User login
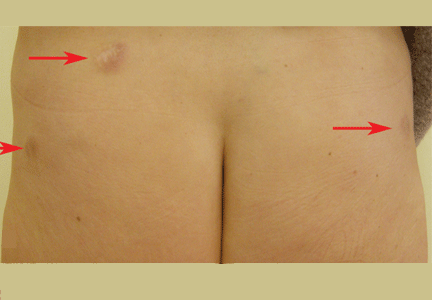
A 52-year-old woman is referred to our dermatology clinic by her primary care physician for swelling and redness of seven old scars. The swelling began 3 months ago.
She is on no regular medications. She has never smoked. She underwent liposuction 7 years ago and appendectomy at age 15.
Laboratory testing shows mild leukopenia, with a white blood cell count of 3.0 × 109/L (reference range 4.2–9.0). Other routine laboratory values are normal, including antinuclear antibody, extractable nuclear antibody, anti-double-stranded DNA, rheumatoid factor, urinalysis, erythrocyte sedimentation rate, C-reactive protein, serum calcium concentration, and liver and renal function tests.
Chest radiography reveals bilateral hilar lymphadenopathy.
Q: What is the next most appropriate diagnostic procedure?
- Skin biopsy
- High-resolution computed tomography (CT) of the chest
- QuantiFERON-TB Gold test
- Ventilatory function tests
- Serum angiotensin-converting enzyme (ACE) level
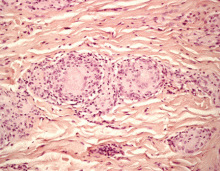
A: All are appropriate at this point. In this case, skin biopsy and high-resolution CT were performed. Histopathologic examination of one of the scars showed multiple well-demarcated, large, noncaseating epitheloid granulomas with histiocytes and multinucleated giant cells. High-resolution CT confirmed bilateral hilar and mediastinal lymphadenopathy and revealed micronodular densities with a bronchovascular and subpleural distribution.
An interferon-gamma-release assay for tuberculosis—QuantiFERON-TB Gold (Cellestis, Carnegie, Australia)—was negative. Ventilatory function tests showed a normal pattern, while the serum ACE level, electrocardiography, and an eye examination revealed no pathologic findings.
Q: What is the diagnosis?
- Keloids
- Scar sarcoidosis
- Paraneoplastic sign
- Dermatofibrosarcoma protuberans
- Tubercolosis
A: Based on the data outlined above, we made the diagnosis of scar sarcoidosis with involvement of hilar and mediastinal lymph nodes. The patient began systemic treatment with oral prednisone 1 mg/kg/day for 6 weeks, which was then gradually withdrawn, until the skin and hilar lesions resolved completely.
SCAR SARCOIDOSIS
Sarcoidosis is a multisystem disorder of unknown cause characterized by the formation of noncaseating granulomas in the affected organs. Patients may present with symptoms related to the specific organ affected, but they may have no symptoms or only general symptoms such as fever or general malaise.
The skin is involved in 25% of cases and presents so many polymorphous manifestations that sarcoidosis has become known as one of the “great imitators” in dermatology.1,2
Although sarcoidosis on liposuction scars has not been reported previously, the reactivation of old scars is well known on sites of previous injections, tattoos, herpes zoster, and burns.2,3
The finding of granuloma is not specific for sarcoidosis (Figure 2). The histologic differential diagnosis of sarcoidosis includes tuberculosis, atypical mycobacteriosis, fungal infection, reaction to a foreign body, rheumatoid nodules, leishmaniasis, Crohn disease, and necrobiosis lipoidica diabeticorum.
The diagnosis of scar sarcoidosis is confirmed only by excluding other conditions via a comprehensive evaluation of clinical manifestations, histology, history, and radiologic and laboratory findings.
It has been suggested that the most satisfying therapy for the patient and physician in sarcoidosis is no treatment at all,4 and in fact sarcoidosis often remits spontaneously. Currently, the choice of treatment depends on the degree of systemic involvement, and the oral corticosteroid prednisone remains the first-line treatment. If the condition does not respond, the use of other systemic agents has been reported, but their effectiveness has not been evaluated in controlled clinical trials.
Recurrence is common after the suspension of treatment; therefore, treatment may need to be continued for several years, with frequent checkups.
Skin lesions are a visible clue to the diagnosis. Reactivation of old scars may be the single manifestation of cutaneous sarcoidosis, but it may also precede or accompany systemic involvement, often representing the main sign of an exacerbation or a relapse of systemic sarcoidosis, as in our patient.5
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol 2007; 25:295–302.
- Tchernev G. Cutaneous sarcoidosis: the “great imitator”: etiopathogenesis, morphology, differential diagnosis, and clinical management. Am J Clin Dermatol 2006; 7:375–382.
- Fernandez-Faith E, McDonnell J. Cutaneous sarcoidosis: differential diagnosis. Clin Dermatol 2007; 25:276–287.
- Baughman RP, Lower EE, du Bois RM. Sarcoidosis. Lancet 2003; 361:1111–1118.
- Sorabjee JS, Garje R. Reactivation of old scars: inevitably sarcoid. Postgrad Med J 2005; 81:60–61.
A 52-year-old woman is referred to our dermatology clinic by her primary care physician for swelling and redness of seven old scars. The swelling began 3 months ago.
She is on no regular medications. She has never smoked. She underwent liposuction 7 years ago and appendectomy at age 15.
Laboratory testing shows mild leukopenia, with a white blood cell count of 3.0 × 109/L (reference range 4.2–9.0). Other routine laboratory values are normal, including antinuclear antibody, extractable nuclear antibody, anti-double-stranded DNA, rheumatoid factor, urinalysis, erythrocyte sedimentation rate, C-reactive protein, serum calcium concentration, and liver and renal function tests.
Chest radiography reveals bilateral hilar lymphadenopathy.
Q: What is the next most appropriate diagnostic procedure?
- Skin biopsy
- High-resolution computed tomography (CT) of the chest
- QuantiFERON-TB Gold test
- Ventilatory function tests
- Serum angiotensin-converting enzyme (ACE) level
A: All are appropriate at this point. In this case, skin biopsy and high-resolution CT were performed. Histopathologic examination of one of the scars showed multiple well-demarcated, large, noncaseating epitheloid granulomas with histiocytes and multinucleated giant cells. High-resolution CT confirmed bilateral hilar and mediastinal lymphadenopathy and revealed micronodular densities with a bronchovascular and subpleural distribution.
An interferon-gamma-release assay for tuberculosis—QuantiFERON-TB Gold (Cellestis, Carnegie, Australia)—was negative. Ventilatory function tests showed a normal pattern, while the serum ACE level, electrocardiography, and an eye examination revealed no pathologic findings.
Q: What is the diagnosis?
- Keloids
- Scar sarcoidosis
- Paraneoplastic sign
- Dermatofibrosarcoma protuberans
- Tubercolosis
A: Based on the data outlined above, we made the diagnosis of scar sarcoidosis with involvement of hilar and mediastinal lymph nodes. The patient began systemic treatment with oral prednisone 1 mg/kg/day for 6 weeks, which was then gradually withdrawn, until the skin and hilar lesions resolved completely.
SCAR SARCOIDOSIS
Sarcoidosis is a multisystem disorder of unknown cause characterized by the formation of noncaseating granulomas in the affected organs. Patients may present with symptoms related to the specific organ affected, but they may have no symptoms or only general symptoms such as fever or general malaise.
The skin is involved in 25% of cases and presents so many polymorphous manifestations that sarcoidosis has become known as one of the “great imitators” in dermatology.1,2
Although sarcoidosis on liposuction scars has not been reported previously, the reactivation of old scars is well known on sites of previous injections, tattoos, herpes zoster, and burns.2,3
The finding of granuloma is not specific for sarcoidosis (Figure 2). The histologic differential diagnosis of sarcoidosis includes tuberculosis, atypical mycobacteriosis, fungal infection, reaction to a foreign body, rheumatoid nodules, leishmaniasis, Crohn disease, and necrobiosis lipoidica diabeticorum.
The diagnosis of scar sarcoidosis is confirmed only by excluding other conditions via a comprehensive evaluation of clinical manifestations, histology, history, and radiologic and laboratory findings.
It has been suggested that the most satisfying therapy for the patient and physician in sarcoidosis is no treatment at all,4 and in fact sarcoidosis often remits spontaneously. Currently, the choice of treatment depends on the degree of systemic involvement, and the oral corticosteroid prednisone remains the first-line treatment. If the condition does not respond, the use of other systemic agents has been reported, but their effectiveness has not been evaluated in controlled clinical trials.
Recurrence is common after the suspension of treatment; therefore, treatment may need to be continued for several years, with frequent checkups.
Skin lesions are a visible clue to the diagnosis. Reactivation of old scars may be the single manifestation of cutaneous sarcoidosis, but it may also precede or accompany systemic involvement, often representing the main sign of an exacerbation or a relapse of systemic sarcoidosis, as in our patient.5
A 52-year-old woman is referred to our dermatology clinic by her primary care physician for swelling and redness of seven old scars. The swelling began 3 months ago.
She is on no regular medications. She has never smoked. She underwent liposuction 7 years ago and appendectomy at age 15.
Laboratory testing shows mild leukopenia, with a white blood cell count of 3.0 × 109/L (reference range 4.2–9.0). Other routine laboratory values are normal, including antinuclear antibody, extractable nuclear antibody, anti-double-stranded DNA, rheumatoid factor, urinalysis, erythrocyte sedimentation rate, C-reactive protein, serum calcium concentration, and liver and renal function tests.
Chest radiography reveals bilateral hilar lymphadenopathy.
Q: What is the next most appropriate diagnostic procedure?
- Skin biopsy
- High-resolution computed tomography (CT) of the chest
- QuantiFERON-TB Gold test
- Ventilatory function tests
- Serum angiotensin-converting enzyme (ACE) level
A: All are appropriate at this point. In this case, skin biopsy and high-resolution CT were performed. Histopathologic examination of one of the scars showed multiple well-demarcated, large, noncaseating epitheloid granulomas with histiocytes and multinucleated giant cells. High-resolution CT confirmed bilateral hilar and mediastinal lymphadenopathy and revealed micronodular densities with a bronchovascular and subpleural distribution.
An interferon-gamma-release assay for tuberculosis—QuantiFERON-TB Gold (Cellestis, Carnegie, Australia)—was negative. Ventilatory function tests showed a normal pattern, while the serum ACE level, electrocardiography, and an eye examination revealed no pathologic findings.
Q: What is the diagnosis?
- Keloids
- Scar sarcoidosis
- Paraneoplastic sign
- Dermatofibrosarcoma protuberans
- Tubercolosis
A: Based on the data outlined above, we made the diagnosis of scar sarcoidosis with involvement of hilar and mediastinal lymph nodes. The patient began systemic treatment with oral prednisone 1 mg/kg/day for 6 weeks, which was then gradually withdrawn, until the skin and hilar lesions resolved completely.
SCAR SARCOIDOSIS
Sarcoidosis is a multisystem disorder of unknown cause characterized by the formation of noncaseating granulomas in the affected organs. Patients may present with symptoms related to the specific organ affected, but they may have no symptoms or only general symptoms such as fever or general malaise.
The skin is involved in 25% of cases and presents so many polymorphous manifestations that sarcoidosis has become known as one of the “great imitators” in dermatology.1,2
Although sarcoidosis on liposuction scars has not been reported previously, the reactivation of old scars is well known on sites of previous injections, tattoos, herpes zoster, and burns.2,3
The finding of granuloma is not specific for sarcoidosis (Figure 2). The histologic differential diagnosis of sarcoidosis includes tuberculosis, atypical mycobacteriosis, fungal infection, reaction to a foreign body, rheumatoid nodules, leishmaniasis, Crohn disease, and necrobiosis lipoidica diabeticorum.
The diagnosis of scar sarcoidosis is confirmed only by excluding other conditions via a comprehensive evaluation of clinical manifestations, histology, history, and radiologic and laboratory findings.
It has been suggested that the most satisfying therapy for the patient and physician in sarcoidosis is no treatment at all,4 and in fact sarcoidosis often remits spontaneously. Currently, the choice of treatment depends on the degree of systemic involvement, and the oral corticosteroid prednisone remains the first-line treatment. If the condition does not respond, the use of other systemic agents has been reported, but their effectiveness has not been evaluated in controlled clinical trials.
Recurrence is common after the suspension of treatment; therefore, treatment may need to be continued for several years, with frequent checkups.
Skin lesions are a visible clue to the diagnosis. Reactivation of old scars may be the single manifestation of cutaneous sarcoidosis, but it may also precede or accompany systemic involvement, often representing the main sign of an exacerbation or a relapse of systemic sarcoidosis, as in our patient.5
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol 2007; 25:295–302.
- Tchernev G. Cutaneous sarcoidosis: the “great imitator”: etiopathogenesis, morphology, differential diagnosis, and clinical management. Am J Clin Dermatol 2006; 7:375–382.
- Fernandez-Faith E, McDonnell J. Cutaneous sarcoidosis: differential diagnosis. Clin Dermatol 2007; 25:276–287.
- Baughman RP, Lower EE, du Bois RM. Sarcoidosis. Lancet 2003; 361:1111–1118.
- Sorabjee JS, Garje R. Reactivation of old scars: inevitably sarcoid. Postgrad Med J 2005; 81:60–61.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol 2007; 25:295–302.
- Tchernev G. Cutaneous sarcoidosis: the “great imitator”: etiopathogenesis, morphology, differential diagnosis, and clinical management. Am J Clin Dermatol 2006; 7:375–382.
- Fernandez-Faith E, McDonnell J. Cutaneous sarcoidosis: differential diagnosis. Clin Dermatol 2007; 25:276–287.
- Baughman RP, Lower EE, du Bois RM. Sarcoidosis. Lancet 2003; 361:1111–1118.
- Sorabjee JS, Garje R. Reactivation of old scars: inevitably sarcoid. Postgrad Med J 2005; 81:60–61.