User login
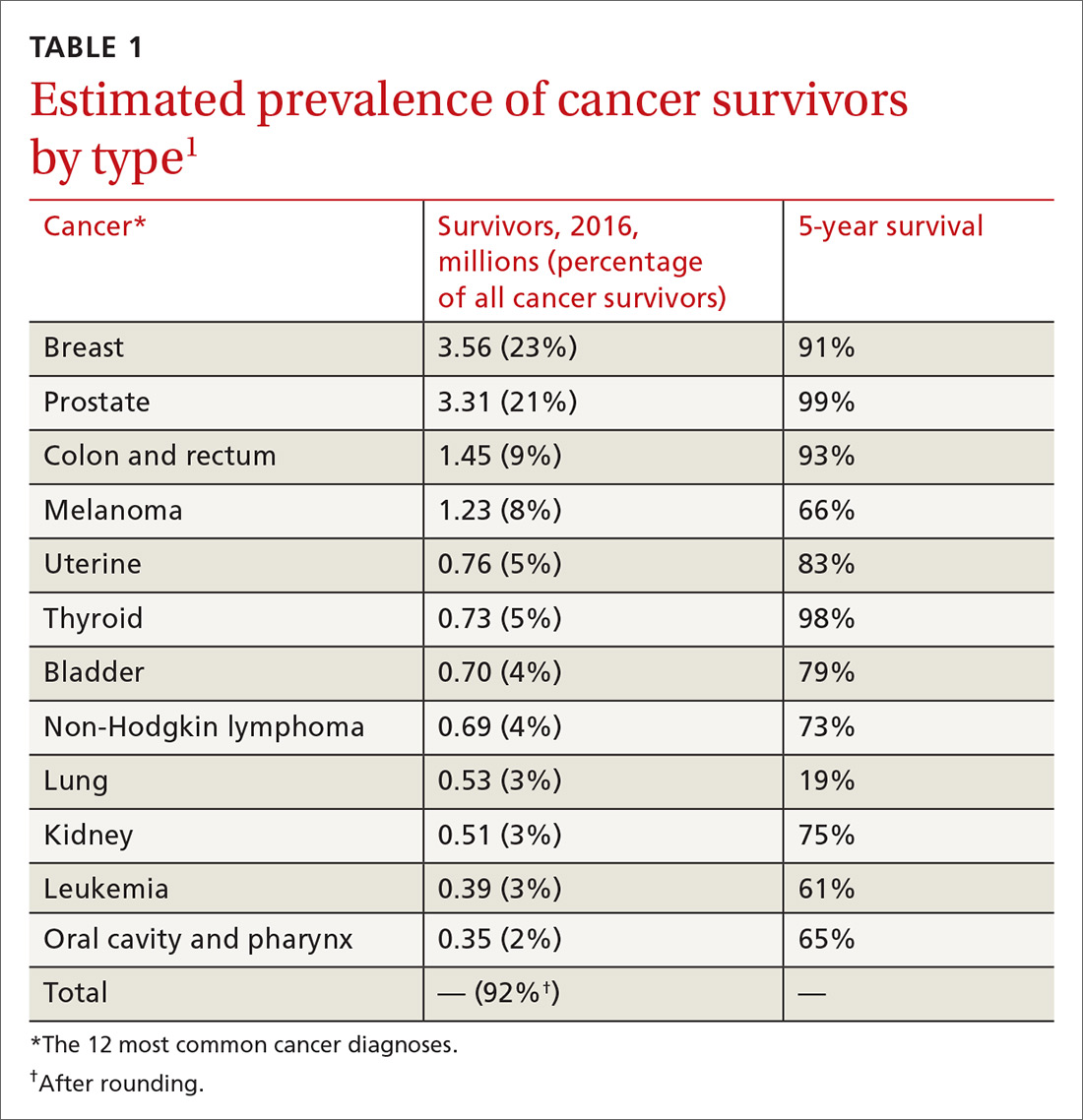
Cancer survivors represent a rapidly increasing population. In 1971, there were 3 million cancer survivors; this number increased to 15.5 million in 2016 and will reach 20 million by 2026.1TABLE 11 shows the percentage of survivors by type of cancer. Cancer survivors tend to be older,* comprising nearly 1 of every 5 people older than 65 years.2
The Institute of Medicine (IOM) identified 3 key characteristics of cancer survivors3:
- Trajectories of survivorship are variable; many cancer patients have periods of relative health between episodes of their disease.
- Survivors require careful cancer monitoring; in addition to the risk that their primary cancer will recur, they have an elevated risk for another, second cancer.
- Both cancer and its treatments increase the risk of other medical and psychiatric problems.
Family physicians (FPs) have optimal skills for navigating the chronic risks and health concerns of the well cancer survivor. This article reviews the primary care management of the functional cancer survivor, focusing on the management of chronic conditions and preventive care.
Survivorship follows any of 6 paths
Cancer survivorship is increasing in importance as treatment has steadily reduced mortality. Six trajectories of cancer survivors have been identified1:
- living cancer-free after treatment with minimal effects
- living cancer-free but suffering serious treatment complications
- Suffering late recurrence
- Developing a second cancer
- Living with intermittent cancer recurrences
- Living with cancer continuously.
Only patients in the last 2 groups are likely to be managed primarily by oncologists.
Survivors look to their FPs for ongoing care
Cancer survivors routinely see their primary care physician after initial treatment. A study of 30,000 Canadian breast cancer survivors demonstrated that follow-up care was limited to an oncologist in only 2%; 84% saw a primary care provider and an oncologist; and 14% saw a primary care provider only.4 A study of colorectal cancer survivors showed that primary care visits increased in each of the 5 years after diagnosis, during which time oncology visits decreased steadily5; in that study, primary care physicians delivered more preventive care than oncologists did.5 Similar to what is done in other chronic conditions, the various effects of cancer are best managed as a whole.
The IOM recommends that cancer survivor care comprise 4 elements2:
- coordination between oncologist and primary care physician
- surveillance for recurrence or spread of existing cancer
- screening for new cancer
- intervention for the effects of cancer and treatment.
Continue to: The following discussion summarizes...
The following discussion summarizes evidence and recommendations for each element of the IOM recommendations for survivor care.
Implementing the 4 elements of cancer survivor care
1. Coordinate care through a unified survivorship care plan
The IOM has noted that the needs of cancer survivors are rarely met2; communication between oncology and primary care is often deficient during transition of care. The IOM has recommended that oncologists provide a survivorship care plan that details the cancer (ie, tumor characteristics), the type of treatment (ie, enrollment in a clinical trial; medical, surgical, or radiation), support services, and follow-up recommendations for the primary care provider. (Examples of elements of a survivorship care plan can be found at www.mskcc.org/hcp-education-training/survivorship/survivorship-care-plan6 and http://sma.org/southern-medical-journal/article/cancer-survivors-history-physical/7).
Regrettably, survivorship care plans have been rarely and poorly employed. Studies show that fewer than one-half of oncologists provide a plan, and that when they do, the plan often lacks recommended information.8,9 Survivorship care plans may soon become common practice, however; the Commission on Cancer of the American College of Surgeons has required their use in all certified cancer centers since 2015.10
2. Provide surveillance of existing cancer
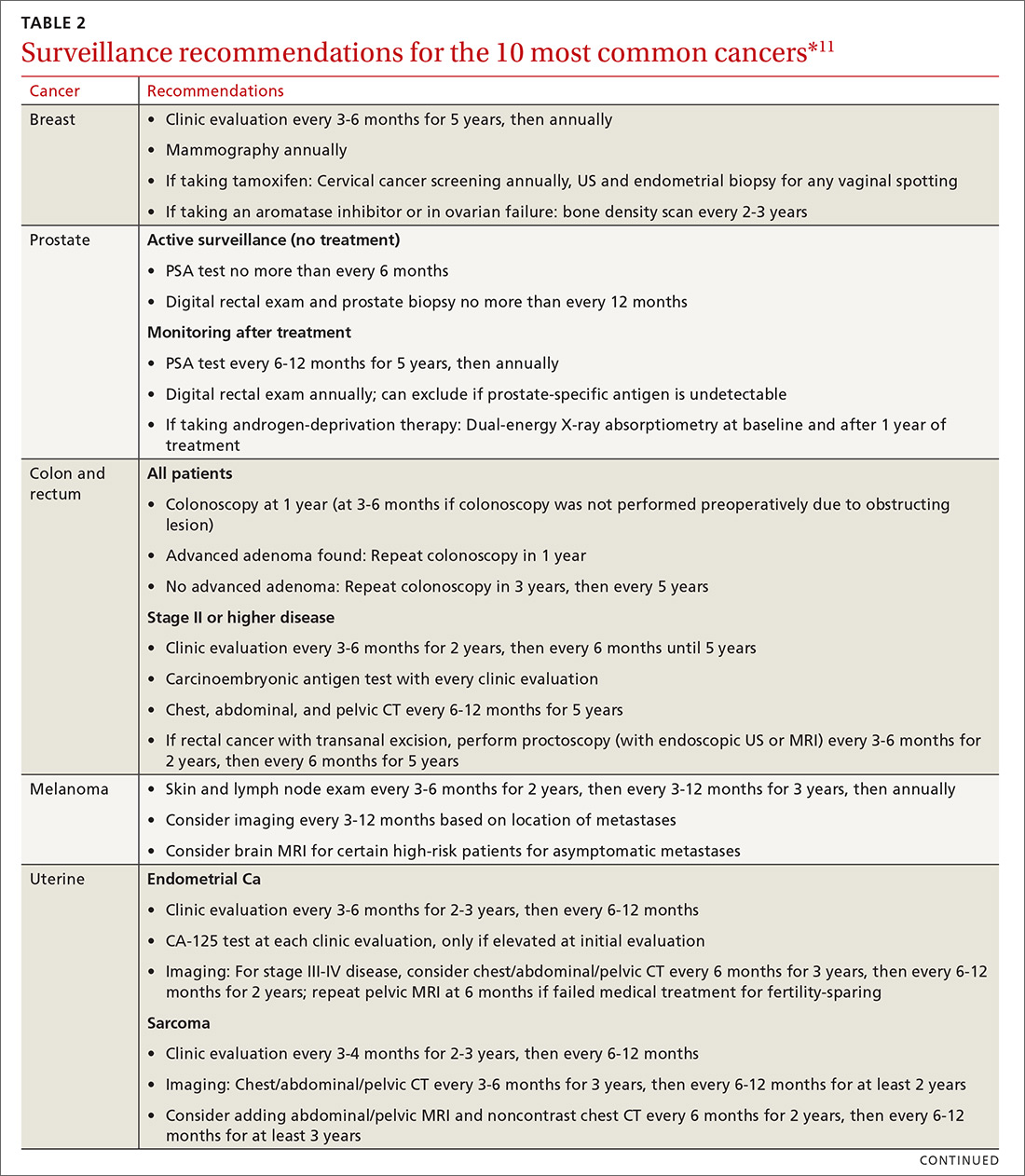
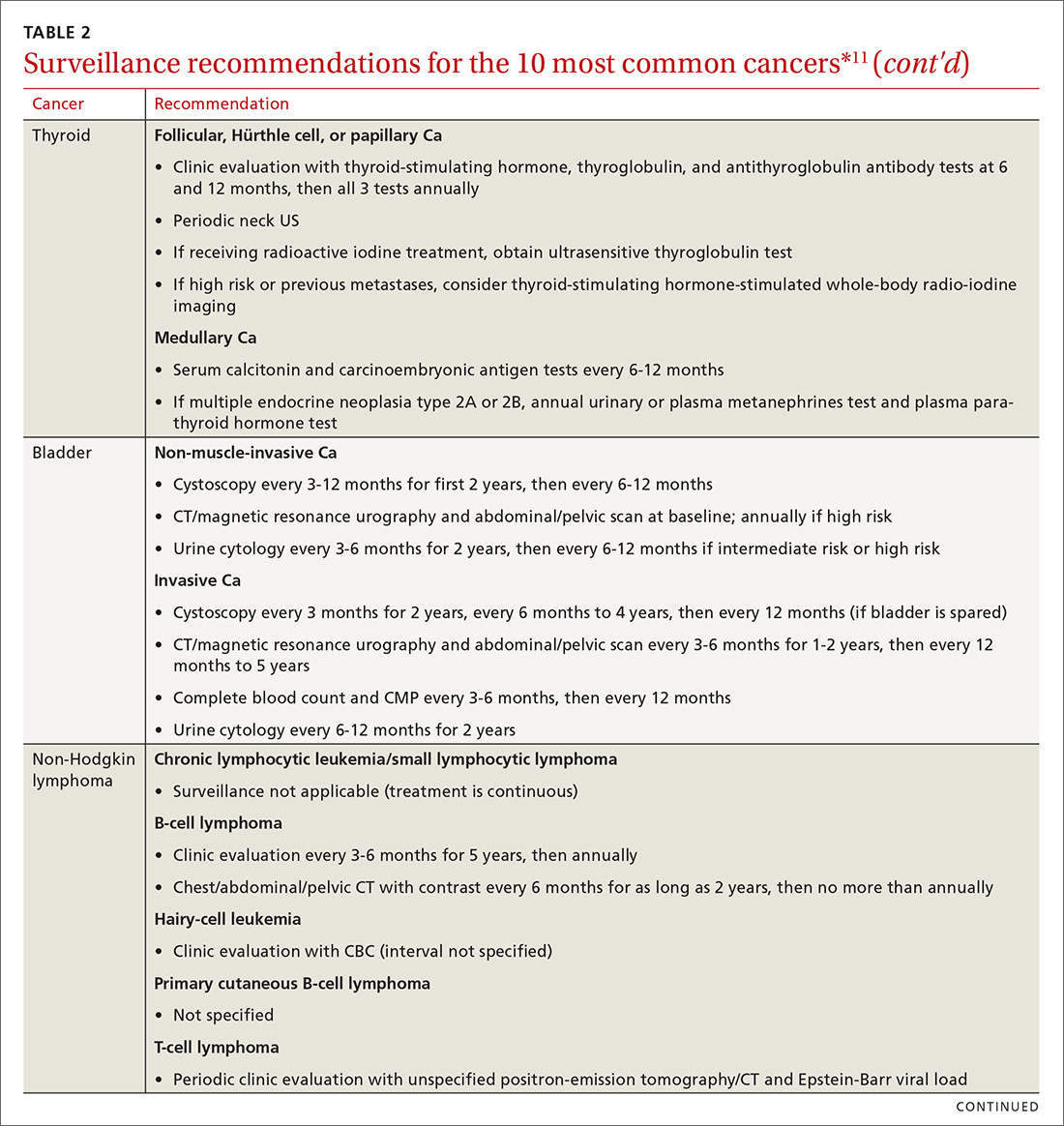
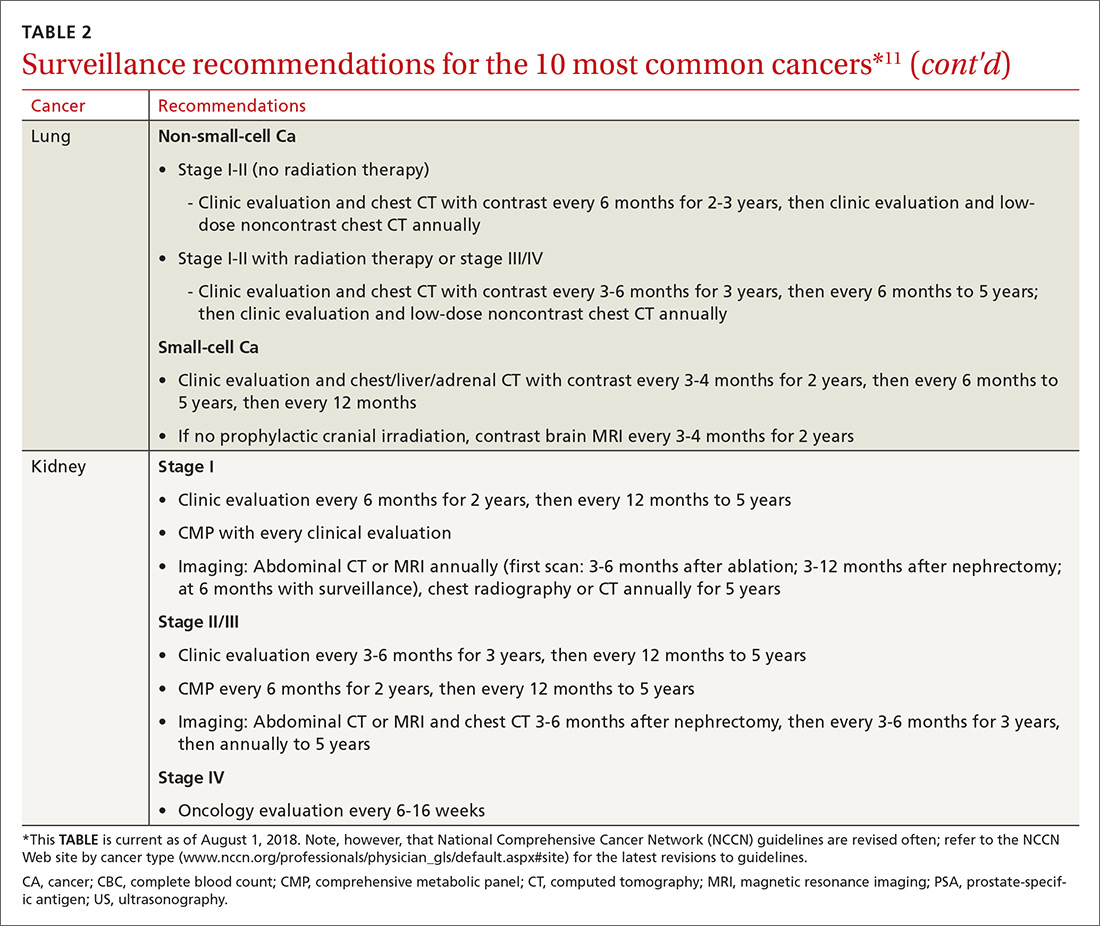
Cancer follow-up is challenging after the initial treatment phase. Although there are many conflicting guidelines for surveillance after cancer, guidelines of the National Comprehensive Cancer Network (NCCN) (summarized in TABLE 211 for the 10 most common cancers in survivors) are the ones generally accepted.12,13
Although individual surveillance recommendations are based on limited evidence, studies confirm the importance of surveillance. A systematic review showed that surveillance mammography after breast cancer reduces breast cancer mortality by 36%.14 A study showed that bladder cancer recurrence diagnosed by surveillance instead of by symptoms led to a 35% increase in 5-year survival.15
Continue to: Yet adherence to cancer surveillance...
Yet adherence to cancer surveillance recommendations is poor. A study of patients with colon cancer demonstrated that only 12% met all recommended surveillance guidelines.16 A study of patients with bladder cancer after radical cystectomy showed that only 9% met recommended surveillance more than 2 years after diagnosis.17 Those dismal statistics may be the result of provider oversight—not patient reluctance.
In the colon cancer study, for example, compliance with follow-up colonoscopy was 80% but compliance with carcinoembryonic antigen testing was only 22%.16 In the bladder cancer study, follow-up urine cytology was obtained in only 23% of patients, although 75% completed recommended imaging.17
Although surveillance remains the oncologist’s responsibility, visits to the FP provide an opportunity to review surveillance and order needed laboratory testing and other studies, including imaging.
3. Screen for new cancers
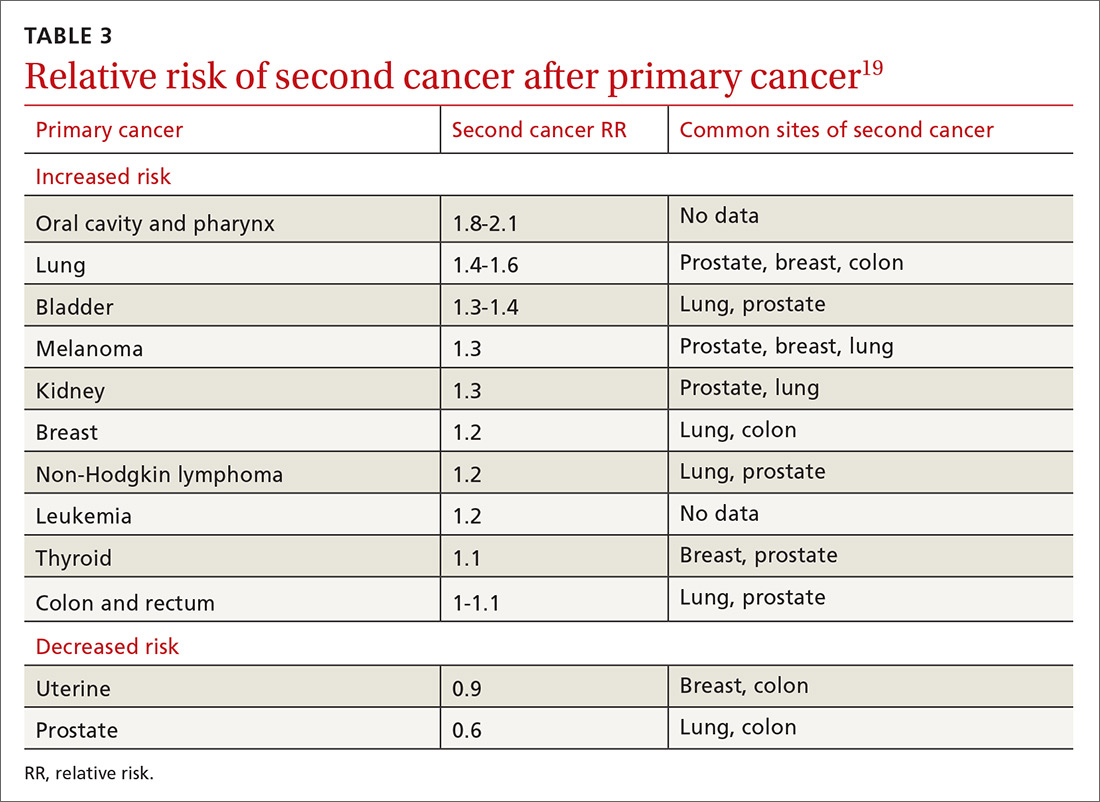
The risk of a second cancer is elevated for cancer survivors compared with the risk of a primary cancer in the healthy general population; some survivors have a lifetime risk of a second cancer as high as 36%.18 Risk varies by cancer type (TABLE 319). Some of this variation is due to the impact of smoking: Smoking-related cancers have the highest risk of second malignancy.19 Genetic predisposition to malignant transformation is also theorized to contribute to increased risk. Second malignancies are dangerous; 55% of patients die of the second cancer compared with only 13% of their initial cancer.19
Studies show that cancer survivors display varying adherence with recommended screening for second cancers. In a study of Latina cancer survivors, depressive symptoms were associated with lower screening compliance.20 A study of survivors of hematologic cancer showed a low rate of cancer screening and high fear of cancer recurrence—suggesting avoidance due to fear.21 Other studies, however, show similar or increased compliance with screening in cancer survivors.22,23 A meta-analysis of 19 studies determined that, overall, cancer survivors receive 25% to 38% more recommended screening than the general population.24
Continue to: Few guidelines exist to guide FPs...
Few guidelines exist to guide FPs in adjusting screening for the cancer survivor. For women who received radiation therapy for a tumor in the chest, for example, the recommendation offered by several groups is to start breast cancer screening 8 to 10 years after treatment or by 30 years of age, and to consider combining magnetic resonance imaging and mammography.25 Recommendations for breast cancer screening do not account for a history of other gynecologic cancers unless genetic markers are present.25 On the other hand, the impact of a history of cancer on the risk of prostate cancer and on screening decisions has not been studied,26 and cervical cancer screening guidelines, which recommend that screening continue after 65 years of age for patients who are immunocompromised, do not address a history of other cancer.27
4. Manage the effects of both the cancer and the treatment
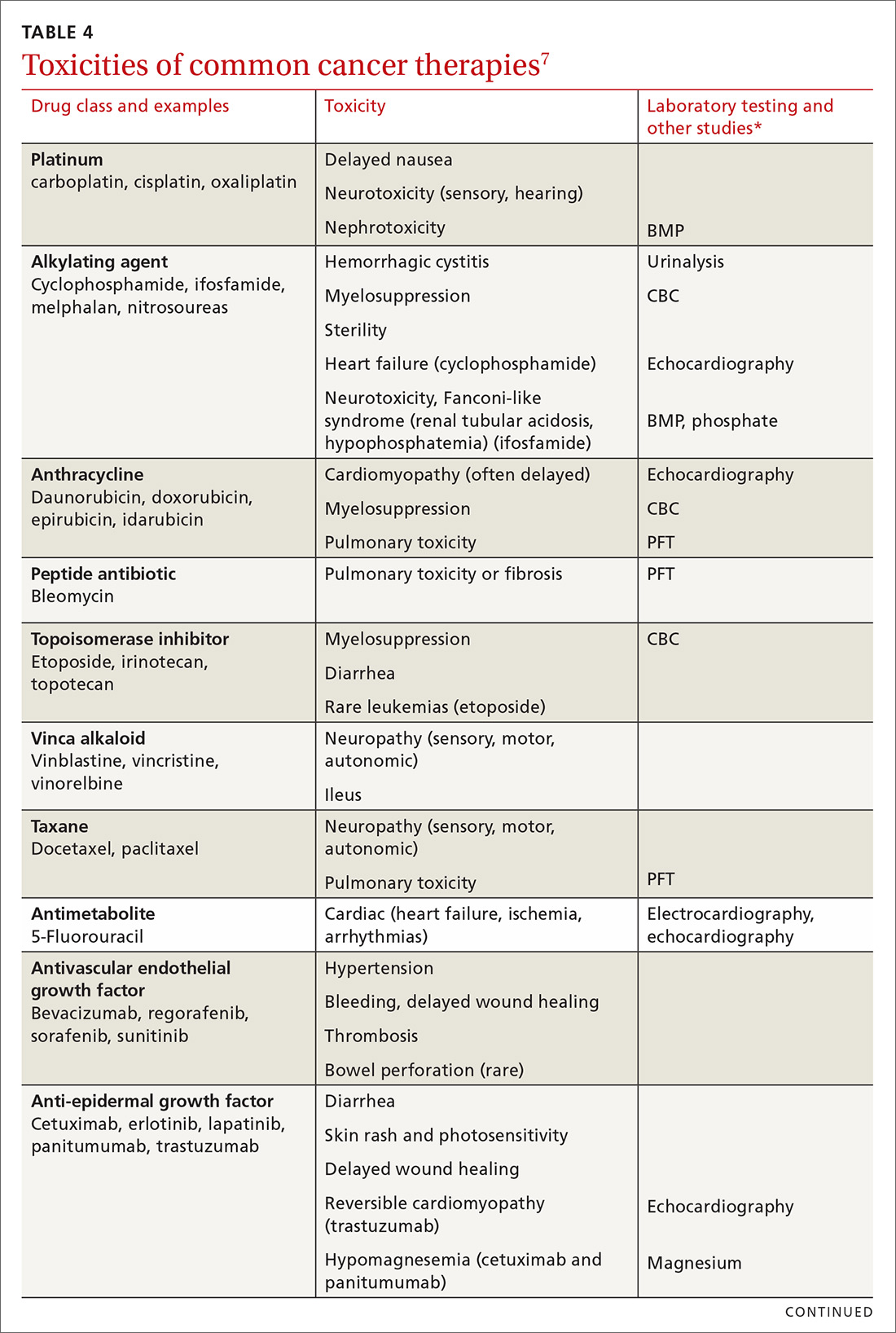
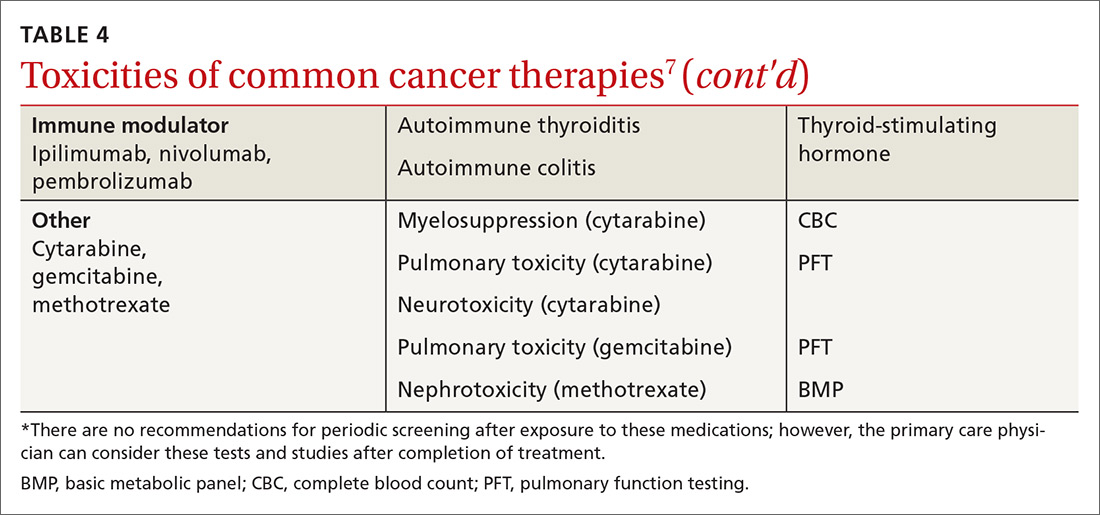
Medical issues faced by cancer survivors are familiar to FPs, but there are some specific recommendations regarding evaluation and treatment that stand in contrast to what would be considered for a healthy, or non-cancer, patient. For example, each chemotherapeutic agent has characteristic adverse effects; TABLE 47 lists the principal adverse effects of common agents and recommendations for testing when these problems develop. Common long-term problems in cancer survivors include fatigue, chronic pain, cognitive dysfunction, psychiatric illness, and cardiovascular disease. Although these symptoms and manifestations are common, the physician must be careful: New or changing symptoms could signal the spread or recurrence of disease. Fear of recurrence can lead patients to exaggerate or minimize symptoms.
Fatigue is the most common symptom seen in cancer survivors during treatment and following remission.28 More than 40% of cancer survivors report significant fatigue.29 Although fatigue is concerning for cancer recurrence, other causes are common in cancer survivors. Both depression and anxiety commonly present with worsened fatigue.30 Sleep disturbances are common, even without a psychiatric diagnosis.31 Effects of treatment, including nausea, anemia, heart failure, and medication adverse effects can cause or worsen fatigue. Pain is associated with fatigue, but to a lesser extent than are depression, anxiety, and nausea.32
Pharmacotherapy of cancer-related fatigue is challenging. Psychostimulants have been most studied. A recent systematic review shows that methylphenidate produces mild or moderate improvement in fatigue, whereas modafanil has minimal effectiveness.33 Antidepressants have not been shown to relieve fatigue.33
A recent meta-analysis showed that nonpharmaceutical treatments for cancer-related fatigue are more effective than pharmacotherapy. In this review, both exercise and pharmacotherapy had a mild-to-moderate effect on fatigue.35 Exercise is best studied in this regard, and has shown the most consistent results.31
Continue to: Chronic pain
Chronic pain. Pain is common in cancer survivors: As many as 40% experience pain for years after initial therapy.36 Treatment of some cancers—eg, thoracotomy (80%), amputation (50%-80%), neck dissection (52%), and surgical management of breast cancer (63%)—increase the likelihood of chronic pain.37 Reports of pain in cancer survivors that should be considered red flags that might signal recurrence of cancer include new or worsening pain; pain worse at night or when recumbent; new neurologic symptoms; and general symptoms of systemic illness37 (TABLE 537).
Management of pain is best approached by its cause, with neurologic, rheumatologic (including myofascial pain and arthralgia), lymphatic, and genital causes most common.37 Across all types of pain, complete relief is unlikely; functional goals provide a more effective target.
For neuropathic cancer pain, duloxetine is the only medication with evidence of benefit; anticonvulsant and topical medications are recommended on the basis of the findings of studies of noncancer pain.38 There are few data on the value of treatments for cancer-related rheumatologic and lymphatic pain, although exercise has shown benefit in both types.38 For dyspareunia and sexual dysfunction (common after gynecologic and nongynecologic cancers), vaginal lubricants and pelvic-floor physiotherapy have shown benefit.39 There is significant overlap in psychiatric comorbidities, sleep, and pain, and addressing all of a patient’s problems can reduce pain and improve function.40
Opioids are often prescribed for pain in cancer survivors. Cancer survivors have a higher rate of opioid prescribing compared with that of non-cancer patients, even 10 years after diagnosis.41 Guidelines of the Centers for Disease Control and Prevention for using opioids to manage chronic pain specifically exclude cancer patients.42 Regrettably, there is no evidence that opioids have long-term efficacy in chronic pain; in fact, evidence is accumulating that chronic opioid therapy exacerbates chronic pain.43
Cognitive dysfunction is present in 17% to 75% of cancer survivors as memory disturbance, psychological disorder, sleep dysfunction, or impairment of executive functioning.44 Cognitive deficits appear to be secondary to both cancer and treatment modalities45; as many as one-third of patients have cognitive dysfunction prior to receiving chemotherapy.46
Continue to: Chemotherapies that are more likely...
Chemotherapies that are more likely to cause cognitive symptoms include methotrexate, 5-fluorouracil, cyclophosphamide, and hormone antagonists.47 More powerful regimens and repetitive chemotherapy regimens tend to cause more cognitive effects.47
Cognitive training interventions show evidence of likely benefit,44,48 leading to recommendations for self-treatment strategies, such as written lists, wordplay, crossword puzzles, jigsaw puzzles, playing a musical instrument, and new hobbies. Small studies suggest a benefit from cognitive behavioral therapy.44,49 A study of breast cancer survivors showed that yoga led to improvement in patient-reported cognitive dysfunction.50 Physical exercise yields cognitive benefit in healthy older adults and is supported by limited evidence in cancer survivors.51
There is no effective pharmacotherapy for cancer- and cancer chemotherapy-related cognitive dysfunction unless a treatable underlying cause is found.44 Symptoms tend to subside with time after completion of chemotherapy, which might be reassuring to patients and families.45
Psychiatric problems. The most common psychiatric issues in cancer survivors are anxiety and depression; the prevalence of anxiety is nearly double that of depression.52 Anxiety often presents as fear of a recurrence of cancer or a feeling of lack of control over present or future circumstances.53 Screening for anxiety and depression is recommended at each visit, using standardized screening questionnaires.54
A small study suggests that psychiatric treatment reduces the risk of early mortality.55 Small studies also suggest that mindfulness-based therapy and cognitive behavioral therapy delivered by telehealth offer benefit.56 A meta-analysis shows that exercise interventions improve depression and anxiety in breast cancer patients.57
Continue to: There are few studies of pharmacotherapy...
There are few studies of pharmacotherapy of anxiety or depression in cancer survivors56; it is known that cancer survivors are nearly twice as likely as the general population to be taking medical therapy for anxiety and depression.58 A Cochrane systematic review of 7 small studies showed uncertain improvement in depressive symptoms in patients with cancer from antidepressant medication; however, an earlier systematic review did show benefit.59,60
In a trial of patients without depression who were being treated for head and neck cancer, escitalopram, 20 mg/d, reduced the risk of subsequent depression compared with placebo.61 A study of 420 breast cancer survivors showed that 300 mg/d and 900 mg/d dosages of gabapentin were both superior to placebo, and nearly equivalent to each other, at reducing anxiety scores.62 In both studies, however, the evidence is nonetheless insufficient to make specific recommendations about these medications.
Cardiac risk
Among chemotherapeutic agents, anthracyclines, such as doxorubicin, cause the most rapid and striking myocyte damage. This damage is dose-dependent and nearly irreversible, with 98% of injury occurring within the first year of chemotherapy.64 More than one half of cancer patients taking an anthracycline have cardiac dysfunction on imaging; 5% will be in overt heart failure 10 to 20 years, or longer, after chemotherapy.63 Following monitoring at 1 year post-therapy, regular cardiac imaging is not recommended in the absence of symptoms.62
Because other cardiotoxic chemotherapeutic agents cause partially reversible damage, imaging is not recommended in the absence of symptoms in patients taking those agents.64
Continue to: Radiation therapy to the chest leads...
Radiation therapy to the chest leads to many cardiac complications, including cardiomyopathy, valvular disease, pericardial disease, and arrhythmias. Development of cardiomyopathy can be delayed 20 to 30 years after radiation; screening echocardiography is therefore recommended every 5 to 10 years after radiation therapy.65 Recent adjustments to the dosages and delivery of radiation therapy should reduce cardiac damage, but will require decades to validate.63
For patients at risk of cardiovascular disease prior to treatment of cancer, there is evidence to support preventive treatment with angiotensin II-receptor antagonists, beta-blockers, and statins to prevent cardiomyopathy.63 Treatment of diagnosed cardiomyopathy and heart failure follows standard guidelines, with significant emphasis on aerobic exercise and smoking cessation.63
Cancer survivorship care: Your critical role
Cancer survivors constitute a large population who frequent the practices of primary care physicians. Primary care visits provide an opportunity to monitor key elements of survivorship, including surveillance of the current cancer and screening for second cancers. Similar to what is seen with diabetes and coronary artery disease, cancer increases cardiac risk, which requires preventive care and chronic management. FPs are well placed to treat common issues in cancer survivors—issues that mirror concerns seen in the general population.
CORRESPONDENCE
Michael J. Arnold, MD, CDR, USN, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, MD 20814; michael.arnold@usuhs.edu.
ACKNOWLEDGEMENT
Kristian Sanchack, MD, and James Higgins, DO, assisted with the editing of the manuscript.
1. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2016-2017. Atlanta, GA: American Cancer Society; 2016. www.cancer.org/research/cancer-facts-statistics/survivor-facts-figures.html. Accessed July 25, 2018.
2. Survivorship. NCCN Guidelines (version 1.2017). Fort Washington, PA: National Comprehensive Cancer Network; 2017. www.nccn.org/professionals/physician_gls/default.aspx#supportive. Accessed July 26, 2018.
3. Kendall C, Decker KM, Groome PA, et al. Use of physician services during the survivorship phase: a multi-province study of women diagnosed with breast cancer. Curr Oncolog. 2017;24:81-89.
4. Snyder CF, Earle CC, Herbert RJ, et al. Preventive care for colorectal cancer survivors: a 5-year longitudinal study. J Clin Oncol. 2008;26:1073-1079.
5. Hewitt M, Greenfield S, Stovall E (eds); Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington DC: The National Academies Press; 2006. www.nap.edu/read/11468/chapter/1. Accessed July 25, 2018.
6. Survivorship care plan. New York, NY: Memorial Sloan Kettering Cancer Center. www.mskcc.org/hcp-education-training/survivorship/survivorship-care-plan. Accessed August 11, 2018.
7. Fuentes AC, Lambird JE, George TJ, et al. Cancer survivor’s history and physical. South Med J. 2017;110:37-44. http://sma.org/southern-medical-journal/article/cancer-survivors-history-physical/. Accessed July 26, 2018.
8. Salz T, Oeffinger KC, McCabe MS, et al. Survivorship care plans in research and practice. CA Cancer J Clin. 2012;62:101-117.
9. Birken SA, Mayer DK, Weiner BJ. Survivorship care plans: prevalence and barriers to use. J Cancer Educ. 2013;28:290-296.
10. American College of Surgeons Commission on Cancer. Cancer program standards 2012: Ensuring patient-centered care. V1.2.1. www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed July 25, 2018.
11. NCCN guidelines for treatment of cancer by site. NCCN Guidelines (version 1.2018). Fort Washington, PA: National Comprehensive Cancer Network; 2018. www.nccn.org/professionals/physician_gls/default.aspx#site. Accessed July 25, 2018.
12. Spronka I, Korevaar JC, Burgers JS, et al. Review of guidance on recurrence risk management for general practitioners in breast cancer, colorectal cancer and melanoma guidelines. Family Pract. 2017;34:154-160.
13. Merkow RP, Korenstein D, Yeahia R, et al. Quality of cancer surveillance clinical practice guidelines: specificity and consistency of recommendations. JAMA Intern Med. 2017;177:701-709.
14. Muradali D, Kennedy EB, Eisen A, et al. Breast screening for survivors of breast cancer: a systematic review. Prev Med. 2017;103:70-75.
15. Giannarini G, Kessler TM, Thoeny HC, et al. Do patients benefit from routine follow-up to detect recurrences after radical cystectomy and ileal orthotopic bladder substitution? Eur Urol. 2010;58:486-494.
16. Sisler JJ, Seo B, Katz A, et al. Concordance with ASCO guidelines for surveillance after colorectal cancer treatment: a population-based analysis. J Oncol Pract. 2012;8:e69-e79.
17. Ehdaie B. Atoria CL, Lowrance WT, et al. Adherence to surveillance guidelines after radical cystectomy: a population-based analysis. Urol Oncol. 2014;32:779-784.
18. Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354-1365.
19. Donin N, Filson C, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer. 2016;122:3075-3086.
20. Holder AE, Ramirez AG, Gallion K. Depressive symptoms in Latina breast cancer survivors: a barrier to cancer screening. Health Psycholog. 2014;33:242-248.
21. Dyer G, Larsen SR, Gilroy N, et al. Adherence to cancer screening guidelines in Australian survivors of allogenic blood and marrow transplantation (BMT). Cancer Med. 2016;5:1702-1716.
22. Mandelzweig L, Chetrit A, Amitai T, et al. Primary prevention and screening practices among long-term breast cancer survivors. Cancer Causes Control. 2017;28:657-666.
23. Bishop MM, Lee SJ, Beaumont JL, et al. The preventive health behaviors of long-term survivors of cancer and hematopoietic stem cell transplantation compared with matched controls. Biol Blood Marrow Transplant. 2010;16:207-214.
24. Uhlig A, Mei J, Baik I, et al. Screening utilization among cancer survivors: a meta-analysis. J Public Health (Oxf). 2018;40:129-137.
25. Hilal T, Rudy DW. Radiation-induced breast cancer: the question of early breast cancer screening in Hodgkin’s lymphoma survivors. Oxf Med Case Reports. 2016;2016:17-18.
26. Lin K, Croswell JM, Koenig H, et al. Prostate-specific antigen-based screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force [Internet]. Evidence Syntheses No. 90. AHRQ Publication No. 12-05160-EF-1. Rockville, MD: Agency for Healthcare Research and Quality (US); October 2011. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0032900/. Accessed July 25, 2018.
27. US Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
28. Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12 Suppl 1:4-10.
29. Jung JY, Lee JM, Kim MS, et al. Comparison of fatigue, depression, and anxiety as factors affecting posttreatment health-related quality of life in lung cancer survivors. Psychooncology. 2018;27:465-470.
30. Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatment. Nat Rev Clin Oncol. 2014;11:597-609.
31. Medysky ME, Temesi J, Culos-Reed SN, et al. Exercise, sleep and cancer-related fatigue: are they related? Neurophysiol Clin. 2017;47:111-122.
32. Oh HS, Sea WS. Systematic review and meta-analysis of the correlates of cancer-related fatigue. Worldviews Evid Based Nurs. 2011;8:191-201.
33. Qu D, Zhang Z, Yu X, et al. Psychotropic drugs for the management of cancer-related fatigue: a systematic review and meta-analysis. Eur J Cancer Care (Engl). 2016;25:970-979.
34. Escalante CP, Manzullo EF. Cancer-related fatigue: the approach and treatment. J Gen Intern Med. 2009;24(suppl 2):S412-S416.
35. Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3:961-968.
36. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32:1739-1747.
37. Davies PS. Chronic pain management in the cancer survivor: tips for primary care providers. Nurse Pract. 2013;39:28-38.
38. Boland EG, Ahmedzai SH. Persistent pain in cancer survivors. Curr Opin Support Palliat Care. 2017;11:181-190.
39. Sears CS, Robinson JW, Walker LM. A comprehensive review of sexual health concerns after cancer treatment and the biopsychosocial treatment options available to female patients. Eur J Cancer Care (Engl). 2017;27:e12738.
40. Schou Bredal I, Smeby NA, Ottesen S, et al. Chronic pain in breast cancer survivors: comparison of psychological, surgical, and medical characteristics between survivors with and without pain. J Pain Symptom Manage. 2014;48:852-862.
41. Sutradhar R, Lokku A, Barbera L. Cancer survivorship and opioid prescribing rates: a population-based matched cohort study among individuals with and without a history of cancer. Cancer. 2017;123:4286-4293.
42. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
43. Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep. 2016;18:71.
44. Von Ah D. Cognitive changes associated with cancer and cancer treatment: state of the science. Clin J Oncol Nurs. 2015;19:47-56.
45. Moore HC. An overview of chemotherapy-related cognitive dysfunction, or ‘chemobrain’. Oncology (Williston Park). 2014;28:797-804.
46. Asher A. Cognitive dysfunction among cancer survivors. Am J Phys Med Rehabil. 2011;90(suppl):S16-S26.
47. Joly F, Rigal O, Noal S, et al. Cognitive dysfunction and cancer: which consequences in terms of disease management? Psychooncology. 2011;20:1251-1258.
48. Attention, thinking or memory problems. American Society of Clinical Oncology Cancer.Net. April 2018. www.cancer.net/navigating-cancer-care/side-effects/attention-thinking-or-memory-problems. Accessed July 25, 2018.
49. Kucherer S, Ferguson RJ. Cognitive behavioral therapy for cancer-related cognitive dysfunction. Curr Opin Support Palliat Care. 2017;11:46-51.
50. Derry HM, Jaremka LM, Bennet JM, et al. Yoga and self-reported cognitive problems in breast cancer survivors: a randomized controlled trial. Psychooncology. 2015;24:958-966.
51. Treanor CJ, McMenamin UC, O’Neill RF, et al. Non-pharmacological interventions for cognitive impairment due to systemic cancer treatment. Cochrane Database Syst Rev. 2016 Aug 16;(8):CD011325.
52. Mitchell AJ, Ferguson DW, Gill J, et al. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. Lancet Oncol. 2013;14:721-732.
53. Inhestern L, Beierlein V, Bultmann JC, et al. Anxiety and depression in working-age cancer survivors: a register-based study. BMC Cancer. 2017;17:347.
54. Partridge AH, Jacobsen PB, Andersen BL. Challenges to standardizing the care for adult cancer survivors: highlighting ASCO’s fatigue and anxiety and depression guidelines. Am Soc Clin Oncol Educ Book. 2015:188-194.
55. Andersen BL, Yang HC, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer. 2008;113:3450-3458.
56. Yi JC, Syrjala KL. Anxiety and depression in cancer survivors. Med Clin N Am. 2017;101:1099-1113.
57. Zhu G, Zhang X, Wang Y, et al. Effects of exercise intervention in breast cancer survivors: a meta-analysis of 33 randomized controlled trials. Onco Targets Ther. 2016;9:2153-2168.
58. Hawkins NA, Soman A, Lunsford N, et al. Use of medications for treating anxiety and depression in cancer survivors in the United States. J Clin Oncol. 2017;35:78-85.
59. Ostuzzi G, Matcham F, Dauchy S, et al. Antidepressants for the treatment of depression in people with cancer. Cochrane Database Syst Rev. 2015 June 1;(6):CD011006.
60. Laoutidis ZG, Mathiak K. Antidepressants in the treatment of depression/depressive symptoms in cancer patients: a systematic review and meta-analysis. BMC Psychiatry. 2013;13:140.
61. Lydiatt WM, Bessette D, Schmid KK, et al. Prevention of depression with escitalopram in patients undergoing treatment for head and neck cancer: randomized, double-blind, placebo-controlled clinical trial. JAMA Otolaryngol Head Neck Surg. 2013;139:678-686.
62. Lavigne JE, Heckler C, Mathews JL, et al. A randomized, controlled, double-blinded clinical trial of gabapentin 300 versus 900 mg versus placebo for anxiety symptoms in breast cancer survivors. Breast Cancer Res Treat. 2012;136:479-486.
63. Okwuosa TM, Anzevino S, Rao R. Cardiovascular disease in cancer survivors. Postgrad Med J. 2017;93:82-90.
64. Plana, JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911-939.
65. Lancellotti, P, Nkomo VT, Badano LP, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:1013-1032.
Cancer survivors represent a rapidly increasing population. In 1971, there were 3 million cancer survivors; this number increased to 15.5 million in 2016 and will reach 20 million by 2026.1TABLE 11 shows the percentage of survivors by type of cancer. Cancer survivors tend to be older,* comprising nearly 1 of every 5 people older than 65 years.2
The Institute of Medicine (IOM) identified 3 key characteristics of cancer survivors3:
- Trajectories of survivorship are variable; many cancer patients have periods of relative health between episodes of their disease.
- Survivors require careful cancer monitoring; in addition to the risk that their primary cancer will recur, they have an elevated risk for another, second cancer.
- Both cancer and its treatments increase the risk of other medical and psychiatric problems.
Family physicians (FPs) have optimal skills for navigating the chronic risks and health concerns of the well cancer survivor. This article reviews the primary care management of the functional cancer survivor, focusing on the management of chronic conditions and preventive care.
Survivorship follows any of 6 paths
Cancer survivorship is increasing in importance as treatment has steadily reduced mortality. Six trajectories of cancer survivors have been identified1:
- living cancer-free after treatment with minimal effects
- living cancer-free but suffering serious treatment complications
- Suffering late recurrence
- Developing a second cancer
- Living with intermittent cancer recurrences
- Living with cancer continuously.
Only patients in the last 2 groups are likely to be managed primarily by oncologists.
Survivors look to their FPs for ongoing care
Cancer survivors routinely see their primary care physician after initial treatment. A study of 30,000 Canadian breast cancer survivors demonstrated that follow-up care was limited to an oncologist in only 2%; 84% saw a primary care provider and an oncologist; and 14% saw a primary care provider only.4 A study of colorectal cancer survivors showed that primary care visits increased in each of the 5 years after diagnosis, during which time oncology visits decreased steadily5; in that study, primary care physicians delivered more preventive care than oncologists did.5 Similar to what is done in other chronic conditions, the various effects of cancer are best managed as a whole.
The IOM recommends that cancer survivor care comprise 4 elements2:
- coordination between oncologist and primary care physician
- surveillance for recurrence or spread of existing cancer
- screening for new cancer
- intervention for the effects of cancer and treatment.
Continue to: The following discussion summarizes...
The following discussion summarizes evidence and recommendations for each element of the IOM recommendations for survivor care.
Implementing the 4 elements of cancer survivor care
1. Coordinate care through a unified survivorship care plan
The IOM has noted that the needs of cancer survivors are rarely met2; communication between oncology and primary care is often deficient during transition of care. The IOM has recommended that oncologists provide a survivorship care plan that details the cancer (ie, tumor characteristics), the type of treatment (ie, enrollment in a clinical trial; medical, surgical, or radiation), support services, and follow-up recommendations for the primary care provider. (Examples of elements of a survivorship care plan can be found at www.mskcc.org/hcp-education-training/survivorship/survivorship-care-plan6 and http://sma.org/southern-medical-journal/article/cancer-survivors-history-physical/7).
Regrettably, survivorship care plans have been rarely and poorly employed. Studies show that fewer than one-half of oncologists provide a plan, and that when they do, the plan often lacks recommended information.8,9 Survivorship care plans may soon become common practice, however; the Commission on Cancer of the American College of Surgeons has required their use in all certified cancer centers since 2015.10
2. Provide surveillance of existing cancer
Cancer follow-up is challenging after the initial treatment phase. Although there are many conflicting guidelines for surveillance after cancer, guidelines of the National Comprehensive Cancer Network (NCCN) (summarized in TABLE 211 for the 10 most common cancers in survivors) are the ones generally accepted.12,13
Although individual surveillance recommendations are based on limited evidence, studies confirm the importance of surveillance. A systematic review showed that surveillance mammography after breast cancer reduces breast cancer mortality by 36%.14 A study showed that bladder cancer recurrence diagnosed by surveillance instead of by symptoms led to a 35% increase in 5-year survival.15
Continue to: Yet adherence to cancer surveillance...
Yet adherence to cancer surveillance recommendations is poor. A study of patients with colon cancer demonstrated that only 12% met all recommended surveillance guidelines.16 A study of patients with bladder cancer after radical cystectomy showed that only 9% met recommended surveillance more than 2 years after diagnosis.17 Those dismal statistics may be the result of provider oversight—not patient reluctance.
In the colon cancer study, for example, compliance with follow-up colonoscopy was 80% but compliance with carcinoembryonic antigen testing was only 22%.16 In the bladder cancer study, follow-up urine cytology was obtained in only 23% of patients, although 75% completed recommended imaging.17
Although surveillance remains the oncologist’s responsibility, visits to the FP provide an opportunity to review surveillance and order needed laboratory testing and other studies, including imaging.
3. Screen for new cancers
The risk of a second cancer is elevated for cancer survivors compared with the risk of a primary cancer in the healthy general population; some survivors have a lifetime risk of a second cancer as high as 36%.18 Risk varies by cancer type (TABLE 319). Some of this variation is due to the impact of smoking: Smoking-related cancers have the highest risk of second malignancy.19 Genetic predisposition to malignant transformation is also theorized to contribute to increased risk. Second malignancies are dangerous; 55% of patients die of the second cancer compared with only 13% of their initial cancer.19
Studies show that cancer survivors display varying adherence with recommended screening for second cancers. In a study of Latina cancer survivors, depressive symptoms were associated with lower screening compliance.20 A study of survivors of hematologic cancer showed a low rate of cancer screening and high fear of cancer recurrence—suggesting avoidance due to fear.21 Other studies, however, show similar or increased compliance with screening in cancer survivors.22,23 A meta-analysis of 19 studies determined that, overall, cancer survivors receive 25% to 38% more recommended screening than the general population.24
Continue to: Few guidelines exist to guide FPs...
Few guidelines exist to guide FPs in adjusting screening for the cancer survivor. For women who received radiation therapy for a tumor in the chest, for example, the recommendation offered by several groups is to start breast cancer screening 8 to 10 years after treatment or by 30 years of age, and to consider combining magnetic resonance imaging and mammography.25 Recommendations for breast cancer screening do not account for a history of other gynecologic cancers unless genetic markers are present.25 On the other hand, the impact of a history of cancer on the risk of prostate cancer and on screening decisions has not been studied,26 and cervical cancer screening guidelines, which recommend that screening continue after 65 years of age for patients who are immunocompromised, do not address a history of other cancer.27
4. Manage the effects of both the cancer and the treatment
Medical issues faced by cancer survivors are familiar to FPs, but there are some specific recommendations regarding evaluation and treatment that stand in contrast to what would be considered for a healthy, or non-cancer, patient. For example, each chemotherapeutic agent has characteristic adverse effects; TABLE 47 lists the principal adverse effects of common agents and recommendations for testing when these problems develop. Common long-term problems in cancer survivors include fatigue, chronic pain, cognitive dysfunction, psychiatric illness, and cardiovascular disease. Although these symptoms and manifestations are common, the physician must be careful: New or changing symptoms could signal the spread or recurrence of disease. Fear of recurrence can lead patients to exaggerate or minimize symptoms.
Fatigue is the most common symptom seen in cancer survivors during treatment and following remission.28 More than 40% of cancer survivors report significant fatigue.29 Although fatigue is concerning for cancer recurrence, other causes are common in cancer survivors. Both depression and anxiety commonly present with worsened fatigue.30 Sleep disturbances are common, even without a psychiatric diagnosis.31 Effects of treatment, including nausea, anemia, heart failure, and medication adverse effects can cause or worsen fatigue. Pain is associated with fatigue, but to a lesser extent than are depression, anxiety, and nausea.32
Pharmacotherapy of cancer-related fatigue is challenging. Psychostimulants have been most studied. A recent systematic review shows that methylphenidate produces mild or moderate improvement in fatigue, whereas modafanil has minimal effectiveness.33 Antidepressants have not been shown to relieve fatigue.33
A recent meta-analysis showed that nonpharmaceutical treatments for cancer-related fatigue are more effective than pharmacotherapy. In this review, both exercise and pharmacotherapy had a mild-to-moderate effect on fatigue.35 Exercise is best studied in this regard, and has shown the most consistent results.31
Continue to: Chronic pain
Chronic pain. Pain is common in cancer survivors: As many as 40% experience pain for years after initial therapy.36 Treatment of some cancers—eg, thoracotomy (80%), amputation (50%-80%), neck dissection (52%), and surgical management of breast cancer (63%)—increase the likelihood of chronic pain.37 Reports of pain in cancer survivors that should be considered red flags that might signal recurrence of cancer include new or worsening pain; pain worse at night or when recumbent; new neurologic symptoms; and general symptoms of systemic illness37 (TABLE 537).
Management of pain is best approached by its cause, with neurologic, rheumatologic (including myofascial pain and arthralgia), lymphatic, and genital causes most common.37 Across all types of pain, complete relief is unlikely; functional goals provide a more effective target.
For neuropathic cancer pain, duloxetine is the only medication with evidence of benefit; anticonvulsant and topical medications are recommended on the basis of the findings of studies of noncancer pain.38 There are few data on the value of treatments for cancer-related rheumatologic and lymphatic pain, although exercise has shown benefit in both types.38 For dyspareunia and sexual dysfunction (common after gynecologic and nongynecologic cancers), vaginal lubricants and pelvic-floor physiotherapy have shown benefit.39 There is significant overlap in psychiatric comorbidities, sleep, and pain, and addressing all of a patient’s problems can reduce pain and improve function.40
Opioids are often prescribed for pain in cancer survivors. Cancer survivors have a higher rate of opioid prescribing compared with that of non-cancer patients, even 10 years after diagnosis.41 Guidelines of the Centers for Disease Control and Prevention for using opioids to manage chronic pain specifically exclude cancer patients.42 Regrettably, there is no evidence that opioids have long-term efficacy in chronic pain; in fact, evidence is accumulating that chronic opioid therapy exacerbates chronic pain.43
Cognitive dysfunction is present in 17% to 75% of cancer survivors as memory disturbance, psychological disorder, sleep dysfunction, or impairment of executive functioning.44 Cognitive deficits appear to be secondary to both cancer and treatment modalities45; as many as one-third of patients have cognitive dysfunction prior to receiving chemotherapy.46
Continue to: Chemotherapies that are more likely...
Chemotherapies that are more likely to cause cognitive symptoms include methotrexate, 5-fluorouracil, cyclophosphamide, and hormone antagonists.47 More powerful regimens and repetitive chemotherapy regimens tend to cause more cognitive effects.47
Cognitive training interventions show evidence of likely benefit,44,48 leading to recommendations for self-treatment strategies, such as written lists, wordplay, crossword puzzles, jigsaw puzzles, playing a musical instrument, and new hobbies. Small studies suggest a benefit from cognitive behavioral therapy.44,49 A study of breast cancer survivors showed that yoga led to improvement in patient-reported cognitive dysfunction.50 Physical exercise yields cognitive benefit in healthy older adults and is supported by limited evidence in cancer survivors.51
There is no effective pharmacotherapy for cancer- and cancer chemotherapy-related cognitive dysfunction unless a treatable underlying cause is found.44 Symptoms tend to subside with time after completion of chemotherapy, which might be reassuring to patients and families.45
Psychiatric problems. The most common psychiatric issues in cancer survivors are anxiety and depression; the prevalence of anxiety is nearly double that of depression.52 Anxiety often presents as fear of a recurrence of cancer or a feeling of lack of control over present or future circumstances.53 Screening for anxiety and depression is recommended at each visit, using standardized screening questionnaires.54
A small study suggests that psychiatric treatment reduces the risk of early mortality.55 Small studies also suggest that mindfulness-based therapy and cognitive behavioral therapy delivered by telehealth offer benefit.56 A meta-analysis shows that exercise interventions improve depression and anxiety in breast cancer patients.57
Continue to: There are few studies of pharmacotherapy...
There are few studies of pharmacotherapy of anxiety or depression in cancer survivors56; it is known that cancer survivors are nearly twice as likely as the general population to be taking medical therapy for anxiety and depression.58 A Cochrane systematic review of 7 small studies showed uncertain improvement in depressive symptoms in patients with cancer from antidepressant medication; however, an earlier systematic review did show benefit.59,60
In a trial of patients without depression who were being treated for head and neck cancer, escitalopram, 20 mg/d, reduced the risk of subsequent depression compared with placebo.61 A study of 420 breast cancer survivors showed that 300 mg/d and 900 mg/d dosages of gabapentin were both superior to placebo, and nearly equivalent to each other, at reducing anxiety scores.62 In both studies, however, the evidence is nonetheless insufficient to make specific recommendations about these medications.
Cardiac risk
Among chemotherapeutic agents, anthracyclines, such as doxorubicin, cause the most rapid and striking myocyte damage. This damage is dose-dependent and nearly irreversible, with 98% of injury occurring within the first year of chemotherapy.64 More than one half of cancer patients taking an anthracycline have cardiac dysfunction on imaging; 5% will be in overt heart failure 10 to 20 years, or longer, after chemotherapy.63 Following monitoring at 1 year post-therapy, regular cardiac imaging is not recommended in the absence of symptoms.62
Because other cardiotoxic chemotherapeutic agents cause partially reversible damage, imaging is not recommended in the absence of symptoms in patients taking those agents.64
Continue to: Radiation therapy to the chest leads...
Radiation therapy to the chest leads to many cardiac complications, including cardiomyopathy, valvular disease, pericardial disease, and arrhythmias. Development of cardiomyopathy can be delayed 20 to 30 years after radiation; screening echocardiography is therefore recommended every 5 to 10 years after radiation therapy.65 Recent adjustments to the dosages and delivery of radiation therapy should reduce cardiac damage, but will require decades to validate.63
For patients at risk of cardiovascular disease prior to treatment of cancer, there is evidence to support preventive treatment with angiotensin II-receptor antagonists, beta-blockers, and statins to prevent cardiomyopathy.63 Treatment of diagnosed cardiomyopathy and heart failure follows standard guidelines, with significant emphasis on aerobic exercise and smoking cessation.63
Cancer survivorship care: Your critical role
Cancer survivors constitute a large population who frequent the practices of primary care physicians. Primary care visits provide an opportunity to monitor key elements of survivorship, including surveillance of the current cancer and screening for second cancers. Similar to what is seen with diabetes and coronary artery disease, cancer increases cardiac risk, which requires preventive care and chronic management. FPs are well placed to treat common issues in cancer survivors—issues that mirror concerns seen in the general population.
CORRESPONDENCE
Michael J. Arnold, MD, CDR, USN, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, MD 20814; michael.arnold@usuhs.edu.
ACKNOWLEDGEMENT
Kristian Sanchack, MD, and James Higgins, DO, assisted with the editing of the manuscript.
Cancer survivors represent a rapidly increasing population. In 1971, there were 3 million cancer survivors; this number increased to 15.5 million in 2016 and will reach 20 million by 2026.1TABLE 11 shows the percentage of survivors by type of cancer. Cancer survivors tend to be older,* comprising nearly 1 of every 5 people older than 65 years.2
The Institute of Medicine (IOM) identified 3 key characteristics of cancer survivors3:
- Trajectories of survivorship are variable; many cancer patients have periods of relative health between episodes of their disease.
- Survivors require careful cancer monitoring; in addition to the risk that their primary cancer will recur, they have an elevated risk for another, second cancer.
- Both cancer and its treatments increase the risk of other medical and psychiatric problems.
Family physicians (FPs) have optimal skills for navigating the chronic risks and health concerns of the well cancer survivor. This article reviews the primary care management of the functional cancer survivor, focusing on the management of chronic conditions and preventive care.
Survivorship follows any of 6 paths
Cancer survivorship is increasing in importance as treatment has steadily reduced mortality. Six trajectories of cancer survivors have been identified1:
- living cancer-free after treatment with minimal effects
- living cancer-free but suffering serious treatment complications
- Suffering late recurrence
- Developing a second cancer
- Living with intermittent cancer recurrences
- Living with cancer continuously.
Only patients in the last 2 groups are likely to be managed primarily by oncologists.
Survivors look to their FPs for ongoing care
Cancer survivors routinely see their primary care physician after initial treatment. A study of 30,000 Canadian breast cancer survivors demonstrated that follow-up care was limited to an oncologist in only 2%; 84% saw a primary care provider and an oncologist; and 14% saw a primary care provider only.4 A study of colorectal cancer survivors showed that primary care visits increased in each of the 5 years after diagnosis, during which time oncology visits decreased steadily5; in that study, primary care physicians delivered more preventive care than oncologists did.5 Similar to what is done in other chronic conditions, the various effects of cancer are best managed as a whole.
The IOM recommends that cancer survivor care comprise 4 elements2:
- coordination between oncologist and primary care physician
- surveillance for recurrence or spread of existing cancer
- screening for new cancer
- intervention for the effects of cancer and treatment.
Continue to: The following discussion summarizes...
The following discussion summarizes evidence and recommendations for each element of the IOM recommendations for survivor care.
Implementing the 4 elements of cancer survivor care
1. Coordinate care through a unified survivorship care plan
The IOM has noted that the needs of cancer survivors are rarely met2; communication between oncology and primary care is often deficient during transition of care. The IOM has recommended that oncologists provide a survivorship care plan that details the cancer (ie, tumor characteristics), the type of treatment (ie, enrollment in a clinical trial; medical, surgical, or radiation), support services, and follow-up recommendations for the primary care provider. (Examples of elements of a survivorship care plan can be found at www.mskcc.org/hcp-education-training/survivorship/survivorship-care-plan6 and http://sma.org/southern-medical-journal/article/cancer-survivors-history-physical/7).
Regrettably, survivorship care plans have been rarely and poorly employed. Studies show that fewer than one-half of oncologists provide a plan, and that when they do, the plan often lacks recommended information.8,9 Survivorship care plans may soon become common practice, however; the Commission on Cancer of the American College of Surgeons has required their use in all certified cancer centers since 2015.10
2. Provide surveillance of existing cancer
Cancer follow-up is challenging after the initial treatment phase. Although there are many conflicting guidelines for surveillance after cancer, guidelines of the National Comprehensive Cancer Network (NCCN) (summarized in TABLE 211 for the 10 most common cancers in survivors) are the ones generally accepted.12,13
Although individual surveillance recommendations are based on limited evidence, studies confirm the importance of surveillance. A systematic review showed that surveillance mammography after breast cancer reduces breast cancer mortality by 36%.14 A study showed that bladder cancer recurrence diagnosed by surveillance instead of by symptoms led to a 35% increase in 5-year survival.15
Continue to: Yet adherence to cancer surveillance...
Yet adherence to cancer surveillance recommendations is poor. A study of patients with colon cancer demonstrated that only 12% met all recommended surveillance guidelines.16 A study of patients with bladder cancer after radical cystectomy showed that only 9% met recommended surveillance more than 2 years after diagnosis.17 Those dismal statistics may be the result of provider oversight—not patient reluctance.
In the colon cancer study, for example, compliance with follow-up colonoscopy was 80% but compliance with carcinoembryonic antigen testing was only 22%.16 In the bladder cancer study, follow-up urine cytology was obtained in only 23% of patients, although 75% completed recommended imaging.17
Although surveillance remains the oncologist’s responsibility, visits to the FP provide an opportunity to review surveillance and order needed laboratory testing and other studies, including imaging.
3. Screen for new cancers
The risk of a second cancer is elevated for cancer survivors compared with the risk of a primary cancer in the healthy general population; some survivors have a lifetime risk of a second cancer as high as 36%.18 Risk varies by cancer type (TABLE 319). Some of this variation is due to the impact of smoking: Smoking-related cancers have the highest risk of second malignancy.19 Genetic predisposition to malignant transformation is also theorized to contribute to increased risk. Second malignancies are dangerous; 55% of patients die of the second cancer compared with only 13% of their initial cancer.19
Studies show that cancer survivors display varying adherence with recommended screening for second cancers. In a study of Latina cancer survivors, depressive symptoms were associated with lower screening compliance.20 A study of survivors of hematologic cancer showed a low rate of cancer screening and high fear of cancer recurrence—suggesting avoidance due to fear.21 Other studies, however, show similar or increased compliance with screening in cancer survivors.22,23 A meta-analysis of 19 studies determined that, overall, cancer survivors receive 25% to 38% more recommended screening than the general population.24
Continue to: Few guidelines exist to guide FPs...
Few guidelines exist to guide FPs in adjusting screening for the cancer survivor. For women who received radiation therapy for a tumor in the chest, for example, the recommendation offered by several groups is to start breast cancer screening 8 to 10 years after treatment or by 30 years of age, and to consider combining magnetic resonance imaging and mammography.25 Recommendations for breast cancer screening do not account for a history of other gynecologic cancers unless genetic markers are present.25 On the other hand, the impact of a history of cancer on the risk of prostate cancer and on screening decisions has not been studied,26 and cervical cancer screening guidelines, which recommend that screening continue after 65 years of age for patients who are immunocompromised, do not address a history of other cancer.27
4. Manage the effects of both the cancer and the treatment
Medical issues faced by cancer survivors are familiar to FPs, but there are some specific recommendations regarding evaluation and treatment that stand in contrast to what would be considered for a healthy, or non-cancer, patient. For example, each chemotherapeutic agent has characteristic adverse effects; TABLE 47 lists the principal adverse effects of common agents and recommendations for testing when these problems develop. Common long-term problems in cancer survivors include fatigue, chronic pain, cognitive dysfunction, psychiatric illness, and cardiovascular disease. Although these symptoms and manifestations are common, the physician must be careful: New or changing symptoms could signal the spread or recurrence of disease. Fear of recurrence can lead patients to exaggerate or minimize symptoms.
Fatigue is the most common symptom seen in cancer survivors during treatment and following remission.28 More than 40% of cancer survivors report significant fatigue.29 Although fatigue is concerning for cancer recurrence, other causes are common in cancer survivors. Both depression and anxiety commonly present with worsened fatigue.30 Sleep disturbances are common, even without a psychiatric diagnosis.31 Effects of treatment, including nausea, anemia, heart failure, and medication adverse effects can cause or worsen fatigue. Pain is associated with fatigue, but to a lesser extent than are depression, anxiety, and nausea.32
Pharmacotherapy of cancer-related fatigue is challenging. Psychostimulants have been most studied. A recent systematic review shows that methylphenidate produces mild or moderate improvement in fatigue, whereas modafanil has minimal effectiveness.33 Antidepressants have not been shown to relieve fatigue.33
A recent meta-analysis showed that nonpharmaceutical treatments for cancer-related fatigue are more effective than pharmacotherapy. In this review, both exercise and pharmacotherapy had a mild-to-moderate effect on fatigue.35 Exercise is best studied in this regard, and has shown the most consistent results.31
Continue to: Chronic pain
Chronic pain. Pain is common in cancer survivors: As many as 40% experience pain for years after initial therapy.36 Treatment of some cancers—eg, thoracotomy (80%), amputation (50%-80%), neck dissection (52%), and surgical management of breast cancer (63%)—increase the likelihood of chronic pain.37 Reports of pain in cancer survivors that should be considered red flags that might signal recurrence of cancer include new or worsening pain; pain worse at night or when recumbent; new neurologic symptoms; and general symptoms of systemic illness37 (TABLE 537).
Management of pain is best approached by its cause, with neurologic, rheumatologic (including myofascial pain and arthralgia), lymphatic, and genital causes most common.37 Across all types of pain, complete relief is unlikely; functional goals provide a more effective target.
For neuropathic cancer pain, duloxetine is the only medication with evidence of benefit; anticonvulsant and topical medications are recommended on the basis of the findings of studies of noncancer pain.38 There are few data on the value of treatments for cancer-related rheumatologic and lymphatic pain, although exercise has shown benefit in both types.38 For dyspareunia and sexual dysfunction (common after gynecologic and nongynecologic cancers), vaginal lubricants and pelvic-floor physiotherapy have shown benefit.39 There is significant overlap in psychiatric comorbidities, sleep, and pain, and addressing all of a patient’s problems can reduce pain and improve function.40
Opioids are often prescribed for pain in cancer survivors. Cancer survivors have a higher rate of opioid prescribing compared with that of non-cancer patients, even 10 years after diagnosis.41 Guidelines of the Centers for Disease Control and Prevention for using opioids to manage chronic pain specifically exclude cancer patients.42 Regrettably, there is no evidence that opioids have long-term efficacy in chronic pain; in fact, evidence is accumulating that chronic opioid therapy exacerbates chronic pain.43
Cognitive dysfunction is present in 17% to 75% of cancer survivors as memory disturbance, psychological disorder, sleep dysfunction, or impairment of executive functioning.44 Cognitive deficits appear to be secondary to both cancer and treatment modalities45; as many as one-third of patients have cognitive dysfunction prior to receiving chemotherapy.46
Continue to: Chemotherapies that are more likely...
Chemotherapies that are more likely to cause cognitive symptoms include methotrexate, 5-fluorouracil, cyclophosphamide, and hormone antagonists.47 More powerful regimens and repetitive chemotherapy regimens tend to cause more cognitive effects.47
Cognitive training interventions show evidence of likely benefit,44,48 leading to recommendations for self-treatment strategies, such as written lists, wordplay, crossword puzzles, jigsaw puzzles, playing a musical instrument, and new hobbies. Small studies suggest a benefit from cognitive behavioral therapy.44,49 A study of breast cancer survivors showed that yoga led to improvement in patient-reported cognitive dysfunction.50 Physical exercise yields cognitive benefit in healthy older adults and is supported by limited evidence in cancer survivors.51
There is no effective pharmacotherapy for cancer- and cancer chemotherapy-related cognitive dysfunction unless a treatable underlying cause is found.44 Symptoms tend to subside with time after completion of chemotherapy, which might be reassuring to patients and families.45
Psychiatric problems. The most common psychiatric issues in cancer survivors are anxiety and depression; the prevalence of anxiety is nearly double that of depression.52 Anxiety often presents as fear of a recurrence of cancer or a feeling of lack of control over present or future circumstances.53 Screening for anxiety and depression is recommended at each visit, using standardized screening questionnaires.54
A small study suggests that psychiatric treatment reduces the risk of early mortality.55 Small studies also suggest that mindfulness-based therapy and cognitive behavioral therapy delivered by telehealth offer benefit.56 A meta-analysis shows that exercise interventions improve depression and anxiety in breast cancer patients.57
Continue to: There are few studies of pharmacotherapy...
There are few studies of pharmacotherapy of anxiety or depression in cancer survivors56; it is known that cancer survivors are nearly twice as likely as the general population to be taking medical therapy for anxiety and depression.58 A Cochrane systematic review of 7 small studies showed uncertain improvement in depressive symptoms in patients with cancer from antidepressant medication; however, an earlier systematic review did show benefit.59,60
In a trial of patients without depression who were being treated for head and neck cancer, escitalopram, 20 mg/d, reduced the risk of subsequent depression compared with placebo.61 A study of 420 breast cancer survivors showed that 300 mg/d and 900 mg/d dosages of gabapentin were both superior to placebo, and nearly equivalent to each other, at reducing anxiety scores.62 In both studies, however, the evidence is nonetheless insufficient to make specific recommendations about these medications.
Cardiac risk
Among chemotherapeutic agents, anthracyclines, such as doxorubicin, cause the most rapid and striking myocyte damage. This damage is dose-dependent and nearly irreversible, with 98% of injury occurring within the first year of chemotherapy.64 More than one half of cancer patients taking an anthracycline have cardiac dysfunction on imaging; 5% will be in overt heart failure 10 to 20 years, or longer, after chemotherapy.63 Following monitoring at 1 year post-therapy, regular cardiac imaging is not recommended in the absence of symptoms.62
Because other cardiotoxic chemotherapeutic agents cause partially reversible damage, imaging is not recommended in the absence of symptoms in patients taking those agents.64
Continue to: Radiation therapy to the chest leads...
Radiation therapy to the chest leads to many cardiac complications, including cardiomyopathy, valvular disease, pericardial disease, and arrhythmias. Development of cardiomyopathy can be delayed 20 to 30 years after radiation; screening echocardiography is therefore recommended every 5 to 10 years after radiation therapy.65 Recent adjustments to the dosages and delivery of radiation therapy should reduce cardiac damage, but will require decades to validate.63
For patients at risk of cardiovascular disease prior to treatment of cancer, there is evidence to support preventive treatment with angiotensin II-receptor antagonists, beta-blockers, and statins to prevent cardiomyopathy.63 Treatment of diagnosed cardiomyopathy and heart failure follows standard guidelines, with significant emphasis on aerobic exercise and smoking cessation.63
Cancer survivorship care: Your critical role
Cancer survivors constitute a large population who frequent the practices of primary care physicians. Primary care visits provide an opportunity to monitor key elements of survivorship, including surveillance of the current cancer and screening for second cancers. Similar to what is seen with diabetes and coronary artery disease, cancer increases cardiac risk, which requires preventive care and chronic management. FPs are well placed to treat common issues in cancer survivors—issues that mirror concerns seen in the general population.
CORRESPONDENCE
Michael J. Arnold, MD, CDR, USN, Uniformed Services University of the Health Sciences, 4301 Jones Bridge Road, Bethesda, MD 20814; michael.arnold@usuhs.edu.
ACKNOWLEDGEMENT
Kristian Sanchack, MD, and James Higgins, DO, assisted with the editing of the manuscript.
1. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2016-2017. Atlanta, GA: American Cancer Society; 2016. www.cancer.org/research/cancer-facts-statistics/survivor-facts-figures.html. Accessed July 25, 2018.
2. Survivorship. NCCN Guidelines (version 1.2017). Fort Washington, PA: National Comprehensive Cancer Network; 2017. www.nccn.org/professionals/physician_gls/default.aspx#supportive. Accessed July 26, 2018.
3. Kendall C, Decker KM, Groome PA, et al. Use of physician services during the survivorship phase: a multi-province study of women diagnosed with breast cancer. Curr Oncolog. 2017;24:81-89.
4. Snyder CF, Earle CC, Herbert RJ, et al. Preventive care for colorectal cancer survivors: a 5-year longitudinal study. J Clin Oncol. 2008;26:1073-1079.
5. Hewitt M, Greenfield S, Stovall E (eds); Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington DC: The National Academies Press; 2006. www.nap.edu/read/11468/chapter/1. Accessed July 25, 2018.
6. Survivorship care plan. New York, NY: Memorial Sloan Kettering Cancer Center. www.mskcc.org/hcp-education-training/survivorship/survivorship-care-plan. Accessed August 11, 2018.
7. Fuentes AC, Lambird JE, George TJ, et al. Cancer survivor’s history and physical. South Med J. 2017;110:37-44. http://sma.org/southern-medical-journal/article/cancer-survivors-history-physical/. Accessed July 26, 2018.
8. Salz T, Oeffinger KC, McCabe MS, et al. Survivorship care plans in research and practice. CA Cancer J Clin. 2012;62:101-117.
9. Birken SA, Mayer DK, Weiner BJ. Survivorship care plans: prevalence and barriers to use. J Cancer Educ. 2013;28:290-296.
10. American College of Surgeons Commission on Cancer. Cancer program standards 2012: Ensuring patient-centered care. V1.2.1. www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed July 25, 2018.
11. NCCN guidelines for treatment of cancer by site. NCCN Guidelines (version 1.2018). Fort Washington, PA: National Comprehensive Cancer Network; 2018. www.nccn.org/professionals/physician_gls/default.aspx#site. Accessed July 25, 2018.
12. Spronka I, Korevaar JC, Burgers JS, et al. Review of guidance on recurrence risk management for general practitioners in breast cancer, colorectal cancer and melanoma guidelines. Family Pract. 2017;34:154-160.
13. Merkow RP, Korenstein D, Yeahia R, et al. Quality of cancer surveillance clinical practice guidelines: specificity and consistency of recommendations. JAMA Intern Med. 2017;177:701-709.
14. Muradali D, Kennedy EB, Eisen A, et al. Breast screening for survivors of breast cancer: a systematic review. Prev Med. 2017;103:70-75.
15. Giannarini G, Kessler TM, Thoeny HC, et al. Do patients benefit from routine follow-up to detect recurrences after radical cystectomy and ileal orthotopic bladder substitution? Eur Urol. 2010;58:486-494.
16. Sisler JJ, Seo B, Katz A, et al. Concordance with ASCO guidelines for surveillance after colorectal cancer treatment: a population-based analysis. J Oncol Pract. 2012;8:e69-e79.
17. Ehdaie B. Atoria CL, Lowrance WT, et al. Adherence to surveillance guidelines after radical cystectomy: a population-based analysis. Urol Oncol. 2014;32:779-784.
18. Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354-1365.
19. Donin N, Filson C, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer. 2016;122:3075-3086.
20. Holder AE, Ramirez AG, Gallion K. Depressive symptoms in Latina breast cancer survivors: a barrier to cancer screening. Health Psycholog. 2014;33:242-248.
21. Dyer G, Larsen SR, Gilroy N, et al. Adherence to cancer screening guidelines in Australian survivors of allogenic blood and marrow transplantation (BMT). Cancer Med. 2016;5:1702-1716.
22. Mandelzweig L, Chetrit A, Amitai T, et al. Primary prevention and screening practices among long-term breast cancer survivors. Cancer Causes Control. 2017;28:657-666.
23. Bishop MM, Lee SJ, Beaumont JL, et al. The preventive health behaviors of long-term survivors of cancer and hematopoietic stem cell transplantation compared with matched controls. Biol Blood Marrow Transplant. 2010;16:207-214.
24. Uhlig A, Mei J, Baik I, et al. Screening utilization among cancer survivors: a meta-analysis. J Public Health (Oxf). 2018;40:129-137.
25. Hilal T, Rudy DW. Radiation-induced breast cancer: the question of early breast cancer screening in Hodgkin’s lymphoma survivors. Oxf Med Case Reports. 2016;2016:17-18.
26. Lin K, Croswell JM, Koenig H, et al. Prostate-specific antigen-based screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force [Internet]. Evidence Syntheses No. 90. AHRQ Publication No. 12-05160-EF-1. Rockville, MD: Agency for Healthcare Research and Quality (US); October 2011. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0032900/. Accessed July 25, 2018.
27. US Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
28. Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12 Suppl 1:4-10.
29. Jung JY, Lee JM, Kim MS, et al. Comparison of fatigue, depression, and anxiety as factors affecting posttreatment health-related quality of life in lung cancer survivors. Psychooncology. 2018;27:465-470.
30. Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatment. Nat Rev Clin Oncol. 2014;11:597-609.
31. Medysky ME, Temesi J, Culos-Reed SN, et al. Exercise, sleep and cancer-related fatigue: are they related? Neurophysiol Clin. 2017;47:111-122.
32. Oh HS, Sea WS. Systematic review and meta-analysis of the correlates of cancer-related fatigue. Worldviews Evid Based Nurs. 2011;8:191-201.
33. Qu D, Zhang Z, Yu X, et al. Psychotropic drugs for the management of cancer-related fatigue: a systematic review and meta-analysis. Eur J Cancer Care (Engl). 2016;25:970-979.
34. Escalante CP, Manzullo EF. Cancer-related fatigue: the approach and treatment. J Gen Intern Med. 2009;24(suppl 2):S412-S416.
35. Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3:961-968.
36. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32:1739-1747.
37. Davies PS. Chronic pain management in the cancer survivor: tips for primary care providers. Nurse Pract. 2013;39:28-38.
38. Boland EG, Ahmedzai SH. Persistent pain in cancer survivors. Curr Opin Support Palliat Care. 2017;11:181-190.
39. Sears CS, Robinson JW, Walker LM. A comprehensive review of sexual health concerns after cancer treatment and the biopsychosocial treatment options available to female patients. Eur J Cancer Care (Engl). 2017;27:e12738.
40. Schou Bredal I, Smeby NA, Ottesen S, et al. Chronic pain in breast cancer survivors: comparison of psychological, surgical, and medical characteristics between survivors with and without pain. J Pain Symptom Manage. 2014;48:852-862.
41. Sutradhar R, Lokku A, Barbera L. Cancer survivorship and opioid prescribing rates: a population-based matched cohort study among individuals with and without a history of cancer. Cancer. 2017;123:4286-4293.
42. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
43. Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep. 2016;18:71.
44. Von Ah D. Cognitive changes associated with cancer and cancer treatment: state of the science. Clin J Oncol Nurs. 2015;19:47-56.
45. Moore HC. An overview of chemotherapy-related cognitive dysfunction, or ‘chemobrain’. Oncology (Williston Park). 2014;28:797-804.
46. Asher A. Cognitive dysfunction among cancer survivors. Am J Phys Med Rehabil. 2011;90(suppl):S16-S26.
47. Joly F, Rigal O, Noal S, et al. Cognitive dysfunction and cancer: which consequences in terms of disease management? Psychooncology. 2011;20:1251-1258.
48. Attention, thinking or memory problems. American Society of Clinical Oncology Cancer.Net. April 2018. www.cancer.net/navigating-cancer-care/side-effects/attention-thinking-or-memory-problems. Accessed July 25, 2018.
49. Kucherer S, Ferguson RJ. Cognitive behavioral therapy for cancer-related cognitive dysfunction. Curr Opin Support Palliat Care. 2017;11:46-51.
50. Derry HM, Jaremka LM, Bennet JM, et al. Yoga and self-reported cognitive problems in breast cancer survivors: a randomized controlled trial. Psychooncology. 2015;24:958-966.
51. Treanor CJ, McMenamin UC, O’Neill RF, et al. Non-pharmacological interventions for cognitive impairment due to systemic cancer treatment. Cochrane Database Syst Rev. 2016 Aug 16;(8):CD011325.
52. Mitchell AJ, Ferguson DW, Gill J, et al. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. Lancet Oncol. 2013;14:721-732.
53. Inhestern L, Beierlein V, Bultmann JC, et al. Anxiety and depression in working-age cancer survivors: a register-based study. BMC Cancer. 2017;17:347.
54. Partridge AH, Jacobsen PB, Andersen BL. Challenges to standardizing the care for adult cancer survivors: highlighting ASCO’s fatigue and anxiety and depression guidelines. Am Soc Clin Oncol Educ Book. 2015:188-194.
55. Andersen BL, Yang HC, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer. 2008;113:3450-3458.
56. Yi JC, Syrjala KL. Anxiety and depression in cancer survivors. Med Clin N Am. 2017;101:1099-1113.
57. Zhu G, Zhang X, Wang Y, et al. Effects of exercise intervention in breast cancer survivors: a meta-analysis of 33 randomized controlled trials. Onco Targets Ther. 2016;9:2153-2168.
58. Hawkins NA, Soman A, Lunsford N, et al. Use of medications for treating anxiety and depression in cancer survivors in the United States. J Clin Oncol. 2017;35:78-85.
59. Ostuzzi G, Matcham F, Dauchy S, et al. Antidepressants for the treatment of depression in people with cancer. Cochrane Database Syst Rev. 2015 June 1;(6):CD011006.
60. Laoutidis ZG, Mathiak K. Antidepressants in the treatment of depression/depressive symptoms in cancer patients: a systematic review and meta-analysis. BMC Psychiatry. 2013;13:140.
61. Lydiatt WM, Bessette D, Schmid KK, et al. Prevention of depression with escitalopram in patients undergoing treatment for head and neck cancer: randomized, double-blind, placebo-controlled clinical trial. JAMA Otolaryngol Head Neck Surg. 2013;139:678-686.
62. Lavigne JE, Heckler C, Mathews JL, et al. A randomized, controlled, double-blinded clinical trial of gabapentin 300 versus 900 mg versus placebo for anxiety symptoms in breast cancer survivors. Breast Cancer Res Treat. 2012;136:479-486.
63. Okwuosa TM, Anzevino S, Rao R. Cardiovascular disease in cancer survivors. Postgrad Med J. 2017;93:82-90.
64. Plana, JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911-939.
65. Lancellotti, P, Nkomo VT, Badano LP, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:1013-1032.
1. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2016-2017. Atlanta, GA: American Cancer Society; 2016. www.cancer.org/research/cancer-facts-statistics/survivor-facts-figures.html. Accessed July 25, 2018.
2. Survivorship. NCCN Guidelines (version 1.2017). Fort Washington, PA: National Comprehensive Cancer Network; 2017. www.nccn.org/professionals/physician_gls/default.aspx#supportive. Accessed July 26, 2018.
3. Kendall C, Decker KM, Groome PA, et al. Use of physician services during the survivorship phase: a multi-province study of women diagnosed with breast cancer. Curr Oncolog. 2017;24:81-89.
4. Snyder CF, Earle CC, Herbert RJ, et al. Preventive care for colorectal cancer survivors: a 5-year longitudinal study. J Clin Oncol. 2008;26:1073-1079.
5. Hewitt M, Greenfield S, Stovall E (eds); Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington DC: The National Academies Press; 2006. www.nap.edu/read/11468/chapter/1. Accessed July 25, 2018.
6. Survivorship care plan. New York, NY: Memorial Sloan Kettering Cancer Center. www.mskcc.org/hcp-education-training/survivorship/survivorship-care-plan. Accessed August 11, 2018.
7. Fuentes AC, Lambird JE, George TJ, et al. Cancer survivor’s history and physical. South Med J. 2017;110:37-44. http://sma.org/southern-medical-journal/article/cancer-survivors-history-physical/. Accessed July 26, 2018.
8. Salz T, Oeffinger KC, McCabe MS, et al. Survivorship care plans in research and practice. CA Cancer J Clin. 2012;62:101-117.
9. Birken SA, Mayer DK, Weiner BJ. Survivorship care plans: prevalence and barriers to use. J Cancer Educ. 2013;28:290-296.
10. American College of Surgeons Commission on Cancer. Cancer program standards 2012: Ensuring patient-centered care. V1.2.1. www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Accessed July 25, 2018.
11. NCCN guidelines for treatment of cancer by site. NCCN Guidelines (version 1.2018). Fort Washington, PA: National Comprehensive Cancer Network; 2018. www.nccn.org/professionals/physician_gls/default.aspx#site. Accessed July 25, 2018.
12. Spronka I, Korevaar JC, Burgers JS, et al. Review of guidance on recurrence risk management for general practitioners in breast cancer, colorectal cancer and melanoma guidelines. Family Pract. 2017;34:154-160.
13. Merkow RP, Korenstein D, Yeahia R, et al. Quality of cancer surveillance clinical practice guidelines: specificity and consistency of recommendations. JAMA Intern Med. 2017;177:701-709.
14. Muradali D, Kennedy EB, Eisen A, et al. Breast screening for survivors of breast cancer: a systematic review. Prev Med. 2017;103:70-75.
15. Giannarini G, Kessler TM, Thoeny HC, et al. Do patients benefit from routine follow-up to detect recurrences after radical cystectomy and ileal orthotopic bladder substitution? Eur Urol. 2010;58:486-494.
16. Sisler JJ, Seo B, Katz A, et al. Concordance with ASCO guidelines for surveillance after colorectal cancer treatment: a population-based analysis. J Oncol Pract. 2012;8:e69-e79.
17. Ehdaie B. Atoria CL, Lowrance WT, et al. Adherence to surveillance guidelines after radical cystectomy: a population-based analysis. Urol Oncol. 2014;32:779-784.
18. Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354-1365.
19. Donin N, Filson C, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer. 2016;122:3075-3086.
20. Holder AE, Ramirez AG, Gallion K. Depressive symptoms in Latina breast cancer survivors: a barrier to cancer screening. Health Psycholog. 2014;33:242-248.
21. Dyer G, Larsen SR, Gilroy N, et al. Adherence to cancer screening guidelines in Australian survivors of allogenic blood and marrow transplantation (BMT). Cancer Med. 2016;5:1702-1716.
22. Mandelzweig L, Chetrit A, Amitai T, et al. Primary prevention and screening practices among long-term breast cancer survivors. Cancer Causes Control. 2017;28:657-666.
23. Bishop MM, Lee SJ, Beaumont JL, et al. The preventive health behaviors of long-term survivors of cancer and hematopoietic stem cell transplantation compared with matched controls. Biol Blood Marrow Transplant. 2010;16:207-214.
24. Uhlig A, Mei J, Baik I, et al. Screening utilization among cancer survivors: a meta-analysis. J Public Health (Oxf). 2018;40:129-137.
25. Hilal T, Rudy DW. Radiation-induced breast cancer: the question of early breast cancer screening in Hodgkin’s lymphoma survivors. Oxf Med Case Reports. 2016;2016:17-18.
26. Lin K, Croswell JM, Koenig H, et al. Prostate-specific antigen-based screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force [Internet]. Evidence Syntheses No. 90. AHRQ Publication No. 12-05160-EF-1. Rockville, MD: Agency for Healthcare Research and Quality (US); October 2011. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0032900/. Accessed July 25, 2018.
27. US Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. JAMA. 2018;320:674-686.
28. Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12 Suppl 1:4-10.
29. Jung JY, Lee JM, Kim MS, et al. Comparison of fatigue, depression, and anxiety as factors affecting posttreatment health-related quality of life in lung cancer survivors. Psychooncology. 2018;27:465-470.
30. Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatment. Nat Rev Clin Oncol. 2014;11:597-609.
31. Medysky ME, Temesi J, Culos-Reed SN, et al. Exercise, sleep and cancer-related fatigue: are they related? Neurophysiol Clin. 2017;47:111-122.
32. Oh HS, Sea WS. Systematic review and meta-analysis of the correlates of cancer-related fatigue. Worldviews Evid Based Nurs. 2011;8:191-201.
33. Qu D, Zhang Z, Yu X, et al. Psychotropic drugs for the management of cancer-related fatigue: a systematic review and meta-analysis. Eur J Cancer Care (Engl). 2016;25:970-979.
34. Escalante CP, Manzullo EF. Cancer-related fatigue: the approach and treatment. J Gen Intern Med. 2009;24(suppl 2):S412-S416.
35. Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3:961-968.
36. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32:1739-1747.
37. Davies PS. Chronic pain management in the cancer survivor: tips for primary care providers. Nurse Pract. 2013;39:28-38.
38. Boland EG, Ahmedzai SH. Persistent pain in cancer survivors. Curr Opin Support Palliat Care. 2017;11:181-190.
39. Sears CS, Robinson JW, Walker LM. A comprehensive review of sexual health concerns after cancer treatment and the biopsychosocial treatment options available to female patients. Eur J Cancer Care (Engl). 2017;27:e12738.
40. Schou Bredal I, Smeby NA, Ottesen S, et al. Chronic pain in breast cancer survivors: comparison of psychological, surgical, and medical characteristics between survivors with and without pain. J Pain Symptom Manage. 2014;48:852-862.
41. Sutradhar R, Lokku A, Barbera L. Cancer survivorship and opioid prescribing rates: a population-based matched cohort study among individuals with and without a history of cancer. Cancer. 2017;123:4286-4293.
42. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
43. Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep. 2016;18:71.
44. Von Ah D. Cognitive changes associated with cancer and cancer treatment: state of the science. Clin J Oncol Nurs. 2015;19:47-56.
45. Moore HC. An overview of chemotherapy-related cognitive dysfunction, or ‘chemobrain’. Oncology (Williston Park). 2014;28:797-804.
46. Asher A. Cognitive dysfunction among cancer survivors. Am J Phys Med Rehabil. 2011;90(suppl):S16-S26.
47. Joly F, Rigal O, Noal S, et al. Cognitive dysfunction and cancer: which consequences in terms of disease management? Psychooncology. 2011;20:1251-1258.
48. Attention, thinking or memory problems. American Society of Clinical Oncology Cancer.Net. April 2018. www.cancer.net/navigating-cancer-care/side-effects/attention-thinking-or-memory-problems. Accessed July 25, 2018.
49. Kucherer S, Ferguson RJ. Cognitive behavioral therapy for cancer-related cognitive dysfunction. Curr Opin Support Palliat Care. 2017;11:46-51.
50. Derry HM, Jaremka LM, Bennet JM, et al. Yoga and self-reported cognitive problems in breast cancer survivors: a randomized controlled trial. Psychooncology. 2015;24:958-966.
51. Treanor CJ, McMenamin UC, O’Neill RF, et al. Non-pharmacological interventions for cognitive impairment due to systemic cancer treatment. Cochrane Database Syst Rev. 2016 Aug 16;(8):CD011325.
52. Mitchell AJ, Ferguson DW, Gill J, et al. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. Lancet Oncol. 2013;14:721-732.
53. Inhestern L, Beierlein V, Bultmann JC, et al. Anxiety and depression in working-age cancer survivors: a register-based study. BMC Cancer. 2017;17:347.
54. Partridge AH, Jacobsen PB, Andersen BL. Challenges to standardizing the care for adult cancer survivors: highlighting ASCO’s fatigue and anxiety and depression guidelines. Am Soc Clin Oncol Educ Book. 2015:188-194.
55. Andersen BL, Yang HC, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer. 2008;113:3450-3458.
56. Yi JC, Syrjala KL. Anxiety and depression in cancer survivors. Med Clin N Am. 2017;101:1099-1113.
57. Zhu G, Zhang X, Wang Y, et al. Effects of exercise intervention in breast cancer survivors: a meta-analysis of 33 randomized controlled trials. Onco Targets Ther. 2016;9:2153-2168.
58. Hawkins NA, Soman A, Lunsford N, et al. Use of medications for treating anxiety and depression in cancer survivors in the United States. J Clin Oncol. 2017;35:78-85.
59. Ostuzzi G, Matcham F, Dauchy S, et al. Antidepressants for the treatment of depression in people with cancer. Cochrane Database Syst Rev. 2015 June 1;(6):CD011006.
60. Laoutidis ZG, Mathiak K. Antidepressants in the treatment of depression/depressive symptoms in cancer patients: a systematic review and meta-analysis. BMC Psychiatry. 2013;13:140.
61. Lydiatt WM, Bessette D, Schmid KK, et al. Prevention of depression with escitalopram in patients undergoing treatment for head and neck cancer: randomized, double-blind, placebo-controlled clinical trial. JAMA Otolaryngol Head Neck Surg. 2013;139:678-686.
62. Lavigne JE, Heckler C, Mathews JL, et al. A randomized, controlled, double-blinded clinical trial of gabapentin 300 versus 900 mg versus placebo for anxiety symptoms in breast cancer survivors. Breast Cancer Res Treat. 2012;136:479-486.
63. Okwuosa TM, Anzevino S, Rao R. Cardiovascular disease in cancer survivors. Postgrad Med J. 2017;93:82-90.
64. Plana, JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911-939.
65. Lancellotti, P, Nkomo VT, Badano LP, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:1013-1032.
PRACTICE RECOMMENDATIONS
› Provide normal age-related cancer screening for cancer survivors because of their high risk of a second cancer. B
› Strongly encourage lifestyle changes for cancer survivors, especially smoking cessation. B
› Recommend exercise, which alleviates pain, depression, anxiety, and (more effectively than any other intervention) fatigue, for cancer survivors. B
› Remain vigilant for the development in cancer survivors of cardiovascular disease, including heart failure, which can appear long after therapy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
*Cancer survivor care in the pediatric patients, including application of a survivorship care plan (also discussed later in this article), is reviewed in “Partnering to optimize care of childhood cancer survivors,” The Journal of Family Practice, April 2017.