User login
In this Update, I report on the latest US Preventive Services Task Force (USPSTF) cervical cancer screening recommendations. In addition, I describe the results of 2 studies, a large prospective multicenter study of the accuracy of sentinel lymph node (SLN) biopsy in endometrial cancer, and a proof-of-concept review of use of checkpoint blockade to increase immune response and of its possible role in endometrial cancer.
hrHPV testing used alone as primary screening for cervical cancer: USPSTF recommendations
US Preventive Services Task Force. Draft recommendation statement: cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Published October 2017. Accessed February 5, 2018.
Despite our rapid advances in understanding the molecular underpinnings of cancer, gynecologic malignancies are still a major cause of morbidity and mortality among women. Cervical cancer stands as an example of how a cancer screening test can be implemented to reduce mortality. In this section, I report on the USPSTF cervical cancer screening recommendations, which were updated in October 2017.
Even with the widespread implementation of screening programs for cervical cancer in the United States, 13,240 women will be diagnosed with the disease in 2018, and 4,170 will die from cervical cancer.1 Most often, cervical cancer occurs in women who have not been adequately screened. It is now recognized that the human papillomavirus (HPV) is the cause of cervical cancer.2
While cervical cytology has long been used as a screening test for cervical cancer, testing for high-risk HPV subtypes (hrHPV testing) also has been used as a screening modality. Traditionally, hrHPV testing is used in combination with cervical cytology, so called cotesting. There is convincing evidence that cervical cytology, as well as strategies that use hrHPV testing, can detect high-grade cervical precancers and cancers and thereby reduce mortality. However, cervical cancer screening is also associated with frequent follow-ups, invasive procedures performed to assess abnormal results, psychological distress, and adverse pregnancy outcomes of treatment for precancerous lesions.
The USPSTF based its new cervical cancer screening recommendations on clinical trial data and decision modeling of various screening strategies, and weighed the benefits and harms of each strategy.
Recommendations from the USPSTF
hrHPV screening for cervical cancer. TheUSPSTF recommends screening with cervical cytology every 3 years for women 21 to 29 years of age. For women 30 to 65 years of age, screening with cytology every 3 years, or hrHPV testing alone used every 5 years, is recommended.
Data from large randomized trials suggest cytologic screening is slightly less sensitive than hrHPV testing in detecting high-grade (grade 2 or 3) cervical intraepithelial neoplasia (CIN). However, hrHPV testing results in more follow-up tests and colposcopies. In a decision model, the USPSTF found that cotesting increased the number of follow-up tests but did not increase detection of grade 3 CIN or invasive cancer. This is the first clinical guideline to recommend hrHPV testing used alone for screening. The American College of Obstetricians and Gynecologists (ACOG) continues to recommend cotesting (cytology in combination with hrHPV) as a primary screening modality in this population.3
Exceptions. According to the USPSTF, 3 populations should not be screened: women over 65 years of age with adequate prior screening who are not otherwise at high risk for cervical cancer; women under 21 years of age; and women who have had a hysterectomy and do not have a history of grade 2 or 3 CIN or cancer.
Summary. The USPSTF recommendations are intended for the general population and are not applicable to women with a history of high-grade CIN or cervical cancer, women with in utero exposure to diethylstilbestrol, and women who are immunocompromised. The remaining USPSTF recommendations are largely in line with guidelines published by ACOG and other groups.3,4
Testing for high-risk HPV alone is a reasonable screening option for cervical cancer. This modality can be used in women 30 to 65 years of age but should not be repeated more frequently than every 5 years in those with a negative result.
Read about SLN biopsy to stage endometrial cancer
SLN biopsy for staging endometrial cancer
Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18(3):384-392.
Surgery is the cornerstone of treatment for most gynecologic cancers. The widespread use of minimally invasive surgical techniques and the introduction of less radical procedures for gynecologic cancers have helped reduce surgical morbidity.
For endometrial cancer, the role of lymphadenectomy is controversial. Data from prospective trials of this procedure suggest an association with increased morbidity and long-term sequelae, such as lymphedema, and no association with improved survival.5,6
SLN biopsy is an important advance and a potential alternative nodal evaluation method that may be associated with decreased morbidity. In this more limited assessment technique, the first nodal drainage basins of a tumor are identified and removed for pathologic evaluation.
Accuracy of SLN biopsy in endometrial cancer was the subject of Rossi and colleagues' recent large prospective multicenter study, the Fluorescence Imaging for Robotic Endometrial Sentinel lymph node biopsy (FIRES) trial.
Details of the study
Rossi and colleagues conducted the FIRES trial to estimate the sensitivity of SLN biopsy in detecting nodal metastases in women with stage I endometrial cancer. Patients (N = 385) from 10 US sites were enrolled in the study. SLN evaluation was performed after cervical injection of indocyanine green followed by robotic-assisted hysterectomy. After identification of the SLN, participants underwent pelvic lymphadenectomy. Performance of para-aortic lymphadenectomy was optional.
Mapping of the SLN was feasible in 86% of patients, including bilateral mapping in 52%. Twelve percent of the participants had nodal metastases. SLN biopsy had a sensitivity of 97% in women who had identification of the SLNs. Similarly, the negative predictive value was high, 99.6%. The procedure was associated with acceptable short-term toxicity with adverse events in 9% of study participants. Common complications included neurologic complications, respiratory distress, nausea and vomiting, and, in 3 patients, bowel injury.
Accurate detection of nodal metastases. Results of the study suggest SLN biopsy is accurate in detecting nodal metastases in women with endometrial cancer. Although long-term toxicity was not examined, other work suggests the lymphedema rates associated with SLN biopsy may be lower than those of lymphadenectomy. While the study described impressive performance characteristics, there remain technical challenges. Even among skilled surgeons trained for the protocol, there was no nodal mapping in nearly half of the women with endometrial cancer. Women without node mapping require full lymphadenectomy thus negating the possible benefits of the procedure.
Given the high accuracy of SLN mapping in endometrial cancer, the procedure likely will become the standard of care for nodal evaluation by gynecologic oncologists.
Read about immunotherapy for gynecologic cancers
Immunotherapy for gynecologic cancers
Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413.
In oncology, precision medicine is rapidly becoming a standard treatment approach. Therapies are being used to target specific genetic alterations in tumors. In cancer immunotherapy, the immune system is being used to facilitate clearance of cancer cells.
The most common mechanism of action of clinically used immunotherapeutic agents is blockade of programmed cell death protein 1 (PD-1), a lymphocyte receptor that prevents the immune system from targeting the body's own cells.7 Cancers that have mutations in the DNA mismatch repair (MMR) proteins display microsatellite instability (MSI) and produce high levels of abnormal proteins.8 These abnormal proteins serve as tumor antigens that can be targeted by the body's normal immune system.
In May 2017, the US Food and Drug Administration (FDA) granted accelerated approval of the PD-1 blocking antibody pembrolizumab for the treatment of unresectable or metastatic MSI-high (MSI-H) or MMR-deficient solid tumors.9 The approval was based on data from 149 patients treated in 5 studies that demonstrated a response rate of 39.6%, including responses that lasted at least 6 months in 78% of participants. This was the first ever cancer drug that received FDA approval based on a tumor's biomarker profile without regard to the site of origin. I describe the results of a study by Le and colleagues that examines the possible role of immunotherapy in a variety of solid tumors in this section.
Details of the study
This study examined the clinical efficacy of PD-1 blockade in 86 patients with advanced, MMR-deficient tumors from 12 different sites. Endometrial cancer was the second most frequent primary tumor site in 17% of patients. Within the cohort, the overall objective response rate was 53%, which included 21% of patients with complete radiographic response (no imaging evidence of cancer). Disease control, either complete or partial response or stable disease, was achieved in 77% of patients. After a median follow-up of 12.5 months, neither the median progression-free survival (PFS) nor median overall survival had been reached. The authors estimated that 2-year overall survival was 64%, substantially higher than expected for patients with advanced solid tumors.
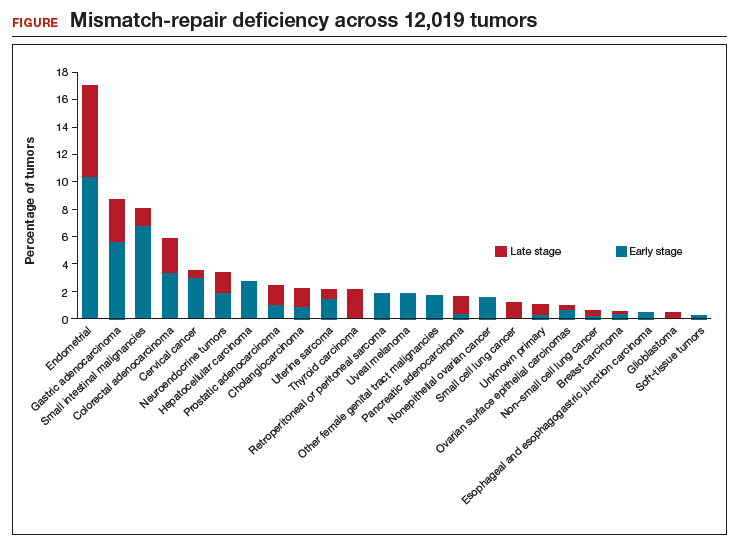
Le and colleagues also performed several in vivo laboratory experiments to explore the mechanisms by which patients responded. In addition, they used sequencing to determine the prevalence of MMR deficiency in 12,019 cancer samples that included 32 distinct tumor types (FIGURE). Endometrial cancer had the highest frequency of MMR deficiency (17%). Four percent of cervical cancers and less than 2% of ovarian cancers were MMR-deficient.
The promise of immunotherapy for endometrial cancer. This study's data and other emerging data have important implications for women with gynecologic cancer, particularly endometrial cancer. First, given the frequency of MMR mutations among women with endometrial cancer, MMR testing should be strongly considered for these patients. Many institutions have protocols for reflex testing with immunohistochemistry for women with endometrial cancer. For women with positive test results, germline sequencing can be performed to determine if they have an inherited MMR deficiency, Lynch syndrome. Presence of an MMR deficiency is an important factor in cancer screening and potential treatment.
Second, the impressive results of PD-1 blockade in patients with MMR-deficient tumors suggest that this treatment strategy may be important for women with recurrent or metastatic endometrial cancer. The ideal timing of immunotherapy for women with endometrial cancer is an area of active ongoing study.
Immunotherapy with PD-1 blockade is an important treatment strategy for women with MMR-deficient or MSI-H gynecologic cancers.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 168: Cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
- Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–1716.
- ASTEC Study Group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125–136.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264.
- Buza N, Ziai J, Hui P. Mismatch repair deficiency testing in clinical practice. Expert Rev Mol Diagn. 2016;16(5):591–604.
- FDA approves first cancer treatment for any solid tumor with a specific genetic feature [news release]. Silver Spring, MD: US Food and Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm560167.htm. Published May 23, 2017. Accessed February 5, 2018.
In this Update, I report on the latest US Preventive Services Task Force (USPSTF) cervical cancer screening recommendations. In addition, I describe the results of 2 studies, a large prospective multicenter study of the accuracy of sentinel lymph node (SLN) biopsy in endometrial cancer, and a proof-of-concept review of use of checkpoint blockade to increase immune response and of its possible role in endometrial cancer.
hrHPV testing used alone as primary screening for cervical cancer: USPSTF recommendations
US Preventive Services Task Force. Draft recommendation statement: cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Published October 2017. Accessed February 5, 2018.
Despite our rapid advances in understanding the molecular underpinnings of cancer, gynecologic malignancies are still a major cause of morbidity and mortality among women. Cervical cancer stands as an example of how a cancer screening test can be implemented to reduce mortality. In this section, I report on the USPSTF cervical cancer screening recommendations, which were updated in October 2017.
Even with the widespread implementation of screening programs for cervical cancer in the United States, 13,240 women will be diagnosed with the disease in 2018, and 4,170 will die from cervical cancer.1 Most often, cervical cancer occurs in women who have not been adequately screened. It is now recognized that the human papillomavirus (HPV) is the cause of cervical cancer.2
While cervical cytology has long been used as a screening test for cervical cancer, testing for high-risk HPV subtypes (hrHPV testing) also has been used as a screening modality. Traditionally, hrHPV testing is used in combination with cervical cytology, so called cotesting. There is convincing evidence that cervical cytology, as well as strategies that use hrHPV testing, can detect high-grade cervical precancers and cancers and thereby reduce mortality. However, cervical cancer screening is also associated with frequent follow-ups, invasive procedures performed to assess abnormal results, psychological distress, and adverse pregnancy outcomes of treatment for precancerous lesions.
The USPSTF based its new cervical cancer screening recommendations on clinical trial data and decision modeling of various screening strategies, and weighed the benefits and harms of each strategy.
Recommendations from the USPSTF
hrHPV screening for cervical cancer. TheUSPSTF recommends screening with cervical cytology every 3 years for women 21 to 29 years of age. For women 30 to 65 years of age, screening with cytology every 3 years, or hrHPV testing alone used every 5 years, is recommended.
Data from large randomized trials suggest cytologic screening is slightly less sensitive than hrHPV testing in detecting high-grade (grade 2 or 3) cervical intraepithelial neoplasia (CIN). However, hrHPV testing results in more follow-up tests and colposcopies. In a decision model, the USPSTF found that cotesting increased the number of follow-up tests but did not increase detection of grade 3 CIN or invasive cancer. This is the first clinical guideline to recommend hrHPV testing used alone for screening. The American College of Obstetricians and Gynecologists (ACOG) continues to recommend cotesting (cytology in combination with hrHPV) as a primary screening modality in this population.3
Exceptions. According to the USPSTF, 3 populations should not be screened: women over 65 years of age with adequate prior screening who are not otherwise at high risk for cervical cancer; women under 21 years of age; and women who have had a hysterectomy and do not have a history of grade 2 or 3 CIN or cancer.
Summary. The USPSTF recommendations are intended for the general population and are not applicable to women with a history of high-grade CIN or cervical cancer, women with in utero exposure to diethylstilbestrol, and women who are immunocompromised. The remaining USPSTF recommendations are largely in line with guidelines published by ACOG and other groups.3,4
Testing for high-risk HPV alone is a reasonable screening option for cervical cancer. This modality can be used in women 30 to 65 years of age but should not be repeated more frequently than every 5 years in those with a negative result.
Read about SLN biopsy to stage endometrial cancer
SLN biopsy for staging endometrial cancer
Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18(3):384-392.
Surgery is the cornerstone of treatment for most gynecologic cancers. The widespread use of minimally invasive surgical techniques and the introduction of less radical procedures for gynecologic cancers have helped reduce surgical morbidity.
For endometrial cancer, the role of lymphadenectomy is controversial. Data from prospective trials of this procedure suggest an association with increased morbidity and long-term sequelae, such as lymphedema, and no association with improved survival.5,6
SLN biopsy is an important advance and a potential alternative nodal evaluation method that may be associated with decreased morbidity. In this more limited assessment technique, the first nodal drainage basins of a tumor are identified and removed for pathologic evaluation.
Accuracy of SLN biopsy in endometrial cancer was the subject of Rossi and colleagues' recent large prospective multicenter study, the Fluorescence Imaging for Robotic Endometrial Sentinel lymph node biopsy (FIRES) trial.
Details of the study
Rossi and colleagues conducted the FIRES trial to estimate the sensitivity of SLN biopsy in detecting nodal metastases in women with stage I endometrial cancer. Patients (N = 385) from 10 US sites were enrolled in the study. SLN evaluation was performed after cervical injection of indocyanine green followed by robotic-assisted hysterectomy. After identification of the SLN, participants underwent pelvic lymphadenectomy. Performance of para-aortic lymphadenectomy was optional.
Mapping of the SLN was feasible in 86% of patients, including bilateral mapping in 52%. Twelve percent of the participants had nodal metastases. SLN biopsy had a sensitivity of 97% in women who had identification of the SLNs. Similarly, the negative predictive value was high, 99.6%. The procedure was associated with acceptable short-term toxicity with adverse events in 9% of study participants. Common complications included neurologic complications, respiratory distress, nausea and vomiting, and, in 3 patients, bowel injury.
Accurate detection of nodal metastases. Results of the study suggest SLN biopsy is accurate in detecting nodal metastases in women with endometrial cancer. Although long-term toxicity was not examined, other work suggests the lymphedema rates associated with SLN biopsy may be lower than those of lymphadenectomy. While the study described impressive performance characteristics, there remain technical challenges. Even among skilled surgeons trained for the protocol, there was no nodal mapping in nearly half of the women with endometrial cancer. Women without node mapping require full lymphadenectomy thus negating the possible benefits of the procedure.
Given the high accuracy of SLN mapping in endometrial cancer, the procedure likely will become the standard of care for nodal evaluation by gynecologic oncologists.
Read about immunotherapy for gynecologic cancers
Immunotherapy for gynecologic cancers
Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413.
In oncology, precision medicine is rapidly becoming a standard treatment approach. Therapies are being used to target specific genetic alterations in tumors. In cancer immunotherapy, the immune system is being used to facilitate clearance of cancer cells.
The most common mechanism of action of clinically used immunotherapeutic agents is blockade of programmed cell death protein 1 (PD-1), a lymphocyte receptor that prevents the immune system from targeting the body's own cells.7 Cancers that have mutations in the DNA mismatch repair (MMR) proteins display microsatellite instability (MSI) and produce high levels of abnormal proteins.8 These abnormal proteins serve as tumor antigens that can be targeted by the body's normal immune system.
In May 2017, the US Food and Drug Administration (FDA) granted accelerated approval of the PD-1 blocking antibody pembrolizumab for the treatment of unresectable or metastatic MSI-high (MSI-H) or MMR-deficient solid tumors.9 The approval was based on data from 149 patients treated in 5 studies that demonstrated a response rate of 39.6%, including responses that lasted at least 6 months in 78% of participants. This was the first ever cancer drug that received FDA approval based on a tumor's biomarker profile without regard to the site of origin. I describe the results of a study by Le and colleagues that examines the possible role of immunotherapy in a variety of solid tumors in this section.
Details of the study
This study examined the clinical efficacy of PD-1 blockade in 86 patients with advanced, MMR-deficient tumors from 12 different sites. Endometrial cancer was the second most frequent primary tumor site in 17% of patients. Within the cohort, the overall objective response rate was 53%, which included 21% of patients with complete radiographic response (no imaging evidence of cancer). Disease control, either complete or partial response or stable disease, was achieved in 77% of patients. After a median follow-up of 12.5 months, neither the median progression-free survival (PFS) nor median overall survival had been reached. The authors estimated that 2-year overall survival was 64%, substantially higher than expected for patients with advanced solid tumors.
Le and colleagues also performed several in vivo laboratory experiments to explore the mechanisms by which patients responded. In addition, they used sequencing to determine the prevalence of MMR deficiency in 12,019 cancer samples that included 32 distinct tumor types (FIGURE). Endometrial cancer had the highest frequency of MMR deficiency (17%). Four percent of cervical cancers and less than 2% of ovarian cancers were MMR-deficient.
The promise of immunotherapy for endometrial cancer. This study's data and other emerging data have important implications for women with gynecologic cancer, particularly endometrial cancer. First, given the frequency of MMR mutations among women with endometrial cancer, MMR testing should be strongly considered for these patients. Many institutions have protocols for reflex testing with immunohistochemistry for women with endometrial cancer. For women with positive test results, germline sequencing can be performed to determine if they have an inherited MMR deficiency, Lynch syndrome. Presence of an MMR deficiency is an important factor in cancer screening and potential treatment.
Second, the impressive results of PD-1 blockade in patients with MMR-deficient tumors suggest that this treatment strategy may be important for women with recurrent or metastatic endometrial cancer. The ideal timing of immunotherapy for women with endometrial cancer is an area of active ongoing study.
Immunotherapy with PD-1 blockade is an important treatment strategy for women with MMR-deficient or MSI-H gynecologic cancers.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In this Update, I report on the latest US Preventive Services Task Force (USPSTF) cervical cancer screening recommendations. In addition, I describe the results of 2 studies, a large prospective multicenter study of the accuracy of sentinel lymph node (SLN) biopsy in endometrial cancer, and a proof-of-concept review of use of checkpoint blockade to increase immune response and of its possible role in endometrial cancer.
hrHPV testing used alone as primary screening for cervical cancer: USPSTF recommendations
US Preventive Services Task Force. Draft recommendation statement: cervical cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2. Published October 2017. Accessed February 5, 2018.
Despite our rapid advances in understanding the molecular underpinnings of cancer, gynecologic malignancies are still a major cause of morbidity and mortality among women. Cervical cancer stands as an example of how a cancer screening test can be implemented to reduce mortality. In this section, I report on the USPSTF cervical cancer screening recommendations, which were updated in October 2017.
Even with the widespread implementation of screening programs for cervical cancer in the United States, 13,240 women will be diagnosed with the disease in 2018, and 4,170 will die from cervical cancer.1 Most often, cervical cancer occurs in women who have not been adequately screened. It is now recognized that the human papillomavirus (HPV) is the cause of cervical cancer.2
While cervical cytology has long been used as a screening test for cervical cancer, testing for high-risk HPV subtypes (hrHPV testing) also has been used as a screening modality. Traditionally, hrHPV testing is used in combination with cervical cytology, so called cotesting. There is convincing evidence that cervical cytology, as well as strategies that use hrHPV testing, can detect high-grade cervical precancers and cancers and thereby reduce mortality. However, cervical cancer screening is also associated with frequent follow-ups, invasive procedures performed to assess abnormal results, psychological distress, and adverse pregnancy outcomes of treatment for precancerous lesions.
The USPSTF based its new cervical cancer screening recommendations on clinical trial data and decision modeling of various screening strategies, and weighed the benefits and harms of each strategy.
Recommendations from the USPSTF
hrHPV screening for cervical cancer. TheUSPSTF recommends screening with cervical cytology every 3 years for women 21 to 29 years of age. For women 30 to 65 years of age, screening with cytology every 3 years, or hrHPV testing alone used every 5 years, is recommended.
Data from large randomized trials suggest cytologic screening is slightly less sensitive than hrHPV testing in detecting high-grade (grade 2 or 3) cervical intraepithelial neoplasia (CIN). However, hrHPV testing results in more follow-up tests and colposcopies. In a decision model, the USPSTF found that cotesting increased the number of follow-up tests but did not increase detection of grade 3 CIN or invasive cancer. This is the first clinical guideline to recommend hrHPV testing used alone for screening. The American College of Obstetricians and Gynecologists (ACOG) continues to recommend cotesting (cytology in combination with hrHPV) as a primary screening modality in this population.3
Exceptions. According to the USPSTF, 3 populations should not be screened: women over 65 years of age with adequate prior screening who are not otherwise at high risk for cervical cancer; women under 21 years of age; and women who have had a hysterectomy and do not have a history of grade 2 or 3 CIN or cancer.
Summary. The USPSTF recommendations are intended for the general population and are not applicable to women with a history of high-grade CIN or cervical cancer, women with in utero exposure to diethylstilbestrol, and women who are immunocompromised. The remaining USPSTF recommendations are largely in line with guidelines published by ACOG and other groups.3,4
Testing for high-risk HPV alone is a reasonable screening option for cervical cancer. This modality can be used in women 30 to 65 years of age but should not be repeated more frequently than every 5 years in those with a negative result.
Read about SLN biopsy to stage endometrial cancer
SLN biopsy for staging endometrial cancer
Rossi EC, Kowalski LD, Scalici J, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017;18(3):384-392.
Surgery is the cornerstone of treatment for most gynecologic cancers. The widespread use of minimally invasive surgical techniques and the introduction of less radical procedures for gynecologic cancers have helped reduce surgical morbidity.
For endometrial cancer, the role of lymphadenectomy is controversial. Data from prospective trials of this procedure suggest an association with increased morbidity and long-term sequelae, such as lymphedema, and no association with improved survival.5,6
SLN biopsy is an important advance and a potential alternative nodal evaluation method that may be associated with decreased morbidity. In this more limited assessment technique, the first nodal drainage basins of a tumor are identified and removed for pathologic evaluation.
Accuracy of SLN biopsy in endometrial cancer was the subject of Rossi and colleagues' recent large prospective multicenter study, the Fluorescence Imaging for Robotic Endometrial Sentinel lymph node biopsy (FIRES) trial.
Details of the study
Rossi and colleagues conducted the FIRES trial to estimate the sensitivity of SLN biopsy in detecting nodal metastases in women with stage I endometrial cancer. Patients (N = 385) from 10 US sites were enrolled in the study. SLN evaluation was performed after cervical injection of indocyanine green followed by robotic-assisted hysterectomy. After identification of the SLN, participants underwent pelvic lymphadenectomy. Performance of para-aortic lymphadenectomy was optional.
Mapping of the SLN was feasible in 86% of patients, including bilateral mapping in 52%. Twelve percent of the participants had nodal metastases. SLN biopsy had a sensitivity of 97% in women who had identification of the SLNs. Similarly, the negative predictive value was high, 99.6%. The procedure was associated with acceptable short-term toxicity with adverse events in 9% of study participants. Common complications included neurologic complications, respiratory distress, nausea and vomiting, and, in 3 patients, bowel injury.
Accurate detection of nodal metastases. Results of the study suggest SLN biopsy is accurate in detecting nodal metastases in women with endometrial cancer. Although long-term toxicity was not examined, other work suggests the lymphedema rates associated with SLN biopsy may be lower than those of lymphadenectomy. While the study described impressive performance characteristics, there remain technical challenges. Even among skilled surgeons trained for the protocol, there was no nodal mapping in nearly half of the women with endometrial cancer. Women without node mapping require full lymphadenectomy thus negating the possible benefits of the procedure.
Given the high accuracy of SLN mapping in endometrial cancer, the procedure likely will become the standard of care for nodal evaluation by gynecologic oncologists.
Read about immunotherapy for gynecologic cancers
Immunotherapy for gynecologic cancers
Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413.
In oncology, precision medicine is rapidly becoming a standard treatment approach. Therapies are being used to target specific genetic alterations in tumors. In cancer immunotherapy, the immune system is being used to facilitate clearance of cancer cells.
The most common mechanism of action of clinically used immunotherapeutic agents is blockade of programmed cell death protein 1 (PD-1), a lymphocyte receptor that prevents the immune system from targeting the body's own cells.7 Cancers that have mutations in the DNA mismatch repair (MMR) proteins display microsatellite instability (MSI) and produce high levels of abnormal proteins.8 These abnormal proteins serve as tumor antigens that can be targeted by the body's normal immune system.
In May 2017, the US Food and Drug Administration (FDA) granted accelerated approval of the PD-1 blocking antibody pembrolizumab for the treatment of unresectable or metastatic MSI-high (MSI-H) or MMR-deficient solid tumors.9 The approval was based on data from 149 patients treated in 5 studies that demonstrated a response rate of 39.6%, including responses that lasted at least 6 months in 78% of participants. This was the first ever cancer drug that received FDA approval based on a tumor's biomarker profile without regard to the site of origin. I describe the results of a study by Le and colleagues that examines the possible role of immunotherapy in a variety of solid tumors in this section.
Details of the study
This study examined the clinical efficacy of PD-1 blockade in 86 patients with advanced, MMR-deficient tumors from 12 different sites. Endometrial cancer was the second most frequent primary tumor site in 17% of patients. Within the cohort, the overall objective response rate was 53%, which included 21% of patients with complete radiographic response (no imaging evidence of cancer). Disease control, either complete or partial response or stable disease, was achieved in 77% of patients. After a median follow-up of 12.5 months, neither the median progression-free survival (PFS) nor median overall survival had been reached. The authors estimated that 2-year overall survival was 64%, substantially higher than expected for patients with advanced solid tumors.
Le and colleagues also performed several in vivo laboratory experiments to explore the mechanisms by which patients responded. In addition, they used sequencing to determine the prevalence of MMR deficiency in 12,019 cancer samples that included 32 distinct tumor types (FIGURE). Endometrial cancer had the highest frequency of MMR deficiency (17%). Four percent of cervical cancers and less than 2% of ovarian cancers were MMR-deficient.
The promise of immunotherapy for endometrial cancer. This study's data and other emerging data have important implications for women with gynecologic cancer, particularly endometrial cancer. First, given the frequency of MMR mutations among women with endometrial cancer, MMR testing should be strongly considered for these patients. Many institutions have protocols for reflex testing with immunohistochemistry for women with endometrial cancer. For women with positive test results, germline sequencing can be performed to determine if they have an inherited MMR deficiency, Lynch syndrome. Presence of an MMR deficiency is an important factor in cancer screening and potential treatment.
Second, the impressive results of PD-1 blockade in patients with MMR-deficient tumors suggest that this treatment strategy may be important for women with recurrent or metastatic endometrial cancer. The ideal timing of immunotherapy for women with endometrial cancer is an area of active ongoing study.
Immunotherapy with PD-1 blockade is an important treatment strategy for women with MMR-deficient or MSI-H gynecologic cancers.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 168: Cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
- Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–1716.
- ASTEC Study Group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125–136.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264.
- Buza N, Ziai J, Hui P. Mismatch repair deficiency testing in clinical practice. Expert Rev Mol Diagn. 2016;16(5):591–604.
- FDA approves first cancer treatment for any solid tumor with a specific genetic feature [news release]. Silver Spring, MD: US Food and Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm560167.htm. Published May 23, 2017. Accessed February 5, 2018.
- American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society; 2018.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No. 168: Cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e130.
- Saslow D, Solomon D, Lawson HW, et al; ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
- Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–1716.
- ASTEC Study Group, Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373(9658):125–136.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264.
- Buza N, Ziai J, Hui P. Mismatch repair deficiency testing in clinical practice. Expert Rev Mol Diagn. 2016;16(5):591–604.
- FDA approves first cancer treatment for any solid tumor with a specific genetic feature [news release]. Silver Spring, MD: US Food and Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm560167.htm. Published May 23, 2017. Accessed February 5, 2018.