User login
India is emerging as one of the world’s largest hotspots for SARS-CoV-2 infection (COVID-19)—second only to the United States—with more than 13,000,000 documented infections since the first case was recorded on January 30, 2020.1,2 Kashmir, a northern territory of India, reported its first case of COVID-19 on March 18, 2020, from the central District Srinagar; this region has accounted for more cases of COVID-19 than any other district throughout the pandemic.3 The large majority of healthcare in District Srinagar is provided by three tertiary care institutions, one district hospital, two subdistrict hospitals, and 70 primary healthcare centers. Potential occupational exposures place healthcare workers (HCWs) at higher risk of acquiring SARS-CoV-2 infection, which in turn may serve as an important source of infection for their families and other community members.4-6 Given the high frequency and geographic variability of asymptomatic infection, growing evidence suggests this hidden reservoir is a source of infection for the general population.7,8
Many countries have started testing for antibodies against SARS-CoV-2, both at the population level and in specific groups, such as HCWs. Seroepidemiological studies are crucial to understanding the dynamics of SARS-CoV-2 infection. Many seroepidemiological studies have been conducted among community populations, but there are insufficient data on HCWs. The World Health Organization also encouraged its member states to conduct seroepidemiological studies to attain a better understanding of COVID-19 infection prevalence and distribution.9-11 Therefore, to quantify the prevalence of SARS-CoV-2 infection among HCWs, we conducted a seroepidemiological study by testing for SARS-CoV-2–specific immunoglobulin (IgG) to gain insight into the extent of infection among specific subgroups of HCWs and to identify risk-factor profiles associated with seropositivity.
METHODS
Study Design and Settings
We conducted this seroepidemiological study to ascertain the presence of IgG antibodies against SARS-CoV-2 among HCWs in the District Srinagar of Kashmir, India. The 2-week period of data collection began on June 15, 2020. As part of healthcare system pandemic preparedness efforts, India’s Ministry of Health provided specific guidelines for health facilities to manage COVID-19. Hospitals were categorized as dedicated COVID and non-COVID hospitals. Dedicated COVID hospitals provided comprehensive care exclusively to patients with COVID-19 and were equipped with fully functional intensive care units, ventilators, and beds with reliable access to oxygen support.12 In addition, infection prevention and control strategies to limit the transmission of SARS-CoV-2 infection were implemented according to guidelines specified by India’s National Center for Disease Control.13 To strengthen service provision, HCWs from other hospitals, including resident physicians, were relocated to these dedicated COVID hospitals. The additional staff were selected by administrative leadership, without input from HCWs.
Study Population and Data Collection
We approached administrative heads of the hospitals in District Srinagar for permission to conduct our study and to invite their HCWs to participate in the study. As Figure 1 shows, we were denied permission by the administrative heads of two tertiary care hospitals. Finally, with a point person serving as a study liaison at each institution, HCWs from three dedicated COVID and seven non-COVID tertiary care hospitals, two subdistrict hospitals, and six primary healthcare centers across the District Srinagar were invited to participate. The sample primary healthcare centers were each selected randomly, after stratification, from six major regions of the district. All frontline HCWs, including physicians, administrative and laboratory personnel, technicians, field workers involved in surveillance activity, and other supporting staff were eligible for the study.

We collected information on an interview form using Epicollect5, a free data-gathering tool widely used in health research.14 Physicians specifically trained in the use of Epicollect5 conducted the face-to-face interview on a prespecified day and recorded the collected information through mobile phones. This information included the participants’ role in providing care to patients with COVID-19 and risk factors for SARS-CoV-2 infection (eg, history of travel since January 1, 2020, symptoms of an influenza-like illness [ILI] in the 4 weeks prior to the interview, close contact with a COVID-19 case). We defined close contact as an unmasked exposure within 6 feet of an infected individual for at least 15 minutes, irrespective of location (ie, community or the hospital).
Following the interview, trained phlebotomists collected 3 to 5 mL of venous blood under aseptic conditions. We strictly adhered to standard operating procedures during collection, transportation, and testing of blood samples. Following collection, the blood samples remained undisturbed for at least 30 minutes before centrifugation, which was performed at the collection site (or at the central laboratory for sites lacking the capability). The samples were then transported for further processing and testing through a cold chain supply line, using vaccine carriers with conditioned icepacks. All testing procedures were conducted with strict adherence to the manufacturers’ guidelines.
Laboratory Procedure
In accordance with the manufacturer’s recommendations, we used a chemiluminescent microparticle immunoassay to detect SARS-CoV-2–specific IgG antibodies in serum samples. The assay is an automated two-step immunoassay for the qualitative detection of IgG antibodies against the nucleocapsid of SARS-CoV-2 in human serum and plasma. The sensitivity and specificity of this test are 100% and 99%, respectively. The test result was considered positive for SARS-CoV-2 IgG if the index value was ≥1.4, consistent with guidance provided by the manufacturer.15
The IgG values were also entered into Epicollect5. Two trained medical interns independently entered the laboratory results in two separate forms. A third medical intern reviewed these forms for discrepancies, in response to which they referenced the source data for adjudication. The information gathered during the interview and the laboratory results were linked with the help of a unique identification number, which was generated at the time of the interview.
Statistical Analysis
We estimated the proportion (and logit-transformed 95% CI) of HCWs with a positive SARS-CoV-2–specific IgG antibody level, the primary outcome of interest. We compared seroprevalence rates by gender, age group, specific occupational group, and type of health facility (dedicated COVID hospital vs non-COVID hospital). Seroprevalence was also
RESULTS
Of the 7,346 HCWs we were granted permission to approach, 2,915 (39.7%) agreed to participate in the study. The participation rate was 49% at the dedicated COVID hospitals (57% physicians and 47% nonphysicians) and 39% at the non-COVID hospitals (46% physicians and 36% nonphysicians). We analyzed information gathered from 2,905 HCWs (Epicollect5 interview forms were missing for nine participants, and the laboratory report was missing for one participant).
The mean age of the participants was 38.6 years, and 35.8% of participants identified as female (Table 1). One third (33.7%) of the participants were physicians, nearly half of whom were residents. In our sample, the overall seroprevalence of SARS-CoV-2–specific antibodies was 2.5% (95% CI, 2.0%-3.1%). 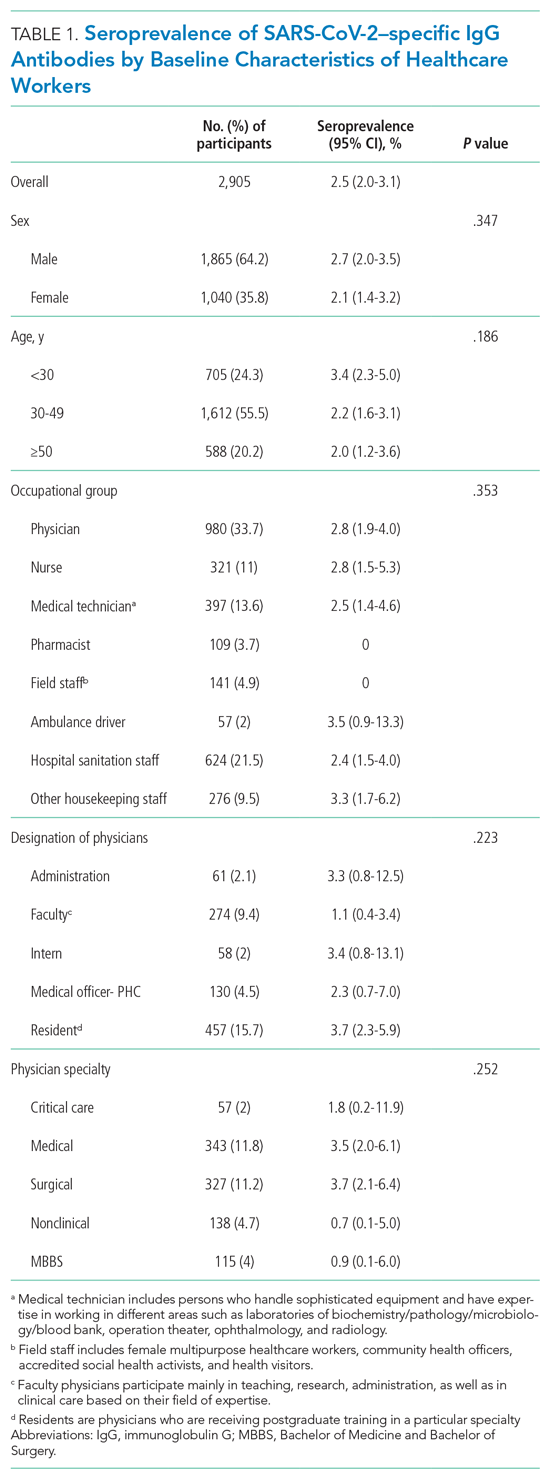

Of the 2,905 participating HCWs, 123 (4.2%) reported an ILI (ie, fever and cough) in the 4 weeks preceding the interview, and 339 (11.7%) reported close contact with a person with COVID-19 (Table 2). A total of 760 (26.2%) HCWs had undergone RT-PCR testing, 29 (3.8%) of whom had a positive result. Stratifying by workplace, history of nasopharyngeal RT-PCR positivity was reported by 4 of 77 (5.1%) participants from dedicated COVID hospitals compared to (3.7%) participants from the non-COVID hospital (P = .528).
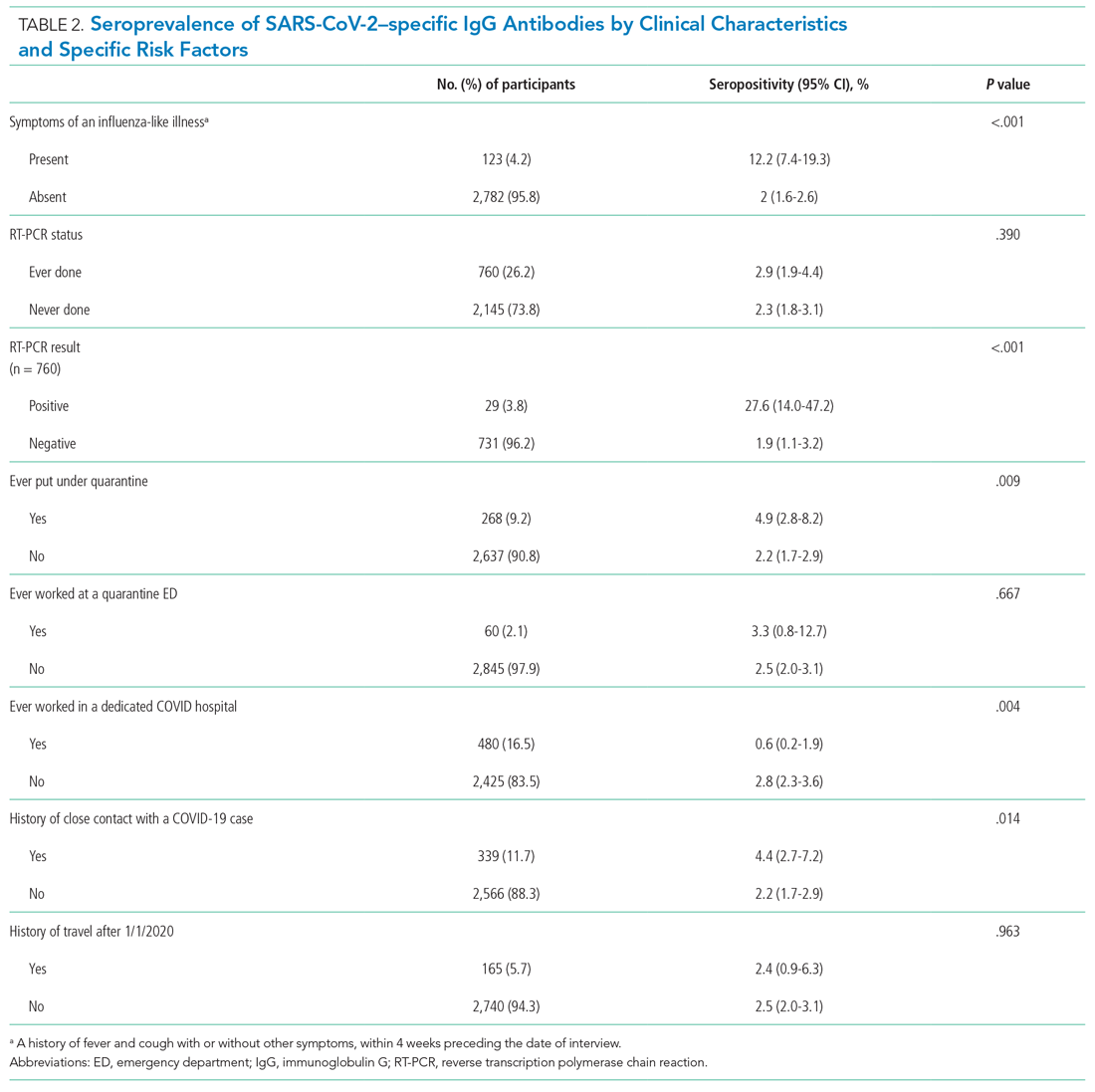
As Table 2 also demonstrates, we found a significantly higher seropositivity rate among HCWs who had a history of ILI (P < .001), a history of positive RT-PCR (P < .001), history of ever being put under quarantine (P = .009), and a self-reported history of close contact with a person with COVID-19 (P = .014). Healthcare workers who had ever worked at a dedicated COVID hospital had a significantly lower seroprevalence of infection (P = .004).
Among HCWs who reported no ILI symptoms in the 4 weeks prior to the interview but who had positive RT-PCR test, 20.8% were seropositive. Of HCWs who reported both ILI and a positive RT-PCR test result, 60.0% were seropositive. Compared to employment at a non-COVID hospital, HCWs working in dedicated COVID hospitals had a reduced multivariate-adjusted risk of seropositivity (odds ratio, 0.21; 95% CI, 0.06-0.66).
DISCUSSION
We aimed to estimate the seroprevalence of SARS-CoV-2 infection in HCWs in different hospital settings in the District Srinagar of Kashmir, India. In general, seroprevalence was low (2.5%), with little difference across gender or occupational group.
Seroprevalence studies of HCWs across divergent workplace environments have revealed estimates ranging from 1% to 10.2%.16-19 Generally, the seroprevalence rates among HCWs are not significantly different from those of the general population, which reflects how different the dynamics of COVID-19 are compared to other infections in healthcare settings. The low seroprevalence observed in our study coincides with the overall low infection rate in the community population. During the study period, District Srinagar reported a median of 28 new infections daily (interquartile range, 17-46), which is indicative of the early phase of the pandemic in the population at the time of the study.20
Among the HCW occupational groups, ambulance drivers and housekeeping staff had the highest seroprevalence rates, followed by nurses and physicians. Possible explanations for higher seropositivity in these groups are improper use or inadequate supply of protective gear and lack of training on the use of personal protective equipment (PPE), resulting in increased exposure risk.21 Concordance of HCW and community infection rates in specific geographic areas suggests that community exposure may be the dominant source of healthcare exposure and infection. Additionally, careful in-hospital behavior of HCWs in dedicated COVID hospitals may have had a spillover effect on their out-of-hospital behavior, which may partially explain our finding that employment at dedicated COVID hospitals was associated with a markedly lower chance of seropositivity. A study of 6,510 HCWs in Chicago, Illinois, showed high seropositivity rates among support service workers, medical assistants, and nurses, with nurses identified as having a markedly higher adjusted odds of seropositivity relative to administrators. The authors of the study concluded that exposure in the community setting plays a crucial role in transmission among HCWs.22 Similarly, higher seroprevalence among housekeeping, nonadministrative staff, and other support service staff has been reported elsewhere.23 Certain underlying factors related to socioeconomic status and lifestyle may also contribute to higher seroprevalence in some occupational groups.24 Nonadherence to masking, social distancing, and proper hand hygiene outside the hospital setting could result in community-acquired infection.
Interestingly, participants who were working in a dedicated COVID hospital or who had ever worked at one had a seroprevalence of 0.6%, much lower than the 2.8% observed among other participants. This difference remained statistically significant after controlling for age, sex, place of work, and occupational group. As these facilities were dedicated to the management and care of patients with COVID-19, the hospital staff strictly adhered to safety precautions, with particular vigilance during patient contact. These hospitals also strictly adhered to infection prevention and control practices based on the latest guidelines released by India’s Ministry of Health and Family Welfare.13
A commitment was made to provide adequate PPE to the dedicated COVID hospitals and staff, commensurate with expected infected patient volumes and associated exposure risks. Healthcare workers were specifically trained on proper donning and doffing of PPE, self-health monitoring, and protocols for reporting symptoms and PPE breaches during patient encounters. Healthcare workers were regularly tested for COVID-19 using nasopharyngeal RT-PCR. Of critical importance, these hospitals implemented a buddy system wherein a team of two or more staff members was responsible for ensuring each other’s safety, proper PPE use, conformance to other protective measures, and reporting breaches of PPE compliance.25 Universal masking was mandatory for all hospital staff and patients at the COVID-focused facilities, with the additional use of N-95 masks, gloves, and face shields during times of patient contact. Administrative measures, including visitor restrictions and environmental sanitation, were rigorously enforced. Also, being a potentially high-risk area for transmission of infection, these facilities implemented staff-rationing to reduce the duration of exposure to the healthcare staff. Third, the HCWs of COVID-dedicated hospitals were provided with separate living accommodations during the period in which they were employed at a dedicated COVID hospital.
In contrast, in non-COVID hospitals, with the exception of HCWs, patients and the hospital visitors were not subject to a masking policy. Moreover, an adequate and timely supply of PPE was not prioritized at the non-COVID facilities due to resource constraints. Further, lack of testing of asymptomatic patients at non-COVID hospitals may have resulted in nosocomial transmission from asymptomatic carriers. Though routine infection prevention and control activities were performed at non-COVID hospitals, we did not assess adherence to infection prevention and control guidelines in the two different categories of hospitals. Our results are also supported by evidence from studies conducted in different hospital settings, the findings of which reiterate the importance of fundamental principles of prevention (eg, proper masking, hand hygiene, and distancing) and are of particular importance in resource-limited settings.17,26,27 The only published study quantifying seroprevalence among HCWs in India was performed in a single hospital setting with separate COVID and non-COVID units. The authors of that study reported a higher seroprevalence among HCWs in the COVID unit. However, this difference seems to be confounded by other factors as revealed by the multivariable analysis result.23
We found a two-fold higher seroprevalence (4.4%) in HCWs who reported close contact with a patient with COVID-19. Respiratory infections pose a greater health risk to HCWs in an occupational setting. Substantial evidence has emerged demonstrating that the respiratory system is the dominant route of SARS-CoV-2 transmission, with proximity and ventilation as key predictive factors.28 Globally, among thousands of HCWs infected with SARS-CoV-2, one of the leading risk factors identified was close contact with a patient with COVID-19; other identified risk factors were lack of PPE, poor infection prevention and control practices, work overload, and a preexisting health condition.29
The seroprevalence estimate among participants who reported an ILI in the 4 weeks preceding the interview was only 12.2%, suggesting an alternative etiology of these symptoms. Among those who reported a previously positive RT-PCR for SARS-CoV-2, only 27.6% showed the presence of SARS-CoV-2–specific IgG antibodies. The inability to mount an antibody-mediated immune response or early conversion to seronegative status during the convalescence phase has been suggested as an explanation for such discordant findings.30 On the contrary, seropositivity among participants who reported having a negative RT-PCR test was 1.9%. There are few plausible explanations for such observations. First, several studies have reported false-negative result rates from RT-PCR testing ranging from 2% to 29%.31-33 Second, the sensitivity of the SARS-CoV-2 assay is influenced by the timing of the test after the onset of symptoms or RT-PCR positivity. The sensitivity of the assay we used varies from 53.1% at day 7 to 100% at day 17 postinfection.34 Variable viral load and differences in duration of viral shedding are other possible reasons for false-negative RT-PCR results.35,36
In our study, seroconversion among asymptomatic HCWs who were RT-PCR-positive was 20.8%. Among HCWs who reported an ILI and were RT-PCR-positive, seropositivity was 60%. In one study, 40% of asymptomatic and 13% of symptomatic patients who tested positive for COVID-19 became seronegative after initial seropositivity—that is, 8 weeks after hospital discharge.37
Serological testing offers insight into both the exposure history and residual COVID-19 susceptibility of HCWs. However, current immunological knowledge does not allow us to conclude that seropositivity conveys high-level immunity against reinfection. As the epidemic evolves, HCWs will continue to be exposed to COVID-19 in the community and the workplace. Serial cross-sectional serosurveys can help monitor the progression of the pandemic within the healthcare setting and guide hospital authorities in resource allocation.
Strengths and Limitations
We used the Abbott Architect SARS-CoV-2 IgG assay, which has exhibited a high level of consistency and performance characteristics when tested in different patient populations. The participation rate was acceptable compared to similar studies, and we included all the major hospitals in the District Srinagar. The findings from our study can therefore be considered representative of the HCWs in the district.
The study results should be interpreted in the context of the following limitations. First, information on risk factors for seropositivity were based on participant report. Also, we did not collect information on the timing of symptoms or the date on which a participant became RT-PCR-positive. Second, information regarding place of exposure (ie, community or hospital setting) was not recorded, limiting conclusions regarding the effect of workplace exposures. Third, given the voluntary nature of participation in the study, there is a possibility of selection bias that may have limited the generalizability of our findings. For example, some HCWs with a recent exposure to COVID-19 or those who were symptomatic at the time of the study might not have participated based on the absence of an individual benefit from IgG testing in the early phase of infection. Conversely, some HCWs who had symptoms in the distant past might have been more likely to have participated in the study. However, we believe that selection bias does not vitiate the validity of the associations based on the plausible assumption that infection risk should be similar between respondents and nonrespondents due to comparable work environments. Finally, with a cross-sectional study design, we cannot ascertain the reconversion from an initial positive-IgG to negative-IgG status, which warrants a cohort study.
CONCLUSION
We conclude that the seroprevalence of SARS-CoV-2 infection was low among HCWs of District Srinagar at the time of the study. Healthcare workers in a dedicated COVID hospital or HCWs who had ever worked in such a facility had lower seroprevalence, suggesting both adherence to and effectiveness of standard protective measures during contact with patients who had COVID-19. Nonetheless, the careful in-hospital behavior of the HCWs at the COVID hospitals may have had a spillover effect on their out-of-hospital behaviors, which lead to community-acquired infection. On the contrary, lack of testing of asymptomatic patients at non-COVID hospitals may have resulted in nosocomial transmission from asymptomatic carriers. We believe that our findings highlight the value of implementing infection prevention and control measures in the hospital setting. Moreover, training and retraining of sanitation and other housekeeping staff on standard hygienic practices and appropriate use of the protective gear may further help reduce their rates of exposure.
Acknowledgments
The authors thank Principal and Dean of the Government Medical College, Srinagar, Professor Samia Rashid, and District Commissioner, Srinagar, Shahid Iqbal Chowdhary for their support. We also acknowledge the support rendered by the Directorate of Health Services, Kashmir; Chief Medical Officer Srinagar; Block Medical Officers; and Zonal Medical Officers of District Srinagar, Kashmir, and extend our appreciation to the medical interns for their efforts in data collection, and to laboratory in-charge Gulzar Ahmad Wani, PhD scholar, Biochemistry, and his staff, who were involved in this study. Finally, we thank the study participants for their understanding of the importance of this study and for their time and participation.
Data availability statement
Data shall be made available on request through the corresponding author.
1. Ministry of Health & Family Welfare. Government of India. Accessed January 11, 2021. https://www.mohfw.gov.in/
2. COVID19 India. Accessed January 11, 2021. https://www.covid19india.org/
3. Government of Jammu & Kashmir. Department of Information & Public Relations. Bulletin on Novel Corona Virus (COVID-19). Accessed January 11, 2021. http://new.jkdirinf.in/NewsDescription.aspx?ID=66598
4. Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418-1420. https://doi.org/10.1016/s0140-6736(20)30917-x
5. Nguyen LH, Drew DA, Graham MS, et al; Coronavirus Pandemic Epidemiology Consortium. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Heal. 2020;5(9):e475-e483. https://doi.org/10.1016/s2468-2667(20)30164-x
6. The Lancet. COVID-19: protecting health-care workers. Lancet. 2020;395(10228):922. https://doi.org/10.1016/s0140-6736(20)30644-9
7. Byambasuren O, Cardona M, Bell K, Clark J, McLaws M-L, Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. Off J Assoc Med Microbiol Infect Dis Canada. 2020;5(4):223-234. https://doi.org/10.3138/jammi-2020-0030
8. Rosenbaum L. Facing Covid-19 in Italy—ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med. 2020;382(20):1873-1875. https://doi.org/10.1056/nejmp2005492
9. World Health Organization. The Unity Studies: WHO Sero-epidemiological Investigations Protocols. Accessed January 11, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/early-investigations
10. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al; ENE-COVID Study Group. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535-544. https://doi.org/10.1016/s0140-6736(20)31483-5
11. Folgueira MD, Muñoz-Ruipérez C, Alonso-López MA, Delgado R. SARS-CoV-2 infection in health care workers in a large public hospital in Madrid, Spain, during March 2020. MedRxiv Web site. Published April 27, 2020. Accessed March 9, 2021. https://doi.org/10.1101/2020.04.07.20055723
12. Ministry of Health & Family Welfare, Directorate General of Health Services, EMR Division. Guidance document on appropriate management of suspect/confirmed cases of COVID-19. Accessed January 11, 2021. https://www.mohfw.gov.in/pdf/FinalGuidanceonMangaementofCovidcasesversion2.pdf
13. Ministry of Health &Family Welfare Government of India. National guidelines for infection prevention and control in healthcare facilities. Accessed January 11, 2021. https://main.mohfw.gov.in/sites/default/files/National%20Guidelines%20for%20IPC%20in%20HCF%20-%20final%281%29.pdf
14. Epicollect5. Accessed January 11, 2021. https://five.epicollect.net/
15. SARS-CoV-2 Immunoassay. Abbott Core Laboratory. Accessed January 11, 2021. https://www.corelaboratory.abbott/us/en/offerings/segments/infectious-disease/sars-cov-2
16. Bendavid E, Mulaney B, Sood N, et al. COVID-19 antibody seroprevalence in Santa Clara County, California. medRxiv. Published online April 30, 2020. Accessed March 9, 2021. https://doi.org/10.1101/2020.04.14.20062463
17. Korth J, Wilde B, Dolff S, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. https://doi.org/10.1016/j.jcv.2020.104437
18. Steensels D, Oris E, Coninx L, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324(2):195-197. https://doi.org/10.1001/jama.2020.11160
19. Behrens GMN, Cossmann A, Stankov M V., et al. Perceived versus proven SARS-CoV-2-specific immune responses in health-care professionals. Infection. 2020;48(4):631-634. https://doi.org/10.1007/s15010-020-01461-0
20. COVID-19 Kashmir Tracker. Accessed January 11, 2021. https://covidkashmir.org/statistics
21. World Health Organization. Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages. Published December 23, 2020. Accessed January 11, 2021. https://www.who.int/publications/i/item/rational-use-of-personal-protective-equipment-for-coronavirus-disease-(covid-19)-and-considerations-during-severe-shortages
22. Wilkins JT, Gray EL, Wallia A, et al. Seroprevalence and correlates of SARS-CoV-2 antibodies in health care workers in Chicago. Open Forum Infect Dis. 2020;8(1):ofaa582. https://doi.org/10.1093/ofid/ofaa582
23. Goenka M, Afzalpurkar S, Goenka U, et al. Seroprevalence of COVID-19 amongst health care workers in a tertiary care hospital of a metropolitan city from India. J Assoc Physicians India. 2020;68(11):14-19. https://doi.org/10.2139/ssrn.3689618
24. Mutambudzi M, Niedwiedz C, Macdonald EB, et al. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK Biobank participants. Occup Environ Med. 2020;oemed-2020-106731. https://doi.org/10.1136/oemed-2020-106731
25. Ministry of Health & Family Welfare, Directorate General of Health Services, EMR Division. Advisory for managing health care workers working in COVID and Non-COVID areas of the hospital. Accessed January 12, 2021. https://cdnbbsr.s3waas.gov.in/s3850af92f8d9903e7a4e0559a98ecc857/uploads/2020/06/2020061949.pdf
26. Rhee C, Baker M, Vaidya V, et al; CDC Prevention Epicenters Program. Incidence of nosocomial COVID-19 in patients hospitalized at a large US academic medical center. JAMA Netw Open. 2020;3(9):e2020498. https://doi.org/10.1001/jamanetworkopen.2020.20498
27. Seidelman J, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-2-CoV)healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41(12):1466-1467. https://doi.org/10.1017/ice.2020.313
28. Meyerowitz EA, Richterman A, Gandhi RT, Sax PE. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med. 2020;174(1):69-79. https://doi.org/10.7326/m20-5008
29. Mhango M, Dzobo M, Chitungo I, Dzinamarira T. COVID-19 risk factors among health workers: a rapid review. Saf Health Work. 2020;11(3):262-265. https://doi.org/10.1016/j.shaw.2020.06.001
30. European Centre for Disease Prevention and Control. Immune responses and immunity to SARS-CoV-2. Updated June 30, 2020. Accessed January 12, 2021. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/immune-responses
31. Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, et al. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. PLoS One. 2020;15(12):e0242958. https://doi.org/10.1371/journal.pone.0242958
32. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(2):E32-E40. https://doi.org/10.1148/radiol.2020200642
33. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection — challenges and implications. N Engl J Med. 2020;383(6):e38. https://doi.org/10.1056/nejmp2015897
34. Bryan A, Pepper G, Wener MH, et al. Performance characteristics of the Abbott Architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58(8):e00941. https://doi.org/10.1128/jcm.00941-20
35. Long Q-X, Liu B-Z, Deng H-J, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845-848. https://doi.org/10.1038/s41591-020-0897-1
36. Tahamtan A, Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020;20(5):453-454. https://doi.org/10.1080/14737159.2020.1757437
37. Long Q-X, Tang X-J, Shi Q-L, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200-1204. https://doi.org/10.1038/s41591-020-0965-6
India is emerging as one of the world’s largest hotspots for SARS-CoV-2 infection (COVID-19)—second only to the United States—with more than 13,000,000 documented infections since the first case was recorded on January 30, 2020.1,2 Kashmir, a northern territory of India, reported its first case of COVID-19 on March 18, 2020, from the central District Srinagar; this region has accounted for more cases of COVID-19 than any other district throughout the pandemic.3 The large majority of healthcare in District Srinagar is provided by three tertiary care institutions, one district hospital, two subdistrict hospitals, and 70 primary healthcare centers. Potential occupational exposures place healthcare workers (HCWs) at higher risk of acquiring SARS-CoV-2 infection, which in turn may serve as an important source of infection for their families and other community members.4-6 Given the high frequency and geographic variability of asymptomatic infection, growing evidence suggests this hidden reservoir is a source of infection for the general population.7,8
Many countries have started testing for antibodies against SARS-CoV-2, both at the population level and in specific groups, such as HCWs. Seroepidemiological studies are crucial to understanding the dynamics of SARS-CoV-2 infection. Many seroepidemiological studies have been conducted among community populations, but there are insufficient data on HCWs. The World Health Organization also encouraged its member states to conduct seroepidemiological studies to attain a better understanding of COVID-19 infection prevalence and distribution.9-11 Therefore, to quantify the prevalence of SARS-CoV-2 infection among HCWs, we conducted a seroepidemiological study by testing for SARS-CoV-2–specific immunoglobulin (IgG) to gain insight into the extent of infection among specific subgroups of HCWs and to identify risk-factor profiles associated with seropositivity.
METHODS
Study Design and Settings
We conducted this seroepidemiological study to ascertain the presence of IgG antibodies against SARS-CoV-2 among HCWs in the District Srinagar of Kashmir, India. The 2-week period of data collection began on June 15, 2020. As part of healthcare system pandemic preparedness efforts, India’s Ministry of Health provided specific guidelines for health facilities to manage COVID-19. Hospitals were categorized as dedicated COVID and non-COVID hospitals. Dedicated COVID hospitals provided comprehensive care exclusively to patients with COVID-19 and were equipped with fully functional intensive care units, ventilators, and beds with reliable access to oxygen support.12 In addition, infection prevention and control strategies to limit the transmission of SARS-CoV-2 infection were implemented according to guidelines specified by India’s National Center for Disease Control.13 To strengthen service provision, HCWs from other hospitals, including resident physicians, were relocated to these dedicated COVID hospitals. The additional staff were selected by administrative leadership, without input from HCWs.
Study Population and Data Collection
We approached administrative heads of the hospitals in District Srinagar for permission to conduct our study and to invite their HCWs to participate in the study. As Figure 1 shows, we were denied permission by the administrative heads of two tertiary care hospitals. Finally, with a point person serving as a study liaison at each institution, HCWs from three dedicated COVID and seven non-COVID tertiary care hospitals, two subdistrict hospitals, and six primary healthcare centers across the District Srinagar were invited to participate. The sample primary healthcare centers were each selected randomly, after stratification, from six major regions of the district. All frontline HCWs, including physicians, administrative and laboratory personnel, technicians, field workers involved in surveillance activity, and other supporting staff were eligible for the study.

We collected information on an interview form using Epicollect5, a free data-gathering tool widely used in health research.14 Physicians specifically trained in the use of Epicollect5 conducted the face-to-face interview on a prespecified day and recorded the collected information through mobile phones. This information included the participants’ role in providing care to patients with COVID-19 and risk factors for SARS-CoV-2 infection (eg, history of travel since January 1, 2020, symptoms of an influenza-like illness [ILI] in the 4 weeks prior to the interview, close contact with a COVID-19 case). We defined close contact as an unmasked exposure within 6 feet of an infected individual for at least 15 minutes, irrespective of location (ie, community or the hospital).
Following the interview, trained phlebotomists collected 3 to 5 mL of venous blood under aseptic conditions. We strictly adhered to standard operating procedures during collection, transportation, and testing of blood samples. Following collection, the blood samples remained undisturbed for at least 30 minutes before centrifugation, which was performed at the collection site (or at the central laboratory for sites lacking the capability). The samples were then transported for further processing and testing through a cold chain supply line, using vaccine carriers with conditioned icepacks. All testing procedures were conducted with strict adherence to the manufacturers’ guidelines.
Laboratory Procedure
In accordance with the manufacturer’s recommendations, we used a chemiluminescent microparticle immunoassay to detect SARS-CoV-2–specific IgG antibodies in serum samples. The assay is an automated two-step immunoassay for the qualitative detection of IgG antibodies against the nucleocapsid of SARS-CoV-2 in human serum and plasma. The sensitivity and specificity of this test are 100% and 99%, respectively. The test result was considered positive for SARS-CoV-2 IgG if the index value was ≥1.4, consistent with guidance provided by the manufacturer.15
The IgG values were also entered into Epicollect5. Two trained medical interns independently entered the laboratory results in two separate forms. A third medical intern reviewed these forms for discrepancies, in response to which they referenced the source data for adjudication. The information gathered during the interview and the laboratory results were linked with the help of a unique identification number, which was generated at the time of the interview.
Statistical Analysis
We estimated the proportion (and logit-transformed 95% CI) of HCWs with a positive SARS-CoV-2–specific IgG antibody level, the primary outcome of interest. We compared seroprevalence rates by gender, age group, specific occupational group, and type of health facility (dedicated COVID hospital vs non-COVID hospital). Seroprevalence was also
RESULTS
Of the 7,346 HCWs we were granted permission to approach, 2,915 (39.7%) agreed to participate in the study. The participation rate was 49% at the dedicated COVID hospitals (57% physicians and 47% nonphysicians) and 39% at the non-COVID hospitals (46% physicians and 36% nonphysicians). We analyzed information gathered from 2,905 HCWs (Epicollect5 interview forms were missing for nine participants, and the laboratory report was missing for one participant).
The mean age of the participants was 38.6 years, and 35.8% of participants identified as female (Table 1). One third (33.7%) of the participants were physicians, nearly half of whom were residents. In our sample, the overall seroprevalence of SARS-CoV-2–specific antibodies was 2.5% (95% CI, 2.0%-3.1%). 

Of the 2,905 participating HCWs, 123 (4.2%) reported an ILI (ie, fever and cough) in the 4 weeks preceding the interview, and 339 (11.7%) reported close contact with a person with COVID-19 (Table 2). A total of 760 (26.2%) HCWs had undergone RT-PCR testing, 29 (3.8%) of whom had a positive result. Stratifying by workplace, history of nasopharyngeal RT-PCR positivity was reported by 4 of 77 (5.1%) participants from dedicated COVID hospitals compared to (3.7%) participants from the non-COVID hospital (P = .528).

As Table 2 also demonstrates, we found a significantly higher seropositivity rate among HCWs who had a history of ILI (P < .001), a history of positive RT-PCR (P < .001), history of ever being put under quarantine (P = .009), and a self-reported history of close contact with a person with COVID-19 (P = .014). Healthcare workers who had ever worked at a dedicated COVID hospital had a significantly lower seroprevalence of infection (P = .004).
Among HCWs who reported no ILI symptoms in the 4 weeks prior to the interview but who had positive RT-PCR test, 20.8% were seropositive. Of HCWs who reported both ILI and a positive RT-PCR test result, 60.0% were seropositive. Compared to employment at a non-COVID hospital, HCWs working in dedicated COVID hospitals had a reduced multivariate-adjusted risk of seropositivity (odds ratio, 0.21; 95% CI, 0.06-0.66).
DISCUSSION
We aimed to estimate the seroprevalence of SARS-CoV-2 infection in HCWs in different hospital settings in the District Srinagar of Kashmir, India. In general, seroprevalence was low (2.5%), with little difference across gender or occupational group.
Seroprevalence studies of HCWs across divergent workplace environments have revealed estimates ranging from 1% to 10.2%.16-19 Generally, the seroprevalence rates among HCWs are not significantly different from those of the general population, which reflects how different the dynamics of COVID-19 are compared to other infections in healthcare settings. The low seroprevalence observed in our study coincides with the overall low infection rate in the community population. During the study period, District Srinagar reported a median of 28 new infections daily (interquartile range, 17-46), which is indicative of the early phase of the pandemic in the population at the time of the study.20
Among the HCW occupational groups, ambulance drivers and housekeeping staff had the highest seroprevalence rates, followed by nurses and physicians. Possible explanations for higher seropositivity in these groups are improper use or inadequate supply of protective gear and lack of training on the use of personal protective equipment (PPE), resulting in increased exposure risk.21 Concordance of HCW and community infection rates in specific geographic areas suggests that community exposure may be the dominant source of healthcare exposure and infection. Additionally, careful in-hospital behavior of HCWs in dedicated COVID hospitals may have had a spillover effect on their out-of-hospital behavior, which may partially explain our finding that employment at dedicated COVID hospitals was associated with a markedly lower chance of seropositivity. A study of 6,510 HCWs in Chicago, Illinois, showed high seropositivity rates among support service workers, medical assistants, and nurses, with nurses identified as having a markedly higher adjusted odds of seropositivity relative to administrators. The authors of the study concluded that exposure in the community setting plays a crucial role in transmission among HCWs.22 Similarly, higher seroprevalence among housekeeping, nonadministrative staff, and other support service staff has been reported elsewhere.23 Certain underlying factors related to socioeconomic status and lifestyle may also contribute to higher seroprevalence in some occupational groups.24 Nonadherence to masking, social distancing, and proper hand hygiene outside the hospital setting could result in community-acquired infection.
Interestingly, participants who were working in a dedicated COVID hospital or who had ever worked at one had a seroprevalence of 0.6%, much lower than the 2.8% observed among other participants. This difference remained statistically significant after controlling for age, sex, place of work, and occupational group. As these facilities were dedicated to the management and care of patients with COVID-19, the hospital staff strictly adhered to safety precautions, with particular vigilance during patient contact. These hospitals also strictly adhered to infection prevention and control practices based on the latest guidelines released by India’s Ministry of Health and Family Welfare.13
A commitment was made to provide adequate PPE to the dedicated COVID hospitals and staff, commensurate with expected infected patient volumes and associated exposure risks. Healthcare workers were specifically trained on proper donning and doffing of PPE, self-health monitoring, and protocols for reporting symptoms and PPE breaches during patient encounters. Healthcare workers were regularly tested for COVID-19 using nasopharyngeal RT-PCR. Of critical importance, these hospitals implemented a buddy system wherein a team of two or more staff members was responsible for ensuring each other’s safety, proper PPE use, conformance to other protective measures, and reporting breaches of PPE compliance.25 Universal masking was mandatory for all hospital staff and patients at the COVID-focused facilities, with the additional use of N-95 masks, gloves, and face shields during times of patient contact. Administrative measures, including visitor restrictions and environmental sanitation, were rigorously enforced. Also, being a potentially high-risk area for transmission of infection, these facilities implemented staff-rationing to reduce the duration of exposure to the healthcare staff. Third, the HCWs of COVID-dedicated hospitals were provided with separate living accommodations during the period in which they were employed at a dedicated COVID hospital.
In contrast, in non-COVID hospitals, with the exception of HCWs, patients and the hospital visitors were not subject to a masking policy. Moreover, an adequate and timely supply of PPE was not prioritized at the non-COVID facilities due to resource constraints. Further, lack of testing of asymptomatic patients at non-COVID hospitals may have resulted in nosocomial transmission from asymptomatic carriers. Though routine infection prevention and control activities were performed at non-COVID hospitals, we did not assess adherence to infection prevention and control guidelines in the two different categories of hospitals. Our results are also supported by evidence from studies conducted in different hospital settings, the findings of which reiterate the importance of fundamental principles of prevention (eg, proper masking, hand hygiene, and distancing) and are of particular importance in resource-limited settings.17,26,27 The only published study quantifying seroprevalence among HCWs in India was performed in a single hospital setting with separate COVID and non-COVID units. The authors of that study reported a higher seroprevalence among HCWs in the COVID unit. However, this difference seems to be confounded by other factors as revealed by the multivariable analysis result.23
We found a two-fold higher seroprevalence (4.4%) in HCWs who reported close contact with a patient with COVID-19. Respiratory infections pose a greater health risk to HCWs in an occupational setting. Substantial evidence has emerged demonstrating that the respiratory system is the dominant route of SARS-CoV-2 transmission, with proximity and ventilation as key predictive factors.28 Globally, among thousands of HCWs infected with SARS-CoV-2, one of the leading risk factors identified was close contact with a patient with COVID-19; other identified risk factors were lack of PPE, poor infection prevention and control practices, work overload, and a preexisting health condition.29
The seroprevalence estimate among participants who reported an ILI in the 4 weeks preceding the interview was only 12.2%, suggesting an alternative etiology of these symptoms. Among those who reported a previously positive RT-PCR for SARS-CoV-2, only 27.6% showed the presence of SARS-CoV-2–specific IgG antibodies. The inability to mount an antibody-mediated immune response or early conversion to seronegative status during the convalescence phase has been suggested as an explanation for such discordant findings.30 On the contrary, seropositivity among participants who reported having a negative RT-PCR test was 1.9%. There are few plausible explanations for such observations. First, several studies have reported false-negative result rates from RT-PCR testing ranging from 2% to 29%.31-33 Second, the sensitivity of the SARS-CoV-2 assay is influenced by the timing of the test after the onset of symptoms or RT-PCR positivity. The sensitivity of the assay we used varies from 53.1% at day 7 to 100% at day 17 postinfection.34 Variable viral load and differences in duration of viral shedding are other possible reasons for false-negative RT-PCR results.35,36
In our study, seroconversion among asymptomatic HCWs who were RT-PCR-positive was 20.8%. Among HCWs who reported an ILI and were RT-PCR-positive, seropositivity was 60%. In one study, 40% of asymptomatic and 13% of symptomatic patients who tested positive for COVID-19 became seronegative after initial seropositivity—that is, 8 weeks after hospital discharge.37
Serological testing offers insight into both the exposure history and residual COVID-19 susceptibility of HCWs. However, current immunological knowledge does not allow us to conclude that seropositivity conveys high-level immunity against reinfection. As the epidemic evolves, HCWs will continue to be exposed to COVID-19 in the community and the workplace. Serial cross-sectional serosurveys can help monitor the progression of the pandemic within the healthcare setting and guide hospital authorities in resource allocation.
Strengths and Limitations
We used the Abbott Architect SARS-CoV-2 IgG assay, which has exhibited a high level of consistency and performance characteristics when tested in different patient populations. The participation rate was acceptable compared to similar studies, and we included all the major hospitals in the District Srinagar. The findings from our study can therefore be considered representative of the HCWs in the district.
The study results should be interpreted in the context of the following limitations. First, information on risk factors for seropositivity were based on participant report. Also, we did not collect information on the timing of symptoms or the date on which a participant became RT-PCR-positive. Second, information regarding place of exposure (ie, community or hospital setting) was not recorded, limiting conclusions regarding the effect of workplace exposures. Third, given the voluntary nature of participation in the study, there is a possibility of selection bias that may have limited the generalizability of our findings. For example, some HCWs with a recent exposure to COVID-19 or those who were symptomatic at the time of the study might not have participated based on the absence of an individual benefit from IgG testing in the early phase of infection. Conversely, some HCWs who had symptoms in the distant past might have been more likely to have participated in the study. However, we believe that selection bias does not vitiate the validity of the associations based on the plausible assumption that infection risk should be similar between respondents and nonrespondents due to comparable work environments. Finally, with a cross-sectional study design, we cannot ascertain the reconversion from an initial positive-IgG to negative-IgG status, which warrants a cohort study.
CONCLUSION
We conclude that the seroprevalence of SARS-CoV-2 infection was low among HCWs of District Srinagar at the time of the study. Healthcare workers in a dedicated COVID hospital or HCWs who had ever worked in such a facility had lower seroprevalence, suggesting both adherence to and effectiveness of standard protective measures during contact with patients who had COVID-19. Nonetheless, the careful in-hospital behavior of the HCWs at the COVID hospitals may have had a spillover effect on their out-of-hospital behaviors, which lead to community-acquired infection. On the contrary, lack of testing of asymptomatic patients at non-COVID hospitals may have resulted in nosocomial transmission from asymptomatic carriers. We believe that our findings highlight the value of implementing infection prevention and control measures in the hospital setting. Moreover, training and retraining of sanitation and other housekeeping staff on standard hygienic practices and appropriate use of the protective gear may further help reduce their rates of exposure.
Acknowledgments
The authors thank Principal and Dean of the Government Medical College, Srinagar, Professor Samia Rashid, and District Commissioner, Srinagar, Shahid Iqbal Chowdhary for their support. We also acknowledge the support rendered by the Directorate of Health Services, Kashmir; Chief Medical Officer Srinagar; Block Medical Officers; and Zonal Medical Officers of District Srinagar, Kashmir, and extend our appreciation to the medical interns for their efforts in data collection, and to laboratory in-charge Gulzar Ahmad Wani, PhD scholar, Biochemistry, and his staff, who were involved in this study. Finally, we thank the study participants for their understanding of the importance of this study and for their time and participation.
Data availability statement
Data shall be made available on request through the corresponding author.
India is emerging as one of the world’s largest hotspots for SARS-CoV-2 infection (COVID-19)—second only to the United States—with more than 13,000,000 documented infections since the first case was recorded on January 30, 2020.1,2 Kashmir, a northern territory of India, reported its first case of COVID-19 on March 18, 2020, from the central District Srinagar; this region has accounted for more cases of COVID-19 than any other district throughout the pandemic.3 The large majority of healthcare in District Srinagar is provided by three tertiary care institutions, one district hospital, two subdistrict hospitals, and 70 primary healthcare centers. Potential occupational exposures place healthcare workers (HCWs) at higher risk of acquiring SARS-CoV-2 infection, which in turn may serve as an important source of infection for their families and other community members.4-6 Given the high frequency and geographic variability of asymptomatic infection, growing evidence suggests this hidden reservoir is a source of infection for the general population.7,8
Many countries have started testing for antibodies against SARS-CoV-2, both at the population level and in specific groups, such as HCWs. Seroepidemiological studies are crucial to understanding the dynamics of SARS-CoV-2 infection. Many seroepidemiological studies have been conducted among community populations, but there are insufficient data on HCWs. The World Health Organization also encouraged its member states to conduct seroepidemiological studies to attain a better understanding of COVID-19 infection prevalence and distribution.9-11 Therefore, to quantify the prevalence of SARS-CoV-2 infection among HCWs, we conducted a seroepidemiological study by testing for SARS-CoV-2–specific immunoglobulin (IgG) to gain insight into the extent of infection among specific subgroups of HCWs and to identify risk-factor profiles associated with seropositivity.
METHODS
Study Design and Settings
We conducted this seroepidemiological study to ascertain the presence of IgG antibodies against SARS-CoV-2 among HCWs in the District Srinagar of Kashmir, India. The 2-week period of data collection began on June 15, 2020. As part of healthcare system pandemic preparedness efforts, India’s Ministry of Health provided specific guidelines for health facilities to manage COVID-19. Hospitals were categorized as dedicated COVID and non-COVID hospitals. Dedicated COVID hospitals provided comprehensive care exclusively to patients with COVID-19 and were equipped with fully functional intensive care units, ventilators, and beds with reliable access to oxygen support.12 In addition, infection prevention and control strategies to limit the transmission of SARS-CoV-2 infection were implemented according to guidelines specified by India’s National Center for Disease Control.13 To strengthen service provision, HCWs from other hospitals, including resident physicians, were relocated to these dedicated COVID hospitals. The additional staff were selected by administrative leadership, without input from HCWs.
Study Population and Data Collection
We approached administrative heads of the hospitals in District Srinagar for permission to conduct our study and to invite their HCWs to participate in the study. As Figure 1 shows, we were denied permission by the administrative heads of two tertiary care hospitals. Finally, with a point person serving as a study liaison at each institution, HCWs from three dedicated COVID and seven non-COVID tertiary care hospitals, two subdistrict hospitals, and six primary healthcare centers across the District Srinagar were invited to participate. The sample primary healthcare centers were each selected randomly, after stratification, from six major regions of the district. All frontline HCWs, including physicians, administrative and laboratory personnel, technicians, field workers involved in surveillance activity, and other supporting staff were eligible for the study.

We collected information on an interview form using Epicollect5, a free data-gathering tool widely used in health research.14 Physicians specifically trained in the use of Epicollect5 conducted the face-to-face interview on a prespecified day and recorded the collected information through mobile phones. This information included the participants’ role in providing care to patients with COVID-19 and risk factors for SARS-CoV-2 infection (eg, history of travel since January 1, 2020, symptoms of an influenza-like illness [ILI] in the 4 weeks prior to the interview, close contact with a COVID-19 case). We defined close contact as an unmasked exposure within 6 feet of an infected individual for at least 15 minutes, irrespective of location (ie, community or the hospital).
Following the interview, trained phlebotomists collected 3 to 5 mL of venous blood under aseptic conditions. We strictly adhered to standard operating procedures during collection, transportation, and testing of blood samples. Following collection, the blood samples remained undisturbed for at least 30 minutes before centrifugation, which was performed at the collection site (or at the central laboratory for sites lacking the capability). The samples were then transported for further processing and testing through a cold chain supply line, using vaccine carriers with conditioned icepacks. All testing procedures were conducted with strict adherence to the manufacturers’ guidelines.
Laboratory Procedure
In accordance with the manufacturer’s recommendations, we used a chemiluminescent microparticle immunoassay to detect SARS-CoV-2–specific IgG antibodies in serum samples. The assay is an automated two-step immunoassay for the qualitative detection of IgG antibodies against the nucleocapsid of SARS-CoV-2 in human serum and plasma. The sensitivity and specificity of this test are 100% and 99%, respectively. The test result was considered positive for SARS-CoV-2 IgG if the index value was ≥1.4, consistent with guidance provided by the manufacturer.15
The IgG values were also entered into Epicollect5. Two trained medical interns independently entered the laboratory results in two separate forms. A third medical intern reviewed these forms for discrepancies, in response to which they referenced the source data for adjudication. The information gathered during the interview and the laboratory results were linked with the help of a unique identification number, which was generated at the time of the interview.
Statistical Analysis
We estimated the proportion (and logit-transformed 95% CI) of HCWs with a positive SARS-CoV-2–specific IgG antibody level, the primary outcome of interest. We compared seroprevalence rates by gender, age group, specific occupational group, and type of health facility (dedicated COVID hospital vs non-COVID hospital). Seroprevalence was also
RESULTS
Of the 7,346 HCWs we were granted permission to approach, 2,915 (39.7%) agreed to participate in the study. The participation rate was 49% at the dedicated COVID hospitals (57% physicians and 47% nonphysicians) and 39% at the non-COVID hospitals (46% physicians and 36% nonphysicians). We analyzed information gathered from 2,905 HCWs (Epicollect5 interview forms were missing for nine participants, and the laboratory report was missing for one participant).
The mean age of the participants was 38.6 years, and 35.8% of participants identified as female (Table 1). One third (33.7%) of the participants were physicians, nearly half of whom were residents. In our sample, the overall seroprevalence of SARS-CoV-2–specific antibodies was 2.5% (95% CI, 2.0%-3.1%). 

Of the 2,905 participating HCWs, 123 (4.2%) reported an ILI (ie, fever and cough) in the 4 weeks preceding the interview, and 339 (11.7%) reported close contact with a person with COVID-19 (Table 2). A total of 760 (26.2%) HCWs had undergone RT-PCR testing, 29 (3.8%) of whom had a positive result. Stratifying by workplace, history of nasopharyngeal RT-PCR positivity was reported by 4 of 77 (5.1%) participants from dedicated COVID hospitals compared to (3.7%) participants from the non-COVID hospital (P = .528).

As Table 2 also demonstrates, we found a significantly higher seropositivity rate among HCWs who had a history of ILI (P < .001), a history of positive RT-PCR (P < .001), history of ever being put under quarantine (P = .009), and a self-reported history of close contact with a person with COVID-19 (P = .014). Healthcare workers who had ever worked at a dedicated COVID hospital had a significantly lower seroprevalence of infection (P = .004).
Among HCWs who reported no ILI symptoms in the 4 weeks prior to the interview but who had positive RT-PCR test, 20.8% were seropositive. Of HCWs who reported both ILI and a positive RT-PCR test result, 60.0% were seropositive. Compared to employment at a non-COVID hospital, HCWs working in dedicated COVID hospitals had a reduced multivariate-adjusted risk of seropositivity (odds ratio, 0.21; 95% CI, 0.06-0.66).
DISCUSSION
We aimed to estimate the seroprevalence of SARS-CoV-2 infection in HCWs in different hospital settings in the District Srinagar of Kashmir, India. In general, seroprevalence was low (2.5%), with little difference across gender or occupational group.
Seroprevalence studies of HCWs across divergent workplace environments have revealed estimates ranging from 1% to 10.2%.16-19 Generally, the seroprevalence rates among HCWs are not significantly different from those of the general population, which reflects how different the dynamics of COVID-19 are compared to other infections in healthcare settings. The low seroprevalence observed in our study coincides with the overall low infection rate in the community population. During the study period, District Srinagar reported a median of 28 new infections daily (interquartile range, 17-46), which is indicative of the early phase of the pandemic in the population at the time of the study.20
Among the HCW occupational groups, ambulance drivers and housekeeping staff had the highest seroprevalence rates, followed by nurses and physicians. Possible explanations for higher seropositivity in these groups are improper use or inadequate supply of protective gear and lack of training on the use of personal protective equipment (PPE), resulting in increased exposure risk.21 Concordance of HCW and community infection rates in specific geographic areas suggests that community exposure may be the dominant source of healthcare exposure and infection. Additionally, careful in-hospital behavior of HCWs in dedicated COVID hospitals may have had a spillover effect on their out-of-hospital behavior, which may partially explain our finding that employment at dedicated COVID hospitals was associated with a markedly lower chance of seropositivity. A study of 6,510 HCWs in Chicago, Illinois, showed high seropositivity rates among support service workers, medical assistants, and nurses, with nurses identified as having a markedly higher adjusted odds of seropositivity relative to administrators. The authors of the study concluded that exposure in the community setting plays a crucial role in transmission among HCWs.22 Similarly, higher seroprevalence among housekeeping, nonadministrative staff, and other support service staff has been reported elsewhere.23 Certain underlying factors related to socioeconomic status and lifestyle may also contribute to higher seroprevalence in some occupational groups.24 Nonadherence to masking, social distancing, and proper hand hygiene outside the hospital setting could result in community-acquired infection.
Interestingly, participants who were working in a dedicated COVID hospital or who had ever worked at one had a seroprevalence of 0.6%, much lower than the 2.8% observed among other participants. This difference remained statistically significant after controlling for age, sex, place of work, and occupational group. As these facilities were dedicated to the management and care of patients with COVID-19, the hospital staff strictly adhered to safety precautions, with particular vigilance during patient contact. These hospitals also strictly adhered to infection prevention and control practices based on the latest guidelines released by India’s Ministry of Health and Family Welfare.13
A commitment was made to provide adequate PPE to the dedicated COVID hospitals and staff, commensurate with expected infected patient volumes and associated exposure risks. Healthcare workers were specifically trained on proper donning and doffing of PPE, self-health monitoring, and protocols for reporting symptoms and PPE breaches during patient encounters. Healthcare workers were regularly tested for COVID-19 using nasopharyngeal RT-PCR. Of critical importance, these hospitals implemented a buddy system wherein a team of two or more staff members was responsible for ensuring each other’s safety, proper PPE use, conformance to other protective measures, and reporting breaches of PPE compliance.25 Universal masking was mandatory for all hospital staff and patients at the COVID-focused facilities, with the additional use of N-95 masks, gloves, and face shields during times of patient contact. Administrative measures, including visitor restrictions and environmental sanitation, were rigorously enforced. Also, being a potentially high-risk area for transmission of infection, these facilities implemented staff-rationing to reduce the duration of exposure to the healthcare staff. Third, the HCWs of COVID-dedicated hospitals were provided with separate living accommodations during the period in which they were employed at a dedicated COVID hospital.
In contrast, in non-COVID hospitals, with the exception of HCWs, patients and the hospital visitors were not subject to a masking policy. Moreover, an adequate and timely supply of PPE was not prioritized at the non-COVID facilities due to resource constraints. Further, lack of testing of asymptomatic patients at non-COVID hospitals may have resulted in nosocomial transmission from asymptomatic carriers. Though routine infection prevention and control activities were performed at non-COVID hospitals, we did not assess adherence to infection prevention and control guidelines in the two different categories of hospitals. Our results are also supported by evidence from studies conducted in different hospital settings, the findings of which reiterate the importance of fundamental principles of prevention (eg, proper masking, hand hygiene, and distancing) and are of particular importance in resource-limited settings.17,26,27 The only published study quantifying seroprevalence among HCWs in India was performed in a single hospital setting with separate COVID and non-COVID units. The authors of that study reported a higher seroprevalence among HCWs in the COVID unit. However, this difference seems to be confounded by other factors as revealed by the multivariable analysis result.23
We found a two-fold higher seroprevalence (4.4%) in HCWs who reported close contact with a patient with COVID-19. Respiratory infections pose a greater health risk to HCWs in an occupational setting. Substantial evidence has emerged demonstrating that the respiratory system is the dominant route of SARS-CoV-2 transmission, with proximity and ventilation as key predictive factors.28 Globally, among thousands of HCWs infected with SARS-CoV-2, one of the leading risk factors identified was close contact with a patient with COVID-19; other identified risk factors were lack of PPE, poor infection prevention and control practices, work overload, and a preexisting health condition.29
The seroprevalence estimate among participants who reported an ILI in the 4 weeks preceding the interview was only 12.2%, suggesting an alternative etiology of these symptoms. Among those who reported a previously positive RT-PCR for SARS-CoV-2, only 27.6% showed the presence of SARS-CoV-2–specific IgG antibodies. The inability to mount an antibody-mediated immune response or early conversion to seronegative status during the convalescence phase has been suggested as an explanation for such discordant findings.30 On the contrary, seropositivity among participants who reported having a negative RT-PCR test was 1.9%. There are few plausible explanations for such observations. First, several studies have reported false-negative result rates from RT-PCR testing ranging from 2% to 29%.31-33 Second, the sensitivity of the SARS-CoV-2 assay is influenced by the timing of the test after the onset of symptoms or RT-PCR positivity. The sensitivity of the assay we used varies from 53.1% at day 7 to 100% at day 17 postinfection.34 Variable viral load and differences in duration of viral shedding are other possible reasons for false-negative RT-PCR results.35,36
In our study, seroconversion among asymptomatic HCWs who were RT-PCR-positive was 20.8%. Among HCWs who reported an ILI and were RT-PCR-positive, seropositivity was 60%. In one study, 40% of asymptomatic and 13% of symptomatic patients who tested positive for COVID-19 became seronegative after initial seropositivity—that is, 8 weeks after hospital discharge.37
Serological testing offers insight into both the exposure history and residual COVID-19 susceptibility of HCWs. However, current immunological knowledge does not allow us to conclude that seropositivity conveys high-level immunity against reinfection. As the epidemic evolves, HCWs will continue to be exposed to COVID-19 in the community and the workplace. Serial cross-sectional serosurveys can help monitor the progression of the pandemic within the healthcare setting and guide hospital authorities in resource allocation.
Strengths and Limitations
We used the Abbott Architect SARS-CoV-2 IgG assay, which has exhibited a high level of consistency and performance characteristics when tested in different patient populations. The participation rate was acceptable compared to similar studies, and we included all the major hospitals in the District Srinagar. The findings from our study can therefore be considered representative of the HCWs in the district.
The study results should be interpreted in the context of the following limitations. First, information on risk factors for seropositivity were based on participant report. Also, we did not collect information on the timing of symptoms or the date on which a participant became RT-PCR-positive. Second, information regarding place of exposure (ie, community or hospital setting) was not recorded, limiting conclusions regarding the effect of workplace exposures. Third, given the voluntary nature of participation in the study, there is a possibility of selection bias that may have limited the generalizability of our findings. For example, some HCWs with a recent exposure to COVID-19 or those who were symptomatic at the time of the study might not have participated based on the absence of an individual benefit from IgG testing in the early phase of infection. Conversely, some HCWs who had symptoms in the distant past might have been more likely to have participated in the study. However, we believe that selection bias does not vitiate the validity of the associations based on the plausible assumption that infection risk should be similar between respondents and nonrespondents due to comparable work environments. Finally, with a cross-sectional study design, we cannot ascertain the reconversion from an initial positive-IgG to negative-IgG status, which warrants a cohort study.
CONCLUSION
We conclude that the seroprevalence of SARS-CoV-2 infection was low among HCWs of District Srinagar at the time of the study. Healthcare workers in a dedicated COVID hospital or HCWs who had ever worked in such a facility had lower seroprevalence, suggesting both adherence to and effectiveness of standard protective measures during contact with patients who had COVID-19. Nonetheless, the careful in-hospital behavior of the HCWs at the COVID hospitals may have had a spillover effect on their out-of-hospital behaviors, which lead to community-acquired infection. On the contrary, lack of testing of asymptomatic patients at non-COVID hospitals may have resulted in nosocomial transmission from asymptomatic carriers. We believe that our findings highlight the value of implementing infection prevention and control measures in the hospital setting. Moreover, training and retraining of sanitation and other housekeeping staff on standard hygienic practices and appropriate use of the protective gear may further help reduce their rates of exposure.
Acknowledgments
The authors thank Principal and Dean of the Government Medical College, Srinagar, Professor Samia Rashid, and District Commissioner, Srinagar, Shahid Iqbal Chowdhary for their support. We also acknowledge the support rendered by the Directorate of Health Services, Kashmir; Chief Medical Officer Srinagar; Block Medical Officers; and Zonal Medical Officers of District Srinagar, Kashmir, and extend our appreciation to the medical interns for their efforts in data collection, and to laboratory in-charge Gulzar Ahmad Wani, PhD scholar, Biochemistry, and his staff, who were involved in this study. Finally, we thank the study participants for their understanding of the importance of this study and for their time and participation.
Data availability statement
Data shall be made available on request through the corresponding author.
1. Ministry of Health & Family Welfare. Government of India. Accessed January 11, 2021. https://www.mohfw.gov.in/
2. COVID19 India. Accessed January 11, 2021. https://www.covid19india.org/
3. Government of Jammu & Kashmir. Department of Information & Public Relations. Bulletin on Novel Corona Virus (COVID-19). Accessed January 11, 2021. http://new.jkdirinf.in/NewsDescription.aspx?ID=66598
4. Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418-1420. https://doi.org/10.1016/s0140-6736(20)30917-x
5. Nguyen LH, Drew DA, Graham MS, et al; Coronavirus Pandemic Epidemiology Consortium. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Heal. 2020;5(9):e475-e483. https://doi.org/10.1016/s2468-2667(20)30164-x
6. The Lancet. COVID-19: protecting health-care workers. Lancet. 2020;395(10228):922. https://doi.org/10.1016/s0140-6736(20)30644-9
7. Byambasuren O, Cardona M, Bell K, Clark J, McLaws M-L, Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. Off J Assoc Med Microbiol Infect Dis Canada. 2020;5(4):223-234. https://doi.org/10.3138/jammi-2020-0030
8. Rosenbaum L. Facing Covid-19 in Italy—ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med. 2020;382(20):1873-1875. https://doi.org/10.1056/nejmp2005492
9. World Health Organization. The Unity Studies: WHO Sero-epidemiological Investigations Protocols. Accessed January 11, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/early-investigations
10. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al; ENE-COVID Study Group. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535-544. https://doi.org/10.1016/s0140-6736(20)31483-5
11. Folgueira MD, Muñoz-Ruipérez C, Alonso-López MA, Delgado R. SARS-CoV-2 infection in health care workers in a large public hospital in Madrid, Spain, during March 2020. MedRxiv Web site. Published April 27, 2020. Accessed March 9, 2021. https://doi.org/10.1101/2020.04.07.20055723
12. Ministry of Health & Family Welfare, Directorate General of Health Services, EMR Division. Guidance document on appropriate management of suspect/confirmed cases of COVID-19. Accessed January 11, 2021. https://www.mohfw.gov.in/pdf/FinalGuidanceonMangaementofCovidcasesversion2.pdf
13. Ministry of Health &Family Welfare Government of India. National guidelines for infection prevention and control in healthcare facilities. Accessed January 11, 2021. https://main.mohfw.gov.in/sites/default/files/National%20Guidelines%20for%20IPC%20in%20HCF%20-%20final%281%29.pdf
14. Epicollect5. Accessed January 11, 2021. https://five.epicollect.net/
15. SARS-CoV-2 Immunoassay. Abbott Core Laboratory. Accessed January 11, 2021. https://www.corelaboratory.abbott/us/en/offerings/segments/infectious-disease/sars-cov-2
16. Bendavid E, Mulaney B, Sood N, et al. COVID-19 antibody seroprevalence in Santa Clara County, California. medRxiv. Published online April 30, 2020. Accessed March 9, 2021. https://doi.org/10.1101/2020.04.14.20062463
17. Korth J, Wilde B, Dolff S, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. https://doi.org/10.1016/j.jcv.2020.104437
18. Steensels D, Oris E, Coninx L, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324(2):195-197. https://doi.org/10.1001/jama.2020.11160
19. Behrens GMN, Cossmann A, Stankov M V., et al. Perceived versus proven SARS-CoV-2-specific immune responses in health-care professionals. Infection. 2020;48(4):631-634. https://doi.org/10.1007/s15010-020-01461-0
20. COVID-19 Kashmir Tracker. Accessed January 11, 2021. https://covidkashmir.org/statistics
21. World Health Organization. Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages. Published December 23, 2020. Accessed January 11, 2021. https://www.who.int/publications/i/item/rational-use-of-personal-protective-equipment-for-coronavirus-disease-(covid-19)-and-considerations-during-severe-shortages
22. Wilkins JT, Gray EL, Wallia A, et al. Seroprevalence and correlates of SARS-CoV-2 antibodies in health care workers in Chicago. Open Forum Infect Dis. 2020;8(1):ofaa582. https://doi.org/10.1093/ofid/ofaa582
23. Goenka M, Afzalpurkar S, Goenka U, et al. Seroprevalence of COVID-19 amongst health care workers in a tertiary care hospital of a metropolitan city from India. J Assoc Physicians India. 2020;68(11):14-19. https://doi.org/10.2139/ssrn.3689618
24. Mutambudzi M, Niedwiedz C, Macdonald EB, et al. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK Biobank participants. Occup Environ Med. 2020;oemed-2020-106731. https://doi.org/10.1136/oemed-2020-106731
25. Ministry of Health & Family Welfare, Directorate General of Health Services, EMR Division. Advisory for managing health care workers working in COVID and Non-COVID areas of the hospital. Accessed January 12, 2021. https://cdnbbsr.s3waas.gov.in/s3850af92f8d9903e7a4e0559a98ecc857/uploads/2020/06/2020061949.pdf
26. Rhee C, Baker M, Vaidya V, et al; CDC Prevention Epicenters Program. Incidence of nosocomial COVID-19 in patients hospitalized at a large US academic medical center. JAMA Netw Open. 2020;3(9):e2020498. https://doi.org/10.1001/jamanetworkopen.2020.20498
27. Seidelman J, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-2-CoV)healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41(12):1466-1467. https://doi.org/10.1017/ice.2020.313
28. Meyerowitz EA, Richterman A, Gandhi RT, Sax PE. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med. 2020;174(1):69-79. https://doi.org/10.7326/m20-5008
29. Mhango M, Dzobo M, Chitungo I, Dzinamarira T. COVID-19 risk factors among health workers: a rapid review. Saf Health Work. 2020;11(3):262-265. https://doi.org/10.1016/j.shaw.2020.06.001
30. European Centre for Disease Prevention and Control. Immune responses and immunity to SARS-CoV-2. Updated June 30, 2020. Accessed January 12, 2021. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/immune-responses
31. Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, et al. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. PLoS One. 2020;15(12):e0242958. https://doi.org/10.1371/journal.pone.0242958
32. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(2):E32-E40. https://doi.org/10.1148/radiol.2020200642
33. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection — challenges and implications. N Engl J Med. 2020;383(6):e38. https://doi.org/10.1056/nejmp2015897
34. Bryan A, Pepper G, Wener MH, et al. Performance characteristics of the Abbott Architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58(8):e00941. https://doi.org/10.1128/jcm.00941-20
35. Long Q-X, Liu B-Z, Deng H-J, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845-848. https://doi.org/10.1038/s41591-020-0897-1
36. Tahamtan A, Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020;20(5):453-454. https://doi.org/10.1080/14737159.2020.1757437
37. Long Q-X, Tang X-J, Shi Q-L, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200-1204. https://doi.org/10.1038/s41591-020-0965-6
1. Ministry of Health & Family Welfare. Government of India. Accessed January 11, 2021. https://www.mohfw.gov.in/
2. COVID19 India. Accessed January 11, 2021. https://www.covid19india.org/
3. Government of Jammu & Kashmir. Department of Information & Public Relations. Bulletin on Novel Corona Virus (COVID-19). Accessed January 11, 2021. http://new.jkdirinf.in/NewsDescription.aspx?ID=66598
4. Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395(10234):1418-1420. https://doi.org/10.1016/s0140-6736(20)30917-x
5. Nguyen LH, Drew DA, Graham MS, et al; Coronavirus Pandemic Epidemiology Consortium. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Heal. 2020;5(9):e475-e483. https://doi.org/10.1016/s2468-2667(20)30164-x
6. The Lancet. COVID-19: protecting health-care workers. Lancet. 2020;395(10228):922. https://doi.org/10.1016/s0140-6736(20)30644-9
7. Byambasuren O, Cardona M, Bell K, Clark J, McLaws M-L, Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. Off J Assoc Med Microbiol Infect Dis Canada. 2020;5(4):223-234. https://doi.org/10.3138/jammi-2020-0030
8. Rosenbaum L. Facing Covid-19 in Italy—ethics, logistics, and therapeutics on the epidemic’s front line. N Engl J Med. 2020;382(20):1873-1875. https://doi.org/10.1056/nejmp2005492
9. World Health Organization. The Unity Studies: WHO Sero-epidemiological Investigations Protocols. Accessed January 11, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/early-investigations
10. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al; ENE-COVID Study Group. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535-544. https://doi.org/10.1016/s0140-6736(20)31483-5
11. Folgueira MD, Muñoz-Ruipérez C, Alonso-López MA, Delgado R. SARS-CoV-2 infection in health care workers in a large public hospital in Madrid, Spain, during March 2020. MedRxiv Web site. Published April 27, 2020. Accessed March 9, 2021. https://doi.org/10.1101/2020.04.07.20055723
12. Ministry of Health & Family Welfare, Directorate General of Health Services, EMR Division. Guidance document on appropriate management of suspect/confirmed cases of COVID-19. Accessed January 11, 2021. https://www.mohfw.gov.in/pdf/FinalGuidanceonMangaementofCovidcasesversion2.pdf
13. Ministry of Health &Family Welfare Government of India. National guidelines for infection prevention and control in healthcare facilities. Accessed January 11, 2021. https://main.mohfw.gov.in/sites/default/files/National%20Guidelines%20for%20IPC%20in%20HCF%20-%20final%281%29.pdf
14. Epicollect5. Accessed January 11, 2021. https://five.epicollect.net/
15. SARS-CoV-2 Immunoassay. Abbott Core Laboratory. Accessed January 11, 2021. https://www.corelaboratory.abbott/us/en/offerings/segments/infectious-disease/sars-cov-2
16. Bendavid E, Mulaney B, Sood N, et al. COVID-19 antibody seroprevalence in Santa Clara County, California. medRxiv. Published online April 30, 2020. Accessed March 9, 2021. https://doi.org/10.1101/2020.04.14.20062463
17. Korth J, Wilde B, Dolff S, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. https://doi.org/10.1016/j.jcv.2020.104437
18. Steensels D, Oris E, Coninx L, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a tertiary center in Belgium. JAMA. 2020;324(2):195-197. https://doi.org/10.1001/jama.2020.11160
19. Behrens GMN, Cossmann A, Stankov M V., et al. Perceived versus proven SARS-CoV-2-specific immune responses in health-care professionals. Infection. 2020;48(4):631-634. https://doi.org/10.1007/s15010-020-01461-0
20. COVID-19 Kashmir Tracker. Accessed January 11, 2021. https://covidkashmir.org/statistics
21. World Health Organization. Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages. Published December 23, 2020. Accessed January 11, 2021. https://www.who.int/publications/i/item/rational-use-of-personal-protective-equipment-for-coronavirus-disease-(covid-19)-and-considerations-during-severe-shortages
22. Wilkins JT, Gray EL, Wallia A, et al. Seroprevalence and correlates of SARS-CoV-2 antibodies in health care workers in Chicago. Open Forum Infect Dis. 2020;8(1):ofaa582. https://doi.org/10.1093/ofid/ofaa582
23. Goenka M, Afzalpurkar S, Goenka U, et al. Seroprevalence of COVID-19 amongst health care workers in a tertiary care hospital of a metropolitan city from India. J Assoc Physicians India. 2020;68(11):14-19. https://doi.org/10.2139/ssrn.3689618
24. Mutambudzi M, Niedwiedz C, Macdonald EB, et al. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK Biobank participants. Occup Environ Med. 2020;oemed-2020-106731. https://doi.org/10.1136/oemed-2020-106731
25. Ministry of Health & Family Welfare, Directorate General of Health Services, EMR Division. Advisory for managing health care workers working in COVID and Non-COVID areas of the hospital. Accessed January 12, 2021. https://cdnbbsr.s3waas.gov.in/s3850af92f8d9903e7a4e0559a98ecc857/uploads/2020/06/2020061949.pdf
26. Rhee C, Baker M, Vaidya V, et al; CDC Prevention Epicenters Program. Incidence of nosocomial COVID-19 in patients hospitalized at a large US academic medical center. JAMA Netw Open. 2020;3(9):e2020498. https://doi.org/10.1001/jamanetworkopen.2020.20498
27. Seidelman J, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-2-CoV)healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol. 2020;41(12):1466-1467. https://doi.org/10.1017/ice.2020.313
28. Meyerowitz EA, Richterman A, Gandhi RT, Sax PE. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med. 2020;174(1):69-79. https://doi.org/10.7326/m20-5008
29. Mhango M, Dzobo M, Chitungo I, Dzinamarira T. COVID-19 risk factors among health workers: a rapid review. Saf Health Work. 2020;11(3):262-265. https://doi.org/10.1016/j.shaw.2020.06.001
30. European Centre for Disease Prevention and Control. Immune responses and immunity to SARS-CoV-2. Updated June 30, 2020. Accessed January 12, 2021. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/immune-responses
31. Arevalo-Rodriguez I, Buitrago-Garcia D, Simancas-Racines D, et al. False-negative results of initial RT-PCR assays for COVID-19: a systematic review. PLoS One. 2020;15(12):e0242958. https://doi.org/10.1371/journal.pone.0242958
32. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(2):E32-E40. https://doi.org/10.1148/radiol.2020200642
33. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection — challenges and implications. N Engl J Med. 2020;383(6):e38. https://doi.org/10.1056/nejmp2015897
34. Bryan A, Pepper G, Wener MH, et al. Performance characteristics of the Abbott Architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58(8):e00941. https://doi.org/10.1128/jcm.00941-20
35. Long Q-X, Liu B-Z, Deng H-J, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845-848. https://doi.org/10.1038/s41591-020-0897-1
36. Tahamtan A, Ardebili A. Real-time RT-PCR in COVID-19 detection: issues affecting the results. Expert Rev Mol Diagn. 2020;20(5):453-454. https://doi.org/10.1080/14737159.2020.1757437
37. Long Q-X, Tang X-J, Shi Q-L, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200-1204. https://doi.org/10.1038/s41591-020-0965-6
©2021 Society of Hospital Medicine