User login
There are currently 7 FDA-approved second-generation long-acting injectable antipsychotics (LAIAs).1-7 These LAIAs provide a unique dosage form that allows patients to receive an antipsychotic without taking oral medications every day, or multiple times per day. This may be an appealing option for patients and clinicians, but because there are several types of LAIAs available, it may be difficult to determine which LAIA characteristics are best for a given patient.
Since the FDA approved the first second-generation LAIA, risperidone long-acting injectable (LAI),1 in 2003, 6 additional second-generation LAIAs have been approved:
- aripiprazole LAI
- aripiprazole lauroxil LAI
- olanzapine pamoate LAI
- paliperidone palmitate monthly injection
- paliperidone palmitate 3-month LAI
- risperidone LAI for subcutaneous (SQ) injection.
When discussing medication options with patients, clinicians need to consider factors that are unique to each LAIA. In this article, I describe the similarities and differences among the second-generation LAIAs, and address common questions about these medications.
A major potential benefit: Increased adherence
One potential benefit of all LAIAs is increased medication adherence compared with oral antipsychotics. One meta-analysis of 21 randomized controlled trials (RCTs) that compared LAIAs with oral antipsychotics and included 5,176 patients found that LAIAs had a similar efficacy to oral antipsychotics in preventing relapse.8 However, a meta-analysis of 25 mirror-image studies comparing LAIAs with oral antipsychotics that included 5,940 patients found that LAIAs were superior in preventing hospitalization.9 In these mirror-image studies, participants received oral antipsychotics first and then switched to LAIAs, and the 2 study periods were compared. Because mirror-image studies are observational, participants do not engage with research teams to the extent that they do in RCTs.9 Although mirror-image studies have limitations, participants in these studies may be a better representation of patients encountered in clinical practice due to the extensive monitoring and follow-up RCT participants typically receive.9
Differences in FDA-approved indications
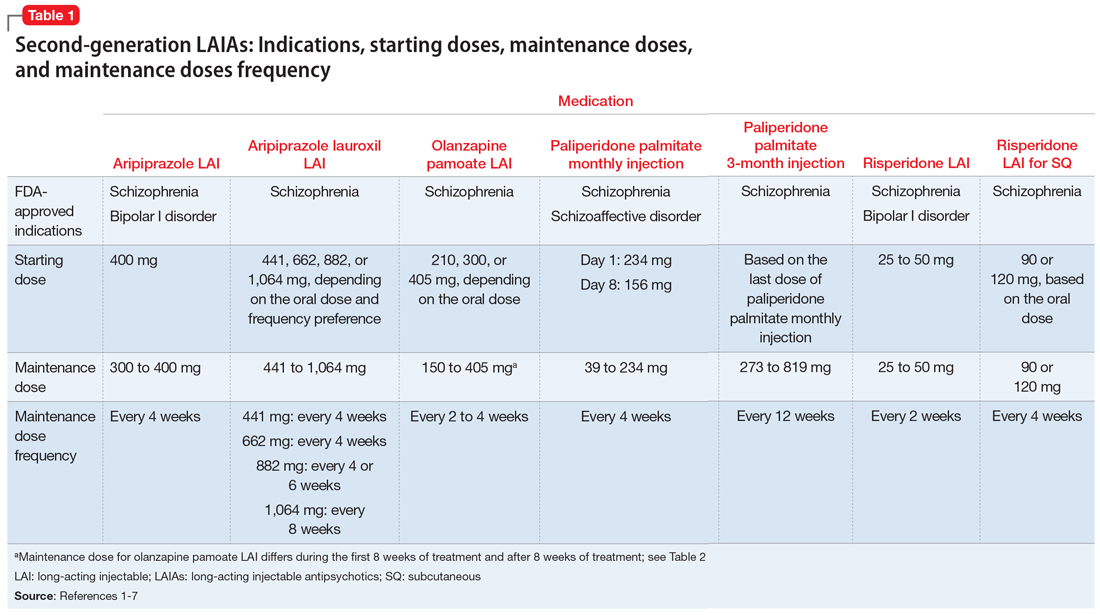
The 7 currently available LAIAs vary in terms of FDA-approved indications, dose options, frequency, need for oral antipsychotic overlap, route of administration, and other factors. Table 11-7 summarizes some of these differences. Although all second-generation LAIAs are approved for schizophrenia,1-7 risperidone LAI and aripiprazole LAI are also approved for bipolar I disorder.1,4 Paliperidone palmitate monthly injection is the only LAIA approved for treating patients with schizoaffective disorder.2
Starting doses
For most LAIAs, the starting dose is the same as the maintenance dose (Table 11-7). One exception is paliperidone palmitate monthly injection, which requires a 234-mg dose on Day 1 followed by a 156-mg dose on Day 8 for all patients, regardless of the maintenance dose required.2 The 156-mg dose may be given 4 days before or after Day 8.2 The first maintenance dose of paliperidone palmitate monthly injection should be administered 5 weeks after the 234-mg dose on Day 1.2 Before starting paliperidone palmitate 3-month injection, patients should be stable on paliperidone palmitate monthly injection for 4 months, and the 2 most recent doses of paliperidone palmitate monthly injection should be the same.3
Maintenance doses
Dosing frequency may be an important factor for some patients when deciding to receive a LAIA. The frequency of the maintenance doses for all second-generation LAIAs varies from every 2 weeks to 12 weeks (Table 11-7). Paliperidone palmitate 3-month LAI is the only LAIA that is administered every 12 weeks.3 Some dosages of aripiprazole lauroxil LAI are administered every 6 or 8 weeks.6 All other second-generation LAIAs are given every 2 to 4 weeks.
Continue to: Start with an oral antipsychotic
Start with an oral antipsychotic
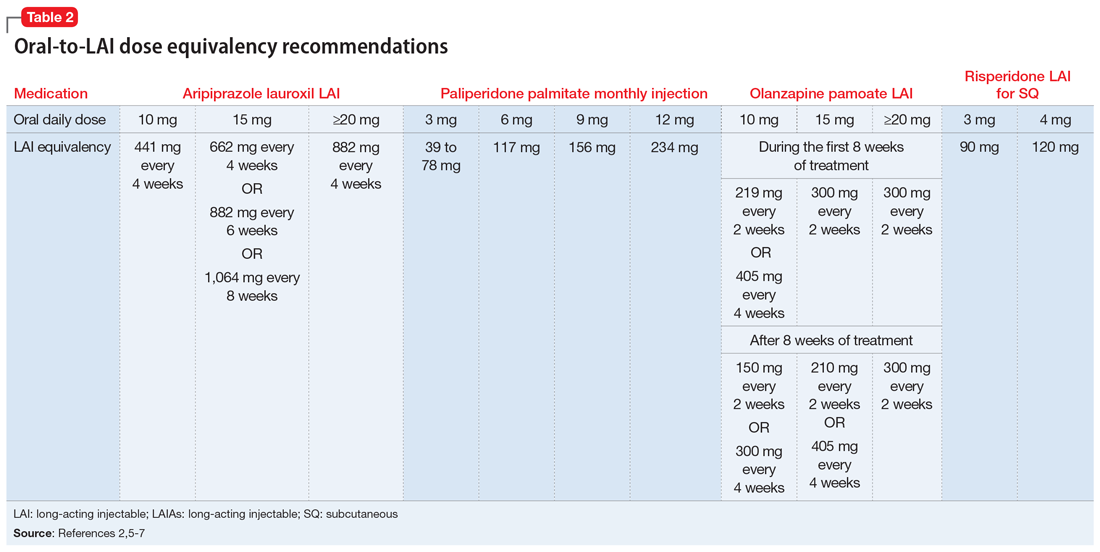
Before starting any LAIA, patients should receive the oral formulation of that antipsychotic to establish tolerability.1-7 Four of the 7 available LAIAs have an oral-to-LAI dose equivalency recommendation in their prescribing information (Table 22,5-7). This can help clinicians estimate the LAIA maintenance dose required to control a patient’s symptoms. If a dose adjustment is needed once a patient starts an LAIA, the dose adjustment can be made when the next injection is due.2
There are 2 important considerations when prescribing olanzapine pamoate LAI. First, the recommended dose for olanzapine pamoate LAI based on oral olanzapine doses differs during the first 8 weeks of treatment compared with after 8 weeks of treatment (Table 22,5-7). Additionally, because there are both short-acting and long-acting injections of olanzapine, it is essential to choose the correct formulation when prescribing this medication.5
Overlap with an oral antipsychotic might be necessary
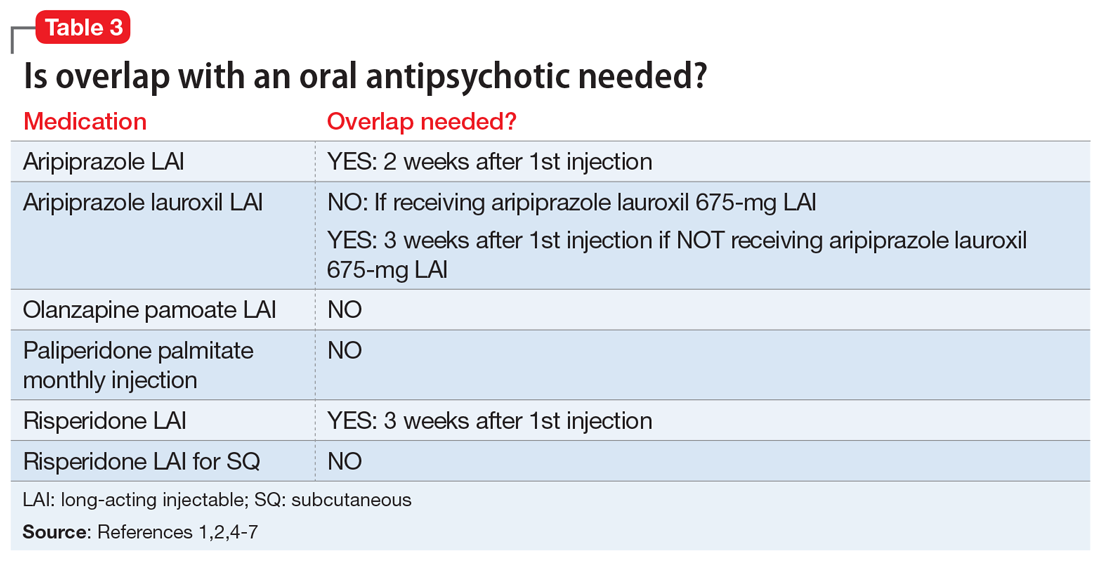
Administration of several of the LAIAs may require overlap with an oral antipsychotic (Table 31,2,4-7). Patients who refuse to take oral medications may benefit from one of the LAIAs that does not require oral overlap—paliperidone palmitate monthly injection, olanzapine pamoate LAI, and risperidone LAI for SQ.2,5,7 Risperidone LAI requires overlap with oral risperidone for 3 weeks.1
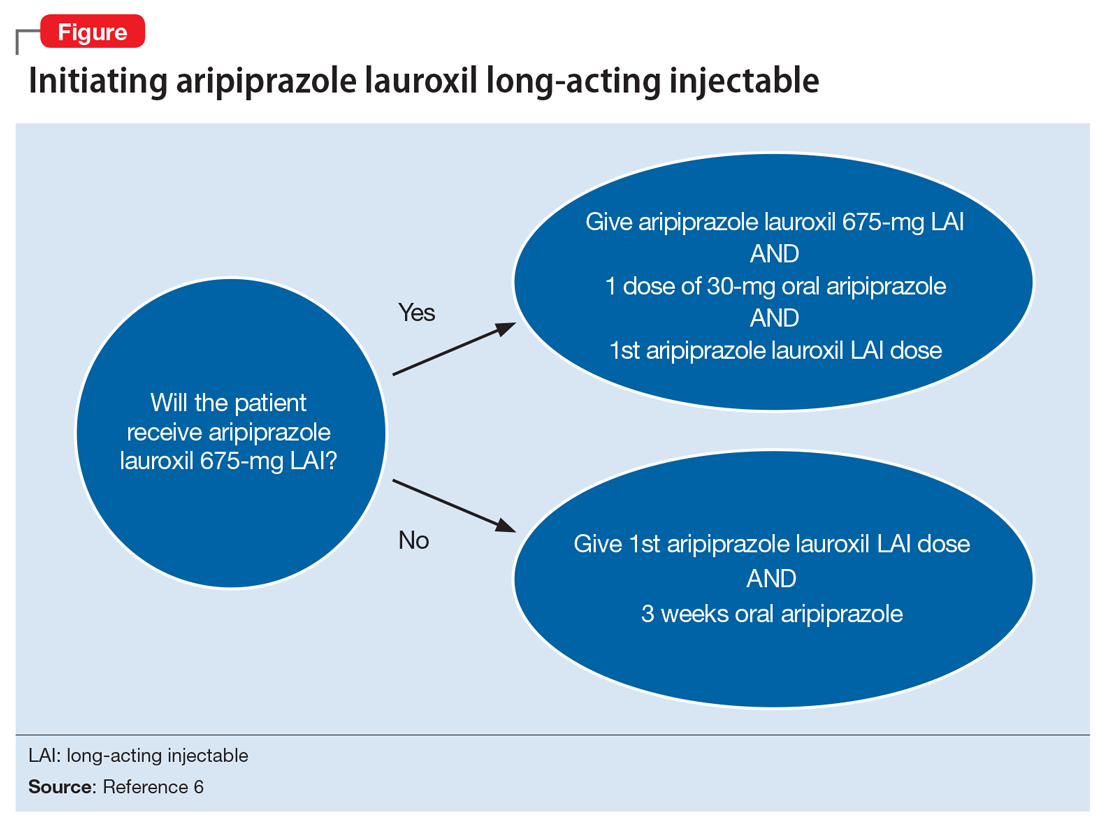
Aripiprazole is available in 2 LAI formulations: aripiprazole LAI and aripiprazole lauroxil LAI. Aripiprazole lauroxil is a prodrug of aripiprazole, and these 2 LAI medications differ in available dose options and dosing frequency.4,6 Aripiprazole LAI requires an oral overlap for 2 weeks after the first injection, whereas aripiprazole lauroxil LAI requires 3 weeks of oral overlap unless aripiprazole lauroxil 675-mg LAI is administered (Figure6).4,6,10
Aripiprazole lauroxil 675-mg LAI is formulated with drug particles that are smaller than those in aripiprazole lauroxil LAI.11 The smaller particle size results in faster dissolution and a more rapid increase in plasma aripiprazole levels. Aripiprazole lauroxil 675-mg LAI is a single injection that should be given with one 30-mg dose of oral aripiprazole.10 This combination results in aripiprazole concentrations that are comparable to aripiprazole lauroxil LAI and oral aripiprazole overlap for 3 weeks after the first injection.10
Continue to: The starting dose of aripiprazole lauroxil LAI...
The starting dose of aripiprazole lauroxil LAI may be administered on the same day as aripiprazole lauroxil 675-mg LAI and the 30-mg oral aripiprazole dose, or it may be administered up to 10 days after.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675-mg LAI are not interchangeable due to differing pharmacokinetic profiles.6,10 Aripiprazole lauroxil 675-mg LAI may be used to re-initiate treatment in a patient who missed doses of aripiprazole lauroxil LAI.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675 mg should not be injected together into the same deltoid or gluteal muscle.
Be mindful of differences in dosing windows
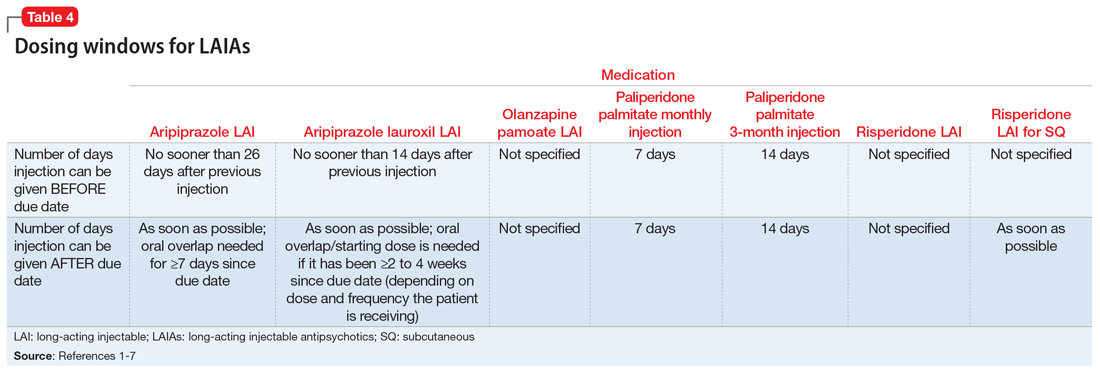
Each LAIA has a specific frequency recommendation, but due to scheduling or other factors, it may not be possible for patients to receive their injection on the specified day. The prescribing information for some LAIAs provides a dosing window (Table 41-7). The prescribing information for risperidone LAI, olanzapine pamoate LAI, and risperidone LAI for SQ does not specify how many days the injection can be administered before or after the due date; however, the prescribing information for risperidone LAI for SQ indicates that if the injection is not given on the due date, it should be administered as soon as possible after that.1,5,7
Paliperidone palmitate monthly injection and paliperidone palmitate 3-month LAI have the clearest recommendations for a dosing window. Paliperidone palmitate monthly injection may be administered 7 days before or after the 4-week due date, and paliperidone palmitate 3-month LAI can be administered 14 days before or after the 12-week due date.2,3
Aripiprazole LAI should not be administered sooner than 26 days after the previous injection, which means that it can be administered up to 2 days before the 4-week due date.4 If administered after the due date, it should be given as soon as possible, although oral overlap is not needed until ≥7 days past the due date.4
Aripiprazole lauroxil LAI has similar recommendations to aripiprazole LAI in that it should not be administered sooner than 14 days after the previous injection.6 If it is given after the due date, it should be administered as soon as possible; oral overlap/starting dose is needed if it has been ≥2 to 4 weeks since the due date, depending on which dose and frequency the patient is receiving.6
Continue to: Recommendations for missed doses
Recommendations for missed doses
Each LAIA has specific recommendations for missed dosing. Carpenter and Wong12 reviewed the recommendations for managing missed LAIA doses in
Consider patient preference
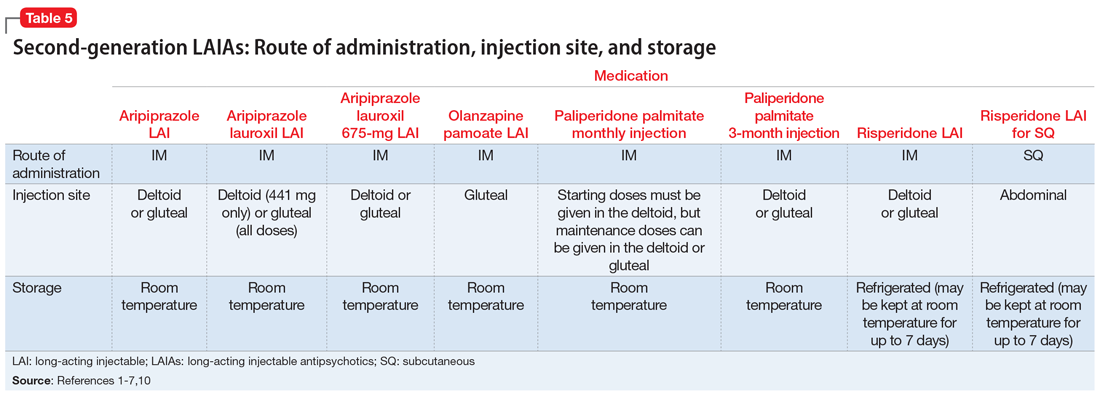
Patient preference for the type and location of the injection may factor into a clinician’s choice of LAIA (Table 51-7,10). Risperidone LAI for SQ is the only LAIA that is administered as an SQ abdominal injection.7 All other LAIAs are IM injections in the deltoid or gluteal muscle.1-6 All doses of risperidone LAI, paliperidone palmitate 3-month LAI, aripiprazole LAI, and aripiprazole lauroxil 675-mg LAI can be administered in the deltoid or gluteal muscle.1,3,4,10 Deltoid administration is required for the 2 starting doses of paliperidone palmitate monthly injection, but maintenance doses can be administered in the deltoid or gluteal muscle. Because administration into the deltoid results in a higher concentration of the drug compared with gluteal administration, administering the 2 starting doses of paliperidone palmitate monthly injection into the deltoid helps to rapidly attain therapeutic concentrations.2 Olanzapine pamoate LAI should be administered only in the gluteal muscle.5 The 441-mg dose of aripiprazole lauroxil LAI may be administered in the deltoid or gluteal muscle, but all other doses of aripiprazole lauroxil LAI should be administered only in the gluteal muscle.6
Storage
Most LAIAs can be stored at room temperature2-6; however, risperidone LAI and risperidone LAI for SQ need to be stored in the refrigerator. Both risperidone LAI and risperidone LAI for SQ may be kept at room temperature for up to 7 days. If they are not used within 7 days at room temperature, they should be discarded.1,7
Clinical pearls for specific LAIAs
Aripiprazole LAI. The recommended starting and maintenance dose for aripiprazole LAI is 400 mg monthly, unless the patient has drug interactions or other factors that require dose adjustment. If patients experience adverse reactions to the 400-mg dose, a reduction to 300 mg monthly could be considered.4
Olanzapine pamoate LAI has a Risk Evaluation and Mitigation Strategy (REMS) due to the potential for post-injection delirium/sedation syndrome (PDSS). Prescribing clinicians, dispensing pharmacies, and administering health care facilities must all be certified to prescribe, dispense, or administer olanzapine pamoate LAI. The patient must also be enrolled in the REMS program.13 Patients must be observed by health care staff for 3 hours after receiving a dose of olanzapine pamoate LAI to monitor for signs and symptoms of PDSS.5
Continue to: Risperidone LAI
Risperidone LAI. When increasing the dose of risperidone LAI, do not expect to see the clinical effects of the new dose earlier than 3 weeks after initiating the higher dose, because the main release of the medication starts at 3 weeks after the injection.1
Risperidone LAI for SQ has specific recommendations for the LAI dose based on whether the patient was stable when receiving 3 or 4 mg/d of oral risperidone. If patients are stable on <3 or >4 mg/d, they may not be candidates for risperidone LAI for SQ.7
Table 61-7,10 lists additional factors to consider when prescribing a specific LAIA.
Bottom Line
Second-generation long-acting injectable antipsychotics (LAIAs) have the potential to increase medication adherence. There are important differences among the 7 currently available LAIAs. For effective prescribing, clinicians need to understand each medication’s unique aspects, including dosing options, frequency, need for oral antipsychotic overlap, and route of administration.
Related Resources
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
- Peters L, Krogmann A, von Hardenberg L, et al. Long-acting injections in schizophrenia: a 3-year update on randomized controlled trials published January 2016-March 2019. Curr Psychiatry Rep. 2019;21(12):124.
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole long-acting injectable • Abilify Maintena
Aripiprazole lauroxil extended-release injectable suspension • Aristada
Aripiprazole lauroxil 675 mg • Aristada Initio
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month injection • Invega Trinza
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Risperidone long-acting injection for SQ • Perseris
1. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
2. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
4. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2019.
5. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; 2019.
6. Aristada [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
7. Perseris [package insert]. North Chesterfield, VA: Indivior, Inc.; 2018.
8. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
9. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
10. Aristada Initio [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
11. Jain R, Meyer J, Wehr A, et al. Size matters: the importance of particle size in a newly developed injectable formulation for the treatment of schizophrenia. CNS Spectr. 2019:1-8.
12. Carpenter J, Wong KK. Long-acting injectable antipsychotics: what to do about missed doses. Current Psychiatry. 2018;17(7):10-12,14-19,56.
13. US Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS) zyprexa relprevv (olanzapine). https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74. Updated April 11, 2019. Accessed January 27, 2020.
There are currently 7 FDA-approved second-generation long-acting injectable antipsychotics (LAIAs).1-7 These LAIAs provide a unique dosage form that allows patients to receive an antipsychotic without taking oral medications every day, or multiple times per day. This may be an appealing option for patients and clinicians, but because there are several types of LAIAs available, it may be difficult to determine which LAIA characteristics are best for a given patient.
Since the FDA approved the first second-generation LAIA, risperidone long-acting injectable (LAI),1 in 2003, 6 additional second-generation LAIAs have been approved:
- aripiprazole LAI
- aripiprazole lauroxil LAI
- olanzapine pamoate LAI
- paliperidone palmitate monthly injection
- paliperidone palmitate 3-month LAI
- risperidone LAI for subcutaneous (SQ) injection.
When discussing medication options with patients, clinicians need to consider factors that are unique to each LAIA. In this article, I describe the similarities and differences among the second-generation LAIAs, and address common questions about these medications.
A major potential benefit: Increased adherence
One potential benefit of all LAIAs is increased medication adherence compared with oral antipsychotics. One meta-analysis of 21 randomized controlled trials (RCTs) that compared LAIAs with oral antipsychotics and included 5,176 patients found that LAIAs had a similar efficacy to oral antipsychotics in preventing relapse.8 However, a meta-analysis of 25 mirror-image studies comparing LAIAs with oral antipsychotics that included 5,940 patients found that LAIAs were superior in preventing hospitalization.9 In these mirror-image studies, participants received oral antipsychotics first and then switched to LAIAs, and the 2 study periods were compared. Because mirror-image studies are observational, participants do not engage with research teams to the extent that they do in RCTs.9 Although mirror-image studies have limitations, participants in these studies may be a better representation of patients encountered in clinical practice due to the extensive monitoring and follow-up RCT participants typically receive.9
Differences in FDA-approved indications
The 7 currently available LAIAs vary in terms of FDA-approved indications, dose options, frequency, need for oral antipsychotic overlap, route of administration, and other factors. Table 11-7 summarizes some of these differences. Although all second-generation LAIAs are approved for schizophrenia,1-7 risperidone LAI and aripiprazole LAI are also approved for bipolar I disorder.1,4 Paliperidone palmitate monthly injection is the only LAIA approved for treating patients with schizoaffective disorder.2
Starting doses
For most LAIAs, the starting dose is the same as the maintenance dose (Table 11-7). One exception is paliperidone palmitate monthly injection, which requires a 234-mg dose on Day 1 followed by a 156-mg dose on Day 8 for all patients, regardless of the maintenance dose required.2 The 156-mg dose may be given 4 days before or after Day 8.2 The first maintenance dose of paliperidone palmitate monthly injection should be administered 5 weeks after the 234-mg dose on Day 1.2 Before starting paliperidone palmitate 3-month injection, patients should be stable on paliperidone palmitate monthly injection for 4 months, and the 2 most recent doses of paliperidone palmitate monthly injection should be the same.3
Maintenance doses
Dosing frequency may be an important factor for some patients when deciding to receive a LAIA. The frequency of the maintenance doses for all second-generation LAIAs varies from every 2 weeks to 12 weeks (Table 11-7). Paliperidone palmitate 3-month LAI is the only LAIA that is administered every 12 weeks.3 Some dosages of aripiprazole lauroxil LAI are administered every 6 or 8 weeks.6 All other second-generation LAIAs are given every 2 to 4 weeks.
Continue to: Start with an oral antipsychotic
Start with an oral antipsychotic
Before starting any LAIA, patients should receive the oral formulation of that antipsychotic to establish tolerability.1-7 Four of the 7 available LAIAs have an oral-to-LAI dose equivalency recommendation in their prescribing information (Table 22,5-7). This can help clinicians estimate the LAIA maintenance dose required to control a patient’s symptoms. If a dose adjustment is needed once a patient starts an LAIA, the dose adjustment can be made when the next injection is due.2
There are 2 important considerations when prescribing olanzapine pamoate LAI. First, the recommended dose for olanzapine pamoate LAI based on oral olanzapine doses differs during the first 8 weeks of treatment compared with after 8 weeks of treatment (Table 22,5-7). Additionally, because there are both short-acting and long-acting injections of olanzapine, it is essential to choose the correct formulation when prescribing this medication.5
Overlap with an oral antipsychotic might be necessary
Administration of several of the LAIAs may require overlap with an oral antipsychotic (Table 31,2,4-7). Patients who refuse to take oral medications may benefit from one of the LAIAs that does not require oral overlap—paliperidone palmitate monthly injection, olanzapine pamoate LAI, and risperidone LAI for SQ.2,5,7 Risperidone LAI requires overlap with oral risperidone for 3 weeks.1
Aripiprazole is available in 2 LAI formulations: aripiprazole LAI and aripiprazole lauroxil LAI. Aripiprazole lauroxil is a prodrug of aripiprazole, and these 2 LAI medications differ in available dose options and dosing frequency.4,6 Aripiprazole LAI requires an oral overlap for 2 weeks after the first injection, whereas aripiprazole lauroxil LAI requires 3 weeks of oral overlap unless aripiprazole lauroxil 675-mg LAI is administered (Figure6).4,6,10
Aripiprazole lauroxil 675-mg LAI is formulated with drug particles that are smaller than those in aripiprazole lauroxil LAI.11 The smaller particle size results in faster dissolution and a more rapid increase in plasma aripiprazole levels. Aripiprazole lauroxil 675-mg LAI is a single injection that should be given with one 30-mg dose of oral aripiprazole.10 This combination results in aripiprazole concentrations that are comparable to aripiprazole lauroxil LAI and oral aripiprazole overlap for 3 weeks after the first injection.10
Continue to: The starting dose of aripiprazole lauroxil LAI...
The starting dose of aripiprazole lauroxil LAI may be administered on the same day as aripiprazole lauroxil 675-mg LAI and the 30-mg oral aripiprazole dose, or it may be administered up to 10 days after.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675-mg LAI are not interchangeable due to differing pharmacokinetic profiles.6,10 Aripiprazole lauroxil 675-mg LAI may be used to re-initiate treatment in a patient who missed doses of aripiprazole lauroxil LAI.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675 mg should not be injected together into the same deltoid or gluteal muscle.
Be mindful of differences in dosing windows
Each LAIA has a specific frequency recommendation, but due to scheduling or other factors, it may not be possible for patients to receive their injection on the specified day. The prescribing information for some LAIAs provides a dosing window (Table 41-7). The prescribing information for risperidone LAI, olanzapine pamoate LAI, and risperidone LAI for SQ does not specify how many days the injection can be administered before or after the due date; however, the prescribing information for risperidone LAI for SQ indicates that if the injection is not given on the due date, it should be administered as soon as possible after that.1,5,7
Paliperidone palmitate monthly injection and paliperidone palmitate 3-month LAI have the clearest recommendations for a dosing window. Paliperidone palmitate monthly injection may be administered 7 days before or after the 4-week due date, and paliperidone palmitate 3-month LAI can be administered 14 days before or after the 12-week due date.2,3
Aripiprazole LAI should not be administered sooner than 26 days after the previous injection, which means that it can be administered up to 2 days before the 4-week due date.4 If administered after the due date, it should be given as soon as possible, although oral overlap is not needed until ≥7 days past the due date.4
Aripiprazole lauroxil LAI has similar recommendations to aripiprazole LAI in that it should not be administered sooner than 14 days after the previous injection.6 If it is given after the due date, it should be administered as soon as possible; oral overlap/starting dose is needed if it has been ≥2 to 4 weeks since the due date, depending on which dose and frequency the patient is receiving.6
Continue to: Recommendations for missed doses
Recommendations for missed doses
Each LAIA has specific recommendations for missed dosing. Carpenter and Wong12 reviewed the recommendations for managing missed LAIA doses in
Consider patient preference
Patient preference for the type and location of the injection may factor into a clinician’s choice of LAIA (Table 51-7,10). Risperidone LAI for SQ is the only LAIA that is administered as an SQ abdominal injection.7 All other LAIAs are IM injections in the deltoid or gluteal muscle.1-6 All doses of risperidone LAI, paliperidone palmitate 3-month LAI, aripiprazole LAI, and aripiprazole lauroxil 675-mg LAI can be administered in the deltoid or gluteal muscle.1,3,4,10 Deltoid administration is required for the 2 starting doses of paliperidone palmitate monthly injection, but maintenance doses can be administered in the deltoid or gluteal muscle. Because administration into the deltoid results in a higher concentration of the drug compared with gluteal administration, administering the 2 starting doses of paliperidone palmitate monthly injection into the deltoid helps to rapidly attain therapeutic concentrations.2 Olanzapine pamoate LAI should be administered only in the gluteal muscle.5 The 441-mg dose of aripiprazole lauroxil LAI may be administered in the deltoid or gluteal muscle, but all other doses of aripiprazole lauroxil LAI should be administered only in the gluteal muscle.6
Storage
Most LAIAs can be stored at room temperature2-6; however, risperidone LAI and risperidone LAI for SQ need to be stored in the refrigerator. Both risperidone LAI and risperidone LAI for SQ may be kept at room temperature for up to 7 days. If they are not used within 7 days at room temperature, they should be discarded.1,7
Clinical pearls for specific LAIAs
Aripiprazole LAI. The recommended starting and maintenance dose for aripiprazole LAI is 400 mg monthly, unless the patient has drug interactions or other factors that require dose adjustment. If patients experience adverse reactions to the 400-mg dose, a reduction to 300 mg monthly could be considered.4
Olanzapine pamoate LAI has a Risk Evaluation and Mitigation Strategy (REMS) due to the potential for post-injection delirium/sedation syndrome (PDSS). Prescribing clinicians, dispensing pharmacies, and administering health care facilities must all be certified to prescribe, dispense, or administer olanzapine pamoate LAI. The patient must also be enrolled in the REMS program.13 Patients must be observed by health care staff for 3 hours after receiving a dose of olanzapine pamoate LAI to monitor for signs and symptoms of PDSS.5
Continue to: Risperidone LAI
Risperidone LAI. When increasing the dose of risperidone LAI, do not expect to see the clinical effects of the new dose earlier than 3 weeks after initiating the higher dose, because the main release of the medication starts at 3 weeks after the injection.1
Risperidone LAI for SQ has specific recommendations for the LAI dose based on whether the patient was stable when receiving 3 or 4 mg/d of oral risperidone. If patients are stable on <3 or >4 mg/d, they may not be candidates for risperidone LAI for SQ.7
Table 61-7,10 lists additional factors to consider when prescribing a specific LAIA.
Bottom Line
Second-generation long-acting injectable antipsychotics (LAIAs) have the potential to increase medication adherence. There are important differences among the 7 currently available LAIAs. For effective prescribing, clinicians need to understand each medication’s unique aspects, including dosing options, frequency, need for oral antipsychotic overlap, and route of administration.
Related Resources
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
- Peters L, Krogmann A, von Hardenberg L, et al. Long-acting injections in schizophrenia: a 3-year update on randomized controlled trials published January 2016-March 2019. Curr Psychiatry Rep. 2019;21(12):124.
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole long-acting injectable • Abilify Maintena
Aripiprazole lauroxil extended-release injectable suspension • Aristada
Aripiprazole lauroxil 675 mg • Aristada Initio
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month injection • Invega Trinza
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Risperidone long-acting injection for SQ • Perseris
There are currently 7 FDA-approved second-generation long-acting injectable antipsychotics (LAIAs).1-7 These LAIAs provide a unique dosage form that allows patients to receive an antipsychotic without taking oral medications every day, or multiple times per day. This may be an appealing option for patients and clinicians, but because there are several types of LAIAs available, it may be difficult to determine which LAIA characteristics are best for a given patient.
Since the FDA approved the first second-generation LAIA, risperidone long-acting injectable (LAI),1 in 2003, 6 additional second-generation LAIAs have been approved:
- aripiprazole LAI
- aripiprazole lauroxil LAI
- olanzapine pamoate LAI
- paliperidone palmitate monthly injection
- paliperidone palmitate 3-month LAI
- risperidone LAI for subcutaneous (SQ) injection.
When discussing medication options with patients, clinicians need to consider factors that are unique to each LAIA. In this article, I describe the similarities and differences among the second-generation LAIAs, and address common questions about these medications.
A major potential benefit: Increased adherence
One potential benefit of all LAIAs is increased medication adherence compared with oral antipsychotics. One meta-analysis of 21 randomized controlled trials (RCTs) that compared LAIAs with oral antipsychotics and included 5,176 patients found that LAIAs had a similar efficacy to oral antipsychotics in preventing relapse.8 However, a meta-analysis of 25 mirror-image studies comparing LAIAs with oral antipsychotics that included 5,940 patients found that LAIAs were superior in preventing hospitalization.9 In these mirror-image studies, participants received oral antipsychotics first and then switched to LAIAs, and the 2 study periods were compared. Because mirror-image studies are observational, participants do not engage with research teams to the extent that they do in RCTs.9 Although mirror-image studies have limitations, participants in these studies may be a better representation of patients encountered in clinical practice due to the extensive monitoring and follow-up RCT participants typically receive.9
Differences in FDA-approved indications
The 7 currently available LAIAs vary in terms of FDA-approved indications, dose options, frequency, need for oral antipsychotic overlap, route of administration, and other factors. Table 11-7 summarizes some of these differences. Although all second-generation LAIAs are approved for schizophrenia,1-7 risperidone LAI and aripiprazole LAI are also approved for bipolar I disorder.1,4 Paliperidone palmitate monthly injection is the only LAIA approved for treating patients with schizoaffective disorder.2
Starting doses
For most LAIAs, the starting dose is the same as the maintenance dose (Table 11-7). One exception is paliperidone palmitate monthly injection, which requires a 234-mg dose on Day 1 followed by a 156-mg dose on Day 8 for all patients, regardless of the maintenance dose required.2 The 156-mg dose may be given 4 days before or after Day 8.2 The first maintenance dose of paliperidone palmitate monthly injection should be administered 5 weeks after the 234-mg dose on Day 1.2 Before starting paliperidone palmitate 3-month injection, patients should be stable on paliperidone palmitate monthly injection for 4 months, and the 2 most recent doses of paliperidone palmitate monthly injection should be the same.3
Maintenance doses
Dosing frequency may be an important factor for some patients when deciding to receive a LAIA. The frequency of the maintenance doses for all second-generation LAIAs varies from every 2 weeks to 12 weeks (Table 11-7). Paliperidone palmitate 3-month LAI is the only LAIA that is administered every 12 weeks.3 Some dosages of aripiprazole lauroxil LAI are administered every 6 or 8 weeks.6 All other second-generation LAIAs are given every 2 to 4 weeks.
Continue to: Start with an oral antipsychotic
Start with an oral antipsychotic
Before starting any LAIA, patients should receive the oral formulation of that antipsychotic to establish tolerability.1-7 Four of the 7 available LAIAs have an oral-to-LAI dose equivalency recommendation in their prescribing information (Table 22,5-7). This can help clinicians estimate the LAIA maintenance dose required to control a patient’s symptoms. If a dose adjustment is needed once a patient starts an LAIA, the dose adjustment can be made when the next injection is due.2
There are 2 important considerations when prescribing olanzapine pamoate LAI. First, the recommended dose for olanzapine pamoate LAI based on oral olanzapine doses differs during the first 8 weeks of treatment compared with after 8 weeks of treatment (Table 22,5-7). Additionally, because there are both short-acting and long-acting injections of olanzapine, it is essential to choose the correct formulation when prescribing this medication.5
Overlap with an oral antipsychotic might be necessary
Administration of several of the LAIAs may require overlap with an oral antipsychotic (Table 31,2,4-7). Patients who refuse to take oral medications may benefit from one of the LAIAs that does not require oral overlap—paliperidone palmitate monthly injection, olanzapine pamoate LAI, and risperidone LAI for SQ.2,5,7 Risperidone LAI requires overlap with oral risperidone for 3 weeks.1
Aripiprazole is available in 2 LAI formulations: aripiprazole LAI and aripiprazole lauroxil LAI. Aripiprazole lauroxil is a prodrug of aripiprazole, and these 2 LAI medications differ in available dose options and dosing frequency.4,6 Aripiprazole LAI requires an oral overlap for 2 weeks after the first injection, whereas aripiprazole lauroxil LAI requires 3 weeks of oral overlap unless aripiprazole lauroxil 675-mg LAI is administered (Figure6).4,6,10
Aripiprazole lauroxil 675-mg LAI is formulated with drug particles that are smaller than those in aripiprazole lauroxil LAI.11 The smaller particle size results in faster dissolution and a more rapid increase in plasma aripiprazole levels. Aripiprazole lauroxil 675-mg LAI is a single injection that should be given with one 30-mg dose of oral aripiprazole.10 This combination results in aripiprazole concentrations that are comparable to aripiprazole lauroxil LAI and oral aripiprazole overlap for 3 weeks after the first injection.10
Continue to: The starting dose of aripiprazole lauroxil LAI...
The starting dose of aripiprazole lauroxil LAI may be administered on the same day as aripiprazole lauroxil 675-mg LAI and the 30-mg oral aripiprazole dose, or it may be administered up to 10 days after.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675-mg LAI are not interchangeable due to differing pharmacokinetic profiles.6,10 Aripiprazole lauroxil 675-mg LAI may be used to re-initiate treatment in a patient who missed doses of aripiprazole lauroxil LAI.10 Aripiprazole lauroxil LAI and aripiprazole lauroxil 675 mg should not be injected together into the same deltoid or gluteal muscle.
Be mindful of differences in dosing windows
Each LAIA has a specific frequency recommendation, but due to scheduling or other factors, it may not be possible for patients to receive their injection on the specified day. The prescribing information for some LAIAs provides a dosing window (Table 41-7). The prescribing information for risperidone LAI, olanzapine pamoate LAI, and risperidone LAI for SQ does not specify how many days the injection can be administered before or after the due date; however, the prescribing information for risperidone LAI for SQ indicates that if the injection is not given on the due date, it should be administered as soon as possible after that.1,5,7
Paliperidone palmitate monthly injection and paliperidone palmitate 3-month LAI have the clearest recommendations for a dosing window. Paliperidone palmitate monthly injection may be administered 7 days before or after the 4-week due date, and paliperidone palmitate 3-month LAI can be administered 14 days before or after the 12-week due date.2,3
Aripiprazole LAI should not be administered sooner than 26 days after the previous injection, which means that it can be administered up to 2 days before the 4-week due date.4 If administered after the due date, it should be given as soon as possible, although oral overlap is not needed until ≥7 days past the due date.4
Aripiprazole lauroxil LAI has similar recommendations to aripiprazole LAI in that it should not be administered sooner than 14 days after the previous injection.6 If it is given after the due date, it should be administered as soon as possible; oral overlap/starting dose is needed if it has been ≥2 to 4 weeks since the due date, depending on which dose and frequency the patient is receiving.6
Continue to: Recommendations for missed doses
Recommendations for missed doses
Each LAIA has specific recommendations for missed dosing. Carpenter and Wong12 reviewed the recommendations for managing missed LAIA doses in
Consider patient preference
Patient preference for the type and location of the injection may factor into a clinician’s choice of LAIA (Table 51-7,10). Risperidone LAI for SQ is the only LAIA that is administered as an SQ abdominal injection.7 All other LAIAs are IM injections in the deltoid or gluteal muscle.1-6 All doses of risperidone LAI, paliperidone palmitate 3-month LAI, aripiprazole LAI, and aripiprazole lauroxil 675-mg LAI can be administered in the deltoid or gluteal muscle.1,3,4,10 Deltoid administration is required for the 2 starting doses of paliperidone palmitate monthly injection, but maintenance doses can be administered in the deltoid or gluteal muscle. Because administration into the deltoid results in a higher concentration of the drug compared with gluteal administration, administering the 2 starting doses of paliperidone palmitate monthly injection into the deltoid helps to rapidly attain therapeutic concentrations.2 Olanzapine pamoate LAI should be administered only in the gluteal muscle.5 The 441-mg dose of aripiprazole lauroxil LAI may be administered in the deltoid or gluteal muscle, but all other doses of aripiprazole lauroxil LAI should be administered only in the gluteal muscle.6
Storage
Most LAIAs can be stored at room temperature2-6; however, risperidone LAI and risperidone LAI for SQ need to be stored in the refrigerator. Both risperidone LAI and risperidone LAI for SQ may be kept at room temperature for up to 7 days. If they are not used within 7 days at room temperature, they should be discarded.1,7
Clinical pearls for specific LAIAs
Aripiprazole LAI. The recommended starting and maintenance dose for aripiprazole LAI is 400 mg monthly, unless the patient has drug interactions or other factors that require dose adjustment. If patients experience adverse reactions to the 400-mg dose, a reduction to 300 mg monthly could be considered.4
Olanzapine pamoate LAI has a Risk Evaluation and Mitigation Strategy (REMS) due to the potential for post-injection delirium/sedation syndrome (PDSS). Prescribing clinicians, dispensing pharmacies, and administering health care facilities must all be certified to prescribe, dispense, or administer olanzapine pamoate LAI. The patient must also be enrolled in the REMS program.13 Patients must be observed by health care staff for 3 hours after receiving a dose of olanzapine pamoate LAI to monitor for signs and symptoms of PDSS.5
Continue to: Risperidone LAI
Risperidone LAI. When increasing the dose of risperidone LAI, do not expect to see the clinical effects of the new dose earlier than 3 weeks after initiating the higher dose, because the main release of the medication starts at 3 weeks after the injection.1
Risperidone LAI for SQ has specific recommendations for the LAI dose based on whether the patient was stable when receiving 3 or 4 mg/d of oral risperidone. If patients are stable on <3 or >4 mg/d, they may not be candidates for risperidone LAI for SQ.7
Table 61-7,10 lists additional factors to consider when prescribing a specific LAIA.
Bottom Line
Second-generation long-acting injectable antipsychotics (LAIAs) have the potential to increase medication adherence. There are important differences among the 7 currently available LAIAs. For effective prescribing, clinicians need to understand each medication’s unique aspects, including dosing options, frequency, need for oral antipsychotic overlap, and route of administration.
Related Resources
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
- Peters L, Krogmann A, von Hardenberg L, et al. Long-acting injections in schizophrenia: a 3-year update on randomized controlled trials published January 2016-March 2019. Curr Psychiatry Rep. 2019;21(12):124.
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole long-acting injectable • Abilify Maintena
Aripiprazole lauroxil extended-release injectable suspension • Aristada
Aripiprazole lauroxil 675 mg • Aristada Initio
Olanzapine pamoate long-acting injection • Zyprexa Relprevv
Paliperidone palmitate monthly long-acting injection • Invega Sustenna
Paliperidone palmitate 3-month injection • Invega Trinza
Risperidone • Risperdal
Risperidone long-acting injection • Risperdal Consta
Risperidone long-acting injection for SQ • Perseris
1. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
2. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
4. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2019.
5. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; 2019.
6. Aristada [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
7. Perseris [package insert]. North Chesterfield, VA: Indivior, Inc.; 2018.
8. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
9. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
10. Aristada Initio [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
11. Jain R, Meyer J, Wehr A, et al. Size matters: the importance of particle size in a newly developed injectable formulation for the treatment of schizophrenia. CNS Spectr. 2019:1-8.
12. Carpenter J, Wong KK. Long-acting injectable antipsychotics: what to do about missed doses. Current Psychiatry. 2018;17(7):10-12,14-19,56.
13. US Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS) zyprexa relprevv (olanzapine). https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74. Updated April 11, 2019. Accessed January 27, 2020.
1. Risperdal Consta [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
2. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
3. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2019.
4. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2019.
5. Zyprexa Relprevv [package insert]. Indianapolis; IN: Eli Lilly and Co.; 2019.
6. Aristada [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
7. Perseris [package insert]. North Chesterfield, VA: Indivior, Inc.; 2018.
8. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213.
9. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965.
10. Aristada Initio [package insert]. Waltham, MA: Alkermes, Inc.; 2019.
11. Jain R, Meyer J, Wehr A, et al. Size matters: the importance of particle size in a newly developed injectable formulation for the treatment of schizophrenia. CNS Spectr. 2019:1-8.
12. Carpenter J, Wong KK. Long-acting injectable antipsychotics: what to do about missed doses. Current Psychiatry. 2018;17(7):10-12,14-19,56.
13. US Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS) zyprexa relprevv (olanzapine). https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74. Updated April 11, 2019. Accessed January 27, 2020.