User login
Cervical cancer screening: Should my practice switch to primary HPV testing?
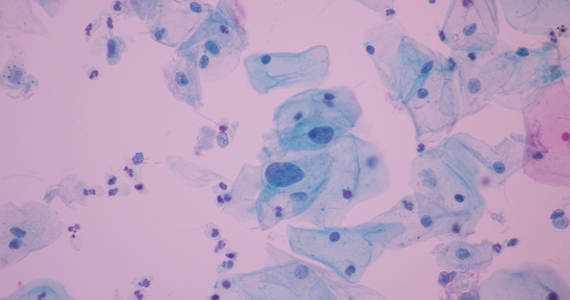
How should I be approaching cervical cancer screening: with primary human papillomavirus (HPV) testing, or cotesting? We get this question all the time from clinicians. Although they have heard of the latest cervical cancer screening guidelines for stand-alone “primary” HPV testing, they are still ordering cervical cytology (Papanicolaou, or Pap, test) for women aged 21 to 29 years and cotesting (cervical cytology with HPV testing) for women with a cervix aged 30 and older.
Changes in cervical cancer testing guidance
Cervical cancer occurs in more than 13,000 women in the United States annually.1 High-risk types of HPV—the known cause of cervical cancer—also cause a large majority of cancers of the anus, vagina, vulva, and oropharynx.2
Cervical cancer screening programs in the United States have markedly decreased the incidence of and mortality from cervical cancer since introduction of the Pap smear in the 1950s. In 2000, HPV testing was approved by the US Food and Drug Administration (FDA) as a reflex test to a Pap smear result of atypical squamous cells of undetermined significance (ASC-US). HPV testing was then approved for use with cytology as a cotest in 2003 and subsequently as a primary stand-alone test in 2014.
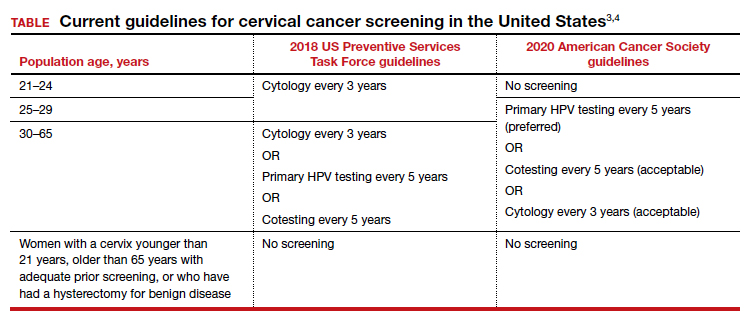
Recently, the American Cancer Society (ACS) released new cervical screening guidelines that depart from prior guidelines.3 They recommend not to screen 21- to 24-year-olds and to start screening at age 25 until age 65 with the preferred strategy of primary HPV testing every 5 years, using an FDA-approved HPV test. Alternative screening strategies are cytology (Pap) every 3 years or cotesting every 5 years.
The 2018 US Preventive Services Task Force (USPSTF) guidelines differ from the ACS guidelines. The USPSTF recommends cytology every 3 years as the preferred method for women with a cervix who are aged 21 to 29 years and, for women with a cervix who are aged 30 to 65 years, the option for cytology every 3 years, primary HPV testing every 5 years, or cotesting every 5 years (TABLE).4
Why the reluctance to switch to HPV testing?
Despite FDA approval in 2014 for primary HPV testing and concurrent professional society guidance to use this testing strategy in women with a cervix who are aged 25 years and older, few practices in the United States have switched over to primary HPV testing for cervical cancer screening.5,6 Several reasons underlie this inertia:
- Many practices currently use HPV tests that are not FDA approved for primary HPV testing.
- Until recently, national screening guidelines did not recommend primary HPV testing as the preferred testing strategy.
- Long-established guidance on the importance of regular cervical cytology screening promoted by the ACS and others (which especially impacts women with a cervix older than age 50 who guide their younger daughters) will rely on significant re-education to move away from the established “Pap smear” cultural icon to a new approach.
- Last but not least, companies that manufacture HPV tests and laboratories integrated to offer such tests not yet approved for primary screening are promoting reliance on the prior proven cotest strategy. They have lobbied to preserve cotesting as a primary test, with some laboratory database studies showing gaps in detection with HPV test screening alone.7-9
Currently, the FDA-approved HPV tests for primary HPV screening include the Cobas HPV test (Roche) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Both are DNA tests for 14 high-risk types of HPV that include genotyping for HPV 16 and 18.
Continue to: Follow the evidence...
Follow the evidence
Several trials in Europe and Canada provide supporting evidence for primary HPV testing, and many European countries have moved to primary HPV testing as their preferred screening method.10,11 The new ACS guidelines put us more in sync with the rest of the world, where HPV testing is the dominant strategy.
It is true that doing additional tests will find more disease; cotesting has been shown to very minimally increase detection of cervical intraepithelial neoplasia grade 2/3 (CIN 2/3) compared with HPV testing alone, but it incurs many more costs and procedures.12 The vast majority of cervical cancer is HPV positive, and cytology still can be used as a triage to primary HPV screening until tests with better sensitivity and/or specificity (such as dual stain and methylation) can be employed to reduce unnecessary “false-positive” driven procedures.
As mentioned, many strong forces are trying to keep cotesting as the preferred strategy. It is important for clinicians to recognize the corporate investment into screening platforms, relationships, and products that underlie some of these efforts so as not to be unfairly influenced by their lobbying. Data from well-conducted, high-quality studies should be the evidence on which one bases a cervical cancer screening strategy.
Innovation catalyzes change
We acknowledge that it is difficult to give up something you have been doing for decades, so there is natural resistance by both patients and clinicians to move the Pap smear into a secondary role. But the data support primary HPV testing as the best screening option from a public health perspective.
At some point, hopefully soon, primary HPV testing will receive approval for self-sampling; this has the potential to reach patients in rural or remote locations who may otherwise not get screened for cervical cancer.13
The 2019 risk-based management guidelines from the ASCCP (American Society for Colposcopy and Cervical Pathology) also incorporate the use of HPV-based screening and surveillance after abnormal tests or colposcopy. Therefore, switching to primary HPV screening will not impact your ability to follow patients appropriately based on clinical guidelines.
Our advice to clinicians is to switch to primary HPV screening now if possible. If that is not feasible, continue your current strategy until you can make the change. And, of course, we recommend that you implement an HPV vaccination program in your practice to maximize primary prevention of HPV-related cancers. ●
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30.
- Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers–United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2016;65:661-666.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- US Preventive Services Task Force; Curry SJ, KristAH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337.
- Cooper CP, Saraiya M. Cervical cancer screening intervals preferred by US women. Am J Prev Med. 2018;55:389-394.
- Austin RM, Onisko A, Zhao C. Enhanced detection of cervical cancer and precancer through use of imaged liquid-based cytology in routine cytology and HPV cotesting. Am J Clin Pathol. 2018;150:385-392.
- Kaufman HW, Alagia DP, Chen Z, et al. Contributions of liquid-based (Papanicolaou) cytology and human papillomavirus testing in cotesting for detection of cervical cancer and precancer in the United States. Am J Clin Pathol. 2020;154:510-516.
- Blatt AJ, Kennedy R, Luff RD, et al. Comparison of cervical cancer screening results among 256,648 women in multiple clinical practices. Cancer Cytopathol. 2015;123:282-288.
- Ronco G, Dillner J, Elfstrom KM, et al; International HPV Screening Working Group. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524-532.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Kim JJ, Burger EA, Regan C, et al. Screening for cervical cancer in primary care: a decision analysis for the US Preventive Services Task Force. JAMA. 2018;320:706-714.
- Arbyn M, Smith SB, Temin S, et al; on behalf of the Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823.
How should I be approaching cervical cancer screening: with primary human papillomavirus (HPV) testing, or cotesting? We get this question all the time from clinicians. Although they have heard of the latest cervical cancer screening guidelines for stand-alone “primary” HPV testing, they are still ordering cervical cytology (Papanicolaou, or Pap, test) for women aged 21 to 29 years and cotesting (cervical cytology with HPV testing) for women with a cervix aged 30 and older.
Changes in cervical cancer testing guidance
Cervical cancer occurs in more than 13,000 women in the United States annually.1 High-risk types of HPV—the known cause of cervical cancer—also cause a large majority of cancers of the anus, vagina, vulva, and oropharynx.2
Cervical cancer screening programs in the United States have markedly decreased the incidence of and mortality from cervical cancer since introduction of the Pap smear in the 1950s. In 2000, HPV testing was approved by the US Food and Drug Administration (FDA) as a reflex test to a Pap smear result of atypical squamous cells of undetermined significance (ASC-US). HPV testing was then approved for use with cytology as a cotest in 2003 and subsequently as a primary stand-alone test in 2014.
Recently, the American Cancer Society (ACS) released new cervical screening guidelines that depart from prior guidelines.3 They recommend not to screen 21- to 24-year-olds and to start screening at age 25 until age 65 with the preferred strategy of primary HPV testing every 5 years, using an FDA-approved HPV test. Alternative screening strategies are cytology (Pap) every 3 years or cotesting every 5 years.
The 2018 US Preventive Services Task Force (USPSTF) guidelines differ from the ACS guidelines. The USPSTF recommends cytology every 3 years as the preferred method for women with a cervix who are aged 21 to 29 years and, for women with a cervix who are aged 30 to 65 years, the option for cytology every 3 years, primary HPV testing every 5 years, or cotesting every 5 years (TABLE).4

Why the reluctance to switch to HPV testing?
Despite FDA approval in 2014 for primary HPV testing and concurrent professional society guidance to use this testing strategy in women with a cervix who are aged 25 years and older, few practices in the United States have switched over to primary HPV testing for cervical cancer screening.5,6 Several reasons underlie this inertia:
- Many practices currently use HPV tests that are not FDA approved for primary HPV testing.
- Until recently, national screening guidelines did not recommend primary HPV testing as the preferred testing strategy.
- Long-established guidance on the importance of regular cervical cytology screening promoted by the ACS and others (which especially impacts women with a cervix older than age 50 who guide their younger daughters) will rely on significant re-education to move away from the established “Pap smear” cultural icon to a new approach.
- Last but not least, companies that manufacture HPV tests and laboratories integrated to offer such tests not yet approved for primary screening are promoting reliance on the prior proven cotest strategy. They have lobbied to preserve cotesting as a primary test, with some laboratory database studies showing gaps in detection with HPV test screening alone.7-9
Currently, the FDA-approved HPV tests for primary HPV screening include the Cobas HPV test (Roche) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Both are DNA tests for 14 high-risk types of HPV that include genotyping for HPV 16 and 18.
Continue to: Follow the evidence...
Follow the evidence
Several trials in Europe and Canada provide supporting evidence for primary HPV testing, and many European countries have moved to primary HPV testing as their preferred screening method.10,11 The new ACS guidelines put us more in sync with the rest of the world, where HPV testing is the dominant strategy.
It is true that doing additional tests will find more disease; cotesting has been shown to very minimally increase detection of cervical intraepithelial neoplasia grade 2/3 (CIN 2/3) compared with HPV testing alone, but it incurs many more costs and procedures.12 The vast majority of cervical cancer is HPV positive, and cytology still can be used as a triage to primary HPV screening until tests with better sensitivity and/or specificity (such as dual stain and methylation) can be employed to reduce unnecessary “false-positive” driven procedures.
As mentioned, many strong forces are trying to keep cotesting as the preferred strategy. It is important for clinicians to recognize the corporate investment into screening platforms, relationships, and products that underlie some of these efforts so as not to be unfairly influenced by their lobbying. Data from well-conducted, high-quality studies should be the evidence on which one bases a cervical cancer screening strategy.
Innovation catalyzes change
We acknowledge that it is difficult to give up something you have been doing for decades, so there is natural resistance by both patients and clinicians to move the Pap smear into a secondary role. But the data support primary HPV testing as the best screening option from a public health perspective.
At some point, hopefully soon, primary HPV testing will receive approval for self-sampling; this has the potential to reach patients in rural or remote locations who may otherwise not get screened for cervical cancer.13
The 2019 risk-based management guidelines from the ASCCP (American Society for Colposcopy and Cervical Pathology) also incorporate the use of HPV-based screening and surveillance after abnormal tests or colposcopy. Therefore, switching to primary HPV screening will not impact your ability to follow patients appropriately based on clinical guidelines.
Our advice to clinicians is to switch to primary HPV screening now if possible. If that is not feasible, continue your current strategy until you can make the change. And, of course, we recommend that you implement an HPV vaccination program in your practice to maximize primary prevention of HPV-related cancers. ●
How should I be approaching cervical cancer screening: with primary human papillomavirus (HPV) testing, or cotesting? We get this question all the time from clinicians. Although they have heard of the latest cervical cancer screening guidelines for stand-alone “primary” HPV testing, they are still ordering cervical cytology (Papanicolaou, or Pap, test) for women aged 21 to 29 years and cotesting (cervical cytology with HPV testing) for women with a cervix aged 30 and older.
Changes in cervical cancer testing guidance
Cervical cancer occurs in more than 13,000 women in the United States annually.1 High-risk types of HPV—the known cause of cervical cancer—also cause a large majority of cancers of the anus, vagina, vulva, and oropharynx.2
Cervical cancer screening programs in the United States have markedly decreased the incidence of and mortality from cervical cancer since introduction of the Pap smear in the 1950s. In 2000, HPV testing was approved by the US Food and Drug Administration (FDA) as a reflex test to a Pap smear result of atypical squamous cells of undetermined significance (ASC-US). HPV testing was then approved for use with cytology as a cotest in 2003 and subsequently as a primary stand-alone test in 2014.
Recently, the American Cancer Society (ACS) released new cervical screening guidelines that depart from prior guidelines.3 They recommend not to screen 21- to 24-year-olds and to start screening at age 25 until age 65 with the preferred strategy of primary HPV testing every 5 years, using an FDA-approved HPV test. Alternative screening strategies are cytology (Pap) every 3 years or cotesting every 5 years.
The 2018 US Preventive Services Task Force (USPSTF) guidelines differ from the ACS guidelines. The USPSTF recommends cytology every 3 years as the preferred method for women with a cervix who are aged 21 to 29 years and, for women with a cervix who are aged 30 to 65 years, the option for cytology every 3 years, primary HPV testing every 5 years, or cotesting every 5 years (TABLE).4

Why the reluctance to switch to HPV testing?
Despite FDA approval in 2014 for primary HPV testing and concurrent professional society guidance to use this testing strategy in women with a cervix who are aged 25 years and older, few practices in the United States have switched over to primary HPV testing for cervical cancer screening.5,6 Several reasons underlie this inertia:
- Many practices currently use HPV tests that are not FDA approved for primary HPV testing.
- Until recently, national screening guidelines did not recommend primary HPV testing as the preferred testing strategy.
- Long-established guidance on the importance of regular cervical cytology screening promoted by the ACS and others (which especially impacts women with a cervix older than age 50 who guide their younger daughters) will rely on significant re-education to move away from the established “Pap smear” cultural icon to a new approach.
- Last but not least, companies that manufacture HPV tests and laboratories integrated to offer such tests not yet approved for primary screening are promoting reliance on the prior proven cotest strategy. They have lobbied to preserve cotesting as a primary test, with some laboratory database studies showing gaps in detection with HPV test screening alone.7-9
Currently, the FDA-approved HPV tests for primary HPV screening include the Cobas HPV test (Roche) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Both are DNA tests for 14 high-risk types of HPV that include genotyping for HPV 16 and 18.
Continue to: Follow the evidence...
Follow the evidence
Several trials in Europe and Canada provide supporting evidence for primary HPV testing, and many European countries have moved to primary HPV testing as their preferred screening method.10,11 The new ACS guidelines put us more in sync with the rest of the world, where HPV testing is the dominant strategy.
It is true that doing additional tests will find more disease; cotesting has been shown to very minimally increase detection of cervical intraepithelial neoplasia grade 2/3 (CIN 2/3) compared with HPV testing alone, but it incurs many more costs and procedures.12 The vast majority of cervical cancer is HPV positive, and cytology still can be used as a triage to primary HPV screening until tests with better sensitivity and/or specificity (such as dual stain and methylation) can be employed to reduce unnecessary “false-positive” driven procedures.
As mentioned, many strong forces are trying to keep cotesting as the preferred strategy. It is important for clinicians to recognize the corporate investment into screening platforms, relationships, and products that underlie some of these efforts so as not to be unfairly influenced by their lobbying. Data from well-conducted, high-quality studies should be the evidence on which one bases a cervical cancer screening strategy.
Innovation catalyzes change
We acknowledge that it is difficult to give up something you have been doing for decades, so there is natural resistance by both patients and clinicians to move the Pap smear into a secondary role. But the data support primary HPV testing as the best screening option from a public health perspective.
At some point, hopefully soon, primary HPV testing will receive approval for self-sampling; this has the potential to reach patients in rural or remote locations who may otherwise not get screened for cervical cancer.13
The 2019 risk-based management guidelines from the ASCCP (American Society for Colposcopy and Cervical Pathology) also incorporate the use of HPV-based screening and surveillance after abnormal tests or colposcopy. Therefore, switching to primary HPV screening will not impact your ability to follow patients appropriately based on clinical guidelines.
Our advice to clinicians is to switch to primary HPV screening now if possible. If that is not feasible, continue your current strategy until you can make the change. And, of course, we recommend that you implement an HPV vaccination program in your practice to maximize primary prevention of HPV-related cancers. ●
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30.
- Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers–United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2016;65:661-666.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- US Preventive Services Task Force; Curry SJ, KristAH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337.
- Cooper CP, Saraiya M. Cervical cancer screening intervals preferred by US women. Am J Prev Med. 2018;55:389-394.
- Austin RM, Onisko A, Zhao C. Enhanced detection of cervical cancer and precancer through use of imaged liquid-based cytology in routine cytology and HPV cotesting. Am J Clin Pathol. 2018;150:385-392.
- Kaufman HW, Alagia DP, Chen Z, et al. Contributions of liquid-based (Papanicolaou) cytology and human papillomavirus testing in cotesting for detection of cervical cancer and precancer in the United States. Am J Clin Pathol. 2020;154:510-516.
- Blatt AJ, Kennedy R, Luff RD, et al. Comparison of cervical cancer screening results among 256,648 women in multiple clinical practices. Cancer Cytopathol. 2015;123:282-288.
- Ronco G, Dillner J, Elfstrom KM, et al; International HPV Screening Working Group. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524-532.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Kim JJ, Burger EA, Regan C, et al. Screening for cervical cancer in primary care: a decision analysis for the US Preventive Services Task Force. JAMA. 2018;320:706-714.
- Arbyn M, Smith SB, Temin S, et al; on behalf of the Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30.
- Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers–United States, 2008-2012. MMWR Morb Mortal Wkly Rep. 2016;65:661-666.
- Fontham ET, Wolf AM, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346.
- US Preventive Services Task Force; Curry SJ, KristAH, Owens DK, et al. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125:330-337.
- Cooper CP, Saraiya M. Cervical cancer screening intervals preferred by US women. Am J Prev Med. 2018;55:389-394.
- Austin RM, Onisko A, Zhao C. Enhanced detection of cervical cancer and precancer through use of imaged liquid-based cytology in routine cytology and HPV cotesting. Am J Clin Pathol. 2018;150:385-392.
- Kaufman HW, Alagia DP, Chen Z, et al. Contributions of liquid-based (Papanicolaou) cytology and human papillomavirus testing in cotesting for detection of cervical cancer and precancer in the United States. Am J Clin Pathol. 2020;154:510-516.
- Blatt AJ, Kennedy R, Luff RD, et al. Comparison of cervical cancer screening results among 256,648 women in multiple clinical practices. Cancer Cytopathol. 2015;123:282-288.
- Ronco G, Dillner J, Elfstrom KM, et al; International HPV Screening Working Group. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524-532.
- Ogilvie GS, van Niekerk D, Krajden M, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43-52.
- Kim JJ, Burger EA, Regan C, et al. Screening for cervical cancer in primary care: a decision analysis for the US Preventive Services Task Force. JAMA. 2018;320:706-714.
- Arbyn M, Smith SB, Temin S, et al; on behalf of the Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018;363:k4823.
Patient-centered risk assessment for ovarian cancer: Individualizing your approach
2 HPV vaccines, 7 questions that you need answered
Not long ago (in medical years), we were still trying to discover the cause of cervical cancer. Today, not only do we know that cause to be persistent human papillomavirus (HPV) infection, but we have two vaccines at our disposal to prevent the primary oncogenic strains of the virus.
We’ve come a long way.
The availability of two vaccines raises questions, however. What kind of data do we have on the bivalent (Cervarix, GlaxoSmithKline) and quadrivalent (Gardasil, Merck) vaccines so far? Is one of them clearly superior to the other? If not, what population is each vaccine best suited for—and how do we counsel patients about their options?
To address these and other questions, OBG Management Contributing Editor Neal M. Lonky, MD, MPH, assembled a panel of physicians who have expertise in cervical disease detection and prevention and asked them to sift the data that have accumulated thus far. In the discussion that follows, they touch on long-term efficacy, the likely impact of the vaccines on cervical cancer screening, and other aspects of disease prevention in the era of HPV vaccination.

Juan C. Felix, MD
Professor of Clinical Pathology and Obstetrics and Gynecology; Director of Cytopathology fellowship; and Chief of Gynecologic Pathology at the Keck School of Medicine, University of Southern California; and Chief of Cytopathology at Los Angeles County and University of Southern California Medical Center in Los Angeles.
Dr. Felix reports that he is a speaker for Merck and GlaxoSmithKline.

Diane M. Harper, MD, MS, MPH
Director of the Gynecologic Cancer Prevention Research Group and Professor of Obstetrics and Gynecology, Community and family Medicine, and Informatics and Personalized Medicine at the University of Missouri–Kansas City School of Medicine.
Dr. Harper reports that she has served as a speaker and advisor for Merck and GlaxoSmithKline, and that the institutions at which she conducted HPV vaccination trials have received funding from Merck and GlaxoSmithKline.

Warner K. Huh, MD
Associate Professor in the Department of Obstetrics and Gynecology, and Associate Scientist at the Comprehensive Cancer Center at the University of Alabama– Birmingham.
Dr. Huh reports that he receives grant or research support from and is a speaker and consultant to Merck and GlaxoSmithKline.

Karen K. Smith-McCune, MD, PhD
John Kerner Endowed Chair of Gynecologic Oncology, Director of the Dysplasia Clinic, and Professor of Obstetrics, Gynecology, and Reproductive Sciences at the University of California–San francisco.
Dr. Smith-McCune reports she has performed unpaid consulting for OncoHealth Inc. and is planning to join its Scientific Advisory Board.
1. How were the vaccines developed?
Neal M. Lonky, MD, MPH: What should clinicians know about the development, function, and mechanism of action of the two HPV vaccines?
Warner K. Huh, MD: The bivalent and quadrivalent vaccines are both excellent products, and their respective Phase-3 trials demonstrate that they provide impressive protection against HPV, particularly among women who test negative (by polymerase chain reaction) for the specific HPV types contained within the vaccines.1-3
Cervarix protects against HPV types 16 and 18, whereas Gardasil is effective against HPV types 6, 11, 16, and 18.
Dr. Lonky: Do the vaccines function similarly?
Diane M. Harper, MD, MS, MPH: Yes. Both stimulate an immediate antibody response in the woman who is not infected with the relevant virus and are effective in preventing cervical intraepithelial neoplasia grade 2 and higher (CIN 2+), as well as persistent infection, caused by vaccine-related and cross-protected HPV types. The quality of the antibody response is best for HPV 16 for both vaccines. The quality of the antibody response for HPV 6, 11, and 18 for Gardasil is much poorer than its response for HPV 16. Cervarix induces an equally high and sustained antibody response for HPV 18 as for HPV 16.
Juan C. Felix, MD: Both vaccines are based on the same virus-like particles (VLP). The functionality of the vaccines is, therefore, mainly dependent on the dosage of VLP and the adjuvant used. Gardasil uses a proprietary aluminum sulfate adjuvant, whereas Cervarix uses aluminum hydroxide and monophosphoryl lipid A.
Karen K. Smith-McCune, MD, PhD: Both adjuvants have an extensive track record of safety and efficacy in other vaccines. Because they have different structures, however, they may have varying effects on many components of the immune response elicited by the L1 antigens.
Dr. Harper: Both adjuvants contain aluminum, which has so far proved to be safe despite the newly established association between high aluminum intake and Alzheimer’s disease.
Dr. Lonky: Were there any notable challenges in developing the vaccines?
Dr. Harper: It was difficult to formulate the appropriate dosages of VLP in Gardasil. Higher dosages of HPV 11 and 16 were needed to prevent cross-inhibition by HPV 6 and 18. As a result, the antigenic protein component of Gardasil that is necessary to effect an immunologic antibody response is high, at 120 μg. In Cervarix, the antigenic VLP load is 20 μg each for HPV 16 and 18.
Dr. Lonky: What is the significance of the different VLP loads?
Dr. Harper: Side effects, such as autoimmune neurologic demyelination, albeit rare, have been associated with a higher antigenic protein load. Multiple reports of autoimmune demyelinating diseases—including paralysis, blindness, and death—have been published by neurologists in regard to Gardasil.4,5 Others have shown that young girls are more at risk than young boys for these neurologic side effects.6
Dr. Felix: Some data suggest that the two vaccine formulations interact differently with the human immune system. In a head-to-head trial funded by GlaxoSmithKline, Cervarix produced higher total and neutralizing antibody titers than Gardasil did.7
Although higher immunogenicity is generally thought to be beneficial, the ultimate determinant of a vaccine’s success is its efficacy—and duration of that efficacy—in clinical trials and follow-up of vaccinated populations. So far, Cervarix has demonstrated efficacy through 8.4 years in its follow-up cohort.8 Similarly, Gardasil has proved to be effective after 5 years of follow-up, with no incident cases of cervical cancer reported in the vaccinated arm.9
2. Does either vaccine offer “extra” immunity?
Dr. Lonky: What is the potential for overlapping immunity to other high-risk viral types with these vaccines?
Dr. Harper: It is quite clear from pivotal trials of both vaccines that Gardasil produces efficacy of 46% against persistent infection caused by HPV 31. Data from the pivotal Phase-3 trial of Gardasil also show that it offers no protection against persistent infection with HPV 45, an important cause of adenocarcinoma.10
In contrast, Cervarix demonstrates substantial efficacy against both persistent infection and CIN 2+ disease caused by HPV 31, 33, and 45.3
These findings mean that Cervarix is 91% effective against HPV types that cause adenocarcinoma and 83% effective overall against squamous cell carcinoma. Compare that with Gardasil, which is 78% effective overall against HPV types that cause adenocarcinoma and 73% effective against HPV types that cause squamous cell carcinoma.
The immune titers tell a supportive story. After vaccination with Gardasil, the antibody titer immediately declines for HPV 6, 11, and 18, reaching the baseline for natural infection within 18 months.7 HPV 18 shows continued, significant loss of seropositivity over time, and antibody titers for HPV 6 and 11 also decline. In the monovalent HPV 16 pre-Gardasil experimental vaccine, 14% of women no longer had measurable titers to HPV 16 after 8.5 years.11
After vaccination with Cervarix, antibody titers for HPV 16 and 18 remain more than seven times and more than four times higher, respectively, than natural infection titers for 8.4 years, with no loss of measurable antibody titer for either type. The antibody titers for HPV 31, 33, and 45 remain substantially higher than natural infection titers for at least 6.4 years. These titers correlate with the vaccine’s very high efficacy against CIN 2+ lesions caused by HPV 16, 18, 31, 33, and 45.
In other words, Cervarix generates an immune response (and efficacy) that indicates robust protection against five of the most common oncogenic HPV types, providing maximal protection against nearly 85% of all cervical cancers. Gardasil protects against 74% of all cervical cancers overall.12 This makes Cervarix the superior cervical cancer vaccine.
Gardasil is the superior vaccine against genital warts, although the duration of its protection is uncertain.
Dr. Huh: I’d just like to point out that there are no head-to-head trials comparing the vaccines in terms of efficacy. Antibody titers are higher with Cervarix than with Gardasil, as you noted, and it may be that, over time, the higher titers are more durable with Cervarix. However, we have yet to fully correlate clinical efficacy with antibody titers. In other words, immunogenicity does not equal clinical efficacy.
Dr. Felix: The data for Gardasil are particularly interesting because there have been no incident HPV-18 lesions detected despite the absence of detectable HPV-18 antibody titers in more than 20% of vaccinated women as soon as 2 years after immunization.9 These data strongly suggest that it is not antibody titer alone that grants protection against HPV-induced lesions of the cervix.
Dr. Harper: This speaks to the difficulty of running a trial to ensure both enough participants and a sufficient attack rate of HPV 18 to cause new lesions to be detected in vaccinated women. In the relevant trial, there were only 112 vaccinated women—not nearly enough women to overcome the very low attack rate of HPV 18 in the trial population—and they were followed for 5 years.9 We cannot be sure that the lack of incident HPV-18 lesions in the vaccinated women is the result of efficacy.
Dr. Felix: As for overlapping immunity to HPV types not included in the vaccines, it has been described for both Cervarix and Gardasil. In the case of Cervarix, the manufacturer demonstrated unexpectedly high rates of protection against all CIN 2+ and CIN 3+ lesions—70% and 87%, respectively. These rates were too high to be explained by protection against types 16, 18, 31, and 45 alone. It is possible, therefore, that Cervarix may protect against other high-risk HPV types.13
Gardasil has proved to be effective against HPV types 31, 33, 52, and others.10 When total protection against CIN 2+ and CIN 3+ lesions is examined from Phase-3 trials of the vaccine, however, the rates are only 42% and 43%, respectively. These data are difficult to interpret because HPV 16 and 18 together are thought to account for 70% of CIN 3. Some reassurance can be gained from the fact that the number of incident cases of CIN 2+ and CIN 3+ caused by HPV 16 and 18 in the vaccinated group in the Gardasil trial was identical to the number seen in the Cervarix trial.3,10 The reason for the discrepancy in total number of cases of CIN 2+ and CIN 3+ between the two trials—and, therefore, between the two vaccines—cannot be explained by cross-protection alone and is probably attributable to differences in study populations. The Gardasil trial had a higher baseline prevalence of HPV 16 and 18 (9% and 4%, respectively) than the Cervarix trial did (5% and 2%, respectively), a fact that may be explained by the different demographics of their respective populations.2,14
Ultimately, it is hazardous to compare trials, particularly when they are conducted in significantly different populations. On this issue, I concur with the World Health Organization (WHO), which recommended that such comparisons be avoided in the determination of which type of HPV vaccine to recommend.15
Dr. Huh: I agree that it would be inappropriate to make cross-trial comparisons, given differences in the way the trials were designed and conducted. To draw conclusions about clinical efficacy of these two excellent vaccines, based on a comparison of their trials, is completely unscientific. Only a true head-to-head study that has efficacy as its endpoint can tell us which vaccine is superior—and such a trial would require thousands (if not tens of thousands) of subjects and a considerable amount of time to complete. In my opinion, such a study would be counterproductive to our goal of vaccination.
Dr. Harper: I disagree. The whole purpose of this roundtable is to compare vaccines. It is not “unscientific” to compare the trials.
Dr. Huh: On the contrary—it is completely inappropriate to directly compare the Phase-3 clinical trials from Merck and GlaxoSmithKline. One can speculate about the differences between them, but any clinical trialist knows that a direct, scientific comparison cannot be made. Only a real head-to-head study powered for efficacy can do this.
- Both the bivalent and quadrivalent vaccines appear to be excellent products. Besides protecting against the main oncogenic strains of human papillomavirus (HPV) (types 16 and 18 for both vaccines, and the genital-wart-associated strains 6 and 11 for the quadrivalent vaccine), both Cervarix and Gardasil offer some degree of cross-protection against additional HPV strains.
- Vaccination of the sexually naïve patient with either vaccine provides significant protection against cervical intraepithelial neoplasia 2 (CIN 2) or worse.
- HPV vaccination is expected to reduce the rate of abnormal Pap tests and the need for common excisional treatments for cervical dysplasia in vaccinated women. It will do the same in the population as a whole if rates of vaccination are sufficient to provide “herd” immunity.
3. Is one vaccine more effective than the other?
Dr. Lonky: How do the vaccines compare in terms of efficacy?
Dr. Smith-McCune: In discussing efficacy, I think we should focus on CIN 3 because it is the immediate surrogate for cancer, whereas CIN 2 lesions can be transient in younger women. I think it is also important to focus on outcomes regardless of the HPV types associated with the lesions. This approach is more clinically relevant, as we don’t perform HPV typing of lesions in clinical practice. Nor do we manage lesions differently depending on the HPV type in the lesion.
That said, it is difficult to compare efficacy of the vaccines for several reasons, a few of which we have already discussed. For example, the bivalent and quadrivalent vaccines were studied in separate randomized trials. Although the study populations were similar, they were not identical. Women in both trials were relatively sexually naïve, but the cutoff for number of lifetime sexual partners was different (5 for Gardasil versus 7 for Cervarix). In trials of Gardasil, women who had a history of abnormal cytology or genital warts were excluded. In trials of Cervarix, women who had a history of colposcopy were excluded. In Gardasil trials, approximately 3% of women were from the Asian Pacific, versus 34% in the Cervarix trials, and so on.3,16
The trials also had different protocols for referral to colposcopy, which would affect disease detection. And the length of follow-up differs between trials.3,9
Dr. Lonky: Can we draw any conclusions about efficacy?
Dr. Smith-McCune: Yes. The trials defined outcomes in several populations of participants. In addition to the overall population (called the “intention-to-treat population” in the Gardasil trials and the “total vaccinated cohort” in the Cervarix trials), the trials defined a subpopulation of women naïve to oncogenic HPV types to gain information about the likely impact of vaccinating girls before the onset of sexual activity. The definitions of these “naïve” populations were slightly different, mainly in the number of HPV types tested, so again, some caution needs to be exercised in making comparisons.
End-of-trial data in the naïve population show a 43% reduction in CIN 3 lesions for Gardasil and 87% for Cervarix (for CIN 3 or worse). By inference, we can tell the sexually naïve patient that vaccination with either vaccine will provide significant protection against CIN 3 lesions, likely to result in significant protection against cervical cancer over time.
We can gather some estimates of efficacy in sexually non-naïve women by looking at results from all trial participants. Gardasil reduced overall CIN 3 lesions by 16% overall; Cervarix reduced CIN 3 or worse by 33%. When counseling an individual patient, if she has had a similarly low number of lifetime sexual partners (e.g., the median number in the Gardasil trials was 2), these results provide an estimate of her likely protection against CIN 3 with vaccination.
Common excisional treatments for cervical dysplasia are known to be associated with adverse perinatal outcomes.17 The ability to reduce the need for these treatments is an important outcome of vaccination. In the HPV-naïve populations, vaccination reduced definitive cervical therapies or excisions by 42% (Gardasil) and 69% (Cervarix). These figures are useful in counseling virginal patients about the long-term benefits of vaccination.
For sexually active patients 26 years and younger, HPV vaccination significantly reduced definitive cervical therapy or excisions by 23% (Gardasil) and 25% (Cervarix). Again, these figures are most applicable for counseling patients who have had relatively few lifetime sexual partners. So the exact extent of protection is likely to vary by the patient’s total number of lifetime sexual partners.
I expect that we will see more data on the effects of vaccination stratified by the number of lifetime sexual partners, because that information would be very useful in counseling individual sexually active women.
Dr. Harper: Both vaccines reduce the rate of abnormal Pap tests by 10% regardless of HPV type in that population of women.9,18
4. Are the two vaccines safe?
Dr. Lonky: What about safety of the vaccines? What do we know?
Dr. Felix: The safety profiles seen in clinical trials of both vaccines are very similar and consist almost entirely of nonserious adverse events.2,9 In the United States, a greater number of Gardasil doses has been administered, owing to its earlier development. As of January 1, 2010, more than 28 million doses had been distributed, and numerous major events had been recorded in the Vaccine Adverse Event Reporting System (VAERS). Of 15,829 adverse events reported, only 8% were considered serious by the CDC. CDC investigation, by expert panels, of all serious adverse events found no evidence linking Gardasil to any of them, including Guillain-Barré syndrome, blood clots, and death.19
Dr. Huh: A few other points to consider:
- The reporting rate for Gardasil is triple that for all other vaccines combined
- Because VAERS is a passive reporting system, under-reporting is distinctly possible
- Post-licensure safety surveillance is still underway
- Both products have pregnancy registries.
Dr. Harper: The current postmarketing commitment between Merck and the FDA is to recognize a rate of serious adverse events that exceeds 2 cases in every 10,000 women in a cohort of 44,000 women who have received all three doses of Gardasil. Although autoimmune neurologic sequelae have occurred after Gardasil administration, regulatory authorities are not required to evaluate these reactions, such as Guillain-Barré syndrome, because the frequency is lower than the agreed-upon threshold. Nevertheless, adverse events could be life threatening to some girls.
Any risk of death—even if it is lower than the agreed-upon threshold—should be presented to women as a possible risk of vaccination with Gardasil. In the United States, the same women could choose a lifetime of Pap screening and be afforded the same protection against cervical cancer as they would get from vaccination.
5. Is quadrivalent better than bivalent?
Dr. Lonky: Why would a clinician choose a bivalent vaccine when the quadrivalent vaccine protects not only against carcinogenic types 16 and 18, but also against HPV-associated genital warts?
Dr. Harper: A smart clinician would ask the patient what she values. The physician is obligated to present the evidence and let her choose!
Dr. Lonky: What does the evidence suggest?
Dr. Felix: A clear recommendation between Cervarix and Gardasil is very difficult to make at this time, for the reasons already stated. Both vaccines provide 98% protection against HPV 16 and 18 for the prevention of CIN 2+ lesions.3,9 As we have discussed, both vaccines also provide protection against high-risk strains of HPV other than types 16 and 18.
In the Cervarix trial, there was an overall reduction of all CIN 2+ lesions that was higher as a percentage of total lesions than the reduction seen in the Gardasil trial.3,9 However, unlike Cervarix, Gardasil significantly reduced the rate of vaginal intraepithelial neoplasia (VaIN) and vulvar intraepithelial neoplasia (VIN).9
Dr. Harper: GlaxoSmithKline is analyzing its data on vulvar and vaginal protection, and it is likely that Cervarix will demonstrate some efficacy in this regard, too. But the economic burden of noncervical cancers is estimated to be only 8% of the economic burden of all HPV-related diseases.20 The prevention of cervical cancer is the dominant clinical and economic force for vaccination.
Dr. Felix: Clearly, the protection against genital warts demonstrated in the Gardasil trial will not be realized with Cervarix, as it does not offer immunization or cross-protection against HPV 6 or 11. Gardasil’s protection against HPV 6 and 11 prompted FDA approval of the vaccine for boys and men.21
According to the WHO, when counseling girls and women about the HPV vaccine, the clinician should weigh the possible value of a deep reduction in total CIN 2+ lesions provided by Cervarix against the reduction in VaIN, VIN, and genital warts provided by Gardasil.15 Boys and men will see clinically proven benefits only from Gardasil for the prevention of external genital warts. Other benefits are strictly theoretical.22,23
Dr. Harper: Vaccine protection must last at least 15 years to reduce the rate of cervical cancer. Otherwise, the development of cervical cancer will only be postponed, if boosters are not implemented.
It is now widely recognized that Cervarix induces high antibody titers, offering 100% efficacy even after 8.4 years, making it very likely that the protection it provides will continue for at least 15 years. It is also widely acknowledged by immunologists that Gardasil-induced titers for HPV 6, 11, and 18 are much shorter-lived, so protection is likely to wane 5 to 10 years after vaccination.
That means that Gardasil provides excellent protection against one cancer-causing type of HPV. In addition, it protects against genital warts caused by HPV types 6 and 11 for at least 5 years. In comparison, Cervarix protects against five cancer-causing types of HPV, thereby preventing about 90% of cervical cancers, and is likely to remain effective for at least 15 years.
There are 10 times as many women who have an abnormal Pap test as there are women who have genital warts, so one would think that Cervarix would be the vaccine of choice in preventing the life-threatening disease of cervical cancer.
Dr. Smith-McCune: I would agree that the choice should be discussed with patients—and with parents. If the objective is primarily to protect against cervical cancer precursors, then the bivalent vaccine may be the better choice, with the caveat that we can’t really compare the results from the vaccine trials for reasons discussed earlier, and there are no data from a randomized head-to-head trial comparing the two vaccines. If the decision involves a desire to reduce genital warts or vulvar and vaginal dysplasia, then the quadrivalent vaccine would be the better choice.
6. What impact do the vaccines have on screening?
Dr. Lonky: Do the vaccines have varying effects on our need to screen for, triage, and treat cervical cancer precursors?
Dr. Harper: The Pap smear has reduced the rate of cervical cancer in the United States by 75%—that rate is now at an all-time low of 8 cases for every 100,000 women. But the Pap smear is not perfect; there is a 30% false-negative rate among women who develop cervical cancer, and a large false-positive rate that involves referral to colposcopy for minimally abnormal cytology reports. And when CIN 2+ disease is detected, treatment is not without risk. Surgery increases the risk of reproductive morbidity in future pregnancies. Having protection against this outcome could be tremendously valuable for some women.
Compare the HPV vaccine, which has probable benefit but also the potential for serious adverse events, including demyelinating diseases that cause blindness, paralysis, and death in a small number of recipients.
If women were to choose to be vaccinated with Gardasil and forgo further Pap screening, the rate of cervical cancer in the United States would rise from 8 to 14 cases for every 100,000 women. If they were to choose Cervarix instead, with no further Pap screening, the rate would rise from 8 to 9.5 cases for every 100,000 women.
If women were to choose both HPV vaccination and continued Pap screening, the rate of cervical cancer still would not decline from its current level of 8 cases for every 100,000 women. Instead, the benefit would be that fewer women have abnormal Pap tests, and fewer women would need to be treated for CIN 2+ disease.
Women and physicians must understand these facts. A woman who chooses to be vaccinated may gain individual protection, but the overall rate of cervical cancer will not be affected.
Dr. Huh: Regardless of the HPV vaccine selected, we need to seriously rethink how we screen women in the United States. One could easily argue that the combination of the vaccine and continued screening is too expensive. It might be wise to consider lengthening the screening interval—and, perhaps, further delaying initial screening to 25 years of age—to make cervical cancer prevention with both modalities more cost-effective.24 The most important thing to recognize is that women still need to be screened, even if they have been vaccinated.
As more women are vaccinated, we expect to see a decline in the prevalence of CIN 2+ and CIN 3+ lesions, and this will ultimately weaken the positive predictive value of cytology. Perhaps it is time to consider the HPV test as a primary screen, with triage to cytology in women who test HPV-positive.25
Dr. Smith-McCune: As we accumulate data over time about the effects of vaccination on the rates of CIN 3 and cancer, modeling will be helpful in determining the best screening algorithm for women who have been vaccinated against HPV.
It is important to remember that approximately 50% of women who are given a diagnosis of cervical cancer in the United States have never been screened. It is vital that we continue to reach out to the under-screened population and focus vaccination efforts on populations of girls who are likely to have limited access to care in the future.
7. Can we vaccinate every woman?
Dr. Lonky: Is universal vaccination of women achievable for either vaccine?
Dr. Felix: Universal vaccination against HPV would be achievable only via school mandates. Without them, vaccination will not approach the 80% threshold needed to produce herd immunity.
Despite the clear benefit of such mandates to the general population—particularly the medically under-served—the issue has become a political football. As a result, school mandates will probably never be realized.
Dr. Harper: I don’t believe it is ethical to mandate vaccination of all girls and women. It is a choice that women and parents, in conversation with their physicians and daughters, must make when considering how to be protected against cervical cancer. Herd immunity is a moot point because we are only vaccinating girls (50% of the population) and can never reach the theoretical 70% threshold for herd immunity to be apparent.
Dr. Lonky: Is the availability of two vaccines a boon or a hindrance?
Dr. Smith-McCune: I think it is always a good thing to have choices in medicine.
Dr. Huh: I see the availability of two vaccines as a boon. That availability means that two companies are now putting forth consistent educational messages about the importance of vaccination and, I hope, stimulating competition that will reduce the overall cost of the vaccine series. Having two vaccines can only promote awareness, access, and greater appreciation of the considerable protection these two vaccines provide.
Despite solid evidence that the quadrivalent (Gardasil) HPV vaccine and the bivalent (Cervarix) HPV vaccine protect against cervical cancer, only about one fifth of the female population between 11 and 26 years of age has received the full series of Gardasil since it won FDA approval in 2006. Barriers to vaccination are not financial alone, as the vaccination rate is similarly low among women who have health insurance.
Why isn’t the vaccination rate higher? I see eight barriers to full implementation:
- Economic disparities. Each vaccine costs roughly $400 (national average) for the full series of injections. Although women who do not get Pap screening are most likely to benefit from the vaccines, they usually cannot afford them. federal childhood immunization programs cover teens and young women until 18 years of age in most states, and until 21 years in a few. that leaves most women who seek vaccination from gynecologists without coverage.
- Fear. Pain at the injection site, syncope, and a slightly elevated incidence of thromboembolism are the adverse events most commonly associated with HPV vaccination in the literature. In the life cycle of a vaccine, reports of sudden death or neurologic injury (Guillain-Barré syndrome) occur in the early years, but are reported at a rate lower than 2 cases in every 10,000 women. Nevertheless, such events may create fear about undergoing immunization.
- Long latency period. Because the outcome of cancer prevention won’t become apparent for 20 to 40 years following vaccination, the need for immunization may seem less than urgent.
- Cultural and religious beliefs. Because carcinogenic HPV strains are sexually transmitted, some families may associate vaccination with the promotion of sexual activity. Even in states that mandate vaccination, the courts have upheld a parent’s right to refuse vaccination on these grounds.
- The premarket push. Aggressive promotion of vaccination by both manufacturers and a push by advocates for legislation to mandate the vaccine prior to completion of Phase-3 trials and gathering of robust safety data may have diminished trust in the vaccine and reduced its acceptance.
- Lack of legislation. In states that do not consider HPV vaccination to be a necessary public health intervention, the lack of mandates and funding reduce the vaccination rate. In addition, some legislators have been more active advocates of vaccination than others.
- Reduced involvement of the obGyn. ObGyns don’t routinely vaccinate patients; pediatricians do. Young women are slipping through the cracks because the conventional ObGyn practice does not have a vaccination program that ensures payment, reimbursement, and completion of the vaccine series. Many ObGyn practices are reluctant to institute such a program because the profit margin is small, there are associated risks, and the time required to counsel the patient and for follow-up is extensive.
- Failure to complete the series. Some women do not complete the full vaccine series, owing to cost or side effects, or both. Solid evidence that a single dose could be as protective as the full series would be compelling. A single-dose vaccine would also be less expensive.
The principal danger of a low vaccination rate is the loss of insurance coverage for immunization against HPV. On one hand, payers may begin to ask whether coverage is justified when so few girls and women are vaccinated, leaving the payer with two burdens: the expense of vaccination and the expense of conventional screening programs and treatment, although the costs of treatment would be reduced with vaccination. On the other hand, Gardasil’s protection against genital warts may provide incentive for payers to cover or discount the vaccine because of the reduction in the need to diagnose, triage, and treat condyloma.
Ultimately, HPV vaccination may become another optional intervention that is paid for by the individual, despite evidence in girls and women that cervical cancer can be prevented.
—NEAL M. LONKY, MD, MPH
We want to hear from you! Tell us what you think.
1. The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915-1927.
2. Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. HPV PATRICIA study group. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161-2170.
3. Paavonen J, Naud P, Salmeron J, et al. For the HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301-314.
4. DiMario FJ, Hajjar M, Ciesielski T. A 16-year-old girl with bilateral visual loss and left hemiparesis following an immunization against human papillomavirus. J Child Neurology. 2010;25(3):321-327.
5. Sutton I, Lahoria R, Tan I, Clouston P, Barnett M. CNS demyelination and quadrivalent HPV vaccination. Multiple Sclerosis. 2009;15(1):116-119.
6. Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10(5):338-349.
7. Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin. 2009;5(10):705-719.
8. GlaxoSmithKline Vaccine HPV-007 Study Group. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 8.4 years. Presented at: 28th Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID); May 4-8, 2010; Nice, France.
9. Muñoz N, Kjaer SK, Sigurdsson K, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102(5):325-339.
10. Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naïve women aged 16-26 years. J Infect Dis. 2009;199(7):926-935.
11. Rowhani-Rahbar A, Mao C, Hughes JP, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine. 2009;27(41):5612-5619.
12. Harper DM, Williams KB. Prophylactic HPV vaccines: current knowledge of impact on gynecologic premalignancies [published online ahead of print July 3, 2010]. Discovery Medicine. 2010;10(50).http://www.discoverymedicine.com/Diane-M-Harper/2010/07/03/prophylactichpv-vaccines-current-knowledge-of-impact-ongynecologic-premalignancies. Accessed July 13, 2010.
13. Cervarix [human papillomavirus bivalent (types 16 and 18) vaccine recombinant]. Food and Drug Administration. Initial US approval: 2009:1–12.
14. Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928-1943.
15. World Health Organization. Human papillomavirus vaccines: WHO position paper. Weekly Epidemiological Record. 2009;84(15):118-131.http://www.who.int/wer/2009/wer8415/en/index.html. Published April 10, 2009. Accessed July 13, 2010.
16. Ault KA. For Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on the risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369(9576):1861-1868.
17. Arbyn M, Kyrgiou M, Simoens C, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ. 2008;337:a1284.-Doi: 10.1136/bmj.a1284.
18. Paavonen J. For the HPV PATRICIA Study Group. Efficacy of HPV 16/18 ASO4-adjuvanted vaccine against abnormal cytology, colposcopy referrals, and cervical procedures. Abstract SS 4–1. Data presented at Cervical Cancer Prevention, EuroGin 2010. European Research Organisation on Genital Infection and Neoplasia (EUROGIN); February 17-20, 2010; Monte Carlo, Monaco.http://www.eurogin.com/2010/programoverview.html. Accessed July 13, 2010.
19. Centers for Disease Control and Prevention. Reports of health concerns following HPV vaccination. 2010;1-3.http://www.cdc.gov/vaccinesafety/Vaccines/HPV/gardasil.html. Published June 21, 2010. Accessed July 13, 2010.
20. Myers ER. The economic impact of HPV vaccines: not just cervical cancer. Am J Obstet Gynecol. 2008;198(5):487-488.
21. Gardasil [human papillomavirus quadrivalent (types 6, 11, 16, and 18) vaccine, recombinant]. Food and Drug Administration; 2009. Initial Approval 2006;1-26.
22. Olsson SE, Kjaer SK, Sigurdsson K, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin. 2009;5(10).
23. Pirotta M, Ung L, Stein A, et al. The psychosocial burden of human papillomavirus related disease and screening interventions. Sex Transm Infect. 2009;85(7):508-513.
24. Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359(8):821-832.
25. Cox JT. Is the HPV test effective as the primary screen for cervical cancer? Examining the Evidence. OBG Management. 2010;22(7):10-11.
Not long ago (in medical years), we were still trying to discover the cause of cervical cancer. Today, not only do we know that cause to be persistent human papillomavirus (HPV) infection, but we have two vaccines at our disposal to prevent the primary oncogenic strains of the virus.
We’ve come a long way.
The availability of two vaccines raises questions, however. What kind of data do we have on the bivalent (Cervarix, GlaxoSmithKline) and quadrivalent (Gardasil, Merck) vaccines so far? Is one of them clearly superior to the other? If not, what population is each vaccine best suited for—and how do we counsel patients about their options?
To address these and other questions, OBG Management Contributing Editor Neal M. Lonky, MD, MPH, assembled a panel of physicians who have expertise in cervical disease detection and prevention and asked them to sift the data that have accumulated thus far. In the discussion that follows, they touch on long-term efficacy, the likely impact of the vaccines on cervical cancer screening, and other aspects of disease prevention in the era of HPV vaccination.

Juan C. Felix, MD
Professor of Clinical Pathology and Obstetrics and Gynecology; Director of Cytopathology fellowship; and Chief of Gynecologic Pathology at the Keck School of Medicine, University of Southern California; and Chief of Cytopathology at Los Angeles County and University of Southern California Medical Center in Los Angeles.
Dr. Felix reports that he is a speaker for Merck and GlaxoSmithKline.

Diane M. Harper, MD, MS, MPH
Director of the Gynecologic Cancer Prevention Research Group and Professor of Obstetrics and Gynecology, Community and family Medicine, and Informatics and Personalized Medicine at the University of Missouri–Kansas City School of Medicine.
Dr. Harper reports that she has served as a speaker and advisor for Merck and GlaxoSmithKline, and that the institutions at which she conducted HPV vaccination trials have received funding from Merck and GlaxoSmithKline.

Warner K. Huh, MD
Associate Professor in the Department of Obstetrics and Gynecology, and Associate Scientist at the Comprehensive Cancer Center at the University of Alabama– Birmingham.
Dr. Huh reports that he receives grant or research support from and is a speaker and consultant to Merck and GlaxoSmithKline.

Karen K. Smith-McCune, MD, PhD
John Kerner Endowed Chair of Gynecologic Oncology, Director of the Dysplasia Clinic, and Professor of Obstetrics, Gynecology, and Reproductive Sciences at the University of California–San francisco.
Dr. Smith-McCune reports she has performed unpaid consulting for OncoHealth Inc. and is planning to join its Scientific Advisory Board.
1. How were the vaccines developed?
Neal M. Lonky, MD, MPH: What should clinicians know about the development, function, and mechanism of action of the two HPV vaccines?
Warner K. Huh, MD: The bivalent and quadrivalent vaccines are both excellent products, and their respective Phase-3 trials demonstrate that they provide impressive protection against HPV, particularly among women who test negative (by polymerase chain reaction) for the specific HPV types contained within the vaccines.1-3
Cervarix protects against HPV types 16 and 18, whereas Gardasil is effective against HPV types 6, 11, 16, and 18.
Dr. Lonky: Do the vaccines function similarly?
Diane M. Harper, MD, MS, MPH: Yes. Both stimulate an immediate antibody response in the woman who is not infected with the relevant virus and are effective in preventing cervical intraepithelial neoplasia grade 2 and higher (CIN 2+), as well as persistent infection, caused by vaccine-related and cross-protected HPV types. The quality of the antibody response is best for HPV 16 for both vaccines. The quality of the antibody response for HPV 6, 11, and 18 for Gardasil is much poorer than its response for HPV 16. Cervarix induces an equally high and sustained antibody response for HPV 18 as for HPV 16.
Juan C. Felix, MD: Both vaccines are based on the same virus-like particles (VLP). The functionality of the vaccines is, therefore, mainly dependent on the dosage of VLP and the adjuvant used. Gardasil uses a proprietary aluminum sulfate adjuvant, whereas Cervarix uses aluminum hydroxide and monophosphoryl lipid A.
Karen K. Smith-McCune, MD, PhD: Both adjuvants have an extensive track record of safety and efficacy in other vaccines. Because they have different structures, however, they may have varying effects on many components of the immune response elicited by the L1 antigens.
Dr. Harper: Both adjuvants contain aluminum, which has so far proved to be safe despite the newly established association between high aluminum intake and Alzheimer’s disease.
Dr. Lonky: Were there any notable challenges in developing the vaccines?
Dr. Harper: It was difficult to formulate the appropriate dosages of VLP in Gardasil. Higher dosages of HPV 11 and 16 were needed to prevent cross-inhibition by HPV 6 and 18. As a result, the antigenic protein component of Gardasil that is necessary to effect an immunologic antibody response is high, at 120 μg. In Cervarix, the antigenic VLP load is 20 μg each for HPV 16 and 18.
Dr. Lonky: What is the significance of the different VLP loads?
Dr. Harper: Side effects, such as autoimmune neurologic demyelination, albeit rare, have been associated with a higher antigenic protein load. Multiple reports of autoimmune demyelinating diseases—including paralysis, blindness, and death—have been published by neurologists in regard to Gardasil.4,5 Others have shown that young girls are more at risk than young boys for these neurologic side effects.6
Dr. Felix: Some data suggest that the two vaccine formulations interact differently with the human immune system. In a head-to-head trial funded by GlaxoSmithKline, Cervarix produced higher total and neutralizing antibody titers than Gardasil did.7
Although higher immunogenicity is generally thought to be beneficial, the ultimate determinant of a vaccine’s success is its efficacy—and duration of that efficacy—in clinical trials and follow-up of vaccinated populations. So far, Cervarix has demonstrated efficacy through 8.4 years in its follow-up cohort.8 Similarly, Gardasil has proved to be effective after 5 years of follow-up, with no incident cases of cervical cancer reported in the vaccinated arm.9

2. Does either vaccine offer “extra” immunity?
Dr. Lonky: What is the potential for overlapping immunity to other high-risk viral types with these vaccines?
Dr. Harper: It is quite clear from pivotal trials of both vaccines that Gardasil produces efficacy of 46% against persistent infection caused by HPV 31. Data from the pivotal Phase-3 trial of Gardasil also show that it offers no protection against persistent infection with HPV 45, an important cause of adenocarcinoma.10
In contrast, Cervarix demonstrates substantial efficacy against both persistent infection and CIN 2+ disease caused by HPV 31, 33, and 45.3
These findings mean that Cervarix is 91% effective against HPV types that cause adenocarcinoma and 83% effective overall against squamous cell carcinoma. Compare that with Gardasil, which is 78% effective overall against HPV types that cause adenocarcinoma and 73% effective against HPV types that cause squamous cell carcinoma.
The immune titers tell a supportive story. After vaccination with Gardasil, the antibody titer immediately declines for HPV 6, 11, and 18, reaching the baseline for natural infection within 18 months.7 HPV 18 shows continued, significant loss of seropositivity over time, and antibody titers for HPV 6 and 11 also decline. In the monovalent HPV 16 pre-Gardasil experimental vaccine, 14% of women no longer had measurable titers to HPV 16 after 8.5 years.11
After vaccination with Cervarix, antibody titers for HPV 16 and 18 remain more than seven times and more than four times higher, respectively, than natural infection titers for 8.4 years, with no loss of measurable antibody titer for either type. The antibody titers for HPV 31, 33, and 45 remain substantially higher than natural infection titers for at least 6.4 years. These titers correlate with the vaccine’s very high efficacy against CIN 2+ lesions caused by HPV 16, 18, 31, 33, and 45.
In other words, Cervarix generates an immune response (and efficacy) that indicates robust protection against five of the most common oncogenic HPV types, providing maximal protection against nearly 85% of all cervical cancers. Gardasil protects against 74% of all cervical cancers overall.12 This makes Cervarix the superior cervical cancer vaccine.
Gardasil is the superior vaccine against genital warts, although the duration of its protection is uncertain.
Dr. Huh: I’d just like to point out that there are no head-to-head trials comparing the vaccines in terms of efficacy. Antibody titers are higher with Cervarix than with Gardasil, as you noted, and it may be that, over time, the higher titers are more durable with Cervarix. However, we have yet to fully correlate clinical efficacy with antibody titers. In other words, immunogenicity does not equal clinical efficacy.
Dr. Felix: The data for Gardasil are particularly interesting because there have been no incident HPV-18 lesions detected despite the absence of detectable HPV-18 antibody titers in more than 20% of vaccinated women as soon as 2 years after immunization.9 These data strongly suggest that it is not antibody titer alone that grants protection against HPV-induced lesions of the cervix.
Dr. Harper: This speaks to the difficulty of running a trial to ensure both enough participants and a sufficient attack rate of HPV 18 to cause new lesions to be detected in vaccinated women. In the relevant trial, there were only 112 vaccinated women—not nearly enough women to overcome the very low attack rate of HPV 18 in the trial population—and they were followed for 5 years.9 We cannot be sure that the lack of incident HPV-18 lesions in the vaccinated women is the result of efficacy.
Dr. Felix: As for overlapping immunity to HPV types not included in the vaccines, it has been described for both Cervarix and Gardasil. In the case of Cervarix, the manufacturer demonstrated unexpectedly high rates of protection against all CIN 2+ and CIN 3+ lesions—70% and 87%, respectively. These rates were too high to be explained by protection against types 16, 18, 31, and 45 alone. It is possible, therefore, that Cervarix may protect against other high-risk HPV types.13
Gardasil has proved to be effective against HPV types 31, 33, 52, and others.10 When total protection against CIN 2+ and CIN 3+ lesions is examined from Phase-3 trials of the vaccine, however, the rates are only 42% and 43%, respectively. These data are difficult to interpret because HPV 16 and 18 together are thought to account for 70% of CIN 3. Some reassurance can be gained from the fact that the number of incident cases of CIN 2+ and CIN 3+ caused by HPV 16 and 18 in the vaccinated group in the Gardasil trial was identical to the number seen in the Cervarix trial.3,10 The reason for the discrepancy in total number of cases of CIN 2+ and CIN 3+ between the two trials—and, therefore, between the two vaccines—cannot be explained by cross-protection alone and is probably attributable to differences in study populations. The Gardasil trial had a higher baseline prevalence of HPV 16 and 18 (9% and 4%, respectively) than the Cervarix trial did (5% and 2%, respectively), a fact that may be explained by the different demographics of their respective populations.2,14
Ultimately, it is hazardous to compare trials, particularly when they are conducted in significantly different populations. On this issue, I concur with the World Health Organization (WHO), which recommended that such comparisons be avoided in the determination of which type of HPV vaccine to recommend.15
Dr. Huh: I agree that it would be inappropriate to make cross-trial comparisons, given differences in the way the trials were designed and conducted. To draw conclusions about clinical efficacy of these two excellent vaccines, based on a comparison of their trials, is completely unscientific. Only a true head-to-head study that has efficacy as its endpoint can tell us which vaccine is superior—and such a trial would require thousands (if not tens of thousands) of subjects and a considerable amount of time to complete. In my opinion, such a study would be counterproductive to our goal of vaccination.
Dr. Harper: I disagree. The whole purpose of this roundtable is to compare vaccines. It is not “unscientific” to compare the trials.
Dr. Huh: On the contrary—it is completely inappropriate to directly compare the Phase-3 clinical trials from Merck and GlaxoSmithKline. One can speculate about the differences between them, but any clinical trialist knows that a direct, scientific comparison cannot be made. Only a real head-to-head study powered for efficacy can do this.
- Both the bivalent and quadrivalent vaccines appear to be excellent products. Besides protecting against the main oncogenic strains of human papillomavirus (HPV) (types 16 and 18 for both vaccines, and the genital-wart-associated strains 6 and 11 for the quadrivalent vaccine), both Cervarix and Gardasil offer some degree of cross-protection against additional HPV strains.
- Vaccination of the sexually naïve patient with either vaccine provides significant protection against cervical intraepithelial neoplasia 2 (CIN 2) or worse.
- HPV vaccination is expected to reduce the rate of abnormal Pap tests and the need for common excisional treatments for cervical dysplasia in vaccinated women. It will do the same in the population as a whole if rates of vaccination are sufficient to provide “herd” immunity.
3. Is one vaccine more effective than the other?
Dr. Lonky: How do the vaccines compare in terms of efficacy?
Dr. Smith-McCune: In discussing efficacy, I think we should focus on CIN 3 because it is the immediate surrogate for cancer, whereas CIN 2 lesions can be transient in younger women. I think it is also important to focus on outcomes regardless of the HPV types associated with the lesions. This approach is more clinically relevant, as we don’t perform HPV typing of lesions in clinical practice. Nor do we manage lesions differently depending on the HPV type in the lesion.
That said, it is difficult to compare efficacy of the vaccines for several reasons, a few of which we have already discussed. For example, the bivalent and quadrivalent vaccines were studied in separate randomized trials. Although the study populations were similar, they were not identical. Women in both trials were relatively sexually naïve, but the cutoff for number of lifetime sexual partners was different (5 for Gardasil versus 7 for Cervarix). In trials of Gardasil, women who had a history of abnormal cytology or genital warts were excluded. In trials of Cervarix, women who had a history of colposcopy were excluded. In Gardasil trials, approximately 3% of women were from the Asian Pacific, versus 34% in the Cervarix trials, and so on.3,16
The trials also had different protocols for referral to colposcopy, which would affect disease detection. And the length of follow-up differs between trials.3,9
Dr. Lonky: Can we draw any conclusions about efficacy?
Dr. Smith-McCune: Yes. The trials defined outcomes in several populations of participants. In addition to the overall population (called the “intention-to-treat population” in the Gardasil trials and the “total vaccinated cohort” in the Cervarix trials), the trials defined a subpopulation of women naïve to oncogenic HPV types to gain information about the likely impact of vaccinating girls before the onset of sexual activity. The definitions of these “naïve” populations were slightly different, mainly in the number of HPV types tested, so again, some caution needs to be exercised in making comparisons.
End-of-trial data in the naïve population show a 43% reduction in CIN 3 lesions for Gardasil and 87% for Cervarix (for CIN 3 or worse). By inference, we can tell the sexually naïve patient that vaccination with either vaccine will provide significant protection against CIN 3 lesions, likely to result in significant protection against cervical cancer over time.
We can gather some estimates of efficacy in sexually non-naïve women by looking at results from all trial participants. Gardasil reduced overall CIN 3 lesions by 16% overall; Cervarix reduced CIN 3 or worse by 33%. When counseling an individual patient, if she has had a similarly low number of lifetime sexual partners (e.g., the median number in the Gardasil trials was 2), these results provide an estimate of her likely protection against CIN 3 with vaccination.
Common excisional treatments for cervical dysplasia are known to be associated with adverse perinatal outcomes.17 The ability to reduce the need for these treatments is an important outcome of vaccination. In the HPV-naïve populations, vaccination reduced definitive cervical therapies or excisions by 42% (Gardasil) and 69% (Cervarix). These figures are useful in counseling virginal patients about the long-term benefits of vaccination.
For sexually active patients 26 years and younger, HPV vaccination significantly reduced definitive cervical therapy or excisions by 23% (Gardasil) and 25% (Cervarix). Again, these figures are most applicable for counseling patients who have had relatively few lifetime sexual partners. So the exact extent of protection is likely to vary by the patient’s total number of lifetime sexual partners.
I expect that we will see more data on the effects of vaccination stratified by the number of lifetime sexual partners, because that information would be very useful in counseling individual sexually active women.
Dr. Harper: Both vaccines reduce the rate of abnormal Pap tests by 10% regardless of HPV type in that population of women.9,18
4. Are the two vaccines safe?
Dr. Lonky: What about safety of the vaccines? What do we know?
Dr. Felix: The safety profiles seen in clinical trials of both vaccines are very similar and consist almost entirely of nonserious adverse events.2,9 In the United States, a greater number of Gardasil doses has been administered, owing to its earlier development. As of January 1, 2010, more than 28 million doses had been distributed, and numerous major events had been recorded in the Vaccine Adverse Event Reporting System (VAERS). Of 15,829 adverse events reported, only 8% were considered serious by the CDC. CDC investigation, by expert panels, of all serious adverse events found no evidence linking Gardasil to any of them, including Guillain-Barré syndrome, blood clots, and death.19
Dr. Huh: A few other points to consider:
- The reporting rate for Gardasil is triple that for all other vaccines combined
- Because VAERS is a passive reporting system, under-reporting is distinctly possible
- Post-licensure safety surveillance is still underway
- Both products have pregnancy registries.
Dr. Harper: The current postmarketing commitment between Merck and the FDA is to recognize a rate of serious adverse events that exceeds 2 cases in every 10,000 women in a cohort of 44,000 women who have received all three doses of Gardasil. Although autoimmune neurologic sequelae have occurred after Gardasil administration, regulatory authorities are not required to evaluate these reactions, such as Guillain-Barré syndrome, because the frequency is lower than the agreed-upon threshold. Nevertheless, adverse events could be life threatening to some girls.
Any risk of death—even if it is lower than the agreed-upon threshold—should be presented to women as a possible risk of vaccination with Gardasil. In the United States, the same women could choose a lifetime of Pap screening and be afforded the same protection against cervical cancer as they would get from vaccination.
5. Is quadrivalent better than bivalent?
Dr. Lonky: Why would a clinician choose a bivalent vaccine when the quadrivalent vaccine protects not only against carcinogenic types 16 and 18, but also against HPV-associated genital warts?
Dr. Harper: A smart clinician would ask the patient what she values. The physician is obligated to present the evidence and let her choose!
Dr. Lonky: What does the evidence suggest?
Dr. Felix: A clear recommendation between Cervarix and Gardasil is very difficult to make at this time, for the reasons already stated. Both vaccines provide 98% protection against HPV 16 and 18 for the prevention of CIN 2+ lesions.3,9 As we have discussed, both vaccines also provide protection against high-risk strains of HPV other than types 16 and 18.
In the Cervarix trial, there was an overall reduction of all CIN 2+ lesions that was higher as a percentage of total lesions than the reduction seen in the Gardasil trial.3,9 However, unlike Cervarix, Gardasil significantly reduced the rate of vaginal intraepithelial neoplasia (VaIN) and vulvar intraepithelial neoplasia (VIN).9
Dr. Harper: GlaxoSmithKline is analyzing its data on vulvar and vaginal protection, and it is likely that Cervarix will demonstrate some efficacy in this regard, too. But the economic burden of noncervical cancers is estimated to be only 8% of the economic burden of all HPV-related diseases.20 The prevention of cervical cancer is the dominant clinical and economic force for vaccination.
Dr. Felix: Clearly, the protection against genital warts demonstrated in the Gardasil trial will not be realized with Cervarix, as it does not offer immunization or cross-protection against HPV 6 or 11. Gardasil’s protection against HPV 6 and 11 prompted FDA approval of the vaccine for boys and men.21
According to the WHO, when counseling girls and women about the HPV vaccine, the clinician should weigh the possible value of a deep reduction in total CIN 2+ lesions provided by Cervarix against the reduction in VaIN, VIN, and genital warts provided by Gardasil.15 Boys and men will see clinically proven benefits only from Gardasil for the prevention of external genital warts. Other benefits are strictly theoretical.22,23
Dr. Harper: Vaccine protection must last at least 15 years to reduce the rate of cervical cancer. Otherwise, the development of cervical cancer will only be postponed, if boosters are not implemented.
It is now widely recognized that Cervarix induces high antibody titers, offering 100% efficacy even after 8.4 years, making it very likely that the protection it provides will continue for at least 15 years. It is also widely acknowledged by immunologists that Gardasil-induced titers for HPV 6, 11, and 18 are much shorter-lived, so protection is likely to wane 5 to 10 years after vaccination.
That means that Gardasil provides excellent protection against one cancer-causing type of HPV. In addition, it protects against genital warts caused by HPV types 6 and 11 for at least 5 years. In comparison, Cervarix protects against five cancer-causing types of HPV, thereby preventing about 90% of cervical cancers, and is likely to remain effective for at least 15 years.
There are 10 times as many women who have an abnormal Pap test as there are women who have genital warts, so one would think that Cervarix would be the vaccine of choice in preventing the life-threatening disease of cervical cancer.
Dr. Smith-McCune: I would agree that the choice should be discussed with patients—and with parents. If the objective is primarily to protect against cervical cancer precursors, then the bivalent vaccine may be the better choice, with the caveat that we can’t really compare the results from the vaccine trials for reasons discussed earlier, and there are no data from a randomized head-to-head trial comparing the two vaccines. If the decision involves a desire to reduce genital warts or vulvar and vaginal dysplasia, then the quadrivalent vaccine would be the better choice.
6. What impact do the vaccines have on screening?
Dr. Lonky: Do the vaccines have varying effects on our need to screen for, triage, and treat cervical cancer precursors?
Dr. Harper: The Pap smear has reduced the rate of cervical cancer in the United States by 75%—that rate is now at an all-time low of 8 cases for every 100,000 women. But the Pap smear is not perfect; there is a 30% false-negative rate among women who develop cervical cancer, and a large false-positive rate that involves referral to colposcopy for minimally abnormal cytology reports. And when CIN 2+ disease is detected, treatment is not without risk. Surgery increases the risk of reproductive morbidity in future pregnancies. Having protection against this outcome could be tremendously valuable for some women.
Compare the HPV vaccine, which has probable benefit but also the potential for serious adverse events, including demyelinating diseases that cause blindness, paralysis, and death in a small number of recipients.
If women were to choose to be vaccinated with Gardasil and forgo further Pap screening, the rate of cervical cancer in the United States would rise from 8 to 14 cases for every 100,000 women. If they were to choose Cervarix instead, with no further Pap screening, the rate would rise from 8 to 9.5 cases for every 100,000 women.
If women were to choose both HPV vaccination and continued Pap screening, the rate of cervical cancer still would not decline from its current level of 8 cases for every 100,000 women. Instead, the benefit would be that fewer women have abnormal Pap tests, and fewer women would need to be treated for CIN 2+ disease.
Women and physicians must understand these facts. A woman who chooses to be vaccinated may gain individual protection, but the overall rate of cervical cancer will not be affected.
Dr. Huh: Regardless of the HPV vaccine selected, we need to seriously rethink how we screen women in the United States. One could easily argue that the combination of the vaccine and continued screening is too expensive. It might be wise to consider lengthening the screening interval—and, perhaps, further delaying initial screening to 25 years of age—to make cervical cancer prevention with both modalities more cost-effective.24 The most important thing to recognize is that women still need to be screened, even if they have been vaccinated.
As more women are vaccinated, we expect to see a decline in the prevalence of CIN 2+ and CIN 3+ lesions, and this will ultimately weaken the positive predictive value of cytology. Perhaps it is time to consider the HPV test as a primary screen, with triage to cytology in women who test HPV-positive.25
Dr. Smith-McCune: As we accumulate data over time about the effects of vaccination on the rates of CIN 3 and cancer, modeling will be helpful in determining the best screening algorithm for women who have been vaccinated against HPV.
It is important to remember that approximately 50% of women who are given a diagnosis of cervical cancer in the United States have never been screened. It is vital that we continue to reach out to the under-screened population and focus vaccination efforts on populations of girls who are likely to have limited access to care in the future.
7. Can we vaccinate every woman?
Dr. Lonky: Is universal vaccination of women achievable for either vaccine?
Dr. Felix: Universal vaccination against HPV would be achievable only via school mandates. Without them, vaccination will not approach the 80% threshold needed to produce herd immunity.
Despite the clear benefit of such mandates to the general population—particularly the medically under-served—the issue has become a political football. As a result, school mandates will probably never be realized.
Dr. Harper: I don’t believe it is ethical to mandate vaccination of all girls and women. It is a choice that women and parents, in conversation with their physicians and daughters, must make when considering how to be protected against cervical cancer. Herd immunity is a moot point because we are only vaccinating girls (50% of the population) and can never reach the theoretical 70% threshold for herd immunity to be apparent.
Dr. Lonky: Is the availability of two vaccines a boon or a hindrance?
Dr. Smith-McCune: I think it is always a good thing to have choices in medicine.
Dr. Huh: I see the availability of two vaccines as a boon. That availability means that two companies are now putting forth consistent educational messages about the importance of vaccination and, I hope, stimulating competition that will reduce the overall cost of the vaccine series. Having two vaccines can only promote awareness, access, and greater appreciation of the considerable protection these two vaccines provide.
Despite solid evidence that the quadrivalent (Gardasil) HPV vaccine and the bivalent (Cervarix) HPV vaccine protect against cervical cancer, only about one fifth of the female population between 11 and 26 years of age has received the full series of Gardasil since it won FDA approval in 2006. Barriers to vaccination are not financial alone, as the vaccination rate is similarly low among women who have health insurance.
Why isn’t the vaccination rate higher? I see eight barriers to full implementation:
- Economic disparities. Each vaccine costs roughly $400 (national average) for the full series of injections. Although women who do not get Pap screening are most likely to benefit from the vaccines, they usually cannot afford them. federal childhood immunization programs cover teens and young women until 18 years of age in most states, and until 21 years in a few. that leaves most women who seek vaccination from gynecologists without coverage.
- Fear. Pain at the injection site, syncope, and a slightly elevated incidence of thromboembolism are the adverse events most commonly associated with HPV vaccination in the literature. In the life cycle of a vaccine, reports of sudden death or neurologic injury (Guillain-Barré syndrome) occur in the early years, but are reported at a rate lower than 2 cases in every 10,000 women. Nevertheless, such events may create fear about undergoing immunization.
- Long latency period. Because the outcome of cancer prevention won’t become apparent for 20 to 40 years following vaccination, the need for immunization may seem less than urgent.
- Cultural and religious beliefs. Because carcinogenic HPV strains are sexually transmitted, some families may associate vaccination with the promotion of sexual activity. Even in states that mandate vaccination, the courts have upheld a parent’s right to refuse vaccination on these grounds.
- The premarket push. Aggressive promotion of vaccination by both manufacturers and a push by advocates for legislation to mandate the vaccine prior to completion of Phase-3 trials and gathering of robust safety data may have diminished trust in the vaccine and reduced its acceptance.
- Lack of legislation. In states that do not consider HPV vaccination to be a necessary public health intervention, the lack of mandates and funding reduce the vaccination rate. In addition, some legislators have been more active advocates of vaccination than others.
- Reduced involvement of the obGyn. ObGyns don’t routinely vaccinate patients; pediatricians do. Young women are slipping through the cracks because the conventional ObGyn practice does not have a vaccination program that ensures payment, reimbursement, and completion of the vaccine series. Many ObGyn practices are reluctant to institute such a program because the profit margin is small, there are associated risks, and the time required to counsel the patient and for follow-up is extensive.
- Failure to complete the series. Some women do not complete the full vaccine series, owing to cost or side effects, or both. Solid evidence that a single dose could be as protective as the full series would be compelling. A single-dose vaccine would also be less expensive.
The principal danger of a low vaccination rate is the loss of insurance coverage for immunization against HPV. On one hand, payers may begin to ask whether coverage is justified when so few girls and women are vaccinated, leaving the payer with two burdens: the expense of vaccination and the expense of conventional screening programs and treatment, although the costs of treatment would be reduced with vaccination. On the other hand, Gardasil’s protection against genital warts may provide incentive for payers to cover or discount the vaccine because of the reduction in the need to diagnose, triage, and treat condyloma.
Ultimately, HPV vaccination may become another optional intervention that is paid for by the individual, despite evidence in girls and women that cervical cancer can be prevented.
—NEAL M. LONKY, MD, MPH
We want to hear from you! Tell us what you think.
Not long ago (in medical years), we were still trying to discover the cause of cervical cancer. Today, not only do we know that cause to be persistent human papillomavirus (HPV) infection, but we have two vaccines at our disposal to prevent the primary oncogenic strains of the virus.
We’ve come a long way.
The availability of two vaccines raises questions, however. What kind of data do we have on the bivalent (Cervarix, GlaxoSmithKline) and quadrivalent (Gardasil, Merck) vaccines so far? Is one of them clearly superior to the other? If not, what population is each vaccine best suited for—and how do we counsel patients about their options?
To address these and other questions, OBG Management Contributing Editor Neal M. Lonky, MD, MPH, assembled a panel of physicians who have expertise in cervical disease detection and prevention and asked them to sift the data that have accumulated thus far. In the discussion that follows, they touch on long-term efficacy, the likely impact of the vaccines on cervical cancer screening, and other aspects of disease prevention in the era of HPV vaccination.

Juan C. Felix, MD
Professor of Clinical Pathology and Obstetrics and Gynecology; Director of Cytopathology fellowship; and Chief of Gynecologic Pathology at the Keck School of Medicine, University of Southern California; and Chief of Cytopathology at Los Angeles County and University of Southern California Medical Center in Los Angeles.
Dr. Felix reports that he is a speaker for Merck and GlaxoSmithKline.

Diane M. Harper, MD, MS, MPH
Director of the Gynecologic Cancer Prevention Research Group and Professor of Obstetrics and Gynecology, Community and family Medicine, and Informatics and Personalized Medicine at the University of Missouri–Kansas City School of Medicine.
Dr. Harper reports that she has served as a speaker and advisor for Merck and GlaxoSmithKline, and that the institutions at which she conducted HPV vaccination trials have received funding from Merck and GlaxoSmithKline.

Warner K. Huh, MD
Associate Professor in the Department of Obstetrics and Gynecology, and Associate Scientist at the Comprehensive Cancer Center at the University of Alabama– Birmingham.
Dr. Huh reports that he receives grant or research support from and is a speaker and consultant to Merck and GlaxoSmithKline.

Karen K. Smith-McCune, MD, PhD
John Kerner Endowed Chair of Gynecologic Oncology, Director of the Dysplasia Clinic, and Professor of Obstetrics, Gynecology, and Reproductive Sciences at the University of California–San francisco.
Dr. Smith-McCune reports she has performed unpaid consulting for OncoHealth Inc. and is planning to join its Scientific Advisory Board.
1. How were the vaccines developed?
Neal M. Lonky, MD, MPH: What should clinicians know about the development, function, and mechanism of action of the two HPV vaccines?
Warner K. Huh, MD: The bivalent and quadrivalent vaccines are both excellent products, and their respective Phase-3 trials demonstrate that they provide impressive protection against HPV, particularly among women who test negative (by polymerase chain reaction) for the specific HPV types contained within the vaccines.1-3
Cervarix protects against HPV types 16 and 18, whereas Gardasil is effective against HPV types 6, 11, 16, and 18.
Dr. Lonky: Do the vaccines function similarly?
Diane M. Harper, MD, MS, MPH: Yes. Both stimulate an immediate antibody response in the woman who is not infected with the relevant virus and are effective in preventing cervical intraepithelial neoplasia grade 2 and higher (CIN 2+), as well as persistent infection, caused by vaccine-related and cross-protected HPV types. The quality of the antibody response is best for HPV 16 for both vaccines. The quality of the antibody response for HPV 6, 11, and 18 for Gardasil is much poorer than its response for HPV 16. Cervarix induces an equally high and sustained antibody response for HPV 18 as for HPV 16.
Juan C. Felix, MD: Both vaccines are based on the same virus-like particles (VLP). The functionality of the vaccines is, therefore, mainly dependent on the dosage of VLP and the adjuvant used. Gardasil uses a proprietary aluminum sulfate adjuvant, whereas Cervarix uses aluminum hydroxide and monophosphoryl lipid A.
Karen K. Smith-McCune, MD, PhD: Both adjuvants have an extensive track record of safety and efficacy in other vaccines. Because they have different structures, however, they may have varying effects on many components of the immune response elicited by the L1 antigens.
Dr. Harper: Both adjuvants contain aluminum, which has so far proved to be safe despite the newly established association between high aluminum intake and Alzheimer’s disease.
Dr. Lonky: Were there any notable challenges in developing the vaccines?
Dr. Harper: It was difficult to formulate the appropriate dosages of VLP in Gardasil. Higher dosages of HPV 11 and 16 were needed to prevent cross-inhibition by HPV 6 and 18. As a result, the antigenic protein component of Gardasil that is necessary to effect an immunologic antibody response is high, at 120 μg. In Cervarix, the antigenic VLP load is 20 μg each for HPV 16 and 18.
Dr. Lonky: What is the significance of the different VLP loads?
Dr. Harper: Side effects, such as autoimmune neurologic demyelination, albeit rare, have been associated with a higher antigenic protein load. Multiple reports of autoimmune demyelinating diseases—including paralysis, blindness, and death—have been published by neurologists in regard to Gardasil.4,5 Others have shown that young girls are more at risk than young boys for these neurologic side effects.6
Dr. Felix: Some data suggest that the two vaccine formulations interact differently with the human immune system. In a head-to-head trial funded by GlaxoSmithKline, Cervarix produced higher total and neutralizing antibody titers than Gardasil did.7
Although higher immunogenicity is generally thought to be beneficial, the ultimate determinant of a vaccine’s success is its efficacy—and duration of that efficacy—in clinical trials and follow-up of vaccinated populations. So far, Cervarix has demonstrated efficacy through 8.4 years in its follow-up cohort.8 Similarly, Gardasil has proved to be effective after 5 years of follow-up, with no incident cases of cervical cancer reported in the vaccinated arm.9

2. Does either vaccine offer “extra” immunity?
Dr. Lonky: What is the potential for overlapping immunity to other high-risk viral types with these vaccines?
Dr. Harper: It is quite clear from pivotal trials of both vaccines that Gardasil produces efficacy of 46% against persistent infection caused by HPV 31. Data from the pivotal Phase-3 trial of Gardasil also show that it offers no protection against persistent infection with HPV 45, an important cause of adenocarcinoma.10
In contrast, Cervarix demonstrates substantial efficacy against both persistent infection and CIN 2+ disease caused by HPV 31, 33, and 45.3
These findings mean that Cervarix is 91% effective against HPV types that cause adenocarcinoma and 83% effective overall against squamous cell carcinoma. Compare that with Gardasil, which is 78% effective overall against HPV types that cause adenocarcinoma and 73% effective against HPV types that cause squamous cell carcinoma.
The immune titers tell a supportive story. After vaccination with Gardasil, the antibody titer immediately declines for HPV 6, 11, and 18, reaching the baseline for natural infection within 18 months.7 HPV 18 shows continued, significant loss of seropositivity over time, and antibody titers for HPV 6 and 11 also decline. In the monovalent HPV 16 pre-Gardasil experimental vaccine, 14% of women no longer had measurable titers to HPV 16 after 8.5 years.11
After vaccination with Cervarix, antibody titers for HPV 16 and 18 remain more than seven times and more than four times higher, respectively, than natural infection titers for 8.4 years, with no loss of measurable antibody titer for either type. The antibody titers for HPV 31, 33, and 45 remain substantially higher than natural infection titers for at least 6.4 years. These titers correlate with the vaccine’s very high efficacy against CIN 2+ lesions caused by HPV 16, 18, 31, 33, and 45.
In other words, Cervarix generates an immune response (and efficacy) that indicates robust protection against five of the most common oncogenic HPV types, providing maximal protection against nearly 85% of all cervical cancers. Gardasil protects against 74% of all cervical cancers overall.12 This makes Cervarix the superior cervical cancer vaccine.
Gardasil is the superior vaccine against genital warts, although the duration of its protection is uncertain.
Dr. Huh: I’d just like to point out that there are no head-to-head trials comparing the vaccines in terms of efficacy. Antibody titers are higher with Cervarix than with Gardasil, as you noted, and it may be that, over time, the higher titers are more durable with Cervarix. However, we have yet to fully correlate clinical efficacy with antibody titers. In other words, immunogenicity does not equal clinical efficacy.
Dr. Felix: The data for Gardasil are particularly interesting because there have been no incident HPV-18 lesions detected despite the absence of detectable HPV-18 antibody titers in more than 20% of vaccinated women as soon as 2 years after immunization.9 These data strongly suggest that it is not antibody titer alone that grants protection against HPV-induced lesions of the cervix.
Dr. Harper: This speaks to the difficulty of running a trial to ensure both enough participants and a sufficient attack rate of HPV 18 to cause new lesions to be detected in vaccinated women. In the relevant trial, there were only 112 vaccinated women—not nearly enough women to overcome the very low attack rate of HPV 18 in the trial population—and they were followed for 5 years.9 We cannot be sure that the lack of incident HPV-18 lesions in the vaccinated women is the result of efficacy.
Dr. Felix: As for overlapping immunity to HPV types not included in the vaccines, it has been described for both Cervarix and Gardasil. In the case of Cervarix, the manufacturer demonstrated unexpectedly high rates of protection against all CIN 2+ and CIN 3+ lesions—70% and 87%, respectively. These rates were too high to be explained by protection against types 16, 18, 31, and 45 alone. It is possible, therefore, that Cervarix may protect against other high-risk HPV types.13
Gardasil has proved to be effective against HPV types 31, 33, 52, and others.10 When total protection against CIN 2+ and CIN 3+ lesions is examined from Phase-3 trials of the vaccine, however, the rates are only 42% and 43%, respectively. These data are difficult to interpret because HPV 16 and 18 together are thought to account for 70% of CIN 3. Some reassurance can be gained from the fact that the number of incident cases of CIN 2+ and CIN 3+ caused by HPV 16 and 18 in the vaccinated group in the Gardasil trial was identical to the number seen in the Cervarix trial.3,10 The reason for the discrepancy in total number of cases of CIN 2+ and CIN 3+ between the two trials—and, therefore, between the two vaccines—cannot be explained by cross-protection alone and is probably attributable to differences in study populations. The Gardasil trial had a higher baseline prevalence of HPV 16 and 18 (9% and 4%, respectively) than the Cervarix trial did (5% and 2%, respectively), a fact that may be explained by the different demographics of their respective populations.2,14
Ultimately, it is hazardous to compare trials, particularly when they are conducted in significantly different populations. On this issue, I concur with the World Health Organization (WHO), which recommended that such comparisons be avoided in the determination of which type of HPV vaccine to recommend.15
Dr. Huh: I agree that it would be inappropriate to make cross-trial comparisons, given differences in the way the trials were designed and conducted. To draw conclusions about clinical efficacy of these two excellent vaccines, based on a comparison of their trials, is completely unscientific. Only a true head-to-head study that has efficacy as its endpoint can tell us which vaccine is superior—and such a trial would require thousands (if not tens of thousands) of subjects and a considerable amount of time to complete. In my opinion, such a study would be counterproductive to our goal of vaccination.
Dr. Harper: I disagree. The whole purpose of this roundtable is to compare vaccines. It is not “unscientific” to compare the trials.
Dr. Huh: On the contrary—it is completely inappropriate to directly compare the Phase-3 clinical trials from Merck and GlaxoSmithKline. One can speculate about the differences between them, but any clinical trialist knows that a direct, scientific comparison cannot be made. Only a real head-to-head study powered for efficacy can do this.
- Both the bivalent and quadrivalent vaccines appear to be excellent products. Besides protecting against the main oncogenic strains of human papillomavirus (HPV) (types 16 and 18 for both vaccines, and the genital-wart-associated strains 6 and 11 for the quadrivalent vaccine), both Cervarix and Gardasil offer some degree of cross-protection against additional HPV strains.
- Vaccination of the sexually naïve patient with either vaccine provides significant protection against cervical intraepithelial neoplasia 2 (CIN 2) or worse.
- HPV vaccination is expected to reduce the rate of abnormal Pap tests and the need for common excisional treatments for cervical dysplasia in vaccinated women. It will do the same in the population as a whole if rates of vaccination are sufficient to provide “herd” immunity.
3. Is one vaccine more effective than the other?
Dr. Lonky: How do the vaccines compare in terms of efficacy?
Dr. Smith-McCune: In discussing efficacy, I think we should focus on CIN 3 because it is the immediate surrogate for cancer, whereas CIN 2 lesions can be transient in younger women. I think it is also important to focus on outcomes regardless of the HPV types associated with the lesions. This approach is more clinically relevant, as we don’t perform HPV typing of lesions in clinical practice. Nor do we manage lesions differently depending on the HPV type in the lesion.
That said, it is difficult to compare efficacy of the vaccines for several reasons, a few of which we have already discussed. For example, the bivalent and quadrivalent vaccines were studied in separate randomized trials. Although the study populations were similar, they were not identical. Women in both trials were relatively sexually naïve, but the cutoff for number of lifetime sexual partners was different (5 for Gardasil versus 7 for Cervarix). In trials of Gardasil, women who had a history of abnormal cytology or genital warts were excluded. In trials of Cervarix, women who had a history of colposcopy were excluded. In Gardasil trials, approximately 3% of women were from the Asian Pacific, versus 34% in the Cervarix trials, and so on.3,16
The trials also had different protocols for referral to colposcopy, which would affect disease detection. And the length of follow-up differs between trials.3,9
Dr. Lonky: Can we draw any conclusions about efficacy?
Dr. Smith-McCune: Yes. The trials defined outcomes in several populations of participants. In addition to the overall population (called the “intention-to-treat population” in the Gardasil trials and the “total vaccinated cohort” in the Cervarix trials), the trials defined a subpopulation of women naïve to oncogenic HPV types to gain information about the likely impact of vaccinating girls before the onset of sexual activity. The definitions of these “naïve” populations were slightly different, mainly in the number of HPV types tested, so again, some caution needs to be exercised in making comparisons.
End-of-trial data in the naïve population show a 43% reduction in CIN 3 lesions for Gardasil and 87% for Cervarix (for CIN 3 or worse). By inference, we can tell the sexually naïve patient that vaccination with either vaccine will provide significant protection against CIN 3 lesions, likely to result in significant protection against cervical cancer over time.
We can gather some estimates of efficacy in sexually non-naïve women by looking at results from all trial participants. Gardasil reduced overall CIN 3 lesions by 16% overall; Cervarix reduced CIN 3 or worse by 33%. When counseling an individual patient, if she has had a similarly low number of lifetime sexual partners (e.g., the median number in the Gardasil trials was 2), these results provide an estimate of her likely protection against CIN 3 with vaccination.
Common excisional treatments for cervical dysplasia are known to be associated with adverse perinatal outcomes.17 The ability to reduce the need for these treatments is an important outcome of vaccination. In the HPV-naïve populations, vaccination reduced definitive cervical therapies or excisions by 42% (Gardasil) and 69% (Cervarix). These figures are useful in counseling virginal patients about the long-term benefits of vaccination.
For sexually active patients 26 years and younger, HPV vaccination significantly reduced definitive cervical therapy or excisions by 23% (Gardasil) and 25% (Cervarix). Again, these figures are most applicable for counseling patients who have had relatively few lifetime sexual partners. So the exact extent of protection is likely to vary by the patient’s total number of lifetime sexual partners.
I expect that we will see more data on the effects of vaccination stratified by the number of lifetime sexual partners, because that information would be very useful in counseling individual sexually active women.
Dr. Harper: Both vaccines reduce the rate of abnormal Pap tests by 10% regardless of HPV type in that population of women.9,18
4. Are the two vaccines safe?
Dr. Lonky: What about safety of the vaccines? What do we know?
Dr. Felix: The safety profiles seen in clinical trials of both vaccines are very similar and consist almost entirely of nonserious adverse events.2,9 In the United States, a greater number of Gardasil doses has been administered, owing to its earlier development. As of January 1, 2010, more than 28 million doses had been distributed, and numerous major events had been recorded in the Vaccine Adverse Event Reporting System (VAERS). Of 15,829 adverse events reported, only 8% were considered serious by the CDC. CDC investigation, by expert panels, of all serious adverse events found no evidence linking Gardasil to any of them, including Guillain-Barré syndrome, blood clots, and death.19
Dr. Huh: A few other points to consider:
- The reporting rate for Gardasil is triple that for all other vaccines combined
- Because VAERS is a passive reporting system, under-reporting is distinctly possible
- Post-licensure safety surveillance is still underway
- Both products have pregnancy registries.
Dr. Harper: The current postmarketing commitment between Merck and the FDA is to recognize a rate of serious adverse events that exceeds 2 cases in every 10,000 women in a cohort of 44,000 women who have received all three doses of Gardasil. Although autoimmune neurologic sequelae have occurred after Gardasil administration, regulatory authorities are not required to evaluate these reactions, such as Guillain-Barré syndrome, because the frequency is lower than the agreed-upon threshold. Nevertheless, adverse events could be life threatening to some girls.
Any risk of death—even if it is lower than the agreed-upon threshold—should be presented to women as a possible risk of vaccination with Gardasil. In the United States, the same women could choose a lifetime of Pap screening and be afforded the same protection against cervical cancer as they would get from vaccination.
5. Is quadrivalent better than bivalent?
Dr. Lonky: Why would a clinician choose a bivalent vaccine when the quadrivalent vaccine protects not only against carcinogenic types 16 and 18, but also against HPV-associated genital warts?
Dr. Harper: A smart clinician would ask the patient what she values. The physician is obligated to present the evidence and let her choose!
Dr. Lonky: What does the evidence suggest?
Dr. Felix: A clear recommendation between Cervarix and Gardasil is very difficult to make at this time, for the reasons already stated. Both vaccines provide 98% protection against HPV 16 and 18 for the prevention of CIN 2+ lesions.3,9 As we have discussed, both vaccines also provide protection against high-risk strains of HPV other than types 16 and 18.
In the Cervarix trial, there was an overall reduction of all CIN 2+ lesions that was higher as a percentage of total lesions than the reduction seen in the Gardasil trial.3,9 However, unlike Cervarix, Gardasil significantly reduced the rate of vaginal intraepithelial neoplasia (VaIN) and vulvar intraepithelial neoplasia (VIN).9
Dr. Harper: GlaxoSmithKline is analyzing its data on vulvar and vaginal protection, and it is likely that Cervarix will demonstrate some efficacy in this regard, too. But the economic burden of noncervical cancers is estimated to be only 8% of the economic burden of all HPV-related diseases.20 The prevention of cervical cancer is the dominant clinical and economic force for vaccination.
Dr. Felix: Clearly, the protection against genital warts demonstrated in the Gardasil trial will not be realized with Cervarix, as it does not offer immunization or cross-protection against HPV 6 or 11. Gardasil’s protection against HPV 6 and 11 prompted FDA approval of the vaccine for boys and men.21
According to the WHO, when counseling girls and women about the HPV vaccine, the clinician should weigh the possible value of a deep reduction in total CIN 2+ lesions provided by Cervarix against the reduction in VaIN, VIN, and genital warts provided by Gardasil.15 Boys and men will see clinically proven benefits only from Gardasil for the prevention of external genital warts. Other benefits are strictly theoretical.22,23
Dr. Harper: Vaccine protection must last at least 15 years to reduce the rate of cervical cancer. Otherwise, the development of cervical cancer will only be postponed, if boosters are not implemented.
It is now widely recognized that Cervarix induces high antibody titers, offering 100% efficacy even after 8.4 years, making it very likely that the protection it provides will continue for at least 15 years. It is also widely acknowledged by immunologists that Gardasil-induced titers for HPV 6, 11, and 18 are much shorter-lived, so protection is likely to wane 5 to 10 years after vaccination.
That means that Gardasil provides excellent protection against one cancer-causing type of HPV. In addition, it protects against genital warts caused by HPV types 6 and 11 for at least 5 years. In comparison, Cervarix protects against five cancer-causing types of HPV, thereby preventing about 90% of cervical cancers, and is likely to remain effective for at least 15 years.
There are 10 times as many women who have an abnormal Pap test as there are women who have genital warts, so one would think that Cervarix would be the vaccine of choice in preventing the life-threatening disease of cervical cancer.
Dr. Smith-McCune: I would agree that the choice should be discussed with patients—and with parents. If the objective is primarily to protect against cervical cancer precursors, then the bivalent vaccine may be the better choice, with the caveat that we can’t really compare the results from the vaccine trials for reasons discussed earlier, and there are no data from a randomized head-to-head trial comparing the two vaccines. If the decision involves a desire to reduce genital warts or vulvar and vaginal dysplasia, then the quadrivalent vaccine would be the better choice.
6. What impact do the vaccines have on screening?
Dr. Lonky: Do the vaccines have varying effects on our need to screen for, triage, and treat cervical cancer precursors?
Dr. Harper: The Pap smear has reduced the rate of cervical cancer in the United States by 75%—that rate is now at an all-time low of 8 cases for every 100,000 women. But the Pap smear is not perfect; there is a 30% false-negative rate among women who develop cervical cancer, and a large false-positive rate that involves referral to colposcopy for minimally abnormal cytology reports. And when CIN 2+ disease is detected, treatment is not without risk. Surgery increases the risk of reproductive morbidity in future pregnancies. Having protection against this outcome could be tremendously valuable for some women.
Compare the HPV vaccine, which has probable benefit but also the potential for serious adverse events, including demyelinating diseases that cause blindness, paralysis, and death in a small number of recipients.
If women were to choose to be vaccinated with Gardasil and forgo further Pap screening, the rate of cervical cancer in the United States would rise from 8 to 14 cases for every 100,000 women. If they were to choose Cervarix instead, with no further Pap screening, the rate would rise from 8 to 9.5 cases for every 100,000 women.
If women were to choose both HPV vaccination and continued Pap screening, the rate of cervical cancer still would not decline from its current level of 8 cases for every 100,000 women. Instead, the benefit would be that fewer women have abnormal Pap tests, and fewer women would need to be treated for CIN 2+ disease.
Women and physicians must understand these facts. A woman who chooses to be vaccinated may gain individual protection, but the overall rate of cervical cancer will not be affected.
Dr. Huh: Regardless of the HPV vaccine selected, we need to seriously rethink how we screen women in the United States. One could easily argue that the combination of the vaccine and continued screening is too expensive. It might be wise to consider lengthening the screening interval—and, perhaps, further delaying initial screening to 25 years of age—to make cervical cancer prevention with both modalities more cost-effective.24 The most important thing to recognize is that women still need to be screened, even if they have been vaccinated.
As more women are vaccinated, we expect to see a decline in the prevalence of CIN 2+ and CIN 3+ lesions, and this will ultimately weaken the positive predictive value of cytology. Perhaps it is time to consider the HPV test as a primary screen, with triage to cytology in women who test HPV-positive.25
Dr. Smith-McCune: As we accumulate data over time about the effects of vaccination on the rates of CIN 3 and cancer, modeling will be helpful in determining the best screening algorithm for women who have been vaccinated against HPV.
It is important to remember that approximately 50% of women who are given a diagnosis of cervical cancer in the United States have never been screened. It is vital that we continue to reach out to the under-screened population and focus vaccination efforts on populations of girls who are likely to have limited access to care in the future.
7. Can we vaccinate every woman?
Dr. Lonky: Is universal vaccination of women achievable for either vaccine?
Dr. Felix: Universal vaccination against HPV would be achievable only via school mandates. Without them, vaccination will not approach the 80% threshold needed to produce herd immunity.
Despite the clear benefit of such mandates to the general population—particularly the medically under-served—the issue has become a political football. As a result, school mandates will probably never be realized.
Dr. Harper: I don’t believe it is ethical to mandate vaccination of all girls and women. It is a choice that women and parents, in conversation with their physicians and daughters, must make when considering how to be protected against cervical cancer. Herd immunity is a moot point because we are only vaccinating girls (50% of the population) and can never reach the theoretical 70% threshold for herd immunity to be apparent.
Dr. Lonky: Is the availability of two vaccines a boon or a hindrance?
Dr. Smith-McCune: I think it is always a good thing to have choices in medicine.
Dr. Huh: I see the availability of two vaccines as a boon. That availability means that two companies are now putting forth consistent educational messages about the importance of vaccination and, I hope, stimulating competition that will reduce the overall cost of the vaccine series. Having two vaccines can only promote awareness, access, and greater appreciation of the considerable protection these two vaccines provide.
Despite solid evidence that the quadrivalent (Gardasil) HPV vaccine and the bivalent (Cervarix) HPV vaccine protect against cervical cancer, only about one fifth of the female population between 11 and 26 years of age has received the full series of Gardasil since it won FDA approval in 2006. Barriers to vaccination are not financial alone, as the vaccination rate is similarly low among women who have health insurance.
Why isn’t the vaccination rate higher? I see eight barriers to full implementation:
- Economic disparities. Each vaccine costs roughly $400 (national average) for the full series of injections. Although women who do not get Pap screening are most likely to benefit from the vaccines, they usually cannot afford them. federal childhood immunization programs cover teens and young women until 18 years of age in most states, and until 21 years in a few. that leaves most women who seek vaccination from gynecologists without coverage.
- Fear. Pain at the injection site, syncope, and a slightly elevated incidence of thromboembolism are the adverse events most commonly associated with HPV vaccination in the literature. In the life cycle of a vaccine, reports of sudden death or neurologic injury (Guillain-Barré syndrome) occur in the early years, but are reported at a rate lower than 2 cases in every 10,000 women. Nevertheless, such events may create fear about undergoing immunization.
- Long latency period. Because the outcome of cancer prevention won’t become apparent for 20 to 40 years following vaccination, the need for immunization may seem less than urgent.
- Cultural and religious beliefs. Because carcinogenic HPV strains are sexually transmitted, some families may associate vaccination with the promotion of sexual activity. Even in states that mandate vaccination, the courts have upheld a parent’s right to refuse vaccination on these grounds.
- The premarket push. Aggressive promotion of vaccination by both manufacturers and a push by advocates for legislation to mandate the vaccine prior to completion of Phase-3 trials and gathering of robust safety data may have diminished trust in the vaccine and reduced its acceptance.
- Lack of legislation. In states that do not consider HPV vaccination to be a necessary public health intervention, the lack of mandates and funding reduce the vaccination rate. In addition, some legislators have been more active advocates of vaccination than others.
- Reduced involvement of the obGyn. ObGyns don’t routinely vaccinate patients; pediatricians do. Young women are slipping through the cracks because the conventional ObGyn practice does not have a vaccination program that ensures payment, reimbursement, and completion of the vaccine series. Many ObGyn practices are reluctant to institute such a program because the profit margin is small, there are associated risks, and the time required to counsel the patient and for follow-up is extensive.
- Failure to complete the series. Some women do not complete the full vaccine series, owing to cost or side effects, or both. Solid evidence that a single dose could be as protective as the full series would be compelling. A single-dose vaccine would also be less expensive.
The principal danger of a low vaccination rate is the loss of insurance coverage for immunization against HPV. On one hand, payers may begin to ask whether coverage is justified when so few girls and women are vaccinated, leaving the payer with two burdens: the expense of vaccination and the expense of conventional screening programs and treatment, although the costs of treatment would be reduced with vaccination. On the other hand, Gardasil’s protection against genital warts may provide incentive for payers to cover or discount the vaccine because of the reduction in the need to diagnose, triage, and treat condyloma.
Ultimately, HPV vaccination may become another optional intervention that is paid for by the individual, despite evidence in girls and women that cervical cancer can be prevented.
—NEAL M. LONKY, MD, MPH
We want to hear from you! Tell us what you think.
1. The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915-1927.
2. Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. HPV PATRICIA study group. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161-2170.
3. Paavonen J, Naud P, Salmeron J, et al. For the HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301-314.
4. DiMario FJ, Hajjar M, Ciesielski T. A 16-year-old girl with bilateral visual loss and left hemiparesis following an immunization against human papillomavirus. J Child Neurology. 2010;25(3):321-327.
5. Sutton I, Lahoria R, Tan I, Clouston P, Barnett M. CNS demyelination and quadrivalent HPV vaccination. Multiple Sclerosis. 2009;15(1):116-119.
6. Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10(5):338-349.
7. Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin. 2009;5(10):705-719.
8. GlaxoSmithKline Vaccine HPV-007 Study Group. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 8.4 years. Presented at: 28th Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID); May 4-8, 2010; Nice, France.
9. Muñoz N, Kjaer SK, Sigurdsson K, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102(5):325-339.
10. Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naïve women aged 16-26 years. J Infect Dis. 2009;199(7):926-935.
11. Rowhani-Rahbar A, Mao C, Hughes JP, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine. 2009;27(41):5612-5619.
12. Harper DM, Williams KB. Prophylactic HPV vaccines: current knowledge of impact on gynecologic premalignancies [published online ahead of print July 3, 2010]. Discovery Medicine. 2010;10(50).http://www.discoverymedicine.com/Diane-M-Harper/2010/07/03/prophylactichpv-vaccines-current-knowledge-of-impact-ongynecologic-premalignancies. Accessed July 13, 2010.
13. Cervarix [human papillomavirus bivalent (types 16 and 18) vaccine recombinant]. Food and Drug Administration. Initial US approval: 2009:1–12.
14. Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928-1943.
15. World Health Organization. Human papillomavirus vaccines: WHO position paper. Weekly Epidemiological Record. 2009;84(15):118-131.http://www.who.int/wer/2009/wer8415/en/index.html. Published April 10, 2009. Accessed July 13, 2010.
16. Ault KA. For Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on the risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369(9576):1861-1868.
17. Arbyn M, Kyrgiou M, Simoens C, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ. 2008;337:a1284.-Doi: 10.1136/bmj.a1284.
18. Paavonen J. For the HPV PATRICIA Study Group. Efficacy of HPV 16/18 ASO4-adjuvanted vaccine against abnormal cytology, colposcopy referrals, and cervical procedures. Abstract SS 4–1. Data presented at Cervical Cancer Prevention, EuroGin 2010. European Research Organisation on Genital Infection and Neoplasia (EUROGIN); February 17-20, 2010; Monte Carlo, Monaco.http://www.eurogin.com/2010/programoverview.html. Accessed July 13, 2010.
19. Centers for Disease Control and Prevention. Reports of health concerns following HPV vaccination. 2010;1-3.http://www.cdc.gov/vaccinesafety/Vaccines/HPV/gardasil.html. Published June 21, 2010. Accessed July 13, 2010.
20. Myers ER. The economic impact of HPV vaccines: not just cervical cancer. Am J Obstet Gynecol. 2008;198(5):487-488.
21. Gardasil [human papillomavirus quadrivalent (types 6, 11, 16, and 18) vaccine, recombinant]. Food and Drug Administration; 2009. Initial Approval 2006;1-26.
22. Olsson SE, Kjaer SK, Sigurdsson K, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin. 2009;5(10).
23. Pirotta M, Ung L, Stein A, et al. The psychosocial burden of human papillomavirus related disease and screening interventions. Sex Transm Infect. 2009;85(7):508-513.
24. Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359(8):821-832.
25. Cox JT. Is the HPV test effective as the primary screen for cervical cancer? Examining the Evidence. OBG Management. 2010;22(7):10-11.
1. The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915-1927.
2. Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. HPV PATRICIA study group. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161-2170.
3. Paavonen J, Naud P, Salmeron J, et al. For the HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301-314.
4. DiMario FJ, Hajjar M, Ciesielski T. A 16-year-old girl with bilateral visual loss and left hemiparesis following an immunization against human papillomavirus. J Child Neurology. 2010;25(3):321-327.
5. Sutton I, Lahoria R, Tan I, Clouston P, Barnett M. CNS demyelination and quadrivalent HPV vaccination. Multiple Sclerosis. 2009;15(1):116-119.
6. Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10(5):338-349.
7. Einstein MH, Baron M, Levin MJ, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin. 2009;5(10):705-719.
8. GlaxoSmithKline Vaccine HPV-007 Study Group. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 8.4 years. Presented at: 28th Annual Meeting of the European Society for Paediatric Infectious Diseases (ESPID); May 4-8, 2010; Nice, France.
9. Muñoz N, Kjaer SK, Sigurdsson K, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102(5):325-339.
10. Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naïve women aged 16-26 years. J Infect Dis. 2009;199(7):926-935.
11. Rowhani-Rahbar A, Mao C, Hughes JP, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine. 2009;27(41):5612-5619.
12. Harper DM, Williams KB. Prophylactic HPV vaccines: current knowledge of impact on gynecologic premalignancies [published online ahead of print July 3, 2010]. Discovery Medicine. 2010;10(50).http://www.discoverymedicine.com/Diane-M-Harper/2010/07/03/prophylactichpv-vaccines-current-knowledge-of-impact-ongynecologic-premalignancies. Accessed July 13, 2010.
13. Cervarix [human papillomavirus bivalent (types 16 and 18) vaccine recombinant]. Food and Drug Administration. Initial US approval: 2009:1–12.
14. Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928-1943.
15. World Health Organization. Human papillomavirus vaccines: WHO position paper. Weekly Epidemiological Record. 2009;84(15):118-131.http://www.who.int/wer/2009/wer8415/en/index.html. Published April 10, 2009. Accessed July 13, 2010.
16. Ault KA. For Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on the risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369(9576):1861-1868.
17. Arbyn M, Kyrgiou M, Simoens C, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ. 2008;337:a1284.-Doi: 10.1136/bmj.a1284.
18. Paavonen J. For the HPV PATRICIA Study Group. Efficacy of HPV 16/18 ASO4-adjuvanted vaccine against abnormal cytology, colposcopy referrals, and cervical procedures. Abstract SS 4–1. Data presented at Cervical Cancer Prevention, EuroGin 2010. European Research Organisation on Genital Infection and Neoplasia (EUROGIN); February 17-20, 2010; Monte Carlo, Monaco.http://www.eurogin.com/2010/programoverview.html. Accessed July 13, 2010.
19. Centers for Disease Control and Prevention. Reports of health concerns following HPV vaccination. 2010;1-3.http://www.cdc.gov/vaccinesafety/Vaccines/HPV/gardasil.html. Published June 21, 2010. Accessed July 13, 2010.
20. Myers ER. The economic impact of HPV vaccines: not just cervical cancer. Am J Obstet Gynecol. 2008;198(5):487-488.
21. Gardasil [human papillomavirus quadrivalent (types 6, 11, 16, and 18) vaccine, recombinant]. Food and Drug Administration; 2009. Initial Approval 2006;1-26.
22. Olsson SE, Kjaer SK, Sigurdsson K, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccin. 2009;5(10).
23. Pirotta M, Ung L, Stein A, et al. The psychosocial burden of human papillomavirus related disease and screening interventions. Sex Transm Infect. 2009;85(7):508-513.
24. Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359(8):821-832.
25. Cox JT. Is the HPV test effective as the primary screen for cervical cancer? Examining the Evidence. OBG Management. 2010;22(7):10-11.
Is hysterectomy definitive treatment for high-grade intraepithelial neoplasia?
The cervix is most susceptible at the transformation zone, but the remainder of the squamous epithelium of the lower genital tract may be at risk in susceptible persons.
What is the residual risk?
Who should we watch?
If the cervix is removed during hysterectomy after CIN 2+ is diagnosed, what is the residual risk to the patient? How should she be managed?
These are key questions, despite the fact that hysterectomy is not as commonly performed to treat high-grade CIN as it is for other indications, such as abnormal bleeding, pain, endometriosis, and fibroids. Hysterectomy for these other conditions without a concomitant history of neoplasia obviates the need for cervical cancer screening via cytology or HPV testing, or both. When CIN is the reason for the hysterectomy, however, or the patient has a history of CIN 2+, there is some residual risk, with the most common sequela being VAIN and, if the VAIN remains undiscovered, vaginal carcinoma. But how do we identify the patient at high risk for VAIN so that we can provide extra resources (screening or colposcopy services)?
Older women merit closer attention
One strength of this study is the fact that it statistically identifiled “older women” as a high-risk subset more likely to develop VAIN 2+. In the study, the mean age of women who were likely to develop VAIN 2+ was 61 years, compared with 46.9 years for women likely to remain free of disease.
The median interval between hysterectomy and diagnosis of VAIN 2+ was 35 months (range, 5–103 months). This information allows us to anticipate an optimal window in which to focus extra screening.
Retrospective design is a weakness
The retrospective design of this review of pathology data from multiple practices in multiple records is a limitation. The exclusion of patients who had coexisting VAIN or a history of VAIN is laudable, but bias is possible. The quality of the documentation of examination of the remainder of the lower genital tract in patients who had abnormal cervical screening during colposcopy (which led to the diagnosis of CIN) is subject to extreme variability, compared with a prospective design that would have defined the elements of vaginal and vulvar colposcopic examination.
In other words, I am concerned that these are truly incident—rather than persistent—lesions following hysterectomy.
How would HPV testing come into play?
This study and the other published studies that address the subject of hysterectomy in the treatment of CIN 2+ did not use HPV testing. If they had, it likely would have provided more information about the true risk of recurrence and helped determine the best screening interval.
The American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines from 2006 do not address this particular scenario, but American College of Obstetricians and Gynecologists (ACOG) guidelines do recommend annual Papanicolaou testing following hysterectomy for CIN until three consecutive tests are negative.1
How reliable are screening methods when the vagina is the target tissue?
Cytologic sampling, HPV test sampling, and colposcopy of the “at-risk” transformation zone or endocervical canal are easier to accomplish than are examination and testing of the entire vaginal vault. The latter are more prone to error and lack evidence-based data to substantiate our practice.
Nevertheless, excellent practice recommendations regarding vaginal colposcopy can be found at the ASCCP Web site at www.asccp.org/edu/practice/vagina.shtml.
We should always carefully examine the entire lower genital tract during colposcopy following referral for abnormal cytology or another abnormal screening test—whether or not the patient has a cervix.-NEAL M.LONKY, MD, MPH
1. Cervical cytology screening. ACOG Practice Bulletin #45. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2003;102:417-427.
The cervix is most susceptible at the transformation zone, but the remainder of the squamous epithelium of the lower genital tract may be at risk in susceptible persons.
What is the residual risk?
Who should we watch?
If the cervix is removed during hysterectomy after CIN 2+ is diagnosed, what is the residual risk to the patient? How should she be managed?
These are key questions, despite the fact that hysterectomy is not as commonly performed to treat high-grade CIN as it is for other indications, such as abnormal bleeding, pain, endometriosis, and fibroids. Hysterectomy for these other conditions without a concomitant history of neoplasia obviates the need for cervical cancer screening via cytology or HPV testing, or both. When CIN is the reason for the hysterectomy, however, or the patient has a history of CIN 2+, there is some residual risk, with the most common sequela being VAIN and, if the VAIN remains undiscovered, vaginal carcinoma. But how do we identify the patient at high risk for VAIN so that we can provide extra resources (screening or colposcopy services)?
Older women merit closer attention
One strength of this study is the fact that it statistically identifiled “older women” as a high-risk subset more likely to develop VAIN 2+. In the study, the mean age of women who were likely to develop VAIN 2+ was 61 years, compared with 46.9 years for women likely to remain free of disease.
The median interval between hysterectomy and diagnosis of VAIN 2+ was 35 months (range, 5–103 months). This information allows us to anticipate an optimal window in which to focus extra screening.
Retrospective design is a weakness
The retrospective design of this review of pathology data from multiple practices in multiple records is a limitation. The exclusion of patients who had coexisting VAIN or a history of VAIN is laudable, but bias is possible. The quality of the documentation of examination of the remainder of the lower genital tract in patients who had abnormal cervical screening during colposcopy (which led to the diagnosis of CIN) is subject to extreme variability, compared with a prospective design that would have defined the elements of vaginal and vulvar colposcopic examination.
In other words, I am concerned that these are truly incident—rather than persistent—lesions following hysterectomy.
How would HPV testing come into play?
This study and the other published studies that address the subject of hysterectomy in the treatment of CIN 2+ did not use HPV testing. If they had, it likely would have provided more information about the true risk of recurrence and helped determine the best screening interval.
The American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines from 2006 do not address this particular scenario, but American College of Obstetricians and Gynecologists (ACOG) guidelines do recommend annual Papanicolaou testing following hysterectomy for CIN until three consecutive tests are negative.1
How reliable are screening methods when the vagina is the target tissue?
Cytologic sampling, HPV test sampling, and colposcopy of the “at-risk” transformation zone or endocervical canal are easier to accomplish than are examination and testing of the entire vaginal vault. The latter are more prone to error and lack evidence-based data to substantiate our practice.
Nevertheless, excellent practice recommendations regarding vaginal colposcopy can be found at the ASCCP Web site at www.asccp.org/edu/practice/vagina.shtml.
We should always carefully examine the entire lower genital tract during colposcopy following referral for abnormal cytology or another abnormal screening test—whether or not the patient has a cervix.-NEAL M.LONKY, MD, MPH
The cervix is most susceptible at the transformation zone, but the remainder of the squamous epithelium of the lower genital tract may be at risk in susceptible persons.
What is the residual risk?
Who should we watch?
If the cervix is removed during hysterectomy after CIN 2+ is diagnosed, what is the residual risk to the patient? How should she be managed?
These are key questions, despite the fact that hysterectomy is not as commonly performed to treat high-grade CIN as it is for other indications, such as abnormal bleeding, pain, endometriosis, and fibroids. Hysterectomy for these other conditions without a concomitant history of neoplasia obviates the need for cervical cancer screening via cytology or HPV testing, or both. When CIN is the reason for the hysterectomy, however, or the patient has a history of CIN 2+, there is some residual risk, with the most common sequela being VAIN and, if the VAIN remains undiscovered, vaginal carcinoma. But how do we identify the patient at high risk for VAIN so that we can provide extra resources (screening or colposcopy services)?
Older women merit closer attention
One strength of this study is the fact that it statistically identifiled “older women” as a high-risk subset more likely to develop VAIN 2+. In the study, the mean age of women who were likely to develop VAIN 2+ was 61 years, compared with 46.9 years for women likely to remain free of disease.
The median interval between hysterectomy and diagnosis of VAIN 2+ was 35 months (range, 5–103 months). This information allows us to anticipate an optimal window in which to focus extra screening.
Retrospective design is a weakness
The retrospective design of this review of pathology data from multiple practices in multiple records is a limitation. The exclusion of patients who had coexisting VAIN or a history of VAIN is laudable, but bias is possible. The quality of the documentation of examination of the remainder of the lower genital tract in patients who had abnormal cervical screening during colposcopy (which led to the diagnosis of CIN) is subject to extreme variability, compared with a prospective design that would have defined the elements of vaginal and vulvar colposcopic examination.
In other words, I am concerned that these are truly incident—rather than persistent—lesions following hysterectomy.
How would HPV testing come into play?
This study and the other published studies that address the subject of hysterectomy in the treatment of CIN 2+ did not use HPV testing. If they had, it likely would have provided more information about the true risk of recurrence and helped determine the best screening interval.
The American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines from 2006 do not address this particular scenario, but American College of Obstetricians and Gynecologists (ACOG) guidelines do recommend annual Papanicolaou testing following hysterectomy for CIN until three consecutive tests are negative.1
How reliable are screening methods when the vagina is the target tissue?
Cytologic sampling, HPV test sampling, and colposcopy of the “at-risk” transformation zone or endocervical canal are easier to accomplish than are examination and testing of the entire vaginal vault. The latter are more prone to error and lack evidence-based data to substantiate our practice.
Nevertheless, excellent practice recommendations regarding vaginal colposcopy can be found at the ASCCP Web site at www.asccp.org/edu/practice/vagina.shtml.
We should always carefully examine the entire lower genital tract during colposcopy following referral for abnormal cytology or another abnormal screening test—whether or not the patient has a cervix.-NEAL M.LONKY, MD, MPH
1. Cervical cytology screening. ACOG Practice Bulletin #45. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2003;102:417-427.
1. Cervical cytology screening. ACOG Practice Bulletin #45. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2003;102:417-427.
Q. Does the HPV vaccine benefit all women in the target age range?
A. No. In the FUTURE I trial, among women who were naïve to all strains of the human papillomavirus (HPV) vaccine at the time of inoculation, the vaccine was 100% effective in preventing anogenital lesions, cervical intraepithelial neoplasia (CIN) 2,3, adenocarcinoma in situ, and cervical cancer. In FUTURE II, among the same population, it was 98% effective in preventing all cervical lesions.
However, in FUTURE I, when the groups comprising HPV-naïve and HPV-positive subjects and those with and without preexisting neoplasia were combined, the vaccine was 20% effective in preventing CIN 2,3, adenocarcinoma in situ, and cervical cancer, and 34% effective in preventing genital warts and vulvar and vaginal intraepithelial neoplasia and cancer due to vaccine and nonvaccine HPV types. In FUTURE II, when the same groups were included, the vaccine was 17% effective in preventing all cervical lesions (regardless of the causal HPV type).
The women involved in these trials fell within the target age range for prophylactic vaccination, with ages from 15 to 26 years.
Expert Commentary
Even females in the target population for HPV vaccination (ages 9 to 26) present with different histories and risk status. An individual may be a candidate for:
- true primary prevention (no evidence of any HPV subtype or neoplasia)
- primary prevention for the vaccine subtypes (ie, negative for HPV type 6, 11, 16, or 18)
- prevention despite the presence of HPV type 6, 11, 16, or 18
- prevention despite the presence of a subtype not covered in the vaccine
- vaccination despite existing vulvar, vaginal, or cervical neoplasia.
The effect of the vaccine on this last group would be considered both prophylactic and therapeutic, defined as secondary prevention of cervical cancer. Secondary prevention (reversal of HPV effect in CIN lesions, leading to regression) has not been demonstrated with the quadrivalent HPV vaccine (Gardasil).
Trials followed women over 3 years
In these randomized trials, both vaccine efficacy (lesions prevented) and safety (side effects) were evaluated.
FUTURE I enrolled nearly 5,500 young women within the advised age range for prophylaxis who were randomized to the quadrivalent HPV vaccine or placebo. Cervical screening and colposcopic biopsy (if indicated) were conducted at baseline, and HPV status was determined. Investigators studied the effect of prophylactic vaccination in women naïve to the four viral subtypes targeted by the vaccine (6, 11, 16, and 18), as well as women in each of the other groups listed above.
The principal aim of FUTURE I was to provide an “ideal” frequent visit schedule with broader referral to colposcopy and determine whether the vaccine would reduce the combined incidence of anogenital warts, vulvar intraepithelial neoplasia (VIN), vaginal intraepithelial neoplasia (VAIN), CIN 1,2,3, and cancer related to HPV subtypes 6, 11, 16, and 18.
FUTURE II involved 12,167 women and a wider visit interval more consistent with clinical practice than FUTURE I. The aim was to determine whether the quadrivalent vaccine would reduce the incidence of CIN 2,3, adenocarcinoma in situ, or invasive carcinoma of the cervix related to HPV subtypes 16 and 18.
In both trials, polymerase chain reaction was used to identify the HPV subtype associated with any CIN, adenocarcinoma in situ, or anogenital lesion that was biopsy-proven, and this information was correlated with the patient’s HPV status before and after vaccination. The baseline and postvaccination serological status against HPV subtypes was also measured.
Safety is not a concern thus far
There were no adverse events that caused death or withdrawal from the studies. The most common side effect was pain at the injection site, which occurred more frequently among vaccinated women.
Bottom line: Keep screening
The quadrivalent vaccine is not equally effective among all female candidates. I recommend the following:
- Encourage vaccination in very young girls and virgins. The vaccine is most effective in females who have not been exposed to any of the four subtypes targeted.
- A lack of type-specific, clinically available serology or lower genital tract screening in a woman who is already sexually active leaves the clinician in the dark about how to counsel her. Because most women I counsel do not harbor all four subtypes covered by the vaccine and lack anogenital disease, I offer the vaccine to all women in the age range that is indicated.
- Counsel women who are already sexually active and who harbor HPV before vaccination, as well as those who acquire the virus during the vaccination period, that even with inoculation they are susceptible to infection and neoplastic transformation, unlike those naïve to the vaccine-specific subtypes. Efficacy is still substantial, however—near 90% overall.
- If type-specific HPV testing becomes commercially available, counsel women who test positive for one of the four HPV subtypes targeted by the vaccine, as well as those with other HPV subtypes, that they are susceptible to neoplastic transformation even with the vaccine.
- Advise women who already have CIN, adenocarcinoma in situ, VIN, VAIN, or benign anogenital disease that the vaccine is not therapeutic and will not prevent the natural progression of these lesions toward cancer.
- Remember that few women harbor all four subtypes targeted by the vaccine. The vaccine may provide some marginal benefit to women already being treated for intraepithelial neoplasia, but each patient should be counseled about the potential lack of benefit.
- Be aware that there is clear benefit in the prevention of anogenital disease independent of the oncogenic risks associated with the virus.
- Don’t forget the other 13 known oncogenic HPV subtypes and the many more nononcogenic types identified so far. The effect of the vaccine is modestly reduced in women who harbor one or more of those subtypes when compared with its efficacy in uninfected recipients and virgins. Therefore, screening for cervical cancer and lower genital tract lesions should continue despite vaccination.
- In an editorial accompanying the FUTURE I and II trials, Sawaya and Smith-McCune observed that oncogenic HPV strains not targeted by the vaccine were responsible for a large number of CIN 2,3 and adenocarcinoma lesions in the FUTURE II trial.1 Although there are no concrete data, the editorialists suggest that, as we vaccinate for known strains, existing strains that are not included or new strains that evolve may “fill the niche” and continue to cause incident cases of neoplasia despite vaccination. This is another reason to screen women cytologically or by direct visual or other in-vivo methods to detect neoplasia.
1. Sawaya GF, Smith-McCune KS. HPV vaccination—more answers, more questions. N Engl J Med. 2007;356:1991-1993.
A. No. In the FUTURE I trial, among women who were naïve to all strains of the human papillomavirus (HPV) vaccine at the time of inoculation, the vaccine was 100% effective in preventing anogenital lesions, cervical intraepithelial neoplasia (CIN) 2,3, adenocarcinoma in situ, and cervical cancer. In FUTURE II, among the same population, it was 98% effective in preventing all cervical lesions.
However, in FUTURE I, when the groups comprising HPV-naïve and HPV-positive subjects and those with and without preexisting neoplasia were combined, the vaccine was 20% effective in preventing CIN 2,3, adenocarcinoma in situ, and cervical cancer, and 34% effective in preventing genital warts and vulvar and vaginal intraepithelial neoplasia and cancer due to vaccine and nonvaccine HPV types. In FUTURE II, when the same groups were included, the vaccine was 17% effective in preventing all cervical lesions (regardless of the causal HPV type).
The women involved in these trials fell within the target age range for prophylactic vaccination, with ages from 15 to 26 years.
Expert Commentary
Even females in the target population for HPV vaccination (ages 9 to 26) present with different histories and risk status. An individual may be a candidate for:
- true primary prevention (no evidence of any HPV subtype or neoplasia)
- primary prevention for the vaccine subtypes (ie, negative for HPV type 6, 11, 16, or 18)
- prevention despite the presence of HPV type 6, 11, 16, or 18
- prevention despite the presence of a subtype not covered in the vaccine
- vaccination despite existing vulvar, vaginal, or cervical neoplasia.
The effect of the vaccine on this last group would be considered both prophylactic and therapeutic, defined as secondary prevention of cervical cancer. Secondary prevention (reversal of HPV effect in CIN lesions, leading to regression) has not been demonstrated with the quadrivalent HPV vaccine (Gardasil).
Trials followed women over 3 years
In these randomized trials, both vaccine efficacy (lesions prevented) and safety (side effects) were evaluated.
FUTURE I enrolled nearly 5,500 young women within the advised age range for prophylaxis who were randomized to the quadrivalent HPV vaccine or placebo. Cervical screening and colposcopic biopsy (if indicated) were conducted at baseline, and HPV status was determined. Investigators studied the effect of prophylactic vaccination in women naïve to the four viral subtypes targeted by the vaccine (6, 11, 16, and 18), as well as women in each of the other groups listed above.
The principal aim of FUTURE I was to provide an “ideal” frequent visit schedule with broader referral to colposcopy and determine whether the vaccine would reduce the combined incidence of anogenital warts, vulvar intraepithelial neoplasia (VIN), vaginal intraepithelial neoplasia (VAIN), CIN 1,2,3, and cancer related to HPV subtypes 6, 11, 16, and 18.
FUTURE II involved 12,167 women and a wider visit interval more consistent with clinical practice than FUTURE I. The aim was to determine whether the quadrivalent vaccine would reduce the incidence of CIN 2,3, adenocarcinoma in situ, or invasive carcinoma of the cervix related to HPV subtypes 16 and 18.
In both trials, polymerase chain reaction was used to identify the HPV subtype associated with any CIN, adenocarcinoma in situ, or anogenital lesion that was biopsy-proven, and this information was correlated with the patient’s HPV status before and after vaccination. The baseline and postvaccination serological status against HPV subtypes was also measured.
Safety is not a concern thus far
There were no adverse events that caused death or withdrawal from the studies. The most common side effect was pain at the injection site, which occurred more frequently among vaccinated women.
Bottom line: Keep screening
The quadrivalent vaccine is not equally effective among all female candidates. I recommend the following:
- Encourage vaccination in very young girls and virgins. The vaccine is most effective in females who have not been exposed to any of the four subtypes targeted.
- A lack of type-specific, clinically available serology or lower genital tract screening in a woman who is already sexually active leaves the clinician in the dark about how to counsel her. Because most women I counsel do not harbor all four subtypes covered by the vaccine and lack anogenital disease, I offer the vaccine to all women in the age range that is indicated.
- Counsel women who are already sexually active and who harbor HPV before vaccination, as well as those who acquire the virus during the vaccination period, that even with inoculation they are susceptible to infection and neoplastic transformation, unlike those naïve to the vaccine-specific subtypes. Efficacy is still substantial, however—near 90% overall.
- If type-specific HPV testing becomes commercially available, counsel women who test positive for one of the four HPV subtypes targeted by the vaccine, as well as those with other HPV subtypes, that they are susceptible to neoplastic transformation even with the vaccine.
- Advise women who already have CIN, adenocarcinoma in situ, VIN, VAIN, or benign anogenital disease that the vaccine is not therapeutic and will not prevent the natural progression of these lesions toward cancer.
- Remember that few women harbor all four subtypes targeted by the vaccine. The vaccine may provide some marginal benefit to women already being treated for intraepithelial neoplasia, but each patient should be counseled about the potential lack of benefit.
- Be aware that there is clear benefit in the prevention of anogenital disease independent of the oncogenic risks associated with the virus.
- Don’t forget the other 13 known oncogenic HPV subtypes and the many more nononcogenic types identified so far. The effect of the vaccine is modestly reduced in women who harbor one or more of those subtypes when compared with its efficacy in uninfected recipients and virgins. Therefore, screening for cervical cancer and lower genital tract lesions should continue despite vaccination.
- In an editorial accompanying the FUTURE I and II trials, Sawaya and Smith-McCune observed that oncogenic HPV strains not targeted by the vaccine were responsible for a large number of CIN 2,3 and adenocarcinoma lesions in the FUTURE II trial.1 Although there are no concrete data, the editorialists suggest that, as we vaccinate for known strains, existing strains that are not included or new strains that evolve may “fill the niche” and continue to cause incident cases of neoplasia despite vaccination. This is another reason to screen women cytologically or by direct visual or other in-vivo methods to detect neoplasia.
A. No. In the FUTURE I trial, among women who were naïve to all strains of the human papillomavirus (HPV) vaccine at the time of inoculation, the vaccine was 100% effective in preventing anogenital lesions, cervical intraepithelial neoplasia (CIN) 2,3, adenocarcinoma in situ, and cervical cancer. In FUTURE II, among the same population, it was 98% effective in preventing all cervical lesions.
However, in FUTURE I, when the groups comprising HPV-naïve and HPV-positive subjects and those with and without preexisting neoplasia were combined, the vaccine was 20% effective in preventing CIN 2,3, adenocarcinoma in situ, and cervical cancer, and 34% effective in preventing genital warts and vulvar and vaginal intraepithelial neoplasia and cancer due to vaccine and nonvaccine HPV types. In FUTURE II, when the same groups were included, the vaccine was 17% effective in preventing all cervical lesions (regardless of the causal HPV type).
The women involved in these trials fell within the target age range for prophylactic vaccination, with ages from 15 to 26 years.
Expert Commentary
Even females in the target population for HPV vaccination (ages 9 to 26) present with different histories and risk status. An individual may be a candidate for:
- true primary prevention (no evidence of any HPV subtype or neoplasia)
- primary prevention for the vaccine subtypes (ie, negative for HPV type 6, 11, 16, or 18)
- prevention despite the presence of HPV type 6, 11, 16, or 18
- prevention despite the presence of a subtype not covered in the vaccine
- vaccination despite existing vulvar, vaginal, or cervical neoplasia.
The effect of the vaccine on this last group would be considered both prophylactic and therapeutic, defined as secondary prevention of cervical cancer. Secondary prevention (reversal of HPV effect in CIN lesions, leading to regression) has not been demonstrated with the quadrivalent HPV vaccine (Gardasil).
Trials followed women over 3 years
In these randomized trials, both vaccine efficacy (lesions prevented) and safety (side effects) were evaluated.
FUTURE I enrolled nearly 5,500 young women within the advised age range for prophylaxis who were randomized to the quadrivalent HPV vaccine or placebo. Cervical screening and colposcopic biopsy (if indicated) were conducted at baseline, and HPV status was determined. Investigators studied the effect of prophylactic vaccination in women naïve to the four viral subtypes targeted by the vaccine (6, 11, 16, and 18), as well as women in each of the other groups listed above.
The principal aim of FUTURE I was to provide an “ideal” frequent visit schedule with broader referral to colposcopy and determine whether the vaccine would reduce the combined incidence of anogenital warts, vulvar intraepithelial neoplasia (VIN), vaginal intraepithelial neoplasia (VAIN), CIN 1,2,3, and cancer related to HPV subtypes 6, 11, 16, and 18.
FUTURE II involved 12,167 women and a wider visit interval more consistent with clinical practice than FUTURE I. The aim was to determine whether the quadrivalent vaccine would reduce the incidence of CIN 2,3, adenocarcinoma in situ, or invasive carcinoma of the cervix related to HPV subtypes 16 and 18.
In both trials, polymerase chain reaction was used to identify the HPV subtype associated with any CIN, adenocarcinoma in situ, or anogenital lesion that was biopsy-proven, and this information was correlated with the patient’s HPV status before and after vaccination. The baseline and postvaccination serological status against HPV subtypes was also measured.
Safety is not a concern thus far
There were no adverse events that caused death or withdrawal from the studies. The most common side effect was pain at the injection site, which occurred more frequently among vaccinated women.
Bottom line: Keep screening
The quadrivalent vaccine is not equally effective among all female candidates. I recommend the following:
- Encourage vaccination in very young girls and virgins. The vaccine is most effective in females who have not been exposed to any of the four subtypes targeted.
- A lack of type-specific, clinically available serology or lower genital tract screening in a woman who is already sexually active leaves the clinician in the dark about how to counsel her. Because most women I counsel do not harbor all four subtypes covered by the vaccine and lack anogenital disease, I offer the vaccine to all women in the age range that is indicated.
- Counsel women who are already sexually active and who harbor HPV before vaccination, as well as those who acquire the virus during the vaccination period, that even with inoculation they are susceptible to infection and neoplastic transformation, unlike those naïve to the vaccine-specific subtypes. Efficacy is still substantial, however—near 90% overall.
- If type-specific HPV testing becomes commercially available, counsel women who test positive for one of the four HPV subtypes targeted by the vaccine, as well as those with other HPV subtypes, that they are susceptible to neoplastic transformation even with the vaccine.
- Advise women who already have CIN, adenocarcinoma in situ, VIN, VAIN, or benign anogenital disease that the vaccine is not therapeutic and will not prevent the natural progression of these lesions toward cancer.
- Remember that few women harbor all four subtypes targeted by the vaccine. The vaccine may provide some marginal benefit to women already being treated for intraepithelial neoplasia, but each patient should be counseled about the potential lack of benefit.
- Be aware that there is clear benefit in the prevention of anogenital disease independent of the oncogenic risks associated with the virus.
- Don’t forget the other 13 known oncogenic HPV subtypes and the many more nononcogenic types identified so far. The effect of the vaccine is modestly reduced in women who harbor one or more of those subtypes when compared with its efficacy in uninfected recipients and virgins. Therefore, screening for cervical cancer and lower genital tract lesions should continue despite vaccination.
- In an editorial accompanying the FUTURE I and II trials, Sawaya and Smith-McCune observed that oncogenic HPV strains not targeted by the vaccine were responsible for a large number of CIN 2,3 and adenocarcinoma lesions in the FUTURE II trial.1 Although there are no concrete data, the editorialists suggest that, as we vaccinate for known strains, existing strains that are not included or new strains that evolve may “fill the niche” and continue to cause incident cases of neoplasia despite vaccination. This is another reason to screen women cytologically or by direct visual or other in-vivo methods to detect neoplasia.
1. Sawaya GF, Smith-McCune KS. HPV vaccination—more answers, more questions. N Engl J Med. 2007;356:1991-1993.
1. Sawaya GF, Smith-McCune KS. HPV vaccination—more answers, more questions. N Engl J Med. 2007;356:1991-1993.
Q Is HPV testing alone a reliable cervical cancer screen?
Strengths of the study include large population
In this massive multicenter trial involving more than 33,000 women, participants were randomly assigned to screening with conventional cytology or to the experimental arm. This was a high-risk population 35 to 60 years of age, of which just slightly over 50% had undergone cervical screening within 4 years. In the first phase of the trial, which is the focus of this study, women in the experimental arm underwent screening with liquid-based cytology and HPV testing, and in the second phase, women were screened using HPV testing alone. The key endpoint for comparison: histology-confirmed CIN2+ after an abnormal screening test. Abnormal screening was defined as cytologic results of atypical squamous cells of undetermined significance or higher (ASCUS+) in the conventional cytology group, and ASCUS+ or a positive HC2 test in the experimental arm.
Because of the large sample size, it was deemed impossible to perform the “gold standard” diagnostic test (colposcopy and biopsy) in women with negative screening, so true sensitivity, specificity, and negative predictive value of the testing strategies could not be determined (TABLE). Because of this, the authors labeled and compared detection rates of CIN2+ in the 2 screening groups as “relative sensitivity.”
TABLE
How to “screen” a screening test
| SCREENING TEST RESULTS | HISTOLOGIC FINDINGS | |
|---|---|---|
| DISEASE PRESENT | DISEASE ABSENT | |
| The ideal screening test focuses on disease that is prevalent. To determine the value of the test, all patients undergo definitive evaluation—in the case of cervical cancer screening, this would entail colposcopy and biopsy—to determine the actual disease rate and the “disease-free” rate. | ||
| Positive | a (true positive) | b (false positive) |
| Negative | c (false negative) | d (true negative) |
| A test’s sensitivity is defined as a/a+c, specificity as d/b+d, positive predictive value as a/a+b, and negative predictive value as d/c+d. | ||
| If the entire screening population is not screened with the “gold-standard” reference test, the values of “c” and “d” cannot be determined. In the study by Ronco and colleagues, the only true rate that can be calculated is positive predictive value. | ||
Expert Commentary
This study leads to several key conclusions:
Practice recommendations
1. Lonky NM, Felix JC, Naidu YM, Wolde-Tsadik G. Triage of atypical squamous cells of undetermined significance with hybrid capture II: colposcopy and histologic human papillomavirus correlations. Obstet Gynecol. 2003;101:481-489.
2. Manos M, Kinney WK, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA. 1999;281:1605-1610.
3. Coste J, et al. Cross-sectional study of conventional cervical smear, monolayer cytology, and human papillomavirus DNA testing for cervical cancer screening. BMJ. 2003;326:733.-
Strengths of the study include large population
In this massive multicenter trial involving more than 33,000 women, participants were randomly assigned to screening with conventional cytology or to the experimental arm. This was a high-risk population 35 to 60 years of age, of which just slightly over 50% had undergone cervical screening within 4 years. In the first phase of the trial, which is the focus of this study, women in the experimental arm underwent screening with liquid-based cytology and HPV testing, and in the second phase, women were screened using HPV testing alone. The key endpoint for comparison: histology-confirmed CIN2+ after an abnormal screening test. Abnormal screening was defined as cytologic results of atypical squamous cells of undetermined significance or higher (ASCUS+) in the conventional cytology group, and ASCUS+ or a positive HC2 test in the experimental arm.
Because of the large sample size, it was deemed impossible to perform the “gold standard” diagnostic test (colposcopy and biopsy) in women with negative screening, so true sensitivity, specificity, and negative predictive value of the testing strategies could not be determined (TABLE). Because of this, the authors labeled and compared detection rates of CIN2+ in the 2 screening groups as “relative sensitivity.”
TABLE
How to “screen” a screening test
| SCREENING TEST RESULTS | HISTOLOGIC FINDINGS | |
|---|---|---|
| DISEASE PRESENT | DISEASE ABSENT | |
| The ideal screening test focuses on disease that is prevalent. To determine the value of the test, all patients undergo definitive evaluation—in the case of cervical cancer screening, this would entail colposcopy and biopsy—to determine the actual disease rate and the “disease-free” rate. | ||
| Positive | a (true positive) | b (false positive) |
| Negative | c (false negative) | d (true negative) |
| A test’s sensitivity is defined as a/a+c, specificity as d/b+d, positive predictive value as a/a+b, and negative predictive value as d/c+d. | ||
| If the entire screening population is not screened with the “gold-standard” reference test, the values of “c” and “d” cannot be determined. In the study by Ronco and colleagues, the only true rate that can be calculated is positive predictive value. | ||
Expert Commentary
This study leads to several key conclusions:
Practice recommendations
Strengths of the study include large population
In this massive multicenter trial involving more than 33,000 women, participants were randomly assigned to screening with conventional cytology or to the experimental arm. This was a high-risk population 35 to 60 years of age, of which just slightly over 50% had undergone cervical screening within 4 years. In the first phase of the trial, which is the focus of this study, women in the experimental arm underwent screening with liquid-based cytology and HPV testing, and in the second phase, women were screened using HPV testing alone. The key endpoint for comparison: histology-confirmed CIN2+ after an abnormal screening test. Abnormal screening was defined as cytologic results of atypical squamous cells of undetermined significance or higher (ASCUS+) in the conventional cytology group, and ASCUS+ or a positive HC2 test in the experimental arm.
Because of the large sample size, it was deemed impossible to perform the “gold standard” diagnostic test (colposcopy and biopsy) in women with negative screening, so true sensitivity, specificity, and negative predictive value of the testing strategies could not be determined (TABLE). Because of this, the authors labeled and compared detection rates of CIN2+ in the 2 screening groups as “relative sensitivity.”
TABLE
How to “screen” a screening test
| SCREENING TEST RESULTS | HISTOLOGIC FINDINGS | |
|---|---|---|
| DISEASE PRESENT | DISEASE ABSENT | |
| The ideal screening test focuses on disease that is prevalent. To determine the value of the test, all patients undergo definitive evaluation—in the case of cervical cancer screening, this would entail colposcopy and biopsy—to determine the actual disease rate and the “disease-free” rate. | ||
| Positive | a (true positive) | b (false positive) |
| Negative | c (false negative) | d (true negative) |
| A test’s sensitivity is defined as a/a+c, specificity as d/b+d, positive predictive value as a/a+b, and negative predictive value as d/c+d. | ||
| If the entire screening population is not screened with the “gold-standard” reference test, the values of “c” and “d” cannot be determined. In the study by Ronco and colleagues, the only true rate that can be calculated is positive predictive value. | ||
Expert Commentary
This study leads to several key conclusions:
Practice recommendations
1. Lonky NM, Felix JC, Naidu YM, Wolde-Tsadik G. Triage of atypical squamous cells of undetermined significance with hybrid capture II: colposcopy and histologic human papillomavirus correlations. Obstet Gynecol. 2003;101:481-489.
2. Manos M, Kinney WK, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA. 1999;281:1605-1610.
3. Coste J, et al. Cross-sectional study of conventional cervical smear, monolayer cytology, and human papillomavirus DNA testing for cervical cancer screening. BMJ. 2003;326:733.-
1. Lonky NM, Felix JC, Naidu YM, Wolde-Tsadik G. Triage of atypical squamous cells of undetermined significance with hybrid capture II: colposcopy and histologic human papillomavirus correlations. Obstet Gynecol. 2003;101:481-489.
2. Manos M, Kinney WK, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA. 1999;281:1605-1610.
3. Coste J, et al. Cross-sectional study of conventional cervical smear, monolayer cytology, and human papillomavirus DNA testing for cervical cancer screening. BMJ. 2003;326:733.-
Q How many Ob/Gyns follow the new rules on Pap testing?
A A minority. Both Ob/Gyns and their patients prefer more frequent screening than the intervals advised by the American Cancer Society and other expert groups, 2 new studies found.
Expert commentary
Less-than-annual screening in women over the age of 30, with 3 or more normal Papanicolaou (Pap) tests, is recommended by 3 expert groups: the American Cancer Society (ACS), the American College of Obstetricians and Gynecologists, and the US Preventive Services Task Force. Other expert groups additionally recommend that we stop screening women who have undergone total hysterectomy for symptomatic fibroids with no history of dysplasia, low-risk women over the age of 70, and virgin girls before age 21.
Neither Ob/Gyns nor patients, however, are inclined to follow these recommendations.
What women want
Most women prefer annual screening, irrespective of evidence. Sirovich and colleagues found that 75% of women 40 or older—most of whom had been regularly screened—prefer annual testing. Once informed of the evidence, 69% said they will persist with annual screening. About half said they will continue being screened up to and after age 80 despite no evidence of benefit.
Only 43% knew of the updated guidelines, and only 10% were being screened at the recommended intervals of 2 to 3 years.
These findings were via a survey conducted concomitant with the 2002 ACS guidelines, and published in February 2005. The nationally representative sample was questioned in a random-digit-dialing telephone survey with a response rate of 75%.
While 40% of the women understood that overscreening is wasteful and might lead to unnecessary further testing and treatment, 50% believed the primary motivation for recommending less frequent screening was cost.
What Ob/Gyns are doing
A majority of Ob/Gyns will screen women annually and indefinitely despite lack of evidence of benefit, Saint and colleagues found. They surveyed 355 randomly selected US Ob/Gyns, with a response rate of 60%.
More than 70% plan to continue screening in a 70-year-old woman with 30 years of negative Pap tests and no sexual intercourse for the past 10 years, and 60% of Ob/Gyns plan to carry on annual screening in a 35-year-old woman with 3 or more consecutive normal tests.
This is surprising, given that 82% of respondents use liquid-based Pap tests and 34% employ combined Pap–human papillomavirus (HPV) testing.
A failure to ask the simple question: “Why?”
It would be fascinating to learn why physicians persist with annual screening. Unfortunately, Saint and colleagues did not ask this question. As is the case in much recent clinical research, we rarely ask the “why” questions that seek to make sense of outcomes analyses. A simple query at the end of the survey asking, “If you screen low-risk patients annually, what is your rationale?” would have helped clarify the issue. The answer probably has little to do with scientific evidence and much to do with tradition, meeting patient expectations, maintaining trust, and possibly fear of litigation from misdiagnosis.
A matter of time
Patients expect the best, and in this competitive era physicians will do what it takes to keep a patient satisfied. If that means annual cervical cancer screening, so be it.
Perhaps the issue boils down to a matter of time. After all, we spent 50 years teaching doctors and patients the importance of annual screening, so it will be no surprise if it takes a long time for both Ob/Gyns and patients to trust that “less is more” in low-risk women.
Change may also hinge on the medical marketplace. Women who expect annual screening link it to more than cervical cancer prevention. They want their Ob/Gyns to focus on the entire health spectrum and will seek other physicians if their expectations are not met.
Despite breakthroughs in cervical cytologic sampling and DNA testing, the new technologies have not yet inspired broad confidence that less frequent screening is good health care.
And a matter of cost
Over the long term, however, paying for the more costly tools and interventions in screening and triage will be possible only with longer screening intervals for screen-negative women.
A A minority. Both Ob/Gyns and their patients prefer more frequent screening than the intervals advised by the American Cancer Society and other expert groups, 2 new studies found.
Expert commentary
Less-than-annual screening in women over the age of 30, with 3 or more normal Papanicolaou (Pap) tests, is recommended by 3 expert groups: the American Cancer Society (ACS), the American College of Obstetricians and Gynecologists, and the US Preventive Services Task Force. Other expert groups additionally recommend that we stop screening women who have undergone total hysterectomy for symptomatic fibroids with no history of dysplasia, low-risk women over the age of 70, and virgin girls before age 21.
Neither Ob/Gyns nor patients, however, are inclined to follow these recommendations.
What women want
Most women prefer annual screening, irrespective of evidence. Sirovich and colleagues found that 75% of women 40 or older—most of whom had been regularly screened—prefer annual testing. Once informed of the evidence, 69% said they will persist with annual screening. About half said they will continue being screened up to and after age 80 despite no evidence of benefit.
Only 43% knew of the updated guidelines, and only 10% were being screened at the recommended intervals of 2 to 3 years.
These findings were via a survey conducted concomitant with the 2002 ACS guidelines, and published in February 2005. The nationally representative sample was questioned in a random-digit-dialing telephone survey with a response rate of 75%.
While 40% of the women understood that overscreening is wasteful and might lead to unnecessary further testing and treatment, 50% believed the primary motivation for recommending less frequent screening was cost.
What Ob/Gyns are doing
A majority of Ob/Gyns will screen women annually and indefinitely despite lack of evidence of benefit, Saint and colleagues found. They surveyed 355 randomly selected US Ob/Gyns, with a response rate of 60%.
More than 70% plan to continue screening in a 70-year-old woman with 30 years of negative Pap tests and no sexual intercourse for the past 10 years, and 60% of Ob/Gyns plan to carry on annual screening in a 35-year-old woman with 3 or more consecutive normal tests.
This is surprising, given that 82% of respondents use liquid-based Pap tests and 34% employ combined Pap–human papillomavirus (HPV) testing.
A failure to ask the simple question: “Why?”
It would be fascinating to learn why physicians persist with annual screening. Unfortunately, Saint and colleagues did not ask this question. As is the case in much recent clinical research, we rarely ask the “why” questions that seek to make sense of outcomes analyses. A simple query at the end of the survey asking, “If you screen low-risk patients annually, what is your rationale?” would have helped clarify the issue. The answer probably has little to do with scientific evidence and much to do with tradition, meeting patient expectations, maintaining trust, and possibly fear of litigation from misdiagnosis.
A matter of time
Patients expect the best, and in this competitive era physicians will do what it takes to keep a patient satisfied. If that means annual cervical cancer screening, so be it.
Perhaps the issue boils down to a matter of time. After all, we spent 50 years teaching doctors and patients the importance of annual screening, so it will be no surprise if it takes a long time for both Ob/Gyns and patients to trust that “less is more” in low-risk women.
Change may also hinge on the medical marketplace. Women who expect annual screening link it to more than cervical cancer prevention. They want their Ob/Gyns to focus on the entire health spectrum and will seek other physicians if their expectations are not met.
Despite breakthroughs in cervical cytologic sampling and DNA testing, the new technologies have not yet inspired broad confidence that less frequent screening is good health care.
And a matter of cost
Over the long term, however, paying for the more costly tools and interventions in screening and triage will be possible only with longer screening intervals for screen-negative women.
A A minority. Both Ob/Gyns and their patients prefer more frequent screening than the intervals advised by the American Cancer Society and other expert groups, 2 new studies found.
Expert commentary
Less-than-annual screening in women over the age of 30, with 3 or more normal Papanicolaou (Pap) tests, is recommended by 3 expert groups: the American Cancer Society (ACS), the American College of Obstetricians and Gynecologists, and the US Preventive Services Task Force. Other expert groups additionally recommend that we stop screening women who have undergone total hysterectomy for symptomatic fibroids with no history of dysplasia, low-risk women over the age of 70, and virgin girls before age 21.
Neither Ob/Gyns nor patients, however, are inclined to follow these recommendations.
What women want
Most women prefer annual screening, irrespective of evidence. Sirovich and colleagues found that 75% of women 40 or older—most of whom had been regularly screened—prefer annual testing. Once informed of the evidence, 69% said they will persist with annual screening. About half said they will continue being screened up to and after age 80 despite no evidence of benefit.
Only 43% knew of the updated guidelines, and only 10% were being screened at the recommended intervals of 2 to 3 years.
These findings were via a survey conducted concomitant with the 2002 ACS guidelines, and published in February 2005. The nationally representative sample was questioned in a random-digit-dialing telephone survey with a response rate of 75%.
While 40% of the women understood that overscreening is wasteful and might lead to unnecessary further testing and treatment, 50% believed the primary motivation for recommending less frequent screening was cost.
What Ob/Gyns are doing
A majority of Ob/Gyns will screen women annually and indefinitely despite lack of evidence of benefit, Saint and colleagues found. They surveyed 355 randomly selected US Ob/Gyns, with a response rate of 60%.
More than 70% plan to continue screening in a 70-year-old woman with 30 years of negative Pap tests and no sexual intercourse for the past 10 years, and 60% of Ob/Gyns plan to carry on annual screening in a 35-year-old woman with 3 or more consecutive normal tests.
This is surprising, given that 82% of respondents use liquid-based Pap tests and 34% employ combined Pap–human papillomavirus (HPV) testing.
A failure to ask the simple question: “Why?”
It would be fascinating to learn why physicians persist with annual screening. Unfortunately, Saint and colleagues did not ask this question. As is the case in much recent clinical research, we rarely ask the “why” questions that seek to make sense of outcomes analyses. A simple query at the end of the survey asking, “If you screen low-risk patients annually, what is your rationale?” would have helped clarify the issue. The answer probably has little to do with scientific evidence and much to do with tradition, meeting patient expectations, maintaining trust, and possibly fear of litigation from misdiagnosis.
A matter of time
Patients expect the best, and in this competitive era physicians will do what it takes to keep a patient satisfied. If that means annual cervical cancer screening, so be it.
Perhaps the issue boils down to a matter of time. After all, we spent 50 years teaching doctors and patients the importance of annual screening, so it will be no surprise if it takes a long time for both Ob/Gyns and patients to trust that “less is more” in low-risk women.
Change may also hinge on the medical marketplace. Women who expect annual screening link it to more than cervical cancer prevention. They want their Ob/Gyns to focus on the entire health spectrum and will seek other physicians if their expectations are not met.
Despite breakthroughs in cervical cytologic sampling and DNA testing, the new technologies have not yet inspired broad confidence that less frequent screening is good health care.
And a matter of cost
Over the long term, however, paying for the more costly tools and interventions in screening and triage will be possible only with longer screening intervals for screen-negative women.