User login
To the Editor:
A 28-year-old recently homeless white man with a history of heroin abuse was admitted with a worsening rash and left ankle pain of 1 week’s duration, as well as subjective fever after 3 weeks of a productive cough, sore throat, hoarse voice, and general malaise. Six days prior to presentation, he developed redness and swelling of the dorsal aspects of both hands with accompanying rash, and 2 days prior to presentation he developed a similar rash on the legs with associated left ankle pain, redness, and swelling. He also reported eye redness, pain, photophobia, crusty eye discharge, and a pins and needles sensation on the soles of both feet. Additionally, he had noted difficulty with urination over several days. He had been homeless for less than 1 month prior to admission.
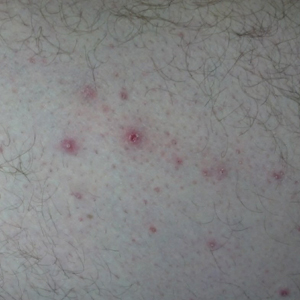
On physical examination, the patient appeared to be well nourished. Skin examination was notable for scattered perifollicular hemorrhagic and hyperkeratotic papules ranging in size from 3 to 6 mm with associated nummular alopecia of the bilateral medial thighs (Figure); well-demarcated desquamated patches on the weight-bearing aspects of the plantar feet; and a 2.0-cm, well-demarcated, thinly raised erythematous patch of the inferolateral penile shaft. Oral examination was notable for multiple discrete areas of ulceration on the lateral aspects of the tongue. Ophthalmic examination revealed conjunctival injection and photophobia. The ankles were edematous and tender (the left ankle more than the right), and range of passive motion was limited by pain.
Laboratory values were remarkable for a hemoglobin count of 13.1 g/dL (reference range, 14.2–18 g/dL), erythrocyte sedimentation rate of 31 mm/h (reference range, 0–10 mm/h), and C-reactive protein level of 5.4 mg/dL (reference range, 0–0.8 mg/dL). Urinalysis was unremarkable, blood cultures were negative, and a chest radiograph was normal. Human immunodeficiency virus and rapid plasma reagin tests were negative, with normal levels of IgG, IgA, and IgM. IgE was elevated at 572 IU/mL (reference range, 0–100 IU/mL). Ultrasonography of the leg was negative for deep vein thrombosis, and a left ankle radiograph was negative for fracture. The patient previously was found to have antinuclear antibodies of 1:40 and negative antineutrophil cytoplasmic antibodies, anti–double-stranded DNA, anti–Sjögren syndrome antigens A and B, and cryoglobulins, as well as normal complement levels. The constellation of rash, arthritis, conjunctivitis, and difficulty with urination raised a high suspicion for reactive arthritis; however, the patient was found to be HLA-B27 negative with a negative urine chlamydia test.
The patient was mildly hypokalemic at 2.9 mmol/L (reference range, 3.5–5.0 mmol/L) and hypoalbuminemic at 3.6 g/dL (reference range, 3.9–5.0 g/dL). He had a slightly elevated international normalized ratio of 1.4 (reference range, 0.9–1.2). Further questioning revealed that his diet consisted mostly of soda and energy drinks; his vitamin C level was subsequently checked and found to be 0 mg/dL (reference range, 0.2–2.0 mg/dL). A diagnosis of scurvy was made, and his symptoms improved at the hospital while maintaining a diet with normal levels of vitamin C. His rash had markedly improved by hospital day 2, joint swelling decreased, and the conjunctival injection and eye pain had resolved. Upon outpatient follow-up, his rash and joint swelling continued to improve, and he had not experienced any further areas of hair loss.
Scurvy, a condition caused by vitamin C deficiency, is a disease of historical importance, as it ravaged ships full of sailors in days past; however, its incidence has decreased drastically since Lind1 first described its treatment using citrus fruits in 1753. Nonetheless, even with modern day access to foods rich in vitamin C, scurvy is far more common than expected in the developed world.
Vitamin C (ascorbic acid) plays a crucial role in human biochemistry. Although many plants and animals can synthesize ascorbic acid, humans and other animals such as guinea pigs lack the required enzyme, making vitamin C an essential nutrient required in dietary intake.2-4 Hypovitaminosis C leads to scurvy when collagen production becomes impaired due to lack of ascorbic acid as a required cofactor for its synthesis, which leads to tissue and capillary fragility, causing hemorrhage and perivascular edema.4 The diagnosis of scurvy is clinical and typically is based on signs such as perivascular hemorrhage, bleeding gums, anemia, impaired wound healing, and ecchymoses in the setting of vitamin C deficiency (<11 μmol/L or <0.2 mg/dL) with rapid resolution upon vitamin C supplementation.5
Important sources of vitamin C include citrus fruits, strawberries, broccoli, spinach, and potatoes. Recommended daily intake is 75 to 90 mg, with smokers requiring 110 to 125 mg daily because of increased oxidative stress.6-9 Although access to these foods in the modern United States is high, as many as 10% of males and 6.9% of females are vitamin C deficient, and in the subset of generally healthy middle-class Americans, as many as 6% are deficient.8,10 The highest risk groups tend to be smokers and individuals with low incomes.8 Although vitamin C deficiency does not automatically equate to scurvy, early studies on experimentally induced scurvy in prisoners showed that signs of scurvy may begin to develop in as few as 29 days of complete vitamin C deprivation, with overt scurvy developing after approximately 40 to 90 days.11,12
Patients with scurvy often pose a diagnostic dilemma for physicians because their presenting symptoms, such as fatigue, anemia, and rash, are nonspecific and can lead physicians down a laborious and costly road of unnecessary tests including vasculitic, infectious, and rheumatologic workups to determine the cause of the symptoms. Increased awareness of the current prevalence of hypovitaminosis C may help to decrease these unnecessary costs by putting scurvy higher on the differential for patients with this spectrum of symptoms.
Scurvy has been called the eternal masquerader because its nonspecific signs and symptoms have often led to misdiagnosis.13 Cases of scurvy mimicking diseases ranging from bone tumors14 to spondyloarthritis15 and vasculitis16 have been reported. The typical patient at risk for scurvy tends to fall in one of the following categories: psychiatric illness, gastrointestinal disorders, malnourishment, chronic alcoholism, drug use, elderly age, infants, restrictive dietary habits or food allergies, or those in developing countries.17-20 Our patient did not fit particularly well into any of the aforementioned high-risk categories; he had only recently become homeless and had a history of intravenous drug use but had not been using drugs in the months prior to the development of scurvy. Additionally, his salient symptoms were more consistent with reactive arthritis than with classic scurvy.
Although he had many symptoms consistent with scurvy such as generalized malaise, perifollicular hemorrhage and hyperkeratosis, spongy edema of the joints, and mild anemia on laboratory testing, he was missing several classic scurvy symptoms. Unlike many patients with scurvy, our patient did not describe any history of bruising easily or dental concerns, and examination was notably absent of ecchymoses as well as spongy or bleeding gums. He did, however, present with eye irritation and photophobia. These symptoms, consistent with keratoconjunctivitis sicca, are lesser known because ocular findings are rarely found in scurvy.21 Patients with scurvy can report eye burning and irritation, redness, blurry vision, and sensitivity to bright light secondary to increased dryness of the corneal surfaces. Horrobin et al22 postulated that this symptom may be mediated by regulation of prostaglandin E1 by vitamin C.
Another less common sign of scurvy found in our patient was patchy alopecia. Alopecia most often is seen in association with concomitant Sjögren syndrome.11,23 The etiology of the hair loss stems from the role of ascorbic acid in disulfide bonding during hair formation. The hair may fracture, coil into a corkscrew hair, or bend in several places, leading to a swan-neck deformity. Although a skin biopsy was not performed in our patient, results typically demonstrate a coiled hair in its follicle.24,25
We present the case of an otherwise generally healthy patient who developed vitamin C deficiency due to a diet consisting mostly of soda and energy drinks. His case presented a diagnostic dilemma, as his symptoms at first seemed most consistent with reactive arthritis and he was missing several of the risk factors and symptoms that would have led to an early diagnosis of scurvy. Vitamin C deficiency is not as uncommon as expected in the developed world; practitioners must be aware of the common as well as the unusual signs of scurvy.
- Lind J. A Treatise of the Scurvy. Edinburgh, Scotland: Sands, Murray, and Cochran; 1753.
- Levine M, Rumsey SC, Daruwala R, et al. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415-1423.
- Jacob RA. Vitamin C. In: Shils ME, Olson JA, Shike M, et al, eds. Modern Nutrition in Health and Disease. Baltimore, MD: William & Wilkins; 1999:467-483.
- Levine M. New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med. 1986;314:892-902.
- Hirschman JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999;41:895-906.
- Bardnard ND, Weissinger R, Jaster BJ, et al, eds. Nutrition Guide for Clinicians. 2nd ed. Washington, DC: Physician’s Committee For Responsible Medicine; 2009:33.
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington DC: National Academic Press; 2000.
- Schleicher RL, Carroll MD, Ford ES, et al. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252-1263.
- Schectman G, Byrd JC, Gruchow HW. The influence of smoking on vitamin C status in adults. Am J Public Health. 1989;79:158-162.
- Johnston CS, Thompson LL. Vitamin C status of an outpatient population. J Am Coll Nutr. 1998;17:366-370.
- Hodges RE, Baker EM, Hood J, et al. Experimental scurvy in man. Am J Clin Nutr. 1969;22:535-548.
- Hodges RE, Hood J, Canham JE, et al. Clinical manifestations of ascorbic acid deficiency in man. Am J Clin Nutr. 1971;24:432-443.
- Gupta P, Taneja K, Iyer PU, et al. Scurvy—the eternal masquerader. Ann Trop Paediatr. 1989;9:118-121.
- Haq RU, Dhammi IK, Jain AK, et al. Infantile scurvy masquerading as bone tumour. Ann Acad Med Singapore. 2013;42:363-365.
- Pazzola G, Possemato N, Germanò G, et al. Scurvy mimicking spondyloarthritis in a young man. Clin Exp Rheumatol. 2013;31:795.
- Friesgaard Christensen A, Clemmensen O, Junker P. Palpable purpura with an unexpected outcome. Case Rep Rheumatol. 2013;2013:678427.
- Des Roches A, Paradis L, Paradis J, et al. Food allergy as a new risk factor for scurvy. Allergy. 2006;61:1487-1488.
- Pimentel L. Scurvy: historical review and current diagnostic approach. Am J Emerg Med. 2003;21:328-332.
- Codreanu F, Jarlot S, Astier C, et al. An apple a day...chronic glossitis in a 4-year-old boy. Eur Ann Allergy Clin Immunol. 2012;44:86-88.
- Stephen R, Utecht T. Scurvy identified in the emergency department: a case report. J Emerg Med. 2001;21:235-237.
- Hood J, Hodges RE. Ocular lesions in scurvy. Am J Clin Nutr. 1969;22:559-567.
- Horrobin DF, Oka M, Manku MS. The regulation of prostaglandin E1 formation: a candidate for one of the fundamental mechanisms involved in the actions of vitamin C. Med Hypotheses. 1979;5:849-858.
- Hood J, Burns CA, Hodges RE. Sjogren’s syndrome in scurvy. N Engl J Med. 1970;282:1120-1124.
- Walter JF. Scurvy resulting from a self-imposed diet. West J Med. 1979;130:177-179.
- Velandia B, Centor RM, McConnell V, et al. Scurvy is still present in developed countries. J Gen Intern Med. 2008;23:1281-1284.
To the Editor:
A 28-year-old recently homeless white man with a history of heroin abuse was admitted with a worsening rash and left ankle pain of 1 week’s duration, as well as subjective fever after 3 weeks of a productive cough, sore throat, hoarse voice, and general malaise. Six days prior to presentation, he developed redness and swelling of the dorsal aspects of both hands with accompanying rash, and 2 days prior to presentation he developed a similar rash on the legs with associated left ankle pain, redness, and swelling. He also reported eye redness, pain, photophobia, crusty eye discharge, and a pins and needles sensation on the soles of both feet. Additionally, he had noted difficulty with urination over several days. He had been homeless for less than 1 month prior to admission.
On physical examination, the patient appeared to be well nourished. Skin examination was notable for scattered perifollicular hemorrhagic and hyperkeratotic papules ranging in size from 3 to 6 mm with associated nummular alopecia of the bilateral medial thighs (Figure); well-demarcated desquamated patches on the weight-bearing aspects of the plantar feet; and a 2.0-cm, well-demarcated, thinly raised erythematous patch of the inferolateral penile shaft. Oral examination was notable for multiple discrete areas of ulceration on the lateral aspects of the tongue. Ophthalmic examination revealed conjunctival injection and photophobia. The ankles were edematous and tender (the left ankle more than the right), and range of passive motion was limited by pain.
Laboratory values were remarkable for a hemoglobin count of 13.1 g/dL (reference range, 14.2–18 g/dL), erythrocyte sedimentation rate of 31 mm/h (reference range, 0–10 mm/h), and C-reactive protein level of 5.4 mg/dL (reference range, 0–0.8 mg/dL). Urinalysis was unremarkable, blood cultures were negative, and a chest radiograph was normal. Human immunodeficiency virus and rapid plasma reagin tests were negative, with normal levels of IgG, IgA, and IgM. IgE was elevated at 572 IU/mL (reference range, 0–100 IU/mL). Ultrasonography of the leg was negative for deep vein thrombosis, and a left ankle radiograph was negative for fracture. The patient previously was found to have antinuclear antibodies of 1:40 and negative antineutrophil cytoplasmic antibodies, anti–double-stranded DNA, anti–Sjögren syndrome antigens A and B, and cryoglobulins, as well as normal complement levels. The constellation of rash, arthritis, conjunctivitis, and difficulty with urination raised a high suspicion for reactive arthritis; however, the patient was found to be HLA-B27 negative with a negative urine chlamydia test.
The patient was mildly hypokalemic at 2.9 mmol/L (reference range, 3.5–5.0 mmol/L) and hypoalbuminemic at 3.6 g/dL (reference range, 3.9–5.0 g/dL). He had a slightly elevated international normalized ratio of 1.4 (reference range, 0.9–1.2). Further questioning revealed that his diet consisted mostly of soda and energy drinks; his vitamin C level was subsequently checked and found to be 0 mg/dL (reference range, 0.2–2.0 mg/dL). A diagnosis of scurvy was made, and his symptoms improved at the hospital while maintaining a diet with normal levels of vitamin C. His rash had markedly improved by hospital day 2, joint swelling decreased, and the conjunctival injection and eye pain had resolved. Upon outpatient follow-up, his rash and joint swelling continued to improve, and he had not experienced any further areas of hair loss.
Scurvy, a condition caused by vitamin C deficiency, is a disease of historical importance, as it ravaged ships full of sailors in days past; however, its incidence has decreased drastically since Lind1 first described its treatment using citrus fruits in 1753. Nonetheless, even with modern day access to foods rich in vitamin C, scurvy is far more common than expected in the developed world.
Vitamin C (ascorbic acid) plays a crucial role in human biochemistry. Although many plants and animals can synthesize ascorbic acid, humans and other animals such as guinea pigs lack the required enzyme, making vitamin C an essential nutrient required in dietary intake.2-4 Hypovitaminosis C leads to scurvy when collagen production becomes impaired due to lack of ascorbic acid as a required cofactor for its synthesis, which leads to tissue and capillary fragility, causing hemorrhage and perivascular edema.4 The diagnosis of scurvy is clinical and typically is based on signs such as perivascular hemorrhage, bleeding gums, anemia, impaired wound healing, and ecchymoses in the setting of vitamin C deficiency (<11 μmol/L or <0.2 mg/dL) with rapid resolution upon vitamin C supplementation.5
Important sources of vitamin C include citrus fruits, strawberries, broccoli, spinach, and potatoes. Recommended daily intake is 75 to 90 mg, with smokers requiring 110 to 125 mg daily because of increased oxidative stress.6-9 Although access to these foods in the modern United States is high, as many as 10% of males and 6.9% of females are vitamin C deficient, and in the subset of generally healthy middle-class Americans, as many as 6% are deficient.8,10 The highest risk groups tend to be smokers and individuals with low incomes.8 Although vitamin C deficiency does not automatically equate to scurvy, early studies on experimentally induced scurvy in prisoners showed that signs of scurvy may begin to develop in as few as 29 days of complete vitamin C deprivation, with overt scurvy developing after approximately 40 to 90 days.11,12
Patients with scurvy often pose a diagnostic dilemma for physicians because their presenting symptoms, such as fatigue, anemia, and rash, are nonspecific and can lead physicians down a laborious and costly road of unnecessary tests including vasculitic, infectious, and rheumatologic workups to determine the cause of the symptoms. Increased awareness of the current prevalence of hypovitaminosis C may help to decrease these unnecessary costs by putting scurvy higher on the differential for patients with this spectrum of symptoms.
Scurvy has been called the eternal masquerader because its nonspecific signs and symptoms have often led to misdiagnosis.13 Cases of scurvy mimicking diseases ranging from bone tumors14 to spondyloarthritis15 and vasculitis16 have been reported. The typical patient at risk for scurvy tends to fall in one of the following categories: psychiatric illness, gastrointestinal disorders, malnourishment, chronic alcoholism, drug use, elderly age, infants, restrictive dietary habits or food allergies, or those in developing countries.17-20 Our patient did not fit particularly well into any of the aforementioned high-risk categories; he had only recently become homeless and had a history of intravenous drug use but had not been using drugs in the months prior to the development of scurvy. Additionally, his salient symptoms were more consistent with reactive arthritis than with classic scurvy.
Although he had many symptoms consistent with scurvy such as generalized malaise, perifollicular hemorrhage and hyperkeratosis, spongy edema of the joints, and mild anemia on laboratory testing, he was missing several classic scurvy symptoms. Unlike many patients with scurvy, our patient did not describe any history of bruising easily or dental concerns, and examination was notably absent of ecchymoses as well as spongy or bleeding gums. He did, however, present with eye irritation and photophobia. These symptoms, consistent with keratoconjunctivitis sicca, are lesser known because ocular findings are rarely found in scurvy.21 Patients with scurvy can report eye burning and irritation, redness, blurry vision, and sensitivity to bright light secondary to increased dryness of the corneal surfaces. Horrobin et al22 postulated that this symptom may be mediated by regulation of prostaglandin E1 by vitamin C.
Another less common sign of scurvy found in our patient was patchy alopecia. Alopecia most often is seen in association with concomitant Sjögren syndrome.11,23 The etiology of the hair loss stems from the role of ascorbic acid in disulfide bonding during hair formation. The hair may fracture, coil into a corkscrew hair, or bend in several places, leading to a swan-neck deformity. Although a skin biopsy was not performed in our patient, results typically demonstrate a coiled hair in its follicle.24,25
We present the case of an otherwise generally healthy patient who developed vitamin C deficiency due to a diet consisting mostly of soda and energy drinks. His case presented a diagnostic dilemma, as his symptoms at first seemed most consistent with reactive arthritis and he was missing several of the risk factors and symptoms that would have led to an early diagnosis of scurvy. Vitamin C deficiency is not as uncommon as expected in the developed world; practitioners must be aware of the common as well as the unusual signs of scurvy.
To the Editor:
A 28-year-old recently homeless white man with a history of heroin abuse was admitted with a worsening rash and left ankle pain of 1 week’s duration, as well as subjective fever after 3 weeks of a productive cough, sore throat, hoarse voice, and general malaise. Six days prior to presentation, he developed redness and swelling of the dorsal aspects of both hands with accompanying rash, and 2 days prior to presentation he developed a similar rash on the legs with associated left ankle pain, redness, and swelling. He also reported eye redness, pain, photophobia, crusty eye discharge, and a pins and needles sensation on the soles of both feet. Additionally, he had noted difficulty with urination over several days. He had been homeless for less than 1 month prior to admission.
On physical examination, the patient appeared to be well nourished. Skin examination was notable for scattered perifollicular hemorrhagic and hyperkeratotic papules ranging in size from 3 to 6 mm with associated nummular alopecia of the bilateral medial thighs (Figure); well-demarcated desquamated patches on the weight-bearing aspects of the plantar feet; and a 2.0-cm, well-demarcated, thinly raised erythematous patch of the inferolateral penile shaft. Oral examination was notable for multiple discrete areas of ulceration on the lateral aspects of the tongue. Ophthalmic examination revealed conjunctival injection and photophobia. The ankles were edematous and tender (the left ankle more than the right), and range of passive motion was limited by pain.
Laboratory values were remarkable for a hemoglobin count of 13.1 g/dL (reference range, 14.2–18 g/dL), erythrocyte sedimentation rate of 31 mm/h (reference range, 0–10 mm/h), and C-reactive protein level of 5.4 mg/dL (reference range, 0–0.8 mg/dL). Urinalysis was unremarkable, blood cultures were negative, and a chest radiograph was normal. Human immunodeficiency virus and rapid plasma reagin tests were negative, with normal levels of IgG, IgA, and IgM. IgE was elevated at 572 IU/mL (reference range, 0–100 IU/mL). Ultrasonography of the leg was negative for deep vein thrombosis, and a left ankle radiograph was negative for fracture. The patient previously was found to have antinuclear antibodies of 1:40 and negative antineutrophil cytoplasmic antibodies, anti–double-stranded DNA, anti–Sjögren syndrome antigens A and B, and cryoglobulins, as well as normal complement levels. The constellation of rash, arthritis, conjunctivitis, and difficulty with urination raised a high suspicion for reactive arthritis; however, the patient was found to be HLA-B27 negative with a negative urine chlamydia test.
The patient was mildly hypokalemic at 2.9 mmol/L (reference range, 3.5–5.0 mmol/L) and hypoalbuminemic at 3.6 g/dL (reference range, 3.9–5.0 g/dL). He had a slightly elevated international normalized ratio of 1.4 (reference range, 0.9–1.2). Further questioning revealed that his diet consisted mostly of soda and energy drinks; his vitamin C level was subsequently checked and found to be 0 mg/dL (reference range, 0.2–2.0 mg/dL). A diagnosis of scurvy was made, and his symptoms improved at the hospital while maintaining a diet with normal levels of vitamin C. His rash had markedly improved by hospital day 2, joint swelling decreased, and the conjunctival injection and eye pain had resolved. Upon outpatient follow-up, his rash and joint swelling continued to improve, and he had not experienced any further areas of hair loss.
Scurvy, a condition caused by vitamin C deficiency, is a disease of historical importance, as it ravaged ships full of sailors in days past; however, its incidence has decreased drastically since Lind1 first described its treatment using citrus fruits in 1753. Nonetheless, even with modern day access to foods rich in vitamin C, scurvy is far more common than expected in the developed world.
Vitamin C (ascorbic acid) plays a crucial role in human biochemistry. Although many plants and animals can synthesize ascorbic acid, humans and other animals such as guinea pigs lack the required enzyme, making vitamin C an essential nutrient required in dietary intake.2-4 Hypovitaminosis C leads to scurvy when collagen production becomes impaired due to lack of ascorbic acid as a required cofactor for its synthesis, which leads to tissue and capillary fragility, causing hemorrhage and perivascular edema.4 The diagnosis of scurvy is clinical and typically is based on signs such as perivascular hemorrhage, bleeding gums, anemia, impaired wound healing, and ecchymoses in the setting of vitamin C deficiency (<11 μmol/L or <0.2 mg/dL) with rapid resolution upon vitamin C supplementation.5
Important sources of vitamin C include citrus fruits, strawberries, broccoli, spinach, and potatoes. Recommended daily intake is 75 to 90 mg, with smokers requiring 110 to 125 mg daily because of increased oxidative stress.6-9 Although access to these foods in the modern United States is high, as many as 10% of males and 6.9% of females are vitamin C deficient, and in the subset of generally healthy middle-class Americans, as many as 6% are deficient.8,10 The highest risk groups tend to be smokers and individuals with low incomes.8 Although vitamin C deficiency does not automatically equate to scurvy, early studies on experimentally induced scurvy in prisoners showed that signs of scurvy may begin to develop in as few as 29 days of complete vitamin C deprivation, with overt scurvy developing after approximately 40 to 90 days.11,12
Patients with scurvy often pose a diagnostic dilemma for physicians because their presenting symptoms, such as fatigue, anemia, and rash, are nonspecific and can lead physicians down a laborious and costly road of unnecessary tests including vasculitic, infectious, and rheumatologic workups to determine the cause of the symptoms. Increased awareness of the current prevalence of hypovitaminosis C may help to decrease these unnecessary costs by putting scurvy higher on the differential for patients with this spectrum of symptoms.
Scurvy has been called the eternal masquerader because its nonspecific signs and symptoms have often led to misdiagnosis.13 Cases of scurvy mimicking diseases ranging from bone tumors14 to spondyloarthritis15 and vasculitis16 have been reported. The typical patient at risk for scurvy tends to fall in one of the following categories: psychiatric illness, gastrointestinal disorders, malnourishment, chronic alcoholism, drug use, elderly age, infants, restrictive dietary habits or food allergies, or those in developing countries.17-20 Our patient did not fit particularly well into any of the aforementioned high-risk categories; he had only recently become homeless and had a history of intravenous drug use but had not been using drugs in the months prior to the development of scurvy. Additionally, his salient symptoms were more consistent with reactive arthritis than with classic scurvy.
Although he had many symptoms consistent with scurvy such as generalized malaise, perifollicular hemorrhage and hyperkeratosis, spongy edema of the joints, and mild anemia on laboratory testing, he was missing several classic scurvy symptoms. Unlike many patients with scurvy, our patient did not describe any history of bruising easily or dental concerns, and examination was notably absent of ecchymoses as well as spongy or bleeding gums. He did, however, present with eye irritation and photophobia. These symptoms, consistent with keratoconjunctivitis sicca, are lesser known because ocular findings are rarely found in scurvy.21 Patients with scurvy can report eye burning and irritation, redness, blurry vision, and sensitivity to bright light secondary to increased dryness of the corneal surfaces. Horrobin et al22 postulated that this symptom may be mediated by regulation of prostaglandin E1 by vitamin C.
Another less common sign of scurvy found in our patient was patchy alopecia. Alopecia most often is seen in association with concomitant Sjögren syndrome.11,23 The etiology of the hair loss stems from the role of ascorbic acid in disulfide bonding during hair formation. The hair may fracture, coil into a corkscrew hair, or bend in several places, leading to a swan-neck deformity. Although a skin biopsy was not performed in our patient, results typically demonstrate a coiled hair in its follicle.24,25
We present the case of an otherwise generally healthy patient who developed vitamin C deficiency due to a diet consisting mostly of soda and energy drinks. His case presented a diagnostic dilemma, as his symptoms at first seemed most consistent with reactive arthritis and he was missing several of the risk factors and symptoms that would have led to an early diagnosis of scurvy. Vitamin C deficiency is not as uncommon as expected in the developed world; practitioners must be aware of the common as well as the unusual signs of scurvy.
- Lind J. A Treatise of the Scurvy. Edinburgh, Scotland: Sands, Murray, and Cochran; 1753.
- Levine M, Rumsey SC, Daruwala R, et al. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415-1423.
- Jacob RA. Vitamin C. In: Shils ME, Olson JA, Shike M, et al, eds. Modern Nutrition in Health and Disease. Baltimore, MD: William & Wilkins; 1999:467-483.
- Levine M. New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med. 1986;314:892-902.
- Hirschman JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999;41:895-906.
- Bardnard ND, Weissinger R, Jaster BJ, et al, eds. Nutrition Guide for Clinicians. 2nd ed. Washington, DC: Physician’s Committee For Responsible Medicine; 2009:33.
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington DC: National Academic Press; 2000.
- Schleicher RL, Carroll MD, Ford ES, et al. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252-1263.
- Schectman G, Byrd JC, Gruchow HW. The influence of smoking on vitamin C status in adults. Am J Public Health. 1989;79:158-162.
- Johnston CS, Thompson LL. Vitamin C status of an outpatient population. J Am Coll Nutr. 1998;17:366-370.
- Hodges RE, Baker EM, Hood J, et al. Experimental scurvy in man. Am J Clin Nutr. 1969;22:535-548.
- Hodges RE, Hood J, Canham JE, et al. Clinical manifestations of ascorbic acid deficiency in man. Am J Clin Nutr. 1971;24:432-443.
- Gupta P, Taneja K, Iyer PU, et al. Scurvy—the eternal masquerader. Ann Trop Paediatr. 1989;9:118-121.
- Haq RU, Dhammi IK, Jain AK, et al. Infantile scurvy masquerading as bone tumour. Ann Acad Med Singapore. 2013;42:363-365.
- Pazzola G, Possemato N, Germanò G, et al. Scurvy mimicking spondyloarthritis in a young man. Clin Exp Rheumatol. 2013;31:795.
- Friesgaard Christensen A, Clemmensen O, Junker P. Palpable purpura with an unexpected outcome. Case Rep Rheumatol. 2013;2013:678427.
- Des Roches A, Paradis L, Paradis J, et al. Food allergy as a new risk factor for scurvy. Allergy. 2006;61:1487-1488.
- Pimentel L. Scurvy: historical review and current diagnostic approach. Am J Emerg Med. 2003;21:328-332.
- Codreanu F, Jarlot S, Astier C, et al. An apple a day...chronic glossitis in a 4-year-old boy. Eur Ann Allergy Clin Immunol. 2012;44:86-88.
- Stephen R, Utecht T. Scurvy identified in the emergency department: a case report. J Emerg Med. 2001;21:235-237.
- Hood J, Hodges RE. Ocular lesions in scurvy. Am J Clin Nutr. 1969;22:559-567.
- Horrobin DF, Oka M, Manku MS. The regulation of prostaglandin E1 formation: a candidate for one of the fundamental mechanisms involved in the actions of vitamin C. Med Hypotheses. 1979;5:849-858.
- Hood J, Burns CA, Hodges RE. Sjogren’s syndrome in scurvy. N Engl J Med. 1970;282:1120-1124.
- Walter JF. Scurvy resulting from a self-imposed diet. West J Med. 1979;130:177-179.
- Velandia B, Centor RM, McConnell V, et al. Scurvy is still present in developed countries. J Gen Intern Med. 2008;23:1281-1284.
- Lind J. A Treatise of the Scurvy. Edinburgh, Scotland: Sands, Murray, and Cochran; 1753.
- Levine M, Rumsey SC, Daruwala R, et al. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415-1423.
- Jacob RA. Vitamin C. In: Shils ME, Olson JA, Shike M, et al, eds. Modern Nutrition in Health and Disease. Baltimore, MD: William & Wilkins; 1999:467-483.
- Levine M. New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med. 1986;314:892-902.
- Hirschman JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol. 1999;41:895-906.
- Bardnard ND, Weissinger R, Jaster BJ, et al, eds. Nutrition Guide for Clinicians. 2nd ed. Washington, DC: Physician’s Committee For Responsible Medicine; 2009:33.
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington DC: National Academic Press; 2000.
- Schleicher RL, Carroll MD, Ford ES, et al. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90:1252-1263.
- Schectman G, Byrd JC, Gruchow HW. The influence of smoking on vitamin C status in adults. Am J Public Health. 1989;79:158-162.
- Johnston CS, Thompson LL. Vitamin C status of an outpatient population. J Am Coll Nutr. 1998;17:366-370.
- Hodges RE, Baker EM, Hood J, et al. Experimental scurvy in man. Am J Clin Nutr. 1969;22:535-548.
- Hodges RE, Hood J, Canham JE, et al. Clinical manifestations of ascorbic acid deficiency in man. Am J Clin Nutr. 1971;24:432-443.
- Gupta P, Taneja K, Iyer PU, et al. Scurvy—the eternal masquerader. Ann Trop Paediatr. 1989;9:118-121.
- Haq RU, Dhammi IK, Jain AK, et al. Infantile scurvy masquerading as bone tumour. Ann Acad Med Singapore. 2013;42:363-365.
- Pazzola G, Possemato N, Germanò G, et al. Scurvy mimicking spondyloarthritis in a young man. Clin Exp Rheumatol. 2013;31:795.
- Friesgaard Christensen A, Clemmensen O, Junker P. Palpable purpura with an unexpected outcome. Case Rep Rheumatol. 2013;2013:678427.
- Des Roches A, Paradis L, Paradis J, et al. Food allergy as a new risk factor for scurvy. Allergy. 2006;61:1487-1488.
- Pimentel L. Scurvy: historical review and current diagnostic approach. Am J Emerg Med. 2003;21:328-332.
- Codreanu F, Jarlot S, Astier C, et al. An apple a day...chronic glossitis in a 4-year-old boy. Eur Ann Allergy Clin Immunol. 2012;44:86-88.
- Stephen R, Utecht T. Scurvy identified in the emergency department: a case report. J Emerg Med. 2001;21:235-237.
- Hood J, Hodges RE. Ocular lesions in scurvy. Am J Clin Nutr. 1969;22:559-567.
- Horrobin DF, Oka M, Manku MS. The regulation of prostaglandin E1 formation: a candidate for one of the fundamental mechanisms involved in the actions of vitamin C. Med Hypotheses. 1979;5:849-858.
- Hood J, Burns CA, Hodges RE. Sjogren’s syndrome in scurvy. N Engl J Med. 1970;282:1120-1124.
- Walter JF. Scurvy resulting from a self-imposed diet. West J Med. 1979;130:177-179.
- Velandia B, Centor RM, McConnell V, et al. Scurvy is still present in developed countries. J Gen Intern Med. 2008;23:1281-1284.
Practice Points
- Patients with scurvy often pose a diagnostic dilemma because their presenting symptoms can lead physicians down a laborious and costly road of unnecessary tests including vasculitic, infectious, and rheumatologic workups.
- The diagnosis of scurvy is clinical and typically is based on signs such as perivascular hemorrhage, bleeding gums, anemia, impaired wound healing, and ecchymoses in the setting of vitamin C deficiency with rapid resolution upon vitamin C supplementation.