User login
OR safety and efficiency: Measuring and monitoring all factors—including surgical volume
The operating room (OR) is a key contributor to a hospital’s profitability. It is a complex environment with ever-advancing technology. A successful surgery completed without complications within an optimal time depends not only on the surgeon’s experience, skills, and knowledge but also on numerous other structural, human, and nontechnical factors over which the surgeon has limited control.
As in any setting that deals with human life, in the OR, team dynamics, communication, and environment play a major role. Research has indicated the benefits of dedicated teams, reduced handoffs, and innovative modalities that continuously and systematically monitor potential breakdowns and propose solutions for the detected problems.
Finally, who should perform your loved one’s hysterectomy? This article also attempts to address the impact of surgeons’ and hospitals’ volume on operative outcomes with a diminishing number of hysterectomies but an increasing number of approaches.
Human factors in the OR
Human factors research was born as a product of the industrial revolution and mass production. It aims to optimize human experience and improve system performance by studying how humans interact with system. The aviation industry, for example, minimized errors significantly by using methods developed by human factors scientists. As another industry with no tolerance for mistakes, the health care sector followed suit. Ultimately, the goal of human factors research in health care is to improve patient safety, optimize work and environment, reduce costs, and enhance employees’ physical and mental health, engagement, comfort, and quality of life (FIGURE 1).1
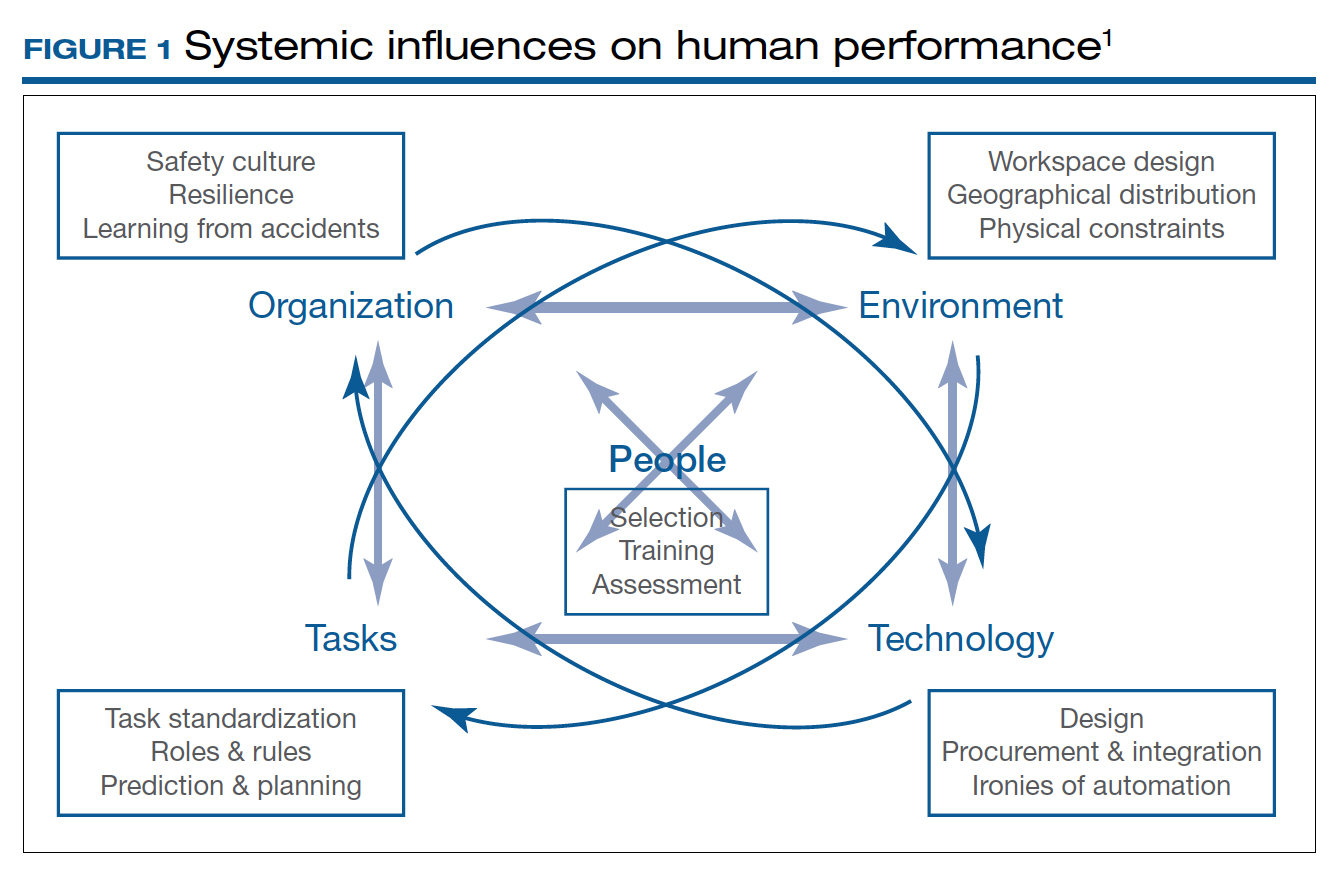
Today’s OR is so complex that it is hard to understand its dynamics without human factors research. Every new OR technology is first tested in controlled and simulated environments to determine “work as imagined.” However, it is necessary to study “work as done” in the real world via direct observation, video recording, questionnaires, and semistructured interviews by an on-site multidisciplinary team. This process not only focuses on surgical skills, process efficiency, and outcomes but also monitors the entire process according to Human Factors and Ergonomics Engineering principles to explore otherwise hidden complexities and latent safety concerns. The Systems Engineering Initiative for Patient Safety (SEIPS) framework is used to study the impact of interactions between people, tasks, technologies, environment, and organization.1
Robot-assisted surgery (RAS), an increasingly popular surgical approach among gynecologic surgeons, recently has been the focus of human factors science. A robotic OR poses unique challenges: the surgeon is not scrubbed, is removed from the operating table, and controls a complex highly technologic device in a crowded and darkened room. These are ideal conditions waiting to be optimized by human factor experts. To demonstrate the importance of human factors in the OR, we review the evidence for RAS.
Continue to: Impact of flow disruptions...
Impact of flow disruptions
Flow disruptions (FDs) were found to be more common in RAS. Catchpole and colleagues identified a mean of 9.62 FDs per hour in 89 robotic procedures, including hysterectomies and sacrocolpopexies, from a variety of fields; FDs occurred more often during the docking stage, followed by the console time, and they mostly were caused by communication breakdown and lack of team familiarity.2
Surgeon experience significantly reduced FDs. Surgeons who had done more than 700 RAS cases experienced 60% fewer FDs than those who had done less than 250 cases (13 vs 8 per hour).2 A study focusing on residents’ impact on RAS outcomes found that each FD increased the total operative time by an average 2.4 minutes, with the number significantly higher when a resident was involved.3 About one-quarter of the training-related FDs were procedure-specific instructions, while one-third were related to instrument and robotic instruction. However, pauses to teach residents did not appear to create significant intraoperative delays. Expectedly, experienced surgeons could anticipate and reduce these disruptions by supporting the whole team.
Human ergonomics, turnover time, and robot-specific skills
In a study of human ergonomics in RAS, Yu and colleagues noted that bedside assistants could experience neck posture problems. Surprisingly, the console could constrain the surgeon’s neck-shoulder region.4 Studies that reported on communication problems in a robotic OR suggest that innovative forms of verbal and nonverbal communication may support successful team communication.5
On the learning curve for RAS, OR turnover time, a key value metric, has been longer. However, turnover time was reduced almost by half from 99.2 to 53.2 minutes over 3 months after concepts from motor racing pit stops were employed, including briefings, leadership, role definition, task allocation, and task sequencing. Average room-ready time also was lowered from 42.2 to 27.2 minutes.6 RAS presents new challenges with sterile instrument processing as well. A successful RAS program, therefore, has organizational needs that include the training of OR and sterile processing staff and appropriate shift management.1
In a robotic OR, not only the surgeon but also the whole team requires robot-specific skills. New training approaches to teamwork, communication, and situation awareness skills are necessary. Robotic equipment, with its data and power cables, 2 consoles, and changing movement paths, necessitate larger rooms with a specific layout.7
In a review of recordings of RAS that used a multidimensional assessment tool to measure team effectiveness and cognitive load, Sexton and colleagues identified anticipation, active team engagement, and higher familiarity scores as the best predictors of team efficiency.8 Several studies emphasized the need for a stable team, especially in the learning phase of robotic surgery.5,9,10 A dedicated robotic team reduced the operative time by 18% during robot-assisted sacrocolpopexy (RASCP).10 RASCP procedures that extended into the afternoon took significantly longer time.9 A dedicated anesthesiologist improved the preoperative time.9 Surgical team handoffs also have reduced OR efficiency.11,12
Studying the impact of human factors is paramount for safe and efficient surgery. It is especially necessary in ORs that are equipped with high technologic instruments such as those used in RAS.
Surgical Black Box: Using data for OR safety and efficiency
Surgical procedures account for more than 50% of medical errors in a hospital setting, many of which are preventable. Postevent analysis with traditional methods, such as “Morbidity and Mortality” meetings held many days later, misses many adverse events in the OR.13 Another challenge with ever-changing and fast-multiplying surgical approaches is the development of effective surgical skill. Reviewing video recording of surgical procedures has been proposed as an instrument for recognizing adverse events and perfecting surgical skills.Recently, an innovative data-capture platform called the OR Black Box, developed by Teodor Grantcharov, MD, PhD, and colleagues, went beyond simple audiovisual recording.14 This high technologic platform not only video records the actual surgical procedure with laparoscopic camera capture (and wearable cameras for open cases) but also monitors the entire OR environment via wide-angle cameras, utilizes sensors, and records both the patient’s and the surgeon’s physiologic parameters.
The OR Black Box generates a holistic view of the OR after synchronization, encryption, and secure storage of all inputs for further analysis by experts and software-based algorithms (FIGURE 2). Computer vision algorithms can recognize improper dissection techniques and complications, such as bleeding. Adverse events are flagged with an automated software on a procedural timeline to facilitate review of procedural steps, disruptive environmental and organizational factors, OR team technical and nontechnical skills, surgeon physiologic stress, and intraoperative errors, events, and rectification processes using validated instruments.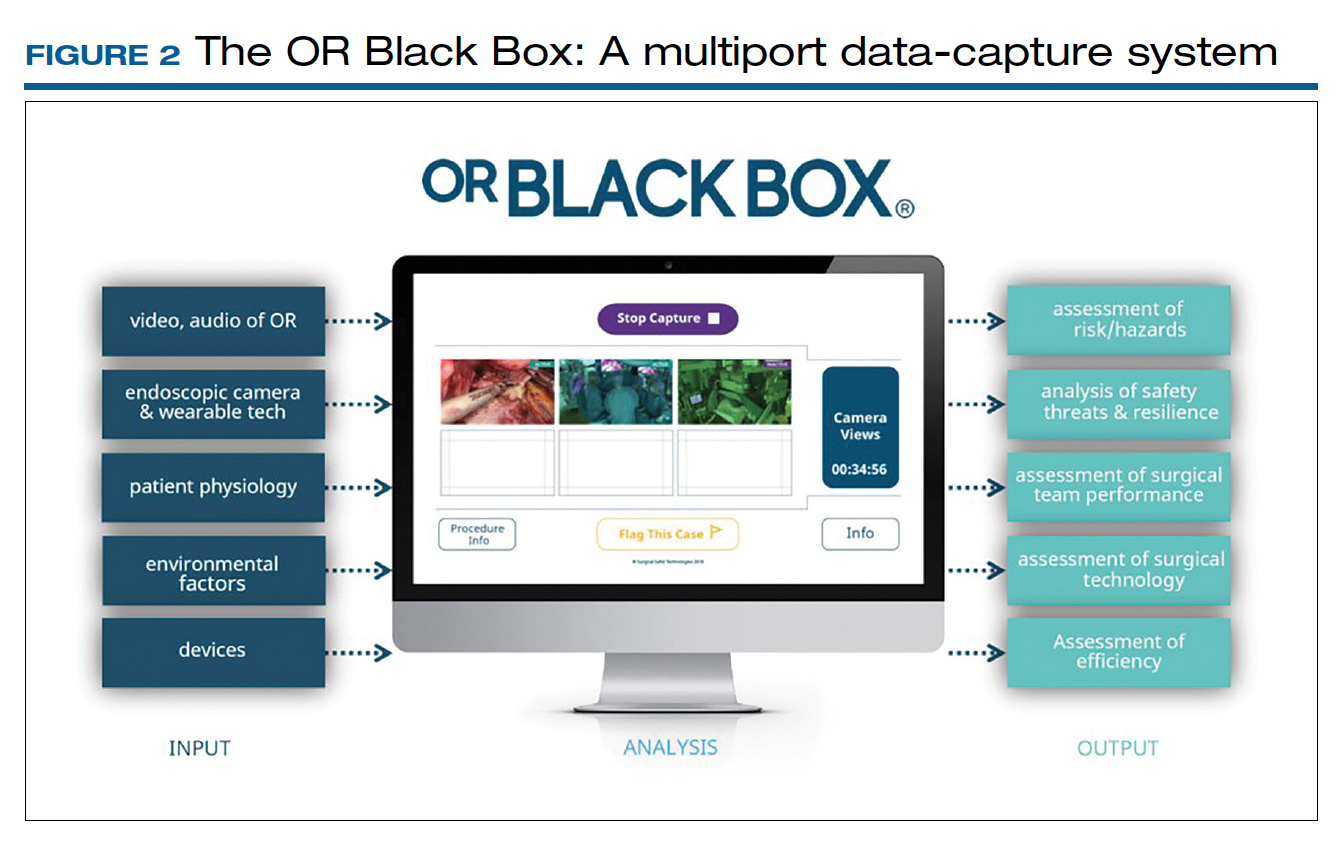
Artificial intelligence built into this platform can automatically extract objective, high-quality, and structured data to generate explainable insights by recognizing adverse events and procedural segments of interest for training and quality improvement and provide a foundation with objective measurements of technical and nontechnical performance for formative and summative assessment. This system, a major step up compared with retrospective review of likely biased medical records and labor-intensive multidisciplinary human observers, has the potential to increase efficiency and reduce costs by studying human factors that include clinical design, technology, and organization. OR efficiency, measured in real time objectively and thoroughly, may save time and resources.
OR Black Box platforms have already started to generate meaningful data. It is not surprising that auditory disruptions—OR doors opening, loud noises, pagers beeping, telephones ringing—were recorded almost every minute during laparoscopic procedures.15 Most technical errors occurred during dissection, resection, and reconstruction and most commonly were associated with improper estimations of force applied to tissue and distance to the target tissue during operative steps of a laparoscopic procedure.16 Another study based on this system showed that technical performance was an independent predictor of postoperative outcomes.17 The OR Black Box identified a device-related interruption in 30% of elective laparoscopic general surgery cases, most commonly in sleeve gastrectomy and oncologic gastrectomy procedures. This sophisticated surgical data recording system also demonstrated a significantly better ability to detect Veress needle injuries (12 vs 3) and near misses (47 vs 0) when compared with traditional chart review.18
Data from the OR Black Box also have been applied to better analyze nontechnical performance, including teamwork and interpersonal dynamics.19 Surgeons most commonly exhibited adept situational awareness and leadership, while the nurse team excelled at task management and situational awareness.19 Of the total care provider team studied, the surgeon and scrub nurse demonstrated the most favorable nontechnical behavior.19 Of note, continuous physiologic monitoring of the surgeon with this system revealed that surgeons under stress had 66% higher adverse events.
The OR Black Box is currently utilized at 20 institutions in North America and Europe. The data compiled from all these institutions revealed that there was a 10% decrease in intraoperative adverse events for each 10-point increase in technical skill score on a scale of 0 to 100 (unpublished data). This centralized data indicated that turnover time ranged widely between 7 and 91 minutes, with variation of cleanup time from 1 to 25 minutes and setup time from 22 to 43 minutes. Institutions can learn from each other using this platform. For example, the information about block time utilization (20%–99%) across institutions provides opportunities for system improvements.
With any revolutionary technology, it is imperative to study its effects on outcomes, training, costs, and privacy before it is widely implemented. We, obstetricians and gynecologists, are very familiar with the impact of electronic fetal monitoring, a great example of a technologic advance that did not improve perinatal outcomes but led to unintended consequences, such as higher rates of cesarean deliveries and lawsuits. Such a tool may lead to potential misrepresentation of intraoperative events unless legal aspects are clearly delineated. As exciting as it is, this disruptive technology requires further exploration with scientific vigor.
Continue to: Surgeon and hospital volume: Surgical outcomes paradigm...
Surgeon and hospital volume: Surgical outcomes paradigm
A landmark study in 1979 that showed decreased mortality in high-volume centers underscored the need for regionalization for certain surgical procedures.20 This association was further substantiated by 2 reports on 2.5 million Medicare beneficiaries that demonstrated significantly lower mortality for all 14 cardiovascular and oncologic procedures for hospitals with larger surgical volume (16% vs 4%) and high-volume surgeons for certain procedures, for example, 15% versus 5% for pancreatic resections for cancer.21,22
A similar association was found for all routes of hysterectomies performed for benign indications. Boyd and colleagues showed that gynecologists who performed fewer than 10 hysterectomies per year had a higher perioperative morbidity rate (16.5%) compared with those who did more (11.7%).23 Specific to vaginal hysterectomy, in a study of more than 6,000 women, surgeons who performed 13 procedures per year had 31% less risk of operative injury than those who did 5.5 procedures per year (2.5% vs 1.7%).24 Overall perioperative complications (5.0% vs 4.0%) and medical complications (5.7% vs 3.9%) were also reduced for higher-volume surgeons. In a cohort of approximately 8,000 women who underwent a laparoscopic hysterectomy, high-volume surgeons had a considerably lower complication rate (4.2% vs 6.2%).25
As expected, lower complication rates of high-volume surgeons led to lower resource utilization, including lower transfusion rates, less intensive care unit utilization, and shorter operative times and, in several studies, length of stay.24,25 Of note, low-volume surgeons were less likely to offer minimally invasive routes and were more likely to convert to laparotomy.26 In addition, significant cost savings have been associated with high surgical volume, which one study showed was 16% ($6,500 vs $5,600) for high-volume surgeons.26 With regard to mortality, a study of 7,800 women found that perioperative mortality increased more than 10-fold for surgeons who performed an average 1 case per year compared with all other surgeons (2.5% vs 0.2%).27
When gynecologic cancers are concerned, arguably, long-term survival outcomes may be more critical than perioperative morbidity and mortality. Higher surgeon and hospital volume are associated with improved perioperative outcomes for endometrial and cervical cancers.28 Importantly, minimally invasive hysterectomy was offered for endometrial cancer significantly more often by surgeons with high volume.28 Survival outcomes were not affected by surgeon or hospital volume, likely due to overall more favorable prognosis for endometrial cancer after treatment.
Although it is intuitive to assume that a surgeon’s skills and experience would make the most impact in procedures for ovarian cancer due to the complexity of ovarian cancer surgery, evidence on short-term outcomes has been mixed. Intriguingly, some studies reported that high-volume institutions had higher complication and readmission rates. However, evidence supports that the surgeon’s volume, and especially hospital volume, improves long-term survival for ovarian cancer, with a negative impact on immediate postoperative morbidity.29 This may suggest that a more aggressive surgical effort improves long-term survival but also can cause more perioperative complications. Further, longer survival may result not only from operative skills but also because of better care by a structured multidisciplinary team at more established high-volume cancer centers.
The association of improved outcomes with higher volume led to public reporting of hospital outcomes. Policy efforts toward regionalization have impacted surgical practice. Based on their analysis of 3.2 million Medicare patients who underwent 1 of 8 different cancer surgeries or cardiovascular operations from 1999 to 2008, Finks and colleagues demonstrated that care was concentrated to fewer hospitals over time for many of these procedures.29 This trend was noted for gynecologic cancer surgery but not for benign gynecologic surgery.
Regionalization of care limits access particularly for minority and underserved communities because of longer travel distances, logistic challenges, and financial strain. An alternative to regionalization of care is targeted quality improvement by rigorous adherence to quality guidelines at low-volume hospitals.
Is there a critical minimum volume that may be used as a requirement for surgeons to maintain their privileges and for hospitals to offer certain procedures? In 2015, minimum volume standards for a number of common procedures were proposed by Johns Hopkins Medicine and Dartmouth-Hitchcock Medical Center, such as 50 hip replacement surgeries per hospital and 25 per physician per year, and 20 pancreatectomies per hospital and 5 per surgeon per year.30 A modeling study for hysterectomy showed that a volume cut point of >1 procedure in the prior year would restrict privileges for a substantial number of surgeons performing abdominal (17.5%), robot-assisted (12.5%), laparoscopic (16.8%), and vaginal (27.6%) hysterectomies.27 This study concluded that minimum-volume standards for hysterectomy for even the lowest volume physicians would restrict a significant number of gynecologic surgeons, including many with outcomes that are better than predicted.
Therefore, while there is good evidence that favors better outcomes in the hands of high-volume surgeons in gynecology, the impact of such policies on gynecologic practice clearly warrants careful monitoring and further study.
- What factors besides the surgeon’s skills influence surgical safety and efficiency?
- Are you ready to have audio, video, and sensor-based recording of everything in the OR?
- Who should perform your loved one’s hysterectomy? Do the surgeon’s and hospital’s volume matter?
- Catchpole K, Bisantz A, Hallbeck MS, et al. Human factors in robotic assisted surgery: lessons from studies ‘in the wild’. Appl Ergon. 2019;78:270-276.
- Catchpole K, Perkins C, Bresee C, et al. Safety, efficiency and learning curves in robotic surgery: a human factors analysis. Surg Endosc. 2016;30:3749-3761.
- Jain M, Fry BT, Hess LW, et al. Barriers to efficiency in robotic surgery: the resident effect. J Surg. Res. 2016;205:296-304.
- Yu D, Dural C, Morrow MM, et al. Intraoperative workload in robotic surgery assessed by wearable motion tracking sensors and questionnaires. Surg Endosc. 2017;31:877-886.
- Randell R, Honey S, Alvarado N, et al. Embedding robotic surgery into routine practice and impacts on communication and decision making: a review of the experience of surgical teams. Cognit Technol Work. 2016;18:423-437.
- Souders CP, Catchpole KR, Wood LN, et al. Reducing operating room turnover time for robotic surgery using a motor racing pit stop model. World J Surg. 2017;4:1943–1949.
- Ahmad N, Hussein AA, Cavuoto L, et al. Ambulatory movements, team dynamics and interactions during robot-assisted surgery. BJU Int. 2016;118:132-139.
- Sexton K, Johnson A, Gotsch A, et al. Anticipation, teamwork, and cognitive load: chasing efficiency during robot-assisted surgery. BMJ Qual Saf. 2018;27:148-154.
- Harmanli O, Solak S, Bayram A, et al. Optimizing the robotic surgery team: an operations management perspective. Int Urogynecol J. 2021;32:1379-1385.
- Carter-Brooks CM, Du AL, Bonidie MJ, et al. The impact of a dedicated robotic team on robotic-assisted sacrocolpopexy outcomes. Female Pelvic Med Reconstr Surg. 2018;24:13-16.
- Giugale LE, Sears S, Lavelle ES, et al. Evaluating the impact of intraoperative surgical team handoffs on patient outcomes. Female Pelvic Med Reconstr Surg. 2017;23:288-292.
- Geynisman-Tan J, Brown O, Mueller M, et al. Operating room efficiency: examining the impact of personnel handoffs. Female Pelvic Med Reconstr Surg. 2018;24:87-89.
- Alsubaie H, Goldenberg M, Grantcharov T. Quantifying recall bias in surgical safety: a need for a modern approach to morbidity and mortality reviews. Can J Surg. 2019;62:39-43.
- Goldenberg MG, Jung J, Grantcharov TP. Using data to enhance performance and improve quality and safety in surgery. JAMA Surg. 2017;152:972-973.
- Jung JJ, Grantcharov TP. The operating room black box: a prospective observational study of the operating room. J Am Coll Surg. 2017;225:S127-S128.
- Jung JJ, Jüni P, Lebovic G, et al. First-year analysis of the operating room black box study. Ann Surg. 2020;271:122-127.
- Jung JJ, Kashfi A, Sharma S, et al. Characterization of device-related interruptions in minimally invasive surgery: need for intraoperative data and effective mitigation strategies. Surg Endosc. 2019;33:717-723.
- Jung JJ, Adams-McGavin RC, Grantcharov TP. Underreporting of Veress needle injuries: comparing direct observation and chart review methods. J Surg Res. 2019;236:266-270.
- Fesco AB, Kuzulugil SS, Babaoglu C, et al. Relationship between intraoperative nontechnical performance and technical events in bariatric surgery. Br J Surg. 2018;105:1044-1050.
- Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364-1369.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137.
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:21172127.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915.
- Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116:1341-1347.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Bretschneider CE, Frazzini Padilla P, Das D, et al. The impact of surgeon volume on perioperative adverse events in women undergoing minimally invasive hysterectomy for the large uterus. Am J Obstet Gynecol. 2018;219:490.e1-490.e8.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Wright JD. The volume-outcome paradigm for gynecologic surgery: clinical and policy implications. Clin Obstet Gynecol. 2020;63:252-265.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high risk surgery. N Engl J Med. 2011;364:21282137.
- Sternberg S. Hospitals move to limit low-volume surgeries. US News & World Report. May 19, 2015. www.usnews.com/news /articles/2015/05/19/hospitals-move-to-limit-low-volume-surgeries. Accessed April 19, 2022.
The operating room (OR) is a key contributor to a hospital’s profitability. It is a complex environment with ever-advancing technology. A successful surgery completed without complications within an optimal time depends not only on the surgeon’s experience, skills, and knowledge but also on numerous other structural, human, and nontechnical factors over which the surgeon has limited control.
As in any setting that deals with human life, in the OR, team dynamics, communication, and environment play a major role. Research has indicated the benefits of dedicated teams, reduced handoffs, and innovative modalities that continuously and systematically monitor potential breakdowns and propose solutions for the detected problems.
Finally, who should perform your loved one’s hysterectomy? This article also attempts to address the impact of surgeons’ and hospitals’ volume on operative outcomes with a diminishing number of hysterectomies but an increasing number of approaches.
Human factors in the OR
Human factors research was born as a product of the industrial revolution and mass production. It aims to optimize human experience and improve system performance by studying how humans interact with system. The aviation industry, for example, minimized errors significantly by using methods developed by human factors scientists. As another industry with no tolerance for mistakes, the health care sector followed suit. Ultimately, the goal of human factors research in health care is to improve patient safety, optimize work and environment, reduce costs, and enhance employees’ physical and mental health, engagement, comfort, and quality of life (FIGURE 1).1

Today’s OR is so complex that it is hard to understand its dynamics without human factors research. Every new OR technology is first tested in controlled and simulated environments to determine “work as imagined.” However, it is necessary to study “work as done” in the real world via direct observation, video recording, questionnaires, and semistructured interviews by an on-site multidisciplinary team. This process not only focuses on surgical skills, process efficiency, and outcomes but also monitors the entire process according to Human Factors and Ergonomics Engineering principles to explore otherwise hidden complexities and latent safety concerns. The Systems Engineering Initiative for Patient Safety (SEIPS) framework is used to study the impact of interactions between people, tasks, technologies, environment, and organization.1
Robot-assisted surgery (RAS), an increasingly popular surgical approach among gynecologic surgeons, recently has been the focus of human factors science. A robotic OR poses unique challenges: the surgeon is not scrubbed, is removed from the operating table, and controls a complex highly technologic device in a crowded and darkened room. These are ideal conditions waiting to be optimized by human factor experts. To demonstrate the importance of human factors in the OR, we review the evidence for RAS.
Continue to: Impact of flow disruptions...
Impact of flow disruptions
Flow disruptions (FDs) were found to be more common in RAS. Catchpole and colleagues identified a mean of 9.62 FDs per hour in 89 robotic procedures, including hysterectomies and sacrocolpopexies, from a variety of fields; FDs occurred more often during the docking stage, followed by the console time, and they mostly were caused by communication breakdown and lack of team familiarity.2
Surgeon experience significantly reduced FDs. Surgeons who had done more than 700 RAS cases experienced 60% fewer FDs than those who had done less than 250 cases (13 vs 8 per hour).2 A study focusing on residents’ impact on RAS outcomes found that each FD increased the total operative time by an average 2.4 minutes, with the number significantly higher when a resident was involved.3 About one-quarter of the training-related FDs were procedure-specific instructions, while one-third were related to instrument and robotic instruction. However, pauses to teach residents did not appear to create significant intraoperative delays. Expectedly, experienced surgeons could anticipate and reduce these disruptions by supporting the whole team.
Human ergonomics, turnover time, and robot-specific skills
In a study of human ergonomics in RAS, Yu and colleagues noted that bedside assistants could experience neck posture problems. Surprisingly, the console could constrain the surgeon’s neck-shoulder region.4 Studies that reported on communication problems in a robotic OR suggest that innovative forms of verbal and nonverbal communication may support successful team communication.5
On the learning curve for RAS, OR turnover time, a key value metric, has been longer. However, turnover time was reduced almost by half from 99.2 to 53.2 minutes over 3 months after concepts from motor racing pit stops were employed, including briefings, leadership, role definition, task allocation, and task sequencing. Average room-ready time also was lowered from 42.2 to 27.2 minutes.6 RAS presents new challenges with sterile instrument processing as well. A successful RAS program, therefore, has organizational needs that include the training of OR and sterile processing staff and appropriate shift management.1
In a robotic OR, not only the surgeon but also the whole team requires robot-specific skills. New training approaches to teamwork, communication, and situation awareness skills are necessary. Robotic equipment, with its data and power cables, 2 consoles, and changing movement paths, necessitate larger rooms with a specific layout.7
In a review of recordings of RAS that used a multidimensional assessment tool to measure team effectiveness and cognitive load, Sexton and colleagues identified anticipation, active team engagement, and higher familiarity scores as the best predictors of team efficiency.8 Several studies emphasized the need for a stable team, especially in the learning phase of robotic surgery.5,9,10 A dedicated robotic team reduced the operative time by 18% during robot-assisted sacrocolpopexy (RASCP).10 RASCP procedures that extended into the afternoon took significantly longer time.9 A dedicated anesthesiologist improved the preoperative time.9 Surgical team handoffs also have reduced OR efficiency.11,12
Studying the impact of human factors is paramount for safe and efficient surgery. It is especially necessary in ORs that are equipped with high technologic instruments such as those used in RAS.
Surgical Black Box: Using data for OR safety and efficiency
Surgical procedures account for more than 50% of medical errors in a hospital setting, many of which are preventable. Postevent analysis with traditional methods, such as “Morbidity and Mortality” meetings held many days later, misses many adverse events in the OR.13 Another challenge with ever-changing and fast-multiplying surgical approaches is the development of effective surgical skill. Reviewing video recording of surgical procedures has been proposed as an instrument for recognizing adverse events and perfecting surgical skills.Recently, an innovative data-capture platform called the OR Black Box, developed by Teodor Grantcharov, MD, PhD, and colleagues, went beyond simple audiovisual recording.14 This high technologic platform not only video records the actual surgical procedure with laparoscopic camera capture (and wearable cameras for open cases) but also monitors the entire OR environment via wide-angle cameras, utilizes sensors, and records both the patient’s and the surgeon’s physiologic parameters.
The OR Black Box generates a holistic view of the OR after synchronization, encryption, and secure storage of all inputs for further analysis by experts and software-based algorithms (FIGURE 2). Computer vision algorithms can recognize improper dissection techniques and complications, such as bleeding. Adverse events are flagged with an automated software on a procedural timeline to facilitate review of procedural steps, disruptive environmental and organizational factors, OR team technical and nontechnical skills, surgeon physiologic stress, and intraoperative errors, events, and rectification processes using validated instruments.
Artificial intelligence built into this platform can automatically extract objective, high-quality, and structured data to generate explainable insights by recognizing adverse events and procedural segments of interest for training and quality improvement and provide a foundation with objective measurements of technical and nontechnical performance for formative and summative assessment. This system, a major step up compared with retrospective review of likely biased medical records and labor-intensive multidisciplinary human observers, has the potential to increase efficiency and reduce costs by studying human factors that include clinical design, technology, and organization. OR efficiency, measured in real time objectively and thoroughly, may save time and resources.
OR Black Box platforms have already started to generate meaningful data. It is not surprising that auditory disruptions—OR doors opening, loud noises, pagers beeping, telephones ringing—were recorded almost every minute during laparoscopic procedures.15 Most technical errors occurred during dissection, resection, and reconstruction and most commonly were associated with improper estimations of force applied to tissue and distance to the target tissue during operative steps of a laparoscopic procedure.16 Another study based on this system showed that technical performance was an independent predictor of postoperative outcomes.17 The OR Black Box identified a device-related interruption in 30% of elective laparoscopic general surgery cases, most commonly in sleeve gastrectomy and oncologic gastrectomy procedures. This sophisticated surgical data recording system also demonstrated a significantly better ability to detect Veress needle injuries (12 vs 3) and near misses (47 vs 0) when compared with traditional chart review.18
Data from the OR Black Box also have been applied to better analyze nontechnical performance, including teamwork and interpersonal dynamics.19 Surgeons most commonly exhibited adept situational awareness and leadership, while the nurse team excelled at task management and situational awareness.19 Of the total care provider team studied, the surgeon and scrub nurse demonstrated the most favorable nontechnical behavior.19 Of note, continuous physiologic monitoring of the surgeon with this system revealed that surgeons under stress had 66% higher adverse events.
The OR Black Box is currently utilized at 20 institutions in North America and Europe. The data compiled from all these institutions revealed that there was a 10% decrease in intraoperative adverse events for each 10-point increase in technical skill score on a scale of 0 to 100 (unpublished data). This centralized data indicated that turnover time ranged widely between 7 and 91 minutes, with variation of cleanup time from 1 to 25 minutes and setup time from 22 to 43 minutes. Institutions can learn from each other using this platform. For example, the information about block time utilization (20%–99%) across institutions provides opportunities for system improvements.
With any revolutionary technology, it is imperative to study its effects on outcomes, training, costs, and privacy before it is widely implemented. We, obstetricians and gynecologists, are very familiar with the impact of electronic fetal monitoring, a great example of a technologic advance that did not improve perinatal outcomes but led to unintended consequences, such as higher rates of cesarean deliveries and lawsuits. Such a tool may lead to potential misrepresentation of intraoperative events unless legal aspects are clearly delineated. As exciting as it is, this disruptive technology requires further exploration with scientific vigor.
Continue to: Surgeon and hospital volume: Surgical outcomes paradigm...
Surgeon and hospital volume: Surgical outcomes paradigm
A landmark study in 1979 that showed decreased mortality in high-volume centers underscored the need for regionalization for certain surgical procedures.20 This association was further substantiated by 2 reports on 2.5 million Medicare beneficiaries that demonstrated significantly lower mortality for all 14 cardiovascular and oncologic procedures for hospitals with larger surgical volume (16% vs 4%) and high-volume surgeons for certain procedures, for example, 15% versus 5% for pancreatic resections for cancer.21,22
A similar association was found for all routes of hysterectomies performed for benign indications. Boyd and colleagues showed that gynecologists who performed fewer than 10 hysterectomies per year had a higher perioperative morbidity rate (16.5%) compared with those who did more (11.7%).23 Specific to vaginal hysterectomy, in a study of more than 6,000 women, surgeons who performed 13 procedures per year had 31% less risk of operative injury than those who did 5.5 procedures per year (2.5% vs 1.7%).24 Overall perioperative complications (5.0% vs 4.0%) and medical complications (5.7% vs 3.9%) were also reduced for higher-volume surgeons. In a cohort of approximately 8,000 women who underwent a laparoscopic hysterectomy, high-volume surgeons had a considerably lower complication rate (4.2% vs 6.2%).25
As expected, lower complication rates of high-volume surgeons led to lower resource utilization, including lower transfusion rates, less intensive care unit utilization, and shorter operative times and, in several studies, length of stay.24,25 Of note, low-volume surgeons were less likely to offer minimally invasive routes and were more likely to convert to laparotomy.26 In addition, significant cost savings have been associated with high surgical volume, which one study showed was 16% ($6,500 vs $5,600) for high-volume surgeons.26 With regard to mortality, a study of 7,800 women found that perioperative mortality increased more than 10-fold for surgeons who performed an average 1 case per year compared with all other surgeons (2.5% vs 0.2%).27
When gynecologic cancers are concerned, arguably, long-term survival outcomes may be more critical than perioperative morbidity and mortality. Higher surgeon and hospital volume are associated with improved perioperative outcomes for endometrial and cervical cancers.28 Importantly, minimally invasive hysterectomy was offered for endometrial cancer significantly more often by surgeons with high volume.28 Survival outcomes were not affected by surgeon or hospital volume, likely due to overall more favorable prognosis for endometrial cancer after treatment.
Although it is intuitive to assume that a surgeon’s skills and experience would make the most impact in procedures for ovarian cancer due to the complexity of ovarian cancer surgery, evidence on short-term outcomes has been mixed. Intriguingly, some studies reported that high-volume institutions had higher complication and readmission rates. However, evidence supports that the surgeon’s volume, and especially hospital volume, improves long-term survival for ovarian cancer, with a negative impact on immediate postoperative morbidity.29 This may suggest that a more aggressive surgical effort improves long-term survival but also can cause more perioperative complications. Further, longer survival may result not only from operative skills but also because of better care by a structured multidisciplinary team at more established high-volume cancer centers.
The association of improved outcomes with higher volume led to public reporting of hospital outcomes. Policy efforts toward regionalization have impacted surgical practice. Based on their analysis of 3.2 million Medicare patients who underwent 1 of 8 different cancer surgeries or cardiovascular operations from 1999 to 2008, Finks and colleagues demonstrated that care was concentrated to fewer hospitals over time for many of these procedures.29 This trend was noted for gynecologic cancer surgery but not for benign gynecologic surgery.
Regionalization of care limits access particularly for minority and underserved communities because of longer travel distances, logistic challenges, and financial strain. An alternative to regionalization of care is targeted quality improvement by rigorous adherence to quality guidelines at low-volume hospitals.
Is there a critical minimum volume that may be used as a requirement for surgeons to maintain their privileges and for hospitals to offer certain procedures? In 2015, minimum volume standards for a number of common procedures were proposed by Johns Hopkins Medicine and Dartmouth-Hitchcock Medical Center, such as 50 hip replacement surgeries per hospital and 25 per physician per year, and 20 pancreatectomies per hospital and 5 per surgeon per year.30 A modeling study for hysterectomy showed that a volume cut point of >1 procedure in the prior year would restrict privileges for a substantial number of surgeons performing abdominal (17.5%), robot-assisted (12.5%), laparoscopic (16.8%), and vaginal (27.6%) hysterectomies.27 This study concluded that minimum-volume standards for hysterectomy for even the lowest volume physicians would restrict a significant number of gynecologic surgeons, including many with outcomes that are better than predicted.
Therefore, while there is good evidence that favors better outcomes in the hands of high-volume surgeons in gynecology, the impact of such policies on gynecologic practice clearly warrants careful monitoring and further study.
- What factors besides the surgeon’s skills influence surgical safety and efficiency?
- Are you ready to have audio, video, and sensor-based recording of everything in the OR?
- Who should perform your loved one’s hysterectomy? Do the surgeon’s and hospital’s volume matter?
The operating room (OR) is a key contributor to a hospital’s profitability. It is a complex environment with ever-advancing technology. A successful surgery completed without complications within an optimal time depends not only on the surgeon’s experience, skills, and knowledge but also on numerous other structural, human, and nontechnical factors over which the surgeon has limited control.
As in any setting that deals with human life, in the OR, team dynamics, communication, and environment play a major role. Research has indicated the benefits of dedicated teams, reduced handoffs, and innovative modalities that continuously and systematically monitor potential breakdowns and propose solutions for the detected problems.
Finally, who should perform your loved one’s hysterectomy? This article also attempts to address the impact of surgeons’ and hospitals’ volume on operative outcomes with a diminishing number of hysterectomies but an increasing number of approaches.
Human factors in the OR
Human factors research was born as a product of the industrial revolution and mass production. It aims to optimize human experience and improve system performance by studying how humans interact with system. The aviation industry, for example, minimized errors significantly by using methods developed by human factors scientists. As another industry with no tolerance for mistakes, the health care sector followed suit. Ultimately, the goal of human factors research in health care is to improve patient safety, optimize work and environment, reduce costs, and enhance employees’ physical and mental health, engagement, comfort, and quality of life (FIGURE 1).1

Today’s OR is so complex that it is hard to understand its dynamics without human factors research. Every new OR technology is first tested in controlled and simulated environments to determine “work as imagined.” However, it is necessary to study “work as done” in the real world via direct observation, video recording, questionnaires, and semistructured interviews by an on-site multidisciplinary team. This process not only focuses on surgical skills, process efficiency, and outcomes but also monitors the entire process according to Human Factors and Ergonomics Engineering principles to explore otherwise hidden complexities and latent safety concerns. The Systems Engineering Initiative for Patient Safety (SEIPS) framework is used to study the impact of interactions between people, tasks, technologies, environment, and organization.1
Robot-assisted surgery (RAS), an increasingly popular surgical approach among gynecologic surgeons, recently has been the focus of human factors science. A robotic OR poses unique challenges: the surgeon is not scrubbed, is removed from the operating table, and controls a complex highly technologic device in a crowded and darkened room. These are ideal conditions waiting to be optimized by human factor experts. To demonstrate the importance of human factors in the OR, we review the evidence for RAS.
Continue to: Impact of flow disruptions...
Impact of flow disruptions
Flow disruptions (FDs) were found to be more common in RAS. Catchpole and colleagues identified a mean of 9.62 FDs per hour in 89 robotic procedures, including hysterectomies and sacrocolpopexies, from a variety of fields; FDs occurred more often during the docking stage, followed by the console time, and they mostly were caused by communication breakdown and lack of team familiarity.2
Surgeon experience significantly reduced FDs. Surgeons who had done more than 700 RAS cases experienced 60% fewer FDs than those who had done less than 250 cases (13 vs 8 per hour).2 A study focusing on residents’ impact on RAS outcomes found that each FD increased the total operative time by an average 2.4 minutes, with the number significantly higher when a resident was involved.3 About one-quarter of the training-related FDs were procedure-specific instructions, while one-third were related to instrument and robotic instruction. However, pauses to teach residents did not appear to create significant intraoperative delays. Expectedly, experienced surgeons could anticipate and reduce these disruptions by supporting the whole team.
Human ergonomics, turnover time, and robot-specific skills
In a study of human ergonomics in RAS, Yu and colleagues noted that bedside assistants could experience neck posture problems. Surprisingly, the console could constrain the surgeon’s neck-shoulder region.4 Studies that reported on communication problems in a robotic OR suggest that innovative forms of verbal and nonverbal communication may support successful team communication.5
On the learning curve for RAS, OR turnover time, a key value metric, has been longer. However, turnover time was reduced almost by half from 99.2 to 53.2 minutes over 3 months after concepts from motor racing pit stops were employed, including briefings, leadership, role definition, task allocation, and task sequencing. Average room-ready time also was lowered from 42.2 to 27.2 minutes.6 RAS presents new challenges with sterile instrument processing as well. A successful RAS program, therefore, has organizational needs that include the training of OR and sterile processing staff and appropriate shift management.1
In a robotic OR, not only the surgeon but also the whole team requires robot-specific skills. New training approaches to teamwork, communication, and situation awareness skills are necessary. Robotic equipment, with its data and power cables, 2 consoles, and changing movement paths, necessitate larger rooms with a specific layout.7
In a review of recordings of RAS that used a multidimensional assessment tool to measure team effectiveness and cognitive load, Sexton and colleagues identified anticipation, active team engagement, and higher familiarity scores as the best predictors of team efficiency.8 Several studies emphasized the need for a stable team, especially in the learning phase of robotic surgery.5,9,10 A dedicated robotic team reduced the operative time by 18% during robot-assisted sacrocolpopexy (RASCP).10 RASCP procedures that extended into the afternoon took significantly longer time.9 A dedicated anesthesiologist improved the preoperative time.9 Surgical team handoffs also have reduced OR efficiency.11,12
Studying the impact of human factors is paramount for safe and efficient surgery. It is especially necessary in ORs that are equipped with high technologic instruments such as those used in RAS.
Surgical Black Box: Using data for OR safety and efficiency
Surgical procedures account for more than 50% of medical errors in a hospital setting, many of which are preventable. Postevent analysis with traditional methods, such as “Morbidity and Mortality” meetings held many days later, misses many adverse events in the OR.13 Another challenge with ever-changing and fast-multiplying surgical approaches is the development of effective surgical skill. Reviewing video recording of surgical procedures has been proposed as an instrument for recognizing adverse events and perfecting surgical skills.Recently, an innovative data-capture platform called the OR Black Box, developed by Teodor Grantcharov, MD, PhD, and colleagues, went beyond simple audiovisual recording.14 This high technologic platform not only video records the actual surgical procedure with laparoscopic camera capture (and wearable cameras for open cases) but also monitors the entire OR environment via wide-angle cameras, utilizes sensors, and records both the patient’s and the surgeon’s physiologic parameters.
The OR Black Box generates a holistic view of the OR after synchronization, encryption, and secure storage of all inputs for further analysis by experts and software-based algorithms (FIGURE 2). Computer vision algorithms can recognize improper dissection techniques and complications, such as bleeding. Adverse events are flagged with an automated software on a procedural timeline to facilitate review of procedural steps, disruptive environmental and organizational factors, OR team technical and nontechnical skills, surgeon physiologic stress, and intraoperative errors, events, and rectification processes using validated instruments.
Artificial intelligence built into this platform can automatically extract objective, high-quality, and structured data to generate explainable insights by recognizing adverse events and procedural segments of interest for training and quality improvement and provide a foundation with objective measurements of technical and nontechnical performance for formative and summative assessment. This system, a major step up compared with retrospective review of likely biased medical records and labor-intensive multidisciplinary human observers, has the potential to increase efficiency and reduce costs by studying human factors that include clinical design, technology, and organization. OR efficiency, measured in real time objectively and thoroughly, may save time and resources.
OR Black Box platforms have already started to generate meaningful data. It is not surprising that auditory disruptions—OR doors opening, loud noises, pagers beeping, telephones ringing—were recorded almost every minute during laparoscopic procedures.15 Most technical errors occurred during dissection, resection, and reconstruction and most commonly were associated with improper estimations of force applied to tissue and distance to the target tissue during operative steps of a laparoscopic procedure.16 Another study based on this system showed that technical performance was an independent predictor of postoperative outcomes.17 The OR Black Box identified a device-related interruption in 30% of elective laparoscopic general surgery cases, most commonly in sleeve gastrectomy and oncologic gastrectomy procedures. This sophisticated surgical data recording system also demonstrated a significantly better ability to detect Veress needle injuries (12 vs 3) and near misses (47 vs 0) when compared with traditional chart review.18
Data from the OR Black Box also have been applied to better analyze nontechnical performance, including teamwork and interpersonal dynamics.19 Surgeons most commonly exhibited adept situational awareness and leadership, while the nurse team excelled at task management and situational awareness.19 Of the total care provider team studied, the surgeon and scrub nurse demonstrated the most favorable nontechnical behavior.19 Of note, continuous physiologic monitoring of the surgeon with this system revealed that surgeons under stress had 66% higher adverse events.
The OR Black Box is currently utilized at 20 institutions in North America and Europe. The data compiled from all these institutions revealed that there was a 10% decrease in intraoperative adverse events for each 10-point increase in technical skill score on a scale of 0 to 100 (unpublished data). This centralized data indicated that turnover time ranged widely between 7 and 91 minutes, with variation of cleanup time from 1 to 25 minutes and setup time from 22 to 43 minutes. Institutions can learn from each other using this platform. For example, the information about block time utilization (20%–99%) across institutions provides opportunities for system improvements.
With any revolutionary technology, it is imperative to study its effects on outcomes, training, costs, and privacy before it is widely implemented. We, obstetricians and gynecologists, are very familiar with the impact of electronic fetal monitoring, a great example of a technologic advance that did not improve perinatal outcomes but led to unintended consequences, such as higher rates of cesarean deliveries and lawsuits. Such a tool may lead to potential misrepresentation of intraoperative events unless legal aspects are clearly delineated. As exciting as it is, this disruptive technology requires further exploration with scientific vigor.
Continue to: Surgeon and hospital volume: Surgical outcomes paradigm...
Surgeon and hospital volume: Surgical outcomes paradigm
A landmark study in 1979 that showed decreased mortality in high-volume centers underscored the need for regionalization for certain surgical procedures.20 This association was further substantiated by 2 reports on 2.5 million Medicare beneficiaries that demonstrated significantly lower mortality for all 14 cardiovascular and oncologic procedures for hospitals with larger surgical volume (16% vs 4%) and high-volume surgeons for certain procedures, for example, 15% versus 5% for pancreatic resections for cancer.21,22
A similar association was found for all routes of hysterectomies performed for benign indications. Boyd and colleagues showed that gynecologists who performed fewer than 10 hysterectomies per year had a higher perioperative morbidity rate (16.5%) compared with those who did more (11.7%).23 Specific to vaginal hysterectomy, in a study of more than 6,000 women, surgeons who performed 13 procedures per year had 31% less risk of operative injury than those who did 5.5 procedures per year (2.5% vs 1.7%).24 Overall perioperative complications (5.0% vs 4.0%) and medical complications (5.7% vs 3.9%) were also reduced for higher-volume surgeons. In a cohort of approximately 8,000 women who underwent a laparoscopic hysterectomy, high-volume surgeons had a considerably lower complication rate (4.2% vs 6.2%).25
As expected, lower complication rates of high-volume surgeons led to lower resource utilization, including lower transfusion rates, less intensive care unit utilization, and shorter operative times and, in several studies, length of stay.24,25 Of note, low-volume surgeons were less likely to offer minimally invasive routes and were more likely to convert to laparotomy.26 In addition, significant cost savings have been associated with high surgical volume, which one study showed was 16% ($6,500 vs $5,600) for high-volume surgeons.26 With regard to mortality, a study of 7,800 women found that perioperative mortality increased more than 10-fold for surgeons who performed an average 1 case per year compared with all other surgeons (2.5% vs 0.2%).27
When gynecologic cancers are concerned, arguably, long-term survival outcomes may be more critical than perioperative morbidity and mortality. Higher surgeon and hospital volume are associated with improved perioperative outcomes for endometrial and cervical cancers.28 Importantly, minimally invasive hysterectomy was offered for endometrial cancer significantly more often by surgeons with high volume.28 Survival outcomes were not affected by surgeon or hospital volume, likely due to overall more favorable prognosis for endometrial cancer after treatment.
Although it is intuitive to assume that a surgeon’s skills and experience would make the most impact in procedures for ovarian cancer due to the complexity of ovarian cancer surgery, evidence on short-term outcomes has been mixed. Intriguingly, some studies reported that high-volume institutions had higher complication and readmission rates. However, evidence supports that the surgeon’s volume, and especially hospital volume, improves long-term survival for ovarian cancer, with a negative impact on immediate postoperative morbidity.29 This may suggest that a more aggressive surgical effort improves long-term survival but also can cause more perioperative complications. Further, longer survival may result not only from operative skills but also because of better care by a structured multidisciplinary team at more established high-volume cancer centers.
The association of improved outcomes with higher volume led to public reporting of hospital outcomes. Policy efforts toward regionalization have impacted surgical practice. Based on their analysis of 3.2 million Medicare patients who underwent 1 of 8 different cancer surgeries or cardiovascular operations from 1999 to 2008, Finks and colleagues demonstrated that care was concentrated to fewer hospitals over time for many of these procedures.29 This trend was noted for gynecologic cancer surgery but not for benign gynecologic surgery.
Regionalization of care limits access particularly for minority and underserved communities because of longer travel distances, logistic challenges, and financial strain. An alternative to regionalization of care is targeted quality improvement by rigorous adherence to quality guidelines at low-volume hospitals.
Is there a critical minimum volume that may be used as a requirement for surgeons to maintain their privileges and for hospitals to offer certain procedures? In 2015, minimum volume standards for a number of common procedures were proposed by Johns Hopkins Medicine and Dartmouth-Hitchcock Medical Center, such as 50 hip replacement surgeries per hospital and 25 per physician per year, and 20 pancreatectomies per hospital and 5 per surgeon per year.30 A modeling study for hysterectomy showed that a volume cut point of >1 procedure in the prior year would restrict privileges for a substantial number of surgeons performing abdominal (17.5%), robot-assisted (12.5%), laparoscopic (16.8%), and vaginal (27.6%) hysterectomies.27 This study concluded that minimum-volume standards for hysterectomy for even the lowest volume physicians would restrict a significant number of gynecologic surgeons, including many with outcomes that are better than predicted.
Therefore, while there is good evidence that favors better outcomes in the hands of high-volume surgeons in gynecology, the impact of such policies on gynecologic practice clearly warrants careful monitoring and further study.
- What factors besides the surgeon’s skills influence surgical safety and efficiency?
- Are you ready to have audio, video, and sensor-based recording of everything in the OR?
- Who should perform your loved one’s hysterectomy? Do the surgeon’s and hospital’s volume matter?
- Catchpole K, Bisantz A, Hallbeck MS, et al. Human factors in robotic assisted surgery: lessons from studies ‘in the wild’. Appl Ergon. 2019;78:270-276.
- Catchpole K, Perkins C, Bresee C, et al. Safety, efficiency and learning curves in robotic surgery: a human factors analysis. Surg Endosc. 2016;30:3749-3761.
- Jain M, Fry BT, Hess LW, et al. Barriers to efficiency in robotic surgery: the resident effect. J Surg. Res. 2016;205:296-304.
- Yu D, Dural C, Morrow MM, et al. Intraoperative workload in robotic surgery assessed by wearable motion tracking sensors and questionnaires. Surg Endosc. 2017;31:877-886.
- Randell R, Honey S, Alvarado N, et al. Embedding robotic surgery into routine practice and impacts on communication and decision making: a review of the experience of surgical teams. Cognit Technol Work. 2016;18:423-437.
- Souders CP, Catchpole KR, Wood LN, et al. Reducing operating room turnover time for robotic surgery using a motor racing pit stop model. World J Surg. 2017;4:1943–1949.
- Ahmad N, Hussein AA, Cavuoto L, et al. Ambulatory movements, team dynamics and interactions during robot-assisted surgery. BJU Int. 2016;118:132-139.
- Sexton K, Johnson A, Gotsch A, et al. Anticipation, teamwork, and cognitive load: chasing efficiency during robot-assisted surgery. BMJ Qual Saf. 2018;27:148-154.
- Harmanli O, Solak S, Bayram A, et al. Optimizing the robotic surgery team: an operations management perspective. Int Urogynecol J. 2021;32:1379-1385.
- Carter-Brooks CM, Du AL, Bonidie MJ, et al. The impact of a dedicated robotic team on robotic-assisted sacrocolpopexy outcomes. Female Pelvic Med Reconstr Surg. 2018;24:13-16.
- Giugale LE, Sears S, Lavelle ES, et al. Evaluating the impact of intraoperative surgical team handoffs on patient outcomes. Female Pelvic Med Reconstr Surg. 2017;23:288-292.
- Geynisman-Tan J, Brown O, Mueller M, et al. Operating room efficiency: examining the impact of personnel handoffs. Female Pelvic Med Reconstr Surg. 2018;24:87-89.
- Alsubaie H, Goldenberg M, Grantcharov T. Quantifying recall bias in surgical safety: a need for a modern approach to morbidity and mortality reviews. Can J Surg. 2019;62:39-43.
- Goldenberg MG, Jung J, Grantcharov TP. Using data to enhance performance and improve quality and safety in surgery. JAMA Surg. 2017;152:972-973.
- Jung JJ, Grantcharov TP. The operating room black box: a prospective observational study of the operating room. J Am Coll Surg. 2017;225:S127-S128.
- Jung JJ, Jüni P, Lebovic G, et al. First-year analysis of the operating room black box study. Ann Surg. 2020;271:122-127.
- Jung JJ, Kashfi A, Sharma S, et al. Characterization of device-related interruptions in minimally invasive surgery: need for intraoperative data and effective mitigation strategies. Surg Endosc. 2019;33:717-723.
- Jung JJ, Adams-McGavin RC, Grantcharov TP. Underreporting of Veress needle injuries: comparing direct observation and chart review methods. J Surg Res. 2019;236:266-270.
- Fesco AB, Kuzulugil SS, Babaoglu C, et al. Relationship between intraoperative nontechnical performance and technical events in bariatric surgery. Br J Surg. 2018;105:1044-1050.
- Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364-1369.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137.
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:21172127.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915.
- Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116:1341-1347.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Bretschneider CE, Frazzini Padilla P, Das D, et al. The impact of surgeon volume on perioperative adverse events in women undergoing minimally invasive hysterectomy for the large uterus. Am J Obstet Gynecol. 2018;219:490.e1-490.e8.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Wright JD. The volume-outcome paradigm for gynecologic surgery: clinical and policy implications. Clin Obstet Gynecol. 2020;63:252-265.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high risk surgery. N Engl J Med. 2011;364:21282137.
- Sternberg S. Hospitals move to limit low-volume surgeries. US News & World Report. May 19, 2015. www.usnews.com/news /articles/2015/05/19/hospitals-move-to-limit-low-volume-surgeries. Accessed April 19, 2022.
- Catchpole K, Bisantz A, Hallbeck MS, et al. Human factors in robotic assisted surgery: lessons from studies ‘in the wild’. Appl Ergon. 2019;78:270-276.
- Catchpole K, Perkins C, Bresee C, et al. Safety, efficiency and learning curves in robotic surgery: a human factors analysis. Surg Endosc. 2016;30:3749-3761.
- Jain M, Fry BT, Hess LW, et al. Barriers to efficiency in robotic surgery: the resident effect. J Surg. Res. 2016;205:296-304.
- Yu D, Dural C, Morrow MM, et al. Intraoperative workload in robotic surgery assessed by wearable motion tracking sensors and questionnaires. Surg Endosc. 2017;31:877-886.
- Randell R, Honey S, Alvarado N, et al. Embedding robotic surgery into routine practice and impacts on communication and decision making: a review of the experience of surgical teams. Cognit Technol Work. 2016;18:423-437.
- Souders CP, Catchpole KR, Wood LN, et al. Reducing operating room turnover time for robotic surgery using a motor racing pit stop model. World J Surg. 2017;4:1943–1949.
- Ahmad N, Hussein AA, Cavuoto L, et al. Ambulatory movements, team dynamics and interactions during robot-assisted surgery. BJU Int. 2016;118:132-139.
- Sexton K, Johnson A, Gotsch A, et al. Anticipation, teamwork, and cognitive load: chasing efficiency during robot-assisted surgery. BMJ Qual Saf. 2018;27:148-154.
- Harmanli O, Solak S, Bayram A, et al. Optimizing the robotic surgery team: an operations management perspective. Int Urogynecol J. 2021;32:1379-1385.
- Carter-Brooks CM, Du AL, Bonidie MJ, et al. The impact of a dedicated robotic team on robotic-assisted sacrocolpopexy outcomes. Female Pelvic Med Reconstr Surg. 2018;24:13-16.
- Giugale LE, Sears S, Lavelle ES, et al. Evaluating the impact of intraoperative surgical team handoffs on patient outcomes. Female Pelvic Med Reconstr Surg. 2017;23:288-292.
- Geynisman-Tan J, Brown O, Mueller M, et al. Operating room efficiency: examining the impact of personnel handoffs. Female Pelvic Med Reconstr Surg. 2018;24:87-89.
- Alsubaie H, Goldenberg M, Grantcharov T. Quantifying recall bias in surgical safety: a need for a modern approach to morbidity and mortality reviews. Can J Surg. 2019;62:39-43.
- Goldenberg MG, Jung J, Grantcharov TP. Using data to enhance performance and improve quality and safety in surgery. JAMA Surg. 2017;152:972-973.
- Jung JJ, Grantcharov TP. The operating room black box: a prospective observational study of the operating room. J Am Coll Surg. 2017;225:S127-S128.
- Jung JJ, Jüni P, Lebovic G, et al. First-year analysis of the operating room black box study. Ann Surg. 2020;271:122-127.
- Jung JJ, Kashfi A, Sharma S, et al. Characterization of device-related interruptions in minimally invasive surgery: need for intraoperative data and effective mitigation strategies. Surg Endosc. 2019;33:717-723.
- Jung JJ, Adams-McGavin RC, Grantcharov TP. Underreporting of Veress needle injuries: comparing direct observation and chart review methods. J Surg Res. 2019;236:266-270.
- Fesco AB, Kuzulugil SS, Babaoglu C, et al. Relationship between intraoperative nontechnical performance and technical events in bariatric surgery. Br J Surg. 2018;105:1044-1050.
- Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364-1369.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137.
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:21172127.
- Boyd LR, Novetsky AP, Curtin JP. Effect of surgical volume on route of hysterectomy and short-term morbidity. Obstet Gynecol. 2010;116:909-915.
- Rogo-Gupta LJ, Lewin SN, Kim JH, et al. The effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116:1341-1347.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Bretschneider CE, Frazzini Padilla P, Das D, et al. The impact of surgeon volume on perioperative adverse events in women undergoing minimally invasive hysterectomy for the large uterus. Am J Obstet Gynecol. 2018;219:490.e1-490.e8.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Wright JD. The volume-outcome paradigm for gynecologic surgery: clinical and policy implications. Clin Obstet Gynecol. 2020;63:252-265.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high risk surgery. N Engl J Med. 2011;364:21282137.
- Sternberg S. Hospitals move to limit low-volume surgeries. US News & World Report. May 19, 2015. www.usnews.com/news /articles/2015/05/19/hospitals-move-to-limit-low-volume-surgeries. Accessed April 19, 2022.