User login
Surgical volume and outcomes for gynecologic surgery: Is more always better?
Over the last 3 decades, abundant evidence has demonstrated the association between surgical volume and outcomes. Patients operated on by high-volume surgeons and at high-volume hospitals have superior outcomes.
Surgical volume in gynecology
The association between both hospital and surgeon volume and outcomes has been explored across a number of gynecologic procedures.3 A meta-analysis that included 741,000 patients found that low-volume surgeons had an increased rate of complications overall, a higher rate of intraoperative complications, and a higher rate of postoperative complications compared with high-volume surgeons. While there was no association between volume and mortality overall, when limited to gynecologic oncology studies, low surgeon volume was associated with increased perioperative mortality.3
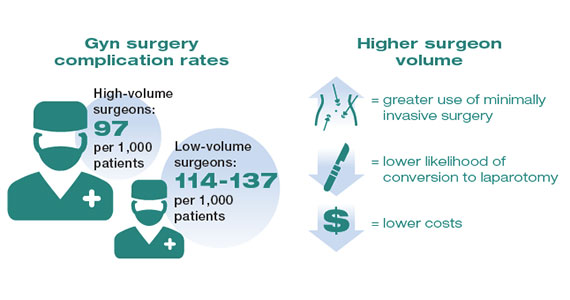
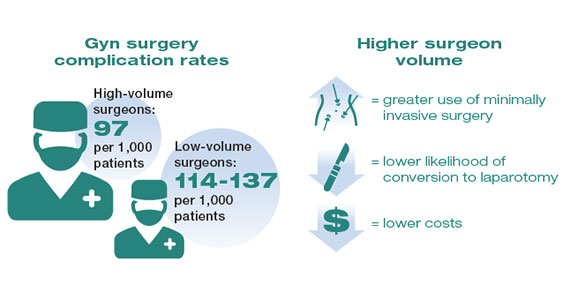
While these studies demonstrated a statistically significant association between surgeon volume and perioperative outcomes, the magnitude of the effect is modest compared with other higher-risk procedures associated with greater perioperative morbidity. For example, in a large study that examined oncologic and cardiovascular surgery, perioperative mortality in patients who underwent pancreatic resection was reduced from 15% for low-volume surgeons to 5% for high-volume surgeons.1 By contrast, for gynecologic surgery, complications occurred in 97 per 1,000 patients operated on by high-volume surgeons compared with between 114 and 137 per 1,000 for low-volume surgeons. Thus, to avoid 1 in-hospital complication, 30 surgeries performed by low-volume surgeons would need to be moved to high-volume surgeons. For intraoperative complications, 38 patients would need to be moved from low- to high-volume surgeons to prevent 1 such complication.3 In addition to morbidity and mortality, higher surgeon volume is associated with greater use of minimally invasive surgery, a lower likelihood of conversion to laparotomy, and lower costs.3
Similarly, hospital volume also has been associated with outcomes for gynecologic surgery.4 In a report of patients who underwent laparoscopic hysterectomy, the authors found that the complication rate was 18% lower for patients at high- versus low-volume hospitals. In addition, cost was lower at the high-volume centers.4 Like surgeon volume, the magnitude of the differential in outcomes between high- and low-volume hospitals is often modest.4
While most studies have focused on short-term outcomes, surgical volume appears also to be associated with longer-term outcomes. For gynecologic cancer, studies have demonstrated an association between hospital volume and survival for ovarian and cervical cancer.5-7 A large report of centers across the United States found that the 5-year survival rate increased from 39% for patients treated at low-volume centers to 51% at the highest-volume hospitals.5 In urogynecology, surgeon volume has been associated with midurethral sling revision. One study noted that after an individual surgeon performed 50 procedures a year, each additional case was associated with a decline in the rate of sling revision.8 One could argue that these longer-term end points may be the measures that matter most to patients.
Although the magnitude of the association between surgical volume and outcomes in gynecology appears to be relatively modest, outcomes for very-low-volume (VLV) surgeons are substantially worse. An analysis of more than 430,000 patients who underwent hysterectomy compared outcomes between VLV surgeons (characterized as surgeons who performed only 1 hysterectomy in the prior year) and other gynecologic surgeons. The overall complication rate was 32% in VLV surgeons compared with 10% among other surgeons, while the perioperative mortality rate was 2.5% versus 0.2% in the 2 groups, respectively. Likely reflecting changing practice patterns in gynecology, a sizable number of surgeons were classified as VLV physicians.9
Continue to: Public health applications of gynecologic surgical volume...
Public health applications of gynecologic surgical volume
The large body of literature on volume and outcomes has led to a number of public health initiatives aimed at reducing perioperative morbidity and mortality. Broadly, these efforts focus on regionalization of care, targeted quality improvement, and the development of minimum volume standards. Each strategy holds promise but also the potential to lead to unwanted consequences.
Regionalization of care
Recognition of the volume-outcomes paradigm has led to efforts to regionalize care for complex procedures to high-volume surgeons and centers.10 A cohort study of surgical patterns of care for Medicare recipients who underwent cancer resections or abdominal aortic aneurysm repair from 1999 to 2008 demonstrated these shifting practice patterns. For example, in 1999–2000, pancreatectomy was performed in 1,308 hospitals, with a median case volume of 5 procedures per year. By 2007–2008, the number of hospitals in which pancreatectomy was performed declined to 978, and the median case volume rose to 16 procedures per year. Importantly, over this time period, risk-adjusted mortality for pancreatectomy declined by 19%, and increased hospital volume was responsible for more than two-thirds of the decline in mortality.10
There has similarly been a gradual concentration of some gynecologic procedures to higher-volume surgeons and centers.11,12 Among patients undergoing hysterectomy for endometrial cancer in New York State, 845 surgeons with a mean case volume of 3 procedures per year treated patients in 2000. By 2014, the number of surgeons who performed these operations declined to 317 while mean annual case volume rose to 10 procedures per year. The number of hospitals in which women with endometrial cancer were treated declined from 182 to 98 over the same time period.11 Similar trends were noted for patients undergoing ovarian cancer resection.12 While patterns of gynecologic care for some surgical procedures have clearly changed, it has been more difficult to link these changes to improvements in outcomes.11,12
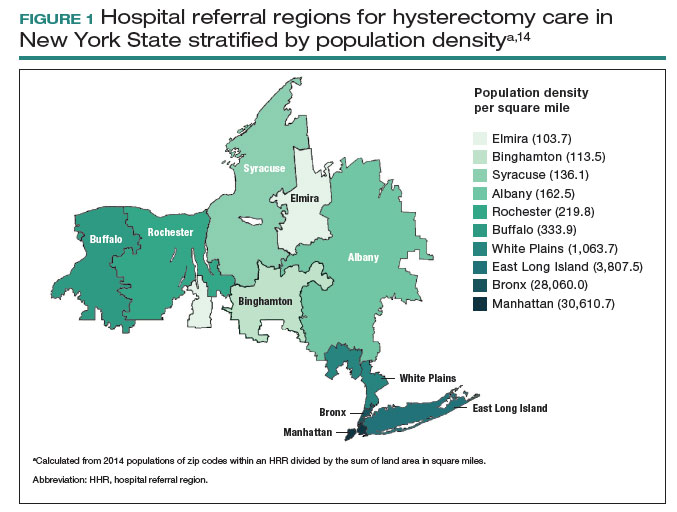
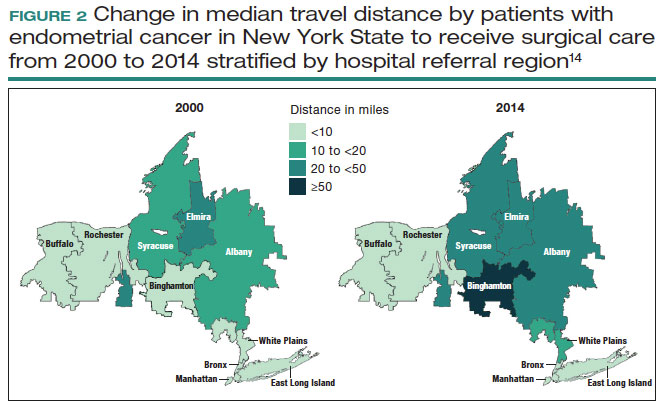
Despite the intuitive appeal of regionalization of surgical care, such a strategy has a number of limitations and practical challenges. Not surprisingly, limiting the number of surgeons and hospitals that perform a given procedure necessitates that patients travel a greater distance to obtain necessary surgical care.13,14 An analysis of endometrial cancer patients in New York State stratified patients based on their area of residence into 10 hospital referral regions (HRRs), which represent health care markets for tertiary medical care. From 2000 to 2014, the distance patients traveled to receive their surgical care increased in all of the HRRs studied. This was most pronounced in 1 of the HRRs in which the median travel distance rose by 47 miles over the 15-year period (FIGURE 1; FIGURE 2).14
Whether patients are willing to travel for care remains a matter of debate and depends on the disease, the surgical procedure, and the anticipated benefit associated with a longer travel distance.15,16 In a discrete choice experiment, 100 participants were given a hypothetical scenario in which they had potentially resectable pancreatic cancer; they were queried on their willingness to travel for care based on varying differences in mortality between a local and regional hospital.15 When mortality at the local hospital was double that of the regional hospital (6% vs 3%), 45% of patients chose to remain at the local hospital. When the differential increased to a 4 times greater mortality at the local hospital (12% vs 3%), 23% of patients still chose to remain at the local hospital.15
A similar study asked patients with ovarian neoplasms whether they would travel 50 miles to a regional center for surgery based on some degree of increased 5-year survival.16 Overall, 79% of patients would travel for a 4% improvement in survival while 97% would travel for a 12% improvement in survival.16
Lastly, a number of studies have shown that regionalization of surgical care disproportionately affects Black and Hispanic patients and those with low socioeconomic status.12,13,17 A simulation study on the effect of regionalizing care for pancreatectomy noted that using a hospital volume threshold of 20 procedures per year, a higher percentage of Black and Hispanic patients than White patients would be required to travel to a higher-volume center.13 Similarly, Medicaid recipients were more likely to be affected.13 Despite the inequities in who must travel for regionalized care, prior work has suggested that regionalization of cancer care to high-volume centers may reduce racial and socioeconomic disparities in survival for some cancers.18
Targeted quality improvement
Realizing the practical limitations of regionalization of care, an alternative strategy is to improve the quality of care at low-volume hospitals.5,19 Quality of care and surgical volume often are correlated, and the delivery of high-quality care can mitigate some of the influence of surgical volume on outcomes.
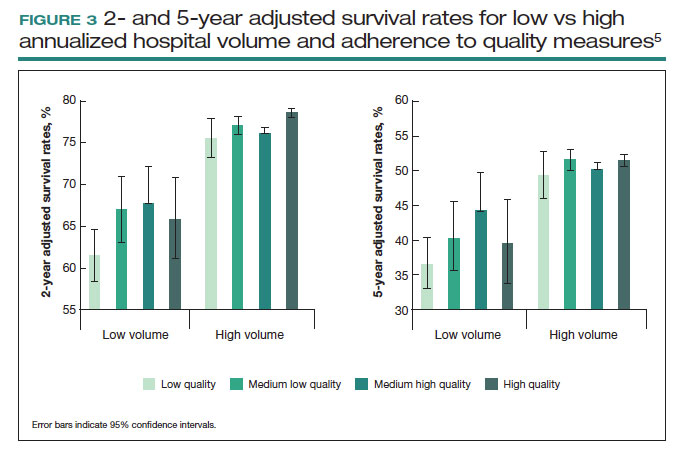
These principles were demonstrated in a study of more than 100,000 patients with ovarian cancer that stratified treating hospitals into volume quintiles.5 As expected, survival (both 2- and 5-year) was highest in the highest-volume quintile hospitals (FIGURE 3).5 Similarly, quality of care, measured through adherence to various process measures, was also highest in the highest-volume quintile hospitals. Interestingly, in the second-fourth volume quintile hospitals, there was substantial variation in adherence to quality metrics. Among hospitals with higher quality care, an improved survival was noted compared with lower quality care hospitals within the same volume quintile. Survival at high-quality, intermediate-volume hospitals approached that of the high-volume quintile hospitals.5
These findings highlight the importance of quality of care as well as the complex interplay of surgical volume and other factors.20 Many have argued that it may be more appropriate to measure quality of care and past performance and outcomes rather than surgical volume.21
Continue to: Minimum volume standards...
Minimum volume standards
While efforts to regionalize surgical care have gradually evolved, calls have been growing to formalize policies that limit the performance of some procedures to surgeons and centers that meet a minimum volume threshold or standard.21 One such effort, based on consensus from 3 academic hospital systems, was a campaign for hospitals to “Take the Volume Pledge.”21 The campaign’s goal is to encourage health care systems to restrict the performance of 10 procedures to surgeons and hospitals within their systems that meet a minimum volume standard for the given operations.21 In essence, procedures would be restricted for low-volume providers and centers and triaged to higher-volume surgeons and hospitals within a given health care system.21
Proponents of the Volume Pledge argue that it is a relatively straightforward way to align patients and providers to optimize outcomes. The Volume Pledge focuses on larger hospital systems and encourages referral within the given system, thus mitigating competitive and financial concerns about referring patients to outside providers. Those who have argued against the Volume Pledge point out that the volume cut points chosen are somewhat arbitrary, that these policies have the potential to negatively impact rural hospitals and those serving smaller communities, and that quality is a more appropriate metric than volume.22 The Volume Pledge does not include any gynecologic procedures, and to date it has met with only limited success.23
Perhaps more directly applicable to gynecologic surgeons are ongoing national trends to base hospital credentialing on surgical volume. In essence, individual surgeons must demonstrate that they have performed a minimum number of procedures to obtain or retain privileges.24,25 While there is strong evidence of the association between volume and outcomes for some complex surgical procedures, linking volume to credentialing has a number of potential pitfalls. Studies of surgical outcomes based on volume represent average performance, and many low-volume providers have better-than-expected outcomes. Volume measures typically represent recent performance; it is difficult to measure the overall experience of individual surgeons. Similarly, surgical outcomes depend on both the surgeon and the system in which the surgeon operates. It is difficult, if not impossible, to account for differences in the environment in which a surgeon works.25
A study of gynecologic surgeons who performed hysterectomy in New York State demonstrates many of the complexities of volume-based credentialing.26 In a cohort of more than55,000 patients who underwent abdominal hysterectomy, there was a strong association between low surgeon volume and a higher-than-expected rate of complications. If one were to consider limiting privileges to even the lowest-volume providers, there would be a significant impact on the surgical workforce. In this cohort, limiting credentialing to the lowest-volume providers, those who performed only 1 abdominal hysterectomy in the prior year would restrict the privileges of 17.5% of the surgeons in the cohort. Further, in this low-volume cohort that performed only 1 abdominal hysterectomy in the prior year, 69% of the surgeons actually had outcomes that were better than predicted.26 These data highlight not only the difficulty of applying averages to individual surgeons but also the profound impact that policy changes could have on the practice of gynecologic surgery.
Volume-outcomes paradigm discussions continue
The association between higher surgeon and hospital procedural volume for gynecologic surgeries and improved outcomes now has been convincingly demonstrated. With this knowledge, over the last decade the patterns of care for patients undergoing gynecologic surgery have clearly shifted, and these operations are now more commonly being performed by a smaller number of physicians and at fewer hospitals.
While efforts to improve quality are clearly important, many policy interventions, such as regionalization of care, have untoward consequences that must be considered. As we move forward, it will be essential to ensure that there is a robust debate among patients, providers, and policymakers on the merits of public health policies based on the volume-outcomes paradigm. ●
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117-2127.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:11281137.
- Mowat A, Maher C, Ballard E. Surgical outcomes for low-volume vs high-volume surgeons in gynecology surgery: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;215:21-33.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Wright JD, Chen L, Hou JY, et al. Association of hospital volume and quality of care with survival for ovarian cancer. Obstet Gynecol. 2017;130:545-553.
- Cliby WA, Powell MA, Al-Hammadi N, et al. Ovarian cancer in the United States: contemporary patterns of care associated with improved survival. Gynecol Oncol. 2015;136:11-17.
- Matsuo K, Shimada M, Yamaguchi S, et al. Association of radical hysterectomy surgical volume and survival for early-stage cervical cancer. Obstet Gynecol. 2019;133:1086-1098.
- Brennand EA, Quan H. Evaluation of the effect of surgeon’s operative volume and specialty on likelihood of revision after mesh midurethral sling placement. Obstet Gynecol. 2019;133:1099-1108.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:21282137.
- Wright JD, Ruiz MP, Chen L, et al. Changes in surgical volume and outcomes over time for women undergoing hysterectomy for endometrial cancer. Obstet Gynecol. 2018;132:59-69.
- Wright JD, Chen L, Buskwofie A, et al. Regionalization of care for women with ovarian cancer. Gynecol Oncol. 2019;154:394-400.
- Fong ZV, Hashimoto DA, Jin G, et al. Simulated volume-based regionalization of complex procedures: impact on spatial access to care. Ann Surg. 2021;274:312-318.
- Knisely A, Huang Y, Melamed A, et al. Effect of regionalization of endometrial cancer care on site of care and patient travel. Am J Obstet Gynecol. 2020;222:58.e1-58.e10.
- Finlayson SR, Birkmeyer JD, Tosteson AN, et al. Patient preferences for location of care: implications for regionalization. Med Care. 1999;37:204-209.
- Shalowitz DI, Nivasch E, Burger RA, et al. Are patients willing to travel for better ovarian cancer care? Gynecol Oncol. 2018;148:42-48.
- Rehmani SS, Liu B, Al-Ayoubi AM, et al. Racial disparity in utilization of high-volume hospitals for surgical treatment of esophageal cancer. Ann Thorac Surg. 2018;106:346-353.
- Nattinger AB, Rademacher N, McGinley EL, et al. Can regionalization of care reduce socioeconomic disparities in breast cancer survival? Med Care. 2021;59:77-81.
- Auerbach AD, Hilton JF, Maselli J, et al. Shop for quality or volume? Volume, quality, and outcomes of coronary artery bypass surgery. Ann Intern Med. 2009;150:696-704.
- Kurlansky PA, Argenziano M, Dunton R, et al. Quality, not volume, determines outcome of coronary artery bypass surgery in a university-based community hospital network. J Thorac Cardiovasc Surg. 2012;143:287-293.
- Urbach DR. Pledging to eliminate low-volume surgery. N Engl J Med. 2015;373:1388-1390.
- Blanco BA, Kothari AN, Blackwell RH, et al. “Take the Volume Pledge” may result in disparity in access to care. Surgery. 2017;161:837-845.
- Farjah F, Grau-Sepulveda MV, Gaissert H, et al. Volume Pledge is not associated with better short-term outcomes after lung cancer resection. J Clin Oncol. 2020;38:3518-3527.
- Tracy EE, Zephyrin LC, Rosman DA, et al. Credentialing based on surgical volume, physician workforce challenges, and patient access. Obstet Gynecol. 2013;122:947-951.
- Statement on credentialing and privileging and volume performance issues. April 1, 2018. American College of Surgeons. Accessed April 10, 2023. https://facs.org/about-acs/statements/credentialing-andprivileging-and-volume-performance-issues/
- Ruiz MP, Chen L, Hou JY, et al. Effect of minimum-volume standards on patient outcomes and surgical practice patterns for hysterectomy. Obstet Gynecol. 2018;132:1229-1237.
Over the last 3 decades, abundant evidence has demonstrated the association between surgical volume and outcomes. Patients operated on by high-volume surgeons and at high-volume hospitals have superior outcomes.
Surgical volume in gynecology
The association between both hospital and surgeon volume and outcomes has been explored across a number of gynecologic procedures.3 A meta-analysis that included 741,000 patients found that low-volume surgeons had an increased rate of complications overall, a higher rate of intraoperative complications, and a higher rate of postoperative complications compared with high-volume surgeons. While there was no association between volume and mortality overall, when limited to gynecologic oncology studies, low surgeon volume was associated with increased perioperative mortality.3
While these studies demonstrated a statistically significant association between surgeon volume and perioperative outcomes, the magnitude of the effect is modest compared with other higher-risk procedures associated with greater perioperative morbidity. For example, in a large study that examined oncologic and cardiovascular surgery, perioperative mortality in patients who underwent pancreatic resection was reduced from 15% for low-volume surgeons to 5% for high-volume surgeons.1 By contrast, for gynecologic surgery, complications occurred in 97 per 1,000 patients operated on by high-volume surgeons compared with between 114 and 137 per 1,000 for low-volume surgeons. Thus, to avoid 1 in-hospital complication, 30 surgeries performed by low-volume surgeons would need to be moved to high-volume surgeons. For intraoperative complications, 38 patients would need to be moved from low- to high-volume surgeons to prevent 1 such complication.3 In addition to morbidity and mortality, higher surgeon volume is associated with greater use of minimally invasive surgery, a lower likelihood of conversion to laparotomy, and lower costs.3

Similarly, hospital volume also has been associated with outcomes for gynecologic surgery.4 In a report of patients who underwent laparoscopic hysterectomy, the authors found that the complication rate was 18% lower for patients at high- versus low-volume hospitals. In addition, cost was lower at the high-volume centers.4 Like surgeon volume, the magnitude of the differential in outcomes between high- and low-volume hospitals is often modest.4
While most studies have focused on short-term outcomes, surgical volume appears also to be associated with longer-term outcomes. For gynecologic cancer, studies have demonstrated an association between hospital volume and survival for ovarian and cervical cancer.5-7 A large report of centers across the United States found that the 5-year survival rate increased from 39% for patients treated at low-volume centers to 51% at the highest-volume hospitals.5 In urogynecology, surgeon volume has been associated with midurethral sling revision. One study noted that after an individual surgeon performed 50 procedures a year, each additional case was associated with a decline in the rate of sling revision.8 One could argue that these longer-term end points may be the measures that matter most to patients.
Although the magnitude of the association between surgical volume and outcomes in gynecology appears to be relatively modest, outcomes for very-low-volume (VLV) surgeons are substantially worse. An analysis of more than 430,000 patients who underwent hysterectomy compared outcomes between VLV surgeons (characterized as surgeons who performed only 1 hysterectomy in the prior year) and other gynecologic surgeons. The overall complication rate was 32% in VLV surgeons compared with 10% among other surgeons, while the perioperative mortality rate was 2.5% versus 0.2% in the 2 groups, respectively. Likely reflecting changing practice patterns in gynecology, a sizable number of surgeons were classified as VLV physicians.9
Continue to: Public health applications of gynecologic surgical volume...
Public health applications of gynecologic surgical volume
The large body of literature on volume and outcomes has led to a number of public health initiatives aimed at reducing perioperative morbidity and mortality. Broadly, these efforts focus on regionalization of care, targeted quality improvement, and the development of minimum volume standards. Each strategy holds promise but also the potential to lead to unwanted consequences.
Regionalization of care
Recognition of the volume-outcomes paradigm has led to efforts to regionalize care for complex procedures to high-volume surgeons and centers.10 A cohort study of surgical patterns of care for Medicare recipients who underwent cancer resections or abdominal aortic aneurysm repair from 1999 to 2008 demonstrated these shifting practice patterns. For example, in 1999–2000, pancreatectomy was performed in 1,308 hospitals, with a median case volume of 5 procedures per year. By 2007–2008, the number of hospitals in which pancreatectomy was performed declined to 978, and the median case volume rose to 16 procedures per year. Importantly, over this time period, risk-adjusted mortality for pancreatectomy declined by 19%, and increased hospital volume was responsible for more than two-thirds of the decline in mortality.10
There has similarly been a gradual concentration of some gynecologic procedures to higher-volume surgeons and centers.11,12 Among patients undergoing hysterectomy for endometrial cancer in New York State, 845 surgeons with a mean case volume of 3 procedures per year treated patients in 2000. By 2014, the number of surgeons who performed these operations declined to 317 while mean annual case volume rose to 10 procedures per year. The number of hospitals in which women with endometrial cancer were treated declined from 182 to 98 over the same time period.11 Similar trends were noted for patients undergoing ovarian cancer resection.12 While patterns of gynecologic care for some surgical procedures have clearly changed, it has been more difficult to link these changes to improvements in outcomes.11,12
Despite the intuitive appeal of regionalization of surgical care, such a strategy has a number of limitations and practical challenges. Not surprisingly, limiting the number of surgeons and hospitals that perform a given procedure necessitates that patients travel a greater distance to obtain necessary surgical care.13,14 An analysis of endometrial cancer patients in New York State stratified patients based on their area of residence into 10 hospital referral regions (HRRs), which represent health care markets for tertiary medical care. From 2000 to 2014, the distance patients traveled to receive their surgical care increased in all of the HRRs studied. This was most pronounced in 1 of the HRRs in which the median travel distance rose by 47 miles over the 15-year period (FIGURE 1; FIGURE 2).14
Whether patients are willing to travel for care remains a matter of debate and depends on the disease, the surgical procedure, and the anticipated benefit associated with a longer travel distance.15,16 In a discrete choice experiment, 100 participants were given a hypothetical scenario in which they had potentially resectable pancreatic cancer; they were queried on their willingness to travel for care based on varying differences in mortality between a local and regional hospital.15 When mortality at the local hospital was double that of the regional hospital (6% vs 3%), 45% of patients chose to remain at the local hospital. When the differential increased to a 4 times greater mortality at the local hospital (12% vs 3%), 23% of patients still chose to remain at the local hospital.15


A similar study asked patients with ovarian neoplasms whether they would travel 50 miles to a regional center for surgery based on some degree of increased 5-year survival.16 Overall, 79% of patients would travel for a 4% improvement in survival while 97% would travel for a 12% improvement in survival.16
Lastly, a number of studies have shown that regionalization of surgical care disproportionately affects Black and Hispanic patients and those with low socioeconomic status.12,13,17 A simulation study on the effect of regionalizing care for pancreatectomy noted that using a hospital volume threshold of 20 procedures per year, a higher percentage of Black and Hispanic patients than White patients would be required to travel to a higher-volume center.13 Similarly, Medicaid recipients were more likely to be affected.13 Despite the inequities in who must travel for regionalized care, prior work has suggested that regionalization of cancer care to high-volume centers may reduce racial and socioeconomic disparities in survival for some cancers.18
Targeted quality improvement
Realizing the practical limitations of regionalization of care, an alternative strategy is to improve the quality of care at low-volume hospitals.5,19 Quality of care and surgical volume often are correlated, and the delivery of high-quality care can mitigate some of the influence of surgical volume on outcomes.
These principles were demonstrated in a study of more than 100,000 patients with ovarian cancer that stratified treating hospitals into volume quintiles.5 As expected, survival (both 2- and 5-year) was highest in the highest-volume quintile hospitals (FIGURE 3).5 Similarly, quality of care, measured through adherence to various process measures, was also highest in the highest-volume quintile hospitals. Interestingly, in the second-fourth volume quintile hospitals, there was substantial variation in adherence to quality metrics. Among hospitals with higher quality care, an improved survival was noted compared with lower quality care hospitals within the same volume quintile. Survival at high-quality, intermediate-volume hospitals approached that of the high-volume quintile hospitals.5

These findings highlight the importance of quality of care as well as the complex interplay of surgical volume and other factors.20 Many have argued that it may be more appropriate to measure quality of care and past performance and outcomes rather than surgical volume.21
Continue to: Minimum volume standards...
Minimum volume standards
While efforts to regionalize surgical care have gradually evolved, calls have been growing to formalize policies that limit the performance of some procedures to surgeons and centers that meet a minimum volume threshold or standard.21 One such effort, based on consensus from 3 academic hospital systems, was a campaign for hospitals to “Take the Volume Pledge.”21 The campaign’s goal is to encourage health care systems to restrict the performance of 10 procedures to surgeons and hospitals within their systems that meet a minimum volume standard for the given operations.21 In essence, procedures would be restricted for low-volume providers and centers and triaged to higher-volume surgeons and hospitals within a given health care system.21
Proponents of the Volume Pledge argue that it is a relatively straightforward way to align patients and providers to optimize outcomes. The Volume Pledge focuses on larger hospital systems and encourages referral within the given system, thus mitigating competitive and financial concerns about referring patients to outside providers. Those who have argued against the Volume Pledge point out that the volume cut points chosen are somewhat arbitrary, that these policies have the potential to negatively impact rural hospitals and those serving smaller communities, and that quality is a more appropriate metric than volume.22 The Volume Pledge does not include any gynecologic procedures, and to date it has met with only limited success.23
Perhaps more directly applicable to gynecologic surgeons are ongoing national trends to base hospital credentialing on surgical volume. In essence, individual surgeons must demonstrate that they have performed a minimum number of procedures to obtain or retain privileges.24,25 While there is strong evidence of the association between volume and outcomes for some complex surgical procedures, linking volume to credentialing has a number of potential pitfalls. Studies of surgical outcomes based on volume represent average performance, and many low-volume providers have better-than-expected outcomes. Volume measures typically represent recent performance; it is difficult to measure the overall experience of individual surgeons. Similarly, surgical outcomes depend on both the surgeon and the system in which the surgeon operates. It is difficult, if not impossible, to account for differences in the environment in which a surgeon works.25
A study of gynecologic surgeons who performed hysterectomy in New York State demonstrates many of the complexities of volume-based credentialing.26 In a cohort of more than55,000 patients who underwent abdominal hysterectomy, there was a strong association between low surgeon volume and a higher-than-expected rate of complications. If one were to consider limiting privileges to even the lowest-volume providers, there would be a significant impact on the surgical workforce. In this cohort, limiting credentialing to the lowest-volume providers, those who performed only 1 abdominal hysterectomy in the prior year would restrict the privileges of 17.5% of the surgeons in the cohort. Further, in this low-volume cohort that performed only 1 abdominal hysterectomy in the prior year, 69% of the surgeons actually had outcomes that were better than predicted.26 These data highlight not only the difficulty of applying averages to individual surgeons but also the profound impact that policy changes could have on the practice of gynecologic surgery.
Volume-outcomes paradigm discussions continue
The association between higher surgeon and hospital procedural volume for gynecologic surgeries and improved outcomes now has been convincingly demonstrated. With this knowledge, over the last decade the patterns of care for patients undergoing gynecologic surgery have clearly shifted, and these operations are now more commonly being performed by a smaller number of physicians and at fewer hospitals.
While efforts to improve quality are clearly important, many policy interventions, such as regionalization of care, have untoward consequences that must be considered. As we move forward, it will be essential to ensure that there is a robust debate among patients, providers, and policymakers on the merits of public health policies based on the volume-outcomes paradigm. ●
Over the last 3 decades, abundant evidence has demonstrated the association between surgical volume and outcomes. Patients operated on by high-volume surgeons and at high-volume hospitals have superior outcomes.
Surgical volume in gynecology
The association between both hospital and surgeon volume and outcomes has been explored across a number of gynecologic procedures.3 A meta-analysis that included 741,000 patients found that low-volume surgeons had an increased rate of complications overall, a higher rate of intraoperative complications, and a higher rate of postoperative complications compared with high-volume surgeons. While there was no association between volume and mortality overall, when limited to gynecologic oncology studies, low surgeon volume was associated with increased perioperative mortality.3
While these studies demonstrated a statistically significant association between surgeon volume and perioperative outcomes, the magnitude of the effect is modest compared with other higher-risk procedures associated with greater perioperative morbidity. For example, in a large study that examined oncologic and cardiovascular surgery, perioperative mortality in patients who underwent pancreatic resection was reduced from 15% for low-volume surgeons to 5% for high-volume surgeons.1 By contrast, for gynecologic surgery, complications occurred in 97 per 1,000 patients operated on by high-volume surgeons compared with between 114 and 137 per 1,000 for low-volume surgeons. Thus, to avoid 1 in-hospital complication, 30 surgeries performed by low-volume surgeons would need to be moved to high-volume surgeons. For intraoperative complications, 38 patients would need to be moved from low- to high-volume surgeons to prevent 1 such complication.3 In addition to morbidity and mortality, higher surgeon volume is associated with greater use of minimally invasive surgery, a lower likelihood of conversion to laparotomy, and lower costs.3

Similarly, hospital volume also has been associated with outcomes for gynecologic surgery.4 In a report of patients who underwent laparoscopic hysterectomy, the authors found that the complication rate was 18% lower for patients at high- versus low-volume hospitals. In addition, cost was lower at the high-volume centers.4 Like surgeon volume, the magnitude of the differential in outcomes between high- and low-volume hospitals is often modest.4
While most studies have focused on short-term outcomes, surgical volume appears also to be associated with longer-term outcomes. For gynecologic cancer, studies have demonstrated an association between hospital volume and survival for ovarian and cervical cancer.5-7 A large report of centers across the United States found that the 5-year survival rate increased from 39% for patients treated at low-volume centers to 51% at the highest-volume hospitals.5 In urogynecology, surgeon volume has been associated with midurethral sling revision. One study noted that after an individual surgeon performed 50 procedures a year, each additional case was associated with a decline in the rate of sling revision.8 One could argue that these longer-term end points may be the measures that matter most to patients.
Although the magnitude of the association between surgical volume and outcomes in gynecology appears to be relatively modest, outcomes for very-low-volume (VLV) surgeons are substantially worse. An analysis of more than 430,000 patients who underwent hysterectomy compared outcomes between VLV surgeons (characterized as surgeons who performed only 1 hysterectomy in the prior year) and other gynecologic surgeons. The overall complication rate was 32% in VLV surgeons compared with 10% among other surgeons, while the perioperative mortality rate was 2.5% versus 0.2% in the 2 groups, respectively. Likely reflecting changing practice patterns in gynecology, a sizable number of surgeons were classified as VLV physicians.9
Continue to: Public health applications of gynecologic surgical volume...
Public health applications of gynecologic surgical volume
The large body of literature on volume and outcomes has led to a number of public health initiatives aimed at reducing perioperative morbidity and mortality. Broadly, these efforts focus on regionalization of care, targeted quality improvement, and the development of minimum volume standards. Each strategy holds promise but also the potential to lead to unwanted consequences.
Regionalization of care
Recognition of the volume-outcomes paradigm has led to efforts to regionalize care for complex procedures to high-volume surgeons and centers.10 A cohort study of surgical patterns of care for Medicare recipients who underwent cancer resections or abdominal aortic aneurysm repair from 1999 to 2008 demonstrated these shifting practice patterns. For example, in 1999–2000, pancreatectomy was performed in 1,308 hospitals, with a median case volume of 5 procedures per year. By 2007–2008, the number of hospitals in which pancreatectomy was performed declined to 978, and the median case volume rose to 16 procedures per year. Importantly, over this time period, risk-adjusted mortality for pancreatectomy declined by 19%, and increased hospital volume was responsible for more than two-thirds of the decline in mortality.10
There has similarly been a gradual concentration of some gynecologic procedures to higher-volume surgeons and centers.11,12 Among patients undergoing hysterectomy for endometrial cancer in New York State, 845 surgeons with a mean case volume of 3 procedures per year treated patients in 2000. By 2014, the number of surgeons who performed these operations declined to 317 while mean annual case volume rose to 10 procedures per year. The number of hospitals in which women with endometrial cancer were treated declined from 182 to 98 over the same time period.11 Similar trends were noted for patients undergoing ovarian cancer resection.12 While patterns of gynecologic care for some surgical procedures have clearly changed, it has been more difficult to link these changes to improvements in outcomes.11,12
Despite the intuitive appeal of regionalization of surgical care, such a strategy has a number of limitations and practical challenges. Not surprisingly, limiting the number of surgeons and hospitals that perform a given procedure necessitates that patients travel a greater distance to obtain necessary surgical care.13,14 An analysis of endometrial cancer patients in New York State stratified patients based on their area of residence into 10 hospital referral regions (HRRs), which represent health care markets for tertiary medical care. From 2000 to 2014, the distance patients traveled to receive their surgical care increased in all of the HRRs studied. This was most pronounced in 1 of the HRRs in which the median travel distance rose by 47 miles over the 15-year period (FIGURE 1; FIGURE 2).14
Whether patients are willing to travel for care remains a matter of debate and depends on the disease, the surgical procedure, and the anticipated benefit associated with a longer travel distance.15,16 In a discrete choice experiment, 100 participants were given a hypothetical scenario in which they had potentially resectable pancreatic cancer; they were queried on their willingness to travel for care based on varying differences in mortality between a local and regional hospital.15 When mortality at the local hospital was double that of the regional hospital (6% vs 3%), 45% of patients chose to remain at the local hospital. When the differential increased to a 4 times greater mortality at the local hospital (12% vs 3%), 23% of patients still chose to remain at the local hospital.15


A similar study asked patients with ovarian neoplasms whether they would travel 50 miles to a regional center for surgery based on some degree of increased 5-year survival.16 Overall, 79% of patients would travel for a 4% improvement in survival while 97% would travel for a 12% improvement in survival.16
Lastly, a number of studies have shown that regionalization of surgical care disproportionately affects Black and Hispanic patients and those with low socioeconomic status.12,13,17 A simulation study on the effect of regionalizing care for pancreatectomy noted that using a hospital volume threshold of 20 procedures per year, a higher percentage of Black and Hispanic patients than White patients would be required to travel to a higher-volume center.13 Similarly, Medicaid recipients were more likely to be affected.13 Despite the inequities in who must travel for regionalized care, prior work has suggested that regionalization of cancer care to high-volume centers may reduce racial and socioeconomic disparities in survival for some cancers.18
Targeted quality improvement
Realizing the practical limitations of regionalization of care, an alternative strategy is to improve the quality of care at low-volume hospitals.5,19 Quality of care and surgical volume often are correlated, and the delivery of high-quality care can mitigate some of the influence of surgical volume on outcomes.
These principles were demonstrated in a study of more than 100,000 patients with ovarian cancer that stratified treating hospitals into volume quintiles.5 As expected, survival (both 2- and 5-year) was highest in the highest-volume quintile hospitals (FIGURE 3).5 Similarly, quality of care, measured through adherence to various process measures, was also highest in the highest-volume quintile hospitals. Interestingly, in the second-fourth volume quintile hospitals, there was substantial variation in adherence to quality metrics. Among hospitals with higher quality care, an improved survival was noted compared with lower quality care hospitals within the same volume quintile. Survival at high-quality, intermediate-volume hospitals approached that of the high-volume quintile hospitals.5

These findings highlight the importance of quality of care as well as the complex interplay of surgical volume and other factors.20 Many have argued that it may be more appropriate to measure quality of care and past performance and outcomes rather than surgical volume.21
Continue to: Minimum volume standards...
Minimum volume standards
While efforts to regionalize surgical care have gradually evolved, calls have been growing to formalize policies that limit the performance of some procedures to surgeons and centers that meet a minimum volume threshold or standard.21 One such effort, based on consensus from 3 academic hospital systems, was a campaign for hospitals to “Take the Volume Pledge.”21 The campaign’s goal is to encourage health care systems to restrict the performance of 10 procedures to surgeons and hospitals within their systems that meet a minimum volume standard for the given operations.21 In essence, procedures would be restricted for low-volume providers and centers and triaged to higher-volume surgeons and hospitals within a given health care system.21
Proponents of the Volume Pledge argue that it is a relatively straightforward way to align patients and providers to optimize outcomes. The Volume Pledge focuses on larger hospital systems and encourages referral within the given system, thus mitigating competitive and financial concerns about referring patients to outside providers. Those who have argued against the Volume Pledge point out that the volume cut points chosen are somewhat arbitrary, that these policies have the potential to negatively impact rural hospitals and those serving smaller communities, and that quality is a more appropriate metric than volume.22 The Volume Pledge does not include any gynecologic procedures, and to date it has met with only limited success.23
Perhaps more directly applicable to gynecologic surgeons are ongoing national trends to base hospital credentialing on surgical volume. In essence, individual surgeons must demonstrate that they have performed a minimum number of procedures to obtain or retain privileges.24,25 While there is strong evidence of the association between volume and outcomes for some complex surgical procedures, linking volume to credentialing has a number of potential pitfalls. Studies of surgical outcomes based on volume represent average performance, and many low-volume providers have better-than-expected outcomes. Volume measures typically represent recent performance; it is difficult to measure the overall experience of individual surgeons. Similarly, surgical outcomes depend on both the surgeon and the system in which the surgeon operates. It is difficult, if not impossible, to account for differences in the environment in which a surgeon works.25
A study of gynecologic surgeons who performed hysterectomy in New York State demonstrates many of the complexities of volume-based credentialing.26 In a cohort of more than55,000 patients who underwent abdominal hysterectomy, there was a strong association between low surgeon volume and a higher-than-expected rate of complications. If one were to consider limiting privileges to even the lowest-volume providers, there would be a significant impact on the surgical workforce. In this cohort, limiting credentialing to the lowest-volume providers, those who performed only 1 abdominal hysterectomy in the prior year would restrict the privileges of 17.5% of the surgeons in the cohort. Further, in this low-volume cohort that performed only 1 abdominal hysterectomy in the prior year, 69% of the surgeons actually had outcomes that were better than predicted.26 These data highlight not only the difficulty of applying averages to individual surgeons but also the profound impact that policy changes could have on the practice of gynecologic surgery.
Volume-outcomes paradigm discussions continue
The association between higher surgeon and hospital procedural volume for gynecologic surgeries and improved outcomes now has been convincingly demonstrated. With this knowledge, over the last decade the patterns of care for patients undergoing gynecologic surgery have clearly shifted, and these operations are now more commonly being performed by a smaller number of physicians and at fewer hospitals.
While efforts to improve quality are clearly important, many policy interventions, such as regionalization of care, have untoward consequences that must be considered. As we move forward, it will be essential to ensure that there is a robust debate among patients, providers, and policymakers on the merits of public health policies based on the volume-outcomes paradigm. ●
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117-2127.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:11281137.
- Mowat A, Maher C, Ballard E. Surgical outcomes for low-volume vs high-volume surgeons in gynecology surgery: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;215:21-33.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Wright JD, Chen L, Hou JY, et al. Association of hospital volume and quality of care with survival for ovarian cancer. Obstet Gynecol. 2017;130:545-553.
- Cliby WA, Powell MA, Al-Hammadi N, et al. Ovarian cancer in the United States: contemporary patterns of care associated with improved survival. Gynecol Oncol. 2015;136:11-17.
- Matsuo K, Shimada M, Yamaguchi S, et al. Association of radical hysterectomy surgical volume and survival for early-stage cervical cancer. Obstet Gynecol. 2019;133:1086-1098.
- Brennand EA, Quan H. Evaluation of the effect of surgeon’s operative volume and specialty on likelihood of revision after mesh midurethral sling placement. Obstet Gynecol. 2019;133:1099-1108.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:21282137.
- Wright JD, Ruiz MP, Chen L, et al. Changes in surgical volume and outcomes over time for women undergoing hysterectomy for endometrial cancer. Obstet Gynecol. 2018;132:59-69.
- Wright JD, Chen L, Buskwofie A, et al. Regionalization of care for women with ovarian cancer. Gynecol Oncol. 2019;154:394-400.
- Fong ZV, Hashimoto DA, Jin G, et al. Simulated volume-based regionalization of complex procedures: impact on spatial access to care. Ann Surg. 2021;274:312-318.
- Knisely A, Huang Y, Melamed A, et al. Effect of regionalization of endometrial cancer care on site of care and patient travel. Am J Obstet Gynecol. 2020;222:58.e1-58.e10.
- Finlayson SR, Birkmeyer JD, Tosteson AN, et al. Patient preferences for location of care: implications for regionalization. Med Care. 1999;37:204-209.
- Shalowitz DI, Nivasch E, Burger RA, et al. Are patients willing to travel for better ovarian cancer care? Gynecol Oncol. 2018;148:42-48.
- Rehmani SS, Liu B, Al-Ayoubi AM, et al. Racial disparity in utilization of high-volume hospitals for surgical treatment of esophageal cancer. Ann Thorac Surg. 2018;106:346-353.
- Nattinger AB, Rademacher N, McGinley EL, et al. Can regionalization of care reduce socioeconomic disparities in breast cancer survival? Med Care. 2021;59:77-81.
- Auerbach AD, Hilton JF, Maselli J, et al. Shop for quality or volume? Volume, quality, and outcomes of coronary artery bypass surgery. Ann Intern Med. 2009;150:696-704.
- Kurlansky PA, Argenziano M, Dunton R, et al. Quality, not volume, determines outcome of coronary artery bypass surgery in a university-based community hospital network. J Thorac Cardiovasc Surg. 2012;143:287-293.
- Urbach DR. Pledging to eliminate low-volume surgery. N Engl J Med. 2015;373:1388-1390.
- Blanco BA, Kothari AN, Blackwell RH, et al. “Take the Volume Pledge” may result in disparity in access to care. Surgery. 2017;161:837-845.
- Farjah F, Grau-Sepulveda MV, Gaissert H, et al. Volume Pledge is not associated with better short-term outcomes after lung cancer resection. J Clin Oncol. 2020;38:3518-3527.
- Tracy EE, Zephyrin LC, Rosman DA, et al. Credentialing based on surgical volume, physician workforce challenges, and patient access. Obstet Gynecol. 2013;122:947-951.
- Statement on credentialing and privileging and volume performance issues. April 1, 2018. American College of Surgeons. Accessed April 10, 2023. https://facs.org/about-acs/statements/credentialing-andprivileging-and-volume-performance-issues/
- Ruiz MP, Chen L, Hou JY, et al. Effect of minimum-volume standards on patient outcomes and surgical practice patterns for hysterectomy. Obstet Gynecol. 2018;132:1229-1237.
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117-2127.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:11281137.
- Mowat A, Maher C, Ballard E. Surgical outcomes for low-volume vs high-volume surgeons in gynecology surgery: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;215:21-33.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Wright JD, Chen L, Hou JY, et al. Association of hospital volume and quality of care with survival for ovarian cancer. Obstet Gynecol. 2017;130:545-553.
- Cliby WA, Powell MA, Al-Hammadi N, et al. Ovarian cancer in the United States: contemporary patterns of care associated with improved survival. Gynecol Oncol. 2015;136:11-17.
- Matsuo K, Shimada M, Yamaguchi S, et al. Association of radical hysterectomy surgical volume and survival for early-stage cervical cancer. Obstet Gynecol. 2019;133:1086-1098.
- Brennand EA, Quan H. Evaluation of the effect of surgeon’s operative volume and specialty on likelihood of revision after mesh midurethral sling placement. Obstet Gynecol. 2019;133:1099-1108.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:21282137.
- Wright JD, Ruiz MP, Chen L, et al. Changes in surgical volume and outcomes over time for women undergoing hysterectomy for endometrial cancer. Obstet Gynecol. 2018;132:59-69.
- Wright JD, Chen L, Buskwofie A, et al. Regionalization of care for women with ovarian cancer. Gynecol Oncol. 2019;154:394-400.
- Fong ZV, Hashimoto DA, Jin G, et al. Simulated volume-based regionalization of complex procedures: impact on spatial access to care. Ann Surg. 2021;274:312-318.
- Knisely A, Huang Y, Melamed A, et al. Effect of regionalization of endometrial cancer care on site of care and patient travel. Am J Obstet Gynecol. 2020;222:58.e1-58.e10.
- Finlayson SR, Birkmeyer JD, Tosteson AN, et al. Patient preferences for location of care: implications for regionalization. Med Care. 1999;37:204-209.
- Shalowitz DI, Nivasch E, Burger RA, et al. Are patients willing to travel for better ovarian cancer care? Gynecol Oncol. 2018;148:42-48.
- Rehmani SS, Liu B, Al-Ayoubi AM, et al. Racial disparity in utilization of high-volume hospitals for surgical treatment of esophageal cancer. Ann Thorac Surg. 2018;106:346-353.
- Nattinger AB, Rademacher N, McGinley EL, et al. Can regionalization of care reduce socioeconomic disparities in breast cancer survival? Med Care. 2021;59:77-81.
- Auerbach AD, Hilton JF, Maselli J, et al. Shop for quality or volume? Volume, quality, and outcomes of coronary artery bypass surgery. Ann Intern Med. 2009;150:696-704.
- Kurlansky PA, Argenziano M, Dunton R, et al. Quality, not volume, determines outcome of coronary artery bypass surgery in a university-based community hospital network. J Thorac Cardiovasc Surg. 2012;143:287-293.
- Urbach DR. Pledging to eliminate low-volume surgery. N Engl J Med. 2015;373:1388-1390.
- Blanco BA, Kothari AN, Blackwell RH, et al. “Take the Volume Pledge” may result in disparity in access to care. Surgery. 2017;161:837-845.
- Farjah F, Grau-Sepulveda MV, Gaissert H, et al. Volume Pledge is not associated with better short-term outcomes after lung cancer resection. J Clin Oncol. 2020;38:3518-3527.
- Tracy EE, Zephyrin LC, Rosman DA, et al. Credentialing based on surgical volume, physician workforce challenges, and patient access. Obstet Gynecol. 2013;122:947-951.
- Statement on credentialing and privileging and volume performance issues. April 1, 2018. American College of Surgeons. Accessed April 10, 2023. https://facs.org/about-acs/statements/credentialing-andprivileging-and-volume-performance-issues/
- Ruiz MP, Chen L, Hou JY, et al. Effect of minimum-volume standards on patient outcomes and surgical practice patterns for hysterectomy. Obstet Gynecol. 2018;132:1229-1237.
OR safety and efficiency: Measuring and monitoring all factors—including surgical volume
The operating room (OR) is a key contributor to a hospital’s profitability. It is a complex environment with ever-advancing technology. A successful surgery completed without complications within an optimal time depends not only on the surgeon’s experience, skills, and knowledge but also on numerous other structural, human, and nontechnical factors over which the surgeon has limited control.
As in any setting that deals with human life, in the OR, team dynamics, communication, and environment play a major role. Research has indicated the benefits of dedicated teams, reduced handoffs, and innovative modalities that continuously and systematically monitor potential breakdowns and propose solutions for the detected problems.
Finally, who should perform your loved one’s hysterectomy? This article also attempts to address the impact of surgeons’ and hospitals’ volume on operative outcomes with a diminishing number of hysterectomies but an increasing number of approaches.
Human factors in the OR
Human factors research was born as a product of the industrial revolution and mass production. It aims to optimize human experience and improve system performance by studying how humans interact with system. The aviation industry, for example, minimized errors significantly by using methods developed by human factors scientists. As another industry with no tolerance for mistakes, the health care sector followed suit. Ultimately, the goal of human factors research in health care is to improve patient safety, optimize work and environment, reduce costs, and enhance employees’ physical and mental health, engagement, comfort, and quality of life (FIGURE 1).1
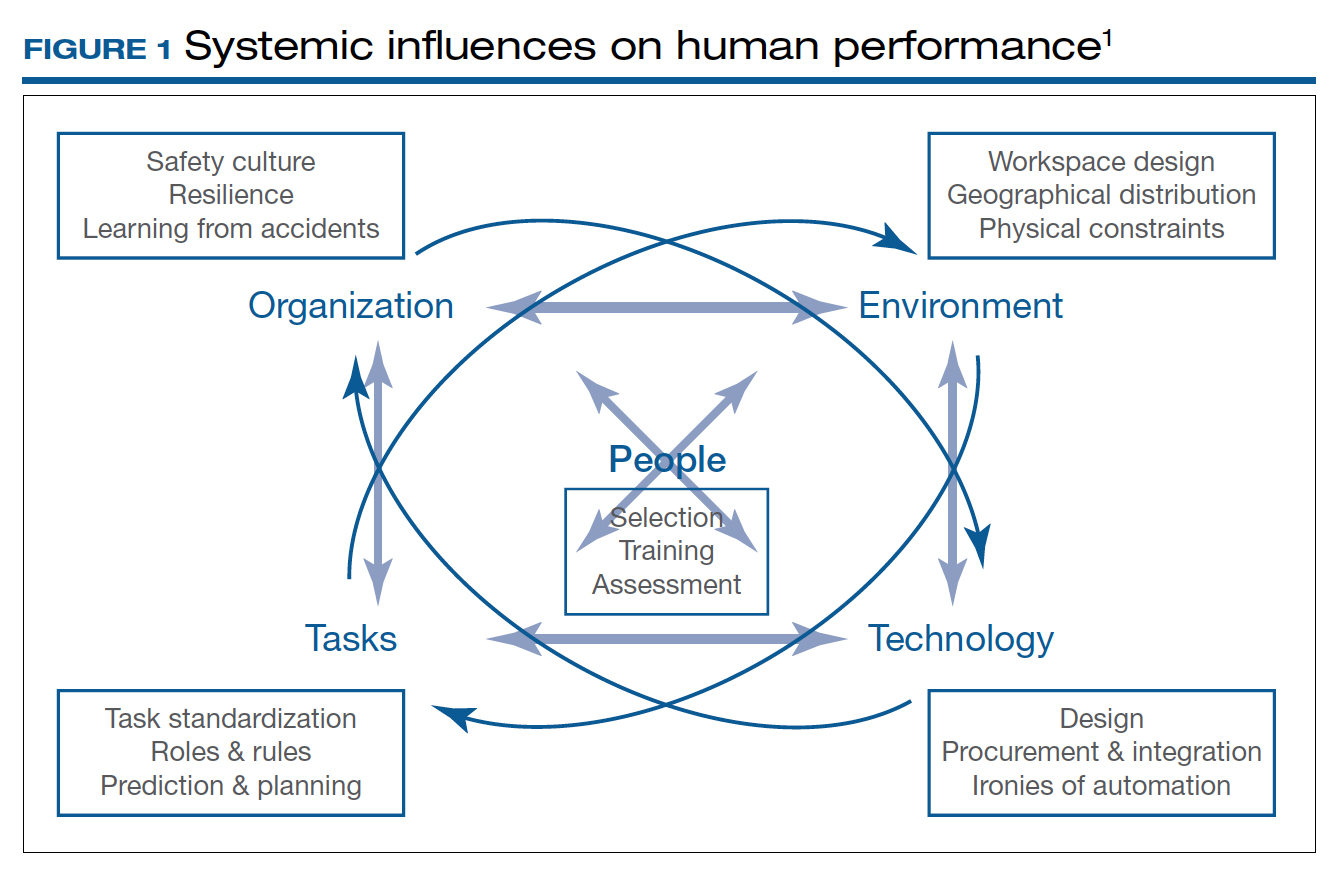
Today’s OR is so complex that it is hard to understand its dynamics without human factors research. Every new OR technology is first tested in controlled and simulated environments to determine “work as imagined.” However, it is necessary to study “work as done” in the real world via direct observation, video recording, questionnaires, and semistructured interviews by an on-site multidisciplinary team. This process not only focuses on surgical skills, process efficiency, and outcomes but also monitors the entire process according to Human Factors and Ergonomics Engineering principles to explore otherwise hidden complexities and latent safety concerns. The Systems Engineering Initiative for Patient Safety (SEIPS) framework is used to study the impact of interactions between people, tasks, technologies, environment, and organization.1
Robot-assisted surgery (RAS), an increasingly popular surgical approach among gynecologic surgeons, recently has been the focus of human factors science. A robotic OR poses unique challenges: the surgeon is not scrubbed, is removed from the operating table, and controls a complex highly technologic device in a crowded and darkened room. These are ideal conditions waiting to be optimized by human factor experts. To demonstrate the importance of human factors in the OR, we review the evidence for RAS.
Continue to: Impact of flow disruptions...
Impact of flow disruptions
Flow disruptions (FDs) were found to be more common in RAS. Catchpole and colleagues identified a mean of 9.62 FDs per hour in 89 robotic procedures, including hysterectomies and sacrocolpopexies, from a variety of fields; FDs occurred more often during the docking stage, followed by the console time, and they mostly were caused by communication breakdown and lack of team familiarity.2
Surgeon experience significantly reduced FDs. Surgeons who had done more than 700 RAS cases experienced 60% fewer FDs than those who had done less than 250 cases (13 vs 8 per hour).2 A study focusing on residents’ impact on RAS outcomes found that each FD increased the total operative time by an average 2.4 minutes, with the number significantly higher when a resident was involved.3 About one-quarter of the training-related FDs were procedure-specific instructions, while one-third were related to instrument and robotic instruction. However, pauses to teach residents did not appear to create significant intraoperative delays. Expectedly, experienced surgeons could anticipate and reduce these disruptions by supporting the whole team.
Human ergonomics, turnover time, and robot-specific skills
In a study of human ergonomics in RAS, Yu and colleagues noted that bedside assistants could experience neck posture problems. Surprisingly, the console could constrain the surgeon’s neck-shoulder region.4 Studies that reported on communication problems in a robotic OR suggest that innovative forms of verbal and nonverbal communication may support successful team communication.5
On the learning curve for RAS, OR turnover time, a key value metric, has been longer. However, turnover time was reduced almost by half from 99.2 to 53.2 minutes over 3 months after concepts from motor racing pit stops were employed, including briefings, leadership, role definition, task allocation, and task sequencing. Average room-ready time also was lowered from 42.2 to 27.2 minutes.6 RAS presents new challenges with sterile instrument processing as well. A successful RAS program, therefore, has organizational needs that include the training of OR and sterile processing staff and appropriate shift management.1
In a robotic OR, not only the surgeon but also the whole team requires robot-specific skills. New training approaches to teamwork, communication, and situation awareness skills are necessary. Robotic equipment, with its data and power cables, 2 consoles, and changing movement paths, necessitate larger rooms with a specific layout.7
In a review of recordings of RAS that used a multidimensional assessment tool to measure team effectiveness and cognitive load, Sexton and colleagues identified anticipation, active team engagement, and higher familiarity scores as the best predictors of team efficiency.8 Several studies emphasized the need for a stable team, especially in the learning phase of robotic surgery.5,9,10 A dedicated robotic team reduced the operative time by 18% during robot-assisted sacrocolpopexy (RASCP).10 RASCP procedures that extended into the afternoon took significantly longer time.9 A dedicated anesthesiologist improved the preoperative time.9 Surgical team handoffs also have reduced OR efficiency.11,12
Studying the impact of human factors is paramount for safe and efficient surgery. It is especially necessary in ORs that are equipped with high technologic instruments such as those used in RAS.
Surgical Black Box: Using data for OR safety and efficiency
Surgical procedures account for more than 50% of medical errors in a hospital setting, many of which are preventable. Postevent analysis with traditional methods, such as “Morbidity and Mortality” meetings held many days later, misses many adverse events in the OR.13 Another challenge with ever-changing and fast-multiplying surgical approaches is the development of effective surgical skill. Reviewing video recording of surgical procedures has been proposed as an instrument for recognizing adverse events and perfecting surgical skills.Recently, an innovative data-capture platform called the OR Black Box, developed by Teodor Grantcharov, MD, PhD, and colleagues, went beyond simple audiovisual recording.14 This high technologic platform not only video records the actual surgical procedure with laparoscopic camera capture (and wearable cameras for open cases) but also monitors the entire OR environment via wide-angle cameras, utilizes sensors, and records both the patient’s and the surgeon’s physiologic parameters.
The OR Black Box generates a holistic view of the OR after synchronization, encryption, and secure storage of all inputs for further analysis by experts and software-based algorithms (FIGURE 2). Computer vision algorithms can recognize improper dissection techniques and complications, such as bleeding. Adverse events are flagged with an automated software on a procedural timeline to facilitate review of procedural steps, disruptive environmental and organizational factors, OR team technical and nontechnical skills, surgeon physiologic stress, and intraoperative errors, events, and rectification processes using validated instruments.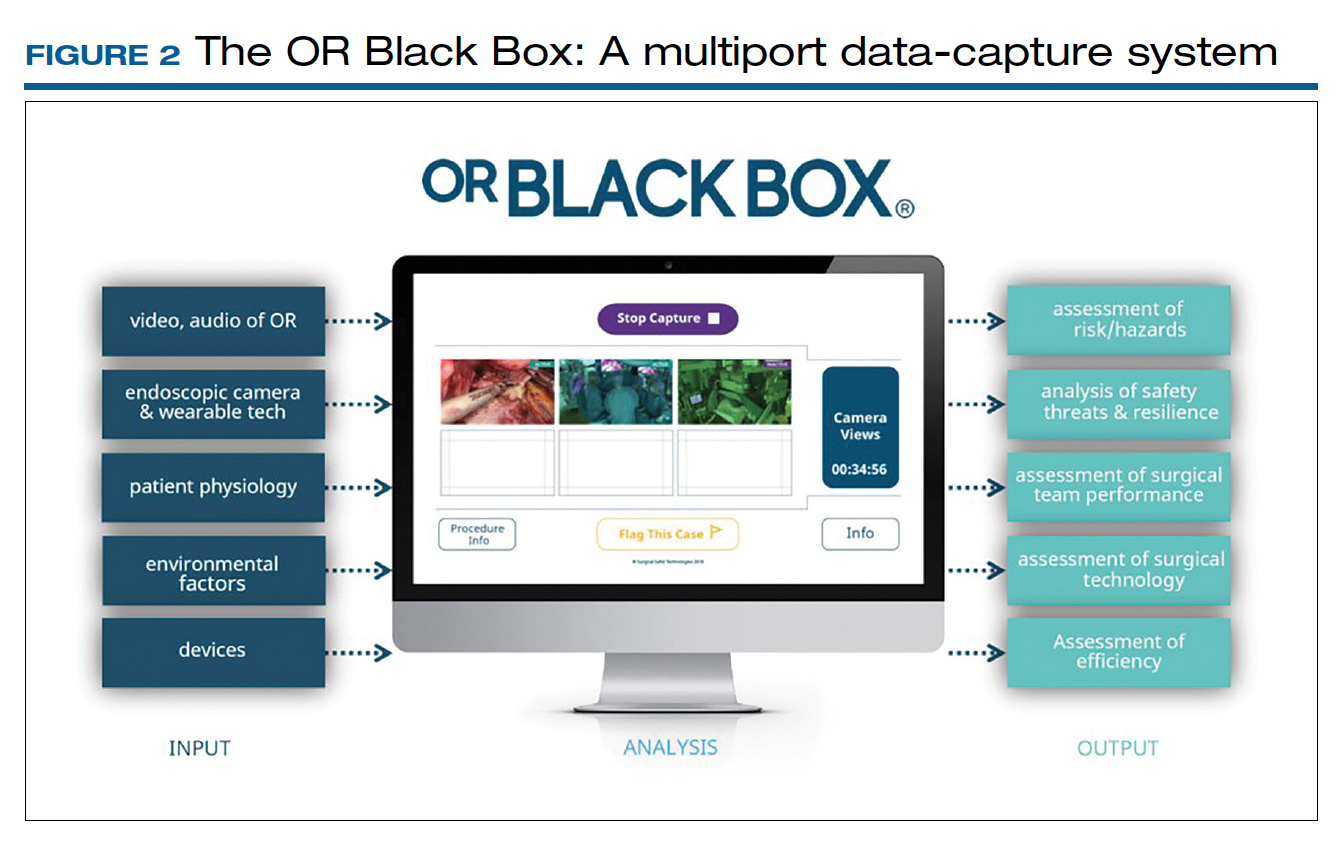
Artificial intelligence built into this platform can automatically extract objective, high-quality, and structured data to generate explainable insights by recognizing adverse events and procedural segments of interest for training and quality improvement and provide a foundation with objective measurements of technical and nontechnical performance for formative and summative assessment. This system, a major step up compared with retrospective review of likely biased medical records and labor-intensive multidisciplinary human observers, has the potential to increase efficiency and reduce costs by studying human factors that include clinical design, technology, and organization. OR efficiency, measured in real time objectively and thoroughly, may save time and resources.
OR Black Box platforms have already started to generate meaningful data. It is not surprising that auditory disruptions—OR doors opening, loud noises, pagers beeping, telephones ringing—were recorded almost every minute during laparoscopic procedures.15 Most technical errors occurred during dissection, resection, and reconstruction and most commonly were associated with improper estimations of force applied to tissue and distance to the target tissue during operative steps of a laparoscopic procedure.16 Another study based on this system showed that technical performance was an independent predictor of postoperative outcomes.17 The OR Black Box identified a device-related interruption in 30% of elective laparoscopic general surgery cases, most commonly in sleeve gastrectomy and oncologic gastrectomy procedures. This sophisticated surgical data recording system also demonstrated a significantly better ability to detect Veress needle injuries (12 vs 3) and near misses (47 vs 0) when compared with traditional chart review.18
Data from the OR Black Box also have been applied to better analyze nontechnical performance, including teamwork and interpersonal dynamics.19 Surgeons most commonly exhibited adept situational awareness and leadership, while the nurse team excelled at task management and situational awareness.19 Of the total care provider team studied, the surgeon and scrub nurse demonstrated the most favorable nontechnical behavior.19 Of note, continuous physiologic monitoring of the surgeon with this system revealed that surgeons under stress had 66% higher adverse events.
The OR Black Box is currently utilized at 20 institutions in North America and Europe. The data compiled from all these institutions revealed that there was a 10% decrease in intraoperative adverse events for each 10-point increase in technical skill score on a scale of 0 to 100 (unpublished data). This centralized data indicated that turnover time ranged widely between 7 and 91 minutes, with variation of cleanup time from 1 to 25 minutes and setup time from 22 to 43 minutes. Institutions can learn from each other using this platform. For example, the information about block time utilization (20%–99%) across institutions provides opportunities for system improvements.
With any revolutionary technology, it is imperative to study its effects on outcomes, training, costs, and privacy before it is widely implemented. We, obstetricians and gynecologists, are very familiar with the impact of electronic fetal monitoring, a great example of a technologic advance that did not improve perinatal outcomes but led to unintended consequences, such as higher rates of cesarean deliveries and lawsuits. Such a tool may lead to potential misrepresentation of intraoperative events unless legal aspects are clearly delineated. As exciting as it is, this disruptive technology requires further exploration with scientific vigor.
Continue to: Surgeon and hospital volume: Surgical outcomes paradigm...
Surgeon and hospital volume: Surgical outcomes paradigm
A landmark study in 1979 that showed decreased mortality in high-volume centers underscored the need for regionalization for certain surgical procedures.20 This association was further substantiated by 2 reports on 2.5 million Medicare beneficiaries that demonstrated significantly lower mortality for all 14 cardiovascular and oncologic procedures for hospitals with larger surgical volume (16% vs 4%) and high-volume surgeons for certain procedures, for example, 15% versus 5% for pancreatic resections for cancer.21,22
A similar association was found for all routes of hysterectomies performed for benign indications. Boyd and colleagues showed that gynecologists who performed fewer than 10 hysterectomies per year had a higher perioperative morbidity rate (16.5%) compared with those who did more (11.7%).23 Specific to vaginal hysterectomy, in a study of more than 6,000 women, surgeons who performed 13 procedures per year had 31% less risk of operative injury than those who did 5.5 procedures per year (2.5% vs 1.7%).24 Overall perioperative complications (5.0% vs 4.0%) and medical complications (5.7% vs 3.9%) were also reduced for higher-volume surgeons. In a cohort of approximately 8,000 women who underwent a laparoscopic hysterectomy, high-volume surgeons had a considerably lower complication rate (4.2% vs 6.2%).25
As expected, lower complication rates of high-volume surgeons led to lower resource utilization, including lower transfusion rates, less intensive care unit utilization, and shorter operative times and, in several studies, length of stay.24,25 Of note, low-volume surgeons were less likely to offer minimally invasive routes and were more likely to convert to laparotomy.26 In addition, significant cost savings have been associated with high surgical volume, which one study showed was 16% ($6,500 vs $5,600) for high-volume surgeons.26 With regard to mortality, a study of 7,800 women found that perioperative mortality increased more than 10-fold for surgeons who performed an average 1 case per year compared with all other surgeons (2.5% vs 0.2%).27
When gynecologic cancers are concerned, arguably, long-term survival outcomes may be more critical than perioperative morbidity and mortality. Higher surgeon and hospital volume are associated with improved perioperative outcomes for endometrial and cervical cancers.28 Importantly, minimally invasive hysterectomy was offered for endometrial cancer significantly more often by surgeons with high volume.28 Survival outcomes were not affected by surgeon or hospital volume, likely due to overall more favorable prognosis for endometrial cancer after treatment.
Although it is intuitive to assume that a surgeon’s skills and experience would make the most impact in procedures for ovarian cancer due to the complexity of ovarian cancer surgery, evidence on short-term outcomes has been mixed. Intriguingly, some studies reported that high-volume institutions had higher complication and readmission rates. However, evidence supports that the surgeon’s volume, and especially hospital volume, improves long-term survival for ovarian cancer, with a negative impact on immediate postoperative morbidity.29 This may suggest that a more aggressive surgical effort improves long-term survival but also can cause more perioperative complications. Further, longer survival may result not only from operative skills but also because of better care by a structured multidisciplinary team at more established high-volume cancer centers.
The association of improved outcomes with higher volume led to public reporting of hospital outcomes. Policy efforts toward regionalization have impacted surgical practice. Based on their analysis of 3.2 million Medicare patients who underwent 1 of 8 different cancer surgeries or cardiovascular operations from 1999 to 2008, Finks and colleagues demonstrated that care was concentrated to fewer hospitals over time for many of these procedures.29 This trend was noted for gynecologic cancer surgery but not for benign gynecologic surgery.
Regionalization of care limits access particularly for minority and underserved communities because of longer travel distances, logistic challenges, and financial strain. An alternative to regionalization of care is targeted quality improvement by rigorous adherence to quality guidelines at low-volume hospitals.
Is there a critical minimum volume that may be used as a requirement for surgeons to maintain their privileges and for hospitals to offer certain procedures? In 2015, minimum volume standards for a number of common procedures were proposed by Johns Hopkins Medicine and Dartmouth-Hitchcock Medical Center, such as 50 hip replacement surgeries per hospital and 25 per physician per year, and 20 pancreatectomies per hospital and 5 per surgeon per year.30 A modeling study for hysterectomy showed that a volume cut point of >1 procedure in the prior year would restrict privileges for a substantial number of surgeons performing abdominal (17.5%), robot-assisted (12.5%), laparoscopic (16.8%), and vaginal (27.6%) hysterectomies.27 This study concluded that minimum-volume standards for hysterectomy for even the lowest volume physicians would restrict a significant number of gynecologic surgeons, including many with outcomes that are better than predicted.
Therefore, while there is good evidence that favors better outcomes in the hands of high-volume surgeons in gynecology, the impact of such policies on gynecologic practice clearly warrants careful monitoring and further study.
- What factors besides the surgeon’s skills influence surgical safety and efficiency?
- Are you ready to have audio, video, and sensor-based recording of everything in the OR?
- Who should perform your loved one’s hysterectomy? Do the surgeon’s and hospital’s volume matter?
- Catchpole K, Bisantz A, Hallbeck MS, et al. Human factors in robotic assisted surgery: lessons from studies ‘in the wild’. Appl Ergon. 2019;78:270-276.
- Catchpole K, Perkins C, Bresee C, et al. Safety, efficiency and learning curves in robotic surgery: a human factors analysis. Surg Endosc. 2016;30:3749-3761.
- Jain M, Fry BT, Hess LW, et al. Barriers to efficiency in robotic surgery: the resident effect. J Surg. Res. 2016;205:296-304.
- Yu D, Dural C, Morrow MM, et al. Intraoperative workload in robotic surgery assessed by wearable motion tracking sensors and questionnaires. Surg Endosc. 2017;31:877-886.
- Randell R, Honey S, Alvarado N, et al. Embedding robotic surgery into routine practice and impacts on communication and decision making: a review of the experience of surgical teams. Cognit Technol Work. 2016;18:423-437.
- Souders CP, Catchpole KR, Wood LN, et al. Reducing operating room turnover time for robotic surgery using a motor racing pit stop model. World J Surg. 2017;4:1943–1949.
- Ahmad N, Hussein AA, Cavuoto L, et al. Ambulatory movements, team dynamics and interactions during robot-assisted surgery. BJU Int. 2016;118:132-139.
- Sexton K, Johnson A, Gotsch A, et al. Anticipation, teamwork, and cognitive load: chasing efficiency during robot-assisted surgery. BMJ Qual Saf. 2018;27:148-154.
- Harmanli O, Solak S, Bayram A, et al. Optimizing the robotic surgery team: an operations management perspective. Int Urogynecol J. 2021;32:1379-1385.
- Carter-Brooks CM, Du AL, Bonidie MJ, et al. The impact of a dedicated robotic team on robotic-assisted sacrocolpopexy outcomes. Female Pelvic Med Reconstr Surg. 2018;24:13-16.
- Giugale LE, Sears S, Lavelle ES, et al. Evaluating the impact of intraoperative surgical team handoffs on patient outcomes. Female Pelvic Med Reconstr Surg. 2017;23:288-292.
- Geynisman-Tan J, Brown O, Mueller M, et al. Operating room efficiency: examining the impact of personnel handoffs. Female Pelvic Med Reconstr Surg. 2018;24:87-89.
- Alsubaie H, Goldenberg M, Grantcharov T. Quantifying recall bias in surgical safety: a need for a modern approach to morbidity and mortality reviews. Can J Surg. 2019;62:39-43.
- Goldenberg MG, Jung J, Grantcharov TP. Using data to enhance performance and improve quality and safety in surgery. JAMA Surg. 2017;152:972-973.
- Jung JJ, Grantcharov TP. The operating room black box: a prospective observational study of the operating room. J Am Coll Surg. 2017;225:S127-S128.
- Jung JJ, Jüni P, Lebovic G, et al. First-year analysis of the operating room black box study. Ann Surg. 2020;271:122-127.
- Jung JJ, Kashfi A, Sharma S, et al. Characterization of device-related interruptions in minimally invasive surgery: need for intraoperative data and effective mitigation strategies. Surg Endosc. 2019;33:717-723.
- Jung JJ, Adams-McGavin RC, Grantcharov TP. Underreporting of Veress needle injuries: comparing direct observation and chart review methods. J Surg Res. 2019;236:266-270.
- Fesco AB, Kuzulugil SS, Babaoglu C, et al. Relationship between intraoperative nontechnical performance and technical events in bariatric surgery. Br J Surg. 2018;105:1044-1050.
- Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364-1369.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137.
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:21172127.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915.
- Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116:1341-1347.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Bretschneider CE, Frazzini Padilla P, Das D, et al. The impact of surgeon volume on perioperative adverse events in women undergoing minimally invasive hysterectomy for the large uterus. Am J Obstet Gynecol. 2018;219:490.e1-490.e8.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Wright JD. The volume-outcome paradigm for gynecologic surgery: clinical and policy implications. Clin Obstet Gynecol. 2020;63:252-265.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high risk surgery. N Engl J Med. 2011;364:21282137.
- Sternberg S. Hospitals move to limit low-volume surgeries. US News & World Report. May 19, 2015. www.usnews.com/news /articles/2015/05/19/hospitals-move-to-limit-low-volume-surgeries. Accessed April 19, 2022.
The operating room (OR) is a key contributor to a hospital’s profitability. It is a complex environment with ever-advancing technology. A successful surgery completed without complications within an optimal time depends not only on the surgeon’s experience, skills, and knowledge but also on numerous other structural, human, and nontechnical factors over which the surgeon has limited control.
As in any setting that deals with human life, in the OR, team dynamics, communication, and environment play a major role. Research has indicated the benefits of dedicated teams, reduced handoffs, and innovative modalities that continuously and systematically monitor potential breakdowns and propose solutions for the detected problems.
Finally, who should perform your loved one’s hysterectomy? This article also attempts to address the impact of surgeons’ and hospitals’ volume on operative outcomes with a diminishing number of hysterectomies but an increasing number of approaches.
Human factors in the OR
Human factors research was born as a product of the industrial revolution and mass production. It aims to optimize human experience and improve system performance by studying how humans interact with system. The aviation industry, for example, minimized errors significantly by using methods developed by human factors scientists. As another industry with no tolerance for mistakes, the health care sector followed suit. Ultimately, the goal of human factors research in health care is to improve patient safety, optimize work and environment, reduce costs, and enhance employees’ physical and mental health, engagement, comfort, and quality of life (FIGURE 1).1

Today’s OR is so complex that it is hard to understand its dynamics without human factors research. Every new OR technology is first tested in controlled and simulated environments to determine “work as imagined.” However, it is necessary to study “work as done” in the real world via direct observation, video recording, questionnaires, and semistructured interviews by an on-site multidisciplinary team. This process not only focuses on surgical skills, process efficiency, and outcomes but also monitors the entire process according to Human Factors and Ergonomics Engineering principles to explore otherwise hidden complexities and latent safety concerns. The Systems Engineering Initiative for Patient Safety (SEIPS) framework is used to study the impact of interactions between people, tasks, technologies, environment, and organization.1
Robot-assisted surgery (RAS), an increasingly popular surgical approach among gynecologic surgeons, recently has been the focus of human factors science. A robotic OR poses unique challenges: the surgeon is not scrubbed, is removed from the operating table, and controls a complex highly technologic device in a crowded and darkened room. These are ideal conditions waiting to be optimized by human factor experts. To demonstrate the importance of human factors in the OR, we review the evidence for RAS.
Continue to: Impact of flow disruptions...
Impact of flow disruptions
Flow disruptions (FDs) were found to be more common in RAS. Catchpole and colleagues identified a mean of 9.62 FDs per hour in 89 robotic procedures, including hysterectomies and sacrocolpopexies, from a variety of fields; FDs occurred more often during the docking stage, followed by the console time, and they mostly were caused by communication breakdown and lack of team familiarity.2
Surgeon experience significantly reduced FDs. Surgeons who had done more than 700 RAS cases experienced 60% fewer FDs than those who had done less than 250 cases (13 vs 8 per hour).2 A study focusing on residents’ impact on RAS outcomes found that each FD increased the total operative time by an average 2.4 minutes, with the number significantly higher when a resident was involved.3 About one-quarter of the training-related FDs were procedure-specific instructions, while one-third were related to instrument and robotic instruction. However, pauses to teach residents did not appear to create significant intraoperative delays. Expectedly, experienced surgeons could anticipate and reduce these disruptions by supporting the whole team.
Human ergonomics, turnover time, and robot-specific skills
In a study of human ergonomics in RAS, Yu and colleagues noted that bedside assistants could experience neck posture problems. Surprisingly, the console could constrain the surgeon’s neck-shoulder region.4 Studies that reported on communication problems in a robotic OR suggest that innovative forms of verbal and nonverbal communication may support successful team communication.5
On the learning curve for RAS, OR turnover time, a key value metric, has been longer. However, turnover time was reduced almost by half from 99.2 to 53.2 minutes over 3 months after concepts from motor racing pit stops were employed, including briefings, leadership, role definition, task allocation, and task sequencing. Average room-ready time also was lowered from 42.2 to 27.2 minutes.6 RAS presents new challenges with sterile instrument processing as well. A successful RAS program, therefore, has organizational needs that include the training of OR and sterile processing staff and appropriate shift management.1
In a robotic OR, not only the surgeon but also the whole team requires robot-specific skills. New training approaches to teamwork, communication, and situation awareness skills are necessary. Robotic equipment, with its data and power cables, 2 consoles, and changing movement paths, necessitate larger rooms with a specific layout.7
In a review of recordings of RAS that used a multidimensional assessment tool to measure team effectiveness and cognitive load, Sexton and colleagues identified anticipation, active team engagement, and higher familiarity scores as the best predictors of team efficiency.8 Several studies emphasized the need for a stable team, especially in the learning phase of robotic surgery.5,9,10 A dedicated robotic team reduced the operative time by 18% during robot-assisted sacrocolpopexy (RASCP).10 RASCP procedures that extended into the afternoon took significantly longer time.9 A dedicated anesthesiologist improved the preoperative time.9 Surgical team handoffs also have reduced OR efficiency.11,12
Studying the impact of human factors is paramount for safe and efficient surgery. It is especially necessary in ORs that are equipped with high technologic instruments such as those used in RAS.
Surgical Black Box: Using data for OR safety and efficiency
Surgical procedures account for more than 50% of medical errors in a hospital setting, many of which are preventable. Postevent analysis with traditional methods, such as “Morbidity and Mortality” meetings held many days later, misses many adverse events in the OR.13 Another challenge with ever-changing and fast-multiplying surgical approaches is the development of effective surgical skill. Reviewing video recording of surgical procedures has been proposed as an instrument for recognizing adverse events and perfecting surgical skills.Recently, an innovative data-capture platform called the OR Black Box, developed by Teodor Grantcharov, MD, PhD, and colleagues, went beyond simple audiovisual recording.14 This high technologic platform not only video records the actual surgical procedure with laparoscopic camera capture (and wearable cameras for open cases) but also monitors the entire OR environment via wide-angle cameras, utilizes sensors, and records both the patient’s and the surgeon’s physiologic parameters.
The OR Black Box generates a holistic view of the OR after synchronization, encryption, and secure storage of all inputs for further analysis by experts and software-based algorithms (FIGURE 2). Computer vision algorithms can recognize improper dissection techniques and complications, such as bleeding. Adverse events are flagged with an automated software on a procedural timeline to facilitate review of procedural steps, disruptive environmental and organizational factors, OR team technical and nontechnical skills, surgeon physiologic stress, and intraoperative errors, events, and rectification processes using validated instruments.
Artificial intelligence built into this platform can automatically extract objective, high-quality, and structured data to generate explainable insights by recognizing adverse events and procedural segments of interest for training and quality improvement and provide a foundation with objective measurements of technical and nontechnical performance for formative and summative assessment. This system, a major step up compared with retrospective review of likely biased medical records and labor-intensive multidisciplinary human observers, has the potential to increase efficiency and reduce costs by studying human factors that include clinical design, technology, and organization. OR efficiency, measured in real time objectively and thoroughly, may save time and resources.
OR Black Box platforms have already started to generate meaningful data. It is not surprising that auditory disruptions—OR doors opening, loud noises, pagers beeping, telephones ringing—were recorded almost every minute during laparoscopic procedures.15 Most technical errors occurred during dissection, resection, and reconstruction and most commonly were associated with improper estimations of force applied to tissue and distance to the target tissue during operative steps of a laparoscopic procedure.16 Another study based on this system showed that technical performance was an independent predictor of postoperative outcomes.17 The OR Black Box identified a device-related interruption in 30% of elective laparoscopic general surgery cases, most commonly in sleeve gastrectomy and oncologic gastrectomy procedures. This sophisticated surgical data recording system also demonstrated a significantly better ability to detect Veress needle injuries (12 vs 3) and near misses (47 vs 0) when compared with traditional chart review.18
Data from the OR Black Box also have been applied to better analyze nontechnical performance, including teamwork and interpersonal dynamics.19 Surgeons most commonly exhibited adept situational awareness and leadership, while the nurse team excelled at task management and situational awareness.19 Of the total care provider team studied, the surgeon and scrub nurse demonstrated the most favorable nontechnical behavior.19 Of note, continuous physiologic monitoring of the surgeon with this system revealed that surgeons under stress had 66% higher adverse events.
The OR Black Box is currently utilized at 20 institutions in North America and Europe. The data compiled from all these institutions revealed that there was a 10% decrease in intraoperative adverse events for each 10-point increase in technical skill score on a scale of 0 to 100 (unpublished data). This centralized data indicated that turnover time ranged widely between 7 and 91 minutes, with variation of cleanup time from 1 to 25 minutes and setup time from 22 to 43 minutes. Institutions can learn from each other using this platform. For example, the information about block time utilization (20%–99%) across institutions provides opportunities for system improvements.
With any revolutionary technology, it is imperative to study its effects on outcomes, training, costs, and privacy before it is widely implemented. We, obstetricians and gynecologists, are very familiar with the impact of electronic fetal monitoring, a great example of a technologic advance that did not improve perinatal outcomes but led to unintended consequences, such as higher rates of cesarean deliveries and lawsuits. Such a tool may lead to potential misrepresentation of intraoperative events unless legal aspects are clearly delineated. As exciting as it is, this disruptive technology requires further exploration with scientific vigor.
Continue to: Surgeon and hospital volume: Surgical outcomes paradigm...
Surgeon and hospital volume: Surgical outcomes paradigm
A landmark study in 1979 that showed decreased mortality in high-volume centers underscored the need for regionalization for certain surgical procedures.20 This association was further substantiated by 2 reports on 2.5 million Medicare beneficiaries that demonstrated significantly lower mortality for all 14 cardiovascular and oncologic procedures for hospitals with larger surgical volume (16% vs 4%) and high-volume surgeons for certain procedures, for example, 15% versus 5% for pancreatic resections for cancer.21,22
A similar association was found for all routes of hysterectomies performed for benign indications. Boyd and colleagues showed that gynecologists who performed fewer than 10 hysterectomies per year had a higher perioperative morbidity rate (16.5%) compared with those who did more (11.7%).23 Specific to vaginal hysterectomy, in a study of more than 6,000 women, surgeons who performed 13 procedures per year had 31% less risk of operative injury than those who did 5.5 procedures per year (2.5% vs 1.7%).24 Overall perioperative complications (5.0% vs 4.0%) and medical complications (5.7% vs 3.9%) were also reduced for higher-volume surgeons. In a cohort of approximately 8,000 women who underwent a laparoscopic hysterectomy, high-volume surgeons had a considerably lower complication rate (4.2% vs 6.2%).25
As expected, lower complication rates of high-volume surgeons led to lower resource utilization, including lower transfusion rates, less intensive care unit utilization, and shorter operative times and, in several studies, length of stay.24,25 Of note, low-volume surgeons were less likely to offer minimally invasive routes and were more likely to convert to laparotomy.26 In addition, significant cost savings have been associated with high surgical volume, which one study showed was 16% ($6,500 vs $5,600) for high-volume surgeons.26 With regard to mortality, a study of 7,800 women found that perioperative mortality increased more than 10-fold for surgeons who performed an average 1 case per year compared with all other surgeons (2.5% vs 0.2%).27
When gynecologic cancers are concerned, arguably, long-term survival outcomes may be more critical than perioperative morbidity and mortality. Higher surgeon and hospital volume are associated with improved perioperative outcomes for endometrial and cervical cancers.28 Importantly, minimally invasive hysterectomy was offered for endometrial cancer significantly more often by surgeons with high volume.28 Survival outcomes were not affected by surgeon or hospital volume, likely due to overall more favorable prognosis for endometrial cancer after treatment.
Although it is intuitive to assume that a surgeon’s skills and experience would make the most impact in procedures for ovarian cancer due to the complexity of ovarian cancer surgery, evidence on short-term outcomes has been mixed. Intriguingly, some studies reported that high-volume institutions had higher complication and readmission rates. However, evidence supports that the surgeon’s volume, and especially hospital volume, improves long-term survival for ovarian cancer, with a negative impact on immediate postoperative morbidity.29 This may suggest that a more aggressive surgical effort improves long-term survival but also can cause more perioperative complications. Further, longer survival may result not only from operative skills but also because of better care by a structured multidisciplinary team at more established high-volume cancer centers.
The association of improved outcomes with higher volume led to public reporting of hospital outcomes. Policy efforts toward regionalization have impacted surgical practice. Based on their analysis of 3.2 million Medicare patients who underwent 1 of 8 different cancer surgeries or cardiovascular operations from 1999 to 2008, Finks and colleagues demonstrated that care was concentrated to fewer hospitals over time for many of these procedures.29 This trend was noted for gynecologic cancer surgery but not for benign gynecologic surgery.
Regionalization of care limits access particularly for minority and underserved communities because of longer travel distances, logistic challenges, and financial strain. An alternative to regionalization of care is targeted quality improvement by rigorous adherence to quality guidelines at low-volume hospitals.
Is there a critical minimum volume that may be used as a requirement for surgeons to maintain their privileges and for hospitals to offer certain procedures? In 2015, minimum volume standards for a number of common procedures were proposed by Johns Hopkins Medicine and Dartmouth-Hitchcock Medical Center, such as 50 hip replacement surgeries per hospital and 25 per physician per year, and 20 pancreatectomies per hospital and 5 per surgeon per year.30 A modeling study for hysterectomy showed that a volume cut point of >1 procedure in the prior year would restrict privileges for a substantial number of surgeons performing abdominal (17.5%), robot-assisted (12.5%), laparoscopic (16.8%), and vaginal (27.6%) hysterectomies.27 This study concluded that minimum-volume standards for hysterectomy for even the lowest volume physicians would restrict a significant number of gynecologic surgeons, including many with outcomes that are better than predicted.
Therefore, while there is good evidence that favors better outcomes in the hands of high-volume surgeons in gynecology, the impact of such policies on gynecologic practice clearly warrants careful monitoring and further study.
- What factors besides the surgeon’s skills influence surgical safety and efficiency?
- Are you ready to have audio, video, and sensor-based recording of everything in the OR?
- Who should perform your loved one’s hysterectomy? Do the surgeon’s and hospital’s volume matter?
The operating room (OR) is a key contributor to a hospital’s profitability. It is a complex environment with ever-advancing technology. A successful surgery completed without complications within an optimal time depends not only on the surgeon’s experience, skills, and knowledge but also on numerous other structural, human, and nontechnical factors over which the surgeon has limited control.
As in any setting that deals with human life, in the OR, team dynamics, communication, and environment play a major role. Research has indicated the benefits of dedicated teams, reduced handoffs, and innovative modalities that continuously and systematically monitor potential breakdowns and propose solutions for the detected problems.
Finally, who should perform your loved one’s hysterectomy? This article also attempts to address the impact of surgeons’ and hospitals’ volume on operative outcomes with a diminishing number of hysterectomies but an increasing number of approaches.
Human factors in the OR
Human factors research was born as a product of the industrial revolution and mass production. It aims to optimize human experience and improve system performance by studying how humans interact with system. The aviation industry, for example, minimized errors significantly by using methods developed by human factors scientists. As another industry with no tolerance for mistakes, the health care sector followed suit. Ultimately, the goal of human factors research in health care is to improve patient safety, optimize work and environment, reduce costs, and enhance employees’ physical and mental health, engagement, comfort, and quality of life (FIGURE 1).1

Today’s OR is so complex that it is hard to understand its dynamics without human factors research. Every new OR technology is first tested in controlled and simulated environments to determine “work as imagined.” However, it is necessary to study “work as done” in the real world via direct observation, video recording, questionnaires, and semistructured interviews by an on-site multidisciplinary team. This process not only focuses on surgical skills, process efficiency, and outcomes but also monitors the entire process according to Human Factors and Ergonomics Engineering principles to explore otherwise hidden complexities and latent safety concerns. The Systems Engineering Initiative for Patient Safety (SEIPS) framework is used to study the impact of interactions between people, tasks, technologies, environment, and organization.1
Robot-assisted surgery (RAS), an increasingly popular surgical approach among gynecologic surgeons, recently has been the focus of human factors science. A robotic OR poses unique challenges: the surgeon is not scrubbed, is removed from the operating table, and controls a complex highly technologic device in a crowded and darkened room. These are ideal conditions waiting to be optimized by human factor experts. To demonstrate the importance of human factors in the OR, we review the evidence for RAS.
Continue to: Impact of flow disruptions...
Impact of flow disruptions
Flow disruptions (FDs) were found to be more common in RAS. Catchpole and colleagues identified a mean of 9.62 FDs per hour in 89 robotic procedures, including hysterectomies and sacrocolpopexies, from a variety of fields; FDs occurred more often during the docking stage, followed by the console time, and they mostly were caused by communication breakdown and lack of team familiarity.2
Surgeon experience significantly reduced FDs. Surgeons who had done more than 700 RAS cases experienced 60% fewer FDs than those who had done less than 250 cases (13 vs 8 per hour).2 A study focusing on residents’ impact on RAS outcomes found that each FD increased the total operative time by an average 2.4 minutes, with the number significantly higher when a resident was involved.3 About one-quarter of the training-related FDs were procedure-specific instructions, while one-third were related to instrument and robotic instruction. However, pauses to teach residents did not appear to create significant intraoperative delays. Expectedly, experienced surgeons could anticipate and reduce these disruptions by supporting the whole team.
Human ergonomics, turnover time, and robot-specific skills
In a study of human ergonomics in RAS, Yu and colleagues noted that bedside assistants could experience neck posture problems. Surprisingly, the console could constrain the surgeon’s neck-shoulder region.4 Studies that reported on communication problems in a robotic OR suggest that innovative forms of verbal and nonverbal communication may support successful team communication.5
On the learning curve for RAS, OR turnover time, a key value metric, has been longer. However, turnover time was reduced almost by half from 99.2 to 53.2 minutes over 3 months after concepts from motor racing pit stops were employed, including briefings, leadership, role definition, task allocation, and task sequencing. Average room-ready time also was lowered from 42.2 to 27.2 minutes.6 RAS presents new challenges with sterile instrument processing as well. A successful RAS program, therefore, has organizational needs that include the training of OR and sterile processing staff and appropriate shift management.1
In a robotic OR, not only the surgeon but also the whole team requires robot-specific skills. New training approaches to teamwork, communication, and situation awareness skills are necessary. Robotic equipment, with its data and power cables, 2 consoles, and changing movement paths, necessitate larger rooms with a specific layout.7
In a review of recordings of RAS that used a multidimensional assessment tool to measure team effectiveness and cognitive load, Sexton and colleagues identified anticipation, active team engagement, and higher familiarity scores as the best predictors of team efficiency.8 Several studies emphasized the need for a stable team, especially in the learning phase of robotic surgery.5,9,10 A dedicated robotic team reduced the operative time by 18% during robot-assisted sacrocolpopexy (RASCP).10 RASCP procedures that extended into the afternoon took significantly longer time.9 A dedicated anesthesiologist improved the preoperative time.9 Surgical team handoffs also have reduced OR efficiency.11,12
Studying the impact of human factors is paramount for safe and efficient surgery. It is especially necessary in ORs that are equipped with high technologic instruments such as those used in RAS.
Surgical Black Box: Using data for OR safety and efficiency
Surgical procedures account for more than 50% of medical errors in a hospital setting, many of which are preventable. Postevent analysis with traditional methods, such as “Morbidity and Mortality” meetings held many days later, misses many adverse events in the OR.13 Another challenge with ever-changing and fast-multiplying surgical approaches is the development of effective surgical skill. Reviewing video recording of surgical procedures has been proposed as an instrument for recognizing adverse events and perfecting surgical skills.Recently, an innovative data-capture platform called the OR Black Box, developed by Teodor Grantcharov, MD, PhD, and colleagues, went beyond simple audiovisual recording.14 This high technologic platform not only video records the actual surgical procedure with laparoscopic camera capture (and wearable cameras for open cases) but also monitors the entire OR environment via wide-angle cameras, utilizes sensors, and records both the patient’s and the surgeon’s physiologic parameters.
The OR Black Box generates a holistic view of the OR after synchronization, encryption, and secure storage of all inputs for further analysis by experts and software-based algorithms (FIGURE 2). Computer vision algorithms can recognize improper dissection techniques and complications, such as bleeding. Adverse events are flagged with an automated software on a procedural timeline to facilitate review of procedural steps, disruptive environmental and organizational factors, OR team technical and nontechnical skills, surgeon physiologic stress, and intraoperative errors, events, and rectification processes using validated instruments.
Artificial intelligence built into this platform can automatically extract objective, high-quality, and structured data to generate explainable insights by recognizing adverse events and procedural segments of interest for training and quality improvement and provide a foundation with objective measurements of technical and nontechnical performance for formative and summative assessment. This system, a major step up compared with retrospective review of likely biased medical records and labor-intensive multidisciplinary human observers, has the potential to increase efficiency and reduce costs by studying human factors that include clinical design, technology, and organization. OR efficiency, measured in real time objectively and thoroughly, may save time and resources.
OR Black Box platforms have already started to generate meaningful data. It is not surprising that auditory disruptions—OR doors opening, loud noises, pagers beeping, telephones ringing—were recorded almost every minute during laparoscopic procedures.15 Most technical errors occurred during dissection, resection, and reconstruction and most commonly were associated with improper estimations of force applied to tissue and distance to the target tissue during operative steps of a laparoscopic procedure.16 Another study based on this system showed that technical performance was an independent predictor of postoperative outcomes.17 The OR Black Box identified a device-related interruption in 30% of elective laparoscopic general surgery cases, most commonly in sleeve gastrectomy and oncologic gastrectomy procedures. This sophisticated surgical data recording system also demonstrated a significantly better ability to detect Veress needle injuries (12 vs 3) and near misses (47 vs 0) when compared with traditional chart review.18
Data from the OR Black Box also have been applied to better analyze nontechnical performance, including teamwork and interpersonal dynamics.19 Surgeons most commonly exhibited adept situational awareness and leadership, while the nurse team excelled at task management and situational awareness.19 Of the total care provider team studied, the surgeon and scrub nurse demonstrated the most favorable nontechnical behavior.19 Of note, continuous physiologic monitoring of the surgeon with this system revealed that surgeons under stress had 66% higher adverse events.
The OR Black Box is currently utilized at 20 institutions in North America and Europe. The data compiled from all these institutions revealed that there was a 10% decrease in intraoperative adverse events for each 10-point increase in technical skill score on a scale of 0 to 100 (unpublished data). This centralized data indicated that turnover time ranged widely between 7 and 91 minutes, with variation of cleanup time from 1 to 25 minutes and setup time from 22 to 43 minutes. Institutions can learn from each other using this platform. For example, the information about block time utilization (20%–99%) across institutions provides opportunities for system improvements.
With any revolutionary technology, it is imperative to study its effects on outcomes, training, costs, and privacy before it is widely implemented. We, obstetricians and gynecologists, are very familiar with the impact of electronic fetal monitoring, a great example of a technologic advance that did not improve perinatal outcomes but led to unintended consequences, such as higher rates of cesarean deliveries and lawsuits. Such a tool may lead to potential misrepresentation of intraoperative events unless legal aspects are clearly delineated. As exciting as it is, this disruptive technology requires further exploration with scientific vigor.
Continue to: Surgeon and hospital volume: Surgical outcomes paradigm...
Surgeon and hospital volume: Surgical outcomes paradigm
A landmark study in 1979 that showed decreased mortality in high-volume centers underscored the need for regionalization for certain surgical procedures.20 This association was further substantiated by 2 reports on 2.5 million Medicare beneficiaries that demonstrated significantly lower mortality for all 14 cardiovascular and oncologic procedures for hospitals with larger surgical volume (16% vs 4%) and high-volume surgeons for certain procedures, for example, 15% versus 5% for pancreatic resections for cancer.21,22
A similar association was found for all routes of hysterectomies performed for benign indications. Boyd and colleagues showed that gynecologists who performed fewer than 10 hysterectomies per year had a higher perioperative morbidity rate (16.5%) compared with those who did more (11.7%).23 Specific to vaginal hysterectomy, in a study of more than 6,000 women, surgeons who performed 13 procedures per year had 31% less risk of operative injury than those who did 5.5 procedures per year (2.5% vs 1.7%).24 Overall perioperative complications (5.0% vs 4.0%) and medical complications (5.7% vs 3.9%) were also reduced for higher-volume surgeons. In a cohort of approximately 8,000 women who underwent a laparoscopic hysterectomy, high-volume surgeons had a considerably lower complication rate (4.2% vs 6.2%).25
As expected, lower complication rates of high-volume surgeons led to lower resource utilization, including lower transfusion rates, less intensive care unit utilization, and shorter operative times and, in several studies, length of stay.24,25 Of note, low-volume surgeons were less likely to offer minimally invasive routes and were more likely to convert to laparotomy.26 In addition, significant cost savings have been associated with high surgical volume, which one study showed was 16% ($6,500 vs $5,600) for high-volume surgeons.26 With regard to mortality, a study of 7,800 women found that perioperative mortality increased more than 10-fold for surgeons who performed an average 1 case per year compared with all other surgeons (2.5% vs 0.2%).27
When gynecologic cancers are concerned, arguably, long-term survival outcomes may be more critical than perioperative morbidity and mortality. Higher surgeon and hospital volume are associated with improved perioperative outcomes for endometrial and cervical cancers.28 Importantly, minimally invasive hysterectomy was offered for endometrial cancer significantly more often by surgeons with high volume.28 Survival outcomes were not affected by surgeon or hospital volume, likely due to overall more favorable prognosis for endometrial cancer after treatment.
Although it is intuitive to assume that a surgeon’s skills and experience would make the most impact in procedures for ovarian cancer due to the complexity of ovarian cancer surgery, evidence on short-term outcomes has been mixed. Intriguingly, some studies reported that high-volume institutions had higher complication and readmission rates. However, evidence supports that the surgeon’s volume, and especially hospital volume, improves long-term survival for ovarian cancer, with a negative impact on immediate postoperative morbidity.29 This may suggest that a more aggressive surgical effort improves long-term survival but also can cause more perioperative complications. Further, longer survival may result not only from operative skills but also because of better care by a structured multidisciplinary team at more established high-volume cancer centers.
The association of improved outcomes with higher volume led to public reporting of hospital outcomes. Policy efforts toward regionalization have impacted surgical practice. Based on their analysis of 3.2 million Medicare patients who underwent 1 of 8 different cancer surgeries or cardiovascular operations from 1999 to 2008, Finks and colleagues demonstrated that care was concentrated to fewer hospitals over time for many of these procedures.29 This trend was noted for gynecologic cancer surgery but not for benign gynecologic surgery.
Regionalization of care limits access particularly for minority and underserved communities because of longer travel distances, logistic challenges, and financial strain. An alternative to regionalization of care is targeted quality improvement by rigorous adherence to quality guidelines at low-volume hospitals.
Is there a critical minimum volume that may be used as a requirement for surgeons to maintain their privileges and for hospitals to offer certain procedures? In 2015, minimum volume standards for a number of common procedures were proposed by Johns Hopkins Medicine and Dartmouth-Hitchcock Medical Center, such as 50 hip replacement surgeries per hospital and 25 per physician per year, and 20 pancreatectomies per hospital and 5 per surgeon per year.30 A modeling study for hysterectomy showed that a volume cut point of >1 procedure in the prior year would restrict privileges for a substantial number of surgeons performing abdominal (17.5%), robot-assisted (12.5%), laparoscopic (16.8%), and vaginal (27.6%) hysterectomies.27 This study concluded that minimum-volume standards for hysterectomy for even the lowest volume physicians would restrict a significant number of gynecologic surgeons, including many with outcomes that are better than predicted.
Therefore, while there is good evidence that favors better outcomes in the hands of high-volume surgeons in gynecology, the impact of such policies on gynecologic practice clearly warrants careful monitoring and further study.
- What factors besides the surgeon’s skills influence surgical safety and efficiency?
- Are you ready to have audio, video, and sensor-based recording of everything in the OR?
- Who should perform your loved one’s hysterectomy? Do the surgeon’s and hospital’s volume matter?
- Catchpole K, Bisantz A, Hallbeck MS, et al. Human factors in robotic assisted surgery: lessons from studies ‘in the wild’. Appl Ergon. 2019;78:270-276.
- Catchpole K, Perkins C, Bresee C, et al. Safety, efficiency and learning curves in robotic surgery: a human factors analysis. Surg Endosc. 2016;30:3749-3761.
- Jain M, Fry BT, Hess LW, et al. Barriers to efficiency in robotic surgery: the resident effect. J Surg. Res. 2016;205:296-304.
- Yu D, Dural C, Morrow MM, et al. Intraoperative workload in robotic surgery assessed by wearable motion tracking sensors and questionnaires. Surg Endosc. 2017;31:877-886.
- Randell R, Honey S, Alvarado N, et al. Embedding robotic surgery into routine practice and impacts on communication and decision making: a review of the experience of surgical teams. Cognit Technol Work. 2016;18:423-437.
- Souders CP, Catchpole KR, Wood LN, et al. Reducing operating room turnover time for robotic surgery using a motor racing pit stop model. World J Surg. 2017;4:1943–1949.
- Ahmad N, Hussein AA, Cavuoto L, et al. Ambulatory movements, team dynamics and interactions during robot-assisted surgery. BJU Int. 2016;118:132-139.
- Sexton K, Johnson A, Gotsch A, et al. Anticipation, teamwork, and cognitive load: chasing efficiency during robot-assisted surgery. BMJ Qual Saf. 2018;27:148-154.
- Harmanli O, Solak S, Bayram A, et al. Optimizing the robotic surgery team: an operations management perspective. Int Urogynecol J. 2021;32:1379-1385.
- Carter-Brooks CM, Du AL, Bonidie MJ, et al. The impact of a dedicated robotic team on robotic-assisted sacrocolpopexy outcomes. Female Pelvic Med Reconstr Surg. 2018;24:13-16.
- Giugale LE, Sears S, Lavelle ES, et al. Evaluating the impact of intraoperative surgical team handoffs on patient outcomes. Female Pelvic Med Reconstr Surg. 2017;23:288-292.
- Geynisman-Tan J, Brown O, Mueller M, et al. Operating room efficiency: examining the impact of personnel handoffs. Female Pelvic Med Reconstr Surg. 2018;24:87-89.
- Alsubaie H, Goldenberg M, Grantcharov T. Quantifying recall bias in surgical safety: a need for a modern approach to morbidity and mortality reviews. Can J Surg. 2019;62:39-43.
- Goldenberg MG, Jung J, Grantcharov TP. Using data to enhance performance and improve quality and safety in surgery. JAMA Surg. 2017;152:972-973.
- Jung JJ, Grantcharov TP. The operating room black box: a prospective observational study of the operating room. J Am Coll Surg. 2017;225:S127-S128.
- Jung JJ, Jüni P, Lebovic G, et al. First-year analysis of the operating room black box study. Ann Surg. 2020;271:122-127.
- Jung JJ, Kashfi A, Sharma S, et al. Characterization of device-related interruptions in minimally invasive surgery: need for intraoperative data and effective mitigation strategies. Surg Endosc. 2019;33:717-723.
- Jung JJ, Adams-McGavin RC, Grantcharov TP. Underreporting of Veress needle injuries: comparing direct observation and chart review methods. J Surg Res. 2019;236:266-270.
- Fesco AB, Kuzulugil SS, Babaoglu C, et al. Relationship between intraoperative nontechnical performance and technical events in bariatric surgery. Br J Surg. 2018;105:1044-1050.
- Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364-1369.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137.
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:21172127.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915.
- Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116:1341-1347.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Bretschneider CE, Frazzini Padilla P, Das D, et al. The impact of surgeon volume on perioperative adverse events in women undergoing minimally invasive hysterectomy for the large uterus. Am J Obstet Gynecol. 2018;219:490.e1-490.e8.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Wright JD. The volume-outcome paradigm for gynecologic surgery: clinical and policy implications. Clin Obstet Gynecol. 2020;63:252-265.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high risk surgery. N Engl J Med. 2011;364:21282137.
- Sternberg S. Hospitals move to limit low-volume surgeries. US News & World Report. May 19, 2015. www.usnews.com/news /articles/2015/05/19/hospitals-move-to-limit-low-volume-surgeries. Accessed April 19, 2022.
- Catchpole K, Bisantz A, Hallbeck MS, et al. Human factors in robotic assisted surgery: lessons from studies ‘in the wild’. Appl Ergon. 2019;78:270-276.
- Catchpole K, Perkins C, Bresee C, et al. Safety, efficiency and learning curves in robotic surgery: a human factors analysis. Surg Endosc. 2016;30:3749-3761.
- Jain M, Fry BT, Hess LW, et al. Barriers to efficiency in robotic surgery: the resident effect. J Surg. Res. 2016;205:296-304.
- Yu D, Dural C, Morrow MM, et al. Intraoperative workload in robotic surgery assessed by wearable motion tracking sensors and questionnaires. Surg Endosc. 2017;31:877-886.
- Randell R, Honey S, Alvarado N, et al. Embedding robotic surgery into routine practice and impacts on communication and decision making: a review of the experience of surgical teams. Cognit Technol Work. 2016;18:423-437.
- Souders CP, Catchpole KR, Wood LN, et al. Reducing operating room turnover time for robotic surgery using a motor racing pit stop model. World J Surg. 2017;4:1943–1949.
- Ahmad N, Hussein AA, Cavuoto L, et al. Ambulatory movements, team dynamics and interactions during robot-assisted surgery. BJU Int. 2016;118:132-139.
- Sexton K, Johnson A, Gotsch A, et al. Anticipation, teamwork, and cognitive load: chasing efficiency during robot-assisted surgery. BMJ Qual Saf. 2018;27:148-154.
- Harmanli O, Solak S, Bayram A, et al. Optimizing the robotic surgery team: an operations management perspective. Int Urogynecol J. 2021;32:1379-1385.
- Carter-Brooks CM, Du AL, Bonidie MJ, et al. The impact of a dedicated robotic team on robotic-assisted sacrocolpopexy outcomes. Female Pelvic Med Reconstr Surg. 2018;24:13-16.
- Giugale LE, Sears S, Lavelle ES, et al. Evaluating the impact of intraoperative surgical team handoffs on patient outcomes. Female Pelvic Med Reconstr Surg. 2017;23:288-292.
- Geynisman-Tan J, Brown O, Mueller M, et al. Operating room efficiency: examining the impact of personnel handoffs. Female Pelvic Med Reconstr Surg. 2018;24:87-89.
- Alsubaie H, Goldenberg M, Grantcharov T. Quantifying recall bias in surgical safety: a need for a modern approach to morbidity and mortality reviews. Can J Surg. 2019;62:39-43.
- Goldenberg MG, Jung J, Grantcharov TP. Using data to enhance performance and improve quality and safety in surgery. JAMA Surg. 2017;152:972-973.
- Jung JJ, Grantcharov TP. The operating room black box: a prospective observational study of the operating room. J Am Coll Surg. 2017;225:S127-S128.
- Jung JJ, Jüni P, Lebovic G, et al. First-year analysis of the operating room black box study. Ann Surg. 2020;271:122-127.
- Jung JJ, Kashfi A, Sharma S, et al. Characterization of device-related interruptions in minimally invasive surgery: need for intraoperative data and effective mitigation strategies. Surg Endosc. 2019;33:717-723.
- Jung JJ, Adams-McGavin RC, Grantcharov TP. Underreporting of Veress needle injuries: comparing direct observation and chart review methods. J Surg Res. 2019;236:266-270.
- Fesco AB, Kuzulugil SS, Babaoglu C, et al. Relationship between intraoperative nontechnical performance and technical events in bariatric surgery. Br J Surg. 2018;105:1044-1050.
- Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364-1369.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137.
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:21172127.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915.
- Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116:1341-1347.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Bretschneider CE, Frazzini Padilla P, Das D, et al. The impact of surgeon volume on perioperative adverse events in women undergoing minimally invasive hysterectomy for the large uterus. Am J Obstet Gynecol. 2018;219:490.e1-490.e8.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Wright JD. The volume-outcome paradigm for gynecologic surgery: clinical and policy implications. Clin Obstet Gynecol. 2020;63:252-265.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high risk surgery. N Engl J Med. 2011;364:21282137.
- Sternberg S. Hospitals move to limit low-volume surgeries. US News & World Report. May 19, 2015. www.usnews.com/news /articles/2015/05/19/hospitals-move-to-limit-low-volume-surgeries. Accessed April 19, 2022.
2021 Update on gynecologic cancer
Gynecologic malignancies continue to be a major cause of cancer-related mortality in women. In 2020, a number of developments changed practice in gynecologic oncology. In this Update, we highlight 3 important articles. The first showed that human papillomavirus (HPV) vaccination reduced the rate of cervical cancer. The next evaluated a novel targeted therapeutic approach using the combination of pembrolizumab and lenvatinib in women with recurrent endometrial carcinoma that progressed after prior systemic therapy. Finally, the third article showed that talcum powder was not associated with an increased risk of ovarian cancer. We provide here a brief overview of the major findings of these studies and how these results are influencing practice.
Evidence establishes that HPV vaccination cuts risk of invasive cervical cancer
Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
HPV infection is associated with 99% of cervical cancers, and approximately 65% to 75% of cases involve HPV 16 or 18.1,2 The quadrivalent HPV (6, 11, 16, 18) vaccine was approved by the US Food and Drug Administration in 2006 for the prevention of cervical intraepithelial lesions and genital warts associated with HPV.3-5 Previous studies of the HPV vaccine showed it to be effective in preventing HPV infection, genital warts, and high-grade precancerous cervical lesions, such as cervical intraepithelial neoplasia grade 2 (CIN2) and grade 3 (CIN3).6-8 While the vaccine offers a number of advantages, the long-term goal of the vaccine—to reduce the incidence of invasive cervical cancer—was not shown until recently.
Large study followed HPV vaccinated and unvaccinated women
Lei and colleagues conducted a registry based cohort study from 2006 through 2017 of women aged 10 to 30 years who were living in Sweden.9 They followed the women from their 10th birthday until they were diagnosed with cervical cancer, died, emigrated from Sweden, were lost to follow-up, or turned 31 years of age. In the study, the unique personal identity numbers assigned to all Swedish residents were linked to a number of large national administrative databases. Beginning in 2007 in Sweden, the quadrivalent vaccine was subsidized for use in girls aged 13 to 17, and a subsequent catch-up period that started in 2012 incorporated women who had not been vaccinated.
Continue to: Cervical cancer rates were lowest in women vaccinated before age 17...
Cervical cancer rates were lowest in women vaccinated before age 17
A total of 1,672,983 women were included in the study; 527,871 received at least one dose of the HPV vaccine. During the study period, cervical cancer was diagnosed in 19 women who had received the quadrivalent HPV vaccine and in 538 women who had not received the vaccine. Women who initiated vaccination before age 17 had the lowest rates of cervical cancer (4 cases per 100,000 persons), followed by women vaccinated after age 17 (54 cases per 100,000 persons) and then those who were not vaccinated (94 cases per 100,000 persons).
After adjusting for confounders, the incidence rate ratio (RR) of cervical cancer was significantly lower among vaccinated women compared with unvaccinated women (RR, 0.37; 95% confidence interval [CI], 0.21– 0.57) (FIGURE 1).9 In addition, women who were vaccinated before age 17 demonstrated the greatest benefit. For those vaccinated before age 17 versus those who were unvaccinated, the RR was 0.12 (95% CI, 0.00–0.34). For women vaccinated between age 17 and 30 versus unvaccinated women, the RR was 0.47 (95% CI, 0.27–0.75).
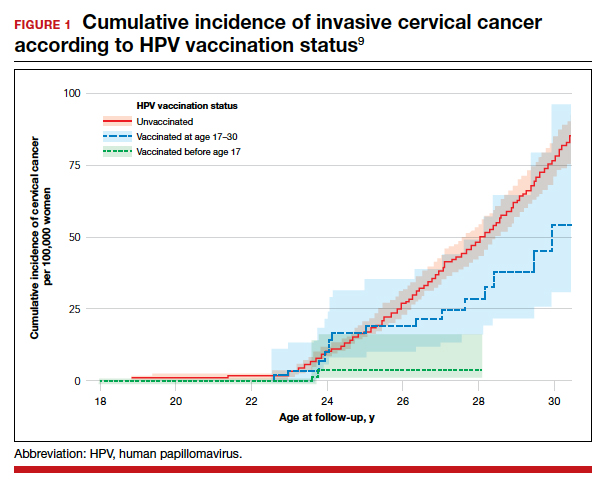
The study by Lei and colleagues showed that HPV vaccination was associated with a substantially lower risk of invasive cervical cancer. While all women who received the vaccine had reduced rates of invasive cervical cancer, those who received the vaccine earlier (before age 17) showed the greatest reduction in invasive cervical cancer. On a population level, this study demonstrates that a program of HPV vaccination can reduce the burden of cervical cancer.
Promising option for patients with advanced endometrial cancer: Lenvatinib plus pembrolizumab
Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
Advanced stage endometrial cancer is associated with a 17% 5-year survival rate.10 Paclitaxel with carboplatin is the standard first-line treatment for advanced, recurrent, and metastatic endometrial cancer; for women who do not respond to this regimen, effective treatment options are limited.11,12
The immunotherapy approach
Immunotherapy is a more recently developed treatment, an approach in which the immune system is activated to target cancer cells. Pembrolizumab is a commonly used agent for many solid tumors.13 This drug binds to the programmed cell death receptor 1 (PD-1) or PD-ligand 1 (PD-L1), a component of the immune checkpoint, which then allows the immune system to target and destroy cancer cells.14
Prembrolizumab is FDA approved for use in the treatment of microsatellite instability-high (MSI-H)/mismatch repair deficient (dMMR) solid tumors that have progressed after prior therapy and for which there are no satisfactory alternative treatment options.15 Endometrial cancers frequently display microsatellite instability and mismatch repair defects.16
Lenvatinib is an oral multikinase inhibitor that targets vascular endothelial growth factor receptors 1, 2, and 3; fibroblast growth factor receptors 1, 2, 3, and 4; and platelet derived growth factor receptor alpha, RET, and KIT.17-19 In a phase 2 study of lenvatinib monotherapy for advanced previously treated endometrial cancer, the response rate was 14.3%.20
While some preclinical studies have examined the combination of immune checkpoint inhibitors with lenvatinib,21-23 a recent study is the first to evaluate this combination in patients with advanced tumors.24
Continue to: Prembrolizumab-lenvatinib combination therapy...
Prembrolizumab-lenvatinib combination therapy
Makker and colleagues conducted an ongoing multinational, open-label, phase 1B/2 study of lenvatinib 20 mg daily orally plus pembrolizumab 200 mg intravenously once every 3 weeks in patients with select solid tumors.24 Women with previously treated endometrial carcinoma (N = 125) were included. Of the study participants, 49% were PD-L1 positive and 10% were MSI-H/dMMR. The primary end point was objective response rate (ORR) at 24 weeks, which was 38.0% (95% CI, 28.8%–47.8%).
The median duration of response was 21.2 months (95% CI, 7.6 months to not estimable). The ORR was similar in patients with PD-L1 expressing tumors (35.8%; 95% CI, 23.1%–50.2%), who are more likely to respond to immunotherapy, compared with those without PD-L1 expression (39.5%; 95% CI, 25.0%–55.6%). For patients with MSI-H/dMMR, there was a higher ORR (63.6%; 95% CI, 30.8%–89.1%, versus 36.2%; 95% CI, 26.5%–46.7%).
Median progression-free survival was 7.4 months (95% CI, 5.3–8.7 months) and median overall survival was 16.7 months (15 months to not estimable). Moderate to severe treatment-related adverse events occurred in 83 patients (66.9%), and 22 patients (17.7%) discontinued 1 or both study drugs because of adverse effects. Two deaths were judged to be treatment related.
This study showed promising results for the combination of pembrolizumab with lenvatinib in women with advanced endometrial carcinoma who have progressed after prior systemic therapy. These data led to an accelerated approval by the FDA for the treatment of women with advanced endometrial carcinoma that is not MSI-H/dMMR, who have disease progression after prior systemic therapy, and who are not candidates for curative surgery or radiation therapy.25 Currently, 2 phase 3 trials of lenvatinib plus pembrolizumab in advanced endometrial carcinoma are underway, which will shed further light on this combination therapy
What is the risk of ovarian cancer in women who use powder in the genital area?
O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
Women apply talcum powder to their genital area to keep skin dry and to prevent rashes. Powder can be applied by direct application, sanitary napkins, diaphragms, or tampons. Most powder products contain the mineral talc. Because it often is found in nature with asbestos, a known carcinogen, talc’s carcinogenic effects have been investigated.26,27
Talc also might ascend through the genital tract and irritate the epithelial lining of the fallopian tubes or ovaries, possibly triggering an inflammatory response that may promote carcinogenesis.28,29 Case-control studies have reported a possible association between genital powder use and ovarian cancer.30,31 Since these studies, talc-related lawsuits and media coverage have increased.32,33
Large prospective cohorts provide data for analysis
In a pooled analysis of 4 large US-based observational cohorts between 1976 and 2017, O’Brien and colleagues noted that 38% of the 252,745 women included in the study self-reported the use of powder in the genital area.34 With a median of 11.2 years of follow-up, 2,168 women developed ovarian cancer (58 cases/100,000 person-years). Among women who reported using genital powder, the incidence of ovarian cancer was 61 cases/100,000 person-years, while for women who reported never using genital powder, the incidence was 55 cases/100,000 person-years. This corresponded to an estimated hazard ratio (HR) of 1.08 (95% CI, 0.99–1.17).
Frequent powder use, long-term use, and never use. Similar findings were seen for those with frequent use versus never use (HR, 1.09; 95% CI, 0.97–1.23) and long-term use versus never use (HR, 1.01; 95% CI, 0.82– 1.25). When restricting the group to women with a patent reproductive tract at baseline, the HR was 1.13 (95% CI, 1.01–1.26), but the P value for interaction comparing women with versus women without a patent reproductive tract was 0.15 (FIGURE 2).34
Bottom line. In contrast to a prior meta-analysis, in this study there was no statistically significant association between the self-reported use of powder in the genital area and the incidence of ovarian cancer. ●
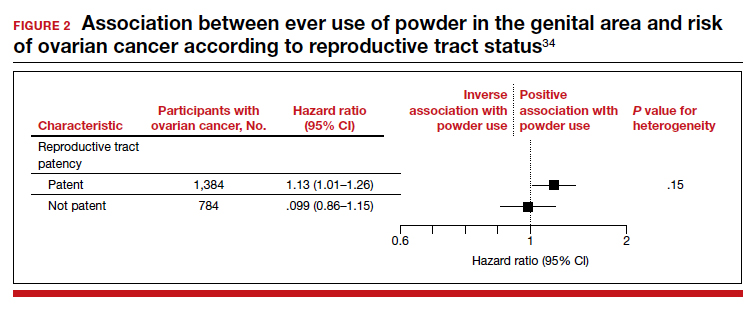
The study by O’Brien and colleagues is the largest study to date with the longest follow-up that examines the possible association between talc-based powder use and ovarian cancer. A strength of this study is the avoidance of recall bias by the selection of administrative data sets that had gathered information on talcum powder use from patients prior to the diagnosis of ovarian cancer. While these findings are reassuring, the study may have been underpowered to identify a small increase in ovarian cancer risk with talc use.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12-19.
- Ault KA; Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861-1868.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928-1943.
- Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against highgrade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693-1702.
- Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5(5):CD009069.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04- adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301-314.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915-1927.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
- American Cancer Society. Survival rates for endometrial cancer. https://www.cancer.org/cancer/endometrial-cancer/ detection-diagnosis-staging/survival-rates.html. Accessed February 9, 2021.
- Miller D, Filiaci V, Fleming G, et al. Late-breaking abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:771.
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: uterine neoplasms. Version 3.2019. https://www.nccn.org/professionals/physician_gls/pdf /uterine.pdf. Accessed February 9, 2021.
- Marcus L, Lemery SJ, Keegan P, et al. FDA approval summary: pembrolizumab for the treatment of microsatellite instabilityhigh solid tumors. Clin Cancer Res. 2019;25:3753-3758.
- Arora E, Masab M, Mittar P, et al. Role of immune checkpoint inhibitors in advanced or recurrent endometrial cancer. Cureus. 2018;10:e2521.
- Keytruda (pembrolizumab). Package insert. Merck Sharp & Dohme; 2018.
- Cancer Genome Atlas Research Network; Kandoth C, Schultz N, Cherniak AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67-73.
- Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664-671.
- Okamoto K, Kodama K, Takase K, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340:97-103.
- Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (E7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014: 638747.
- Vergote I, Teneriello M, Powell MA, et al. A phase II trial of lenvatinib in patients with advanced or recurrent endometrial cancer: angiopoietin-2 as a predictive marker for clinical outcomes. J Clin Oncol. 2013;31(15 suppl): abstract 5520.
- Kimura T, Kato Y, Ozawa Y, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109:3993-4002.
- Kato Y, Tabata K, Hori Y, et al. Effects of lenvatinib on tumorassociated macrophages enhance antitumor activity of PD-1 signal inhibitors. Mol Cancer Ther. 2015;14(12 suppl 2): abstract A92.
- Kato Y, Bao X, Macgrath S, et al. Lenvatinib mesilate (LEN) enhanced antitumor activity of a PD-1 blockade agent by potentiating Th1 immune response. Ann Oncol. 2016;27(suppl 6): abstract 2PD.
- Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
- Lenvima (lenvatinib). Package insert. Woodcliff Lake, NJ: Eisai; 2019.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Carbon black, titanium dioxide, and talc. IARC Monogr Eval Carcinog Risks Hum. 2010;93:1-413.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, metals, fibres, and dusts. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt C):11-465.
- Erickson BK, Conner MG, Landen CN Jr. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209:409-414.
- Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459-1467.
- Terry KL, Karageorgi S, Shvetsov YB, et al; Ovarian Cancer Association Consortium. Genital powder use and risk of ovarian cancer: a pooled analysis of 8,525 cases and 9,859 controls. Cancer Prev Res. 2013;6:811-821.
- Penninkilampi R, Eslick GD. Perineal talc use and ovarian cancer: a systematic review and meta-analysis. Epidemiology. 2018;29:41-49.
- Hsu T. Johnson & Johnson told to pay $4.7 billion in baby powder lawsuit. New York Times. July 12, 2018. Accessed February 18, 2021. https://www.nytimes.com/2018/07/12 /business/johnson-johnson-talcum-powder.html.
- McGinley L. Does talcum powder cause ovarian cancer? Washington Post. August 25, 2017. Accessed February 18, 2021. https://www.washingtonpost.com/news/to-your -health/wp/2017/08/23/does-talcum-powder-cause -ovarian-cancer-experts-are-divided/.
- O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.

Gynecologic malignancies continue to be a major cause of cancer-related mortality in women. In 2020, a number of developments changed practice in gynecologic oncology. In this Update, we highlight 3 important articles. The first showed that human papillomavirus (HPV) vaccination reduced the rate of cervical cancer. The next evaluated a novel targeted therapeutic approach using the combination of pembrolizumab and lenvatinib in women with recurrent endometrial carcinoma that progressed after prior systemic therapy. Finally, the third article showed that talcum powder was not associated with an increased risk of ovarian cancer. We provide here a brief overview of the major findings of these studies and how these results are influencing practice.
Evidence establishes that HPV vaccination cuts risk of invasive cervical cancer
Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
HPV infection is associated with 99% of cervical cancers, and approximately 65% to 75% of cases involve HPV 16 or 18.1,2 The quadrivalent HPV (6, 11, 16, 18) vaccine was approved by the US Food and Drug Administration in 2006 for the prevention of cervical intraepithelial lesions and genital warts associated with HPV.3-5 Previous studies of the HPV vaccine showed it to be effective in preventing HPV infection, genital warts, and high-grade precancerous cervical lesions, such as cervical intraepithelial neoplasia grade 2 (CIN2) and grade 3 (CIN3).6-8 While the vaccine offers a number of advantages, the long-term goal of the vaccine—to reduce the incidence of invasive cervical cancer—was not shown until recently.
Large study followed HPV vaccinated and unvaccinated women
Lei and colleagues conducted a registry based cohort study from 2006 through 2017 of women aged 10 to 30 years who were living in Sweden.9 They followed the women from their 10th birthday until they were diagnosed with cervical cancer, died, emigrated from Sweden, were lost to follow-up, or turned 31 years of age. In the study, the unique personal identity numbers assigned to all Swedish residents were linked to a number of large national administrative databases. Beginning in 2007 in Sweden, the quadrivalent vaccine was subsidized for use in girls aged 13 to 17, and a subsequent catch-up period that started in 2012 incorporated women who had not been vaccinated.
Continue to: Cervical cancer rates were lowest in women vaccinated before age 17...
Cervical cancer rates were lowest in women vaccinated before age 17
A total of 1,672,983 women were included in the study; 527,871 received at least one dose of the HPV vaccine. During the study period, cervical cancer was diagnosed in 19 women who had received the quadrivalent HPV vaccine and in 538 women who had not received the vaccine. Women who initiated vaccination before age 17 had the lowest rates of cervical cancer (4 cases per 100,000 persons), followed by women vaccinated after age 17 (54 cases per 100,000 persons) and then those who were not vaccinated (94 cases per 100,000 persons).
After adjusting for confounders, the incidence rate ratio (RR) of cervical cancer was significantly lower among vaccinated women compared with unvaccinated women (RR, 0.37; 95% confidence interval [CI], 0.21– 0.57) (FIGURE 1).9 In addition, women who were vaccinated before age 17 demonstrated the greatest benefit. For those vaccinated before age 17 versus those who were unvaccinated, the RR was 0.12 (95% CI, 0.00–0.34). For women vaccinated between age 17 and 30 versus unvaccinated women, the RR was 0.47 (95% CI, 0.27–0.75).

The study by Lei and colleagues showed that HPV vaccination was associated with a substantially lower risk of invasive cervical cancer. While all women who received the vaccine had reduced rates of invasive cervical cancer, those who received the vaccine earlier (before age 17) showed the greatest reduction in invasive cervical cancer. On a population level, this study demonstrates that a program of HPV vaccination can reduce the burden of cervical cancer.
Promising option for patients with advanced endometrial cancer: Lenvatinib plus pembrolizumab
Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
Advanced stage endometrial cancer is associated with a 17% 5-year survival rate.10 Paclitaxel with carboplatin is the standard first-line treatment for advanced, recurrent, and metastatic endometrial cancer; for women who do not respond to this regimen, effective treatment options are limited.11,12
The immunotherapy approach
Immunotherapy is a more recently developed treatment, an approach in which the immune system is activated to target cancer cells. Pembrolizumab is a commonly used agent for many solid tumors.13 This drug binds to the programmed cell death receptor 1 (PD-1) or PD-ligand 1 (PD-L1), a component of the immune checkpoint, which then allows the immune system to target and destroy cancer cells.14
Prembrolizumab is FDA approved for use in the treatment of microsatellite instability-high (MSI-H)/mismatch repair deficient (dMMR) solid tumors that have progressed after prior therapy and for which there are no satisfactory alternative treatment options.15 Endometrial cancers frequently display microsatellite instability and mismatch repair defects.16
Lenvatinib is an oral multikinase inhibitor that targets vascular endothelial growth factor receptors 1, 2, and 3; fibroblast growth factor receptors 1, 2, 3, and 4; and platelet derived growth factor receptor alpha, RET, and KIT.17-19 In a phase 2 study of lenvatinib monotherapy for advanced previously treated endometrial cancer, the response rate was 14.3%.20
While some preclinical studies have examined the combination of immune checkpoint inhibitors with lenvatinib,21-23 a recent study is the first to evaluate this combination in patients with advanced tumors.24
Continue to: Prembrolizumab-lenvatinib combination therapy...
Prembrolizumab-lenvatinib combination therapy
Makker and colleagues conducted an ongoing multinational, open-label, phase 1B/2 study of lenvatinib 20 mg daily orally plus pembrolizumab 200 mg intravenously once every 3 weeks in patients with select solid tumors.24 Women with previously treated endometrial carcinoma (N = 125) were included. Of the study participants, 49% were PD-L1 positive and 10% were MSI-H/dMMR. The primary end point was objective response rate (ORR) at 24 weeks, which was 38.0% (95% CI, 28.8%–47.8%).
The median duration of response was 21.2 months (95% CI, 7.6 months to not estimable). The ORR was similar in patients with PD-L1 expressing tumors (35.8%; 95% CI, 23.1%–50.2%), who are more likely to respond to immunotherapy, compared with those without PD-L1 expression (39.5%; 95% CI, 25.0%–55.6%). For patients with MSI-H/dMMR, there was a higher ORR (63.6%; 95% CI, 30.8%–89.1%, versus 36.2%; 95% CI, 26.5%–46.7%).
Median progression-free survival was 7.4 months (95% CI, 5.3–8.7 months) and median overall survival was 16.7 months (15 months to not estimable). Moderate to severe treatment-related adverse events occurred in 83 patients (66.9%), and 22 patients (17.7%) discontinued 1 or both study drugs because of adverse effects. Two deaths were judged to be treatment related.
This study showed promising results for the combination of pembrolizumab with lenvatinib in women with advanced endometrial carcinoma who have progressed after prior systemic therapy. These data led to an accelerated approval by the FDA for the treatment of women with advanced endometrial carcinoma that is not MSI-H/dMMR, who have disease progression after prior systemic therapy, and who are not candidates for curative surgery or radiation therapy.25 Currently, 2 phase 3 trials of lenvatinib plus pembrolizumab in advanced endometrial carcinoma are underway, which will shed further light on this combination therapy
What is the risk of ovarian cancer in women who use powder in the genital area?
O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
Women apply talcum powder to their genital area to keep skin dry and to prevent rashes. Powder can be applied by direct application, sanitary napkins, diaphragms, or tampons. Most powder products contain the mineral talc. Because it often is found in nature with asbestos, a known carcinogen, talc’s carcinogenic effects have been investigated.26,27
Talc also might ascend through the genital tract and irritate the epithelial lining of the fallopian tubes or ovaries, possibly triggering an inflammatory response that may promote carcinogenesis.28,29 Case-control studies have reported a possible association between genital powder use and ovarian cancer.30,31 Since these studies, talc-related lawsuits and media coverage have increased.32,33
Large prospective cohorts provide data for analysis
In a pooled analysis of 4 large US-based observational cohorts between 1976 and 2017, O’Brien and colleagues noted that 38% of the 252,745 women included in the study self-reported the use of powder in the genital area.34 With a median of 11.2 years of follow-up, 2,168 women developed ovarian cancer (58 cases/100,000 person-years). Among women who reported using genital powder, the incidence of ovarian cancer was 61 cases/100,000 person-years, while for women who reported never using genital powder, the incidence was 55 cases/100,000 person-years. This corresponded to an estimated hazard ratio (HR) of 1.08 (95% CI, 0.99–1.17).
Frequent powder use, long-term use, and never use. Similar findings were seen for those with frequent use versus never use (HR, 1.09; 95% CI, 0.97–1.23) and long-term use versus never use (HR, 1.01; 95% CI, 0.82– 1.25). When restricting the group to women with a patent reproductive tract at baseline, the HR was 1.13 (95% CI, 1.01–1.26), but the P value for interaction comparing women with versus women without a patent reproductive tract was 0.15 (FIGURE 2).34
Bottom line. In contrast to a prior meta-analysis, in this study there was no statistically significant association between the self-reported use of powder in the genital area and the incidence of ovarian cancer. ●

The study by O’Brien and colleagues is the largest study to date with the longest follow-up that examines the possible association between talc-based powder use and ovarian cancer. A strength of this study is the avoidance of recall bias by the selection of administrative data sets that had gathered information on talcum powder use from patients prior to the diagnosis of ovarian cancer. While these findings are reassuring, the study may have been underpowered to identify a small increase in ovarian cancer risk with talc use.

Gynecologic malignancies continue to be a major cause of cancer-related mortality in women. In 2020, a number of developments changed practice in gynecologic oncology. In this Update, we highlight 3 important articles. The first showed that human papillomavirus (HPV) vaccination reduced the rate of cervical cancer. The next evaluated a novel targeted therapeutic approach using the combination of pembrolizumab and lenvatinib in women with recurrent endometrial carcinoma that progressed after prior systemic therapy. Finally, the third article showed that talcum powder was not associated with an increased risk of ovarian cancer. We provide here a brief overview of the major findings of these studies and how these results are influencing practice.
Evidence establishes that HPV vaccination cuts risk of invasive cervical cancer
Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
HPV infection is associated with 99% of cervical cancers, and approximately 65% to 75% of cases involve HPV 16 or 18.1,2 The quadrivalent HPV (6, 11, 16, 18) vaccine was approved by the US Food and Drug Administration in 2006 for the prevention of cervical intraepithelial lesions and genital warts associated with HPV.3-5 Previous studies of the HPV vaccine showed it to be effective in preventing HPV infection, genital warts, and high-grade precancerous cervical lesions, such as cervical intraepithelial neoplasia grade 2 (CIN2) and grade 3 (CIN3).6-8 While the vaccine offers a number of advantages, the long-term goal of the vaccine—to reduce the incidence of invasive cervical cancer—was not shown until recently.
Large study followed HPV vaccinated and unvaccinated women
Lei and colleagues conducted a registry based cohort study from 2006 through 2017 of women aged 10 to 30 years who were living in Sweden.9 They followed the women from their 10th birthday until they were diagnosed with cervical cancer, died, emigrated from Sweden, were lost to follow-up, or turned 31 years of age. In the study, the unique personal identity numbers assigned to all Swedish residents were linked to a number of large national administrative databases. Beginning in 2007 in Sweden, the quadrivalent vaccine was subsidized for use in girls aged 13 to 17, and a subsequent catch-up period that started in 2012 incorporated women who had not been vaccinated.
Continue to: Cervical cancer rates were lowest in women vaccinated before age 17...
Cervical cancer rates were lowest in women vaccinated before age 17
A total of 1,672,983 women were included in the study; 527,871 received at least one dose of the HPV vaccine. During the study period, cervical cancer was diagnosed in 19 women who had received the quadrivalent HPV vaccine and in 538 women who had not received the vaccine. Women who initiated vaccination before age 17 had the lowest rates of cervical cancer (4 cases per 100,000 persons), followed by women vaccinated after age 17 (54 cases per 100,000 persons) and then those who were not vaccinated (94 cases per 100,000 persons).
After adjusting for confounders, the incidence rate ratio (RR) of cervical cancer was significantly lower among vaccinated women compared with unvaccinated women (RR, 0.37; 95% confidence interval [CI], 0.21– 0.57) (FIGURE 1).9 In addition, women who were vaccinated before age 17 demonstrated the greatest benefit. For those vaccinated before age 17 versus those who were unvaccinated, the RR was 0.12 (95% CI, 0.00–0.34). For women vaccinated between age 17 and 30 versus unvaccinated women, the RR was 0.47 (95% CI, 0.27–0.75).

The study by Lei and colleagues showed that HPV vaccination was associated with a substantially lower risk of invasive cervical cancer. While all women who received the vaccine had reduced rates of invasive cervical cancer, those who received the vaccine earlier (before age 17) showed the greatest reduction in invasive cervical cancer. On a population level, this study demonstrates that a program of HPV vaccination can reduce the burden of cervical cancer.
Promising option for patients with advanced endometrial cancer: Lenvatinib plus pembrolizumab
Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
Advanced stage endometrial cancer is associated with a 17% 5-year survival rate.10 Paclitaxel with carboplatin is the standard first-line treatment for advanced, recurrent, and metastatic endometrial cancer; for women who do not respond to this regimen, effective treatment options are limited.11,12
The immunotherapy approach
Immunotherapy is a more recently developed treatment, an approach in which the immune system is activated to target cancer cells. Pembrolizumab is a commonly used agent for many solid tumors.13 This drug binds to the programmed cell death receptor 1 (PD-1) or PD-ligand 1 (PD-L1), a component of the immune checkpoint, which then allows the immune system to target and destroy cancer cells.14
Prembrolizumab is FDA approved for use in the treatment of microsatellite instability-high (MSI-H)/mismatch repair deficient (dMMR) solid tumors that have progressed after prior therapy and for which there are no satisfactory alternative treatment options.15 Endometrial cancers frequently display microsatellite instability and mismatch repair defects.16
Lenvatinib is an oral multikinase inhibitor that targets vascular endothelial growth factor receptors 1, 2, and 3; fibroblast growth factor receptors 1, 2, 3, and 4; and platelet derived growth factor receptor alpha, RET, and KIT.17-19 In a phase 2 study of lenvatinib monotherapy for advanced previously treated endometrial cancer, the response rate was 14.3%.20
While some preclinical studies have examined the combination of immune checkpoint inhibitors with lenvatinib,21-23 a recent study is the first to evaluate this combination in patients with advanced tumors.24
Continue to: Prembrolizumab-lenvatinib combination therapy...
Prembrolizumab-lenvatinib combination therapy
Makker and colleagues conducted an ongoing multinational, open-label, phase 1B/2 study of lenvatinib 20 mg daily orally plus pembrolizumab 200 mg intravenously once every 3 weeks in patients with select solid tumors.24 Women with previously treated endometrial carcinoma (N = 125) were included. Of the study participants, 49% were PD-L1 positive and 10% were MSI-H/dMMR. The primary end point was objective response rate (ORR) at 24 weeks, which was 38.0% (95% CI, 28.8%–47.8%).
The median duration of response was 21.2 months (95% CI, 7.6 months to not estimable). The ORR was similar in patients with PD-L1 expressing tumors (35.8%; 95% CI, 23.1%–50.2%), who are more likely to respond to immunotherapy, compared with those without PD-L1 expression (39.5%; 95% CI, 25.0%–55.6%). For patients with MSI-H/dMMR, there was a higher ORR (63.6%; 95% CI, 30.8%–89.1%, versus 36.2%; 95% CI, 26.5%–46.7%).
Median progression-free survival was 7.4 months (95% CI, 5.3–8.7 months) and median overall survival was 16.7 months (15 months to not estimable). Moderate to severe treatment-related adverse events occurred in 83 patients (66.9%), and 22 patients (17.7%) discontinued 1 or both study drugs because of adverse effects. Two deaths were judged to be treatment related.
This study showed promising results for the combination of pembrolizumab with lenvatinib in women with advanced endometrial carcinoma who have progressed after prior systemic therapy. These data led to an accelerated approval by the FDA for the treatment of women with advanced endometrial carcinoma that is not MSI-H/dMMR, who have disease progression after prior systemic therapy, and who are not candidates for curative surgery or radiation therapy.25 Currently, 2 phase 3 trials of lenvatinib plus pembrolizumab in advanced endometrial carcinoma are underway, which will shed further light on this combination therapy
What is the risk of ovarian cancer in women who use powder in the genital area?
O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
Women apply talcum powder to their genital area to keep skin dry and to prevent rashes. Powder can be applied by direct application, sanitary napkins, diaphragms, or tampons. Most powder products contain the mineral talc. Because it often is found in nature with asbestos, a known carcinogen, talc’s carcinogenic effects have been investigated.26,27
Talc also might ascend through the genital tract and irritate the epithelial lining of the fallopian tubes or ovaries, possibly triggering an inflammatory response that may promote carcinogenesis.28,29 Case-control studies have reported a possible association between genital powder use and ovarian cancer.30,31 Since these studies, talc-related lawsuits and media coverage have increased.32,33
Large prospective cohorts provide data for analysis
In a pooled analysis of 4 large US-based observational cohorts between 1976 and 2017, O’Brien and colleagues noted that 38% of the 252,745 women included in the study self-reported the use of powder in the genital area.34 With a median of 11.2 years of follow-up, 2,168 women developed ovarian cancer (58 cases/100,000 person-years). Among women who reported using genital powder, the incidence of ovarian cancer was 61 cases/100,000 person-years, while for women who reported never using genital powder, the incidence was 55 cases/100,000 person-years. This corresponded to an estimated hazard ratio (HR) of 1.08 (95% CI, 0.99–1.17).
Frequent powder use, long-term use, and never use. Similar findings were seen for those with frequent use versus never use (HR, 1.09; 95% CI, 0.97–1.23) and long-term use versus never use (HR, 1.01; 95% CI, 0.82– 1.25). When restricting the group to women with a patent reproductive tract at baseline, the HR was 1.13 (95% CI, 1.01–1.26), but the P value for interaction comparing women with versus women without a patent reproductive tract was 0.15 (FIGURE 2).34
Bottom line. In contrast to a prior meta-analysis, in this study there was no statistically significant association between the self-reported use of powder in the genital area and the incidence of ovarian cancer. ●

The study by O’Brien and colleagues is the largest study to date with the longest follow-up that examines the possible association between talc-based powder use and ovarian cancer. A strength of this study is the avoidance of recall bias by the selection of administrative data sets that had gathered information on talcum powder use from patients prior to the diagnosis of ovarian cancer. While these findings are reassuring, the study may have been underpowered to identify a small increase in ovarian cancer risk with talc use.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12-19.
- Ault KA; Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861-1868.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928-1943.
- Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against highgrade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693-1702.
- Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5(5):CD009069.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04- adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301-314.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915-1927.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
- American Cancer Society. Survival rates for endometrial cancer. https://www.cancer.org/cancer/endometrial-cancer/ detection-diagnosis-staging/survival-rates.html. Accessed February 9, 2021.
- Miller D, Filiaci V, Fleming G, et al. Late-breaking abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:771.
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: uterine neoplasms. Version 3.2019. https://www.nccn.org/professionals/physician_gls/pdf /uterine.pdf. Accessed February 9, 2021.
- Marcus L, Lemery SJ, Keegan P, et al. FDA approval summary: pembrolizumab for the treatment of microsatellite instabilityhigh solid tumors. Clin Cancer Res. 2019;25:3753-3758.
- Arora E, Masab M, Mittar P, et al. Role of immune checkpoint inhibitors in advanced or recurrent endometrial cancer. Cureus. 2018;10:e2521.
- Keytruda (pembrolizumab). Package insert. Merck Sharp & Dohme; 2018.
- Cancer Genome Atlas Research Network; Kandoth C, Schultz N, Cherniak AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67-73.
- Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664-671.
- Okamoto K, Kodama K, Takase K, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340:97-103.
- Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (E7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014: 638747.
- Vergote I, Teneriello M, Powell MA, et al. A phase II trial of lenvatinib in patients with advanced or recurrent endometrial cancer: angiopoietin-2 as a predictive marker for clinical outcomes. J Clin Oncol. 2013;31(15 suppl): abstract 5520.
- Kimura T, Kato Y, Ozawa Y, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109:3993-4002.
- Kato Y, Tabata K, Hori Y, et al. Effects of lenvatinib on tumorassociated macrophages enhance antitumor activity of PD-1 signal inhibitors. Mol Cancer Ther. 2015;14(12 suppl 2): abstract A92.
- Kato Y, Bao X, Macgrath S, et al. Lenvatinib mesilate (LEN) enhanced antitumor activity of a PD-1 blockade agent by potentiating Th1 immune response. Ann Oncol. 2016;27(suppl 6): abstract 2PD.
- Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
- Lenvima (lenvatinib). Package insert. Woodcliff Lake, NJ: Eisai; 2019.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Carbon black, titanium dioxide, and talc. IARC Monogr Eval Carcinog Risks Hum. 2010;93:1-413.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, metals, fibres, and dusts. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt C):11-465.
- Erickson BK, Conner MG, Landen CN Jr. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209:409-414.
- Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459-1467.
- Terry KL, Karageorgi S, Shvetsov YB, et al; Ovarian Cancer Association Consortium. Genital powder use and risk of ovarian cancer: a pooled analysis of 8,525 cases and 9,859 controls. Cancer Prev Res. 2013;6:811-821.
- Penninkilampi R, Eslick GD. Perineal talc use and ovarian cancer: a systematic review and meta-analysis. Epidemiology. 2018;29:41-49.
- Hsu T. Johnson & Johnson told to pay $4.7 billion in baby powder lawsuit. New York Times. July 12, 2018. Accessed February 18, 2021. https://www.nytimes.com/2018/07/12 /business/johnson-johnson-talcum-powder.html.
- McGinley L. Does talcum powder cause ovarian cancer? Washington Post. August 25, 2017. Accessed February 18, 2021. https://www.washingtonpost.com/news/to-your -health/wp/2017/08/23/does-talcum-powder-cause -ovarian-cancer-experts-are-divided/.
- O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-1056.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12-19.
- Ault KA; Future II Study Group. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861-1868.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928-1943.
- Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against highgrade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693-1702.
- Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5(5):CD009069.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04- adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301-314.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915-1927.
- Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340-1348.
- American Cancer Society. Survival rates for endometrial cancer. https://www.cancer.org/cancer/endometrial-cancer/ detection-diagnosis-staging/survival-rates.html. Accessed February 9, 2021.
- Miller D, Filiaci V, Fleming G, et al. Late-breaking abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:771.
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: uterine neoplasms. Version 3.2019. https://www.nccn.org/professionals/physician_gls/pdf /uterine.pdf. Accessed February 9, 2021.
- Marcus L, Lemery SJ, Keegan P, et al. FDA approval summary: pembrolizumab for the treatment of microsatellite instabilityhigh solid tumors. Clin Cancer Res. 2019;25:3753-3758.
- Arora E, Masab M, Mittar P, et al. Role of immune checkpoint inhibitors in advanced or recurrent endometrial cancer. Cureus. 2018;10:e2521.
- Keytruda (pembrolizumab). Package insert. Merck Sharp & Dohme; 2018.
- Cancer Genome Atlas Research Network; Kandoth C, Schultz N, Cherniak AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67-73.
- Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664-671.
- Okamoto K, Kodama K, Takase K, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340:97-103.
- Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (E7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014: 638747.
- Vergote I, Teneriello M, Powell MA, et al. A phase II trial of lenvatinib in patients with advanced or recurrent endometrial cancer: angiopoietin-2 as a predictive marker for clinical outcomes. J Clin Oncol. 2013;31(15 suppl): abstract 5520.
- Kimura T, Kato Y, Ozawa Y, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109:3993-4002.
- Kato Y, Tabata K, Hori Y, et al. Effects of lenvatinib on tumorassociated macrophages enhance antitumor activity of PD-1 signal inhibitors. Mol Cancer Ther. 2015;14(12 suppl 2): abstract A92.
- Kato Y, Bao X, Macgrath S, et al. Lenvatinib mesilate (LEN) enhanced antitumor activity of a PD-1 blockade agent by potentiating Th1 immune response. Ann Oncol. 2016;27(suppl 6): abstract 2PD.
- Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
- Lenvima (lenvatinib). Package insert. Woodcliff Lake, NJ: Eisai; 2019.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Carbon black, titanium dioxide, and talc. IARC Monogr Eval Carcinog Risks Hum. 2010;93:1-413.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Arsenic, metals, fibres, and dusts. IARC Monogr Eval Carcinog Risks Hum. 2012;100(pt C):11-465.
- Erickson BK, Conner MG, Landen CN Jr. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209:409-414.
- Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91:1459-1467.
- Terry KL, Karageorgi S, Shvetsov YB, et al; Ovarian Cancer Association Consortium. Genital powder use and risk of ovarian cancer: a pooled analysis of 8,525 cases and 9,859 controls. Cancer Prev Res. 2013;6:811-821.
- Penninkilampi R, Eslick GD. Perineal talc use and ovarian cancer: a systematic review and meta-analysis. Epidemiology. 2018;29:41-49.
- Hsu T. Johnson & Johnson told to pay $4.7 billion in baby powder lawsuit. New York Times. July 12, 2018. Accessed February 18, 2021. https://www.nytimes.com/2018/07/12 /business/johnson-johnson-talcum-powder.html.
- McGinley L. Does talcum powder cause ovarian cancer? Washington Post. August 25, 2017. Accessed February 18, 2021. https://www.washingtonpost.com/news/to-your -health/wp/2017/08/23/does-talcum-powder-cause -ovarian-cancer-experts-are-divided/.
- O’Brien KM, Tworoger SS, Harris HR, et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA. 2020;323:49-59.
2020 Update on gynecologic cancer
Over the past year, major strides have been made in the treatment of gynecologic malignancies. In this Update, we highlight 3 notable studies. The first is a phase 3, multicenter, international, randomized clinical trial that demonstrated a significant improvement in both overall and failure-free survival with the use of adjuvant chemoradiation versus radiotherapy alone in patients with stage III or high-risk uterine cancer. Additionally, we describe the results of 2 phase 3, multicenter, international, randomized clinical trials in ovarian cancer treatment: use of poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitors in combination with platinum and taxane-based chemotherapy followed by the PARP inhibitor as maintenance therapy, and secondary cytoreductive surgery in platinum-sensitive, recurrent ovarian cancer.
We provide a brief overview of current treatment strategies, summarize the key findings of these trials, and establish how these findings have changed our management of these gynecologic malignancies.
Adjuvant chemotherapy and radiotherapy improves survival in women with high-risk endometrial cancer
de Boer SM, Powell ME, Mileshkin L, et al; on behalf of the PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;1273-1285.
In the United States, it is estimated that more than 61,000 women were diagnosed with endometrial cancer in 2019.1 Women with endometrial cancer usually have a favorable prognosis; more than 65% are diagnosed with early-stage disease, which is associated with a 95% 5-year survival rate.1 However, 15% to 20% of patients have disease with high-risk features, including advanced stage (stage II-IV), high tumor grade, lymphovascular space invasion, deep myometrial invasion, or nonendometrioid histologic subtypes (serous or clear cell).2 The presence of these high-risk disease features is associated with an increased incidence of distant metastases and cancer-related death.
Adjuvant therapy in high-risk endometrial cancer
To date, the optimal adjuvant therapy for patients with high-risk endometrial cancer remains controversial. Prior data from Gynecologic Oncology Group (GOG) protocol 122 demonstrated that chemotherapy significantly improved progression-free survival and overall survival when compared with radiotherapy in patients with advanced-stage endometrial cancer.3 As such, chemotherapy now is frequently used in this population, often in combination with radiation, although data describing the benefit of chemoradiation are limited.4 For women with earlier-stage disease with high-risk features, the value of chemotherapy plus radiation is uncertain.5,6
Continue to: Benefit observed with adjuvant chemoradiotherapy...
Benefit observed with adjuvant chemoradiotherapy
In a multicenter, international, randomized phase 3 trial, known as the PORTEC-3 trial, de Boer and colleagues sought to determine if combined adjuvant chemoradiation improved overall survival (OS) and failure-free survival when compared with external-beam radiation therapy (EBRT) alone in the treatment of women with high-risk endometrial cancer.7 Women were eligible for the study if they had histologically confirmed stage I, grade 3 endometrioid endometrial cancer with deep invasion and/or lymphovascular space invasion, stage II or III disease, or stage I-III disease with serous or clear cell histology.
Participants were randomly assigned in a 1:1 ratio; 330 women received adjuvant EBRT alone (total dose of 48.6 Gy administered in 27 fractions), and 330 received adjuvant chemotherapy during and after radiation therapy (CTRT) (2 cycles of cisplatin 50 mg/m2 IV given on days 1 and 22 of EBRT followed by 4 cycles of carboplatin AUC 5 and paclitaxel 175 mg/m2 IV every 3 weeks).
At a median follow-up of 73 months, treatment with adjuvant CTRT, compared with adjuvant EBRT alone, was associated with a significant improvement in both overall survival (5-year OS: 81.4% vs 76.1%, P = .034 [FIGURE]) and failure-free survival (5-year failure-free survival: 76.5% vs 69.1%, P = .016).

The greatest absolute benefit of adjuvant CTRT, compared with EBRT alone, in survival was among women with stage III endometrial cancer (5-year OS: 78.5% vs 68.5%, P = .043) or serous cancers (19% absolute improvement in 5-year OS), or both. Significant differences in 5-year OS and failure-free survival in women with stage I-II cancer were not observed with adjuvant CTRT when compared with adjuvant EBRT alone. At 5 years, significantly more adverse events of grade 2 or worse were reported in the adjuvant CTRT arm.
Results from similar trials
Since the publication of results from the updated analysis of PORTEC-3, results from 2 pertinent trials have been published.8,9 In the GOG 249 trial, women with stage I-II endometrial cancer with high-risk features were randomly assigned to receive 3 cycles of carboplatin-paclitaxel chemotherapy with vaginal brachytherapy or EBRT.8 There was no difference in survival, but a significant increase in both pelvic and para-aortic recurrences were seen after the combination of chemotherapy and vaginal brachytherapy.8
In GOG 258, women with stage III-IVA endometrial cancer were randomly assigned to receive chemotherapy alone (carboplatin-paclitaxel) or adjuvant chemotherapy after EBRT.9 No differences in recurrence-free or overall survival were noted, but there was a significant increase in the number of vaginal and pelvic or para-aortic recurrences in patients in the chemotherapy-only arm.9
The conflicting data regarding the ideal adjuvant therapy for endometrial cancer suggests that treatment decisions should be individualized. Pelvic EBRT with concurrent adjuvant chemotherapy should be considered in women with stage III endometrial cancer or serous cancers as combination therapy improves survival, although dual modality treatment is associated with increased toxicity. Chemoradiation appears to have less benefit for women with stage I–II cancers with other pathologic risk factors.
Role for PARP inhibitor plus first-line chemotherapy, and as maintenance therapy, in ovarian cancer treatment
Coleman RL, Fleming GF, Brady MF, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
Ovarian cancer is the leading cause of gynecologic cancer-related deaths among women in the United States.10 Treatment consists of cytoreductive surgery combined with platinum and taxane-based chemotherapy.11 Despite favorable initial responses, more than 80% of patients experience a recurrence, with an 18-month median time to progression.12 As a result, recent efforts have focused on finding novel therapeutic approaches to improve treatment outcomes and mitigate the risk of disease recurrence.
Continue to: PARP inhibitors are changing the face of treatment...
PARP inhibitors are changing the face of treatment
Poly(adenosine diphosphate-ribose) polymerases (PARPs) are a family of enzymes that play a critical role in DNA damage repair. These enzymes promote DNA repair by recruiting proteins involved in repairing single-strand and double-strand DNA breaks and in protecting and restarting stalled DNA replication forks.13 The predominant mechanisms of action of PARP inhibitors in cells with homologous-recombination deficiency (HRD) include inhibiting repair of single-strand DNA breaks and trapping PARP-DNA complexes at stalled DNA replication forks.14
Germline or somatic BRCA1/2 mutations and genetic alterations resulting in HRD are present in about 20% and 30% of ovarian carcinomas, respectively, and increase the susceptibility of tumors to platinum-based agents and PARP inhibitors.15,16 Based on multiple clinical trials that demonstrated the efficacy of single-agent PARP in the treatment of recurrent ovarian carcinoma and as maintenance therapy after an initial response to platinum-based therapy, the US Food and Drug Administration approved olaparib, niraparib, and rucaparib for the treatment of high-grade epithelial ovarian cancer.17-19 Only olaparib is approved for maintenance therapy after initial adjuvant therapy in patients with BRCA mutations.20
Given the robust response to PARP inhibitors, there has been great interest in using these agents earlier in the disease course in combination with chemotherapy.
Efficacy of veliparib with chemotherapy and as maintenance monotherapy
In a randomized, double-blind, placebo-controlled phase 3 trial, Coleman and colleagues sought to determine the efficacy of the PARP inhibitor veliparib when administered with first-line carboplatin and paclitaxel induction chemotherapy and subsequently continued as maintenance monotherapy.21
Women with stage III or IV high-grade epithelial ovarian, fallopian tube, or primary peritoneal carcinoma were eligible for the study. Cytoreductive surgery could be performed prior to the initiation of trial treatment or after 3 cycles of chemotherapy.
Participants were randomized in a 1:1:1 ratio: 371 women received carboplatin and paclitaxel plus placebo followed by placebo maintenance (control arm); 376 received chemotherapy plus veliparib followed by placebo maintenance (veliparib combination-only arm); and 377 received chemotherapy plus veliparib followed by veliparib maintenance therapy (veliparib-throughout arm). Combination chemotherapy consisted of 6 cycles, and maintenance therapy was an additional 30 cycles.
Progression-free survival extended
At a median follow-up of 28 months, investigators observed a significant improvement in progression-free survival in the veliparib-throughout (initial and maintenance therapy) arm compared with the control arm in 3 cohorts: the BRCA-mutation cohort, the HRD cohort, and the intention-to-treat population (all participants undergoing randomization).
In the BRCA-mutation cohort, the median progression-free survival was 12.7 months longer in the veliparib-throughout arm than in the control arm. Similarly, in the HRD cohort, the median progression-free survival was 11.4 months longer in the veliparib-throughout arm than in the control group. In the intention-to-treat population, the median progression-free survival increased from 17.3 to 23.5 months in the veliparib-throughout arm compared with the control arm.
Women who received veliparib experienced increased rates of nausea, anemia, and fatigue and were more likely to require dose reductions and treatment interruptions. Myelodysplastic syndrome was reported in 1 patient (BRCA1 positive) in the veliparib combination-only arm.
For women with newly diagnosed, previously untreated stage III or IV high-grade serous ovarian carcinoma, carboplatin, paclitaxel, and veliparib induction therapy followed by single-agent veliparib maintenance therapy resulted in a significant improvement in median progression-free survival compared with induction chemotherapy alone. However, veliparib use was also associated with a higher incidence of adverse effects that required dose reduction and/or interruption during both the combination and maintenance phases of treatment.
Secondary cytoreductive surgery or chemotherapy alone for platinum-sensitive recurrent ovarian carcinoma?
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
Primary surgical cytoreduction combined with platinum and taxane-based chemotherapy remains the mainstay of ovarian cancer treatment.11 The role of surgery for women with recurrent ovarian cancer, so-called secondary cytoreduction, remains controversial.22
Data have shown that among women who undergo secondary surgery, those with little or no postoperative residual disease benefit the most from a secondary debulking.23-26 Prior work largely is based on small retrospective reports and is limited by substantial bias in the selection of patients undergoing surgery. Additionally, with the availability of targeted therapies such as bevacizumab and PARP inhibitors as maintenance—medical interventions with a demonstrated benefit in progression-free survival17-19,27—the role of secondary cytoreduction in the treatment of ovarian carcinoma needs to be clarified.
Continue to: Overall survival after secondary cytoreduction followed by chemotherapy...
Overall survival after secondary cytoreduction followed by chemotherapy
Coleman and colleagues conducted a prospective, multicenter, international, randomized phase 3 trial to assess whether secondary cytoreductive surgery followed by chemotherapy would improve overall survival versus chemotherapy alone among women with resectable platinum-sensitive, recurrent ovarian cancer.22 Platinum sensitivity was defined as a disease-free interval of at least 6 months after the last cycle of platinum-based chemotherapy.
All women had recurrent epithelial ovarian carcinoma considered to be amenable to complete gross surgical resection by the investigator and a history of complete response to at least 3 cycles of platinum-based chemotherapy as determined by a normal CA-125 value or negative imaging studies (if obtained).
Participants were randomly assigned 1:1, with 240 women assigned to secondary surgical cytoreduction followed by platinum-based chemotherapy, and 245 assigned to chemotherapy alone. The type of adjuvant chemotherapy used (carboplatin-paclitaxel or carboplatin-gemcitabine) and whether or not bevacizumab was administered were at the investigators' discretion.
Shorter survival, decline in quality of life
Among the participants assigned to and who underwent surgery, complete gross resection was achieved in 67%. Eighty-four percent of the entire study population received platinum-based chemotherapy with bevacizumab followed by bevacizumab maintenance therapy, which was equally distributed between the 2 study arms.
At a median follow-up of 48.1 months, median overall survival was 50.6 months in the surgery arm compared with 64.7 months in the chemotherapy arm, corresponding to a hazard ratio (HR) for death of 1.29 (95% confidence interval [CI], 0.97-1.72; P = .08). This effect was unchanged after adjusting for platinum-free interval, chemotherapy choice, and restricting the analysis to women who had a complete gross resection.
Similarly, the adjusted HR for disease progression or death was 0.82 (95% CI, 0.66-1.01) and corresponded to a median progression-free survival of 18.9 months for the surgery group and 16.2 months for the chemotherapy group. Surgical morbidity was reported in 9% of patients who underwent surgery, and 1 patient (0.4%) died from postoperative complications.
While a significant decline in both quality of life and patient-reported outcomes was reported immediately after surgery, significant differences were not noted between the 2 groups after the initial postoperative recovery period.
For women with platinum-sensitive, recurrent ovarian cancer, a secondary cytoreductive surgery followed by chemotherapy was not associated with an improvement in overall survival when compared with chemotherapy alone. Secondary cytoreductive surgery should not be used routinely in women with recurrent ovarian cancer.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;20:7-34.
- Colombo N, Creutzberg C, Amant F, et al; ESMO-ESGOESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27:16-41.
- Randall ME, Filiaci VL, Muss H, et al; Gynecologic Oncology Group Study. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2006;24:36-44.
- Syeda S, Chen L, Hou JY, et al. Chemotherapy, radiation, or combination therapy for stage III uterine cancer. Obstet Gynecol. 2019;134:17-29.
- Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266-271.
- Susumu N, Sagae S, Udagawa Y, et al; Japanese Gynecologic Oncology Group. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226-233.
- de Boer SM, Powell ME, Mileshkin L, et al; PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20:1273-1285.
- Randall ME, Filiaci V, McMeekin DS, et al. Phase III trial: adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early stage endometrial cancer. J Clin Oncol. 2019;37:1810-1818.
- Matei D, Filiaci V, Randall ME, et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med. 2019;380:2317-2326.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. NCCN guidelines insights: ovarian cancer, version 1.2019. J Natl Compr Canc Netw. 2019;17:896-909.
- Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi24-vi32.
- Moore KN, Mirza MR, Matulonis UA. The poly (ADP ribose) polymerase inhibitor niraparib: management of toxicities. Gynecol Oncol. 2018;149:214-220.
- Konstantinopoulos PA, Matulonis UA. PARP inhibitors in ovarian cancer: a trailblazing and transformative journey. Clin Cancer Res. 2018;24:4062-4065.
- Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764-775.
- Mukhopadhyay A, Plummer ER, Elattar A, et al. Clinicopathological features of homologous recombination-deficient epithelial ovarian cancers: sensitivity to PARP inhibitors, platinum, and survival. Cancer Res. 2012;72:5675-5682.
- Mirza MR, Monk BJ, Herrstedt J, et al; ENGOT-OV16/ NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-2164.
- Pujade-Lauraine E, Ledermann JA, Selle F, et al; SOLO2/ ENGOT-Ov21 Investigators. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274-1284.
- Coleman RL, Oza AM, Lorusso D, et al; ARIEL3 Investigators. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949-1961.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Coleman RL, Fleming GF, Brady MF, et al. Veliparib with firstline chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Bommert M, Harter P, Heitz F, et al. When should surgery be used for recurrent ovarian carcinoma? Clin Oncol (R Coll Radiol). 2018;30:493-497.
- Santillan A, Karam AK, Li AJ, et al. Secondary cytoreductive surgery for isolated nodal recurrence in patients with epithelial ovarian cancer. Gynecol Oncol. 2007;104:686-690.
- Zang RY, Harter P, Chi DS, et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br J Cancer. 2011;105:890-896.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
- Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30: 2039-2045.
Over the past year, major strides have been made in the treatment of gynecologic malignancies. In this Update, we highlight 3 notable studies. The first is a phase 3, multicenter, international, randomized clinical trial that demonstrated a significant improvement in both overall and failure-free survival with the use of adjuvant chemoradiation versus radiotherapy alone in patients with stage III or high-risk uterine cancer. Additionally, we describe the results of 2 phase 3, multicenter, international, randomized clinical trials in ovarian cancer treatment: use of poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitors in combination with platinum and taxane-based chemotherapy followed by the PARP inhibitor as maintenance therapy, and secondary cytoreductive surgery in platinum-sensitive, recurrent ovarian cancer.
We provide a brief overview of current treatment strategies, summarize the key findings of these trials, and establish how these findings have changed our management of these gynecologic malignancies.
Adjuvant chemotherapy and radiotherapy improves survival in women with high-risk endometrial cancer
de Boer SM, Powell ME, Mileshkin L, et al; on behalf of the PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;1273-1285.
In the United States, it is estimated that more than 61,000 women were diagnosed with endometrial cancer in 2019.1 Women with endometrial cancer usually have a favorable prognosis; more than 65% are diagnosed with early-stage disease, which is associated with a 95% 5-year survival rate.1 However, 15% to 20% of patients have disease with high-risk features, including advanced stage (stage II-IV), high tumor grade, lymphovascular space invasion, deep myometrial invasion, or nonendometrioid histologic subtypes (serous or clear cell).2 The presence of these high-risk disease features is associated with an increased incidence of distant metastases and cancer-related death.
Adjuvant therapy in high-risk endometrial cancer
To date, the optimal adjuvant therapy for patients with high-risk endometrial cancer remains controversial. Prior data from Gynecologic Oncology Group (GOG) protocol 122 demonstrated that chemotherapy significantly improved progression-free survival and overall survival when compared with radiotherapy in patients with advanced-stage endometrial cancer.3 As such, chemotherapy now is frequently used in this population, often in combination with radiation, although data describing the benefit of chemoradiation are limited.4 For women with earlier-stage disease with high-risk features, the value of chemotherapy plus radiation is uncertain.5,6
Continue to: Benefit observed with adjuvant chemoradiotherapy...
Benefit observed with adjuvant chemoradiotherapy
In a multicenter, international, randomized phase 3 trial, known as the PORTEC-3 trial, de Boer and colleagues sought to determine if combined adjuvant chemoradiation improved overall survival (OS) and failure-free survival when compared with external-beam radiation therapy (EBRT) alone in the treatment of women with high-risk endometrial cancer.7 Women were eligible for the study if they had histologically confirmed stage I, grade 3 endometrioid endometrial cancer with deep invasion and/or lymphovascular space invasion, stage II or III disease, or stage I-III disease with serous or clear cell histology.
Participants were randomly assigned in a 1:1 ratio; 330 women received adjuvant EBRT alone (total dose of 48.6 Gy administered in 27 fractions), and 330 received adjuvant chemotherapy during and after radiation therapy (CTRT) (2 cycles of cisplatin 50 mg/m2 IV given on days 1 and 22 of EBRT followed by 4 cycles of carboplatin AUC 5 and paclitaxel 175 mg/m2 IV every 3 weeks).
At a median follow-up of 73 months, treatment with adjuvant CTRT, compared with adjuvant EBRT alone, was associated with a significant improvement in both overall survival (5-year OS: 81.4% vs 76.1%, P = .034 [FIGURE]) and failure-free survival (5-year failure-free survival: 76.5% vs 69.1%, P = .016).

The greatest absolute benefit of adjuvant CTRT, compared with EBRT alone, in survival was among women with stage III endometrial cancer (5-year OS: 78.5% vs 68.5%, P = .043) or serous cancers (19% absolute improvement in 5-year OS), or both. Significant differences in 5-year OS and failure-free survival in women with stage I-II cancer were not observed with adjuvant CTRT when compared with adjuvant EBRT alone. At 5 years, significantly more adverse events of grade 2 or worse were reported in the adjuvant CTRT arm.
Results from similar trials
Since the publication of results from the updated analysis of PORTEC-3, results from 2 pertinent trials have been published.8,9 In the GOG 249 trial, women with stage I-II endometrial cancer with high-risk features were randomly assigned to receive 3 cycles of carboplatin-paclitaxel chemotherapy with vaginal brachytherapy or EBRT.8 There was no difference in survival, but a significant increase in both pelvic and para-aortic recurrences were seen after the combination of chemotherapy and vaginal brachytherapy.8
In GOG 258, women with stage III-IVA endometrial cancer were randomly assigned to receive chemotherapy alone (carboplatin-paclitaxel) or adjuvant chemotherapy after EBRT.9 No differences in recurrence-free or overall survival were noted, but there was a significant increase in the number of vaginal and pelvic or para-aortic recurrences in patients in the chemotherapy-only arm.9
The conflicting data regarding the ideal adjuvant therapy for endometrial cancer suggests that treatment decisions should be individualized. Pelvic EBRT with concurrent adjuvant chemotherapy should be considered in women with stage III endometrial cancer or serous cancers as combination therapy improves survival, although dual modality treatment is associated with increased toxicity. Chemoradiation appears to have less benefit for women with stage I–II cancers with other pathologic risk factors.
Role for PARP inhibitor plus first-line chemotherapy, and as maintenance therapy, in ovarian cancer treatment
Coleman RL, Fleming GF, Brady MF, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
Ovarian cancer is the leading cause of gynecologic cancer-related deaths among women in the United States.10 Treatment consists of cytoreductive surgery combined with platinum and taxane-based chemotherapy.11 Despite favorable initial responses, more than 80% of patients experience a recurrence, with an 18-month median time to progression.12 As a result, recent efforts have focused on finding novel therapeutic approaches to improve treatment outcomes and mitigate the risk of disease recurrence.
Continue to: PARP inhibitors are changing the face of treatment...
PARP inhibitors are changing the face of treatment
Poly(adenosine diphosphate-ribose) polymerases (PARPs) are a family of enzymes that play a critical role in DNA damage repair. These enzymes promote DNA repair by recruiting proteins involved in repairing single-strand and double-strand DNA breaks and in protecting and restarting stalled DNA replication forks.13 The predominant mechanisms of action of PARP inhibitors in cells with homologous-recombination deficiency (HRD) include inhibiting repair of single-strand DNA breaks and trapping PARP-DNA complexes at stalled DNA replication forks.14
Germline or somatic BRCA1/2 mutations and genetic alterations resulting in HRD are present in about 20% and 30% of ovarian carcinomas, respectively, and increase the susceptibility of tumors to platinum-based agents and PARP inhibitors.15,16 Based on multiple clinical trials that demonstrated the efficacy of single-agent PARP in the treatment of recurrent ovarian carcinoma and as maintenance therapy after an initial response to platinum-based therapy, the US Food and Drug Administration approved olaparib, niraparib, and rucaparib for the treatment of high-grade epithelial ovarian cancer.17-19 Only olaparib is approved for maintenance therapy after initial adjuvant therapy in patients with BRCA mutations.20
Given the robust response to PARP inhibitors, there has been great interest in using these agents earlier in the disease course in combination with chemotherapy.
Efficacy of veliparib with chemotherapy and as maintenance monotherapy
In a randomized, double-blind, placebo-controlled phase 3 trial, Coleman and colleagues sought to determine the efficacy of the PARP inhibitor veliparib when administered with first-line carboplatin and paclitaxel induction chemotherapy and subsequently continued as maintenance monotherapy.21
Women with stage III or IV high-grade epithelial ovarian, fallopian tube, or primary peritoneal carcinoma were eligible for the study. Cytoreductive surgery could be performed prior to the initiation of trial treatment or after 3 cycles of chemotherapy.
Participants were randomized in a 1:1:1 ratio: 371 women received carboplatin and paclitaxel plus placebo followed by placebo maintenance (control arm); 376 received chemotherapy plus veliparib followed by placebo maintenance (veliparib combination-only arm); and 377 received chemotherapy plus veliparib followed by veliparib maintenance therapy (veliparib-throughout arm). Combination chemotherapy consisted of 6 cycles, and maintenance therapy was an additional 30 cycles.
Progression-free survival extended
At a median follow-up of 28 months, investigators observed a significant improvement in progression-free survival in the veliparib-throughout (initial and maintenance therapy) arm compared with the control arm in 3 cohorts: the BRCA-mutation cohort, the HRD cohort, and the intention-to-treat population (all participants undergoing randomization).
In the BRCA-mutation cohort, the median progression-free survival was 12.7 months longer in the veliparib-throughout arm than in the control arm. Similarly, in the HRD cohort, the median progression-free survival was 11.4 months longer in the veliparib-throughout arm than in the control group. In the intention-to-treat population, the median progression-free survival increased from 17.3 to 23.5 months in the veliparib-throughout arm compared with the control arm.
Women who received veliparib experienced increased rates of nausea, anemia, and fatigue and were more likely to require dose reductions and treatment interruptions. Myelodysplastic syndrome was reported in 1 patient (BRCA1 positive) in the veliparib combination-only arm.
For women with newly diagnosed, previously untreated stage III or IV high-grade serous ovarian carcinoma, carboplatin, paclitaxel, and veliparib induction therapy followed by single-agent veliparib maintenance therapy resulted in a significant improvement in median progression-free survival compared with induction chemotherapy alone. However, veliparib use was also associated with a higher incidence of adverse effects that required dose reduction and/or interruption during both the combination and maintenance phases of treatment.
Secondary cytoreductive surgery or chemotherapy alone for platinum-sensitive recurrent ovarian carcinoma?
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
Primary surgical cytoreduction combined with platinum and taxane-based chemotherapy remains the mainstay of ovarian cancer treatment.11 The role of surgery for women with recurrent ovarian cancer, so-called secondary cytoreduction, remains controversial.22
Data have shown that among women who undergo secondary surgery, those with little or no postoperative residual disease benefit the most from a secondary debulking.23-26 Prior work largely is based on small retrospective reports and is limited by substantial bias in the selection of patients undergoing surgery. Additionally, with the availability of targeted therapies such as bevacizumab and PARP inhibitors as maintenance—medical interventions with a demonstrated benefit in progression-free survival17-19,27—the role of secondary cytoreduction in the treatment of ovarian carcinoma needs to be clarified.
Continue to: Overall survival after secondary cytoreduction followed by chemotherapy...
Overall survival after secondary cytoreduction followed by chemotherapy
Coleman and colleagues conducted a prospective, multicenter, international, randomized phase 3 trial to assess whether secondary cytoreductive surgery followed by chemotherapy would improve overall survival versus chemotherapy alone among women with resectable platinum-sensitive, recurrent ovarian cancer.22 Platinum sensitivity was defined as a disease-free interval of at least 6 months after the last cycle of platinum-based chemotherapy.
All women had recurrent epithelial ovarian carcinoma considered to be amenable to complete gross surgical resection by the investigator and a history of complete response to at least 3 cycles of platinum-based chemotherapy as determined by a normal CA-125 value or negative imaging studies (if obtained).
Participants were randomly assigned 1:1, with 240 women assigned to secondary surgical cytoreduction followed by platinum-based chemotherapy, and 245 assigned to chemotherapy alone. The type of adjuvant chemotherapy used (carboplatin-paclitaxel or carboplatin-gemcitabine) and whether or not bevacizumab was administered were at the investigators' discretion.
Shorter survival, decline in quality of life
Among the participants assigned to and who underwent surgery, complete gross resection was achieved in 67%. Eighty-four percent of the entire study population received platinum-based chemotherapy with bevacizumab followed by bevacizumab maintenance therapy, which was equally distributed between the 2 study arms.
At a median follow-up of 48.1 months, median overall survival was 50.6 months in the surgery arm compared with 64.7 months in the chemotherapy arm, corresponding to a hazard ratio (HR) for death of 1.29 (95% confidence interval [CI], 0.97-1.72; P = .08). This effect was unchanged after adjusting for platinum-free interval, chemotherapy choice, and restricting the analysis to women who had a complete gross resection.
Similarly, the adjusted HR for disease progression or death was 0.82 (95% CI, 0.66-1.01) and corresponded to a median progression-free survival of 18.9 months for the surgery group and 16.2 months for the chemotherapy group. Surgical morbidity was reported in 9% of patients who underwent surgery, and 1 patient (0.4%) died from postoperative complications.
While a significant decline in both quality of life and patient-reported outcomes was reported immediately after surgery, significant differences were not noted between the 2 groups after the initial postoperative recovery period.
For women with platinum-sensitive, recurrent ovarian cancer, a secondary cytoreductive surgery followed by chemotherapy was not associated with an improvement in overall survival when compared with chemotherapy alone. Secondary cytoreductive surgery should not be used routinely in women with recurrent ovarian cancer.
Over the past year, major strides have been made in the treatment of gynecologic malignancies. In this Update, we highlight 3 notable studies. The first is a phase 3, multicenter, international, randomized clinical trial that demonstrated a significant improvement in both overall and failure-free survival with the use of adjuvant chemoradiation versus radiotherapy alone in patients with stage III or high-risk uterine cancer. Additionally, we describe the results of 2 phase 3, multicenter, international, randomized clinical trials in ovarian cancer treatment: use of poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitors in combination with platinum and taxane-based chemotherapy followed by the PARP inhibitor as maintenance therapy, and secondary cytoreductive surgery in platinum-sensitive, recurrent ovarian cancer.
We provide a brief overview of current treatment strategies, summarize the key findings of these trials, and establish how these findings have changed our management of these gynecologic malignancies.
Adjuvant chemotherapy and radiotherapy improves survival in women with high-risk endometrial cancer
de Boer SM, Powell ME, Mileshkin L, et al; on behalf of the PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;1273-1285.
In the United States, it is estimated that more than 61,000 women were diagnosed with endometrial cancer in 2019.1 Women with endometrial cancer usually have a favorable prognosis; more than 65% are diagnosed with early-stage disease, which is associated with a 95% 5-year survival rate.1 However, 15% to 20% of patients have disease with high-risk features, including advanced stage (stage II-IV), high tumor grade, lymphovascular space invasion, deep myometrial invasion, or nonendometrioid histologic subtypes (serous or clear cell).2 The presence of these high-risk disease features is associated with an increased incidence of distant metastases and cancer-related death.
Adjuvant therapy in high-risk endometrial cancer
To date, the optimal adjuvant therapy for patients with high-risk endometrial cancer remains controversial. Prior data from Gynecologic Oncology Group (GOG) protocol 122 demonstrated that chemotherapy significantly improved progression-free survival and overall survival when compared with radiotherapy in patients with advanced-stage endometrial cancer.3 As such, chemotherapy now is frequently used in this population, often in combination with radiation, although data describing the benefit of chemoradiation are limited.4 For women with earlier-stage disease with high-risk features, the value of chemotherapy plus radiation is uncertain.5,6
Continue to: Benefit observed with adjuvant chemoradiotherapy...
Benefit observed with adjuvant chemoradiotherapy
In a multicenter, international, randomized phase 3 trial, known as the PORTEC-3 trial, de Boer and colleagues sought to determine if combined adjuvant chemoradiation improved overall survival (OS) and failure-free survival when compared with external-beam radiation therapy (EBRT) alone in the treatment of women with high-risk endometrial cancer.7 Women were eligible for the study if they had histologically confirmed stage I, grade 3 endometrioid endometrial cancer with deep invasion and/or lymphovascular space invasion, stage II or III disease, or stage I-III disease with serous or clear cell histology.
Participants were randomly assigned in a 1:1 ratio; 330 women received adjuvant EBRT alone (total dose of 48.6 Gy administered in 27 fractions), and 330 received adjuvant chemotherapy during and after radiation therapy (CTRT) (2 cycles of cisplatin 50 mg/m2 IV given on days 1 and 22 of EBRT followed by 4 cycles of carboplatin AUC 5 and paclitaxel 175 mg/m2 IV every 3 weeks).
At a median follow-up of 73 months, treatment with adjuvant CTRT, compared with adjuvant EBRT alone, was associated with a significant improvement in both overall survival (5-year OS: 81.4% vs 76.1%, P = .034 [FIGURE]) and failure-free survival (5-year failure-free survival: 76.5% vs 69.1%, P = .016).

The greatest absolute benefit of adjuvant CTRT, compared with EBRT alone, in survival was among women with stage III endometrial cancer (5-year OS: 78.5% vs 68.5%, P = .043) or serous cancers (19% absolute improvement in 5-year OS), or both. Significant differences in 5-year OS and failure-free survival in women with stage I-II cancer were not observed with adjuvant CTRT when compared with adjuvant EBRT alone. At 5 years, significantly more adverse events of grade 2 or worse were reported in the adjuvant CTRT arm.
Results from similar trials
Since the publication of results from the updated analysis of PORTEC-3, results from 2 pertinent trials have been published.8,9 In the GOG 249 trial, women with stage I-II endometrial cancer with high-risk features were randomly assigned to receive 3 cycles of carboplatin-paclitaxel chemotherapy with vaginal brachytherapy or EBRT.8 There was no difference in survival, but a significant increase in both pelvic and para-aortic recurrences were seen after the combination of chemotherapy and vaginal brachytherapy.8
In GOG 258, women with stage III-IVA endometrial cancer were randomly assigned to receive chemotherapy alone (carboplatin-paclitaxel) or adjuvant chemotherapy after EBRT.9 No differences in recurrence-free or overall survival were noted, but there was a significant increase in the number of vaginal and pelvic or para-aortic recurrences in patients in the chemotherapy-only arm.9
The conflicting data regarding the ideal adjuvant therapy for endometrial cancer suggests that treatment decisions should be individualized. Pelvic EBRT with concurrent adjuvant chemotherapy should be considered in women with stage III endometrial cancer or serous cancers as combination therapy improves survival, although dual modality treatment is associated with increased toxicity. Chemoradiation appears to have less benefit for women with stage I–II cancers with other pathologic risk factors.
Role for PARP inhibitor plus first-line chemotherapy, and as maintenance therapy, in ovarian cancer treatment
Coleman RL, Fleming GF, Brady MF, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
Ovarian cancer is the leading cause of gynecologic cancer-related deaths among women in the United States.10 Treatment consists of cytoreductive surgery combined with platinum and taxane-based chemotherapy.11 Despite favorable initial responses, more than 80% of patients experience a recurrence, with an 18-month median time to progression.12 As a result, recent efforts have focused on finding novel therapeutic approaches to improve treatment outcomes and mitigate the risk of disease recurrence.
Continue to: PARP inhibitors are changing the face of treatment...
PARP inhibitors are changing the face of treatment
Poly(adenosine diphosphate-ribose) polymerases (PARPs) are a family of enzymes that play a critical role in DNA damage repair. These enzymes promote DNA repair by recruiting proteins involved in repairing single-strand and double-strand DNA breaks and in protecting and restarting stalled DNA replication forks.13 The predominant mechanisms of action of PARP inhibitors in cells with homologous-recombination deficiency (HRD) include inhibiting repair of single-strand DNA breaks and trapping PARP-DNA complexes at stalled DNA replication forks.14
Germline or somatic BRCA1/2 mutations and genetic alterations resulting in HRD are present in about 20% and 30% of ovarian carcinomas, respectively, and increase the susceptibility of tumors to platinum-based agents and PARP inhibitors.15,16 Based on multiple clinical trials that demonstrated the efficacy of single-agent PARP in the treatment of recurrent ovarian carcinoma and as maintenance therapy after an initial response to platinum-based therapy, the US Food and Drug Administration approved olaparib, niraparib, and rucaparib for the treatment of high-grade epithelial ovarian cancer.17-19 Only olaparib is approved for maintenance therapy after initial adjuvant therapy in patients with BRCA mutations.20
Given the robust response to PARP inhibitors, there has been great interest in using these agents earlier in the disease course in combination with chemotherapy.
Efficacy of veliparib with chemotherapy and as maintenance monotherapy
In a randomized, double-blind, placebo-controlled phase 3 trial, Coleman and colleagues sought to determine the efficacy of the PARP inhibitor veliparib when administered with first-line carboplatin and paclitaxel induction chemotherapy and subsequently continued as maintenance monotherapy.21
Women with stage III or IV high-grade epithelial ovarian, fallopian tube, or primary peritoneal carcinoma were eligible for the study. Cytoreductive surgery could be performed prior to the initiation of trial treatment or after 3 cycles of chemotherapy.
Participants were randomized in a 1:1:1 ratio: 371 women received carboplatin and paclitaxel plus placebo followed by placebo maintenance (control arm); 376 received chemotherapy plus veliparib followed by placebo maintenance (veliparib combination-only arm); and 377 received chemotherapy plus veliparib followed by veliparib maintenance therapy (veliparib-throughout arm). Combination chemotherapy consisted of 6 cycles, and maintenance therapy was an additional 30 cycles.
Progression-free survival extended
At a median follow-up of 28 months, investigators observed a significant improvement in progression-free survival in the veliparib-throughout (initial and maintenance therapy) arm compared with the control arm in 3 cohorts: the BRCA-mutation cohort, the HRD cohort, and the intention-to-treat population (all participants undergoing randomization).
In the BRCA-mutation cohort, the median progression-free survival was 12.7 months longer in the veliparib-throughout arm than in the control arm. Similarly, in the HRD cohort, the median progression-free survival was 11.4 months longer in the veliparib-throughout arm than in the control group. In the intention-to-treat population, the median progression-free survival increased from 17.3 to 23.5 months in the veliparib-throughout arm compared with the control arm.
Women who received veliparib experienced increased rates of nausea, anemia, and fatigue and were more likely to require dose reductions and treatment interruptions. Myelodysplastic syndrome was reported in 1 patient (BRCA1 positive) in the veliparib combination-only arm.
For women with newly diagnosed, previously untreated stage III or IV high-grade serous ovarian carcinoma, carboplatin, paclitaxel, and veliparib induction therapy followed by single-agent veliparib maintenance therapy resulted in a significant improvement in median progression-free survival compared with induction chemotherapy alone. However, veliparib use was also associated with a higher incidence of adverse effects that required dose reduction and/or interruption during both the combination and maintenance phases of treatment.
Secondary cytoreductive surgery or chemotherapy alone for platinum-sensitive recurrent ovarian carcinoma?
Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
Primary surgical cytoreduction combined with platinum and taxane-based chemotherapy remains the mainstay of ovarian cancer treatment.11 The role of surgery for women with recurrent ovarian cancer, so-called secondary cytoreduction, remains controversial.22
Data have shown that among women who undergo secondary surgery, those with little or no postoperative residual disease benefit the most from a secondary debulking.23-26 Prior work largely is based on small retrospective reports and is limited by substantial bias in the selection of patients undergoing surgery. Additionally, with the availability of targeted therapies such as bevacizumab and PARP inhibitors as maintenance—medical interventions with a demonstrated benefit in progression-free survival17-19,27—the role of secondary cytoreduction in the treatment of ovarian carcinoma needs to be clarified.
Continue to: Overall survival after secondary cytoreduction followed by chemotherapy...
Overall survival after secondary cytoreduction followed by chemotherapy
Coleman and colleagues conducted a prospective, multicenter, international, randomized phase 3 trial to assess whether secondary cytoreductive surgery followed by chemotherapy would improve overall survival versus chemotherapy alone among women with resectable platinum-sensitive, recurrent ovarian cancer.22 Platinum sensitivity was defined as a disease-free interval of at least 6 months after the last cycle of platinum-based chemotherapy.
All women had recurrent epithelial ovarian carcinoma considered to be amenable to complete gross surgical resection by the investigator and a history of complete response to at least 3 cycles of platinum-based chemotherapy as determined by a normal CA-125 value or negative imaging studies (if obtained).
Participants were randomly assigned 1:1, with 240 women assigned to secondary surgical cytoreduction followed by platinum-based chemotherapy, and 245 assigned to chemotherapy alone. The type of adjuvant chemotherapy used (carboplatin-paclitaxel or carboplatin-gemcitabine) and whether or not bevacizumab was administered were at the investigators' discretion.
Shorter survival, decline in quality of life
Among the participants assigned to and who underwent surgery, complete gross resection was achieved in 67%. Eighty-four percent of the entire study population received platinum-based chemotherapy with bevacizumab followed by bevacizumab maintenance therapy, which was equally distributed between the 2 study arms.
At a median follow-up of 48.1 months, median overall survival was 50.6 months in the surgery arm compared with 64.7 months in the chemotherapy arm, corresponding to a hazard ratio (HR) for death of 1.29 (95% confidence interval [CI], 0.97-1.72; P = .08). This effect was unchanged after adjusting for platinum-free interval, chemotherapy choice, and restricting the analysis to women who had a complete gross resection.
Similarly, the adjusted HR for disease progression or death was 0.82 (95% CI, 0.66-1.01) and corresponded to a median progression-free survival of 18.9 months for the surgery group and 16.2 months for the chemotherapy group. Surgical morbidity was reported in 9% of patients who underwent surgery, and 1 patient (0.4%) died from postoperative complications.
While a significant decline in both quality of life and patient-reported outcomes was reported immediately after surgery, significant differences were not noted between the 2 groups after the initial postoperative recovery period.
For women with platinum-sensitive, recurrent ovarian cancer, a secondary cytoreductive surgery followed by chemotherapy was not associated with an improvement in overall survival when compared with chemotherapy alone. Secondary cytoreductive surgery should not be used routinely in women with recurrent ovarian cancer.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;20:7-34.
- Colombo N, Creutzberg C, Amant F, et al; ESMO-ESGOESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27:16-41.
- Randall ME, Filiaci VL, Muss H, et al; Gynecologic Oncology Group Study. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2006;24:36-44.
- Syeda S, Chen L, Hou JY, et al. Chemotherapy, radiation, or combination therapy for stage III uterine cancer. Obstet Gynecol. 2019;134:17-29.
- Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266-271.
- Susumu N, Sagae S, Udagawa Y, et al; Japanese Gynecologic Oncology Group. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226-233.
- de Boer SM, Powell ME, Mileshkin L, et al; PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20:1273-1285.
- Randall ME, Filiaci V, McMeekin DS, et al. Phase III trial: adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early stage endometrial cancer. J Clin Oncol. 2019;37:1810-1818.
- Matei D, Filiaci V, Randall ME, et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med. 2019;380:2317-2326.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. NCCN guidelines insights: ovarian cancer, version 1.2019. J Natl Compr Canc Netw. 2019;17:896-909.
- Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi24-vi32.
- Moore KN, Mirza MR, Matulonis UA. The poly (ADP ribose) polymerase inhibitor niraparib: management of toxicities. Gynecol Oncol. 2018;149:214-220.
- Konstantinopoulos PA, Matulonis UA. PARP inhibitors in ovarian cancer: a trailblazing and transformative journey. Clin Cancer Res. 2018;24:4062-4065.
- Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764-775.
- Mukhopadhyay A, Plummer ER, Elattar A, et al. Clinicopathological features of homologous recombination-deficient epithelial ovarian cancers: sensitivity to PARP inhibitors, platinum, and survival. Cancer Res. 2012;72:5675-5682.
- Mirza MR, Monk BJ, Herrstedt J, et al; ENGOT-OV16/ NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-2164.
- Pujade-Lauraine E, Ledermann JA, Selle F, et al; SOLO2/ ENGOT-Ov21 Investigators. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274-1284.
- Coleman RL, Oza AM, Lorusso D, et al; ARIEL3 Investigators. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949-1961.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Coleman RL, Fleming GF, Brady MF, et al. Veliparib with firstline chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Bommert M, Harter P, Heitz F, et al. When should surgery be used for recurrent ovarian carcinoma? Clin Oncol (R Coll Radiol). 2018;30:493-497.
- Santillan A, Karam AK, Li AJ, et al. Secondary cytoreductive surgery for isolated nodal recurrence in patients with epithelial ovarian cancer. Gynecol Oncol. 2007;104:686-690.
- Zang RY, Harter P, Chi DS, et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br J Cancer. 2011;105:890-896.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
- Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30: 2039-2045.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;20:7-34.
- Colombo N, Creutzberg C, Amant F, et al; ESMO-ESGOESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol. 2016;27:16-41.
- Randall ME, Filiaci VL, Muss H, et al; Gynecologic Oncology Group Study. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2006;24:36-44.
- Syeda S, Chen L, Hou JY, et al. Chemotherapy, radiation, or combination therapy for stage III uterine cancer. Obstet Gynecol. 2019;134:17-29.
- Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266-271.
- Susumu N, Sagae S, Udagawa Y, et al; Japanese Gynecologic Oncology Group. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226-233.
- de Boer SM, Powell ME, Mileshkin L, et al; PORTEC Study Group. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019;20:1273-1285.
- Randall ME, Filiaci V, McMeekin DS, et al. Phase III trial: adjuvant pelvic radiation therapy versus vaginal brachytherapy plus paclitaxel/carboplatin in high-intermediate and high-risk early stage endometrial cancer. J Clin Oncol. 2019;37:1810-1818.
- Matei D, Filiaci V, Randall ME, et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med. 2019;380:2317-2326.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, et al. NCCN guidelines insights: ovarian cancer, version 1.2019. J Natl Compr Canc Netw. 2019;17:896-909.
- Ledermann JA, Raja FA, Fotopoulou C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi24-vi32.
- Moore KN, Mirza MR, Matulonis UA. The poly (ADP ribose) polymerase inhibitor niraparib: management of toxicities. Gynecol Oncol. 2018;149:214-220.
- Konstantinopoulos PA, Matulonis UA. PARP inhibitors in ovarian cancer: a trailblazing and transformative journey. Clin Cancer Res. 2018;24:4062-4065.
- Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764-775.
- Mukhopadhyay A, Plummer ER, Elattar A, et al. Clinicopathological features of homologous recombination-deficient epithelial ovarian cancers: sensitivity to PARP inhibitors, platinum, and survival. Cancer Res. 2012;72:5675-5682.
- Mirza MR, Monk BJ, Herrstedt J, et al; ENGOT-OV16/ NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154-2164.
- Pujade-Lauraine E, Ledermann JA, Selle F, et al; SOLO2/ ENGOT-Ov21 Investigators. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274-1284.
- Coleman RL, Oza AM, Lorusso D, et al; ARIEL3 Investigators. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949-1961.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Coleman RL, Fleming GF, Brady MF, et al. Veliparib with firstline chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403-2415.
- Coleman RL, Spirtos NM, Enserro D, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929-1939.
- Bommert M, Harter P, Heitz F, et al. When should surgery be used for recurrent ovarian carcinoma? Clin Oncol (R Coll Radiol). 2018;30:493-497.
- Santillan A, Karam AK, Li AJ, et al. Secondary cytoreductive surgery for isolated nodal recurrence in patients with epithelial ovarian cancer. Gynecol Oncol. 2007;104:686-690.
- Zang RY, Harter P, Chi DS, et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br J Cancer. 2011;105:890-896.
- Chi DS, McCaughty K, Diaz JP, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933-1939.
- Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30: 2039-2045.
2019 Update on gynecologic cancer
Of the major developments in 2018 that changed practice in gynecologic oncology, we highlight 3 here.
First, a trial on the use of hyperthermic intraperitoneal chemotherapy (HIPEC) for patients with ovarian cancer after neoadjuvant chemotherapy demonstrated an overall survival benefit of 12 months for patients treated with HIPEC. Second, a trial on polyadenosine diphosphate-ribose polymerase (PARP) inhibitors as maintenance therapy after adjuvant chemotherapy showed that women with a BRCA mutation had a progression-free survival benefit of nearly 3 years. Third, the Laparoscopic Approach to Cervical Cancer trial revealed a significant decrease in survival in women with early-stage cervical cancer who underwent minimally invasive radical hysterectomy compared with those who had the traditional open approach. In addition, a retrospective study that analyzed information from large cancer databases showed that national survival rates decreased for patients with cervical cancer as the use of laparoscopic radical hysterectomy rose.
In this Update, we summarize the major findings of these trials, provide background on treatment strategies, and discuss how our practice as cancer specialists has changed in light of these studies' findings.
HIPEC improves overall survival in advanced ovarian cancer—by a lot
Van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378:230-240.
In the United States, women with advanced-stage ovarian cancer typically are treated with primary cytoreductive (debulking) surgery followed by platinum- and taxane-based chemotherapy. The goal of cytoreductive surgery is the resection of all grossly visible tumor. While associated with favorable oncologic outcomes, cytoreductive surgery also is accompanied by significant morbidity, and surgery is not always feasible.
Neoadjuvant chemotherapy (NACT) has emerged as an alternative treatment strategy to primary cytoreductive surgery. Women treated with NACT typically undergo 3 to 4 cycles of platinum- and taxane-based chemotherapy, receive interval cytoreduction, and then are treated with an additional 3 to 4 cycles of chemotherapy postoperatively. Several large, randomized controlled trials have demonstrated that survival is similar for women with advanced-stage ovarian cancer treated with either primary cytoreduction or NACT.1,2 Importantly, perioperative morbidity is substantially lower with NACT and the rate of complete tumor resection is improved. Use of NACT for ovarian cancer has increased substantially in recent years.3
Rationale for intraperitoneal chemotherapy
Intraperitoneal (IP) chemotherapy has long been utilized in the treatment of ovarian cancer.4 Given that the abdomen is the most common site of metastatic spread for ovarian cancer, there is a strong rationale for direct infusion of chemotherapy into the abdominal cavity. Several early trials showed that adjuvant IP chemotherapy improves survival compared with intravenous chemotherapy alone.5,6 Yet complete adoption of IP chemotherapy has been limited by evidence of moderately increased toxicities, such as pain, infections, and bowel obstructions, as well as IP catheter complications.5,7
Heated IP chemotherapy for recurrent ovarian cancer
More recently, interest has focused on HIPEC. In this approach, chemotherapy is heated to 42°C and administered into the abdominal cavity immediately after cytoreductive surgery; a temperature of 40°C to 41°C is maintained for total perfusion over a 90-minute period. The increased temperature induces apoptosis and protein degeneration, leading to greater penetration by the chemotherapy along peritoneal surfaces.8
For ovarian cancer, HIPEC has been explored in a number of small studies, predominately for women with recurrent disease.9 These studies demonstrated that HIPEC increased toxicities with gastrointestinal and renal complications but improved overall and disease-free survival.
HIPEC for primary treatment
Van Driel and colleagues explored the safety and efficacy of HIPEC for the primary treatment of ovarian cancer.10 In their multicenter trial, the authors sought to determine if there was a survival benefit with HIPEC in patients with stage III ovarian, fallopian tube, or peritoneal cancer treated with NACT. Eligible participants initially were treated with 3 cycles of chemotherapy with carboplatin and paclitaxel. Two-hundred forty-five patients who had a response or stable disease were then randomly assigned to undergo either interval cytoreductive surgery alone or surgery with HIPEC using cisplatin. Both groups received 3 additional cycles of carboplatin and paclitaxel after surgery.
Results. Treatment with HIPEC was associated with a 3.5-month improvement in recurrence-free survival compared with surgery alone (14.2 vs 10.7 months) and a 12-month improvement in overall survival (45.7 vs 33.9 months). After a median follow-up of 4.7 years, 62% of patients in the surgery group and 50% of the patients in the HIPEC group had died.
Adverse events. Rates of grade 3 and 4 adverse events were similar for both treatment arms (25% in the surgery group vs 27% in the HIPEC plus surgery group), and there was no significant difference in hospital length of stay (8 vs 10 days, which included a mandatory 1-night stay in the intensive care unit for HIPEC-treated patients).
For carefully selected women with advanced ovarian cancer treated with neoadjuvant chemotherapy, HIPEC at the time of interval cytoreductive surgery may improve survival by a year.
Continue to: PARP inhibitors extend survival in ovarian cancer...
PARP inhibitors extend survival in ovarian cancer, especially for women with a BRCA mutation
Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
Ovarian cancer is the deadliest malignancy affecting women in the United States. While patients are likely to respond to their initial chemotherapy and surgery, there is a significant risk for cancer recurrence, from which the high mortality rates arise.
Maintenance therapy has considerable potential for preventing recurrences. Based on the results of a large Gynecologic Oncology Group study,11 in 2017 the US Food and Drug Administration (FDA) approved bevacizumab for use in combination with and following standard carboplatin and paclitaxel chemotherapy for women with advanced ovarian cancer. In the trial, maintenance therapy with 10 months of bevacizumab improved progression-free survival by 4 months; however, it did not improve overall survival, and adverse events included bowel perforations and hypertension.11 Alternative targets for maintenance therapy to prevent or minimize the risk of recurrence in women with ovarian cancer have been actively investigated.
PARP inhibitors work by damaging cancer cell DNA
PARP is a key enzyme that repairs DNA damage within cells. Drugs that inhibit PARP trap this enzyme at the site of single-strand breaks, disrupting single-strand repair and inducing double-strand breaks. Since the homologous recombination pathway used to repair double-strand DNA breaks does not function in BRCA-mutated tissues, PARP inhibitors ultimately induce targeted DNA damage and apoptosis in both germline and somatic BRCA mutation carriers.12
In the United States, 3 PARP inhibitors (olaparib, niraparib, and rucaparib) are FDA approved as maintenance therapy for use in women with recurrent ovarian cancer that had responded completely or partially to platinum-based chemotherapy, regardless of BRCA status. PARP inhibitors also have been approved for treatment of advanced ovarian cancer in BRCA mutation carriers who have received 3 or more lines of platinum-based chemotherapy. Because of their efficacy in the treatment of recurrent ovarian cancer, there is great interest in using PARP inhibitors earlier in the disease course.
Olaparib is effective in women with BRCA mutations
In an international, randomized, double-blind, phase 3 trial, Moore and colleagues sought to determine the efficacy of the PARP inhibitor olaparib administered as maintenance therapy in women with germline or somatic BRCA mutations.13 Women were eligible if they had BRCA1 or BRCA2 mutations with newly diagnosed advanced (stage III or IV) ovarian, fallopian tube, or peritoneal cancer and a complete or partial response to platinum-based chemotherapy after cytoreduction.
Women were randomly assigned in a 2:1 ratio, with 260 participants receiving twice daily olaparib and 131 receiving placebo.
Results. After 41 months of follow-up, the disease-free survival rate was 60% in the olaparib group, compared with 27% in the placebo arm. Progression-free survival was 36 months longer in the olaparib maintenance group than in the placebo group.
Adverse events. While 21% of women treated with olaparib experienced serious adverse events (compared with 12% in the placebo group), most were related to anemia. Acute myeloid leukemia occurred in 3 (1%) of the 260 patients receiving olaparib.
For women with deleterious BRCA1 and/or BRCA2 mutations, administering PARP inhibitors as a maintenance therapy following primary treatment with the standard platinum-based chemotherapy improves progression-free survival by at least 3 years.
Continue to: Is MIS radical hysterectomy (vs open) for cervical cancer safe?
Is MIS radical hysterectomy (vs open) for cervical cancer safe?
Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
For various procedures, minimally invasive surgery (MIS) is associated with decreased blood loss, shorter postoperative stay, and decreased postoperative complications and readmission rates. In oncology, MIS has demonstrated equivalent outcomes compared with open procedures for colorectal and endometrial cancers.14,15
Increasing use of MIS in cervical cancer
For patients with cervical cancer, minimally invasive radical hysterectomy has more favorable perioperative outcomes, less morbidity, and decreased costs than open radical hysterectomy.16-20 However, many of the studies used to justify these benefits were small, lacked adequate follow-up, and were not adequately powered to detect a true survival difference. Some trials compared contemporary MIS enrollees to historical open surgery controls, who may have had more advanced-stage disease and may have been treated with different adjuvant chemoradiation.
Despite these major limitations, minimally invasive radical hysterectomy became an acceptable—and often preferable—alternative to open radical hysterectomy for early-stage cervical cancer. This acceptance was written into National Comprehensive Cancer Network guidelines,21 and minimally invasive radical hysterectomy rapidly gained popularity, increasing from 1.8% in 2006 to 31% in 2010.22
Randomized trial revealed surprising findings
Ramirez and colleagues recently published the results of the Laparoscopic Approach to Cervical Cancer (LACC) trial, a randomized controlled trial that compared open with minimally invasive radical hysterectomy in women with stage IA1-IB1 cervical cancer.23 The study was designed as a noninferiority trial in which researchers set a threshold of -7.2% for how much worse the survival of MIS patients could be compared with open surgery before MIS could be declared an inferior treatment. A total of 631 patients were enrolled at 33 centers worldwide. After an interim analysis demonstrated a safety signal in the MIS radical hysterectomy cohort, the study was closed before completion of enrollment.
Overall, 91% of patients randomly assigned to treatment had stage IB1 tumors. At the time of analysis, nearly 60% of enrollees had survival data at 4.5 years to provide adequate power for full analysis.
Results. Disease-free survival (the time from randomization to recurrence or death from cervical cancer) was 86.0% in the MIS group and 96.5% in the open hysterectomy group. At 4.5 years, 27 MIS patients had recurrent disease, compared with 7 patients who underwent abdominal radical hysterectomy. There were 14 cancer-related deaths in the MIS group, compared with 2 in the open group.
Three-year disease-free survival was 91.2% in the MIS group versus 97.1% in the abdominal radical hysterectomy group (hazard ratio, 3.74; 95% confidence interval, 1.63-8.58) The overall 3-year survival was 93.8% in the MIS group, compared with 99.0% in the open group.23
Retrospective cohort study had similar results
Concurrent with publication of the LACC trial results, Melamed and colleagues published an observational study on the safety of MIS radical hysterectomy for early-stage cervical cancer.22 They used data from the National Cancer Database to examine 2,461 women with stage IA2-IB1 cervical cancer who underwent radical hysterectomy from 2010 to 2013. Approximately half of the women (49.8%) underwent minimally invasive radical hysterectomy.
Results. After a median follow-up of 45 months, the 4-year mortality rate was 9.1% among women who underwent MIS radical hysterectomy, compared with 5.3% for those who had an abdominal radical hysterectomy.
Using the complimentary Surveillance, Epidemiology, and End Results (SEER) registry dataset, the authors examined population-level trends in use of MIS radical hysterectomy and survival. From 2000 to 2006, when MIS radical hysterectomy was rarely utilized, 4-year survival for cervical cancer was relatively stable. After adoption of MIS radical hysterectomy in 2006, 4-year relative survival declined by 0.8% annually for cervical cancer (FIGURE).22
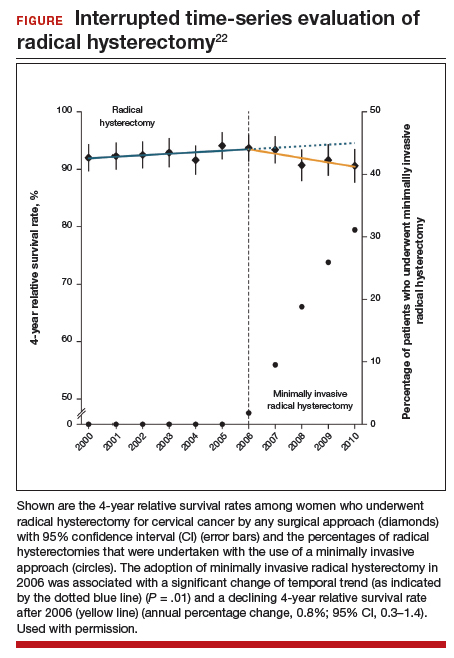
Both a randomized controlled trial and a large observational study demonstrated decreased survival for women with early-stage cervical cancer who underwent minimally invasive radical hysterectomy. Use of minimally invasive radical hysterectomy should be used with caution in women with early-stage cervical cancer.
- Vergote I, Trope CG, Amant F, et al; European Organization for Research and Treatment of Cancer–Gynaecological Cancer Group; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943-953.
- Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249-257.
- Melamed A, Hinchcliff EM, Clemmer JT, et al. Trends in the use of neoadjuvant chemotherapy for advanced ovarian cancer in the United States. Gynecol Oncol. 2016;143:236-240.
- Markman M. Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol. 2003;4:277-283.
- Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001-1007.
- Armstrong DK, Bundy B, Wenzel L, et al; Gynecologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43.
- Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950-1955.
- van de Vaart PJ, van der Vange N, Zoetmulder FA, et al. Intraperitoneal cisplatin with regional hyperthermia in advanced ovarian cancer: pharmacokinetics and cisplatin-DNA adduct formation in patients and ovarian cancer cell lines. Eur J Cancer. 1998;34:148-154.
- Bakrin N, Cotte E, Golfier F, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for persistent and recurrent advanced ovarian carcinoma: a multicenter, prospective study of 246 patients. Ann Surg Oncol. 2012;19:4052-4058.
- van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018;378:230-240.
- Burger RA, Brady MF, Bookman MA, et al; Gynecologic Oncology Group. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473-2483.
- Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917-921.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012;30:695-700.
- Clinical Outcomes of Surgical Therapy Study Group, Nelson H, Sargent DJ, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050-2059.
- Lee EJ, Kang H, Kim DH. A comparative study of laparoscopic radical hysterectomy with radical abdominal hysterectomy for early-stage cervical cancer: a long-term follow-up study. Eur J Obstet Gynecol Reprod Biol. 2011;156:83-86.
- Malzoni M, Tinelli R, Cosentino F, et al. Total laparoscopic radical hysterectomy versus abdominal radical hysterectomy with lymphadenectomy in patients with early cervical cancer: our experience. Ann Surg Oncol. 2009;16:1316-1323.
- Nam JH, Park JY, Kim DY, et al. Laparoscopic versus open radical hysterectomy in early-stage cervical cancer: long-term survival outcomes in a matched cohort study. Ann Oncol. 2012;23:903-911.
- Obermair A, Gebski V, Frumovitz M, et al. A phase III randomized clinical trial comparing laparoscopic or robotic radical hysterectomy with abdominal radical hysterectomy in patients with early stage cervical cancer. J Minim Invasive Gynecol. 2008;15:584-588.
- Mendivil AA, Rettenmaier MA, Abaid LN, et al. Survival rate comparisons amongst cervical cancer patients treated with an open, robotic-assisted or laparoscopic radical hysterectomy: a five year experience. Surg Oncol. 2016;25:66-71.
- National Comprehensive Care Network. NCCN clinical practice guidelines in oncology: cervical cancer, version 1.2018. http://oncolife.com.ua/doc/nccn/Cervical_Cancer.pdf. Accessed February 11, 2019.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
Of the major developments in 2018 that changed practice in gynecologic oncology, we highlight 3 here.
First, a trial on the use of hyperthermic intraperitoneal chemotherapy (HIPEC) for patients with ovarian cancer after neoadjuvant chemotherapy demonstrated an overall survival benefit of 12 months for patients treated with HIPEC. Second, a trial on polyadenosine diphosphate-ribose polymerase (PARP) inhibitors as maintenance therapy after adjuvant chemotherapy showed that women with a BRCA mutation had a progression-free survival benefit of nearly 3 years. Third, the Laparoscopic Approach to Cervical Cancer trial revealed a significant decrease in survival in women with early-stage cervical cancer who underwent minimally invasive radical hysterectomy compared with those who had the traditional open approach. In addition, a retrospective study that analyzed information from large cancer databases showed that national survival rates decreased for patients with cervical cancer as the use of laparoscopic radical hysterectomy rose.
In this Update, we summarize the major findings of these trials, provide background on treatment strategies, and discuss how our practice as cancer specialists has changed in light of these studies' findings.
HIPEC improves overall survival in advanced ovarian cancer—by a lot
Van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378:230-240.
In the United States, women with advanced-stage ovarian cancer typically are treated with primary cytoreductive (debulking) surgery followed by platinum- and taxane-based chemotherapy. The goal of cytoreductive surgery is the resection of all grossly visible tumor. While associated with favorable oncologic outcomes, cytoreductive surgery also is accompanied by significant morbidity, and surgery is not always feasible.
Neoadjuvant chemotherapy (NACT) has emerged as an alternative treatment strategy to primary cytoreductive surgery. Women treated with NACT typically undergo 3 to 4 cycles of platinum- and taxane-based chemotherapy, receive interval cytoreduction, and then are treated with an additional 3 to 4 cycles of chemotherapy postoperatively. Several large, randomized controlled trials have demonstrated that survival is similar for women with advanced-stage ovarian cancer treated with either primary cytoreduction or NACT.1,2 Importantly, perioperative morbidity is substantially lower with NACT and the rate of complete tumor resection is improved. Use of NACT for ovarian cancer has increased substantially in recent years.3
Rationale for intraperitoneal chemotherapy
Intraperitoneal (IP) chemotherapy has long been utilized in the treatment of ovarian cancer.4 Given that the abdomen is the most common site of metastatic spread for ovarian cancer, there is a strong rationale for direct infusion of chemotherapy into the abdominal cavity. Several early trials showed that adjuvant IP chemotherapy improves survival compared with intravenous chemotherapy alone.5,6 Yet complete adoption of IP chemotherapy has been limited by evidence of moderately increased toxicities, such as pain, infections, and bowel obstructions, as well as IP catheter complications.5,7
Heated IP chemotherapy for recurrent ovarian cancer
More recently, interest has focused on HIPEC. In this approach, chemotherapy is heated to 42°C and administered into the abdominal cavity immediately after cytoreductive surgery; a temperature of 40°C to 41°C is maintained for total perfusion over a 90-minute period. The increased temperature induces apoptosis and protein degeneration, leading to greater penetration by the chemotherapy along peritoneal surfaces.8
For ovarian cancer, HIPEC has been explored in a number of small studies, predominately for women with recurrent disease.9 These studies demonstrated that HIPEC increased toxicities with gastrointestinal and renal complications but improved overall and disease-free survival.
HIPEC for primary treatment
Van Driel and colleagues explored the safety and efficacy of HIPEC for the primary treatment of ovarian cancer.10 In their multicenter trial, the authors sought to determine if there was a survival benefit with HIPEC in patients with stage III ovarian, fallopian tube, or peritoneal cancer treated with NACT. Eligible participants initially were treated with 3 cycles of chemotherapy with carboplatin and paclitaxel. Two-hundred forty-five patients who had a response or stable disease were then randomly assigned to undergo either interval cytoreductive surgery alone or surgery with HIPEC using cisplatin. Both groups received 3 additional cycles of carboplatin and paclitaxel after surgery.
Results. Treatment with HIPEC was associated with a 3.5-month improvement in recurrence-free survival compared with surgery alone (14.2 vs 10.7 months) and a 12-month improvement in overall survival (45.7 vs 33.9 months). After a median follow-up of 4.7 years, 62% of patients in the surgery group and 50% of the patients in the HIPEC group had died.
Adverse events. Rates of grade 3 and 4 adverse events were similar for both treatment arms (25% in the surgery group vs 27% in the HIPEC plus surgery group), and there was no significant difference in hospital length of stay (8 vs 10 days, which included a mandatory 1-night stay in the intensive care unit for HIPEC-treated patients).
For carefully selected women with advanced ovarian cancer treated with neoadjuvant chemotherapy, HIPEC at the time of interval cytoreductive surgery may improve survival by a year.
Continue to: PARP inhibitors extend survival in ovarian cancer...
PARP inhibitors extend survival in ovarian cancer, especially for women with a BRCA mutation
Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
Ovarian cancer is the deadliest malignancy affecting women in the United States. While patients are likely to respond to their initial chemotherapy and surgery, there is a significant risk for cancer recurrence, from which the high mortality rates arise.
Maintenance therapy has considerable potential for preventing recurrences. Based on the results of a large Gynecologic Oncology Group study,11 in 2017 the US Food and Drug Administration (FDA) approved bevacizumab for use in combination with and following standard carboplatin and paclitaxel chemotherapy for women with advanced ovarian cancer. In the trial, maintenance therapy with 10 months of bevacizumab improved progression-free survival by 4 months; however, it did not improve overall survival, and adverse events included bowel perforations and hypertension.11 Alternative targets for maintenance therapy to prevent or minimize the risk of recurrence in women with ovarian cancer have been actively investigated.
PARP inhibitors work by damaging cancer cell DNA
PARP is a key enzyme that repairs DNA damage within cells. Drugs that inhibit PARP trap this enzyme at the site of single-strand breaks, disrupting single-strand repair and inducing double-strand breaks. Since the homologous recombination pathway used to repair double-strand DNA breaks does not function in BRCA-mutated tissues, PARP inhibitors ultimately induce targeted DNA damage and apoptosis in both germline and somatic BRCA mutation carriers.12
In the United States, 3 PARP inhibitors (olaparib, niraparib, and rucaparib) are FDA approved as maintenance therapy for use in women with recurrent ovarian cancer that had responded completely or partially to platinum-based chemotherapy, regardless of BRCA status. PARP inhibitors also have been approved for treatment of advanced ovarian cancer in BRCA mutation carriers who have received 3 or more lines of platinum-based chemotherapy. Because of their efficacy in the treatment of recurrent ovarian cancer, there is great interest in using PARP inhibitors earlier in the disease course.
Olaparib is effective in women with BRCA mutations
In an international, randomized, double-blind, phase 3 trial, Moore and colleagues sought to determine the efficacy of the PARP inhibitor olaparib administered as maintenance therapy in women with germline or somatic BRCA mutations.13 Women were eligible if they had BRCA1 or BRCA2 mutations with newly diagnosed advanced (stage III or IV) ovarian, fallopian tube, or peritoneal cancer and a complete or partial response to platinum-based chemotherapy after cytoreduction.
Women were randomly assigned in a 2:1 ratio, with 260 participants receiving twice daily olaparib and 131 receiving placebo.
Results. After 41 months of follow-up, the disease-free survival rate was 60% in the olaparib group, compared with 27% in the placebo arm. Progression-free survival was 36 months longer in the olaparib maintenance group than in the placebo group.
Adverse events. While 21% of women treated with olaparib experienced serious adverse events (compared with 12% in the placebo group), most were related to anemia. Acute myeloid leukemia occurred in 3 (1%) of the 260 patients receiving olaparib.
For women with deleterious BRCA1 and/or BRCA2 mutations, administering PARP inhibitors as a maintenance therapy following primary treatment with the standard platinum-based chemotherapy improves progression-free survival by at least 3 years.
Continue to: Is MIS radical hysterectomy (vs open) for cervical cancer safe?
Is MIS radical hysterectomy (vs open) for cervical cancer safe?
Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
For various procedures, minimally invasive surgery (MIS) is associated with decreased blood loss, shorter postoperative stay, and decreased postoperative complications and readmission rates. In oncology, MIS has demonstrated equivalent outcomes compared with open procedures for colorectal and endometrial cancers.14,15
Increasing use of MIS in cervical cancer
For patients with cervical cancer, minimally invasive radical hysterectomy has more favorable perioperative outcomes, less morbidity, and decreased costs than open radical hysterectomy.16-20 However, many of the studies used to justify these benefits were small, lacked adequate follow-up, and were not adequately powered to detect a true survival difference. Some trials compared contemporary MIS enrollees to historical open surgery controls, who may have had more advanced-stage disease and may have been treated with different adjuvant chemoradiation.
Despite these major limitations, minimally invasive radical hysterectomy became an acceptable—and often preferable—alternative to open radical hysterectomy for early-stage cervical cancer. This acceptance was written into National Comprehensive Cancer Network guidelines,21 and minimally invasive radical hysterectomy rapidly gained popularity, increasing from 1.8% in 2006 to 31% in 2010.22
Randomized trial revealed surprising findings
Ramirez and colleagues recently published the results of the Laparoscopic Approach to Cervical Cancer (LACC) trial, a randomized controlled trial that compared open with minimally invasive radical hysterectomy in women with stage IA1-IB1 cervical cancer.23 The study was designed as a noninferiority trial in which researchers set a threshold of -7.2% for how much worse the survival of MIS patients could be compared with open surgery before MIS could be declared an inferior treatment. A total of 631 patients were enrolled at 33 centers worldwide. After an interim analysis demonstrated a safety signal in the MIS radical hysterectomy cohort, the study was closed before completion of enrollment.
Overall, 91% of patients randomly assigned to treatment had stage IB1 tumors. At the time of analysis, nearly 60% of enrollees had survival data at 4.5 years to provide adequate power for full analysis.
Results. Disease-free survival (the time from randomization to recurrence or death from cervical cancer) was 86.0% in the MIS group and 96.5% in the open hysterectomy group. At 4.5 years, 27 MIS patients had recurrent disease, compared with 7 patients who underwent abdominal radical hysterectomy. There were 14 cancer-related deaths in the MIS group, compared with 2 in the open group.
Three-year disease-free survival was 91.2% in the MIS group versus 97.1% in the abdominal radical hysterectomy group (hazard ratio, 3.74; 95% confidence interval, 1.63-8.58) The overall 3-year survival was 93.8% in the MIS group, compared with 99.0% in the open group.23
Retrospective cohort study had similar results
Concurrent with publication of the LACC trial results, Melamed and colleagues published an observational study on the safety of MIS radical hysterectomy for early-stage cervical cancer.22 They used data from the National Cancer Database to examine 2,461 women with stage IA2-IB1 cervical cancer who underwent radical hysterectomy from 2010 to 2013. Approximately half of the women (49.8%) underwent minimally invasive radical hysterectomy.
Results. After a median follow-up of 45 months, the 4-year mortality rate was 9.1% among women who underwent MIS radical hysterectomy, compared with 5.3% for those who had an abdominal radical hysterectomy.
Using the complimentary Surveillance, Epidemiology, and End Results (SEER) registry dataset, the authors examined population-level trends in use of MIS radical hysterectomy and survival. From 2000 to 2006, when MIS radical hysterectomy was rarely utilized, 4-year survival for cervical cancer was relatively stable. After adoption of MIS radical hysterectomy in 2006, 4-year relative survival declined by 0.8% annually for cervical cancer (FIGURE).22

Both a randomized controlled trial and a large observational study demonstrated decreased survival for women with early-stage cervical cancer who underwent minimally invasive radical hysterectomy. Use of minimally invasive radical hysterectomy should be used with caution in women with early-stage cervical cancer.
Of the major developments in 2018 that changed practice in gynecologic oncology, we highlight 3 here.
First, a trial on the use of hyperthermic intraperitoneal chemotherapy (HIPEC) for patients with ovarian cancer after neoadjuvant chemotherapy demonstrated an overall survival benefit of 12 months for patients treated with HIPEC. Second, a trial on polyadenosine diphosphate-ribose polymerase (PARP) inhibitors as maintenance therapy after adjuvant chemotherapy showed that women with a BRCA mutation had a progression-free survival benefit of nearly 3 years. Third, the Laparoscopic Approach to Cervical Cancer trial revealed a significant decrease in survival in women with early-stage cervical cancer who underwent minimally invasive radical hysterectomy compared with those who had the traditional open approach. In addition, a retrospective study that analyzed information from large cancer databases showed that national survival rates decreased for patients with cervical cancer as the use of laparoscopic radical hysterectomy rose.
In this Update, we summarize the major findings of these trials, provide background on treatment strategies, and discuss how our practice as cancer specialists has changed in light of these studies' findings.
HIPEC improves overall survival in advanced ovarian cancer—by a lot
Van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378:230-240.
In the United States, women with advanced-stage ovarian cancer typically are treated with primary cytoreductive (debulking) surgery followed by platinum- and taxane-based chemotherapy. The goal of cytoreductive surgery is the resection of all grossly visible tumor. While associated with favorable oncologic outcomes, cytoreductive surgery also is accompanied by significant morbidity, and surgery is not always feasible.
Neoadjuvant chemotherapy (NACT) has emerged as an alternative treatment strategy to primary cytoreductive surgery. Women treated with NACT typically undergo 3 to 4 cycles of platinum- and taxane-based chemotherapy, receive interval cytoreduction, and then are treated with an additional 3 to 4 cycles of chemotherapy postoperatively. Several large, randomized controlled trials have demonstrated that survival is similar for women with advanced-stage ovarian cancer treated with either primary cytoreduction or NACT.1,2 Importantly, perioperative morbidity is substantially lower with NACT and the rate of complete tumor resection is improved. Use of NACT for ovarian cancer has increased substantially in recent years.3
Rationale for intraperitoneal chemotherapy
Intraperitoneal (IP) chemotherapy has long been utilized in the treatment of ovarian cancer.4 Given that the abdomen is the most common site of metastatic spread for ovarian cancer, there is a strong rationale for direct infusion of chemotherapy into the abdominal cavity. Several early trials showed that adjuvant IP chemotherapy improves survival compared with intravenous chemotherapy alone.5,6 Yet complete adoption of IP chemotherapy has been limited by evidence of moderately increased toxicities, such as pain, infections, and bowel obstructions, as well as IP catheter complications.5,7
Heated IP chemotherapy for recurrent ovarian cancer
More recently, interest has focused on HIPEC. In this approach, chemotherapy is heated to 42°C and administered into the abdominal cavity immediately after cytoreductive surgery; a temperature of 40°C to 41°C is maintained for total perfusion over a 90-minute period. The increased temperature induces apoptosis and protein degeneration, leading to greater penetration by the chemotherapy along peritoneal surfaces.8
For ovarian cancer, HIPEC has been explored in a number of small studies, predominately for women with recurrent disease.9 These studies demonstrated that HIPEC increased toxicities with gastrointestinal and renal complications but improved overall and disease-free survival.
HIPEC for primary treatment
Van Driel and colleagues explored the safety and efficacy of HIPEC for the primary treatment of ovarian cancer.10 In their multicenter trial, the authors sought to determine if there was a survival benefit with HIPEC in patients with stage III ovarian, fallopian tube, or peritoneal cancer treated with NACT. Eligible participants initially were treated with 3 cycles of chemotherapy with carboplatin and paclitaxel. Two-hundred forty-five patients who had a response or stable disease were then randomly assigned to undergo either interval cytoreductive surgery alone or surgery with HIPEC using cisplatin. Both groups received 3 additional cycles of carboplatin and paclitaxel after surgery.
Results. Treatment with HIPEC was associated with a 3.5-month improvement in recurrence-free survival compared with surgery alone (14.2 vs 10.7 months) and a 12-month improvement in overall survival (45.7 vs 33.9 months). After a median follow-up of 4.7 years, 62% of patients in the surgery group and 50% of the patients in the HIPEC group had died.
Adverse events. Rates of grade 3 and 4 adverse events were similar for both treatment arms (25% in the surgery group vs 27% in the HIPEC plus surgery group), and there was no significant difference in hospital length of stay (8 vs 10 days, which included a mandatory 1-night stay in the intensive care unit for HIPEC-treated patients).
For carefully selected women with advanced ovarian cancer treated with neoadjuvant chemotherapy, HIPEC at the time of interval cytoreductive surgery may improve survival by a year.
Continue to: PARP inhibitors extend survival in ovarian cancer...
PARP inhibitors extend survival in ovarian cancer, especially for women with a BRCA mutation
Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
Ovarian cancer is the deadliest malignancy affecting women in the United States. While patients are likely to respond to their initial chemotherapy and surgery, there is a significant risk for cancer recurrence, from which the high mortality rates arise.
Maintenance therapy has considerable potential for preventing recurrences. Based on the results of a large Gynecologic Oncology Group study,11 in 2017 the US Food and Drug Administration (FDA) approved bevacizumab for use in combination with and following standard carboplatin and paclitaxel chemotherapy for women with advanced ovarian cancer. In the trial, maintenance therapy with 10 months of bevacizumab improved progression-free survival by 4 months; however, it did not improve overall survival, and adverse events included bowel perforations and hypertension.11 Alternative targets for maintenance therapy to prevent or minimize the risk of recurrence in women with ovarian cancer have been actively investigated.
PARP inhibitors work by damaging cancer cell DNA
PARP is a key enzyme that repairs DNA damage within cells. Drugs that inhibit PARP trap this enzyme at the site of single-strand breaks, disrupting single-strand repair and inducing double-strand breaks. Since the homologous recombination pathway used to repair double-strand DNA breaks does not function in BRCA-mutated tissues, PARP inhibitors ultimately induce targeted DNA damage and apoptosis in both germline and somatic BRCA mutation carriers.12
In the United States, 3 PARP inhibitors (olaparib, niraparib, and rucaparib) are FDA approved as maintenance therapy for use in women with recurrent ovarian cancer that had responded completely or partially to platinum-based chemotherapy, regardless of BRCA status. PARP inhibitors also have been approved for treatment of advanced ovarian cancer in BRCA mutation carriers who have received 3 or more lines of platinum-based chemotherapy. Because of their efficacy in the treatment of recurrent ovarian cancer, there is great interest in using PARP inhibitors earlier in the disease course.
Olaparib is effective in women with BRCA mutations
In an international, randomized, double-blind, phase 3 trial, Moore and colleagues sought to determine the efficacy of the PARP inhibitor olaparib administered as maintenance therapy in women with germline or somatic BRCA mutations.13 Women were eligible if they had BRCA1 or BRCA2 mutations with newly diagnosed advanced (stage III or IV) ovarian, fallopian tube, or peritoneal cancer and a complete or partial response to platinum-based chemotherapy after cytoreduction.
Women were randomly assigned in a 2:1 ratio, with 260 participants receiving twice daily olaparib and 131 receiving placebo.
Results. After 41 months of follow-up, the disease-free survival rate was 60% in the olaparib group, compared with 27% in the placebo arm. Progression-free survival was 36 months longer in the olaparib maintenance group than in the placebo group.
Adverse events. While 21% of women treated with olaparib experienced serious adverse events (compared with 12% in the placebo group), most were related to anemia. Acute myeloid leukemia occurred in 3 (1%) of the 260 patients receiving olaparib.
For women with deleterious BRCA1 and/or BRCA2 mutations, administering PARP inhibitors as a maintenance therapy following primary treatment with the standard platinum-based chemotherapy improves progression-free survival by at least 3 years.
Continue to: Is MIS radical hysterectomy (vs open) for cervical cancer safe?
Is MIS radical hysterectomy (vs open) for cervical cancer safe?
Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
For various procedures, minimally invasive surgery (MIS) is associated with decreased blood loss, shorter postoperative stay, and decreased postoperative complications and readmission rates. In oncology, MIS has demonstrated equivalent outcomes compared with open procedures for colorectal and endometrial cancers.14,15
Increasing use of MIS in cervical cancer
For patients with cervical cancer, minimally invasive radical hysterectomy has more favorable perioperative outcomes, less morbidity, and decreased costs than open radical hysterectomy.16-20 However, many of the studies used to justify these benefits were small, lacked adequate follow-up, and were not adequately powered to detect a true survival difference. Some trials compared contemporary MIS enrollees to historical open surgery controls, who may have had more advanced-stage disease and may have been treated with different adjuvant chemoradiation.
Despite these major limitations, minimally invasive radical hysterectomy became an acceptable—and often preferable—alternative to open radical hysterectomy for early-stage cervical cancer. This acceptance was written into National Comprehensive Cancer Network guidelines,21 and minimally invasive radical hysterectomy rapidly gained popularity, increasing from 1.8% in 2006 to 31% in 2010.22
Randomized trial revealed surprising findings
Ramirez and colleagues recently published the results of the Laparoscopic Approach to Cervical Cancer (LACC) trial, a randomized controlled trial that compared open with minimally invasive radical hysterectomy in women with stage IA1-IB1 cervical cancer.23 The study was designed as a noninferiority trial in which researchers set a threshold of -7.2% for how much worse the survival of MIS patients could be compared with open surgery before MIS could be declared an inferior treatment. A total of 631 patients were enrolled at 33 centers worldwide. After an interim analysis demonstrated a safety signal in the MIS radical hysterectomy cohort, the study was closed before completion of enrollment.
Overall, 91% of patients randomly assigned to treatment had stage IB1 tumors. At the time of analysis, nearly 60% of enrollees had survival data at 4.5 years to provide adequate power for full analysis.
Results. Disease-free survival (the time from randomization to recurrence or death from cervical cancer) was 86.0% in the MIS group and 96.5% in the open hysterectomy group. At 4.5 years, 27 MIS patients had recurrent disease, compared with 7 patients who underwent abdominal radical hysterectomy. There were 14 cancer-related deaths in the MIS group, compared with 2 in the open group.
Three-year disease-free survival was 91.2% in the MIS group versus 97.1% in the abdominal radical hysterectomy group (hazard ratio, 3.74; 95% confidence interval, 1.63-8.58) The overall 3-year survival was 93.8% in the MIS group, compared with 99.0% in the open group.23
Retrospective cohort study had similar results
Concurrent with publication of the LACC trial results, Melamed and colleagues published an observational study on the safety of MIS radical hysterectomy for early-stage cervical cancer.22 They used data from the National Cancer Database to examine 2,461 women with stage IA2-IB1 cervical cancer who underwent radical hysterectomy from 2010 to 2013. Approximately half of the women (49.8%) underwent minimally invasive radical hysterectomy.
Results. After a median follow-up of 45 months, the 4-year mortality rate was 9.1% among women who underwent MIS radical hysterectomy, compared with 5.3% for those who had an abdominal radical hysterectomy.
Using the complimentary Surveillance, Epidemiology, and End Results (SEER) registry dataset, the authors examined population-level trends in use of MIS radical hysterectomy and survival. From 2000 to 2006, when MIS radical hysterectomy was rarely utilized, 4-year survival for cervical cancer was relatively stable. After adoption of MIS radical hysterectomy in 2006, 4-year relative survival declined by 0.8% annually for cervical cancer (FIGURE).22

Both a randomized controlled trial and a large observational study demonstrated decreased survival for women with early-stage cervical cancer who underwent minimally invasive radical hysterectomy. Use of minimally invasive radical hysterectomy should be used with caution in women with early-stage cervical cancer.
- Vergote I, Trope CG, Amant F, et al; European Organization for Research and Treatment of Cancer–Gynaecological Cancer Group; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943-953.
- Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249-257.
- Melamed A, Hinchcliff EM, Clemmer JT, et al. Trends in the use of neoadjuvant chemotherapy for advanced ovarian cancer in the United States. Gynecol Oncol. 2016;143:236-240.
- Markman M. Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol. 2003;4:277-283.
- Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001-1007.
- Armstrong DK, Bundy B, Wenzel L, et al; Gynecologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43.
- Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950-1955.
- van de Vaart PJ, van der Vange N, Zoetmulder FA, et al. Intraperitoneal cisplatin with regional hyperthermia in advanced ovarian cancer: pharmacokinetics and cisplatin-DNA adduct formation in patients and ovarian cancer cell lines. Eur J Cancer. 1998;34:148-154.
- Bakrin N, Cotte E, Golfier F, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for persistent and recurrent advanced ovarian carcinoma: a multicenter, prospective study of 246 patients. Ann Surg Oncol. 2012;19:4052-4058.
- van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018;378:230-240.
- Burger RA, Brady MF, Bookman MA, et al; Gynecologic Oncology Group. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473-2483.
- Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917-921.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012;30:695-700.
- Clinical Outcomes of Surgical Therapy Study Group, Nelson H, Sargent DJ, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050-2059.
- Lee EJ, Kang H, Kim DH. A comparative study of laparoscopic radical hysterectomy with radical abdominal hysterectomy for early-stage cervical cancer: a long-term follow-up study. Eur J Obstet Gynecol Reprod Biol. 2011;156:83-86.
- Malzoni M, Tinelli R, Cosentino F, et al. Total laparoscopic radical hysterectomy versus abdominal radical hysterectomy with lymphadenectomy in patients with early cervical cancer: our experience. Ann Surg Oncol. 2009;16:1316-1323.
- Nam JH, Park JY, Kim DY, et al. Laparoscopic versus open radical hysterectomy in early-stage cervical cancer: long-term survival outcomes in a matched cohort study. Ann Oncol. 2012;23:903-911.
- Obermair A, Gebski V, Frumovitz M, et al. A phase III randomized clinical trial comparing laparoscopic or robotic radical hysterectomy with abdominal radical hysterectomy in patients with early stage cervical cancer. J Minim Invasive Gynecol. 2008;15:584-588.
- Mendivil AA, Rettenmaier MA, Abaid LN, et al. Survival rate comparisons amongst cervical cancer patients treated with an open, robotic-assisted or laparoscopic radical hysterectomy: a five year experience. Surg Oncol. 2016;25:66-71.
- National Comprehensive Care Network. NCCN clinical practice guidelines in oncology: cervical cancer, version 1.2018. http://oncolife.com.ua/doc/nccn/Cervical_Cancer.pdf. Accessed February 11, 2019.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Vergote I, Trope CG, Amant F, et al; European Organization for Research and Treatment of Cancer–Gynaecological Cancer Group; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943-953.
- Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249-257.
- Melamed A, Hinchcliff EM, Clemmer JT, et al. Trends in the use of neoadjuvant chemotherapy for advanced ovarian cancer in the United States. Gynecol Oncol. 2016;143:236-240.
- Markman M. Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol. 2003;4:277-283.
- Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001-1007.
- Armstrong DK, Bundy B, Wenzel L, et al; Gynecologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43.
- Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950-1955.
- van de Vaart PJ, van der Vange N, Zoetmulder FA, et al. Intraperitoneal cisplatin with regional hyperthermia in advanced ovarian cancer: pharmacokinetics and cisplatin-DNA adduct formation in patients and ovarian cancer cell lines. Eur J Cancer. 1998;34:148-154.
- Bakrin N, Cotte E, Golfier F, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for persistent and recurrent advanced ovarian carcinoma: a multicenter, prospective study of 246 patients. Ann Surg Oncol. 2012;19:4052-4058.
- van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018;378:230-240.
- Burger RA, Brady MF, Bookman MA, et al; Gynecologic Oncology Group. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473-2483.
- Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917-921.
- Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012;30:695-700.
- Clinical Outcomes of Surgical Therapy Study Group, Nelson H, Sargent DJ, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050-2059.
- Lee EJ, Kang H, Kim DH. A comparative study of laparoscopic radical hysterectomy with radical abdominal hysterectomy for early-stage cervical cancer: a long-term follow-up study. Eur J Obstet Gynecol Reprod Biol. 2011;156:83-86.
- Malzoni M, Tinelli R, Cosentino F, et al. Total laparoscopic radical hysterectomy versus abdominal radical hysterectomy with lymphadenectomy in patients with early cervical cancer: our experience. Ann Surg Oncol. 2009;16:1316-1323.
- Nam JH, Park JY, Kim DY, et al. Laparoscopic versus open radical hysterectomy in early-stage cervical cancer: long-term survival outcomes in a matched cohort study. Ann Oncol. 2012;23:903-911.
- Obermair A, Gebski V, Frumovitz M, et al. A phase III randomized clinical trial comparing laparoscopic or robotic radical hysterectomy with abdominal radical hysterectomy in patients with early stage cervical cancer. J Minim Invasive Gynecol. 2008;15:584-588.
- Mendivil AA, Rettenmaier MA, Abaid LN, et al. Survival rate comparisons amongst cervical cancer patients treated with an open, robotic-assisted or laparoscopic radical hysterectomy: a five year experience. Surg Oncol. 2016;25:66-71.
- National Comprehensive Care Network. NCCN clinical practice guidelines in oncology: cervical cancer, version 1.2018. http://oncolife.com.ua/doc/nccn/Cervical_Cancer.pdf. Accessed February 11, 2019.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
2018 Update on gynecologic cancer
In this Update, I report on the latest US Preventive Services Task Force (USPSTF) cervical cancer screening recommendations. In addition, I describe the results of 2 studies, a large prospective multicenter study of the accuracy of sentinel lymph node (SLN) biopsy in endometrial cancer, and a proof-of-concept review of use of checkpoint blockade to increase immune response and of its possible role in endometrial cancer.
hrHPV testing used alone as primary screening for cervical cancer: USPSTF recommendations
US Preventive Services Task Force. Draft recommendation statement: cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Published October 2017. Accessed February 5, 2018.
Despite our rapid advances in understanding the molecular underpinnings of cancer, gynecologic malignancies are still a major cause of morbidity and mortality among women. Cervical cancer stands as an example of how a cancer screening test can be implemented to reduce mortality. In this section, I report on the USPSTF cervical cancer screening recommendations, which were updated in October 2017.
Even with the widespread implementation of screening programs for cervical cancer in the United States, 13,240 women will be diagnosed with the disease in 2018, and 4,170 will die from cervical cancer.1 Most often, cervical cancer occurs in women who have not been adequately screened. It is now recognized that the human papillomavirus (HPV) is the cause of cervical cancer.2
While cervical cytology has long been used as a screening test for cervical cancer, testing for high-risk HPV subtypes (hrHPV testing) also has been used as a screening modality. Traditionally, hrHPV testing is used in combination with cervical cytology, so called cotesting. There is convincing evidence that cervical cytology, as well as strategies that use hrHPV testing, can detect high-grade cervical precancers and cancers and thereby reduce mortality. However, cervical cancer screening is also associated with frequent follow-ups, invasive procedures performed to assess abnormal results, psychological distress, and adverse pregnancy outcomes of treatment for precancerous lesions.
The USPSTF based its new cervical cancer screening recommendations on clinical trial data and decision modeling of various screening strategies, and weighed the benefits and harms of each strategy.
Recommendations from the USPSTF
hrHPV screening for cervical cancer. TheUSPSTF recommends screening with cervical cytology every 3 years for women 21 to 29 years of age. For women 30 to 65 years of age, screening with cytology every 3 years, or hrHPV testing alone used every 5 years, is recommended.
Data from large randomized trials suggest cytologic screening is slightly less sensitive than hrHPV testing in detecting high-grade (grade 2 or 3) cervical intraepithelial neoplasia (CIN). However, hrHPV testing results in more follow-up tests and colposcopies. In a decision model, the USPSTF found that cotesting increased the number of follow-up tests but did not increase detection of grade 3 CIN or invasive cancer. This is the first clinical guideline to recommend hrHPV testing used alone for screening. The American College of Obstetricians and Gynecologists (ACOG) continues to recommend cotesting (cytology in combination with hrHPV) as a primary screening modality in this population.3
Exceptions. According to the USPSTF, 3 populations should not be screened: women over 65 years of age with adequate prior screening who are not otherwise at high risk for cervical cancer; women under 21 years of age; and women who have had a hysterectomy and do not have a history of grade 2 or 3 CIN or cancer.
Summary. The USPSTF recommendations are intended for the general population and are not applicable to women with a history of high-grade CIN or cervical cancer, women with in utero exposure to diethylstilbestrol, and women who are immunocompromised. The remaining USPSTF recommendations are largely in line with guidelines published by ACOG and other groups.3,4
Testing for high-risk HPV alone is a reasonable screening option for cervical cancer. This modality can be used in women 30 to 65 years of age but should not be repeated more frequently than every 5 years in those with a negative result.
Read about SLN biopsy to stage endometrial cancer
SLN biopsy for staging endometrial cancer
Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18(3):384-392.
Surgery is the cornerstone of treatment for most gynecologic cancers. The widespread use of minimally invasive surgical techniques and the introduction of less radical procedures for gynecologic cancers have helped reduce surgical morbidity.
For endometrial cancer, the role of lymphadenectomy is controversial. Data from prospective trials of this procedure suggest an association with increased morbidity and long-term sequelae, such as lymphedema, and no association with improved survival.5,6
SLN biopsy is an important advance and a potential alternative nodal evaluation method that may be associated with decreased morbidity. In this more limited assessment technique, the first nodal drainage basins of a tumor are identified and removed for pathologic evaluation.
Accuracy of SLN biopsy in endometrial cancer was the subject of Rossi and colleagues' recent large prospective multicenter study, the Fluorescence Imaging for Robotic Endometrial Sentinel lymph node biopsy (FIRES) trial.
Details of the study
Rossi and colleagues conducted the FIRES trial to estimate the sensitivity of SLN biopsy in detecting nodal metastases in women with stage I endometrial cancer. Patients (N = 385) from 10 US sites were enrolled in the study. SLN evaluation was performed after cervical injection of indocyanine green followed by robotic-assisted hysterectomy. After identification of the SLN, participants underwent pelvic lymphadenectomy. Performance of para-aortic lymphadenectomy was optional.
Mapping of the SLN was feasible in 86% of patients, including bilateral mapping in 52%. Twelve percent of the participants had nodal metastases. SLN biopsy had a sensitivity of 97% in women who had identification of the SLNs. Similarly, the negative predictive value was high, 99.6%. The procedure was associated with acceptable short-term toxicity with adverse events in 9% of study participants. Common complications included neurologic complications, respiratory distress, nausea and vomiting, and, in 3 patients, bowel injury.
Accurate detection of nodal metastases. Results of the study suggest SLN biopsy is accurate in detecting nodal metastases in women with endometrial cancer. Although long-term toxicity was not examined, other work suggests the lymphedema rates associated with SLN biopsy may be lower than those of lymphadenectomy. While the study described impressive performance characteristics, there remain technical challenges. Even among skilled surgeons trained for the protocol, there was no nodal mapping in nearly half of the women with endometrial cancer. Women without node mapping require full lymphadenectomy thus negating the possible benefits of the procedure.
Given the high accuracy of SLN mapping in endometrial cancer, the procedure likely will become the standard of care for nodal evaluation by gynecologic oncologists.
Read about immunotherapy for gynecologic cancers
Immunotherapy for gynecologic cancers
Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413.
In oncology, precision medicine is rapidly becoming a standard treatment approach. Therapies are being used to target specific genetic alterations in tumors. In cancer immunotherapy, the immune system is being used to facilitate clearance of cancer cells.
The most common mechanism of action of clinically used immunotherapeutic agents is blockade of programmed cell death protein 1 (PD-1), a lymphocyte receptor that prevents the immune system from targeting the body's own cells.7 Cancers that have mutations in the DNA mismatch repair (MMR) proteins display microsatellite instability (MSI) and produce high levels of abnormal proteins.8 These abnormal proteins serve as tumor antigens that can be targeted by the body's normal immune system.
In May 2017, the US Food and Drug Administration (FDA) granted accelerated approval of the PD-1 blocking antibody pembrolizumab for the treatment of unresectable or metastatic MSI-high (MSI-H) or MMR-deficient solid tumors.9 The approval was based on data from 149 patients treated in 5 studies that demonstrated a response rate of 39.6%, including responses that lasted at least 6 months in 78% of participants. This was the first ever cancer drug that received FDA approval based on a tumor's biomarker profile without regard to the site of origin. I describe the results of a study by Le and colleagues that examines the possible role of immunotherapy in a variety of solid tumors in this section.
Details of the study
This study examined the clinical efficacy of PD-1 blockade in 86 patients with advanced, MMR-deficient tumors from 12 different sites. Endometrial cancer was the second most frequent primary tumor site in 17% of patients. Within the cohort, the overall objective response rate was 53%, which included 21% of patients with complete radiographic response (no imaging evidence of cancer). Disease control, either complete or partial response or stable disease, was achieved in 77% of patients. After a median follow-up of 12.5 months, neither the median progression-free survival (PFS) nor median overall survival had been reached. The authors estimated that 2-year overall survival was 64%, substantially higher than expected for patients with advanced solid tumors.
Le and colleagues also performed several in vivo laboratory experiments to explore the mechanisms by which patients responded. In addition, they used sequencing to determine the prevalence of MMR deficiency in 12,019 cancer samples that included 32 distinct tumor types (FIGURE). Endometrial cancer had the highest frequency of MMR deficiency (17%). Four percent of cervical cancers and less than 2% of ovarian cancers were MMR-deficient.
The promise of immunotherapy for endometrial cancer. This study's data and other emerging data have important implications for women with gynecologic cancer, particularly endometrial cancer. First, given the frequency of MMR mutations among women with endometrial cancer, MMR testing should be strongly considered for these patients. Many institutions have protocols for reflex testing with immunohistochemistry for women with endometrial cancer. For women with positive test results, germline sequencing can be performed to determine if they have an inherited MMR deficiency, Lynch syndrome. Presence of an MMR deficiency is an important factor in cancer screening and potential treatment.
Second, the impressive results of PD-1 blockade in patients with MMR-deficient tumors suggest that this treatment strategy may be important for women with recurrent or metastatic endometrial cancer. The ideal timing of immunotherapy for women with endometrial cancer is an area of active ongoing study.
Immunotherapy with PD-1 blockade is an important treatment strategy for women with MMR-deficient or MSI-H gynecologic cancers.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 168: Cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
- Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–1716.
- ASTEC Study Group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125–136.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264.
- Buza N, Ziai J, Hui P. Mismatch repair deficiency testing in clinical practice. Expert Rev Mol Diagn. 2016;16(5):591–604.
- FDA approves first cancer treatment for any solid tumor with a specific genetic feature [news release]. Silver Spring, MD: US Food and Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm560167.htm. Published May 23, 2017. Accessed February 5, 2018.
In this Update, I report on the latest US Preventive Services Task Force (USPSTF) cervical cancer screening recommendations. In addition, I describe the results of 2 studies, a large prospective multicenter study of the accuracy of sentinel lymph node (SLN) biopsy in endometrial cancer, and a proof-of-concept review of use of checkpoint blockade to increase immune response and of its possible role in endometrial cancer.
hrHPV testing used alone as primary screening for cervical cancer: USPSTF recommendations
US Preventive Services Task Force. Draft recommendation statement: cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Published October 2017. Accessed February 5, 2018.
Despite our rapid advances in understanding the molecular underpinnings of cancer, gynecologic malignancies are still a major cause of morbidity and mortality among women. Cervical cancer stands as an example of how a cancer screening test can be implemented to reduce mortality. In this section, I report on the USPSTF cervical cancer screening recommendations, which were updated in October 2017.
Even with the widespread implementation of screening programs for cervical cancer in the United States, 13,240 women will be diagnosed with the disease in 2018, and 4,170 will die from cervical cancer.1 Most often, cervical cancer occurs in women who have not been adequately screened. It is now recognized that the human papillomavirus (HPV) is the cause of cervical cancer.2
While cervical cytology has long been used as a screening test for cervical cancer, testing for high-risk HPV subtypes (hrHPV testing) also has been used as a screening modality. Traditionally, hrHPV testing is used in combination with cervical cytology, so called cotesting. There is convincing evidence that cervical cytology, as well as strategies that use hrHPV testing, can detect high-grade cervical precancers and cancers and thereby reduce mortality. However, cervical cancer screening is also associated with frequent follow-ups, invasive procedures performed to assess abnormal results, psychological distress, and adverse pregnancy outcomes of treatment for precancerous lesions.
The USPSTF based its new cervical cancer screening recommendations on clinical trial data and decision modeling of various screening strategies, and weighed the benefits and harms of each strategy.
Recommendations from the USPSTF
hrHPV screening for cervical cancer. TheUSPSTF recommends screening with cervical cytology every 3 years for women 21 to 29 years of age. For women 30 to 65 years of age, screening with cytology every 3 years, or hrHPV testing alone used every 5 years, is recommended.
Data from large randomized trials suggest cytologic screening is slightly less sensitive than hrHPV testing in detecting high-grade (grade 2 or 3) cervical intraepithelial neoplasia (CIN). However, hrHPV testing results in more follow-up tests and colposcopies. In a decision model, the USPSTF found that cotesting increased the number of follow-up tests but did not increase detection of grade 3 CIN or invasive cancer. This is the first clinical guideline to recommend hrHPV testing used alone for screening. The American College of Obstetricians and Gynecologists (ACOG) continues to recommend cotesting (cytology in combination with hrHPV) as a primary screening modality in this population.3
Exceptions. According to the USPSTF, 3 populations should not be screened: women over 65 years of age with adequate prior screening who are not otherwise at high risk for cervical cancer; women under 21 years of age; and women who have had a hysterectomy and do not have a history of grade 2 or 3 CIN or cancer.
Summary. The USPSTF recommendations are intended for the general population and are not applicable to women with a history of high-grade CIN or cervical cancer, women with in utero exposure to diethylstilbestrol, and women who are immunocompromised. The remaining USPSTF recommendations are largely in line with guidelines published by ACOG and other groups.3,4
Testing for high-risk HPV alone is a reasonable screening option for cervical cancer. This modality can be used in women 30 to 65 years of age but should not be repeated more frequently than every 5 years in those with a negative result.
Read about SLN biopsy to stage endometrial cancer
SLN biopsy for staging endometrial cancer
Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18(3):384-392.
Surgery is the cornerstone of treatment for most gynecologic cancers. The widespread use of minimally invasive surgical techniques and the introduction of less radical procedures for gynecologic cancers have helped reduce surgical morbidity.
For endometrial cancer, the role of lymphadenectomy is controversial. Data from prospective trials of this procedure suggest an association with increased morbidity and long-term sequelae, such as lymphedema, and no association with improved survival.5,6
SLN biopsy is an important advance and a potential alternative nodal evaluation method that may be associated with decreased morbidity. In this more limited assessment technique, the first nodal drainage basins of a tumor are identified and removed for pathologic evaluation.
Accuracy of SLN biopsy in endometrial cancer was the subject of Rossi and colleagues' recent large prospective multicenter study, the Fluorescence Imaging for Robotic Endometrial Sentinel lymph node biopsy (FIRES) trial.
Details of the study
Rossi and colleagues conducted the FIRES trial to estimate the sensitivity of SLN biopsy in detecting nodal metastases in women with stage I endometrial cancer. Patients (N = 385) from 10 US sites were enrolled in the study. SLN evaluation was performed after cervical injection of indocyanine green followed by robotic-assisted hysterectomy. After identification of the SLN, participants underwent pelvic lymphadenectomy. Performance of para-aortic lymphadenectomy was optional.
Mapping of the SLN was feasible in 86% of patients, including bilateral mapping in 52%. Twelve percent of the participants had nodal metastases. SLN biopsy had a sensitivity of 97% in women who had identification of the SLNs. Similarly, the negative predictive value was high, 99.6%. The procedure was associated with acceptable short-term toxicity with adverse events in 9% of study participants. Common complications included neurologic complications, respiratory distress, nausea and vomiting, and, in 3 patients, bowel injury.
Accurate detection of nodal metastases. Results of the study suggest SLN biopsy is accurate in detecting nodal metastases in women with endometrial cancer. Although long-term toxicity was not examined, other work suggests the lymphedema rates associated with SLN biopsy may be lower than those of lymphadenectomy. While the study described impressive performance characteristics, there remain technical challenges. Even among skilled surgeons trained for the protocol, there was no nodal mapping in nearly half of the women with endometrial cancer. Women without node mapping require full lymphadenectomy thus negating the possible benefits of the procedure.
Given the high accuracy of SLN mapping in endometrial cancer, the procedure likely will become the standard of care for nodal evaluation by gynecologic oncologists.
Read about immunotherapy for gynecologic cancers
Immunotherapy for gynecologic cancers
Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413.
In oncology, precision medicine is rapidly becoming a standard treatment approach. Therapies are being used to target specific genetic alterations in tumors. In cancer immunotherapy, the immune system is being used to facilitate clearance of cancer cells.
The most common mechanism of action of clinically used immunotherapeutic agents is blockade of programmed cell death protein 1 (PD-1), a lymphocyte receptor that prevents the immune system from targeting the body's own cells.7 Cancers that have mutations in the DNA mismatch repair (MMR) proteins display microsatellite instability (MSI) and produce high levels of abnormal proteins.8 These abnormal proteins serve as tumor antigens that can be targeted by the body's normal immune system.
In May 2017, the US Food and Drug Administration (FDA) granted accelerated approval of the PD-1 blocking antibody pembrolizumab for the treatment of unresectable or metastatic MSI-high (MSI-H) or MMR-deficient solid tumors.9 The approval was based on data from 149 patients treated in 5 studies that demonstrated a response rate of 39.6%, including responses that lasted at least 6 months in 78% of participants. This was the first ever cancer drug that received FDA approval based on a tumor's biomarker profile without regard to the site of origin. I describe the results of a study by Le and colleagues that examines the possible role of immunotherapy in a variety of solid tumors in this section.
Details of the study
This study examined the clinical efficacy of PD-1 blockade in 86 patients with advanced, MMR-deficient tumors from 12 different sites. Endometrial cancer was the second most frequent primary tumor site in 17% of patients. Within the cohort, the overall objective response rate was 53%, which included 21% of patients with complete radiographic response (no imaging evidence of cancer). Disease control, either complete or partial response or stable disease, was achieved in 77% of patients. After a median follow-up of 12.5 months, neither the median progression-free survival (PFS) nor median overall survival had been reached. The authors estimated that 2-year overall survival was 64%, substantially higher than expected for patients with advanced solid tumors.
Le and colleagues also performed several in vivo laboratory experiments to explore the mechanisms by which patients responded. In addition, they used sequencing to determine the prevalence of MMR deficiency in 12,019 cancer samples that included 32 distinct tumor types (FIGURE). Endometrial cancer had the highest frequency of MMR deficiency (17%). Four percent of cervical cancers and less than 2% of ovarian cancers were MMR-deficient.
The promise of immunotherapy for endometrial cancer. This study's data and other emerging data have important implications for women with gynecologic cancer, particularly endometrial cancer. First, given the frequency of MMR mutations among women with endometrial cancer, MMR testing should be strongly considered for these patients. Many institutions have protocols for reflex testing with immunohistochemistry for women with endometrial cancer. For women with positive test results, germline sequencing can be performed to determine if they have an inherited MMR deficiency, Lynch syndrome. Presence of an MMR deficiency is an important factor in cancer screening and potential treatment.
Second, the impressive results of PD-1 blockade in patients with MMR-deficient tumors suggest that this treatment strategy may be important for women with recurrent or metastatic endometrial cancer. The ideal timing of immunotherapy for women with endometrial cancer is an area of active ongoing study.
Immunotherapy with PD-1 blockade is an important treatment strategy for women with MMR-deficient or MSI-H gynecologic cancers.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In this Update, I report on the latest US Preventive Services Task Force (USPSTF) cervical cancer screening recommendations. In addition, I describe the results of 2 studies, a large prospective multicenter study of the accuracy of sentinel lymph node (SLN) biopsy in endometrial cancer, and a proof-of-concept review of use of checkpoint blockade to increase immune response and of its possible role in endometrial cancer.
hrHPV testing used alone as primary screening for cervical cancer: USPSTF recommendations
US Preventive Services Task Force. Draft recommendation statement: cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Published October 2017. Accessed February 5, 2018.
Despite our rapid advances in understanding the molecular underpinnings of cancer, gynecologic malignancies are still a major cause of morbidity and mortality among women. Cervical cancer stands as an example of how a cancer screening test can be implemented to reduce mortality. In this section, I report on the USPSTF cervical cancer screening recommendations, which were updated in October 2017.
Even with the widespread implementation of screening programs for cervical cancer in the United States, 13,240 women will be diagnosed with the disease in 2018, and 4,170 will die from cervical cancer.1 Most often, cervical cancer occurs in women who have not been adequately screened. It is now recognized that the human papillomavirus (HPV) is the cause of cervical cancer.2
While cervical cytology has long been used as a screening test for cervical cancer, testing for high-risk HPV subtypes (hrHPV testing) also has been used as a screening modality. Traditionally, hrHPV testing is used in combination with cervical cytology, so called cotesting. There is convincing evidence that cervical cytology, as well as strategies that use hrHPV testing, can detect high-grade cervical precancers and cancers and thereby reduce mortality. However, cervical cancer screening is also associated with frequent follow-ups, invasive procedures performed to assess abnormal results, psychological distress, and adverse pregnancy outcomes of treatment for precancerous lesions.
The USPSTF based its new cervical cancer screening recommendations on clinical trial data and decision modeling of various screening strategies, and weighed the benefits and harms of each strategy.
Recommendations from the USPSTF
hrHPV screening for cervical cancer. TheUSPSTF recommends screening with cervical cytology every 3 years for women 21 to 29 years of age. For women 30 to 65 years of age, screening with cytology every 3 years, or hrHPV testing alone used every 5 years, is recommended.
Data from large randomized trials suggest cytologic screening is slightly less sensitive than hrHPV testing in detecting high-grade (grade 2 or 3) cervical intraepithelial neoplasia (CIN). However, hrHPV testing results in more follow-up tests and colposcopies. In a decision model, the USPSTF found that cotesting increased the number of follow-up tests but did not increase detection of grade 3 CIN or invasive cancer. This is the first clinical guideline to recommend hrHPV testing used alone for screening. The American College of Obstetricians and Gynecologists (ACOG) continues to recommend cotesting (cytology in combination with hrHPV) as a primary screening modality in this population.3
Exceptions. According to the USPSTF, 3 populations should not be screened: women over 65 years of age with adequate prior screening who are not otherwise at high risk for cervical cancer; women under 21 years of age; and women who have had a hysterectomy and do not have a history of grade 2 or 3 CIN or cancer.
Summary. The USPSTF recommendations are intended for the general population and are not applicable to women with a history of high-grade CIN or cervical cancer, women with in utero exposure to diethylstilbestrol, and women who are immunocompromised. The remaining USPSTF recommendations are largely in line with guidelines published by ACOG and other groups.3,4
Testing for high-risk HPV alone is a reasonable screening option for cervical cancer. This modality can be used in women 30 to 65 years of age but should not be repeated more frequently than every 5 years in those with a negative result.
Read about SLN biopsy to stage endometrial cancer
SLN biopsy for staging endometrial cancer
Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18(3):384-392.
Surgery is the cornerstone of treatment for most gynecologic cancers. The widespread use of minimally invasive surgical techniques and the introduction of less radical procedures for gynecologic cancers have helped reduce surgical morbidity.
For endometrial cancer, the role of lymphadenectomy is controversial. Data from prospective trials of this procedure suggest an association with increased morbidity and long-term sequelae, such as lymphedema, and no association with improved survival.5,6
SLN biopsy is an important advance and a potential alternative nodal evaluation method that may be associated with decreased morbidity. In this more limited assessment technique, the first nodal drainage basins of a tumor are identified and removed for pathologic evaluation.
Accuracy of SLN biopsy in endometrial cancer was the subject of Rossi and colleagues' recent large prospective multicenter study, the Fluorescence Imaging for Robotic Endometrial Sentinel lymph node biopsy (FIRES) trial.
Details of the study
Rossi and colleagues conducted the FIRES trial to estimate the sensitivity of SLN biopsy in detecting nodal metastases in women with stage I endometrial cancer. Patients (N = 385) from 10 US sites were enrolled in the study. SLN evaluation was performed after cervical injection of indocyanine green followed by robotic-assisted hysterectomy. After identification of the SLN, participants underwent pelvic lymphadenectomy. Performance of para-aortic lymphadenectomy was optional.
Mapping of the SLN was feasible in 86% of patients, including bilateral mapping in 52%. Twelve percent of the participants had nodal metastases. SLN biopsy had a sensitivity of 97% in women who had identification of the SLNs. Similarly, the negative predictive value was high, 99.6%. The procedure was associated with acceptable short-term toxicity with adverse events in 9% of study participants. Common complications included neurologic complications, respiratory distress, nausea and vomiting, and, in 3 patients, bowel injury.
Accurate detection of nodal metastases. Results of the study suggest SLN biopsy is accurate in detecting nodal metastases in women with endometrial cancer. Although long-term toxicity was not examined, other work suggests the lymphedema rates associated with SLN biopsy may be lower than those of lymphadenectomy. While the study described impressive performance characteristics, there remain technical challenges. Even among skilled surgeons trained for the protocol, there was no nodal mapping in nearly half of the women with endometrial cancer. Women without node mapping require full lymphadenectomy thus negating the possible benefits of the procedure.
Given the high accuracy of SLN mapping in endometrial cancer, the procedure likely will become the standard of care for nodal evaluation by gynecologic oncologists.
Read about immunotherapy for gynecologic cancers
Immunotherapy for gynecologic cancers
Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413.
In oncology, precision medicine is rapidly becoming a standard treatment approach. Therapies are being used to target specific genetic alterations in tumors. In cancer immunotherapy, the immune system is being used to facilitate clearance of cancer cells.
The most common mechanism of action of clinically used immunotherapeutic agents is blockade of programmed cell death protein 1 (PD-1), a lymphocyte receptor that prevents the immune system from targeting the body's own cells.7 Cancers that have mutations in the DNA mismatch repair (MMR) proteins display microsatellite instability (MSI) and produce high levels of abnormal proteins.8 These abnormal proteins serve as tumor antigens that can be targeted by the body's normal immune system.
In May 2017, the US Food and Drug Administration (FDA) granted accelerated approval of the PD-1 blocking antibody pembrolizumab for the treatment of unresectable or metastatic MSI-high (MSI-H) or MMR-deficient solid tumors.9 The approval was based on data from 149 patients treated in 5 studies that demonstrated a response rate of 39.6%, including responses that lasted at least 6 months in 78% of participants. This was the first ever cancer drug that received FDA approval based on a tumor's biomarker profile without regard to the site of origin. I describe the results of a study by Le and colleagues that examines the possible role of immunotherapy in a variety of solid tumors in this section.
Details of the study
This study examined the clinical efficacy of PD-1 blockade in 86 patients with advanced, MMR-deficient tumors from 12 different sites. Endometrial cancer was the second most frequent primary tumor site in 17% of patients. Within the cohort, the overall objective response rate was 53%, which included 21% of patients with complete radiographic response (no imaging evidence of cancer). Disease control, either complete or partial response or stable disease, was achieved in 77% of patients. After a median follow-up of 12.5 months, neither the median progression-free survival (PFS) nor median overall survival had been reached. The authors estimated that 2-year overall survival was 64%, substantially higher than expected for patients with advanced solid tumors.
Le and colleagues also performed several in vivo laboratory experiments to explore the mechanisms by which patients responded. In addition, they used sequencing to determine the prevalence of MMR deficiency in 12,019 cancer samples that included 32 distinct tumor types (FIGURE). Endometrial cancer had the highest frequency of MMR deficiency (17%). Four percent of cervical cancers and less than 2% of ovarian cancers were MMR-deficient.
The promise of immunotherapy for endometrial cancer. This study's data and other emerging data have important implications for women with gynecologic cancer, particularly endometrial cancer. First, given the frequency of MMR mutations among women with endometrial cancer, MMR testing should be strongly considered for these patients. Many institutions have protocols for reflex testing with immunohistochemistry for women with endometrial cancer. For women with positive test results, germline sequencing can be performed to determine if they have an inherited MMR deficiency, Lynch syndrome. Presence of an MMR deficiency is an important factor in cancer screening and potential treatment.
Second, the impressive results of PD-1 blockade in patients with MMR-deficient tumors suggest that this treatment strategy may be important for women with recurrent or metastatic endometrial cancer. The ideal timing of immunotherapy for women with endometrial cancer is an area of active ongoing study.
Immunotherapy with PD-1 blockade is an important treatment strategy for women with MMR-deficient or MSI-H gynecologic cancers.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 168: Cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
- Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–1716.
- ASTEC Study Group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125–136.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264.
- Buza N, Ziai J, Hui P. Mismatch repair deficiency testing in clinical practice. Expert Rev Mol Diagn. 2016;16(5):591–604.
- FDA approves first cancer treatment for any solid tumor with a specific genetic feature [news release]. Silver Spring, MD: US Food and Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm560167.htm. Published May 23, 2017. Accessed February 5, 2018.
- American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 168: Cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
- Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–1716.
- ASTEC Study Group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125–136.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264.
- Buza N, Ziai J, Hui P. Mismatch repair deficiency testing in clinical practice. Expert Rev Mol Diagn. 2016;16(5):591–604.
- FDA approves first cancer treatment for any solid tumor with a specific genetic feature [news release]. Silver Spring, MD: US Food and Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm560167.htm. Published May 23, 2017. Accessed February 5, 2018.
Patient-centered risk assessment for ovarian cancer: Individualizing your approach
Is sentinel lymph node mapping associated with acceptable performance characteristics for the detection of nodal metastases in women with endometrial cancer?
EXPERT COMMENTARY
The role of lymphadenectomy for endometrial cancer has evolved considerably over the last 30 years. While pathologic assessment of the nodes provides important information to tailor adjuvant therapy, 2 randomized trials both reported no survival benefit in women who underwent lymphadenectomy compared with hysterectomy alone.1,2 Further, these trials revealed that lymphadenectomy was associated with significant short- and long-term sequelae.
SLN biopsy, a procedure in which a small number of nodes that represent the first drainage basins of a primary tumor are removed, has been proposed as an alternative to traditional lymphadenectomy. Although SLN biopsy is commonly used for other solid tumors, few large, multicenter studies have been conducted to evaluate the technique’s safety in endometrial cancer.
Related article:
2016 Update on cancer
Details of the study
The Fluorescence Imaging for Robotic Endometrial Sentinel lymph node biopsy (FIRES) trial was a prospective trial evaluating the performance characteristics of SLN biopsy in women with clinical stage 1 endometrial cancer at 10 sites in the United States. After cervical injection of indocyanine green, patients underwent robot-assisted hysterectomy with SLN biopsy followed by pelvic lymphadenectomy. Para-aortic lymphadenectomy was performed at the discretion of the attending surgeon. The study’s primary end point was sensitivity of SLN biopsy for detecting metastatic disease in women who had mapping.
Over approximately 3 years, 385 patients were enrolled. Overall, 86% of patients had mapping of at least 1 SLN and 52% had bilateral mapping. Positive nodes were found in 12% of the study population. Among women who had SLNs identified, 35 of 36 nodal metastases were identified (97% sensitivity). Negative SLNs correctly predicted the absence of metastases (negative predictive value) in 99.6% of patients.
Overall, the procedure was well tolerated. Adverse events were noted in 9% of patients, and approximately two-thirds were considered serious adverse events. The most common adverse events were neurologic complications, respiratory distress, nausea and vomiting, and bowel injury in 3 patients. One ureteral injury occurred during SLN biopsy.
Related article:
Does laparoscopic versus open abdominal surgery for stage I endometrial cancer affect oncologic outcomes?
Study strengths and weaknesses
The FIRES study provides strong evidence for the effectiveness of SLN biopsy in women with apparent early stage endometrial cancer. The procedure not only was highly accurate in identifying nodal disease but it also had acceptable adverse events. Further, many of the benefits of SLN biopsy, such as a reduction in lymphedema, will require long-term follow-up.
Consider study results in context. As oncologists consider the role of SLN biopsy in practice, this work should be interpreted in the context of the study design. The study was performed by only 18 surgeons at 10 centers. Prior to study initiation, each site and surgeon underwent formal training and observation to ensure that the technique for SLN biopsy was adequate. Clearly, there will be a learning curve for SLN biopsy, and this study’s results may not immediately be generalizable.
Despite rigorous quality control procedures, there was no nodal mapping in 48% of the hemi-pelvises. In practice, these patients require lymph node dissection. The authors estimated that 50% of patients would still require lymphadenectomy (40% unilateral, 10% bilateral) if SLN mapping was used in routine practice. In addition, while the FIRES trial included women with high-risk histologies, the majority of patients had low-risk, endometrioid tumors. Further study will help to define performance of SLN biopsy in populations at higher risk for nodal metastases.
--Jason D. Wright, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–1716.
- ASTEC Study Group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125–136.
EXPERT COMMENTARY
The role of lymphadenectomy for endometrial cancer has evolved considerably over the last 30 years. While pathologic assessment of the nodes provides important information to tailor adjuvant therapy, 2 randomized trials both reported no survival benefit in women who underwent lymphadenectomy compared with hysterectomy alone.1,2 Further, these trials revealed that lymphadenectomy was associated with significant short- and long-term sequelae.
SLN biopsy, a procedure in which a small number of nodes that represent the first drainage basins of a primary tumor are removed, has been proposed as an alternative to traditional lymphadenectomy. Although SLN biopsy is commonly used for other solid tumors, few large, multicenter studies have been conducted to evaluate the technique’s safety in endometrial cancer.
Related article:
2016 Update on cancer
Details of the study
The Fluorescence Imaging for Robotic Endometrial Sentinel lymph node biopsy (FIRES) trial was a prospective trial evaluating the performance characteristics of SLN biopsy in women with clinical stage 1 endometrial cancer at 10 sites in the United States. After cervical injection of indocyanine green, patients underwent robot-assisted hysterectomy with SLN biopsy followed by pelvic lymphadenectomy. Para-aortic lymphadenectomy was performed at the discretion of the attending surgeon. The study’s primary end point was sensitivity of SLN biopsy for detecting metastatic disease in women who had mapping.
Over approximately 3 years, 385 patients were enrolled. Overall, 86% of patients had mapping of at least 1 SLN and 52% had bilateral mapping. Positive nodes were found in 12% of the study population. Among women who had SLNs identified, 35 of 36 nodal metastases were identified (97% sensitivity). Negative SLNs correctly predicted the absence of metastases (negative predictive value) in 99.6% of patients.
Overall, the procedure was well tolerated. Adverse events were noted in 9% of patients, and approximately two-thirds were considered serious adverse events. The most common adverse events were neurologic complications, respiratory distress, nausea and vomiting, and bowel injury in 3 patients. One ureteral injury occurred during SLN biopsy.
Related article:
Does laparoscopic versus open abdominal surgery for stage I endometrial cancer affect oncologic outcomes?
Study strengths and weaknesses
The FIRES study provides strong evidence for the effectiveness of SLN biopsy in women with apparent early stage endometrial cancer. The procedure not only was highly accurate in identifying nodal disease but it also had acceptable adverse events. Further, many of the benefits of SLN biopsy, such as a reduction in lymphedema, will require long-term follow-up.
Consider study results in context. As oncologists consider the role of SLN biopsy in practice, this work should be interpreted in the context of the study design. The study was performed by only 18 surgeons at 10 centers. Prior to study initiation, each site and surgeon underwent formal training and observation to ensure that the technique for SLN biopsy was adequate. Clearly, there will be a learning curve for SLN biopsy, and this study’s results may not immediately be generalizable.
Despite rigorous quality control procedures, there was no nodal mapping in 48% of the hemi-pelvises. In practice, these patients require lymph node dissection. The authors estimated that 50% of patients would still require lymphadenectomy (40% unilateral, 10% bilateral) if SLN mapping was used in routine practice. In addition, while the FIRES trial included women with high-risk histologies, the majority of patients had low-risk, endometrioid tumors. Further study will help to define performance of SLN biopsy in populations at higher risk for nodal metastases.
--Jason D. Wright, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
The role of lymphadenectomy for endometrial cancer has evolved considerably over the last 30 years. While pathologic assessment of the nodes provides important information to tailor adjuvant therapy, 2 randomized trials both reported no survival benefit in women who underwent lymphadenectomy compared with hysterectomy alone.1,2 Further, these trials revealed that lymphadenectomy was associated with significant short- and long-term sequelae.
SLN biopsy, a procedure in which a small number of nodes that represent the first drainage basins of a primary tumor are removed, has been proposed as an alternative to traditional lymphadenectomy. Although SLN biopsy is commonly used for other solid tumors, few large, multicenter studies have been conducted to evaluate the technique’s safety in endometrial cancer.
Related article:
2016 Update on cancer
Details of the study
The Fluorescence Imaging for Robotic Endometrial Sentinel lymph node biopsy (FIRES) trial was a prospective trial evaluating the performance characteristics of SLN biopsy in women with clinical stage 1 endometrial cancer at 10 sites in the United States. After cervical injection of indocyanine green, patients underwent robot-assisted hysterectomy with SLN biopsy followed by pelvic lymphadenectomy. Para-aortic lymphadenectomy was performed at the discretion of the attending surgeon. The study’s primary end point was sensitivity of SLN biopsy for detecting metastatic disease in women who had mapping.
Over approximately 3 years, 385 patients were enrolled. Overall, 86% of patients had mapping of at least 1 SLN and 52% had bilateral mapping. Positive nodes were found in 12% of the study population. Among women who had SLNs identified, 35 of 36 nodal metastases were identified (97% sensitivity). Negative SLNs correctly predicted the absence of metastases (negative predictive value) in 99.6% of patients.
Overall, the procedure was well tolerated. Adverse events were noted in 9% of patients, and approximately two-thirds were considered serious adverse events. The most common adverse events were neurologic complications, respiratory distress, nausea and vomiting, and bowel injury in 3 patients. One ureteral injury occurred during SLN biopsy.
Related article:
Does laparoscopic versus open abdominal surgery for stage I endometrial cancer affect oncologic outcomes?
Study strengths and weaknesses
The FIRES study provides strong evidence for the effectiveness of SLN biopsy in women with apparent early stage endometrial cancer. The procedure not only was highly accurate in identifying nodal disease but it also had acceptable adverse events. Further, many of the benefits of SLN biopsy, such as a reduction in lymphedema, will require long-term follow-up.
Consider study results in context. As oncologists consider the role of SLN biopsy in practice, this work should be interpreted in the context of the study design. The study was performed by only 18 surgeons at 10 centers. Prior to study initiation, each site and surgeon underwent formal training and observation to ensure that the technique for SLN biopsy was adequate. Clearly, there will be a learning curve for SLN biopsy, and this study’s results may not immediately be generalizable.
Despite rigorous quality control procedures, there was no nodal mapping in 48% of the hemi-pelvises. In practice, these patients require lymph node dissection. The authors estimated that 50% of patients would still require lymphadenectomy (40% unilateral, 10% bilateral) if SLN mapping was used in routine practice. In addition, while the FIRES trial included women with high-risk histologies, the majority of patients had low-risk, endometrioid tumors. Further study will help to define performance of SLN biopsy in populations at higher risk for nodal metastases.
--Jason D. Wright, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–1716.
- ASTEC Study Group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125–136.
- Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–1716.
- ASTEC Study Group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125–136.
2017 Update on ovarian cancer
In 2017, an estimated 22,240 women will be diagnosed with ovarian cancer, and 14,080 women will die of the disease.1 The high mortality associated with ovarian cancer is due largely to the inability to detect the disease early and the lack of effective therapeutics for women with recurrent disease. In this Update, we review important advances in the diagnosis and treatment of ovarian cancer.
Development of an effective screening tool for women at average risk has been an elusive challenge. The United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) examined the efficacy of transvaginal ultrasound and cancer antigen 125 (CA 125) monitoring for ovarian cancer in a large cohort of women.
For women diagnosed with ovarian cancer, treatment paradigms for the initial management of the disease have shifted dramatically. Based on data from multiple randomized controlled trials, neoadjuvant chemotherapy (NACT) is being used more frequently. The American Society of Clinical Oncology and the Society of Gynecologic Oncology developed consensus recommendations for the appropriate use of NACT and primary cytoreductive surgery for women with ovarian cancer.
Finally, all of oncology has moved toward incorporating molecularly targeted therapeutics directed toward individual genetic abnormalities in tumors, so-called precision medicine. In ovarian cancer, poly(adenosine diphosphate [ADP]–ribose) polymerase (PARP) has emerged as an important target, particularly for women with BRCA gene pathway mutations. We describe a recently published randomized controlled trial of the PARP inhibitor niraparib.
Read about ovarian cancer screening tests
Is CA 125 or ultrasound screening appropriate for the general population?
Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387(10022):945-956.
In the United States, the overall ovarian cancer 5-year survival rate is 46.2%, resulting in more than 14,000 deaths annually.2 The poor prognosis associated with this malignancy is largely attributable to the fact that almost 75% of women have stage III or stage IV disease at the time of diagnosis.2 Ovarian cancer is usually associated with vague, nonspecific symptoms as it progresses, which contributes to delayed diagnosis and increased mortality.
Multiple studies have examined pelvic ultrasonography and tumor markers, such as CA 125, as possible screening tools to increase early detection in asymptomatic women. However, neither modality alone or in combination has sufficient sensitivity or specificity to recommend it for use in the general population.3,4 Nevertheless, the search for an appropriate screening tool continues, and the UKCTOCS trial results have reinvigorated this discussion.5
The UKCTOCS findings
The UKCTOCS was a multicenter, randomized controlled trial in the United Kingdom in which researchers allocated 202,638 women aged 50 to 74 years to 1 of 3 groups: annual multimodal screening (MMS) with serum CA 125 interpreted with the use of the risk of ovarian cancer algorithm, annual transvaginal ultrasound screening (USS), or no screening. The median follow-up was more than 11 years.
The investigators found that equivalent rates of ovarian cancer were diagnosed in each group: 0.7% in the MMS group, 0.6% in the USS group, and 0.6% in the no-screening group. Overall, there was no significant reduction in the mortality rate from ovarian cancer in either of the 2 screening groups compared with the no-screening group.5
An important subset discovery
However, in a prespecified subset analysis excluding "prevalent cases" (women with ovarian cancer thought to be present prior to randomization and subsequent screening), ovarian cancer mortality was significantly lower in the MMS group compared with the no-screening group (P = .021). Compared with no screening, MMS was associated with a 20% reduction in mortality rate from ovarian cancer over time, with the most pronounced effects occurring at years 7 to 14 of follow-up, suggesting the possible increased effectiveness of screening over time.5
Related article:
Can CA 125 screening reduce mortality from ovarian cancer?
Concordance with other screening trials
While impressive in study magnitude and scope, the UKCTOCS results did not demonstrate a significant mortality benefit associated with MMS or USS when compared with no screening. Although the screening complications were low (<1% in both screening groups), the authors did note a false-positive surgery rate of 14 per 10,000 screens for the MMS group and 50 per 10,000 screens for the USS group. Based on the performance of screening in this trial, 641 women would need to be screened annually using MMS for 14 years to prevent 1 ovarian cancer death.
Like the UKCTOCS, the ovarian cancer-screening arm of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial in the United States was also unable to demonstrate a reduction in mortality rate with screening with CA 125 and transvaginal ultrasound. Importantly, more than one-third of women with a false-positive screen underwent surgery and 15% of them experienced a major complication.6 Based on these findings, the US Preventive Services Task Force grades screening for ovarian cancer as D, suggesting that the harms of screening may outweigh the benefits.7
While screening for ovarian cancer remains an important need, there is currently no evidence to suggest that serum tumor marker or ultrasound screening is appropriate in the general population. Studies using more specific screening tests or strategies targeted to higher-risk women are ongoing.
Read about patient selection for neoadjuvant chemotherapy
New clinical practice guideline advises neoadjuvant chemotherapy for certain women with ovarian cancer
Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(28):3460-3473.
It has long been held as a central dogma that primary cytoreductive surgery (PCS) is the preferred initial treatment for women with newly diagnosed ovarian cancer.8 However, PCS is associated with substantial morbidity, and the ability to achieve optimal cytoreduction (<1 cm of residual disease), an important prognostic factor, is often compromised in women with significant tumor burden.9,10
Neoadjuvant chemotherapy, in which chemotherapy is administered prior to surgical cytoreduction, challenges the traditional treatment paradigm for advanced-stage ovarian cancer. Several randomized controlled trials have reported equivalent survival for primary surgical cytoreduction and NACT. Importantly, women who received NACT had fewer complications and were more likely to have optimal cytoreduction at the time of surgery.11,12 These studies have limitations, however, and the role of NACT remains uncertain.
To help guide clinicians, the Society of Gynecologic Oncology and the American Society of Clinical Oncology convened an expert panel to provide recommendations and guidance on the evaluation of women for and the use of NACT in the setting of advanced ovarian cancer.13
Related article:
2015 Update on cancer
Recommendation: Clinical evaluation and patient selection
Strong clinical evidence supports that all women with suspected stage IIIC or stage IV ovarian cancer should be evaluated by a gynecologic oncologist prior to the initiation of therapy. The evaluation should include at least a computed tomography scan of the chest, abdomen, and pelvis to assess the extent of disease and resectability. A preoperative risk assessment should be performed to assess risk factors for increased morbidity and mortality.
Women who have a high perioperative risk profile or a low likelihood of achieving cytoreduction to 1 cm or less of residual tumor should receive NACT. Prior to the initiation of NACT, histologic confirmation of ovarian cancer should be obtained.13
Outcomes for neoadjuvant chemotherapy versus primary cytoreduction
Four phase 3 randomized controlled trials (EORTC 55971, CHORUS, JCOG0602, and SCORPION) suggest that NACT is noninferior to PCS with regard to progression-free survival and overall survival. NACT is associated with less perioperative and postoperative morbidity and mortality and is associated with shorter hospital stays.
To date, complete data are available only from the EORTC and CHORUS trials, which both demonstrated similar progression-free survival and overall survival for NACT and PCS. Critics have noted, however, that both trials have shorter median overall survival for the PCS groups than were previously reported in other phase 3 studies in the United States, suggesting the possibility of different patient populations or less aggressive "surgical effort." Thus, PCS remains the preferred management strategy for women with advanced-stage ovarian cancer in whom there is a high likelihood of optimal cytoreduction.13
Recommendation: Use of neoadjuvant chemotherapy
Patients who are appropriate candidates for NACT should be treated with a platinum and taxane doublet and should receive interval cytoreduction following 3 to 4 cycles of therapy if a favorable response is noted. Patients whose disease progresses despite NACT have a poor prognosis, and there is little role for surgical treatment with the exception of palliative purposes.13
Neoadjuvant chemotherapy is a noninferior and appropriate treatment option for women who are poor surgical candidates or who have a low likelihood of optimal cytoreduction. When optimal cytoreduction is possible, however, PCS is preferred (see FIGURE). The data on the efficacy of NACT for ovarian cancer have led to increased use of this treatment in the United States.
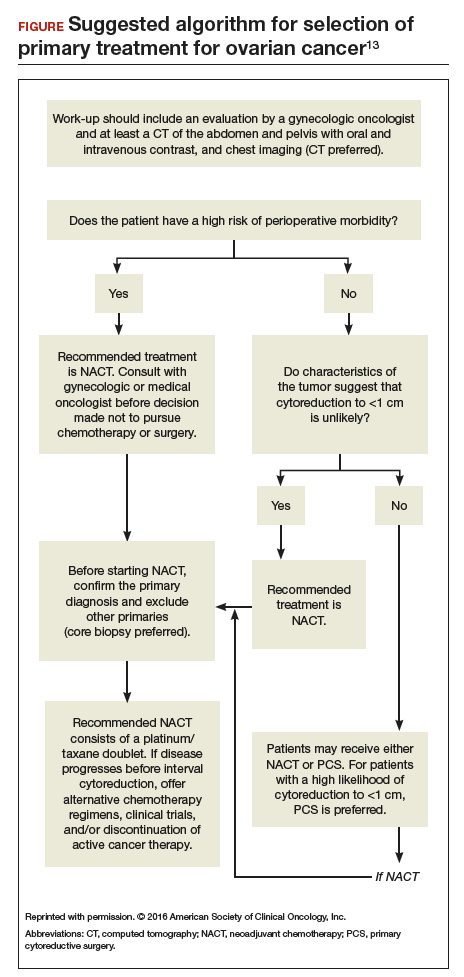
Read about a new PARP inhibitor for maintenance therapy
Niraparib is promising as maintenance therapy in ovarian cancer
Mirza MR, Monk BJ, Herrstedt J, et al; for the ENGOT-OV16/NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154-2164.
Approximately 85% of women with ovarian cancer will develop recurrent disease. Women with ovarian cancer are commonly treated with a range of antineoplastic agents over the course of their lifetime. As such, there is a great need for additional active therapeutic agents in this setting. Recently, substantial effort has been directed toward "precision" or "personalized medicine" in oncology.
Precision medicine, targeted therapies in oncology
Precision medicine refers to the customization of medical therapy based on the genetic characterization of the individual patient or the molecular profile of the patient's tumor. As a result of large-scale molecular profiling from projects such as the International Cancer Genome Consortium and The Cancer Genome Atlas, an abundance of molecular data has been generated through the characterization of multiple tumor types. This has led to the discovery of key cancer drivers, alterations, and specific molecular profiles that have distinct prognostic and treatment implications. These data, in combination with the commercial availability of molecular profiling tests, has made precision medicine a reality for women with ovarian cancer.
This wealth of new information has led to development of targeted therapeutics that block the growth and spread of cancer by acting on specific molecules or molecular pathways. Targeted therapies approved for cancer treatment include hormonal therapies, signal transduction inhibitors, gene expression modulators, apoptosis inducers, angiogenesis inhibitors, and immunotherapies.14
How PARP inhibitors work
PARP inhibitors are a class of agents that are emerging as important therapies for ovarian cancer. These agents block the nuclear protein PARP, which functions to detect and repair single-strand DNA breaks with the resulting accumulation of double-stranded DNA breaks.15 In the setting of DNA damage, the homologous recombination repair pathway is activated for repair. However, homologous recombination deficiencies (HRD) can arise as a result of BRCA1 or BRCA2 mutations or BRCA-independent pathways, which effectively disable this DNA repair pathway. As a result, when PARP inhibitors are used in patients with HRD, the cell cannot repair double-stranded DNA breaks and this leads to "synthetic lethality."16
Understanding this molecular mechanism of PARP inhibitors as well as the frequent abnormalities in the BRCA genes and HRD pathways in ovarian cancer has provided an important potential therapeutic target in ovarian cancer. A number of PARP inhibitors are now commercially available and are undergoing testing in ovarian cancer.
Related article:
Is a minimally invasive approach to hysterectomy for Gyn cancer utilized equally in all racial and income groups?
Niraparib for ovarian cancer
In a randomized, double-blind, phase 3 trial by Mizra and colleagues, 553 women with platinum-sensitive recurrent ovarian cancer who responded to therapy were divided according to the presence or absence of a germline BRCA (gBRCA) mutation and randomly assigned to niraparib 300 mg or placebo once daily. Women in the niraparib group had a significantly longer median duration of progression-free survival than did those in the placebo group. This was most pronounced in women in the gBRCA cohort (21.0 vs 5.5 months). Importantly, niraparib was associated with improved progression-free survival in HRD-positive patients without gBRCA mutations (12.9 vs 3.8 months) as well as in the HRD-negative subgroup (6.9 vs 3.8 months).17
Overall, niraparib was well tolerated. About 15% of women discontinued the drug due to toxicity. Significant (grade 3 or 4) adverse events were seen in three-quarters of women treated with niraparib, and they most commonly consisted of hematologic toxicities. Patient-reported outcomes were similar for both groups, indicating no significant effect from niraparib on quality of life.17
This study's results suggest that niraparib has clinical activity against ovarian cancer. Importantly, niraparib was active in women with gBRCA mutations, in those with HRD without a gBRCA mutation, and potentially in women without HRD. If approved by the US Food and Drug Administration, niraparib will join olaparib and rucaparib as a newly approved therapeutic agent for ovarian cancer. This study provides important evidence that suggests niraparib maintenance therapy may be an efficacious and important addition to the treatment armamentarium for platinum-sensitive ovarian cancer.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: ovarian cancer. https://seer.cancer.gov/statfacts/html/ovary.html. Accessed January 20, 2017.
- Jacobs I, Davies AP, Bridges J, et al. Prevalence screening for ovarian cancer in postmenopausal women by CA 125 measurement and ultrasonography. BMJ. 1993;306(6884):1030–1034.
- van Nagell JR Jr, Pavlik EJ. Ovarian cancer screening. Clin Obstet Gynecol. 2012;55:43–51.
- Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387(10022):945–956.
- Buys SS, Partridge E, Black A, et al; PLCO Project Team. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening randomized controlled trial. JAMA. 2011;305(22):2295–2303.
- Moyer VA, US Preventive Services Task Force. Screening for ovarian cancer: US Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2012;157(12):900–904.
- Schorge JO, McCann C, Del Carmen MG. Surgical debulking of ovarian cancer: what difference does it make? Rev Obstet Gynecol. 2010;3(3):111–117.
- Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170(4):974–979; discussion 979–980.
- Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–1259.
- Vergote I, Trope CG, Amant F, et al; European Organization for Research and Treatment of Cancer–Gynaecological Cancer Goup; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953.
- Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386(9990):249–257.
- Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(28):3460–3473.
- National Cancer Institute. Targeted cancer therapies. https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet. Updated April 25, 2014. Accessed January 21, 2017.
- Drean A, Lord CJ, Ashworth A. PARP inhibitor combination therapy. Crit Rev Oncol Hematol. 2016;108:73–85.
- Ledermann JA, El-Khouly F. PARP inhibitors in ovarian cancer: clinical evidence for informed treatment decisions. Br J Cancer. 2015;113(suppl 1):S10–S16.
- Mirza MR, Monk BJ, Herrstedt J, et al; ENGOT-OV16/NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–2164.
In 2017, an estimated 22,240 women will be diagnosed with ovarian cancer, and 14,080 women will die of the disease.1 The high mortality associated with ovarian cancer is due largely to the inability to detect the disease early and the lack of effective therapeutics for women with recurrent disease. In this Update, we review important advances in the diagnosis and treatment of ovarian cancer.
Development of an effective screening tool for women at average risk has been an elusive challenge. The United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) examined the efficacy of transvaginal ultrasound and cancer antigen 125 (CA 125) monitoring for ovarian cancer in a large cohort of women.
For women diagnosed with ovarian cancer, treatment paradigms for the initial management of the disease have shifted dramatically. Based on data from multiple randomized controlled trials, neoadjuvant chemotherapy (NACT) is being used more frequently. The American Society of Clinical Oncology and the Society of Gynecologic Oncology developed consensus recommendations for the appropriate use of NACT and primary cytoreductive surgery for women with ovarian cancer.
Finally, all of oncology has moved toward incorporating molecularly targeted therapeutics directed toward individual genetic abnormalities in tumors, so-called precision medicine. In ovarian cancer, poly(adenosine diphosphate [ADP]–ribose) polymerase (PARP) has emerged as an important target, particularly for women with BRCA gene pathway mutations. We describe a recently published randomized controlled trial of the PARP inhibitor niraparib.
Read about ovarian cancer screening tests
Is CA 125 or ultrasound screening appropriate for the general population?
Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387(10022):945-956.
In the United States, the overall ovarian cancer 5-year survival rate is 46.2%, resulting in more than 14,000 deaths annually.2 The poor prognosis associated with this malignancy is largely attributable to the fact that almost 75% of women have stage III or stage IV disease at the time of diagnosis.2 Ovarian cancer is usually associated with vague, nonspecific symptoms as it progresses, which contributes to delayed diagnosis and increased mortality.
Multiple studies have examined pelvic ultrasonography and tumor markers, such as CA 125, as possible screening tools to increase early detection in asymptomatic women. However, neither modality alone or in combination has sufficient sensitivity or specificity to recommend it for use in the general population.3,4 Nevertheless, the search for an appropriate screening tool continues, and the UKCTOCS trial results have reinvigorated this discussion.5

The UKCTOCS findings
The UKCTOCS was a multicenter, randomized controlled trial in the United Kingdom in which researchers allocated 202,638 women aged 50 to 74 years to 1 of 3 groups: annual multimodal screening (MMS) with serum CA 125 interpreted with the use of the risk of ovarian cancer algorithm, annual transvaginal ultrasound screening (USS), or no screening. The median follow-up was more than 11 years.
The investigators found that equivalent rates of ovarian cancer were diagnosed in each group: 0.7% in the MMS group, 0.6% in the USS group, and 0.6% in the no-screening group. Overall, there was no significant reduction in the mortality rate from ovarian cancer in either of the 2 screening groups compared with the no-screening group.5
An important subset discovery
However, in a prespecified subset analysis excluding "prevalent cases" (women with ovarian cancer thought to be present prior to randomization and subsequent screening), ovarian cancer mortality was significantly lower in the MMS group compared with the no-screening group (P = .021). Compared with no screening, MMS was associated with a 20% reduction in mortality rate from ovarian cancer over time, with the most pronounced effects occurring at years 7 to 14 of follow-up, suggesting the possible increased effectiveness of screening over time.5
Related article:
Can CA 125 screening reduce mortality from ovarian cancer?
Concordance with other screening trials
While impressive in study magnitude and scope, the UKCTOCS results did not demonstrate a significant mortality benefit associated with MMS or USS when compared with no screening. Although the screening complications were low (<1% in both screening groups), the authors did note a false-positive surgery rate of 14 per 10,000 screens for the MMS group and 50 per 10,000 screens for the USS group. Based on the performance of screening in this trial, 641 women would need to be screened annually using MMS for 14 years to prevent 1 ovarian cancer death.
Like the UKCTOCS, the ovarian cancer-screening arm of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial in the United States was also unable to demonstrate a reduction in mortality rate with screening with CA 125 and transvaginal ultrasound. Importantly, more than one-third of women with a false-positive screen underwent surgery and 15% of them experienced a major complication.6 Based on these findings, the US Preventive Services Task Force grades screening for ovarian cancer as D, suggesting that the harms of screening may outweigh the benefits.7
While screening for ovarian cancer remains an important need, there is currently no evidence to suggest that serum tumor marker or ultrasound screening is appropriate in the general population. Studies using more specific screening tests or strategies targeted to higher-risk women are ongoing.
Read about patient selection for neoadjuvant chemotherapy
New clinical practice guideline advises neoadjuvant chemotherapy for certain women with ovarian cancer
Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(28):3460-3473.
It has long been held as a central dogma that primary cytoreductive surgery (PCS) is the preferred initial treatment for women with newly diagnosed ovarian cancer.8 However, PCS is associated with substantial morbidity, and the ability to achieve optimal cytoreduction (<1 cm of residual disease), an important prognostic factor, is often compromised in women with significant tumor burden.9,10
Neoadjuvant chemotherapy, in which chemotherapy is administered prior to surgical cytoreduction, challenges the traditional treatment paradigm for advanced-stage ovarian cancer. Several randomized controlled trials have reported equivalent survival for primary surgical cytoreduction and NACT. Importantly, women who received NACT had fewer complications and were more likely to have optimal cytoreduction at the time of surgery.11,12 These studies have limitations, however, and the role of NACT remains uncertain.
To help guide clinicians, the Society of Gynecologic Oncology and the American Society of Clinical Oncology convened an expert panel to provide recommendations and guidance on the evaluation of women for and the use of NACT in the setting of advanced ovarian cancer.13
Related article:
2015 Update on cancer
Recommendation: Clinical evaluation and patient selection
Strong clinical evidence supports that all women with suspected stage IIIC or stage IV ovarian cancer should be evaluated by a gynecologic oncologist prior to the initiation of therapy. The evaluation should include at least a computed tomography scan of the chest, abdomen, and pelvis to assess the extent of disease and resectability. A preoperative risk assessment should be performed to assess risk factors for increased morbidity and mortality.
Women who have a high perioperative risk profile or a low likelihood of achieving cytoreduction to 1 cm or less of residual tumor should receive NACT. Prior to the initiation of NACT, histologic confirmation of ovarian cancer should be obtained.13
Outcomes for neoadjuvant chemotherapy versus primary cytoreduction
Four phase 3 randomized controlled trials (EORTC 55971, CHORUS, JCOG0602, and SCORPION) suggest that NACT is noninferior to PCS with regard to progression-free survival and overall survival. NACT is associated with less perioperative and postoperative morbidity and mortality and is associated with shorter hospital stays.
To date, complete data are available only from the EORTC and CHORUS trials, which both demonstrated similar progression-free survival and overall survival for NACT and PCS. Critics have noted, however, that both trials have shorter median overall survival for the PCS groups than were previously reported in other phase 3 studies in the United States, suggesting the possibility of different patient populations or less aggressive "surgical effort." Thus, PCS remains the preferred management strategy for women with advanced-stage ovarian cancer in whom there is a high likelihood of optimal cytoreduction.13
Recommendation: Use of neoadjuvant chemotherapy
Patients who are appropriate candidates for NACT should be treated with a platinum and taxane doublet and should receive interval cytoreduction following 3 to 4 cycles of therapy if a favorable response is noted. Patients whose disease progresses despite NACT have a poor prognosis, and there is little role for surgical treatment with the exception of palliative purposes.13
Neoadjuvant chemotherapy is a noninferior and appropriate treatment option for women who are poor surgical candidates or who have a low likelihood of optimal cytoreduction. When optimal cytoreduction is possible, however, PCS is preferred (see FIGURE). The data on the efficacy of NACT for ovarian cancer have led to increased use of this treatment in the United States.

Read about a new PARP inhibitor for maintenance therapy
Niraparib is promising as maintenance therapy in ovarian cancer
Mirza MR, Monk BJ, Herrstedt J, et al; for the ENGOT-OV16/NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154-2164.
Approximately 85% of women with ovarian cancer will develop recurrent disease. Women with ovarian cancer are commonly treated with a range of antineoplastic agents over the course of their lifetime. As such, there is a great need for additional active therapeutic agents in this setting. Recently, substantial effort has been directed toward "precision" or "personalized medicine" in oncology.
Precision medicine, targeted therapies in oncology
Precision medicine refers to the customization of medical therapy based on the genetic characterization of the individual patient or the molecular profile of the patient's tumor. As a result of large-scale molecular profiling from projects such as the International Cancer Genome Consortium and The Cancer Genome Atlas, an abundance of molecular data has been generated through the characterization of multiple tumor types. This has led to the discovery of key cancer drivers, alterations, and specific molecular profiles that have distinct prognostic and treatment implications. These data, in combination with the commercial availability of molecular profiling tests, has made precision medicine a reality for women with ovarian cancer.
This wealth of new information has led to development of targeted therapeutics that block the growth and spread of cancer by acting on specific molecules or molecular pathways. Targeted therapies approved for cancer treatment include hormonal therapies, signal transduction inhibitors, gene expression modulators, apoptosis inducers, angiogenesis inhibitors, and immunotherapies.14
How PARP inhibitors work
PARP inhibitors are a class of agents that are emerging as important therapies for ovarian cancer. These agents block the nuclear protein PARP, which functions to detect and repair single-strand DNA breaks with the resulting accumulation of double-stranded DNA breaks.15 In the setting of DNA damage, the homologous recombination repair pathway is activated for repair. However, homologous recombination deficiencies (HRD) can arise as a result of BRCA1 or BRCA2 mutations or BRCA-independent pathways, which effectively disable this DNA repair pathway. As a result, when PARP inhibitors are used in patients with HRD, the cell cannot repair double-stranded DNA breaks and this leads to "synthetic lethality."16
Understanding this molecular mechanism of PARP inhibitors as well as the frequent abnormalities in the BRCA genes and HRD pathways in ovarian cancer has provided an important potential therapeutic target in ovarian cancer. A number of PARP inhibitors are now commercially available and are undergoing testing in ovarian cancer.
Related article:
Is a minimally invasive approach to hysterectomy for Gyn cancer utilized equally in all racial and income groups?
Niraparib for ovarian cancer
In a randomized, double-blind, phase 3 trial by Mizra and colleagues, 553 women with platinum-sensitive recurrent ovarian cancer who responded to therapy were divided according to the presence or absence of a germline BRCA (gBRCA) mutation and randomly assigned to niraparib 300 mg or placebo once daily. Women in the niraparib group had a significantly longer median duration of progression-free survival than did those in the placebo group. This was most pronounced in women in the gBRCA cohort (21.0 vs 5.5 months). Importantly, niraparib was associated with improved progression-free survival in HRD-positive patients without gBRCA mutations (12.9 vs 3.8 months) as well as in the HRD-negative subgroup (6.9 vs 3.8 months).17
Overall, niraparib was well tolerated. About 15% of women discontinued the drug due to toxicity. Significant (grade 3 or 4) adverse events were seen in three-quarters of women treated with niraparib, and they most commonly consisted of hematologic toxicities. Patient-reported outcomes were similar for both groups, indicating no significant effect from niraparib on quality of life.17
This study's results suggest that niraparib has clinical activity against ovarian cancer. Importantly, niraparib was active in women with gBRCA mutations, in those with HRD without a gBRCA mutation, and potentially in women without HRD. If approved by the US Food and Drug Administration, niraparib will join olaparib and rucaparib as a newly approved therapeutic agent for ovarian cancer. This study provides important evidence that suggests niraparib maintenance therapy may be an efficacious and important addition to the treatment armamentarium for platinum-sensitive ovarian cancer.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In 2017, an estimated 22,240 women will be diagnosed with ovarian cancer, and 14,080 women will die of the disease.1 The high mortality associated with ovarian cancer is due largely to the inability to detect the disease early and the lack of effective therapeutics for women with recurrent disease. In this Update, we review important advances in the diagnosis and treatment of ovarian cancer.
Development of an effective screening tool for women at average risk has been an elusive challenge. The United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) examined the efficacy of transvaginal ultrasound and cancer antigen 125 (CA 125) monitoring for ovarian cancer in a large cohort of women.
For women diagnosed with ovarian cancer, treatment paradigms for the initial management of the disease have shifted dramatically. Based on data from multiple randomized controlled trials, neoadjuvant chemotherapy (NACT) is being used more frequently. The American Society of Clinical Oncology and the Society of Gynecologic Oncology developed consensus recommendations for the appropriate use of NACT and primary cytoreductive surgery for women with ovarian cancer.
Finally, all of oncology has moved toward incorporating molecularly targeted therapeutics directed toward individual genetic abnormalities in tumors, so-called precision medicine. In ovarian cancer, poly(adenosine diphosphate [ADP]–ribose) polymerase (PARP) has emerged as an important target, particularly for women with BRCA gene pathway mutations. We describe a recently published randomized controlled trial of the PARP inhibitor niraparib.
Read about ovarian cancer screening tests
Is CA 125 or ultrasound screening appropriate for the general population?
Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387(10022):945-956.
In the United States, the overall ovarian cancer 5-year survival rate is 46.2%, resulting in more than 14,000 deaths annually.2 The poor prognosis associated with this malignancy is largely attributable to the fact that almost 75% of women have stage III or stage IV disease at the time of diagnosis.2 Ovarian cancer is usually associated with vague, nonspecific symptoms as it progresses, which contributes to delayed diagnosis and increased mortality.
Multiple studies have examined pelvic ultrasonography and tumor markers, such as CA 125, as possible screening tools to increase early detection in asymptomatic women. However, neither modality alone or in combination has sufficient sensitivity or specificity to recommend it for use in the general population.3,4 Nevertheless, the search for an appropriate screening tool continues, and the UKCTOCS trial results have reinvigorated this discussion.5

The UKCTOCS findings
The UKCTOCS was a multicenter, randomized controlled trial in the United Kingdom in which researchers allocated 202,638 women aged 50 to 74 years to 1 of 3 groups: annual multimodal screening (MMS) with serum CA 125 interpreted with the use of the risk of ovarian cancer algorithm, annual transvaginal ultrasound screening (USS), or no screening. The median follow-up was more than 11 years.
The investigators found that equivalent rates of ovarian cancer were diagnosed in each group: 0.7% in the MMS group, 0.6% in the USS group, and 0.6% in the no-screening group. Overall, there was no significant reduction in the mortality rate from ovarian cancer in either of the 2 screening groups compared with the no-screening group.5
An important subset discovery
However, in a prespecified subset analysis excluding "prevalent cases" (women with ovarian cancer thought to be present prior to randomization and subsequent screening), ovarian cancer mortality was significantly lower in the MMS group compared with the no-screening group (P = .021). Compared with no screening, MMS was associated with a 20% reduction in mortality rate from ovarian cancer over time, with the most pronounced effects occurring at years 7 to 14 of follow-up, suggesting the possible increased effectiveness of screening over time.5
Related article:
Can CA 125 screening reduce mortality from ovarian cancer?
Concordance with other screening trials
While impressive in study magnitude and scope, the UKCTOCS results did not demonstrate a significant mortality benefit associated with MMS or USS when compared with no screening. Although the screening complications were low (<1% in both screening groups), the authors did note a false-positive surgery rate of 14 per 10,000 screens for the MMS group and 50 per 10,000 screens for the USS group. Based on the performance of screening in this trial, 641 women would need to be screened annually using MMS for 14 years to prevent 1 ovarian cancer death.
Like the UKCTOCS, the ovarian cancer-screening arm of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial in the United States was also unable to demonstrate a reduction in mortality rate with screening with CA 125 and transvaginal ultrasound. Importantly, more than one-third of women with a false-positive screen underwent surgery and 15% of them experienced a major complication.6 Based on these findings, the US Preventive Services Task Force grades screening for ovarian cancer as D, suggesting that the harms of screening may outweigh the benefits.7
While screening for ovarian cancer remains an important need, there is currently no evidence to suggest that serum tumor marker or ultrasound screening is appropriate in the general population. Studies using more specific screening tests or strategies targeted to higher-risk women are ongoing.
Read about patient selection for neoadjuvant chemotherapy
New clinical practice guideline advises neoadjuvant chemotherapy for certain women with ovarian cancer
Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(28):3460-3473.
It has long been held as a central dogma that primary cytoreductive surgery (PCS) is the preferred initial treatment for women with newly diagnosed ovarian cancer.8 However, PCS is associated with substantial morbidity, and the ability to achieve optimal cytoreduction (<1 cm of residual disease), an important prognostic factor, is often compromised in women with significant tumor burden.9,10
Neoadjuvant chemotherapy, in which chemotherapy is administered prior to surgical cytoreduction, challenges the traditional treatment paradigm for advanced-stage ovarian cancer. Several randomized controlled trials have reported equivalent survival for primary surgical cytoreduction and NACT. Importantly, women who received NACT had fewer complications and were more likely to have optimal cytoreduction at the time of surgery.11,12 These studies have limitations, however, and the role of NACT remains uncertain.
To help guide clinicians, the Society of Gynecologic Oncology and the American Society of Clinical Oncology convened an expert panel to provide recommendations and guidance on the evaluation of women for and the use of NACT in the setting of advanced ovarian cancer.13
Related article:
2015 Update on cancer
Recommendation: Clinical evaluation and patient selection
Strong clinical evidence supports that all women with suspected stage IIIC or stage IV ovarian cancer should be evaluated by a gynecologic oncologist prior to the initiation of therapy. The evaluation should include at least a computed tomography scan of the chest, abdomen, and pelvis to assess the extent of disease and resectability. A preoperative risk assessment should be performed to assess risk factors for increased morbidity and mortality.
Women who have a high perioperative risk profile or a low likelihood of achieving cytoreduction to 1 cm or less of residual tumor should receive NACT. Prior to the initiation of NACT, histologic confirmation of ovarian cancer should be obtained.13
Outcomes for neoadjuvant chemotherapy versus primary cytoreduction
Four phase 3 randomized controlled trials (EORTC 55971, CHORUS, JCOG0602, and SCORPION) suggest that NACT is noninferior to PCS with regard to progression-free survival and overall survival. NACT is associated with less perioperative and postoperative morbidity and mortality and is associated with shorter hospital stays.
To date, complete data are available only from the EORTC and CHORUS trials, which both demonstrated similar progression-free survival and overall survival for NACT and PCS. Critics have noted, however, that both trials have shorter median overall survival for the PCS groups than were previously reported in other phase 3 studies in the United States, suggesting the possibility of different patient populations or less aggressive "surgical effort." Thus, PCS remains the preferred management strategy for women with advanced-stage ovarian cancer in whom there is a high likelihood of optimal cytoreduction.13
Recommendation: Use of neoadjuvant chemotherapy
Patients who are appropriate candidates for NACT should be treated with a platinum and taxane doublet and should receive interval cytoreduction following 3 to 4 cycles of therapy if a favorable response is noted. Patients whose disease progresses despite NACT have a poor prognosis, and there is little role for surgical treatment with the exception of palliative purposes.13
Neoadjuvant chemotherapy is a noninferior and appropriate treatment option for women who are poor surgical candidates or who have a low likelihood of optimal cytoreduction. When optimal cytoreduction is possible, however, PCS is preferred (see FIGURE). The data on the efficacy of NACT for ovarian cancer have led to increased use of this treatment in the United States.

Read about a new PARP inhibitor for maintenance therapy
Niraparib is promising as maintenance therapy in ovarian cancer
Mirza MR, Monk BJ, Herrstedt J, et al; for the ENGOT-OV16/NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154-2164.
Approximately 85% of women with ovarian cancer will develop recurrent disease. Women with ovarian cancer are commonly treated with a range of antineoplastic agents over the course of their lifetime. As such, there is a great need for additional active therapeutic agents in this setting. Recently, substantial effort has been directed toward "precision" or "personalized medicine" in oncology.
Precision medicine, targeted therapies in oncology
Precision medicine refers to the customization of medical therapy based on the genetic characterization of the individual patient or the molecular profile of the patient's tumor. As a result of large-scale molecular profiling from projects such as the International Cancer Genome Consortium and The Cancer Genome Atlas, an abundance of molecular data has been generated through the characterization of multiple tumor types. This has led to the discovery of key cancer drivers, alterations, and specific molecular profiles that have distinct prognostic and treatment implications. These data, in combination with the commercial availability of molecular profiling tests, has made precision medicine a reality for women with ovarian cancer.
This wealth of new information has led to development of targeted therapeutics that block the growth and spread of cancer by acting on specific molecules or molecular pathways. Targeted therapies approved for cancer treatment include hormonal therapies, signal transduction inhibitors, gene expression modulators, apoptosis inducers, angiogenesis inhibitors, and immunotherapies.14
How PARP inhibitors work
PARP inhibitors are a class of agents that are emerging as important therapies for ovarian cancer. These agents block the nuclear protein PARP, which functions to detect and repair single-strand DNA breaks with the resulting accumulation of double-stranded DNA breaks.15 In the setting of DNA damage, the homologous recombination repair pathway is activated for repair. However, homologous recombination deficiencies (HRD) can arise as a result of BRCA1 or BRCA2 mutations or BRCA-independent pathways, which effectively disable this DNA repair pathway. As a result, when PARP inhibitors are used in patients with HRD, the cell cannot repair double-stranded DNA breaks and this leads to "synthetic lethality."16
Understanding this molecular mechanism of PARP inhibitors as well as the frequent abnormalities in the BRCA genes and HRD pathways in ovarian cancer has provided an important potential therapeutic target in ovarian cancer. A number of PARP inhibitors are now commercially available and are undergoing testing in ovarian cancer.
Related article:
Is a minimally invasive approach to hysterectomy for Gyn cancer utilized equally in all racial and income groups?
Niraparib for ovarian cancer
In a randomized, double-blind, phase 3 trial by Mizra and colleagues, 553 women with platinum-sensitive recurrent ovarian cancer who responded to therapy were divided according to the presence or absence of a germline BRCA (gBRCA) mutation and randomly assigned to niraparib 300 mg or placebo once daily. Women in the niraparib group had a significantly longer median duration of progression-free survival than did those in the placebo group. This was most pronounced in women in the gBRCA cohort (21.0 vs 5.5 months). Importantly, niraparib was associated with improved progression-free survival in HRD-positive patients without gBRCA mutations (12.9 vs 3.8 months) as well as in the HRD-negative subgroup (6.9 vs 3.8 months).17
Overall, niraparib was well tolerated. About 15% of women discontinued the drug due to toxicity. Significant (grade 3 or 4) adverse events were seen in three-quarters of women treated with niraparib, and they most commonly consisted of hematologic toxicities. Patient-reported outcomes were similar for both groups, indicating no significant effect from niraparib on quality of life.17
This study's results suggest that niraparib has clinical activity against ovarian cancer. Importantly, niraparib was active in women with gBRCA mutations, in those with HRD without a gBRCA mutation, and potentially in women without HRD. If approved by the US Food and Drug Administration, niraparib will join olaparib and rucaparib as a newly approved therapeutic agent for ovarian cancer. This study provides important evidence that suggests niraparib maintenance therapy may be an efficacious and important addition to the treatment armamentarium for platinum-sensitive ovarian cancer.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: ovarian cancer. https://seer.cancer.gov/statfacts/html/ovary.html. Accessed January 20, 2017.
- Jacobs I, Davies AP, Bridges J, et al. Prevalence screening for ovarian cancer in postmenopausal women by CA 125 measurement and ultrasonography. BMJ. 1993;306(6884):1030–1034.
- van Nagell JR Jr, Pavlik EJ. Ovarian cancer screening. Clin Obstet Gynecol. 2012;55:43–51.
- Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387(10022):945–956.
- Buys SS, Partridge E, Black A, et al; PLCO Project Team. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening randomized controlled trial. JAMA. 2011;305(22):2295–2303.
- Moyer VA, US Preventive Services Task Force. Screening for ovarian cancer: US Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2012;157(12):900–904.
- Schorge JO, McCann C, Del Carmen MG. Surgical debulking of ovarian cancer: what difference does it make? Rev Obstet Gynecol. 2010;3(3):111–117.
- Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170(4):974–979; discussion 979–980.
- Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–1259.
- Vergote I, Trope CG, Amant F, et al; European Organization for Research and Treatment of Cancer–Gynaecological Cancer Goup; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953.
- Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386(9990):249–257.
- Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(28):3460–3473.
- National Cancer Institute. Targeted cancer therapies. https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet. Updated April 25, 2014. Accessed January 21, 2017.
- Drean A, Lord CJ, Ashworth A. PARP inhibitor combination therapy. Crit Rev Oncol Hematol. 2016;108:73–85.
- Ledermann JA, El-Khouly F. PARP inhibitors in ovarian cancer: clinical evidence for informed treatment decisions. Br J Cancer. 2015;113(suppl 1):S10–S16.
- Mirza MR, Monk BJ, Herrstedt J, et al; ENGOT-OV16/NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–2164.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: ovarian cancer. https://seer.cancer.gov/statfacts/html/ovary.html. Accessed January 20, 2017.
- Jacobs I, Davies AP, Bridges J, et al. Prevalence screening for ovarian cancer in postmenopausal women by CA 125 measurement and ultrasonography. BMJ. 1993;306(6884):1030–1034.
- van Nagell JR Jr, Pavlik EJ. Ovarian cancer screening. Clin Obstet Gynecol. 2012;55:43–51.
- Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387(10022):945–956.
- Buys SS, Partridge E, Black A, et al; PLCO Project Team. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening randomized controlled trial. JAMA. 2011;305(22):2295–2303.
- Moyer VA, US Preventive Services Task Force. Screening for ovarian cancer: US Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2012;157(12):900–904.
- Schorge JO, McCann C, Del Carmen MG. Surgical debulking of ovarian cancer: what difference does it make? Rev Obstet Gynecol. 2010;3(3):111–117.
- Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170(4):974–979; discussion 979–980.
- Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–1259.
- Vergote I, Trope CG, Amant F, et al; European Organization for Research and Treatment of Cancer–Gynaecological Cancer Goup; NCIC Clinical Trials Group. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953.
- Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386(9990):249–257.
- Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(28):3460–3473.
- National Cancer Institute. Targeted cancer therapies. https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet. Updated April 25, 2014. Accessed January 21, 2017.
- Drean A, Lord CJ, Ashworth A. PARP inhibitor combination therapy. Crit Rev Oncol Hematol. 2016;108:73–85.
- Ledermann JA, El-Khouly F. PARP inhibitors in ovarian cancer: clinical evidence for informed treatment decisions. Br J Cancer. 2015;113(suppl 1):S10–S16.
- Mirza MR, Monk BJ, Herrstedt J, et al; ENGOT-OV16/NOVA Investigators. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–2164.