User login
- Engage patients in shared decision making by discussing the benefits and risks of prostate cancer screening. Patients who review educational pamphlets before an office visit engage more fully in the decision-making process. (B)
- If performing prostate cancer screening, limit to men with greater than 10 years life expectancy. (B)
- Because the lead time of a diagnosis based on PSA screening is estimated to be 5 to 7 years, PSA screening every other year is unlikely to cause a loss of sensitivity. (B)
- Men with tumors with a Gleason score less than 5 are the best candidates for “watchful waiting,” having a favorable 20-year survival. (B)
Prostate cancer screening in asymptomatic men remains controversial, and it is difficult to present its benefits and risks quickly in a way that is understandable to patients. Yet many expert groups agree that physicians should enter into a mutual decision-making process with patients.1-3
This article reviews the latest information relevant to the controversy, offers “talking points” for family physicians to use when discussing screening with patients, and lists websites that patients may find helpful when making a decision about prostate cancer screening.
For this review, we searched for recent articles that are generalizable to a primary care population and of the highest evidence level available. We preferentially discuss population-based studies, studies from randomized trials of screening, and meta-analyses, rather than results that are hospital- or clinic-based. For a complete systematic review of this topic (from 2002), readers are referred to one conducted for the Agency for Healthcare Research and Quality.4 (See Scope of the problem).
Adenocarcinoma of the prostate is a significant public health burden. Age, family history, and race are the only known risk factors. Most cancers (86%) are diagnosed while still confined to the prostate; however, invasion beyond the capsule is sometimes not apparent until surgery.
Incidence. In 2005, there will be approximately 232,090 new cases of prostate cancer.5 American men have a 17% chance of being diagnosed with prostate cancer; African Americans have a 65% greater risk of developing prostate cancer than Caucasians.6 In fact, African Americans have the highest prostate cancer annual age-adjusted incidence rates in the world: 272/100,000 compared with 164/100,000 for Caucasian Americans.6 The rate for US Asian/Pacific Islander and Hispanic men is less than that for Caucasian Americans.
Mortality. There will be approximately 30,350 prostate cancer deaths in 2004.5 There is 3% chance of dying from prostate cancer; however, the risk of death is about 55% higher for African American men than Caucasian American men.6
Risk factors other than race. Risk of prostate cancer diagnosis increases with age: 1 in 48 men aged 40 to 59 years will be diagnosed with prostate cancer, while 1 in 8 men aged 60 to 79 years are at risk.7 A man who has a first-degree relative with prostate cancer is 2.4 times as likely to be diagnosed with prostate cancer as a man with no affected relatives.8
Key components of the controversy
How effective is screening?
A good screening test does 2 things. First, it detects a disease earlier than it would be detected with no screening at all, and it does so with sufficient accuracy to avoid a large number of false-positive and false-negative results.
Second, it leads to treatment of early disease that will likely produce a more favorable health outcome than waiting to treat patients who have signs and symptoms of disease.9 Unfortunately it is still unclear whether screening tests for prostate cancer meet these 2 criteria.
Skewed numbers. Yes, estimates of false-positive and false-negative results are available from numerous studies of different populations. However, in most studies, only men with abnormal test results receive a biopsy. Men with normal screening test results are not biopsied. Therefore the number of false negatives (and true negatives) is unknown. Furthermore, these estimates are often based on patients from urology clinics, a group more likely to have disease, thereby increasing the positive predictive value of a given screening test.
Whether current screening methods—in particular, prostate-specific antigen (PSA)—identify prostate cancers destined to become clinically relevant is also unknown. If screening does identify such cancers, a decrease in prostate cancer mortality among men who were screened is expected. If it does not, overdiagnosis and treatment of clinically insignificant cancers will negatively impact the quality of men’s lives without extending their life spans.
Clinical variability of the disease
The natural history of prostate cancer is uncertain. If cancer is left unidentified or untreated, more men will die with prostate cancer than of prostate cancer.
Clinically, prostate cancer ranges from an asymptomatic slow-growing tumor to an aggressive cancer with painful metastases. Treatment may be unnecessary at one end of the spectrum and palliative at the other.
The goal of screening is to identify slow-growing tumors destined to extend beyond the prostate while they are confined to the prostate and amenable to treatment, thereby decreasing the risk of prostate cancer morbidity and death.
Usually low morbidity. For a 50-year-old man, the risk of being diagnosed with prostate cancer by age 80 years is 15%; however, the same man has a 1.4% chance of dying of prostate cancer over that 30-year period.7 This 10-fold difference shows that prostate cancer usually is not a fatal illness. Another indication of the often benign nature of the disease is the high percentage of prostate cancers identified at surgery for bladder cancer; up to 40% of specimens contain unsuspected prostate cancer (level of evidence [LOE]: 2c).10,11
Most clinical diagnoses (80%) and prostate cancer deaths (90%) occur among men older than 65 years;6 the median age at diagnosis is 72 years.12 More than 75% of men older than 85 years will have histological prostate cancer (LOE: 2c).13 Many men live with their disease for more than 10 years, but do not die of it (LOE: 2c).12 Additionally, a review of several decision analyses indicates that men 75 years of age and older are not likely to benefit from screening and aggressive treatment (LOE: 2a).14
Hence the recommendation: if performing prostate cancer screening, limit to men with greater than 10 years life expectancy.15
Details of screening tests
Digital rectal examination insufficient
Digital rectal examination (DRE)—palpating the prostate gland to determine size and consistency—is one screening tool for prostate cancer, usually performed in conjunction with PSA testing.15 It is not a difficult or expensive test, but its reproducibility is only fair, even among experienced urologists (LOE: 2b).16
The sensitivity and specificity of DRE can only be estimated because men with a normal finding on DRE are not routinely biopsied in any studies (TABLE 1).17,18 One of two pertinent meta-analyses18 included studies that followed men with a negative DRE finding for development of prostate cancer (LOE: 2a). Both meta-analyses of DRE as a screening tool found that the included studies were heterogeneous in their study populations and definition of abnormal DRE test result (LOE: 2a). The positive predictive value (PPV) of DRE was 28% in one meta-analysis and 18% in the other.
TABLE 1
Characteristics of screening tests for prostate cancer
| TEST/SOURCE | DESCRIPTION (LEVEL OF EVIDENCE) | SN ESTIMATE (%) | SP ESTIMATE (%) | LIKELIHOOD RATIO | PPV (%) |
|---|---|---|---|---|---|
| Abnormal DRE18 | Meta-analysis of studies that performed biopsies for abnormal DRE (2a) | 59 (51%–67%) | 94 (91%–96%) | + 9.8 – 0.4 | 28 (20–36) |
| Abnormal DRE17 | Meta-analysis of studies that performed biopsies for abnormal DRE or PSA (2a) | 53.2 | 83.6 | + 3.24 – 0.56 | 17.8 |
| PSA >4 ng/mL12 | Review of multiple cohort studies with diverse populations, not systematic | Mean = 71 | Mean = 75 | + 2.8 – 0.30 | 37 |
| PSA >4 ng/mL19 | Longitudinal retrospective study of prostate cancers that developed within 2 years of PSA test (3b) | 73.2 | 85.4 | + 5.0 – 0.2 | |
| PSA >4 ng/mL20 | Nested case-control study of prostate cancers that developed within 5 years of PSA test in a cohort study (3b) | 86 | 94 | + 14.3 – 0.15 | |
| PSA > 4 ng/mL17 | Meta-analysis of studies that performed biopsies for abnormal PSA or DRE (2a) | 72.1 | 93.2 | + 10.2 – 0.30 | 25.1 |
| Sn, sensitivity; Sp, specificity; PPV, positive predictive value; | |||||
| PSA, prostate-specific antigen; DRE, digital rectal examination. | |||||
Prostate-specific antigen: Improving its clinical usefulness
Prostate-specific antigen (PSA), first detected in serum in 1979, is a protein produced by prostate epithelial cells. It was originally used to follow men treated for prostate cancer for evidence of recurrence. In the late 1980s, it became widely used in the US to screen for prostate cancer.
An elevated PSA level is suggestive but not diagnostic of prostate cancer. Elevated levels also occur with advancing age, increased prostate size, and prostatitis, and following ejaculation. Prostate manipulation such as biopsy and surgery (but not digital examination) also elevates PSA.
Customary cutpoint. An abnormal serum PSA level is commonly regarded as 4 ng/mL or greater. At this cutpoint, most studies report sensitivity for cancer of around 70%, with more variability in specificity (TABLE 1). Again, in most studies that report sensitivity and specificity, men with a PSA level less than 4 ng/mL did not undergo prostate biopsy; therefore, the number of false negatives and true negatives are only estimates.
In nested case-control studies of longitudinal cohorts, eligible cases were defined by the length of time between an abnormal PSA result and prostate cancer diagnosis, while controls were men who were not diagnosed with prostate cancer during the same time period (however, a biopsy was not usually performed). The PPV of an abnormal PSA level is estimated at 25% to 37%. As a comparison, the PPV of a positive mammogram finding for women 50 to 59 years is 4% to 9%, and 10% to 19% for women age 60 to 69.21
Suggested strategies to improve PSA accuracy. Approximately 70% of men with an elevated serum PSA level do not have cancer. To decrease the number of unnecessary biopsies, experts have suggested several strategies:
- using DRE with PSA
- calculating a ratio of free PSA (unbound to protein) or complexed PSA (bound to protein) to total PSA
- measuring PSA density, which incorporates the volume of the prostate gland and is subject to observer variability in prostate volume measurement
- recording PSA velocity, which is the annual rate of change in PSA level and requires 3 or more measurements.
Revise the cutpoint? The cutpoint of 4 ng/mL has been deemed too high by some clinicians. A recent report of men enrolled in a large prostate cancer prevention trial found that among the 9459 men receiving placebo, 2950 had a PSA level of 4 ng/mL or less and had normal prostates on DRE (LOE: 1b).22 All 2950 underwent a prostate biopsy; 449 (15.2%) were positive for cancer. Nearly 30% of those had a PSA level of 2 ng/mL or less.
Approximately 25% of tumors in men referred for biopsy because of abnormal PSA or DRE findings are thought to be discovered by chance due to the biopsy procedure and the high prevalence of disease (LOE: 2c).23 Furthermore, a recent study indicates that annual PSA fluctuation is substantial; 45% of men who initially had a value of 4 ng/mL or greater, subsequently had a normal level (LOE: 2b).24 Isolated abnormal levels should be confirmed before referral for biopsy.
Identifying clinically relevant cancers
The continued controversy around PSA screening relates to the potential over-diagnosis of localized prostate cancers that are not likely to become clinically significant. The ideal screening test would predictably identify cancers likely to progress.
Screening over-detects
Several computer simulations have estimated the amount of over-detection of prostate cancer. The National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) registry data demonstrated that the proportion of prostate cancer found through PSA testing that otherwise would not have been diagnosed in the patient’s lifetime was 29% for white men and 44% for black men (LOE: 2c).25
An Italian study found that the proportional excess of cancers detected by screening over those that would have been expected in the absence of screening is greater than 50% (LOE: 2c).26
Finally, a model based on results from the European randomized study of prostate cancer screening estimated that a screening program with a 4-year interval from age 55 to 67 has an over-detection rate of 48% (cancers that would not have been diagnosed in the absence of screening) (LOE: 2c).27 Additionally, the authors determined that such screening might advance prostate cancer diagnosis by at least 10 years. Previous estimates of diagnosis lead time have been in the 5- to 7-year range (LOE: 2b).28 These findings indicate that screening intervals of 2 to 4 years are unlikely to cause a loss of sensitivity.
Spread of tumor
Spread of the tumor beyond the prostate capsule is a poor prognostic sign.29 Unfortunately, this occurrence is often not known until surgery, and the result usually is an “up-staging” between clinical diagnosis and pathological diagnosis. Nevertheless, most cancers (86%) diagnosed between 1992 and 1999 were localized to the prostate.6 Because these cancers have not spread beyond the prostate at the time of diagnosis, they are more likely to be curable. They are also more likely to represent tumors that may grow so slowly that the host will die of something other than prostate cancer.
Gleason score
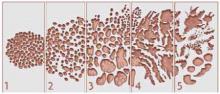
Gleason score is another predictor of cancers destined to become clinically relevant (FIGURE 1). A Gleason score of 7 or greater denotes moderate to poor cellular differentiation and indicates a greater potential for progression than lower Gleason values.29 A recent long-term follow-up report on a cohort of men with localized cancer treated conservatively demonstrated that men with low-grade tumors (Gleason score 2–4) have a minimal risk of dying from prostate cancer after 20 years, while men with high-grade tumors (Gleason score of 8–10) have high probability of prostate cancer death within 10 years of diagnosis (LOE: 2b).30 Ecological31 and clinical studies32 indicate that a substantial proportion of PSA-detected cancers are moderately differentiated. This is especially true in a first round of screening; as in the European randomized study of screening, where 36% of cancers were Gleason score 7 or higher (LOE: 1b).33
FIGURE 1
Calculating the Gleason score
The Gleason score is based on the level of differentiation and growth pattern of prostate cancer cells. Cancer cells that closely resemble the normal prostate cells when viewed under low-power magnification are well differentiated. Cancer cells that do not retain the structure of the surrounding normal cells are poorly differentiated. Scores range from 1 to 5.
In examining histologic samples of a patient’s prostate tissue, the pathologist will identify the 2 most commonly occurring patterns (types of differentiation) among the cancer cells and assign a numerical value to each pattern. The 2 numbers are then added to yield the final Gleason score. If a single pattern dominates, the pathologist will simply double the corresponding value.
Total scores range from 2 to 10. Scores in the range of 2–4 are considered well-differentiated, 5–7 are moderately differentiated, and 8–10 are poorly differentiated. In general, the higher the score, the worse the prognosis. Men with well-differentiated tumors that are treated conservatively have minimal risk of dying from prostate cancer.
Is declining mortality a sign of screening success?
Prostate cancer mortality has been declining since the mid-1990s in numerous parts of the world; the US,6,34 Canada,35 Australia,36 and the United Kingdom37 have all reported a reduction in the rate of prostate cancer deaths. Advocates of PSA screening point to this trend as evidence of the effectiveness of screening. But such ecological data are difficult to interpret. For instance, although much less PSA screening is performed in the UK, mortality trends are similar to those in the US where PSA testing has been used more widely.38
Aggressive screening not necessarily the reason. In the US, 2 geographic areas—Seattle, Washington and Connecticut—provided a natural experiment to compare the effect of aggressive screening on prostate cancer mortality (LOE: 2c).39 Although more aggressive screening and treatment took place in the Seattle area, prostate cancer mortality rates were similar to those in Connecticut over 11 years of follow-up. Similarly, in a study in British Columbia, prostate cancer mortality from 1985 to 1999 was not associated with the intensity of PSA screening (LOE: 2c).40
Other possible explanations. If the mortality decrease is not related to PSA screening, what could cause it? One explanation is “attribution bias.” Death certificate misattribution of cause of death from prostate cancer may partially explain the pattern of increasing, then decreasing mortality rates (LOE: 2c).41 Improvement in prostate cancer treatment, especially for advanced stage, and in particular hormone therapy, is another possible explanation for the decreasing prostate cancer mortality (LOE: 2c).14,42
Benefits of screening
The benefit of any effective screening test is a decrease in the risk of the screened-disease mortality. The best way to demonstrate decreased risk is through a randomized controlled study of the screening test, and 2 such trials are underway for prostate cancer. In the meantime, a decision model estimates that aggressive treatment of organ-confined disease potentially adds 3 years of life for men in their fifties, 1.5 years for men in their sixties, and 0.4 years for men in their seventies (LOE: 2c).3
Others have concluded that 25 men with clinically detected prostate cancer would need to be treated with surgery to prevent 1 prostate cancer death during a 6-year period, without evidence that quality of life is improved (LOE: 2c).43
Consider quality of life. With uncertainty surrounding improvement in the quantity of life as a result of prostate cancer screening, improved quality of life may be an issue for patients. Focus group research has demonstrated that some patients believe it is better to know if a cancer is present than to wonder if it will be diagnosed when it is too late for cure.44
General quality of life has been found to be similar among men treated for prostate cancer and age-matched controls without prostate cancer; however, urinary, sexual, and bowel function vary substantially between treated and untreated men and by treatment type (LOE: 3b) (TABLE 2).45,46 In general, men treated with radical prostatectomy and brachytherapy often report better general quality of life than men who undergo radiation treatment, despite having more urinary and sexual problems (LOE: 2b).47,48
TABLE 2
Estimates of risk associated with specific prostate cancer treatments 12 months or more after treatment
| TREATMENT OUTCOMES | RADICAL PROSTATECTOMY (%) | EXTERNAL BEAM RADIATION (%) | BRACHY-THERAPY* (%) | ANDROGEN DEPRIVATION THERAPY (%) | UNTREATED (%) |
|---|---|---|---|---|---|
| Death within 2 months of treatment | 0.5–0.7 | 0.2–0.5 | 0.2–0.5 | ||
| Urinary problems: | |||||
| Incontinence | 10–50 | 2–16 | 6–16 | ||
| Wearing pads | 5–32 | 2–12 | 2–16 | ||
| Urinary bother† | 4–20 | 3–15 | 3–16 | ||
| Sexual problems: | |||||
| Impotence | 50–80‡ | 30–60 | 20–60** | 70–92 | 20–50 |
| Sexual bother | 10–40 | 10–30 | 10–18 | 25–38 | 10–32 |
| Bowel problems: | |||||
| Bowel problems§ | 9–15 | 6–35 | 4–20 | ||
| Loose stools/diarrhea | 15–21 | 6–37 | 4–10 | ||
| Bowel bother | 1–3 | 4–12 | 2–10 | ||
| Other symptoms | Breast swelling: 5–25 | ||||
| Hot flashes: 50–60 | |||||
| * Fewer studies on brachytherapy are available, especially those with long-term follow-up; therefore, these findings are less certain than other entries. | |||||
| ‡ Includes nerve-sparing prostatectomy. | |||||
| † EBRT and brachytherapy patients are more likely to experience irritative voiding symptoms (i.e. dysuria, urgency and hesitancy and noctoria), while RP patients are more likely to experience incontinence. | |||||
| ** Impotence risk gradually increases with time after treatment. | |||||
| § Includes symptoms such as painful bowel movement and urgency | |||||
| Sources:references 14, 50–53, 65–71. | |||||
Harms of screening
The chances of undergoing a biopsy based on an abnormal screening PSA are estimated at 15% to 40% depending on the patient’s age (FIGURE 2).3 There are adverse effects associated with transrectal biopsy of the prostate. In 2 large population-basedstudies of screening, the most frequent complications were hematuria and hematospermia (LOE: 1b, 2b) (TABLE 3), with more serious consequences such as sepsis and hospitalization occurring in fewer than 1% of patients. A study of 100 screened men with an abnormal PSA who underwent prostate biopsy found that although 69% felt moderate to severe pain with the biopsy, 80% would be willing to undergo a repeat biopsy (LOE: 1b).49
Treatment options. If the biopsy result is positive, the most common treatment options for localized cancer—which represents over 80% of all prostate cancers diagnosed6—include radical prostatectomy, external beam radiation therapy, brachytherapy (internal radiation therapy) or expectant management (watchful waiting). Population-based studies have reported outcomes for these treatment options (TABLE 2). Outcomes derived from hospital-based series of other prostate cancer treatments, such as cryotherapy and 3-dimensional radiation, are available, but the estimates often reflect the experience of only a few hospitals and are not representative of other facilities. Androgen ablation is the standard treatment for metastatic prostate cancer.
Untoward effects of treatment. Approximately 60% of radical prostatectomy patients report some incontinence 12 months or more after surgery (LOE: 2b),50,51 and about 30% of patients need to wear pads for urine leakage (LOE: 2b).50-53 Men undergoing radiation therapy have less urinary incontinence, but about 30% complain of diarrhea and loose stools (LOE: 2b).51,52 Both therapies are associated with a high percentage of erectile dysfunction: approximately 60% of radiation therapy patients and 75% of surgery patients report their erections are not firm enough for intercourse (LOE: 2b).51,52
Expectant management (following the cancer with regular PSA and ultrasound testing) is sometimes difficult to “sell” to patients whose fear of cancer dictates that the only logical response is to “cut it out.”44 A recent randomized trial indicated that radical prostatectomy lowers prostate cancer mortality, local progression, distant metastasis, and overall survival as compared with watchful waiting over a median of 8.2 years of follow-up (LOE: 1b).54 However, these results may have little relevance to prostate cancer screening since only 5% of the cancers were screen-detected and 76% were palpable.
FIGURE 2
Yield of screening 1000 men for prostate cancer
TABLE 3
Percentage of patients with specific complication of transrectal prostate biopsy
| CONDITION | TYROL STUDY63 | EUROPEAN RANDOMIZED STUDY OF SCREENING 64 |
|---|---|---|
| Gross hematuria >1 day | 12.5% | 22.6% |
| Hematospermia | 29.8% | 50.4% |
| Significant pain | 4.0% | 7.5% |
| Rectal bleeding | 0.6% | 1.3% |
| Nausea | 0.8% | 0.3% |
| Fever >38.5°C | 0.8% | 3.5% |
| Epididymitis | 0.7% | 0.07% |
| Sepsis | 0.3% | Not available |
| Hospitalization | Not available | 0.5% |
| Tyrol study63: LOE: 2b, N=6024 biopsies; ERSS study64: LOE: 1b, N=5802 biopsies. | ||
Recommendations from expert groups
Different expert groups have conflicting recommendations. Both the American Urological Association and the American Cancer Society recommend annual PSA screening starting at age 50 for most men; younger if risk factors are present. Groups that are evidence based tend to recommend a shared decision making process with patients. The AAFP and American College of Physicians advise physicians to counsel men on the known risks and uncertain benefits of screening for prostate cancer. The US Preventive Services Task Force 2002 update concluded that evidence is insufficient to recommend for or against routine screening for prostate cancer using PSA or DRE. The National Cancer Institute cites a lack of evidence to determine a net benefit for PSA or DRE screening.
When will we know more?
Only 1 randomized controlled trial of prostate cancer screening has been completed55: 46,193 men were randomized to either PSA and DRE or no screening from 1989 to 1996. The study had methodological problems; for instance, only 23% of the group randomized to screening was screened. The investigators in the trial have interpreted its results as demonstrating a decrease in prostate cancer deaths in the screened group compared with the unscreened group (15 vs 48.7 per 100,000 man-years).55 Others have criticized the statistical analysis and calculated the results using an “intent to screen” analysis, finding no difference in prostate cancer deaths between the 2 groups.3,56
Two randomized controlled trials of screening are ongoing: the National Cancer Institute’s Prostate, Lung, Colon, Ovarian (PLCO) Screening Trial57 and the European Randomized Study of Screening for Prostate Cancer.58 Both were started in the mid-1990s and will not have results available for a few more years. Also underway is a randomized trial of intervention (radical prostatectomy) versus expectant management, called the Prostate Cancer Intervention Versus Observation Trial (PIVOT).59
Counseling recommendations
However, providing men with information on prostate cancer screening before they discussed it with their family physician, rather than after the visit, resulted in patients having a significantly more active role in making a screening decision, and lower levels of decisional conflict (LOE: 2b).61 Informational pamphlets are available through the AAFP and CDC websites listed in TABLE 4. Additional websites containing prostate cancer screening information are found in TABLE 4. We also provide a bullet item list of key points for discussion with patients (TABLE 5), which can be used along with the balance sheet provided here (TABLE 2).
Shared decision-making is not an easy or quick process. Yet, the majority of patients will benefit from the discussion, regardless of the final decision. Of course, there are instances when a shared decision-making process is well-documented, and still results in an undesirable outcome;62 however, while the evidence for screening remains controversial, patients have the right to know that those controversies exist and why they exist.
TABLE 4
Useful websites for patients to find prostate cancer screening information
| CENTERS FOR DISEASE CONTROL AND PREVENTION |
| www.cdc.gov/cancer/prostate/decisionguide/index.htm |
| 10th grade reading level* |
| Good coverage of screening and treatment controversies |
| Offers downloadable PDF version |
| NATIONAL CANCER INSTITUTE |
| cis.nci.nih.gov/asp/FactSheetPub/AlphaSubList.asp?alpha=47 |
| 10th grade reading level |
| FAQ format |
| Offers Spanish version |
| AMERICAN CANCER SOCIETY |
| www.cancer.org/docroot/CRI/content/CRI_2_4_3X_Can_prostate_ cancer_be_found_early_36.asp |
| 12th grade reading level |
| Lacks discussion of treatment options and their side effects |
| Biased in favor of screening but acknowledges that other distinguished organizations are not |
| AMERICAN UROLOGICAL ASSOCIATION |
| www.urologyhealth.org/adult/index.cfm?cat=09 |
| 12th grade reading level |
| Easy to navigate among screening and specific treatment pages |
| Biased in favor of PSA screening |
| AMERICAN ACADEMY OF FAMILY PHYSICIANS |
| familydoctor.org/healthfacts/361/ |
| 11th grade reading level |
| Question/answer format |
| Very straightforward, lacks depth |
| www.aafp.org/x19519.xml |
| 7th grade reading level |
| Separate information sheet for patients and physicians |
| Presents possible outcomes of PSA test and prostate cancer treatment in easy-to-follow format |
| WEBMD |
| my.webmd.com/medical_information/condition_centers/prostate_cancer/default.htm |
| 9th grade reading level |
| Question/answer format |
| Specifically addresses false negative and positives with current estimates |
| DARTMOUTH CENTER FOR SHARED DECISION MAKING |
| www.dhmc.org/dhmc-internet-upload/file_collection/PSA.pdf |
| 6th grade reading level |
| Well-designed, simple presentation of pros and cons of PSA testing |
| *Fleish-Kincaid grade level score based on average sentence length and average number of syllables per word. |
TABLE 5
Talking points for patients and physicians
| Prostate cancer is an important men’s health problem |
| Screening may prevent early prostate cancer death |
| DRE alone has little value as a screening test |
| Age, prostate size, prostatitis, ejaculation, prostate biopsy, and prostate surgery can cause a falsely elevated PSA test |
| Approximately 70% of men with an elevated serum PSA do not have cancer |
| The percentage of PSA screening false negatives ranges from 10%–22% in large studies |
| If the test is abnormal, a biopsy will be recommended |
| If the biopsy is positive, treatment options will be given |
| Many men experience long-term urinary incontinence and impotence related to their treatment |
CORRESPONDING AUTHOR
Kendra Schwartz, MD, MSPH, 101 E. Alexandrine, Detroit, MI 48201, E-mail: kensch@med.wayne.edu
1. American Academy of Family Physicians. Summary of recommendation for periodic health examinations. August 2002. Available at: www.aafp.org/PreBuilt/PHERev5.30802.pdf. Accessed on June 10, 2005.
2. US Preventive Services Task Force. Screening for Prostate Cancer. 2002. Available at: www.ahrq.gov/clinic/uspstf/uspsprca.htm. Accessed on June 10, 2005.
3. American College of Physicians. Screening for prostate cancer. Position paper. Clinical Guideline: Part III. Ann Intern Med 1997;126:480-484.
4. Harris R, Lohr K, Beck R, Fink K, Godley P, Bunton A. Screening for prostate cancer. Systematic Evidence Review for AHRQ. Available at: www.ahrq.gov/uspstfix.htm. Accessed on June 10, 2005.
5. American Cancer Society. Cancer statistics 2005. CA Cancer J Clin 2005;55:10-30.
6. Ries L, Eisner M, Kosary C, et al. SEER Cancer Statistics Review, 1975–2000. Bethesda, Md: National Cancer Institute; 2003. Available at seer.cancer.gov/csr/1975_2002/. Accessed on June 10, 2005.
7. DevCan: Probability of dying of cancer [computer program]. Version 5.1. Bethesda, Md: National Cancer Instititute; 2003. Available at: srab.cancer.gov/devcan/. Accessed on June 10, 2005.
8. Neal DE, Leung HY, Powell PH, Hamdy FC, Donovan JL. Unanswered questions in screening for prostate cancer. Eur J Cancer 2000;36:1316-1321.
9. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Alexandria, Va: International Medical Publishing; 1996.
10. Kabalin J, McNeal J, Price H, Freiha F, Stamey T. Unsuspected adenocarcinoma of the prostate in patients undergooing cystoprostatectomy for other causes: incidence, histology and morphometric observations. J Urol 1989;141:1091-1094.
11. Montie J, Wood DJ, Pontes J, Boyett J, Levin H. Adenocarcinoma of the prostate in cystoprostatectomy specimens removed for bladder cancer. Cancer 1989;63:381-385.
12. Bunting PS. Screening for prostate cancer with prostate-specific antigen: beware the biases. Clin Chim Acta 2002;315:71-97.
13. Gronberg H. Prostate cancer epidemiology. Lancet 2003;361:859-864.
14. Harris R, Lohr KN. Screening for prostate cancer: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137:917-929.
15. American Cancer Society. ACS Cancer Detection Guidelines. Available at: www.cancer.org/docroot/PED/content/PED_2_3X_ACS_Cancer_Detection_Guidelines _36.asp. Accessed June 10, 2005.
16. Smith D, Catalona W. Interexaminer variability of digital rectal examination in detecting prostate cancer. Urology 1995;45:70-74.
17. Kishor M, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract 2003;16:95-101.
18. Hoogendam A, Buntix F, deVet HCW. The diagnostic value of digital rectal examination in primary care screening for prostate cancer: a meta-analysis. Fam Pract 1999;16:621-626.
19. Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA 1995;273:289-294.
20. Hakama M, Stenman UH, Aromaa A, Leinonen J, Hakulinen T, Knekt I. Validity of the prostate specific antigen test for prostate cancer screening: followup study with a bank of 21,000 sera in Finland. J Urol 2001;166:2189-2191.
21. Humphrey L, Helfand M, Chan B, Woolf S. Breast cancer screening: summary of the evidence. Ann Intern Med 2002;137:344-346.
22. Thompson I, Pauler D, Goodman P, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level <4 ng per milliliter. N Engl J Med 2004;350:2239-2246.
23. Collins MM, Ransohoff D, MJ Barry. Early detection of prostate cancer-serendipity strikes again. JAMA 1997;278:1516-1519.
24. Eastham J, Riedel E, Scardino P, et al. Variation of serum prostate-specific antigen levels. An evaluation of year-to-year fluctuations. JAMA 2003;289:2695-2700.
25. Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst 2002;94:981-990.
26. Zappa M, Ciatto S, Bonardi R, Mazzotta A. Overdiagnosis of prostate carcinoma by screening: an estimate based on the results of the Florence Screening Pilot Study. Ann Oncol 1998;9:1297-1300.
27. Draisma G, Boer R, Otto S, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European randomized study of screening for prostate cancer. J Natl Cancer Inst 2003;95:868-878.
28. Auvin A, Maattanen L, Stenman UH, et al. Lead-time in prostate cancer screening (Finland). Cancer Causes Control 2002;13:279-285.
29. Gleason D, Mellinger G. Group at VACUR. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 1974;111:58-64.
30. Albertson PC, Hanley JA, Fine J. 20-Year outcomes following conservative management of clinically localized prostate cancer. JAMA 2005;293:2095-2101.
31. Schwartz K, Grignon D, Sakr W, Wood DJ. Prostate cancer histologic trends in the metropolitan Detroit are, 1982 to 1996. Urology 1999;53:769-774.
32. Smith D, Catalona W. The nature of prostate cancer detected through prostate specific antigen based screening. J Urol 1994;152:1732-1736.
33. Hoedemaeker RF, van der Kwast T, Boer R, et al. Pathological features of prostate cancer found at population-based screening with a four-year interval. J Natl Cancer Inst 2001;93:1153-1158.
34. Chu KC, Tarone RE, Freeman HP. Trends in prostate cancer mortality among black men and white men in the United States. Cancer 2003;97:1507-1516.
35. National Cancer Institute of Canada. Canadian cancer statistics 2001. Toronto: National Cancer Institute of Canada; 2001. Available at: www.ncic.cancer.ca. Accessed June 10, 2005.
36. Coory M, Baade P. Mortality from prostate cancer is decreasing. Med J Aust 2002;176:345-345.
37. Majeed A, Babb P, Jones J, Quinn M. Trends in prostate cancer incidence, mortality and survival in England and Wales, 1971–1998. BJU Int 2000;85:1058-1062.
38. Oliver S, Gunnell D, Donovan J. Comparison of trends in prostate-cancer mortality in England and Wales and the USA. Lancet 2000;355:1788-1789.
39. Lu-Yao G, Albertsen PC, Stanford JL, Stukel TA, Walker-Corkery ES, Barry MJ. Natural experiment examining impact of aggressive screening and treatment on prostate cancer mortality in two fixed cohorts from Seattle area and Connecticut. BMJ 2002;325:740.-
40. Coldman A, Phillips N, Pickles T. Trends in prostate cancer incidence and mortality: an analysis of mortality change by screening intensity. CMAJ 2003;168:31-35.
41. Feuer EJ, Merrill RM, Hankey BF. Cancer surveillance series: interpreting trends in prostate cancer—part II: Cause of death misclassification and the recent rise and fall in prostate cancer mortality. J Natl Cancer Inst 1999;91:1025-1032.
42. Frankel S, Smith GD, Donovan J, Neal D. Screening for prostate cancer. Lancet 2003;361:1122-1128.
43. Partin MR, Wilt TJ. Informing patients about prostate cancer screening: identifying and meeting the challenges while the evidence remains uncertain. Am J Med 2002;113:691-693.
44. McFall SL, Hamm RM. Interpretation of prostate cancer screening events and outcomes: a focus group study. Patient Educ Couns 2003;49:207-218.
45. Litwin MS, Hays RD, Fink A, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA 1995;273:129-135.
46. Penson DF, Litwin MS. Quality of life after treatment for prostate cancer. Curr Urol Rep 2003;4:185-195.
47. Lubeck DP, Litwin MS, Henning JM, Stoddard ML, Flanders SC, Carroll PR. Changes in the health-related quality of life in the first year after treatment for prostate cancer: results from CaPSURE. Urology 1999;53:180-186.
48. Bacon C, Giovannucci E, Testa M, Kawachi I. The impact of cancer treatment on quality of life outcomes for patients with localized prostate cancer. J Urol 2001;166:1804-1810.
49. Makinen T, Auvinen A, Hakama M, Stenman UH, Tammela TL. Acceptability and complications of prostate biopsy in population-based PSA screening versus routine clinical practice: a prospective, controlled study. Urology 2002;60:846-850.
50. Fowler FJ, Jr, Barry MJ, Lu-Yao G, Roman A, Wasson J, Wennberg JE. Patient-reported complications and follow-up treatment after radical prostatectomy. The National Medicare Experience: 1988-1990 (updated June 1993). Urology 1993;42:622-629.
51. Schwartz K, Bunner S, Bearer R, Severson RK. Complications from treatment for prostate carcinoma among men in the Detroit area. Cancer 2002;95:82-89.
52. Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst 2000;92:1582-1592.
53. Sebesta M, Cespedes RD, Luhman E, Optenberg S, Thompson IM. Questionnaire-based outcomes of urinary incontinence and satisfaction rates after radical prostatectomy in a national study population. Urology 2002;60:1055-1058.
54. Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005;352:1977-1184.
55. Labrie F, Candas B, Dupont A, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate 1999;38:83-91.
56. Alexander FE, Prescott RJ. Reply to Labrie et al. Results of the mortality analysis of the Quebec randomized controlled trial (RCT). Prostate 1999;40:135-137.
57. Prorok P, Andriole G, Bresalier R, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials 2000;21:273S-309S.
58. Standaert B, Denis L. The European Randomized Study of Screening for Prostate Cancer: an update. Cancer 1997;80:1830-1834.
59. Wilt TJ, Brawer M. The Prostate Cancer Intervention Versus Observation Trial: a randomized trial comparing radical prostatectomy versus expectant management for the treatment of clinically localized prostate cancer. J Urol 1994;152:1910-1914.
60. Schapira M, VanRuiswyk J. The effect of an illustrated pamphlet decision-aid on the use of prostate cancer screening tests. J Fam Pract 2000;49:418-424.
61. Davison B, Kirk P, Degner L, Hassard T. Information and patient participation in screening for prostate cancer. Patient Educ Couns 1999;37:255-263.
62. Merenstein D. Winners and losers. JAMA 2004;291:15-16.
63. Horninger W, Berger A, Pelzer A, et al. Screening for prostate cancer: updated experience from the Tyrol study. Current Urol Reports 2004;5:220-225.
64. Raaijmakers R, Kirkels WJ, Roobol MJ, Wildhagen MF, Schrder FH. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology 2002;60:826-830.
65. Fowler F, Barry MJ, Lu-Yao G, Wasson J, Bin L. Outcomes of external beam radiation therapy for prostate cancer: a study of Medicare beneficiaries in three Surveillance, Epidemiology, and End Results areas. J Clin Oncol 1996;14:2258-2265.
66. Hollenbeck BK, Dunn RL, Wei JT, Sandler HM, Sanda MG. Sexual health recovery after prostatectomy, external radiation, or brachytherapy for early stage prostate cancer. Curr Urol Rep 2004;5:212-219.
67. Potosky AL, Knopf K, Clegg LX, et al. Quality-of-life outcomes after primary androgen deprivation therapy: results from the Prostate Cancer Outcomes Study. J Clin Oncol 2001;19:3750-3757.
68. Lee R, Penson DF. Treatment outcomes in localized prostate cancer: a patient-oriented approach. Semin Urol Oncol 2002;20:63-73.
69. Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA 2000;283:354-360.
70. Robinson JW, Moritz S, Fung T. Meta-analysis of rates of erectile function after treatment of localized prostate carcinoma. Int J Radiat Oncol Biol Phys 2002;54:1063-1068.
71. Coley CM, Barry MJ, Fleming C, Fahs MC, Mulley AG. Early detection of prostate cancer. Part II: Estimating the risks, benefits, and costs. American College of Physicians. Ann Intern Med 1997;126:468-479.
- Engage patients in shared decision making by discussing the benefits and risks of prostate cancer screening. Patients who review educational pamphlets before an office visit engage more fully in the decision-making process. (B)
- If performing prostate cancer screening, limit to men with greater than 10 years life expectancy. (B)
- Because the lead time of a diagnosis based on PSA screening is estimated to be 5 to 7 years, PSA screening every other year is unlikely to cause a loss of sensitivity. (B)
- Men with tumors with a Gleason score less than 5 are the best candidates for “watchful waiting,” having a favorable 20-year survival. (B)
Prostate cancer screening in asymptomatic men remains controversial, and it is difficult to present its benefits and risks quickly in a way that is understandable to patients. Yet many expert groups agree that physicians should enter into a mutual decision-making process with patients.1-3
This article reviews the latest information relevant to the controversy, offers “talking points” for family physicians to use when discussing screening with patients, and lists websites that patients may find helpful when making a decision about prostate cancer screening.
For this review, we searched for recent articles that are generalizable to a primary care population and of the highest evidence level available. We preferentially discuss population-based studies, studies from randomized trials of screening, and meta-analyses, rather than results that are hospital- or clinic-based. For a complete systematic review of this topic (from 2002), readers are referred to one conducted for the Agency for Healthcare Research and Quality.4 (See Scope of the problem).
Adenocarcinoma of the prostate is a significant public health burden. Age, family history, and race are the only known risk factors. Most cancers (86%) are diagnosed while still confined to the prostate; however, invasion beyond the capsule is sometimes not apparent until surgery.
Incidence. In 2005, there will be approximately 232,090 new cases of prostate cancer.5 American men have a 17% chance of being diagnosed with prostate cancer; African Americans have a 65% greater risk of developing prostate cancer than Caucasians.6 In fact, African Americans have the highest prostate cancer annual age-adjusted incidence rates in the world: 272/100,000 compared with 164/100,000 for Caucasian Americans.6 The rate for US Asian/Pacific Islander and Hispanic men is less than that for Caucasian Americans.
Mortality. There will be approximately 30,350 prostate cancer deaths in 2004.5 There is 3% chance of dying from prostate cancer; however, the risk of death is about 55% higher for African American men than Caucasian American men.6
Risk factors other than race. Risk of prostate cancer diagnosis increases with age: 1 in 48 men aged 40 to 59 years will be diagnosed with prostate cancer, while 1 in 8 men aged 60 to 79 years are at risk.7 A man who has a first-degree relative with prostate cancer is 2.4 times as likely to be diagnosed with prostate cancer as a man with no affected relatives.8
Key components of the controversy
How effective is screening?
A good screening test does 2 things. First, it detects a disease earlier than it would be detected with no screening at all, and it does so with sufficient accuracy to avoid a large number of false-positive and false-negative results.
Second, it leads to treatment of early disease that will likely produce a more favorable health outcome than waiting to treat patients who have signs and symptoms of disease.9 Unfortunately it is still unclear whether screening tests for prostate cancer meet these 2 criteria.
Skewed numbers. Yes, estimates of false-positive and false-negative results are available from numerous studies of different populations. However, in most studies, only men with abnormal test results receive a biopsy. Men with normal screening test results are not biopsied. Therefore the number of false negatives (and true negatives) is unknown. Furthermore, these estimates are often based on patients from urology clinics, a group more likely to have disease, thereby increasing the positive predictive value of a given screening test.
Whether current screening methods—in particular, prostate-specific antigen (PSA)—identify prostate cancers destined to become clinically relevant is also unknown. If screening does identify such cancers, a decrease in prostate cancer mortality among men who were screened is expected. If it does not, overdiagnosis and treatment of clinically insignificant cancers will negatively impact the quality of men’s lives without extending their life spans.
Clinical variability of the disease
The natural history of prostate cancer is uncertain. If cancer is left unidentified or untreated, more men will die with prostate cancer than of prostate cancer.
Clinically, prostate cancer ranges from an asymptomatic slow-growing tumor to an aggressive cancer with painful metastases. Treatment may be unnecessary at one end of the spectrum and palliative at the other.
The goal of screening is to identify slow-growing tumors destined to extend beyond the prostate while they are confined to the prostate and amenable to treatment, thereby decreasing the risk of prostate cancer morbidity and death.
Usually low morbidity. For a 50-year-old man, the risk of being diagnosed with prostate cancer by age 80 years is 15%; however, the same man has a 1.4% chance of dying of prostate cancer over that 30-year period.7 This 10-fold difference shows that prostate cancer usually is not a fatal illness. Another indication of the often benign nature of the disease is the high percentage of prostate cancers identified at surgery for bladder cancer; up to 40% of specimens contain unsuspected prostate cancer (level of evidence [LOE]: 2c).10,11
Most clinical diagnoses (80%) and prostate cancer deaths (90%) occur among men older than 65 years;6 the median age at diagnosis is 72 years.12 More than 75% of men older than 85 years will have histological prostate cancer (LOE: 2c).13 Many men live with their disease for more than 10 years, but do not die of it (LOE: 2c).12 Additionally, a review of several decision analyses indicates that men 75 years of age and older are not likely to benefit from screening and aggressive treatment (LOE: 2a).14
Hence the recommendation: if performing prostate cancer screening, limit to men with greater than 10 years life expectancy.15
Details of screening tests
Digital rectal examination insufficient
Digital rectal examination (DRE)—palpating the prostate gland to determine size and consistency—is one screening tool for prostate cancer, usually performed in conjunction with PSA testing.15 It is not a difficult or expensive test, but its reproducibility is only fair, even among experienced urologists (LOE: 2b).16
The sensitivity and specificity of DRE can only be estimated because men with a normal finding on DRE are not routinely biopsied in any studies (TABLE 1).17,18 One of two pertinent meta-analyses18 included studies that followed men with a negative DRE finding for development of prostate cancer (LOE: 2a). Both meta-analyses of DRE as a screening tool found that the included studies were heterogeneous in their study populations and definition of abnormal DRE test result (LOE: 2a). The positive predictive value (PPV) of DRE was 28% in one meta-analysis and 18% in the other.
TABLE 1
Characteristics of screening tests for prostate cancer
| TEST/SOURCE | DESCRIPTION (LEVEL OF EVIDENCE) | SN ESTIMATE (%) | SP ESTIMATE (%) | LIKELIHOOD RATIO | PPV (%) |
|---|---|---|---|---|---|
| Abnormal DRE18 | Meta-analysis of studies that performed biopsies for abnormal DRE (2a) | 59 (51%–67%) | 94 (91%–96%) | + 9.8 – 0.4 | 28 (20–36) |
| Abnormal DRE17 | Meta-analysis of studies that performed biopsies for abnormal DRE or PSA (2a) | 53.2 | 83.6 | + 3.24 – 0.56 | 17.8 |
| PSA >4 ng/mL12 | Review of multiple cohort studies with diverse populations, not systematic | Mean = 71 | Mean = 75 | + 2.8 – 0.30 | 37 |
| PSA >4 ng/mL19 | Longitudinal retrospective study of prostate cancers that developed within 2 years of PSA test (3b) | 73.2 | 85.4 | + 5.0 – 0.2 | |
| PSA >4 ng/mL20 | Nested case-control study of prostate cancers that developed within 5 years of PSA test in a cohort study (3b) | 86 | 94 | + 14.3 – 0.15 | |
| PSA > 4 ng/mL17 | Meta-analysis of studies that performed biopsies for abnormal PSA or DRE (2a) | 72.1 | 93.2 | + 10.2 – 0.30 | 25.1 |
| Sn, sensitivity; Sp, specificity; PPV, positive predictive value; | |||||
| PSA, prostate-specific antigen; DRE, digital rectal examination. | |||||
Prostate-specific antigen: Improving its clinical usefulness
Prostate-specific antigen (PSA), first detected in serum in 1979, is a protein produced by prostate epithelial cells. It was originally used to follow men treated for prostate cancer for evidence of recurrence. In the late 1980s, it became widely used in the US to screen for prostate cancer.
An elevated PSA level is suggestive but not diagnostic of prostate cancer. Elevated levels also occur with advancing age, increased prostate size, and prostatitis, and following ejaculation. Prostate manipulation such as biopsy and surgery (but not digital examination) also elevates PSA.
Customary cutpoint. An abnormal serum PSA level is commonly regarded as 4 ng/mL or greater. At this cutpoint, most studies report sensitivity for cancer of around 70%, with more variability in specificity (TABLE 1). Again, in most studies that report sensitivity and specificity, men with a PSA level less than 4 ng/mL did not undergo prostate biopsy; therefore, the number of false negatives and true negatives are only estimates.
In nested case-control studies of longitudinal cohorts, eligible cases were defined by the length of time between an abnormal PSA result and prostate cancer diagnosis, while controls were men who were not diagnosed with prostate cancer during the same time period (however, a biopsy was not usually performed). The PPV of an abnormal PSA level is estimated at 25% to 37%. As a comparison, the PPV of a positive mammogram finding for women 50 to 59 years is 4% to 9%, and 10% to 19% for women age 60 to 69.21
Suggested strategies to improve PSA accuracy. Approximately 70% of men with an elevated serum PSA level do not have cancer. To decrease the number of unnecessary biopsies, experts have suggested several strategies:
- using DRE with PSA
- calculating a ratio of free PSA (unbound to protein) or complexed PSA (bound to protein) to total PSA
- measuring PSA density, which incorporates the volume of the prostate gland and is subject to observer variability in prostate volume measurement
- recording PSA velocity, which is the annual rate of change in PSA level and requires 3 or more measurements.
Revise the cutpoint? The cutpoint of 4 ng/mL has been deemed too high by some clinicians. A recent report of men enrolled in a large prostate cancer prevention trial found that among the 9459 men receiving placebo, 2950 had a PSA level of 4 ng/mL or less and had normal prostates on DRE (LOE: 1b).22 All 2950 underwent a prostate biopsy; 449 (15.2%) were positive for cancer. Nearly 30% of those had a PSA level of 2 ng/mL or less.
Approximately 25% of tumors in men referred for biopsy because of abnormal PSA or DRE findings are thought to be discovered by chance due to the biopsy procedure and the high prevalence of disease (LOE: 2c).23 Furthermore, a recent study indicates that annual PSA fluctuation is substantial; 45% of men who initially had a value of 4 ng/mL or greater, subsequently had a normal level (LOE: 2b).24 Isolated abnormal levels should be confirmed before referral for biopsy.
Identifying clinically relevant cancers
The continued controversy around PSA screening relates to the potential over-diagnosis of localized prostate cancers that are not likely to become clinically significant. The ideal screening test would predictably identify cancers likely to progress.
Screening over-detects
Several computer simulations have estimated the amount of over-detection of prostate cancer. The National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) registry data demonstrated that the proportion of prostate cancer found through PSA testing that otherwise would not have been diagnosed in the patient’s lifetime was 29% for white men and 44% for black men (LOE: 2c).25
An Italian study found that the proportional excess of cancers detected by screening over those that would have been expected in the absence of screening is greater than 50% (LOE: 2c).26
Finally, a model based on results from the European randomized study of prostate cancer screening estimated that a screening program with a 4-year interval from age 55 to 67 has an over-detection rate of 48% (cancers that would not have been diagnosed in the absence of screening) (LOE: 2c).27 Additionally, the authors determined that such screening might advance prostate cancer diagnosis by at least 10 years. Previous estimates of diagnosis lead time have been in the 5- to 7-year range (LOE: 2b).28 These findings indicate that screening intervals of 2 to 4 years are unlikely to cause a loss of sensitivity.
Spread of tumor
Spread of the tumor beyond the prostate capsule is a poor prognostic sign.29 Unfortunately, this occurrence is often not known until surgery, and the result usually is an “up-staging” between clinical diagnosis and pathological diagnosis. Nevertheless, most cancers (86%) diagnosed between 1992 and 1999 were localized to the prostate.6 Because these cancers have not spread beyond the prostate at the time of diagnosis, they are more likely to be curable. They are also more likely to represent tumors that may grow so slowly that the host will die of something other than prostate cancer.
Gleason score
Gleason score is another predictor of cancers destined to become clinically relevant (FIGURE 1). A Gleason score of 7 or greater denotes moderate to poor cellular differentiation and indicates a greater potential for progression than lower Gleason values.29 A recent long-term follow-up report on a cohort of men with localized cancer treated conservatively demonstrated that men with low-grade tumors (Gleason score 2–4) have a minimal risk of dying from prostate cancer after 20 years, while men with high-grade tumors (Gleason score of 8–10) have high probability of prostate cancer death within 10 years of diagnosis (LOE: 2b).30 Ecological31 and clinical studies32 indicate that a substantial proportion of PSA-detected cancers are moderately differentiated. This is especially true in a first round of screening; as in the European randomized study of screening, where 36% of cancers were Gleason score 7 or higher (LOE: 1b).33
FIGURE 1
Calculating the Gleason score
The Gleason score is based on the level of differentiation and growth pattern of prostate cancer cells. Cancer cells that closely resemble the normal prostate cells when viewed under low-power magnification are well differentiated. Cancer cells that do not retain the structure of the surrounding normal cells are poorly differentiated. Scores range from 1 to 5.
In examining histologic samples of a patient’s prostate tissue, the pathologist will identify the 2 most commonly occurring patterns (types of differentiation) among the cancer cells and assign a numerical value to each pattern. The 2 numbers are then added to yield the final Gleason score. If a single pattern dominates, the pathologist will simply double the corresponding value.
Total scores range from 2 to 10. Scores in the range of 2–4 are considered well-differentiated, 5–7 are moderately differentiated, and 8–10 are poorly differentiated. In general, the higher the score, the worse the prognosis. Men with well-differentiated tumors that are treated conservatively have minimal risk of dying from prostate cancer.
Is declining mortality a sign of screening success?
Prostate cancer mortality has been declining since the mid-1990s in numerous parts of the world; the US,6,34 Canada,35 Australia,36 and the United Kingdom37 have all reported a reduction in the rate of prostate cancer deaths. Advocates of PSA screening point to this trend as evidence of the effectiveness of screening. But such ecological data are difficult to interpret. For instance, although much less PSA screening is performed in the UK, mortality trends are similar to those in the US where PSA testing has been used more widely.38
Aggressive screening not necessarily the reason. In the US, 2 geographic areas—Seattle, Washington and Connecticut—provided a natural experiment to compare the effect of aggressive screening on prostate cancer mortality (LOE: 2c).39 Although more aggressive screening and treatment took place in the Seattle area, prostate cancer mortality rates were similar to those in Connecticut over 11 years of follow-up. Similarly, in a study in British Columbia, prostate cancer mortality from 1985 to 1999 was not associated with the intensity of PSA screening (LOE: 2c).40
Other possible explanations. If the mortality decrease is not related to PSA screening, what could cause it? One explanation is “attribution bias.” Death certificate misattribution of cause of death from prostate cancer may partially explain the pattern of increasing, then decreasing mortality rates (LOE: 2c).41 Improvement in prostate cancer treatment, especially for advanced stage, and in particular hormone therapy, is another possible explanation for the decreasing prostate cancer mortality (LOE: 2c).14,42
Benefits of screening
The benefit of any effective screening test is a decrease in the risk of the screened-disease mortality. The best way to demonstrate decreased risk is through a randomized controlled study of the screening test, and 2 such trials are underway for prostate cancer. In the meantime, a decision model estimates that aggressive treatment of organ-confined disease potentially adds 3 years of life for men in their fifties, 1.5 years for men in their sixties, and 0.4 years for men in their seventies (LOE: 2c).3
Others have concluded that 25 men with clinically detected prostate cancer would need to be treated with surgery to prevent 1 prostate cancer death during a 6-year period, without evidence that quality of life is improved (LOE: 2c).43
Consider quality of life. With uncertainty surrounding improvement in the quantity of life as a result of prostate cancer screening, improved quality of life may be an issue for patients. Focus group research has demonstrated that some patients believe it is better to know if a cancer is present than to wonder if it will be diagnosed when it is too late for cure.44
General quality of life has been found to be similar among men treated for prostate cancer and age-matched controls without prostate cancer; however, urinary, sexual, and bowel function vary substantially between treated and untreated men and by treatment type (LOE: 3b) (TABLE 2).45,46 In general, men treated with radical prostatectomy and brachytherapy often report better general quality of life than men who undergo radiation treatment, despite having more urinary and sexual problems (LOE: 2b).47,48
TABLE 2
Estimates of risk associated with specific prostate cancer treatments 12 months or more after treatment
| TREATMENT OUTCOMES | RADICAL PROSTATECTOMY (%) | EXTERNAL BEAM RADIATION (%) | BRACHY-THERAPY* (%) | ANDROGEN DEPRIVATION THERAPY (%) | UNTREATED (%) |
|---|---|---|---|---|---|
| Death within 2 months of treatment | 0.5–0.7 | 0.2–0.5 | 0.2–0.5 | ||
| Urinary problems: | |||||
| Incontinence | 10–50 | 2–16 | 6–16 | ||
| Wearing pads | 5–32 | 2–12 | 2–16 | ||
| Urinary bother† | 4–20 | 3–15 | 3–16 | ||
| Sexual problems: | |||||
| Impotence | 50–80‡ | 30–60 | 20–60** | 70–92 | 20–50 |
| Sexual bother | 10–40 | 10–30 | 10–18 | 25–38 | 10–32 |
| Bowel problems: | |||||
| Bowel problems§ | 9–15 | 6–35 | 4–20 | ||
| Loose stools/diarrhea | 15–21 | 6–37 | 4–10 | ||
| Bowel bother | 1–3 | 4–12 | 2–10 | ||
| Other symptoms | Breast swelling: 5–25 | ||||
| Hot flashes: 50–60 | |||||
| * Fewer studies on brachytherapy are available, especially those with long-term follow-up; therefore, these findings are less certain than other entries. | |||||
| ‡ Includes nerve-sparing prostatectomy. | |||||
| † EBRT and brachytherapy patients are more likely to experience irritative voiding symptoms (i.e. dysuria, urgency and hesitancy and noctoria), while RP patients are more likely to experience incontinence. | |||||
| ** Impotence risk gradually increases with time after treatment. | |||||
| § Includes symptoms such as painful bowel movement and urgency | |||||
| Sources:references 14, 50–53, 65–71. | |||||
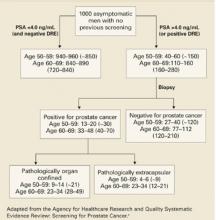
Harms of screening
The chances of undergoing a biopsy based on an abnormal screening PSA are estimated at 15% to 40% depending on the patient’s age (FIGURE 2).3 There are adverse effects associated with transrectal biopsy of the prostate. In 2 large population-basedstudies of screening, the most frequent complications were hematuria and hematospermia (LOE: 1b, 2b) (TABLE 3), with more serious consequences such as sepsis and hospitalization occurring in fewer than 1% of patients. A study of 100 screened men with an abnormal PSA who underwent prostate biopsy found that although 69% felt moderate to severe pain with the biopsy, 80% would be willing to undergo a repeat biopsy (LOE: 1b).49
Treatment options. If the biopsy result is positive, the most common treatment options for localized cancer—which represents over 80% of all prostate cancers diagnosed6—include radical prostatectomy, external beam radiation therapy, brachytherapy (internal radiation therapy) or expectant management (watchful waiting). Population-based studies have reported outcomes for these treatment options (TABLE 2). Outcomes derived from hospital-based series of other prostate cancer treatments, such as cryotherapy and 3-dimensional radiation, are available, but the estimates often reflect the experience of only a few hospitals and are not representative of other facilities. Androgen ablation is the standard treatment for metastatic prostate cancer.
Untoward effects of treatment. Approximately 60% of radical prostatectomy patients report some incontinence 12 months or more after surgery (LOE: 2b),50,51 and about 30% of patients need to wear pads for urine leakage (LOE: 2b).50-53 Men undergoing radiation therapy have less urinary incontinence, but about 30% complain of diarrhea and loose stools (LOE: 2b).51,52 Both therapies are associated with a high percentage of erectile dysfunction: approximately 60% of radiation therapy patients and 75% of surgery patients report their erections are not firm enough for intercourse (LOE: 2b).51,52
Expectant management (following the cancer with regular PSA and ultrasound testing) is sometimes difficult to “sell” to patients whose fear of cancer dictates that the only logical response is to “cut it out.”44 A recent randomized trial indicated that radical prostatectomy lowers prostate cancer mortality, local progression, distant metastasis, and overall survival as compared with watchful waiting over a median of 8.2 years of follow-up (LOE: 1b).54 However, these results may have little relevance to prostate cancer screening since only 5% of the cancers were screen-detected and 76% were palpable.
FIGURE 2
Yield of screening 1000 men for prostate cancer
TABLE 3
Percentage of patients with specific complication of transrectal prostate biopsy
| CONDITION | TYROL STUDY63 | EUROPEAN RANDOMIZED STUDY OF SCREENING 64 |
|---|---|---|
| Gross hematuria >1 day | 12.5% | 22.6% |
| Hematospermia | 29.8% | 50.4% |
| Significant pain | 4.0% | 7.5% |
| Rectal bleeding | 0.6% | 1.3% |
| Nausea | 0.8% | 0.3% |
| Fever >38.5°C | 0.8% | 3.5% |
| Epididymitis | 0.7% | 0.07% |
| Sepsis | 0.3% | Not available |
| Hospitalization | Not available | 0.5% |
| Tyrol study63: LOE: 2b, N=6024 biopsies; ERSS study64: LOE: 1b, N=5802 biopsies. | ||
Recommendations from expert groups
Different expert groups have conflicting recommendations. Both the American Urological Association and the American Cancer Society recommend annual PSA screening starting at age 50 for most men; younger if risk factors are present. Groups that are evidence based tend to recommend a shared decision making process with patients. The AAFP and American College of Physicians advise physicians to counsel men on the known risks and uncertain benefits of screening for prostate cancer. The US Preventive Services Task Force 2002 update concluded that evidence is insufficient to recommend for or against routine screening for prostate cancer using PSA or DRE. The National Cancer Institute cites a lack of evidence to determine a net benefit for PSA or DRE screening.
When will we know more?
Only 1 randomized controlled trial of prostate cancer screening has been completed55: 46,193 men were randomized to either PSA and DRE or no screening from 1989 to 1996. The study had methodological problems; for instance, only 23% of the group randomized to screening was screened. The investigators in the trial have interpreted its results as demonstrating a decrease in prostate cancer deaths in the screened group compared with the unscreened group (15 vs 48.7 per 100,000 man-years).55 Others have criticized the statistical analysis and calculated the results using an “intent to screen” analysis, finding no difference in prostate cancer deaths between the 2 groups.3,56
Two randomized controlled trials of screening are ongoing: the National Cancer Institute’s Prostate, Lung, Colon, Ovarian (PLCO) Screening Trial57 and the European Randomized Study of Screening for Prostate Cancer.58 Both were started in the mid-1990s and will not have results available for a few more years. Also underway is a randomized trial of intervention (radical prostatectomy) versus expectant management, called the Prostate Cancer Intervention Versus Observation Trial (PIVOT).59
Counseling recommendations
However, providing men with information on prostate cancer screening before they discussed it with their family physician, rather than after the visit, resulted in patients having a significantly more active role in making a screening decision, and lower levels of decisional conflict (LOE: 2b).61 Informational pamphlets are available through the AAFP and CDC websites listed in TABLE 4. Additional websites containing prostate cancer screening information are found in TABLE 4. We also provide a bullet item list of key points for discussion with patients (TABLE 5), which can be used along with the balance sheet provided here (TABLE 2).
Shared decision-making is not an easy or quick process. Yet, the majority of patients will benefit from the discussion, regardless of the final decision. Of course, there are instances when a shared decision-making process is well-documented, and still results in an undesirable outcome;62 however, while the evidence for screening remains controversial, patients have the right to know that those controversies exist and why they exist.
TABLE 4
Useful websites for patients to find prostate cancer screening information
| CENTERS FOR DISEASE CONTROL AND PREVENTION |
| www.cdc.gov/cancer/prostate/decisionguide/index.htm |
| 10th grade reading level* |
| Good coverage of screening and treatment controversies |
| Offers downloadable PDF version |
| NATIONAL CANCER INSTITUTE |
| cis.nci.nih.gov/asp/FactSheetPub/AlphaSubList.asp?alpha=47 |
| 10th grade reading level |
| FAQ format |
| Offers Spanish version |
| AMERICAN CANCER SOCIETY |
| www.cancer.org/docroot/CRI/content/CRI_2_4_3X_Can_prostate_ cancer_be_found_early_36.asp |
| 12th grade reading level |
| Lacks discussion of treatment options and their side effects |
| Biased in favor of screening but acknowledges that other distinguished organizations are not |
| AMERICAN UROLOGICAL ASSOCIATION |
| www.urologyhealth.org/adult/index.cfm?cat=09 |
| 12th grade reading level |
| Easy to navigate among screening and specific treatment pages |
| Biased in favor of PSA screening |
| AMERICAN ACADEMY OF FAMILY PHYSICIANS |
| familydoctor.org/healthfacts/361/ |
| 11th grade reading level |
| Question/answer format |
| Very straightforward, lacks depth |
| www.aafp.org/x19519.xml |
| 7th grade reading level |
| Separate information sheet for patients and physicians |
| Presents possible outcomes of PSA test and prostate cancer treatment in easy-to-follow format |
| WEBMD |
| my.webmd.com/medical_information/condition_centers/prostate_cancer/default.htm |
| 9th grade reading level |
| Question/answer format |
| Specifically addresses false negative and positives with current estimates |
| DARTMOUTH CENTER FOR SHARED DECISION MAKING |
| www.dhmc.org/dhmc-internet-upload/file_collection/PSA.pdf |
| 6th grade reading level |
| Well-designed, simple presentation of pros and cons of PSA testing |
| *Fleish-Kincaid grade level score based on average sentence length and average number of syllables per word. |
TABLE 5
Talking points for patients and physicians
| Prostate cancer is an important men’s health problem |
| Screening may prevent early prostate cancer death |
| DRE alone has little value as a screening test |
| Age, prostate size, prostatitis, ejaculation, prostate biopsy, and prostate surgery can cause a falsely elevated PSA test |
| Approximately 70% of men with an elevated serum PSA do not have cancer |
| The percentage of PSA screening false negatives ranges from 10%–22% in large studies |
| If the test is abnormal, a biopsy will be recommended |
| If the biopsy is positive, treatment options will be given |
| Many men experience long-term urinary incontinence and impotence related to their treatment |
CORRESPONDING AUTHOR
Kendra Schwartz, MD, MSPH, 101 E. Alexandrine, Detroit, MI 48201, E-mail: kensch@med.wayne.edu
- Engage patients in shared decision making by discussing the benefits and risks of prostate cancer screening. Patients who review educational pamphlets before an office visit engage more fully in the decision-making process. (B)
- If performing prostate cancer screening, limit to men with greater than 10 years life expectancy. (B)
- Because the lead time of a diagnosis based on PSA screening is estimated to be 5 to 7 years, PSA screening every other year is unlikely to cause a loss of sensitivity. (B)
- Men with tumors with a Gleason score less than 5 are the best candidates for “watchful waiting,” having a favorable 20-year survival. (B)
Prostate cancer screening in asymptomatic men remains controversial, and it is difficult to present its benefits and risks quickly in a way that is understandable to patients. Yet many expert groups agree that physicians should enter into a mutual decision-making process with patients.1-3
This article reviews the latest information relevant to the controversy, offers “talking points” for family physicians to use when discussing screening with patients, and lists websites that patients may find helpful when making a decision about prostate cancer screening.
For this review, we searched for recent articles that are generalizable to a primary care population and of the highest evidence level available. We preferentially discuss population-based studies, studies from randomized trials of screening, and meta-analyses, rather than results that are hospital- or clinic-based. For a complete systematic review of this topic (from 2002), readers are referred to one conducted for the Agency for Healthcare Research and Quality.4 (See Scope of the problem).
Adenocarcinoma of the prostate is a significant public health burden. Age, family history, and race are the only known risk factors. Most cancers (86%) are diagnosed while still confined to the prostate; however, invasion beyond the capsule is sometimes not apparent until surgery.
Incidence. In 2005, there will be approximately 232,090 new cases of prostate cancer.5 American men have a 17% chance of being diagnosed with prostate cancer; African Americans have a 65% greater risk of developing prostate cancer than Caucasians.6 In fact, African Americans have the highest prostate cancer annual age-adjusted incidence rates in the world: 272/100,000 compared with 164/100,000 for Caucasian Americans.6 The rate for US Asian/Pacific Islander and Hispanic men is less than that for Caucasian Americans.
Mortality. There will be approximately 30,350 prostate cancer deaths in 2004.5 There is 3% chance of dying from prostate cancer; however, the risk of death is about 55% higher for African American men than Caucasian American men.6
Risk factors other than race. Risk of prostate cancer diagnosis increases with age: 1 in 48 men aged 40 to 59 years will be diagnosed with prostate cancer, while 1 in 8 men aged 60 to 79 years are at risk.7 A man who has a first-degree relative with prostate cancer is 2.4 times as likely to be diagnosed with prostate cancer as a man with no affected relatives.8
Key components of the controversy
How effective is screening?
A good screening test does 2 things. First, it detects a disease earlier than it would be detected with no screening at all, and it does so with sufficient accuracy to avoid a large number of false-positive and false-negative results.
Second, it leads to treatment of early disease that will likely produce a more favorable health outcome than waiting to treat patients who have signs and symptoms of disease.9 Unfortunately it is still unclear whether screening tests for prostate cancer meet these 2 criteria.
Skewed numbers. Yes, estimates of false-positive and false-negative results are available from numerous studies of different populations. However, in most studies, only men with abnormal test results receive a biopsy. Men with normal screening test results are not biopsied. Therefore the number of false negatives (and true negatives) is unknown. Furthermore, these estimates are often based on patients from urology clinics, a group more likely to have disease, thereby increasing the positive predictive value of a given screening test.
Whether current screening methods—in particular, prostate-specific antigen (PSA)—identify prostate cancers destined to become clinically relevant is also unknown. If screening does identify such cancers, a decrease in prostate cancer mortality among men who were screened is expected. If it does not, overdiagnosis and treatment of clinically insignificant cancers will negatively impact the quality of men’s lives without extending their life spans.
Clinical variability of the disease
The natural history of prostate cancer is uncertain. If cancer is left unidentified or untreated, more men will die with prostate cancer than of prostate cancer.
Clinically, prostate cancer ranges from an asymptomatic slow-growing tumor to an aggressive cancer with painful metastases. Treatment may be unnecessary at one end of the spectrum and palliative at the other.
The goal of screening is to identify slow-growing tumors destined to extend beyond the prostate while they are confined to the prostate and amenable to treatment, thereby decreasing the risk of prostate cancer morbidity and death.
Usually low morbidity. For a 50-year-old man, the risk of being diagnosed with prostate cancer by age 80 years is 15%; however, the same man has a 1.4% chance of dying of prostate cancer over that 30-year period.7 This 10-fold difference shows that prostate cancer usually is not a fatal illness. Another indication of the often benign nature of the disease is the high percentage of prostate cancers identified at surgery for bladder cancer; up to 40% of specimens contain unsuspected prostate cancer (level of evidence [LOE]: 2c).10,11
Most clinical diagnoses (80%) and prostate cancer deaths (90%) occur among men older than 65 years;6 the median age at diagnosis is 72 years.12 More than 75% of men older than 85 years will have histological prostate cancer (LOE: 2c).13 Many men live with their disease for more than 10 years, but do not die of it (LOE: 2c).12 Additionally, a review of several decision analyses indicates that men 75 years of age and older are not likely to benefit from screening and aggressive treatment (LOE: 2a).14
Hence the recommendation: if performing prostate cancer screening, limit to men with greater than 10 years life expectancy.15
Details of screening tests
Digital rectal examination insufficient
Digital rectal examination (DRE)—palpating the prostate gland to determine size and consistency—is one screening tool for prostate cancer, usually performed in conjunction with PSA testing.15 It is not a difficult or expensive test, but its reproducibility is only fair, even among experienced urologists (LOE: 2b).16
The sensitivity and specificity of DRE can only be estimated because men with a normal finding on DRE are not routinely biopsied in any studies (TABLE 1).17,18 One of two pertinent meta-analyses18 included studies that followed men with a negative DRE finding for development of prostate cancer (LOE: 2a). Both meta-analyses of DRE as a screening tool found that the included studies were heterogeneous in their study populations and definition of abnormal DRE test result (LOE: 2a). The positive predictive value (PPV) of DRE was 28% in one meta-analysis and 18% in the other.
TABLE 1
Characteristics of screening tests for prostate cancer
| TEST/SOURCE | DESCRIPTION (LEVEL OF EVIDENCE) | SN ESTIMATE (%) | SP ESTIMATE (%) | LIKELIHOOD RATIO | PPV (%) |
|---|---|---|---|---|---|
| Abnormal DRE18 | Meta-analysis of studies that performed biopsies for abnormal DRE (2a) | 59 (51%–67%) | 94 (91%–96%) | + 9.8 – 0.4 | 28 (20–36) |
| Abnormal DRE17 | Meta-analysis of studies that performed biopsies for abnormal DRE or PSA (2a) | 53.2 | 83.6 | + 3.24 – 0.56 | 17.8 |
| PSA >4 ng/mL12 | Review of multiple cohort studies with diverse populations, not systematic | Mean = 71 | Mean = 75 | + 2.8 – 0.30 | 37 |
| PSA >4 ng/mL19 | Longitudinal retrospective study of prostate cancers that developed within 2 years of PSA test (3b) | 73.2 | 85.4 | + 5.0 – 0.2 | |
| PSA >4 ng/mL20 | Nested case-control study of prostate cancers that developed within 5 years of PSA test in a cohort study (3b) | 86 | 94 | + 14.3 – 0.15 | |
| PSA > 4 ng/mL17 | Meta-analysis of studies that performed biopsies for abnormal PSA or DRE (2a) | 72.1 | 93.2 | + 10.2 – 0.30 | 25.1 |
| Sn, sensitivity; Sp, specificity; PPV, positive predictive value; | |||||
| PSA, prostate-specific antigen; DRE, digital rectal examination. | |||||
Prostate-specific antigen: Improving its clinical usefulness
Prostate-specific antigen (PSA), first detected in serum in 1979, is a protein produced by prostate epithelial cells. It was originally used to follow men treated for prostate cancer for evidence of recurrence. In the late 1980s, it became widely used in the US to screen for prostate cancer.
An elevated PSA level is suggestive but not diagnostic of prostate cancer. Elevated levels also occur with advancing age, increased prostate size, and prostatitis, and following ejaculation. Prostate manipulation such as biopsy and surgery (but not digital examination) also elevates PSA.
Customary cutpoint. An abnormal serum PSA level is commonly regarded as 4 ng/mL or greater. At this cutpoint, most studies report sensitivity for cancer of around 70%, with more variability in specificity (TABLE 1). Again, in most studies that report sensitivity and specificity, men with a PSA level less than 4 ng/mL did not undergo prostate biopsy; therefore, the number of false negatives and true negatives are only estimates.
In nested case-control studies of longitudinal cohorts, eligible cases were defined by the length of time between an abnormal PSA result and prostate cancer diagnosis, while controls were men who were not diagnosed with prostate cancer during the same time period (however, a biopsy was not usually performed). The PPV of an abnormal PSA level is estimated at 25% to 37%. As a comparison, the PPV of a positive mammogram finding for women 50 to 59 years is 4% to 9%, and 10% to 19% for women age 60 to 69.21
Suggested strategies to improve PSA accuracy. Approximately 70% of men with an elevated serum PSA level do not have cancer. To decrease the number of unnecessary biopsies, experts have suggested several strategies:
- using DRE with PSA
- calculating a ratio of free PSA (unbound to protein) or complexed PSA (bound to protein) to total PSA
- measuring PSA density, which incorporates the volume of the prostate gland and is subject to observer variability in prostate volume measurement
- recording PSA velocity, which is the annual rate of change in PSA level and requires 3 or more measurements.
Revise the cutpoint? The cutpoint of 4 ng/mL has been deemed too high by some clinicians. A recent report of men enrolled in a large prostate cancer prevention trial found that among the 9459 men receiving placebo, 2950 had a PSA level of 4 ng/mL or less and had normal prostates on DRE (LOE: 1b).22 All 2950 underwent a prostate biopsy; 449 (15.2%) were positive for cancer. Nearly 30% of those had a PSA level of 2 ng/mL or less.
Approximately 25% of tumors in men referred for biopsy because of abnormal PSA or DRE findings are thought to be discovered by chance due to the biopsy procedure and the high prevalence of disease (LOE: 2c).23 Furthermore, a recent study indicates that annual PSA fluctuation is substantial; 45% of men who initially had a value of 4 ng/mL or greater, subsequently had a normal level (LOE: 2b).24 Isolated abnormal levels should be confirmed before referral for biopsy.
Identifying clinically relevant cancers
The continued controversy around PSA screening relates to the potential over-diagnosis of localized prostate cancers that are not likely to become clinically significant. The ideal screening test would predictably identify cancers likely to progress.
Screening over-detects
Several computer simulations have estimated the amount of over-detection of prostate cancer. The National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) registry data demonstrated that the proportion of prostate cancer found through PSA testing that otherwise would not have been diagnosed in the patient’s lifetime was 29% for white men and 44% for black men (LOE: 2c).25
An Italian study found that the proportional excess of cancers detected by screening over those that would have been expected in the absence of screening is greater than 50% (LOE: 2c).26
Finally, a model based on results from the European randomized study of prostate cancer screening estimated that a screening program with a 4-year interval from age 55 to 67 has an over-detection rate of 48% (cancers that would not have been diagnosed in the absence of screening) (LOE: 2c).27 Additionally, the authors determined that such screening might advance prostate cancer diagnosis by at least 10 years. Previous estimates of diagnosis lead time have been in the 5- to 7-year range (LOE: 2b).28 These findings indicate that screening intervals of 2 to 4 years are unlikely to cause a loss of sensitivity.
Spread of tumor
Spread of the tumor beyond the prostate capsule is a poor prognostic sign.29 Unfortunately, this occurrence is often not known until surgery, and the result usually is an “up-staging” between clinical diagnosis and pathological diagnosis. Nevertheless, most cancers (86%) diagnosed between 1992 and 1999 were localized to the prostate.6 Because these cancers have not spread beyond the prostate at the time of diagnosis, they are more likely to be curable. They are also more likely to represent tumors that may grow so slowly that the host will die of something other than prostate cancer.
Gleason score
Gleason score is another predictor of cancers destined to become clinically relevant (FIGURE 1). A Gleason score of 7 or greater denotes moderate to poor cellular differentiation and indicates a greater potential for progression than lower Gleason values.29 A recent long-term follow-up report on a cohort of men with localized cancer treated conservatively demonstrated that men with low-grade tumors (Gleason score 2–4) have a minimal risk of dying from prostate cancer after 20 years, while men with high-grade tumors (Gleason score of 8–10) have high probability of prostate cancer death within 10 years of diagnosis (LOE: 2b).30 Ecological31 and clinical studies32 indicate that a substantial proportion of PSA-detected cancers are moderately differentiated. This is especially true in a first round of screening; as in the European randomized study of screening, where 36% of cancers were Gleason score 7 or higher (LOE: 1b).33
FIGURE 1
Calculating the Gleason score
The Gleason score is based on the level of differentiation and growth pattern of prostate cancer cells. Cancer cells that closely resemble the normal prostate cells when viewed under low-power magnification are well differentiated. Cancer cells that do not retain the structure of the surrounding normal cells are poorly differentiated. Scores range from 1 to 5.
In examining histologic samples of a patient’s prostate tissue, the pathologist will identify the 2 most commonly occurring patterns (types of differentiation) among the cancer cells and assign a numerical value to each pattern. The 2 numbers are then added to yield the final Gleason score. If a single pattern dominates, the pathologist will simply double the corresponding value.
Total scores range from 2 to 10. Scores in the range of 2–4 are considered well-differentiated, 5–7 are moderately differentiated, and 8–10 are poorly differentiated. In general, the higher the score, the worse the prognosis. Men with well-differentiated tumors that are treated conservatively have minimal risk of dying from prostate cancer.
Is declining mortality a sign of screening success?
Prostate cancer mortality has been declining since the mid-1990s in numerous parts of the world; the US,6,34 Canada,35 Australia,36 and the United Kingdom37 have all reported a reduction in the rate of prostate cancer deaths. Advocates of PSA screening point to this trend as evidence of the effectiveness of screening. But such ecological data are difficult to interpret. For instance, although much less PSA screening is performed in the UK, mortality trends are similar to those in the US where PSA testing has been used more widely.38
Aggressive screening not necessarily the reason. In the US, 2 geographic areas—Seattle, Washington and Connecticut—provided a natural experiment to compare the effect of aggressive screening on prostate cancer mortality (LOE: 2c).39 Although more aggressive screening and treatment took place in the Seattle area, prostate cancer mortality rates were similar to those in Connecticut over 11 years of follow-up. Similarly, in a study in British Columbia, prostate cancer mortality from 1985 to 1999 was not associated with the intensity of PSA screening (LOE: 2c).40
Other possible explanations. If the mortality decrease is not related to PSA screening, what could cause it? One explanation is “attribution bias.” Death certificate misattribution of cause of death from prostate cancer may partially explain the pattern of increasing, then decreasing mortality rates (LOE: 2c).41 Improvement in prostate cancer treatment, especially for advanced stage, and in particular hormone therapy, is another possible explanation for the decreasing prostate cancer mortality (LOE: 2c).14,42
Benefits of screening
The benefit of any effective screening test is a decrease in the risk of the screened-disease mortality. The best way to demonstrate decreased risk is through a randomized controlled study of the screening test, and 2 such trials are underway for prostate cancer. In the meantime, a decision model estimates that aggressive treatment of organ-confined disease potentially adds 3 years of life for men in their fifties, 1.5 years for men in their sixties, and 0.4 years for men in their seventies (LOE: 2c).3
Others have concluded that 25 men with clinically detected prostate cancer would need to be treated with surgery to prevent 1 prostate cancer death during a 6-year period, without evidence that quality of life is improved (LOE: 2c).43
Consider quality of life. With uncertainty surrounding improvement in the quantity of life as a result of prostate cancer screening, improved quality of life may be an issue for patients. Focus group research has demonstrated that some patients believe it is better to know if a cancer is present than to wonder if it will be diagnosed when it is too late for cure.44
General quality of life has been found to be similar among men treated for prostate cancer and age-matched controls without prostate cancer; however, urinary, sexual, and bowel function vary substantially between treated and untreated men and by treatment type (LOE: 3b) (TABLE 2).45,46 In general, men treated with radical prostatectomy and brachytherapy often report better general quality of life than men who undergo radiation treatment, despite having more urinary and sexual problems (LOE: 2b).47,48
TABLE 2
Estimates of risk associated with specific prostate cancer treatments 12 months or more after treatment
| TREATMENT OUTCOMES | RADICAL PROSTATECTOMY (%) | EXTERNAL BEAM RADIATION (%) | BRACHY-THERAPY* (%) | ANDROGEN DEPRIVATION THERAPY (%) | UNTREATED (%) |
|---|---|---|---|---|---|
| Death within 2 months of treatment | 0.5–0.7 | 0.2–0.5 | 0.2–0.5 | ||
| Urinary problems: | |||||
| Incontinence | 10–50 | 2–16 | 6–16 | ||
| Wearing pads | 5–32 | 2–12 | 2–16 | ||
| Urinary bother† | 4–20 | 3–15 | 3–16 | ||
| Sexual problems: | |||||
| Impotence | 50–80‡ | 30–60 | 20–60** | 70–92 | 20–50 |
| Sexual bother | 10–40 | 10–30 | 10–18 | 25–38 | 10–32 |
| Bowel problems: | |||||
| Bowel problems§ | 9–15 | 6–35 | 4–20 | ||
| Loose stools/diarrhea | 15–21 | 6–37 | 4–10 | ||
| Bowel bother | 1–3 | 4–12 | 2–10 | ||
| Other symptoms | Breast swelling: 5–25 | ||||
| Hot flashes: 50–60 | |||||
| * Fewer studies on brachytherapy are available, especially those with long-term follow-up; therefore, these findings are less certain than other entries. | |||||
| ‡ Includes nerve-sparing prostatectomy. | |||||
| † EBRT and brachytherapy patients are more likely to experience irritative voiding symptoms (i.e. dysuria, urgency and hesitancy and noctoria), while RP patients are more likely to experience incontinence. | |||||
| ** Impotence risk gradually increases with time after treatment. | |||||
| § Includes symptoms such as painful bowel movement and urgency | |||||
| Sources:references 14, 50–53, 65–71. | |||||
Harms of screening
The chances of undergoing a biopsy based on an abnormal screening PSA are estimated at 15% to 40% depending on the patient’s age (FIGURE 2).3 There are adverse effects associated with transrectal biopsy of the prostate. In 2 large population-basedstudies of screening, the most frequent complications were hematuria and hematospermia (LOE: 1b, 2b) (TABLE 3), with more serious consequences such as sepsis and hospitalization occurring in fewer than 1% of patients. A study of 100 screened men with an abnormal PSA who underwent prostate biopsy found that although 69% felt moderate to severe pain with the biopsy, 80% would be willing to undergo a repeat biopsy (LOE: 1b).49
Treatment options. If the biopsy result is positive, the most common treatment options for localized cancer—which represents over 80% of all prostate cancers diagnosed6—include radical prostatectomy, external beam radiation therapy, brachytherapy (internal radiation therapy) or expectant management (watchful waiting). Population-based studies have reported outcomes for these treatment options (TABLE 2). Outcomes derived from hospital-based series of other prostate cancer treatments, such as cryotherapy and 3-dimensional radiation, are available, but the estimates often reflect the experience of only a few hospitals and are not representative of other facilities. Androgen ablation is the standard treatment for metastatic prostate cancer.
Untoward effects of treatment. Approximately 60% of radical prostatectomy patients report some incontinence 12 months or more after surgery (LOE: 2b),50,51 and about 30% of patients need to wear pads for urine leakage (LOE: 2b).50-53 Men undergoing radiation therapy have less urinary incontinence, but about 30% complain of diarrhea and loose stools (LOE: 2b).51,52 Both therapies are associated with a high percentage of erectile dysfunction: approximately 60% of radiation therapy patients and 75% of surgery patients report their erections are not firm enough for intercourse (LOE: 2b).51,52
Expectant management (following the cancer with regular PSA and ultrasound testing) is sometimes difficult to “sell” to patients whose fear of cancer dictates that the only logical response is to “cut it out.”44 A recent randomized trial indicated that radical prostatectomy lowers prostate cancer mortality, local progression, distant metastasis, and overall survival as compared with watchful waiting over a median of 8.2 years of follow-up (LOE: 1b).54 However, these results may have little relevance to prostate cancer screening since only 5% of the cancers were screen-detected and 76% were palpable.
FIGURE 2
Yield of screening 1000 men for prostate cancer
TABLE 3
Percentage of patients with specific complication of transrectal prostate biopsy
| CONDITION | TYROL STUDY63 | EUROPEAN RANDOMIZED STUDY OF SCREENING 64 |
|---|---|---|
| Gross hematuria >1 day | 12.5% | 22.6% |
| Hematospermia | 29.8% | 50.4% |
| Significant pain | 4.0% | 7.5% |
| Rectal bleeding | 0.6% | 1.3% |
| Nausea | 0.8% | 0.3% |
| Fever >38.5°C | 0.8% | 3.5% |
| Epididymitis | 0.7% | 0.07% |
| Sepsis | 0.3% | Not available |
| Hospitalization | Not available | 0.5% |
| Tyrol study63: LOE: 2b, N=6024 biopsies; ERSS study64: LOE: 1b, N=5802 biopsies. | ||
Recommendations from expert groups
Different expert groups have conflicting recommendations. Both the American Urological Association and the American Cancer Society recommend annual PSA screening starting at age 50 for most men; younger if risk factors are present. Groups that are evidence based tend to recommend a shared decision making process with patients. The AAFP and American College of Physicians advise physicians to counsel men on the known risks and uncertain benefits of screening for prostate cancer. The US Preventive Services Task Force 2002 update concluded that evidence is insufficient to recommend for or against routine screening for prostate cancer using PSA or DRE. The National Cancer Institute cites a lack of evidence to determine a net benefit for PSA or DRE screening.
When will we know more?
Only 1 randomized controlled trial of prostate cancer screening has been completed55: 46,193 men were randomized to either PSA and DRE or no screening from 1989 to 1996. The study had methodological problems; for instance, only 23% of the group randomized to screening was screened. The investigators in the trial have interpreted its results as demonstrating a decrease in prostate cancer deaths in the screened group compared with the unscreened group (15 vs 48.7 per 100,000 man-years).55 Others have criticized the statistical analysis and calculated the results using an “intent to screen” analysis, finding no difference in prostate cancer deaths between the 2 groups.3,56
Two randomized controlled trials of screening are ongoing: the National Cancer Institute’s Prostate, Lung, Colon, Ovarian (PLCO) Screening Trial57 and the European Randomized Study of Screening for Prostate Cancer.58 Both were started in the mid-1990s and will not have results available for a few more years. Also underway is a randomized trial of intervention (radical prostatectomy) versus expectant management, called the Prostate Cancer Intervention Versus Observation Trial (PIVOT).59
Counseling recommendations
However, providing men with information on prostate cancer screening before they discussed it with their family physician, rather than after the visit, resulted in patients having a significantly more active role in making a screening decision, and lower levels of decisional conflict (LOE: 2b).61 Informational pamphlets are available through the AAFP and CDC websites listed in TABLE 4. Additional websites containing prostate cancer screening information are found in TABLE 4. We also provide a bullet item list of key points for discussion with patients (TABLE 5), which can be used along with the balance sheet provided here (TABLE 2).
Shared decision-making is not an easy or quick process. Yet, the majority of patients will benefit from the discussion, regardless of the final decision. Of course, there are instances when a shared decision-making process is well-documented, and still results in an undesirable outcome;62 however, while the evidence for screening remains controversial, patients have the right to know that those controversies exist and why they exist.
TABLE 4
Useful websites for patients to find prostate cancer screening information
| CENTERS FOR DISEASE CONTROL AND PREVENTION |
| www.cdc.gov/cancer/prostate/decisionguide/index.htm |
| 10th grade reading level* |
| Good coverage of screening and treatment controversies |
| Offers downloadable PDF version |
| NATIONAL CANCER INSTITUTE |
| cis.nci.nih.gov/asp/FactSheetPub/AlphaSubList.asp?alpha=47 |
| 10th grade reading level |
| FAQ format |
| Offers Spanish version |
| AMERICAN CANCER SOCIETY |
| www.cancer.org/docroot/CRI/content/CRI_2_4_3X_Can_prostate_ cancer_be_found_early_36.asp |
| 12th grade reading level |
| Lacks discussion of treatment options and their side effects |
| Biased in favor of screening but acknowledges that other distinguished organizations are not |
| AMERICAN UROLOGICAL ASSOCIATION |
| www.urologyhealth.org/adult/index.cfm?cat=09 |
| 12th grade reading level |
| Easy to navigate among screening and specific treatment pages |
| Biased in favor of PSA screening |
| AMERICAN ACADEMY OF FAMILY PHYSICIANS |
| familydoctor.org/healthfacts/361/ |
| 11th grade reading level |
| Question/answer format |
| Very straightforward, lacks depth |
| www.aafp.org/x19519.xml |
| 7th grade reading level |
| Separate information sheet for patients and physicians |
| Presents possible outcomes of PSA test and prostate cancer treatment in easy-to-follow format |
| WEBMD |
| my.webmd.com/medical_information/condition_centers/prostate_cancer/default.htm |
| 9th grade reading level |
| Question/answer format |
| Specifically addresses false negative and positives with current estimates |
| DARTMOUTH CENTER FOR SHARED DECISION MAKING |
| www.dhmc.org/dhmc-internet-upload/file_collection/PSA.pdf |
| 6th grade reading level |
| Well-designed, simple presentation of pros and cons of PSA testing |
| *Fleish-Kincaid grade level score based on average sentence length and average number of syllables per word. |
TABLE 5
Talking points for patients and physicians
| Prostate cancer is an important men’s health problem |
| Screening may prevent early prostate cancer death |
| DRE alone has little value as a screening test |
| Age, prostate size, prostatitis, ejaculation, prostate biopsy, and prostate surgery can cause a falsely elevated PSA test |
| Approximately 70% of men with an elevated serum PSA do not have cancer |
| The percentage of PSA screening false negatives ranges from 10%–22% in large studies |
| If the test is abnormal, a biopsy will be recommended |
| If the biopsy is positive, treatment options will be given |
| Many men experience long-term urinary incontinence and impotence related to their treatment |
CORRESPONDING AUTHOR
Kendra Schwartz, MD, MSPH, 101 E. Alexandrine, Detroit, MI 48201, E-mail: kensch@med.wayne.edu
1. American Academy of Family Physicians. Summary of recommendation for periodic health examinations. August 2002. Available at: www.aafp.org/PreBuilt/PHERev5.30802.pdf. Accessed on June 10, 2005.
2. US Preventive Services Task Force. Screening for Prostate Cancer. 2002. Available at: www.ahrq.gov/clinic/uspstf/uspsprca.htm. Accessed on June 10, 2005.
3. American College of Physicians. Screening for prostate cancer. Position paper. Clinical Guideline: Part III. Ann Intern Med 1997;126:480-484.
4. Harris R, Lohr K, Beck R, Fink K, Godley P, Bunton A. Screening for prostate cancer. Systematic Evidence Review for AHRQ. Available at: www.ahrq.gov/uspstfix.htm. Accessed on June 10, 2005.
5. American Cancer Society. Cancer statistics 2005. CA Cancer J Clin 2005;55:10-30.
6. Ries L, Eisner M, Kosary C, et al. SEER Cancer Statistics Review, 1975–2000. Bethesda, Md: National Cancer Institute; 2003. Available at seer.cancer.gov/csr/1975_2002/. Accessed on June 10, 2005.
7. DevCan: Probability of dying of cancer [computer program]. Version 5.1. Bethesda, Md: National Cancer Instititute; 2003. Available at: srab.cancer.gov/devcan/. Accessed on June 10, 2005.
8. Neal DE, Leung HY, Powell PH, Hamdy FC, Donovan JL. Unanswered questions in screening for prostate cancer. Eur J Cancer 2000;36:1316-1321.
9. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Alexandria, Va: International Medical Publishing; 1996.
10. Kabalin J, McNeal J, Price H, Freiha F, Stamey T. Unsuspected adenocarcinoma of the prostate in patients undergooing cystoprostatectomy for other causes: incidence, histology and morphometric observations. J Urol 1989;141:1091-1094.
11. Montie J, Wood DJ, Pontes J, Boyett J, Levin H. Adenocarcinoma of the prostate in cystoprostatectomy specimens removed for bladder cancer. Cancer 1989;63:381-385.
12. Bunting PS. Screening for prostate cancer with prostate-specific antigen: beware the biases. Clin Chim Acta 2002;315:71-97.
13. Gronberg H. Prostate cancer epidemiology. Lancet 2003;361:859-864.
14. Harris R, Lohr KN. Screening for prostate cancer: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137:917-929.
15. American Cancer Society. ACS Cancer Detection Guidelines. Available at: www.cancer.org/docroot/PED/content/PED_2_3X_ACS_Cancer_Detection_Guidelines _36.asp. Accessed June 10, 2005.
16. Smith D, Catalona W. Interexaminer variability of digital rectal examination in detecting prostate cancer. Urology 1995;45:70-74.
17. Kishor M, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract 2003;16:95-101.
18. Hoogendam A, Buntix F, deVet HCW. The diagnostic value of digital rectal examination in primary care screening for prostate cancer: a meta-analysis. Fam Pract 1999;16:621-626.
19. Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA 1995;273:289-294.
20. Hakama M, Stenman UH, Aromaa A, Leinonen J, Hakulinen T, Knekt I. Validity of the prostate specific antigen test for prostate cancer screening: followup study with a bank of 21,000 sera in Finland. J Urol 2001;166:2189-2191.
21. Humphrey L, Helfand M, Chan B, Woolf S. Breast cancer screening: summary of the evidence. Ann Intern Med 2002;137:344-346.
22. Thompson I, Pauler D, Goodman P, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level <4 ng per milliliter. N Engl J Med 2004;350:2239-2246.
23. Collins MM, Ransohoff D, MJ Barry. Early detection of prostate cancer-serendipity strikes again. JAMA 1997;278:1516-1519.
24. Eastham J, Riedel E, Scardino P, et al. Variation of serum prostate-specific antigen levels. An evaluation of year-to-year fluctuations. JAMA 2003;289:2695-2700.
25. Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst 2002;94:981-990.
26. Zappa M, Ciatto S, Bonardi R, Mazzotta A. Overdiagnosis of prostate carcinoma by screening: an estimate based on the results of the Florence Screening Pilot Study. Ann Oncol 1998;9:1297-1300.
27. Draisma G, Boer R, Otto S, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European randomized study of screening for prostate cancer. J Natl Cancer Inst 2003;95:868-878.
28. Auvin A, Maattanen L, Stenman UH, et al. Lead-time in prostate cancer screening (Finland). Cancer Causes Control 2002;13:279-285.
29. Gleason D, Mellinger G. Group at VACUR. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 1974;111:58-64.
30. Albertson PC, Hanley JA, Fine J. 20-Year outcomes following conservative management of clinically localized prostate cancer. JAMA 2005;293:2095-2101.
31. Schwartz K, Grignon D, Sakr W, Wood DJ. Prostate cancer histologic trends in the metropolitan Detroit are, 1982 to 1996. Urology 1999;53:769-774.
32. Smith D, Catalona W. The nature of prostate cancer detected through prostate specific antigen based screening. J Urol 1994;152:1732-1736.
33. Hoedemaeker RF, van der Kwast T, Boer R, et al. Pathological features of prostate cancer found at population-based screening with a four-year interval. J Natl Cancer Inst 2001;93:1153-1158.
34. Chu KC, Tarone RE, Freeman HP. Trends in prostate cancer mortality among black men and white men in the United States. Cancer 2003;97:1507-1516.
35. National Cancer Institute of Canada. Canadian cancer statistics 2001. Toronto: National Cancer Institute of Canada; 2001. Available at: www.ncic.cancer.ca. Accessed June 10, 2005.
36. Coory M, Baade P. Mortality from prostate cancer is decreasing. Med J Aust 2002;176:345-345.
37. Majeed A, Babb P, Jones J, Quinn M. Trends in prostate cancer incidence, mortality and survival in England and Wales, 1971–1998. BJU Int 2000;85:1058-1062.
38. Oliver S, Gunnell D, Donovan J. Comparison of trends in prostate-cancer mortality in England and Wales and the USA. Lancet 2000;355:1788-1789.
39. Lu-Yao G, Albertsen PC, Stanford JL, Stukel TA, Walker-Corkery ES, Barry MJ. Natural experiment examining impact of aggressive screening and treatment on prostate cancer mortality in two fixed cohorts from Seattle area and Connecticut. BMJ 2002;325:740.-
40. Coldman A, Phillips N, Pickles T. Trends in prostate cancer incidence and mortality: an analysis of mortality change by screening intensity. CMAJ 2003;168:31-35.
41. Feuer EJ, Merrill RM, Hankey BF. Cancer surveillance series: interpreting trends in prostate cancer—part II: Cause of death misclassification and the recent rise and fall in prostate cancer mortality. J Natl Cancer Inst 1999;91:1025-1032.
42. Frankel S, Smith GD, Donovan J, Neal D. Screening for prostate cancer. Lancet 2003;361:1122-1128.
43. Partin MR, Wilt TJ. Informing patients about prostate cancer screening: identifying and meeting the challenges while the evidence remains uncertain. Am J Med 2002;113:691-693.
44. McFall SL, Hamm RM. Interpretation of prostate cancer screening events and outcomes: a focus group study. Patient Educ Couns 2003;49:207-218.
45. Litwin MS, Hays RD, Fink A, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA 1995;273:129-135.
46. Penson DF, Litwin MS. Quality of life after treatment for prostate cancer. Curr Urol Rep 2003;4:185-195.
47. Lubeck DP, Litwin MS, Henning JM, Stoddard ML, Flanders SC, Carroll PR. Changes in the health-related quality of life in the first year after treatment for prostate cancer: results from CaPSURE. Urology 1999;53:180-186.
48. Bacon C, Giovannucci E, Testa M, Kawachi I. The impact of cancer treatment on quality of life outcomes for patients with localized prostate cancer. J Urol 2001;166:1804-1810.
49. Makinen T, Auvinen A, Hakama M, Stenman UH, Tammela TL. Acceptability and complications of prostate biopsy in population-based PSA screening versus routine clinical practice: a prospective, controlled study. Urology 2002;60:846-850.
50. Fowler FJ, Jr, Barry MJ, Lu-Yao G, Roman A, Wasson J, Wennberg JE. Patient-reported complications and follow-up treatment after radical prostatectomy. The National Medicare Experience: 1988-1990 (updated June 1993). Urology 1993;42:622-629.
51. Schwartz K, Bunner S, Bearer R, Severson RK. Complications from treatment for prostate carcinoma among men in the Detroit area. Cancer 2002;95:82-89.
52. Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst 2000;92:1582-1592.
53. Sebesta M, Cespedes RD, Luhman E, Optenberg S, Thompson IM. Questionnaire-based outcomes of urinary incontinence and satisfaction rates after radical prostatectomy in a national study population. Urology 2002;60:1055-1058.
54. Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005;352:1977-1184.
55. Labrie F, Candas B, Dupont A, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate 1999;38:83-91.
56. Alexander FE, Prescott RJ. Reply to Labrie et al. Results of the mortality analysis of the Quebec randomized controlled trial (RCT). Prostate 1999;40:135-137.
57. Prorok P, Andriole G, Bresalier R, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials 2000;21:273S-309S.
58. Standaert B, Denis L. The European Randomized Study of Screening for Prostate Cancer: an update. Cancer 1997;80:1830-1834.
59. Wilt TJ, Brawer M. The Prostate Cancer Intervention Versus Observation Trial: a randomized trial comparing radical prostatectomy versus expectant management for the treatment of clinically localized prostate cancer. J Urol 1994;152:1910-1914.
60. Schapira M, VanRuiswyk J. The effect of an illustrated pamphlet decision-aid on the use of prostate cancer screening tests. J Fam Pract 2000;49:418-424.
61. Davison B, Kirk P, Degner L, Hassard T. Information and patient participation in screening for prostate cancer. Patient Educ Couns 1999;37:255-263.
62. Merenstein D. Winners and losers. JAMA 2004;291:15-16.
63. Horninger W, Berger A, Pelzer A, et al. Screening for prostate cancer: updated experience from the Tyrol study. Current Urol Reports 2004;5:220-225.
64. Raaijmakers R, Kirkels WJ, Roobol MJ, Wildhagen MF, Schrder FH. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology 2002;60:826-830.
65. Fowler F, Barry MJ, Lu-Yao G, Wasson J, Bin L. Outcomes of external beam radiation therapy for prostate cancer: a study of Medicare beneficiaries in three Surveillance, Epidemiology, and End Results areas. J Clin Oncol 1996;14:2258-2265.
66. Hollenbeck BK, Dunn RL, Wei JT, Sandler HM, Sanda MG. Sexual health recovery after prostatectomy, external radiation, or brachytherapy for early stage prostate cancer. Curr Urol Rep 2004;5:212-219.
67. Potosky AL, Knopf K, Clegg LX, et al. Quality-of-life outcomes after primary androgen deprivation therapy: results from the Prostate Cancer Outcomes Study. J Clin Oncol 2001;19:3750-3757.
68. Lee R, Penson DF. Treatment outcomes in localized prostate cancer: a patient-oriented approach. Semin Urol Oncol 2002;20:63-73.
69. Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA 2000;283:354-360.
70. Robinson JW, Moritz S, Fung T. Meta-analysis of rates of erectile function after treatment of localized prostate carcinoma. Int J Radiat Oncol Biol Phys 2002;54:1063-1068.
71. Coley CM, Barry MJ, Fleming C, Fahs MC, Mulley AG. Early detection of prostate cancer. Part II: Estimating the risks, benefits, and costs. American College of Physicians. Ann Intern Med 1997;126:468-479.
1. American Academy of Family Physicians. Summary of recommendation for periodic health examinations. August 2002. Available at: www.aafp.org/PreBuilt/PHERev5.30802.pdf. Accessed on June 10, 2005.
2. US Preventive Services Task Force. Screening for Prostate Cancer. 2002. Available at: www.ahrq.gov/clinic/uspstf/uspsprca.htm. Accessed on June 10, 2005.
3. American College of Physicians. Screening for prostate cancer. Position paper. Clinical Guideline: Part III. Ann Intern Med 1997;126:480-484.
4. Harris R, Lohr K, Beck R, Fink K, Godley P, Bunton A. Screening for prostate cancer. Systematic Evidence Review for AHRQ. Available at: www.ahrq.gov/uspstfix.htm. Accessed on June 10, 2005.
5. American Cancer Society. Cancer statistics 2005. CA Cancer J Clin 2005;55:10-30.
6. Ries L, Eisner M, Kosary C, et al. SEER Cancer Statistics Review, 1975–2000. Bethesda, Md: National Cancer Institute; 2003. Available at seer.cancer.gov/csr/1975_2002/. Accessed on June 10, 2005.
7. DevCan: Probability of dying of cancer [computer program]. Version 5.1. Bethesda, Md: National Cancer Instititute; 2003. Available at: srab.cancer.gov/devcan/. Accessed on June 10, 2005.
8. Neal DE, Leung HY, Powell PH, Hamdy FC, Donovan JL. Unanswered questions in screening for prostate cancer. Eur J Cancer 2000;36:1316-1321.
9. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Alexandria, Va: International Medical Publishing; 1996.
10. Kabalin J, McNeal J, Price H, Freiha F, Stamey T. Unsuspected adenocarcinoma of the prostate in patients undergooing cystoprostatectomy for other causes: incidence, histology and morphometric observations. J Urol 1989;141:1091-1094.
11. Montie J, Wood DJ, Pontes J, Boyett J, Levin H. Adenocarcinoma of the prostate in cystoprostatectomy specimens removed for bladder cancer. Cancer 1989;63:381-385.
12. Bunting PS. Screening for prostate cancer with prostate-specific antigen: beware the biases. Clin Chim Acta 2002;315:71-97.
13. Gronberg H. Prostate cancer epidemiology. Lancet 2003;361:859-864.
14. Harris R, Lohr KN. Screening for prostate cancer: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137:917-929.
15. American Cancer Society. ACS Cancer Detection Guidelines. Available at: www.cancer.org/docroot/PED/content/PED_2_3X_ACS_Cancer_Detection_Guidelines _36.asp. Accessed June 10, 2005.
16. Smith D, Catalona W. Interexaminer variability of digital rectal examination in detecting prostate cancer. Urology 1995;45:70-74.
17. Kishor M, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract 2003;16:95-101.
18. Hoogendam A, Buntix F, deVet HCW. The diagnostic value of digital rectal examination in primary care screening for prostate cancer: a meta-analysis. Fam Pract 1999;16:621-626.
19. Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA 1995;273:289-294.
20. Hakama M, Stenman UH, Aromaa A, Leinonen J, Hakulinen T, Knekt I. Validity of the prostate specific antigen test for prostate cancer screening: followup study with a bank of 21,000 sera in Finland. J Urol 2001;166:2189-2191.
21. Humphrey L, Helfand M, Chan B, Woolf S. Breast cancer screening: summary of the evidence. Ann Intern Med 2002;137:344-346.
22. Thompson I, Pauler D, Goodman P, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level <4 ng per milliliter. N Engl J Med 2004;350:2239-2246.
23. Collins MM, Ransohoff D, MJ Barry. Early detection of prostate cancer-serendipity strikes again. JAMA 1997;278:1516-1519.
24. Eastham J, Riedel E, Scardino P, et al. Variation of serum prostate-specific antigen levels. An evaluation of year-to-year fluctuations. JAMA 2003;289:2695-2700.
25. Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst 2002;94:981-990.
26. Zappa M, Ciatto S, Bonardi R, Mazzotta A. Overdiagnosis of prostate carcinoma by screening: an estimate based on the results of the Florence Screening Pilot Study. Ann Oncol 1998;9:1297-1300.
27. Draisma G, Boer R, Otto S, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European randomized study of screening for prostate cancer. J Natl Cancer Inst 2003;95:868-878.
28. Auvin A, Maattanen L, Stenman UH, et al. Lead-time in prostate cancer screening (Finland). Cancer Causes Control 2002;13:279-285.
29. Gleason D, Mellinger G. Group at VACUR. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 1974;111:58-64.
30. Albertson PC, Hanley JA, Fine J. 20-Year outcomes following conservative management of clinically localized prostate cancer. JAMA 2005;293:2095-2101.
31. Schwartz K, Grignon D, Sakr W, Wood DJ. Prostate cancer histologic trends in the metropolitan Detroit are, 1982 to 1996. Urology 1999;53:769-774.
32. Smith D, Catalona W. The nature of prostate cancer detected through prostate specific antigen based screening. J Urol 1994;152:1732-1736.
33. Hoedemaeker RF, van der Kwast T, Boer R, et al. Pathological features of prostate cancer found at population-based screening with a four-year interval. J Natl Cancer Inst 2001;93:1153-1158.
34. Chu KC, Tarone RE, Freeman HP. Trends in prostate cancer mortality among black men and white men in the United States. Cancer 2003;97:1507-1516.
35. National Cancer Institute of Canada. Canadian cancer statistics 2001. Toronto: National Cancer Institute of Canada; 2001. Available at: www.ncic.cancer.ca. Accessed June 10, 2005.
36. Coory M, Baade P. Mortality from prostate cancer is decreasing. Med J Aust 2002;176:345-345.
37. Majeed A, Babb P, Jones J, Quinn M. Trends in prostate cancer incidence, mortality and survival in England and Wales, 1971–1998. BJU Int 2000;85:1058-1062.
38. Oliver S, Gunnell D, Donovan J. Comparison of trends in prostate-cancer mortality in England and Wales and the USA. Lancet 2000;355:1788-1789.
39. Lu-Yao G, Albertsen PC, Stanford JL, Stukel TA, Walker-Corkery ES, Barry MJ. Natural experiment examining impact of aggressive screening and treatment on prostate cancer mortality in two fixed cohorts from Seattle area and Connecticut. BMJ 2002;325:740.-
40. Coldman A, Phillips N, Pickles T. Trends in prostate cancer incidence and mortality: an analysis of mortality change by screening intensity. CMAJ 2003;168:31-35.
41. Feuer EJ, Merrill RM, Hankey BF. Cancer surveillance series: interpreting trends in prostate cancer—part II: Cause of death misclassification and the recent rise and fall in prostate cancer mortality. J Natl Cancer Inst 1999;91:1025-1032.
42. Frankel S, Smith GD, Donovan J, Neal D. Screening for prostate cancer. Lancet 2003;361:1122-1128.
43. Partin MR, Wilt TJ. Informing patients about prostate cancer screening: identifying and meeting the challenges while the evidence remains uncertain. Am J Med 2002;113:691-693.
44. McFall SL, Hamm RM. Interpretation of prostate cancer screening events and outcomes: a focus group study. Patient Educ Couns 2003;49:207-218.
45. Litwin MS, Hays RD, Fink A, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA 1995;273:129-135.
46. Penson DF, Litwin MS. Quality of life after treatment for prostate cancer. Curr Urol Rep 2003;4:185-195.
47. Lubeck DP, Litwin MS, Henning JM, Stoddard ML, Flanders SC, Carroll PR. Changes in the health-related quality of life in the first year after treatment for prostate cancer: results from CaPSURE. Urology 1999;53:180-186.
48. Bacon C, Giovannucci E, Testa M, Kawachi I. The impact of cancer treatment on quality of life outcomes for patients with localized prostate cancer. J Urol 2001;166:1804-1810.
49. Makinen T, Auvinen A, Hakama M, Stenman UH, Tammela TL. Acceptability and complications of prostate biopsy in population-based PSA screening versus routine clinical practice: a prospective, controlled study. Urology 2002;60:846-850.
50. Fowler FJ, Jr, Barry MJ, Lu-Yao G, Roman A, Wasson J, Wennberg JE. Patient-reported complications and follow-up treatment after radical prostatectomy. The National Medicare Experience: 1988-1990 (updated June 1993). Urology 1993;42:622-629.
51. Schwartz K, Bunner S, Bearer R, Severson RK. Complications from treatment for prostate carcinoma among men in the Detroit area. Cancer 2002;95:82-89.
52. Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst 2000;92:1582-1592.
53. Sebesta M, Cespedes RD, Luhman E, Optenberg S, Thompson IM. Questionnaire-based outcomes of urinary incontinence and satisfaction rates after radical prostatectomy in a national study population. Urology 2002;60:1055-1058.
54. Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005;352:1977-1184.
55. Labrie F, Candas B, Dupont A, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate 1999;38:83-91.
56. Alexander FE, Prescott RJ. Reply to Labrie et al. Results of the mortality analysis of the Quebec randomized controlled trial (RCT). Prostate 1999;40:135-137.
57. Prorok P, Andriole G, Bresalier R, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials 2000;21:273S-309S.
58. Standaert B, Denis L. The European Randomized Study of Screening for Prostate Cancer: an update. Cancer 1997;80:1830-1834.
59. Wilt TJ, Brawer M. The Prostate Cancer Intervention Versus Observation Trial: a randomized trial comparing radical prostatectomy versus expectant management for the treatment of clinically localized prostate cancer. J Urol 1994;152:1910-1914.
60. Schapira M, VanRuiswyk J. The effect of an illustrated pamphlet decision-aid on the use of prostate cancer screening tests. J Fam Pract 2000;49:418-424.
61. Davison B, Kirk P, Degner L, Hassard T. Information and patient participation in screening for prostate cancer. Patient Educ Couns 1999;37:255-263.
62. Merenstein D. Winners and losers. JAMA 2004;291:15-16.
63. Horninger W, Berger A, Pelzer A, et al. Screening for prostate cancer: updated experience from the Tyrol study. Current Urol Reports 2004;5:220-225.
64. Raaijmakers R, Kirkels WJ, Roobol MJ, Wildhagen MF, Schrder FH. Complication rates and risk factors of 5802 transrectal ultrasound-guided sextant biopsies of the prostate within a population-based screening program. Urology 2002;60:826-830.
65. Fowler F, Barry MJ, Lu-Yao G, Wasson J, Bin L. Outcomes of external beam radiation therapy for prostate cancer: a study of Medicare beneficiaries in three Surveillance, Epidemiology, and End Results areas. J Clin Oncol 1996;14:2258-2265.
66. Hollenbeck BK, Dunn RL, Wei JT, Sandler HM, Sanda MG. Sexual health recovery after prostatectomy, external radiation, or brachytherapy for early stage prostate cancer. Curr Urol Rep 2004;5:212-219.
67. Potosky AL, Knopf K, Clegg LX, et al. Quality-of-life outcomes after primary androgen deprivation therapy: results from the Prostate Cancer Outcomes Study. J Clin Oncol 2001;19:3750-3757.
68. Lee R, Penson DF. Treatment outcomes in localized prostate cancer: a patient-oriented approach. Semin Urol Oncol 2002;20:63-73.
69. Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA 2000;283:354-360.
70. Robinson JW, Moritz S, Fung T. Meta-analysis of rates of erectile function after treatment of localized prostate carcinoma. Int J Radiat Oncol Biol Phys 2002;54:1063-1068.
71. Coley CM, Barry MJ, Fleming C, Fahs MC, Mulley AG. Early detection of prostate cancer. Part II: Estimating the risks, benefits, and costs. American College of Physicians. Ann Intern Med 1997;126:468-479.