User login
Despite more than 2 decades of significant advances in human immunodeficiency virus (HIV) testing and treatment and major HIV‐oriented public health initiatives, the Centers for Disease Control and Prevention (CDC) reports that the incidence of new HIV cases in the United States has remained stable at about 40 000 cases annually.1 CDC estimates indicate that 252 000312 000 of the 1 039 0001 185 000 people in the United States with HIV infection do not know their serostatus,2 and it appears that these unaware individuals may play a significant role in HIV transmission to others.3, 4 In an effort to promote testing for HIV, the CDC initiated a program called Advancing HIV Prevention: New Strategies for a Changing Epidemic in 2003.1 This program recommends incorporating HIV testing into routine medical care.
A decade before Advancing HIV Prevention was published, the CDC directly addressed the issue of HIV testing of hospitalized patients by recommending that hospitals with an HIV seroprevalence rate of at least 1% or an AIDS diagnosis rate 1.0 per 1000 discharges should strongly consider adopting a policy of offering HIV counseling and testing routinely to patients ages 1554 years.5 Despite the information on discharge diagnosis rates often being easily available from hospital databases, even if seroprevalence rates may not, routine HIV testing of hospitalized patients has not occurred.
In 2005 the United States Preventive Services Taskforce (USPSTF) recommendations stated that there was fair evidence that screening adolescents and adults not known to be at increased risk for HIV can detect additional individuals with HIV.6 Their statement reflects data from Chen et al., who identified that self‐reported risk factordirected testing strategies would have missed nearly three quarters of the HIV infections in their clinic setting,7 and from Peterman et al., who demonstrated that 2026% of HIV‐positive patients acknowledged no HIV‐associated risk factors.8
Despite the prior CDC recommendations,1, 5 Chen and Peterman's data,7, 8 and acknowledgment of the high accuracy of the new HIV antibody tests, making false‐positive test results quite rare, the published recommendations of the USPSTF do not support routinely testing individuals who are not at increased risk for acquiring the infection because of the relatively low yield and concern about anxiety and related consequences of HIV testing.
Hospitalists are poised to offer inpatient HIV testing to all inpatients at hospitals that meet the CDC guidelines in an effort to reduce the numbers of patients who have undiagnosed HIV infection. This article examines inpatient HIV testing including barriers that may exist to routine testing and reviews the available rapid HIV tests, which may assist in overcoming some of these barriers.
HIV Testing in the Hospital
Patients diagnosed with HIV infection often have had multiple contacts with the medical community, both inpatient and outpatient, prior to their HIV diagnosis, during which HIV testing had not been offered, thus delaying diagnosis.9 Though clinicians often identify and document triggers that should prompt HIV testing, patients with HIV infection are still not diagnosed in a timely manner. In addition, according to previously published data on inpatient testing from urban institutions, the targeted testing of patients based on traditional risk factors also misses a large proportion of HIV‐infected patients.10 Thus, routine nontargeted inpatient testing, as the CDC suggests, is the preferred strategy.
More than a quarter of patients with HIV in the United States are diagnosed in hospital settings, often in conjunction with an illness that prompts specific testing.11 An important recent study by Brady evaluated the HIV seroprevalence on the medicine and trauma medicine services of 2 hospitals during 2 seasons. The study was blinded and used leftover blood samples taken for other reasons. It found seroprevalence rates varying between 1.4% and 3.7%.12 Two points are noteworthy about this study. First, having excluded those from patients with known HIV disease, a significant proportion of the samples identified as seropositive likely represented unidentified HIV cases. Second, although the seroprevalence varied depending on the season during which testing was done and the service from which blood was obtained, even the lower percentage (1.4%) is higher than the CDC's threshold for offering routine HIV testing.5
With the average length of a hospital stay declining to less than 5 days,13 many patients who undergo nonrapid HIV testing while hospitalized will not receive their results prior to discharge. Though no data specifying the rates of HIV test result follow‐up after hospital discharge have been published, the experience in the outpatient setting suggests a significant number of patients never receive their test results. The CDC estimates that 31% of patients who tested positive for HIV did not return to receive their test results.14 State‐funded, community‐based programs also have highly variable rates of return, with published reports of 2548% of patients never receiving their results.1517 Fortunately, new and highly accurate rapid HIV tests are now available in the United States, almost eliminating the problem of loss to follow‐up18 (see Rapid HIV Antibody Tests, below).
Barriers to Implementing HIV Testing
There are numerous potential barriers to instituting broad‐based screening of hospitalized patients for HIV in addition to the follow‐up issues with standard HIV tests illustrated above. These include the cost and cost effectiveness of the program; the logistics of test performance and counseling on the ward; the risk of offending patients; and the culture changes required of inpatient caregivers and hospital administrators. Each of these is addressed briefly.
Cost
Two cost effectiveness analyses examining routine HIV testing have been published recently. The first, by Sanders,20 assumed a 1% seroprevalence of undiagnosed HIV infection in accordance with CDC recommendations5 and found a one‐time testing cost of $15 078 (2004 dollars) per quality‐adjusted life‐year (QALY) including the benefit accrued to sexual partners of the tested patient. This cost/QALY rose to nearly $40 000/QALY with a seroprevalence of only 0.1%. The second study, by Paltiel,21 demonstrated that the cost/QALY of one‐time testing of patients with a 1% seroprevalence to be $38 000.
A few points must be noted about these studies. First, they are not based on inpatient testing specifically. Nonetheless, the Brady study, above,12 as well as our own experience with routine inpatient testing (unpublished data), suggests that the prevalence may be similar in many inpatient populations. Second, the cost/QALY is very consistent with other routine screening efforts broadly accepted.22 Finally, although both analyses cited moderately to significantly higher costs/QALY for recurrent (eg, every 35 years) routine testing, the relevance of this to routine inpatient testing is less clear.
Another study compared hospitalized patients newly testing HIV positive with a rapid HIV test kit, performed in an emergency department, with those testing HIV positive with conventional HIV tests performed on an inpatient unit.23 Though it was not designed as a cost analysis, the length of stay of the group that received the rapid test was 7 days shorter than that of the group that received the conventional test (6 vs. 13 days; P < .001), with type of HIV testing used identified as an independent effect on length of stay in multivariate regression analysis.
Despite what these analyses reported, start‐up costs for HIV testing services can be substantial, and, at present, insurance reimbursement for HIV counseling does not exist. If physicians offer HIV counseling, they may bill for their time as an extended service, when appropriate. Laboratory fees can be billed, which may help to cover materials and processing costs. Grants through the CDC or the Department of Public Health may be available to support programs that operationalize routine HIV testing.
Logistics of Routine Testing on the Ward
An inpatient unit is a difficult place to do HIV counseling. Issues of patient privacy are substantial, especially in shared rooms or when family or friends are present. Physicians and counselors must be cognizant of these issues and be flexible in the timing and structure of the counseling offered to maximize patient comfort and minimize interruptions. Educating inpatient staff about HIV counseling may help to avoid embarrassing situations and interruptions.
In addition, the time required to do HIV testing properly could significantly slow a busy physician's work flow if offered to every patient. Dedicated HIV counseling and testing staff members can be of great assistance in the process and can remove the time barrier from the physician by performing the tests themselves. Such staff members require training in HIV testing procedures if they are to perform point‐of‐care tests at the bedside. This type of program, coordinated with the leadership of the inpatient service, is ideal for providing routine screening of all admissions as recommended by the CDC.5 In addition, considerations about minimizing or eliminating pretest counseling are ongoing, with counseling only offered during the posttest phase.1, 24 This plan would also reduce the impact of this process on work flow.
An advantage of using an inpatient service as a site for HIV testing is the ability to mobilize a hospital's resources should a patient be diagnosed as HIV positive. Addressing the medical, psychological, and psychosocial needs of newly diagnosed (or previously diagnosed but medically disconnected) patient requires using a multidisciplinary team approach, including inpatient caregivers, social workers, case managers, mental health providers, and HIV specialists.
Avoiding Offending Patients and Changing Hospital Culture
An inpatient unit is an unusual place for routine screening, which usually is relegated to the ambulatory setting. Moreover, with the stigma of HIV still present, despite efforts to quell it,25 inpatient caregivers and hospital administrators may be uncomfortable in approaching or having a trained counselor approach all patients on an inpatient service to discuss HIV counseling and testing.
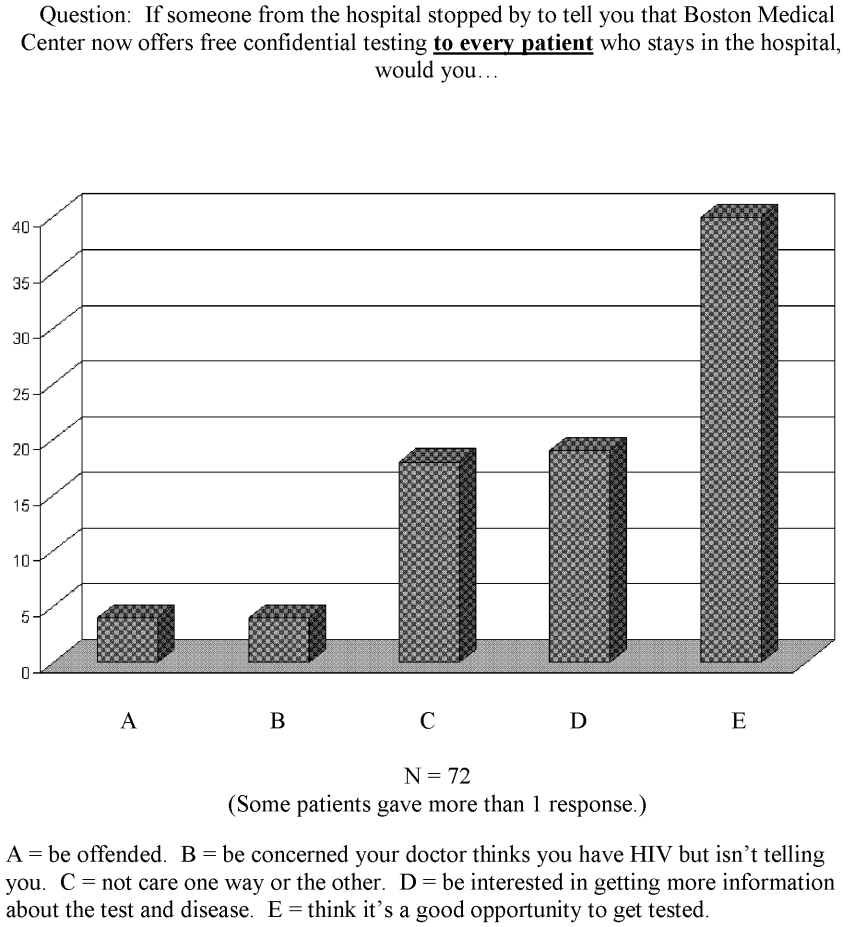
No studies have been published on inpatient attitudes toward routinely being offered HIV testing. Our HIV testing service faced this question when we wanted to expand our inpatient testing from risk‐factor‐directed and physician‐referral‐based testing to routine testing. To assess patient responses, we asked 72 medical inpatients how they would feel about an unsolicited offer to be tested for HIV while they were inpatients. The results, displayed in Figure 1, demonstrated that only 11% of the patients had an unfavorable response. Of note, the study did not permit further explanations to be given to dispel the concerns of those whose response was unfavorable. With this information, our administration permitted expanded testing to commence.
From the experiences of our testing program, with several thousand patients having been approached, we have found that patients are very rarely offended or upset by being offered HIV testing.
Rapid HIV Antibody Tests in the United States
As noted, a substantial proportion of patients fail to return to obtain results.1517 As with other posthospitalization test follow‐ups,26 significant complications may occur if follow‐up of HIV test results is inadequate. Rapid HIV antibody tests may offer programs a way to ensure that the vast majority of patients learn their test results.
There are currently 4 rapid HIV tests that have been approved for use in the United States by the Food and Drug Administration (FDA). Two of these, the OraQuick ADVANCE Rapid HIV‐1/2 Antibody Test (OraSure Technologies, Inc., Bethlehem, PA)27 and the Uni‐Gold Recombigen HIV Test (Trinity Biotech, Bray, County Wicklow, Ireland),28 have received a waiver from the Clinical Laboratories Improvement Amendment (CLIA), which means they may be used outside a laboratory setting.29 Such a waiver means these tests may be used at the bedside of a patient in a point‐of‐care (POC) fashion similar to that of blood sugar monitoring.
It must be noted, however, that extensive quality assurance and quality control are involved with the use of these POC tests.30 Despite the CLIA waiver, a relationship with the hospital laboratory is required, as the test kits may only be used by an agent of the laboratory. An agent is an individual who the laboratory deems capable and qualified to perform the test competently.
Two additional rapid HIV tests are FDA approved but not CLIA waived. These tests, the Reveal G2 Rapid HIV‐1 Antibody Test (MedMira, Bayers Lake Park, Halifax, Nova Scotia)31 and the Multispot HIV‐1/HIV‐2 Rapid Test (Bio‐Rad Laboratories, Redmond, Washington),32 must be performed in a laboratory (see Table 1).
| Rapid HIV Test | Specimen Type | Sensitivity (95% CI) | Specificity (95% CI) | CLIA Category | Cost |
|---|---|---|---|---|---|
| |||||
| OraQuick Advance Rapid HIV1/2 Antibody Test | Oral fluid | 99.3% (98.499.7) | 99.8% (99.699.9) | Waived | $17.50 |
| Whole blood (finger stick or venipuncture) | 99.6% (98.599.9) | 100% (99.7100) | Waived | ||
| Plasma | 99.6% (98.999.8) | 99.9% (99.699.9) | Moderate complexity | ||
| Reveal G‐2 Rapid HIV‐1 Antibody Test | Serum | 99.8% (99.5100) | 99.1% (98.899.4) | Moderate complexity | $14.50 |
| Plasma | 99.8% (99.5100) | 98.6% (98.498.8) | Moderate complexity | ||
| Uni‐Gold Recombigen HIV Test | Whole blood (finger stick or venipuncture) | 100% (99.5100) | 99.7% (99.0100) | Waived | $15.75 |
| Serum and plasma | 100% (99.5100) | 99.8% (99.3100) | Moderate complexity | ||
| Multispot HIV‐1/HIV‐2 Rapid Test | Serum | 100% (99.94100) | 99.93% (99.79100) | Moderate complexity | $25.00 |
| Plasma | 100% (99.94100) | 99.91% (99.77100) | Moderate complexity | ||
All 4 tests have sensitivities and specificities similar to those of commercially available standard HIV enzyme immunosorbent assays (EIA) for HIV. As the tests are extremely sensitive, no confirmatory testing is required for nonreactive rapid test results. These tests should be considered negative. False negatives may occur if the patient has had a recent HIV exposure. Thus, as with standard EIA tests, it is important to recommend retesting in 6 weeks for all patients who test HIV negative but who have had a high‐risk exposure in the last 3 months. Also, very rarely, patients receiving antiretroviral therapy who have successfully suppressed their viral replication below detectable limits for long periods may also have false‐negative results. Therefore, with all patients, it is important to reinforce the idea that it is not appropriate to retest for HIV if a patient already knows he or she is HIV positive.
All reactive rapid HIV tests require confirmation. This process is most commonly done with a Western Blot assay and must be completed before a patient is told that he or she has confirmed HIV infection. Although uncommon, false‐positive rapid tests do occur, reinforcing the need for confirmatory testing before a formal diagnosis of HIV infection can be made. Currently, no FDA‐approved rapid confirmatory HIV test is available, so standard laboratory delays may be unavoidable for these patients. It is therefore critical that hospitals providing rapid HIV testing have access to medical and social support systems that may be rapidly mobilized for patients with reactive and confirmed positive tests.
Hospitalists at the Helm of Routine Inpatient HIV Testing
Putting a hospitalist in charge of implementing inpatient HIV testing has several advantages. First, as experts in the hospital systems in which they work, hospitalists are prime candidates to organize a multidisciplinary team involving those from nursing, laboratory medicine, mental health, and social work, as well as HIV specialists. If dedicated HIV counselors are available to participate, they, too, should be included. A hospitalist with an interest in HIV makes an ideal director of such a multidisciplinary program.
Second, hospitalists are on the front line of clinical care and see patients during the earliest hours of their clinical evaluation. By making HIV testing a routine part of all admissions, the hospitalist may act as a role model in the process and will also be able to explain to patients that they are not being singled out, as all patients are encouraged to undergo testing.
Finally, with the demonstrated added value of hospitalist programs33 and the recent literature demonstrating the cost effectiveness of routine HIV testing,20, 21 hospitalists are well suited to demonstrate leadership in the acquisition of the resources required to make routine inpatient HIV testing possible.
Future Directions
To make routine testing a broadly accepted reality, several developments must begin to take place. These include: increasing education about HIV disease as a chronic disease rather than a rapidly terminal illness;34 reducing the stigma of HIV disease (a stigma that has impaired testing rates),25 which should include discussions of eliminating the need for separate HIV test consent forms, not required for testing for other sexually transmitted diseases (eg, syphilis) or life‐threatening diseases (eg, hepatitis C);1 examining the experience and impact of the universal HIV testing recommendations for pregnant women;35, 36 reducing1, 24 or entirely eliminating37 the requirements for extensive pretest counselingwhich may be a low‐yield38 time barrierwith a greater focus on case‐specific post‐test risk reduction;1 and broadening the realization that targeted testing based on traditional HIV risk factors fails to identify a significant number of HIV cases.10, 39
CONCLUSIONS
Though it has been more than a decade since the original CDC recommendations on inpatient HIV testing were released,5 it remains quite clear that routine inpatient HIV testing can and should be a reality in many hospitals in the United States. As the literature12 and our institution's experience suggest, those in an inpatient service may be a population with a higher prevalence of HIV disease, and as such, an inpatient service should be a venue where routine HIV testing is offered. The U.S. Preventive Services Taskforce's conclusion that the benefit of screening adolescents and adults without risk factors for HIV is too small relative to potential harms to justify a general recommendation6 may not apply to the inpatient services where HIV disease may be more common than in the general population. However, because of time constraints, busy clinicians may require the assistance of an HIV counseling and testing service to make this kind of program a reality.
Clearly, using targeted testing strategies based on traditional HIV risk factors fails to identify a significant proportion of undiagnosed HIV cases.7, 8 New, FDA‐approved rapid HIV antibody tests can help to reduce the issue of loss to follow‐up as a barrier to having successful testing programs, and the cost effectiveness of such HIV testing programs has been suggested in recent literature. Although studies are needed to elucidate the differences between routinely tested inpatients and those tested in more traditional ambulatory sites, hospitalists have the opportunity to take the lead in dramatically increasing testing and in substantially decreasing the number of patients unaware of their HIV status.
- Centers for Disease Control and Prevention.Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003.MMWR Morb Mortal Wkly Rep.2003;52:329–332.
- ,.Estimated HIV prevalence in the United States at the end of 2003. 2005 National HIV Prevention Conference; June 12–15,2005; Atlanta, Ga. Abstract T1–B110.
- ,,, et al.Understanding delay to medical care for HIV infection: the long‐term non‐presenter.AIDS2001;15:77–85.
- ,,, et al.HIV prevalence and associated risks in young men who have sex with men. Young Men's Survey Study Group.JAMA.2000;284:198–204.
- Centers for Disease Control and Prevention.Recommendations for HIV testing services for inpatients and outpatients in acute‐care hospital settings.MMWR Recomm Rep.1993;42(RR‐2):1–6.
- US Preventive Services Taskforce.Screening for HIV: recommendation statement.Ann Intern Med.2005;143(1):32–37.
- ,,,.Risk assessment to improve targeting of HIV counseling and testing services for STD clinic patients.Sex Transm Dis.1998;25:539–543.
- ,,.Opportunities of targeting publicly funded human immunodeficiency virus counseling and testing.J Acquir Immune Defic Syndr Hum Retrovirol.1996;12:69–74.
- ,,,,,.Assessing missed opportunities for HIV testing in medical settings.J Gen Intern Med.2004;19:349–356.
- ,,,.Identifying undiagnosed human immunodeficiency virus: the yield of routine, voluntary, inpatient testing.Arch Intern Med.2002;162:887–892.
- .Learning more about the HIV‐infected population not in care in the US. Poster TuPeG 5690, presented at: XIV International AIDS Conference; July2002; Barcelona, Spain.
- ,,, et al.Seasonal variation in undiagnosed HIV infection on the general medicine and trauma services of two urban hospitals.JGIM.2005;20:324–330.
- ,.2001 National Hospital Discharge Survey. Advance data from vital and health statistics; no 332.Hyattsville, Md:National Center for Health Statistics;2003.
- HIV counseling and testing in publicly funded sites. Annual report, 1997 and 1998.Centers for Disease Control and Prevention [CDC Web site]. Available at: http://www.cdc.gov/hiv/pubs/cts98.pdf. Accessed February 17,2005.
- ,.Rapid hiv testing in urban outreach: a strategy for improving posttest counseling rates.AIDS Educ Prev. Dec2001;13(6):541–550.
- Update: HIV counseling and testing using rapid tests—United States, 1995.MMWR Morb Mortal Wkly Rep.1998;47:211–215.
- ,,, et al.HIV testing among young adults and older adolescents in the setting of acute substance abuse treatment.J Acquir Immune Defic Syndr.2001;27:135–142.
- ,.Rapid HIV testing in the era of OraQuick®.Todays Ther Trends.2003;21:307–344.
- ,,,.A rapid review of rapid HIV antibody tests.Curr Inf Dis Repts.2006;8:125–131.
- ,,, et al.Cost‐effectiveness of screening for HIV in the era of highly active antiretroviral therapy.New Eng J Med.2005;352:570–585.
- ,,, et al.Expanded screening for HIV in the United States—an analysis of cost effectiveness.New Eng J Med.2005;352:586–595.
- Harvard Center for Risk Analysis: The CEA Registry. Cost‐utility analyses published from 1976 to 2001, with ratios converted to 2002 US dollars. Available at: http://www.hsph.harvard.edu/cearegistry/data/1976‐2001_CEratios_comprehensive_4‐7‐2004.pdf. Accessed August 15,2005.
- ,,, et al.The role of rapid vs conventional human immunodeficiency virus testing for inpatients: effects on quality of care.Arch Intern Med.2005;165:1956 The role of rapid vs. conventional Human Immunodeficiency Virus testing for inpatients 1960.
- CDC.Revised guidelines for HIV counseling, testing, and referral.MMWR Recomm Rep.2001;50(RR19);1–58.
- Health Resources and Services Administration. Stigma and HIV/AIDS: a review of the literature. Available at: http://hab.hrsa.gov/publications/stigma/introduction.htm. Accessed August 15,2005.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- Orasure Technologies, Inc. Bethlehem, Pa. OraQuick Advance rapid HIV 1/2 rapid antibody test [package insert]. Available at: http://www.orasure.com/uploaded/398.pdf?1389(suppl 1).
- ,.AIDS as a chronic illness: psychosocial implications.AIDS.2002;16(suppl 4):S69–S76.
- ,,,,.Prenatal screening for HIV: a review of the evidence for the U.S. Preventive Services Taskforce.Ann Intern Med2005;143:38–54.
- CDC.Revised recommendations for HIV screening of pregnant women.MMWR Recomm Rep.2001;50(RR19):59–86.
- ,.HIV testing should no longer be accorded any special status.BMJ.2005;330:492–493.
- The EXPLORE Study Team.Effects of a behavioral intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomized controlled study.Lancet.2004;364:41–50.
- ,.Think HIV. Why physicians should lower their threshold for HIV testing.Arch Intern Med.1999;159:1994–2000.
Despite more than 2 decades of significant advances in human immunodeficiency virus (HIV) testing and treatment and major HIV‐oriented public health initiatives, the Centers for Disease Control and Prevention (CDC) reports that the incidence of new HIV cases in the United States has remained stable at about 40 000 cases annually.1 CDC estimates indicate that 252 000312 000 of the 1 039 0001 185 000 people in the United States with HIV infection do not know their serostatus,2 and it appears that these unaware individuals may play a significant role in HIV transmission to others.3, 4 In an effort to promote testing for HIV, the CDC initiated a program called Advancing HIV Prevention: New Strategies for a Changing Epidemic in 2003.1 This program recommends incorporating HIV testing into routine medical care.
A decade before Advancing HIV Prevention was published, the CDC directly addressed the issue of HIV testing of hospitalized patients by recommending that hospitals with an HIV seroprevalence rate of at least 1% or an AIDS diagnosis rate 1.0 per 1000 discharges should strongly consider adopting a policy of offering HIV counseling and testing routinely to patients ages 1554 years.5 Despite the information on discharge diagnosis rates often being easily available from hospital databases, even if seroprevalence rates may not, routine HIV testing of hospitalized patients has not occurred.
In 2005 the United States Preventive Services Taskforce (USPSTF) recommendations stated that there was fair evidence that screening adolescents and adults not known to be at increased risk for HIV can detect additional individuals with HIV.6 Their statement reflects data from Chen et al., who identified that self‐reported risk factordirected testing strategies would have missed nearly three quarters of the HIV infections in their clinic setting,7 and from Peterman et al., who demonstrated that 2026% of HIV‐positive patients acknowledged no HIV‐associated risk factors.8
Despite the prior CDC recommendations,1, 5 Chen and Peterman's data,7, 8 and acknowledgment of the high accuracy of the new HIV antibody tests, making false‐positive test results quite rare, the published recommendations of the USPSTF do not support routinely testing individuals who are not at increased risk for acquiring the infection because of the relatively low yield and concern about anxiety and related consequences of HIV testing.
Hospitalists are poised to offer inpatient HIV testing to all inpatients at hospitals that meet the CDC guidelines in an effort to reduce the numbers of patients who have undiagnosed HIV infection. This article examines inpatient HIV testing including barriers that may exist to routine testing and reviews the available rapid HIV tests, which may assist in overcoming some of these barriers.
HIV Testing in the Hospital
Patients diagnosed with HIV infection often have had multiple contacts with the medical community, both inpatient and outpatient, prior to their HIV diagnosis, during which HIV testing had not been offered, thus delaying diagnosis.9 Though clinicians often identify and document triggers that should prompt HIV testing, patients with HIV infection are still not diagnosed in a timely manner. In addition, according to previously published data on inpatient testing from urban institutions, the targeted testing of patients based on traditional risk factors also misses a large proportion of HIV‐infected patients.10 Thus, routine nontargeted inpatient testing, as the CDC suggests, is the preferred strategy.
More than a quarter of patients with HIV in the United States are diagnosed in hospital settings, often in conjunction with an illness that prompts specific testing.11 An important recent study by Brady evaluated the HIV seroprevalence on the medicine and trauma medicine services of 2 hospitals during 2 seasons. The study was blinded and used leftover blood samples taken for other reasons. It found seroprevalence rates varying between 1.4% and 3.7%.12 Two points are noteworthy about this study. First, having excluded those from patients with known HIV disease, a significant proportion of the samples identified as seropositive likely represented unidentified HIV cases. Second, although the seroprevalence varied depending on the season during which testing was done and the service from which blood was obtained, even the lower percentage (1.4%) is higher than the CDC's threshold for offering routine HIV testing.5
With the average length of a hospital stay declining to less than 5 days,13 many patients who undergo nonrapid HIV testing while hospitalized will not receive their results prior to discharge. Though no data specifying the rates of HIV test result follow‐up after hospital discharge have been published, the experience in the outpatient setting suggests a significant number of patients never receive their test results. The CDC estimates that 31% of patients who tested positive for HIV did not return to receive their test results.14 State‐funded, community‐based programs also have highly variable rates of return, with published reports of 2548% of patients never receiving their results.1517 Fortunately, new and highly accurate rapid HIV tests are now available in the United States, almost eliminating the problem of loss to follow‐up18 (see Rapid HIV Antibody Tests, below).
Barriers to Implementing HIV Testing
There are numerous potential barriers to instituting broad‐based screening of hospitalized patients for HIV in addition to the follow‐up issues with standard HIV tests illustrated above. These include the cost and cost effectiveness of the program; the logistics of test performance and counseling on the ward; the risk of offending patients; and the culture changes required of inpatient caregivers and hospital administrators. Each of these is addressed briefly.
Cost
Two cost effectiveness analyses examining routine HIV testing have been published recently. The first, by Sanders,20 assumed a 1% seroprevalence of undiagnosed HIV infection in accordance with CDC recommendations5 and found a one‐time testing cost of $15 078 (2004 dollars) per quality‐adjusted life‐year (QALY) including the benefit accrued to sexual partners of the tested patient. This cost/QALY rose to nearly $40 000/QALY with a seroprevalence of only 0.1%. The second study, by Paltiel,21 demonstrated that the cost/QALY of one‐time testing of patients with a 1% seroprevalence to be $38 000.
A few points must be noted about these studies. First, they are not based on inpatient testing specifically. Nonetheless, the Brady study, above,12 as well as our own experience with routine inpatient testing (unpublished data), suggests that the prevalence may be similar in many inpatient populations. Second, the cost/QALY is very consistent with other routine screening efforts broadly accepted.22 Finally, although both analyses cited moderately to significantly higher costs/QALY for recurrent (eg, every 35 years) routine testing, the relevance of this to routine inpatient testing is less clear.
Another study compared hospitalized patients newly testing HIV positive with a rapid HIV test kit, performed in an emergency department, with those testing HIV positive with conventional HIV tests performed on an inpatient unit.23 Though it was not designed as a cost analysis, the length of stay of the group that received the rapid test was 7 days shorter than that of the group that received the conventional test (6 vs. 13 days; P < .001), with type of HIV testing used identified as an independent effect on length of stay in multivariate regression analysis.
Despite what these analyses reported, start‐up costs for HIV testing services can be substantial, and, at present, insurance reimbursement for HIV counseling does not exist. If physicians offer HIV counseling, they may bill for their time as an extended service, when appropriate. Laboratory fees can be billed, which may help to cover materials and processing costs. Grants through the CDC or the Department of Public Health may be available to support programs that operationalize routine HIV testing.
Logistics of Routine Testing on the Ward
An inpatient unit is a difficult place to do HIV counseling. Issues of patient privacy are substantial, especially in shared rooms or when family or friends are present. Physicians and counselors must be cognizant of these issues and be flexible in the timing and structure of the counseling offered to maximize patient comfort and minimize interruptions. Educating inpatient staff about HIV counseling may help to avoid embarrassing situations and interruptions.
In addition, the time required to do HIV testing properly could significantly slow a busy physician's work flow if offered to every patient. Dedicated HIV counseling and testing staff members can be of great assistance in the process and can remove the time barrier from the physician by performing the tests themselves. Such staff members require training in HIV testing procedures if they are to perform point‐of‐care tests at the bedside. This type of program, coordinated with the leadership of the inpatient service, is ideal for providing routine screening of all admissions as recommended by the CDC.5 In addition, considerations about minimizing or eliminating pretest counseling are ongoing, with counseling only offered during the posttest phase.1, 24 This plan would also reduce the impact of this process on work flow.
An advantage of using an inpatient service as a site for HIV testing is the ability to mobilize a hospital's resources should a patient be diagnosed as HIV positive. Addressing the medical, psychological, and psychosocial needs of newly diagnosed (or previously diagnosed but medically disconnected) patient requires using a multidisciplinary team approach, including inpatient caregivers, social workers, case managers, mental health providers, and HIV specialists.
Avoiding Offending Patients and Changing Hospital Culture
An inpatient unit is an unusual place for routine screening, which usually is relegated to the ambulatory setting. Moreover, with the stigma of HIV still present, despite efforts to quell it,25 inpatient caregivers and hospital administrators may be uncomfortable in approaching or having a trained counselor approach all patients on an inpatient service to discuss HIV counseling and testing.
No studies have been published on inpatient attitudes toward routinely being offered HIV testing. Our HIV testing service faced this question when we wanted to expand our inpatient testing from risk‐factor‐directed and physician‐referral‐based testing to routine testing. To assess patient responses, we asked 72 medical inpatients how they would feel about an unsolicited offer to be tested for HIV while they were inpatients. The results, displayed in Figure 1, demonstrated that only 11% of the patients had an unfavorable response. Of note, the study did not permit further explanations to be given to dispel the concerns of those whose response was unfavorable. With this information, our administration permitted expanded testing to commence.

From the experiences of our testing program, with several thousand patients having been approached, we have found that patients are very rarely offended or upset by being offered HIV testing.
Rapid HIV Antibody Tests in the United States
As noted, a substantial proportion of patients fail to return to obtain results.1517 As with other posthospitalization test follow‐ups,26 significant complications may occur if follow‐up of HIV test results is inadequate. Rapid HIV antibody tests may offer programs a way to ensure that the vast majority of patients learn their test results.
There are currently 4 rapid HIV tests that have been approved for use in the United States by the Food and Drug Administration (FDA). Two of these, the OraQuick ADVANCE Rapid HIV‐1/2 Antibody Test (OraSure Technologies, Inc., Bethlehem, PA)27 and the Uni‐Gold Recombigen HIV Test (Trinity Biotech, Bray, County Wicklow, Ireland),28 have received a waiver from the Clinical Laboratories Improvement Amendment (CLIA), which means they may be used outside a laboratory setting.29 Such a waiver means these tests may be used at the bedside of a patient in a point‐of‐care (POC) fashion similar to that of blood sugar monitoring.
It must be noted, however, that extensive quality assurance and quality control are involved with the use of these POC tests.30 Despite the CLIA waiver, a relationship with the hospital laboratory is required, as the test kits may only be used by an agent of the laboratory. An agent is an individual who the laboratory deems capable and qualified to perform the test competently.
Two additional rapid HIV tests are FDA approved but not CLIA waived. These tests, the Reveal G2 Rapid HIV‐1 Antibody Test (MedMira, Bayers Lake Park, Halifax, Nova Scotia)31 and the Multispot HIV‐1/HIV‐2 Rapid Test (Bio‐Rad Laboratories, Redmond, Washington),32 must be performed in a laboratory (see Table 1).
| Rapid HIV Test | Specimen Type | Sensitivity (95% CI) | Specificity (95% CI) | CLIA Category | Cost |
|---|---|---|---|---|---|
| |||||
| OraQuick Advance Rapid HIV1/2 Antibody Test | Oral fluid | 99.3% (98.499.7) | 99.8% (99.699.9) | Waived | $17.50 |
| Whole blood (finger stick or venipuncture) | 99.6% (98.599.9) | 100% (99.7100) | Waived | ||
| Plasma | 99.6% (98.999.8) | 99.9% (99.699.9) | Moderate complexity | ||
| Reveal G‐2 Rapid HIV‐1 Antibody Test | Serum | 99.8% (99.5100) | 99.1% (98.899.4) | Moderate complexity | $14.50 |
| Plasma | 99.8% (99.5100) | 98.6% (98.498.8) | Moderate complexity | ||
| Uni‐Gold Recombigen HIV Test | Whole blood (finger stick or venipuncture) | 100% (99.5100) | 99.7% (99.0100) | Waived | $15.75 |
| Serum and plasma | 100% (99.5100) | 99.8% (99.3100) | Moderate complexity | ||
| Multispot HIV‐1/HIV‐2 Rapid Test | Serum | 100% (99.94100) | 99.93% (99.79100) | Moderate complexity | $25.00 |
| Plasma | 100% (99.94100) | 99.91% (99.77100) | Moderate complexity | ||
All 4 tests have sensitivities and specificities similar to those of commercially available standard HIV enzyme immunosorbent assays (EIA) for HIV. As the tests are extremely sensitive, no confirmatory testing is required for nonreactive rapid test results. These tests should be considered negative. False negatives may occur if the patient has had a recent HIV exposure. Thus, as with standard EIA tests, it is important to recommend retesting in 6 weeks for all patients who test HIV negative but who have had a high‐risk exposure in the last 3 months. Also, very rarely, patients receiving antiretroviral therapy who have successfully suppressed their viral replication below detectable limits for long periods may also have false‐negative results. Therefore, with all patients, it is important to reinforce the idea that it is not appropriate to retest for HIV if a patient already knows he or she is HIV positive.
All reactive rapid HIV tests require confirmation. This process is most commonly done with a Western Blot assay and must be completed before a patient is told that he or she has confirmed HIV infection. Although uncommon, false‐positive rapid tests do occur, reinforcing the need for confirmatory testing before a formal diagnosis of HIV infection can be made. Currently, no FDA‐approved rapid confirmatory HIV test is available, so standard laboratory delays may be unavoidable for these patients. It is therefore critical that hospitals providing rapid HIV testing have access to medical and social support systems that may be rapidly mobilized for patients with reactive and confirmed positive tests.
Hospitalists at the Helm of Routine Inpatient HIV Testing
Putting a hospitalist in charge of implementing inpatient HIV testing has several advantages. First, as experts in the hospital systems in which they work, hospitalists are prime candidates to organize a multidisciplinary team involving those from nursing, laboratory medicine, mental health, and social work, as well as HIV specialists. If dedicated HIV counselors are available to participate, they, too, should be included. A hospitalist with an interest in HIV makes an ideal director of such a multidisciplinary program.
Second, hospitalists are on the front line of clinical care and see patients during the earliest hours of their clinical evaluation. By making HIV testing a routine part of all admissions, the hospitalist may act as a role model in the process and will also be able to explain to patients that they are not being singled out, as all patients are encouraged to undergo testing.
Finally, with the demonstrated added value of hospitalist programs33 and the recent literature demonstrating the cost effectiveness of routine HIV testing,20, 21 hospitalists are well suited to demonstrate leadership in the acquisition of the resources required to make routine inpatient HIV testing possible.
Future Directions
To make routine testing a broadly accepted reality, several developments must begin to take place. These include: increasing education about HIV disease as a chronic disease rather than a rapidly terminal illness;34 reducing the stigma of HIV disease (a stigma that has impaired testing rates),25 which should include discussions of eliminating the need for separate HIV test consent forms, not required for testing for other sexually transmitted diseases (eg, syphilis) or life‐threatening diseases (eg, hepatitis C);1 examining the experience and impact of the universal HIV testing recommendations for pregnant women;35, 36 reducing1, 24 or entirely eliminating37 the requirements for extensive pretest counselingwhich may be a low‐yield38 time barrierwith a greater focus on case‐specific post‐test risk reduction;1 and broadening the realization that targeted testing based on traditional HIV risk factors fails to identify a significant number of HIV cases.10, 39
CONCLUSIONS
Though it has been more than a decade since the original CDC recommendations on inpatient HIV testing were released,5 it remains quite clear that routine inpatient HIV testing can and should be a reality in many hospitals in the United States. As the literature12 and our institution's experience suggest, those in an inpatient service may be a population with a higher prevalence of HIV disease, and as such, an inpatient service should be a venue where routine HIV testing is offered. The U.S. Preventive Services Taskforce's conclusion that the benefit of screening adolescents and adults without risk factors for HIV is too small relative to potential harms to justify a general recommendation6 may not apply to the inpatient services where HIV disease may be more common than in the general population. However, because of time constraints, busy clinicians may require the assistance of an HIV counseling and testing service to make this kind of program a reality.
Clearly, using targeted testing strategies based on traditional HIV risk factors fails to identify a significant proportion of undiagnosed HIV cases.7, 8 New, FDA‐approved rapid HIV antibody tests can help to reduce the issue of loss to follow‐up as a barrier to having successful testing programs, and the cost effectiveness of such HIV testing programs has been suggested in recent literature. Although studies are needed to elucidate the differences between routinely tested inpatients and those tested in more traditional ambulatory sites, hospitalists have the opportunity to take the lead in dramatically increasing testing and in substantially decreasing the number of patients unaware of their HIV status.
Despite more than 2 decades of significant advances in human immunodeficiency virus (HIV) testing and treatment and major HIV‐oriented public health initiatives, the Centers for Disease Control and Prevention (CDC) reports that the incidence of new HIV cases in the United States has remained stable at about 40 000 cases annually.1 CDC estimates indicate that 252 000312 000 of the 1 039 0001 185 000 people in the United States with HIV infection do not know their serostatus,2 and it appears that these unaware individuals may play a significant role in HIV transmission to others.3, 4 In an effort to promote testing for HIV, the CDC initiated a program called Advancing HIV Prevention: New Strategies for a Changing Epidemic in 2003.1 This program recommends incorporating HIV testing into routine medical care.
A decade before Advancing HIV Prevention was published, the CDC directly addressed the issue of HIV testing of hospitalized patients by recommending that hospitals with an HIV seroprevalence rate of at least 1% or an AIDS diagnosis rate 1.0 per 1000 discharges should strongly consider adopting a policy of offering HIV counseling and testing routinely to patients ages 1554 years.5 Despite the information on discharge diagnosis rates often being easily available from hospital databases, even if seroprevalence rates may not, routine HIV testing of hospitalized patients has not occurred.
In 2005 the United States Preventive Services Taskforce (USPSTF) recommendations stated that there was fair evidence that screening adolescents and adults not known to be at increased risk for HIV can detect additional individuals with HIV.6 Their statement reflects data from Chen et al., who identified that self‐reported risk factordirected testing strategies would have missed nearly three quarters of the HIV infections in their clinic setting,7 and from Peterman et al., who demonstrated that 2026% of HIV‐positive patients acknowledged no HIV‐associated risk factors.8
Despite the prior CDC recommendations,1, 5 Chen and Peterman's data,7, 8 and acknowledgment of the high accuracy of the new HIV antibody tests, making false‐positive test results quite rare, the published recommendations of the USPSTF do not support routinely testing individuals who are not at increased risk for acquiring the infection because of the relatively low yield and concern about anxiety and related consequences of HIV testing.
Hospitalists are poised to offer inpatient HIV testing to all inpatients at hospitals that meet the CDC guidelines in an effort to reduce the numbers of patients who have undiagnosed HIV infection. This article examines inpatient HIV testing including barriers that may exist to routine testing and reviews the available rapid HIV tests, which may assist in overcoming some of these barriers.
HIV Testing in the Hospital
Patients diagnosed with HIV infection often have had multiple contacts with the medical community, both inpatient and outpatient, prior to their HIV diagnosis, during which HIV testing had not been offered, thus delaying diagnosis.9 Though clinicians often identify and document triggers that should prompt HIV testing, patients with HIV infection are still not diagnosed in a timely manner. In addition, according to previously published data on inpatient testing from urban institutions, the targeted testing of patients based on traditional risk factors also misses a large proportion of HIV‐infected patients.10 Thus, routine nontargeted inpatient testing, as the CDC suggests, is the preferred strategy.
More than a quarter of patients with HIV in the United States are diagnosed in hospital settings, often in conjunction with an illness that prompts specific testing.11 An important recent study by Brady evaluated the HIV seroprevalence on the medicine and trauma medicine services of 2 hospitals during 2 seasons. The study was blinded and used leftover blood samples taken for other reasons. It found seroprevalence rates varying between 1.4% and 3.7%.12 Two points are noteworthy about this study. First, having excluded those from patients with known HIV disease, a significant proportion of the samples identified as seropositive likely represented unidentified HIV cases. Second, although the seroprevalence varied depending on the season during which testing was done and the service from which blood was obtained, even the lower percentage (1.4%) is higher than the CDC's threshold for offering routine HIV testing.5
With the average length of a hospital stay declining to less than 5 days,13 many patients who undergo nonrapid HIV testing while hospitalized will not receive their results prior to discharge. Though no data specifying the rates of HIV test result follow‐up after hospital discharge have been published, the experience in the outpatient setting suggests a significant number of patients never receive their test results. The CDC estimates that 31% of patients who tested positive for HIV did not return to receive their test results.14 State‐funded, community‐based programs also have highly variable rates of return, with published reports of 2548% of patients never receiving their results.1517 Fortunately, new and highly accurate rapid HIV tests are now available in the United States, almost eliminating the problem of loss to follow‐up18 (see Rapid HIV Antibody Tests, below).
Barriers to Implementing HIV Testing
There are numerous potential barriers to instituting broad‐based screening of hospitalized patients for HIV in addition to the follow‐up issues with standard HIV tests illustrated above. These include the cost and cost effectiveness of the program; the logistics of test performance and counseling on the ward; the risk of offending patients; and the culture changes required of inpatient caregivers and hospital administrators. Each of these is addressed briefly.
Cost
Two cost effectiveness analyses examining routine HIV testing have been published recently. The first, by Sanders,20 assumed a 1% seroprevalence of undiagnosed HIV infection in accordance with CDC recommendations5 and found a one‐time testing cost of $15 078 (2004 dollars) per quality‐adjusted life‐year (QALY) including the benefit accrued to sexual partners of the tested patient. This cost/QALY rose to nearly $40 000/QALY with a seroprevalence of only 0.1%. The second study, by Paltiel,21 demonstrated that the cost/QALY of one‐time testing of patients with a 1% seroprevalence to be $38 000.
A few points must be noted about these studies. First, they are not based on inpatient testing specifically. Nonetheless, the Brady study, above,12 as well as our own experience with routine inpatient testing (unpublished data), suggests that the prevalence may be similar in many inpatient populations. Second, the cost/QALY is very consistent with other routine screening efforts broadly accepted.22 Finally, although both analyses cited moderately to significantly higher costs/QALY for recurrent (eg, every 35 years) routine testing, the relevance of this to routine inpatient testing is less clear.
Another study compared hospitalized patients newly testing HIV positive with a rapid HIV test kit, performed in an emergency department, with those testing HIV positive with conventional HIV tests performed on an inpatient unit.23 Though it was not designed as a cost analysis, the length of stay of the group that received the rapid test was 7 days shorter than that of the group that received the conventional test (6 vs. 13 days; P < .001), with type of HIV testing used identified as an independent effect on length of stay in multivariate regression analysis.
Despite what these analyses reported, start‐up costs for HIV testing services can be substantial, and, at present, insurance reimbursement for HIV counseling does not exist. If physicians offer HIV counseling, they may bill for their time as an extended service, when appropriate. Laboratory fees can be billed, which may help to cover materials and processing costs. Grants through the CDC or the Department of Public Health may be available to support programs that operationalize routine HIV testing.
Logistics of Routine Testing on the Ward
An inpatient unit is a difficult place to do HIV counseling. Issues of patient privacy are substantial, especially in shared rooms or when family or friends are present. Physicians and counselors must be cognizant of these issues and be flexible in the timing and structure of the counseling offered to maximize patient comfort and minimize interruptions. Educating inpatient staff about HIV counseling may help to avoid embarrassing situations and interruptions.
In addition, the time required to do HIV testing properly could significantly slow a busy physician's work flow if offered to every patient. Dedicated HIV counseling and testing staff members can be of great assistance in the process and can remove the time barrier from the physician by performing the tests themselves. Such staff members require training in HIV testing procedures if they are to perform point‐of‐care tests at the bedside. This type of program, coordinated with the leadership of the inpatient service, is ideal for providing routine screening of all admissions as recommended by the CDC.5 In addition, considerations about minimizing or eliminating pretest counseling are ongoing, with counseling only offered during the posttest phase.1, 24 This plan would also reduce the impact of this process on work flow.
An advantage of using an inpatient service as a site for HIV testing is the ability to mobilize a hospital's resources should a patient be diagnosed as HIV positive. Addressing the medical, psychological, and psychosocial needs of newly diagnosed (or previously diagnosed but medically disconnected) patient requires using a multidisciplinary team approach, including inpatient caregivers, social workers, case managers, mental health providers, and HIV specialists.
Avoiding Offending Patients and Changing Hospital Culture
An inpatient unit is an unusual place for routine screening, which usually is relegated to the ambulatory setting. Moreover, with the stigma of HIV still present, despite efforts to quell it,25 inpatient caregivers and hospital administrators may be uncomfortable in approaching or having a trained counselor approach all patients on an inpatient service to discuss HIV counseling and testing.
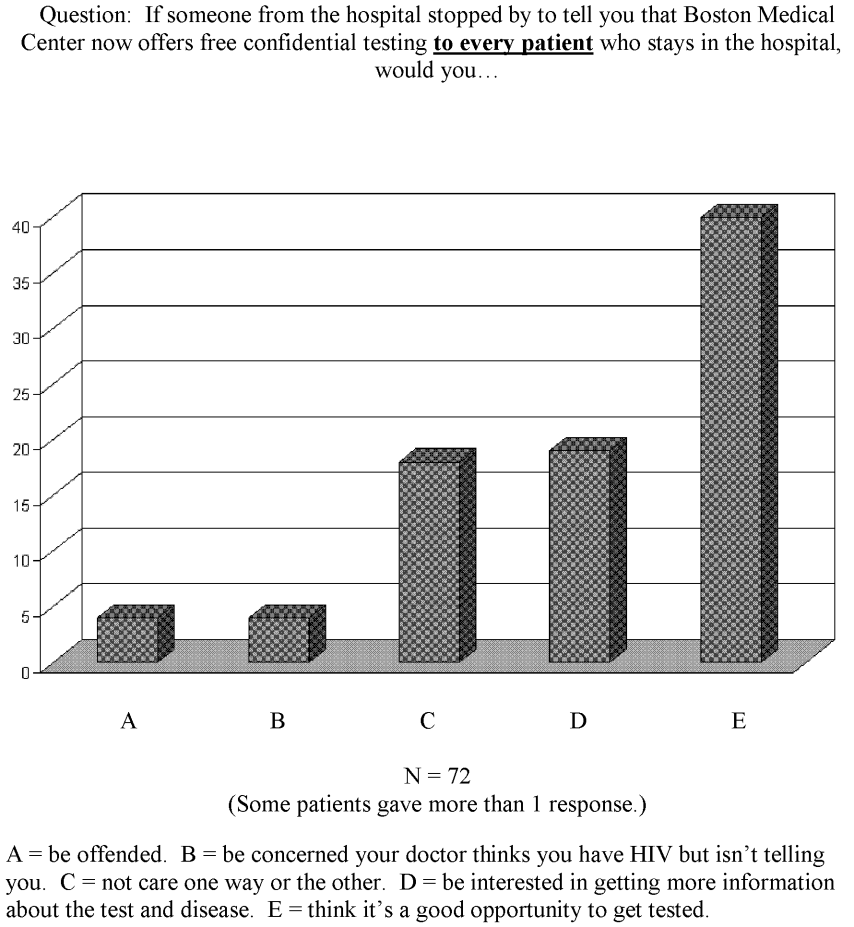
No studies have been published on inpatient attitudes toward routinely being offered HIV testing. Our HIV testing service faced this question when we wanted to expand our inpatient testing from risk‐factor‐directed and physician‐referral‐based testing to routine testing. To assess patient responses, we asked 72 medical inpatients how they would feel about an unsolicited offer to be tested for HIV while they were inpatients. The results, displayed in Figure 1, demonstrated that only 11% of the patients had an unfavorable response. Of note, the study did not permit further explanations to be given to dispel the concerns of those whose response was unfavorable. With this information, our administration permitted expanded testing to commence.

From the experiences of our testing program, with several thousand patients having been approached, we have found that patients are very rarely offended or upset by being offered HIV testing.
Rapid HIV Antibody Tests in the United States
As noted, a substantial proportion of patients fail to return to obtain results.1517 As with other posthospitalization test follow‐ups,26 significant complications may occur if follow‐up of HIV test results is inadequate. Rapid HIV antibody tests may offer programs a way to ensure that the vast majority of patients learn their test results.
There are currently 4 rapid HIV tests that have been approved for use in the United States by the Food and Drug Administration (FDA). Two of these, the OraQuick ADVANCE Rapid HIV‐1/2 Antibody Test (OraSure Technologies, Inc., Bethlehem, PA)27 and the Uni‐Gold Recombigen HIV Test (Trinity Biotech, Bray, County Wicklow, Ireland),28 have received a waiver from the Clinical Laboratories Improvement Amendment (CLIA), which means they may be used outside a laboratory setting.29 Such a waiver means these tests may be used at the bedside of a patient in a point‐of‐care (POC) fashion similar to that of blood sugar monitoring.
It must be noted, however, that extensive quality assurance and quality control are involved with the use of these POC tests.30 Despite the CLIA waiver, a relationship with the hospital laboratory is required, as the test kits may only be used by an agent of the laboratory. An agent is an individual who the laboratory deems capable and qualified to perform the test competently.
Two additional rapid HIV tests are FDA approved but not CLIA waived. These tests, the Reveal G2 Rapid HIV‐1 Antibody Test (MedMira, Bayers Lake Park, Halifax, Nova Scotia)31 and the Multispot HIV‐1/HIV‐2 Rapid Test (Bio‐Rad Laboratories, Redmond, Washington),32 must be performed in a laboratory (see Table 1).
| Rapid HIV Test | Specimen Type | Sensitivity (95% CI) | Specificity (95% CI) | CLIA Category | Cost |
|---|---|---|---|---|---|
| |||||
| OraQuick Advance Rapid HIV1/2 Antibody Test | Oral fluid | 99.3% (98.499.7) | 99.8% (99.699.9) | Waived | $17.50 |
| Whole blood (finger stick or venipuncture) | 99.6% (98.599.9) | 100% (99.7100) | Waived | ||
| Plasma | 99.6% (98.999.8) | 99.9% (99.699.9) | Moderate complexity | ||
| Reveal G‐2 Rapid HIV‐1 Antibody Test | Serum | 99.8% (99.5100) | 99.1% (98.899.4) | Moderate complexity | $14.50 |
| Plasma | 99.8% (99.5100) | 98.6% (98.498.8) | Moderate complexity | ||
| Uni‐Gold Recombigen HIV Test | Whole blood (finger stick or venipuncture) | 100% (99.5100) | 99.7% (99.0100) | Waived | $15.75 |
| Serum and plasma | 100% (99.5100) | 99.8% (99.3100) | Moderate complexity | ||
| Multispot HIV‐1/HIV‐2 Rapid Test | Serum | 100% (99.94100) | 99.93% (99.79100) | Moderate complexity | $25.00 |
| Plasma | 100% (99.94100) | 99.91% (99.77100) | Moderate complexity | ||
All 4 tests have sensitivities and specificities similar to those of commercially available standard HIV enzyme immunosorbent assays (EIA) for HIV. As the tests are extremely sensitive, no confirmatory testing is required for nonreactive rapid test results. These tests should be considered negative. False negatives may occur if the patient has had a recent HIV exposure. Thus, as with standard EIA tests, it is important to recommend retesting in 6 weeks for all patients who test HIV negative but who have had a high‐risk exposure in the last 3 months. Also, very rarely, patients receiving antiretroviral therapy who have successfully suppressed their viral replication below detectable limits for long periods may also have false‐negative results. Therefore, with all patients, it is important to reinforce the idea that it is not appropriate to retest for HIV if a patient already knows he or she is HIV positive.
All reactive rapid HIV tests require confirmation. This process is most commonly done with a Western Blot assay and must be completed before a patient is told that he or she has confirmed HIV infection. Although uncommon, false‐positive rapid tests do occur, reinforcing the need for confirmatory testing before a formal diagnosis of HIV infection can be made. Currently, no FDA‐approved rapid confirmatory HIV test is available, so standard laboratory delays may be unavoidable for these patients. It is therefore critical that hospitals providing rapid HIV testing have access to medical and social support systems that may be rapidly mobilized for patients with reactive and confirmed positive tests.
Hospitalists at the Helm of Routine Inpatient HIV Testing
Putting a hospitalist in charge of implementing inpatient HIV testing has several advantages. First, as experts in the hospital systems in which they work, hospitalists are prime candidates to organize a multidisciplinary team involving those from nursing, laboratory medicine, mental health, and social work, as well as HIV specialists. If dedicated HIV counselors are available to participate, they, too, should be included. A hospitalist with an interest in HIV makes an ideal director of such a multidisciplinary program.
Second, hospitalists are on the front line of clinical care and see patients during the earliest hours of their clinical evaluation. By making HIV testing a routine part of all admissions, the hospitalist may act as a role model in the process and will also be able to explain to patients that they are not being singled out, as all patients are encouraged to undergo testing.
Finally, with the demonstrated added value of hospitalist programs33 and the recent literature demonstrating the cost effectiveness of routine HIV testing,20, 21 hospitalists are well suited to demonstrate leadership in the acquisition of the resources required to make routine inpatient HIV testing possible.
Future Directions
To make routine testing a broadly accepted reality, several developments must begin to take place. These include: increasing education about HIV disease as a chronic disease rather than a rapidly terminal illness;34 reducing the stigma of HIV disease (a stigma that has impaired testing rates),25 which should include discussions of eliminating the need for separate HIV test consent forms, not required for testing for other sexually transmitted diseases (eg, syphilis) or life‐threatening diseases (eg, hepatitis C);1 examining the experience and impact of the universal HIV testing recommendations for pregnant women;35, 36 reducing1, 24 or entirely eliminating37 the requirements for extensive pretest counselingwhich may be a low‐yield38 time barrierwith a greater focus on case‐specific post‐test risk reduction;1 and broadening the realization that targeted testing based on traditional HIV risk factors fails to identify a significant number of HIV cases.10, 39
CONCLUSIONS
Though it has been more than a decade since the original CDC recommendations on inpatient HIV testing were released,5 it remains quite clear that routine inpatient HIV testing can and should be a reality in many hospitals in the United States. As the literature12 and our institution's experience suggest, those in an inpatient service may be a population with a higher prevalence of HIV disease, and as such, an inpatient service should be a venue where routine HIV testing is offered. The U.S. Preventive Services Taskforce's conclusion that the benefit of screening adolescents and adults without risk factors for HIV is too small relative to potential harms to justify a general recommendation6 may not apply to the inpatient services where HIV disease may be more common than in the general population. However, because of time constraints, busy clinicians may require the assistance of an HIV counseling and testing service to make this kind of program a reality.
Clearly, using targeted testing strategies based on traditional HIV risk factors fails to identify a significant proportion of undiagnosed HIV cases.7, 8 New, FDA‐approved rapid HIV antibody tests can help to reduce the issue of loss to follow‐up as a barrier to having successful testing programs, and the cost effectiveness of such HIV testing programs has been suggested in recent literature. Although studies are needed to elucidate the differences between routinely tested inpatients and those tested in more traditional ambulatory sites, hospitalists have the opportunity to take the lead in dramatically increasing testing and in substantially decreasing the number of patients unaware of their HIV status.
- Centers for Disease Control and Prevention.Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003.MMWR Morb Mortal Wkly Rep.2003;52:329–332.
- ,.Estimated HIV prevalence in the United States at the end of 2003. 2005 National HIV Prevention Conference; June 12–15,2005; Atlanta, Ga. Abstract T1–B110.
- ,,, et al.Understanding delay to medical care for HIV infection: the long‐term non‐presenter.AIDS2001;15:77–85.
- ,,, et al.HIV prevalence and associated risks in young men who have sex with men. Young Men's Survey Study Group.JAMA.2000;284:198–204.
- Centers for Disease Control and Prevention.Recommendations for HIV testing services for inpatients and outpatients in acute‐care hospital settings.MMWR Recomm Rep.1993;42(RR‐2):1–6.
- US Preventive Services Taskforce.Screening for HIV: recommendation statement.Ann Intern Med.2005;143(1):32–37.
- ,,,.Risk assessment to improve targeting of HIV counseling and testing services for STD clinic patients.Sex Transm Dis.1998;25:539–543.
- ,,.Opportunities of targeting publicly funded human immunodeficiency virus counseling and testing.J Acquir Immune Defic Syndr Hum Retrovirol.1996;12:69–74.
- ,,,,,.Assessing missed opportunities for HIV testing in medical settings.J Gen Intern Med.2004;19:349–356.
- ,,,.Identifying undiagnosed human immunodeficiency virus: the yield of routine, voluntary, inpatient testing.Arch Intern Med.2002;162:887–892.
- .Learning more about the HIV‐infected population not in care in the US. Poster TuPeG 5690, presented at: XIV International AIDS Conference; July2002; Barcelona, Spain.
- ,,, et al.Seasonal variation in undiagnosed HIV infection on the general medicine and trauma services of two urban hospitals.JGIM.2005;20:324–330.
- ,.2001 National Hospital Discharge Survey. Advance data from vital and health statistics; no 332.Hyattsville, Md:National Center for Health Statistics;2003.
- HIV counseling and testing in publicly funded sites. Annual report, 1997 and 1998.Centers for Disease Control and Prevention [CDC Web site]. Available at: http://www.cdc.gov/hiv/pubs/cts98.pdf. Accessed February 17,2005.
- ,.Rapid hiv testing in urban outreach: a strategy for improving posttest counseling rates.AIDS Educ Prev. Dec2001;13(6):541–550.
- Update: HIV counseling and testing using rapid tests—United States, 1995.MMWR Morb Mortal Wkly Rep.1998;47:211–215.
- ,,, et al.HIV testing among young adults and older adolescents in the setting of acute substance abuse treatment.J Acquir Immune Defic Syndr.2001;27:135–142.
- ,.Rapid HIV testing in the era of OraQuick®.Todays Ther Trends.2003;21:307–344.
- ,,,.A rapid review of rapid HIV antibody tests.Curr Inf Dis Repts.2006;8:125–131.
- ,,, et al.Cost‐effectiveness of screening for HIV in the era of highly active antiretroviral therapy.New Eng J Med.2005;352:570–585.
- ,,, et al.Expanded screening for HIV in the United States—an analysis of cost effectiveness.New Eng J Med.2005;352:586–595.
- Harvard Center for Risk Analysis: The CEA Registry. Cost‐utility analyses published from 1976 to 2001, with ratios converted to 2002 US dollars. Available at: http://www.hsph.harvard.edu/cearegistry/data/1976‐2001_CEratios_comprehensive_4‐7‐2004.pdf. Accessed August 15,2005.
- ,,, et al.The role of rapid vs conventional human immunodeficiency virus testing for inpatients: effects on quality of care.Arch Intern Med.2005;165:1956 The role of rapid vs. conventional Human Immunodeficiency Virus testing for inpatients 1960.
- CDC.Revised guidelines for HIV counseling, testing, and referral.MMWR Recomm Rep.2001;50(RR19);1–58.
- Health Resources and Services Administration. Stigma and HIV/AIDS: a review of the literature. Available at: http://hab.hrsa.gov/publications/stigma/introduction.htm. Accessed August 15,2005.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- Orasure Technologies, Inc. Bethlehem, Pa. OraQuick Advance rapid HIV 1/2 rapid antibody test [package insert]. Available at: http://www.orasure.com/uploaded/398.pdf?1389(suppl 1).
- ,.AIDS as a chronic illness: psychosocial implications.AIDS.2002;16(suppl 4):S69–S76.
- ,,,,.Prenatal screening for HIV: a review of the evidence for the U.S. Preventive Services Taskforce.Ann Intern Med2005;143:38–54.
- CDC.Revised recommendations for HIV screening of pregnant women.MMWR Recomm Rep.2001;50(RR19):59–86.
- ,.HIV testing should no longer be accorded any special status.BMJ.2005;330:492–493.
- The EXPLORE Study Team.Effects of a behavioral intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomized controlled study.Lancet.2004;364:41–50.
- ,.Think HIV. Why physicians should lower their threshold for HIV testing.Arch Intern Med.1999;159:1994–2000.
- Centers for Disease Control and Prevention.Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003.MMWR Morb Mortal Wkly Rep.2003;52:329–332.
- ,.Estimated HIV prevalence in the United States at the end of 2003. 2005 National HIV Prevention Conference; June 12–15,2005; Atlanta, Ga. Abstract T1–B110.
- ,,, et al.Understanding delay to medical care for HIV infection: the long‐term non‐presenter.AIDS2001;15:77–85.
- ,,, et al.HIV prevalence and associated risks in young men who have sex with men. Young Men's Survey Study Group.JAMA.2000;284:198–204.
- Centers for Disease Control and Prevention.Recommendations for HIV testing services for inpatients and outpatients in acute‐care hospital settings.MMWR Recomm Rep.1993;42(RR‐2):1–6.
- US Preventive Services Taskforce.Screening for HIV: recommendation statement.Ann Intern Med.2005;143(1):32–37.
- ,,,.Risk assessment to improve targeting of HIV counseling and testing services for STD clinic patients.Sex Transm Dis.1998;25:539–543.
- ,,.Opportunities of targeting publicly funded human immunodeficiency virus counseling and testing.J Acquir Immune Defic Syndr Hum Retrovirol.1996;12:69–74.
- ,,,,,.Assessing missed opportunities for HIV testing in medical settings.J Gen Intern Med.2004;19:349–356.
- ,,,.Identifying undiagnosed human immunodeficiency virus: the yield of routine, voluntary, inpatient testing.Arch Intern Med.2002;162:887–892.
- .Learning more about the HIV‐infected population not in care in the US. Poster TuPeG 5690, presented at: XIV International AIDS Conference; July2002; Barcelona, Spain.
- ,,, et al.Seasonal variation in undiagnosed HIV infection on the general medicine and trauma services of two urban hospitals.JGIM.2005;20:324–330.
- ,.2001 National Hospital Discharge Survey. Advance data from vital and health statistics; no 332.Hyattsville, Md:National Center for Health Statistics;2003.
- HIV counseling and testing in publicly funded sites. Annual report, 1997 and 1998.Centers for Disease Control and Prevention [CDC Web site]. Available at: http://www.cdc.gov/hiv/pubs/cts98.pdf. Accessed February 17,2005.
- ,.Rapid hiv testing in urban outreach: a strategy for improving posttest counseling rates.AIDS Educ Prev. Dec2001;13(6):541–550.
- Update: HIV counseling and testing using rapid tests—United States, 1995.MMWR Morb Mortal Wkly Rep.1998;47:211–215.
- ,,, et al.HIV testing among young adults and older adolescents in the setting of acute substance abuse treatment.J Acquir Immune Defic Syndr.2001;27:135–142.
- ,.Rapid HIV testing in the era of OraQuick®.Todays Ther Trends.2003;21:307–344.
- ,,,.A rapid review of rapid HIV antibody tests.Curr Inf Dis Repts.2006;8:125–131.
- ,,, et al.Cost‐effectiveness of screening for HIV in the era of highly active antiretroviral therapy.New Eng J Med.2005;352:570–585.
- ,,, et al.Expanded screening for HIV in the United States—an analysis of cost effectiveness.New Eng J Med.2005;352:586–595.
- Harvard Center for Risk Analysis: The CEA Registry. Cost‐utility analyses published from 1976 to 2001, with ratios converted to 2002 US dollars. Available at: http://www.hsph.harvard.edu/cearegistry/data/1976‐2001_CEratios_comprehensive_4‐7‐2004.pdf. Accessed August 15,2005.
- ,,, et al.The role of rapid vs conventional human immunodeficiency virus testing for inpatients: effects on quality of care.Arch Intern Med.2005;165:1956 The role of rapid vs. conventional Human Immunodeficiency Virus testing for inpatients 1960.
- CDC.Revised guidelines for HIV counseling, testing, and referral.MMWR Recomm Rep.2001;50(RR19);1–58.
- Health Resources and Services Administration. Stigma and HIV/AIDS: a review of the literature. Available at: http://hab.hrsa.gov/publications/stigma/introduction.htm. Accessed August 15,2005.
- ,,, et al.Patient safety concerns arising from test results that return after hospital discharge.Ann Intern Med.2005;143:121–128.
- Orasure Technologies, Inc. Bethlehem, Pa. OraQuick Advance rapid HIV 1/2 rapid antibody test [package insert]. Available at: http://www.orasure.com/uploaded/398.pdf?1389(suppl 1).
- ,.AIDS as a chronic illness: psychosocial implications.AIDS.2002;16(suppl 4):S69–S76.
- ,,,,.Prenatal screening for HIV: a review of the evidence for the U.S. Preventive Services Taskforce.Ann Intern Med2005;143:38–54.
- CDC.Revised recommendations for HIV screening of pregnant women.MMWR Recomm Rep.2001;50(RR19):59–86.
- ,.HIV testing should no longer be accorded any special status.BMJ.2005;330:492–493.
- The EXPLORE Study Team.Effects of a behavioral intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomized controlled study.Lancet.2004;364:41–50.
- ,.Think HIV. Why physicians should lower their threshold for HIV testing.Arch Intern Med.1999;159:1994–2000.