadvertent placement of mesh through important structures such as bowel, bladder, or ureters. If this were to happen and be diagnosed intraoperatively, what would you recommend that the surgeon do—abort the procedure or simply remove the mesh or trocar and attempt to pass it again safely?
DR. LUCENTE: That is a difficult question to answer because so much depends on various intraoperative factors.
I am much more comfortable proceeding with surgery after intraoperative bladder injury than after bowel or rectal injury. We have successfully corrected cystotomies that were small, did not encroach on the ureter, and were easily repaired without tension—and we have seen no fistula formation as a result.
The key is to maintain a high index of suspicion throughout the procedure. We have always diagnosed injuries before mesh is delivered—either during dissection or during passage of the needle or trocar. We have not experienced any ureteral injuries aside from “kinking” of one ureter, which was easily corrected with simple readjustment of the mesh.
If, at any time, we were concerned about potential infection, fistula, or a more severe complication that would be aggravated by proceeding with the operation, we would abort the procedure. However, we would be likely to proceed with an alternative operation to address the pelvic-support defect so that the patient would not awaken with intraoperative injury and no surgical treatment for her primary complaint.
We conduct informed consent in such a way as to preserve our flexibility to adapt the surgical plan to execute the reparative work that is necessary despite the development of a non–life-threatening complication during surgery. In the event of any injury to the bowel that would involve gross spillage of fecal material, of course, I would abort placement of synthetic mesh.
DR. WALTERS: If I placed one of the trocars through the bladder or bowel, I would probably remove it, reposition it, and continue with the surgery. With bladder perforation, this approach is generally no problem, but I would usually leave a Foley catheter in place for 1 week of continuous bladder drainage.
If I placed the trocar through the rectum, I would probably oversew the proctotomy, irrigate the space, and continue with the mesh repair. If I had an outright laceration in the bladder or rectum as part of the dissection, I would repair it and consider converting the surgery to prolapse repair without mesh.
The most dreaded complication: the foreshortened vagina
DR. KARRAM: It would seem that the most difficult complication to deal with is the foreshortened, firm, painful vagina. A patient who has these problems may be perceived, at times, as a pelvic “cripple.” Is this an accepted, albeit rare, complication? Or can it be avoided?
DR. LUCENTE: This is the most feared complication arising from the use of synthetic mesh. I do believe it can almost always be avoided—but I never say never. The key is to pay full attention to considerations of vaginal length before surgery, including, first, preservation of the cervix, and, second, placing the mesh loosely, properly sized, and attached with optimization of apical support to preserve vaginal length.
I also believe that use of second-generation meshes that are lighter, more elastic, and more flexible helps reduce this complication when the mesh is properly placed by a surgeon well trained in the technique.
When the vagina is foreshortened, the sooner it is revised, the better the chance that pain will resolve, whether the mesh is removed or released.
DR. RAZ: Mesh infection, capsular formation, dissection of a thin vaginal wall, and excess vaginal-wall excision lead to the short, firm, and painful vagina. The use and abuse of mesh has created a new subspecialty to manage mesh complications. The PFS syndrome (painful, firm, and short vagina) is one of the most difficult complications to treat because, in many cases, it cannot be reversed without major surgery.
DR. WALTERS: Women who have a foreshortened, firm, or painful vagina after mesh augmentation almost always need to have the mesh removed with reconstruction of the vaginal canal. I have never seen a successful outcome in this type of patient without complete or near-complete removal of the mesh.
1. van Raalte H, Lucente V, Haff R, Murphy M. Prolift: an innovative delivery system for transvaginal placement of synthetic grafts for the repair of pelvic organ prolapse. J Pelvic Med Surg .2007;13:351-360.
2. Murphy M, Raders JL, Haff R, Yeager M, Lucente V. Early U.S. experience with vaginal extraperitoneal colpopexy using propylene graft (Prolift) for the treatment of pelvic organ prolapse. J Pelvic Med Surg .2006;12:104-105.
3. Nguyen JM, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111:891-898.
4. Nieminen K, Hiltunen R, Heiskanen E, et al. Symptom resolution and sexual function after anterior vaginal wall repair with or without polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1611-1616.
5. Brubaker L, Nygaard I, Richter HE, et al. Two-year outcomes after sacrocolpopexy with and without Burch to prevent stress urinary incontinence. Obstet Gynecol. 2008;112:49-55.
1. van Raalte H, Lucente V, Haff R, Murphy M. Prolift: an innovative delivery system for transvaginal placement of synthetic grafts for the repair of pelvic organ prolapse. J Pelvic Med Surg .2007;13:351-360.
2. Murphy M, Raders JL, Haff R, Yeager M, Lucente V. Early U.S. experience with vaginal extraperitoneal colpopexy using propylene graft (Prolift) for the treatment of pelvic organ prolapse. J Pelvic Med Surg .2006;12:104-105.
3. Nguyen JM, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111:891-898.
4. Nieminen K, Hiltunen R, Heiskanen E, et al. Symptom resolution and sexual function after anterior vaginal wall repair with or without polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1611-1616.
5. Brubaker L, Nygaard I, Richter HE, et al. Two-year outcomes after sacrocolpopexy with and without Burch to prevent stress urinary incontinence. Obstet Gynecol. 2008;112:49-55.
ROUNDTABLE: PART 1 OF 2: Using mesh to repair prolapse calls for more than a kit—it takes skill
MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.
SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.
VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.
MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
Mesh kits for repairing prolapse are proliferating like crazy, just as they did for midurethral sling procedures. But mesh augmentation of prolapse surgeries requires more than a prepackaged assortment of tools and materials. In this article, moderator Mickey M. Karram, MD, and a panel of nationally recognized urogynecologists and urologists describe the literature on mesh augmentation and discuss indications, contraindications, techniques, applicable cases, and the considerable training required.
In Part 2, which will appear in the February issue of OBG Management, the panel tackles the thorny topic of complications, including erosion, extrusion, foreshortening of the vagina, dyspareunia, and pain. Their discussion focuses on ways to avoid these problems, and methods for correcting them.
Do we have enough data?
DR. KARRAM: To start, let’s quickly review the peer-reviewed literature on the use of mesh augmentation during surgery for pelvic organ prolapse.
DR. WALTERS: Until recently, most data concerned open abdominal sacrocolpopexy (ASC) using polypropylene or Merseline mesh. There is significant clinical experience with this operation, and multiple cohort studies show long-term cure rates of 78% to 100% for apical prolapse.1
At least two randomized controlled trials have compared open ASC with sutured vaginal colpopexy procedures, and ASC is certainly equal to—perhaps better than—all transvaginal sutured repairs.2,3
With ASC, most recurrences affect the distal half of the vagina and involve one or more of the following:
- anterior or posterior vaginal wall prolapse (or both)
- stress urinary incontinence (SUI)
- distal rectocele.1,3
Mesh erosion occurs in 3.4% of cases and is usually easily managed.1 Other complications, including bowel injury, tend to be related to access, regardless of whether the operation is performed via laparotomy or laparoscopy.
Robotic sacrocolpopexy has become popular in recent years, and we will probably see data on this approach as we gain experience.
When it comes to vaginal mesh kits, the peer-reviewed literature is just beginning to expand, with many studies being presented at international meetings. For anterior and, possibly, apical vaginal prolapse, the cure rate after use of a mesh kit appears to be as high as, or higher than, the rate for sutured repairs.4 This high rate of anatomic cure is balanced somewhat by additional cost and complications involving mesh and the kits.
For posterior vaginal wall prolapse and rectocele, I firmly believe, based on our research and that of others, that sutured repairs are superior to graft-augmented surgery.5
DR. KARRAM: What are the indications and contraindications for mesh augmentation of prolapse repair ( FIGURES 1 and 2 )?
DR. LUCENTE: I believe mesh is indicated in any patient in need of surgical repair of pelvic organ prolapse who is seeking optimal durability and is willing to accept the known risks of the surgery.
The issue becomes more complex when it comes to contraindications. Absolute contraindications are fairly obvious; they include medically unstable patients and those who may have an inactive infectious process within the pelvis or even undiagnosed abnormal uterine bleeding.
At our center, because the potential for dyspareunia and pelvic discomfort is our biggest concern, we have developed a profile of the patient who is more likely to develop these complaints. The profile includes any patient who has a chronic pain disorder of any type, but especially chronic pelvic pain disorders such as endometriosis and vulvodynia. Other risk factors appear to be a history of pelvic surgery involving any permanent material, suture or mesh, and young age.
So if we have a patient in her late 30s who has undergone reconstructive surgery using permanent sutures and who has an element of chronic pelvic pain, we would counsel her strongly to consider surgical options other than the use of synthetic mesh.
DR. WALTERS: The main indications for mesh-augmented prolapse repair are recurrent posthysterectomy vaginal vault prolapse, for which I usually perform ASC, and recurrent cystocele or anteriorapical prolapse, for which I use one of the anterior mesh kits.
I still think sutured repairs—by that, I mean uterosacral ligament or sacrospinous colpopexy with sutured rectocele repair—work best for recurrent posterior wall and posteriorapical prolapse. I don’t use mesh augmentation for rectocele.
The main contraindication to mesh augmentation, as I see it, is a history of mesh complications. If I am repairing a mesh complication such as erosion or pain, I do not place another mesh.
Medical issues that might increase mesh complications, such as diabetes, steroid use, or severe vaginal atrophy, would, at the very least, make me consider carefully whether mesh augmentation is appropriate. The literature is not clear on this, so mesh could still be used if the surgeon thinks it is necessary.
DR. KARRAM: I haven’t found a definitive indication for mesh augmentation. We have used biologic meshes empirically, but I am not convinced that they really add long-term durability, regardless of whether they are used in the anterior or posterior vaginal segment.
Our published durability rate for traditional suture-type repairs is in the range of 85% at 5 years out.6 Even if I assumed that mesh would give me 100% 5-year durability, this rate would have to be at the expense of some erosion, pain, and other complications unique to mesh. I do not think that the potential improvement in durability is worth these potential complications.
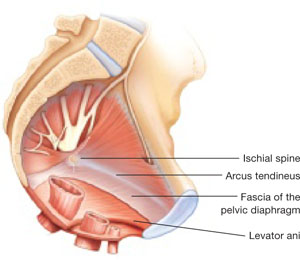
FIGURE 1 When the pelvic support system is intact, prolapse is rare
In the normal pelvis, organs are supported by a complex web of muscles, fascia, and connective tissue. 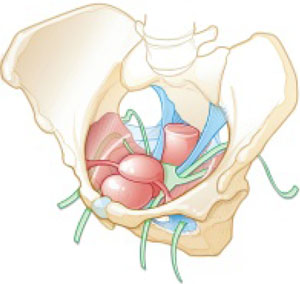
FIGURE 2 Mesh augmentation seeks to enhance the durability of repair
One type of mesh in final position. Mesh-augmented repair restores the vaginal apex and lends support to the walls of the vagina.
DR. KARRAM: If you are doing a lot of mesh repairs, you are obviously content with the results and feel that the few complications you are seeing are outweighed by the advantages mesh confers. How do you avoid extrusion and avert creation of a painful vagina?
DR. RAZ: Most of our cases are recurrent prolapse after failed vaginal or abdominal repair. I am indeed using a significant amount of soft polypropylene mesh for reconstructive procedures. As with the use of any other synthetic material, low-grade infection can develop after a few weeks or months. I use copious irrigation with antibiotic solution during reconstruction.
To avoid extrusion, I perform deep, rather than superficial, dissection of the vaginal wall to allow for better coverage of the mesh. For posterior mesh reconstruction, I cover the mesh with pararectal fascia to prevent erosion.
For mesh-augmented procedures, I cut the mesh myself in the operating room ( FIGURE 3 ). For a sling, I use a 10 cm × 1 cm soft polypropylene mesh. For a grade 3 or 4 cystocele, I use a trapezoid of soft polypropylene mesh with several points of fixation:
- at the sacrouterine ligament
- lateral to the obturator fascia
- distal to the bladder neck.
I always repair the vault at the same time.
For vault prolapse, I use a segment of soft polypropylene mesh in the shape of an apron with two arms (1 cm × 4 cm) and a central segment (4 cm × 7 cm). I support the vault using number 1-0 delayed absorbable suture and mesh. From outside the vaginal wall, in the posterolateral deep vaginal wall (inside the peritoneum), I incorporate the origin of the sacrouterine ligament and one arm of the mesh in the groove between the colon and levator ani, 15 cm from the introitus. I bring the suture 1 cm from the original entrance. A separate set of sutures brings the perirectal fascia together with the sacrouterine ligaments and perivesical fascia to close the peritoneal cavity. I tie the vault-suspension sutures, providing support to the cuff in a high posterior position (12 to 15 cm from the introitus).
In selected cases of significant recurrent rectocele, I use a rectangle of soft polypropylene mesh anchored to the origin of the sacrouterine ligament and distal to the perineal membrane. The mesh is covered by the pararectal fascia.
We have not seen vaginal, urethral, or bladder erosion in 1,800 cases of our distal urethral Prolene sling procedure using 10 cm × 1 cm soft mesh. In patients who have significant cystocele, vault prolapse, and recurrent rectocele, our vaginal erosion rate is 3%. We have never encountered rectal, bladder, or bowel perforation using our technique.
DR. LUCENTE: We often use mesh and are more than simply content with our results—we are extremely pleased, and so are our patients. Having said that, our techniques have definitely evolved over the past few years, as we’ve focused on how to decrease exposure and, more recently, optimize sexual function and vaginal comfort.
First, to avoid exposure, the most critical step is precise hydrodissection and distention of the true vesicovaginal space. This step can only be achieved through careful tactile guidance of the needle tip into the space, where it should remain while hydrodissection is performed. Always remember, sharp dissection “follows” hydrodissection. If you place the needle bevel within the vaginal wall, you will “split” the vaginal wall—as during standard colporrhaphy—which will lead to a high exposure rate.
Second, to avoid dyspareunia, it’s essential to pay close attention to POP-Q measurements, especially vaginal length, to ensure that the reconstruction restores the same length without foreshortening. This approach entails leaving the cervix in most patients who have a shorter vagina, and making sure that the mesh is secured above the ischial spine in younger, sexually active patients who have demonstrated a higher risk of postoperative deep, penetrating dyspareunia, compared with older, less sexually active patients.
Also paramount is to ensure that you have manually displaced the vagina inwardly as much as possible before deploying or setting the mesh. If you simply try to suture secure the mesh with the vagina incised open, without the ability to deploy the mesh with a closed, displaced vagina (to mimic deep penetration), it is difficult, if not impossible, to properly set the mesh for optimal comfort.
In the early days of midurethral pubovaginal slings using polypropylene, the adage was “looser is better than tighter.” This is even truer for transvaginal mesh.
DR. KARRAM: Dr. Walters, please describe your current surgical procedure of choice without mesh and explain why you haven’t adopted mesh for routine repairs.
DR. WALTERS: About 20% of my prolapse surgeries—usually for posthysterectomy or recurrent vaginal vault prolapse—involve ASC with placement of polypropylene mesh. I perform most of these cases through a Pfannenstiel incision, but I’ve also done them laparoscopically. Several of my partners perform ASC laparoscopically and robotically.
For the other 80% of my patients who have prolapse, I perform repairs transvaginally, usually using high bilateral uterosacralligament vaginal-vault suspension. We have learned to suture higher and slightly more medial on the uterosacral ligaments to attain greater vaginal depth and minimize ureteral obstruction. We use two or three sutures on each uterosacral ligament, usually a combination of permanent and delayed absorbable sutures.
I am also performing more sacrospinous ligament suspensions because this operation is being studied by the Pelvic Floor Disorders Network. Properly performed, it is an excellent surgery for apical prolapse. But, as with most of our surgeries for prolapse, recurrent anterior wall prolapse remains a problem.
Like you, Dr. Karram, we’ve studied our group’s anatomic and functional outcomes very carefully for more than 10 years and are mostly satisfied with our cure and complication rates. Although our anatomic outcomes with these surgeries are not always perfect, our reoperation rate for prolapse is only about 5%, with a high level of satisfaction in 88% to 92% of patients.
DR. RAZ: Unaugmented reconstruction fails in more than 30% of cases. Some patients who have significant prolapse and attenuated tissue think that this tissue will become healthier or stronger after reconstructive surgery, but that isn’t the case. In these situations, excision and plication make no clinical sense.
The problem is that we have yet to identify the ideal surrogate for poor-quality tissue. Most of us use polypropylene mesh in different variations. We need a better material that will be nonimmunogenic, well tolerated, and easily incorporated without erosion. Xenograft-like derivatives of dermis, or allografts such as cadaveric fascia, have failed over the long term because the body reabsorbs the graft without forming any new connective tissue.
FIGURE 3 Mesh can be cut in the OR to custom-fit a patient
Hand-cut mesh and points of placement.
PHOTO: SHLOMO RAZ, MD
Is a kit a valuable aid?
DR. KARRAM: If a surgeon wants to augment a repair, what are the advantages of a packaged mesh kit, compared with simply cutting the mesh and performing surgery without a kit?
DR. WALTERS: The advantages of a packaged mesh kit are the convenience involved and the ability to consistently perform the same operation with the same product. That facilitates learning, teaching, and research. It also helps us understand the published literature a little better because “custom” prolapse repairs are operator-dependent and difficult to apply generally to a population of surgeons.
These advantages are most clearly apparent with midurethral sling mesh kits, which have almost revolutionized surgery for stress incontinence. I don’t believe mesh kits for prolapse are there yet, but they certainly have potential.
DR. RAZ: I’m opposed to the use of kits. They are industry-driven. One company has made $1 billion selling them. Imagine a patient who undergoes placement of a sling kit ($1,000), cystocele kit ($1,500), and posterior mesh kit ($1,500). How can our healthcare system sustain this burden, especially when there is no real evidence that a kit improves the operation, and given the incredible complication rate that we see?
Moreover, the kits contain a single-use needle and passer and a precut segment of polypropylene mesh. But every patient is different and requires a unique size or shape of mesh. I don’t believe that a surgeon who knows pelvic anatomy needs a kit to perform mesh-augmented reconstruction. We can buy the same segment of mesh for $200 to $400, cut it as needed, and perform the same operation advertised by industry.
For surgeons who prefer a kit, the tools that are included should be made reusable.
DR. LUCENTE: In my opinion, the primary advantage of a commercially available transvaginal mesh delivery system—notice, I avoided the word “kit,” because I think there are plenty of negative connotations associated with it—is the ability to deliver the mesh in a “tension”-free manner.
One alternative that many people pursue is cutting the mesh to size and using sutures to hold it in place while tissue ingrowth occurs. However, the hernia literature suggests that suturing mesh in place increases the risk of postoperative discomfort at the site of implantation. The true cause of the discomfort remains unclear, but it is thought to arise from nerve tethering or traction at the pre-committed points of attachment before the host tissue and mesh interface have adjusted or settled with tissue ingrowth.
All neuropathic complications of mesh implantation have been shown in the current hernia literature to be increased with the use of sutures.7 Also, as previously mentioned, it is extremely difficult to set or adjust the mesh with the vaginal incision remaining “open,” which is a downside to suture techniques.
What training is necessary to use a kit?
DR. KARRAM: Mesh kits are aggressively promoted by industry, with close to half a dozen different kits to be available soon. What is the minimum amount of training one should have before utilizing these kits?
DR. WALTERS: The surgeon should at least know how to perform traditional sutured prolapse repairs and SUI surgery and be able to perform cystoscopy. Ideally, the surgeon should undergo training on a cadaver with a skilled and experienced user of the mesh kit. The surgeon also should carefully review the risks and benefits of mesh kits with the patient and inform the patient that he or she is in the early learning curve of a particular surgery. The informed patient should have a right to refuse mesh-augmented prolapse surgery after the consent process.
DR. LUCENTE: I’m glad you asked this question. I strongly believe that surgical expertise and proficiency within gynecology need to be more effectively addressed by us all. We have a situation in our field in which techniques and technology are widening the gap between what is possible and what the surgeon is comfortable doing safely.
It’s incumbent on all of us, especially those who are in a leadership position as a chairperson or chief of a division, to work with our physician staff and faculty to optimize surgical skill and patient outcomes, including safety, with new technologies.
As for the minimal amount of training needed, that’s extremely variable. It depends on the current skill set of the physician and his or her ability to pick up the mechanics of the surgery as it is taught through a cadaver lab or preceptorship. It’s regrettable that some physicians lack the objectivity and insight to judge their own skill set. This, again, is the time for a chairperson or chief of a division to step up to the plate and ensure proper credentialing and demonstration of proficiency.
It is unrealistic to expect industry to decide who should or should not utilize this truly breakthrough technology. That is our responsibility as physicians.
DR. KARRAM: At a minimum, I think any surgeon utilizing a kit should have a firm understanding of pelvic floor anatomy and experience performing traditional repairs:
- intraperitoneal procedures such as Mc-Call culdoplasty and uterosacral suspension
- sacrospinous suspension
- retropubic procedures and anti-incontinence operations such as pubovaginal slings.
This three-dimensional understanding of the pelvic floor is mandatory if one is to assume that blind passage of trocars through potentially dangerous spaces is the wave of the future.
DR. RAZ: You need to be a pelvic surgeon, know your anatomy, and know how to manage complications if you are going to use one of these kits. You should stick to the surgery that works best in your hands. Industry cannot teach you to be a good pelvic surgeon; it takes lifelong experience.
DR. KARRAM: If you have a patient who is sexually inactive with pelvic organ prolapse, would you prefer a mesh repair or an obliterative procedure? And why?
DR. WALTERS: If the patient is sexually inactive—especially if she is older and definitely will not be in the future—it makes absolutely no sense to perform a mesh-augmented repair. A traditional, somewhat tight, sutured repair works fine in this setting and carries very low risk.
In fact, our group and others have found that, in carefully selected patients, partial colpectomy and colpocleisis procedures (without grafts) have among the highest cure and satisfaction rates of all surgeries we perform for prolapse; they also have relatively low risk.8 Recurrent prolapse after an obliterative procedure is rare; most of the dissatisfaction relates to postoperative voiding difficulties or persistent or de novo urinary incontinence.
DR. KARRAM: I also prefer an obliterative procedure. I see no reason to bring in the cost and potential for complications that mesh repair entails. An obliterative procedure should produce an anatomic success rate close to 100%, with minimal complications. It also can be performed quickly with minimal anesthesia and convalescence.
DR. LUCENTE: My response is based on a clinical study that my associate, Dr. Miles Murphy, has performed, comparing a transvaginal mesh procedure with a LaForte operation for severe pelvic organ prolapse.9 Both patient groups were well satisfied with the result, and success rates were comparable. However, the group that underwent the transvaginal mesh procedure had a shorter operative time.
As a result of these studies, we tend to prefer transvaginal mesh repair. Even though the woman may be sexually inactive, the procedure preserves vaginal function, and we all know that life has a way of being unpredictable. Her situation may change so that she once again desires sexual function.
However, for a very elderly woman—one in her late 80s or 90s—who has severe or extreme prolapse with a very large procidentia and vaginal length measuring, say, 13 cm beyond the introitus, I do prefer an obliterative procedure.
DR. RAZ: I agree. I would not offer a sexually inactive patient an obliterative procedure. You never know what the future will hold.
Mesh repair can be performed safely, provided the surgeon has good knowledge of anatomic landmarks and knows how to manage any potential complications that may arise.
1. Nygaard IE, McCreery R, Brubaker L, et al. Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805-823.
2. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1421;discussion 1421-1422.
3. Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol. 2004;190:20-26.
4. Murphy M. Society of Gynecologic Surgeons Systematic Review Group. Clinical practice guidelines on vaginal graft use from the Society of Gynecologic Surgeons. Obstet Gynecol. 2008;112:1123-1130.
5. Paraiso MF, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical procedures including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
6. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol 2006;108:255-263.
7. EU Hernia Trialists Collaboration. Repair of groin hernia with synthetic mesh: meta-analysis of randomized controlled trials. Ann Surg. 2002;235:322-332.
8. Barber MD, Amundsen C, Paraiso MFR, Weidner A, Romero A, Walters MD. Quality of life after surgery for genital prolapse in elderly women: obliterative and reconstructive surgery. Int Urogynecol J. 2007;18:799-806.
9. Murphy M, van Raalte H, Mercurio E, Haff R, Wiseman B, Lucente VR. Incontinence-related quality of life and sexual function following the tension-free vaginal tape vs the “inside-out” tension-free vaginal tape obturator. Int Urogynecol J. 2008;19:481-487.
MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.
SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.
VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.
MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
Mesh kits for repairing prolapse are proliferating like crazy, just as they did for midurethral sling procedures. But mesh augmentation of prolapse surgeries requires more than a prepackaged assortment of tools and materials. In this article, moderator Mickey M. Karram, MD, and a panel of nationally recognized urogynecologists and urologists describe the literature on mesh augmentation and discuss indications, contraindications, techniques, applicable cases, and the considerable training required.
In Part 2, which will appear in the February issue of OBG Management, the panel tackles the thorny topic of complications, including erosion, extrusion, foreshortening of the vagina, dyspareunia, and pain. Their discussion focuses on ways to avoid these problems, and methods for correcting them.
Do we have enough data?
DR. KARRAM: To start, let’s quickly review the peer-reviewed literature on the use of mesh augmentation during surgery for pelvic organ prolapse.
DR. WALTERS: Until recently, most data concerned open abdominal sacrocolpopexy (ASC) using polypropylene or Merseline mesh. There is significant clinical experience with this operation, and multiple cohort studies show long-term cure rates of 78% to 100% for apical prolapse.1
At least two randomized controlled trials have compared open ASC with sutured vaginal colpopexy procedures, and ASC is certainly equal to—perhaps better than—all transvaginal sutured repairs.2,3
With ASC, most recurrences affect the distal half of the vagina and involve one or more of the following:
- anterior or posterior vaginal wall prolapse (or both)
- stress urinary incontinence (SUI)
- distal rectocele.1,3
Mesh erosion occurs in 3.4% of cases and is usually easily managed.1 Other complications, including bowel injury, tend to be related to access, regardless of whether the operation is performed via laparotomy or laparoscopy.
Robotic sacrocolpopexy has become popular in recent years, and we will probably see data on this approach as we gain experience.
When it comes to vaginal mesh kits, the peer-reviewed literature is just beginning to expand, with many studies being presented at international meetings. For anterior and, possibly, apical vaginal prolapse, the cure rate after use of a mesh kit appears to be as high as, or higher than, the rate for sutured repairs.4 This high rate of anatomic cure is balanced somewhat by additional cost and complications involving mesh and the kits.
For posterior vaginal wall prolapse and rectocele, I firmly believe, based on our research and that of others, that sutured repairs are superior to graft-augmented surgery.5
DR. KARRAM: What are the indications and contraindications for mesh augmentation of prolapse repair ( FIGURES 1 and 2 )?
DR. LUCENTE: I believe mesh is indicated in any patient in need of surgical repair of pelvic organ prolapse who is seeking optimal durability and is willing to accept the known risks of the surgery.
The issue becomes more complex when it comes to contraindications. Absolute contraindications are fairly obvious; they include medically unstable patients and those who may have an inactive infectious process within the pelvis or even undiagnosed abnormal uterine bleeding.
At our center, because the potential for dyspareunia and pelvic discomfort is our biggest concern, we have developed a profile of the patient who is more likely to develop these complaints. The profile includes any patient who has a chronic pain disorder of any type, but especially chronic pelvic pain disorders such as endometriosis and vulvodynia. Other risk factors appear to be a history of pelvic surgery involving any permanent material, suture or mesh, and young age.
So if we have a patient in her late 30s who has undergone reconstructive surgery using permanent sutures and who has an element of chronic pelvic pain, we would counsel her strongly to consider surgical options other than the use of synthetic mesh.
DR. WALTERS: The main indications for mesh-augmented prolapse repair are recurrent posthysterectomy vaginal vault prolapse, for which I usually perform ASC, and recurrent cystocele or anteriorapical prolapse, for which I use one of the anterior mesh kits.
I still think sutured repairs—by that, I mean uterosacral ligament or sacrospinous colpopexy with sutured rectocele repair—work best for recurrent posterior wall and posteriorapical prolapse. I don’t use mesh augmentation for rectocele.
The main contraindication to mesh augmentation, as I see it, is a history of mesh complications. If I am repairing a mesh complication such as erosion or pain, I do not place another mesh.
Medical issues that might increase mesh complications, such as diabetes, steroid use, or severe vaginal atrophy, would, at the very least, make me consider carefully whether mesh augmentation is appropriate. The literature is not clear on this, so mesh could still be used if the surgeon thinks it is necessary.
DR. KARRAM: I haven’t found a definitive indication for mesh augmentation. We have used biologic meshes empirically, but I am not convinced that they really add long-term durability, regardless of whether they are used in the anterior or posterior vaginal segment.
Our published durability rate for traditional suture-type repairs is in the range of 85% at 5 years out.6 Even if I assumed that mesh would give me 100% 5-year durability, this rate would have to be at the expense of some erosion, pain, and other complications unique to mesh. I do not think that the potential improvement in durability is worth these potential complications.

FIGURE 1 When the pelvic support system is intact, prolapse is rare
In the normal pelvis, organs are supported by a complex web of muscles, fascia, and connective tissue. 
FIGURE 2 Mesh augmentation seeks to enhance the durability of repair
One type of mesh in final position. Mesh-augmented repair restores the vaginal apex and lends support to the walls of the vagina.
DR. KARRAM: If you are doing a lot of mesh repairs, you are obviously content with the results and feel that the few complications you are seeing are outweighed by the advantages mesh confers. How do you avoid extrusion and avert creation of a painful vagina?
DR. RAZ: Most of our cases are recurrent prolapse after failed vaginal or abdominal repair. I am indeed using a significant amount of soft polypropylene mesh for reconstructive procedures. As with the use of any other synthetic material, low-grade infection can develop after a few weeks or months. I use copious irrigation with antibiotic solution during reconstruction.
To avoid extrusion, I perform deep, rather than superficial, dissection of the vaginal wall to allow for better coverage of the mesh. For posterior mesh reconstruction, I cover the mesh with pararectal fascia to prevent erosion.
For mesh-augmented procedures, I cut the mesh myself in the operating room ( FIGURE 3 ). For a sling, I use a 10 cm × 1 cm soft polypropylene mesh. For a grade 3 or 4 cystocele, I use a trapezoid of soft polypropylene mesh with several points of fixation:
- at the sacrouterine ligament
- lateral to the obturator fascia
- distal to the bladder neck.
I always repair the vault at the same time.
For vault prolapse, I use a segment of soft polypropylene mesh in the shape of an apron with two arms (1 cm × 4 cm) and a central segment (4 cm × 7 cm). I support the vault using number 1-0 delayed absorbable suture and mesh. From outside the vaginal wall, in the posterolateral deep vaginal wall (inside the peritoneum), I incorporate the origin of the sacrouterine ligament and one arm of the mesh in the groove between the colon and levator ani, 15 cm from the introitus. I bring the suture 1 cm from the original entrance. A separate set of sutures brings the perirectal fascia together with the sacrouterine ligaments and perivesical fascia to close the peritoneal cavity. I tie the vault-suspension sutures, providing support to the cuff in a high posterior position (12 to 15 cm from the introitus).
In selected cases of significant recurrent rectocele, I use a rectangle of soft polypropylene mesh anchored to the origin of the sacrouterine ligament and distal to the perineal membrane. The mesh is covered by the pararectal fascia.
We have not seen vaginal, urethral, or bladder erosion in 1,800 cases of our distal urethral Prolene sling procedure using 10 cm × 1 cm soft mesh. In patients who have significant cystocele, vault prolapse, and recurrent rectocele, our vaginal erosion rate is 3%. We have never encountered rectal, bladder, or bowel perforation using our technique.
DR. LUCENTE: We often use mesh and are more than simply content with our results—we are extremely pleased, and so are our patients. Having said that, our techniques have definitely evolved over the past few years, as we’ve focused on how to decrease exposure and, more recently, optimize sexual function and vaginal comfort.
First, to avoid exposure, the most critical step is precise hydrodissection and distention of the true vesicovaginal space. This step can only be achieved through careful tactile guidance of the needle tip into the space, where it should remain while hydrodissection is performed. Always remember, sharp dissection “follows” hydrodissection. If you place the needle bevel within the vaginal wall, you will “split” the vaginal wall—as during standard colporrhaphy—which will lead to a high exposure rate.
Second, to avoid dyspareunia, it’s essential to pay close attention to POP-Q measurements, especially vaginal length, to ensure that the reconstruction restores the same length without foreshortening. This approach entails leaving the cervix in most patients who have a shorter vagina, and making sure that the mesh is secured above the ischial spine in younger, sexually active patients who have demonstrated a higher risk of postoperative deep, penetrating dyspareunia, compared with older, less sexually active patients.
Also paramount is to ensure that you have manually displaced the vagina inwardly as much as possible before deploying or setting the mesh. If you simply try to suture secure the mesh with the vagina incised open, without the ability to deploy the mesh with a closed, displaced vagina (to mimic deep penetration), it is difficult, if not impossible, to properly set the mesh for optimal comfort.
In the early days of midurethral pubovaginal slings using polypropylene, the adage was “looser is better than tighter.” This is even truer for transvaginal mesh.
DR. KARRAM: Dr. Walters, please describe your current surgical procedure of choice without mesh and explain why you haven’t adopted mesh for routine repairs.
DR. WALTERS: About 20% of my prolapse surgeries—usually for posthysterectomy or recurrent vaginal vault prolapse—involve ASC with placement of polypropylene mesh. I perform most of these cases through a Pfannenstiel incision, but I’ve also done them laparoscopically. Several of my partners perform ASC laparoscopically and robotically.
For the other 80% of my patients who have prolapse, I perform repairs transvaginally, usually using high bilateral uterosacralligament vaginal-vault suspension. We have learned to suture higher and slightly more medial on the uterosacral ligaments to attain greater vaginal depth and minimize ureteral obstruction. We use two or three sutures on each uterosacral ligament, usually a combination of permanent and delayed absorbable sutures.
I am also performing more sacrospinous ligament suspensions because this operation is being studied by the Pelvic Floor Disorders Network. Properly performed, it is an excellent surgery for apical prolapse. But, as with most of our surgeries for prolapse, recurrent anterior wall prolapse remains a problem.
Like you, Dr. Karram, we’ve studied our group’s anatomic and functional outcomes very carefully for more than 10 years and are mostly satisfied with our cure and complication rates. Although our anatomic outcomes with these surgeries are not always perfect, our reoperation rate for prolapse is only about 5%, with a high level of satisfaction in 88% to 92% of patients.
DR. RAZ: Unaugmented reconstruction fails in more than 30% of cases. Some patients who have significant prolapse and attenuated tissue think that this tissue will become healthier or stronger after reconstructive surgery, but that isn’t the case. In these situations, excision and plication make no clinical sense.
The problem is that we have yet to identify the ideal surrogate for poor-quality tissue. Most of us use polypropylene mesh in different variations. We need a better material that will be nonimmunogenic, well tolerated, and easily incorporated without erosion. Xenograft-like derivatives of dermis, or allografts such as cadaveric fascia, have failed over the long term because the body reabsorbs the graft without forming any new connective tissue.

FIGURE 3 Mesh can be cut in the OR to custom-fit a patient
Hand-cut mesh and points of placement.
PHOTO: SHLOMO RAZ, MD
Is a kit a valuable aid?
DR. KARRAM: If a surgeon wants to augment a repair, what are the advantages of a packaged mesh kit, compared with simply cutting the mesh and performing surgery without a kit?
DR. WALTERS: The advantages of a packaged mesh kit are the convenience involved and the ability to consistently perform the same operation with the same product. That facilitates learning, teaching, and research. It also helps us understand the published literature a little better because “custom” prolapse repairs are operator-dependent and difficult to apply generally to a population of surgeons.
These advantages are most clearly apparent with midurethral sling mesh kits, which have almost revolutionized surgery for stress incontinence. I don’t believe mesh kits for prolapse are there yet, but they certainly have potential.
DR. RAZ: I’m opposed to the use of kits. They are industry-driven. One company has made $1 billion selling them. Imagine a patient who undergoes placement of a sling kit ($1,000), cystocele kit ($1,500), and posterior mesh kit ($1,500). How can our healthcare system sustain this burden, especially when there is no real evidence that a kit improves the operation, and given the incredible complication rate that we see?
Moreover, the kits contain a single-use needle and passer and a precut segment of polypropylene mesh. But every patient is different and requires a unique size or shape of mesh. I don’t believe that a surgeon who knows pelvic anatomy needs a kit to perform mesh-augmented reconstruction. We can buy the same segment of mesh for $200 to $400, cut it as needed, and perform the same operation advertised by industry.
For surgeons who prefer a kit, the tools that are included should be made reusable.
DR. LUCENTE: In my opinion, the primary advantage of a commercially available transvaginal mesh delivery system—notice, I avoided the word “kit,” because I think there are plenty of negative connotations associated with it—is the ability to deliver the mesh in a “tension”-free manner.
One alternative that many people pursue is cutting the mesh to size and using sutures to hold it in place while tissue ingrowth occurs. However, the hernia literature suggests that suturing mesh in place increases the risk of postoperative discomfort at the site of implantation. The true cause of the discomfort remains unclear, but it is thought to arise from nerve tethering or traction at the pre-committed points of attachment before the host tissue and mesh interface have adjusted or settled with tissue ingrowth.
All neuropathic complications of mesh implantation have been shown in the current hernia literature to be increased with the use of sutures.7 Also, as previously mentioned, it is extremely difficult to set or adjust the mesh with the vaginal incision remaining “open,” which is a downside to suture techniques.
What training is necessary to use a kit?
DR. KARRAM: Mesh kits are aggressively promoted by industry, with close to half a dozen different kits to be available soon. What is the minimum amount of training one should have before utilizing these kits?
DR. WALTERS: The surgeon should at least know how to perform traditional sutured prolapse repairs and SUI surgery and be able to perform cystoscopy. Ideally, the surgeon should undergo training on a cadaver with a skilled and experienced user of the mesh kit. The surgeon also should carefully review the risks and benefits of mesh kits with the patient and inform the patient that he or she is in the early learning curve of a particular surgery. The informed patient should have a right to refuse mesh-augmented prolapse surgery after the consent process.
DR. LUCENTE: I’m glad you asked this question. I strongly believe that surgical expertise and proficiency within gynecology need to be more effectively addressed by us all. We have a situation in our field in which techniques and technology are widening the gap between what is possible and what the surgeon is comfortable doing safely.
It’s incumbent on all of us, especially those who are in a leadership position as a chairperson or chief of a division, to work with our physician staff and faculty to optimize surgical skill and patient outcomes, including safety, with new technologies.
As for the minimal amount of training needed, that’s extremely variable. It depends on the current skill set of the physician and his or her ability to pick up the mechanics of the surgery as it is taught through a cadaver lab or preceptorship. It’s regrettable that some physicians lack the objectivity and insight to judge their own skill set. This, again, is the time for a chairperson or chief of a division to step up to the plate and ensure proper credentialing and demonstration of proficiency.
It is unrealistic to expect industry to decide who should or should not utilize this truly breakthrough technology. That is our responsibility as physicians.
DR. KARRAM: At a minimum, I think any surgeon utilizing a kit should have a firm understanding of pelvic floor anatomy and experience performing traditional repairs:
- intraperitoneal procedures such as Mc-Call culdoplasty and uterosacral suspension
- sacrospinous suspension
- retropubic procedures and anti-incontinence operations such as pubovaginal slings.
This three-dimensional understanding of the pelvic floor is mandatory if one is to assume that blind passage of trocars through potentially dangerous spaces is the wave of the future.
DR. RAZ: You need to be a pelvic surgeon, know your anatomy, and know how to manage complications if you are going to use one of these kits. You should stick to the surgery that works best in your hands. Industry cannot teach you to be a good pelvic surgeon; it takes lifelong experience.
DR. KARRAM: If you have a patient who is sexually inactive with pelvic organ prolapse, would you prefer a mesh repair or an obliterative procedure? And why?
DR. WALTERS: If the patient is sexually inactive—especially if she is older and definitely will not be in the future—it makes absolutely no sense to perform a mesh-augmented repair. A traditional, somewhat tight, sutured repair works fine in this setting and carries very low risk.
In fact, our group and others have found that, in carefully selected patients, partial colpectomy and colpocleisis procedures (without grafts) have among the highest cure and satisfaction rates of all surgeries we perform for prolapse; they also have relatively low risk.8 Recurrent prolapse after an obliterative procedure is rare; most of the dissatisfaction relates to postoperative voiding difficulties or persistent or de novo urinary incontinence.
DR. KARRAM: I also prefer an obliterative procedure. I see no reason to bring in the cost and potential for complications that mesh repair entails. An obliterative procedure should produce an anatomic success rate close to 100%, with minimal complications. It also can be performed quickly with minimal anesthesia and convalescence.
DR. LUCENTE: My response is based on a clinical study that my associate, Dr. Miles Murphy, has performed, comparing a transvaginal mesh procedure with a LaForte operation for severe pelvic organ prolapse.9 Both patient groups were well satisfied with the result, and success rates were comparable. However, the group that underwent the transvaginal mesh procedure had a shorter operative time.
As a result of these studies, we tend to prefer transvaginal mesh repair. Even though the woman may be sexually inactive, the procedure preserves vaginal function, and we all know that life has a way of being unpredictable. Her situation may change so that she once again desires sexual function.
However, for a very elderly woman—one in her late 80s or 90s—who has severe or extreme prolapse with a very large procidentia and vaginal length measuring, say, 13 cm beyond the introitus, I do prefer an obliterative procedure.
DR. RAZ: I agree. I would not offer a sexually inactive patient an obliterative procedure. You never know what the future will hold.
Mesh repair can be performed safely, provided the surgeon has good knowledge of anatomic landmarks and knows how to manage any potential complications that may arise.
MICKEY M. KARRAM, MD, moderator, is Director of Urogynecology at Good Samaritan Hospital and Voluntary Professor of ObGyn at the University of Cincinnati School of Medicine in Cincinnati, Ohio.
SHLOMO RAZ, MD, is Professor of Urology and Chief of Pelvic Medicine and Reconstructive Urology at UCLA School of Medicine in Los Angeles.
VINCENT LUCENTE, MD, MBA, is Founder and Director of the Institute for Female Pelvic Medicine and Reconstructive Surgery in Allentown, Pa, and Clinical Professor of ObGyn at Temple University School of Medicine in Philadelphia.
MARK D. WALTERS, MD, is Professor and Vice Chair of Gynecology, Section of Urogynecology and Reconstructive Pelvic Surgery, Department of Obstetrics and Gynecology, at the Cleveland Clinic in Cleveland, Ohio.
Mesh kits for repairing prolapse are proliferating like crazy, just as they did for midurethral sling procedures. But mesh augmentation of prolapse surgeries requires more than a prepackaged assortment of tools and materials. In this article, moderator Mickey M. Karram, MD, and a panel of nationally recognized urogynecologists and urologists describe the literature on mesh augmentation and discuss indications, contraindications, techniques, applicable cases, and the considerable training required.
In Part 2, which will appear in the February issue of OBG Management, the panel tackles the thorny topic of complications, including erosion, extrusion, foreshortening of the vagina, dyspareunia, and pain. Their discussion focuses on ways to avoid these problems, and methods for correcting them.
Do we have enough data?
DR. KARRAM: To start, let’s quickly review the peer-reviewed literature on the use of mesh augmentation during surgery for pelvic organ prolapse.
DR. WALTERS: Until recently, most data concerned open abdominal sacrocolpopexy (ASC) using polypropylene or Merseline mesh. There is significant clinical experience with this operation, and multiple cohort studies show long-term cure rates of 78% to 100% for apical prolapse.1
At least two randomized controlled trials have compared open ASC with sutured vaginal colpopexy procedures, and ASC is certainly equal to—perhaps better than—all transvaginal sutured repairs.2,3
With ASC, most recurrences affect the distal half of the vagina and involve one or more of the following:
- anterior or posterior vaginal wall prolapse (or both)
- stress urinary incontinence (SUI)
- distal rectocele.1,3
Mesh erosion occurs in 3.4% of cases and is usually easily managed.1 Other complications, including bowel injury, tend to be related to access, regardless of whether the operation is performed via laparotomy or laparoscopy.
Robotic sacrocolpopexy has become popular in recent years, and we will probably see data on this approach as we gain experience.
When it comes to vaginal mesh kits, the peer-reviewed literature is just beginning to expand, with many studies being presented at international meetings. For anterior and, possibly, apical vaginal prolapse, the cure rate after use of a mesh kit appears to be as high as, or higher than, the rate for sutured repairs.4 This high rate of anatomic cure is balanced somewhat by additional cost and complications involving mesh and the kits.
For posterior vaginal wall prolapse and rectocele, I firmly believe, based on our research and that of others, that sutured repairs are superior to graft-augmented surgery.5
DR. KARRAM: What are the indications and contraindications for mesh augmentation of prolapse repair ( FIGURES 1 and 2 )?
DR. LUCENTE: I believe mesh is indicated in any patient in need of surgical repair of pelvic organ prolapse who is seeking optimal durability and is willing to accept the known risks of the surgery.
The issue becomes more complex when it comes to contraindications. Absolute contraindications are fairly obvious; they include medically unstable patients and those who may have an inactive infectious process within the pelvis or even undiagnosed abnormal uterine bleeding.
At our center, because the potential for dyspareunia and pelvic discomfort is our biggest concern, we have developed a profile of the patient who is more likely to develop these complaints. The profile includes any patient who has a chronic pain disorder of any type, but especially chronic pelvic pain disorders such as endometriosis and vulvodynia. Other risk factors appear to be a history of pelvic surgery involving any permanent material, suture or mesh, and young age.
So if we have a patient in her late 30s who has undergone reconstructive surgery using permanent sutures and who has an element of chronic pelvic pain, we would counsel her strongly to consider surgical options other than the use of synthetic mesh.
DR. WALTERS: The main indications for mesh-augmented prolapse repair are recurrent posthysterectomy vaginal vault prolapse, for which I usually perform ASC, and recurrent cystocele or anteriorapical prolapse, for which I use one of the anterior mesh kits.
I still think sutured repairs—by that, I mean uterosacral ligament or sacrospinous colpopexy with sutured rectocele repair—work best for recurrent posterior wall and posteriorapical prolapse. I don’t use mesh augmentation for rectocele.
The main contraindication to mesh augmentation, as I see it, is a history of mesh complications. If I am repairing a mesh complication such as erosion or pain, I do not place another mesh.
Medical issues that might increase mesh complications, such as diabetes, steroid use, or severe vaginal atrophy, would, at the very least, make me consider carefully whether mesh augmentation is appropriate. The literature is not clear on this, so mesh could still be used if the surgeon thinks it is necessary.
DR. KARRAM: I haven’t found a definitive indication for mesh augmentation. We have used biologic meshes empirically, but I am not convinced that they really add long-term durability, regardless of whether they are used in the anterior or posterior vaginal segment.
Our published durability rate for traditional suture-type repairs is in the range of 85% at 5 years out.6 Even if I assumed that mesh would give me 100% 5-year durability, this rate would have to be at the expense of some erosion, pain, and other complications unique to mesh. I do not think that the potential improvement in durability is worth these potential complications.

FIGURE 1 When the pelvic support system is intact, prolapse is rare
In the normal pelvis, organs are supported by a complex web of muscles, fascia, and connective tissue. 
FIGURE 2 Mesh augmentation seeks to enhance the durability of repair
One type of mesh in final position. Mesh-augmented repair restores the vaginal apex and lends support to the walls of the vagina.
DR. KARRAM: If you are doing a lot of mesh repairs, you are obviously content with the results and feel that the few complications you are seeing are outweighed by the advantages mesh confers. How do you avoid extrusion and avert creation of a painful vagina?
DR. RAZ: Most of our cases are recurrent prolapse after failed vaginal or abdominal repair. I am indeed using a significant amount of soft polypropylene mesh for reconstructive procedures. As with the use of any other synthetic material, low-grade infection can develop after a few weeks or months. I use copious irrigation with antibiotic solution during reconstruction.
To avoid extrusion, I perform deep, rather than superficial, dissection of the vaginal wall to allow for better coverage of the mesh. For posterior mesh reconstruction, I cover the mesh with pararectal fascia to prevent erosion.
For mesh-augmented procedures, I cut the mesh myself in the operating room ( FIGURE 3 ). For a sling, I use a 10 cm × 1 cm soft polypropylene mesh. For a grade 3 or 4 cystocele, I use a trapezoid of soft polypropylene mesh with several points of fixation:
- at the sacrouterine ligament
- lateral to the obturator fascia
- distal to the bladder neck.
I always repair the vault at the same time.
For vault prolapse, I use a segment of soft polypropylene mesh in the shape of an apron with two arms (1 cm × 4 cm) and a central segment (4 cm × 7 cm). I support the vault using number 1-0 delayed absorbable suture and mesh. From outside the vaginal wall, in the posterolateral deep vaginal wall (inside the peritoneum), I incorporate the origin of the sacrouterine ligament and one arm of the mesh in the groove between the colon and levator ani, 15 cm from the introitus. I bring the suture 1 cm from the original entrance. A separate set of sutures brings the perirectal fascia together with the sacrouterine ligaments and perivesical fascia to close the peritoneal cavity. I tie the vault-suspension sutures, providing support to the cuff in a high posterior position (12 to 15 cm from the introitus).
In selected cases of significant recurrent rectocele, I use a rectangle of soft polypropylene mesh anchored to the origin of the sacrouterine ligament and distal to the perineal membrane. The mesh is covered by the pararectal fascia.
We have not seen vaginal, urethral, or bladder erosion in 1,800 cases of our distal urethral Prolene sling procedure using 10 cm × 1 cm soft mesh. In patients who have significant cystocele, vault prolapse, and recurrent rectocele, our vaginal erosion rate is 3%. We have never encountered rectal, bladder, or bowel perforation using our technique.
DR. LUCENTE: We often use mesh and are more than simply content with our results—we are extremely pleased, and so are our patients. Having said that, our techniques have definitely evolved over the past few years, as we’ve focused on how to decrease exposure and, more recently, optimize sexual function and vaginal comfort.
First, to avoid exposure, the most critical step is precise hydrodissection and distention of the true vesicovaginal space. This step can only be achieved through careful tactile guidance of the needle tip into the space, where it should remain while hydrodissection is performed. Always remember, sharp dissection “follows” hydrodissection. If you place the needle bevel within the vaginal wall, you will “split” the vaginal wall—as during standard colporrhaphy—which will lead to a high exposure rate.
Second, to avoid dyspareunia, it’s essential to pay close attention to POP-Q measurements, especially vaginal length, to ensure that the reconstruction restores the same length without foreshortening. This approach entails leaving the cervix in most patients who have a shorter vagina, and making sure that the mesh is secured above the ischial spine in younger, sexually active patients who have demonstrated a higher risk of postoperative deep, penetrating dyspareunia, compared with older, less sexually active patients.
Also paramount is to ensure that you have manually displaced the vagina inwardly as much as possible before deploying or setting the mesh. If you simply try to suture secure the mesh with the vagina incised open, without the ability to deploy the mesh with a closed, displaced vagina (to mimic deep penetration), it is difficult, if not impossible, to properly set the mesh for optimal comfort.
In the early days of midurethral pubovaginal slings using polypropylene, the adage was “looser is better than tighter.” This is even truer for transvaginal mesh.
DR. KARRAM: Dr. Walters, please describe your current surgical procedure of choice without mesh and explain why you haven’t adopted mesh for routine repairs.
DR. WALTERS: About 20% of my prolapse surgeries—usually for posthysterectomy or recurrent vaginal vault prolapse—involve ASC with placement of polypropylene mesh. I perform most of these cases through a Pfannenstiel incision, but I’ve also done them laparoscopically. Several of my partners perform ASC laparoscopically and robotically.
For the other 80% of my patients who have prolapse, I perform repairs transvaginally, usually using high bilateral uterosacralligament vaginal-vault suspension. We have learned to suture higher and slightly more medial on the uterosacral ligaments to attain greater vaginal depth and minimize ureteral obstruction. We use two or three sutures on each uterosacral ligament, usually a combination of permanent and delayed absorbable sutures.
I am also performing more sacrospinous ligament suspensions because this operation is being studied by the Pelvic Floor Disorders Network. Properly performed, it is an excellent surgery for apical prolapse. But, as with most of our surgeries for prolapse, recurrent anterior wall prolapse remains a problem.
Like you, Dr. Karram, we’ve studied our group’s anatomic and functional outcomes very carefully for more than 10 years and are mostly satisfied with our cure and complication rates. Although our anatomic outcomes with these surgeries are not always perfect, our reoperation rate for prolapse is only about 5%, with a high level of satisfaction in 88% to 92% of patients.
DR. RAZ: Unaugmented reconstruction fails in more than 30% of cases. Some patients who have significant prolapse and attenuated tissue think that this tissue will become healthier or stronger after reconstructive surgery, but that isn’t the case. In these situations, excision and plication make no clinical sense.
The problem is that we have yet to identify the ideal surrogate for poor-quality tissue. Most of us use polypropylene mesh in different variations. We need a better material that will be nonimmunogenic, well tolerated, and easily incorporated without erosion. Xenograft-like derivatives of dermis, or allografts such as cadaveric fascia, have failed over the long term because the body reabsorbs the graft without forming any new connective tissue.

FIGURE 3 Mesh can be cut in the OR to custom-fit a patient
Hand-cut mesh and points of placement.
PHOTO: SHLOMO RAZ, MD
Is a kit a valuable aid?
DR. KARRAM: If a surgeon wants to augment a repair, what are the advantages of a packaged mesh kit, compared with simply cutting the mesh and performing surgery without a kit?
DR. WALTERS: The advantages of a packaged mesh kit are the convenience involved and the ability to consistently perform the same operation with the same product. That facilitates learning, teaching, and research. It also helps us understand the published literature a little better because “custom” prolapse repairs are operator-dependent and difficult to apply generally to a population of surgeons.
These advantages are most clearly apparent with midurethral sling mesh kits, which have almost revolutionized surgery for stress incontinence. I don’t believe mesh kits for prolapse are there yet, but they certainly have potential.
DR. RAZ: I’m opposed to the use of kits. They are industry-driven. One company has made $1 billion selling them. Imagine a patient who undergoes placement of a sling kit ($1,000), cystocele kit ($1,500), and posterior mesh kit ($1,500). How can our healthcare system sustain this burden, especially when there is no real evidence that a kit improves the operation, and given the incredible complication rate that we see?
Moreover, the kits contain a single-use needle and passer and a precut segment of polypropylene mesh. But every patient is different and requires a unique size or shape of mesh. I don’t believe that a surgeon who knows pelvic anatomy needs a kit to perform mesh-augmented reconstruction. We can buy the same segment of mesh for $200 to $400, cut it as needed, and perform the same operation advertised by industry.
For surgeons who prefer a kit, the tools that are included should be made reusable.
DR. LUCENTE: In my opinion, the primary advantage of a commercially available transvaginal mesh delivery system—notice, I avoided the word “kit,” because I think there are plenty of negative connotations associated with it—is the ability to deliver the mesh in a “tension”-free manner.
One alternative that many people pursue is cutting the mesh to size and using sutures to hold it in place while tissue ingrowth occurs. However, the hernia literature suggests that suturing mesh in place increases the risk of postoperative discomfort at the site of implantation. The true cause of the discomfort remains unclear, but it is thought to arise from nerve tethering or traction at the pre-committed points of attachment before the host tissue and mesh interface have adjusted or settled with tissue ingrowth.
All neuropathic complications of mesh implantation have been shown in the current hernia literature to be increased with the use of sutures.7 Also, as previously mentioned, it is extremely difficult to set or adjust the mesh with the vaginal incision remaining “open,” which is a downside to suture techniques.
What training is necessary to use a kit?
DR. KARRAM: Mesh kits are aggressively promoted by industry, with close to half a dozen different kits to be available soon. What is the minimum amount of training one should have before utilizing these kits?
DR. WALTERS: The surgeon should at least know how to perform traditional sutured prolapse repairs and SUI surgery and be able to perform cystoscopy. Ideally, the surgeon should undergo training on a cadaver with a skilled and experienced user of the mesh kit. The surgeon also should carefully review the risks and benefits of mesh kits with the patient and inform the patient that he or she is in the early learning curve of a particular surgery. The informed patient should have a right to refuse mesh-augmented prolapse surgery after the consent process.
DR. LUCENTE: I’m glad you asked this question. I strongly believe that surgical expertise and proficiency within gynecology need to be more effectively addressed by us all. We have a situation in our field in which techniques and technology are widening the gap between what is possible and what the surgeon is comfortable doing safely.
It’s incumbent on all of us, especially those who are in a leadership position as a chairperson or chief of a division, to work with our physician staff and faculty to optimize surgical skill and patient outcomes, including safety, with new technologies.
As for the minimal amount of training needed, that’s extremely variable. It depends on the current skill set of the physician and his or her ability to pick up the mechanics of the surgery as it is taught through a cadaver lab or preceptorship. It’s regrettable that some physicians lack the objectivity and insight to judge their own skill set. This, again, is the time for a chairperson or chief of a division to step up to the plate and ensure proper credentialing and demonstration of proficiency.
It is unrealistic to expect industry to decide who should or should not utilize this truly breakthrough technology. That is our responsibility as physicians.
DR. KARRAM: At a minimum, I think any surgeon utilizing a kit should have a firm understanding of pelvic floor anatomy and experience performing traditional repairs:
- intraperitoneal procedures such as Mc-Call culdoplasty and uterosacral suspension
- sacrospinous suspension
- retropubic procedures and anti-incontinence operations such as pubovaginal slings.
This three-dimensional understanding of the pelvic floor is mandatory if one is to assume that blind passage of trocars through potentially dangerous spaces is the wave of the future.
DR. RAZ: You need to be a pelvic surgeon, know your anatomy, and know how to manage complications if you are going to use one of these kits. You should stick to the surgery that works best in your hands. Industry cannot teach you to be a good pelvic surgeon; it takes lifelong experience.
DR. KARRAM: If you have a patient who is sexually inactive with pelvic organ prolapse, would you prefer a mesh repair or an obliterative procedure? And why?
DR. WALTERS: If the patient is sexually inactive—especially if she is older and definitely will not be in the future—it makes absolutely no sense to perform a mesh-augmented repair. A traditional, somewhat tight, sutured repair works fine in this setting and carries very low risk.
In fact, our group and others have found that, in carefully selected patients, partial colpectomy and colpocleisis procedures (without grafts) have among the highest cure and satisfaction rates of all surgeries we perform for prolapse; they also have relatively low risk.8 Recurrent prolapse after an obliterative procedure is rare; most of the dissatisfaction relates to postoperative voiding difficulties or persistent or de novo urinary incontinence.
DR. KARRAM: I also prefer an obliterative procedure. I see no reason to bring in the cost and potential for complications that mesh repair entails. An obliterative procedure should produce an anatomic success rate close to 100%, with minimal complications. It also can be performed quickly with minimal anesthesia and convalescence.
DR. LUCENTE: My response is based on a clinical study that my associate, Dr. Miles Murphy, has performed, comparing a transvaginal mesh procedure with a LaForte operation for severe pelvic organ prolapse.9 Both patient groups were well satisfied with the result, and success rates were comparable. However, the group that underwent the transvaginal mesh procedure had a shorter operative time.
As a result of these studies, we tend to prefer transvaginal mesh repair. Even though the woman may be sexually inactive, the procedure preserves vaginal function, and we all know that life has a way of being unpredictable. Her situation may change so that she once again desires sexual function.
However, for a very elderly woman—one in her late 80s or 90s—who has severe or extreme prolapse with a very large procidentia and vaginal length measuring, say, 13 cm beyond the introitus, I do prefer an obliterative procedure.
DR. RAZ: I agree. I would not offer a sexually inactive patient an obliterative procedure. You never know what the future will hold.
Mesh repair can be performed safely, provided the surgeon has good knowledge of anatomic landmarks and knows how to manage any potential complications that may arise.
1. Nygaard IE, McCreery R, Brubaker L, et al. Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805-823.
2. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1421;discussion 1421-1422.
3. Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol. 2004;190:20-26.
4. Murphy M. Society of Gynecologic Surgeons Systematic Review Group. Clinical practice guidelines on vaginal graft use from the Society of Gynecologic Surgeons. Obstet Gynecol. 2008;112:1123-1130.
5. Paraiso MF, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical procedures including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
6. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol 2006;108:255-263.
7. EU Hernia Trialists Collaboration. Repair of groin hernia with synthetic mesh: meta-analysis of randomized controlled trials. Ann Surg. 2002;235:322-332.
8. Barber MD, Amundsen C, Paraiso MFR, Weidner A, Romero A, Walters MD. Quality of life after surgery for genital prolapse in elderly women: obliterative and reconstructive surgery. Int Urogynecol J. 2007;18:799-806.
9. Murphy M, van Raalte H, Mercurio E, Haff R, Wiseman B, Lucente VR. Incontinence-related quality of life and sexual function following the tension-free vaginal tape vs the “inside-out” tension-free vaginal tape obturator. Int Urogynecol J. 2008;19:481-487.
1. Nygaard IE, McCreery R, Brubaker L, et al. Pelvic Floor Disorders Network. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805-823.
2. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1421;discussion 1421-1422.
3. Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol. 2004;190:20-26.
4. Murphy M. Society of Gynecologic Surgeons Systematic Review Group. Clinical practice guidelines on vaginal graft use from the Society of Gynecologic Surgeons. Obstet Gynecol. 2008;112:1123-1130.
5. Paraiso MF, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical procedures including graft augmentation. Am J Obstet Gynecol. 2006;195:1762-1771.
6. Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM. Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol 2006;108:255-263.
7. EU Hernia Trialists Collaboration. Repair of groin hernia with synthetic mesh: meta-analysis of randomized controlled trials. Ann Surg. 2002;235:322-332.
8. Barber MD, Amundsen C, Paraiso MFR, Weidner A, Romero A, Walters MD. Quality of life after surgery for genital prolapse in elderly women: obliterative and reconstructive surgery. Int Urogynecol J. 2007;18:799-806.
9. Murphy M, van Raalte H, Mercurio E, Haff R, Wiseman B, Lucente VR. Incontinence-related quality of life and sexual function following the tension-free vaginal tape vs the “inside-out” tension-free vaginal tape obturator. Int Urogynecol J. 2008;19:481-487.
